User login
New technologies show promise for treating pigmented lesions
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
FROM MOA 2020
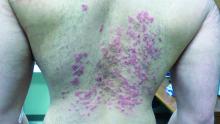
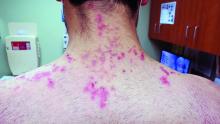
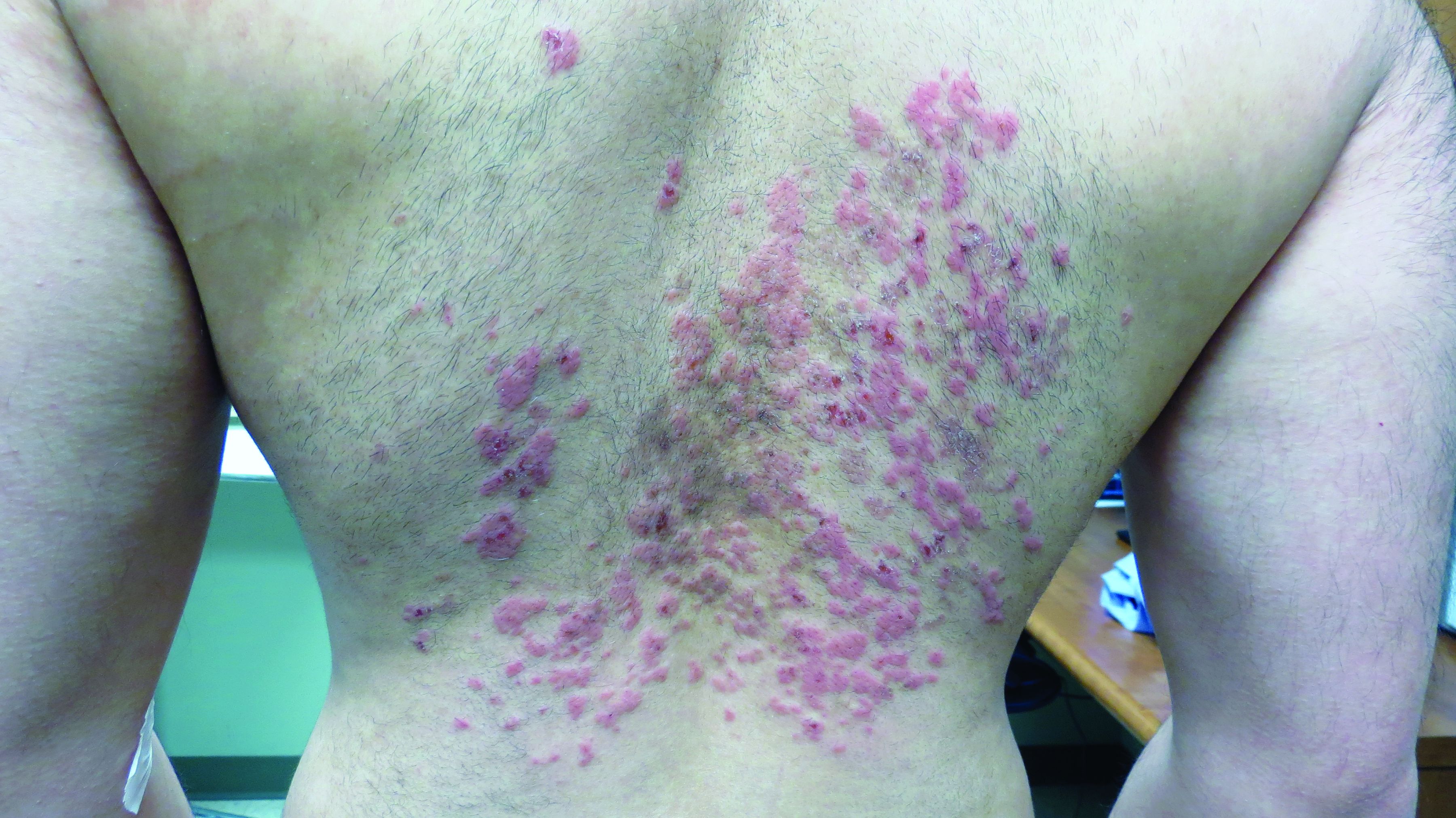
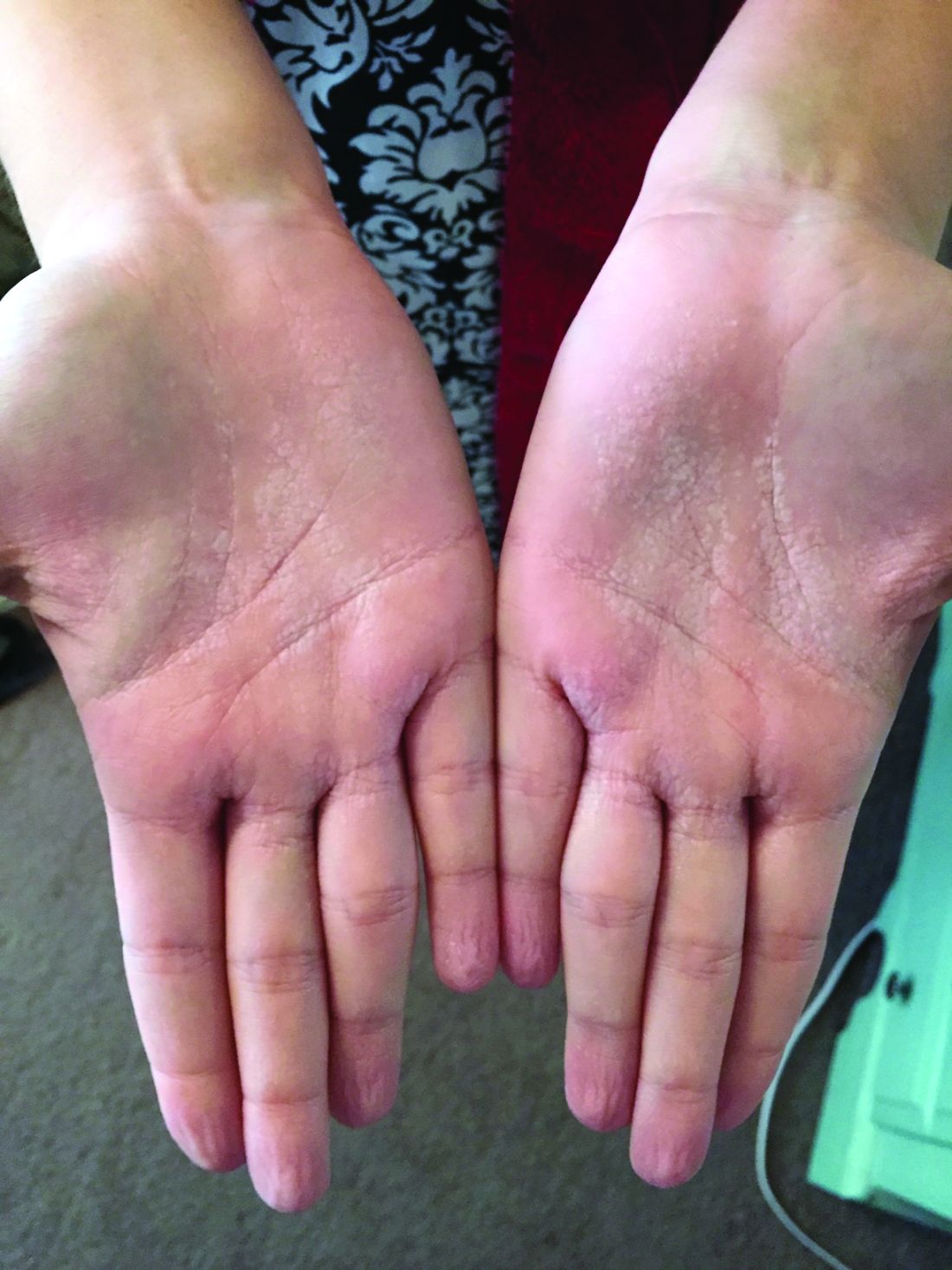
A 31-year-old with a 3-week history of a waxing and waning, mildly pruritic eruption on his neck, chest, and back
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
Don’t discount ultrapotent topical corticosteroids for vulvar lichen sclerosus
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
EXPERT ANALYSIS FROM THE ISSVD BIENNIAL CONFERENCE
New lupus classification criteria perform well in children, young adults
, according to results from a single-center, retrospective study.
However, the 2019 criteria, which were developed using cohorts of adult patients with SLE, were statistically no better than the 1997 ACR criteria at identifying those without the disease, first author Najla Aljaberi, MBBS, of the Cincinnati Children’s Hospital Medical Center, and colleagues reported in Arthritis Care & Research.
The 2019 criteria were especially good at correctly classifying SLE in non-White youths, but the two sets of criteria performed equally well among male and female youths with SLE and across age groups.
“Our study confirms superior sensitivity of the new criteria over the 1997-ACR criteria in youths with SLE. The difference in sensitivity estimates between the two criteria sets (2019-EULAR/ACR vs. 1997-ACR) may be explained by a higher weight being assigned to immunologic criteria, less strict hematologic criteria (not requiring >2 occurrences), and the inclusion of subjective features of arthritis. Notably, our estimates of the sensitivity of the 2019-EULAR/ACR criteria were similar to those reported from a Brazilian pediatric study by Fonseca et al. (87.7%) that also used physician diagnosis as reference standard,” the researchers wrote.
Dr. Aljaberi and colleagues reviewed electronic medical records of 112 patients with SLE aged 2-21 years and 105 controls aged 1-19 years at Cincinnati Children’s Hospital Medical Center during 2008-2019. Patients identified in the records at the center were considered to have SLE based on ICD-10 codes assigned by experienced pediatric rheumatologists. The control patients included 69 (66%) with juvenile dermatomyositis and 36 with juvenile scleroderma/systemic sclerosis, based on corresponding ICD-10 codes.
Among the SLE cases, 57% were White and 81% were female, while Whites represented 83% and females 71% of control patients. Young adults aged 18-21 years represented a minority of SLE cases (18%) and controls (7%).
The 2019 criteria had significantly higher sensitivity than did the 1997 criteria (85% vs. 72%, respectively; P = .023) but similar specificity (83% vs. 87%; P = .456). A total of 17 out of the 112 SLE cases failed to meet the 2019 criteria, 13 (76%) of whom were White. Overall, 31 SLE cases did not meet the 1997 criteria, but 15 of those fulfilled the 2019 criteria. While there was no statistically significant difference in the sensitivity of the 2019 criteria between non-White and White cases (92% vs. 80%, respectively; P = .08), the difference in sensitivity was significant with the 1997 criteria (83% vs. 64%; P < .02).
The 2019 criteria had similar sensitivity in males and females (86% vs. 81%, respectively), as well as specificity (81% vs. 87%). The 1997 criteria also provided similar sensitivity between males and females (71% vs. 76%) as well as specificity (85% vs. 90%).
In only four instances did SLE cases meet 2019 criteria before ICD-10 diagnosis of SLE, whereas in the other 108 cases the ICD-10 diagnosis coincided with reaching the threshold for meeting 2019 criteria.
There was no funding secured for the study, and the authors had no conflicts of interest to disclose.
SOURCE: Aljaberi N et al. Arthritis Care Res. 2020 Aug 25. doi: 10.1002/acr.24430.
, according to results from a single-center, retrospective study.
However, the 2019 criteria, which were developed using cohorts of adult patients with SLE, were statistically no better than the 1997 ACR criteria at identifying those without the disease, first author Najla Aljaberi, MBBS, of the Cincinnati Children’s Hospital Medical Center, and colleagues reported in Arthritis Care & Research.
The 2019 criteria were especially good at correctly classifying SLE in non-White youths, but the two sets of criteria performed equally well among male and female youths with SLE and across age groups.
“Our study confirms superior sensitivity of the new criteria over the 1997-ACR criteria in youths with SLE. The difference in sensitivity estimates between the two criteria sets (2019-EULAR/ACR vs. 1997-ACR) may be explained by a higher weight being assigned to immunologic criteria, less strict hematologic criteria (not requiring >2 occurrences), and the inclusion of subjective features of arthritis. Notably, our estimates of the sensitivity of the 2019-EULAR/ACR criteria were similar to those reported from a Brazilian pediatric study by Fonseca et al. (87.7%) that also used physician diagnosis as reference standard,” the researchers wrote.
Dr. Aljaberi and colleagues reviewed electronic medical records of 112 patients with SLE aged 2-21 years and 105 controls aged 1-19 years at Cincinnati Children’s Hospital Medical Center during 2008-2019. Patients identified in the records at the center were considered to have SLE based on ICD-10 codes assigned by experienced pediatric rheumatologists. The control patients included 69 (66%) with juvenile dermatomyositis and 36 with juvenile scleroderma/systemic sclerosis, based on corresponding ICD-10 codes.
Among the SLE cases, 57% were White and 81% were female, while Whites represented 83% and females 71% of control patients. Young adults aged 18-21 years represented a minority of SLE cases (18%) and controls (7%).
The 2019 criteria had significantly higher sensitivity than did the 1997 criteria (85% vs. 72%, respectively; P = .023) but similar specificity (83% vs. 87%; P = .456). A total of 17 out of the 112 SLE cases failed to meet the 2019 criteria, 13 (76%) of whom were White. Overall, 31 SLE cases did not meet the 1997 criteria, but 15 of those fulfilled the 2019 criteria. While there was no statistically significant difference in the sensitivity of the 2019 criteria between non-White and White cases (92% vs. 80%, respectively; P = .08), the difference in sensitivity was significant with the 1997 criteria (83% vs. 64%; P < .02).
The 2019 criteria had similar sensitivity in males and females (86% vs. 81%, respectively), as well as specificity (81% vs. 87%). The 1997 criteria also provided similar sensitivity between males and females (71% vs. 76%) as well as specificity (85% vs. 90%).
In only four instances did SLE cases meet 2019 criteria before ICD-10 diagnosis of SLE, whereas in the other 108 cases the ICD-10 diagnosis coincided with reaching the threshold for meeting 2019 criteria.
There was no funding secured for the study, and the authors had no conflicts of interest to disclose.
SOURCE: Aljaberi N et al. Arthritis Care Res. 2020 Aug 25. doi: 10.1002/acr.24430.
, according to results from a single-center, retrospective study.
However, the 2019 criteria, which were developed using cohorts of adult patients with SLE, were statistically no better than the 1997 ACR criteria at identifying those without the disease, first author Najla Aljaberi, MBBS, of the Cincinnati Children’s Hospital Medical Center, and colleagues reported in Arthritis Care & Research.
The 2019 criteria were especially good at correctly classifying SLE in non-White youths, but the two sets of criteria performed equally well among male and female youths with SLE and across age groups.
“Our study confirms superior sensitivity of the new criteria over the 1997-ACR criteria in youths with SLE. The difference in sensitivity estimates between the two criteria sets (2019-EULAR/ACR vs. 1997-ACR) may be explained by a higher weight being assigned to immunologic criteria, less strict hematologic criteria (not requiring >2 occurrences), and the inclusion of subjective features of arthritis. Notably, our estimates of the sensitivity of the 2019-EULAR/ACR criteria were similar to those reported from a Brazilian pediatric study by Fonseca et al. (87.7%) that also used physician diagnosis as reference standard,” the researchers wrote.
Dr. Aljaberi and colleagues reviewed electronic medical records of 112 patients with SLE aged 2-21 years and 105 controls aged 1-19 years at Cincinnati Children’s Hospital Medical Center during 2008-2019. Patients identified in the records at the center were considered to have SLE based on ICD-10 codes assigned by experienced pediatric rheumatologists. The control patients included 69 (66%) with juvenile dermatomyositis and 36 with juvenile scleroderma/systemic sclerosis, based on corresponding ICD-10 codes.
Among the SLE cases, 57% were White and 81% were female, while Whites represented 83% and females 71% of control patients. Young adults aged 18-21 years represented a minority of SLE cases (18%) and controls (7%).
The 2019 criteria had significantly higher sensitivity than did the 1997 criteria (85% vs. 72%, respectively; P = .023) but similar specificity (83% vs. 87%; P = .456). A total of 17 out of the 112 SLE cases failed to meet the 2019 criteria, 13 (76%) of whom were White. Overall, 31 SLE cases did not meet the 1997 criteria, but 15 of those fulfilled the 2019 criteria. While there was no statistically significant difference in the sensitivity of the 2019 criteria between non-White and White cases (92% vs. 80%, respectively; P = .08), the difference in sensitivity was significant with the 1997 criteria (83% vs. 64%; P < .02).
The 2019 criteria had similar sensitivity in males and females (86% vs. 81%, respectively), as well as specificity (81% vs. 87%). The 1997 criteria also provided similar sensitivity between males and females (71% vs. 76%) as well as specificity (85% vs. 90%).
In only four instances did SLE cases meet 2019 criteria before ICD-10 diagnosis of SLE, whereas in the other 108 cases the ICD-10 diagnosis coincided with reaching the threshold for meeting 2019 criteria.
There was no funding secured for the study, and the authors had no conflicts of interest to disclose.
SOURCE: Aljaberi N et al. Arthritis Care Res. 2020 Aug 25. doi: 10.1002/acr.24430.
FROM ARTHRITIS CARE & RESEARCH
Hidradenitis suppurativa therapy options should be patient guided
of their most challenging symptoms, according to an expert summary presented at the Skin of Color Update 2020.
“If your patient is only focused on the appearance of the lesions or the presence of sinus tracts, they might not think your treatment is working,” said Ginette A. Okoye, MD, professor and chair, department of dermatology, Howard University, Washington.
Instead, she advised working with patients to define priorities, allowing them to measure and appreciate improvement. The most difficult symptoms for one patient, such as pain or persistent abscess drainage, might not be the same for another.
There is a large array of treatment options for HS. These were once typically employed in stepwise manner, moving from steroids to hormonal therapies, antibiotics, and on to biologics and lasers, but Dr. Okoye reported that she layers on treatments, guided by patient priorities and responses. “Most of my patients are not on just one treatment at a time,” she said.
In addition to patient goals, her treatment choices are also influenced by the presence of comorbidities such as metabolic syndrome, polycystic ovarian syndrome (PCOS), or inflammatory bowel disease (IBD). For example, she reported she is more likely to include metformin among treatment options in patients with central obesity or insulin resistance, whereas she moves more quickly to a biologic for those with another systemic inflammatory disease such as IBD.
Although multiple factors appear to contribute to the symptoms of HS, the pathophysiology remains incompletely understood, but follicular occlusion is often “a primary inciting event,” Dr. Okoye said.
For this reason, laser hair removal can provide substantial benefit, she noted. Not only does it eliminate the occlusion, but the heat generated by the laser eliminates some of the pathogens, such as Porphyromonas gingivalis, associated with HS.
“Lasers work well for preventing new lesions from forming but also in making active lesions go away faster,” said Dr. Okoye, who relies on the Nd:YAG laser when treating this disease in darker skin. She has found lasers to be particularly effective in mild to moderate disease.
When using lasers, one challenge is third-party insurance, according to Dr. Okoye, who reported that she has tried repeatedly to convince payers that this treatment is medically indicated for HS, but claims have been routinely denied. As a result, she has had to significantly discount the cost of laser at her center in order to provide access to “a modality that actually works.”
Incision and drainage of inflamed painful lesions is a common intervention in HS, but Dr. Okoye discourages this approach. Because of the high recurrence rates, the benefits are temporary. Instead, she recommends an intralesional injection of triamcinolone acetonide diluted with equal amounts of lidocaine.
With this injection, “there is immediate pain relief followed by significant resolution of the inflammation,” she said. Because of the likelihood that patients seeking care in the emergency department for acutely inflamed lesions will receive surgical treatment, Dr. Okoye recommends offering patients urgent appointments for steroid injections when painful and inflamed lesions need immediate attention.
In contrast, marsupialization of abscesses or sinus tracts, often called deroofing, is associated with a relatively low risk of recurrence, can be done under local anesthesia in an office, and can lead to resolution of persistent nodules in patients with mild disease.
“This is an easy procedure that takes relatively little time,” advised Dr. Okoye, who provided CPT codes (10060 and 10061) that will provide reimbursement as long as procedural notes describe the rationale.
Metformin is an attractive adjunctive therapy for HS in patients with type 2 diabetes or features that suggest metabolic disturbances, such as central obesity, hypercholesterolemia, hypertension, or hypertriglyceridemia. It should also be considered in patients with PCOS because metformin decreases ovarian androgen production, she said.
When prescribing metformin in HS, which is an off-label indication, “I prefer the extended release formulation. It has a better profile in regard to gastrointestinal side effects and it can be taken once-daily,” Dr. Okoye said.
Citing a study that suggests patients with HS have even worse quality of life scores than do patients with diabetes, Dr. Okoye also emphasized the importance of psychosocial support and lifestyle modification as part of a holistic approach. With multiple manifestations of varying severity, individualizing therapy to control symptoms that the patient finds most bothersome is essential for optimizing patient well being.
Tien Viet Nguyen, MD, who practices dermatology and conducts clinical research in Bellevue, Wash., agrees that a comprehensive treatment program is needed. First author of a recent review article on HS, Dr. Nguyen agreed that common comorbidities like IBD, PCOS, and diabetes are accompanied frequently by a host of mental health and behavioral issues that contribute to impaired quality of life, such as depression, low self-esteem, sexual dysfunction, impaired sleep, and substance use disorders.
“Therefore, addressing these important comorbidities and quality of life issues with other health care professionals as a team is the best approach to improving health outcomes,” he said in an interview.
Dr. Nguyen also recently authored a chapter on quality of life issues associated with HS in the soon-to-be-published Comprehensive Guide to Hidradenitis Suppurativa (1st Edition, Dermatology Clinics). He agreed that optimal outcomes are achieved by an interdisciplinary team of health care providers who can address the sometimes independent but often interrelated comorbidities associated with this disorder.
Dr. Okoye has financial relationships with Pfizer and Unilver, but neither is relevant to this topic.
of their most challenging symptoms, according to an expert summary presented at the Skin of Color Update 2020.
“If your patient is only focused on the appearance of the lesions or the presence of sinus tracts, they might not think your treatment is working,” said Ginette A. Okoye, MD, professor and chair, department of dermatology, Howard University, Washington.
Instead, she advised working with patients to define priorities, allowing them to measure and appreciate improvement. The most difficult symptoms for one patient, such as pain or persistent abscess drainage, might not be the same for another.
There is a large array of treatment options for HS. These were once typically employed in stepwise manner, moving from steroids to hormonal therapies, antibiotics, and on to biologics and lasers, but Dr. Okoye reported that she layers on treatments, guided by patient priorities and responses. “Most of my patients are not on just one treatment at a time,” she said.
In addition to patient goals, her treatment choices are also influenced by the presence of comorbidities such as metabolic syndrome, polycystic ovarian syndrome (PCOS), or inflammatory bowel disease (IBD). For example, she reported she is more likely to include metformin among treatment options in patients with central obesity or insulin resistance, whereas she moves more quickly to a biologic for those with another systemic inflammatory disease such as IBD.
Although multiple factors appear to contribute to the symptoms of HS, the pathophysiology remains incompletely understood, but follicular occlusion is often “a primary inciting event,” Dr. Okoye said.
For this reason, laser hair removal can provide substantial benefit, she noted. Not only does it eliminate the occlusion, but the heat generated by the laser eliminates some of the pathogens, such as Porphyromonas gingivalis, associated with HS.
“Lasers work well for preventing new lesions from forming but also in making active lesions go away faster,” said Dr. Okoye, who relies on the Nd:YAG laser when treating this disease in darker skin. She has found lasers to be particularly effective in mild to moderate disease.
When using lasers, one challenge is third-party insurance, according to Dr. Okoye, who reported that she has tried repeatedly to convince payers that this treatment is medically indicated for HS, but claims have been routinely denied. As a result, she has had to significantly discount the cost of laser at her center in order to provide access to “a modality that actually works.”
Incision and drainage of inflamed painful lesions is a common intervention in HS, but Dr. Okoye discourages this approach. Because of the high recurrence rates, the benefits are temporary. Instead, she recommends an intralesional injection of triamcinolone acetonide diluted with equal amounts of lidocaine.
With this injection, “there is immediate pain relief followed by significant resolution of the inflammation,” she said. Because of the likelihood that patients seeking care in the emergency department for acutely inflamed lesions will receive surgical treatment, Dr. Okoye recommends offering patients urgent appointments for steroid injections when painful and inflamed lesions need immediate attention.
In contrast, marsupialization of abscesses or sinus tracts, often called deroofing, is associated with a relatively low risk of recurrence, can be done under local anesthesia in an office, and can lead to resolution of persistent nodules in patients with mild disease.
“This is an easy procedure that takes relatively little time,” advised Dr. Okoye, who provided CPT codes (10060 and 10061) that will provide reimbursement as long as procedural notes describe the rationale.
Metformin is an attractive adjunctive therapy for HS in patients with type 2 diabetes or features that suggest metabolic disturbances, such as central obesity, hypercholesterolemia, hypertension, or hypertriglyceridemia. It should also be considered in patients with PCOS because metformin decreases ovarian androgen production, she said.
When prescribing metformin in HS, which is an off-label indication, “I prefer the extended release formulation. It has a better profile in regard to gastrointestinal side effects and it can be taken once-daily,” Dr. Okoye said.
Citing a study that suggests patients with HS have even worse quality of life scores than do patients with diabetes, Dr. Okoye also emphasized the importance of psychosocial support and lifestyle modification as part of a holistic approach. With multiple manifestations of varying severity, individualizing therapy to control symptoms that the patient finds most bothersome is essential for optimizing patient well being.
Tien Viet Nguyen, MD, who practices dermatology and conducts clinical research in Bellevue, Wash., agrees that a comprehensive treatment program is needed. First author of a recent review article on HS, Dr. Nguyen agreed that common comorbidities like IBD, PCOS, and diabetes are accompanied frequently by a host of mental health and behavioral issues that contribute to impaired quality of life, such as depression, low self-esteem, sexual dysfunction, impaired sleep, and substance use disorders.
“Therefore, addressing these important comorbidities and quality of life issues with other health care professionals as a team is the best approach to improving health outcomes,” he said in an interview.
Dr. Nguyen also recently authored a chapter on quality of life issues associated with HS in the soon-to-be-published Comprehensive Guide to Hidradenitis Suppurativa (1st Edition, Dermatology Clinics). He agreed that optimal outcomes are achieved by an interdisciplinary team of health care providers who can address the sometimes independent but often interrelated comorbidities associated with this disorder.
Dr. Okoye has financial relationships with Pfizer and Unilver, but neither is relevant to this topic.
of their most challenging symptoms, according to an expert summary presented at the Skin of Color Update 2020.
“If your patient is only focused on the appearance of the lesions or the presence of sinus tracts, they might not think your treatment is working,” said Ginette A. Okoye, MD, professor and chair, department of dermatology, Howard University, Washington.
Instead, she advised working with patients to define priorities, allowing them to measure and appreciate improvement. The most difficult symptoms for one patient, such as pain or persistent abscess drainage, might not be the same for another.
There is a large array of treatment options for HS. These were once typically employed in stepwise manner, moving from steroids to hormonal therapies, antibiotics, and on to biologics and lasers, but Dr. Okoye reported that she layers on treatments, guided by patient priorities and responses. “Most of my patients are not on just one treatment at a time,” she said.
In addition to patient goals, her treatment choices are also influenced by the presence of comorbidities such as metabolic syndrome, polycystic ovarian syndrome (PCOS), or inflammatory bowel disease (IBD). For example, she reported she is more likely to include metformin among treatment options in patients with central obesity or insulin resistance, whereas she moves more quickly to a biologic for those with another systemic inflammatory disease such as IBD.
Although multiple factors appear to contribute to the symptoms of HS, the pathophysiology remains incompletely understood, but follicular occlusion is often “a primary inciting event,” Dr. Okoye said.
For this reason, laser hair removal can provide substantial benefit, she noted. Not only does it eliminate the occlusion, but the heat generated by the laser eliminates some of the pathogens, such as Porphyromonas gingivalis, associated with HS.
“Lasers work well for preventing new lesions from forming but also in making active lesions go away faster,” said Dr. Okoye, who relies on the Nd:YAG laser when treating this disease in darker skin. She has found lasers to be particularly effective in mild to moderate disease.
When using lasers, one challenge is third-party insurance, according to Dr. Okoye, who reported that she has tried repeatedly to convince payers that this treatment is medically indicated for HS, but claims have been routinely denied. As a result, she has had to significantly discount the cost of laser at her center in order to provide access to “a modality that actually works.”
Incision and drainage of inflamed painful lesions is a common intervention in HS, but Dr. Okoye discourages this approach. Because of the high recurrence rates, the benefits are temporary. Instead, she recommends an intralesional injection of triamcinolone acetonide diluted with equal amounts of lidocaine.
With this injection, “there is immediate pain relief followed by significant resolution of the inflammation,” she said. Because of the likelihood that patients seeking care in the emergency department for acutely inflamed lesions will receive surgical treatment, Dr. Okoye recommends offering patients urgent appointments for steroid injections when painful and inflamed lesions need immediate attention.
In contrast, marsupialization of abscesses or sinus tracts, often called deroofing, is associated with a relatively low risk of recurrence, can be done under local anesthesia in an office, and can lead to resolution of persistent nodules in patients with mild disease.
“This is an easy procedure that takes relatively little time,” advised Dr. Okoye, who provided CPT codes (10060 and 10061) that will provide reimbursement as long as procedural notes describe the rationale.
Metformin is an attractive adjunctive therapy for HS in patients with type 2 diabetes or features that suggest metabolic disturbances, such as central obesity, hypercholesterolemia, hypertension, or hypertriglyceridemia. It should also be considered in patients with PCOS because metformin decreases ovarian androgen production, she said.
When prescribing metformin in HS, which is an off-label indication, “I prefer the extended release formulation. It has a better profile in regard to gastrointestinal side effects and it can be taken once-daily,” Dr. Okoye said.
Citing a study that suggests patients with HS have even worse quality of life scores than do patients with diabetes, Dr. Okoye also emphasized the importance of psychosocial support and lifestyle modification as part of a holistic approach. With multiple manifestations of varying severity, individualizing therapy to control symptoms that the patient finds most bothersome is essential for optimizing patient well being.
Tien Viet Nguyen, MD, who practices dermatology and conducts clinical research in Bellevue, Wash., agrees that a comprehensive treatment program is needed. First author of a recent review article on HS, Dr. Nguyen agreed that common comorbidities like IBD, PCOS, and diabetes are accompanied frequently by a host of mental health and behavioral issues that contribute to impaired quality of life, such as depression, low self-esteem, sexual dysfunction, impaired sleep, and substance use disorders.
“Therefore, addressing these important comorbidities and quality of life issues with other health care professionals as a team is the best approach to improving health outcomes,” he said in an interview.
Dr. Nguyen also recently authored a chapter on quality of life issues associated with HS in the soon-to-be-published Comprehensive Guide to Hidradenitis Suppurativa (1st Edition, Dermatology Clinics). He agreed that optimal outcomes are achieved by an interdisciplinary team of health care providers who can address the sometimes independent but often interrelated comorbidities associated with this disorder.
Dr. Okoye has financial relationships with Pfizer and Unilver, but neither is relevant to this topic.
FROM SOC 2020
How Kodak Gold contributed to bias in dermatology
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.
Promising drugs line up for lupus treatment
Systemic lupus erythematosus remains a treatment challenge, but a variety of drugs in the pipeline are set to target type I interferons, cytokines, and B cells, according to Richard Furie, MD, chief of the division of rheumatology at Northwell Health and professor of medicine at Hofstra University, Hempstead, N.Y.
In general, when treating patients with systemic lupus erythematosus (SLE), “we just don’t see satisfactory response rates,” Dr. Furie said in an online presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“I think the greatest unmet need is in lupus nephritis,” he said. The data show that not even one-third of patients are adequately responding to standard of care treatment. “We need to do better in lupus nephritis but also for those patients with moderate-severe manifestations outside the kidney.”
Patients with SLE have elevated levels of interferon-alpha, Dr. Furie noted. Data from recent studies show that interferon inhibitors can reduce clinical activity in SLE patients, he said.
“About two-thirds to three-quarters of lupus patients have evidence of interferon pathway activation,” he said. There are three types of interferons, and five subtypes of type I interferon, and all five subtypes of type I interferon bind to the same receptor, which is an important strategy for drug development.
In particular, recent phase 2 and 3 trials have focused on targeting type I interferons with anifrolumab, which blocks all five subtypes.
Dr. Furie cited “very robust results” from a phase 2 study. Results of two phase 3 trials of anifrolumab led to a split decision, but the totality of the data collected across the phase 2 and 3 studies points to a drug that is effective for patients with SLE. The two phase 3 studies were published in Lancet Rheumatology and the New England Journal of Medicine.
Dr. Furie also identified recent studies of baricitinib (Olumiant), which has the ability to target several different cytokines. A phase 2 study in 2018 showed a significant difference in SLE Responder Index between lupus patients who received 4 mg of baricitinib or placebo, and a phase 3 study is underway, he said.
For lupus nephritis, Dr. Furie cited the BLISS-LN trial, a 104-week, randomized trial of patients with active lupus nephritis. The group of patients who received belimumab (Benlysta), a monoclonal antibody that targets B-cell activating factor, in addition to standard therapy had significant improvements in renal responses, compared with standard therapy alone (43.0% vs. 32.3%). The outcome measure was Primary Efficacy Renal Response, defined as urinary protein/creatinine ratio <0.7, eGFR ≥60 mL/min per 1.73 m2, confirmation on consecutive visits, and required tapering of background glucocorticoids.
Although belimumab was approved for SLE in 2011, the BLISS-LN study focused on SLE patients with active kidney disease. “Neutralizing B-cell activating factor and down-regulating autoreactive B-cell function in kidneys represented a compelling therapeutic approach to lupus nephritis,” he explained.
Voclosporin, distinct from cyclosporine, has also been studied in lupus nephritis, Dr. Furie said. Voclosporin offers several benefits over cyclosporine, including greater potency and a lower drug and metabolite load, as well as a more consistent pharmacokinetic and pharmacodynamic relationship, he said. In the phase 3 AURORA study, presented at this year’s EULAR congress, 40% of patients with lupus nephritis met the primary endpoint of a renal response at 52 weeks, compared with 22.5% of placebo patients.
Looking ahead to the treatment of SLE in 2021, “I feel very strongly that voclosporin will be approved for lupus nephritis,” he said. He also predicted that the use of belimumab will be officially extended for lupus nephritis and that anifrolumab will receive an approval for SLE patients.
In addition, the future may witness the increased use of biomarkers and development of more individualized therapy. These breakthroughs will yield better outcomes for all lupus patients, he said.
Dr. Furie disclosed relationships with GlaxoSmithKline, Genentech/Roche, Aurinia Pharmaceuticals, AstraZeneca/MedImmune, and Eli Lilly. Global Academy for Medical Education and this news organization are owned by the same parent company.
Systemic lupus erythematosus remains a treatment challenge, but a variety of drugs in the pipeline are set to target type I interferons, cytokines, and B cells, according to Richard Furie, MD, chief of the division of rheumatology at Northwell Health and professor of medicine at Hofstra University, Hempstead, N.Y.
In general, when treating patients with systemic lupus erythematosus (SLE), “we just don’t see satisfactory response rates,” Dr. Furie said in an online presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“I think the greatest unmet need is in lupus nephritis,” he said. The data show that not even one-third of patients are adequately responding to standard of care treatment. “We need to do better in lupus nephritis but also for those patients with moderate-severe manifestations outside the kidney.”
Patients with SLE have elevated levels of interferon-alpha, Dr. Furie noted. Data from recent studies show that interferon inhibitors can reduce clinical activity in SLE patients, he said.
“About two-thirds to three-quarters of lupus patients have evidence of interferon pathway activation,” he said. There are three types of interferons, and five subtypes of type I interferon, and all five subtypes of type I interferon bind to the same receptor, which is an important strategy for drug development.
In particular, recent phase 2 and 3 trials have focused on targeting type I interferons with anifrolumab, which blocks all five subtypes.
Dr. Furie cited “very robust results” from a phase 2 study. Results of two phase 3 trials of anifrolumab led to a split decision, but the totality of the data collected across the phase 2 and 3 studies points to a drug that is effective for patients with SLE. The two phase 3 studies were published in Lancet Rheumatology and the New England Journal of Medicine.
Dr. Furie also identified recent studies of baricitinib (Olumiant), which has the ability to target several different cytokines. A phase 2 study in 2018 showed a significant difference in SLE Responder Index between lupus patients who received 4 mg of baricitinib or placebo, and a phase 3 study is underway, he said.
For lupus nephritis, Dr. Furie cited the BLISS-LN trial, a 104-week, randomized trial of patients with active lupus nephritis. The group of patients who received belimumab (Benlysta), a monoclonal antibody that targets B-cell activating factor, in addition to standard therapy had significant improvements in renal responses, compared with standard therapy alone (43.0% vs. 32.3%). The outcome measure was Primary Efficacy Renal Response, defined as urinary protein/creatinine ratio <0.7, eGFR ≥60 mL/min per 1.73 m2, confirmation on consecutive visits, and required tapering of background glucocorticoids.
Although belimumab was approved for SLE in 2011, the BLISS-LN study focused on SLE patients with active kidney disease. “Neutralizing B-cell activating factor and down-regulating autoreactive B-cell function in kidneys represented a compelling therapeutic approach to lupus nephritis,” he explained.
Voclosporin, distinct from cyclosporine, has also been studied in lupus nephritis, Dr. Furie said. Voclosporin offers several benefits over cyclosporine, including greater potency and a lower drug and metabolite load, as well as a more consistent pharmacokinetic and pharmacodynamic relationship, he said. In the phase 3 AURORA study, presented at this year’s EULAR congress, 40% of patients with lupus nephritis met the primary endpoint of a renal response at 52 weeks, compared with 22.5% of placebo patients.
Looking ahead to the treatment of SLE in 2021, “I feel very strongly that voclosporin will be approved for lupus nephritis,” he said. He also predicted that the use of belimumab will be officially extended for lupus nephritis and that anifrolumab will receive an approval for SLE patients.
In addition, the future may witness the increased use of biomarkers and development of more individualized therapy. These breakthroughs will yield better outcomes for all lupus patients, he said.
Dr. Furie disclosed relationships with GlaxoSmithKline, Genentech/Roche, Aurinia Pharmaceuticals, AstraZeneca/MedImmune, and Eli Lilly. Global Academy for Medical Education and this news organization are owned by the same parent company.
Systemic lupus erythematosus remains a treatment challenge, but a variety of drugs in the pipeline are set to target type I interferons, cytokines, and B cells, according to Richard Furie, MD, chief of the division of rheumatology at Northwell Health and professor of medicine at Hofstra University, Hempstead, N.Y.
In general, when treating patients with systemic lupus erythematosus (SLE), “we just don’t see satisfactory response rates,” Dr. Furie said in an online presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“I think the greatest unmet need is in lupus nephritis,” he said. The data show that not even one-third of patients are adequately responding to standard of care treatment. “We need to do better in lupus nephritis but also for those patients with moderate-severe manifestations outside the kidney.”
Patients with SLE have elevated levels of interferon-alpha, Dr. Furie noted. Data from recent studies show that interferon inhibitors can reduce clinical activity in SLE patients, he said.
“About two-thirds to three-quarters of lupus patients have evidence of interferon pathway activation,” he said. There are three types of interferons, and five subtypes of type I interferon, and all five subtypes of type I interferon bind to the same receptor, which is an important strategy for drug development.
In particular, recent phase 2 and 3 trials have focused on targeting type I interferons with anifrolumab, which blocks all five subtypes.
Dr. Furie cited “very robust results” from a phase 2 study. Results of two phase 3 trials of anifrolumab led to a split decision, but the totality of the data collected across the phase 2 and 3 studies points to a drug that is effective for patients with SLE. The two phase 3 studies were published in Lancet Rheumatology and the New England Journal of Medicine.
Dr. Furie also identified recent studies of baricitinib (Olumiant), which has the ability to target several different cytokines. A phase 2 study in 2018 showed a significant difference in SLE Responder Index between lupus patients who received 4 mg of baricitinib or placebo, and a phase 3 study is underway, he said.
For lupus nephritis, Dr. Furie cited the BLISS-LN trial, a 104-week, randomized trial of patients with active lupus nephritis. The group of patients who received belimumab (Benlysta), a monoclonal antibody that targets B-cell activating factor, in addition to standard therapy had significant improvements in renal responses, compared with standard therapy alone (43.0% vs. 32.3%). The outcome measure was Primary Efficacy Renal Response, defined as urinary protein/creatinine ratio <0.7, eGFR ≥60 mL/min per 1.73 m2, confirmation on consecutive visits, and required tapering of background glucocorticoids.
Although belimumab was approved for SLE in 2011, the BLISS-LN study focused on SLE patients with active kidney disease. “Neutralizing B-cell activating factor and down-regulating autoreactive B-cell function in kidneys represented a compelling therapeutic approach to lupus nephritis,” he explained.
Voclosporin, distinct from cyclosporine, has also been studied in lupus nephritis, Dr. Furie said. Voclosporin offers several benefits over cyclosporine, including greater potency and a lower drug and metabolite load, as well as a more consistent pharmacokinetic and pharmacodynamic relationship, he said. In the phase 3 AURORA study, presented at this year’s EULAR congress, 40% of patients with lupus nephritis met the primary endpoint of a renal response at 52 weeks, compared with 22.5% of placebo patients.
Looking ahead to the treatment of SLE in 2021, “I feel very strongly that voclosporin will be approved for lupus nephritis,” he said. He also predicted that the use of belimumab will be officially extended for lupus nephritis and that anifrolumab will receive an approval for SLE patients.
In addition, the future may witness the increased use of biomarkers and development of more individualized therapy. These breakthroughs will yield better outcomes for all lupus patients, he said.
Dr. Furie disclosed relationships with GlaxoSmithKline, Genentech/Roche, Aurinia Pharmaceuticals, AstraZeneca/MedImmune, and Eli Lilly. Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM PRD 2020
A woman with an asymptomatic eruption on her palms after exposure to water
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
Phone outreach intervention feasible to reduce SLE readmissions
A nurse-led intervention aimed at reducing hospital readmission rates for systemic lupus erythematosus (SLE) is feasible but the jury is out as to whether it can achieve its primary goal, a study has found.
A paper published in Arthritis Care & Research presents the outcomes of a retrospective study using electronic health records that looked at the effect of a quality improvement initiative at the University of Colorado Hospital on readmission rates in two cohorts of 48 and 56 individuals with SLE.
Emily Bowers, MD, of the department of rheumatology at the University of Colorado at Denver, Aurora, and coauthors wrote that hospital readmission rates for SLE are as high as 36% for 30-day readmission. They are significantly higher than for other common chronic diseases such as heart failure, COPD, and diabetes. Readmission for SLE is associated with young age, ethnic or racial diversity, public health insurance, multiorgan involvement, and other comorbidities.
The intervention involved first alerting clinic nurses via the patient’s electronic medical record when the patient was discharged from hospital. The nurses would then call the patient within 48 hours to answer any questions and review their discharge information, and then consult with a rheumatologist on on-call if needed. This call was documented in the patient’s medical record.
In the preintervention cohort, there were 59 hospitalizations among 48 patients, 29% of which were followed by readmission within 30 days; 53% of these readmissions were lupus related. In the cohort that followed introduction of the intervention, there were 73 hospitalizations among 56 individuals, and 19% were followed by readmission within 30 days, 29% of which were lupus related.
After accounting for gender, age, race, and insurance type, the researchers calculated that there was an 89% higher odds of readmission in the nonintervention group than in the intervention group, but the difference was not statistically significant.
The authors noted that although the results were not statistically significant, the low cost of the intervention – requiring around 30 minutes of nursing time – meant even small reductions in the number of emergency department or hospital admissions would make it a cost-effective approach.
“Telephone outreach is an excellent method of providing additional support to patients, assessing clinical needs, reinforcing education about SLE, medications, and common complications such as drug side effects and infections, and allows for patients to ask pertinent questions to RN providers with expertise in the management of lupus,” the authors wrote.
The nurses also recorded qualitative information about the calls, which picked up some patient issues that could be addressed. For example, a patient was discharged with the wrong amount of prednisone, which the nurse was able to fix by adjusting the order and sending it to the pharmacy. Two other patients were confused by their medication instructions and were taking the medication incorrectly; the nurse arranged for the patients to come in for educational session. In another case, the nurse was able to arrange an infusion for the patient, and for one patient with concerns about infection, the nurse was able to advise that person on symptoms and how to seek care.
“To increase implementation of the intervention, we have discussed creating a discharge order set, which would include an automatic EMR message to the nurses,” the authors wrote. “Future studies should explore alternative ways of communicating with our patients after discharge, such as the use of text messaging, messaging through the patient portal in the EMR, or telehealth.”
The authors had no financial disclosures, and there was no outside financial support for the study.
SOURCE: Bowers E et al. Arthritis Care Res. 2020 Aug 29. doi: 10.1002/acr.24435.
A nurse-led intervention aimed at reducing hospital readmission rates for systemic lupus erythematosus (SLE) is feasible but the jury is out as to whether it can achieve its primary goal, a study has found.
A paper published in Arthritis Care & Research presents the outcomes of a retrospective study using electronic health records that looked at the effect of a quality improvement initiative at the University of Colorado Hospital on readmission rates in two cohorts of 48 and 56 individuals with SLE.
Emily Bowers, MD, of the department of rheumatology at the University of Colorado at Denver, Aurora, and coauthors wrote that hospital readmission rates for SLE are as high as 36% for 30-day readmission. They are significantly higher than for other common chronic diseases such as heart failure, COPD, and diabetes. Readmission for SLE is associated with young age, ethnic or racial diversity, public health insurance, multiorgan involvement, and other comorbidities.
The intervention involved first alerting clinic nurses via the patient’s electronic medical record when the patient was discharged from hospital. The nurses would then call the patient within 48 hours to answer any questions and review their discharge information, and then consult with a rheumatologist on on-call if needed. This call was documented in the patient’s medical record.
In the preintervention cohort, there were 59 hospitalizations among 48 patients, 29% of which were followed by readmission within 30 days; 53% of these readmissions were lupus related. In the cohort that followed introduction of the intervention, there were 73 hospitalizations among 56 individuals, and 19% were followed by readmission within 30 days, 29% of which were lupus related.
After accounting for gender, age, race, and insurance type, the researchers calculated that there was an 89% higher odds of readmission in the nonintervention group than in the intervention group, but the difference was not statistically significant.
The authors noted that although the results were not statistically significant, the low cost of the intervention – requiring around 30 minutes of nursing time – meant even small reductions in the number of emergency department or hospital admissions would make it a cost-effective approach.
“Telephone outreach is an excellent method of providing additional support to patients, assessing clinical needs, reinforcing education about SLE, medications, and common complications such as drug side effects and infections, and allows for patients to ask pertinent questions to RN providers with expertise in the management of lupus,” the authors wrote.
The nurses also recorded qualitative information about the calls, which picked up some patient issues that could be addressed. For example, a patient was discharged with the wrong amount of prednisone, which the nurse was able to fix by adjusting the order and sending it to the pharmacy. Two other patients were confused by their medication instructions and were taking the medication incorrectly; the nurse arranged for the patients to come in for educational session. In another case, the nurse was able to arrange an infusion for the patient, and for one patient with concerns about infection, the nurse was able to advise that person on symptoms and how to seek care.
“To increase implementation of the intervention, we have discussed creating a discharge order set, which would include an automatic EMR message to the nurses,” the authors wrote. “Future studies should explore alternative ways of communicating with our patients after discharge, such as the use of text messaging, messaging through the patient portal in the EMR, or telehealth.”
The authors had no financial disclosures, and there was no outside financial support for the study.
SOURCE: Bowers E et al. Arthritis Care Res. 2020 Aug 29. doi: 10.1002/acr.24435.
A nurse-led intervention aimed at reducing hospital readmission rates for systemic lupus erythematosus (SLE) is feasible but the jury is out as to whether it can achieve its primary goal, a study has found.
A paper published in Arthritis Care & Research presents the outcomes of a retrospective study using electronic health records that looked at the effect of a quality improvement initiative at the University of Colorado Hospital on readmission rates in two cohorts of 48 and 56 individuals with SLE.
Emily Bowers, MD, of the department of rheumatology at the University of Colorado at Denver, Aurora, and coauthors wrote that hospital readmission rates for SLE are as high as 36% for 30-day readmission. They are significantly higher than for other common chronic diseases such as heart failure, COPD, and diabetes. Readmission for SLE is associated with young age, ethnic or racial diversity, public health insurance, multiorgan involvement, and other comorbidities.
The intervention involved first alerting clinic nurses via the patient’s electronic medical record when the patient was discharged from hospital. The nurses would then call the patient within 48 hours to answer any questions and review their discharge information, and then consult with a rheumatologist on on-call if needed. This call was documented in the patient’s medical record.
In the preintervention cohort, there were 59 hospitalizations among 48 patients, 29% of which were followed by readmission within 30 days; 53% of these readmissions were lupus related. In the cohort that followed introduction of the intervention, there were 73 hospitalizations among 56 individuals, and 19% were followed by readmission within 30 days, 29% of which were lupus related.
After accounting for gender, age, race, and insurance type, the researchers calculated that there was an 89% higher odds of readmission in the nonintervention group than in the intervention group, but the difference was not statistically significant.
The authors noted that although the results were not statistically significant, the low cost of the intervention – requiring around 30 minutes of nursing time – meant even small reductions in the number of emergency department or hospital admissions would make it a cost-effective approach.
“Telephone outreach is an excellent method of providing additional support to patients, assessing clinical needs, reinforcing education about SLE, medications, and common complications such as drug side effects and infections, and allows for patients to ask pertinent questions to RN providers with expertise in the management of lupus,” the authors wrote.
The nurses also recorded qualitative information about the calls, which picked up some patient issues that could be addressed. For example, a patient was discharged with the wrong amount of prednisone, which the nurse was able to fix by adjusting the order and sending it to the pharmacy. Two other patients were confused by their medication instructions and were taking the medication incorrectly; the nurse arranged for the patients to come in for educational session. In another case, the nurse was able to arrange an infusion for the patient, and for one patient with concerns about infection, the nurse was able to advise that person on symptoms and how to seek care.
“To increase implementation of the intervention, we have discussed creating a discharge order set, which would include an automatic EMR message to the nurses,” the authors wrote. “Future studies should explore alternative ways of communicating with our patients after discharge, such as the use of text messaging, messaging through the patient portal in the EMR, or telehealth.”
The authors had no financial disclosures, and there was no outside financial support for the study.
SOURCE: Bowers E et al. Arthritis Care Res. 2020 Aug 29. doi: 10.1002/acr.24435.
FROM ARTHRITIS CARE & RESEARCH
Age, smoking among leading cancer risk factors for SLE patients
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
FROM ARTHRITIS CARE & RESEARCH