User login
Psychedelic therapy tied to reduced depression, anxiety
TOPLINE:
Perhaps most surprising to investigators, however, was that treatment was also associated with improved cognitive scores in the veterans, many of whom had traumatic brain injuries.
METHODOLOGY:
- Investigators reviewed clinical charts of 86 SOFVs who received psychedelic-assisted treatment at a therapeutic program in Mexico, 86% of whom sustained head injuries during deployment.
- On the first day of the study, participants received a single oral dose (10 mg/kg) of ibogaine hydrochloride in a group setting with two to five other attendees and spent the next day reflecting on their experience with program staff.
- On the third day, participants inhaled 5-MeO-DMT in three incremental doses for a total of 50 mg and were then invited to reflect on their experience both individually and with the group of peers who shared the experience.
- Follow-up surveys at 1, 3, and 6 months posttreatment between September 2019 to March 2021 measured symptoms of posttraumatic stress disorder, cognitive functioning, generalized anxiety disorder, depression, and quality of life.
TAKEAWAY:
- There were significant improvements in self-reported PTSD symptoms, depression, anxiety, insomnia severity, anger, and a large improvement in self-reported satisfaction with life (P < .001 for all).
- Participants reported significant increases in psychological flexibility (P < .001), cognitive functioning (P < .001), and postconcussive symptoms (P < .001).
- Treatment was also associated with a significant reduction in suicidal ideation from pretreatment to 1-month follow-up (P < .01).
IN PRACTICE:
“If consistently replicated, this could have major implications for the landscape of mental health care if people are able to experience significant and sustained healing with 3 days of intensive treatment, relative to our traditionally available interventions that require 8-12 weeks of weekly therapy (for example, gold standard talk therapies such as [prolonged exposure] or [cognitive processing therapy]), or daily use of a pharmacotherapy such as [a selective serotonin reuptake inhibitor] for months to years,” study authors write.
SOURCE:
Alan Kooi Davis, PhD, of the Center for Psychedelic Drug Research and Education at Ohio State University, led the study, which was published online in the American Journal of Drug and Alcohol Abuse.
LIMITATIONS:
Study assessments are based solely on self-report measures. Future research should implement carefully designed batteries that include both self-report and gold-standard clinician-administered measures to better capture symptom improvement and other information. The sample also lacked diversity with regard to race, religion, and socioeconomic status.
DISCLOSURES:
The study was funded by Veterans Exploring Treatment Solutions. Dr. Davis is a board member at Source Resource Foundation and a lead trainer at Fluence. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
Perhaps most surprising to investigators, however, was that treatment was also associated with improved cognitive scores in the veterans, many of whom had traumatic brain injuries.
METHODOLOGY:
- Investigators reviewed clinical charts of 86 SOFVs who received psychedelic-assisted treatment at a therapeutic program in Mexico, 86% of whom sustained head injuries during deployment.
- On the first day of the study, participants received a single oral dose (10 mg/kg) of ibogaine hydrochloride in a group setting with two to five other attendees and spent the next day reflecting on their experience with program staff.
- On the third day, participants inhaled 5-MeO-DMT in three incremental doses for a total of 50 mg and were then invited to reflect on their experience both individually and with the group of peers who shared the experience.
- Follow-up surveys at 1, 3, and 6 months posttreatment between September 2019 to March 2021 measured symptoms of posttraumatic stress disorder, cognitive functioning, generalized anxiety disorder, depression, and quality of life.
TAKEAWAY:
- There were significant improvements in self-reported PTSD symptoms, depression, anxiety, insomnia severity, anger, and a large improvement in self-reported satisfaction with life (P < .001 for all).
- Participants reported significant increases in psychological flexibility (P < .001), cognitive functioning (P < .001), and postconcussive symptoms (P < .001).
- Treatment was also associated with a significant reduction in suicidal ideation from pretreatment to 1-month follow-up (P < .01).
IN PRACTICE:
“If consistently replicated, this could have major implications for the landscape of mental health care if people are able to experience significant and sustained healing with 3 days of intensive treatment, relative to our traditionally available interventions that require 8-12 weeks of weekly therapy (for example, gold standard talk therapies such as [prolonged exposure] or [cognitive processing therapy]), or daily use of a pharmacotherapy such as [a selective serotonin reuptake inhibitor] for months to years,” study authors write.
SOURCE:
Alan Kooi Davis, PhD, of the Center for Psychedelic Drug Research and Education at Ohio State University, led the study, which was published online in the American Journal of Drug and Alcohol Abuse.
LIMITATIONS:
Study assessments are based solely on self-report measures. Future research should implement carefully designed batteries that include both self-report and gold-standard clinician-administered measures to better capture symptom improvement and other information. The sample also lacked diversity with regard to race, religion, and socioeconomic status.
DISCLOSURES:
The study was funded by Veterans Exploring Treatment Solutions. Dr. Davis is a board member at Source Resource Foundation and a lead trainer at Fluence. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
Perhaps most surprising to investigators, however, was that treatment was also associated with improved cognitive scores in the veterans, many of whom had traumatic brain injuries.
METHODOLOGY:
- Investigators reviewed clinical charts of 86 SOFVs who received psychedelic-assisted treatment at a therapeutic program in Mexico, 86% of whom sustained head injuries during deployment.
- On the first day of the study, participants received a single oral dose (10 mg/kg) of ibogaine hydrochloride in a group setting with two to five other attendees and spent the next day reflecting on their experience with program staff.
- On the third day, participants inhaled 5-MeO-DMT in three incremental doses for a total of 50 mg and were then invited to reflect on their experience both individually and with the group of peers who shared the experience.
- Follow-up surveys at 1, 3, and 6 months posttreatment between September 2019 to March 2021 measured symptoms of posttraumatic stress disorder, cognitive functioning, generalized anxiety disorder, depression, and quality of life.
TAKEAWAY:
- There were significant improvements in self-reported PTSD symptoms, depression, anxiety, insomnia severity, anger, and a large improvement in self-reported satisfaction with life (P < .001 for all).
- Participants reported significant increases in psychological flexibility (P < .001), cognitive functioning (P < .001), and postconcussive symptoms (P < .001).
- Treatment was also associated with a significant reduction in suicidal ideation from pretreatment to 1-month follow-up (P < .01).
IN PRACTICE:
“If consistently replicated, this could have major implications for the landscape of mental health care if people are able to experience significant and sustained healing with 3 days of intensive treatment, relative to our traditionally available interventions that require 8-12 weeks of weekly therapy (for example, gold standard talk therapies such as [prolonged exposure] or [cognitive processing therapy]), or daily use of a pharmacotherapy such as [a selective serotonin reuptake inhibitor] for months to years,” study authors write.
SOURCE:
Alan Kooi Davis, PhD, of the Center for Psychedelic Drug Research and Education at Ohio State University, led the study, which was published online in the American Journal of Drug and Alcohol Abuse.
LIMITATIONS:
Study assessments are based solely on self-report measures. Future research should implement carefully designed batteries that include both self-report and gold-standard clinician-administered measures to better capture symptom improvement and other information. The sample also lacked diversity with regard to race, religion, and socioeconomic status.
DISCLOSURES:
The study was funded by Veterans Exploring Treatment Solutions. Dr. Davis is a board member at Source Resource Foundation and a lead trainer at Fluence. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF DRUG AND ALCOHOL ABUSE
Air pollution tied to postpartum depression
TOPLINE:
METHODOLOGY:
- Researchers analyzed data on 340,679 women who had singleton live births at Kaiser Permanente Southern California facilities between 2008 and 2016.
- Ambient air pollution exposures were assessed based on maternal residential addresses using monthly averages of particulate matter ≤ 2.5 mcm (PM2.5), PM ≤ 10 mcm (PM10), nitrogen dioxide, and ozone from Environmental Protection Agency monitoring stations.
- Constituents of PM2.5 (sulfate, nitrate, ammonium, organic matter, and black carbon) were obtained from models based on satellite, ground-based monitor, and chemical transport modeling data.
- Women with an Edinburgh Postnatal Depression Scale score of at least 10 during the first 6 months postpartum were referred for further assessment, including diagnosis and treatment.
TAKEAWAY:
- A total of 25,674 women had PPD (7.5%).
- Positive associations were observed between PPD ozone (adjusted odds ratio, 1.09), PM10 (aOR, 1.02), and PM2.5 (aOR, 1.02), with no statistically significant association with nitrogen dioxide.
- Among PM2.5 constituents, black carbon had the strongest association with PPD (OR 1.04).
- Overall, a higher risk of PPD was associated with ozone exposure during the entire pregnancy and postpartum periods and with PM exposure during the late pregnancy and postpartum periods.
IN PRACTICE:
“These findings suggest that long-term antepartum and postpartum air pollution exposure is a potentially modifiable environmental risk factor for PPD and an important public health issue to address for improved maternal mental health,” the authors wrote.
SOURCE:
The study, with first author Yi Sun, PhD, Chinese Academy of Medical Sciences and Peking Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Postpartum exposures were estimated using only maternal address at delivery, which may have led to exposure misclassification. Potential exposure misclassifications may also exist since indoor and personal exposure levels could not be estimated. Although several covariates were adjusted for, some residual or unmeasured covariates were inevitable due to data unavailability, such as psychiatric history, adverse life events, and marital status, which may affect mental health.
DISCLOSURES:
This study was supported by a grant from the National Institute of Environmental Health Sciences. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed data on 340,679 women who had singleton live births at Kaiser Permanente Southern California facilities between 2008 and 2016.
- Ambient air pollution exposures were assessed based on maternal residential addresses using monthly averages of particulate matter ≤ 2.5 mcm (PM2.5), PM ≤ 10 mcm (PM10), nitrogen dioxide, and ozone from Environmental Protection Agency monitoring stations.
- Constituents of PM2.5 (sulfate, nitrate, ammonium, organic matter, and black carbon) were obtained from models based on satellite, ground-based monitor, and chemical transport modeling data.
- Women with an Edinburgh Postnatal Depression Scale score of at least 10 during the first 6 months postpartum were referred for further assessment, including diagnosis and treatment.
TAKEAWAY:
- A total of 25,674 women had PPD (7.5%).
- Positive associations were observed between PPD ozone (adjusted odds ratio, 1.09), PM10 (aOR, 1.02), and PM2.5 (aOR, 1.02), with no statistically significant association with nitrogen dioxide.
- Among PM2.5 constituents, black carbon had the strongest association with PPD (OR 1.04).
- Overall, a higher risk of PPD was associated with ozone exposure during the entire pregnancy and postpartum periods and with PM exposure during the late pregnancy and postpartum periods.
IN PRACTICE:
“These findings suggest that long-term antepartum and postpartum air pollution exposure is a potentially modifiable environmental risk factor for PPD and an important public health issue to address for improved maternal mental health,” the authors wrote.
SOURCE:
The study, with first author Yi Sun, PhD, Chinese Academy of Medical Sciences and Peking Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Postpartum exposures were estimated using only maternal address at delivery, which may have led to exposure misclassification. Potential exposure misclassifications may also exist since indoor and personal exposure levels could not be estimated. Although several covariates were adjusted for, some residual or unmeasured covariates were inevitable due to data unavailability, such as psychiatric history, adverse life events, and marital status, which may affect mental health.
DISCLOSURES:
This study was supported by a grant from the National Institute of Environmental Health Sciences. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed data on 340,679 women who had singleton live births at Kaiser Permanente Southern California facilities between 2008 and 2016.
- Ambient air pollution exposures were assessed based on maternal residential addresses using monthly averages of particulate matter ≤ 2.5 mcm (PM2.5), PM ≤ 10 mcm (PM10), nitrogen dioxide, and ozone from Environmental Protection Agency monitoring stations.
- Constituents of PM2.5 (sulfate, nitrate, ammonium, organic matter, and black carbon) were obtained from models based on satellite, ground-based monitor, and chemical transport modeling data.
- Women with an Edinburgh Postnatal Depression Scale score of at least 10 during the first 6 months postpartum were referred for further assessment, including diagnosis and treatment.
TAKEAWAY:
- A total of 25,674 women had PPD (7.5%).
- Positive associations were observed between PPD ozone (adjusted odds ratio, 1.09), PM10 (aOR, 1.02), and PM2.5 (aOR, 1.02), with no statistically significant association with nitrogen dioxide.
- Among PM2.5 constituents, black carbon had the strongest association with PPD (OR 1.04).
- Overall, a higher risk of PPD was associated with ozone exposure during the entire pregnancy and postpartum periods and with PM exposure during the late pregnancy and postpartum periods.
IN PRACTICE:
“These findings suggest that long-term antepartum and postpartum air pollution exposure is a potentially modifiable environmental risk factor for PPD and an important public health issue to address for improved maternal mental health,” the authors wrote.
SOURCE:
The study, with first author Yi Sun, PhD, Chinese Academy of Medical Sciences and Peking Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Postpartum exposures were estimated using only maternal address at delivery, which may have led to exposure misclassification. Potential exposure misclassifications may also exist since indoor and personal exposure levels could not be estimated. Although several covariates were adjusted for, some residual or unmeasured covariates were inevitable due to data unavailability, such as psychiatric history, adverse life events, and marital status, which may affect mental health.
DISCLOSURES:
This study was supported by a grant from the National Institute of Environmental Health Sciences. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Lag in antidepressant treatment response explained?
BARCELONA – , new imaging data suggest.
In a double-blind study, more than 30 volunteers were randomly assigned to the SSRI escitalopram or placebo for 3-5 weeks. Using PET imaging, the investigators found that over time, synaptic density significantly increased significantly in the neocortex and hippocampus but only in patients taking the active drug.
The results point to two conclusions, said study investigator Gitta Moos Knudsen, MD, PhD, clinical professor and chief physician at the department of clinical medicine, neurology, psychiatry and sensory sciences at Copenhagen (Denmark) University Hospital.
First, they indicate that SSRIs increase synaptic density in brain areas critically involved in depression, a finding that would go some way to indicating that the synaptic density in the brain may be involved in how antidepressants function, “which would give us a target for developing novel drugs against depression,” said Dr. Knudsen.
“Secondly, our data suggest synapses build up over a period of weeks, which would explain why the effects of these drugs take time to kick in,” she added.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress and simultaneously published online in Molecular Psychiatry.
Marked increase in synaptic density
SSRIs are widely used for depression as well as anxiety and obsessive-compulsive disorder. It is thought that they act via neuroplasticity and synaptic remodeling to improve cognition and emotion processing. However, the investigators note clinical evidence is lacking.
For the study, the researchers randomly assigned healthy individuals to either 20-mg escitalopram or placebo for 3-5 weeks.
They performed PET with the 11C-UCB-J tracer, which allows imaging of the synaptic vesicle glycoprotein 2A (SV2A) in the brain, synaptic density, as well as changes in density over time, in the hippocampus and neocortex.
Between May 2020 and October 2021, 17 individuals were assigned to escitalopram and 15 to placebo. There were no significant differences between two groups in terms of age, sex, and PET-related variables. Serum escitalopram measurements confirmed that all participants in the active drug group were compliant.
When synaptic density was assessed at a single time point, an average of 29 days after the intervention, there were no significant differences between the escitalopram and placebo groups in either the neocortex (P = .41) or in the hippocampus (P = .26).
However, when they performed a secondary analysis of the time-dependent effect on SV2A levels, they found a marked difference between the two study groups.
Compared with the placebo group, participants taking escitalopram had a marked increase in synaptic density in both the neocortex (rp value, 0.58; P = .003) and the hippocampus (rp value, 0.41; P = .048).
In contrast, there were no significant changes in synaptic density in either the neocortex (rp value, –0.01; P = .95) or the hippocampus (rp value, –0.06; P = .62) in the hippocampus.
“That is consistent with our clinical observation that it takes time to evolve synaptic density, along with clinical improvement. Does that mean that the increase in synaptic density is a precondition for improvement in symptoms? We don’t know,” said Dr. Knudsen.
Exciting but not conclusive
Session co-chair Oliver Howes, MD, PhD, professor of molecular psychiatry, King’s College London, agreed that the results do not prove the gradual increase in synaptic density the treatment response lag with SSRIs.
“We definitely don’t yet have all the data to know one way or the other,” he said in an interview.
Another potential hypothesis, he said, is that SSRIs are causing shifts in underlying brain circuits that lead to cognitive changes before there is a discernable improvement in mood.
Indeed, Dr. Howes suggested that increases in synaptic density and cognitive changes related to SSRI use are not necessarily dependent on each other and could even be unrelated.
Also commenting on the research, David Nutt, MD, PhD, Edmond J. Safra professor of neuropsychopharmacology at Imperial College London, said that the “delay in therapeutic action of antidepressants has been a puzzle to psychiatrists ever since they were first discerned over 50 years ago. So, these new data in humans, that use cutting edge brain imaging to demonstrate an increase in brain connections developing over the period that the depression lifts, are very exciting.”
Dr. Nutt added that the results provide further evidence that “enhancing serotonin function in the brain can have enduring health benefits.”
Funding support was provided by the Danish Council for Independent Research, the Lundbeck Foundation, Rigshospitalet, and the Swedish Research Council. Open access funding provided by Royal Library, Copenhagen University Library.
Dr. Knudsen declares relationships with Sage Biogen, H. Lundbeck, Onsero, Pangea, Gilgamesh, Abbvie, and PureTechHealth. Another author declares relationships with Cambridge Cognition and PopReach via Cambridge Enterprise.
A version of this article first appeared on Medscape.com.
BARCELONA – , new imaging data suggest.
In a double-blind study, more than 30 volunteers were randomly assigned to the SSRI escitalopram or placebo for 3-5 weeks. Using PET imaging, the investigators found that over time, synaptic density significantly increased significantly in the neocortex and hippocampus but only in patients taking the active drug.
The results point to two conclusions, said study investigator Gitta Moos Knudsen, MD, PhD, clinical professor and chief physician at the department of clinical medicine, neurology, psychiatry and sensory sciences at Copenhagen (Denmark) University Hospital.
First, they indicate that SSRIs increase synaptic density in brain areas critically involved in depression, a finding that would go some way to indicating that the synaptic density in the brain may be involved in how antidepressants function, “which would give us a target for developing novel drugs against depression,” said Dr. Knudsen.
“Secondly, our data suggest synapses build up over a period of weeks, which would explain why the effects of these drugs take time to kick in,” she added.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress and simultaneously published online in Molecular Psychiatry.
Marked increase in synaptic density
SSRIs are widely used for depression as well as anxiety and obsessive-compulsive disorder. It is thought that they act via neuroplasticity and synaptic remodeling to improve cognition and emotion processing. However, the investigators note clinical evidence is lacking.
For the study, the researchers randomly assigned healthy individuals to either 20-mg escitalopram or placebo for 3-5 weeks.
They performed PET with the 11C-UCB-J tracer, which allows imaging of the synaptic vesicle glycoprotein 2A (SV2A) in the brain, synaptic density, as well as changes in density over time, in the hippocampus and neocortex.
Between May 2020 and October 2021, 17 individuals were assigned to escitalopram and 15 to placebo. There were no significant differences between two groups in terms of age, sex, and PET-related variables. Serum escitalopram measurements confirmed that all participants in the active drug group were compliant.
When synaptic density was assessed at a single time point, an average of 29 days after the intervention, there were no significant differences between the escitalopram and placebo groups in either the neocortex (P = .41) or in the hippocampus (P = .26).
However, when they performed a secondary analysis of the time-dependent effect on SV2A levels, they found a marked difference between the two study groups.
Compared with the placebo group, participants taking escitalopram had a marked increase in synaptic density in both the neocortex (rp value, 0.58; P = .003) and the hippocampus (rp value, 0.41; P = .048).
In contrast, there were no significant changes in synaptic density in either the neocortex (rp value, –0.01; P = .95) or the hippocampus (rp value, –0.06; P = .62) in the hippocampus.
“That is consistent with our clinical observation that it takes time to evolve synaptic density, along with clinical improvement. Does that mean that the increase in synaptic density is a precondition for improvement in symptoms? We don’t know,” said Dr. Knudsen.
Exciting but not conclusive
Session co-chair Oliver Howes, MD, PhD, professor of molecular psychiatry, King’s College London, agreed that the results do not prove the gradual increase in synaptic density the treatment response lag with SSRIs.
“We definitely don’t yet have all the data to know one way or the other,” he said in an interview.
Another potential hypothesis, he said, is that SSRIs are causing shifts in underlying brain circuits that lead to cognitive changes before there is a discernable improvement in mood.
Indeed, Dr. Howes suggested that increases in synaptic density and cognitive changes related to SSRI use are not necessarily dependent on each other and could even be unrelated.
Also commenting on the research, David Nutt, MD, PhD, Edmond J. Safra professor of neuropsychopharmacology at Imperial College London, said that the “delay in therapeutic action of antidepressants has been a puzzle to psychiatrists ever since they were first discerned over 50 years ago. So, these new data in humans, that use cutting edge brain imaging to demonstrate an increase in brain connections developing over the period that the depression lifts, are very exciting.”
Dr. Nutt added that the results provide further evidence that “enhancing serotonin function in the brain can have enduring health benefits.”
Funding support was provided by the Danish Council for Independent Research, the Lundbeck Foundation, Rigshospitalet, and the Swedish Research Council. Open access funding provided by Royal Library, Copenhagen University Library.
Dr. Knudsen declares relationships with Sage Biogen, H. Lundbeck, Onsero, Pangea, Gilgamesh, Abbvie, and PureTechHealth. Another author declares relationships with Cambridge Cognition and PopReach via Cambridge Enterprise.
A version of this article first appeared on Medscape.com.
BARCELONA – , new imaging data suggest.
In a double-blind study, more than 30 volunteers were randomly assigned to the SSRI escitalopram or placebo for 3-5 weeks. Using PET imaging, the investigators found that over time, synaptic density significantly increased significantly in the neocortex and hippocampus but only in patients taking the active drug.
The results point to two conclusions, said study investigator Gitta Moos Knudsen, MD, PhD, clinical professor and chief physician at the department of clinical medicine, neurology, psychiatry and sensory sciences at Copenhagen (Denmark) University Hospital.
First, they indicate that SSRIs increase synaptic density in brain areas critically involved in depression, a finding that would go some way to indicating that the synaptic density in the brain may be involved in how antidepressants function, “which would give us a target for developing novel drugs against depression,” said Dr. Knudsen.
“Secondly, our data suggest synapses build up over a period of weeks, which would explain why the effects of these drugs take time to kick in,” she added.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress and simultaneously published online in Molecular Psychiatry.
Marked increase in synaptic density
SSRIs are widely used for depression as well as anxiety and obsessive-compulsive disorder. It is thought that they act via neuroplasticity and synaptic remodeling to improve cognition and emotion processing. However, the investigators note clinical evidence is lacking.
For the study, the researchers randomly assigned healthy individuals to either 20-mg escitalopram or placebo for 3-5 weeks.
They performed PET with the 11C-UCB-J tracer, which allows imaging of the synaptic vesicle glycoprotein 2A (SV2A) in the brain, synaptic density, as well as changes in density over time, in the hippocampus and neocortex.
Between May 2020 and October 2021, 17 individuals were assigned to escitalopram and 15 to placebo. There were no significant differences between two groups in terms of age, sex, and PET-related variables. Serum escitalopram measurements confirmed that all participants in the active drug group were compliant.
When synaptic density was assessed at a single time point, an average of 29 days after the intervention, there were no significant differences between the escitalopram and placebo groups in either the neocortex (P = .41) or in the hippocampus (P = .26).
However, when they performed a secondary analysis of the time-dependent effect on SV2A levels, they found a marked difference between the two study groups.
Compared with the placebo group, participants taking escitalopram had a marked increase in synaptic density in both the neocortex (rp value, 0.58; P = .003) and the hippocampus (rp value, 0.41; P = .048).
In contrast, there were no significant changes in synaptic density in either the neocortex (rp value, –0.01; P = .95) or the hippocampus (rp value, –0.06; P = .62) in the hippocampus.
“That is consistent with our clinical observation that it takes time to evolve synaptic density, along with clinical improvement. Does that mean that the increase in synaptic density is a precondition for improvement in symptoms? We don’t know,” said Dr. Knudsen.
Exciting but not conclusive
Session co-chair Oliver Howes, MD, PhD, professor of molecular psychiatry, King’s College London, agreed that the results do not prove the gradual increase in synaptic density the treatment response lag with SSRIs.
“We definitely don’t yet have all the data to know one way or the other,” he said in an interview.
Another potential hypothesis, he said, is that SSRIs are causing shifts in underlying brain circuits that lead to cognitive changes before there is a discernable improvement in mood.
Indeed, Dr. Howes suggested that increases in synaptic density and cognitive changes related to SSRI use are not necessarily dependent on each other and could even be unrelated.
Also commenting on the research, David Nutt, MD, PhD, Edmond J. Safra professor of neuropsychopharmacology at Imperial College London, said that the “delay in therapeutic action of antidepressants has been a puzzle to psychiatrists ever since they were first discerned over 50 years ago. So, these new data in humans, that use cutting edge brain imaging to demonstrate an increase in brain connections developing over the period that the depression lifts, are very exciting.”
Dr. Nutt added that the results provide further evidence that “enhancing serotonin function in the brain can have enduring health benefits.”
Funding support was provided by the Danish Council for Independent Research, the Lundbeck Foundation, Rigshospitalet, and the Swedish Research Council. Open access funding provided by Royal Library, Copenhagen University Library.
Dr. Knudsen declares relationships with Sage Biogen, H. Lundbeck, Onsero, Pangea, Gilgamesh, Abbvie, and PureTechHealth. Another author declares relationships with Cambridge Cognition and PopReach via Cambridge Enterprise.
A version of this article first appeared on Medscape.com.
AT ECNP 2023
Esketamine bests quetiapine for severe depression in head-to-head trial
BARCELONA – (TRD), results of a large, multicenter, head-to-head phase 3 trial show.
Results from the ESCAPE-TRD study, which included 675 participants with TRD, show that esketamine was associated with significantly increased rates of both depression and functional remission, compared with quetiapine.
More than 675 patients were randomly assigned to receive one of the two drugs along with ongoing treatment with an SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI).
Esketamine increased remission rates at 2 and 8 months over quetiapine by 72% and raised functional remission rates at 8 months by 88% while decreasing adverse event rates.
The findings were presented at the annual meeting of the European College of Neuropsychopharmacology and were published online in the New England Journal of Medicine.
New hope
The results provide “some hope for our patients suffering from TRD, which, given the data, is somewhat of a misnomer,” said study investigator Andreas Reif, MD, professor of psychiatry, psychosomatic medicine, and psychotherapy, University Hospital Frankfurt–Goethe University, Frankfurt am Main, Germany, and president-elect of the ECNP.
“These patients are not resistant, they just have resistance to monoaminergic drugs,” he added. Esketamine, he said, is a “new weapon in our armamentarium.”
Dr. Reif said TRD is a serious condition that affects approximately 20%-30% of those with major depressive disorder and has “substantial impact” on patients’ lives, including quality of life and level of functioning.
“We know that esketamine nasal spray is effective in TRD. However, up to now, there were only placebo-controlled trials in addition to ongoing antidepressant treatment,” Dr. Reif noted. Consequently, he added, a head-to-head comparison with an active agent with proven efficacy was “urgently needed.”
For the trial, patients from 171 sites in 24 countries with TRD, defined as a less than 25% improvement in symptoms with two or more consecutive treatments of adequate dosage and duration, were randomly assigned to receive esketamine nasal spray (n = 336) or quetiapine (n = 340) extended release together with ongoing SSRI or SNRI therapy.
Both esketamine and quetiapine were flexibly dosed. The primary endpoint was rates of remission at week 8 on the Montgomery-Åsberg Depression Rating Scale (MADRS). After week 8, patients entered a maintenance phase that lasted to week 32.
Dr. Reif said the study population was representative of a typical TRD population.
The average duration of the current depression episode was more than 5 years, and the average MADRS score was above 30.
Key findings
Results showed that those who received esketamine in combination with an SSRI or SNRI experienced a significantly higher rate of remission at week 8, compared with those treated with quetiapine (27.1% vs. 17.6%; P = .003). This equated to an adjusted odds ratio for remission of 1.74 (P = .003).
Use of esketamine was also associated with a higher rate of remission at week 8, and patients remained relapse free at week 32 (21.7% vs. 14.1% with quetiapine; odds ratio, 1.72; P = .008).
At every time point through the study, the proportion of patients experiencing remission was significantly greater with esketamine than with quetiapine. The absolute rate of remission at week 32 was 55.0%, versus 37.0% (P < .001).
Dr. Reif noted that the definition of remission used in the study was a MADRS score of less than or equal to 10, but if the “more lenient” definition of less than or equal to 12, which has been used previously, were to be applied, the absolute remission rates would rise to 65.1%, versus 46.7%.
Dr. Reif also presented results on functional remission rates beyond 32 weeks – data that were not included in the study as published in NEJM.
While remission rates increased over time in both study arms, the functional remission rate at week 32 was, again, significantly higher with esketamine than with quetiapine (38.1% vs. 25.0%; OR, 1.88; P < .001).
The safety data revealed no new signals, Dr. Reif said. Use of esketamine was associated with a lower rate of treatment-emergent adverse events that led to treatment discontinuation, at 4.2% vs. 11.0% with quetiapine.
Among patients given the ketamine-derived drug, there were lower rates of nervous system disorders, and there were no incidences of weight gain, fatigue, or hangover.
Dr. Reif said the results show that esketamine nasal spray was superior to quetiapine in achieving remission over time and that it “greatly improves patients’ functional impairment” while achieving “generally lower” adverse event rates.
He added that they are currently running a significant number of secondary analyses “to give us a better grasp of which patient benefits most” from esketamine therapy over quetiapine. The results may potentially be used to guide patient selection.
‘Tremendous advance’
Session co-chair Mark Weiser, MD, chairman at the department of psychiatry, Tel Aviv (Israel) University, said in an interview that the results are “very exciting” and offer “further proof of a tremendous advance in our field.”
Dr. Weiser, who was not involved in the study, added that demonstrating functional improvement with esketamine was key.
“It’s great to improve symptoms,” he said, “but to have patients show an improvement in their functionality is really the bottom line of this. Not only do you feel better, but you function better, and that’s of extreme importance and makes us feel very optimistic about the future.”
Josep Antoni Ramos-Quiroga, MD, PhD, head of psychiatry, Vall Hebron University Hospital and Autonomous University of Barcelona, welcomed the findings.
“The results of this study show the superior response and safety of esketamine nasal spray when compared with quetiapine,” he said in a release. “This gives people with treatment-resistant depression more safe treatment options.”
The study was funded by Janssen EMEA. Dr. Reif has relationships with Boehringer Ingelheim, COMPASS, Janssen Pharmaceuticals, LivaNova USA, Medice, Saga Therapeutics, and Shire. Other authors have disclosed numerous relationships with industry.
A version of this article first appeared on Medscape.com.
BARCELONA – (TRD), results of a large, multicenter, head-to-head phase 3 trial show.
Results from the ESCAPE-TRD study, which included 675 participants with TRD, show that esketamine was associated with significantly increased rates of both depression and functional remission, compared with quetiapine.
More than 675 patients were randomly assigned to receive one of the two drugs along with ongoing treatment with an SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI).
Esketamine increased remission rates at 2 and 8 months over quetiapine by 72% and raised functional remission rates at 8 months by 88% while decreasing adverse event rates.
The findings were presented at the annual meeting of the European College of Neuropsychopharmacology and were published online in the New England Journal of Medicine.
New hope
The results provide “some hope for our patients suffering from TRD, which, given the data, is somewhat of a misnomer,” said study investigator Andreas Reif, MD, professor of psychiatry, psychosomatic medicine, and psychotherapy, University Hospital Frankfurt–Goethe University, Frankfurt am Main, Germany, and president-elect of the ECNP.
“These patients are not resistant, they just have resistance to monoaminergic drugs,” he added. Esketamine, he said, is a “new weapon in our armamentarium.”
Dr. Reif said TRD is a serious condition that affects approximately 20%-30% of those with major depressive disorder and has “substantial impact” on patients’ lives, including quality of life and level of functioning.
“We know that esketamine nasal spray is effective in TRD. However, up to now, there were only placebo-controlled trials in addition to ongoing antidepressant treatment,” Dr. Reif noted. Consequently, he added, a head-to-head comparison with an active agent with proven efficacy was “urgently needed.”
For the trial, patients from 171 sites in 24 countries with TRD, defined as a less than 25% improvement in symptoms with two or more consecutive treatments of adequate dosage and duration, were randomly assigned to receive esketamine nasal spray (n = 336) or quetiapine (n = 340) extended release together with ongoing SSRI or SNRI therapy.
Both esketamine and quetiapine were flexibly dosed. The primary endpoint was rates of remission at week 8 on the Montgomery-Åsberg Depression Rating Scale (MADRS). After week 8, patients entered a maintenance phase that lasted to week 32.
Dr. Reif said the study population was representative of a typical TRD population.
The average duration of the current depression episode was more than 5 years, and the average MADRS score was above 30.
Key findings
Results showed that those who received esketamine in combination with an SSRI or SNRI experienced a significantly higher rate of remission at week 8, compared with those treated with quetiapine (27.1% vs. 17.6%; P = .003). This equated to an adjusted odds ratio for remission of 1.74 (P = .003).
Use of esketamine was also associated with a higher rate of remission at week 8, and patients remained relapse free at week 32 (21.7% vs. 14.1% with quetiapine; odds ratio, 1.72; P = .008).
At every time point through the study, the proportion of patients experiencing remission was significantly greater with esketamine than with quetiapine. The absolute rate of remission at week 32 was 55.0%, versus 37.0% (P < .001).
Dr. Reif noted that the definition of remission used in the study was a MADRS score of less than or equal to 10, but if the “more lenient” definition of less than or equal to 12, which has been used previously, were to be applied, the absolute remission rates would rise to 65.1%, versus 46.7%.
Dr. Reif also presented results on functional remission rates beyond 32 weeks – data that were not included in the study as published in NEJM.
While remission rates increased over time in both study arms, the functional remission rate at week 32 was, again, significantly higher with esketamine than with quetiapine (38.1% vs. 25.0%; OR, 1.88; P < .001).
The safety data revealed no new signals, Dr. Reif said. Use of esketamine was associated with a lower rate of treatment-emergent adverse events that led to treatment discontinuation, at 4.2% vs. 11.0% with quetiapine.
Among patients given the ketamine-derived drug, there were lower rates of nervous system disorders, and there were no incidences of weight gain, fatigue, or hangover.
Dr. Reif said the results show that esketamine nasal spray was superior to quetiapine in achieving remission over time and that it “greatly improves patients’ functional impairment” while achieving “generally lower” adverse event rates.
He added that they are currently running a significant number of secondary analyses “to give us a better grasp of which patient benefits most” from esketamine therapy over quetiapine. The results may potentially be used to guide patient selection.
‘Tremendous advance’
Session co-chair Mark Weiser, MD, chairman at the department of psychiatry, Tel Aviv (Israel) University, said in an interview that the results are “very exciting” and offer “further proof of a tremendous advance in our field.”
Dr. Weiser, who was not involved in the study, added that demonstrating functional improvement with esketamine was key.
“It’s great to improve symptoms,” he said, “but to have patients show an improvement in their functionality is really the bottom line of this. Not only do you feel better, but you function better, and that’s of extreme importance and makes us feel very optimistic about the future.”
Josep Antoni Ramos-Quiroga, MD, PhD, head of psychiatry, Vall Hebron University Hospital and Autonomous University of Barcelona, welcomed the findings.
“The results of this study show the superior response and safety of esketamine nasal spray when compared with quetiapine,” he said in a release. “This gives people with treatment-resistant depression more safe treatment options.”
The study was funded by Janssen EMEA. Dr. Reif has relationships with Boehringer Ingelheim, COMPASS, Janssen Pharmaceuticals, LivaNova USA, Medice, Saga Therapeutics, and Shire. Other authors have disclosed numerous relationships with industry.
A version of this article first appeared on Medscape.com.
BARCELONA – (TRD), results of a large, multicenter, head-to-head phase 3 trial show.
Results from the ESCAPE-TRD study, which included 675 participants with TRD, show that esketamine was associated with significantly increased rates of both depression and functional remission, compared with quetiapine.
More than 675 patients were randomly assigned to receive one of the two drugs along with ongoing treatment with an SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI).
Esketamine increased remission rates at 2 and 8 months over quetiapine by 72% and raised functional remission rates at 8 months by 88% while decreasing adverse event rates.
The findings were presented at the annual meeting of the European College of Neuropsychopharmacology and were published online in the New England Journal of Medicine.
New hope
The results provide “some hope for our patients suffering from TRD, which, given the data, is somewhat of a misnomer,” said study investigator Andreas Reif, MD, professor of psychiatry, psychosomatic medicine, and psychotherapy, University Hospital Frankfurt–Goethe University, Frankfurt am Main, Germany, and president-elect of the ECNP.
“These patients are not resistant, they just have resistance to monoaminergic drugs,” he added. Esketamine, he said, is a “new weapon in our armamentarium.”
Dr. Reif said TRD is a serious condition that affects approximately 20%-30% of those with major depressive disorder and has “substantial impact” on patients’ lives, including quality of life and level of functioning.
“We know that esketamine nasal spray is effective in TRD. However, up to now, there were only placebo-controlled trials in addition to ongoing antidepressant treatment,” Dr. Reif noted. Consequently, he added, a head-to-head comparison with an active agent with proven efficacy was “urgently needed.”
For the trial, patients from 171 sites in 24 countries with TRD, defined as a less than 25% improvement in symptoms with two or more consecutive treatments of adequate dosage and duration, were randomly assigned to receive esketamine nasal spray (n = 336) or quetiapine (n = 340) extended release together with ongoing SSRI or SNRI therapy.
Both esketamine and quetiapine were flexibly dosed. The primary endpoint was rates of remission at week 8 on the Montgomery-Åsberg Depression Rating Scale (MADRS). After week 8, patients entered a maintenance phase that lasted to week 32.
Dr. Reif said the study population was representative of a typical TRD population.
The average duration of the current depression episode was more than 5 years, and the average MADRS score was above 30.
Key findings
Results showed that those who received esketamine in combination with an SSRI or SNRI experienced a significantly higher rate of remission at week 8, compared with those treated with quetiapine (27.1% vs. 17.6%; P = .003). This equated to an adjusted odds ratio for remission of 1.74 (P = .003).
Use of esketamine was also associated with a higher rate of remission at week 8, and patients remained relapse free at week 32 (21.7% vs. 14.1% with quetiapine; odds ratio, 1.72; P = .008).
At every time point through the study, the proportion of patients experiencing remission was significantly greater with esketamine than with quetiapine. The absolute rate of remission at week 32 was 55.0%, versus 37.0% (P < .001).
Dr. Reif noted that the definition of remission used in the study was a MADRS score of less than or equal to 10, but if the “more lenient” definition of less than or equal to 12, which has been used previously, were to be applied, the absolute remission rates would rise to 65.1%, versus 46.7%.
Dr. Reif also presented results on functional remission rates beyond 32 weeks – data that were not included in the study as published in NEJM.
While remission rates increased over time in both study arms, the functional remission rate at week 32 was, again, significantly higher with esketamine than with quetiapine (38.1% vs. 25.0%; OR, 1.88; P < .001).
The safety data revealed no new signals, Dr. Reif said. Use of esketamine was associated with a lower rate of treatment-emergent adverse events that led to treatment discontinuation, at 4.2% vs. 11.0% with quetiapine.
Among patients given the ketamine-derived drug, there were lower rates of nervous system disorders, and there were no incidences of weight gain, fatigue, or hangover.
Dr. Reif said the results show that esketamine nasal spray was superior to quetiapine in achieving remission over time and that it “greatly improves patients’ functional impairment” while achieving “generally lower” adverse event rates.
He added that they are currently running a significant number of secondary analyses “to give us a better grasp of which patient benefits most” from esketamine therapy over quetiapine. The results may potentially be used to guide patient selection.
‘Tremendous advance’
Session co-chair Mark Weiser, MD, chairman at the department of psychiatry, Tel Aviv (Israel) University, said in an interview that the results are “very exciting” and offer “further proof of a tremendous advance in our field.”
Dr. Weiser, who was not involved in the study, added that demonstrating functional improvement with esketamine was key.
“It’s great to improve symptoms,” he said, “but to have patients show an improvement in their functionality is really the bottom line of this. Not only do you feel better, but you function better, and that’s of extreme importance and makes us feel very optimistic about the future.”
Josep Antoni Ramos-Quiroga, MD, PhD, head of psychiatry, Vall Hebron University Hospital and Autonomous University of Barcelona, welcomed the findings.
“The results of this study show the superior response and safety of esketamine nasal spray when compared with quetiapine,” he said in a release. “This gives people with treatment-resistant depression more safe treatment options.”
The study was funded by Janssen EMEA. Dr. Reif has relationships with Boehringer Ingelheim, COMPASS, Janssen Pharmaceuticals, LivaNova USA, Medice, Saga Therapeutics, and Shire. Other authors have disclosed numerous relationships with industry.
A version of this article first appeared on Medscape.com.
AT ECNP 2023
Zuranolone: FAQs for clinicians and patients
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
Online CBT aids remission of anxiety, depression in students
, according to a study published in JAMA Psychiatry . The intervention was developed by researchers from the United States, Mexico, and Colombia and studied in undergraduate university students.
The research included 1,319 students with anxiety and depression. The students were randomly assigned to three groups that received either remote (internet-based) cognitive behavioral therapy guided by a therapist, self-guided cognitive behavioral therapy (without support from a therapist), or standard treatment provided by the health care services within their community (the control condition).
Students who received guided cognitive behavioral therapy had higher combined rates of remission of these disorders (51.8%) than students who received self-guided therapy (37.8%) or conventional therapy (40%). These differences were not significant for remission of anxiety, however.
Guided cognitive behavioral therapy was associated with the highest probability of remission of anxiety and depression in 91.7% of students, the highest probability of remission of anxiety in all students, and the highest probability of remission of depression in 71.5% of participants.
The results of this analysis could be used to improve psychological care by optimizing how different treatment methods are assigned, especially in mental health institutions where available technical and human resources are limited, according to the investigators.
“We started designing this study before COVID-19 with the idea of optimizing care for these mental health problems,” said study author Corina Benjet Miner, PhD, an epidemiological and psychosocial researcher at the Ramón de la Fuente National Institute of Psychiatry, Mexico City. “We wanted to find additional strategies to achieve better care. The pandemic helped us because, even though this has been undergoing research for many years, internet-delivered interventions were not as well accepted. But during the pandemic, there weren’t any other options.”
Given the high prevalence of mental disorders before and after the pandemic, no health care system in the world would be able to provide in-person care to each patient with depression or anxiety, said Dr. Benjet Miner. “So, the idea is to look for other cost-effective strategies that can ramp up our interventions and reach a greater number of people without negatively impacting the quality of care,” she explained.
“I believe that [the precision model] is an excellent proposal that can save financial resources and avoid transfers,” said Juana Olvera Méndez, PhD, research professor working with the cognitive behavioral approach at the Iztacala Faculty of Higher Studies (FESI) of the National Autonomous University of Mexico, Mexico City. “It also makes it possible to provide patients with immediate care, in contrast to when someone has to go in for [in-person] therapy, which will depend a lot on how the therapist approaches the situation.”
Students from seven universities in Colombia and Mexico were included in the study. They were aged 18 years or older and had a score of 10 or greater on the self-administered Generalized Anxiety Disorder scale-7 test, or had depression with scores of 10 or greater on the nine-item Patient Health Questionnaire, which is also self-administered.
The study’s exclusion criteria included a history of bipolar disorder, nonaffective psychosis, or suicidal ideation with suicide attempts. The investigators used 284 prescription predictors to anticipate the differential response to antianxiety and antidepression therapy.
By grouping these predictors into 11 conceptual categories (such as demographic characteristics, COVID-19–linked stressors, or mental disorder comorbidities) and using machine learning algorithms, the investigators were able to predict in an individualized manner the probability of remission for participants in each of the groups.
“For depression, we found that 28.5% of patients could experience better or equivalent effects from the self-guided program (in comparison to the guided program). Once you have this program, it doesn’t cost anything, so there could be a massive number of people who could benefit from a cost-free therapy,” said Dr. Benjet Miner.
While numerous studies in precision medicine have tried to determine the most appropriate treatment for each patient, “they don’t have the high number of predictors that we used in this research, and I feel like this gives us a significant edge,” she added.
She also explained that they found no differences in user satisfaction between the guided and unguided version of the therapy, so now they must discover why the guided version works better. One notable point is that patients accessed (online) the guided program twice as many times as those who used the self-guided version, but the number of times used is not enough to explain the better outcomes.
“We believe that patients develop some sort of connection with the guides, who are not providing therapy but only making recommendations in brief interactions with patients once a week. It has something to do with that connection, in addition to the longer time spent interacting with the platform, which provides better results with the guided version,” stated Dr. Benjet Miner.
One of the main limitations of this study is that, though it compares three treatment methods, the third one (standard care) is not homogeneous, because each of the seven universities from which the students were selected has different resources for this purpose. “Some universities, like the National Autonomous University of Mexico, have very formal services, with teams of psychologists and psychiatrists, while others don’t have this type of service, or they cover additional aspects, like vocational counseling. So, it’s very difficult to determine exactly what kind of care patients are receiving, because it’s not homogeneous,” she said.
As many as nine assessments using psychometric tests are sometimes required before the intervention can be evaluated, said Dr. Méndez. “This study doesn’t go into too much detail in that area, focusing rather on treatment. So, it would be important to know the diagnoses of the users, who may be experiencing different degrees of depression or anxiety. It would be worth asking what happens if a user requires psychiatric treatment or support.”
Dr. Méndez, who provides psychological therapy in person and online at the Student Support and Counselling Center at FESI, pointed out that it would be important to provide close follow-up on these results to see whether they are sustained in the short and long terms. In her opinion, this model could be presented to other users requiring treatment for anxiety or depression, provided that they can use information and communication technologies.
This precision model, which can also be supported on mobile phones or tablets, could be transferred to primary care facilities or vulnerable populations in rural areas, said Dr. Benjet Miner. “The idea is to reach a point where these algorithms become accurate enough and have a really strong predictive power so that clinicians can use them. The goal is always to find the best treatment at the lowest cost, so that it’s sustainable,” she concluded.
This study was funded by grant number R01MH120648 from the National Institute of Mental Health and the Fogarty International Center. Dr. Benjet Miner reports no relevant financial relationships; the declarations of the remaining authors can be found at the publication’s website.
This article was translated from Medscape’s Spanish Edition and a version first appeared on Medscape.com.
, according to a study published in JAMA Psychiatry . The intervention was developed by researchers from the United States, Mexico, and Colombia and studied in undergraduate university students.
The research included 1,319 students with anxiety and depression. The students were randomly assigned to three groups that received either remote (internet-based) cognitive behavioral therapy guided by a therapist, self-guided cognitive behavioral therapy (without support from a therapist), or standard treatment provided by the health care services within their community (the control condition).
Students who received guided cognitive behavioral therapy had higher combined rates of remission of these disorders (51.8%) than students who received self-guided therapy (37.8%) or conventional therapy (40%). These differences were not significant for remission of anxiety, however.
Guided cognitive behavioral therapy was associated with the highest probability of remission of anxiety and depression in 91.7% of students, the highest probability of remission of anxiety in all students, and the highest probability of remission of depression in 71.5% of participants.
The results of this analysis could be used to improve psychological care by optimizing how different treatment methods are assigned, especially in mental health institutions where available technical and human resources are limited, according to the investigators.
“We started designing this study before COVID-19 with the idea of optimizing care for these mental health problems,” said study author Corina Benjet Miner, PhD, an epidemiological and psychosocial researcher at the Ramón de la Fuente National Institute of Psychiatry, Mexico City. “We wanted to find additional strategies to achieve better care. The pandemic helped us because, even though this has been undergoing research for many years, internet-delivered interventions were not as well accepted. But during the pandemic, there weren’t any other options.”
Given the high prevalence of mental disorders before and after the pandemic, no health care system in the world would be able to provide in-person care to each patient with depression or anxiety, said Dr. Benjet Miner. “So, the idea is to look for other cost-effective strategies that can ramp up our interventions and reach a greater number of people without negatively impacting the quality of care,” she explained.
“I believe that [the precision model] is an excellent proposal that can save financial resources and avoid transfers,” said Juana Olvera Méndez, PhD, research professor working with the cognitive behavioral approach at the Iztacala Faculty of Higher Studies (FESI) of the National Autonomous University of Mexico, Mexico City. “It also makes it possible to provide patients with immediate care, in contrast to when someone has to go in for [in-person] therapy, which will depend a lot on how the therapist approaches the situation.”
Students from seven universities in Colombia and Mexico were included in the study. They were aged 18 years or older and had a score of 10 or greater on the self-administered Generalized Anxiety Disorder scale-7 test, or had depression with scores of 10 or greater on the nine-item Patient Health Questionnaire, which is also self-administered.
The study’s exclusion criteria included a history of bipolar disorder, nonaffective psychosis, or suicidal ideation with suicide attempts. The investigators used 284 prescription predictors to anticipate the differential response to antianxiety and antidepression therapy.
By grouping these predictors into 11 conceptual categories (such as demographic characteristics, COVID-19–linked stressors, or mental disorder comorbidities) and using machine learning algorithms, the investigators were able to predict in an individualized manner the probability of remission for participants in each of the groups.
“For depression, we found that 28.5% of patients could experience better or equivalent effects from the self-guided program (in comparison to the guided program). Once you have this program, it doesn’t cost anything, so there could be a massive number of people who could benefit from a cost-free therapy,” said Dr. Benjet Miner.
While numerous studies in precision medicine have tried to determine the most appropriate treatment for each patient, “they don’t have the high number of predictors that we used in this research, and I feel like this gives us a significant edge,” she added.
She also explained that they found no differences in user satisfaction between the guided and unguided version of the therapy, so now they must discover why the guided version works better. One notable point is that patients accessed (online) the guided program twice as many times as those who used the self-guided version, but the number of times used is not enough to explain the better outcomes.
“We believe that patients develop some sort of connection with the guides, who are not providing therapy but only making recommendations in brief interactions with patients once a week. It has something to do with that connection, in addition to the longer time spent interacting with the platform, which provides better results with the guided version,” stated Dr. Benjet Miner.
One of the main limitations of this study is that, though it compares three treatment methods, the third one (standard care) is not homogeneous, because each of the seven universities from which the students were selected has different resources for this purpose. “Some universities, like the National Autonomous University of Mexico, have very formal services, with teams of psychologists and psychiatrists, while others don’t have this type of service, or they cover additional aspects, like vocational counseling. So, it’s very difficult to determine exactly what kind of care patients are receiving, because it’s not homogeneous,” she said.
As many as nine assessments using psychometric tests are sometimes required before the intervention can be evaluated, said Dr. Méndez. “This study doesn’t go into too much detail in that area, focusing rather on treatment. So, it would be important to know the diagnoses of the users, who may be experiencing different degrees of depression or anxiety. It would be worth asking what happens if a user requires psychiatric treatment or support.”
Dr. Méndez, who provides psychological therapy in person and online at the Student Support and Counselling Center at FESI, pointed out that it would be important to provide close follow-up on these results to see whether they are sustained in the short and long terms. In her opinion, this model could be presented to other users requiring treatment for anxiety or depression, provided that they can use information and communication technologies.
This precision model, which can also be supported on mobile phones or tablets, could be transferred to primary care facilities or vulnerable populations in rural areas, said Dr. Benjet Miner. “The idea is to reach a point where these algorithms become accurate enough and have a really strong predictive power so that clinicians can use them. The goal is always to find the best treatment at the lowest cost, so that it’s sustainable,” she concluded.
This study was funded by grant number R01MH120648 from the National Institute of Mental Health and the Fogarty International Center. Dr. Benjet Miner reports no relevant financial relationships; the declarations of the remaining authors can be found at the publication’s website.
This article was translated from Medscape’s Spanish Edition and a version first appeared on Medscape.com.
, according to a study published in JAMA Psychiatry . The intervention was developed by researchers from the United States, Mexico, and Colombia and studied in undergraduate university students.
The research included 1,319 students with anxiety and depression. The students were randomly assigned to three groups that received either remote (internet-based) cognitive behavioral therapy guided by a therapist, self-guided cognitive behavioral therapy (without support from a therapist), or standard treatment provided by the health care services within their community (the control condition).
Students who received guided cognitive behavioral therapy had higher combined rates of remission of these disorders (51.8%) than students who received self-guided therapy (37.8%) or conventional therapy (40%). These differences were not significant for remission of anxiety, however.
Guided cognitive behavioral therapy was associated with the highest probability of remission of anxiety and depression in 91.7% of students, the highest probability of remission of anxiety in all students, and the highest probability of remission of depression in 71.5% of participants.
The results of this analysis could be used to improve psychological care by optimizing how different treatment methods are assigned, especially in mental health institutions where available technical and human resources are limited, according to the investigators.
“We started designing this study before COVID-19 with the idea of optimizing care for these mental health problems,” said study author Corina Benjet Miner, PhD, an epidemiological and psychosocial researcher at the Ramón de la Fuente National Institute of Psychiatry, Mexico City. “We wanted to find additional strategies to achieve better care. The pandemic helped us because, even though this has been undergoing research for many years, internet-delivered interventions were not as well accepted. But during the pandemic, there weren’t any other options.”
Given the high prevalence of mental disorders before and after the pandemic, no health care system in the world would be able to provide in-person care to each patient with depression or anxiety, said Dr. Benjet Miner. “So, the idea is to look for other cost-effective strategies that can ramp up our interventions and reach a greater number of people without negatively impacting the quality of care,” she explained.
“I believe that [the precision model] is an excellent proposal that can save financial resources and avoid transfers,” said Juana Olvera Méndez, PhD, research professor working with the cognitive behavioral approach at the Iztacala Faculty of Higher Studies (FESI) of the National Autonomous University of Mexico, Mexico City. “It also makes it possible to provide patients with immediate care, in contrast to when someone has to go in for [in-person] therapy, which will depend a lot on how the therapist approaches the situation.”
Students from seven universities in Colombia and Mexico were included in the study. They were aged 18 years or older and had a score of 10 or greater on the self-administered Generalized Anxiety Disorder scale-7 test, or had depression with scores of 10 or greater on the nine-item Patient Health Questionnaire, which is also self-administered.
The study’s exclusion criteria included a history of bipolar disorder, nonaffective psychosis, or suicidal ideation with suicide attempts. The investigators used 284 prescription predictors to anticipate the differential response to antianxiety and antidepression therapy.
By grouping these predictors into 11 conceptual categories (such as demographic characteristics, COVID-19–linked stressors, or mental disorder comorbidities) and using machine learning algorithms, the investigators were able to predict in an individualized manner the probability of remission for participants in each of the groups.
“For depression, we found that 28.5% of patients could experience better or equivalent effects from the self-guided program (in comparison to the guided program). Once you have this program, it doesn’t cost anything, so there could be a massive number of people who could benefit from a cost-free therapy,” said Dr. Benjet Miner.
While numerous studies in precision medicine have tried to determine the most appropriate treatment for each patient, “they don’t have the high number of predictors that we used in this research, and I feel like this gives us a significant edge,” she added.
She also explained that they found no differences in user satisfaction between the guided and unguided version of the therapy, so now they must discover why the guided version works better. One notable point is that patients accessed (online) the guided program twice as many times as those who used the self-guided version, but the number of times used is not enough to explain the better outcomes.
“We believe that patients develop some sort of connection with the guides, who are not providing therapy but only making recommendations in brief interactions with patients once a week. It has something to do with that connection, in addition to the longer time spent interacting with the platform, which provides better results with the guided version,” stated Dr. Benjet Miner.
One of the main limitations of this study is that, though it compares three treatment methods, the third one (standard care) is not homogeneous, because each of the seven universities from which the students were selected has different resources for this purpose. “Some universities, like the National Autonomous University of Mexico, have very formal services, with teams of psychologists and psychiatrists, while others don’t have this type of service, or they cover additional aspects, like vocational counseling. So, it’s very difficult to determine exactly what kind of care patients are receiving, because it’s not homogeneous,” she said.
As many as nine assessments using psychometric tests are sometimes required before the intervention can be evaluated, said Dr. Méndez. “This study doesn’t go into too much detail in that area, focusing rather on treatment. So, it would be important to know the diagnoses of the users, who may be experiencing different degrees of depression or anxiety. It would be worth asking what happens if a user requires psychiatric treatment or support.”
Dr. Méndez, who provides psychological therapy in person and online at the Student Support and Counselling Center at FESI, pointed out that it would be important to provide close follow-up on these results to see whether they are sustained in the short and long terms. In her opinion, this model could be presented to other users requiring treatment for anxiety or depression, provided that they can use information and communication technologies.
This precision model, which can also be supported on mobile phones or tablets, could be transferred to primary care facilities or vulnerable populations in rural areas, said Dr. Benjet Miner. “The idea is to reach a point where these algorithms become accurate enough and have a really strong predictive power so that clinicians can use them. The goal is always to find the best treatment at the lowest cost, so that it’s sustainable,” she concluded.
This study was funded by grant number R01MH120648 from the National Institute of Mental Health and the Fogarty International Center. Dr. Benjet Miner reports no relevant financial relationships; the declarations of the remaining authors can be found at the publication’s website.
This article was translated from Medscape’s Spanish Edition and a version first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
The cult of the suicide risk assessment
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Psilocybin reduces symptoms, disability in major depression
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
FROM JAMA
Suicidal behavior tied to increased all-cause mortality in MDD
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.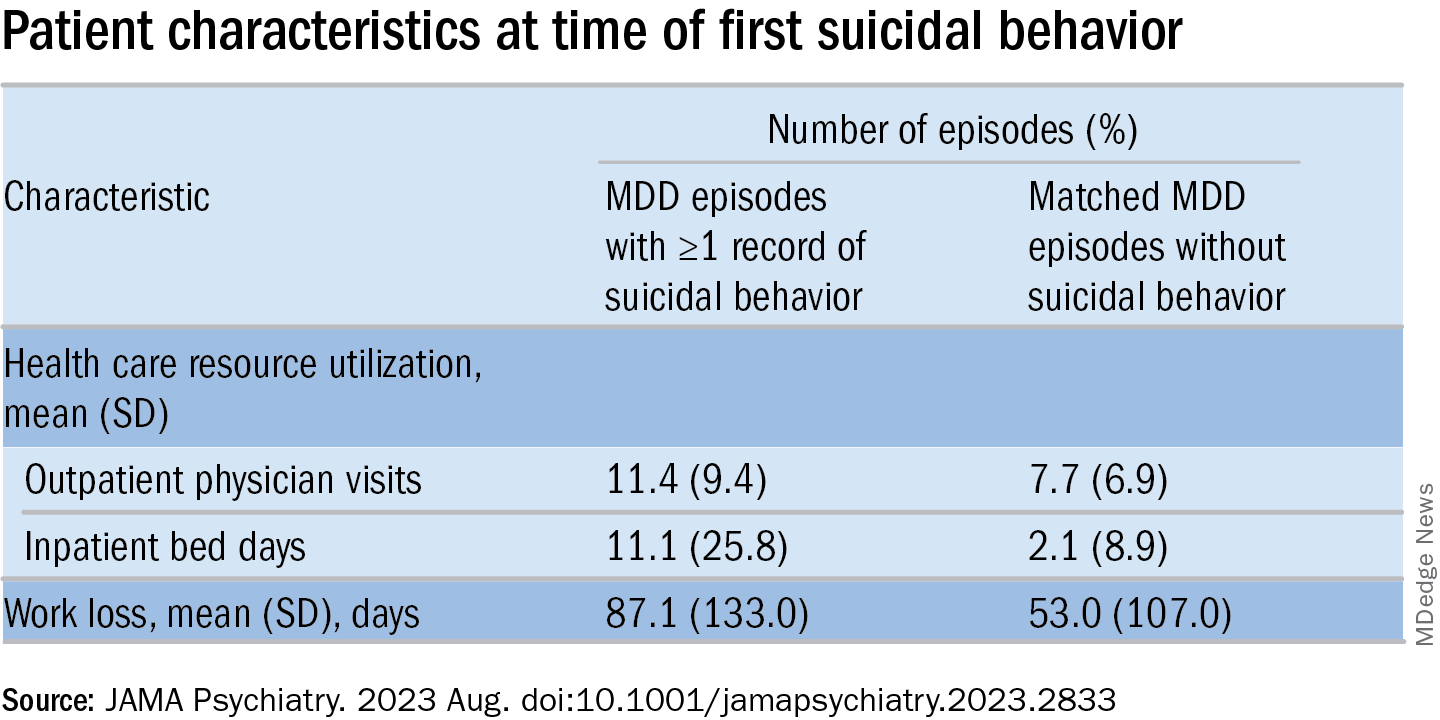
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
The three pillars of perinatal care: Babies, parents, dyadic relationships
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.

















