User login
Finerenone benefits T2D across spectrum of renal function
Treatment with finerenone produced roughly similar reductions in heart failure–related outcomes in people with type 2 diabetes and chronic kidney disease (CKD) across the spectrum of kidney function, compared with placebo, including those who had albuminuria but a preserved estimated glomerular filtration rate (eGFR), in a post hoc analysis of pooled data from more than 13,000 people.
The findings, from the two pivotal trials for the agent, “reinforce the importance of routine eGFR and UACR [urinary albumin-to-creatinine ratio] screening” in people with type 2 diabetes to identify new candidates for treatment with finerenone (Kerendia), Gerasimos Filippatos, MD, and coauthors said in a report published online in JACC: Heart Failure.
Among the 13,026 patients in the two combined trials, 40% had a preserved eGFR of greater than 60 mL/min per 1.73 m2 despite also having albuminuria with a UACR of at least 30 mg/g, showing how often this combination occurs. But many clinicians “do not follow the guidelines” and fail to measure the UACR in these patients in routine practice, noted Dr. Filippatos at the annual congress of the European Society of Cardiology in August.
“We now have something to do for these patients,” treat them with finerenone, said Dr. Filippatos, professor and director of heart failure at the Attikon University Hospital, Athens.
The availability of finerenone following its U.S. approval in 2021 means clinicians “must get used to measuring UACR” in people with type 2 diabetes even when their eGFR is normal, especially people with type 2 diabetes plus high cardiovascular disease risk, he said.
The Food and Drug Administration approved finerenone, a nonsteroidal mineralocorticoid receptor antagonist, for treating people with type 2 diabetes and CKD in July 2021, but its uptake has been slow, experts say. In a talk in September 2022 during the annual meeting of the European Association for the Study of Diabetes, Jennifer B. Green, MD, estimated that U.S. uptake of finerenone for appropriate people with type 2 diabetes had not advanced beyond 10%.
A recent review also noted that uptake of screening for elevated UACR in U.S. patients with type 2 diabetes was in the range of 10%-40% during 2017-2019, a “shockingly low rate,” said Dr. Green, a professor and diabetes specialist at Duke University, Durham, N.C.
A new reason to screen for albuminuria
“It’s an extremely important message,” Johann Bauersachs, MD, commented in an interview. Results from “many studies have shown that albuminuria is an excellent additional marker for cardiovascular disease risk. But measurement of albuminuria is not widely done, despite guidelines that recommend annual albuminuria testing in people with type 2 diabetes,” said Dr. Bauersachs, professor and head of the department of cardiology at Hannover (Germany) Medical School.
“Even before there was finerenone, there were reasons to measure UACR, but I hope adding finerenone will help, and more clinicians will incorporate UACR into their routine practice,” said Dr. Bauersachs, who was not involved with the finerenone studies.
The analyses reported by Dr. Filippatos and coauthors used data from two related trials of finerenone, FIDELIO-DKD and FIGARO-DKD, combined by prespecified design into a single dataset, FIDELITY, with a total of 13,026 participants eligible for analysis and followed for a median of 3 years. All had type 2 diabetes and CKD based on having a UACR of at least 30 mg/g. Their eGFR levels could run as high as 74 mL/min per 1.73 m2 in FIDELIO-DKD, and as high as 90 mL/min/1.73m2 in FIGARO-DKD. The two trials excluded people with heart failure with reduced ejection fraction, and those with a serum potassium greater than 4.8 mmol/L.
In the FIDELITY dataset treatment with finerenone led to a significant 17% reduction in the combined incidence of cardiovascular death or first hospitalization for heart failure relative to those who received placebo. This relative risk reduction was not affected by either eGFR or UACR values at baseline, the new analysis showed.
The analysis also demonstrated a nonsignificant trend toward greater reductions in heart failure–related outcomes among study participants who began with an eGFR in the normal range of at least 60 mL/min per 1.73 m2. The researchers also found a nonsignificant trend to a greater reduction in heart failure–related events among those with a UACR of less than 300 mg/g.
Finerenone favors patients with less advanced CKD
In short “the magnitude of the treatment benefit tended to favor patients with less advanced CKD,” concluded the researchers, suggesting that “earlier intervention [with finerenone] in the CKD course is likely to provide the greatest long-term benefit on heart failure–related outcomes.” This led them to further infer “the importance of not only routine assessing eGFR, but also perhaps more importantly, routinely screening for UACR to facilitate early diagnosis and early intervention in patients with type 2 diabetes.”
Findings from FIDELIO-DKD and FIGARO-DKD led to recent guideline additions for finerenone by several medical groups. In August 2022, the American Association of Clinical Endocrinologists released an update to its guideline for managing people with diabetes that recommended treating people with type 2 diabetes with finerenone when they have a UACR of at least 30 mg/g if they are already treated with a maximum-tolerated dose of a renin-angiotensin system inhibitor, have a normal serum potassium level, and have an eGFR of at least 25 mL/min per 1.73 m2. The identical recommendation also appeared in a Consensus Report from the American Diabetes Association and KDIGO, an international organization promoting evidence-based management of patients with CKD.
“Finerenone provides a very important contribution because it improves prognosis even in very well managed patients” with type 2 diabetes, commented Lars Rydén, MD, professor of cardiology at the Karolinska Institute in Stockholm, as designated discussant for the report by Dr. Filippatos at the ESC congress.
The findings from the FIDELITY analysis are “trustworthy, and clinically important,” Dr. Rydén said. When left untreated, diabetic kidney disease “reduces life expectancy by an average of 16 years.”
The finerenone trials were sponsored by Bayer, which markets finerenone (Kerendia). Dr. Filippatos has received lecture fees from Bayer as well as from Amgen, Medtronic, Novartis, Servier, and Vifor. Dr. Green has financial ties to Bayer as well as to Anji, AstraZeneca, Boehringer Ingelheim/Lilly, Hawthorne Effect/Omada, Merck, Novo Nordisk, Pfizer, Roche, Sanofi/Lexicon, and Valo. Dr. Bauersachs has been a consultant to Bayer as well as to Amgen, AstraZeneca, Boehringer Ingelheim, Cardior, Cervia, CVRx, Novartis, Pfizer, and Vifor, and he has received research funding from Abiomed. Dr. Rydén has financial ties to Bayer, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
Treatment with finerenone produced roughly similar reductions in heart failure–related outcomes in people with type 2 diabetes and chronic kidney disease (CKD) across the spectrum of kidney function, compared with placebo, including those who had albuminuria but a preserved estimated glomerular filtration rate (eGFR), in a post hoc analysis of pooled data from more than 13,000 people.
The findings, from the two pivotal trials for the agent, “reinforce the importance of routine eGFR and UACR [urinary albumin-to-creatinine ratio] screening” in people with type 2 diabetes to identify new candidates for treatment with finerenone (Kerendia), Gerasimos Filippatos, MD, and coauthors said in a report published online in JACC: Heart Failure.
Among the 13,026 patients in the two combined trials, 40% had a preserved eGFR of greater than 60 mL/min per 1.73 m2 despite also having albuminuria with a UACR of at least 30 mg/g, showing how often this combination occurs. But many clinicians “do not follow the guidelines” and fail to measure the UACR in these patients in routine practice, noted Dr. Filippatos at the annual congress of the European Society of Cardiology in August.
“We now have something to do for these patients,” treat them with finerenone, said Dr. Filippatos, professor and director of heart failure at the Attikon University Hospital, Athens.
The availability of finerenone following its U.S. approval in 2021 means clinicians “must get used to measuring UACR” in people with type 2 diabetes even when their eGFR is normal, especially people with type 2 diabetes plus high cardiovascular disease risk, he said.
The Food and Drug Administration approved finerenone, a nonsteroidal mineralocorticoid receptor antagonist, for treating people with type 2 diabetes and CKD in July 2021, but its uptake has been slow, experts say. In a talk in September 2022 during the annual meeting of the European Association for the Study of Diabetes, Jennifer B. Green, MD, estimated that U.S. uptake of finerenone for appropriate people with type 2 diabetes had not advanced beyond 10%.
A recent review also noted that uptake of screening for elevated UACR in U.S. patients with type 2 diabetes was in the range of 10%-40% during 2017-2019, a “shockingly low rate,” said Dr. Green, a professor and diabetes specialist at Duke University, Durham, N.C.
A new reason to screen for albuminuria
“It’s an extremely important message,” Johann Bauersachs, MD, commented in an interview. Results from “many studies have shown that albuminuria is an excellent additional marker for cardiovascular disease risk. But measurement of albuminuria is not widely done, despite guidelines that recommend annual albuminuria testing in people with type 2 diabetes,” said Dr. Bauersachs, professor and head of the department of cardiology at Hannover (Germany) Medical School.
“Even before there was finerenone, there were reasons to measure UACR, but I hope adding finerenone will help, and more clinicians will incorporate UACR into their routine practice,” said Dr. Bauersachs, who was not involved with the finerenone studies.
The analyses reported by Dr. Filippatos and coauthors used data from two related trials of finerenone, FIDELIO-DKD and FIGARO-DKD, combined by prespecified design into a single dataset, FIDELITY, with a total of 13,026 participants eligible for analysis and followed for a median of 3 years. All had type 2 diabetes and CKD based on having a UACR of at least 30 mg/g. Their eGFR levels could run as high as 74 mL/min per 1.73 m2 in FIDELIO-DKD, and as high as 90 mL/min/1.73m2 in FIGARO-DKD. The two trials excluded people with heart failure with reduced ejection fraction, and those with a serum potassium greater than 4.8 mmol/L.
In the FIDELITY dataset treatment with finerenone led to a significant 17% reduction in the combined incidence of cardiovascular death or first hospitalization for heart failure relative to those who received placebo. This relative risk reduction was not affected by either eGFR or UACR values at baseline, the new analysis showed.
The analysis also demonstrated a nonsignificant trend toward greater reductions in heart failure–related outcomes among study participants who began with an eGFR in the normal range of at least 60 mL/min per 1.73 m2. The researchers also found a nonsignificant trend to a greater reduction in heart failure–related events among those with a UACR of less than 300 mg/g.
Finerenone favors patients with less advanced CKD
In short “the magnitude of the treatment benefit tended to favor patients with less advanced CKD,” concluded the researchers, suggesting that “earlier intervention [with finerenone] in the CKD course is likely to provide the greatest long-term benefit on heart failure–related outcomes.” This led them to further infer “the importance of not only routine assessing eGFR, but also perhaps more importantly, routinely screening for UACR to facilitate early diagnosis and early intervention in patients with type 2 diabetes.”
Findings from FIDELIO-DKD and FIGARO-DKD led to recent guideline additions for finerenone by several medical groups. In August 2022, the American Association of Clinical Endocrinologists released an update to its guideline for managing people with diabetes that recommended treating people with type 2 diabetes with finerenone when they have a UACR of at least 30 mg/g if they are already treated with a maximum-tolerated dose of a renin-angiotensin system inhibitor, have a normal serum potassium level, and have an eGFR of at least 25 mL/min per 1.73 m2. The identical recommendation also appeared in a Consensus Report from the American Diabetes Association and KDIGO, an international organization promoting evidence-based management of patients with CKD.
“Finerenone provides a very important contribution because it improves prognosis even in very well managed patients” with type 2 diabetes, commented Lars Rydén, MD, professor of cardiology at the Karolinska Institute in Stockholm, as designated discussant for the report by Dr. Filippatos at the ESC congress.
The findings from the FIDELITY analysis are “trustworthy, and clinically important,” Dr. Rydén said. When left untreated, diabetic kidney disease “reduces life expectancy by an average of 16 years.”
The finerenone trials were sponsored by Bayer, which markets finerenone (Kerendia). Dr. Filippatos has received lecture fees from Bayer as well as from Amgen, Medtronic, Novartis, Servier, and Vifor. Dr. Green has financial ties to Bayer as well as to Anji, AstraZeneca, Boehringer Ingelheim/Lilly, Hawthorne Effect/Omada, Merck, Novo Nordisk, Pfizer, Roche, Sanofi/Lexicon, and Valo. Dr. Bauersachs has been a consultant to Bayer as well as to Amgen, AstraZeneca, Boehringer Ingelheim, Cardior, Cervia, CVRx, Novartis, Pfizer, and Vifor, and he has received research funding from Abiomed. Dr. Rydén has financial ties to Bayer, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
Treatment with finerenone produced roughly similar reductions in heart failure–related outcomes in people with type 2 diabetes and chronic kidney disease (CKD) across the spectrum of kidney function, compared with placebo, including those who had albuminuria but a preserved estimated glomerular filtration rate (eGFR), in a post hoc analysis of pooled data from more than 13,000 people.
The findings, from the two pivotal trials for the agent, “reinforce the importance of routine eGFR and UACR [urinary albumin-to-creatinine ratio] screening” in people with type 2 diabetes to identify new candidates for treatment with finerenone (Kerendia), Gerasimos Filippatos, MD, and coauthors said in a report published online in JACC: Heart Failure.
Among the 13,026 patients in the two combined trials, 40% had a preserved eGFR of greater than 60 mL/min per 1.73 m2 despite also having albuminuria with a UACR of at least 30 mg/g, showing how often this combination occurs. But many clinicians “do not follow the guidelines” and fail to measure the UACR in these patients in routine practice, noted Dr. Filippatos at the annual congress of the European Society of Cardiology in August.
“We now have something to do for these patients,” treat them with finerenone, said Dr. Filippatos, professor and director of heart failure at the Attikon University Hospital, Athens.
The availability of finerenone following its U.S. approval in 2021 means clinicians “must get used to measuring UACR” in people with type 2 diabetes even when their eGFR is normal, especially people with type 2 diabetes plus high cardiovascular disease risk, he said.
The Food and Drug Administration approved finerenone, a nonsteroidal mineralocorticoid receptor antagonist, for treating people with type 2 diabetes and CKD in July 2021, but its uptake has been slow, experts say. In a talk in September 2022 during the annual meeting of the European Association for the Study of Diabetes, Jennifer B. Green, MD, estimated that U.S. uptake of finerenone for appropriate people with type 2 diabetes had not advanced beyond 10%.
A recent review also noted that uptake of screening for elevated UACR in U.S. patients with type 2 diabetes was in the range of 10%-40% during 2017-2019, a “shockingly low rate,” said Dr. Green, a professor and diabetes specialist at Duke University, Durham, N.C.
A new reason to screen for albuminuria
“It’s an extremely important message,” Johann Bauersachs, MD, commented in an interview. Results from “many studies have shown that albuminuria is an excellent additional marker for cardiovascular disease risk. But measurement of albuminuria is not widely done, despite guidelines that recommend annual albuminuria testing in people with type 2 diabetes,” said Dr. Bauersachs, professor and head of the department of cardiology at Hannover (Germany) Medical School.
“Even before there was finerenone, there were reasons to measure UACR, but I hope adding finerenone will help, and more clinicians will incorporate UACR into their routine practice,” said Dr. Bauersachs, who was not involved with the finerenone studies.
The analyses reported by Dr. Filippatos and coauthors used data from two related trials of finerenone, FIDELIO-DKD and FIGARO-DKD, combined by prespecified design into a single dataset, FIDELITY, with a total of 13,026 participants eligible for analysis and followed for a median of 3 years. All had type 2 diabetes and CKD based on having a UACR of at least 30 mg/g. Their eGFR levels could run as high as 74 mL/min per 1.73 m2 in FIDELIO-DKD, and as high as 90 mL/min/1.73m2 in FIGARO-DKD. The two trials excluded people with heart failure with reduced ejection fraction, and those with a serum potassium greater than 4.8 mmol/L.
In the FIDELITY dataset treatment with finerenone led to a significant 17% reduction in the combined incidence of cardiovascular death or first hospitalization for heart failure relative to those who received placebo. This relative risk reduction was not affected by either eGFR or UACR values at baseline, the new analysis showed.
The analysis also demonstrated a nonsignificant trend toward greater reductions in heart failure–related outcomes among study participants who began with an eGFR in the normal range of at least 60 mL/min per 1.73 m2. The researchers also found a nonsignificant trend to a greater reduction in heart failure–related events among those with a UACR of less than 300 mg/g.
Finerenone favors patients with less advanced CKD
In short “the magnitude of the treatment benefit tended to favor patients with less advanced CKD,” concluded the researchers, suggesting that “earlier intervention [with finerenone] in the CKD course is likely to provide the greatest long-term benefit on heart failure–related outcomes.” This led them to further infer “the importance of not only routine assessing eGFR, but also perhaps more importantly, routinely screening for UACR to facilitate early diagnosis and early intervention in patients with type 2 diabetes.”
Findings from FIDELIO-DKD and FIGARO-DKD led to recent guideline additions for finerenone by several medical groups. In August 2022, the American Association of Clinical Endocrinologists released an update to its guideline for managing people with diabetes that recommended treating people with type 2 diabetes with finerenone when they have a UACR of at least 30 mg/g if they are already treated with a maximum-tolerated dose of a renin-angiotensin system inhibitor, have a normal serum potassium level, and have an eGFR of at least 25 mL/min per 1.73 m2. The identical recommendation also appeared in a Consensus Report from the American Diabetes Association and KDIGO, an international organization promoting evidence-based management of patients with CKD.
“Finerenone provides a very important contribution because it improves prognosis even in very well managed patients” with type 2 diabetes, commented Lars Rydén, MD, professor of cardiology at the Karolinska Institute in Stockholm, as designated discussant for the report by Dr. Filippatos at the ESC congress.
The findings from the FIDELITY analysis are “trustworthy, and clinically important,” Dr. Rydén said. When left untreated, diabetic kidney disease “reduces life expectancy by an average of 16 years.”
The finerenone trials were sponsored by Bayer, which markets finerenone (Kerendia). Dr. Filippatos has received lecture fees from Bayer as well as from Amgen, Medtronic, Novartis, Servier, and Vifor. Dr. Green has financial ties to Bayer as well as to Anji, AstraZeneca, Boehringer Ingelheim/Lilly, Hawthorne Effect/Omada, Merck, Novo Nordisk, Pfizer, Roche, Sanofi/Lexicon, and Valo. Dr. Bauersachs has been a consultant to Bayer as well as to Amgen, AstraZeneca, Boehringer Ingelheim, Cardior, Cervia, CVRx, Novartis, Pfizer, and Vifor, and he has received research funding from Abiomed. Dr. Rydén has financial ties to Bayer, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
FROM JACC: HEART FAILURE
Ultrasonic renal denervation passes 2-month test in uncontrolled HTN: RADIANCE II
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.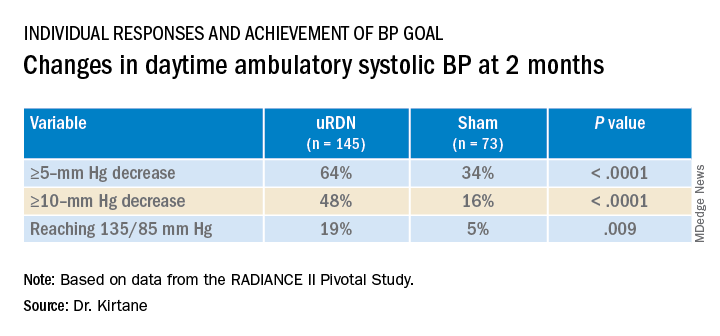
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).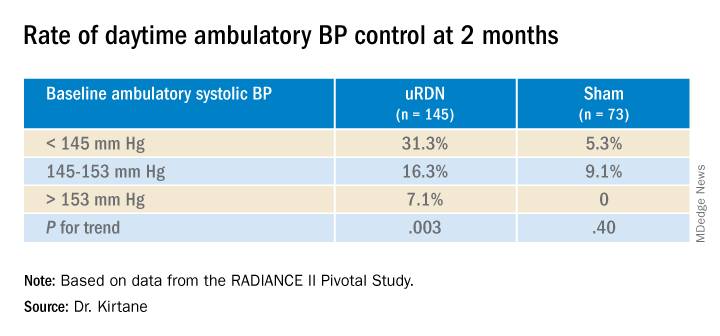
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
No invasive strategy benefit at 5 years in ISCHEMIA-CKD extension study
A trip to the cath lab for possible revascularization after a positive stress test, compared with a wait-and-see approach backed by optimal medications, did not improve 5-year survival for patients with advanced chronic kidney disease (CKD) in the ISCHEMIA-CKD trial’s extension study, ISCHEMIA-CKD EXTEND.
The long-term results, from the same 777 patients followed for an average of 2.2 years in the main trial, are consistent with the overall findings of no survival advantage with an initially invasive strategy, compared with one that is initially conservative. The finding applies to patients like those in the trial who had moderate to severe ischemia at stress testing and whose CKD put them in an especially high-risk and little-studied coronary artery disease (CAD) category.
Indeed, in a reflection of that high-risk status, 5-year all-cause mortality reached about 40% and cardiovascular (CV) mortality approached 30%, with no significant differences between patients in the invasive- and conservative-strategy groups.
Those numbers arguably put CKD’s effect on CAD survival in about the same league as an ejection fraction (EF) of 35% or less. For context, all-cause mortality over 3-4 years was about 32% in the REVIVED-BCIS2 trial of such patients with ischemic reduced-EF cardiomyopathy, whether or not they were revascularized, observed Sripal Bangalore, MD, MHA.
Yet in ISCHEMIA-CKD EXTEND, “you’re seeing in a group of patients, with largely preserved EF but advanced CKD, a mortality rate close to 40% at 5 years,” said Dr. Bangalore of New York University.
Although the study doesn’t show benefit from the initially invasive approach in CKD patients with stable CAD, for those with acute coronary syndromes (ACS), it seems to suggest that “at least the invasive strategy is safe,” Dr. Bangalore said during a press conference preceding his presentation of the study Aug. 29 at the annual congress of the European Society of Cardiology, held in Barcelona.
REVIVED-BCIS2 was also presented at the ESC sessions on Aug. 27, as reported by this news organization.
ISCHEMIA-CKD EXTEND “is a large trial and a very well-done trial. The results are robust, and they should influence clinical practice,” Deepak L. Bhatt, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said as the invited discussant after Dr. Bangalore’s presentation.
“The main message here, really, is don’t just go looking for ischemia, at least with the modalities used in this trial, in your CKD patients as a routine practice, and then try to stomp out that ischemia with revascularization,” Dr. Bhatt said. “The right thing to do in these high-risk patients is to focus on lifestyle modification and intensive medical therapy.”
A caveat, he said, is that the trial’s results don’t apply to the types of patients excluded from it, including those with recent ACS and those who are highly symptomatic or have an EF of less than 35%.
“Those CKD patients likely benefit from an invasive strategy with anatomically appropriate revascularization,” whether percutaneous coronary intervention (PCI) or coronary bypass surgery, Dr. Bhatt said.
At a median follow-up of 5 years in the extension study, the rates of death from any cause were 40.6% for patients in the invasive-strategy group and 37.4% for those in the conservative-strategy group. That yielded a hazard ratio of 1.12 (95% confidence interval, 0.89-1.41; P = .32) after adjustment for age, sex, diabetes status, EF, dialysis status, and – for patients not on dialysis – baseline estimated glomerular filtration rate.
The rates of CV death were 29% for patients managed invasively and 27% for those initially managed conservatively, for a similarly adjusted HR of 1.04 (95% CI, 0.80-1.37; P = .75).
In subgroup analyses, Dr. Bangalore reported, there were no significant differences in all-cause or CV mortality by diabetes status, by severity of baseline ischemia, or by whether the patient had recently experienced new or more frequent angina at study entry, was on guideline-directed medical therapy at baseline, or was on dialysis.
Among the contributions of ISCHEMIA-CKD and its 5-year extension study, Dr. Bangalore told this news organization, is that the relative safety of revascularization they showed may help to counter “renalism,” that is, the aversion to invasive intervention in patients with advanced CKD in clinical practice.
For example, if a patient with advanced CKD presents with an acute myocardial infarction, “people are hesitant to take them to the cath lab,” Dr. Bangalore said. But “if you follow protocols, if you follow strategies to minimize the risk, you can safely go ahead and do it.”
But in patients with stable CAD, as the ISCHEMIA-CKD studies show, “routinely revascularizing them may not have significant benefits.”
ISCHEMIC-CKD and its extension study were funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore discloses receiving research grants from NHLBI and serving as a consultant for Abbott Vascular, Biotronik, Boston Scientific, Amgen, Pfizer, Merck, and Reata. Dr. Bhatt has disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article first appeared on Medscape.com.
A trip to the cath lab for possible revascularization after a positive stress test, compared with a wait-and-see approach backed by optimal medications, did not improve 5-year survival for patients with advanced chronic kidney disease (CKD) in the ISCHEMIA-CKD trial’s extension study, ISCHEMIA-CKD EXTEND.
The long-term results, from the same 777 patients followed for an average of 2.2 years in the main trial, are consistent with the overall findings of no survival advantage with an initially invasive strategy, compared with one that is initially conservative. The finding applies to patients like those in the trial who had moderate to severe ischemia at stress testing and whose CKD put them in an especially high-risk and little-studied coronary artery disease (CAD) category.
Indeed, in a reflection of that high-risk status, 5-year all-cause mortality reached about 40% and cardiovascular (CV) mortality approached 30%, with no significant differences between patients in the invasive- and conservative-strategy groups.
Those numbers arguably put CKD’s effect on CAD survival in about the same league as an ejection fraction (EF) of 35% or less. For context, all-cause mortality over 3-4 years was about 32% in the REVIVED-BCIS2 trial of such patients with ischemic reduced-EF cardiomyopathy, whether or not they were revascularized, observed Sripal Bangalore, MD, MHA.
Yet in ISCHEMIA-CKD EXTEND, “you’re seeing in a group of patients, with largely preserved EF but advanced CKD, a mortality rate close to 40% at 5 years,” said Dr. Bangalore of New York University.
Although the study doesn’t show benefit from the initially invasive approach in CKD patients with stable CAD, for those with acute coronary syndromes (ACS), it seems to suggest that “at least the invasive strategy is safe,” Dr. Bangalore said during a press conference preceding his presentation of the study Aug. 29 at the annual congress of the European Society of Cardiology, held in Barcelona.
REVIVED-BCIS2 was also presented at the ESC sessions on Aug. 27, as reported by this news organization.
ISCHEMIA-CKD EXTEND “is a large trial and a very well-done trial. The results are robust, and they should influence clinical practice,” Deepak L. Bhatt, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said as the invited discussant after Dr. Bangalore’s presentation.
“The main message here, really, is don’t just go looking for ischemia, at least with the modalities used in this trial, in your CKD patients as a routine practice, and then try to stomp out that ischemia with revascularization,” Dr. Bhatt said. “The right thing to do in these high-risk patients is to focus on lifestyle modification and intensive medical therapy.”
A caveat, he said, is that the trial’s results don’t apply to the types of patients excluded from it, including those with recent ACS and those who are highly symptomatic or have an EF of less than 35%.
“Those CKD patients likely benefit from an invasive strategy with anatomically appropriate revascularization,” whether percutaneous coronary intervention (PCI) or coronary bypass surgery, Dr. Bhatt said.
At a median follow-up of 5 years in the extension study, the rates of death from any cause were 40.6% for patients in the invasive-strategy group and 37.4% for those in the conservative-strategy group. That yielded a hazard ratio of 1.12 (95% confidence interval, 0.89-1.41; P = .32) after adjustment for age, sex, diabetes status, EF, dialysis status, and – for patients not on dialysis – baseline estimated glomerular filtration rate.
The rates of CV death were 29% for patients managed invasively and 27% for those initially managed conservatively, for a similarly adjusted HR of 1.04 (95% CI, 0.80-1.37; P = .75).
In subgroup analyses, Dr. Bangalore reported, there were no significant differences in all-cause or CV mortality by diabetes status, by severity of baseline ischemia, or by whether the patient had recently experienced new or more frequent angina at study entry, was on guideline-directed medical therapy at baseline, or was on dialysis.
Among the contributions of ISCHEMIA-CKD and its 5-year extension study, Dr. Bangalore told this news organization, is that the relative safety of revascularization they showed may help to counter “renalism,” that is, the aversion to invasive intervention in patients with advanced CKD in clinical practice.
For example, if a patient with advanced CKD presents with an acute myocardial infarction, “people are hesitant to take them to the cath lab,” Dr. Bangalore said. But “if you follow protocols, if you follow strategies to minimize the risk, you can safely go ahead and do it.”
But in patients with stable CAD, as the ISCHEMIA-CKD studies show, “routinely revascularizing them may not have significant benefits.”
ISCHEMIC-CKD and its extension study were funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore discloses receiving research grants from NHLBI and serving as a consultant for Abbott Vascular, Biotronik, Boston Scientific, Amgen, Pfizer, Merck, and Reata. Dr. Bhatt has disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article first appeared on Medscape.com.
A trip to the cath lab for possible revascularization after a positive stress test, compared with a wait-and-see approach backed by optimal medications, did not improve 5-year survival for patients with advanced chronic kidney disease (CKD) in the ISCHEMIA-CKD trial’s extension study, ISCHEMIA-CKD EXTEND.
The long-term results, from the same 777 patients followed for an average of 2.2 years in the main trial, are consistent with the overall findings of no survival advantage with an initially invasive strategy, compared with one that is initially conservative. The finding applies to patients like those in the trial who had moderate to severe ischemia at stress testing and whose CKD put them in an especially high-risk and little-studied coronary artery disease (CAD) category.
Indeed, in a reflection of that high-risk status, 5-year all-cause mortality reached about 40% and cardiovascular (CV) mortality approached 30%, with no significant differences between patients in the invasive- and conservative-strategy groups.
Those numbers arguably put CKD’s effect on CAD survival in about the same league as an ejection fraction (EF) of 35% or less. For context, all-cause mortality over 3-4 years was about 32% in the REVIVED-BCIS2 trial of such patients with ischemic reduced-EF cardiomyopathy, whether or not they were revascularized, observed Sripal Bangalore, MD, MHA.
Yet in ISCHEMIA-CKD EXTEND, “you’re seeing in a group of patients, with largely preserved EF but advanced CKD, a mortality rate close to 40% at 5 years,” said Dr. Bangalore of New York University.
Although the study doesn’t show benefit from the initially invasive approach in CKD patients with stable CAD, for those with acute coronary syndromes (ACS), it seems to suggest that “at least the invasive strategy is safe,” Dr. Bangalore said during a press conference preceding his presentation of the study Aug. 29 at the annual congress of the European Society of Cardiology, held in Barcelona.
REVIVED-BCIS2 was also presented at the ESC sessions on Aug. 27, as reported by this news organization.
ISCHEMIA-CKD EXTEND “is a large trial and a very well-done trial. The results are robust, and they should influence clinical practice,” Deepak L. Bhatt, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said as the invited discussant after Dr. Bangalore’s presentation.
“The main message here, really, is don’t just go looking for ischemia, at least with the modalities used in this trial, in your CKD patients as a routine practice, and then try to stomp out that ischemia with revascularization,” Dr. Bhatt said. “The right thing to do in these high-risk patients is to focus on lifestyle modification and intensive medical therapy.”
A caveat, he said, is that the trial’s results don’t apply to the types of patients excluded from it, including those with recent ACS and those who are highly symptomatic or have an EF of less than 35%.
“Those CKD patients likely benefit from an invasive strategy with anatomically appropriate revascularization,” whether percutaneous coronary intervention (PCI) or coronary bypass surgery, Dr. Bhatt said.
At a median follow-up of 5 years in the extension study, the rates of death from any cause were 40.6% for patients in the invasive-strategy group and 37.4% for those in the conservative-strategy group. That yielded a hazard ratio of 1.12 (95% confidence interval, 0.89-1.41; P = .32) after adjustment for age, sex, diabetes status, EF, dialysis status, and – for patients not on dialysis – baseline estimated glomerular filtration rate.
The rates of CV death were 29% for patients managed invasively and 27% for those initially managed conservatively, for a similarly adjusted HR of 1.04 (95% CI, 0.80-1.37; P = .75).
In subgroup analyses, Dr. Bangalore reported, there were no significant differences in all-cause or CV mortality by diabetes status, by severity of baseline ischemia, or by whether the patient had recently experienced new or more frequent angina at study entry, was on guideline-directed medical therapy at baseline, or was on dialysis.
Among the contributions of ISCHEMIA-CKD and its 5-year extension study, Dr. Bangalore told this news organization, is that the relative safety of revascularization they showed may help to counter “renalism,” that is, the aversion to invasive intervention in patients with advanced CKD in clinical practice.
For example, if a patient with advanced CKD presents with an acute myocardial infarction, “people are hesitant to take them to the cath lab,” Dr. Bangalore said. But “if you follow protocols, if you follow strategies to minimize the risk, you can safely go ahead and do it.”
But in patients with stable CAD, as the ISCHEMIA-CKD studies show, “routinely revascularizing them may not have significant benefits.”
ISCHEMIC-CKD and its extension study were funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore discloses receiving research grants from NHLBI and serving as a consultant for Abbott Vascular, Biotronik, Boston Scientific, Amgen, Pfizer, Merck, and Reata. Dr. Bhatt has disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Gastrointestinal Stromal Tumor Arising From the Small Intestine in a Heart Transplant Recipient on Hemodialysis and Chronic Immunosuppression: A Case Report
Background
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors with worse prognosis if arising from the small bowel. Surgery remains the mainstay of treatment for patients with resectable tumors. Imatinib has become the standard treatment in cKIT-positive GISTs with significant morbidity in neoadjuvant, adjuvant, and palliative settings. There are limited data on efficacy and safety of imatinib in dialysis patients, and chemotherapy dosing is challenging in dialysis patients with multiple comorbidities.
Presentation
A 68-year-old male with a history of orthotopic heart transplantation on sirolimus with prednisone, cardiac allograft vasculopathy, plus ESRD on peritoneal dialysis (PD), presented with lower abdominal pain and fever. Abdominal imaging revealed a right lower quadrant (RLQ) mass with concern for bowel perforation.
Diagnosis and Treatment
The patient underwent exploratory laparoscopy with small bowel resection, excision of the mesenteric small bowel mass, drainage and washout of intraabdominal abscess, removal of PD catheter, and transition to hemodialysis. Pathology revealed a 14.5-cm high-grade GIST with mixed spindle and epithelioid types involving the ileal wall and mesentery, consistent with pT4 primary tumor and stage IIIB disease. Molecular testing was positive for c-KIT and DOG-1 mutations.
After a prolonged recovery, repeat abdominal imaging demonstrated metastatic liver disease and a new RLQ lesion. The patient was started on palliative imatinib 100 mg daily with subsequent increase to 200 mg daily. He was monitored closely for toxicities but reported only mild nausea controlled with ondansetron. Hemodialysis was continued 3 times per week. Follow up scans 3 months later showed improvement in RLQ mass and hepatic lesions. The patient remains on the current dose 15 months after the diagnosis.
Conclusion
To our knowledge, this is the first case of a small intestinal GIST in a heart transplant recipient treated with dose-reduced imatinib with concurrent dialysis and immunosuppression. Treatment decision-making was complex given concern for cardiotoxicity with pre-existing cardiac disease and drug-drug interactions with immunosuppressive agents. While some literature suggests standard dose imatinib with dialysis, no large-scale studies evaluated pharmacokinetics of imatinib with creatinine clearance < 20 mL/min. There is a need for further studies to determine dosing strategies for such patients.
Background
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors with worse prognosis if arising from the small bowel. Surgery remains the mainstay of treatment for patients with resectable tumors. Imatinib has become the standard treatment in cKIT-positive GISTs with significant morbidity in neoadjuvant, adjuvant, and palliative settings. There are limited data on efficacy and safety of imatinib in dialysis patients, and chemotherapy dosing is challenging in dialysis patients with multiple comorbidities.
Presentation
A 68-year-old male with a history of orthotopic heart transplantation on sirolimus with prednisone, cardiac allograft vasculopathy, plus ESRD on peritoneal dialysis (PD), presented with lower abdominal pain and fever. Abdominal imaging revealed a right lower quadrant (RLQ) mass with concern for bowel perforation.
Diagnosis and Treatment
The patient underwent exploratory laparoscopy with small bowel resection, excision of the mesenteric small bowel mass, drainage and washout of intraabdominal abscess, removal of PD catheter, and transition to hemodialysis. Pathology revealed a 14.5-cm high-grade GIST with mixed spindle and epithelioid types involving the ileal wall and mesentery, consistent with pT4 primary tumor and stage IIIB disease. Molecular testing was positive for c-KIT and DOG-1 mutations.
After a prolonged recovery, repeat abdominal imaging demonstrated metastatic liver disease and a new RLQ lesion. The patient was started on palliative imatinib 100 mg daily with subsequent increase to 200 mg daily. He was monitored closely for toxicities but reported only mild nausea controlled with ondansetron. Hemodialysis was continued 3 times per week. Follow up scans 3 months later showed improvement in RLQ mass and hepatic lesions. The patient remains on the current dose 15 months after the diagnosis.
Conclusion
To our knowledge, this is the first case of a small intestinal GIST in a heart transplant recipient treated with dose-reduced imatinib with concurrent dialysis and immunosuppression. Treatment decision-making was complex given concern for cardiotoxicity with pre-existing cardiac disease and drug-drug interactions with immunosuppressive agents. While some literature suggests standard dose imatinib with dialysis, no large-scale studies evaluated pharmacokinetics of imatinib with creatinine clearance < 20 mL/min. There is a need for further studies to determine dosing strategies for such patients.
Background
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors with worse prognosis if arising from the small bowel. Surgery remains the mainstay of treatment for patients with resectable tumors. Imatinib has become the standard treatment in cKIT-positive GISTs with significant morbidity in neoadjuvant, adjuvant, and palliative settings. There are limited data on efficacy and safety of imatinib in dialysis patients, and chemotherapy dosing is challenging in dialysis patients with multiple comorbidities.
Presentation
A 68-year-old male with a history of orthotopic heart transplantation on sirolimus with prednisone, cardiac allograft vasculopathy, plus ESRD on peritoneal dialysis (PD), presented with lower abdominal pain and fever. Abdominal imaging revealed a right lower quadrant (RLQ) mass with concern for bowel perforation.
Diagnosis and Treatment
The patient underwent exploratory laparoscopy with small bowel resection, excision of the mesenteric small bowel mass, drainage and washout of intraabdominal abscess, removal of PD catheter, and transition to hemodialysis. Pathology revealed a 14.5-cm high-grade GIST with mixed spindle and epithelioid types involving the ileal wall and mesentery, consistent with pT4 primary tumor and stage IIIB disease. Molecular testing was positive for c-KIT and DOG-1 mutations.
After a prolonged recovery, repeat abdominal imaging demonstrated metastatic liver disease and a new RLQ lesion. The patient was started on palliative imatinib 100 mg daily with subsequent increase to 200 mg daily. He was monitored closely for toxicities but reported only mild nausea controlled with ondansetron. Hemodialysis was continued 3 times per week. Follow up scans 3 months later showed improvement in RLQ mass and hepatic lesions. The patient remains on the current dose 15 months after the diagnosis.
Conclusion
To our knowledge, this is the first case of a small intestinal GIST in a heart transplant recipient treated with dose-reduced imatinib with concurrent dialysis and immunosuppression. Treatment decision-making was complex given concern for cardiotoxicity with pre-existing cardiac disease and drug-drug interactions with immunosuppressive agents. While some literature suggests standard dose imatinib with dialysis, no large-scale studies evaluated pharmacokinetics of imatinib with creatinine clearance < 20 mL/min. There is a need for further studies to determine dosing strategies for such patients.
Albuminuria linked to higher CVD risk in diabetes
BARCELONA – Fewer than half the adults in Denmark with type 2 diabetes in 2015 had a recent assessment for albuminuria, and those who underwent testing and had albuminuria had a greater than 50% increased rate of incident heart failure, MI, stroke, or all-cause death during 4-year follow-up, in a study of more than 74,000 Danish residents.
Even those in this study with type 2 diabetes but without albuminuria had a 19% rate of these adverse outcomes, highlighting the “substantial” cardiovascular disease risk faced by people with type 2 diabetes even without a clear indication of nephropathy, Saaima Parveen, MD, a cardiology researcher at Herlev and Gentofte University Hospital in Copenhagen, said at the annual congress of the European Society of Cardiology.
This high rate of heart failure, MI, stroke, or death even in the absence of what is conventionally defined as albuminuria – a urinary albumin-to-creatinine ratio (UACR) of at least 30 mg/g – suggests that this threshold for albuminuria may be too high, commented Luis M. Ruilope, MD, professor of public health and preventive medicine at Autonoma University, Madrid, who was not involved with the Danish study.
The study reported by Dr. Parveen “is very important because it shows that the risk of events is high not only in people with diabetes with albuminuria, but also in those without albuminuria,” Dr. Ruilope said in an interview.
The profile of albuminuria as a risk marker for people with type 2 diabetes spiked following the 2021 U.S. approval of finerenone (Kerendia) as an agent specifically targeted to adults with type 2 diabetes and albuminuria. (Finerenone gained marketing approval by in Europe in February 2022 under the same brand name.)
A lower threshold for albuminuria?
“Even patients with a UACR of 10-29 mg/g have risk and should be considered for finerenone treatment, said Dr. Ruilope. “People with type 2 diabetes with a UACR of 10-29 mg/g could explain” the high background risk shown by Dr. Parveen in her reported data. “In people with type 2 diabetes and a UACR of 10-29 mg/g we also see progression of kidney disease, but it’s slower” than in those who meet the current, standard threshold for albuminuria.
Dr. Ruilope was a coinvestigator for both of the finerenone pivotal trials, FIDELIO-DKD and FIGARO-DKD. Although the design of both these studies specified enrollment of people with type 2 diabetes and a UACR of at least 30 mg/g, a few hundred of the total combined enrollment of more than 13,000 patients had UACR values below this level, and analysis of this subgroup could provide some important insights into the value of finerenone for people with “high normal” albuminuria, he said.
The study led by Dr. Parveen used data routinely collected in Danish national records and focused on all Danish adults diagnosed with type 2 diabetes as of Jan. 1, 2015, who also had information in their records for a UACR and an estimated glomerular filtration rate (eGFR) within the preceding year.
The records showed that only 47% of these people had a UACR value during this time frame, and that 57% had a recent measure of their eGFR, despite prevailing recommendations for routine and regular measurements of these parameters for all people with type 2 diabetes.
Dr. Parveen hypothesized that UACR measurement may lag for several reasons, such as reliance by primary care physicians on urine dipstick assessments, which preclude calculation of a UACR, poor adherence to regular medical assessment by people in low socioeconomic groups, and medical examination done outside of morning time periods, which is the best time of day for assessing UACR.
More albuminuria measurement needed in primary care
“Measurement of albuminuria in people with type 2 diabetes is improving in Europe, but is not yet at the level that’s needed,” commented Dr. Ruilope. “We are pushing to have it done more often in primary care practices,” he said.
Among the 74,014 people with type 2 diabetes who had the measurement records that allowed for their inclusion in the study, 40% had albuminuria and 60% did not.
During 4 years of follow-up, the incidence of heart failure, MI, stroke, or all-cause death was 28.6% in those with albuminuria and 18.7% among those without albuminuria, reported Dr. Parveen.
The rates for each event type in those with albuminuria were 7.0% for heart failure, 4.4% for MI, 7.6% for stroke, and 16.6% for all-cause death (each patient could tally more than one type of event). Among those without albuminuria, the rates were 4.0%, 3.2%, 5.5%, and 9.3%, respectively.
The study received no commercial funding. Dr. Parveen and Dr. Ruilope had no disclosures.
BARCELONA – Fewer than half the adults in Denmark with type 2 diabetes in 2015 had a recent assessment for albuminuria, and those who underwent testing and had albuminuria had a greater than 50% increased rate of incident heart failure, MI, stroke, or all-cause death during 4-year follow-up, in a study of more than 74,000 Danish residents.
Even those in this study with type 2 diabetes but without albuminuria had a 19% rate of these adverse outcomes, highlighting the “substantial” cardiovascular disease risk faced by people with type 2 diabetes even without a clear indication of nephropathy, Saaima Parveen, MD, a cardiology researcher at Herlev and Gentofte University Hospital in Copenhagen, said at the annual congress of the European Society of Cardiology.
This high rate of heart failure, MI, stroke, or death even in the absence of what is conventionally defined as albuminuria – a urinary albumin-to-creatinine ratio (UACR) of at least 30 mg/g – suggests that this threshold for albuminuria may be too high, commented Luis M. Ruilope, MD, professor of public health and preventive medicine at Autonoma University, Madrid, who was not involved with the Danish study.
The study reported by Dr. Parveen “is very important because it shows that the risk of events is high not only in people with diabetes with albuminuria, but also in those without albuminuria,” Dr. Ruilope said in an interview.
The profile of albuminuria as a risk marker for people with type 2 diabetes spiked following the 2021 U.S. approval of finerenone (Kerendia) as an agent specifically targeted to adults with type 2 diabetes and albuminuria. (Finerenone gained marketing approval by in Europe in February 2022 under the same brand name.)
A lower threshold for albuminuria?
“Even patients with a UACR of 10-29 mg/g have risk and should be considered for finerenone treatment, said Dr. Ruilope. “People with type 2 diabetes with a UACR of 10-29 mg/g could explain” the high background risk shown by Dr. Parveen in her reported data. “In people with type 2 diabetes and a UACR of 10-29 mg/g we also see progression of kidney disease, but it’s slower” than in those who meet the current, standard threshold for albuminuria.
Dr. Ruilope was a coinvestigator for both of the finerenone pivotal trials, FIDELIO-DKD and FIGARO-DKD. Although the design of both these studies specified enrollment of people with type 2 diabetes and a UACR of at least 30 mg/g, a few hundred of the total combined enrollment of more than 13,000 patients had UACR values below this level, and analysis of this subgroup could provide some important insights into the value of finerenone for people with “high normal” albuminuria, he said.
The study led by Dr. Parveen used data routinely collected in Danish national records and focused on all Danish adults diagnosed with type 2 diabetes as of Jan. 1, 2015, who also had information in their records for a UACR and an estimated glomerular filtration rate (eGFR) within the preceding year.
The records showed that only 47% of these people had a UACR value during this time frame, and that 57% had a recent measure of their eGFR, despite prevailing recommendations for routine and regular measurements of these parameters for all people with type 2 diabetes.
Dr. Parveen hypothesized that UACR measurement may lag for several reasons, such as reliance by primary care physicians on urine dipstick assessments, which preclude calculation of a UACR, poor adherence to regular medical assessment by people in low socioeconomic groups, and medical examination done outside of morning time periods, which is the best time of day for assessing UACR.
More albuminuria measurement needed in primary care
“Measurement of albuminuria in people with type 2 diabetes is improving in Europe, but is not yet at the level that’s needed,” commented Dr. Ruilope. “We are pushing to have it done more often in primary care practices,” he said.
Among the 74,014 people with type 2 diabetes who had the measurement records that allowed for their inclusion in the study, 40% had albuminuria and 60% did not.
During 4 years of follow-up, the incidence of heart failure, MI, stroke, or all-cause death was 28.6% in those with albuminuria and 18.7% among those without albuminuria, reported Dr. Parveen.
The rates for each event type in those with albuminuria were 7.0% for heart failure, 4.4% for MI, 7.6% for stroke, and 16.6% for all-cause death (each patient could tally more than one type of event). Among those without albuminuria, the rates were 4.0%, 3.2%, 5.5%, and 9.3%, respectively.
The study received no commercial funding. Dr. Parveen and Dr. Ruilope had no disclosures.
BARCELONA – Fewer than half the adults in Denmark with type 2 diabetes in 2015 had a recent assessment for albuminuria, and those who underwent testing and had albuminuria had a greater than 50% increased rate of incident heart failure, MI, stroke, or all-cause death during 4-year follow-up, in a study of more than 74,000 Danish residents.
Even those in this study with type 2 diabetes but without albuminuria had a 19% rate of these adverse outcomes, highlighting the “substantial” cardiovascular disease risk faced by people with type 2 diabetes even without a clear indication of nephropathy, Saaima Parveen, MD, a cardiology researcher at Herlev and Gentofte University Hospital in Copenhagen, said at the annual congress of the European Society of Cardiology.
This high rate of heart failure, MI, stroke, or death even in the absence of what is conventionally defined as albuminuria – a urinary albumin-to-creatinine ratio (UACR) of at least 30 mg/g – suggests that this threshold for albuminuria may be too high, commented Luis M. Ruilope, MD, professor of public health and preventive medicine at Autonoma University, Madrid, who was not involved with the Danish study.
The study reported by Dr. Parveen “is very important because it shows that the risk of events is high not only in people with diabetes with albuminuria, but also in those without albuminuria,” Dr. Ruilope said in an interview.
The profile of albuminuria as a risk marker for people with type 2 diabetes spiked following the 2021 U.S. approval of finerenone (Kerendia) as an agent specifically targeted to adults with type 2 diabetes and albuminuria. (Finerenone gained marketing approval by in Europe in February 2022 under the same brand name.)
A lower threshold for albuminuria?
“Even patients with a UACR of 10-29 mg/g have risk and should be considered for finerenone treatment, said Dr. Ruilope. “People with type 2 diabetes with a UACR of 10-29 mg/g could explain” the high background risk shown by Dr. Parveen in her reported data. “In people with type 2 diabetes and a UACR of 10-29 mg/g we also see progression of kidney disease, but it’s slower” than in those who meet the current, standard threshold for albuminuria.
Dr. Ruilope was a coinvestigator for both of the finerenone pivotal trials, FIDELIO-DKD and FIGARO-DKD. Although the design of both these studies specified enrollment of people with type 2 diabetes and a UACR of at least 30 mg/g, a few hundred of the total combined enrollment of more than 13,000 patients had UACR values below this level, and analysis of this subgroup could provide some important insights into the value of finerenone for people with “high normal” albuminuria, he said.
The study led by Dr. Parveen used data routinely collected in Danish national records and focused on all Danish adults diagnosed with type 2 diabetes as of Jan. 1, 2015, who also had information in their records for a UACR and an estimated glomerular filtration rate (eGFR) within the preceding year.
The records showed that only 47% of these people had a UACR value during this time frame, and that 57% had a recent measure of their eGFR, despite prevailing recommendations for routine and regular measurements of these parameters for all people with type 2 diabetes.
Dr. Parveen hypothesized that UACR measurement may lag for several reasons, such as reliance by primary care physicians on urine dipstick assessments, which preclude calculation of a UACR, poor adherence to regular medical assessment by people in low socioeconomic groups, and medical examination done outside of morning time periods, which is the best time of day for assessing UACR.
More albuminuria measurement needed in primary care
“Measurement of albuminuria in people with type 2 diabetes is improving in Europe, but is not yet at the level that’s needed,” commented Dr. Ruilope. “We are pushing to have it done more often in primary care practices,” he said.
Among the 74,014 people with type 2 diabetes who had the measurement records that allowed for their inclusion in the study, 40% had albuminuria and 60% did not.
During 4 years of follow-up, the incidence of heart failure, MI, stroke, or all-cause death was 28.6% in those with albuminuria and 18.7% among those without albuminuria, reported Dr. Parveen.
The rates for each event type in those with albuminuria were 7.0% for heart failure, 4.4% for MI, 7.6% for stroke, and 16.6% for all-cause death (each patient could tally more than one type of event). Among those without albuminuria, the rates were 4.0%, 3.2%, 5.5%, and 9.3%, respectively.
The study received no commercial funding. Dr. Parveen and Dr. Ruilope had no disclosures.
AT ESC CONGRESS 2022
Low calcium, potassium key risk factors for kidney stones
as well as their symptomatic recurrence, a population-based study of dietary factors shows.
“Our research is of particular importance as recommendations for preventing symptomatic recurrence of kidney stones has largely been based on dietary factors associated with the incidence rather than the recurrence of stone formation,” Api Chewcharat, MD, Mayo Clinic, Rochester, Minn., said in a video discussing the study.
“We recommend a daily intake of calcium of approximately 1,200 mg and a diet that is high in potassium, especially high in fruits and vegetables, in order to prevent both incident and recurrent symptomatic kidney stone formation,” he stressed.
The study was published online in Mayo Clinic Proceedings.
Lower dietary calcium, potassium, and fluid associated with increased incidence
Some 411 patients with incident symptomatic kidney stone formation were recruited. Diets were compared between them and 384 controls. Patients were seen at the Mayo Clinic in either Minnesota or Florida between Jan. 1, 2009, and Aug. 31, 2018. “Dietary factors were based on a Viocare food frequency questionnaire administered during a baseline in-person study visit,” Dr. Chewcharat and colleagues observed.
During a median follow-up of 4.1 years, 73 patients experienced a symptomatic recurrence. In a fully adjusted analysis, a dietary calcium intake less than 1,200 mg/d was associated with incident stone formation. Similarly, among participants with a fluid intake less than 3,400 mL/d – about nine 12-oz glasses of fluid – was also associated with incident stone formation, as was a lower intake of dietary potassium, caffeine, and phytate. Phytate is an antioxidant found in whole grains, nuts, and other foods that can increase calcium absorption and urinary calcium excretion.
After excluding patients who were taking either a thiazide diuretic or a calcium supplement, lower dietary calcium and potassium, fluid, and phytate intake remained significantly associated with incident stone formation.
However, only lower dietary calcium intake was associated with a higher risk for symptomatic recurrence, although a lower dietary potassium intake was also associated with a higher risk for symptomatic recurrence in an analysis that adjusted for body mass index, fluid, and energy intake.
As the authors suggested, patients may be less keen to adjust their diet to prevent the development of incident kidney stones. On the other hand, they may be much more willing to adjust their diet to prevent their symptomatic recurrence. The Department of Agriculture currently recommends that individuals get approximately 1,200 mg/d of dietary calcium which, given the study results, appears to be justified for the prevention of symptomatic stone recurrence.
A higher-calcium diet is associated with a higher urinary pH, and citrate confers an alkali load which helps protect against the formation of calcium oxalate stones. Foods that are high in potassium also contain more fluid, citrate, and phytate, which, again, have been reported to be protective against kidney stones. “Changing your diet to prevent kidney stones can be very difficult,” Andrew Rule, MD, a nephrologist at the Mayo Clinic said in a statement.
“Thus, knowing the dietary factors that are most important for preventing kidney stone recurrence can help patients and providers know what to prioritize,” he added.
The authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
as well as their symptomatic recurrence, a population-based study of dietary factors shows.
“Our research is of particular importance as recommendations for preventing symptomatic recurrence of kidney stones has largely been based on dietary factors associated with the incidence rather than the recurrence of stone formation,” Api Chewcharat, MD, Mayo Clinic, Rochester, Minn., said in a video discussing the study.
“We recommend a daily intake of calcium of approximately 1,200 mg and a diet that is high in potassium, especially high in fruits and vegetables, in order to prevent both incident and recurrent symptomatic kidney stone formation,” he stressed.
The study was published online in Mayo Clinic Proceedings.
Lower dietary calcium, potassium, and fluid associated with increased incidence
Some 411 patients with incident symptomatic kidney stone formation were recruited. Diets were compared between them and 384 controls. Patients were seen at the Mayo Clinic in either Minnesota or Florida between Jan. 1, 2009, and Aug. 31, 2018. “Dietary factors were based on a Viocare food frequency questionnaire administered during a baseline in-person study visit,” Dr. Chewcharat and colleagues observed.
During a median follow-up of 4.1 years, 73 patients experienced a symptomatic recurrence. In a fully adjusted analysis, a dietary calcium intake less than 1,200 mg/d was associated with incident stone formation. Similarly, among participants with a fluid intake less than 3,400 mL/d – about nine 12-oz glasses of fluid – was also associated with incident stone formation, as was a lower intake of dietary potassium, caffeine, and phytate. Phytate is an antioxidant found in whole grains, nuts, and other foods that can increase calcium absorption and urinary calcium excretion.
After excluding patients who were taking either a thiazide diuretic or a calcium supplement, lower dietary calcium and potassium, fluid, and phytate intake remained significantly associated with incident stone formation.
However, only lower dietary calcium intake was associated with a higher risk for symptomatic recurrence, although a lower dietary potassium intake was also associated with a higher risk for symptomatic recurrence in an analysis that adjusted for body mass index, fluid, and energy intake.
As the authors suggested, patients may be less keen to adjust their diet to prevent the development of incident kidney stones. On the other hand, they may be much more willing to adjust their diet to prevent their symptomatic recurrence. The Department of Agriculture currently recommends that individuals get approximately 1,200 mg/d of dietary calcium which, given the study results, appears to be justified for the prevention of symptomatic stone recurrence.
A higher-calcium diet is associated with a higher urinary pH, and citrate confers an alkali load which helps protect against the formation of calcium oxalate stones. Foods that are high in potassium also contain more fluid, citrate, and phytate, which, again, have been reported to be protective against kidney stones. “Changing your diet to prevent kidney stones can be very difficult,” Andrew Rule, MD, a nephrologist at the Mayo Clinic said in a statement.
“Thus, knowing the dietary factors that are most important for preventing kidney stone recurrence can help patients and providers know what to prioritize,” he added.
The authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
as well as their symptomatic recurrence, a population-based study of dietary factors shows.
“Our research is of particular importance as recommendations for preventing symptomatic recurrence of kidney stones has largely been based on dietary factors associated with the incidence rather than the recurrence of stone formation,” Api Chewcharat, MD, Mayo Clinic, Rochester, Minn., said in a video discussing the study.
“We recommend a daily intake of calcium of approximately 1,200 mg and a diet that is high in potassium, especially high in fruits and vegetables, in order to prevent both incident and recurrent symptomatic kidney stone formation,” he stressed.
The study was published online in Mayo Clinic Proceedings.
Lower dietary calcium, potassium, and fluid associated with increased incidence
Some 411 patients with incident symptomatic kidney stone formation were recruited. Diets were compared between them and 384 controls. Patients were seen at the Mayo Clinic in either Minnesota or Florida between Jan. 1, 2009, and Aug. 31, 2018. “Dietary factors were based on a Viocare food frequency questionnaire administered during a baseline in-person study visit,” Dr. Chewcharat and colleagues observed.
During a median follow-up of 4.1 years, 73 patients experienced a symptomatic recurrence. In a fully adjusted analysis, a dietary calcium intake less than 1,200 mg/d was associated with incident stone formation. Similarly, among participants with a fluid intake less than 3,400 mL/d – about nine 12-oz glasses of fluid – was also associated with incident stone formation, as was a lower intake of dietary potassium, caffeine, and phytate. Phytate is an antioxidant found in whole grains, nuts, and other foods that can increase calcium absorption and urinary calcium excretion.
After excluding patients who were taking either a thiazide diuretic or a calcium supplement, lower dietary calcium and potassium, fluid, and phytate intake remained significantly associated with incident stone formation.
However, only lower dietary calcium intake was associated with a higher risk for symptomatic recurrence, although a lower dietary potassium intake was also associated with a higher risk for symptomatic recurrence in an analysis that adjusted for body mass index, fluid, and energy intake.
As the authors suggested, patients may be less keen to adjust their diet to prevent the development of incident kidney stones. On the other hand, they may be much more willing to adjust their diet to prevent their symptomatic recurrence. The Department of Agriculture currently recommends that individuals get approximately 1,200 mg/d of dietary calcium which, given the study results, appears to be justified for the prevention of symptomatic stone recurrence.
A higher-calcium diet is associated with a higher urinary pH, and citrate confers an alkali load which helps protect against the formation of calcium oxalate stones. Foods that are high in potassium also contain more fluid, citrate, and phytate, which, again, have been reported to be protective against kidney stones. “Changing your diet to prevent kidney stones can be very difficult,” Andrew Rule, MD, a nephrologist at the Mayo Clinic said in a statement.
“Thus, knowing the dietary factors that are most important for preventing kidney stone recurrence can help patients and providers know what to prioritize,” he added.
The authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
FROM MAYO CLINIC PRECEEDINGS
Supporting Patients on Complex Care Journeys: How Technology Can Bridge the Gaps
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; [email protected]
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; [email protected]
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; [email protected]
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
FDA approves belimumab for children with lupus nephritis
The Food and Drug Administration has approved belimumab (Benlysta) for treating active lupus nephritis (LN) in children aged 5-17 years. The drug can now be used to treat adult and pediatric patients with systemic lupus erythematosus (SLE) and LN. The decision expands therapeutic options for the estimated 1.5 million Americans currently living with lupus.
“This approval marks a significant step forward in providing treatment options to these children at risk of incurring kidney damage early on in life,” Stevan W. Gibson, president and CEO of the Lupus Foundation of America, said in a press release issued by the manufacturer, GlaxoSmithKline. LN is a condition that sometimes develops in people with lupus. In LN, the autoimmune cells produced by the disease attack the kidney. Roughly 40% of people with SLE experience LN.
Damage to the kidneys causes the body to have difficulty processing waste and toxins. This can create a host of problems, including end-stage kidney disease, which may be treated only with dialysis or kidney transplant. These situations significantly increase mortality among people with lupus, especially children.
Prior to the approval, the only treatment pathway for children with active LN included immunosuppressants and corticosteroids. While they may be effective, use of these classes of drugs may come with many side effects, including susceptibility to other diseases and infections. Belimumab, by contrast, is a B-lymphocyte stimulator protein inhibitor. It inhibits the survival of B cells, which are thought to play a role in the disease’s pathophysiology.
Belimumab was first approved to treat patients with SLE in 2011. It was approved for children with SLE 8 years later. The drug’s indications were expanded to include adults with LN in 2020.
Organizations within the lupus research community have communicated their support of the FDA’s decision. “Our community has much to celebrate with the approval of the first and much-needed treatment for children with lupus nephritis,” Lupus Research Alliance President and CEO Kenneth M. Farber said in a release from the organization.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved belimumab (Benlysta) for treating active lupus nephritis (LN) in children aged 5-17 years. The drug can now be used to treat adult and pediatric patients with systemic lupus erythematosus (SLE) and LN. The decision expands therapeutic options for the estimated 1.5 million Americans currently living with lupus.
“This approval marks a significant step forward in providing treatment options to these children at risk of incurring kidney damage early on in life,” Stevan W. Gibson, president and CEO of the Lupus Foundation of America, said in a press release issued by the manufacturer, GlaxoSmithKline. LN is a condition that sometimes develops in people with lupus. In LN, the autoimmune cells produced by the disease attack the kidney. Roughly 40% of people with SLE experience LN.
Damage to the kidneys causes the body to have difficulty processing waste and toxins. This can create a host of problems, including end-stage kidney disease, which may be treated only with dialysis or kidney transplant. These situations significantly increase mortality among people with lupus, especially children.
Prior to the approval, the only treatment pathway for children with active LN included immunosuppressants and corticosteroids. While they may be effective, use of these classes of drugs may come with many side effects, including susceptibility to other diseases and infections. Belimumab, by contrast, is a B-lymphocyte stimulator protein inhibitor. It inhibits the survival of B cells, which are thought to play a role in the disease’s pathophysiology.
Belimumab was first approved to treat patients with SLE in 2011. It was approved for children with SLE 8 years later. The drug’s indications were expanded to include adults with LN in 2020.
Organizations within the lupus research community have communicated their support of the FDA’s decision. “Our community has much to celebrate with the approval of the first and much-needed treatment for children with lupus nephritis,” Lupus Research Alliance President and CEO Kenneth M. Farber said in a release from the organization.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved belimumab (Benlysta) for treating active lupus nephritis (LN) in children aged 5-17 years. The drug can now be used to treat adult and pediatric patients with systemic lupus erythematosus (SLE) and LN. The decision expands therapeutic options for the estimated 1.5 million Americans currently living with lupus.
“This approval marks a significant step forward in providing treatment options to these children at risk of incurring kidney damage early on in life,” Stevan W. Gibson, president and CEO of the Lupus Foundation of America, said in a press release issued by the manufacturer, GlaxoSmithKline. LN is a condition that sometimes develops in people with lupus. In LN, the autoimmune cells produced by the disease attack the kidney. Roughly 40% of people with SLE experience LN.
Damage to the kidneys causes the body to have difficulty processing waste and toxins. This can create a host of problems, including end-stage kidney disease, which may be treated only with dialysis or kidney transplant. These situations significantly increase mortality among people with lupus, especially children.
Prior to the approval, the only treatment pathway for children with active LN included immunosuppressants and corticosteroids. While they may be effective, use of these classes of drugs may come with many side effects, including susceptibility to other diseases and infections. Belimumab, by contrast, is a B-lymphocyte stimulator protein inhibitor. It inhibits the survival of B cells, which are thought to play a role in the disease’s pathophysiology.
Belimumab was first approved to treat patients with SLE in 2011. It was approved for children with SLE 8 years later. The drug’s indications were expanded to include adults with LN in 2020.
Organizations within the lupus research community have communicated their support of the FDA’s decision. “Our community has much to celebrate with the approval of the first and much-needed treatment for children with lupus nephritis,” Lupus Research Alliance President and CEO Kenneth M. Farber said in a release from the organization.
A version of this article first appeared on Medscape.com.
Rosuvastatin again linked with risks to kidneys
Rosuvastatin for cholesterol lowering was associated with slightly greater risks for kidney harm than atorvastatin, risks that were greater at higher-dose levels, in a large retrospective cohort study.
The most potent statin on the market, rosuvastatin has been linked with excess risk for kidney damage compared with atorvastatin in case reports and small trials, but there has been little surveillance of the issue following its approval in 2003.
The current analysis “is one of the first and largest real-world studies” examining rosuvastatin versus atorvastatin for risk for hematuria, proteinuria, and kidney failure with replacement therapy – dialysis or transplantation – across a range of estimated glomerular filtration rates (eGFR) in a heterogeneous population, the researchers write.
“Our findings suggest the need for greater care in prescribing and monitoring of rosuvastatin, particularly in patients who are receiving high doses” or have severe chronic kidney disease (CKD), they concluded in their report published online in the Journal of the American Society of Nephrology.
The analysis included close to 1 million patients in the United States who were newly prescribed rosuvastatin or atorvastatin from 2011 through 2019; they were followed a median of 3.1 years. Among the findings:
- Users of rosuvastatin had an 8% higher risk for hematuria, a 17% higher risk for proteinuria, and a 15% higher risk for kidney failure with replacement therapy, compared with those on atorvastatin
- The two groups avoided MI and stroke to similar extents
- About 44% of patients with severe CKD G4+ (eGFR < 30 mL/min per 1.73 m2) were prescribed a higher rosuvastatin dosage than the maximum 10 mg/day recommended for such patients by the Food and Drug Administration.
From this study, “we do not know why the adherence of FDA dosing recommendation for rosuvastatin in patients with severe CKD is low,” lead author Jung-Im Shin, MD, PhD, said in an interview.
“It is likely that not many clinicians are aware of rosuvastatin’s dosing recommendations [in severe CKD], or potential risks of hematuria or proteinuria,” speculated Dr. Shin, assistant professor at Johns Hopkins University, Baltimore.
“High-dose rosuvastatin [and its cardiovascular benefits] may not merit the risk, even if small, particularly in low eGFR,” she said. “Our study provides the opportunity to increase awareness of this clinical issue.”
“Future studies are warranted to shed light on the discrepancy between real-world practice and FDA dosing recommendations for high-dose rosuvastatin,” the researchers noted.
‘Greater awareness and education are key’
Invited to comment, Swapnil Hiremath, MD, a nephrologist at the Ottawa Hospital Research Institute, noted that the higher risk for nephrotoxicity with high-dose rosuvastatin versus high-dose atorvastatin was shown in the PLANET 1 trial published in 2015 and in, for example, a case report published in 2016 – which the researchers also mention.
“I was personally surprised” at the high proportion of patients with severe CKD who received higher than recommended doses of rosuvastatin, said Dr. Hiremath, who is also an associate professor at the University of Ottawa and a Freely Filtered podcaster, and not associated with the current study.
“We do see this occasionally,” he continued, “but either because someone is targeting LDL [cholesterol] and hasn’t noted the GFR, or possibly the patient was started on a high dose a long time ago and the kidney function has declined, and no one has noted the high dose.”
“Greater awareness and education are key,” observed Dr. Hiremath. “My personal bias is to have renal pharmacists involved in multidisciplinary clinics when GFR [is] less than 30 or so,” he said. “There are so many other tricky medicine/interaction issues” in patients with kidney disease.
Nevertheless, “I would be careful in drawing too many conclusions from an observational study,” Dr. Hiremath added. “There’s always the threat of residual confounding and selection bias,” which the researchers acknowledge, “and especially competing risks.”
For example, “if there is less cardiovascular death with rosuvastatin, then more people will remain alive to develop kidney failure.”
Dosing in practice unclear
Atorvastatin at 40-mg and 80-mg dosages and rosuvastatin at 20 mg and 40 mg are the only two statins considered high-intensity, the researchers noted.
Development of an 80-mg dosage for rosuvastatin was dropped because of hematuria and proteinuria safety signals highlighted at the time of rosuvastatin’s FDA approval.
However, there has been little postmarketing surveillance to assess real-world risk from high-intensity rosuvastatin, and it remains unclear whether and to what extent clinical practice adheres to the starting dosage recommended by the FDA in severe CKD, 5 mg/day with a maximum of 10 mg/day, the report noted.
The researchers analyzed deidentified electronic health record data from 40 health care organizations in the United States from the OptumLabs Data Warehouse database. They entered 152,101 new rosuvastatin users and 795,799 new atorvastatin users, and excluded patients with a history of rhabdomyolysis.
Patients in the two groups were similar with respect to CKD prevalence, cardiovascular risk factors, and demographics. Their age averaged 60 years, 48% were women, and 82% were White.
Hematuria was defined as dipstick hematuria > + or the presence of more than 3 red blood cells per high-power field in urine microscopy, at least twice. Proteinuria was defined as dipstick proteinuria > ++ or urine albumin-to-creatinine ratio greater than 300 mg/g at least twice.
Overall, 2.9% of patients had hematuria (3.4% of the rosuvastatin group and 2.8% of those taking atorvastatin) and 1% of patients had proteinuria (1.2% and 0.9%, respectively).
After balancing baseline characteristics in both groups using inverse probability of treatment weighting, rosuvastatin treatment, compared with atorvastatin, was associated with significantly greater risks for hematuria (hazard ratio, 1.08), proteinuria (HR, 1.17), and kidney failure requiring replacement therapy (HR, 1.15).
Patients with eGFR less than 30 mL/min per 1.73 m2 had an approximately twofold higher risk for hematuria and ninefold higher risk for proteinuria during the follow-up compared with patients with eGFR of at least 60 mL/min per 1.73 m2.
Patients with eGFR less than 30 mL/min per 1.73 m2 were commonly prescribed high-dose rosuvastatin (29.9% received the 20-mg dose and 14% the 40-mg dose), contrary to the labeling recommendation.
Dr. Shin reported receiving research Funding from the National Institutes of Health and Merck; disclosures for the other authors are in the report. Dr. Hiremath reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rosuvastatin for cholesterol lowering was associated with slightly greater risks for kidney harm than atorvastatin, risks that were greater at higher-dose levels, in a large retrospective cohort study.
The most potent statin on the market, rosuvastatin has been linked with excess risk for kidney damage compared with atorvastatin in case reports and small trials, but there has been little surveillance of the issue following its approval in 2003.
The current analysis “is one of the first and largest real-world studies” examining rosuvastatin versus atorvastatin for risk for hematuria, proteinuria, and kidney failure with replacement therapy – dialysis or transplantation – across a range of estimated glomerular filtration rates (eGFR) in a heterogeneous population, the researchers write.
“Our findings suggest the need for greater care in prescribing and monitoring of rosuvastatin, particularly in patients who are receiving high doses” or have severe chronic kidney disease (CKD), they concluded in their report published online in the Journal of the American Society of Nephrology.
The analysis included close to 1 million patients in the United States who were newly prescribed rosuvastatin or atorvastatin from 2011 through 2019; they were followed a median of 3.1 years. Among the findings:
- Users of rosuvastatin had an 8% higher risk for hematuria, a 17% higher risk for proteinuria, and a 15% higher risk for kidney failure with replacement therapy, compared with those on atorvastatin
- The two groups avoided MI and stroke to similar extents
- About 44% of patients with severe CKD G4+ (eGFR < 30 mL/min per 1.73 m2) were prescribed a higher rosuvastatin dosage than the maximum 10 mg/day recommended for such patients by the Food and Drug Administration.
From this study, “we do not know why the adherence of FDA dosing recommendation for rosuvastatin in patients with severe CKD is low,” lead author Jung-Im Shin, MD, PhD, said in an interview.
“It is likely that not many clinicians are aware of rosuvastatin’s dosing recommendations [in severe CKD], or potential risks of hematuria or proteinuria,” speculated Dr. Shin, assistant professor at Johns Hopkins University, Baltimore.
“High-dose rosuvastatin [and its cardiovascular benefits] may not merit the risk, even if small, particularly in low eGFR,” she said. “Our study provides the opportunity to increase awareness of this clinical issue.”
“Future studies are warranted to shed light on the discrepancy between real-world practice and FDA dosing recommendations for high-dose rosuvastatin,” the researchers noted.
‘Greater awareness and education are key’
Invited to comment, Swapnil Hiremath, MD, a nephrologist at the Ottawa Hospital Research Institute, noted that the higher risk for nephrotoxicity with high-dose rosuvastatin versus high-dose atorvastatin was shown in the PLANET 1 trial published in 2015 and in, for example, a case report published in 2016 – which the researchers also mention.
“I was personally surprised” at the high proportion of patients with severe CKD who received higher than recommended doses of rosuvastatin, said Dr. Hiremath, who is also an associate professor at the University of Ottawa and a Freely Filtered podcaster, and not associated with the current study.
“We do see this occasionally,” he continued, “but either because someone is targeting LDL [cholesterol] and hasn’t noted the GFR, or possibly the patient was started on a high dose a long time ago and the kidney function has declined, and no one has noted the high dose.”
“Greater awareness and education are key,” observed Dr. Hiremath. “My personal bias is to have renal pharmacists involved in multidisciplinary clinics when GFR [is] less than 30 or so,” he said. “There are so many other tricky medicine/interaction issues” in patients with kidney disease.
Nevertheless, “I would be careful in drawing too many conclusions from an observational study,” Dr. Hiremath added. “There’s always the threat of residual confounding and selection bias,” which the researchers acknowledge, “and especially competing risks.”
For example, “if there is less cardiovascular death with rosuvastatin, then more people will remain alive to develop kidney failure.”
Dosing in practice unclear
Atorvastatin at 40-mg and 80-mg dosages and rosuvastatin at 20 mg and 40 mg are the only two statins considered high-intensity, the researchers noted.
Development of an 80-mg dosage for rosuvastatin was dropped because of hematuria and proteinuria safety signals highlighted at the time of rosuvastatin’s FDA approval.
However, there has been little postmarketing surveillance to assess real-world risk from high-intensity rosuvastatin, and it remains unclear whether and to what extent clinical practice adheres to the starting dosage recommended by the FDA in severe CKD, 5 mg/day with a maximum of 10 mg/day, the report noted.
The researchers analyzed deidentified electronic health record data from 40 health care organizations in the United States from the OptumLabs Data Warehouse database. They entered 152,101 new rosuvastatin users and 795,799 new atorvastatin users, and excluded patients with a history of rhabdomyolysis.
Patients in the two groups were similar with respect to CKD prevalence, cardiovascular risk factors, and demographics. Their age averaged 60 years, 48% were women, and 82% were White.
Hematuria was defined as dipstick hematuria > + or the presence of more than 3 red blood cells per high-power field in urine microscopy, at least twice. Proteinuria was defined as dipstick proteinuria > ++ or urine albumin-to-creatinine ratio greater than 300 mg/g at least twice.
Overall, 2.9% of patients had hematuria (3.4% of the rosuvastatin group and 2.8% of those taking atorvastatin) and 1% of patients had proteinuria (1.2% and 0.9%, respectively).
After balancing baseline characteristics in both groups using inverse probability of treatment weighting, rosuvastatin treatment, compared with atorvastatin, was associated with significantly greater risks for hematuria (hazard ratio, 1.08), proteinuria (HR, 1.17), and kidney failure requiring replacement therapy (HR, 1.15).
Patients with eGFR less than 30 mL/min per 1.73 m2 had an approximately twofold higher risk for hematuria and ninefold higher risk for proteinuria during the follow-up compared with patients with eGFR of at least 60 mL/min per 1.73 m2.
Patients with eGFR less than 30 mL/min per 1.73 m2 were commonly prescribed high-dose rosuvastatin (29.9% received the 20-mg dose and 14% the 40-mg dose), contrary to the labeling recommendation.
Dr. Shin reported receiving research Funding from the National Institutes of Health and Merck; disclosures for the other authors are in the report. Dr. Hiremath reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rosuvastatin for cholesterol lowering was associated with slightly greater risks for kidney harm than atorvastatin, risks that were greater at higher-dose levels, in a large retrospective cohort study.
The most potent statin on the market, rosuvastatin has been linked with excess risk for kidney damage compared with atorvastatin in case reports and small trials, but there has been little surveillance of the issue following its approval in 2003.
The current analysis “is one of the first and largest real-world studies” examining rosuvastatin versus atorvastatin for risk for hematuria, proteinuria, and kidney failure with replacement therapy – dialysis or transplantation – across a range of estimated glomerular filtration rates (eGFR) in a heterogeneous population, the researchers write.
“Our findings suggest the need for greater care in prescribing and monitoring of rosuvastatin, particularly in patients who are receiving high doses” or have severe chronic kidney disease (CKD), they concluded in their report published online in the Journal of the American Society of Nephrology.
The analysis included close to 1 million patients in the United States who were newly prescribed rosuvastatin or atorvastatin from 2011 through 2019; they were followed a median of 3.1 years. Among the findings:
- Users of rosuvastatin had an 8% higher risk for hematuria, a 17% higher risk for proteinuria, and a 15% higher risk for kidney failure with replacement therapy, compared with those on atorvastatin
- The two groups avoided MI and stroke to similar extents
- About 44% of patients with severe CKD G4+ (eGFR < 30 mL/min per 1.73 m2) were prescribed a higher rosuvastatin dosage than the maximum 10 mg/day recommended for such patients by the Food and Drug Administration.
From this study, “we do not know why the adherence of FDA dosing recommendation for rosuvastatin in patients with severe CKD is low,” lead author Jung-Im Shin, MD, PhD, said in an interview.
“It is likely that not many clinicians are aware of rosuvastatin’s dosing recommendations [in severe CKD], or potential risks of hematuria or proteinuria,” speculated Dr. Shin, assistant professor at Johns Hopkins University, Baltimore.
“High-dose rosuvastatin [and its cardiovascular benefits] may not merit the risk, even if small, particularly in low eGFR,” she said. “Our study provides the opportunity to increase awareness of this clinical issue.”
“Future studies are warranted to shed light on the discrepancy between real-world practice and FDA dosing recommendations for high-dose rosuvastatin,” the researchers noted.
‘Greater awareness and education are key’
Invited to comment, Swapnil Hiremath, MD, a nephrologist at the Ottawa Hospital Research Institute, noted that the higher risk for nephrotoxicity with high-dose rosuvastatin versus high-dose atorvastatin was shown in the PLANET 1 trial published in 2015 and in, for example, a case report published in 2016 – which the researchers also mention.
“I was personally surprised” at the high proportion of patients with severe CKD who received higher than recommended doses of rosuvastatin, said Dr. Hiremath, who is also an associate professor at the University of Ottawa and a Freely Filtered podcaster, and not associated with the current study.
“We do see this occasionally,” he continued, “but either because someone is targeting LDL [cholesterol] and hasn’t noted the GFR, or possibly the patient was started on a high dose a long time ago and the kidney function has declined, and no one has noted the high dose.”
“Greater awareness and education are key,” observed Dr. Hiremath. “My personal bias is to have renal pharmacists involved in multidisciplinary clinics when GFR [is] less than 30 or so,” he said. “There are so many other tricky medicine/interaction issues” in patients with kidney disease.
Nevertheless, “I would be careful in drawing too many conclusions from an observational study,” Dr. Hiremath added. “There’s always the threat of residual confounding and selection bias,” which the researchers acknowledge, “and especially competing risks.”
For example, “if there is less cardiovascular death with rosuvastatin, then more people will remain alive to develop kidney failure.”
Dosing in practice unclear
Atorvastatin at 40-mg and 80-mg dosages and rosuvastatin at 20 mg and 40 mg are the only two statins considered high-intensity, the researchers noted.
Development of an 80-mg dosage for rosuvastatin was dropped because of hematuria and proteinuria safety signals highlighted at the time of rosuvastatin’s FDA approval.
However, there has been little postmarketing surveillance to assess real-world risk from high-intensity rosuvastatin, and it remains unclear whether and to what extent clinical practice adheres to the starting dosage recommended by the FDA in severe CKD, 5 mg/day with a maximum of 10 mg/day, the report noted.
The researchers analyzed deidentified electronic health record data from 40 health care organizations in the United States from the OptumLabs Data Warehouse database. They entered 152,101 new rosuvastatin users and 795,799 new atorvastatin users, and excluded patients with a history of rhabdomyolysis.
Patients in the two groups were similar with respect to CKD prevalence, cardiovascular risk factors, and demographics. Their age averaged 60 years, 48% were women, and 82% were White.
Hematuria was defined as dipstick hematuria > + or the presence of more than 3 red blood cells per high-power field in urine microscopy, at least twice. Proteinuria was defined as dipstick proteinuria > ++ or urine albumin-to-creatinine ratio greater than 300 mg/g at least twice.
Overall, 2.9% of patients had hematuria (3.4% of the rosuvastatin group and 2.8% of those taking atorvastatin) and 1% of patients had proteinuria (1.2% and 0.9%, respectively).
After balancing baseline characteristics in both groups using inverse probability of treatment weighting, rosuvastatin treatment, compared with atorvastatin, was associated with significantly greater risks for hematuria (hazard ratio, 1.08), proteinuria (HR, 1.17), and kidney failure requiring replacement therapy (HR, 1.15).
Patients with eGFR less than 30 mL/min per 1.73 m2 had an approximately twofold higher risk for hematuria and ninefold higher risk for proteinuria during the follow-up compared with patients with eGFR of at least 60 mL/min per 1.73 m2.
Patients with eGFR less than 30 mL/min per 1.73 m2 were commonly prescribed high-dose rosuvastatin (29.9% received the 20-mg dose and 14% the 40-mg dose), contrary to the labeling recommendation.
Dr. Shin reported receiving research Funding from the National Institutes of Health and Merck; disclosures for the other authors are in the report. Dr. Hiremath reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY
Could a type 2 diabetes drug tackle kidney stones?
than patients who received placebo during a median 1.5 years of treatment.
These findings are from an analysis of pooled data from phase 1-4 clinical trials of empagliflozin for blood glucose control in 15,081 patients with type 2 diabetes.
Priyadarshini Balasubramanian, MD, presented the study as a poster at the annual meeting of the Endocrine Society; the study also was published online in the Journal of Clinical Endocrinology & Metabolism.
The researchers acknowledge this was a retrospective, post-hoc analysis and that urolithiasis – a stone in the urinary tract, which includes nephrolithiasis, a kidney stone – was an adverse event, not a primary or secondary outcome.
Also, the stone composition, which might help explain how the drug may affect stone formation, is unknown.
Therefore, “dedicated randomized prospective clinical trials are needed to confirm these initial observations in patients both with and without type 2 diabetes,” said Dr. Balasubramanian, a clinical fellow in the section of endocrinology & metabolism, department of internal medicine at Yale University, New Haven, Conn.
However, “if this association is proven, empagliflozin may be used to decrease the risk of kidney stones at least in those with type 2 diabetes, but maybe also in those without diabetes,” Dr. Balasubramanian said in an interview.
Further trials are also needed to determine if this is a class effect, which is likely, she speculated, and to unravel the potential mechanism.
This is important because of the prevalence of kidney stones, which affect up to 15% of the general population and 15%-20% of patients with diabetes, she explained.
‘Provocative’ earlier findings
The current study was prompted by a recent observational study by Kasper B. Kristensen, MD, PhD, and colleagues.
Because SGLT2 inhibitors increase urinary glucose excretion through reduced renal reabsorption of glucose leading to osmotic diuresis and increased urinary flow, they hypothesized that these therapies “may reduce the risk of upper urinary tract stones (nephrolithiasis) by reducing the concentration of lithogenic substances in urine.”
Using data from Danish Health registries, they matched 12,325 individuals newly prescribed an SGLT2 inhibitor 1:1 with patients newly prescribed a glucagonlike peptide-1 (GLP1) agonist, another new class of drugs for treating type 2 diabetes.
They found a hazard ratio of 0.51 (95% confidence interval, 0.37-0.71) for incident nephrolithiasis and a hazard ratio of 0.68 (95% CI, 0.48-0.97) for recurrent nephrolithiasis for patients taking SGLT2 inhibitors versus GLP-1 agonists.
These findings are “striking,” according to Dr. Balasubramanian and colleagues. However, “these data, while provocative, were entirely retrospective and therefore possibly prone to bias,” they add.
Pooled data from 20 trials
The current study analyzed data from 20 randomized controlled trials of glycemic control in type 2 diabetes, in which 10,177 patients had received empagliflozin 10 mg or 25 mg and 4,904 patients had received placebo.
Most patients (46.5%) had participated in the EMPA-REG OUTCOMES trial, which also had the longest follow-up (2.6 years).
The researchers identified patients with a new stone from the urinary tract (including the kidney, ureter, and urethra). Patients had received either the study drug for a median of 543 days or placebo for a median of 549 days.
During treatment, 104 of 10,177 patients in the pooled empagliflozin groups and 79 of 4,904 patients in the pooled placebo groups developed a stone in the urinary tract.
This was equivalent to 0.63 new urinary-tract stones per 100 patient-years in the pooled empagliflozin groups versus 1.01 new urinary-tract stones per 100 patient-years in the pooled placebo groups.
The incidence rate ratio was 0.64 (95% CI, 0.48-0.86), in favor of empagliflozin.
When the analysis was restricted to new kidney stones, the results were similar: 75 of 10,177 patients in the pooled empagliflozin groups and 57 of 4,904 patients in the pooled placebo groups developed a kidney stone.
This was equivalent to 0.45 new kidney stones per 100 patient-years in the pooled empagliflozin groups versus 0.72 new kidney stones per 100 patient-years in the pooled placebo groups.
The IRR was 0.65 (95% CI, 0.46-0.92), in favor of empagliflozin.
Upcoming small RCT in adults without diabetes
Invited to comment on the new study, Dr. Kristensen said: “The reduced risk of SGLT2 inhibitors towards nephrolithiasis is now reported in at least two studies with different methodology, different populations, and different exposure and outcome definitions.”
“I agree that randomized clinical trials designed specifically to confirm these findings appear warranted,” added Dr. Kristensen, from the Institute of Public Health, Clinical Pharmacology, Pharmacy, and Environmental Medicine, University of Southern Denmark in Odense.
There is a need for studies in patients with and without diabetes, he added, especially ones that focus on prevention of nephrolithiasis in patients with kidney stone disease.
A new trial should shed further light on this.
Results are expected by the end of 2022 for SWEETSTONE (Impact of the SGLT2 Inhibitor Empagliflozin on Urinary Supersaturations in Kidney Stone Formers), a randomized, double-blind crossover exploratory study in 46 patients without diabetes.
This should provide preliminary data to “establish the relevance for larger trials assessing the prophylactic potential of empagliflozin in kidney stone disease,” according to an article on the trial protocol recently published in BMJ.
The trials included in the pooled dataset were funded by Boehringer Ingelheim or the Boehringer Ingelheim and Eli Lilly Diabetes Alliance. Dr. Balasubramanian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than patients who received placebo during a median 1.5 years of treatment.
These findings are from an analysis of pooled data from phase 1-4 clinical trials of empagliflozin for blood glucose control in 15,081 patients with type 2 diabetes.
Priyadarshini Balasubramanian, MD, presented the study as a poster at the annual meeting of the Endocrine Society; the study also was published online in the Journal of Clinical Endocrinology & Metabolism.
The researchers acknowledge this was a retrospective, post-hoc analysis and that urolithiasis – a stone in the urinary tract, which includes nephrolithiasis, a kidney stone – was an adverse event, not a primary or secondary outcome.
Also, the stone composition, which might help explain how the drug may affect stone formation, is unknown.
Therefore, “dedicated randomized prospective clinical trials are needed to confirm these initial observations in patients both with and without type 2 diabetes,” said Dr. Balasubramanian, a clinical fellow in the section of endocrinology & metabolism, department of internal medicine at Yale University, New Haven, Conn.
However, “if this association is proven, empagliflozin may be used to decrease the risk of kidney stones at least in those with type 2 diabetes, but maybe also in those without diabetes,” Dr. Balasubramanian said in an interview.
Further trials are also needed to determine if this is a class effect, which is likely, she speculated, and to unravel the potential mechanism.
This is important because of the prevalence of kidney stones, which affect up to 15% of the general population and 15%-20% of patients with diabetes, she explained.
‘Provocative’ earlier findings
The current study was prompted by a recent observational study by Kasper B. Kristensen, MD, PhD, and colleagues.
Because SGLT2 inhibitors increase urinary glucose excretion through reduced renal reabsorption of glucose leading to osmotic diuresis and increased urinary flow, they hypothesized that these therapies “may reduce the risk of upper urinary tract stones (nephrolithiasis) by reducing the concentration of lithogenic substances in urine.”
Using data from Danish Health registries, they matched 12,325 individuals newly prescribed an SGLT2 inhibitor 1:1 with patients newly prescribed a glucagonlike peptide-1 (GLP1) agonist, another new class of drugs for treating type 2 diabetes.
They found a hazard ratio of 0.51 (95% confidence interval, 0.37-0.71) for incident nephrolithiasis and a hazard ratio of 0.68 (95% CI, 0.48-0.97) for recurrent nephrolithiasis for patients taking SGLT2 inhibitors versus GLP-1 agonists.
These findings are “striking,” according to Dr. Balasubramanian and colleagues. However, “these data, while provocative, were entirely retrospective and therefore possibly prone to bias,” they add.
Pooled data from 20 trials
The current study analyzed data from 20 randomized controlled trials of glycemic control in type 2 diabetes, in which 10,177 patients had received empagliflozin 10 mg or 25 mg and 4,904 patients had received placebo.
Most patients (46.5%) had participated in the EMPA-REG OUTCOMES trial, which also had the longest follow-up (2.6 years).
The researchers identified patients with a new stone from the urinary tract (including the kidney, ureter, and urethra). Patients had received either the study drug for a median of 543 days or placebo for a median of 549 days.
During treatment, 104 of 10,177 patients in the pooled empagliflozin groups and 79 of 4,904 patients in the pooled placebo groups developed a stone in the urinary tract.
This was equivalent to 0.63 new urinary-tract stones per 100 patient-years in the pooled empagliflozin groups versus 1.01 new urinary-tract stones per 100 patient-years in the pooled placebo groups.
The incidence rate ratio was 0.64 (95% CI, 0.48-0.86), in favor of empagliflozin.
When the analysis was restricted to new kidney stones, the results were similar: 75 of 10,177 patients in the pooled empagliflozin groups and 57 of 4,904 patients in the pooled placebo groups developed a kidney stone.
This was equivalent to 0.45 new kidney stones per 100 patient-years in the pooled empagliflozin groups versus 0.72 new kidney stones per 100 patient-years in the pooled placebo groups.
The IRR was 0.65 (95% CI, 0.46-0.92), in favor of empagliflozin.
Upcoming small RCT in adults without diabetes
Invited to comment on the new study, Dr. Kristensen said: “The reduced risk of SGLT2 inhibitors towards nephrolithiasis is now reported in at least two studies with different methodology, different populations, and different exposure and outcome definitions.”
“I agree that randomized clinical trials designed specifically to confirm these findings appear warranted,” added Dr. Kristensen, from the Institute of Public Health, Clinical Pharmacology, Pharmacy, and Environmental Medicine, University of Southern Denmark in Odense.
There is a need for studies in patients with and without diabetes, he added, especially ones that focus on prevention of nephrolithiasis in patients with kidney stone disease.
A new trial should shed further light on this.
Results are expected by the end of 2022 for SWEETSTONE (Impact of the SGLT2 Inhibitor Empagliflozin on Urinary Supersaturations in Kidney Stone Formers), a randomized, double-blind crossover exploratory study in 46 patients without diabetes.
This should provide preliminary data to “establish the relevance for larger trials assessing the prophylactic potential of empagliflozin in kidney stone disease,” according to an article on the trial protocol recently published in BMJ.
The trials included in the pooled dataset were funded by Boehringer Ingelheim or the Boehringer Ingelheim and Eli Lilly Diabetes Alliance. Dr. Balasubramanian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than patients who received placebo during a median 1.5 years of treatment.
These findings are from an analysis of pooled data from phase 1-4 clinical trials of empagliflozin for blood glucose control in 15,081 patients with type 2 diabetes.
Priyadarshini Balasubramanian, MD, presented the study as a poster at the annual meeting of the Endocrine Society; the study also was published online in the Journal of Clinical Endocrinology & Metabolism.
The researchers acknowledge this was a retrospective, post-hoc analysis and that urolithiasis – a stone in the urinary tract, which includes nephrolithiasis, a kidney stone – was an adverse event, not a primary or secondary outcome.
Also, the stone composition, which might help explain how the drug may affect stone formation, is unknown.
Therefore, “dedicated randomized prospective clinical trials are needed to confirm these initial observations in patients both with and without type 2 diabetes,” said Dr. Balasubramanian, a clinical fellow in the section of endocrinology & metabolism, department of internal medicine at Yale University, New Haven, Conn.
However, “if this association is proven, empagliflozin may be used to decrease the risk of kidney stones at least in those with type 2 diabetes, but maybe also in those without diabetes,” Dr. Balasubramanian said in an interview.
Further trials are also needed to determine if this is a class effect, which is likely, she speculated, and to unravel the potential mechanism.
This is important because of the prevalence of kidney stones, which affect up to 15% of the general population and 15%-20% of patients with diabetes, she explained.
‘Provocative’ earlier findings
The current study was prompted by a recent observational study by Kasper B. Kristensen, MD, PhD, and colleagues.
Because SGLT2 inhibitors increase urinary glucose excretion through reduced renal reabsorption of glucose leading to osmotic diuresis and increased urinary flow, they hypothesized that these therapies “may reduce the risk of upper urinary tract stones (nephrolithiasis) by reducing the concentration of lithogenic substances in urine.”
Using data from Danish Health registries, they matched 12,325 individuals newly prescribed an SGLT2 inhibitor 1:1 with patients newly prescribed a glucagonlike peptide-1 (GLP1) agonist, another new class of drugs for treating type 2 diabetes.
They found a hazard ratio of 0.51 (95% confidence interval, 0.37-0.71) for incident nephrolithiasis and a hazard ratio of 0.68 (95% CI, 0.48-0.97) for recurrent nephrolithiasis for patients taking SGLT2 inhibitors versus GLP-1 agonists.
These findings are “striking,” according to Dr. Balasubramanian and colleagues. However, “these data, while provocative, were entirely retrospective and therefore possibly prone to bias,” they add.
Pooled data from 20 trials
The current study analyzed data from 20 randomized controlled trials of glycemic control in type 2 diabetes, in which 10,177 patients had received empagliflozin 10 mg or 25 mg and 4,904 patients had received placebo.
Most patients (46.5%) had participated in the EMPA-REG OUTCOMES trial, which also had the longest follow-up (2.6 years).
The researchers identified patients with a new stone from the urinary tract (including the kidney, ureter, and urethra). Patients had received either the study drug for a median of 543 days or placebo for a median of 549 days.
During treatment, 104 of 10,177 patients in the pooled empagliflozin groups and 79 of 4,904 patients in the pooled placebo groups developed a stone in the urinary tract.
This was equivalent to 0.63 new urinary-tract stones per 100 patient-years in the pooled empagliflozin groups versus 1.01 new urinary-tract stones per 100 patient-years in the pooled placebo groups.
The incidence rate ratio was 0.64 (95% CI, 0.48-0.86), in favor of empagliflozin.
When the analysis was restricted to new kidney stones, the results were similar: 75 of 10,177 patients in the pooled empagliflozin groups and 57 of 4,904 patients in the pooled placebo groups developed a kidney stone.
This was equivalent to 0.45 new kidney stones per 100 patient-years in the pooled empagliflozin groups versus 0.72 new kidney stones per 100 patient-years in the pooled placebo groups.
The IRR was 0.65 (95% CI, 0.46-0.92), in favor of empagliflozin.
Upcoming small RCT in adults without diabetes
Invited to comment on the new study, Dr. Kristensen said: “The reduced risk of SGLT2 inhibitors towards nephrolithiasis is now reported in at least two studies with different methodology, different populations, and different exposure and outcome definitions.”
“I agree that randomized clinical trials designed specifically to confirm these findings appear warranted,” added Dr. Kristensen, from the Institute of Public Health, Clinical Pharmacology, Pharmacy, and Environmental Medicine, University of Southern Denmark in Odense.
There is a need for studies in patients with and without diabetes, he added, especially ones that focus on prevention of nephrolithiasis in patients with kidney stone disease.
A new trial should shed further light on this.
Results are expected by the end of 2022 for SWEETSTONE (Impact of the SGLT2 Inhibitor Empagliflozin on Urinary Supersaturations in Kidney Stone Formers), a randomized, double-blind crossover exploratory study in 46 patients without diabetes.
This should provide preliminary data to “establish the relevance for larger trials assessing the prophylactic potential of empagliflozin in kidney stone disease,” according to an article on the trial protocol recently published in BMJ.
The trials included in the pooled dataset were funded by Boehringer Ingelheim or the Boehringer Ingelheim and Eli Lilly Diabetes Alliance. Dr. Balasubramanian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022













