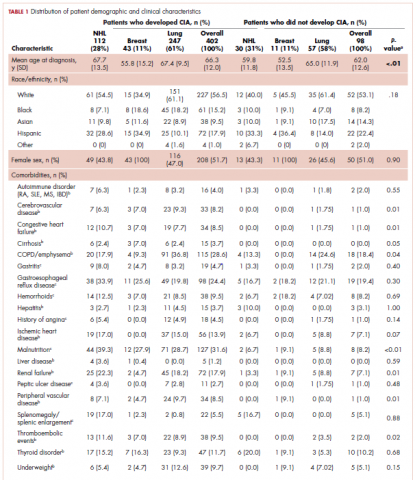
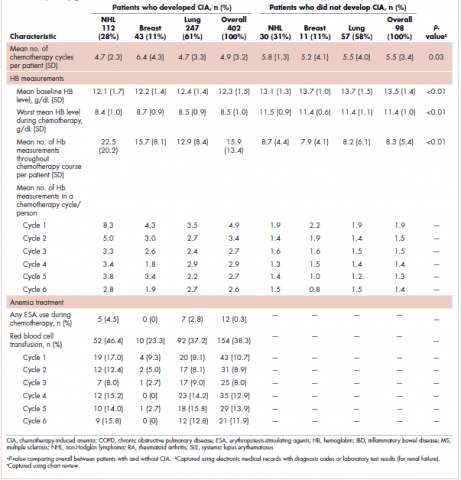
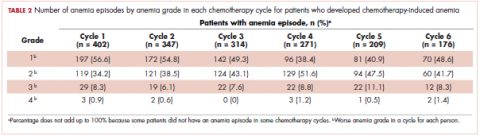
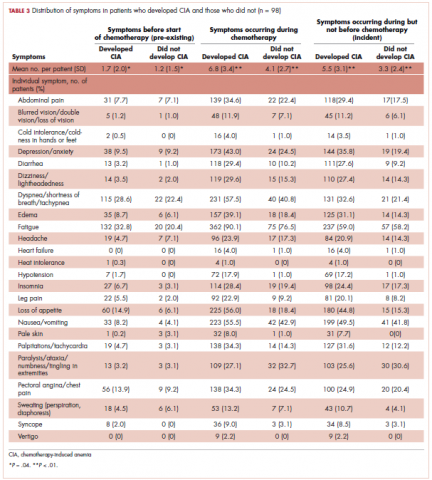
User login
MAIA: Daratumumab plus len-dex improves myeloma PFS
SAN DIEGO – Patients with newly diagnosed multiple myeloma who were ineligible for transplant had a 44% reduction in the risk of disease progression or death when they were treated with the anti-CD38 monoclonal antibody daratumumab (Darzalex) added to lenalidomide (Revlimid) and dexamethasone, compared with lenalidomide-dexamethasone alone, an interim analysis from the MAIA trial showed.
Among 737 patients in a phase 3 trial, median progression-free survival – the primary endpoint – had not been reached after a median follow-up of 28 months for patients randomized to daratumumab, lenalidomide, and dexamethasone (DRd), versus 31.9 months for patients randomized to lenalidomide and dexamethasone (Rd).
The 30-month PFS rate in the DRd arm was 71%, compared with 56% for the Rd arm. This difference translated into a hazard ratio (HR) for progression of 0.56 (P less than .0001), reported Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille, France.
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” he said at the annual meeting of the American Society of Hematology.
Dr. Facon and his colleagues had previously shown in the FIRST trial that in newly diagnosed transplant-ineligible patients, continuous treatment with lenalidomide and low-dose dexamethasone was associated with significant overall survival and PFS benefits, compared with melphalan-prednisone-thalidomide.
In the POLLUX trial, investigators reported that in patients with multiple myeloma that was refractory or had relapsed after at least one prior line of therapy, the DRd combination was associated with a 63% reduction in the risk for disease progression or death, compared with Rd alone.
MAIA details
The MAIA trial is a pivotal, phase 3 study pitting DRd against Rd in transplant-ineligible patients with newly diagnosed multiple myeloma.
Patients with untreated disease who had Eastern Cooperative Oncology Group (ECOG) status of 0-2 and creatinine clearance rates of at least 30 mL/min were enrolled. Patients were randomly assigned to either DRd, with intravenous daratumumab 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression, plus lenalidomide 25 mg orally per day on days 1-21 until disease progression, and dexamethasone 40 mg orally or intravenously weekly until disease progression; or to the same regimen without daratumumab.
The median patient age was 73 years and 99% of all patients were aged 65 years or older. Demographic and clinical characteristics were well balanced between the treatment arms.
The primary endpoint of progression-free survival was superior with DRd.
DRd also was associated with a significantly higher overall response rate (93% vs. 81%), complete response rate (48% vs. 25%), very good partial response or better rate (79% vs. 53%), and minimal residual disease (MRD) negativity rate (24% vs. 7%; P less than .0001 for all comparisons).
The DRd combination was associated with infusion-related reactions in 41% of patients, but only 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events (TEAE) grade 3 or greater that were more common with DRd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%). Conversely, thrombocytopenia (7% vs. 9%, grade 3 or 4) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic TEAEs that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia. Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the study arms.
Janssen funded the study. Dr. Facon reported speakers bureau and advisory board participation for Celgene, Janssen, and Takeda; and advisory board participation for Sanofi, Amgen, Karyopharm, and Oncopeptides.
SOURCE: Facon T et al. ASH 2018, Abstract LBA-2.
SAN DIEGO – Patients with newly diagnosed multiple myeloma who were ineligible for transplant had a 44% reduction in the risk of disease progression or death when they were treated with the anti-CD38 monoclonal antibody daratumumab (Darzalex) added to lenalidomide (Revlimid) and dexamethasone, compared with lenalidomide-dexamethasone alone, an interim analysis from the MAIA trial showed.
Among 737 patients in a phase 3 trial, median progression-free survival – the primary endpoint – had not been reached after a median follow-up of 28 months for patients randomized to daratumumab, lenalidomide, and dexamethasone (DRd), versus 31.9 months for patients randomized to lenalidomide and dexamethasone (Rd).
The 30-month PFS rate in the DRd arm was 71%, compared with 56% for the Rd arm. This difference translated into a hazard ratio (HR) for progression of 0.56 (P less than .0001), reported Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille, France.
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” he said at the annual meeting of the American Society of Hematology.
Dr. Facon and his colleagues had previously shown in the FIRST trial that in newly diagnosed transplant-ineligible patients, continuous treatment with lenalidomide and low-dose dexamethasone was associated with significant overall survival and PFS benefits, compared with melphalan-prednisone-thalidomide.
In the POLLUX trial, investigators reported that in patients with multiple myeloma that was refractory or had relapsed after at least one prior line of therapy, the DRd combination was associated with a 63% reduction in the risk for disease progression or death, compared with Rd alone.
MAIA details
The MAIA trial is a pivotal, phase 3 study pitting DRd against Rd in transplant-ineligible patients with newly diagnosed multiple myeloma.
Patients with untreated disease who had Eastern Cooperative Oncology Group (ECOG) status of 0-2 and creatinine clearance rates of at least 30 mL/min were enrolled. Patients were randomly assigned to either DRd, with intravenous daratumumab 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression, plus lenalidomide 25 mg orally per day on days 1-21 until disease progression, and dexamethasone 40 mg orally or intravenously weekly until disease progression; or to the same regimen without daratumumab.
The median patient age was 73 years and 99% of all patients were aged 65 years or older. Demographic and clinical characteristics were well balanced between the treatment arms.
The primary endpoint of progression-free survival was superior with DRd.
DRd also was associated with a significantly higher overall response rate (93% vs. 81%), complete response rate (48% vs. 25%), very good partial response or better rate (79% vs. 53%), and minimal residual disease (MRD) negativity rate (24% vs. 7%; P less than .0001 for all comparisons).
The DRd combination was associated with infusion-related reactions in 41% of patients, but only 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events (TEAE) grade 3 or greater that were more common with DRd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%). Conversely, thrombocytopenia (7% vs. 9%, grade 3 or 4) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic TEAEs that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia. Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the study arms.
Janssen funded the study. Dr. Facon reported speakers bureau and advisory board participation for Celgene, Janssen, and Takeda; and advisory board participation for Sanofi, Amgen, Karyopharm, and Oncopeptides.
SOURCE: Facon T et al. ASH 2018, Abstract LBA-2.
SAN DIEGO – Patients with newly diagnosed multiple myeloma who were ineligible for transplant had a 44% reduction in the risk of disease progression or death when they were treated with the anti-CD38 monoclonal antibody daratumumab (Darzalex) added to lenalidomide (Revlimid) and dexamethasone, compared with lenalidomide-dexamethasone alone, an interim analysis from the MAIA trial showed.
Among 737 patients in a phase 3 trial, median progression-free survival – the primary endpoint – had not been reached after a median follow-up of 28 months for patients randomized to daratumumab, lenalidomide, and dexamethasone (DRd), versus 31.9 months for patients randomized to lenalidomide and dexamethasone (Rd).
The 30-month PFS rate in the DRd arm was 71%, compared with 56% for the Rd arm. This difference translated into a hazard ratio (HR) for progression of 0.56 (P less than .0001), reported Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille, France.
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” he said at the annual meeting of the American Society of Hematology.
Dr. Facon and his colleagues had previously shown in the FIRST trial that in newly diagnosed transplant-ineligible patients, continuous treatment with lenalidomide and low-dose dexamethasone was associated with significant overall survival and PFS benefits, compared with melphalan-prednisone-thalidomide.
In the POLLUX trial, investigators reported that in patients with multiple myeloma that was refractory or had relapsed after at least one prior line of therapy, the DRd combination was associated with a 63% reduction in the risk for disease progression or death, compared with Rd alone.
MAIA details
The MAIA trial is a pivotal, phase 3 study pitting DRd against Rd in transplant-ineligible patients with newly diagnosed multiple myeloma.
Patients with untreated disease who had Eastern Cooperative Oncology Group (ECOG) status of 0-2 and creatinine clearance rates of at least 30 mL/min were enrolled. Patients were randomly assigned to either DRd, with intravenous daratumumab 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression, plus lenalidomide 25 mg orally per day on days 1-21 until disease progression, and dexamethasone 40 mg orally or intravenously weekly until disease progression; or to the same regimen without daratumumab.
The median patient age was 73 years and 99% of all patients were aged 65 years or older. Demographic and clinical characteristics were well balanced between the treatment arms.
The primary endpoint of progression-free survival was superior with DRd.
DRd also was associated with a significantly higher overall response rate (93% vs. 81%), complete response rate (48% vs. 25%), very good partial response or better rate (79% vs. 53%), and minimal residual disease (MRD) negativity rate (24% vs. 7%; P less than .0001 for all comparisons).
The DRd combination was associated with infusion-related reactions in 41% of patients, but only 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events (TEAE) grade 3 or greater that were more common with DRd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%). Conversely, thrombocytopenia (7% vs. 9%, grade 3 or 4) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic TEAEs that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia. Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the study arms.
Janssen funded the study. Dr. Facon reported speakers bureau and advisory board participation for Celgene, Janssen, and Takeda; and advisory board participation for Sanofi, Amgen, Karyopharm, and Oncopeptides.
SOURCE: Facon T et al. ASH 2018, Abstract LBA-2.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: At 30 months of follow-up, DRd was associated with a 44% reduction in the risk of death, compared with Rd.
Study details: Randomized phase 3 trial of 737 patients with newly diagnosed multiple myeloma who were ineligible for transplant.
Disclosures: Janssen funded the study. Dr. Facon reported speakers bureau and advisory board participation for Celgene, Janssen, and Takeda; and advisory board participation for Sanofi, Amgen, Karyopharm, and Oncopeptides.
Source: Facon T et al. ASH 2018, Abstract LBA-2.
Combo bests standard care in younger CLL patients
SAN DIEGO—In a phase 3 trial, ibrutinib plus rituximab (IR) improved survival when compared with standard chemoimmunotherapy in patients younger than 70 with untreated chronic lymphocytic leukemia (CLL).
Patients who received IR had superior progression-free survival (PFS) and overall survival compared to patients who received fludarabine, cyclophosphamide, and rituximab (FCR).
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” said Tait D. Shanafelt, MD, of Stanford University in California.
In fact, the study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
He presented the results during the late-breaking abstract session at the 2018 ASH Annual Meeting (abstract LBA-4*).
The trial (NCT02048813) included 529 patients age 70 or younger with previously untreated CLL. They were randomized on a 2:1 basis to either six cycles of FCR according to standard protocols (n=175) or IR (n=354).
IR consisted of ibrutinib given at 420 mg daily for each 28-day cycle and rituximab given at 50 mg/m2 on day 1 of cycle 2, at 325 mg/m2 on day 2 of cycle 2, and at 500 mg/m2 on day 1 for cycles 3 to 7.
From cycle 8 on, patients in the IR arm received daily ibrutinib at 420 mg until disease progression.
Dr. Shanafelt said patient characteristics were well-balanced between the treatment arms.
He presented results from both an intent-to-treat (ITT) analysis and a per-protocol analysis excluding 22 patients in the IR arm and nine patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
PFS
In the ITT analysis, there were 37 cases of progression or death in the IR arm and 40 cases in the FCR arm. This difference translated into a hazard ratio (HR) for progression or death of 0.35 with IR (P<0.00001).
In the per-protocol analysis, there were 33 cases of progression or death in the IR arm and 39 cases in the FCR arm. The HR was 0.32 favoring IR (P<0.00001).
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was significantly better with IR in patients with unmutated IGHV (HR= 0.26, P<0.00001) but not in patients with mutated IGHV (HR=0.44, P=0.07).
Overall survival
In the ITT analysis, there were four deaths in the IR arm and 10 in the FCR arm (HR=0.17, P<0.0003).
In the per-protocol analysis, there were three deaths in the IR arm and 10 deaths in the FCR arm (HR=0.13, P<0.0001).
Dr. Shanafelt noted that, although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning the rate of death in the FCR arm was five-fold higher than in the IR arm.
Safety and cost
Grade 3 or greater treatment-related adverse events (AEs) occurred in 58.5% of patients in the IR arm and 72.1% of patients in the FCR arm (P=0.004).
Specific AEs that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P<0.001 for all comparisons).
AEs that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P=0.04) and hypertension (7.4% vs. 1.9%, P=0.01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost. The monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, AbbVie, Pharmacyclics, and Janssen.
*Data in the abstract differ from the presentation.
SAN DIEGO—In a phase 3 trial, ibrutinib plus rituximab (IR) improved survival when compared with standard chemoimmunotherapy in patients younger than 70 with untreated chronic lymphocytic leukemia (CLL).
Patients who received IR had superior progression-free survival (PFS) and overall survival compared to patients who received fludarabine, cyclophosphamide, and rituximab (FCR).
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” said Tait D. Shanafelt, MD, of Stanford University in California.
In fact, the study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
He presented the results during the late-breaking abstract session at the 2018 ASH Annual Meeting (abstract LBA-4*).
The trial (NCT02048813) included 529 patients age 70 or younger with previously untreated CLL. They were randomized on a 2:1 basis to either six cycles of FCR according to standard protocols (n=175) or IR (n=354).
IR consisted of ibrutinib given at 420 mg daily for each 28-day cycle and rituximab given at 50 mg/m2 on day 1 of cycle 2, at 325 mg/m2 on day 2 of cycle 2, and at 500 mg/m2 on day 1 for cycles 3 to 7.
From cycle 8 on, patients in the IR arm received daily ibrutinib at 420 mg until disease progression.
Dr. Shanafelt said patient characteristics were well-balanced between the treatment arms.
He presented results from both an intent-to-treat (ITT) analysis and a per-protocol analysis excluding 22 patients in the IR arm and nine patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
PFS
In the ITT analysis, there were 37 cases of progression or death in the IR arm and 40 cases in the FCR arm. This difference translated into a hazard ratio (HR) for progression or death of 0.35 with IR (P<0.00001).
In the per-protocol analysis, there were 33 cases of progression or death in the IR arm and 39 cases in the FCR arm. The HR was 0.32 favoring IR (P<0.00001).
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was significantly better with IR in patients with unmutated IGHV (HR= 0.26, P<0.00001) but not in patients with mutated IGHV (HR=0.44, P=0.07).
Overall survival
In the ITT analysis, there were four deaths in the IR arm and 10 in the FCR arm (HR=0.17, P<0.0003).
In the per-protocol analysis, there were three deaths in the IR arm and 10 deaths in the FCR arm (HR=0.13, P<0.0001).
Dr. Shanafelt noted that, although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning the rate of death in the FCR arm was five-fold higher than in the IR arm.
Safety and cost
Grade 3 or greater treatment-related adverse events (AEs) occurred in 58.5% of patients in the IR arm and 72.1% of patients in the FCR arm (P=0.004).
Specific AEs that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P<0.001 for all comparisons).
AEs that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P=0.04) and hypertension (7.4% vs. 1.9%, P=0.01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost. The monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, AbbVie, Pharmacyclics, and Janssen.
*Data in the abstract differ from the presentation.
SAN DIEGO—In a phase 3 trial, ibrutinib plus rituximab (IR) improved survival when compared with standard chemoimmunotherapy in patients younger than 70 with untreated chronic lymphocytic leukemia (CLL).
Patients who received IR had superior progression-free survival (PFS) and overall survival compared to patients who received fludarabine, cyclophosphamide, and rituximab (FCR).
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” said Tait D. Shanafelt, MD, of Stanford University in California.
In fact, the study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
He presented the results during the late-breaking abstract session at the 2018 ASH Annual Meeting (abstract LBA-4*).
The trial (NCT02048813) included 529 patients age 70 or younger with previously untreated CLL. They were randomized on a 2:1 basis to either six cycles of FCR according to standard protocols (n=175) or IR (n=354).
IR consisted of ibrutinib given at 420 mg daily for each 28-day cycle and rituximab given at 50 mg/m2 on day 1 of cycle 2, at 325 mg/m2 on day 2 of cycle 2, and at 500 mg/m2 on day 1 for cycles 3 to 7.
From cycle 8 on, patients in the IR arm received daily ibrutinib at 420 mg until disease progression.
Dr. Shanafelt said patient characteristics were well-balanced between the treatment arms.
He presented results from both an intent-to-treat (ITT) analysis and a per-protocol analysis excluding 22 patients in the IR arm and nine patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
PFS
In the ITT analysis, there were 37 cases of progression or death in the IR arm and 40 cases in the FCR arm. This difference translated into a hazard ratio (HR) for progression or death of 0.35 with IR (P<0.00001).
In the per-protocol analysis, there were 33 cases of progression or death in the IR arm and 39 cases in the FCR arm. The HR was 0.32 favoring IR (P<0.00001).
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was significantly better with IR in patients with unmutated IGHV (HR= 0.26, P<0.00001) but not in patients with mutated IGHV (HR=0.44, P=0.07).
Overall survival
In the ITT analysis, there were four deaths in the IR arm and 10 in the FCR arm (HR=0.17, P<0.0003).
In the per-protocol analysis, there were three deaths in the IR arm and 10 deaths in the FCR arm (HR=0.13, P<0.0001).
Dr. Shanafelt noted that, although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning the rate of death in the FCR arm was five-fold higher than in the IR arm.
Safety and cost
Grade 3 or greater treatment-related adverse events (AEs) occurred in 58.5% of patients in the IR arm and 72.1% of patients in the FCR arm (P=0.004).
Specific AEs that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P<0.001 for all comparisons).
AEs that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P=0.04) and hypertension (7.4% vs. 1.9%, P=0.01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost. The monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, AbbVie, Pharmacyclics, and Janssen.
*Data in the abstract differ from the presentation.
Tom Brokaw opens up on surviving multiple myeloma
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
EXPERT ANALYSIS FROM ASH 2018
Lymphodepletion improves efficacy of CAR T cells in HL
SAN DIEGO—A phase 1 study suggests lymphodepletion can improve the efficacy of CD30-directed chimeric antigen receptor (CAR) T-cell therapy in patients with Hodgkin lymphoma (HL).
Researchers observed improved responses in HL patients treated with fludarabine and cyclophosphamide prior to CD30.CAR T-cell therapy.
This lymphodepleting regimen was also associated with increased toxicity, compared to no lymphodepletion. However, researchers consider the regimen safe.
Carlos A. Ramos, MD, of Baylor College of Medicine in Houston, Texas, presented these results at the 2018 ASH Annual Meeting (abstract 680*).
Without lymphodepletion
Dr. Ramos first discussed a previous phase 1 trial (NCT01316146), which was published in The Journal of Clinical Investigation in 2017.
In this trial, he and his colleagues had tested CD30.CAR T-cell therapy in patients with relapsed/refractory, CD30+ HL or T-cell non-Hodgkin lymphoma. None of these patients underwent lymphodepletion.
There were no dose-limiting toxicities in this trial—including no neurotoxicity or cytokine release syndrome—but responses were “limited,” according to Dr. Ramos.
Three patients achieved a complete response (CR), three had stable disease, and three progressed.
“Although we saw no significant toxicities and some good clinical responses . . ., the bottom line is that the responses were still quite limited, with several patients having, at most, stable disease or progressive disease,” Dr. Ramos said.
With lymphodepletion
Results from the previous trial prompted Dr. Ramos and his colleagues to conduct the RELY-30 trial (NCT02917083) and investigate whether lymphodepletion would improve responses to CD30.CAR T-cell therapy.
Thus far, 11 patients have been treated on this trial. All had relapsed, CD30+ HL at baseline. Six patients are male, and their median age at baseline was 30 (range, 17-69).
The patients had a median of 5 prior treatments (range, 2-9). This included PD-1 inhibitors (n=10), brentuximab vedotin (n=8), and transplant (n=6).
All patients received lymphodepletion with cyclophosphamide at 500 mg/m2 and fludarabine at 30 mg/m2 daily for 3 days. They then received CD30.CAR T-cell therapy at 2×107 cells/m2 or 1×108 cells/m2.
Dr. Ramos noted that CD30.CAR T-cell expansion was dose-dependent and increased by lymphodepleting chemotherapy.
“The peak expansion is much higher [with lymphodepletion], probably in the order of two to three logs higher than what we see without lymphodepleting chemotherapy,” he said. “So chemotherapy makes a difference.”
Increased CD30.CAR T-cell expansion was associated with improved response. Of the nine evaluable patients, six achieved a CR, and three progressed.
Four complete responders were still in CR at last follow-up, one of them for more than a year. However, two complete responders ultimately progressed.
In addition to improved responses, the researchers observed increased toxicity in this trial. Dr. Ramos said some of these toxicities are “probably attributable” to the lymphodepleting chemotherapy.
Toxicities included grade 1 cytokine release syndrome (no tocilizumab required), maculopapular rash, transient cytopenias, nausea, vomiting, and alopecia.
Dr. Ramos said these results suggest adoptive transfer of CD30.CAR T cells is “safe, even with chemotherapy.”
He noted that the duration of response with this treatment is unknown, but trial enrollment and follow-up are ongoing.
RELY-30 was sponsored by Baylor College of Medicine. Dr. Ramos reported relationships with Novartis, Celgene, Bluebird Bio, and Tessa Therapeutics.
*Data in the abstract differ from the presentation.
SAN DIEGO—A phase 1 study suggests lymphodepletion can improve the efficacy of CD30-directed chimeric antigen receptor (CAR) T-cell therapy in patients with Hodgkin lymphoma (HL).
Researchers observed improved responses in HL patients treated with fludarabine and cyclophosphamide prior to CD30.CAR T-cell therapy.
This lymphodepleting regimen was also associated with increased toxicity, compared to no lymphodepletion. However, researchers consider the regimen safe.
Carlos A. Ramos, MD, of Baylor College of Medicine in Houston, Texas, presented these results at the 2018 ASH Annual Meeting (abstract 680*).
Without lymphodepletion
Dr. Ramos first discussed a previous phase 1 trial (NCT01316146), which was published in The Journal of Clinical Investigation in 2017.
In this trial, he and his colleagues had tested CD30.CAR T-cell therapy in patients with relapsed/refractory, CD30+ HL or T-cell non-Hodgkin lymphoma. None of these patients underwent lymphodepletion.
There were no dose-limiting toxicities in this trial—including no neurotoxicity or cytokine release syndrome—but responses were “limited,” according to Dr. Ramos.
Three patients achieved a complete response (CR), three had stable disease, and three progressed.
“Although we saw no significant toxicities and some good clinical responses . . ., the bottom line is that the responses were still quite limited, with several patients having, at most, stable disease or progressive disease,” Dr. Ramos said.
With lymphodepletion
Results from the previous trial prompted Dr. Ramos and his colleagues to conduct the RELY-30 trial (NCT02917083) and investigate whether lymphodepletion would improve responses to CD30.CAR T-cell therapy.
Thus far, 11 patients have been treated on this trial. All had relapsed, CD30+ HL at baseline. Six patients are male, and their median age at baseline was 30 (range, 17-69).
The patients had a median of 5 prior treatments (range, 2-9). This included PD-1 inhibitors (n=10), brentuximab vedotin (n=8), and transplant (n=6).
All patients received lymphodepletion with cyclophosphamide at 500 mg/m2 and fludarabine at 30 mg/m2 daily for 3 days. They then received CD30.CAR T-cell therapy at 2×107 cells/m2 or 1×108 cells/m2.
Dr. Ramos noted that CD30.CAR T-cell expansion was dose-dependent and increased by lymphodepleting chemotherapy.
“The peak expansion is much higher [with lymphodepletion], probably in the order of two to three logs higher than what we see without lymphodepleting chemotherapy,” he said. “So chemotherapy makes a difference.”
Increased CD30.CAR T-cell expansion was associated with improved response. Of the nine evaluable patients, six achieved a CR, and three progressed.
Four complete responders were still in CR at last follow-up, one of them for more than a year. However, two complete responders ultimately progressed.
In addition to improved responses, the researchers observed increased toxicity in this trial. Dr. Ramos said some of these toxicities are “probably attributable” to the lymphodepleting chemotherapy.
Toxicities included grade 1 cytokine release syndrome (no tocilizumab required), maculopapular rash, transient cytopenias, nausea, vomiting, and alopecia.
Dr. Ramos said these results suggest adoptive transfer of CD30.CAR T cells is “safe, even with chemotherapy.”
He noted that the duration of response with this treatment is unknown, but trial enrollment and follow-up are ongoing.
RELY-30 was sponsored by Baylor College of Medicine. Dr. Ramos reported relationships with Novartis, Celgene, Bluebird Bio, and Tessa Therapeutics.
*Data in the abstract differ from the presentation.
SAN DIEGO—A phase 1 study suggests lymphodepletion can improve the efficacy of CD30-directed chimeric antigen receptor (CAR) T-cell therapy in patients with Hodgkin lymphoma (HL).
Researchers observed improved responses in HL patients treated with fludarabine and cyclophosphamide prior to CD30.CAR T-cell therapy.
This lymphodepleting regimen was also associated with increased toxicity, compared to no lymphodepletion. However, researchers consider the regimen safe.
Carlos A. Ramos, MD, of Baylor College of Medicine in Houston, Texas, presented these results at the 2018 ASH Annual Meeting (abstract 680*).
Without lymphodepletion
Dr. Ramos first discussed a previous phase 1 trial (NCT01316146), which was published in The Journal of Clinical Investigation in 2017.
In this trial, he and his colleagues had tested CD30.CAR T-cell therapy in patients with relapsed/refractory, CD30+ HL or T-cell non-Hodgkin lymphoma. None of these patients underwent lymphodepletion.
There were no dose-limiting toxicities in this trial—including no neurotoxicity or cytokine release syndrome—but responses were “limited,” according to Dr. Ramos.
Three patients achieved a complete response (CR), three had stable disease, and three progressed.
“Although we saw no significant toxicities and some good clinical responses . . ., the bottom line is that the responses were still quite limited, with several patients having, at most, stable disease or progressive disease,” Dr. Ramos said.
With lymphodepletion
Results from the previous trial prompted Dr. Ramos and his colleagues to conduct the RELY-30 trial (NCT02917083) and investigate whether lymphodepletion would improve responses to CD30.CAR T-cell therapy.
Thus far, 11 patients have been treated on this trial. All had relapsed, CD30+ HL at baseline. Six patients are male, and their median age at baseline was 30 (range, 17-69).
The patients had a median of 5 prior treatments (range, 2-9). This included PD-1 inhibitors (n=10), brentuximab vedotin (n=8), and transplant (n=6).
All patients received lymphodepletion with cyclophosphamide at 500 mg/m2 and fludarabine at 30 mg/m2 daily for 3 days. They then received CD30.CAR T-cell therapy at 2×107 cells/m2 or 1×108 cells/m2.
Dr. Ramos noted that CD30.CAR T-cell expansion was dose-dependent and increased by lymphodepleting chemotherapy.
“The peak expansion is much higher [with lymphodepletion], probably in the order of two to three logs higher than what we see without lymphodepleting chemotherapy,” he said. “So chemotherapy makes a difference.”
Increased CD30.CAR T-cell expansion was associated with improved response. Of the nine evaluable patients, six achieved a CR, and three progressed.
Four complete responders were still in CR at last follow-up, one of them for more than a year. However, two complete responders ultimately progressed.
In addition to improved responses, the researchers observed increased toxicity in this trial. Dr. Ramos said some of these toxicities are “probably attributable” to the lymphodepleting chemotherapy.
Toxicities included grade 1 cytokine release syndrome (no tocilizumab required), maculopapular rash, transient cytopenias, nausea, vomiting, and alopecia.
Dr. Ramos said these results suggest adoptive transfer of CD30.CAR T cells is “safe, even with chemotherapy.”
He noted that the duration of response with this treatment is unknown, but trial enrollment and follow-up are ongoing.
RELY-30 was sponsored by Baylor College of Medicine. Dr. Ramos reported relationships with Novartis, Celgene, Bluebird Bio, and Tessa Therapeutics.
*Data in the abstract differ from the presentation.
Fludarabine deemed important for CD30.CAR T-cell therapy
SAN DIEGO—Fludarabine is “very important” for lymphodepletion prior to CD30-directed chimeric antigen receptor (CAR) T-cell therapy, according to a presentation at the 2018 ASH Annual Meeting.
A phase 1/2 study showed that bendamustine alone was not sufficient as lymphodepletion.
However, adding fludarabine to bendamustine could enhance responses to CD30.CAR T-cell therapy and improve progression-free survival (PFS) in patients with Hodgkin or non-Hodgkin lymphoma.
Natalie S. Grover, MD, of the University of North Carolina in Chapel Hill, presented these results as abstract 681.*
This trial (NCT02690545) included patients with relapsed/refractory, CD30+ Hodgkin lymphoma or T-cell non-Hodgkin lymphoma.
Twenty-four adult patients have been treated thus far. Twenty-two had classical Hodgkin lymphoma, one had Sézary syndrome, and one had enteropathy-associated T-cell lymphoma.
The patients’ median age at baseline was 34.5 years (range, 23-69), and they had received a median of 7.5 prior lines of therapy (range, 3-17).
Prior treatments included brentuximab vedotin (n=23), checkpoint inhibitors (n=16), autologous transplant (n=17), and allogeneic transplant (n=7).
In this trial, patients could receive bridging therapy while their T cells were being processed. They then underwent lymphodepletion and received CAR T-cell therapy at one of two doses.
Bendamustine alone
Eight patients received lymphodepletion with 2 days of bendamustine at 90 mg/m2. Three of these patients received CD30.CAR T-cell therapy at 1×108 cells/m2, and all three progressed.
Of the five patients who received CAR T-cell therapy at a dose of 2×108 cells/m2, one progressed, one had stable disease, and three had a complete response (CR).
However, all three complete responders were in CR prior to lymphodepletion as a result of bridging therapy.
“Responses were more modest than what we were hoping for with lymphodepletion,” Dr. Grover noted. “We looked at the cytokine levels in patients getting bendamustine lymphodepletion and saw that bendamustine wasn’t supporting an ideal cytokine milieu. IL-7 and IL-15 are important for T-cell expansion, and these levels were not increased in patients post-bendamustine.”
When the researchers added fludarabine to the lymphodepleting regimen, they observed an increase in T-cell expansion.
Bendamustine plus fludarabine
Sixteen patients received bendamustine plus fludarabine prior to CAR T-cell therapy. The regimen consisted of 3 days of bendamustine at 70 mg/m2 and fludarabine at 30 mg/m2.
All 16 patients received CAR T cells at 2×108 cells/m2, which was the recommended phase 2 dose.
“Responses were more impressive in the bendamustine-fludarabine cohort,” Dr. Grover noted.
Twelve of the 16 patients achieved a CR, although two patients were already in CR prior to lymphodepletion.
Two patients had a partial response, one had stable disease, and one progressed.
PFS and toxicity
Dr. Grover and her colleagues also assessed PFS. At a median follow-up of 100 days, the median PFS was 164 days for the entire cohort, excluding patients who were in CR prior to lymphodepletion.
The median PFS was 396 days for the bendamustine-fludarabine cohort and 55 days for patients in the bendamustine-alone cohort (P=0.001).
There was no neurotoxicity in this trial.
Three patients developed cytokine release syndrome (CRS). Two patients had grade 1 CRS that resolved spontaneously, and one patient had grade 2 CRS, which responded to tocilizumab. Two of the patients with CRS had T-cell lymphoma. The Sézary patient had grade 2 CRS.
Eight patients had a mild rash, one of whom had a rash at baseline.
“CAR T cells against CD30 preceded by lymphodepletion with bendamustine and fludarabine have promising efficacy and a good safety profile in treating patients with relapsed/refractory, CD30+ lymphomas,” Dr. Grover said in closing.
“Fludarabine is very important in enhancing cytokines for improved growth and persistence of CAR T cells.”
This trial was sponsored by UNC Lineberger Comprehensive Cancer Center. Dr. Grover reported consulting for Seattle Genetics.
*Data in the abstract differ from the presentation.
SAN DIEGO—Fludarabine is “very important” for lymphodepletion prior to CD30-directed chimeric antigen receptor (CAR) T-cell therapy, according to a presentation at the 2018 ASH Annual Meeting.
A phase 1/2 study showed that bendamustine alone was not sufficient as lymphodepletion.
However, adding fludarabine to bendamustine could enhance responses to CD30.CAR T-cell therapy and improve progression-free survival (PFS) in patients with Hodgkin or non-Hodgkin lymphoma.
Natalie S. Grover, MD, of the University of North Carolina in Chapel Hill, presented these results as abstract 681.*
This trial (NCT02690545) included patients with relapsed/refractory, CD30+ Hodgkin lymphoma or T-cell non-Hodgkin lymphoma.
Twenty-four adult patients have been treated thus far. Twenty-two had classical Hodgkin lymphoma, one had Sézary syndrome, and one had enteropathy-associated T-cell lymphoma.
The patients’ median age at baseline was 34.5 years (range, 23-69), and they had received a median of 7.5 prior lines of therapy (range, 3-17).
Prior treatments included brentuximab vedotin (n=23), checkpoint inhibitors (n=16), autologous transplant (n=17), and allogeneic transplant (n=7).
In this trial, patients could receive bridging therapy while their T cells were being processed. They then underwent lymphodepletion and received CAR T-cell therapy at one of two doses.
Bendamustine alone
Eight patients received lymphodepletion with 2 days of bendamustine at 90 mg/m2. Three of these patients received CD30.CAR T-cell therapy at 1×108 cells/m2, and all three progressed.
Of the five patients who received CAR T-cell therapy at a dose of 2×108 cells/m2, one progressed, one had stable disease, and three had a complete response (CR).
However, all three complete responders were in CR prior to lymphodepletion as a result of bridging therapy.
“Responses were more modest than what we were hoping for with lymphodepletion,” Dr. Grover noted. “We looked at the cytokine levels in patients getting bendamustine lymphodepletion and saw that bendamustine wasn’t supporting an ideal cytokine milieu. IL-7 and IL-15 are important for T-cell expansion, and these levels were not increased in patients post-bendamustine.”
When the researchers added fludarabine to the lymphodepleting regimen, they observed an increase in T-cell expansion.
Bendamustine plus fludarabine
Sixteen patients received bendamustine plus fludarabine prior to CAR T-cell therapy. The regimen consisted of 3 days of bendamustine at 70 mg/m2 and fludarabine at 30 mg/m2.
All 16 patients received CAR T cells at 2×108 cells/m2, which was the recommended phase 2 dose.
“Responses were more impressive in the bendamustine-fludarabine cohort,” Dr. Grover noted.
Twelve of the 16 patients achieved a CR, although two patients were already in CR prior to lymphodepletion.
Two patients had a partial response, one had stable disease, and one progressed.
PFS and toxicity
Dr. Grover and her colleagues also assessed PFS. At a median follow-up of 100 days, the median PFS was 164 days for the entire cohort, excluding patients who were in CR prior to lymphodepletion.
The median PFS was 396 days for the bendamustine-fludarabine cohort and 55 days for patients in the bendamustine-alone cohort (P=0.001).
There was no neurotoxicity in this trial.
Three patients developed cytokine release syndrome (CRS). Two patients had grade 1 CRS that resolved spontaneously, and one patient had grade 2 CRS, which responded to tocilizumab. Two of the patients with CRS had T-cell lymphoma. The Sézary patient had grade 2 CRS.
Eight patients had a mild rash, one of whom had a rash at baseline.
“CAR T cells against CD30 preceded by lymphodepletion with bendamustine and fludarabine have promising efficacy and a good safety profile in treating patients with relapsed/refractory, CD30+ lymphomas,” Dr. Grover said in closing.
“Fludarabine is very important in enhancing cytokines for improved growth and persistence of CAR T cells.”
This trial was sponsored by UNC Lineberger Comprehensive Cancer Center. Dr. Grover reported consulting for Seattle Genetics.
*Data in the abstract differ from the presentation.
SAN DIEGO—Fludarabine is “very important” for lymphodepletion prior to CD30-directed chimeric antigen receptor (CAR) T-cell therapy, according to a presentation at the 2018 ASH Annual Meeting.
A phase 1/2 study showed that bendamustine alone was not sufficient as lymphodepletion.
However, adding fludarabine to bendamustine could enhance responses to CD30.CAR T-cell therapy and improve progression-free survival (PFS) in patients with Hodgkin or non-Hodgkin lymphoma.
Natalie S. Grover, MD, of the University of North Carolina in Chapel Hill, presented these results as abstract 681.*
This trial (NCT02690545) included patients with relapsed/refractory, CD30+ Hodgkin lymphoma or T-cell non-Hodgkin lymphoma.
Twenty-four adult patients have been treated thus far. Twenty-two had classical Hodgkin lymphoma, one had Sézary syndrome, and one had enteropathy-associated T-cell lymphoma.
The patients’ median age at baseline was 34.5 years (range, 23-69), and they had received a median of 7.5 prior lines of therapy (range, 3-17).
Prior treatments included brentuximab vedotin (n=23), checkpoint inhibitors (n=16), autologous transplant (n=17), and allogeneic transplant (n=7).
In this trial, patients could receive bridging therapy while their T cells were being processed. They then underwent lymphodepletion and received CAR T-cell therapy at one of two doses.
Bendamustine alone
Eight patients received lymphodepletion with 2 days of bendamustine at 90 mg/m2. Three of these patients received CD30.CAR T-cell therapy at 1×108 cells/m2, and all three progressed.
Of the five patients who received CAR T-cell therapy at a dose of 2×108 cells/m2, one progressed, one had stable disease, and three had a complete response (CR).
However, all three complete responders were in CR prior to lymphodepletion as a result of bridging therapy.
“Responses were more modest than what we were hoping for with lymphodepletion,” Dr. Grover noted. “We looked at the cytokine levels in patients getting bendamustine lymphodepletion and saw that bendamustine wasn’t supporting an ideal cytokine milieu. IL-7 and IL-15 are important for T-cell expansion, and these levels were not increased in patients post-bendamustine.”
When the researchers added fludarabine to the lymphodepleting regimen, they observed an increase in T-cell expansion.
Bendamustine plus fludarabine
Sixteen patients received bendamustine plus fludarabine prior to CAR T-cell therapy. The regimen consisted of 3 days of bendamustine at 70 mg/m2 and fludarabine at 30 mg/m2.
All 16 patients received CAR T cells at 2×108 cells/m2, which was the recommended phase 2 dose.
“Responses were more impressive in the bendamustine-fludarabine cohort,” Dr. Grover noted.
Twelve of the 16 patients achieved a CR, although two patients were already in CR prior to lymphodepletion.
Two patients had a partial response, one had stable disease, and one progressed.
PFS and toxicity
Dr. Grover and her colleagues also assessed PFS. At a median follow-up of 100 days, the median PFS was 164 days for the entire cohort, excluding patients who were in CR prior to lymphodepletion.
The median PFS was 396 days for the bendamustine-fludarabine cohort and 55 days for patients in the bendamustine-alone cohort (P=0.001).
There was no neurotoxicity in this trial.
Three patients developed cytokine release syndrome (CRS). Two patients had grade 1 CRS that resolved spontaneously, and one patient had grade 2 CRS, which responded to tocilizumab. Two of the patients with CRS had T-cell lymphoma. The Sézary patient had grade 2 CRS.
Eight patients had a mild rash, one of whom had a rash at baseline.
“CAR T cells against CD30 preceded by lymphodepletion with bendamustine and fludarabine have promising efficacy and a good safety profile in treating patients with relapsed/refractory, CD30+ lymphomas,” Dr. Grover said in closing.
“Fludarabine is very important in enhancing cytokines for improved growth and persistence of CAR T cells.”
This trial was sponsored by UNC Lineberger Comprehensive Cancer Center. Dr. Grover reported consulting for Seattle Genetics.
*Data in the abstract differ from the presentation.
Mutation confers resistance to venetoclax in CLL
SAN DIEGO—A recurrent mutation in BCL2, the therapeutic target of venetoclax, appears to be a major contributor to drug resistance in patients with chronic lymphocytic leukemia (CLL), investigators reported.
The mutation has been detected in some patients with CLL up to 2 years before resistance to venetoclax actually develops, according to Piers Blombery, MBBS, of the Peter MacCallum Cancer Center in Melbourne, Victoria, Australia.
“We have identified the first acquired BCL2 mutation developed in patients clinically treated with venetoclax,” he said during the late-breaking abstracts session at the 2018 ASH Annual Meeting.
The mutation, which the investigators have labeled BCL2 Gly101Val, “is a recurrent and frequent mediator of resistance and may be detected years before clinical relapse occurs,” Dr. Blombery added.
A paper on the mutation was published in Cancer Discovery to coincide with the presentation at ASH (abstract LBA-7).
Despite the demonstrated efficacy of venetoclax as continuous therapy in patients with relapsed or refractory CLL, the majority of patients experience disease progression, prompting the investigators to explore molecular mechanisms of secondary resistance.
To do this, they analyzed paired samples from 15 patients with CLL, enrolled in clinical trials of venetoclax, collected both before the start of venetoclax therapy and at the time of disease progression.
In seven patients, the investigators identified a novel mutation that showed up at the time of progression but was absent from the pre-venetoclax samples.
The mutation first became detectable from about 19 to 42 months after the start of therapy and preceded clinical progression by as much as 25 months, the investigators found.
They pinned the mutation down to the BH3-binding groove on BCL2, the same molecular site targeted by venetoclax. They found the mutation was not present in samples from 96 patients with venetoclax-naive CLL nor in any other B-cell malignancies.
Searches for references to the mutation in both a cancer database (COSMIC) and a population database (gnomAD) came up empty.
In other experiments, the investigators determined that cell lines overexpressing BCL2 Gly101Val are resistant to venetoclax, and, in the presence of venetoclax in vitro, BCL2 Gly101Val-expressing cells have a growth advantage compared with wild-type cells.
Additionally, they showed that the mutation results in impaired venetoclax binding in vitro.
“BCL2 Gly101Val is observed subclonally, implicating multiple mechanisms of venetoclax resistance in the same patient,” Dr. Blombery said.
He added that the identification of the resistance mutation is a strong rationale for using combination therapy to treat patients with relapsed or refractory CLL to help prevent or attenuate selection pressures that lead to resistance.
Dr. Blombery reported having no relevant disclosures. The investigators were supported by the Wilson Center for Lymphoma Genomics, Snowdome Foundation, National Health Medical Research Council, Leukemia and Lymphoma Society, Leukemia Foundation, Cancer Council of Victoria, and Australian Cancer Research Foundation.
SAN DIEGO—A recurrent mutation in BCL2, the therapeutic target of venetoclax, appears to be a major contributor to drug resistance in patients with chronic lymphocytic leukemia (CLL), investigators reported.
The mutation has been detected in some patients with CLL up to 2 years before resistance to venetoclax actually develops, according to Piers Blombery, MBBS, of the Peter MacCallum Cancer Center in Melbourne, Victoria, Australia.
“We have identified the first acquired BCL2 mutation developed in patients clinically treated with venetoclax,” he said during the late-breaking abstracts session at the 2018 ASH Annual Meeting.
The mutation, which the investigators have labeled BCL2 Gly101Val, “is a recurrent and frequent mediator of resistance and may be detected years before clinical relapse occurs,” Dr. Blombery added.
A paper on the mutation was published in Cancer Discovery to coincide with the presentation at ASH (abstract LBA-7).
Despite the demonstrated efficacy of venetoclax as continuous therapy in patients with relapsed or refractory CLL, the majority of patients experience disease progression, prompting the investigators to explore molecular mechanisms of secondary resistance.
To do this, they analyzed paired samples from 15 patients with CLL, enrolled in clinical trials of venetoclax, collected both before the start of venetoclax therapy and at the time of disease progression.
In seven patients, the investigators identified a novel mutation that showed up at the time of progression but was absent from the pre-venetoclax samples.
The mutation first became detectable from about 19 to 42 months after the start of therapy and preceded clinical progression by as much as 25 months, the investigators found.
They pinned the mutation down to the BH3-binding groove on BCL2, the same molecular site targeted by venetoclax. They found the mutation was not present in samples from 96 patients with venetoclax-naive CLL nor in any other B-cell malignancies.
Searches for references to the mutation in both a cancer database (COSMIC) and a population database (gnomAD) came up empty.
In other experiments, the investigators determined that cell lines overexpressing BCL2 Gly101Val are resistant to venetoclax, and, in the presence of venetoclax in vitro, BCL2 Gly101Val-expressing cells have a growth advantage compared with wild-type cells.
Additionally, they showed that the mutation results in impaired venetoclax binding in vitro.
“BCL2 Gly101Val is observed subclonally, implicating multiple mechanisms of venetoclax resistance in the same patient,” Dr. Blombery said.
He added that the identification of the resistance mutation is a strong rationale for using combination therapy to treat patients with relapsed or refractory CLL to help prevent or attenuate selection pressures that lead to resistance.
Dr. Blombery reported having no relevant disclosures. The investigators were supported by the Wilson Center for Lymphoma Genomics, Snowdome Foundation, National Health Medical Research Council, Leukemia and Lymphoma Society, Leukemia Foundation, Cancer Council of Victoria, and Australian Cancer Research Foundation.
SAN DIEGO—A recurrent mutation in BCL2, the therapeutic target of venetoclax, appears to be a major contributor to drug resistance in patients with chronic lymphocytic leukemia (CLL), investigators reported.
The mutation has been detected in some patients with CLL up to 2 years before resistance to venetoclax actually develops, according to Piers Blombery, MBBS, of the Peter MacCallum Cancer Center in Melbourne, Victoria, Australia.
“We have identified the first acquired BCL2 mutation developed in patients clinically treated with venetoclax,” he said during the late-breaking abstracts session at the 2018 ASH Annual Meeting.
The mutation, which the investigators have labeled BCL2 Gly101Val, “is a recurrent and frequent mediator of resistance and may be detected years before clinical relapse occurs,” Dr. Blombery added.
A paper on the mutation was published in Cancer Discovery to coincide with the presentation at ASH (abstract LBA-7).
Despite the demonstrated efficacy of venetoclax as continuous therapy in patients with relapsed or refractory CLL, the majority of patients experience disease progression, prompting the investigators to explore molecular mechanisms of secondary resistance.
To do this, they analyzed paired samples from 15 patients with CLL, enrolled in clinical trials of venetoclax, collected both before the start of venetoclax therapy and at the time of disease progression.
In seven patients, the investigators identified a novel mutation that showed up at the time of progression but was absent from the pre-venetoclax samples.
The mutation first became detectable from about 19 to 42 months after the start of therapy and preceded clinical progression by as much as 25 months, the investigators found.
They pinned the mutation down to the BH3-binding groove on BCL2, the same molecular site targeted by venetoclax. They found the mutation was not present in samples from 96 patients with venetoclax-naive CLL nor in any other B-cell malignancies.
Searches for references to the mutation in both a cancer database (COSMIC) and a population database (gnomAD) came up empty.
In other experiments, the investigators determined that cell lines overexpressing BCL2 Gly101Val are resistant to venetoclax, and, in the presence of venetoclax in vitro, BCL2 Gly101Val-expressing cells have a growth advantage compared with wild-type cells.
Additionally, they showed that the mutation results in impaired venetoclax binding in vitro.
“BCL2 Gly101Val is observed subclonally, implicating multiple mechanisms of venetoclax resistance in the same patient,” Dr. Blombery said.
He added that the identification of the resistance mutation is a strong rationale for using combination therapy to treat patients with relapsed or refractory CLL to help prevent or attenuate selection pressures that lead to resistance.
Dr. Blombery reported having no relevant disclosures. The investigators were supported by the Wilson Center for Lymphoma Genomics, Snowdome Foundation, National Health Medical Research Council, Leukemia and Lymphoma Society, Leukemia Foundation, Cancer Council of Victoria, and Australian Cancer Research Foundation.
Intravascular large B-cell lymphoma: an elusive diagnosis with challenging management
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
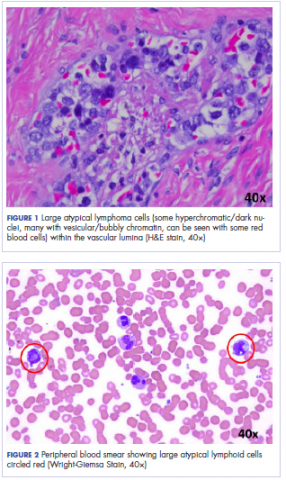
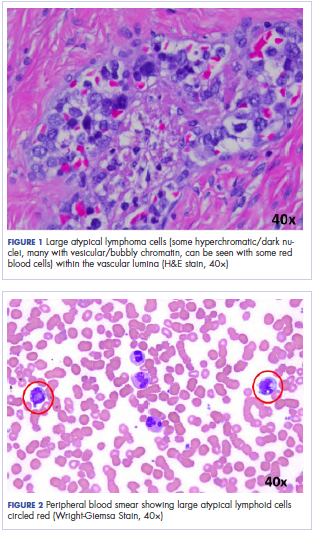
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
Two-drug combo deemed ‘very promising’ for PMBCL
SAN DIEGO—Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising . . . to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president and head of medical at Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid also noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the 2018 ASH Annual Meeting (abstract 1691).
Rationale
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients, but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
Patients and treatment
The interim analysis of this trial included 30 patients with relapsed/refractory PMCBL. Their median age at enrollment was 35.5 (range, 19 to 83), and 57% of patients were female.
Sixty percent of patients had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was 2 (range, 1-5). Thirteen percent of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit (n=9), disease progression (n=7), AEs unrelated to treatment (n=2), patient request (n=1), and “other” reasons (n=1).
Safety
“There were no new safety signals,” Dr. Eid said. “The adverse events reflected the two agents’ profiles.”
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment-related. There were three other deaths in the trial, but they were considered unrelated to treatment.
Response
The complete response rate was 27% (n=8), and the partial response rate was 43% (n=13), for an overall response rate of 70% (n=21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n=3) had stable disease, 13% (n=4) progressed, and investigators were unable to determine the status for 7% of patients (n=2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
“The results are very, very promising,” Dr. Eid said.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics.
SAN DIEGO—Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising . . . to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president and head of medical at Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid also noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the 2018 ASH Annual Meeting (abstract 1691).
Rationale
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients, but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
Patients and treatment
The interim analysis of this trial included 30 patients with relapsed/refractory PMCBL. Their median age at enrollment was 35.5 (range, 19 to 83), and 57% of patients were female.
Sixty percent of patients had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was 2 (range, 1-5). Thirteen percent of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit (n=9), disease progression (n=7), AEs unrelated to treatment (n=2), patient request (n=1), and “other” reasons (n=1).
Safety
“There were no new safety signals,” Dr. Eid said. “The adverse events reflected the two agents’ profiles.”
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment-related. There were three other deaths in the trial, but they were considered unrelated to treatment.
Response
The complete response rate was 27% (n=8), and the partial response rate was 43% (n=13), for an overall response rate of 70% (n=21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n=3) had stable disease, 13% (n=4) progressed, and investigators were unable to determine the status for 7% of patients (n=2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
“The results are very, very promising,” Dr. Eid said.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics.
SAN DIEGO—Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising . . . to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president and head of medical at Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid also noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the 2018 ASH Annual Meeting (abstract 1691).
Rationale
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients, but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
Patients and treatment
The interim analysis of this trial included 30 patients with relapsed/refractory PMCBL. Their median age at enrollment was 35.5 (range, 19 to 83), and 57% of patients were female.
Sixty percent of patients had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was 2 (range, 1-5). Thirteen percent of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit (n=9), disease progression (n=7), AEs unrelated to treatment (n=2), patient request (n=1), and “other” reasons (n=1).
Safety
“There were no new safety signals,” Dr. Eid said. “The adverse events reflected the two agents’ profiles.”
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment-related. There were three other deaths in the trial, but they were considered unrelated to treatment.
Response
The complete response rate was 27% (n=8), and the partial response rate was 43% (n=13), for an overall response rate of 70% (n=21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n=3) had stable disease, 13% (n=4) progressed, and investigators were unable to determine the status for 7% of patients (n=2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
“The results are very, very promising,” Dr. Eid said.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics.
Symptom burdens related to chemotherapy-induced anemia in stage IV cancer
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
LCAR-B38M CAR T therapy appears durable in myeloma
SAN DIEGO – The chimeric antigen receptor (CAR) T-cell therapy LCAR-B38M is in the race for approval in multiple myeloma following encouraging phase 1 results reported at the annual meeting of the American Society of Hematology.
In the LEGEND-2 phase 1/2 open study of 57 patients with advanced relapsed/refractory multiple myeloma treated with the investigational CAR T therapy, the overall response rate was 88% and the complete response rate was 74%. Among 42 patients who achieved complete response, 39 (68%) were negative for minimal residual disease (MRD).
With a median follow-up of 12 months, the median duration of response was 16 months and progression-free survival was 15 months. But in patients who achieved MRD-negative complete response, the median progression-free survival was extended to 24 months.
Pyrexia and cytokine release syndrome were reported in 90% or more of patients. Thrombocytopenia and leukopenia were reported in nearly half of patients.
The phase 1 study was conducted by researchers from the Second Affiliated Hospital of Xi’an Jiaotong University in Xi’an, China. The B-cell maturation antigen (BCMA)–directed CAR T-cell therapy is being jointly developed by Nanjing Legend Biotech and Janssen. A phase 2 study is currently being planned in China for LCAR-B38M. In parallel, Janssen and Legend are enrolling patients in a phase 1b/2 trial of the agent (also known as JNJ-68284528) in the United States.
The therapy joins a growing field of anti-BCMA CAR T-cell agents with promising initial trial results, including bb2121.
In a video interview at ASH, Sen Zhuang, MD, PhD, vice president of oncology clinical development at Janssen Research & Development, said this class of CAR T agents offers the potential for “very long remissions” and possibly even a “cure” for myeloma.
The LEGEND-2 study is sponsored by Nanjing Legend Biotech and two of the investigators reported employment with the company.
SAN DIEGO – The chimeric antigen receptor (CAR) T-cell therapy LCAR-B38M is in the race for approval in multiple myeloma following encouraging phase 1 results reported at the annual meeting of the American Society of Hematology.
In the LEGEND-2 phase 1/2 open study of 57 patients with advanced relapsed/refractory multiple myeloma treated with the investigational CAR T therapy, the overall response rate was 88% and the complete response rate was 74%. Among 42 patients who achieved complete response, 39 (68%) were negative for minimal residual disease (MRD).
With a median follow-up of 12 months, the median duration of response was 16 months and progression-free survival was 15 months. But in patients who achieved MRD-negative complete response, the median progression-free survival was extended to 24 months.
Pyrexia and cytokine release syndrome were reported in 90% or more of patients. Thrombocytopenia and leukopenia were reported in nearly half of patients.
The phase 1 study was conducted by researchers from the Second Affiliated Hospital of Xi’an Jiaotong University in Xi’an, China. The B-cell maturation antigen (BCMA)–directed CAR T-cell therapy is being jointly developed by Nanjing Legend Biotech and Janssen. A phase 2 study is currently being planned in China for LCAR-B38M. In parallel, Janssen and Legend are enrolling patients in a phase 1b/2 trial of the agent (also known as JNJ-68284528) in the United States.
The therapy joins a growing field of anti-BCMA CAR T-cell agents with promising initial trial results, including bb2121.
In a video interview at ASH, Sen Zhuang, MD, PhD, vice president of oncology clinical development at Janssen Research & Development, said this class of CAR T agents offers the potential for “very long remissions” and possibly even a “cure” for myeloma.
The LEGEND-2 study is sponsored by Nanjing Legend Biotech and two of the investigators reported employment with the company.
SAN DIEGO – The chimeric antigen receptor (CAR) T-cell therapy LCAR-B38M is in the race for approval in multiple myeloma following encouraging phase 1 results reported at the annual meeting of the American Society of Hematology.
In the LEGEND-2 phase 1/2 open study of 57 patients with advanced relapsed/refractory multiple myeloma treated with the investigational CAR T therapy, the overall response rate was 88% and the complete response rate was 74%. Among 42 patients who achieved complete response, 39 (68%) were negative for minimal residual disease (MRD).
With a median follow-up of 12 months, the median duration of response was 16 months and progression-free survival was 15 months. But in patients who achieved MRD-negative complete response, the median progression-free survival was extended to 24 months.
Pyrexia and cytokine release syndrome were reported in 90% or more of patients. Thrombocytopenia and leukopenia were reported in nearly half of patients.
The phase 1 study was conducted by researchers from the Second Affiliated Hospital of Xi’an Jiaotong University in Xi’an, China. The B-cell maturation antigen (BCMA)–directed CAR T-cell therapy is being jointly developed by Nanjing Legend Biotech and Janssen. A phase 2 study is currently being planned in China for LCAR-B38M. In parallel, Janssen and Legend are enrolling patients in a phase 1b/2 trial of the agent (also known as JNJ-68284528) in the United States.
The therapy joins a growing field of anti-BCMA CAR T-cell agents with promising initial trial results, including bb2121.
In a video interview at ASH, Sen Zhuang, MD, PhD, vice president of oncology clinical development at Janssen Research & Development, said this class of CAR T agents offers the potential for “very long remissions” and possibly even a “cure” for myeloma.
The LEGEND-2 study is sponsored by Nanjing Legend Biotech and two of the investigators reported employment with the company.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The complete response rate was 74% with median progression-free survival of 15 months.
Study details: A phase 1/2 study of 57 patients with advanced relapsed/refractory multiple myeloma.
Disclosures: The study is sponsored by Nanjing Legend Biotech. Two of the investigators reported employment with the company.