User login
Updates on Kidney Donation
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Kidney Donation & HIV
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Polymyxin use generates flood of acute kidney injuries
LAS VEGAS – Polymyxins are back on the scene due to the worrisome increase in multidrug-resistant gram-negative bacterial infections.
"These drugs were taken off the shelf many years ago because of their severe nephrotoxicity, but they’ve entered the arena again because they’re very effective – and their toxicity has reemerged as well," Dr. Mark A. Perazella warned at a meeting sponsored by the National Kidney Foundation.
Drug-induced renal injury accounts for 18%-27% of all acute kidney injury in hospital cases. Antimicrobial agents are among the top causes of drug-related nephropathy, particularly in the hospitalized population, he added.
The incidence of acute kidney injury associated with polymyxin B and polymyxin E (colistin) in contemporary practice is a whopping 30%-60%, depending upon the cumulative dose and duration of therapy, comorbid conditions, and whether other nephrotoxic medications are being used concomitantly. Often an individual’s risk of acute kidney injury is at the high end of this range because the polymyxins are viewed as agents of last resort, and vancomycin – a nephrotoxin in its own right, albeit a less potent one – is commonly on board.
Plus, patients with a serious multidrug-resistant gram-negative infection often have multiple comorbidities and are in a weakened state, further predisposing them to acute kidney injury upon exposure to a nephrotoxin, added Dr. Perazella, professor of medicine and director of the acute dialysis unit at Yale University in New Haven, Conn.
There are no randomized trial data to define polymyxin dosing parameters that maximize efficacy and minimize renal risk, which is clearly dose and duration dependent. For example, in a retrospective study of 173 adults on polymyxin B, the incidence of acute kidney injury was 60%; 10% required dialysis, and 14% had to discontinue treatment because of nephrotoxicity. The median cumulative dose of polymyxin B in patients with acute kidney injury was 1,578 mg, as compared with 800 mg in those without acute kidney injury. Concomitant vancomycin was used by 82% of patients with and by 55% without acute kidney injury (J. Infect. 2012;65:80-7).
The reported incidence of acute kidney injury in patients on vancomycin is most often in the 10%-20% range. Risk is higher in patients with multiple comorbidities and increases with higher cumulative doses and longer-duration therapy. In an effort to improve cure rates, the target vancomycin trough level has increase from 10-20 mg/dL to 15-20 mg/dL. That higher trough level means more cases of acute kidney injury, according to Dr. Perazella.
The randomized prospective ZEPHYR study highlighted fixed-dose linezolid as an attractive alternative to dose-optimized vancomycin for treatment of hospital-acquired methicillin-resistant Staphylococcus aureus pneumonia. In a randomized study of 165 participants, the clinical cure rate was significantly better with linezolid by a margin of 58%-47%. The incidence of nephrotoxicity was 8% in the linezolid group, compared with 18% with vancomycin.
Dr. Perazella reported having no financial conflicts.
LAS VEGAS – Polymyxins are back on the scene due to the worrisome increase in multidrug-resistant gram-negative bacterial infections.
"These drugs were taken off the shelf many years ago because of their severe nephrotoxicity, but they’ve entered the arena again because they’re very effective – and their toxicity has reemerged as well," Dr. Mark A. Perazella warned at a meeting sponsored by the National Kidney Foundation.
Drug-induced renal injury accounts for 18%-27% of all acute kidney injury in hospital cases. Antimicrobial agents are among the top causes of drug-related nephropathy, particularly in the hospitalized population, he added.
The incidence of acute kidney injury associated with polymyxin B and polymyxin E (colistin) in contemporary practice is a whopping 30%-60%, depending upon the cumulative dose and duration of therapy, comorbid conditions, and whether other nephrotoxic medications are being used concomitantly. Often an individual’s risk of acute kidney injury is at the high end of this range because the polymyxins are viewed as agents of last resort, and vancomycin – a nephrotoxin in its own right, albeit a less potent one – is commonly on board.
Plus, patients with a serious multidrug-resistant gram-negative infection often have multiple comorbidities and are in a weakened state, further predisposing them to acute kidney injury upon exposure to a nephrotoxin, added Dr. Perazella, professor of medicine and director of the acute dialysis unit at Yale University in New Haven, Conn.
There are no randomized trial data to define polymyxin dosing parameters that maximize efficacy and minimize renal risk, which is clearly dose and duration dependent. For example, in a retrospective study of 173 adults on polymyxin B, the incidence of acute kidney injury was 60%; 10% required dialysis, and 14% had to discontinue treatment because of nephrotoxicity. The median cumulative dose of polymyxin B in patients with acute kidney injury was 1,578 mg, as compared with 800 mg in those without acute kidney injury. Concomitant vancomycin was used by 82% of patients with and by 55% without acute kidney injury (J. Infect. 2012;65:80-7).
The reported incidence of acute kidney injury in patients on vancomycin is most often in the 10%-20% range. Risk is higher in patients with multiple comorbidities and increases with higher cumulative doses and longer-duration therapy. In an effort to improve cure rates, the target vancomycin trough level has increase from 10-20 mg/dL to 15-20 mg/dL. That higher trough level means more cases of acute kidney injury, according to Dr. Perazella.
The randomized prospective ZEPHYR study highlighted fixed-dose linezolid as an attractive alternative to dose-optimized vancomycin for treatment of hospital-acquired methicillin-resistant Staphylococcus aureus pneumonia. In a randomized study of 165 participants, the clinical cure rate was significantly better with linezolid by a margin of 58%-47%. The incidence of nephrotoxicity was 8% in the linezolid group, compared with 18% with vancomycin.
Dr. Perazella reported having no financial conflicts.
LAS VEGAS – Polymyxins are back on the scene due to the worrisome increase in multidrug-resistant gram-negative bacterial infections.
"These drugs were taken off the shelf many years ago because of their severe nephrotoxicity, but they’ve entered the arena again because they’re very effective – and their toxicity has reemerged as well," Dr. Mark A. Perazella warned at a meeting sponsored by the National Kidney Foundation.
Drug-induced renal injury accounts for 18%-27% of all acute kidney injury in hospital cases. Antimicrobial agents are among the top causes of drug-related nephropathy, particularly in the hospitalized population, he added.
The incidence of acute kidney injury associated with polymyxin B and polymyxin E (colistin) in contemporary practice is a whopping 30%-60%, depending upon the cumulative dose and duration of therapy, comorbid conditions, and whether other nephrotoxic medications are being used concomitantly. Often an individual’s risk of acute kidney injury is at the high end of this range because the polymyxins are viewed as agents of last resort, and vancomycin – a nephrotoxin in its own right, albeit a less potent one – is commonly on board.
Plus, patients with a serious multidrug-resistant gram-negative infection often have multiple comorbidities and are in a weakened state, further predisposing them to acute kidney injury upon exposure to a nephrotoxin, added Dr. Perazella, professor of medicine and director of the acute dialysis unit at Yale University in New Haven, Conn.
There are no randomized trial data to define polymyxin dosing parameters that maximize efficacy and minimize renal risk, which is clearly dose and duration dependent. For example, in a retrospective study of 173 adults on polymyxin B, the incidence of acute kidney injury was 60%; 10% required dialysis, and 14% had to discontinue treatment because of nephrotoxicity. The median cumulative dose of polymyxin B in patients with acute kidney injury was 1,578 mg, as compared with 800 mg in those without acute kidney injury. Concomitant vancomycin was used by 82% of patients with and by 55% without acute kidney injury (J. Infect. 2012;65:80-7).
The reported incidence of acute kidney injury in patients on vancomycin is most often in the 10%-20% range. Risk is higher in patients with multiple comorbidities and increases with higher cumulative doses and longer-duration therapy. In an effort to improve cure rates, the target vancomycin trough level has increase from 10-20 mg/dL to 15-20 mg/dL. That higher trough level means more cases of acute kidney injury, according to Dr. Perazella.
The randomized prospective ZEPHYR study highlighted fixed-dose linezolid as an attractive alternative to dose-optimized vancomycin for treatment of hospital-acquired methicillin-resistant Staphylococcus aureus pneumonia. In a randomized study of 165 participants, the clinical cure rate was significantly better with linezolid by a margin of 58%-47%. The incidence of nephrotoxicity was 8% in the linezolid group, compared with 18% with vancomycin.
Dr. Perazella reported having no financial conflicts.
EXPERT ANALYSIS FROM SCM 14
Renal denervation proceeds as U.S. trial’s flaws emerge
PARIS – At least three different factors undermined the SYMPLICITY HTN-3 trial that earlier this year did not show a significant difference in blood pressure lowering between renal denervation and a sham-control procedure, most notably the failure of the vast majority of operators in the study to follow ablation instructions and produce thorough and reliable interruptions of sympathetic innervation of the kidneys, according to new data released by the trial’s investigators.
As the full range of problems with the U.S.-based SYMPLICITY HTN-3 trial, which had its main results reported in April (N. Engl. J. Med. 2014;370:1393-1401), became apparent in a report at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, many top European practitioners and supporters of renal denervation voiced their belief that the treatment is an effective and safe option for many patients with true drug-resistant, severe hypertension.
The only qualifications they now add are that renal denervation is not easily performed and must be done carefully and in a more targeted way, with an ongoing need to find the patients best suited for treatment and the best methods for delivering treatment.
During the meeting, Dr. Felix Mahfoud, an interventional cardiologist at the University Hospital of Saarland in Homburg/Saar, Germany, joined with hypertension specialist Dr. Konstantinos Tsioufis of the University of Athens and Dr. William Wijns, codirector of EuroPCR, in an official statement from the meeting that despite the SYMPLICITY HTN-3 results they continued to support renal denervation as a treatment option for selected patients with drug-resistant, severe hypertension.
Their sentiment echoed another endorsement made a few weeks earlier for continued use and study of renal denervation from the European Society of Hypertension (ESH) in reaction to the SYMPLICITY HTN-3 results.
The ESH "sticks to its statement" from 2013 on using renal denervation in appropriate patients with treatment-resistant, severe hypertension (Eurointervention 2013;9:R58-R66), said Dr. Roland E. Schmieder, first author for the 2013 ESH position paper and a leader in European use of renal denervation.
"We need more studies to prove that renal denervation works, and in particular to get more precise information on which patients get the greatest benefit," Dr. Schmieder said in a separate talk at the meeting. For the time being, he said he was comfortable with routine use of renal denervation in patients with an office systolic BP of at least 160 mm Hg that remains at this level despite maximally tolerated treatment with at least three antihypertensive drugs, including a diuretic, the use endorsed by current European guidelines. It remains appropriate to investigate the impact of renal denervation on other disorders, such as heart failure, arrhythmia, metabolic syndrome, and depressed renal function, said Dr. Schmieder, professor and head of hypertension and vascular medicine research at University Hospital in Erlangen, Germany.
The problems with SYMPLICITY HTN-3
While much speculation swirled around what had gone wrong in the SYMPLICITY HTN-3 trial after researchers on the study gave their first report on the results early in the spring, the full extent of the study’s problems didn’t flesh out until a follow-up report during EuroPCR by coinvestigator Dr. David E. Kandzari. In his analysis, Dr. Kandzari highlighted three distinct problems with the trial that he and his associates identified in a series of post hoc analyses:
• The failure of a large minority of enrolled patients in both arms of the study to remain on a stable medical regimen during the 6 months of follow-up before the primary efficacy outcomes were measured.
• The inexplicably large reduction in BP among the sham-control patients, especially among African American patients, who made up a quarter of the trial’s population.
• The vastly incomplete nerve-ablation treatment that most patients received, treatments that usually failed to meet the standards specified in the trial’s protocol.
The background medical regimens that patients received proved unstable during SYMPLICITY HTN-3 even though the study design mandated that patients be on a stable regimen for at least 2 weeks before entering the study. Roughly 80% of enrolled patients in both the denervation and sham-control arms of the study had been on a stable regimen for at least 6 weeks before they entered. Despite that, during the 6 months of follow-up, 211 (39%) of patients in the study underwent a change in their medication regimen. The changes occurred at virtually identical rates in both study arms, and in more than two-thirds of cases were driven by medical necessity.
"The pattern of drug changes challenges the notion of maximally tolerated therapy," Dr. Kandzari said during his report. "Can this [maximally tolerated therapy] be sustained in a randomized, controlled trial?" It also raised the issues of how trial design can better limit drug changes.
Even though it remains unclear why blood pressure reduction was so pronounced among the African Americans in the sham-control group, the impact of this unexpected effect substantially upended the trial’s endpoints. Among the 49 African Americans randomized to sham treatment, office-measured systolic pressure dropped by an average of 17.8 mm Hg, far exceeding the 8.6–mm Hg decline seen among the non–African Americans in the control arm and even exceeding the average 15.5–mm Hg drop in office systolic BP among African Americans treated with renal denervation.
"The absolute reduction in blood pressure by renal denervation in African Americans was identical to non–African Americans." The problem that arose "related more to what happened in the sham-control group of African Americans, who had a nearly 18–mm Hg reduction in blood pressure," said Dr. Kandzari, chief scientific officer and director of interventional cardiology at Piedmont Heart Institute in Atlanta.
The low rate at which patients assigned to receive renal denervation actually received the type of treatment spelled out in the study’s protocol may have been the biggest problem of all, although Dr. Kandzari stressed that, in his opinion "no single factor led to the neutral efficacy seen in the study."
The supplementary methods section of the SYMPLICITY HTN-3 report published in April explicitly called for patients to receive "4-6 ablations" per side, delivering them in a spiral, circumferential pattern starting distally in each renal artery. That meant each patient was to receive a minimum of eight total ablations.
But analysis of data recorded independently by the research nurse and by the proctor during each procedure, as well as cineangiography films made and submitted by the operator for each ablation, clearly showed that many patients did not receive the treatment that the protocol spelled out. Synthesis of the data collected by the three methods showed that about half of the 364 patients randomized to renal denervation received at least eight ablations, while the other half did not receive this minimum number.
The three separate sets of ablation records also contained information on whether ablations occurred in the anterior, posterior, superior, or inferior quadrants of each renal artery. Full circumferential ablation, what the protocol prescribed, required an ablation in at least one of each of these quadrants per side. What actually happened was that 253 patients (70%) received no circumferential ablations, 68 patients (19%) received circumferential ablation on just one side, and 19 patients (5%) received the bilateral circumferential ablations that the protocol called for. Data for the remaining 24 patients treated with renal denervation were not amenable to analysis for this parameter.
As might be expected, greater ablation number and completeness strongly linked with a robust blood pressure effect.
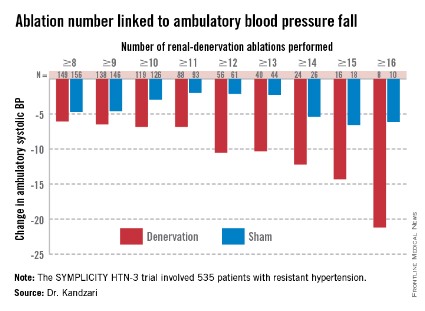
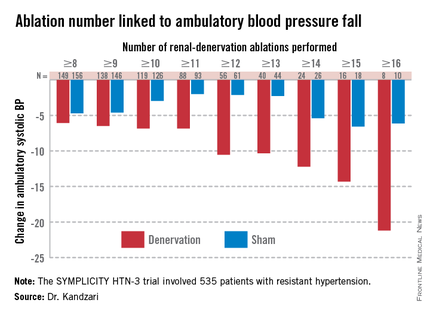
Among patients who received at least eight ablations, office systolic pressure fell by an average 13.1 mm Hg. But among the nine patients who received 16 or more ablations, the average systolic BP reduction at 6 months was 30.9 mm Hg. Among the 18 patients who received at least 15 ablations, the average systolic pressure reduction was 25.4 mm Hg. A very similar relationship occurred for BPs measured by ambulatory monitoring (see graphic), and the data also suggested a positive link between an increasing number of ablations and an increased effect on heart rate. The consistency of the association across all three measures lent further support to this as a real relationship, Dr. Kandzari noted.
Circumferentiality of the ablations showed a similar pattern. The average office systolic pressure fall in patients with no circumferential ablations was 14.2 mm Hg, and it was 16.1 mm Hg in patients who received just one circumferential ablation. But in the 19 patients who received circumferential ablations bilaterally, the average office systolic pressure reduction was 24.3 mm Hg, with a similar pattern seen for ambulatory measures as well as for home-based BP measurements.
"All patients randomized to renal denervation received renal denervation, but they may not have received it in a fashion that seemed to translate into a greater blood pressure reduction," Dr. Kandzari concluded.
Who to treat, where to treat, how to treat
"One result of the neutral HTN-3 result was a call to revisit the basic science behind renal denervation. The clinical enthusiasm had exceeded the science behind renal denervation," Dr. Kandzari observed.
Renal denervation’s many European advocates seem to agree, and have begun the process of determining characteristics of the best patients to receive renal denervation and where and how ablations are best delivered within the renal artery to achieve interruption of sympathetic innervation, although the targeting information they have right now is rudimentary.
"Probably most important is patient selection. You must be sure to get the right patient, one with high sympathetic activity, because the treatment lowers sympathetic activity," said Dr. Atul Pathak, an interventional cardiologist at Paul Sabatier University in Toulouse, France.
Some clues for patient selection have come from the Global SYMPLICITY Registry, which is enrolling patients treated with renal denervation at more than 200 experienced centers worldwide, many of them in Germany but also elsewhere in Europe, Australia, Canada, Korea, and other locations. Initial findings from the first 1,000 patients entered into the registry and followed for 6 months came out in March at the annual meeting of the American College of Cardiology, and Dr. Mahfoud presented new analyses of the data at EuroPCR.
"The major concern we had when we started renal denervation was its safety. I believe the safety issue is now answered," especially with the data collected in the global registry as well as in the SYMPLICITY HTN-3 trial, by far the largest trial completed for the procedure, said Dr. Thomas Zeller, professor and head of clinical and interventional angiology at the Heart Center in Bad Krozingen, Germany. "I was concerned that we might harm the renal arteries with long-lasting stenosis or embolic showers, but this does not happen, at least with the Symplicity catheter," he said during a talk at the meeting.
"The number of patients suitable for renal denervation is potentially much smaller than we initially expected. Real drug resistance is rare, poor adherence is common, and the Symplicity catheter is technically challenging and not effective in every patient. It is hard to rotate the catheter in the tortuous iliac arteries that some patients with hypertension have; the anatomic conditions of hypertension may not be suited to the Symplicity flex catheter," said Dr. Zeller, who added that he has performed renal denervations with the Symplicity catheter since 2009.
"We should focus on the patients that the HTN-3 trial identified as responders, including patients younger than 65, and patients on an aldosterone antagonist," he suggested in a talk at the meeting.
Finding the right patients and the right ablation targets
In the SYMPLICITY HTN-3 trial, 123 (23%) of the 535 patients remained severely hypertensive despite treatment with an aldosterone antagonist such as spironolactone at the time of entry into the study. In this subgroup, renal denervation produced an average 8.1–mm Hg additional reduction in office systolic BP compared with the average reduction seen among the sham-control patients, a much larger effect than the average 3.2–mm Hg incremental reduction by renal denervation over control seen in the patients who were not on an aldosterone antagonist at baseline, Dr. Manesh Patel reported in a talk at the meeting.
One possible explanation for this effect is that "these patients were resistant to an aldosterone antagonist and hence have a good chance of having high sympathetic activity," explained Dr. Patel, director of interventional cardiology at Duke University in Durham, N.C., and a coinvestigator on the SYMPLICITY HTN-3 trial. Another possibility is that "aldosterone antagonist use is a marker for patients who have been treated in a hypertension clinic to receive this fourth-line agent," and hence are more likely to have true drug-resistant hypertension, he added. More recent analyses of the HTN-3 results also showed that the 38% of patients who entered the study while on treatment with a vasodilator had absolutely no added benefit from renal denervation compared with the sham controls, while in the patients not on a vasodilator renal denervation produced an average 6.7–mm Hg reduction in office systolic BP compared with control patients, a statistically significant difference.
"We must accept that currently denervation is a ‘black box’ procedure. You deliver energy and you hope blood pressure goes down, but the main confounder is we are not sure if we have damaged the nerve fibers," Dr. Mahfoud said.
According to data he compiled, the depth of ablation penetration varies by device, with several devices including the Symplicity producing an ablation depth of 3 mm, while a few other systems produce ablation depths of 4 mm or even 6 mm.
Results from autopsy studies he analyzed suggested that afferent nerve density closer to the renal-artery lumen is highest in the distal section of the renal artery compared with the more proximal side, and that the posterior and anterior quadrants of the distal renal artery harbor a higher concentration of nerve fibers closer to the lumen than the superior and inferior quadrants.
This information begins to define the "sweet spot" for applying denervation energy, Dr. Mahfoud said. When he performs renal denervation today "we go even more distally, into the branches [off the distal renal arteries] if they are large enough" to accommodate the catheter. "Nerves are not equally distributed over the entire renal artery," and ideally this information should help guide ablation placements, he said.
The global divide in renal denervation use
The inability of the SYMPLICITY HTN-3 trial to prove the treatment’s efficacy has further divided use of renal denervation by geography. The technology remains unapproved for U.S. use, and will remain that way until another large, sham-controlled trial finishes and shows a clear benefit for BP reduction. In contrast, the procedure’s use in Europe seems on track to continue and grow further, although European thought leaders urge caution and further research to identify the best denervation techniques and optimal patients.
European leaders such as Dr. Mahfoud and Dr. Schmieder also see great promise in using renal denervation for other types of patients, such as those with heart failure or arrhythmias. Just one example of the wide-ranging effects examined for renal denervation was a report Dr. Mahfoud cited published earlier this year that focused on changes in left ventricular mass in 55 patients with resistant hypertension who underwent renal denervation. The results collected by Dr. Mahfoud and his associates showed that even when patients experienced little or no change in their systolic BP they often had substantial reductions in left ventricular mass (Eur. Heart J. 2014 March 6 [doi:10.1093/eurheartj/ehu093]).
"Reducing systolic blood pressure by 10 mm Hg [in patients with severe, drug-resistant hypertension] would have a massive impact, so renal denervation remains an important tool for potentially benefiting patients with uncontrolled hypertension," Dr. Wijns, codirector of the Cardiovascular Center in Aalst, Belgium, said in an interview.
But the renal denervation tool that is increasingly seen as important by the cardiovascular disease leadership in Europe will remain beyond the reach of U.S. physicians for some time to come.
The SYMPLICITY HTN-3 trial and the Global SYMPLICITY Registry were sponsored by Medtronic, which markets the Symplicity catheter. All of the sources for this article have received speaker fees, consulting fees, and/or research grants from Medtronic and numerous other medical device, drug, or biotechnology companies.
On Twitter @mitchelzoler
PARIS – At least three different factors undermined the SYMPLICITY HTN-3 trial that earlier this year did not show a significant difference in blood pressure lowering between renal denervation and a sham-control procedure, most notably the failure of the vast majority of operators in the study to follow ablation instructions and produce thorough and reliable interruptions of sympathetic innervation of the kidneys, according to new data released by the trial’s investigators.
As the full range of problems with the U.S.-based SYMPLICITY HTN-3 trial, which had its main results reported in April (N. Engl. J. Med. 2014;370:1393-1401), became apparent in a report at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, many top European practitioners and supporters of renal denervation voiced their belief that the treatment is an effective and safe option for many patients with true drug-resistant, severe hypertension.

The only qualifications they now add are that renal denervation is not easily performed and must be done carefully and in a more targeted way, with an ongoing need to find the patients best suited for treatment and the best methods for delivering treatment.
During the meeting, Dr. Felix Mahfoud, an interventional cardiologist at the University Hospital of Saarland in Homburg/Saar, Germany, joined with hypertension specialist Dr. Konstantinos Tsioufis of the University of Athens and Dr. William Wijns, codirector of EuroPCR, in an official statement from the meeting that despite the SYMPLICITY HTN-3 results they continued to support renal denervation as a treatment option for selected patients with drug-resistant, severe hypertension.
Their sentiment echoed another endorsement made a few weeks earlier for continued use and study of renal denervation from the European Society of Hypertension (ESH) in reaction to the SYMPLICITY HTN-3 results.
The ESH "sticks to its statement" from 2013 on using renal denervation in appropriate patients with treatment-resistant, severe hypertension (Eurointervention 2013;9:R58-R66), said Dr. Roland E. Schmieder, first author for the 2013 ESH position paper and a leader in European use of renal denervation.
"We need more studies to prove that renal denervation works, and in particular to get more precise information on which patients get the greatest benefit," Dr. Schmieder said in a separate talk at the meeting. For the time being, he said he was comfortable with routine use of renal denervation in patients with an office systolic BP of at least 160 mm Hg that remains at this level despite maximally tolerated treatment with at least three antihypertensive drugs, including a diuretic, the use endorsed by current European guidelines. It remains appropriate to investigate the impact of renal denervation on other disorders, such as heart failure, arrhythmia, metabolic syndrome, and depressed renal function, said Dr. Schmieder, professor and head of hypertension and vascular medicine research at University Hospital in Erlangen, Germany.
The problems with SYMPLICITY HTN-3
While much speculation swirled around what had gone wrong in the SYMPLICITY HTN-3 trial after researchers on the study gave their first report on the results early in the spring, the full extent of the study’s problems didn’t flesh out until a follow-up report during EuroPCR by coinvestigator Dr. David E. Kandzari. In his analysis, Dr. Kandzari highlighted three distinct problems with the trial that he and his associates identified in a series of post hoc analyses:
• The failure of a large minority of enrolled patients in both arms of the study to remain on a stable medical regimen during the 6 months of follow-up before the primary efficacy outcomes were measured.
• The inexplicably large reduction in BP among the sham-control patients, especially among African American patients, who made up a quarter of the trial’s population.
• The vastly incomplete nerve-ablation treatment that most patients received, treatments that usually failed to meet the standards specified in the trial’s protocol.
The background medical regimens that patients received proved unstable during SYMPLICITY HTN-3 even though the study design mandated that patients be on a stable regimen for at least 2 weeks before entering the study. Roughly 80% of enrolled patients in both the denervation and sham-control arms of the study had been on a stable regimen for at least 6 weeks before they entered. Despite that, during the 6 months of follow-up, 211 (39%) of patients in the study underwent a change in their medication regimen. The changes occurred at virtually identical rates in both study arms, and in more than two-thirds of cases were driven by medical necessity.
"The pattern of drug changes challenges the notion of maximally tolerated therapy," Dr. Kandzari said during his report. "Can this [maximally tolerated therapy] be sustained in a randomized, controlled trial?" It also raised the issues of how trial design can better limit drug changes.
Even though it remains unclear why blood pressure reduction was so pronounced among the African Americans in the sham-control group, the impact of this unexpected effect substantially upended the trial’s endpoints. Among the 49 African Americans randomized to sham treatment, office-measured systolic pressure dropped by an average of 17.8 mm Hg, far exceeding the 8.6–mm Hg decline seen among the non–African Americans in the control arm and even exceeding the average 15.5–mm Hg drop in office systolic BP among African Americans treated with renal denervation.
"The absolute reduction in blood pressure by renal denervation in African Americans was identical to non–African Americans." The problem that arose "related more to what happened in the sham-control group of African Americans, who had a nearly 18–mm Hg reduction in blood pressure," said Dr. Kandzari, chief scientific officer and director of interventional cardiology at Piedmont Heart Institute in Atlanta.
The low rate at which patients assigned to receive renal denervation actually received the type of treatment spelled out in the study’s protocol may have been the biggest problem of all, although Dr. Kandzari stressed that, in his opinion "no single factor led to the neutral efficacy seen in the study."
The supplementary methods section of the SYMPLICITY HTN-3 report published in April explicitly called for patients to receive "4-6 ablations" per side, delivering them in a spiral, circumferential pattern starting distally in each renal artery. That meant each patient was to receive a minimum of eight total ablations.
But analysis of data recorded independently by the research nurse and by the proctor during each procedure, as well as cineangiography films made and submitted by the operator for each ablation, clearly showed that many patients did not receive the treatment that the protocol spelled out. Synthesis of the data collected by the three methods showed that about half of the 364 patients randomized to renal denervation received at least eight ablations, while the other half did not receive this minimum number.
The three separate sets of ablation records also contained information on whether ablations occurred in the anterior, posterior, superior, or inferior quadrants of each renal artery. Full circumferential ablation, what the protocol prescribed, required an ablation in at least one of each of these quadrants per side. What actually happened was that 253 patients (70%) received no circumferential ablations, 68 patients (19%) received circumferential ablation on just one side, and 19 patients (5%) received the bilateral circumferential ablations that the protocol called for. Data for the remaining 24 patients treated with renal denervation were not amenable to analysis for this parameter.
As might be expected, greater ablation number and completeness strongly linked with a robust blood pressure effect.
Among patients who received at least eight ablations, office systolic pressure fell by an average 13.1 mm Hg. But among the nine patients who received 16 or more ablations, the average systolic BP reduction at 6 months was 30.9 mm Hg. Among the 18 patients who received at least 15 ablations, the average systolic pressure reduction was 25.4 mm Hg. A very similar relationship occurred for BPs measured by ambulatory monitoring (see graphic), and the data also suggested a positive link between an increasing number of ablations and an increased effect on heart rate. The consistency of the association across all three measures lent further support to this as a real relationship, Dr. Kandzari noted.
Circumferentiality of the ablations showed a similar pattern. The average office systolic pressure fall in patients with no circumferential ablations was 14.2 mm Hg, and it was 16.1 mm Hg in patients who received just one circumferential ablation. But in the 19 patients who received circumferential ablations bilaterally, the average office systolic pressure reduction was 24.3 mm Hg, with a similar pattern seen for ambulatory measures as well as for home-based BP measurements.
"All patients randomized to renal denervation received renal denervation, but they may not have received it in a fashion that seemed to translate into a greater blood pressure reduction," Dr. Kandzari concluded.
Who to treat, where to treat, how to treat
"One result of the neutral HTN-3 result was a call to revisit the basic science behind renal denervation. The clinical enthusiasm had exceeded the science behind renal denervation," Dr. Kandzari observed.
Renal denervation’s many European advocates seem to agree, and have begun the process of determining characteristics of the best patients to receive renal denervation and where and how ablations are best delivered within the renal artery to achieve interruption of sympathetic innervation, although the targeting information they have right now is rudimentary.
"Probably most important is patient selection. You must be sure to get the right patient, one with high sympathetic activity, because the treatment lowers sympathetic activity," said Dr. Atul Pathak, an interventional cardiologist at Paul Sabatier University in Toulouse, France.
Some clues for patient selection have come from the Global SYMPLICITY Registry, which is enrolling patients treated with renal denervation at more than 200 experienced centers worldwide, many of them in Germany but also elsewhere in Europe, Australia, Canada, Korea, and other locations. Initial findings from the first 1,000 patients entered into the registry and followed for 6 months came out in March at the annual meeting of the American College of Cardiology, and Dr. Mahfoud presented new analyses of the data at EuroPCR.
"The major concern we had when we started renal denervation was its safety. I believe the safety issue is now answered," especially with the data collected in the global registry as well as in the SYMPLICITY HTN-3 trial, by far the largest trial completed for the procedure, said Dr. Thomas Zeller, professor and head of clinical and interventional angiology at the Heart Center in Bad Krozingen, Germany. "I was concerned that we might harm the renal arteries with long-lasting stenosis or embolic showers, but this does not happen, at least with the Symplicity catheter," he said during a talk at the meeting.
"The number of patients suitable for renal denervation is potentially much smaller than we initially expected. Real drug resistance is rare, poor adherence is common, and the Symplicity catheter is technically challenging and not effective in every patient. It is hard to rotate the catheter in the tortuous iliac arteries that some patients with hypertension have; the anatomic conditions of hypertension may not be suited to the Symplicity flex catheter," said Dr. Zeller, who added that he has performed renal denervations with the Symplicity catheter since 2009.
"We should focus on the patients that the HTN-3 trial identified as responders, including patients younger than 65, and patients on an aldosterone antagonist," he suggested in a talk at the meeting.
Finding the right patients and the right ablation targets
In the SYMPLICITY HTN-3 trial, 123 (23%) of the 535 patients remained severely hypertensive despite treatment with an aldosterone antagonist such as spironolactone at the time of entry into the study. In this subgroup, renal denervation produced an average 8.1–mm Hg additional reduction in office systolic BP compared with the average reduction seen among the sham-control patients, a much larger effect than the average 3.2–mm Hg incremental reduction by renal denervation over control seen in the patients who were not on an aldosterone antagonist at baseline, Dr. Manesh Patel reported in a talk at the meeting.
One possible explanation for this effect is that "these patients were resistant to an aldosterone antagonist and hence have a good chance of having high sympathetic activity," explained Dr. Patel, director of interventional cardiology at Duke University in Durham, N.C., and a coinvestigator on the SYMPLICITY HTN-3 trial. Another possibility is that "aldosterone antagonist use is a marker for patients who have been treated in a hypertension clinic to receive this fourth-line agent," and hence are more likely to have true drug-resistant hypertension, he added. More recent analyses of the HTN-3 results also showed that the 38% of patients who entered the study while on treatment with a vasodilator had absolutely no added benefit from renal denervation compared with the sham controls, while in the patients not on a vasodilator renal denervation produced an average 6.7–mm Hg reduction in office systolic BP compared with control patients, a statistically significant difference.
"We must accept that currently denervation is a ‘black box’ procedure. You deliver energy and you hope blood pressure goes down, but the main confounder is we are not sure if we have damaged the nerve fibers," Dr. Mahfoud said.
According to data he compiled, the depth of ablation penetration varies by device, with several devices including the Symplicity producing an ablation depth of 3 mm, while a few other systems produce ablation depths of 4 mm or even 6 mm.
Results from autopsy studies he analyzed suggested that afferent nerve density closer to the renal-artery lumen is highest in the distal section of the renal artery compared with the more proximal side, and that the posterior and anterior quadrants of the distal renal artery harbor a higher concentration of nerve fibers closer to the lumen than the superior and inferior quadrants.
This information begins to define the "sweet spot" for applying denervation energy, Dr. Mahfoud said. When he performs renal denervation today "we go even more distally, into the branches [off the distal renal arteries] if they are large enough" to accommodate the catheter. "Nerves are not equally distributed over the entire renal artery," and ideally this information should help guide ablation placements, he said.
The global divide in renal denervation use
The inability of the SYMPLICITY HTN-3 trial to prove the treatment’s efficacy has further divided use of renal denervation by geography. The technology remains unapproved for U.S. use, and will remain that way until another large, sham-controlled trial finishes and shows a clear benefit for BP reduction. In contrast, the procedure’s use in Europe seems on track to continue and grow further, although European thought leaders urge caution and further research to identify the best denervation techniques and optimal patients.
European leaders such as Dr. Mahfoud and Dr. Schmieder also see great promise in using renal denervation for other types of patients, such as those with heart failure or arrhythmias. Just one example of the wide-ranging effects examined for renal denervation was a report Dr. Mahfoud cited published earlier this year that focused on changes in left ventricular mass in 55 patients with resistant hypertension who underwent renal denervation. The results collected by Dr. Mahfoud and his associates showed that even when patients experienced little or no change in their systolic BP they often had substantial reductions in left ventricular mass (Eur. Heart J. 2014 March 6 [doi:10.1093/eurheartj/ehu093]).
"Reducing systolic blood pressure by 10 mm Hg [in patients with severe, drug-resistant hypertension] would have a massive impact, so renal denervation remains an important tool for potentially benefiting patients with uncontrolled hypertension," Dr. Wijns, codirector of the Cardiovascular Center in Aalst, Belgium, said in an interview.
But the renal denervation tool that is increasingly seen as important by the cardiovascular disease leadership in Europe will remain beyond the reach of U.S. physicians for some time to come.
The SYMPLICITY HTN-3 trial and the Global SYMPLICITY Registry were sponsored by Medtronic, which markets the Symplicity catheter. All of the sources for this article have received speaker fees, consulting fees, and/or research grants from Medtronic and numerous other medical device, drug, or biotechnology companies.
On Twitter @mitchelzoler
PARIS – At least three different factors undermined the SYMPLICITY HTN-3 trial that earlier this year did not show a significant difference in blood pressure lowering between renal denervation and a sham-control procedure, most notably the failure of the vast majority of operators in the study to follow ablation instructions and produce thorough and reliable interruptions of sympathetic innervation of the kidneys, according to new data released by the trial’s investigators.
As the full range of problems with the U.S.-based SYMPLICITY HTN-3 trial, which had its main results reported in April (N. Engl. J. Med. 2014;370:1393-1401), became apparent in a report at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, many top European practitioners and supporters of renal denervation voiced their belief that the treatment is an effective and safe option for many patients with true drug-resistant, severe hypertension.

The only qualifications they now add are that renal denervation is not easily performed and must be done carefully and in a more targeted way, with an ongoing need to find the patients best suited for treatment and the best methods for delivering treatment.
During the meeting, Dr. Felix Mahfoud, an interventional cardiologist at the University Hospital of Saarland in Homburg/Saar, Germany, joined with hypertension specialist Dr. Konstantinos Tsioufis of the University of Athens and Dr. William Wijns, codirector of EuroPCR, in an official statement from the meeting that despite the SYMPLICITY HTN-3 results they continued to support renal denervation as a treatment option for selected patients with drug-resistant, severe hypertension.
Their sentiment echoed another endorsement made a few weeks earlier for continued use and study of renal denervation from the European Society of Hypertension (ESH) in reaction to the SYMPLICITY HTN-3 results.
The ESH "sticks to its statement" from 2013 on using renal denervation in appropriate patients with treatment-resistant, severe hypertension (Eurointervention 2013;9:R58-R66), said Dr. Roland E. Schmieder, first author for the 2013 ESH position paper and a leader in European use of renal denervation.
"We need more studies to prove that renal denervation works, and in particular to get more precise information on which patients get the greatest benefit," Dr. Schmieder said in a separate talk at the meeting. For the time being, he said he was comfortable with routine use of renal denervation in patients with an office systolic BP of at least 160 mm Hg that remains at this level despite maximally tolerated treatment with at least three antihypertensive drugs, including a diuretic, the use endorsed by current European guidelines. It remains appropriate to investigate the impact of renal denervation on other disorders, such as heart failure, arrhythmia, metabolic syndrome, and depressed renal function, said Dr. Schmieder, professor and head of hypertension and vascular medicine research at University Hospital in Erlangen, Germany.
The problems with SYMPLICITY HTN-3
While much speculation swirled around what had gone wrong in the SYMPLICITY HTN-3 trial after researchers on the study gave their first report on the results early in the spring, the full extent of the study’s problems didn’t flesh out until a follow-up report during EuroPCR by coinvestigator Dr. David E. Kandzari. In his analysis, Dr. Kandzari highlighted three distinct problems with the trial that he and his associates identified in a series of post hoc analyses:
• The failure of a large minority of enrolled patients in both arms of the study to remain on a stable medical regimen during the 6 months of follow-up before the primary efficacy outcomes were measured.
• The inexplicably large reduction in BP among the sham-control patients, especially among African American patients, who made up a quarter of the trial’s population.
• The vastly incomplete nerve-ablation treatment that most patients received, treatments that usually failed to meet the standards specified in the trial’s protocol.
The background medical regimens that patients received proved unstable during SYMPLICITY HTN-3 even though the study design mandated that patients be on a stable regimen for at least 2 weeks before entering the study. Roughly 80% of enrolled patients in both the denervation and sham-control arms of the study had been on a stable regimen for at least 6 weeks before they entered. Despite that, during the 6 months of follow-up, 211 (39%) of patients in the study underwent a change in their medication regimen. The changes occurred at virtually identical rates in both study arms, and in more than two-thirds of cases were driven by medical necessity.
"The pattern of drug changes challenges the notion of maximally tolerated therapy," Dr. Kandzari said during his report. "Can this [maximally tolerated therapy] be sustained in a randomized, controlled trial?" It also raised the issues of how trial design can better limit drug changes.
Even though it remains unclear why blood pressure reduction was so pronounced among the African Americans in the sham-control group, the impact of this unexpected effect substantially upended the trial’s endpoints. Among the 49 African Americans randomized to sham treatment, office-measured systolic pressure dropped by an average of 17.8 mm Hg, far exceeding the 8.6–mm Hg decline seen among the non–African Americans in the control arm and even exceeding the average 15.5–mm Hg drop in office systolic BP among African Americans treated with renal denervation.
"The absolute reduction in blood pressure by renal denervation in African Americans was identical to non–African Americans." The problem that arose "related more to what happened in the sham-control group of African Americans, who had a nearly 18–mm Hg reduction in blood pressure," said Dr. Kandzari, chief scientific officer and director of interventional cardiology at Piedmont Heart Institute in Atlanta.
The low rate at which patients assigned to receive renal denervation actually received the type of treatment spelled out in the study’s protocol may have been the biggest problem of all, although Dr. Kandzari stressed that, in his opinion "no single factor led to the neutral efficacy seen in the study."
The supplementary methods section of the SYMPLICITY HTN-3 report published in April explicitly called for patients to receive "4-6 ablations" per side, delivering them in a spiral, circumferential pattern starting distally in each renal artery. That meant each patient was to receive a minimum of eight total ablations.
But analysis of data recorded independently by the research nurse and by the proctor during each procedure, as well as cineangiography films made and submitted by the operator for each ablation, clearly showed that many patients did not receive the treatment that the protocol spelled out. Synthesis of the data collected by the three methods showed that about half of the 364 patients randomized to renal denervation received at least eight ablations, while the other half did not receive this minimum number.
The three separate sets of ablation records also contained information on whether ablations occurred in the anterior, posterior, superior, or inferior quadrants of each renal artery. Full circumferential ablation, what the protocol prescribed, required an ablation in at least one of each of these quadrants per side. What actually happened was that 253 patients (70%) received no circumferential ablations, 68 patients (19%) received circumferential ablation on just one side, and 19 patients (5%) received the bilateral circumferential ablations that the protocol called for. Data for the remaining 24 patients treated with renal denervation were not amenable to analysis for this parameter.
As might be expected, greater ablation number and completeness strongly linked with a robust blood pressure effect.
Among patients who received at least eight ablations, office systolic pressure fell by an average 13.1 mm Hg. But among the nine patients who received 16 or more ablations, the average systolic BP reduction at 6 months was 30.9 mm Hg. Among the 18 patients who received at least 15 ablations, the average systolic pressure reduction was 25.4 mm Hg. A very similar relationship occurred for BPs measured by ambulatory monitoring (see graphic), and the data also suggested a positive link between an increasing number of ablations and an increased effect on heart rate. The consistency of the association across all three measures lent further support to this as a real relationship, Dr. Kandzari noted.
Circumferentiality of the ablations showed a similar pattern. The average office systolic pressure fall in patients with no circumferential ablations was 14.2 mm Hg, and it was 16.1 mm Hg in patients who received just one circumferential ablation. But in the 19 patients who received circumferential ablations bilaterally, the average office systolic pressure reduction was 24.3 mm Hg, with a similar pattern seen for ambulatory measures as well as for home-based BP measurements.
"All patients randomized to renal denervation received renal denervation, but they may not have received it in a fashion that seemed to translate into a greater blood pressure reduction," Dr. Kandzari concluded.
Who to treat, where to treat, how to treat
"One result of the neutral HTN-3 result was a call to revisit the basic science behind renal denervation. The clinical enthusiasm had exceeded the science behind renal denervation," Dr. Kandzari observed.
Renal denervation’s many European advocates seem to agree, and have begun the process of determining characteristics of the best patients to receive renal denervation and where and how ablations are best delivered within the renal artery to achieve interruption of sympathetic innervation, although the targeting information they have right now is rudimentary.
"Probably most important is patient selection. You must be sure to get the right patient, one with high sympathetic activity, because the treatment lowers sympathetic activity," said Dr. Atul Pathak, an interventional cardiologist at Paul Sabatier University in Toulouse, France.
Some clues for patient selection have come from the Global SYMPLICITY Registry, which is enrolling patients treated with renal denervation at more than 200 experienced centers worldwide, many of them in Germany but also elsewhere in Europe, Australia, Canada, Korea, and other locations. Initial findings from the first 1,000 patients entered into the registry and followed for 6 months came out in March at the annual meeting of the American College of Cardiology, and Dr. Mahfoud presented new analyses of the data at EuroPCR.
"The major concern we had when we started renal denervation was its safety. I believe the safety issue is now answered," especially with the data collected in the global registry as well as in the SYMPLICITY HTN-3 trial, by far the largest trial completed for the procedure, said Dr. Thomas Zeller, professor and head of clinical and interventional angiology at the Heart Center in Bad Krozingen, Germany. "I was concerned that we might harm the renal arteries with long-lasting stenosis or embolic showers, but this does not happen, at least with the Symplicity catheter," he said during a talk at the meeting.
"The number of patients suitable for renal denervation is potentially much smaller than we initially expected. Real drug resistance is rare, poor adherence is common, and the Symplicity catheter is technically challenging and not effective in every patient. It is hard to rotate the catheter in the tortuous iliac arteries that some patients with hypertension have; the anatomic conditions of hypertension may not be suited to the Symplicity flex catheter," said Dr. Zeller, who added that he has performed renal denervations with the Symplicity catheter since 2009.
"We should focus on the patients that the HTN-3 trial identified as responders, including patients younger than 65, and patients on an aldosterone antagonist," he suggested in a talk at the meeting.
Finding the right patients and the right ablation targets
In the SYMPLICITY HTN-3 trial, 123 (23%) of the 535 patients remained severely hypertensive despite treatment with an aldosterone antagonist such as spironolactone at the time of entry into the study. In this subgroup, renal denervation produced an average 8.1–mm Hg additional reduction in office systolic BP compared with the average reduction seen among the sham-control patients, a much larger effect than the average 3.2–mm Hg incremental reduction by renal denervation over control seen in the patients who were not on an aldosterone antagonist at baseline, Dr. Manesh Patel reported in a talk at the meeting.
One possible explanation for this effect is that "these patients were resistant to an aldosterone antagonist and hence have a good chance of having high sympathetic activity," explained Dr. Patel, director of interventional cardiology at Duke University in Durham, N.C., and a coinvestigator on the SYMPLICITY HTN-3 trial. Another possibility is that "aldosterone antagonist use is a marker for patients who have been treated in a hypertension clinic to receive this fourth-line agent," and hence are more likely to have true drug-resistant hypertension, he added. More recent analyses of the HTN-3 results also showed that the 38% of patients who entered the study while on treatment with a vasodilator had absolutely no added benefit from renal denervation compared with the sham controls, while in the patients not on a vasodilator renal denervation produced an average 6.7–mm Hg reduction in office systolic BP compared with control patients, a statistically significant difference.
"We must accept that currently denervation is a ‘black box’ procedure. You deliver energy and you hope blood pressure goes down, but the main confounder is we are not sure if we have damaged the nerve fibers," Dr. Mahfoud said.
According to data he compiled, the depth of ablation penetration varies by device, with several devices including the Symplicity producing an ablation depth of 3 mm, while a few other systems produce ablation depths of 4 mm or even 6 mm.
Results from autopsy studies he analyzed suggested that afferent nerve density closer to the renal-artery lumen is highest in the distal section of the renal artery compared with the more proximal side, and that the posterior and anterior quadrants of the distal renal artery harbor a higher concentration of nerve fibers closer to the lumen than the superior and inferior quadrants.
This information begins to define the "sweet spot" for applying denervation energy, Dr. Mahfoud said. When he performs renal denervation today "we go even more distally, into the branches [off the distal renal arteries] if they are large enough" to accommodate the catheter. "Nerves are not equally distributed over the entire renal artery," and ideally this information should help guide ablation placements, he said.
The global divide in renal denervation use
The inability of the SYMPLICITY HTN-3 trial to prove the treatment’s efficacy has further divided use of renal denervation by geography. The technology remains unapproved for U.S. use, and will remain that way until another large, sham-controlled trial finishes and shows a clear benefit for BP reduction. In contrast, the procedure’s use in Europe seems on track to continue and grow further, although European thought leaders urge caution and further research to identify the best denervation techniques and optimal patients.
European leaders such as Dr. Mahfoud and Dr. Schmieder also see great promise in using renal denervation for other types of patients, such as those with heart failure or arrhythmias. Just one example of the wide-ranging effects examined for renal denervation was a report Dr. Mahfoud cited published earlier this year that focused on changes in left ventricular mass in 55 patients with resistant hypertension who underwent renal denervation. The results collected by Dr. Mahfoud and his associates showed that even when patients experienced little or no change in their systolic BP they often had substantial reductions in left ventricular mass (Eur. Heart J. 2014 March 6 [doi:10.1093/eurheartj/ehu093]).
"Reducing systolic blood pressure by 10 mm Hg [in patients with severe, drug-resistant hypertension] would have a massive impact, so renal denervation remains an important tool for potentially benefiting patients with uncontrolled hypertension," Dr. Wijns, codirector of the Cardiovascular Center in Aalst, Belgium, said in an interview.
But the renal denervation tool that is increasingly seen as important by the cardiovascular disease leadership in Europe will remain beyond the reach of U.S. physicians for some time to come.
The SYMPLICITY HTN-3 trial and the Global SYMPLICITY Registry were sponsored by Medtronic, which markets the Symplicity catheter. All of the sources for this article have received speaker fees, consulting fees, and/or research grants from Medtronic and numerous other medical device, drug, or biotechnology companies.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM EUROPCR 2014
ZS-9 may be a safer hyperkalemia therapy
LAS VEGAS – A novel, highly selective, oral potassium ion–binding agent called ZS-9 effectively reversed hyperkalemia in patients with underlying diabetes, heart failure, or chronic kidney disease.
ZS-9’s safety and tolerability matched those of placebo in the double-blind trial, which was the largest phase III treatment trial ever conducted in patients with hyperkalemia, Dr. Bhupinder Singh said at a meeting sponsored by the National Kidney Foundation.
ZS-9 is an inorganic, microporous crystal composed of zirconium silicate. It is insoluble, highly stable, and not systemically absorbed. In vitro it is more than 125 times more selective for potassium ions than is sodium polystyrene sulfate (SPS, brand name Kayexalate), the resin-based therapy traditionally used in hyperkalemia. ZS-9 also has nine times greater potassium ion binding capacity than SPS, according to Dr. Singh, of Apex Research in Riverside, Calif.
He reported on 753 patients with serum potassium levels of 5.0-6.5 mEq/L; 60% had chronic kidney disease, 40% had heart failure, and 60% had diabetes. Two-thirds of the patients were on an ACE inhibitor or an angiotensin receptor blocker (ARB). Importantly, 28% of subjects had an estimated glomerular filtration rate below 29 mL/min per 1.73 m2.
Subjects were randomized to one of four dosing regimens of ZS-9 or to placebo. For the first 48 hours of the study, patients received oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g t.i.d. or placebo. Patients whose serum potassium levels normalized after 48 hours were then switched to once-daily doses of the same strengths or to placebo for 12 days in order to evaluate ZS-9’s safety and efficacy as a maintenance therapy.
The average reduction from baseline at 48 hours in serum potassium (as measured 14 hours after the last dose) was 0.46 mEq/L in the 2.5-g t.i.d. group, 0.54 mEq/L in 5-mg t.i.d. group, and 0.73 mEq/L in the 10-mg t.i.d. group. Normokalemia was attained at a similar rate in all patient subgroups, regardless of their underlying hyperkalemia-promoting disease. ZS-9 at 1.25 g t.i.d. wasn’t significantly more effective than placebo.
The higher a patient’s baseline serum potassium, the larger the absolute drop in response to a given dose. At 10 mg t.i.d., for example, patients with a baseline serum potassium level of 5.3 mEq/L or less averaged a 0.58-mEq/L reduction. Those who started at 5.4-5.5 mEq/L averaged a 0.98-mEq/L decrease, and patients with a baseline serum potassium level in excess of 5.5 mEq/L had a 1.10-mEq/L reduction.
Of patients who were on ZS-9 at 10 mg t.i.d. for the acute phase of the study, 99% were normokalemic at 48 hours.
All told, 542 patients entered the 12-day maintenance therapy phase. The two higher dosing regimens – 5 and 10 mg once daily – provided clinically and statistically significant benefit, with serum potassium levels remaining flat throughout the study period. Those who were switched double-blind to placebo after 12 days on ZS-9 immediately experienced a rise in their serum potassium. For example, patients with a serum potassium at 4.6 mEq/L during 12 days on ZS-9 at 10 mg climbed on average to a serum potassium of 5.0 mEq/L after 1 week on placebo.
Serum sodium, magnesium, and calcium did not change, nor did blood pressure or body weight in ZS-9-treated patients. There were no cases of significant hypokalemia (values below 3.0 mEq/L) in the study. Two of 11,000 serum potassium measures were less than 3.5 mEq/L. The side-effect profile of ZS-9 was basically the same as that of placebo. The incidence of nausea, vomiting, and other gastrointestinal adverse effects was actually more than twice as high with placebo than it was with active therapy, according to Dr. Singh.
Ongoing and planned studies of ZS-9 include an open-label, 12-month study and a 1-month, randomized treatment withdrawal with long-term extension.
Dr. Singh cited two major candidates for ZS-9 in clinical practice. One is in the large subgroup of patients with heart failure, diabetic kidney disease, or proteinuric nephropathy who become hyperkalemic on optimal doses of guideline-recommended therapy with an ACE inhibitor or ARB. The other potential beneficiaries are patients who would like to follow a healthy diet emphasizing vegetables, fruits, and low-fat dairy products, many of which are quite high in potassium content.
Dr. Singh reported serving as a consultant to ZS Pharma, which is developing ZS-9, as well as to Keryx Biopharmaceuticals and Concert.

|
|
The phase III ZS-9 results are extremely encouraging. There is a major unmet need for a safe and effective agent for acute and chronic management of hyperkalemia. Concern is mounting regarding chronic use of sodium polystyrene sulfate. A systematic review of published reports highlighted the risk of potentially fatal colonic necrosis and other gastrointestinal injuries with this traditional therapy (Am. J. Med. 2013;126:264.e9-e.24).
This information calls into question the use of SPS as a chronic therapy. So we need other options, and we perhaps have some on the horizon with ZS-9. Patiromer is another novel therapy well along in the developmental pipeline. This high-capacity, nonabsorbed, oral ion-exchange polymer also performed well in a phase III randomized trial presented by Dr. Matthew Weir of the University of Maryland, Baltimore, last fall at the annual meeting of the American Society of Nephrology.
Dr. John P. Middleton, a nephrologist at Duke University in Durham, N.C., made these remarks in a separate presentation at the meeting. Dr. Middleton reported having no financial conflicts.

|
|
The phase III ZS-9 results are extremely encouraging. There is a major unmet need for a safe and effective agent for acute and chronic management of hyperkalemia. Concern is mounting regarding chronic use of sodium polystyrene sulfate. A systematic review of published reports highlighted the risk of potentially fatal colonic necrosis and other gastrointestinal injuries with this traditional therapy (Am. J. Med. 2013;126:264.e9-e.24).
This information calls into question the use of SPS as a chronic therapy. So we need other options, and we perhaps have some on the horizon with ZS-9. Patiromer is another novel therapy well along in the developmental pipeline. This high-capacity, nonabsorbed, oral ion-exchange polymer also performed well in a phase III randomized trial presented by Dr. Matthew Weir of the University of Maryland, Baltimore, last fall at the annual meeting of the American Society of Nephrology.
Dr. John P. Middleton, a nephrologist at Duke University in Durham, N.C., made these remarks in a separate presentation at the meeting. Dr. Middleton reported having no financial conflicts.

|
|
The phase III ZS-9 results are extremely encouraging. There is a major unmet need for a safe and effective agent for acute and chronic management of hyperkalemia. Concern is mounting regarding chronic use of sodium polystyrene sulfate. A systematic review of published reports highlighted the risk of potentially fatal colonic necrosis and other gastrointestinal injuries with this traditional therapy (Am. J. Med. 2013;126:264.e9-e.24).
This information calls into question the use of SPS as a chronic therapy. So we need other options, and we perhaps have some on the horizon with ZS-9. Patiromer is another novel therapy well along in the developmental pipeline. This high-capacity, nonabsorbed, oral ion-exchange polymer also performed well in a phase III randomized trial presented by Dr. Matthew Weir of the University of Maryland, Baltimore, last fall at the annual meeting of the American Society of Nephrology.
Dr. John P. Middleton, a nephrologist at Duke University in Durham, N.C., made these remarks in a separate presentation at the meeting. Dr. Middleton reported having no financial conflicts.
LAS VEGAS – A novel, highly selective, oral potassium ion–binding agent called ZS-9 effectively reversed hyperkalemia in patients with underlying diabetes, heart failure, or chronic kidney disease.
ZS-9’s safety and tolerability matched those of placebo in the double-blind trial, which was the largest phase III treatment trial ever conducted in patients with hyperkalemia, Dr. Bhupinder Singh said at a meeting sponsored by the National Kidney Foundation.
ZS-9 is an inorganic, microporous crystal composed of zirconium silicate. It is insoluble, highly stable, and not systemically absorbed. In vitro it is more than 125 times more selective for potassium ions than is sodium polystyrene sulfate (SPS, brand name Kayexalate), the resin-based therapy traditionally used in hyperkalemia. ZS-9 also has nine times greater potassium ion binding capacity than SPS, according to Dr. Singh, of Apex Research in Riverside, Calif.
He reported on 753 patients with serum potassium levels of 5.0-6.5 mEq/L; 60% had chronic kidney disease, 40% had heart failure, and 60% had diabetes. Two-thirds of the patients were on an ACE inhibitor or an angiotensin receptor blocker (ARB). Importantly, 28% of subjects had an estimated glomerular filtration rate below 29 mL/min per 1.73 m2.
Subjects were randomized to one of four dosing regimens of ZS-9 or to placebo. For the first 48 hours of the study, patients received oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g t.i.d. or placebo. Patients whose serum potassium levels normalized after 48 hours were then switched to once-daily doses of the same strengths or to placebo for 12 days in order to evaluate ZS-9’s safety and efficacy as a maintenance therapy.
The average reduction from baseline at 48 hours in serum potassium (as measured 14 hours after the last dose) was 0.46 mEq/L in the 2.5-g t.i.d. group, 0.54 mEq/L in 5-mg t.i.d. group, and 0.73 mEq/L in the 10-mg t.i.d. group. Normokalemia was attained at a similar rate in all patient subgroups, regardless of their underlying hyperkalemia-promoting disease. ZS-9 at 1.25 g t.i.d. wasn’t significantly more effective than placebo.
The higher a patient’s baseline serum potassium, the larger the absolute drop in response to a given dose. At 10 mg t.i.d., for example, patients with a baseline serum potassium level of 5.3 mEq/L or less averaged a 0.58-mEq/L reduction. Those who started at 5.4-5.5 mEq/L averaged a 0.98-mEq/L decrease, and patients with a baseline serum potassium level in excess of 5.5 mEq/L had a 1.10-mEq/L reduction.
Of patients who were on ZS-9 at 10 mg t.i.d. for the acute phase of the study, 99% were normokalemic at 48 hours.
All told, 542 patients entered the 12-day maintenance therapy phase. The two higher dosing regimens – 5 and 10 mg once daily – provided clinically and statistically significant benefit, with serum potassium levels remaining flat throughout the study period. Those who were switched double-blind to placebo after 12 days on ZS-9 immediately experienced a rise in their serum potassium. For example, patients with a serum potassium at 4.6 mEq/L during 12 days on ZS-9 at 10 mg climbed on average to a serum potassium of 5.0 mEq/L after 1 week on placebo.
Serum sodium, magnesium, and calcium did not change, nor did blood pressure or body weight in ZS-9-treated patients. There were no cases of significant hypokalemia (values below 3.0 mEq/L) in the study. Two of 11,000 serum potassium measures were less than 3.5 mEq/L. The side-effect profile of ZS-9 was basically the same as that of placebo. The incidence of nausea, vomiting, and other gastrointestinal adverse effects was actually more than twice as high with placebo than it was with active therapy, according to Dr. Singh.
Ongoing and planned studies of ZS-9 include an open-label, 12-month study and a 1-month, randomized treatment withdrawal with long-term extension.
Dr. Singh cited two major candidates for ZS-9 in clinical practice. One is in the large subgroup of patients with heart failure, diabetic kidney disease, or proteinuric nephropathy who become hyperkalemic on optimal doses of guideline-recommended therapy with an ACE inhibitor or ARB. The other potential beneficiaries are patients who would like to follow a healthy diet emphasizing vegetables, fruits, and low-fat dairy products, many of which are quite high in potassium content.
Dr. Singh reported serving as a consultant to ZS Pharma, which is developing ZS-9, as well as to Keryx Biopharmaceuticals and Concert.
LAS VEGAS – A novel, highly selective, oral potassium ion–binding agent called ZS-9 effectively reversed hyperkalemia in patients with underlying diabetes, heart failure, or chronic kidney disease.
ZS-9’s safety and tolerability matched those of placebo in the double-blind trial, which was the largest phase III treatment trial ever conducted in patients with hyperkalemia, Dr. Bhupinder Singh said at a meeting sponsored by the National Kidney Foundation.
ZS-9 is an inorganic, microporous crystal composed of zirconium silicate. It is insoluble, highly stable, and not systemically absorbed. In vitro it is more than 125 times more selective for potassium ions than is sodium polystyrene sulfate (SPS, brand name Kayexalate), the resin-based therapy traditionally used in hyperkalemia. ZS-9 also has nine times greater potassium ion binding capacity than SPS, according to Dr. Singh, of Apex Research in Riverside, Calif.
He reported on 753 patients with serum potassium levels of 5.0-6.5 mEq/L; 60% had chronic kidney disease, 40% had heart failure, and 60% had diabetes. Two-thirds of the patients were on an ACE inhibitor or an angiotensin receptor blocker (ARB). Importantly, 28% of subjects had an estimated glomerular filtration rate below 29 mL/min per 1.73 m2.
Subjects were randomized to one of four dosing regimens of ZS-9 or to placebo. For the first 48 hours of the study, patients received oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g t.i.d. or placebo. Patients whose serum potassium levels normalized after 48 hours were then switched to once-daily doses of the same strengths or to placebo for 12 days in order to evaluate ZS-9’s safety and efficacy as a maintenance therapy.
The average reduction from baseline at 48 hours in serum potassium (as measured 14 hours after the last dose) was 0.46 mEq/L in the 2.5-g t.i.d. group, 0.54 mEq/L in 5-mg t.i.d. group, and 0.73 mEq/L in the 10-mg t.i.d. group. Normokalemia was attained at a similar rate in all patient subgroups, regardless of their underlying hyperkalemia-promoting disease. ZS-9 at 1.25 g t.i.d. wasn’t significantly more effective than placebo.
The higher a patient’s baseline serum potassium, the larger the absolute drop in response to a given dose. At 10 mg t.i.d., for example, patients with a baseline serum potassium level of 5.3 mEq/L or less averaged a 0.58-mEq/L reduction. Those who started at 5.4-5.5 mEq/L averaged a 0.98-mEq/L decrease, and patients with a baseline serum potassium level in excess of 5.5 mEq/L had a 1.10-mEq/L reduction.
Of patients who were on ZS-9 at 10 mg t.i.d. for the acute phase of the study, 99% were normokalemic at 48 hours.
All told, 542 patients entered the 12-day maintenance therapy phase. The two higher dosing regimens – 5 and 10 mg once daily – provided clinically and statistically significant benefit, with serum potassium levels remaining flat throughout the study period. Those who were switched double-blind to placebo after 12 days on ZS-9 immediately experienced a rise in their serum potassium. For example, patients with a serum potassium at 4.6 mEq/L during 12 days on ZS-9 at 10 mg climbed on average to a serum potassium of 5.0 mEq/L after 1 week on placebo.
Serum sodium, magnesium, and calcium did not change, nor did blood pressure or body weight in ZS-9-treated patients. There were no cases of significant hypokalemia (values below 3.0 mEq/L) in the study. Two of 11,000 serum potassium measures were less than 3.5 mEq/L. The side-effect profile of ZS-9 was basically the same as that of placebo. The incidence of nausea, vomiting, and other gastrointestinal adverse effects was actually more than twice as high with placebo than it was with active therapy, according to Dr. Singh.
Ongoing and planned studies of ZS-9 include an open-label, 12-month study and a 1-month, randomized treatment withdrawal with long-term extension.
Dr. Singh cited two major candidates for ZS-9 in clinical practice. One is in the large subgroup of patients with heart failure, diabetic kidney disease, or proteinuric nephropathy who become hyperkalemic on optimal doses of guideline-recommended therapy with an ACE inhibitor or ARB. The other potential beneficiaries are patients who would like to follow a healthy diet emphasizing vegetables, fruits, and low-fat dairy products, many of which are quite high in potassium content.
Dr. Singh reported serving as a consultant to ZS Pharma, which is developing ZS-9, as well as to Keryx Biopharmaceuticals and Concert.
AT SCM 14
Key clinical point: ZS-9 may become a safer treatment alternative to sodium polystyrene sulfate.
Major finding: After 48 hours on the investigational selective potassium ion trapper known as ZS-9 at 10 mg t.i.d., 99% of initially hyperkalemic patients with chronic kidney disease, diabetes, and/or heart failure were normokalemic.
Data source: This phase III, double-blind trial included 753 hyperkalemic patients randomized to one of four doses of ZS-9 or to placebo.
Disclosures: The study was sponsored by ZS Pharma. Dr. Singh reported serving as a consultant to ZS Pharma, which is developing ZS-9.
Depression linked to increased mortality in dialysis patients
LAS VEGAS – Depression in patients with chronic kidney disease is common, underrecognized, and undertreated, Dr. Daniel E. Weiner asserted at a meeting sponsored by the National Kidney Foundation.
Moreover, a recent meta-analysis by University of Toronto researchers demonstrated that the presence of depressive symptoms in patients on long-term dialysis was independently associated with a 51% increase in mortality, noted Dr. Weiner, a nephrologist at Tufts University, Boston.
The meta-analysis included 12 observational studies in which formal depression scales were utilized in more than 21,000 dialysis patients. Even after adjusting for potential publication bias, the presence of depressive symptoms was associated with a 45% increased risk of mortality (Am. J. Kidney Dis. 2014;63:623-35).
The prevalence of depression is elevated among patients across the spectrum of chronic kidney disease (CKD) severity. This was highlighted in a study in which 272 consecutive non–dialysis dependent patients with stage 2-5 CKD underwent screening for depression using the structured Mini International Neuropsychiatric Interview. One in five met criteria for an ongoing major depressive episode. The prevalence of depression didn’t vary significantly by CKD stage (Am. J. Kidney Dis. 2009;54:424-32).
Depression is even more common among patients on dialysis for end-stage renal disease, with prevalence rates of up to 42% reported, Dr. Weiner continued.
He urged physicians to routinely screen for depression in patients with CKD. Studies indicate that’s not widely being done at present, probably in part because the evidence for therapeutic benefit is spotty because the large randomized controlled trials of antidepressant medications have generally excluded patients with advanced CKD.
Dr. Weiner, however, argued that routine screening in patients with CKD is warranted given that the yield is markedly higher than in the general population, depression is associated with increased mortality in dialysis-dependent patients, and it is a treatable disorder. There is evidence from small studies that the selective serotonin reuptake inhibitors (SSRIs) are safe in patients with CKD. Cognitive-behavioral therapy and exercise-based treatment regimens also are backed by supporting evidence.
Dr. Weiner reported having no financial conflicts regarding his presentation.
LAS VEGAS – Depression in patients with chronic kidney disease is common, underrecognized, and undertreated, Dr. Daniel E. Weiner asserted at a meeting sponsored by the National Kidney Foundation.
Moreover, a recent meta-analysis by University of Toronto researchers demonstrated that the presence of depressive symptoms in patients on long-term dialysis was independently associated with a 51% increase in mortality, noted Dr. Weiner, a nephrologist at Tufts University, Boston.
The meta-analysis included 12 observational studies in which formal depression scales were utilized in more than 21,000 dialysis patients. Even after adjusting for potential publication bias, the presence of depressive symptoms was associated with a 45% increased risk of mortality (Am. J. Kidney Dis. 2014;63:623-35).
The prevalence of depression is elevated among patients across the spectrum of chronic kidney disease (CKD) severity. This was highlighted in a study in which 272 consecutive non–dialysis dependent patients with stage 2-5 CKD underwent screening for depression using the structured Mini International Neuropsychiatric Interview. One in five met criteria for an ongoing major depressive episode. The prevalence of depression didn’t vary significantly by CKD stage (Am. J. Kidney Dis. 2009;54:424-32).
Depression is even more common among patients on dialysis for end-stage renal disease, with prevalence rates of up to 42% reported, Dr. Weiner continued.
He urged physicians to routinely screen for depression in patients with CKD. Studies indicate that’s not widely being done at present, probably in part because the evidence for therapeutic benefit is spotty because the large randomized controlled trials of antidepressant medications have generally excluded patients with advanced CKD.
Dr. Weiner, however, argued that routine screening in patients with CKD is warranted given that the yield is markedly higher than in the general population, depression is associated with increased mortality in dialysis-dependent patients, and it is a treatable disorder. There is evidence from small studies that the selective serotonin reuptake inhibitors (SSRIs) are safe in patients with CKD. Cognitive-behavioral therapy and exercise-based treatment regimens also are backed by supporting evidence.
Dr. Weiner reported having no financial conflicts regarding his presentation.
LAS VEGAS – Depression in patients with chronic kidney disease is common, underrecognized, and undertreated, Dr. Daniel E. Weiner asserted at a meeting sponsored by the National Kidney Foundation.
Moreover, a recent meta-analysis by University of Toronto researchers demonstrated that the presence of depressive symptoms in patients on long-term dialysis was independently associated with a 51% increase in mortality, noted Dr. Weiner, a nephrologist at Tufts University, Boston.
The meta-analysis included 12 observational studies in which formal depression scales were utilized in more than 21,000 dialysis patients. Even after adjusting for potential publication bias, the presence of depressive symptoms was associated with a 45% increased risk of mortality (Am. J. Kidney Dis. 2014;63:623-35).
The prevalence of depression is elevated among patients across the spectrum of chronic kidney disease (CKD) severity. This was highlighted in a study in which 272 consecutive non–dialysis dependent patients with stage 2-5 CKD underwent screening for depression using the structured Mini International Neuropsychiatric Interview. One in five met criteria for an ongoing major depressive episode. The prevalence of depression didn’t vary significantly by CKD stage (Am. J. Kidney Dis. 2009;54:424-32).
Depression is even more common among patients on dialysis for end-stage renal disease, with prevalence rates of up to 42% reported, Dr. Weiner continued.
He urged physicians to routinely screen for depression in patients with CKD. Studies indicate that’s not widely being done at present, probably in part because the evidence for therapeutic benefit is spotty because the large randomized controlled trials of antidepressant medications have generally excluded patients with advanced CKD.
Dr. Weiner, however, argued that routine screening in patients with CKD is warranted given that the yield is markedly higher than in the general population, depression is associated with increased mortality in dialysis-dependent patients, and it is a treatable disorder. There is evidence from small studies that the selective serotonin reuptake inhibitors (SSRIs) are safe in patients with CKD. Cognitive-behavioral therapy and exercise-based treatment regimens also are backed by supporting evidence.
Dr. Weiner reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM SCM 14
CKD considered a type 2 diabetes risk equivalent in CAD patients
SAN FRANCISCO – Among patients with coronary artery disease, those who have both chronic kidney disease and type 2 diabetes face a significantly increased risk of cardiovascular events, compared with those who have either condition separately, results from a novel study showed.
"Type 2 diabetes is a paramount risk factor for cardiovascular disease, in particular among patients with established coronary artery disease," Dr. Christoph H. Saely said at the annual scientific sessions of the American Diabetes Association. "Similarly, chronic kidney disease [CKD] confers a high risk of cardiovascular events in CAD patients. Whether type 2 diabetes or CKD has a more severe impact on event risk in this population is unknown in CAD patients. Whether type 2 diabetes or CKD has a more severe impact on event risk in this population is unknown. We aimed at investigating the single and joint effects of type 2 diabetes and of CKD on cardiovascular risk in patients with angiographically proven CAD."
Dr. Saely, of the Academic Teaching Hospital of Feldkirch, Austria, and his associates prospectively recorded cardiovascular events in a cohort of 1,423 patients with coronary artery disease as evidenced by angiography and followed them for 8 years. They defined CKD as an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m² and defined type 2 diabetes with ADA criteria.
At baseline, the mean age of patients was 65 years, 72% were male, and 66% had hypertension.
The researchers found that the risk of cardiovascular events was significantly higher in patients with type 2 diabetes than in nondiabetic subjects, at 39% and 29%, respectively. That risk was also significantly higher in patients with CKD than in those without it, at 47% and 29%, respectively.
Of the 1,423 patients, 841 had neither type 2 diabetes nor CKD, 336 had type 2 diabetes but not CKD, 145 had CKD but not diabetes, and 101 had both conditions. Dr. Saely reported that the rate of cardiovascular events among patients with neither type 2 diabetes nor CKD was 26%. In contrast, the rates of cardiovascular events were significantly higher in patients with type 2 diabetes who did not have CKD, at 35%; and in nondiabetic patients with CKD, at 43%; and were highest in patients with both conditions, at 54%.
The researchers also found that patients with type 2 diabetes and CKD faced a significantly higher risk of cardiovascular events, compared with those who had type 2 diabetes but no CKD, and in those without type 2 diabetes but with CKD. Event rates were similar in patients with type 2 diabetes but not CKD and in nondiabetic patients with CKD.
Dr. Saely said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk
SAN FRANCISCO – Among patients with coronary artery disease, those who have both chronic kidney disease and type 2 diabetes face a significantly increased risk of cardiovascular events, compared with those who have either condition separately, results from a novel study showed.
"Type 2 diabetes is a paramount risk factor for cardiovascular disease, in particular among patients with established coronary artery disease," Dr. Christoph H. Saely said at the annual scientific sessions of the American Diabetes Association. "Similarly, chronic kidney disease [CKD] confers a high risk of cardiovascular events in CAD patients. Whether type 2 diabetes or CKD has a more severe impact on event risk in this population is unknown in CAD patients. Whether type 2 diabetes or CKD has a more severe impact on event risk in this population is unknown. We aimed at investigating the single and joint effects of type 2 diabetes and of CKD on cardiovascular risk in patients with angiographically proven CAD."
Dr. Saely, of the Academic Teaching Hospital of Feldkirch, Austria, and his associates prospectively recorded cardiovascular events in a cohort of 1,423 patients with coronary artery disease as evidenced by angiography and followed them for 8 years. They defined CKD as an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m² and defined type 2 diabetes with ADA criteria.
At baseline, the mean age of patients was 65 years, 72% were male, and 66% had hypertension.
The researchers found that the risk of cardiovascular events was significantly higher in patients with type 2 diabetes than in nondiabetic subjects, at 39% and 29%, respectively. That risk was also significantly higher in patients with CKD than in those without it, at 47% and 29%, respectively.
Of the 1,423 patients, 841 had neither type 2 diabetes nor CKD, 336 had type 2 diabetes but not CKD, 145 had CKD but not diabetes, and 101 had both conditions. Dr. Saely reported that the rate of cardiovascular events among patients with neither type 2 diabetes nor CKD was 26%. In contrast, the rates of cardiovascular events were significantly higher in patients with type 2 diabetes who did not have CKD, at 35%; and in nondiabetic patients with CKD, at 43%; and were highest in patients with both conditions, at 54%.
The researchers also found that patients with type 2 diabetes and CKD faced a significantly higher risk of cardiovascular events, compared with those who had type 2 diabetes but no CKD, and in those without type 2 diabetes but with CKD. Event rates were similar in patients with type 2 diabetes but not CKD and in nondiabetic patients with CKD.
Dr. Saely said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk
SAN FRANCISCO – Among patients with coronary artery disease, those who have both chronic kidney disease and type 2 diabetes face a significantly increased risk of cardiovascular events, compared with those who have either condition separately, results from a novel study showed.
"Type 2 diabetes is a paramount risk factor for cardiovascular disease, in particular among patients with established coronary artery disease," Dr. Christoph H. Saely said at the annual scientific sessions of the American Diabetes Association. "Similarly, chronic kidney disease [CKD] confers a high risk of cardiovascular events in CAD patients. Whether type 2 diabetes or CKD has a more severe impact on event risk in this population is unknown in CAD patients. Whether type 2 diabetes or CKD has a more severe impact on event risk in this population is unknown. We aimed at investigating the single and joint effects of type 2 diabetes and of CKD on cardiovascular risk in patients with angiographically proven CAD."
Dr. Saely, of the Academic Teaching Hospital of Feldkirch, Austria, and his associates prospectively recorded cardiovascular events in a cohort of 1,423 patients with coronary artery disease as evidenced by angiography and followed them for 8 years. They defined CKD as an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m² and defined type 2 diabetes with ADA criteria.
At baseline, the mean age of patients was 65 years, 72% were male, and 66% had hypertension.
The researchers found that the risk of cardiovascular events was significantly higher in patients with type 2 diabetes than in nondiabetic subjects, at 39% and 29%, respectively. That risk was also significantly higher in patients with CKD than in those without it, at 47% and 29%, respectively.
Of the 1,423 patients, 841 had neither type 2 diabetes nor CKD, 336 had type 2 diabetes but not CKD, 145 had CKD but not diabetes, and 101 had both conditions. Dr. Saely reported that the rate of cardiovascular events among patients with neither type 2 diabetes nor CKD was 26%. In contrast, the rates of cardiovascular events were significantly higher in patients with type 2 diabetes who did not have CKD, at 35%; and in nondiabetic patients with CKD, at 43%; and were highest in patients with both conditions, at 54%.
The researchers also found that patients with type 2 diabetes and CKD faced a significantly higher risk of cardiovascular events, compared with those who had type 2 diabetes but no CKD, and in those without type 2 diabetes but with CKD. Event rates were similar in patients with type 2 diabetes but not CKD and in nondiabetic patients with CKD.
Dr. Saely said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Type 2 diabetes and chronic kidney disease contribute synergistically to cardiac event risk in patients with established coronary artery disease.
Major finding: Among patients with CAD, the rate of cardiovascular events was 35% in those with type 2 diabetes but not CKD, 43% in nondiabetic patients with CKD, and 54% in those who had both diabetes and CKD.
Data source: A prospective study of 1,423 patients with CAD as evidenced by angiography.
Disclosures: Dr. Saely had no relevant financial conflicts to disclose.
New definition of clinically meaningful CKD progression
LAS VEGAS – A landmark study has provided physicians with an improved definition of clinically significant progression of chronic kidney disease for use as a major endpoint in clinical trials as well as in daily medical practice.
Specifically, it’s now clear from the study conducted by the global CKD Prognosis Consortium that a 30% decline in estimated glomerular filtration rate (eGFR) over the course of 2 years portends a 5- to 6-fold increased risk of developing end-stage renal disease (ESRD) during the next 2-3 years, Dr. Josef Coresh reported at a meeting sponsored by the National Kidney Foundation.
The magnitude of increased risk of ESRD in patients with a 30% 2-year decline in eGFR was similar regardless of their baseline stage of CKD. That is, patients with a low baseline eGFR of less than 60 mL/min per 1.73 m2 had an adjusted 5.4-fold increased risk if they had a 30% drop in eGFR, compared with those whose eGFR remained unchanged over a 2-year period, while those with a 30% eGFR drop starting from a higher baseline of 60 mL/min per 1.73 m2 or more had a 6-fold increased risk, said Dr. Coresh, professor of epidemiology and director of the George W. Comstock Center for Public Health Research and Prevention at Johns Hopkins University, Baltimore.
He presented an individual-level meta-analysis of 1.5 million participants in 28 cohorts. Among roughly half a million patients in the 19 cohorts with an initial eGFR below 60 mL/min per 1.73 m2, there were 7,523 ESRD events during a mean 2.4 years of follow-up beginning at the close of the 2-year baseline period. An additional 1,009 ESRD events occurred during the follow-up period in participants with an initial eGFR of 60 mL/min per 1.73 m2 or more.
This study grew out of a collaboration between the National Kidney Foundation and the Food and Drug Administration. Officials at the regulatory agency now accept that the established endpoint used to document CKD progression in clinical trials – that is, a doubling of serum creatinine concentration from baseline – has held back therapeutic progress in the field. That’s because this FDA-mandated surrogate endpoint is a late event and thus requires studies with large sample sizes and long follow-up times. The agency is eager for evidence in support of a better surrogate endpoint, explained Dr. Coresh, who is also director of the cardiovascular epidemiology training program at Johns Hopkins.
The new meta-analysis confirmed that a doubling of serum creatinine concentration over the course of 2 years is indeed a valid endpoint for CKD progression. It was associated with a 32-fold increased risk of developing ESRD in patients who started with an eGFR below 60 mL/min per 1.73 m2 and a 57-fold increased risk in patients with a higher starting eGFR. However, a doubling of serum creatinine in a 2-year time frame was also a rare outcome, occurring in less than 1% of patients with a low initial eGFR and in 0.1% of those with a higher initial value. In contrast, a 30% drop in eGFR over a 2-year period was 10 times more common.
"A doubling of serum creatinine captures only 10% of the excess or population-attributable risk of ESRD during follow-up, whereas a 30% decline in eGFR captures 44% of the population with ESRD in the baseline low-eGFR group and 28% in those with a baseline eGFR of 60 mL/min per 1.73 m2 or greater," Dr. Coresh said.
An impressive level of consistency in the results was seen across the 28 studies included in the meta-analysis, which featured adjustment for confounders.
"I think this level of remarkable consistency makes it safer to think that in many settings, if you have a 30% decline in eGFR over 2 years, you will have a substantially increased risk of ESRD of approximately 5-fold," according to the physician.
He stressed that the meta-analysis demonstrated that it’s not just the change in eGFR over the course of 2 years that’s important in determining risk, but that the starting eGFR level and duration of follow-up are also key in determining absolute risk.
"For example, someone who starts at 50 mL/min per 1.73 m2 and is stable for 2 years has a subsequent risk of ESRD 10 years later of only 5%. If, on the other hand, that patient had a 30% decline in eGFR during 2 years, the 10-year risk becomes 21%. And if you start out at an eGFR of 35 mL/min per 1.73 m2, your 10-year risk of ESRD goes from 18% if your eGFR is unchanged for 2 years to 64% if your eGFR falls by 30%. So within the first 2 years, you have the ability to detect things that will happen a long time from now, with pretty good power," Dr. Coresh said.
The findings held true regardless of patient age, the presence or absence of diabetes, and albuminuria.
This meta-analysis, which was supported by the National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases, will result in six publications during the next year. The first was published online on June 3 (JAMA 2014 [doi:10.1001/jama.2014.6634]). The JAMA report goes beyond Dr. Coresh’s time-limited Las Vegas presentation in that it also includes data on all-cause mortality risk according to change in eGFR. He noted this is important because the majority of patients with CKD die of cardiovascular and other causes without ever reaching ESRD. In the meta-analysis, a 30% decline in eGFR over the course of 2 years was associated with an 80% increased risk in all-cause mortality.
Dr. Coresh reported having no relevant financial conflicts.
LAS VEGAS – A landmark study has provided physicians with an improved definition of clinically significant progression of chronic kidney disease for use as a major endpoint in clinical trials as well as in daily medical practice.
Specifically, it’s now clear from the study conducted by the global CKD Prognosis Consortium that a 30% decline in estimated glomerular filtration rate (eGFR) over the course of 2 years portends a 5- to 6-fold increased risk of developing end-stage renal disease (ESRD) during the next 2-3 years, Dr. Josef Coresh reported at a meeting sponsored by the National Kidney Foundation.
The magnitude of increased risk of ESRD in patients with a 30% 2-year decline in eGFR was similar regardless of their baseline stage of CKD. That is, patients with a low baseline eGFR of less than 60 mL/min per 1.73 m2 had an adjusted 5.4-fold increased risk if they had a 30% drop in eGFR, compared with those whose eGFR remained unchanged over a 2-year period, while those with a 30% eGFR drop starting from a higher baseline of 60 mL/min per 1.73 m2 or more had a 6-fold increased risk, said Dr. Coresh, professor of epidemiology and director of the George W. Comstock Center for Public Health Research and Prevention at Johns Hopkins University, Baltimore.
He presented an individual-level meta-analysis of 1.5 million participants in 28 cohorts. Among roughly half a million patients in the 19 cohorts with an initial eGFR below 60 mL/min per 1.73 m2, there were 7,523 ESRD events during a mean 2.4 years of follow-up beginning at the close of the 2-year baseline period. An additional 1,009 ESRD events occurred during the follow-up period in participants with an initial eGFR of 60 mL/min per 1.73 m2 or more.
This study grew out of a collaboration between the National Kidney Foundation and the Food and Drug Administration. Officials at the regulatory agency now accept that the established endpoint used to document CKD progression in clinical trials – that is, a doubling of serum creatinine concentration from baseline – has held back therapeutic progress in the field. That’s because this FDA-mandated surrogate endpoint is a late event and thus requires studies with large sample sizes and long follow-up times. The agency is eager for evidence in support of a better surrogate endpoint, explained Dr. Coresh, who is also director of the cardiovascular epidemiology training program at Johns Hopkins.
The new meta-analysis confirmed that a doubling of serum creatinine concentration over the course of 2 years is indeed a valid endpoint for CKD progression. It was associated with a 32-fold increased risk of developing ESRD in patients who started with an eGFR below 60 mL/min per 1.73 m2 and a 57-fold increased risk in patients with a higher starting eGFR. However, a doubling of serum creatinine in a 2-year time frame was also a rare outcome, occurring in less than 1% of patients with a low initial eGFR and in 0.1% of those with a higher initial value. In contrast, a 30% drop in eGFR over a 2-year period was 10 times more common.
"A doubling of serum creatinine captures only 10% of the excess or population-attributable risk of ESRD during follow-up, whereas a 30% decline in eGFR captures 44% of the population with ESRD in the baseline low-eGFR group and 28% in those with a baseline eGFR of 60 mL/min per 1.73 m2 or greater," Dr. Coresh said.
An impressive level of consistency in the results was seen across the 28 studies included in the meta-analysis, which featured adjustment for confounders.
"I think this level of remarkable consistency makes it safer to think that in many settings, if you have a 30% decline in eGFR over 2 years, you will have a substantially increased risk of ESRD of approximately 5-fold," according to the physician.
He stressed that the meta-analysis demonstrated that it’s not just the change in eGFR over the course of 2 years that’s important in determining risk, but that the starting eGFR level and duration of follow-up are also key in determining absolute risk.
"For example, someone who starts at 50 mL/min per 1.73 m2 and is stable for 2 years has a subsequent risk of ESRD 10 years later of only 5%. If, on the other hand, that patient had a 30% decline in eGFR during 2 years, the 10-year risk becomes 21%. And if you start out at an eGFR of 35 mL/min per 1.73 m2, your 10-year risk of ESRD goes from 18% if your eGFR is unchanged for 2 years to 64% if your eGFR falls by 30%. So within the first 2 years, you have the ability to detect things that will happen a long time from now, with pretty good power," Dr. Coresh said.
The findings held true regardless of patient age, the presence or absence of diabetes, and albuminuria.
This meta-analysis, which was supported by the National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases, will result in six publications during the next year. The first was published online on June 3 (JAMA 2014 [doi:10.1001/jama.2014.6634]). The JAMA report goes beyond Dr. Coresh’s time-limited Las Vegas presentation in that it also includes data on all-cause mortality risk according to change in eGFR. He noted this is important because the majority of patients with CKD die of cardiovascular and other causes without ever reaching ESRD. In the meta-analysis, a 30% decline in eGFR over the course of 2 years was associated with an 80% increased risk in all-cause mortality.
Dr. Coresh reported having no relevant financial conflicts.
LAS VEGAS – A landmark study has provided physicians with an improved definition of clinically significant progression of chronic kidney disease for use as a major endpoint in clinical trials as well as in daily medical practice.
Specifically, it’s now clear from the study conducted by the global CKD Prognosis Consortium that a 30% decline in estimated glomerular filtration rate (eGFR) over the course of 2 years portends a 5- to 6-fold increased risk of developing end-stage renal disease (ESRD) during the next 2-3 years, Dr. Josef Coresh reported at a meeting sponsored by the National Kidney Foundation.
The magnitude of increased risk of ESRD in patients with a 30% 2-year decline in eGFR was similar regardless of their baseline stage of CKD. That is, patients with a low baseline eGFR of less than 60 mL/min per 1.73 m2 had an adjusted 5.4-fold increased risk if they had a 30% drop in eGFR, compared with those whose eGFR remained unchanged over a 2-year period, while those with a 30% eGFR drop starting from a higher baseline of 60 mL/min per 1.73 m2 or more had a 6-fold increased risk, said Dr. Coresh, professor of epidemiology and director of the George W. Comstock Center for Public Health Research and Prevention at Johns Hopkins University, Baltimore.
He presented an individual-level meta-analysis of 1.5 million participants in 28 cohorts. Among roughly half a million patients in the 19 cohorts with an initial eGFR below 60 mL/min per 1.73 m2, there were 7,523 ESRD events during a mean 2.4 years of follow-up beginning at the close of the 2-year baseline period. An additional 1,009 ESRD events occurred during the follow-up period in participants with an initial eGFR of 60 mL/min per 1.73 m2 or more.
This study grew out of a collaboration between the National Kidney Foundation and the Food and Drug Administration. Officials at the regulatory agency now accept that the established endpoint used to document CKD progression in clinical trials – that is, a doubling of serum creatinine concentration from baseline – has held back therapeutic progress in the field. That’s because this FDA-mandated surrogate endpoint is a late event and thus requires studies with large sample sizes and long follow-up times. The agency is eager for evidence in support of a better surrogate endpoint, explained Dr. Coresh, who is also director of the cardiovascular epidemiology training program at Johns Hopkins.
The new meta-analysis confirmed that a doubling of serum creatinine concentration over the course of 2 years is indeed a valid endpoint for CKD progression. It was associated with a 32-fold increased risk of developing ESRD in patients who started with an eGFR below 60 mL/min per 1.73 m2 and a 57-fold increased risk in patients with a higher starting eGFR. However, a doubling of serum creatinine in a 2-year time frame was also a rare outcome, occurring in less than 1% of patients with a low initial eGFR and in 0.1% of those with a higher initial value. In contrast, a 30% drop in eGFR over a 2-year period was 10 times more common.
"A doubling of serum creatinine captures only 10% of the excess or population-attributable risk of ESRD during follow-up, whereas a 30% decline in eGFR captures 44% of the population with ESRD in the baseline low-eGFR group and 28% in those with a baseline eGFR of 60 mL/min per 1.73 m2 or greater," Dr. Coresh said.
An impressive level of consistency in the results was seen across the 28 studies included in the meta-analysis, which featured adjustment for confounders.
"I think this level of remarkable consistency makes it safer to think that in many settings, if you have a 30% decline in eGFR over 2 years, you will have a substantially increased risk of ESRD of approximately 5-fold," according to the physician.
He stressed that the meta-analysis demonstrated that it’s not just the change in eGFR over the course of 2 years that’s important in determining risk, but that the starting eGFR level and duration of follow-up are also key in determining absolute risk.
"For example, someone who starts at 50 mL/min per 1.73 m2 and is stable for 2 years has a subsequent risk of ESRD 10 years later of only 5%. If, on the other hand, that patient had a 30% decline in eGFR during 2 years, the 10-year risk becomes 21%. And if you start out at an eGFR of 35 mL/min per 1.73 m2, your 10-year risk of ESRD goes from 18% if your eGFR is unchanged for 2 years to 64% if your eGFR falls by 30%. So within the first 2 years, you have the ability to detect things that will happen a long time from now, with pretty good power," Dr. Coresh said.
The findings held true regardless of patient age, the presence or absence of diabetes, and albuminuria.
This meta-analysis, which was supported by the National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases, will result in six publications during the next year. The first was published online on June 3 (JAMA 2014 [doi:10.1001/jama.2014.6634]). The JAMA report goes beyond Dr. Coresh’s time-limited Las Vegas presentation in that it also includes data on all-cause mortality risk according to change in eGFR. He noted this is important because the majority of patients with CKD die of cardiovascular and other causes without ever reaching ESRD. In the meta-analysis, a 30% decline in eGFR over the course of 2 years was associated with an 80% increased risk in all-cause mortality.
Dr. Coresh reported having no relevant financial conflicts.
AT SCM 14
Key clinical point: A decline of 30% in eGFR over the course of 2 years helps identify those who will progress to end-stage renal disease in the next 2-3 years.
Major finding: Patients with a 30% drop in estimated glomerular filtration rate during a 2-year period had a 5- to 6-fold increased risk of developing end-stage renal disease during a subsequent mean 2.4 years of follow-up, compared with patients whose eGFR remained unchanged.
Data source: An individual-level meta-analysis of 28 studies involving roughly 1.5 million patients with chronic kidney disease.
Disclosures: The study was supported by the National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. The presenter reported having no relevant financial conflicts.
Midurethral sling study: At 5 years satisfaction remained high, though continence declined
ORLANDO – Success rates at 5 years were similar in women treated with a transobturator midurethral sling and women treated with a retropubic midurethral sling, but the transobturator patients were more likely to report an easing of symptoms, according to an extension of the Trial of Mid-Urethral Slings.
Overall satisfaction was similar in the two groups, Dr. Leslie M. Rickey reported during a Best Abstract session at the annual meeting of the American Urological Association.
Of 597 subjects in the Trial of Midurethral Slings (TOMUS), 404 participated in the extension phase (E-TOMUS). There was no significant difference in success rates – which declined over time – between those in the transobturator group and those in the retropubic group (43% vs. 51%). However, treatment success for retropubic midurethral slings was nearly 8% higher and did not meet equivalency criteria at 1 or 5 years, said Dr. Rickey, a urologist at Yale University, New Haven, Conn., who presented on behalf of Dr. Kimberly S. Kenton and the Urinary Incontinence Treatment Network.
Stress incontinence symptoms continued to increase over time in both groups but did not differ between the groups at 5 years; urge symptoms also increased over time but were reported significantly more often in the retropubic midurethral sling group.
"Overall urgency symptoms and sexual dysfunction, although still improved from baseline, did worsen over time," Dr. Rickey said, noting that they worsened significantly more overall in the retropubic group.
However, the proportion of patients who were completely or mostly satisfied remained similar in the groups (although it decreased from 92% and 93% to 80% and 85% in the groups, respectively, over time). Those in the transobturator group were almost twice as likely to report improvement in their urinary condition as measured by the Patient Global Impression of Improvement scale (odds ratio, 1.94), Dr. Rickey said.
With respect to adverse events, 10% of women overall experienced an adverse event (mainly urinary tract infections or mesh exposure) or serious adverse event; the rate did not differ between the groups. There were six serious adverse events requiring intervention, including one case of mesh erosion in each group and four urinary tract infections in the retropubic midurethral sling group.
"Importantly, there were seven new mesh exposures in years 3-5 after surgery," Dr. Rickey said, noting that three occurred in the retropubic midurethral sling group and four occurred in the transobturator midurethral sling group.
Women included in the extension phase were TOMUS participants who did not undergo surgical retreatment for stress urinary incontinence after the initial procedure. Participants completed annual in-person visits, including pelvic examination, symptom, mesh, and quality of life questionnaires.
Treatment success was defined as no retreatment for stress urinary incontinence, and no self-reported stress urinary incontinence symptoms using the Medical, Epidemiological, and Social Aspects of Aging questionnaire, she said.
"In conclusion, the 5-year continence rates did decline after either retropubic midurethral sling or transobturator sling, and did not meet prespecified criteria for equivalency. Satisfaction does remain high in both groups. In general, urinary symptoms and quality of life were more improved at 5 years after the transobturator approach, and mesh erosion rates remain low at 1.7%," she said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors reported having no disclosures.
ORLANDO – Success rates at 5 years were similar in women treated with a transobturator midurethral sling and women treated with a retropubic midurethral sling, but the transobturator patients were more likely to report an easing of symptoms, according to an extension of the Trial of Mid-Urethral Slings.
Overall satisfaction was similar in the two groups, Dr. Leslie M. Rickey reported during a Best Abstract session at the annual meeting of the American Urological Association.
Of 597 subjects in the Trial of Midurethral Slings (TOMUS), 404 participated in the extension phase (E-TOMUS). There was no significant difference in success rates – which declined over time – between those in the transobturator group and those in the retropubic group (43% vs. 51%). However, treatment success for retropubic midurethral slings was nearly 8% higher and did not meet equivalency criteria at 1 or 5 years, said Dr. Rickey, a urologist at Yale University, New Haven, Conn., who presented on behalf of Dr. Kimberly S. Kenton and the Urinary Incontinence Treatment Network.
Stress incontinence symptoms continued to increase over time in both groups but did not differ between the groups at 5 years; urge symptoms also increased over time but were reported significantly more often in the retropubic midurethral sling group.
"Overall urgency symptoms and sexual dysfunction, although still improved from baseline, did worsen over time," Dr. Rickey said, noting that they worsened significantly more overall in the retropubic group.
However, the proportion of patients who were completely or mostly satisfied remained similar in the groups (although it decreased from 92% and 93% to 80% and 85% in the groups, respectively, over time). Those in the transobturator group were almost twice as likely to report improvement in their urinary condition as measured by the Patient Global Impression of Improvement scale (odds ratio, 1.94), Dr. Rickey said.
With respect to adverse events, 10% of women overall experienced an adverse event (mainly urinary tract infections or mesh exposure) or serious adverse event; the rate did not differ between the groups. There were six serious adverse events requiring intervention, including one case of mesh erosion in each group and four urinary tract infections in the retropubic midurethral sling group.
"Importantly, there were seven new mesh exposures in years 3-5 after surgery," Dr. Rickey said, noting that three occurred in the retropubic midurethral sling group and four occurred in the transobturator midurethral sling group.
Women included in the extension phase were TOMUS participants who did not undergo surgical retreatment for stress urinary incontinence after the initial procedure. Participants completed annual in-person visits, including pelvic examination, symptom, mesh, and quality of life questionnaires.
Treatment success was defined as no retreatment for stress urinary incontinence, and no self-reported stress urinary incontinence symptoms using the Medical, Epidemiological, and Social Aspects of Aging questionnaire, she said.
"In conclusion, the 5-year continence rates did decline after either retropubic midurethral sling or transobturator sling, and did not meet prespecified criteria for equivalency. Satisfaction does remain high in both groups. In general, urinary symptoms and quality of life were more improved at 5 years after the transobturator approach, and mesh erosion rates remain low at 1.7%," she said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors reported having no disclosures.
ORLANDO – Success rates at 5 years were similar in women treated with a transobturator midurethral sling and women treated with a retropubic midurethral sling, but the transobturator patients were more likely to report an easing of symptoms, according to an extension of the Trial of Mid-Urethral Slings.
Overall satisfaction was similar in the two groups, Dr. Leslie M. Rickey reported during a Best Abstract session at the annual meeting of the American Urological Association.
Of 597 subjects in the Trial of Midurethral Slings (TOMUS), 404 participated in the extension phase (E-TOMUS). There was no significant difference in success rates – which declined over time – between those in the transobturator group and those in the retropubic group (43% vs. 51%). However, treatment success for retropubic midurethral slings was nearly 8% higher and did not meet equivalency criteria at 1 or 5 years, said Dr. Rickey, a urologist at Yale University, New Haven, Conn., who presented on behalf of Dr. Kimberly S. Kenton and the Urinary Incontinence Treatment Network.
Stress incontinence symptoms continued to increase over time in both groups but did not differ between the groups at 5 years; urge symptoms also increased over time but were reported significantly more often in the retropubic midurethral sling group.
"Overall urgency symptoms and sexual dysfunction, although still improved from baseline, did worsen over time," Dr. Rickey said, noting that they worsened significantly more overall in the retropubic group.
However, the proportion of patients who were completely or mostly satisfied remained similar in the groups (although it decreased from 92% and 93% to 80% and 85% in the groups, respectively, over time). Those in the transobturator group were almost twice as likely to report improvement in their urinary condition as measured by the Patient Global Impression of Improvement scale (odds ratio, 1.94), Dr. Rickey said.
With respect to adverse events, 10% of women overall experienced an adverse event (mainly urinary tract infections or mesh exposure) or serious adverse event; the rate did not differ between the groups. There were six serious adverse events requiring intervention, including one case of mesh erosion in each group and four urinary tract infections in the retropubic midurethral sling group.
"Importantly, there were seven new mesh exposures in years 3-5 after surgery," Dr. Rickey said, noting that three occurred in the retropubic midurethral sling group and four occurred in the transobturator midurethral sling group.
Women included in the extension phase were TOMUS participants who did not undergo surgical retreatment for stress urinary incontinence after the initial procedure. Participants completed annual in-person visits, including pelvic examination, symptom, mesh, and quality of life questionnaires.
Treatment success was defined as no retreatment for stress urinary incontinence, and no self-reported stress urinary incontinence symptoms using the Medical, Epidemiological, and Social Aspects of Aging questionnaire, she said.
"In conclusion, the 5-year continence rates did decline after either retropubic midurethral sling or transobturator sling, and did not meet prespecified criteria for equivalency. Satisfaction does remain high in both groups. In general, urinary symptoms and quality of life were more improved at 5 years after the transobturator approach, and mesh erosion rates remain low at 1.7%," she said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors reported having no disclosures.
AT THE AUA ANNUAL MEETING
Key clinical point: The benefits of midurethral slings persisted 5 years in the majority of women.
Major finding: Overall rates of satisfaction at 5 years were 79% and 85% with retropubic midurethral and transobturator midurethral slings, respectively.
Data source: Extended follow-up of 404 patients from the TOMUS trial (E-TOMUS).
Disclosures: This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors reported having no disclosures.
Off-pump CABG reduces acute kidney injury, but not long-term function loss
Compared with on-pump coronary artery bypass graft surgery, off-pump CABG reduced the risk of postoperative acute kidney injury, but was not associated with better-preserved kidney function at 1 year in a substudy of the randomized CORONARY trial.
Acute kidney injury within 30 days of surgery occurred in 17.5% of 1,472 patients in the off-pump group, compared with 20.8% of 1,460 in the on-pump group (relative risk, 0.83). Loss of kidney function at 1 year occurred in 17.1% and 15.3% of patients in the groups, respectively (relative risk, 1.10), Dr. Amit X. Garg of the London (Ontario)Health Sciences Center, and his colleagues reported on behalf of the CORONARY (Coronary Artery Bypass Grafting Surgery Off- or On-pump Revascularization Study) investigators.
The observed relative risks for both acute kidney injury and long-term loss of kidney function were consistent when the data were reanalyzed using multiple alternate definitions of the two outcomes, as well as when using the composite outcomes of acute kidney injury or death, or kidney function loss or death, respectively, the authors said.
The findings were presented at the annual European Renal Association-European Dialysis and Transplant Association Congress, and were published simultaneously in JAMA.
CORONARY participants were 4,732 adults undergoing first isolated CABG surgery at 79 sites in 19 countries. Previously published results from the CORONARY trial showed no significant difference between on- and off-pump CABG with respect to a composite outcome of death, nonfatal myocardial infarction, stroke or new dialysis for kidney failure (or in any of these individual components) within 30 days or at 1 year postrandomization. Those included in the current analysis were 2,932 patients from 63 sites in 16 countries who were enrolled into the kidney function substudy between January, 2010 and November, 2011.
Acute kidney injury was defined as an increase of at least 50% in serum creatinine concentration from prerandomization. Loss of kidney function at 1 year was defined as at least a 20% loss in estimated glomerular filtration rate from prerandomization level, the investigators said (JAMA 2014 June 2[doi:10.1001/jama.2014.4952]).
The current findings, along with those from a recent meta-analysis of 22 prior randomized, controlled trials provide convincing evidence that off-pump vs. on-pump CABG surgery reduces the risk of mild to moderate acute kidney injury, particularly in those with preoperative chronic kidney disease, they said.
Mild or moderate acute kidney injury affects about 30% of patients after cardiac surgery, with about 1% requiring acute dialysis for severe kidney injury, but the effects of mild or moderate acute kidney injury on long-term kidney function are not clear.
Animal studies have suggested a causal link, and several human observational studies have shown a link as early as 3 months after injury, but "it remains unproven in a randomized clinical trial that an intervention that prevents such acute kidney injury better preserves long-term kidney function," they said.
The lack of an effect on long-term kidney function in the CORONARY trial may reflect inadequate follow-up, errors with serum creatinine concentration as a measure of kidney function, nonacute kidney injury effects of off-pump CABG surgery or differential care in follow-up between the groups, small magnitude of injury reduction with off-pump CABG, or lack of association between mild to moderate acute kidney injury and substantial chronic kidney disease.
"Regardless of the reason attributed to the observed 1-year kidney results from the CORONARY trial, the findings emphasize proof is needed to claim an intervention that reduces the risk of mild acute kidney injury better preserves long-term kidney function for the group that received it," the investigators wrote, adding that this has implications for the development, testing, and use of interventions to prevent acute kidney injury.
The findings support the current position of regulatory agencies – including the Food and Drug Administration – that no intervention will be approved based solely on its ability to prevent modest acute kidney injury, but rather that proof is required that the intervention has an effect on long-term permanent kidney function or other clinically meaningful events, they explained.
"This provides pause for interventions such as N-acetylcysteine and intravenous sodium bicarbonate, which have been broadly adopted because smaller trials demonstrated a reduced risk of modest acute kidney injury without proof of an effect on permanent kidney function. Our results also have implications for trials currently examining intervention effects on mild acute kidney injury without assessing long-term kidney function," they concluded. Furthermore, future trials should consider multiple measures of kidney function over time, examine trajectories of kidney function loss, and use new markers of kidney function or injury, and enroll a greater number of patients with baseline chronic kidney disease to better ascertain whether a causal relationship exists between acute kidney injury and long-term kidney function, they said.
CORONARY and the kidney substudy were supported by grants from the Canadian Institutes of Health Research.
Dr. Garg reported receiving grant funding from Astellas, Roche, and Pfizer. Another author (Dr. P.J. Devereaux) reported receiving grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Roche Diagnostics, and Stryker, and one (Dr. Chirag R. Parikh) reported participating on a data monitoring committee for a phase II trial sponsored by AbbVie. The remaining authors reported having no disclosures.
Compared with on-pump coronary artery bypass graft surgery, off-pump CABG reduced the risk of postoperative acute kidney injury, but was not associated with better-preserved kidney function at 1 year in a substudy of the randomized CORONARY trial.
Acute kidney injury within 30 days of surgery occurred in 17.5% of 1,472 patients in the off-pump group, compared with 20.8% of 1,460 in the on-pump group (relative risk, 0.83). Loss of kidney function at 1 year occurred in 17.1% and 15.3% of patients in the groups, respectively (relative risk, 1.10), Dr. Amit X. Garg of the London (Ontario)Health Sciences Center, and his colleagues reported on behalf of the CORONARY (Coronary Artery Bypass Grafting Surgery Off- or On-pump Revascularization Study) investigators.
The observed relative risks for both acute kidney injury and long-term loss of kidney function were consistent when the data were reanalyzed using multiple alternate definitions of the two outcomes, as well as when using the composite outcomes of acute kidney injury or death, or kidney function loss or death, respectively, the authors said.
The findings were presented at the annual European Renal Association-European Dialysis and Transplant Association Congress, and were published simultaneously in JAMA.
CORONARY participants were 4,732 adults undergoing first isolated CABG surgery at 79 sites in 19 countries. Previously published results from the CORONARY trial showed no significant difference between on- and off-pump CABG with respect to a composite outcome of death, nonfatal myocardial infarction, stroke or new dialysis for kidney failure (or in any of these individual components) within 30 days or at 1 year postrandomization. Those included in the current analysis were 2,932 patients from 63 sites in 16 countries who were enrolled into the kidney function substudy between January, 2010 and November, 2011.
Acute kidney injury was defined as an increase of at least 50% in serum creatinine concentration from prerandomization. Loss of kidney function at 1 year was defined as at least a 20% loss in estimated glomerular filtration rate from prerandomization level, the investigators said (JAMA 2014 June 2[doi:10.1001/jama.2014.4952]).
The current findings, along with those from a recent meta-analysis of 22 prior randomized, controlled trials provide convincing evidence that off-pump vs. on-pump CABG surgery reduces the risk of mild to moderate acute kidney injury, particularly in those with preoperative chronic kidney disease, they said.
Mild or moderate acute kidney injury affects about 30% of patients after cardiac surgery, with about 1% requiring acute dialysis for severe kidney injury, but the effects of mild or moderate acute kidney injury on long-term kidney function are not clear.
Animal studies have suggested a causal link, and several human observational studies have shown a link as early as 3 months after injury, but "it remains unproven in a randomized clinical trial that an intervention that prevents such acute kidney injury better preserves long-term kidney function," they said.
The lack of an effect on long-term kidney function in the CORONARY trial may reflect inadequate follow-up, errors with serum creatinine concentration as a measure of kidney function, nonacute kidney injury effects of off-pump CABG surgery or differential care in follow-up between the groups, small magnitude of injury reduction with off-pump CABG, or lack of association between mild to moderate acute kidney injury and substantial chronic kidney disease.
"Regardless of the reason attributed to the observed 1-year kidney results from the CORONARY trial, the findings emphasize proof is needed to claim an intervention that reduces the risk of mild acute kidney injury better preserves long-term kidney function for the group that received it," the investigators wrote, adding that this has implications for the development, testing, and use of interventions to prevent acute kidney injury.
The findings support the current position of regulatory agencies – including the Food and Drug Administration – that no intervention will be approved based solely on its ability to prevent modest acute kidney injury, but rather that proof is required that the intervention has an effect on long-term permanent kidney function or other clinically meaningful events, they explained.
"This provides pause for interventions such as N-acetylcysteine and intravenous sodium bicarbonate, which have been broadly adopted because smaller trials demonstrated a reduced risk of modest acute kidney injury without proof of an effect on permanent kidney function. Our results also have implications for trials currently examining intervention effects on mild acute kidney injury without assessing long-term kidney function," they concluded. Furthermore, future trials should consider multiple measures of kidney function over time, examine trajectories of kidney function loss, and use new markers of kidney function or injury, and enroll a greater number of patients with baseline chronic kidney disease to better ascertain whether a causal relationship exists between acute kidney injury and long-term kidney function, they said.
CORONARY and the kidney substudy were supported by grants from the Canadian Institutes of Health Research.
Dr. Garg reported receiving grant funding from Astellas, Roche, and Pfizer. Another author (Dr. P.J. Devereaux) reported receiving grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Roche Diagnostics, and Stryker, and one (Dr. Chirag R. Parikh) reported participating on a data monitoring committee for a phase II trial sponsored by AbbVie. The remaining authors reported having no disclosures.
Compared with on-pump coronary artery bypass graft surgery, off-pump CABG reduced the risk of postoperative acute kidney injury, but was not associated with better-preserved kidney function at 1 year in a substudy of the randomized CORONARY trial.
Acute kidney injury within 30 days of surgery occurred in 17.5% of 1,472 patients in the off-pump group, compared with 20.8% of 1,460 in the on-pump group (relative risk, 0.83). Loss of kidney function at 1 year occurred in 17.1% and 15.3% of patients in the groups, respectively (relative risk, 1.10), Dr. Amit X. Garg of the London (Ontario)Health Sciences Center, and his colleagues reported on behalf of the CORONARY (Coronary Artery Bypass Grafting Surgery Off- or On-pump Revascularization Study) investigators.
The observed relative risks for both acute kidney injury and long-term loss of kidney function were consistent when the data were reanalyzed using multiple alternate definitions of the two outcomes, as well as when using the composite outcomes of acute kidney injury or death, or kidney function loss or death, respectively, the authors said.
The findings were presented at the annual European Renal Association-European Dialysis and Transplant Association Congress, and were published simultaneously in JAMA.
CORONARY participants were 4,732 adults undergoing first isolated CABG surgery at 79 sites in 19 countries. Previously published results from the CORONARY trial showed no significant difference between on- and off-pump CABG with respect to a composite outcome of death, nonfatal myocardial infarction, stroke or new dialysis for kidney failure (or in any of these individual components) within 30 days or at 1 year postrandomization. Those included in the current analysis were 2,932 patients from 63 sites in 16 countries who were enrolled into the kidney function substudy between January, 2010 and November, 2011.
Acute kidney injury was defined as an increase of at least 50% in serum creatinine concentration from prerandomization. Loss of kidney function at 1 year was defined as at least a 20% loss in estimated glomerular filtration rate from prerandomization level, the investigators said (JAMA 2014 June 2[doi:10.1001/jama.2014.4952]).
The current findings, along with those from a recent meta-analysis of 22 prior randomized, controlled trials provide convincing evidence that off-pump vs. on-pump CABG surgery reduces the risk of mild to moderate acute kidney injury, particularly in those with preoperative chronic kidney disease, they said.
Mild or moderate acute kidney injury affects about 30% of patients after cardiac surgery, with about 1% requiring acute dialysis for severe kidney injury, but the effects of mild or moderate acute kidney injury on long-term kidney function are not clear.
Animal studies have suggested a causal link, and several human observational studies have shown a link as early as 3 months after injury, but "it remains unproven in a randomized clinical trial that an intervention that prevents such acute kidney injury better preserves long-term kidney function," they said.
The lack of an effect on long-term kidney function in the CORONARY trial may reflect inadequate follow-up, errors with serum creatinine concentration as a measure of kidney function, nonacute kidney injury effects of off-pump CABG surgery or differential care in follow-up between the groups, small magnitude of injury reduction with off-pump CABG, or lack of association between mild to moderate acute kidney injury and substantial chronic kidney disease.
"Regardless of the reason attributed to the observed 1-year kidney results from the CORONARY trial, the findings emphasize proof is needed to claim an intervention that reduces the risk of mild acute kidney injury better preserves long-term kidney function for the group that received it," the investigators wrote, adding that this has implications for the development, testing, and use of interventions to prevent acute kidney injury.
The findings support the current position of regulatory agencies – including the Food and Drug Administration – that no intervention will be approved based solely on its ability to prevent modest acute kidney injury, but rather that proof is required that the intervention has an effect on long-term permanent kidney function or other clinically meaningful events, they explained.
"This provides pause for interventions such as N-acetylcysteine and intravenous sodium bicarbonate, which have been broadly adopted because smaller trials demonstrated a reduced risk of modest acute kidney injury without proof of an effect on permanent kidney function. Our results also have implications for trials currently examining intervention effects on mild acute kidney injury without assessing long-term kidney function," they concluded. Furthermore, future trials should consider multiple measures of kidney function over time, examine trajectories of kidney function loss, and use new markers of kidney function or injury, and enroll a greater number of patients with baseline chronic kidney disease to better ascertain whether a causal relationship exists between acute kidney injury and long-term kidney function, they said.
CORONARY and the kidney substudy were supported by grants from the Canadian Institutes of Health Research.
Dr. Garg reported receiving grant funding from Astellas, Roche, and Pfizer. Another author (Dr. P.J. Devereaux) reported receiving grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Roche Diagnostics, and Stryker, and one (Dr. Chirag R. Parikh) reported participating on a data monitoring committee for a phase II trial sponsored by AbbVie. The remaining authors reported having no disclosures.
FROM THE ERA-EDTA ANNUAL CONGRESS
Key clinical point: Preservation of kidney function is not a reason to choose off-pump CABG over on-pump.
Major finding: Relative risk of acute kidney injury, 0.83; relative risk of kidney function loss at 1 year, 1.10 with off- vs. on-pump CABG.
Data source: A substudy of 2,932 patients from the randomized CORONARY trial.
Disclosures: CORONARY and the kidney substudy were supported by grants from the Canadian Institutes of Health Research. Dr. Garg reported receiving grant funding from Astellas, Roche, and Pfizer. Another author (Dr. P.J. Devereaux) reported receiving grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Roche Diagnostics, and Stryker, and one (Dr. Chirag R. Parikh) reported participating on a data monitoring committee for a phase II trial sponsored by AbbVie. The remaining authors reported having no disclosures.