User login
Researchers link two genes to Raynaud’s disease
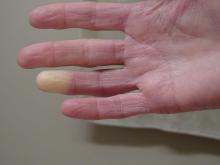
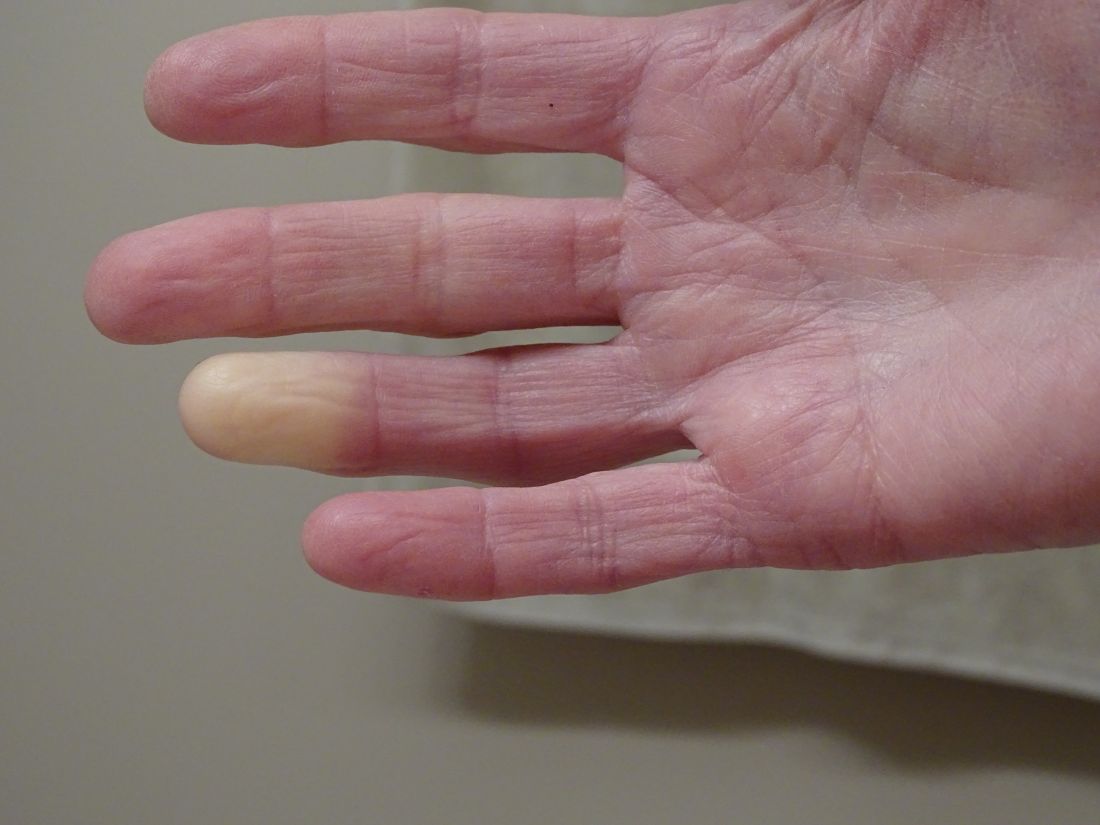
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Approximately 20% of U.S. adults are diagnosed with arthritis
TOPLINE:
The prevalence of reported diagnosed arthritis in the United States is highest overall in older adults with comorbid chronic conditions.
METHODOLOGY:
- Researchers reviewed data from the National Health Interview Survey (NHIS) from 2019 to 2021 to update the prevalence of self-reported arthritis in the United States.
- The sample sizes for the 2019, 2020, and 2021 NHIS were 31,997, 21,153, and 29,482, with survey response rates of 59.1%, 48.9%, and 50.9%, respectively.
- The unadjusted and age-standardized prevalence estimates were calculated for adults aged 18 years and older and based on self-reported health and demographic data.
TAKEAWAY:
- Overall, arthritis was diagnosed in 53.2 million adults aged 18 years and older in the United States; of these, 88.3% were aged 45 years and older and 48.3% were 65 years and older.
- Age-standardized prevalence of arthritis was higher in women vs men and among veterans vs nonveterans (20.9% vs 16.3% and 24.2% vs 18.5%, respectively).
- When categorized by race, age-standardized prevalence of arthritis was higher among non-Hispanic White individuals, compared with Hispanic or Latino individuals or non-Hispanic Asian individuals (20.1%, 14.7%, and 10.3%, respectively).
- The prevalence of arthritis also was higher among individuals with self-reported diagnosis of chronic conditions including dementia, chronic obstructive pulmonary disease, stroke, heart disease, diabetes, and cancer than in those without these conditions; approximately half of adults aged 65 years and older with arthritis reported at least one of these conditions.
IN PRACTICE:
“These prevalence estimates can be used to guide public health policies and activities to increase equitable access to physical activity opportunities within the built environment and other community-based, arthritis-appropriate, evidence-based interventions,” the authors write.
SOURCE:
The study was led by Elizabeth A. Fallon, PhD, of the Centers for Disease Control and Prevention, Atlanta, Georgia. The data were published online in the CDC’s Morbidity and Mortality Weekly Report.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality between individual characteristics and arthritis diagnosis; other limitations included the reliance on self-reports, possible response bias, and the inability to calculate prevalence of arthritis subtypes.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of reported diagnosed arthritis in the United States is highest overall in older adults with comorbid chronic conditions.
METHODOLOGY:
- Researchers reviewed data from the National Health Interview Survey (NHIS) from 2019 to 2021 to update the prevalence of self-reported arthritis in the United States.
- The sample sizes for the 2019, 2020, and 2021 NHIS were 31,997, 21,153, and 29,482, with survey response rates of 59.1%, 48.9%, and 50.9%, respectively.
- The unadjusted and age-standardized prevalence estimates were calculated for adults aged 18 years and older and based on self-reported health and demographic data.
TAKEAWAY:
- Overall, arthritis was diagnosed in 53.2 million adults aged 18 years and older in the United States; of these, 88.3% were aged 45 years and older and 48.3% were 65 years and older.
- Age-standardized prevalence of arthritis was higher in women vs men and among veterans vs nonveterans (20.9% vs 16.3% and 24.2% vs 18.5%, respectively).
- When categorized by race, age-standardized prevalence of arthritis was higher among non-Hispanic White individuals, compared with Hispanic or Latino individuals or non-Hispanic Asian individuals (20.1%, 14.7%, and 10.3%, respectively).
- The prevalence of arthritis also was higher among individuals with self-reported diagnosis of chronic conditions including dementia, chronic obstructive pulmonary disease, stroke, heart disease, diabetes, and cancer than in those without these conditions; approximately half of adults aged 65 years and older with arthritis reported at least one of these conditions.
IN PRACTICE:
“These prevalence estimates can be used to guide public health policies and activities to increase equitable access to physical activity opportunities within the built environment and other community-based, arthritis-appropriate, evidence-based interventions,” the authors write.
SOURCE:
The study was led by Elizabeth A. Fallon, PhD, of the Centers for Disease Control and Prevention, Atlanta, Georgia. The data were published online in the CDC’s Morbidity and Mortality Weekly Report.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality between individual characteristics and arthritis diagnosis; other limitations included the reliance on self-reports, possible response bias, and the inability to calculate prevalence of arthritis subtypes.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of reported diagnosed arthritis in the United States is highest overall in older adults with comorbid chronic conditions.
METHODOLOGY:
- Researchers reviewed data from the National Health Interview Survey (NHIS) from 2019 to 2021 to update the prevalence of self-reported arthritis in the United States.
- The sample sizes for the 2019, 2020, and 2021 NHIS were 31,997, 21,153, and 29,482, with survey response rates of 59.1%, 48.9%, and 50.9%, respectively.
- The unadjusted and age-standardized prevalence estimates were calculated for adults aged 18 years and older and based on self-reported health and demographic data.
TAKEAWAY:
- Overall, arthritis was diagnosed in 53.2 million adults aged 18 years and older in the United States; of these, 88.3% were aged 45 years and older and 48.3% were 65 years and older.
- Age-standardized prevalence of arthritis was higher in women vs men and among veterans vs nonveterans (20.9% vs 16.3% and 24.2% vs 18.5%, respectively).
- When categorized by race, age-standardized prevalence of arthritis was higher among non-Hispanic White individuals, compared with Hispanic or Latino individuals or non-Hispanic Asian individuals (20.1%, 14.7%, and 10.3%, respectively).
- The prevalence of arthritis also was higher among individuals with self-reported diagnosis of chronic conditions including dementia, chronic obstructive pulmonary disease, stroke, heart disease, diabetes, and cancer than in those without these conditions; approximately half of adults aged 65 years and older with arthritis reported at least one of these conditions.
IN PRACTICE:
“These prevalence estimates can be used to guide public health policies and activities to increase equitable access to physical activity opportunities within the built environment and other community-based, arthritis-appropriate, evidence-based interventions,” the authors write.
SOURCE:
The study was led by Elizabeth A. Fallon, PhD, of the Centers for Disease Control and Prevention, Atlanta, Georgia. The data were published online in the CDC’s Morbidity and Mortality Weekly Report.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality between individual characteristics and arthritis diagnosis; other limitations included the reliance on self-reports, possible response bias, and the inability to calculate prevalence of arthritis subtypes.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Anemia, iron deficit common in rheumatic disease pregnancy
TOPLINE:
, according to findings from a longitudinal cohort study.
METHODOLOGY:
- Researchers analyzed data from 368 pregnancies in women with rheumatic diseases during the period 2014-2022; nearly two-thirds (62%) had a connective tissue disease, 16% had rheumatoid arthritis or juvenile idiopathic arthritis, 14% had spondyloarthritis, 3% had vasculitis, and 7% had other diseases.
- Patients were aged 17-44 years, with a median age of 32 years at the time of birth.
- Researchers examined the frequency of anemia and iron deficiency and the impact of anemia on adverse maternal and child outcomes.
TAKEAWAY:
- The prevalence of iron deficiency was 28%, 51%, and 62% in the first, second, and third trimesters, respectively.
- The prevalence of anemia was 18%, 27%, and 33% in the first, second, and third trimesters, respectively.
- There was an increased risk for fetal complications such as malformation, infections, small for gestational age, neonatal lupus, preterm birth, and abortion or stillbirth in association with maternal connective tissue disease (odds ratio, 2.14) and also with low maternal hemoglobin levels and maternal iron deficiency (ORs, 0.52 and 0.86, respectively).
- Lower maternal hemoglobin levels were associated with an increased risk for maternal complications (OR, 1.47) such as flare with adaption of rheumatic medication and pregnancy-related adverse events (preeclampsia, gestational diabetes, bleeding complications, and thromboembolism), but patients with connective tissue disease had a lower risk for maternal complications (OR, 0.51); mean serum ferritin had no significant impact on maternal complications (OR, 1.02).
IN PRACTICE:
“Patients with rheumatic diseases suffer more often and already in early pregnancy from iron deficiency,” the researchers write. Therefore, early identification of anemia and iron deficiency in this population could inform prepregnancy counseling.
SOURCE:
The lead author on the study was Ann-Christin Pecher, MD, of University Hospital Tübingen, Germany. The study was published online in Joint Bone Spine.
LIMITATIONS:
The findings were limited by the use of a single dataset that might not be representative of all pregnant patients with rheumatic diseases. Other limitations included the lack of a standardized approach to iron supplementation.
DISCLOSURES:
The study was supported by a grant from the Medical Faculty of Tübingen Clinician-Scientist to the lead author. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to findings from a longitudinal cohort study.
METHODOLOGY:
- Researchers analyzed data from 368 pregnancies in women with rheumatic diseases during the period 2014-2022; nearly two-thirds (62%) had a connective tissue disease, 16% had rheumatoid arthritis or juvenile idiopathic arthritis, 14% had spondyloarthritis, 3% had vasculitis, and 7% had other diseases.
- Patients were aged 17-44 years, with a median age of 32 years at the time of birth.
- Researchers examined the frequency of anemia and iron deficiency and the impact of anemia on adverse maternal and child outcomes.
TAKEAWAY:
- The prevalence of iron deficiency was 28%, 51%, and 62% in the first, second, and third trimesters, respectively.
- The prevalence of anemia was 18%, 27%, and 33% in the first, second, and third trimesters, respectively.
- There was an increased risk for fetal complications such as malformation, infections, small for gestational age, neonatal lupus, preterm birth, and abortion or stillbirth in association with maternal connective tissue disease (odds ratio, 2.14) and also with low maternal hemoglobin levels and maternal iron deficiency (ORs, 0.52 and 0.86, respectively).
- Lower maternal hemoglobin levels were associated with an increased risk for maternal complications (OR, 1.47) such as flare with adaption of rheumatic medication and pregnancy-related adverse events (preeclampsia, gestational diabetes, bleeding complications, and thromboembolism), but patients with connective tissue disease had a lower risk for maternal complications (OR, 0.51); mean serum ferritin had no significant impact on maternal complications (OR, 1.02).
IN PRACTICE:
“Patients with rheumatic diseases suffer more often and already in early pregnancy from iron deficiency,” the researchers write. Therefore, early identification of anemia and iron deficiency in this population could inform prepregnancy counseling.
SOURCE:
The lead author on the study was Ann-Christin Pecher, MD, of University Hospital Tübingen, Germany. The study was published online in Joint Bone Spine.
LIMITATIONS:
The findings were limited by the use of a single dataset that might not be representative of all pregnant patients with rheumatic diseases. Other limitations included the lack of a standardized approach to iron supplementation.
DISCLOSURES:
The study was supported by a grant from the Medical Faculty of Tübingen Clinician-Scientist to the lead author. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to findings from a longitudinal cohort study.
METHODOLOGY:
- Researchers analyzed data from 368 pregnancies in women with rheumatic diseases during the period 2014-2022; nearly two-thirds (62%) had a connective tissue disease, 16% had rheumatoid arthritis or juvenile idiopathic arthritis, 14% had spondyloarthritis, 3% had vasculitis, and 7% had other diseases.
- Patients were aged 17-44 years, with a median age of 32 years at the time of birth.
- Researchers examined the frequency of anemia and iron deficiency and the impact of anemia on adverse maternal and child outcomes.
TAKEAWAY:
- The prevalence of iron deficiency was 28%, 51%, and 62% in the first, second, and third trimesters, respectively.
- The prevalence of anemia was 18%, 27%, and 33% in the first, second, and third trimesters, respectively.
- There was an increased risk for fetal complications such as malformation, infections, small for gestational age, neonatal lupus, preterm birth, and abortion or stillbirth in association with maternal connective tissue disease (odds ratio, 2.14) and also with low maternal hemoglobin levels and maternal iron deficiency (ORs, 0.52 and 0.86, respectively).
- Lower maternal hemoglobin levels were associated with an increased risk for maternal complications (OR, 1.47) such as flare with adaption of rheumatic medication and pregnancy-related adverse events (preeclampsia, gestational diabetes, bleeding complications, and thromboembolism), but patients with connective tissue disease had a lower risk for maternal complications (OR, 0.51); mean serum ferritin had no significant impact on maternal complications (OR, 1.02).
IN PRACTICE:
“Patients with rheumatic diseases suffer more often and already in early pregnancy from iron deficiency,” the researchers write. Therefore, early identification of anemia and iron deficiency in this population could inform prepregnancy counseling.
SOURCE:
The lead author on the study was Ann-Christin Pecher, MD, of University Hospital Tübingen, Germany. The study was published online in Joint Bone Spine.
LIMITATIONS:
The findings were limited by the use of a single dataset that might not be representative of all pregnant patients with rheumatic diseases. Other limitations included the lack of a standardized approach to iron supplementation.
DISCLOSURES:
The study was supported by a grant from the Medical Faculty of Tübingen Clinician-Scientist to the lead author. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anti-acid meds lower strength of systemic sclerosis drug
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Are women and men with rheumatism treated equally?
LEIPZIG, GERMANY – Women eat more healthily, visit their physician more often, and accept offers of prophylactic treatment more frequently than their male counterparts. Nevertheless, they are generally diagnosed with a rheumatic disease much later. “With systemic sclerosis for example, diagnosis occurs a whole year later than for male patients,” said Uta Kiltz, MD, senior physician at the Ruhrgebiet Rheumatism Center in Bochum, Germany, at a press conference for the annual congress of the German Society for Rheumatology.
In addition, certain markers and antibodies can be detected earlier in men’s blood – for example in systemic sclerosis. “What’s more, women exhibit a more diverse array of symptoms, which can make an unequivocal diagnosis difficult,” Dr. Kiltz explained.
Differences between the sexes in terms of disease progression and clinical presentation have been described for most rheumatic diseases. Roughly speaking, women often exhibit a much wider range of symptoms and report a higher disease burden, whereas men tend to experience a more severe progression of the disease.
Comorbidities also occur at different rates between the sexes. Whereas women with rheumatoid arthritis suffer more frequently from osteoporosis and depression, men are more likely to develop cardiovascular diseases and diabetes.
Gender-sensitive approach
Like Dr. Kiltz, Susanna Späthling-Mestekemper, MD, PhD, of the Munich-Pasing (Germany) Rheumatology Practice, also advocates a gender-sensitive approach to diagnosis and therapy. Dr. Späthling-Mestekemper referred to this during the conference, stating that women are still treated more poorly than men. The difference in treatment quality results from gaps in knowledge in the following areas:
- Sex-specific differences in the diagnosis and therapy of rheumatic diseases and in basic and clinical research
- Sex-specific differences in communication between male and female patients and between male and female physicians.
Dr. Späthling-Mestekemper used axial spondyloarthritis (axSpA) as a “prominent example” of false diagnoses. “Men more commonly fulfill the modified New York criteria – involvement of the axial skeleton, the lumbar spine, and increasing radiological progression.”
In contrast, women with axSpA exhibit the following differences:
- It is more likely for the cervical spine to be affected.
- Women are more likely to suffer from peripheral joint involvement.
- They suffer more from whole body pain.
- They have fatigue and exhaustion.
- They exhibit fewer humoral signs of inflammation (lower C-reactive protein).
- They are rarely HLA-B27 positive.
“We also have to completely rethink how we make the diagnosis in women,” said Dr. Späthling-Mestekemper. The current approach leads to women with axSpA being diagnosed much later than men. “Depending on the study, the difference can range from 7 months to 2 years,” according to Dr. Späthling-Mestekemper.
A 2018 Spanish study reported that the most common incorrect diagnoses in women with axSpA were sciatica, osteoarthritis, and fibromyalgia.
However, it is not just in axSpA that there are significant differences between men and women. There is evidence that women with systemic lupus erythematosus suffer more from musculoskeletal symptoms, while men with lupus exhibit more severe organ involvement (especially more serositis and nephritis).
For systemic sclerosis, women have the higher survival rate. They also exhibit skin involvement more frequently. Men, however, are more likely to have organ involvement, especially with the lungs.
TNF blockers
Using the example of axSpA, Dr. Späthling-Mestekemper also showed that men and women respond differently to tumor necrosis factor (TNF) blocker therapy. “The duration of therapy with TNF blockers is shorter for women: 33.4 months versus 44.9 months. They respond less to this therapy; they stop and change more frequently.”
Data from March 2023 show that, in contrast, there is no evidence of a difference in response to Janus kinase inhibitor treatment.
The presence of enthesitis has been discussed as one reason for the worse response to TNF blockers in women, since they have it more often than men do. “In fact, a better response to TNF blockers is associated with HLA-B27 positivity, with the absence of enthesitis and with TNF blocker naivety. In women, higher fat-mass index could also play a part, or even abdominal weight gain, which also increases in women after menopause,” said Dr. Späthling-Mestekemper.
She mentioned the following other potential reasons for a delayed therapy response to biological drugs in women:
- Genetic, physical, or hormonal causes
- Widespread pain or fibromyalgia
- Late diagnosis or late application of therapy, which lowers the chances of remission.
Even the science itself has shown the following sex-specific shortcomings:
- Disregarding sex-specific differences in animal-experimental studies (which, until recently, were only conducted in male mice to avoid hormone fluctuations)
- Women in clinical studies are still underrepresented: only 37% of the populations in phase 3 studies are women; 64% of studies do not describe any sex-specific differences
- Most of the data come from epidemiological analyses (not from basic research)
- Gaps in medical textbooks
Communication differences
Female patients are looking for explanations, whereas male patients describe specific symptoms. Female physicians talk, while male physicians treat. They sound like stereotypes, but they have been substantiated in multiple studies, said Dr. Späthling-Mestekemper. In general, the study results show that male patients behave in the following ways:
- Describe their symptoms in terms of specifics
- Do not like to admit having mental health issues
- Are three to five times more likely to commit suicide because of depression than women
On the other hand, female patients behave in the following ways:
- Look for an explanation for their symptoms
- Often do not have their physical symptoms taken seriously
- Are often pushed in a psychosomatic direction.
Female physicians focus on the following questions:
- Prevention, communication, shared decision-making, open-ended questions, “positive” discussions, patient self-management (chronic diseases such as diabetes: female physicians are better at reaching the therapy goals set by the ADA guidelines than male physicians)
- Psychosocial situations: consultations last 1 minute longer (10%).
Male physicians focus on the following questions:
- Medical history
- Physical examination (cardiac catheterizations after a heart attack are arranged much more commonly by male rather than female physicians)
- Diagnostics
Recognition and training
A large-scale surgical study in 2021 made a few waves. The study analyzed whether it makes a difference if women are operated on by men or by women. The results showed that women who had been operated on by men exhibited a higher level of risk after the surgery, compared with men who had been operated on by men or by women. The risk took the following forms:
- 15% higher risk for a worse surgery result
- 16% higher risk for complications
- 11% higher risk for repeat hospitalization
- 20% higher risk for a longer period of hospitalization
- 32% higher risk for mortality
The study authors provided the following potential reasons for these differences:
- Male physicians underestimate the severity of symptoms in their female patients
- Women are less comfortable indicating their postoperative pain to a male physician
- Different working style and treatment decisions between female and male physicians
- Unconsciously incorporated role patterns and preconceptions
“Our potential solutions are recognition and training. We need a personalized style of medicine; we need to have a closer look. We owe our male and female patients as much,” said Dr. Späthling-Mestekemper.
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
LEIPZIG, GERMANY – Women eat more healthily, visit their physician more often, and accept offers of prophylactic treatment more frequently than their male counterparts. Nevertheless, they are generally diagnosed with a rheumatic disease much later. “With systemic sclerosis for example, diagnosis occurs a whole year later than for male patients,” said Uta Kiltz, MD, senior physician at the Ruhrgebiet Rheumatism Center in Bochum, Germany, at a press conference for the annual congress of the German Society for Rheumatology.
In addition, certain markers and antibodies can be detected earlier in men’s blood – for example in systemic sclerosis. “What’s more, women exhibit a more diverse array of symptoms, which can make an unequivocal diagnosis difficult,” Dr. Kiltz explained.
Differences between the sexes in terms of disease progression and clinical presentation have been described for most rheumatic diseases. Roughly speaking, women often exhibit a much wider range of symptoms and report a higher disease burden, whereas men tend to experience a more severe progression of the disease.
Comorbidities also occur at different rates between the sexes. Whereas women with rheumatoid arthritis suffer more frequently from osteoporosis and depression, men are more likely to develop cardiovascular diseases and diabetes.
Gender-sensitive approach
Like Dr. Kiltz, Susanna Späthling-Mestekemper, MD, PhD, of the Munich-Pasing (Germany) Rheumatology Practice, also advocates a gender-sensitive approach to diagnosis and therapy. Dr. Späthling-Mestekemper referred to this during the conference, stating that women are still treated more poorly than men. The difference in treatment quality results from gaps in knowledge in the following areas:
- Sex-specific differences in the diagnosis and therapy of rheumatic diseases and in basic and clinical research
- Sex-specific differences in communication between male and female patients and between male and female physicians.
Dr. Späthling-Mestekemper used axial spondyloarthritis (axSpA) as a “prominent example” of false diagnoses. “Men more commonly fulfill the modified New York criteria – involvement of the axial skeleton, the lumbar spine, and increasing radiological progression.”
In contrast, women with axSpA exhibit the following differences:
- It is more likely for the cervical spine to be affected.
- Women are more likely to suffer from peripheral joint involvement.
- They suffer more from whole body pain.
- They have fatigue and exhaustion.
- They exhibit fewer humoral signs of inflammation (lower C-reactive protein).
- They are rarely HLA-B27 positive.
“We also have to completely rethink how we make the diagnosis in women,” said Dr. Späthling-Mestekemper. The current approach leads to women with axSpA being diagnosed much later than men. “Depending on the study, the difference can range from 7 months to 2 years,” according to Dr. Späthling-Mestekemper.
A 2018 Spanish study reported that the most common incorrect diagnoses in women with axSpA were sciatica, osteoarthritis, and fibromyalgia.
However, it is not just in axSpA that there are significant differences between men and women. There is evidence that women with systemic lupus erythematosus suffer more from musculoskeletal symptoms, while men with lupus exhibit more severe organ involvement (especially more serositis and nephritis).
For systemic sclerosis, women have the higher survival rate. They also exhibit skin involvement more frequently. Men, however, are more likely to have organ involvement, especially with the lungs.
TNF blockers
Using the example of axSpA, Dr. Späthling-Mestekemper also showed that men and women respond differently to tumor necrosis factor (TNF) blocker therapy. “The duration of therapy with TNF blockers is shorter for women: 33.4 months versus 44.9 months. They respond less to this therapy; they stop and change more frequently.”
Data from March 2023 show that, in contrast, there is no evidence of a difference in response to Janus kinase inhibitor treatment.
The presence of enthesitis has been discussed as one reason for the worse response to TNF blockers in women, since they have it more often than men do. “In fact, a better response to TNF blockers is associated with HLA-B27 positivity, with the absence of enthesitis and with TNF blocker naivety. In women, higher fat-mass index could also play a part, or even abdominal weight gain, which also increases in women after menopause,” said Dr. Späthling-Mestekemper.
She mentioned the following other potential reasons for a delayed therapy response to biological drugs in women:
- Genetic, physical, or hormonal causes
- Widespread pain or fibromyalgia
- Late diagnosis or late application of therapy, which lowers the chances of remission.
Even the science itself has shown the following sex-specific shortcomings:
- Disregarding sex-specific differences in animal-experimental studies (which, until recently, were only conducted in male mice to avoid hormone fluctuations)
- Women in clinical studies are still underrepresented: only 37% of the populations in phase 3 studies are women; 64% of studies do not describe any sex-specific differences
- Most of the data come from epidemiological analyses (not from basic research)
- Gaps in medical textbooks
Communication differences
Female patients are looking for explanations, whereas male patients describe specific symptoms. Female physicians talk, while male physicians treat. They sound like stereotypes, but they have been substantiated in multiple studies, said Dr. Späthling-Mestekemper. In general, the study results show that male patients behave in the following ways:
- Describe their symptoms in terms of specifics
- Do not like to admit having mental health issues
- Are three to five times more likely to commit suicide because of depression than women
On the other hand, female patients behave in the following ways:
- Look for an explanation for their symptoms
- Often do not have their physical symptoms taken seriously
- Are often pushed in a psychosomatic direction.
Female physicians focus on the following questions:
- Prevention, communication, shared decision-making, open-ended questions, “positive” discussions, patient self-management (chronic diseases such as diabetes: female physicians are better at reaching the therapy goals set by the ADA guidelines than male physicians)
- Psychosocial situations: consultations last 1 minute longer (10%).
Male physicians focus on the following questions:
- Medical history
- Physical examination (cardiac catheterizations after a heart attack are arranged much more commonly by male rather than female physicians)
- Diagnostics
Recognition and training
A large-scale surgical study in 2021 made a few waves. The study analyzed whether it makes a difference if women are operated on by men or by women. The results showed that women who had been operated on by men exhibited a higher level of risk after the surgery, compared with men who had been operated on by men or by women. The risk took the following forms:
- 15% higher risk for a worse surgery result
- 16% higher risk for complications
- 11% higher risk for repeat hospitalization
- 20% higher risk for a longer period of hospitalization
- 32% higher risk for mortality
The study authors provided the following potential reasons for these differences:
- Male physicians underestimate the severity of symptoms in their female patients
- Women are less comfortable indicating their postoperative pain to a male physician
- Different working style and treatment decisions between female and male physicians
- Unconsciously incorporated role patterns and preconceptions
“Our potential solutions are recognition and training. We need a personalized style of medicine; we need to have a closer look. We owe our male and female patients as much,” said Dr. Späthling-Mestekemper.
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
LEIPZIG, GERMANY – Women eat more healthily, visit their physician more often, and accept offers of prophylactic treatment more frequently than their male counterparts. Nevertheless, they are generally diagnosed with a rheumatic disease much later. “With systemic sclerosis for example, diagnosis occurs a whole year later than for male patients,” said Uta Kiltz, MD, senior physician at the Ruhrgebiet Rheumatism Center in Bochum, Germany, at a press conference for the annual congress of the German Society for Rheumatology.
In addition, certain markers and antibodies can be detected earlier in men’s blood – for example in systemic sclerosis. “What’s more, women exhibit a more diverse array of symptoms, which can make an unequivocal diagnosis difficult,” Dr. Kiltz explained.
Differences between the sexes in terms of disease progression and clinical presentation have been described for most rheumatic diseases. Roughly speaking, women often exhibit a much wider range of symptoms and report a higher disease burden, whereas men tend to experience a more severe progression of the disease.
Comorbidities also occur at different rates between the sexes. Whereas women with rheumatoid arthritis suffer more frequently from osteoporosis and depression, men are more likely to develop cardiovascular diseases and diabetes.
Gender-sensitive approach
Like Dr. Kiltz, Susanna Späthling-Mestekemper, MD, PhD, of the Munich-Pasing (Germany) Rheumatology Practice, also advocates a gender-sensitive approach to diagnosis and therapy. Dr. Späthling-Mestekemper referred to this during the conference, stating that women are still treated more poorly than men. The difference in treatment quality results from gaps in knowledge in the following areas:
- Sex-specific differences in the diagnosis and therapy of rheumatic diseases and in basic and clinical research
- Sex-specific differences in communication between male and female patients and between male and female physicians.
Dr. Späthling-Mestekemper used axial spondyloarthritis (axSpA) as a “prominent example” of false diagnoses. “Men more commonly fulfill the modified New York criteria – involvement of the axial skeleton, the lumbar spine, and increasing radiological progression.”
In contrast, women with axSpA exhibit the following differences:
- It is more likely for the cervical spine to be affected.
- Women are more likely to suffer from peripheral joint involvement.
- They suffer more from whole body pain.
- They have fatigue and exhaustion.
- They exhibit fewer humoral signs of inflammation (lower C-reactive protein).
- They are rarely HLA-B27 positive.
“We also have to completely rethink how we make the diagnosis in women,” said Dr. Späthling-Mestekemper. The current approach leads to women with axSpA being diagnosed much later than men. “Depending on the study, the difference can range from 7 months to 2 years,” according to Dr. Späthling-Mestekemper.
A 2018 Spanish study reported that the most common incorrect diagnoses in women with axSpA were sciatica, osteoarthritis, and fibromyalgia.
However, it is not just in axSpA that there are significant differences between men and women. There is evidence that women with systemic lupus erythematosus suffer more from musculoskeletal symptoms, while men with lupus exhibit more severe organ involvement (especially more serositis and nephritis).
For systemic sclerosis, women have the higher survival rate. They also exhibit skin involvement more frequently. Men, however, are more likely to have organ involvement, especially with the lungs.
TNF blockers
Using the example of axSpA, Dr. Späthling-Mestekemper also showed that men and women respond differently to tumor necrosis factor (TNF) blocker therapy. “The duration of therapy with TNF blockers is shorter for women: 33.4 months versus 44.9 months. They respond less to this therapy; they stop and change more frequently.”
Data from March 2023 show that, in contrast, there is no evidence of a difference in response to Janus kinase inhibitor treatment.
The presence of enthesitis has been discussed as one reason for the worse response to TNF blockers in women, since they have it more often than men do. “In fact, a better response to TNF blockers is associated with HLA-B27 positivity, with the absence of enthesitis and with TNF blocker naivety. In women, higher fat-mass index could also play a part, or even abdominal weight gain, which also increases in women after menopause,” said Dr. Späthling-Mestekemper.
She mentioned the following other potential reasons for a delayed therapy response to biological drugs in women:
- Genetic, physical, or hormonal causes
- Widespread pain or fibromyalgia
- Late diagnosis or late application of therapy, which lowers the chances of remission.
Even the science itself has shown the following sex-specific shortcomings:
- Disregarding sex-specific differences in animal-experimental studies (which, until recently, were only conducted in male mice to avoid hormone fluctuations)
- Women in clinical studies are still underrepresented: only 37% of the populations in phase 3 studies are women; 64% of studies do not describe any sex-specific differences
- Most of the data come from epidemiological analyses (not from basic research)
- Gaps in medical textbooks
Communication differences
Female patients are looking for explanations, whereas male patients describe specific symptoms. Female physicians talk, while male physicians treat. They sound like stereotypes, but they have been substantiated in multiple studies, said Dr. Späthling-Mestekemper. In general, the study results show that male patients behave in the following ways:
- Describe their symptoms in terms of specifics
- Do not like to admit having mental health issues
- Are three to five times more likely to commit suicide because of depression than women
On the other hand, female patients behave in the following ways:
- Look for an explanation for their symptoms
- Often do not have their physical symptoms taken seriously
- Are often pushed in a psychosomatic direction.
Female physicians focus on the following questions:
- Prevention, communication, shared decision-making, open-ended questions, “positive” discussions, patient self-management (chronic diseases such as diabetes: female physicians are better at reaching the therapy goals set by the ADA guidelines than male physicians)
- Psychosocial situations: consultations last 1 minute longer (10%).
Male physicians focus on the following questions:
- Medical history
- Physical examination (cardiac catheterizations after a heart attack are arranged much more commonly by male rather than female physicians)
- Diagnostics
Recognition and training
A large-scale surgical study in 2021 made a few waves. The study analyzed whether it makes a difference if women are operated on by men or by women. The results showed that women who had been operated on by men exhibited a higher level of risk after the surgery, compared with men who had been operated on by men or by women. The risk took the following forms:
- 15% higher risk for a worse surgery result
- 16% higher risk for complications
- 11% higher risk for repeat hospitalization
- 20% higher risk for a longer period of hospitalization
- 32% higher risk for mortality
The study authors provided the following potential reasons for these differences:
- Male physicians underestimate the severity of symptoms in their female patients
- Women are less comfortable indicating their postoperative pain to a male physician
- Different working style and treatment decisions between female and male physicians
- Unconsciously incorporated role patterns and preconceptions
“Our potential solutions are recognition and training. We need a personalized style of medicine; we need to have a closer look. We owe our male and female patients as much,” said Dr. Späthling-Mestekemper.
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
AT THE GERMAN RHEUMATOLOGY CONGRESS 2023
More evidence shows COVID-19’s link to risk for autoimmune disease
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
PET scan at diagnosis may help to predict aneurysm risk in patients with giant cell arteritis
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Tapering lupus drugs in stable patients: Large study outlines risks, benefits
The question looms large for patients with stable systemic lupus erythematosus (SLE): to taper or not to taper corticosteroids or immunosuppressive therapy? For patients and the physicians treating them, the evidence points in both directions. Flares are exacerbated by tapering, but simultaneously organ damage is tempered. Where is the balance? What competing factors together inform decision-making?
A recent multinational, observational cohort study conducted by Jiacai Cho, MBBS, of National University Hospital, Singapore, and colleagues, and published in The Lancet Rheumatology concluded that, given the odds of excess flares associated with tapering of corticosteroids and immunosuppressive therapy in patients with stable SLE, drug tapering warrants careful consideration of risks and benefits and is best reserved for those in complete clinical and serological remission with stable disease for at least 6 months. However, in an accompanying editorial, Yann Nguyen, MD, MPH, and Nathalie Costedoat-Chalumeau, MD, PhD, of the National Referral Center for Rare Autoimmune and Systemic Diseases at Cochin Hospital, Paris, and the Center for Research in Epidemiology and Statistics at Paris City University, argued for tipping the scale back from some of those expressed cautions.
In interviews, experts in the field expressed both strong appreciation for the cohort study and, like the editorialists, cognizance of its limitations.
Dr. Cho and colleagues recruited 3,002 adult patients with SLE (92.2% female, median age 39.5 years), from 25 sites across 13 Asia-Pacific countries. They were receiving routine clinical care and had achieved stable disease in at least one of two or more visits. Stable disease was defined by meeting criteria for Lupus Low Disease Activity State (LLDAS; SLE Disease Activity Index 2000 [SLEDAI-2K] score ≤ 4, Physician Global Assessment [PGA] ≤ 1, and prednisolone ≤ 7.5 mg/day), the 2021 DORIS definition of remission (clinical SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day), or DORIS complete remission on therapy (SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day). Any decrease in dose of corticosteroids or immunosuppressive therapy (mycophenolate mofetil, calcineurin inhibitors, azathioprine, leflunomide, or methotrexate) defined tapering. The investigators compared the odds of disease flares (SELENA-SLEDAI Flare Index) at the visit following tapering among those with tapering versus those who had continued the same drug doses.
Higher odds of flare with tapering
Tapering, compared with continuing with the same dose, was clearly associated with higher odds of flare at the next visit (11.4% with continuing vs. 17.0% with tapering; odds ratio, 1.24; 95% confidence interval, 1.10-1.39; P = .0005). Flares among patients who tapered were also slightly more often severe than with continuing the same dose (21.5% of flares vs. 19.7%). The level of remission at the time of tapering also mattered. Of 2,095 continuous tapering attempts, 860 (41.1%) were initiated in LLDAS, 596 (28.4%) in remission, and 639 (30.5%) in complete remission. Tapering when in LLDAS or remission, compared with complete remission, was associated with a higher likelihood of flare by 1 year (LLDAS: OR, 1.37; 95% CI, 1.03-1.81; P = .029; and remission: OR, 1.45; 95% CI, 1.08-1.94; P = .013). Time to first flare followed the same pattern. Also, sustained LLDAS, remission, or complete remission for at least 6 months just before the time of taper was associated with lower odds of flare at next visit and flares in 1 year, and longer time to flare.
Take baseline disease status, hydroxychloroquine’s effect into account
Dr. Nguyen and Dr. Costedoat-Chalumeau underscored several factors that may soften the risk for flares seen with tapering. They pointed to higher baseline doses of prednisone and immunosuppressants (and thus likely more severe disease that is more likely to flare) in the patients with tapering. Also, the SELENA-SLEDAI Flare Index used in the study classifies some clinically insignificant flares as mild to moderate and ignores the benefit of tapering. (It classifies patients as having a severe flare even when starting a new immunosuppressant prescription, such as azathioprine, methotrexate, or both, in an effort to reduce corticosteroid use.) They wrote that the study did not assess the rate of clinically meaningful flares (“essentially renal flares”), nor did it highlight that the “tiny” increase in absolute risk of severe flares (from 2.2% to 3.7%) could be further contextualized by the offset of the smaller, unmeasured rate of clinically significant flares and the “extremely relevant” risk of concomitant damage from prolonged treatment.
Dr. Nguyen and Dr. Costedoat-Chalumeau urged hydroxychloroquine use for all patients unless clearly contraindicated. In their own research, they have detailed hydroxychloroquine benefits in reducing not only flare risk, but also comorbidities, damage, and mortality. In the current study, the prevalence of hydroxychloroquine use in all the patient visits was only 63.3%. “We can assume that if more patients had been treated with hydroxychloroquine, both the number of flares and the difference between the two strategies would have been lower,” they wrote. They cited findings from a study of patients in remission for 2 years or longer in the Toronto Lupus Cohort in which a gradual taper of corticosteroids over 1 year was safe and feasible and resulted in less damage accrual at 24 months than not tapering. Optimizing tapering can minimize flare risk, they concluded.
Tapering SLE medications always involves some chance of flare and has to be considered a calculated risk, Sasha Bernatsky, MD, the James McGill professor of medicine in the division of rheumatology at McGill University, Montreal, said in an interview. “Long-term prednisone is not good for patients. I have heard it called ‘the miracle drug from hell’ – meaning that, yes, it controls disease, but at a cost of long-term complications. So we must be conscientious about tapering prednisone.” She observed that in the short-term, there may not be a huge risk to keeping a patient on an antimalarial and counseling patients to stay on it because their risk of flare is higher if they taper. Rheumatologists usually agree, however, that after 10 years or more, there is a real chance of retinal toxicity. “In our Montreal cohort, the risk of retinal toxicity was 5% after an average of 12.8 years of antimalarial use. My concern is that if a patient develops SLE in their 20s, how do we decide if we should keep them on an antimalarial for the next 60 or 70 years? If we keep them on the drug from age 25 to 45, and they then get retinal toxicity, they would essentially never be able to be on the drug again. So I do try to keep patients on the lowest dose of an antimalarial that is possible.”
Dr. Bernatsky pointed out further, “We think about tapering other immunosuppressants (such as methotrexate or mycophenolate or azathioprine) quite differently than prednisone tapering. We take our time a bit more, since many patients will tolerate being on standard doses of these drugs fairly well. If or when we do consider tapering these drugs, both our intuition and the literature suggests that someone with worse baseline disease activity or severity, who has needed a lot of steroids and multiple combinations of drugs to control disease, has a higher chance of flaring than someone with milder disease. As the editorial points out, lupus physicians (and their patients) need to think carefully about the patient’s risk profile, and be sure to tailor follow-up based on flare risk.”
Frank discussions with patients about the risks of tapering are needed, she said. “On one hand, there is consensus about how some aspects of lupus should be managed (for example, aggressive treatment of severe nephritis), but on the other hand, when it comes to long-term management and especially discussing tapering, we must have good discussions with patients. When a patient asks if they can taper a drug – many just lower or stop their drugs without asking – I am as honest as I can be, but ultimately have to admit any taper could be associated with a flare. It’s helpful to have actual figures to discuss with patients.”
No surprises
“This is an interesting study, which did not produce any surprises,” Dafna D. Gladman, MD, professor of medicine at University of Toronto and senior scientist at the university’s Schroeder Arthritis Institute, said when asked to comment. “We already knew from previous studies that abrupt withdrawal is not a good idea, and that if you taper when a patient is under conditions of remission, the rate of flare is actually lower than the usual rate of flare that occurs in people who continue on these medications. But the major limitation is that they did not specifically look at those who we would taper in clinical practice. In addition, they do not specify that the patients had to be on low-dose glucocorticoids before tapering, and they combined both immunosuppressive and steroids. It is not clear from the study what the excess flare rate was, or whether the flares were mild or severe. Most flares in patients with SLE are mild, consisting of skin and joint manifestations, while only a few patients have flares in kidney or neurologic manifestations.”
Dr. Gladman described her approach to tapering: “We aim for our patients to be taking no more than 5 mg of prednisone and to be in at least clinical remission with a SLEDAI-2K of 0 for at least 2 years before we would taper to glucocorticoids withdrawal. We always withdraw glucocorticoids first and immunosuppressives later, and keep patients on antimalarials the longest, unless there are specific side effects to the immunosuppressive or antimalarials which require their cessation earlier.”
Uncertainty persists
Other SLE experts weighing in confirmed the view that future research should aim to achieve clarity about the relative risks and benefits of tapering SLE drug regimens to maintain disease remission while minimizing potential for organ damage.
“Steroids are our friend and our enemy,” Joan T. Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “If a person with lupus is in a lot of trouble, corticosteroids are almost universally a good option to get them out. But for too many decades, for too many patients, despite all the improvements we have made in better understanding the disease and developing some promising new treatments, we have yet to shed the inexorable toxicity in multiple organs of steroid dependence.” She continued, “Corticosteroids, even at low dose, may have broad-spectrum effects. But, in fact, so do many of the more ‘targeted’ agents. If all patients were lined up at the beginning of a study while being given azathioprine or a calcineurin inhibitor or belimumab at a stable, tolerable dose, you might see the same data if you tapered that agent down. What we really need is improved individualized guidance about when and how fast to remove immune modulators from stable patients with lupus without disturbing the balance that had been achieved in such a quiescent patient.”
That enduring uncertainty was echoed by Daniel J. Wallace, MD, professor of medicine at Cedars-Sinai Medical Center, Los Angeles: “The take-home message from this interesting paper,” he commented, “is that current lupus biomarkers are not adequate. They do not guide the practitioner well enough, so that all too often medication regimens are tapered even though the risks are not really well known. Also, there is evidence in the literature that fibrosis and ‘damage’ progress even if acute phase reactants such as sedimentation rate, [C-reactive protein], complement 3 and 4, and anti-dsDNA are normal. We don’t have a good metric to detect them.”
Dr. Cho and colleagues’ study was funded by AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Serono, GlaxoSmithKline, and UCB. Dr. Gladman disclosed consulting and/or research support from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
The question looms large for patients with stable systemic lupus erythematosus (SLE): to taper or not to taper corticosteroids or immunosuppressive therapy? For patients and the physicians treating them, the evidence points in both directions. Flares are exacerbated by tapering, but simultaneously organ damage is tempered. Where is the balance? What competing factors together inform decision-making?
A recent multinational, observational cohort study conducted by Jiacai Cho, MBBS, of National University Hospital, Singapore, and colleagues, and published in The Lancet Rheumatology concluded that, given the odds of excess flares associated with tapering of corticosteroids and immunosuppressive therapy in patients with stable SLE, drug tapering warrants careful consideration of risks and benefits and is best reserved for those in complete clinical and serological remission with stable disease for at least 6 months. However, in an accompanying editorial, Yann Nguyen, MD, MPH, and Nathalie Costedoat-Chalumeau, MD, PhD, of the National Referral Center for Rare Autoimmune and Systemic Diseases at Cochin Hospital, Paris, and the Center for Research in Epidemiology and Statistics at Paris City University, argued for tipping the scale back from some of those expressed cautions.
In interviews, experts in the field expressed both strong appreciation for the cohort study and, like the editorialists, cognizance of its limitations.
Dr. Cho and colleagues recruited 3,002 adult patients with SLE (92.2% female, median age 39.5 years), from 25 sites across 13 Asia-Pacific countries. They were receiving routine clinical care and had achieved stable disease in at least one of two or more visits. Stable disease was defined by meeting criteria for Lupus Low Disease Activity State (LLDAS; SLE Disease Activity Index 2000 [SLEDAI-2K] score ≤ 4, Physician Global Assessment [PGA] ≤ 1, and prednisolone ≤ 7.5 mg/day), the 2021 DORIS definition of remission (clinical SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day), or DORIS complete remission on therapy (SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day). Any decrease in dose of corticosteroids or immunosuppressive therapy (mycophenolate mofetil, calcineurin inhibitors, azathioprine, leflunomide, or methotrexate) defined tapering. The investigators compared the odds of disease flares (SELENA-SLEDAI Flare Index) at the visit following tapering among those with tapering versus those who had continued the same drug doses.
Higher odds of flare with tapering
Tapering, compared with continuing with the same dose, was clearly associated with higher odds of flare at the next visit (11.4% with continuing vs. 17.0% with tapering; odds ratio, 1.24; 95% confidence interval, 1.10-1.39; P = .0005). Flares among patients who tapered were also slightly more often severe than with continuing the same dose (21.5% of flares vs. 19.7%). The level of remission at the time of tapering also mattered. Of 2,095 continuous tapering attempts, 860 (41.1%) were initiated in LLDAS, 596 (28.4%) in remission, and 639 (30.5%) in complete remission. Tapering when in LLDAS or remission, compared with complete remission, was associated with a higher likelihood of flare by 1 year (LLDAS: OR, 1.37; 95% CI, 1.03-1.81; P = .029; and remission: OR, 1.45; 95% CI, 1.08-1.94; P = .013). Time to first flare followed the same pattern. Also, sustained LLDAS, remission, or complete remission for at least 6 months just before the time of taper was associated with lower odds of flare at next visit and flares in 1 year, and longer time to flare.
Take baseline disease status, hydroxychloroquine’s effect into account
Dr. Nguyen and Dr. Costedoat-Chalumeau underscored several factors that may soften the risk for flares seen with tapering. They pointed to higher baseline doses of prednisone and immunosuppressants (and thus likely more severe disease that is more likely to flare) in the patients with tapering. Also, the SELENA-SLEDAI Flare Index used in the study classifies some clinically insignificant flares as mild to moderate and ignores the benefit of tapering. (It classifies patients as having a severe flare even when starting a new immunosuppressant prescription, such as azathioprine, methotrexate, or both, in an effort to reduce corticosteroid use.) They wrote that the study did not assess the rate of clinically meaningful flares (“essentially renal flares”), nor did it highlight that the “tiny” increase in absolute risk of severe flares (from 2.2% to 3.7%) could be further contextualized by the offset of the smaller, unmeasured rate of clinically significant flares and the “extremely relevant” risk of concomitant damage from prolonged treatment.
Dr. Nguyen and Dr. Costedoat-Chalumeau urged hydroxychloroquine use for all patients unless clearly contraindicated. In their own research, they have detailed hydroxychloroquine benefits in reducing not only flare risk, but also comorbidities, damage, and mortality. In the current study, the prevalence of hydroxychloroquine use in all the patient visits was only 63.3%. “We can assume that if more patients had been treated with hydroxychloroquine, both the number of flares and the difference between the two strategies would have been lower,” they wrote. They cited findings from a study of patients in remission for 2 years or longer in the Toronto Lupus Cohort in which a gradual taper of corticosteroids over 1 year was safe and feasible and resulted in less damage accrual at 24 months than not tapering. Optimizing tapering can minimize flare risk, they concluded.
Tapering SLE medications always involves some chance of flare and has to be considered a calculated risk, Sasha Bernatsky, MD, the James McGill professor of medicine in the division of rheumatology at McGill University, Montreal, said in an interview. “Long-term prednisone is not good for patients. I have heard it called ‘the miracle drug from hell’ – meaning that, yes, it controls disease, but at a cost of long-term complications. So we must be conscientious about tapering prednisone.” She observed that in the short-term, there may not be a huge risk to keeping a patient on an antimalarial and counseling patients to stay on it because their risk of flare is higher if they taper. Rheumatologists usually agree, however, that after 10 years or more, there is a real chance of retinal toxicity. “In our Montreal cohort, the risk of retinal toxicity was 5% after an average of 12.8 years of antimalarial use. My concern is that if a patient develops SLE in their 20s, how do we decide if we should keep them on an antimalarial for the next 60 or 70 years? If we keep them on the drug from age 25 to 45, and they then get retinal toxicity, they would essentially never be able to be on the drug again. So I do try to keep patients on the lowest dose of an antimalarial that is possible.”
Dr. Bernatsky pointed out further, “We think about tapering other immunosuppressants (such as methotrexate or mycophenolate or azathioprine) quite differently than prednisone tapering. We take our time a bit more, since many patients will tolerate being on standard doses of these drugs fairly well. If or when we do consider tapering these drugs, both our intuition and the literature suggests that someone with worse baseline disease activity or severity, who has needed a lot of steroids and multiple combinations of drugs to control disease, has a higher chance of flaring than someone with milder disease. As the editorial points out, lupus physicians (and their patients) need to think carefully about the patient’s risk profile, and be sure to tailor follow-up based on flare risk.”
Frank discussions with patients about the risks of tapering are needed, she said. “On one hand, there is consensus about how some aspects of lupus should be managed (for example, aggressive treatment of severe nephritis), but on the other hand, when it comes to long-term management and especially discussing tapering, we must have good discussions with patients. When a patient asks if they can taper a drug – many just lower or stop their drugs without asking – I am as honest as I can be, but ultimately have to admit any taper could be associated with a flare. It’s helpful to have actual figures to discuss with patients.”
No surprises
“This is an interesting study, which did not produce any surprises,” Dafna D. Gladman, MD, professor of medicine at University of Toronto and senior scientist at the university’s Schroeder Arthritis Institute, said when asked to comment. “We already knew from previous studies that abrupt withdrawal is not a good idea, and that if you taper when a patient is under conditions of remission, the rate of flare is actually lower than the usual rate of flare that occurs in people who continue on these medications. But the major limitation is that they did not specifically look at those who we would taper in clinical practice. In addition, they do not specify that the patients had to be on low-dose glucocorticoids before tapering, and they combined both immunosuppressive and steroids. It is not clear from the study what the excess flare rate was, or whether the flares were mild or severe. Most flares in patients with SLE are mild, consisting of skin and joint manifestations, while only a few patients have flares in kidney or neurologic manifestations.”
Dr. Gladman described her approach to tapering: “We aim for our patients to be taking no more than 5 mg of prednisone and to be in at least clinical remission with a SLEDAI-2K of 0 for at least 2 years before we would taper to glucocorticoids withdrawal. We always withdraw glucocorticoids first and immunosuppressives later, and keep patients on antimalarials the longest, unless there are specific side effects to the immunosuppressive or antimalarials which require their cessation earlier.”
Uncertainty persists
Other SLE experts weighing in confirmed the view that future research should aim to achieve clarity about the relative risks and benefits of tapering SLE drug regimens to maintain disease remission while minimizing potential for organ damage.
“Steroids are our friend and our enemy,” Joan T. Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “If a person with lupus is in a lot of trouble, corticosteroids are almost universally a good option to get them out. But for too many decades, for too many patients, despite all the improvements we have made in better understanding the disease and developing some promising new treatments, we have yet to shed the inexorable toxicity in multiple organs of steroid dependence.” She continued, “Corticosteroids, even at low dose, may have broad-spectrum effects. But, in fact, so do many of the more ‘targeted’ agents. If all patients were lined up at the beginning of a study while being given azathioprine or a calcineurin inhibitor or belimumab at a stable, tolerable dose, you might see the same data if you tapered that agent down. What we really need is improved individualized guidance about when and how fast to remove immune modulators from stable patients with lupus without disturbing the balance that had been achieved in such a quiescent patient.”
That enduring uncertainty was echoed by Daniel J. Wallace, MD, professor of medicine at Cedars-Sinai Medical Center, Los Angeles: “The take-home message from this interesting paper,” he commented, “is that current lupus biomarkers are not adequate. They do not guide the practitioner well enough, so that all too often medication regimens are tapered even though the risks are not really well known. Also, there is evidence in the literature that fibrosis and ‘damage’ progress even if acute phase reactants such as sedimentation rate, [C-reactive protein], complement 3 and 4, and anti-dsDNA are normal. We don’t have a good metric to detect them.”
Dr. Cho and colleagues’ study was funded by AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Serono, GlaxoSmithKline, and UCB. Dr. Gladman disclosed consulting and/or research support from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
The question looms large for patients with stable systemic lupus erythematosus (SLE): to taper or not to taper corticosteroids or immunosuppressive therapy? For patients and the physicians treating them, the evidence points in both directions. Flares are exacerbated by tapering, but simultaneously organ damage is tempered. Where is the balance? What competing factors together inform decision-making?
A recent multinational, observational cohort study conducted by Jiacai Cho, MBBS, of National University Hospital, Singapore, and colleagues, and published in The Lancet Rheumatology concluded that, given the odds of excess flares associated with tapering of corticosteroids and immunosuppressive therapy in patients with stable SLE, drug tapering warrants careful consideration of risks and benefits and is best reserved for those in complete clinical and serological remission with stable disease for at least 6 months. However, in an accompanying editorial, Yann Nguyen, MD, MPH, and Nathalie Costedoat-Chalumeau, MD, PhD, of the National Referral Center for Rare Autoimmune and Systemic Diseases at Cochin Hospital, Paris, and the Center for Research in Epidemiology and Statistics at Paris City University, argued for tipping the scale back from some of those expressed cautions.
In interviews, experts in the field expressed both strong appreciation for the cohort study and, like the editorialists, cognizance of its limitations.
Dr. Cho and colleagues recruited 3,002 adult patients with SLE (92.2% female, median age 39.5 years), from 25 sites across 13 Asia-Pacific countries. They were receiving routine clinical care and had achieved stable disease in at least one of two or more visits. Stable disease was defined by meeting criteria for Lupus Low Disease Activity State (LLDAS; SLE Disease Activity Index 2000 [SLEDAI-2K] score ≤ 4, Physician Global Assessment [PGA] ≤ 1, and prednisolone ≤ 7.5 mg/day), the 2021 DORIS definition of remission (clinical SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day), or DORIS complete remission on therapy (SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day). Any decrease in dose of corticosteroids or immunosuppressive therapy (mycophenolate mofetil, calcineurin inhibitors, azathioprine, leflunomide, or methotrexate) defined tapering. The investigators compared the odds of disease flares (SELENA-SLEDAI Flare Index) at the visit following tapering among those with tapering versus those who had continued the same drug doses.
Higher odds of flare with tapering
Tapering, compared with continuing with the same dose, was clearly associated with higher odds of flare at the next visit (11.4% with continuing vs. 17.0% with tapering; odds ratio, 1.24; 95% confidence interval, 1.10-1.39; P = .0005). Flares among patients who tapered were also slightly more often severe than with continuing the same dose (21.5% of flares vs. 19.7%). The level of remission at the time of tapering also mattered. Of 2,095 continuous tapering attempts, 860 (41.1%) were initiated in LLDAS, 596 (28.4%) in remission, and 639 (30.5%) in complete remission. Tapering when in LLDAS or remission, compared with complete remission, was associated with a higher likelihood of flare by 1 year (LLDAS: OR, 1.37; 95% CI, 1.03-1.81; P = .029; and remission: OR, 1.45; 95% CI, 1.08-1.94; P = .013). Time to first flare followed the same pattern. Also, sustained LLDAS, remission, or complete remission for at least 6 months just before the time of taper was associated with lower odds of flare at next visit and flares in 1 year, and longer time to flare.
Take baseline disease status, hydroxychloroquine’s effect into account
Dr. Nguyen and Dr. Costedoat-Chalumeau underscored several factors that may soften the risk for flares seen with tapering. They pointed to higher baseline doses of prednisone and immunosuppressants (and thus likely more severe disease that is more likely to flare) in the patients with tapering. Also, the SELENA-SLEDAI Flare Index used in the study classifies some clinically insignificant flares as mild to moderate and ignores the benefit of tapering. (It classifies patients as having a severe flare even when starting a new immunosuppressant prescription, such as azathioprine, methotrexate, or both, in an effort to reduce corticosteroid use.) They wrote that the study did not assess the rate of clinically meaningful flares (“essentially renal flares”), nor did it highlight that the “tiny” increase in absolute risk of severe flares (from 2.2% to 3.7%) could be further contextualized by the offset of the smaller, unmeasured rate of clinically significant flares and the “extremely relevant” risk of concomitant damage from prolonged treatment.
Dr. Nguyen and Dr. Costedoat-Chalumeau urged hydroxychloroquine use for all patients unless clearly contraindicated. In their own research, they have detailed hydroxychloroquine benefits in reducing not only flare risk, but also comorbidities, damage, and mortality. In the current study, the prevalence of hydroxychloroquine use in all the patient visits was only 63.3%. “We can assume that if more patients had been treated with hydroxychloroquine, both the number of flares and the difference between the two strategies would have been lower,” they wrote. They cited findings from a study of patients in remission for 2 years or longer in the Toronto Lupus Cohort in which a gradual taper of corticosteroids over 1 year was safe and feasible and resulted in less damage accrual at 24 months than not tapering. Optimizing tapering can minimize flare risk, they concluded.
Tapering SLE medications always involves some chance of flare and has to be considered a calculated risk, Sasha Bernatsky, MD, the James McGill professor of medicine in the division of rheumatology at McGill University, Montreal, said in an interview. “Long-term prednisone is not good for patients. I have heard it called ‘the miracle drug from hell’ – meaning that, yes, it controls disease, but at a cost of long-term complications. So we must be conscientious about tapering prednisone.” She observed that in the short-term, there may not be a huge risk to keeping a patient on an antimalarial and counseling patients to stay on it because their risk of flare is higher if they taper. Rheumatologists usually agree, however, that after 10 years or more, there is a real chance of retinal toxicity. “In our Montreal cohort, the risk of retinal toxicity was 5% after an average of 12.8 years of antimalarial use. My concern is that if a patient develops SLE in their 20s, how do we decide if we should keep them on an antimalarial for the next 60 or 70 years? If we keep them on the drug from age 25 to 45, and they then get retinal toxicity, they would essentially never be able to be on the drug again. So I do try to keep patients on the lowest dose of an antimalarial that is possible.”
Dr. Bernatsky pointed out further, “We think about tapering other immunosuppressants (such as methotrexate or mycophenolate or azathioprine) quite differently than prednisone tapering. We take our time a bit more, since many patients will tolerate being on standard doses of these drugs fairly well. If or when we do consider tapering these drugs, both our intuition and the literature suggests that someone with worse baseline disease activity or severity, who has needed a lot of steroids and multiple combinations of drugs to control disease, has a higher chance of flaring than someone with milder disease. As the editorial points out, lupus physicians (and their patients) need to think carefully about the patient’s risk profile, and be sure to tailor follow-up based on flare risk.”
Frank discussions with patients about the risks of tapering are needed, she said. “On one hand, there is consensus about how some aspects of lupus should be managed (for example, aggressive treatment of severe nephritis), but on the other hand, when it comes to long-term management and especially discussing tapering, we must have good discussions with patients. When a patient asks if they can taper a drug – many just lower or stop their drugs without asking – I am as honest as I can be, but ultimately have to admit any taper could be associated with a flare. It’s helpful to have actual figures to discuss with patients.”
No surprises
“This is an interesting study, which did not produce any surprises,” Dafna D. Gladman, MD, professor of medicine at University of Toronto and senior scientist at the university’s Schroeder Arthritis Institute, said when asked to comment. “We already knew from previous studies that abrupt withdrawal is not a good idea, and that if you taper when a patient is under conditions of remission, the rate of flare is actually lower than the usual rate of flare that occurs in people who continue on these medications. But the major limitation is that they did not specifically look at those who we would taper in clinical practice. In addition, they do not specify that the patients had to be on low-dose glucocorticoids before tapering, and they combined both immunosuppressive and steroids. It is not clear from the study what the excess flare rate was, or whether the flares were mild or severe. Most flares in patients with SLE are mild, consisting of skin and joint manifestations, while only a few patients have flares in kidney or neurologic manifestations.”
Dr. Gladman described her approach to tapering: “We aim for our patients to be taking no more than 5 mg of prednisone and to be in at least clinical remission with a SLEDAI-2K of 0 for at least 2 years before we would taper to glucocorticoids withdrawal. We always withdraw glucocorticoids first and immunosuppressives later, and keep patients on antimalarials the longest, unless there are specific side effects to the immunosuppressive or antimalarials which require their cessation earlier.”
Uncertainty persists
Other SLE experts weighing in confirmed the view that future research should aim to achieve clarity about the relative risks and benefits of tapering SLE drug regimens to maintain disease remission while minimizing potential for organ damage.
“Steroids are our friend and our enemy,” Joan T. Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “If a person with lupus is in a lot of trouble, corticosteroids are almost universally a good option to get them out. But for too many decades, for too many patients, despite all the improvements we have made in better understanding the disease and developing some promising new treatments, we have yet to shed the inexorable toxicity in multiple organs of steroid dependence.” She continued, “Corticosteroids, even at low dose, may have broad-spectrum effects. But, in fact, so do many of the more ‘targeted’ agents. If all patients were lined up at the beginning of a study while being given azathioprine or a calcineurin inhibitor or belimumab at a stable, tolerable dose, you might see the same data if you tapered that agent down. What we really need is improved individualized guidance about when and how fast to remove immune modulators from stable patients with lupus without disturbing the balance that had been achieved in such a quiescent patient.”
That enduring uncertainty was echoed by Daniel J. Wallace, MD, professor of medicine at Cedars-Sinai Medical Center, Los Angeles: “The take-home message from this interesting paper,” he commented, “is that current lupus biomarkers are not adequate. They do not guide the practitioner well enough, so that all too often medication regimens are tapered even though the risks are not really well known. Also, there is evidence in the literature that fibrosis and ‘damage’ progress even if acute phase reactants such as sedimentation rate, [C-reactive protein], complement 3 and 4, and anti-dsDNA are normal. We don’t have a good metric to detect them.”
Dr. Cho and colleagues’ study was funded by AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Serono, GlaxoSmithKline, and UCB. Dr. Gladman disclosed consulting and/or research support from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
FROM THE LANCET RHEUMATOLOGY
Are cellular therapies the future of autoimmune disease?
A revolutionary treatment for cancers may also be able to treat and reset the immune system to provide long-term remission or possibly even cure certain autoimmune diseases.
Chimeric antigen receptor (CAR) T-cell therapy has offered a novel approach to treating hematologic cancers since 2017, but there are early signs that these cellular immunotherapies could be repurposed for B-cell mediated autoimmune diseases.
In September of last year, researchers in Germany reported that five patients with refractory systemic lupus erythematosus (SLE) treated with CAR T-cell therapy all achieved drug-free remission. At the time of publication, no patients had relapsed for up to 17 months after treatment. The authors described seroconversion of antinuclear antibodies in two patients with the longest follow-up, “indicating that abrogation of autoimmune B-cell clones may lead to a more widespread correction of autoimmunity,” the researchers write.
In another case study published in June, researchers used CD-19 targeted CAR-T cells to treat a 41-year-old man with refractory antisynthetase syndrome with progressive myositis and interstitial lung disease. Six months after treatment, there were no signs of myositis on MRI and a chest CT scan showed full regression of alveolitis.
Since then, two biotechnology companies – Cabaletta Bio in Philadelphia and Kyverna Therapeutics in Emeryville, Calif. – have already been granted fast-track designations from the U.S. Food and Drug Administration for CAR T-cell therapy for SLE and lupus nephritis. Bristol-Myers Squibb is also conducting a phase 1 trial in patients with severe, refractory SLE. Several biotechnology companies and hospitals in China are also conducting clinical trials for SLE. But this is only the tip of the iceberg regarding cellular therapies for autoimmune disease, said Max Konig, MD, PhD, an assistant professor of medicine in the division of rheumatology at Johns Hopkins University, Baltimore.
“It’s an incredibly exciting time. It’s unprecedented in the history of autoimmunity,” he noted.
A ‘reboot’ for the immune system
B-cell targeted therapies have been around since the early 2000s with drugs like rituximab, a monoclonal antibody medication that targets CD20, an antigen expressed on the surface of B cells. The CAR T cells currently available target another surface antigen, CD19, and are a much more potent therapy. Both are effective at depleting B cells in blood, but these engineered CD19-targeted T cells can reach B cells sitting in tissues in a way that antibody therapies cannot, Dr. Konig explained.
“If you have a patient with myositis, for example, where autoreactive B cells are sitting in the inflamed muscle, or a patient with rheumatoid arthritis, where you have disease-relevant B cells in hard-to-reach tissues like the synovium, those cells are much harder to deplete with an antibody, compared to a T cell that evolved to surveil and effectively kill in all tissues,” he explained.
In this process, T cells are collected from patients via leukapheresis and then re-engineered to express chimeric antigen receptors. A few days before these modified T cells are infused back into the patient, the patients are given a low-dose chemotherapy (lymphodepletion) regimen to help increase the effectiveness of the therapy. The one-time infusion is generally given on an inpatient basis, and patients are then monitored in hospital for side effects.
Once B cells are depleted, disease symptoms improve. But in the case studies published to date, once B cells re-emerge, they are naïve and no longer producing autoreactive B cells.
“Maybe it’s like a tabula rasa: You wipe [the B cells] out and start with a clean slate. Then, the immune system reboots, and now it’s working, whereas before it was messed up,” said Carl June, MD, who directs the Center for Cellular Immunotherapies at the at the University of Pennsylvania, Philadelphia. Dr. June and his research team led the development of CAR T-cell therapies for blood cancers.
The findings suggest that autoantibodies “might not be hardwired into the immune system,” he said.
But Dr. Konig stressed that we are still in the early days of clinical trials, and more research is necessary to understand the safety and efficacy of these therapies.
“There’s an incredible buzz around CAR T cells at the moment in rheumatology, which is great because I think that’s where the future is,” he said. “But we still need to learn how to appropriately apply these therapies in randomized, controlled trials.”
So far, the evidence behind CD19 CAR T-cell therapies in autoimmune disease is from case studies and phase 1 trials in a very small number of selected patients. (The upcoming Cabaletta and Kyverna trials in lupus will also be small, consisting of 12 patients each.)
Risks of intensive therapy
But while these therapies show promise, the process is very intensive. The lymphodepleting regimen increases the risk for infection and patients are commonly hospitalized for a week or more following infusion for toxicity monitoring. Serious adverse events such as cytokine release syndrome (CRS) can occur days to weeks after CAR T-cell infusion. In the five-patient case series reported in 2022, patients were hospitalized for 10 days following treatment.
The patient with antisynthetase syndrome, as well as three of five patients in the SLE case series study experienced mild CRS following infusion. Patients are also at a high risk for infection, as the engineered T cells target all B cells, not just the autoreactive immune cells.
The inability to differentiate between disease-causing and protective immune cells is an issue for all currently available drugs treating autoimmune disease, Dr. Konig said. But scientists are already working on how to make these potent cellular therapies safer and more precise.
Alternatives to standard CAR T-cell therapies
Engineering T cells with RNA is a new approach to limit the side effects and toxicity of CAR T-cell therapy, said Chris Jewell, PhD, the chief scientific officer at Cartesian Therapeutics, a biotechnology company based in Gaithersburg, Md. The company’s RNA CAR T-cell (rCAR-T) therapy – called DESCARTES-08 – is in phase 2 clinical trials for treatment of myasthenia gravis. Once these rCAR-T cells are infused in patients, as they divide, the RNAnaturally decays, he explained, meaning that after a certain point, the CAR is no longer expressed.
DESCARTES-08 targets B-cell maturation antigen (BCMA), which is primarily expressed on plasma cells, rather than all B cells, Dr. Jewell said.
“Targeting BCMA, we actually have a more selective profile,” he explained. “We are targeting the cells primarily responsible for the pathogenicity; many plasma cells – such as long-lived plasma cells – also take a long time to repopulate.”
This therapy also does not require lymphodepletion prior to infusion and can be done in an outpatient setting. The therapy is given in multiple infusions, once per week.
In the most recent clinical trial, patients with myasthenia gravis received six infusions over 6 weeks and experienced notable decreases in myasthenia gravis severity scale at up to 9 months of follow-up.
While standard CAR T-cell therapies under clinical investigational up to now all use effector T cells, regulatory T cells (Tregs) can also be engineered to target autoimmune disease. Abata Therapeutics, based in Boston, is using this approach for therapies for progressive multiple sclerosis and type 1 diabetes. These engineered Tregs express a T-cell receptor (TCR) that recognizes tissue-specific antigens and suppress inflammation at the site of the disease. “Treg-based cell therapies are really harnessing the natural power of regulatory cells to reset immune tolerance and recalibrate the immune system,” said their chief medical officer, Leonard Dragone, MD, PhD.
These therapies are derived from terminally differentiated cells that have limited capacity to produce pro-inflammatory cytokines including interleukin-2 or interferon gamma, Dr. Dragone explained. “CRS is difficult to envision from engineered Treg products and hasn’t been observed in any clinical experience with polyclonal Tregs,” he said.
This approach also does not require lymphodepletion prior to treatment. The company’s Treg cellular therapy for progressive MS is currently in investigational new drug-enabling studies, and they aim to dose their first patients in 2024.
Precision immunotherapy
For B-cell driven autoimmune diseases where the autoantibody is known, researchers have begun to re-engineer T cells to recognize only autoreactive B cells. While CD19 CAR T cells act more like a sledgehammer, these precision cellular immunotherapies are “like a razor’s strike,” Dr. June said.
“The chimeric autoantibody receptor (CAAR) approach targets autoantibodies that are expressed only on the surface of autoimmune B cells and are not expressed on normal B cells, which ideally should lead to precision targeting of just the cells that cause autoimmune disease,” explained Aimee Payne, MD, PhD, professor of dermatology and director of the Penn Clinical Autoimmunity Center of Excellence at the University of Pennsylvania, Philadelphia.
She and her research team used this approach to develop a treatment for mucosal pemphigus vulgaris, an autoimmune blistering disease of mucous membranes driven by autoantibodies against desmoglein 3.
“The current standard of care for pemphigus is to treat with steroids and rituximab, an infusion therapy that results in global, but temporary, B-cell depletion,” she said. “By expressing desmoglein 3 (DSG3) on the surface of the CAAR T-cell therapy, we target just the anti-DSG3 B cells that cause disease in mucosal pemphigus vulgaris and spare the healthy B cells.”
The therapy – called DSG3-CAART – is being developed by Cabaletta Bio and is now in phase 1 clinical trials. The approach is also being investigated to treat certain types of myasthenia gravis and membranous nephropathy.
Dr. Konig’s lab at Johns Hopkins developed and is now exploring a new precision cellular immunotherapy approach, chimeric autoantigen-T cell receptor (CATCR) T-cell therapy, to treat antiphospholipid syndrome, which is in preclinical stages. In this approach, Dr. Konig and his team are “re-engineering the natural T-cell receptor to selectively kill disease-causing B cells that drive antiphospholipid syndrome,” he explained.
He anticipates the CD19 CAR T-cell therapies currently in clinical trials will help to pave the way for this new generation of precision cellular therapies. The ultimate goal of these therapies, he said, is to uncouple therapeutic potency from infection risk.
“That’s really the holy grail in the treatment of autoimmune diseases. It’s tantalizingly close, but we’re not there yet.”
Dr. June is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives license revenue from such licenses. Dr. June is a scientific founder of Tmunity Therapeutics and Capstan Therapeutics and is a member of the scientific advisory boards of AC Immune SA, Alaunos, BlueSphere Bio, Cabaletta, Carisma, Cartography Biosciences, Cellares, Celldex, Decheng Capital, Poseida, Verismo, and WIRB-Copernicus Group. Dr. Konig is a consultant for argenx and Revel and is listed as inventor for patent applications filed by John Hopkins University. Dr. Payne holds equity, grants, payments, and patent licensing from Cabaletta Bio and consults for Janssen.
A version of this article first appeared on Medscape.com.
A revolutionary treatment for cancers may also be able to treat and reset the immune system to provide long-term remission or possibly even cure certain autoimmune diseases.
Chimeric antigen receptor (CAR) T-cell therapy has offered a novel approach to treating hematologic cancers since 2017, but there are early signs that these cellular immunotherapies could be repurposed for B-cell mediated autoimmune diseases.
In September of last year, researchers in Germany reported that five patients with refractory systemic lupus erythematosus (SLE) treated with CAR T-cell therapy all achieved drug-free remission. At the time of publication, no patients had relapsed for up to 17 months after treatment. The authors described seroconversion of antinuclear antibodies in two patients with the longest follow-up, “indicating that abrogation of autoimmune B-cell clones may lead to a more widespread correction of autoimmunity,” the researchers write.
In another case study published in June, researchers used CD-19 targeted CAR-T cells to treat a 41-year-old man with refractory antisynthetase syndrome with progressive myositis and interstitial lung disease. Six months after treatment, there were no signs of myositis on MRI and a chest CT scan showed full regression of alveolitis.
Since then, two biotechnology companies – Cabaletta Bio in Philadelphia and Kyverna Therapeutics in Emeryville, Calif. – have already been granted fast-track designations from the U.S. Food and Drug Administration for CAR T-cell therapy for SLE and lupus nephritis. Bristol-Myers Squibb is also conducting a phase 1 trial in patients with severe, refractory SLE. Several biotechnology companies and hospitals in China are also conducting clinical trials for SLE. But this is only the tip of the iceberg regarding cellular therapies for autoimmune disease, said Max Konig, MD, PhD, an assistant professor of medicine in the division of rheumatology at Johns Hopkins University, Baltimore.
“It’s an incredibly exciting time. It’s unprecedented in the history of autoimmunity,” he noted.
A ‘reboot’ for the immune system
B-cell targeted therapies have been around since the early 2000s with drugs like rituximab, a monoclonal antibody medication that targets CD20, an antigen expressed on the surface of B cells. The CAR T cells currently available target another surface antigen, CD19, and are a much more potent therapy. Both are effective at depleting B cells in blood, but these engineered CD19-targeted T cells can reach B cells sitting in tissues in a way that antibody therapies cannot, Dr. Konig explained.
“If you have a patient with myositis, for example, where autoreactive B cells are sitting in the inflamed muscle, or a patient with rheumatoid arthritis, where you have disease-relevant B cells in hard-to-reach tissues like the synovium, those cells are much harder to deplete with an antibody, compared to a T cell that evolved to surveil and effectively kill in all tissues,” he explained.
In this process, T cells are collected from patients via leukapheresis and then re-engineered to express chimeric antigen receptors. A few days before these modified T cells are infused back into the patient, the patients are given a low-dose chemotherapy (lymphodepletion) regimen to help increase the effectiveness of the therapy. The one-time infusion is generally given on an inpatient basis, and patients are then monitored in hospital for side effects.
Once B cells are depleted, disease symptoms improve. But in the case studies published to date, once B cells re-emerge, they are naïve and no longer producing autoreactive B cells.
“Maybe it’s like a tabula rasa: You wipe [the B cells] out and start with a clean slate. Then, the immune system reboots, and now it’s working, whereas before it was messed up,” said Carl June, MD, who directs the Center for Cellular Immunotherapies at the at the University of Pennsylvania, Philadelphia. Dr. June and his research team led the development of CAR T-cell therapies for blood cancers.
The findings suggest that autoantibodies “might not be hardwired into the immune system,” he said.
But Dr. Konig stressed that we are still in the early days of clinical trials, and more research is necessary to understand the safety and efficacy of these therapies.
“There’s an incredible buzz around CAR T cells at the moment in rheumatology, which is great because I think that’s where the future is,” he said. “But we still need to learn how to appropriately apply these therapies in randomized, controlled trials.”
So far, the evidence behind CD19 CAR T-cell therapies in autoimmune disease is from case studies and phase 1 trials in a very small number of selected patients. (The upcoming Cabaletta and Kyverna trials in lupus will also be small, consisting of 12 patients each.)
Risks of intensive therapy
But while these therapies show promise, the process is very intensive. The lymphodepleting regimen increases the risk for infection and patients are commonly hospitalized for a week or more following infusion for toxicity monitoring. Serious adverse events such as cytokine release syndrome (CRS) can occur days to weeks after CAR T-cell infusion. In the five-patient case series reported in 2022, patients were hospitalized for 10 days following treatment.
The patient with antisynthetase syndrome, as well as three of five patients in the SLE case series study experienced mild CRS following infusion. Patients are also at a high risk for infection, as the engineered T cells target all B cells, not just the autoreactive immune cells.
The inability to differentiate between disease-causing and protective immune cells is an issue for all currently available drugs treating autoimmune disease, Dr. Konig said. But scientists are already working on how to make these potent cellular therapies safer and more precise.
Alternatives to standard CAR T-cell therapies
Engineering T cells with RNA is a new approach to limit the side effects and toxicity of CAR T-cell therapy, said Chris Jewell, PhD, the chief scientific officer at Cartesian Therapeutics, a biotechnology company based in Gaithersburg, Md. The company’s RNA CAR T-cell (rCAR-T) therapy – called DESCARTES-08 – is in phase 2 clinical trials for treatment of myasthenia gravis. Once these rCAR-T cells are infused in patients, as they divide, the RNAnaturally decays, he explained, meaning that after a certain point, the CAR is no longer expressed.
DESCARTES-08 targets B-cell maturation antigen (BCMA), which is primarily expressed on plasma cells, rather than all B cells, Dr. Jewell said.
“Targeting BCMA, we actually have a more selective profile,” he explained. “We are targeting the cells primarily responsible for the pathogenicity; many plasma cells – such as long-lived plasma cells – also take a long time to repopulate.”
This therapy also does not require lymphodepletion prior to infusion and can be done in an outpatient setting. The therapy is given in multiple infusions, once per week.
In the most recent clinical trial, patients with myasthenia gravis received six infusions over 6 weeks and experienced notable decreases in myasthenia gravis severity scale at up to 9 months of follow-up.
While standard CAR T-cell therapies under clinical investigational up to now all use effector T cells, regulatory T cells (Tregs) can also be engineered to target autoimmune disease. Abata Therapeutics, based in Boston, is using this approach for therapies for progressive multiple sclerosis and type 1 diabetes. These engineered Tregs express a T-cell receptor (TCR) that recognizes tissue-specific antigens and suppress inflammation at the site of the disease. “Treg-based cell therapies are really harnessing the natural power of regulatory cells to reset immune tolerance and recalibrate the immune system,” said their chief medical officer, Leonard Dragone, MD, PhD.
These therapies are derived from terminally differentiated cells that have limited capacity to produce pro-inflammatory cytokines including interleukin-2 or interferon gamma, Dr. Dragone explained. “CRS is difficult to envision from engineered Treg products and hasn’t been observed in any clinical experience with polyclonal Tregs,” he said.
This approach also does not require lymphodepletion prior to treatment. The company’s Treg cellular therapy for progressive MS is currently in investigational new drug-enabling studies, and they aim to dose their first patients in 2024.
Precision immunotherapy
For B-cell driven autoimmune diseases where the autoantibody is known, researchers have begun to re-engineer T cells to recognize only autoreactive B cells. While CD19 CAR T cells act more like a sledgehammer, these precision cellular immunotherapies are “like a razor’s strike,” Dr. June said.
“The chimeric autoantibody receptor (CAAR) approach targets autoantibodies that are expressed only on the surface of autoimmune B cells and are not expressed on normal B cells, which ideally should lead to precision targeting of just the cells that cause autoimmune disease,” explained Aimee Payne, MD, PhD, professor of dermatology and director of the Penn Clinical Autoimmunity Center of Excellence at the University of Pennsylvania, Philadelphia.
She and her research team used this approach to develop a treatment for mucosal pemphigus vulgaris, an autoimmune blistering disease of mucous membranes driven by autoantibodies against desmoglein 3.
“The current standard of care for pemphigus is to treat with steroids and rituximab, an infusion therapy that results in global, but temporary, B-cell depletion,” she said. “By expressing desmoglein 3 (DSG3) on the surface of the CAAR T-cell therapy, we target just the anti-DSG3 B cells that cause disease in mucosal pemphigus vulgaris and spare the healthy B cells.”
The therapy – called DSG3-CAART – is being developed by Cabaletta Bio and is now in phase 1 clinical trials. The approach is also being investigated to treat certain types of myasthenia gravis and membranous nephropathy.
Dr. Konig’s lab at Johns Hopkins developed and is now exploring a new precision cellular immunotherapy approach, chimeric autoantigen-T cell receptor (CATCR) T-cell therapy, to treat antiphospholipid syndrome, which is in preclinical stages. In this approach, Dr. Konig and his team are “re-engineering the natural T-cell receptor to selectively kill disease-causing B cells that drive antiphospholipid syndrome,” he explained.
He anticipates the CD19 CAR T-cell therapies currently in clinical trials will help to pave the way for this new generation of precision cellular therapies. The ultimate goal of these therapies, he said, is to uncouple therapeutic potency from infection risk.
“That’s really the holy grail in the treatment of autoimmune diseases. It’s tantalizingly close, but we’re not there yet.”
Dr. June is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives license revenue from such licenses. Dr. June is a scientific founder of Tmunity Therapeutics and Capstan Therapeutics and is a member of the scientific advisory boards of AC Immune SA, Alaunos, BlueSphere Bio, Cabaletta, Carisma, Cartography Biosciences, Cellares, Celldex, Decheng Capital, Poseida, Verismo, and WIRB-Copernicus Group. Dr. Konig is a consultant for argenx and Revel and is listed as inventor for patent applications filed by John Hopkins University. Dr. Payne holds equity, grants, payments, and patent licensing from Cabaletta Bio and consults for Janssen.
A version of this article first appeared on Medscape.com.
A revolutionary treatment for cancers may also be able to treat and reset the immune system to provide long-term remission or possibly even cure certain autoimmune diseases.
Chimeric antigen receptor (CAR) T-cell therapy has offered a novel approach to treating hematologic cancers since 2017, but there are early signs that these cellular immunotherapies could be repurposed for B-cell mediated autoimmune diseases.
In September of last year, researchers in Germany reported that five patients with refractory systemic lupus erythematosus (SLE) treated with CAR T-cell therapy all achieved drug-free remission. At the time of publication, no patients had relapsed for up to 17 months after treatment. The authors described seroconversion of antinuclear antibodies in two patients with the longest follow-up, “indicating that abrogation of autoimmune B-cell clones may lead to a more widespread correction of autoimmunity,” the researchers write.
In another case study published in June, researchers used CD-19 targeted CAR-T cells to treat a 41-year-old man with refractory antisynthetase syndrome with progressive myositis and interstitial lung disease. Six months after treatment, there were no signs of myositis on MRI and a chest CT scan showed full regression of alveolitis.
Since then, two biotechnology companies – Cabaletta Bio in Philadelphia and Kyverna Therapeutics in Emeryville, Calif. – have already been granted fast-track designations from the U.S. Food and Drug Administration for CAR T-cell therapy for SLE and lupus nephritis. Bristol-Myers Squibb is also conducting a phase 1 trial in patients with severe, refractory SLE. Several biotechnology companies and hospitals in China are also conducting clinical trials for SLE. But this is only the tip of the iceberg regarding cellular therapies for autoimmune disease, said Max Konig, MD, PhD, an assistant professor of medicine in the division of rheumatology at Johns Hopkins University, Baltimore.
“It’s an incredibly exciting time. It’s unprecedented in the history of autoimmunity,” he noted.
A ‘reboot’ for the immune system
B-cell targeted therapies have been around since the early 2000s with drugs like rituximab, a monoclonal antibody medication that targets CD20, an antigen expressed on the surface of B cells. The CAR T cells currently available target another surface antigen, CD19, and are a much more potent therapy. Both are effective at depleting B cells in blood, but these engineered CD19-targeted T cells can reach B cells sitting in tissues in a way that antibody therapies cannot, Dr. Konig explained.
“If you have a patient with myositis, for example, where autoreactive B cells are sitting in the inflamed muscle, or a patient with rheumatoid arthritis, where you have disease-relevant B cells in hard-to-reach tissues like the synovium, those cells are much harder to deplete with an antibody, compared to a T cell that evolved to surveil and effectively kill in all tissues,” he explained.
In this process, T cells are collected from patients via leukapheresis and then re-engineered to express chimeric antigen receptors. A few days before these modified T cells are infused back into the patient, the patients are given a low-dose chemotherapy (lymphodepletion) regimen to help increase the effectiveness of the therapy. The one-time infusion is generally given on an inpatient basis, and patients are then monitored in hospital for side effects.
Once B cells are depleted, disease symptoms improve. But in the case studies published to date, once B cells re-emerge, they are naïve and no longer producing autoreactive B cells.
“Maybe it’s like a tabula rasa: You wipe [the B cells] out and start with a clean slate. Then, the immune system reboots, and now it’s working, whereas before it was messed up,” said Carl June, MD, who directs the Center for Cellular Immunotherapies at the at the University of Pennsylvania, Philadelphia. Dr. June and his research team led the development of CAR T-cell therapies for blood cancers.
The findings suggest that autoantibodies “might not be hardwired into the immune system,” he said.
But Dr. Konig stressed that we are still in the early days of clinical trials, and more research is necessary to understand the safety and efficacy of these therapies.
“There’s an incredible buzz around CAR T cells at the moment in rheumatology, which is great because I think that’s where the future is,” he said. “But we still need to learn how to appropriately apply these therapies in randomized, controlled trials.”
So far, the evidence behind CD19 CAR T-cell therapies in autoimmune disease is from case studies and phase 1 trials in a very small number of selected patients. (The upcoming Cabaletta and Kyverna trials in lupus will also be small, consisting of 12 patients each.)
Risks of intensive therapy
But while these therapies show promise, the process is very intensive. The lymphodepleting regimen increases the risk for infection and patients are commonly hospitalized for a week or more following infusion for toxicity monitoring. Serious adverse events such as cytokine release syndrome (CRS) can occur days to weeks after CAR T-cell infusion. In the five-patient case series reported in 2022, patients were hospitalized for 10 days following treatment.
The patient with antisynthetase syndrome, as well as three of five patients in the SLE case series study experienced mild CRS following infusion. Patients are also at a high risk for infection, as the engineered T cells target all B cells, not just the autoreactive immune cells.
The inability to differentiate between disease-causing and protective immune cells is an issue for all currently available drugs treating autoimmune disease, Dr. Konig said. But scientists are already working on how to make these potent cellular therapies safer and more precise.
Alternatives to standard CAR T-cell therapies
Engineering T cells with RNA is a new approach to limit the side effects and toxicity of CAR T-cell therapy, said Chris Jewell, PhD, the chief scientific officer at Cartesian Therapeutics, a biotechnology company based in Gaithersburg, Md. The company’s RNA CAR T-cell (rCAR-T) therapy – called DESCARTES-08 – is in phase 2 clinical trials for treatment of myasthenia gravis. Once these rCAR-T cells are infused in patients, as they divide, the RNAnaturally decays, he explained, meaning that after a certain point, the CAR is no longer expressed.
DESCARTES-08 targets B-cell maturation antigen (BCMA), which is primarily expressed on plasma cells, rather than all B cells, Dr. Jewell said.
“Targeting BCMA, we actually have a more selective profile,” he explained. “We are targeting the cells primarily responsible for the pathogenicity; many plasma cells – such as long-lived plasma cells – also take a long time to repopulate.”
This therapy also does not require lymphodepletion prior to infusion and can be done in an outpatient setting. The therapy is given in multiple infusions, once per week.
In the most recent clinical trial, patients with myasthenia gravis received six infusions over 6 weeks and experienced notable decreases in myasthenia gravis severity scale at up to 9 months of follow-up.
While standard CAR T-cell therapies under clinical investigational up to now all use effector T cells, regulatory T cells (Tregs) can also be engineered to target autoimmune disease. Abata Therapeutics, based in Boston, is using this approach for therapies for progressive multiple sclerosis and type 1 diabetes. These engineered Tregs express a T-cell receptor (TCR) that recognizes tissue-specific antigens and suppress inflammation at the site of the disease. “Treg-based cell therapies are really harnessing the natural power of regulatory cells to reset immune tolerance and recalibrate the immune system,” said their chief medical officer, Leonard Dragone, MD, PhD.
These therapies are derived from terminally differentiated cells that have limited capacity to produce pro-inflammatory cytokines including interleukin-2 or interferon gamma, Dr. Dragone explained. “CRS is difficult to envision from engineered Treg products and hasn’t been observed in any clinical experience with polyclonal Tregs,” he said.
This approach also does not require lymphodepletion prior to treatment. The company’s Treg cellular therapy for progressive MS is currently in investigational new drug-enabling studies, and they aim to dose their first patients in 2024.
Precision immunotherapy
For B-cell driven autoimmune diseases where the autoantibody is known, researchers have begun to re-engineer T cells to recognize only autoreactive B cells. While CD19 CAR T cells act more like a sledgehammer, these precision cellular immunotherapies are “like a razor’s strike,” Dr. June said.
“The chimeric autoantibody receptor (CAAR) approach targets autoantibodies that are expressed only on the surface of autoimmune B cells and are not expressed on normal B cells, which ideally should lead to precision targeting of just the cells that cause autoimmune disease,” explained Aimee Payne, MD, PhD, professor of dermatology and director of the Penn Clinical Autoimmunity Center of Excellence at the University of Pennsylvania, Philadelphia.
She and her research team used this approach to develop a treatment for mucosal pemphigus vulgaris, an autoimmune blistering disease of mucous membranes driven by autoantibodies against desmoglein 3.
“The current standard of care for pemphigus is to treat with steroids and rituximab, an infusion therapy that results in global, but temporary, B-cell depletion,” she said. “By expressing desmoglein 3 (DSG3) on the surface of the CAAR T-cell therapy, we target just the anti-DSG3 B cells that cause disease in mucosal pemphigus vulgaris and spare the healthy B cells.”
The therapy – called DSG3-CAART – is being developed by Cabaletta Bio and is now in phase 1 clinical trials. The approach is also being investigated to treat certain types of myasthenia gravis and membranous nephropathy.
Dr. Konig’s lab at Johns Hopkins developed and is now exploring a new precision cellular immunotherapy approach, chimeric autoantigen-T cell receptor (CATCR) T-cell therapy, to treat antiphospholipid syndrome, which is in preclinical stages. In this approach, Dr. Konig and his team are “re-engineering the natural T-cell receptor to selectively kill disease-causing B cells that drive antiphospholipid syndrome,” he explained.
He anticipates the CD19 CAR T-cell therapies currently in clinical trials will help to pave the way for this new generation of precision cellular therapies. The ultimate goal of these therapies, he said, is to uncouple therapeutic potency from infection risk.
“That’s really the holy grail in the treatment of autoimmune diseases. It’s tantalizingly close, but we’re not there yet.”
Dr. June is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives license revenue from such licenses. Dr. June is a scientific founder of Tmunity Therapeutics and Capstan Therapeutics and is a member of the scientific advisory boards of AC Immune SA, Alaunos, BlueSphere Bio, Cabaletta, Carisma, Cartography Biosciences, Cellares, Celldex, Decheng Capital, Poseida, Verismo, and WIRB-Copernicus Group. Dr. Konig is a consultant for argenx and Revel and is listed as inventor for patent applications filed by John Hopkins University. Dr. Payne holds equity, grants, payments, and patent licensing from Cabaletta Bio and consults for Janssen.
A version of this article first appeared on Medscape.com.
Ginger consumption may mitigate neutrophil dysfunction and inflammation
TOPLINE:
Blood samples from healthy adults show an inhibition of neutrophil extracellular trap formation (NET) after 1 week of daily ginger supplements.
METHODOLOGY:
- Researchers recruited nine healthy adults aged 18-38 years to receive a 100-mg oral ginger supplement daily for 7 consecutive days.
- Blood samples were collected at baseline and on days 7 and 14, with isolation of neutrophils, peripheral blood mononuclear cells, and plasma.
- The researchers measured NET formation (NETosis) as a way to show the effect of ginger on inflammation.
TAKEAWAY:
- Measures of neutrophil cyclic AMP (cAMP) were significantly higher after 7 days of ginger supplements, compared with baseline levels, although these levels returned to near baseline by 1 week after discontinuing ginger consumption.
- Oral ginger supplements reduced neutrophil phosphodiesterase (PDE) activity by 40% from baseline, similar to results seen with synthetic PDE4 inhibitors.
- The results build on previous studies showing inhibition of neutrophil hyperactivity in mice with antiphospholipid syndrome and lupus after injection with a purified ginger preparation.
- Researchers replicated the results showing effects of oral ginger on neutrophils in eight additional healthy adults who also showed reduced NETosis and increased cAMP after 1 week of ginger supplements.
IN PRACTICE:
The results show biologic support for the potential of ginger to affect neutrophil function in humans; therefore, “ginger may have a real ability to complement treatment programs that are already underway,” said corresponding author Jason Knight, MD, of the University of Michigan, Ann Arbor, in a press release.
SOURCE:
First author Ramadan A. Ali, MD, of the University of Michigan, Ann Arbor, and colleagues reported their study in JCI Insight.
LIMITATIONS:
More research is needed in humans with inflammatory and autoimmune diseases to confirm the findings and explore ginger as an adjuvant therapeutic intervention.
DISCLOSURES:
The study received no outside funding. The researchers report no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
Blood samples from healthy adults show an inhibition of neutrophil extracellular trap formation (NET) after 1 week of daily ginger supplements.
METHODOLOGY:
- Researchers recruited nine healthy adults aged 18-38 years to receive a 100-mg oral ginger supplement daily for 7 consecutive days.
- Blood samples were collected at baseline and on days 7 and 14, with isolation of neutrophils, peripheral blood mononuclear cells, and plasma.
- The researchers measured NET formation (NETosis) as a way to show the effect of ginger on inflammation.
TAKEAWAY:
- Measures of neutrophil cyclic AMP (cAMP) were significantly higher after 7 days of ginger supplements, compared with baseline levels, although these levels returned to near baseline by 1 week after discontinuing ginger consumption.
- Oral ginger supplements reduced neutrophil phosphodiesterase (PDE) activity by 40% from baseline, similar to results seen with synthetic PDE4 inhibitors.
- The results build on previous studies showing inhibition of neutrophil hyperactivity in mice with antiphospholipid syndrome and lupus after injection with a purified ginger preparation.
- Researchers replicated the results showing effects of oral ginger on neutrophils in eight additional healthy adults who also showed reduced NETosis and increased cAMP after 1 week of ginger supplements.
IN PRACTICE:
The results show biologic support for the potential of ginger to affect neutrophil function in humans; therefore, “ginger may have a real ability to complement treatment programs that are already underway,” said corresponding author Jason Knight, MD, of the University of Michigan, Ann Arbor, in a press release.
SOURCE:
First author Ramadan A. Ali, MD, of the University of Michigan, Ann Arbor, and colleagues reported their study in JCI Insight.
LIMITATIONS:
More research is needed in humans with inflammatory and autoimmune diseases to confirm the findings and explore ginger as an adjuvant therapeutic intervention.
DISCLOSURES:
The study received no outside funding. The researchers report no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
Blood samples from healthy adults show an inhibition of neutrophil extracellular trap formation (NET) after 1 week of daily ginger supplements.
METHODOLOGY:
- Researchers recruited nine healthy adults aged 18-38 years to receive a 100-mg oral ginger supplement daily for 7 consecutive days.
- Blood samples were collected at baseline and on days 7 and 14, with isolation of neutrophils, peripheral blood mononuclear cells, and plasma.
- The researchers measured NET formation (NETosis) as a way to show the effect of ginger on inflammation.
TAKEAWAY:
- Measures of neutrophil cyclic AMP (cAMP) were significantly higher after 7 days of ginger supplements, compared with baseline levels, although these levels returned to near baseline by 1 week after discontinuing ginger consumption.
- Oral ginger supplements reduced neutrophil phosphodiesterase (PDE) activity by 40% from baseline, similar to results seen with synthetic PDE4 inhibitors.
- The results build on previous studies showing inhibition of neutrophil hyperactivity in mice with antiphospholipid syndrome and lupus after injection with a purified ginger preparation.
- Researchers replicated the results showing effects of oral ginger on neutrophils in eight additional healthy adults who also showed reduced NETosis and increased cAMP after 1 week of ginger supplements.
IN PRACTICE:
The results show biologic support for the potential of ginger to affect neutrophil function in humans; therefore, “ginger may have a real ability to complement treatment programs that are already underway,” said corresponding author Jason Knight, MD, of the University of Michigan, Ann Arbor, in a press release.
SOURCE:
First author Ramadan A. Ali, MD, of the University of Michigan, Ann Arbor, and colleagues reported their study in JCI Insight.
LIMITATIONS:
More research is needed in humans with inflammatory and autoimmune diseases to confirm the findings and explore ginger as an adjuvant therapeutic intervention.
DISCLOSURES:
The study received no outside funding. The researchers report no relevant financial relationships.
A version of this article appeared on Medscape.com.