User login
Tanners Risk Sunbed Use Despite Knowing the Dangers
CANCUN, Mexico — Knowledge doesn't necessarily mean power when it comes to tanning beds.
The results of a small British survey show that the majority of patients in a dermatology clinic who used tanning beds for purely cosmetic reasons were well acquainted with the risks.
Ninety-four percent of the patients surveyed were aware of the link between tanning bed use and skin cancer, and even volunteered that they knew other risks, Dr. Tina Ninan said at Wonca 2010, the conference of the World Organization of Family Doctors. "Knowing the risks does not deter patients from engaging in this risky behavior," said Dr. Ninan, a general practice physician in Newcastle upon Tyne, England.
She distributed some simple surveys to 102 patients waiting in a dermatology clinic at the University Hospital of North Durham. Most of the respondents (65%) were women, and most (60%) were older than 40 years.
Thirty-four patients admitted to using tanning beds. Most of these (27) were women, meaning that 42% of the women surveyed admitted to using the devices. But they weren't alone: 18% of the men surveyed also said they used tanning beds.
Most of the users (88%) said that they had started visiting tanning salons before they turned 35 years old—a period considered crucial in developing an increased risk of skin cancers. "More disturbingly, 58% said they began tanning at age 17-25, and 18% of the users started at younger than 16 years," Dr. Ninan said.
Although most reported occasional use, 38% of the tanners said they visited a tanning bed weekly. Most used tanning beds at their local beauty salon, but 9% said they had the devices at home. "Six percent also said they tanned at their gym. Having [tanning] beds at gyms and other health centers really sends a mixed message about their health benefits," Dr. Ninan said.
Coin-operated tanning beds were also popular among the group, with 16% reporting use. This trend is particularly disturbing, Dr. Ninan said, because it circumvents what little control there may be over exposure. And although the United Kingdom recently passed a law forbidding teens to use tanning beds, no one can prevent them from accessing the coin-operated types, she added. Additionally, there is no guarantee that unsupervised tanners use the correct eye protection.
Looking good was the main motivation for tanning. Most tanners (64%) said that they just like the look of tanned skin, although 14% said they were trying to treat a skin condition, including acne, rosacea, and psoriasis. Seventeen percent of the tanners were getting ready for a beach vacation and thought a base tan would help protect them from sunburn. "Unfortunately, it's been shown that going away on vacation with a tan only offers an SPF of about 2-3," Dr. Ninan said.
The motivations for tanning were apparently stronger than worries about its risks.
Almost everyone (94%) was aware of the connection between tanning bed use and skin cancer. Respondents were also free to write in other risks they knew of— most frequently listed were wrinkles and eye damage.
Although she admitted that understanding risks won't necessarily change behavior, Dr. Ninan encouraged physicians to find "teachable moments" to educate their patients about tanning bed use. "For example, I frequently have patients asking me to check their moles. This is always the chance I take to ask about [tanning] bed use and its risks."
Dr. Ninan disclosed that she had no financial conflicts.
CANCUN, Mexico — Knowledge doesn't necessarily mean power when it comes to tanning beds.
The results of a small British survey show that the majority of patients in a dermatology clinic who used tanning beds for purely cosmetic reasons were well acquainted with the risks.
Ninety-four percent of the patients surveyed were aware of the link between tanning bed use and skin cancer, and even volunteered that they knew other risks, Dr. Tina Ninan said at Wonca 2010, the conference of the World Organization of Family Doctors. "Knowing the risks does not deter patients from engaging in this risky behavior," said Dr. Ninan, a general practice physician in Newcastle upon Tyne, England.
She distributed some simple surveys to 102 patients waiting in a dermatology clinic at the University Hospital of North Durham. Most of the respondents (65%) were women, and most (60%) were older than 40 years.
Thirty-four patients admitted to using tanning beds. Most of these (27) were women, meaning that 42% of the women surveyed admitted to using the devices. But they weren't alone: 18% of the men surveyed also said they used tanning beds.
Most of the users (88%) said that they had started visiting tanning salons before they turned 35 years old—a period considered crucial in developing an increased risk of skin cancers. "More disturbingly, 58% said they began tanning at age 17-25, and 18% of the users started at younger than 16 years," Dr. Ninan said.
Although most reported occasional use, 38% of the tanners said they visited a tanning bed weekly. Most used tanning beds at their local beauty salon, but 9% said they had the devices at home. "Six percent also said they tanned at their gym. Having [tanning] beds at gyms and other health centers really sends a mixed message about their health benefits," Dr. Ninan said.
Coin-operated tanning beds were also popular among the group, with 16% reporting use. This trend is particularly disturbing, Dr. Ninan said, because it circumvents what little control there may be over exposure. And although the United Kingdom recently passed a law forbidding teens to use tanning beds, no one can prevent them from accessing the coin-operated types, she added. Additionally, there is no guarantee that unsupervised tanners use the correct eye protection.
Looking good was the main motivation for tanning. Most tanners (64%) said that they just like the look of tanned skin, although 14% said they were trying to treat a skin condition, including acne, rosacea, and psoriasis. Seventeen percent of the tanners were getting ready for a beach vacation and thought a base tan would help protect them from sunburn. "Unfortunately, it's been shown that going away on vacation with a tan only offers an SPF of about 2-3," Dr. Ninan said.
The motivations for tanning were apparently stronger than worries about its risks.
Almost everyone (94%) was aware of the connection between tanning bed use and skin cancer. Respondents were also free to write in other risks they knew of— most frequently listed were wrinkles and eye damage.
Although she admitted that understanding risks won't necessarily change behavior, Dr. Ninan encouraged physicians to find "teachable moments" to educate their patients about tanning bed use. "For example, I frequently have patients asking me to check their moles. This is always the chance I take to ask about [tanning] bed use and its risks."
Dr. Ninan disclosed that she had no financial conflicts.
CANCUN, Mexico — Knowledge doesn't necessarily mean power when it comes to tanning beds.
The results of a small British survey show that the majority of patients in a dermatology clinic who used tanning beds for purely cosmetic reasons were well acquainted with the risks.
Ninety-four percent of the patients surveyed were aware of the link between tanning bed use and skin cancer, and even volunteered that they knew other risks, Dr. Tina Ninan said at Wonca 2010, the conference of the World Organization of Family Doctors. "Knowing the risks does not deter patients from engaging in this risky behavior," said Dr. Ninan, a general practice physician in Newcastle upon Tyne, England.
She distributed some simple surveys to 102 patients waiting in a dermatology clinic at the University Hospital of North Durham. Most of the respondents (65%) were women, and most (60%) were older than 40 years.
Thirty-four patients admitted to using tanning beds. Most of these (27) were women, meaning that 42% of the women surveyed admitted to using the devices. But they weren't alone: 18% of the men surveyed also said they used tanning beds.
Most of the users (88%) said that they had started visiting tanning salons before they turned 35 years old—a period considered crucial in developing an increased risk of skin cancers. "More disturbingly, 58% said they began tanning at age 17-25, and 18% of the users started at younger than 16 years," Dr. Ninan said.
Although most reported occasional use, 38% of the tanners said they visited a tanning bed weekly. Most used tanning beds at their local beauty salon, but 9% said they had the devices at home. "Six percent also said they tanned at their gym. Having [tanning] beds at gyms and other health centers really sends a mixed message about their health benefits," Dr. Ninan said.
Coin-operated tanning beds were also popular among the group, with 16% reporting use. This trend is particularly disturbing, Dr. Ninan said, because it circumvents what little control there may be over exposure. And although the United Kingdom recently passed a law forbidding teens to use tanning beds, no one can prevent them from accessing the coin-operated types, she added. Additionally, there is no guarantee that unsupervised tanners use the correct eye protection.
Looking good was the main motivation for tanning. Most tanners (64%) said that they just like the look of tanned skin, although 14% said they were trying to treat a skin condition, including acne, rosacea, and psoriasis. Seventeen percent of the tanners were getting ready for a beach vacation and thought a base tan would help protect them from sunburn. "Unfortunately, it's been shown that going away on vacation with a tan only offers an SPF of about 2-3," Dr. Ninan said.
The motivations for tanning were apparently stronger than worries about its risks.
Almost everyone (94%) was aware of the connection between tanning bed use and skin cancer. Respondents were also free to write in other risks they knew of— most frequently listed were wrinkles and eye damage.
Although she admitted that understanding risks won't necessarily change behavior, Dr. Ninan encouraged physicians to find "teachable moments" to educate their patients about tanning bed use. "For example, I frequently have patients asking me to check their moles. This is always the chance I take to ask about [tanning] bed use and its risks."
Dr. Ninan disclosed that she had no financial conflicts.
PET/CT Found Best for Lymph Node Scans in Merkel Cell Carcinoma
NEW YORK — Use of positron emission tomography/computed tomography to assess lymph node involvement in Merkel cell carcinoma gives the best sensitivity and equal specificity, compared with more traditional imaging modalities.
"CT or MRI have been the imaging modalities of choice when staging patients with Merkel cell carcinoma," Dr. Michael B. Colgan said at the annual meeting of the American College of Mohs Surgery "We also know these techniques have shortcomings."
However, "We know from our colleagues in oncology … that PET/CT has become their go-to for staging and oftentimes restaging of disease."
Dr. Colgan, of the dermatology department at the Mayo Clinic in Rochester, Minn., looked at patients from three centers diagnosed with primary Merkel cell carcinoma between 1986 and 2008.
All patients had, as part of their cancer staging, a documented imaging study of their regional lymph node basin. Overall, 75 patients underwent a CT scan for this purpose; 34 had a PET/CT scan; and 10 patients had an MRI.
According to Dr. Colgan, the MRI results, when compared to the preferred method of histopathologic confirmation, were "dismal." The sensitivity in these cases was 0%; the specificity was 86%, the positive predictive value 0%, and the negative predictive value 67% in "detecting nodal basin involvement."
The CT scan results were slightly better - 54% sensitivity, said Dr. Colgan. "But if my radiologist tells me the CT is negative, 3 out of 10 times it's probably not the case," he said, for a negative predictive value of 70%. The specificity was 95%, and the positive predictive value was 90%.
The PET/CT results, on the other hand, offered a sensitivity of 77% - significantly higher than the other two modalities - a specificity of 95%, a positive predictive value of 91%, and a negative predictive value of 87% in detecting regional lymph node involvement.
"PET/CT can affect the stage and ultimately the clinical plan for these patients," he said.
He added that in three cases of PET/CT false negatives, there was either single node involvement or a small micrometastasis that went undetected.
"The big question when we're dealing with Merkel cell carcinoma is, if PET/CT still misses micromets [microscopic metastases], how are these scans best utilized?" asked Dr. Colgan. "That's a debate we need to have."
He pointed to some limitation of the study, including its retrospective design and the possibility of sampling bias, given that "all these patients went to get scanned."
A prospective study, of the "same patient, same node, at the same point in time" is needed.
Nevertheless, he said, especially compared with MRI, "If you were going to look at these two imaging modalities side by side, my choice is the PET/CT."
In an interview following the meeting, Dr. Colgan was asked whether PET/CT scans ought to be the standard for detecting lymph node involvement in other cancers, too.
"I would hesitate to extrapolate these results to other tumors, as each tumor has a unique metabolic signature and route of metastasis," he said.
"It is a remarkable imaging technique, but the studies need to be done."
Dr. Colgan stated that he had no conflicts of interest to disclose in relation to this study.
NEW YORK — Use of positron emission tomography/computed tomography to assess lymph node involvement in Merkel cell carcinoma gives the best sensitivity and equal specificity, compared with more traditional imaging modalities.
"CT or MRI have been the imaging modalities of choice when staging patients with Merkel cell carcinoma," Dr. Michael B. Colgan said at the annual meeting of the American College of Mohs Surgery "We also know these techniques have shortcomings."
However, "We know from our colleagues in oncology … that PET/CT has become their go-to for staging and oftentimes restaging of disease."
Dr. Colgan, of the dermatology department at the Mayo Clinic in Rochester, Minn., looked at patients from three centers diagnosed with primary Merkel cell carcinoma between 1986 and 2008.
All patients had, as part of their cancer staging, a documented imaging study of their regional lymph node basin. Overall, 75 patients underwent a CT scan for this purpose; 34 had a PET/CT scan; and 10 patients had an MRI.
According to Dr. Colgan, the MRI results, when compared to the preferred method of histopathologic confirmation, were "dismal." The sensitivity in these cases was 0%; the specificity was 86%, the positive predictive value 0%, and the negative predictive value 67% in "detecting nodal basin involvement."
The CT scan results were slightly better - 54% sensitivity, said Dr. Colgan. "But if my radiologist tells me the CT is negative, 3 out of 10 times it's probably not the case," he said, for a negative predictive value of 70%. The specificity was 95%, and the positive predictive value was 90%.
The PET/CT results, on the other hand, offered a sensitivity of 77% - significantly higher than the other two modalities - a specificity of 95%, a positive predictive value of 91%, and a negative predictive value of 87% in detecting regional lymph node involvement.
"PET/CT can affect the stage and ultimately the clinical plan for these patients," he said.
He added that in three cases of PET/CT false negatives, there was either single node involvement or a small micrometastasis that went undetected.
"The big question when we're dealing with Merkel cell carcinoma is, if PET/CT still misses micromets [microscopic metastases], how are these scans best utilized?" asked Dr. Colgan. "That's a debate we need to have."
He pointed to some limitation of the study, including its retrospective design and the possibility of sampling bias, given that "all these patients went to get scanned."
A prospective study, of the "same patient, same node, at the same point in time" is needed.
Nevertheless, he said, especially compared with MRI, "If you were going to look at these two imaging modalities side by side, my choice is the PET/CT."
In an interview following the meeting, Dr. Colgan was asked whether PET/CT scans ought to be the standard for detecting lymph node involvement in other cancers, too.
"I would hesitate to extrapolate these results to other tumors, as each tumor has a unique metabolic signature and route of metastasis," he said.
"It is a remarkable imaging technique, but the studies need to be done."
Dr. Colgan stated that he had no conflicts of interest to disclose in relation to this study.
NEW YORK — Use of positron emission tomography/computed tomography to assess lymph node involvement in Merkel cell carcinoma gives the best sensitivity and equal specificity, compared with more traditional imaging modalities.
"CT or MRI have been the imaging modalities of choice when staging patients with Merkel cell carcinoma," Dr. Michael B. Colgan said at the annual meeting of the American College of Mohs Surgery "We also know these techniques have shortcomings."
However, "We know from our colleagues in oncology … that PET/CT has become their go-to for staging and oftentimes restaging of disease."
Dr. Colgan, of the dermatology department at the Mayo Clinic in Rochester, Minn., looked at patients from three centers diagnosed with primary Merkel cell carcinoma between 1986 and 2008.
All patients had, as part of their cancer staging, a documented imaging study of their regional lymph node basin. Overall, 75 patients underwent a CT scan for this purpose; 34 had a PET/CT scan; and 10 patients had an MRI.
According to Dr. Colgan, the MRI results, when compared to the preferred method of histopathologic confirmation, were "dismal." The sensitivity in these cases was 0%; the specificity was 86%, the positive predictive value 0%, and the negative predictive value 67% in "detecting nodal basin involvement."
The CT scan results were slightly better - 54% sensitivity, said Dr. Colgan. "But if my radiologist tells me the CT is negative, 3 out of 10 times it's probably not the case," he said, for a negative predictive value of 70%. The specificity was 95%, and the positive predictive value was 90%.
The PET/CT results, on the other hand, offered a sensitivity of 77% - significantly higher than the other two modalities - a specificity of 95%, a positive predictive value of 91%, and a negative predictive value of 87% in detecting regional lymph node involvement.
"PET/CT can affect the stage and ultimately the clinical plan for these patients," he said.
He added that in three cases of PET/CT false negatives, there was either single node involvement or a small micrometastasis that went undetected.
"The big question when we're dealing with Merkel cell carcinoma is, if PET/CT still misses micromets [microscopic metastases], how are these scans best utilized?" asked Dr. Colgan. "That's a debate we need to have."
He pointed to some limitation of the study, including its retrospective design and the possibility of sampling bias, given that "all these patients went to get scanned."
A prospective study, of the "same patient, same node, at the same point in time" is needed.
Nevertheless, he said, especially compared with MRI, "If you were going to look at these two imaging modalities side by side, my choice is the PET/CT."
In an interview following the meeting, Dr. Colgan was asked whether PET/CT scans ought to be the standard for detecting lymph node involvement in other cancers, too.
"I would hesitate to extrapolate these results to other tumors, as each tumor has a unique metabolic signature and route of metastasis," he said.
"It is a remarkable imaging technique, but the studies need to be done."
Dr. Colgan stated that he had no conflicts of interest to disclose in relation to this study.
ABD Retreat Seeks Consensus on Procedural Dermatology Exam
MONTEREY, Calif. — An American Board of Dermatology retreat on June 12 will attempt to reach peace between conflicting factions within the specialty on the issue of a certifying exam in procedural dermatology.
The gathering in Chicago will bring together leaders of various dermatologic associations who favor or oppose an idea first presented in 2008 by the American Board of Dermatology (ABD) to create a subspecialty certifying exam in procedural dermatology.
The proposal drew fire from the American Society for Mohs Surgery, the American Academy of Dermatology, and others who feared that eventually only fellowship-trained physicians would be eligible to take the exam and that insurers would refuse payments for procedures by noncertified physicians, thus restricting access to patient care.
The controversy prompted the ABD to slow consideration of the exam, and to schedule the upcoming retreat to obtain input from dermatologic organizations, Dr. Lee Portnoff, president of the American Society for Mohs Surgery (ASMS), said in an interview at the Society's annual meeting. The ASMS, which does not require fellowship training for membership, opposes the exam proposal. The American College of Mohs Surgery (ACMS), which requires members to have completed a 1- to 2-year fellowship, favors the proposal.
Under the ABD's original proposal, dermatologists who had completed a procedural dermatology fellowship would have the option of sitting for the certification exam and - for a limited time - physicians who had not completed a fellowship but who had significant surgical experience would be "grandfathered" in.
"I don't know if the current proposal differs significantly enough from last year's proposal that any of the dermatology organizations would support it, and I don't know how seriously the ABD would incorporate any comments at the retreat," Dr. Portnoff said.
"Our [ASMS's] position is that sufficient dermatologic surgery training should be included in all dermatology residencies so that certification by the ABD would assume competence in dermatologic surgery," he added.
"One of the large questions is, who will be deemed qualified to sit for the exam? That very question may be the dividing point, and is part of the main controversy," Dr. Matthew M. Goodman said at the meeting.
"We feel, in this Society [ASMS], that if you're appropriately trained - which can occur in residency or post residency - you can be competent to do Mohs surgery in a quality way for the best care of your patients without having done a fellowship or passed a certifying exam," added Dr. Goodman, the founding president of the Society and a Mohs surgeon in private practice in Santa Ana, Calif. "There's nothing against fellowships or against a certifying exam per se. We just would rather not see those as divisive forces."
Dr. Leonard M. Dzubow, president of the ACMS and a former director of the ABD, noted in an interview that procedural dermatology includes not just Mohs surgery but the entire spectrum of dermatologic surgery, so subspecialty certification in the field should be of interest to all dermatologists with advanced experience.
The ACMS's mission includes fostering excellence in treatment and in patient education about treatment options and different subspecialties. "The concept of certification will include several pathways for qualification, which will make it available to all physicians with sufficient experience and knowledge," said Dr. Dzubow, professor of dermatology at the University of Pennsylvania, Philadelphia.
Dr. Randall K. Roenigk, a past president of the ABD, said in an interview that the impetus for the exam was to give residents and fellows who complete training programs accredited by the Accreditation Council for Graduate Medical Education the option of being board certified.
"Just like all 24 core specialties and 117 subspecialties associated with the American Board of Medical Specialties, the mission of the ABD is to assure the public that physicians who hold themselves out as specialists are competent in a defined body of knowledge," said Dr. Roenigk, chairman of the department of dermatology at the Mayo Clinic in Rochester, Minn.
Dr. Elaine C. Siegfried, current president of the ABD and professor of pediatrics and dermatology at St. Louis University, said the ABD would have no comment on the topic until after the retreat.
If the ABD creates the certifying exam, procedural dermatology would join dermatopathology, pediatric dermatology, and clinical and laboratory dermatologic immunology as board-certified subspecialties.
MONTEREY, Calif. — An American Board of Dermatology retreat on June 12 will attempt to reach peace between conflicting factions within the specialty on the issue of a certifying exam in procedural dermatology.
The gathering in Chicago will bring together leaders of various dermatologic associations who favor or oppose an idea first presented in 2008 by the American Board of Dermatology (ABD) to create a subspecialty certifying exam in procedural dermatology.
The proposal drew fire from the American Society for Mohs Surgery, the American Academy of Dermatology, and others who feared that eventually only fellowship-trained physicians would be eligible to take the exam and that insurers would refuse payments for procedures by noncertified physicians, thus restricting access to patient care.
The controversy prompted the ABD to slow consideration of the exam, and to schedule the upcoming retreat to obtain input from dermatologic organizations, Dr. Lee Portnoff, president of the American Society for Mohs Surgery (ASMS), said in an interview at the Society's annual meeting. The ASMS, which does not require fellowship training for membership, opposes the exam proposal. The American College of Mohs Surgery (ACMS), which requires members to have completed a 1- to 2-year fellowship, favors the proposal.
Under the ABD's original proposal, dermatologists who had completed a procedural dermatology fellowship would have the option of sitting for the certification exam and - for a limited time - physicians who had not completed a fellowship but who had significant surgical experience would be "grandfathered" in.
"I don't know if the current proposal differs significantly enough from last year's proposal that any of the dermatology organizations would support it, and I don't know how seriously the ABD would incorporate any comments at the retreat," Dr. Portnoff said.
"Our [ASMS's] position is that sufficient dermatologic surgery training should be included in all dermatology residencies so that certification by the ABD would assume competence in dermatologic surgery," he added.
"One of the large questions is, who will be deemed qualified to sit for the exam? That very question may be the dividing point, and is part of the main controversy," Dr. Matthew M. Goodman said at the meeting.
"We feel, in this Society [ASMS], that if you're appropriately trained - which can occur in residency or post residency - you can be competent to do Mohs surgery in a quality way for the best care of your patients without having done a fellowship or passed a certifying exam," added Dr. Goodman, the founding president of the Society and a Mohs surgeon in private practice in Santa Ana, Calif. "There's nothing against fellowships or against a certifying exam per se. We just would rather not see those as divisive forces."
Dr. Leonard M. Dzubow, president of the ACMS and a former director of the ABD, noted in an interview that procedural dermatology includes not just Mohs surgery but the entire spectrum of dermatologic surgery, so subspecialty certification in the field should be of interest to all dermatologists with advanced experience.
The ACMS's mission includes fostering excellence in treatment and in patient education about treatment options and different subspecialties. "The concept of certification will include several pathways for qualification, which will make it available to all physicians with sufficient experience and knowledge," said Dr. Dzubow, professor of dermatology at the University of Pennsylvania, Philadelphia.
Dr. Randall K. Roenigk, a past president of the ABD, said in an interview that the impetus for the exam was to give residents and fellows who complete training programs accredited by the Accreditation Council for Graduate Medical Education the option of being board certified.
"Just like all 24 core specialties and 117 subspecialties associated with the American Board of Medical Specialties, the mission of the ABD is to assure the public that physicians who hold themselves out as specialists are competent in a defined body of knowledge," said Dr. Roenigk, chairman of the department of dermatology at the Mayo Clinic in Rochester, Minn.
Dr. Elaine C. Siegfried, current president of the ABD and professor of pediatrics and dermatology at St. Louis University, said the ABD would have no comment on the topic until after the retreat.
If the ABD creates the certifying exam, procedural dermatology would join dermatopathology, pediatric dermatology, and clinical and laboratory dermatologic immunology as board-certified subspecialties.
MONTEREY, Calif. — An American Board of Dermatology retreat on June 12 will attempt to reach peace between conflicting factions within the specialty on the issue of a certifying exam in procedural dermatology.
The gathering in Chicago will bring together leaders of various dermatologic associations who favor or oppose an idea first presented in 2008 by the American Board of Dermatology (ABD) to create a subspecialty certifying exam in procedural dermatology.
The proposal drew fire from the American Society for Mohs Surgery, the American Academy of Dermatology, and others who feared that eventually only fellowship-trained physicians would be eligible to take the exam and that insurers would refuse payments for procedures by noncertified physicians, thus restricting access to patient care.
The controversy prompted the ABD to slow consideration of the exam, and to schedule the upcoming retreat to obtain input from dermatologic organizations, Dr. Lee Portnoff, president of the American Society for Mohs Surgery (ASMS), said in an interview at the Society's annual meeting. The ASMS, which does not require fellowship training for membership, opposes the exam proposal. The American College of Mohs Surgery (ACMS), which requires members to have completed a 1- to 2-year fellowship, favors the proposal.
Under the ABD's original proposal, dermatologists who had completed a procedural dermatology fellowship would have the option of sitting for the certification exam and - for a limited time - physicians who had not completed a fellowship but who had significant surgical experience would be "grandfathered" in.
"I don't know if the current proposal differs significantly enough from last year's proposal that any of the dermatology organizations would support it, and I don't know how seriously the ABD would incorporate any comments at the retreat," Dr. Portnoff said.
"Our [ASMS's] position is that sufficient dermatologic surgery training should be included in all dermatology residencies so that certification by the ABD would assume competence in dermatologic surgery," he added.
"One of the large questions is, who will be deemed qualified to sit for the exam? That very question may be the dividing point, and is part of the main controversy," Dr. Matthew M. Goodman said at the meeting.
"We feel, in this Society [ASMS], that if you're appropriately trained - which can occur in residency or post residency - you can be competent to do Mohs surgery in a quality way for the best care of your patients without having done a fellowship or passed a certifying exam," added Dr. Goodman, the founding president of the Society and a Mohs surgeon in private practice in Santa Ana, Calif. "There's nothing against fellowships or against a certifying exam per se. We just would rather not see those as divisive forces."
Dr. Leonard M. Dzubow, president of the ACMS and a former director of the ABD, noted in an interview that procedural dermatology includes not just Mohs surgery but the entire spectrum of dermatologic surgery, so subspecialty certification in the field should be of interest to all dermatologists with advanced experience.
The ACMS's mission includes fostering excellence in treatment and in patient education about treatment options and different subspecialties. "The concept of certification will include several pathways for qualification, which will make it available to all physicians with sufficient experience and knowledge," said Dr. Dzubow, professor of dermatology at the University of Pennsylvania, Philadelphia.
Dr. Randall K. Roenigk, a past president of the ABD, said in an interview that the impetus for the exam was to give residents and fellows who complete training programs accredited by the Accreditation Council for Graduate Medical Education the option of being board certified.
"Just like all 24 core specialties and 117 subspecialties associated with the American Board of Medical Specialties, the mission of the ABD is to assure the public that physicians who hold themselves out as specialists are competent in a defined body of knowledge," said Dr. Roenigk, chairman of the department of dermatology at the Mayo Clinic in Rochester, Minn.
Dr. Elaine C. Siegfried, current president of the ABD and professor of pediatrics and dermatology at St. Louis University, said the ABD would have no comment on the topic until after the retreat.
If the ABD creates the certifying exam, procedural dermatology would join dermatopathology, pediatric dermatology, and clinical and laboratory dermatologic immunology as board-certified subspecialties.
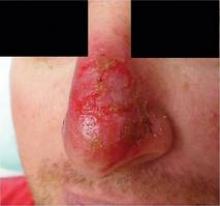
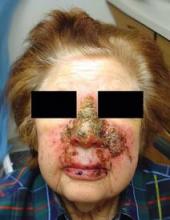
Imiquimod Before Mohs Is No Help for Nodular, Nasal BCC
MONTEREY, Calif. — Using imiquimod on nodular basal cell carcinomas on the nose before Mohs surgery failed to simplify the surgery or reduce costs and significantly increased local adverse reactions, in a randomized, controlled study of 28 patients.
"Doctors across the country are using imiquimod 'off label' to treat BCCs on the nose, and they should know these findings before doing that," said Dr. David F. Butler.
The study was inspired by a patient who refused surgery or radiation for her nodular, nasal BCC and was treated with good results using imiquimod, he said at the annual meeting of the American Society for Mohs Surgery. Perhaps, he reasoned, adjunctive imiquimod might reduce similar tumors before treatment with Mohs surgery.
"I was surprised that imiquimod did not reduce the number of stages, reduce the cost of Mohs surgery, or reduce the cost of repair," said Dr. Butler, chair of dermatology at the Scott and White Clinic and professor of internal medicine at Texas A&M University, both in Temple, Tex.
Of the 31 patients who enrolled in the study, 3 dropped out, 2 because of local adverse events and 1 because of other illness. Among the 28 patients who completed the study, 10 of 12 (83%) in the imiquimod arm and 4 of 16 (25%) in the control arm developed local adverse events after 3 weeks of treatment, including redness, blisters, erosions, and crusting. After 6 weeks, the same number in the imiquimod group and two patients in the control group (12%) had local adverse reactions. The differences between the groups were statistically significant.
The frequency of local adverse events limits the usefulness of imiquimod as adjunctive therapy in these cases, he said.
Only one stage of Mohs surgery was needed for 11 patients in each group. Two stages were needed for one patient in the imiquimod arm (8%) and five patients in the control arm (31%). Surgical defect sizes averaged 88 mm2 in the imiquimod arm and 100 mm2 in the control arm (Dermatol. Surg. 2009;35:24-9).
A larger study might show a difference, he acknowledged. The current study did not include the cost of imiquimod, so even if a larger study finds that adjunctive imiquimod therapy reduces the number of Mohs stages needed, the medication cost might negate any cost savings in the surgery.
Patients applied 5% imiquimod cream or vehicle to the tumor five nights a week for 6 weeks and covered it with a bandage supplied by investigators. One month after stopping the imiquimod, they underwent Mohs surgery.
Dr. Butler said in an interview that he was surprised by the "inordinately high" proportion of patients with tumor remaining at the time of Mohs surgery after imiquimod treatment.
Only 5 of 12 patients (42%) in the treatment group had complete clearance of the tumor after imiquimod therapy, with tumor absent (presumably destroyed) in the first stage block. "That's a relatively low number when you compare it to the 80% cure rate that you get when using imiquimod to treat superficial BCCs on the trunk or extremities," he said. "My concern is that nodular BCCs on the nose may be a different problem."
Imiquimod was approved in 2004 to treat superficial BCC on the trunk and extremities, with histologic cure rates of 79%-82% at those sites, previous studies have shown. Clearance rates have been lower, however, for nodular BCCs, with reports ranging from 65% to 76%, he noted. In addition, the most common site for BCCs is not the trunk or extremities but the nose, accounting for 25%-30%.
Dr. Butler advised against using imiquimod as a stand-alone therapy for nodular BCCs on the nose, but said it may be a reasonable option for patients who cannot or will not undergo other treatments.
"We do recommend, however, that if you're going to use imiquimod as a single therapy for nodular BCCs on the nose, that once you finish the treatment, that you go back and do a little biopsy of the area to document that the cancer is gone," he added.
Graceway Pharmaceuticals and 3M funded the study. Dr. Butler reported that he had no conflicts of interest.
MONTEREY, Calif. — Using imiquimod on nodular basal cell carcinomas on the nose before Mohs surgery failed to simplify the surgery or reduce costs and significantly increased local adverse reactions, in a randomized, controlled study of 28 patients.
"Doctors across the country are using imiquimod 'off label' to treat BCCs on the nose, and they should know these findings before doing that," said Dr. David F. Butler.
The study was inspired by a patient who refused surgery or radiation for her nodular, nasal BCC and was treated with good results using imiquimod, he said at the annual meeting of the American Society for Mohs Surgery. Perhaps, he reasoned, adjunctive imiquimod might reduce similar tumors before treatment with Mohs surgery.
"I was surprised that imiquimod did not reduce the number of stages, reduce the cost of Mohs surgery, or reduce the cost of repair," said Dr. Butler, chair of dermatology at the Scott and White Clinic and professor of internal medicine at Texas A&M University, both in Temple, Tex.
Of the 31 patients who enrolled in the study, 3 dropped out, 2 because of local adverse events and 1 because of other illness. Among the 28 patients who completed the study, 10 of 12 (83%) in the imiquimod arm and 4 of 16 (25%) in the control arm developed local adverse events after 3 weeks of treatment, including redness, blisters, erosions, and crusting. After 6 weeks, the same number in the imiquimod group and two patients in the control group (12%) had local adverse reactions. The differences between the groups were statistically significant.
The frequency of local adverse events limits the usefulness of imiquimod as adjunctive therapy in these cases, he said.
Only one stage of Mohs surgery was needed for 11 patients in each group. Two stages were needed for one patient in the imiquimod arm (8%) and five patients in the control arm (31%). Surgical defect sizes averaged 88 mm2 in the imiquimod arm and 100 mm2 in the control arm (Dermatol. Surg. 2009;35:24-9).
A larger study might show a difference, he acknowledged. The current study did not include the cost of imiquimod, so even if a larger study finds that adjunctive imiquimod therapy reduces the number of Mohs stages needed, the medication cost might negate any cost savings in the surgery.
Patients applied 5% imiquimod cream or vehicle to the tumor five nights a week for 6 weeks and covered it with a bandage supplied by investigators. One month after stopping the imiquimod, they underwent Mohs surgery.
Dr. Butler said in an interview that he was surprised by the "inordinately high" proportion of patients with tumor remaining at the time of Mohs surgery after imiquimod treatment.
Only 5 of 12 patients (42%) in the treatment group had complete clearance of the tumor after imiquimod therapy, with tumor absent (presumably destroyed) in the first stage block. "That's a relatively low number when you compare it to the 80% cure rate that you get when using imiquimod to treat superficial BCCs on the trunk or extremities," he said. "My concern is that nodular BCCs on the nose may be a different problem."
Imiquimod was approved in 2004 to treat superficial BCC on the trunk and extremities, with histologic cure rates of 79%-82% at those sites, previous studies have shown. Clearance rates have been lower, however, for nodular BCCs, with reports ranging from 65% to 76%, he noted. In addition, the most common site for BCCs is not the trunk or extremities but the nose, accounting for 25%-30%.
Dr. Butler advised against using imiquimod as a stand-alone therapy for nodular BCCs on the nose, but said it may be a reasonable option for patients who cannot or will not undergo other treatments.
"We do recommend, however, that if you're going to use imiquimod as a single therapy for nodular BCCs on the nose, that once you finish the treatment, that you go back and do a little biopsy of the area to document that the cancer is gone," he added.
Graceway Pharmaceuticals and 3M funded the study. Dr. Butler reported that he had no conflicts of interest.
MONTEREY, Calif. — Using imiquimod on nodular basal cell carcinomas on the nose before Mohs surgery failed to simplify the surgery or reduce costs and significantly increased local adverse reactions, in a randomized, controlled study of 28 patients.
"Doctors across the country are using imiquimod 'off label' to treat BCCs on the nose, and they should know these findings before doing that," said Dr. David F. Butler.
The study was inspired by a patient who refused surgery or radiation for her nodular, nasal BCC and was treated with good results using imiquimod, he said at the annual meeting of the American Society for Mohs Surgery. Perhaps, he reasoned, adjunctive imiquimod might reduce similar tumors before treatment with Mohs surgery.
"I was surprised that imiquimod did not reduce the number of stages, reduce the cost of Mohs surgery, or reduce the cost of repair," said Dr. Butler, chair of dermatology at the Scott and White Clinic and professor of internal medicine at Texas A&M University, both in Temple, Tex.
Of the 31 patients who enrolled in the study, 3 dropped out, 2 because of local adverse events and 1 because of other illness. Among the 28 patients who completed the study, 10 of 12 (83%) in the imiquimod arm and 4 of 16 (25%) in the control arm developed local adverse events after 3 weeks of treatment, including redness, blisters, erosions, and crusting. After 6 weeks, the same number in the imiquimod group and two patients in the control group (12%) had local adverse reactions. The differences between the groups were statistically significant.
The frequency of local adverse events limits the usefulness of imiquimod as adjunctive therapy in these cases, he said.
Only one stage of Mohs surgery was needed for 11 patients in each group. Two stages were needed for one patient in the imiquimod arm (8%) and five patients in the control arm (31%). Surgical defect sizes averaged 88 mm2 in the imiquimod arm and 100 mm2 in the control arm (Dermatol. Surg. 2009;35:24-9).
A larger study might show a difference, he acknowledged. The current study did not include the cost of imiquimod, so even if a larger study finds that adjunctive imiquimod therapy reduces the number of Mohs stages needed, the medication cost might negate any cost savings in the surgery.
Patients applied 5% imiquimod cream or vehicle to the tumor five nights a week for 6 weeks and covered it with a bandage supplied by investigators. One month after stopping the imiquimod, they underwent Mohs surgery.
Dr. Butler said in an interview that he was surprised by the "inordinately high" proportion of patients with tumor remaining at the time of Mohs surgery after imiquimod treatment.
Only 5 of 12 patients (42%) in the treatment group had complete clearance of the tumor after imiquimod therapy, with tumor absent (presumably destroyed) in the first stage block. "That's a relatively low number when you compare it to the 80% cure rate that you get when using imiquimod to treat superficial BCCs on the trunk or extremities," he said. "My concern is that nodular BCCs on the nose may be a different problem."
Imiquimod was approved in 2004 to treat superficial BCC on the trunk and extremities, with histologic cure rates of 79%-82% at those sites, previous studies have shown. Clearance rates have been lower, however, for nodular BCCs, with reports ranging from 65% to 76%, he noted. In addition, the most common site for BCCs is not the trunk or extremities but the nose, accounting for 25%-30%.
Dr. Butler advised against using imiquimod as a stand-alone therapy for nodular BCCs on the nose, but said it may be a reasonable option for patients who cannot or will not undergo other treatments.
"We do recommend, however, that if you're going to use imiquimod as a single therapy for nodular BCCs on the nose, that once you finish the treatment, that you go back and do a little biopsy of the area to document that the cancer is gone," he added.
Graceway Pharmaceuticals and 3M funded the study. Dr. Butler reported that he had no conflicts of interest.
ASCO, FDA Team Up to Help Patients Access Investigational Therapies
CHICAGO — A new online resource will help seriously ill patients and their physicians request expanded access to investigational therapies when they have exhausted all other treatment options and the patients are not eligible for a clinical trial, it was announced June 4 at a joint news conference of the American Society of Clinical Oncology and the Food and Drug Administration.
"Most clinical trials have quite stringent eligibility criteria, and many patients are not eligible to enroll," said ASCO President-Elect George W. Sledge Jr., of Indiana University Simon Cancer Center, Indianapolis.
"In those instances when preliminary scientific evidence suggests that treatment with an unapproved therapy might be beneficial, the FDA's expanded access regulations allow physicians to request access to an investigational agent, outside of the clinical trial setting, for seriously ill patients," he said.
Expanded access has been allowed since the 1970s, but many physicians have been uncertain about how to request it, what regulations apply, and the legal ramifications. All of these issues are clarified in the new Web site.
"It's been clear to the agency that there was room for progress," said FDA Principal Deputy Commissioner Dr. Joshua M. Sharfstein, addressing the press conference on the opening day of ASCO's annual meeting.
"This is about the opportunity for the FDA to work with a medical specialty society, in this case ASCO, in what really should be a model for the agency - and work together to develop a program to reach out to clinicians and help them understand something that can be a little confusing at first," he said.
The new resources are available immediately to all patients and physicians, free of charge, through ASCO's physician education Web site, ASCO University.
The material is presented in three online modules, which introduce the expanded access regulations, outline the process for requesting expanded access, and explain the physicians' legal responsibilities for treating patients with an investigational agent outside of a clinical trial.
The modules also contain resources such as a glossary, an expanded access request checklist, and various templates (letter to manufacturer, consent form, etc.) to help physicians with the paperwork.
In August 2009, the FDA issued updated regulations and attempted to clarify the rules in two publications, "Expanded Access to Investigational Drugs for Treatment Use," and "Charging for Investigational Drugs." The new Web site goes a step further.
To clarify the new rules for patients and the medical community, ASCO consulted the main stakeholders, including representatives from patient advocacy groups, manufacturers, institutional review boards, and the FDA, as well as physicians.
Although these tools were made with the oncology community in mind, these resources should be helpful to doctors from other medical specialties as well.
CHICAGO — A new online resource will help seriously ill patients and their physicians request expanded access to investigational therapies when they have exhausted all other treatment options and the patients are not eligible for a clinical trial, it was announced June 4 at a joint news conference of the American Society of Clinical Oncology and the Food and Drug Administration.
"Most clinical trials have quite stringent eligibility criteria, and many patients are not eligible to enroll," said ASCO President-Elect George W. Sledge Jr., of Indiana University Simon Cancer Center, Indianapolis.
"In those instances when preliminary scientific evidence suggests that treatment with an unapproved therapy might be beneficial, the FDA's expanded access regulations allow physicians to request access to an investigational agent, outside of the clinical trial setting, for seriously ill patients," he said.
Expanded access has been allowed since the 1970s, but many physicians have been uncertain about how to request it, what regulations apply, and the legal ramifications. All of these issues are clarified in the new Web site.
"It's been clear to the agency that there was room for progress," said FDA Principal Deputy Commissioner Dr. Joshua M. Sharfstein, addressing the press conference on the opening day of ASCO's annual meeting.
"This is about the opportunity for the FDA to work with a medical specialty society, in this case ASCO, in what really should be a model for the agency - and work together to develop a program to reach out to clinicians and help them understand something that can be a little confusing at first," he said.
The new resources are available immediately to all patients and physicians, free of charge, through ASCO's physician education Web site, ASCO University.
The material is presented in three online modules, which introduce the expanded access regulations, outline the process for requesting expanded access, and explain the physicians' legal responsibilities for treating patients with an investigational agent outside of a clinical trial.
The modules also contain resources such as a glossary, an expanded access request checklist, and various templates (letter to manufacturer, consent form, etc.) to help physicians with the paperwork.
In August 2009, the FDA issued updated regulations and attempted to clarify the rules in two publications, "Expanded Access to Investigational Drugs for Treatment Use," and "Charging for Investigational Drugs." The new Web site goes a step further.
To clarify the new rules for patients and the medical community, ASCO consulted the main stakeholders, including representatives from patient advocacy groups, manufacturers, institutional review boards, and the FDA, as well as physicians.
Although these tools were made with the oncology community in mind, these resources should be helpful to doctors from other medical specialties as well.
CHICAGO — A new online resource will help seriously ill patients and their physicians request expanded access to investigational therapies when they have exhausted all other treatment options and the patients are not eligible for a clinical trial, it was announced June 4 at a joint news conference of the American Society of Clinical Oncology and the Food and Drug Administration.
"Most clinical trials have quite stringent eligibility criteria, and many patients are not eligible to enroll," said ASCO President-Elect George W. Sledge Jr., of Indiana University Simon Cancer Center, Indianapolis.
"In those instances when preliminary scientific evidence suggests that treatment with an unapproved therapy might be beneficial, the FDA's expanded access regulations allow physicians to request access to an investigational agent, outside of the clinical trial setting, for seriously ill patients," he said.
Expanded access has been allowed since the 1970s, but many physicians have been uncertain about how to request it, what regulations apply, and the legal ramifications. All of these issues are clarified in the new Web site.
"It's been clear to the agency that there was room for progress," said FDA Principal Deputy Commissioner Dr. Joshua M. Sharfstein, addressing the press conference on the opening day of ASCO's annual meeting.
"This is about the opportunity for the FDA to work with a medical specialty society, in this case ASCO, in what really should be a model for the agency - and work together to develop a program to reach out to clinicians and help them understand something that can be a little confusing at first," he said.
The new resources are available immediately to all patients and physicians, free of charge, through ASCO's physician education Web site, ASCO University.
The material is presented in three online modules, which introduce the expanded access regulations, outline the process for requesting expanded access, and explain the physicians' legal responsibilities for treating patients with an investigational agent outside of a clinical trial.
The modules also contain resources such as a glossary, an expanded access request checklist, and various templates (letter to manufacturer, consent form, etc.) to help physicians with the paperwork.
In August 2009, the FDA issued updated regulations and attempted to clarify the rules in two publications, "Expanded Access to Investigational Drugs for Treatment Use," and "Charging for Investigational Drugs." The new Web site goes a step further.
To clarify the new rules for patients and the medical community, ASCO consulted the main stakeholders, including representatives from patient advocacy groups, manufacturers, institutional review boards, and the FDA, as well as physicians.
Although these tools were made with the oncology community in mind, these resources should be helpful to doctors from other medical specialties as well.
ASCO: Ipilimumab Emerges as First Treatment to Improve Melanoma Survival
CHICAGO — After a 30-year drought during which 70 randomized trials failed to improve outcomes in advanced melanoma, the first agent in a new class of drugs has prolonged survival of advanced, pretreated patients in a large international phase III trial.
“This is the first time we have shown a survival benefit in metastatic melanoma,” Dr. Steven O’Day, the lead investigator, announced at the annual meeting of the American Society of Clinical Oncology.
The new agent, ipilimumab, is a monoclonal antibody targeting the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a gene that limits the ability of T cells to attack cancerous cells.
Median overall survival reached 10.1 months in 137 patients whose only active treatment was ipilimumab and 10 months in 403 patients given ipilimumab with an experimental vaccine. It was 6.4 months in 136 patients who received the vaccine without ipilimumab – a length of time that falls within the 6-9 month life expectancy for patients with metastatic melanoma, according to Dr. O’Day, chief of research and director of the melanoma program at the Angeles Clinic and Research Institute in Los Angeles.
The difference between the study arms treated with ipilimumab and patients who received only the vaccine was highly significant with a hazard ratio of 0.68 (P= .0004).
The one-year survival rate was nearly twice as high in the ipilimumab arms (46% vs. 25%), as was the two-year rate (24% vs. 14%). Some long-term survivors continue to be followed 4.5 years after treatment.
Disease-control rates were also significantly higher in the two ipilimumab arms (28.5% with ipilimumab alone and 20.1% with ipilimumab plus vaccine vs. 11% with the vaccine alone). Best overall response rates likewise were higher (10.9% and 5.7%, respectively, vs. 1.5%).
Addition of the GP 100 peptide vaccine did not appear to improve outcomes, Dr. O’Day noted. The investigators chose it for the control arm because it had drawn responses in a previous trial, and there is no standard of care for these patients. Dacarbazine (DTIC) has long and often been used, but no randomized trial has ever proven it superior to best supportive care.
Disclosures included that research funding was provided by Medarex and Bristol-Myers Squibb, which is developing ipilimumab.
CHICAGO — After a 30-year drought during which 70 randomized trials failed to improve outcomes in advanced melanoma, the first agent in a new class of drugs has prolonged survival of advanced, pretreated patients in a large international phase III trial.
“This is the first time we have shown a survival benefit in metastatic melanoma,” Dr. Steven O’Day, the lead investigator, announced at the annual meeting of the American Society of Clinical Oncology.
The new agent, ipilimumab, is a monoclonal antibody targeting the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a gene that limits the ability of T cells to attack cancerous cells.
Median overall survival reached 10.1 months in 137 patients whose only active treatment was ipilimumab and 10 months in 403 patients given ipilimumab with an experimental vaccine. It was 6.4 months in 136 patients who received the vaccine without ipilimumab – a length of time that falls within the 6-9 month life expectancy for patients with metastatic melanoma, according to Dr. O’Day, chief of research and director of the melanoma program at the Angeles Clinic and Research Institute in Los Angeles.
The difference between the study arms treated with ipilimumab and patients who received only the vaccine was highly significant with a hazard ratio of 0.68 (P= .0004).
The one-year survival rate was nearly twice as high in the ipilimumab arms (46% vs. 25%), as was the two-year rate (24% vs. 14%). Some long-term survivors continue to be followed 4.5 years after treatment.
Disease-control rates were also significantly higher in the two ipilimumab arms (28.5% with ipilimumab alone and 20.1% with ipilimumab plus vaccine vs. 11% with the vaccine alone). Best overall response rates likewise were higher (10.9% and 5.7%, respectively, vs. 1.5%).
Addition of the GP 100 peptide vaccine did not appear to improve outcomes, Dr. O’Day noted. The investigators chose it for the control arm because it had drawn responses in a previous trial, and there is no standard of care for these patients. Dacarbazine (DTIC) has long and often been used, but no randomized trial has ever proven it superior to best supportive care.
Disclosures included that research funding was provided by Medarex and Bristol-Myers Squibb, which is developing ipilimumab.
CHICAGO — After a 30-year drought during which 70 randomized trials failed to improve outcomes in advanced melanoma, the first agent in a new class of drugs has prolonged survival of advanced, pretreated patients in a large international phase III trial.
“This is the first time we have shown a survival benefit in metastatic melanoma,” Dr. Steven O’Day, the lead investigator, announced at the annual meeting of the American Society of Clinical Oncology.
The new agent, ipilimumab, is a monoclonal antibody targeting the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a gene that limits the ability of T cells to attack cancerous cells.
Median overall survival reached 10.1 months in 137 patients whose only active treatment was ipilimumab and 10 months in 403 patients given ipilimumab with an experimental vaccine. It was 6.4 months in 136 patients who received the vaccine without ipilimumab – a length of time that falls within the 6-9 month life expectancy for patients with metastatic melanoma, according to Dr. O’Day, chief of research and director of the melanoma program at the Angeles Clinic and Research Institute in Los Angeles.
The difference between the study arms treated with ipilimumab and patients who received only the vaccine was highly significant with a hazard ratio of 0.68 (P= .0004).
The one-year survival rate was nearly twice as high in the ipilimumab arms (46% vs. 25%), as was the two-year rate (24% vs. 14%). Some long-term survivors continue to be followed 4.5 years after treatment.
Disease-control rates were also significantly higher in the two ipilimumab arms (28.5% with ipilimumab alone and 20.1% with ipilimumab plus vaccine vs. 11% with the vaccine alone). Best overall response rates likewise were higher (10.9% and 5.7%, respectively, vs. 1.5%).
Addition of the GP 100 peptide vaccine did not appear to improve outcomes, Dr. O’Day noted. The investigators chose it for the control arm because it had drawn responses in a previous trial, and there is no standard of care for these patients. Dacarbazine (DTIC) has long and often been used, but no randomized trial has ever proven it superior to best supportive care.
Disclosures included that research funding was provided by Medarex and Bristol-Myers Squibb, which is developing ipilimumab.
Genetic Testing Has Mixed Impact on Skin Self-Exam Behavior
SEATTLE — The impact of genetic testing on skin self-examination behavior among individuals at high risk for melanoma varies with personal history of the disease and test results, according to the first prospective study of this issue in a tested population.
Of 37 individuals at high risk for melanoma because of family history, those who had previously had the disease and who learned that they carried a mutation that sharply increased risk did not alter their skin self-exam behavior. Both before testing and 2 years afterward, 73% were doing these exams about every month, as is recommended, or more often.
Individuals who had not had melanoma but who learned that they carried the mutation stepped up their skin self-exam behavior: Only 30% were doing these exams at least monthly before testing, but 60% were doing so 2 years afterward.
By contrast, individuals who had not had melanoma and who learned that they did not carry the mutation had little change in their behavior, even though regular skin self-exams are also recommended for this group: 38% were doing these exams roughly once a month or more often before testing, and 44% were doing so at follow-up.
"Researchers and genetic counselors believe that learning one's objective risk will actually motivate behavior change," said lead investigator Jennifer M. Taber at the annual meeting of the Society of behavioral Medicine.
There are several concerns, however.
"One is that individuals who test negative will feel that their risk is so low that they don't need to engage in prevention or screening behaviors anymore, that they might feel a false sense of security and not change their behavior," she explained.
"Another concern is that for those who test positive, they will feel a sense of fatalism—that there is nothing they can do, their risk is so high anyway, so why bother engaging in the behaviors," she added.
Ms. Taber, a graduate student in psychology at the University of Utah in Salt Lake City, and her coinvestigators studied 37 adults from families with very high rates of melanoma. All underwent genetic testing for the p16 mutation, which sharply increases melanoma risk, and were followed for 2 years.
Nearly a third (30%) of participants were affected carriers, meaning they had a history of melanoma and had the mutation; 27% were unaffected carriers, meaning they did not have a history of the disease and did have the mutation; and 43% were noncarriers who did not have a history of the disease and did not have the mutation.
Monthly skin self-exams are recommended for all individuals from families with high rates of melanoma, regardless of their genetic test results, Ms. Taber noted, because even those with a negative result have a lifetime probability of the disease twice that of the general population.
The investigators classified the participants' skin self-exam behavior, according to the number of these exams performed in a 6-month period, as being on target (four to eight exams); overscreening (more than eight), which may actually hamper detection of changes; and underscreening (fewer than four), which may lead to missed lesions.
Two years after testing, the percentage of participants who were either on target or overscreening remained at the same high baseline level among affected carriers (73%) and had doubled among unaffected carriers (from 30% to 60%), but had increased only slightly among noncarriers (from 38% to 44%), Ms. Taber reported.
When the results were viewed another way, the percentage of participants who improved their skin self-exam practice during the 2-year period—to comply with the once-a-month recommendation—was 46% in the affected carrier group, 60% in the unaffected carrier group, and 25% in the noncarrier group.
Compared with participants who did not improve, those who did improve reported feeling that they had more control over detecting melanoma early (4.4 vs. 3.7 points on a 5-point scale).
In a subanalysis of the noncarriers, those who were underscreening at 2 years gave as their reason being busy or forgetful, feeling unqualified to perform the exams, and/or believing that their risk was not high enough.
In addition, noncarriers who improved their skin self-exam performance had a gain in their perceived control over early detection during follow-up, whereas those failing to improve did not.
The consistent finding of a link between an improvement in self-exam behavior and perceived control over detecting melanoma early has implications for strategies to increase this behavior, Ms. Taber said.
Perhaps physicians should target control perceptions over detecting a melanoma or perhaps skin self-examination competence, she commented.
Similarly, Ms. Taber noted, the barriers cited by noncarriers who were underscreening provide valuable insight specifically for individuals having negative genetic test results.
Perhaps counseling sessions should "target perceived importance of skin self-exams to make sure that individuals realize that their risk is high enough that they should be performing these behaviors, and perhaps do something like reminder or booster sessions for these individuals," she said.
Ms. Taber reported having no relevant conflicts of interest.
SEATTLE — The impact of genetic testing on skin self-examination behavior among individuals at high risk for melanoma varies with personal history of the disease and test results, according to the first prospective study of this issue in a tested population.
Of 37 individuals at high risk for melanoma because of family history, those who had previously had the disease and who learned that they carried a mutation that sharply increased risk did not alter their skin self-exam behavior. Both before testing and 2 years afterward, 73% were doing these exams about every month, as is recommended, or more often.
Individuals who had not had melanoma but who learned that they carried the mutation stepped up their skin self-exam behavior: Only 30% were doing these exams at least monthly before testing, but 60% were doing so 2 years afterward.
By contrast, individuals who had not had melanoma and who learned that they did not carry the mutation had little change in their behavior, even though regular skin self-exams are also recommended for this group: 38% were doing these exams roughly once a month or more often before testing, and 44% were doing so at follow-up.
"Researchers and genetic counselors believe that learning one's objective risk will actually motivate behavior change," said lead investigator Jennifer M. Taber at the annual meeting of the Society of behavioral Medicine.
There are several concerns, however.
"One is that individuals who test negative will feel that their risk is so low that they don't need to engage in prevention or screening behaviors anymore, that they might feel a false sense of security and not change their behavior," she explained.
"Another concern is that for those who test positive, they will feel a sense of fatalism—that there is nothing they can do, their risk is so high anyway, so why bother engaging in the behaviors," she added.
Ms. Taber, a graduate student in psychology at the University of Utah in Salt Lake City, and her coinvestigators studied 37 adults from families with very high rates of melanoma. All underwent genetic testing for the p16 mutation, which sharply increases melanoma risk, and were followed for 2 years.
Nearly a third (30%) of participants were affected carriers, meaning they had a history of melanoma and had the mutation; 27% were unaffected carriers, meaning they did not have a history of the disease and did have the mutation; and 43% were noncarriers who did not have a history of the disease and did not have the mutation.
Monthly skin self-exams are recommended for all individuals from families with high rates of melanoma, regardless of their genetic test results, Ms. Taber noted, because even those with a negative result have a lifetime probability of the disease twice that of the general population.
The investigators classified the participants' skin self-exam behavior, according to the number of these exams performed in a 6-month period, as being on target (four to eight exams); overscreening (more than eight), which may actually hamper detection of changes; and underscreening (fewer than four), which may lead to missed lesions.
Two years after testing, the percentage of participants who were either on target or overscreening remained at the same high baseline level among affected carriers (73%) and had doubled among unaffected carriers (from 30% to 60%), but had increased only slightly among noncarriers (from 38% to 44%), Ms. Taber reported.
When the results were viewed another way, the percentage of participants who improved their skin self-exam practice during the 2-year period—to comply with the once-a-month recommendation—was 46% in the affected carrier group, 60% in the unaffected carrier group, and 25% in the noncarrier group.
Compared with participants who did not improve, those who did improve reported feeling that they had more control over detecting melanoma early (4.4 vs. 3.7 points on a 5-point scale).
In a subanalysis of the noncarriers, those who were underscreening at 2 years gave as their reason being busy or forgetful, feeling unqualified to perform the exams, and/or believing that their risk was not high enough.
In addition, noncarriers who improved their skin self-exam performance had a gain in their perceived control over early detection during follow-up, whereas those failing to improve did not.
The consistent finding of a link between an improvement in self-exam behavior and perceived control over detecting melanoma early has implications for strategies to increase this behavior, Ms. Taber said.
Perhaps physicians should target control perceptions over detecting a melanoma or perhaps skin self-examination competence, she commented.
Similarly, Ms. Taber noted, the barriers cited by noncarriers who were underscreening provide valuable insight specifically for individuals having negative genetic test results.
Perhaps counseling sessions should "target perceived importance of skin self-exams to make sure that individuals realize that their risk is high enough that they should be performing these behaviors, and perhaps do something like reminder or booster sessions for these individuals," she said.
Ms. Taber reported having no relevant conflicts of interest.
SEATTLE — The impact of genetic testing on skin self-examination behavior among individuals at high risk for melanoma varies with personal history of the disease and test results, according to the first prospective study of this issue in a tested population.
Of 37 individuals at high risk for melanoma because of family history, those who had previously had the disease and who learned that they carried a mutation that sharply increased risk did not alter their skin self-exam behavior. Both before testing and 2 years afterward, 73% were doing these exams about every month, as is recommended, or more often.
Individuals who had not had melanoma but who learned that they carried the mutation stepped up their skin self-exam behavior: Only 30% were doing these exams at least monthly before testing, but 60% were doing so 2 years afterward.
By contrast, individuals who had not had melanoma and who learned that they did not carry the mutation had little change in their behavior, even though regular skin self-exams are also recommended for this group: 38% were doing these exams roughly once a month or more often before testing, and 44% were doing so at follow-up.
"Researchers and genetic counselors believe that learning one's objective risk will actually motivate behavior change," said lead investigator Jennifer M. Taber at the annual meeting of the Society of behavioral Medicine.
There are several concerns, however.
"One is that individuals who test negative will feel that their risk is so low that they don't need to engage in prevention or screening behaviors anymore, that they might feel a false sense of security and not change their behavior," she explained.
"Another concern is that for those who test positive, they will feel a sense of fatalism—that there is nothing they can do, their risk is so high anyway, so why bother engaging in the behaviors," she added.
Ms. Taber, a graduate student in psychology at the University of Utah in Salt Lake City, and her coinvestigators studied 37 adults from families with very high rates of melanoma. All underwent genetic testing for the p16 mutation, which sharply increases melanoma risk, and were followed for 2 years.
Nearly a third (30%) of participants were affected carriers, meaning they had a history of melanoma and had the mutation; 27% were unaffected carriers, meaning they did not have a history of the disease and did have the mutation; and 43% were noncarriers who did not have a history of the disease and did not have the mutation.
Monthly skin self-exams are recommended for all individuals from families with high rates of melanoma, regardless of their genetic test results, Ms. Taber noted, because even those with a negative result have a lifetime probability of the disease twice that of the general population.
The investigators classified the participants' skin self-exam behavior, according to the number of these exams performed in a 6-month period, as being on target (four to eight exams); overscreening (more than eight), which may actually hamper detection of changes; and underscreening (fewer than four), which may lead to missed lesions.
Two years after testing, the percentage of participants who were either on target or overscreening remained at the same high baseline level among affected carriers (73%) and had doubled among unaffected carriers (from 30% to 60%), but had increased only slightly among noncarriers (from 38% to 44%), Ms. Taber reported.
When the results were viewed another way, the percentage of participants who improved their skin self-exam practice during the 2-year period—to comply with the once-a-month recommendation—was 46% in the affected carrier group, 60% in the unaffected carrier group, and 25% in the noncarrier group.
Compared with participants who did not improve, those who did improve reported feeling that they had more control over detecting melanoma early (4.4 vs. 3.7 points on a 5-point scale).
In a subanalysis of the noncarriers, those who were underscreening at 2 years gave as their reason being busy or forgetful, feeling unqualified to perform the exams, and/or believing that their risk was not high enough.
In addition, noncarriers who improved their skin self-exam performance had a gain in their perceived control over early detection during follow-up, whereas those failing to improve did not.
The consistent finding of a link between an improvement in self-exam behavior and perceived control over detecting melanoma early has implications for strategies to increase this behavior, Ms. Taber said.
Perhaps physicians should target control perceptions over detecting a melanoma or perhaps skin self-examination competence, she commented.
Similarly, Ms. Taber noted, the barriers cited by noncarriers who were underscreening provide valuable insight specifically for individuals having negative genetic test results.
Perhaps counseling sessions should "target perceived importance of skin self-exams to make sure that individuals realize that their risk is high enough that they should be performing these behaviors, and perhaps do something like reminder or booster sessions for these individuals," she said.
Ms. Taber reported having no relevant conflicts of interest.
Electrochemotherapy Provides Cost-Effective Palliation in Advanced Melanoma
MADRID — Electrochemotherapy is gaining traction in Europe as palliative therapy for cutaneous metastases in patients with stage III melanoma.
Electrochemotherapy was the subject of numerous presentations at the World Congress on Cancers of the Skin sponsored by the Skin Cancer Foundation, including a plenary overview by Dr. Josep Malvehy of the University of Barcelona.
“Electrochemotherapy is simple, highly effective, safe, and cost effective since it can be done on an outpatient basis. No specific skill is required. A 1-day training at an experienced center is sufficient to let a physician feel confident with the treatment,” he said.
The therapy is based upon the phenomenon of electroporation, in which brief, intense electric pulses are applied to a treatment area to temporarily increase the permeability of cell membranes. This allows entry of previously administered low-dose chemotherapy drugs into the tumor cells. Roughly 20 minutes after application of the electric pulses the channels reseal, trapping the drug within the cells. Intralesional or intravenous bleomycin or intralesional cisplatin are typically used, he explained.
An Italian company, IGEA, has received European marketing approval for its Cliniporator electrochemotherapy system and is now seeking Food and Drug Administration clearance.
In a pivotal European clinical trial—the European Standard Operating Procedures of Electrochemotherapy (ESOPE) study—41 patients were treated for 171 symptomatic cutaneous metastases of melanoma, sarcoma, or carcinoma. There was a 74% complete response and a 10% partial response rate with no significant toxicity (Eur. J. Cancer Supplements 2006;4:3-13).
Those are stellar results for palliation in difficult cases, and they have subsequently been replicated in other studies and born out in clinical experience, Dr. Malvehy said.
He turns to electrochemotherapy for palliation in melanoma patients with inoperable skin or subcutaneous metastases, for metastatic lesions that have progressed despite conventional high-dose chemotherapy, and in patients with truncal lesions or who are otherwise unsuitable for isolated limb perfusion.
The therapy’s major limitation is that efficacy drops off substantially for tumors larger than 3 cm.
Dr. Malvehy noted that, in a cost-effectiveness analysis funded by IGEA, investigators utilizing Italian national health care system data determined that a course of electrochemotherapy for control of cutaneous and subcutaneous tumors averaged 1,408 euro, compared with 1,124 euro for radiotherapy, 14,052 euro for interferon-alpha, 18,530 euro for isolated limb perfusion, and 2,089 euro for hyperthermia in combination with chemotherapy and radiotherapy (Ther. Clin. Risk Manag. 2008;4:541-8).
“In our clinic we’re now using electrochemotherapy to take on difficult head and neck cancers that are inoperable and have no response to chemotherapy or radiotherapy. Our preliminary results are really very good,” he said.<[qm]>
Dr. Malvehy reported no financial conflicts.
MADRID — Electrochemotherapy is gaining traction in Europe as palliative therapy for cutaneous metastases in patients with stage III melanoma.
Electrochemotherapy was the subject of numerous presentations at the World Congress on Cancers of the Skin sponsored by the Skin Cancer Foundation, including a plenary overview by Dr. Josep Malvehy of the University of Barcelona.
“Electrochemotherapy is simple, highly effective, safe, and cost effective since it can be done on an outpatient basis. No specific skill is required. A 1-day training at an experienced center is sufficient to let a physician feel confident with the treatment,” he said.
The therapy is based upon the phenomenon of electroporation, in which brief, intense electric pulses are applied to a treatment area to temporarily increase the permeability of cell membranes. This allows entry of previously administered low-dose chemotherapy drugs into the tumor cells. Roughly 20 minutes after application of the electric pulses the channels reseal, trapping the drug within the cells. Intralesional or intravenous bleomycin or intralesional cisplatin are typically used, he explained.
An Italian company, IGEA, has received European marketing approval for its Cliniporator electrochemotherapy system and is now seeking Food and Drug Administration clearance.
In a pivotal European clinical trial—the European Standard Operating Procedures of Electrochemotherapy (ESOPE) study—41 patients were treated for 171 symptomatic cutaneous metastases of melanoma, sarcoma, or carcinoma. There was a 74% complete response and a 10% partial response rate with no significant toxicity (Eur. J. Cancer Supplements 2006;4:3-13).
Those are stellar results for palliation in difficult cases, and they have subsequently been replicated in other studies and born out in clinical experience, Dr. Malvehy said.
He turns to electrochemotherapy for palliation in melanoma patients with inoperable skin or subcutaneous metastases, for metastatic lesions that have progressed despite conventional high-dose chemotherapy, and in patients with truncal lesions or who are otherwise unsuitable for isolated limb perfusion.
The therapy’s major limitation is that efficacy drops off substantially for tumors larger than 3 cm.
Dr. Malvehy noted that, in a cost-effectiveness analysis funded by IGEA, investigators utilizing Italian national health care system data determined that a course of electrochemotherapy for control of cutaneous and subcutaneous tumors averaged 1,408 euro, compared with 1,124 euro for radiotherapy, 14,052 euro for interferon-alpha, 18,530 euro for isolated limb perfusion, and 2,089 euro for hyperthermia in combination with chemotherapy and radiotherapy (Ther. Clin. Risk Manag. 2008;4:541-8).
“In our clinic we’re now using electrochemotherapy to take on difficult head and neck cancers that are inoperable and have no response to chemotherapy or radiotherapy. Our preliminary results are really very good,” he said.<[qm]>
Dr. Malvehy reported no financial conflicts.
MADRID — Electrochemotherapy is gaining traction in Europe as palliative therapy for cutaneous metastases in patients with stage III melanoma.
Electrochemotherapy was the subject of numerous presentations at the World Congress on Cancers of the Skin sponsored by the Skin Cancer Foundation, including a plenary overview by Dr. Josep Malvehy of the University of Barcelona.
“Electrochemotherapy is simple, highly effective, safe, and cost effective since it can be done on an outpatient basis. No specific skill is required. A 1-day training at an experienced center is sufficient to let a physician feel confident with the treatment,” he said.
The therapy is based upon the phenomenon of electroporation, in which brief, intense electric pulses are applied to a treatment area to temporarily increase the permeability of cell membranes. This allows entry of previously administered low-dose chemotherapy drugs into the tumor cells. Roughly 20 minutes after application of the electric pulses the channels reseal, trapping the drug within the cells. Intralesional or intravenous bleomycin or intralesional cisplatin are typically used, he explained.
An Italian company, IGEA, has received European marketing approval for its Cliniporator electrochemotherapy system and is now seeking Food and Drug Administration clearance.
In a pivotal European clinical trial—the European Standard Operating Procedures of Electrochemotherapy (ESOPE) study—41 patients were treated for 171 symptomatic cutaneous metastases of melanoma, sarcoma, or carcinoma. There was a 74% complete response and a 10% partial response rate with no significant toxicity (Eur. J. Cancer Supplements 2006;4:3-13).
Those are stellar results for palliation in difficult cases, and they have subsequently been replicated in other studies and born out in clinical experience, Dr. Malvehy said.
He turns to electrochemotherapy for palliation in melanoma patients with inoperable skin or subcutaneous metastases, for metastatic lesions that have progressed despite conventional high-dose chemotherapy, and in patients with truncal lesions or who are otherwise unsuitable for isolated limb perfusion.
The therapy’s major limitation is that efficacy drops off substantially for tumors larger than 3 cm.
Dr. Malvehy noted that, in a cost-effectiveness analysis funded by IGEA, investigators utilizing Italian national health care system data determined that a course of electrochemotherapy for control of cutaneous and subcutaneous tumors averaged 1,408 euro, compared with 1,124 euro for radiotherapy, 14,052 euro for interferon-alpha, 18,530 euro for isolated limb perfusion, and 2,089 euro for hyperthermia in combination with chemotherapy and radiotherapy (Ther. Clin. Risk Manag. 2008;4:541-8).
“In our clinic we’re now using electrochemotherapy to take on difficult head and neck cancers that are inoperable and have no response to chemotherapy or radiotherapy. Our preliminary results are really very good,” he said.<[qm]>
Dr. Malvehy reported no financial conflicts.
Large Congenital Melanocytic Nevi: Associated Risks and Management Considerations
Jordan B. Slutsky, MD, Jeffrey M. Barr, MD, Alisa N. Femia, MD, and Ashfaq A. Marghoob, MD
Large congenital melanocytic nevi (LCMN) in neonates can cause considerable concern for parents, family members, and physicians. A detailed understanding of the medical risks, including cutaneous melanoma (CM), extracutaneous melanoma (ECM), and neurocutaneous melanocytosis (NCM), as well as the psychological stress that these lesions can cause in patients, will guide informed management decisions as well as provide comfort to parents. Current data indicate that LCMN greater than 20 cm, and more likely greater than 40 to 60 cm, are the lesions at greatest risk for complications such as CM, ECM, and NCM. Additionally, lesions on the trunk are at greater risk for developing CM, and LCMN in association with numerous satellite nevi are at greatest risk for NCM. Individualized management plans, including clinical observation, magnetic resonance imaging (MRI), and possibly surgery should be based on the risk versus benefit ratio, taking into account the size of the LCMN, its location, the number of satellite nevi, symptoms, and numerous other factors which will be reviewed. This paper will provide a detailed analysis of the risks associated with LCMN, as well as a discussion regarding management and treatment options.
*For a PDF of the full article, click on the link to the left of this introduction.
Jordan B. Slutsky, MD, Jeffrey M. Barr, MD, Alisa N. Femia, MD, and Ashfaq A. Marghoob, MD
Large congenital melanocytic nevi (LCMN) in neonates can cause considerable concern for parents, family members, and physicians. A detailed understanding of the medical risks, including cutaneous melanoma (CM), extracutaneous melanoma (ECM), and neurocutaneous melanocytosis (NCM), as well as the psychological stress that these lesions can cause in patients, will guide informed management decisions as well as provide comfort to parents. Current data indicate that LCMN greater than 20 cm, and more likely greater than 40 to 60 cm, are the lesions at greatest risk for complications such as CM, ECM, and NCM. Additionally, lesions on the trunk are at greater risk for developing CM, and LCMN in association with numerous satellite nevi are at greatest risk for NCM. Individualized management plans, including clinical observation, magnetic resonance imaging (MRI), and possibly surgery should be based on the risk versus benefit ratio, taking into account the size of the LCMN, its location, the number of satellite nevi, symptoms, and numerous other factors which will be reviewed. This paper will provide a detailed analysis of the risks associated with LCMN, as well as a discussion regarding management and treatment options.
*For a PDF of the full article, click on the link to the left of this introduction.
Jordan B. Slutsky, MD, Jeffrey M. Barr, MD, Alisa N. Femia, MD, and Ashfaq A. Marghoob, MD
Large congenital melanocytic nevi (LCMN) in neonates can cause considerable concern for parents, family members, and physicians. A detailed understanding of the medical risks, including cutaneous melanoma (CM), extracutaneous melanoma (ECM), and neurocutaneous melanocytosis (NCM), as well as the psychological stress that these lesions can cause in patients, will guide informed management decisions as well as provide comfort to parents. Current data indicate that LCMN greater than 20 cm, and more likely greater than 40 to 60 cm, are the lesions at greatest risk for complications such as CM, ECM, and NCM. Additionally, lesions on the trunk are at greater risk for developing CM, and LCMN in association with numerous satellite nevi are at greatest risk for NCM. Individualized management plans, including clinical observation, magnetic resonance imaging (MRI), and possibly surgery should be based on the risk versus benefit ratio, taking into account the size of the LCMN, its location, the number of satellite nevi, symptoms, and numerous other factors which will be reviewed. This paper will provide a detailed analysis of the risks associated with LCMN, as well as a discussion regarding management and treatment options.
*For a PDF of the full article, click on the link to the left of this introduction.