User login
Renal failure in HCV cirrhosis
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with hepatitis C 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns such as hypertension or diabetes.
Two weeks ago, routine laboratory tests in the clinic showed that his serum creatinine level had increased from baseline. He was asked to stop his diuretics and increase his fluid intake. Nevertheless, his kidney function continued to decline (Table 1), and he was admitted to the hospital for further evaluation.
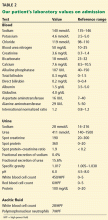
On admission, he appeared comfortable. He denied recent use of any medications, including nonsteroidal anti-inflammatory drugs, antibiotics, and diuretics, and he had no genitourinary symptoms. His temperature was normal, blood pressure 170/90 mm Hg, pulse rate 72 per minute, and respiratory rate 16. His skin and sclerae were not jaundiced; his abdomen was not tender, but it was grossly distended with ascites. He also had +3 pedal edema (on a scale of 4) extending to both knees. The rest of his physical examination was unremarkable. Results of further laboratory tests are shown in in Table 2.
Ultrasonography of the liver demonstrated cirrhosis with patent flow through the shunt, and ultrasonography of the kidneys showed that both were slightly enlarged with increased cortical echogenicity but no hydronephrosis or obstruction.
EXPLORING THE CAUSE OF RENAL FAILURE
1. Given this information, what is the likely cause of our patient’s renal failure?
- Volume depletion
- Acute tubular necrosis
- Hepatorenal syndrome
- HCV glomerulopathy
Renal failure is a common complication in cirrhosis and portends a higher risk of death.1 The differential diagnosis is broad, but a systematic approach incorporating data from the history, physical examination, and laboratory tests can help identify the cause and is essential in determining the prognosis and proper treatment.
Volume depletion
Volume depletion is a common cause of renal failure in cirrhotic patients. Common precipitants are excessive diuresis and gastrointestinal fluid loss from bleeding, vomiting, and diarrhea. Despite having ascites and edema, patients may have low fluid volume in the vascular space. Therefore, the first step in a patient with acute kidney injury is to withhold diuretics and give fluids. The renal failure usually rapidly reverses if the patient does not have renal parenchymal disease.2
Our patient did not present with any fluid losses, and his high blood pressure and normal heart rate did not suggest volume depletion. And most importantly, withholding his diuretics and giving fluids did not reverse his renal failure. Thus, volume depletion was an unlikely cause.
Acute tubular necrosis
The altered hemodynamics caused by cirrhosis predispose patients to acute tubular necrosis. Classically, this presents as muddy brown casts and renal tubular epithelial cells on urinalysis and as a fractional excretion of sodium greater than 2%.1 However, these microscopic findings lack sensitivity, and patients with cirrhosis may have marked sodium avidity and low urine sodium excretion despite tubular injury.3
This diagnosis must still be considered in patients with renal failure, especially after an insult such as hemorrhagic or septic shock or intake of nephrotoxins. However, because our patient did not have a history of any of these and because his renal failure had been progressing over weeks, acute tubular necrosis was considered unlikely.
Hepatorenal syndrome
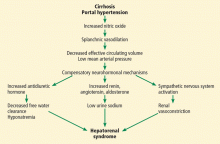
Hepatorenal syndrome is characterized by progressive renal failure in the absence of renal parenchymal disease. It is a functional disorder, ie, the decreased glomerular filtration rate results from renal vasoconstriction, which in turn is due to decreased systemic vascular resistance and increased compensatory activity of the renin-angiotensin-aldosterone axis and of antiduretic hormone release (Figure 1).
Hepatorenal syndrome often occurs in patients with advanced liver disease. These patients typically have a hyperdynamic circulation (systemic vasodilation, low blood pressure, and increased blood volume) with a low mean arterial pressure and increased renin and norepinephrine levels. Other frequent findings include hyponatremia, low urinary sodium excretion (< 2 mmol/day), and low free water clearance,4 all of which mark the high systemic levels of antidiuretic hormone and aldosterone.
Importantly, while hepatorenal syndrome is always considered in the differential diagnosis because of its unique prognosis and therapy, it remains a diagnosis of exclusion. The International Ascites Club5 has provided diagnostic criteria for hepatorenal syndrome:
- Cirrhosis and ascites
- Serum creatinine greater than 1.5 mg/dL
- Failure of serum creatinine to fall to less than 1.5 mg/dL after at least 48 hours of diuretic withdrawal and volume expansion with albumin (recommended dose 1 g/kg body weight per day up to a maximum of 100 g per day)
- Absence of shock
- No current or recent treatment with nephrotoxic drugs
- No signs of parenchymal kidney disease such as proteinuria (protein excretion > 500 mg/day), microhematuria (> 50 red blood cells per high-power field), or abnormalities on renal ultrasonography.
While these criteria are not perfect,6 they remind clinicians that there are other important causes of renal insufficiency in cirrhosis.
Clinically, our patient had no evidence of a hyperdynamic circulation and was instead hypertensive. He was eunatremic and did not have marked renal sodium avidity. His pyuria, proteinuria (his protein excretion was approximately 1.9 g/day as determined by urine spot protein-to-creatinine ratio), and results of ultrasonography also suggested underlying renal parenchymal disease. Therefore, hepatorenal syndrome was not the likely diagnosis.
HCV glomerulopathy
Intrinsic renal disease is likely, given our patient’s proteinuria, active urine sediment (ie, containing red blood cells, white blood cells, and protein), and abnormal findings on ultrasonography. In patients with HCV infection and no other cause of intrinsic kidney disease, immune complex deposition leading to glomerulonephritis is the most common pattern.7 Despite the intrinsic renal disease, fractional excretion of sodium may be less than 1% in glomerulonephritis. Hypertension in a patient such as ours with cirrhosis and renal insufficiency raises suspicion for glomerular disease, as hypertension is unlikely in advanced cirrhosis.8
Glomerulonephritis in patients with cirrhosis is often clinically silent and may be highly prevalent; some studies have shown glomerular involvement in 55% to 83% of patients with cirrhosis.9,10 This increases the risk of end-stage renal disease, and the Kidney Disease Improving Global Outcomes guideline recommends that HCV-infected patients be tested at least once a year for proteinuria, hematuria, and estimated glomerular filtration rate to detect possible HCV-associated kidney disease.11 According to current guidelines of the Infectious Diseases Society of America (IDSA) and American Association for the Study of Liver Diseases (AASLD) , detection of glomerulonephritis in HCV patients puts them in the highest priority class for treatment of HCV.12
HISTOLOGIC FINDINGS
Because of the high likelihood of glomerulopathy, our patient underwent renal biopsy.
2. What is the classic pathologic finding in HCV kidney disease?
- Focal segmental glomerulosclerosis
- Crescentic glomerulonephritis
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
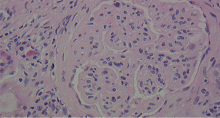
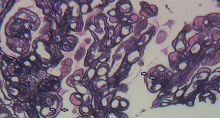
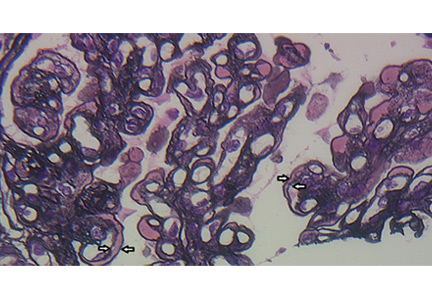
A number of pathologic patterns have been described in HCV kidney disease, including membranous glomerulonephritis, immunoglobulin A nephropathy, and focal segmental glomerulosclerosis. However, by far the most common pattern is type 1 membranoproliferative glomerulonephritis.13 (Types 2 and 3 are much less common, and we will not discuss them here.) In type 1, light microscopy shows increased mesangial cells and thickened capillary walls (lobular glomeruli), staining of the basement membrane reveals double contours (“tram tracking”) or splitting due to mesangial deposition, and immunofluorescence demonstrates immunoglobulin G and complement C3 deposition. All of these findings were seen in our patient (Figure 2, Figure 3).
Membranoproliferative glomerulonephritis in patients with HCV is most commonly associated with cryoglobulins, a mixture of monoclonal or polyclonal immunoglobulin (Ig) M that have antiglobulin (rheumatoid factor) activity and bind to polyclonal IgG. They reversibly precipitate at less than 37°C, (98.6°F), hence their name. Only 50% to 70% of patients with cryoglobulinemic membranoproliferative glomerulonephritis have detectable serum cryoglobulins; however, kidney biopsy may show globular accumulations of eosinophilic material and prominent hypercellularity due to infiltration of glomerular capillaries with mononuclear and polymorphonuclear leukocytes.
Noncryoglobulinemic membranoproliferative glomerulonephritis is also found in patients with HCV infection. Its histologic features are similar, but on biopsy, there is less prominent leukocytic infiltration and no eosinophilic material. Although the pathogenesis of glomerulonephritis in HCV infection is poorly understood, it is thought to result from deposition of circulating immune complexes of HCV, anti-HCV, and rheumatoid factor in the glomeruli.
3. What laboratory finding is often seen in membranoproliferative glomerulonephritis?
- Positive cytoplasmic antineutrophil cytoplasmic antibody
- serum complement Low levels
- Antiphospholipase A2 receptor antibodies
Cytoplasmic antineutrophil cytoplasmic antibody is seen in granulomatosis with polyangiitis, while antiphospholipid A2 receptor antibodies are seen in idiopathic membranous nephritis.
Low serum complement levels are frequently found in membranoproliferative glomerulonephritis. It is believed that immune complex deposition leads to glomerular damage through activation of the complement pathway and the subsequent influx of inflammatory cells, release of cytokines and proteases, and damage to capillary walls. When repair ensues, new mesangial matrix and basement membrane are deposited, leading to mesangial expansion and duplicated basement membrane.14
In cryoglobulinemic membranoproliferative glomerulonephritis, the complement C4 level is often much lower than C3, but in noncryoglobulinemic forms C3 is lower. A mnemonic to remember nephritic syndromes with low complement levels is “hy-PO-CO-MP-L-EM-ents”; PO for postinfectious, CO for cryoglobulins, MP for membranoproliferative glomerulonephritis, L for lupus, and EM for embolic.
BACK TO OUR PATIENT
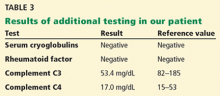
In addition to kidney biopsy, we tested our patient for serum cryoglobulins, rheumatoid factor, and serum complements. Results from these tests (Table 3), in addition to the lack of cryoglobulins on his biopsy, led to the conclusion that he had noncryoglobulinemic membranoproliferative glomerulonephritis.
WHO SHOULD RECEIVE TREATMENT FOR HCV?
4. According to the current IDSA/AASLD guidelines, which of the following patients should not receive direct-acting antiviral therapy for HCV?
- Patients with HCV and only low-stage fibrosis
- Patients with decompensated cirrhosis
- Patients with a glomerular filtration rate less than 30 mL/minute
- None of the above—nearly all patients with HCV infection should receive treatment for it
While certain patients have compelling indications for HCV treatment, such as advanced fibrosis, severe extrahepatic manifestations of HCV (eg, glomerulonephritis, cryoglobulinemia), and posttransplant status, current guidelines recommend treatment for nearly all patients with HCV, including those with low-stage fibrosis.12
Patients with Child-Pugh grade B or C decompensated cirrhosis, even with hepatocellular carcinoma, may be considered for treatment. Multiple studies have demonstrated the efficacy and safety of direct-acting antiviral drugs in this patient population. In one randomized controlled trial,15 the combination of ledipasvir, sofosbuvir, and ribavirin resulted in high sustained virologic response rates at 12 weeks in patients infected with HCV genotype 1 or 4 with advanced liver disease, irrespective of transplant status (86% to 89% of patients were pretransplant). Sustained virologic response was associated with improvements in Model for End-Stage Liver Disease and Child-Pugh scores largely due to decreases in bilirubin and improvement in synthetic function (ie, albumin).
Similarly, even patients with a glomerular filtration rate less than 30 mL/min are candidates for treatment. Those with a glomerular filtration rate above 30 mL/min need no dosage adjustments for the most common regimens, while regimens are also available for those with a rate less than 30 mL/min. Although patients with low baseline renal function have a higher frequency of anemia (especially with ribavirin), worsening renal dysfunction, and more severe adverse events, treatment responses remain high and comparable to those without renal impairment.
The Hepatitis C Therapeutic Registry and Research Network (HCV-TARGET) is conducting an ongoing prospective study evaluating real-world use of direct-acting antiviral agents. The study has reported the safety and efficacy of sofosbuvir-containing regimens in patients with varying severities of kidney disease, including glomerular filtration rates less than 30 mL/min). The patients received different regimens that included sofosbuvir. The regimens were reportedly tolerated, and the rate of sustained viral response at 12 weeks remained high.16
The efficacy of direct-acting antiviral agents for HCV-associated glomerulonephritis remains to be studied but is promising. Earlier studies found that antiviral therapy based on interferon alfa with or without ribavirin can significantly decrease proteinuria and stabilize renal function.17–20 HCV RNA clearance has been found to best predict renal improvement.
OUR PATIENT’S COURSE
Unfortunately, our patient’s kidney function declined further over the next 3 months, and he is currently on dialysis awaiting simultaneous liver and kidney transplant.
- Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009; 361:1279–1290.
- Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am 2009; 93:855–869.
- Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol 2006; 1:1066–1079.
- Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993; 105:229–236.
- Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007; 56:1310–1318.
- Watt K, Uhanova J, Minuk GY. Hepatorenal syndrome: diagnostic accuracy, clinical features, and outcome in a tertiary care center. Am J Gastroenterol 2002; 97:2046–2050.
- Graupera I, Cardenas A. Diagnostic approach to renal failure in cirrhosis. Clin Liver Dis 2013; 2:128–131.
- Dash SC, Bhowmik D. Glomerulopathy with liver disease: patterns and management. Saudi J Kidney Dis Transpl 2000; 11:414–420.
- Arase Y, Ikeda K, Murashima N, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med 1998; 37:836–840.
- McGuire BM, Julian BA, Bynon JS, et al. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med 2006; 144:735–741.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008; 109:S1–S99.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed July 10, 2016.
- Lai KN. Hepatitis-related renal disease. Future Virology 2011; 6:1361–1376.
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 2012; 366:1119–1131.
- Charlton M, Everson GT, Flamm SL, et al; SOLAR-1 Investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149:649–659.
- Saxena V, Koraishy FM, Sise ME, et al; HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int 2016; 36:807–816.
- Feng B, Eknoyan G, Guo ZS, et al. Effect of interferon alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012; 27:640–646.
- Bruchfeld A, Lindahl K, Ståhle L, Söderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003; 18:1573–1580.
- Rossi P, Bertani T, Baio P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis. Long-term remission after antiviral therapy. Kidney Int 2003; 63:2236–2241.
- Alric L, Plaisier E, Thebault S, et al. Influence of antiviral therapy in hepatitis C virus associated cryoglobulinemic MPGN. Am J Kidney Dis 2004; 43:617–623.
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with hepatitis C 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns such as hypertension or diabetes.
Two weeks ago, routine laboratory tests in the clinic showed that his serum creatinine level had increased from baseline. He was asked to stop his diuretics and increase his fluid intake. Nevertheless, his kidney function continued to decline (Table 1), and he was admitted to the hospital for further evaluation.
On admission, he appeared comfortable. He denied recent use of any medications, including nonsteroidal anti-inflammatory drugs, antibiotics, and diuretics, and he had no genitourinary symptoms. His temperature was normal, blood pressure 170/90 mm Hg, pulse rate 72 per minute, and respiratory rate 16. His skin and sclerae were not jaundiced; his abdomen was not tender, but it was grossly distended with ascites. He also had +3 pedal edema (on a scale of 4) extending to both knees. The rest of his physical examination was unremarkable. Results of further laboratory tests are shown in in Table 2.
Ultrasonography of the liver demonstrated cirrhosis with patent flow through the shunt, and ultrasonography of the kidneys showed that both were slightly enlarged with increased cortical echogenicity but no hydronephrosis or obstruction.
EXPLORING THE CAUSE OF RENAL FAILURE
1. Given this information, what is the likely cause of our patient’s renal failure?
- Volume depletion
- Acute tubular necrosis
- Hepatorenal syndrome
- HCV glomerulopathy
Renal failure is a common complication in cirrhosis and portends a higher risk of death.1 The differential diagnosis is broad, but a systematic approach incorporating data from the history, physical examination, and laboratory tests can help identify the cause and is essential in determining the prognosis and proper treatment.
Volume depletion
Volume depletion is a common cause of renal failure in cirrhotic patients. Common precipitants are excessive diuresis and gastrointestinal fluid loss from bleeding, vomiting, and diarrhea. Despite having ascites and edema, patients may have low fluid volume in the vascular space. Therefore, the first step in a patient with acute kidney injury is to withhold diuretics and give fluids. The renal failure usually rapidly reverses if the patient does not have renal parenchymal disease.2
Our patient did not present with any fluid losses, and his high blood pressure and normal heart rate did not suggest volume depletion. And most importantly, withholding his diuretics and giving fluids did not reverse his renal failure. Thus, volume depletion was an unlikely cause.
Acute tubular necrosis
The altered hemodynamics caused by cirrhosis predispose patients to acute tubular necrosis. Classically, this presents as muddy brown casts and renal tubular epithelial cells on urinalysis and as a fractional excretion of sodium greater than 2%.1 However, these microscopic findings lack sensitivity, and patients with cirrhosis may have marked sodium avidity and low urine sodium excretion despite tubular injury.3
This diagnosis must still be considered in patients with renal failure, especially after an insult such as hemorrhagic or septic shock or intake of nephrotoxins. However, because our patient did not have a history of any of these and because his renal failure had been progressing over weeks, acute tubular necrosis was considered unlikely.
Hepatorenal syndrome
Hepatorenal syndrome is characterized by progressive renal failure in the absence of renal parenchymal disease. It is a functional disorder, ie, the decreased glomerular filtration rate results from renal vasoconstriction, which in turn is due to decreased systemic vascular resistance and increased compensatory activity of the renin-angiotensin-aldosterone axis and of antiduretic hormone release (Figure 1).
Hepatorenal syndrome often occurs in patients with advanced liver disease. These patients typically have a hyperdynamic circulation (systemic vasodilation, low blood pressure, and increased blood volume) with a low mean arterial pressure and increased renin and norepinephrine levels. Other frequent findings include hyponatremia, low urinary sodium excretion (< 2 mmol/day), and low free water clearance,4 all of which mark the high systemic levels of antidiuretic hormone and aldosterone.
Importantly, while hepatorenal syndrome is always considered in the differential diagnosis because of its unique prognosis and therapy, it remains a diagnosis of exclusion. The International Ascites Club5 has provided diagnostic criteria for hepatorenal syndrome:
- Cirrhosis and ascites
- Serum creatinine greater than 1.5 mg/dL
- Failure of serum creatinine to fall to less than 1.5 mg/dL after at least 48 hours of diuretic withdrawal and volume expansion with albumin (recommended dose 1 g/kg body weight per day up to a maximum of 100 g per day)
- Absence of shock
- No current or recent treatment with nephrotoxic drugs
- No signs of parenchymal kidney disease such as proteinuria (protein excretion > 500 mg/day), microhematuria (> 50 red blood cells per high-power field), or abnormalities on renal ultrasonography.
While these criteria are not perfect,6 they remind clinicians that there are other important causes of renal insufficiency in cirrhosis.
Clinically, our patient had no evidence of a hyperdynamic circulation and was instead hypertensive. He was eunatremic and did not have marked renal sodium avidity. His pyuria, proteinuria (his protein excretion was approximately 1.9 g/day as determined by urine spot protein-to-creatinine ratio), and results of ultrasonography also suggested underlying renal parenchymal disease. Therefore, hepatorenal syndrome was not the likely diagnosis.
HCV glomerulopathy
Intrinsic renal disease is likely, given our patient’s proteinuria, active urine sediment (ie, containing red blood cells, white blood cells, and protein), and abnormal findings on ultrasonography. In patients with HCV infection and no other cause of intrinsic kidney disease, immune complex deposition leading to glomerulonephritis is the most common pattern.7 Despite the intrinsic renal disease, fractional excretion of sodium may be less than 1% in glomerulonephritis. Hypertension in a patient such as ours with cirrhosis and renal insufficiency raises suspicion for glomerular disease, as hypertension is unlikely in advanced cirrhosis.8
Glomerulonephritis in patients with cirrhosis is often clinically silent and may be highly prevalent; some studies have shown glomerular involvement in 55% to 83% of patients with cirrhosis.9,10 This increases the risk of end-stage renal disease, and the Kidney Disease Improving Global Outcomes guideline recommends that HCV-infected patients be tested at least once a year for proteinuria, hematuria, and estimated glomerular filtration rate to detect possible HCV-associated kidney disease.11 According to current guidelines of the Infectious Diseases Society of America (IDSA) and American Association for the Study of Liver Diseases (AASLD) , detection of glomerulonephritis in HCV patients puts them in the highest priority class for treatment of HCV.12
HISTOLOGIC FINDINGS
Because of the high likelihood of glomerulopathy, our patient underwent renal biopsy.
2. What is the classic pathologic finding in HCV kidney disease?
- Focal segmental glomerulosclerosis
- Crescentic glomerulonephritis
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
A number of pathologic patterns have been described in HCV kidney disease, including membranous glomerulonephritis, immunoglobulin A nephropathy, and focal segmental glomerulosclerosis. However, by far the most common pattern is type 1 membranoproliferative glomerulonephritis.13 (Types 2 and 3 are much less common, and we will not discuss them here.) In type 1, light microscopy shows increased mesangial cells and thickened capillary walls (lobular glomeruli), staining of the basement membrane reveals double contours (“tram tracking”) or splitting due to mesangial deposition, and immunofluorescence demonstrates immunoglobulin G and complement C3 deposition. All of these findings were seen in our patient (Figure 2, Figure 3).
Membranoproliferative glomerulonephritis in patients with HCV is most commonly associated with cryoglobulins, a mixture of monoclonal or polyclonal immunoglobulin (Ig) M that have antiglobulin (rheumatoid factor) activity and bind to polyclonal IgG. They reversibly precipitate at less than 37°C, (98.6°F), hence their name. Only 50% to 70% of patients with cryoglobulinemic membranoproliferative glomerulonephritis have detectable serum cryoglobulins; however, kidney biopsy may show globular accumulations of eosinophilic material and prominent hypercellularity due to infiltration of glomerular capillaries with mononuclear and polymorphonuclear leukocytes.
Noncryoglobulinemic membranoproliferative glomerulonephritis is also found in patients with HCV infection. Its histologic features are similar, but on biopsy, there is less prominent leukocytic infiltration and no eosinophilic material. Although the pathogenesis of glomerulonephritis in HCV infection is poorly understood, it is thought to result from deposition of circulating immune complexes of HCV, anti-HCV, and rheumatoid factor in the glomeruli.
3. What laboratory finding is often seen in membranoproliferative glomerulonephritis?
- Positive cytoplasmic antineutrophil cytoplasmic antibody
- serum complement Low levels
- Antiphospholipase A2 receptor antibodies
Cytoplasmic antineutrophil cytoplasmic antibody is seen in granulomatosis with polyangiitis, while antiphospholipid A2 receptor antibodies are seen in idiopathic membranous nephritis.
Low serum complement levels are frequently found in membranoproliferative glomerulonephritis. It is believed that immune complex deposition leads to glomerular damage through activation of the complement pathway and the subsequent influx of inflammatory cells, release of cytokines and proteases, and damage to capillary walls. When repair ensues, new mesangial matrix and basement membrane are deposited, leading to mesangial expansion and duplicated basement membrane.14
In cryoglobulinemic membranoproliferative glomerulonephritis, the complement C4 level is often much lower than C3, but in noncryoglobulinemic forms C3 is lower. A mnemonic to remember nephritic syndromes with low complement levels is “hy-PO-CO-MP-L-EM-ents”; PO for postinfectious, CO for cryoglobulins, MP for membranoproliferative glomerulonephritis, L for lupus, and EM for embolic.
BACK TO OUR PATIENT
In addition to kidney biopsy, we tested our patient for serum cryoglobulins, rheumatoid factor, and serum complements. Results from these tests (Table 3), in addition to the lack of cryoglobulins on his biopsy, led to the conclusion that he had noncryoglobulinemic membranoproliferative glomerulonephritis.
WHO SHOULD RECEIVE TREATMENT FOR HCV?
4. According to the current IDSA/AASLD guidelines, which of the following patients should not receive direct-acting antiviral therapy for HCV?
- Patients with HCV and only low-stage fibrosis
- Patients with decompensated cirrhosis
- Patients with a glomerular filtration rate less than 30 mL/minute
- None of the above—nearly all patients with HCV infection should receive treatment for it
While certain patients have compelling indications for HCV treatment, such as advanced fibrosis, severe extrahepatic manifestations of HCV (eg, glomerulonephritis, cryoglobulinemia), and posttransplant status, current guidelines recommend treatment for nearly all patients with HCV, including those with low-stage fibrosis.12
Patients with Child-Pugh grade B or C decompensated cirrhosis, even with hepatocellular carcinoma, may be considered for treatment. Multiple studies have demonstrated the efficacy and safety of direct-acting antiviral drugs in this patient population. In one randomized controlled trial,15 the combination of ledipasvir, sofosbuvir, and ribavirin resulted in high sustained virologic response rates at 12 weeks in patients infected with HCV genotype 1 or 4 with advanced liver disease, irrespective of transplant status (86% to 89% of patients were pretransplant). Sustained virologic response was associated with improvements in Model for End-Stage Liver Disease and Child-Pugh scores largely due to decreases in bilirubin and improvement in synthetic function (ie, albumin).
Similarly, even patients with a glomerular filtration rate less than 30 mL/min are candidates for treatment. Those with a glomerular filtration rate above 30 mL/min need no dosage adjustments for the most common regimens, while regimens are also available for those with a rate less than 30 mL/min. Although patients with low baseline renal function have a higher frequency of anemia (especially with ribavirin), worsening renal dysfunction, and more severe adverse events, treatment responses remain high and comparable to those without renal impairment.
The Hepatitis C Therapeutic Registry and Research Network (HCV-TARGET) is conducting an ongoing prospective study evaluating real-world use of direct-acting antiviral agents. The study has reported the safety and efficacy of sofosbuvir-containing regimens in patients with varying severities of kidney disease, including glomerular filtration rates less than 30 mL/min). The patients received different regimens that included sofosbuvir. The regimens were reportedly tolerated, and the rate of sustained viral response at 12 weeks remained high.16
The efficacy of direct-acting antiviral agents for HCV-associated glomerulonephritis remains to be studied but is promising. Earlier studies found that antiviral therapy based on interferon alfa with or without ribavirin can significantly decrease proteinuria and stabilize renal function.17–20 HCV RNA clearance has been found to best predict renal improvement.
OUR PATIENT’S COURSE
Unfortunately, our patient’s kidney function declined further over the next 3 months, and he is currently on dialysis awaiting simultaneous liver and kidney transplant.
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with hepatitis C 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns such as hypertension or diabetes.
Two weeks ago, routine laboratory tests in the clinic showed that his serum creatinine level had increased from baseline. He was asked to stop his diuretics and increase his fluid intake. Nevertheless, his kidney function continued to decline (Table 1), and he was admitted to the hospital for further evaluation.
On admission, he appeared comfortable. He denied recent use of any medications, including nonsteroidal anti-inflammatory drugs, antibiotics, and diuretics, and he had no genitourinary symptoms. His temperature was normal, blood pressure 170/90 mm Hg, pulse rate 72 per minute, and respiratory rate 16. His skin and sclerae were not jaundiced; his abdomen was not tender, but it was grossly distended with ascites. He also had +3 pedal edema (on a scale of 4) extending to both knees. The rest of his physical examination was unremarkable. Results of further laboratory tests are shown in in Table 2.
Ultrasonography of the liver demonstrated cirrhosis with patent flow through the shunt, and ultrasonography of the kidneys showed that both were slightly enlarged with increased cortical echogenicity but no hydronephrosis or obstruction.
EXPLORING THE CAUSE OF RENAL FAILURE
1. Given this information, what is the likely cause of our patient’s renal failure?
- Volume depletion
- Acute tubular necrosis
- Hepatorenal syndrome
- HCV glomerulopathy
Renal failure is a common complication in cirrhosis and portends a higher risk of death.1 The differential diagnosis is broad, but a systematic approach incorporating data from the history, physical examination, and laboratory tests can help identify the cause and is essential in determining the prognosis and proper treatment.
Volume depletion
Volume depletion is a common cause of renal failure in cirrhotic patients. Common precipitants are excessive diuresis and gastrointestinal fluid loss from bleeding, vomiting, and diarrhea. Despite having ascites and edema, patients may have low fluid volume in the vascular space. Therefore, the first step in a patient with acute kidney injury is to withhold diuretics and give fluids. The renal failure usually rapidly reverses if the patient does not have renal parenchymal disease.2
Our patient did not present with any fluid losses, and his high blood pressure and normal heart rate did not suggest volume depletion. And most importantly, withholding his diuretics and giving fluids did not reverse his renal failure. Thus, volume depletion was an unlikely cause.
Acute tubular necrosis
The altered hemodynamics caused by cirrhosis predispose patients to acute tubular necrosis. Classically, this presents as muddy brown casts and renal tubular epithelial cells on urinalysis and as a fractional excretion of sodium greater than 2%.1 However, these microscopic findings lack sensitivity, and patients with cirrhosis may have marked sodium avidity and low urine sodium excretion despite tubular injury.3
This diagnosis must still be considered in patients with renal failure, especially after an insult such as hemorrhagic or septic shock or intake of nephrotoxins. However, because our patient did not have a history of any of these and because his renal failure had been progressing over weeks, acute tubular necrosis was considered unlikely.
Hepatorenal syndrome
Hepatorenal syndrome is characterized by progressive renal failure in the absence of renal parenchymal disease. It is a functional disorder, ie, the decreased glomerular filtration rate results from renal vasoconstriction, which in turn is due to decreased systemic vascular resistance and increased compensatory activity of the renin-angiotensin-aldosterone axis and of antiduretic hormone release (Figure 1).
Hepatorenal syndrome often occurs in patients with advanced liver disease. These patients typically have a hyperdynamic circulation (systemic vasodilation, low blood pressure, and increased blood volume) with a low mean arterial pressure and increased renin and norepinephrine levels. Other frequent findings include hyponatremia, low urinary sodium excretion (< 2 mmol/day), and low free water clearance,4 all of which mark the high systemic levels of antidiuretic hormone and aldosterone.
Importantly, while hepatorenal syndrome is always considered in the differential diagnosis because of its unique prognosis and therapy, it remains a diagnosis of exclusion. The International Ascites Club5 has provided diagnostic criteria for hepatorenal syndrome:
- Cirrhosis and ascites
- Serum creatinine greater than 1.5 mg/dL
- Failure of serum creatinine to fall to less than 1.5 mg/dL after at least 48 hours of diuretic withdrawal and volume expansion with albumin (recommended dose 1 g/kg body weight per day up to a maximum of 100 g per day)
- Absence of shock
- No current or recent treatment with nephrotoxic drugs
- No signs of parenchymal kidney disease such as proteinuria (protein excretion > 500 mg/day), microhematuria (> 50 red blood cells per high-power field), or abnormalities on renal ultrasonography.
While these criteria are not perfect,6 they remind clinicians that there are other important causes of renal insufficiency in cirrhosis.
Clinically, our patient had no evidence of a hyperdynamic circulation and was instead hypertensive. He was eunatremic and did not have marked renal sodium avidity. His pyuria, proteinuria (his protein excretion was approximately 1.9 g/day as determined by urine spot protein-to-creatinine ratio), and results of ultrasonography also suggested underlying renal parenchymal disease. Therefore, hepatorenal syndrome was not the likely diagnosis.
HCV glomerulopathy
Intrinsic renal disease is likely, given our patient’s proteinuria, active urine sediment (ie, containing red blood cells, white blood cells, and protein), and abnormal findings on ultrasonography. In patients with HCV infection and no other cause of intrinsic kidney disease, immune complex deposition leading to glomerulonephritis is the most common pattern.7 Despite the intrinsic renal disease, fractional excretion of sodium may be less than 1% in glomerulonephritis. Hypertension in a patient such as ours with cirrhosis and renal insufficiency raises suspicion for glomerular disease, as hypertension is unlikely in advanced cirrhosis.8
Glomerulonephritis in patients with cirrhosis is often clinically silent and may be highly prevalent; some studies have shown glomerular involvement in 55% to 83% of patients with cirrhosis.9,10 This increases the risk of end-stage renal disease, and the Kidney Disease Improving Global Outcomes guideline recommends that HCV-infected patients be tested at least once a year for proteinuria, hematuria, and estimated glomerular filtration rate to detect possible HCV-associated kidney disease.11 According to current guidelines of the Infectious Diseases Society of America (IDSA) and American Association for the Study of Liver Diseases (AASLD) , detection of glomerulonephritis in HCV patients puts them in the highest priority class for treatment of HCV.12
HISTOLOGIC FINDINGS
Because of the high likelihood of glomerulopathy, our patient underwent renal biopsy.
2. What is the classic pathologic finding in HCV kidney disease?
- Focal segmental glomerulosclerosis
- Crescentic glomerulonephritis
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
A number of pathologic patterns have been described in HCV kidney disease, including membranous glomerulonephritis, immunoglobulin A nephropathy, and focal segmental glomerulosclerosis. However, by far the most common pattern is type 1 membranoproliferative glomerulonephritis.13 (Types 2 and 3 are much less common, and we will not discuss them here.) In type 1, light microscopy shows increased mesangial cells and thickened capillary walls (lobular glomeruli), staining of the basement membrane reveals double contours (“tram tracking”) or splitting due to mesangial deposition, and immunofluorescence demonstrates immunoglobulin G and complement C3 deposition. All of these findings were seen in our patient (Figure 2, Figure 3).
Membranoproliferative glomerulonephritis in patients with HCV is most commonly associated with cryoglobulins, a mixture of monoclonal or polyclonal immunoglobulin (Ig) M that have antiglobulin (rheumatoid factor) activity and bind to polyclonal IgG. They reversibly precipitate at less than 37°C, (98.6°F), hence their name. Only 50% to 70% of patients with cryoglobulinemic membranoproliferative glomerulonephritis have detectable serum cryoglobulins; however, kidney biopsy may show globular accumulations of eosinophilic material and prominent hypercellularity due to infiltration of glomerular capillaries with mononuclear and polymorphonuclear leukocytes.
Noncryoglobulinemic membranoproliferative glomerulonephritis is also found in patients with HCV infection. Its histologic features are similar, but on biopsy, there is less prominent leukocytic infiltration and no eosinophilic material. Although the pathogenesis of glomerulonephritis in HCV infection is poorly understood, it is thought to result from deposition of circulating immune complexes of HCV, anti-HCV, and rheumatoid factor in the glomeruli.
3. What laboratory finding is often seen in membranoproliferative glomerulonephritis?
- Positive cytoplasmic antineutrophil cytoplasmic antibody
- serum complement Low levels
- Antiphospholipase A2 receptor antibodies
Cytoplasmic antineutrophil cytoplasmic antibody is seen in granulomatosis with polyangiitis, while antiphospholipid A2 receptor antibodies are seen in idiopathic membranous nephritis.
Low serum complement levels are frequently found in membranoproliferative glomerulonephritis. It is believed that immune complex deposition leads to glomerular damage through activation of the complement pathway and the subsequent influx of inflammatory cells, release of cytokines and proteases, and damage to capillary walls. When repair ensues, new mesangial matrix and basement membrane are deposited, leading to mesangial expansion and duplicated basement membrane.14
In cryoglobulinemic membranoproliferative glomerulonephritis, the complement C4 level is often much lower than C3, but in noncryoglobulinemic forms C3 is lower. A mnemonic to remember nephritic syndromes with low complement levels is “hy-PO-CO-MP-L-EM-ents”; PO for postinfectious, CO for cryoglobulins, MP for membranoproliferative glomerulonephritis, L for lupus, and EM for embolic.
BACK TO OUR PATIENT
In addition to kidney biopsy, we tested our patient for serum cryoglobulins, rheumatoid factor, and serum complements. Results from these tests (Table 3), in addition to the lack of cryoglobulins on his biopsy, led to the conclusion that he had noncryoglobulinemic membranoproliferative glomerulonephritis.
WHO SHOULD RECEIVE TREATMENT FOR HCV?
4. According to the current IDSA/AASLD guidelines, which of the following patients should not receive direct-acting antiviral therapy for HCV?
- Patients with HCV and only low-stage fibrosis
- Patients with decompensated cirrhosis
- Patients with a glomerular filtration rate less than 30 mL/minute
- None of the above—nearly all patients with HCV infection should receive treatment for it
While certain patients have compelling indications for HCV treatment, such as advanced fibrosis, severe extrahepatic manifestations of HCV (eg, glomerulonephritis, cryoglobulinemia), and posttransplant status, current guidelines recommend treatment for nearly all patients with HCV, including those with low-stage fibrosis.12
Patients with Child-Pugh grade B or C decompensated cirrhosis, even with hepatocellular carcinoma, may be considered for treatment. Multiple studies have demonstrated the efficacy and safety of direct-acting antiviral drugs in this patient population. In one randomized controlled trial,15 the combination of ledipasvir, sofosbuvir, and ribavirin resulted in high sustained virologic response rates at 12 weeks in patients infected with HCV genotype 1 or 4 with advanced liver disease, irrespective of transplant status (86% to 89% of patients were pretransplant). Sustained virologic response was associated with improvements in Model for End-Stage Liver Disease and Child-Pugh scores largely due to decreases in bilirubin and improvement in synthetic function (ie, albumin).
Similarly, even patients with a glomerular filtration rate less than 30 mL/min are candidates for treatment. Those with a glomerular filtration rate above 30 mL/min need no dosage adjustments for the most common regimens, while regimens are also available for those with a rate less than 30 mL/min. Although patients with low baseline renal function have a higher frequency of anemia (especially with ribavirin), worsening renal dysfunction, and more severe adverse events, treatment responses remain high and comparable to those without renal impairment.
The Hepatitis C Therapeutic Registry and Research Network (HCV-TARGET) is conducting an ongoing prospective study evaluating real-world use of direct-acting antiviral agents. The study has reported the safety and efficacy of sofosbuvir-containing regimens in patients with varying severities of kidney disease, including glomerular filtration rates less than 30 mL/min). The patients received different regimens that included sofosbuvir. The regimens were reportedly tolerated, and the rate of sustained viral response at 12 weeks remained high.16
The efficacy of direct-acting antiviral agents for HCV-associated glomerulonephritis remains to be studied but is promising. Earlier studies found that antiviral therapy based on interferon alfa with or without ribavirin can significantly decrease proteinuria and stabilize renal function.17–20 HCV RNA clearance has been found to best predict renal improvement.
OUR PATIENT’S COURSE
Unfortunately, our patient’s kidney function declined further over the next 3 months, and he is currently on dialysis awaiting simultaneous liver and kidney transplant.
- Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009; 361:1279–1290.
- Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am 2009; 93:855–869.
- Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol 2006; 1:1066–1079.
- Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993; 105:229–236.
- Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007; 56:1310–1318.
- Watt K, Uhanova J, Minuk GY. Hepatorenal syndrome: diagnostic accuracy, clinical features, and outcome in a tertiary care center. Am J Gastroenterol 2002; 97:2046–2050.
- Graupera I, Cardenas A. Diagnostic approach to renal failure in cirrhosis. Clin Liver Dis 2013; 2:128–131.
- Dash SC, Bhowmik D. Glomerulopathy with liver disease: patterns and management. Saudi J Kidney Dis Transpl 2000; 11:414–420.
- Arase Y, Ikeda K, Murashima N, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med 1998; 37:836–840.
- McGuire BM, Julian BA, Bynon JS, et al. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med 2006; 144:735–741.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008; 109:S1–S99.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed July 10, 2016.
- Lai KN. Hepatitis-related renal disease. Future Virology 2011; 6:1361–1376.
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 2012; 366:1119–1131.
- Charlton M, Everson GT, Flamm SL, et al; SOLAR-1 Investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149:649–659.
- Saxena V, Koraishy FM, Sise ME, et al; HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int 2016; 36:807–816.
- Feng B, Eknoyan G, Guo ZS, et al. Effect of interferon alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012; 27:640–646.
- Bruchfeld A, Lindahl K, Ståhle L, Söderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003; 18:1573–1580.
- Rossi P, Bertani T, Baio P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis. Long-term remission after antiviral therapy. Kidney Int 2003; 63:2236–2241.
- Alric L, Plaisier E, Thebault S, et al. Influence of antiviral therapy in hepatitis C virus associated cryoglobulinemic MPGN. Am J Kidney Dis 2004; 43:617–623.
- Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009; 361:1279–1290.
- Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am 2009; 93:855–869.
- Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol 2006; 1:1066–1079.
- Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993; 105:229–236.
- Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007; 56:1310–1318.
- Watt K, Uhanova J, Minuk GY. Hepatorenal syndrome: diagnostic accuracy, clinical features, and outcome in a tertiary care center. Am J Gastroenterol 2002; 97:2046–2050.
- Graupera I, Cardenas A. Diagnostic approach to renal failure in cirrhosis. Clin Liver Dis 2013; 2:128–131.
- Dash SC, Bhowmik D. Glomerulopathy with liver disease: patterns and management. Saudi J Kidney Dis Transpl 2000; 11:414–420.
- Arase Y, Ikeda K, Murashima N, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med 1998; 37:836–840.
- McGuire BM, Julian BA, Bynon JS, et al. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med 2006; 144:735–741.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008; 109:S1–S99.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed July 10, 2016.
- Lai KN. Hepatitis-related renal disease. Future Virology 2011; 6:1361–1376.
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 2012; 366:1119–1131.
- Charlton M, Everson GT, Flamm SL, et al; SOLAR-1 Investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149:649–659.
- Saxena V, Koraishy FM, Sise ME, et al; HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int 2016; 36:807–816.
- Feng B, Eknoyan G, Guo ZS, et al. Effect of interferon alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012; 27:640–646.
- Bruchfeld A, Lindahl K, Ståhle L, Söderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003; 18:1573–1580.
- Rossi P, Bertani T, Baio P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis. Long-term remission after antiviral therapy. Kidney Int 2003; 63:2236–2241.
- Alric L, Plaisier E, Thebault S, et al. Influence of antiviral therapy in hepatitis C virus associated cryoglobulinemic MPGN. Am J Kidney Dis 2004; 43:617–623.
Newer Insulin Glargine Formula Curbs Nocturnal Hypoglycemia
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Newer insulin glargine formula curbs nocturnal hypoglycemia
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Insulin glargine 300 U/mL provided glycemic control comparable to insulin glargine 100 U/mL, but it reduced the risk of nocturnal hypoglycemia by a greater margin, regardless of renal function.
Major finding: The risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group.
Data source: A post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies.
Disclosures: The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
Blood pressure targets
To the Editor: I read with great interest the article by Thomas et al, “Interpreting SPRINT: How low should you go?”1
Hypertension is the most prevalent modifiable risk factor, affecting almost one in every three people in the United States.2 Moreover, only half of people with hypertension have their blood pressure under control to the current standard of lower than 140/90 mm Hg.2 The Systolic Blood Pressure Intervention Trial (SPRINT) tested a lower goal systolic pressure, ie, less than 120 mm Hg, and found it more beneficial than the standard goal of less than 140 mm Hg.3
A drawback of SPRINT that Thomas et al did not address in their interpretation of the trial is that the two study groups were not homogeneous in terms of the antihypertensive drugs used. Antihypertensive drugs do not only lower blood pressure—some of them have additional pleiotropic effects, making their use more advantageous in special situations. For example, renin-angiotensin-aldosterone system (RAAS) blockers—ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoid receptor antagonists—are disease-modfying drugs in heart failure, as are certain beta-blockers.4 The cardiovascular benefit seen in the intensive-treatment group in SPRINT compared with the standard-therapy group was primarily due to a reduction in heart failure (a 38% relative risk reduction, P = .0002),3 for which RAAS blockers and beta-adrenergic blocking drugs have been shown consistently to be beneficial. But the intensive- and standard-therapy groups were not homogeneous in terms of the use of RAAS blockers and beta-blockers.
So, was the cardiovascular benefit attained in the intensive-treatment group in SPRINT due to the benefit of lower blood pressure or to the drugs used?
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013 Oct;(133):1–8.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
To the Editor: I read with great interest the article by Thomas et al, “Interpreting SPRINT: How low should you go?”1
Hypertension is the most prevalent modifiable risk factor, affecting almost one in every three people in the United States.2 Moreover, only half of people with hypertension have their blood pressure under control to the current standard of lower than 140/90 mm Hg.2 The Systolic Blood Pressure Intervention Trial (SPRINT) tested a lower goal systolic pressure, ie, less than 120 mm Hg, and found it more beneficial than the standard goal of less than 140 mm Hg.3
A drawback of SPRINT that Thomas et al did not address in their interpretation of the trial is that the two study groups were not homogeneous in terms of the antihypertensive drugs used. Antihypertensive drugs do not only lower blood pressure—some of them have additional pleiotropic effects, making their use more advantageous in special situations. For example, renin-angiotensin-aldosterone system (RAAS) blockers—ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoid receptor antagonists—are disease-modfying drugs in heart failure, as are certain beta-blockers.4 The cardiovascular benefit seen in the intensive-treatment group in SPRINT compared with the standard-therapy group was primarily due to a reduction in heart failure (a 38% relative risk reduction, P = .0002),3 for which RAAS blockers and beta-adrenergic blocking drugs have been shown consistently to be beneficial. But the intensive- and standard-therapy groups were not homogeneous in terms of the use of RAAS blockers and beta-blockers.
So, was the cardiovascular benefit attained in the intensive-treatment group in SPRINT due to the benefit of lower blood pressure or to the drugs used?
To the Editor: I read with great interest the article by Thomas et al, “Interpreting SPRINT: How low should you go?”1
Hypertension is the most prevalent modifiable risk factor, affecting almost one in every three people in the United States.2 Moreover, only half of people with hypertension have their blood pressure under control to the current standard of lower than 140/90 mm Hg.2 The Systolic Blood Pressure Intervention Trial (SPRINT) tested a lower goal systolic pressure, ie, less than 120 mm Hg, and found it more beneficial than the standard goal of less than 140 mm Hg.3
A drawback of SPRINT that Thomas et al did not address in their interpretation of the trial is that the two study groups were not homogeneous in terms of the antihypertensive drugs used. Antihypertensive drugs do not only lower blood pressure—some of them have additional pleiotropic effects, making their use more advantageous in special situations. For example, renin-angiotensin-aldosterone system (RAAS) blockers—ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoid receptor antagonists—are disease-modfying drugs in heart failure, as are certain beta-blockers.4 The cardiovascular benefit seen in the intensive-treatment group in SPRINT compared with the standard-therapy group was primarily due to a reduction in heart failure (a 38% relative risk reduction, P = .0002),3 for which RAAS blockers and beta-adrenergic blocking drugs have been shown consistently to be beneficial. But the intensive- and standard-therapy groups were not homogeneous in terms of the use of RAAS blockers and beta-blockers.
So, was the cardiovascular benefit attained in the intensive-treatment group in SPRINT due to the benefit of lower blood pressure or to the drugs used?
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013 Oct;(133):1–8.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013 Oct;(133):1–8.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
Blood pressure targets
To the Editor: In their review,1 Thomas et al noted that the benefits of intensive blood pressure lowering seen in the SPRINT study2 were not observed in the Action to Control Cardiovascular Risk in Diabetes-Blood pressure (ACCORD BP) trial3 or in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial.4 In addition to the reasons discussed in their review, the discrepancy may be due to the surprisingly low rate of statin use in the patients enrolled in SPRINT. Even though 61% of the patients in SPRINT had a 10-year Framingham risk score greater than 15%, only 44% of the patients were on statin therapy. In comparison, rates of statin use in ACCORD BP and SPS3 were 65% and 83%, respectively.
A possible interaction between statin use and intensive blood pressure lowering is consistent with previous data on angiotensin-converting enzyme (ACE) inhibitor use in high-risk populations.
The Heart Outcomes Prevention Evaluation (HOPE) trial,5 in which only 29% of patients received lipid-lowering therapy, found that ACE inhibitor use was associated with a significant reduction in a composite cardiovascular outcome, whereas the Prevention of Events With Angiotensin-Converting Enzyme Inhibitor Therapy (PEACE) trial,6 in which 70% of patients were on lipid-lowering therapy, did not show a benefit for ACE inhibitor therapy. In addition, there are many drug interactions between statins and calcium channel blockers, potentially limiting options for simultaneous aggressive treatment of lipid levels and blood pressure.
In summary, aggressive use of statins may confer sufficient cardiovascular protection when aggressive antihypertensive therapy provides little or no incremental benefit. Hopefully, further analyses of these trials will shed light on this important question.
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- ACCORD Study Group; Cushma WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342:145–153.
- The PEACE Trial Investigators. Angiotensin-converting–enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351:2058–2068.
To the Editor: In their review,1 Thomas et al noted that the benefits of intensive blood pressure lowering seen in the SPRINT study2 were not observed in the Action to Control Cardiovascular Risk in Diabetes-Blood pressure (ACCORD BP) trial3 or in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial.4 In addition to the reasons discussed in their review, the discrepancy may be due to the surprisingly low rate of statin use in the patients enrolled in SPRINT. Even though 61% of the patients in SPRINT had a 10-year Framingham risk score greater than 15%, only 44% of the patients were on statin therapy. In comparison, rates of statin use in ACCORD BP and SPS3 were 65% and 83%, respectively.
A possible interaction between statin use and intensive blood pressure lowering is consistent with previous data on angiotensin-converting enzyme (ACE) inhibitor use in high-risk populations.
The Heart Outcomes Prevention Evaluation (HOPE) trial,5 in which only 29% of patients received lipid-lowering therapy, found that ACE inhibitor use was associated with a significant reduction in a composite cardiovascular outcome, whereas the Prevention of Events With Angiotensin-Converting Enzyme Inhibitor Therapy (PEACE) trial,6 in which 70% of patients were on lipid-lowering therapy, did not show a benefit for ACE inhibitor therapy. In addition, there are many drug interactions between statins and calcium channel blockers, potentially limiting options for simultaneous aggressive treatment of lipid levels and blood pressure.
In summary, aggressive use of statins may confer sufficient cardiovascular protection when aggressive antihypertensive therapy provides little or no incremental benefit. Hopefully, further analyses of these trials will shed light on this important question.
To the Editor: In their review,1 Thomas et al noted that the benefits of intensive blood pressure lowering seen in the SPRINT study2 were not observed in the Action to Control Cardiovascular Risk in Diabetes-Blood pressure (ACCORD BP) trial3 or in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial.4 In addition to the reasons discussed in their review, the discrepancy may be due to the surprisingly low rate of statin use in the patients enrolled in SPRINT. Even though 61% of the patients in SPRINT had a 10-year Framingham risk score greater than 15%, only 44% of the patients were on statin therapy. In comparison, rates of statin use in ACCORD BP and SPS3 were 65% and 83%, respectively.
A possible interaction between statin use and intensive blood pressure lowering is consistent with previous data on angiotensin-converting enzyme (ACE) inhibitor use in high-risk populations.
The Heart Outcomes Prevention Evaluation (HOPE) trial,5 in which only 29% of patients received lipid-lowering therapy, found that ACE inhibitor use was associated with a significant reduction in a composite cardiovascular outcome, whereas the Prevention of Events With Angiotensin-Converting Enzyme Inhibitor Therapy (PEACE) trial,6 in which 70% of patients were on lipid-lowering therapy, did not show a benefit for ACE inhibitor therapy. In addition, there are many drug interactions between statins and calcium channel blockers, potentially limiting options for simultaneous aggressive treatment of lipid levels and blood pressure.
In summary, aggressive use of statins may confer sufficient cardiovascular protection when aggressive antihypertensive therapy provides little or no incremental benefit. Hopefully, further analyses of these trials will shed light on this important question.
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- ACCORD Study Group; Cushma WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342:145–153.
- The PEACE Trial Investigators. Angiotensin-converting–enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351:2058–2068.
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- ACCORD Study Group; Cushma WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342:145–153.
- The PEACE Trial Investigators. Angiotensin-converting–enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351:2058–2068.
In reply: Blood pressure targets
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
European ANCA-associated vasculitis guidance gets first makeover since 2009
LONDON – Updated management recommendations for patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis from the European League Against Rheumatism and the European Renal Association-European Dialysis and Transplant Association aim to provide clinicians with reliable guidance on the best approach to treatment.
The update, presented at the European Congress of Rheumatology and recently published online in Annals of the Rheumatic Diseases (Ann Rheum Dis. 2016 Jun 23. doi:10.1136/annrheumdis-2016-209133), reassessed items in the 2009 recommendations for the management of primary systemic vasculitis and focused only on the management of ANCA-associated vasculitis (AAV), according to recommendations task force member Dr. Max Yates.
“In the past 5 years, 1,691 papers have been published on primary systemic vasculitis in internal medicine, rheumatology, and nephrology journals. Together with the licensing of rituximab for AAV, it was an opportune time to update the recommendations with an AAV focus,” Dr. Yates explained. The revised guidance is based on a systematic literature review from January 2007 to February 2015, focusing in particular on specific items that needed updating, such as the importance of ANCA testing and biopsy in diagnosis and follow-up, disease staging at diagnosis, the choices for remission-induction and remission-maintenance therapies, and the drug choices for relapsing and refractory disease. The task force considered for the first time the choice of immunosuppressive drugs and biologic agents (principally rituximab) and immunologic monitoring. They identified patient education as another priority.
“These updated recommendations provide a framework of practice and should apply to the majority of patients with AAV,” added Dr. Yates, who is a clinical fellow at Norwich Medical School at the University of East Anglia and works in the department of rheumatology at the Norfolk and Norwich (England) University Hospital.
The 22-member task force included rheumatologists, internists, nephrologists, a clinical immunologist, an otorhinolaryngologist, a chest physician, an ophthalmologist, a vasculitis nurse, and a patient with vasculitis from 11 countries in Europe and the United States. The task force was convened by rheumatologist Dr. Chetan Mukhtyar of the Norfolk and Norwich University Hospital on behalf of EULAR and by vasculitis and renal specialist Dr. David Jayne of Addenbrooke’s Hospital in Cambridge (England) on behalf of the European Renal Association-European Dialysis and Transplant Association.
The recommendations now contain one single, simple overarching principle, Dr. Mukhtyar said at the congress. That is, the need for shared decision making between the patient and the clinician. This principle is also included as the first point in many of the other recently updated EULAR recommendations on the management of rheumatic diseases.
Both previous and updated versions of the vasculitis recommendations contain 15 recommendations, with some changed and others combined. One key recommendation is about who should treat patients with AAV; it states that patients “should be managed in close collaboration with, or at, centers of expertise,” Dr. Mukhtyar said.“Patients with ANCA-associated vasculitis have often very complex presentations that involve several different specialties, and it is always worthwhile that these patients are looked after by people who commonly see them, because these are rare conditions,” he observed.
Deciding when to perform a biopsy is also covered, with the recommendation being that it can be used to establish a new diagnosis and to further evaluate cases of suspected relapsing vasculitis. “When do you do a biopsy?” Dr. Mukhtyar asked. “Well, every time you can, every time it is clinically feasible,” he suggested.
As for treatment, there are different recommendations depending on whether the aim is to induce or maintain remission and whether there has been a major relapse. In patients with organ- or life-threatening disease, for example, the advice is to use glucocorticoids and either cyclophosphamide or rituximab to induce remission, Dr. Mukhtyar said. The specific dosing or administration of glucocorticoids is not specified as this will depend on the clinical situation, but the advice is to taper down when possible, somewhere between month 3 and 5.
For remission induction in less severe (non–organ threatening) disease, the recommendation is to use glucocorticoids plus either methotrexate or mycophenolate mofetil. Situations when methotrexate or mycophenolate mofetil should and should not be used are specified, notably when cyclophosphamide or rituximab are not available or are contraindicated.
For maintenance of remission, the task force advised using low-dose glucocorticoids plus azathioprine, rituximab, methotrexate, or mycophenolate mofetil.
Guidance on when to use plasma exchange is given for patients with severe disease and options following failure of remission-induction therapy, and when to switch therapy is also covered.
There are also several follow-up recommendations, such as the periodic assessment of cardiovascular risk, and patient-focused recommendations on awareness of the nature, benefits, and risks of therapy.
The recommendations should provide clinicians with reliable guidance on the best approach to treating AAV, according to Dr. Yates. “From the patients’ point of view, these recommendations should provide useful insight into which treatments they are likely to be offered and when. They also emphasize that as a patient, you should have a voice in your treatment and if you have any questions or concerns, be sure to speak with your specialist.”
Dr. Yates and Dr. Mukhtyar did not report having any relevant disclosures.
The prior 2009 EULAR recommendations were very much in need of updating given the plethora of studies in the past 7 years addressing ANCA-associated vasculitis (AAV). The emergence of rituximab as an effective therapy in AAV had to be considered and included in these newer guidelines. Its potential role in both remission induction, as well as remission maintenance of AAV, is addressed.
The recommendations are somewhat complicated, particularly as eosinophilic granulomatosis with polyangiitis (EGPA, previously referred to as Churg-Strauss syndrome) has been included, but most of the well-done prospective clinical trials addressing remission induction and remission maintenance in AAV were limited to patients with granulomatosis with polyangiitis or microscopic polyangiitis and did not include patients with EGPA. The role of plasma exchange is also discussed, but the results of the PEXIVAS trial, which will address that more definitively, are not yet forthcoming. Those results are anticipated in the not too distant future and will much better define that component of management in those most severely ill patients with AAV.

|
| Dr. Robert Spiera |
These recommendations serve as a framework for helping clinicians understand what is widely accepted as standard of care for these diseases but in no way can define individual treatment decisions as the authors acknowledge. Such decisions must become very personalized in relation to details of the patient’s individual comorbidities and other features of their medical and even socioeconomic status. For example, when choosing between rituximab and cyclophosphamide for remission induction in a young woman (or man, for that matter), future fertility concerns (which cyclophosphamide could potentially compromise) are very relevant. Moreover, the costs of rituximab are substantial, and the lack of superiority of rituximab over cyclophosphamide in many situations, particularly in patients with new severe disease, could be an important factor to consider when choosing which immunosuppressive will be used.
Many of the unanswered questions await results of ongoing or upcoming trials, including some addressing the relative efficacy of various remission maintenance regimens (rituximab vs. azathioprine) or the role of plasmapheresis. Many questions in AAV are not easily addressable in clinical trials, such as whether there are some groups of patients in whom remission maintenance therapy should never be withdrawn. However, such questions may be addressed through observational studies of the well-defined patient cohorts and registries that have been developed in the United States and Europe.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, N.Y. He is also professor of clinical medicine at Cornell University, N.Y. He has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
The prior 2009 EULAR recommendations were very much in need of updating given the plethora of studies in the past 7 years addressing ANCA-associated vasculitis (AAV). The emergence of rituximab as an effective therapy in AAV had to be considered and included in these newer guidelines. Its potential role in both remission induction, as well as remission maintenance of AAV, is addressed.
The recommendations are somewhat complicated, particularly as eosinophilic granulomatosis with polyangiitis (EGPA, previously referred to as Churg-Strauss syndrome) has been included, but most of the well-done prospective clinical trials addressing remission induction and remission maintenance in AAV were limited to patients with granulomatosis with polyangiitis or microscopic polyangiitis and did not include patients with EGPA. The role of plasma exchange is also discussed, but the results of the PEXIVAS trial, which will address that more definitively, are not yet forthcoming. Those results are anticipated in the not too distant future and will much better define that component of management in those most severely ill patients with AAV.

|
| Dr. Robert Spiera |
These recommendations serve as a framework for helping clinicians understand what is widely accepted as standard of care for these diseases but in no way can define individual treatment decisions as the authors acknowledge. Such decisions must become very personalized in relation to details of the patient’s individual comorbidities and other features of their medical and even socioeconomic status. For example, when choosing between rituximab and cyclophosphamide for remission induction in a young woman (or man, for that matter), future fertility concerns (which cyclophosphamide could potentially compromise) are very relevant. Moreover, the costs of rituximab are substantial, and the lack of superiority of rituximab over cyclophosphamide in many situations, particularly in patients with new severe disease, could be an important factor to consider when choosing which immunosuppressive will be used.
Many of the unanswered questions await results of ongoing or upcoming trials, including some addressing the relative efficacy of various remission maintenance regimens (rituximab vs. azathioprine) or the role of plasmapheresis. Many questions in AAV are not easily addressable in clinical trials, such as whether there are some groups of patients in whom remission maintenance therapy should never be withdrawn. However, such questions may be addressed through observational studies of the well-defined patient cohorts and registries that have been developed in the United States and Europe.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, N.Y. He is also professor of clinical medicine at Cornell University, N.Y. He has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
The prior 2009 EULAR recommendations were very much in need of updating given the plethora of studies in the past 7 years addressing ANCA-associated vasculitis (AAV). The emergence of rituximab as an effective therapy in AAV had to be considered and included in these newer guidelines. Its potential role in both remission induction, as well as remission maintenance of AAV, is addressed.
The recommendations are somewhat complicated, particularly as eosinophilic granulomatosis with polyangiitis (EGPA, previously referred to as Churg-Strauss syndrome) has been included, but most of the well-done prospective clinical trials addressing remission induction and remission maintenance in AAV were limited to patients with granulomatosis with polyangiitis or microscopic polyangiitis and did not include patients with EGPA. The role of plasma exchange is also discussed, but the results of the PEXIVAS trial, which will address that more definitively, are not yet forthcoming. Those results are anticipated in the not too distant future and will much better define that component of management in those most severely ill patients with AAV.

|
| Dr. Robert Spiera |
These recommendations serve as a framework for helping clinicians understand what is widely accepted as standard of care for these diseases but in no way can define individual treatment decisions as the authors acknowledge. Such decisions must become very personalized in relation to details of the patient’s individual comorbidities and other features of their medical and even socioeconomic status. For example, when choosing between rituximab and cyclophosphamide for remission induction in a young woman (or man, for that matter), future fertility concerns (which cyclophosphamide could potentially compromise) are very relevant. Moreover, the costs of rituximab are substantial, and the lack of superiority of rituximab over cyclophosphamide in many situations, particularly in patients with new severe disease, could be an important factor to consider when choosing which immunosuppressive will be used.
Many of the unanswered questions await results of ongoing or upcoming trials, including some addressing the relative efficacy of various remission maintenance regimens (rituximab vs. azathioprine) or the role of plasmapheresis. Many questions in AAV are not easily addressable in clinical trials, such as whether there are some groups of patients in whom remission maintenance therapy should never be withdrawn. However, such questions may be addressed through observational studies of the well-defined patient cohorts and registries that have been developed in the United States and Europe.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, N.Y. He is also professor of clinical medicine at Cornell University, N.Y. He has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
LONDON – Updated management recommendations for patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis from the European League Against Rheumatism and the European Renal Association-European Dialysis and Transplant Association aim to provide clinicians with reliable guidance on the best approach to treatment.
The update, presented at the European Congress of Rheumatology and recently published online in Annals of the Rheumatic Diseases (Ann Rheum Dis. 2016 Jun 23. doi:10.1136/annrheumdis-2016-209133), reassessed items in the 2009 recommendations for the management of primary systemic vasculitis and focused only on the management of ANCA-associated vasculitis (AAV), according to recommendations task force member Dr. Max Yates.
“In the past 5 years, 1,691 papers have been published on primary systemic vasculitis in internal medicine, rheumatology, and nephrology journals. Together with the licensing of rituximab for AAV, it was an opportune time to update the recommendations with an AAV focus,” Dr. Yates explained. The revised guidance is based on a systematic literature review from January 2007 to February 2015, focusing in particular on specific items that needed updating, such as the importance of ANCA testing and biopsy in diagnosis and follow-up, disease staging at diagnosis, the choices for remission-induction and remission-maintenance therapies, and the drug choices for relapsing and refractory disease. The task force considered for the first time the choice of immunosuppressive drugs and biologic agents (principally rituximab) and immunologic monitoring. They identified patient education as another priority.
“These updated recommendations provide a framework of practice and should apply to the majority of patients with AAV,” added Dr. Yates, who is a clinical fellow at Norwich Medical School at the University of East Anglia and works in the department of rheumatology at the Norfolk and Norwich (England) University Hospital.
The 22-member task force included rheumatologists, internists, nephrologists, a clinical immunologist, an otorhinolaryngologist, a chest physician, an ophthalmologist, a vasculitis nurse, and a patient with vasculitis from 11 countries in Europe and the United States. The task force was convened by rheumatologist Dr. Chetan Mukhtyar of the Norfolk and Norwich University Hospital on behalf of EULAR and by vasculitis and renal specialist Dr. David Jayne of Addenbrooke’s Hospital in Cambridge (England) on behalf of the European Renal Association-European Dialysis and Transplant Association.
The recommendations now contain one single, simple overarching principle, Dr. Mukhtyar said at the congress. That is, the need for shared decision making between the patient and the clinician. This principle is also included as the first point in many of the other recently updated EULAR recommendations on the management of rheumatic diseases.
Both previous and updated versions of the vasculitis recommendations contain 15 recommendations, with some changed and others combined. One key recommendation is about who should treat patients with AAV; it states that patients “should be managed in close collaboration with, or at, centers of expertise,” Dr. Mukhtyar said.“Patients with ANCA-associated vasculitis have often very complex presentations that involve several different specialties, and it is always worthwhile that these patients are looked after by people who commonly see them, because these are rare conditions,” he observed.
Deciding when to perform a biopsy is also covered, with the recommendation being that it can be used to establish a new diagnosis and to further evaluate cases of suspected relapsing vasculitis. “When do you do a biopsy?” Dr. Mukhtyar asked. “Well, every time you can, every time it is clinically feasible,” he suggested.
As for treatment, there are different recommendations depending on whether the aim is to induce or maintain remission and whether there has been a major relapse. In patients with organ- or life-threatening disease, for example, the advice is to use glucocorticoids and either cyclophosphamide or rituximab to induce remission, Dr. Mukhtyar said. The specific dosing or administration of glucocorticoids is not specified as this will depend on the clinical situation, but the advice is to taper down when possible, somewhere between month 3 and 5.
For remission induction in less severe (non–organ threatening) disease, the recommendation is to use glucocorticoids plus either methotrexate or mycophenolate mofetil. Situations when methotrexate or mycophenolate mofetil should and should not be used are specified, notably when cyclophosphamide or rituximab are not available or are contraindicated.
For maintenance of remission, the task force advised using low-dose glucocorticoids plus azathioprine, rituximab, methotrexate, or mycophenolate mofetil.
Guidance on when to use plasma exchange is given for patients with severe disease and options following failure of remission-induction therapy, and when to switch therapy is also covered.
There are also several follow-up recommendations, such as the periodic assessment of cardiovascular risk, and patient-focused recommendations on awareness of the nature, benefits, and risks of therapy.
The recommendations should provide clinicians with reliable guidance on the best approach to treating AAV, according to Dr. Yates. “From the patients’ point of view, these recommendations should provide useful insight into which treatments they are likely to be offered and when. They also emphasize that as a patient, you should have a voice in your treatment and if you have any questions or concerns, be sure to speak with your specialist.”
Dr. Yates and Dr. Mukhtyar did not report having any relevant disclosures.
LONDON – Updated management recommendations for patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis from the European League Against Rheumatism and the European Renal Association-European Dialysis and Transplant Association aim to provide clinicians with reliable guidance on the best approach to treatment.
The update, presented at the European Congress of Rheumatology and recently published online in Annals of the Rheumatic Diseases (Ann Rheum Dis. 2016 Jun 23. doi:10.1136/annrheumdis-2016-209133), reassessed items in the 2009 recommendations for the management of primary systemic vasculitis and focused only on the management of ANCA-associated vasculitis (AAV), according to recommendations task force member Dr. Max Yates.
“In the past 5 years, 1,691 papers have been published on primary systemic vasculitis in internal medicine, rheumatology, and nephrology journals. Together with the licensing of rituximab for AAV, it was an opportune time to update the recommendations with an AAV focus,” Dr. Yates explained. The revised guidance is based on a systematic literature review from January 2007 to February 2015, focusing in particular on specific items that needed updating, such as the importance of ANCA testing and biopsy in diagnosis and follow-up, disease staging at diagnosis, the choices for remission-induction and remission-maintenance therapies, and the drug choices for relapsing and refractory disease. The task force considered for the first time the choice of immunosuppressive drugs and biologic agents (principally rituximab) and immunologic monitoring. They identified patient education as another priority.
“These updated recommendations provide a framework of practice and should apply to the majority of patients with AAV,” added Dr. Yates, who is a clinical fellow at Norwich Medical School at the University of East Anglia and works in the department of rheumatology at the Norfolk and Norwich (England) University Hospital.
The 22-member task force included rheumatologists, internists, nephrologists, a clinical immunologist, an otorhinolaryngologist, a chest physician, an ophthalmologist, a vasculitis nurse, and a patient with vasculitis from 11 countries in Europe and the United States. The task force was convened by rheumatologist Dr. Chetan Mukhtyar of the Norfolk and Norwich University Hospital on behalf of EULAR and by vasculitis and renal specialist Dr. David Jayne of Addenbrooke’s Hospital in Cambridge (England) on behalf of the European Renal Association-European Dialysis and Transplant Association.
The recommendations now contain one single, simple overarching principle, Dr. Mukhtyar said at the congress. That is, the need for shared decision making between the patient and the clinician. This principle is also included as the first point in many of the other recently updated EULAR recommendations on the management of rheumatic diseases.
Both previous and updated versions of the vasculitis recommendations contain 15 recommendations, with some changed and others combined. One key recommendation is about who should treat patients with AAV; it states that patients “should be managed in close collaboration with, or at, centers of expertise,” Dr. Mukhtyar said.“Patients with ANCA-associated vasculitis have often very complex presentations that involve several different specialties, and it is always worthwhile that these patients are looked after by people who commonly see them, because these are rare conditions,” he observed.
Deciding when to perform a biopsy is also covered, with the recommendation being that it can be used to establish a new diagnosis and to further evaluate cases of suspected relapsing vasculitis. “When do you do a biopsy?” Dr. Mukhtyar asked. “Well, every time you can, every time it is clinically feasible,” he suggested.
As for treatment, there are different recommendations depending on whether the aim is to induce or maintain remission and whether there has been a major relapse. In patients with organ- or life-threatening disease, for example, the advice is to use glucocorticoids and either cyclophosphamide or rituximab to induce remission, Dr. Mukhtyar said. The specific dosing or administration of glucocorticoids is not specified as this will depend on the clinical situation, but the advice is to taper down when possible, somewhere between month 3 and 5.
For remission induction in less severe (non–organ threatening) disease, the recommendation is to use glucocorticoids plus either methotrexate or mycophenolate mofetil. Situations when methotrexate or mycophenolate mofetil should and should not be used are specified, notably when cyclophosphamide or rituximab are not available or are contraindicated.
For maintenance of remission, the task force advised using low-dose glucocorticoids plus azathioprine, rituximab, methotrexate, or mycophenolate mofetil.
Guidance on when to use plasma exchange is given for patients with severe disease and options following failure of remission-induction therapy, and when to switch therapy is also covered.
There are also several follow-up recommendations, such as the periodic assessment of cardiovascular risk, and patient-focused recommendations on awareness of the nature, benefits, and risks of therapy.
The recommendations should provide clinicians with reliable guidance on the best approach to treating AAV, according to Dr. Yates. “From the patients’ point of view, these recommendations should provide useful insight into which treatments they are likely to be offered and when. They also emphasize that as a patient, you should have a voice in your treatment and if you have any questions or concerns, be sure to speak with your specialist.”
Dr. Yates and Dr. Mukhtyar did not report having any relevant disclosures.
AT THE EULAR 2016 CONGRESS
Expanding Treatment Options
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Nutrition Guidelines for CKD
Q) I see patients with diabetes, hypertension, chronic kidney disease (CKD), obesity ... often all within the same patient! I keep hearing that the DASH diet is best for these patients. Is this true? Do you have any suggestions (or handouts) for teaching good eating habits in a 15-minute office visit?
It is always nice to focus on what patients can do, rather than what they can’t. Patients with diabetes, kidney disease, heart disease, and obesity hear a lot of “can’t” messages, making “can” messages particularly important to emphasize.
Healthy diets for diabetes, heart, and kidney patients include foods low in trans and saturated fats and sodium. Not all CKD patients are required to follow a low-potassium diet; dietary restrictions are based on laboratory values, medications, and other factors. As we know, adding an ACE inhibitor or an angiotensin receptor blocker (ARB) to the treatment regimen can cause an elevation in serum potassium.
For adults with CKD, it is recommended that sodium intake be restricted to < 2,000 mg/d.4 And in this population, salt substitutes are not recommended, since they often contain large amounts of potassium chloride, which increases risk for hyperkalemia.5 Other spices (eg, garlic, pepper, lemon) are better substitutes for salt.
The late Paul Prudhomme, an award-winning chef from New Orleans, struggled with obesity and health issues for years. He developed wonderful, kidney-friendly spices free of salt and potassium. His line of spices, Magic Seasoning Blends, is sold in many grocery stores. You can recommend them without worry.
Studies have shown that the usual Western diet (which features an abundance of processed foods, fats, and sugars) contributes to kidney disease.6 The DASH (Dietary Approaches to Stop Hypertension) diet, developed by cardio experts, replaces these foods with healthier alternatives.
Recent research has shown that the DASH diet does, in fact, slow the progression of kidney disease.7 It also lowers blood pressure and decreases kidney stone formation, which are risk factors for kidney disease.
So, the DASH diet is protective for your patients (from both a kidney and a cardiac standpoint)—but how do you explain this in a 15-minute office visit?
Here are a few quick tips:
• Increase fruit and vegetable intake to include all colors on your plate (and no, tan is not really a color)
• If you eat meat, the cooking method should start with “B” (ie, bake, boil, broil, barbeque [without salty sauce]) ... Note that “fried” does not start with “B”!
• Use a smaller plate and you will not eat as much
• Use technology in your favor. There are great apps and downloads you can recommend (see Table). —CC
Christine Corbett, MSN, APRN, FNP-BC, CNN-NP
Kansas City Veterans Affairs, Kansas City, Missouri
References
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;(3):1-150.
5. National Kidney Disease Education Program. Potassium: tips for people with chronic kidney disease (CKD). www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/nutrition-potassium/Documents/nutrition-potassium-508.pdf. Accessed June 20, 2016.
6. Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol. 2011;301(5):F919-F931.
7. Steiber A. DASH-style diet effective in preventing, delaying CKD progression. Renal and Urology News. 2012.
Q) I see patients with diabetes, hypertension, chronic kidney disease (CKD), obesity ... often all within the same patient! I keep hearing that the DASH diet is best for these patients. Is this true? Do you have any suggestions (or handouts) for teaching good eating habits in a 15-minute office visit?
It is always nice to focus on what patients can do, rather than what they can’t. Patients with diabetes, kidney disease, heart disease, and obesity hear a lot of “can’t” messages, making “can” messages particularly important to emphasize.
Healthy diets for diabetes, heart, and kidney patients include foods low in trans and saturated fats and sodium. Not all CKD patients are required to follow a low-potassium diet; dietary restrictions are based on laboratory values, medications, and other factors. As we know, adding an ACE inhibitor or an angiotensin receptor blocker (ARB) to the treatment regimen can cause an elevation in serum potassium.
For adults with CKD, it is recommended that sodium intake be restricted to < 2,000 mg/d.4 And in this population, salt substitutes are not recommended, since they often contain large amounts of potassium chloride, which increases risk for hyperkalemia.5 Other spices (eg, garlic, pepper, lemon) are better substitutes for salt.
The late Paul Prudhomme, an award-winning chef from New Orleans, struggled with obesity and health issues for years. He developed wonderful, kidney-friendly spices free of salt and potassium. His line of spices, Magic Seasoning Blends, is sold in many grocery stores. You can recommend them without worry.
Studies have shown that the usual Western diet (which features an abundance of processed foods, fats, and sugars) contributes to kidney disease.6 The DASH (Dietary Approaches to Stop Hypertension) diet, developed by cardio experts, replaces these foods with healthier alternatives.
Recent research has shown that the DASH diet does, in fact, slow the progression of kidney disease.7 It also lowers blood pressure and decreases kidney stone formation, which are risk factors for kidney disease.
So, the DASH diet is protective for your patients (from both a kidney and a cardiac standpoint)—but how do you explain this in a 15-minute office visit?
Here are a few quick tips:
• Increase fruit and vegetable intake to include all colors on your plate (and no, tan is not really a color)
• If you eat meat, the cooking method should start with “B” (ie, bake, boil, broil, barbeque [without salty sauce]) ... Note that “fried” does not start with “B”!
• Use a smaller plate and you will not eat as much
• Use technology in your favor. There are great apps and downloads you can recommend (see Table). —CC
Christine Corbett, MSN, APRN, FNP-BC, CNN-NP
Kansas City Veterans Affairs, Kansas City, Missouri
References
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;(3):1-150.
5. National Kidney Disease Education Program. Potassium: tips for people with chronic kidney disease (CKD). www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/nutrition-potassium/Documents/nutrition-potassium-508.pdf. Accessed June 20, 2016.
6. Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol. 2011;301(5):F919-F931.
7. Steiber A. DASH-style diet effective in preventing, delaying CKD progression. Renal and Urology News. 2012.
Q) I see patients with diabetes, hypertension, chronic kidney disease (CKD), obesity ... often all within the same patient! I keep hearing that the DASH diet is best for these patients. Is this true? Do you have any suggestions (or handouts) for teaching good eating habits in a 15-minute office visit?
It is always nice to focus on what patients can do, rather than what they can’t. Patients with diabetes, kidney disease, heart disease, and obesity hear a lot of “can’t” messages, making “can” messages particularly important to emphasize.
Healthy diets for diabetes, heart, and kidney patients include foods low in trans and saturated fats and sodium. Not all CKD patients are required to follow a low-potassium diet; dietary restrictions are based on laboratory values, medications, and other factors. As we know, adding an ACE inhibitor or an angiotensin receptor blocker (ARB) to the treatment regimen can cause an elevation in serum potassium.
For adults with CKD, it is recommended that sodium intake be restricted to < 2,000 mg/d.4 And in this population, salt substitutes are not recommended, since they often contain large amounts of potassium chloride, which increases risk for hyperkalemia.5 Other spices (eg, garlic, pepper, lemon) are better substitutes for salt.
The late Paul Prudhomme, an award-winning chef from New Orleans, struggled with obesity and health issues for years. He developed wonderful, kidney-friendly spices free of salt and potassium. His line of spices, Magic Seasoning Blends, is sold in many grocery stores. You can recommend them without worry.
Studies have shown that the usual Western diet (which features an abundance of processed foods, fats, and sugars) contributes to kidney disease.6 The DASH (Dietary Approaches to Stop Hypertension) diet, developed by cardio experts, replaces these foods with healthier alternatives.
Recent research has shown that the DASH diet does, in fact, slow the progression of kidney disease.7 It also lowers blood pressure and decreases kidney stone formation, which are risk factors for kidney disease.
So, the DASH diet is protective for your patients (from both a kidney and a cardiac standpoint)—but how do you explain this in a 15-minute office visit?
Here are a few quick tips:
• Increase fruit and vegetable intake to include all colors on your plate (and no, tan is not really a color)
• If you eat meat, the cooking method should start with “B” (ie, bake, boil, broil, barbeque [without salty sauce]) ... Note that “fried” does not start with “B”!
• Use a smaller plate and you will not eat as much
• Use technology in your favor. There are great apps and downloads you can recommend (see Table). —CC
Christine Corbett, MSN, APRN, FNP-BC, CNN-NP
Kansas City Veterans Affairs, Kansas City, Missouri
References
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;(3):1-150.
5. National Kidney Disease Education Program. Potassium: tips for people with chronic kidney disease (CKD). www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/nutrition-potassium/Documents/nutrition-potassium-508.pdf. Accessed June 20, 2016.
6. Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol. 2011;301(5):F919-F931.
7. Steiber A. DASH-style diet effective in preventing, delaying CKD progression. Renal and Urology News. 2012.
FDA Strengthens Kidney Warnings on Two Diabetes Drugs
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”