User login
Lower thyroid hormone levels a red flag for elevated suicide risk?
Patients with comorbid anxiety and mood disorders who have reduced, albeit “normal” serum levels of thyroid-stimulating hormone (TSH) may be at increased risk for suicidal ideation, new research suggests.
In a cross-sectional study, clinical data on diagnosis, medication use, and symptom scores were gathered, along with assessments of blood levels of thyroid axis hormones, in patients with both anxiety and mood disorders.
After investigators accounted for age, gender, symptoms, medication use, and other potential confounders, patients with suicidal ideation were 54% less likely to have higher TSH levels. There was no association found with other thyroid hormones.
Based on the results, the assessment of thyroid hormone levels “may be important for suicide prevention and might allow clinicians to evaluate the potential of the suicidal ideation risk in individuals with [anxiety and mood disorders],” co-investigator Vilma Liaugaudaite, PhD student, Neuroscience Institute of the Lithuanian University of Health Sciences, Palanga, and colleagues note.
The findings were presented at the 34th European College of Neuropsychopharmacology (ECNP) Congress.
‘Complex mechanism’
Ms. Liaugaudaite told this news organization that thyroid hormones are known to have a “profound” effect on mood and behavior.
Recent studies show “various degrees of hypothalamic-pituitary-thyroid axis dysregulation are associated with suicidal behavior” in patients with depression, she added.
Noting that disturbances in the serotonin system “constitute the most common biochemical abnormality associated with suicidal behavior,” Ms. Liaugaudaite said it is thought thyroid hormones “are involved in a complex compensatory mechanism to correct reduced central 5-hydroxytryptamine activity” via lower TSH levels.
In addition, hypersecretion of thyrotropin-releasing hormone, which stimulates the release of TSH, “has been considered a compensatory mechanism to maintain normal thyroid hormone secretion and normalize serotonin activity in depressed patients,” she said.
To investigate associations between thyroid axis hormones and suicidality in individuals with comorbid anxiety and mood disorders, the researchers assessed consecutive patients attending a stress disorders clinic.
Sociodemographic and clinical information was gathered, and patients completed the Mini International Neuropsychiatric Interview, the Patient Health Questionnaire-9 (PHQ-9), and the General Anxiety Disorder-7 (GAD-7) scale.
Fasting blood samples were also tested for free thyroxine (FT4), free triiodothyronine (FT3), and TSH levels.
Significant association
Suicidal ideation was identified in 42 participants. Serum FT4, FT3, and TSH levels were within the normal range.
There were no significant differences between patients with and without suicidal ideation in terms of age, gender, education, obesity, smoking, and medication use.
Suicidal ideation was associated with higher scores on the PHQ-9 (15.5 vs. 13.3; P = .085), and with lower TSH levels (1.54 IU/L vs. 2.04 IU/L; P = .092).
The association between serum TSH levels and suicidal ideation was significant after multivariate logistic regression analysis accounted for age, gender, PHQ-9 and GAD-7 scores, education, body mass index, smoking, and use of antidepressants, tranquilizers, mood stabilizers, and neuroleptics.
Specifically, patients with suicidal ideation were significantly less likely to have higher TSH levels than those without, at an odds ratio of 0.46 (P = .027).
There were no significant associations between serum FT4 and FT3 levels and suicidal ideation.
Interesting, but preliminary
Commenting on the findings, Sanjeev Sockalingam, MD, vice chair and professor of psychiatry at the University of Toronto, said it is an “interesting study” because the literature on trying to identify individuals at risk for suicidal ideation or behaviors is “quite mixed, in terms of the results.”
However, it was a cross-sectional study with a relatively small sample size, and studies of this nature typically include patients with hypothyroidism “who end up having suicidal thoughts,” said Dr. Sockalingam, who was not involved with the research.
“I do wonder, given the sample size and patient population, if there may be other factors that may have been related to this,” he added.
Dr. Sockalingam noted that he would like to see more data on the medications the patients were taking, and he underlined that the thyroid levels were in the normal range, “so it’s a bit difficult to untangle what that means in terms of these subtle changes in thyroid levels.”
Robert Levitan, MD, Cameron Wilson Chair in Depression Research at the Centre for Addiction and Mental Health, Toronto, also emphasized that the thyroid levels were in the normal range.
He commented that it therefore “seems unlikely that there’s going to be some biological effect that’s going to affect the brain in a significant enough way” to influence suicidal ideation.
Dr. Levitan continued, “What’s probably happening is there’s some other clinical issue here that they just haven’t picked up on that’s leading in one direction to the suicidal ideation and perhaps affecting the TSH to some extent.”
Although the study is, therefore, “preliminary,” the findings are nevertheless “interesting,” he concluded.
The study received no funding. Ms. Liaugaudaite, Dr. Sockalingam, and Dr. Levitan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with comorbid anxiety and mood disorders who have reduced, albeit “normal” serum levels of thyroid-stimulating hormone (TSH) may be at increased risk for suicidal ideation, new research suggests.
In a cross-sectional study, clinical data on diagnosis, medication use, and symptom scores were gathered, along with assessments of blood levels of thyroid axis hormones, in patients with both anxiety and mood disorders.
After investigators accounted for age, gender, symptoms, medication use, and other potential confounders, patients with suicidal ideation were 54% less likely to have higher TSH levels. There was no association found with other thyroid hormones.
Based on the results, the assessment of thyroid hormone levels “may be important for suicide prevention and might allow clinicians to evaluate the potential of the suicidal ideation risk in individuals with [anxiety and mood disorders],” co-investigator Vilma Liaugaudaite, PhD student, Neuroscience Institute of the Lithuanian University of Health Sciences, Palanga, and colleagues note.
The findings were presented at the 34th European College of Neuropsychopharmacology (ECNP) Congress.
‘Complex mechanism’
Ms. Liaugaudaite told this news organization that thyroid hormones are known to have a “profound” effect on mood and behavior.
Recent studies show “various degrees of hypothalamic-pituitary-thyroid axis dysregulation are associated with suicidal behavior” in patients with depression, she added.
Noting that disturbances in the serotonin system “constitute the most common biochemical abnormality associated with suicidal behavior,” Ms. Liaugaudaite said it is thought thyroid hormones “are involved in a complex compensatory mechanism to correct reduced central 5-hydroxytryptamine activity” via lower TSH levels.
In addition, hypersecretion of thyrotropin-releasing hormone, which stimulates the release of TSH, “has been considered a compensatory mechanism to maintain normal thyroid hormone secretion and normalize serotonin activity in depressed patients,” she said.
To investigate associations between thyroid axis hormones and suicidality in individuals with comorbid anxiety and mood disorders, the researchers assessed consecutive patients attending a stress disorders clinic.
Sociodemographic and clinical information was gathered, and patients completed the Mini International Neuropsychiatric Interview, the Patient Health Questionnaire-9 (PHQ-9), and the General Anxiety Disorder-7 (GAD-7) scale.
Fasting blood samples were also tested for free thyroxine (FT4), free triiodothyronine (FT3), and TSH levels.
Significant association
Suicidal ideation was identified in 42 participants. Serum FT4, FT3, and TSH levels were within the normal range.
There were no significant differences between patients with and without suicidal ideation in terms of age, gender, education, obesity, smoking, and medication use.
Suicidal ideation was associated with higher scores on the PHQ-9 (15.5 vs. 13.3; P = .085), and with lower TSH levels (1.54 IU/L vs. 2.04 IU/L; P = .092).
The association between serum TSH levels and suicidal ideation was significant after multivariate logistic regression analysis accounted for age, gender, PHQ-9 and GAD-7 scores, education, body mass index, smoking, and use of antidepressants, tranquilizers, mood stabilizers, and neuroleptics.
Specifically, patients with suicidal ideation were significantly less likely to have higher TSH levels than those without, at an odds ratio of 0.46 (P = .027).
There were no significant associations between serum FT4 and FT3 levels and suicidal ideation.
Interesting, but preliminary
Commenting on the findings, Sanjeev Sockalingam, MD, vice chair and professor of psychiatry at the University of Toronto, said it is an “interesting study” because the literature on trying to identify individuals at risk for suicidal ideation or behaviors is “quite mixed, in terms of the results.”
However, it was a cross-sectional study with a relatively small sample size, and studies of this nature typically include patients with hypothyroidism “who end up having suicidal thoughts,” said Dr. Sockalingam, who was not involved with the research.
“I do wonder, given the sample size and patient population, if there may be other factors that may have been related to this,” he added.
Dr. Sockalingam noted that he would like to see more data on the medications the patients were taking, and he underlined that the thyroid levels were in the normal range, “so it’s a bit difficult to untangle what that means in terms of these subtle changes in thyroid levels.”
Robert Levitan, MD, Cameron Wilson Chair in Depression Research at the Centre for Addiction and Mental Health, Toronto, also emphasized that the thyroid levels were in the normal range.
He commented that it therefore “seems unlikely that there’s going to be some biological effect that’s going to affect the brain in a significant enough way” to influence suicidal ideation.
Dr. Levitan continued, “What’s probably happening is there’s some other clinical issue here that they just haven’t picked up on that’s leading in one direction to the suicidal ideation and perhaps affecting the TSH to some extent.”
Although the study is, therefore, “preliminary,” the findings are nevertheless “interesting,” he concluded.
The study received no funding. Ms. Liaugaudaite, Dr. Sockalingam, and Dr. Levitan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with comorbid anxiety and mood disorders who have reduced, albeit “normal” serum levels of thyroid-stimulating hormone (TSH) may be at increased risk for suicidal ideation, new research suggests.
In a cross-sectional study, clinical data on diagnosis, medication use, and symptom scores were gathered, along with assessments of blood levels of thyroid axis hormones, in patients with both anxiety and mood disorders.
After investigators accounted for age, gender, symptoms, medication use, and other potential confounders, patients with suicidal ideation were 54% less likely to have higher TSH levels. There was no association found with other thyroid hormones.
Based on the results, the assessment of thyroid hormone levels “may be important for suicide prevention and might allow clinicians to evaluate the potential of the suicidal ideation risk in individuals with [anxiety and mood disorders],” co-investigator Vilma Liaugaudaite, PhD student, Neuroscience Institute of the Lithuanian University of Health Sciences, Palanga, and colleagues note.
The findings were presented at the 34th European College of Neuropsychopharmacology (ECNP) Congress.
‘Complex mechanism’
Ms. Liaugaudaite told this news organization that thyroid hormones are known to have a “profound” effect on mood and behavior.
Recent studies show “various degrees of hypothalamic-pituitary-thyroid axis dysregulation are associated with suicidal behavior” in patients with depression, she added.
Noting that disturbances in the serotonin system “constitute the most common biochemical abnormality associated with suicidal behavior,” Ms. Liaugaudaite said it is thought thyroid hormones “are involved in a complex compensatory mechanism to correct reduced central 5-hydroxytryptamine activity” via lower TSH levels.
In addition, hypersecretion of thyrotropin-releasing hormone, which stimulates the release of TSH, “has been considered a compensatory mechanism to maintain normal thyroid hormone secretion and normalize serotonin activity in depressed patients,” she said.
To investigate associations between thyroid axis hormones and suicidality in individuals with comorbid anxiety and mood disorders, the researchers assessed consecutive patients attending a stress disorders clinic.
Sociodemographic and clinical information was gathered, and patients completed the Mini International Neuropsychiatric Interview, the Patient Health Questionnaire-9 (PHQ-9), and the General Anxiety Disorder-7 (GAD-7) scale.
Fasting blood samples were also tested for free thyroxine (FT4), free triiodothyronine (FT3), and TSH levels.
Significant association
Suicidal ideation was identified in 42 participants. Serum FT4, FT3, and TSH levels were within the normal range.
There were no significant differences between patients with and without suicidal ideation in terms of age, gender, education, obesity, smoking, and medication use.
Suicidal ideation was associated with higher scores on the PHQ-9 (15.5 vs. 13.3; P = .085), and with lower TSH levels (1.54 IU/L vs. 2.04 IU/L; P = .092).
The association between serum TSH levels and suicidal ideation was significant after multivariate logistic regression analysis accounted for age, gender, PHQ-9 and GAD-7 scores, education, body mass index, smoking, and use of antidepressants, tranquilizers, mood stabilizers, and neuroleptics.
Specifically, patients with suicidal ideation were significantly less likely to have higher TSH levels than those without, at an odds ratio of 0.46 (P = .027).
There were no significant associations between serum FT4 and FT3 levels and suicidal ideation.
Interesting, but preliminary
Commenting on the findings, Sanjeev Sockalingam, MD, vice chair and professor of psychiatry at the University of Toronto, said it is an “interesting study” because the literature on trying to identify individuals at risk for suicidal ideation or behaviors is “quite mixed, in terms of the results.”
However, it was a cross-sectional study with a relatively small sample size, and studies of this nature typically include patients with hypothyroidism “who end up having suicidal thoughts,” said Dr. Sockalingam, who was not involved with the research.
“I do wonder, given the sample size and patient population, if there may be other factors that may have been related to this,” he added.
Dr. Sockalingam noted that he would like to see more data on the medications the patients were taking, and he underlined that the thyroid levels were in the normal range, “so it’s a bit difficult to untangle what that means in terms of these subtle changes in thyroid levels.”
Robert Levitan, MD, Cameron Wilson Chair in Depression Research at the Centre for Addiction and Mental Health, Toronto, also emphasized that the thyroid levels were in the normal range.
He commented that it therefore “seems unlikely that there’s going to be some biological effect that’s going to affect the brain in a significant enough way” to influence suicidal ideation.
Dr. Levitan continued, “What’s probably happening is there’s some other clinical issue here that they just haven’t picked up on that’s leading in one direction to the suicidal ideation and perhaps affecting the TSH to some extent.”
Although the study is, therefore, “preliminary,” the findings are nevertheless “interesting,” he concluded.
The study received no funding. Ms. Liaugaudaite, Dr. Sockalingam, and Dr. Levitan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECNP 2021
What turns wandering thoughts into something worse?
With all the lockdowns and social distancing of the pandemic, millions of people have had a lot of time to themselves. Many may have filled that time with baking, long walks, or video games, but minds wandering during these periods was inevitable. Coincident with these experiences were increases in depression and anxiety, which could be linked to the same brain network that is thought to support a meandering mind, called the default mode network.
Scientists interested in this network wanted to understand how wandering thoughts can lead some people to a state of brooding in which the same negative thoughts resurface repeatedly. To gain some insight into these patterns, they recorded more than 2,000 thoughts spoken aloud by 78 study participants who did nothing but let their minds wander for 10 minutes.
Senior researcher Jessica Andrews-Hanna, PhD, assistant professor of psychology, University of Arizona, Tucson, and colleagues hoped that analyzing these stream-of-consciousness thoughts could yield insights into how people become stuck in negative mental spirals.
They found that most participants thought about the present or future in words that were neither particularly negative nor positive. Almost three-quarters of the thoughts were focused inward on the person or were imaginative.
Negativity breeds negativity
But the investigators found an interesting pattern with regard to negative thoughts. The more negative someone’s thoughts became, the more likely that their next idea would be related to their previous one. In other words, negative thoughts created a chain reaction of more negative thoughts.
, indicating true mental meandering. The pattern suggested that negativity tends to narrow the range of thoughts, whereas positivity tends to expand it during periods in which the mind wanders.
The researchers also found, unsurprisingly, that negative thoughts that were focused on the self and on the past were more likely to result in brooding and that positive thoughts were less likely to arise.
Most study participants were young and educated and may have only said things that they were comfortable allowing the researchers to hear. And because the authors didn’t ask participants about their moods, the investigators could not associate specific patterns of thought with any mental health conditions.
Although the findings, published in Scientific Reports, do not on their own point to solutions for depression or anxiety, they may offer a starting point for future research into how negative trains of thoughts begin – and perhaps how to derail them.
A version of this article first appeared on Medscape.com.
With all the lockdowns and social distancing of the pandemic, millions of people have had a lot of time to themselves. Many may have filled that time with baking, long walks, or video games, but minds wandering during these periods was inevitable. Coincident with these experiences were increases in depression and anxiety, which could be linked to the same brain network that is thought to support a meandering mind, called the default mode network.
Scientists interested in this network wanted to understand how wandering thoughts can lead some people to a state of brooding in which the same negative thoughts resurface repeatedly. To gain some insight into these patterns, they recorded more than 2,000 thoughts spoken aloud by 78 study participants who did nothing but let their minds wander for 10 minutes.
Senior researcher Jessica Andrews-Hanna, PhD, assistant professor of psychology, University of Arizona, Tucson, and colleagues hoped that analyzing these stream-of-consciousness thoughts could yield insights into how people become stuck in negative mental spirals.
They found that most participants thought about the present or future in words that were neither particularly negative nor positive. Almost three-quarters of the thoughts were focused inward on the person or were imaginative.
Negativity breeds negativity
But the investigators found an interesting pattern with regard to negative thoughts. The more negative someone’s thoughts became, the more likely that their next idea would be related to their previous one. In other words, negative thoughts created a chain reaction of more negative thoughts.
, indicating true mental meandering. The pattern suggested that negativity tends to narrow the range of thoughts, whereas positivity tends to expand it during periods in which the mind wanders.
The researchers also found, unsurprisingly, that negative thoughts that were focused on the self and on the past were more likely to result in brooding and that positive thoughts were less likely to arise.
Most study participants were young and educated and may have only said things that they were comfortable allowing the researchers to hear. And because the authors didn’t ask participants about their moods, the investigators could not associate specific patterns of thought with any mental health conditions.
Although the findings, published in Scientific Reports, do not on their own point to solutions for depression or anxiety, they may offer a starting point for future research into how negative trains of thoughts begin – and perhaps how to derail them.
A version of this article first appeared on Medscape.com.
With all the lockdowns and social distancing of the pandemic, millions of people have had a lot of time to themselves. Many may have filled that time with baking, long walks, or video games, but minds wandering during these periods was inevitable. Coincident with these experiences were increases in depression and anxiety, which could be linked to the same brain network that is thought to support a meandering mind, called the default mode network.
Scientists interested in this network wanted to understand how wandering thoughts can lead some people to a state of brooding in which the same negative thoughts resurface repeatedly. To gain some insight into these patterns, they recorded more than 2,000 thoughts spoken aloud by 78 study participants who did nothing but let their minds wander for 10 minutes.
Senior researcher Jessica Andrews-Hanna, PhD, assistant professor of psychology, University of Arizona, Tucson, and colleagues hoped that analyzing these stream-of-consciousness thoughts could yield insights into how people become stuck in negative mental spirals.
They found that most participants thought about the present or future in words that were neither particularly negative nor positive. Almost three-quarters of the thoughts were focused inward on the person or were imaginative.
Negativity breeds negativity
But the investigators found an interesting pattern with regard to negative thoughts. The more negative someone’s thoughts became, the more likely that their next idea would be related to their previous one. In other words, negative thoughts created a chain reaction of more negative thoughts.
, indicating true mental meandering. The pattern suggested that negativity tends to narrow the range of thoughts, whereas positivity tends to expand it during periods in which the mind wanders.
The researchers also found, unsurprisingly, that negative thoughts that were focused on the self and on the past were more likely to result in brooding and that positive thoughts were less likely to arise.
Most study participants were young and educated and may have only said things that they were comfortable allowing the researchers to hear. And because the authors didn’t ask participants about their moods, the investigators could not associate specific patterns of thought with any mental health conditions.
Although the findings, published in Scientific Reports, do not on their own point to solutions for depression or anxiety, they may offer a starting point for future research into how negative trains of thoughts begin – and perhaps how to derail them.
A version of this article first appeared on Medscape.com.
Anxiety, depression symptoms rose and fell with new COVID cases
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.
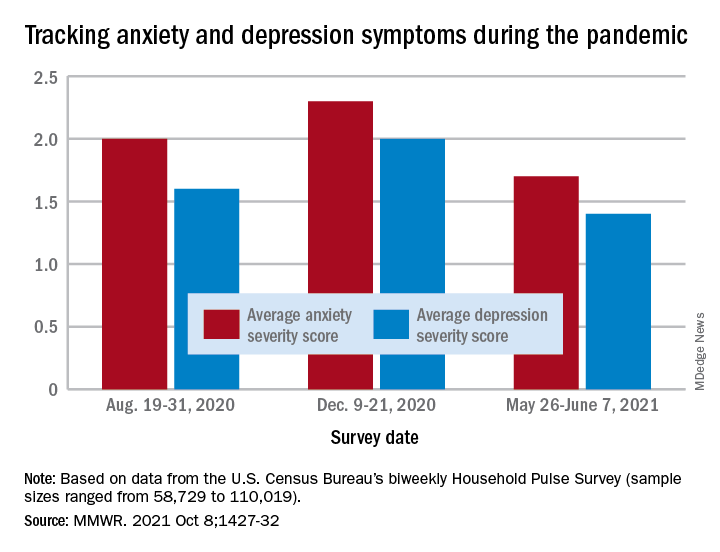
“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.

“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.

“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
FROM THE MMWR
Steroid a promising short-term treatment option for major depression?
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
FROM ECNP 2021
Depression rates up threefold since start of COVID-19
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
FROM LANCET REGIONAL HEALTH – AMERICAS
Customized brain stimulation: New hope for severe depression
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Should clinicians recommend vitamin D for psychiatric patients during COVID-19?
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Treating major depressive disorder after limited response to an initial agent
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
Antipsychotic effective for bipolar depression in phase 3 trial
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Dopamine and reward: The story of social media
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.