User login
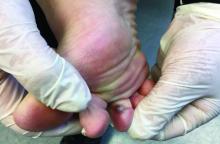
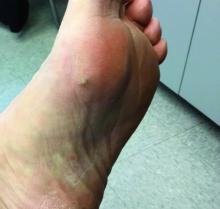
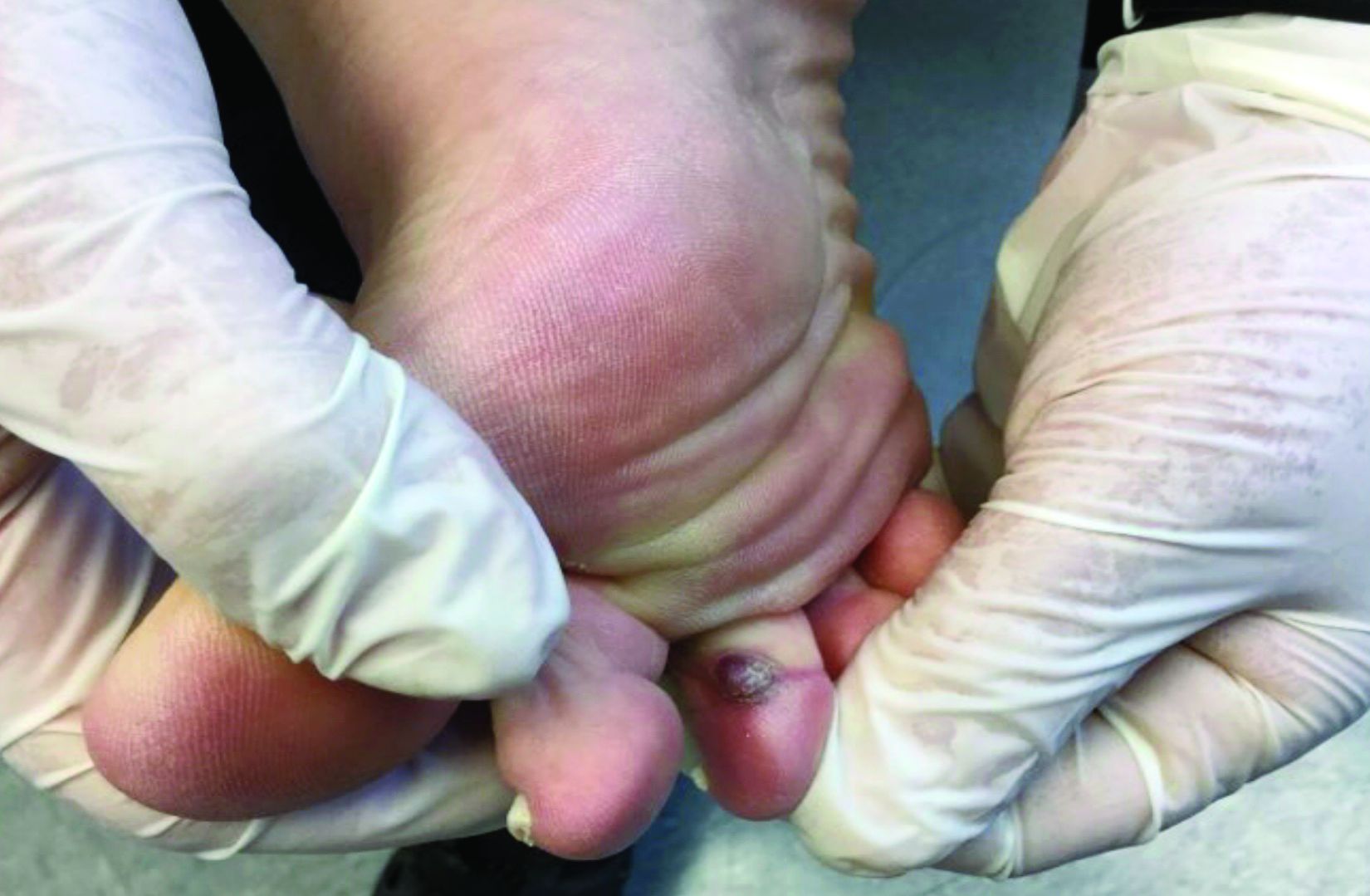
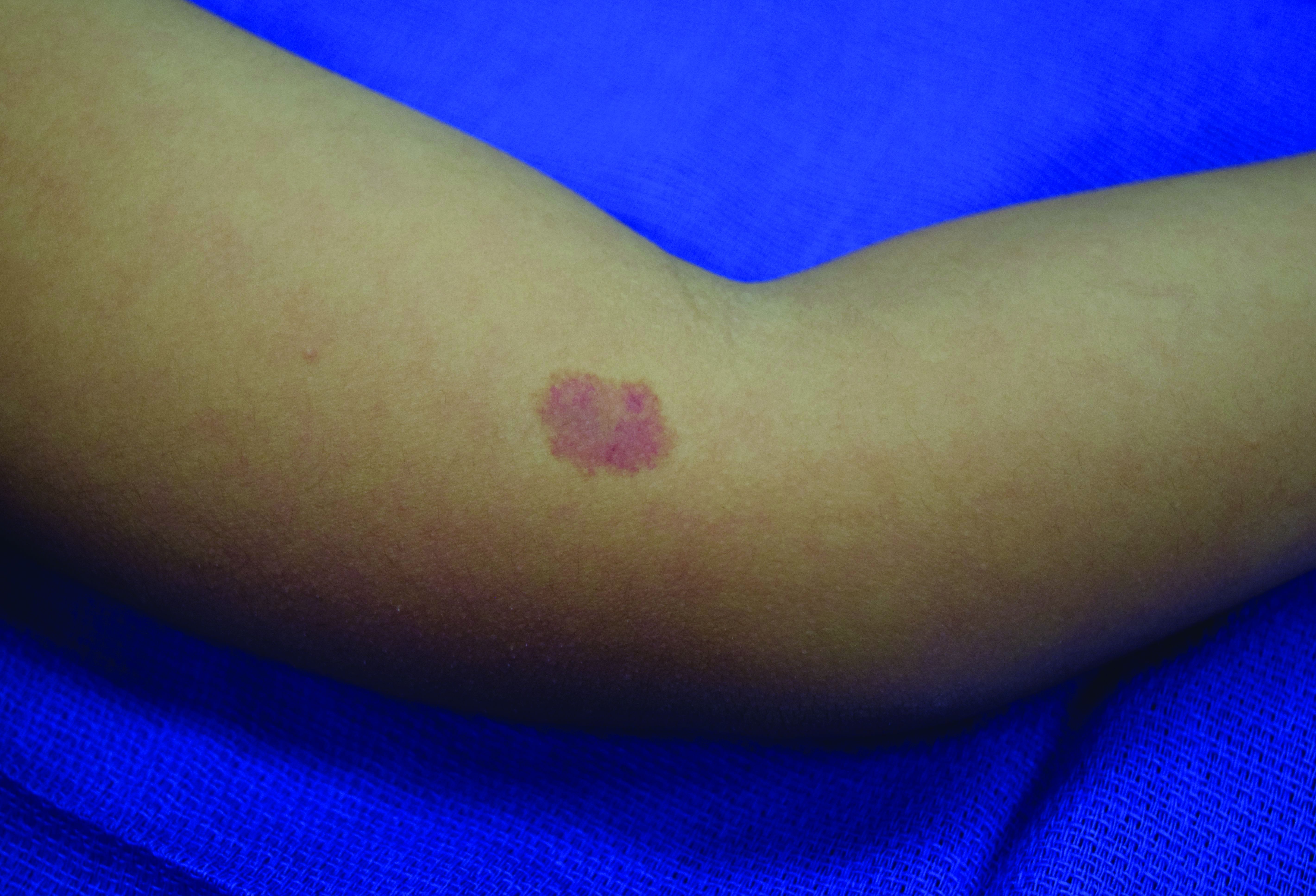
A 70-year-old presented with a 3-week history of asymptomatic violaceous papules on his feet
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Photosensitivity diagnosis made simple
of the University of Southern California, Los Angeles.
“When a patient comes in who makes you suspect a photosensitivity, there will be two different presentations,” he said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In some cases, the patient presents with a reaction they believe is sun related, although they don’t have a rash currently, he said. In other cases, “you as a good clinician suspect photosensitivity because the eruption is in a photo distribution,” although the patient may or may not relate it to sun exposure, he added.
Dr. DeLeo noted a few key points to include when taking the history in patients with likely photosensitivity, whether or not they present with a rash.
“I always ask patients when did the episode occur? Is it chronic?” Also ask about timing: Does the reaction occur in the sun, or later? Does it occur quickly and go away within hours, or occur within days or weeks of exposure?
“Always take a good drug history, as photosensitivity can often be related to drugs,” Dr. DeLeo noted. For example, approximately 50% of individuals on amiodarone will have some type of photosensitivity, he said.
Other drug-induced photosensitive conditions include drug-induced subacute cutaneous lupus and pseudoporphyria from NSAIDs, as well as hyperpigmentation from diltiazem, which most often occurs in Black women, he said.
“Photodrug reactions are usually related to UVA radiation, and that is important because you can develop it through the window while driving in your car”: The car windows do not protect against UVA, Dr. DeLeo said. If you have a patient who tells you about a photosensitivity or has a rash and they are on a photosensitizing drug, first rule out connective tissue disease, then discontinue the drug in collaboration with the patient’s internist and wait for the reaction to disappear, and it should, he said.
Some photosensitivity rashes have characteristic patterns, notably connective tissue disease patterns in lupus and dermatomyositis patients, bullous eruptions in cases of porphyria or phototoxic contact dermatitis, and eczematous eruptions, Dr. DeLeo noted.
Patients who present without a rash, but report a history of a reaction that they believe is related to sun exposure, fall into two categories: some had a rash that occurred while in the sun and disappeared quickly, and some had one that occurred hours or days after exposure and lasted a few days to weeks, said Dr. DeLeo.
The differential diagnosis in the patient with immediate photosensitivity is fairly clear: These patients usually have solar urticaria, he said. However, some lupus patients may report this reaction so it is important to rule out connective tissue disease. The diagnosis can be made with phototesting or do a simple test by having the patient sit out in the sunshine, he said.
For the patient who has a delayed reactivity after sun exposure, and doesn’t have the reaction when they come to the office, the differential diagnosis in a simply applied way is that, if the reaction spared the face, it is likely polymorphous light eruption (PMLE); but if the face is involved, the patient likely has photoallergic contact dermatitis, Dr. DeLeo explained. However, always consider the alternatives of connective tissue disease, drug reactions, and contact dermatitis that is not photoallergic, he noted.
PMLE “is the most common photosensitivity reaction that we see in the United States,” and it almost always occurs when people are away from home, usually on vacation, said Dr. DeLeo. The differential diagnosis for patients with recurrent or delayed rash involving the face could be photoallergic contact dermatitis, but rule out airborne contact dermatitis, personal care product contact dermatitis, and chronic actinic dermatitis, he said. A work-up for these patients could include a photo test, photopatch test, or patch test.
Dr. DeLeo disclosed serving as a consultant for Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
of the University of Southern California, Los Angeles.
“When a patient comes in who makes you suspect a photosensitivity, there will be two different presentations,” he said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In some cases, the patient presents with a reaction they believe is sun related, although they don’t have a rash currently, he said. In other cases, “you as a good clinician suspect photosensitivity because the eruption is in a photo distribution,” although the patient may or may not relate it to sun exposure, he added.
Dr. DeLeo noted a few key points to include when taking the history in patients with likely photosensitivity, whether or not they present with a rash.
“I always ask patients when did the episode occur? Is it chronic?” Also ask about timing: Does the reaction occur in the sun, or later? Does it occur quickly and go away within hours, or occur within days or weeks of exposure?
“Always take a good drug history, as photosensitivity can often be related to drugs,” Dr. DeLeo noted. For example, approximately 50% of individuals on amiodarone will have some type of photosensitivity, he said.
Other drug-induced photosensitive conditions include drug-induced subacute cutaneous lupus and pseudoporphyria from NSAIDs, as well as hyperpigmentation from diltiazem, which most often occurs in Black women, he said.
“Photodrug reactions are usually related to UVA radiation, and that is important because you can develop it through the window while driving in your car”: The car windows do not protect against UVA, Dr. DeLeo said. If you have a patient who tells you about a photosensitivity or has a rash and they are on a photosensitizing drug, first rule out connective tissue disease, then discontinue the drug in collaboration with the patient’s internist and wait for the reaction to disappear, and it should, he said.
Some photosensitivity rashes have characteristic patterns, notably connective tissue disease patterns in lupus and dermatomyositis patients, bullous eruptions in cases of porphyria or phototoxic contact dermatitis, and eczematous eruptions, Dr. DeLeo noted.
Patients who present without a rash, but report a history of a reaction that they believe is related to sun exposure, fall into two categories: some had a rash that occurred while in the sun and disappeared quickly, and some had one that occurred hours or days after exposure and lasted a few days to weeks, said Dr. DeLeo.
The differential diagnosis in the patient with immediate photosensitivity is fairly clear: These patients usually have solar urticaria, he said. However, some lupus patients may report this reaction so it is important to rule out connective tissue disease. The diagnosis can be made with phototesting or do a simple test by having the patient sit out in the sunshine, he said.
For the patient who has a delayed reactivity after sun exposure, and doesn’t have the reaction when they come to the office, the differential diagnosis in a simply applied way is that, if the reaction spared the face, it is likely polymorphous light eruption (PMLE); but if the face is involved, the patient likely has photoallergic contact dermatitis, Dr. DeLeo explained. However, always consider the alternatives of connective tissue disease, drug reactions, and contact dermatitis that is not photoallergic, he noted.
PMLE “is the most common photosensitivity reaction that we see in the United States,” and it almost always occurs when people are away from home, usually on vacation, said Dr. DeLeo. The differential diagnosis for patients with recurrent or delayed rash involving the face could be photoallergic contact dermatitis, but rule out airborne contact dermatitis, personal care product contact dermatitis, and chronic actinic dermatitis, he said. A work-up for these patients could include a photo test, photopatch test, or patch test.
Dr. DeLeo disclosed serving as a consultant for Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
of the University of Southern California, Los Angeles.
“When a patient comes in who makes you suspect a photosensitivity, there will be two different presentations,” he said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In some cases, the patient presents with a reaction they believe is sun related, although they don’t have a rash currently, he said. In other cases, “you as a good clinician suspect photosensitivity because the eruption is in a photo distribution,” although the patient may or may not relate it to sun exposure, he added.
Dr. DeLeo noted a few key points to include when taking the history in patients with likely photosensitivity, whether or not they present with a rash.
“I always ask patients when did the episode occur? Is it chronic?” Also ask about timing: Does the reaction occur in the sun, or later? Does it occur quickly and go away within hours, or occur within days or weeks of exposure?
“Always take a good drug history, as photosensitivity can often be related to drugs,” Dr. DeLeo noted. For example, approximately 50% of individuals on amiodarone will have some type of photosensitivity, he said.
Other drug-induced photosensitive conditions include drug-induced subacute cutaneous lupus and pseudoporphyria from NSAIDs, as well as hyperpigmentation from diltiazem, which most often occurs in Black women, he said.
“Photodrug reactions are usually related to UVA radiation, and that is important because you can develop it through the window while driving in your car”: The car windows do not protect against UVA, Dr. DeLeo said. If you have a patient who tells you about a photosensitivity or has a rash and they are on a photosensitizing drug, first rule out connective tissue disease, then discontinue the drug in collaboration with the patient’s internist and wait for the reaction to disappear, and it should, he said.
Some photosensitivity rashes have characteristic patterns, notably connective tissue disease patterns in lupus and dermatomyositis patients, bullous eruptions in cases of porphyria or phototoxic contact dermatitis, and eczematous eruptions, Dr. DeLeo noted.
Patients who present without a rash, but report a history of a reaction that they believe is related to sun exposure, fall into two categories: some had a rash that occurred while in the sun and disappeared quickly, and some had one that occurred hours or days after exposure and lasted a few days to weeks, said Dr. DeLeo.
The differential diagnosis in the patient with immediate photosensitivity is fairly clear: These patients usually have solar urticaria, he said. However, some lupus patients may report this reaction so it is important to rule out connective tissue disease. The diagnosis can be made with phototesting or do a simple test by having the patient sit out in the sunshine, he said.
For the patient who has a delayed reactivity after sun exposure, and doesn’t have the reaction when they come to the office, the differential diagnosis in a simply applied way is that, if the reaction spared the face, it is likely polymorphous light eruption (PMLE); but if the face is involved, the patient likely has photoallergic contact dermatitis, Dr. DeLeo explained. However, always consider the alternatives of connective tissue disease, drug reactions, and contact dermatitis that is not photoallergic, he noted.
PMLE “is the most common photosensitivity reaction that we see in the United States,” and it almost always occurs when people are away from home, usually on vacation, said Dr. DeLeo. The differential diagnosis for patients with recurrent or delayed rash involving the face could be photoallergic contact dermatitis, but rule out airborne contact dermatitis, personal care product contact dermatitis, and chronic actinic dermatitis, he said. A work-up for these patients could include a photo test, photopatch test, or patch test.
Dr. DeLeo disclosed serving as a consultant for Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Sunscreen myths, controversies continue
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Expert shares key facts about keloid therapy
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
GLIMMER of hope for itch in primary biliary cholangitis
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
GLIMMER of hope for itch in primary biliary cholangitis
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A 4-year-old presented to our pediatric dermatology clinic for evaluation of asymptomatic "brown spots."
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.
Systemic sclerosis patients share their perspectives and needs in treatment trials
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
FROM AN FDA PATIENT-FOCUSED DRUG DEVELOPMENT MEETING
Low IgA levels associated with increased infection risk in SLE patients
A new study of immunoglobulin levels in adult patients with systemic lupus erythematosus (SLE) has found that acquired low levels of IgA are associated with a higher risk of infection.
To the knowledge of first author Ibrahim Almaghlouth, MD, of the division of rheumatology at the University of Toronto, and colleagues, “this is the first dedicated study to examine the relationship between acquired low immunoglobulins and infection risk in adult patients with SLE.” But as to whether there may be a “protective role for immunoglobulins and the potential effect of immunoglobulin replacement in a setting of recurrent or severe infection among SLE patients requires further study.”
To determine if the risk of infection was tied to acquired low immunoglobulin levels, the researchers launched a retrospective analysis of data from a prospective cohort study of adult SLE patients from a Toronto lupus cohort that was established in 1970. The study was published in Rheumatology.
A total of 448 patients with at least two low immunoglobulin tests were matched with 656 SLE patients with no low immunoglobulins according to enrollment decade. The average age of the low-immunoglobulin group was 41.8 years, compared with 39.3 years in the control group. Average disease duration was 11.2 years in the low-immunoglobulin group and 7.6 years in the control group.
Of the patients in the low-immunoglobulin group, 221 had consecutive low tests and 227 had nonconsecutive low tests. Overall, 98 of those patients had low IgG, 251 patients had low IgM, and 51 patients had low IgA. Only 48 patients had overlapping low levels, including 5 with all three.
Average levels among the low-immunoglobulin group at baseline were 11.5 (standard deviation, 6.1) g/L of IgG, 0.8 (1.1) g/L of IgM, and 2.4 (1.6) g/L of IgA, while average levels among the control group were 16.3 (6.4) g/L of IgG, 1.8 (1.2) g/L of IgM, and 3.2 (1.5) g/L of IgA. In the primary analysis, after adjustment using propensity scoring, there were 97 infections: 47 in the low-immunoglobulin group and 50 in the control group. The most common types were respiratory and urinary tract infections, and the rate of infection was higher in patients with low IgA. The IgA level associated with risk of infection was less than 0.75 g/L.
After Cox regression analysis, the only variable that significantly increased infection risk was a low IgA level (hazard ratio, 3.19; 95% confidence interval, 1.17-8.71), not a low IgG level (HR, 1.87; 95% CI, 0.77-4.54) or low IgM level (HR, 0.63; 95% CI, 0.34-1.17). In regard to recovery among the low-immunoglobulin group, 11 patients (2.5%) recovered from low immunoglobulins within in the first year, followed by 36 (8.2%) in the second year, 44 (10.1%) in the third year, and 80 (18.4%) in the fourth year. All told, 60% (263) of patients with acquired hypogammaglobulinemia recovered over a 4-year period.
Is there clinical relevance to low IgA?
“I don’t see us using this clinically immediately,” Karen Costenbader, MD, a rheumatologist at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said in an interview. “We do test immunoglobulins often, especially in patients who’ve had biologic therapy. Will we start thinking about their IgA levels? It’s not clear, and the researchers leave it up in the air as to what this means, beyond them being at high risk.”
That said, she added, “IgA levels are interesting, especially in a time of COVID, because they’re associated with mucosal immunity. Is this subset of patients going to be at particularly high risk for the coronavirus?”
She also noted that, though immunoglobulin replacement has been helpful in her patients, it’s an expensive therapy to recommend for low IgA levels without knowing exactly what is causing these deficiencies. “My question is, would it be useful to follow these levels in lupus patients, even we don’t know what to do about them?” she asked. “We know there are a lot of risk factors for infections, so is the IgA going to be useful above and beyond that, and then what can we do about it?”
The authors acknowledged their study’s potential limitations, including low infection rates and yearly measurements of immunoglobulin levels, which could’ve led to misclassifying a lab error as true low immunoglobulin. They also highlighted its strengths, including using various methods to reduce selection and confounding bias while also reporting consistent results after examining multiple definitions of low immunoglobulins and outcomes.
The study received no specific funding, and the authors reported no potential conflicts of interest.
SOURCE: Almaghlouth I et al. Rheumatology. 2020 Oct 2. doi: 10.1093/rheumatology/keaa641.
A new study of immunoglobulin levels in adult patients with systemic lupus erythematosus (SLE) has found that acquired low levels of IgA are associated with a higher risk of infection.
To the knowledge of first author Ibrahim Almaghlouth, MD, of the division of rheumatology at the University of Toronto, and colleagues, “this is the first dedicated study to examine the relationship between acquired low immunoglobulins and infection risk in adult patients with SLE.” But as to whether there may be a “protective role for immunoglobulins and the potential effect of immunoglobulin replacement in a setting of recurrent or severe infection among SLE patients requires further study.”
To determine if the risk of infection was tied to acquired low immunoglobulin levels, the researchers launched a retrospective analysis of data from a prospective cohort study of adult SLE patients from a Toronto lupus cohort that was established in 1970. The study was published in Rheumatology.
A total of 448 patients with at least two low immunoglobulin tests were matched with 656 SLE patients with no low immunoglobulins according to enrollment decade. The average age of the low-immunoglobulin group was 41.8 years, compared with 39.3 years in the control group. Average disease duration was 11.2 years in the low-immunoglobulin group and 7.6 years in the control group.
Of the patients in the low-immunoglobulin group, 221 had consecutive low tests and 227 had nonconsecutive low tests. Overall, 98 of those patients had low IgG, 251 patients had low IgM, and 51 patients had low IgA. Only 48 patients had overlapping low levels, including 5 with all three.
Average levels among the low-immunoglobulin group at baseline were 11.5 (standard deviation, 6.1) g/L of IgG, 0.8 (1.1) g/L of IgM, and 2.4 (1.6) g/L of IgA, while average levels among the control group were 16.3 (6.4) g/L of IgG, 1.8 (1.2) g/L of IgM, and 3.2 (1.5) g/L of IgA. In the primary analysis, after adjustment using propensity scoring, there were 97 infections: 47 in the low-immunoglobulin group and 50 in the control group. The most common types were respiratory and urinary tract infections, and the rate of infection was higher in patients with low IgA. The IgA level associated with risk of infection was less than 0.75 g/L.
After Cox regression analysis, the only variable that significantly increased infection risk was a low IgA level (hazard ratio, 3.19; 95% confidence interval, 1.17-8.71), not a low IgG level (HR, 1.87; 95% CI, 0.77-4.54) or low IgM level (HR, 0.63; 95% CI, 0.34-1.17). In regard to recovery among the low-immunoglobulin group, 11 patients (2.5%) recovered from low immunoglobulins within in the first year, followed by 36 (8.2%) in the second year, 44 (10.1%) in the third year, and 80 (18.4%) in the fourth year. All told, 60% (263) of patients with acquired hypogammaglobulinemia recovered over a 4-year period.
Is there clinical relevance to low IgA?
“I don’t see us using this clinically immediately,” Karen Costenbader, MD, a rheumatologist at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said in an interview. “We do test immunoglobulins often, especially in patients who’ve had biologic therapy. Will we start thinking about their IgA levels? It’s not clear, and the researchers leave it up in the air as to what this means, beyond them being at high risk.”
That said, she added, “IgA levels are interesting, especially in a time of COVID, because they’re associated with mucosal immunity. Is this subset of patients going to be at particularly high risk for the coronavirus?”
She also noted that, though immunoglobulin replacement has been helpful in her patients, it’s an expensive therapy to recommend for low IgA levels without knowing exactly what is causing these deficiencies. “My question is, would it be useful to follow these levels in lupus patients, even we don’t know what to do about them?” she asked. “We know there are a lot of risk factors for infections, so is the IgA going to be useful above and beyond that, and then what can we do about it?”
The authors acknowledged their study’s potential limitations, including low infection rates and yearly measurements of immunoglobulin levels, which could’ve led to misclassifying a lab error as true low immunoglobulin. They also highlighted its strengths, including using various methods to reduce selection and confounding bias while also reporting consistent results after examining multiple definitions of low immunoglobulins and outcomes.
The study received no specific funding, and the authors reported no potential conflicts of interest.
SOURCE: Almaghlouth I et al. Rheumatology. 2020 Oct 2. doi: 10.1093/rheumatology/keaa641.
A new study of immunoglobulin levels in adult patients with systemic lupus erythematosus (SLE) has found that acquired low levels of IgA are associated with a higher risk of infection.
To the knowledge of first author Ibrahim Almaghlouth, MD, of the division of rheumatology at the University of Toronto, and colleagues, “this is the first dedicated study to examine the relationship between acquired low immunoglobulins and infection risk in adult patients with SLE.” But as to whether there may be a “protective role for immunoglobulins and the potential effect of immunoglobulin replacement in a setting of recurrent or severe infection among SLE patients requires further study.”
To determine if the risk of infection was tied to acquired low immunoglobulin levels, the researchers launched a retrospective analysis of data from a prospective cohort study of adult SLE patients from a Toronto lupus cohort that was established in 1970. The study was published in Rheumatology.
A total of 448 patients with at least two low immunoglobulin tests were matched with 656 SLE patients with no low immunoglobulins according to enrollment decade. The average age of the low-immunoglobulin group was 41.8 years, compared with 39.3 years in the control group. Average disease duration was 11.2 years in the low-immunoglobulin group and 7.6 years in the control group.
Of the patients in the low-immunoglobulin group, 221 had consecutive low tests and 227 had nonconsecutive low tests. Overall, 98 of those patients had low IgG, 251 patients had low IgM, and 51 patients had low IgA. Only 48 patients had overlapping low levels, including 5 with all three.
Average levels among the low-immunoglobulin group at baseline were 11.5 (standard deviation, 6.1) g/L of IgG, 0.8 (1.1) g/L of IgM, and 2.4 (1.6) g/L of IgA, while average levels among the control group were 16.3 (6.4) g/L of IgG, 1.8 (1.2) g/L of IgM, and 3.2 (1.5) g/L of IgA. In the primary analysis, after adjustment using propensity scoring, there were 97 infections: 47 in the low-immunoglobulin group and 50 in the control group. The most common types were respiratory and urinary tract infections, and the rate of infection was higher in patients with low IgA. The IgA level associated with risk of infection was less than 0.75 g/L.
After Cox regression analysis, the only variable that significantly increased infection risk was a low IgA level (hazard ratio, 3.19; 95% confidence interval, 1.17-8.71), not a low IgG level (HR, 1.87; 95% CI, 0.77-4.54) or low IgM level (HR, 0.63; 95% CI, 0.34-1.17). In regard to recovery among the low-immunoglobulin group, 11 patients (2.5%) recovered from low immunoglobulins within in the first year, followed by 36 (8.2%) in the second year, 44 (10.1%) in the third year, and 80 (18.4%) in the fourth year. All told, 60% (263) of patients with acquired hypogammaglobulinemia recovered over a 4-year period.
Is there clinical relevance to low IgA?
“I don’t see us using this clinically immediately,” Karen Costenbader, MD, a rheumatologist at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said in an interview. “We do test immunoglobulins often, especially in patients who’ve had biologic therapy. Will we start thinking about their IgA levels? It’s not clear, and the researchers leave it up in the air as to what this means, beyond them being at high risk.”
That said, she added, “IgA levels are interesting, especially in a time of COVID, because they’re associated with mucosal immunity. Is this subset of patients going to be at particularly high risk for the coronavirus?”
She also noted that, though immunoglobulin replacement has been helpful in her patients, it’s an expensive therapy to recommend for low IgA levels without knowing exactly what is causing these deficiencies. “My question is, would it be useful to follow these levels in lupus patients, even we don’t know what to do about them?” she asked. “We know there are a lot of risk factors for infections, so is the IgA going to be useful above and beyond that, and then what can we do about it?”
The authors acknowledged their study’s potential limitations, including low infection rates and yearly measurements of immunoglobulin levels, which could’ve led to misclassifying a lab error as true low immunoglobulin. They also highlighted its strengths, including using various methods to reduce selection and confounding bias while also reporting consistent results after examining multiple definitions of low immunoglobulins and outcomes.
The study received no specific funding, and the authors reported no potential conflicts of interest.
SOURCE: Almaghlouth I et al. Rheumatology. 2020 Oct 2. doi: 10.1093/rheumatology/keaa641.
FROM RHEUMATOLOGY
New technologies show promise for treating pigmented lesions
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
FROM MOA 2020