User login
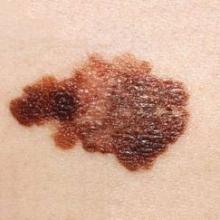
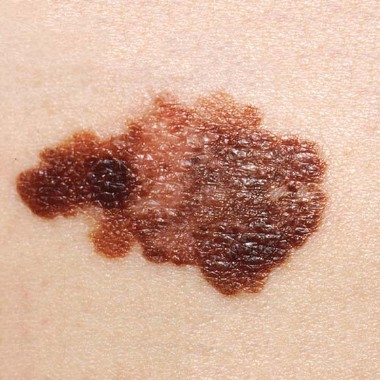
Substantial delay seen in melanoma surgery for Medicare patients
DENVER – As many as 25% of Medicare patients with melanoma will experience treatment delays of up to 1.5 months, and 18% may have treatment delays as long as 3 months after a lesion is biopsied, especially if a nondermatologist is performing the excision, based on data from a review of more than 32,000 patients.
Patients who saw dermatologists for their surgery were much less likely to experience a delay in treatment, Dr. Jason Lott said at the annual meeting of the American Academy of Dermatology.
"If a primary care provider was doing the surgery – and many still do their own surgery – patients were 1.6 times more likely to have a delay of more than 1.5 months, and twice as likely to have a delay of more than 3 months," said Dr. Lott of Yale University, New Haven, Conn. "I am making the argument that specialty matters."
Other factors significantly associated with treatment delay included older patient age, comorbidities, tumor stage, and lesion location.
To examine factors contributing to a surgical delay in the United States, Dr. Lott and his colleagues conducted a population-based study of treatment waiting times in more than 32,501 Medicare patients who had been diagnosed with melanoma from 2000 to 2009. The primary outcome was the time between biopsy and surgical treatment.
The most common lesion sites were the head and neck (40%), followed by the extremities and trunk. Compared with head/neck lesions, surgery on the trunk and extremities was significantly less likely to be delayed up to 1.5 months (odds ratio, 0.74).
Melanomas were about equally divided between in situ and localized disease (48% and 44%), with the remainder having distant occurrence. Compared with in situ disease, regional and distant disease were significantly more likely to be treated later, with odds ratios ranging from 1.31 for a delay of up to 1.5 months (regional disease) to 2.15 for a delay of more than 3 months (distant disease).
Older patients had greater delays than did younger patients, with the biggest disparities between those aged 80-84 years and those aged 70-74 years, In the younger group, 54% were treated within 1 month, compared with 50% of the older patients. A delay of up to 3 months occurred in 38% of the younger group and 41% of the older group.
Patients with no medical comorbidities received significantly prompter treatment, with 54% being treated within 1 month compared with 47% of those with three comorbidities.
Expeditious treatment is certainly important to patients, Dr. Lott said, and likely important for achieving optimal outcomes. "Patients don’t like walking around knowing they have a malignancy that’s not treated, with even a marginally increased chance that something bad will happen," he noted.
That risk is not unfounded, Dr. Lott said. The United Kingdom’s "2-week" rule, implemented in 2000, mandates an urgent consultation for suspected malignancies. Patients with suspected melanomas are seen in a special pigmented lesions clinic, and often treated on the same day. In 2007, this practice was shown to positively impact melanoma survival.
Dr. Lott described a retrospective study conducted in the United Kingdom that examined outcomes in 4,399 patients, all of whom were evaluated at a pigmented lesions clinic within 2 weeks of a primary care identification of a suspicious lesion. During the study period, 96 melanomas were identified, and 96% of those were treated within 2 weeks of the primary referral. Most (74%) were excised on the day of the clinic visit.
The authors compared these results with those of 78 melanoma patients who were diagnosed in the 2 years before urgent referral became standard. These patients waited up to 34 days for a referral and up to 74 days for treatment. Patients seen in the clinic had significantly thinner tumors (Breslow thickness 1.68 vs. 2.39 mm). In addition to melanomas, 748 nonmelanoma skin cancers were treated at the clinics.
Among the melanoma patients diagnosed in the clinics, the 5-year survival rate was 82%, compared with 62% in patients diagnosed before the urgent referral.
"Earlier treatment does matter," Dr. Lott said.
Dr. Lott said he had no relevant financial conflicts to disclose.
On Twitter @alz_gal
DENVER – As many as 25% of Medicare patients with melanoma will experience treatment delays of up to 1.5 months, and 18% may have treatment delays as long as 3 months after a lesion is biopsied, especially if a nondermatologist is performing the excision, based on data from a review of more than 32,000 patients.
Patients who saw dermatologists for their surgery were much less likely to experience a delay in treatment, Dr. Jason Lott said at the annual meeting of the American Academy of Dermatology.
"If a primary care provider was doing the surgery – and many still do their own surgery – patients were 1.6 times more likely to have a delay of more than 1.5 months, and twice as likely to have a delay of more than 3 months," said Dr. Lott of Yale University, New Haven, Conn. "I am making the argument that specialty matters."
Other factors significantly associated with treatment delay included older patient age, comorbidities, tumor stage, and lesion location.
To examine factors contributing to a surgical delay in the United States, Dr. Lott and his colleagues conducted a population-based study of treatment waiting times in more than 32,501 Medicare patients who had been diagnosed with melanoma from 2000 to 2009. The primary outcome was the time between biopsy and surgical treatment.
The most common lesion sites were the head and neck (40%), followed by the extremities and trunk. Compared with head/neck lesions, surgery on the trunk and extremities was significantly less likely to be delayed up to 1.5 months (odds ratio, 0.74).
Melanomas were about equally divided between in situ and localized disease (48% and 44%), with the remainder having distant occurrence. Compared with in situ disease, regional and distant disease were significantly more likely to be treated later, with odds ratios ranging from 1.31 for a delay of up to 1.5 months (regional disease) to 2.15 for a delay of more than 3 months (distant disease).
Older patients had greater delays than did younger patients, with the biggest disparities between those aged 80-84 years and those aged 70-74 years, In the younger group, 54% were treated within 1 month, compared with 50% of the older patients. A delay of up to 3 months occurred in 38% of the younger group and 41% of the older group.
Patients with no medical comorbidities received significantly prompter treatment, with 54% being treated within 1 month compared with 47% of those with three comorbidities.
Expeditious treatment is certainly important to patients, Dr. Lott said, and likely important for achieving optimal outcomes. "Patients don’t like walking around knowing they have a malignancy that’s not treated, with even a marginally increased chance that something bad will happen," he noted.
That risk is not unfounded, Dr. Lott said. The United Kingdom’s "2-week" rule, implemented in 2000, mandates an urgent consultation for suspected malignancies. Patients with suspected melanomas are seen in a special pigmented lesions clinic, and often treated on the same day. In 2007, this practice was shown to positively impact melanoma survival.
Dr. Lott described a retrospective study conducted in the United Kingdom that examined outcomes in 4,399 patients, all of whom were evaluated at a pigmented lesions clinic within 2 weeks of a primary care identification of a suspicious lesion. During the study period, 96 melanomas were identified, and 96% of those were treated within 2 weeks of the primary referral. Most (74%) were excised on the day of the clinic visit.
The authors compared these results with those of 78 melanoma patients who were diagnosed in the 2 years before urgent referral became standard. These patients waited up to 34 days for a referral and up to 74 days for treatment. Patients seen in the clinic had significantly thinner tumors (Breslow thickness 1.68 vs. 2.39 mm). In addition to melanomas, 748 nonmelanoma skin cancers were treated at the clinics.
Among the melanoma patients diagnosed in the clinics, the 5-year survival rate was 82%, compared with 62% in patients diagnosed before the urgent referral.
"Earlier treatment does matter," Dr. Lott said.
Dr. Lott said he had no relevant financial conflicts to disclose.
On Twitter @alz_gal
DENVER – As many as 25% of Medicare patients with melanoma will experience treatment delays of up to 1.5 months, and 18% may have treatment delays as long as 3 months after a lesion is biopsied, especially if a nondermatologist is performing the excision, based on data from a review of more than 32,000 patients.
Patients who saw dermatologists for their surgery were much less likely to experience a delay in treatment, Dr. Jason Lott said at the annual meeting of the American Academy of Dermatology.
"If a primary care provider was doing the surgery – and many still do their own surgery – patients were 1.6 times more likely to have a delay of more than 1.5 months, and twice as likely to have a delay of more than 3 months," said Dr. Lott of Yale University, New Haven, Conn. "I am making the argument that specialty matters."
Other factors significantly associated with treatment delay included older patient age, comorbidities, tumor stage, and lesion location.
To examine factors contributing to a surgical delay in the United States, Dr. Lott and his colleagues conducted a population-based study of treatment waiting times in more than 32,501 Medicare patients who had been diagnosed with melanoma from 2000 to 2009. The primary outcome was the time between biopsy and surgical treatment.
The most common lesion sites were the head and neck (40%), followed by the extremities and trunk. Compared with head/neck lesions, surgery on the trunk and extremities was significantly less likely to be delayed up to 1.5 months (odds ratio, 0.74).
Melanomas were about equally divided between in situ and localized disease (48% and 44%), with the remainder having distant occurrence. Compared with in situ disease, regional and distant disease were significantly more likely to be treated later, with odds ratios ranging from 1.31 for a delay of up to 1.5 months (regional disease) to 2.15 for a delay of more than 3 months (distant disease).
Older patients had greater delays than did younger patients, with the biggest disparities between those aged 80-84 years and those aged 70-74 years, In the younger group, 54% were treated within 1 month, compared with 50% of the older patients. A delay of up to 3 months occurred in 38% of the younger group and 41% of the older group.
Patients with no medical comorbidities received significantly prompter treatment, with 54% being treated within 1 month compared with 47% of those with three comorbidities.
Expeditious treatment is certainly important to patients, Dr. Lott said, and likely important for achieving optimal outcomes. "Patients don’t like walking around knowing they have a malignancy that’s not treated, with even a marginally increased chance that something bad will happen," he noted.
That risk is not unfounded, Dr. Lott said. The United Kingdom’s "2-week" rule, implemented in 2000, mandates an urgent consultation for suspected malignancies. Patients with suspected melanomas are seen in a special pigmented lesions clinic, and often treated on the same day. In 2007, this practice was shown to positively impact melanoma survival.
Dr. Lott described a retrospective study conducted in the United Kingdom that examined outcomes in 4,399 patients, all of whom were evaluated at a pigmented lesions clinic within 2 weeks of a primary care identification of a suspicious lesion. During the study period, 96 melanomas were identified, and 96% of those were treated within 2 weeks of the primary referral. Most (74%) were excised on the day of the clinic visit.
The authors compared these results with those of 78 melanoma patients who were diagnosed in the 2 years before urgent referral became standard. These patients waited up to 34 days for a referral and up to 74 days for treatment. Patients seen in the clinic had significantly thinner tumors (Breslow thickness 1.68 vs. 2.39 mm). In addition to melanomas, 748 nonmelanoma skin cancers were treated at the clinics.
Among the melanoma patients diagnosed in the clinics, the 5-year survival rate was 82%, compared with 62% in patients diagnosed before the urgent referral.
"Earlier treatment does matter," Dr. Lott said.
Dr. Lott said he had no relevant financial conflicts to disclose.
On Twitter @alz_gal
AT THE AAD ANNUAL MEETING
Major finding: Melanoma excision delays were significantly less common when the diagnostic physician was a dermatologist, rather than a nondermatologist (OR, 0.67 for more than 1.5 months; 0.58 for more than 3 months).
Data source: A Medicare database study involving more than 32,000 patients with melanoma.
Disclosures: Dr. Lott said he had no relevant financial disclosures.
What’s old is new again for actinic keratoses treatment
DENVER – Changes in health insurance coverage are prompting a resurgence in the use of 5-fluorouracil to treat actinic keratoses, according to Dr. Linda Susan Marcus.
Not only is 5-fluorouracil (5-FU) effective, "but it has become increasingly difficult to get some of the newer topical agents covered by health insurance plans, especially Medicare. This is the reality now," Dr. Marcus said at the annual meeting of the American Academy of Dermatology.
Dr. Marcus, a dermatologist in Wyckoff, N.J., noted that 5-FU blocks methylation of deoxyuridylic acid to thymidylic acid in DNA, altering only fast-dividing cancerous cells. The agent is available in 1%, 2%, and 5% solutions, and in 1% and 5% creams. "We don’t really use the solutions much anymore; they’re very irritating," Dr. Marcus said. "The 1% cream is less irritating, but 5% cream is really the gold standard." Her approach is to have patients apply the 5% 5-FU cream to the affected area twice a day for 3 weeks. Another option is a 0.5% 5-FU cream with a microsphere delivery system "that traps the active ingredients in the skin surface to increase efficacy and decrease irritancy," she said. "Some people use this for maintenance or cycle therapy prior to cryosurgery."
As for side effects, 5-FU elicits erythema, scaliness, and crusting (which can be avoided with the milder preparations); but these conditions are self-limited, Dr. Marcus noted. Some dermatologists use topical steroids or hyaluronic acid gels "to make the erythema go away faster," she added. "There are studies that say if you use these topical steroids, it curtails efficacy and you might lose some efficacy. That might be true. However, you have to make it user-friendly for the patient. Use your clinical judgment."
Other topical preparations for actinic keratoses on the market include:
• Diclofenac sodium 3% in 2.5% hyaluronic acid gel. This colorless agent is designed to be applied twice a day for 2-3 months. "That can pose a compliance issue for some patients," Dr. Marcus said. "The mechanism is unknown, but it probably functions as an NSAID that may involve prostaglandin levels in UV exposed skin and upregulation of COX-2, which may promote proliferation. Cyclooxygenase is the rate-limiting enzyme step in prostaglandin synthesis."
Dr. Marcus said that that diclofenac sodium 3% in 2.5% hyaluronic acid gel may be best suited for patients with mild lesions and for pre- or post cryosurgery.
• Imiquimod. A 5% formulation of imiquimod "is becoming the new gold standard of topical therapies, but it can be irritating," Dr. Marcus said. A 3.75% formulation is available that is designed to be used for 2 weeks, followed by a 2-week break, and then the patient repeats the cycle, Dr. Marcus said, adding that she uses the 3.75% formulation most often for her patients with actinic keratoses.
Dr. Marcus described imiquimod as an immune response modifier that induces mRNA encoding cytokines like alpha-interferon, TNF, and interleukin-12 for a cytotoxic T-lymphocyte response.
"There’s a direct proapoptotic effect in changing cancerous cells as a result of bypassing transduction paths activating caspase-3 downstream of membrane-bound death receptor activation," she said. "You can get a severe reaction, but there shouldn’t be a lot of pain. You get excellent cosmetic results upon healing."
Dermatologists often tweak the frequency of application, she added, and results from some studies suggest that outcomes with imiquimod are similar to those obtained with 5-FU, while others hint that imiquimod may provide longer-lasting results. "Field-directed therapy is the advantage since it brings out subclinical lesions, but you need a lot of hand holding to encourage patients with this phenomenon," Dr. Marcus said.
• Ingenol mebutate (PEP005). Approved as a gel in January of 2012, ingenol mebutate is a natural diterpene from the Euphorbia peplus flowering plant in Southeast Asia. The agent is believed to augment neutrophil-killing ability on abnormal cells via damaging mitochondria, and its antiangiogenic properties promote healing and skin regeneration. "It’s proven histologically in superficial basal cell epithelioma, which is interesting, because this drug is approved only in the United States and the indication is only for actinic keratosis and not for superficial basal cells," Dr. Marcus said.
In early studies, a 0.0025% preparation resulted in 38% clearance and was tolerable, while a 0.125% preparation gave 100% clearance but was too irritating. The approved form of ingenol mebutate gel is a 0.015% formulation applied daily for 3 days to head areas and ingenol mebutate gel 0.05% applied daily for 2 days to body areas. "You apply it for 3 days, but the reaction actually peaks on the 4th day, so when the patients are not applying it, they are still going to get a bit of redness before they’re into the healing phase," Dr. Marcus said. "The advantage is that you’re only applying it for 2-3 days, and then you’re done, so it increases compliance."
Photodynamic therapy is useful for field therapy, and it is done in one office visit, so compliance is not an issue, Dr. Marcus said. Photodynamic therapy also is covered by Medicare. "It may illicit some burning and require hand holding, but is effective," she said. "The key is combination therapy."
Dr. Marcus disclosed that she has received honoraria, grants, and research support from numerous pharmaceutical companies.
DENVER – Changes in health insurance coverage are prompting a resurgence in the use of 5-fluorouracil to treat actinic keratoses, according to Dr. Linda Susan Marcus.
Not only is 5-fluorouracil (5-FU) effective, "but it has become increasingly difficult to get some of the newer topical agents covered by health insurance plans, especially Medicare. This is the reality now," Dr. Marcus said at the annual meeting of the American Academy of Dermatology.
Dr. Marcus, a dermatologist in Wyckoff, N.J., noted that 5-FU blocks methylation of deoxyuridylic acid to thymidylic acid in DNA, altering only fast-dividing cancerous cells. The agent is available in 1%, 2%, and 5% solutions, and in 1% and 5% creams. "We don’t really use the solutions much anymore; they’re very irritating," Dr. Marcus said. "The 1% cream is less irritating, but 5% cream is really the gold standard." Her approach is to have patients apply the 5% 5-FU cream to the affected area twice a day for 3 weeks. Another option is a 0.5% 5-FU cream with a microsphere delivery system "that traps the active ingredients in the skin surface to increase efficacy and decrease irritancy," she said. "Some people use this for maintenance or cycle therapy prior to cryosurgery."
As for side effects, 5-FU elicits erythema, scaliness, and crusting (which can be avoided with the milder preparations); but these conditions are self-limited, Dr. Marcus noted. Some dermatologists use topical steroids or hyaluronic acid gels "to make the erythema go away faster," she added. "There are studies that say if you use these topical steroids, it curtails efficacy and you might lose some efficacy. That might be true. However, you have to make it user-friendly for the patient. Use your clinical judgment."
Other topical preparations for actinic keratoses on the market include:
• Diclofenac sodium 3% in 2.5% hyaluronic acid gel. This colorless agent is designed to be applied twice a day for 2-3 months. "That can pose a compliance issue for some patients," Dr. Marcus said. "The mechanism is unknown, but it probably functions as an NSAID that may involve prostaglandin levels in UV exposed skin and upregulation of COX-2, which may promote proliferation. Cyclooxygenase is the rate-limiting enzyme step in prostaglandin synthesis."
Dr. Marcus said that that diclofenac sodium 3% in 2.5% hyaluronic acid gel may be best suited for patients with mild lesions and for pre- or post cryosurgery.
• Imiquimod. A 5% formulation of imiquimod "is becoming the new gold standard of topical therapies, but it can be irritating," Dr. Marcus said. A 3.75% formulation is available that is designed to be used for 2 weeks, followed by a 2-week break, and then the patient repeats the cycle, Dr. Marcus said, adding that she uses the 3.75% formulation most often for her patients with actinic keratoses.
Dr. Marcus described imiquimod as an immune response modifier that induces mRNA encoding cytokines like alpha-interferon, TNF, and interleukin-12 for a cytotoxic T-lymphocyte response.
"There’s a direct proapoptotic effect in changing cancerous cells as a result of bypassing transduction paths activating caspase-3 downstream of membrane-bound death receptor activation," she said. "You can get a severe reaction, but there shouldn’t be a lot of pain. You get excellent cosmetic results upon healing."
Dermatologists often tweak the frequency of application, she added, and results from some studies suggest that outcomes with imiquimod are similar to those obtained with 5-FU, while others hint that imiquimod may provide longer-lasting results. "Field-directed therapy is the advantage since it brings out subclinical lesions, but you need a lot of hand holding to encourage patients with this phenomenon," Dr. Marcus said.
• Ingenol mebutate (PEP005). Approved as a gel in January of 2012, ingenol mebutate is a natural diterpene from the Euphorbia peplus flowering plant in Southeast Asia. The agent is believed to augment neutrophil-killing ability on abnormal cells via damaging mitochondria, and its antiangiogenic properties promote healing and skin regeneration. "It’s proven histologically in superficial basal cell epithelioma, which is interesting, because this drug is approved only in the United States and the indication is only for actinic keratosis and not for superficial basal cells," Dr. Marcus said.
In early studies, a 0.0025% preparation resulted in 38% clearance and was tolerable, while a 0.125% preparation gave 100% clearance but was too irritating. The approved form of ingenol mebutate gel is a 0.015% formulation applied daily for 3 days to head areas and ingenol mebutate gel 0.05% applied daily for 2 days to body areas. "You apply it for 3 days, but the reaction actually peaks on the 4th day, so when the patients are not applying it, they are still going to get a bit of redness before they’re into the healing phase," Dr. Marcus said. "The advantage is that you’re only applying it for 2-3 days, and then you’re done, so it increases compliance."
Photodynamic therapy is useful for field therapy, and it is done in one office visit, so compliance is not an issue, Dr. Marcus said. Photodynamic therapy also is covered by Medicare. "It may illicit some burning and require hand holding, but is effective," she said. "The key is combination therapy."
Dr. Marcus disclosed that she has received honoraria, grants, and research support from numerous pharmaceutical companies.
DENVER – Changes in health insurance coverage are prompting a resurgence in the use of 5-fluorouracil to treat actinic keratoses, according to Dr. Linda Susan Marcus.
Not only is 5-fluorouracil (5-FU) effective, "but it has become increasingly difficult to get some of the newer topical agents covered by health insurance plans, especially Medicare. This is the reality now," Dr. Marcus said at the annual meeting of the American Academy of Dermatology.
Dr. Marcus, a dermatologist in Wyckoff, N.J., noted that 5-FU blocks methylation of deoxyuridylic acid to thymidylic acid in DNA, altering only fast-dividing cancerous cells. The agent is available in 1%, 2%, and 5% solutions, and in 1% and 5% creams. "We don’t really use the solutions much anymore; they’re very irritating," Dr. Marcus said. "The 1% cream is less irritating, but 5% cream is really the gold standard." Her approach is to have patients apply the 5% 5-FU cream to the affected area twice a day for 3 weeks. Another option is a 0.5% 5-FU cream with a microsphere delivery system "that traps the active ingredients in the skin surface to increase efficacy and decrease irritancy," she said. "Some people use this for maintenance or cycle therapy prior to cryosurgery."
As for side effects, 5-FU elicits erythema, scaliness, and crusting (which can be avoided with the milder preparations); but these conditions are self-limited, Dr. Marcus noted. Some dermatologists use topical steroids or hyaluronic acid gels "to make the erythema go away faster," she added. "There are studies that say if you use these topical steroids, it curtails efficacy and you might lose some efficacy. That might be true. However, you have to make it user-friendly for the patient. Use your clinical judgment."
Other topical preparations for actinic keratoses on the market include:
• Diclofenac sodium 3% in 2.5% hyaluronic acid gel. This colorless agent is designed to be applied twice a day for 2-3 months. "That can pose a compliance issue for some patients," Dr. Marcus said. "The mechanism is unknown, but it probably functions as an NSAID that may involve prostaglandin levels in UV exposed skin and upregulation of COX-2, which may promote proliferation. Cyclooxygenase is the rate-limiting enzyme step in prostaglandin synthesis."
Dr. Marcus said that that diclofenac sodium 3% in 2.5% hyaluronic acid gel may be best suited for patients with mild lesions and for pre- or post cryosurgery.
• Imiquimod. A 5% formulation of imiquimod "is becoming the new gold standard of topical therapies, but it can be irritating," Dr. Marcus said. A 3.75% formulation is available that is designed to be used for 2 weeks, followed by a 2-week break, and then the patient repeats the cycle, Dr. Marcus said, adding that she uses the 3.75% formulation most often for her patients with actinic keratoses.
Dr. Marcus described imiquimod as an immune response modifier that induces mRNA encoding cytokines like alpha-interferon, TNF, and interleukin-12 for a cytotoxic T-lymphocyte response.
"There’s a direct proapoptotic effect in changing cancerous cells as a result of bypassing transduction paths activating caspase-3 downstream of membrane-bound death receptor activation," she said. "You can get a severe reaction, but there shouldn’t be a lot of pain. You get excellent cosmetic results upon healing."
Dermatologists often tweak the frequency of application, she added, and results from some studies suggest that outcomes with imiquimod are similar to those obtained with 5-FU, while others hint that imiquimod may provide longer-lasting results. "Field-directed therapy is the advantage since it brings out subclinical lesions, but you need a lot of hand holding to encourage patients with this phenomenon," Dr. Marcus said.
• Ingenol mebutate (PEP005). Approved as a gel in January of 2012, ingenol mebutate is a natural diterpene from the Euphorbia peplus flowering plant in Southeast Asia. The agent is believed to augment neutrophil-killing ability on abnormal cells via damaging mitochondria, and its antiangiogenic properties promote healing and skin regeneration. "It’s proven histologically in superficial basal cell epithelioma, which is interesting, because this drug is approved only in the United States and the indication is only for actinic keratosis and not for superficial basal cells," Dr. Marcus said.
In early studies, a 0.0025% preparation resulted in 38% clearance and was tolerable, while a 0.125% preparation gave 100% clearance but was too irritating. The approved form of ingenol mebutate gel is a 0.015% formulation applied daily for 3 days to head areas and ingenol mebutate gel 0.05% applied daily for 2 days to body areas. "You apply it for 3 days, but the reaction actually peaks on the 4th day, so when the patients are not applying it, they are still going to get a bit of redness before they’re into the healing phase," Dr. Marcus said. "The advantage is that you’re only applying it for 2-3 days, and then you’re done, so it increases compliance."
Photodynamic therapy is useful for field therapy, and it is done in one office visit, so compliance is not an issue, Dr. Marcus said. Photodynamic therapy also is covered by Medicare. "It may illicit some burning and require hand holding, but is effective," she said. "The key is combination therapy."
Dr. Marcus disclosed that she has received honoraria, grants, and research support from numerous pharmaceutical companies.
EXPERT ANALYSIS FROM THE AAD ANNUAL MEETING
New study supports imiquimod for lentigo maligna
WAIKOLOA, HAWAII – The topical immunomodulator imiquimod appears to be beneficial therapy in selected patients with lentigo maligna or lentigo maligna melanoma, according to the largest case series to date.
"Based upon our experience, imiquimod 5% cream is a viable option as primary or adjunctive therapy of lentigo maligna and the in situ component of lentigo maligna melanoma in patients where surgery is really not feasible, or you’ve optimized surgical margins without achieving a clear histologic margin. I emphasize this should only be considered for in situ melanoma; we’re not using it to treat invasive melanoma," Dr. Susan M. Swetter said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
A cautionary note: Imiquimod (Aldara) for lentigo maligna (LM) is off-label therapy. It’s crucial to discuss with candidates the limitations of nonsurgical treatment for LM and lentigo maligna melanoma (LMM), which includes increased risk of local recurrence because of the lack of margin control, the possibility of a missed invasive melanoma, and the lack of supporting evidence from randomized trials with long-term follow-up.
"When we’re talking about imiquimod as potential treatment for lentigo maligna – and I can’t emphasize this enough – it requires close clinical follow-up, [carefully] documented discussion with the patient, and strong patient compliance. We have had cases of lentigo maligna melanoma where there have been metastases, not because of the in situ component, but because of the initial invasive tumor. You’ve got to follow these patients long term like a hawk. I follow them with Wood’s lamp and dermoscopy, and I biopsy any pigmentation," said Dr. Swetter, professor of dermatology and director of the pigmented lesion and cutaneous melanoma clinic at Stanford (Calif.) University Medical Center.
That being said, there are many scenarios in which surgical excision – the recommended first-line treatment for LM and LMM – might not be feasible. These include the sizable numbers of patients of advanced age who have limiting comorbid medical conditions or cognitive impairment. Also, LM has a strong predilection for the head and neck, and some affected patients avidly wish to avoid potentially disfiguring surgery.
Dr. Swetter presented a retrospective study of 60 patients with 62 LM or LMM lesions treated with imiquimod 5% cream at Stanford or the Veterans Affairs Palo Alto Health Care System. Most she treated personally. Imiquimod was primary therapy in 20 cases and adjuvant therapy following failed surgical attempts to achieve histologic clearance in the rest.
Pathologically, 45% of the lesions were LM, 29% were atypical intraepidermal melanocytic proliferations or poorly evolving LM, and 26% were LMM with histologic transection of LM and excision of the invasive melanoma component. Three-quarters of treated lesions were on the head or neck.
The lesions had a mean Breslow depth of 1.17 mm. Prior to imiquimod, half of the patients had one excision and another 19% had two or more. The mean duration of imiquimod therapy was 10.9 weeks, with a mean post treatment follow-up of 38 months.
"I typically treat to a 2-cm margin around the lesion," the dermatologist noted.
The overall clinical or histologic clearance rate was 86%. This is strikingly similar to the collective 82% rate in 46 reports involving 264 treated patients described in a published review by dermatologists at the University of Colorado, Denver (Dermatol. Surg. 2012;38:937-46).
An inflammatory response to imiquimod was predictive of favorable treatment outcome, especially in patients using the topical agent as primary therapy. Of the 20 patients who used imiquimod as primary therapy, all 16 who experienced clinical and/or histologic clearance had an initial inflammatory response. In contrast, none of the four clinical nonresponders showed an inflammatory response.
Outcomes weren’t quite as good in patients using imiquimod as adjuvant therapy.
"This is likely related to more challenging cases in the adjuvant setting, where the patients may have failed multiple attempts at wide local excision for histologic clearance," Dr. Swetter surmised.
Although the use of imiquimod as treatment for LM remains off label, it’s worth noting that it receives support in major practice guidelines. National Comprehensive Cancer Network guidelines recommend consideration of topical imiquimod or radiotherapy as adjunctive treatment for LM in selected patients after optimal surgery, with a level 2B recommendation. And the American Academy of Dermatology lists imiquimod as among the adjunctive options (J. Am. Acad. Dermatol. 2009; 61:865-7). What’s really needed now, according to Dr. Swetter, is a randomized controlled trial to firmly demonstrate benefit.
Session chair Dr. Allan C. Halpern said he and his colleagues have also been using topical imiquimod to treat LM with impressive results.
"What we’ve been doing, without literature to support it, is a broad shave biopsy, essentially removing the lesion, and then going right in with imiquimod before the patient heals. We’ve gotten some really wonderful clinical results," said Dr. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center in New York.
"That said, I think it’s important to remind people that – even with surgery – we do see occasional recurrences of desmoplastic melanomas in association with prior lentigo maligna. There’s no reason to think that’s going to be any less common in the imiquimod era. It’s going to happen to us," he cautioned.
Dr. Swetter reported having no financial conflicts. Dr. Halpern has received research grants from SciBase, DermTech, Caliber, and Canfield.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The topical immunomodulator imiquimod appears to be beneficial therapy in selected patients with lentigo maligna or lentigo maligna melanoma, according to the largest case series to date.
"Based upon our experience, imiquimod 5% cream is a viable option as primary or adjunctive therapy of lentigo maligna and the in situ component of lentigo maligna melanoma in patients where surgery is really not feasible, or you’ve optimized surgical margins without achieving a clear histologic margin. I emphasize this should only be considered for in situ melanoma; we’re not using it to treat invasive melanoma," Dr. Susan M. Swetter said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
A cautionary note: Imiquimod (Aldara) for lentigo maligna (LM) is off-label therapy. It’s crucial to discuss with candidates the limitations of nonsurgical treatment for LM and lentigo maligna melanoma (LMM), which includes increased risk of local recurrence because of the lack of margin control, the possibility of a missed invasive melanoma, and the lack of supporting evidence from randomized trials with long-term follow-up.
"When we’re talking about imiquimod as potential treatment for lentigo maligna – and I can’t emphasize this enough – it requires close clinical follow-up, [carefully] documented discussion with the patient, and strong patient compliance. We have had cases of lentigo maligna melanoma where there have been metastases, not because of the in situ component, but because of the initial invasive tumor. You’ve got to follow these patients long term like a hawk. I follow them with Wood’s lamp and dermoscopy, and I biopsy any pigmentation," said Dr. Swetter, professor of dermatology and director of the pigmented lesion and cutaneous melanoma clinic at Stanford (Calif.) University Medical Center.
That being said, there are many scenarios in which surgical excision – the recommended first-line treatment for LM and LMM – might not be feasible. These include the sizable numbers of patients of advanced age who have limiting comorbid medical conditions or cognitive impairment. Also, LM has a strong predilection for the head and neck, and some affected patients avidly wish to avoid potentially disfiguring surgery.
Dr. Swetter presented a retrospective study of 60 patients with 62 LM or LMM lesions treated with imiquimod 5% cream at Stanford or the Veterans Affairs Palo Alto Health Care System. Most she treated personally. Imiquimod was primary therapy in 20 cases and adjuvant therapy following failed surgical attempts to achieve histologic clearance in the rest.
Pathologically, 45% of the lesions were LM, 29% were atypical intraepidermal melanocytic proliferations or poorly evolving LM, and 26% were LMM with histologic transection of LM and excision of the invasive melanoma component. Three-quarters of treated lesions were on the head or neck.
The lesions had a mean Breslow depth of 1.17 mm. Prior to imiquimod, half of the patients had one excision and another 19% had two or more. The mean duration of imiquimod therapy was 10.9 weeks, with a mean post treatment follow-up of 38 months.
"I typically treat to a 2-cm margin around the lesion," the dermatologist noted.
The overall clinical or histologic clearance rate was 86%. This is strikingly similar to the collective 82% rate in 46 reports involving 264 treated patients described in a published review by dermatologists at the University of Colorado, Denver (Dermatol. Surg. 2012;38:937-46).
An inflammatory response to imiquimod was predictive of favorable treatment outcome, especially in patients using the topical agent as primary therapy. Of the 20 patients who used imiquimod as primary therapy, all 16 who experienced clinical and/or histologic clearance had an initial inflammatory response. In contrast, none of the four clinical nonresponders showed an inflammatory response.
Outcomes weren’t quite as good in patients using imiquimod as adjuvant therapy.
"This is likely related to more challenging cases in the adjuvant setting, where the patients may have failed multiple attempts at wide local excision for histologic clearance," Dr. Swetter surmised.
Although the use of imiquimod as treatment for LM remains off label, it’s worth noting that it receives support in major practice guidelines. National Comprehensive Cancer Network guidelines recommend consideration of topical imiquimod or radiotherapy as adjunctive treatment for LM in selected patients after optimal surgery, with a level 2B recommendation. And the American Academy of Dermatology lists imiquimod as among the adjunctive options (J. Am. Acad. Dermatol. 2009; 61:865-7). What’s really needed now, according to Dr. Swetter, is a randomized controlled trial to firmly demonstrate benefit.
Session chair Dr. Allan C. Halpern said he and his colleagues have also been using topical imiquimod to treat LM with impressive results.
"What we’ve been doing, without literature to support it, is a broad shave biopsy, essentially removing the lesion, and then going right in with imiquimod before the patient heals. We’ve gotten some really wonderful clinical results," said Dr. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center in New York.
"That said, I think it’s important to remind people that – even with surgery – we do see occasional recurrences of desmoplastic melanomas in association with prior lentigo maligna. There’s no reason to think that’s going to be any less common in the imiquimod era. It’s going to happen to us," he cautioned.
Dr. Swetter reported having no financial conflicts. Dr. Halpern has received research grants from SciBase, DermTech, Caliber, and Canfield.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The topical immunomodulator imiquimod appears to be beneficial therapy in selected patients with lentigo maligna or lentigo maligna melanoma, according to the largest case series to date.
"Based upon our experience, imiquimod 5% cream is a viable option as primary or adjunctive therapy of lentigo maligna and the in situ component of lentigo maligna melanoma in patients where surgery is really not feasible, or you’ve optimized surgical margins without achieving a clear histologic margin. I emphasize this should only be considered for in situ melanoma; we’re not using it to treat invasive melanoma," Dr. Susan M. Swetter said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
A cautionary note: Imiquimod (Aldara) for lentigo maligna (LM) is off-label therapy. It’s crucial to discuss with candidates the limitations of nonsurgical treatment for LM and lentigo maligna melanoma (LMM), which includes increased risk of local recurrence because of the lack of margin control, the possibility of a missed invasive melanoma, and the lack of supporting evidence from randomized trials with long-term follow-up.
"When we’re talking about imiquimod as potential treatment for lentigo maligna – and I can’t emphasize this enough – it requires close clinical follow-up, [carefully] documented discussion with the patient, and strong patient compliance. We have had cases of lentigo maligna melanoma where there have been metastases, not because of the in situ component, but because of the initial invasive tumor. You’ve got to follow these patients long term like a hawk. I follow them with Wood’s lamp and dermoscopy, and I biopsy any pigmentation," said Dr. Swetter, professor of dermatology and director of the pigmented lesion and cutaneous melanoma clinic at Stanford (Calif.) University Medical Center.
That being said, there are many scenarios in which surgical excision – the recommended first-line treatment for LM and LMM – might not be feasible. These include the sizable numbers of patients of advanced age who have limiting comorbid medical conditions or cognitive impairment. Also, LM has a strong predilection for the head and neck, and some affected patients avidly wish to avoid potentially disfiguring surgery.
Dr. Swetter presented a retrospective study of 60 patients with 62 LM or LMM lesions treated with imiquimod 5% cream at Stanford or the Veterans Affairs Palo Alto Health Care System. Most she treated personally. Imiquimod was primary therapy in 20 cases and adjuvant therapy following failed surgical attempts to achieve histologic clearance in the rest.
Pathologically, 45% of the lesions were LM, 29% were atypical intraepidermal melanocytic proliferations or poorly evolving LM, and 26% were LMM with histologic transection of LM and excision of the invasive melanoma component. Three-quarters of treated lesions were on the head or neck.
The lesions had a mean Breslow depth of 1.17 mm. Prior to imiquimod, half of the patients had one excision and another 19% had two or more. The mean duration of imiquimod therapy was 10.9 weeks, with a mean post treatment follow-up of 38 months.
"I typically treat to a 2-cm margin around the lesion," the dermatologist noted.
The overall clinical or histologic clearance rate was 86%. This is strikingly similar to the collective 82% rate in 46 reports involving 264 treated patients described in a published review by dermatologists at the University of Colorado, Denver (Dermatol. Surg. 2012;38:937-46).
An inflammatory response to imiquimod was predictive of favorable treatment outcome, especially in patients using the topical agent as primary therapy. Of the 20 patients who used imiquimod as primary therapy, all 16 who experienced clinical and/or histologic clearance had an initial inflammatory response. In contrast, none of the four clinical nonresponders showed an inflammatory response.
Outcomes weren’t quite as good in patients using imiquimod as adjuvant therapy.
"This is likely related to more challenging cases in the adjuvant setting, where the patients may have failed multiple attempts at wide local excision for histologic clearance," Dr. Swetter surmised.
Although the use of imiquimod as treatment for LM remains off label, it’s worth noting that it receives support in major practice guidelines. National Comprehensive Cancer Network guidelines recommend consideration of topical imiquimod or radiotherapy as adjunctive treatment for LM in selected patients after optimal surgery, with a level 2B recommendation. And the American Academy of Dermatology lists imiquimod as among the adjunctive options (J. Am. Acad. Dermatol. 2009; 61:865-7). What’s really needed now, according to Dr. Swetter, is a randomized controlled trial to firmly demonstrate benefit.
Session chair Dr. Allan C. Halpern said he and his colleagues have also been using topical imiquimod to treat LM with impressive results.
"What we’ve been doing, without literature to support it, is a broad shave biopsy, essentially removing the lesion, and then going right in with imiquimod before the patient heals. We’ve gotten some really wonderful clinical results," said Dr. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center in New York.
"That said, I think it’s important to remind people that – even with surgery – we do see occasional recurrences of desmoplastic melanomas in association with prior lentigo maligna. There’s no reason to think that’s going to be any less common in the imiquimod era. It’s going to happen to us," he cautioned.
Dr. Swetter reported having no financial conflicts. Dr. Halpern has received research grants from SciBase, DermTech, Caliber, and Canfield.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Immunotherapy for melanoma progresses with some interesting response patterns
HOLLYWOOD, FLA. – Tremendous progress has been made in recent years in the area of immunotherapy for the treatment of melanoma, and new agents and combinations continue to emerge, with some interesting response patterns, according to Dr. John A. Thompson.
Ipilimumab, for example, was added as a category 1 first-line systemic treatment option for melanoma to the 2012 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Melanoma. The change was based largely on two trials showing prolonged survival in patients treated with the monoclonal antibody.
In one study (the MDX010-20 trial), 650 patients with previously treated metastatic melanoma were randomized to receive either ipilimumab and a gp100 vaccine, ipilimumab alone, or vaccine alone. Those who received ipilimumab had better overall survival (10 months, 10.1 months, and 6.4 months, respectively). The hazard ratios for death were 0.68 and 0.66 for ipilimumab plus gp100 vs. gp100 alone, and for ipilimumab alone vs. gp100 alone, respectively (N. Engl. J. Med. 2010;363:711-23).
A plateau on the survival curve after about 2.5 years out to about 5 years suggests that ipilimumab-treated patients may have prolonged survival, said Dr. Thompson, codirector of the Seattle Cancer Care Alliance Melanoma Clinic and a member of the NCCN Guidelines Panel on Melanoma.
The second trial (CA184-024) was a randomized controlled trial of ipilimumab as first-line therapy in about 500 patients with metastatic melanoma who were randomized to receive ipilimumab and dacarbazine or placebo and dacarbazine. The ipilimumab group had significantly better overall survival (11.2 vs. 9.1 months), and survival was higher among the ipilimumab-treated patients at 1 year (47.3% vs. 36.3%), 2 years (28.5% vs. 17.9%, and 3 years (20.8% vs. 12.2%), with a hazard ratio for death of 0.72 (N. Engl. J. Med. 2011;364:2517-26).
It is important to keep in mind that ipilimumab can be associated with an "interesting" response pattern, and that response may be delayed, Dr. Thompson noted at the annual conference of the National Comprehensive Cancer Network.
He described one case involving a patient with extensive disease considered to be nonresectable. After 12 weeks, having received four doses, the patient’s disease appeared to progress with swelling and an increase in the size of tumors.
However, without further therapy, the disease began to regress by week 14, and by 2 years it was completely eliminated, Dr. Thompson said.
"So we have to be patient and wait for the generation of an immune response to ipilimumab," he said.
In a more recent study, ipilimumab was shown to be useful for the treatment of uveal melanoma. The response rate in 39 patients with metastatic uveal melanoma who were included in the multicenter, retrospective study and who were treated with either 3 mg/kg (34 patients) or 10 mg/kg (5 patients), was 2.6% at 12 weeks, and the response plus stable disease rate was 46% (Cancer 2013;119:3687).
At week 23, the response rate was 2.6% and the response plus stable disease rate was 28.2%. One patient had a complete response, and 1 had a partial response at 100 weeks, for an immune-related response rate of 5.1%. The median overall survival from first dose was 9.6 months.
Treatment in all three trials was associated with significant toxicity, Dr. Thompson said.
"We have to be very careful in managing the potential for immune-related adverse events. I think everyone using this agent or similar immune-checkpoint inhibitor drugs should educate themselves about the types of toxicities and how they should be handled," he said, noting that useful information about the risks of serious immune-mediated adverse reactions, along with algorithms for managing them, can be found at www.yervoy.com/hcp/rems.
He also said he routinely gives patients a "wallet card" that lists potential side effects, which can be helpful for identifying drug-related effects and for providing valuable information during doctor or emergency department visits.
Toxicities generally involve the skin (pruritus, rash), the gastrointestinal tract (diarrhea, abdominal pain, blood in stool, bowel perforation, peritoneal signs), the liver (elevated aspartate aminotransferase/alanine aminotransferase or bilirubin), the endocrine system (fatigue, headache, mental status changes, hypotension, abnormal thyroid function tests/serum chemistries), and the neurological system (unilateral or bilateral weakness, sensory alterations, paresthesias).
Toxicities affecting the skin typically appear around the time of the second dose, and GI effects tend to begin around the time of the third dose, he said. The GI toxicities affect up to 25% of patients, can progress rapidly, and require active intervention; patients should be advised not to ignore symptoms or write them off as a result of "something they ate."
Endocrine toxicities tend to occur toward the end of treatment, and some can be subtle in onset and difficult to diagnose without careful monitoring.
In general, mild toxicities should prompt evaluation for other causes of the symptoms, and can be managed with symptomatic therapy. Moderate toxicities (four to six stools per day over baseline, abdominal pain, blood in the stool, for example) should prompt withholding of ipilimumab, and treatment with prednisone or an equivalent at 0.5 mg/kg day if the symptoms persist for more than a week.
For severe toxicity (seven or more stools per day over baseline, peritoneal signs along with signs of perforation, ileus, and fever, for example), discontinue ipilimumab; evaluate for bowel perforation; consider endoscopy; and give steroids at 1-2 mg/kg per day until the patient improves, with tapering over a month, he said.
As for other emerging immunotherapy drugs, anti–programmed death-1 (anti-PD-1) antibodies now in development are showing great promise in melanoma. One recent study showed that combining an anti-PD-1 (nivolumab) and ipilimumab was effective for the treatment of advanced melanoma (N. Engl. J. Med. 2013;369:122-33). Concurrent treatment with both agents was associated with a "very encouraging" 53% objective response rate in 53 patients, "albeit with a high rate of grade III/IV toxicity," he said.
"The findings are quite striking in terms of the degree of suppression in tumor measurement," he added, noting that the studies are ongoing.
Compared with targeted therapy for melanoma, which tends to have early and dramatic results, with tumor shrinkage and delayed tumor progression early, but with a plateau over the long term ("the answer is still out" on long-term efficacy, Dr. Thompson noted), immunotherapy tends to have little effect early in the course of therapy but is associated with a "promising tail on the survival curve, where there’s a subset of patients who have very durable response and survival," he said.
A simplified treatment algorithm can be used to help select the appropriate treatment, he added.
For patients with low-volume BRAF wild-type disease (who thus are not thought to be eligible for BRAF-directed therapy), consider clinical trial enrollment or high-dose interleukin-2, ipilimumab, or an anti-PD1 (expected to be available soon, according to Dr. Thompson).
For those with BRAF wild-type and symptomatic bulky disease, the choice is more difficult given the delayed immune response with ipilimumab. Consider a clinical trial; combining cytotoxic agents with an immune checkpoint inhibitor may also be appropriate in these patients, he said.
The decision is also complicated for those with documented BRAF mutation and low-volume disease, as going straight to targeted therapy is an option, but trying an immune checkpoint drug first, and moving to targeted therapy if the patient fails to respond, is also an option.
Targeted therapy is recommended for those with BRAF mutation and bulky disease, he said.
As for patients who have undergone resection for stage 3 or certain high-risk stage 2 disease, the guidelines call for consideration of adjuvant therapy. Interferon is an approved therapy for these patients, but there has been a lot of disagreement among NCCN Melanoma Panel members regarding its use because of the side-effect profile.
A study looking at ipilimumab vs. placebo in these patients is underway, as is a trial comparing low- and high-dose ipilimumab and interferon.
"We are eagerly awaiting the results," he said, noting that other areas of interest with respect to immunotherapy include T-cell therapy (including cells with engineered immune receptors); lymphokines (such as IL-15 and IL-21), either alone or in combination with vaccines or immune checkpoint inhibitors; receptor-directed cytokines; and combinations of targeted agents with immunomodulators.
Dr. Thompson reported receiving grant or research support from Bristol-Myers Squibb, Exelixis, Genentech, and GlaxoSmithKline.
HOLLYWOOD, FLA. – Tremendous progress has been made in recent years in the area of immunotherapy for the treatment of melanoma, and new agents and combinations continue to emerge, with some interesting response patterns, according to Dr. John A. Thompson.
Ipilimumab, for example, was added as a category 1 first-line systemic treatment option for melanoma to the 2012 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Melanoma. The change was based largely on two trials showing prolonged survival in patients treated with the monoclonal antibody.
In one study (the MDX010-20 trial), 650 patients with previously treated metastatic melanoma were randomized to receive either ipilimumab and a gp100 vaccine, ipilimumab alone, or vaccine alone. Those who received ipilimumab had better overall survival (10 months, 10.1 months, and 6.4 months, respectively). The hazard ratios for death were 0.68 and 0.66 for ipilimumab plus gp100 vs. gp100 alone, and for ipilimumab alone vs. gp100 alone, respectively (N. Engl. J. Med. 2010;363:711-23).
A plateau on the survival curve after about 2.5 years out to about 5 years suggests that ipilimumab-treated patients may have prolonged survival, said Dr. Thompson, codirector of the Seattle Cancer Care Alliance Melanoma Clinic and a member of the NCCN Guidelines Panel on Melanoma.
The second trial (CA184-024) was a randomized controlled trial of ipilimumab as first-line therapy in about 500 patients with metastatic melanoma who were randomized to receive ipilimumab and dacarbazine or placebo and dacarbazine. The ipilimumab group had significantly better overall survival (11.2 vs. 9.1 months), and survival was higher among the ipilimumab-treated patients at 1 year (47.3% vs. 36.3%), 2 years (28.5% vs. 17.9%, and 3 years (20.8% vs. 12.2%), with a hazard ratio for death of 0.72 (N. Engl. J. Med. 2011;364:2517-26).
It is important to keep in mind that ipilimumab can be associated with an "interesting" response pattern, and that response may be delayed, Dr. Thompson noted at the annual conference of the National Comprehensive Cancer Network.
He described one case involving a patient with extensive disease considered to be nonresectable. After 12 weeks, having received four doses, the patient’s disease appeared to progress with swelling and an increase in the size of tumors.
However, without further therapy, the disease began to regress by week 14, and by 2 years it was completely eliminated, Dr. Thompson said.
"So we have to be patient and wait for the generation of an immune response to ipilimumab," he said.
In a more recent study, ipilimumab was shown to be useful for the treatment of uveal melanoma. The response rate in 39 patients with metastatic uveal melanoma who were included in the multicenter, retrospective study and who were treated with either 3 mg/kg (34 patients) or 10 mg/kg (5 patients), was 2.6% at 12 weeks, and the response plus stable disease rate was 46% (Cancer 2013;119:3687).
At week 23, the response rate was 2.6% and the response plus stable disease rate was 28.2%. One patient had a complete response, and 1 had a partial response at 100 weeks, for an immune-related response rate of 5.1%. The median overall survival from first dose was 9.6 months.
Treatment in all three trials was associated with significant toxicity, Dr. Thompson said.
"We have to be very careful in managing the potential for immune-related adverse events. I think everyone using this agent or similar immune-checkpoint inhibitor drugs should educate themselves about the types of toxicities and how they should be handled," he said, noting that useful information about the risks of serious immune-mediated adverse reactions, along with algorithms for managing them, can be found at www.yervoy.com/hcp/rems.
He also said he routinely gives patients a "wallet card" that lists potential side effects, which can be helpful for identifying drug-related effects and for providing valuable information during doctor or emergency department visits.
Toxicities generally involve the skin (pruritus, rash), the gastrointestinal tract (diarrhea, abdominal pain, blood in stool, bowel perforation, peritoneal signs), the liver (elevated aspartate aminotransferase/alanine aminotransferase or bilirubin), the endocrine system (fatigue, headache, mental status changes, hypotension, abnormal thyroid function tests/serum chemistries), and the neurological system (unilateral or bilateral weakness, sensory alterations, paresthesias).
Toxicities affecting the skin typically appear around the time of the second dose, and GI effects tend to begin around the time of the third dose, he said. The GI toxicities affect up to 25% of patients, can progress rapidly, and require active intervention; patients should be advised not to ignore symptoms or write them off as a result of "something they ate."
Endocrine toxicities tend to occur toward the end of treatment, and some can be subtle in onset and difficult to diagnose without careful monitoring.
In general, mild toxicities should prompt evaluation for other causes of the symptoms, and can be managed with symptomatic therapy. Moderate toxicities (four to six stools per day over baseline, abdominal pain, blood in the stool, for example) should prompt withholding of ipilimumab, and treatment with prednisone or an equivalent at 0.5 mg/kg day if the symptoms persist for more than a week.
For severe toxicity (seven or more stools per day over baseline, peritoneal signs along with signs of perforation, ileus, and fever, for example), discontinue ipilimumab; evaluate for bowel perforation; consider endoscopy; and give steroids at 1-2 mg/kg per day until the patient improves, with tapering over a month, he said.
As for other emerging immunotherapy drugs, anti–programmed death-1 (anti-PD-1) antibodies now in development are showing great promise in melanoma. One recent study showed that combining an anti-PD-1 (nivolumab) and ipilimumab was effective for the treatment of advanced melanoma (N. Engl. J. Med. 2013;369:122-33). Concurrent treatment with both agents was associated with a "very encouraging" 53% objective response rate in 53 patients, "albeit with a high rate of grade III/IV toxicity," he said.
"The findings are quite striking in terms of the degree of suppression in tumor measurement," he added, noting that the studies are ongoing.
Compared with targeted therapy for melanoma, which tends to have early and dramatic results, with tumor shrinkage and delayed tumor progression early, but with a plateau over the long term ("the answer is still out" on long-term efficacy, Dr. Thompson noted), immunotherapy tends to have little effect early in the course of therapy but is associated with a "promising tail on the survival curve, where there’s a subset of patients who have very durable response and survival," he said.
A simplified treatment algorithm can be used to help select the appropriate treatment, he added.
For patients with low-volume BRAF wild-type disease (who thus are not thought to be eligible for BRAF-directed therapy), consider clinical trial enrollment or high-dose interleukin-2, ipilimumab, or an anti-PD1 (expected to be available soon, according to Dr. Thompson).
For those with BRAF wild-type and symptomatic bulky disease, the choice is more difficult given the delayed immune response with ipilimumab. Consider a clinical trial; combining cytotoxic agents with an immune checkpoint inhibitor may also be appropriate in these patients, he said.
The decision is also complicated for those with documented BRAF mutation and low-volume disease, as going straight to targeted therapy is an option, but trying an immune checkpoint drug first, and moving to targeted therapy if the patient fails to respond, is also an option.
Targeted therapy is recommended for those with BRAF mutation and bulky disease, he said.
As for patients who have undergone resection for stage 3 or certain high-risk stage 2 disease, the guidelines call for consideration of adjuvant therapy. Interferon is an approved therapy for these patients, but there has been a lot of disagreement among NCCN Melanoma Panel members regarding its use because of the side-effect profile.
A study looking at ipilimumab vs. placebo in these patients is underway, as is a trial comparing low- and high-dose ipilimumab and interferon.
"We are eagerly awaiting the results," he said, noting that other areas of interest with respect to immunotherapy include T-cell therapy (including cells with engineered immune receptors); lymphokines (such as IL-15 and IL-21), either alone or in combination with vaccines or immune checkpoint inhibitors; receptor-directed cytokines; and combinations of targeted agents with immunomodulators.
Dr. Thompson reported receiving grant or research support from Bristol-Myers Squibb, Exelixis, Genentech, and GlaxoSmithKline.
HOLLYWOOD, FLA. – Tremendous progress has been made in recent years in the area of immunotherapy for the treatment of melanoma, and new agents and combinations continue to emerge, with some interesting response patterns, according to Dr. John A. Thompson.
Ipilimumab, for example, was added as a category 1 first-line systemic treatment option for melanoma to the 2012 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Melanoma. The change was based largely on two trials showing prolonged survival in patients treated with the monoclonal antibody.
In one study (the MDX010-20 trial), 650 patients with previously treated metastatic melanoma were randomized to receive either ipilimumab and a gp100 vaccine, ipilimumab alone, or vaccine alone. Those who received ipilimumab had better overall survival (10 months, 10.1 months, and 6.4 months, respectively). The hazard ratios for death were 0.68 and 0.66 for ipilimumab plus gp100 vs. gp100 alone, and for ipilimumab alone vs. gp100 alone, respectively (N. Engl. J. Med. 2010;363:711-23).
A plateau on the survival curve after about 2.5 years out to about 5 years suggests that ipilimumab-treated patients may have prolonged survival, said Dr. Thompson, codirector of the Seattle Cancer Care Alliance Melanoma Clinic and a member of the NCCN Guidelines Panel on Melanoma.
The second trial (CA184-024) was a randomized controlled trial of ipilimumab as first-line therapy in about 500 patients with metastatic melanoma who were randomized to receive ipilimumab and dacarbazine or placebo and dacarbazine. The ipilimumab group had significantly better overall survival (11.2 vs. 9.1 months), and survival was higher among the ipilimumab-treated patients at 1 year (47.3% vs. 36.3%), 2 years (28.5% vs. 17.9%, and 3 years (20.8% vs. 12.2%), with a hazard ratio for death of 0.72 (N. Engl. J. Med. 2011;364:2517-26).
It is important to keep in mind that ipilimumab can be associated with an "interesting" response pattern, and that response may be delayed, Dr. Thompson noted at the annual conference of the National Comprehensive Cancer Network.
He described one case involving a patient with extensive disease considered to be nonresectable. After 12 weeks, having received four doses, the patient’s disease appeared to progress with swelling and an increase in the size of tumors.
However, without further therapy, the disease began to regress by week 14, and by 2 years it was completely eliminated, Dr. Thompson said.
"So we have to be patient and wait for the generation of an immune response to ipilimumab," he said.
In a more recent study, ipilimumab was shown to be useful for the treatment of uveal melanoma. The response rate in 39 patients with metastatic uveal melanoma who were included in the multicenter, retrospective study and who were treated with either 3 mg/kg (34 patients) or 10 mg/kg (5 patients), was 2.6% at 12 weeks, and the response plus stable disease rate was 46% (Cancer 2013;119:3687).
At week 23, the response rate was 2.6% and the response plus stable disease rate was 28.2%. One patient had a complete response, and 1 had a partial response at 100 weeks, for an immune-related response rate of 5.1%. The median overall survival from first dose was 9.6 months.
Treatment in all three trials was associated with significant toxicity, Dr. Thompson said.
"We have to be very careful in managing the potential for immune-related adverse events. I think everyone using this agent or similar immune-checkpoint inhibitor drugs should educate themselves about the types of toxicities and how they should be handled," he said, noting that useful information about the risks of serious immune-mediated adverse reactions, along with algorithms for managing them, can be found at www.yervoy.com/hcp/rems.
He also said he routinely gives patients a "wallet card" that lists potential side effects, which can be helpful for identifying drug-related effects and for providing valuable information during doctor or emergency department visits.
Toxicities generally involve the skin (pruritus, rash), the gastrointestinal tract (diarrhea, abdominal pain, blood in stool, bowel perforation, peritoneal signs), the liver (elevated aspartate aminotransferase/alanine aminotransferase or bilirubin), the endocrine system (fatigue, headache, mental status changes, hypotension, abnormal thyroid function tests/serum chemistries), and the neurological system (unilateral or bilateral weakness, sensory alterations, paresthesias).
Toxicities affecting the skin typically appear around the time of the second dose, and GI effects tend to begin around the time of the third dose, he said. The GI toxicities affect up to 25% of patients, can progress rapidly, and require active intervention; patients should be advised not to ignore symptoms or write them off as a result of "something they ate."
Endocrine toxicities tend to occur toward the end of treatment, and some can be subtle in onset and difficult to diagnose without careful monitoring.
In general, mild toxicities should prompt evaluation for other causes of the symptoms, and can be managed with symptomatic therapy. Moderate toxicities (four to six stools per day over baseline, abdominal pain, blood in the stool, for example) should prompt withholding of ipilimumab, and treatment with prednisone or an equivalent at 0.5 mg/kg day if the symptoms persist for more than a week.
For severe toxicity (seven or more stools per day over baseline, peritoneal signs along with signs of perforation, ileus, and fever, for example), discontinue ipilimumab; evaluate for bowel perforation; consider endoscopy; and give steroids at 1-2 mg/kg per day until the patient improves, with tapering over a month, he said.
As for other emerging immunotherapy drugs, anti–programmed death-1 (anti-PD-1) antibodies now in development are showing great promise in melanoma. One recent study showed that combining an anti-PD-1 (nivolumab) and ipilimumab was effective for the treatment of advanced melanoma (N. Engl. J. Med. 2013;369:122-33). Concurrent treatment with both agents was associated with a "very encouraging" 53% objective response rate in 53 patients, "albeit with a high rate of grade III/IV toxicity," he said.
"The findings are quite striking in terms of the degree of suppression in tumor measurement," he added, noting that the studies are ongoing.
Compared with targeted therapy for melanoma, which tends to have early and dramatic results, with tumor shrinkage and delayed tumor progression early, but with a plateau over the long term ("the answer is still out" on long-term efficacy, Dr. Thompson noted), immunotherapy tends to have little effect early in the course of therapy but is associated with a "promising tail on the survival curve, where there’s a subset of patients who have very durable response and survival," he said.
A simplified treatment algorithm can be used to help select the appropriate treatment, he added.
For patients with low-volume BRAF wild-type disease (who thus are not thought to be eligible for BRAF-directed therapy), consider clinical trial enrollment or high-dose interleukin-2, ipilimumab, or an anti-PD1 (expected to be available soon, according to Dr. Thompson).
For those with BRAF wild-type and symptomatic bulky disease, the choice is more difficult given the delayed immune response with ipilimumab. Consider a clinical trial; combining cytotoxic agents with an immune checkpoint inhibitor may also be appropriate in these patients, he said.
The decision is also complicated for those with documented BRAF mutation and low-volume disease, as going straight to targeted therapy is an option, but trying an immune checkpoint drug first, and moving to targeted therapy if the patient fails to respond, is also an option.
Targeted therapy is recommended for those with BRAF mutation and bulky disease, he said.
As for patients who have undergone resection for stage 3 or certain high-risk stage 2 disease, the guidelines call for consideration of adjuvant therapy. Interferon is an approved therapy for these patients, but there has been a lot of disagreement among NCCN Melanoma Panel members regarding its use because of the side-effect profile.
A study looking at ipilimumab vs. placebo in these patients is underway, as is a trial comparing low- and high-dose ipilimumab and interferon.
"We are eagerly awaiting the results," he said, noting that other areas of interest with respect to immunotherapy include T-cell therapy (including cells with engineered immune receptors); lymphokines (such as IL-15 and IL-21), either alone or in combination with vaccines or immune checkpoint inhibitors; receptor-directed cytokines; and combinations of targeted agents with immunomodulators.
Dr. Thompson reported receiving grant or research support from Bristol-Myers Squibb, Exelixis, Genentech, and GlaxoSmithKline.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE
Delayed response in ipilimumab therapy
Metastatic melanoma is a deadly disease with a 5-year survival rate lower than 20%.1 In 2011, ipilimumab, a fully humanized antibody that binds to cytotoxic T-lymphocyte–associated antigen 4 (CTLA4) was approved by the US Food and Drug Administration based on improved survival in a pivotal trial.2 CTLA4 is a molecule on cytotoxic T-lymphocytes that plays a critical role in attenuating immune responses. Ipilimumab blocks the binding of B7, the ligand of CTLA4, thereby blocking the activation of CTLA4 and sustaining antitumor immune responses. The time course to response can be variable with immunotherapeutics. We report on a patient who experienced a considerable delay before responding to ipilimumab.
Click on the PDF icon at the top of this introduction to read the full article.
Metastatic melanoma is a deadly disease with a 5-year survival rate lower than 20%.1 In 2011, ipilimumab, a fully humanized antibody that binds to cytotoxic T-lymphocyte–associated antigen 4 (CTLA4) was approved by the US Food and Drug Administration based on improved survival in a pivotal trial.2 CTLA4 is a molecule on cytotoxic T-lymphocytes that plays a critical role in attenuating immune responses. Ipilimumab blocks the binding of B7, the ligand of CTLA4, thereby blocking the activation of CTLA4 and sustaining antitumor immune responses. The time course to response can be variable with immunotherapeutics. We report on a patient who experienced a considerable delay before responding to ipilimumab.
Click on the PDF icon at the top of this introduction to read the full article.
Metastatic melanoma is a deadly disease with a 5-year survival rate lower than 20%.1 In 2011, ipilimumab, a fully humanized antibody that binds to cytotoxic T-lymphocyte–associated antigen 4 (CTLA4) was approved by the US Food and Drug Administration based on improved survival in a pivotal trial.2 CTLA4 is a molecule on cytotoxic T-lymphocytes that plays a critical role in attenuating immune responses. Ipilimumab blocks the binding of B7, the ligand of CTLA4, thereby blocking the activation of CTLA4 and sustaining antitumor immune responses. The time course to response can be variable with immunotherapeutics. We report on a patient who experienced a considerable delay before responding to ipilimumab.
Click on the PDF icon at the top of this introduction to read the full article.
Melanomas were less invasive at diagnosis in patients with established dermatologist
People diagnosed with melanoma were significantly more likely to have melanoma in situ and to have thinner invasive melanomas if they had a regular dermatologist, compared with those who did not have a regular dermatologist, in a retrospective study of 388 patients diagnosed at an academic dermatology department.
These findings were statistically significant for those who had detected the melanomas themselves, but not for those whose dermatologists had detected the melanomas. Self-detected melanomas were in situ in 36 of 61 (59.0%) patients with an established dermatologist vs. 40 of 108 (37.0%) patients without an established dermatologist (P =.006), reported Michelle Cheng and her associates at the University of Pittsburgh.
The time needed to wait to see a dermatologist and the time since the last dermatologic examination were not associated with the invasiveness or the depth of the melanomas at the time of the diagnosis, the investigators said.
These findings "may be explained by a high benefit associated with a first dermatologic visit because of patient education about melanoma detection and/or having a dermatologist to call when the patient finds a suspicious lesion," they wrote (J. Am. Acad. Dermatol. 2014 March [doi:10.1016/j.jaad.2013.10.060]).
The study addressed the uncertainties about the impact of different factors on the invasiveness and depth of melanoma: having had a previous physical exam by a dermatologist before the diagnosis, the recency of that exam, and the time needed to wait for an appointment. The 388 adults (mean age, 55 years) were diagnosed with melanoma at the University of Pittsburgh Medical Center between February 2003 and December 2010. Of these patients, 51% had detected the melanoma themselves and 37% had had a dermatologic exam within the previous year at the university. Of the 388 melanomas diagnosed, 44% (171) were invasive with a mean Breslow depth of 0.96 mm. About 18% (71 patients), had a history of melanoma, and about 37% had seen a dermatologist within the previous year.
Of the 317 with no previous history of melanoma, almost 64% (103 of 162) of those with an established dermatologist were diagnosed with melanoma in situ vs. 44.5% (69 of 155) of those without an established dermatologist, a statistically significant difference (P = .001). The depths of the lesions were also significantly lower among those with an established dermatologist than among those with no dermatologist (median depth, 0.48 mm vs. 0.61 mm, respectively; P = .003).
Among the patients with self-detected melanoma, 41% of those with an established dermatologist had invasive disease, vs. 63% of those who did not have an established dermatologist (P = .006). But among the patients whose melanomas were detected by the dermatologist, 31% of those with an established dermatologist had invasive disease, vs. 40% of those with no established dermatologist, which was not statistically significant (P = .323).
When considering that a skin cancer screen is cost effective, the authors concluded, the results of this study "highlight the value of having even a single dermatologic examination and suggest that educating patients to detect their own melanomas is an important part of improving early detection of melanoma."
All of the patients were from one part of Pennsylvania and were treated at the same medical center, which was a limitation of the study, but the patients were heterogenous and were treated at four dermatology clinics by different attending dermatologists, the authors said. They could not confirm that each patient received the same level of education about melanoma at their visits, but added that most of the dermatologists in the clinics teach the ABCDEs of melanoma and provide counseling in skin self-exams and AAD skin cancer brochures.
The study included clinical research fellowship funding from the Doris Duke Charitable Foundation for one of the authors, UL1/NIH funding for another author, and funding for the statistics consultation from the National Institutes of Health. The authors declared no conflicts of interest.
People diagnosed with melanoma were significantly more likely to have melanoma in situ and to have thinner invasive melanomas if they had a regular dermatologist, compared with those who did not have a regular dermatologist, in a retrospective study of 388 patients diagnosed at an academic dermatology department.
These findings were statistically significant for those who had detected the melanomas themselves, but not for those whose dermatologists had detected the melanomas. Self-detected melanomas were in situ in 36 of 61 (59.0%) patients with an established dermatologist vs. 40 of 108 (37.0%) patients without an established dermatologist (P =.006), reported Michelle Cheng and her associates at the University of Pittsburgh.
The time needed to wait to see a dermatologist and the time since the last dermatologic examination were not associated with the invasiveness or the depth of the melanomas at the time of the diagnosis, the investigators said.
These findings "may be explained by a high benefit associated with a first dermatologic visit because of patient education about melanoma detection and/or having a dermatologist to call when the patient finds a suspicious lesion," they wrote (J. Am. Acad. Dermatol. 2014 March [doi:10.1016/j.jaad.2013.10.060]).
The study addressed the uncertainties about the impact of different factors on the invasiveness and depth of melanoma: having had a previous physical exam by a dermatologist before the diagnosis, the recency of that exam, and the time needed to wait for an appointment. The 388 adults (mean age, 55 years) were diagnosed with melanoma at the University of Pittsburgh Medical Center between February 2003 and December 2010. Of these patients, 51% had detected the melanoma themselves and 37% had had a dermatologic exam within the previous year at the university. Of the 388 melanomas diagnosed, 44% (171) were invasive with a mean Breslow depth of 0.96 mm. About 18% (71 patients), had a history of melanoma, and about 37% had seen a dermatologist within the previous year.
Of the 317 with no previous history of melanoma, almost 64% (103 of 162) of those with an established dermatologist were diagnosed with melanoma in situ vs. 44.5% (69 of 155) of those without an established dermatologist, a statistically significant difference (P = .001). The depths of the lesions were also significantly lower among those with an established dermatologist than among those with no dermatologist (median depth, 0.48 mm vs. 0.61 mm, respectively; P = .003).
Among the patients with self-detected melanoma, 41% of those with an established dermatologist had invasive disease, vs. 63% of those who did not have an established dermatologist (P = .006). But among the patients whose melanomas were detected by the dermatologist, 31% of those with an established dermatologist had invasive disease, vs. 40% of those with no established dermatologist, which was not statistically significant (P = .323).
When considering that a skin cancer screen is cost effective, the authors concluded, the results of this study "highlight the value of having even a single dermatologic examination and suggest that educating patients to detect their own melanomas is an important part of improving early detection of melanoma."
All of the patients were from one part of Pennsylvania and were treated at the same medical center, which was a limitation of the study, but the patients were heterogenous and were treated at four dermatology clinics by different attending dermatologists, the authors said. They could not confirm that each patient received the same level of education about melanoma at their visits, but added that most of the dermatologists in the clinics teach the ABCDEs of melanoma and provide counseling in skin self-exams and AAD skin cancer brochures.
The study included clinical research fellowship funding from the Doris Duke Charitable Foundation for one of the authors, UL1/NIH funding for another author, and funding for the statistics consultation from the National Institutes of Health. The authors declared no conflicts of interest.
People diagnosed with melanoma were significantly more likely to have melanoma in situ and to have thinner invasive melanomas if they had a regular dermatologist, compared with those who did not have a regular dermatologist, in a retrospective study of 388 patients diagnosed at an academic dermatology department.
These findings were statistically significant for those who had detected the melanomas themselves, but not for those whose dermatologists had detected the melanomas. Self-detected melanomas were in situ in 36 of 61 (59.0%) patients with an established dermatologist vs. 40 of 108 (37.0%) patients without an established dermatologist (P =.006), reported Michelle Cheng and her associates at the University of Pittsburgh.
The time needed to wait to see a dermatologist and the time since the last dermatologic examination were not associated with the invasiveness or the depth of the melanomas at the time of the diagnosis, the investigators said.
These findings "may be explained by a high benefit associated with a first dermatologic visit because of patient education about melanoma detection and/or having a dermatologist to call when the patient finds a suspicious lesion," they wrote (J. Am. Acad. Dermatol. 2014 March [doi:10.1016/j.jaad.2013.10.060]).
The study addressed the uncertainties about the impact of different factors on the invasiveness and depth of melanoma: having had a previous physical exam by a dermatologist before the diagnosis, the recency of that exam, and the time needed to wait for an appointment. The 388 adults (mean age, 55 years) were diagnosed with melanoma at the University of Pittsburgh Medical Center between February 2003 and December 2010. Of these patients, 51% had detected the melanoma themselves and 37% had had a dermatologic exam within the previous year at the university. Of the 388 melanomas diagnosed, 44% (171) were invasive with a mean Breslow depth of 0.96 mm. About 18% (71 patients), had a history of melanoma, and about 37% had seen a dermatologist within the previous year.
Of the 317 with no previous history of melanoma, almost 64% (103 of 162) of those with an established dermatologist were diagnosed with melanoma in situ vs. 44.5% (69 of 155) of those without an established dermatologist, a statistically significant difference (P = .001). The depths of the lesions were also significantly lower among those with an established dermatologist than among those with no dermatologist (median depth, 0.48 mm vs. 0.61 mm, respectively; P = .003).
Among the patients with self-detected melanoma, 41% of those with an established dermatologist had invasive disease, vs. 63% of those who did not have an established dermatologist (P = .006). But among the patients whose melanomas were detected by the dermatologist, 31% of those with an established dermatologist had invasive disease, vs. 40% of those with no established dermatologist, which was not statistically significant (P = .323).
When considering that a skin cancer screen is cost effective, the authors concluded, the results of this study "highlight the value of having even a single dermatologic examination and suggest that educating patients to detect their own melanomas is an important part of improving early detection of melanoma."
All of the patients were from one part of Pennsylvania and were treated at the same medical center, which was a limitation of the study, but the patients were heterogenous and were treated at four dermatology clinics by different attending dermatologists, the authors said. They could not confirm that each patient received the same level of education about melanoma at their visits, but added that most of the dermatologists in the clinics teach the ABCDEs of melanoma and provide counseling in skin self-exams and AAD skin cancer brochures.
The study included clinical research fellowship funding from the Doris Duke Charitable Foundation for one of the authors, UL1/NIH funding for another author, and funding for the statistics consultation from the National Institutes of Health. The authors declared no conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major finding: In a study of 388 patients diagnosed with melanoma, those who had an established dermatologist were significantly more likely to be diagnosed with melanoma in situ than those with no dermatologist (64% vs. 44.5%; P = .001), and had thinner invasive melanoma-findings (median depth, 0.48 mm vs. 0.61 mm; P = .003).
Data source: A retrospective study of 388 patients with biopsy-confirmed melanomas at an academic medical center, which evaluated the impact of having an established dermatologist and other factors on the depth of the melanoma when diagnosed.
Disclosures: The study included Clinical Research Fellowship funding from the Doris Duke Charitable Foundation for one of the authors, UL1/NIH funding for another author, and funding for the statistics consultation from the National Institutes of Health. The authors declared no conflicts of interest.
Cell-penetrating domain improves therapeutic cancer vaccine potency
Fusing a tumor-specific antigen with a cell-penetrating domain can generate a more potent therapeutic dendritic cell–based cancer vaccine by enhancing intracellular bioavailability without altering the dendritic cell surface antigens, researchers reported online March 26 in JAMA Surgery.
The immune system can eliminate tumor cells through the generation of cytotoxic T lymphocytes, a process known as immune surveillance, but cancer cells can evade this process, and immunotherapy with dendritic cells is a possible means of reengaging the immune system.
Cancer-testis antigens are ideal candidates for tumor immunotherapy because they are restricted to gonadal germ cells and are unexpressed in healthy adult tissue. Melanoma antigen family A, 3 (MAGE-A3) is a cancer-testis antigen that has attracted attention because it is expressed in a wide variety of cancer types.
A team led by Ramesh B. Batchu, Ph.D., of Wayne State University, Detroit, cloned and purified MAGE-A3 with an amino acid sequence that can transport large proteins across the plasma membrane, to address the problem of inadequate cytoplasmic expression of tumor-specific antigens, and thus enhance production of cytotoxic T lymphocytes.
In a series of laboratory experiments, the cell-penetrating, domain-fused melanoma antigen did show more rapid and efficient penetration of the dendritic cell membrane compared with the normal antigen, Dr. Batchu and his associates reported online (JAMA Surgery 2014 March 26 [doi: 10.1001/jamasurg.2013.4113]).
"We have demonstrated for the first time, to our knowledge, that cloning and purifying MAGE-A3 with CPD [cell-penetrating domain] enhances its cytosolic bioavailability in DCs [dendritic cells] without altering cell surface antigens required for T-cell activation, potentially making it a more potent therapeutic cancer vaccine compared with existing MAGE-A3 protein and peptide vaccines," the authors wrote.
Dr. Batchu and his colleagues reported that they had no conflicts of interest.
Fusing a tumor-specific antigen with a cell-penetrating domain can generate a more potent therapeutic dendritic cell–based cancer vaccine by enhancing intracellular bioavailability without altering the dendritic cell surface antigens, researchers reported online March 26 in JAMA Surgery.
The immune system can eliminate tumor cells through the generation of cytotoxic T lymphocytes, a process known as immune surveillance, but cancer cells can evade this process, and immunotherapy with dendritic cells is a possible means of reengaging the immune system.
Cancer-testis antigens are ideal candidates for tumor immunotherapy because they are restricted to gonadal germ cells and are unexpressed in healthy adult tissue. Melanoma antigen family A, 3 (MAGE-A3) is a cancer-testis antigen that has attracted attention because it is expressed in a wide variety of cancer types.
A team led by Ramesh B. Batchu, Ph.D., of Wayne State University, Detroit, cloned and purified MAGE-A3 with an amino acid sequence that can transport large proteins across the plasma membrane, to address the problem of inadequate cytoplasmic expression of tumor-specific antigens, and thus enhance production of cytotoxic T lymphocytes.
In a series of laboratory experiments, the cell-penetrating, domain-fused melanoma antigen did show more rapid and efficient penetration of the dendritic cell membrane compared with the normal antigen, Dr. Batchu and his associates reported online (JAMA Surgery 2014 March 26 [doi: 10.1001/jamasurg.2013.4113]).
"We have demonstrated for the first time, to our knowledge, that cloning and purifying MAGE-A3 with CPD [cell-penetrating domain] enhances its cytosolic bioavailability in DCs [dendritic cells] without altering cell surface antigens required for T-cell activation, potentially making it a more potent therapeutic cancer vaccine compared with existing MAGE-A3 protein and peptide vaccines," the authors wrote.
Dr. Batchu and his colleagues reported that they had no conflicts of interest.
Fusing a tumor-specific antigen with a cell-penetrating domain can generate a more potent therapeutic dendritic cell–based cancer vaccine by enhancing intracellular bioavailability without altering the dendritic cell surface antigens, researchers reported online March 26 in JAMA Surgery.
The immune system can eliminate tumor cells through the generation of cytotoxic T lymphocytes, a process known as immune surveillance, but cancer cells can evade this process, and immunotherapy with dendritic cells is a possible means of reengaging the immune system.
Cancer-testis antigens are ideal candidates for tumor immunotherapy because they are restricted to gonadal germ cells and are unexpressed in healthy adult tissue. Melanoma antigen family A, 3 (MAGE-A3) is a cancer-testis antigen that has attracted attention because it is expressed in a wide variety of cancer types.
A team led by Ramesh B. Batchu, Ph.D., of Wayne State University, Detroit, cloned and purified MAGE-A3 with an amino acid sequence that can transport large proteins across the plasma membrane, to address the problem of inadequate cytoplasmic expression of tumor-specific antigens, and thus enhance production of cytotoxic T lymphocytes.
In a series of laboratory experiments, the cell-penetrating, domain-fused melanoma antigen did show more rapid and efficient penetration of the dendritic cell membrane compared with the normal antigen, Dr. Batchu and his associates reported online (JAMA Surgery 2014 March 26 [doi: 10.1001/jamasurg.2013.4113]).
"We have demonstrated for the first time, to our knowledge, that cloning and purifying MAGE-A3 with CPD [cell-penetrating domain] enhances its cytosolic bioavailability in DCs [dendritic cells] without altering cell surface antigens required for T-cell activation, potentially making it a more potent therapeutic cancer vaccine compared with existing MAGE-A3 protein and peptide vaccines," the authors wrote.
Dr. Batchu and his colleagues reported that they had no conflicts of interest.
FROM JAMA SURGERY
Major finding: A cell-penetrating, domain-fused melanoma antigen showed more rapid and efficient penetration of the dendritic cell membrane than did the normal antigen.
Data source: Laboratory experiments on dendritic cell penetration by tumor-specific MAGE-A3.
Disclosures: Dr. Batchu and his colleagues reported that they had no conflicts of interest.
In situ melanoma high risk for subsequent diagnosis
In situ melanoma patients have a significant, substantially elevated risk for subsequent invasive or in situ melanoma – nearly as high as the risk for patients presenting with more invasive disease.
The finding means that "education and continued surveillance are paramount not only for persons with an invasive melanoma but also for persons with an in situ melanoma," wrote Danny R. Youlden in JAMA Dermatology, published online March 19 (doi:10.1001/jamadermatol.2013.9852).
Mr. Youlden, a biostatistician with Cancer Council Queensland, in Brisbane, Australia, and his colleagues looked at 39,668 cases of first primary invasive melanoma, and 22,845 patients with first primary in situ melanoma, diagnosed between 1982 and 2005.
Patients were followed for a median of more than 9 years, during which time there were 5,358 subsequent primary invasive melanomas; 3,520 (66%) of these occurred in patients with previous invasive melanomas.
Compared with the general Australia population, that amounted to a standardized incidence ratio for primary invasive melanoma of 5.42 for patients with previous invasive melanoma diagnoses (95% CI, 5.23-5.61) and 4.59 for patients with previous in situ melanoma (95% CI, 4.37-4.82).
Moreover, the authors found that the body site of the second melanoma was typically the same as for the first invasive or in situ diagnosis, especially on the head and lower extremities; in particular, females with a first primary invasive melanoma on the head had a standardized incidence ratio of 13.32 for a second primary invasive melanoma of the head, compared with the general population (95% CI, 10.28-16.98).
The authors disclosed no conflicts of interest; several reported grants from the National Health and Medical Research Council.
In situ melanoma patients have a significant, substantially elevated risk for subsequent invasive or in situ melanoma – nearly as high as the risk for patients presenting with more invasive disease.
The finding means that "education and continued surveillance are paramount not only for persons with an invasive melanoma but also for persons with an in situ melanoma," wrote Danny R. Youlden in JAMA Dermatology, published online March 19 (doi:10.1001/jamadermatol.2013.9852).
Mr. Youlden, a biostatistician with Cancer Council Queensland, in Brisbane, Australia, and his colleagues looked at 39,668 cases of first primary invasive melanoma, and 22,845 patients with first primary in situ melanoma, diagnosed between 1982 and 2005.
Patients were followed for a median of more than 9 years, during which time there were 5,358 subsequent primary invasive melanomas; 3,520 (66%) of these occurred in patients with previous invasive melanomas.
Compared with the general Australia population, that amounted to a standardized incidence ratio for primary invasive melanoma of 5.42 for patients with previous invasive melanoma diagnoses (95% CI, 5.23-5.61) and 4.59 for patients with previous in situ melanoma (95% CI, 4.37-4.82).
Moreover, the authors found that the body site of the second melanoma was typically the same as for the first invasive or in situ diagnosis, especially on the head and lower extremities; in particular, females with a first primary invasive melanoma on the head had a standardized incidence ratio of 13.32 for a second primary invasive melanoma of the head, compared with the general population (95% CI, 10.28-16.98).
The authors disclosed no conflicts of interest; several reported grants from the National Health and Medical Research Council.
In situ melanoma patients have a significant, substantially elevated risk for subsequent invasive or in situ melanoma – nearly as high as the risk for patients presenting with more invasive disease.
The finding means that "education and continued surveillance are paramount not only for persons with an invasive melanoma but also for persons with an in situ melanoma," wrote Danny R. Youlden in JAMA Dermatology, published online March 19 (doi:10.1001/jamadermatol.2013.9852).
Mr. Youlden, a biostatistician with Cancer Council Queensland, in Brisbane, Australia, and his colleagues looked at 39,668 cases of first primary invasive melanoma, and 22,845 patients with first primary in situ melanoma, diagnosed between 1982 and 2005.
Patients were followed for a median of more than 9 years, during which time there were 5,358 subsequent primary invasive melanomas; 3,520 (66%) of these occurred in patients with previous invasive melanomas.
Compared with the general Australia population, that amounted to a standardized incidence ratio for primary invasive melanoma of 5.42 for patients with previous invasive melanoma diagnoses (95% CI, 5.23-5.61) and 4.59 for patients with previous in situ melanoma (95% CI, 4.37-4.82).
Moreover, the authors found that the body site of the second melanoma was typically the same as for the first invasive or in situ diagnosis, especially on the head and lower extremities; in particular, females with a first primary invasive melanoma on the head had a standardized incidence ratio of 13.32 for a second primary invasive melanoma of the head, compared with the general population (95% CI, 10.28-16.98).
The authors disclosed no conflicts of interest; several reported grants from the National Health and Medical Research Council.
FROM JAMA DERMATOLOGY
Major finding: In situ melanoma patients have a nearly fivefold greater risk of subsequent primary invasive melanoma, compared with the general population.
Data source: A retrospective cohort study of the Queensland Cancer Registry.
Disclosures: The authors disclosed no conflicts of interest; several reported grants from the National Health and Medical Research Council.
VIDEO: The DecisionDx-Melanoma test can predict metastasis of sentinel node-negative melanomas
DENVER – A 134-patient study of patients with stage I, II, or III cutaneous melanoma found that the DecisionDx-Melanoma test was useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results.
In a video interview, Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University Skin Cancer Institute, Chicago, explains the best uses for the test and its patient management advantages.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
DENVER – A 134-patient study of patients with stage I, II, or III cutaneous melanoma found that the DecisionDx-Melanoma test was useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results.
In a video interview, Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University Skin Cancer Institute, Chicago, explains the best uses for the test and its patient management advantages.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
DENVER – A 134-patient study of patients with stage I, II, or III cutaneous melanoma found that the DecisionDx-Melanoma test was useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results.
In a video interview, Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University Skin Cancer Institute, Chicago, explains the best uses for the test and its patient management advantages.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
AT THE AAD ANNUAL MEETING
Gene test predicts metastasis of sentinel node-negative melanomas
DENVER – A gene expression profile test was an independent predictor of metastasis of primary cutaneous melanomas in patients with negative sentinel lymph node biopsies.
The DecisionDx-Melanoma test is useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results, said Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University, Chicago, Skin Cancer Institute. The test "is an independent predictor of metastasis and death, and significantly improves upon sentinel lymph node biopsy for staging melanoma patients."
The results of the DecisionDx-Melanoma test, a noninvasive 31-gene expression profile (GEP) test, were compared with the results of sentinel lymph node biopsy (SLNB) in 134 patients who had stage I, II, or III cutaneous melanoma and underwent a documented sentinel lymph node procedure. Of the 134 patients, 28 had positive sentinel lymph nodes and 91 had positive (class 2, high risk) GEP results.
Metastases developed over a subsequent 5-year period in 18 of the 28 patients with positive SLNB and in 62 of the 91 patients with positive GEP results. Metastases developed in 51 of the 106 patients with negative SLNB and in 7 of 43 patients with negative (class 1, low risk) GEP results.
While the positive predictive value of the two tests were comparable, the ability of GEP to predict negative outcomes was significantly better than that of SLNB (P less than .0001), Dr. Gerami reported at the annual meeting of the American Academy of Dermatology.
The rate of 5-year metastasis-free survival (MFS) was 55% for 106 patients with negative SLNB, compared to 37% for 28 patients with positive SLNB (P = .003). The GEP test results showed improved prognostic accuracy in these same patients with an MFS of 87% for the 43 patients with negative GEP (class 1, low risk) results and of 31% for the 91 patients with positive GEP (class 2, high risk) results (P less than .0001).
Differences in overall survival (OS) paralleled the MFS rates, with SLNB-negative patients having a 5-year OS of 67% and SLNB-positive patients having a 5-year OS of 55% (P = .024). OS for negative GEP (class 1, low risk) patients was 92% and for positive GEP (high risk, class 2) was 49% (P less than .0001).
Use of the GEP test also was analyzed in combination with SLNB status. As expected, the 20% of patients (n = 27) who had high-risk results for both tests (GEP class 2 and SLNB-positive findings) had lower survival rates (MFS, 34%; OS, 53%). Similarly, the 31% of patients (n = 42) who had low-risk results for both tests (GEP class 1 and SLNB-negative findings) had higher survival rates (MFS, 82%; OS, 92%).
Importantly, the MFS was 31% and the OS was 49% at 5 years in the 64 patients who had SLNB-negative results but class 2 GEP test results, Dr. Gerami said. Cox multivariate analysis comparing the GEP test to SLNB showed the GEP test to be the only independent and highly significant prognostic factor in this analysis (P less than .000003).
Dr. Gerami has been a consultant to Castle Biosciences. The DecisionDx-Melanoma test is a product of Castle Biosciences, the sponsor of the study. More information about the test can be found at www.skinmelanoma.com.
On Twitter @maryjodales
DENVER – A gene expression profile test was an independent predictor of metastasis of primary cutaneous melanomas in patients with negative sentinel lymph node biopsies.
The DecisionDx-Melanoma test is useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results, said Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University, Chicago, Skin Cancer Institute. The test "is an independent predictor of metastasis and death, and significantly improves upon sentinel lymph node biopsy for staging melanoma patients."
The results of the DecisionDx-Melanoma test, a noninvasive 31-gene expression profile (GEP) test, were compared with the results of sentinel lymph node biopsy (SLNB) in 134 patients who had stage I, II, or III cutaneous melanoma and underwent a documented sentinel lymph node procedure. Of the 134 patients, 28 had positive sentinel lymph nodes and 91 had positive (class 2, high risk) GEP results.
Metastases developed over a subsequent 5-year period in 18 of the 28 patients with positive SLNB and in 62 of the 91 patients with positive GEP results. Metastases developed in 51 of the 106 patients with negative SLNB and in 7 of 43 patients with negative (class 1, low risk) GEP results.
While the positive predictive value of the two tests were comparable, the ability of GEP to predict negative outcomes was significantly better than that of SLNB (P less than .0001), Dr. Gerami reported at the annual meeting of the American Academy of Dermatology.
The rate of 5-year metastasis-free survival (MFS) was 55% for 106 patients with negative SLNB, compared to 37% for 28 patients with positive SLNB (P = .003). The GEP test results showed improved prognostic accuracy in these same patients with an MFS of 87% for the 43 patients with negative GEP (class 1, low risk) results and of 31% for the 91 patients with positive GEP (class 2, high risk) results (P less than .0001).
Differences in overall survival (OS) paralleled the MFS rates, with SLNB-negative patients having a 5-year OS of 67% and SLNB-positive patients having a 5-year OS of 55% (P = .024). OS for negative GEP (class 1, low risk) patients was 92% and for positive GEP (high risk, class 2) was 49% (P less than .0001).
Use of the GEP test also was analyzed in combination with SLNB status. As expected, the 20% of patients (n = 27) who had high-risk results for both tests (GEP class 2 and SLNB-positive findings) had lower survival rates (MFS, 34%; OS, 53%). Similarly, the 31% of patients (n = 42) who had low-risk results for both tests (GEP class 1 and SLNB-negative findings) had higher survival rates (MFS, 82%; OS, 92%).
Importantly, the MFS was 31% and the OS was 49% at 5 years in the 64 patients who had SLNB-negative results but class 2 GEP test results, Dr. Gerami said. Cox multivariate analysis comparing the GEP test to SLNB showed the GEP test to be the only independent and highly significant prognostic factor in this analysis (P less than .000003).
Dr. Gerami has been a consultant to Castle Biosciences. The DecisionDx-Melanoma test is a product of Castle Biosciences, the sponsor of the study. More information about the test can be found at www.skinmelanoma.com.
On Twitter @maryjodales
DENVER – A gene expression profile test was an independent predictor of metastasis of primary cutaneous melanomas in patients with negative sentinel lymph node biopsies.
The DecisionDx-Melanoma test is useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results, said Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University, Chicago, Skin Cancer Institute. The test "is an independent predictor of metastasis and death, and significantly improves upon sentinel lymph node biopsy for staging melanoma patients."
The results of the DecisionDx-Melanoma test, a noninvasive 31-gene expression profile (GEP) test, were compared with the results of sentinel lymph node biopsy (SLNB) in 134 patients who had stage I, II, or III cutaneous melanoma and underwent a documented sentinel lymph node procedure. Of the 134 patients, 28 had positive sentinel lymph nodes and 91 had positive (class 2, high risk) GEP results.
Metastases developed over a subsequent 5-year period in 18 of the 28 patients with positive SLNB and in 62 of the 91 patients with positive GEP results. Metastases developed in 51 of the 106 patients with negative SLNB and in 7 of 43 patients with negative (class 1, low risk) GEP results.
While the positive predictive value of the two tests were comparable, the ability of GEP to predict negative outcomes was significantly better than that of SLNB (P less than .0001), Dr. Gerami reported at the annual meeting of the American Academy of Dermatology.
The rate of 5-year metastasis-free survival (MFS) was 55% for 106 patients with negative SLNB, compared to 37% for 28 patients with positive SLNB (P = .003). The GEP test results showed improved prognostic accuracy in these same patients with an MFS of 87% for the 43 patients with negative GEP (class 1, low risk) results and of 31% for the 91 patients with positive GEP (class 2, high risk) results (P less than .0001).
Differences in overall survival (OS) paralleled the MFS rates, with SLNB-negative patients having a 5-year OS of 67% and SLNB-positive patients having a 5-year OS of 55% (P = .024). OS for negative GEP (class 1, low risk) patients was 92% and for positive GEP (high risk, class 2) was 49% (P less than .0001).
Use of the GEP test also was analyzed in combination with SLNB status. As expected, the 20% of patients (n = 27) who had high-risk results for both tests (GEP class 2 and SLNB-positive findings) had lower survival rates (MFS, 34%; OS, 53%). Similarly, the 31% of patients (n = 42) who had low-risk results for both tests (GEP class 1 and SLNB-negative findings) had higher survival rates (MFS, 82%; OS, 92%).
Importantly, the MFS was 31% and the OS was 49% at 5 years in the 64 patients who had SLNB-negative results but class 2 GEP test results, Dr. Gerami said. Cox multivariate analysis comparing the GEP test to SLNB showed the GEP test to be the only independent and highly significant prognostic factor in this analysis (P less than .000003).
Dr. Gerami has been a consultant to Castle Biosciences. The DecisionDx-Melanoma test is a product of Castle Biosciences, the sponsor of the study. More information about the test can be found at www.skinmelanoma.com.
On Twitter @maryjodales
AT THE AAD ANNUAL MEETING
Major finding: Metastasis-free survival was 31% and overall survival was 49% at 5 years in the 64 patients who had negative sentinel node lymph biopsy results and high-risk, class 2 gene expression profile test results.
Data source: A study of 134 patients who had stage I, II, or III cutaneous melanoma and underwent a documented sentinel lymph node procedure.
Disclosures: Dr. Gerami has been a consultant to Castle Biosciences. The DecisionDx-Melanoma test is a product of Castle Biosciences, the study sponsor.