User login
Updates in aspirin use, aducanumab, and CKD diagnostic criteria in geriatric medicine
I selected these topics as they were among the most discussed by my colleagues in geriatric medicine and inquired about by my primary care patients in geriatric medicine clinic. I hope that these updates provide primary care clinicians who care for older adults with more context and background information regarding new Alzheimer’s disease therapy to better answer patient inquiries, and to feel empowered to deprescribe aspirin and reframe the diagnostic criteria of chronic kidney disease (CKD).
Aspirin for primary prevention
It was welcome news in the geriatrics community when the United States Preventive Services Task Force updated their guidelines in April 2022 to recommend against the initiation of aspirin for primary prevention in adults aged 60 or older. This recommendation was based on studies that found that net benefits of CVD prevention in older adults are outweighed by risk of bleeding.1
The risk of bleeding increases with age and can occur in individuals without common risk factors for bleeding, such as prior gastrointestinal bleeding, peptic ulcer disease, concurrent NSAID use, or corticosteroid use.
While it may be easier to not initiate aspirin for primary prevention, deprescribing aspirin for patients who have been on aspirin long term for primary prevention presents more of a challenge. Modeling data from the USPTSF suggest stopping aspirin at age 75 for those taking aspirin for primary prevention.2
Behavioral change, particularly for patients who have been on aspirin for decades, can be difficult. A 2021 study by Green et al. found that language that resonates the most with older adults when deprescribing emphasized the side effects rather than statements such as “this will not help you” or “do not need anymore.”3
Aducanumab for mild cognitive impairment and mild Alzheimer’s dementia
One of the most discussed topics this past year is the Food and Drug Administration approval of aducanumab (brand name Aduhelm) in June 2021. Aducanumab is the first approved disease-modifying therapy for Alzheimer’s disease and the first drug approved for the treatment of Alzheimer’s disease since 2003. Aducanumab is an antiamyloid monoclonal antibody that was developed to reduce amyloid plaque in the brain, one of the features of Alzheimer’s disease pathology.
Uptake of aducanumab by dementia providers has been limited for several reasons. Firstly, the clinical significance of the drug remains in question. ENGAGE and EMERGE were the two main randomized clinical trials that studied the effect of aducanumab on amyloid burden and clinical stages of dementia over 18 months. While both studies demonstrated that aducanumab reduced amyloid burden based on neuroimaging and in cerebrospinal fluid, the ENGAGE trial found no difference in the stage of dementia. The EMERGE trial did note a small, statistically significant difference in stage of dementia, however the participants of the EMERGE trial had a faster rate of progression of dementia than the placebo participants in the ENGAGE trial, which could have contributed to the difference detected.4
Additionally, exclusion criteria for both trials call into question the generalizability of this study. Participants over age 85, with CKD, prior stroke, or transient ischemic attacks, or on anticoagulation were excluded. One of the drivers for the exclusion criteria is the increased risk of macro and microhemorrhages.
Thirty-five percent of research participants were incidentally noted to have brain edema, an abnormality called amyloid-related imaging abnormality or ARIA-E, that necessitated serial monitoring with brain MRIs. It is also important to highlight that inclusion of African American, Hispanic, and Latinx participants in these studies was less than 5%, despite a higher incidence of Alzheimer’s disease in these populations.5
Lastly, economic implications for the U.S. health care system with increased uptake of aducanumab could be enormous. Originally quoted at $56,000 yearly, Biogen, the maker of aducanumab, recently reduced annual costs to $28,200 per patient.
In April 2022, CMS released a statement that antiamyloid monoclonal antibodies and related services, including PET scans, would be covered under Medicare for those with mild cognitive impairment and mild Alzheimer’s dementia with confirmed presence of amyloid. A study by Mafi et al. estimated that aducanumab could cost Medicare between $7 billion and $37.4 billion annually based on lower and upper bound estimates of eligible Medicare beneficiaries.6
Overdiagnosis of CKD in older adults
The current diagnostic criteria of CKD, which is based on an estimated glomerular filtration rate (eGFR) of less than 60, has been up for debate, as glomerular filtration rate (GFR) physiologically decreases with age. Fixed thresholds can lead to underdiagnosis of CKD in younger adults and overdiagnosis of CKD in older adults. Age-adapted thresholds for the diagnosis of CKD have been proposed, with the suggestion of an eGFR threshold of 45mL/min/1.73 m2 for adults aged 65 and older.7
The clinical implication of using an age-adapted eGFR threshold definition was investigated in a 2021 cohort study by Liu et al.8 In this study, outcomes of adults diagnosed with CKD using a fixed threshold versus age-adapted threshold were compared with a healthy cohort.
A fixed threshold led to a 60% higher incidence of CKD diagnosis. However, incidence of renal failure and all-cause mortality in older adults with an eGFR between 45-59 /min/1.73 m2 with normal or mild albuminuria was of similar magnitude to the healthy cohort at 5 years of follow-up.
These findings support the use of age-adapted thresholds for the diagnosis of CKD in older adults, as an earlier diagnosis of mild CKD does not equate to clinical benefits, but could lead to harms of unnecessary interventions and patient anxiety.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. Selak Vet al. Predicting bleeding risk to guide aspirin use for the primary prevention of cardiovascular disease: A cohort study. Ann Intern Med. 2019;170(6):357-68. doi: 10.7326/M18-2808.
2. US Preventive Services Task Force. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-84. doi: 10.1001/jama.2022.4983.
3. Green AR et al. Assessment of patient-preferred language to achieve goal-aligned deprescribing in older adults. JAMA Netw Open. 2021;4(4):e212633. doi: 10.1001/jamanetworkopen.2021.2633.
4. Oh ES. Use of anti-amyloid therapy for Alzheimer’s disease in clinical practice. An update on Alzheimer’s disease diagnosis and therapeutics. Presentation at American Geriatrics Society Meeting, 2022. Orlando.
5. Amjad H. Issues of Access and Marginalization. An update on Alzheimer’s disease diagnosis and therapeutics. Presentation at: American Geriatrics Society Meeting, 2022. Orlando.
6. Mafi JN et al. Estimated annual spending on aducanumab in the U.S. Medicare program. JAMA Health Forum. 2022;3(1):e214495. doi: 10.1001/jamahealthforum.2021.4495.
7. Delanaye P et al. CKD: A call for an age-adapted definition. J Am Soc Nephrol. 2019;30(10):1785-1805. doi: 10.1681/ASN.2019030238.
8. Liu Pet al. Accounting for age in the definition of chronic kidney disease. JAMA Intern Med. 2021;181(10):1359-66. doi: 10.1001/jamainternmed.2021.4813.
I selected these topics as they were among the most discussed by my colleagues in geriatric medicine and inquired about by my primary care patients in geriatric medicine clinic. I hope that these updates provide primary care clinicians who care for older adults with more context and background information regarding new Alzheimer’s disease therapy to better answer patient inquiries, and to feel empowered to deprescribe aspirin and reframe the diagnostic criteria of chronic kidney disease (CKD).
Aspirin for primary prevention
It was welcome news in the geriatrics community when the United States Preventive Services Task Force updated their guidelines in April 2022 to recommend against the initiation of aspirin for primary prevention in adults aged 60 or older. This recommendation was based on studies that found that net benefits of CVD prevention in older adults are outweighed by risk of bleeding.1
The risk of bleeding increases with age and can occur in individuals without common risk factors for bleeding, such as prior gastrointestinal bleeding, peptic ulcer disease, concurrent NSAID use, or corticosteroid use.
While it may be easier to not initiate aspirin for primary prevention, deprescribing aspirin for patients who have been on aspirin long term for primary prevention presents more of a challenge. Modeling data from the USPTSF suggest stopping aspirin at age 75 for those taking aspirin for primary prevention.2
Behavioral change, particularly for patients who have been on aspirin for decades, can be difficult. A 2021 study by Green et al. found that language that resonates the most with older adults when deprescribing emphasized the side effects rather than statements such as “this will not help you” or “do not need anymore.”3
Aducanumab for mild cognitive impairment and mild Alzheimer’s dementia
One of the most discussed topics this past year is the Food and Drug Administration approval of aducanumab (brand name Aduhelm) in June 2021. Aducanumab is the first approved disease-modifying therapy for Alzheimer’s disease and the first drug approved for the treatment of Alzheimer’s disease since 2003. Aducanumab is an antiamyloid monoclonal antibody that was developed to reduce amyloid plaque in the brain, one of the features of Alzheimer’s disease pathology.
Uptake of aducanumab by dementia providers has been limited for several reasons. Firstly, the clinical significance of the drug remains in question. ENGAGE and EMERGE were the two main randomized clinical trials that studied the effect of aducanumab on amyloid burden and clinical stages of dementia over 18 months. While both studies demonstrated that aducanumab reduced amyloid burden based on neuroimaging and in cerebrospinal fluid, the ENGAGE trial found no difference in the stage of dementia. The EMERGE trial did note a small, statistically significant difference in stage of dementia, however the participants of the EMERGE trial had a faster rate of progression of dementia than the placebo participants in the ENGAGE trial, which could have contributed to the difference detected.4
Additionally, exclusion criteria for both trials call into question the generalizability of this study. Participants over age 85, with CKD, prior stroke, or transient ischemic attacks, or on anticoagulation were excluded. One of the drivers for the exclusion criteria is the increased risk of macro and microhemorrhages.
Thirty-five percent of research participants were incidentally noted to have brain edema, an abnormality called amyloid-related imaging abnormality or ARIA-E, that necessitated serial monitoring with brain MRIs. It is also important to highlight that inclusion of African American, Hispanic, and Latinx participants in these studies was less than 5%, despite a higher incidence of Alzheimer’s disease in these populations.5
Lastly, economic implications for the U.S. health care system with increased uptake of aducanumab could be enormous. Originally quoted at $56,000 yearly, Biogen, the maker of aducanumab, recently reduced annual costs to $28,200 per patient.
In April 2022, CMS released a statement that antiamyloid monoclonal antibodies and related services, including PET scans, would be covered under Medicare for those with mild cognitive impairment and mild Alzheimer’s dementia with confirmed presence of amyloid. A study by Mafi et al. estimated that aducanumab could cost Medicare between $7 billion and $37.4 billion annually based on lower and upper bound estimates of eligible Medicare beneficiaries.6
Overdiagnosis of CKD in older adults
The current diagnostic criteria of CKD, which is based on an estimated glomerular filtration rate (eGFR) of less than 60, has been up for debate, as glomerular filtration rate (GFR) physiologically decreases with age. Fixed thresholds can lead to underdiagnosis of CKD in younger adults and overdiagnosis of CKD in older adults. Age-adapted thresholds for the diagnosis of CKD have been proposed, with the suggestion of an eGFR threshold of 45mL/min/1.73 m2 for adults aged 65 and older.7
The clinical implication of using an age-adapted eGFR threshold definition was investigated in a 2021 cohort study by Liu et al.8 In this study, outcomes of adults diagnosed with CKD using a fixed threshold versus age-adapted threshold were compared with a healthy cohort.
A fixed threshold led to a 60% higher incidence of CKD diagnosis. However, incidence of renal failure and all-cause mortality in older adults with an eGFR between 45-59 /min/1.73 m2 with normal or mild albuminuria was of similar magnitude to the healthy cohort at 5 years of follow-up.
These findings support the use of age-adapted thresholds for the diagnosis of CKD in older adults, as an earlier diagnosis of mild CKD does not equate to clinical benefits, but could lead to harms of unnecessary interventions and patient anxiety.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. Selak Vet al. Predicting bleeding risk to guide aspirin use for the primary prevention of cardiovascular disease: A cohort study. Ann Intern Med. 2019;170(6):357-68. doi: 10.7326/M18-2808.
2. US Preventive Services Task Force. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-84. doi: 10.1001/jama.2022.4983.
3. Green AR et al. Assessment of patient-preferred language to achieve goal-aligned deprescribing in older adults. JAMA Netw Open. 2021;4(4):e212633. doi: 10.1001/jamanetworkopen.2021.2633.
4. Oh ES. Use of anti-amyloid therapy for Alzheimer’s disease in clinical practice. An update on Alzheimer’s disease diagnosis and therapeutics. Presentation at American Geriatrics Society Meeting, 2022. Orlando.
5. Amjad H. Issues of Access and Marginalization. An update on Alzheimer’s disease diagnosis and therapeutics. Presentation at: American Geriatrics Society Meeting, 2022. Orlando.
6. Mafi JN et al. Estimated annual spending on aducanumab in the U.S. Medicare program. JAMA Health Forum. 2022;3(1):e214495. doi: 10.1001/jamahealthforum.2021.4495.
7. Delanaye P et al. CKD: A call for an age-adapted definition. J Am Soc Nephrol. 2019;30(10):1785-1805. doi: 10.1681/ASN.2019030238.
8. Liu Pet al. Accounting for age in the definition of chronic kidney disease. JAMA Intern Med. 2021;181(10):1359-66. doi: 10.1001/jamainternmed.2021.4813.
I selected these topics as they were among the most discussed by my colleagues in geriatric medicine and inquired about by my primary care patients in geriatric medicine clinic. I hope that these updates provide primary care clinicians who care for older adults with more context and background information regarding new Alzheimer’s disease therapy to better answer patient inquiries, and to feel empowered to deprescribe aspirin and reframe the diagnostic criteria of chronic kidney disease (CKD).
Aspirin for primary prevention
It was welcome news in the geriatrics community when the United States Preventive Services Task Force updated their guidelines in April 2022 to recommend against the initiation of aspirin for primary prevention in adults aged 60 or older. This recommendation was based on studies that found that net benefits of CVD prevention in older adults are outweighed by risk of bleeding.1
The risk of bleeding increases with age and can occur in individuals without common risk factors for bleeding, such as prior gastrointestinal bleeding, peptic ulcer disease, concurrent NSAID use, or corticosteroid use.
While it may be easier to not initiate aspirin for primary prevention, deprescribing aspirin for patients who have been on aspirin long term for primary prevention presents more of a challenge. Modeling data from the USPTSF suggest stopping aspirin at age 75 for those taking aspirin for primary prevention.2
Behavioral change, particularly for patients who have been on aspirin for decades, can be difficult. A 2021 study by Green et al. found that language that resonates the most with older adults when deprescribing emphasized the side effects rather than statements such as “this will not help you” or “do not need anymore.”3
Aducanumab for mild cognitive impairment and mild Alzheimer’s dementia
One of the most discussed topics this past year is the Food and Drug Administration approval of aducanumab (brand name Aduhelm) in June 2021. Aducanumab is the first approved disease-modifying therapy for Alzheimer’s disease and the first drug approved for the treatment of Alzheimer’s disease since 2003. Aducanumab is an antiamyloid monoclonal antibody that was developed to reduce amyloid plaque in the brain, one of the features of Alzheimer’s disease pathology.
Uptake of aducanumab by dementia providers has been limited for several reasons. Firstly, the clinical significance of the drug remains in question. ENGAGE and EMERGE were the two main randomized clinical trials that studied the effect of aducanumab on amyloid burden and clinical stages of dementia over 18 months. While both studies demonstrated that aducanumab reduced amyloid burden based on neuroimaging and in cerebrospinal fluid, the ENGAGE trial found no difference in the stage of dementia. The EMERGE trial did note a small, statistically significant difference in stage of dementia, however the participants of the EMERGE trial had a faster rate of progression of dementia than the placebo participants in the ENGAGE trial, which could have contributed to the difference detected.4
Additionally, exclusion criteria for both trials call into question the generalizability of this study. Participants over age 85, with CKD, prior stroke, or transient ischemic attacks, or on anticoagulation were excluded. One of the drivers for the exclusion criteria is the increased risk of macro and microhemorrhages.
Thirty-five percent of research participants were incidentally noted to have brain edema, an abnormality called amyloid-related imaging abnormality or ARIA-E, that necessitated serial monitoring with brain MRIs. It is also important to highlight that inclusion of African American, Hispanic, and Latinx participants in these studies was less than 5%, despite a higher incidence of Alzheimer’s disease in these populations.5
Lastly, economic implications for the U.S. health care system with increased uptake of aducanumab could be enormous. Originally quoted at $56,000 yearly, Biogen, the maker of aducanumab, recently reduced annual costs to $28,200 per patient.
In April 2022, CMS released a statement that antiamyloid monoclonal antibodies and related services, including PET scans, would be covered under Medicare for those with mild cognitive impairment and mild Alzheimer’s dementia with confirmed presence of amyloid. A study by Mafi et al. estimated that aducanumab could cost Medicare between $7 billion and $37.4 billion annually based on lower and upper bound estimates of eligible Medicare beneficiaries.6
Overdiagnosis of CKD in older adults
The current diagnostic criteria of CKD, which is based on an estimated glomerular filtration rate (eGFR) of less than 60, has been up for debate, as glomerular filtration rate (GFR) physiologically decreases with age. Fixed thresholds can lead to underdiagnosis of CKD in younger adults and overdiagnosis of CKD in older adults. Age-adapted thresholds for the diagnosis of CKD have been proposed, with the suggestion of an eGFR threshold of 45mL/min/1.73 m2 for adults aged 65 and older.7
The clinical implication of using an age-adapted eGFR threshold definition was investigated in a 2021 cohort study by Liu et al.8 In this study, outcomes of adults diagnosed with CKD using a fixed threshold versus age-adapted threshold were compared with a healthy cohort.
A fixed threshold led to a 60% higher incidence of CKD diagnosis. However, incidence of renal failure and all-cause mortality in older adults with an eGFR between 45-59 /min/1.73 m2 with normal or mild albuminuria was of similar magnitude to the healthy cohort at 5 years of follow-up.
These findings support the use of age-adapted thresholds for the diagnosis of CKD in older adults, as an earlier diagnosis of mild CKD does not equate to clinical benefits, but could lead to harms of unnecessary interventions and patient anxiety.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. Selak Vet al. Predicting bleeding risk to guide aspirin use for the primary prevention of cardiovascular disease: A cohort study. Ann Intern Med. 2019;170(6):357-68. doi: 10.7326/M18-2808.
2. US Preventive Services Task Force. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-84. doi: 10.1001/jama.2022.4983.
3. Green AR et al. Assessment of patient-preferred language to achieve goal-aligned deprescribing in older adults. JAMA Netw Open. 2021;4(4):e212633. doi: 10.1001/jamanetworkopen.2021.2633.
4. Oh ES. Use of anti-amyloid therapy for Alzheimer’s disease in clinical practice. An update on Alzheimer’s disease diagnosis and therapeutics. Presentation at American Geriatrics Society Meeting, 2022. Orlando.
5. Amjad H. Issues of Access and Marginalization. An update on Alzheimer’s disease diagnosis and therapeutics. Presentation at: American Geriatrics Society Meeting, 2022. Orlando.
6. Mafi JN et al. Estimated annual spending on aducanumab in the U.S. Medicare program. JAMA Health Forum. 2022;3(1):e214495. doi: 10.1001/jamahealthforum.2021.4495.
7. Delanaye P et al. CKD: A call for an age-adapted definition. J Am Soc Nephrol. 2019;30(10):1785-1805. doi: 10.1681/ASN.2019030238.
8. Liu Pet al. Accounting for age in the definition of chronic kidney disease. JAMA Intern Med. 2021;181(10):1359-66. doi: 10.1001/jamainternmed.2021.4813.
Experts elevate new drugs for diabetic kidney disease
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
AT ADA 2022
SGLT2 inhibitors cut AFib risk in real-word analysis
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
AT ADA 2022
ADA updates on finerenone, SGLT2 inhibitors, and race-based eGFR
As it gears up for the first in-person scientific sessions for 3 years, the American Diabetes Association has issued an addendum to its most recent annual clinical practice recommendations published in December 2021, the 2022 Standards of Medical Care in Diabetes, based on recent trial evidence and consensus.
The update informs clinicians about:
- The effect of the nonsteroidal mineralocorticoid antagonist (Kerendia) on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease.
- The effect of a sodium-glucose cotransporter 2 (SGLT2) inhibitor on heart failure and renal outcomes in patients with type 2 diabetes.
The National Kidney Foundation and the American Society of Nephrology Task Force recommendation to remove race in the formula for calculating estimated glomerular filtration rate (eGFR).
“This is the fifth year that we are able to update the Standards of Care after it has been published through our Living Standards of Care updates, making it possible to give diabetes care providers the most important information and the latest evidence relevant to their practice,” Robert A. Gabbay, MD, PhD, ADA chief scientific and medical officer, said in a press release from the organization.
The addendum, entitled, “Living Standards of Care,” updates Section 10, “Cardiovascular Disease and Risk Management,” and Section 11, “Chronic Kidney Disease and Risk Management” of the 2022 Standards of Medical Care in Diabetes.
The amendments were approved by the ADA Professional Practice Committee, which is responsible for developing the Standards of Care. The American College of Cardiology reviewed and endorsed the section on CVD and risk management.
The Living Standards Update was published online in Diabetes Care.
CVD and risk management
In the Addendum to Section 10, “Cardiovascular Disease and Risk Management,” the committee writes:
- “For patients with type 2 diabetes and chronic kidney disease treated with maximum tolerated doses of ACE inhibitors or angiotensin receptor blockers, addition of finerenone should be considered to improve cardiovascular outcomes and reduce the risk of chronic kidney disease progression. A”
- “Patients with type 2 diabetes and chronic kidney disease should be considered for treatment with finerenone to reduce cardiovascular outcomes and the risk of chronic kidney disease progression.”
- “In patients with type 2 diabetes and established heart failure with either preserved or reduced ejection fraction, an SGLT2 inhibitor [with proven benefit in this patient population] is recommended to reduce risk of worsening heart failure, hospitalizations for heart failure, and cardiovascular death. ”
In the section “Statin Treatment,” the addendum no longer states that “a prospective trial of a newer fibrate ... is ongoing,” because that trial investigating pemafibrate (Kowa), a novel selective peroxisome proliferator-activated receptor alpha modulator (or fibrate), has been discontinued.
Chronic kidney disease and risk management
In the Addendum to Section 11, “Chronic Kidney Disease and Risk Management,” the committee writes:
- “Traditionally, eGFR is calculated from serum creatinine using a validated formula. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is preferred. ... Historically, a correction factor for muscle mass was included in a modified equation for African Americans; however, due to various issues with inequities, it was decided to the equation such that it applies to all. Hence, a committee was convened, resulting in the recommendation for immediate implementation of the CKD-EPI creatinine equation refit without the race variable in all laboratories in the U.S.” (This is based on an National Kidney Foundation and American Society of Nephrology Task Force recommendation.)
- “Additionally, increased use of cystatin C, especially to confirm estimated GFR in adults who are at risk for or have chronic kidney disease, because combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone.”
Evidence from clinical trials
The update is based on findings from the following clinical trials:
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD)
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD)
- FIDELITY, a prespecified pooled analysis of FIDELIO-DKD and FIGARO-DKD
- Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved)
- Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients with PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF)
- Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT).
A version of this article first appeared on Medscape.com.
As it gears up for the first in-person scientific sessions for 3 years, the American Diabetes Association has issued an addendum to its most recent annual clinical practice recommendations published in December 2021, the 2022 Standards of Medical Care in Diabetes, based on recent trial evidence and consensus.
The update informs clinicians about:
- The effect of the nonsteroidal mineralocorticoid antagonist (Kerendia) on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease.
- The effect of a sodium-glucose cotransporter 2 (SGLT2) inhibitor on heart failure and renal outcomes in patients with type 2 diabetes.
The National Kidney Foundation and the American Society of Nephrology Task Force recommendation to remove race in the formula for calculating estimated glomerular filtration rate (eGFR).
“This is the fifth year that we are able to update the Standards of Care after it has been published through our Living Standards of Care updates, making it possible to give diabetes care providers the most important information and the latest evidence relevant to their practice,” Robert A. Gabbay, MD, PhD, ADA chief scientific and medical officer, said in a press release from the organization.
The addendum, entitled, “Living Standards of Care,” updates Section 10, “Cardiovascular Disease and Risk Management,” and Section 11, “Chronic Kidney Disease and Risk Management” of the 2022 Standards of Medical Care in Diabetes.
The amendments were approved by the ADA Professional Practice Committee, which is responsible for developing the Standards of Care. The American College of Cardiology reviewed and endorsed the section on CVD and risk management.
The Living Standards Update was published online in Diabetes Care.
CVD and risk management
In the Addendum to Section 10, “Cardiovascular Disease and Risk Management,” the committee writes:
- “For patients with type 2 diabetes and chronic kidney disease treated with maximum tolerated doses of ACE inhibitors or angiotensin receptor blockers, addition of finerenone should be considered to improve cardiovascular outcomes and reduce the risk of chronic kidney disease progression. A”
- “Patients with type 2 diabetes and chronic kidney disease should be considered for treatment with finerenone to reduce cardiovascular outcomes and the risk of chronic kidney disease progression.”
- “In patients with type 2 diabetes and established heart failure with either preserved or reduced ejection fraction, an SGLT2 inhibitor [with proven benefit in this patient population] is recommended to reduce risk of worsening heart failure, hospitalizations for heart failure, and cardiovascular death. ”
In the section “Statin Treatment,” the addendum no longer states that “a prospective trial of a newer fibrate ... is ongoing,” because that trial investigating pemafibrate (Kowa), a novel selective peroxisome proliferator-activated receptor alpha modulator (or fibrate), has been discontinued.
Chronic kidney disease and risk management
In the Addendum to Section 11, “Chronic Kidney Disease and Risk Management,” the committee writes:
- “Traditionally, eGFR is calculated from serum creatinine using a validated formula. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is preferred. ... Historically, a correction factor for muscle mass was included in a modified equation for African Americans; however, due to various issues with inequities, it was decided to the equation such that it applies to all. Hence, a committee was convened, resulting in the recommendation for immediate implementation of the CKD-EPI creatinine equation refit without the race variable in all laboratories in the U.S.” (This is based on an National Kidney Foundation and American Society of Nephrology Task Force recommendation.)
- “Additionally, increased use of cystatin C, especially to confirm estimated GFR in adults who are at risk for or have chronic kidney disease, because combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone.”
Evidence from clinical trials
The update is based on findings from the following clinical trials:
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD)
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD)
- FIDELITY, a prespecified pooled analysis of FIDELIO-DKD and FIGARO-DKD
- Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved)
- Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients with PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF)
- Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT).
A version of this article first appeared on Medscape.com.
As it gears up for the first in-person scientific sessions for 3 years, the American Diabetes Association has issued an addendum to its most recent annual clinical practice recommendations published in December 2021, the 2022 Standards of Medical Care in Diabetes, based on recent trial evidence and consensus.
The update informs clinicians about:
- The effect of the nonsteroidal mineralocorticoid antagonist (Kerendia) on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease.
- The effect of a sodium-glucose cotransporter 2 (SGLT2) inhibitor on heart failure and renal outcomes in patients with type 2 diabetes.
The National Kidney Foundation and the American Society of Nephrology Task Force recommendation to remove race in the formula for calculating estimated glomerular filtration rate (eGFR).
“This is the fifth year that we are able to update the Standards of Care after it has been published through our Living Standards of Care updates, making it possible to give diabetes care providers the most important information and the latest evidence relevant to their practice,” Robert A. Gabbay, MD, PhD, ADA chief scientific and medical officer, said in a press release from the organization.
The addendum, entitled, “Living Standards of Care,” updates Section 10, “Cardiovascular Disease and Risk Management,” and Section 11, “Chronic Kidney Disease and Risk Management” of the 2022 Standards of Medical Care in Diabetes.
The amendments were approved by the ADA Professional Practice Committee, which is responsible for developing the Standards of Care. The American College of Cardiology reviewed and endorsed the section on CVD and risk management.
The Living Standards Update was published online in Diabetes Care.
CVD and risk management
In the Addendum to Section 10, “Cardiovascular Disease and Risk Management,” the committee writes:
- “For patients with type 2 diabetes and chronic kidney disease treated with maximum tolerated doses of ACE inhibitors or angiotensin receptor blockers, addition of finerenone should be considered to improve cardiovascular outcomes and reduce the risk of chronic kidney disease progression. A”
- “Patients with type 2 diabetes and chronic kidney disease should be considered for treatment with finerenone to reduce cardiovascular outcomes and the risk of chronic kidney disease progression.”
- “In patients with type 2 diabetes and established heart failure with either preserved or reduced ejection fraction, an SGLT2 inhibitor [with proven benefit in this patient population] is recommended to reduce risk of worsening heart failure, hospitalizations for heart failure, and cardiovascular death. ”
In the section “Statin Treatment,” the addendum no longer states that “a prospective trial of a newer fibrate ... is ongoing,” because that trial investigating pemafibrate (Kowa), a novel selective peroxisome proliferator-activated receptor alpha modulator (or fibrate), has been discontinued.
Chronic kidney disease and risk management
In the Addendum to Section 11, “Chronic Kidney Disease and Risk Management,” the committee writes:
- “Traditionally, eGFR is calculated from serum creatinine using a validated formula. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is preferred. ... Historically, a correction factor for muscle mass was included in a modified equation for African Americans; however, due to various issues with inequities, it was decided to the equation such that it applies to all. Hence, a committee was convened, resulting in the recommendation for immediate implementation of the CKD-EPI creatinine equation refit without the race variable in all laboratories in the U.S.” (This is based on an National Kidney Foundation and American Society of Nephrology Task Force recommendation.)
- “Additionally, increased use of cystatin C, especially to confirm estimated GFR in adults who are at risk for or have chronic kidney disease, because combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone.”
Evidence from clinical trials
The update is based on findings from the following clinical trials:
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD)
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD)
- FIDELITY, a prespecified pooled analysis of FIDELIO-DKD and FIGARO-DKD
- Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved)
- Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients with PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF)
- Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT).
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Stopping immunosuppressives in lupus nephritis isn’t noninferior to continuing
COPENHAGEN – Discontinuing maintenance immunosuppressive therapy (IST) in patients with proliferative lupus nephritis in remission proved less effective than continuing it in terms of the rates of renal relapse and severe systemic lupus erythematosus (SLE) flare, results from the WIN-Lupus trial show.
Lead researcher Noemie Jourde-Chiche, MD, assistant professor at Aix-Marseille (France) University, presented the results at the annual European Congress of Rheumatology.
The randomized, controlled trial aimed to determine the optimal duration of maintenance IST for proliferative lupus nephritis, by asking whether discontinuation of such therapy after 2-3 years was noninferior to IST continuation for 2 more years.
“This is the first randomized IST trial in proliferative lupus nephritis,” Dr. Jourde-Chiche reported. “We found that noninferiority of IST discontinuation was not demonstrated, and those who discontinued had a higher risk of SLE flares, but the majority of patients who discontinued did not experience flare.”
Regarding the incidence of renal relapse, no statistically significant difference was found between patients who continued versus those who discontinued IST.
Rheumatologist Christophe Richez, MD, of the Groupe Hospitalier Pellegrin-CHU de Bordeaux (France) welcomed the trial. “The work does not find a significant difference but suggests that with more power, the difference would have been significant,” he said in an interview.
He added that a significant number of patients refused to enter the study for several reasons, including scheduling a pregnancy and fear of relapse after stopping the treatment. “This in itself shows that we need this type of study to know if we can stop the treatment to plan a pregnancy or if a relay treatment is necessary. These data also mean we can now better inform our patients about the benefit-risk [profile] of continuing treatment or not.”
Dr. Richez, who was not involved in the trial, noted that the data provide information that strongly suggest more research is needed to better determine which patients are at risk of relapse, and consequently, for which patients is discontinuation possible. “There’s also a need for further analysis of tolerance to the immunosuppressive drugs according to the strategy.”
Finally, he referred to the issue around therapeutic adherence that is often faced with management of lupus. “The data from this study will allow us to better explain to patients the need to continue or discontinue their treatments, including hydroxychloroquine, and also the possibility of decreasing the immunosuppressive drugs to half-dose.”
Maintenance therapy in lupus nephritis: To continue or not?
Conducted in 28 French centers, participants had class III or IV lupus nephritis with active lesions and had previously received induction IST of cyclophosphamide or mycophenolate mofetil with hydroxychloroquine and glucocorticoids. Maintenance IST was azathioprine or mycophenolate mofetil, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day).
A total of 96 patients were randomized into two groups: continuation of maintenance therapy (azathioprine or mycophenolate mofetil for 2-3 more years, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day), or discontinuation of maintenance therapy (azathioprine or mycophenolate mofetil) over 3 months.
Both study arms were similar with a mean age of 36-37 years, and 82%-86% of patients were female, 59%-67% were White. They had a mean disease duration of 7-9 years, and 72%-80% had experienced their first flare of proliferative nephritis. A total of 54%-65% had received cyclophosphamide induction therapy, and 75%-81% were on mycophenolate mofetil maintenance therapy that had been ongoing for a mean of 2.8 years. Tests of patients’ kidney function revealed a mean serum creatinine of 67-72 micromol/L and estimated glomerular filtration rate (eGFR) of 94-101 mL/min per 1.73 m2. The mean SLE Disease Activity Index score was around 2.
Follow-up visits were conducted every 3 months for 2 years, and the trial had a primary end point of renal relapse rate at 24 months (confirmed by kidney biopsy), while secondary endpoints included rate of severe SLE flares, survival without renal relapse, or severe flare and adverse events, among others.
Patients were excluded if they were not taking hydroxychloroquine, had extrarenal SLE, an eGFR less than 30 mL/min per 1.73 m2 or stage VI disease. After some participants did not finish the trial because of pregnancy, wish for pregnancy, or adverse events, data from a total of 40 in the IST continuation group and 44 in the discontinuation group were analyzed.
Noninferiority of discontinuation versus continuation not shown
A total of 12.5% in the continuation arm experienced renal relapse over 24 months, compared with 27.3% in the discontinuation arm (P = .079).
“The endpoint of noninferiority of discontinuation of immunosuppressive therapy was not shown, but no statistically significant difference was found between groups for renal relapse,” Dr. Jourde-Chiche said.
Severe SLE flares (renal or extrarenal) occurred significantly less often over 24 months in the continuation arm at 12.5% versus 31.8% in the discontinuation arm (P = .034).
However, Dr. Jourde-Chiche pointed out that “the majority of patients who discontinued treatment did not experience a flare.”
No differences were seen between groups with respect to any secondary endpoints. “Fortunately, no patients died or reached end-stage renal disease, and overall, the adverse events did not differ between groups,” she reported.
The study identified several risk factors for renal relapse, among which were low complement component 3 and higher baseline urinary protein to creatinine ratio.
Longer immunosuppressive therapy prediscontinuation leads to better results
Gabriella Moroni, MD, of the nephrology unit at Ospedale Maggiore Policlinico, Milan, authored a review of studies that attempted to interrupt glucocorticoids and other immunosuppressive agents in lupus nephritis and in SLE. Her review concluded that “the available data suggest that therapy withdrawal is feasible at least in patients enjoying a complete clinical remission after a prolonged therapy.”
Asked to comment on the French study, Dr. Moroni said the trial was very welcome. “In our long-term experience, we have stopped IST in 73 patients with lupus nephritis, followed for a mean of 23 years. Of these, 32 never reassumed therapy and 20 had at least one flare.”
Dr. Moroni noted that those participants who did not experience flares had significantly longer IST and longer remission before discontinuation and took hydroxychloroquine more frequently.
“We feel that, to prevent severe flares, firstly, lupus nephritis should be in complete remission for at least 3 years, and secondly, patients should have received IST for at least 5 years before discontinuation; immunosuppressive drugs should be tapered off very slowly and after strict clinical surveillance, and finally, hydroxychloroquine can prevent extrarenal flares.
Dr. Jourde-Chiche reported serving on a speakers bureau for Vifor Pharma and receiving grant/research support from Fresenius Medical Care. Some coauthors reported financial ties to many pharmaceutical companies. Dr. Richez said he has received fees for lectures or boards from GlaxoSmithKline, AstraZeneca, and Novartis. Dr. Moroni had no relevant disclosures.
COPENHAGEN – Discontinuing maintenance immunosuppressive therapy (IST) in patients with proliferative lupus nephritis in remission proved less effective than continuing it in terms of the rates of renal relapse and severe systemic lupus erythematosus (SLE) flare, results from the WIN-Lupus trial show.
Lead researcher Noemie Jourde-Chiche, MD, assistant professor at Aix-Marseille (France) University, presented the results at the annual European Congress of Rheumatology.
The randomized, controlled trial aimed to determine the optimal duration of maintenance IST for proliferative lupus nephritis, by asking whether discontinuation of such therapy after 2-3 years was noninferior to IST continuation for 2 more years.
“This is the first randomized IST trial in proliferative lupus nephritis,” Dr. Jourde-Chiche reported. “We found that noninferiority of IST discontinuation was not demonstrated, and those who discontinued had a higher risk of SLE flares, but the majority of patients who discontinued did not experience flare.”
Regarding the incidence of renal relapse, no statistically significant difference was found between patients who continued versus those who discontinued IST.
Rheumatologist Christophe Richez, MD, of the Groupe Hospitalier Pellegrin-CHU de Bordeaux (France) welcomed the trial. “The work does not find a significant difference but suggests that with more power, the difference would have been significant,” he said in an interview.
He added that a significant number of patients refused to enter the study for several reasons, including scheduling a pregnancy and fear of relapse after stopping the treatment. “This in itself shows that we need this type of study to know if we can stop the treatment to plan a pregnancy or if a relay treatment is necessary. These data also mean we can now better inform our patients about the benefit-risk [profile] of continuing treatment or not.”
Dr. Richez, who was not involved in the trial, noted that the data provide information that strongly suggest more research is needed to better determine which patients are at risk of relapse, and consequently, for which patients is discontinuation possible. “There’s also a need for further analysis of tolerance to the immunosuppressive drugs according to the strategy.”
Finally, he referred to the issue around therapeutic adherence that is often faced with management of lupus. “The data from this study will allow us to better explain to patients the need to continue or discontinue their treatments, including hydroxychloroquine, and also the possibility of decreasing the immunosuppressive drugs to half-dose.”
Maintenance therapy in lupus nephritis: To continue or not?
Conducted in 28 French centers, participants had class III or IV lupus nephritis with active lesions and had previously received induction IST of cyclophosphamide or mycophenolate mofetil with hydroxychloroquine and glucocorticoids. Maintenance IST was azathioprine or mycophenolate mofetil, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day).
A total of 96 patients were randomized into two groups: continuation of maintenance therapy (azathioprine or mycophenolate mofetil for 2-3 more years, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day), or discontinuation of maintenance therapy (azathioprine or mycophenolate mofetil) over 3 months.
Both study arms were similar with a mean age of 36-37 years, and 82%-86% of patients were female, 59%-67% were White. They had a mean disease duration of 7-9 years, and 72%-80% had experienced their first flare of proliferative nephritis. A total of 54%-65% had received cyclophosphamide induction therapy, and 75%-81% were on mycophenolate mofetil maintenance therapy that had been ongoing for a mean of 2.8 years. Tests of patients’ kidney function revealed a mean serum creatinine of 67-72 micromol/L and estimated glomerular filtration rate (eGFR) of 94-101 mL/min per 1.73 m2. The mean SLE Disease Activity Index score was around 2.
Follow-up visits were conducted every 3 months for 2 years, and the trial had a primary end point of renal relapse rate at 24 months (confirmed by kidney biopsy), while secondary endpoints included rate of severe SLE flares, survival without renal relapse, or severe flare and adverse events, among others.
Patients were excluded if they were not taking hydroxychloroquine, had extrarenal SLE, an eGFR less than 30 mL/min per 1.73 m2 or stage VI disease. After some participants did not finish the trial because of pregnancy, wish for pregnancy, or adverse events, data from a total of 40 in the IST continuation group and 44 in the discontinuation group were analyzed.
Noninferiority of discontinuation versus continuation not shown
A total of 12.5% in the continuation arm experienced renal relapse over 24 months, compared with 27.3% in the discontinuation arm (P = .079).
“The endpoint of noninferiority of discontinuation of immunosuppressive therapy was not shown, but no statistically significant difference was found between groups for renal relapse,” Dr. Jourde-Chiche said.
Severe SLE flares (renal or extrarenal) occurred significantly less often over 24 months in the continuation arm at 12.5% versus 31.8% in the discontinuation arm (P = .034).
However, Dr. Jourde-Chiche pointed out that “the majority of patients who discontinued treatment did not experience a flare.”
No differences were seen between groups with respect to any secondary endpoints. “Fortunately, no patients died or reached end-stage renal disease, and overall, the adverse events did not differ between groups,” she reported.
The study identified several risk factors for renal relapse, among which were low complement component 3 and higher baseline urinary protein to creatinine ratio.
Longer immunosuppressive therapy prediscontinuation leads to better results
Gabriella Moroni, MD, of the nephrology unit at Ospedale Maggiore Policlinico, Milan, authored a review of studies that attempted to interrupt glucocorticoids and other immunosuppressive agents in lupus nephritis and in SLE. Her review concluded that “the available data suggest that therapy withdrawal is feasible at least in patients enjoying a complete clinical remission after a prolonged therapy.”
Asked to comment on the French study, Dr. Moroni said the trial was very welcome. “In our long-term experience, we have stopped IST in 73 patients with lupus nephritis, followed for a mean of 23 years. Of these, 32 never reassumed therapy and 20 had at least one flare.”
Dr. Moroni noted that those participants who did not experience flares had significantly longer IST and longer remission before discontinuation and took hydroxychloroquine more frequently.
“We feel that, to prevent severe flares, firstly, lupus nephritis should be in complete remission for at least 3 years, and secondly, patients should have received IST for at least 5 years before discontinuation; immunosuppressive drugs should be tapered off very slowly and after strict clinical surveillance, and finally, hydroxychloroquine can prevent extrarenal flares.
Dr. Jourde-Chiche reported serving on a speakers bureau for Vifor Pharma and receiving grant/research support from Fresenius Medical Care. Some coauthors reported financial ties to many pharmaceutical companies. Dr. Richez said he has received fees for lectures or boards from GlaxoSmithKline, AstraZeneca, and Novartis. Dr. Moroni had no relevant disclosures.
COPENHAGEN – Discontinuing maintenance immunosuppressive therapy (IST) in patients with proliferative lupus nephritis in remission proved less effective than continuing it in terms of the rates of renal relapse and severe systemic lupus erythematosus (SLE) flare, results from the WIN-Lupus trial show.
Lead researcher Noemie Jourde-Chiche, MD, assistant professor at Aix-Marseille (France) University, presented the results at the annual European Congress of Rheumatology.
The randomized, controlled trial aimed to determine the optimal duration of maintenance IST for proliferative lupus nephritis, by asking whether discontinuation of such therapy after 2-3 years was noninferior to IST continuation for 2 more years.
“This is the first randomized IST trial in proliferative lupus nephritis,” Dr. Jourde-Chiche reported. “We found that noninferiority of IST discontinuation was not demonstrated, and those who discontinued had a higher risk of SLE flares, but the majority of patients who discontinued did not experience flare.”
Regarding the incidence of renal relapse, no statistically significant difference was found between patients who continued versus those who discontinued IST.
Rheumatologist Christophe Richez, MD, of the Groupe Hospitalier Pellegrin-CHU de Bordeaux (France) welcomed the trial. “The work does not find a significant difference but suggests that with more power, the difference would have been significant,” he said in an interview.
He added that a significant number of patients refused to enter the study for several reasons, including scheduling a pregnancy and fear of relapse after stopping the treatment. “This in itself shows that we need this type of study to know if we can stop the treatment to plan a pregnancy or if a relay treatment is necessary. These data also mean we can now better inform our patients about the benefit-risk [profile] of continuing treatment or not.”
Dr. Richez, who was not involved in the trial, noted that the data provide information that strongly suggest more research is needed to better determine which patients are at risk of relapse, and consequently, for which patients is discontinuation possible. “There’s also a need for further analysis of tolerance to the immunosuppressive drugs according to the strategy.”
Finally, he referred to the issue around therapeutic adherence that is often faced with management of lupus. “The data from this study will allow us to better explain to patients the need to continue or discontinue their treatments, including hydroxychloroquine, and also the possibility of decreasing the immunosuppressive drugs to half-dose.”
Maintenance therapy in lupus nephritis: To continue or not?
Conducted in 28 French centers, participants had class III or IV lupus nephritis with active lesions and had previously received induction IST of cyclophosphamide or mycophenolate mofetil with hydroxychloroquine and glucocorticoids. Maintenance IST was azathioprine or mycophenolate mofetil, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day).
A total of 96 patients were randomized into two groups: continuation of maintenance therapy (azathioprine or mycophenolate mofetil for 2-3 more years, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day), or discontinuation of maintenance therapy (azathioprine or mycophenolate mofetil) over 3 months.
Both study arms were similar with a mean age of 36-37 years, and 82%-86% of patients were female, 59%-67% were White. They had a mean disease duration of 7-9 years, and 72%-80% had experienced their first flare of proliferative nephritis. A total of 54%-65% had received cyclophosphamide induction therapy, and 75%-81% were on mycophenolate mofetil maintenance therapy that had been ongoing for a mean of 2.8 years. Tests of patients’ kidney function revealed a mean serum creatinine of 67-72 micromol/L and estimated glomerular filtration rate (eGFR) of 94-101 mL/min per 1.73 m2. The mean SLE Disease Activity Index score was around 2.
Follow-up visits were conducted every 3 months for 2 years, and the trial had a primary end point of renal relapse rate at 24 months (confirmed by kidney biopsy), while secondary endpoints included rate of severe SLE flares, survival without renal relapse, or severe flare and adverse events, among others.
Patients were excluded if they were not taking hydroxychloroquine, had extrarenal SLE, an eGFR less than 30 mL/min per 1.73 m2 or stage VI disease. After some participants did not finish the trial because of pregnancy, wish for pregnancy, or adverse events, data from a total of 40 in the IST continuation group and 44 in the discontinuation group were analyzed.
Noninferiority of discontinuation versus continuation not shown
A total of 12.5% in the continuation arm experienced renal relapse over 24 months, compared with 27.3% in the discontinuation arm (P = .079).
“The endpoint of noninferiority of discontinuation of immunosuppressive therapy was not shown, but no statistically significant difference was found between groups for renal relapse,” Dr. Jourde-Chiche said.
Severe SLE flares (renal or extrarenal) occurred significantly less often over 24 months in the continuation arm at 12.5% versus 31.8% in the discontinuation arm (P = .034).
However, Dr. Jourde-Chiche pointed out that “the majority of patients who discontinued treatment did not experience a flare.”
No differences were seen between groups with respect to any secondary endpoints. “Fortunately, no patients died or reached end-stage renal disease, and overall, the adverse events did not differ between groups,” she reported.
The study identified several risk factors for renal relapse, among which were low complement component 3 and higher baseline urinary protein to creatinine ratio.
Longer immunosuppressive therapy prediscontinuation leads to better results
Gabriella Moroni, MD, of the nephrology unit at Ospedale Maggiore Policlinico, Milan, authored a review of studies that attempted to interrupt glucocorticoids and other immunosuppressive agents in lupus nephritis and in SLE. Her review concluded that “the available data suggest that therapy withdrawal is feasible at least in patients enjoying a complete clinical remission after a prolonged therapy.”
Asked to comment on the French study, Dr. Moroni said the trial was very welcome. “In our long-term experience, we have stopped IST in 73 patients with lupus nephritis, followed for a mean of 23 years. Of these, 32 never reassumed therapy and 20 had at least one flare.”
Dr. Moroni noted that those participants who did not experience flares had significantly longer IST and longer remission before discontinuation and took hydroxychloroquine more frequently.
“We feel that, to prevent severe flares, firstly, lupus nephritis should be in complete remission for at least 3 years, and secondly, patients should have received IST for at least 5 years before discontinuation; immunosuppressive drugs should be tapered off very slowly and after strict clinical surveillance, and finally, hydroxychloroquine can prevent extrarenal flares.
Dr. Jourde-Chiche reported serving on a speakers bureau for Vifor Pharma and receiving grant/research support from Fresenius Medical Care. Some coauthors reported financial ties to many pharmaceutical companies. Dr. Richez said he has received fees for lectures or boards from GlaxoSmithKline, AstraZeneca, and Novartis. Dr. Moroni had no relevant disclosures.
AT THE EULAR 2022 CONGRESS
Will tirzepatide slow kidney function decline in type 2 diabetes?
The “twincretin” tirzepatide might become part of the “arsenal” against diabetic kidney disease, new research suggests. Notably, the drug significantly reduced the likelihood of macroalbuminuria, in a prespecified subanalysis of the SURPASS-4 clinical trial.
“Once-per-week tirzepatide compared to [daily] insulin glargine treatment resulted in a meaningful improvement in estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (UACR) and the risk of end stage kidney disease (ESKD) – with low risk of clinically relevant hypoglycemia in participants with type 2 diabetes at high cardiovascular risk and varying degrees of chronic kidney disease (CKD),” lead investigator Hiddo J. L. Heerspink, PhD, PharmD, summarized in an email to this news organization.
The U.S. Food and Drug Administration has just approved tirzepatide (Mounjaro, Eli Lilly) – a novel, glucose-dependent insulinotropic polypeptide (GIP) combined with a glucagonlike peptide-1 (GLP-1) receptor agonist – to treat glycemia in patients with type 2 diabetes, based on five pivotal SURPASS trials.
Dr. Heerspink presented the new findings about tirzepatide’s impact on kidney function in an oral session at the annual scientific sessions of the American Diabetes Association.
40% reduced risk of kidney function decline
The main results of SURPASS-4 were published in the Lancet in October 2021, and showed that tirzepatide appeared superior to insulin glargine in lowering hemoglobin A1c in patients with type 2 diabetes at high cardiovascular risk who were inadequately controlled on oral diabetes treatments.
Now, Dr. Heerspink has shown that patients who received tirzepatide as opposed to insulin glargine were significantly less likely to have kidney function decline that included new-onset macroalbuminuria (hazard ratio, 0.59; P < .05).
“These are very large benefits and clearly indicate the potential of tirzepatide to be a very strong kidney protective drug,” said Dr. Heerspink, from the department of clinical pharmacy and pharmacology, University Medical Center Groningen (the Netherlands).
“Based on results from the SURPASS-4 trial, tirzepatide has significant kidney-protective effects in adults with type 2 diabetes with high cardiovascular risk and largely normal kidney function,” Christine Limonte, MD, chair of the session in which the analysis was presented, agreed, in an email to this news organization.
The approximate 40% reduced risk of kidney function decline in this population “is important because it suggests that this novel agent may contribute to the growing arsenal for preventing and treating diabetic kidney disease,” added Dr. Limonte, a clinical research fellow in the division of nephrology, University of Washington, Seattle.
“Over the last several years,” she noted, “sodium glucose cotransporter-2 [SGLT2] inhibitors and GLP-1 receptor agonists have been identified as having significant kidney-protective effects in type 2 diabetes, and as such are becoming first-line agents in the treatment of diabetic kidney disease.”
Additional studies are needed, she added, to assess the impacts of tirzepatide compared to these agents (particularly GLP-1 receptor agonists, which overlap in their mechanism of action).
“With the growing number of therapeutic options for diabetic kidney disease, future research should also focus on identifying combinations of agents which benefit individuals in a ‘targeted’ manner,” according to Dr. Limonte.
“Ensuring accessibility to kidney-protective agents by promoting access to health care and reducing drug costs is essential to improving outcomes in diabetic kidney disease,” she added.
Strongest reduction seen in risk of new macroalbuminuria
One in three adults with diabetes has CKD, according to a press release issued by the ADA. Therefore, there is a need for therapies to reduce the development and progression of CKD in patients with type 2 diabetes.
The prespecified analysis of SUPRESS-4 investigated potential renoprotective effects of tirzepatide.
The trial enrolled 1,995 patients with type 2 diabetes who were at increased risk of cardiovascular disease. The patients had a mean age of 63.6 years and a mean hemoglobin A1c of 8.5%.
Most patients had normal kidney function. The mean eGFR based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was 81.3 mL/min per 1.73 m2.
Few patients (17%) had moderately or severely reduced kidney function (eGFR <60 mL/min per 1.73 m2). Around a quarter of the patients (28%) had microalbuminuria (UACR 30-300 mg/g) and 8% had macroalbuminuria (UACR >300 mg/g).
The patients were randomized to receive a weekly injection of 5, 10, or 15 mg tirzepatide or a daily individualized injection of insulin glargine starting at 10 IU/day at bedtime, titrated to a fasting blood glucose <100 mg/dL, in addition to existing oral glucose-lowering agents. The primary outcomes in the subanalysis were:
- Endpoint 1: a composite of ≥40% decline in eGFR from baseline, renal death, progression to ESKD, and new-onset macroalbuminuria.
- Endpoint 2: the same as endpoint 1 excluding new-onset macroalbuminuria.
During a median follow up of 85 weeks and up to 104 weeks, patients who received tirzepatide versus insulin glargine were significantly less likely to reach endpoint 1 but not endpoint 2.
In addition, tirzepatide “very strongly” reduced the risk of new-onset macroalbuminuria, compared to insulin glargine, by approximately 60% in the complete study cohort (hazard ratio, 0.41; P < .05), Dr. Limonte noted.
Tirzepatide also reduced the risk of a >40% decline in eGFR, but this effect was not statistically significant, possibly because this outcome was underpowered. There were also too few kidney deaths and progressions to ESKD to meaningfully assess the effects of tirzepatide on these outcomes.
Therefore, Dr. Limonte noted, “it is likely that tirzepatide’s significant benefit on composite endpoint 1 was largely driven by this agent’s impact on reducing macroalbuminuria onset [explaining why a significant benefit was not seen with composite endpoint 2, which excluded new-onset macroalbuminuria].”
The study was funded by Eli Lilly. Dr. Heerspink disclosed that he is a consultant for AstraZeneca, Bayer AG, Boehringer Ingelheim, Chinook Therapeutics, CSL Behring, Gilead Sciences, Goldfinch Bio, Janssen Research & Development, Mitsubishi Tanabe Pharma, Mundipharma, and Traveere Pharmaceuticals, and has received research support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk.
Dr. Limonte disclosed that she receives funds from the American Kidney Fund’s Clinical Scientist in Nephrology Award.
A version of this article first appeared on Medscape.com.
The “twincretin” tirzepatide might become part of the “arsenal” against diabetic kidney disease, new research suggests. Notably, the drug significantly reduced the likelihood of macroalbuminuria, in a prespecified subanalysis of the SURPASS-4 clinical trial.
“Once-per-week tirzepatide compared to [daily] insulin glargine treatment resulted in a meaningful improvement in estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (UACR) and the risk of end stage kidney disease (ESKD) – with low risk of clinically relevant hypoglycemia in participants with type 2 diabetes at high cardiovascular risk and varying degrees of chronic kidney disease (CKD),” lead investigator Hiddo J. L. Heerspink, PhD, PharmD, summarized in an email to this news organization.
The U.S. Food and Drug Administration has just approved tirzepatide (Mounjaro, Eli Lilly) – a novel, glucose-dependent insulinotropic polypeptide (GIP) combined with a glucagonlike peptide-1 (GLP-1) receptor agonist – to treat glycemia in patients with type 2 diabetes, based on five pivotal SURPASS trials.
Dr. Heerspink presented the new findings about tirzepatide’s impact on kidney function in an oral session at the annual scientific sessions of the American Diabetes Association.
40% reduced risk of kidney function decline
The main results of SURPASS-4 were published in the Lancet in October 2021, and showed that tirzepatide appeared superior to insulin glargine in lowering hemoglobin A1c in patients with type 2 diabetes at high cardiovascular risk who were inadequately controlled on oral diabetes treatments.
Now, Dr. Heerspink has shown that patients who received tirzepatide as opposed to insulin glargine were significantly less likely to have kidney function decline that included new-onset macroalbuminuria (hazard ratio, 0.59; P < .05).
“These are very large benefits and clearly indicate the potential of tirzepatide to be a very strong kidney protective drug,” said Dr. Heerspink, from the department of clinical pharmacy and pharmacology, University Medical Center Groningen (the Netherlands).
“Based on results from the SURPASS-4 trial, tirzepatide has significant kidney-protective effects in adults with type 2 diabetes with high cardiovascular risk and largely normal kidney function,” Christine Limonte, MD, chair of the session in which the analysis was presented, agreed, in an email to this news organization.
The approximate 40% reduced risk of kidney function decline in this population “is important because it suggests that this novel agent may contribute to the growing arsenal for preventing and treating diabetic kidney disease,” added Dr. Limonte, a clinical research fellow in the division of nephrology, University of Washington, Seattle.
“Over the last several years,” she noted, “sodium glucose cotransporter-2 [SGLT2] inhibitors and GLP-1 receptor agonists have been identified as having significant kidney-protective effects in type 2 diabetes, and as such are becoming first-line agents in the treatment of diabetic kidney disease.”
Additional studies are needed, she added, to assess the impacts of tirzepatide compared to these agents (particularly GLP-1 receptor agonists, which overlap in their mechanism of action).
“With the growing number of therapeutic options for diabetic kidney disease, future research should also focus on identifying combinations of agents which benefit individuals in a ‘targeted’ manner,” according to Dr. Limonte.
“Ensuring accessibility to kidney-protective agents by promoting access to health care and reducing drug costs is essential to improving outcomes in diabetic kidney disease,” she added.
Strongest reduction seen in risk of new macroalbuminuria
One in three adults with diabetes has CKD, according to a press release issued by the ADA. Therefore, there is a need for therapies to reduce the development and progression of CKD in patients with type 2 diabetes.
The prespecified analysis of SUPRESS-4 investigated potential renoprotective effects of tirzepatide.
The trial enrolled 1,995 patients with type 2 diabetes who were at increased risk of cardiovascular disease. The patients had a mean age of 63.6 years and a mean hemoglobin A1c of 8.5%.
Most patients had normal kidney function. The mean eGFR based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was 81.3 mL/min per 1.73 m2.
Few patients (17%) had moderately or severely reduced kidney function (eGFR <60 mL/min per 1.73 m2). Around a quarter of the patients (28%) had microalbuminuria (UACR 30-300 mg/g) and 8% had macroalbuminuria (UACR >300 mg/g).
The patients were randomized to receive a weekly injection of 5, 10, or 15 mg tirzepatide or a daily individualized injection of insulin glargine starting at 10 IU/day at bedtime, titrated to a fasting blood glucose <100 mg/dL, in addition to existing oral glucose-lowering agents. The primary outcomes in the subanalysis were:
- Endpoint 1: a composite of ≥40% decline in eGFR from baseline, renal death, progression to ESKD, and new-onset macroalbuminuria.
- Endpoint 2: the same as endpoint 1 excluding new-onset macroalbuminuria.
During a median follow up of 85 weeks and up to 104 weeks, patients who received tirzepatide versus insulin glargine were significantly less likely to reach endpoint 1 but not endpoint 2.
In addition, tirzepatide “very strongly” reduced the risk of new-onset macroalbuminuria, compared to insulin glargine, by approximately 60% in the complete study cohort (hazard ratio, 0.41; P < .05), Dr. Limonte noted.
Tirzepatide also reduced the risk of a >40% decline in eGFR, but this effect was not statistically significant, possibly because this outcome was underpowered. There were also too few kidney deaths and progressions to ESKD to meaningfully assess the effects of tirzepatide on these outcomes.
Therefore, Dr. Limonte noted, “it is likely that tirzepatide’s significant benefit on composite endpoint 1 was largely driven by this agent’s impact on reducing macroalbuminuria onset [explaining why a significant benefit was not seen with composite endpoint 2, which excluded new-onset macroalbuminuria].”
The study was funded by Eli Lilly. Dr. Heerspink disclosed that he is a consultant for AstraZeneca, Bayer AG, Boehringer Ingelheim, Chinook Therapeutics, CSL Behring, Gilead Sciences, Goldfinch Bio, Janssen Research & Development, Mitsubishi Tanabe Pharma, Mundipharma, and Traveere Pharmaceuticals, and has received research support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk.
Dr. Limonte disclosed that she receives funds from the American Kidney Fund’s Clinical Scientist in Nephrology Award.
A version of this article first appeared on Medscape.com.
The “twincretin” tirzepatide might become part of the “arsenal” against diabetic kidney disease, new research suggests. Notably, the drug significantly reduced the likelihood of macroalbuminuria, in a prespecified subanalysis of the SURPASS-4 clinical trial.
“Once-per-week tirzepatide compared to [daily] insulin glargine treatment resulted in a meaningful improvement in estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (UACR) and the risk of end stage kidney disease (ESKD) – with low risk of clinically relevant hypoglycemia in participants with type 2 diabetes at high cardiovascular risk and varying degrees of chronic kidney disease (CKD),” lead investigator Hiddo J. L. Heerspink, PhD, PharmD, summarized in an email to this news organization.
The U.S. Food and Drug Administration has just approved tirzepatide (Mounjaro, Eli Lilly) – a novel, glucose-dependent insulinotropic polypeptide (GIP) combined with a glucagonlike peptide-1 (GLP-1) receptor agonist – to treat glycemia in patients with type 2 diabetes, based on five pivotal SURPASS trials.
Dr. Heerspink presented the new findings about tirzepatide’s impact on kidney function in an oral session at the annual scientific sessions of the American Diabetes Association.
40% reduced risk of kidney function decline
The main results of SURPASS-4 were published in the Lancet in October 2021, and showed that tirzepatide appeared superior to insulin glargine in lowering hemoglobin A1c in patients with type 2 diabetes at high cardiovascular risk who were inadequately controlled on oral diabetes treatments.
Now, Dr. Heerspink has shown that patients who received tirzepatide as opposed to insulin glargine were significantly less likely to have kidney function decline that included new-onset macroalbuminuria (hazard ratio, 0.59; P < .05).
“These are very large benefits and clearly indicate the potential of tirzepatide to be a very strong kidney protective drug,” said Dr. Heerspink, from the department of clinical pharmacy and pharmacology, University Medical Center Groningen (the Netherlands).
“Based on results from the SURPASS-4 trial, tirzepatide has significant kidney-protective effects in adults with type 2 diabetes with high cardiovascular risk and largely normal kidney function,” Christine Limonte, MD, chair of the session in which the analysis was presented, agreed, in an email to this news organization.
The approximate 40% reduced risk of kidney function decline in this population “is important because it suggests that this novel agent may contribute to the growing arsenal for preventing and treating diabetic kidney disease,” added Dr. Limonte, a clinical research fellow in the division of nephrology, University of Washington, Seattle.
“Over the last several years,” she noted, “sodium glucose cotransporter-2 [SGLT2] inhibitors and GLP-1 receptor agonists have been identified as having significant kidney-protective effects in type 2 diabetes, and as such are becoming first-line agents in the treatment of diabetic kidney disease.”
Additional studies are needed, she added, to assess the impacts of tirzepatide compared to these agents (particularly GLP-1 receptor agonists, which overlap in their mechanism of action).
“With the growing number of therapeutic options for diabetic kidney disease, future research should also focus on identifying combinations of agents which benefit individuals in a ‘targeted’ manner,” according to Dr. Limonte.
“Ensuring accessibility to kidney-protective agents by promoting access to health care and reducing drug costs is essential to improving outcomes in diabetic kidney disease,” she added.
Strongest reduction seen in risk of new macroalbuminuria
One in three adults with diabetes has CKD, according to a press release issued by the ADA. Therefore, there is a need for therapies to reduce the development and progression of CKD in patients with type 2 diabetes.
The prespecified analysis of SUPRESS-4 investigated potential renoprotective effects of tirzepatide.
The trial enrolled 1,995 patients with type 2 diabetes who were at increased risk of cardiovascular disease. The patients had a mean age of 63.6 years and a mean hemoglobin A1c of 8.5%.
Most patients had normal kidney function. The mean eGFR based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was 81.3 mL/min per 1.73 m2.
Few patients (17%) had moderately or severely reduced kidney function (eGFR <60 mL/min per 1.73 m2). Around a quarter of the patients (28%) had microalbuminuria (UACR 30-300 mg/g) and 8% had macroalbuminuria (UACR >300 mg/g).
The patients were randomized to receive a weekly injection of 5, 10, or 15 mg tirzepatide or a daily individualized injection of insulin glargine starting at 10 IU/day at bedtime, titrated to a fasting blood glucose <100 mg/dL, in addition to existing oral glucose-lowering agents. The primary outcomes in the subanalysis were:
- Endpoint 1: a composite of ≥40% decline in eGFR from baseline, renal death, progression to ESKD, and new-onset macroalbuminuria.
- Endpoint 2: the same as endpoint 1 excluding new-onset macroalbuminuria.
During a median follow up of 85 weeks and up to 104 weeks, patients who received tirzepatide versus insulin glargine were significantly less likely to reach endpoint 1 but not endpoint 2.
In addition, tirzepatide “very strongly” reduced the risk of new-onset macroalbuminuria, compared to insulin glargine, by approximately 60% in the complete study cohort (hazard ratio, 0.41; P < .05), Dr. Limonte noted.
Tirzepatide also reduced the risk of a >40% decline in eGFR, but this effect was not statistically significant, possibly because this outcome was underpowered. There were also too few kidney deaths and progressions to ESKD to meaningfully assess the effects of tirzepatide on these outcomes.
Therefore, Dr. Limonte noted, “it is likely that tirzepatide’s significant benefit on composite endpoint 1 was largely driven by this agent’s impact on reducing macroalbuminuria onset [explaining why a significant benefit was not seen with composite endpoint 2, which excluded new-onset macroalbuminuria].”
The study was funded by Eli Lilly. Dr. Heerspink disclosed that he is a consultant for AstraZeneca, Bayer AG, Boehringer Ingelheim, Chinook Therapeutics, CSL Behring, Gilead Sciences, Goldfinch Bio, Janssen Research & Development, Mitsubishi Tanabe Pharma, Mundipharma, and Traveere Pharmaceuticals, and has received research support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk.
Dr. Limonte disclosed that she receives funds from the American Kidney Fund’s Clinical Scientist in Nephrology Award.
A version of this article first appeared on Medscape.com.
FROM ADA 2022
AUA 2022: A report from the trenches
The annual meeting of the American Urological Association took place recently at the Ernest N. Morial Convention Center in New Orleans. Hundreds of talks and abstracts were presented over the 4 days in New Orleans; below is a summary of what I found to be the key scientific highlights.
1. Updates to the AUA’s guidelines for management of localized kidney cancer
The AUA’s recommendations for the treatment of localized kidney cancer have changed dramatically over the past few decades. Gone are the days of simply removing the entire kidney every time a mass is found. Today, a partial nephrectomy is preferred in most situations.
Our understanding that the prevalence of familial kidney cancer is much higher than previously thought has led to a change in the guidelines regarding which patients should receive genetic counseling. For the first time, the guidelines include the use of adjuvant medical treatment, such as pembrolizumab. A 2021 study in the New England Journal of Medicine showed a survival benefit for patients with high-risk disease who receive such therapies, so it›s not surprising that such treatments are now recommended.
The development of new second- and third-generation gadolinium contrast agents that spare the kidneys has dramatically increased the role for MRIs for patients with severe or even end-stage renal disease. As a result, the guidelines were updated to recommend the use of these agents. The role of a renal biopsy, which has always been limited, given the ability of cross-sectional imaging to diagnosis this disease, has further been constrained and should now be performed only when the results would clearly change a clinical decision, such as whether or not the lesion in question is a metastasis.
2. New and better ureteroscope technology
No one likes kidney stones, not the patient who deals with the incredible pain, nor the surgeon who has to remove them, given that these cases often present in the wee hours of the morning. The preferred surgical approach has changed dramatically over the past decade, moving away from extracorporeal shockwave lithotripsy toward flexible ureteroscope-based technology, which has a higher clearance rate and is more widely and more immediately available. Flexible ureteroscopy has been held back by technological barriers, including limited scope deflection and low laser power. The exceptionally high cost of repair and the tendency of the instruments to break haven’t helped, either. Although single-use ureteroscopes have been available for some time, it wasn’t until the recently introduced second-generation scopes became widely available that they have become popular. These new scopes have small external diameters, great optics, and can easily be used. Newer high-powered lasers and the change from holmium:YAG-based lasers to thulium technology is greatly increasing the size of stones that can be safely addressed ureteroscopically. The cost analysis of single-use technology versus reusable scopes tends to be site dependent but can be appealing in certain situations. Also, on the technology forefront, a new robotically assisted ureteroscope is being introduced that offers the chance for improved intrapelvic mobility and better ergonomics for the surgeon.
3. New options for the treatment of clinically localized prostate cancer
Since the guidelines were last updated in 2017, the definitive management of localized prostate cancer has changed dramatically. Although radical prostatectomy and radiotherapy remain the preferred options for men who choose treatment for their disease, the updated guidelines state that active surveillance is now the preferred approach for men with low-risk cancers.
Although the preferred surveillance protocol is still being debated, the consensus is that almost all men with low-risk disease can be safely monitored for some period. The imaging technology available to monitor patients is also radically changing with the rollout of prostate-specific membrane antigen–based PET technology. The increased sensitivity and specificity of this modality opens the door not only for better up-front staging of newly diagnosed patients with prostate cancer but also may allow clinicians to earlier identify and treat men with metastatic disease. The guidelines for the first time address the use of genetic markers to individualize treatment of men with advanced or metastatic prostate cancer. Exactly which treatments these patients need is still being debated, but the ability to use patient-specific genetic mutation information to customize treatment is potentially groundbreaking.
4. New treatment options for patients with high-grade non–muscle-invasive bladder cancer (NMIBC) refractory to bacille Calmette-Guérin (BCG) therapy
Patients with NMIBC who do not respond to BCG therapy are in a tough position. Cystectomy remains the preferred option as a second-line strategy, but the procedure has a complication rate approaching 30%. Further, many patients are not willing to have their bladder removed because of the life-altering changes that go along with having an urostomy or a neobladder. While intravesical treatments such as valrubicin, docetaxel, or gemcitabine have been available for many years, the success rates of those options are limited. The Food and Drug Administration recently approved the use of the immunotherapy-based treatment pembrolizumab. While none of these options is perfect, the fact that we now have at least some alternatives is a huge step in the right direction.
5. It’s all about the patient: Involving patients in designing the health care delivery system
Although it seems like an obvious concept, patients themselves have traditionally not been involved in designing the health care delivery system on which they rely. Research presented at the AUA shows that many health care outcomes improve when patients are actively involved in the process. For example, Angela Smith, MD, of the University of North Carolina at Chapel Hill, presented a study showing that including patients in the identification of possible research topics helps them feel engaged and more likely to participate in studies. Patients who are involved in advisory councils at the local hospital level are more likely to report having received high-quality care. And surveying patients on the goals of national health care policy helps them feel that the outcomes are more equitable.
As a small-town urologist who spends his days in the trenches of urology, I think the next time my group considers participating in new cancer research, I may talk to the local cancer support group first. If Dr. Smith’s data are correct, not only would our patients be better served, but we would also have an easier time filling the trial!
The 2023 AUA conference is going to be held in Chicago next spring. I hope to see you there!
A version of this article first appeared on Medscape.com.
The annual meeting of the American Urological Association took place recently at the Ernest N. Morial Convention Center in New Orleans. Hundreds of talks and abstracts were presented over the 4 days in New Orleans; below is a summary of what I found to be the key scientific highlights.
1. Updates to the AUA’s guidelines for management of localized kidney cancer
The AUA’s recommendations for the treatment of localized kidney cancer have changed dramatically over the past few decades. Gone are the days of simply removing the entire kidney every time a mass is found. Today, a partial nephrectomy is preferred in most situations.
Our understanding that the prevalence of familial kidney cancer is much higher than previously thought has led to a change in the guidelines regarding which patients should receive genetic counseling. For the first time, the guidelines include the use of adjuvant medical treatment, such as pembrolizumab. A 2021 study in the New England Journal of Medicine showed a survival benefit for patients with high-risk disease who receive such therapies, so it›s not surprising that such treatments are now recommended.
The development of new second- and third-generation gadolinium contrast agents that spare the kidneys has dramatically increased the role for MRIs for patients with severe or even end-stage renal disease. As a result, the guidelines were updated to recommend the use of these agents. The role of a renal biopsy, which has always been limited, given the ability of cross-sectional imaging to diagnosis this disease, has further been constrained and should now be performed only when the results would clearly change a clinical decision, such as whether or not the lesion in question is a metastasis.
2. New and better ureteroscope technology
No one likes kidney stones, not the patient who deals with the incredible pain, nor the surgeon who has to remove them, given that these cases often present in the wee hours of the morning. The preferred surgical approach has changed dramatically over the past decade, moving away from extracorporeal shockwave lithotripsy toward flexible ureteroscope-based technology, which has a higher clearance rate and is more widely and more immediately available. Flexible ureteroscopy has been held back by technological barriers, including limited scope deflection and low laser power. The exceptionally high cost of repair and the tendency of the instruments to break haven’t helped, either. Although single-use ureteroscopes have been available for some time, it wasn’t until the recently introduced second-generation scopes became widely available that they have become popular. These new scopes have small external diameters, great optics, and can easily be used. Newer high-powered lasers and the change from holmium:YAG-based lasers to thulium technology is greatly increasing the size of stones that can be safely addressed ureteroscopically. The cost analysis of single-use technology versus reusable scopes tends to be site dependent but can be appealing in certain situations. Also, on the technology forefront, a new robotically assisted ureteroscope is being introduced that offers the chance for improved intrapelvic mobility and better ergonomics for the surgeon.
3. New options for the treatment of clinically localized prostate cancer
Since the guidelines were last updated in 2017, the definitive management of localized prostate cancer has changed dramatically. Although radical prostatectomy and radiotherapy remain the preferred options for men who choose treatment for their disease, the updated guidelines state that active surveillance is now the preferred approach for men with low-risk cancers.
Although the preferred surveillance protocol is still being debated, the consensus is that almost all men with low-risk disease can be safely monitored for some period. The imaging technology available to monitor patients is also radically changing with the rollout of prostate-specific membrane antigen–based PET technology. The increased sensitivity and specificity of this modality opens the door not only for better up-front staging of newly diagnosed patients with prostate cancer but also may allow clinicians to earlier identify and treat men with metastatic disease. The guidelines for the first time address the use of genetic markers to individualize treatment of men with advanced or metastatic prostate cancer. Exactly which treatments these patients need is still being debated, but the ability to use patient-specific genetic mutation information to customize treatment is potentially groundbreaking.
4. New treatment options for patients with high-grade non–muscle-invasive bladder cancer (NMIBC) refractory to bacille Calmette-Guérin (BCG) therapy
Patients with NMIBC who do not respond to BCG therapy are in a tough position. Cystectomy remains the preferred option as a second-line strategy, but the procedure has a complication rate approaching 30%. Further, many patients are not willing to have their bladder removed because of the life-altering changes that go along with having an urostomy or a neobladder. While intravesical treatments such as valrubicin, docetaxel, or gemcitabine have been available for many years, the success rates of those options are limited. The Food and Drug Administration recently approved the use of the immunotherapy-based treatment pembrolizumab. While none of these options is perfect, the fact that we now have at least some alternatives is a huge step in the right direction.
5. It’s all about the patient: Involving patients in designing the health care delivery system
Although it seems like an obvious concept, patients themselves have traditionally not been involved in designing the health care delivery system on which they rely. Research presented at the AUA shows that many health care outcomes improve when patients are actively involved in the process. For example, Angela Smith, MD, of the University of North Carolina at Chapel Hill, presented a study showing that including patients in the identification of possible research topics helps them feel engaged and more likely to participate in studies. Patients who are involved in advisory councils at the local hospital level are more likely to report having received high-quality care. And surveying patients on the goals of national health care policy helps them feel that the outcomes are more equitable.
As a small-town urologist who spends his days in the trenches of urology, I think the next time my group considers participating in new cancer research, I may talk to the local cancer support group first. If Dr. Smith’s data are correct, not only would our patients be better served, but we would also have an easier time filling the trial!
The 2023 AUA conference is going to be held in Chicago next spring. I hope to see you there!
A version of this article first appeared on Medscape.com.
The annual meeting of the American Urological Association took place recently at the Ernest N. Morial Convention Center in New Orleans. Hundreds of talks and abstracts were presented over the 4 days in New Orleans; below is a summary of what I found to be the key scientific highlights.
1. Updates to the AUA’s guidelines for management of localized kidney cancer
The AUA’s recommendations for the treatment of localized kidney cancer have changed dramatically over the past few decades. Gone are the days of simply removing the entire kidney every time a mass is found. Today, a partial nephrectomy is preferred in most situations.
Our understanding that the prevalence of familial kidney cancer is much higher than previously thought has led to a change in the guidelines regarding which patients should receive genetic counseling. For the first time, the guidelines include the use of adjuvant medical treatment, such as pembrolizumab. A 2021 study in the New England Journal of Medicine showed a survival benefit for patients with high-risk disease who receive such therapies, so it›s not surprising that such treatments are now recommended.
The development of new second- and third-generation gadolinium contrast agents that spare the kidneys has dramatically increased the role for MRIs for patients with severe or even end-stage renal disease. As a result, the guidelines were updated to recommend the use of these agents. The role of a renal biopsy, which has always been limited, given the ability of cross-sectional imaging to diagnosis this disease, has further been constrained and should now be performed only when the results would clearly change a clinical decision, such as whether or not the lesion in question is a metastasis.
2. New and better ureteroscope technology
No one likes kidney stones, not the patient who deals with the incredible pain, nor the surgeon who has to remove them, given that these cases often present in the wee hours of the morning. The preferred surgical approach has changed dramatically over the past decade, moving away from extracorporeal shockwave lithotripsy toward flexible ureteroscope-based technology, which has a higher clearance rate and is more widely and more immediately available. Flexible ureteroscopy has been held back by technological barriers, including limited scope deflection and low laser power. The exceptionally high cost of repair and the tendency of the instruments to break haven’t helped, either. Although single-use ureteroscopes have been available for some time, it wasn’t until the recently introduced second-generation scopes became widely available that they have become popular. These new scopes have small external diameters, great optics, and can easily be used. Newer high-powered lasers and the change from holmium:YAG-based lasers to thulium technology is greatly increasing the size of stones that can be safely addressed ureteroscopically. The cost analysis of single-use technology versus reusable scopes tends to be site dependent but can be appealing in certain situations. Also, on the technology forefront, a new robotically assisted ureteroscope is being introduced that offers the chance for improved intrapelvic mobility and better ergonomics for the surgeon.
3. New options for the treatment of clinically localized prostate cancer
Since the guidelines were last updated in 2017, the definitive management of localized prostate cancer has changed dramatically. Although radical prostatectomy and radiotherapy remain the preferred options for men who choose treatment for their disease, the updated guidelines state that active surveillance is now the preferred approach for men with low-risk cancers.
Although the preferred surveillance protocol is still being debated, the consensus is that almost all men with low-risk disease can be safely monitored for some period. The imaging technology available to monitor patients is also radically changing with the rollout of prostate-specific membrane antigen–based PET technology. The increased sensitivity and specificity of this modality opens the door not only for better up-front staging of newly diagnosed patients with prostate cancer but also may allow clinicians to earlier identify and treat men with metastatic disease. The guidelines for the first time address the use of genetic markers to individualize treatment of men with advanced or metastatic prostate cancer. Exactly which treatments these patients need is still being debated, but the ability to use patient-specific genetic mutation information to customize treatment is potentially groundbreaking.
4. New treatment options for patients with high-grade non–muscle-invasive bladder cancer (NMIBC) refractory to bacille Calmette-Guérin (BCG) therapy
Patients with NMIBC who do not respond to BCG therapy are in a tough position. Cystectomy remains the preferred option as a second-line strategy, but the procedure has a complication rate approaching 30%. Further, many patients are not willing to have their bladder removed because of the life-altering changes that go along with having an urostomy or a neobladder. While intravesical treatments such as valrubicin, docetaxel, or gemcitabine have been available for many years, the success rates of those options are limited. The Food and Drug Administration recently approved the use of the immunotherapy-based treatment pembrolizumab. While none of these options is perfect, the fact that we now have at least some alternatives is a huge step in the right direction.
5. It’s all about the patient: Involving patients in designing the health care delivery system
Although it seems like an obvious concept, patients themselves have traditionally not been involved in designing the health care delivery system on which they rely. Research presented at the AUA shows that many health care outcomes improve when patients are actively involved in the process. For example, Angela Smith, MD, of the University of North Carolina at Chapel Hill, presented a study showing that including patients in the identification of possible research topics helps them feel engaged and more likely to participate in studies. Patients who are involved in advisory councils at the local hospital level are more likely to report having received high-quality care. And surveying patients on the goals of national health care policy helps them feel that the outcomes are more equitable.
As a small-town urologist who spends his days in the trenches of urology, I think the next time my group considers participating in new cancer research, I may talk to the local cancer support group first. If Dr. Smith’s data are correct, not only would our patients be better served, but we would also have an easier time filling the trial!
The 2023 AUA conference is going to be held in Chicago next spring. I hope to see you there!
A version of this article first appeared on Medscape.com.
FROM AUA 2022
Mixing BP meds with NSAID may be ‘triple whammy’ for kidneys
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM MATHEMATICAL BIOSCIENCES
Substantially enlarged cardiac silhouette
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.
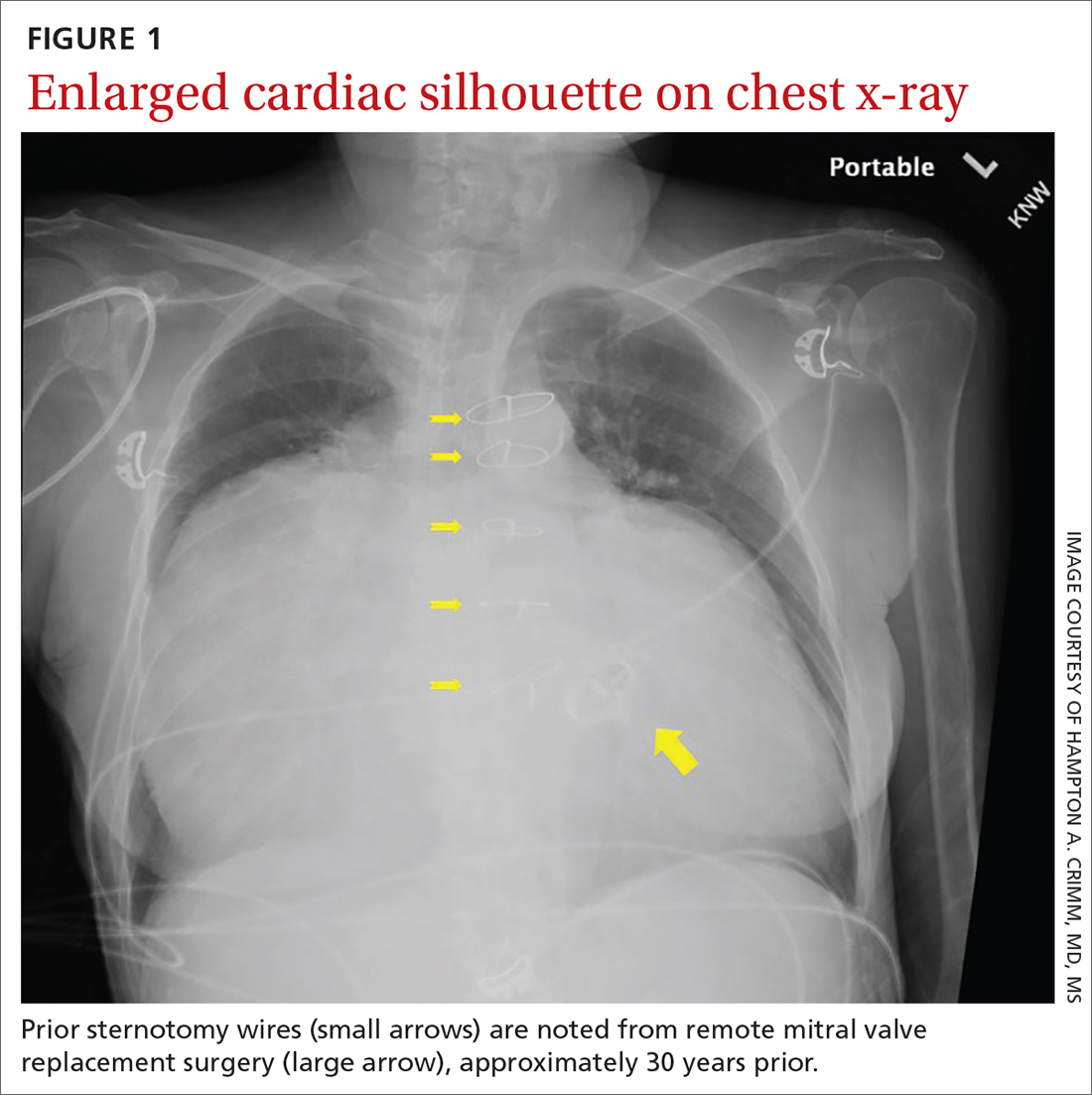
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.
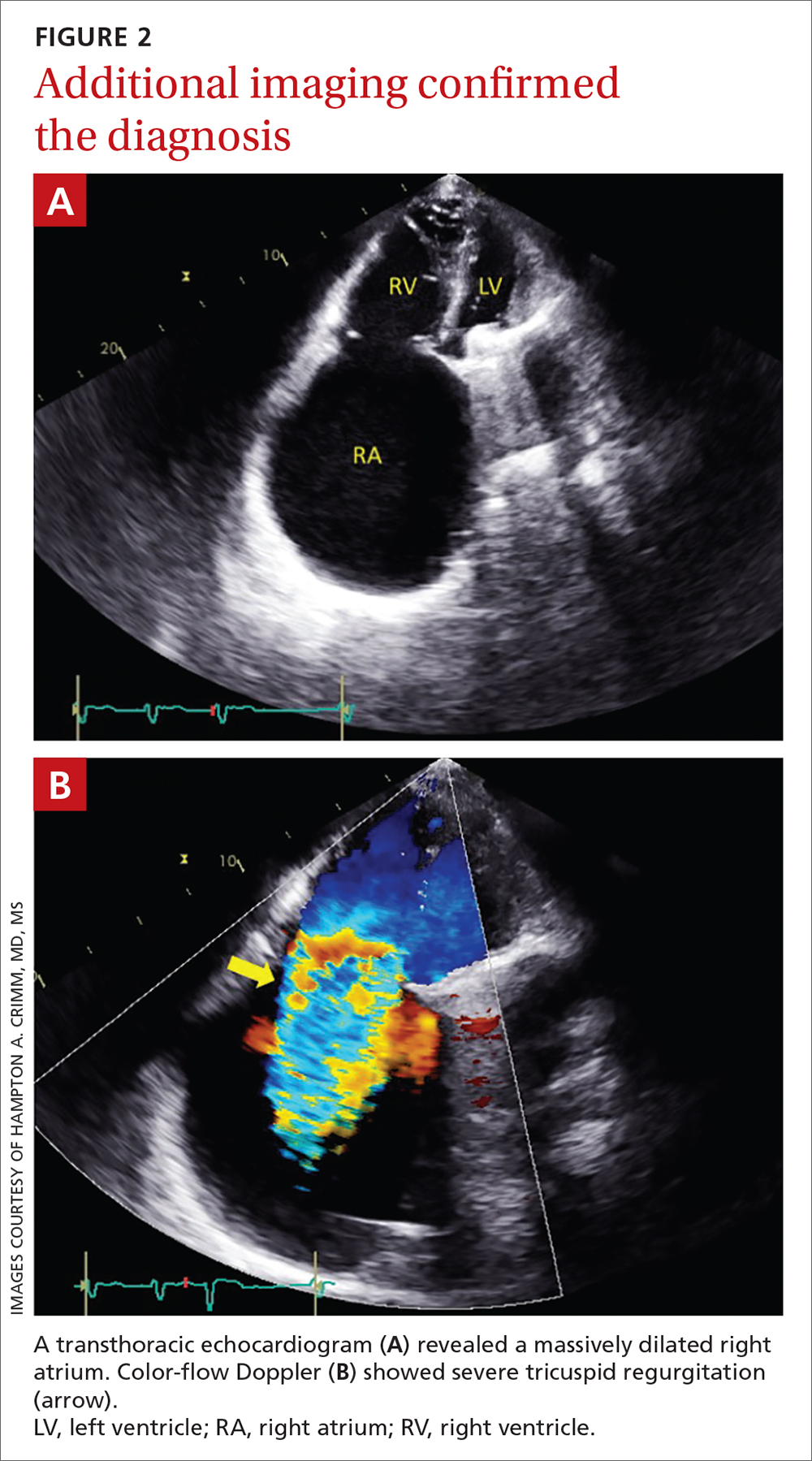
RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.

RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.

RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
Lowering BP according to newest guidance would cut CV events
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY