User login
Key driver of fish oil’s antidepressant effects revealed
A key molecular mechanism underpinning the anti-inflammatory, antidepressant, and neuroprotective effects of omega-3 fatty acids has been identified. In findings that could lead to the development of new treatments for depression, the research provides the “first evidence” that hippocampal neurons are able to produce two key lipid metabolites of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – lipoxygenase and cytochrome P450, lead investigator Alessandra Borsini, PhD, told this news organization.
This is how EPA and DHA exert their anti-inflammatory and neurogenic properties in vitro, as well as antidepressant properties in patients with depression, said Dr. Borsini, from King’s College London.
“Indeed, we found evidence for a correlation between increased levels of these metabolites and a decrease in severity of depressive symptoms in patients with major depressive disorder,” Dr. Borsini said.
The study was published online June 16 in Molecular Psychiatry.
‘Depression in a dish’
Despite the known role of inflammation in depression, there remains a lack of data showing anti-inflammatory strategies that are effective, safe for everyday use, and with a clear mechanism of action, the researchers note.
Dr. Borsini and colleagues tested the theory that when EPA and DHA are metabolized, some of their metabolites, or lipid mediators, can protect the brain from the harmful effects of inflammation. They used a validated “depression in a dish” in vitro human hippocampal cell model to test their theory.
They found that treating human hippocampal cells with EPA or DHA before exposing them to cytokines prevented increased cell death and decreased neurogenesis. Both these impacts had been previously observed in cells exposed to cytokines alone.
They confirmed that these effects were mediated by the formation of several key lipid mediators produced by EPA and DHA – namely hydroxyeicosapentaenoic acid, hydroxydocosahexaenoic acid, epoxyeicosatetraenoic acid (EpETE), and epoxydocosapentaenoic acid (EpDPA).
It’s the first time these lipid mediators were detected in human hippocampal neurons, the researchers say.
They also found that treating the neurons with an enzyme inhibitor increased the availability of two of these metabolites (EpETE and EpDPA), suggesting a possible way by which future treatments could be optimized.
The findings were replicated in 22 patients with major depression given either EPA (3 g/day) or DHA (1.4 g/day) for 12 weeks. In both groups, EPA or DHA treatment was associated with an increase in their respective metabolites and significant improvement in depressive symptoms.
The average reduction in symptom scores was 64% and 71% in the EPA and DHA groups, respectively, and there was some evidence that higher levels of the same metabolites correlated with less severe depressive symptoms.
“For some time we have known that omega-3 [polyunsaturated fatty acid (PUFA)] can induce antidepressant and anti-inflammatory effects, but, without further understanding of how this happens in the human brain, it has been difficult to develop treatments,” Dr. Borsini said in a news release.
“ which can inform the development of potential new treatments for depression using omega-3 PUFA,” Dr. Borsini added.
“We need to be cautious when interpreting data generated from the correlation between levels of metabolites and depressive symptoms as findings require further validation in a bigger sample of patients,” Dr. Borsini said.
“It is important to highlight that our research has not shown that by simply increasing omega-3 fatty acids in our diets or through taking nutritional supplements we can reduce inflammation or depression,” study author Carmine Pariante, MD, PhD, from King’s College London, said in the news release.
“The mechanisms behind the associations between depression and omega-3 PUFA are complicated and require further research and clinical trials to fully understand how they work and inform future therapeutic approaches,” Dr. Pariante said.
No clinical implications
Weighing in on this research in a Science Media Centre statement, Kevin McConway, emeritus professor of applied statistics, The Open University, Milton Keynes, United Kingdom, said, “The point of the study was to throw some light on the mechanisms in the body by which omega-3 fatty acids might work to reduce inflammation or depression.”
“The research mostly involved cells in laboratory dishes, but it also involved treating a small sample of patients with major depression by giving them supplements of one or other of the two omega-3 acids under investigation for 12 weeks,” he noted.
“The researchers found that the patients’ average scores on a standard set of questions, used to diagnose and measure depression, improved over that 12-week period, for each of the two fatty acids.
While depression symptoms improved over 12 weeks with omega-3 fatty acid treatment, “depression symptoms change over time anyway, for many reasons,” and depressive symptoms might have improved over 12 weeks even if the patients had not been given the omega-3 acids, Dr. McConway said.
“We just can’t tell since every patient got omega-3 fatty acids. So these results can hint that omega-3 fatty acids might help in depression, but it comes nowhere near showing that this is the case with a reasonable degree of certainty,” he cautioned.
“Indeed the researchers did not carry out this part of their study to see whether the omega-3 supplements help with depression – they did it to see whether the biochemical changes that they had seen in cell cultures in the lab might also occur in human bodies,” he noted.
This research was funded in part by grants to the investigators from the U.K. Medical Research Council, the European Commission Horizon 2020, and the National Institute for Health Research (NIHR), Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King’s College London. Dr. Borsini has received research funding from Johnson & Johnson for research on depression and inflammation. Dr. McConway is a trustee of the Science Media Centre and a member of its advisory committee.
A version of this article first appeared on Medscape.com.
A key molecular mechanism underpinning the anti-inflammatory, antidepressant, and neuroprotective effects of omega-3 fatty acids has been identified. In findings that could lead to the development of new treatments for depression, the research provides the “first evidence” that hippocampal neurons are able to produce two key lipid metabolites of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – lipoxygenase and cytochrome P450, lead investigator Alessandra Borsini, PhD, told this news organization.
This is how EPA and DHA exert their anti-inflammatory and neurogenic properties in vitro, as well as antidepressant properties in patients with depression, said Dr. Borsini, from King’s College London.
“Indeed, we found evidence for a correlation between increased levels of these metabolites and a decrease in severity of depressive symptoms in patients with major depressive disorder,” Dr. Borsini said.
The study was published online June 16 in Molecular Psychiatry.
‘Depression in a dish’
Despite the known role of inflammation in depression, there remains a lack of data showing anti-inflammatory strategies that are effective, safe for everyday use, and with a clear mechanism of action, the researchers note.
Dr. Borsini and colleagues tested the theory that when EPA and DHA are metabolized, some of their metabolites, or lipid mediators, can protect the brain from the harmful effects of inflammation. They used a validated “depression in a dish” in vitro human hippocampal cell model to test their theory.
They found that treating human hippocampal cells with EPA or DHA before exposing them to cytokines prevented increased cell death and decreased neurogenesis. Both these impacts had been previously observed in cells exposed to cytokines alone.
They confirmed that these effects were mediated by the formation of several key lipid mediators produced by EPA and DHA – namely hydroxyeicosapentaenoic acid, hydroxydocosahexaenoic acid, epoxyeicosatetraenoic acid (EpETE), and epoxydocosapentaenoic acid (EpDPA).
It’s the first time these lipid mediators were detected in human hippocampal neurons, the researchers say.
They also found that treating the neurons with an enzyme inhibitor increased the availability of two of these metabolites (EpETE and EpDPA), suggesting a possible way by which future treatments could be optimized.
The findings were replicated in 22 patients with major depression given either EPA (3 g/day) or DHA (1.4 g/day) for 12 weeks. In both groups, EPA or DHA treatment was associated with an increase in their respective metabolites and significant improvement in depressive symptoms.
The average reduction in symptom scores was 64% and 71% in the EPA and DHA groups, respectively, and there was some evidence that higher levels of the same metabolites correlated with less severe depressive symptoms.
“For some time we have known that omega-3 [polyunsaturated fatty acid (PUFA)] can induce antidepressant and anti-inflammatory effects, but, without further understanding of how this happens in the human brain, it has been difficult to develop treatments,” Dr. Borsini said in a news release.
“ which can inform the development of potential new treatments for depression using omega-3 PUFA,” Dr. Borsini added.
“We need to be cautious when interpreting data generated from the correlation between levels of metabolites and depressive symptoms as findings require further validation in a bigger sample of patients,” Dr. Borsini said.
“It is important to highlight that our research has not shown that by simply increasing omega-3 fatty acids in our diets or through taking nutritional supplements we can reduce inflammation or depression,” study author Carmine Pariante, MD, PhD, from King’s College London, said in the news release.
“The mechanisms behind the associations between depression and omega-3 PUFA are complicated and require further research and clinical trials to fully understand how they work and inform future therapeutic approaches,” Dr. Pariante said.
No clinical implications
Weighing in on this research in a Science Media Centre statement, Kevin McConway, emeritus professor of applied statistics, The Open University, Milton Keynes, United Kingdom, said, “The point of the study was to throw some light on the mechanisms in the body by which omega-3 fatty acids might work to reduce inflammation or depression.”
“The research mostly involved cells in laboratory dishes, but it also involved treating a small sample of patients with major depression by giving them supplements of one or other of the two omega-3 acids under investigation for 12 weeks,” he noted.
“The researchers found that the patients’ average scores on a standard set of questions, used to diagnose and measure depression, improved over that 12-week period, for each of the two fatty acids.
While depression symptoms improved over 12 weeks with omega-3 fatty acid treatment, “depression symptoms change over time anyway, for many reasons,” and depressive symptoms might have improved over 12 weeks even if the patients had not been given the omega-3 acids, Dr. McConway said.
“We just can’t tell since every patient got omega-3 fatty acids. So these results can hint that omega-3 fatty acids might help in depression, but it comes nowhere near showing that this is the case with a reasonable degree of certainty,” he cautioned.
“Indeed the researchers did not carry out this part of their study to see whether the omega-3 supplements help with depression – they did it to see whether the biochemical changes that they had seen in cell cultures in the lab might also occur in human bodies,” he noted.
This research was funded in part by grants to the investigators from the U.K. Medical Research Council, the European Commission Horizon 2020, and the National Institute for Health Research (NIHR), Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King’s College London. Dr. Borsini has received research funding from Johnson & Johnson for research on depression and inflammation. Dr. McConway is a trustee of the Science Media Centre and a member of its advisory committee.
A version of this article first appeared on Medscape.com.
A key molecular mechanism underpinning the anti-inflammatory, antidepressant, and neuroprotective effects of omega-3 fatty acids has been identified. In findings that could lead to the development of new treatments for depression, the research provides the “first evidence” that hippocampal neurons are able to produce two key lipid metabolites of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – lipoxygenase and cytochrome P450, lead investigator Alessandra Borsini, PhD, told this news organization.
This is how EPA and DHA exert their anti-inflammatory and neurogenic properties in vitro, as well as antidepressant properties in patients with depression, said Dr. Borsini, from King’s College London.
“Indeed, we found evidence for a correlation between increased levels of these metabolites and a decrease in severity of depressive symptoms in patients with major depressive disorder,” Dr. Borsini said.
The study was published online June 16 in Molecular Psychiatry.
‘Depression in a dish’
Despite the known role of inflammation in depression, there remains a lack of data showing anti-inflammatory strategies that are effective, safe for everyday use, and with a clear mechanism of action, the researchers note.
Dr. Borsini and colleagues tested the theory that when EPA and DHA are metabolized, some of their metabolites, or lipid mediators, can protect the brain from the harmful effects of inflammation. They used a validated “depression in a dish” in vitro human hippocampal cell model to test their theory.
They found that treating human hippocampal cells with EPA or DHA before exposing them to cytokines prevented increased cell death and decreased neurogenesis. Both these impacts had been previously observed in cells exposed to cytokines alone.
They confirmed that these effects were mediated by the formation of several key lipid mediators produced by EPA and DHA – namely hydroxyeicosapentaenoic acid, hydroxydocosahexaenoic acid, epoxyeicosatetraenoic acid (EpETE), and epoxydocosapentaenoic acid (EpDPA).
It’s the first time these lipid mediators were detected in human hippocampal neurons, the researchers say.
They also found that treating the neurons with an enzyme inhibitor increased the availability of two of these metabolites (EpETE and EpDPA), suggesting a possible way by which future treatments could be optimized.
The findings were replicated in 22 patients with major depression given either EPA (3 g/day) or DHA (1.4 g/day) for 12 weeks. In both groups, EPA or DHA treatment was associated with an increase in their respective metabolites and significant improvement in depressive symptoms.
The average reduction in symptom scores was 64% and 71% in the EPA and DHA groups, respectively, and there was some evidence that higher levels of the same metabolites correlated with less severe depressive symptoms.
“For some time we have known that omega-3 [polyunsaturated fatty acid (PUFA)] can induce antidepressant and anti-inflammatory effects, but, without further understanding of how this happens in the human brain, it has been difficult to develop treatments,” Dr. Borsini said in a news release.
“ which can inform the development of potential new treatments for depression using omega-3 PUFA,” Dr. Borsini added.
“We need to be cautious when interpreting data generated from the correlation between levels of metabolites and depressive symptoms as findings require further validation in a bigger sample of patients,” Dr. Borsini said.
“It is important to highlight that our research has not shown that by simply increasing omega-3 fatty acids in our diets or through taking nutritional supplements we can reduce inflammation or depression,” study author Carmine Pariante, MD, PhD, from King’s College London, said in the news release.
“The mechanisms behind the associations between depression and omega-3 PUFA are complicated and require further research and clinical trials to fully understand how they work and inform future therapeutic approaches,” Dr. Pariante said.
No clinical implications
Weighing in on this research in a Science Media Centre statement, Kevin McConway, emeritus professor of applied statistics, The Open University, Milton Keynes, United Kingdom, said, “The point of the study was to throw some light on the mechanisms in the body by which omega-3 fatty acids might work to reduce inflammation or depression.”
“The research mostly involved cells in laboratory dishes, but it also involved treating a small sample of patients with major depression by giving them supplements of one or other of the two omega-3 acids under investigation for 12 weeks,” he noted.
“The researchers found that the patients’ average scores on a standard set of questions, used to diagnose and measure depression, improved over that 12-week period, for each of the two fatty acids.
While depression symptoms improved over 12 weeks with omega-3 fatty acid treatment, “depression symptoms change over time anyway, for many reasons,” and depressive symptoms might have improved over 12 weeks even if the patients had not been given the omega-3 acids, Dr. McConway said.
“We just can’t tell since every patient got omega-3 fatty acids. So these results can hint that omega-3 fatty acids might help in depression, but it comes nowhere near showing that this is the case with a reasonable degree of certainty,” he cautioned.
“Indeed the researchers did not carry out this part of their study to see whether the omega-3 supplements help with depression – they did it to see whether the biochemical changes that they had seen in cell cultures in the lab might also occur in human bodies,” he noted.
This research was funded in part by grants to the investigators from the U.K. Medical Research Council, the European Commission Horizon 2020, and the National Institute for Health Research (NIHR), Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King’s College London. Dr. Borsini has received research funding from Johnson & Johnson for research on depression and inflammation. Dr. McConway is a trustee of the Science Media Centre and a member of its advisory committee.
A version of this article first appeared on Medscape.com.
Risk to infant may warrant drug treatment for postpartum depression
If moderate to severe postpartum depression poses a risk to child development, that argues in favor of pharmacologic therapy, according to a detailed risk-benefit assessment presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“It is important to consider that there are potential risks of antidepressant drugs, but there are also potential risks from not providing effective treatment,” reported Neha Hudepohl, MD, at the virtual meeting, presented by MedscapeLive.
On a website maintained by the Centers for Disease Control and Prevention, the question of whether antidepressants pose a risk to breast-feeding children is answered with a “maybe.” Although many of these drugs can be detected in breast milk, according to the CDC, “most have little or no effect on milk supply or on infant well-being.”
This is enough uncertainty that antidepressants are not first-line intervention when postpartum depression is mild, said Dr. Hudepohl, director of women’s mental health at Prisma Health Upstate, Greenville, S.C. However, she noted that the American College of Obstetricians and Gynecologists is among the organizations that recommend drug treatment if symptoms are moderate to severe.
“Depression in the mother affects interactions in feeding practices, sleep routines and patterns, and safety practices,” said Dr. Hudepohl, citing published studies supporting each of these consequences.
For the child, there is some degree of uncertainty about risk from untreated maternal depression as well as from breast mild exposure to antidepressants. Conclusive statements are not offered by ACOG and others.
“Some but not all studies have shown an impact of either antenatal or postnatal depression on speech recognition in infancy of native versus nonnative languages, IQ, and cognitive development, and reduction in left frontal brain electrical activity associated with impaired positive emotions,” Dr. Hudepohl reported.
Sifting through published data, Dr. Hudepohl cited studies associating persistent postpartum depression with a more than fourfold risk of behavioral problems at the age of 3.5 years, lower grades in mathematics at age 16, and a higher prevalence of depression at age 18. Among children who had depressed mothers in infancy, there have also been studies showing a higher reactivity to stressors and higher baseline cortisol levels.
“The good news is that Dr. Hudepohl said. In fact, she cited evidence of a correlation between improvement in maternal symptoms and a reduction in the complications in children, such as behavioral problems.
Postpartum depression, which can develop anytime in the first 12 months after childbirth, is not uncommon, occurring in approximately 15% of women, according to Dr. Hudepohl. Risk factors include personal or family history of depression, anxiety or depression during pregnancy, and a prior history of postpartum depression.
Postpartum depression increases the risk of maternal suicide by about 70-fold, Dr. Hudepohl reported. She noted that the peaks in suicide attempts in the 1st and 12th month after delivery. Adverse infant outcomes are not a predictor of increase risk of attempts, but fetal or infant death are.
According to one study, about 40% of mothers with postpartum depression have intrusive thoughts that involve harming their child. About 15% fear being alone with their infant. Behaviors such as decreased playfulness, less talking, or other interactions with the child, and inconsistent response to the child are all likely to contribute to impaired maternal-child bonding, Dr. Hudepohl reported.
For women who discontinued antidepressants for pregnancy but have now developed significant postpartum depression, Dr. Hudepohl recommended using “what has worked in the past.” She considered monotherapy preferable if possible, but severe symptoms warrant more aggressive intervention. Dr. Hudepohl pointed out that the risks of antidepressants taken by the breast-feeding mother to the infant remain unclear despite multiple studies attempting to establish and quantify risk.
“Antidepressants are the most researched medication in pregnancy,” she said.
Conversely, the risks of untreated symptoms to the mother are significant, and the potential risks to the infant and family – if variable – are not insignificant.
Overall, “nonpharmacologic treatment is preferred first line for mild symptoms,” Dr. Hudepohl, but she and others consider a risk-benefit ratio growing increasingly in favor of drug therapy when this approach is the best option for bringing moderate to severe symptoms under control.
Whether depression arises during pregnancy or in the postpartum period, “psychotherapy is generally considered first-line treatment,” agreed Nancy Byatt, DO, MS, MBA, professor of psychiatry and of obstetrics and gynecology at the University of Massachusetts, Worcester.
Dr. Byatt, who has published frequently on this topic, further agreed that risks to the mother and the child increase with uncontrolled depression in the postpartum period. With symptoms of greater intensity, the uncertain risks of medication are outweighed by substantial potential benefits.
“When a pregnant or postpartum individual has moderate to severe illness, treatment with medication is typically recommended, because the benefits are thought to outweigh the risks,” she said, echoing a consensus opinion among experts and organized medicine.
MedscapeLive and this news organization are owned by the same parent company. Dr. Hudepohl and Dr. Byatt reported no potential financial conflicts of interest.
If moderate to severe postpartum depression poses a risk to child development, that argues in favor of pharmacologic therapy, according to a detailed risk-benefit assessment presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“It is important to consider that there are potential risks of antidepressant drugs, but there are also potential risks from not providing effective treatment,” reported Neha Hudepohl, MD, at the virtual meeting, presented by MedscapeLive.
On a website maintained by the Centers for Disease Control and Prevention, the question of whether antidepressants pose a risk to breast-feeding children is answered with a “maybe.” Although many of these drugs can be detected in breast milk, according to the CDC, “most have little or no effect on milk supply or on infant well-being.”
This is enough uncertainty that antidepressants are not first-line intervention when postpartum depression is mild, said Dr. Hudepohl, director of women’s mental health at Prisma Health Upstate, Greenville, S.C. However, she noted that the American College of Obstetricians and Gynecologists is among the organizations that recommend drug treatment if symptoms are moderate to severe.
“Depression in the mother affects interactions in feeding practices, sleep routines and patterns, and safety practices,” said Dr. Hudepohl, citing published studies supporting each of these consequences.
For the child, there is some degree of uncertainty about risk from untreated maternal depression as well as from breast mild exposure to antidepressants. Conclusive statements are not offered by ACOG and others.
“Some but not all studies have shown an impact of either antenatal or postnatal depression on speech recognition in infancy of native versus nonnative languages, IQ, and cognitive development, and reduction in left frontal brain electrical activity associated with impaired positive emotions,” Dr. Hudepohl reported.
Sifting through published data, Dr. Hudepohl cited studies associating persistent postpartum depression with a more than fourfold risk of behavioral problems at the age of 3.5 years, lower grades in mathematics at age 16, and a higher prevalence of depression at age 18. Among children who had depressed mothers in infancy, there have also been studies showing a higher reactivity to stressors and higher baseline cortisol levels.
“The good news is that Dr. Hudepohl said. In fact, she cited evidence of a correlation between improvement in maternal symptoms and a reduction in the complications in children, such as behavioral problems.
Postpartum depression, which can develop anytime in the first 12 months after childbirth, is not uncommon, occurring in approximately 15% of women, according to Dr. Hudepohl. Risk factors include personal or family history of depression, anxiety or depression during pregnancy, and a prior history of postpartum depression.
Postpartum depression increases the risk of maternal suicide by about 70-fold, Dr. Hudepohl reported. She noted that the peaks in suicide attempts in the 1st and 12th month after delivery. Adverse infant outcomes are not a predictor of increase risk of attempts, but fetal or infant death are.
According to one study, about 40% of mothers with postpartum depression have intrusive thoughts that involve harming their child. About 15% fear being alone with their infant. Behaviors such as decreased playfulness, less talking, or other interactions with the child, and inconsistent response to the child are all likely to contribute to impaired maternal-child bonding, Dr. Hudepohl reported.
For women who discontinued antidepressants for pregnancy but have now developed significant postpartum depression, Dr. Hudepohl recommended using “what has worked in the past.” She considered monotherapy preferable if possible, but severe symptoms warrant more aggressive intervention. Dr. Hudepohl pointed out that the risks of antidepressants taken by the breast-feeding mother to the infant remain unclear despite multiple studies attempting to establish and quantify risk.
“Antidepressants are the most researched medication in pregnancy,” she said.
Conversely, the risks of untreated symptoms to the mother are significant, and the potential risks to the infant and family – if variable – are not insignificant.
Overall, “nonpharmacologic treatment is preferred first line for mild symptoms,” Dr. Hudepohl, but she and others consider a risk-benefit ratio growing increasingly in favor of drug therapy when this approach is the best option for bringing moderate to severe symptoms under control.
Whether depression arises during pregnancy or in the postpartum period, “psychotherapy is generally considered first-line treatment,” agreed Nancy Byatt, DO, MS, MBA, professor of psychiatry and of obstetrics and gynecology at the University of Massachusetts, Worcester.
Dr. Byatt, who has published frequently on this topic, further agreed that risks to the mother and the child increase with uncontrolled depression in the postpartum period. With symptoms of greater intensity, the uncertain risks of medication are outweighed by substantial potential benefits.
“When a pregnant or postpartum individual has moderate to severe illness, treatment with medication is typically recommended, because the benefits are thought to outweigh the risks,” she said, echoing a consensus opinion among experts and organized medicine.
MedscapeLive and this news organization are owned by the same parent company. Dr. Hudepohl and Dr. Byatt reported no potential financial conflicts of interest.
If moderate to severe postpartum depression poses a risk to child development, that argues in favor of pharmacologic therapy, according to a detailed risk-benefit assessment presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“It is important to consider that there are potential risks of antidepressant drugs, but there are also potential risks from not providing effective treatment,” reported Neha Hudepohl, MD, at the virtual meeting, presented by MedscapeLive.
On a website maintained by the Centers for Disease Control and Prevention, the question of whether antidepressants pose a risk to breast-feeding children is answered with a “maybe.” Although many of these drugs can be detected in breast milk, according to the CDC, “most have little or no effect on milk supply or on infant well-being.”
This is enough uncertainty that antidepressants are not first-line intervention when postpartum depression is mild, said Dr. Hudepohl, director of women’s mental health at Prisma Health Upstate, Greenville, S.C. However, she noted that the American College of Obstetricians and Gynecologists is among the organizations that recommend drug treatment if symptoms are moderate to severe.
“Depression in the mother affects interactions in feeding practices, sleep routines and patterns, and safety practices,” said Dr. Hudepohl, citing published studies supporting each of these consequences.
For the child, there is some degree of uncertainty about risk from untreated maternal depression as well as from breast mild exposure to antidepressants. Conclusive statements are not offered by ACOG and others.
“Some but not all studies have shown an impact of either antenatal or postnatal depression on speech recognition in infancy of native versus nonnative languages, IQ, and cognitive development, and reduction in left frontal brain electrical activity associated with impaired positive emotions,” Dr. Hudepohl reported.
Sifting through published data, Dr. Hudepohl cited studies associating persistent postpartum depression with a more than fourfold risk of behavioral problems at the age of 3.5 years, lower grades in mathematics at age 16, and a higher prevalence of depression at age 18. Among children who had depressed mothers in infancy, there have also been studies showing a higher reactivity to stressors and higher baseline cortisol levels.
“The good news is that Dr. Hudepohl said. In fact, she cited evidence of a correlation between improvement in maternal symptoms and a reduction in the complications in children, such as behavioral problems.
Postpartum depression, which can develop anytime in the first 12 months after childbirth, is not uncommon, occurring in approximately 15% of women, according to Dr. Hudepohl. Risk factors include personal or family history of depression, anxiety or depression during pregnancy, and a prior history of postpartum depression.
Postpartum depression increases the risk of maternal suicide by about 70-fold, Dr. Hudepohl reported. She noted that the peaks in suicide attempts in the 1st and 12th month after delivery. Adverse infant outcomes are not a predictor of increase risk of attempts, but fetal or infant death are.
According to one study, about 40% of mothers with postpartum depression have intrusive thoughts that involve harming their child. About 15% fear being alone with their infant. Behaviors such as decreased playfulness, less talking, or other interactions with the child, and inconsistent response to the child are all likely to contribute to impaired maternal-child bonding, Dr. Hudepohl reported.
For women who discontinued antidepressants for pregnancy but have now developed significant postpartum depression, Dr. Hudepohl recommended using “what has worked in the past.” She considered monotherapy preferable if possible, but severe symptoms warrant more aggressive intervention. Dr. Hudepohl pointed out that the risks of antidepressants taken by the breast-feeding mother to the infant remain unclear despite multiple studies attempting to establish and quantify risk.
“Antidepressants are the most researched medication in pregnancy,” she said.
Conversely, the risks of untreated symptoms to the mother are significant, and the potential risks to the infant and family – if variable – are not insignificant.
Overall, “nonpharmacologic treatment is preferred first line for mild symptoms,” Dr. Hudepohl, but she and others consider a risk-benefit ratio growing increasingly in favor of drug therapy when this approach is the best option for bringing moderate to severe symptoms under control.
Whether depression arises during pregnancy or in the postpartum period, “psychotherapy is generally considered first-line treatment,” agreed Nancy Byatt, DO, MS, MBA, professor of psychiatry and of obstetrics and gynecology at the University of Massachusetts, Worcester.
Dr. Byatt, who has published frequently on this topic, further agreed that risks to the mother and the child increase with uncontrolled depression in the postpartum period. With symptoms of greater intensity, the uncertain risks of medication are outweighed by substantial potential benefits.
“When a pregnant or postpartum individual has moderate to severe illness, treatment with medication is typically recommended, because the benefits are thought to outweigh the risks,” she said, echoing a consensus opinion among experts and organized medicine.
MedscapeLive and this news organization are owned by the same parent company. Dr. Hudepohl and Dr. Byatt reported no potential financial conflicts of interest.
FROM CP/AACP PSYCHIATRY UPDATE
Depression remains common among dystonia patients
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
FROM NEUROSCIENCE AND BIOBEHAVIORAL REVIEWS
High rates of work-related trauma, PTSD in intern physicians
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”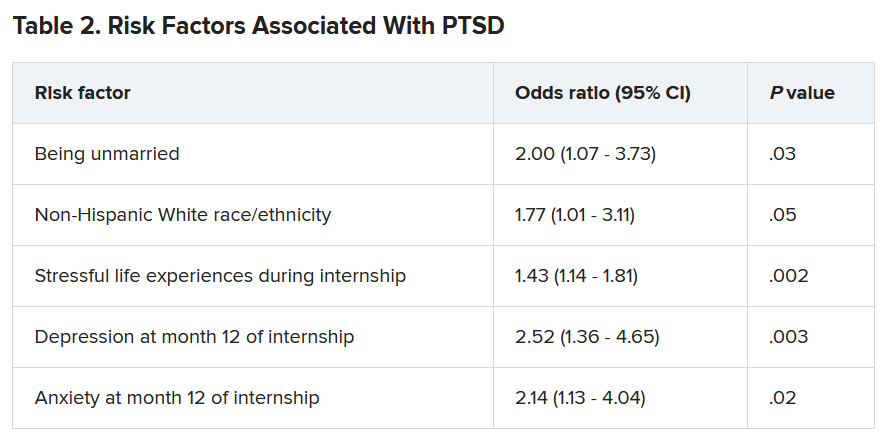
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neurodegeneration complicates psychiatric care for Parkinson’s patients
Managing depression and anxiety in Parkinson’s disease should start with a review of medications and involve multidisciplinary care, according to a recent summary of evidence.
“Depression and anxiety have a complex relationship with the disease and while the exact mechanism for this association is unknown, both disturbances occur with increased prevalence across the disease course and when present earlier in life, increase the risk of PD by about twofold,” wrote Gregory M. Pontone, MD, of Johns Hopkins University, Baltimore, and colleagues.
Randomized trials to guide treatment of anxiety and depression in patients with Parkinson’s disease (PD) are limited, the researchers noted. However, data from a longitudinal study showed that PD patients whose depression remitted spontaneously or responded to treatment were able to attain a level of function similar to that of never-depressed PD patients, Dr. Pontone and colleagues said.
The researchers offered a pair of treatment algorithms to help guide clinicians in managing depression and anxiety in PD. However, a caveat to keep in mind is that “the benefit of antidepressant medications, used for depression or anxiety, can be confounded when motor symptoms are not optimally treated,” the researchers emphasized.
For depression, the researchers advised starting with some lab work; “at a minimum we suggest checking a complete blood count, metabolic panel, TSH, B12, and folate,” they noted. They recommended an antidepressant, cognitive-behavioral therapy, or both, as a first-line treatment, such as monotherapy with selective norepinephrine reuptake inhibitors or selective serotonin reuptake inhibitors. They advised titrating the chosen monotherapy to a minimum effective dose over a 2- to 3-week period to assess response.
“We recommend continuing antidepressant therapy for at least 1 year based on literature in non-PD populations and anecdotal clinical experience. At 1 year, if not in remission, consider continuing treatment or augmenting to improve response,” the researchers said.
, and they recommended using anxiety rating scales to diagnose anxiety in PD. “Given the high prevalence of atypical anxiety syndromes in PD and their potential association with both motor and nonmotor symptoms of the disease, working with the neurologist to achieve optimal control of PD is an essential first step to alleviating anxiety,” they emphasized.
The researchers also advised addressing comorbidities, including cardiovascular disease, chronic pain, diabetes, gastrointestinal issues, hyperthyroidism, and lung disease, all of which can be associated with anxiety. Once comorbidities are addressed, they advised caution given the lack of evidence for efficacy of both pharmacologic and nonpharmacologic anxiety treatments for PD patients. However, first-tier treatment for anxiety could include monotherapy with serotonin-norepinephrine reuptake inhibitors or selective serotonin reuptake inhibitors, they said.
PD patients with depression and anxiety also may benefit from nonpharmacologic interventions, including exercise, mindfulness, relaxation therapy, and cognitive behavioral therapy the researchers said.
Although the algorithm may not differ significantly from current treatment protocols, it highlights aspects unique to PD patients, the researchers said. In particular, the algorithm shows “that interventions used for motor symptoms, for example, dopamine agonists, may be especially potent for mood in the PD population and that augmentation strategies, such as antipsychotics and lithium, may not be well tolerated given their outsized risk of adverse events in PD,” they said.
“While an article of this kind cannot hope to address the gap in knowledge on comparative efficacy between interventions, it can guide readers on the best strategies for implementation and risk mitigation in PD – essentially focusing more on effectiveness,” they concluded.
The study received no outside funding. Dr. Pontone disclosed serving as a consultant for Acadia Pharmaceuticals and Concert Pharmaceuticals.
Managing depression and anxiety in Parkinson’s disease should start with a review of medications and involve multidisciplinary care, according to a recent summary of evidence.
“Depression and anxiety have a complex relationship with the disease and while the exact mechanism for this association is unknown, both disturbances occur with increased prevalence across the disease course and when present earlier in life, increase the risk of PD by about twofold,” wrote Gregory M. Pontone, MD, of Johns Hopkins University, Baltimore, and colleagues.
Randomized trials to guide treatment of anxiety and depression in patients with Parkinson’s disease (PD) are limited, the researchers noted. However, data from a longitudinal study showed that PD patients whose depression remitted spontaneously or responded to treatment were able to attain a level of function similar to that of never-depressed PD patients, Dr. Pontone and colleagues said.
The researchers offered a pair of treatment algorithms to help guide clinicians in managing depression and anxiety in PD. However, a caveat to keep in mind is that “the benefit of antidepressant medications, used for depression or anxiety, can be confounded when motor symptoms are not optimally treated,” the researchers emphasized.
For depression, the researchers advised starting with some lab work; “at a minimum we suggest checking a complete blood count, metabolic panel, TSH, B12, and folate,” they noted. They recommended an antidepressant, cognitive-behavioral therapy, or both, as a first-line treatment, such as monotherapy with selective norepinephrine reuptake inhibitors or selective serotonin reuptake inhibitors. They advised titrating the chosen monotherapy to a minimum effective dose over a 2- to 3-week period to assess response.
“We recommend continuing antidepressant therapy for at least 1 year based on literature in non-PD populations and anecdotal clinical experience. At 1 year, if not in remission, consider continuing treatment or augmenting to improve response,” the researchers said.
, and they recommended using anxiety rating scales to diagnose anxiety in PD. “Given the high prevalence of atypical anxiety syndromes in PD and their potential association with both motor and nonmotor symptoms of the disease, working with the neurologist to achieve optimal control of PD is an essential first step to alleviating anxiety,” they emphasized.
The researchers also advised addressing comorbidities, including cardiovascular disease, chronic pain, diabetes, gastrointestinal issues, hyperthyroidism, and lung disease, all of which can be associated with anxiety. Once comorbidities are addressed, they advised caution given the lack of evidence for efficacy of both pharmacologic and nonpharmacologic anxiety treatments for PD patients. However, first-tier treatment for anxiety could include monotherapy with serotonin-norepinephrine reuptake inhibitors or selective serotonin reuptake inhibitors, they said.
PD patients with depression and anxiety also may benefit from nonpharmacologic interventions, including exercise, mindfulness, relaxation therapy, and cognitive behavioral therapy the researchers said.
Although the algorithm may not differ significantly from current treatment protocols, it highlights aspects unique to PD patients, the researchers said. In particular, the algorithm shows “that interventions used for motor symptoms, for example, dopamine agonists, may be especially potent for mood in the PD population and that augmentation strategies, such as antipsychotics and lithium, may not be well tolerated given their outsized risk of adverse events in PD,” they said.
“While an article of this kind cannot hope to address the gap in knowledge on comparative efficacy between interventions, it can guide readers on the best strategies for implementation and risk mitigation in PD – essentially focusing more on effectiveness,” they concluded.
The study received no outside funding. Dr. Pontone disclosed serving as a consultant for Acadia Pharmaceuticals and Concert Pharmaceuticals.
Managing depression and anxiety in Parkinson’s disease should start with a review of medications and involve multidisciplinary care, according to a recent summary of evidence.
“Depression and anxiety have a complex relationship with the disease and while the exact mechanism for this association is unknown, both disturbances occur with increased prevalence across the disease course and when present earlier in life, increase the risk of PD by about twofold,” wrote Gregory M. Pontone, MD, of Johns Hopkins University, Baltimore, and colleagues.
Randomized trials to guide treatment of anxiety and depression in patients with Parkinson’s disease (PD) are limited, the researchers noted. However, data from a longitudinal study showed that PD patients whose depression remitted spontaneously or responded to treatment were able to attain a level of function similar to that of never-depressed PD patients, Dr. Pontone and colleagues said.
The researchers offered a pair of treatment algorithms to help guide clinicians in managing depression and anxiety in PD. However, a caveat to keep in mind is that “the benefit of antidepressant medications, used for depression or anxiety, can be confounded when motor symptoms are not optimally treated,” the researchers emphasized.
For depression, the researchers advised starting with some lab work; “at a minimum we suggest checking a complete blood count, metabolic panel, TSH, B12, and folate,” they noted. They recommended an antidepressant, cognitive-behavioral therapy, or both, as a first-line treatment, such as monotherapy with selective norepinephrine reuptake inhibitors or selective serotonin reuptake inhibitors. They advised titrating the chosen monotherapy to a minimum effective dose over a 2- to 3-week period to assess response.
“We recommend continuing antidepressant therapy for at least 1 year based on literature in non-PD populations and anecdotal clinical experience. At 1 year, if not in remission, consider continuing treatment or augmenting to improve response,” the researchers said.
, and they recommended using anxiety rating scales to diagnose anxiety in PD. “Given the high prevalence of atypical anxiety syndromes in PD and their potential association with both motor and nonmotor symptoms of the disease, working with the neurologist to achieve optimal control of PD is an essential first step to alleviating anxiety,” they emphasized.
The researchers also advised addressing comorbidities, including cardiovascular disease, chronic pain, diabetes, gastrointestinal issues, hyperthyroidism, and lung disease, all of which can be associated with anxiety. Once comorbidities are addressed, they advised caution given the lack of evidence for efficacy of both pharmacologic and nonpharmacologic anxiety treatments for PD patients. However, first-tier treatment for anxiety could include monotherapy with serotonin-norepinephrine reuptake inhibitors or selective serotonin reuptake inhibitors, they said.
PD patients with depression and anxiety also may benefit from nonpharmacologic interventions, including exercise, mindfulness, relaxation therapy, and cognitive behavioral therapy the researchers said.
Although the algorithm may not differ significantly from current treatment protocols, it highlights aspects unique to PD patients, the researchers said. In particular, the algorithm shows “that interventions used for motor symptoms, for example, dopamine agonists, may be especially potent for mood in the PD population and that augmentation strategies, such as antipsychotics and lithium, may not be well tolerated given their outsized risk of adverse events in PD,” they said.
“While an article of this kind cannot hope to address the gap in knowledge on comparative efficacy between interventions, it can guide readers on the best strategies for implementation and risk mitigation in PD – essentially focusing more on effectiveness,” they concluded.
The study received no outside funding. Dr. Pontone disclosed serving as a consultant for Acadia Pharmaceuticals and Concert Pharmaceuticals.
FROM THE AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Low-dose nitrous oxide shows benefit for resistant depression
A 1-hour treatment with a low concentration of nitrous oxide, commonly known as “laughing gas,” appears to relieve symptoms of treatment-resistant major depression (TRMD), with effects lasting as long as several weeks, new research suggests.
In a trial with a crossover design, investigators randomly assigned 28 patients with severe TRMD to receive a single 1-hour inhalation of placebo or nitrous oxide once a month over a 3-month period. Participants received an inhalation of placebo; a 25% concentration of nitrous oxide; and a 50% concentration of nitrous oxide. Sessions were conducted 4 weeks apart.
Both doses of nitrous oxide were associated with substantial improvement in depressive symptoms for roughly 85% of participants. However, the 25% concentration had a lower risk for adverse effects, which included sedation, nausea, and mild dissociation, compared to the 50% concentration.
“Twenty-five percent nitrous oxide has similar efficacy, compared to 50% nitrous oxide, and reduced side effects fourfold,” lead author Peter Nagele, MD, MSc, chair and professor of anesthesia and critical care, University of Chicago, said in an interview.
“We also observed that many patients had a 2-week improvement of depressive symptoms after a nitrous oxide treatment,” said Dr. Nagele, who also is professor of psychiatry and behavioral neuroscience.
The study was published online June 9 in Science Translational Medicine.
Further refinement
A previous proof-of-principle study conducted by the same researchers demonstrated that a 1-hour inhalation of 50% nitrous oxide had rapid antidepressant effects for patients with TRMD.
The current phase 2 trial “is a follow-up study to our earlier 2015 pilot trial and was designed to further refine the dose of nitrous oxide needed for antidepressant efficacy,” Dr. Nagele said.
“An important secondary aim [of the current study] was to determine whether a lower dose – 25% – would reduce side effects, and a third aim was to determine how long the antidepressants effects last,” he explained.
To investigate, the researchers enrolled 28 patients (median [interquartile range (IQR)] age 39 years [26-68 years]; 71% women; 96% White) to have three inhalation sessions (placebo, 25%, and 50% nitrous oxide) at 4-week intervals. Twenty patients completed all three inhalation sessions, and four completed ≥1 treatment.
Participants had “sustained and refractory depressive illness,” with a mean illness lifetime duration of 17.5 years and an extensive history of antidepressant drug failure (median, 4.5 [2-10] adequate-dose/duration antidepressants).
Some patients had undergone vagus nerve stimulation, electroconvulsive therapy, or repetitive transcranial magnetic stimulation, or had received ketamine (4%, 8%, 13%, and 8%, respectively).
The primary outcome was improvement on the 21-item Hamilton Depression rating Scale (HDRS-21) score over a 2-week observation period.
‘Stronger evidence’
Compared to placebo, nitrous oxide significantly improved depressive symptoms (P = .01). There was no significant difference between the 25% and the 50% concentrations (P = .58).
The estimated difference in HDRS-21 scores between the placebo and various treatment groups are shown in the following table.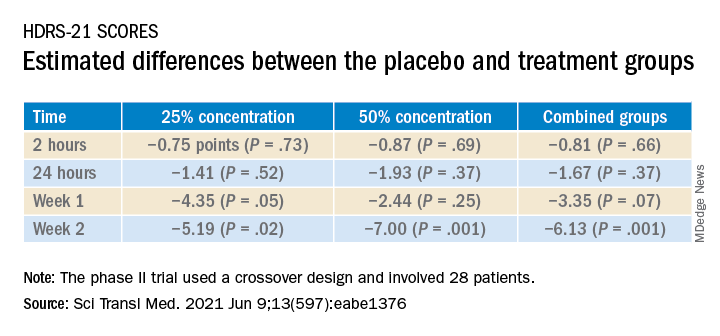
To ensure there where were carryover effects between the two doses, the researchers performed an analysis to ascertain whether order of receipt of the higher dose was related to the 2-week HDRS-21 score; they found no significant effect of trial order (P = .22).
The 20 patients who completed the entire course of treatment “experienced a clinically significant improvement in depressive symptoms from a median baseline HDRS-21 score of 20.5 (IQR, 19.0 to 25.5) to 8.5 (IQR, 2.0 to 16.0) at study completion, corresponding to a median change of −11.0 points (IQR, −3.3 to −14.0 points; P < .0001) after the 3-month study period,” the investigators noted.
The types of treatment response and improvement in depressive symptoms from baseline to study completion are listed in the table below.
There were statistically significant differences in adverse events between the two treatment doses; 47 events occurred following inhalation of the 50% concentration, compared to 11 after inhalation of the 25% concentration. There were six adverse events after inhalation of placebo (P < .0001).
“None of the adverse events were serious, and nearly all occurred either during or immediately after the treatment session and resolved within several hours,” the authors reported.
“We need to be remindful that – despite the exciting results of the study – the study was small and cannot be considered definitive evidence; as such, it is too early to advocate for the use of nitrous oxide in everyday clinical practice,” Dr. Nagele said.
Nevertheless, on the basis of the current findings, he stated.
Rapid-acting antidepressants
Commenting on the study in an interview, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at Toronto Western Hospital, noted that the research into nitrous oxide is “part of an interest in rapid-acting antidepressants.”
Dr. McIntyre, also the chairman and executive director of the Brain and Cognition Discovery Foundation, Toronto, who was not involved with the study, found it “interesting” that “almost 20% of the sample had previously had suboptimal outcomes to ketamine and/or neurostimulation, meaning these patients had serious refractory illness, but the benefit [of nitrous oxide] was sustained at 2 weeks.”
Studies of the use of nitrous oxide for patients with bipolar depression “would be warranted, since it appears generally safe and well tolerated,” said Dr. McIntyre, director of the Depression and Bipolar Support Alliance.
The study was sponsored by an award to Dr. Nagele from the NARSAD Independent Investigator Award from the Brain and Behavior Research Foundation and an award to Dr. Nagele and other coauthors from the Taylor Family Institute for Innovative Psychiatric Research at Washington University in St. Louis. Dr. Nagele receives funding from the National Institute of Mental Health the American Foundation for Prevention of Suicide, and the Brain and Behavior Research Foundation; has received research funding and honoraria from Roche Diagnostics and Abbott Diagnostics; and has previously filed for intellectual property protection related to the use of nitrous oxide in major depression. The other authors’ disclosures are listed on the original article. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and Abbvie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.
A 1-hour treatment with a low concentration of nitrous oxide, commonly known as “laughing gas,” appears to relieve symptoms of treatment-resistant major depression (TRMD), with effects lasting as long as several weeks, new research suggests.
In a trial with a crossover design, investigators randomly assigned 28 patients with severe TRMD to receive a single 1-hour inhalation of placebo or nitrous oxide once a month over a 3-month period. Participants received an inhalation of placebo; a 25% concentration of nitrous oxide; and a 50% concentration of nitrous oxide. Sessions were conducted 4 weeks apart.
Both doses of nitrous oxide were associated with substantial improvement in depressive symptoms for roughly 85% of participants. However, the 25% concentration had a lower risk for adverse effects, which included sedation, nausea, and mild dissociation, compared to the 50% concentration.
“Twenty-five percent nitrous oxide has similar efficacy, compared to 50% nitrous oxide, and reduced side effects fourfold,” lead author Peter Nagele, MD, MSc, chair and professor of anesthesia and critical care, University of Chicago, said in an interview.
“We also observed that many patients had a 2-week improvement of depressive symptoms after a nitrous oxide treatment,” said Dr. Nagele, who also is professor of psychiatry and behavioral neuroscience.
The study was published online June 9 in Science Translational Medicine.
Further refinement
A previous proof-of-principle study conducted by the same researchers demonstrated that a 1-hour inhalation of 50% nitrous oxide had rapid antidepressant effects for patients with TRMD.
The current phase 2 trial “is a follow-up study to our earlier 2015 pilot trial and was designed to further refine the dose of nitrous oxide needed for antidepressant efficacy,” Dr. Nagele said.
“An important secondary aim [of the current study] was to determine whether a lower dose – 25% – would reduce side effects, and a third aim was to determine how long the antidepressants effects last,” he explained.
To investigate, the researchers enrolled 28 patients (median [interquartile range (IQR)] age 39 years [26-68 years]; 71% women; 96% White) to have three inhalation sessions (placebo, 25%, and 50% nitrous oxide) at 4-week intervals. Twenty patients completed all three inhalation sessions, and four completed ≥1 treatment.
Participants had “sustained and refractory depressive illness,” with a mean illness lifetime duration of 17.5 years and an extensive history of antidepressant drug failure (median, 4.5 [2-10] adequate-dose/duration antidepressants).
Some patients had undergone vagus nerve stimulation, electroconvulsive therapy, or repetitive transcranial magnetic stimulation, or had received ketamine (4%, 8%, 13%, and 8%, respectively).
The primary outcome was improvement on the 21-item Hamilton Depression rating Scale (HDRS-21) score over a 2-week observation period.
‘Stronger evidence’
Compared to placebo, nitrous oxide significantly improved depressive symptoms (P = .01). There was no significant difference between the 25% and the 50% concentrations (P = .58).
The estimated difference in HDRS-21 scores between the placebo and various treatment groups are shown in the following table.
To ensure there where were carryover effects between the two doses, the researchers performed an analysis to ascertain whether order of receipt of the higher dose was related to the 2-week HDRS-21 score; they found no significant effect of trial order (P = .22).
The 20 patients who completed the entire course of treatment “experienced a clinically significant improvement in depressive symptoms from a median baseline HDRS-21 score of 20.5 (IQR, 19.0 to 25.5) to 8.5 (IQR, 2.0 to 16.0) at study completion, corresponding to a median change of −11.0 points (IQR, −3.3 to −14.0 points; P < .0001) after the 3-month study period,” the investigators noted.
The types of treatment response and improvement in depressive symptoms from baseline to study completion are listed in the table below.
There were statistically significant differences in adverse events between the two treatment doses; 47 events occurred following inhalation of the 50% concentration, compared to 11 after inhalation of the 25% concentration. There were six adverse events after inhalation of placebo (P < .0001).
“None of the adverse events were serious, and nearly all occurred either during or immediately after the treatment session and resolved within several hours,” the authors reported.
“We need to be remindful that – despite the exciting results of the study – the study was small and cannot be considered definitive evidence; as such, it is too early to advocate for the use of nitrous oxide in everyday clinical practice,” Dr. Nagele said.
Nevertheless, on the basis of the current findings, he stated.
Rapid-acting antidepressants
Commenting on the study in an interview, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at Toronto Western Hospital, noted that the research into nitrous oxide is “part of an interest in rapid-acting antidepressants.”
Dr. McIntyre, also the chairman and executive director of the Brain and Cognition Discovery Foundation, Toronto, who was not involved with the study, found it “interesting” that “almost 20% of the sample had previously had suboptimal outcomes to ketamine and/or neurostimulation, meaning these patients had serious refractory illness, but the benefit [of nitrous oxide] was sustained at 2 weeks.”
Studies of the use of nitrous oxide for patients with bipolar depression “would be warranted, since it appears generally safe and well tolerated,” said Dr. McIntyre, director of the Depression and Bipolar Support Alliance.
The study was sponsored by an award to Dr. Nagele from the NARSAD Independent Investigator Award from the Brain and Behavior Research Foundation and an award to Dr. Nagele and other coauthors from the Taylor Family Institute for Innovative Psychiatric Research at Washington University in St. Louis. Dr. Nagele receives funding from the National Institute of Mental Health the American Foundation for Prevention of Suicide, and the Brain and Behavior Research Foundation; has received research funding and honoraria from Roche Diagnostics and Abbott Diagnostics; and has previously filed for intellectual property protection related to the use of nitrous oxide in major depression. The other authors’ disclosures are listed on the original article. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and Abbvie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.
A 1-hour treatment with a low concentration of nitrous oxide, commonly known as “laughing gas,” appears to relieve symptoms of treatment-resistant major depression (TRMD), with effects lasting as long as several weeks, new research suggests.
In a trial with a crossover design, investigators randomly assigned 28 patients with severe TRMD to receive a single 1-hour inhalation of placebo or nitrous oxide once a month over a 3-month period. Participants received an inhalation of placebo; a 25% concentration of nitrous oxide; and a 50% concentration of nitrous oxide. Sessions were conducted 4 weeks apart.
Both doses of nitrous oxide were associated with substantial improvement in depressive symptoms for roughly 85% of participants. However, the 25% concentration had a lower risk for adverse effects, which included sedation, nausea, and mild dissociation, compared to the 50% concentration.
“Twenty-five percent nitrous oxide has similar efficacy, compared to 50% nitrous oxide, and reduced side effects fourfold,” lead author Peter Nagele, MD, MSc, chair and professor of anesthesia and critical care, University of Chicago, said in an interview.
“We also observed that many patients had a 2-week improvement of depressive symptoms after a nitrous oxide treatment,” said Dr. Nagele, who also is professor of psychiatry and behavioral neuroscience.
The study was published online June 9 in Science Translational Medicine.
Further refinement
A previous proof-of-principle study conducted by the same researchers demonstrated that a 1-hour inhalation of 50% nitrous oxide had rapid antidepressant effects for patients with TRMD.
The current phase 2 trial “is a follow-up study to our earlier 2015 pilot trial and was designed to further refine the dose of nitrous oxide needed for antidepressant efficacy,” Dr. Nagele said.
“An important secondary aim [of the current study] was to determine whether a lower dose – 25% – would reduce side effects, and a third aim was to determine how long the antidepressants effects last,” he explained.
To investigate, the researchers enrolled 28 patients (median [interquartile range (IQR)] age 39 years [26-68 years]; 71% women; 96% White) to have three inhalation sessions (placebo, 25%, and 50% nitrous oxide) at 4-week intervals. Twenty patients completed all three inhalation sessions, and four completed ≥1 treatment.
Participants had “sustained and refractory depressive illness,” with a mean illness lifetime duration of 17.5 years and an extensive history of antidepressant drug failure (median, 4.5 [2-10] adequate-dose/duration antidepressants).
Some patients had undergone vagus nerve stimulation, electroconvulsive therapy, or repetitive transcranial magnetic stimulation, or had received ketamine (4%, 8%, 13%, and 8%, respectively).
The primary outcome was improvement on the 21-item Hamilton Depression rating Scale (HDRS-21) score over a 2-week observation period.
‘Stronger evidence’
Compared to placebo, nitrous oxide significantly improved depressive symptoms (P = .01). There was no significant difference between the 25% and the 50% concentrations (P = .58).
The estimated difference in HDRS-21 scores between the placebo and various treatment groups are shown in the following table.
To ensure there where were carryover effects between the two doses, the researchers performed an analysis to ascertain whether order of receipt of the higher dose was related to the 2-week HDRS-21 score; they found no significant effect of trial order (P = .22).
The 20 patients who completed the entire course of treatment “experienced a clinically significant improvement in depressive symptoms from a median baseline HDRS-21 score of 20.5 (IQR, 19.0 to 25.5) to 8.5 (IQR, 2.0 to 16.0) at study completion, corresponding to a median change of −11.0 points (IQR, −3.3 to −14.0 points; P < .0001) after the 3-month study period,” the investigators noted.
The types of treatment response and improvement in depressive symptoms from baseline to study completion are listed in the table below.
There were statistically significant differences in adverse events between the two treatment doses; 47 events occurred following inhalation of the 50% concentration, compared to 11 after inhalation of the 25% concentration. There were six adverse events after inhalation of placebo (P < .0001).
“None of the adverse events were serious, and nearly all occurred either during or immediately after the treatment session and resolved within several hours,” the authors reported.
“We need to be remindful that – despite the exciting results of the study – the study was small and cannot be considered definitive evidence; as such, it is too early to advocate for the use of nitrous oxide in everyday clinical practice,” Dr. Nagele said.
Nevertheless, on the basis of the current findings, he stated.
Rapid-acting antidepressants
Commenting on the study in an interview, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at Toronto Western Hospital, noted that the research into nitrous oxide is “part of an interest in rapid-acting antidepressants.”
Dr. McIntyre, also the chairman and executive director of the Brain and Cognition Discovery Foundation, Toronto, who was not involved with the study, found it “interesting” that “almost 20% of the sample had previously had suboptimal outcomes to ketamine and/or neurostimulation, meaning these patients had serious refractory illness, but the benefit [of nitrous oxide] was sustained at 2 weeks.”
Studies of the use of nitrous oxide for patients with bipolar depression “would be warranted, since it appears generally safe and well tolerated,” said Dr. McIntyre, director of the Depression and Bipolar Support Alliance.
The study was sponsored by an award to Dr. Nagele from the NARSAD Independent Investigator Award from the Brain and Behavior Research Foundation and an award to Dr. Nagele and other coauthors from the Taylor Family Institute for Innovative Psychiatric Research at Washington University in St. Louis. Dr. Nagele receives funding from the National Institute of Mental Health the American Foundation for Prevention of Suicide, and the Brain and Behavior Research Foundation; has received research funding and honoraria from Roche Diagnostics and Abbott Diagnostics; and has previously filed for intellectual property protection related to the use of nitrous oxide in major depression. The other authors’ disclosures are listed on the original article. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and Abbvie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.
How a community-based program for SMI pivoted during the pandemic
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
COVID-19 tied to spike in suspected suicide attempts by girls
Suspected suicide attempts by teenage girls have increased significantly during the COVID-19 pandemic, according to data released today by the U.S. Centers for Disease Control and Prevention.
Among children and adolescents aged 12-17 years, the average weekly number of emergency department visits for suspected suicide attempts was 22.3% higher during summer 2020 and 39.1% higher during winter 2021 than during the corresponding periods in 2019.
The increase was most evident among young girls.
Between Feb. 21 and March 20, 2021, the number of ED visits for suspected suicide attempts was about 51% higher among girls aged 12-17 years than during the same period in 2019. Among boys aged 12-17 years, ED visits for suspected suicide attempts increased 4%, the CDC reports.
“Young persons might represent a group at high risk because they might have been particularly affected by mitigation measures, such as physical distancing (including a lack of connectedness to schools, teachers, and peers); barriers to mental health treatment; increases in substance use; and anxiety about family health and economic problems, which are all risk factors for suicide,” write the authors, led by Ellen Yard, PhD, with the CDC’s National Center for Injury Prevention and Control.
In addition, the findings from this study suggest there has been “more severe distress among young females than has been identified in previous reports during the pandemic, reinforcing the need for increased attention to, and prevention for, this population,” they point out.
The results were published June 11 in Morbidity and Mortality Weekly Report.
The findings are based on data for ED visits for suspected suicide from the National Syndromic Surveillance Program, which includes about 71% of the nation’s EDs in 49 states (all except Hawaii) and the District of Columbia.
Earlier data reported by the CDC showed that the proportion of mental health–related ED visits among children and adolescents aged 12-17 years increased by 31% during 2020, compared with 2019.
‘Time for action is now’
These new findings underscore the “enormous impact the COVID-19 pandemic is having on our country’s overall emotional wellbeing, especially among young people,” the National Action Alliance for Suicide Prevention (Action Alliance) Media Messaging Work Group said in a statement responding to the newly released data.
“Just as we have taken steps to protect our physical health throughout the pandemic, we must also take steps to protect our mental and emotional health,” the group says.
The data, the group says, specifically speak to the importance of improving suicide care both during and after ED visits by scaling up the adoption of best practices, such as the Recommended Standard Care for People with Suicide Risk: Making Health Care Suicide Safe and Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.
“These and other evidence-based best practices must be adopted by health care systems nationwide to ensure safe, effective suicide care for all,” the group says.
“However, health care systems cannot address this issue alone. Suicide is a complex public health issue that also requires a comprehensive, community-based approach to addressing it. We must ensure suicide prevention is infused into a variety of community-based settings – such as schools, workplaces, and places of worship – to ensure people are connected with prevention activities and resources before a crisis occurs,” the group says.
It also highlights the crucial role of social connectedness as a protective factor against suicide.
“Research indicates that a sense of belonging and social connectedness improves physical, mental, and emotional wellbeing. Everyone can play a role in being there for each other and helping to build resiliency. Having real, honest conversations about our own mental health opens the door for connection and social support,” the group says.
It calls on leaders from all sectors and industries to make suicide prevention “a national priority by becoming engaged in the issue and bringing resources to bear. The time for action is now.”
A version of this article first appeared on Medscape.com.
Suspected suicide attempts by teenage girls have increased significantly during the COVID-19 pandemic, according to data released today by the U.S. Centers for Disease Control and Prevention.
Among children and adolescents aged 12-17 years, the average weekly number of emergency department visits for suspected suicide attempts was 22.3% higher during summer 2020 and 39.1% higher during winter 2021 than during the corresponding periods in 2019.
The increase was most evident among young girls.
Between Feb. 21 and March 20, 2021, the number of ED visits for suspected suicide attempts was about 51% higher among girls aged 12-17 years than during the same period in 2019. Among boys aged 12-17 years, ED visits for suspected suicide attempts increased 4%, the CDC reports.
“Young persons might represent a group at high risk because they might have been particularly affected by mitigation measures, such as physical distancing (including a lack of connectedness to schools, teachers, and peers); barriers to mental health treatment; increases in substance use; and anxiety about family health and economic problems, which are all risk factors for suicide,” write the authors, led by Ellen Yard, PhD, with the CDC’s National Center for Injury Prevention and Control.
In addition, the findings from this study suggest there has been “more severe distress among young females than has been identified in previous reports during the pandemic, reinforcing the need for increased attention to, and prevention for, this population,” they point out.
The results were published June 11 in Morbidity and Mortality Weekly Report.
The findings are based on data for ED visits for suspected suicide from the National Syndromic Surveillance Program, which includes about 71% of the nation’s EDs in 49 states (all except Hawaii) and the District of Columbia.
Earlier data reported by the CDC showed that the proportion of mental health–related ED visits among children and adolescents aged 12-17 years increased by 31% during 2020, compared with 2019.
‘Time for action is now’
These new findings underscore the “enormous impact the COVID-19 pandemic is having on our country’s overall emotional wellbeing, especially among young people,” the National Action Alliance for Suicide Prevention (Action Alliance) Media Messaging Work Group said in a statement responding to the newly released data.
“Just as we have taken steps to protect our physical health throughout the pandemic, we must also take steps to protect our mental and emotional health,” the group says.
The data, the group says, specifically speak to the importance of improving suicide care both during and after ED visits by scaling up the adoption of best practices, such as the Recommended Standard Care for People with Suicide Risk: Making Health Care Suicide Safe and Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.
“These and other evidence-based best practices must be adopted by health care systems nationwide to ensure safe, effective suicide care for all,” the group says.
“However, health care systems cannot address this issue alone. Suicide is a complex public health issue that also requires a comprehensive, community-based approach to addressing it. We must ensure suicide prevention is infused into a variety of community-based settings – such as schools, workplaces, and places of worship – to ensure people are connected with prevention activities and resources before a crisis occurs,” the group says.
It also highlights the crucial role of social connectedness as a protective factor against suicide.
“Research indicates that a sense of belonging and social connectedness improves physical, mental, and emotional wellbeing. Everyone can play a role in being there for each other and helping to build resiliency. Having real, honest conversations about our own mental health opens the door for connection and social support,” the group says.
It calls on leaders from all sectors and industries to make suicide prevention “a national priority by becoming engaged in the issue and bringing resources to bear. The time for action is now.”
A version of this article first appeared on Medscape.com.
Suspected suicide attempts by teenage girls have increased significantly during the COVID-19 pandemic, according to data released today by the U.S. Centers for Disease Control and Prevention.
Among children and adolescents aged 12-17 years, the average weekly number of emergency department visits for suspected suicide attempts was 22.3% higher during summer 2020 and 39.1% higher during winter 2021 than during the corresponding periods in 2019.
The increase was most evident among young girls.
Between Feb. 21 and March 20, 2021, the number of ED visits for suspected suicide attempts was about 51% higher among girls aged 12-17 years than during the same period in 2019. Among boys aged 12-17 years, ED visits for suspected suicide attempts increased 4%, the CDC reports.
“Young persons might represent a group at high risk because they might have been particularly affected by mitigation measures, such as physical distancing (including a lack of connectedness to schools, teachers, and peers); barriers to mental health treatment; increases in substance use; and anxiety about family health and economic problems, which are all risk factors for suicide,” write the authors, led by Ellen Yard, PhD, with the CDC’s National Center for Injury Prevention and Control.
In addition, the findings from this study suggest there has been “more severe distress among young females than has been identified in previous reports during the pandemic, reinforcing the need for increased attention to, and prevention for, this population,” they point out.
The results were published June 11 in Morbidity and Mortality Weekly Report.
The findings are based on data for ED visits for suspected suicide from the National Syndromic Surveillance Program, which includes about 71% of the nation’s EDs in 49 states (all except Hawaii) and the District of Columbia.
Earlier data reported by the CDC showed that the proportion of mental health–related ED visits among children and adolescents aged 12-17 years increased by 31% during 2020, compared with 2019.
‘Time for action is now’
These new findings underscore the “enormous impact the COVID-19 pandemic is having on our country’s overall emotional wellbeing, especially among young people,” the National Action Alliance for Suicide Prevention (Action Alliance) Media Messaging Work Group said in a statement responding to the newly released data.
“Just as we have taken steps to protect our physical health throughout the pandemic, we must also take steps to protect our mental and emotional health,” the group says.
The data, the group says, specifically speak to the importance of improving suicide care both during and after ED visits by scaling up the adoption of best practices, such as the Recommended Standard Care for People with Suicide Risk: Making Health Care Suicide Safe and Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.
“These and other evidence-based best practices must be adopted by health care systems nationwide to ensure safe, effective suicide care for all,” the group says.
“However, health care systems cannot address this issue alone. Suicide is a complex public health issue that also requires a comprehensive, community-based approach to addressing it. We must ensure suicide prevention is infused into a variety of community-based settings – such as schools, workplaces, and places of worship – to ensure people are connected with prevention activities and resources before a crisis occurs,” the group says.
It also highlights the crucial role of social connectedness as a protective factor against suicide.
“Research indicates that a sense of belonging and social connectedness improves physical, mental, and emotional wellbeing. Everyone can play a role in being there for each other and helping to build resiliency. Having real, honest conversations about our own mental health opens the door for connection and social support,” the group says.
It calls on leaders from all sectors and industries to make suicide prevention “a national priority by becoming engaged in the issue and bringing resources to bear. The time for action is now.”
A version of this article first appeared on Medscape.com.
Schizophrenia meds a key contributor to cognitive impairment
Anticholinergic medication burden from antipsychotics, antidepressants, and other psychotropics has a cumulative effect of worsening cognitive function in patients with schizophrenia, new research indicates.
“The link between long-term use of anticholinergic medications and cognitive impairment is well-known and growing,” lead researcher Yash Joshi, MD, department of psychiatry, University of California, San Diego, said in an interview.
“While this association is relevant for everyone, it is particularly important for those living with schizophrenia, who often struggle with cognitive difficulties conferred by the illness itself,” said Dr. Joshi.
“Brain health in schizophrenia is a game of inches, and even small negative effects on cognitive functioning through anticholinergic medication burden may have large impacts on patients’ lives,” he added.
The study was published online May 14 in the American Journal of Psychiatry.
‘Striking’ results
Dr. Joshi and colleagues set out to comprehensively characterize how the cumulative anticholinergic burden from different classes of medications affect cognition in patients with schizophrenia.
They assessed medical records, including all prescribed medications, for 1,120 adults with a diagnosis of schizophrenia or schizoaffective disorder.
For each participant, prescribed medications were rated and summed using a modified anticholinergic cognitive burden (ACB) scale. Cognitive functioning was assessed by performance on domains of the Penn Computerized Neurocognitive Battery (PCNB).
The investigators found that 63% of participants had an ACB score of at least 3, which is “striking,” said Dr. Joshi, given that previous studies have shown that an ACB score of 3 in a healthy, older adult is associated with cognitive dysfunction and a 50% increased risk of developing dementia.
About one-quarter of participants had an ACB score of 6 or higher.
Yet, these high ACB scores are not hard to achieve in routine psychiatric care, the researchers note.
For example, a patient taking olanzapine daily to ease symptoms of psychosis would have an ACB score of 3; if hydroxyzine was added for anxiety or insomnia, the patient’s ACB score would rise to 6, they point out.
Lightening the load
Antipsychotics contributed more than half of the anticholinergic burden, while traditional anticholinergics, antidepressants, mood stabilizers, and benzodiazepines accounted for the remainder.
“It is easy even for well-meaning clinicians to inadvertently contribute to anticholinergic medication burden through routine and appropriate care. The unique finding here is that this burden comes from medications we don’t usually think of as typical anticholinergic agents,” senior author Gregory Light, PhD, with University of California, San Diego, said in a news release.
Anticholinergic medication burden was significantly associated with generalized impairments in cognitive functioning across all cognitive domains on the PCNB with comparable magnitude and after controlling for multiple proxies of functioning or disease severity.
Higher anticholinergic medication burden was associated with worse cognitive performance. The PCNB global cognitive averages for none, low, average, high, and very high anticholinergic burdens were, respectively (in z values), -0.51, -0.70, -0.85, -0.96, and -1.15.
The results suggest “total cumulative anticholinergic burden – rather than anticholinergic burden attributable to a specific antipsychotic or psychotropic medication class – is a key contributor to cognitive impairment in schizophrenia,” the researchers write.
“The results imply that if it is clinically safe and practical,” said Dr. Joshi.
“This may be accomplished by reducing overall polypharmacy or transitioning to equivalent medications with lower overall anticholinergic burden. While ‘traditional’ anticholinergic medications should always be scrutinized, all medications should be carefully evaluated to understand whether they contribute to cumulative anticholinergic medication burden,” he added.
Confirmatory findings
Commenting on the study for this news organization, Jessica Gannon, MD, assistant professor of psychiatry, University of Pittsburgh, said the author’s findings “aren’t surprising, but the work that they did was pretty comprehensive [and] further fleshed out some of our concerns about the impact of anticholinergics on cognitive function in patients with schizophrenia.”
“We certainly have to use some of these medications for patients, like antipsychotics that do have some anticholinergic burden associated with them. We don’t really have other options,” Dr. Gannon said.
“But certainly I think this calls us to be better stewards of medication in general. And when we prescribe for comorbid conditions, like depression and anxiety, we should be careful in our prescribing practices, try not to prescribe an anticholinergic medication, and, if they have been prescribed, to deprescribe them,” Dr. Gannon added.
The study was supported by grants from the National Institute of Mental Health; the Sidney R. Baer, Jr. Foundation; the Brain and Behavior Research Foundation; the VISN-22 Mental Illness Research, Education, and Clinical Center; and the Department of Veterans Affairs. Dr. Joshi and Dr. Gannon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anticholinergic medication burden from antipsychotics, antidepressants, and other psychotropics has a cumulative effect of worsening cognitive function in patients with schizophrenia, new research indicates.
“The link between long-term use of anticholinergic medications and cognitive impairment is well-known and growing,” lead researcher Yash Joshi, MD, department of psychiatry, University of California, San Diego, said in an interview.
“While this association is relevant for everyone, it is particularly important for those living with schizophrenia, who often struggle with cognitive difficulties conferred by the illness itself,” said Dr. Joshi.
“Brain health in schizophrenia is a game of inches, and even small negative effects on cognitive functioning through anticholinergic medication burden may have large impacts on patients’ lives,” he added.
The study was published online May 14 in the American Journal of Psychiatry.
‘Striking’ results
Dr. Joshi and colleagues set out to comprehensively characterize how the cumulative anticholinergic burden from different classes of medications affect cognition in patients with schizophrenia.
They assessed medical records, including all prescribed medications, for 1,120 adults with a diagnosis of schizophrenia or schizoaffective disorder.
For each participant, prescribed medications were rated and summed using a modified anticholinergic cognitive burden (ACB) scale. Cognitive functioning was assessed by performance on domains of the Penn Computerized Neurocognitive Battery (PCNB).
The investigators found that 63% of participants had an ACB score of at least 3, which is “striking,” said Dr. Joshi, given that previous studies have shown that an ACB score of 3 in a healthy, older adult is associated with cognitive dysfunction and a 50% increased risk of developing dementia.
About one-quarter of participants had an ACB score of 6 or higher.
Yet, these high ACB scores are not hard to achieve in routine psychiatric care, the researchers note.
For example, a patient taking olanzapine daily to ease symptoms of psychosis would have an ACB score of 3; if hydroxyzine was added for anxiety or insomnia, the patient’s ACB score would rise to 6, they point out.
Lightening the load
Antipsychotics contributed more than half of the anticholinergic burden, while traditional anticholinergics, antidepressants, mood stabilizers, and benzodiazepines accounted for the remainder.
“It is easy even for well-meaning clinicians to inadvertently contribute to anticholinergic medication burden through routine and appropriate care. The unique finding here is that this burden comes from medications we don’t usually think of as typical anticholinergic agents,” senior author Gregory Light, PhD, with University of California, San Diego, said in a news release.
Anticholinergic medication burden was significantly associated with generalized impairments in cognitive functioning across all cognitive domains on the PCNB with comparable magnitude and after controlling for multiple proxies of functioning or disease severity.
Higher anticholinergic medication burden was associated with worse cognitive performance. The PCNB global cognitive averages for none, low, average, high, and very high anticholinergic burdens were, respectively (in z values), -0.51, -0.70, -0.85, -0.96, and -1.15.
The results suggest “total cumulative anticholinergic burden – rather than anticholinergic burden attributable to a specific antipsychotic or psychotropic medication class – is a key contributor to cognitive impairment in schizophrenia,” the researchers write.
“The results imply that if it is clinically safe and practical,” said Dr. Joshi.
“This may be accomplished by reducing overall polypharmacy or transitioning to equivalent medications with lower overall anticholinergic burden. While ‘traditional’ anticholinergic medications should always be scrutinized, all medications should be carefully evaluated to understand whether they contribute to cumulative anticholinergic medication burden,” he added.
Confirmatory findings
Commenting on the study for this news organization, Jessica Gannon, MD, assistant professor of psychiatry, University of Pittsburgh, said the author’s findings “aren’t surprising, but the work that they did was pretty comprehensive [and] further fleshed out some of our concerns about the impact of anticholinergics on cognitive function in patients with schizophrenia.”
“We certainly have to use some of these medications for patients, like antipsychotics that do have some anticholinergic burden associated with them. We don’t really have other options,” Dr. Gannon said.
“But certainly I think this calls us to be better stewards of medication in general. And when we prescribe for comorbid conditions, like depression and anxiety, we should be careful in our prescribing practices, try not to prescribe an anticholinergic medication, and, if they have been prescribed, to deprescribe them,” Dr. Gannon added.
The study was supported by grants from the National Institute of Mental Health; the Sidney R. Baer, Jr. Foundation; the Brain and Behavior Research Foundation; the VISN-22 Mental Illness Research, Education, and Clinical Center; and the Department of Veterans Affairs. Dr. Joshi and Dr. Gannon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anticholinergic medication burden from antipsychotics, antidepressants, and other psychotropics has a cumulative effect of worsening cognitive function in patients with schizophrenia, new research indicates.
“The link between long-term use of anticholinergic medications and cognitive impairment is well-known and growing,” lead researcher Yash Joshi, MD, department of psychiatry, University of California, San Diego, said in an interview.
“While this association is relevant for everyone, it is particularly important for those living with schizophrenia, who often struggle with cognitive difficulties conferred by the illness itself,” said Dr. Joshi.
“Brain health in schizophrenia is a game of inches, and even small negative effects on cognitive functioning through anticholinergic medication burden may have large impacts on patients’ lives,” he added.
The study was published online May 14 in the American Journal of Psychiatry.
‘Striking’ results
Dr. Joshi and colleagues set out to comprehensively characterize how the cumulative anticholinergic burden from different classes of medications affect cognition in patients with schizophrenia.
They assessed medical records, including all prescribed medications, for 1,120 adults with a diagnosis of schizophrenia or schizoaffective disorder.
For each participant, prescribed medications were rated and summed using a modified anticholinergic cognitive burden (ACB) scale. Cognitive functioning was assessed by performance on domains of the Penn Computerized Neurocognitive Battery (PCNB).
The investigators found that 63% of participants had an ACB score of at least 3, which is “striking,” said Dr. Joshi, given that previous studies have shown that an ACB score of 3 in a healthy, older adult is associated with cognitive dysfunction and a 50% increased risk of developing dementia.
About one-quarter of participants had an ACB score of 6 or higher.
Yet, these high ACB scores are not hard to achieve in routine psychiatric care, the researchers note.
For example, a patient taking olanzapine daily to ease symptoms of psychosis would have an ACB score of 3; if hydroxyzine was added for anxiety or insomnia, the patient’s ACB score would rise to 6, they point out.
Lightening the load
Antipsychotics contributed more than half of the anticholinergic burden, while traditional anticholinergics, antidepressants, mood stabilizers, and benzodiazepines accounted for the remainder.
“It is easy even for well-meaning clinicians to inadvertently contribute to anticholinergic medication burden through routine and appropriate care. The unique finding here is that this burden comes from medications we don’t usually think of as typical anticholinergic agents,” senior author Gregory Light, PhD, with University of California, San Diego, said in a news release.
Anticholinergic medication burden was significantly associated with generalized impairments in cognitive functioning across all cognitive domains on the PCNB with comparable magnitude and after controlling for multiple proxies of functioning or disease severity.
Higher anticholinergic medication burden was associated with worse cognitive performance. The PCNB global cognitive averages for none, low, average, high, and very high anticholinergic burdens were, respectively (in z values), -0.51, -0.70, -0.85, -0.96, and -1.15.
The results suggest “total cumulative anticholinergic burden – rather than anticholinergic burden attributable to a specific antipsychotic or psychotropic medication class – is a key contributor to cognitive impairment in schizophrenia,” the researchers write.
“The results imply that if it is clinically safe and practical,” said Dr. Joshi.
“This may be accomplished by reducing overall polypharmacy or transitioning to equivalent medications with lower overall anticholinergic burden. While ‘traditional’ anticholinergic medications should always be scrutinized, all medications should be carefully evaluated to understand whether they contribute to cumulative anticholinergic medication burden,” he added.
Confirmatory findings
Commenting on the study for this news organization, Jessica Gannon, MD, assistant professor of psychiatry, University of Pittsburgh, said the author’s findings “aren’t surprising, but the work that they did was pretty comprehensive [and] further fleshed out some of our concerns about the impact of anticholinergics on cognitive function in patients with schizophrenia.”
“We certainly have to use some of these medications for patients, like antipsychotics that do have some anticholinergic burden associated with them. We don’t really have other options,” Dr. Gannon said.
“But certainly I think this calls us to be better stewards of medication in general. And when we prescribe for comorbid conditions, like depression and anxiety, we should be careful in our prescribing practices, try not to prescribe an anticholinergic medication, and, if they have been prescribed, to deprescribe them,” Dr. Gannon added.
The study was supported by grants from the National Institute of Mental Health; the Sidney R. Baer, Jr. Foundation; the Brain and Behavior Research Foundation; the VISN-22 Mental Illness Research, Education, and Clinical Center; and the Department of Veterans Affairs. Dr. Joshi and Dr. Gannon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Remarkable’ response to diabetes drug in resistant bipolar depression
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.








