User login
Psychological/neuropsychological testing: When to refer for reexamination
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
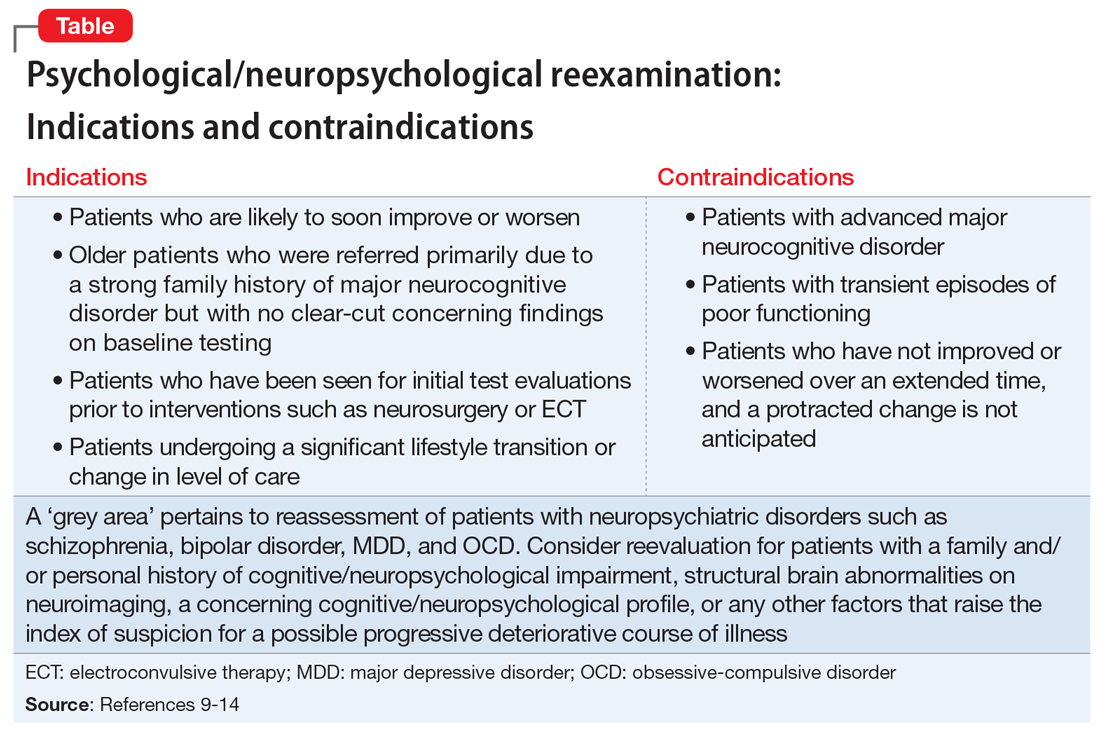
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.
Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.

Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.

Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
EDs saw more benzodiazepine overdoses, but fewer patients overall, in 2020
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.
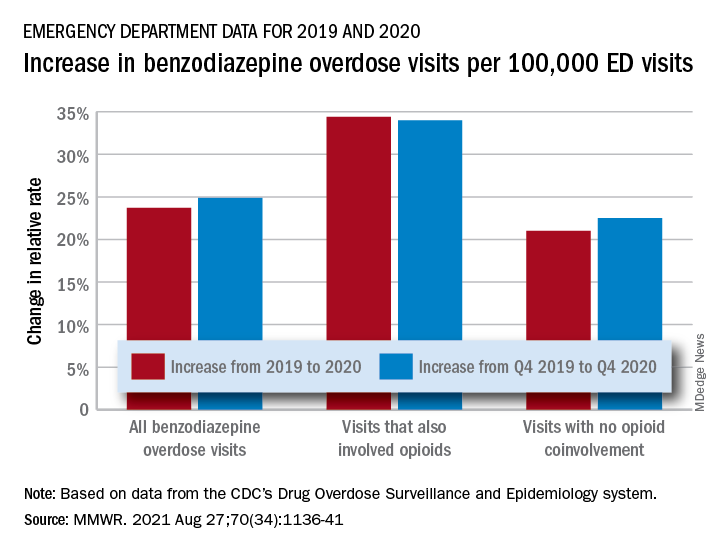
The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
FROM MMWR
FDA OKs IV Briviact for seizures in kids as young as 1 month
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
ACST-2: Carotid stenting, surgery on par in asymptomatic patients
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Angiography can wait for cardiac arrest without ST-elevation
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
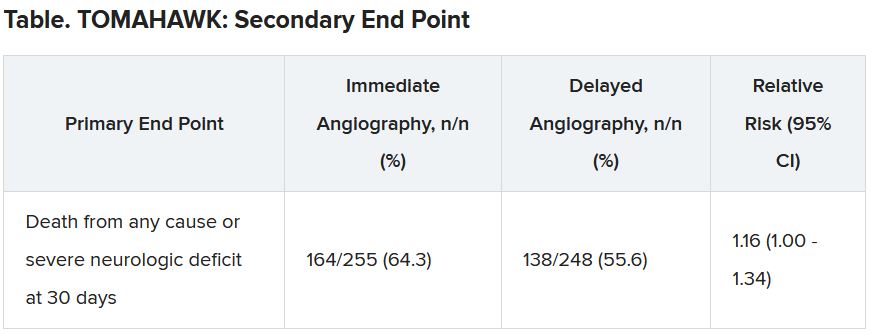
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Human brain patterns may help build a better AI system
new research suggests. “This work opens new opportunities to discover how the network organization of the brain optimizes cognitive capacity,” wrote researchers from The Neuro (Montreal Neurological Institute–Hospital) and the Quebec Artificial Intelligence Institute.
Senior investigator Bratislav Misic, PhD, said the research has potential clinical application for studying diseases of the brain, which is something his team is actively working on. “For example, using MRI techniques, we can measure different patterns of atrophy in neurodegenerative diseases such as Alzheimer’s disease,” he said.
“We can use these disease patterns from real patients to artificially lesion these connectomes and to ask how a particular disease causes a particular pattern of symptoms and cognitive deficits,” he added.
The findings were published online in Nature Machine Intelligence.
Unique approach
Using brain imaging data, the investigators reconstructed a human brain connectivity pattern and applied it to an artificial neural network. After training, the artificial neural network successfully performed a working memory task more flexibly and efficiently than other “benchmark” AI systems.
The researchers noted that their approach is unique because previous work on brain connectivity, also known as connectomics, has focused on describing brain organization without regard to how it actually functions.
Traditional artificial neural network have arbitrary structures that do not reflect how real brain networks are organized. Integrating brain connectomics into the construction of artificial neural network can reveal how the wiring of the brain supports specific cognitive skills, the investigators wrote.
“Up until now, if you look at how neural networks are constructed, the architectures that are used are very ad hoc and very problem specific,” Dr. Misic said. “But the connectomics revolution that’s happened in neuroscience over the past 20 years or so has given us the ability to really measure and trace out connection patterns in a variety of organisms, including the human brain.”
He noted that the researchers took wiring patterns of the real human brain and implemented it as an artificial neural network. They then “trained that network to perform a very simple cognitive task, and when you compare it to other benchmark architectures, it actually does better.”
This shows that there is “something fundamentally different about how the human brain is wired up and that the design principles that we can see in the human brain could be used to potentially build better artificial networks,” Dr. Misic concluded.
Funding for the research was provided by the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains, Healthy Lives initiative, and by the Natural Sciences and Engineering Research Council of Canada, Fonds de Recherche du Quebec – Santé, the Canadian Institute for Advanced Research, Canada Research Chairs, Fonds de Recherche du Quebec – Nature et Technologies, and the Centre UNIQUE (Union of Neuroscience and Artificial Intelligence). The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests. “This work opens new opportunities to discover how the network organization of the brain optimizes cognitive capacity,” wrote researchers from The Neuro (Montreal Neurological Institute–Hospital) and the Quebec Artificial Intelligence Institute.
Senior investigator Bratislav Misic, PhD, said the research has potential clinical application for studying diseases of the brain, which is something his team is actively working on. “For example, using MRI techniques, we can measure different patterns of atrophy in neurodegenerative diseases such as Alzheimer’s disease,” he said.
“We can use these disease patterns from real patients to artificially lesion these connectomes and to ask how a particular disease causes a particular pattern of symptoms and cognitive deficits,” he added.
The findings were published online in Nature Machine Intelligence.
Unique approach
Using brain imaging data, the investigators reconstructed a human brain connectivity pattern and applied it to an artificial neural network. After training, the artificial neural network successfully performed a working memory task more flexibly and efficiently than other “benchmark” AI systems.
The researchers noted that their approach is unique because previous work on brain connectivity, also known as connectomics, has focused on describing brain organization without regard to how it actually functions.
Traditional artificial neural network have arbitrary structures that do not reflect how real brain networks are organized. Integrating brain connectomics into the construction of artificial neural network can reveal how the wiring of the brain supports specific cognitive skills, the investigators wrote.
“Up until now, if you look at how neural networks are constructed, the architectures that are used are very ad hoc and very problem specific,” Dr. Misic said. “But the connectomics revolution that’s happened in neuroscience over the past 20 years or so has given us the ability to really measure and trace out connection patterns in a variety of organisms, including the human brain.”
He noted that the researchers took wiring patterns of the real human brain and implemented it as an artificial neural network. They then “trained that network to perform a very simple cognitive task, and when you compare it to other benchmark architectures, it actually does better.”
This shows that there is “something fundamentally different about how the human brain is wired up and that the design principles that we can see in the human brain could be used to potentially build better artificial networks,” Dr. Misic concluded.
Funding for the research was provided by the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains, Healthy Lives initiative, and by the Natural Sciences and Engineering Research Council of Canada, Fonds de Recherche du Quebec – Santé, the Canadian Institute for Advanced Research, Canada Research Chairs, Fonds de Recherche du Quebec – Nature et Technologies, and the Centre UNIQUE (Union of Neuroscience and Artificial Intelligence). The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests. “This work opens new opportunities to discover how the network organization of the brain optimizes cognitive capacity,” wrote researchers from The Neuro (Montreal Neurological Institute–Hospital) and the Quebec Artificial Intelligence Institute.
Senior investigator Bratislav Misic, PhD, said the research has potential clinical application for studying diseases of the brain, which is something his team is actively working on. “For example, using MRI techniques, we can measure different patterns of atrophy in neurodegenerative diseases such as Alzheimer’s disease,” he said.
“We can use these disease patterns from real patients to artificially lesion these connectomes and to ask how a particular disease causes a particular pattern of symptoms and cognitive deficits,” he added.
The findings were published online in Nature Machine Intelligence.
Unique approach
Using brain imaging data, the investigators reconstructed a human brain connectivity pattern and applied it to an artificial neural network. After training, the artificial neural network successfully performed a working memory task more flexibly and efficiently than other “benchmark” AI systems.
The researchers noted that their approach is unique because previous work on brain connectivity, also known as connectomics, has focused on describing brain organization without regard to how it actually functions.
Traditional artificial neural network have arbitrary structures that do not reflect how real brain networks are organized. Integrating brain connectomics into the construction of artificial neural network can reveal how the wiring of the brain supports specific cognitive skills, the investigators wrote.
“Up until now, if you look at how neural networks are constructed, the architectures that are used are very ad hoc and very problem specific,” Dr. Misic said. “But the connectomics revolution that’s happened in neuroscience over the past 20 years or so has given us the ability to really measure and trace out connection patterns in a variety of organisms, including the human brain.”
He noted that the researchers took wiring patterns of the real human brain and implemented it as an artificial neural network. They then “trained that network to perform a very simple cognitive task, and when you compare it to other benchmark architectures, it actually does better.”
This shows that there is “something fundamentally different about how the human brain is wired up and that the design principles that we can see in the human brain could be used to potentially build better artificial networks,” Dr. Misic concluded.
Funding for the research was provided by the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains, Healthy Lives initiative, and by the Natural Sciences and Engineering Research Council of Canada, Fonds de Recherche du Quebec – Santé, the Canadian Institute for Advanced Research, Canada Research Chairs, Fonds de Recherche du Quebec – Nature et Technologies, and the Centre UNIQUE (Union of Neuroscience and Artificial Intelligence). The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MACHINE INTELLIGENCE
NIH to study COVID vaccine booster in people with autoimmune disease
In the wake of the Centers for Disease Control and Prevention’s recommendation for a third COVID-19 mRNA vaccine dose for immunocompromised people and the Food and Drug Administration’s authorization of the third dose, the according to an announcement.
The investigators of the trial, called COVID‐19 Booster Vaccine in Autoimmune Disease Non‐Responders, also want to determine if pausing immunosuppressive therapy for autoimmune disease improves the antibody response to an extra dose of a COVID-19 vaccine.
The trial will specifically look at the effects of mycophenolate mofetil (MMF) or mycophenolic acid (MPA), and methotrexate (MTX), or receipt of B cell–depletion therapy such as rituximab within the past 12 months on immune response to a booster dose in people with systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, systemic sclerosis, or pemphigus. They have to have either no serologic response to their initial COVID-19 vaccine regimen or a suboptimal response, defined as a Roche Elecsys Anti-SARS-CoV-2 S (RBD) result greater than or equal to 50 U/mL.
The results of studies conducted in solid-organ transplant recipients who take immunosuppressants showed that an extra dose of vaccine could improve the immune response to the vaccine in many of the individuals, which suggests that the same approach might work in people with autoimmune disease who need treatment with immunosuppressive drugs. Improving the immune response of people with autoimmune disease to COVID-19 vaccines is important because higher rates of severe COVID-19 and death have been reported in this group of patients than in the general population, and it is unclear whether this is attributable to the autoimmune disease, the immunosuppressive medications taken to treat it, or both.
The open-label trial, conducted by the NIAID-funded Autoimmunity Centers of Excellence, aims to enroll 600 people aged 18 years and older with those conditions at 15-20 sites in the United States.
Because medications commonly taken by people with these conditions have been associated with poorer immune responses to vaccines, the trial will randomize the following two cohorts to stop or continue taking their immunosuppressive medication(s) or stop them before and after the booster according to protocol:
- Cohort 1 includes people who are taking MMF or MPA, without additional B cell–depleting medications or MTX.
- Cohort 2 includes people who are taking MTX without additional B cell–depleting medications or MMF/MPA.
A third, nonrandomized cohort consists of people who have received B cell–depletion therapy within the past 12 months regardless of whether they are also taking MMF/MPA or MTX.
Besides the cohort-specific exclusions, other rheumatic disease medications, including biologics, are allowed in the groups.
The primary outcome of the trial is the proportion of participants who have a protective antibody response at week 4. Secondary outcomes will examine various antibody responses at intervals, changes in disease activity across autoimmune diseases, adverse events, and SARS-CoV-2 infections out to 48 weeks.
Study participants will be followed for a total of 13 months. Preliminary results are expected in November 2021, according to the National Institutes of Health.
The trial is being led by Judith James, MD, PhD; Meggan Mackay, MD, MS; Dinesh Khanna, MBBS, MSc; and Amit Bar-Or, MD.
In the wake of the Centers for Disease Control and Prevention’s recommendation for a third COVID-19 mRNA vaccine dose for immunocompromised people and the Food and Drug Administration’s authorization of the third dose, the according to an announcement.
The investigators of the trial, called COVID‐19 Booster Vaccine in Autoimmune Disease Non‐Responders, also want to determine if pausing immunosuppressive therapy for autoimmune disease improves the antibody response to an extra dose of a COVID-19 vaccine.
The trial will specifically look at the effects of mycophenolate mofetil (MMF) or mycophenolic acid (MPA), and methotrexate (MTX), or receipt of B cell–depletion therapy such as rituximab within the past 12 months on immune response to a booster dose in people with systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, systemic sclerosis, or pemphigus. They have to have either no serologic response to their initial COVID-19 vaccine regimen or a suboptimal response, defined as a Roche Elecsys Anti-SARS-CoV-2 S (RBD) result greater than or equal to 50 U/mL.
The results of studies conducted in solid-organ transplant recipients who take immunosuppressants showed that an extra dose of vaccine could improve the immune response to the vaccine in many of the individuals, which suggests that the same approach might work in people with autoimmune disease who need treatment with immunosuppressive drugs. Improving the immune response of people with autoimmune disease to COVID-19 vaccines is important because higher rates of severe COVID-19 and death have been reported in this group of patients than in the general population, and it is unclear whether this is attributable to the autoimmune disease, the immunosuppressive medications taken to treat it, or both.
The open-label trial, conducted by the NIAID-funded Autoimmunity Centers of Excellence, aims to enroll 600 people aged 18 years and older with those conditions at 15-20 sites in the United States.
Because medications commonly taken by people with these conditions have been associated with poorer immune responses to vaccines, the trial will randomize the following two cohorts to stop or continue taking their immunosuppressive medication(s) or stop them before and after the booster according to protocol:
- Cohort 1 includes people who are taking MMF or MPA, without additional B cell–depleting medications or MTX.
- Cohort 2 includes people who are taking MTX without additional B cell–depleting medications or MMF/MPA.
A third, nonrandomized cohort consists of people who have received B cell–depletion therapy within the past 12 months regardless of whether they are also taking MMF/MPA or MTX.
Besides the cohort-specific exclusions, other rheumatic disease medications, including biologics, are allowed in the groups.
The primary outcome of the trial is the proportion of participants who have a protective antibody response at week 4. Secondary outcomes will examine various antibody responses at intervals, changes in disease activity across autoimmune diseases, adverse events, and SARS-CoV-2 infections out to 48 weeks.
Study participants will be followed for a total of 13 months. Preliminary results are expected in November 2021, according to the National Institutes of Health.
The trial is being led by Judith James, MD, PhD; Meggan Mackay, MD, MS; Dinesh Khanna, MBBS, MSc; and Amit Bar-Or, MD.
In the wake of the Centers for Disease Control and Prevention’s recommendation for a third COVID-19 mRNA vaccine dose for immunocompromised people and the Food and Drug Administration’s authorization of the third dose, the according to an announcement.
The investigators of the trial, called COVID‐19 Booster Vaccine in Autoimmune Disease Non‐Responders, also want to determine if pausing immunosuppressive therapy for autoimmune disease improves the antibody response to an extra dose of a COVID-19 vaccine.
The trial will specifically look at the effects of mycophenolate mofetil (MMF) or mycophenolic acid (MPA), and methotrexate (MTX), or receipt of B cell–depletion therapy such as rituximab within the past 12 months on immune response to a booster dose in people with systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, systemic sclerosis, or pemphigus. They have to have either no serologic response to their initial COVID-19 vaccine regimen or a suboptimal response, defined as a Roche Elecsys Anti-SARS-CoV-2 S (RBD) result greater than or equal to 50 U/mL.
The results of studies conducted in solid-organ transplant recipients who take immunosuppressants showed that an extra dose of vaccine could improve the immune response to the vaccine in many of the individuals, which suggests that the same approach might work in people with autoimmune disease who need treatment with immunosuppressive drugs. Improving the immune response of people with autoimmune disease to COVID-19 vaccines is important because higher rates of severe COVID-19 and death have been reported in this group of patients than in the general population, and it is unclear whether this is attributable to the autoimmune disease, the immunosuppressive medications taken to treat it, or both.
The open-label trial, conducted by the NIAID-funded Autoimmunity Centers of Excellence, aims to enroll 600 people aged 18 years and older with those conditions at 15-20 sites in the United States.
Because medications commonly taken by people with these conditions have been associated with poorer immune responses to vaccines, the trial will randomize the following two cohorts to stop or continue taking their immunosuppressive medication(s) or stop them before and after the booster according to protocol:
- Cohort 1 includes people who are taking MMF or MPA, without additional B cell–depleting medications or MTX.
- Cohort 2 includes people who are taking MTX without additional B cell–depleting medications or MMF/MPA.
A third, nonrandomized cohort consists of people who have received B cell–depletion therapy within the past 12 months regardless of whether they are also taking MMF/MPA or MTX.
Besides the cohort-specific exclusions, other rheumatic disease medications, including biologics, are allowed in the groups.
The primary outcome of the trial is the proportion of participants who have a protective antibody response at week 4. Secondary outcomes will examine various antibody responses at intervals, changes in disease activity across autoimmune diseases, adverse events, and SARS-CoV-2 infections out to 48 weeks.
Study participants will be followed for a total of 13 months. Preliminary results are expected in November 2021, according to the National Institutes of Health.
The trial is being led by Judith James, MD, PhD; Meggan Mackay, MD, MS; Dinesh Khanna, MBBS, MSc; and Amit Bar-Or, MD.
Young Black and White athletes differ in how they recover from concussions
, according to a new study on racial differences in concussion recovery.
“The findings from this study provide novel evidence that the recovery experience following sport-related concussion likely differs between Black and White athletes, and understanding these differences may serve to provide better and more personalized intervention and management strategies,” wrote lead author Aaron M. Yengo-Kahn, MD, of Vanderbilt University Medical Center in Nashville, Tenn. The study was published in the Journal of Neurosurgery: Pediatrics.
To assess how postconcussion experiences and recovery time differ among young White and Black athletes, the researchers launched a retrospective cohort study of youths between the ages of 12 and 23 from the middle Tennessee, northern Alabama, and southern Kentucky regions who had been treated for sport-related concussion. Using data from the Vanderbilt Sports Concussion Center’s outcome registry, they examined the records of 247 student-athletes, 211 of whom were White and 36 of whom were Black.
The majority of the athletes were male – 58% of the White group and 78% of the Black group – and their average age across groups was roughly 16 years. Thirty-three percent of the Black athletes were on public insurance, compared with just 6% of the White athletes, and 41% of the Black athletes lived in low–median income areas while 55% of the White athletes lived in areas with a high median income. Approximately 90% of each group played contact sports.
The median time to symptom resolution was 21 days (interquartile range, 10.5-61.0) for White athletes but just 12.3 days (IQR, 6.8-28.0) for Black athletes. Multivariable regression confirmed that Black athletes reached asymptomatic status sooner than White athletes (hazard ratio, 1.497; 95% confidence interval, 1.014-2.209; P = .042). “The observed shorter symptom resolution among the Black athletes may be explained by a complex interplay among race, concussion knowledge, attitudes toward sport-related concussion, reporting behavior, and sociodemographic disparities,” the authors noted.
The median time until returning to school post injury was 2 school days (IQR, 0-5) for White athletes and 0 school days (IQR, 0-2) for Black athletes. After multivariable analysis, being Black was indeed associated with returning to school sooner, compared with being White (HR, 1.522; 95% CI, 1.02-2,27; P = .040). Being Black was also associated with being less likely to a report a change in daily activity post concussion (odds ratio, 0.368; 95% CI, 0.136-0.996; P = .049).
Adding race to research
To make headway toward understanding race’s impact on concussion research, the authors proposed three immediate steps: Work directly with schools instead of clinics or emergency departments, match the diversity of study cohorts with the racial makeup of the surrounding community, and consider race as a covariate during study design.
“In our work with concussions, there is very little reported on race or racism or how racism affects how patients are navigating these spaces,” said coauthor Jessica Wallace, PhD, of the department of health science at the University of Alabama in Tuscaloosa, Ala., in an interview. “But we have so many athletes at the youth level, adolescent level, even the collegiate level; it’s such a diverse array of patients. We need to have data representative of all of our groups so that we know where we need to be intentional about reducing disparities and closing gaps.”
Dr. Wallace, who recently authored a study on the underreporting of concussions among Black and White high school athletes, emphasized the need for concussion research to be a true collaboration across disciplines.
“I approach this work from this public health and athletic training lens, whereas a lot of my collaborators are in neurosurgery and neurology,” she said. “Moving forward, we as a scientific clinical community have to do interdisciplinary work and be very intentional about how we go about closing these gaps. We have to recognize that there are differences in knowledge and in care, and they’re unacceptable, and we have to work collaboratively in providing resources to communities equitably to decrease them.”
The authors acknowledged their study’s limitations, including the retrospective nature of the study, using zip codes to determine median household income, and an unbalanced number of White and Black athletes. They did add, however, that the ratio of participants “generally aligns with census data in the surrounding metropolitan and county areas.” That said, they also surmised that the scarcity of Black athletes could indicate a deeper disparity in health care system usage and asked future researchers to “consider enrolling athletes directly from schools rather than from within the concussion clinic only.”
Dr. Yengo-Kahn disclosed holding a compensated position on the scientific advisory board of BlinkTBI, but the authors noted that the company had no role in the study and its products were not used. No other conflicts of interest were reported.
, according to a new study on racial differences in concussion recovery.
“The findings from this study provide novel evidence that the recovery experience following sport-related concussion likely differs between Black and White athletes, and understanding these differences may serve to provide better and more personalized intervention and management strategies,” wrote lead author Aaron M. Yengo-Kahn, MD, of Vanderbilt University Medical Center in Nashville, Tenn. The study was published in the Journal of Neurosurgery: Pediatrics.
To assess how postconcussion experiences and recovery time differ among young White and Black athletes, the researchers launched a retrospective cohort study of youths between the ages of 12 and 23 from the middle Tennessee, northern Alabama, and southern Kentucky regions who had been treated for sport-related concussion. Using data from the Vanderbilt Sports Concussion Center’s outcome registry, they examined the records of 247 student-athletes, 211 of whom were White and 36 of whom were Black.
The majority of the athletes were male – 58% of the White group and 78% of the Black group – and their average age across groups was roughly 16 years. Thirty-three percent of the Black athletes were on public insurance, compared with just 6% of the White athletes, and 41% of the Black athletes lived in low–median income areas while 55% of the White athletes lived in areas with a high median income. Approximately 90% of each group played contact sports.
The median time to symptom resolution was 21 days (interquartile range, 10.5-61.0) for White athletes but just 12.3 days (IQR, 6.8-28.0) for Black athletes. Multivariable regression confirmed that Black athletes reached asymptomatic status sooner than White athletes (hazard ratio, 1.497; 95% confidence interval, 1.014-2.209; P = .042). “The observed shorter symptom resolution among the Black athletes may be explained by a complex interplay among race, concussion knowledge, attitudes toward sport-related concussion, reporting behavior, and sociodemographic disparities,” the authors noted.
The median time until returning to school post injury was 2 school days (IQR, 0-5) for White athletes and 0 school days (IQR, 0-2) for Black athletes. After multivariable analysis, being Black was indeed associated with returning to school sooner, compared with being White (HR, 1.522; 95% CI, 1.02-2,27; P = .040). Being Black was also associated with being less likely to a report a change in daily activity post concussion (odds ratio, 0.368; 95% CI, 0.136-0.996; P = .049).
Adding race to research
To make headway toward understanding race’s impact on concussion research, the authors proposed three immediate steps: Work directly with schools instead of clinics or emergency departments, match the diversity of study cohorts with the racial makeup of the surrounding community, and consider race as a covariate during study design.
“In our work with concussions, there is very little reported on race or racism or how racism affects how patients are navigating these spaces,” said coauthor Jessica Wallace, PhD, of the department of health science at the University of Alabama in Tuscaloosa, Ala., in an interview. “But we have so many athletes at the youth level, adolescent level, even the collegiate level; it’s such a diverse array of patients. We need to have data representative of all of our groups so that we know where we need to be intentional about reducing disparities and closing gaps.”
Dr. Wallace, who recently authored a study on the underreporting of concussions among Black and White high school athletes, emphasized the need for concussion research to be a true collaboration across disciplines.
“I approach this work from this public health and athletic training lens, whereas a lot of my collaborators are in neurosurgery and neurology,” she said. “Moving forward, we as a scientific clinical community have to do interdisciplinary work and be very intentional about how we go about closing these gaps. We have to recognize that there are differences in knowledge and in care, and they’re unacceptable, and we have to work collaboratively in providing resources to communities equitably to decrease them.”
The authors acknowledged their study’s limitations, including the retrospective nature of the study, using zip codes to determine median household income, and an unbalanced number of White and Black athletes. They did add, however, that the ratio of participants “generally aligns with census data in the surrounding metropolitan and county areas.” That said, they also surmised that the scarcity of Black athletes could indicate a deeper disparity in health care system usage and asked future researchers to “consider enrolling athletes directly from schools rather than from within the concussion clinic only.”
Dr. Yengo-Kahn disclosed holding a compensated position on the scientific advisory board of BlinkTBI, but the authors noted that the company had no role in the study and its products were not used. No other conflicts of interest were reported.
, according to a new study on racial differences in concussion recovery.
“The findings from this study provide novel evidence that the recovery experience following sport-related concussion likely differs between Black and White athletes, and understanding these differences may serve to provide better and more personalized intervention and management strategies,” wrote lead author Aaron M. Yengo-Kahn, MD, of Vanderbilt University Medical Center in Nashville, Tenn. The study was published in the Journal of Neurosurgery: Pediatrics.
To assess how postconcussion experiences and recovery time differ among young White and Black athletes, the researchers launched a retrospective cohort study of youths between the ages of 12 and 23 from the middle Tennessee, northern Alabama, and southern Kentucky regions who had been treated for sport-related concussion. Using data from the Vanderbilt Sports Concussion Center’s outcome registry, they examined the records of 247 student-athletes, 211 of whom were White and 36 of whom were Black.
The majority of the athletes were male – 58% of the White group and 78% of the Black group – and their average age across groups was roughly 16 years. Thirty-three percent of the Black athletes were on public insurance, compared with just 6% of the White athletes, and 41% of the Black athletes lived in low–median income areas while 55% of the White athletes lived in areas with a high median income. Approximately 90% of each group played contact sports.
The median time to symptom resolution was 21 days (interquartile range, 10.5-61.0) for White athletes but just 12.3 days (IQR, 6.8-28.0) for Black athletes. Multivariable regression confirmed that Black athletes reached asymptomatic status sooner than White athletes (hazard ratio, 1.497; 95% confidence interval, 1.014-2.209; P = .042). “The observed shorter symptom resolution among the Black athletes may be explained by a complex interplay among race, concussion knowledge, attitudes toward sport-related concussion, reporting behavior, and sociodemographic disparities,” the authors noted.
The median time until returning to school post injury was 2 school days (IQR, 0-5) for White athletes and 0 school days (IQR, 0-2) for Black athletes. After multivariable analysis, being Black was indeed associated with returning to school sooner, compared with being White (HR, 1.522; 95% CI, 1.02-2,27; P = .040). Being Black was also associated with being less likely to a report a change in daily activity post concussion (odds ratio, 0.368; 95% CI, 0.136-0.996; P = .049).
Adding race to research
To make headway toward understanding race’s impact on concussion research, the authors proposed three immediate steps: Work directly with schools instead of clinics or emergency departments, match the diversity of study cohorts with the racial makeup of the surrounding community, and consider race as a covariate during study design.
“In our work with concussions, there is very little reported on race or racism or how racism affects how patients are navigating these spaces,” said coauthor Jessica Wallace, PhD, of the department of health science at the University of Alabama in Tuscaloosa, Ala., in an interview. “But we have so many athletes at the youth level, adolescent level, even the collegiate level; it’s such a diverse array of patients. We need to have data representative of all of our groups so that we know where we need to be intentional about reducing disparities and closing gaps.”
Dr. Wallace, who recently authored a study on the underreporting of concussions among Black and White high school athletes, emphasized the need for concussion research to be a true collaboration across disciplines.
“I approach this work from this public health and athletic training lens, whereas a lot of my collaborators are in neurosurgery and neurology,” she said. “Moving forward, we as a scientific clinical community have to do interdisciplinary work and be very intentional about how we go about closing these gaps. We have to recognize that there are differences in knowledge and in care, and they’re unacceptable, and we have to work collaboratively in providing resources to communities equitably to decrease them.”
The authors acknowledged their study’s limitations, including the retrospective nature of the study, using zip codes to determine median household income, and an unbalanced number of White and Black athletes. They did add, however, that the ratio of participants “generally aligns with census data in the surrounding metropolitan and county areas.” That said, they also surmised that the scarcity of Black athletes could indicate a deeper disparity in health care system usage and asked future researchers to “consider enrolling athletes directly from schools rather than from within the concussion clinic only.”
Dr. Yengo-Kahn disclosed holding a compensated position on the scientific advisory board of BlinkTBI, but the authors noted that the company had no role in the study and its products were not used. No other conflicts of interest were reported.
FROM THE JOURNAL OF NEUROSURGERY: PEDIATRICS
Atogepant reduces migraine days: ADVANCE trial results published
full results from a phase 3 trial suggest.
AbbVie, the company developing the oral therapy, announced topline results of the ADVANCE trial of atogepant last year. Safety results were presented in April at the 2021 annual meeting of the American Academy of Neurology.
The full results were published online Aug. 19 in the New England Journal of Medicine ahead of the upcoming target action date of the U.S. Food and Drug Administration.
The multicenter study included nearly 900 patients who were randomly assigned to receive either placebo or one of three doses of atogepant for 12 weeks. The mean number of monthly migraine days decreased by about 4 for all three doses of the active treatment, compared with a reduction of 2.5 days with placebo.
“Overall, this study showed us that atogepant was safe and surprisingly seems to be pretty effective regardless of the dose,” said lead author Jessica Ailani, MD, director of MedStar Georgetown Headache Center and associate professor of neurology at Georgetown University, Washington.
All doses effective
The study included 873 patients with episodic migraine with or without aura. Patients who were not assigned to the placebo control group received either 10 mg, 30 mg, or 60 mg of atogepant once daily.
After a 4-week screening period, all patients received treatment for 12 weeks and then entered a 4-week safety follow-up period. In total, the participants completed eight scheduled clinical visits.
The mean reduction from baseline in the mean number of migraine days per month was 3.7 with the 10-mg dose of atogepant, 3.9 with the 30-mg dose, 4.2 with the 60-mg dose, and 2.5 with placebo. The differences between each active dose and placebo was statistically significant (P < .001).
Treatment with the CGRP inhibitor was also associated with a reduction in the mean number of headache days per month. The mean reduction from baseline was 3.9 days for the 10-mg dose, 4.0 days for the 30-mg dose, 4.2 days for the 60-mg dose, and 2.5 days for placebo (P < .001 for all comparisons with placebo).
In addition, for 55.6% of the 10-mg group, 58.7% of the 30-mg group, 60.8% of the 60-mg group, and 29.0% of the control group, there was a reduction of at least 50% in the 3-month average number of migraine days per month (P < .001 for each vs. placebo).
The most commonly reported adverse events (AEs) among patients who received atogepant were constipation (6.9%-7.7% across doses), nausea (4.4%-6.1%), and upper respiratory tract infection (1.4%-3.9%). Frequency of AEs did not differ between the active-treatment groups and the control group, and no relationships between AEs and atogepant dose were observed.
Multidose flexibility
“Side effects were pretty even across the board,” said Dr. Ailani. She noted that the reported AEs were expected because of atogepant’s mechanism of action. In addition, the rate of discontinuation in the study was low.
The proportion of participants who experienced a reduction in monthly migraine days of at least 50% grew as time passed. “By the end of this study, your chance of having a greater than 50% response is about 75%,” Dr. Ailani said.
“Imagine telling your patient, ‘You stick on this drug for 3 months, and I can almost guarantee you that you’re going to get better,’” she added.
Although the treatment has no drug-drug contraindications, drug-drug interactions may occur. “The availability of various doses would allow clinicians to adjust treatment to avoid potential drug-drug interactions,” said Dr. Ailani. “That multidose flexibility is very important.”
An FDA decision on atogepant could be made in the coming months. “I’m hopeful, as a clinician, that it is positive news, because we really have waited a long time for something like this,” Dr. Ailani said.
“You can easily identify patients who would do well on this medication,” she added.
In a different study of atogepant among patients with chronic migraine, there were recruitment delays because of the pandemic. That study is now almost complete, Dr. Ailani reported.
“Well-conducted study”
Commenting on the findings, Kathleen B. Digre, MD, chief of the division of headache and neuro-ophthalmology at the University of Utah Health, Salt Lake City, expressed enthusiasm for the experimental drug. “I’m excited to see another treatment modality for migraine,” said Dr. Digre, who was not involved with the research. “It was a very well-conducted study,” she added.
The treatment arms were almost identical in regard to disease severity, and all the doses showed an effect. Although the difference in reduction of monthly migraine days in comparison with placebo was numerically small, “for people who have frequent migraine, it’s important,” Dr. Digre said.
The results for atogepant should be viewed in a larger context, however. “Even though it’s a treatment that works better than placebo for well-matched controls, it may not be a medication that everybody’s going to respond to,” she noted. “And we can’t generalize it for some of the most disabled people, which is for chronic migraine,” she said.
It is significant that the study was published in the New England Journal of Medicine, Dr. Digre noted. “Sometimes migraine is dismissed as not important and not affecting people’s lives,” she said. “That makes me very happy to see migraine being taken seriously by our major journals.”
In addition, she noted that the prospects for FDA approval of atogepant seem favorable. “I’m hopeful that they will approve it, because it’s got a low side-effect profile, plus it’s effective.”
Migraine-specific preventive therapy has emerged only in the past few years. “I’m so excited to see this surge of preventive medicine for migraine,” Dr. Digre said. “It’s so important, because we see so many people who are disabled by migraine,” she added.
The study was funded by Allergan before atogepant was acquired by AbbVie. Dr. Ailani has received honoraria from AbbVie for consulting, has received compensation from Allergan and AbbVie for participating in a speakers’ bureau, and has received clinical trial grants from Allergan. Dr. Digre has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
full results from a phase 3 trial suggest.
AbbVie, the company developing the oral therapy, announced topline results of the ADVANCE trial of atogepant last year. Safety results were presented in April at the 2021 annual meeting of the American Academy of Neurology.
The full results were published online Aug. 19 in the New England Journal of Medicine ahead of the upcoming target action date of the U.S. Food and Drug Administration.
The multicenter study included nearly 900 patients who were randomly assigned to receive either placebo or one of three doses of atogepant for 12 weeks. The mean number of monthly migraine days decreased by about 4 for all three doses of the active treatment, compared with a reduction of 2.5 days with placebo.
“Overall, this study showed us that atogepant was safe and surprisingly seems to be pretty effective regardless of the dose,” said lead author Jessica Ailani, MD, director of MedStar Georgetown Headache Center and associate professor of neurology at Georgetown University, Washington.
All doses effective
The study included 873 patients with episodic migraine with or without aura. Patients who were not assigned to the placebo control group received either 10 mg, 30 mg, or 60 mg of atogepant once daily.
After a 4-week screening period, all patients received treatment for 12 weeks and then entered a 4-week safety follow-up period. In total, the participants completed eight scheduled clinical visits.
The mean reduction from baseline in the mean number of migraine days per month was 3.7 with the 10-mg dose of atogepant, 3.9 with the 30-mg dose, 4.2 with the 60-mg dose, and 2.5 with placebo. The differences between each active dose and placebo was statistically significant (P < .001).
Treatment with the CGRP inhibitor was also associated with a reduction in the mean number of headache days per month. The mean reduction from baseline was 3.9 days for the 10-mg dose, 4.0 days for the 30-mg dose, 4.2 days for the 60-mg dose, and 2.5 days for placebo (P < .001 for all comparisons with placebo).
In addition, for 55.6% of the 10-mg group, 58.7% of the 30-mg group, 60.8% of the 60-mg group, and 29.0% of the control group, there was a reduction of at least 50% in the 3-month average number of migraine days per month (P < .001 for each vs. placebo).
The most commonly reported adverse events (AEs) among patients who received atogepant were constipation (6.9%-7.7% across doses), nausea (4.4%-6.1%), and upper respiratory tract infection (1.4%-3.9%). Frequency of AEs did not differ between the active-treatment groups and the control group, and no relationships between AEs and atogepant dose were observed.
Multidose flexibility
“Side effects were pretty even across the board,” said Dr. Ailani. She noted that the reported AEs were expected because of atogepant’s mechanism of action. In addition, the rate of discontinuation in the study was low.
The proportion of participants who experienced a reduction in monthly migraine days of at least 50% grew as time passed. “By the end of this study, your chance of having a greater than 50% response is about 75%,” Dr. Ailani said.
“Imagine telling your patient, ‘You stick on this drug for 3 months, and I can almost guarantee you that you’re going to get better,’” she added.
Although the treatment has no drug-drug contraindications, drug-drug interactions may occur. “The availability of various doses would allow clinicians to adjust treatment to avoid potential drug-drug interactions,” said Dr. Ailani. “That multidose flexibility is very important.”
An FDA decision on atogepant could be made in the coming months. “I’m hopeful, as a clinician, that it is positive news, because we really have waited a long time for something like this,” Dr. Ailani said.
“You can easily identify patients who would do well on this medication,” she added.
In a different study of atogepant among patients with chronic migraine, there were recruitment delays because of the pandemic. That study is now almost complete, Dr. Ailani reported.
“Well-conducted study”
Commenting on the findings, Kathleen B. Digre, MD, chief of the division of headache and neuro-ophthalmology at the University of Utah Health, Salt Lake City, expressed enthusiasm for the experimental drug. “I’m excited to see another treatment modality for migraine,” said Dr. Digre, who was not involved with the research. “It was a very well-conducted study,” she added.
The treatment arms were almost identical in regard to disease severity, and all the doses showed an effect. Although the difference in reduction of monthly migraine days in comparison with placebo was numerically small, “for people who have frequent migraine, it’s important,” Dr. Digre said.
The results for atogepant should be viewed in a larger context, however. “Even though it’s a treatment that works better than placebo for well-matched controls, it may not be a medication that everybody’s going to respond to,” she noted. “And we can’t generalize it for some of the most disabled people, which is for chronic migraine,” she said.
It is significant that the study was published in the New England Journal of Medicine, Dr. Digre noted. “Sometimes migraine is dismissed as not important and not affecting people’s lives,” she said. “That makes me very happy to see migraine being taken seriously by our major journals.”
In addition, she noted that the prospects for FDA approval of atogepant seem favorable. “I’m hopeful that they will approve it, because it’s got a low side-effect profile, plus it’s effective.”
Migraine-specific preventive therapy has emerged only in the past few years. “I’m so excited to see this surge of preventive medicine for migraine,” Dr. Digre said. “It’s so important, because we see so many people who are disabled by migraine,” she added.
The study was funded by Allergan before atogepant was acquired by AbbVie. Dr. Ailani has received honoraria from AbbVie for consulting, has received compensation from Allergan and AbbVie for participating in a speakers’ bureau, and has received clinical trial grants from Allergan. Dr. Digre has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
full results from a phase 3 trial suggest.
AbbVie, the company developing the oral therapy, announced topline results of the ADVANCE trial of atogepant last year. Safety results were presented in April at the 2021 annual meeting of the American Academy of Neurology.
The full results were published online Aug. 19 in the New England Journal of Medicine ahead of the upcoming target action date of the U.S. Food and Drug Administration.
The multicenter study included nearly 900 patients who were randomly assigned to receive either placebo or one of three doses of atogepant for 12 weeks. The mean number of monthly migraine days decreased by about 4 for all three doses of the active treatment, compared with a reduction of 2.5 days with placebo.
“Overall, this study showed us that atogepant was safe and surprisingly seems to be pretty effective regardless of the dose,” said lead author Jessica Ailani, MD, director of MedStar Georgetown Headache Center and associate professor of neurology at Georgetown University, Washington.
All doses effective
The study included 873 patients with episodic migraine with or without aura. Patients who were not assigned to the placebo control group received either 10 mg, 30 mg, or 60 mg of atogepant once daily.
After a 4-week screening period, all patients received treatment for 12 weeks and then entered a 4-week safety follow-up period. In total, the participants completed eight scheduled clinical visits.
The mean reduction from baseline in the mean number of migraine days per month was 3.7 with the 10-mg dose of atogepant, 3.9 with the 30-mg dose, 4.2 with the 60-mg dose, and 2.5 with placebo. The differences between each active dose and placebo was statistically significant (P < .001).
Treatment with the CGRP inhibitor was also associated with a reduction in the mean number of headache days per month. The mean reduction from baseline was 3.9 days for the 10-mg dose, 4.0 days for the 30-mg dose, 4.2 days for the 60-mg dose, and 2.5 days for placebo (P < .001 for all comparisons with placebo).
In addition, for 55.6% of the 10-mg group, 58.7% of the 30-mg group, 60.8% of the 60-mg group, and 29.0% of the control group, there was a reduction of at least 50% in the 3-month average number of migraine days per month (P < .001 for each vs. placebo).
The most commonly reported adverse events (AEs) among patients who received atogepant were constipation (6.9%-7.7% across doses), nausea (4.4%-6.1%), and upper respiratory tract infection (1.4%-3.9%). Frequency of AEs did not differ between the active-treatment groups and the control group, and no relationships between AEs and atogepant dose were observed.
Multidose flexibility
“Side effects were pretty even across the board,” said Dr. Ailani. She noted that the reported AEs were expected because of atogepant’s mechanism of action. In addition, the rate of discontinuation in the study was low.
The proportion of participants who experienced a reduction in monthly migraine days of at least 50% grew as time passed. “By the end of this study, your chance of having a greater than 50% response is about 75%,” Dr. Ailani said.
“Imagine telling your patient, ‘You stick on this drug for 3 months, and I can almost guarantee you that you’re going to get better,’” she added.
Although the treatment has no drug-drug contraindications, drug-drug interactions may occur. “The availability of various doses would allow clinicians to adjust treatment to avoid potential drug-drug interactions,” said Dr. Ailani. “That multidose flexibility is very important.”
An FDA decision on atogepant could be made in the coming months. “I’m hopeful, as a clinician, that it is positive news, because we really have waited a long time for something like this,” Dr. Ailani said.
“You can easily identify patients who would do well on this medication,” she added.
In a different study of atogepant among patients with chronic migraine, there were recruitment delays because of the pandemic. That study is now almost complete, Dr. Ailani reported.
“Well-conducted study”
Commenting on the findings, Kathleen B. Digre, MD, chief of the division of headache and neuro-ophthalmology at the University of Utah Health, Salt Lake City, expressed enthusiasm for the experimental drug. “I’m excited to see another treatment modality for migraine,” said Dr. Digre, who was not involved with the research. “It was a very well-conducted study,” she added.
The treatment arms were almost identical in regard to disease severity, and all the doses showed an effect. Although the difference in reduction of monthly migraine days in comparison with placebo was numerically small, “for people who have frequent migraine, it’s important,” Dr. Digre said.
The results for atogepant should be viewed in a larger context, however. “Even though it’s a treatment that works better than placebo for well-matched controls, it may not be a medication that everybody’s going to respond to,” she noted. “And we can’t generalize it for some of the most disabled people, which is for chronic migraine,” she said.
It is significant that the study was published in the New England Journal of Medicine, Dr. Digre noted. “Sometimes migraine is dismissed as not important and not affecting people’s lives,” she said. “That makes me very happy to see migraine being taken seriously by our major journals.”
In addition, she noted that the prospects for FDA approval of atogepant seem favorable. “I’m hopeful that they will approve it, because it’s got a low side-effect profile, plus it’s effective.”
Migraine-specific preventive therapy has emerged only in the past few years. “I’m so excited to see this surge of preventive medicine for migraine,” Dr. Digre said. “It’s so important, because we see so many people who are disabled by migraine,” she added.
The study was funded by Allergan before atogepant was acquired by AbbVie. Dr. Ailani has received honoraria from AbbVie for consulting, has received compensation from Allergan and AbbVie for participating in a speakers’ bureau, and has received clinical trial grants from Allergan. Dr. Digre has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Efficacy of gabapentin for treatment of alcohol use disorders
Background: Up to 30 million people in the United States meet criteria for alcohol use disorder. Gabapentin addresses symptoms of protracted withdrawal such as insomnia, irritability, difficulty with attention, dysphoria, and anxiety. It does that by acting on voltage-gated calcium channels and, in turn, influencing GABA and glutamate tone and activity.
Study design: Double-blind, placebo-controlled, randomized clinical trial.
Settings: Academic ambulatory setting at the Medical University of South Carolina.
Synopsis: A total of 96 community-recruited participants were randomly assigned to gabapentin and placebo arm then treated and followed for a total of 16 weeks. The gabapentin arm received gradual increments of gabapentin reaching up to 1,200 mg/day by day 5. The control group received placebo in blister packs. Individuals in the gabapentin arm, compared with those in the placebo arm, showed 18.6% (P = .02) more no heavy–drinking days, with a number needed to treat (NNT) of 5.4, and 13.8% (P = .04) more total abstinence days, with an NNT of 6.2. The prestudy high–alcohol withdrawal group in particular had significantly less relapse to heavy drinking (P = .02; NNT, 3.1) and more total abstinence (P = .03; NNT, 2.7) when treated with gabapentin.
A couple of study limitations were a significant noncompletion rate (30% in gabapentin arm and 39% in the placebo arm) and self-reported alcohol withdrawal symptoms prior to entry into the study.
Bottom line: Gabapentin helps in reducing drinking and maintaining alcohol abstinence in individuals with alcohol use disorder, especially those with high–alcohol withdrawal symptoms.
Citation: Anton RF et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: A randomized clinical trial. JAMA Intern Med. 2020 Mar 9;180(5):728-36.
Dr. Gaddam is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Up to 30 million people in the United States meet criteria for alcohol use disorder. Gabapentin addresses symptoms of protracted withdrawal such as insomnia, irritability, difficulty with attention, dysphoria, and anxiety. It does that by acting on voltage-gated calcium channels and, in turn, influencing GABA and glutamate tone and activity.
Study design: Double-blind, placebo-controlled, randomized clinical trial.
Settings: Academic ambulatory setting at the Medical University of South Carolina.
Synopsis: A total of 96 community-recruited participants were randomly assigned to gabapentin and placebo arm then treated and followed for a total of 16 weeks. The gabapentin arm received gradual increments of gabapentin reaching up to 1,200 mg/day by day 5. The control group received placebo in blister packs. Individuals in the gabapentin arm, compared with those in the placebo arm, showed 18.6% (P = .02) more no heavy–drinking days, with a number needed to treat (NNT) of 5.4, and 13.8% (P = .04) more total abstinence days, with an NNT of 6.2. The prestudy high–alcohol withdrawal group in particular had significantly less relapse to heavy drinking (P = .02; NNT, 3.1) and more total abstinence (P = .03; NNT, 2.7) when treated with gabapentin.
A couple of study limitations were a significant noncompletion rate (30% in gabapentin arm and 39% in the placebo arm) and self-reported alcohol withdrawal symptoms prior to entry into the study.
Bottom line: Gabapentin helps in reducing drinking and maintaining alcohol abstinence in individuals with alcohol use disorder, especially those with high–alcohol withdrawal symptoms.
Citation: Anton RF et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: A randomized clinical trial. JAMA Intern Med. 2020 Mar 9;180(5):728-36.
Dr. Gaddam is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Up to 30 million people in the United States meet criteria for alcohol use disorder. Gabapentin addresses symptoms of protracted withdrawal such as insomnia, irritability, difficulty with attention, dysphoria, and anxiety. It does that by acting on voltage-gated calcium channels and, in turn, influencing GABA and glutamate tone and activity.
Study design: Double-blind, placebo-controlled, randomized clinical trial.
Settings: Academic ambulatory setting at the Medical University of South Carolina.
Synopsis: A total of 96 community-recruited participants were randomly assigned to gabapentin and placebo arm then treated and followed for a total of 16 weeks. The gabapentin arm received gradual increments of gabapentin reaching up to 1,200 mg/day by day 5. The control group received placebo in blister packs. Individuals in the gabapentin arm, compared with those in the placebo arm, showed 18.6% (P = .02) more no heavy–drinking days, with a number needed to treat (NNT) of 5.4, and 13.8% (P = .04) more total abstinence days, with an NNT of 6.2. The prestudy high–alcohol withdrawal group in particular had significantly less relapse to heavy drinking (P = .02; NNT, 3.1) and more total abstinence (P = .03; NNT, 2.7) when treated with gabapentin.
A couple of study limitations were a significant noncompletion rate (30% in gabapentin arm and 39% in the placebo arm) and self-reported alcohol withdrawal symptoms prior to entry into the study.
Bottom line: Gabapentin helps in reducing drinking and maintaining alcohol abstinence in individuals with alcohol use disorder, especially those with high–alcohol withdrawal symptoms.
Citation: Anton RF et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: A randomized clinical trial. JAMA Intern Med. 2020 Mar 9;180(5):728-36.
Dr. Gaddam is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.