User login
Most clinicians undertreat childhood lichen sclerosus
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
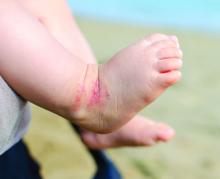
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
FROM SPD 2020
For suspected hair disorders, consider trichoscopy before biopsy
In the clinical experience of Bianca Maria Piraccini, MD,
Dermoscopic imaging, also known as trichoscopy, “avoids invasive procedures and provides immediate results,” Dr. Piraccini, of the University of Bologna’s division of dermatology in the department of experimental, diagnostic, and specialty medicine, said during the virtual annual meeting of the Society for Pediatric Dermatology. “It is helpful for diagnosing all sorts of alopecia, starting with those that appear at birth, such as aplasia cutis congenita to those that appear in adolescence, such as androgenetic alopecia.”
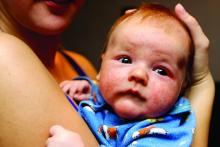
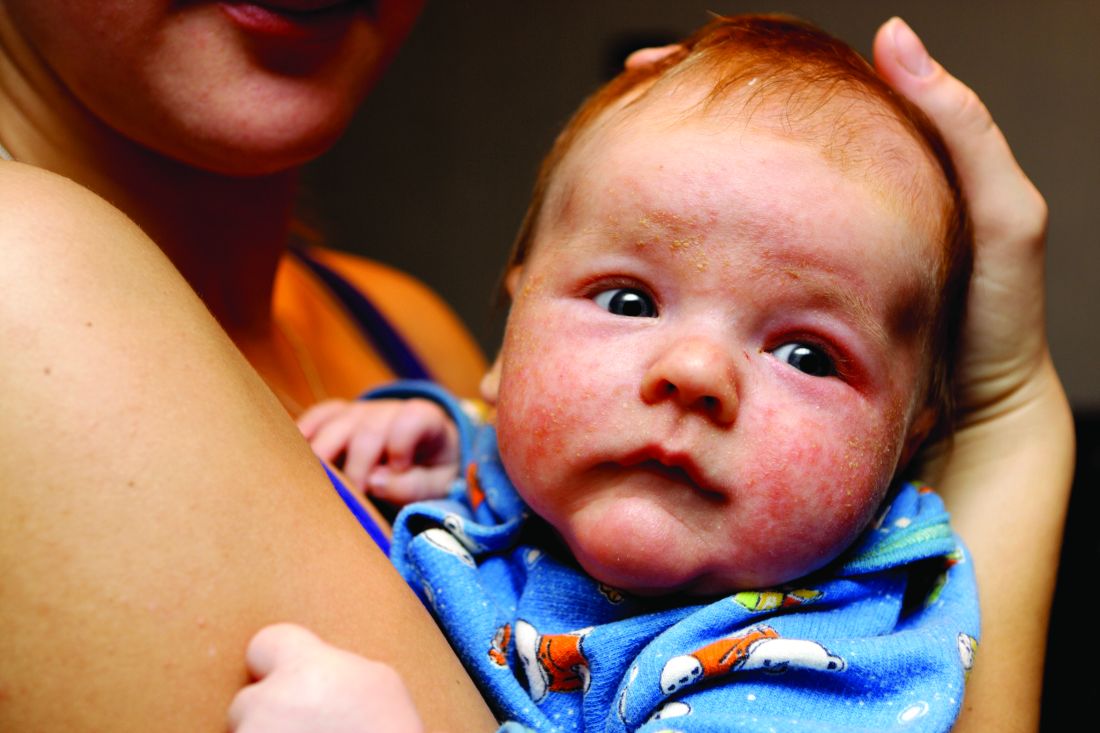
Dr. Piraccini noted that lanugo hair is produced at 16-20 weeks’ gestation and is shed in utero and replaced by thicker hair at 32-36 weeks. “The speed of transition from vellus to intermediate and terminal hair varies from child to child,” she said. “The scalp at birth presents with thin, intermediate, or thick hair.”
In a dermoscopic evaluation of hair in 45 neonates during their first 30 days of life, Dr. Piraccini and colleagues found that 70% had low density hair while the remaining 30% had high density hair (Br J Dermatol 2013; 169:896-900). Two neonates presented a frontal-temporal pattern of hair loss. Trichoscopy revealed that nine neonates, all in the poor hair density group, had a particular hair shaft dermoscopic feature, characterized by the presence of widespread thin hair.
In some children, she continued, hair in the occipital area does not enter the telogen phase until after birth. These hairs remain on the scalp for 8-12 weeks and then fall out, resulting in neonatal occipital alopecia, which is the most common form of transient neonatal hair loss. Neonatal occipital alopecia is characterized by a band-like shape or oval alopecic patch with a sharp lower margin, but it often goes unnoticed by parents.
“It occurs with higher prevalence in infants born to mothers younger than age 34, in those with a non-cesarean birth, and in those with a gestational age greater than 37 weeks,” Dr. Piraccini said. “There are different degrees of severity. On trichoscopy, the condition appears as thin regrowing hair. The outcome is totally benign, with normal hair growth within the first year of life.”
Any aspect of alopecia in the occipital area in young children may be a sign of hair shaft disorders, which are characterized by increased hair fragility. “Trichoscopy is diagnostic,” she said. “When applied to the hair you see monilethrix, a rare inherited disorder characterized by sparse, brittle hair that often breaks before reaching a few inches in length. As the child grows, the hair gradually acquires the characteristics it will have in adulthood. “It may remain thin and with a short anagen phase for several years, but acute shedding is rare,” she said.
When an older child presents with increased hair shedding, the first exam to perform is the pull test. If it results in painless traction of several anagen hair without sheaths and with ragged cuticles, think about loose anagen hair syndrome. This condition affects females more than males, usually occurs between the ages of 2 and 5, and is characterized by a defective anchoring of the hair shaft to the hair follicle. The three clinical types of loose anagen hair syndrome are short, rough sparse hair; increased shedding; and areas of alopecia. The syndrome “tends to be inherited but spontaneously improves with aging,” Dr. Piraccini said.
Alopecia areata, another common pediatric hair disorder, occurs in 20% of patients younger than 16 years of age and 9% of those with Down syndrome, and is associated with a family history of the condition. Young age at onset is a negative prognostic factor. “On trichoscopy, common features of alopecia areata are yellow dots, black dots, exclamation mark hairs, and broken hair,” she said. “Trichoscopy can also help you distinguish acute from chronic alopecia areata. The risk of relapse is common, and psychological support is mandatory, because it is very stressful for children.”
Another form of patchy alopecia, trichotillomania, occurs mainly in school-aged children and appears as irregular patches of alopecia with hairs broken at different lengths. “The pull test is negative because all telogen hairs have been pulled out by the patient,” Dr. Piraccini said. “Parents often do not accept the diagnosis as they do not see the child touching his or her hair. It has a good prognosis.”
Trichoscopic signs of trichotillomania include black dots, hair broken at different length, flame hair, clots of hair, and tulip hair. Treatment typically consists of psychological counseling and N-acetylcysteine 600-2,400 g/day.
Dr. Piraccini reported having no relevant financial disclosures.
In the clinical experience of Bianca Maria Piraccini, MD,
Dermoscopic imaging, also known as trichoscopy, “avoids invasive procedures and provides immediate results,” Dr. Piraccini, of the University of Bologna’s division of dermatology in the department of experimental, diagnostic, and specialty medicine, said during the virtual annual meeting of the Society for Pediatric Dermatology. “It is helpful for diagnosing all sorts of alopecia, starting with those that appear at birth, such as aplasia cutis congenita to those that appear in adolescence, such as androgenetic alopecia.”
Dr. Piraccini noted that lanugo hair is produced at 16-20 weeks’ gestation and is shed in utero and replaced by thicker hair at 32-36 weeks. “The speed of transition from vellus to intermediate and terminal hair varies from child to child,” she said. “The scalp at birth presents with thin, intermediate, or thick hair.”
In a dermoscopic evaluation of hair in 45 neonates during their first 30 days of life, Dr. Piraccini and colleagues found that 70% had low density hair while the remaining 30% had high density hair (Br J Dermatol 2013; 169:896-900). Two neonates presented a frontal-temporal pattern of hair loss. Trichoscopy revealed that nine neonates, all in the poor hair density group, had a particular hair shaft dermoscopic feature, characterized by the presence of widespread thin hair.
In some children, she continued, hair in the occipital area does not enter the telogen phase until after birth. These hairs remain on the scalp for 8-12 weeks and then fall out, resulting in neonatal occipital alopecia, which is the most common form of transient neonatal hair loss. Neonatal occipital alopecia is characterized by a band-like shape or oval alopecic patch with a sharp lower margin, but it often goes unnoticed by parents.
“It occurs with higher prevalence in infants born to mothers younger than age 34, in those with a non-cesarean birth, and in those with a gestational age greater than 37 weeks,” Dr. Piraccini said. “There are different degrees of severity. On trichoscopy, the condition appears as thin regrowing hair. The outcome is totally benign, with normal hair growth within the first year of life.”
Any aspect of alopecia in the occipital area in young children may be a sign of hair shaft disorders, which are characterized by increased hair fragility. “Trichoscopy is diagnostic,” she said. “When applied to the hair you see monilethrix, a rare inherited disorder characterized by sparse, brittle hair that often breaks before reaching a few inches in length. As the child grows, the hair gradually acquires the characteristics it will have in adulthood. “It may remain thin and with a short anagen phase for several years, but acute shedding is rare,” she said.
When an older child presents with increased hair shedding, the first exam to perform is the pull test. If it results in painless traction of several anagen hair without sheaths and with ragged cuticles, think about loose anagen hair syndrome. This condition affects females more than males, usually occurs between the ages of 2 and 5, and is characterized by a defective anchoring of the hair shaft to the hair follicle. The three clinical types of loose anagen hair syndrome are short, rough sparse hair; increased shedding; and areas of alopecia. The syndrome “tends to be inherited but spontaneously improves with aging,” Dr. Piraccini said.
Alopecia areata, another common pediatric hair disorder, occurs in 20% of patients younger than 16 years of age and 9% of those with Down syndrome, and is associated with a family history of the condition. Young age at onset is a negative prognostic factor. “On trichoscopy, common features of alopecia areata are yellow dots, black dots, exclamation mark hairs, and broken hair,” she said. “Trichoscopy can also help you distinguish acute from chronic alopecia areata. The risk of relapse is common, and psychological support is mandatory, because it is very stressful for children.”
Another form of patchy alopecia, trichotillomania, occurs mainly in school-aged children and appears as irregular patches of alopecia with hairs broken at different lengths. “The pull test is negative because all telogen hairs have been pulled out by the patient,” Dr. Piraccini said. “Parents often do not accept the diagnosis as they do not see the child touching his or her hair. It has a good prognosis.”
Trichoscopic signs of trichotillomania include black dots, hair broken at different length, flame hair, clots of hair, and tulip hair. Treatment typically consists of psychological counseling and N-acetylcysteine 600-2,400 g/day.
Dr. Piraccini reported having no relevant financial disclosures.
In the clinical experience of Bianca Maria Piraccini, MD,
Dermoscopic imaging, also known as trichoscopy, “avoids invasive procedures and provides immediate results,” Dr. Piraccini, of the University of Bologna’s division of dermatology in the department of experimental, diagnostic, and specialty medicine, said during the virtual annual meeting of the Society for Pediatric Dermatology. “It is helpful for diagnosing all sorts of alopecia, starting with those that appear at birth, such as aplasia cutis congenita to those that appear in adolescence, such as androgenetic alopecia.”
Dr. Piraccini noted that lanugo hair is produced at 16-20 weeks’ gestation and is shed in utero and replaced by thicker hair at 32-36 weeks. “The speed of transition from vellus to intermediate and terminal hair varies from child to child,” she said. “The scalp at birth presents with thin, intermediate, or thick hair.”
In a dermoscopic evaluation of hair in 45 neonates during their first 30 days of life, Dr. Piraccini and colleagues found that 70% had low density hair while the remaining 30% had high density hair (Br J Dermatol 2013; 169:896-900). Two neonates presented a frontal-temporal pattern of hair loss. Trichoscopy revealed that nine neonates, all in the poor hair density group, had a particular hair shaft dermoscopic feature, characterized by the presence of widespread thin hair.
In some children, she continued, hair in the occipital area does not enter the telogen phase until after birth. These hairs remain on the scalp for 8-12 weeks and then fall out, resulting in neonatal occipital alopecia, which is the most common form of transient neonatal hair loss. Neonatal occipital alopecia is characterized by a band-like shape or oval alopecic patch with a sharp lower margin, but it often goes unnoticed by parents.
“It occurs with higher prevalence in infants born to mothers younger than age 34, in those with a non-cesarean birth, and in those with a gestational age greater than 37 weeks,” Dr. Piraccini said. “There are different degrees of severity. On trichoscopy, the condition appears as thin regrowing hair. The outcome is totally benign, with normal hair growth within the first year of life.”
Any aspect of alopecia in the occipital area in young children may be a sign of hair shaft disorders, which are characterized by increased hair fragility. “Trichoscopy is diagnostic,” she said. “When applied to the hair you see monilethrix, a rare inherited disorder characterized by sparse, brittle hair that often breaks before reaching a few inches in length. As the child grows, the hair gradually acquires the characteristics it will have in adulthood. “It may remain thin and with a short anagen phase for several years, but acute shedding is rare,” she said.
When an older child presents with increased hair shedding, the first exam to perform is the pull test. If it results in painless traction of several anagen hair without sheaths and with ragged cuticles, think about loose anagen hair syndrome. This condition affects females more than males, usually occurs between the ages of 2 and 5, and is characterized by a defective anchoring of the hair shaft to the hair follicle. The three clinical types of loose anagen hair syndrome are short, rough sparse hair; increased shedding; and areas of alopecia. The syndrome “tends to be inherited but spontaneously improves with aging,” Dr. Piraccini said.
Alopecia areata, another common pediatric hair disorder, occurs in 20% of patients younger than 16 years of age and 9% of those with Down syndrome, and is associated with a family history of the condition. Young age at onset is a negative prognostic factor. “On trichoscopy, common features of alopecia areata are yellow dots, black dots, exclamation mark hairs, and broken hair,” she said. “Trichoscopy can also help you distinguish acute from chronic alopecia areata. The risk of relapse is common, and psychological support is mandatory, because it is very stressful for children.”
Another form of patchy alopecia, trichotillomania, occurs mainly in school-aged children and appears as irregular patches of alopecia with hairs broken at different lengths. “The pull test is negative because all telogen hairs have been pulled out by the patient,” Dr. Piraccini said. “Parents often do not accept the diagnosis as they do not see the child touching his or her hair. It has a good prognosis.”
Trichoscopic signs of trichotillomania include black dots, hair broken at different length, flame hair, clots of hair, and tulip hair. Treatment typically consists of psychological counseling and N-acetylcysteine 600-2,400 g/day.
Dr. Piraccini reported having no relevant financial disclosures.
FROM SPD 2020
So, you’ve been sued. What now?
By the time physicians turn 65 years old, more than 75% of those in low-risk specialties such as pediatric dermatology have been named in a lawsuit, compared with 99% of those in high-risk specialties such as obstetrics and gynecology, according to Ilona J. Frieden, MD.
“We all know there’s a possibility that we could get named in a lawsuit,” she said during the virtual annual meeting of the Society for Pediatric Dermatology. “It could happen to any of us. Lawsuits are not uncommon, but few of us have received any kind of training for how to handle them.”
Based on her experience being named in a malpractice/wrongful death lawsuit, Dr. Frieden, who has had a nearly 4-decade career as a pediatric dermatologist at the University of California, San Francisco, offered the following tips for clinicians facing practice-related litigation:
First, immediately inform the risk management representatives at your institution or your malpractice insurance carrier. “Tell them about the situation and arrange to talk to a lawyer,” she advised.
Second, prepare to confront a range of emotions. “Depending on the circumstances, [that could be] fear, anger, dread, and defensiveness,” said Dr. Frieden, professor of dermatology and pediatrics, at UCSF. “What surprised me was this sort of physical sensation. I felt like I had been kicked in the stomach. In retrospect, this is not such a surprising finding. It really is an assault on your professional identity, so it made sense to me as I thought about this.”
Third, slow yourself down. The litigation process typically takes 2-5 years, “so this is a marathon; this is not a sprint,” she said. “While you are waiting you will be told, ‘Don’t discuss this case with anyone.’ While this may be true for the specific details of the case, it isn’t true about what you are feeling and how this affects you. You can and you should talk to a trusted friend, to a spouse, or even to a therapist so that you can process what you’re going through and not feel alone.”
Fourth, try to focus on the patients that you help. Having a patient in your pediatric dermatology practice die “is a rare event,” she said. “Try to not let such an event define you in terms of your professional identity. Meanwhile [remember that] you’re helping lots and lots of people.”
Fifth, be humble, both for yourself and the experts you might turn to for advice when you’re facing a complex case. “Though I have decades of experience, I find myself feeling more willing rather than less willing to ask for help,” Dr. Frieden said. “Also, the culture has changed. We email colleagues all the time to say, ‘This doesn’t make sense. Can you please tell me what your thoughts are?’ ”
She closed her remarks by noting that physicians “put ourselves in harm’s way in the process of trying to do the best we can for patients. That is something we have to accept.” She reported having no financial disclosures.
By the time physicians turn 65 years old, more than 75% of those in low-risk specialties such as pediatric dermatology have been named in a lawsuit, compared with 99% of those in high-risk specialties such as obstetrics and gynecology, according to Ilona J. Frieden, MD.
“We all know there’s a possibility that we could get named in a lawsuit,” she said during the virtual annual meeting of the Society for Pediatric Dermatology. “It could happen to any of us. Lawsuits are not uncommon, but few of us have received any kind of training for how to handle them.”
Based on her experience being named in a malpractice/wrongful death lawsuit, Dr. Frieden, who has had a nearly 4-decade career as a pediatric dermatologist at the University of California, San Francisco, offered the following tips for clinicians facing practice-related litigation:
First, immediately inform the risk management representatives at your institution or your malpractice insurance carrier. “Tell them about the situation and arrange to talk to a lawyer,” she advised.
Second, prepare to confront a range of emotions. “Depending on the circumstances, [that could be] fear, anger, dread, and defensiveness,” said Dr. Frieden, professor of dermatology and pediatrics, at UCSF. “What surprised me was this sort of physical sensation. I felt like I had been kicked in the stomach. In retrospect, this is not such a surprising finding. It really is an assault on your professional identity, so it made sense to me as I thought about this.”
Third, slow yourself down. The litigation process typically takes 2-5 years, “so this is a marathon; this is not a sprint,” she said. “While you are waiting you will be told, ‘Don’t discuss this case with anyone.’ While this may be true for the specific details of the case, it isn’t true about what you are feeling and how this affects you. You can and you should talk to a trusted friend, to a spouse, or even to a therapist so that you can process what you’re going through and not feel alone.”
Fourth, try to focus on the patients that you help. Having a patient in your pediatric dermatology practice die “is a rare event,” she said. “Try to not let such an event define you in terms of your professional identity. Meanwhile [remember that] you’re helping lots and lots of people.”
Fifth, be humble, both for yourself and the experts you might turn to for advice when you’re facing a complex case. “Though I have decades of experience, I find myself feeling more willing rather than less willing to ask for help,” Dr. Frieden said. “Also, the culture has changed. We email colleagues all the time to say, ‘This doesn’t make sense. Can you please tell me what your thoughts are?’ ”
She closed her remarks by noting that physicians “put ourselves in harm’s way in the process of trying to do the best we can for patients. That is something we have to accept.” She reported having no financial disclosures.
By the time physicians turn 65 years old, more than 75% of those in low-risk specialties such as pediatric dermatology have been named in a lawsuit, compared with 99% of those in high-risk specialties such as obstetrics and gynecology, according to Ilona J. Frieden, MD.
“We all know there’s a possibility that we could get named in a lawsuit,” she said during the virtual annual meeting of the Society for Pediatric Dermatology. “It could happen to any of us. Lawsuits are not uncommon, but few of us have received any kind of training for how to handle them.”
Based on her experience being named in a malpractice/wrongful death lawsuit, Dr. Frieden, who has had a nearly 4-decade career as a pediatric dermatologist at the University of California, San Francisco, offered the following tips for clinicians facing practice-related litigation:
First, immediately inform the risk management representatives at your institution or your malpractice insurance carrier. “Tell them about the situation and arrange to talk to a lawyer,” she advised.
Second, prepare to confront a range of emotions. “Depending on the circumstances, [that could be] fear, anger, dread, and defensiveness,” said Dr. Frieden, professor of dermatology and pediatrics, at UCSF. “What surprised me was this sort of physical sensation. I felt like I had been kicked in the stomach. In retrospect, this is not such a surprising finding. It really is an assault on your professional identity, so it made sense to me as I thought about this.”
Third, slow yourself down. The litigation process typically takes 2-5 years, “so this is a marathon; this is not a sprint,” she said. “While you are waiting you will be told, ‘Don’t discuss this case with anyone.’ While this may be true for the specific details of the case, it isn’t true about what you are feeling and how this affects you. You can and you should talk to a trusted friend, to a spouse, or even to a therapist so that you can process what you’re going through and not feel alone.”
Fourth, try to focus on the patients that you help. Having a patient in your pediatric dermatology practice die “is a rare event,” she said. “Try to not let such an event define you in terms of your professional identity. Meanwhile [remember that] you’re helping lots and lots of people.”
Fifth, be humble, both for yourself and the experts you might turn to for advice when you’re facing a complex case. “Though I have decades of experience, I find myself feeling more willing rather than less willing to ask for help,” Dr. Frieden said. “Also, the culture has changed. We email colleagues all the time to say, ‘This doesn’t make sense. Can you please tell me what your thoughts are?’ ”
She closed her remarks by noting that physicians “put ourselves in harm’s way in the process of trying to do the best we can for patients. That is something we have to accept.” She reported having no financial disclosures.
FROM SPD 2020
Memphis clinic created to care for children and adolescents diagnosed with melanoma
Pediatric melanoma remains a rare diagnosis – representing just 1%-4% of all melanomas – and it continues to be poorly understood.
“There are many questions about its biology, histopathology, and clinical behavior,” Teresa S. Wright, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “This diagnosis can be very difficult to establish. These lesions can be very unusual and require several different expert opinions to arrive at a diagnosis. Oftentimes, there may be an initial misdiagnosis or disagreement about diagnosis. This frequently results in a delay of treatment.”
Dr. Wright, chief of pediatric dermatology at LeBonheur Children’s Hospital and associate professor of dermatology at the University of Tennessee Health Science Center, Memphis, added that once a diagnosis of pediatric melanoma has been established, things don’t get any easier because of the lack of evidence-based guidelines for management. “There are really no standard recommendations regarding the workup, treatment, or follow-up for these patients,” she said.
Referral Clinic Launched
In 2016, under the direction of Alberto Pappo, MD, director of the solid tumor division at St. Jude Children’s Research Hospital in Memphis, Dr. Wright and several colleagues at “As a group, we address questions surrounding the diagnosis and pathology of the patient’s lesion, as well as therapy and follow-up for each individual patient,” Dr. Wright said.
Members of the clinic team include a pediatric oncologist, an adult oncologist, and a surgical oncologist (all with melanoma expertise); a pediatric surgeon, a pediatric dermatologist, a pediatric radiologist, a pathologist, and a nursing team, which includes a pediatric nurse practitioner, three registered nurses, and other support staff, including those that provide genetic counseling and child life specialists. To be eligible for the clinic, which typically is scheduled in April and November every year, patients must be no older than 21 years, must be referred by a physician, and must have a diagnosis of melanoma or Spitzoid melanoma, not including ocular melanoma. They must be currently undergoing treatment or followed by a physician who requests or supports a consult to optimize clinical management of the patient. St. Jude foots the bill for all travel, housing, and meal expenses. All pertinent materials are collected in advance of the 2-day clinic, including medical records, lab results, histology slides, tissue samples, and radiographic studies. The pathologist performs an initial review of the histology slides and additional genomic studies are performed based on the pathologist’s diagnosis.
Patients typically arrive on a Wednesday evening and have their first clinic visit Thursday morning. First, the oncology team performs a thorough history and physical examination, then Dr. Wright performs a thorough skin examination and a professional photographer captures images of relevant skin lesions. That afternoon, members of the multidisciplinary team meet to review each patient’s entire course, including previous surgeries and any medical therapies.
“We review their pathology, including histology slides and results of any genomic studies,” Dr. Wright said. “We also review all the radiographic studies they’ve had, which may include plain films, CT scans, PET scans, MRIs, and ultrasounds. Then we form a consensus opinion regarding a diagnosis. Sometimes we feel a change in diagnosis is warranted.” For example, she added, “we have had a number of patients referred to us with an initial diagnosis of Spitzoid melanoma where, after review, we felt that a diagnosis of atypical Spitzoid tumor was more appropriate for them. We also talk about any treatment they’ve had in the past and decide if any additional surgical or medical treatment is indicated at this time. Lastly, we make recommendations for follow-up or surveillance.”
On Thursday evening, the clinic sponsors a casual dinner for families, which features an educational presentation by one or more faculty members. Topics covered in the past include sun protection, melanoma in children, and an overview of melanoma research.
The next morning, each family meets with the panel of specialists. “The team members introduce themselves and describe their roles within the team, and family members introduce themselves and tell their child’s story. “Then, each team member describes their findings and gives their overall assessment. The family receives recommendations for any additional testing, therapy, and follow-up, and the patient and family’s questions are answered.”
Families are also offered the opportunity to participate in research. “They can donate samples to a tissue bank, and patients may qualify for future clinical trials at St. Jude Children’s Research Hospital,” Dr. Wright said.
To date, 20 female and 18 male patients have traveled to the Pediatric and Adolescent Melanoma Referral Clinic from 21 states and Puerto Rico for assessment and consultation. They ranged in age from 6 months to 18 years, and their average age is 9 years. Members of the clinic team have seen 13 patients with a diagnosis of Spitzoid melanoma, 10 with malignant melanoma, 8 with atypical melanocytic neoplasm, 3 with congenital melanoma, 3 with atypical Spitz tumor, and 1 with congenital melanocytic nevus.
The median age at diagnosis was 12 years for malignant melanoma and 9 years for Spitzoid melanoma; and the male to female ratio is 7:3 for malignant melanoma and 4:9 for Spitzoid melanoma. These are the patients who have come to the multidisciplinary clinic, these specialists see other patients with a diagnosis of pediatric or adolescent melanoma at other times of the year, Dr. Wright noted.
A common refrain she hears from pediatric melanoma patients and their families is that the initial skin lesion appears to be unremarkable. “Many times, this is a skin-colored or pink papule, which starts out looking very much like a molluscum or a wart or an insect bite, or something else that nobody’s worried about,” Dr. Wright said. “But over time, something happens, and the common factor is rapid growth. Time and again when I ask parents, ‘What changed? What got your attention?’ The answer is nearly always rapid growth.”
She emphasized that patients frequently arrive at the clinic with multiple opinions about their diagnosis. “It’s not unusual for a significant amount of time to pass between the initial biopsy and the final diagnosis,” she said. “Given the lack of evidence-based guidelines for children, a delay in diagnosis can make decisions about management even more difficult. Because pediatric melanoma is so rare, and there are no standard guidelines for management, there’s a major lack of consistency in terms of how patients are evaluated, treated, and followed.”
Dr. Wright said the team’s goals are to continue the biannual clinic and collect more data and tissue samples for further genomic studies on pediatric melanoma. “Ultimately, we would like to hold a consensus summit meeting of experts to develop and publish evidence-based guidelines for the management of pediatric and adolescent melanoma.”
Dr. Wright reported having no relevant disclosures.
Pediatric melanoma remains a rare diagnosis – representing just 1%-4% of all melanomas – and it continues to be poorly understood.
“There are many questions about its biology, histopathology, and clinical behavior,” Teresa S. Wright, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “This diagnosis can be very difficult to establish. These lesions can be very unusual and require several different expert opinions to arrive at a diagnosis. Oftentimes, there may be an initial misdiagnosis or disagreement about diagnosis. This frequently results in a delay of treatment.”
Dr. Wright, chief of pediatric dermatology at LeBonheur Children’s Hospital and associate professor of dermatology at the University of Tennessee Health Science Center, Memphis, added that once a diagnosis of pediatric melanoma has been established, things don’t get any easier because of the lack of evidence-based guidelines for management. “There are really no standard recommendations regarding the workup, treatment, or follow-up for these patients,” she said.
Referral Clinic Launched
In 2016, under the direction of Alberto Pappo, MD, director of the solid tumor division at St. Jude Children’s Research Hospital in Memphis, Dr. Wright and several colleagues at “As a group, we address questions surrounding the diagnosis and pathology of the patient’s lesion, as well as therapy and follow-up for each individual patient,” Dr. Wright said.
Members of the clinic team include a pediatric oncologist, an adult oncologist, and a surgical oncologist (all with melanoma expertise); a pediatric surgeon, a pediatric dermatologist, a pediatric radiologist, a pathologist, and a nursing team, which includes a pediatric nurse practitioner, three registered nurses, and other support staff, including those that provide genetic counseling and child life specialists. To be eligible for the clinic, which typically is scheduled in April and November every year, patients must be no older than 21 years, must be referred by a physician, and must have a diagnosis of melanoma or Spitzoid melanoma, not including ocular melanoma. They must be currently undergoing treatment or followed by a physician who requests or supports a consult to optimize clinical management of the patient. St. Jude foots the bill for all travel, housing, and meal expenses. All pertinent materials are collected in advance of the 2-day clinic, including medical records, lab results, histology slides, tissue samples, and radiographic studies. The pathologist performs an initial review of the histology slides and additional genomic studies are performed based on the pathologist’s diagnosis.
Patients typically arrive on a Wednesday evening and have their first clinic visit Thursday morning. First, the oncology team performs a thorough history and physical examination, then Dr. Wright performs a thorough skin examination and a professional photographer captures images of relevant skin lesions. That afternoon, members of the multidisciplinary team meet to review each patient’s entire course, including previous surgeries and any medical therapies.
“We review their pathology, including histology slides and results of any genomic studies,” Dr. Wright said. “We also review all the radiographic studies they’ve had, which may include plain films, CT scans, PET scans, MRIs, and ultrasounds. Then we form a consensus opinion regarding a diagnosis. Sometimes we feel a change in diagnosis is warranted.” For example, she added, “we have had a number of patients referred to us with an initial diagnosis of Spitzoid melanoma where, after review, we felt that a diagnosis of atypical Spitzoid tumor was more appropriate for them. We also talk about any treatment they’ve had in the past and decide if any additional surgical or medical treatment is indicated at this time. Lastly, we make recommendations for follow-up or surveillance.”
On Thursday evening, the clinic sponsors a casual dinner for families, which features an educational presentation by one or more faculty members. Topics covered in the past include sun protection, melanoma in children, and an overview of melanoma research.
The next morning, each family meets with the panel of specialists. “The team members introduce themselves and describe their roles within the team, and family members introduce themselves and tell their child’s story. “Then, each team member describes their findings and gives their overall assessment. The family receives recommendations for any additional testing, therapy, and follow-up, and the patient and family’s questions are answered.”
Families are also offered the opportunity to participate in research. “They can donate samples to a tissue bank, and patients may qualify for future clinical trials at St. Jude Children’s Research Hospital,” Dr. Wright said.
To date, 20 female and 18 male patients have traveled to the Pediatric and Adolescent Melanoma Referral Clinic from 21 states and Puerto Rico for assessment and consultation. They ranged in age from 6 months to 18 years, and their average age is 9 years. Members of the clinic team have seen 13 patients with a diagnosis of Spitzoid melanoma, 10 with malignant melanoma, 8 with atypical melanocytic neoplasm, 3 with congenital melanoma, 3 with atypical Spitz tumor, and 1 with congenital melanocytic nevus.
The median age at diagnosis was 12 years for malignant melanoma and 9 years for Spitzoid melanoma; and the male to female ratio is 7:3 for malignant melanoma and 4:9 for Spitzoid melanoma. These are the patients who have come to the multidisciplinary clinic, these specialists see other patients with a diagnosis of pediatric or adolescent melanoma at other times of the year, Dr. Wright noted.
A common refrain she hears from pediatric melanoma patients and their families is that the initial skin lesion appears to be unremarkable. “Many times, this is a skin-colored or pink papule, which starts out looking very much like a molluscum or a wart or an insect bite, or something else that nobody’s worried about,” Dr. Wright said. “But over time, something happens, and the common factor is rapid growth. Time and again when I ask parents, ‘What changed? What got your attention?’ The answer is nearly always rapid growth.”
She emphasized that patients frequently arrive at the clinic with multiple opinions about their diagnosis. “It’s not unusual for a significant amount of time to pass between the initial biopsy and the final diagnosis,” she said. “Given the lack of evidence-based guidelines for children, a delay in diagnosis can make decisions about management even more difficult. Because pediatric melanoma is so rare, and there are no standard guidelines for management, there’s a major lack of consistency in terms of how patients are evaluated, treated, and followed.”
Dr. Wright said the team’s goals are to continue the biannual clinic and collect more data and tissue samples for further genomic studies on pediatric melanoma. “Ultimately, we would like to hold a consensus summit meeting of experts to develop and publish evidence-based guidelines for the management of pediatric and adolescent melanoma.”
Dr. Wright reported having no relevant disclosures.
Pediatric melanoma remains a rare diagnosis – representing just 1%-4% of all melanomas – and it continues to be poorly understood.
“There are many questions about its biology, histopathology, and clinical behavior,” Teresa S. Wright, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “This diagnosis can be very difficult to establish. These lesions can be very unusual and require several different expert opinions to arrive at a diagnosis. Oftentimes, there may be an initial misdiagnosis or disagreement about diagnosis. This frequently results in a delay of treatment.”
Dr. Wright, chief of pediatric dermatology at LeBonheur Children’s Hospital and associate professor of dermatology at the University of Tennessee Health Science Center, Memphis, added that once a diagnosis of pediatric melanoma has been established, things don’t get any easier because of the lack of evidence-based guidelines for management. “There are really no standard recommendations regarding the workup, treatment, or follow-up for these patients,” she said.
Referral Clinic Launched
In 2016, under the direction of Alberto Pappo, MD, director of the solid tumor division at St. Jude Children’s Research Hospital in Memphis, Dr. Wright and several colleagues at “As a group, we address questions surrounding the diagnosis and pathology of the patient’s lesion, as well as therapy and follow-up for each individual patient,” Dr. Wright said.
Members of the clinic team include a pediatric oncologist, an adult oncologist, and a surgical oncologist (all with melanoma expertise); a pediatric surgeon, a pediatric dermatologist, a pediatric radiologist, a pathologist, and a nursing team, which includes a pediatric nurse practitioner, three registered nurses, and other support staff, including those that provide genetic counseling and child life specialists. To be eligible for the clinic, which typically is scheduled in April and November every year, patients must be no older than 21 years, must be referred by a physician, and must have a diagnosis of melanoma or Spitzoid melanoma, not including ocular melanoma. They must be currently undergoing treatment or followed by a physician who requests or supports a consult to optimize clinical management of the patient. St. Jude foots the bill for all travel, housing, and meal expenses. All pertinent materials are collected in advance of the 2-day clinic, including medical records, lab results, histology slides, tissue samples, and radiographic studies. The pathologist performs an initial review of the histology slides and additional genomic studies are performed based on the pathologist’s diagnosis.
Patients typically arrive on a Wednesday evening and have their first clinic visit Thursday morning. First, the oncology team performs a thorough history and physical examination, then Dr. Wright performs a thorough skin examination and a professional photographer captures images of relevant skin lesions. That afternoon, members of the multidisciplinary team meet to review each patient’s entire course, including previous surgeries and any medical therapies.
“We review their pathology, including histology slides and results of any genomic studies,” Dr. Wright said. “We also review all the radiographic studies they’ve had, which may include plain films, CT scans, PET scans, MRIs, and ultrasounds. Then we form a consensus opinion regarding a diagnosis. Sometimes we feel a change in diagnosis is warranted.” For example, she added, “we have had a number of patients referred to us with an initial diagnosis of Spitzoid melanoma where, after review, we felt that a diagnosis of atypical Spitzoid tumor was more appropriate for them. We also talk about any treatment they’ve had in the past and decide if any additional surgical or medical treatment is indicated at this time. Lastly, we make recommendations for follow-up or surveillance.”
On Thursday evening, the clinic sponsors a casual dinner for families, which features an educational presentation by one or more faculty members. Topics covered in the past include sun protection, melanoma in children, and an overview of melanoma research.
The next morning, each family meets with the panel of specialists. “The team members introduce themselves and describe their roles within the team, and family members introduce themselves and tell their child’s story. “Then, each team member describes their findings and gives their overall assessment. The family receives recommendations for any additional testing, therapy, and follow-up, and the patient and family’s questions are answered.”
Families are also offered the opportunity to participate in research. “They can donate samples to a tissue bank, and patients may qualify for future clinical trials at St. Jude Children’s Research Hospital,” Dr. Wright said.
To date, 20 female and 18 male patients have traveled to the Pediatric and Adolescent Melanoma Referral Clinic from 21 states and Puerto Rico for assessment and consultation. They ranged in age from 6 months to 18 years, and their average age is 9 years. Members of the clinic team have seen 13 patients with a diagnosis of Spitzoid melanoma, 10 with malignant melanoma, 8 with atypical melanocytic neoplasm, 3 with congenital melanoma, 3 with atypical Spitz tumor, and 1 with congenital melanocytic nevus.
The median age at diagnosis was 12 years for malignant melanoma and 9 years for Spitzoid melanoma; and the male to female ratio is 7:3 for malignant melanoma and 4:9 for Spitzoid melanoma. These are the patients who have come to the multidisciplinary clinic, these specialists see other patients with a diagnosis of pediatric or adolescent melanoma at other times of the year, Dr. Wright noted.
A common refrain she hears from pediatric melanoma patients and their families is that the initial skin lesion appears to be unremarkable. “Many times, this is a skin-colored or pink papule, which starts out looking very much like a molluscum or a wart or an insect bite, or something else that nobody’s worried about,” Dr. Wright said. “But over time, something happens, and the common factor is rapid growth. Time and again when I ask parents, ‘What changed? What got your attention?’ The answer is nearly always rapid growth.”
She emphasized that patients frequently arrive at the clinic with multiple opinions about their diagnosis. “It’s not unusual for a significant amount of time to pass between the initial biopsy and the final diagnosis,” she said. “Given the lack of evidence-based guidelines for children, a delay in diagnosis can make decisions about management even more difficult. Because pediatric melanoma is so rare, and there are no standard guidelines for management, there’s a major lack of consistency in terms of how patients are evaluated, treated, and followed.”
Dr. Wright said the team’s goals are to continue the biannual clinic and collect more data and tissue samples for further genomic studies on pediatric melanoma. “Ultimately, we would like to hold a consensus summit meeting of experts to develop and publish evidence-based guidelines for the management of pediatric and adolescent melanoma.”
Dr. Wright reported having no relevant disclosures.
FROM SPD 2020
Database offers snapshot of common causes of pediatric allergic contact dermatitis
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
FROM SPD 2020
Lenalidomide may be an answer for refractory cutaneous lupus
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
FROM SPD 2020
Patch testing in children: An evolving science
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
FROM SPD 2020
Nine states have no board-certified pediatric dermatologist, analysis reveals
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
FROM SPD 2020
No link between topical steroids and fracture risk found in children with atopic dermatitis
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
FROM SPD 2020
Racial differences in rates of atopic dermatitis observed early in life
, results from a single-center retrospective study found.
“Atopic dermatitis is a very common pediatric skin condition with significant morbidity for patients and their families,” lead study author Reesa L. Monir, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “Existing studies show increased disease prevalence in Black and Asian children relative to White children, with conflicting data for Hispanic children. The methodology behind many of these existing studies, however, is somewhat questionable. Many were survey-based studies asking parents to remember a diagnosis of eczema or even asking parents to just report an itchy rash and using that as a diagnosis.”
For the current study, Dr. Monir and colleagues reviewed the records of 4,016 infants born between June 1, 2011, and April 30, 2017, who were followed in the University of Florida’s health care system. The researchers defined this as having two or more well-child visits after birth and at least one visit at 300 days of life or later, and the used documentation of specific ICD-9 or ICD-10 codes to capture an objective diagnosis of atopic dermatitis (AD). Of the 4,016 patients, 39.2% were Black, 38.5% were White, 7.1% were Hispanic, 5.3% were Asian, 6.5% were from other racial backgrounds, and 3.4% were multiracial.
Dr. Monir, who is a resident in the department of dermatology at the University of Florida, Gainesville, reported that Black infants had the highest prevalence of AD at 37%, followed by Asian infants (25.8%), Hispanic infants (24.1%), multiracial infants (23%), infants from other racial backgrounds (19.1%), and non-Hispanic White infants (17.9%). Compared with White infants, the odds ratio estimates for AD was highest for Black infants (OR, 2.62), followed by Asian infants (OR, 1.55), multiracial infants (OR, 1.42), Hispanic infants (OR, 1.41), and infants from other racial backgrounds (OR, .97).
On unadjusted analysis, the following factors were significantly associated with race: delivery mode (P = .006), insurance type (P less than .001), NICU stay (P less than .001), and gestational age (P less than .0001). However, on multivariate logistic regression, only two factors were significantly associated with the diagnosis of AD: race (P less than .0001) and NICU stay (P = .0385).
“When we looked at the early childhood period specifically, we found striking racial differences in the rates of AD arising early in life,” Dr. Monir concluded. “The diagnosis was independently associated with race and NICU stay. We suggest that further investigation into these disparities and ways we can mitigate them should focus on this early childhood period.”
The researchers reported having no relevant financial disclosures.
, results from a single-center retrospective study found.
“Atopic dermatitis is a very common pediatric skin condition with significant morbidity for patients and their families,” lead study author Reesa L. Monir, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “Existing studies show increased disease prevalence in Black and Asian children relative to White children, with conflicting data for Hispanic children. The methodology behind many of these existing studies, however, is somewhat questionable. Many were survey-based studies asking parents to remember a diagnosis of eczema or even asking parents to just report an itchy rash and using that as a diagnosis.”
For the current study, Dr. Monir and colleagues reviewed the records of 4,016 infants born between June 1, 2011, and April 30, 2017, who were followed in the University of Florida’s health care system. The researchers defined this as having two or more well-child visits after birth and at least one visit at 300 days of life or later, and the used documentation of specific ICD-9 or ICD-10 codes to capture an objective diagnosis of atopic dermatitis (AD). Of the 4,016 patients, 39.2% were Black, 38.5% were White, 7.1% were Hispanic, 5.3% were Asian, 6.5% were from other racial backgrounds, and 3.4% were multiracial.
Dr. Monir, who is a resident in the department of dermatology at the University of Florida, Gainesville, reported that Black infants had the highest prevalence of AD at 37%, followed by Asian infants (25.8%), Hispanic infants (24.1%), multiracial infants (23%), infants from other racial backgrounds (19.1%), and non-Hispanic White infants (17.9%). Compared with White infants, the odds ratio estimates for AD was highest for Black infants (OR, 2.62), followed by Asian infants (OR, 1.55), multiracial infants (OR, 1.42), Hispanic infants (OR, 1.41), and infants from other racial backgrounds (OR, .97).
On unadjusted analysis, the following factors were significantly associated with race: delivery mode (P = .006), insurance type (P less than .001), NICU stay (P less than .001), and gestational age (P less than .0001). However, on multivariate logistic regression, only two factors were significantly associated with the diagnosis of AD: race (P less than .0001) and NICU stay (P = .0385).
“When we looked at the early childhood period specifically, we found striking racial differences in the rates of AD arising early in life,” Dr. Monir concluded. “The diagnosis was independently associated with race and NICU stay. We suggest that further investigation into these disparities and ways we can mitigate them should focus on this early childhood period.”
The researchers reported having no relevant financial disclosures.
, results from a single-center retrospective study found.
“Atopic dermatitis is a very common pediatric skin condition with significant morbidity for patients and their families,” lead study author Reesa L. Monir, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “Existing studies show increased disease prevalence in Black and Asian children relative to White children, with conflicting data for Hispanic children. The methodology behind many of these existing studies, however, is somewhat questionable. Many were survey-based studies asking parents to remember a diagnosis of eczema or even asking parents to just report an itchy rash and using that as a diagnosis.”
For the current study, Dr. Monir and colleagues reviewed the records of 4,016 infants born between June 1, 2011, and April 30, 2017, who were followed in the University of Florida’s health care system. The researchers defined this as having two or more well-child visits after birth and at least one visit at 300 days of life or later, and the used documentation of specific ICD-9 or ICD-10 codes to capture an objective diagnosis of atopic dermatitis (AD). Of the 4,016 patients, 39.2% were Black, 38.5% were White, 7.1% were Hispanic, 5.3% were Asian, 6.5% were from other racial backgrounds, and 3.4% were multiracial.
Dr. Monir, who is a resident in the department of dermatology at the University of Florida, Gainesville, reported that Black infants had the highest prevalence of AD at 37%, followed by Asian infants (25.8%), Hispanic infants (24.1%), multiracial infants (23%), infants from other racial backgrounds (19.1%), and non-Hispanic White infants (17.9%). Compared with White infants, the odds ratio estimates for AD was highest for Black infants (OR, 2.62), followed by Asian infants (OR, 1.55), multiracial infants (OR, 1.42), Hispanic infants (OR, 1.41), and infants from other racial backgrounds (OR, .97).
On unadjusted analysis, the following factors were significantly associated with race: delivery mode (P = .006), insurance type (P less than .001), NICU stay (P less than .001), and gestational age (P less than .0001). However, on multivariate logistic regression, only two factors were significantly associated with the diagnosis of AD: race (P less than .0001) and NICU stay (P = .0385).
“When we looked at the early childhood period specifically, we found striking racial differences in the rates of AD arising early in life,” Dr. Monir concluded. “The diagnosis was independently associated with race and NICU stay. We suggest that further investigation into these disparities and ways we can mitigate them should focus on this early childhood period.”
The researchers reported having no relevant financial disclosures.
FROM SPD 2020