User login
Routine weight counseling urged for women at midlife
Midlife women who are of normal weight or are overweight should routinely receive counseling aimed at limiting weight gain and preventing obesity and its associated health risks, a new clinical guideline states.
The recommendation, issued by the Women’s Preventive Services Initiative (WPSI) of the American College of Obstetricians and Gynecologists (ACOG), supports regular lifestyle counseling for women aged 40-60 years with normal or overweight body mass index of 18.5-29.9 kg/m2. Counseling could include individualized discussion of healthy eating and physical activity initiated by health professionals involved in preventive care.
Published online in Annals of Internal Medicine, the guideline addresses the prevalence and health burdens of obesity in U.S. women of middle age and seeks to reduce the known harms of obesity with an intervention of minimal anticipated harms. High BMI increases the risk for many chronic conditions including hypertension, dyslipidemia, type 2 diabetes, coronary artery disease, stroke, and all-cause mortality.
The best way to counsel, however, remains unclear. “Although the optimal approach could not be discerned from existing trials, a range of interventions of varying duration, frequency, and intensity showed benefit with potential clinical significance,” wrote the WPSI guideline panel, led by David P. Chelmow, MD, chair of the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond.
The guideline rests on a systematic literature review led by family doctor Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center, at Oregon Health & Science University in Portland, suggesting moderate reductions in weight could be achieved by offering advice to this age group.
The federally supported WPSI was launched by ACOG in 2016. The guideline fills a gap in current recommendations in that it targets a specific risk group and specifies individual counseling based on its effectiveness and applicability in primary care settings.
In another benefit of routine counseling, the panel stated, “Normalizing counseling about healthy diet and physical activity by providing it to all midlife women may also mitigate concerns about weight stigma resulting from only counseling women with obesity.”
The panelists noted that during 2017-2018, the prevalence of obesity (BMI ≥ 30.0 kg/m2) was 43.3% among U.S. women aged 40-59 years, while the prevalence of severe obesity (BMI ≥ 40.0 kg/m2) was highest in this age group at 11.5%. “Midlife women gain weight at an average of approximately 1.5 pounds per year, which increases their risk for transitioning from normal or overweight to obese BMI,” the panelists wrote.
The review
Dr. Cantor’s group analyzed seven randomized controlled trials (RCTs) published up to October 2021 from 12 publications involving 51,638 participants. Although the trials were largely small and heterogeneous, they suggested that counseling may result in modest differences in weight change without causing important harms.
Four RCTs showed significant favorable weight changes for counseling over no-counseling control groups, with a mean difference of 0.87 to 2.5 kg, whereas one trial of counseling and two trials of exercise showed no differences. One of two RCTs reported improved quality-of-life measures.
As for harms, while interventions did not increase measures of depression or stress in one trial, self-reported falls (37% vs. 29%, P < .001) and injuries (19% vs. 14%, P = .03) were more frequent with exercise counseling in one trial.
“More research is needed to determine optimal content, frequency, length, and number of sessions required and should include additional patient populations,” Dr. Cantor and associates wrote.
In terms of limitations, the authors acknowledged that trials of behavioral interventions in maintaining or reducing weight in midlife women demonstrate small magnitudes of effect.
Offering a nonparticipant’s perspective on the WPSI guideline for this news organization, JoAnn E. Manson, MD, DrPH, MACP, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, said its message is of prime importance for women of middle age and it goes beyond concern about pounds lost or gained.
“Midlife and the transition to menopause are high-risk periods for women in terms of typical changes in body composition that increase the risk of adverse cardiometabolic outcomes,” said Dr. Manson, professor of women’s health at Harvard Medical School, Boston. “Counseling women should be a priority for physicians in clinical practice. And it’s not just whether weight gain is reflected on the scales or not but whether there’s an increase in central abdominal fat, a decrease in lean muscle mass, and an increase in adverse glucose tolerance.”
It is essential for women to be vigilant at this time, she added, and their exercise regimens should include strength and resistance training to preserve lean muscle mass and boost metabolic rate. Dr. Manson’s group has issued several statements stressing how important it is for clinicians to take decisive action on the counseling front and how they can do this in very little time during routine practice.
Also in full support of the guideline is Mary L. Rosser, MD, PhD, assistant professor of women’s health in obstetrics and gynecology at Columbia University Irving Medical Center in New York. “Midlife is a wonderful opportunity to encourage patients to assess their overall health status and make changes to impact their future health. Women in middle age tend to experience weight gain due to a variety of factors including aging and lifestyle,” said Dr. Rosser, who was not involved in the writing of the review or guideline.
While aging and genetics cannot be altered, behaviors can, and in her view, favorable behaviors would also include stress reduction and adequate sleep.
“The importance of reducing obesity with early intervention and prevention must focus on all women,” Dr. Rosser said. “We must narrow the inequities gap in care especially for high-risk minority groups and underserved populations. This will reduce disease and death and provide women the gift of active living and feeling better.”
The WPSI authors have made available a summary of the review and guideline for patients.
The systematic review and clinical guideline were funded by the federal Health Resources and Services Administration through ACOG. The authors of the guideline and the review authors disclosed no relevant financial conflicts of interest. Dr. Manson and Dr. Rosser disclosed no relevant competing interests with regard to their comments.
Midlife women who are of normal weight or are overweight should routinely receive counseling aimed at limiting weight gain and preventing obesity and its associated health risks, a new clinical guideline states.
The recommendation, issued by the Women’s Preventive Services Initiative (WPSI) of the American College of Obstetricians and Gynecologists (ACOG), supports regular lifestyle counseling for women aged 40-60 years with normal or overweight body mass index of 18.5-29.9 kg/m2. Counseling could include individualized discussion of healthy eating and physical activity initiated by health professionals involved in preventive care.
Published online in Annals of Internal Medicine, the guideline addresses the prevalence and health burdens of obesity in U.S. women of middle age and seeks to reduce the known harms of obesity with an intervention of minimal anticipated harms. High BMI increases the risk for many chronic conditions including hypertension, dyslipidemia, type 2 diabetes, coronary artery disease, stroke, and all-cause mortality.
The best way to counsel, however, remains unclear. “Although the optimal approach could not be discerned from existing trials, a range of interventions of varying duration, frequency, and intensity showed benefit with potential clinical significance,” wrote the WPSI guideline panel, led by David P. Chelmow, MD, chair of the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond.
The guideline rests on a systematic literature review led by family doctor Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center, at Oregon Health & Science University in Portland, suggesting moderate reductions in weight could be achieved by offering advice to this age group.
The federally supported WPSI was launched by ACOG in 2016. The guideline fills a gap in current recommendations in that it targets a specific risk group and specifies individual counseling based on its effectiveness and applicability in primary care settings.
In another benefit of routine counseling, the panel stated, “Normalizing counseling about healthy diet and physical activity by providing it to all midlife women may also mitigate concerns about weight stigma resulting from only counseling women with obesity.”
The panelists noted that during 2017-2018, the prevalence of obesity (BMI ≥ 30.0 kg/m2) was 43.3% among U.S. women aged 40-59 years, while the prevalence of severe obesity (BMI ≥ 40.0 kg/m2) was highest in this age group at 11.5%. “Midlife women gain weight at an average of approximately 1.5 pounds per year, which increases their risk for transitioning from normal or overweight to obese BMI,” the panelists wrote.
The review
Dr. Cantor’s group analyzed seven randomized controlled trials (RCTs) published up to October 2021 from 12 publications involving 51,638 participants. Although the trials were largely small and heterogeneous, they suggested that counseling may result in modest differences in weight change without causing important harms.
Four RCTs showed significant favorable weight changes for counseling over no-counseling control groups, with a mean difference of 0.87 to 2.5 kg, whereas one trial of counseling and two trials of exercise showed no differences. One of two RCTs reported improved quality-of-life measures.
As for harms, while interventions did not increase measures of depression or stress in one trial, self-reported falls (37% vs. 29%, P < .001) and injuries (19% vs. 14%, P = .03) were more frequent with exercise counseling in one trial.
“More research is needed to determine optimal content, frequency, length, and number of sessions required and should include additional patient populations,” Dr. Cantor and associates wrote.
In terms of limitations, the authors acknowledged that trials of behavioral interventions in maintaining or reducing weight in midlife women demonstrate small magnitudes of effect.
Offering a nonparticipant’s perspective on the WPSI guideline for this news organization, JoAnn E. Manson, MD, DrPH, MACP, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, said its message is of prime importance for women of middle age and it goes beyond concern about pounds lost or gained.
“Midlife and the transition to menopause are high-risk periods for women in terms of typical changes in body composition that increase the risk of adverse cardiometabolic outcomes,” said Dr. Manson, professor of women’s health at Harvard Medical School, Boston. “Counseling women should be a priority for physicians in clinical practice. And it’s not just whether weight gain is reflected on the scales or not but whether there’s an increase in central abdominal fat, a decrease in lean muscle mass, and an increase in adverse glucose tolerance.”
It is essential for women to be vigilant at this time, she added, and their exercise regimens should include strength and resistance training to preserve lean muscle mass and boost metabolic rate. Dr. Manson’s group has issued several statements stressing how important it is for clinicians to take decisive action on the counseling front and how they can do this in very little time during routine practice.
Also in full support of the guideline is Mary L. Rosser, MD, PhD, assistant professor of women’s health in obstetrics and gynecology at Columbia University Irving Medical Center in New York. “Midlife is a wonderful opportunity to encourage patients to assess their overall health status and make changes to impact their future health. Women in middle age tend to experience weight gain due to a variety of factors including aging and lifestyle,” said Dr. Rosser, who was not involved in the writing of the review or guideline.
While aging and genetics cannot be altered, behaviors can, and in her view, favorable behaviors would also include stress reduction and adequate sleep.
“The importance of reducing obesity with early intervention and prevention must focus on all women,” Dr. Rosser said. “We must narrow the inequities gap in care especially for high-risk minority groups and underserved populations. This will reduce disease and death and provide women the gift of active living and feeling better.”
The WPSI authors have made available a summary of the review and guideline for patients.
The systematic review and clinical guideline were funded by the federal Health Resources and Services Administration through ACOG. The authors of the guideline and the review authors disclosed no relevant financial conflicts of interest. Dr. Manson and Dr. Rosser disclosed no relevant competing interests with regard to their comments.
Midlife women who are of normal weight or are overweight should routinely receive counseling aimed at limiting weight gain and preventing obesity and its associated health risks, a new clinical guideline states.
The recommendation, issued by the Women’s Preventive Services Initiative (WPSI) of the American College of Obstetricians and Gynecologists (ACOG), supports regular lifestyle counseling for women aged 40-60 years with normal or overweight body mass index of 18.5-29.9 kg/m2. Counseling could include individualized discussion of healthy eating and physical activity initiated by health professionals involved in preventive care.
Published online in Annals of Internal Medicine, the guideline addresses the prevalence and health burdens of obesity in U.S. women of middle age and seeks to reduce the known harms of obesity with an intervention of minimal anticipated harms. High BMI increases the risk for many chronic conditions including hypertension, dyslipidemia, type 2 diabetes, coronary artery disease, stroke, and all-cause mortality.
The best way to counsel, however, remains unclear. “Although the optimal approach could not be discerned from existing trials, a range of interventions of varying duration, frequency, and intensity showed benefit with potential clinical significance,” wrote the WPSI guideline panel, led by David P. Chelmow, MD, chair of the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond.
The guideline rests on a systematic literature review led by family doctor Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center, at Oregon Health & Science University in Portland, suggesting moderate reductions in weight could be achieved by offering advice to this age group.
The federally supported WPSI was launched by ACOG in 2016. The guideline fills a gap in current recommendations in that it targets a specific risk group and specifies individual counseling based on its effectiveness and applicability in primary care settings.
In another benefit of routine counseling, the panel stated, “Normalizing counseling about healthy diet and physical activity by providing it to all midlife women may also mitigate concerns about weight stigma resulting from only counseling women with obesity.”
The panelists noted that during 2017-2018, the prevalence of obesity (BMI ≥ 30.0 kg/m2) was 43.3% among U.S. women aged 40-59 years, while the prevalence of severe obesity (BMI ≥ 40.0 kg/m2) was highest in this age group at 11.5%. “Midlife women gain weight at an average of approximately 1.5 pounds per year, which increases their risk for transitioning from normal or overweight to obese BMI,” the panelists wrote.
The review
Dr. Cantor’s group analyzed seven randomized controlled trials (RCTs) published up to October 2021 from 12 publications involving 51,638 participants. Although the trials were largely small and heterogeneous, they suggested that counseling may result in modest differences in weight change without causing important harms.
Four RCTs showed significant favorable weight changes for counseling over no-counseling control groups, with a mean difference of 0.87 to 2.5 kg, whereas one trial of counseling and two trials of exercise showed no differences. One of two RCTs reported improved quality-of-life measures.
As for harms, while interventions did not increase measures of depression or stress in one trial, self-reported falls (37% vs. 29%, P < .001) and injuries (19% vs. 14%, P = .03) were more frequent with exercise counseling in one trial.
“More research is needed to determine optimal content, frequency, length, and number of sessions required and should include additional patient populations,” Dr. Cantor and associates wrote.
In terms of limitations, the authors acknowledged that trials of behavioral interventions in maintaining or reducing weight in midlife women demonstrate small magnitudes of effect.
Offering a nonparticipant’s perspective on the WPSI guideline for this news organization, JoAnn E. Manson, MD, DrPH, MACP, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, said its message is of prime importance for women of middle age and it goes beyond concern about pounds lost or gained.
“Midlife and the transition to menopause are high-risk periods for women in terms of typical changes in body composition that increase the risk of adverse cardiometabolic outcomes,” said Dr. Manson, professor of women’s health at Harvard Medical School, Boston. “Counseling women should be a priority for physicians in clinical practice. And it’s not just whether weight gain is reflected on the scales or not but whether there’s an increase in central abdominal fat, a decrease in lean muscle mass, and an increase in adverse glucose tolerance.”
It is essential for women to be vigilant at this time, she added, and their exercise regimens should include strength and resistance training to preserve lean muscle mass and boost metabolic rate. Dr. Manson’s group has issued several statements stressing how important it is for clinicians to take decisive action on the counseling front and how they can do this in very little time during routine practice.
Also in full support of the guideline is Mary L. Rosser, MD, PhD, assistant professor of women’s health in obstetrics and gynecology at Columbia University Irving Medical Center in New York. “Midlife is a wonderful opportunity to encourage patients to assess their overall health status and make changes to impact their future health. Women in middle age tend to experience weight gain due to a variety of factors including aging and lifestyle,” said Dr. Rosser, who was not involved in the writing of the review or guideline.
While aging and genetics cannot be altered, behaviors can, and in her view, favorable behaviors would also include stress reduction and adequate sleep.
“The importance of reducing obesity with early intervention and prevention must focus on all women,” Dr. Rosser said. “We must narrow the inequities gap in care especially for high-risk minority groups and underserved populations. This will reduce disease and death and provide women the gift of active living and feeling better.”
The WPSI authors have made available a summary of the review and guideline for patients.
The systematic review and clinical guideline were funded by the federal Health Resources and Services Administration through ACOG. The authors of the guideline and the review authors disclosed no relevant financial conflicts of interest. Dr. Manson and Dr. Rosser disclosed no relevant competing interests with regard to their comments.
FROM ANNALS OF INTERNAL MEDICINE
Hormone therapy didn’t increase recurrence or mortality in women treated for breast cancer
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
2022 Update on menopause
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
Hormone therapy and breast cancer: An overview
It is projected that by 2050, 1.6 billion women in the world will have reached menopause or the postmenopausal period, a significant increase, compared with a billion women in 2020. Of all menopausal women, around 75% are affected by troublesome menopause symptoms, such as hot flashes and night sweats.
Around 84% of postmenopausal women experience genitourinary symptoms, such as vulvovaginal atrophy and incontinence.
Menopausal hormone therapy (MHT) is the most effective treatment for managing these symptoms; however, its effects on numerous aspects of female health remain uncertain, in particular with regard to breast cancer. The influence of MHT on breast cancer remains unsettled, with discordant findings from observational studies and randomized clinical trials, a factor that affects the decisions made by doctors concerning hormone therapy in menopausal women.
Background
Conjugated equine estrogens (CEEs) were introduced into clinical practice in the 1940s. For decades, MHT was the main treatment in conventional medicine for the symptoms of menopause. MHT was used in Western countries for about 600 million women starting from 1970, and it progressively increased during the 1990s. Professional organizations recommended MHT for the prevention of osteoporosis and chronic heart disease (CHD), and a third of prescriptions were for women older than 60 years.
Against this background, the National Institutes of Health launched randomized trials of MHT through the Women’s Health Initiative (WHI) to test whether the association with reduced risk for CHD found in observational studies was real and to obtain reliable information on the overall risks and benefits regarding the prevention of chronic disease for postmenopausal women aged 50-79 years.
The WHI trials tested standard-dose oral CEEs with and without standard-dose continuous medroxyprogesterone acetate (EPT). In 2002, the results of the WHI studies raised a series of concerns about the long-term safety of MHT, in particular the finding of an increased risk of breast cancer for women undergoing therapy. That risk exceeded the benefits from reductions in hip fractures and colorectal cancer.
The WHI findings received wide attention. Prescriptions for MHT dropped precipitously after 2002 and continued to decline in subsequent years. Declines were most marked for standard-dose EPT and in older women. The results of the CEE study were less negative, compared with those for EPT, as they showed no effect on CHD, a nonsignificant reduction in the risk of breast cancer, and a more favorable risk-benefit ratio for younger women, compared with older women. A decade later, it had become widely accepted that MHT should not be used for the prevention of chronic disease in older women; however, short-term use for treatment of vasomotor symptoms remains an accepted indication.
Risks and outcomes
Emerging from a series of WHI reports are complex models on the effect of hormonal therapy on the risk and outcome of breast cancer. In one study, women with an intact uterus received CEEs plus medroxyprogesterone acetate (MPA). An increase in the risk of breast cancer was observed over a median of 5.6 years of treatment, followed by a moderate reduction, with the risk increasing after 13 years of cumulative follow-up. For women treated with CEE alone, the reduction in risk observed over an average of 7.2 years of treatment was maintained for 13 years of follow-up.
Results from observational studies contrast with those from randomized controlled trials, particularly those concerning the use of estrogens only. A meta-analysis by the Collaborative Group on Hormonal Factors in Breast Cancer showed that both EPT and CEE were associated with a higher risk of breast neoplasia. Results of the Million Women Study showed a higher death rate.
Treatment methods and duration
Information from prospective studies on the effects of commencing MHT at various ages between 40 and 59 years show that for women who commenced treatment at any time within this age range, the relative risk was similar and was highly significant for all ages. Few women had started MHT treatment well after menopause at ages 60-69 years, and their excess risks during years 5-14 of current use were significant for estrogen-progestogen but not for estrogen-only MHT.
If these associations are largely causal, then for women of average weight in developed countries, 5 years of MHT, starting at age 50 years, would increase breast cancer incidence at ages 50-69 years by about 1 in every 50 users of estrogen plus daily progestogen preparations; 1 in every 70 users of estrogen plus intermittent progestogen preparations; and 1 in every 200 users of estrogen-only preparations. The corresponding excesses from 10 years of MHT would be about twice as great.
During 5-14 years of MHT use, the RRs were similarly increased if MHT use had started at ages 40-44 years, 45-49 years, 50-54 years, and 55-59 years; RRs appeared to be attenuated if MHT use had started after age 60 years. They were also attenuated by adiposity, particularly for estrogen-only MHT (which had little effect in obese women). After MHT use ceased, some excess risk of breast cancer persisted for more than a decade; this is directly correlated with the duration of treatment.
Therefore, it can be expected that the effects of MHT may vary between participants on the basis of age or time since menopause, as well as treatments (MHT type, dose, formulation, duration of use, and route of administration). Regarding formulation effects on the risk of breast cancer, new evidence shows an increased risk of 28%. Progestogens appeared to be differentially associated with breast cancer (micronized progesterone: odds ratio, 0.99; 95% confidence interval 0.55-1.79; synthetic progestin: OR, 1.28; 95% CI, 1.22-1.35). When prescribing MHT, micronized progesterone may be the safer progestogen to use.
In conclusion, MHT has a complex balance of benefits and risk on various health outcomes. Some effects differ qualitatively between ET and EPT. Regarding use of MHT, consideration should be given to the full range of effects, along with patients’ values and preferences. The overall quality of existing systematic reviews is moderate to poor. Clinicians should evaluate their scientific strength before considering applying their results in clinical practice. Regarding use of any hormone therapy regimen, consideration should be given to the full range of risk and benefits and should involve shared decisionmaking with the patient. It should be recognized that risk-benefit balance is altered by factors such as age, time from menopause, oophorectomy status, and prior hysterectomy and that some outcomes persist and there is some attenuation after stopping use.
This article was translated from Univadis Italy.
A version of the article appeared on Medscape.com.
It is projected that by 2050, 1.6 billion women in the world will have reached menopause or the postmenopausal period, a significant increase, compared with a billion women in 2020. Of all menopausal women, around 75% are affected by troublesome menopause symptoms, such as hot flashes and night sweats.
Around 84% of postmenopausal women experience genitourinary symptoms, such as vulvovaginal atrophy and incontinence.
Menopausal hormone therapy (MHT) is the most effective treatment for managing these symptoms; however, its effects on numerous aspects of female health remain uncertain, in particular with regard to breast cancer. The influence of MHT on breast cancer remains unsettled, with discordant findings from observational studies and randomized clinical trials, a factor that affects the decisions made by doctors concerning hormone therapy in menopausal women.
Background
Conjugated equine estrogens (CEEs) were introduced into clinical practice in the 1940s. For decades, MHT was the main treatment in conventional medicine for the symptoms of menopause. MHT was used in Western countries for about 600 million women starting from 1970, and it progressively increased during the 1990s. Professional organizations recommended MHT for the prevention of osteoporosis and chronic heart disease (CHD), and a third of prescriptions were for women older than 60 years.
Against this background, the National Institutes of Health launched randomized trials of MHT through the Women’s Health Initiative (WHI) to test whether the association with reduced risk for CHD found in observational studies was real and to obtain reliable information on the overall risks and benefits regarding the prevention of chronic disease for postmenopausal women aged 50-79 years.
The WHI trials tested standard-dose oral CEEs with and without standard-dose continuous medroxyprogesterone acetate (EPT). In 2002, the results of the WHI studies raised a series of concerns about the long-term safety of MHT, in particular the finding of an increased risk of breast cancer for women undergoing therapy. That risk exceeded the benefits from reductions in hip fractures and colorectal cancer.
The WHI findings received wide attention. Prescriptions for MHT dropped precipitously after 2002 and continued to decline in subsequent years. Declines were most marked for standard-dose EPT and in older women. The results of the CEE study were less negative, compared with those for EPT, as they showed no effect on CHD, a nonsignificant reduction in the risk of breast cancer, and a more favorable risk-benefit ratio for younger women, compared with older women. A decade later, it had become widely accepted that MHT should not be used for the prevention of chronic disease in older women; however, short-term use for treatment of vasomotor symptoms remains an accepted indication.
Risks and outcomes
Emerging from a series of WHI reports are complex models on the effect of hormonal therapy on the risk and outcome of breast cancer. In one study, women with an intact uterus received CEEs plus medroxyprogesterone acetate (MPA). An increase in the risk of breast cancer was observed over a median of 5.6 years of treatment, followed by a moderate reduction, with the risk increasing after 13 years of cumulative follow-up. For women treated with CEE alone, the reduction in risk observed over an average of 7.2 years of treatment was maintained for 13 years of follow-up.
Results from observational studies contrast with those from randomized controlled trials, particularly those concerning the use of estrogens only. A meta-analysis by the Collaborative Group on Hormonal Factors in Breast Cancer showed that both EPT and CEE were associated with a higher risk of breast neoplasia. Results of the Million Women Study showed a higher death rate.
Treatment methods and duration
Information from prospective studies on the effects of commencing MHT at various ages between 40 and 59 years show that for women who commenced treatment at any time within this age range, the relative risk was similar and was highly significant for all ages. Few women had started MHT treatment well after menopause at ages 60-69 years, and their excess risks during years 5-14 of current use were significant for estrogen-progestogen but not for estrogen-only MHT.
If these associations are largely causal, then for women of average weight in developed countries, 5 years of MHT, starting at age 50 years, would increase breast cancer incidence at ages 50-69 years by about 1 in every 50 users of estrogen plus daily progestogen preparations; 1 in every 70 users of estrogen plus intermittent progestogen preparations; and 1 in every 200 users of estrogen-only preparations. The corresponding excesses from 10 years of MHT would be about twice as great.
During 5-14 years of MHT use, the RRs were similarly increased if MHT use had started at ages 40-44 years, 45-49 years, 50-54 years, and 55-59 years; RRs appeared to be attenuated if MHT use had started after age 60 years. They were also attenuated by adiposity, particularly for estrogen-only MHT (which had little effect in obese women). After MHT use ceased, some excess risk of breast cancer persisted for more than a decade; this is directly correlated with the duration of treatment.
Therefore, it can be expected that the effects of MHT may vary between participants on the basis of age or time since menopause, as well as treatments (MHT type, dose, formulation, duration of use, and route of administration). Regarding formulation effects on the risk of breast cancer, new evidence shows an increased risk of 28%. Progestogens appeared to be differentially associated with breast cancer (micronized progesterone: odds ratio, 0.99; 95% confidence interval 0.55-1.79; synthetic progestin: OR, 1.28; 95% CI, 1.22-1.35). When prescribing MHT, micronized progesterone may be the safer progestogen to use.
In conclusion, MHT has a complex balance of benefits and risk on various health outcomes. Some effects differ qualitatively between ET and EPT. Regarding use of MHT, consideration should be given to the full range of effects, along with patients’ values and preferences. The overall quality of existing systematic reviews is moderate to poor. Clinicians should evaluate their scientific strength before considering applying their results in clinical practice. Regarding use of any hormone therapy regimen, consideration should be given to the full range of risk and benefits and should involve shared decisionmaking with the patient. It should be recognized that risk-benefit balance is altered by factors such as age, time from menopause, oophorectomy status, and prior hysterectomy and that some outcomes persist and there is some attenuation after stopping use.
This article was translated from Univadis Italy.
A version of the article appeared on Medscape.com.
It is projected that by 2050, 1.6 billion women in the world will have reached menopause or the postmenopausal period, a significant increase, compared with a billion women in 2020. Of all menopausal women, around 75% are affected by troublesome menopause symptoms, such as hot flashes and night sweats.
Around 84% of postmenopausal women experience genitourinary symptoms, such as vulvovaginal atrophy and incontinence.
Menopausal hormone therapy (MHT) is the most effective treatment for managing these symptoms; however, its effects on numerous aspects of female health remain uncertain, in particular with regard to breast cancer. The influence of MHT on breast cancer remains unsettled, with discordant findings from observational studies and randomized clinical trials, a factor that affects the decisions made by doctors concerning hormone therapy in menopausal women.
Background
Conjugated equine estrogens (CEEs) were introduced into clinical practice in the 1940s. For decades, MHT was the main treatment in conventional medicine for the symptoms of menopause. MHT was used in Western countries for about 600 million women starting from 1970, and it progressively increased during the 1990s. Professional organizations recommended MHT for the prevention of osteoporosis and chronic heart disease (CHD), and a third of prescriptions were for women older than 60 years.
Against this background, the National Institutes of Health launched randomized trials of MHT through the Women’s Health Initiative (WHI) to test whether the association with reduced risk for CHD found in observational studies was real and to obtain reliable information on the overall risks and benefits regarding the prevention of chronic disease for postmenopausal women aged 50-79 years.
The WHI trials tested standard-dose oral CEEs with and without standard-dose continuous medroxyprogesterone acetate (EPT). In 2002, the results of the WHI studies raised a series of concerns about the long-term safety of MHT, in particular the finding of an increased risk of breast cancer for women undergoing therapy. That risk exceeded the benefits from reductions in hip fractures and colorectal cancer.
The WHI findings received wide attention. Prescriptions for MHT dropped precipitously after 2002 and continued to decline in subsequent years. Declines were most marked for standard-dose EPT and in older women. The results of the CEE study were less negative, compared with those for EPT, as they showed no effect on CHD, a nonsignificant reduction in the risk of breast cancer, and a more favorable risk-benefit ratio for younger women, compared with older women. A decade later, it had become widely accepted that MHT should not be used for the prevention of chronic disease in older women; however, short-term use for treatment of vasomotor symptoms remains an accepted indication.
Risks and outcomes
Emerging from a series of WHI reports are complex models on the effect of hormonal therapy on the risk and outcome of breast cancer. In one study, women with an intact uterus received CEEs plus medroxyprogesterone acetate (MPA). An increase in the risk of breast cancer was observed over a median of 5.6 years of treatment, followed by a moderate reduction, with the risk increasing after 13 years of cumulative follow-up. For women treated with CEE alone, the reduction in risk observed over an average of 7.2 years of treatment was maintained for 13 years of follow-up.
Results from observational studies contrast with those from randomized controlled trials, particularly those concerning the use of estrogens only. A meta-analysis by the Collaborative Group on Hormonal Factors in Breast Cancer showed that both EPT and CEE were associated with a higher risk of breast neoplasia. Results of the Million Women Study showed a higher death rate.
Treatment methods and duration
Information from prospective studies on the effects of commencing MHT at various ages between 40 and 59 years show that for women who commenced treatment at any time within this age range, the relative risk was similar and was highly significant for all ages. Few women had started MHT treatment well after menopause at ages 60-69 years, and their excess risks during years 5-14 of current use were significant for estrogen-progestogen but not for estrogen-only MHT.
If these associations are largely causal, then for women of average weight in developed countries, 5 years of MHT, starting at age 50 years, would increase breast cancer incidence at ages 50-69 years by about 1 in every 50 users of estrogen plus daily progestogen preparations; 1 in every 70 users of estrogen plus intermittent progestogen preparations; and 1 in every 200 users of estrogen-only preparations. The corresponding excesses from 10 years of MHT would be about twice as great.
During 5-14 years of MHT use, the RRs were similarly increased if MHT use had started at ages 40-44 years, 45-49 years, 50-54 years, and 55-59 years; RRs appeared to be attenuated if MHT use had started after age 60 years. They were also attenuated by adiposity, particularly for estrogen-only MHT (which had little effect in obese women). After MHT use ceased, some excess risk of breast cancer persisted for more than a decade; this is directly correlated with the duration of treatment.
Therefore, it can be expected that the effects of MHT may vary between participants on the basis of age or time since menopause, as well as treatments (MHT type, dose, formulation, duration of use, and route of administration). Regarding formulation effects on the risk of breast cancer, new evidence shows an increased risk of 28%. Progestogens appeared to be differentially associated with breast cancer (micronized progesterone: odds ratio, 0.99; 95% confidence interval 0.55-1.79; synthetic progestin: OR, 1.28; 95% CI, 1.22-1.35). When prescribing MHT, micronized progesterone may be the safer progestogen to use.
In conclusion, MHT has a complex balance of benefits and risk on various health outcomes. Some effects differ qualitatively between ET and EPT. Regarding use of MHT, consideration should be given to the full range of effects, along with patients’ values and preferences. The overall quality of existing systematic reviews is moderate to poor. Clinicians should evaluate their scientific strength before considering applying their results in clinical practice. Regarding use of any hormone therapy regimen, consideration should be given to the full range of risk and benefits and should involve shared decisionmaking with the patient. It should be recognized that risk-benefit balance is altered by factors such as age, time from menopause, oophorectomy status, and prior hysterectomy and that some outcomes persist and there is some attenuation after stopping use.
This article was translated from Univadis Italy.
A version of the article appeared on Medscape.com.
COVID-19 Pandemic stress affected ovulation, not menstruation
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ENDO 2022
Why do we treat menopause as a disease?
Menopause gets a bad rap in medical literature and throughout society, say authors of a new analysis. And they argue that the negativity undermines women’s health outlook in the years that should be a natural life transition.
Menopause has been medicalized over centuries and talked about as if it were a disease, they say, and that may increase women’s anxiety and apprehension about the midlife stage.
It’s time to change the narrative, says Martha Hickey, MD, with the department of obstetrics and gynaecology at the Royal Women’s Hospital in Victoria, Australia, and her coauthors. Their analysis was published online in the BMJ.
“The message that menopause signals decay and decline, which can potentially be delayed or reversed by hormonal treatments, persists and is reinforced by the media, medical literature, and information for women, often driven by marketing interests,” they write.
Such messages may chip away at women’s confidence. Dr. Hickey and colleagues cite surveys in the United States and Ireland that found that most women (65%-77%) feel unprepared for menopause.
“Together with limited public discussion and education and shame attached to ageing in women, this may contribute to embarrassment and negative expectations about menopause,” the authors write.
The ‘untold misery of oestrogen-starved women’
These messages have deep roots. Take for instance, gynecologist Robert Wilson’s words in his 1966 book “Feminine Forever.” The authors note he recommended estrogen for all menopausal women “to treat their ‘serious, painful and often crippling disease’ and avoid the ‘untold misery of alcoholism, drug addiction, divorce, and broken
homes caused by these unstable, oestrogen-starved women.’ ”
Women experience menopause in very different ways. Experience with menopause also differs by country, the authors explain. “Women’s experience of menopause is also strongly influenced by social values around reproduction and ageing, with positive or negative ramifications,” they write.
“For example, women tend to have worse experiences of menopause in countries where their value is predicated on youth and reproductive capacity and ageing is associated with decline.”
The authors argue that the medicalization of menopause has condensed the wide range of women’s experiences at a typical age into “a narrowly defined disease requiring treatment.”
Promoting exercise, stopping smoking among positive messages
An editorial by Haitham Hamoda, MD, and Sara Moger, with the British Menopause Society, notes that more than 75% of women experiencing menopause report symptoms, and more than 25% describe severe symptoms.
The editorialists point out that the National Institute of Health and Care Excellence and others recommend an individualized approach to addressing menopause that includes a comprehensive approach – advice on exercise, weight management, stopping smoking, and reducing alcohol as well as options such as hormone therapy (HT).
The literature says the main indication for HT is for severe symptoms and not as a preventive measure. “Evidence does not support use of HT to reduce the risk of dementia,” they point out.
While some women may benefit from HT, that should not be explored to the exclusion of other avenues of help, Dr. Hickey and colleagues write. Risks must also be considered.
Menopause blamed in a difficult time of life
Jennifer Howell, MD, an obstetrician/gynecologist and certified menopause provider at Duke University in Durham, N.C., told this news organization that menopause is often blamed in a time of life when women naturally are experiencing an array of stressful and emotional changes.
It often coincides with children heading to college, navigating midlife challenges in marriage, helping aging parents, managing demanding careers, and health issues.
People want a reason for changes women experience, and too often the finger gets pointed at menopause, Dr. Howell said.
The message women hear has always been, “It’s got to be your hormones. And people want to hear that there’s a hormonal solution.”
Making menopause the target also has led to nonevidence-based “snake-oil” type remedies sold in unregulated powders, creams, and pellets, Dr. Howell noted.
Dr. Howell has treated thousands of menopausal women in her clinic and she says she spends a good deal of time with them explaining a holistic view of the process, much like what the authors describe, with lifestyle changes and treatment options.
Sometimes HT is the solution, Dr. Howell says, but “it’s become a crutch. Hormones are not a panacea.”
She is frustrated with the amount of disinformation circulating online. Groups like the North American Menopause Society put out reliable evidence-based information, but they compete “with a lot of nonsense,” she says.
The message that women should hear, she says is that “[menopause] is a natural part of aging and there may or may not be symptoms that come along with it. If there are, there are things we can do,” she says.
Menopause gets a bad rap in medical literature and throughout society, say authors of a new analysis. And they argue that the negativity undermines women’s health outlook in the years that should be a natural life transition.
Menopause has been medicalized over centuries and talked about as if it were a disease, they say, and that may increase women’s anxiety and apprehension about the midlife stage.
It’s time to change the narrative, says Martha Hickey, MD, with the department of obstetrics and gynaecology at the Royal Women’s Hospital in Victoria, Australia, and her coauthors. Their analysis was published online in the BMJ.
“The message that menopause signals decay and decline, which can potentially be delayed or reversed by hormonal treatments, persists and is reinforced by the media, medical literature, and information for women, often driven by marketing interests,” they write.
Such messages may chip away at women’s confidence. Dr. Hickey and colleagues cite surveys in the United States and Ireland that found that most women (65%-77%) feel unprepared for menopause.
“Together with limited public discussion and education and shame attached to ageing in women, this may contribute to embarrassment and negative expectations about menopause,” the authors write.
The ‘untold misery of oestrogen-starved women’
These messages have deep roots. Take for instance, gynecologist Robert Wilson’s words in his 1966 book “Feminine Forever.” The authors note he recommended estrogen for all menopausal women “to treat their ‘serious, painful and often crippling disease’ and avoid the ‘untold misery of alcoholism, drug addiction, divorce, and broken
homes caused by these unstable, oestrogen-starved women.’ ”
Women experience menopause in very different ways. Experience with menopause also differs by country, the authors explain. “Women’s experience of menopause is also strongly influenced by social values around reproduction and ageing, with positive or negative ramifications,” they write.
“For example, women tend to have worse experiences of menopause in countries where their value is predicated on youth and reproductive capacity and ageing is associated with decline.”
The authors argue that the medicalization of menopause has condensed the wide range of women’s experiences at a typical age into “a narrowly defined disease requiring treatment.”
Promoting exercise, stopping smoking among positive messages
An editorial by Haitham Hamoda, MD, and Sara Moger, with the British Menopause Society, notes that more than 75% of women experiencing menopause report symptoms, and more than 25% describe severe symptoms.
The editorialists point out that the National Institute of Health and Care Excellence and others recommend an individualized approach to addressing menopause that includes a comprehensive approach – advice on exercise, weight management, stopping smoking, and reducing alcohol as well as options such as hormone therapy (HT).
The literature says the main indication for HT is for severe symptoms and not as a preventive measure. “Evidence does not support use of HT to reduce the risk of dementia,” they point out.
While some women may benefit from HT, that should not be explored to the exclusion of other avenues of help, Dr. Hickey and colleagues write. Risks must also be considered.
Menopause blamed in a difficult time of life
Jennifer Howell, MD, an obstetrician/gynecologist and certified menopause provider at Duke University in Durham, N.C., told this news organization that menopause is often blamed in a time of life when women naturally are experiencing an array of stressful and emotional changes.
It often coincides with children heading to college, navigating midlife challenges in marriage, helping aging parents, managing demanding careers, and health issues.
People want a reason for changes women experience, and too often the finger gets pointed at menopause, Dr. Howell said.
The message women hear has always been, “It’s got to be your hormones. And people want to hear that there’s a hormonal solution.”
Making menopause the target also has led to nonevidence-based “snake-oil” type remedies sold in unregulated powders, creams, and pellets, Dr. Howell noted.
Dr. Howell has treated thousands of menopausal women in her clinic and she says she spends a good deal of time with them explaining a holistic view of the process, much like what the authors describe, with lifestyle changes and treatment options.
Sometimes HT is the solution, Dr. Howell says, but “it’s become a crutch. Hormones are not a panacea.”
She is frustrated with the amount of disinformation circulating online. Groups like the North American Menopause Society put out reliable evidence-based information, but they compete “with a lot of nonsense,” she says.
The message that women should hear, she says is that “[menopause] is a natural part of aging and there may or may not be symptoms that come along with it. If there are, there are things we can do,” she says.
Menopause gets a bad rap in medical literature and throughout society, say authors of a new analysis. And they argue that the negativity undermines women’s health outlook in the years that should be a natural life transition.
Menopause has been medicalized over centuries and talked about as if it were a disease, they say, and that may increase women’s anxiety and apprehension about the midlife stage.
It’s time to change the narrative, says Martha Hickey, MD, with the department of obstetrics and gynaecology at the Royal Women’s Hospital in Victoria, Australia, and her coauthors. Their analysis was published online in the BMJ.
“The message that menopause signals decay and decline, which can potentially be delayed or reversed by hormonal treatments, persists and is reinforced by the media, medical literature, and information for women, often driven by marketing interests,” they write.
Such messages may chip away at women’s confidence. Dr. Hickey and colleagues cite surveys in the United States and Ireland that found that most women (65%-77%) feel unprepared for menopause.
“Together with limited public discussion and education and shame attached to ageing in women, this may contribute to embarrassment and negative expectations about menopause,” the authors write.
The ‘untold misery of oestrogen-starved women’
These messages have deep roots. Take for instance, gynecologist Robert Wilson’s words in his 1966 book “Feminine Forever.” The authors note he recommended estrogen for all menopausal women “to treat their ‘serious, painful and often crippling disease’ and avoid the ‘untold misery of alcoholism, drug addiction, divorce, and broken
homes caused by these unstable, oestrogen-starved women.’ ”
Women experience menopause in very different ways. Experience with menopause also differs by country, the authors explain. “Women’s experience of menopause is also strongly influenced by social values around reproduction and ageing, with positive or negative ramifications,” they write.
“For example, women tend to have worse experiences of menopause in countries where their value is predicated on youth and reproductive capacity and ageing is associated with decline.”
The authors argue that the medicalization of menopause has condensed the wide range of women’s experiences at a typical age into “a narrowly defined disease requiring treatment.”
Promoting exercise, stopping smoking among positive messages
An editorial by Haitham Hamoda, MD, and Sara Moger, with the British Menopause Society, notes that more than 75% of women experiencing menopause report symptoms, and more than 25% describe severe symptoms.
The editorialists point out that the National Institute of Health and Care Excellence and others recommend an individualized approach to addressing menopause that includes a comprehensive approach – advice on exercise, weight management, stopping smoking, and reducing alcohol as well as options such as hormone therapy (HT).
The literature says the main indication for HT is for severe symptoms and not as a preventive measure. “Evidence does not support use of HT to reduce the risk of dementia,” they point out.
While some women may benefit from HT, that should not be explored to the exclusion of other avenues of help, Dr. Hickey and colleagues write. Risks must also be considered.
Menopause blamed in a difficult time of life
Jennifer Howell, MD, an obstetrician/gynecologist and certified menopause provider at Duke University in Durham, N.C., told this news organization that menopause is often blamed in a time of life when women naturally are experiencing an array of stressful and emotional changes.
It often coincides with children heading to college, navigating midlife challenges in marriage, helping aging parents, managing demanding careers, and health issues.
People want a reason for changes women experience, and too often the finger gets pointed at menopause, Dr. Howell said.
The message women hear has always been, “It’s got to be your hormones. And people want to hear that there’s a hormonal solution.”
Making menopause the target also has led to nonevidence-based “snake-oil” type remedies sold in unregulated powders, creams, and pellets, Dr. Howell noted.
Dr. Howell has treated thousands of menopausal women in her clinic and she says she spends a good deal of time with them explaining a holistic view of the process, much like what the authors describe, with lifestyle changes and treatment options.
Sometimes HT is the solution, Dr. Howell says, but “it’s become a crutch. Hormones are not a panacea.”
She is frustrated with the amount of disinformation circulating online. Groups like the North American Menopause Society put out reliable evidence-based information, but they compete “with a lot of nonsense,” she says.
The message that women should hear, she says is that “[menopause] is a natural part of aging and there may or may not be symptoms that come along with it. If there are, there are things we can do,” she says.
FROM BMJ
Nonhormonal drug for menopause symptoms passes phase 3 test
A phase 3 trial has associated the neurokinin-3 (NK3)–receptor inhibitor fezolinetant, an oral therapy taken once daily, with substantial control over the symptoms of menopause, according to results of the randomized SKYLIGHT 2 trial.
The nonhormonal therapy has the potential to address an important unmet need, Genevieve Neal-Perry, MD, PhD, said at the annual meeting of the Endocrine Society.
The health risks of hormone therapy (HT) have “caused quite a few women to consider whether hormone replacement is right for them, and, in addition, there are other individuals who have hormone-responsive cancers or other disorders that might prohibit them [from using HT],” Dr. Neal-Perry said.
The NK3 receptor stimulates the thermoregulatory center in the hypothalamus. By blocking the NK3 receptor, vasodilation and other downstream effects are inhibited, explained Dr. Neal-Perry. She credited relatively recent advances in understanding the mechanisms of menopausal symptoms for identifying this and other potentially targetable mediators.
SKYLIGHT 2 trial: Two phases
In the double-blind multinational phase 3 SKYLIGHT 2 trial, 484 otherwise healthy symptomatic menopausal women were randomized to 30 mg of fezolinetant, 45 mg of fezolinetant, or placebo. The 120 participating centers were in North American and Europe.
In the first phase, safety and efficacy were evaluated over 12 weeks. In a second extension phase, placebo patients were rerandomized to one of the fezolinetant study doses. Those on active therapy remained in their assigned groups. All patients were then followed for an additional 40 weeks.
The coprimary endpoints were frequency and severity of moderate to severe vasomotor symptoms as reported by patients using an electronic diary. There were several secondary endpoints, including patient-reported outcomes regarding sleep quality.
As expected from other controlled trials, placebo patients achieved about a 40% reduction in moderate to severe vasomotor symptom frequency over the first 12 weeks. Relative to placebo, symptom frequency declined more quickly and steeply on fezolinetant. By week 12, both achieved reductions of about 60%. Statistical P values for the differences in the three arms were not provided, but Dr. Neal-Perry reported they were significant.
Vasomotor severity, like frequency, is reduced
The change in vasomotor severity, which subjects in the trial rated as better or worse, was also significant. The differences in the severity curves were less, but they separated in favor of the two active treatment arms by about 2 weeks, and the curves continued to show an advantage for fezolinetant over both the first 12 weeks and then the remaining 40 weeks.
Overall, the decline in vasomotor symptom frequency remained on a persistent downward slope on both doses of fezolinetant for the full 52 weeks of the study, so that the reduction at 52 weeks was on the order of 25% greater than that seen at 12 weeks.
At 52 weeks, “you can see that individuals on placebo who were crossed over to an active treatment had a significant reduction in their hot flashes and look very much like those who were randomized to fezolinetant at the beginning of the study,” said Dr. Neal-Perry, who is chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
Other outcomes also favored fezolinetant over placebo. For example, a reduction in sleep disturbance observed at 12 weeks was sustained over the full 52 weeks of the study. The reduction in sleep symptoms appeared to be slightly greater on the higher dose, but the benefit at 52 weeks among patients after the crossover was similar on either active arm.
No serious side effects identified
There were no serious drug-related treatment-emergent adverse events in any treatment group. One patient in the placebo arm (< 1%), two patients in the 30-mg fezolinetant arm (1.2%), and five patients in the 45-mg arm (3%) discontinued therapy for an adverse event considered to be treatment related.
“The most common side effect associated with fezolinetant was headache. There were no other side effects that led patients to pull out of the study,” Dr. Neal-Perry reported at the meeting, which was held in Atlanta and virtually.
According to Dr. Neal-Perry the vasomotor symptoms relative to menopause, which occur in almost all women, are moderate to severe in an estimated 35%-45%. Some groups, such as those with an elevated body mass index and African Americans, appear to be at even greater risk. Study enrollment was specifically designed to include these high-risk groups, but the subgroup efficacy data have not yet been analyzed.
Other drugs with a similar mechanism of action have not been brought forward because of concern about elevated liver enzymes, but Dr. Neal-Perry said that this does not appear to be an issue for fezolinetant, which was designed with greater specificity for the NK3 target than previous treatments.
If fezolinetant is approved, Dr. Neal-Perry expects this agent to fulfill an important unmet need because of the limitations of other nonhormonal solutions for control of menopause symptoms.
HT alternatives limited
For control of many menopause symptoms, particularly hot flashes, hormone therapy (HT) is the most efficacious, but Richard J. Santen, MD, emeritus professor and an endocrinologist at the University of Virginia, Charlottesville, agreed there is a need for alternatives.
In addition to those who have contraindications for HT, Dr. Santen said in an interview that this option is not acceptable to others “for a variety of reasons.” The problem is that the alternatives are limited.
“The SSRI agents and gabapentin are alternative nonhormonal agents, but they have side effects and are not as effective,” he said. Hot flashes “can be a major disruptor of quality of life,” so he is intrigued with the positive results achieved with fezolinetant.
“A new drug such as reported at the Endocrine Society meeting would be an important new addition to the armamentarium,” he said.
Dr. Neal-Perry reports no conflicts of interest.
A phase 3 trial has associated the neurokinin-3 (NK3)–receptor inhibitor fezolinetant, an oral therapy taken once daily, with substantial control over the symptoms of menopause, according to results of the randomized SKYLIGHT 2 trial.
The nonhormonal therapy has the potential to address an important unmet need, Genevieve Neal-Perry, MD, PhD, said at the annual meeting of the Endocrine Society.
The health risks of hormone therapy (HT) have “caused quite a few women to consider whether hormone replacement is right for them, and, in addition, there are other individuals who have hormone-responsive cancers or other disorders that might prohibit them [from using HT],” Dr. Neal-Perry said.
The NK3 receptor stimulates the thermoregulatory center in the hypothalamus. By blocking the NK3 receptor, vasodilation and other downstream effects are inhibited, explained Dr. Neal-Perry. She credited relatively recent advances in understanding the mechanisms of menopausal symptoms for identifying this and other potentially targetable mediators.
SKYLIGHT 2 trial: Two phases
In the double-blind multinational phase 3 SKYLIGHT 2 trial, 484 otherwise healthy symptomatic menopausal women were randomized to 30 mg of fezolinetant, 45 mg of fezolinetant, or placebo. The 120 participating centers were in North American and Europe.
In the first phase, safety and efficacy were evaluated over 12 weeks. In a second extension phase, placebo patients were rerandomized to one of the fezolinetant study doses. Those on active therapy remained in their assigned groups. All patients were then followed for an additional 40 weeks.
The coprimary endpoints were frequency and severity of moderate to severe vasomotor symptoms as reported by patients using an electronic diary. There were several secondary endpoints, including patient-reported outcomes regarding sleep quality.
As expected from other controlled trials, placebo patients achieved about a 40% reduction in moderate to severe vasomotor symptom frequency over the first 12 weeks. Relative to placebo, symptom frequency declined more quickly and steeply on fezolinetant. By week 12, both achieved reductions of about 60%. Statistical P values for the differences in the three arms were not provided, but Dr. Neal-Perry reported they were significant.
Vasomotor severity, like frequency, is reduced
The change in vasomotor severity, which subjects in the trial rated as better or worse, was also significant. The differences in the severity curves were less, but they separated in favor of the two active treatment arms by about 2 weeks, and the curves continued to show an advantage for fezolinetant over both the first 12 weeks and then the remaining 40 weeks.
Overall, the decline in vasomotor symptom frequency remained on a persistent downward slope on both doses of fezolinetant for the full 52 weeks of the study, so that the reduction at 52 weeks was on the order of 25% greater than that seen at 12 weeks.
At 52 weeks, “you can see that individuals on placebo who were crossed over to an active treatment had a significant reduction in their hot flashes and look very much like those who were randomized to fezolinetant at the beginning of the study,” said Dr. Neal-Perry, who is chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
Other outcomes also favored fezolinetant over placebo. For example, a reduction in sleep disturbance observed at 12 weeks was sustained over the full 52 weeks of the study. The reduction in sleep symptoms appeared to be slightly greater on the higher dose, but the benefit at 52 weeks among patients after the crossover was similar on either active arm.
No serious side effects identified
There were no serious drug-related treatment-emergent adverse events in any treatment group. One patient in the placebo arm (< 1%), two patients in the 30-mg fezolinetant arm (1.2%), and five patients in the 45-mg arm (3%) discontinued therapy for an adverse event considered to be treatment related.
“The most common side effect associated with fezolinetant was headache. There were no other side effects that led patients to pull out of the study,” Dr. Neal-Perry reported at the meeting, which was held in Atlanta and virtually.
According to Dr. Neal-Perry the vasomotor symptoms relative to menopause, which occur in almost all women, are moderate to severe in an estimated 35%-45%. Some groups, such as those with an elevated body mass index and African Americans, appear to be at even greater risk. Study enrollment was specifically designed to include these high-risk groups, but the subgroup efficacy data have not yet been analyzed.
Other drugs with a similar mechanism of action have not been brought forward because of concern about elevated liver enzymes, but Dr. Neal-Perry said that this does not appear to be an issue for fezolinetant, which was designed with greater specificity for the NK3 target than previous treatments.
If fezolinetant is approved, Dr. Neal-Perry expects this agent to fulfill an important unmet need because of the limitations of other nonhormonal solutions for control of menopause symptoms.
HT alternatives limited
For control of many menopause symptoms, particularly hot flashes, hormone therapy (HT) is the most efficacious, but Richard J. Santen, MD, emeritus professor and an endocrinologist at the University of Virginia, Charlottesville, agreed there is a need for alternatives.
In addition to those who have contraindications for HT, Dr. Santen said in an interview that this option is not acceptable to others “for a variety of reasons.” The problem is that the alternatives are limited.
“The SSRI agents and gabapentin are alternative nonhormonal agents, but they have side effects and are not as effective,” he said. Hot flashes “can be a major disruptor of quality of life,” so he is intrigued with the positive results achieved with fezolinetant.
“A new drug such as reported at the Endocrine Society meeting would be an important new addition to the armamentarium,” he said.
Dr. Neal-Perry reports no conflicts of interest.
A phase 3 trial has associated the neurokinin-3 (NK3)–receptor inhibitor fezolinetant, an oral therapy taken once daily, with substantial control over the symptoms of menopause, according to results of the randomized SKYLIGHT 2 trial.
The nonhormonal therapy has the potential to address an important unmet need, Genevieve Neal-Perry, MD, PhD, said at the annual meeting of the Endocrine Society.
The health risks of hormone therapy (HT) have “caused quite a few women to consider whether hormone replacement is right for them, and, in addition, there are other individuals who have hormone-responsive cancers or other disorders that might prohibit them [from using HT],” Dr. Neal-Perry said.
The NK3 receptor stimulates the thermoregulatory center in the hypothalamus. By blocking the NK3 receptor, vasodilation and other downstream effects are inhibited, explained Dr. Neal-Perry. She credited relatively recent advances in understanding the mechanisms of menopausal symptoms for identifying this and other potentially targetable mediators.
SKYLIGHT 2 trial: Two phases
In the double-blind multinational phase 3 SKYLIGHT 2 trial, 484 otherwise healthy symptomatic menopausal women were randomized to 30 mg of fezolinetant, 45 mg of fezolinetant, or placebo. The 120 participating centers were in North American and Europe.
In the first phase, safety and efficacy were evaluated over 12 weeks. In a second extension phase, placebo patients were rerandomized to one of the fezolinetant study doses. Those on active therapy remained in their assigned groups. All patients were then followed for an additional 40 weeks.
The coprimary endpoints were frequency and severity of moderate to severe vasomotor symptoms as reported by patients using an electronic diary. There were several secondary endpoints, including patient-reported outcomes regarding sleep quality.
As expected from other controlled trials, placebo patients achieved about a 40% reduction in moderate to severe vasomotor symptom frequency over the first 12 weeks. Relative to placebo, symptom frequency declined more quickly and steeply on fezolinetant. By week 12, both achieved reductions of about 60%. Statistical P values for the differences in the three arms were not provided, but Dr. Neal-Perry reported they were significant.
Vasomotor severity, like frequency, is reduced
The change in vasomotor severity, which subjects in the trial rated as better or worse, was also significant. The differences in the severity curves were less, but they separated in favor of the two active treatment arms by about 2 weeks, and the curves continued to show an advantage for fezolinetant over both the first 12 weeks and then the remaining 40 weeks.
Overall, the decline in vasomotor symptom frequency remained on a persistent downward slope on both doses of fezolinetant for the full 52 weeks of the study, so that the reduction at 52 weeks was on the order of 25% greater than that seen at 12 weeks.
At 52 weeks, “you can see that individuals on placebo who were crossed over to an active treatment had a significant reduction in their hot flashes and look very much like those who were randomized to fezolinetant at the beginning of the study,” said Dr. Neal-Perry, who is chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
Other outcomes also favored fezolinetant over placebo. For example, a reduction in sleep disturbance observed at 12 weeks was sustained over the full 52 weeks of the study. The reduction in sleep symptoms appeared to be slightly greater on the higher dose, but the benefit at 52 weeks among patients after the crossover was similar on either active arm.
No serious side effects identified
There were no serious drug-related treatment-emergent adverse events in any treatment group. One patient in the placebo arm (< 1%), two patients in the 30-mg fezolinetant arm (1.2%), and five patients in the 45-mg arm (3%) discontinued therapy for an adverse event considered to be treatment related.
“The most common side effect associated with fezolinetant was headache. There were no other side effects that led patients to pull out of the study,” Dr. Neal-Perry reported at the meeting, which was held in Atlanta and virtually.
According to Dr. Neal-Perry the vasomotor symptoms relative to menopause, which occur in almost all women, are moderate to severe in an estimated 35%-45%. Some groups, such as those with an elevated body mass index and African Americans, appear to be at even greater risk. Study enrollment was specifically designed to include these high-risk groups, but the subgroup efficacy data have not yet been analyzed.
Other drugs with a similar mechanism of action have not been brought forward because of concern about elevated liver enzymes, but Dr. Neal-Perry said that this does not appear to be an issue for fezolinetant, which was designed with greater specificity for the NK3 target than previous treatments.
If fezolinetant is approved, Dr. Neal-Perry expects this agent to fulfill an important unmet need because of the limitations of other nonhormonal solutions for control of menopause symptoms.
HT alternatives limited
For control of many menopause symptoms, particularly hot flashes, hormone therapy (HT) is the most efficacious, but Richard J. Santen, MD, emeritus professor and an endocrinologist at the University of Virginia, Charlottesville, agreed there is a need for alternatives.
In addition to those who have contraindications for HT, Dr. Santen said in an interview that this option is not acceptable to others “for a variety of reasons.” The problem is that the alternatives are limited.
“The SSRI agents and gabapentin are alternative nonhormonal agents, but they have side effects and are not as effective,” he said. Hot flashes “can be a major disruptor of quality of life,” so he is intrigued with the positive results achieved with fezolinetant.
“A new drug such as reported at the Endocrine Society meeting would be an important new addition to the armamentarium,” he said.
Dr. Neal-Perry reports no conflicts of interest.
FROM ENDO 2022
Surgeons may underestimate recovery from incontinence operation
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AUGS 2022
Knowledge gaps and challenges in care for menopausal women
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5
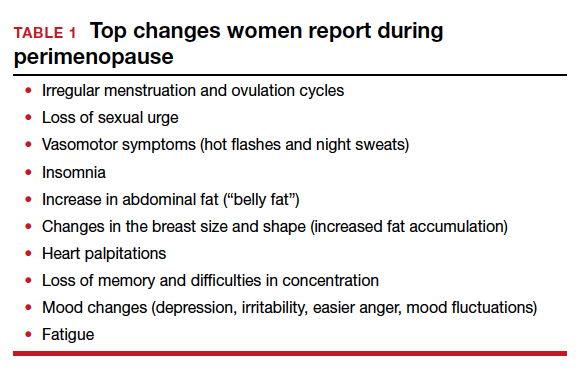
Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17
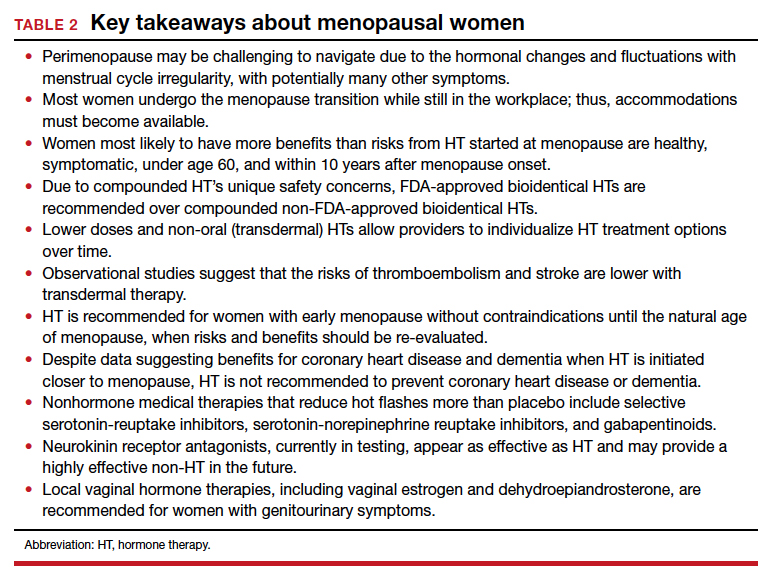
Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22
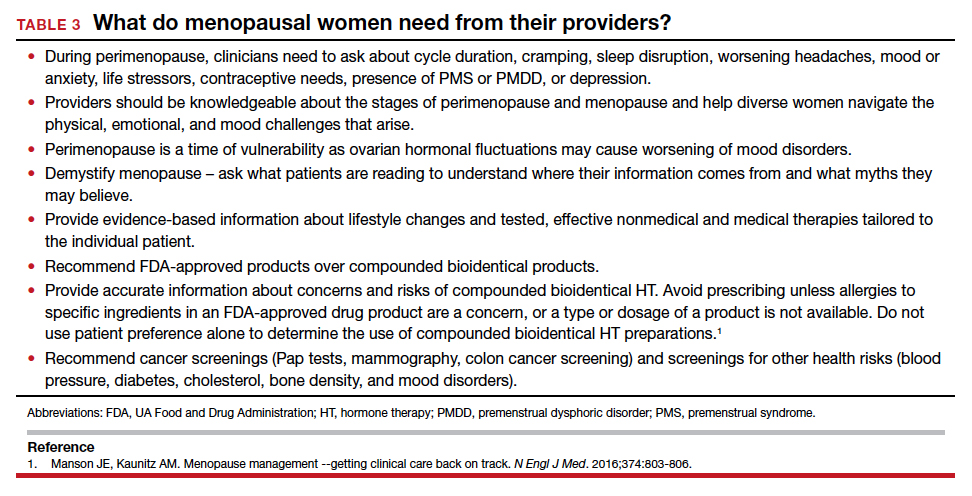
Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5

Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17

Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22

Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5

Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17

Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22

Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.
Hormones account for 10% of lipid changes after menopause
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY