User login
Look for nephrotoxicity in adult survivors of childhood cancer
LAS VEGAS – Adult survivors of childhood cancer treated with high-dose cisplatin or high-dose ifosfamide are at markedly increased risk for chronic renal impairment, according to a large Dutch study with a median 18.3-year follow-up.
Long-term treatment-related nephrotoxicity was also seen in the adult survivors of childhood cancer who underwent unilateral nephrectomy combined with abdominal radiation therapy.
"This study is perhaps a warning to us when we’re seeing these adult survivors of childhood cancer in clinic, particularly if they have a history of ifosfamide or cisplatin use, to pay closer attention to the development of chronic kidney disease so they can be managed better in the future," Dr. Anushree C. Shirali said at a meeting sponsored by the National Kidney Foundation.
Drug-induced acute kidney injury is a common event during cancer therapy. Its mechanisms and treatments are well studied. In contrast, the long-term nephrotoxicity of the powerful therapies used in treating childhood cancers has received much less scrutiny. But it’s an increasingly relevant issue because childhood cancer survival rates have improved substantially. Indeed, as the Dutch investigators observed, today 1 in 570 young adults is a childhood cancer survivor (Clin. J. Am. Soc. Nephrol. 2013;8:922-9).
Dr. Shirali, a nephrologist at Yale University in New Haven, Conn., highlighted the Dutch study of 763 adult survivors of childhood cancer because of its unusually long and complete follow-up. The investigators included nearly 90% of all adult survivors treated at Erasmus University in Rotterdam, the Netherlands, during 1964-2005.
High-dose ifosfamide was associated with a 6.2-fold greater likelihood of an increased urinary beta2-microglobulin/creatinine ratio indicative of persisting tubular dysfunction, compared with cancer survivors not receiving that therapy. High-dose cisplatin was associated with a 5.2-fold increased risk of albuminuria. The estimated glomerular filtration rate in adult survivors who had received high-dose ifosfamide was 88 mL/min per 1.73 m2, significantly lower than the 98 mL/min per 1.73 m2 in others. Similarly, patients who had received high-dose cisplatin had an average estimated glomerular filtration rate of 83 mL/min per 1.73 m2, compared with 101 mL/min per 1.73 m2 in survivors not treated with high-dose cisplatin.
In contrast to ifosfamide, its isomer cyclophosphamide was not associated with long-term nephrotoxicity; neither was carboplatin, a cisplatin analogue, or methotrexate. Although methotrexate is known to cause acute nephrotoxicity, this phenomenon appears to be completely reversible, since methotrexate-treated, long-term cancer survivors didn’t develop tubular or glomerular dysfunction.
This long-term study was supported by the Dutch Kidney Foundation. Dr. Shirali, who was not involved in the study, reported having no financial conflicts.
LAS VEGAS – Adult survivors of childhood cancer treated with high-dose cisplatin or high-dose ifosfamide are at markedly increased risk for chronic renal impairment, according to a large Dutch study with a median 18.3-year follow-up.
Long-term treatment-related nephrotoxicity was also seen in the adult survivors of childhood cancer who underwent unilateral nephrectomy combined with abdominal radiation therapy.
"This study is perhaps a warning to us when we’re seeing these adult survivors of childhood cancer in clinic, particularly if they have a history of ifosfamide or cisplatin use, to pay closer attention to the development of chronic kidney disease so they can be managed better in the future," Dr. Anushree C. Shirali said at a meeting sponsored by the National Kidney Foundation.
Drug-induced acute kidney injury is a common event during cancer therapy. Its mechanisms and treatments are well studied. In contrast, the long-term nephrotoxicity of the powerful therapies used in treating childhood cancers has received much less scrutiny. But it’s an increasingly relevant issue because childhood cancer survival rates have improved substantially. Indeed, as the Dutch investigators observed, today 1 in 570 young adults is a childhood cancer survivor (Clin. J. Am. Soc. Nephrol. 2013;8:922-9).
Dr. Shirali, a nephrologist at Yale University in New Haven, Conn., highlighted the Dutch study of 763 adult survivors of childhood cancer because of its unusually long and complete follow-up. The investigators included nearly 90% of all adult survivors treated at Erasmus University in Rotterdam, the Netherlands, during 1964-2005.
High-dose ifosfamide was associated with a 6.2-fold greater likelihood of an increased urinary beta2-microglobulin/creatinine ratio indicative of persisting tubular dysfunction, compared with cancer survivors not receiving that therapy. High-dose cisplatin was associated with a 5.2-fold increased risk of albuminuria. The estimated glomerular filtration rate in adult survivors who had received high-dose ifosfamide was 88 mL/min per 1.73 m2, significantly lower than the 98 mL/min per 1.73 m2 in others. Similarly, patients who had received high-dose cisplatin had an average estimated glomerular filtration rate of 83 mL/min per 1.73 m2, compared with 101 mL/min per 1.73 m2 in survivors not treated with high-dose cisplatin.
In contrast to ifosfamide, its isomer cyclophosphamide was not associated with long-term nephrotoxicity; neither was carboplatin, a cisplatin analogue, or methotrexate. Although methotrexate is known to cause acute nephrotoxicity, this phenomenon appears to be completely reversible, since methotrexate-treated, long-term cancer survivors didn’t develop tubular or glomerular dysfunction.
This long-term study was supported by the Dutch Kidney Foundation. Dr. Shirali, who was not involved in the study, reported having no financial conflicts.
LAS VEGAS – Adult survivors of childhood cancer treated with high-dose cisplatin or high-dose ifosfamide are at markedly increased risk for chronic renal impairment, according to a large Dutch study with a median 18.3-year follow-up.
Long-term treatment-related nephrotoxicity was also seen in the adult survivors of childhood cancer who underwent unilateral nephrectomy combined with abdominal radiation therapy.
"This study is perhaps a warning to us when we’re seeing these adult survivors of childhood cancer in clinic, particularly if they have a history of ifosfamide or cisplatin use, to pay closer attention to the development of chronic kidney disease so they can be managed better in the future," Dr. Anushree C. Shirali said at a meeting sponsored by the National Kidney Foundation.
Drug-induced acute kidney injury is a common event during cancer therapy. Its mechanisms and treatments are well studied. In contrast, the long-term nephrotoxicity of the powerful therapies used in treating childhood cancers has received much less scrutiny. But it’s an increasingly relevant issue because childhood cancer survival rates have improved substantially. Indeed, as the Dutch investigators observed, today 1 in 570 young adults is a childhood cancer survivor (Clin. J. Am. Soc. Nephrol. 2013;8:922-9).
Dr. Shirali, a nephrologist at Yale University in New Haven, Conn., highlighted the Dutch study of 763 adult survivors of childhood cancer because of its unusually long and complete follow-up. The investigators included nearly 90% of all adult survivors treated at Erasmus University in Rotterdam, the Netherlands, during 1964-2005.
High-dose ifosfamide was associated with a 6.2-fold greater likelihood of an increased urinary beta2-microglobulin/creatinine ratio indicative of persisting tubular dysfunction, compared with cancer survivors not receiving that therapy. High-dose cisplatin was associated with a 5.2-fold increased risk of albuminuria. The estimated glomerular filtration rate in adult survivors who had received high-dose ifosfamide was 88 mL/min per 1.73 m2, significantly lower than the 98 mL/min per 1.73 m2 in others. Similarly, patients who had received high-dose cisplatin had an average estimated glomerular filtration rate of 83 mL/min per 1.73 m2, compared with 101 mL/min per 1.73 m2 in survivors not treated with high-dose cisplatin.
In contrast to ifosfamide, its isomer cyclophosphamide was not associated with long-term nephrotoxicity; neither was carboplatin, a cisplatin analogue, or methotrexate. Although methotrexate is known to cause acute nephrotoxicity, this phenomenon appears to be completely reversible, since methotrexate-treated, long-term cancer survivors didn’t develop tubular or glomerular dysfunction.
This long-term study was supported by the Dutch Kidney Foundation. Dr. Shirali, who was not involved in the study, reported having no financial conflicts.
AT SCM 14
Key clinical point: Today 1 in 570 young adults is a childhood cancer survivor, and many are on the road to chronic kidney disease.
Major finding: Adult survivors of childhood cancer treated with high-dose cisplatin or high-dose ifosfamide had a significantly lower estimated glomerular filtration rate than those who were not.
Data source: This was a retrospective, single-center study involving 763 adult survivors of childhood cancer with a median 18.3 years of follow-up.
Disclosures: This long-term study was supported by the Dutch Kidney Foundation. Dr. Shirali, who was not involved in the study, reported having no financial conflicts.
High total and LDL cholesterol levels increased risk of chronic kidney disease
MELBOURNE – Elevated total cholesterol and low-density lipoprotein cholesterol levels in patients with coronary heart disease were significantly associated with an increased risk of chronic kidney disease, according to a retrospective analysis of data from two large, randomized, controlled trials.
Data presented at the World Congress of Cardiology 2014 showed total cholesterol levels above 240 mg/dL were associated with a significant 78% increase in the risk of chronic kidney disease, while LDL cholesterol greater than 190 mg/dL was associated with a 72% increase in risk.
Elevated non–high-density lipoprotein cholesterol levels and the ratio of total cholesterol to HDL cholesterol were both associated with elevated risk of chronic kidney disease, but reduced HDL cholesterol and the ratio of apolipoprotein B/apolipoprotein A did not significantly affect risk.
Dyslipidemia is present in around 60% of patients with chronic kidney disease, noted presenter Dr. Prakash Deedwania, professor of medicine at the University of California, San Francisco. Previous studies in patients with coronary heart disease and chronic kidney disease also have shown that statins have a renoprotective effect.
However, Dr. Deedwania said there has been little exploration of the impact of baseline lipid parameters on renal function.
"We have found that there is a significant increase in not only the prevalence but also the morbidity and mortality in people with chronic kidney disease with coronary events and other cardiovascular events," Dr. Deedwania said in an interview.
Using data from the Treating to New Targets (TNT) study and Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) study, in which patients were treated with either atorvastatin or simvastatin, researchers were able to examine the relationship between baseline lipoprotein parameters and kidney function in a combined cohort of more than 19,000 patients with coronary heart disease.
"That showed very good relationship between total cholesterol and LDL cholesterol with progressive decline in renal function, which then helped me explain what I had observed earlier in terms of improvement in kidney function in early-stage patients with statins," Dr. Deedwania said at the meeting, which was sponsored by the World Heart Federation.
The relationship between lipoprotein parameters and chronic kidney disease persisted even after adjustment for age, body mass index, smoking status, diabetes history and status, hypertension, and treatment assignment.
The study defined chronic kidney disease as an estimated glomerular filtration rate below 60 mL/min per 1.73 m2. However, Dr. Deedwania said no patients achieved stage 4 kidney disease, and all eGFR measurements fell between 45 and 60 mL/min per 1.73 m2.
The analysis used a relatively early definition of kidney disease as the outcome of interest, observed session chair Dr. Vlado Perkovic, professor of medicine at the University of Sydney (Australia).
Dr. Deedwania later said he believed the key was to focus on early-stage interventions rather than waiting until the disease progressed and suggested some interventional trials had failed to achieve an effect because they were done in more advanced patients.
The researchers declared a range of speakers fees, consultancies, and honoraria from the pharmaceutical industry; two of the authors were employees of Pfizer.
MELBOURNE – Elevated total cholesterol and low-density lipoprotein cholesterol levels in patients with coronary heart disease were significantly associated with an increased risk of chronic kidney disease, according to a retrospective analysis of data from two large, randomized, controlled trials.
Data presented at the World Congress of Cardiology 2014 showed total cholesterol levels above 240 mg/dL were associated with a significant 78% increase in the risk of chronic kidney disease, while LDL cholesterol greater than 190 mg/dL was associated with a 72% increase in risk.
Elevated non–high-density lipoprotein cholesterol levels and the ratio of total cholesterol to HDL cholesterol were both associated with elevated risk of chronic kidney disease, but reduced HDL cholesterol and the ratio of apolipoprotein B/apolipoprotein A did not significantly affect risk.
Dyslipidemia is present in around 60% of patients with chronic kidney disease, noted presenter Dr. Prakash Deedwania, professor of medicine at the University of California, San Francisco. Previous studies in patients with coronary heart disease and chronic kidney disease also have shown that statins have a renoprotective effect.
However, Dr. Deedwania said there has been little exploration of the impact of baseline lipid parameters on renal function.
"We have found that there is a significant increase in not only the prevalence but also the morbidity and mortality in people with chronic kidney disease with coronary events and other cardiovascular events," Dr. Deedwania said in an interview.
Using data from the Treating to New Targets (TNT) study and Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) study, in which patients were treated with either atorvastatin or simvastatin, researchers were able to examine the relationship between baseline lipoprotein parameters and kidney function in a combined cohort of more than 19,000 patients with coronary heart disease.
"That showed very good relationship between total cholesterol and LDL cholesterol with progressive decline in renal function, which then helped me explain what I had observed earlier in terms of improvement in kidney function in early-stage patients with statins," Dr. Deedwania said at the meeting, which was sponsored by the World Heart Federation.
The relationship between lipoprotein parameters and chronic kidney disease persisted even after adjustment for age, body mass index, smoking status, diabetes history and status, hypertension, and treatment assignment.
The study defined chronic kidney disease as an estimated glomerular filtration rate below 60 mL/min per 1.73 m2. However, Dr. Deedwania said no patients achieved stage 4 kidney disease, and all eGFR measurements fell between 45 and 60 mL/min per 1.73 m2.
The analysis used a relatively early definition of kidney disease as the outcome of interest, observed session chair Dr. Vlado Perkovic, professor of medicine at the University of Sydney (Australia).
Dr. Deedwania later said he believed the key was to focus on early-stage interventions rather than waiting until the disease progressed and suggested some interventional trials had failed to achieve an effect because they were done in more advanced patients.
The researchers declared a range of speakers fees, consultancies, and honoraria from the pharmaceutical industry; two of the authors were employees of Pfizer.
MELBOURNE – Elevated total cholesterol and low-density lipoprotein cholesterol levels in patients with coronary heart disease were significantly associated with an increased risk of chronic kidney disease, according to a retrospective analysis of data from two large, randomized, controlled trials.
Data presented at the World Congress of Cardiology 2014 showed total cholesterol levels above 240 mg/dL were associated with a significant 78% increase in the risk of chronic kidney disease, while LDL cholesterol greater than 190 mg/dL was associated with a 72% increase in risk.
Elevated non–high-density lipoprotein cholesterol levels and the ratio of total cholesterol to HDL cholesterol were both associated with elevated risk of chronic kidney disease, but reduced HDL cholesterol and the ratio of apolipoprotein B/apolipoprotein A did not significantly affect risk.
Dyslipidemia is present in around 60% of patients with chronic kidney disease, noted presenter Dr. Prakash Deedwania, professor of medicine at the University of California, San Francisco. Previous studies in patients with coronary heart disease and chronic kidney disease also have shown that statins have a renoprotective effect.
However, Dr. Deedwania said there has been little exploration of the impact of baseline lipid parameters on renal function.
"We have found that there is a significant increase in not only the prevalence but also the morbidity and mortality in people with chronic kidney disease with coronary events and other cardiovascular events," Dr. Deedwania said in an interview.
Using data from the Treating to New Targets (TNT) study and Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) study, in which patients were treated with either atorvastatin or simvastatin, researchers were able to examine the relationship between baseline lipoprotein parameters and kidney function in a combined cohort of more than 19,000 patients with coronary heart disease.
"That showed very good relationship between total cholesterol and LDL cholesterol with progressive decline in renal function, which then helped me explain what I had observed earlier in terms of improvement in kidney function in early-stage patients with statins," Dr. Deedwania said at the meeting, which was sponsored by the World Heart Federation.
The relationship between lipoprotein parameters and chronic kidney disease persisted even after adjustment for age, body mass index, smoking status, diabetes history and status, hypertension, and treatment assignment.
The study defined chronic kidney disease as an estimated glomerular filtration rate below 60 mL/min per 1.73 m2. However, Dr. Deedwania said no patients achieved stage 4 kidney disease, and all eGFR measurements fell between 45 and 60 mL/min per 1.73 m2.
The analysis used a relatively early definition of kidney disease as the outcome of interest, observed session chair Dr. Vlado Perkovic, professor of medicine at the University of Sydney (Australia).
Dr. Deedwania later said he believed the key was to focus on early-stage interventions rather than waiting until the disease progressed and suggested some interventional trials had failed to achieve an effect because they were done in more advanced patients.
The researchers declared a range of speakers fees, consultancies, and honoraria from the pharmaceutical industry; two of the authors were employees of Pfizer.
AT WCC 2014
Key clinical point: Patients with high total cholesterol levels or high LDL cholesterol may be at risk for chronic kidney disease.
Major finding: Total cholesterol levels above 240 mg/dL are associated with a significant 78% increase in the risk of chronic kidney disease among patients with coronary heart disease, while LDL cholesterol greater than 190 mg/dL was associated with a 72% increase in risk.
Data source: Retrospective analysis of data from more than 19,000 patients enrolled in two randomized, controlled trials of statin therapy in patients with coronary heart disease.
Disclosures: Researchers declared a range of speakers fees, consultancies, and honorariums from the pharmaceutical industry; two authors were employees of Pfizer.
FDA Approves Blood Test for Membranous Glomerulonephritis
The Food and Drug Administration has approved a noninvasive test to determine whether a chronic kidney disease is caused by an autoimmune disease or another cause such as infection.
The EUROIMMUN Anti- PLA2R IFA blood test detects an antibody that is specific to primary membranous glomerulonephritis (pMGN). MGN, a chronic kidney disease, damages the glomeruli; it can lead to kidney failure and transplant. Symptoms include swelling, hypercholesterolemia, hypertension, and an increased predisposition to blood clots.
The condition mostly affects white men. It occurs in 2 of every 10,000 people and is more common after age 40, according to the National Library of Medicine. Risk factors include cancers, especially lung and colon cancer; exposure to toxins, including gold and mercury; infections, including hepatitis B, malaria, syphilis, and endocarditis; certain medications, including penicillamine, trimethadione, and skin-lightening creams; and systemic lupus erythematosus, rheumatoid arthritis, Graves’ disease, and other autoimmune disorders.
"Treatment of MGN depends on the underlying cause of the disease," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement. "This test can help patients get a timely diagnosis for their MGN and aid with earlier treatment."
Test manufacturer EUROIMMUN US submitted data that compared 275 blood samples from patients with presumed pMGN, with 285 samples from patients diagnosed with other kidney diseases including secondary MGN (sMGN) and autoimmune diseases. The test detected pMGN in 77% of the presumed pMGN samples, and gave a false-positive result in less than 1% of the other samples.
The diagnostic test helped distinguish pMGN from sMGN in most of the patients.
The FDA said that the test should not be used alone to diagnose pMGN, but that patients’ symptoms and other laboratory test results should also be considered. A kidney biopsy is required for confirmation, according to the FDA.
On Twitter @aliciaault
The Food and Drug Administration has approved a noninvasive test to determine whether a chronic kidney disease is caused by an autoimmune disease or another cause such as infection.
The EUROIMMUN Anti- PLA2R IFA blood test detects an antibody that is specific to primary membranous glomerulonephritis (pMGN). MGN, a chronic kidney disease, damages the glomeruli; it can lead to kidney failure and transplant. Symptoms include swelling, hypercholesterolemia, hypertension, and an increased predisposition to blood clots.
The condition mostly affects white men. It occurs in 2 of every 10,000 people and is more common after age 40, according to the National Library of Medicine. Risk factors include cancers, especially lung and colon cancer; exposure to toxins, including gold and mercury; infections, including hepatitis B, malaria, syphilis, and endocarditis; certain medications, including penicillamine, trimethadione, and skin-lightening creams; and systemic lupus erythematosus, rheumatoid arthritis, Graves’ disease, and other autoimmune disorders.
"Treatment of MGN depends on the underlying cause of the disease," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement. "This test can help patients get a timely diagnosis for their MGN and aid with earlier treatment."
Test manufacturer EUROIMMUN US submitted data that compared 275 blood samples from patients with presumed pMGN, with 285 samples from patients diagnosed with other kidney diseases including secondary MGN (sMGN) and autoimmune diseases. The test detected pMGN in 77% of the presumed pMGN samples, and gave a false-positive result in less than 1% of the other samples.
The diagnostic test helped distinguish pMGN from sMGN in most of the patients.
The FDA said that the test should not be used alone to diagnose pMGN, but that patients’ symptoms and other laboratory test results should also be considered. A kidney biopsy is required for confirmation, according to the FDA.
On Twitter @aliciaault
The Food and Drug Administration has approved a noninvasive test to determine whether a chronic kidney disease is caused by an autoimmune disease or another cause such as infection.
The EUROIMMUN Anti- PLA2R IFA blood test detects an antibody that is specific to primary membranous glomerulonephritis (pMGN). MGN, a chronic kidney disease, damages the glomeruli; it can lead to kidney failure and transplant. Symptoms include swelling, hypercholesterolemia, hypertension, and an increased predisposition to blood clots.
The condition mostly affects white men. It occurs in 2 of every 10,000 people and is more common after age 40, according to the National Library of Medicine. Risk factors include cancers, especially lung and colon cancer; exposure to toxins, including gold and mercury; infections, including hepatitis B, malaria, syphilis, and endocarditis; certain medications, including penicillamine, trimethadione, and skin-lightening creams; and systemic lupus erythematosus, rheumatoid arthritis, Graves’ disease, and other autoimmune disorders.
"Treatment of MGN depends on the underlying cause of the disease," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement. "This test can help patients get a timely diagnosis for their MGN and aid with earlier treatment."
Test manufacturer EUROIMMUN US submitted data that compared 275 blood samples from patients with presumed pMGN, with 285 samples from patients diagnosed with other kidney diseases including secondary MGN (sMGN) and autoimmune diseases. The test detected pMGN in 77% of the presumed pMGN samples, and gave a false-positive result in less than 1% of the other samples.
The diagnostic test helped distinguish pMGN from sMGN in most of the patients.
The FDA said that the test should not be used alone to diagnose pMGN, but that patients’ symptoms and other laboratory test results should also be considered. A kidney biopsy is required for confirmation, according to the FDA.
On Twitter @aliciaault
Rituximab yielded long-term benefit in lupus nephritis
PARIS – Treatment with rituximab in patients with refractory lupus nephritis was associated with significant long-term improvement in symptoms, histology, and laboratory markers in a prospective study.
Over a median follow-up period of 18 months (interquartile range, 12-36 months), the drug normalized proteinuria and was associated with a median reduction in glucocorticoid use of 28 mg/day, according to Dr. Maria Tsanyan of the Nasonova Research Institute of Rheumatology, Moscow.
She and her colleagues followed 60 patients with refractory lupus nephritis who had a median age of 26 years and median disease duration of 37.5 months. At baseline, the patients’ median glomerular filtration rate was 46 mL/min, and their median amount of proteinuria was 1.83 g/day. A total of 14 patients (23%) had nephrotic syndrome.
All patients had failed prior therapy. These treatments included high-dose glucocorticoids (45%), pulsed high-dose glucocorticoid and cyclophosphamide (90%), mycophenolate mofetil (17%), azathioprine (7%), hydroxychloroquine (17%), and cyclosporine A (8%).
Treatment with rituximab varied according to clinical status and response. The initial doses were 1,000 mg in 25 patients and 2,000 mg in 30 patients. Five patients did not receive a full initial course of rituximab because of the development of end-stage renal disease, including four who received only 500 mg and one who received 1,500 mg.
Most (37) required more than one course: 21 needed two courses, 11 needed three, 4 needed four courses, and 1 received five courses of the drug.
In response to an audience member’s question about the basis on which the investigators decided to re-treat patients with rituximab, Dr. Tsanyan said that they tried to achieve complete responses in patients with disease exacerbation or partial response at 6 months or 12 months after the initial dose.
Based on the Systemic Lupus Erythematosus International Collaborating Clinics renal activity/response exercise criteria, 58% had a complete response, 22% had a partial response, 12% were nonresponders, and 8% died of acute renal failure. Overall, 28% of patients had an exacerbation of disease during treatment.
There was no difference in response between patients who received 1,000 mg and 2,000 mg rituximab, she said at the annual European Congress of Rheumatology.
By 6 months of treatment, the median Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score had decreased from 20 to 4. This improvement held at 12 months. By 42 months, the median score had dropped to 2. By SLEDAI-2K index, 50% were considered to be complete responders, 30% partial responders, and 12% nonresponders.
Antibodies to double-stranded DNA fell from a mean of 67.7 U/mL at baseline to 34.5 U/mL at 42 months. Daily glucocorticoid use dropped from 35 mg to 7.5 mg.
C3 and C4 complement components also improved. C3 rose from 0.7 to 0.98 g/L, and C4 from 0.1 g/L to 0.24 g/L. Daily proteinuria fell from 1.6 g/day to 0.01 g/day and serum total protein rose from 59 g/L to 72 g/L.
Sixteen patients had renal biopsies both at baseline and at the end of their follow-up period. Of 14 patients who had World Health Organization class IV nephritis at baseline, 5 were unchanged, 3 moved to class III, 5 to class II, and 1 to class I. Two class III patients improved to class I.
There were 13 adverse events, all of which occurred during the first 3 months of rituximab therapy. These included pneumonia (4), aspergillosis (1), bronchitis (1), cystitis (2), shingles (3), furunculosis (1) and pityriasis versicolor (1).
Dr. Tsanyan did not have any financial disclosures.
PARIS – Treatment with rituximab in patients with refractory lupus nephritis was associated with significant long-term improvement in symptoms, histology, and laboratory markers in a prospective study.
Over a median follow-up period of 18 months (interquartile range, 12-36 months), the drug normalized proteinuria and was associated with a median reduction in glucocorticoid use of 28 mg/day, according to Dr. Maria Tsanyan of the Nasonova Research Institute of Rheumatology, Moscow.
She and her colleagues followed 60 patients with refractory lupus nephritis who had a median age of 26 years and median disease duration of 37.5 months. At baseline, the patients’ median glomerular filtration rate was 46 mL/min, and their median amount of proteinuria was 1.83 g/day. A total of 14 patients (23%) had nephrotic syndrome.
All patients had failed prior therapy. These treatments included high-dose glucocorticoids (45%), pulsed high-dose glucocorticoid and cyclophosphamide (90%), mycophenolate mofetil (17%), azathioprine (7%), hydroxychloroquine (17%), and cyclosporine A (8%).
Treatment with rituximab varied according to clinical status and response. The initial doses were 1,000 mg in 25 patients and 2,000 mg in 30 patients. Five patients did not receive a full initial course of rituximab because of the development of end-stage renal disease, including four who received only 500 mg and one who received 1,500 mg.
Most (37) required more than one course: 21 needed two courses, 11 needed three, 4 needed four courses, and 1 received five courses of the drug.
In response to an audience member’s question about the basis on which the investigators decided to re-treat patients with rituximab, Dr. Tsanyan said that they tried to achieve complete responses in patients with disease exacerbation or partial response at 6 months or 12 months after the initial dose.
Based on the Systemic Lupus Erythematosus International Collaborating Clinics renal activity/response exercise criteria, 58% had a complete response, 22% had a partial response, 12% were nonresponders, and 8% died of acute renal failure. Overall, 28% of patients had an exacerbation of disease during treatment.
There was no difference in response between patients who received 1,000 mg and 2,000 mg rituximab, she said at the annual European Congress of Rheumatology.
By 6 months of treatment, the median Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score had decreased from 20 to 4. This improvement held at 12 months. By 42 months, the median score had dropped to 2. By SLEDAI-2K index, 50% were considered to be complete responders, 30% partial responders, and 12% nonresponders.
Antibodies to double-stranded DNA fell from a mean of 67.7 U/mL at baseline to 34.5 U/mL at 42 months. Daily glucocorticoid use dropped from 35 mg to 7.5 mg.
C3 and C4 complement components also improved. C3 rose from 0.7 to 0.98 g/L, and C4 from 0.1 g/L to 0.24 g/L. Daily proteinuria fell from 1.6 g/day to 0.01 g/day and serum total protein rose from 59 g/L to 72 g/L.
Sixteen patients had renal biopsies both at baseline and at the end of their follow-up period. Of 14 patients who had World Health Organization class IV nephritis at baseline, 5 were unchanged, 3 moved to class III, 5 to class II, and 1 to class I. Two class III patients improved to class I.
There were 13 adverse events, all of which occurred during the first 3 months of rituximab therapy. These included pneumonia (4), aspergillosis (1), bronchitis (1), cystitis (2), shingles (3), furunculosis (1) and pityriasis versicolor (1).
Dr. Tsanyan did not have any financial disclosures.
PARIS – Treatment with rituximab in patients with refractory lupus nephritis was associated with significant long-term improvement in symptoms, histology, and laboratory markers in a prospective study.
Over a median follow-up period of 18 months (interquartile range, 12-36 months), the drug normalized proteinuria and was associated with a median reduction in glucocorticoid use of 28 mg/day, according to Dr. Maria Tsanyan of the Nasonova Research Institute of Rheumatology, Moscow.
She and her colleagues followed 60 patients with refractory lupus nephritis who had a median age of 26 years and median disease duration of 37.5 months. At baseline, the patients’ median glomerular filtration rate was 46 mL/min, and their median amount of proteinuria was 1.83 g/day. A total of 14 patients (23%) had nephrotic syndrome.
All patients had failed prior therapy. These treatments included high-dose glucocorticoids (45%), pulsed high-dose glucocorticoid and cyclophosphamide (90%), mycophenolate mofetil (17%), azathioprine (7%), hydroxychloroquine (17%), and cyclosporine A (8%).
Treatment with rituximab varied according to clinical status and response. The initial doses were 1,000 mg in 25 patients and 2,000 mg in 30 patients. Five patients did not receive a full initial course of rituximab because of the development of end-stage renal disease, including four who received only 500 mg and one who received 1,500 mg.
Most (37) required more than one course: 21 needed two courses, 11 needed three, 4 needed four courses, and 1 received five courses of the drug.
In response to an audience member’s question about the basis on which the investigators decided to re-treat patients with rituximab, Dr. Tsanyan said that they tried to achieve complete responses in patients with disease exacerbation or partial response at 6 months or 12 months after the initial dose.
Based on the Systemic Lupus Erythematosus International Collaborating Clinics renal activity/response exercise criteria, 58% had a complete response, 22% had a partial response, 12% were nonresponders, and 8% died of acute renal failure. Overall, 28% of patients had an exacerbation of disease during treatment.
There was no difference in response between patients who received 1,000 mg and 2,000 mg rituximab, she said at the annual European Congress of Rheumatology.
By 6 months of treatment, the median Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score had decreased from 20 to 4. This improvement held at 12 months. By 42 months, the median score had dropped to 2. By SLEDAI-2K index, 50% were considered to be complete responders, 30% partial responders, and 12% nonresponders.
Antibodies to double-stranded DNA fell from a mean of 67.7 U/mL at baseline to 34.5 U/mL at 42 months. Daily glucocorticoid use dropped from 35 mg to 7.5 mg.
C3 and C4 complement components also improved. C3 rose from 0.7 to 0.98 g/L, and C4 from 0.1 g/L to 0.24 g/L. Daily proteinuria fell from 1.6 g/day to 0.01 g/day and serum total protein rose from 59 g/L to 72 g/L.
Sixteen patients had renal biopsies both at baseline and at the end of their follow-up period. Of 14 patients who had World Health Organization class IV nephritis at baseline, 5 were unchanged, 3 moved to class III, 5 to class II, and 1 to class I. Two class III patients improved to class I.
There were 13 adverse events, all of which occurred during the first 3 months of rituximab therapy. These included pneumonia (4), aspergillosis (1), bronchitis (1), cystitis (2), shingles (3), furunculosis (1) and pityriasis versicolor (1).
Dr. Tsanyan did not have any financial disclosures.
AT THE EULAR CONGRESS 2014
Key clinical point: Rituximab delivered long-term results for patients with refractory lupus nephritis.
Major finding: During a median follow-up period of 18 months, the drug normalized proteinuria, improved glomerular filtration rate, and was associated with a median steroid reduction of 28 mg/day.
Data source: An open-label, prospective study of 60 patients with refractory lupus nephritis.
Disclosures: Dr. Maria Tsanyan had no financial disclosures.
Hyponatremia linked to osteoporosis, fragility fractures
CHICAGO – Hyponatremia quadruples the risk of osteoporosis and fragility fractures, according to a retrospective database study presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
A team from Georgetown University in Washington matched 30,517 patients diagnosed with osteoporosis to 30,517 controls for age, race, sex, and how long they had been in the database of the MedStar Health System, which serves Baltimore and Washington.
The investigators found that patients with chronic hyponatremia – at least two sodium values below 135 mmol/L at least 1 year apart – were far more likely to be later diagnosed with osteoporosis (adjusted odds ratio, 3.99). Recent hyponatremia – at least one value below 135 mmol/L in the previous 30 days – also increased the risk (aOR, 3.08). In contrast, glucocorticoid use, a known osteoporosis risk factor, was associated with a far lower risk (aOR, 1.4), the team reported in a poster session at the meeting.
The researchers had similar results when they matched 46,256 patients with fragility fractures to 46,256 without: The fracture risk was substantially increased in patients with chronic hyponatremia (aOR, 4.71) and recent hyponatremia (aOR, 3.08). A previous diagnosis of osteoporosis – again, a known risk factor – increased the risk only moderately (aOR, 1.8).
The severity of hyponatremia played a role, too; patients with at least one value below 125 mmol/L had the highest risk for osteoporosis and fragility fractures.
The findings were all statistically significant.
"The results of this study support the hypothesis that hyponatremia is a significant and clinically important risk factor for both osteoporosis and bone fractures in inpatients and outpatients," the team concluded.
"We were surprised by the odds ratios and how strong a factor this was. Right now, hyponatremia is not an indication for bone mineral density [testing] because it’s never been considered to be a risk factor. It ought to be added as an indication. Patients with hyponatremia beyond an isolated single event should be evaluated for their bone density and fracture risk" no matter their age or sex, said senior investigator Dr. Joseph Verbalis, chief of the division on endocrinology and metabolism at Georgetown.
Based on the findings, "we [speculate] that early treatment of hyponatremia will prevent progression of bone disease and decrease fracture risk," and perhaps even obviate the need for bisphosphonates. "It’s an implication that needs to be followed up with definitive studies," he said.
Chronic hyponatremia increases osteoclast proliferation and activity, while recent hyponatremia reduces reaction time and makes it less likely people will catch themselves if they stumble. Elderly people are most at risk, either from overzealous salt restriction, sodium-depleting drugs like thiazide diuretics, or the syndrome of inappropriate antidiuretic hormone secretion (Indian J. Endocrinol. Metab. 2011;15(Suppl3):S208-S215).
The mean age of subjects in the osteoporosis analysis was about 75 years, and almost 90% were women. In the fragility fracture analysis, the mean age was about 60 years, and just over half the subjects were women.
Among the roughly 3 million patients the team initially sampled at the start of their work, there was a more than twofold increase in the prevalence of osteoporosis in hyponatremic (4.6%) vs. nonhyponatremic (1.8%) subjects, and a similar increase in vertebral or long-bone fractures (9.5% vs. 3.7%).
Dr. Verbalis is a consultant, investigator, speaker, and adviser for Otsuka, the maker of the hyponatremia drug tolvaptan. He is also a consultant for Cornerstone Therapeutics and Ferring Pharmaceuticals. The study had no outside funding.
CHICAGO – Hyponatremia quadruples the risk of osteoporosis and fragility fractures, according to a retrospective database study presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
A team from Georgetown University in Washington matched 30,517 patients diagnosed with osteoporosis to 30,517 controls for age, race, sex, and how long they had been in the database of the MedStar Health System, which serves Baltimore and Washington.
The investigators found that patients with chronic hyponatremia – at least two sodium values below 135 mmol/L at least 1 year apart – were far more likely to be later diagnosed with osteoporosis (adjusted odds ratio, 3.99). Recent hyponatremia – at least one value below 135 mmol/L in the previous 30 days – also increased the risk (aOR, 3.08). In contrast, glucocorticoid use, a known osteoporosis risk factor, was associated with a far lower risk (aOR, 1.4), the team reported in a poster session at the meeting.
The researchers had similar results when they matched 46,256 patients with fragility fractures to 46,256 without: The fracture risk was substantially increased in patients with chronic hyponatremia (aOR, 4.71) and recent hyponatremia (aOR, 3.08). A previous diagnosis of osteoporosis – again, a known risk factor – increased the risk only moderately (aOR, 1.8).
The severity of hyponatremia played a role, too; patients with at least one value below 125 mmol/L had the highest risk for osteoporosis and fragility fractures.
The findings were all statistically significant.
"The results of this study support the hypothesis that hyponatremia is a significant and clinically important risk factor for both osteoporosis and bone fractures in inpatients and outpatients," the team concluded.
"We were surprised by the odds ratios and how strong a factor this was. Right now, hyponatremia is not an indication for bone mineral density [testing] because it’s never been considered to be a risk factor. It ought to be added as an indication. Patients with hyponatremia beyond an isolated single event should be evaluated for their bone density and fracture risk" no matter their age or sex, said senior investigator Dr. Joseph Verbalis, chief of the division on endocrinology and metabolism at Georgetown.
Based on the findings, "we [speculate] that early treatment of hyponatremia will prevent progression of bone disease and decrease fracture risk," and perhaps even obviate the need for bisphosphonates. "It’s an implication that needs to be followed up with definitive studies," he said.
Chronic hyponatremia increases osteoclast proliferation and activity, while recent hyponatremia reduces reaction time and makes it less likely people will catch themselves if they stumble. Elderly people are most at risk, either from overzealous salt restriction, sodium-depleting drugs like thiazide diuretics, or the syndrome of inappropriate antidiuretic hormone secretion (Indian J. Endocrinol. Metab. 2011;15(Suppl3):S208-S215).
The mean age of subjects in the osteoporosis analysis was about 75 years, and almost 90% were women. In the fragility fracture analysis, the mean age was about 60 years, and just over half the subjects were women.
Among the roughly 3 million patients the team initially sampled at the start of their work, there was a more than twofold increase in the prevalence of osteoporosis in hyponatremic (4.6%) vs. nonhyponatremic (1.8%) subjects, and a similar increase in vertebral or long-bone fractures (9.5% vs. 3.7%).
Dr. Verbalis is a consultant, investigator, speaker, and adviser for Otsuka, the maker of the hyponatremia drug tolvaptan. He is also a consultant for Cornerstone Therapeutics and Ferring Pharmaceuticals. The study had no outside funding.
CHICAGO – Hyponatremia quadruples the risk of osteoporosis and fragility fractures, according to a retrospective database study presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
A team from Georgetown University in Washington matched 30,517 patients diagnosed with osteoporosis to 30,517 controls for age, race, sex, and how long they had been in the database of the MedStar Health System, which serves Baltimore and Washington.
The investigators found that patients with chronic hyponatremia – at least two sodium values below 135 mmol/L at least 1 year apart – were far more likely to be later diagnosed with osteoporosis (adjusted odds ratio, 3.99). Recent hyponatremia – at least one value below 135 mmol/L in the previous 30 days – also increased the risk (aOR, 3.08). In contrast, glucocorticoid use, a known osteoporosis risk factor, was associated with a far lower risk (aOR, 1.4), the team reported in a poster session at the meeting.
The researchers had similar results when they matched 46,256 patients with fragility fractures to 46,256 without: The fracture risk was substantially increased in patients with chronic hyponatremia (aOR, 4.71) and recent hyponatremia (aOR, 3.08). A previous diagnosis of osteoporosis – again, a known risk factor – increased the risk only moderately (aOR, 1.8).
The severity of hyponatremia played a role, too; patients with at least one value below 125 mmol/L had the highest risk for osteoporosis and fragility fractures.
The findings were all statistically significant.
"The results of this study support the hypothesis that hyponatremia is a significant and clinically important risk factor for both osteoporosis and bone fractures in inpatients and outpatients," the team concluded.
"We were surprised by the odds ratios and how strong a factor this was. Right now, hyponatremia is not an indication for bone mineral density [testing] because it’s never been considered to be a risk factor. It ought to be added as an indication. Patients with hyponatremia beyond an isolated single event should be evaluated for their bone density and fracture risk" no matter their age or sex, said senior investigator Dr. Joseph Verbalis, chief of the division on endocrinology and metabolism at Georgetown.
Based on the findings, "we [speculate] that early treatment of hyponatremia will prevent progression of bone disease and decrease fracture risk," and perhaps even obviate the need for bisphosphonates. "It’s an implication that needs to be followed up with definitive studies," he said.
Chronic hyponatremia increases osteoclast proliferation and activity, while recent hyponatremia reduces reaction time and makes it less likely people will catch themselves if they stumble. Elderly people are most at risk, either from overzealous salt restriction, sodium-depleting drugs like thiazide diuretics, or the syndrome of inappropriate antidiuretic hormone secretion (Indian J. Endocrinol. Metab. 2011;15(Suppl3):S208-S215).
The mean age of subjects in the osteoporosis analysis was about 75 years, and almost 90% were women. In the fragility fracture analysis, the mean age was about 60 years, and just over half the subjects were women.
Among the roughly 3 million patients the team initially sampled at the start of their work, there was a more than twofold increase in the prevalence of osteoporosis in hyponatremic (4.6%) vs. nonhyponatremic (1.8%) subjects, and a similar increase in vertebral or long-bone fractures (9.5% vs. 3.7%).
Dr. Verbalis is a consultant, investigator, speaker, and adviser for Otsuka, the maker of the hyponatremia drug tolvaptan. He is also a consultant for Cornerstone Therapeutics and Ferring Pharmaceuticals. The study had no outside funding.
AT ICE/ENDO 2014
Key clinical point: Early treatment of hyponatremia may one day prove to slow the progression of osteoporosis and reduce the need for bisphosphonates.
Major finding: In patients with chronic hyponatremia, the adjusted odds ratio for developing osteoporosis was 3.99, compared with those without.
Data Source: Retrospective case-control study involving more than 150,000 subjects.
Disclosures: Dr. Verbalis is a consultant, investigator, speaker, and adviser for Otsuka, the maker of the hyponatremia drug tolvaptan. The study had no outside funding.
Trial of sirukumab for lupus nephritis falls flat
PARIS – The investigational interleukin-6 blocker sirukumab provided no benefit to patients with lupus nephritis but put them at a very high risk of developing a serious infection in a small, randomized, placebo-controlled trial.
Based on the study results, the study sponsor, Janssen, has shut down its program for the lupus nephritis indication, Dr. Ronald van Vollenhoven said at the annual European Congress of Rheumatology.
"While a few patients did experience a reduction in proteinuria, we had an unacceptably high rate of adverse events," said Dr. van Vollenhoven, chief of the Unit for Clinical Therapy Research, Inflammatory Diseases, at the Karolinska Institute, Stockholm. "Based on these findings, we will not be advancing any further investigation of sirukumab for patients with active lupus nephritis."
The company continues to develop the drug for rheumatoid arthritis.
In the trial, 21 patients received intravenous sirukumab 10 mg/kg once every 4 weeks for 24 weeks, and 4 received placebo. During the trial and a 16-week safety observation follow-up period, 48% of those who took the investigational IL-6 blocker developed a serious adverse event, including infections serious enough to require hospital admission.
While not specifying the infections, which occurred in 10 patients, Dr. van Vollenhoven noted that five patients taking the drug discontinued it because of adverse events, which included Haemophilus influenzae pneumonia, elevated liver enzymes, anaphylactic reaction after the first dose, worsening nephritis, and severe neutropenia.
Overall, 19 patients completed the study. In addition to the five who quit because of adverse events, one additional patient withdrew voluntarily. All had active lupus nephritis of about 50 months’ duration, with a mean daily proteinuria of more than 2 g; about a third of the group had nephrotic proteinuria. The mean Systemic Lupus Erythematosus Disease Activity Index 2000 was about 16. All were taking concomitant mycophenolate mofetil or azathioprine.
At the end of the treatment period, there was no significant between-group difference in proteinuria, Dr. van Vollenhoven said. Four of those in the active group experienced at least a 50% reduction in proteinuria over baseline, while none of those in the placebo group experienced this change. However, this difference was not statistically significant. There were no significant changes in the patient or physical global assessment for either group.
Serious adverse events occurred in 48% of those taking the drug, including serious infections (30%) as well as renal/urinary (19%), blood (9.5%), and gastrointestinal (9.5%) events. None occurred in the placebo group.
Among patients in the sirukumab group, there was one grade 4 lymphocytopenia, one grade 4 neutropenia, and two grade 3 neutropenias. One patient had a grade 2 liver enzyme elevation.
Dr. van Vollenhoven was on the study steering committee and is a consultant and speaker for Janssen, as well as other drug manufacturers. Four coauthors are employees of Janssen.
PARIS – The investigational interleukin-6 blocker sirukumab provided no benefit to patients with lupus nephritis but put them at a very high risk of developing a serious infection in a small, randomized, placebo-controlled trial.
Based on the study results, the study sponsor, Janssen, has shut down its program for the lupus nephritis indication, Dr. Ronald van Vollenhoven said at the annual European Congress of Rheumatology.
"While a few patients did experience a reduction in proteinuria, we had an unacceptably high rate of adverse events," said Dr. van Vollenhoven, chief of the Unit for Clinical Therapy Research, Inflammatory Diseases, at the Karolinska Institute, Stockholm. "Based on these findings, we will not be advancing any further investigation of sirukumab for patients with active lupus nephritis."
The company continues to develop the drug for rheumatoid arthritis.
In the trial, 21 patients received intravenous sirukumab 10 mg/kg once every 4 weeks for 24 weeks, and 4 received placebo. During the trial and a 16-week safety observation follow-up period, 48% of those who took the investigational IL-6 blocker developed a serious adverse event, including infections serious enough to require hospital admission.
While not specifying the infections, which occurred in 10 patients, Dr. van Vollenhoven noted that five patients taking the drug discontinued it because of adverse events, which included Haemophilus influenzae pneumonia, elevated liver enzymes, anaphylactic reaction after the first dose, worsening nephritis, and severe neutropenia.
Overall, 19 patients completed the study. In addition to the five who quit because of adverse events, one additional patient withdrew voluntarily. All had active lupus nephritis of about 50 months’ duration, with a mean daily proteinuria of more than 2 g; about a third of the group had nephrotic proteinuria. The mean Systemic Lupus Erythematosus Disease Activity Index 2000 was about 16. All were taking concomitant mycophenolate mofetil or azathioprine.
At the end of the treatment period, there was no significant between-group difference in proteinuria, Dr. van Vollenhoven said. Four of those in the active group experienced at least a 50% reduction in proteinuria over baseline, while none of those in the placebo group experienced this change. However, this difference was not statistically significant. There were no significant changes in the patient or physical global assessment for either group.
Serious adverse events occurred in 48% of those taking the drug, including serious infections (30%) as well as renal/urinary (19%), blood (9.5%), and gastrointestinal (9.5%) events. None occurred in the placebo group.
Among patients in the sirukumab group, there was one grade 4 lymphocytopenia, one grade 4 neutropenia, and two grade 3 neutropenias. One patient had a grade 2 liver enzyme elevation.
Dr. van Vollenhoven was on the study steering committee and is a consultant and speaker for Janssen, as well as other drug manufacturers. Four coauthors are employees of Janssen.
PARIS – The investigational interleukin-6 blocker sirukumab provided no benefit to patients with lupus nephritis but put them at a very high risk of developing a serious infection in a small, randomized, placebo-controlled trial.
Based on the study results, the study sponsor, Janssen, has shut down its program for the lupus nephritis indication, Dr. Ronald van Vollenhoven said at the annual European Congress of Rheumatology.
"While a few patients did experience a reduction in proteinuria, we had an unacceptably high rate of adverse events," said Dr. van Vollenhoven, chief of the Unit for Clinical Therapy Research, Inflammatory Diseases, at the Karolinska Institute, Stockholm. "Based on these findings, we will not be advancing any further investigation of sirukumab for patients with active lupus nephritis."
The company continues to develop the drug for rheumatoid arthritis.
In the trial, 21 patients received intravenous sirukumab 10 mg/kg once every 4 weeks for 24 weeks, and 4 received placebo. During the trial and a 16-week safety observation follow-up period, 48% of those who took the investigational IL-6 blocker developed a serious adverse event, including infections serious enough to require hospital admission.
While not specifying the infections, which occurred in 10 patients, Dr. van Vollenhoven noted that five patients taking the drug discontinued it because of adverse events, which included Haemophilus influenzae pneumonia, elevated liver enzymes, anaphylactic reaction after the first dose, worsening nephritis, and severe neutropenia.
Overall, 19 patients completed the study. In addition to the five who quit because of adverse events, one additional patient withdrew voluntarily. All had active lupus nephritis of about 50 months’ duration, with a mean daily proteinuria of more than 2 g; about a third of the group had nephrotic proteinuria. The mean Systemic Lupus Erythematosus Disease Activity Index 2000 was about 16. All were taking concomitant mycophenolate mofetil or azathioprine.
At the end of the treatment period, there was no significant between-group difference in proteinuria, Dr. van Vollenhoven said. Four of those in the active group experienced at least a 50% reduction in proteinuria over baseline, while none of those in the placebo group experienced this change. However, this difference was not statistically significant. There were no significant changes in the patient or physical global assessment for either group.
Serious adverse events occurred in 48% of those taking the drug, including serious infections (30%) as well as renal/urinary (19%), blood (9.5%), and gastrointestinal (9.5%) events. None occurred in the placebo group.
Among patients in the sirukumab group, there was one grade 4 lymphocytopenia, one grade 4 neutropenia, and two grade 3 neutropenias. One patient had a grade 2 liver enzyme elevation.
Dr. van Vollenhoven was on the study steering committee and is a consultant and speaker for Janssen, as well as other drug manufacturers. Four coauthors are employees of Janssen.
AT THE EULAR CONGRESS 2014
Key clinical point: Janssen has discontinued its investigation of sirukumab for lupus nephritis.
Major finding: Almost half of those who took sirukumab developed a serious adverse event, including infections requiring hospitalization.
Data source: A randomized, placebo-controlled trial of 21 patients.
Disclosures: The study was sponsored by Janssen. Dr. van Vollenhoven was on the study steering committee and is a consultant and speaker for the company, as well as other drug manufacturers. Four coauthors are employees of Janssen.
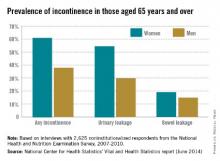
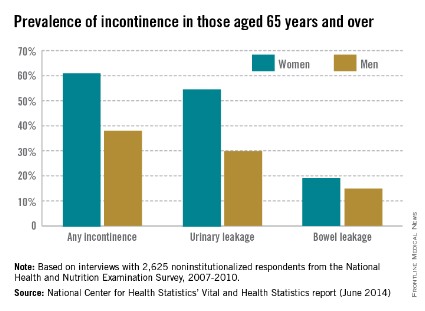
More than half of older women have experienced incontinence
Among noninstitutionalized Americans aged 65 years and older, 61.2% of women and 38% of men experience at least occasional urinary or bowel incontinence, the National Center for Health Statistics reported.
Data from the 2007-2010 National Health and Nutrition Examination Survey (NHANES) show that 54.8% of women and 29.9% of men had urinary leakage at least a few times a month, while 19.2% of women and 14.9% of men had accidental bowel leakage of mucus, liquid stool, or solid stool at least one to three times a month, according to the NCHS (Vital Health Stat. 2014;3[36]).
Among women aged 65-74 years, 60.6% had some type of incontinence, compared with 61.9% of women aged 75 years and over. The difference was larger for men, however, with 34.1% of those aged 65-74 years reporting incontinence, compared with 42.4% of men aged 75 years and over, the report showed.
The NHANES data are based on in-home interviews with a nationally representative sample of 2,625 respondents.
Among noninstitutionalized Americans aged 65 years and older, 61.2% of women and 38% of men experience at least occasional urinary or bowel incontinence, the National Center for Health Statistics reported.
Data from the 2007-2010 National Health and Nutrition Examination Survey (NHANES) show that 54.8% of women and 29.9% of men had urinary leakage at least a few times a month, while 19.2% of women and 14.9% of men had accidental bowel leakage of mucus, liquid stool, or solid stool at least one to three times a month, according to the NCHS (Vital Health Stat. 2014;3[36]).
Among women aged 65-74 years, 60.6% had some type of incontinence, compared with 61.9% of women aged 75 years and over. The difference was larger for men, however, with 34.1% of those aged 65-74 years reporting incontinence, compared with 42.4% of men aged 75 years and over, the report showed.
The NHANES data are based on in-home interviews with a nationally representative sample of 2,625 respondents.
Among noninstitutionalized Americans aged 65 years and older, 61.2% of women and 38% of men experience at least occasional urinary or bowel incontinence, the National Center for Health Statistics reported.
Data from the 2007-2010 National Health and Nutrition Examination Survey (NHANES) show that 54.8% of women and 29.9% of men had urinary leakage at least a few times a month, while 19.2% of women and 14.9% of men had accidental bowel leakage of mucus, liquid stool, or solid stool at least one to three times a month, according to the NCHS (Vital Health Stat. 2014;3[36]).
Among women aged 65-74 years, 60.6% had some type of incontinence, compared with 61.9% of women aged 75 years and over. The difference was larger for men, however, with 34.1% of those aged 65-74 years reporting incontinence, compared with 42.4% of men aged 75 years and over, the report showed.
The NHANES data are based on in-home interviews with a nationally representative sample of 2,625 respondents.
Debate continues on antibiotic prophylaxis for UTIs in children with reflux
ORLANDO – Antibiotic prophylaxis was shown in the recently published RIVUR trial to halve the risk of recurrent febrile urinary tract infection in infants and young children with vesicoureteral reflux. Conversely, a meta-analysis of six controlled trials concluded there is little to no benefit of antibiotic prophylaxis in this population.
The conflicting data highlight the ongoing debate regarding the best approach for preventing and managing febrile urinary tract infections (UTIs) in children with vesicoureteral reflux (VUR).
The RIVUR (Randomized Intervention for Children with Vesicoureteral Reflux) trial findings, which were published in May in the New England Journal of Medicine, showed that among children with VUR who were aged 2-71 months, 2 years of treatment with trimethoprim-sulfamethoxazole halved the risk of febrile or symptomatic UTI recurrence (N. Eng. J. Med. 2014;370:2367-76). The difference between those who received prophylaxis and those who did not emerged early and increased over the 2-year study period, Dr. Saul Greenfield said during an update on the trial results at the annual meeting of the American Urological Association.
"The magnitude of treatment effect warrants serious consideration of prophylaxis in these children. We also feel that these results warrant reconsideration of the recent guideline recommendations by the American Academy of Pediatrics in 2011 ... advising against evaluation of children with a VCUG [voiding cystourethrogram] after their first UTI," said Dr. Greenfield, director of pediatric urology at Women and Children’s Hospital of Buffalo, N.Y. and one of the RIVUR investigators.
That AAP recommendation was based on a number of studies that showed no benefit from antibiotic prophylaxis in children with VUR, which suggests there is little value in diagnosing VUR (Pediatrics 2011;28:595-610).
In fact, a meta-analysis of six controlled trials, which also was presented at the AUA meeting, suggested that the benefit of antibiotic prophylaxis for preventing febrile UTIs in children with VUR is small at best, and evidence to support its use is lacking.
Pooled data for 986 patients included in the trials showed that in 417 with dilating VUR, the risk of recurrent febrile UTI was 22.46% in those who received antibiotics, and 29.79% in controls. The relative risk of treatment failure with antibiotic prophylaxis was 0.75, and the absolute risk reduction was 7.33%, Dr. José Netto reported.
The number needed to treat to prevent one UTI was 13.64, according to Dr. Netto of State University of Feira de Santana in Brazil.
In 515 patients with nondilating VUR, the risk of febrile UTI was 5.31% in patients who received prophylactic antibiotics, and 6.09% in those who did not. The relative risk of treatment failure was 0.87, and the absolute risk reduction was 0.78%. The number needed to treat was 129, Dr. Netto said.
The studies included in the meta-analysis were published prior to August 2013. Only one was placebo-controlled. Patients included in the study included 663 girls (67.2%), and the median age of all patients was 21 months.
The studies all used co-trimoxazole in standard doses, and three of the six also used co-amoxiclav or nitrofurantoin as alternative treatments.
Antibiotic prophylaxis is often used in children with VUR, but several recent studies have failed to demonstrate the usefulness of this approach with respect to reducing the risk of febrile UTI, Dr. Netto said, noting that the studies included heterogeneous populations with distinct grades of VUR and varying rates of recurrent UTI.
Based on the current findings, it remains uncertain whether antibiotic prophylaxis reduces the risk of febrile UTI in children with VUR, although it is possible – given the differences seen between those with dilating VUR and those with nondilating VUR – that specific subgroups of children with dilating VUR may benefit from prophylaxis, he concluded.
The conflicting findings between this meta-analysis, and the RIVUR trial underscore the importance of ongoing evaluation of antibiotic prophylaxis.
"The best ways to prevent and treat febrile urinary tract infections in children with VUR are still very much up for discussion," Dr. Anthony Atala, W.H. Boyce Professor and chair of the urology department at Wake Forest Baptist Medical Center in Winston-Salem, N.C., said in an AUA press statement.
"Examining and reexamining the AAP guidelines and practices that guide our work are critical to better understand the condition and improve the lives of those children who are living with the condition each day," said Dr. Atala, who is also director of the Wake Forest Institute for Regenerative Medicine.
Dr. Atala reported serving in a leadership position at Plureon Corp. Dr. Greenfield and Dr. Netto reported having no disclosures.
ORLANDO – Antibiotic prophylaxis was shown in the recently published RIVUR trial to halve the risk of recurrent febrile urinary tract infection in infants and young children with vesicoureteral reflux. Conversely, a meta-analysis of six controlled trials concluded there is little to no benefit of antibiotic prophylaxis in this population.
The conflicting data highlight the ongoing debate regarding the best approach for preventing and managing febrile urinary tract infections (UTIs) in children with vesicoureteral reflux (VUR).
The RIVUR (Randomized Intervention for Children with Vesicoureteral Reflux) trial findings, which were published in May in the New England Journal of Medicine, showed that among children with VUR who were aged 2-71 months, 2 years of treatment with trimethoprim-sulfamethoxazole halved the risk of febrile or symptomatic UTI recurrence (N. Eng. J. Med. 2014;370:2367-76). The difference between those who received prophylaxis and those who did not emerged early and increased over the 2-year study period, Dr. Saul Greenfield said during an update on the trial results at the annual meeting of the American Urological Association.
"The magnitude of treatment effect warrants serious consideration of prophylaxis in these children. We also feel that these results warrant reconsideration of the recent guideline recommendations by the American Academy of Pediatrics in 2011 ... advising against evaluation of children with a VCUG [voiding cystourethrogram] after their first UTI," said Dr. Greenfield, director of pediatric urology at Women and Children’s Hospital of Buffalo, N.Y. and one of the RIVUR investigators.
That AAP recommendation was based on a number of studies that showed no benefit from antibiotic prophylaxis in children with VUR, which suggests there is little value in diagnosing VUR (Pediatrics 2011;28:595-610).
In fact, a meta-analysis of six controlled trials, which also was presented at the AUA meeting, suggested that the benefit of antibiotic prophylaxis for preventing febrile UTIs in children with VUR is small at best, and evidence to support its use is lacking.
Pooled data for 986 patients included in the trials showed that in 417 with dilating VUR, the risk of recurrent febrile UTI was 22.46% in those who received antibiotics, and 29.79% in controls. The relative risk of treatment failure with antibiotic prophylaxis was 0.75, and the absolute risk reduction was 7.33%, Dr. José Netto reported.
The number needed to treat to prevent one UTI was 13.64, according to Dr. Netto of State University of Feira de Santana in Brazil.
In 515 patients with nondilating VUR, the risk of febrile UTI was 5.31% in patients who received prophylactic antibiotics, and 6.09% in those who did not. The relative risk of treatment failure was 0.87, and the absolute risk reduction was 0.78%. The number needed to treat was 129, Dr. Netto said.
The studies included in the meta-analysis were published prior to August 2013. Only one was placebo-controlled. Patients included in the study included 663 girls (67.2%), and the median age of all patients was 21 months.
The studies all used co-trimoxazole in standard doses, and three of the six also used co-amoxiclav or nitrofurantoin as alternative treatments.
Antibiotic prophylaxis is often used in children with VUR, but several recent studies have failed to demonstrate the usefulness of this approach with respect to reducing the risk of febrile UTI, Dr. Netto said, noting that the studies included heterogeneous populations with distinct grades of VUR and varying rates of recurrent UTI.
Based on the current findings, it remains uncertain whether antibiotic prophylaxis reduces the risk of febrile UTI in children with VUR, although it is possible – given the differences seen between those with dilating VUR and those with nondilating VUR – that specific subgroups of children with dilating VUR may benefit from prophylaxis, he concluded.
The conflicting findings between this meta-analysis, and the RIVUR trial underscore the importance of ongoing evaluation of antibiotic prophylaxis.
"The best ways to prevent and treat febrile urinary tract infections in children with VUR are still very much up for discussion," Dr. Anthony Atala, W.H. Boyce Professor and chair of the urology department at Wake Forest Baptist Medical Center in Winston-Salem, N.C., said in an AUA press statement.
"Examining and reexamining the AAP guidelines and practices that guide our work are critical to better understand the condition and improve the lives of those children who are living with the condition each day," said Dr. Atala, who is also director of the Wake Forest Institute for Regenerative Medicine.
Dr. Atala reported serving in a leadership position at Plureon Corp. Dr. Greenfield and Dr. Netto reported having no disclosures.
ORLANDO – Antibiotic prophylaxis was shown in the recently published RIVUR trial to halve the risk of recurrent febrile urinary tract infection in infants and young children with vesicoureteral reflux. Conversely, a meta-analysis of six controlled trials concluded there is little to no benefit of antibiotic prophylaxis in this population.
The conflicting data highlight the ongoing debate regarding the best approach for preventing and managing febrile urinary tract infections (UTIs) in children with vesicoureteral reflux (VUR).
The RIVUR (Randomized Intervention for Children with Vesicoureteral Reflux) trial findings, which were published in May in the New England Journal of Medicine, showed that among children with VUR who were aged 2-71 months, 2 years of treatment with trimethoprim-sulfamethoxazole halved the risk of febrile or symptomatic UTI recurrence (N. Eng. J. Med. 2014;370:2367-76). The difference between those who received prophylaxis and those who did not emerged early and increased over the 2-year study period, Dr. Saul Greenfield said during an update on the trial results at the annual meeting of the American Urological Association.
"The magnitude of treatment effect warrants serious consideration of prophylaxis in these children. We also feel that these results warrant reconsideration of the recent guideline recommendations by the American Academy of Pediatrics in 2011 ... advising against evaluation of children with a VCUG [voiding cystourethrogram] after their first UTI," said Dr. Greenfield, director of pediatric urology at Women and Children’s Hospital of Buffalo, N.Y. and one of the RIVUR investigators.
That AAP recommendation was based on a number of studies that showed no benefit from antibiotic prophylaxis in children with VUR, which suggests there is little value in diagnosing VUR (Pediatrics 2011;28:595-610).
In fact, a meta-analysis of six controlled trials, which also was presented at the AUA meeting, suggested that the benefit of antibiotic prophylaxis for preventing febrile UTIs in children with VUR is small at best, and evidence to support its use is lacking.
Pooled data for 986 patients included in the trials showed that in 417 with dilating VUR, the risk of recurrent febrile UTI was 22.46% in those who received antibiotics, and 29.79% in controls. The relative risk of treatment failure with antibiotic prophylaxis was 0.75, and the absolute risk reduction was 7.33%, Dr. José Netto reported.
The number needed to treat to prevent one UTI was 13.64, according to Dr. Netto of State University of Feira de Santana in Brazil.
In 515 patients with nondilating VUR, the risk of febrile UTI was 5.31% in patients who received prophylactic antibiotics, and 6.09% in those who did not. The relative risk of treatment failure was 0.87, and the absolute risk reduction was 0.78%. The number needed to treat was 129, Dr. Netto said.
The studies included in the meta-analysis were published prior to August 2013. Only one was placebo-controlled. Patients included in the study included 663 girls (67.2%), and the median age of all patients was 21 months.
The studies all used co-trimoxazole in standard doses, and three of the six also used co-amoxiclav or nitrofurantoin as alternative treatments.
Antibiotic prophylaxis is often used in children with VUR, but several recent studies have failed to demonstrate the usefulness of this approach with respect to reducing the risk of febrile UTI, Dr. Netto said, noting that the studies included heterogeneous populations with distinct grades of VUR and varying rates of recurrent UTI.
Based on the current findings, it remains uncertain whether antibiotic prophylaxis reduces the risk of febrile UTI in children with VUR, although it is possible – given the differences seen between those with dilating VUR and those with nondilating VUR – that specific subgroups of children with dilating VUR may benefit from prophylaxis, he concluded.
The conflicting findings between this meta-analysis, and the RIVUR trial underscore the importance of ongoing evaluation of antibiotic prophylaxis.
"The best ways to prevent and treat febrile urinary tract infections in children with VUR are still very much up for discussion," Dr. Anthony Atala, W.H. Boyce Professor and chair of the urology department at Wake Forest Baptist Medical Center in Winston-Salem, N.C., said in an AUA press statement.
"Examining and reexamining the AAP guidelines and practices that guide our work are critical to better understand the condition and improve the lives of those children who are living with the condition each day," said Dr. Atala, who is also director of the Wake Forest Institute for Regenerative Medicine.
Dr. Atala reported serving in a leadership position at Plureon Corp. Dr. Greenfield and Dr. Netto reported having no disclosures.
EXPERT ANALYSIS FROM THE AUA ANNUAL MEETING
Key clinical point: It remains unclear whether antibiotic prophylaxis is beneficial for febrile UTI in children with vesicoureteral reflux.
Major finding: In the RIVUR trial, antibiotic prophylaxis halved the risk of recurrent UTI; in the meta-analysis, prophylaxis was associated with only small benefit.
Data source: The randomized controlled RIVUR trial of 607 children, and a meta-analysis of six controlled studies involving 986 children.
Disclosures: Dr. Atala reported serving in a leadership position at Plureon Corp. Dr. Greenfield and Dr. Netto reported having no disclosures.
Promoting higher blood pressure targets for frail older adults: A consensus guideline from Canada
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
KEY POINTS
- For frail elderly patients, consider starting treatment if the systolic blood pressure is 160 mm Hg or higher.
- An appropriate target in this population is a seated systolic pressure between 140 and 160 mm Hg, as long as there is no orthostatic drop to less than 140 mm Hg upon standing from a lying position and treatment does not adversely affect quality of life.
- The blood pressure target does not need to be lower if the patient has diabetes. If the patient is severely frail and has a short life expectancy, a systolic target of 160 to 190 mm Hg may be reasonable.
- If the systolic pressure is below 140 mm Hg, antihypertensive medications can be reduced as long as they are not indicated for other conditions.
- In general, one should prescribe no more than two antihypertensive medications.
Primary prevention of diabetic kidney disease: Thumbs up/down
LAS VEGAS – Contrary to conventional wisdom, neither ACE inhibitors nor angiotensin receptor blockers have any role to play in primary prevention of diabetic kidney disease, according to Dr. Robert C. Stanton, chief of nephrology at the Harvard University’s Joslin Diabetes Center, Boston.
"I don’t see any unique indication for ACE inhibitors and ARBs for the primary prevention of kidney disease in diabetic patients, especially given that around 70% of diabetes patients will never develop kidney disease. They’re perfectly fine blood pressure pills. But as a magic kidney disease prevention drug, I don’t see any evidence for that. Of course, patients with proteinuria are another issue entirely. Those drugs absolutely are beneficial in that setting," he said at a meeting sponsored by the National Kidney Foundation.
When Dr. Stanton polled his audience electronically during the course of his talk, however, the majority of physicians indicated that they believe ACE inhibitors and ARBs are indeed useful for primary prevention of diabetic kidney disease. The evidence, Dr. Stanton emphasized, shows otherwise.
For example, a well-conducted, randomized, multicenter, placebo-controlled, 5-year clinical trial showed no benefit for enalapril or losartan in preventing kidney disease in patients with type 1 diabetes (N. Engl. J. Med. 2009;361:40-51). And three randomized controlled trials showed no primary preventive benefit for candesartan in more than 5,000 patients with type 1 or type 2 diabetes (Ann. Intern. Med. 2009;151:11-20).
Dr. Stanton noted that lots of other interventions have been proposed for the primary prevention of kidney disease in diabetes patients. Some are supported by solid evidence of benefit, others are not.
Here is his view of the preventive landscape:
• Intensive blood glucose control. "This is the easy one," he said. "A lot of us in the diabetes world feel that a hemoglobin A1c of 7% is the appropriate target for preventing many complications. It’s a reasonable target and should be achieved whether you’re talking about type 1 or type 2 patients."
The nephrologist noted that recent 25-year follow-up data from the landmark Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study showed that fully 18 years after the intervention ended, patients assigned to intensive blood glucose control still showed highly impressive 50% reductions in the cumulative incidence of both microalbuminuria and end-stage renal disease compared with patients placed on less intensive control (Diabetes Care 2014;37:24-30).
• Smoking cessation. Smoking has been linked to a several-fold increased risk of diabetic kidney disease. "I think of diabetes as an endothelial cell disease, and smoking is the greatest endothelial cell poison we’ve come up with. So stopping smoking is something well worth doing," Dr. Stanton said.
• Blood pressure control. No question exists regarding its renoprotective effect. But recent guidelines are dizzyingly all over the map in terms of target pressure recommendations.
"I’m getting a major headache reading these articles right now. I can show you the data. Good luck! I personally like a target of 130/80 mm Hg or less, particularly when it’s not that hard to get there. But I’d let you decide what particular target you favor," he said.
He prefers 130/80 mm Hg as a target blood pressure for primary prevention of diabetic kidney disease in large part because of a meta-analysis showing that it was associated with a 10% reduction in the risk of developing microalbuminuria and an 11% decrease in end-stage renal disease (PloS Med 2012;9(8):e1001293).
• Weight loss. The growing bariatric surgery literature supports weight loss as a primary preventive strategy.
• Protein intake. There is no role for a low-protein diet – say, less than 0.8 g/kg per day – for primary prevention of kidney disease in diabetes patients. And Dr. Stanton believes a high-protein diet in the range of more than 1.5 or 2 g/kg per day is best avoided in patients with diabetes, although he stressed that the evidence on this score remains sketchy.
Still, "I would not go on a body-building diet or an Atkins-type diet," he cautioned.
• Targeting glomerular hyperfiltration. Studies have shown conflicting results. "For me, there’s no clear role for targeting hyperfiltration," said Dr. Stanton, who cited a comprehensive review that he finds persuasive (Diabetologia 2010;53:2093-104).
The key to developing more effective primary prevention strategies, according to Dr. Stanton, will be first to establish markers that clearly identify the 30% or so of diabetes patients who will go on to develop renal disease, then test novel interventions specifically in that high-risk group.
Promising biomarkers include circulating tumor necrosis factor alpha receptor levels, von Willebrand factor, monocyte chemoattractant factor, asymmetrical dimethylarginine, interleukin-6 and -8, and Fas receptor.
For example, one study showed that patients with type 2 diabetes in the top quartile for circulating TNF receptor 1 had a cumulative 12-year incidence of end-stage renal disease of 54%, compared to just 3% in patients in the other quartiles (J. Am. Soc. Nephrol. 2012;23:507-15).
"Lots of companies are looking at these now. These markers may be coming our way as indicators of people with diabetes who are likely to progress to kidney disease," Dr. Stanton said.
He reported serving as a consultant to Boehringer Ingelheim.
LAS VEGAS – Contrary to conventional wisdom, neither ACE inhibitors nor angiotensin receptor blockers have any role to play in primary prevention of diabetic kidney disease, according to Dr. Robert C. Stanton, chief of nephrology at the Harvard University’s Joslin Diabetes Center, Boston.
"I don’t see any unique indication for ACE inhibitors and ARBs for the primary prevention of kidney disease in diabetic patients, especially given that around 70% of diabetes patients will never develop kidney disease. They’re perfectly fine blood pressure pills. But as a magic kidney disease prevention drug, I don’t see any evidence for that. Of course, patients with proteinuria are another issue entirely. Those drugs absolutely are beneficial in that setting," he said at a meeting sponsored by the National Kidney Foundation.
When Dr. Stanton polled his audience electronically during the course of his talk, however, the majority of physicians indicated that they believe ACE inhibitors and ARBs are indeed useful for primary prevention of diabetic kidney disease. The evidence, Dr. Stanton emphasized, shows otherwise.
For example, a well-conducted, randomized, multicenter, placebo-controlled, 5-year clinical trial showed no benefit for enalapril or losartan in preventing kidney disease in patients with type 1 diabetes (N. Engl. J. Med. 2009;361:40-51). And three randomized controlled trials showed no primary preventive benefit for candesartan in more than 5,000 patients with type 1 or type 2 diabetes (Ann. Intern. Med. 2009;151:11-20).
Dr. Stanton noted that lots of other interventions have been proposed for the primary prevention of kidney disease in diabetes patients. Some are supported by solid evidence of benefit, others are not.
Here is his view of the preventive landscape:
• Intensive blood glucose control. "This is the easy one," he said. "A lot of us in the diabetes world feel that a hemoglobin A1c of 7% is the appropriate target for preventing many complications. It’s a reasonable target and should be achieved whether you’re talking about type 1 or type 2 patients."
The nephrologist noted that recent 25-year follow-up data from the landmark Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study showed that fully 18 years after the intervention ended, patients assigned to intensive blood glucose control still showed highly impressive 50% reductions in the cumulative incidence of both microalbuminuria and end-stage renal disease compared with patients placed on less intensive control (Diabetes Care 2014;37:24-30).
• Smoking cessation. Smoking has been linked to a several-fold increased risk of diabetic kidney disease. "I think of diabetes as an endothelial cell disease, and smoking is the greatest endothelial cell poison we’ve come up with. So stopping smoking is something well worth doing," Dr. Stanton said.
• Blood pressure control. No question exists regarding its renoprotective effect. But recent guidelines are dizzyingly all over the map in terms of target pressure recommendations.
"I’m getting a major headache reading these articles right now. I can show you the data. Good luck! I personally like a target of 130/80 mm Hg or less, particularly when it’s not that hard to get there. But I’d let you decide what particular target you favor," he said.
He prefers 130/80 mm Hg as a target blood pressure for primary prevention of diabetic kidney disease in large part because of a meta-analysis showing that it was associated with a 10% reduction in the risk of developing microalbuminuria and an 11% decrease in end-stage renal disease (PloS Med 2012;9(8):e1001293).
• Weight loss. The growing bariatric surgery literature supports weight loss as a primary preventive strategy.
• Protein intake. There is no role for a low-protein diet – say, less than 0.8 g/kg per day – for primary prevention of kidney disease in diabetes patients. And Dr. Stanton believes a high-protein diet in the range of more than 1.5 or 2 g/kg per day is best avoided in patients with diabetes, although he stressed that the evidence on this score remains sketchy.
Still, "I would not go on a body-building diet or an Atkins-type diet," he cautioned.
• Targeting glomerular hyperfiltration. Studies have shown conflicting results. "For me, there’s no clear role for targeting hyperfiltration," said Dr. Stanton, who cited a comprehensive review that he finds persuasive (Diabetologia 2010;53:2093-104).
The key to developing more effective primary prevention strategies, according to Dr. Stanton, will be first to establish markers that clearly identify the 30% or so of diabetes patients who will go on to develop renal disease, then test novel interventions specifically in that high-risk group.
Promising biomarkers include circulating tumor necrosis factor alpha receptor levels, von Willebrand factor, monocyte chemoattractant factor, asymmetrical dimethylarginine, interleukin-6 and -8, and Fas receptor.
For example, one study showed that patients with type 2 diabetes in the top quartile for circulating TNF receptor 1 had a cumulative 12-year incidence of end-stage renal disease of 54%, compared to just 3% in patients in the other quartiles (J. Am. Soc. Nephrol. 2012;23:507-15).
"Lots of companies are looking at these now. These markers may be coming our way as indicators of people with diabetes who are likely to progress to kidney disease," Dr. Stanton said.
He reported serving as a consultant to Boehringer Ingelheim.
LAS VEGAS – Contrary to conventional wisdom, neither ACE inhibitors nor angiotensin receptor blockers have any role to play in primary prevention of diabetic kidney disease, according to Dr. Robert C. Stanton, chief of nephrology at the Harvard University’s Joslin Diabetes Center, Boston.
"I don’t see any unique indication for ACE inhibitors and ARBs for the primary prevention of kidney disease in diabetic patients, especially given that around 70% of diabetes patients will never develop kidney disease. They’re perfectly fine blood pressure pills. But as a magic kidney disease prevention drug, I don’t see any evidence for that. Of course, patients with proteinuria are another issue entirely. Those drugs absolutely are beneficial in that setting," he said at a meeting sponsored by the National Kidney Foundation.
When Dr. Stanton polled his audience electronically during the course of his talk, however, the majority of physicians indicated that they believe ACE inhibitors and ARBs are indeed useful for primary prevention of diabetic kidney disease. The evidence, Dr. Stanton emphasized, shows otherwise.
For example, a well-conducted, randomized, multicenter, placebo-controlled, 5-year clinical trial showed no benefit for enalapril or losartan in preventing kidney disease in patients with type 1 diabetes (N. Engl. J. Med. 2009;361:40-51). And three randomized controlled trials showed no primary preventive benefit for candesartan in more than 5,000 patients with type 1 or type 2 diabetes (Ann. Intern. Med. 2009;151:11-20).
Dr. Stanton noted that lots of other interventions have been proposed for the primary prevention of kidney disease in diabetes patients. Some are supported by solid evidence of benefit, others are not.
Here is his view of the preventive landscape:
• Intensive blood glucose control. "This is the easy one," he said. "A lot of us in the diabetes world feel that a hemoglobin A1c of 7% is the appropriate target for preventing many complications. It’s a reasonable target and should be achieved whether you’re talking about type 1 or type 2 patients."
The nephrologist noted that recent 25-year follow-up data from the landmark Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study showed that fully 18 years after the intervention ended, patients assigned to intensive blood glucose control still showed highly impressive 50% reductions in the cumulative incidence of both microalbuminuria and end-stage renal disease compared with patients placed on less intensive control (Diabetes Care 2014;37:24-30).
• Smoking cessation. Smoking has been linked to a several-fold increased risk of diabetic kidney disease. "I think of diabetes as an endothelial cell disease, and smoking is the greatest endothelial cell poison we’ve come up with. So stopping smoking is something well worth doing," Dr. Stanton said.
• Blood pressure control. No question exists regarding its renoprotective effect. But recent guidelines are dizzyingly all over the map in terms of target pressure recommendations.
"I’m getting a major headache reading these articles right now. I can show you the data. Good luck! I personally like a target of 130/80 mm Hg or less, particularly when it’s not that hard to get there. But I’d let you decide what particular target you favor," he said.
He prefers 130/80 mm Hg as a target blood pressure for primary prevention of diabetic kidney disease in large part because of a meta-analysis showing that it was associated with a 10% reduction in the risk of developing microalbuminuria and an 11% decrease in end-stage renal disease (PloS Med 2012;9(8):e1001293).
• Weight loss. The growing bariatric surgery literature supports weight loss as a primary preventive strategy.
• Protein intake. There is no role for a low-protein diet – say, less than 0.8 g/kg per day – for primary prevention of kidney disease in diabetes patients. And Dr. Stanton believes a high-protein diet in the range of more than 1.5 or 2 g/kg per day is best avoided in patients with diabetes, although he stressed that the evidence on this score remains sketchy.
Still, "I would not go on a body-building diet or an Atkins-type diet," he cautioned.
• Targeting glomerular hyperfiltration. Studies have shown conflicting results. "For me, there’s no clear role for targeting hyperfiltration," said Dr. Stanton, who cited a comprehensive review that he finds persuasive (Diabetologia 2010;53:2093-104).
The key to developing more effective primary prevention strategies, according to Dr. Stanton, will be first to establish markers that clearly identify the 30% or so of diabetes patients who will go on to develop renal disease, then test novel interventions specifically in that high-risk group.
Promising biomarkers include circulating tumor necrosis factor alpha receptor levels, von Willebrand factor, monocyte chemoattractant factor, asymmetrical dimethylarginine, interleukin-6 and -8, and Fas receptor.
For example, one study showed that patients with type 2 diabetes in the top quartile for circulating TNF receptor 1 had a cumulative 12-year incidence of end-stage renal disease of 54%, compared to just 3% in patients in the other quartiles (J. Am. Soc. Nephrol. 2012;23:507-15).
"Lots of companies are looking at these now. These markers may be coming our way as indicators of people with diabetes who are likely to progress to kidney disease," Dr. Stanton said.
He reported serving as a consultant to Boehringer Ingelheim.
EXPERT ANALYSIS FROM SCM 14