User login
Older NHL survivors show worse cognitive decline
Older long-term survivors of non-Hodgkin lymphoma (NHL) may have worse cognitive outcomes compared with the noncancer aging population, according to a cross-sectional study.
The findings suggest additional research is needed to better understand cognitive decline in older survivors of NHL.
“The aim of the present study was to examine the difference in cognitive status between a group of long-term older survivors of NHL compared with a group of noncancer controls of the same age,” wrote Domenico La Carpia, MD, of Fondazione ANT Italia Onlus, Florence, Italy, and colleagues.
The researchers conducted a multicenter cross-sectional cohort study involving 63 long-term survivors of NHL and 61 age-matched controls. Their report was published in the Journal of Geriatric Oncology.
Eligible survivors and controls were aged 65 years and older. Among both groups, the mean age of study participants was 74 years, and most survivors were women (58.7%).
While cognitive decline was assessed via standardized neuropsychological testing, the team also evaluated polypharmacy, functional status, and level of multimorbidity in the cohort.
Other clinical data, including the time from complete remission, type of treatment received, and histopathological type of tumor, were collected from patient charts and included in the analysis.
After analysis, the researchers found that NHL survivors had a higher mean number of chronic conditions (3.4 vs. 2.3; P = .003), were receiving more medications (3.4 vs. 2.3; P = .03), and had worse functional status compared with controls.
In addition, survivors had impaired executive functioning compared with control subjects (Trail Making Test B-A, 47.9 vs. 32.1; P = .04), but scores on the Mini Mental State Examination (MMSE) did not differ between the groups.
“A small, statistically significant difference was also observed in verbal memory scores between the two groups,” they reported.
The researchers acknowledged that a key limitation was the cross-sectional nature of the study; hence, causality cannot be inferred from the data.
“Comprehensive geriatric assessment for older cancer survivors is advisable to identify those individuals who are at highest risk of developing disability and to implement tailored early interventions,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: La Carpia D et al. J Geriatr Oncol. 2020 Jan 31. doi: 10.1016/j.jgo.2020.01.007.
Older long-term survivors of non-Hodgkin lymphoma (NHL) may have worse cognitive outcomes compared with the noncancer aging population, according to a cross-sectional study.
The findings suggest additional research is needed to better understand cognitive decline in older survivors of NHL.
“The aim of the present study was to examine the difference in cognitive status between a group of long-term older survivors of NHL compared with a group of noncancer controls of the same age,” wrote Domenico La Carpia, MD, of Fondazione ANT Italia Onlus, Florence, Italy, and colleagues.
The researchers conducted a multicenter cross-sectional cohort study involving 63 long-term survivors of NHL and 61 age-matched controls. Their report was published in the Journal of Geriatric Oncology.
Eligible survivors and controls were aged 65 years and older. Among both groups, the mean age of study participants was 74 years, and most survivors were women (58.7%).
While cognitive decline was assessed via standardized neuropsychological testing, the team also evaluated polypharmacy, functional status, and level of multimorbidity in the cohort.
Other clinical data, including the time from complete remission, type of treatment received, and histopathological type of tumor, were collected from patient charts and included in the analysis.
After analysis, the researchers found that NHL survivors had a higher mean number of chronic conditions (3.4 vs. 2.3; P = .003), were receiving more medications (3.4 vs. 2.3; P = .03), and had worse functional status compared with controls.
In addition, survivors had impaired executive functioning compared with control subjects (Trail Making Test B-A, 47.9 vs. 32.1; P = .04), but scores on the Mini Mental State Examination (MMSE) did not differ between the groups.
“A small, statistically significant difference was also observed in verbal memory scores between the two groups,” they reported.
The researchers acknowledged that a key limitation was the cross-sectional nature of the study; hence, causality cannot be inferred from the data.
“Comprehensive geriatric assessment for older cancer survivors is advisable to identify those individuals who are at highest risk of developing disability and to implement tailored early interventions,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: La Carpia D et al. J Geriatr Oncol. 2020 Jan 31. doi: 10.1016/j.jgo.2020.01.007.
Older long-term survivors of non-Hodgkin lymphoma (NHL) may have worse cognitive outcomes compared with the noncancer aging population, according to a cross-sectional study.
The findings suggest additional research is needed to better understand cognitive decline in older survivors of NHL.
“The aim of the present study was to examine the difference in cognitive status between a group of long-term older survivors of NHL compared with a group of noncancer controls of the same age,” wrote Domenico La Carpia, MD, of Fondazione ANT Italia Onlus, Florence, Italy, and colleagues.
The researchers conducted a multicenter cross-sectional cohort study involving 63 long-term survivors of NHL and 61 age-matched controls. Their report was published in the Journal of Geriatric Oncology.
Eligible survivors and controls were aged 65 years and older. Among both groups, the mean age of study participants was 74 years, and most survivors were women (58.7%).
While cognitive decline was assessed via standardized neuropsychological testing, the team also evaluated polypharmacy, functional status, and level of multimorbidity in the cohort.
Other clinical data, including the time from complete remission, type of treatment received, and histopathological type of tumor, were collected from patient charts and included in the analysis.
After analysis, the researchers found that NHL survivors had a higher mean number of chronic conditions (3.4 vs. 2.3; P = .003), were receiving more medications (3.4 vs. 2.3; P = .03), and had worse functional status compared with controls.
In addition, survivors had impaired executive functioning compared with control subjects (Trail Making Test B-A, 47.9 vs. 32.1; P = .04), but scores on the Mini Mental State Examination (MMSE) did not differ between the groups.
“A small, statistically significant difference was also observed in verbal memory scores between the two groups,” they reported.
The researchers acknowledged that a key limitation was the cross-sectional nature of the study; hence, causality cannot be inferred from the data.
“Comprehensive geriatric assessment for older cancer survivors is advisable to identify those individuals who are at highest risk of developing disability and to implement tailored early interventions,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: La Carpia D et al. J Geriatr Oncol. 2020 Jan 31. doi: 10.1016/j.jgo.2020.01.007.
FROM THE JOURNAL OF GERIATRIC ONCOLOGY
CRISPR-engineered T cells may be safe for cancer, but do they work?
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
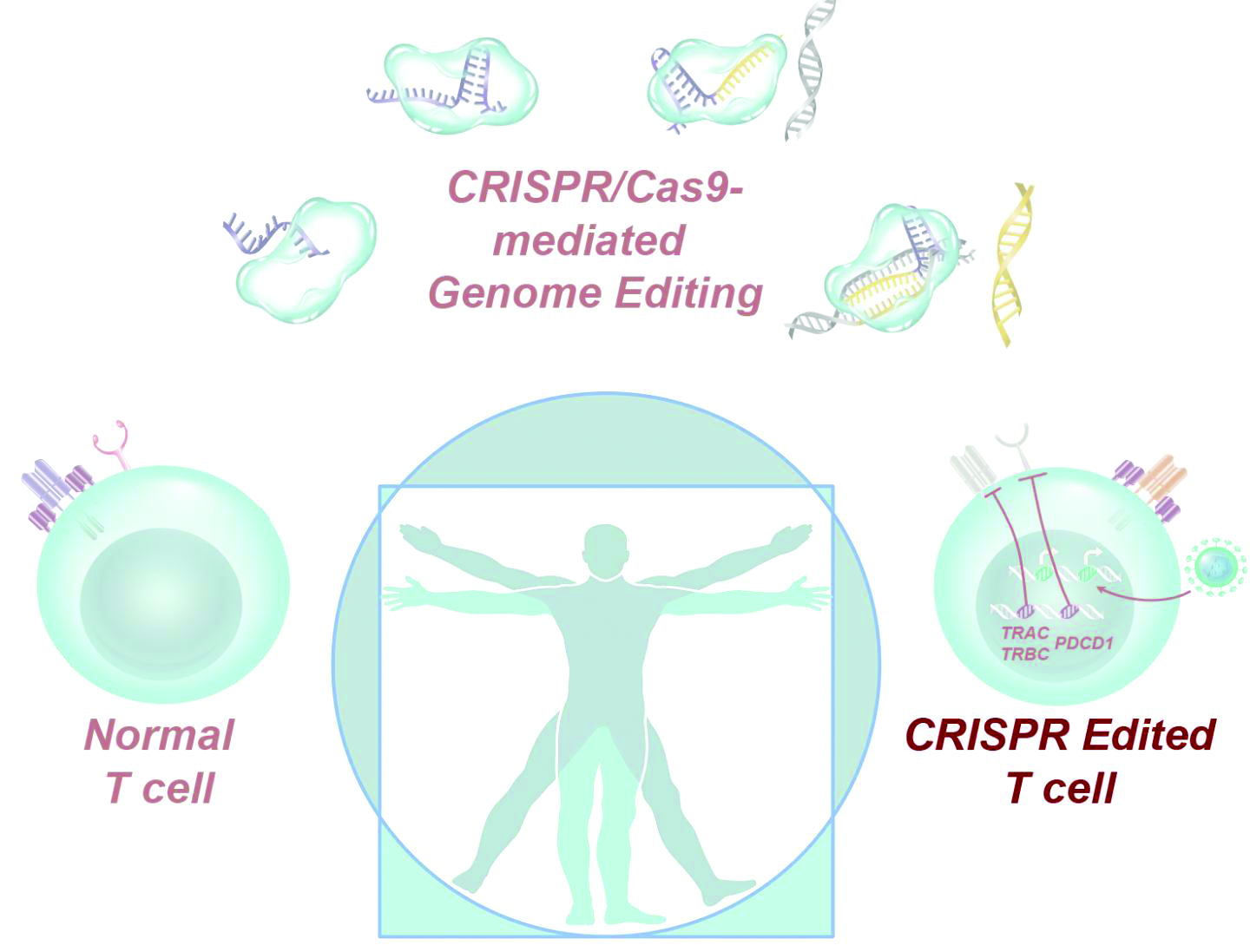
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
FROM SCIENCE
Global project reveals cancer’s genomic playbook
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE
ECHELON-1 update: A+AVD bests ABVD in Hodgkin lymphoma
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
FROM BLOOD
Adding lymphopenia component ‘improves’ FLIPI
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
FROM BLOOD CANCER JOURNAL
Experts break down latest CAR T-cell advances in lymphoma
ORLANDO – There’s now mature data surrounding the use of chimeric antigen receptor (CAR) T-cell therapy in lymphoma, and the annual meeting of the American Society of Hematology brought forth additional information from real-world studies, insights about what is driving relapse, and promising data on mantle cell lymphoma.

The roundtable participants included Brian Hill, MD, of the Cleveland Clinic Taussig Cancer Center; Frederick L. Locke, MD, of the Moffit Cancer Center in Tampa, Fla.; and Peter Riedell, MD, of the University of Chicago.
Among the studies highlighted by the panel was the Transcend NHL 001 study (Abstract 241), which looked at third-line use of lisocabtagene maraleucel (liso-cel) in patients with diffuse large B-cell lymphoma, transformed follicular lymphoma, and other indolent non-Hodgkin lymphoma subtypes. More than 300 patients were enrolled, and liso-cel met all primary and secondary efficacy endpoints, with an overall response rate of more than 70%. The notable take-home point from the study was the safety profile, Dr. Riedell noted. Liso-cel was associated with a lower rate of cytokine release syndrome and neurologic toxicity, compared with the currently approved products.
Since patients in the study had a lower incidence and later onset of cytokine release syndrome, liso-cel could be a candidate for outpatient administration, Dr. Locke said. However, doing that would require “significant infrastructure” in hospitals and clinics to properly support patients, especially given that the treatment-related mortality on the study was similar to approved CAR T-cell products at about 3%. “You have to be ready to admit the patient to the hospital very rapidly, and you have to have the providers and the nurses who are vigilant when the patient is not in the hospital,” he said.
Another notable study presented at ASH examined the characteristics and outcomes of patients receiving bridging therapy while awaiting treatment with axicabtagene ciloleucel (Abstract 245). This real-world study adds interesting information to the field because, in some of the studies that were pivotal to the approval of CAR T-cell therapy, bridging therapy was not allowed, Dr. Locke said.
In this analysis, researchers found that the overall survival was worse among patients who received bridging. This finding suggests that patients who received bridging therapy had a different biology or that the therapy itself may have had an effect on the host or tumor microenvironment that affected the efficacy of the CAR T-cell therapy, the researchers reported.
The panel also highlighted the Zuma-2 study, which looked at KTE-X19, an anti-CD19 CAR T-cell therapy, among more than 70 patients with relapsed/refractory mantle cell lymphoma who had failed treatment with a Bruton’s tyrosine kinase inhibitor (Abstract 754). “This was, I thought, kind of a sleeper study at ASH,” said Dr. Hill, who was one of the authors of the study.
The overall response rate was 93% with about two-thirds of patients achieving a complete response. Researchers found that the response was consistent across subgroups, including Ki-67 and patients with prior use of steroids or bridging therapy. Dr. Locke, who was also a study author, said the results are a “game changer.”
“I’m very excited about it,” Dr. Riedell said, noting that these are patients without a lot of treatment options.
The panel also discussed other studies from ASH, including an analysis of tumor tissue samples from patients in the ZUMA-1 trial who had responded and subsequently relapsed (Abstract 203); a multicenter prospective analysis of circulating tumor DNA in diffuse large B-cell lymphoma patients who had relapsed after treatment with axicabtagene ciloleucel (Abstract 884); and the early use of corticosteroids to prevent toxicities in patients in cohort 4 of the ZUMA-1 trial (Abstract 243).
Dr. Hill reported consulting with Juno/Celgene/BMS and Novartis and research and consulting for Kite/Gilead. Dr. Locke reported consulting for Cellular Biomedicine Group and being a scientific adviser to Kite/Gilead, Novartis, Celgene/BMS, GammaDelta Therapeutics, Calibr, and Allogene. Dr. Riedell reported consulting for Bayer and Verastem, consulting for and research funding from Novartis and BMS/Celgene, and consulting for, research funding from, and speaking for Kite.
ORLANDO – There’s now mature data surrounding the use of chimeric antigen receptor (CAR) T-cell therapy in lymphoma, and the annual meeting of the American Society of Hematology brought forth additional information from real-world studies, insights about what is driving relapse, and promising data on mantle cell lymphoma.

The roundtable participants included Brian Hill, MD, of the Cleveland Clinic Taussig Cancer Center; Frederick L. Locke, MD, of the Moffit Cancer Center in Tampa, Fla.; and Peter Riedell, MD, of the University of Chicago.
Among the studies highlighted by the panel was the Transcend NHL 001 study (Abstract 241), which looked at third-line use of lisocabtagene maraleucel (liso-cel) in patients with diffuse large B-cell lymphoma, transformed follicular lymphoma, and other indolent non-Hodgkin lymphoma subtypes. More than 300 patients were enrolled, and liso-cel met all primary and secondary efficacy endpoints, with an overall response rate of more than 70%. The notable take-home point from the study was the safety profile, Dr. Riedell noted. Liso-cel was associated with a lower rate of cytokine release syndrome and neurologic toxicity, compared with the currently approved products.
Since patients in the study had a lower incidence and later onset of cytokine release syndrome, liso-cel could be a candidate for outpatient administration, Dr. Locke said. However, doing that would require “significant infrastructure” in hospitals and clinics to properly support patients, especially given that the treatment-related mortality on the study was similar to approved CAR T-cell products at about 3%. “You have to be ready to admit the patient to the hospital very rapidly, and you have to have the providers and the nurses who are vigilant when the patient is not in the hospital,” he said.
Another notable study presented at ASH examined the characteristics and outcomes of patients receiving bridging therapy while awaiting treatment with axicabtagene ciloleucel (Abstract 245). This real-world study adds interesting information to the field because, in some of the studies that were pivotal to the approval of CAR T-cell therapy, bridging therapy was not allowed, Dr. Locke said.
In this analysis, researchers found that the overall survival was worse among patients who received bridging. This finding suggests that patients who received bridging therapy had a different biology or that the therapy itself may have had an effect on the host or tumor microenvironment that affected the efficacy of the CAR T-cell therapy, the researchers reported.
The panel also highlighted the Zuma-2 study, which looked at KTE-X19, an anti-CD19 CAR T-cell therapy, among more than 70 patients with relapsed/refractory mantle cell lymphoma who had failed treatment with a Bruton’s tyrosine kinase inhibitor (Abstract 754). “This was, I thought, kind of a sleeper study at ASH,” said Dr. Hill, who was one of the authors of the study.
The overall response rate was 93% with about two-thirds of patients achieving a complete response. Researchers found that the response was consistent across subgroups, including Ki-67 and patients with prior use of steroids or bridging therapy. Dr. Locke, who was also a study author, said the results are a “game changer.”
“I’m very excited about it,” Dr. Riedell said, noting that these are patients without a lot of treatment options.
The panel also discussed other studies from ASH, including an analysis of tumor tissue samples from patients in the ZUMA-1 trial who had responded and subsequently relapsed (Abstract 203); a multicenter prospective analysis of circulating tumor DNA in diffuse large B-cell lymphoma patients who had relapsed after treatment with axicabtagene ciloleucel (Abstract 884); and the early use of corticosteroids to prevent toxicities in patients in cohort 4 of the ZUMA-1 trial (Abstract 243).
Dr. Hill reported consulting with Juno/Celgene/BMS and Novartis and research and consulting for Kite/Gilead. Dr. Locke reported consulting for Cellular Biomedicine Group and being a scientific adviser to Kite/Gilead, Novartis, Celgene/BMS, GammaDelta Therapeutics, Calibr, and Allogene. Dr. Riedell reported consulting for Bayer and Verastem, consulting for and research funding from Novartis and BMS/Celgene, and consulting for, research funding from, and speaking for Kite.
ORLANDO – There’s now mature data surrounding the use of chimeric antigen receptor (CAR) T-cell therapy in lymphoma, and the annual meeting of the American Society of Hematology brought forth additional information from real-world studies, insights about what is driving relapse, and promising data on mantle cell lymphoma.

The roundtable participants included Brian Hill, MD, of the Cleveland Clinic Taussig Cancer Center; Frederick L. Locke, MD, of the Moffit Cancer Center in Tampa, Fla.; and Peter Riedell, MD, of the University of Chicago.
Among the studies highlighted by the panel was the Transcend NHL 001 study (Abstract 241), which looked at third-line use of lisocabtagene maraleucel (liso-cel) in patients with diffuse large B-cell lymphoma, transformed follicular lymphoma, and other indolent non-Hodgkin lymphoma subtypes. More than 300 patients were enrolled, and liso-cel met all primary and secondary efficacy endpoints, with an overall response rate of more than 70%. The notable take-home point from the study was the safety profile, Dr. Riedell noted. Liso-cel was associated with a lower rate of cytokine release syndrome and neurologic toxicity, compared with the currently approved products.
Since patients in the study had a lower incidence and later onset of cytokine release syndrome, liso-cel could be a candidate for outpatient administration, Dr. Locke said. However, doing that would require “significant infrastructure” in hospitals and clinics to properly support patients, especially given that the treatment-related mortality on the study was similar to approved CAR T-cell products at about 3%. “You have to be ready to admit the patient to the hospital very rapidly, and you have to have the providers and the nurses who are vigilant when the patient is not in the hospital,” he said.
Another notable study presented at ASH examined the characteristics and outcomes of patients receiving bridging therapy while awaiting treatment with axicabtagene ciloleucel (Abstract 245). This real-world study adds interesting information to the field because, in some of the studies that were pivotal to the approval of CAR T-cell therapy, bridging therapy was not allowed, Dr. Locke said.
In this analysis, researchers found that the overall survival was worse among patients who received bridging. This finding suggests that patients who received bridging therapy had a different biology or that the therapy itself may have had an effect on the host or tumor microenvironment that affected the efficacy of the CAR T-cell therapy, the researchers reported.
The panel also highlighted the Zuma-2 study, which looked at KTE-X19, an anti-CD19 CAR T-cell therapy, among more than 70 patients with relapsed/refractory mantle cell lymphoma who had failed treatment with a Bruton’s tyrosine kinase inhibitor (Abstract 754). “This was, I thought, kind of a sleeper study at ASH,” said Dr. Hill, who was one of the authors of the study.
The overall response rate was 93% with about two-thirds of patients achieving a complete response. Researchers found that the response was consistent across subgroups, including Ki-67 and patients with prior use of steroids or bridging therapy. Dr. Locke, who was also a study author, said the results are a “game changer.”
“I’m very excited about it,” Dr. Riedell said, noting that these are patients without a lot of treatment options.
The panel also discussed other studies from ASH, including an analysis of tumor tissue samples from patients in the ZUMA-1 trial who had responded and subsequently relapsed (Abstract 203); a multicenter prospective analysis of circulating tumor DNA in diffuse large B-cell lymphoma patients who had relapsed after treatment with axicabtagene ciloleucel (Abstract 884); and the early use of corticosteroids to prevent toxicities in patients in cohort 4 of the ZUMA-1 trial (Abstract 243).
Dr. Hill reported consulting with Juno/Celgene/BMS and Novartis and research and consulting for Kite/Gilead. Dr. Locke reported consulting for Cellular Biomedicine Group and being a scientific adviser to Kite/Gilead, Novartis, Celgene/BMS, GammaDelta Therapeutics, Calibr, and Allogene. Dr. Riedell reported consulting for Bayer and Verastem, consulting for and research funding from Novartis and BMS/Celgene, and consulting for, research funding from, and speaking for Kite.
EXPERT ANALYSIS FROM ASH 2019
Start of myeloma therapy may be delayed for women, minorities
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
FROM JCO ONCOLOGY PRACTICE
Key clinical point:
Major finding: Patients with delays in treatment were more likely to be women (odds ratio, 1.15) and more likely to be non-Hispanic blacks (OR, 1.21).
Study details: Retrospective analysis of 74,722 patients in the National Cancer Database diagnosed with multiple myeloma between 2004 and 2015.
Disclosures: Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
Source: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
CAR T-cell therapy may worsen mental health in some patients
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Adult survivors of childhood cancer are experiencing fewer major cardiac events
Adult survivors of pediatric cancers appear to be experiencing fewer major cardiac events in adulthood partly because of reduced radiotherapy exposure, especially among survivors of Hodgkin lymphoma, recent research published in BMJ has shown.
“Contemporary cancer treatment has focused on advancing cure rates while attempting to minimize long term adverse effects,” Daniel A. Mulrooney, MD, of the Division of Cancer Survivorship, Department of Oncology, at St. Jude Children’s Research Hospital, Arlington, Va., and colleagues wrote. “Patterns of exposure to cardiotoxic treatment have changed over time, with fewer children receiving chest directed radiation, with lower doses and smaller volumes for those who do, and an increased use of anthracyclines, albeit with reduced cumulative doses as the risk for late-onset heart failure became apparent.”
Although research has been published on improved survival rates of children who underwent cancer treatment in the 1990s, compared with those who received treatment in the 1980s and 1970s, Dr. Mulrooney and colleagues set out to determine whether cardiac outcomes were reduced as well. They conducted a retrospective study of 23,462 5-year survivors of pediatric cancer, which consisted of leukemia, brain cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft-tissue sarcomas, and bone sarcomas diagnosed between January 1970 and December 1999. Researchers compared the cardiac outcomes of the survivors, including heart failure, coronary artery disease, valvular heart disease, pericardial disease, and arrhythmias, with a comparison group of their siblings (n = 5,057) separated by decade. The adult survivors tended to be women (46% vs. 40%) with a median age of 6.1 years at diagnosis and 27.7 years at final follow-up.
Of the 6,193 participants treated for cancer in the 1970s, the 20-year cumulative incidence of heart failure was 0.69%, while the 9,363 participants treated in the 1980s had an incidence of 0.74%, and 7,906 participants in the 1990s had a cumulative incidence of 0.54% over 20 years. The 20-year cumulative incidence for coronary artery disease (CAD) was 0.38% for participants in the 1970s, 0.24% for participants in the 1980s, and 0.19% for participants in the 1990s (P less than .01). Researchers noted the 20-year cumulative incidence of valvular disease, pericardial disease, and arrhythmias did not decrease between the 1970s and the 1990s.
When comparing the rate of major cardiac events of participants in the 1980s and 1990s with those of the 1970s, CAD diagnoses significantly decreased in the 1980s (hazard ratio, 0.65; 95% confidence interval, 0.45-0.92) and 1990s (HR, 0.53; 95% CI, 0.36-0.77), while there was no significant decrease in heart failure or valvular heart disease risk over time. After adjusting for cardiac radiation, overall risk for CAD was attenuated (HR, 0.90; 0.78-1.05), and Hodgkin lymphoma survivors saw the greatest change between unadjusted (HR, 0.77; 95% CI, 0.66-0.89) and adjusted risk (HR, 0.87; 95% CI, 0.69-1.10) when accounting for cardiac radiation.
“While additional longitudinal follow-up is needed to establish whether similar reductions in the cumulative incidence of heart failure can be confirmed in multivariable analysis, these results suggest that efforts to modify cancer therapies in children and promote health surveillance for survivors are beginning to show benefits not only in overall survival but also in late adverse cardiac effects,” the researchers concluded.
In a related editorial, Mike Hawkins, DPhil, of the Centre for Childhood Cancer Survivor Studies, Institute of Applied Health Research at the University of Birmingham (England), and colleagues said that, while measuring cardiotoxicity is important for this patient population, traditional risk factors with independent associations to cardiac outcomes should also be studied. Guidelines on follow-up for these patients are also needed to inform clinical practice, such as those produced by the International Late Effects of Childhood Cancer Guideline Harmonization Group, they added.
“Survivorship issues are extremely important to patients, their families, and their doctors,” they said. “In two research priority setting initiatives in the United Kingdom, detailed consultation with patients with cancer, survivors, families, friends, and healthcare professionals identified further research into the consequences of cancer as a top priority.”
This study was funded by grants from the National Cancer Institute, Cancer Center Support (CORE) to St. Jude Children’s Research Hospital and American Lebanese Syrian Associated Charities. The authors of the study and the editorial reported no relevant conflicts of interest.
SOURCE: Mulrooney A et al. BMJ. 2020. doi: 10.1136/bmj.l6794.
Adult survivors of pediatric cancers appear to be experiencing fewer major cardiac events in adulthood partly because of reduced radiotherapy exposure, especially among survivors of Hodgkin lymphoma, recent research published in BMJ has shown.
“Contemporary cancer treatment has focused on advancing cure rates while attempting to minimize long term adverse effects,” Daniel A. Mulrooney, MD, of the Division of Cancer Survivorship, Department of Oncology, at St. Jude Children’s Research Hospital, Arlington, Va., and colleagues wrote. “Patterns of exposure to cardiotoxic treatment have changed over time, with fewer children receiving chest directed radiation, with lower doses and smaller volumes for those who do, and an increased use of anthracyclines, albeit with reduced cumulative doses as the risk for late-onset heart failure became apparent.”
Although research has been published on improved survival rates of children who underwent cancer treatment in the 1990s, compared with those who received treatment in the 1980s and 1970s, Dr. Mulrooney and colleagues set out to determine whether cardiac outcomes were reduced as well. They conducted a retrospective study of 23,462 5-year survivors of pediatric cancer, which consisted of leukemia, brain cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft-tissue sarcomas, and bone sarcomas diagnosed between January 1970 and December 1999. Researchers compared the cardiac outcomes of the survivors, including heart failure, coronary artery disease, valvular heart disease, pericardial disease, and arrhythmias, with a comparison group of their siblings (n = 5,057) separated by decade. The adult survivors tended to be women (46% vs. 40%) with a median age of 6.1 years at diagnosis and 27.7 years at final follow-up.
Of the 6,193 participants treated for cancer in the 1970s, the 20-year cumulative incidence of heart failure was 0.69%, while the 9,363 participants treated in the 1980s had an incidence of 0.74%, and 7,906 participants in the 1990s had a cumulative incidence of 0.54% over 20 years. The 20-year cumulative incidence for coronary artery disease (CAD) was 0.38% for participants in the 1970s, 0.24% for participants in the 1980s, and 0.19% for participants in the 1990s (P less than .01). Researchers noted the 20-year cumulative incidence of valvular disease, pericardial disease, and arrhythmias did not decrease between the 1970s and the 1990s.
When comparing the rate of major cardiac events of participants in the 1980s and 1990s with those of the 1970s, CAD diagnoses significantly decreased in the 1980s (hazard ratio, 0.65; 95% confidence interval, 0.45-0.92) and 1990s (HR, 0.53; 95% CI, 0.36-0.77), while there was no significant decrease in heart failure or valvular heart disease risk over time. After adjusting for cardiac radiation, overall risk for CAD was attenuated (HR, 0.90; 0.78-1.05), and Hodgkin lymphoma survivors saw the greatest change between unadjusted (HR, 0.77; 95% CI, 0.66-0.89) and adjusted risk (HR, 0.87; 95% CI, 0.69-1.10) when accounting for cardiac radiation.
“While additional longitudinal follow-up is needed to establish whether similar reductions in the cumulative incidence of heart failure can be confirmed in multivariable analysis, these results suggest that efforts to modify cancer therapies in children and promote health surveillance for survivors are beginning to show benefits not only in overall survival but also in late adverse cardiac effects,” the researchers concluded.
In a related editorial, Mike Hawkins, DPhil, of the Centre for Childhood Cancer Survivor Studies, Institute of Applied Health Research at the University of Birmingham (England), and colleagues said that, while measuring cardiotoxicity is important for this patient population, traditional risk factors with independent associations to cardiac outcomes should also be studied. Guidelines on follow-up for these patients are also needed to inform clinical practice, such as those produced by the International Late Effects of Childhood Cancer Guideline Harmonization Group, they added.
“Survivorship issues are extremely important to patients, their families, and their doctors,” they said. “In two research priority setting initiatives in the United Kingdom, detailed consultation with patients with cancer, survivors, families, friends, and healthcare professionals identified further research into the consequences of cancer as a top priority.”
This study was funded by grants from the National Cancer Institute, Cancer Center Support (CORE) to St. Jude Children’s Research Hospital and American Lebanese Syrian Associated Charities. The authors of the study and the editorial reported no relevant conflicts of interest.
SOURCE: Mulrooney A et al. BMJ. 2020. doi: 10.1136/bmj.l6794.
Adult survivors of pediatric cancers appear to be experiencing fewer major cardiac events in adulthood partly because of reduced radiotherapy exposure, especially among survivors of Hodgkin lymphoma, recent research published in BMJ has shown.
“Contemporary cancer treatment has focused on advancing cure rates while attempting to minimize long term adverse effects,” Daniel A. Mulrooney, MD, of the Division of Cancer Survivorship, Department of Oncology, at St. Jude Children’s Research Hospital, Arlington, Va., and colleagues wrote. “Patterns of exposure to cardiotoxic treatment have changed over time, with fewer children receiving chest directed radiation, with lower doses and smaller volumes for those who do, and an increased use of anthracyclines, albeit with reduced cumulative doses as the risk for late-onset heart failure became apparent.”
Although research has been published on improved survival rates of children who underwent cancer treatment in the 1990s, compared with those who received treatment in the 1980s and 1970s, Dr. Mulrooney and colleagues set out to determine whether cardiac outcomes were reduced as well. They conducted a retrospective study of 23,462 5-year survivors of pediatric cancer, which consisted of leukemia, brain cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft-tissue sarcomas, and bone sarcomas diagnosed between January 1970 and December 1999. Researchers compared the cardiac outcomes of the survivors, including heart failure, coronary artery disease, valvular heart disease, pericardial disease, and arrhythmias, with a comparison group of their siblings (n = 5,057) separated by decade. The adult survivors tended to be women (46% vs. 40%) with a median age of 6.1 years at diagnosis and 27.7 years at final follow-up.
Of the 6,193 participants treated for cancer in the 1970s, the 20-year cumulative incidence of heart failure was 0.69%, while the 9,363 participants treated in the 1980s had an incidence of 0.74%, and 7,906 participants in the 1990s had a cumulative incidence of 0.54% over 20 years. The 20-year cumulative incidence for coronary artery disease (CAD) was 0.38% for participants in the 1970s, 0.24% for participants in the 1980s, and 0.19% for participants in the 1990s (P less than .01). Researchers noted the 20-year cumulative incidence of valvular disease, pericardial disease, and arrhythmias did not decrease between the 1970s and the 1990s.
When comparing the rate of major cardiac events of participants in the 1980s and 1990s with those of the 1970s, CAD diagnoses significantly decreased in the 1980s (hazard ratio, 0.65; 95% confidence interval, 0.45-0.92) and 1990s (HR, 0.53; 95% CI, 0.36-0.77), while there was no significant decrease in heart failure or valvular heart disease risk over time. After adjusting for cardiac radiation, overall risk for CAD was attenuated (HR, 0.90; 0.78-1.05), and Hodgkin lymphoma survivors saw the greatest change between unadjusted (HR, 0.77; 95% CI, 0.66-0.89) and adjusted risk (HR, 0.87; 95% CI, 0.69-1.10) when accounting for cardiac radiation.
“While additional longitudinal follow-up is needed to establish whether similar reductions in the cumulative incidence of heart failure can be confirmed in multivariable analysis, these results suggest that efforts to modify cancer therapies in children and promote health surveillance for survivors are beginning to show benefits not only in overall survival but also in late adverse cardiac effects,” the researchers concluded.
In a related editorial, Mike Hawkins, DPhil, of the Centre for Childhood Cancer Survivor Studies, Institute of Applied Health Research at the University of Birmingham (England), and colleagues said that, while measuring cardiotoxicity is important for this patient population, traditional risk factors with independent associations to cardiac outcomes should also be studied. Guidelines on follow-up for these patients are also needed to inform clinical practice, such as those produced by the International Late Effects of Childhood Cancer Guideline Harmonization Group, they added.
“Survivorship issues are extremely important to patients, their families, and their doctors,” they said. “In two research priority setting initiatives in the United Kingdom, detailed consultation with patients with cancer, survivors, families, friends, and healthcare professionals identified further research into the consequences of cancer as a top priority.”
This study was funded by grants from the National Cancer Institute, Cancer Center Support (CORE) to St. Jude Children’s Research Hospital and American Lebanese Syrian Associated Charities. The authors of the study and the editorial reported no relevant conflicts of interest.
SOURCE: Mulrooney A et al. BMJ. 2020. doi: 10.1136/bmj.l6794.
FROM BMJ
REGN1979 shows good activity in pretreated aggressive B-NHL
ORLANDO – A novel bispecific antibody directed against CD20 and CD3 was associated in a phase 1 trial with a high overall response rate among patients with relapsed or refractory B-cell non-Hodgkin lymphomas in early clinical trials, including patients with diffuse large B-cell lymphoma (DLBCL) that had progressed following chimeric antigen receptor (CAR) T-cell therapy.
Among 22 patients previously treated for relapsed/refractory follicular lymphoma of grade 1-3a, there were 21 responses (95%), including 17 complete responses (CR) and 4 partial responses (PR), with the remaining patient having stable disease at 12 weeks of follow-up, reported Rajat Bannerji, MD, PhD, from the Rutgers Cancer Institute of New Jersey in New Brunswick.
“We had activity that was fairly robust in this heavily pretreated population with follicular lymphoma, large-cell patients who had not received CAR T and large-cell patients who had received CAR T, mantle cell, and marginal zone [lymphoma],” he said at the annual meeting of the American Society of Hematology.
REGN1979 is an anti-CD20 and anti-CD3 bispecific IgG4 antibody. It is designed to cross-link and activate CD3-expressing T cells on contact with CD20-positive B cells to kill CD20-positive tumor cells independent of T-cell receptor recognition.
The antibody is administered via an escalating dose schedule consisting of initial, intermediate, and step-up doses.
In addition to the follicular lymphoma response rates noted before, patients with heavily pretreated DLBCL who received the antibody at a dose of 80 mg or higher had an overall response rate of 57.9% (11 patients) including 42.1% CR (8 patients), and 15.8% PR (3 patients). Two patients had stable disease at the 12-week assessment, three had disease progression, and three were not available for assessment.
Among seven patients with DLBCL treated at 80 mg or above who had not received CAR T therapy, five had a CR, one had stable disease, and one had disease progression. Of 12 patients with prior CAR T exposure, 3 had complete responses, 3 had partial responses, 1 had stable disease, 2 had progressive disease, and 3 were not available for assessment.
Among six patients with mantle cell lymphoma and six with marginal zone lymphoma treated across all disease levels, the ORR in each cohort was 67%, with two of six patients in each cohort having a complete response, and two having a partial response.
The safety analysis of all 110 patients enrolled showed that no patients experience a dose-limiting toxicity during the escalation phase, and no maximum tolerated doses were identified.
The most common treatment-related adverse events (AEs) were pyrexia in 88 patients, cytokine release syndrome in 65, chills in 56, fatigue in 40, and anemia in 39.
The most common grade 3-4 AEs were anemia in 24, and hypophosphatemia, lymphopenia, and neutropenia in 21 patients each.
Neurologic AEs were transient and did not require treatment discontinuation, and there were no grade 4 neurologic AEs or deaths from neurologic side effects.
Six patients discontinued the study drug because of treatment-related AEs that included cytomegalovirus infection, grade 3 hemolysis, fatigue, pneumonia, and toxoplasmosis.
A total of 15 patients died during the study, 10 of which were caused by progressive disease, with other deaths caused by gastric perforation, cardiac arrest, lung infection, pneumonia, and 1 from fungal pneumonia 7 months after treatment discontinuation. In addition, after the data cutoff, one patient with mantle cell lymphoma blastoid variant with bone-marrow involvement and bulky disease who was enrolled in an expansion cohort died from tumor lysis syndrome.
The dose-escalation portion of the trial has been completed and expansion cohorts are being enrolled. In addition, REGN1979 is being investigated in a phase 2 global multiarm trial.
The study is supported by Regeneron Pharmaceuticals. Dr. Bannerji reported research funding, travel support, and consulting fees from Regeneron and others.
SOURCE: Bannerji R et al. ASH 2019, Abstract 762.
ORLANDO – A novel bispecific antibody directed against CD20 and CD3 was associated in a phase 1 trial with a high overall response rate among patients with relapsed or refractory B-cell non-Hodgkin lymphomas in early clinical trials, including patients with diffuse large B-cell lymphoma (DLBCL) that had progressed following chimeric antigen receptor (CAR) T-cell therapy.
Among 22 patients previously treated for relapsed/refractory follicular lymphoma of grade 1-3a, there were 21 responses (95%), including 17 complete responses (CR) and 4 partial responses (PR), with the remaining patient having stable disease at 12 weeks of follow-up, reported Rajat Bannerji, MD, PhD, from the Rutgers Cancer Institute of New Jersey in New Brunswick.
“We had activity that was fairly robust in this heavily pretreated population with follicular lymphoma, large-cell patients who had not received CAR T and large-cell patients who had received CAR T, mantle cell, and marginal zone [lymphoma],” he said at the annual meeting of the American Society of Hematology.
REGN1979 is an anti-CD20 and anti-CD3 bispecific IgG4 antibody. It is designed to cross-link and activate CD3-expressing T cells on contact with CD20-positive B cells to kill CD20-positive tumor cells independent of T-cell receptor recognition.
The antibody is administered via an escalating dose schedule consisting of initial, intermediate, and step-up doses.
In addition to the follicular lymphoma response rates noted before, patients with heavily pretreated DLBCL who received the antibody at a dose of 80 mg or higher had an overall response rate of 57.9% (11 patients) including 42.1% CR (8 patients), and 15.8% PR (3 patients). Two patients had stable disease at the 12-week assessment, three had disease progression, and three were not available for assessment.
Among seven patients with DLBCL treated at 80 mg or above who had not received CAR T therapy, five had a CR, one had stable disease, and one had disease progression. Of 12 patients with prior CAR T exposure, 3 had complete responses, 3 had partial responses, 1 had stable disease, 2 had progressive disease, and 3 were not available for assessment.
Among six patients with mantle cell lymphoma and six with marginal zone lymphoma treated across all disease levels, the ORR in each cohort was 67%, with two of six patients in each cohort having a complete response, and two having a partial response.
The safety analysis of all 110 patients enrolled showed that no patients experience a dose-limiting toxicity during the escalation phase, and no maximum tolerated doses were identified.
The most common treatment-related adverse events (AEs) were pyrexia in 88 patients, cytokine release syndrome in 65, chills in 56, fatigue in 40, and anemia in 39.
The most common grade 3-4 AEs were anemia in 24, and hypophosphatemia, lymphopenia, and neutropenia in 21 patients each.
Neurologic AEs were transient and did not require treatment discontinuation, and there were no grade 4 neurologic AEs or deaths from neurologic side effects.
Six patients discontinued the study drug because of treatment-related AEs that included cytomegalovirus infection, grade 3 hemolysis, fatigue, pneumonia, and toxoplasmosis.
A total of 15 patients died during the study, 10 of which were caused by progressive disease, with other deaths caused by gastric perforation, cardiac arrest, lung infection, pneumonia, and 1 from fungal pneumonia 7 months after treatment discontinuation. In addition, after the data cutoff, one patient with mantle cell lymphoma blastoid variant with bone-marrow involvement and bulky disease who was enrolled in an expansion cohort died from tumor lysis syndrome.
The dose-escalation portion of the trial has been completed and expansion cohorts are being enrolled. In addition, REGN1979 is being investigated in a phase 2 global multiarm trial.
The study is supported by Regeneron Pharmaceuticals. Dr. Bannerji reported research funding, travel support, and consulting fees from Regeneron and others.
SOURCE: Bannerji R et al. ASH 2019, Abstract 762.
ORLANDO – A novel bispecific antibody directed against CD20 and CD3 was associated in a phase 1 trial with a high overall response rate among patients with relapsed or refractory B-cell non-Hodgkin lymphomas in early clinical trials, including patients with diffuse large B-cell lymphoma (DLBCL) that had progressed following chimeric antigen receptor (CAR) T-cell therapy.
Among 22 patients previously treated for relapsed/refractory follicular lymphoma of grade 1-3a, there were 21 responses (95%), including 17 complete responses (CR) and 4 partial responses (PR), with the remaining patient having stable disease at 12 weeks of follow-up, reported Rajat Bannerji, MD, PhD, from the Rutgers Cancer Institute of New Jersey in New Brunswick.
“We had activity that was fairly robust in this heavily pretreated population with follicular lymphoma, large-cell patients who had not received CAR T and large-cell patients who had received CAR T, mantle cell, and marginal zone [lymphoma],” he said at the annual meeting of the American Society of Hematology.
REGN1979 is an anti-CD20 and anti-CD3 bispecific IgG4 antibody. It is designed to cross-link and activate CD3-expressing T cells on contact with CD20-positive B cells to kill CD20-positive tumor cells independent of T-cell receptor recognition.
The antibody is administered via an escalating dose schedule consisting of initial, intermediate, and step-up doses.
In addition to the follicular lymphoma response rates noted before, patients with heavily pretreated DLBCL who received the antibody at a dose of 80 mg or higher had an overall response rate of 57.9% (11 patients) including 42.1% CR (8 patients), and 15.8% PR (3 patients). Two patients had stable disease at the 12-week assessment, three had disease progression, and three were not available for assessment.
Among seven patients with DLBCL treated at 80 mg or above who had not received CAR T therapy, five had a CR, one had stable disease, and one had disease progression. Of 12 patients with prior CAR T exposure, 3 had complete responses, 3 had partial responses, 1 had stable disease, 2 had progressive disease, and 3 were not available for assessment.
Among six patients with mantle cell lymphoma and six with marginal zone lymphoma treated across all disease levels, the ORR in each cohort was 67%, with two of six patients in each cohort having a complete response, and two having a partial response.
The safety analysis of all 110 patients enrolled showed that no patients experience a dose-limiting toxicity during the escalation phase, and no maximum tolerated doses were identified.
The most common treatment-related adverse events (AEs) were pyrexia in 88 patients, cytokine release syndrome in 65, chills in 56, fatigue in 40, and anemia in 39.
The most common grade 3-4 AEs were anemia in 24, and hypophosphatemia, lymphopenia, and neutropenia in 21 patients each.
Neurologic AEs were transient and did not require treatment discontinuation, and there were no grade 4 neurologic AEs or deaths from neurologic side effects.
Six patients discontinued the study drug because of treatment-related AEs that included cytomegalovirus infection, grade 3 hemolysis, fatigue, pneumonia, and toxoplasmosis.
A total of 15 patients died during the study, 10 of which were caused by progressive disease, with other deaths caused by gastric perforation, cardiac arrest, lung infection, pneumonia, and 1 from fungal pneumonia 7 months after treatment discontinuation. In addition, after the data cutoff, one patient with mantle cell lymphoma blastoid variant with bone-marrow involvement and bulky disease who was enrolled in an expansion cohort died from tumor lysis syndrome.
The dose-escalation portion of the trial has been completed and expansion cohorts are being enrolled. In addition, REGN1979 is being investigated in a phase 2 global multiarm trial.
The study is supported by Regeneron Pharmaceuticals. Dr. Bannerji reported research funding, travel support, and consulting fees from Regeneron and others.
SOURCE: Bannerji R et al. ASH 2019, Abstract 762.
REPORTING FROM ASH 2019