User login
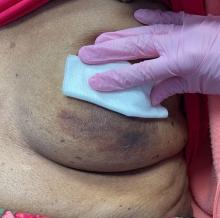
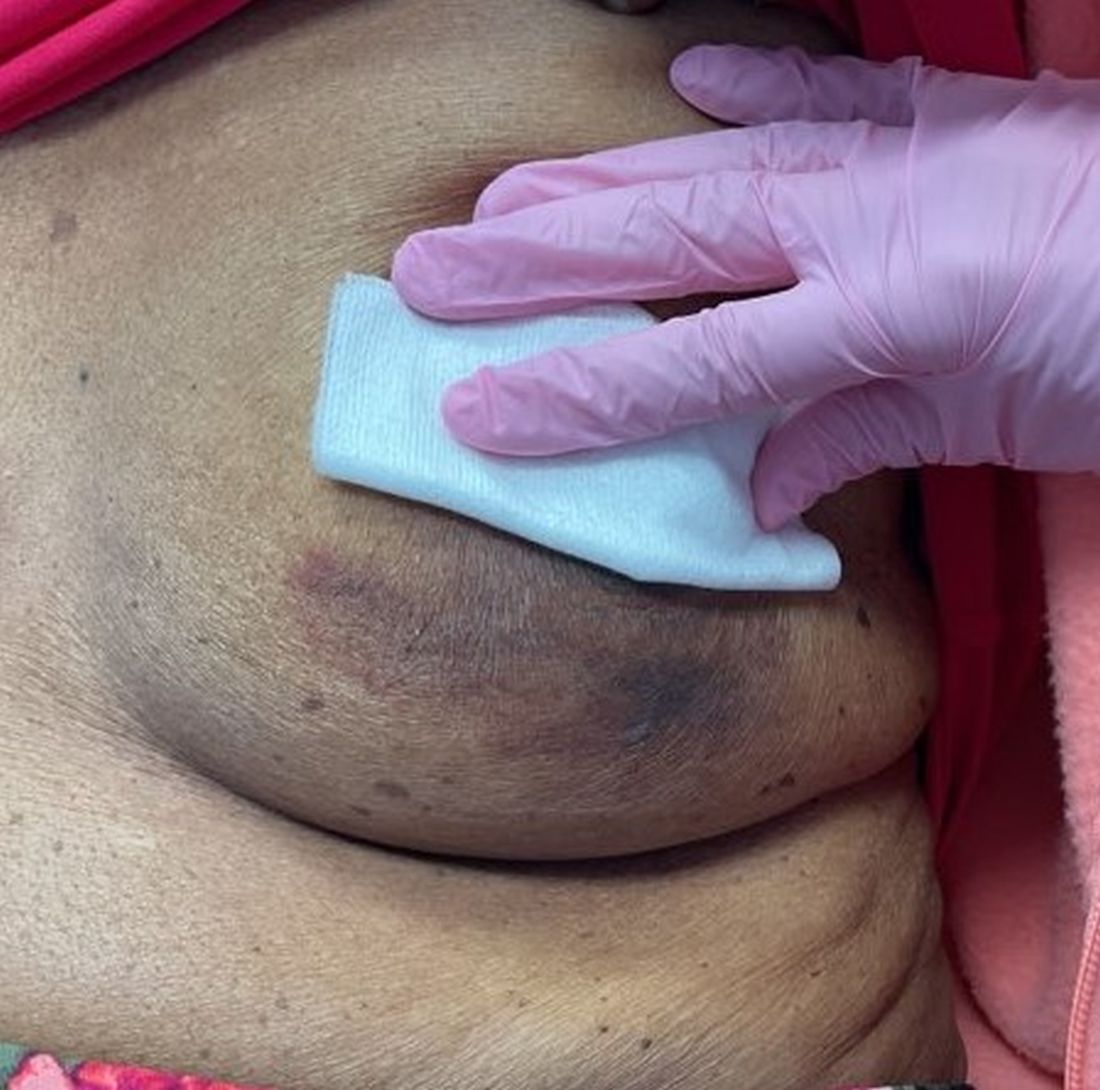
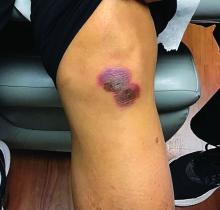
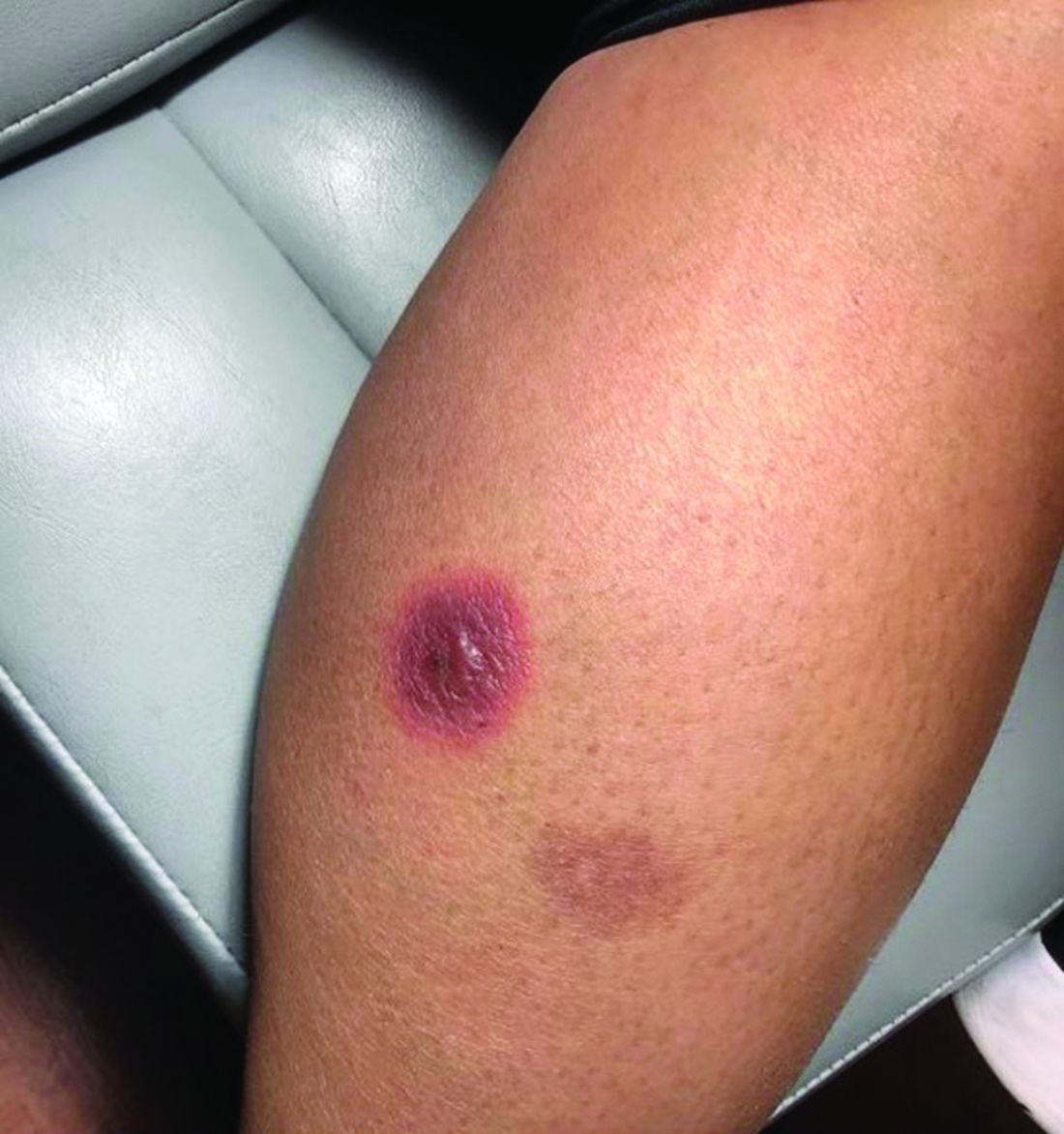
An 88-year-old Black woman presented with 3 months duration of asymptomatic, violaceous patches on the left breast
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
More phase 3 data support use of nemolizumab for prurigo nodularis
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
FDA approves second treatment for adults with hidradenitis suppurativa
The U.S.
The development, which was announced Oct. 31, makes secukinumab the first and only interleukin (IL)–17A inhibitor approved by the FDA for HS, which affects an estimated 1% of the worldwide population. It joins the tumor necrosis factor blocker adalimumab as the only FDA-approved treatment options for HS.
Secukinumab (Cosentyx) was previously approved by the FDA for treatment of moderate-to-severe plaque psoriasis in adults, and several other indications including psoriatic arthritis and ankylosing spondylitis.
Approval for HS was based on the pivotal phase 3 SUNSHINE and SUNRISE trials, which had a combined enrollment of more than 1,000 patients with HS in 40 countries. The studies evaluated efficacy, safety, and tolerability of two dose regimens of the drug in adults with moderate to severe HS at 16 weeks and up to 52 weeks.
According to a press release from Novartis announcing the approval, results at week 16 showed that a significantly higher proportion of patients achieved a Hidradenitis Suppurativa Clinical Response (HiSCR) when treated with secukinumab 300 mg every 2 weeks, compared with placebo: 44.5% vs. 29.4%, respectively, in the SUNSHINE trial and 38.3.% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
Similarly, results at week 16 showed that a significantly higher proportion of patients achieved an HiSCR when treated with secukinumab 300 mg every 4 weeks, compared with placebo: 41.3% vs. 29.4% in the SUNSHINE trial and 42.5% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
In addition, in an exploratory analysis out to 52 weeks, HiSCR values observed at week 16 following either dose regimen of secukinumab were improved over time up to week 52. In SUNSHINE, the values improved by 56.4% in patients treated with secukinumab every 3 weeks and by 56.3% in those treated with secukinumab every 4 weeks. In SUNRISE, the values improved by 65% in patients who were treated with secukinumab every 2 weeks and by 62.2% in those who were treated with the drug every 4 weeks.
In an interview, Haley Naik, MD, a dermatologist who directs the hidradenitis suppurativa program at the University of California, San Francisco, characterized the approval as a win for HS patients. “Patients now not only have a second option for approved therapy for HS, but also an option that raises the bar for what we can expect from therapeutic response,” she told this news organization. “I am excited to see a novel therapy that improves HS and quality of life for patients make it through the regulatory pipeline.”
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, Union Chimique Belge, and Novartis; investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
A version of this article first appeared on Medscape.com.
The U.S.
The development, which was announced Oct. 31, makes secukinumab the first and only interleukin (IL)–17A inhibitor approved by the FDA for HS, which affects an estimated 1% of the worldwide population. It joins the tumor necrosis factor blocker adalimumab as the only FDA-approved treatment options for HS.
Secukinumab (Cosentyx) was previously approved by the FDA for treatment of moderate-to-severe plaque psoriasis in adults, and several other indications including psoriatic arthritis and ankylosing spondylitis.
Approval for HS was based on the pivotal phase 3 SUNSHINE and SUNRISE trials, which had a combined enrollment of more than 1,000 patients with HS in 40 countries. The studies evaluated efficacy, safety, and tolerability of two dose regimens of the drug in adults with moderate to severe HS at 16 weeks and up to 52 weeks.
According to a press release from Novartis announcing the approval, results at week 16 showed that a significantly higher proportion of patients achieved a Hidradenitis Suppurativa Clinical Response (HiSCR) when treated with secukinumab 300 mg every 2 weeks, compared with placebo: 44.5% vs. 29.4%, respectively, in the SUNSHINE trial and 38.3.% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
Similarly, results at week 16 showed that a significantly higher proportion of patients achieved an HiSCR when treated with secukinumab 300 mg every 4 weeks, compared with placebo: 41.3% vs. 29.4% in the SUNSHINE trial and 42.5% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
In addition, in an exploratory analysis out to 52 weeks, HiSCR values observed at week 16 following either dose regimen of secukinumab were improved over time up to week 52. In SUNSHINE, the values improved by 56.4% in patients treated with secukinumab every 3 weeks and by 56.3% in those treated with secukinumab every 4 weeks. In SUNRISE, the values improved by 65% in patients who were treated with secukinumab every 2 weeks and by 62.2% in those who were treated with the drug every 4 weeks.
In an interview, Haley Naik, MD, a dermatologist who directs the hidradenitis suppurativa program at the University of California, San Francisco, characterized the approval as a win for HS patients. “Patients now not only have a second option for approved therapy for HS, but also an option that raises the bar for what we can expect from therapeutic response,” she told this news organization. “I am excited to see a novel therapy that improves HS and quality of life for patients make it through the regulatory pipeline.”
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, Union Chimique Belge, and Novartis; investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
A version of this article first appeared on Medscape.com.
The U.S.
The development, which was announced Oct. 31, makes secukinumab the first and only interleukin (IL)–17A inhibitor approved by the FDA for HS, which affects an estimated 1% of the worldwide population. It joins the tumor necrosis factor blocker adalimumab as the only FDA-approved treatment options for HS.
Secukinumab (Cosentyx) was previously approved by the FDA for treatment of moderate-to-severe plaque psoriasis in adults, and several other indications including psoriatic arthritis and ankylosing spondylitis.
Approval for HS was based on the pivotal phase 3 SUNSHINE and SUNRISE trials, which had a combined enrollment of more than 1,000 patients with HS in 40 countries. The studies evaluated efficacy, safety, and tolerability of two dose regimens of the drug in adults with moderate to severe HS at 16 weeks and up to 52 weeks.
According to a press release from Novartis announcing the approval, results at week 16 showed that a significantly higher proportion of patients achieved a Hidradenitis Suppurativa Clinical Response (HiSCR) when treated with secukinumab 300 mg every 2 weeks, compared with placebo: 44.5% vs. 29.4%, respectively, in the SUNSHINE trial and 38.3.% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
Similarly, results at week 16 showed that a significantly higher proportion of patients achieved an HiSCR when treated with secukinumab 300 mg every 4 weeks, compared with placebo: 41.3% vs. 29.4% in the SUNSHINE trial and 42.5% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
In addition, in an exploratory analysis out to 52 weeks, HiSCR values observed at week 16 following either dose regimen of secukinumab were improved over time up to week 52. In SUNSHINE, the values improved by 56.4% in patients treated with secukinumab every 3 weeks and by 56.3% in those treated with secukinumab every 4 weeks. In SUNRISE, the values improved by 65% in patients who were treated with secukinumab every 2 weeks and by 62.2% in those who were treated with the drug every 4 weeks.
In an interview, Haley Naik, MD, a dermatologist who directs the hidradenitis suppurativa program at the University of California, San Francisco, characterized the approval as a win for HS patients. “Patients now not only have a second option for approved therapy for HS, but also an option that raises the bar for what we can expect from therapeutic response,” she told this news organization. “I am excited to see a novel therapy that improves HS and quality of life for patients make it through the regulatory pipeline.”
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, Union Chimique Belge, and Novartis; investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
A version of this article first appeared on Medscape.com.
Review finds no CV or VTE risk signal with use of JAK inhibitors for skin indications
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
FROM JAMA DERMATOLOGY
Multicenter study aims to find new treatments for hidradenitis suppurativa
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
Teledermatology model takes hold with grants to underserved areas
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
Researchers link two genes to Raynaud’s disease
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
A 42-year-old woman presented with a few days of erosions on her buccal mucosa, tongue, and soft palate
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
Spironolactone safe, effective option for women with hidradenitis suppurativa
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
AT CALDERM 2023
Hidradenitis suppurativa experts reach consensus on treatment outcome measures
TOPLINE:
Hidradenitis suppurativa (HS) experts collaborated to reach consensus on a core set of outcome measures, with the intent of improving the management of HS in clinical practice.
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
TOPLINE:
Hidradenitis suppurativa (HS) experts collaborated to reach consensus on a core set of outcome measures, with the intent of improving the management of HS in clinical practice.
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
TOPLINE:
Hidradenitis suppurativa (HS) experts collaborated to reach consensus on a core set of outcome measures, with the intent of improving the management of HS in clinical practice.
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY