User login
Vigilance is Required to Avoid Misdiagnosed Melanomas
Misdiagnosis of melanoma is a major cause of litigation against both dermatologists and dermatopathologists.
The majority of claims filed between 1985 and 2001 involving the misdiagnosis of melanoma were because of a false negative diagnosis, which may translate to a reduced chance of survival for some patients, Dr. Ashfaq A. Marghoob reported at the seminar
Two important strategies can help minimize missing melanoma, he said. First, remain vigilant and remember that many melanomas lack the classic ABCD features.
"Questioning yourself and your pathologist regarding the diagnosis will help towards identifying many of these melanomas. In other words, remain skeptical of lesions lacking clinical-dermoscopy correlation or lesions lacking dermoscopy-histopathology correlation. Second, engage patients in their own care by having them share the responsibility of detecting early melanoma by encouraging them to examine their own skin on a regular basis," said Dr. Marghoob, who is a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
Some melanomas may not manifest concerning features, and can mimic benign lesions. To assure that a malignant melanoma will eventually be found, periodic total body examinations by a physician, and regular patient self-examinations, are key. He stressed that physician examinations and self-skin exams are complementary.
While it is widely accepted that early detection means better prognosis, modest delays of up to 6 months have not been shown to affect ultimate outcomes. However, there is one exception. Nodular melanoma can grow rapidly, and even small delays in diagnosis can have serious consequences.
The most common scenarios in melanoma litigation cases include nodular melanoma being misdiagnosed by a clinician or pathologist; a partial biopsy not capturing the most diagnostically relevant part of the lesion; malignant melanoma being misdiagnosed as a dysplastic or spitz nevus; unrecognized desmoplastic malignant melanoma; and metastatic malignant melanoma with an unknown primary or recurrence of melanoma (Am. J. Surg. Pathol. 2003;27:1278-83).
Dr. Marghoob discussed each of these cases in detail.
Misdiagnosis of nodular melanoma as nevus by a clinician or pathologist. Many nodular melanomas lack helpful diagnostic features, such as those in the ABCD criteria for malignant melanoma, which can lead to a misdiagnosis. However, the ABCDE criteria that take lesion evolution into account may be of some help, noted Dr. Marghoob.
In order to track lesion evolution, ask patients about the history of changes and symptoms. Total body photography may help on rare occasions to detect new lesions, some of which may be subtle. In addition, dermoscopy results may persuade the clinician to obtain a biopsy of a clinically banal appearing lesion that is in fact a nodular melanoma.
Partial biopsy issues. If a biopsy is performed of a lesion that clinically looks like melanoma and the pathology diagnosis is nevus, it is imperative that the clinician and pathologist reconcile the difference. In cases where there is discordance, consider asking for step-sectioning, special stains, or--in very rare instances--fluorescence in situ hybridization to look for signature chromosomal aberrations. In addition, a partial biopsy may not be representative of the rest of the lesion. If a partial biopsy was performed, re-excise the lesion, said Dr. Marghoob. Remember that a pathology report should never be read in a vacuum.
Excisional biopsy is the preferred method for melanocytic lesions, when possible, because partial biopsy may sample nondiagnostic areas or miss the prognostically-worse portion of the lesion.
"Partial biopsy assumes that a clinician can consistently predict the portion of a suspicious pigmented lesion that will have the worst representative histology," said Dr. Marghoob. In one study, 40% of excised melanomas had worse pathology, compared with initial punch biopsy, and 20% of melanomas revealed invasion, which was not seen in initial punch biopsy (Arch. Dermatol. 1996;132:1297-1302).
The ideal biopsy is excisional with a 2- to 3-mm margin, is oriented along the lines of lymphatic drainage, and is step sectioned. This limits sampling error, removes dysplastic nevus completely (preventing recurrence), and better predicts the Breslow depth if the lesion proves to be a melanoma, said Dr. Marghoob.
Even when step sectioned, less than 2% of the lesion is evaluated. In one study, thorough block sampling resulted in identification of increased tumor thickness in 43% of cases by a mean of 0.16 mm (Arch. Dermatol. 2005;141:734-6).
Misdiagnosis of a melanoma as dysplastic or spitz nevus. When a partial biopsy reveals dysplastic or spitz nevus, it is important to completely excise the lesion. Malignant melanoma can sometimes masquerade as a spitz nevus, and focus of malignant melanoma may have been missed on the biopsy. Many dermatologists are of the opinion that spitz nevi should be completely excised--at least in adults, said Dr. Marghoob.
Unrecognized desmoplastic malignant melanoma. Desmoplastic melanoma can be banal in appearance, with 70% appearing amelanotic, he said. These lesions may only present as firmness in the subcutaneous tissue. For "banal" appearing firm lesions on chronically sun-damaged skin, suspicion should be raised if the lesions are symptomatic, growing, are associated with a lentigo maligna, or reveal irregular vessels with dermoscopy, said Dr. Marghoob.
Metastatic melanoma with unknown primary or recurrence of melanoma. Whenever possible, do not remove seemingly benign lesions and discard them, he said. Also, be careful and selective about the use of liquid nitrogen or a laser on lesions that have not been confirmed to be benign through biopsy.
He noted that cases of assumed benign lesions that recur after ablation (via liquid nitrogen, curettage, or laser), may ultimately prove to be melanoma on histopathology. Furthermore, in the unlikely event that a patient develops metastatic melanoma with an unknown primary, it may be presumed that one of the ablated lesions may have been the primary.
Dr. Marghoob disclosed having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
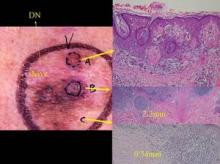
A partial biopsy was performed of this lesion which had clinical features of melanoma. The pathology analysis was reported as a Clark’s nevus (this area is the white scar on the left upper portion of the lesion). Due the clinical-pathology discordance the clinician decided to excise the lesion. As can be seen from the histology, this lesion was a melanoma and depending on the location of a partial biopsy the results can range from a Clark’s nevus to melanoma in situ to microinvasive to deeply invasive melanoma. Photo courtesy Dr. Ashfaq Marghoob.
Misdiagnosis of melanoma is a major cause of litigation against both dermatologists and dermatopathologists.
The majority of claims filed between 1985 and 2001 involving the misdiagnosis of melanoma were because of a false negative diagnosis, which may translate to a reduced chance of survival for some patients, Dr. Ashfaq A. Marghoob reported at the seminar
Two important strategies can help minimize missing melanoma, he said. First, remain vigilant and remember that many melanomas lack the classic ABCD features.
"Questioning yourself and your pathologist regarding the diagnosis will help towards identifying many of these melanomas. In other words, remain skeptical of lesions lacking clinical-dermoscopy correlation or lesions lacking dermoscopy-histopathology correlation. Second, engage patients in their own care by having them share the responsibility of detecting early melanoma by encouraging them to examine their own skin on a regular basis," said Dr. Marghoob, who is a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
Some melanomas may not manifest concerning features, and can mimic benign lesions. To assure that a malignant melanoma will eventually be found, periodic total body examinations by a physician, and regular patient self-examinations, are key. He stressed that physician examinations and self-skin exams are complementary.
While it is widely accepted that early detection means better prognosis, modest delays of up to 6 months have not been shown to affect ultimate outcomes. However, there is one exception. Nodular melanoma can grow rapidly, and even small delays in diagnosis can have serious consequences.
The most common scenarios in melanoma litigation cases include nodular melanoma being misdiagnosed by a clinician or pathologist; a partial biopsy not capturing the most diagnostically relevant part of the lesion; malignant melanoma being misdiagnosed as a dysplastic or spitz nevus; unrecognized desmoplastic malignant melanoma; and metastatic malignant melanoma with an unknown primary or recurrence of melanoma (Am. J. Surg. Pathol. 2003;27:1278-83).
Dr. Marghoob discussed each of these cases in detail.
Misdiagnosis of nodular melanoma as nevus by a clinician or pathologist. Many nodular melanomas lack helpful diagnostic features, such as those in the ABCD criteria for malignant melanoma, which can lead to a misdiagnosis. However, the ABCDE criteria that take lesion evolution into account may be of some help, noted Dr. Marghoob.
In order to track lesion evolution, ask patients about the history of changes and symptoms. Total body photography may help on rare occasions to detect new lesions, some of which may be subtle. In addition, dermoscopy results may persuade the clinician to obtain a biopsy of a clinically banal appearing lesion that is in fact a nodular melanoma.
Partial biopsy issues. If a biopsy is performed of a lesion that clinically looks like melanoma and the pathology diagnosis is nevus, it is imperative that the clinician and pathologist reconcile the difference. In cases where there is discordance, consider asking for step-sectioning, special stains, or--in very rare instances--fluorescence in situ hybridization to look for signature chromosomal aberrations. In addition, a partial biopsy may not be representative of the rest of the lesion. If a partial biopsy was performed, re-excise the lesion, said Dr. Marghoob. Remember that a pathology report should never be read in a vacuum.
Excisional biopsy is the preferred method for melanocytic lesions, when possible, because partial biopsy may sample nondiagnostic areas or miss the prognostically-worse portion of the lesion.
"Partial biopsy assumes that a clinician can consistently predict the portion of a suspicious pigmented lesion that will have the worst representative histology," said Dr. Marghoob. In one study, 40% of excised melanomas had worse pathology, compared with initial punch biopsy, and 20% of melanomas revealed invasion, which was not seen in initial punch biopsy (Arch. Dermatol. 1996;132:1297-1302).
The ideal biopsy is excisional with a 2- to 3-mm margin, is oriented along the lines of lymphatic drainage, and is step sectioned. This limits sampling error, removes dysplastic nevus completely (preventing recurrence), and better predicts the Breslow depth if the lesion proves to be a melanoma, said Dr. Marghoob.
Even when step sectioned, less than 2% of the lesion is evaluated. In one study, thorough block sampling resulted in identification of increased tumor thickness in 43% of cases by a mean of 0.16 mm (Arch. Dermatol. 2005;141:734-6).
Misdiagnosis of a melanoma as dysplastic or spitz nevus. When a partial biopsy reveals dysplastic or spitz nevus, it is important to completely excise the lesion. Malignant melanoma can sometimes masquerade as a spitz nevus, and focus of malignant melanoma may have been missed on the biopsy. Many dermatologists are of the opinion that spitz nevi should be completely excised--at least in adults, said Dr. Marghoob.
Unrecognized desmoplastic malignant melanoma. Desmoplastic melanoma can be banal in appearance, with 70% appearing amelanotic, he said. These lesions may only present as firmness in the subcutaneous tissue. For "banal" appearing firm lesions on chronically sun-damaged skin, suspicion should be raised if the lesions are symptomatic, growing, are associated with a lentigo maligna, or reveal irregular vessels with dermoscopy, said Dr. Marghoob.
Metastatic melanoma with unknown primary or recurrence of melanoma. Whenever possible, do not remove seemingly benign lesions and discard them, he said. Also, be careful and selective about the use of liquid nitrogen or a laser on lesions that have not been confirmed to be benign through biopsy.
He noted that cases of assumed benign lesions that recur after ablation (via liquid nitrogen, curettage, or laser), may ultimately prove to be melanoma on histopathology. Furthermore, in the unlikely event that a patient develops metastatic melanoma with an unknown primary, it may be presumed that one of the ablated lesions may have been the primary.
Dr. Marghoob disclosed having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
A partial biopsy was performed of this lesion which had clinical features of melanoma. The pathology analysis was reported as a Clark’s nevus (this area is the white scar on the left upper portion of the lesion). Due the clinical-pathology discordance the clinician decided to excise the lesion. As can be seen from the histology, this lesion was a melanoma and depending on the location of a partial biopsy the results can range from a Clark’s nevus to melanoma in situ to microinvasive to deeply invasive melanoma. Photo courtesy Dr. Ashfaq Marghoob.
Misdiagnosis of melanoma is a major cause of litigation against both dermatologists and dermatopathologists.
The majority of claims filed between 1985 and 2001 involving the misdiagnosis of melanoma were because of a false negative diagnosis, which may translate to a reduced chance of survival for some patients, Dr. Ashfaq A. Marghoob reported at the seminar
Two important strategies can help minimize missing melanoma, he said. First, remain vigilant and remember that many melanomas lack the classic ABCD features.
"Questioning yourself and your pathologist regarding the diagnosis will help towards identifying many of these melanomas. In other words, remain skeptical of lesions lacking clinical-dermoscopy correlation or lesions lacking dermoscopy-histopathology correlation. Second, engage patients in their own care by having them share the responsibility of detecting early melanoma by encouraging them to examine their own skin on a regular basis," said Dr. Marghoob, who is a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
Some melanomas may not manifest concerning features, and can mimic benign lesions. To assure that a malignant melanoma will eventually be found, periodic total body examinations by a physician, and regular patient self-examinations, are key. He stressed that physician examinations and self-skin exams are complementary.
While it is widely accepted that early detection means better prognosis, modest delays of up to 6 months have not been shown to affect ultimate outcomes. However, there is one exception. Nodular melanoma can grow rapidly, and even small delays in diagnosis can have serious consequences.
The most common scenarios in melanoma litigation cases include nodular melanoma being misdiagnosed by a clinician or pathologist; a partial biopsy not capturing the most diagnostically relevant part of the lesion; malignant melanoma being misdiagnosed as a dysplastic or spitz nevus; unrecognized desmoplastic malignant melanoma; and metastatic malignant melanoma with an unknown primary or recurrence of melanoma (Am. J. Surg. Pathol. 2003;27:1278-83).
Dr. Marghoob discussed each of these cases in detail.
Misdiagnosis of nodular melanoma as nevus by a clinician or pathologist. Many nodular melanomas lack helpful diagnostic features, such as those in the ABCD criteria for malignant melanoma, which can lead to a misdiagnosis. However, the ABCDE criteria that take lesion evolution into account may be of some help, noted Dr. Marghoob.
In order to track lesion evolution, ask patients about the history of changes and symptoms. Total body photography may help on rare occasions to detect new lesions, some of which may be subtle. In addition, dermoscopy results may persuade the clinician to obtain a biopsy of a clinically banal appearing lesion that is in fact a nodular melanoma.
Partial biopsy issues. If a biopsy is performed of a lesion that clinically looks like melanoma and the pathology diagnosis is nevus, it is imperative that the clinician and pathologist reconcile the difference. In cases where there is discordance, consider asking for step-sectioning, special stains, or--in very rare instances--fluorescence in situ hybridization to look for signature chromosomal aberrations. In addition, a partial biopsy may not be representative of the rest of the lesion. If a partial biopsy was performed, re-excise the lesion, said Dr. Marghoob. Remember that a pathology report should never be read in a vacuum.
Excisional biopsy is the preferred method for melanocytic lesions, when possible, because partial biopsy may sample nondiagnostic areas or miss the prognostically-worse portion of the lesion.
"Partial biopsy assumes that a clinician can consistently predict the portion of a suspicious pigmented lesion that will have the worst representative histology," said Dr. Marghoob. In one study, 40% of excised melanomas had worse pathology, compared with initial punch biopsy, and 20% of melanomas revealed invasion, which was not seen in initial punch biopsy (Arch. Dermatol. 1996;132:1297-1302).
The ideal biopsy is excisional with a 2- to 3-mm margin, is oriented along the lines of lymphatic drainage, and is step sectioned. This limits sampling error, removes dysplastic nevus completely (preventing recurrence), and better predicts the Breslow depth if the lesion proves to be a melanoma, said Dr. Marghoob.
Even when step sectioned, less than 2% of the lesion is evaluated. In one study, thorough block sampling resulted in identification of increased tumor thickness in 43% of cases by a mean of 0.16 mm (Arch. Dermatol. 2005;141:734-6).
Misdiagnosis of a melanoma as dysplastic or spitz nevus. When a partial biopsy reveals dysplastic or spitz nevus, it is important to completely excise the lesion. Malignant melanoma can sometimes masquerade as a spitz nevus, and focus of malignant melanoma may have been missed on the biopsy. Many dermatologists are of the opinion that spitz nevi should be completely excised--at least in adults, said Dr. Marghoob.
Unrecognized desmoplastic malignant melanoma. Desmoplastic melanoma can be banal in appearance, with 70% appearing amelanotic, he said. These lesions may only present as firmness in the subcutaneous tissue. For "banal" appearing firm lesions on chronically sun-damaged skin, suspicion should be raised if the lesions are symptomatic, growing, are associated with a lentigo maligna, or reveal irregular vessels with dermoscopy, said Dr. Marghoob.
Metastatic melanoma with unknown primary or recurrence of melanoma. Whenever possible, do not remove seemingly benign lesions and discard them, he said. Also, be careful and selective about the use of liquid nitrogen or a laser on lesions that have not been confirmed to be benign through biopsy.
He noted that cases of assumed benign lesions that recur after ablation (via liquid nitrogen, curettage, or laser), may ultimately prove to be melanoma on histopathology. Furthermore, in the unlikely event that a patient develops metastatic melanoma with an unknown primary, it may be presumed that one of the ablated lesions may have been the primary.
Dr. Marghoob disclosed having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
A partial biopsy was performed of this lesion which had clinical features of melanoma. The pathology analysis was reported as a Clark’s nevus (this area is the white scar on the left upper portion of the lesion). Due the clinical-pathology discordance the clinician decided to excise the lesion. As can be seen from the histology, this lesion was a melanoma and depending on the location of a partial biopsy the results can range from a Clark’s nevus to melanoma in situ to microinvasive to deeply invasive melanoma. Photo courtesy Dr. Ashfaq Marghoob.
Recognizing Risk Factors Can Help Catch Melanomas Earlier
Not all patients are equal when it comes to melanoma risk.
Knowing which patients may be more likely to develop melanoma can serve as a prompt to increase suspicion during examination, said Dr. James M. Grichnik. Be on the lookout for patients with the following features and characteristics, he suggested at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
Nevi
The presence of even one atypical (dysplastic) nevus independently predicts up to a 60% greater risk for melanoma, said Dr. Grichnik, director of the melanoma program and professor of dermatology at the University of Miami.
Higher numbers of common nevi also increase risk. Nevi counts are associated with UV exposure, and can be used as a surrogate marker for UV-induced cutaneous damage. Melanoma risk increases 47% with 16-40 common nevi on the whole body, he noted. Risk increases as mole count climbs, with nearly a seven-fold increased risk associated with nevi counts of 101 and higher (Euro. J. Ca. 2005;41:28-44).
Phenotype
Light-colored skin, hair, and eyes are associated with an increased melanoma risk. In 60 studies published before Sept. 2002, melanoma risk was twice as high with skin type I, compared with skin type IV; with fair skin color, compared with dark skin color; and with a high density rather than a low density of freckles. Compared with dark eyes, blue eyes were associated with a 47% higher risk. And melanoma risk was more than three times higher with red hair, compared with dark hair (Euro. J. Ca. 2005;41:28-44).
UV Exposure
Use of tanning beds increases melanoma risk 75% if the first exposure occurs before age 35 years, according to Dr. Grichnik. Multiple studies report that melanoma risk is higher in people who have experienced short, intense episodes of burning sun exposure (especially during childhood). The effects of chronic sun exposure are more difficult to determine, but may be associated with lentigo maligna-type melanoma.
Occupation
Airline crews, especially pilots, have a higher-than expected incidence of melanoma (BMJ 2002;325:567). This may be due to greater sun-exposed activities between flights in areas of the world with high sun exposure levels, he speculated.
Socioeconomics
Wealthier people are more likely to develop melanoma, compared with age-matched populations - the opposite of trends for squamous cell carcinoma, according to studies. Again, the higher risk for melanoma may be associated with sunny holidays in winter, and sun-exposed recreational activities enabled by affluence, Dr. Grichnik suggested.
History
Taking a good personal history can identify some of the risk factors listed above. Family history is important too - a significant proportion of patients with melanoma will have genetic risk factors for the disease, he said.
Thirteen percent of melanoma patients have at least one first-degree relative with melanoma (Hum. Genet. 2009;126:499-510). The disease appears to be twice as common in people who have a parent with a history of melanoma, three times as common if a sibling has had melanoma, and nine times as common if both a parent and a sibling have had melanoma, compared with people who have no family history of melanoma.
Once a melanoma is identifed, a variety of tools can help discern whether it is deadly, Dr. Grichnik added. These include the American Joint Committee on Cancer criteria for staging and classification of melanomas, the mitotic rate, nodal status, circulating tumor cell status, molecular marker status, and a history of prior nonmelanoma skin cancer or other cancers.
Dr. Grichnik is a founder and shareholder in DigitalDerm Inc. and has been a consultant and researcher for Electro-Optical Systems Inc. and Spectral Image Inc. SDEF and this news organization are owned by Elsevier.
Young girl having sun cream administered to her shoulder by her mother.
©Mark Swallow/iStockphoto.com
Not all patients are equal when it comes to melanoma risk.
Knowing which patients may be more likely to develop melanoma can serve as a prompt to increase suspicion during examination, said Dr. James M. Grichnik. Be on the lookout for patients with the following features and characteristics, he suggested at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
Nevi
The presence of even one atypical (dysplastic) nevus independently predicts up to a 60% greater risk for melanoma, said Dr. Grichnik, director of the melanoma program and professor of dermatology at the University of Miami.
Higher numbers of common nevi also increase risk. Nevi counts are associated with UV exposure, and can be used as a surrogate marker for UV-induced cutaneous damage. Melanoma risk increases 47% with 16-40 common nevi on the whole body, he noted. Risk increases as mole count climbs, with nearly a seven-fold increased risk associated with nevi counts of 101 and higher (Euro. J. Ca. 2005;41:28-44).
Phenotype
Light-colored skin, hair, and eyes are associated with an increased melanoma risk. In 60 studies published before Sept. 2002, melanoma risk was twice as high with skin type I, compared with skin type IV; with fair skin color, compared with dark skin color; and with a high density rather than a low density of freckles. Compared with dark eyes, blue eyes were associated with a 47% higher risk. And melanoma risk was more than three times higher with red hair, compared with dark hair (Euro. J. Ca. 2005;41:28-44).
UV Exposure
Use of tanning beds increases melanoma risk 75% if the first exposure occurs before age 35 years, according to Dr. Grichnik. Multiple studies report that melanoma risk is higher in people who have experienced short, intense episodes of burning sun exposure (especially during childhood). The effects of chronic sun exposure are more difficult to determine, but may be associated with lentigo maligna-type melanoma.
Occupation
Airline crews, especially pilots, have a higher-than expected incidence of melanoma (BMJ 2002;325:567). This may be due to greater sun-exposed activities between flights in areas of the world with high sun exposure levels, he speculated.
Socioeconomics
Wealthier people are more likely to develop melanoma, compared with age-matched populations - the opposite of trends for squamous cell carcinoma, according to studies. Again, the higher risk for melanoma may be associated with sunny holidays in winter, and sun-exposed recreational activities enabled by affluence, Dr. Grichnik suggested.
History
Taking a good personal history can identify some of the risk factors listed above. Family history is important too - a significant proportion of patients with melanoma will have genetic risk factors for the disease, he said.
Thirteen percent of melanoma patients have at least one first-degree relative with melanoma (Hum. Genet. 2009;126:499-510). The disease appears to be twice as common in people who have a parent with a history of melanoma, three times as common if a sibling has had melanoma, and nine times as common if both a parent and a sibling have had melanoma, compared with people who have no family history of melanoma.
Once a melanoma is identifed, a variety of tools can help discern whether it is deadly, Dr. Grichnik added. These include the American Joint Committee on Cancer criteria for staging and classification of melanomas, the mitotic rate, nodal status, circulating tumor cell status, molecular marker status, and a history of prior nonmelanoma skin cancer or other cancers.
Dr. Grichnik is a founder and shareholder in DigitalDerm Inc. and has been a consultant and researcher for Electro-Optical Systems Inc. and Spectral Image Inc. SDEF and this news organization are owned by Elsevier.
Young girl having sun cream administered to her shoulder by her mother.
©Mark Swallow/iStockphoto.com
Not all patients are equal when it comes to melanoma risk.
Knowing which patients may be more likely to develop melanoma can serve as a prompt to increase suspicion during examination, said Dr. James M. Grichnik. Be on the lookout for patients with the following features and characteristics, he suggested at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
Nevi
The presence of even one atypical (dysplastic) nevus independently predicts up to a 60% greater risk for melanoma, said Dr. Grichnik, director of the melanoma program and professor of dermatology at the University of Miami.
Higher numbers of common nevi also increase risk. Nevi counts are associated with UV exposure, and can be used as a surrogate marker for UV-induced cutaneous damage. Melanoma risk increases 47% with 16-40 common nevi on the whole body, he noted. Risk increases as mole count climbs, with nearly a seven-fold increased risk associated with nevi counts of 101 and higher (Euro. J. Ca. 2005;41:28-44).
Phenotype
Light-colored skin, hair, and eyes are associated with an increased melanoma risk. In 60 studies published before Sept. 2002, melanoma risk was twice as high with skin type I, compared with skin type IV; with fair skin color, compared with dark skin color; and with a high density rather than a low density of freckles. Compared with dark eyes, blue eyes were associated with a 47% higher risk. And melanoma risk was more than three times higher with red hair, compared with dark hair (Euro. J. Ca. 2005;41:28-44).
UV Exposure
Use of tanning beds increases melanoma risk 75% if the first exposure occurs before age 35 years, according to Dr. Grichnik. Multiple studies report that melanoma risk is higher in people who have experienced short, intense episodes of burning sun exposure (especially during childhood). The effects of chronic sun exposure are more difficult to determine, but may be associated with lentigo maligna-type melanoma.
Occupation
Airline crews, especially pilots, have a higher-than expected incidence of melanoma (BMJ 2002;325:567). This may be due to greater sun-exposed activities between flights in areas of the world with high sun exposure levels, he speculated.
Socioeconomics
Wealthier people are more likely to develop melanoma, compared with age-matched populations - the opposite of trends for squamous cell carcinoma, according to studies. Again, the higher risk for melanoma may be associated with sunny holidays in winter, and sun-exposed recreational activities enabled by affluence, Dr. Grichnik suggested.
History
Taking a good personal history can identify some of the risk factors listed above. Family history is important too - a significant proportion of patients with melanoma will have genetic risk factors for the disease, he said.
Thirteen percent of melanoma patients have at least one first-degree relative with melanoma (Hum. Genet. 2009;126:499-510). The disease appears to be twice as common in people who have a parent with a history of melanoma, three times as common if a sibling has had melanoma, and nine times as common if both a parent and a sibling have had melanoma, compared with people who have no family history of melanoma.
Once a melanoma is identifed, a variety of tools can help discern whether it is deadly, Dr. Grichnik added. These include the American Joint Committee on Cancer criteria for staging and classification of melanomas, the mitotic rate, nodal status, circulating tumor cell status, molecular marker status, and a history of prior nonmelanoma skin cancer or other cancers.
Dr. Grichnik is a founder and shareholder in DigitalDerm Inc. and has been a consultant and researcher for Electro-Optical Systems Inc. and Spectral Image Inc. SDEF and this news organization are owned by Elsevier.
Young girl having sun cream administered to her shoulder by her mother.
©Mark Swallow/iStockphoto.com
Ethnic Disparities in Malignant Melanoma
The lifetime risk of white patients developing melanoma is currently estimated at 1 in 50, compared with 1 in 1,000 for black patients. Although darker-pigmented populations are consistently reported to have lower melanoma risk--possibly related to protection from ultraviolet radiation provided by melanin--darker skinned patients have been consistently shown to have worse outcomes than whites.
While less common in blacks, malignant melanoma is nonetheless an aggressive, deadly disease and presents at a more advanced stage at diagnosis and with a decreased chance of survival than in white patints. Underlying etiologies for the disparity in prognosis are still poorly understood.
Many of the public health interventions for melanoma prevention have focused predominantly on whites. However, recent studies in blacks and Hispanics that have elucidated advanced stage of melanoma at presentation and a higher mortality rate have led to increased efforts to characterize melanoma in ethnic minorities.
One such study found that ethnic disparities persist in the incidence of melanoma and its stage at diagnosis (Arch Dermatol. 2009;145:1369-74). Researchers analyzed information in the Florida Cancer Data System, a statewide, population-based cancer registry. They looked at three 5-year periods: 1990 to 1994, 1995 to 1999, and 2000 to 2004.
Of 41,072 cases of melanoma diagnosed from 1990 to 2004, 39,670 were in whites, 1,148 in Hispanics, and 254 in blacks. Advanced melanoma diagnosed at either regional or distant stages were seen in 26.4% of black patients, compared with 17.8% of Hispanics and 11.6% of whites. Blacks and Hispanic patients had advanced cancer odd ratios of 2.7 and 1.6, respectively, compared with whites.
The authors of the study concluded that public health efforts are one of the primary sources of ethnic disparities. However the question still remains: is race (and in essence a more aggressive tumor biology) an independent prognostic indicator?
Multiple studies have elucidated worse prognosis in blacks even when controlling for socioeconomic status, stage, tumor thickness, ulceration, anatomic site, and histological type. This data has been refuted in other studies that show no survival advantage in whites; however, the controversy still remains.
This study is a much needed reminder that increased surveillance is needed, as well as more targeted public health efforts, prevention models, and educational programs specific to the culture and languages of ethnic groups. We also need to continue to investigate the unique genetic and biologic markers of melanoma in different ethnic populations to improve clinical detection and develop targeted therapies.
The lifetime risk of white patients developing melanoma is currently estimated at 1 in 50, compared with 1 in 1,000 for black patients. Although darker-pigmented populations are consistently reported to have lower melanoma risk--possibly related to protection from ultraviolet radiation provided by melanin--darker skinned patients have been consistently shown to have worse outcomes than whites.
While less common in blacks, malignant melanoma is nonetheless an aggressive, deadly disease and presents at a more advanced stage at diagnosis and with a decreased chance of survival than in white patints. Underlying etiologies for the disparity in prognosis are still poorly understood.
Many of the public health interventions for melanoma prevention have focused predominantly on whites. However, recent studies in blacks and Hispanics that have elucidated advanced stage of melanoma at presentation and a higher mortality rate have led to increased efforts to characterize melanoma in ethnic minorities.
One such study found that ethnic disparities persist in the incidence of melanoma and its stage at diagnosis (Arch Dermatol. 2009;145:1369-74). Researchers analyzed information in the Florida Cancer Data System, a statewide, population-based cancer registry. They looked at three 5-year periods: 1990 to 1994, 1995 to 1999, and 2000 to 2004.
Of 41,072 cases of melanoma diagnosed from 1990 to 2004, 39,670 were in whites, 1,148 in Hispanics, and 254 in blacks. Advanced melanoma diagnosed at either regional or distant stages were seen in 26.4% of black patients, compared with 17.8% of Hispanics and 11.6% of whites. Blacks and Hispanic patients had advanced cancer odd ratios of 2.7 and 1.6, respectively, compared with whites.
The authors of the study concluded that public health efforts are one of the primary sources of ethnic disparities. However the question still remains: is race (and in essence a more aggressive tumor biology) an independent prognostic indicator?
Multiple studies have elucidated worse prognosis in blacks even when controlling for socioeconomic status, stage, tumor thickness, ulceration, anatomic site, and histological type. This data has been refuted in other studies that show no survival advantage in whites; however, the controversy still remains.
This study is a much needed reminder that increased surveillance is needed, as well as more targeted public health efforts, prevention models, and educational programs specific to the culture and languages of ethnic groups. We also need to continue to investigate the unique genetic and biologic markers of melanoma in different ethnic populations to improve clinical detection and develop targeted therapies.
The lifetime risk of white patients developing melanoma is currently estimated at 1 in 50, compared with 1 in 1,000 for black patients. Although darker-pigmented populations are consistently reported to have lower melanoma risk--possibly related to protection from ultraviolet radiation provided by melanin--darker skinned patients have been consistently shown to have worse outcomes than whites.
While less common in blacks, malignant melanoma is nonetheless an aggressive, deadly disease and presents at a more advanced stage at diagnosis and with a decreased chance of survival than in white patints. Underlying etiologies for the disparity in prognosis are still poorly understood.
Many of the public health interventions for melanoma prevention have focused predominantly on whites. However, recent studies in blacks and Hispanics that have elucidated advanced stage of melanoma at presentation and a higher mortality rate have led to increased efforts to characterize melanoma in ethnic minorities.
One such study found that ethnic disparities persist in the incidence of melanoma and its stage at diagnosis (Arch Dermatol. 2009;145:1369-74). Researchers analyzed information in the Florida Cancer Data System, a statewide, population-based cancer registry. They looked at three 5-year periods: 1990 to 1994, 1995 to 1999, and 2000 to 2004.
Of 41,072 cases of melanoma diagnosed from 1990 to 2004, 39,670 were in whites, 1,148 in Hispanics, and 254 in blacks. Advanced melanoma diagnosed at either regional or distant stages were seen in 26.4% of black patients, compared with 17.8% of Hispanics and 11.6% of whites. Blacks and Hispanic patients had advanced cancer odd ratios of 2.7 and 1.6, respectively, compared with whites.
The authors of the study concluded that public health efforts are one of the primary sources of ethnic disparities. However the question still remains: is race (and in essence a more aggressive tumor biology) an independent prognostic indicator?
Multiple studies have elucidated worse prognosis in blacks even when controlling for socioeconomic status, stage, tumor thickness, ulceration, anatomic site, and histological type. This data has been refuted in other studies that show no survival advantage in whites; however, the controversy still remains.
This study is a much needed reminder that increased surveillance is needed, as well as more targeted public health efforts, prevention models, and educational programs specific to the culture and languages of ethnic groups. We also need to continue to investigate the unique genetic and biologic markers of melanoma in different ethnic populations to improve clinical detection and develop targeted therapies.
Multiple Melanomas in the Same Patient a Real Phenomenon
SAN DIEGO - The chances of a patient developing multiple primary melanomas over a lifetime is a real phenomenon, with an incidence ranging from 2% to 8% among patients who have had a first melanoma, or an average of about 5%.
Of patients who develop additional melanomas, about 80% develop one in addition to the original, 15% develop two, and the remainder develop three or more. "In my practice I have about four people I follow who have had five or six primary melanomas," said Dr. Burrows, who has practiced dermatology for nearly 40 years and is currently with the division of dermatology at Scripps Clinic Rancho Bernardo in San Diego. "We've become the primary care physicians for patients with melanoma."
He went on to note that the risk of multiple primary melanomas is twofold higher among men, and that the majority of subsequent primary melanomas (70%) occur on a different anatomical site, while 30% occur on the same site.
"They have the same distribution as melanomas in general," he said.
The majority of subsequent primary melanomas occur after 2 months, while 30% occur within 2 months or less.
Depth of invasion is similar to national statistics for all primary melanomas. "But the second primary melanoma tends to be thinner than the first one, which makes sense," Dr. Burrows commented. "After the first primary melanoma we raise our index of suspicion on lesions that are irregular. In addition, the patient has a significant level of worry."
Recognized risk factors for multiple melanomas include presence of atypical/dysplastic nevi, family history, and early age of onset.
According to a retrospective review of 1,258 melanoma patients treated at the Scripps Clinic between 1990 and 2000, 149 (12%) developed multiple primary lesions, which is more than double the national incidence. "This could be due to one of two things," Dr. Burrows said. "One is the criteria that are used in making the diagnosis of melanoma in situ. I wonder if we [at Scripps] diagnose melanoma in situ more often, as opposed to others who might sign it out as atypical melanocytic hyperplasia or another worrisome diagnosis."
The other possibility is that incidence of melanoma is rising. "We know that we are making the diagnosis of primary melanomas at a younger age than we did 20 years ago," Dr. Burrows said.
In the Scripps series, 75% of patients had two primary melanomas, 15% had three, and the remainder had four or more. The average age at initial primary melanoma diagnosis was 64 among men and 56 among women.
Nearly half of the patients (49%) developed subsequent melanomas less than 3 years after their initial primary melanoma diagnoses.
At this point, Dr. Burrows does not recommend testing for the CDKN2A and CDK4 gene mutations in most patients. "It's not a good screening tool for the general population or fair-skinned population with multiple nevi, but it has potential use in screening patients with a family history of melanoma," he said.
"You want to know if they have a 75%-100% risk of developing melanoma. But in my opinion, this test is not helpful in people who have had one primary melanoma. It doesn't pick out the people at risk of getting a second primary melanoma."
As for managing patients with multiple melanomas, a full skin exam during initial workup and follow-up intervals is essential, he said. "Follow-up should be lifelong."
Dr. Burrows had no conflicts to disclose.
(Image above is of Dr. William M. Burrows/Photo credit: Doug Brunk)
SAN DIEGO - The chances of a patient developing multiple primary melanomas over a lifetime is a real phenomenon, with an incidence ranging from 2% to 8% among patients who have had a first melanoma, or an average of about 5%.
Of patients who develop additional melanomas, about 80% develop one in addition to the original, 15% develop two, and the remainder develop three or more. "In my practice I have about four people I follow who have had five or six primary melanomas," said Dr. Burrows, who has practiced dermatology for nearly 40 years and is currently with the division of dermatology at Scripps Clinic Rancho Bernardo in San Diego. "We've become the primary care physicians for patients with melanoma."
He went on to note that the risk of multiple primary melanomas is twofold higher among men, and that the majority of subsequent primary melanomas (70%) occur on a different anatomical site, while 30% occur on the same site.
"They have the same distribution as melanomas in general," he said.
The majority of subsequent primary melanomas occur after 2 months, while 30% occur within 2 months or less.
Depth of invasion is similar to national statistics for all primary melanomas. "But the second primary melanoma tends to be thinner than the first one, which makes sense," Dr. Burrows commented. "After the first primary melanoma we raise our index of suspicion on lesions that are irregular. In addition, the patient has a significant level of worry."
Recognized risk factors for multiple melanomas include presence of atypical/dysplastic nevi, family history, and early age of onset.
According to a retrospective review of 1,258 melanoma patients treated at the Scripps Clinic between 1990 and 2000, 149 (12%) developed multiple primary lesions, which is more than double the national incidence. "This could be due to one of two things," Dr. Burrows said. "One is the criteria that are used in making the diagnosis of melanoma in situ. I wonder if we [at Scripps] diagnose melanoma in situ more often, as opposed to others who might sign it out as atypical melanocytic hyperplasia or another worrisome diagnosis."
The other possibility is that incidence of melanoma is rising. "We know that we are making the diagnosis of primary melanomas at a younger age than we did 20 years ago," Dr. Burrows said.
In the Scripps series, 75% of patients had two primary melanomas, 15% had three, and the remainder had four or more. The average age at initial primary melanoma diagnosis was 64 among men and 56 among women.
Nearly half of the patients (49%) developed subsequent melanomas less than 3 years after their initial primary melanoma diagnoses.
At this point, Dr. Burrows does not recommend testing for the CDKN2A and CDK4 gene mutations in most patients. "It's not a good screening tool for the general population or fair-skinned population with multiple nevi, but it has potential use in screening patients with a family history of melanoma," he said.
"You want to know if they have a 75%-100% risk of developing melanoma. But in my opinion, this test is not helpful in people who have had one primary melanoma. It doesn't pick out the people at risk of getting a second primary melanoma."
As for managing patients with multiple melanomas, a full skin exam during initial workup and follow-up intervals is essential, he said. "Follow-up should be lifelong."
Dr. Burrows had no conflicts to disclose.
(Image above is of Dr. William M. Burrows/Photo credit: Doug Brunk)
SAN DIEGO - The chances of a patient developing multiple primary melanomas over a lifetime is a real phenomenon, with an incidence ranging from 2% to 8% among patients who have had a first melanoma, or an average of about 5%.
Of patients who develop additional melanomas, about 80% develop one in addition to the original, 15% develop two, and the remainder develop three or more. "In my practice I have about four people I follow who have had five or six primary melanomas," said Dr. Burrows, who has practiced dermatology for nearly 40 years and is currently with the division of dermatology at Scripps Clinic Rancho Bernardo in San Diego. "We've become the primary care physicians for patients with melanoma."
He went on to note that the risk of multiple primary melanomas is twofold higher among men, and that the majority of subsequent primary melanomas (70%) occur on a different anatomical site, while 30% occur on the same site.
"They have the same distribution as melanomas in general," he said.
The majority of subsequent primary melanomas occur after 2 months, while 30% occur within 2 months or less.
Depth of invasion is similar to national statistics for all primary melanomas. "But the second primary melanoma tends to be thinner than the first one, which makes sense," Dr. Burrows commented. "After the first primary melanoma we raise our index of suspicion on lesions that are irregular. In addition, the patient has a significant level of worry."
Recognized risk factors for multiple melanomas include presence of atypical/dysplastic nevi, family history, and early age of onset.
According to a retrospective review of 1,258 melanoma patients treated at the Scripps Clinic between 1990 and 2000, 149 (12%) developed multiple primary lesions, which is more than double the national incidence. "This could be due to one of two things," Dr. Burrows said. "One is the criteria that are used in making the diagnosis of melanoma in situ. I wonder if we [at Scripps] diagnose melanoma in situ more often, as opposed to others who might sign it out as atypical melanocytic hyperplasia or another worrisome diagnosis."
The other possibility is that incidence of melanoma is rising. "We know that we are making the diagnosis of primary melanomas at a younger age than we did 20 years ago," Dr. Burrows said.
In the Scripps series, 75% of patients had two primary melanomas, 15% had three, and the remainder had four or more. The average age at initial primary melanoma diagnosis was 64 among men and 56 among women.
Nearly half of the patients (49%) developed subsequent melanomas less than 3 years after their initial primary melanoma diagnoses.
At this point, Dr. Burrows does not recommend testing for the CDKN2A and CDK4 gene mutations in most patients. "It's not a good screening tool for the general population or fair-skinned population with multiple nevi, but it has potential use in screening patients with a family history of melanoma," he said.
"You want to know if they have a 75%-100% risk of developing melanoma. But in my opinion, this test is not helpful in people who have had one primary melanoma. It doesn't pick out the people at risk of getting a second primary melanoma."
As for managing patients with multiple melanomas, a full skin exam during initial workup and follow-up intervals is essential, he said. "Follow-up should be lifelong."
Dr. Burrows had no conflicts to disclose.
(Image above is of Dr. William M. Burrows/Photo credit: Doug Brunk)
Expert Recommends Snapping Photos of Every Lesion Biopsied
SAN DIEGO — The way Dr. Charles H. Miller sees it, dermatologists should have a digital camera at the ready to take photos of every lesion they biopsy.
"You should jump in and start doing this as part of your daily practice, because the future of dermatology is going to be significantly impacted by how we use digital images," Dr. Miller, chief of dermatology at Kaiser Permanente, San Diego, said at a melanoma update sponsored by the Scripps Clinic.
He and his associates routinely send along digital images of relevant lesions when they refer their patients to other specialists. "Anytime we send a patient for Mohs surgery, head and neck surgery, general surgery, or plastic surgery, we'll upload photos," he explained. "The surgeons love it because they can get an idea of what to expect before they see the patient. They're well prepared for what's to come, oftentimes saving a preop appointment."
He emphasized the importance of choosing a digital camera that fits you and your style of practice. Some can be had for less than $200, but the most important characteristic of a camera is not price. It's "that it's one you'll use often," Dr. Miller said.
"Some people wear white coats; others don't. Some people see 60-80 patients a day; other people see 20 a day. If you're seeing a lot more than 30 patients a day, make sure you have the camera with you at all times or else you're just not going to take the time to snap a picture."
In general, digital cameras with a resolution of at least 11 megapixels (MP) take pictures that are just as clear as those taken with the old 35-mm Kodachrome film technology. And when viewed on computer monitors, photos of much lower resolutions are acceptable. He divided his favorite cameras currently on the market into one of three groups based on size: "shirt pocket," "pants pocket," and "white-coat pocket" cameras "The actual measurements won't vary much," just an inch or so, "but it's enough" to make a difference, Dr. Miller said. Prices are approximate.
His recommended shirt-pocket cameras, which are "usually no bigger than a deck of cards," include the Canon SD790 (8 MP, $225), the Nikon S210 (8 MP, $150), the Sony T700 (10 MP, $350), and the Panasonic FX37 (10 MP, $270).
His recommended pants-pocket cameras include the Fujifilm 200EXR (12 MP, $360), the Canon A470 (8 MP, $145), the Canon S90 (10 MP, $399), the Canon SD780 IS (12 MP, $250), the Panasonic FX150 (15 MP, $300), and the Sony W300 (14 MP, $300).
Dr. Miller's recommended white coat-pocket cameras include the Panasonic LX3 (10 MP, $425), the Nikon P6000 (14 MP, $450), and the Canon G11 (10 MP, $459).
For even higher resolution, consider digital single lens reflex (DSLR) cameras, which accept macro lenses that are more optically correct and have less distortion than lenses in the smaller cameras listed above. Dr. Miller's suggested cameras in this category include the Nikon D40x (10 MP, $499), the Canon EOS Rebel T1i (15 MP, $720), the Nikon D90 (12 MP, $900), and the Nikon D700 (12 MP, $2,450).
"A lot of these cameras now include video capability as well," he noted. "That's something that dermatologists will start to consider in the future. It may be easier to take a quick video of a patient if it's at high-enough resolution, as opposed to taking single pictures. But that's something that needs to be more thoroughly evaluated."
Dr. Miller also advises dermatologists to think of digital cameras as disposable items that they'll want to replace every 3-5 years. "Once you get into that mind-set, it's not nearly as hard to jump in, because you realize that every few years you're going to grant yourself the benefit of upgrading," he said. He is on his sixth digital camera in 13 years.
Disclosures: Dr. Miller had no relevant conflicts to disclose.
SAN DIEGO — The way Dr. Charles H. Miller sees it, dermatologists should have a digital camera at the ready to take photos of every lesion they biopsy.
"You should jump in and start doing this as part of your daily practice, because the future of dermatology is going to be significantly impacted by how we use digital images," Dr. Miller, chief of dermatology at Kaiser Permanente, San Diego, said at a melanoma update sponsored by the Scripps Clinic.
He and his associates routinely send along digital images of relevant lesions when they refer their patients to other specialists. "Anytime we send a patient for Mohs surgery, head and neck surgery, general surgery, or plastic surgery, we'll upload photos," he explained. "The surgeons love it because they can get an idea of what to expect before they see the patient. They're well prepared for what's to come, oftentimes saving a preop appointment."
He emphasized the importance of choosing a digital camera that fits you and your style of practice. Some can be had for less than $200, but the most important characteristic of a camera is not price. It's "that it's one you'll use often," Dr. Miller said.
"Some people wear white coats; others don't. Some people see 60-80 patients a day; other people see 20 a day. If you're seeing a lot more than 30 patients a day, make sure you have the camera with you at all times or else you're just not going to take the time to snap a picture."
In general, digital cameras with a resolution of at least 11 megapixels (MP) take pictures that are just as clear as those taken with the old 35-mm Kodachrome film technology. And when viewed on computer monitors, photos of much lower resolutions are acceptable. He divided his favorite cameras currently on the market into one of three groups based on size: "shirt pocket," "pants pocket," and "white-coat pocket" cameras "The actual measurements won't vary much," just an inch or so, "but it's enough" to make a difference, Dr. Miller said. Prices are approximate.
His recommended shirt-pocket cameras, which are "usually no bigger than a deck of cards," include the Canon SD790 (8 MP, $225), the Nikon S210 (8 MP, $150), the Sony T700 (10 MP, $350), and the Panasonic FX37 (10 MP, $270).
His recommended pants-pocket cameras include the Fujifilm 200EXR (12 MP, $360), the Canon A470 (8 MP, $145), the Canon S90 (10 MP, $399), the Canon SD780 IS (12 MP, $250), the Panasonic FX150 (15 MP, $300), and the Sony W300 (14 MP, $300).
Dr. Miller's recommended white coat-pocket cameras include the Panasonic LX3 (10 MP, $425), the Nikon P6000 (14 MP, $450), and the Canon G11 (10 MP, $459).
For even higher resolution, consider digital single lens reflex (DSLR) cameras, which accept macro lenses that are more optically correct and have less distortion than lenses in the smaller cameras listed above. Dr. Miller's suggested cameras in this category include the Nikon D40x (10 MP, $499), the Canon EOS Rebel T1i (15 MP, $720), the Nikon D90 (12 MP, $900), and the Nikon D700 (12 MP, $2,450).
"A lot of these cameras now include video capability as well," he noted. "That's something that dermatologists will start to consider in the future. It may be easier to take a quick video of a patient if it's at high-enough resolution, as opposed to taking single pictures. But that's something that needs to be more thoroughly evaluated."
Dr. Miller also advises dermatologists to think of digital cameras as disposable items that they'll want to replace every 3-5 years. "Once you get into that mind-set, it's not nearly as hard to jump in, because you realize that every few years you're going to grant yourself the benefit of upgrading," he said. He is on his sixth digital camera in 13 years.
Disclosures: Dr. Miller had no relevant conflicts to disclose.
SAN DIEGO — The way Dr. Charles H. Miller sees it, dermatologists should have a digital camera at the ready to take photos of every lesion they biopsy.
"You should jump in and start doing this as part of your daily practice, because the future of dermatology is going to be significantly impacted by how we use digital images," Dr. Miller, chief of dermatology at Kaiser Permanente, San Diego, said at a melanoma update sponsored by the Scripps Clinic.
He and his associates routinely send along digital images of relevant lesions when they refer their patients to other specialists. "Anytime we send a patient for Mohs surgery, head and neck surgery, general surgery, or plastic surgery, we'll upload photos," he explained. "The surgeons love it because they can get an idea of what to expect before they see the patient. They're well prepared for what's to come, oftentimes saving a preop appointment."
He emphasized the importance of choosing a digital camera that fits you and your style of practice. Some can be had for less than $200, but the most important characteristic of a camera is not price. It's "that it's one you'll use often," Dr. Miller said.
"Some people wear white coats; others don't. Some people see 60-80 patients a day; other people see 20 a day. If you're seeing a lot more than 30 patients a day, make sure you have the camera with you at all times or else you're just not going to take the time to snap a picture."
In general, digital cameras with a resolution of at least 11 megapixels (MP) take pictures that are just as clear as those taken with the old 35-mm Kodachrome film technology. And when viewed on computer monitors, photos of much lower resolutions are acceptable. He divided his favorite cameras currently on the market into one of three groups based on size: "shirt pocket," "pants pocket," and "white-coat pocket" cameras "The actual measurements won't vary much," just an inch or so, "but it's enough" to make a difference, Dr. Miller said. Prices are approximate.
His recommended shirt-pocket cameras, which are "usually no bigger than a deck of cards," include the Canon SD790 (8 MP, $225), the Nikon S210 (8 MP, $150), the Sony T700 (10 MP, $350), and the Panasonic FX37 (10 MP, $270).
His recommended pants-pocket cameras include the Fujifilm 200EXR (12 MP, $360), the Canon A470 (8 MP, $145), the Canon S90 (10 MP, $399), the Canon SD780 IS (12 MP, $250), the Panasonic FX150 (15 MP, $300), and the Sony W300 (14 MP, $300).
Dr. Miller's recommended white coat-pocket cameras include the Panasonic LX3 (10 MP, $425), the Nikon P6000 (14 MP, $450), and the Canon G11 (10 MP, $459).
For even higher resolution, consider digital single lens reflex (DSLR) cameras, which accept macro lenses that are more optically correct and have less distortion than lenses in the smaller cameras listed above. Dr. Miller's suggested cameras in this category include the Nikon D40x (10 MP, $499), the Canon EOS Rebel T1i (15 MP, $720), the Nikon D90 (12 MP, $900), and the Nikon D700 (12 MP, $2,450).
"A lot of these cameras now include video capability as well," he noted. "That's something that dermatologists will start to consider in the future. It may be easier to take a quick video of a patient if it's at high-enough resolution, as opposed to taking single pictures. But that's something that needs to be more thoroughly evaluated."
Dr. Miller also advises dermatologists to think of digital cameras as disposable items that they'll want to replace every 3-5 years. "Once you get into that mind-set, it's not nearly as hard to jump in, because you realize that every few years you're going to grant yourself the benefit of upgrading," he said. He is on his sixth digital camera in 13 years.
Disclosures: Dr. Miller had no relevant conflicts to disclose.
Presidential Conspiracy Alert!
Malignant melanoma killed FDR! That is the working theory powering a book out this year entitled “FDR’s Deadly Secret” (New York: PublicAffairs, 2010), by Dr. Steven Lomazow and Eric Fettmann.
His heavy drinking was legion. Only British Prime Minister Winston Churchill seemed able to drink him under the table. And it would take more than the laps he swam in the White House pool and at his “Little White House” retreat in Warm Springs, Ga., to make up for how sedentary his life became after he was diagnosed with polio.
And let’s not even talk about his diet, which was a recipe for cerebrovascular disease. But, according to the authors, his weight loss and generally increasingly frail appearance in the final months of his life was not because of a combination of the stress induced by years of world war and poor lifestyle choices. Rather, if one accepts the book’s premises, FDR’s rapid decline was due to the metastasis of a melanoma from over his left eye brow to his brain and bowel.
The putative melanoma first appeared in photos in the 1920s, grew in size throughout the 1930s, and disappeared from view in 1940, by which time it was large enough to not look out of place over Aaron Neville’s eyebrow.
What’s the basis for this call to rewrite medical history? It’s not FDR’s medical records, which disappeared decades ago. And his personal physician never spoke of his patient’s rapid decline. Dr. Lomazow, a neurologist, and Mr. Fettmann, an editor at the New York Post, based their diagnosis on diary notes of people who worked in the White House.
Whatever the truth may be, never has this blog—“The Mole” —been more aptly named.
Sally Kubetin
Senior Editor
Photo courtesy Center for the Study of Intelligence, Central Intelligence Agency
Malignant melanoma killed FDR! That is the working theory powering a book out this year entitled “FDR’s Deadly Secret” (New York: PublicAffairs, 2010), by Dr. Steven Lomazow and Eric Fettmann.
His heavy drinking was legion. Only British Prime Minister Winston Churchill seemed able to drink him under the table. And it would take more than the laps he swam in the White House pool and at his “Little White House” retreat in Warm Springs, Ga., to make up for how sedentary his life became after he was diagnosed with polio.
And let’s not even talk about his diet, which was a recipe for cerebrovascular disease. But, according to the authors, his weight loss and generally increasingly frail appearance in the final months of his life was not because of a combination of the stress induced by years of world war and poor lifestyle choices. Rather, if one accepts the book’s premises, FDR’s rapid decline was due to the metastasis of a melanoma from over his left eye brow to his brain and bowel.
The putative melanoma first appeared in photos in the 1920s, grew in size throughout the 1930s, and disappeared from view in 1940, by which time it was large enough to not look out of place over Aaron Neville’s eyebrow.
What’s the basis for this call to rewrite medical history? It’s not FDR’s medical records, which disappeared decades ago. And his personal physician never spoke of his patient’s rapid decline. Dr. Lomazow, a neurologist, and Mr. Fettmann, an editor at the New York Post, based their diagnosis on diary notes of people who worked in the White House.
Whatever the truth may be, never has this blog—“The Mole” —been more aptly named.
Sally Kubetin
Senior Editor
Photo courtesy Center for the Study of Intelligence, Central Intelligence Agency
Malignant melanoma killed FDR! That is the working theory powering a book out this year entitled “FDR’s Deadly Secret” (New York: PublicAffairs, 2010), by Dr. Steven Lomazow and Eric Fettmann.
His heavy drinking was legion. Only British Prime Minister Winston Churchill seemed able to drink him under the table. And it would take more than the laps he swam in the White House pool and at his “Little White House” retreat in Warm Springs, Ga., to make up for how sedentary his life became after he was diagnosed with polio.
And let’s not even talk about his diet, which was a recipe for cerebrovascular disease. But, according to the authors, his weight loss and generally increasingly frail appearance in the final months of his life was not because of a combination of the stress induced by years of world war and poor lifestyle choices. Rather, if one accepts the book’s premises, FDR’s rapid decline was due to the metastasis of a melanoma from over his left eye brow to his brain and bowel.
The putative melanoma first appeared in photos in the 1920s, grew in size throughout the 1930s, and disappeared from view in 1940, by which time it was large enough to not look out of place over Aaron Neville’s eyebrow.
What’s the basis for this call to rewrite medical history? It’s not FDR’s medical records, which disappeared decades ago. And his personal physician never spoke of his patient’s rapid decline. Dr. Lomazow, a neurologist, and Mr. Fettmann, an editor at the New York Post, based their diagnosis on diary notes of people who worked in the White House.
Whatever the truth may be, never has this blog—“The Mole” —been more aptly named.
Sally Kubetin
Senior Editor
Photo courtesy Center for the Study of Intelligence, Central Intelligence Agency
Facial Skin Damage from Sun Exposure and an Unsuccessful Cosmetic Procedure
What Is Your Diagnosis? Metastatic Adenocarcinoma
Indoor Tanning: Just Say No [editorial]
Architectural Features Help Establish Melanoma Diagnosis
SAN DIEGO — Many times the most important feature in establishing the correct diagnosis of melanoma is evaluating the lesion's overall growth pattern, referred to as its architecture or silhouette, said Dr. David J. Barnette Jr.
"These architectural features include size, symmetry, and circumscription," Dr. Barnette, said at a melanoma update sponsored by the Scripps Clinic. "Accurate prognosis relies on assessing tumor depth, whereas making the correct diagnosis may require evaluation of the lateral aspects of the lesion."
After you perform a biopsy and send it to a pathologist, the results should fall under one of three categories: "benign"- a nevus or one of its variants; "malignant"- a melanoma; or "not sure"- either the diagnosis or biologic behavior of the lesion is unknown, said Dr. Barnette, a dermatopathologist and dermatologist at the Scripps Clinic, La Jolla, Calif. A pathology report may convey the latter diagnosis with terms such as atypical nevus, atypical melanocytic lesion, atypical melanocytosis, atypical melanocytic hyperplasia (AMH), or melanocytic tumor of uncertain malignant potential.
"These terms are not problematic if the clinician and pathologist are on the same page as to what they mean and how to best treat the patient," Dr. Barnette said, noting that he and his associates use the term AMH when the diagnosis is unclear.
"If the lesion is not completely excised, we include a recommendation for re-excision," he said. "This is appropriate treatment for either an atypical nevus or an evolving melanoma in situ. Communication between the pathologist and clinician and good clinicopathologic correlation are critical in preventing over- and under-treatment."
A biopsy report for an established diagnosis of melanoma should include a comment regarding its Breslow tumor depth, mitotic rate, presence or ulceration if applicable, and an assessment of the surgical margins, especially if an excisional biopsy was performed. Other elements to consider including in a biopsy report include site of lesion, type of biopsy, presence of angiolymphatic invasion (if present), margin status, melanoma subtype, regression (if present), and host response, including plasma cells and tumor-infiltrating lymphocytes (if present).
In November 2003, the Association of Directors of Anatomic and Surgical Pathology established a diagnostic checklist for skin melanoma.
Dr. Barnette disclosed no conflicts of interests.
SAN DIEGO — Many times the most important feature in establishing the correct diagnosis of melanoma is evaluating the lesion's overall growth pattern, referred to as its architecture or silhouette, said Dr. David J. Barnette Jr.
"These architectural features include size, symmetry, and circumscription," Dr. Barnette, said at a melanoma update sponsored by the Scripps Clinic. "Accurate prognosis relies on assessing tumor depth, whereas making the correct diagnosis may require evaluation of the lateral aspects of the lesion."
After you perform a biopsy and send it to a pathologist, the results should fall under one of three categories: "benign"- a nevus or one of its variants; "malignant"- a melanoma; or "not sure"- either the diagnosis or biologic behavior of the lesion is unknown, said Dr. Barnette, a dermatopathologist and dermatologist at the Scripps Clinic, La Jolla, Calif. A pathology report may convey the latter diagnosis with terms such as atypical nevus, atypical melanocytic lesion, atypical melanocytosis, atypical melanocytic hyperplasia (AMH), or melanocytic tumor of uncertain malignant potential.
"These terms are not problematic if the clinician and pathologist are on the same page as to what they mean and how to best treat the patient," Dr. Barnette said, noting that he and his associates use the term AMH when the diagnosis is unclear.
"If the lesion is not completely excised, we include a recommendation for re-excision," he said. "This is appropriate treatment for either an atypical nevus or an evolving melanoma in situ. Communication between the pathologist and clinician and good clinicopathologic correlation are critical in preventing over- and under-treatment."
A biopsy report for an established diagnosis of melanoma should include a comment regarding its Breslow tumor depth, mitotic rate, presence or ulceration if applicable, and an assessment of the surgical margins, especially if an excisional biopsy was performed. Other elements to consider including in a biopsy report include site of lesion, type of biopsy, presence of angiolymphatic invasion (if present), margin status, melanoma subtype, regression (if present), and host response, including plasma cells and tumor-infiltrating lymphocytes (if present).
In November 2003, the Association of Directors of Anatomic and Surgical Pathology established a diagnostic checklist for skin melanoma.
Dr. Barnette disclosed no conflicts of interests.
SAN DIEGO — Many times the most important feature in establishing the correct diagnosis of melanoma is evaluating the lesion's overall growth pattern, referred to as its architecture or silhouette, said Dr. David J. Barnette Jr.
"These architectural features include size, symmetry, and circumscription," Dr. Barnette, said at a melanoma update sponsored by the Scripps Clinic. "Accurate prognosis relies on assessing tumor depth, whereas making the correct diagnosis may require evaluation of the lateral aspects of the lesion."
After you perform a biopsy and send it to a pathologist, the results should fall under one of three categories: "benign"- a nevus or one of its variants; "malignant"- a melanoma; or "not sure"- either the diagnosis or biologic behavior of the lesion is unknown, said Dr. Barnette, a dermatopathologist and dermatologist at the Scripps Clinic, La Jolla, Calif. A pathology report may convey the latter diagnosis with terms such as atypical nevus, atypical melanocytic lesion, atypical melanocytosis, atypical melanocytic hyperplasia (AMH), or melanocytic tumor of uncertain malignant potential.
"These terms are not problematic if the clinician and pathologist are on the same page as to what they mean and how to best treat the patient," Dr. Barnette said, noting that he and his associates use the term AMH when the diagnosis is unclear.
"If the lesion is not completely excised, we include a recommendation for re-excision," he said. "This is appropriate treatment for either an atypical nevus or an evolving melanoma in situ. Communication between the pathologist and clinician and good clinicopathologic correlation are critical in preventing over- and under-treatment."
A biopsy report for an established diagnosis of melanoma should include a comment regarding its Breslow tumor depth, mitotic rate, presence or ulceration if applicable, and an assessment of the surgical margins, especially if an excisional biopsy was performed. Other elements to consider including in a biopsy report include site of lesion, type of biopsy, presence of angiolymphatic invasion (if present), margin status, melanoma subtype, regression (if present), and host response, including plasma cells and tumor-infiltrating lymphocytes (if present).
In November 2003, the Association of Directors of Anatomic and Surgical Pathology established a diagnostic checklist for skin melanoma.
Dr. Barnette disclosed no conflicts of interests.