User login
Role of Confocal Microscopy in Lesion Management
In an interview, Dr. Lawrence T. Wang discusses the role of confocal microscopy for the management of pigmented lesions, including melanoma.
In an interview, Dr. Lawrence T. Wang discusses the role of confocal microscopy for the management of pigmented lesions, including melanoma.
In an interview, Dr. Lawrence T. Wang discusses the role of confocal microscopy for the management of pigmented lesions, including melanoma.
AAD: Skin Toxicities From Anticancer Treatment Prove Costly
MIAMI - The cost of treating dermatologic toxicities associated with targeted anticancer therapies contributes significantly to the overall financial burden of cancer care, cost analysis data from 131 affected patients suggest.
The overall cost of caring for treatment-related dermatologic toxicities in the 30-month study was more than $337,409, or $2,576 per patient, Dr. Judy H. Borovicka reported at the annual meeting of the American Academy of Dermatology.
The analysis included the cost of clinic visits, laboratory tests, diagnostic and therapeutic procedures, medications, and supplies, said Dr. Borovicka, a clinical trial investigator at Northwestern University, Chicago.
Patients had a total of 481 visits (for an average of 3.7 per person) to a subspecialty clinic developed to treat skin and eye reactions to inhibitors of epidermal growth-factor receptor and kinases (the SERIES Clinic in Chicago) between November 2005 and April 2008, for a mean cost per visit of $701. All were diagnosed with 1 of 15 primary cancer types, and were treated with rituximab, erlotinib, imatinib, lapatinib, panitumumab, sorafenib, or sunitinib.
The more common dermatologic toxicities associated with these drugs included papulopustular rash, periungual inflammation, xerosis, alopecia, and ocular changes. Papulopustular rash was the most common toxicity, occurring in 45%-100% of patients.
Sorafenib induced the most costly dermatologic toxicities, with a mean overall patient cost of $2,974; lapatinib and imatinib were associated with the least costly toxicities, with mean overall costs of $1,275 and $1,490, respectively, Dr. Borovicka said, noting that the differences in cost between sorafenib and lapatinib/imatinib were statistically significant.
Toxicity severity was predictive of increased patient cost; however, age, gender, race/ethnicity, cancer type, and cancer severity were not predictors of patient cost, Dr. Borovicka said.
"Toxicities impact the quality of life of patients undergoing treatment with targeted anticancer therapies, and they carry significant financial implications... management of untoward effects is paramount to minimizing the disruption of therapy," she concluded.
Dr. Borovicka stated that she has no relevant financial or other disclosures related to this study.
MIAMI - The cost of treating dermatologic toxicities associated with targeted anticancer therapies contributes significantly to the overall financial burden of cancer care, cost analysis data from 131 affected patients suggest.
The overall cost of caring for treatment-related dermatologic toxicities in the 30-month study was more than $337,409, or $2,576 per patient, Dr. Judy H. Borovicka reported at the annual meeting of the American Academy of Dermatology.
The analysis included the cost of clinic visits, laboratory tests, diagnostic and therapeutic procedures, medications, and supplies, said Dr. Borovicka, a clinical trial investigator at Northwestern University, Chicago.
Patients had a total of 481 visits (for an average of 3.7 per person) to a subspecialty clinic developed to treat skin and eye reactions to inhibitors of epidermal growth-factor receptor and kinases (the SERIES Clinic in Chicago) between November 2005 and April 2008, for a mean cost per visit of $701. All were diagnosed with 1 of 15 primary cancer types, and were treated with rituximab, erlotinib, imatinib, lapatinib, panitumumab, sorafenib, or sunitinib.
The more common dermatologic toxicities associated with these drugs included papulopustular rash, periungual inflammation, xerosis, alopecia, and ocular changes. Papulopustular rash was the most common toxicity, occurring in 45%-100% of patients.
Sorafenib induced the most costly dermatologic toxicities, with a mean overall patient cost of $2,974; lapatinib and imatinib were associated with the least costly toxicities, with mean overall costs of $1,275 and $1,490, respectively, Dr. Borovicka said, noting that the differences in cost between sorafenib and lapatinib/imatinib were statistically significant.
Toxicity severity was predictive of increased patient cost; however, age, gender, race/ethnicity, cancer type, and cancer severity were not predictors of patient cost, Dr. Borovicka said.
"Toxicities impact the quality of life of patients undergoing treatment with targeted anticancer therapies, and they carry significant financial implications... management of untoward effects is paramount to minimizing the disruption of therapy," she concluded.
Dr. Borovicka stated that she has no relevant financial or other disclosures related to this study.
MIAMI - The cost of treating dermatologic toxicities associated with targeted anticancer therapies contributes significantly to the overall financial burden of cancer care, cost analysis data from 131 affected patients suggest.
The overall cost of caring for treatment-related dermatologic toxicities in the 30-month study was more than $337,409, or $2,576 per patient, Dr. Judy H. Borovicka reported at the annual meeting of the American Academy of Dermatology.
The analysis included the cost of clinic visits, laboratory tests, diagnostic and therapeutic procedures, medications, and supplies, said Dr. Borovicka, a clinical trial investigator at Northwestern University, Chicago.
Patients had a total of 481 visits (for an average of 3.7 per person) to a subspecialty clinic developed to treat skin and eye reactions to inhibitors of epidermal growth-factor receptor and kinases (the SERIES Clinic in Chicago) between November 2005 and April 2008, for a mean cost per visit of $701. All were diagnosed with 1 of 15 primary cancer types, and were treated with rituximab, erlotinib, imatinib, lapatinib, panitumumab, sorafenib, or sunitinib.
The more common dermatologic toxicities associated with these drugs included papulopustular rash, periungual inflammation, xerosis, alopecia, and ocular changes. Papulopustular rash was the most common toxicity, occurring in 45%-100% of patients.
Sorafenib induced the most costly dermatologic toxicities, with a mean overall patient cost of $2,974; lapatinib and imatinib were associated with the least costly toxicities, with mean overall costs of $1,275 and $1,490, respectively, Dr. Borovicka said, noting that the differences in cost between sorafenib and lapatinib/imatinib were statistically significant.
Toxicity severity was predictive of increased patient cost; however, age, gender, race/ethnicity, cancer type, and cancer severity were not predictors of patient cost, Dr. Borovicka said.
"Toxicities impact the quality of life of patients undergoing treatment with targeted anticancer therapies, and they carry significant financial implications... management of untoward effects is paramount to minimizing the disruption of therapy," she concluded.
Dr. Borovicka stated that she has no relevant financial or other disclosures related to this study.
ACGME Releases Revised Requirements for Procedural Derm Fellows
Procedural dermatology fellows will soon have new requirements, including specifics about how many surgical cases they must perform.
The requirement is one of the changes put forward by the Accreditation Council for Graduate Medical Education as part of a regular review of the graduate medical education program requirements for procedural dermatology. This is the first revision to the program requirements since the subspecialty was first accredited in 2003.
The new requirements are scheduled to go into effect in July, but there may be some additional revisions before then as the group receives last-minute comments on the document, according to ACGME. The current requirements and revisions are posted online at www.acgme.org/acWebsite/RRC_080/080_prIndex.asp.
Under the revised document, ACGME is keeping the requirement that fellowship programs provide a sufficient volume and variety of surgical cases for fellows, specifically that at least 1,000 dermatologic surgical procedures per fellow are available and that at least 500 of that total must be Mohs micrographic surgery procedures. ACGME, however, is now also specifying case requirements for individual fellows. Fellows must perform, not simply observe, at least 400 surgical cases, of which at least 200 have to be Mohs surgery procedures.
ACGME is also adding requirements that frozen section slides for Mohs surgery must be reviewed and approved as part of an ongoing quality assurance program by an appropriate peer-reviewed organization. Facilities also will be required to have their frozen section laboratory and examination areas accredited by appropriate oversight bodies.
The biggest change in the document is the way it is organized, but much of the content is the same, said Dr. Randall K. Roenigk, professor of dermatology at the Mayo Clinic in Rochester, Minn., and chair of ACGME's Residency Review Committee for Dermatology. For example, the three major components of procedural dermatology fellowship training--Mohs micrographic surgery, reconstruction, and cosmetic surgery--remain the same, he said.
The regular revisions to the program requirements by ACGME are really a way to assure the public. "It's really an attempt to demonstrate to the public that we're doing our best to train the best doctors," Dr. Roenigk said.
But Dr. Lee S. Portnoff, a full-time Mohs surgeon in St. Louis and president of the American Society for Mohs Surgery, said that he is concerned about some of the changes ACGME has made.
The requirement to perform at least 400 surgical cases, of which at least 200 are Mohs procedures, would allow some fellows to complete the requirements without getting sufficient experience in non-Mohs surgeries, he said. If fellows are allowed to count Mohs-related repairs toward their overall surgical requirement, they could easily meet the requirements without doing much in the way of other types of procedures.
"This may be just a Mohs fellowship by another name," Dr. Portnoff said.
Procedural dermatology fellows will soon have new requirements, including specifics about how many surgical cases they must perform.
The requirement is one of the changes put forward by the Accreditation Council for Graduate Medical Education as part of a regular review of the graduate medical education program requirements for procedural dermatology. This is the first revision to the program requirements since the subspecialty was first accredited in 2003.
The new requirements are scheduled to go into effect in July, but there may be some additional revisions before then as the group receives last-minute comments on the document, according to ACGME. The current requirements and revisions are posted online at www.acgme.org/acWebsite/RRC_080/080_prIndex.asp.
Under the revised document, ACGME is keeping the requirement that fellowship programs provide a sufficient volume and variety of surgical cases for fellows, specifically that at least 1,000 dermatologic surgical procedures per fellow are available and that at least 500 of that total must be Mohs micrographic surgery procedures. ACGME, however, is now also specifying case requirements for individual fellows. Fellows must perform, not simply observe, at least 400 surgical cases, of which at least 200 have to be Mohs surgery procedures.
ACGME is also adding requirements that frozen section slides for Mohs surgery must be reviewed and approved as part of an ongoing quality assurance program by an appropriate peer-reviewed organization. Facilities also will be required to have their frozen section laboratory and examination areas accredited by appropriate oversight bodies.
The biggest change in the document is the way it is organized, but much of the content is the same, said Dr. Randall K. Roenigk, professor of dermatology at the Mayo Clinic in Rochester, Minn., and chair of ACGME's Residency Review Committee for Dermatology. For example, the three major components of procedural dermatology fellowship training--Mohs micrographic surgery, reconstruction, and cosmetic surgery--remain the same, he said.
The regular revisions to the program requirements by ACGME are really a way to assure the public. "It's really an attempt to demonstrate to the public that we're doing our best to train the best doctors," Dr. Roenigk said.
But Dr. Lee S. Portnoff, a full-time Mohs surgeon in St. Louis and president of the American Society for Mohs Surgery, said that he is concerned about some of the changes ACGME has made.
The requirement to perform at least 400 surgical cases, of which at least 200 are Mohs procedures, would allow some fellows to complete the requirements without getting sufficient experience in non-Mohs surgeries, he said. If fellows are allowed to count Mohs-related repairs toward their overall surgical requirement, they could easily meet the requirements without doing much in the way of other types of procedures.
"This may be just a Mohs fellowship by another name," Dr. Portnoff said.
Procedural dermatology fellows will soon have new requirements, including specifics about how many surgical cases they must perform.
The requirement is one of the changes put forward by the Accreditation Council for Graduate Medical Education as part of a regular review of the graduate medical education program requirements for procedural dermatology. This is the first revision to the program requirements since the subspecialty was first accredited in 2003.
The new requirements are scheduled to go into effect in July, but there may be some additional revisions before then as the group receives last-minute comments on the document, according to ACGME. The current requirements and revisions are posted online at www.acgme.org/acWebsite/RRC_080/080_prIndex.asp.
Under the revised document, ACGME is keeping the requirement that fellowship programs provide a sufficient volume and variety of surgical cases for fellows, specifically that at least 1,000 dermatologic surgical procedures per fellow are available and that at least 500 of that total must be Mohs micrographic surgery procedures. ACGME, however, is now also specifying case requirements for individual fellows. Fellows must perform, not simply observe, at least 400 surgical cases, of which at least 200 have to be Mohs surgery procedures.
ACGME is also adding requirements that frozen section slides for Mohs surgery must be reviewed and approved as part of an ongoing quality assurance program by an appropriate peer-reviewed organization. Facilities also will be required to have their frozen section laboratory and examination areas accredited by appropriate oversight bodies.
The biggest change in the document is the way it is organized, but much of the content is the same, said Dr. Randall K. Roenigk, professor of dermatology at the Mayo Clinic in Rochester, Minn., and chair of ACGME's Residency Review Committee for Dermatology. For example, the three major components of procedural dermatology fellowship training--Mohs micrographic surgery, reconstruction, and cosmetic surgery--remain the same, he said.
The regular revisions to the program requirements by ACGME are really a way to assure the public. "It's really an attempt to demonstrate to the public that we're doing our best to train the best doctors," Dr. Roenigk said.
But Dr. Lee S. Portnoff, a full-time Mohs surgeon in St. Louis and president of the American Society for Mohs Surgery, said that he is concerned about some of the changes ACGME has made.
The requirement to perform at least 400 surgical cases, of which at least 200 are Mohs procedures, would allow some fellows to complete the requirements without getting sufficient experience in non-Mohs surgeries, he said. If fellows are allowed to count Mohs-related repairs toward their overall surgical requirement, they could easily meet the requirements without doing much in the way of other types of procedures.
"This may be just a Mohs fellowship by another name," Dr. Portnoff said.
New Model Dramatically Revises Skin Cancer Prevalence Estimates
Twelve to 15 million white patients living in the United States have had at least one nonmelanoma skin cancer in their lifetimes, according to estimates based on a new mathematical model reported in the March issue of the Archives of Dermatology.
This figure is approximately twice that of previous estimates based on patient surveys, such as the estimate calculated in the U.S. National Health Interview Study, according to Dr. Robert S. Stern of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
This difference can be attributed in part to people often incorrectly reporting their skin cancer histories when surveyed, falsely believing that basal cell and squamous cell lesions are not cancerous or that all skin cancers can be considered melanoma.
Dr. Stern devised his mathematical model using the same basic data available from national samples, such as the Surveillance, Epidemiology, and End Results (SEER) studies and information from the National Cancer Institute. His model, however, took into consideration several factors that had not been accounted for in previous estimates, such as the likelihood that patients develop numerous nonmelanoma skin cancers over the course of several years and that “a substantial proportion” of patients with melanoma also have nonmalignant skin cancers.
According to his model, “about 13 million white non-Hispanic U.S. residents (6%) have had more than 22 million nonmelanoma skin cancers” (Arch. Dermatol. 2010;146:279-82).
This puts the prevalence of a skin cancer history at a level far higher than that of any other cancer – prevalence that “exceeds that of all other cancers diagnosed since 1975,” he added. “Recent population-based data concerning skin cancer incidence, morbidity, and cost of care are lacking for the most common types of skin cancer, [basal cell carcinoma] and [squamous cell carcinoma].”
Regarding patients’ inaccurate reporting of skin cancer histories in surveys and interviews, Dr. Stern noted that many patients equate skin cancer with melanoma. “Hundreds of thousands of patients may be unnecessarily burdened with the belief that they have had a potentially lethal cancer (melanoma) when in fact they have had a skin tumor that is very unlikely to be lethal,he wrote.
In contrast, patients with breast or prostate cancer are much more likely to accurately report their cancer histories, so incidence and prevalence estimates for these tumors are much more accurate than those for skin cancer, Dr. Stern noted.
This study was funded in part by the National Institutes of Health. Dr. Stern reported being a consultant for Johnson & Johnson, Vertex, and Takeda and an expert witness for Alphapharm, Mutual Pharm, and Johnson & Johnson.
Twelve to 15 million white patients living in the United States have had at least one nonmelanoma skin cancer in their lifetimes, according to estimates based on a new mathematical model reported in the March issue of the Archives of Dermatology.
This figure is approximately twice that of previous estimates based on patient surveys, such as the estimate calculated in the U.S. National Health Interview Study, according to Dr. Robert S. Stern of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
This difference can be attributed in part to people often incorrectly reporting their skin cancer histories when surveyed, falsely believing that basal cell and squamous cell lesions are not cancerous or that all skin cancers can be considered melanoma.
Dr. Stern devised his mathematical model using the same basic data available from national samples, such as the Surveillance, Epidemiology, and End Results (SEER) studies and information from the National Cancer Institute. His model, however, took into consideration several factors that had not been accounted for in previous estimates, such as the likelihood that patients develop numerous nonmelanoma skin cancers over the course of several years and that “a substantial proportion” of patients with melanoma also have nonmalignant skin cancers.
According to his model, “about 13 million white non-Hispanic U.S. residents (6%) have had more than 22 million nonmelanoma skin cancers” (Arch. Dermatol. 2010;146:279-82).
This puts the prevalence of a skin cancer history at a level far higher than that of any other cancer – prevalence that “exceeds that of all other cancers diagnosed since 1975,” he added. “Recent population-based data concerning skin cancer incidence, morbidity, and cost of care are lacking for the most common types of skin cancer, [basal cell carcinoma] and [squamous cell carcinoma].”
Regarding patients’ inaccurate reporting of skin cancer histories in surveys and interviews, Dr. Stern noted that many patients equate skin cancer with melanoma. “Hundreds of thousands of patients may be unnecessarily burdened with the belief that they have had a potentially lethal cancer (melanoma) when in fact they have had a skin tumor that is very unlikely to be lethal,he wrote.
In contrast, patients with breast or prostate cancer are much more likely to accurately report their cancer histories, so incidence and prevalence estimates for these tumors are much more accurate than those for skin cancer, Dr. Stern noted.
This study was funded in part by the National Institutes of Health. Dr. Stern reported being a consultant for Johnson & Johnson, Vertex, and Takeda and an expert witness for Alphapharm, Mutual Pharm, and Johnson & Johnson.
Twelve to 15 million white patients living in the United States have had at least one nonmelanoma skin cancer in their lifetimes, according to estimates based on a new mathematical model reported in the March issue of the Archives of Dermatology.
This figure is approximately twice that of previous estimates based on patient surveys, such as the estimate calculated in the U.S. National Health Interview Study, according to Dr. Robert S. Stern of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
This difference can be attributed in part to people often incorrectly reporting their skin cancer histories when surveyed, falsely believing that basal cell and squamous cell lesions are not cancerous or that all skin cancers can be considered melanoma.
Dr. Stern devised his mathematical model using the same basic data available from national samples, such as the Surveillance, Epidemiology, and End Results (SEER) studies and information from the National Cancer Institute. His model, however, took into consideration several factors that had not been accounted for in previous estimates, such as the likelihood that patients develop numerous nonmelanoma skin cancers over the course of several years and that “a substantial proportion” of patients with melanoma also have nonmalignant skin cancers.
According to his model, “about 13 million white non-Hispanic U.S. residents (6%) have had more than 22 million nonmelanoma skin cancers” (Arch. Dermatol. 2010;146:279-82).
This puts the prevalence of a skin cancer history at a level far higher than that of any other cancer – prevalence that “exceeds that of all other cancers diagnosed since 1975,” he added. “Recent population-based data concerning skin cancer incidence, morbidity, and cost of care are lacking for the most common types of skin cancer, [basal cell carcinoma] and [squamous cell carcinoma].”
Regarding patients’ inaccurate reporting of skin cancer histories in surveys and interviews, Dr. Stern noted that many patients equate skin cancer with melanoma. “Hundreds of thousands of patients may be unnecessarily burdened with the belief that they have had a potentially lethal cancer (melanoma) when in fact they have had a skin tumor that is very unlikely to be lethal,he wrote.
In contrast, patients with breast or prostate cancer are much more likely to accurately report their cancer histories, so incidence and prevalence estimates for these tumors are much more accurate than those for skin cancer, Dr. Stern noted.
This study was funded in part by the National Institutes of Health. Dr. Stern reported being a consultant for Johnson & Johnson, Vertex, and Takeda and an expert witness for Alphapharm, Mutual Pharm, and Johnson & Johnson.
Prevention, Detection of Early Melanoma Urged to Curb Expenses
Treatment for melanoma costs the U.S. Medicare program about $249 million annually, but effective prevention and early detection could reduce expenses by up to 60%, according to a new study reported in the March issue of the Archives of Dermatology.
Treatment expenses for each patient who died from melanoma totaled more than $28,000 on average from the time of diagnosis until death, according to Dr. Anne M. Seidler and her colleagues. Policymakers should consider crafting guidelines for melanoma screening that reflect increased risks for patients older than age 65 years.
Although relatively few elderly patients die of melanoma, per-patient expenses are particularly high in cases of advanced disease, noted Dr. Seidler of the department of dermatology at Emory University in Atlanta and her associates.
“The majority of consumption is attributable to advanced-stage disease and the terminal phase of treatment,” they wrote. “If all patients were diagnosed and effectively treated in stage 0 or I, we estimate that the annual direct costs for the population 65 years or older would be between $99 million and $161 million, or 40% to 65% of their current value of $249 million.”
The researchers used Surveillance, Epidemiology, and End Results (SEER) data from 1,858 Medicare beneficiaries with a confirmed melanoma diagnosis and calculated cost by stage and treatment phase (Arch. Dermatol. 2010;146:249-56).
Average monthly per-patient melanoma charges were $2,194 during the initial 4 months of treatment. After this initial treatment phase, monthly costs fell to $902, but then increased to $3,933 if the cancer spread and became terminal, according to the investigators. Total costs may be higher than found based on how much patients spent on copayments and deductibles.
A total of 263 patients died of melanoma during the 6 years studied. These patients lived an average of 26 months after diagnosis, and their care cost an average of $13,020 per year, the study reported.
Early-stage melanoma costs appeared similar to those of prostate cancer, while late-stage melanoma costs resembled those of colon cancer, which generally is more expensive to treat. “The lack of definitive, effective therapy for melanoma, which may result in utilization of multiple chemotherapeutic agents in these later stages, likely drives up the costs,” the investigators noted.
The authors reported no financial conflicts of interest.
Treatment for melanoma costs the U.S. Medicare program about $249 million annually, but effective prevention and early detection could reduce expenses by up to 60%, according to a new study reported in the March issue of the Archives of Dermatology.
Treatment expenses for each patient who died from melanoma totaled more than $28,000 on average from the time of diagnosis until death, according to Dr. Anne M. Seidler and her colleagues. Policymakers should consider crafting guidelines for melanoma screening that reflect increased risks for patients older than age 65 years.
Although relatively few elderly patients die of melanoma, per-patient expenses are particularly high in cases of advanced disease, noted Dr. Seidler of the department of dermatology at Emory University in Atlanta and her associates.
“The majority of consumption is attributable to advanced-stage disease and the terminal phase of treatment,” they wrote. “If all patients were diagnosed and effectively treated in stage 0 or I, we estimate that the annual direct costs for the population 65 years or older would be between $99 million and $161 million, or 40% to 65% of their current value of $249 million.”
The researchers used Surveillance, Epidemiology, and End Results (SEER) data from 1,858 Medicare beneficiaries with a confirmed melanoma diagnosis and calculated cost by stage and treatment phase (Arch. Dermatol. 2010;146:249-56).
Average monthly per-patient melanoma charges were $2,194 during the initial 4 months of treatment. After this initial treatment phase, monthly costs fell to $902, but then increased to $3,933 if the cancer spread and became terminal, according to the investigators. Total costs may be higher than found based on how much patients spent on copayments and deductibles.
A total of 263 patients died of melanoma during the 6 years studied. These patients lived an average of 26 months after diagnosis, and their care cost an average of $13,020 per year, the study reported.
Early-stage melanoma costs appeared similar to those of prostate cancer, while late-stage melanoma costs resembled those of colon cancer, which generally is more expensive to treat. “The lack of definitive, effective therapy for melanoma, which may result in utilization of multiple chemotherapeutic agents in these later stages, likely drives up the costs,” the investigators noted.
The authors reported no financial conflicts of interest.
Treatment for melanoma costs the U.S. Medicare program about $249 million annually, but effective prevention and early detection could reduce expenses by up to 60%, according to a new study reported in the March issue of the Archives of Dermatology.
Treatment expenses for each patient who died from melanoma totaled more than $28,000 on average from the time of diagnosis until death, according to Dr. Anne M. Seidler and her colleagues. Policymakers should consider crafting guidelines for melanoma screening that reflect increased risks for patients older than age 65 years.
Although relatively few elderly patients die of melanoma, per-patient expenses are particularly high in cases of advanced disease, noted Dr. Seidler of the department of dermatology at Emory University in Atlanta and her associates.
“The majority of consumption is attributable to advanced-stage disease and the terminal phase of treatment,” they wrote. “If all patients were diagnosed and effectively treated in stage 0 or I, we estimate that the annual direct costs for the population 65 years or older would be between $99 million and $161 million, or 40% to 65% of their current value of $249 million.”
The researchers used Surveillance, Epidemiology, and End Results (SEER) data from 1,858 Medicare beneficiaries with a confirmed melanoma diagnosis and calculated cost by stage and treatment phase (Arch. Dermatol. 2010;146:249-56).
Average monthly per-patient melanoma charges were $2,194 during the initial 4 months of treatment. After this initial treatment phase, monthly costs fell to $902, but then increased to $3,933 if the cancer spread and became terminal, according to the investigators. Total costs may be higher than found based on how much patients spent on copayments and deductibles.
A total of 263 patients died of melanoma during the 6 years studied. These patients lived an average of 26 months after diagnosis, and their care cost an average of $13,020 per year, the study reported.
Early-stage melanoma costs appeared similar to those of prostate cancer, while late-stage melanoma costs resembled those of colon cancer, which generally is more expensive to treat. “The lack of definitive, effective therapy for melanoma, which may result in utilization of multiple chemotherapeutic agents in these later stages, likely drives up the costs,” the investigators noted.
The authors reported no financial conflicts of interest.
ACGME Approves Revised Procedural Dermatology Program Requirements
The American College of Graduate Medical Education recently approved revised program requirements for procedural dermatology, they announced in a March 1 e-communication. The revised requirements are available at http://www.acgme.org/acWebsite/RRC_080/080_prIndex.asp.
Story to follow.
The American College of Graduate Medical Education recently approved revised program requirements for procedural dermatology, they announced in a March 1 e-communication. The revised requirements are available at http://www.acgme.org/acWebsite/RRC_080/080_prIndex.asp.
Story to follow.
The American College of Graduate Medical Education recently approved revised program requirements for procedural dermatology, they announced in a March 1 e-communication. The revised requirements are available at http://www.acgme.org/acWebsite/RRC_080/080_prIndex.asp.
Story to follow.
Calm Parental Fears About Sunscreens for Kids
"The benefits of sun protection clearly outweigh the risks," said Dr. Sheila Fallon Friedlander, but the debate continues over how to balance the benefits of outdoor activities for children with protection against skin cancer.
Some studies suggest outdoor activity is protective against melanoma, Dr. Friedlander said She cited a case-control study of 583 cases of cutaneous malignant melanoma and 608 controls (Int. J. Epidemiol. 1999;28:418-27). Intermittent sun exposure, such as beach vacations during adolescence and the use of tanning beds and sunlamps, was associated with a significantly greater risk for melanoma, whereas chronic exposure, indicated by days of outdoor activity during adolescence and by outdoor jobs in adulthood, was associated with a significantly reduced risk for melanoma.
But sun protection and patient education does appear to play a role in reducing the number of nevi in children. Dr. Friedlander, clinical professor of pediatrics and medicine at the University of California, San Diego, cited another study in which 458 children in first to fourth grade in Canada were randomized to receive sunscreen and counseling about sun protection. Three years later, the sunscreen group had significantly fewer new nevi compared with the control group (JAMA 2000;283:2955-60).
What's a dermatologist to do? "Common sense prevails," Dr. Friedlander said. She advised dermatologists to counsel children and their parents to protect against sunburns by using sunscreens and sun-protective clothing. Also, "identify high-risk patients and follow them" so that any problems can be spotted early, she said.
But once parents and children are on board with sun protection, what should they use? The Environmental Working Group (EWG), a nonprofit organization that reviews and disseminates information about contaminants in consumer products and the environment, has come down in favor of physical sunscreens, based on a review of 400 studies and 2,000 sunscreens, Dr. Friedlander said.
Some parents and children prefer organic sunscreens, which contain oxybenzones, but the EWG rates these products as more dangerous than physical sunscreens. Some research suggests that oxybenzones can be absorbed into the skin in a way that the nano-particles of zinc oxide and titanium dioxide in physical sunscreens cannot, Dr. Friedlander said.
Be aware of what sunscreen characteristics raise concerns in your patients and their parents, Dr. Friedlander said. Share information with them, refer them to the EWG Web (www.ewg.org) and come up with a reasonable plan for sun protection.
Dr. Friedlander has served as a clinical investigator for Johnson & Johnson, which manufactures sunscreen products. SDEF and this news organization are owned by Elsevier.
"The benefits of sun protection clearly outweigh the risks," said Dr. Sheila Fallon Friedlander, but the debate continues over how to balance the benefits of outdoor activities for children with protection against skin cancer.
Some studies suggest outdoor activity is protective against melanoma, Dr. Friedlander said She cited a case-control study of 583 cases of cutaneous malignant melanoma and 608 controls (Int. J. Epidemiol. 1999;28:418-27). Intermittent sun exposure, such as beach vacations during adolescence and the use of tanning beds and sunlamps, was associated with a significantly greater risk for melanoma, whereas chronic exposure, indicated by days of outdoor activity during adolescence and by outdoor jobs in adulthood, was associated with a significantly reduced risk for melanoma.
But sun protection and patient education does appear to play a role in reducing the number of nevi in children. Dr. Friedlander, clinical professor of pediatrics and medicine at the University of California, San Diego, cited another study in which 458 children in first to fourth grade in Canada were randomized to receive sunscreen and counseling about sun protection. Three years later, the sunscreen group had significantly fewer new nevi compared with the control group (JAMA 2000;283:2955-60).
What's a dermatologist to do? "Common sense prevails," Dr. Friedlander said. She advised dermatologists to counsel children and their parents to protect against sunburns by using sunscreens and sun-protective clothing. Also, "identify high-risk patients and follow them" so that any problems can be spotted early, she said.
But once parents and children are on board with sun protection, what should they use? The Environmental Working Group (EWG), a nonprofit organization that reviews and disseminates information about contaminants in consumer products and the environment, has come down in favor of physical sunscreens, based on a review of 400 studies and 2,000 sunscreens, Dr. Friedlander said.
Some parents and children prefer organic sunscreens, which contain oxybenzones, but the EWG rates these products as more dangerous than physical sunscreens. Some research suggests that oxybenzones can be absorbed into the skin in a way that the nano-particles of zinc oxide and titanium dioxide in physical sunscreens cannot, Dr. Friedlander said.
Be aware of what sunscreen characteristics raise concerns in your patients and their parents, Dr. Friedlander said. Share information with them, refer them to the EWG Web (www.ewg.org) and come up with a reasonable plan for sun protection.
Dr. Friedlander has served as a clinical investigator for Johnson & Johnson, which manufactures sunscreen products. SDEF and this news organization are owned by Elsevier.
"The benefits of sun protection clearly outweigh the risks," said Dr. Sheila Fallon Friedlander, but the debate continues over how to balance the benefits of outdoor activities for children with protection against skin cancer.
Some studies suggest outdoor activity is protective against melanoma, Dr. Friedlander said She cited a case-control study of 583 cases of cutaneous malignant melanoma and 608 controls (Int. J. Epidemiol. 1999;28:418-27). Intermittent sun exposure, such as beach vacations during adolescence and the use of tanning beds and sunlamps, was associated with a significantly greater risk for melanoma, whereas chronic exposure, indicated by days of outdoor activity during adolescence and by outdoor jobs in adulthood, was associated with a significantly reduced risk for melanoma.
But sun protection and patient education does appear to play a role in reducing the number of nevi in children. Dr. Friedlander, clinical professor of pediatrics and medicine at the University of California, San Diego, cited another study in which 458 children in first to fourth grade in Canada were randomized to receive sunscreen and counseling about sun protection. Three years later, the sunscreen group had significantly fewer new nevi compared with the control group (JAMA 2000;283:2955-60).
What's a dermatologist to do? "Common sense prevails," Dr. Friedlander said. She advised dermatologists to counsel children and their parents to protect against sunburns by using sunscreens and sun-protective clothing. Also, "identify high-risk patients and follow them" so that any problems can be spotted early, she said.
But once parents and children are on board with sun protection, what should they use? The Environmental Working Group (EWG), a nonprofit organization that reviews and disseminates information about contaminants in consumer products and the environment, has come down in favor of physical sunscreens, based on a review of 400 studies and 2,000 sunscreens, Dr. Friedlander said.
Some parents and children prefer organic sunscreens, which contain oxybenzones, but the EWG rates these products as more dangerous than physical sunscreens. Some research suggests that oxybenzones can be absorbed into the skin in a way that the nano-particles of zinc oxide and titanium dioxide in physical sunscreens cannot, Dr. Friedlander said.
Be aware of what sunscreen characteristics raise concerns in your patients and their parents, Dr. Friedlander said. Share information with them, refer them to the EWG Web (www.ewg.org) and come up with a reasonable plan for sun protection.
Dr. Friedlander has served as a clinical investigator for Johnson & Johnson, which manufactures sunscreen products. SDEF and this news organization are owned by Elsevier.
Three Factors Help Estimate Metastasis Risk of Thin Melanomas
Three clinical factors help estimate which thin melanomas are likely to harbor occult nodal metastases, and thus aid in selecting which patients would benefit most from sentinel node biopsy, according to a report in the February issue of the Archives of Surgery.
The three factors--patient sex, patient age at diagnosis, and lesion Breslow thickness--were used to develop a scoring system for estimating the risk of nodal recurrence. "This risk assessment is not intended to mandate what risk level is appropriate for sentinel node evaluation, but it allows for a better informed discussion with the patient newly diagnosed as having melanoma.
"Such information could be used to reassure extremely low-risk patients who may be anxious about the possibility of metastases or convince patients at higher risk of the need to consider biopsy," said Dr. Mark B. Faries of the John Wayne Cancer Institute, Santa Monica, Calif., and his associates (Arch. Surg. 2010;145:137-42) .
The use of sentinel node biopsy in patients with thin melanoma is controversial, since most such cases are at low risk for metastasis but the disease is usually fatal for the few patients who do develop recurrence. Doing the procedure in every case "would be prohibitively expensive and would expose a large number of patients with an extremely low risk of nodal disease to the real, albeit low, risk of toxic effects related to the procedure," they noted.
Dr. Faries and his colleagues reviewed the records in a prospective database of over 13,000 cases of thin (<1 mm) melanoma treated in 1971-2005 with wide excision but no nodal staging or lymphadenectomy. A total of 1,732 subjects were enrolled in the study. They were followed every 3 months for 2 years, every 4-6 months for the next 3 years, and annually thereafter.
During a median follow-up of 13 years, 51 patients (3%) developed nodal metastases. The median time to such recurrence was 38 months.
A variety of clinical factors were assessed to identify which ones were the strongest predictors of metastasis. Male sex, younger age at diagnosis, and greater tumor Breslow thickness were strongly predictive of metastasis, while factors such as tumor site and Clark level were not.
The investigators developed a scoring system using these 3 factors. Applying it to the study subjects, they found that the system predicted a 0.1% risk of metastasis in the lowest-risk subjects, compared with predicting a 17% risk of metastasis in the highest-risk subjects.
Tumor ulceration was predictive of nodal recurrence, with an 8% rate of metastasis in cases of ulcerated lesions compared to a 3% rate in cases without ulceration. However, ulceration was not included in the scoring system because information on ulceration often was not included in patient records, and a small proportion (approximately 2%) of this subset of patients showed lesion ulceration.
"Although such ulcerated lesions clearly deserve greater concern and evaluation, the rarity of this finding decreases the utility of including it in a prediction scheme," Dr. Faries and his associates said.
This study was funded in part by the National Cancer Institute and the Amyx Foundation. No financial conflicts of interest were reported.
Three clinical factors help estimate which thin melanomas are likely to harbor occult nodal metastases, and thus aid in selecting which patients would benefit most from sentinel node biopsy, according to a report in the February issue of the Archives of Surgery.
The three factors--patient sex, patient age at diagnosis, and lesion Breslow thickness--were used to develop a scoring system for estimating the risk of nodal recurrence. "This risk assessment is not intended to mandate what risk level is appropriate for sentinel node evaluation, but it allows for a better informed discussion with the patient newly diagnosed as having melanoma.
"Such information could be used to reassure extremely low-risk patients who may be anxious about the possibility of metastases or convince patients at higher risk of the need to consider biopsy," said Dr. Mark B. Faries of the John Wayne Cancer Institute, Santa Monica, Calif., and his associates (Arch. Surg. 2010;145:137-42) .
The use of sentinel node biopsy in patients with thin melanoma is controversial, since most such cases are at low risk for metastasis but the disease is usually fatal for the few patients who do develop recurrence. Doing the procedure in every case "would be prohibitively expensive and would expose a large number of patients with an extremely low risk of nodal disease to the real, albeit low, risk of toxic effects related to the procedure," they noted.
Dr. Faries and his colleagues reviewed the records in a prospective database of over 13,000 cases of thin (<1 mm) melanoma treated in 1971-2005 with wide excision but no nodal staging or lymphadenectomy. A total of 1,732 subjects were enrolled in the study. They were followed every 3 months for 2 years, every 4-6 months for the next 3 years, and annually thereafter.
During a median follow-up of 13 years, 51 patients (3%) developed nodal metastases. The median time to such recurrence was 38 months.
A variety of clinical factors were assessed to identify which ones were the strongest predictors of metastasis. Male sex, younger age at diagnosis, and greater tumor Breslow thickness were strongly predictive of metastasis, while factors such as tumor site and Clark level were not.
The investigators developed a scoring system using these 3 factors. Applying it to the study subjects, they found that the system predicted a 0.1% risk of metastasis in the lowest-risk subjects, compared with predicting a 17% risk of metastasis in the highest-risk subjects.
Tumor ulceration was predictive of nodal recurrence, with an 8% rate of metastasis in cases of ulcerated lesions compared to a 3% rate in cases without ulceration. However, ulceration was not included in the scoring system because information on ulceration often was not included in patient records, and a small proportion (approximately 2%) of this subset of patients showed lesion ulceration.
"Although such ulcerated lesions clearly deserve greater concern and evaluation, the rarity of this finding decreases the utility of including it in a prediction scheme," Dr. Faries and his associates said.
This study was funded in part by the National Cancer Institute and the Amyx Foundation. No financial conflicts of interest were reported.
Three clinical factors help estimate which thin melanomas are likely to harbor occult nodal metastases, and thus aid in selecting which patients would benefit most from sentinel node biopsy, according to a report in the February issue of the Archives of Surgery.
The three factors--patient sex, patient age at diagnosis, and lesion Breslow thickness--were used to develop a scoring system for estimating the risk of nodal recurrence. "This risk assessment is not intended to mandate what risk level is appropriate for sentinel node evaluation, but it allows for a better informed discussion with the patient newly diagnosed as having melanoma.
"Such information could be used to reassure extremely low-risk patients who may be anxious about the possibility of metastases or convince patients at higher risk of the need to consider biopsy," said Dr. Mark B. Faries of the John Wayne Cancer Institute, Santa Monica, Calif., and his associates (Arch. Surg. 2010;145:137-42) .
The use of sentinel node biopsy in patients with thin melanoma is controversial, since most such cases are at low risk for metastasis but the disease is usually fatal for the few patients who do develop recurrence. Doing the procedure in every case "would be prohibitively expensive and would expose a large number of patients with an extremely low risk of nodal disease to the real, albeit low, risk of toxic effects related to the procedure," they noted.
Dr. Faries and his colleagues reviewed the records in a prospective database of over 13,000 cases of thin (<1 mm) melanoma treated in 1971-2005 with wide excision but no nodal staging or lymphadenectomy. A total of 1,732 subjects were enrolled in the study. They were followed every 3 months for 2 years, every 4-6 months for the next 3 years, and annually thereafter.
During a median follow-up of 13 years, 51 patients (3%) developed nodal metastases. The median time to such recurrence was 38 months.
A variety of clinical factors were assessed to identify which ones were the strongest predictors of metastasis. Male sex, younger age at diagnosis, and greater tumor Breslow thickness were strongly predictive of metastasis, while factors such as tumor site and Clark level were not.
The investigators developed a scoring system using these 3 factors. Applying it to the study subjects, they found that the system predicted a 0.1% risk of metastasis in the lowest-risk subjects, compared with predicting a 17% risk of metastasis in the highest-risk subjects.
Tumor ulceration was predictive of nodal recurrence, with an 8% rate of metastasis in cases of ulcerated lesions compared to a 3% rate in cases without ulceration. However, ulceration was not included in the scoring system because information on ulceration often was not included in patient records, and a small proportion (approximately 2%) of this subset of patients showed lesion ulceration.
"Although such ulcerated lesions clearly deserve greater concern and evaluation, the rarity of this finding decreases the utility of including it in a prediction scheme," Dr. Faries and his associates said.
This study was funded in part by the National Cancer Institute and the Amyx Foundation. No financial conflicts of interest were reported.
NSAIDs Fail to Cut Cutaneous Squamous Cell Carcinoma Risk
The use of nonsteroidal anti-inflammatory drugs does not reduce the risk of cutaneous squamous cell carcinoma, according to a study published online Feb. 15 in the Archives of Dermatology.
In a retrospective case-control study involving more than 800 subjects, the dose, duration, and type of NSAID exposure exerted "no clear effect" on the risk of developing squamous cell carcinoma (SCC), said Dr. Maryam M. Asgari of Kaiser Permanente of Northern California, Oakland, and associates.
NSAIDs block the synthesis of proinflammatory prostaglandins and "inhibit neoplastic proliferation by inducing apoptosis and inhibiting angiogenesis," the authors wrote. They have been reported to protect against colorectal, breast, prostate, and lung cancer, and have shown activity against SCCs both in animal and in vitro studies. However, human studies have produced conflicting results.
Dr. Asgari and her colleagues assessed NSAID use during the preceding decade in 415 patients with pathologically confirmed SCC who were diagnosed in 2004 and 415 control subjects matched for age, sex, and race. Only cases of extragenital and nonmucosal SCC were included.
The subjects (aged 43-85 years) completed 3-page questionnaires detailing medication use, health history, skin cancer history, and risk factors. NSAID use was analyzed by four categories: any NSAIDs, aspirin, ibuprofen, and nonaspirin NSAIDs. Regular use was defined as taking the medication at least once per week for at least 1 year.
Most of the subjects (61%) reported regular use of NSAIDs at some time during the preceding 10 years, most commonly aspirin (48%), followed by ibuprofen (18%), naproxen (5%), and nabumetone (4%).
There was no association between self-reported NSAID use and SCC risk.
This finding was validated in a separate analysis of pharmacy records of NSAIDs dispensed to the same group of subjects, the investigators said (Arch. Dermatol. 2010 Feb. 15 [doi:10.1001/archdermatol.2009.374]).
Dose and duration of exposure had no effect on the results.
"Our results are largely consistent with" three of the four published articles examining the association of NSAIDs with SCC risk, they added.
Disclosures: This study was funded by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases and the National Cancer Institute. One of Dr. Asgari's associates reported serving on an advisory committee for Roche Laboratories and consulting for law firms serving both plaintiffs and Ortho-McNeil-Janssen Pharmaceuticals in legal cases. No other potential conflicts of interest were reported.
The use of nonsteroidal anti-inflammatory drugs does not reduce the risk of cutaneous squamous cell carcinoma, according to a study published online Feb. 15 in the Archives of Dermatology.
In a retrospective case-control study involving more than 800 subjects, the dose, duration, and type of NSAID exposure exerted "no clear effect" on the risk of developing squamous cell carcinoma (SCC), said Dr. Maryam M. Asgari of Kaiser Permanente of Northern California, Oakland, and associates.
NSAIDs block the synthesis of proinflammatory prostaglandins and "inhibit neoplastic proliferation by inducing apoptosis and inhibiting angiogenesis," the authors wrote. They have been reported to protect against colorectal, breast, prostate, and lung cancer, and have shown activity against SCCs both in animal and in vitro studies. However, human studies have produced conflicting results.
Dr. Asgari and her colleagues assessed NSAID use during the preceding decade in 415 patients with pathologically confirmed SCC who were diagnosed in 2004 and 415 control subjects matched for age, sex, and race. Only cases of extragenital and nonmucosal SCC were included.
The subjects (aged 43-85 years) completed 3-page questionnaires detailing medication use, health history, skin cancer history, and risk factors. NSAID use was analyzed by four categories: any NSAIDs, aspirin, ibuprofen, and nonaspirin NSAIDs. Regular use was defined as taking the medication at least once per week for at least 1 year.
Most of the subjects (61%) reported regular use of NSAIDs at some time during the preceding 10 years, most commonly aspirin (48%), followed by ibuprofen (18%), naproxen (5%), and nabumetone (4%).
There was no association between self-reported NSAID use and SCC risk.
This finding was validated in a separate analysis of pharmacy records of NSAIDs dispensed to the same group of subjects, the investigators said (Arch. Dermatol. 2010 Feb. 15 [doi:10.1001/archdermatol.2009.374]).
Dose and duration of exposure had no effect on the results.
"Our results are largely consistent with" three of the four published articles examining the association of NSAIDs with SCC risk, they added.
Disclosures: This study was funded by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases and the National Cancer Institute. One of Dr. Asgari's associates reported serving on an advisory committee for Roche Laboratories and consulting for law firms serving both plaintiffs and Ortho-McNeil-Janssen Pharmaceuticals in legal cases. No other potential conflicts of interest were reported.
The use of nonsteroidal anti-inflammatory drugs does not reduce the risk of cutaneous squamous cell carcinoma, according to a study published online Feb. 15 in the Archives of Dermatology.
In a retrospective case-control study involving more than 800 subjects, the dose, duration, and type of NSAID exposure exerted "no clear effect" on the risk of developing squamous cell carcinoma (SCC), said Dr. Maryam M. Asgari of Kaiser Permanente of Northern California, Oakland, and associates.
NSAIDs block the synthesis of proinflammatory prostaglandins and "inhibit neoplastic proliferation by inducing apoptosis and inhibiting angiogenesis," the authors wrote. They have been reported to protect against colorectal, breast, prostate, and lung cancer, and have shown activity against SCCs both in animal and in vitro studies. However, human studies have produced conflicting results.
Dr. Asgari and her colleagues assessed NSAID use during the preceding decade in 415 patients with pathologically confirmed SCC who were diagnosed in 2004 and 415 control subjects matched for age, sex, and race. Only cases of extragenital and nonmucosal SCC were included.
The subjects (aged 43-85 years) completed 3-page questionnaires detailing medication use, health history, skin cancer history, and risk factors. NSAID use was analyzed by four categories: any NSAIDs, aspirin, ibuprofen, and nonaspirin NSAIDs. Regular use was defined as taking the medication at least once per week for at least 1 year.
Most of the subjects (61%) reported regular use of NSAIDs at some time during the preceding 10 years, most commonly aspirin (48%), followed by ibuprofen (18%), naproxen (5%), and nabumetone (4%).
There was no association between self-reported NSAID use and SCC risk.
This finding was validated in a separate analysis of pharmacy records of NSAIDs dispensed to the same group of subjects, the investigators said (Arch. Dermatol. 2010 Feb. 15 [doi:10.1001/archdermatol.2009.374]).
Dose and duration of exposure had no effect on the results.
"Our results are largely consistent with" three of the four published articles examining the association of NSAIDs with SCC risk, they added.
Disclosures: This study was funded by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases and the National Cancer Institute. One of Dr. Asgari's associates reported serving on an advisory committee for Roche Laboratories and consulting for law firms serving both plaintiffs and Ortho-McNeil-Janssen Pharmaceuticals in legal cases. No other potential conflicts of interest were reported.
Plant Extract Under Study for Treating AKs
A number of new therapies for cutaneous diseases are helping dermatologists improve patient care.
One such agent, ingenol mebutate, is a plant extract being used to treat actinic keratoses, Dr. J. Mark Jackson explained at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
An active component of radium weed, the extract is believed to induce primary necrosis by a neutrophil-mediated, antibody-dependent cellular toxicity. Ingenol mebutate is widely used in Australia to treat actinic keratoses, said Dr. Jackson, a clinical professor of medicine and dermatology at the University of Louisville in Kentucky.
Phase III trial results were released at press time by LEO Pharma, the manufacturer of ingenol mebutate. A 0.05% concentration of ingenol mebutate was used for 2 consecutive days in 250 patients with actinic keratoses on non-head locations. According to the company's press release, the gel met its primary clinical endpoint of complete lesion clearance. The study's full findings are expected to be presented at the upcoming meeting of the American Academy of Dermatology.
In the phase II, randomized, double-blind, placebo-controlled trial, 58 patients with 5 biopsy-proven actinic keratoses were treated with ingenol mebutate gel or placebo on days 1 and 2 or days 1 and 8 (Australasian J. Dermatol. 2009;50:16-22).
Complete clearance was achieved in 71% of actinic keratoses treated with the 0.05% formulation of ingenol mebutate, compared with 25% of those treated with the 0.01% formulation, 40% of those treated with the 0.0025% formulation, and 32% of those treated with the placebo gel.
Side effects were mild to moderate, including four cases of hypopigmentation at the treatment sites and one case of persistent scabbing and pain that extended beyond the study period in three of five actinic keratoses.
The use of itraconazole for treating palmoplantar pustulosis is another advance, he said. In a recent open-label study, six patients with palmoplantar pustulosis and no other evidence of psoriasis received 100 mg itraconazole daily for 1 month, followed by every other day for 1 month (Dermatol. Ther. 2009;22:85-9).
Three patients achieved complete response, with improvement after 2 weeks of treatment. The remaining three patients developed no new pustules but continued to have some disease activity. All patients experienced relapse, but retreatment controlled disease in two patients.
During a discussion of retapamulin, which is approved for the topical treatment of impetigo due to Staphylococcus aureus (methicillin-susceptible S. aureus only) and S. pyogenes in patients older than 9 months of age, Dr. Jackson noted that the agent can cause irritation to the nasal mucosa "and is, therefore, not a good treatment for many patients with nasal carriage of MRSA. If nasal carriage is resistant to mupirocin, you may try retapamulin, but you should warn the patient in advance to try a test area first."
Cutaneous reactions can occur in response to epidermal growth factor receptors (EGFRs), Dr. Jackson warned. A prospective study of 30 patients taking either cetuximab or erlotinib for cancer noted acneiform pustule and follicular eruptions in a seborrheic distribution 7-10 days after therapy (J. Am. Acad. Dermatol. 2006;55:429-37).
"Palmoplantar eruptions have also been reported," he said. "So have xerosis, pyogenic granulomas, and paronychia." Cutaneous reactions to EGFRs are dose dependent and recur with subsequent treatment.
Be aware of the procoagulant effects of thalidomide, which is approved for the treatment of chronic recurrent and severe erythema nodosum leprosum and is used for many other inflammatory dermatologic conditions, he said. A study of 25 patients found that deep vein thrombosis occurred in 20% of patients taking thalidomide for various inflammatory conditions (J. Dermatolog. Treat. 2007;18:335-40).
Patients at particular risk include those with systemic lupus erythematosus, a malignancy, antiphospholipid syndrome, smokers, women on oral contraceptives, patients with hyperhomocysteinemia, and patients withdrawing from antimalarials, he added.
Dr. Jackson advised that dermatologists should have a basic understanding of interstitial granulomatous dermatitis, first described in 1993. Also known as granulomatous dermatitis with associated arthritis, the condition "generally presents as erythematous, violaceous, hyperpigmented, annular plaques on the trunk and thighs," he said. "Sometimes there is an acral distribution."
Histopathology reveals dense inflammatory mixed cell infiltrate, necrobiosis, sparse mucin deposition, and no vasculitis.
Associated diseases include rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune conditions. The disease can also be triggered by infections and certain medications, including ACE inhibitors, calcium channel blockers, beta blockers, and lipid lowering agents.
Treatment options include glucocorticoids, NSAIDs, and TNF-alpha inhibitors.
Several new therapies are under study to help physicians treat cutaneous diseases such as actinic keratoses (shown here on an elbow). Photo courtesy Dr. Roger I. Ceilley.
Dr. Jackson disclosed that he has received research, honoraria, consulting or other support from Abbott, Amgen, Biogen Idec, Centocor Ortho Biotech, Ferndale Laboratories, Galderma, Genentech, Medicis, Novartis, Promius Pharma, Quinnova Pharmaceuticals, Roche, and Stiefel.
SDEF and this news organization are owned by Elsevier.
A number of new therapies for cutaneous diseases are helping dermatologists improve patient care.
One such agent, ingenol mebutate, is a plant extract being used to treat actinic keratoses, Dr. J. Mark Jackson explained at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
An active component of radium weed, the extract is believed to induce primary necrosis by a neutrophil-mediated, antibody-dependent cellular toxicity. Ingenol mebutate is widely used in Australia to treat actinic keratoses, said Dr. Jackson, a clinical professor of medicine and dermatology at the University of Louisville in Kentucky.
Phase III trial results were released at press time by LEO Pharma, the manufacturer of ingenol mebutate. A 0.05% concentration of ingenol mebutate was used for 2 consecutive days in 250 patients with actinic keratoses on non-head locations. According to the company's press release, the gel met its primary clinical endpoint of complete lesion clearance. The study's full findings are expected to be presented at the upcoming meeting of the American Academy of Dermatology.
In the phase II, randomized, double-blind, placebo-controlled trial, 58 patients with 5 biopsy-proven actinic keratoses were treated with ingenol mebutate gel or placebo on days 1 and 2 or days 1 and 8 (Australasian J. Dermatol. 2009;50:16-22).
Complete clearance was achieved in 71% of actinic keratoses treated with the 0.05% formulation of ingenol mebutate, compared with 25% of those treated with the 0.01% formulation, 40% of those treated with the 0.0025% formulation, and 32% of those treated with the placebo gel.
Side effects were mild to moderate, including four cases of hypopigmentation at the treatment sites and one case of persistent scabbing and pain that extended beyond the study period in three of five actinic keratoses.
The use of itraconazole for treating palmoplantar pustulosis is another advance, he said. In a recent open-label study, six patients with palmoplantar pustulosis and no other evidence of psoriasis received 100 mg itraconazole daily for 1 month, followed by every other day for 1 month (Dermatol. Ther. 2009;22:85-9).
Three patients achieved complete response, with improvement after 2 weeks of treatment. The remaining three patients developed no new pustules but continued to have some disease activity. All patients experienced relapse, but retreatment controlled disease in two patients.
During a discussion of retapamulin, which is approved for the topical treatment of impetigo due to Staphylococcus aureus (methicillin-susceptible S. aureus only) and S. pyogenes in patients older than 9 months of age, Dr. Jackson noted that the agent can cause irritation to the nasal mucosa "and is, therefore, not a good treatment for many patients with nasal carriage of MRSA. If nasal carriage is resistant to mupirocin, you may try retapamulin, but you should warn the patient in advance to try a test area first."
Cutaneous reactions can occur in response to epidermal growth factor receptors (EGFRs), Dr. Jackson warned. A prospective study of 30 patients taking either cetuximab or erlotinib for cancer noted acneiform pustule and follicular eruptions in a seborrheic distribution 7-10 days after therapy (J. Am. Acad. Dermatol. 2006;55:429-37).
"Palmoplantar eruptions have also been reported," he said. "So have xerosis, pyogenic granulomas, and paronychia." Cutaneous reactions to EGFRs are dose dependent and recur with subsequent treatment.
Be aware of the procoagulant effects of thalidomide, which is approved for the treatment of chronic recurrent and severe erythema nodosum leprosum and is used for many other inflammatory dermatologic conditions, he said. A study of 25 patients found that deep vein thrombosis occurred in 20% of patients taking thalidomide for various inflammatory conditions (J. Dermatolog. Treat. 2007;18:335-40).
Patients at particular risk include those with systemic lupus erythematosus, a malignancy, antiphospholipid syndrome, smokers, women on oral contraceptives, patients with hyperhomocysteinemia, and patients withdrawing from antimalarials, he added.
Dr. Jackson advised that dermatologists should have a basic understanding of interstitial granulomatous dermatitis, first described in 1993. Also known as granulomatous dermatitis with associated arthritis, the condition "generally presents as erythematous, violaceous, hyperpigmented, annular plaques on the trunk and thighs," he said. "Sometimes there is an acral distribution."
Histopathology reveals dense inflammatory mixed cell infiltrate, necrobiosis, sparse mucin deposition, and no vasculitis.
Associated diseases include rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune conditions. The disease can also be triggered by infections and certain medications, including ACE inhibitors, calcium channel blockers, beta blockers, and lipid lowering agents.
Treatment options include glucocorticoids, NSAIDs, and TNF-alpha inhibitors.
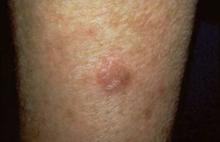
Several new therapies are under study to help physicians treat cutaneous diseases such as actinic keratoses (shown here on an elbow). Photo courtesy Dr. Roger I. Ceilley.
Dr. Jackson disclosed that he has received research, honoraria, consulting or other support from Abbott, Amgen, Biogen Idec, Centocor Ortho Biotech, Ferndale Laboratories, Galderma, Genentech, Medicis, Novartis, Promius Pharma, Quinnova Pharmaceuticals, Roche, and Stiefel.
SDEF and this news organization are owned by Elsevier.
A number of new therapies for cutaneous diseases are helping dermatologists improve patient care.
One such agent, ingenol mebutate, is a plant extract being used to treat actinic keratoses, Dr. J. Mark Jackson explained at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
An active component of radium weed, the extract is believed to induce primary necrosis by a neutrophil-mediated, antibody-dependent cellular toxicity. Ingenol mebutate is widely used in Australia to treat actinic keratoses, said Dr. Jackson, a clinical professor of medicine and dermatology at the University of Louisville in Kentucky.
Phase III trial results were released at press time by LEO Pharma, the manufacturer of ingenol mebutate. A 0.05% concentration of ingenol mebutate was used for 2 consecutive days in 250 patients with actinic keratoses on non-head locations. According to the company's press release, the gel met its primary clinical endpoint of complete lesion clearance. The study's full findings are expected to be presented at the upcoming meeting of the American Academy of Dermatology.
In the phase II, randomized, double-blind, placebo-controlled trial, 58 patients with 5 biopsy-proven actinic keratoses were treated with ingenol mebutate gel or placebo on days 1 and 2 or days 1 and 8 (Australasian J. Dermatol. 2009;50:16-22).
Complete clearance was achieved in 71% of actinic keratoses treated with the 0.05% formulation of ingenol mebutate, compared with 25% of those treated with the 0.01% formulation, 40% of those treated with the 0.0025% formulation, and 32% of those treated with the placebo gel.
Side effects were mild to moderate, including four cases of hypopigmentation at the treatment sites and one case of persistent scabbing and pain that extended beyond the study period in three of five actinic keratoses.
The use of itraconazole for treating palmoplantar pustulosis is another advance, he said. In a recent open-label study, six patients with palmoplantar pustulosis and no other evidence of psoriasis received 100 mg itraconazole daily for 1 month, followed by every other day for 1 month (Dermatol. Ther. 2009;22:85-9).
Three patients achieved complete response, with improvement after 2 weeks of treatment. The remaining three patients developed no new pustules but continued to have some disease activity. All patients experienced relapse, but retreatment controlled disease in two patients.
During a discussion of retapamulin, which is approved for the topical treatment of impetigo due to Staphylococcus aureus (methicillin-susceptible S. aureus only) and S. pyogenes in patients older than 9 months of age, Dr. Jackson noted that the agent can cause irritation to the nasal mucosa "and is, therefore, not a good treatment for many patients with nasal carriage of MRSA. If nasal carriage is resistant to mupirocin, you may try retapamulin, but you should warn the patient in advance to try a test area first."
Cutaneous reactions can occur in response to epidermal growth factor receptors (EGFRs), Dr. Jackson warned. A prospective study of 30 patients taking either cetuximab or erlotinib for cancer noted acneiform pustule and follicular eruptions in a seborrheic distribution 7-10 days after therapy (J. Am. Acad. Dermatol. 2006;55:429-37).
"Palmoplantar eruptions have also been reported," he said. "So have xerosis, pyogenic granulomas, and paronychia." Cutaneous reactions to EGFRs are dose dependent and recur with subsequent treatment.
Be aware of the procoagulant effects of thalidomide, which is approved for the treatment of chronic recurrent and severe erythema nodosum leprosum and is used for many other inflammatory dermatologic conditions, he said. A study of 25 patients found that deep vein thrombosis occurred in 20% of patients taking thalidomide for various inflammatory conditions (J. Dermatolog. Treat. 2007;18:335-40).
Patients at particular risk include those with systemic lupus erythematosus, a malignancy, antiphospholipid syndrome, smokers, women on oral contraceptives, patients with hyperhomocysteinemia, and patients withdrawing from antimalarials, he added.
Dr. Jackson advised that dermatologists should have a basic understanding of interstitial granulomatous dermatitis, first described in 1993. Also known as granulomatous dermatitis with associated arthritis, the condition "generally presents as erythematous, violaceous, hyperpigmented, annular plaques on the trunk and thighs," he said. "Sometimes there is an acral distribution."
Histopathology reveals dense inflammatory mixed cell infiltrate, necrobiosis, sparse mucin deposition, and no vasculitis.
Associated diseases include rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune conditions. The disease can also be triggered by infections and certain medications, including ACE inhibitors, calcium channel blockers, beta blockers, and lipid lowering agents.
Treatment options include glucocorticoids, NSAIDs, and TNF-alpha inhibitors.
Several new therapies are under study to help physicians treat cutaneous diseases such as actinic keratoses (shown here on an elbow). Photo courtesy Dr. Roger I. Ceilley.
Dr. Jackson disclosed that he has received research, honoraria, consulting or other support from Abbott, Amgen, Biogen Idec, Centocor Ortho Biotech, Ferndale Laboratories, Galderma, Genentech, Medicis, Novartis, Promius Pharma, Quinnova Pharmaceuticals, Roche, and Stiefel.
SDEF and this news organization are owned by Elsevier.