User login
Nonhormonal drug fezolinetant found safe for hot flashes in yearlong study
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
FROM NAMS 2022
Menopause symptoms negatively affect women’s work
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
FROM NAMS 2022
Poor evidence for vaginal laser therapy
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
FROM NAMS 2022
Hormone changes: The star of every stage in women’s sleep
MADRID – Because of the hormone changes that occur throughout their lives, women experience sleep problems that differ significantly from those experienced by men. Indeed, 75%-84% of pregnant women don’t sleep well during the third trimester, and up to 80% of women in menopause have symptoms that prevent them from getting a good night’s rest. For those seeking a precision medicine approach, the challenge is to identify the relationship between the different sex-related phenotypes and the sleep conditions.
Irene Cano, MD, PhD, is the coordinator of the sleep department at the Spanish Society of Pulmonology and Thoracic Surgery. She spoke with this news organization about the significant impact of hormones on sleep disorders in women.
“Reproductive hormones like estrogen and progesterone play a meaningful role in brain functions – not only those linked to the regulation of reproduction but also other physiological processes related to the regulation of circadian rhythms, cognitive performance, mood, and sleep. In addition, other hormones – for example, prolactin, growth hormone, cortisol, and melatonin – have sex-dependent effects on sleep,” Dr. Cano said.
Girls start puberty at a younger age than boys. As girls enter adolescence, they go to bed later and waking up earlier. So, girls are getting less than the 10 hours of sleep that they should be getting at this stage of life. The result is sleep debt, which gives rise to various problems: poor academic performance, ADHD, obesity, and metabolic problems, to name a few. As Ariadna Farré, RN, a sleep unit nurse, noted at SEPAR’s Joint Winter Meeting, “schools would have to start morning classes later to get adolescents to perform well academically. As the situation is now, half of the kids are falling asleep at their desks.”
Influencing sleep quality
Dr. Cano explained the issue as follows: “In adolescence, along with changes in young women’s hormone levels, we begin to see differences between the sexes. The changes in levels of estrogens and progesterone are what’s responsible for the changes that, to some extent, cause those disturbances in the quality of our sleep and in the stages of our sleep.”
Thus, sleep can be affected by the changes in hormone level that occur during a menstrual cycle. Estrogens, which increase during the follicular phase, are associated with REM sleep, while progesterone, which increases during the luteal phase, increases non-REM sleep. “In the 3-6 days prior to menstruation, it’s quite common for a woman to report difficulties falling asleep and staying asleep, in connection with a decline in the percentage of time she spends in REM sleep, in the context of premenstrual syndrome. In addition,” Dr. Cano pointed out, “menstrual bleeding, that loss of blood, is associated with a drop in iron levels, making it more likely that the woman will experience restless legs syndrome.”
Cardiovascular system
This news organization also spoke with Milagros Merino, MD, PhD, president of the Spanish Sleep Society. “The consequences that lack of sleep have on the cardiovascular system – we’re essentially talking about certain arrhythmias, high blood pressure, thrombosis in some cases, stroke, and heart attack. Lack of sleep also gives rise to endocrine and metabolic issues, like overweight and being at a greater risk of developing diabetes. And as for mental health, we see, among other things, attention and memory problems, emotional lability, and irascibility. Numerous studies have confirmed all of this.”
Sleep apnea also deserves mention, Dr. Merino added. “Although this disorder is more common in men, we’re seeing it more and more now in women, along with the cardiovascular issues that it brings about.”
Another cardiovascular risk factor is insomnia, said Dr. Merino. “This sleep disorder is more prevalent in women. As hormones constantly change, the ways women sleep constantly change, from one stage of life to the next. They sleep one way in childhood, another way in adolescence, and yet another way in menopause.”
Sleep in pregnancy
During pregnancy, hormone changes are much more pronounced. During the first trimester, progesterone levels increase, making the woman drowsy. On top of that, her sleep is interrupted by more frequent visits to the bathroom as well as greater general discomfort.
In the second trimester, sleep interruptions persist but are not as bad as they were during the first 3 months. In the third trimester, 75%-84% of pregnant women find it difficult to sleep because of aches and pains, the need to urinate during the night, cramps, and heartburn.
“Major physical changes are happening. When the bladder gets compressed, the woman has to get up and go to the bathroom. There’s an interruption in her sleep,” Ms. Farré explained. In addition, as the pregnancy progresses, the woman gains weight and her body mass index (BMI) increases, which can bring on obstructive sleep apnea, high blood pressure, preeclampsia, and diabetes, if not closely monitored.
Other factors include concomitant treatments, such as contraceptives, and the stages of life, such as pregnancy and lactation. “When a woman of childbearing age has restless legs syndrome, more often than not, this means that she has an iron deficiency that needs to be treated with oral iron supplements,” said Dr. Merino. “However, there are few medications that can be given to a pregnant woman – and RLS is relatively common during pregnancy. So, we have to turn to oral or intravenous iron supplements. Yet another matter is narcolepsy. In these cases, all medications have to be stopped during pregnancy and lactation, as they can be harmful to the baby.”
Sleep apnea
While one in five menopausal women are asymptomatic, the others experience mild to severe symptoms of apnea that frequently interrupt their sleep. In this stage of life, which begins around age 50 years, the hormones that had provided protection against sleep disruptions start to decrease. As a result, there is a rise in sleep problems, especially insomnia, breathing-related sleep disorders (for example, apnea), and restless legs syndrome.
The prevalence of breathing-related sleep disorders during menopause is attributable to weight gain, the drop in levels of estrogens, and the redistribution of adipose tissue in the body. Other factors also increase a woman’s risk of experiencing apnea. They range from stress, depression, and other psychological and psychiatric conditions to health status, medication use, and simply the fact of getting older. “Sleep apnea is more common in men than in premenopausal women. The numbers even out, though, when we compare men against menopausal women,” Dr. Cano noted.
In women, symptoms of sleep apnea are frequently attributed to menopause. There is some overlap: insomnia, headache, irritability, low mood, decreased libido, fatigue during the day, and feeling sleepy. Only much later is the woman’s condition correctly diagnosed as sleep apnea. So, even though presenting with the same complaints, a man will be diagnosed with sleep apnea sooner than a woman will – in some cases, around 10 years sooner.
“On the other hand, we’d always thought that, in menopause, insomnia was characterized by awakenings occurring throughout the second half of the night. But perhaps what happens more often is that women are regularly waking up repeatedly over the course of the entire night, as opposed to experiencing a wakefulness that starts early and lasts throughout the night or having a problem falling asleep to begin with,” said Dr. Merino. “The good news is that hormone replacement therapy can get things back to the way they were. And getting better sleep will help to overcome insomnia.”
Socioeconomic status
Insomnia is the most common sleep disorder. It affects 10%-20% of people, mostly women. “The fact that sleep problems are more prevalent in women can be explained by the fact that among women, there is a higher incidence of conditions that disrupt sleep, such as depression,” said Dr. Cano.
“Insomnia is much more common in adult women than adult men. And at menopause, women find that the insomnia only gets worse,” Dr. Merino added. “But around that same age, 50 years old, what we start to see more frequently in men is REM sleep behavior disorder, a type of parasomnia that’s a risk marker of degenerative nerve diseases.”
Dr. Cano emphasized one finding that, though basic, is not well known. “After adjusting for socioeconomic characteristics, the difference between the sexes in reporting sleep problems is cut in half. This suggests that an important factor that explains why there are differences in sleep problems between the sexes is that women’s socioeconomic status is generally lower than men’s.
“As for sleep apnea in particular,” Dr. Cano continued, “the kinds of symptoms that women have can be different from the classic ones seen in men – snoring, pauses in breathing, and daytime sleepiness; women are being underdiagnosed, and when they are diagnosed, that’s happening at a later age and at a higher BMI.”
So, it’s alarming that, as reported by SEPAR, 90% of women with obstructive sleep apnea are not being diagnosed.
Precision medicine approach
“The majority of research studies on sleep apnea have focused on men – given the prevalence of cases – and the results have been extrapolated to women. This is why there’s still a lot of work to be done in terms of better defining the characteristics specific to each sleep disorder and how they relate to each sex,” said Dr. Cano. “Being able to identify the relationship between the different sex-related phenotypes and each condition will allow us to take a precision medicine approach tailored to a patient’s particular characteristics.”
As Dr. Merino put it: “The approach to sleep disorders is always personalized. The patient’s sex, in and of itself, doesn’t have that great of an impact on this approach. What does have a great impact are women’s life stages. There are some subtle differences here and there, such as types of continuous positive airway pressure machines. The ones that are designed for women have masks that are better suited to their facial features, which differ from men’s.”
A precision medicine approach can be taken to treat any sleep disorder. For insomnia, the approach allows healthcare professionals to employ an appropriate cognitive-behavioral therapy plan or to determine which drugs would be more effective – all on the basis of symptoms and the characteristics of the particular case. Regarding sleep apnea, Dr. Cano explained, “taking into account the different anatomical characteristics or the higher prevalence of positional apnea will also allow us to offer different therapeutic alternatives to continuous positive airway pressure, such as mandibular advancement devices or positional therapy devices.”
Women should be encouraged to develop good sleep habits. These include taking circadian rhythms into account and aligning lifestyles accordingly. It also means going to bed earlier than the men in the household. For menopausal women, recommended sleep habits range from keeping their bedroom at an ideal temperature, following a diet rich in vegetables to avoid becoming overweight, and exercising daily. While this advice may be more applicable to teenagers, adults can benefit from it as well: Electronic devices should be turned off well before bedtime. Whether from a phone screen, a tablet screen, or a TV screen, the light emitted can keep one awake, which can be harmful to one’s health.
Dr. Cano and Dr. Merino disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MADRID – Because of the hormone changes that occur throughout their lives, women experience sleep problems that differ significantly from those experienced by men. Indeed, 75%-84% of pregnant women don’t sleep well during the third trimester, and up to 80% of women in menopause have symptoms that prevent them from getting a good night’s rest. For those seeking a precision medicine approach, the challenge is to identify the relationship between the different sex-related phenotypes and the sleep conditions.
Irene Cano, MD, PhD, is the coordinator of the sleep department at the Spanish Society of Pulmonology and Thoracic Surgery. She spoke with this news organization about the significant impact of hormones on sleep disorders in women.
“Reproductive hormones like estrogen and progesterone play a meaningful role in brain functions – not only those linked to the regulation of reproduction but also other physiological processes related to the regulation of circadian rhythms, cognitive performance, mood, and sleep. In addition, other hormones – for example, prolactin, growth hormone, cortisol, and melatonin – have sex-dependent effects on sleep,” Dr. Cano said.
Girls start puberty at a younger age than boys. As girls enter adolescence, they go to bed later and waking up earlier. So, girls are getting less than the 10 hours of sleep that they should be getting at this stage of life. The result is sleep debt, which gives rise to various problems: poor academic performance, ADHD, obesity, and metabolic problems, to name a few. As Ariadna Farré, RN, a sleep unit nurse, noted at SEPAR’s Joint Winter Meeting, “schools would have to start morning classes later to get adolescents to perform well academically. As the situation is now, half of the kids are falling asleep at their desks.”
Influencing sleep quality
Dr. Cano explained the issue as follows: “In adolescence, along with changes in young women’s hormone levels, we begin to see differences between the sexes. The changes in levels of estrogens and progesterone are what’s responsible for the changes that, to some extent, cause those disturbances in the quality of our sleep and in the stages of our sleep.”
Thus, sleep can be affected by the changes in hormone level that occur during a menstrual cycle. Estrogens, which increase during the follicular phase, are associated with REM sleep, while progesterone, which increases during the luteal phase, increases non-REM sleep. “In the 3-6 days prior to menstruation, it’s quite common for a woman to report difficulties falling asleep and staying asleep, in connection with a decline in the percentage of time she spends in REM sleep, in the context of premenstrual syndrome. In addition,” Dr. Cano pointed out, “menstrual bleeding, that loss of blood, is associated with a drop in iron levels, making it more likely that the woman will experience restless legs syndrome.”
Cardiovascular system
This news organization also spoke with Milagros Merino, MD, PhD, president of the Spanish Sleep Society. “The consequences that lack of sleep have on the cardiovascular system – we’re essentially talking about certain arrhythmias, high blood pressure, thrombosis in some cases, stroke, and heart attack. Lack of sleep also gives rise to endocrine and metabolic issues, like overweight and being at a greater risk of developing diabetes. And as for mental health, we see, among other things, attention and memory problems, emotional lability, and irascibility. Numerous studies have confirmed all of this.”
Sleep apnea also deserves mention, Dr. Merino added. “Although this disorder is more common in men, we’re seeing it more and more now in women, along with the cardiovascular issues that it brings about.”
Another cardiovascular risk factor is insomnia, said Dr. Merino. “This sleep disorder is more prevalent in women. As hormones constantly change, the ways women sleep constantly change, from one stage of life to the next. They sleep one way in childhood, another way in adolescence, and yet another way in menopause.”
Sleep in pregnancy
During pregnancy, hormone changes are much more pronounced. During the first trimester, progesterone levels increase, making the woman drowsy. On top of that, her sleep is interrupted by more frequent visits to the bathroom as well as greater general discomfort.
In the second trimester, sleep interruptions persist but are not as bad as they were during the first 3 months. In the third trimester, 75%-84% of pregnant women find it difficult to sleep because of aches and pains, the need to urinate during the night, cramps, and heartburn.
“Major physical changes are happening. When the bladder gets compressed, the woman has to get up and go to the bathroom. There’s an interruption in her sleep,” Ms. Farré explained. In addition, as the pregnancy progresses, the woman gains weight and her body mass index (BMI) increases, which can bring on obstructive sleep apnea, high blood pressure, preeclampsia, and diabetes, if not closely monitored.
Other factors include concomitant treatments, such as contraceptives, and the stages of life, such as pregnancy and lactation. “When a woman of childbearing age has restless legs syndrome, more often than not, this means that she has an iron deficiency that needs to be treated with oral iron supplements,” said Dr. Merino. “However, there are few medications that can be given to a pregnant woman – and RLS is relatively common during pregnancy. So, we have to turn to oral or intravenous iron supplements. Yet another matter is narcolepsy. In these cases, all medications have to be stopped during pregnancy and lactation, as they can be harmful to the baby.”
Sleep apnea
While one in five menopausal women are asymptomatic, the others experience mild to severe symptoms of apnea that frequently interrupt their sleep. In this stage of life, which begins around age 50 years, the hormones that had provided protection against sleep disruptions start to decrease. As a result, there is a rise in sleep problems, especially insomnia, breathing-related sleep disorders (for example, apnea), and restless legs syndrome.
The prevalence of breathing-related sleep disorders during menopause is attributable to weight gain, the drop in levels of estrogens, and the redistribution of adipose tissue in the body. Other factors also increase a woman’s risk of experiencing apnea. They range from stress, depression, and other psychological and psychiatric conditions to health status, medication use, and simply the fact of getting older. “Sleep apnea is more common in men than in premenopausal women. The numbers even out, though, when we compare men against menopausal women,” Dr. Cano noted.
In women, symptoms of sleep apnea are frequently attributed to menopause. There is some overlap: insomnia, headache, irritability, low mood, decreased libido, fatigue during the day, and feeling sleepy. Only much later is the woman’s condition correctly diagnosed as sleep apnea. So, even though presenting with the same complaints, a man will be diagnosed with sleep apnea sooner than a woman will – in some cases, around 10 years sooner.
“On the other hand, we’d always thought that, in menopause, insomnia was characterized by awakenings occurring throughout the second half of the night. But perhaps what happens more often is that women are regularly waking up repeatedly over the course of the entire night, as opposed to experiencing a wakefulness that starts early and lasts throughout the night or having a problem falling asleep to begin with,” said Dr. Merino. “The good news is that hormone replacement therapy can get things back to the way they were. And getting better sleep will help to overcome insomnia.”
Socioeconomic status
Insomnia is the most common sleep disorder. It affects 10%-20% of people, mostly women. “The fact that sleep problems are more prevalent in women can be explained by the fact that among women, there is a higher incidence of conditions that disrupt sleep, such as depression,” said Dr. Cano.
“Insomnia is much more common in adult women than adult men. And at menopause, women find that the insomnia only gets worse,” Dr. Merino added. “But around that same age, 50 years old, what we start to see more frequently in men is REM sleep behavior disorder, a type of parasomnia that’s a risk marker of degenerative nerve diseases.”
Dr. Cano emphasized one finding that, though basic, is not well known. “After adjusting for socioeconomic characteristics, the difference between the sexes in reporting sleep problems is cut in half. This suggests that an important factor that explains why there are differences in sleep problems between the sexes is that women’s socioeconomic status is generally lower than men’s.
“As for sleep apnea in particular,” Dr. Cano continued, “the kinds of symptoms that women have can be different from the classic ones seen in men – snoring, pauses in breathing, and daytime sleepiness; women are being underdiagnosed, and when they are diagnosed, that’s happening at a later age and at a higher BMI.”
So, it’s alarming that, as reported by SEPAR, 90% of women with obstructive sleep apnea are not being diagnosed.
Precision medicine approach
“The majority of research studies on sleep apnea have focused on men – given the prevalence of cases – and the results have been extrapolated to women. This is why there’s still a lot of work to be done in terms of better defining the characteristics specific to each sleep disorder and how they relate to each sex,” said Dr. Cano. “Being able to identify the relationship between the different sex-related phenotypes and each condition will allow us to take a precision medicine approach tailored to a patient’s particular characteristics.”
As Dr. Merino put it: “The approach to sleep disorders is always personalized. The patient’s sex, in and of itself, doesn’t have that great of an impact on this approach. What does have a great impact are women’s life stages. There are some subtle differences here and there, such as types of continuous positive airway pressure machines. The ones that are designed for women have masks that are better suited to their facial features, which differ from men’s.”
A precision medicine approach can be taken to treat any sleep disorder. For insomnia, the approach allows healthcare professionals to employ an appropriate cognitive-behavioral therapy plan or to determine which drugs would be more effective – all on the basis of symptoms and the characteristics of the particular case. Regarding sleep apnea, Dr. Cano explained, “taking into account the different anatomical characteristics or the higher prevalence of positional apnea will also allow us to offer different therapeutic alternatives to continuous positive airway pressure, such as mandibular advancement devices or positional therapy devices.”
Women should be encouraged to develop good sleep habits. These include taking circadian rhythms into account and aligning lifestyles accordingly. It also means going to bed earlier than the men in the household. For menopausal women, recommended sleep habits range from keeping their bedroom at an ideal temperature, following a diet rich in vegetables to avoid becoming overweight, and exercising daily. While this advice may be more applicable to teenagers, adults can benefit from it as well: Electronic devices should be turned off well before bedtime. Whether from a phone screen, a tablet screen, or a TV screen, the light emitted can keep one awake, which can be harmful to one’s health.
Dr. Cano and Dr. Merino disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MADRID – Because of the hormone changes that occur throughout their lives, women experience sleep problems that differ significantly from those experienced by men. Indeed, 75%-84% of pregnant women don’t sleep well during the third trimester, and up to 80% of women in menopause have symptoms that prevent them from getting a good night’s rest. For those seeking a precision medicine approach, the challenge is to identify the relationship between the different sex-related phenotypes and the sleep conditions.
Irene Cano, MD, PhD, is the coordinator of the sleep department at the Spanish Society of Pulmonology and Thoracic Surgery. She spoke with this news organization about the significant impact of hormones on sleep disorders in women.
“Reproductive hormones like estrogen and progesterone play a meaningful role in brain functions – not only those linked to the regulation of reproduction but also other physiological processes related to the regulation of circadian rhythms, cognitive performance, mood, and sleep. In addition, other hormones – for example, prolactin, growth hormone, cortisol, and melatonin – have sex-dependent effects on sleep,” Dr. Cano said.
Girls start puberty at a younger age than boys. As girls enter adolescence, they go to bed later and waking up earlier. So, girls are getting less than the 10 hours of sleep that they should be getting at this stage of life. The result is sleep debt, which gives rise to various problems: poor academic performance, ADHD, obesity, and metabolic problems, to name a few. As Ariadna Farré, RN, a sleep unit nurse, noted at SEPAR’s Joint Winter Meeting, “schools would have to start morning classes later to get adolescents to perform well academically. As the situation is now, half of the kids are falling asleep at their desks.”
Influencing sleep quality
Dr. Cano explained the issue as follows: “In adolescence, along with changes in young women’s hormone levels, we begin to see differences between the sexes. The changes in levels of estrogens and progesterone are what’s responsible for the changes that, to some extent, cause those disturbances in the quality of our sleep and in the stages of our sleep.”
Thus, sleep can be affected by the changes in hormone level that occur during a menstrual cycle. Estrogens, which increase during the follicular phase, are associated with REM sleep, while progesterone, which increases during the luteal phase, increases non-REM sleep. “In the 3-6 days prior to menstruation, it’s quite common for a woman to report difficulties falling asleep and staying asleep, in connection with a decline in the percentage of time she spends in REM sleep, in the context of premenstrual syndrome. In addition,” Dr. Cano pointed out, “menstrual bleeding, that loss of blood, is associated with a drop in iron levels, making it more likely that the woman will experience restless legs syndrome.”
Cardiovascular system
This news organization also spoke with Milagros Merino, MD, PhD, president of the Spanish Sleep Society. “The consequences that lack of sleep have on the cardiovascular system – we’re essentially talking about certain arrhythmias, high blood pressure, thrombosis in some cases, stroke, and heart attack. Lack of sleep also gives rise to endocrine and metabolic issues, like overweight and being at a greater risk of developing diabetes. And as for mental health, we see, among other things, attention and memory problems, emotional lability, and irascibility. Numerous studies have confirmed all of this.”
Sleep apnea also deserves mention, Dr. Merino added. “Although this disorder is more common in men, we’re seeing it more and more now in women, along with the cardiovascular issues that it brings about.”
Another cardiovascular risk factor is insomnia, said Dr. Merino. “This sleep disorder is more prevalent in women. As hormones constantly change, the ways women sleep constantly change, from one stage of life to the next. They sleep one way in childhood, another way in adolescence, and yet another way in menopause.”
Sleep in pregnancy
During pregnancy, hormone changes are much more pronounced. During the first trimester, progesterone levels increase, making the woman drowsy. On top of that, her sleep is interrupted by more frequent visits to the bathroom as well as greater general discomfort.
In the second trimester, sleep interruptions persist but are not as bad as they were during the first 3 months. In the third trimester, 75%-84% of pregnant women find it difficult to sleep because of aches and pains, the need to urinate during the night, cramps, and heartburn.
“Major physical changes are happening. When the bladder gets compressed, the woman has to get up and go to the bathroom. There’s an interruption in her sleep,” Ms. Farré explained. In addition, as the pregnancy progresses, the woman gains weight and her body mass index (BMI) increases, which can bring on obstructive sleep apnea, high blood pressure, preeclampsia, and diabetes, if not closely monitored.
Other factors include concomitant treatments, such as contraceptives, and the stages of life, such as pregnancy and lactation. “When a woman of childbearing age has restless legs syndrome, more often than not, this means that she has an iron deficiency that needs to be treated with oral iron supplements,” said Dr. Merino. “However, there are few medications that can be given to a pregnant woman – and RLS is relatively common during pregnancy. So, we have to turn to oral or intravenous iron supplements. Yet another matter is narcolepsy. In these cases, all medications have to be stopped during pregnancy and lactation, as they can be harmful to the baby.”
Sleep apnea
While one in five menopausal women are asymptomatic, the others experience mild to severe symptoms of apnea that frequently interrupt their sleep. In this stage of life, which begins around age 50 years, the hormones that had provided protection against sleep disruptions start to decrease. As a result, there is a rise in sleep problems, especially insomnia, breathing-related sleep disorders (for example, apnea), and restless legs syndrome.
The prevalence of breathing-related sleep disorders during menopause is attributable to weight gain, the drop in levels of estrogens, and the redistribution of adipose tissue in the body. Other factors also increase a woman’s risk of experiencing apnea. They range from stress, depression, and other psychological and psychiatric conditions to health status, medication use, and simply the fact of getting older. “Sleep apnea is more common in men than in premenopausal women. The numbers even out, though, when we compare men against menopausal women,” Dr. Cano noted.
In women, symptoms of sleep apnea are frequently attributed to menopause. There is some overlap: insomnia, headache, irritability, low mood, decreased libido, fatigue during the day, and feeling sleepy. Only much later is the woman’s condition correctly diagnosed as sleep apnea. So, even though presenting with the same complaints, a man will be diagnosed with sleep apnea sooner than a woman will – in some cases, around 10 years sooner.
“On the other hand, we’d always thought that, in menopause, insomnia was characterized by awakenings occurring throughout the second half of the night. But perhaps what happens more often is that women are regularly waking up repeatedly over the course of the entire night, as opposed to experiencing a wakefulness that starts early and lasts throughout the night or having a problem falling asleep to begin with,” said Dr. Merino. “The good news is that hormone replacement therapy can get things back to the way they were. And getting better sleep will help to overcome insomnia.”
Socioeconomic status
Insomnia is the most common sleep disorder. It affects 10%-20% of people, mostly women. “The fact that sleep problems are more prevalent in women can be explained by the fact that among women, there is a higher incidence of conditions that disrupt sleep, such as depression,” said Dr. Cano.
“Insomnia is much more common in adult women than adult men. And at menopause, women find that the insomnia only gets worse,” Dr. Merino added. “But around that same age, 50 years old, what we start to see more frequently in men is REM sleep behavior disorder, a type of parasomnia that’s a risk marker of degenerative nerve diseases.”
Dr. Cano emphasized one finding that, though basic, is not well known. “After adjusting for socioeconomic characteristics, the difference between the sexes in reporting sleep problems is cut in half. This suggests that an important factor that explains why there are differences in sleep problems between the sexes is that women’s socioeconomic status is generally lower than men’s.
“As for sleep apnea in particular,” Dr. Cano continued, “the kinds of symptoms that women have can be different from the classic ones seen in men – snoring, pauses in breathing, and daytime sleepiness; women are being underdiagnosed, and when they are diagnosed, that’s happening at a later age and at a higher BMI.”
So, it’s alarming that, as reported by SEPAR, 90% of women with obstructive sleep apnea are not being diagnosed.
Precision medicine approach
“The majority of research studies on sleep apnea have focused on men – given the prevalence of cases – and the results have been extrapolated to women. This is why there’s still a lot of work to be done in terms of better defining the characteristics specific to each sleep disorder and how they relate to each sex,” said Dr. Cano. “Being able to identify the relationship between the different sex-related phenotypes and each condition will allow us to take a precision medicine approach tailored to a patient’s particular characteristics.”
As Dr. Merino put it: “The approach to sleep disorders is always personalized. The patient’s sex, in and of itself, doesn’t have that great of an impact on this approach. What does have a great impact are women’s life stages. There are some subtle differences here and there, such as types of continuous positive airway pressure machines. The ones that are designed for women have masks that are better suited to their facial features, which differ from men’s.”
A precision medicine approach can be taken to treat any sleep disorder. For insomnia, the approach allows healthcare professionals to employ an appropriate cognitive-behavioral therapy plan or to determine which drugs would be more effective – all on the basis of symptoms and the characteristics of the particular case. Regarding sleep apnea, Dr. Cano explained, “taking into account the different anatomical characteristics or the higher prevalence of positional apnea will also allow us to offer different therapeutic alternatives to continuous positive airway pressure, such as mandibular advancement devices or positional therapy devices.”
Women should be encouraged to develop good sleep habits. These include taking circadian rhythms into account and aligning lifestyles accordingly. It also means going to bed earlier than the men in the household. For menopausal women, recommended sleep habits range from keeping their bedroom at an ideal temperature, following a diet rich in vegetables to avoid becoming overweight, and exercising daily. While this advice may be more applicable to teenagers, adults can benefit from it as well: Electronic devices should be turned off well before bedtime. Whether from a phone screen, a tablet screen, or a TV screen, the light emitted can keep one awake, which can be harmful to one’s health.
Dr. Cano and Dr. Merino disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
Early or delayed menopause and irregular periods tied to new-onset atrial fibrillation
Takeaway
- Early or delayed menopause and a history of irregular menstrual cycles were significantly associated with a greater risk of new-onset atrial fibrillation (AF) in women.
- Women with nulliparity and multiparity had a greater risk of new-onset AF compared with those with one to two live births.
Why this matters
- Findings highlight the significance of considering the reproductive history of women while developing tailored screening and prevention strategies for AF.
Study design
- A population-based cohort study of 235,191 women (age, 40-69 years) without AF and a history of hysterectomy and/or bilateral oophorectomy, identified from the UK Biobank (2006-2010).
- Funding: Gender and Prevention Grant from ZonMw and other.
Key results
- During a median follow-up of 11.6 years, 4,629 (2.0%) women were diagnosed with new-onset AF.
- A history of irregular menstrual cycle was associated with higher risk of new-onset AF (adjusted HR, 1.34; 95% confidence interval, 1.01-1.79; P = .04).
- Compared with women who experienced menarche at the age of 12 years, the risk of new-onset AF was significantly higher in those who experienced menarche:
- –Earlier between the ages of 7 and 11 years (aHR, 1.10; 95% CI, 1.00-1.21; P = .04) and
- –Later between the ages of 13 and 18 years (aHR, 1.08; 95% CI, 1.00-1.17; P = .05).
- The risk of new-onset AF was significantly higher in women who experienced menopause:
- –At the age of < 35 years (aHR, 2.25; 95% CI, 1.48-3.43; P < .001);
- –Between the ages of 35 and 44 years (aHR, 1.24; 95% CI, 1.10-1.39; P < .001); and
- –At the age of ≥ 60 years (aHR, 1.34; 95% CI, 1.10-1.78; P = .04).
- Women with no live births (aHR, 1.13; 95% CI, 1.04-1.24; P < .01), four to six live births (aHR, 1.12; 95% CI, 1.01-1.24; P = .04), and ≥ seven live births (aHR, 1.67; 95% CI, 1.03-2.70; P = .03) vs. those with one to two live births had a significantly higher risk of new-onset AF.
Limitations
- Observational design.
A version of this article first appeared on Medscape UK.
Reference
Lu Z, Aribas E, Geurts S, Roeters van Lennep JE, Ikram MA, Bos MM, de Groot NMS, Kavousi M. Association Between Sex-Specific Risk Factors and Risk of New-Onset Atrial Fibrillation Among Women. JAMA Netw Open. 2022;5(9):e2229716. doi: 10.1001/jamanetworkopen.2022.29716. PMID: 36048441.
Takeaway
- Early or delayed menopause and a history of irregular menstrual cycles were significantly associated with a greater risk of new-onset atrial fibrillation (AF) in women.
- Women with nulliparity and multiparity had a greater risk of new-onset AF compared with those with one to two live births.
Why this matters
- Findings highlight the significance of considering the reproductive history of women while developing tailored screening and prevention strategies for AF.
Study design
- A population-based cohort study of 235,191 women (age, 40-69 years) without AF and a history of hysterectomy and/or bilateral oophorectomy, identified from the UK Biobank (2006-2010).
- Funding: Gender and Prevention Grant from ZonMw and other.
Key results
- During a median follow-up of 11.6 years, 4,629 (2.0%) women were diagnosed with new-onset AF.
- A history of irregular menstrual cycle was associated with higher risk of new-onset AF (adjusted HR, 1.34; 95% confidence interval, 1.01-1.79; P = .04).
- Compared with women who experienced menarche at the age of 12 years, the risk of new-onset AF was significantly higher in those who experienced menarche:
- –Earlier between the ages of 7 and 11 years (aHR, 1.10; 95% CI, 1.00-1.21; P = .04) and
- –Later between the ages of 13 and 18 years (aHR, 1.08; 95% CI, 1.00-1.17; P = .05).
- The risk of new-onset AF was significantly higher in women who experienced menopause:
- –At the age of < 35 years (aHR, 2.25; 95% CI, 1.48-3.43; P < .001);
- –Between the ages of 35 and 44 years (aHR, 1.24; 95% CI, 1.10-1.39; P < .001); and
- –At the age of ≥ 60 years (aHR, 1.34; 95% CI, 1.10-1.78; P = .04).
- Women with no live births (aHR, 1.13; 95% CI, 1.04-1.24; P < .01), four to six live births (aHR, 1.12; 95% CI, 1.01-1.24; P = .04), and ≥ seven live births (aHR, 1.67; 95% CI, 1.03-2.70; P = .03) vs. those with one to two live births had a significantly higher risk of new-onset AF.
Limitations
- Observational design.
A version of this article first appeared on Medscape UK.
Reference
Lu Z, Aribas E, Geurts S, Roeters van Lennep JE, Ikram MA, Bos MM, de Groot NMS, Kavousi M. Association Between Sex-Specific Risk Factors and Risk of New-Onset Atrial Fibrillation Among Women. JAMA Netw Open. 2022;5(9):e2229716. doi: 10.1001/jamanetworkopen.2022.29716. PMID: 36048441.
Takeaway
- Early or delayed menopause and a history of irregular menstrual cycles were significantly associated with a greater risk of new-onset atrial fibrillation (AF) in women.
- Women with nulliparity and multiparity had a greater risk of new-onset AF compared with those with one to two live births.
Why this matters
- Findings highlight the significance of considering the reproductive history of women while developing tailored screening and prevention strategies for AF.
Study design
- A population-based cohort study of 235,191 women (age, 40-69 years) without AF and a history of hysterectomy and/or bilateral oophorectomy, identified from the UK Biobank (2006-2010).
- Funding: Gender and Prevention Grant from ZonMw and other.
Key results
- During a median follow-up of 11.6 years, 4,629 (2.0%) women were diagnosed with new-onset AF.
- A history of irregular menstrual cycle was associated with higher risk of new-onset AF (adjusted HR, 1.34; 95% confidence interval, 1.01-1.79; P = .04).
- Compared with women who experienced menarche at the age of 12 years, the risk of new-onset AF was significantly higher in those who experienced menarche:
- –Earlier between the ages of 7 and 11 years (aHR, 1.10; 95% CI, 1.00-1.21; P = .04) and
- –Later between the ages of 13 and 18 years (aHR, 1.08; 95% CI, 1.00-1.17; P = .05).
- The risk of new-onset AF was significantly higher in women who experienced menopause:
- –At the age of < 35 years (aHR, 2.25; 95% CI, 1.48-3.43; P < .001);
- –Between the ages of 35 and 44 years (aHR, 1.24; 95% CI, 1.10-1.39; P < .001); and
- –At the age of ≥ 60 years (aHR, 1.34; 95% CI, 1.10-1.78; P = .04).
- Women with no live births (aHR, 1.13; 95% CI, 1.04-1.24; P < .01), four to six live births (aHR, 1.12; 95% CI, 1.01-1.24; P = .04), and ≥ seven live births (aHR, 1.67; 95% CI, 1.03-2.70; P = .03) vs. those with one to two live births had a significantly higher risk of new-onset AF.
Limitations
- Observational design.
A version of this article first appeared on Medscape UK.
Reference
Lu Z, Aribas E, Geurts S, Roeters van Lennep JE, Ikram MA, Bos MM, de Groot NMS, Kavousi M. Association Between Sex-Specific Risk Factors and Risk of New-Onset Atrial Fibrillation Among Women. JAMA Netw Open. 2022;5(9):e2229716. doi: 10.1001/jamanetworkopen.2022.29716. PMID: 36048441.
FROM JAMA NETWORK OPEN
New ovulatory disorder classifications from FIGO replace 50-year-old system
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
FROM INTERNATIONAL JOURNAL OF GYNECOLOGY AND OBSTETRICS
Early menopause linked with increased risk of heart problems
SEOUL, South Korea – Menopause before age 40 is associated with elevated risk of heart failure and atrial fibrillation, according to a study published in European Heart Journal, from the European Society of Cardiology (ESC). The study of more than 1.4 million women revealed that the younger the age at menopause, the higher the risk of heart failure and atrial fibrillation.
“Women with premature menopause should be aware that they may be more likely to develop heart failure or atrial fibrillation than their peers,” said study author Ga Eun Nam, MD, PhD, of Korea University College of Medicine, Seoul. “This may be good motivation to improve lifestyle habits known to be linked with heart disease, such as quitting smoking and exercising.”
Cardiovascular disease typically occurs up to 10 years later in women than men. Premenopausal women are thought to benefit from estrogen’s protective effect on the cardiovascular system. The cessation of menstruation and subsequent decline of estrogen levels may make women more vulnerable to cardiovascular disease.
A national population
Premature menopause affects 1% of women younger than 40 years, the ESC press release stated. Prior studies have found a link between premature (before age 40 years) and early (before age 45 years) menopause and cardiovascular disease overall, but the evidence for heart failure or atrial fibrillation alone is limited. This study examined the associations between premature menopause, age at menopause, and incident heart failure and atrial fibrillation. Data were obtained from the Korean National Health Insurance System (NHIS), which provides health screening at least every 2 years and includes 97% of the population.
The study included 1,401,175 postmenopausal women aged 30 years and older who completed the NHIS health checkup in 2009. Participants were monitored until the end of 2018 for new-onset heart failure and atrial fibrillation. Information was collected on demographics, health behaviors, and reproductive factors, including age at menopause and use of hormone replacement therapy (HRT). Age at menopause was split into four categories: younger than 40 years, 40-44 years, 45-49 years, and 50 years or older. Premature menopause was defined as having the final menstrual period before age 40 years.
Some 28,111 (2%) participants had a history of premature menopause. For these women, the average age at menopause was 36.7 years. The average age at study enrollment for women with and for those without a history of premature menopause was 60 and 61.5 years, respectively. During an average follow-up of 9.1 years, 42,699 (3.0%) developed heart failure, and 44,834 (3.2%) developed atrial fibrillation.
The researchers analyzed the association between history of premature menopause and incident heart failure and atrial fibrillation after adjusting for age, smoking, alcohol use, physical activity, income, body mass index, hypertension, type 2 diabetes, dyslipidemia, chronic kidney disease, coronary heart disease, HRT, and age at menarche. Women who experienced premature menopause had a 33% higher risk for heart failure and 9% higher risk for atrial fibrillation, compared with those who did not.
Reproductive history
The researchers then analyzed the associations between age at menopause and incidence of heart failure and atrial fibrillation after adjusting for the same factors as in the previous analyses. The risk for incident heart failure increased as the age at menopause decreased. Compared with women aged 50 years and older at menopause, those aged 45-49 years, 40-44 years, and younger than 40 years at menopause had 11%, 23%, and 39% greater risk for incident heart failure, respectively. Similarly, the risk for incident atrial fibrillation increased as the age at menopause decreased; the risk was 4%, 10%, and 11% higher for those aged 45-49 years, 40-44 years, and younger than 40 years at menopause, respectively, compared with women aged 50 years and older at menopause.
The authors said that several factors may explain the associations between menopausal age, heart failure, and atrial fibrillation, such as the drop in estrogen levels and changes in body fat distribution.
Dr. Nam concluded, “The misconception that heart disease primarily affects men has meant that sex-specific risk factors have been largely ignored. Evidence is growing that undergoing menopause before the age of 40 years may increase the likelihood of heart disease later in life. Our study indicates that reproductive history should be routinely considered in addition to traditional risk factors such as smoking when evaluating the future likelihood of heart failure and atrial fibrillation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
SEOUL, South Korea – Menopause before age 40 is associated with elevated risk of heart failure and atrial fibrillation, according to a study published in European Heart Journal, from the European Society of Cardiology (ESC). The study of more than 1.4 million women revealed that the younger the age at menopause, the higher the risk of heart failure and atrial fibrillation.
“Women with premature menopause should be aware that they may be more likely to develop heart failure or atrial fibrillation than their peers,” said study author Ga Eun Nam, MD, PhD, of Korea University College of Medicine, Seoul. “This may be good motivation to improve lifestyle habits known to be linked with heart disease, such as quitting smoking and exercising.”
Cardiovascular disease typically occurs up to 10 years later in women than men. Premenopausal women are thought to benefit from estrogen’s protective effect on the cardiovascular system. The cessation of menstruation and subsequent decline of estrogen levels may make women more vulnerable to cardiovascular disease.
A national population
Premature menopause affects 1% of women younger than 40 years, the ESC press release stated. Prior studies have found a link between premature (before age 40 years) and early (before age 45 years) menopause and cardiovascular disease overall, but the evidence for heart failure or atrial fibrillation alone is limited. This study examined the associations between premature menopause, age at menopause, and incident heart failure and atrial fibrillation. Data were obtained from the Korean National Health Insurance System (NHIS), which provides health screening at least every 2 years and includes 97% of the population.
The study included 1,401,175 postmenopausal women aged 30 years and older who completed the NHIS health checkup in 2009. Participants were monitored until the end of 2018 for new-onset heart failure and atrial fibrillation. Information was collected on demographics, health behaviors, and reproductive factors, including age at menopause and use of hormone replacement therapy (HRT). Age at menopause was split into four categories: younger than 40 years, 40-44 years, 45-49 years, and 50 years or older. Premature menopause was defined as having the final menstrual period before age 40 years.
Some 28,111 (2%) participants had a history of premature menopause. For these women, the average age at menopause was 36.7 years. The average age at study enrollment for women with and for those without a history of premature menopause was 60 and 61.5 years, respectively. During an average follow-up of 9.1 years, 42,699 (3.0%) developed heart failure, and 44,834 (3.2%) developed atrial fibrillation.
The researchers analyzed the association between history of premature menopause and incident heart failure and atrial fibrillation after adjusting for age, smoking, alcohol use, physical activity, income, body mass index, hypertension, type 2 diabetes, dyslipidemia, chronic kidney disease, coronary heart disease, HRT, and age at menarche. Women who experienced premature menopause had a 33% higher risk for heart failure and 9% higher risk for atrial fibrillation, compared with those who did not.
Reproductive history
The researchers then analyzed the associations between age at menopause and incidence of heart failure and atrial fibrillation after adjusting for the same factors as in the previous analyses. The risk for incident heart failure increased as the age at menopause decreased. Compared with women aged 50 years and older at menopause, those aged 45-49 years, 40-44 years, and younger than 40 years at menopause had 11%, 23%, and 39% greater risk for incident heart failure, respectively. Similarly, the risk for incident atrial fibrillation increased as the age at menopause decreased; the risk was 4%, 10%, and 11% higher for those aged 45-49 years, 40-44 years, and younger than 40 years at menopause, respectively, compared with women aged 50 years and older at menopause.
The authors said that several factors may explain the associations between menopausal age, heart failure, and atrial fibrillation, such as the drop in estrogen levels and changes in body fat distribution.
Dr. Nam concluded, “The misconception that heart disease primarily affects men has meant that sex-specific risk factors have been largely ignored. Evidence is growing that undergoing menopause before the age of 40 years may increase the likelihood of heart disease later in life. Our study indicates that reproductive history should be routinely considered in addition to traditional risk factors such as smoking when evaluating the future likelihood of heart failure and atrial fibrillation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
SEOUL, South Korea – Menopause before age 40 is associated with elevated risk of heart failure and atrial fibrillation, according to a study published in European Heart Journal, from the European Society of Cardiology (ESC). The study of more than 1.4 million women revealed that the younger the age at menopause, the higher the risk of heart failure and atrial fibrillation.
“Women with premature menopause should be aware that they may be more likely to develop heart failure or atrial fibrillation than their peers,” said study author Ga Eun Nam, MD, PhD, of Korea University College of Medicine, Seoul. “This may be good motivation to improve lifestyle habits known to be linked with heart disease, such as quitting smoking and exercising.”
Cardiovascular disease typically occurs up to 10 years later in women than men. Premenopausal women are thought to benefit from estrogen’s protective effect on the cardiovascular system. The cessation of menstruation and subsequent decline of estrogen levels may make women more vulnerable to cardiovascular disease.
A national population
Premature menopause affects 1% of women younger than 40 years, the ESC press release stated. Prior studies have found a link between premature (before age 40 years) and early (before age 45 years) menopause and cardiovascular disease overall, but the evidence for heart failure or atrial fibrillation alone is limited. This study examined the associations between premature menopause, age at menopause, and incident heart failure and atrial fibrillation. Data were obtained from the Korean National Health Insurance System (NHIS), which provides health screening at least every 2 years and includes 97% of the population.
The study included 1,401,175 postmenopausal women aged 30 years and older who completed the NHIS health checkup in 2009. Participants were monitored until the end of 2018 for new-onset heart failure and atrial fibrillation. Information was collected on demographics, health behaviors, and reproductive factors, including age at menopause and use of hormone replacement therapy (HRT). Age at menopause was split into four categories: younger than 40 years, 40-44 years, 45-49 years, and 50 years or older. Premature menopause was defined as having the final menstrual period before age 40 years.
Some 28,111 (2%) participants had a history of premature menopause. For these women, the average age at menopause was 36.7 years. The average age at study enrollment for women with and for those without a history of premature menopause was 60 and 61.5 years, respectively. During an average follow-up of 9.1 years, 42,699 (3.0%) developed heart failure, and 44,834 (3.2%) developed atrial fibrillation.
The researchers analyzed the association between history of premature menopause and incident heart failure and atrial fibrillation after adjusting for age, smoking, alcohol use, physical activity, income, body mass index, hypertension, type 2 diabetes, dyslipidemia, chronic kidney disease, coronary heart disease, HRT, and age at menarche. Women who experienced premature menopause had a 33% higher risk for heart failure and 9% higher risk for atrial fibrillation, compared with those who did not.
Reproductive history
The researchers then analyzed the associations between age at menopause and incidence of heart failure and atrial fibrillation after adjusting for the same factors as in the previous analyses. The risk for incident heart failure increased as the age at menopause decreased. Compared with women aged 50 years and older at menopause, those aged 45-49 years, 40-44 years, and younger than 40 years at menopause had 11%, 23%, and 39% greater risk for incident heart failure, respectively. Similarly, the risk for incident atrial fibrillation increased as the age at menopause decreased; the risk was 4%, 10%, and 11% higher for those aged 45-49 years, 40-44 years, and younger than 40 years at menopause, respectively, compared with women aged 50 years and older at menopause.
The authors said that several factors may explain the associations between menopausal age, heart failure, and atrial fibrillation, such as the drop in estrogen levels and changes in body fat distribution.
Dr. Nam concluded, “The misconception that heart disease primarily affects men has meant that sex-specific risk factors have been largely ignored. Evidence is growing that undergoing menopause before the age of 40 years may increase the likelihood of heart disease later in life. Our study indicates that reproductive history should be routinely considered in addition to traditional risk factors such as smoking when evaluating the future likelihood of heart failure and atrial fibrillation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
How should you advise your 54-year-old patient about the use of HT?
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16
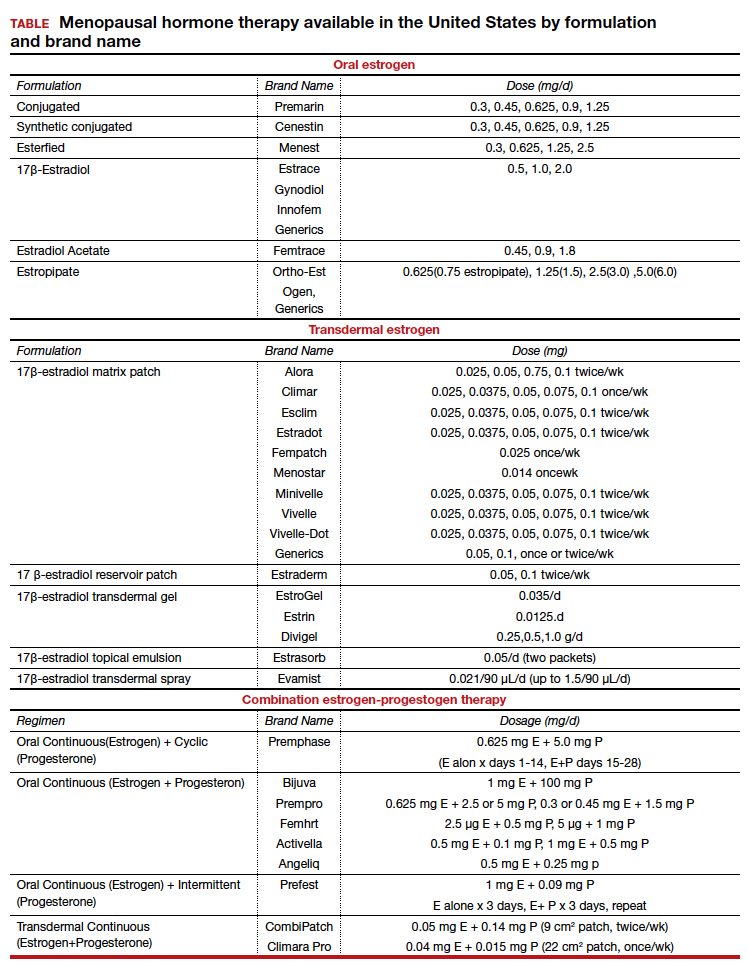
Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16

Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16

Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
Does cannabis help with menopause symptoms?
Many women with symptoms of menopause are turning to cannabis for help, researchers have found, despite a lack of evidence that the drug works for these issues.
In a survey of perimenopausal and menopausal women who said they’ve used cannabis, nearly 80% said they use medical marijuana to alleviate symptoms such as sleep disturbances, hot flashes, and mood swings.
“Increasingly, we see greater numbers of individuals exploiting the use of cannabis and cannabinoids for lots of conditions. We realized there was no long-term data on how women were treating themselves for conditions like menopause,” said Staci Gruber, PhD, director of the Marijuana Investigations for Neuroscientific Discovery (MIND) program at McLean Hospital, an affiliate of Harvard Medical School, Boston, who led the study.
Dr. Gruber and her colleagues surveyed 131 perimenopausal and 127 postmenopausal women about their use of cannabis, identifying them through targeted advertising and social media platforms such as Twitter, Facebook, and Reddit.
The survey, published in Menopause, found 83.5% reported habitual cannabis use and 86% said they were current users. Around half of the women reported mixed medical/recreational use; 30.8% reported recreational use only and 17.7% said they only used medical forms of the drug.
The three most common modes of cannabis use were smoking a joint, bowl, or bong (84.3%); using edibles (78.3%);, and vaping oils (52.6%).
The researchers found that women in perimenopause reported markedly worse symptoms than did those in menopause, and these women tended to use a wider variety of cannabis products.
Dr. Gruber said clinicians should be asking their menopausal patients if they use cannabis to alleviate their symptoms.
Stephanie Faubion, MD, MBA, a women’s health expert at Mayo Clinic in Rochester, Minn., and Jacksonville, Fla., said the looming question is whether cannabis in fact works in these patients.
“What we need is to figure out whether it works for women, and that hasn’t been studied yet,” she said.
Dr. Faubion, medical director for the North American Menopause Society, said the society is now conducting a review of worldwide data on nonhormonal treatments for symptoms of menopause. The report, which will examine the most current research on the effects of cannabis, hypnosis, diet, exercise, acupuncture, yoga, and meditation, will be released in 2023, she said.
Dr. Gruber said she hopes her group’s research will open the doors to more detailed explorations of how strains of cannabis and their levels of cannabidiol, a chemical compound in cannabis plants, and tetrahydrocannabinol, the main psychoactive component in cannabis, affect the symptoms women experience from menopause. Clinical trials for products aimed at specific symptoms also will be important, she added.
“We have a paucity of data from primary care clinicians,” Dr. Gruber said. “We, as researchers and clinicians, should be providing women with the research to make informed choices.”
The study was supported by private donations to the MIND program at McLean Hospital. No funding sources were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Gruber reported grants from the National Institute on Drug Abuse, Foria/Praxis Ventures, and Charlotte’s Web. She reported personal fees from the Coalition for Cannabis Policy, Education and Regulation; Beth Israel Deaconess; Fenway Health; Greenwich Biosciences Cannabis Education Working Group; and National Academy of Neuropsychology outside the submitted work. Dr. Faubion reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many women with symptoms of menopause are turning to cannabis for help, researchers have found, despite a lack of evidence that the drug works for these issues.
In a survey of perimenopausal and menopausal women who said they’ve used cannabis, nearly 80% said they use medical marijuana to alleviate symptoms such as sleep disturbances, hot flashes, and mood swings.
“Increasingly, we see greater numbers of individuals exploiting the use of cannabis and cannabinoids for lots of conditions. We realized there was no long-term data on how women were treating themselves for conditions like menopause,” said Staci Gruber, PhD, director of the Marijuana Investigations for Neuroscientific Discovery (MIND) program at McLean Hospital, an affiliate of Harvard Medical School, Boston, who led the study.
Dr. Gruber and her colleagues surveyed 131 perimenopausal and 127 postmenopausal women about their use of cannabis, identifying them through targeted advertising and social media platforms such as Twitter, Facebook, and Reddit.
The survey, published in Menopause, found 83.5% reported habitual cannabis use and 86% said they were current users. Around half of the women reported mixed medical/recreational use; 30.8% reported recreational use only and 17.7% said they only used medical forms of the drug.
The three most common modes of cannabis use were smoking a joint, bowl, or bong (84.3%); using edibles (78.3%);, and vaping oils (52.6%).
The researchers found that women in perimenopause reported markedly worse symptoms than did those in menopause, and these women tended to use a wider variety of cannabis products.
Dr. Gruber said clinicians should be asking their menopausal patients if they use cannabis to alleviate their symptoms.
Stephanie Faubion, MD, MBA, a women’s health expert at Mayo Clinic in Rochester, Minn., and Jacksonville, Fla., said the looming question is whether cannabis in fact works in these patients.
“What we need is to figure out whether it works for women, and that hasn’t been studied yet,” she said.
Dr. Faubion, medical director for the North American Menopause Society, said the society is now conducting a review of worldwide data on nonhormonal treatments for symptoms of menopause. The report, which will examine the most current research on the effects of cannabis, hypnosis, diet, exercise, acupuncture, yoga, and meditation, will be released in 2023, she said.
Dr. Gruber said she hopes her group’s research will open the doors to more detailed explorations of how strains of cannabis and their levels of cannabidiol, a chemical compound in cannabis plants, and tetrahydrocannabinol, the main psychoactive component in cannabis, affect the symptoms women experience from menopause. Clinical trials for products aimed at specific symptoms also will be important, she added.
“We have a paucity of data from primary care clinicians,” Dr. Gruber said. “We, as researchers and clinicians, should be providing women with the research to make informed choices.”
The study was supported by private donations to the MIND program at McLean Hospital. No funding sources were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Gruber reported grants from the National Institute on Drug Abuse, Foria/Praxis Ventures, and Charlotte’s Web. She reported personal fees from the Coalition for Cannabis Policy, Education and Regulation; Beth Israel Deaconess; Fenway Health; Greenwich Biosciences Cannabis Education Working Group; and National Academy of Neuropsychology outside the submitted work. Dr. Faubion reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many women with symptoms of menopause are turning to cannabis for help, researchers have found, despite a lack of evidence that the drug works for these issues.
In a survey of perimenopausal and menopausal women who said they’ve used cannabis, nearly 80% said they use medical marijuana to alleviate symptoms such as sleep disturbances, hot flashes, and mood swings.
“Increasingly, we see greater numbers of individuals exploiting the use of cannabis and cannabinoids for lots of conditions. We realized there was no long-term data on how women were treating themselves for conditions like menopause,” said Staci Gruber, PhD, director of the Marijuana Investigations for Neuroscientific Discovery (MIND) program at McLean Hospital, an affiliate of Harvard Medical School, Boston, who led the study.
Dr. Gruber and her colleagues surveyed 131 perimenopausal and 127 postmenopausal women about their use of cannabis, identifying them through targeted advertising and social media platforms such as Twitter, Facebook, and Reddit.
The survey, published in Menopause, found 83.5% reported habitual cannabis use and 86% said they were current users. Around half of the women reported mixed medical/recreational use; 30.8% reported recreational use only and 17.7% said they only used medical forms of the drug.
The three most common modes of cannabis use were smoking a joint, bowl, or bong (84.3%); using edibles (78.3%);, and vaping oils (52.6%).
The researchers found that women in perimenopause reported markedly worse symptoms than did those in menopause, and these women tended to use a wider variety of cannabis products.
Dr. Gruber said clinicians should be asking their menopausal patients if they use cannabis to alleviate their symptoms.
Stephanie Faubion, MD, MBA, a women’s health expert at Mayo Clinic in Rochester, Minn., and Jacksonville, Fla., said the looming question is whether cannabis in fact works in these patients.
“What we need is to figure out whether it works for women, and that hasn’t been studied yet,” she said.
Dr. Faubion, medical director for the North American Menopause Society, said the society is now conducting a review of worldwide data on nonhormonal treatments for symptoms of menopause. The report, which will examine the most current research on the effects of cannabis, hypnosis, diet, exercise, acupuncture, yoga, and meditation, will be released in 2023, she said.
Dr. Gruber said she hopes her group’s research will open the doors to more detailed explorations of how strains of cannabis and their levels of cannabidiol, a chemical compound in cannabis plants, and tetrahydrocannabinol, the main psychoactive component in cannabis, affect the symptoms women experience from menopause. Clinical trials for products aimed at specific symptoms also will be important, she added.
“We have a paucity of data from primary care clinicians,” Dr. Gruber said. “We, as researchers and clinicians, should be providing women with the research to make informed choices.”
The study was supported by private donations to the MIND program at McLean Hospital. No funding sources were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Gruber reported grants from the National Institute on Drug Abuse, Foria/Praxis Ventures, and Charlotte’s Web. She reported personal fees from the Coalition for Cannabis Policy, Education and Regulation; Beth Israel Deaconess; Fenway Health; Greenwich Biosciences Cannabis Education Working Group; and National Academy of Neuropsychology outside the submitted work. Dr. Faubion reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NAMS affirms value of hormone therapy for menopausal women
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
FROM MENOPAUSE