User login
Radiologically Isolated Syndrome: A condition that often precedes an MS diagnosis in children
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227
ECTRIMS 2021: Disease-Modifying Therapies for Relapsing-Remitting Multiple Sclerosis
Dr Joseph Berger of the Perelman School of Medicine in Philadelphia discusses abstracts from ECTRIMS 2021 focusing on the use of disease-modifying therapies (DMTs) in patients with relapsing-remitting multiple sclerosis.
Dr Berger discusses ULTIMATE I and ULTIMATE II results, in which ublituximab — a novel monoclonal antibody — improved annualized relapse rates, Multiple Sclerosis Functional Composite scores, and percentages of patients with no evidence of disease activity compared to teriflunomide.
Dr Berger also highlights a study that examined the association between serum neurofilament light (NfL) levels and disease progression in patients on natalizumab. Although NfL levels were significantly reduced after initiation of therapy, no differences were evident between progressors and nonprogressors.
Next, he examines 3-year data from the CASTING study, which assessed ocrelizumab in patients who had a suboptimal response to one or two previous DMTs. Follow-up analysis showed that patients who received ocrelizumab had consistently low disease activity throughout the study period; mean Expanded Disability Status Scale (EDSS) scores, annualized relapse rates, and no evidence of disease activity were also stable.
Dr Berger concludes with a comparative analysis of patients who started on or switched to dimethyl fumarate or teriflunomide. Dimethyl fumarate showed more favorable outcomes in time to relapse, time to EDSS worsening, and sensitivity analysis.
--
Joseph R. Berger, MD, Professor; Associate Chief, Department of Neurology, Multiple Sclerosis Division, University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania
Joseph R. Berger, MD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Roche/Genentech
Received income in an amount equal to or greater than $250 from: Amgen; Biogen; Bristol-Myers Squibb; Celgene; Genzyme; Excision Bio; Dr. Reddy; Serono; Morphic; Novartis; Inhibikase; Morphic; Encycle; Merck; Mapi
Dr Joseph Berger of the Perelman School of Medicine in Philadelphia discusses abstracts from ECTRIMS 2021 focusing on the use of disease-modifying therapies (DMTs) in patients with relapsing-remitting multiple sclerosis.
Dr Berger discusses ULTIMATE I and ULTIMATE II results, in which ublituximab — a novel monoclonal antibody — improved annualized relapse rates, Multiple Sclerosis Functional Composite scores, and percentages of patients with no evidence of disease activity compared to teriflunomide.
Dr Berger also highlights a study that examined the association between serum neurofilament light (NfL) levels and disease progression in patients on natalizumab. Although NfL levels were significantly reduced after initiation of therapy, no differences were evident between progressors and nonprogressors.
Next, he examines 3-year data from the CASTING study, which assessed ocrelizumab in patients who had a suboptimal response to one or two previous DMTs. Follow-up analysis showed that patients who received ocrelizumab had consistently low disease activity throughout the study period; mean Expanded Disability Status Scale (EDSS) scores, annualized relapse rates, and no evidence of disease activity were also stable.
Dr Berger concludes with a comparative analysis of patients who started on or switched to dimethyl fumarate or teriflunomide. Dimethyl fumarate showed more favorable outcomes in time to relapse, time to EDSS worsening, and sensitivity analysis.
--
Joseph R. Berger, MD, Professor; Associate Chief, Department of Neurology, Multiple Sclerosis Division, University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania
Joseph R. Berger, MD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Roche/Genentech
Received income in an amount equal to or greater than $250 from: Amgen; Biogen; Bristol-Myers Squibb; Celgene; Genzyme; Excision Bio; Dr. Reddy; Serono; Morphic; Novartis; Inhibikase; Morphic; Encycle; Merck; Mapi
Dr Joseph Berger of the Perelman School of Medicine in Philadelphia discusses abstracts from ECTRIMS 2021 focusing on the use of disease-modifying therapies (DMTs) in patients with relapsing-remitting multiple sclerosis.
Dr Berger discusses ULTIMATE I and ULTIMATE II results, in which ublituximab — a novel monoclonal antibody — improved annualized relapse rates, Multiple Sclerosis Functional Composite scores, and percentages of patients with no evidence of disease activity compared to teriflunomide.
Dr Berger also highlights a study that examined the association between serum neurofilament light (NfL) levels and disease progression in patients on natalizumab. Although NfL levels were significantly reduced after initiation of therapy, no differences were evident between progressors and nonprogressors.
Next, he examines 3-year data from the CASTING study, which assessed ocrelizumab in patients who had a suboptimal response to one or two previous DMTs. Follow-up analysis showed that patients who received ocrelizumab had consistently low disease activity throughout the study period; mean Expanded Disability Status Scale (EDSS) scores, annualized relapse rates, and no evidence of disease activity were also stable.
Dr Berger concludes with a comparative analysis of patients who started on or switched to dimethyl fumarate or teriflunomide. Dimethyl fumarate showed more favorable outcomes in time to relapse, time to EDSS worsening, and sensitivity analysis.
--
Joseph R. Berger, MD, Professor; Associate Chief, Department of Neurology, Multiple Sclerosis Division, University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania
Joseph R. Berger, MD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Roche/Genentech
Received income in an amount equal to or greater than $250 from: Amgen; Biogen; Bristol-Myers Squibb; Celgene; Genzyme; Excision Bio; Dr. Reddy; Serono; Morphic; Novartis; Inhibikase; Morphic; Encycle; Merck; Mapi

Cannabis use common for MS-related spasticity
, new research suggests. Findings from a survey conducted through a large registry in 2020 showed that 31% of patients with MS reported trying cannabis to treat their symptoms – and 20% reported regular use.
Spasticity was reported by 80% as the reason why they used cannabis, while pain was cited as the reason by 69% and sleep problems/insomnia was cited by 61%.
Investigators noted that the new data reflect the latest patterns of use amid sweeping changes in recreational and medical marijuana laws.
“Interest in the use of cannabis for managing MS symptoms continues to increase as more data become available and access becomes easier,” co-investigator Amber Salter, PhD, associate professor, UT Southwestern Medical Center, Dallas, told attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Administration routes vary
The survey was conducted through the longitudinal North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, a voluntary, self-report registry for patients with MS. Of 6,934 registry participants invited to participate, 3,249 (47%) responded. The majority of responders were women (79%) and the mean age was 61 years. About 63% were being treated with disease-modifying therapies.
Overall, 31% of respondents reported having used cannabis to treat their MS symptoms. In addition, 20% reported regular current cannabis use, with an average use of 20 days in the past month. As many as 40% of the current users reported using cannabis daily.
“In general we saw some small differences in current users, who tended to include more males; have higher spasticity, pain, and sleep symptoms; and [were] more likely to be unemployed and younger,” Dr. Salter said.
The most common forms of cannabis administration were smoking (33%) and eating (20%). In addition, 12% reported vaporizing cannabis with a highly concentrated material, 11% administered cannabis sublingually, and 11% reported swallowing it.
Further, 8% reported vaporizing cannabis as a dried flower, 5% used it topically, and 1% reported drinking it.
Of note, the definition of “cannabis/marijuana” in the study excluded hemp cannabidiol (CBD) or products marketed as CBD only.
Consistent use
The most common reason for use by far was spasticity (80%). This was followed by for pain (69%) and sleep/insomnia problems (61%). Among users, 37% reported doing so to treat all three of those problems.
Regarding other symptoms, 36% used cannabis for anxiety, 24% for depression, 18% for overactive bladder, 17% for nausea or gastrointestinal problems, 16% for migraine or headaches, 14% for tremors, and 6% for other purposes.
The vast majority (95%) reported cannabis to be very or somewhat helpful for their symptoms.
Among the 69% of respondents who reported not using cannabis for their MS symptoms, the most commonly cited reasons were a lack of evidence on efficacy (40%) or safety (27%), concerns of legality (25%), lack of insurance coverage (22%), prohibitive cost (18%), and adverse side effects.
Surprisingly, the dramatic shift in the legalization of cannabis use in many states does not appear to be reflected in changes in cannabis use for MS, Dr. Salter said.
“We conducted an anonymous NARCOMS survey a couple of years prior to this survey, and our results are generally consistent. There’s been a small increase in the use and an acceptance or willingness to consider cannabis, but it’s relatively consistent,” she said.
“Despite the changes in access, the landscape hasn’t really changed very much in terms of evidence of the effects on MS symptoms, so that could be why,” Dr. Salter added.
Most patients appear to feel comfortable discussing their cannabis use with their physician, with 75% reporting doing so. However, the most common primary source of medical guidance for treating MS with cannabis was “nobody/self”; for 20%, the source for medical guidance was a dispensary professional.
As many as 62% of respondents reported obtaining their cannabis products from dispensaries, while other sources included family/friend (18%) or an acquaintance (13%). About 31% reported their most preferred type of cannabis to be equal parts THC and cannabidiol, while 30% preferred high THC/low cannabidiol (30%).
Mirrors clinical practice findings
Commenting on the study, Laura T. Safar, MD, vice chair of Psychiatry at Lahey Hospital and Medical Center and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings generally fall in line with cannabis use among patients with MS in her practice.
“This is [consistent] with my general experience: A high percentage of my patients with MS are using cannabis with the goal of addressing their MS symptoms that way,” said Dr. Safar, who was not involved with the research.
One notable recent change in patients’ inquiries about cannabis is their apparent confidence in the information they’re getting, she noted. This is a sign of the ever-expanding sources of information – but from sources who may or may not have an understanding of effects in MS, she added.
“What seems new is a certain level of specificity in the information patients state – regardless of its accuracy. There is more technical information widely available about cannabis online and in the dispensaries,” said Dr. Safar.
“A lot of that information may not have been tested scientifically, but it is presented with an aura of truth,” she said.
While misconceptions about cannabis use in MS may not be new, “the conviction with which they are stated and believed seems stronger,” even though they have been validated by questionably expert sources, Dr. Safar noted.
She pointed out that psychiatric effects are among her patients’ notable concerns of cannabis use in MS.
“Cannabis use, especially daily use in moderate to large amounts, can have negative cognitive side effects,” she said. “In addition, it can have other psychiatric side effects: worsening of mood and anxiety, apathy, and anhedonia, a lack of pleasure or enjoyment, and a flattening of the emotional experience.”
Countering misinformation
Dr. Safar said she works to counter misinformation and provide more reliable, evidence-based recommendations.
“I educate my patients about what we know from scientific trials about the potential benefits, including possible help with pain, excluding central pain, and with spasticity,” she said. Dr. Safar added that she also discusses possible risks, such as worsening of cognition, mood, and anxiety.
On the basis of an individual’s presentation, and working in collaboration with their neurologist as appropriate, Dr. Safar said she discusses the following issues with the patient:
- Does cannabis make sense for the symptoms being presented?
- Has the patient received benefit so far?
- Are there side effects they may be experiencing?
- Would it be appropriate to lower the cannabis dose/frequency of its use?
- If a patient is using cannabis with an objective that is not backed up by the literature, such as depression, are they open to information about other treatment options?
The study was sponsored by GW Research. Dr. Salter has conducted research for GW Pharmaceuticals companies. Dr. Safar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Findings from a survey conducted through a large registry in 2020 showed that 31% of patients with MS reported trying cannabis to treat their symptoms – and 20% reported regular use.
Spasticity was reported by 80% as the reason why they used cannabis, while pain was cited as the reason by 69% and sleep problems/insomnia was cited by 61%.
Investigators noted that the new data reflect the latest patterns of use amid sweeping changes in recreational and medical marijuana laws.
“Interest in the use of cannabis for managing MS symptoms continues to increase as more data become available and access becomes easier,” co-investigator Amber Salter, PhD, associate professor, UT Southwestern Medical Center, Dallas, told attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Administration routes vary
The survey was conducted through the longitudinal North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, a voluntary, self-report registry for patients with MS. Of 6,934 registry participants invited to participate, 3,249 (47%) responded. The majority of responders were women (79%) and the mean age was 61 years. About 63% were being treated with disease-modifying therapies.
Overall, 31% of respondents reported having used cannabis to treat their MS symptoms. In addition, 20% reported regular current cannabis use, with an average use of 20 days in the past month. As many as 40% of the current users reported using cannabis daily.
“In general we saw some small differences in current users, who tended to include more males; have higher spasticity, pain, and sleep symptoms; and [were] more likely to be unemployed and younger,” Dr. Salter said.
The most common forms of cannabis administration were smoking (33%) and eating (20%). In addition, 12% reported vaporizing cannabis with a highly concentrated material, 11% administered cannabis sublingually, and 11% reported swallowing it.
Further, 8% reported vaporizing cannabis as a dried flower, 5% used it topically, and 1% reported drinking it.
Of note, the definition of “cannabis/marijuana” in the study excluded hemp cannabidiol (CBD) or products marketed as CBD only.
Consistent use
The most common reason for use by far was spasticity (80%). This was followed by for pain (69%) and sleep/insomnia problems (61%). Among users, 37% reported doing so to treat all three of those problems.
Regarding other symptoms, 36% used cannabis for anxiety, 24% for depression, 18% for overactive bladder, 17% for nausea or gastrointestinal problems, 16% for migraine or headaches, 14% for tremors, and 6% for other purposes.
The vast majority (95%) reported cannabis to be very or somewhat helpful for their symptoms.
Among the 69% of respondents who reported not using cannabis for their MS symptoms, the most commonly cited reasons were a lack of evidence on efficacy (40%) or safety (27%), concerns of legality (25%), lack of insurance coverage (22%), prohibitive cost (18%), and adverse side effects.
Surprisingly, the dramatic shift in the legalization of cannabis use in many states does not appear to be reflected in changes in cannabis use for MS, Dr. Salter said.
“We conducted an anonymous NARCOMS survey a couple of years prior to this survey, and our results are generally consistent. There’s been a small increase in the use and an acceptance or willingness to consider cannabis, but it’s relatively consistent,” she said.
“Despite the changes in access, the landscape hasn’t really changed very much in terms of evidence of the effects on MS symptoms, so that could be why,” Dr. Salter added.
Most patients appear to feel comfortable discussing their cannabis use with their physician, with 75% reporting doing so. However, the most common primary source of medical guidance for treating MS with cannabis was “nobody/self”; for 20%, the source for medical guidance was a dispensary professional.
As many as 62% of respondents reported obtaining their cannabis products from dispensaries, while other sources included family/friend (18%) or an acquaintance (13%). About 31% reported their most preferred type of cannabis to be equal parts THC and cannabidiol, while 30% preferred high THC/low cannabidiol (30%).
Mirrors clinical practice findings
Commenting on the study, Laura T. Safar, MD, vice chair of Psychiatry at Lahey Hospital and Medical Center and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings generally fall in line with cannabis use among patients with MS in her practice.
“This is [consistent] with my general experience: A high percentage of my patients with MS are using cannabis with the goal of addressing their MS symptoms that way,” said Dr. Safar, who was not involved with the research.
One notable recent change in patients’ inquiries about cannabis is their apparent confidence in the information they’re getting, she noted. This is a sign of the ever-expanding sources of information – but from sources who may or may not have an understanding of effects in MS, she added.
“What seems new is a certain level of specificity in the information patients state – regardless of its accuracy. There is more technical information widely available about cannabis online and in the dispensaries,” said Dr. Safar.
“A lot of that information may not have been tested scientifically, but it is presented with an aura of truth,” she said.
While misconceptions about cannabis use in MS may not be new, “the conviction with which they are stated and believed seems stronger,” even though they have been validated by questionably expert sources, Dr. Safar noted.
She pointed out that psychiatric effects are among her patients’ notable concerns of cannabis use in MS.
“Cannabis use, especially daily use in moderate to large amounts, can have negative cognitive side effects,” she said. “In addition, it can have other psychiatric side effects: worsening of mood and anxiety, apathy, and anhedonia, a lack of pleasure or enjoyment, and a flattening of the emotional experience.”
Countering misinformation
Dr. Safar said she works to counter misinformation and provide more reliable, evidence-based recommendations.
“I educate my patients about what we know from scientific trials about the potential benefits, including possible help with pain, excluding central pain, and with spasticity,” she said. Dr. Safar added that she also discusses possible risks, such as worsening of cognition, mood, and anxiety.
On the basis of an individual’s presentation, and working in collaboration with their neurologist as appropriate, Dr. Safar said she discusses the following issues with the patient:
- Does cannabis make sense for the symptoms being presented?
- Has the patient received benefit so far?
- Are there side effects they may be experiencing?
- Would it be appropriate to lower the cannabis dose/frequency of its use?
- If a patient is using cannabis with an objective that is not backed up by the literature, such as depression, are they open to information about other treatment options?
The study was sponsored by GW Research. Dr. Salter has conducted research for GW Pharmaceuticals companies. Dr. Safar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Findings from a survey conducted through a large registry in 2020 showed that 31% of patients with MS reported trying cannabis to treat their symptoms – and 20% reported regular use.
Spasticity was reported by 80% as the reason why they used cannabis, while pain was cited as the reason by 69% and sleep problems/insomnia was cited by 61%.
Investigators noted that the new data reflect the latest patterns of use amid sweeping changes in recreational and medical marijuana laws.
“Interest in the use of cannabis for managing MS symptoms continues to increase as more data become available and access becomes easier,” co-investigator Amber Salter, PhD, associate professor, UT Southwestern Medical Center, Dallas, told attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Administration routes vary
The survey was conducted through the longitudinal North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, a voluntary, self-report registry for patients with MS. Of 6,934 registry participants invited to participate, 3,249 (47%) responded. The majority of responders were women (79%) and the mean age was 61 years. About 63% were being treated with disease-modifying therapies.
Overall, 31% of respondents reported having used cannabis to treat their MS symptoms. In addition, 20% reported regular current cannabis use, with an average use of 20 days in the past month. As many as 40% of the current users reported using cannabis daily.
“In general we saw some small differences in current users, who tended to include more males; have higher spasticity, pain, and sleep symptoms; and [were] more likely to be unemployed and younger,” Dr. Salter said.
The most common forms of cannabis administration were smoking (33%) and eating (20%). In addition, 12% reported vaporizing cannabis with a highly concentrated material, 11% administered cannabis sublingually, and 11% reported swallowing it.
Further, 8% reported vaporizing cannabis as a dried flower, 5% used it topically, and 1% reported drinking it.
Of note, the definition of “cannabis/marijuana” in the study excluded hemp cannabidiol (CBD) or products marketed as CBD only.
Consistent use
The most common reason for use by far was spasticity (80%). This was followed by for pain (69%) and sleep/insomnia problems (61%). Among users, 37% reported doing so to treat all three of those problems.
Regarding other symptoms, 36% used cannabis for anxiety, 24% for depression, 18% for overactive bladder, 17% for nausea or gastrointestinal problems, 16% for migraine or headaches, 14% for tremors, and 6% for other purposes.
The vast majority (95%) reported cannabis to be very or somewhat helpful for their symptoms.
Among the 69% of respondents who reported not using cannabis for their MS symptoms, the most commonly cited reasons were a lack of evidence on efficacy (40%) or safety (27%), concerns of legality (25%), lack of insurance coverage (22%), prohibitive cost (18%), and adverse side effects.
Surprisingly, the dramatic shift in the legalization of cannabis use in many states does not appear to be reflected in changes in cannabis use for MS, Dr. Salter said.
“We conducted an anonymous NARCOMS survey a couple of years prior to this survey, and our results are generally consistent. There’s been a small increase in the use and an acceptance or willingness to consider cannabis, but it’s relatively consistent,” she said.
“Despite the changes in access, the landscape hasn’t really changed very much in terms of evidence of the effects on MS symptoms, so that could be why,” Dr. Salter added.
Most patients appear to feel comfortable discussing their cannabis use with their physician, with 75% reporting doing so. However, the most common primary source of medical guidance for treating MS with cannabis was “nobody/self”; for 20%, the source for medical guidance was a dispensary professional.
As many as 62% of respondents reported obtaining their cannabis products from dispensaries, while other sources included family/friend (18%) or an acquaintance (13%). About 31% reported their most preferred type of cannabis to be equal parts THC and cannabidiol, while 30% preferred high THC/low cannabidiol (30%).
Mirrors clinical practice findings
Commenting on the study, Laura T. Safar, MD, vice chair of Psychiatry at Lahey Hospital and Medical Center and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings generally fall in line with cannabis use among patients with MS in her practice.
“This is [consistent] with my general experience: A high percentage of my patients with MS are using cannabis with the goal of addressing their MS symptoms that way,” said Dr. Safar, who was not involved with the research.
One notable recent change in patients’ inquiries about cannabis is their apparent confidence in the information they’re getting, she noted. This is a sign of the ever-expanding sources of information – but from sources who may or may not have an understanding of effects in MS, she added.
“What seems new is a certain level of specificity in the information patients state – regardless of its accuracy. There is more technical information widely available about cannabis online and in the dispensaries,” said Dr. Safar.
“A lot of that information may not have been tested scientifically, but it is presented with an aura of truth,” she said.
While misconceptions about cannabis use in MS may not be new, “the conviction with which they are stated and believed seems stronger,” even though they have been validated by questionably expert sources, Dr. Safar noted.
She pointed out that psychiatric effects are among her patients’ notable concerns of cannabis use in MS.
“Cannabis use, especially daily use in moderate to large amounts, can have negative cognitive side effects,” she said. “In addition, it can have other psychiatric side effects: worsening of mood and anxiety, apathy, and anhedonia, a lack of pleasure or enjoyment, and a flattening of the emotional experience.”
Countering misinformation
Dr. Safar said she works to counter misinformation and provide more reliable, evidence-based recommendations.
“I educate my patients about what we know from scientific trials about the potential benefits, including possible help with pain, excluding central pain, and with spasticity,” she said. Dr. Safar added that she also discusses possible risks, such as worsening of cognition, mood, and anxiety.
On the basis of an individual’s presentation, and working in collaboration with their neurologist as appropriate, Dr. Safar said she discusses the following issues with the patient:
- Does cannabis make sense for the symptoms being presented?
- Has the patient received benefit so far?
- Are there side effects they may be experiencing?
- Would it be appropriate to lower the cannabis dose/frequency of its use?
- If a patient is using cannabis with an objective that is not backed up by the literature, such as depression, are they open to information about other treatment options?
The study was sponsored by GW Research. Dr. Salter has conducted research for GW Pharmaceuticals companies. Dr. Safar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021
New consensus guideline on clinical MRI use in MS
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021
The impact of modifiable risk factors such as diet and obesity in Pediatric MS patients
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
Multiple DMTs linked to alopecia, especially in women
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
FROM CMSC 2021
Certain DMTs in MS linked to more psoriasis
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
FROM CMSC 2021
Which agent is best for neuromyelitis optica?
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.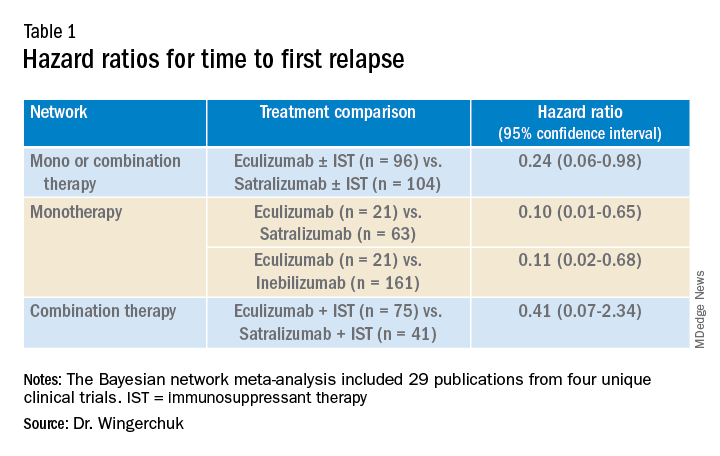
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).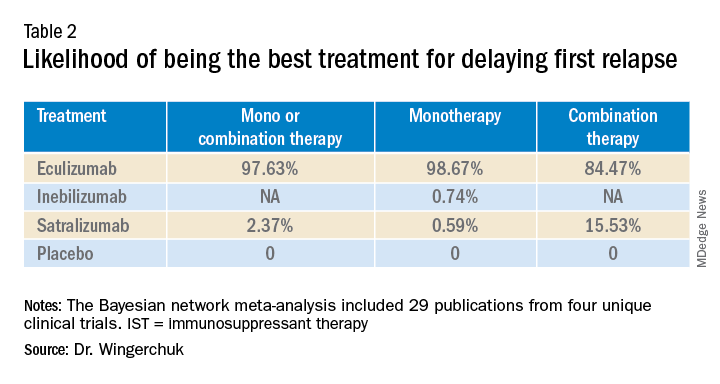
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
Two diets linked to improved cognition, fatigue in MS
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021
MS and COVID: Docs switched DMTs but maybe didn’t need to
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
FROM CMSC 2021



