User login
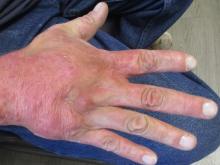
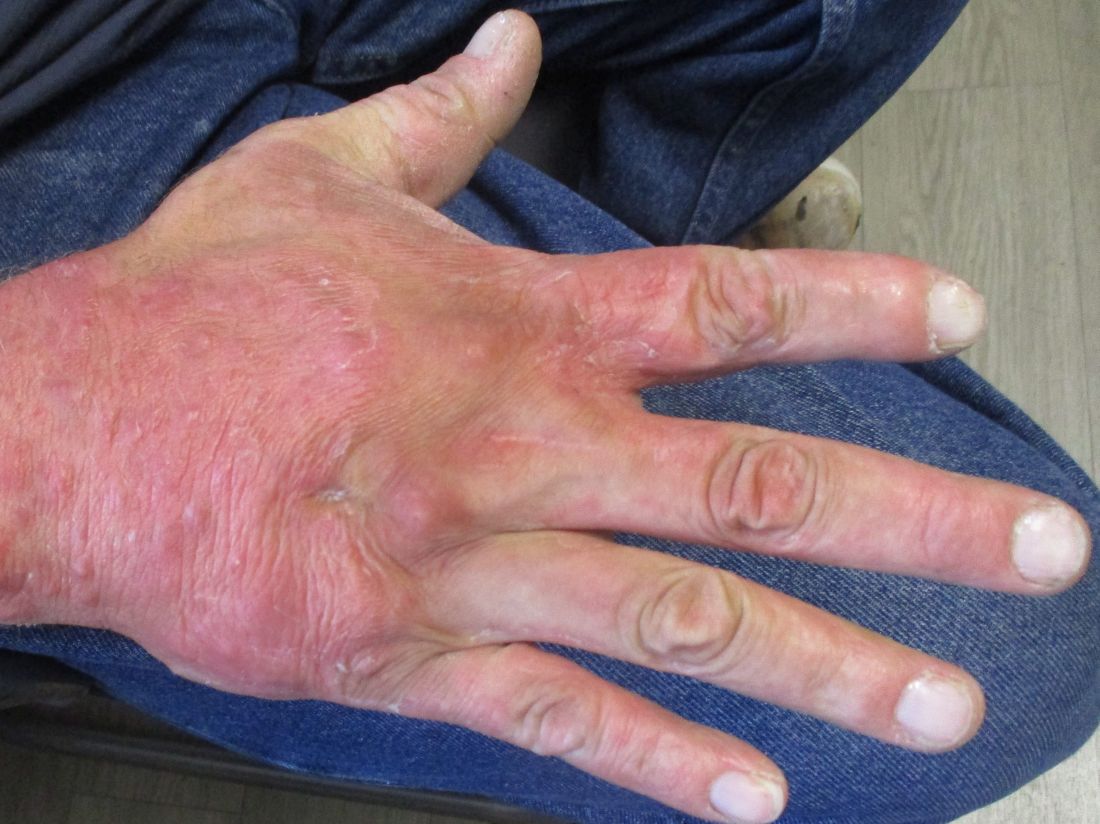
A 36-year-old presents with a mildly pruritic rash consisting of pink papules on his hand
. MG is a dermatophytic folliculitis that classically presents as folliculocentric plaque, in which there are papules, pustules, and nodules, usually found on the lower leg and almost exclusively in adults.1 Wrists are commonly affected as well.
MG is typically caused by mechanical disruption of hair follicles that allows fungi to penetrate deep into dermal tissue.2 Quite often, the source of infection is typically the patient’s skin or nails. Associated risk factors include longstanding fungal infection, shaving or other cutaneous trauma, topical steroids, and immunosuppressive therapy.3,4 Although MG can be caused by other fungal species, it is most often caused by Trichophyton rubrum or Trichophyton tonsurans.1 There are two types of MG, the perifollicular papular form, which is localized and typically occurs in healthy individuals, and the deep subcutaneous plaque or nodular forms that usually occur in immunocompromised individuals.5
MG is an important clinical manifestation to be familiar with because of the increase in the numbers of solid-organ transplants and patients on immunosuppressive therapies. These patients are highly predisposed to opportunistic infections with aggressive clinical courses and will usually require prolonged treatment as relapses are common.3,5
Tissue culture and skin biopsy are often needed to establish the diagnosis. If a topical antifungal has been used, KOH (potassium hydroxide) and culture may be negative. This patient’s tissue culture was positive for T. rubrum. The histopathology revealed hyperkeratosis and acanthosis with focal parakeratosis and a lymphohistiocytic infiltrate in the dermis. On PAS (Periodic acid–Schiff ) stain, PAS-positive hyphae were identified in the keratin layer, confirming a diagnosis of tinea infection.
First line treatment includes systemic antifungals such as griseofulvin, ketoconazole, itraconazole, and terbinafine. Duration of therapy is typically 4-8 weeks or until all lesions are cleared.3,5
This case and photo were submitted by Mr. Hakimi of University of California San Diego School of Medicine and Dr. Sateesh of San Diego Family Dermatology. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1.“Fitzpatrick’s Dermatology in General Medicine” (New York: McGraw-Hill Medical, 2012).
2. Bonifaz A et al. Gac Med Mex. Sep-Oct 2008;144(5):427-33.
3. Romero FA et al. Transpl Infect Dis. 2011 Aug;13(4):424-3. doi:10.1111/j.1399-3062.2010.00596.x
4. Chou WY, Hsu CJ. Medicine (Baltimore). 2016 Jan;95(2):e2245. doi: 10.1097/MD.0000000000002245.
5. Ilkit M et al. Med Mycol. 2102 Jul;50(5):449-57.
. MG is a dermatophytic folliculitis that classically presents as folliculocentric plaque, in which there are papules, pustules, and nodules, usually found on the lower leg and almost exclusively in adults.1 Wrists are commonly affected as well.
MG is typically caused by mechanical disruption of hair follicles that allows fungi to penetrate deep into dermal tissue.2 Quite often, the source of infection is typically the patient’s skin or nails. Associated risk factors include longstanding fungal infection, shaving or other cutaneous trauma, topical steroids, and immunosuppressive therapy.3,4 Although MG can be caused by other fungal species, it is most often caused by Trichophyton rubrum or Trichophyton tonsurans.1 There are two types of MG, the perifollicular papular form, which is localized and typically occurs in healthy individuals, and the deep subcutaneous plaque or nodular forms that usually occur in immunocompromised individuals.5
MG is an important clinical manifestation to be familiar with because of the increase in the numbers of solid-organ transplants and patients on immunosuppressive therapies. These patients are highly predisposed to opportunistic infections with aggressive clinical courses and will usually require prolonged treatment as relapses are common.3,5
Tissue culture and skin biopsy are often needed to establish the diagnosis. If a topical antifungal has been used, KOH (potassium hydroxide) and culture may be negative. This patient’s tissue culture was positive for T. rubrum. The histopathology revealed hyperkeratosis and acanthosis with focal parakeratosis and a lymphohistiocytic infiltrate in the dermis. On PAS (Periodic acid–Schiff ) stain, PAS-positive hyphae were identified in the keratin layer, confirming a diagnosis of tinea infection.
First line treatment includes systemic antifungals such as griseofulvin, ketoconazole, itraconazole, and terbinafine. Duration of therapy is typically 4-8 weeks or until all lesions are cleared.3,5
This case and photo were submitted by Mr. Hakimi of University of California San Diego School of Medicine and Dr. Sateesh of San Diego Family Dermatology. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1.“Fitzpatrick’s Dermatology in General Medicine” (New York: McGraw-Hill Medical, 2012).
2. Bonifaz A et al. Gac Med Mex. Sep-Oct 2008;144(5):427-33.
3. Romero FA et al. Transpl Infect Dis. 2011 Aug;13(4):424-3. doi:10.1111/j.1399-3062.2010.00596.x
4. Chou WY, Hsu CJ. Medicine (Baltimore). 2016 Jan;95(2):e2245. doi: 10.1097/MD.0000000000002245.
5. Ilkit M et al. Med Mycol. 2102 Jul;50(5):449-57.
. MG is a dermatophytic folliculitis that classically presents as folliculocentric plaque, in which there are papules, pustules, and nodules, usually found on the lower leg and almost exclusively in adults.1 Wrists are commonly affected as well.
MG is typically caused by mechanical disruption of hair follicles that allows fungi to penetrate deep into dermal tissue.2 Quite often, the source of infection is typically the patient’s skin or nails. Associated risk factors include longstanding fungal infection, shaving or other cutaneous trauma, topical steroids, and immunosuppressive therapy.3,4 Although MG can be caused by other fungal species, it is most often caused by Trichophyton rubrum or Trichophyton tonsurans.1 There are two types of MG, the perifollicular papular form, which is localized and typically occurs in healthy individuals, and the deep subcutaneous plaque or nodular forms that usually occur in immunocompromised individuals.5
MG is an important clinical manifestation to be familiar with because of the increase in the numbers of solid-organ transplants and patients on immunosuppressive therapies. These patients are highly predisposed to opportunistic infections with aggressive clinical courses and will usually require prolonged treatment as relapses are common.3,5
Tissue culture and skin biopsy are often needed to establish the diagnosis. If a topical antifungal has been used, KOH (potassium hydroxide) and culture may be negative. This patient’s tissue culture was positive for T. rubrum. The histopathology revealed hyperkeratosis and acanthosis with focal parakeratosis and a lymphohistiocytic infiltrate in the dermis. On PAS (Periodic acid–Schiff ) stain, PAS-positive hyphae were identified in the keratin layer, confirming a diagnosis of tinea infection.
First line treatment includes systemic antifungals such as griseofulvin, ketoconazole, itraconazole, and terbinafine. Duration of therapy is typically 4-8 weeks or until all lesions are cleared.3,5
This case and photo were submitted by Mr. Hakimi of University of California San Diego School of Medicine and Dr. Sateesh of San Diego Family Dermatology. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1.“Fitzpatrick’s Dermatology in General Medicine” (New York: McGraw-Hill Medical, 2012).
2. Bonifaz A et al. Gac Med Mex. Sep-Oct 2008;144(5):427-33.
3. Romero FA et al. Transpl Infect Dis. 2011 Aug;13(4):424-3. doi:10.1111/j.1399-3062.2010.00596.x
4. Chou WY, Hsu CJ. Medicine (Baltimore). 2016 Jan;95(2):e2245. doi: 10.1097/MD.0000000000002245.
5. Ilkit M et al. Med Mycol. 2102 Jul;50(5):449-57.
Most clinicians undertreat childhood lichen sclerosus
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
FROM SPD 2020
Cachexia affects more than half of lupus patients
Cachexia developed in 56% of adults with systemic lupus erythematosus over a 5-year period, and 18% did not recover their weight, based on data from more than 2,000 patients.
Although weight loss is common in patients with systemic lupus erythematosus (SLE), cachexia, a disorder of involuntary weight loss, is largely undescribed in SLE patients, wrote George Stojan, MD, of Johns Hopkins University, Baltimore, and colleagues. Cachexia has been described in a range of disorders, including heart failure, renal disease, and rheumatoid arthritis, they said. “Cachexia has been shown to lead to progressive functional impairment, treatment-related complications, poor quality of life, and increased mortality,” they added.
In a study published in Arthritis Care & Research, the investigators reviewed data from the Hopkins Lupus Cohort, consisting of all SLE patients seen at a single center who are followed at least quarterly.
The study population included 2,452 SLE patients older than 18 years who had their weight assessed at each clinic visit. The average follow-up period was 7.75 years, and the average number of weight measurements per patient was nearly 24.
Cachexia was defined as a 5% stable weight loss in 6 months without starvation relative to the average weight in all prior cohort visits; and/or weight loss of more than 2% without starvation relative to the average weight in all prior cohort visits in addition to a body mass index less than 20 kg/m2.
Overall, the risk for cachexia within 5 years of entering the study was significantly higher in patients with a BMI less than 20, current steroid use, vasculitis, lupus nephritis, serositis, hematologic lupus, positive anti-double strand DNA (anti-dsDNA), anti-Smith (anti-Sm), and antiribonucleoprotein (anti-RNP), the researchers noted. After adjustment for prednisone use, cachexia remained significantly associated with lupus nephritis, vasculitis, serositis, and hematologic lupus.
Future organ damage including cataracts, retinal change or optic atrophy, cognitive impairment, cerebrovascular accidents, cranial or peripheral neuropathy, pulmonary hypertension, pleural fibrosis, angina or coronary bypass, bowel infarction or resection, osteoporosis, avascular necrosis, and premature gonadal failure were significantly more likely among patients with intermittent cachexia, compared with those with continuous or no cachexia. Patients with continuous cachexia were significantly more likely to experience an estimated glomerular filtration rate less than 50 mL/min/1.73 m2, proteinuria greater than 3.5 g/day, and end-stage renal disease.
The patients who never developed cachexia were significantly less likely to develop malignancies, diabetes, valvular disease, or cardiomyopathy than were those who did have cachexia, the researchers said.
The mechanisms of action for cachexia in SLE remain unclear, but studies in cancer patients may provide some guidance, the researchers noted. “Tumors secrete a range of procachexia factors thought to be unique to cancer-related cachexia, and colloquially termed the ‘tumor secretome,’ ” they said. “Every single proinflammatory cytokine mentioned as part of the tumor secretome has a role in lupus pathogenesis,” suggesting a possible common pathway to cachexia across different diseases, they said.
The study findings were limited by several factors, mainly the use of BMI to measure weight “since BMI is a rather poor indicator of percent of body fat,” the researchers noted. “Ideally, cachexia would be defined as sarcopenia based on body composition evaluation with a dual x-ray absorptiometry,” they wrote.
The study was supported by the National Institutes of Health and the NIH Roadmap for Medical Research. The researchers had no financial conflicts to disclose.
SOURCE: Stojan G et al. Arthritis Care Res. 2020 Aug 2. doi: 10.1002/acr.24395.
Cachexia developed in 56% of adults with systemic lupus erythematosus over a 5-year period, and 18% did not recover their weight, based on data from more than 2,000 patients.
Although weight loss is common in patients with systemic lupus erythematosus (SLE), cachexia, a disorder of involuntary weight loss, is largely undescribed in SLE patients, wrote George Stojan, MD, of Johns Hopkins University, Baltimore, and colleagues. Cachexia has been described in a range of disorders, including heart failure, renal disease, and rheumatoid arthritis, they said. “Cachexia has been shown to lead to progressive functional impairment, treatment-related complications, poor quality of life, and increased mortality,” they added.
In a study published in Arthritis Care & Research, the investigators reviewed data from the Hopkins Lupus Cohort, consisting of all SLE patients seen at a single center who are followed at least quarterly.
The study population included 2,452 SLE patients older than 18 years who had their weight assessed at each clinic visit. The average follow-up period was 7.75 years, and the average number of weight measurements per patient was nearly 24.
Cachexia was defined as a 5% stable weight loss in 6 months without starvation relative to the average weight in all prior cohort visits; and/or weight loss of more than 2% without starvation relative to the average weight in all prior cohort visits in addition to a body mass index less than 20 kg/m2.
Overall, the risk for cachexia within 5 years of entering the study was significantly higher in patients with a BMI less than 20, current steroid use, vasculitis, lupus nephritis, serositis, hematologic lupus, positive anti-double strand DNA (anti-dsDNA), anti-Smith (anti-Sm), and antiribonucleoprotein (anti-RNP), the researchers noted. After adjustment for prednisone use, cachexia remained significantly associated with lupus nephritis, vasculitis, serositis, and hematologic lupus.
Future organ damage including cataracts, retinal change or optic atrophy, cognitive impairment, cerebrovascular accidents, cranial or peripheral neuropathy, pulmonary hypertension, pleural fibrosis, angina or coronary bypass, bowel infarction or resection, osteoporosis, avascular necrosis, and premature gonadal failure were significantly more likely among patients with intermittent cachexia, compared with those with continuous or no cachexia. Patients with continuous cachexia were significantly more likely to experience an estimated glomerular filtration rate less than 50 mL/min/1.73 m2, proteinuria greater than 3.5 g/day, and end-stage renal disease.
The patients who never developed cachexia were significantly less likely to develop malignancies, diabetes, valvular disease, or cardiomyopathy than were those who did have cachexia, the researchers said.
The mechanisms of action for cachexia in SLE remain unclear, but studies in cancer patients may provide some guidance, the researchers noted. “Tumors secrete a range of procachexia factors thought to be unique to cancer-related cachexia, and colloquially termed the ‘tumor secretome,’ ” they said. “Every single proinflammatory cytokine mentioned as part of the tumor secretome has a role in lupus pathogenesis,” suggesting a possible common pathway to cachexia across different diseases, they said.
The study findings were limited by several factors, mainly the use of BMI to measure weight “since BMI is a rather poor indicator of percent of body fat,” the researchers noted. “Ideally, cachexia would be defined as sarcopenia based on body composition evaluation with a dual x-ray absorptiometry,” they wrote.
The study was supported by the National Institutes of Health and the NIH Roadmap for Medical Research. The researchers had no financial conflicts to disclose.
SOURCE: Stojan G et al. Arthritis Care Res. 2020 Aug 2. doi: 10.1002/acr.24395.
Cachexia developed in 56% of adults with systemic lupus erythematosus over a 5-year period, and 18% did not recover their weight, based on data from more than 2,000 patients.
Although weight loss is common in patients with systemic lupus erythematosus (SLE), cachexia, a disorder of involuntary weight loss, is largely undescribed in SLE patients, wrote George Stojan, MD, of Johns Hopkins University, Baltimore, and colleagues. Cachexia has been described in a range of disorders, including heart failure, renal disease, and rheumatoid arthritis, they said. “Cachexia has been shown to lead to progressive functional impairment, treatment-related complications, poor quality of life, and increased mortality,” they added.
In a study published in Arthritis Care & Research, the investigators reviewed data from the Hopkins Lupus Cohort, consisting of all SLE patients seen at a single center who are followed at least quarterly.
The study population included 2,452 SLE patients older than 18 years who had their weight assessed at each clinic visit. The average follow-up period was 7.75 years, and the average number of weight measurements per patient was nearly 24.
Cachexia was defined as a 5% stable weight loss in 6 months without starvation relative to the average weight in all prior cohort visits; and/or weight loss of more than 2% without starvation relative to the average weight in all prior cohort visits in addition to a body mass index less than 20 kg/m2.
Overall, the risk for cachexia within 5 years of entering the study was significantly higher in patients with a BMI less than 20, current steroid use, vasculitis, lupus nephritis, serositis, hematologic lupus, positive anti-double strand DNA (anti-dsDNA), anti-Smith (anti-Sm), and antiribonucleoprotein (anti-RNP), the researchers noted. After adjustment for prednisone use, cachexia remained significantly associated with lupus nephritis, vasculitis, serositis, and hematologic lupus.
Future organ damage including cataracts, retinal change or optic atrophy, cognitive impairment, cerebrovascular accidents, cranial or peripheral neuropathy, pulmonary hypertension, pleural fibrosis, angina or coronary bypass, bowel infarction or resection, osteoporosis, avascular necrosis, and premature gonadal failure were significantly more likely among patients with intermittent cachexia, compared with those with continuous or no cachexia. Patients with continuous cachexia were significantly more likely to experience an estimated glomerular filtration rate less than 50 mL/min/1.73 m2, proteinuria greater than 3.5 g/day, and end-stage renal disease.
The patients who never developed cachexia were significantly less likely to develop malignancies, diabetes, valvular disease, or cardiomyopathy than were those who did have cachexia, the researchers said.
The mechanisms of action for cachexia in SLE remain unclear, but studies in cancer patients may provide some guidance, the researchers noted. “Tumors secrete a range of procachexia factors thought to be unique to cancer-related cachexia, and colloquially termed the ‘tumor secretome,’ ” they said. “Every single proinflammatory cytokine mentioned as part of the tumor secretome has a role in lupus pathogenesis,” suggesting a possible common pathway to cachexia across different diseases, they said.
The study findings were limited by several factors, mainly the use of BMI to measure weight “since BMI is a rather poor indicator of percent of body fat,” the researchers noted. “Ideally, cachexia would be defined as sarcopenia based on body composition evaluation with a dual x-ray absorptiometry,” they wrote.
The study was supported by the National Institutes of Health and the NIH Roadmap for Medical Research. The researchers had no financial conflicts to disclose.
SOURCE: Stojan G et al. Arthritis Care Res. 2020 Aug 2. doi: 10.1002/acr.24395.
FROM ARTHRITIS CARE & RESEARCH
Dermatology atlas will profile disease in all skin types
An atlas that displays the unique nuances between different skin types across the spectrum of dermatologic disease is scheduled for release this coming winter.
Available as an e-book or physical text, Dermatologists need to know what skin diseases look like on all types of skin, said Adam Friedman, MD, who is developing this e-book with Misty Eleryan, MD, MS.
From the SARS-CoV-2 pandemic, multiple nonviral pandemics have rapidly emerged, “most notably the persistent and well-masked racism that maintains disparities in all facets of life, from economics to health care,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview.
In dermatology, “clear disparities in workforce representation and trainee/practitioner education have become more apparent than ever before,” he added.
The project is a collaboration of George Washington University and education publishers Sanova Works and Educational Testing & Assessment Systems.
As a person of color who recently completed her residency in dermatology at George Washington University, Dr. Eleryan had noticed a lack of diversity in photos of common dermatoses. This can contribute to misdiagnoses and delays in treatment in patients of color, Dr. Eleryan, who is now a micrographic surgery and dermatologic oncology fellow at the University of California, Los Angeles, said in an interview.
“We recognized the gap, which is the lack of diversity/variation of skin tones in our dermatology textbooks and atlases,” she added.
The project was several years in the making, Dr. Friedman said. To do this right, “you need resources, funding, and a collaborative and galvanized team of experts.” That involved coordinating with several medical publishers and amassing a team of medical photographers and an expert panel that will assist in evaluating and securing difficult-to-access clinical images.
The atlas is one of several initiatives in the dermatology field to address racial disparities in patient care.
Noticing similar information gaps about clinical presentations in darker skin, a medical student in the United Kingdom, Malone Mukwende, created “Mind the Gap,” a handbook that presents side-by-side images of diseases and illnesses in light and dark skin. This project “highlights how far behind we are, that a medical student with minimal dermatology exposure and experience not only recognized the need but was ready to do something about it,” Dr. Friedman noted.
Others in the field have spotlighted disparities in the medical literature. The SARS-CoV-2 pandemic has especially brought this out, Graeme M. Lipper, MD wrote in a recent editorial for this news organization.
He referred to a literature review of 46 articles describing COVID-19–associated skin manifestations, which included mostly (92%) images in patients with skin types I-III (92%) and none in patients with skin types V or VI.
“These investigators have identified a damning lack of images of COVID-19–associated skin manifestations in patients with darker skin,” added Dr. Lipper, an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital.
For now, Dr. Friedman said that the atlas won’t contain a specific section on COVID-19 skin manifestations, although viral-associated skin reactions like morbilliform eruptions, urticaria, and retiform purpura will be displayed. Overall, the atlas will address 60-70 skin conditions.
Physicians who fail to educate themselves on the variations of skin conditions in all skin types may potentially harm patients of color, Dr. Eleryan said. As Dr. Lipper noted in his editorial, nearly half of all dermatologists feel they haven’t had adequate exposure to diseases in skin of color.
“Our atlas will fill that void and hopefully assist in closing the gap in health disparities among patients of color, who are often misdiagnosed or rendered diagnoses very late in the disease process,” Dr. Eleryan said.
An atlas that displays the unique nuances between different skin types across the spectrum of dermatologic disease is scheduled for release this coming winter.
Available as an e-book or physical text, Dermatologists need to know what skin diseases look like on all types of skin, said Adam Friedman, MD, who is developing this e-book with Misty Eleryan, MD, MS.
From the SARS-CoV-2 pandemic, multiple nonviral pandemics have rapidly emerged, “most notably the persistent and well-masked racism that maintains disparities in all facets of life, from economics to health care,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview.
In dermatology, “clear disparities in workforce representation and trainee/practitioner education have become more apparent than ever before,” he added.
The project is a collaboration of George Washington University and education publishers Sanova Works and Educational Testing & Assessment Systems.
As a person of color who recently completed her residency in dermatology at George Washington University, Dr. Eleryan had noticed a lack of diversity in photos of common dermatoses. This can contribute to misdiagnoses and delays in treatment in patients of color, Dr. Eleryan, who is now a micrographic surgery and dermatologic oncology fellow at the University of California, Los Angeles, said in an interview.
“We recognized the gap, which is the lack of diversity/variation of skin tones in our dermatology textbooks and atlases,” she added.
The project was several years in the making, Dr. Friedman said. To do this right, “you need resources, funding, and a collaborative and galvanized team of experts.” That involved coordinating with several medical publishers and amassing a team of medical photographers and an expert panel that will assist in evaluating and securing difficult-to-access clinical images.
The atlas is one of several initiatives in the dermatology field to address racial disparities in patient care.
Noticing similar information gaps about clinical presentations in darker skin, a medical student in the United Kingdom, Malone Mukwende, created “Mind the Gap,” a handbook that presents side-by-side images of diseases and illnesses in light and dark skin. This project “highlights how far behind we are, that a medical student with minimal dermatology exposure and experience not only recognized the need but was ready to do something about it,” Dr. Friedman noted.
Others in the field have spotlighted disparities in the medical literature. The SARS-CoV-2 pandemic has especially brought this out, Graeme M. Lipper, MD wrote in a recent editorial for this news organization.
He referred to a literature review of 46 articles describing COVID-19–associated skin manifestations, which included mostly (92%) images in patients with skin types I-III (92%) and none in patients with skin types V or VI.
“These investigators have identified a damning lack of images of COVID-19–associated skin manifestations in patients with darker skin,” added Dr. Lipper, an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital.
For now, Dr. Friedman said that the atlas won’t contain a specific section on COVID-19 skin manifestations, although viral-associated skin reactions like morbilliform eruptions, urticaria, and retiform purpura will be displayed. Overall, the atlas will address 60-70 skin conditions.
Physicians who fail to educate themselves on the variations of skin conditions in all skin types may potentially harm patients of color, Dr. Eleryan said. As Dr. Lipper noted in his editorial, nearly half of all dermatologists feel they haven’t had adequate exposure to diseases in skin of color.
“Our atlas will fill that void and hopefully assist in closing the gap in health disparities among patients of color, who are often misdiagnosed or rendered diagnoses very late in the disease process,” Dr. Eleryan said.
An atlas that displays the unique nuances between different skin types across the spectrum of dermatologic disease is scheduled for release this coming winter.
Available as an e-book or physical text, Dermatologists need to know what skin diseases look like on all types of skin, said Adam Friedman, MD, who is developing this e-book with Misty Eleryan, MD, MS.
From the SARS-CoV-2 pandemic, multiple nonviral pandemics have rapidly emerged, “most notably the persistent and well-masked racism that maintains disparities in all facets of life, from economics to health care,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview.
In dermatology, “clear disparities in workforce representation and trainee/practitioner education have become more apparent than ever before,” he added.
The project is a collaboration of George Washington University and education publishers Sanova Works and Educational Testing & Assessment Systems.
As a person of color who recently completed her residency in dermatology at George Washington University, Dr. Eleryan had noticed a lack of diversity in photos of common dermatoses. This can contribute to misdiagnoses and delays in treatment in patients of color, Dr. Eleryan, who is now a micrographic surgery and dermatologic oncology fellow at the University of California, Los Angeles, said in an interview.
“We recognized the gap, which is the lack of diversity/variation of skin tones in our dermatology textbooks and atlases,” she added.
The project was several years in the making, Dr. Friedman said. To do this right, “you need resources, funding, and a collaborative and galvanized team of experts.” That involved coordinating with several medical publishers and amassing a team of medical photographers and an expert panel that will assist in evaluating and securing difficult-to-access clinical images.
The atlas is one of several initiatives in the dermatology field to address racial disparities in patient care.
Noticing similar information gaps about clinical presentations in darker skin, a medical student in the United Kingdom, Malone Mukwende, created “Mind the Gap,” a handbook that presents side-by-side images of diseases and illnesses in light and dark skin. This project “highlights how far behind we are, that a medical student with minimal dermatology exposure and experience not only recognized the need but was ready to do something about it,” Dr. Friedman noted.
Others in the field have spotlighted disparities in the medical literature. The SARS-CoV-2 pandemic has especially brought this out, Graeme M. Lipper, MD wrote in a recent editorial for this news organization.
He referred to a literature review of 46 articles describing COVID-19–associated skin manifestations, which included mostly (92%) images in patients with skin types I-III (92%) and none in patients with skin types V or VI.
“These investigators have identified a damning lack of images of COVID-19–associated skin manifestations in patients with darker skin,” added Dr. Lipper, an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital.
For now, Dr. Friedman said that the atlas won’t contain a specific section on COVID-19 skin manifestations, although viral-associated skin reactions like morbilliform eruptions, urticaria, and retiform purpura will be displayed. Overall, the atlas will address 60-70 skin conditions.
Physicians who fail to educate themselves on the variations of skin conditions in all skin types may potentially harm patients of color, Dr. Eleryan said. As Dr. Lipper noted in his editorial, nearly half of all dermatologists feel they haven’t had adequate exposure to diseases in skin of color.
“Our atlas will fill that void and hopefully assist in closing the gap in health disparities among patients of color, who are often misdiagnosed or rendered diagnoses very late in the disease process,” Dr. Eleryan said.
New topicals for excessive sweating are in sight
Safe and effective of novel agents presented at the virtual annual meeting of the American Academy of Dermatology.
Both investigational topical anticholinergic agents – 5% sofpironium bromide (SPB) gel and 1% glycopyrronium bromide (GPB) cream – met all of the efficacy and safety endpoints required by the Food and Drug Administration.
Primary axillary hyperhidrosis, or symmetrical bilateral excessive armpit sweating, has a prevalence worldwide of 1%-16%, with 5%-6% the most frequently cited numbers. The condition has a strong adverse impact on quality of life. Primary axillary hyperhidrosis is not caused by a disorder of the sweat glands; rather, it’s actually a dysregulation of the autonomic nervous system leading to disproportionate sweating, explained Christoph Abels, MD, PhD, medical director at Dr. August Wolff in Bielefeld, Germany.
“What’s surprising is that more than 50% of patients do not receive appropriate treatment, very likely due to lack of awareness or embarrassment,” he added.
Also, many patients are put off by the systemic side effects of the oral anticholinergic agents, which are the current off-label treatment mainstay for patients with moderate or severe disease, according to Tomoko Fujimoto, MD, PhD, director of Ikebukuro Nishiguchi Fukurou Dermatology, near Tokyo.
Sofpironium bromide gel
Dr. Fujimoto presented the results of a phase 3, double-blind, multicenter, 6-week, vehicle-controlled clinical trial conducted in 281 Japanese patients with moderate to severe primary axillary hyperhidrosis as defined by a baseline score of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS). Participants were randomized to self-application of 5% SPB gel or its vehicle once daily before bedtime.
Sofpironium bromide blocks the cholinergic response mediated by the M3 muscarinic receptor subtype expressed on eccrine sweat glands, thereby inhibiting sweating. The drug then undergoes breakdown into an inactive metabolite after reaching the blood.
An important aspect of both SPB gel and GPB cream is that these agents are rolled onto the axillae using a dedicated applicator. Patients never touch the medications with their hands, thus avoiding accidental exposure to the mucous membranes. This largely prevents problems with mydriasis and blurred vision as anticholinergic side effects, which has been an issue with glycopyrronium tosylate topical cloth wipes (Qbrexza), the first FDA-approved treatment for primary axillary hyperhidrosis.
The primary endpoint in the Japanese study was at least a 1-point improvement on the HDSS plus at least a 50% reduction in gravimetric sweat production between baseline and week 6. This composite outcome was achieved in 53.9% of patients in the active treatment arm, compared with 36.4% of controls.
The secondary endpoint consisting of a week-6 HDSS score of 1 or 2 – that is, underarm sweating that’s either never noticeable or is tolerable – occurred in 60.3% of the sofpironium bromide group and 47.9% of controls, a between-group difference that achieved statistical significance by week 2, when the rates were 46.8% and 28.2%.
The reduction in total gravimetric weight of axillary sweat from a mean baseline of 227 mg collected over 5 minutes was also significantly greater in the SPB group: a decrease of 157.6 mg, compared with 127.6 mg in controls; a between-group difference that also was significant by week 2. The mean Dermatology Life Quality Index score dropped by 6.8 points in the active-treatment arm from a baseline of 11.3, a significant improvement over the mean 4.5-point drop in controls.
A new 5-point measure of subjective symptoms of primary axillary hyperhidrosis – the Hyperhidrosis Disease Severity Measure–Axilla (HDSM-Ax) – improved by 1.41 points in the SPB group, significantly better than the 0.93 points in vehicle-treated controls. About 48% of patients on SBP experienced at least a 1.5-point reduction on the HDSM-Ax, compared with 26% of controls.
Regarding safety, there was a 2% incidence of application-site itch or scale in the SBP group. Anticholinergic side effects consisted of a single case of mydriasis, another of constipation, and two complaints of thirst, all mild, none resulting in treatment discontinuation. There were no reports of headache or blurred vision.
“These results indicate that the safety risks of sofpironium bromide can be considered small and controllable,” Dr. Fujimoto said. “Moreover, sofpironium bromide is a topical agent that patients can use by themselves, so it is highly convenient, unlike, say, botulinum toxin type A injections.”
Glycopyrronium bromide cream
Following on the heels of a recently published dose-ranging study (Br J Dermatol. 2020 Jan;182[1]:229-231), Dr. Abels presented the 4-week outcomes of a phase 3a, double-blind, randomized, five-country trial of once-daily 1% GPB cream or placebo in 171 patients with moderate or severe primary axillary hyperhidrosis. A phase 3b, open-label, 72-week, long-term safety trial is ongoing in 516 patients.
The primary endpoint of the 4-week trial was the reduction in gravimetric sweat production from day 1 to day 29. A reduction of 50% or more was documented in 57.5% of the patients on GBP and 34.5% of controls. A 75% or greater reduction occurred in 32.2% of the active-treatment group and 16.7% of those on placebo. And a decrease of at least 90% was seen in 23% of patients on topical GBP, compared with 9.5% of controls. All these between-group differences were significant.
The FDA now requires a quality of life measurement as a coprimary endpoint in phase 3 hyperhidrosis studies, and the phase 3 GBP trial also served as the successful validation study for a new patient-reported quality of life instrument designed specifically for this purpose. The new tool, known as the Hyperhydrosis Quality of Life questionnaire (HidroQol), proved much more sensitive than the HDSS or DLQI for evaluating clinical improvement in response to treatment (Br J Dermatol. 2020 Jun 8. doi: 10.1111/bjd.19300).
Initial results from the long-term phase 3b safety study should be available this fall on the first 100 patients followed on topical GBP for 1 year and for 300 followed for 6 months, Dr. Abels said.
Dr. Fujimoto reported serving as a paid consultant to and speaker for Kaken Pharmaceutical, which is developing SBP gel with Brickell Biotech. Dr. Abels is an employee of the company that is developing GPB cream.
Safe and effective of novel agents presented at the virtual annual meeting of the American Academy of Dermatology.
Both investigational topical anticholinergic agents – 5% sofpironium bromide (SPB) gel and 1% glycopyrronium bromide (GPB) cream – met all of the efficacy and safety endpoints required by the Food and Drug Administration.
Primary axillary hyperhidrosis, or symmetrical bilateral excessive armpit sweating, has a prevalence worldwide of 1%-16%, with 5%-6% the most frequently cited numbers. The condition has a strong adverse impact on quality of life. Primary axillary hyperhidrosis is not caused by a disorder of the sweat glands; rather, it’s actually a dysregulation of the autonomic nervous system leading to disproportionate sweating, explained Christoph Abels, MD, PhD, medical director at Dr. August Wolff in Bielefeld, Germany.
“What’s surprising is that more than 50% of patients do not receive appropriate treatment, very likely due to lack of awareness or embarrassment,” he added.
Also, many patients are put off by the systemic side effects of the oral anticholinergic agents, which are the current off-label treatment mainstay for patients with moderate or severe disease, according to Tomoko Fujimoto, MD, PhD, director of Ikebukuro Nishiguchi Fukurou Dermatology, near Tokyo.
Sofpironium bromide gel
Dr. Fujimoto presented the results of a phase 3, double-blind, multicenter, 6-week, vehicle-controlled clinical trial conducted in 281 Japanese patients with moderate to severe primary axillary hyperhidrosis as defined by a baseline score of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS). Participants were randomized to self-application of 5% SPB gel or its vehicle once daily before bedtime.
Sofpironium bromide blocks the cholinergic response mediated by the M3 muscarinic receptor subtype expressed on eccrine sweat glands, thereby inhibiting sweating. The drug then undergoes breakdown into an inactive metabolite after reaching the blood.
An important aspect of both SPB gel and GPB cream is that these agents are rolled onto the axillae using a dedicated applicator. Patients never touch the medications with their hands, thus avoiding accidental exposure to the mucous membranes. This largely prevents problems with mydriasis and blurred vision as anticholinergic side effects, which has been an issue with glycopyrronium tosylate topical cloth wipes (Qbrexza), the first FDA-approved treatment for primary axillary hyperhidrosis.
The primary endpoint in the Japanese study was at least a 1-point improvement on the HDSS plus at least a 50% reduction in gravimetric sweat production between baseline and week 6. This composite outcome was achieved in 53.9% of patients in the active treatment arm, compared with 36.4% of controls.
The secondary endpoint consisting of a week-6 HDSS score of 1 or 2 – that is, underarm sweating that’s either never noticeable or is tolerable – occurred in 60.3% of the sofpironium bromide group and 47.9% of controls, a between-group difference that achieved statistical significance by week 2, when the rates were 46.8% and 28.2%.
The reduction in total gravimetric weight of axillary sweat from a mean baseline of 227 mg collected over 5 minutes was also significantly greater in the SPB group: a decrease of 157.6 mg, compared with 127.6 mg in controls; a between-group difference that also was significant by week 2. The mean Dermatology Life Quality Index score dropped by 6.8 points in the active-treatment arm from a baseline of 11.3, a significant improvement over the mean 4.5-point drop in controls.
A new 5-point measure of subjective symptoms of primary axillary hyperhidrosis – the Hyperhidrosis Disease Severity Measure–Axilla (HDSM-Ax) – improved by 1.41 points in the SPB group, significantly better than the 0.93 points in vehicle-treated controls. About 48% of patients on SBP experienced at least a 1.5-point reduction on the HDSM-Ax, compared with 26% of controls.
Regarding safety, there was a 2% incidence of application-site itch or scale in the SBP group. Anticholinergic side effects consisted of a single case of mydriasis, another of constipation, and two complaints of thirst, all mild, none resulting in treatment discontinuation. There were no reports of headache or blurred vision.
“These results indicate that the safety risks of sofpironium bromide can be considered small and controllable,” Dr. Fujimoto said. “Moreover, sofpironium bromide is a topical agent that patients can use by themselves, so it is highly convenient, unlike, say, botulinum toxin type A injections.”
Glycopyrronium bromide cream
Following on the heels of a recently published dose-ranging study (Br J Dermatol. 2020 Jan;182[1]:229-231), Dr. Abels presented the 4-week outcomes of a phase 3a, double-blind, randomized, five-country trial of once-daily 1% GPB cream or placebo in 171 patients with moderate or severe primary axillary hyperhidrosis. A phase 3b, open-label, 72-week, long-term safety trial is ongoing in 516 patients.
The primary endpoint of the 4-week trial was the reduction in gravimetric sweat production from day 1 to day 29. A reduction of 50% or more was documented in 57.5% of the patients on GBP and 34.5% of controls. A 75% or greater reduction occurred in 32.2% of the active-treatment group and 16.7% of those on placebo. And a decrease of at least 90% was seen in 23% of patients on topical GBP, compared with 9.5% of controls. All these between-group differences were significant.
The FDA now requires a quality of life measurement as a coprimary endpoint in phase 3 hyperhidrosis studies, and the phase 3 GBP trial also served as the successful validation study for a new patient-reported quality of life instrument designed specifically for this purpose. The new tool, known as the Hyperhydrosis Quality of Life questionnaire (HidroQol), proved much more sensitive than the HDSS or DLQI for evaluating clinical improvement in response to treatment (Br J Dermatol. 2020 Jun 8. doi: 10.1111/bjd.19300).
Initial results from the long-term phase 3b safety study should be available this fall on the first 100 patients followed on topical GBP for 1 year and for 300 followed for 6 months, Dr. Abels said.
Dr. Fujimoto reported serving as a paid consultant to and speaker for Kaken Pharmaceutical, which is developing SBP gel with Brickell Biotech. Dr. Abels is an employee of the company that is developing GPB cream.
Safe and effective of novel agents presented at the virtual annual meeting of the American Academy of Dermatology.
Both investigational topical anticholinergic agents – 5% sofpironium bromide (SPB) gel and 1% glycopyrronium bromide (GPB) cream – met all of the efficacy and safety endpoints required by the Food and Drug Administration.
Primary axillary hyperhidrosis, or symmetrical bilateral excessive armpit sweating, has a prevalence worldwide of 1%-16%, with 5%-6% the most frequently cited numbers. The condition has a strong adverse impact on quality of life. Primary axillary hyperhidrosis is not caused by a disorder of the sweat glands; rather, it’s actually a dysregulation of the autonomic nervous system leading to disproportionate sweating, explained Christoph Abels, MD, PhD, medical director at Dr. August Wolff in Bielefeld, Germany.
“What’s surprising is that more than 50% of patients do not receive appropriate treatment, very likely due to lack of awareness or embarrassment,” he added.
Also, many patients are put off by the systemic side effects of the oral anticholinergic agents, which are the current off-label treatment mainstay for patients with moderate or severe disease, according to Tomoko Fujimoto, MD, PhD, director of Ikebukuro Nishiguchi Fukurou Dermatology, near Tokyo.
Sofpironium bromide gel
Dr. Fujimoto presented the results of a phase 3, double-blind, multicenter, 6-week, vehicle-controlled clinical trial conducted in 281 Japanese patients with moderate to severe primary axillary hyperhidrosis as defined by a baseline score of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS). Participants were randomized to self-application of 5% SPB gel or its vehicle once daily before bedtime.
Sofpironium bromide blocks the cholinergic response mediated by the M3 muscarinic receptor subtype expressed on eccrine sweat glands, thereby inhibiting sweating. The drug then undergoes breakdown into an inactive metabolite after reaching the blood.
An important aspect of both SPB gel and GPB cream is that these agents are rolled onto the axillae using a dedicated applicator. Patients never touch the medications with their hands, thus avoiding accidental exposure to the mucous membranes. This largely prevents problems with mydriasis and blurred vision as anticholinergic side effects, which has been an issue with glycopyrronium tosylate topical cloth wipes (Qbrexza), the first FDA-approved treatment for primary axillary hyperhidrosis.
The primary endpoint in the Japanese study was at least a 1-point improvement on the HDSS plus at least a 50% reduction in gravimetric sweat production between baseline and week 6. This composite outcome was achieved in 53.9% of patients in the active treatment arm, compared with 36.4% of controls.
The secondary endpoint consisting of a week-6 HDSS score of 1 or 2 – that is, underarm sweating that’s either never noticeable or is tolerable – occurred in 60.3% of the sofpironium bromide group and 47.9% of controls, a between-group difference that achieved statistical significance by week 2, when the rates were 46.8% and 28.2%.
The reduction in total gravimetric weight of axillary sweat from a mean baseline of 227 mg collected over 5 minutes was also significantly greater in the SPB group: a decrease of 157.6 mg, compared with 127.6 mg in controls; a between-group difference that also was significant by week 2. The mean Dermatology Life Quality Index score dropped by 6.8 points in the active-treatment arm from a baseline of 11.3, a significant improvement over the mean 4.5-point drop in controls.
A new 5-point measure of subjective symptoms of primary axillary hyperhidrosis – the Hyperhidrosis Disease Severity Measure–Axilla (HDSM-Ax) – improved by 1.41 points in the SPB group, significantly better than the 0.93 points in vehicle-treated controls. About 48% of patients on SBP experienced at least a 1.5-point reduction on the HDSM-Ax, compared with 26% of controls.
Regarding safety, there was a 2% incidence of application-site itch or scale in the SBP group. Anticholinergic side effects consisted of a single case of mydriasis, another of constipation, and two complaints of thirst, all mild, none resulting in treatment discontinuation. There were no reports of headache or blurred vision.
“These results indicate that the safety risks of sofpironium bromide can be considered small and controllable,” Dr. Fujimoto said. “Moreover, sofpironium bromide is a topical agent that patients can use by themselves, so it is highly convenient, unlike, say, botulinum toxin type A injections.”
Glycopyrronium bromide cream
Following on the heels of a recently published dose-ranging study (Br J Dermatol. 2020 Jan;182[1]:229-231), Dr. Abels presented the 4-week outcomes of a phase 3a, double-blind, randomized, five-country trial of once-daily 1% GPB cream or placebo in 171 patients with moderate or severe primary axillary hyperhidrosis. A phase 3b, open-label, 72-week, long-term safety trial is ongoing in 516 patients.
The primary endpoint of the 4-week trial was the reduction in gravimetric sweat production from day 1 to day 29. A reduction of 50% or more was documented in 57.5% of the patients on GBP and 34.5% of controls. A 75% or greater reduction occurred in 32.2% of the active-treatment group and 16.7% of those on placebo. And a decrease of at least 90% was seen in 23% of patients on topical GBP, compared with 9.5% of controls. All these between-group differences were significant.
The FDA now requires a quality of life measurement as a coprimary endpoint in phase 3 hyperhidrosis studies, and the phase 3 GBP trial also served as the successful validation study for a new patient-reported quality of life instrument designed specifically for this purpose. The new tool, known as the Hyperhydrosis Quality of Life questionnaire (HidroQol), proved much more sensitive than the HDSS or DLQI for evaluating clinical improvement in response to treatment (Br J Dermatol. 2020 Jun 8. doi: 10.1111/bjd.19300).
Initial results from the long-term phase 3b safety study should be available this fall on the first 100 patients followed on topical GBP for 1 year and for 300 followed for 6 months, Dr. Abels said.
Dr. Fujimoto reported serving as a paid consultant to and speaker for Kaken Pharmaceutical, which is developing SBP gel with Brickell Biotech. Dr. Abels is an employee of the company that is developing GPB cream.
FROM AAD 20
Medical student in the UK creates handbook of clinical signs on darker skin
.
Malone Mukwende, a second year student at St. George’s, University of London, had the idea after only being taught about clinical signs and symptoms on White skin.
The handbook is called Mind the Gap. It contains side-by-side images demonstrating how illnesses and diseases can present in light and dark skin.
He hopes the handbook will help future doctors spot and diagnose potentially life-threatening diseases on Black, Asian, and Minority Ethnic (BAME) people.
It comes as nearly 200,000 people have signed a petition calling for medical schools to include BAME representation in clinical teaching.
It points to Kawasaki disease, a rare condition affecting young children. On white skin it appears as a red rash but on darker skin it shows up differently and is much harder to spot.
Medscape UK asked Malone Mukwende about the handbook.
Q&A
Where did the idea come from for Mind the Gap?
On arrival at medical school I noticed the lack of teaching on darker skins. We were often being taught to look for symptoms such as red rashes. I was aware that this would not appear as described in my own skin. When flagging to tutors it was clear that they didn’t know of any other way to describe these conditions and I knew that I had to make a change to that. After extensively asking peer tutors and also lecturers it was clear there was a major gap in the current medical education and a lot of the time I was being told to go and look for it myself.
Following on from that I undertook a staff-student partnership at my university with two members of staff who helped me to create Mind the Gap.
Who did you collaborate with at St. George’s?
I worked with Margot Turner, a senior lecturer in diversity, and Dr. Peter Tamony, a clinical lecturer. We were a dynamic team that had a common goal in mind.
When will the handbook be available?
We are currently working on the best way of disseminating the work to the public. There has been an incredible response since I posted it on my social media, with posts being seen over 3 million times, as well as numerous press features. I am hoping to provide a further update on when the book will be out toward the end of July.
What do you think of the petition to medical schools to include more teaching of the effects of illness and diseases on Black, Asian, and Minority Ethnic people?
The petition closely ties in with the work that I am doing. It is clear that there is an urgent need to increase the medical education on darker skins so that the profession can serve the patient population. We saw in the recent COVID-19 pandemic that the worst affected group of people were from a BAME background.
There are a host of reasons as to why this may have been the case. However another factor may be that healthcare professionals weren’t able to identify these signs and symptoms in time. Some of the coronavirus guidance from royal colleges stated information such as looking for patients to be ‘blue around the lips’. This may have led to slower identification of coronavirus.
To see over 180,000 signatures on the petition was a positive step in the right direction. It is clear to see that this is a big issue. If we fail to act now that the issue has been identified, we run the risk of lives being lost.
A version of this article originally appeared on Medscape.com.
.
Malone Mukwende, a second year student at St. George’s, University of London, had the idea after only being taught about clinical signs and symptoms on White skin.
The handbook is called Mind the Gap. It contains side-by-side images demonstrating how illnesses and diseases can present in light and dark skin.
He hopes the handbook will help future doctors spot and diagnose potentially life-threatening diseases on Black, Asian, and Minority Ethnic (BAME) people.
It comes as nearly 200,000 people have signed a petition calling for medical schools to include BAME representation in clinical teaching.
It points to Kawasaki disease, a rare condition affecting young children. On white skin it appears as a red rash but on darker skin it shows up differently and is much harder to spot.
Medscape UK asked Malone Mukwende about the handbook.
Q&A
Where did the idea come from for Mind the Gap?
On arrival at medical school I noticed the lack of teaching on darker skins. We were often being taught to look for symptoms such as red rashes. I was aware that this would not appear as described in my own skin. When flagging to tutors it was clear that they didn’t know of any other way to describe these conditions and I knew that I had to make a change to that. After extensively asking peer tutors and also lecturers it was clear there was a major gap in the current medical education and a lot of the time I was being told to go and look for it myself.
Following on from that I undertook a staff-student partnership at my university with two members of staff who helped me to create Mind the Gap.
Who did you collaborate with at St. George’s?
I worked with Margot Turner, a senior lecturer in diversity, and Dr. Peter Tamony, a clinical lecturer. We were a dynamic team that had a common goal in mind.
When will the handbook be available?
We are currently working on the best way of disseminating the work to the public. There has been an incredible response since I posted it on my social media, with posts being seen over 3 million times, as well as numerous press features. I am hoping to provide a further update on when the book will be out toward the end of July.
What do you think of the petition to medical schools to include more teaching of the effects of illness and diseases on Black, Asian, and Minority Ethnic people?
The petition closely ties in with the work that I am doing. It is clear that there is an urgent need to increase the medical education on darker skins so that the profession can serve the patient population. We saw in the recent COVID-19 pandemic that the worst affected group of people were from a BAME background.
There are a host of reasons as to why this may have been the case. However another factor may be that healthcare professionals weren’t able to identify these signs and symptoms in time. Some of the coronavirus guidance from royal colleges stated information such as looking for patients to be ‘blue around the lips’. This may have led to slower identification of coronavirus.
To see over 180,000 signatures on the petition was a positive step in the right direction. It is clear to see that this is a big issue. If we fail to act now that the issue has been identified, we run the risk of lives being lost.
A version of this article originally appeared on Medscape.com.
.
Malone Mukwende, a second year student at St. George’s, University of London, had the idea after only being taught about clinical signs and symptoms on White skin.
The handbook is called Mind the Gap. It contains side-by-side images demonstrating how illnesses and diseases can present in light and dark skin.
He hopes the handbook will help future doctors spot and diagnose potentially life-threatening diseases on Black, Asian, and Minority Ethnic (BAME) people.
It comes as nearly 200,000 people have signed a petition calling for medical schools to include BAME representation in clinical teaching.
It points to Kawasaki disease, a rare condition affecting young children. On white skin it appears as a red rash but on darker skin it shows up differently and is much harder to spot.
Medscape UK asked Malone Mukwende about the handbook.
Q&A
Where did the idea come from for Mind the Gap?
On arrival at medical school I noticed the lack of teaching on darker skins. We were often being taught to look for symptoms such as red rashes. I was aware that this would not appear as described in my own skin. When flagging to tutors it was clear that they didn’t know of any other way to describe these conditions and I knew that I had to make a change to that. After extensively asking peer tutors and also lecturers it was clear there was a major gap in the current medical education and a lot of the time I was being told to go and look for it myself.
Following on from that I undertook a staff-student partnership at my university with two members of staff who helped me to create Mind the Gap.
Who did you collaborate with at St. George’s?
I worked with Margot Turner, a senior lecturer in diversity, and Dr. Peter Tamony, a clinical lecturer. We were a dynamic team that had a common goal in mind.
When will the handbook be available?
We are currently working on the best way of disseminating the work to the public. There has been an incredible response since I posted it on my social media, with posts being seen over 3 million times, as well as numerous press features. I am hoping to provide a further update on when the book will be out toward the end of July.
What do you think of the petition to medical schools to include more teaching of the effects of illness and diseases on Black, Asian, and Minority Ethnic people?
The petition closely ties in with the work that I am doing. It is clear that there is an urgent need to increase the medical education on darker skins so that the profession can serve the patient population. We saw in the recent COVID-19 pandemic that the worst affected group of people were from a BAME background.
There are a host of reasons as to why this may have been the case. However another factor may be that healthcare professionals weren’t able to identify these signs and symptoms in time. Some of the coronavirus guidance from royal colleges stated information such as looking for patients to be ‘blue around the lips’. This may have led to slower identification of coronavirus.
To see over 180,000 signatures on the petition was a positive step in the right direction. It is clear to see that this is a big issue. If we fail to act now that the issue has been identified, we run the risk of lives being lost.
A version of this article originally appeared on Medscape.com.
How to set up your hyperhidrosis patients for treatment success
When children and adolescents first present to George Hightower, MD, PhD, with suspected primary hyperhidrosis, he tries to gauge their level of impairment and distress.
“I ask my patients directly: ‘Does this get in the way of doing things you enjoy?’ ” Dr. Hightower said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. If they say yes, he then asks, “‘What are those things that it gets in the way of?’ Also, so that I can develop a rapport with them, I ask, ‘Is it causing you to view yourself negatively?’ I also ask them how they anticipate treatment is going to change that.”
Dr. Hightower, of the departments of dermatology and pediatrics, University of California, San Diego, and a pediatric dermatologist at Rady Children’s Hospital, defined focal primary hyperhidrosis as focal, visible, excessive sweating for at least 6 months without an apparent cause, plus at least two of the following characteristics: bilateral and relatively symmetric, sweating that impairs daily activities, onset before age 25, at least one episode per week, family history of idiopathic hyperhidrosis, and focal sweating that stops during sleep.
“Based on their prominence in the popular media, armpits relative to body surface area play an oversized role in our patients’ perception of well-being,” he said. “Most of all, patients’ concerns regarding their armpits include one more of the following symptoms: smelly, sweaty, red, and itchy or painful.”
Topical antiperspirants are the preferred initial treatment. “They’re widely available, inexpensive, and well-tolerated therapies,” Dr. Hightower said. Most commercially available antiperspirants contain low-dose aluminum or other metal that keeps the sweat gland ducts from opening.
“Most patients referred to me have failed to improve with over-the-counter antiperspirants or aluminum chloride 20%,” he said. “We start by reviewing the appropriate use of aluminum chloride 20%. If they’re using it appropriately and fail to achieve adequate control, I open the discussion to use glycopyrronium tosylate cloth 2.4%, applied daily. This can be cost prohibitive or not covered by insurance.” Other options include glycopyrrolate 1-6 mg daily and microwave-based procedural intervention.
In a post hoc analysis, researchers examined the efficacy and safety findings by age from two phase three randomized, controlled trials of glycopyrronium tosylate in pediatric primary axillary hyperhidrosis (Pediatr Dermatol. 2019 Jan-Feb;36[1]:89-99). It was well tolerated in the 19 patients aged 9-16 years. “No patients discontinued from the study in this age group [because of] symptomatology,” said Dr. Hightower, who was not involved with the study. “The concerns related to this medication are related to anticholinergic effects such as blurry vision and dry mouth, but overall, randomized clinical trial data support the benefit of this medication in helping patients improve the symptoms of hyperhidrosis.”
In an earlier study, researchers retrospectively studied children with hyperhidrosis who were treated with a mean dosage of 2 mg glycopyrronium tosylate daily (J Am Acad Dermatol 2012 Nov;67[5]:918-23). The average age of patients was 15 years. Most (90%) experienced some improvement and 71% of those who responded saw major improvement. This occurred within hours of administration and disappeared within a day of discontinuation. The two most common side effects were dry mouth (26%) and dry eyes (10%). More worrisome side effects were associated with higher dosing, including blurring of vision (3%) and sensation of palpitations (3%).
When patients return for their first follow-up appointment after starting a treatment plan, Dr. Hightower revisits their level of impairment and distress with hyperhidrosis. “I ask, ‘Remember that activity that you were doing before that this was getting in the way of? Are you doing that more? Do you feel like you can do that in a way that you weren’t able to do before, whether it’s playing an instrument or spending time with friends?’ ”
He also sets expectations with patients and their families with comments such as, “If this treatment does not work for you after 2 months, the next option I would consider is ...” and, “for most people there is no cure, but treatment is helpful.” He also emphasizes the importance of follow-up care, so they “come back to assess the next steps.”
Dr. Hightower reported having no financial disclosures.
When children and adolescents first present to George Hightower, MD, PhD, with suspected primary hyperhidrosis, he tries to gauge their level of impairment and distress.
“I ask my patients directly: ‘Does this get in the way of doing things you enjoy?’ ” Dr. Hightower said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. If they say yes, he then asks, “‘What are those things that it gets in the way of?’ Also, so that I can develop a rapport with them, I ask, ‘Is it causing you to view yourself negatively?’ I also ask them how they anticipate treatment is going to change that.”
Dr. Hightower, of the departments of dermatology and pediatrics, University of California, San Diego, and a pediatric dermatologist at Rady Children’s Hospital, defined focal primary hyperhidrosis as focal, visible, excessive sweating for at least 6 months without an apparent cause, plus at least two of the following characteristics: bilateral and relatively symmetric, sweating that impairs daily activities, onset before age 25, at least one episode per week, family history of idiopathic hyperhidrosis, and focal sweating that stops during sleep.
“Based on their prominence in the popular media, armpits relative to body surface area play an oversized role in our patients’ perception of well-being,” he said. “Most of all, patients’ concerns regarding their armpits include one more of the following symptoms: smelly, sweaty, red, and itchy or painful.”
Topical antiperspirants are the preferred initial treatment. “They’re widely available, inexpensive, and well-tolerated therapies,” Dr. Hightower said. Most commercially available antiperspirants contain low-dose aluminum or other metal that keeps the sweat gland ducts from opening.
“Most patients referred to me have failed to improve with over-the-counter antiperspirants or aluminum chloride 20%,” he said. “We start by reviewing the appropriate use of aluminum chloride 20%. If they’re using it appropriately and fail to achieve adequate control, I open the discussion to use glycopyrronium tosylate cloth 2.4%, applied daily. This can be cost prohibitive or not covered by insurance.” Other options include glycopyrrolate 1-6 mg daily and microwave-based procedural intervention.
In a post hoc analysis, researchers examined the efficacy and safety findings by age from two phase three randomized, controlled trials of glycopyrronium tosylate in pediatric primary axillary hyperhidrosis (Pediatr Dermatol. 2019 Jan-Feb;36[1]:89-99). It was well tolerated in the 19 patients aged 9-16 years. “No patients discontinued from the study in this age group [because of] symptomatology,” said Dr. Hightower, who was not involved with the study. “The concerns related to this medication are related to anticholinergic effects such as blurry vision and dry mouth, but overall, randomized clinical trial data support the benefit of this medication in helping patients improve the symptoms of hyperhidrosis.”
In an earlier study, researchers retrospectively studied children with hyperhidrosis who were treated with a mean dosage of 2 mg glycopyrronium tosylate daily (J Am Acad Dermatol 2012 Nov;67[5]:918-23). The average age of patients was 15 years. Most (90%) experienced some improvement and 71% of those who responded saw major improvement. This occurred within hours of administration and disappeared within a day of discontinuation. The two most common side effects were dry mouth (26%) and dry eyes (10%). More worrisome side effects were associated with higher dosing, including blurring of vision (3%) and sensation of palpitations (3%).
When patients return for their first follow-up appointment after starting a treatment plan, Dr. Hightower revisits their level of impairment and distress with hyperhidrosis. “I ask, ‘Remember that activity that you were doing before that this was getting in the way of? Are you doing that more? Do you feel like you can do that in a way that you weren’t able to do before, whether it’s playing an instrument or spending time with friends?’ ”
He also sets expectations with patients and their families with comments such as, “If this treatment does not work for you after 2 months, the next option I would consider is ...” and, “for most people there is no cure, but treatment is helpful.” He also emphasizes the importance of follow-up care, so they “come back to assess the next steps.”
Dr. Hightower reported having no financial disclosures.
When children and adolescents first present to George Hightower, MD, PhD, with suspected primary hyperhidrosis, he tries to gauge their level of impairment and distress.
“I ask my patients directly: ‘Does this get in the way of doing things you enjoy?’ ” Dr. Hightower said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. If they say yes, he then asks, “‘What are those things that it gets in the way of?’ Also, so that I can develop a rapport with them, I ask, ‘Is it causing you to view yourself negatively?’ I also ask them how they anticipate treatment is going to change that.”
Dr. Hightower, of the departments of dermatology and pediatrics, University of California, San Diego, and a pediatric dermatologist at Rady Children’s Hospital, defined focal primary hyperhidrosis as focal, visible, excessive sweating for at least 6 months without an apparent cause, plus at least two of the following characteristics: bilateral and relatively symmetric, sweating that impairs daily activities, onset before age 25, at least one episode per week, family history of idiopathic hyperhidrosis, and focal sweating that stops during sleep.
“Based on their prominence in the popular media, armpits relative to body surface area play an oversized role in our patients’ perception of well-being,” he said. “Most of all, patients’ concerns regarding their armpits include one more of the following symptoms: smelly, sweaty, red, and itchy or painful.”
Topical antiperspirants are the preferred initial treatment. “They’re widely available, inexpensive, and well-tolerated therapies,” Dr. Hightower said. Most commercially available antiperspirants contain low-dose aluminum or other metal that keeps the sweat gland ducts from opening.
“Most patients referred to me have failed to improve with over-the-counter antiperspirants or aluminum chloride 20%,” he said. “We start by reviewing the appropriate use of aluminum chloride 20%. If they’re using it appropriately and fail to achieve adequate control, I open the discussion to use glycopyrronium tosylate cloth 2.4%, applied daily. This can be cost prohibitive or not covered by insurance.” Other options include glycopyrrolate 1-6 mg daily and microwave-based procedural intervention.
In a post hoc analysis, researchers examined the efficacy and safety findings by age from two phase three randomized, controlled trials of glycopyrronium tosylate in pediatric primary axillary hyperhidrosis (Pediatr Dermatol. 2019 Jan-Feb;36[1]:89-99). It was well tolerated in the 19 patients aged 9-16 years. “No patients discontinued from the study in this age group [because of] symptomatology,” said Dr. Hightower, who was not involved with the study. “The concerns related to this medication are related to anticholinergic effects such as blurry vision and dry mouth, but overall, randomized clinical trial data support the benefit of this medication in helping patients improve the symptoms of hyperhidrosis.”
In an earlier study, researchers retrospectively studied children with hyperhidrosis who were treated with a mean dosage of 2 mg glycopyrronium tosylate daily (J Am Acad Dermatol 2012 Nov;67[5]:918-23). The average age of patients was 15 years. Most (90%) experienced some improvement and 71% of those who responded saw major improvement. This occurred within hours of administration and disappeared within a day of discontinuation. The two most common side effects were dry mouth (26%) and dry eyes (10%). More worrisome side effects were associated with higher dosing, including blurring of vision (3%) and sensation of palpitations (3%).
When patients return for their first follow-up appointment after starting a treatment plan, Dr. Hightower revisits their level of impairment and distress with hyperhidrosis. “I ask, ‘Remember that activity that you were doing before that this was getting in the way of? Are you doing that more? Do you feel like you can do that in a way that you weren’t able to do before, whether it’s playing an instrument or spending time with friends?’ ”
He also sets expectations with patients and their families with comments such as, “If this treatment does not work for you after 2 months, the next option I would consider is ...” and, “for most people there is no cure, but treatment is helpful.” He also emphasizes the importance of follow-up care, so they “come back to assess the next steps.”
Dr. Hightower reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY 2020
Ixekizumab deemed effective for pityriasis rubra pilaris
Teri Greiling, MD, PhD, said at the virtual annual meeting of the American Academy of Dermatology.
The interleukin-17A inhibitor induced long-term remission of pityriasis rubra pilaris (PRP) in 4 of the 11 participants in her open-label, single-arm, 24-week clinical trial of ixekizumab (Taltz) at the Food and Drug Administration–approved dosing for psoriasis. Of 11 patients, 7 experienced at least a 50% reduction in signs and symptoms of the disease. Marked quality-of-life improvements occurred as well, reported Dr. Greiling, a dermatologist at Oregon Health & Science University, Portland.
PRP is a rare papulosquamous disorder that’s challenging to diagnosis and often difficult to treat. Indeed, there is no FDA-approved treatment. The disorder is characterized by widespread follicular keratotic plaques with scale and notable islands of sparing, often with an accompanying waxy yellow palmoplantar keratoderma with fissuring. There are adult- and childhood-onset forms of PRP, as well as sporadic and familial subtypes. Some cases are associated with variants in the CARD14 gene, known to also play a role in familial psoriasis.
Patients with PRP say the disorder has a major adverse impact on their quality of life. Itching and pain are often prominent features.
The 11 study participants, drawn from throughout the United States, were adults with a mean 12-year history of symptoms. All were required to have both clinical and biopsy evidence of PRP. Five had classic adult-onset PRP, five had atypical adult-onset PRP, and one had classic juvenile-onset PRP. All had moderate or severe disease as reflected in a Physician’s Global Assessment score of 3 or 4 on the 0-4 scale. Of the 11, 10 had previously received various systemic therapies for their PRP without success.
Since there is as yet no established PRP severity grading tool, Dr. Greiling applied the principles of the Psoriasis Area and Severity Index (PASI). Participants had a mean baseline PASI score of 24.6, a Dermatology Life Quality Index (DLQI) score of 18, and itch and pain scores of 7 and 6, respectively, on a self-rated 10-point scale.
The primary outcome in the study was change in PASI score at week 24, which was 4 weeks after the final dose of ixekizumab. The mean score improved from 24.8 at week 0 to 9.7 at week 24. Five patients achieved at least a 75% decrease in PASI score, or PASI 75 response, and 2 had a PASI 90 response. The DLQI improved from 18 to 3, and both itch and pain scores dropped to a median of 1. There was no association between response to treatment and PRP clinical subtype.
The four top responders – those with a PASI 89 or better response at week 24 – remained clear or almost clear at week 36, fully 16 weeks after their last dose of ixekizumab. Patients with an intermediate week 24 response – that is, a 50%-85% reduction in PASI score – all relapsed before week 36. The patient with the worst PASI score at both baseline and 24 weeks decided to continue on ixekizumab dosed every 2 weeks independent of the study, rather than at the FDA-approved dosing every 4 weeks for psoriasis, with a resultant drop to a PASI score of 8 at week 36.
To look at mechanism of benefit, Dr. Greiling used quantitative polymerase chain reaction to examine key cytokine expression in the epidermis and dermis. Not surprisingly, IL-17A expression was markedly reduced at both sites, suggesting the importance of the Th17 axis in the pathophysiology of PRP. In contrast, there was no significant change in IL-23 expression.
No serious or unexpected adverse events occurred in the 24-week study.
“In terms of ixekizumab, compared to other treatments, I definitely think it is more effective than any conventional therapies, such as topical steroids, methotrexate, or acitretin,” she said in an interview.
Asked about other biologics, Dr. Greiling said she hasn’t found tumor necrosis factor inhibitors very helpful in her patients with PRP. A formal trial of the IL-17A inhibitor secukinumab (Cosentyx) has been done elsewhere, and although the results haven’t yet been published, her understanding is that the efficacy was similar to her ixekizumab trial.
“I’ve had some of my ixekizumab patients switch to secukinumab, for insurance reasons, though, and had it not be quite as effective, although still helpful,” she said.
Dr. Greiling is now enrolling patients with PRP in a trial of the IL-23 inhibitor guselkumab (Tremfya). It’s her early impression that this may prove to be another therapeutic option.
“I have not yet used brodalumab [Siliq], but I wonder if it would also be helpful, since it seems to have a stronger blockade, working on the IL-17 receptor A,” she said.
She cited two pressing needs that would advance PRP research: the lack of standard criteria for disease diagnosis and the absence of PRP-specific disease measurement tools. “We’re trying to remedy that,” the dermatologist said.
Her study was funded by Eli Lilly. She reported receiving research funding from that company and Janssen.
Teri Greiling, MD, PhD, said at the virtual annual meeting of the American Academy of Dermatology.
The interleukin-17A inhibitor induced long-term remission of pityriasis rubra pilaris (PRP) in 4 of the 11 participants in her open-label, single-arm, 24-week clinical trial of ixekizumab (Taltz) at the Food and Drug Administration–approved dosing for psoriasis. Of 11 patients, 7 experienced at least a 50% reduction in signs and symptoms of the disease. Marked quality-of-life improvements occurred as well, reported Dr. Greiling, a dermatologist at Oregon Health & Science University, Portland.
PRP is a rare papulosquamous disorder that’s challenging to diagnosis and often difficult to treat. Indeed, there is no FDA-approved treatment. The disorder is characterized by widespread follicular keratotic plaques with scale and notable islands of sparing, often with an accompanying waxy yellow palmoplantar keratoderma with fissuring. There are adult- and childhood-onset forms of PRP, as well as sporadic and familial subtypes. Some cases are associated with variants in the CARD14 gene, known to also play a role in familial psoriasis.
Patients with PRP say the disorder has a major adverse impact on their quality of life. Itching and pain are often prominent features.
The 11 study participants, drawn from throughout the United States, were adults with a mean 12-year history of symptoms. All were required to have both clinical and biopsy evidence of PRP. Five had classic adult-onset PRP, five had atypical adult-onset PRP, and one had classic juvenile-onset PRP. All had moderate or severe disease as reflected in a Physician’s Global Assessment score of 3 or 4 on the 0-4 scale. Of the 11, 10 had previously received various systemic therapies for their PRP without success.
Since there is as yet no established PRP severity grading tool, Dr. Greiling applied the principles of the Psoriasis Area and Severity Index (PASI). Participants had a mean baseline PASI score of 24.6, a Dermatology Life Quality Index (DLQI) score of 18, and itch and pain scores of 7 and 6, respectively, on a self-rated 10-point scale.
The primary outcome in the study was change in PASI score at week 24, which was 4 weeks after the final dose of ixekizumab. The mean score improved from 24.8 at week 0 to 9.7 at week 24. Five patients achieved at least a 75% decrease in PASI score, or PASI 75 response, and 2 had a PASI 90 response. The DLQI improved from 18 to 3, and both itch and pain scores dropped to a median of 1. There was no association between response to treatment and PRP clinical subtype.
The four top responders – those with a PASI 89 or better response at week 24 – remained clear or almost clear at week 36, fully 16 weeks after their last dose of ixekizumab. Patients with an intermediate week 24 response – that is, a 50%-85% reduction in PASI score – all relapsed before week 36. The patient with the worst PASI score at both baseline and 24 weeks decided to continue on ixekizumab dosed every 2 weeks independent of the study, rather than at the FDA-approved dosing every 4 weeks for psoriasis, with a resultant drop to a PASI score of 8 at week 36.
To look at mechanism of benefit, Dr. Greiling used quantitative polymerase chain reaction to examine key cytokine expression in the epidermis and dermis. Not surprisingly, IL-17A expression was markedly reduced at both sites, suggesting the importance of the Th17 axis in the pathophysiology of PRP. In contrast, there was no significant change in IL-23 expression.
No serious or unexpected adverse events occurred in the 24-week study.
“In terms of ixekizumab, compared to other treatments, I definitely think it is more effective than any conventional therapies, such as topical steroids, methotrexate, or acitretin,” she said in an interview.
Asked about other biologics, Dr. Greiling said she hasn’t found tumor necrosis factor inhibitors very helpful in her patients with PRP. A formal trial of the IL-17A inhibitor secukinumab (Cosentyx) has been done elsewhere, and although the results haven’t yet been published, her understanding is that the efficacy was similar to her ixekizumab trial.
“I’ve had some of my ixekizumab patients switch to secukinumab, for insurance reasons, though, and had it not be quite as effective, although still helpful,” she said.
Dr. Greiling is now enrolling patients with PRP in a trial of the IL-23 inhibitor guselkumab (Tremfya). It’s her early impression that this may prove to be another therapeutic option.
“I have not yet used brodalumab [Siliq], but I wonder if it would also be helpful, since it seems to have a stronger blockade, working on the IL-17 receptor A,” she said.
She cited two pressing needs that would advance PRP research: the lack of standard criteria for disease diagnosis and the absence of PRP-specific disease measurement tools. “We’re trying to remedy that,” the dermatologist said.
Her study was funded by Eli Lilly. She reported receiving research funding from that company and Janssen.
Teri Greiling, MD, PhD, said at the virtual annual meeting of the American Academy of Dermatology.
The interleukin-17A inhibitor induced long-term remission of pityriasis rubra pilaris (PRP) in 4 of the 11 participants in her open-label, single-arm, 24-week clinical trial of ixekizumab (Taltz) at the Food and Drug Administration–approved dosing for psoriasis. Of 11 patients, 7 experienced at least a 50% reduction in signs and symptoms of the disease. Marked quality-of-life improvements occurred as well, reported Dr. Greiling, a dermatologist at Oregon Health & Science University, Portland.
PRP is a rare papulosquamous disorder that’s challenging to diagnosis and often difficult to treat. Indeed, there is no FDA-approved treatment. The disorder is characterized by widespread follicular keratotic plaques with scale and notable islands of sparing, often with an accompanying waxy yellow palmoplantar keratoderma with fissuring. There are adult- and childhood-onset forms of PRP, as well as sporadic and familial subtypes. Some cases are associated with variants in the CARD14 gene, known to also play a role in familial psoriasis.
Patients with PRP say the disorder has a major adverse impact on their quality of life. Itching and pain are often prominent features.
The 11 study participants, drawn from throughout the United States, were adults with a mean 12-year history of symptoms. All were required to have both clinical and biopsy evidence of PRP. Five had classic adult-onset PRP, five had atypical adult-onset PRP, and one had classic juvenile-onset PRP. All had moderate or severe disease as reflected in a Physician’s Global Assessment score of 3 or 4 on the 0-4 scale. Of the 11, 10 had previously received various systemic therapies for their PRP without success.
Since there is as yet no established PRP severity grading tool, Dr. Greiling applied the principles of the Psoriasis Area and Severity Index (PASI). Participants had a mean baseline PASI score of 24.6, a Dermatology Life Quality Index (DLQI) score of 18, and itch and pain scores of 7 and 6, respectively, on a self-rated 10-point scale.
The primary outcome in the study was change in PASI score at week 24, which was 4 weeks after the final dose of ixekizumab. The mean score improved from 24.8 at week 0 to 9.7 at week 24. Five patients achieved at least a 75% decrease in PASI score, or PASI 75 response, and 2 had a PASI 90 response. The DLQI improved from 18 to 3, and both itch and pain scores dropped to a median of 1. There was no association between response to treatment and PRP clinical subtype.
The four top responders – those with a PASI 89 or better response at week 24 – remained clear or almost clear at week 36, fully 16 weeks after their last dose of ixekizumab. Patients with an intermediate week 24 response – that is, a 50%-85% reduction in PASI score – all relapsed before week 36. The patient with the worst PASI score at both baseline and 24 weeks decided to continue on ixekizumab dosed every 2 weeks independent of the study, rather than at the FDA-approved dosing every 4 weeks for psoriasis, with a resultant drop to a PASI score of 8 at week 36.
To look at mechanism of benefit, Dr. Greiling used quantitative polymerase chain reaction to examine key cytokine expression in the epidermis and dermis. Not surprisingly, IL-17A expression was markedly reduced at both sites, suggesting the importance of the Th17 axis in the pathophysiology of PRP. In contrast, there was no significant change in IL-23 expression.
No serious or unexpected adverse events occurred in the 24-week study.
“In terms of ixekizumab, compared to other treatments, I definitely think it is more effective than any conventional therapies, such as topical steroids, methotrexate, or acitretin,” she said in an interview.
Asked about other biologics, Dr. Greiling said she hasn’t found tumor necrosis factor inhibitors very helpful in her patients with PRP. A formal trial of the IL-17A inhibitor secukinumab (Cosentyx) has been done elsewhere, and although the results haven’t yet been published, her understanding is that the efficacy was similar to her ixekizumab trial.
“I’ve had some of my ixekizumab patients switch to secukinumab, for insurance reasons, though, and had it not be quite as effective, although still helpful,” she said.
Dr. Greiling is now enrolling patients with PRP in a trial of the IL-23 inhibitor guselkumab (Tremfya). It’s her early impression that this may prove to be another therapeutic option.
“I have not yet used brodalumab [Siliq], but I wonder if it would also be helpful, since it seems to have a stronger blockade, working on the IL-17 receptor A,” she said.
She cited two pressing needs that would advance PRP research: the lack of standard criteria for disease diagnosis and the absence of PRP-specific disease measurement tools. “We’re trying to remedy that,” the dermatologist said.
Her study was funded by Eli Lilly. She reported receiving research funding from that company and Janssen.
FROM AAD 2020
Lenalidomide may be an answer for refractory cutaneous lupus
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
FROM SPD 2020
Are laser treatments better than steroids for lichen sclerosus?
Laser treatment for lichen sclerosus was noninferior to steroid therapy after 6 months and may lead to better outcomes on various patient- and physician-reported measures, according to trial results presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
Patients with lichen sclerosus often present with itching, burning, and dysuria. Untreated, the vulvar dystrophy can cause architectural changes and is associated with an increased risk of vulvar malignancies.
Topical steroids are the standard treatment. To assess whether fractional CO2 laser treatment is noninferior to clobetasol propionate at 6 months, Linda Burkett, MD, and colleagues conducted a randomized controlled trial. Dr. Burkett is affiliated with MedStar Washington Hospital Center and Georgetown University in Washington and UPMC Magee-Womens Hospital in Pittsburgh.
The researchers enrolled 52 postmenopausal women with biopsy-proven lichen sclerosus. Patients had to have significant symptoms reflected by a score of at least 21 on the Skindex-29.
Twenty-seven women were assigned to receive laser therapy, and 25 were assigned to receive steroids. One patient in the steroid arm was lost to follow-up. About half of the patients in each group had prior clobetasol propionate exposure.
Patients in the steroid arm were started on 0.05% clobetasol propionate used nightly for 4 weeks, then three times per week for 8 weeks, and then as needed. They had a phone call follow-up at 2 weeks to confirm compliance and an optional in-person appointment at 3 months.
Patients in the laser arm received three laser treatments 4-6 weeks apart.
At 6 months, all patients returned for repeat assessments. The primary outcome was the Skindex-29, a dermatologic questionnaire. Secondary outcomes included a patient visual analog scale of bothersome vulvar symptoms, a provider visual assessment score, the Vaginal Health Index, the Vulvovaginal Symptom Questionnaire, the Patient Global Impression of Improvement, and the Patient Global Impression of Satisfaction.
Average Skindex-29 scores from baseline to 6 months improved more in the laser treatment group, compared with the steroid group, for all health-related quality of life categories: overall, emotional, functional, and symptoms. “At 6 months across all scores, patients reported very little bother,” Dr. Burkett said.
Differences between the groups were statistically significant for all but the functional subscore.
Average scores on subjective secondary outcomes improved more in the laser treatment group, compared with the steroid treatment group. The between-group differences were statistically significant for irritation and the Vulvovaginal Symptom Questionnaire.
For provider-based scores, patients in the laser group had greater improvement on all measures except perianal involvement, relative to patients in the steroid group. In addition, fusion of the labia minora and phimosis worsened in the steroid group.
Differences between the groups were statistically significant for phimosis, erosion, and the Vaginal Health Index.
Significantly more patients in the laser group than in the steroid group were satisfied or very satisfied with the results at 6 months (81% vs. 41%). Patients in the laser group were more likely to report that they were better or much better (89% vs. 62%), though the difference was not statistically significant.
There were no major adverse events.
The trial – the first randomized controlled study of energy-based treatment for lichen sclerosus – was conducted at a single center, and treatment was not blinded, Dr. Burkett noted.
“The treatment effect was pretty significant in favor of laser therapy,” said Cecile A. Ferrando, MD, MPH, of the Center for Urogynecology and Pelvic Reconstructive Surgery at Cleveland Clinic, commenting on the research.
“Compliance issues with clobetasol aside,” the findings raise the question of whether laser therapy should be offered as first-line treatment, Dr. Ferrando said.
The study might have been more robust had it excluded patients with previous clobetasol propionate exposure, Dr. Ferrando added.
Dr. Burkett noted that future studies may incorporate multiple centers, histology measures, and sham laser treatments and include only women who have not previously received clobetasol propionate.
The researchers had no relevant financial disclosures. Dr. Ferrando disclosed authorship royalties from UpToDate.
SOURCE: Burkett L et al. SGS 2020, Abstract 09.
Laser treatment for lichen sclerosus was noninferior to steroid therapy after 6 months and may lead to better outcomes on various patient- and physician-reported measures, according to trial results presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
Patients with lichen sclerosus often present with itching, burning, and dysuria. Untreated, the vulvar dystrophy can cause architectural changes and is associated with an increased risk of vulvar malignancies.
Topical steroids are the standard treatment. To assess whether fractional CO2 laser treatment is noninferior to clobetasol propionate at 6 months, Linda Burkett, MD, and colleagues conducted a randomized controlled trial. Dr. Burkett is affiliated with MedStar Washington Hospital Center and Georgetown University in Washington and UPMC Magee-Womens Hospital in Pittsburgh.
The researchers enrolled 52 postmenopausal women with biopsy-proven lichen sclerosus. Patients had to have significant symptoms reflected by a score of at least 21 on the Skindex-29.
Twenty-seven women were assigned to receive laser therapy, and 25 were assigned to receive steroids. One patient in the steroid arm was lost to follow-up. About half of the patients in each group had prior clobetasol propionate exposure.
Patients in the steroid arm were started on 0.05% clobetasol propionate used nightly for 4 weeks, then three times per week for 8 weeks, and then as needed. They had a phone call follow-up at 2 weeks to confirm compliance and an optional in-person appointment at 3 months.
Patients in the laser arm received three laser treatments 4-6 weeks apart.
At 6 months, all patients returned for repeat assessments. The primary outcome was the Skindex-29, a dermatologic questionnaire. Secondary outcomes included a patient visual analog scale of bothersome vulvar symptoms, a provider visual assessment score, the Vaginal Health Index, the Vulvovaginal Symptom Questionnaire, the Patient Global Impression of Improvement, and the Patient Global Impression of Satisfaction.
Average Skindex-29 scores from baseline to 6 months improved more in the laser treatment group, compared with the steroid group, for all health-related quality of life categories: overall, emotional, functional, and symptoms. “At 6 months across all scores, patients reported very little bother,” Dr. Burkett said.
Differences between the groups were statistically significant for all but the functional subscore.
Average scores on subjective secondary outcomes improved more in the laser treatment group, compared with the steroid treatment group. The between-group differences were statistically significant for irritation and the Vulvovaginal Symptom Questionnaire.
For provider-based scores, patients in the laser group had greater improvement on all measures except perianal involvement, relative to patients in the steroid group. In addition, fusion of the labia minora and phimosis worsened in the steroid group.
Differences between the groups were statistically significant for phimosis, erosion, and the Vaginal Health Index.
Significantly more patients in the laser group than in the steroid group were satisfied or very satisfied with the results at 6 months (81% vs. 41%). Patients in the laser group were more likely to report that they were better or much better (89% vs. 62%), though the difference was not statistically significant.
There were no major adverse events.
The trial – the first randomized controlled study of energy-based treatment for lichen sclerosus – was conducted at a single center, and treatment was not blinded, Dr. Burkett noted.
“The treatment effect was pretty significant in favor of laser therapy,” said Cecile A. Ferrando, MD, MPH, of the Center for Urogynecology and Pelvic Reconstructive Surgery at Cleveland Clinic, commenting on the research.
“Compliance issues with clobetasol aside,” the findings raise the question of whether laser therapy should be offered as first-line treatment, Dr. Ferrando said.
The study might have been more robust had it excluded patients with previous clobetasol propionate exposure, Dr. Ferrando added.
Dr. Burkett noted that future studies may incorporate multiple centers, histology measures, and sham laser treatments and include only women who have not previously received clobetasol propionate.
The researchers had no relevant financial disclosures. Dr. Ferrando disclosed authorship royalties from UpToDate.
SOURCE: Burkett L et al. SGS 2020, Abstract 09.
Laser treatment for lichen sclerosus was noninferior to steroid therapy after 6 months and may lead to better outcomes on various patient- and physician-reported measures, according to trial results presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
Patients with lichen sclerosus often present with itching, burning, and dysuria. Untreated, the vulvar dystrophy can cause architectural changes and is associated with an increased risk of vulvar malignancies.
Topical steroids are the standard treatment. To assess whether fractional CO2 laser treatment is noninferior to clobetasol propionate at 6 months, Linda Burkett, MD, and colleagues conducted a randomized controlled trial. Dr. Burkett is affiliated with MedStar Washington Hospital Center and Georgetown University in Washington and UPMC Magee-Womens Hospital in Pittsburgh.
The researchers enrolled 52 postmenopausal women with biopsy-proven lichen sclerosus. Patients had to have significant symptoms reflected by a score of at least 21 on the Skindex-29.
Twenty-seven women were assigned to receive laser therapy, and 25 were assigned to receive steroids. One patient in the steroid arm was lost to follow-up. About half of the patients in each group had prior clobetasol propionate exposure.
Patients in the steroid arm were started on 0.05% clobetasol propionate used nightly for 4 weeks, then three times per week for 8 weeks, and then as needed. They had a phone call follow-up at 2 weeks to confirm compliance and an optional in-person appointment at 3 months.
Patients in the laser arm received three laser treatments 4-6 weeks apart.
At 6 months, all patients returned for repeat assessments. The primary outcome was the Skindex-29, a dermatologic questionnaire. Secondary outcomes included a patient visual analog scale of bothersome vulvar symptoms, a provider visual assessment score, the Vaginal Health Index, the Vulvovaginal Symptom Questionnaire, the Patient Global Impression of Improvement, and the Patient Global Impression of Satisfaction.
Average Skindex-29 scores from baseline to 6 months improved more in the laser treatment group, compared with the steroid group, for all health-related quality of life categories: overall, emotional, functional, and symptoms. “At 6 months across all scores, patients reported very little bother,” Dr. Burkett said.
Differences between the groups were statistically significant for all but the functional subscore.
Average scores on subjective secondary outcomes improved more in the laser treatment group, compared with the steroid treatment group. The between-group differences were statistically significant for irritation and the Vulvovaginal Symptom Questionnaire.
For provider-based scores, patients in the laser group had greater improvement on all measures except perianal involvement, relative to patients in the steroid group. In addition, fusion of the labia minora and phimosis worsened in the steroid group.
Differences between the groups were statistically significant for phimosis, erosion, and the Vaginal Health Index.
Significantly more patients in the laser group than in the steroid group were satisfied or very satisfied with the results at 6 months (81% vs. 41%). Patients in the laser group were more likely to report that they were better or much better (89% vs. 62%), though the difference was not statistically significant.
There were no major adverse events.
The trial – the first randomized controlled study of energy-based treatment for lichen sclerosus – was conducted at a single center, and treatment was not blinded, Dr. Burkett noted.
“The treatment effect was pretty significant in favor of laser therapy,” said Cecile A. Ferrando, MD, MPH, of the Center for Urogynecology and Pelvic Reconstructive Surgery at Cleveland Clinic, commenting on the research.
“Compliance issues with clobetasol aside,” the findings raise the question of whether laser therapy should be offered as first-line treatment, Dr. Ferrando said.
The study might have been more robust had it excluded patients with previous clobetasol propionate exposure, Dr. Ferrando added.
Dr. Burkett noted that future studies may incorporate multiple centers, histology measures, and sham laser treatments and include only women who have not previously received clobetasol propionate.
The researchers had no relevant financial disclosures. Dr. Ferrando disclosed authorship royalties from UpToDate.
SOURCE: Burkett L et al. SGS 2020, Abstract 09.
FROM SGS 2020