User login
Patient-reported outcomes for patients with chronic liver disease
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).
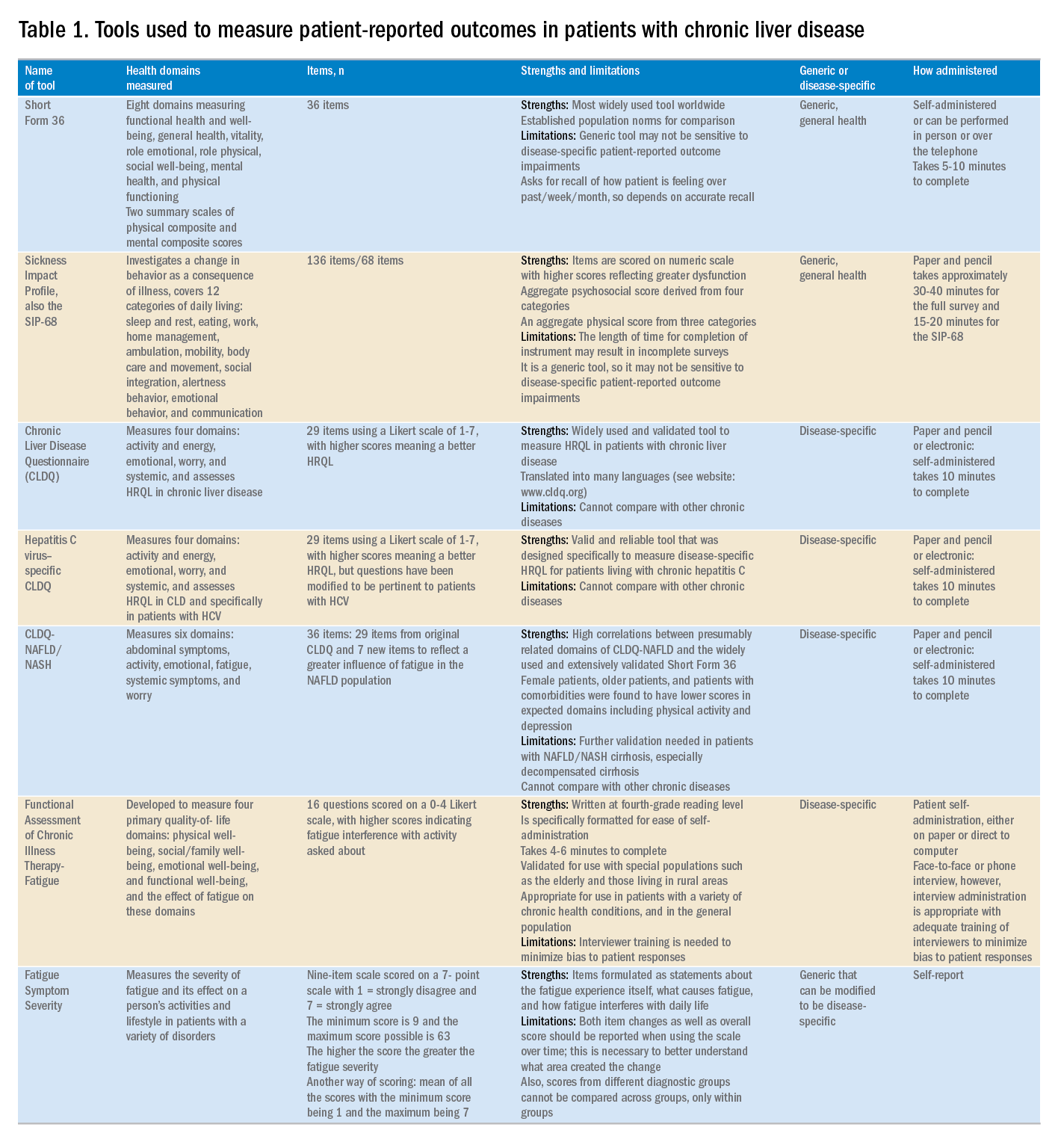
Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).

Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).

Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Biomarker algorithm may offer noninvasive look at liver fibrosis
Serum biomarkers may enable a noninvasive method of detecting advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD), according to results from a recent study.
An algorithm created by the investigators distinguished NAFLD patients with advanced liver fibrosis from those with mild to moderate fibrosis, reported lead author Rohit Loomba, MD, of the University of California at San Diego and his colleagues.
“Liver biopsy is currently the gold standard for diagnosing NASH [nonalcoholic steatohepatitis] and staging liver fibrosis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, it is a costly and invasive procedure with an all-cause mortality risk of approximately 0.2%. Liver biopsy typically samples only 1/50,000th of the organ, and it is liable to sampling error with an error rate of 25% for diagnosis of hepatic fibrosis.”
Existing serum-based tests are reliable for diagnosing nonfibrotic NAFLD, but they may misdiagnosis patients with advanced fibrosis. Although imaging-based techniques may provide better diagnostic accuracy, some are available only for subgroups of patients, while others come with a high financial burden. Diagnostic shortcomings may have a major effect on patient outcomes, particularly when risk groups are considered.
“Fibrosis stages F3 and F4 (advanced fibrosis) are primary predictors of liver-related morbidity and mortality, with 11%-22% of NASH patients reported to have advanced fibrosis,” the investigators noted.
The investigators therefore aimed to distinguish such high-risk NAFLD patients from those with mild or moderate liver fibrosis. Three biomarkers were included: hyaluronic acid (HA), TIMP metallopeptidase inhibitor 1 (TIMP-1), and alpha2-macroglobulin (A2M). Each biomarker has documented associations with liver fibrosis. For instance, higher A2M concentrations inhibit fibrinolysis, HA is associated with excessive extracellular matrix and fibrotic tissue, and TIMP-1 is a known liver fibrosis marker and inhibitor of extracellular matrix degradation. The relative strengths of each in detecting advanced liver fibrosis was determined through an algorithm.
The investigators relied on archived serum samples from Duke University, Durham, N.C., (n = 792) and University of California at San Diego (n = 244) that were collected within 11 days of liver biopsy. Biopsies were performed with 15- to 16-gauge needles using at least eight portal tracts, and these samples were used to diagnose NAFLD. Patients with alcoholic liver disease or hepatitis C virus were excluded.
Algorithm training was based on serum measurements from 396 patients treated at Duke University. Samples were divided into mild to moderate (F0-F2) or advanced (F3-F4) fibrosis and split into 10 subsets. The logical regression model was trained on nine subsets and tested on the 10th, with iterations 10 times through this sequence until all 10 samples were tested. This process was repeated 10,000 times. Using the median coefficients from 100,000 logistical regression models, the samples were scored using the algorithm from 0 to 100, with higher numbers representing more advanced fibrosis, and the relative weights of each biomarker measurement were determined.
A noninferiority protocol was used to validate the algorithm, through which the area under the receiver operating characteristic (AUROC) curve was calculated. The AUROC curve of the validation samples was 0.856, with 0.5 being the score for a random algorithm. The algorithm correctly classified 90.0% of F0 cases, 75.0% of F1 cases, 53.8% of F2 cases, 77.4% of F3 cases, and 94.4% of F4 cases. The sensitivity was 79.7% and the specificity was 75.7%.
The algorithm was superior to Fibrosis-4 (FIB-4) and NAFLD Fibrosis Score (NFS) in two validation cohorts. In a combination of validation cohorts, the algorithm correctly identified 79.5% of F3-F4 patients, compared with rates of 25.8% and 28.0% from FIB-4 and NFS, respectively. The investigators noted that the algorithm was unaffected by sex or age. In contrast, FIB-4 is biased toward females, and both FIB-4 and NFS are less accurate with patients aged 35 years or younger.
“Performance of the training and validation sets was robust and well matched, enabling the reliable differentiation of NAFLD patients with and without advanced fibrosis,” the investigators concluded.
The study was supported by Prometheus Laboratories. Authors not employed by Prometheus Laboratories were employed by Duke University or the University of California, San Diego; each institution received funding from Prometheus Laboratories.
SOURCE: Loomba R et al. Clin Gastroenterol Hepatol. 2018 Nov 15. doi: 10.1016/j.cgh.2018.11.004.
Serum biomarkers may enable a noninvasive method of detecting advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD), according to results from a recent study.
An algorithm created by the investigators distinguished NAFLD patients with advanced liver fibrosis from those with mild to moderate fibrosis, reported lead author Rohit Loomba, MD, of the University of California at San Diego and his colleagues.
“Liver biopsy is currently the gold standard for diagnosing NASH [nonalcoholic steatohepatitis] and staging liver fibrosis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, it is a costly and invasive procedure with an all-cause mortality risk of approximately 0.2%. Liver biopsy typically samples only 1/50,000th of the organ, and it is liable to sampling error with an error rate of 25% for diagnosis of hepatic fibrosis.”
Existing serum-based tests are reliable for diagnosing nonfibrotic NAFLD, but they may misdiagnosis patients with advanced fibrosis. Although imaging-based techniques may provide better diagnostic accuracy, some are available only for subgroups of patients, while others come with a high financial burden. Diagnostic shortcomings may have a major effect on patient outcomes, particularly when risk groups are considered.
“Fibrosis stages F3 and F4 (advanced fibrosis) are primary predictors of liver-related morbidity and mortality, with 11%-22% of NASH patients reported to have advanced fibrosis,” the investigators noted.
The investigators therefore aimed to distinguish such high-risk NAFLD patients from those with mild or moderate liver fibrosis. Three biomarkers were included: hyaluronic acid (HA), TIMP metallopeptidase inhibitor 1 (TIMP-1), and alpha2-macroglobulin (A2M). Each biomarker has documented associations with liver fibrosis. For instance, higher A2M concentrations inhibit fibrinolysis, HA is associated with excessive extracellular matrix and fibrotic tissue, and TIMP-1 is a known liver fibrosis marker and inhibitor of extracellular matrix degradation. The relative strengths of each in detecting advanced liver fibrosis was determined through an algorithm.
The investigators relied on archived serum samples from Duke University, Durham, N.C., (n = 792) and University of California at San Diego (n = 244) that were collected within 11 days of liver biopsy. Biopsies were performed with 15- to 16-gauge needles using at least eight portal tracts, and these samples were used to diagnose NAFLD. Patients with alcoholic liver disease or hepatitis C virus were excluded.
Algorithm training was based on serum measurements from 396 patients treated at Duke University. Samples were divided into mild to moderate (F0-F2) or advanced (F3-F4) fibrosis and split into 10 subsets. The logical regression model was trained on nine subsets and tested on the 10th, with iterations 10 times through this sequence until all 10 samples were tested. This process was repeated 10,000 times. Using the median coefficients from 100,000 logistical regression models, the samples were scored using the algorithm from 0 to 100, with higher numbers representing more advanced fibrosis, and the relative weights of each biomarker measurement were determined.
A noninferiority protocol was used to validate the algorithm, through which the area under the receiver operating characteristic (AUROC) curve was calculated. The AUROC curve of the validation samples was 0.856, with 0.5 being the score for a random algorithm. The algorithm correctly classified 90.0% of F0 cases, 75.0% of F1 cases, 53.8% of F2 cases, 77.4% of F3 cases, and 94.4% of F4 cases. The sensitivity was 79.7% and the specificity was 75.7%.
The algorithm was superior to Fibrosis-4 (FIB-4) and NAFLD Fibrosis Score (NFS) in two validation cohorts. In a combination of validation cohorts, the algorithm correctly identified 79.5% of F3-F4 patients, compared with rates of 25.8% and 28.0% from FIB-4 and NFS, respectively. The investigators noted that the algorithm was unaffected by sex or age. In contrast, FIB-4 is biased toward females, and both FIB-4 and NFS are less accurate with patients aged 35 years or younger.
“Performance of the training and validation sets was robust and well matched, enabling the reliable differentiation of NAFLD patients with and without advanced fibrosis,” the investigators concluded.
The study was supported by Prometheus Laboratories. Authors not employed by Prometheus Laboratories were employed by Duke University or the University of California, San Diego; each institution received funding from Prometheus Laboratories.
SOURCE: Loomba R et al. Clin Gastroenterol Hepatol. 2018 Nov 15. doi: 10.1016/j.cgh.2018.11.004.
Serum biomarkers may enable a noninvasive method of detecting advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD), according to results from a recent study.
An algorithm created by the investigators distinguished NAFLD patients with advanced liver fibrosis from those with mild to moderate fibrosis, reported lead author Rohit Loomba, MD, of the University of California at San Diego and his colleagues.
“Liver biopsy is currently the gold standard for diagnosing NASH [nonalcoholic steatohepatitis] and staging liver fibrosis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, it is a costly and invasive procedure with an all-cause mortality risk of approximately 0.2%. Liver biopsy typically samples only 1/50,000th of the organ, and it is liable to sampling error with an error rate of 25% for diagnosis of hepatic fibrosis.”
Existing serum-based tests are reliable for diagnosing nonfibrotic NAFLD, but they may misdiagnosis patients with advanced fibrosis. Although imaging-based techniques may provide better diagnostic accuracy, some are available only for subgroups of patients, while others come with a high financial burden. Diagnostic shortcomings may have a major effect on patient outcomes, particularly when risk groups are considered.
“Fibrosis stages F3 and F4 (advanced fibrosis) are primary predictors of liver-related morbidity and mortality, with 11%-22% of NASH patients reported to have advanced fibrosis,” the investigators noted.
The investigators therefore aimed to distinguish such high-risk NAFLD patients from those with mild or moderate liver fibrosis. Three biomarkers were included: hyaluronic acid (HA), TIMP metallopeptidase inhibitor 1 (TIMP-1), and alpha2-macroglobulin (A2M). Each biomarker has documented associations with liver fibrosis. For instance, higher A2M concentrations inhibit fibrinolysis, HA is associated with excessive extracellular matrix and fibrotic tissue, and TIMP-1 is a known liver fibrosis marker and inhibitor of extracellular matrix degradation. The relative strengths of each in detecting advanced liver fibrosis was determined through an algorithm.
The investigators relied on archived serum samples from Duke University, Durham, N.C., (n = 792) and University of California at San Diego (n = 244) that were collected within 11 days of liver biopsy. Biopsies were performed with 15- to 16-gauge needles using at least eight portal tracts, and these samples were used to diagnose NAFLD. Patients with alcoholic liver disease or hepatitis C virus were excluded.
Algorithm training was based on serum measurements from 396 patients treated at Duke University. Samples were divided into mild to moderate (F0-F2) or advanced (F3-F4) fibrosis and split into 10 subsets. The logical regression model was trained on nine subsets and tested on the 10th, with iterations 10 times through this sequence until all 10 samples were tested. This process was repeated 10,000 times. Using the median coefficients from 100,000 logistical regression models, the samples were scored using the algorithm from 0 to 100, with higher numbers representing more advanced fibrosis, and the relative weights of each biomarker measurement were determined.
A noninferiority protocol was used to validate the algorithm, through which the area under the receiver operating characteristic (AUROC) curve was calculated. The AUROC curve of the validation samples was 0.856, with 0.5 being the score for a random algorithm. The algorithm correctly classified 90.0% of F0 cases, 75.0% of F1 cases, 53.8% of F2 cases, 77.4% of F3 cases, and 94.4% of F4 cases. The sensitivity was 79.7% and the specificity was 75.7%.
The algorithm was superior to Fibrosis-4 (FIB-4) and NAFLD Fibrosis Score (NFS) in two validation cohorts. In a combination of validation cohorts, the algorithm correctly identified 79.5% of F3-F4 patients, compared with rates of 25.8% and 28.0% from FIB-4 and NFS, respectively. The investigators noted that the algorithm was unaffected by sex or age. In contrast, FIB-4 is biased toward females, and both FIB-4 and NFS are less accurate with patients aged 35 years or younger.
“Performance of the training and validation sets was robust and well matched, enabling the reliable differentiation of NAFLD patients with and without advanced fibrosis,” the investigators concluded.
The study was supported by Prometheus Laboratories. Authors not employed by Prometheus Laboratories were employed by Duke University or the University of California, San Diego; each institution received funding from Prometheus Laboratories.
SOURCE: Loomba R et al. Clin Gastroenterol Hepatol. 2018 Nov 15. doi: 10.1016/j.cgh.2018.11.004.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: A serum biomarker–based algorithm may provide a noninvasive method of detecting advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD).
Major finding: The area under the receiver operator characteristic (AUROC) curve for a combination of validation samples was 0.856.
Study details: A retrospective study of liver fibrosis serum markers and clinical data from 396 patients with NAFLD and various stages of fibrosis.
Disclosures: The study was supported by Prometheus Laboratories. Authors not employed by Prometheus Laboratories were employed by Duke University or the University of California, San Diego; each institution received funding from Prometheus Laboratories.
Source: Loomba R et al. Clin Gastroenterol Hepatol. 2018 Nov 15. doi: 10.1016/j.cgh.2018.11.004.
HCC screening linked with improved tumor detection
“Our study’s aim was to characterize utilization of HCC screening receipt and its association with early tumor detection and improved survival in a nationally representative cohort of patients in the United States,” wrote Debra T. Choi, PhD, MPH, of Baylor College of Medicine, Houston, and her colleagues.
The researchers retrospectively studied a cohort of 13,174 patients with HCC from 2003 to 2013 included in the Surveillance, Epidemiology, and End Results Program–Medicare database. They examined the acquisition of HCC in the 3 years leading up to HCC diagnosis using three separate categories: consistent, inconsistent, or no screening. Dr. Choi and her colleagues studied the associations between receiving HCC screening and subsequent effects on overall survival.
“HCC prognosis depends on tumor stage at the time of diagnosis, with curative treatment options only available for patients diagnosed at an early stage,” the researchers wrote. “Patients with early-stage HCC can achieve 5-year survival rates of 70% if they undergo surgical resection or liver transplantation, compared to a median survival of 1 year for patients with advanced HCC,” they added.
After multivariable analysis, the investigators found that 51.1% of patients with cirrhosis did not receive screening in the 3 years leading up to HCC diagnosis. In addition, they went on to report that only 6.8% of patients were consistently screened on an annual basis.
“HCC screening receipt was associated with early tumor detection and potentially improved overall survival, with attenuated benefits in those with inconsistent screening compared to those who had received consistent screening,” they explained.
In terms of efficacy, consistent screening was associated with an increased rate of early-stage tumor detection (odds ratio, 1.98, 95% confidence interval, 1.68-2.33) and decreased risk of death (hazard ratio, 0.76, 95% CI, 0.70-0.83) after adjustment for lead time bias. Given these results, Dr. Choi and her colleagues said that early HCC screening may help with hepatic tumor detection at later disease stages.
“Given the demonstrated benefits of HCC screening, [it] is an important step to reverse the high rates of late stage diagnosis and poor survival,” they added.
The investigators noted that several patient-specific factors may be driving these associations. In particular, they found a link between female sex and receiving HCC screening. Dr. Choi and her colleagues suggested this association is not related to the perceived benefits from screening.
“Studies have suggested females may be more likely to adhere to screening recommendations; however, patient adherence is not a common barrier to HCC screening completion and therefore it is unclear if this is the sole driver of this association,” they acknowledged.
Moving forward, the researchers highlighted the importance of educating primary care providers about the benefits of screening. Moreover, they said that screening receipt is currently on the rise, which has shown positive effects on overall survival.
The Center for Innovations in Quality, Effectiveness, and Safety funded the study. Additional support was provided by the Texas A&M Health Science Center Engineering Experiment Station big data seed grant program. The authors reported no conflicts of interest.
SOURCE: Choi DT et al. Clin Gastroenterol Hepatol. 2018 Oct 25. doi: 10.1016/j.cgh.2018.10.031.
“Our study’s aim was to characterize utilization of HCC screening receipt and its association with early tumor detection and improved survival in a nationally representative cohort of patients in the United States,” wrote Debra T. Choi, PhD, MPH, of Baylor College of Medicine, Houston, and her colleagues.
The researchers retrospectively studied a cohort of 13,174 patients with HCC from 2003 to 2013 included in the Surveillance, Epidemiology, and End Results Program–Medicare database. They examined the acquisition of HCC in the 3 years leading up to HCC diagnosis using three separate categories: consistent, inconsistent, or no screening. Dr. Choi and her colleagues studied the associations between receiving HCC screening and subsequent effects on overall survival.
“HCC prognosis depends on tumor stage at the time of diagnosis, with curative treatment options only available for patients diagnosed at an early stage,” the researchers wrote. “Patients with early-stage HCC can achieve 5-year survival rates of 70% if they undergo surgical resection or liver transplantation, compared to a median survival of 1 year for patients with advanced HCC,” they added.
After multivariable analysis, the investigators found that 51.1% of patients with cirrhosis did not receive screening in the 3 years leading up to HCC diagnosis. In addition, they went on to report that only 6.8% of patients were consistently screened on an annual basis.
“HCC screening receipt was associated with early tumor detection and potentially improved overall survival, with attenuated benefits in those with inconsistent screening compared to those who had received consistent screening,” they explained.
In terms of efficacy, consistent screening was associated with an increased rate of early-stage tumor detection (odds ratio, 1.98, 95% confidence interval, 1.68-2.33) and decreased risk of death (hazard ratio, 0.76, 95% CI, 0.70-0.83) after adjustment for lead time bias. Given these results, Dr. Choi and her colleagues said that early HCC screening may help with hepatic tumor detection at later disease stages.
“Given the demonstrated benefits of HCC screening, [it] is an important step to reverse the high rates of late stage diagnosis and poor survival,” they added.
The investigators noted that several patient-specific factors may be driving these associations. In particular, they found a link between female sex and receiving HCC screening. Dr. Choi and her colleagues suggested this association is not related to the perceived benefits from screening.
“Studies have suggested females may be more likely to adhere to screening recommendations; however, patient adherence is not a common barrier to HCC screening completion and therefore it is unclear if this is the sole driver of this association,” they acknowledged.
Moving forward, the researchers highlighted the importance of educating primary care providers about the benefits of screening. Moreover, they said that screening receipt is currently on the rise, which has shown positive effects on overall survival.
The Center for Innovations in Quality, Effectiveness, and Safety funded the study. Additional support was provided by the Texas A&M Health Science Center Engineering Experiment Station big data seed grant program. The authors reported no conflicts of interest.
SOURCE: Choi DT et al. Clin Gastroenterol Hepatol. 2018 Oct 25. doi: 10.1016/j.cgh.2018.10.031.
“Our study’s aim was to characterize utilization of HCC screening receipt and its association with early tumor detection and improved survival in a nationally representative cohort of patients in the United States,” wrote Debra T. Choi, PhD, MPH, of Baylor College of Medicine, Houston, and her colleagues.
The researchers retrospectively studied a cohort of 13,174 patients with HCC from 2003 to 2013 included in the Surveillance, Epidemiology, and End Results Program–Medicare database. They examined the acquisition of HCC in the 3 years leading up to HCC diagnosis using three separate categories: consistent, inconsistent, or no screening. Dr. Choi and her colleagues studied the associations between receiving HCC screening and subsequent effects on overall survival.
“HCC prognosis depends on tumor stage at the time of diagnosis, with curative treatment options only available for patients diagnosed at an early stage,” the researchers wrote. “Patients with early-stage HCC can achieve 5-year survival rates of 70% if they undergo surgical resection or liver transplantation, compared to a median survival of 1 year for patients with advanced HCC,” they added.
After multivariable analysis, the investigators found that 51.1% of patients with cirrhosis did not receive screening in the 3 years leading up to HCC diagnosis. In addition, they went on to report that only 6.8% of patients were consistently screened on an annual basis.
“HCC screening receipt was associated with early tumor detection and potentially improved overall survival, with attenuated benefits in those with inconsistent screening compared to those who had received consistent screening,” they explained.
In terms of efficacy, consistent screening was associated with an increased rate of early-stage tumor detection (odds ratio, 1.98, 95% confidence interval, 1.68-2.33) and decreased risk of death (hazard ratio, 0.76, 95% CI, 0.70-0.83) after adjustment for lead time bias. Given these results, Dr. Choi and her colleagues said that early HCC screening may help with hepatic tumor detection at later disease stages.
“Given the demonstrated benefits of HCC screening, [it] is an important step to reverse the high rates of late stage diagnosis and poor survival,” they added.
The investigators noted that several patient-specific factors may be driving these associations. In particular, they found a link between female sex and receiving HCC screening. Dr. Choi and her colleagues suggested this association is not related to the perceived benefits from screening.
“Studies have suggested females may be more likely to adhere to screening recommendations; however, patient adherence is not a common barrier to HCC screening completion and therefore it is unclear if this is the sole driver of this association,” they acknowledged.
Moving forward, the researchers highlighted the importance of educating primary care providers about the benefits of screening. Moreover, they said that screening receipt is currently on the rise, which has shown positive effects on overall survival.
The Center for Innovations in Quality, Effectiveness, and Safety funded the study. Additional support was provided by the Texas A&M Health Science Center Engineering Experiment Station big data seed grant program. The authors reported no conflicts of interest.
SOURCE: Choi DT et al. Clin Gastroenterol Hepatol. 2018 Oct 25. doi: 10.1016/j.cgh.2018.10.031.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Hepatocellular carcinoma (HCC) screening for patients with cirrhosis remains an underutilized technique to detect early-stage malignancy.
Major finding: Regular HCC screening was associated with an increased rate of early-stage tumor detection (odds ratio, 1.98).
Study details: Retrospective analysis of 13,174 patients with HCC who were screened 3 years prior to cancer diagnosis.
Disclosures: The Center for Innovations in Quality, Effectiveness, and Safety funded the study. Additional support was provided by the Texas A&M Health Science Center Engineering Experiment Station big data seed grant program. The authors reported no conflicts of interest.
Source: Choi DT et al. Clin Gastroenterol Hepatol. 2018 Oct 25. doi: 10.1016/j.cgh.2018.10.031.
Hepatitis C debrief: Therapy has matured, access issues remain
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
In the GI Patient Center, AGA offers resources to help ensure patients are receiving the best possible care and living their best life.
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
In the GI Patient Center, AGA offers resources to help ensure patients are receiving the best possible care and living their best life.
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
In the GI Patient Center, AGA offers resources to help ensure patients are receiving the best possible care and living their best life.
REPORTING FROM THE LIVER MEETING 2018
Early treatment with direct-acting antivirals linked to reduced medical costs in noncirrhotic HCV
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Noncirrhotic chronic hepatitis C patients incur high medical costs after their first diagnosis if left untreated.
Major finding: In the first 3 years post index, the average medical costs incurred in the direct-acting antiviral–treated and untreated groups were $28,392 and $42,914, respectively.
Study details: A database sample of 3,069 adults first diagnosed with chronic hepatitis C in or after 2013.
Disclosures: AbbVie provided funding for the study. The company employs Dr. Bao and two of the study coauthors.
Normothermic machine perfusion found to salvage fatty livers for transplantation
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
AT THE LIVER MEETING 2018
Key clinical point: The addition of apheresis filtration and defatting agents to normothermic machine perfusion led to significant improvements in liver function.
Major finding: Livers which received apheresis filtration and defatting agents fared better than those that did not.
Study details: An analysis of 18 discarded human steatotic livers that were perfused on a normothermic, blood-based circuit for 48 hours.
Disclosures: Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
Skin rashes often accompany drug-induced liver injury
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Researchers hope the findings will shed light on the mechanism of injury.
Major finding: 28% of patients with DILI also had a skin rash.
Study details: Retrospective analysis of 921 DILI patients.
Disclosures: No source of funding was disclosed. Dr. Devarbhavi disclosed no relevant conflicts.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
Medicaid patients have higher MELD scores at time of liver transplantation
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
AT THE LIVER MEETING 2018
Key clinical point: Significant insurance-specific disparities in MELD scores at time of wait-list registration were observed among patients with and without hepatocellular carcinoma.
Major finding: Among patients without hepatocellular carcinoma, those with Medicaid coverage were 2.45 times more likely to have higher MELD scores at wait-list registration, compared with those covered by commercial or private insurance (P less than .01).
Study details: A retrospective analysis of 88,542 liver transplantation wait-list registrants.
Disclosures: Dr. Robinson reported having no disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
The Liver Meeting 2018: Hepatitis B novel therapies debrief – key abstracts
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
REPORTING FROM THE LIVER MEETING 2018
Routine markers predicted histologic response to obeticholic acid in NASH
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
FROM GASTROENTEROLOGY
Key clinical point: Routine clinical and laboratory parameters were significant correlates of histologic response to obeticholic acid therapy in patients with nonalcoholic steatohepatitis.
Major finding: Significant predictors included baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides up to 154 mg/dL, baseline international normalized ratio up to 1, baseline aspartate aminotransferase up to 49 U/L, and at least a 17-U/L decrease in alanine aminotransferase at week 24.
Study details: Secondary analysis of data from 200 patients with nonalcoholic steatohepatitis in the randomized, double-blind FLINT trial.
Disclosures: The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
Source: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.