User login
USPSTF: Screening pregnant women for asymptomatic bacteriuria cuts pyelonephritis risk
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
FROM JAMA
Type of renal dysfunction affects liver cirrhosis mortality risk
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
ACE inhibitor and ARB therapy: Practical recommendations
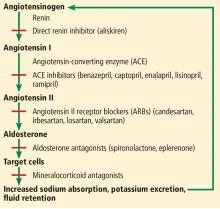
Inhibition of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) is widely used in the treatment of heart failure, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction.
In this issue, Momoniat et al1 review the benefits of ACE inhibitors and ARBs and how to manage adverse effects. I would like to add some of my own observations.
ARE ACE INHIBITORS REALLY BETTER THAN ARBs?
ACE inhibitors have been the cornerstone of treatment for patients with heart failure with reduced ejection fraction (HFrEF), in whom their use is associated with reduced rates of morbidity and death.2,3 The use of ARBs in these patients is also associated with decreased rates of morbidity and death4,5; however, in early comparisons, ACE inhibitors were deemed more effective in decreasing the incidence of myocardial infarction, cardiovascular death, and all-cause mortality in patients with hypertension, diabetes, and increased cardiovascular risk,6 and all-cause mortality in patients with HFrEF.7
This presumed superiority of ACE inhibitors over ARBs was thought to be a result of a greater vasodilatory effect caused by inhibiting the degradation of bradykinin and leading to increased levels of nitric oxide and vasoactive prostaglandins.8 Another proposed explanation was that because ARBs block angiotensin II AT1 receptors but not AT2 receptors, the increased stimulation of markedly upregulated AT2 receptors in atheromatous plaques in response to elevated serum levels of angiotensin II was deleterious.6 Therefore, ACE inhibitors have been recommended as first-line therapy by most guidelines, whereas ARBs are recommended as second-line therapy, when patients are unable to tolerate ACE inhibitors.
Nevertheless, the much debated differences in outcomes between ACE inhibitors and ARBs do not seem to be real and may have originated from a generational gap in the trials.
The ACE inhibitor trials were performed a decade earlier than the ARB trials. Indirect comparisons of their respective placebo-controlled trials assumed that the placebo groups used for comparison in the 2 sets of trials were similar.9,10 Actually, the rate of cardiovascular disease decreased nearly 50% between the decades of 1990 to 2000 and 2000 to 2010, the likely result of aggressive primary and secondary prevention strategies in clinical practice, including revascularization and lipid-lowering therapy.10
In fact, a meta-regression analysis showed that the differences between ACE inhibitors and ARBs compared with placebo were due to higher event rates in the placebo groups in the ACE inhibitor trials than in the ARB trials for the outcomes of death, cardiovascular death, and myocardial infarction.11 Sensitivity analyses restricted to trials published after 2000 to control for this generational gap showed similar efficacy with ACE inhibitors vs placebo and with ARBs vs placebo for all clinical outcomes.11 Moreover, recent studies have shown that ARBs produce a greater decrease in cardiovascular events than ACE inhibitors, especially in patients with established cardiovascular disease.12,13
An advantage of ARBs over ACE inhibitors is fewer adverse effects: in general, ARBs are better tolerated than ACE inhibitors.14 There are also ethnic differences in the risks of adverse reactions to these medications. African Americans have a higher risk of developing angioedema with ACE inhibitors compared with the rest of the US population, and Chinese Americans have a higher risk than whites of developing cough with ACE inhibitors.9,15
HOW I MANAGE THESE MEDICATIONS
In my medical practice, I try to make sure patients with HFrEF, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction receive an inhibitor of the renin-angiotensin-aldosterone system.
Which agent?
I prefer ARBs because patients tolerate them better. I continue ACE inhibitors in patients who are already taking them without adverse effects, and I change to ARBs in patients who later become unable to tolerate ACE inhibitors.
Most antihypertensive agents increase the risk of incident gout, except for calcium channel blockers and losartan.16 Losartan is the only ARB with a uricosuric effect, although a mild one,17,18 due to inhibition of the urate transporter 1,19 and therefore I prefer to use it instead of other ARBs or ACE inhibitors in patients who have a concomitant diagnosis of gout.
Which combinations of agents?
The addition of beta-blockers and mineralocorticoid receptor blockers to ACE inhibitors or ARBs is associated with a further decrease in the mortality risk for patients with HFrEF,20–22 but some patients cannot tolerate these combinations or optimized doses of these medications because of worsening hypotension or increased risk of developing acute kidney injury or hyperkalemia.
In most cases, I try not to combine ACE inhibitors with ARBs. This combination may be useful in nondiabetic patients with proteinuria refractory to maximum treatment with 1 class of these agents, but it is associated with an increased risk of hyperkalemia or acute kidney injury in patients with diabetic nephropathy without improving rates of the clinical outcomes of death or cardiovascular events.23 I prefer adding a daily low dose of a mineralocorticoid receptor blocker to an ACE inhibitor or an ARB, which is more effective in controlling refractory proteinuria.24 This regimen is associated with decreased rates of mortality, cardiovascular mortality, and hospitalization for heart failure in patients with HFrEF,22 although it can lead to a higher frequency of hyperkalemia,25 and patients on it require frequent dietary education and monitoring of serum potassium.
I avoid combining direct renin inhibitors with ACE inhibitors or ARBs, since this combination has been contraindicated by the US Food and Drug Administration due to lack of reduction in target-organ damage and an associated increased risk of hypotension, hyperkalemia, and kidney failure, and a slight increase in the risk of stroke or death in patients with diabetic nephropathy.26
Valsartan-sacubitril
Neprilysin is a membrane-bound endopeptidase that degrades vasoactive peptides, including B-type natriuretic peptide and atrial natriuretic peptide.27 The combination of the ARB valsartan and the neprilysin inhibitor sacubitril is associated with a 20% further decrease in rates of cardiovascular mortality and hospitalization and a 16% decrease in total mortality for patients with HFrEF compared with an ACE inhibitor, although there can also be more hypotension and angioedema with the combination.27,28
Very importantly, an ACE inhibitor cannot be used together with valsartan-sacubitril due to increased risk of angioedema and cough. I change ACE inhibitors or ARBs to valsartan-sacubitril in patients with HFrEF who still have symptoms of heart failure. Interestingly, a network meta-analysis showed that the combination of valsartan-sacubitril plus a mineralocorticoid receptor blocker and a beta-blocker resulted in the greatest mortality reduction in patients with HFrEF.7 A word of caution, though: one can also expect an increased risk of hypotension, hyperkalemia, and kidney failure.
Monitoring
It is crucial to monitor blood pressure, serum potassium, and renal function in patients receiving ACE inhibitors, ARBs, mineralocorticoid receptor blockers, valsartan-sacubitril, or combinations of these medications, particularly in elderly patients, who are more susceptible to complications. I use a multidisciplinary approach in my clinic: a patient educator, dietitian, pharmacist, and advanced practice nurse play key roles in educating and monitoring patients for the development of possible complications from this therapy or interactions with other medications.
A recent population-based cohort study found an association of ACE inhibitor use with a 14% relative increase in lung cancer incidence after 10 years of use, compared with ARBs,29 but this may not represent a large absolute risk (calculated number needed to harm of 2,970 after 10 years of ACE inhibitor use) and should be balanced against the improvement in morbidity and mortality gained with use of an ACE inhibitor. Additional studies with long-term follow-up are needed to investigate this possible association.
TAKE-HOME POINTS
- Blockade of the renin-angiotensin-aldosterone system is a cornerstone in the therapy of cardiovascular disease.
- ARBs are as effective as ACE inhibitors and have a better tolerability profile.
- ACE inhibitors cause more angioedema in African Americans and more cough in Chinese Americans than in the rest of the population.
- ACE inhibitors and most ARBs (except for losartan) increase the risk of gout.
- The combination of beta-blockers and mineralocorticoid receptor blockers with ACE inhibitors or ARBs and, lately, the use of the valsartan-sacubitril combination have been increasingly beneficial for patients with HFrEF.
- Momoniat T, Ilyas D, Bhandari S. ACE inhibitors and ARBs: managing potassium and renal function. Cleve Clin J Med 2019; 86(9):601–607. doi:10.3949/ccjm.86a.18024
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316(23):1429–1435. doi:10.1056/NEJM198706043162301
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Young JB, Dunlap ME, Pfeffer MA, et al; Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004; 110(17):2618–2626. doi:10.1161/01.CIR.0000146819.43235.A9
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345(23):1667–1675. doi:10.1056/NEJMoa010713
- Straus MH, Hall AS. Angiotensin receptor blockers do not reduce risk of myocardial infarction, cardiovascular death, or total mortality: further evidence for the ARB-MI paradox. Circulation 2017; 135(22):2088–2090. doi:10.1161/CIRCULATIONAHA.117.026112
- Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction. A network meta-analysis. Circ Heart Fail 2017; 10(1). pii:e003529. doi:10.1161/CIRCHEARTFAILURE.116.003529
- Chobanian AV. Editorial: angiotensin inhibition. N Engl J Med 1974; 291(16):844–845. doi:10.1056/NEJM197410172911611
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol 2018; 71(13):1474–1482. doi:10.1016/j.jacc.2018.01.058
- Messerli FH, Bangalore S. Angiotensin receptor blockers reduce cardiovascular events, including the risk of myocardial infarction. Circulation 2017; 135(22):2085–2087. doi:10.1161/CIRCULATIONAHA.116.025950
- Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc 2016; 91(1):51–60. doi:10.1016/j.mayocp.2015.10.019
- Potier L, Roussel R, Elbez Y, et al; REACH Registry Investigators. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart 2017; 103(17):1339–1346. doi:10.1136/heartjnl-2016-310705
- Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147,020 patients from randomized trials. BMJ 2011; 342:d2234. doi:10.1136/bmj.d2234
- Saglimbene V, Palmer SC, Ruospo M, et al; Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) Investigators. The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): a randomized, controlled trial. J Am Soc Nephrol 2018; 29(12):2890–2899. doi:10.1681/ASN.2018040443
- McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ 2006; 332(7551):1177–1181. doi:10.1136/bmj.38803.528113.55
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190. doi:10.1136/bmj.d8190
- Wolff ML, Cruz JL, Vanderman AJ, Brown JN. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis 2015; 6(6):339–346. doi:10.1177/2040622315596119
- Schmidt A, Gruber U, Böhmig G, Köller E, Mayer G. The effect of ACE inhibitor and angiotensin II receptor antagonist therapy on serum uric acid levels and potassium homeostasis in hypertensive renal transplant recipients treated with CsA. Nephrol Dial Transplant 2001; 16(5):1034–1037. pmid:11328912
- Hamada T, Ichida K, Hosoyamada M, et al. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT1) in hypertensive patients. Am J Hypertens 2008; 21(10):1157–1162. doi:10.1038/ajh.2008.245
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344(22):1651–1658. doi:10.1056/NEJM200105313442201
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11-21. doi:10.1056/NEJMoa1009492
- Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903. doi:10.1056/NEJMoa1303154
- Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1(2):256–262. doi:10.2215/CJN.01040905
- Abbas S, Ihle P, Harder S, Schubert I. Risk of hyperkalemia and combined use of spironolactone and long-term ACE inhibitor/angiotensin receptor blocker therapy in heart failure using real-life data: a population- and insurance-based cohort. Pharmacoepidemiol Drug Saf 2015; 24(4):406–413. doi:10.1002/pds.3748
- US Food and Drug Administration. FDA drug safety communication: new warning and contraindication for blood pressure medicines containing aliskiren (Tekturna). www.fda.gov/Drugs/DrugSafety/ucm300889.htm. Accessed March 8, 2019.
- Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016; 102(17):1342–1347. doi:10.1136/heartjnl-2014-306775
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018; 363:k4209. doi:10.1136/bmj.k4209
Inhibition of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) is widely used in the treatment of heart failure, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction.
In this issue, Momoniat et al1 review the benefits of ACE inhibitors and ARBs and how to manage adverse effects. I would like to add some of my own observations.
ARE ACE INHIBITORS REALLY BETTER THAN ARBs?
ACE inhibitors have been the cornerstone of treatment for patients with heart failure with reduced ejection fraction (HFrEF), in whom their use is associated with reduced rates of morbidity and death.2,3 The use of ARBs in these patients is also associated with decreased rates of morbidity and death4,5; however, in early comparisons, ACE inhibitors were deemed more effective in decreasing the incidence of myocardial infarction, cardiovascular death, and all-cause mortality in patients with hypertension, diabetes, and increased cardiovascular risk,6 and all-cause mortality in patients with HFrEF.7
This presumed superiority of ACE inhibitors over ARBs was thought to be a result of a greater vasodilatory effect caused by inhibiting the degradation of bradykinin and leading to increased levels of nitric oxide and vasoactive prostaglandins.8 Another proposed explanation was that because ARBs block angiotensin II AT1 receptors but not AT2 receptors, the increased stimulation of markedly upregulated AT2 receptors in atheromatous plaques in response to elevated serum levels of angiotensin II was deleterious.6 Therefore, ACE inhibitors have been recommended as first-line therapy by most guidelines, whereas ARBs are recommended as second-line therapy, when patients are unable to tolerate ACE inhibitors.
Nevertheless, the much debated differences in outcomes between ACE inhibitors and ARBs do not seem to be real and may have originated from a generational gap in the trials.
The ACE inhibitor trials were performed a decade earlier than the ARB trials. Indirect comparisons of their respective placebo-controlled trials assumed that the placebo groups used for comparison in the 2 sets of trials were similar.9,10 Actually, the rate of cardiovascular disease decreased nearly 50% between the decades of 1990 to 2000 and 2000 to 2010, the likely result of aggressive primary and secondary prevention strategies in clinical practice, including revascularization and lipid-lowering therapy.10
In fact, a meta-regression analysis showed that the differences between ACE inhibitors and ARBs compared with placebo were due to higher event rates in the placebo groups in the ACE inhibitor trials than in the ARB trials for the outcomes of death, cardiovascular death, and myocardial infarction.11 Sensitivity analyses restricted to trials published after 2000 to control for this generational gap showed similar efficacy with ACE inhibitors vs placebo and with ARBs vs placebo for all clinical outcomes.11 Moreover, recent studies have shown that ARBs produce a greater decrease in cardiovascular events than ACE inhibitors, especially in patients with established cardiovascular disease.12,13
An advantage of ARBs over ACE inhibitors is fewer adverse effects: in general, ARBs are better tolerated than ACE inhibitors.14 There are also ethnic differences in the risks of adverse reactions to these medications. African Americans have a higher risk of developing angioedema with ACE inhibitors compared with the rest of the US population, and Chinese Americans have a higher risk than whites of developing cough with ACE inhibitors.9,15
HOW I MANAGE THESE MEDICATIONS
In my medical practice, I try to make sure patients with HFrEF, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction receive an inhibitor of the renin-angiotensin-aldosterone system.
Which agent?
I prefer ARBs because patients tolerate them better. I continue ACE inhibitors in patients who are already taking them without adverse effects, and I change to ARBs in patients who later become unable to tolerate ACE inhibitors.
Most antihypertensive agents increase the risk of incident gout, except for calcium channel blockers and losartan.16 Losartan is the only ARB with a uricosuric effect, although a mild one,17,18 due to inhibition of the urate transporter 1,19 and therefore I prefer to use it instead of other ARBs or ACE inhibitors in patients who have a concomitant diagnosis of gout.
Which combinations of agents?
The addition of beta-blockers and mineralocorticoid receptor blockers to ACE inhibitors or ARBs is associated with a further decrease in the mortality risk for patients with HFrEF,20–22 but some patients cannot tolerate these combinations or optimized doses of these medications because of worsening hypotension or increased risk of developing acute kidney injury or hyperkalemia.
In most cases, I try not to combine ACE inhibitors with ARBs. This combination may be useful in nondiabetic patients with proteinuria refractory to maximum treatment with 1 class of these agents, but it is associated with an increased risk of hyperkalemia or acute kidney injury in patients with diabetic nephropathy without improving rates of the clinical outcomes of death or cardiovascular events.23 I prefer adding a daily low dose of a mineralocorticoid receptor blocker to an ACE inhibitor or an ARB, which is more effective in controlling refractory proteinuria.24 This regimen is associated with decreased rates of mortality, cardiovascular mortality, and hospitalization for heart failure in patients with HFrEF,22 although it can lead to a higher frequency of hyperkalemia,25 and patients on it require frequent dietary education and monitoring of serum potassium.
I avoid combining direct renin inhibitors with ACE inhibitors or ARBs, since this combination has been contraindicated by the US Food and Drug Administration due to lack of reduction in target-organ damage and an associated increased risk of hypotension, hyperkalemia, and kidney failure, and a slight increase in the risk of stroke or death in patients with diabetic nephropathy.26
Valsartan-sacubitril
Neprilysin is a membrane-bound endopeptidase that degrades vasoactive peptides, including B-type natriuretic peptide and atrial natriuretic peptide.27 The combination of the ARB valsartan and the neprilysin inhibitor sacubitril is associated with a 20% further decrease in rates of cardiovascular mortality and hospitalization and a 16% decrease in total mortality for patients with HFrEF compared with an ACE inhibitor, although there can also be more hypotension and angioedema with the combination.27,28
Very importantly, an ACE inhibitor cannot be used together with valsartan-sacubitril due to increased risk of angioedema and cough. I change ACE inhibitors or ARBs to valsartan-sacubitril in patients with HFrEF who still have symptoms of heart failure. Interestingly, a network meta-analysis showed that the combination of valsartan-sacubitril plus a mineralocorticoid receptor blocker and a beta-blocker resulted in the greatest mortality reduction in patients with HFrEF.7 A word of caution, though: one can also expect an increased risk of hypotension, hyperkalemia, and kidney failure.
Monitoring
It is crucial to monitor blood pressure, serum potassium, and renal function in patients receiving ACE inhibitors, ARBs, mineralocorticoid receptor blockers, valsartan-sacubitril, or combinations of these medications, particularly in elderly patients, who are more susceptible to complications. I use a multidisciplinary approach in my clinic: a patient educator, dietitian, pharmacist, and advanced practice nurse play key roles in educating and monitoring patients for the development of possible complications from this therapy or interactions with other medications.
A recent population-based cohort study found an association of ACE inhibitor use with a 14% relative increase in lung cancer incidence after 10 years of use, compared with ARBs,29 but this may not represent a large absolute risk (calculated number needed to harm of 2,970 after 10 years of ACE inhibitor use) and should be balanced against the improvement in morbidity and mortality gained with use of an ACE inhibitor. Additional studies with long-term follow-up are needed to investigate this possible association.
TAKE-HOME POINTS
- Blockade of the renin-angiotensin-aldosterone system is a cornerstone in the therapy of cardiovascular disease.
- ARBs are as effective as ACE inhibitors and have a better tolerability profile.
- ACE inhibitors cause more angioedema in African Americans and more cough in Chinese Americans than in the rest of the population.
- ACE inhibitors and most ARBs (except for losartan) increase the risk of gout.
- The combination of beta-blockers and mineralocorticoid receptor blockers with ACE inhibitors or ARBs and, lately, the use of the valsartan-sacubitril combination have been increasingly beneficial for patients with HFrEF.
Inhibition of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) is widely used in the treatment of heart failure, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction.
In this issue, Momoniat et al1 review the benefits of ACE inhibitors and ARBs and how to manage adverse effects. I would like to add some of my own observations.
ARE ACE INHIBITORS REALLY BETTER THAN ARBs?
ACE inhibitors have been the cornerstone of treatment for patients with heart failure with reduced ejection fraction (HFrEF), in whom their use is associated with reduced rates of morbidity and death.2,3 The use of ARBs in these patients is also associated with decreased rates of morbidity and death4,5; however, in early comparisons, ACE inhibitors were deemed more effective in decreasing the incidence of myocardial infarction, cardiovascular death, and all-cause mortality in patients with hypertension, diabetes, and increased cardiovascular risk,6 and all-cause mortality in patients with HFrEF.7
This presumed superiority of ACE inhibitors over ARBs was thought to be a result of a greater vasodilatory effect caused by inhibiting the degradation of bradykinin and leading to increased levels of nitric oxide and vasoactive prostaglandins.8 Another proposed explanation was that because ARBs block angiotensin II AT1 receptors but not AT2 receptors, the increased stimulation of markedly upregulated AT2 receptors in atheromatous plaques in response to elevated serum levels of angiotensin II was deleterious.6 Therefore, ACE inhibitors have been recommended as first-line therapy by most guidelines, whereas ARBs are recommended as second-line therapy, when patients are unable to tolerate ACE inhibitors.
Nevertheless, the much debated differences in outcomes between ACE inhibitors and ARBs do not seem to be real and may have originated from a generational gap in the trials.
The ACE inhibitor trials were performed a decade earlier than the ARB trials. Indirect comparisons of their respective placebo-controlled trials assumed that the placebo groups used for comparison in the 2 sets of trials were similar.9,10 Actually, the rate of cardiovascular disease decreased nearly 50% between the decades of 1990 to 2000 and 2000 to 2010, the likely result of aggressive primary and secondary prevention strategies in clinical practice, including revascularization and lipid-lowering therapy.10
In fact, a meta-regression analysis showed that the differences between ACE inhibitors and ARBs compared with placebo were due to higher event rates in the placebo groups in the ACE inhibitor trials than in the ARB trials for the outcomes of death, cardiovascular death, and myocardial infarction.11 Sensitivity analyses restricted to trials published after 2000 to control for this generational gap showed similar efficacy with ACE inhibitors vs placebo and with ARBs vs placebo for all clinical outcomes.11 Moreover, recent studies have shown that ARBs produce a greater decrease in cardiovascular events than ACE inhibitors, especially in patients with established cardiovascular disease.12,13
An advantage of ARBs over ACE inhibitors is fewer adverse effects: in general, ARBs are better tolerated than ACE inhibitors.14 There are also ethnic differences in the risks of adverse reactions to these medications. African Americans have a higher risk of developing angioedema with ACE inhibitors compared with the rest of the US population, and Chinese Americans have a higher risk than whites of developing cough with ACE inhibitors.9,15
HOW I MANAGE THESE MEDICATIONS
In my medical practice, I try to make sure patients with HFrEF, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction receive an inhibitor of the renin-angiotensin-aldosterone system.
Which agent?
I prefer ARBs because patients tolerate them better. I continue ACE inhibitors in patients who are already taking them without adverse effects, and I change to ARBs in patients who later become unable to tolerate ACE inhibitors.
Most antihypertensive agents increase the risk of incident gout, except for calcium channel blockers and losartan.16 Losartan is the only ARB with a uricosuric effect, although a mild one,17,18 due to inhibition of the urate transporter 1,19 and therefore I prefer to use it instead of other ARBs or ACE inhibitors in patients who have a concomitant diagnosis of gout.
Which combinations of agents?
The addition of beta-blockers and mineralocorticoid receptor blockers to ACE inhibitors or ARBs is associated with a further decrease in the mortality risk for patients with HFrEF,20–22 but some patients cannot tolerate these combinations or optimized doses of these medications because of worsening hypotension or increased risk of developing acute kidney injury or hyperkalemia.
In most cases, I try not to combine ACE inhibitors with ARBs. This combination may be useful in nondiabetic patients with proteinuria refractory to maximum treatment with 1 class of these agents, but it is associated with an increased risk of hyperkalemia or acute kidney injury in patients with diabetic nephropathy without improving rates of the clinical outcomes of death or cardiovascular events.23 I prefer adding a daily low dose of a mineralocorticoid receptor blocker to an ACE inhibitor or an ARB, which is more effective in controlling refractory proteinuria.24 This regimen is associated with decreased rates of mortality, cardiovascular mortality, and hospitalization for heart failure in patients with HFrEF,22 although it can lead to a higher frequency of hyperkalemia,25 and patients on it require frequent dietary education and monitoring of serum potassium.
I avoid combining direct renin inhibitors with ACE inhibitors or ARBs, since this combination has been contraindicated by the US Food and Drug Administration due to lack of reduction in target-organ damage and an associated increased risk of hypotension, hyperkalemia, and kidney failure, and a slight increase in the risk of stroke or death in patients with diabetic nephropathy.26
Valsartan-sacubitril
Neprilysin is a membrane-bound endopeptidase that degrades vasoactive peptides, including B-type natriuretic peptide and atrial natriuretic peptide.27 The combination of the ARB valsartan and the neprilysin inhibitor sacubitril is associated with a 20% further decrease in rates of cardiovascular mortality and hospitalization and a 16% decrease in total mortality for patients with HFrEF compared with an ACE inhibitor, although there can also be more hypotension and angioedema with the combination.27,28
Very importantly, an ACE inhibitor cannot be used together with valsartan-sacubitril due to increased risk of angioedema and cough. I change ACE inhibitors or ARBs to valsartan-sacubitril in patients with HFrEF who still have symptoms of heart failure. Interestingly, a network meta-analysis showed that the combination of valsartan-sacubitril plus a mineralocorticoid receptor blocker and a beta-blocker resulted in the greatest mortality reduction in patients with HFrEF.7 A word of caution, though: one can also expect an increased risk of hypotension, hyperkalemia, and kidney failure.
Monitoring
It is crucial to monitor blood pressure, serum potassium, and renal function in patients receiving ACE inhibitors, ARBs, mineralocorticoid receptor blockers, valsartan-sacubitril, or combinations of these medications, particularly in elderly patients, who are more susceptible to complications. I use a multidisciplinary approach in my clinic: a patient educator, dietitian, pharmacist, and advanced practice nurse play key roles in educating and monitoring patients for the development of possible complications from this therapy or interactions with other medications.
A recent population-based cohort study found an association of ACE inhibitor use with a 14% relative increase in lung cancer incidence after 10 years of use, compared with ARBs,29 but this may not represent a large absolute risk (calculated number needed to harm of 2,970 after 10 years of ACE inhibitor use) and should be balanced against the improvement in morbidity and mortality gained with use of an ACE inhibitor. Additional studies with long-term follow-up are needed to investigate this possible association.
TAKE-HOME POINTS
- Blockade of the renin-angiotensin-aldosterone system is a cornerstone in the therapy of cardiovascular disease.
- ARBs are as effective as ACE inhibitors and have a better tolerability profile.
- ACE inhibitors cause more angioedema in African Americans and more cough in Chinese Americans than in the rest of the population.
- ACE inhibitors and most ARBs (except for losartan) increase the risk of gout.
- The combination of beta-blockers and mineralocorticoid receptor blockers with ACE inhibitors or ARBs and, lately, the use of the valsartan-sacubitril combination have been increasingly beneficial for patients with HFrEF.
- Momoniat T, Ilyas D, Bhandari S. ACE inhibitors and ARBs: managing potassium and renal function. Cleve Clin J Med 2019; 86(9):601–607. doi:10.3949/ccjm.86a.18024
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316(23):1429–1435. doi:10.1056/NEJM198706043162301
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Young JB, Dunlap ME, Pfeffer MA, et al; Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004; 110(17):2618–2626. doi:10.1161/01.CIR.0000146819.43235.A9
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345(23):1667–1675. doi:10.1056/NEJMoa010713
- Straus MH, Hall AS. Angiotensin receptor blockers do not reduce risk of myocardial infarction, cardiovascular death, or total mortality: further evidence for the ARB-MI paradox. Circulation 2017; 135(22):2088–2090. doi:10.1161/CIRCULATIONAHA.117.026112
- Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction. A network meta-analysis. Circ Heart Fail 2017; 10(1). pii:e003529. doi:10.1161/CIRCHEARTFAILURE.116.003529
- Chobanian AV. Editorial: angiotensin inhibition. N Engl J Med 1974; 291(16):844–845. doi:10.1056/NEJM197410172911611
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol 2018; 71(13):1474–1482. doi:10.1016/j.jacc.2018.01.058
- Messerli FH, Bangalore S. Angiotensin receptor blockers reduce cardiovascular events, including the risk of myocardial infarction. Circulation 2017; 135(22):2085–2087. doi:10.1161/CIRCULATIONAHA.116.025950
- Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc 2016; 91(1):51–60. doi:10.1016/j.mayocp.2015.10.019
- Potier L, Roussel R, Elbez Y, et al; REACH Registry Investigators. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart 2017; 103(17):1339–1346. doi:10.1136/heartjnl-2016-310705
- Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147,020 patients from randomized trials. BMJ 2011; 342:d2234. doi:10.1136/bmj.d2234
- Saglimbene V, Palmer SC, Ruospo M, et al; Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) Investigators. The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): a randomized, controlled trial. J Am Soc Nephrol 2018; 29(12):2890–2899. doi:10.1681/ASN.2018040443
- McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ 2006; 332(7551):1177–1181. doi:10.1136/bmj.38803.528113.55
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190. doi:10.1136/bmj.d8190
- Wolff ML, Cruz JL, Vanderman AJ, Brown JN. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis 2015; 6(6):339–346. doi:10.1177/2040622315596119
- Schmidt A, Gruber U, Böhmig G, Köller E, Mayer G. The effect of ACE inhibitor and angiotensin II receptor antagonist therapy on serum uric acid levels and potassium homeostasis in hypertensive renal transplant recipients treated with CsA. Nephrol Dial Transplant 2001; 16(5):1034–1037. pmid:11328912
- Hamada T, Ichida K, Hosoyamada M, et al. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT1) in hypertensive patients. Am J Hypertens 2008; 21(10):1157–1162. doi:10.1038/ajh.2008.245
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344(22):1651–1658. doi:10.1056/NEJM200105313442201
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11-21. doi:10.1056/NEJMoa1009492
- Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903. doi:10.1056/NEJMoa1303154
- Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1(2):256–262. doi:10.2215/CJN.01040905
- Abbas S, Ihle P, Harder S, Schubert I. Risk of hyperkalemia and combined use of spironolactone and long-term ACE inhibitor/angiotensin receptor blocker therapy in heart failure using real-life data: a population- and insurance-based cohort. Pharmacoepidemiol Drug Saf 2015; 24(4):406–413. doi:10.1002/pds.3748
- US Food and Drug Administration. FDA drug safety communication: new warning and contraindication for blood pressure medicines containing aliskiren (Tekturna). www.fda.gov/Drugs/DrugSafety/ucm300889.htm. Accessed March 8, 2019.
- Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016; 102(17):1342–1347. doi:10.1136/heartjnl-2014-306775
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018; 363:k4209. doi:10.1136/bmj.k4209
- Momoniat T, Ilyas D, Bhandari S. ACE inhibitors and ARBs: managing potassium and renal function. Cleve Clin J Med 2019; 86(9):601–607. doi:10.3949/ccjm.86a.18024
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316(23):1429–1435. doi:10.1056/NEJM198706043162301
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Young JB, Dunlap ME, Pfeffer MA, et al; Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004; 110(17):2618–2626. doi:10.1161/01.CIR.0000146819.43235.A9
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345(23):1667–1675. doi:10.1056/NEJMoa010713
- Straus MH, Hall AS. Angiotensin receptor blockers do not reduce risk of myocardial infarction, cardiovascular death, or total mortality: further evidence for the ARB-MI paradox. Circulation 2017; 135(22):2088–2090. doi:10.1161/CIRCULATIONAHA.117.026112
- Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction. A network meta-analysis. Circ Heart Fail 2017; 10(1). pii:e003529. doi:10.1161/CIRCHEARTFAILURE.116.003529
- Chobanian AV. Editorial: angiotensin inhibition. N Engl J Med 1974; 291(16):844–845. doi:10.1056/NEJM197410172911611
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol 2018; 71(13):1474–1482. doi:10.1016/j.jacc.2018.01.058
- Messerli FH, Bangalore S. Angiotensin receptor blockers reduce cardiovascular events, including the risk of myocardial infarction. Circulation 2017; 135(22):2085–2087. doi:10.1161/CIRCULATIONAHA.116.025950
- Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc 2016; 91(1):51–60. doi:10.1016/j.mayocp.2015.10.019
- Potier L, Roussel R, Elbez Y, et al; REACH Registry Investigators. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart 2017; 103(17):1339–1346. doi:10.1136/heartjnl-2016-310705
- Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147,020 patients from randomized trials. BMJ 2011; 342:d2234. doi:10.1136/bmj.d2234
- Saglimbene V, Palmer SC, Ruospo M, et al; Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) Investigators. The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): a randomized, controlled trial. J Am Soc Nephrol 2018; 29(12):2890–2899. doi:10.1681/ASN.2018040443
- McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ 2006; 332(7551):1177–1181. doi:10.1136/bmj.38803.528113.55
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190. doi:10.1136/bmj.d8190
- Wolff ML, Cruz JL, Vanderman AJ, Brown JN. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis 2015; 6(6):339–346. doi:10.1177/2040622315596119
- Schmidt A, Gruber U, Böhmig G, Köller E, Mayer G. The effect of ACE inhibitor and angiotensin II receptor antagonist therapy on serum uric acid levels and potassium homeostasis in hypertensive renal transplant recipients treated with CsA. Nephrol Dial Transplant 2001; 16(5):1034–1037. pmid:11328912
- Hamada T, Ichida K, Hosoyamada M, et al. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT1) in hypertensive patients. Am J Hypertens 2008; 21(10):1157–1162. doi:10.1038/ajh.2008.245
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344(22):1651–1658. doi:10.1056/NEJM200105313442201
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11-21. doi:10.1056/NEJMoa1009492
- Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903. doi:10.1056/NEJMoa1303154
- Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1(2):256–262. doi:10.2215/CJN.01040905
- Abbas S, Ihle P, Harder S, Schubert I. Risk of hyperkalemia and combined use of spironolactone and long-term ACE inhibitor/angiotensin receptor blocker therapy in heart failure using real-life data: a population- and insurance-based cohort. Pharmacoepidemiol Drug Saf 2015; 24(4):406–413. doi:10.1002/pds.3748
- US Food and Drug Administration. FDA drug safety communication: new warning and contraindication for blood pressure medicines containing aliskiren (Tekturna). www.fda.gov/Drugs/DrugSafety/ucm300889.htm. Accessed March 8, 2019.
- Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016; 102(17):1342–1347. doi:10.1136/heartjnl-2014-306775
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018; 363:k4209. doi:10.1136/bmj.k4209
ACE inhibitors and ARBs: Managing potassium and renal function
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
KEY POINTS
- ACE inhibitors and ARBs reduce proteinuria by lowering the intraglomerular pressure, reducing hyperfiltration.
- These drugs tend to raise the serum potassium level and reduce the glomerular filtration rate (GFR). Monitoring the serum potassium and creatinine levels and the GFR is therefore imperative.
- Despite the benefits, concern for adverse effects including hyperkalemia and a rise in serum creatinine has led to reluctance to prescribe these drugs, and they are underused in the patients who may derive the greatest benefit.
Farxiga gets Fast Track status from FDA
The Food and Drug Administration has given Fast Track designation to the development of dapagliflozin (Farxiga) to delay progression of renal failure and to prevent cardiovascular and renal death in patients with chronic kidney disease with and without type 2 diabetes, according to a release from AstraZeneca.
The Fast Track designation is meant to accelerate the development and review process for the treatment of serious conditions that have unmet therapeutic needs.
Dapagliflozin, an oral daily sodium-glucose transporter 2 inhibitor, is approved both as a monotherapy and a component of combination therapy for the improvement of glycemic control in patients with type 2 diabetes, according to the release. It is given as an adjunct to diet and exercise, and has also shown additional benefits of weight loss and reduction in blood pressure.
A phase 3, randomized, placebo-controlled trial, DAPA-CVD (NCT03036150), is currently underway to evaluate the drug’s efficacy specifically in terms of renal outcomes and cardiovascular mortality in patients with chronic kidney disease, with and without type 2 diabetes. Participants receive once-daily dapagliflozin or placebo in addition to standard care.
Taking dapagliflozin carries risks of hypotension, renal impairment, hypoglycemia, and other concerns. The most common adverse reactions (5% or greater incidence) include female genital mycotic infections, nasopharyngitis, and urinary tract infections. Full prescribing information can be found on the agency’s website.
The Food and Drug Administration has given Fast Track designation to the development of dapagliflozin (Farxiga) to delay progression of renal failure and to prevent cardiovascular and renal death in patients with chronic kidney disease with and without type 2 diabetes, according to a release from AstraZeneca.
The Fast Track designation is meant to accelerate the development and review process for the treatment of serious conditions that have unmet therapeutic needs.
Dapagliflozin, an oral daily sodium-glucose transporter 2 inhibitor, is approved both as a monotherapy and a component of combination therapy for the improvement of glycemic control in patients with type 2 diabetes, according to the release. It is given as an adjunct to diet and exercise, and has also shown additional benefits of weight loss and reduction in blood pressure.
A phase 3, randomized, placebo-controlled trial, DAPA-CVD (NCT03036150), is currently underway to evaluate the drug’s efficacy specifically in terms of renal outcomes and cardiovascular mortality in patients with chronic kidney disease, with and without type 2 diabetes. Participants receive once-daily dapagliflozin or placebo in addition to standard care.
Taking dapagliflozin carries risks of hypotension, renal impairment, hypoglycemia, and other concerns. The most common adverse reactions (5% or greater incidence) include female genital mycotic infections, nasopharyngitis, and urinary tract infections. Full prescribing information can be found on the agency’s website.
The Food and Drug Administration has given Fast Track designation to the development of dapagliflozin (Farxiga) to delay progression of renal failure and to prevent cardiovascular and renal death in patients with chronic kidney disease with and without type 2 diabetes, according to a release from AstraZeneca.
The Fast Track designation is meant to accelerate the development and review process for the treatment of serious conditions that have unmet therapeutic needs.
Dapagliflozin, an oral daily sodium-glucose transporter 2 inhibitor, is approved both as a monotherapy and a component of combination therapy for the improvement of glycemic control in patients with type 2 diabetes, according to the release. It is given as an adjunct to diet and exercise, and has also shown additional benefits of weight loss and reduction in blood pressure.
A phase 3, randomized, placebo-controlled trial, DAPA-CVD (NCT03036150), is currently underway to evaluate the drug’s efficacy specifically in terms of renal outcomes and cardiovascular mortality in patients with chronic kidney disease, with and without type 2 diabetes. Participants receive once-daily dapagliflozin or placebo in addition to standard care.
Taking dapagliflozin carries risks of hypotension, renal impairment, hypoglycemia, and other concerns. The most common adverse reactions (5% or greater incidence) include female genital mycotic infections, nasopharyngitis, and urinary tract infections. Full prescribing information can be found on the agency’s website.
Anticoagulant therapy for AFib in patients with end-stage renal disease
Warfarin or apixaban are sensible options
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
Warfarin or apixaban are sensible options
Warfarin or apixaban are sensible options
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
Urine albumin testing is crucial for patients at risk for CKD, but drop the 24-hour urine test
SAN DIEGO – Whatever you do, don’t order a 24-hour urine test. Do encourage “pork holidays.” And choose between an ACE inhibitor and an ARB – don’t give them both to a single patient, according to Kim Zuber, PA-C, MS, a nephrology physician assistant from St. Petersburg, Fla., in a presentation about kidney disease, hypertension, and diabetes at the Metabolic & Endocrine Disease Summit, sponsored by Global Academy for Medical Education.
Ms. Zuber, who is the executive director of the American Academy of Nephrology PAs and the outreach chair of the National Kidney Foundation, outlined some approaches for the diagnosis, management, and treatment of chronic kidney disease (CKD) with comorbid hypertension and diabetes.
- Use the right urine test. Ms. Zuber said, although it’s often not performed. In fact, research suggests that most Medicare patients with diabetes, hypertension, or both do not have this test, she said. Order a urine albumin-to-creatinine ratio (UACR) test at least once a year in at-risk patients, she recommended, and more frequently if they show signs of abnormal values. But be aware, she said, some labs might refer to the test as microalbuminuria instead of UACR, and be prepared to calculate the UACR yourself if your institution provides only albumin and creatinine levels. Also watch out for mix-ups regarding UACR measurements. Nephrotic-range proteinuria starts at 3 g/dL or 3,000 mg/dL, she said, and residents often confuse those two sets of units. “Many have gotten in trouble with that,” she said.
- Don’t go near a 24-hour urine test. Thinking about ordering a 24-hour urine test that requires a patients to collect all their urine for a day? Think again. “We’ve been telling you almost 20 years not to do this,” Ms. Zuber said. These tests “are unreliable, and they don’t work.”
- Don’t focus on tight blood pressure control. Studies provide little insight into the ideal blood pressure readings for patients with diabetes and CKD, according to Ms. Zuber, but some findings suggest that tight control can be harmful to the kidneys. She urges her patients to treat hypertension in part by embracing lifestyle change. “I tell them that if you improve your lifestyle, you can give up one of your drugs. When they average 15 drugs a day, that becomes popular.” Physical activity, the DASH diet, salt restriction, moderate alcohol consumption, weight loss, stress reduction, and smoking cessation can all lower blood pressure, she added.
- Talk up the “pork holiday.” For patients with hypertension, “sodium restriction is huge,” Ms. Zuber said, especially among black patients. She urges her patients to take “pork holidays”, that is, eat pork only four times a year, on holidays such as the Fourth of July. She also urges them to prepare food in ways that begin with B, as in bake, boil, and barbecue. “You’ll notice that ‘fry’ doesn’t start with a B.”
- Try an ACE inhibitor or an ARB, but not both. In patients with hypertension plus diabetes and/or CKD, Ms. Zuber suggests using an angiotensin-converting enzyme inhibitor or angiotensin receptor blockers, but not both. One or the other can improve albuminuria, she said, but together they can boost risk of CKD, hyperkalemia, and hypotension.
Consider factors such as formularies and personal experience when trying to decide which drug to use, she said. If a patient still has hypertension, consider a diuretic and then move to a calcium channel or beta blocker. However, she cautioned, although beta blockers, they can cause erectile dysfunction.
Global Academy for Medical Education and this news organization are owned by the same parent company. Ms. Zuber reported no disclosures.
SAN DIEGO – Whatever you do, don’t order a 24-hour urine test. Do encourage “pork holidays.” And choose between an ACE inhibitor and an ARB – don’t give them both to a single patient, according to Kim Zuber, PA-C, MS, a nephrology physician assistant from St. Petersburg, Fla., in a presentation about kidney disease, hypertension, and diabetes at the Metabolic & Endocrine Disease Summit, sponsored by Global Academy for Medical Education.
Ms. Zuber, who is the executive director of the American Academy of Nephrology PAs and the outreach chair of the National Kidney Foundation, outlined some approaches for the diagnosis, management, and treatment of chronic kidney disease (CKD) with comorbid hypertension and diabetes.
- Use the right urine test. Ms. Zuber said, although it’s often not performed. In fact, research suggests that most Medicare patients with diabetes, hypertension, or both do not have this test, she said. Order a urine albumin-to-creatinine ratio (UACR) test at least once a year in at-risk patients, she recommended, and more frequently if they show signs of abnormal values. But be aware, she said, some labs might refer to the test as microalbuminuria instead of UACR, and be prepared to calculate the UACR yourself if your institution provides only albumin and creatinine levels. Also watch out for mix-ups regarding UACR measurements. Nephrotic-range proteinuria starts at 3 g/dL or 3,000 mg/dL, she said, and residents often confuse those two sets of units. “Many have gotten in trouble with that,” she said.
- Don’t go near a 24-hour urine test. Thinking about ordering a 24-hour urine test that requires a patients to collect all their urine for a day? Think again. “We’ve been telling you almost 20 years not to do this,” Ms. Zuber said. These tests “are unreliable, and they don’t work.”
- Don’t focus on tight blood pressure control. Studies provide little insight into the ideal blood pressure readings for patients with diabetes and CKD, according to Ms. Zuber, but some findings suggest that tight control can be harmful to the kidneys. She urges her patients to treat hypertension in part by embracing lifestyle change. “I tell them that if you improve your lifestyle, you can give up one of your drugs. When they average 15 drugs a day, that becomes popular.” Physical activity, the DASH diet, salt restriction, moderate alcohol consumption, weight loss, stress reduction, and smoking cessation can all lower blood pressure, she added.
- Talk up the “pork holiday.” For patients with hypertension, “sodium restriction is huge,” Ms. Zuber said, especially among black patients. She urges her patients to take “pork holidays”, that is, eat pork only four times a year, on holidays such as the Fourth of July. She also urges them to prepare food in ways that begin with B, as in bake, boil, and barbecue. “You’ll notice that ‘fry’ doesn’t start with a B.”
- Try an ACE inhibitor or an ARB, but not both. In patients with hypertension plus diabetes and/or CKD, Ms. Zuber suggests using an angiotensin-converting enzyme inhibitor or angiotensin receptor blockers, but not both. One or the other can improve albuminuria, she said, but together they can boost risk of CKD, hyperkalemia, and hypotension.
Consider factors such as formularies and personal experience when trying to decide which drug to use, she said. If a patient still has hypertension, consider a diuretic and then move to a calcium channel or beta blocker. However, she cautioned, although beta blockers, they can cause erectile dysfunction.
Global Academy for Medical Education and this news organization are owned by the same parent company. Ms. Zuber reported no disclosures.
SAN DIEGO – Whatever you do, don’t order a 24-hour urine test. Do encourage “pork holidays.” And choose between an ACE inhibitor and an ARB – don’t give them both to a single patient, according to Kim Zuber, PA-C, MS, a nephrology physician assistant from St. Petersburg, Fla., in a presentation about kidney disease, hypertension, and diabetes at the Metabolic & Endocrine Disease Summit, sponsored by Global Academy for Medical Education.
Ms. Zuber, who is the executive director of the American Academy of Nephrology PAs and the outreach chair of the National Kidney Foundation, outlined some approaches for the diagnosis, management, and treatment of chronic kidney disease (CKD) with comorbid hypertension and diabetes.
- Use the right urine test. Ms. Zuber said, although it’s often not performed. In fact, research suggests that most Medicare patients with diabetes, hypertension, or both do not have this test, she said. Order a urine albumin-to-creatinine ratio (UACR) test at least once a year in at-risk patients, she recommended, and more frequently if they show signs of abnormal values. But be aware, she said, some labs might refer to the test as microalbuminuria instead of UACR, and be prepared to calculate the UACR yourself if your institution provides only albumin and creatinine levels. Also watch out for mix-ups regarding UACR measurements. Nephrotic-range proteinuria starts at 3 g/dL or 3,000 mg/dL, she said, and residents often confuse those two sets of units. “Many have gotten in trouble with that,” she said.
- Don’t go near a 24-hour urine test. Thinking about ordering a 24-hour urine test that requires a patients to collect all their urine for a day? Think again. “We’ve been telling you almost 20 years not to do this,” Ms. Zuber said. These tests “are unreliable, and they don’t work.”
- Don’t focus on tight blood pressure control. Studies provide little insight into the ideal blood pressure readings for patients with diabetes and CKD, according to Ms. Zuber, but some findings suggest that tight control can be harmful to the kidneys. She urges her patients to treat hypertension in part by embracing lifestyle change. “I tell them that if you improve your lifestyle, you can give up one of your drugs. When they average 15 drugs a day, that becomes popular.” Physical activity, the DASH diet, salt restriction, moderate alcohol consumption, weight loss, stress reduction, and smoking cessation can all lower blood pressure, she added.
- Talk up the “pork holiday.” For patients with hypertension, “sodium restriction is huge,” Ms. Zuber said, especially among black patients. She urges her patients to take “pork holidays”, that is, eat pork only four times a year, on holidays such as the Fourth of July. She also urges them to prepare food in ways that begin with B, as in bake, boil, and barbecue. “You’ll notice that ‘fry’ doesn’t start with a B.”
- Try an ACE inhibitor or an ARB, but not both. In patients with hypertension plus diabetes and/or CKD, Ms. Zuber suggests using an angiotensin-converting enzyme inhibitor or angiotensin receptor blockers, but not both. One or the other can improve albuminuria, she said, but together they can boost risk of CKD, hyperkalemia, and hypotension.
Consider factors such as formularies and personal experience when trying to decide which drug to use, she said. If a patient still has hypertension, consider a diuretic and then move to a calcium channel or beta blocker. However, she cautioned, although beta blockers, they can cause erectile dysfunction.
Global Academy for Medical Education and this news organization are owned by the same parent company. Ms. Zuber reported no disclosures.
EXPERT ANALYSIS FROM MEDS 2019
Deciding when a picture is worth a thousand words and several thousand dollars
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
When does acute pyelonephritis require imaging?
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
No increase in UTI risk with SGLT-2 inhibitors
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Sodium-glucose cotransporter 2 inhibitors are not associated with a greater risk of urinary tract infections, compared with dipeptidyl peptidase-4 inhibitors or glucagonlike peptide–1 receptor agonists.
Major finding: The incidence of severe urinary tract infections is similar among patients taking sodium-glucose cotransporter 2 inhibitors, dipeptidyl peptidase–4, inhibitors or glucagonlike peptide–1 receptor agonists.
Study details: A population-based, propensity-matched cohort study in 235,730 individuals with type 2 diabetes.
Disclosures: The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
Source: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.