User login
Blood test predicts Merkel cell carcinoma metastases
DENVER – A newly-available test based on the results of a simple blood draw has proved useful for detecting early recurrences of Merkel cell carcinomas in those patients who produce antibodies to the Merkel polyomavirus oncoprotein at initial diagnosis.
Known as AMERK, the test can be used at diagnosis to determine which patients have the oncoprotein antibodies and performed at routine followups as an indicator of recurrence in asymptomatic, antibody-positive patients.
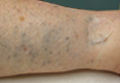
The test already has been shown to alert clinicians to the presence of surgically manageable metastases that would have otherwise gone undetected until symptoms prompted a tomographic scan, Dr. Astrid Blom reported at the annual meeting of the American Academy of Dermatology. She presented two cases of surgically operable metastatic Merkel cell tumors, one metastatic to the kidney and the other to the pancreas; both were detected via increasing titers of oncoprotein antibodies in otherwise asymptomatic patients.
Nearly 40% of Merkel cell carcinomas recur. Performing a predictive blood test at routine followups can be a reassuring measure in those patients with negative findings. It also can be an early indicator of metastasis in otherwise asymptomatic patients who made oncoprotein antibodies at diagnosis and were successfully treated for Merkel call carcinoma, she said.
Merkel cell polyomavirus drives about 80% of the approximately 2,000 Merkel cell carcinomas that are diagnosed each year. About half of affected patients produce oncoprotein antibodies to the polyomavirus, which are detectable at diagnosis. The AMERK test is useful only for followup of those patients with oncoprotein antibodies. Patients who lack initially detectable levels of oncoprotein antibodies at diagnosis do not later produce antibodies should their disease recur, reported Dr. Blom of the University of Washington, Seattle, where the test was developed and is performed.
The clinical utility of AMERK, a 75-step assay that takes nearly 2 days to perform, was verified using 1,342 samples from 104 controls and 519 patients from around the world, with data correlating to 3,018 status updates. The data analysis was limited to the 217 patients with adequate follow-up data.
A simple blood draw was collected and sent for analysis at the University of Washington, Seattle. Oncoprotein antibodies were detected in 52% of 217 patients with incident cases of Merkel cell carcinoma and in 2% of 530 control subjects. The antibody titers in controls were barely detectable, however, unlike the levels seen in the patients. The sensitivity of the test was 82%, and the specificity was 98%; the negative predictive value was 99%, and the positive predictive value was 78%.
Levels decrease by 90% or more over the course of the year after successful surgical treatment of Merkel cell carcinomas. When the cancers recur, at least a 10-fold increase in oncoprotein antibody titers are noted.
Support for the development of the AMERK test came from the National Institutes of Health, the National Cancer Institute, and the American Cancer Society and from private donors and patients.
RESOURCES:
Information about the test is available online at http://www.merkelcell.org/sero/
A video that discusses the antibody test can be viewed here.
Dr. Kelly Paulsen, et.al. published the scientific paper that first described the test (Cancer Res. 70(21): 8388-97).
How to order the Merkel virus serology test
Physicians and staff based outside of the University of Washington system should contact their local laboratory where the patient will have their blood sample collected to initiate the process.
The local laboratory "send out test coordinator" should contact the UW Reference Laboratory Services Call Center at 206-685-6066. UW Reference Laboratory Services will set up an account for the ordering physician or clinic; collect the required billing and reporting information; and provide requirements for sample collection, processing and shipping from the local laboratory. Once this one-time administrative process is complete, patient samples can be collected routinely from that facility for the Merkel antibody test (name of test is "AMERK").
On Twitter @maryjodales
DENVER – A newly-available test based on the results of a simple blood draw has proved useful for detecting early recurrences of Merkel cell carcinomas in those patients who produce antibodies to the Merkel polyomavirus oncoprotein at initial diagnosis.
Known as AMERK, the test can be used at diagnosis to determine which patients have the oncoprotein antibodies and performed at routine followups as an indicator of recurrence in asymptomatic, antibody-positive patients.
The test already has been shown to alert clinicians to the presence of surgically manageable metastases that would have otherwise gone undetected until symptoms prompted a tomographic scan, Dr. Astrid Blom reported at the annual meeting of the American Academy of Dermatology. She presented two cases of surgically operable metastatic Merkel cell tumors, one metastatic to the kidney and the other to the pancreas; both were detected via increasing titers of oncoprotein antibodies in otherwise asymptomatic patients.
Nearly 40% of Merkel cell carcinomas recur. Performing a predictive blood test at routine followups can be a reassuring measure in those patients with negative findings. It also can be an early indicator of metastasis in otherwise asymptomatic patients who made oncoprotein antibodies at diagnosis and were successfully treated for Merkel call carcinoma, she said.
Merkel cell polyomavirus drives about 80% of the approximately 2,000 Merkel cell carcinomas that are diagnosed each year. About half of affected patients produce oncoprotein antibodies to the polyomavirus, which are detectable at diagnosis. The AMERK test is useful only for followup of those patients with oncoprotein antibodies. Patients who lack initially detectable levels of oncoprotein antibodies at diagnosis do not later produce antibodies should their disease recur, reported Dr. Blom of the University of Washington, Seattle, where the test was developed and is performed.
The clinical utility of AMERK, a 75-step assay that takes nearly 2 days to perform, was verified using 1,342 samples from 104 controls and 519 patients from around the world, with data correlating to 3,018 status updates. The data analysis was limited to the 217 patients with adequate follow-up data.
A simple blood draw was collected and sent for analysis at the University of Washington, Seattle. Oncoprotein antibodies were detected in 52% of 217 patients with incident cases of Merkel cell carcinoma and in 2% of 530 control subjects. The antibody titers in controls were barely detectable, however, unlike the levels seen in the patients. The sensitivity of the test was 82%, and the specificity was 98%; the negative predictive value was 99%, and the positive predictive value was 78%.
Levels decrease by 90% or more over the course of the year after successful surgical treatment of Merkel cell carcinomas. When the cancers recur, at least a 10-fold increase in oncoprotein antibody titers are noted.
Support for the development of the AMERK test came from the National Institutes of Health, the National Cancer Institute, and the American Cancer Society and from private donors and patients.
RESOURCES:
Information about the test is available online at http://www.merkelcell.org/sero/
A video that discusses the antibody test can be viewed here.
Dr. Kelly Paulsen, et.al. published the scientific paper that first described the test (Cancer Res. 70(21): 8388-97).
How to order the Merkel virus serology test
Physicians and staff based outside of the University of Washington system should contact their local laboratory where the patient will have their blood sample collected to initiate the process.
The local laboratory "send out test coordinator" should contact the UW Reference Laboratory Services Call Center at 206-685-6066. UW Reference Laboratory Services will set up an account for the ordering physician or clinic; collect the required billing and reporting information; and provide requirements for sample collection, processing and shipping from the local laboratory. Once this one-time administrative process is complete, patient samples can be collected routinely from that facility for the Merkel antibody test (name of test is "AMERK").
On Twitter @maryjodales
DENVER – A newly-available test based on the results of a simple blood draw has proved useful for detecting early recurrences of Merkel cell carcinomas in those patients who produce antibodies to the Merkel polyomavirus oncoprotein at initial diagnosis.
Known as AMERK, the test can be used at diagnosis to determine which patients have the oncoprotein antibodies and performed at routine followups as an indicator of recurrence in asymptomatic, antibody-positive patients.
The test already has been shown to alert clinicians to the presence of surgically manageable metastases that would have otherwise gone undetected until symptoms prompted a tomographic scan, Dr. Astrid Blom reported at the annual meeting of the American Academy of Dermatology. She presented two cases of surgically operable metastatic Merkel cell tumors, one metastatic to the kidney and the other to the pancreas; both were detected via increasing titers of oncoprotein antibodies in otherwise asymptomatic patients.
Nearly 40% of Merkel cell carcinomas recur. Performing a predictive blood test at routine followups can be a reassuring measure in those patients with negative findings. It also can be an early indicator of metastasis in otherwise asymptomatic patients who made oncoprotein antibodies at diagnosis and were successfully treated for Merkel call carcinoma, she said.
Merkel cell polyomavirus drives about 80% of the approximately 2,000 Merkel cell carcinomas that are diagnosed each year. About half of affected patients produce oncoprotein antibodies to the polyomavirus, which are detectable at diagnosis. The AMERK test is useful only for followup of those patients with oncoprotein antibodies. Patients who lack initially detectable levels of oncoprotein antibodies at diagnosis do not later produce antibodies should their disease recur, reported Dr. Blom of the University of Washington, Seattle, where the test was developed and is performed.
The clinical utility of AMERK, a 75-step assay that takes nearly 2 days to perform, was verified using 1,342 samples from 104 controls and 519 patients from around the world, with data correlating to 3,018 status updates. The data analysis was limited to the 217 patients with adequate follow-up data.
A simple blood draw was collected and sent for analysis at the University of Washington, Seattle. Oncoprotein antibodies were detected in 52% of 217 patients with incident cases of Merkel cell carcinoma and in 2% of 530 control subjects. The antibody titers in controls were barely detectable, however, unlike the levels seen in the patients. The sensitivity of the test was 82%, and the specificity was 98%; the negative predictive value was 99%, and the positive predictive value was 78%.
Levels decrease by 90% or more over the course of the year after successful surgical treatment of Merkel cell carcinomas. When the cancers recur, at least a 10-fold increase in oncoprotein antibody titers are noted.
Support for the development of the AMERK test came from the National Institutes of Health, the National Cancer Institute, and the American Cancer Society and from private donors and patients.
RESOURCES:
Information about the test is available online at http://www.merkelcell.org/sero/
A video that discusses the antibody test can be viewed here.
Dr. Kelly Paulsen, et.al. published the scientific paper that first described the test (Cancer Res. 70(21): 8388-97).
How to order the Merkel virus serology test
Physicians and staff based outside of the University of Washington system should contact their local laboratory where the patient will have their blood sample collected to initiate the process.
The local laboratory "send out test coordinator" should contact the UW Reference Laboratory Services Call Center at 206-685-6066. UW Reference Laboratory Services will set up an account for the ordering physician or clinic; collect the required billing and reporting information; and provide requirements for sample collection, processing and shipping from the local laboratory. Once this one-time administrative process is complete, patient samples can be collected routinely from that facility for the Merkel antibody test (name of test is "AMERK").
On Twitter @maryjodales
AT THE AAD ANNUAL MEETING
Major finding: Two cases of surgically operable, metastatic Merkel cell tumors, one metastatic to the kidney and the other to the pancreas, were detected via increasing serum titers of oncoprotein antibodies as part of the followup of otherwise asymptomatic patients.
Data source: Followup study of the 52% of 217 patients who had incident cases of Merkel cell carcinoma and had positive titers for oncoprotein antibodies.
Disclosures: Support for the development of the AMERK test came from the National Institutes of Health, the National Cancer Institute, the American Cancer Society, and from private donors and patients.
New tanning bed technology no safer than the old
Tanning bed technology circa the year 2000, when the industry began using lamps that emit larger doses of long-wave ultraviolet A (between 335 and 400 nm), is no safer than tanning beds pre-2000, a meta-analysis of the association between melanoma and tanning beds has shown.
Globally, people who had been exposed to tanning beds were 16% more likely to have melanoma; in the United States, where tanning bed intensity is unrestricted, the risk from exposure is 23% higher than in the general population. In Europe and in Australia and New Zealand, where the intensity is limited to an ultraviolet index of 12 and 36 respectively, the increased risk was not significant, reported investigators in the March issue of the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2013.11.050).
Further, tanning bed use before the age of 25 years additionally increased a person’s melanoma odds to 35% compared with 11% in those who had indoor tanning exposure after the age of 25 years. Sophia Colantonia of the University of Ottawa and her coauthors also found a threshold effect of 10 or more tanning bed sessions being associated with the highest risk of melanoma.
"Assessing and communicating health risk to patients in an easily understood metric based on number of tanning bed sessions could be helpful to clinical practice," wrote the investigators.
For the meta-analysis, the investigators searched Scopus, MEDLINE, and the Cumulative Index to Nursing and Allied Health Literature for studies published before Aug. 14, 2013. In all, 31 studies that included 14,956 cases of melanoma and 233,106 controls were analyzed. In those who had ever used a tanning bed, the odds ratio (OR) for melanoma was 1.16 (95% CI, 1.05-1.28). Tanning bed use after 2000 had an OR of 1.22 (95% CI, 1.03-1.45). For individuals who had used 10 or more tanning bed sessions, the OR was 1.34 (95% CI, 1.05-1.71). North Americans were found to have higher rates of melanoma (OR, 1.23; 95% CI, 1.03-1.47) vs. Europeans and Oceanianians for whom the OR was not significant. Ten or more tanning sessions in a lifetime was associated with the highest risk of melanoma (OR, 1.34; 95% CI, 1.05-1.71).
Limitations to the meta-analysis included that not all of the studies used the same age range for their study groups, and evidence quality ranged from mediocre to poor, the investigators noted.
The investigators reported that there were no funding sources, and no conflicts of interest were declared.
Tanning bed technology circa the year 2000, when the industry began using lamps that emit larger doses of long-wave ultraviolet A (between 335 and 400 nm), is no safer than tanning beds pre-2000, a meta-analysis of the association between melanoma and tanning beds has shown.
Globally, people who had been exposed to tanning beds were 16% more likely to have melanoma; in the United States, where tanning bed intensity is unrestricted, the risk from exposure is 23% higher than in the general population. In Europe and in Australia and New Zealand, where the intensity is limited to an ultraviolet index of 12 and 36 respectively, the increased risk was not significant, reported investigators in the March issue of the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2013.11.050).
Further, tanning bed use before the age of 25 years additionally increased a person’s melanoma odds to 35% compared with 11% in those who had indoor tanning exposure after the age of 25 years. Sophia Colantonia of the University of Ottawa and her coauthors also found a threshold effect of 10 or more tanning bed sessions being associated with the highest risk of melanoma.
"Assessing and communicating health risk to patients in an easily understood metric based on number of tanning bed sessions could be helpful to clinical practice," wrote the investigators.
For the meta-analysis, the investigators searched Scopus, MEDLINE, and the Cumulative Index to Nursing and Allied Health Literature for studies published before Aug. 14, 2013. In all, 31 studies that included 14,956 cases of melanoma and 233,106 controls were analyzed. In those who had ever used a tanning bed, the odds ratio (OR) for melanoma was 1.16 (95% CI, 1.05-1.28). Tanning bed use after 2000 had an OR of 1.22 (95% CI, 1.03-1.45). For individuals who had used 10 or more tanning bed sessions, the OR was 1.34 (95% CI, 1.05-1.71). North Americans were found to have higher rates of melanoma (OR, 1.23; 95% CI, 1.03-1.47) vs. Europeans and Oceanianians for whom the OR was not significant. Ten or more tanning sessions in a lifetime was associated with the highest risk of melanoma (OR, 1.34; 95% CI, 1.05-1.71).
Limitations to the meta-analysis included that not all of the studies used the same age range for their study groups, and evidence quality ranged from mediocre to poor, the investigators noted.
The investigators reported that there were no funding sources, and no conflicts of interest were declared.
Tanning bed technology circa the year 2000, when the industry began using lamps that emit larger doses of long-wave ultraviolet A (between 335 and 400 nm), is no safer than tanning beds pre-2000, a meta-analysis of the association between melanoma and tanning beds has shown.
Globally, people who had been exposed to tanning beds were 16% more likely to have melanoma; in the United States, where tanning bed intensity is unrestricted, the risk from exposure is 23% higher than in the general population. In Europe and in Australia and New Zealand, where the intensity is limited to an ultraviolet index of 12 and 36 respectively, the increased risk was not significant, reported investigators in the March issue of the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2013.11.050).
Further, tanning bed use before the age of 25 years additionally increased a person’s melanoma odds to 35% compared with 11% in those who had indoor tanning exposure after the age of 25 years. Sophia Colantonia of the University of Ottawa and her coauthors also found a threshold effect of 10 or more tanning bed sessions being associated with the highest risk of melanoma.
"Assessing and communicating health risk to patients in an easily understood metric based on number of tanning bed sessions could be helpful to clinical practice," wrote the investigators.
For the meta-analysis, the investigators searched Scopus, MEDLINE, and the Cumulative Index to Nursing and Allied Health Literature for studies published before Aug. 14, 2013. In all, 31 studies that included 14,956 cases of melanoma and 233,106 controls were analyzed. In those who had ever used a tanning bed, the odds ratio (OR) for melanoma was 1.16 (95% CI, 1.05-1.28). Tanning bed use after 2000 had an OR of 1.22 (95% CI, 1.03-1.45). For individuals who had used 10 or more tanning bed sessions, the OR was 1.34 (95% CI, 1.05-1.71). North Americans were found to have higher rates of melanoma (OR, 1.23; 95% CI, 1.03-1.47) vs. Europeans and Oceanianians for whom the OR was not significant. Ten or more tanning sessions in a lifetime was associated with the highest risk of melanoma (OR, 1.34; 95% CI, 1.05-1.71).
Limitations to the meta-analysis included that not all of the studies used the same age range for their study groups, and evidence quality ranged from mediocre to poor, the investigators noted.
The investigators reported that there were no funding sources, and no conflicts of interest were declared.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major finding: Exposure to tanning bed technology, new or old, led to a 16% increased risk of melanoma worldwide.
Data source: A meta-analysis of 31 studies with 14,956 melanoma cases and 233,106 controls.
Disclosures: The investigators reported that there were no funding sources, and no conflicts of interest were declared.
Genomic testing refined accuracy of melanoma risk prediction
ARLINGTON, VA. – Combining test data for melanocortin-1 receptor gene variants and common genomic variants with nongenetic screening resulted in better melanoma risk prediction, Ann Cust, Ph.D., reported at the annual meeting of the American Society of Preventive Oncology.
"We’ve shown in this study that measuring genetic factors to determine who’s at high risk for melanoma can play an important role in prediction," said Dr. Cust of the University of Sydney, Australia, in an interview. "This gives us a good model for looking at whether genetic factors can improve the way we target preventive behaviors."
In the United States, melanoma accounts for only about 2% of all skin cancers, but the most skin cancer–related deaths. Fair skin, light hair, freckling, and a family history of melanoma are known risk factors for the disease. Australia, where the study was conducted, has the highest incidence of melanoma in the world.
To date, skin cancer prevention efforts in both countries have largely relied upon mass media sun protection campaigns and identifying people at high risk based on their skin and hair pigmentation, Dr. Cust said. "But some people don’t know they are at high risk, and so they aren’t taking precautions," she said.
"Some of the genetic factors for melanoma are linked to pigmentation, but there are variations in genes involved in DNA repair and other biological pathways that occur in people whose pigmentation wouldn’t suggest they are at high risk, but they are."
For the study, Dr. Cust and her colleagues genotyped common variants in 18 different genes in a study group of 552 Australians, aged 18-39 years, all of whom had confirmed cases of invasive cutaneous melanoma, and also in a control group of 405 Australians of European ancestry without melanoma. The study was a population-based, case-control family study that assessed traditional melanoma risk factors such as hair color, moles, family history of melanoma, use of indoor tanning, and tendency to sunburn. They then performed genomewide association studies to identify the 18 specific gene regions that have common genomic variants that influence melanoma risk.
The investigators found that the area under the curve (AUC) of the predicted risk in the controls went from 0.76 (95% confidence interval, 0.73-0.79) using demographic and nongenetic factors, to 0.81 (95% CI, 0.78 -0.84) when MC1R genotype and novel common genomic variant data were added. They also found that the combined contribution to the AUC of the novel common variants was similar to that of the established common variants of MC1R and CDKN2A.
By combining the genetic and nongenetic data, the quartile classification of predicted risk improved a net 17% (95% CI, 9-242), compared with the nongenetic predictive model alone.
"This is just the first step," said Dr. Cust. "The next thing we have to work out is how this knowledge of melanoma risk translates into behavior change." To that end, Dr. Cust is currently seeking a grant to study a range of implications inherent in this study.
"We need to address the ethical implications such as to do with informed consent, and the psychosocial implications, as well as the economic ones," she said. Other concerns she noted include whether it will interfere with one’s life insurance coverage.
As for the findings’ effect on practice, Dr. Cust believes that general practitioners are already caught up in the tide of genomic testing that has become part of a consumer-driven market in the last decade. "Some patients already obtain direct-to-consumer genetic tests and bring their results in to their doctors," said Dr. Cust.
Whether primary care physicians will need to learn how to administer the test and at what cost also remains to be settled.
Although she said her next study will evaluate the cost of genomics for prevention compared with the cost of intervention, there is no turning back, according to Dr. Cust. "We are part of a genomic revolution. We already use genomics for individualized treatment of cancer, so I think it’s inevitable that one day it will become part of preventive care. It’s just been slower to get into that realm."
Dr. Cust is funded by fellowships from the Australian National Health and Medical Research Council and the Cancer Institute New South Wales, Australia.
ARLINGTON, VA. – Combining test data for melanocortin-1 receptor gene variants and common genomic variants with nongenetic screening resulted in better melanoma risk prediction, Ann Cust, Ph.D., reported at the annual meeting of the American Society of Preventive Oncology.
"We’ve shown in this study that measuring genetic factors to determine who’s at high risk for melanoma can play an important role in prediction," said Dr. Cust of the University of Sydney, Australia, in an interview. "This gives us a good model for looking at whether genetic factors can improve the way we target preventive behaviors."
In the United States, melanoma accounts for only about 2% of all skin cancers, but the most skin cancer–related deaths. Fair skin, light hair, freckling, and a family history of melanoma are known risk factors for the disease. Australia, where the study was conducted, has the highest incidence of melanoma in the world.
To date, skin cancer prevention efforts in both countries have largely relied upon mass media sun protection campaigns and identifying people at high risk based on their skin and hair pigmentation, Dr. Cust said. "But some people don’t know they are at high risk, and so they aren’t taking precautions," she said.
"Some of the genetic factors for melanoma are linked to pigmentation, but there are variations in genes involved in DNA repair and other biological pathways that occur in people whose pigmentation wouldn’t suggest they are at high risk, but they are."
For the study, Dr. Cust and her colleagues genotyped common variants in 18 different genes in a study group of 552 Australians, aged 18-39 years, all of whom had confirmed cases of invasive cutaneous melanoma, and also in a control group of 405 Australians of European ancestry without melanoma. The study was a population-based, case-control family study that assessed traditional melanoma risk factors such as hair color, moles, family history of melanoma, use of indoor tanning, and tendency to sunburn. They then performed genomewide association studies to identify the 18 specific gene regions that have common genomic variants that influence melanoma risk.
The investigators found that the area under the curve (AUC) of the predicted risk in the controls went from 0.76 (95% confidence interval, 0.73-0.79) using demographic and nongenetic factors, to 0.81 (95% CI, 0.78 -0.84) when MC1R genotype and novel common genomic variant data were added. They also found that the combined contribution to the AUC of the novel common variants was similar to that of the established common variants of MC1R and CDKN2A.
By combining the genetic and nongenetic data, the quartile classification of predicted risk improved a net 17% (95% CI, 9-242), compared with the nongenetic predictive model alone.
"This is just the first step," said Dr. Cust. "The next thing we have to work out is how this knowledge of melanoma risk translates into behavior change." To that end, Dr. Cust is currently seeking a grant to study a range of implications inherent in this study.
"We need to address the ethical implications such as to do with informed consent, and the psychosocial implications, as well as the economic ones," she said. Other concerns she noted include whether it will interfere with one’s life insurance coverage.
As for the findings’ effect on practice, Dr. Cust believes that general practitioners are already caught up in the tide of genomic testing that has become part of a consumer-driven market in the last decade. "Some patients already obtain direct-to-consumer genetic tests and bring their results in to their doctors," said Dr. Cust.
Whether primary care physicians will need to learn how to administer the test and at what cost also remains to be settled.
Although she said her next study will evaluate the cost of genomics for prevention compared with the cost of intervention, there is no turning back, according to Dr. Cust. "We are part of a genomic revolution. We already use genomics for individualized treatment of cancer, so I think it’s inevitable that one day it will become part of preventive care. It’s just been slower to get into that realm."
Dr. Cust is funded by fellowships from the Australian National Health and Medical Research Council and the Cancer Institute New South Wales, Australia.
ARLINGTON, VA. – Combining test data for melanocortin-1 receptor gene variants and common genomic variants with nongenetic screening resulted in better melanoma risk prediction, Ann Cust, Ph.D., reported at the annual meeting of the American Society of Preventive Oncology.
"We’ve shown in this study that measuring genetic factors to determine who’s at high risk for melanoma can play an important role in prediction," said Dr. Cust of the University of Sydney, Australia, in an interview. "This gives us a good model for looking at whether genetic factors can improve the way we target preventive behaviors."
In the United States, melanoma accounts for only about 2% of all skin cancers, but the most skin cancer–related deaths. Fair skin, light hair, freckling, and a family history of melanoma are known risk factors for the disease. Australia, where the study was conducted, has the highest incidence of melanoma in the world.
To date, skin cancer prevention efforts in both countries have largely relied upon mass media sun protection campaigns and identifying people at high risk based on their skin and hair pigmentation, Dr. Cust said. "But some people don’t know they are at high risk, and so they aren’t taking precautions," she said.
"Some of the genetic factors for melanoma are linked to pigmentation, but there are variations in genes involved in DNA repair and other biological pathways that occur in people whose pigmentation wouldn’t suggest they are at high risk, but they are."
For the study, Dr. Cust and her colleagues genotyped common variants in 18 different genes in a study group of 552 Australians, aged 18-39 years, all of whom had confirmed cases of invasive cutaneous melanoma, and also in a control group of 405 Australians of European ancestry without melanoma. The study was a population-based, case-control family study that assessed traditional melanoma risk factors such as hair color, moles, family history of melanoma, use of indoor tanning, and tendency to sunburn. They then performed genomewide association studies to identify the 18 specific gene regions that have common genomic variants that influence melanoma risk.
The investigators found that the area under the curve (AUC) of the predicted risk in the controls went from 0.76 (95% confidence interval, 0.73-0.79) using demographic and nongenetic factors, to 0.81 (95% CI, 0.78 -0.84) when MC1R genotype and novel common genomic variant data were added. They also found that the combined contribution to the AUC of the novel common variants was similar to that of the established common variants of MC1R and CDKN2A.
By combining the genetic and nongenetic data, the quartile classification of predicted risk improved a net 17% (95% CI, 9-242), compared with the nongenetic predictive model alone.
"This is just the first step," said Dr. Cust. "The next thing we have to work out is how this knowledge of melanoma risk translates into behavior change." To that end, Dr. Cust is currently seeking a grant to study a range of implications inherent in this study.
"We need to address the ethical implications such as to do with informed consent, and the psychosocial implications, as well as the economic ones," she said. Other concerns she noted include whether it will interfere with one’s life insurance coverage.
As for the findings’ effect on practice, Dr. Cust believes that general practitioners are already caught up in the tide of genomic testing that has become part of a consumer-driven market in the last decade. "Some patients already obtain direct-to-consumer genetic tests and bring their results in to their doctors," said Dr. Cust.
Whether primary care physicians will need to learn how to administer the test and at what cost also remains to be settled.
Although she said her next study will evaluate the cost of genomics for prevention compared with the cost of intervention, there is no turning back, according to Dr. Cust. "We are part of a genomic revolution. We already use genomics for individualized treatment of cancer, so I think it’s inevitable that one day it will become part of preventive care. It’s just been slower to get into that realm."
Dr. Cust is funded by fellowships from the Australian National Health and Medical Research Council and the Cancer Institute New South Wales, Australia.
AT THE ASPO ANNUAL MEETING
Major finding: Adding genetic testing for MC1R and other novel, common genomic variants, to nongenetic screening methods improved melanoma risk prediction by 17% (95% CI, 9-24).
Data source: Combined genetic and nongenetic melanoma risk analysis of 552 Australians aged 18-39 years with confirmed invasive cutaneous melanoma, compared with that in 405 controls from an Australian, population-based, case-control family study.
Disclosures: Dr. Cust is funded by fellowships from the Australian National Health and Medical Research Council and the Cancer Institute New South Wales, Australia.
The push for smaller, smarter cancer trials
The American Society of Clinical Oncology is pressing cancer researchers to rethink the design of future clinical trials to achieve larger gains in four common cancers.
The final recommendations, which come after months of deliberations and public comment, try to hit the sweet spot between proposing guidelines that are not obtainable, and thus ignored, and having ambitious yet realistic goals.
For pancreatic cancer, for example, the experts recommended that clinical trials seek to improve median overall survival by 50%, or 4-5 months, for patients eligible for FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) and by 3-4 months for those eligible for gemcitabine (Gemzar) with or without nab-paclitaxel (Abraxane).
Overall survival (OS) was selected over progression-free survival as the primary endpoint, although it was acknowledged that OS poses challenges such as the need for longer follow-up, the potential confounding effect of post-study therapies, and use of second-line therapies for secondary mutations identified after progression during first-line targeted therapy.
Ultimately, an improvement in median OS of 2.5-6 months, depending on the setting, was identified as the minimum incremental improvement over standard therapy that would define a clinically meaningful outcome.
The recommendations, published March 17 in the Journal of Clinical Oncology (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.53.8009]), also note that incremental improvements should be accompanied by little to no added toxicity over current treatments, and that a highly toxic regimen should produce the greatest OS gains to be considered clinically meaningful.
"We expect that sponsors will appreciate the need for raising the bar with regard to clinical trial goals, but that they will be conservative in their adoption of the recommendations," Dr. Lee M. Ellis, committee chair and professor of surgery at the University of Texas M.D. Anderson Cancer Center, Houston, said in an interview. "Trials designed with less ambitious goals may still be of benefit to individual patients if trial endpoints are met and if we can develop methods to identify patients most likely to benefit from the intervention."
Achieving the "smaller and smarter" trials envisioned by the committee rests on the ability to select patients for targeted therapy based on the molecular drivers of their tumors, rather than enrolling all comers. Unfortunately, in many cases, targeted agents continue to be developed without complete understanding of the drug target and, therefore, companion diagnostics to aid in patient selection, the experts observed.
"It is difficult to hit a target when it is not certain where it is or if it is valid," agreed Dr. David M. Dilts, codirector of the Center for Management Research in Healthcare, Oregon Health & Science University, Portland, in an accompanying editorial (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.5277]). "This, not insubstantial risk, should be ameliorated in the near future as major clinical research organizations are banking specimens, some of which are highly annotated, and as technology to analyze such specimens becomes faster, better, and cheaper."
To further this goal, the expert committee calls on trial sponsors to develop comprehensive biospecimen banks for each trial.
"Obstacles to developing these banks include cost and the willingness and ability of trial sponsors to foot the bill," Dr. Ellis said. "However, we believe the investment will pay off in increasing our ability to understand the molecular drivers of cancer and, as a result, more appropriate targeted therapies for people with cancer."
QOL
Though quality of life was a common theme that arose in all working group discussions, the recommendations lack hard targets in this area. Instead, the working groups cited the 2011 approval of the Janus kinase 1 and 2 inhibitor ruxolitinib (Jakavi) for myelofibrosis as an example of how serial assessment of specific cancer-related symptoms can define a clinically meaningful outcome for patients.
"It is not enough to just mention how important quality of life is. A clinical trial must be designed with a suite of thoughtful, feasible, validated patient-reported outcome measures that capture clinical benefit," Ms. Musa Mayer, a long-time advocate for patients with metastatic breast cancer, said in an interview. "Observed adverse events can never fully account for the lived experience of a given treatment."
Breast cancer
For breast cancer, the committee selected metastatic triple-negative breast cancer that was previously untreated for metastatic disease. They recommend clinical trials aim for an increase in OS of 4.5-6 months, although it was noted that consensus was not achieved by the breast cancer group on the magnitude of the benefit that would be considered clinically meaningful. The current median overall survival in this poor-prognosis population is 18 months.
Lung cancer
The committee addressed two lung cancer populations: nonsquamous cell carcinoma and squamous cell carcinoma. They recommend clinical trials seek to improve OS by 3.25-4 months and by 2.5-3 months, respectively. Current baseline median OS in these groups is 13 and 10 months.
Colon cancer
The recommendations for colon cancer target patients with disease progression with all prior therapies, or who are not candidates for standard second- or third-line options. Here, the goal is to improve OS by 3-5 months over the current baseline median OS of 4-6 months.
Notably, the cost of delivering the recommended targets for all four cancers was not addressed by the committee. The ASCO Value of Cancer Care Task Force, however, is already tasked with evaluating the efficacy, toxicity, and cost of specific oncology treatments.
"The working group provided thoughtful recommendations for the topics considered, although the specific recommendations were limited," Ms. Patricia Haugen, breast cancer survivor and current member and previous chair of the Department of Defense Congressionally Directed Breast Cancer Research Program Integration Panel, said in an interview.
She is hopeful that the new recommendations will be followed, but said there needs to be broad support and commitment to changes that produce more meaningful clinical benefit. "That commitment must be real and must come from all parties involved in the clinical trials process, so that clinical trials that do not meet a high bar are not considered, funded, nor implemented," she said.
Editorialist Dr. Dilts agreed that advocates from many areas are needed if the recommended goals are to be reached and suggested what might be required is "a more DARPA [Defense Advanced Research Projects Agency] approach, where answering high-risk questions are fostered and supported."
Dr. Ellis reported a consultant/advisory role with Genentech, Roche, Imclone, Eli Lilly, and Amgen. Ms. Mayer, Ms. Haugen, and Dr. Dilts reported no potential conflicts of interest.
The American Society of Clinical Oncology is pressing cancer researchers to rethink the design of future clinical trials to achieve larger gains in four common cancers.
The final recommendations, which come after months of deliberations and public comment, try to hit the sweet spot between proposing guidelines that are not obtainable, and thus ignored, and having ambitious yet realistic goals.
For pancreatic cancer, for example, the experts recommended that clinical trials seek to improve median overall survival by 50%, or 4-5 months, for patients eligible for FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) and by 3-4 months for those eligible for gemcitabine (Gemzar) with or without nab-paclitaxel (Abraxane).
Overall survival (OS) was selected over progression-free survival as the primary endpoint, although it was acknowledged that OS poses challenges such as the need for longer follow-up, the potential confounding effect of post-study therapies, and use of second-line therapies for secondary mutations identified after progression during first-line targeted therapy.
Ultimately, an improvement in median OS of 2.5-6 months, depending on the setting, was identified as the minimum incremental improvement over standard therapy that would define a clinically meaningful outcome.
The recommendations, published March 17 in the Journal of Clinical Oncology (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.53.8009]), also note that incremental improvements should be accompanied by little to no added toxicity over current treatments, and that a highly toxic regimen should produce the greatest OS gains to be considered clinically meaningful.
"We expect that sponsors will appreciate the need for raising the bar with regard to clinical trial goals, but that they will be conservative in their adoption of the recommendations," Dr. Lee M. Ellis, committee chair and professor of surgery at the University of Texas M.D. Anderson Cancer Center, Houston, said in an interview. "Trials designed with less ambitious goals may still be of benefit to individual patients if trial endpoints are met and if we can develop methods to identify patients most likely to benefit from the intervention."
Achieving the "smaller and smarter" trials envisioned by the committee rests on the ability to select patients for targeted therapy based on the molecular drivers of their tumors, rather than enrolling all comers. Unfortunately, in many cases, targeted agents continue to be developed without complete understanding of the drug target and, therefore, companion diagnostics to aid in patient selection, the experts observed.
"It is difficult to hit a target when it is not certain where it is or if it is valid," agreed Dr. David M. Dilts, codirector of the Center for Management Research in Healthcare, Oregon Health & Science University, Portland, in an accompanying editorial (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.5277]). "This, not insubstantial risk, should be ameliorated in the near future as major clinical research organizations are banking specimens, some of which are highly annotated, and as technology to analyze such specimens becomes faster, better, and cheaper."
To further this goal, the expert committee calls on trial sponsors to develop comprehensive biospecimen banks for each trial.
"Obstacles to developing these banks include cost and the willingness and ability of trial sponsors to foot the bill," Dr. Ellis said. "However, we believe the investment will pay off in increasing our ability to understand the molecular drivers of cancer and, as a result, more appropriate targeted therapies for people with cancer."
QOL
Though quality of life was a common theme that arose in all working group discussions, the recommendations lack hard targets in this area. Instead, the working groups cited the 2011 approval of the Janus kinase 1 and 2 inhibitor ruxolitinib (Jakavi) for myelofibrosis as an example of how serial assessment of specific cancer-related symptoms can define a clinically meaningful outcome for patients.
"It is not enough to just mention how important quality of life is. A clinical trial must be designed with a suite of thoughtful, feasible, validated patient-reported outcome measures that capture clinical benefit," Ms. Musa Mayer, a long-time advocate for patients with metastatic breast cancer, said in an interview. "Observed adverse events can never fully account for the lived experience of a given treatment."
Breast cancer
For breast cancer, the committee selected metastatic triple-negative breast cancer that was previously untreated for metastatic disease. They recommend clinical trials aim for an increase in OS of 4.5-6 months, although it was noted that consensus was not achieved by the breast cancer group on the magnitude of the benefit that would be considered clinically meaningful. The current median overall survival in this poor-prognosis population is 18 months.
Lung cancer
The committee addressed two lung cancer populations: nonsquamous cell carcinoma and squamous cell carcinoma. They recommend clinical trials seek to improve OS by 3.25-4 months and by 2.5-3 months, respectively. Current baseline median OS in these groups is 13 and 10 months.
Colon cancer
The recommendations for colon cancer target patients with disease progression with all prior therapies, or who are not candidates for standard second- or third-line options. Here, the goal is to improve OS by 3-5 months over the current baseline median OS of 4-6 months.
Notably, the cost of delivering the recommended targets for all four cancers was not addressed by the committee. The ASCO Value of Cancer Care Task Force, however, is already tasked with evaluating the efficacy, toxicity, and cost of specific oncology treatments.
"The working group provided thoughtful recommendations for the topics considered, although the specific recommendations were limited," Ms. Patricia Haugen, breast cancer survivor and current member and previous chair of the Department of Defense Congressionally Directed Breast Cancer Research Program Integration Panel, said in an interview.
She is hopeful that the new recommendations will be followed, but said there needs to be broad support and commitment to changes that produce more meaningful clinical benefit. "That commitment must be real and must come from all parties involved in the clinical trials process, so that clinical trials that do not meet a high bar are not considered, funded, nor implemented," she said.
Editorialist Dr. Dilts agreed that advocates from many areas are needed if the recommended goals are to be reached and suggested what might be required is "a more DARPA [Defense Advanced Research Projects Agency] approach, where answering high-risk questions are fostered and supported."
Dr. Ellis reported a consultant/advisory role with Genentech, Roche, Imclone, Eli Lilly, and Amgen. Ms. Mayer, Ms. Haugen, and Dr. Dilts reported no potential conflicts of interest.
The American Society of Clinical Oncology is pressing cancer researchers to rethink the design of future clinical trials to achieve larger gains in four common cancers.
The final recommendations, which come after months of deliberations and public comment, try to hit the sweet spot between proposing guidelines that are not obtainable, and thus ignored, and having ambitious yet realistic goals.
For pancreatic cancer, for example, the experts recommended that clinical trials seek to improve median overall survival by 50%, or 4-5 months, for patients eligible for FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) and by 3-4 months for those eligible for gemcitabine (Gemzar) with or without nab-paclitaxel (Abraxane).
Overall survival (OS) was selected over progression-free survival as the primary endpoint, although it was acknowledged that OS poses challenges such as the need for longer follow-up, the potential confounding effect of post-study therapies, and use of second-line therapies for secondary mutations identified after progression during first-line targeted therapy.
Ultimately, an improvement in median OS of 2.5-6 months, depending on the setting, was identified as the minimum incremental improvement over standard therapy that would define a clinically meaningful outcome.
The recommendations, published March 17 in the Journal of Clinical Oncology (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.53.8009]), also note that incremental improvements should be accompanied by little to no added toxicity over current treatments, and that a highly toxic regimen should produce the greatest OS gains to be considered clinically meaningful.
"We expect that sponsors will appreciate the need for raising the bar with regard to clinical trial goals, but that they will be conservative in their adoption of the recommendations," Dr. Lee M. Ellis, committee chair and professor of surgery at the University of Texas M.D. Anderson Cancer Center, Houston, said in an interview. "Trials designed with less ambitious goals may still be of benefit to individual patients if trial endpoints are met and if we can develop methods to identify patients most likely to benefit from the intervention."
Achieving the "smaller and smarter" trials envisioned by the committee rests on the ability to select patients for targeted therapy based on the molecular drivers of their tumors, rather than enrolling all comers. Unfortunately, in many cases, targeted agents continue to be developed without complete understanding of the drug target and, therefore, companion diagnostics to aid in patient selection, the experts observed.
"It is difficult to hit a target when it is not certain where it is or if it is valid," agreed Dr. David M. Dilts, codirector of the Center for Management Research in Healthcare, Oregon Health & Science University, Portland, in an accompanying editorial (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.5277]). "This, not insubstantial risk, should be ameliorated in the near future as major clinical research organizations are banking specimens, some of which are highly annotated, and as technology to analyze such specimens becomes faster, better, and cheaper."
To further this goal, the expert committee calls on trial sponsors to develop comprehensive biospecimen banks for each trial.
"Obstacles to developing these banks include cost and the willingness and ability of trial sponsors to foot the bill," Dr. Ellis said. "However, we believe the investment will pay off in increasing our ability to understand the molecular drivers of cancer and, as a result, more appropriate targeted therapies for people with cancer."
QOL
Though quality of life was a common theme that arose in all working group discussions, the recommendations lack hard targets in this area. Instead, the working groups cited the 2011 approval of the Janus kinase 1 and 2 inhibitor ruxolitinib (Jakavi) for myelofibrosis as an example of how serial assessment of specific cancer-related symptoms can define a clinically meaningful outcome for patients.
"It is not enough to just mention how important quality of life is. A clinical trial must be designed with a suite of thoughtful, feasible, validated patient-reported outcome measures that capture clinical benefit," Ms. Musa Mayer, a long-time advocate for patients with metastatic breast cancer, said in an interview. "Observed adverse events can never fully account for the lived experience of a given treatment."
Breast cancer
For breast cancer, the committee selected metastatic triple-negative breast cancer that was previously untreated for metastatic disease. They recommend clinical trials aim for an increase in OS of 4.5-6 months, although it was noted that consensus was not achieved by the breast cancer group on the magnitude of the benefit that would be considered clinically meaningful. The current median overall survival in this poor-prognosis population is 18 months.
Lung cancer
The committee addressed two lung cancer populations: nonsquamous cell carcinoma and squamous cell carcinoma. They recommend clinical trials seek to improve OS by 3.25-4 months and by 2.5-3 months, respectively. Current baseline median OS in these groups is 13 and 10 months.
Colon cancer
The recommendations for colon cancer target patients with disease progression with all prior therapies, or who are not candidates for standard second- or third-line options. Here, the goal is to improve OS by 3-5 months over the current baseline median OS of 4-6 months.
Notably, the cost of delivering the recommended targets for all four cancers was not addressed by the committee. The ASCO Value of Cancer Care Task Force, however, is already tasked with evaluating the efficacy, toxicity, and cost of specific oncology treatments.
"The working group provided thoughtful recommendations for the topics considered, although the specific recommendations were limited," Ms. Patricia Haugen, breast cancer survivor and current member and previous chair of the Department of Defense Congressionally Directed Breast Cancer Research Program Integration Panel, said in an interview.
She is hopeful that the new recommendations will be followed, but said there needs to be broad support and commitment to changes that produce more meaningful clinical benefit. "That commitment must be real and must come from all parties involved in the clinical trials process, so that clinical trials that do not meet a high bar are not considered, funded, nor implemented," she said.
Editorialist Dr. Dilts agreed that advocates from many areas are needed if the recommended goals are to be reached and suggested what might be required is "a more DARPA [Defense Advanced Research Projects Agency] approach, where answering high-risk questions are fostered and supported."
Dr. Ellis reported a consultant/advisory role with Genentech, Roche, Imclone, Eli Lilly, and Amgen. Ms. Mayer, Ms. Haugen, and Dr. Dilts reported no potential conflicts of interest.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
AAD 2014 sessions offer something for everyone
The American Academy’s 2014 annual meeting in Denver will feature new CME sessions and updates on the latest dermatology research.
This year’s program features expert commentary on key issues in medical dermatology, including "Melanoma Multidisciplinary Care 2014: What You Need to Know" on Sunday, March 23, from 1 p.m. to 3 p.m. in Room 705/707 and "Dermatologic Manifestations of New Oncology Drugs," also on Sunday, March 23, from 1 p.m. to 3 p.m. in the Mile High Ballroom 3B. Looking for the latest in aesthetic dermatology? Check out the "Advanced Botulinum Toxin" live demonstration session on Saturday, March 22, from 2 p.m. to 5 p.m. in the Bellco Theater.
There will be expert sessions on pregnancy dermatoses, cutaneous T-cell lymphoma, pediatric dermatology, skin of color, and the latest on treatments for hair and nail conditions. The full scientific session list is available online.
A series of practice management lectures includes topics such as "How to Have an Unforgettably Positive Office Visit" on Saturday, March 22, from 10:00 a.m. to 12:00 p.m. in Room 709/7111 and "Hot Buttons: Recognizing What Sets You Off and Managing Your Triggers" on Sunday, March 23, from 1:00 p.m. to 3:00 p.m. in Room 702.
There is also a mobile device app that meeting attendees can download that contains session schedules, exhibitor and attendee lists, and more.
Can’t attend the meeting? Visit www.eDermatologyNews.com for live conference coverage.
On Twitter @Sknews
The American Academy’s 2014 annual meeting in Denver will feature new CME sessions and updates on the latest dermatology research.
This year’s program features expert commentary on key issues in medical dermatology, including "Melanoma Multidisciplinary Care 2014: What You Need to Know" on Sunday, March 23, from 1 p.m. to 3 p.m. in Room 705/707 and "Dermatologic Manifestations of New Oncology Drugs," also on Sunday, March 23, from 1 p.m. to 3 p.m. in the Mile High Ballroom 3B. Looking for the latest in aesthetic dermatology? Check out the "Advanced Botulinum Toxin" live demonstration session on Saturday, March 22, from 2 p.m. to 5 p.m. in the Bellco Theater.
There will be expert sessions on pregnancy dermatoses, cutaneous T-cell lymphoma, pediatric dermatology, skin of color, and the latest on treatments for hair and nail conditions. The full scientific session list is available online.
A series of practice management lectures includes topics such as "How to Have an Unforgettably Positive Office Visit" on Saturday, March 22, from 10:00 a.m. to 12:00 p.m. in Room 709/7111 and "Hot Buttons: Recognizing What Sets You Off and Managing Your Triggers" on Sunday, March 23, from 1:00 p.m. to 3:00 p.m. in Room 702.
There is also a mobile device app that meeting attendees can download that contains session schedules, exhibitor and attendee lists, and more.
Can’t attend the meeting? Visit www.eDermatologyNews.com for live conference coverage.
On Twitter @Sknews
The American Academy’s 2014 annual meeting in Denver will feature new CME sessions and updates on the latest dermatology research.
This year’s program features expert commentary on key issues in medical dermatology, including "Melanoma Multidisciplinary Care 2014: What You Need to Know" on Sunday, March 23, from 1 p.m. to 3 p.m. in Room 705/707 and "Dermatologic Manifestations of New Oncology Drugs," also on Sunday, March 23, from 1 p.m. to 3 p.m. in the Mile High Ballroom 3B. Looking for the latest in aesthetic dermatology? Check out the "Advanced Botulinum Toxin" live demonstration session on Saturday, March 22, from 2 p.m. to 5 p.m. in the Bellco Theater.
There will be expert sessions on pregnancy dermatoses, cutaneous T-cell lymphoma, pediatric dermatology, skin of color, and the latest on treatments for hair and nail conditions. The full scientific session list is available online.
A series of practice management lectures includes topics such as "How to Have an Unforgettably Positive Office Visit" on Saturday, March 22, from 10:00 a.m. to 12:00 p.m. in Room 709/7111 and "Hot Buttons: Recognizing What Sets You Off and Managing Your Triggers" on Sunday, March 23, from 1:00 p.m. to 3:00 p.m. in Room 702.
There is also a mobile device app that meeting attendees can download that contains session schedules, exhibitor and attendee lists, and more.
Can’t attend the meeting? Visit www.eDermatologyNews.com for live conference coverage.
On Twitter @Sknews
VIDEO: Photodynamic therapy pearls can improve results
CHAMPIONSGATE, FLA. – Do you use photodynamic therapy to treat actinic keratoses? Dr. Joel Cohen has some tips for you. At the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Cohen spoke to us about current strategies for making the most of PDT, and which treatment plans work best for certain patients. What’s next for PDT? Dr. Cohen of the department of dermatology at the University of Colorado, Denver, highlights promising preliminary studies involving different types of combination therapies that can yield cosmetic and medical benefits.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHAMPIONSGATE, FLA. – Do you use photodynamic therapy to treat actinic keratoses? Dr. Joel Cohen has some tips for you. At the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Cohen spoke to us about current strategies for making the most of PDT, and which treatment plans work best for certain patients. What’s next for PDT? Dr. Cohen of the department of dermatology at the University of Colorado, Denver, highlights promising preliminary studies involving different types of combination therapies that can yield cosmetic and medical benefits.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHAMPIONSGATE, FLA. – Do you use photodynamic therapy to treat actinic keratoses? Dr. Joel Cohen has some tips for you. At the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Cohen spoke to us about current strategies for making the most of PDT, and which treatment plans work best for certain patients. What’s next for PDT? Dr. Cohen of the department of dermatology at the University of Colorado, Denver, highlights promising preliminary studies involving different types of combination therapies that can yield cosmetic and medical benefits.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM ODAC
Nivolumab extends survival in advanced melanoma
An immunomodulator that targets the PD-1 pathway effected sustained remission in 31% of patients who took it for advanced melanoma.
Remission lasted a median of 2 years, with 1- and 2-year overall survival of 62% and 43% respectively, wrote Dr. Suzanne Topalian and colleagues.
But perhaps even more exciting, they said, is that the benefit of nivolumab persisted for at least 16 weeks in all patients who had to discontinue therapy.
The report appeared in the March 3 issue of the Journal of Clinical Oncology.
"The persistence of partial tumor regression and stable disease ... suggests that PD-1 blockade may reset the immune equilibrium between tumor and host," wrote Dr. Topalian, director of the melanoma program at the Sidney Kimmel Comprehensive Cancer Center, Baltimore, and her coauthors.
The findings "are consistent with a mechanism by which an effective tumor-selective immune memory response may have been established in some patients, similar to immune memory against specific infectious organisms after antigen exposure," the investigators wrote (J. Clin. Oncol. 2014 March [doi:10.1200/JCO.2013.53.0105]).
PD-1 is an immune checkpoint that helps regulate the autoimmune action of activated T cells. Tumor cells can co-opt this pathway, using it to hide from T cell attack. Blocking PD-1 unmasks tumor cells, allowing the immune system to target them more effectively.
The 2-year follow-up study tracked response rates in 107 patients with advanced melanoma, who were part of a larger study of 306 patients with various solid-tumor cancers. The initial results of the phase I trial were reported at the 2013 meeting of the American Society of Clinical Oncology.
The new report focuses only on the patients with melanoma, who have completed treatment for at least 14 months, and up to 4 years. All of the patients had undergone two to five prior systemic treatments. They received up to 96 weeks of biweekly nivolumab infusions in varying doses.
Median progression-free survival was 3.7 months; 1- and 2-year progression-free survival rates were 36% and 27%, respectively.
About a third of the patients (33; 31%) experienced an objective response to the drug, with a median duration of 2 years. Fifteen of these (45%) occurred within the first 8 weeks of treatment, the investigators wrote. Of these responding patients, 19 (58%) were still responding when the data were analyzed, the authors noted.
Tumors regressed at various sites, including, in one patient, an unresectable lesion that had eroded through the zygomatic arch into the orbit. Response occurred within 4 months of treatment initiation. This lesion remains in remission. A biopsy showed fibrosis and infiltrating lymphocytes, but no tumor cells. This finding hints that residual tumor seen in some patients’ imaging may in fact be scarring. If that is true, the researchers said, they could have underestimated the real response rate.
Seventeen of the 33 patients who responded discontinued treatment for reasons other than progression. Among these, 12 (71%) maintained their response for 16-56 weeks; in eight of these, response was ongoing at the time of data analysis. Among these is a patient who had metastatic lesions in the adrenal gland, small bowel, and mesenteric lymph nodes.
An additional 7% (7 patients) experienced stable disease for at least 24 weeks at doses of 0.1 to 10 mg/kg. "Unconventional" response patterns not meeting RECIST criteria occurred in four patients, but there was no associated dose pattern; three had received 1 mg/kg and the fourth, 10 mg/kg. Disease progressed in 11 patients treated at doses of 0.1 to 03 mg/kg; none of these responded to a dose escalation, the investigators said.
Nivolumab’s safety data were excellent when compared with other cancer treatments, they noted. Most adverse events occurred within the first 6 months – a hint that close observation and early intervention could minimize their impact. The most common were fatigue (32%), rash (23%), and diarrhea (18%). Grades 3 and 4 adverse events occurred in 24 patients. Treatment-related select adverse events of any grade occurred in 58 (54%). The most common were skin disorders (36%), gastrointestinal events (18%), and endocrinopathies (13%) – not unexpected in an immunomodulating drug. There were no drug-related deaths in the study.
Although this study examined nivolumab as monotherapy, others are looking at a possibly synergistic effect when it’s given with other immunomodulators, and with chemotherapy, kinase inhibitors, or cancer vaccines.
"Innovative, rational treatment combinations based on preclinical evidence may be needed to realize the full therapeutic potential of PD-1 blockade," the investigators said.
Dr. Topalian and several of her coauthors received research funding or had other financial relationships with Bristol-Myers Squibb, sponsor of the study.
Immunotherapy treatments for cancer might "raise survival curves to new heights," Dr. Geraldine O’Sullivan Coyne wrote in an editorial.
"The continued demonstration of promising clinical outcomes with the use of modern immunotherapy strategies such as nivolumab may allow medical oncologists to have raised expectations for patients with metastatic cancer," wrote Dr. Coyne and her colleagues.
Immune checkpoint inhibitors, including ipilimumab and nivolumab, hold much promise, as exemplified by their large number of complete and near-complete responses, the colleagues wrote. Both drugs showed a flattening of overall survival curves after 2 years, suggesting that at least some patients could derive considerable benefit from longer-term treatment.
"This tail in the curve raises questions about how to increase the number of patients who receive long-term clinical benefit, and how to predict who those patients might be."
Combining one of these drugs with another treatment might even compound its effect, they said. "One promising strategy, referred to as immunogenic intensification, combines one immune checkpoint inhibitor with a different [one] or a therapeutic vaccine ... Hypothetically, vaccine therapy could have a priming or steering effect on the immune system, potentially reducing the need for higher doses of immune checkpoint inhibition or multiple immune checkpoint inhibitors, and lessening the risk of inducing autoimmunity. Such approaches may have the potential to improve clinical outcomes while minimizing adverse effects."
There’s nowhere to go but up for the kinds of cancers being investigated with these drugs, they said.
"Current treatment for metastatic solid tumors ... remains palliative, but perhaps immune checkpoint inhibitors can be part of a strategy to enhance the number of patients who can enjoy sustained, durable responses. ... If future clinical trials validate the findings that have been seen in recent years, perhaps immunotherapy will not just raise expectations, but the all-important survival tail of Kaplan-Meier curves as well."
Dr. Geraldine O’Sullivan Coyne is a medical oncologist at the National Cancer Institute. Neither she nor her co-authors had any financial disclosures.
Immunotherapy treatments for cancer might "raise survival curves to new heights," Dr. Geraldine O’Sullivan Coyne wrote in an editorial.
"The continued demonstration of promising clinical outcomes with the use of modern immunotherapy strategies such as nivolumab may allow medical oncologists to have raised expectations for patients with metastatic cancer," wrote Dr. Coyne and her colleagues.
Immune checkpoint inhibitors, including ipilimumab and nivolumab, hold much promise, as exemplified by their large number of complete and near-complete responses, the colleagues wrote. Both drugs showed a flattening of overall survival curves after 2 years, suggesting that at least some patients could derive considerable benefit from longer-term treatment.
"This tail in the curve raises questions about how to increase the number of patients who receive long-term clinical benefit, and how to predict who those patients might be."
Combining one of these drugs with another treatment might even compound its effect, they said. "One promising strategy, referred to as immunogenic intensification, combines one immune checkpoint inhibitor with a different [one] or a therapeutic vaccine ... Hypothetically, vaccine therapy could have a priming or steering effect on the immune system, potentially reducing the need for higher doses of immune checkpoint inhibition or multiple immune checkpoint inhibitors, and lessening the risk of inducing autoimmunity. Such approaches may have the potential to improve clinical outcomes while minimizing adverse effects."
There’s nowhere to go but up for the kinds of cancers being investigated with these drugs, they said.
"Current treatment for metastatic solid tumors ... remains palliative, but perhaps immune checkpoint inhibitors can be part of a strategy to enhance the number of patients who can enjoy sustained, durable responses. ... If future clinical trials validate the findings that have been seen in recent years, perhaps immunotherapy will not just raise expectations, but the all-important survival tail of Kaplan-Meier curves as well."
Dr. Geraldine O’Sullivan Coyne is a medical oncologist at the National Cancer Institute. Neither she nor her co-authors had any financial disclosures.
Immunotherapy treatments for cancer might "raise survival curves to new heights," Dr. Geraldine O’Sullivan Coyne wrote in an editorial.
"The continued demonstration of promising clinical outcomes with the use of modern immunotherapy strategies such as nivolumab may allow medical oncologists to have raised expectations for patients with metastatic cancer," wrote Dr. Coyne and her colleagues.
Immune checkpoint inhibitors, including ipilimumab and nivolumab, hold much promise, as exemplified by their large number of complete and near-complete responses, the colleagues wrote. Both drugs showed a flattening of overall survival curves after 2 years, suggesting that at least some patients could derive considerable benefit from longer-term treatment.
"This tail in the curve raises questions about how to increase the number of patients who receive long-term clinical benefit, and how to predict who those patients might be."
Combining one of these drugs with another treatment might even compound its effect, they said. "One promising strategy, referred to as immunogenic intensification, combines one immune checkpoint inhibitor with a different [one] or a therapeutic vaccine ... Hypothetically, vaccine therapy could have a priming or steering effect on the immune system, potentially reducing the need for higher doses of immune checkpoint inhibition or multiple immune checkpoint inhibitors, and lessening the risk of inducing autoimmunity. Such approaches may have the potential to improve clinical outcomes while minimizing adverse effects."
There’s nowhere to go but up for the kinds of cancers being investigated with these drugs, they said.
"Current treatment for metastatic solid tumors ... remains palliative, but perhaps immune checkpoint inhibitors can be part of a strategy to enhance the number of patients who can enjoy sustained, durable responses. ... If future clinical trials validate the findings that have been seen in recent years, perhaps immunotherapy will not just raise expectations, but the all-important survival tail of Kaplan-Meier curves as well."
Dr. Geraldine O’Sullivan Coyne is a medical oncologist at the National Cancer Institute. Neither she nor her co-authors had any financial disclosures.
An immunomodulator that targets the PD-1 pathway effected sustained remission in 31% of patients who took it for advanced melanoma.
Remission lasted a median of 2 years, with 1- and 2-year overall survival of 62% and 43% respectively, wrote Dr. Suzanne Topalian and colleagues.
But perhaps even more exciting, they said, is that the benefit of nivolumab persisted for at least 16 weeks in all patients who had to discontinue therapy.
The report appeared in the March 3 issue of the Journal of Clinical Oncology.
"The persistence of partial tumor regression and stable disease ... suggests that PD-1 blockade may reset the immune equilibrium between tumor and host," wrote Dr. Topalian, director of the melanoma program at the Sidney Kimmel Comprehensive Cancer Center, Baltimore, and her coauthors.
The findings "are consistent with a mechanism by which an effective tumor-selective immune memory response may have been established in some patients, similar to immune memory against specific infectious organisms after antigen exposure," the investigators wrote (J. Clin. Oncol. 2014 March [doi:10.1200/JCO.2013.53.0105]).
PD-1 is an immune checkpoint that helps regulate the autoimmune action of activated T cells. Tumor cells can co-opt this pathway, using it to hide from T cell attack. Blocking PD-1 unmasks tumor cells, allowing the immune system to target them more effectively.
The 2-year follow-up study tracked response rates in 107 patients with advanced melanoma, who were part of a larger study of 306 patients with various solid-tumor cancers. The initial results of the phase I trial were reported at the 2013 meeting of the American Society of Clinical Oncology.
The new report focuses only on the patients with melanoma, who have completed treatment for at least 14 months, and up to 4 years. All of the patients had undergone two to five prior systemic treatments. They received up to 96 weeks of biweekly nivolumab infusions in varying doses.
Median progression-free survival was 3.7 months; 1- and 2-year progression-free survival rates were 36% and 27%, respectively.
About a third of the patients (33; 31%) experienced an objective response to the drug, with a median duration of 2 years. Fifteen of these (45%) occurred within the first 8 weeks of treatment, the investigators wrote. Of these responding patients, 19 (58%) were still responding when the data were analyzed, the authors noted.
Tumors regressed at various sites, including, in one patient, an unresectable lesion that had eroded through the zygomatic arch into the orbit. Response occurred within 4 months of treatment initiation. This lesion remains in remission. A biopsy showed fibrosis and infiltrating lymphocytes, but no tumor cells. This finding hints that residual tumor seen in some patients’ imaging may in fact be scarring. If that is true, the researchers said, they could have underestimated the real response rate.
Seventeen of the 33 patients who responded discontinued treatment for reasons other than progression. Among these, 12 (71%) maintained their response for 16-56 weeks; in eight of these, response was ongoing at the time of data analysis. Among these is a patient who had metastatic lesions in the adrenal gland, small bowel, and mesenteric lymph nodes.
An additional 7% (7 patients) experienced stable disease for at least 24 weeks at doses of 0.1 to 10 mg/kg. "Unconventional" response patterns not meeting RECIST criteria occurred in four patients, but there was no associated dose pattern; three had received 1 mg/kg and the fourth, 10 mg/kg. Disease progressed in 11 patients treated at doses of 0.1 to 03 mg/kg; none of these responded to a dose escalation, the investigators said.
Nivolumab’s safety data were excellent when compared with other cancer treatments, they noted. Most adverse events occurred within the first 6 months – a hint that close observation and early intervention could minimize their impact. The most common were fatigue (32%), rash (23%), and diarrhea (18%). Grades 3 and 4 adverse events occurred in 24 patients. Treatment-related select adverse events of any grade occurred in 58 (54%). The most common were skin disorders (36%), gastrointestinal events (18%), and endocrinopathies (13%) – not unexpected in an immunomodulating drug. There were no drug-related deaths in the study.
Although this study examined nivolumab as monotherapy, others are looking at a possibly synergistic effect when it’s given with other immunomodulators, and with chemotherapy, kinase inhibitors, or cancer vaccines.
"Innovative, rational treatment combinations based on preclinical evidence may be needed to realize the full therapeutic potential of PD-1 blockade," the investigators said.
Dr. Topalian and several of her coauthors received research funding or had other financial relationships with Bristol-Myers Squibb, sponsor of the study.
An immunomodulator that targets the PD-1 pathway effected sustained remission in 31% of patients who took it for advanced melanoma.
Remission lasted a median of 2 years, with 1- and 2-year overall survival of 62% and 43% respectively, wrote Dr. Suzanne Topalian and colleagues.
But perhaps even more exciting, they said, is that the benefit of nivolumab persisted for at least 16 weeks in all patients who had to discontinue therapy.
The report appeared in the March 3 issue of the Journal of Clinical Oncology.
"The persistence of partial tumor regression and stable disease ... suggests that PD-1 blockade may reset the immune equilibrium between tumor and host," wrote Dr. Topalian, director of the melanoma program at the Sidney Kimmel Comprehensive Cancer Center, Baltimore, and her coauthors.
The findings "are consistent with a mechanism by which an effective tumor-selective immune memory response may have been established in some patients, similar to immune memory against specific infectious organisms after antigen exposure," the investigators wrote (J. Clin. Oncol. 2014 March [doi:10.1200/JCO.2013.53.0105]).
PD-1 is an immune checkpoint that helps regulate the autoimmune action of activated T cells. Tumor cells can co-opt this pathway, using it to hide from T cell attack. Blocking PD-1 unmasks tumor cells, allowing the immune system to target them more effectively.
The 2-year follow-up study tracked response rates in 107 patients with advanced melanoma, who were part of a larger study of 306 patients with various solid-tumor cancers. The initial results of the phase I trial were reported at the 2013 meeting of the American Society of Clinical Oncology.
The new report focuses only on the patients with melanoma, who have completed treatment for at least 14 months, and up to 4 years. All of the patients had undergone two to five prior systemic treatments. They received up to 96 weeks of biweekly nivolumab infusions in varying doses.
Median progression-free survival was 3.7 months; 1- and 2-year progression-free survival rates were 36% and 27%, respectively.
About a third of the patients (33; 31%) experienced an objective response to the drug, with a median duration of 2 years. Fifteen of these (45%) occurred within the first 8 weeks of treatment, the investigators wrote. Of these responding patients, 19 (58%) were still responding when the data were analyzed, the authors noted.
Tumors regressed at various sites, including, in one patient, an unresectable lesion that had eroded through the zygomatic arch into the orbit. Response occurred within 4 months of treatment initiation. This lesion remains in remission. A biopsy showed fibrosis and infiltrating lymphocytes, but no tumor cells. This finding hints that residual tumor seen in some patients’ imaging may in fact be scarring. If that is true, the researchers said, they could have underestimated the real response rate.
Seventeen of the 33 patients who responded discontinued treatment for reasons other than progression. Among these, 12 (71%) maintained their response for 16-56 weeks; in eight of these, response was ongoing at the time of data analysis. Among these is a patient who had metastatic lesions in the adrenal gland, small bowel, and mesenteric lymph nodes.
An additional 7% (7 patients) experienced stable disease for at least 24 weeks at doses of 0.1 to 10 mg/kg. "Unconventional" response patterns not meeting RECIST criteria occurred in four patients, but there was no associated dose pattern; three had received 1 mg/kg and the fourth, 10 mg/kg. Disease progressed in 11 patients treated at doses of 0.1 to 03 mg/kg; none of these responded to a dose escalation, the investigators said.
Nivolumab’s safety data were excellent when compared with other cancer treatments, they noted. Most adverse events occurred within the first 6 months – a hint that close observation and early intervention could minimize their impact. The most common were fatigue (32%), rash (23%), and diarrhea (18%). Grades 3 and 4 adverse events occurred in 24 patients. Treatment-related select adverse events of any grade occurred in 58 (54%). The most common were skin disorders (36%), gastrointestinal events (18%), and endocrinopathies (13%) – not unexpected in an immunomodulating drug. There were no drug-related deaths in the study.
Although this study examined nivolumab as monotherapy, others are looking at a possibly synergistic effect when it’s given with other immunomodulators, and with chemotherapy, kinase inhibitors, or cancer vaccines.
"Innovative, rational treatment combinations based on preclinical evidence may be needed to realize the full therapeutic potential of PD-1 blockade," the investigators said.
Dr. Topalian and several of her coauthors received research funding or had other financial relationships with Bristol-Myers Squibb, sponsor of the study.
Major finding: The PD-1 receptor inhibitor nivolumab effected sustained response in 31% of those who took it for advanced melanoma.
Data source: A retrospective study of 107 patients with advanced melanoma.
Disclosures: Dr. Topalian and several coauthors received research funding or had other financial relationships with Bristol-Myers Squibb, sponsor of the study.
Vemurafenib improved survival in BRAF mutation–positive metastatic melanoma
Vemurafenib had a significant survival benefit compared with dacarbazine in adults with treatment-naive, BRAFV600E and BRAFV600K mutation–positive metastatic melanoma, investigators reported in the March issue of Lancet Oncology.
In the phase III trial, 675 patients with BRAFV600 mutations were randomized to receive BRAF inhibitor vemurafenib (960 mg orally b.i.d.) or dacarbazine (1,000 mg/m2 IV every 3 weeks). The median overall survival was 13.6 months among 337 patients randomized to receive vemurafenib, compared with 9.7 months for the 338 patients randomized to receive dacarbazine (hazard ratio, 0.70; 95% confidence interval, 0.57-0.87, P = .0008), reported Dr. Grant McArthur of Peter MacCallum Cancer Centre, East Melbourne, Australia, and his associates.
Researchers followed vemurafenib and dacarbazine patients for a median of 12.5 and 9.5 months, respectively. One-quarter of dacarbazine patients later crossed over to vemurafenib (Lancet Oncol. 2014;15:323-32).
Median progression-free survival times also favored vemurafenib (6.9 months vs. 1.6 months for dacarbazine; HR for vemurafenib, 0.38; 95% CI, 0.32-0.46, P less than .0001). Among those patients available for follow-up at 18 months, after crossover had occurred, the difference in overall survival was insignificant (39% for vemurafenib vs. 34% for dacarbazine), the investigators reported.
Although BRAFV600E is the most common mutation in patients with metastatic melanoma, a substantial proportion of patients carry the BRAFV600K mutation (8.6% in the current study), and data suggest that these patients might be at increased risk for brain and lung metastases and might have a shorter time from diagnosis to metastasis and death than patients with a BRAFVV600E mutation, the investigators said.
Benefit from vemurafenib was found for both types of BRAF mutations. For the 598 patients with BRAFV600E disease (91%), the median overall survival in the vemurafenib group was 13.3 months compared with 10.0 months in the dacarbazine group (HR, 0.75; 95% CI, 0.60-0.93, P = .0085). For the 57 patients with BRAFV600K disease (9%), the median overall survival in the vemurafenib group was 14.5 months (95% CI, 11.2 to not estimable) compared with 7.6 months in the dacarbazine group (HR, 0.43; 95% CI, 0.21-0.90, P = .024), the investigators reported.
Grade 3-4 events included cutaneous squamous cell carcinoma (19%), keratoacanthomas (10%), rash (9%), and abnormal liver function tests (11%) in the vemurafenib group, and neutropenia (9%) in the dacarbazine group. Eight patients (2%) in the vemurafenib group developed new primary melanomas. The death rate due to adverse events was 2% in each group.
F Hoffman/La Roche/Genentech funded the trial. Dr. McArthur reported providing uncompensated services to Roche/Genentech. Sixteen of his associates reported receiving support from or serving as consultants or advisers to Roche, Roche/Genentech, or Genentech. Three other associates are employees of Roche/Genentech, Roche Molecular Systems, or F Hoffman/La Roche.
Vemurafenib had a significant survival benefit compared with dacarbazine in adults with treatment-naive, BRAFV600E and BRAFV600K mutation–positive metastatic melanoma, investigators reported in the March issue of Lancet Oncology.
In the phase III trial, 675 patients with BRAFV600 mutations were randomized to receive BRAF inhibitor vemurafenib (960 mg orally b.i.d.) or dacarbazine (1,000 mg/m2 IV every 3 weeks). The median overall survival was 13.6 months among 337 patients randomized to receive vemurafenib, compared with 9.7 months for the 338 patients randomized to receive dacarbazine (hazard ratio, 0.70; 95% confidence interval, 0.57-0.87, P = .0008), reported Dr. Grant McArthur of Peter MacCallum Cancer Centre, East Melbourne, Australia, and his associates.
Researchers followed vemurafenib and dacarbazine patients for a median of 12.5 and 9.5 months, respectively. One-quarter of dacarbazine patients later crossed over to vemurafenib (Lancet Oncol. 2014;15:323-32).
Median progression-free survival times also favored vemurafenib (6.9 months vs. 1.6 months for dacarbazine; HR for vemurafenib, 0.38; 95% CI, 0.32-0.46, P less than .0001). Among those patients available for follow-up at 18 months, after crossover had occurred, the difference in overall survival was insignificant (39% for vemurafenib vs. 34% for dacarbazine), the investigators reported.
Although BRAFV600E is the most common mutation in patients with metastatic melanoma, a substantial proportion of patients carry the BRAFV600K mutation (8.6% in the current study), and data suggest that these patients might be at increased risk for brain and lung metastases and might have a shorter time from diagnosis to metastasis and death than patients with a BRAFVV600E mutation, the investigators said.
Benefit from vemurafenib was found for both types of BRAF mutations. For the 598 patients with BRAFV600E disease (91%), the median overall survival in the vemurafenib group was 13.3 months compared with 10.0 months in the dacarbazine group (HR, 0.75; 95% CI, 0.60-0.93, P = .0085). For the 57 patients with BRAFV600K disease (9%), the median overall survival in the vemurafenib group was 14.5 months (95% CI, 11.2 to not estimable) compared with 7.6 months in the dacarbazine group (HR, 0.43; 95% CI, 0.21-0.90, P = .024), the investigators reported.
Grade 3-4 events included cutaneous squamous cell carcinoma (19%), keratoacanthomas (10%), rash (9%), and abnormal liver function tests (11%) in the vemurafenib group, and neutropenia (9%) in the dacarbazine group. Eight patients (2%) in the vemurafenib group developed new primary melanomas. The death rate due to adverse events was 2% in each group.
F Hoffman/La Roche/Genentech funded the trial. Dr. McArthur reported providing uncompensated services to Roche/Genentech. Sixteen of his associates reported receiving support from or serving as consultants or advisers to Roche, Roche/Genentech, or Genentech. Three other associates are employees of Roche/Genentech, Roche Molecular Systems, or F Hoffman/La Roche.
Vemurafenib had a significant survival benefit compared with dacarbazine in adults with treatment-naive, BRAFV600E and BRAFV600K mutation–positive metastatic melanoma, investigators reported in the March issue of Lancet Oncology.
In the phase III trial, 675 patients with BRAFV600 mutations were randomized to receive BRAF inhibitor vemurafenib (960 mg orally b.i.d.) or dacarbazine (1,000 mg/m2 IV every 3 weeks). The median overall survival was 13.6 months among 337 patients randomized to receive vemurafenib, compared with 9.7 months for the 338 patients randomized to receive dacarbazine (hazard ratio, 0.70; 95% confidence interval, 0.57-0.87, P = .0008), reported Dr. Grant McArthur of Peter MacCallum Cancer Centre, East Melbourne, Australia, and his associates.
Researchers followed vemurafenib and dacarbazine patients for a median of 12.5 and 9.5 months, respectively. One-quarter of dacarbazine patients later crossed over to vemurafenib (Lancet Oncol. 2014;15:323-32).
Median progression-free survival times also favored vemurafenib (6.9 months vs. 1.6 months for dacarbazine; HR for vemurafenib, 0.38; 95% CI, 0.32-0.46, P less than .0001). Among those patients available for follow-up at 18 months, after crossover had occurred, the difference in overall survival was insignificant (39% for vemurafenib vs. 34% for dacarbazine), the investigators reported.
Although BRAFV600E is the most common mutation in patients with metastatic melanoma, a substantial proportion of patients carry the BRAFV600K mutation (8.6% in the current study), and data suggest that these patients might be at increased risk for brain and lung metastases and might have a shorter time from diagnosis to metastasis and death than patients with a BRAFVV600E mutation, the investigators said.
Benefit from vemurafenib was found for both types of BRAF mutations. For the 598 patients with BRAFV600E disease (91%), the median overall survival in the vemurafenib group was 13.3 months compared with 10.0 months in the dacarbazine group (HR, 0.75; 95% CI, 0.60-0.93, P = .0085). For the 57 patients with BRAFV600K disease (9%), the median overall survival in the vemurafenib group was 14.5 months (95% CI, 11.2 to not estimable) compared with 7.6 months in the dacarbazine group (HR, 0.43; 95% CI, 0.21-0.90, P = .024), the investigators reported.
Grade 3-4 events included cutaneous squamous cell carcinoma (19%), keratoacanthomas (10%), rash (9%), and abnormal liver function tests (11%) in the vemurafenib group, and neutropenia (9%) in the dacarbazine group. Eight patients (2%) in the vemurafenib group developed new primary melanomas. The death rate due to adverse events was 2% in each group.
F Hoffman/La Roche/Genentech funded the trial. Dr. McArthur reported providing uncompensated services to Roche/Genentech. Sixteen of his associates reported receiving support from or serving as consultants or advisers to Roche, Roche/Genentech, or Genentech. Three other associates are employees of Roche/Genentech, Roche Molecular Systems, or F Hoffman/La Roche.
FROM LANCET ONCOLOGY
Major finding: The median overall survival was 13.6 months for patients receiving vemurafenib compared with 9.7 months for patients receiving dacarbazine (hazard ratio, 0.70; 95% confidence interval, 0.57-0.87, P = .0008).
Data source: A phase III randomized, open-label trial of 675 adults with treatment-naive, BRAFV600E and BRAFV600K mutation–positive metastatic melanoma.
Disclosures: F Hoffman/La Roche/Genentech funded the trial. Dr. McArthur reported providing uncompensated services to Roche/Genentech. Sixteen of his associates reported receiving support from or serving as consultants or advisers to Roche, Roche/Genentech, or Genentech. Three other associates are employees of Roche/Genentech, Roche Molecular Systems, or F Hoffman/La Roche.