User login
Perioperative statins for cardiac surgery didn’t reduce kidney injury
SAN DIEGO – High-dose perioperative atorvastatin treatment did not reduce acute kidney injury following elective cardiac surgery, and it may increase risk in patients with chronic kidney disease (CKD) who are naive to statin treatment, results from a large, randomized trial showed.
“Despite advances in patient management that have reduced mortality during cardiac surgery, acute kidney injury continues to complicate the postoperative course in 20%-30% of patients,” Dr. Frederic Tremaine Billings said during a press briefing at a meeting sponsored by the American Society of Nephrology.
“Its diagnosis is independently associated with a fivefold increase in mortality following the surgery,” Dr. Tremaine added. “Statins affect several mechanisms underlying postoperative acute kidney injury. Widely prescribed to reduce cholesterol synthesis, these drugs also reduce lipid modification of intracellular signaling molecules, which have been shown to improve perfusion and reduce oxidative stress – both mechanisms important in acute kidney injury following cardiac surgery.”
Dr. Billings of the department of anesthesiology and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., and his associates tested the hypothesis that short-term, high-dose perioperative (preoperative, intraoperative, and postoperative) atorvastatin reduces acute kidney injury (AKI) following elective cardiac surgery.
The researchers randomly assigned preoperative statin-naive patients to 80 mg of atorvastatin on the morning before surgery, 40 mg on the morning of surgery, and 40 mg daily throughout hospitalization, or to a matching placebo regimen. In addition, they randomly assigned patients who were already using statins prior to surgery to 80 mg of atorvastatin the morning of surgery, and 40 mg on the morning after surgery, or to a matching placebo regimen.
“We felt it was important to not withhold statin treatment in patients already using statins prior to surgery, beyond what is typically done in clinical practice,” Dr. Billings explained. “For this reason, preoperative statin–using subjects continued their statin up until the day before surgery, and then resumed their statin use on postoperative day 2.”
The primary endpoint of the study was the incidence of AKI as determined by Acute Kidney Injury Network criteria (a 0.3 mg/dL increase in serum creatinine concentrations within 48 hours of surgery). Secondary endpoints included the maximum creatinine increase from baseline to 48 hours after surgery, ICU delirium diagnosed by the Confusion Assessment Method for the ICU, myocardial injury, and the incidence of atrial fibrillation, pneumonia, and stroke. Safety endpoints included liver toxicity, muscle toxicity, and adverse events.
The study was limited to adults having elective cardiac surgery and excluded those with statin intolerance, acute coronary syndrome, liver dysfunction, use of CYP3A4 inhibitors, kidney transplant recipients, those currently on dialysis, and those who were pregnant.
From November 2009 to October 2014, the researchers recruited 653 patients. But the trial was halted on recommendation of Vanderbilt’s data and safety monitoring board because of futility and an increased incidence of AKI among statin-naive patients with CKD randomized to atorvastatin.
Among all patients, AKI occurred in 20.8% of those randomized to atorvastatin, compared with 19.5% of those randomized to placebo, a difference that was neither clinically nor statistically significant (P = .75), Dr. Billings reported.
However, among the 199 patients who were statin naive, AKI occurred in 21.6% of those randomized to atorvastatin, compared with 13.4% of those randomized to placebo (P = .14). “An 8% difference in the incidence of AKI is of clinical importance, if true,” he said.
Among the 36 statin-naive patients with CKD, AKI occurred in 52.9% of those randomized to atorvastatin, compared with 15.8% of those randomized to placebo (P = .03). “While the number of patients in this subgroup is small, the magnitude of effect is striking,” Dr. Billings said.
Among the 416 patients who were using statins prior to surgery, AKI occurred in 20.4% of those randomized to atorvastatin, compared with 22.4% of those randomized to placebo (P = .63). Results were similar among the subset of those patients who had CKD (31.3% vs. 36.3%; P = .59).
Safety endpoints were similar between the two groups.
Strengths of the study, Dr. Billings said, include the fact that it’s the largest randomized, controlled trial to date to test this hypothesis, the pragmatic design of the protocol, and rigorous methodology.
Limitations include the “small number of patients in the statin-naive CKD subgroup,” he noted. “And the short duration of treatment among prestudy statin-using patients could limit the observation that short-term withdrawal is not harmful – although we felt it appropriate not to limit statins beyond what’s typical in clinical practice, based on prior reports that even short-term statin withdrawal may be harmful.”
The National Institutes of Health and the department of anesthesiology at Vanderbilt University supported the study. Dr. Billings reported having no financial disclosures.
SAN DIEGO – High-dose perioperative atorvastatin treatment did not reduce acute kidney injury following elective cardiac surgery, and it may increase risk in patients with chronic kidney disease (CKD) who are naive to statin treatment, results from a large, randomized trial showed.
“Despite advances in patient management that have reduced mortality during cardiac surgery, acute kidney injury continues to complicate the postoperative course in 20%-30% of patients,” Dr. Frederic Tremaine Billings said during a press briefing at a meeting sponsored by the American Society of Nephrology.
“Its diagnosis is independently associated with a fivefold increase in mortality following the surgery,” Dr. Tremaine added. “Statins affect several mechanisms underlying postoperative acute kidney injury. Widely prescribed to reduce cholesterol synthesis, these drugs also reduce lipid modification of intracellular signaling molecules, which have been shown to improve perfusion and reduce oxidative stress – both mechanisms important in acute kidney injury following cardiac surgery.”
Dr. Billings of the department of anesthesiology and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., and his associates tested the hypothesis that short-term, high-dose perioperative (preoperative, intraoperative, and postoperative) atorvastatin reduces acute kidney injury (AKI) following elective cardiac surgery.
The researchers randomly assigned preoperative statin-naive patients to 80 mg of atorvastatin on the morning before surgery, 40 mg on the morning of surgery, and 40 mg daily throughout hospitalization, or to a matching placebo regimen. In addition, they randomly assigned patients who were already using statins prior to surgery to 80 mg of atorvastatin the morning of surgery, and 40 mg on the morning after surgery, or to a matching placebo regimen.
“We felt it was important to not withhold statin treatment in patients already using statins prior to surgery, beyond what is typically done in clinical practice,” Dr. Billings explained. “For this reason, preoperative statin–using subjects continued their statin up until the day before surgery, and then resumed their statin use on postoperative day 2.”
The primary endpoint of the study was the incidence of AKI as determined by Acute Kidney Injury Network criteria (a 0.3 mg/dL increase in serum creatinine concentrations within 48 hours of surgery). Secondary endpoints included the maximum creatinine increase from baseline to 48 hours after surgery, ICU delirium diagnosed by the Confusion Assessment Method for the ICU, myocardial injury, and the incidence of atrial fibrillation, pneumonia, and stroke. Safety endpoints included liver toxicity, muscle toxicity, and adverse events.
The study was limited to adults having elective cardiac surgery and excluded those with statin intolerance, acute coronary syndrome, liver dysfunction, use of CYP3A4 inhibitors, kidney transplant recipients, those currently on dialysis, and those who were pregnant.
From November 2009 to October 2014, the researchers recruited 653 patients. But the trial was halted on recommendation of Vanderbilt’s data and safety monitoring board because of futility and an increased incidence of AKI among statin-naive patients with CKD randomized to atorvastatin.
Among all patients, AKI occurred in 20.8% of those randomized to atorvastatin, compared with 19.5% of those randomized to placebo, a difference that was neither clinically nor statistically significant (P = .75), Dr. Billings reported.
However, among the 199 patients who were statin naive, AKI occurred in 21.6% of those randomized to atorvastatin, compared with 13.4% of those randomized to placebo (P = .14). “An 8% difference in the incidence of AKI is of clinical importance, if true,” he said.
Among the 36 statin-naive patients with CKD, AKI occurred in 52.9% of those randomized to atorvastatin, compared with 15.8% of those randomized to placebo (P = .03). “While the number of patients in this subgroup is small, the magnitude of effect is striking,” Dr. Billings said.
Among the 416 patients who were using statins prior to surgery, AKI occurred in 20.4% of those randomized to atorvastatin, compared with 22.4% of those randomized to placebo (P = .63). Results were similar among the subset of those patients who had CKD (31.3% vs. 36.3%; P = .59).
Safety endpoints were similar between the two groups.
Strengths of the study, Dr. Billings said, include the fact that it’s the largest randomized, controlled trial to date to test this hypothesis, the pragmatic design of the protocol, and rigorous methodology.
Limitations include the “small number of patients in the statin-naive CKD subgroup,” he noted. “And the short duration of treatment among prestudy statin-using patients could limit the observation that short-term withdrawal is not harmful – although we felt it appropriate not to limit statins beyond what’s typical in clinical practice, based on prior reports that even short-term statin withdrawal may be harmful.”
The National Institutes of Health and the department of anesthesiology at Vanderbilt University supported the study. Dr. Billings reported having no financial disclosures.
SAN DIEGO – High-dose perioperative atorvastatin treatment did not reduce acute kidney injury following elective cardiac surgery, and it may increase risk in patients with chronic kidney disease (CKD) who are naive to statin treatment, results from a large, randomized trial showed.
“Despite advances in patient management that have reduced mortality during cardiac surgery, acute kidney injury continues to complicate the postoperative course in 20%-30% of patients,” Dr. Frederic Tremaine Billings said during a press briefing at a meeting sponsored by the American Society of Nephrology.
“Its diagnosis is independently associated with a fivefold increase in mortality following the surgery,” Dr. Tremaine added. “Statins affect several mechanisms underlying postoperative acute kidney injury. Widely prescribed to reduce cholesterol synthesis, these drugs also reduce lipid modification of intracellular signaling molecules, which have been shown to improve perfusion and reduce oxidative stress – both mechanisms important in acute kidney injury following cardiac surgery.”
Dr. Billings of the department of anesthesiology and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., and his associates tested the hypothesis that short-term, high-dose perioperative (preoperative, intraoperative, and postoperative) atorvastatin reduces acute kidney injury (AKI) following elective cardiac surgery.
The researchers randomly assigned preoperative statin-naive patients to 80 mg of atorvastatin on the morning before surgery, 40 mg on the morning of surgery, and 40 mg daily throughout hospitalization, or to a matching placebo regimen. In addition, they randomly assigned patients who were already using statins prior to surgery to 80 mg of atorvastatin the morning of surgery, and 40 mg on the morning after surgery, or to a matching placebo regimen.
“We felt it was important to not withhold statin treatment in patients already using statins prior to surgery, beyond what is typically done in clinical practice,” Dr. Billings explained. “For this reason, preoperative statin–using subjects continued their statin up until the day before surgery, and then resumed their statin use on postoperative day 2.”
The primary endpoint of the study was the incidence of AKI as determined by Acute Kidney Injury Network criteria (a 0.3 mg/dL increase in serum creatinine concentrations within 48 hours of surgery). Secondary endpoints included the maximum creatinine increase from baseline to 48 hours after surgery, ICU delirium diagnosed by the Confusion Assessment Method for the ICU, myocardial injury, and the incidence of atrial fibrillation, pneumonia, and stroke. Safety endpoints included liver toxicity, muscle toxicity, and adverse events.
The study was limited to adults having elective cardiac surgery and excluded those with statin intolerance, acute coronary syndrome, liver dysfunction, use of CYP3A4 inhibitors, kidney transplant recipients, those currently on dialysis, and those who were pregnant.
From November 2009 to October 2014, the researchers recruited 653 patients. But the trial was halted on recommendation of Vanderbilt’s data and safety monitoring board because of futility and an increased incidence of AKI among statin-naive patients with CKD randomized to atorvastatin.
Among all patients, AKI occurred in 20.8% of those randomized to atorvastatin, compared with 19.5% of those randomized to placebo, a difference that was neither clinically nor statistically significant (P = .75), Dr. Billings reported.
However, among the 199 patients who were statin naive, AKI occurred in 21.6% of those randomized to atorvastatin, compared with 13.4% of those randomized to placebo (P = .14). “An 8% difference in the incidence of AKI is of clinical importance, if true,” he said.
Among the 36 statin-naive patients with CKD, AKI occurred in 52.9% of those randomized to atorvastatin, compared with 15.8% of those randomized to placebo (P = .03). “While the number of patients in this subgroup is small, the magnitude of effect is striking,” Dr. Billings said.
Among the 416 patients who were using statins prior to surgery, AKI occurred in 20.4% of those randomized to atorvastatin, compared with 22.4% of those randomized to placebo (P = .63). Results were similar among the subset of those patients who had CKD (31.3% vs. 36.3%; P = .59).
Safety endpoints were similar between the two groups.
Strengths of the study, Dr. Billings said, include the fact that it’s the largest randomized, controlled trial to date to test this hypothesis, the pragmatic design of the protocol, and rigorous methodology.
Limitations include the “small number of patients in the statin-naive CKD subgroup,” he noted. “And the short duration of treatment among prestudy statin-using patients could limit the observation that short-term withdrawal is not harmful – although we felt it appropriate not to limit statins beyond what’s typical in clinical practice, based on prior reports that even short-term statin withdrawal may be harmful.”
The National Institutes of Health and the department of anesthesiology at Vanderbilt University supported the study. Dr. Billings reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: The use of high-dose perioperative atorvastatin did not reduce acute kidney injury in patients undergoing elective cardiac surgery.
Major finding: Among all patients, acute kidney injury occurred in 20.8% of those randomized to atorvastatin, compared with 19.5% of those randomized to placebo, a difference that is neither clinically nor statistically significant (P = .75).
Data source: A randomized, controlled trial of 653 patients to test the hypothesis that short-term, high-dose perioperative atorvastatin reduces acute kidney injury following elective cardiac surgery.
Disclosures: The National Institutes of Health and the department of anesthesiology at Vanderbilt University supported the study. Dr. Billings reported having no financial disclosures.
Hormone Treatment Associated With Better Kidney Function
SAN DIEGO – The use of hormone therapy was associated with a lower urine albumin-to-creatinine ratio and a decreased risk of albuminuria, results from a cross-sectional study suggest.
“This may be useful information for providers taking care of menopausal women who are considering the use of hormone therapy for vasomotor symptoms and are worried about the systemic effects of these medications,” lead study author Dr. Andrea G. Kattah said in an interview after the annual meeting of the American Society of Nephrology. “Though our data only show an association and not cause and effect, hormone therapy is associated with better kidney function in this study.”
Results from many animal studies suggest that estrogen can have beneficial effects on the kidneys, noted Dr. Kattah, who conducted the research with Dr. Vesna D. Garovic and colleagues in the division of hypertension and nephrology at the Mayo Clinic, Rochester, Minn.
“In addition, in human studies of chronic kidney disease, premenopausal women tend to have slower progression of kidney disease than men,” she said. “Studies on the effects of hormone therapy on kidney function in women have had variable results. We wanted to look at the association of hormone therapy and renal function in a large, multiethnic cohort with well-defined health conditions that may confound the relationship between hormone therapy and renal disease.”
Study participants included 2,217 women enrolled in the Family Blood Pressure Program, a multinetwork effort to study the genetics of hypertension. During a study visit between 2000 and 2004, the women completed questionnaires about medical history, menopausal status, and use of hormone therapy (HT) in the past month. Clinicians also took their blood pressure, measured their body mass index, and drew blood to determine levels of serum creatinine and urine albumin-to-creatinine ratio (UACR).
Of the 2,217 women, 673 were on HT and 1,544 were not, and their mean ages were 60 years and 63 years, respectively.
In unadjusted analysis, Dr. Kattah and her associates found that UACR was significantly lower in those on HT, compared with those who were not (3.5 mg/g creatinine vs. 5.2 mg/g creatinine, respectively, P less than .001), as was the number of women with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2 (7% vs. 10%, P = .003).
After adjusting for renal and cardiovascular risk factors including age, race, smoking, diabetes, hypertension, and family history of hypertension, the use of HT was still significantly associated with a lower UACR and decreased risk of microalbuminuria (odds ratio, 0.61). The association between HT and eGFR of less than 60 mL/min per 1.73 m2 was no longer significant after adjustment, but there was a trend toward higher eGFR and fewer women with an eGFR of less than 60 mL/min per 1.73 m2 among those on HT.
“Not surprisingly, the women taking hormone therapy were different than those who were not, and they generally had fewer health problems, such as diabetes and hyperlipidemia,” Dr. Kattah said. “However, after taking these differences into account in our models, we still found a significant decrease in the risk of having microalbuminuria in those on hormone therapy.”
Dr. Kattah acknowledged certain limitations of the study, including the fact that its design is “cross-sectional and cannot answer the question of whether or not hormone therapy can improve kidney function.
In addition, “we do not have data on how long women were taking hormone therapy, which other studies have suggested is an important factor.”
The researchers reported having no financial disclosures.
SAN DIEGO – The use of hormone therapy was associated with a lower urine albumin-to-creatinine ratio and a decreased risk of albuminuria, results from a cross-sectional study suggest.
“This may be useful information for providers taking care of menopausal women who are considering the use of hormone therapy for vasomotor symptoms and are worried about the systemic effects of these medications,” lead study author Dr. Andrea G. Kattah said in an interview after the annual meeting of the American Society of Nephrology. “Though our data only show an association and not cause and effect, hormone therapy is associated with better kidney function in this study.”
Results from many animal studies suggest that estrogen can have beneficial effects on the kidneys, noted Dr. Kattah, who conducted the research with Dr. Vesna D. Garovic and colleagues in the division of hypertension and nephrology at the Mayo Clinic, Rochester, Minn.
“In addition, in human studies of chronic kidney disease, premenopausal women tend to have slower progression of kidney disease than men,” she said. “Studies on the effects of hormone therapy on kidney function in women have had variable results. We wanted to look at the association of hormone therapy and renal function in a large, multiethnic cohort with well-defined health conditions that may confound the relationship between hormone therapy and renal disease.”
Study participants included 2,217 women enrolled in the Family Blood Pressure Program, a multinetwork effort to study the genetics of hypertension. During a study visit between 2000 and 2004, the women completed questionnaires about medical history, menopausal status, and use of hormone therapy (HT) in the past month. Clinicians also took their blood pressure, measured their body mass index, and drew blood to determine levels of serum creatinine and urine albumin-to-creatinine ratio (UACR).
Of the 2,217 women, 673 were on HT and 1,544 were not, and their mean ages were 60 years and 63 years, respectively.
In unadjusted analysis, Dr. Kattah and her associates found that UACR was significantly lower in those on HT, compared with those who were not (3.5 mg/g creatinine vs. 5.2 mg/g creatinine, respectively, P less than .001), as was the number of women with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2 (7% vs. 10%, P = .003).
After adjusting for renal and cardiovascular risk factors including age, race, smoking, diabetes, hypertension, and family history of hypertension, the use of HT was still significantly associated with a lower UACR and decreased risk of microalbuminuria (odds ratio, 0.61). The association between HT and eGFR of less than 60 mL/min per 1.73 m2 was no longer significant after adjustment, but there was a trend toward higher eGFR and fewer women with an eGFR of less than 60 mL/min per 1.73 m2 among those on HT.
“Not surprisingly, the women taking hormone therapy were different than those who were not, and they generally had fewer health problems, such as diabetes and hyperlipidemia,” Dr. Kattah said. “However, after taking these differences into account in our models, we still found a significant decrease in the risk of having microalbuminuria in those on hormone therapy.”
Dr. Kattah acknowledged certain limitations of the study, including the fact that its design is “cross-sectional and cannot answer the question of whether or not hormone therapy can improve kidney function.
In addition, “we do not have data on how long women were taking hormone therapy, which other studies have suggested is an important factor.”
The researchers reported having no financial disclosures.
SAN DIEGO – The use of hormone therapy was associated with a lower urine albumin-to-creatinine ratio and a decreased risk of albuminuria, results from a cross-sectional study suggest.
“This may be useful information for providers taking care of menopausal women who are considering the use of hormone therapy for vasomotor symptoms and are worried about the systemic effects of these medications,” lead study author Dr. Andrea G. Kattah said in an interview after the annual meeting of the American Society of Nephrology. “Though our data only show an association and not cause and effect, hormone therapy is associated with better kidney function in this study.”
Results from many animal studies suggest that estrogen can have beneficial effects on the kidneys, noted Dr. Kattah, who conducted the research with Dr. Vesna D. Garovic and colleagues in the division of hypertension and nephrology at the Mayo Clinic, Rochester, Minn.
“In addition, in human studies of chronic kidney disease, premenopausal women tend to have slower progression of kidney disease than men,” she said. “Studies on the effects of hormone therapy on kidney function in women have had variable results. We wanted to look at the association of hormone therapy and renal function in a large, multiethnic cohort with well-defined health conditions that may confound the relationship between hormone therapy and renal disease.”
Study participants included 2,217 women enrolled in the Family Blood Pressure Program, a multinetwork effort to study the genetics of hypertension. During a study visit between 2000 and 2004, the women completed questionnaires about medical history, menopausal status, and use of hormone therapy (HT) in the past month. Clinicians also took their blood pressure, measured their body mass index, and drew blood to determine levels of serum creatinine and urine albumin-to-creatinine ratio (UACR).
Of the 2,217 women, 673 were on HT and 1,544 were not, and their mean ages were 60 years and 63 years, respectively.
In unadjusted analysis, Dr. Kattah and her associates found that UACR was significantly lower in those on HT, compared with those who were not (3.5 mg/g creatinine vs. 5.2 mg/g creatinine, respectively, P less than .001), as was the number of women with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2 (7% vs. 10%, P = .003).
After adjusting for renal and cardiovascular risk factors including age, race, smoking, diabetes, hypertension, and family history of hypertension, the use of HT was still significantly associated with a lower UACR and decreased risk of microalbuminuria (odds ratio, 0.61). The association between HT and eGFR of less than 60 mL/min per 1.73 m2 was no longer significant after adjustment, but there was a trend toward higher eGFR and fewer women with an eGFR of less than 60 mL/min per 1.73 m2 among those on HT.
“Not surprisingly, the women taking hormone therapy were different than those who were not, and they generally had fewer health problems, such as diabetes and hyperlipidemia,” Dr. Kattah said. “However, after taking these differences into account in our models, we still found a significant decrease in the risk of having microalbuminuria in those on hormone therapy.”
Dr. Kattah acknowledged certain limitations of the study, including the fact that its design is “cross-sectional and cannot answer the question of whether or not hormone therapy can improve kidney function.
In addition, “we do not have data on how long women were taking hormone therapy, which other studies have suggested is an important factor.”
The researchers reported having no financial disclosures.
AT KIDNEY WEEK 2015
Hormone treatment associated with better kidney function
SAN DIEGO – The use of hormone therapy was associated with a lower urine albumin-to-creatinine ratio and a decreased risk of albuminuria, results from a cross-sectional study suggest.
“This may be useful information for providers taking care of menopausal women who are considering the use of hormone therapy for vasomotor symptoms and are worried about the systemic effects of these medications,” lead study author Dr. Andrea G. Kattah said in an interview after the annual meeting of the American Society of Nephrology. “Though our data only show an association and not cause and effect, hormone therapy is associated with better kidney function in this study.”
Results from many animal studies suggest that estrogen can have beneficial effects on the kidneys, noted Dr. Kattah, who conducted the research with Dr. Vesna D. Garovic and colleagues in the division of hypertension and nephrology at the Mayo Clinic, Rochester, Minn.
“In addition, in human studies of chronic kidney disease, premenopausal women tend to have slower progression of kidney disease than men,” she said. “Studies on the effects of hormone therapy on kidney function in women have had variable results. We wanted to look at the association of hormone therapy and renal function in a large, multiethnic cohort with well-defined health conditions that may confound the relationship between hormone therapy and renal disease.”
Study participants included 2,217 women enrolled in the Family Blood Pressure Program, a multinetwork effort to study the genetics of hypertension. During a study visit between 2000 and 2004, the women completed questionnaires about medical history, menopausal status, and use of hormone therapy (HT) in the past month. Clinicians also took their blood pressure, measured their body mass index, and drew blood to determine levels of serum creatinine and urine albumin-to-creatinine ratio (UACR).
Of the 2,217 women, 673 were on HT and 1,544 were not, and their mean ages were 60 years and 63 years, respectively.
In unadjusted analysis, Dr. Kattah and her associates found that UACR was significantly lower in those on HT, compared with those who were not (3.5 mg/g creatinine vs. 5.2 mg/g creatinine, respectively, P less than .001), as was the number of women with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2 (7% vs. 10%, P = .003).
After adjusting for renal and cardiovascular risk factors including age, race, smoking, diabetes, hypertension, and family history of hypertension, the use of HT was still significantly associated with a lower UACR and decreased risk of microalbuminuria (odds ratio, 0.61). The association between HT and eGFR of less than 60 mL/min per 1.73 m2 was no longer significant after adjustment, but there was a trend toward higher eGFR and fewer women with an eGFR of less than 60 mL/min per 1.73 m2 among those on HT.
“Not surprisingly, the women taking hormone therapy were different than those who were not, and they generally had fewer health problems, such as diabetes and hyperlipidemia,” Dr. Kattah said. “However, after taking these differences into account in our models, we still found a significant decrease in the risk of having microalbuminuria in those on hormone therapy.”
Dr. Kattah acknowledged certain limitations of the study, including the fact that its design is “cross-sectional and cannot answer the question of whether or not hormone therapy can improve kidney function.
In addition, “we do not have data on how long women were taking hormone therapy, which other studies have suggested is an important factor.”
The researchers reported having no financial disclosures.
SAN DIEGO – The use of hormone therapy was associated with a lower urine albumin-to-creatinine ratio and a decreased risk of albuminuria, results from a cross-sectional study suggest.
“This may be useful information for providers taking care of menopausal women who are considering the use of hormone therapy for vasomotor symptoms and are worried about the systemic effects of these medications,” lead study author Dr. Andrea G. Kattah said in an interview after the annual meeting of the American Society of Nephrology. “Though our data only show an association and not cause and effect, hormone therapy is associated with better kidney function in this study.”
Results from many animal studies suggest that estrogen can have beneficial effects on the kidneys, noted Dr. Kattah, who conducted the research with Dr. Vesna D. Garovic and colleagues in the division of hypertension and nephrology at the Mayo Clinic, Rochester, Minn.
“In addition, in human studies of chronic kidney disease, premenopausal women tend to have slower progression of kidney disease than men,” she said. “Studies on the effects of hormone therapy on kidney function in women have had variable results. We wanted to look at the association of hormone therapy and renal function in a large, multiethnic cohort with well-defined health conditions that may confound the relationship between hormone therapy and renal disease.”
Study participants included 2,217 women enrolled in the Family Blood Pressure Program, a multinetwork effort to study the genetics of hypertension. During a study visit between 2000 and 2004, the women completed questionnaires about medical history, menopausal status, and use of hormone therapy (HT) in the past month. Clinicians also took their blood pressure, measured their body mass index, and drew blood to determine levels of serum creatinine and urine albumin-to-creatinine ratio (UACR).
Of the 2,217 women, 673 were on HT and 1,544 were not, and their mean ages were 60 years and 63 years, respectively.
In unadjusted analysis, Dr. Kattah and her associates found that UACR was significantly lower in those on HT, compared with those who were not (3.5 mg/g creatinine vs. 5.2 mg/g creatinine, respectively, P less than .001), as was the number of women with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2 (7% vs. 10%, P = .003).
After adjusting for renal and cardiovascular risk factors including age, race, smoking, diabetes, hypertension, and family history of hypertension, the use of HT was still significantly associated with a lower UACR and decreased risk of microalbuminuria (odds ratio, 0.61). The association between HT and eGFR of less than 60 mL/min per 1.73 m2 was no longer significant after adjustment, but there was a trend toward higher eGFR and fewer women with an eGFR of less than 60 mL/min per 1.73 m2 among those on HT.
“Not surprisingly, the women taking hormone therapy were different than those who were not, and they generally had fewer health problems, such as diabetes and hyperlipidemia,” Dr. Kattah said. “However, after taking these differences into account in our models, we still found a significant decrease in the risk of having microalbuminuria in those on hormone therapy.”
Dr. Kattah acknowledged certain limitations of the study, including the fact that its design is “cross-sectional and cannot answer the question of whether or not hormone therapy can improve kidney function.
In addition, “we do not have data on how long women were taking hormone therapy, which other studies have suggested is an important factor.”
The researchers reported having no financial disclosures.
SAN DIEGO – The use of hormone therapy was associated with a lower urine albumin-to-creatinine ratio and a decreased risk of albuminuria, results from a cross-sectional study suggest.
“This may be useful information for providers taking care of menopausal women who are considering the use of hormone therapy for vasomotor symptoms and are worried about the systemic effects of these medications,” lead study author Dr. Andrea G. Kattah said in an interview after the annual meeting of the American Society of Nephrology. “Though our data only show an association and not cause and effect, hormone therapy is associated with better kidney function in this study.”
Results from many animal studies suggest that estrogen can have beneficial effects on the kidneys, noted Dr. Kattah, who conducted the research with Dr. Vesna D. Garovic and colleagues in the division of hypertension and nephrology at the Mayo Clinic, Rochester, Minn.
“In addition, in human studies of chronic kidney disease, premenopausal women tend to have slower progression of kidney disease than men,” she said. “Studies on the effects of hormone therapy on kidney function in women have had variable results. We wanted to look at the association of hormone therapy and renal function in a large, multiethnic cohort with well-defined health conditions that may confound the relationship between hormone therapy and renal disease.”
Study participants included 2,217 women enrolled in the Family Blood Pressure Program, a multinetwork effort to study the genetics of hypertension. During a study visit between 2000 and 2004, the women completed questionnaires about medical history, menopausal status, and use of hormone therapy (HT) in the past month. Clinicians also took their blood pressure, measured their body mass index, and drew blood to determine levels of serum creatinine and urine albumin-to-creatinine ratio (UACR).
Of the 2,217 women, 673 were on HT and 1,544 were not, and their mean ages were 60 years and 63 years, respectively.
In unadjusted analysis, Dr. Kattah and her associates found that UACR was significantly lower in those on HT, compared with those who were not (3.5 mg/g creatinine vs. 5.2 mg/g creatinine, respectively, P less than .001), as was the number of women with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2 (7% vs. 10%, P = .003).
After adjusting for renal and cardiovascular risk factors including age, race, smoking, diabetes, hypertension, and family history of hypertension, the use of HT was still significantly associated with a lower UACR and decreased risk of microalbuminuria (odds ratio, 0.61). The association between HT and eGFR of less than 60 mL/min per 1.73 m2 was no longer significant after adjustment, but there was a trend toward higher eGFR and fewer women with an eGFR of less than 60 mL/min per 1.73 m2 among those on HT.
“Not surprisingly, the women taking hormone therapy were different than those who were not, and they generally had fewer health problems, such as diabetes and hyperlipidemia,” Dr. Kattah said. “However, after taking these differences into account in our models, we still found a significant decrease in the risk of having microalbuminuria in those on hormone therapy.”
Dr. Kattah acknowledged certain limitations of the study, including the fact that its design is “cross-sectional and cannot answer the question of whether or not hormone therapy can improve kidney function.
In addition, “we do not have data on how long women were taking hormone therapy, which other studies have suggested is an important factor.”
The researchers reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: Women using hormone therapy had a significantly lower urine albumin-to-creatinine ratio and decreased risk of microalbuminuria, compared with those who did not.
Major finding: After adjusting for renal and cardiovascular risk factors, hormone therapy was significantly associated with a lower urine albumin-to-creatinine ratio and a decreased risk of microalbuminuria (OR, 0.61).
Data source: An analysis of 2,217 women enrolled in the Family Blood Pressure Program, a multinetwork effort to study the genetics of hypertension.
Disclosures: The researchers reported having no financial disclosures.
Early-stage kidney disease benefits most from intensive glucose control
VANCOUVER – Intensive glucose control needs to begin early in the course of kidney disease to have its greatest renal protective effect in patients with type 2 diabetes, suggests a study reported at the World Diabetes Congress.
The conclusions are based on long-term follow-up of patients with type 2 diabetes and high vascular risk who were treated on an international randomized trial for about 5 years with either intensive or standard glucose control.
Results for the 8,494 patients at a median total follow-up of nearly a decade showed that patients with lesser stages of chronic kidney disease (CKD) at the start of the study benefited more from intensive over standard glucose control in reducing the risk of onset of dialysis or renal transplantation, reported Dr. Sophia Zoungas.
Reassuringly, intensive glucose lowering was not associated with an increase in the risk of death or cardiovascular death, regardless of patients’ CKD stage at baseline. And the higher relative risk of severe hypoglycemia with the intensive strategy was likewise similar across baseline CKD stages.
“We think that the commencement of intensive glucose control is particularly important early in the disease process, and hopefully we can get in before the development of significant kidney disease,” said Dr. Zoungas, an endocrinologist with the Monash University School of Public Health and Preventive Medicine in Clayton, Australia.
“The lesser benefit in those with moderately reduced kidney function (CKD stage 3 or greater) may indicate that the glucose-independent mechanisms of renal progression may predominate in the later stages of the disease,” she speculated.
These new data are helpful for real-world clinical care, according to session co-moderator Dr. David A. D’Alessio, a professor of medicine and director of the division of endocrinology, metabolism, and nutrition at Duke University, Durham, N.C.
“I think this study was really important. It shows the strength of doing a good trial and then not stopping at 5 years, but continuing to collect data,” he said in an interview. “These studies end up being really useful to practitioners because we oftentimes see patients whom we’ve followed for up to 10 years.”
The patients studied had participated in the ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation), which tested both intensive glucose control and blood pressure–lowering therapy. After the 5-year trial, patients returned to their usual practitioners’ care but had continued collection of data. The ADVANCE post-trial observational study (ADVANCE-ON) analyses were conducted after a median total follow-up of 9.9 years. Main findings were previously published (N Engl J Med. 2014;371:1392-406).
The greater reduction in hemoglobin A1c levels seen with intensive glucose control versus standard control on the trial was lost after the trial ended, Dr. Zoungas noted. “So if there was any effect in ADVANCE-ON, it would be attributable to the difference achieved during the in-trial period,” she said.
Compared with peers in the standard control group, patients in the intensive control group had a reduction in the risk of end-stage kidney disease during the entire follow-up (hazard ratio, 0.54) similar to that seen while they were on the trial (hazard ratio, 0.35).
In subgroup analyses, the lower the baseline stage of CKD, the greater the edge of intensive glucose control over standard glucose control for reducing the risk of end-stage kidney disease. The hazard ratio was 0.16 for patients with no CKD at baseline, 0.34 for those with stage 1 or 2, and 0.89 for patients with stage 3 or higher (P = .04). The number needed to treat for 5 years to prevent one case of end-stage kidney disease during the entire follow-up was 259, 109, and 393, respectively.
“What’s interesting to note here is that during the overall 9.9 years of follow-up, we have smaller numbers needed to treat for those with no evidence of CKD or early-stage CKD,” Dr. Zoungas said.
The investigators explored treatment impact on all-cause mortality and cardiovascular mortality according to baseline CKD stage, prompted by findings from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial showing increased risks of these outcomes in patients with mild to moderate CKD at baseline who received intensive glucose control (Kidney Int. 2015;87:649-59).
ADVANCE-ON results, in contrast, showed no significant elevation in risk with intensive control, either while patients were on the trial or for the total 9.9 years of follow-up even in those at the more advanced stages of CKD, Dr. Zoungas said.
The relative risk of severe hypoglycemia was similarly elevated with intensive control, compared with standard control across CKD subgroups, although the absolute risk was higher for those with stage 3 or higher disease.
Dr. Zoungas disclosed that she receives honoraria from Servier for speaking about ADVANCE and ADVANCE-ON at scientific meetings, and that she is on the speakers bureau and advisory boards for and receives travel support from Merck Sharp & Dohme and AstraZeneca/Bristol-Myers Squibb. The study was funded in part by Servier.
VANCOUVER – Intensive glucose control needs to begin early in the course of kidney disease to have its greatest renal protective effect in patients with type 2 diabetes, suggests a study reported at the World Diabetes Congress.
The conclusions are based on long-term follow-up of patients with type 2 diabetes and high vascular risk who were treated on an international randomized trial for about 5 years with either intensive or standard glucose control.
Results for the 8,494 patients at a median total follow-up of nearly a decade showed that patients with lesser stages of chronic kidney disease (CKD) at the start of the study benefited more from intensive over standard glucose control in reducing the risk of onset of dialysis or renal transplantation, reported Dr. Sophia Zoungas.
Reassuringly, intensive glucose lowering was not associated with an increase in the risk of death or cardiovascular death, regardless of patients’ CKD stage at baseline. And the higher relative risk of severe hypoglycemia with the intensive strategy was likewise similar across baseline CKD stages.
“We think that the commencement of intensive glucose control is particularly important early in the disease process, and hopefully we can get in before the development of significant kidney disease,” said Dr. Zoungas, an endocrinologist with the Monash University School of Public Health and Preventive Medicine in Clayton, Australia.
“The lesser benefit in those with moderately reduced kidney function (CKD stage 3 or greater) may indicate that the glucose-independent mechanisms of renal progression may predominate in the later stages of the disease,” she speculated.
These new data are helpful for real-world clinical care, according to session co-moderator Dr. David A. D’Alessio, a professor of medicine and director of the division of endocrinology, metabolism, and nutrition at Duke University, Durham, N.C.
“I think this study was really important. It shows the strength of doing a good trial and then not stopping at 5 years, but continuing to collect data,” he said in an interview. “These studies end up being really useful to practitioners because we oftentimes see patients whom we’ve followed for up to 10 years.”
The patients studied had participated in the ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation), which tested both intensive glucose control and blood pressure–lowering therapy. After the 5-year trial, patients returned to their usual practitioners’ care but had continued collection of data. The ADVANCE post-trial observational study (ADVANCE-ON) analyses were conducted after a median total follow-up of 9.9 years. Main findings were previously published (N Engl J Med. 2014;371:1392-406).
The greater reduction in hemoglobin A1c levels seen with intensive glucose control versus standard control on the trial was lost after the trial ended, Dr. Zoungas noted. “So if there was any effect in ADVANCE-ON, it would be attributable to the difference achieved during the in-trial period,” she said.
Compared with peers in the standard control group, patients in the intensive control group had a reduction in the risk of end-stage kidney disease during the entire follow-up (hazard ratio, 0.54) similar to that seen while they were on the trial (hazard ratio, 0.35).
In subgroup analyses, the lower the baseline stage of CKD, the greater the edge of intensive glucose control over standard glucose control for reducing the risk of end-stage kidney disease. The hazard ratio was 0.16 for patients with no CKD at baseline, 0.34 for those with stage 1 or 2, and 0.89 for patients with stage 3 or higher (P = .04). The number needed to treat for 5 years to prevent one case of end-stage kidney disease during the entire follow-up was 259, 109, and 393, respectively.
“What’s interesting to note here is that during the overall 9.9 years of follow-up, we have smaller numbers needed to treat for those with no evidence of CKD or early-stage CKD,” Dr. Zoungas said.
The investigators explored treatment impact on all-cause mortality and cardiovascular mortality according to baseline CKD stage, prompted by findings from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial showing increased risks of these outcomes in patients with mild to moderate CKD at baseline who received intensive glucose control (Kidney Int. 2015;87:649-59).
ADVANCE-ON results, in contrast, showed no significant elevation in risk with intensive control, either while patients were on the trial or for the total 9.9 years of follow-up even in those at the more advanced stages of CKD, Dr. Zoungas said.
The relative risk of severe hypoglycemia was similarly elevated with intensive control, compared with standard control across CKD subgroups, although the absolute risk was higher for those with stage 3 or higher disease.
Dr. Zoungas disclosed that she receives honoraria from Servier for speaking about ADVANCE and ADVANCE-ON at scientific meetings, and that she is on the speakers bureau and advisory boards for and receives travel support from Merck Sharp & Dohme and AstraZeneca/Bristol-Myers Squibb. The study was funded in part by Servier.
VANCOUVER – Intensive glucose control needs to begin early in the course of kidney disease to have its greatest renal protective effect in patients with type 2 diabetes, suggests a study reported at the World Diabetes Congress.
The conclusions are based on long-term follow-up of patients with type 2 diabetes and high vascular risk who were treated on an international randomized trial for about 5 years with either intensive or standard glucose control.
Results for the 8,494 patients at a median total follow-up of nearly a decade showed that patients with lesser stages of chronic kidney disease (CKD) at the start of the study benefited more from intensive over standard glucose control in reducing the risk of onset of dialysis or renal transplantation, reported Dr. Sophia Zoungas.
Reassuringly, intensive glucose lowering was not associated with an increase in the risk of death or cardiovascular death, regardless of patients’ CKD stage at baseline. And the higher relative risk of severe hypoglycemia with the intensive strategy was likewise similar across baseline CKD stages.
“We think that the commencement of intensive glucose control is particularly important early in the disease process, and hopefully we can get in before the development of significant kidney disease,” said Dr. Zoungas, an endocrinologist with the Monash University School of Public Health and Preventive Medicine in Clayton, Australia.
“The lesser benefit in those with moderately reduced kidney function (CKD stage 3 or greater) may indicate that the glucose-independent mechanisms of renal progression may predominate in the later stages of the disease,” she speculated.
These new data are helpful for real-world clinical care, according to session co-moderator Dr. David A. D’Alessio, a professor of medicine and director of the division of endocrinology, metabolism, and nutrition at Duke University, Durham, N.C.
“I think this study was really important. It shows the strength of doing a good trial and then not stopping at 5 years, but continuing to collect data,” he said in an interview. “These studies end up being really useful to practitioners because we oftentimes see patients whom we’ve followed for up to 10 years.”
The patients studied had participated in the ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation), which tested both intensive glucose control and blood pressure–lowering therapy. After the 5-year trial, patients returned to their usual practitioners’ care but had continued collection of data. The ADVANCE post-trial observational study (ADVANCE-ON) analyses were conducted after a median total follow-up of 9.9 years. Main findings were previously published (N Engl J Med. 2014;371:1392-406).
The greater reduction in hemoglobin A1c levels seen with intensive glucose control versus standard control on the trial was lost after the trial ended, Dr. Zoungas noted. “So if there was any effect in ADVANCE-ON, it would be attributable to the difference achieved during the in-trial period,” she said.
Compared with peers in the standard control group, patients in the intensive control group had a reduction in the risk of end-stage kidney disease during the entire follow-up (hazard ratio, 0.54) similar to that seen while they were on the trial (hazard ratio, 0.35).
In subgroup analyses, the lower the baseline stage of CKD, the greater the edge of intensive glucose control over standard glucose control for reducing the risk of end-stage kidney disease. The hazard ratio was 0.16 for patients with no CKD at baseline, 0.34 for those with stage 1 or 2, and 0.89 for patients with stage 3 or higher (P = .04). The number needed to treat for 5 years to prevent one case of end-stage kidney disease during the entire follow-up was 259, 109, and 393, respectively.
“What’s interesting to note here is that during the overall 9.9 years of follow-up, we have smaller numbers needed to treat for those with no evidence of CKD or early-stage CKD,” Dr. Zoungas said.
The investigators explored treatment impact on all-cause mortality and cardiovascular mortality according to baseline CKD stage, prompted by findings from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial showing increased risks of these outcomes in patients with mild to moderate CKD at baseline who received intensive glucose control (Kidney Int. 2015;87:649-59).
ADVANCE-ON results, in contrast, showed no significant elevation in risk with intensive control, either while patients were on the trial or for the total 9.9 years of follow-up even in those at the more advanced stages of CKD, Dr. Zoungas said.
The relative risk of severe hypoglycemia was similarly elevated with intensive control, compared with standard control across CKD subgroups, although the absolute risk was higher for those with stage 3 or higher disease.
Dr. Zoungas disclosed that she receives honoraria from Servier for speaking about ADVANCE and ADVANCE-ON at scientific meetings, and that she is on the speakers bureau and advisory boards for and receives travel support from Merck Sharp & Dohme and AstraZeneca/Bristol-Myers Squibb. The study was funded in part by Servier.
AT THE WORLD DIABETES CONGRESS
Key clinical point: The benefit of intensive glucose control in reducing the risk of end-stage kidney disease is greatest when started before patients develop renal disease.
Major finding: The lower the stage of chronic kidney disease at baseline, the greater the relative reduction in risk of end-stage kidney disease with intensive vs. standard glucose control (P = .04).
Data source: A post-trial observational study of 8,494 patients with type 2 diabetes and high vascular risk (ADVANCE-ON study).
Disclosures: Dr. Zoungas disclosed that she receives honoraria from Servier for speaking about ADVANCE and ADVANCE-ON at scientific meetings, and that she is on the speakers bureau and advisory boards for and receives travel support from Merck Sharp & Dohme and AstraZeneca/Bristol-Myers Squibb. The study was funded in part by Servier.
Vitamin D improved vascular function in kidney disease
SAN DIEGO – Raising levels of vitamin D, deficient in many patients with chronic kidney disease, improved vascular function and reduced inflammation and vessel wall stiffness after 16 weeks, according to results of a study in a small group of early kidney disease patients in India.
“This tells us that vitamin D has the potential, at least over the short- to intermediate term, to improve vascular function,” with a promise of reducing or preventing cardiovascular outcomes in a larger study, said Dr. Vivekanand Jha, of the George Institute for Global Health in New Delhi.
“Patients with very early kidney disease are at very high risk of developing cardiovascular complications such as heart attacks, strokes, and peripheral vascular disease – and that’s what kills them before they get to the point where they need dialysis,” Dr. Jha said. “So, many die before they need dialysis. But we don’t know the factors that cause these cardiovascular complications.”
His group hypothesized that because vitamin D has biological action on blood vessels, “if we supplemented patients with vitamin D, their vascular function would improve.”
Participants were randomized to receive directly observed oral doses of 300,000 IU of cholecalciferol at baseline and at 8 weeks, or directly observed doses of a placebo in a matched control arm.
“We measured several parameters to look at vascular function after 16 weeks and found that patients who received vitamin D showed normalized vitamin D and decreases in levels of parathyroid hormone,” Dr. Jha said at the meeting sponsored by the American Society of Nephrology.
“But most importantly, we found that vitamin D recipients showed improvement in their vascular function on two or three tests; in flow-mediated dilation, which suggests improved endothelial function; and improved augmentation index, which looks at the stiffness of blood vessels,” he explained.
The next step is a much larger study designed to show that vitamin D supplements in this population can prevent cardiovascular events. “But that’s going to be a long-term study,” Dr. Jha noted.
“I don’t want to get ahead of myself and say that this is going to cure cardiovascular problems,” Dr. Jha cautioned. “But now we know we need to do a definitive study to look at these events, and prove whether supplementation with vitamin D does or does not significantly improve those endpoints.”
SAN DIEGO – Raising levels of vitamin D, deficient in many patients with chronic kidney disease, improved vascular function and reduced inflammation and vessel wall stiffness after 16 weeks, according to results of a study in a small group of early kidney disease patients in India.
“This tells us that vitamin D has the potential, at least over the short- to intermediate term, to improve vascular function,” with a promise of reducing or preventing cardiovascular outcomes in a larger study, said Dr. Vivekanand Jha, of the George Institute for Global Health in New Delhi.
“Patients with very early kidney disease are at very high risk of developing cardiovascular complications such as heart attacks, strokes, and peripheral vascular disease – and that’s what kills them before they get to the point where they need dialysis,” Dr. Jha said. “So, many die before they need dialysis. But we don’t know the factors that cause these cardiovascular complications.”
His group hypothesized that because vitamin D has biological action on blood vessels, “if we supplemented patients with vitamin D, their vascular function would improve.”
Participants were randomized to receive directly observed oral doses of 300,000 IU of cholecalciferol at baseline and at 8 weeks, or directly observed doses of a placebo in a matched control arm.
“We measured several parameters to look at vascular function after 16 weeks and found that patients who received vitamin D showed normalized vitamin D and decreases in levels of parathyroid hormone,” Dr. Jha said at the meeting sponsored by the American Society of Nephrology.
“But most importantly, we found that vitamin D recipients showed improvement in their vascular function on two or three tests; in flow-mediated dilation, which suggests improved endothelial function; and improved augmentation index, which looks at the stiffness of blood vessels,” he explained.
The next step is a much larger study designed to show that vitamin D supplements in this population can prevent cardiovascular events. “But that’s going to be a long-term study,” Dr. Jha noted.
“I don’t want to get ahead of myself and say that this is going to cure cardiovascular problems,” Dr. Jha cautioned. “But now we know we need to do a definitive study to look at these events, and prove whether supplementation with vitamin D does or does not significantly improve those endpoints.”
SAN DIEGO – Raising levels of vitamin D, deficient in many patients with chronic kidney disease, improved vascular function and reduced inflammation and vessel wall stiffness after 16 weeks, according to results of a study in a small group of early kidney disease patients in India.
“This tells us that vitamin D has the potential, at least over the short- to intermediate term, to improve vascular function,” with a promise of reducing or preventing cardiovascular outcomes in a larger study, said Dr. Vivekanand Jha, of the George Institute for Global Health in New Delhi.
“Patients with very early kidney disease are at very high risk of developing cardiovascular complications such as heart attacks, strokes, and peripheral vascular disease – and that’s what kills them before they get to the point where they need dialysis,” Dr. Jha said. “So, many die before they need dialysis. But we don’t know the factors that cause these cardiovascular complications.”
His group hypothesized that because vitamin D has biological action on blood vessels, “if we supplemented patients with vitamin D, their vascular function would improve.”
Participants were randomized to receive directly observed oral doses of 300,000 IU of cholecalciferol at baseline and at 8 weeks, or directly observed doses of a placebo in a matched control arm.
“We measured several parameters to look at vascular function after 16 weeks and found that patients who received vitamin D showed normalized vitamin D and decreases in levels of parathyroid hormone,” Dr. Jha said at the meeting sponsored by the American Society of Nephrology.
“But most importantly, we found that vitamin D recipients showed improvement in their vascular function on two or three tests; in flow-mediated dilation, which suggests improved endothelial function; and improved augmentation index, which looks at the stiffness of blood vessels,” he explained.
The next step is a much larger study designed to show that vitamin D supplements in this population can prevent cardiovascular events. “But that’s going to be a long-term study,” Dr. Jha noted.
“I don’t want to get ahead of myself and say that this is going to cure cardiovascular problems,” Dr. Jha cautioned. “But now we know we need to do a definitive study to look at these events, and prove whether supplementation with vitamin D does or does not significantly improve those endpoints.”
AT KIDNEY WEEK 2015
Key clinical point: Vitamin D supplements may improve vascular function in patients with stage 3 and 4 chronic kidney disease who did not have diabetes.
Major finding: 70% of patients in the treatment group achieved a 40% change in endothelial-dependent flow-mediated dilation and vascular function after 16 weeks. All patients achieved sufficient vitamin D levels.
Data source: A randomized, double-blind, placebo-controlled trial of 120 patients in New Delhi.
Disclosures: The researchers reported grant support from the Indian government and the department of biotechnology at the George Institute for Global Health in New Delhi.
AHA: Three measures risk stratify acute heart failure
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
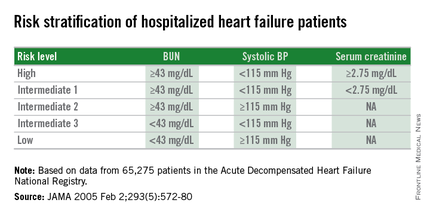
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.
The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.

The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.

The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Dichotomous cutoffs for BUN, systolic BP, and serum creatinine together robustly risk stratified patients hospitalized for acute decompensated heart failure.
Major finding: Three baseline measures together stratified patients over an eightfold range for 30-day mortality following hospital discharge.
Data source: Application of the risk-stratification formula to 3,628 heart failure patients hospitalized at one U.S. center during 2000-2013.
Disclosures: Dr. Win had no disclosures.
When it's beneficial to defer dialysis
THE CASE
A 94-year-old Hispanic man with hypertension, congestive heart failure (CHF), anemia of chronic disease, and end-stage renal disease (ESRD) presented to our facility with weakness and shortness of breath. We diagnosed a CHF exacerbation. Initially, he exhibited some respiratory distress that required observation in the coronary care unit and bi-level positive airway pressure therapy to maintain oxygen saturation. Our patient was then moved to a step-down unit where his primary caregiver, his granddaughter, told the medical team that he was limited at home in some of his instrumental activities of daily living. Specifically, he was unable to prepare meals or manage his finances on his own.
Nephrology was consulted for consideration of hemodialysis (HD) because our patient’s creatinine on admission was 7.2 mg/dL (normal for men is 0.7-1.3 mg/dL) and his estimated glomerular filtration rate (GFR) was 7 mL/min (normal is 90-120 mL/min). The patient’s family was conflicted over whether or not to start HD. Palliative Care was consulted to help establish goals of care.
A decision is made. In light of the patient’s limited functional status and his expressed desire to stay at home with his family and receive limited medical care there, the Nephrology and Palliative Care teams recommended delaying HD despite the patient’s worsening renal function. The patient was discharged home with home care services, and he and the family were instructed to follow up with Nephrology for supportive renal management.
DISCUSSION
The decision to delay HD in patients with ESRD is a difficult one that requires shared decision-making between patients and medical providers. Palliative Care consultation services are often involved in this process.
Recent literature supports an “intent-to-defer” based on an evaluation of the patient’s functionality. This represents a paradigm shift from the previous “intent-to-start-early” treatment strategy. In fact, rather than starting early, the Canadian Society of Nephrology recommends delaying initiation of HD in patients with a GFR <15 mL/min.1 Close monitoring of these patients by both a primary care physician and nephrologist is essential.
When considering initiation of HD, it’s important to look at the overall benefit of this intervention in light of the patient’s mortality risk and quality of life. Many patients who receive HD—especially the elderly—report that it takes more than 6 hours to recover following a dialysis treatment.2
Not surprisingly, depression is common in elderly HD patients. Compared to their younger cohorts, older HD patients have a 62% increased risk of developing depression.3 Also, patients who are considered frail and are receiving HD have more than 3 times the mortality risk within one year than those who are not (hazard ratio=3.42; 95% confidence interval, 2.45-4.76).4 (The researchers’ definition of frailty included poor self-reported physical function, exhaustion/fatigue, low physical activity, and undernutrition.4)
Functional status. Although a patient’s age should not be a limiting factor for HD referral, functional status should be considered. Patients with limited functionality and significant dependence have an increased risk of death during the first year of HD.5
Palliative approach gains acceptance. It is becoming more accepted within the nephrology community to consider a palliative approach to patients with ESRD. Organizations such as the Renal Physicians Association recommend effective prognostication, early advanced care planning, forgoing HD in patients with a poor prognosis, and involving Palliative Care early in the decision-making process.6 Aligning the patient’s goals of care with the appropriate treatment method—particularly in patients with comorbid conditions—is an important practice when caring for those with limited life expectancy and functionality.7
THE TAKEAWAY
Intent-to-defer HD may be a preferred strategy when caring for many patients with ESRD. Taking into consideration a patient’s comorbidities and functional status, while considering mortality risk and quality of life are essential. Involving palliative care and nephrology specialists can help patients and families understand HD and make an educated decision regarding when to start it.
1. Nesrallah GE, Mustafa RA, Clark WF, et al; Canadian Society of Nephrology. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186:112-117.
2. Rayner HC, Zepel L, Fuller DS, et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2014;64:86-94.
3. Canaud B, Tong L, Tentori F, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2011;6:1651-1662.
4. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967.
5. Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012-1021.
6. Renal Physicians Association. Shared decision-making in the appropriate initiation of and withdrawal from dialysis: clinical practice guideline. 2nd ed. Rockville, MD: Renal Physicians Association; 2010.
7. Grubbs V, Moss AH, Cohen LM, et al; Dialysis Advisory Group of the American Society of Nephrology. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9:2203-2209.
THE CASE
A 94-year-old Hispanic man with hypertension, congestive heart failure (CHF), anemia of chronic disease, and end-stage renal disease (ESRD) presented to our facility with weakness and shortness of breath. We diagnosed a CHF exacerbation. Initially, he exhibited some respiratory distress that required observation in the coronary care unit and bi-level positive airway pressure therapy to maintain oxygen saturation. Our patient was then moved to a step-down unit where his primary caregiver, his granddaughter, told the medical team that he was limited at home in some of his instrumental activities of daily living. Specifically, he was unable to prepare meals or manage his finances on his own.
Nephrology was consulted for consideration of hemodialysis (HD) because our patient’s creatinine on admission was 7.2 mg/dL (normal for men is 0.7-1.3 mg/dL) and his estimated glomerular filtration rate (GFR) was 7 mL/min (normal is 90-120 mL/min). The patient’s family was conflicted over whether or not to start HD. Palliative Care was consulted to help establish goals of care.
A decision is made. In light of the patient’s limited functional status and his expressed desire to stay at home with his family and receive limited medical care there, the Nephrology and Palliative Care teams recommended delaying HD despite the patient’s worsening renal function. The patient was discharged home with home care services, and he and the family were instructed to follow up with Nephrology for supportive renal management.
DISCUSSION
The decision to delay HD in patients with ESRD is a difficult one that requires shared decision-making between patients and medical providers. Palliative Care consultation services are often involved in this process.
Recent literature supports an “intent-to-defer” based on an evaluation of the patient’s functionality. This represents a paradigm shift from the previous “intent-to-start-early” treatment strategy. In fact, rather than starting early, the Canadian Society of Nephrology recommends delaying initiation of HD in patients with a GFR <15 mL/min.1 Close monitoring of these patients by both a primary care physician and nephrologist is essential.
When considering initiation of HD, it’s important to look at the overall benefit of this intervention in light of the patient’s mortality risk and quality of life. Many patients who receive HD—especially the elderly—report that it takes more than 6 hours to recover following a dialysis treatment.2
Not surprisingly, depression is common in elderly HD patients. Compared to their younger cohorts, older HD patients have a 62% increased risk of developing depression.3 Also, patients who are considered frail and are receiving HD have more than 3 times the mortality risk within one year than those who are not (hazard ratio=3.42; 95% confidence interval, 2.45-4.76).4 (The researchers’ definition of frailty included poor self-reported physical function, exhaustion/fatigue, low physical activity, and undernutrition.4)
Functional status. Although a patient’s age should not be a limiting factor for HD referral, functional status should be considered. Patients with limited functionality and significant dependence have an increased risk of death during the first year of HD.5
Palliative approach gains acceptance. It is becoming more accepted within the nephrology community to consider a palliative approach to patients with ESRD. Organizations such as the Renal Physicians Association recommend effective prognostication, early advanced care planning, forgoing HD in patients with a poor prognosis, and involving Palliative Care early in the decision-making process.6 Aligning the patient’s goals of care with the appropriate treatment method—particularly in patients with comorbid conditions—is an important practice when caring for those with limited life expectancy and functionality.7
THE TAKEAWAY
Intent-to-defer HD may be a preferred strategy when caring for many patients with ESRD. Taking into consideration a patient’s comorbidities and functional status, while considering mortality risk and quality of life are essential. Involving palliative care and nephrology specialists can help patients and families understand HD and make an educated decision regarding when to start it.
THE CASE
A 94-year-old Hispanic man with hypertension, congestive heart failure (CHF), anemia of chronic disease, and end-stage renal disease (ESRD) presented to our facility with weakness and shortness of breath. We diagnosed a CHF exacerbation. Initially, he exhibited some respiratory distress that required observation in the coronary care unit and bi-level positive airway pressure therapy to maintain oxygen saturation. Our patient was then moved to a step-down unit where his primary caregiver, his granddaughter, told the medical team that he was limited at home in some of his instrumental activities of daily living. Specifically, he was unable to prepare meals or manage his finances on his own.
Nephrology was consulted for consideration of hemodialysis (HD) because our patient’s creatinine on admission was 7.2 mg/dL (normal for men is 0.7-1.3 mg/dL) and his estimated glomerular filtration rate (GFR) was 7 mL/min (normal is 90-120 mL/min). The patient’s family was conflicted over whether or not to start HD. Palliative Care was consulted to help establish goals of care.
A decision is made. In light of the patient’s limited functional status and his expressed desire to stay at home with his family and receive limited medical care there, the Nephrology and Palliative Care teams recommended delaying HD despite the patient’s worsening renal function. The patient was discharged home with home care services, and he and the family were instructed to follow up with Nephrology for supportive renal management.
DISCUSSION
The decision to delay HD in patients with ESRD is a difficult one that requires shared decision-making between patients and medical providers. Palliative Care consultation services are often involved in this process.
Recent literature supports an “intent-to-defer” based on an evaluation of the patient’s functionality. This represents a paradigm shift from the previous “intent-to-start-early” treatment strategy. In fact, rather than starting early, the Canadian Society of Nephrology recommends delaying initiation of HD in patients with a GFR <15 mL/min.1 Close monitoring of these patients by both a primary care physician and nephrologist is essential.
When considering initiation of HD, it’s important to look at the overall benefit of this intervention in light of the patient’s mortality risk and quality of life. Many patients who receive HD—especially the elderly—report that it takes more than 6 hours to recover following a dialysis treatment.2
Not surprisingly, depression is common in elderly HD patients. Compared to their younger cohorts, older HD patients have a 62% increased risk of developing depression.3 Also, patients who are considered frail and are receiving HD have more than 3 times the mortality risk within one year than those who are not (hazard ratio=3.42; 95% confidence interval, 2.45-4.76).4 (The researchers’ definition of frailty included poor self-reported physical function, exhaustion/fatigue, low physical activity, and undernutrition.4)
Functional status. Although a patient’s age should not be a limiting factor for HD referral, functional status should be considered. Patients with limited functionality and significant dependence have an increased risk of death during the first year of HD.5
Palliative approach gains acceptance. It is becoming more accepted within the nephrology community to consider a palliative approach to patients with ESRD. Organizations such as the Renal Physicians Association recommend effective prognostication, early advanced care planning, forgoing HD in patients with a poor prognosis, and involving Palliative Care early in the decision-making process.6 Aligning the patient’s goals of care with the appropriate treatment method—particularly in patients with comorbid conditions—is an important practice when caring for those with limited life expectancy and functionality.7
THE TAKEAWAY
Intent-to-defer HD may be a preferred strategy when caring for many patients with ESRD. Taking into consideration a patient’s comorbidities and functional status, while considering mortality risk and quality of life are essential. Involving palliative care and nephrology specialists can help patients and families understand HD and make an educated decision regarding when to start it.
1. Nesrallah GE, Mustafa RA, Clark WF, et al; Canadian Society of Nephrology. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186:112-117.
2. Rayner HC, Zepel L, Fuller DS, et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2014;64:86-94.
3. Canaud B, Tong L, Tentori F, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2011;6:1651-1662.
4. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967.
5. Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012-1021.
6. Renal Physicians Association. Shared decision-making in the appropriate initiation of and withdrawal from dialysis: clinical practice guideline. 2nd ed. Rockville, MD: Renal Physicians Association; 2010.
7. Grubbs V, Moss AH, Cohen LM, et al; Dialysis Advisory Group of the American Society of Nephrology. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9:2203-2209.
1. Nesrallah GE, Mustafa RA, Clark WF, et al; Canadian Society of Nephrology. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186:112-117.
2. Rayner HC, Zepel L, Fuller DS, et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2014;64:86-94.
3. Canaud B, Tong L, Tentori F, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2011;6:1651-1662.
4. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967.
5. Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012-1021.
6. Renal Physicians Association. Shared decision-making in the appropriate initiation of and withdrawal from dialysis: clinical practice guideline. 2nd ed. Rockville, MD: Renal Physicians Association; 2010.
7. Grubbs V, Moss AH, Cohen LM, et al; Dialysis Advisory Group of the American Society of Nephrology. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9:2203-2209.
Brown tumor of the pelvis
A 39-year-old man presented with acute left hip pain and inability to bear weight following a minor trauma. The patient had a history of polycystic kidney disease and was on dialysis. Five years ago he had undergone bilateral nephrectomy and a renal transplantation that subsequently failed.
On examination, the active and passive range of motion of the left hip were limited due to pain. His serum laboratory values were:
- Parathyroid hormone 259.7 pmol/L (reference range 1.5–9.3)
- Calcium 2.32 mmol/L (1.15–1.32)
- Phosphate 3.26 mmol/L (0.8–1.45).
Computed tomography of the pelvis revealed an exophytic calcified lesion with multiple cystic spaces and fluid-fluid levels centered on the left pubis, extending medially into the right pubis and laterally into the left adductor muscle group. An acute pathologic fracture was documented in the left inferior pubic ramus (Figure 1). Other radiographic signs of long-standing hyperparathyroidism were present, including subperiosteal bone resorption at the radial side of the middle phalanges and the clavicle epiphysis.
The differential diagnosis of the pelvic lesion included giant cell tumor of bone with aneurysmal bone-cyst-like changes, osteitis fibrosa cystica, and, less likely, metastatic bone disease. Biopsy of the lesion showed clusters of osteoclast-type giant cells on a background of spindle cells and fibrous stroma that in this clinical context was consistent with the diagnosis of brown tumor (Figure 2).1
BROWN TUMOR
Brown tumor has been reported in fewer than 2% of patients with primary hyperparathyroidism and in 1.5% to 1.7% of those with secondary hyperparathyroidism (ie, from chronic renal failure, malabsorption, vitamin D deficiency, or hypocalcemia).2–4 An excess of parathyroid hormone increases the number and activity of osteoclasts, which are responsible for the lytic lesions. Brown tumor is the localized form of osteitis fibrosa cystica and is the most characteristic of the many skeletal changes that accompany secondary hyperparathyroidism.
Brown tumor is named for its color, which results from hemorrhages with accumulation of hemosiderin within the vascularized fibrous tissue. The tumor most commonly affects the pelvis, ribs, long-bone shafts, clavicle, and mandible.5 Clinical symptoms are nonspecific and depend on the size and location of the lesion.
Medical management of secondary hyperparathyroidism in dialysis patients involves some combination of phosphate binders (either calcium-containing or non-calcium-containing binders), calcitriol or synthetic vitamin D analogs, and a calcimimetic. Parathyroidectomy is required if drug therapy is ineffective. Surgical excision of brown tumor should be considered in patients who have large bone defects with spontaneous fracture risk or increasing pain. Our patient declined surgical intervention.
- Davies AM, Evans N, Mangham DC, Grimer RJ. MR imaging of brown tumour with fluid-fluid levels: a report of three cases. Eur Radiol 2001; 11:1445–1449.
- Silverberg SJ, Bilezikian JP. Evaluation and management of primary hyperparathyroidism. J Clin Endocrinol Metab 1996; 81:2036–2040.
- Bohlman ME, Kim YC, Eagan J, Spees EK. Brown tumor in secondary hyperparathyroidism causing acute paraplegia. Am J Med 1986; 81:545–547.
- Demay MB, Rosenthal DI, Deshpande V. Case records of the Massachusetts General Hospital. Case 16-2008. A 46-year-old woman with bone pain. N Engl J Med 2008; 358:2266–2274.
- Perlman JS, Pletcher SD, Schmidt BL, Eisele DW. Pathology quiz case 2. Giant cell lesion (brown tumor) of the mandible, associated with primary hyperparathyroidism (HPT). Arch Otolaryngol Head Neck Surg 2004; 130:793–794.
A 39-year-old man presented with acute left hip pain and inability to bear weight following a minor trauma. The patient had a history of polycystic kidney disease and was on dialysis. Five years ago he had undergone bilateral nephrectomy and a renal transplantation that subsequently failed.
On examination, the active and passive range of motion of the left hip were limited due to pain. His serum laboratory values were:
- Parathyroid hormone 259.7 pmol/L (reference range 1.5–9.3)
- Calcium 2.32 mmol/L (1.15–1.32)
- Phosphate 3.26 mmol/L (0.8–1.45).
Computed tomography of the pelvis revealed an exophytic calcified lesion with multiple cystic spaces and fluid-fluid levels centered on the left pubis, extending medially into the right pubis and laterally into the left adductor muscle group. An acute pathologic fracture was documented in the left inferior pubic ramus (Figure 1). Other radiographic signs of long-standing hyperparathyroidism were present, including subperiosteal bone resorption at the radial side of the middle phalanges and the clavicle epiphysis.
The differential diagnosis of the pelvic lesion included giant cell tumor of bone with aneurysmal bone-cyst-like changes, osteitis fibrosa cystica, and, less likely, metastatic bone disease. Biopsy of the lesion showed clusters of osteoclast-type giant cells on a background of spindle cells and fibrous stroma that in this clinical context was consistent with the diagnosis of brown tumor (Figure 2).1
BROWN TUMOR
Brown tumor has been reported in fewer than 2% of patients with primary hyperparathyroidism and in 1.5% to 1.7% of those with secondary hyperparathyroidism (ie, from chronic renal failure, malabsorption, vitamin D deficiency, or hypocalcemia).2–4 An excess of parathyroid hormone increases the number and activity of osteoclasts, which are responsible for the lytic lesions. Brown tumor is the localized form of osteitis fibrosa cystica and is the most characteristic of the many skeletal changes that accompany secondary hyperparathyroidism.
Brown tumor is named for its color, which results from hemorrhages with accumulation of hemosiderin within the vascularized fibrous tissue. The tumor most commonly affects the pelvis, ribs, long-bone shafts, clavicle, and mandible.5 Clinical symptoms are nonspecific and depend on the size and location of the lesion.
Medical management of secondary hyperparathyroidism in dialysis patients involves some combination of phosphate binders (either calcium-containing or non-calcium-containing binders), calcitriol or synthetic vitamin D analogs, and a calcimimetic. Parathyroidectomy is required if drug therapy is ineffective. Surgical excision of brown tumor should be considered in patients who have large bone defects with spontaneous fracture risk or increasing pain. Our patient declined surgical intervention.
A 39-year-old man presented with acute left hip pain and inability to bear weight following a minor trauma. The patient had a history of polycystic kidney disease and was on dialysis. Five years ago he had undergone bilateral nephrectomy and a renal transplantation that subsequently failed.
On examination, the active and passive range of motion of the left hip were limited due to pain. His serum laboratory values were:
- Parathyroid hormone 259.7 pmol/L (reference range 1.5–9.3)
- Calcium 2.32 mmol/L (1.15–1.32)
- Phosphate 3.26 mmol/L (0.8–1.45).
Computed tomography of the pelvis revealed an exophytic calcified lesion with multiple cystic spaces and fluid-fluid levels centered on the left pubis, extending medially into the right pubis and laterally into the left adductor muscle group. An acute pathologic fracture was documented in the left inferior pubic ramus (Figure 1). Other radiographic signs of long-standing hyperparathyroidism were present, including subperiosteal bone resorption at the radial side of the middle phalanges and the clavicle epiphysis.
The differential diagnosis of the pelvic lesion included giant cell tumor of bone with aneurysmal bone-cyst-like changes, osteitis fibrosa cystica, and, less likely, metastatic bone disease. Biopsy of the lesion showed clusters of osteoclast-type giant cells on a background of spindle cells and fibrous stroma that in this clinical context was consistent with the diagnosis of brown tumor (Figure 2).1
BROWN TUMOR
Brown tumor has been reported in fewer than 2% of patients with primary hyperparathyroidism and in 1.5% to 1.7% of those with secondary hyperparathyroidism (ie, from chronic renal failure, malabsorption, vitamin D deficiency, or hypocalcemia).2–4 An excess of parathyroid hormone increases the number and activity of osteoclasts, which are responsible for the lytic lesions. Brown tumor is the localized form of osteitis fibrosa cystica and is the most characteristic of the many skeletal changes that accompany secondary hyperparathyroidism.
Brown tumor is named for its color, which results from hemorrhages with accumulation of hemosiderin within the vascularized fibrous tissue. The tumor most commonly affects the pelvis, ribs, long-bone shafts, clavicle, and mandible.5 Clinical symptoms are nonspecific and depend on the size and location of the lesion.
Medical management of secondary hyperparathyroidism in dialysis patients involves some combination of phosphate binders (either calcium-containing or non-calcium-containing binders), calcitriol or synthetic vitamin D analogs, and a calcimimetic. Parathyroidectomy is required if drug therapy is ineffective. Surgical excision of brown tumor should be considered in patients who have large bone defects with spontaneous fracture risk or increasing pain. Our patient declined surgical intervention.
- Davies AM, Evans N, Mangham DC, Grimer RJ. MR imaging of brown tumour with fluid-fluid levels: a report of three cases. Eur Radiol 2001; 11:1445–1449.
- Silverberg SJ, Bilezikian JP. Evaluation and management of primary hyperparathyroidism. J Clin Endocrinol Metab 1996; 81:2036–2040.
- Bohlman ME, Kim YC, Eagan J, Spees EK. Brown tumor in secondary hyperparathyroidism causing acute paraplegia. Am J Med 1986; 81:545–547.
- Demay MB, Rosenthal DI, Deshpande V. Case records of the Massachusetts General Hospital. Case 16-2008. A 46-year-old woman with bone pain. N Engl J Med 2008; 358:2266–2274.
- Perlman JS, Pletcher SD, Schmidt BL, Eisele DW. Pathology quiz case 2. Giant cell lesion (brown tumor) of the mandible, associated with primary hyperparathyroidism (HPT). Arch Otolaryngol Head Neck Surg 2004; 130:793–794.
- Davies AM, Evans N, Mangham DC, Grimer RJ. MR imaging of brown tumour with fluid-fluid levels: a report of three cases. Eur Radiol 2001; 11:1445–1449.
- Silverberg SJ, Bilezikian JP. Evaluation and management of primary hyperparathyroidism. J Clin Endocrinol Metab 1996; 81:2036–2040.
- Bohlman ME, Kim YC, Eagan J, Spees EK. Brown tumor in secondary hyperparathyroidism causing acute paraplegia. Am J Med 1986; 81:545–547.
- Demay MB, Rosenthal DI, Deshpande V. Case records of the Massachusetts General Hospital. Case 16-2008. A 46-year-old woman with bone pain. N Engl J Med 2008; 358:2266–2274.
- Perlman JS, Pletcher SD, Schmidt BL, Eisele DW. Pathology quiz case 2. Giant cell lesion (brown tumor) of the mandible, associated with primary hyperparathyroidism (HPT). Arch Otolaryngol Head Neck Surg 2004; 130:793–794.
Blood Pressure Above 140/80 Worsens Proteinuric Diabetic Kidney Disease
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
AT KIDNEY WEEK 2015
Blood pressure above 140/80 worsens proteinuric diabetic kidney disease
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: In patients with proteinuric diabetic kidney disease, a mean systolic BP greater than 140 mm Hg and a mean diastolic BP greater than 80 mm Hg were linked to worse renal outcomes.
Major finding: A mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes (hazard ratios of 1.51 and 1.54, respectively).
Data source: Blood pressure data from 1,448 patients who were randomized to the VA NEPHRON-D trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone.
Disclosures: The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.