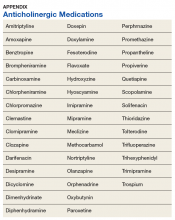
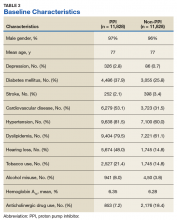
User login
I’m getting old (and it’s costing me)
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
Huntington’s disease biomarkers appear 24 years before clinical symptoms
, according to a study published in the June Lancet Neurology. The data come from the Huntington’s disease Young Adult Study (HD-YAS) conducted in the United Kingdom.
The genetic cause of Huntington’s disease provides a potential target for biomarker treatment, wrote joint first authors Rachael I. Scahill, PhD, and Paul Zeun, BMBS, of University College London and colleagues.
“A detailed characterization of the premanifest period in Huntington’s disease is crucial for disease staging, informing the optimum time to initiate treatments, and identifying biomarkers for future trials in people with premanifest Huntington’s disease (preHD),” they said.
Identifying biomarkers of pre-Huntington’s disease
For their study, the researchers recruited 64 young adults with presymptomatic Huntington’s disease (preHD) and 67 controls, with an average age of 29 years. Brain imaging was conducted between Aug. 2, 2017, and April 25, 2019. Individuals with preexisting measurable cognitive and psychiatric disorders were excluded.
The researchers found no significant evidence of cognitive or psychiatric impairment in the preHD group at 23.6 years from the predicted onset of symptoms. The preHD group showed smaller putamen volumes, compared with controls, but this difference had no apparent relation to the timing of symptom onset, the researchers said.
Brain imaging revealed elevations in the CSF mutant huntingtin, neurofilament light protein (NfL), YKL-40, and plasma NfL among individuals with preHD, compared with controls. Of these, CSF NfL showed the highest effect size of measures in the study and showed a significant increasing association with estimated years to the onset of clinical symptoms of HD carriers. Overall, 53% of individuals with preHD had CSF NfL values in the normal range, and 47% had elevated values, compared with controls.
“NfL is therefore a potential candidate to provide a measure of disease progression in early preHD and might eventually be used as a marker of response to treatment in future preventive trials,” the researchers said.
The study findings were limited by several factors including potential underpowering to detect associations with age and CAG gene segment repeats, the researchers noted.
However, “By identifying a cohort of individuals with preHD and no detectable functional impairment but who begin to exhibit subtle elevations in select biological measures of neurodegeneration, we have highlighted a crucial point early in the disease process,” they concluded.
“Intervening at this stage might offer the prospect of delaying or preventing further neurodegeneration while function is intact, giving gene carriers many more years of life without impairment,” they added.
What is the best window for treatment?
The study is “particularly important since the absence of any subclinical symptoms in preHD individuals far from onset shows that the abnormal developmental aspect of Huntington’s disease has no substantial effect on adults’ clinical pattern,” wrote Anne-Catherine Bachoud-Lévi, MD, of Université Paris Est, Créteil, France, in an accompanying comment.
“The most robust findings of [the study] are the sensitiveness of NfL, compared with mutant huntingtin in CSF of individuals with preHD, and that degenerative rather than developmental disorders are clinically relevant,” she said. However, potential limitations to the study include the exclusion absence of language and calculation as part of the cognitive assessments, she noted. “Ideally, more sensitive cognitive tasks including these domains should be designed for preHD participants.”
In addition, the risks versus benefits of any long-term treatment must be considered, Dr. Bachoud-Lévi noted.
“The best window for treatment should instead target the time when a detectable subclinical slope of cognitive performance allows for predicting disease onset within a few years,” she said. “Turning to machine learning methodology, such as that in oncology, might also permit combining the best window and the best disease-modifying therapy for individuals with preHD,” she added.
The study was supported by the Wellcome Trust, CHDI Foundation. The researchers had no financial conflicts to disclose. Dr. Bachoud-Lévi disclosed grants and personal fees from Roche, and grants from the French Ministry of Health and Direction de la Recherche Clinique.
SOURCES: Scahill RI et al. Lancet Neurol. 2020 June;19:502-12; Bachoud-Lévi A-C. Lancet Neurol. 2020 June;19:473-5.
, according to a study published in the June Lancet Neurology. The data come from the Huntington’s disease Young Adult Study (HD-YAS) conducted in the United Kingdom.
The genetic cause of Huntington’s disease provides a potential target for biomarker treatment, wrote joint first authors Rachael I. Scahill, PhD, and Paul Zeun, BMBS, of University College London and colleagues.
“A detailed characterization of the premanifest period in Huntington’s disease is crucial for disease staging, informing the optimum time to initiate treatments, and identifying biomarkers for future trials in people with premanifest Huntington’s disease (preHD),” they said.
Identifying biomarkers of pre-Huntington’s disease
For their study, the researchers recruited 64 young adults with presymptomatic Huntington’s disease (preHD) and 67 controls, with an average age of 29 years. Brain imaging was conducted between Aug. 2, 2017, and April 25, 2019. Individuals with preexisting measurable cognitive and psychiatric disorders were excluded.
The researchers found no significant evidence of cognitive or psychiatric impairment in the preHD group at 23.6 years from the predicted onset of symptoms. The preHD group showed smaller putamen volumes, compared with controls, but this difference had no apparent relation to the timing of symptom onset, the researchers said.
Brain imaging revealed elevations in the CSF mutant huntingtin, neurofilament light protein (NfL), YKL-40, and plasma NfL among individuals with preHD, compared with controls. Of these, CSF NfL showed the highest effect size of measures in the study and showed a significant increasing association with estimated years to the onset of clinical symptoms of HD carriers. Overall, 53% of individuals with preHD had CSF NfL values in the normal range, and 47% had elevated values, compared with controls.
“NfL is therefore a potential candidate to provide a measure of disease progression in early preHD and might eventually be used as a marker of response to treatment in future preventive trials,” the researchers said.
The study findings were limited by several factors including potential underpowering to detect associations with age and CAG gene segment repeats, the researchers noted.
However, “By identifying a cohort of individuals with preHD and no detectable functional impairment but who begin to exhibit subtle elevations in select biological measures of neurodegeneration, we have highlighted a crucial point early in the disease process,” they concluded.
“Intervening at this stage might offer the prospect of delaying or preventing further neurodegeneration while function is intact, giving gene carriers many more years of life without impairment,” they added.
What is the best window for treatment?
The study is “particularly important since the absence of any subclinical symptoms in preHD individuals far from onset shows that the abnormal developmental aspect of Huntington’s disease has no substantial effect on adults’ clinical pattern,” wrote Anne-Catherine Bachoud-Lévi, MD, of Université Paris Est, Créteil, France, in an accompanying comment.
“The most robust findings of [the study] are the sensitiveness of NfL, compared with mutant huntingtin in CSF of individuals with preHD, and that degenerative rather than developmental disorders are clinically relevant,” she said. However, potential limitations to the study include the exclusion absence of language and calculation as part of the cognitive assessments, she noted. “Ideally, more sensitive cognitive tasks including these domains should be designed for preHD participants.”
In addition, the risks versus benefits of any long-term treatment must be considered, Dr. Bachoud-Lévi noted.
“The best window for treatment should instead target the time when a detectable subclinical slope of cognitive performance allows for predicting disease onset within a few years,” she said. “Turning to machine learning methodology, such as that in oncology, might also permit combining the best window and the best disease-modifying therapy for individuals with preHD,” she added.
The study was supported by the Wellcome Trust, CHDI Foundation. The researchers had no financial conflicts to disclose. Dr. Bachoud-Lévi disclosed grants and personal fees from Roche, and grants from the French Ministry of Health and Direction de la Recherche Clinique.
SOURCES: Scahill RI et al. Lancet Neurol. 2020 June;19:502-12; Bachoud-Lévi A-C. Lancet Neurol. 2020 June;19:473-5.
, according to a study published in the June Lancet Neurology. The data come from the Huntington’s disease Young Adult Study (HD-YAS) conducted in the United Kingdom.
The genetic cause of Huntington’s disease provides a potential target for biomarker treatment, wrote joint first authors Rachael I. Scahill, PhD, and Paul Zeun, BMBS, of University College London and colleagues.
“A detailed characterization of the premanifest period in Huntington’s disease is crucial for disease staging, informing the optimum time to initiate treatments, and identifying biomarkers for future trials in people with premanifest Huntington’s disease (preHD),” they said.
Identifying biomarkers of pre-Huntington’s disease
For their study, the researchers recruited 64 young adults with presymptomatic Huntington’s disease (preHD) and 67 controls, with an average age of 29 years. Brain imaging was conducted between Aug. 2, 2017, and April 25, 2019. Individuals with preexisting measurable cognitive and psychiatric disorders were excluded.
The researchers found no significant evidence of cognitive or psychiatric impairment in the preHD group at 23.6 years from the predicted onset of symptoms. The preHD group showed smaller putamen volumes, compared with controls, but this difference had no apparent relation to the timing of symptom onset, the researchers said.
Brain imaging revealed elevations in the CSF mutant huntingtin, neurofilament light protein (NfL), YKL-40, and plasma NfL among individuals with preHD, compared with controls. Of these, CSF NfL showed the highest effect size of measures in the study and showed a significant increasing association with estimated years to the onset of clinical symptoms of HD carriers. Overall, 53% of individuals with preHD had CSF NfL values in the normal range, and 47% had elevated values, compared with controls.
“NfL is therefore a potential candidate to provide a measure of disease progression in early preHD and might eventually be used as a marker of response to treatment in future preventive trials,” the researchers said.
The study findings were limited by several factors including potential underpowering to detect associations with age and CAG gene segment repeats, the researchers noted.
However, “By identifying a cohort of individuals with preHD and no detectable functional impairment but who begin to exhibit subtle elevations in select biological measures of neurodegeneration, we have highlighted a crucial point early in the disease process,” they concluded.
“Intervening at this stage might offer the prospect of delaying or preventing further neurodegeneration while function is intact, giving gene carriers many more years of life without impairment,” they added.
What is the best window for treatment?
The study is “particularly important since the absence of any subclinical symptoms in preHD individuals far from onset shows that the abnormal developmental aspect of Huntington’s disease has no substantial effect on adults’ clinical pattern,” wrote Anne-Catherine Bachoud-Lévi, MD, of Université Paris Est, Créteil, France, in an accompanying comment.
“The most robust findings of [the study] are the sensitiveness of NfL, compared with mutant huntingtin in CSF of individuals with preHD, and that degenerative rather than developmental disorders are clinically relevant,” she said. However, potential limitations to the study include the exclusion absence of language and calculation as part of the cognitive assessments, she noted. “Ideally, more sensitive cognitive tasks including these domains should be designed for preHD participants.”
In addition, the risks versus benefits of any long-term treatment must be considered, Dr. Bachoud-Lévi noted.
“The best window for treatment should instead target the time when a detectable subclinical slope of cognitive performance allows for predicting disease onset within a few years,” she said. “Turning to machine learning methodology, such as that in oncology, might also permit combining the best window and the best disease-modifying therapy for individuals with preHD,” she added.
The study was supported by the Wellcome Trust, CHDI Foundation. The researchers had no financial conflicts to disclose. Dr. Bachoud-Lévi disclosed grants and personal fees from Roche, and grants from the French Ministry of Health and Direction de la Recherche Clinique.
SOURCES: Scahill RI et al. Lancet Neurol. 2020 June;19:502-12; Bachoud-Lévi A-C. Lancet Neurol. 2020 June;19:473-5.
FROM LANCET NEUROLOGY
COVID-19 neurologic effects: Does the virus directly attack the brain?
A new review article summarizes what is known so far, and what clinicians need to look out for.
“We frequently see neurological conditions in people with COVID-19, but we understand very little about these effects. Is it the virus entering the brain/nerves or are they a result of a general inflammation or immune response – a bystander effect of people being severely ill. It is probably a combination of both,” said senior author Serena Spudich, MD, Gilbert H. Glaser Professor of Neurology; division chief of neurological infections & global neurology; and codirector of the Center for Neuroepidemiology and Clinical Neurological Research at Yale University, New Haven, Conn.
“Our message is that there are fairly frequent neurological sequelae of COVID-19 and we need to be alert to these, and to try to understand the potential long-term consequences,” she said.
The review was published online May 29 in JAMA Neurology.
Brain changes linked to loss of smell
In a separate article also published online in JAMA Neurology the same day, an Italian group describes a COVID-19 patient with anosmia (loss of sense of smell) who showed brain abnormalities on MRI in the areas associated with smell – the right gyrus rectus and the olfactory bulbs. These changes were resolved on later scan and the patient recovered her sense of smell.
“Based on the MRI findings, we can speculate that SARS-CoV-2 might invade the brain through the olfactory pathway,” conclude the researchers, led by first author Letterio S. Politi, MD, of the department of neuroradiology at IRCCS Istituto Clinico Humanitas and Humanitas University, Milan, Italy.
Can coronaviruses enter the CNS?
Dr. Spudich described this case report as “compelling evidence suggesting that loss of smell is a neurologic effect.”
“Loss of smell and/or taste is a common symptom in COVID-19, so this may suggest that an awful lot of people have some neurological involvement,” Dr. Spudich commented. “While a transient loss of smell or taste is not serious, if the virus has infected brain tissue the question is could this then spread to other parts of the brain and cause other more serious neurological effects,” she added.
In their review article, Dr. Spudich and colleagues present evidence showing that coronaviruses can enter the CNS.
“We know that SARS-1 and MERS have been shown to enter the nervous system and several coronaviruses have been shown to cause direct brain effects,” she said. “There is also some evidence that SARS-CoV-2 can do this too. As well as these latest MRI findings linked to loss of smell, there is a report of the virus being found in endothelial cells in the brain and a French autopsy study has also detected virus in the brain.”
Complications of other systemic effects?
Dr. Spudich is a neurologist specializing in neurologic consequences of infectious disease. “We don’t normally have such vast numbers of patients but in the last 3 months there has been an avalanche,” she says. From her personal experience, she believes the majority of neurologic symptoms in COVID-19 patients are most probably complications of other systemic effects, such as kidney, heart, or liver problems. But there is likely also a direct viral effect on the CNS in some patients.
“Reports from China suggested that serious neurologic effects were present in about one-third of hospitalized COVID-19 patients. I would say in our experience the figure would be less than that – maybe around 10%,” she noted.
Some COVID-19 patients are presenting with primary neurologic symptoms. For example, an elderly person may first develop confusion rather than a cough or shortness of breath; others have had severe headache as an initial COVID-19 symptom, Dr. Spudich reported. “Medical staff need to be aware of this – a severe headache in a patient who doesn’t normally get headaches could be a sign of the virus.”
Some of the neurologic symptoms could be caused by autoimmunity. Dr. Spudich explained that, in acute HIV infection a small proportion of patients can first present with autoimmune neurologic effects such as Guillain-Barré syndrome, an autoimmune condition of the nerves which causes a tingling sensation in the hands and feet. “This is well described in HIV, but we are also now seeing this in COVID-19 patients too,” she said. “A panoply of conditions can be caused by autoimmunity.”
On the increase in strokes that has been reported in COVID-19 patients, Dr. Spudich said, “this could be due to direct effects of the virus (e.g., causing an increase in coagulation or infecting the endothelial cells in the brain) or it could just be the final trigger for patients who were at risk of stroke anyway.”
There have been some very high-profile reports of younger patients with major strokes, she said, “but we haven’t seen that in our hospital. For the most part in my experience, strokes are happening in older COVID-19 patients with stroke risk factors such as AF [atrial fibrillation], hypertension, and diabetes. We haven’t seen a preponderance of strokes in young, otherwise healthy people.”
Even in patients who have neurologic effects as the first sign of COVID-19 infection, it is not known whether these symptoms are caused directly by the virus.
“We know that flu can cause people to have headaches, but that is because of an increase in inflammatory cytokines. On the other hand, patients with acute HIV infection often have headaches as a result of the virus getting into the brain. We don’t know where in this [cluster] COVID-19 virus falls,” Dr. Spudich said.
Much is still unknown
“The information we have is very sparse at this point. We need far more systematic information on this from CSF samples and imaging.” Dr. Spudich urged clinicians to try to collect such information in patients with neurologic symptoms.
Acknowledging that fewer such tests are being done at present because of concerns over infection risk, Dr. Spudich suggested that some changes in procedure may help. “In our hospital we have a portable MRI scanner which can be brought to the patient. This means the patient does not have to move across the hospital for a scan. This helps us to decide whether the patient has had a stroke, which can be missed when patients are on a ventilator.”
It is also unclear whether the neurologic effects seen during COVID-19 infection will last long term.
Dr. Spudich noted that there have been reports of COVID-19 patients discharged from intensive care having difficulty with higher cognitive function for some time thereafter. “This can happen after being in ICU but is it more pronounced in COVID-19 patients? An ongoing study is underway to look at this,” she said.
This article first appeared on Medscape.com.
A new review article summarizes what is known so far, and what clinicians need to look out for.
“We frequently see neurological conditions in people with COVID-19, but we understand very little about these effects. Is it the virus entering the brain/nerves or are they a result of a general inflammation or immune response – a bystander effect of people being severely ill. It is probably a combination of both,” said senior author Serena Spudich, MD, Gilbert H. Glaser Professor of Neurology; division chief of neurological infections & global neurology; and codirector of the Center for Neuroepidemiology and Clinical Neurological Research at Yale University, New Haven, Conn.
“Our message is that there are fairly frequent neurological sequelae of COVID-19 and we need to be alert to these, and to try to understand the potential long-term consequences,” she said.
The review was published online May 29 in JAMA Neurology.
Brain changes linked to loss of smell
In a separate article also published online in JAMA Neurology the same day, an Italian group describes a COVID-19 patient with anosmia (loss of sense of smell) who showed brain abnormalities on MRI in the areas associated with smell – the right gyrus rectus and the olfactory bulbs. These changes were resolved on later scan and the patient recovered her sense of smell.
“Based on the MRI findings, we can speculate that SARS-CoV-2 might invade the brain through the olfactory pathway,” conclude the researchers, led by first author Letterio S. Politi, MD, of the department of neuroradiology at IRCCS Istituto Clinico Humanitas and Humanitas University, Milan, Italy.
Can coronaviruses enter the CNS?
Dr. Spudich described this case report as “compelling evidence suggesting that loss of smell is a neurologic effect.”
“Loss of smell and/or taste is a common symptom in COVID-19, so this may suggest that an awful lot of people have some neurological involvement,” Dr. Spudich commented. “While a transient loss of smell or taste is not serious, if the virus has infected brain tissue the question is could this then spread to other parts of the brain and cause other more serious neurological effects,” she added.
In their review article, Dr. Spudich and colleagues present evidence showing that coronaviruses can enter the CNS.
“We know that SARS-1 and MERS have been shown to enter the nervous system and several coronaviruses have been shown to cause direct brain effects,” she said. “There is also some evidence that SARS-CoV-2 can do this too. As well as these latest MRI findings linked to loss of smell, there is a report of the virus being found in endothelial cells in the brain and a French autopsy study has also detected virus in the brain.”
Complications of other systemic effects?
Dr. Spudich is a neurologist specializing in neurologic consequences of infectious disease. “We don’t normally have such vast numbers of patients but in the last 3 months there has been an avalanche,” she says. From her personal experience, she believes the majority of neurologic symptoms in COVID-19 patients are most probably complications of other systemic effects, such as kidney, heart, or liver problems. But there is likely also a direct viral effect on the CNS in some patients.
“Reports from China suggested that serious neurologic effects were present in about one-third of hospitalized COVID-19 patients. I would say in our experience the figure would be less than that – maybe around 10%,” she noted.
Some COVID-19 patients are presenting with primary neurologic symptoms. For example, an elderly person may first develop confusion rather than a cough or shortness of breath; others have had severe headache as an initial COVID-19 symptom, Dr. Spudich reported. “Medical staff need to be aware of this – a severe headache in a patient who doesn’t normally get headaches could be a sign of the virus.”
Some of the neurologic symptoms could be caused by autoimmunity. Dr. Spudich explained that, in acute HIV infection a small proportion of patients can first present with autoimmune neurologic effects such as Guillain-Barré syndrome, an autoimmune condition of the nerves which causes a tingling sensation in the hands and feet. “This is well described in HIV, but we are also now seeing this in COVID-19 patients too,” she said. “A panoply of conditions can be caused by autoimmunity.”
On the increase in strokes that has been reported in COVID-19 patients, Dr. Spudich said, “this could be due to direct effects of the virus (e.g., causing an increase in coagulation or infecting the endothelial cells in the brain) or it could just be the final trigger for patients who were at risk of stroke anyway.”
There have been some very high-profile reports of younger patients with major strokes, she said, “but we haven’t seen that in our hospital. For the most part in my experience, strokes are happening in older COVID-19 patients with stroke risk factors such as AF [atrial fibrillation], hypertension, and diabetes. We haven’t seen a preponderance of strokes in young, otherwise healthy people.”
Even in patients who have neurologic effects as the first sign of COVID-19 infection, it is not known whether these symptoms are caused directly by the virus.
“We know that flu can cause people to have headaches, but that is because of an increase in inflammatory cytokines. On the other hand, patients with acute HIV infection often have headaches as a result of the virus getting into the brain. We don’t know where in this [cluster] COVID-19 virus falls,” Dr. Spudich said.
Much is still unknown
“The information we have is very sparse at this point. We need far more systematic information on this from CSF samples and imaging.” Dr. Spudich urged clinicians to try to collect such information in patients with neurologic symptoms.
Acknowledging that fewer such tests are being done at present because of concerns over infection risk, Dr. Spudich suggested that some changes in procedure may help. “In our hospital we have a portable MRI scanner which can be brought to the patient. This means the patient does not have to move across the hospital for a scan. This helps us to decide whether the patient has had a stroke, which can be missed when patients are on a ventilator.”
It is also unclear whether the neurologic effects seen during COVID-19 infection will last long term.
Dr. Spudich noted that there have been reports of COVID-19 patients discharged from intensive care having difficulty with higher cognitive function for some time thereafter. “This can happen after being in ICU but is it more pronounced in COVID-19 patients? An ongoing study is underway to look at this,” she said.
This article first appeared on Medscape.com.
A new review article summarizes what is known so far, and what clinicians need to look out for.
“We frequently see neurological conditions in people with COVID-19, but we understand very little about these effects. Is it the virus entering the brain/nerves or are they a result of a general inflammation or immune response – a bystander effect of people being severely ill. It is probably a combination of both,” said senior author Serena Spudich, MD, Gilbert H. Glaser Professor of Neurology; division chief of neurological infections & global neurology; and codirector of the Center for Neuroepidemiology and Clinical Neurological Research at Yale University, New Haven, Conn.
“Our message is that there are fairly frequent neurological sequelae of COVID-19 and we need to be alert to these, and to try to understand the potential long-term consequences,” she said.
The review was published online May 29 in JAMA Neurology.
Brain changes linked to loss of smell
In a separate article also published online in JAMA Neurology the same day, an Italian group describes a COVID-19 patient with anosmia (loss of sense of smell) who showed brain abnormalities on MRI in the areas associated with smell – the right gyrus rectus and the olfactory bulbs. These changes were resolved on later scan and the patient recovered her sense of smell.
“Based on the MRI findings, we can speculate that SARS-CoV-2 might invade the brain through the olfactory pathway,” conclude the researchers, led by first author Letterio S. Politi, MD, of the department of neuroradiology at IRCCS Istituto Clinico Humanitas and Humanitas University, Milan, Italy.
Can coronaviruses enter the CNS?
Dr. Spudich described this case report as “compelling evidence suggesting that loss of smell is a neurologic effect.”
“Loss of smell and/or taste is a common symptom in COVID-19, so this may suggest that an awful lot of people have some neurological involvement,” Dr. Spudich commented. “While a transient loss of smell or taste is not serious, if the virus has infected brain tissue the question is could this then spread to other parts of the brain and cause other more serious neurological effects,” she added.
In their review article, Dr. Spudich and colleagues present evidence showing that coronaviruses can enter the CNS.
“We know that SARS-1 and MERS have been shown to enter the nervous system and several coronaviruses have been shown to cause direct brain effects,” she said. “There is also some evidence that SARS-CoV-2 can do this too. As well as these latest MRI findings linked to loss of smell, there is a report of the virus being found in endothelial cells in the brain and a French autopsy study has also detected virus in the brain.”
Complications of other systemic effects?
Dr. Spudich is a neurologist specializing in neurologic consequences of infectious disease. “We don’t normally have such vast numbers of patients but in the last 3 months there has been an avalanche,” she says. From her personal experience, she believes the majority of neurologic symptoms in COVID-19 patients are most probably complications of other systemic effects, such as kidney, heart, or liver problems. But there is likely also a direct viral effect on the CNS in some patients.
“Reports from China suggested that serious neurologic effects were present in about one-third of hospitalized COVID-19 patients. I would say in our experience the figure would be less than that – maybe around 10%,” she noted.
Some COVID-19 patients are presenting with primary neurologic symptoms. For example, an elderly person may first develop confusion rather than a cough or shortness of breath; others have had severe headache as an initial COVID-19 symptom, Dr. Spudich reported. “Medical staff need to be aware of this – a severe headache in a patient who doesn’t normally get headaches could be a sign of the virus.”
Some of the neurologic symptoms could be caused by autoimmunity. Dr. Spudich explained that, in acute HIV infection a small proportion of patients can first present with autoimmune neurologic effects such as Guillain-Barré syndrome, an autoimmune condition of the nerves which causes a tingling sensation in the hands and feet. “This is well described in HIV, but we are also now seeing this in COVID-19 patients too,” she said. “A panoply of conditions can be caused by autoimmunity.”
On the increase in strokes that has been reported in COVID-19 patients, Dr. Spudich said, “this could be due to direct effects of the virus (e.g., causing an increase in coagulation or infecting the endothelial cells in the brain) or it could just be the final trigger for patients who were at risk of stroke anyway.”
There have been some very high-profile reports of younger patients with major strokes, she said, “but we haven’t seen that in our hospital. For the most part in my experience, strokes are happening in older COVID-19 patients with stroke risk factors such as AF [atrial fibrillation], hypertension, and diabetes. We haven’t seen a preponderance of strokes in young, otherwise healthy people.”
Even in patients who have neurologic effects as the first sign of COVID-19 infection, it is not known whether these symptoms are caused directly by the virus.
“We know that flu can cause people to have headaches, but that is because of an increase in inflammatory cytokines. On the other hand, patients with acute HIV infection often have headaches as a result of the virus getting into the brain. We don’t know where in this [cluster] COVID-19 virus falls,” Dr. Spudich said.
Much is still unknown
“The information we have is very sparse at this point. We need far more systematic information on this from CSF samples and imaging.” Dr. Spudich urged clinicians to try to collect such information in patients with neurologic symptoms.
Acknowledging that fewer such tests are being done at present because of concerns over infection risk, Dr. Spudich suggested that some changes in procedure may help. “In our hospital we have a portable MRI scanner which can be brought to the patient. This means the patient does not have to move across the hospital for a scan. This helps us to decide whether the patient has had a stroke, which can be missed when patients are on a ventilator.”
It is also unclear whether the neurologic effects seen during COVID-19 infection will last long term.
Dr. Spudich noted that there have been reports of COVID-19 patients discharged from intensive care having difficulty with higher cognitive function for some time thereafter. “This can happen after being in ICU but is it more pronounced in COVID-19 patients? An ongoing study is underway to look at this,” she said.
This article first appeared on Medscape.com.
Antenatal corticosteroids may increase risk for mental and behavioral disorders
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
FROM JAMA
Key clinical point: Exposure to maternal antenatal corticosteroid treatment is significantly associated with mental and behavioral disorders in children, compared with nonexposure.
Major finding: After adjustment for such variables as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure (HR, 1.33). Among children born at term, the adjusted HR was 1.47.
Study details: A population-based retrospective cohort study that included 670,097 children in Finland.
Disclosures: The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The authors had no conflict of interest disclosures.
Source: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
Newest oral DMTs haven’t yet made a big impact in the MS world
So far, it’s more like a ripple, according to a study of neurologists’ prescribing patterns. “The recently approved therapies will initially be niched as later-line options,” predicted Virginia R. Schobel, MSc, nephrology franchise head at Spherix Global Insights, an independent market intelligence firm in Exton, Pa.
At the virtual annual meeting of the Consortium of Multiple Sclerosis Centers, Ms. Schobel presented the results of a retrospective chart audit Spherix conducted in February 2020 of 1,006 patients with MS who were switched to a new DMT by 199 U.S. participating neurologists within the previous 3 months. About 72% of the switchers had relapsing remitting MS (RRMS).
Assessing the three new oral DMTs
The purpose of the study was to gain an understanding of the early adoption patterns for the three recently approved oral DMTs: siponimod (Mayzent), cladribine (Mavenclad), and diroximel fumarate (Vumerity).
The first surprise was that only 41% of medication switches to a new DMT among the RRMS group were to oral DMTs; that’s a substantially lower proportion than in prior Spherix chart audits. Instead, the most popular switch was to ocrelizumab (Ocrevus), a monoclonal antibody.
“Things to keep in mind when we see the switch shares for the newer products are just how crowded this market has become and how much Ocrevus has really changed the market,” Ms. Schobel explained in an interview. “Ocrevus has become increasingly dominant in the RRMS segment, so that now there are six oral DMTs competing among themselves for a relatively limited pool of patients.”
Because of grandfathering by the Food and Drug Administration, most of the oral DMTs now share identical indications for clinically isolated syndrome, RRMS, and active secondary progressive MS. Ocrevus, she noted, has the same indications.
Only 1% of MS patients who switched to a different DMT in late 2019 or early 2020 moved to diroximel fumarate. Three percent switched to siponimod, and another 3% switched to cladribine. Switches to the three older, established oral DMTs were collectively five times more common, with 15% of patients moving to dimethyl fumarate (Tecfidera), 11% to fingolimod (Gilenya), and 9% to teriflunomide (Aubagio).
Ms. Schobel said that the three latest oral DMTs offer advantages over the older ones in terms of various combinations of efficacy, dosing schedule, and/or tolerability, which may make them attractive options as first-line therapy. She predicted that, over time as neurologists gain increasing familiarity with these drugs as first line, they will also gradually become more comfortable in turning to them as switch options.
First-time switches to an oral DMT among patients with RRMS were most often made in search of improved efficacy. Neurologists cited this as their main reason for 73% of switches to cladribine and 36% of switches to teriflunomide, with the other oral agents falling at various points in between. A switch to fingolimod was most often driven by a wish for a high-efficacy DMT with once-daily oral dosing. Improved tolerability figured prominently in switches to teriflunomide, and even more so in the relatively few changes to diroximel fumarate.
Drug switching in the pandemic era
Ms. Schobel said Spherix has been serially tracking neurologists’ prescribing for MS during the COVID-19 pandemic, which has clearly had an enormous dampening effect on medication switching. In mid-April, neurologists’ switching volume was down by 70%, compared with prepandemic figures. A slow recovery began in May, but by the end of the month prescription-switching volume was still down by 52%.
Of the neurologist prescriptions that are being run for switching thus far during the pandemic, 82% are being done via telemedicine. Therein hangs a tale, since neurology doesn’t readily lend itself to practice by telemedicine. Indeed, neurologists are using telemedicine to a lesser extent than physicians in the other specialties that Spherix monitors, according to Ms. Schobel. “COVID is definitely changing the MS world. Within MS, drug switching is now much more likely to involve a switch to a DMT that doesn’t impact the immune response and is not immunosuppressant, such as an injectable interferon or glatiramer acetate,” she said. “In this COVID world, safety and conservatism may end up trumping the move toward ‘time is brain’ which we’ve been talking so much about in recent years: the importance of getting patients on high-efficacy DMTs from the start in order to give them the best chance for positive outcomes.”
Ms. Schobel noted that Spherix received no industry funding to conduct these studies.
So far, it’s more like a ripple, according to a study of neurologists’ prescribing patterns. “The recently approved therapies will initially be niched as later-line options,” predicted Virginia R. Schobel, MSc, nephrology franchise head at Spherix Global Insights, an independent market intelligence firm in Exton, Pa.
At the virtual annual meeting of the Consortium of Multiple Sclerosis Centers, Ms. Schobel presented the results of a retrospective chart audit Spherix conducted in February 2020 of 1,006 patients with MS who were switched to a new DMT by 199 U.S. participating neurologists within the previous 3 months. About 72% of the switchers had relapsing remitting MS (RRMS).
Assessing the three new oral DMTs
The purpose of the study was to gain an understanding of the early adoption patterns for the three recently approved oral DMTs: siponimod (Mayzent), cladribine (Mavenclad), and diroximel fumarate (Vumerity).
The first surprise was that only 41% of medication switches to a new DMT among the RRMS group were to oral DMTs; that’s a substantially lower proportion than in prior Spherix chart audits. Instead, the most popular switch was to ocrelizumab (Ocrevus), a monoclonal antibody.
“Things to keep in mind when we see the switch shares for the newer products are just how crowded this market has become and how much Ocrevus has really changed the market,” Ms. Schobel explained in an interview. “Ocrevus has become increasingly dominant in the RRMS segment, so that now there are six oral DMTs competing among themselves for a relatively limited pool of patients.”
Because of grandfathering by the Food and Drug Administration, most of the oral DMTs now share identical indications for clinically isolated syndrome, RRMS, and active secondary progressive MS. Ocrevus, she noted, has the same indications.
Only 1% of MS patients who switched to a different DMT in late 2019 or early 2020 moved to diroximel fumarate. Three percent switched to siponimod, and another 3% switched to cladribine. Switches to the three older, established oral DMTs were collectively five times more common, with 15% of patients moving to dimethyl fumarate (Tecfidera), 11% to fingolimod (Gilenya), and 9% to teriflunomide (Aubagio).
Ms. Schobel said that the three latest oral DMTs offer advantages over the older ones in terms of various combinations of efficacy, dosing schedule, and/or tolerability, which may make them attractive options as first-line therapy. She predicted that, over time as neurologists gain increasing familiarity with these drugs as first line, they will also gradually become more comfortable in turning to them as switch options.
First-time switches to an oral DMT among patients with RRMS were most often made in search of improved efficacy. Neurologists cited this as their main reason for 73% of switches to cladribine and 36% of switches to teriflunomide, with the other oral agents falling at various points in between. A switch to fingolimod was most often driven by a wish for a high-efficacy DMT with once-daily oral dosing. Improved tolerability figured prominently in switches to teriflunomide, and even more so in the relatively few changes to diroximel fumarate.
Drug switching in the pandemic era
Ms. Schobel said Spherix has been serially tracking neurologists’ prescribing for MS during the COVID-19 pandemic, which has clearly had an enormous dampening effect on medication switching. In mid-April, neurologists’ switching volume was down by 70%, compared with prepandemic figures. A slow recovery began in May, but by the end of the month prescription-switching volume was still down by 52%.
Of the neurologist prescriptions that are being run for switching thus far during the pandemic, 82% are being done via telemedicine. Therein hangs a tale, since neurology doesn’t readily lend itself to practice by telemedicine. Indeed, neurologists are using telemedicine to a lesser extent than physicians in the other specialties that Spherix monitors, according to Ms. Schobel. “COVID is definitely changing the MS world. Within MS, drug switching is now much more likely to involve a switch to a DMT that doesn’t impact the immune response and is not immunosuppressant, such as an injectable interferon or glatiramer acetate,” she said. “In this COVID world, safety and conservatism may end up trumping the move toward ‘time is brain’ which we’ve been talking so much about in recent years: the importance of getting patients on high-efficacy DMTs from the start in order to give them the best chance for positive outcomes.”
Ms. Schobel noted that Spherix received no industry funding to conduct these studies.
So far, it’s more like a ripple, according to a study of neurologists’ prescribing patterns. “The recently approved therapies will initially be niched as later-line options,” predicted Virginia R. Schobel, MSc, nephrology franchise head at Spherix Global Insights, an independent market intelligence firm in Exton, Pa.
At the virtual annual meeting of the Consortium of Multiple Sclerosis Centers, Ms. Schobel presented the results of a retrospective chart audit Spherix conducted in February 2020 of 1,006 patients with MS who were switched to a new DMT by 199 U.S. participating neurologists within the previous 3 months. About 72% of the switchers had relapsing remitting MS (RRMS).
Assessing the three new oral DMTs
The purpose of the study was to gain an understanding of the early adoption patterns for the three recently approved oral DMTs: siponimod (Mayzent), cladribine (Mavenclad), and diroximel fumarate (Vumerity).
The first surprise was that only 41% of medication switches to a new DMT among the RRMS group were to oral DMTs; that’s a substantially lower proportion than in prior Spherix chart audits. Instead, the most popular switch was to ocrelizumab (Ocrevus), a monoclonal antibody.
“Things to keep in mind when we see the switch shares for the newer products are just how crowded this market has become and how much Ocrevus has really changed the market,” Ms. Schobel explained in an interview. “Ocrevus has become increasingly dominant in the RRMS segment, so that now there are six oral DMTs competing among themselves for a relatively limited pool of patients.”
Because of grandfathering by the Food and Drug Administration, most of the oral DMTs now share identical indications for clinically isolated syndrome, RRMS, and active secondary progressive MS. Ocrevus, she noted, has the same indications.
Only 1% of MS patients who switched to a different DMT in late 2019 or early 2020 moved to diroximel fumarate. Three percent switched to siponimod, and another 3% switched to cladribine. Switches to the three older, established oral DMTs were collectively five times more common, with 15% of patients moving to dimethyl fumarate (Tecfidera), 11% to fingolimod (Gilenya), and 9% to teriflunomide (Aubagio).
Ms. Schobel said that the three latest oral DMTs offer advantages over the older ones in terms of various combinations of efficacy, dosing schedule, and/or tolerability, which may make them attractive options as first-line therapy. She predicted that, over time as neurologists gain increasing familiarity with these drugs as first line, they will also gradually become more comfortable in turning to them as switch options.
First-time switches to an oral DMT among patients with RRMS were most often made in search of improved efficacy. Neurologists cited this as their main reason for 73% of switches to cladribine and 36% of switches to teriflunomide, with the other oral agents falling at various points in between. A switch to fingolimod was most often driven by a wish for a high-efficacy DMT with once-daily oral dosing. Improved tolerability figured prominently in switches to teriflunomide, and even more so in the relatively few changes to diroximel fumarate.
Drug switching in the pandemic era
Ms. Schobel said Spherix has been serially tracking neurologists’ prescribing for MS during the COVID-19 pandemic, which has clearly had an enormous dampening effect on medication switching. In mid-April, neurologists’ switching volume was down by 70%, compared with prepandemic figures. A slow recovery began in May, but by the end of the month prescription-switching volume was still down by 52%.
Of the neurologist prescriptions that are being run for switching thus far during the pandemic, 82% are being done via telemedicine. Therein hangs a tale, since neurology doesn’t readily lend itself to practice by telemedicine. Indeed, neurologists are using telemedicine to a lesser extent than physicians in the other specialties that Spherix monitors, according to Ms. Schobel. “COVID is definitely changing the MS world. Within MS, drug switching is now much more likely to involve a switch to a DMT that doesn’t impact the immune response and is not immunosuppressant, such as an injectable interferon or glatiramer acetate,” she said. “In this COVID world, safety and conservatism may end up trumping the move toward ‘time is brain’ which we’ve been talking so much about in recent years: the importance of getting patients on high-efficacy DMTs from the start in order to give them the best chance for positive outcomes.”
Ms. Schobel noted that Spherix received no industry funding to conduct these studies.
FROM CMSC 2020
Is cannabis gaining acceptance as a treatment for neuropathic pain?
, a recent debate on the topic suggests. During the debate, one expert argued for, and another against, there being sufficient evidence for the use of cannabis to treat neuropathic pain, but in the end, they agreed that some patients do benefit.
The discussion took place at the Congress of the European Academy of Neurology (EAN) 2020, which transitioned to a virtual online meeting because of the COVID-19 pandemic.
The cannabis plant has 460 constituents. The two main components are tetrahydrocannabinol (THC) and cannabidiol (CBD). It can be consumed by swallowing oil extracts, by the sublingual route, or by smoking or eating the plant. Cannabis medications already in use include oral THC (nabilone, dronabinol) and an oral mucosal spray, nabiximols (Sativex).
Arguing that therapeutic cannabis is helpful for neuropathic pain, Elon Eisenberg, MD, professor of neurology and pain medicine, Israel Institute of Technology, Haifa, cited a number of encouraging randomized, controlled trials and meta-analyses of studies on the subject.
Opioid substitute
Dr. Eisenberg discussed three relevant articles. One was a 2016 viewpoint article published in JAMA that concluded that “cannabis seems to be a substitute, a rather good one, for opioids,” said Dr. Eisenberg.
A “comprehensive” 440-page review, published by the National Academies Press in 2017, evaluated the evidence to that point and “came to the conclusion there is substantial evidence that cannabis is an effective treatment for chronic pain in adults,” said Dr. Eisenberg.
And a 2018 position paper from the European Pain Federation determined that “the quantity and quality of evidence is such that cannabis-based medicines may be reasonably considered for chronic neuropathic pain,” he said.
He noted that the most recent results from an Israeli prospective cohort registry study that is following more than 851 patients who are taking cannabis over 1 year are positive. Analyses show a steady reduction in pain intensity and improvements in catastrophizing and disability. Importantly, he said, participants are using fewer opioids. However, about 40% of patients in that registry study experienced some adverse event, although most were not serious, said Dr. Eisenberg.
Not convinced
Arguing on the other side – that therapeutic cannabis is not helpful for neuropathic pain – was Nadine Attal, MD, PhD, professor of therapeutics and pain at the University Versailles Saint Quentin, France. She questioned the quality of some of the research to date and stressed that studies should consider neuropathic pain as a primary outcome – not spasticity or pain in general. They should also be double-blind, randomized, and placebo controlled, she said.
In addition, she said these studies should enroll at least 10 patients per group and should continue for 3 weeks or longer.
Dr. Attal wondered which of the many plant derivatives (phytocannabinoids) are used in cannabis studies.
She discussed four meta-analyses or reviews on the topic, some of which she said are “heterogeneous” and don’t provide convincing evidence for cannabis use in neuropathic pain.
For example, one review examined only marijuana, and all studies in it were short term. One of the studies in this review was of spasticity. Another review included two studies of cancer pain, and the most positive study in NP used short-term inhaled THC.
“There is no evidence to date that cannabinoids, including nabiximols or oral THC, administered for at least 3 weeks are more effective than placebo in neuropathic pain,” she concluded.
Some responders
However, Dr. Attal acknowledged that cannabis might be effective for some patients. In her experience, which has been borne out by some observational studies, patients with paroxysmal pain, or sudden stabbing pain, seem to get more relief from cannabis. “It’s absolutely possible that there’s a subgroup of symptoms or a subgroup of patients with specific symptoms who are much better responders to cannabis than others,” she said.
Asked if patients experience increased pain after withdrawing from cannabis, Dr. Eisenberg said he has observed that many patients stop taking cannabis when they start feeling better, but he hasn’t seen severe withdrawal symptoms.
However, there are other concerns related to cannabis use, said Dr. Eisenberg. A major concern regards driving a vehicle. In Israel, getting behind the wheel is prohibited within 6 hours of using cannabis.
But Dr. Eisenberg pointed out that published data on the safety of cannabis and driving were based on recreational users. “We need to keep in mind that recreational users typically use other substances, so we’re not sure the data is accurate,” he said.
There are increasing reports of stroke, transient ischemic attack, and MI among cannabis users. This is especially concerning because many of these cases involve young male adults who have no risk factors, said Dr. Eisenberg.
One conference delegate asked whether legal issues make it difficult to properly investigate cannabis in large studies. Dr. Eisenberg noted that legal concerns may help explain why there have not been any new randomized, controlled trials for about 2 years. “In the U.S., you can’t do clinical trials; cannabis is still regarded as schedule I substance,” he said.
Some physicians “are reluctant to deal with cannabis unless they get better data,” he said. “Doing research on cannabis seems to be somehow out of the mainstream.” Moreover, the research is difficult to carry out, owing to the complexity of the cannabis plant, which has many constituents. Perhaps it’s a matter of identifying and adding particular components to better demonstrate reduced pain, said Dr. Eisenberg.
Another complicating factor is that bioavailability differs considerably from one patient to another, “sometimes even by 10-fold,” he said.
Dr. Attal’s group will be starting a study next January that will enroll a large sample of patients with neuropathic pain or spasticity. In that study, cannabis will be dispensed through pharmacies and primary care. The aim of the study is “to see how it works in a real-life setting,” she said
Those participating in the virtual session were asked to vote on which side they agreed with. About 57% voted in favor of cannabis use, 14% voted against, and 28% had no opinion.
Dr. Eisenberg has received research grants from Rafa Laboratories, Saga Medical Ltd., Israel Pain Association, and Teva Israel. Dr. Attal has received support from Merck Sharp & Dohme, Sanofi, Ipsen, Novartis, Aptinyx, Air Liquide, Lilly, and Grunenthal.
A version of this article originally appeared on Medscape.com.
, a recent debate on the topic suggests. During the debate, one expert argued for, and another against, there being sufficient evidence for the use of cannabis to treat neuropathic pain, but in the end, they agreed that some patients do benefit.
The discussion took place at the Congress of the European Academy of Neurology (EAN) 2020, which transitioned to a virtual online meeting because of the COVID-19 pandemic.
The cannabis plant has 460 constituents. The two main components are tetrahydrocannabinol (THC) and cannabidiol (CBD). It can be consumed by swallowing oil extracts, by the sublingual route, or by smoking or eating the plant. Cannabis medications already in use include oral THC (nabilone, dronabinol) and an oral mucosal spray, nabiximols (Sativex).
Arguing that therapeutic cannabis is helpful for neuropathic pain, Elon Eisenberg, MD, professor of neurology and pain medicine, Israel Institute of Technology, Haifa, cited a number of encouraging randomized, controlled trials and meta-analyses of studies on the subject.
Opioid substitute
Dr. Eisenberg discussed three relevant articles. One was a 2016 viewpoint article published in JAMA that concluded that “cannabis seems to be a substitute, a rather good one, for opioids,” said Dr. Eisenberg.
A “comprehensive” 440-page review, published by the National Academies Press in 2017, evaluated the evidence to that point and “came to the conclusion there is substantial evidence that cannabis is an effective treatment for chronic pain in adults,” said Dr. Eisenberg.
And a 2018 position paper from the European Pain Federation determined that “the quantity and quality of evidence is such that cannabis-based medicines may be reasonably considered for chronic neuropathic pain,” he said.
He noted that the most recent results from an Israeli prospective cohort registry study that is following more than 851 patients who are taking cannabis over 1 year are positive. Analyses show a steady reduction in pain intensity and improvements in catastrophizing and disability. Importantly, he said, participants are using fewer opioids. However, about 40% of patients in that registry study experienced some adverse event, although most were not serious, said Dr. Eisenberg.
Not convinced
Arguing on the other side – that therapeutic cannabis is not helpful for neuropathic pain – was Nadine Attal, MD, PhD, professor of therapeutics and pain at the University Versailles Saint Quentin, France. She questioned the quality of some of the research to date and stressed that studies should consider neuropathic pain as a primary outcome – not spasticity or pain in general. They should also be double-blind, randomized, and placebo controlled, she said.
In addition, she said these studies should enroll at least 10 patients per group and should continue for 3 weeks or longer.
Dr. Attal wondered which of the many plant derivatives (phytocannabinoids) are used in cannabis studies.
She discussed four meta-analyses or reviews on the topic, some of which she said are “heterogeneous” and don’t provide convincing evidence for cannabis use in neuropathic pain.
For example, one review examined only marijuana, and all studies in it were short term. One of the studies in this review was of spasticity. Another review included two studies of cancer pain, and the most positive study in NP used short-term inhaled THC.
“There is no evidence to date that cannabinoids, including nabiximols or oral THC, administered for at least 3 weeks are more effective than placebo in neuropathic pain,” she concluded.
Some responders
However, Dr. Attal acknowledged that cannabis might be effective for some patients. In her experience, which has been borne out by some observational studies, patients with paroxysmal pain, or sudden stabbing pain, seem to get more relief from cannabis. “It’s absolutely possible that there’s a subgroup of symptoms or a subgroup of patients with specific symptoms who are much better responders to cannabis than others,” she said.
Asked if patients experience increased pain after withdrawing from cannabis, Dr. Eisenberg said he has observed that many patients stop taking cannabis when they start feeling better, but he hasn’t seen severe withdrawal symptoms.
However, there are other concerns related to cannabis use, said Dr. Eisenberg. A major concern regards driving a vehicle. In Israel, getting behind the wheel is prohibited within 6 hours of using cannabis.
But Dr. Eisenberg pointed out that published data on the safety of cannabis and driving were based on recreational users. “We need to keep in mind that recreational users typically use other substances, so we’re not sure the data is accurate,” he said.
There are increasing reports of stroke, transient ischemic attack, and MI among cannabis users. This is especially concerning because many of these cases involve young male adults who have no risk factors, said Dr. Eisenberg.
One conference delegate asked whether legal issues make it difficult to properly investigate cannabis in large studies. Dr. Eisenberg noted that legal concerns may help explain why there have not been any new randomized, controlled trials for about 2 years. “In the U.S., you can’t do clinical trials; cannabis is still regarded as schedule I substance,” he said.
Some physicians “are reluctant to deal with cannabis unless they get better data,” he said. “Doing research on cannabis seems to be somehow out of the mainstream.” Moreover, the research is difficult to carry out, owing to the complexity of the cannabis plant, which has many constituents. Perhaps it’s a matter of identifying and adding particular components to better demonstrate reduced pain, said Dr. Eisenberg.
Another complicating factor is that bioavailability differs considerably from one patient to another, “sometimes even by 10-fold,” he said.
Dr. Attal’s group will be starting a study next January that will enroll a large sample of patients with neuropathic pain or spasticity. In that study, cannabis will be dispensed through pharmacies and primary care. The aim of the study is “to see how it works in a real-life setting,” she said
Those participating in the virtual session were asked to vote on which side they agreed with. About 57% voted in favor of cannabis use, 14% voted against, and 28% had no opinion.
Dr. Eisenberg has received research grants from Rafa Laboratories, Saga Medical Ltd., Israel Pain Association, and Teva Israel. Dr. Attal has received support from Merck Sharp & Dohme, Sanofi, Ipsen, Novartis, Aptinyx, Air Liquide, Lilly, and Grunenthal.
A version of this article originally appeared on Medscape.com.
, a recent debate on the topic suggests. During the debate, one expert argued for, and another against, there being sufficient evidence for the use of cannabis to treat neuropathic pain, but in the end, they agreed that some patients do benefit.
The discussion took place at the Congress of the European Academy of Neurology (EAN) 2020, which transitioned to a virtual online meeting because of the COVID-19 pandemic.
The cannabis plant has 460 constituents. The two main components are tetrahydrocannabinol (THC) and cannabidiol (CBD). It can be consumed by swallowing oil extracts, by the sublingual route, or by smoking or eating the plant. Cannabis medications already in use include oral THC (nabilone, dronabinol) and an oral mucosal spray, nabiximols (Sativex).
Arguing that therapeutic cannabis is helpful for neuropathic pain, Elon Eisenberg, MD, professor of neurology and pain medicine, Israel Institute of Technology, Haifa, cited a number of encouraging randomized, controlled trials and meta-analyses of studies on the subject.
Opioid substitute
Dr. Eisenberg discussed three relevant articles. One was a 2016 viewpoint article published in JAMA that concluded that “cannabis seems to be a substitute, a rather good one, for opioids,” said Dr. Eisenberg.
A “comprehensive” 440-page review, published by the National Academies Press in 2017, evaluated the evidence to that point and “came to the conclusion there is substantial evidence that cannabis is an effective treatment for chronic pain in adults,” said Dr. Eisenberg.
And a 2018 position paper from the European Pain Federation determined that “the quantity and quality of evidence is such that cannabis-based medicines may be reasonably considered for chronic neuropathic pain,” he said.
He noted that the most recent results from an Israeli prospective cohort registry study that is following more than 851 patients who are taking cannabis over 1 year are positive. Analyses show a steady reduction in pain intensity and improvements in catastrophizing and disability. Importantly, he said, participants are using fewer opioids. However, about 40% of patients in that registry study experienced some adverse event, although most were not serious, said Dr. Eisenberg.
Not convinced
Arguing on the other side – that therapeutic cannabis is not helpful for neuropathic pain – was Nadine Attal, MD, PhD, professor of therapeutics and pain at the University Versailles Saint Quentin, France. She questioned the quality of some of the research to date and stressed that studies should consider neuropathic pain as a primary outcome – not spasticity or pain in general. They should also be double-blind, randomized, and placebo controlled, she said.
In addition, she said these studies should enroll at least 10 patients per group and should continue for 3 weeks or longer.
Dr. Attal wondered which of the many plant derivatives (phytocannabinoids) are used in cannabis studies.
She discussed four meta-analyses or reviews on the topic, some of which she said are “heterogeneous” and don’t provide convincing evidence for cannabis use in neuropathic pain.
For example, one review examined only marijuana, and all studies in it were short term. One of the studies in this review was of spasticity. Another review included two studies of cancer pain, and the most positive study in NP used short-term inhaled THC.
“There is no evidence to date that cannabinoids, including nabiximols or oral THC, administered for at least 3 weeks are more effective than placebo in neuropathic pain,” she concluded.
Some responders
However, Dr. Attal acknowledged that cannabis might be effective for some patients. In her experience, which has been borne out by some observational studies, patients with paroxysmal pain, or sudden stabbing pain, seem to get more relief from cannabis. “It’s absolutely possible that there’s a subgroup of symptoms or a subgroup of patients with specific symptoms who are much better responders to cannabis than others,” she said.
Asked if patients experience increased pain after withdrawing from cannabis, Dr. Eisenberg said he has observed that many patients stop taking cannabis when they start feeling better, but he hasn’t seen severe withdrawal symptoms.
However, there are other concerns related to cannabis use, said Dr. Eisenberg. A major concern regards driving a vehicle. In Israel, getting behind the wheel is prohibited within 6 hours of using cannabis.
But Dr. Eisenberg pointed out that published data on the safety of cannabis and driving were based on recreational users. “We need to keep in mind that recreational users typically use other substances, so we’re not sure the data is accurate,” he said.
There are increasing reports of stroke, transient ischemic attack, and MI among cannabis users. This is especially concerning because many of these cases involve young male adults who have no risk factors, said Dr. Eisenberg.
One conference delegate asked whether legal issues make it difficult to properly investigate cannabis in large studies. Dr. Eisenberg noted that legal concerns may help explain why there have not been any new randomized, controlled trials for about 2 years. “In the U.S., you can’t do clinical trials; cannabis is still regarded as schedule I substance,” he said.
Some physicians “are reluctant to deal with cannabis unless they get better data,” he said. “Doing research on cannabis seems to be somehow out of the mainstream.” Moreover, the research is difficult to carry out, owing to the complexity of the cannabis plant, which has many constituents. Perhaps it’s a matter of identifying and adding particular components to better demonstrate reduced pain, said Dr. Eisenberg.
Another complicating factor is that bioavailability differs considerably from one patient to another, “sometimes even by 10-fold,” he said.
Dr. Attal’s group will be starting a study next January that will enroll a large sample of patients with neuropathic pain or spasticity. In that study, cannabis will be dispensed through pharmacies and primary care. The aim of the study is “to see how it works in a real-life setting,” she said
Those participating in the virtual session were asked to vote on which side they agreed with. About 57% voted in favor of cannabis use, 14% voted against, and 28% had no opinion.
Dr. Eisenberg has received research grants from Rafa Laboratories, Saga Medical Ltd., Israel Pain Association, and Teva Israel. Dr. Attal has received support from Merck Sharp & Dohme, Sanofi, Ipsen, Novartis, Aptinyx, Air Liquide, Lilly, and Grunenthal.
A version of this article originally appeared on Medscape.com.
FROM EAN 2020
Most adult epilepsy-related deaths could be avoided
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, clinical research fellow, Muir Maxwell Epilepsy Center, the University of Edinburgh, Scotland, told a press briefing.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2020, which is being conducted as a virtual/online meeting because of the COVID-19 pandemic.
As his PhD dissertation, Dr. Mbizvo is investigating the rates, causes, and risk factors for epilepsy-related deaths and the percentage of these that are potentially avoidable.
The National Health Service of Scotland contains various linked administrative data sets. Each resident of Scotland has a unique identifier that facilitates investigations across the health system.
Dr. Mbizvo investigated adults and adolescents aged 16 years and older who died because of epilepsy during 2009-2016. He compared this group to patients of similar age who were living with epilepsy to identify risk factors that might help focus resources. During the study period, 2,149 epilepsy-related deaths occurred. Nearly 60% involved at least one seizure-related hospital admission.
Heavy burden
Of the patients who died because of epilepsy, 24% were seen in an outpatient neurologic clinic. “So there’s this heavy burden of admissions not translating to neurology follow-up,” said Dr. Mbizvo.
During the study period, there was no reduction in mortality “despite advances in medical care,” said Dr. Mbizvo.
Younger people with epilepsy were found to be more likely to die. The standardized mortality rate was 6/100,000 (95% confidence interval, 2.3-9.7) among those aged 16-24 years. By contrast, among those aged 45-54 years, the rate was 2/100,000 (95% CI, 1.1-2.1); it was lower in older age groups.
“The overall mortality is not reducing; people are dying young, and neurologists are really not getting involved,” Dr. Mbizvo said.
Among the almost 600 deaths of those aged 16-54 years, 58% were from Scotland’s “most deprived areas,” he noted.
From medical records and antiepileptic drug (AED) use, Dr. Mbizvo looked for risk factors that may have contributed to these epilepsy-related deaths. The most common cause of death in the group aged 16- 54 years was sudden unexpected death in epilepsy (SUDEP), followed by respiratory disorders, such as aspiration pneumonia.
“We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” said Dr. Mbizvo.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” said Dr. Mbizvo.
Worrisome group
Mental and behavioral disorders, largely alcohol related, were the next most common cause of death.
“This is a group I worry about,” said Dr. Mbizvo. “I think they’re seen in the acute services and discharged as alcohol-withdrawal seizures. It’s possible that some have epilepsy and are never referred to a neurologist, and this may translate into increased mortality.”
Dr. Mbizvo is analyzing how these results differ from what is seen in the general population of Scotland among those younger than 75 years.
The top cause of death in the general population is neoplasm of the lungs. Aspiration of the lung is near the top for those who died from epilepsy, but the mechanisms leading to lung-related deaths in these populations may differ, said Dr. Mbizvo.
By applying coding methodology from fields unrelated to epilepsy where this approach has been tried, he determined that 78% of epilepsy-related deaths among those younger than 55 years were potentially avoidable.
“As a method, this is still in its infancy and will require validation, but we see this as a start,” Dr. Mbizvo said.
He provided examples from medical records that illustrate avoidable factors that could contribute to death. These included cases in which patients were discharged with the wrong dose of AED and in which patients drowned in a bath after having not been appropriately educated about seizure safety.
Can’t plug in
Patients with a first seizure are typically referred quickly to an appropriate service, but Dr. Mbizvo is concerned about those with chronic, stable epilepsy. “These people may at some point decompensate, and there’s no channel to plug them back into neurology services to make it easy for them to access a neurologist,” he said.
Currently, experts tell discharged patients to call if a problem occurs, but the system “is rather ad hoc,” said Dr. Mbizvo.
Because of the COVID-19 crisis, the use of telemedicine is increasing. This is helping to improve the system. “We may be able to build a virtual community for people who are on antiepileptic drugs and who suddenly begin to experience seizures again, to enable them to quickly get help, alongside a defined pathway to an epilepsy specialist,” said Dr. Mbizvo.
He hopes to develop a risk index for epilepsy patients similar to one used in cardiology that assesses risks such as smoking, high cholesterol level, and obesity. Although such a risk score might be similar to the SUDEP risk indices being developed, it will take into account death from any epilepsy-related cause, said Dr. Mbizvo. “Having not yet completed the analysis, I’m not sure which aspects will confer the greatest risk,” he said.
He added that, anecdotally, he has noticed a slight trend toward high mortality among patients with epilepsy who present multiple times at emergency departments in a year.
If this trend is statistically valid, “it could help create a traffic light flagging system on A&Es [accident and emergency departments] in which individuals with epilepsy who, for example, have two or more attendances to A&E in a year become flagged as high risk of death and are plugged into a rapid access epilepsy specialist clinic,” he said.
For their part, neurologists should recognize drug-resistant epilepsy early and refer such patients for assessment for resective surgery. If successful, such surgery reduces the risk for premature mortality, said Dr. Mbizvo.
Patients should not become discouraged by drug resistance, either. Research shows that, with careful reassessment of epilepsy type and drug changes, some patients whose condition is thought to be intractable could experience significant improvement in seizure frequency or seizures could be stopped.
“We need to talk to our patients more about the importance of adherence and encourage them to be honest with us if they don’t like the drugs we’re giving them and, as a result, are not taking them as recommended,” Dr. Mbizvo said.
Physicians also need to screen for mood disorders, especially suicidal ideation. Increasingly, specialists are recognizing mental health as an important area of epilepsy care.
They should also conduct a “safety briefing” perhaps twice a year in which they discuss, for example, SUDEP risk, driving concerns, showering instead of bathing, ensuring that a life guard is present at a swimming pool, and other measures.
Commenting on the study, Josemir W. (Ley) Sander, MD, PhD, professor of neurology and clinical epilepsy at University College London, said he welcomes any effort that highlights the problem of premature death among people with epilepsy and that offers possible ways to mitigate it.
Although the study “shows that premature death among people with epilepsy is a major issue,” many health care providers are not fully aware of the extent of this problem, said Dr. Sander. “For many, epilepsy is just a benign condition in which people have seizures,” he said. A risk score that could identify those at high risk for death and establishing preventive measures “would go a long way to decrease the burden of epilepsy,” he noted.
The study was supported by Epilepsy Research UK and the Juliet Bergqvist Memorial Fund. Dr. Mbizvo and Dr. Sander have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, clinical research fellow, Muir Maxwell Epilepsy Center, the University of Edinburgh, Scotland, told a press briefing.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2020, which is being conducted as a virtual/online meeting because of the COVID-19 pandemic.
As his PhD dissertation, Dr. Mbizvo is investigating the rates, causes, and risk factors for epilepsy-related deaths and the percentage of these that are potentially avoidable.
The National Health Service of Scotland contains various linked administrative data sets. Each resident of Scotland has a unique identifier that facilitates investigations across the health system.
Dr. Mbizvo investigated adults and adolescents aged 16 years and older who died because of epilepsy during 2009-2016. He compared this group to patients of similar age who were living with epilepsy to identify risk factors that might help focus resources. During the study period, 2,149 epilepsy-related deaths occurred. Nearly 60% involved at least one seizure-related hospital admission.
Heavy burden
Of the patients who died because of epilepsy, 24% were seen in an outpatient neurologic clinic. “So there’s this heavy burden of admissions not translating to neurology follow-up,” said Dr. Mbizvo.
During the study period, there was no reduction in mortality “despite advances in medical care,” said Dr. Mbizvo.
Younger people with epilepsy were found to be more likely to die. The standardized mortality rate was 6/100,000 (95% confidence interval, 2.3-9.7) among those aged 16-24 years. By contrast, among those aged 45-54 years, the rate was 2/100,000 (95% CI, 1.1-2.1); it was lower in older age groups.
“The overall mortality is not reducing; people are dying young, and neurologists are really not getting involved,” Dr. Mbizvo said.
Among the almost 600 deaths of those aged 16-54 years, 58% were from Scotland’s “most deprived areas,” he noted.
From medical records and antiepileptic drug (AED) use, Dr. Mbizvo looked for risk factors that may have contributed to these epilepsy-related deaths. The most common cause of death in the group aged 16- 54 years was sudden unexpected death in epilepsy (SUDEP), followed by respiratory disorders, such as aspiration pneumonia.
“We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” said Dr. Mbizvo.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” said Dr. Mbizvo.
Worrisome group
Mental and behavioral disorders, largely alcohol related, were the next most common cause of death.
“This is a group I worry about,” said Dr. Mbizvo. “I think they’re seen in the acute services and discharged as alcohol-withdrawal seizures. It’s possible that some have epilepsy and are never referred to a neurologist, and this may translate into increased mortality.”
Dr. Mbizvo is analyzing how these results differ from what is seen in the general population of Scotland among those younger than 75 years.
The top cause of death in the general population is neoplasm of the lungs. Aspiration of the lung is near the top for those who died from epilepsy, but the mechanisms leading to lung-related deaths in these populations may differ, said Dr. Mbizvo.
By applying coding methodology from fields unrelated to epilepsy where this approach has been tried, he determined that 78% of epilepsy-related deaths among those younger than 55 years were potentially avoidable.
“As a method, this is still in its infancy and will require validation, but we see this as a start,” Dr. Mbizvo said.
He provided examples from medical records that illustrate avoidable factors that could contribute to death. These included cases in which patients were discharged with the wrong dose of AED and in which patients drowned in a bath after having not been appropriately educated about seizure safety.
Can’t plug in
Patients with a first seizure are typically referred quickly to an appropriate service, but Dr. Mbizvo is concerned about those with chronic, stable epilepsy. “These people may at some point decompensate, and there’s no channel to plug them back into neurology services to make it easy for them to access a neurologist,” he said.
Currently, experts tell discharged patients to call if a problem occurs, but the system “is rather ad hoc,” said Dr. Mbizvo.
Because of the COVID-19 crisis, the use of telemedicine is increasing. This is helping to improve the system. “We may be able to build a virtual community for people who are on antiepileptic drugs and who suddenly begin to experience seizures again, to enable them to quickly get help, alongside a defined pathway to an epilepsy specialist,” said Dr. Mbizvo.
He hopes to develop a risk index for epilepsy patients similar to one used in cardiology that assesses risks such as smoking, high cholesterol level, and obesity. Although such a risk score might be similar to the SUDEP risk indices being developed, it will take into account death from any epilepsy-related cause, said Dr. Mbizvo. “Having not yet completed the analysis, I’m not sure which aspects will confer the greatest risk,” he said.
He added that, anecdotally, he has noticed a slight trend toward high mortality among patients with epilepsy who present multiple times at emergency departments in a year.
If this trend is statistically valid, “it could help create a traffic light flagging system on A&Es [accident and emergency departments] in which individuals with epilepsy who, for example, have two or more attendances to A&E in a year become flagged as high risk of death and are plugged into a rapid access epilepsy specialist clinic,” he said.
For their part, neurologists should recognize drug-resistant epilepsy early and refer such patients for assessment for resective surgery. If successful, such surgery reduces the risk for premature mortality, said Dr. Mbizvo.
Patients should not become discouraged by drug resistance, either. Research shows that, with careful reassessment of epilepsy type and drug changes, some patients whose condition is thought to be intractable could experience significant improvement in seizure frequency or seizures could be stopped.
“We need to talk to our patients more about the importance of adherence and encourage them to be honest with us if they don’t like the drugs we’re giving them and, as a result, are not taking them as recommended,” Dr. Mbizvo said.
Physicians also need to screen for mood disorders, especially suicidal ideation. Increasingly, specialists are recognizing mental health as an important area of epilepsy care.
They should also conduct a “safety briefing” perhaps twice a year in which they discuss, for example, SUDEP risk, driving concerns, showering instead of bathing, ensuring that a life guard is present at a swimming pool, and other measures.
Commenting on the study, Josemir W. (Ley) Sander, MD, PhD, professor of neurology and clinical epilepsy at University College London, said he welcomes any effort that highlights the problem of premature death among people with epilepsy and that offers possible ways to mitigate it.
Although the study “shows that premature death among people with epilepsy is a major issue,” many health care providers are not fully aware of the extent of this problem, said Dr. Sander. “For many, epilepsy is just a benign condition in which people have seizures,” he said. A risk score that could identify those at high risk for death and establishing preventive measures “would go a long way to decrease the burden of epilepsy,” he noted.
The study was supported by Epilepsy Research UK and the Juliet Bergqvist Memorial Fund. Dr. Mbizvo and Dr. Sander have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, clinical research fellow, Muir Maxwell Epilepsy Center, the University of Edinburgh, Scotland, told a press briefing.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2020, which is being conducted as a virtual/online meeting because of the COVID-19 pandemic.
As his PhD dissertation, Dr. Mbizvo is investigating the rates, causes, and risk factors for epilepsy-related deaths and the percentage of these that are potentially avoidable.
The National Health Service of Scotland contains various linked administrative data sets. Each resident of Scotland has a unique identifier that facilitates investigations across the health system.
Dr. Mbizvo investigated adults and adolescents aged 16 years and older who died because of epilepsy during 2009-2016. He compared this group to patients of similar age who were living with epilepsy to identify risk factors that might help focus resources. During the study period, 2,149 epilepsy-related deaths occurred. Nearly 60% involved at least one seizure-related hospital admission.
Heavy burden
Of the patients who died because of epilepsy, 24% were seen in an outpatient neurologic clinic. “So there’s this heavy burden of admissions not translating to neurology follow-up,” said Dr. Mbizvo.
During the study period, there was no reduction in mortality “despite advances in medical care,” said Dr. Mbizvo.
Younger people with epilepsy were found to be more likely to die. The standardized mortality rate was 6/100,000 (95% confidence interval, 2.3-9.7) among those aged 16-24 years. By contrast, among those aged 45-54 years, the rate was 2/100,000 (95% CI, 1.1-2.1); it was lower in older age groups.
“The overall mortality is not reducing; people are dying young, and neurologists are really not getting involved,” Dr. Mbizvo said.
Among the almost 600 deaths of those aged 16-54 years, 58% were from Scotland’s “most deprived areas,” he noted.
From medical records and antiepileptic drug (AED) use, Dr. Mbizvo looked for risk factors that may have contributed to these epilepsy-related deaths. The most common cause of death in the group aged 16- 54 years was sudden unexpected death in epilepsy (SUDEP), followed by respiratory disorders, such as aspiration pneumonia.
“We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” said Dr. Mbizvo.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” said Dr. Mbizvo.
Worrisome group
Mental and behavioral disorders, largely alcohol related, were the next most common cause of death.
“This is a group I worry about,” said Dr. Mbizvo. “I think they’re seen in the acute services and discharged as alcohol-withdrawal seizures. It’s possible that some have epilepsy and are never referred to a neurologist, and this may translate into increased mortality.”
Dr. Mbizvo is analyzing how these results differ from what is seen in the general population of Scotland among those younger than 75 years.
The top cause of death in the general population is neoplasm of the lungs. Aspiration of the lung is near the top for those who died from epilepsy, but the mechanisms leading to lung-related deaths in these populations may differ, said Dr. Mbizvo.
By applying coding methodology from fields unrelated to epilepsy where this approach has been tried, he determined that 78% of epilepsy-related deaths among those younger than 55 years were potentially avoidable.
“As a method, this is still in its infancy and will require validation, but we see this as a start,” Dr. Mbizvo said.
He provided examples from medical records that illustrate avoidable factors that could contribute to death. These included cases in which patients were discharged with the wrong dose of AED and in which patients drowned in a bath after having not been appropriately educated about seizure safety.
Can’t plug in
Patients with a first seizure are typically referred quickly to an appropriate service, but Dr. Mbizvo is concerned about those with chronic, stable epilepsy. “These people may at some point decompensate, and there’s no channel to plug them back into neurology services to make it easy for them to access a neurologist,” he said.
Currently, experts tell discharged patients to call if a problem occurs, but the system “is rather ad hoc,” said Dr. Mbizvo.
Because of the COVID-19 crisis, the use of telemedicine is increasing. This is helping to improve the system. “We may be able to build a virtual community for people who are on antiepileptic drugs and who suddenly begin to experience seizures again, to enable them to quickly get help, alongside a defined pathway to an epilepsy specialist,” said Dr. Mbizvo.
He hopes to develop a risk index for epilepsy patients similar to one used in cardiology that assesses risks such as smoking, high cholesterol level, and obesity. Although such a risk score might be similar to the SUDEP risk indices being developed, it will take into account death from any epilepsy-related cause, said Dr. Mbizvo. “Having not yet completed the analysis, I’m not sure which aspects will confer the greatest risk,” he said.
He added that, anecdotally, he has noticed a slight trend toward high mortality among patients with epilepsy who present multiple times at emergency departments in a year.
If this trend is statistically valid, “it could help create a traffic light flagging system on A&Es [accident and emergency departments] in which individuals with epilepsy who, for example, have two or more attendances to A&E in a year become flagged as high risk of death and are plugged into a rapid access epilepsy specialist clinic,” he said.
For their part, neurologists should recognize drug-resistant epilepsy early and refer such patients for assessment for resective surgery. If successful, such surgery reduces the risk for premature mortality, said Dr. Mbizvo.
Patients should not become discouraged by drug resistance, either. Research shows that, with careful reassessment of epilepsy type and drug changes, some patients whose condition is thought to be intractable could experience significant improvement in seizure frequency or seizures could be stopped.
“We need to talk to our patients more about the importance of adherence and encourage them to be honest with us if they don’t like the drugs we’re giving them and, as a result, are not taking them as recommended,” Dr. Mbizvo said.
Physicians also need to screen for mood disorders, especially suicidal ideation. Increasingly, specialists are recognizing mental health as an important area of epilepsy care.
They should also conduct a “safety briefing” perhaps twice a year in which they discuss, for example, SUDEP risk, driving concerns, showering instead of bathing, ensuring that a life guard is present at a swimming pool, and other measures.
Commenting on the study, Josemir W. (Ley) Sander, MD, PhD, professor of neurology and clinical epilepsy at University College London, said he welcomes any effort that highlights the problem of premature death among people with epilepsy and that offers possible ways to mitigate it.
Although the study “shows that premature death among people with epilepsy is a major issue,” many health care providers are not fully aware of the extent of this problem, said Dr. Sander. “For many, epilepsy is just a benign condition in which people have seizures,” he said. A risk score that could identify those at high risk for death and establishing preventive measures “would go a long way to decrease the burden of epilepsy,” he noted.
The study was supported by Epilepsy Research UK and the Juliet Bergqvist Memorial Fund. Dr. Mbizvo and Dr. Sander have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM EAN 2020
No benefit of three commonly used medications for MS fatigue
The TRIUMPHANT study found no difference between the effects of amantadine, modafinil, methylphenidate, and placebo in the Modified Fatigue Impact Scale (MFIS) in a study involving 141 patients with MS.
There was also no difference between any of the drugs and placebo in any of the preplanned subgroups which included different Expanded Disability Status Scale scores, depressive scores, use of disease-modifying therapy, or type of MS (relapsing remitting or progressive).
The research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
“These three drugs are used very commonly used for MS fatigue by neurologists, psychiatrists, and primary care doctors, but they don’t seem to be any better than placebo. They were all associated with increased side effects compared with placebo even with short-term use,” said lead investigator Bardia Nourbakhsh, MD, assistant professor of neurology at Johns Hopkins University, Baltimore.
However, in a post hoc analysis there was an improvement in daytime sleepiness with two of the drugs – methylphenidate and modafinil. “These two agents reduced daytime sleepiness in patients with high daytime sleepiness scores at baseline, with about a 4-point difference versus placebo, which was significant. But as this was not a preplanned analysis, we have to be cautious in its interpretation,” Dr. Nourbakhsh said. “However, this finding may not be too surprising as both these drugs are licensed as stimulants for use in narcolepsy patients with excessive daytime sleepiness.”
“Our recommendations are that as amantadine was not better than placebo in any subgroup its use should be discouraged in MS fatigue,” Dr. Nourbakhsh commented. “Modafinil and methylphenidate may possibly be considered for MS patients with excessive daytime sleepiness, but this should really be confirmed in further studies.”
Fatigue is a common and debilitating symptom of MS, occurring in about 70%-80% of patients with MS. There is no approved drug treatment. However nonpharmacologic therapies have shown some success: studies of exercise and cognitive-behavioral therapy (CBT) have shown these may be effective without causing side effects, Dr. Nourbakhsh noted. “So we should be getting patients to try exercise and CBT before jumping to medication.”
Dr. Nourbakhsh said he was disappointed with the results of the study but not terribly surprised. “We use these three medications frequently in the clinic and we have not been seeing great benefits so we wondered whether they were actually effective.”
He said that the trial was adequately powered and the question has been answered. “These are valuable results – they will hopefully encourage doctors to think twice before prescribing these medications that could be harmful and have no clear benefit,” Dr. Nourbakhsh concluded.
For the randomized, double-blind, placebo-controlled, four-sequence, four-period crossover trial, 141 patients with MS and fatigue received twice-daily oral amantadine (maximum 200 mg/day), modafinil (maximum 200 mg/day), methylphenidate (maximum 20 mg/day), or placebo, each given for up to 6 weeks with a 2-week washout between each medication.
Patients had a mean baseline MFIS score of 51.3 and were randomly assigned to one of four medication administration sequences. Data from 136 participants were available for the analysis of the primary outcome (change in MFIS score), and 111 participants completed all four medication periods.
In the intent-to-treat analysis, the least-squares means of total MFIS scores at the maximally tolerated dose were as follows: 40.7 with placebo, 41.2 with amantadine, 39.0 with modafinil, and 38.7 with methylphenidate (P = .20 for the overall medication effect; P > .05 for all pairwise comparisons). “All medications and placebo reduced the MS fatigue score by 10-12 points from baseline, so there was quite a substantial placebo effect,” Dr. Nourbakhsh noted. There was no statistically significant difference in the physical and cognitive subscales of MFIS and quality of life measures between any of the study medications and placebo. All three drugs were associated with an increase in adverse effects versus placebo.
Dr. Nourbakhsh says he is hopeful that this negative study may stimulate further research into new targets and medications for MS fatigue.
His group has recently conducted a pilot study of intravenous ketamine in MS fatigue with some encouraging results, but he stressed it needs to be tested in a larger study before it can be recommended for use in clinical practice. “While an IV medication is not ideal, the effect did seem to be quite long-lived with a difference still evident at 28 days, so it could perhaps be dosed once a month, which could be feasible,” he said.
Commenting on the TRIUMPHANT study, Jeffrey Cohen, MD, of the Cleveland Clinic, said that “fatigue is a common, often disabling, symptom of MS. It is poorly understood and probably encompasses several mechanisms. There currently is no generally effective treatment for MS-related fatigue.”
“These results are not surprising and confirm previous studies,” Dr. Cohen said. “Despite no benefit from these medicines for patients as a group, they are occasionally helpful for individual patients, so they are frequently tried empirically.
“It also is important to address any factors besides MS that may be causing or contributing to fatigue, for example, sleep disruption, medication side effects, depression, other medical conditions such as anemia or hypothyroidism,” he added.
Dr. Nourbakhsh has reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities for Jazz Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
The TRIUMPHANT study found no difference between the effects of amantadine, modafinil, methylphenidate, and placebo in the Modified Fatigue Impact Scale (MFIS) in a study involving 141 patients with MS.
There was also no difference between any of the drugs and placebo in any of the preplanned subgroups which included different Expanded Disability Status Scale scores, depressive scores, use of disease-modifying therapy, or type of MS (relapsing remitting or progressive).
The research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
“These three drugs are used very commonly used for MS fatigue by neurologists, psychiatrists, and primary care doctors, but they don’t seem to be any better than placebo. They were all associated with increased side effects compared with placebo even with short-term use,” said lead investigator Bardia Nourbakhsh, MD, assistant professor of neurology at Johns Hopkins University, Baltimore.
However, in a post hoc analysis there was an improvement in daytime sleepiness with two of the drugs – methylphenidate and modafinil. “These two agents reduced daytime sleepiness in patients with high daytime sleepiness scores at baseline, with about a 4-point difference versus placebo, which was significant. But as this was not a preplanned analysis, we have to be cautious in its interpretation,” Dr. Nourbakhsh said. “However, this finding may not be too surprising as both these drugs are licensed as stimulants for use in narcolepsy patients with excessive daytime sleepiness.”
“Our recommendations are that as amantadine was not better than placebo in any subgroup its use should be discouraged in MS fatigue,” Dr. Nourbakhsh commented. “Modafinil and methylphenidate may possibly be considered for MS patients with excessive daytime sleepiness, but this should really be confirmed in further studies.”
Fatigue is a common and debilitating symptom of MS, occurring in about 70%-80% of patients with MS. There is no approved drug treatment. However nonpharmacologic therapies have shown some success: studies of exercise and cognitive-behavioral therapy (CBT) have shown these may be effective without causing side effects, Dr. Nourbakhsh noted. “So we should be getting patients to try exercise and CBT before jumping to medication.”
Dr. Nourbakhsh said he was disappointed with the results of the study but not terribly surprised. “We use these three medications frequently in the clinic and we have not been seeing great benefits so we wondered whether they were actually effective.”
He said that the trial was adequately powered and the question has been answered. “These are valuable results – they will hopefully encourage doctors to think twice before prescribing these medications that could be harmful and have no clear benefit,” Dr. Nourbakhsh concluded.
For the randomized, double-blind, placebo-controlled, four-sequence, four-period crossover trial, 141 patients with MS and fatigue received twice-daily oral amantadine (maximum 200 mg/day), modafinil (maximum 200 mg/day), methylphenidate (maximum 20 mg/day), or placebo, each given for up to 6 weeks with a 2-week washout between each medication.
Patients had a mean baseline MFIS score of 51.3 and were randomly assigned to one of four medication administration sequences. Data from 136 participants were available for the analysis of the primary outcome (change in MFIS score), and 111 participants completed all four medication periods.
In the intent-to-treat analysis, the least-squares means of total MFIS scores at the maximally tolerated dose were as follows: 40.7 with placebo, 41.2 with amantadine, 39.0 with modafinil, and 38.7 with methylphenidate (P = .20 for the overall medication effect; P > .05 for all pairwise comparisons). “All medications and placebo reduced the MS fatigue score by 10-12 points from baseline, so there was quite a substantial placebo effect,” Dr. Nourbakhsh noted. There was no statistically significant difference in the physical and cognitive subscales of MFIS and quality of life measures between any of the study medications and placebo. All three drugs were associated with an increase in adverse effects versus placebo.
Dr. Nourbakhsh says he is hopeful that this negative study may stimulate further research into new targets and medications for MS fatigue.
His group has recently conducted a pilot study of intravenous ketamine in MS fatigue with some encouraging results, but he stressed it needs to be tested in a larger study before it can be recommended for use in clinical practice. “While an IV medication is not ideal, the effect did seem to be quite long-lived with a difference still evident at 28 days, so it could perhaps be dosed once a month, which could be feasible,” he said.
Commenting on the TRIUMPHANT study, Jeffrey Cohen, MD, of the Cleveland Clinic, said that “fatigue is a common, often disabling, symptom of MS. It is poorly understood and probably encompasses several mechanisms. There currently is no generally effective treatment for MS-related fatigue.”
“These results are not surprising and confirm previous studies,” Dr. Cohen said. “Despite no benefit from these medicines for patients as a group, they are occasionally helpful for individual patients, so they are frequently tried empirically.
“It also is important to address any factors besides MS that may be causing or contributing to fatigue, for example, sleep disruption, medication side effects, depression, other medical conditions such as anemia or hypothyroidism,” he added.
Dr. Nourbakhsh has reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities for Jazz Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
The TRIUMPHANT study found no difference between the effects of amantadine, modafinil, methylphenidate, and placebo in the Modified Fatigue Impact Scale (MFIS) in a study involving 141 patients with MS.
There was also no difference between any of the drugs and placebo in any of the preplanned subgroups which included different Expanded Disability Status Scale scores, depressive scores, use of disease-modifying therapy, or type of MS (relapsing remitting or progressive).
The research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
“These three drugs are used very commonly used for MS fatigue by neurologists, psychiatrists, and primary care doctors, but they don’t seem to be any better than placebo. They were all associated with increased side effects compared with placebo even with short-term use,” said lead investigator Bardia Nourbakhsh, MD, assistant professor of neurology at Johns Hopkins University, Baltimore.
However, in a post hoc analysis there was an improvement in daytime sleepiness with two of the drugs – methylphenidate and modafinil. “These two agents reduced daytime sleepiness in patients with high daytime sleepiness scores at baseline, with about a 4-point difference versus placebo, which was significant. But as this was not a preplanned analysis, we have to be cautious in its interpretation,” Dr. Nourbakhsh said. “However, this finding may not be too surprising as both these drugs are licensed as stimulants for use in narcolepsy patients with excessive daytime sleepiness.”
“Our recommendations are that as amantadine was not better than placebo in any subgroup its use should be discouraged in MS fatigue,” Dr. Nourbakhsh commented. “Modafinil and methylphenidate may possibly be considered for MS patients with excessive daytime sleepiness, but this should really be confirmed in further studies.”
Fatigue is a common and debilitating symptom of MS, occurring in about 70%-80% of patients with MS. There is no approved drug treatment. However nonpharmacologic therapies have shown some success: studies of exercise and cognitive-behavioral therapy (CBT) have shown these may be effective without causing side effects, Dr. Nourbakhsh noted. “So we should be getting patients to try exercise and CBT before jumping to medication.”
Dr. Nourbakhsh said he was disappointed with the results of the study but not terribly surprised. “We use these three medications frequently in the clinic and we have not been seeing great benefits so we wondered whether they were actually effective.”
He said that the trial was adequately powered and the question has been answered. “These are valuable results – they will hopefully encourage doctors to think twice before prescribing these medications that could be harmful and have no clear benefit,” Dr. Nourbakhsh concluded.
For the randomized, double-blind, placebo-controlled, four-sequence, four-period crossover trial, 141 patients with MS and fatigue received twice-daily oral amantadine (maximum 200 mg/day), modafinil (maximum 200 mg/day), methylphenidate (maximum 20 mg/day), or placebo, each given for up to 6 weeks with a 2-week washout between each medication.
Patients had a mean baseline MFIS score of 51.3 and were randomly assigned to one of four medication administration sequences. Data from 136 participants were available for the analysis of the primary outcome (change in MFIS score), and 111 participants completed all four medication periods.
In the intent-to-treat analysis, the least-squares means of total MFIS scores at the maximally tolerated dose were as follows: 40.7 with placebo, 41.2 with amantadine, 39.0 with modafinil, and 38.7 with methylphenidate (P = .20 for the overall medication effect; P > .05 for all pairwise comparisons). “All medications and placebo reduced the MS fatigue score by 10-12 points from baseline, so there was quite a substantial placebo effect,” Dr. Nourbakhsh noted. There was no statistically significant difference in the physical and cognitive subscales of MFIS and quality of life measures between any of the study medications and placebo. All three drugs were associated with an increase in adverse effects versus placebo.
Dr. Nourbakhsh says he is hopeful that this negative study may stimulate further research into new targets and medications for MS fatigue.
His group has recently conducted a pilot study of intravenous ketamine in MS fatigue with some encouraging results, but he stressed it needs to be tested in a larger study before it can be recommended for use in clinical practice. “While an IV medication is not ideal, the effect did seem to be quite long-lived with a difference still evident at 28 days, so it could perhaps be dosed once a month, which could be feasible,” he said.
Commenting on the TRIUMPHANT study, Jeffrey Cohen, MD, of the Cleveland Clinic, said that “fatigue is a common, often disabling, symptom of MS. It is poorly understood and probably encompasses several mechanisms. There currently is no generally effective treatment for MS-related fatigue.”
“These results are not surprising and confirm previous studies,” Dr. Cohen said. “Despite no benefit from these medicines for patients as a group, they are occasionally helpful for individual patients, so they are frequently tried empirically.
“It also is important to address any factors besides MS that may be causing or contributing to fatigue, for example, sleep disruption, medication side effects, depression, other medical conditions such as anemia or hypothyroidism,” he added.
Dr. Nourbakhsh has reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities for Jazz Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Research News: Neurologic Disorders (FULL)
Modest Evidence for Benefit in Studies of Cannabis in MS
While several dozen studies have been conducted into cannabis-based treatments for symptoms of multiple sclerosis (MS), a new systematic review deems most to be of fair to poor quality. Reviewers found modest evidence of benefit and plenty of room for more research.
“Cannabis-based medicine may be useful for refractory MS symptoms, especially spasticity and pain, and side effects are usually well tolerated,” study lead author Natasha Breward, a graduate student at the College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, said in an interview. Breward spoke prior to the presentation of the study findings at the 2019 meeting of the Consortium of Multiple Sclerosis Centers.
For the review, Breward and colleagues focused on 60 studies—26 randomized controlled trials and 34 trials with other designs. Forty of the studies used nabiximols, an oromucosal spray that is derived from the cannabis sativa plant and approved for use in multiple countries but not yet in the US.
According to Breward, some of the other treatments included dried cannabis that is smoked or eaten and cannabidiol that’s typically delivered with tetrahydrocannabinol (THC) either oromucosally or as an oral capsule.
MS symptoms treated in the studies included spasticity (n = 29), pain (n = 8), and cognition (n = 6). The researchers considered 22 studies to be poor quality, 14 to be fair quality, and 24 to be good/excellent quality.
The researchers found that the cannabis-based medicine “significantly reduced spasticity and pain in several individual good-quality studies,” Breward said. The drugs seem to work by inhibiting neurotransmitter release via cannabinoids. “However, the variability in study quality—and in the products and regimens studied—make it hard to draw any conclusions about specific products and doses that may have the most potential benefit,” she added.
“Further research should focus on the use of different products and formulations of cannabis-based medicine such as cannabis oil and cannabidiol-prominent products, as no studies have focused on this area,” she said. “Research should also look at the potential of cannabis-based medicine for the treatment of disease progression, as cannabinoids are anti-inflammatory and immunomodulatory. Finally, more research regarding the potentially synergistic effects of cannabis-based medicine administered with current MS medications would also be useful.”
Randy Dotinga, MDedge.com/neurology
Brain Volumes After TBI Correlate With Clinical Features
Brain volumes of specific regions of interest can be used to classify traumatic brain injury subjects that fall into predetermined symptom categories, according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computed cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI.
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite (surfer.nmr.mgh.harvard.edu), which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations. Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction.
In an abstract, the researchers noted that their study is ongoing. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc; BrainBox Solutions, Inc; and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc and has received research support from BrainBox Solutions. The other authors reported no other disclosures.
Glenn S. Williams, MDedge.com/neurology
What Other Drugs Do Patients Take When They Start MS Therapy?
Concomitant medication use is common when patients with multiple sclerosis (MS) start disease-modifying drugs (DMDs), according to research presented at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA Real-World Data Adjudicated Claims–US database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with ≥ 2 MS diagnosis claims and at least 1 DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18 to 63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th revisions, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson Comorbidity Index was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged ≥ 55 years. Hyperlipidemia, hypertension, and diabetes mellitus were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
Jake Rem
Depression, Fatigue, Pain, and Anxiety Are Common in the Year After MS Diagnosis
In the 12 months after diagnosis, pain, fatigue, depression, and anxiety are common among patients with multiple sclerosis (MS), researchers reported at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and about 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for 1 condition, 21% for 2, 19% for 3, and 17% for all 4. Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
Jake Remaly, MDedge.com/neurology
Experts Propose New Definition and Recommendations for Alzheimer-like Disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain. “As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer, are seen in > 20% of individuals aged > 80 years, according to large, community-based autopsy series. It coexists with Alzheimer disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and colleagues noted.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer disease neuropathologic changes.
The authors proposed a 3-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus. The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E ∑ 4 allele, known to be a risk factor for Alzheimer disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report. LATE also could obscure the effects of potentially disease-modifying agents being tested in Alzheimer disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote. Dr. Nelson and coauthors reported no author disclosures.
Source: Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527.
Andrew D. Bowser, MDedge.com/neurology
Modest Evidence for Benefit in Studies of Cannabis in MS
While several dozen studies have been conducted into cannabis-based treatments for symptoms of multiple sclerosis (MS), a new systematic review deems most to be of fair to poor quality. Reviewers found modest evidence of benefit and plenty of room for more research.
“Cannabis-based medicine may be useful for refractory MS symptoms, especially spasticity and pain, and side effects are usually well tolerated,” study lead author Natasha Breward, a graduate student at the College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, said in an interview. Breward spoke prior to the presentation of the study findings at the 2019 meeting of the Consortium of Multiple Sclerosis Centers.
For the review, Breward and colleagues focused on 60 studies—26 randomized controlled trials and 34 trials with other designs. Forty of the studies used nabiximols, an oromucosal spray that is derived from the cannabis sativa plant and approved for use in multiple countries but not yet in the US.
According to Breward, some of the other treatments included dried cannabis that is smoked or eaten and cannabidiol that’s typically delivered with tetrahydrocannabinol (THC) either oromucosally or as an oral capsule.
MS symptoms treated in the studies included spasticity (n = 29), pain (n = 8), and cognition (n = 6). The researchers considered 22 studies to be poor quality, 14 to be fair quality, and 24 to be good/excellent quality.
The researchers found that the cannabis-based medicine “significantly reduced spasticity and pain in several individual good-quality studies,” Breward said. The drugs seem to work by inhibiting neurotransmitter release via cannabinoids. “However, the variability in study quality—and in the products and regimens studied—make it hard to draw any conclusions about specific products and doses that may have the most potential benefit,” she added.
“Further research should focus on the use of different products and formulations of cannabis-based medicine such as cannabis oil and cannabidiol-prominent products, as no studies have focused on this area,” she said. “Research should also look at the potential of cannabis-based medicine for the treatment of disease progression, as cannabinoids are anti-inflammatory and immunomodulatory. Finally, more research regarding the potentially synergistic effects of cannabis-based medicine administered with current MS medications would also be useful.”
Randy Dotinga, MDedge.com/neurology
Brain Volumes After TBI Correlate With Clinical Features
Brain volumes of specific regions of interest can be used to classify traumatic brain injury subjects that fall into predetermined symptom categories, according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computed cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI.
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite (surfer.nmr.mgh.harvard.edu), which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations. Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction.
In an abstract, the researchers noted that their study is ongoing. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc; BrainBox Solutions, Inc; and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc and has received research support from BrainBox Solutions. The other authors reported no other disclosures.
Glenn S. Williams, MDedge.com/neurology
What Other Drugs Do Patients Take When They Start MS Therapy?
Concomitant medication use is common when patients with multiple sclerosis (MS) start disease-modifying drugs (DMDs), according to research presented at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA Real-World Data Adjudicated Claims–US database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with ≥ 2 MS diagnosis claims and at least 1 DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18 to 63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th revisions, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson Comorbidity Index was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged ≥ 55 years. Hyperlipidemia, hypertension, and diabetes mellitus were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
Jake Rem
Depression, Fatigue, Pain, and Anxiety Are Common in the Year After MS Diagnosis
In the 12 months after diagnosis, pain, fatigue, depression, and anxiety are common among patients with multiple sclerosis (MS), researchers reported at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and about 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for 1 condition, 21% for 2, 19% for 3, and 17% for all 4. Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
Jake Remaly, MDedge.com/neurology
Experts Propose New Definition and Recommendations for Alzheimer-like Disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain. “As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer, are seen in > 20% of individuals aged > 80 years, according to large, community-based autopsy series. It coexists with Alzheimer disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and colleagues noted.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer disease neuropathologic changes.
The authors proposed a 3-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus. The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E ∑ 4 allele, known to be a risk factor for Alzheimer disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report. LATE also could obscure the effects of potentially disease-modifying agents being tested in Alzheimer disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote. Dr. Nelson and coauthors reported no author disclosures.
Source: Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527.
Andrew D. Bowser, MDedge.com/neurology
Modest Evidence for Benefit in Studies of Cannabis in MS
While several dozen studies have been conducted into cannabis-based treatments for symptoms of multiple sclerosis (MS), a new systematic review deems most to be of fair to poor quality. Reviewers found modest evidence of benefit and plenty of room for more research.
“Cannabis-based medicine may be useful for refractory MS symptoms, especially spasticity and pain, and side effects are usually well tolerated,” study lead author Natasha Breward, a graduate student at the College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, said in an interview. Breward spoke prior to the presentation of the study findings at the 2019 meeting of the Consortium of Multiple Sclerosis Centers.
For the review, Breward and colleagues focused on 60 studies—26 randomized controlled trials and 34 trials with other designs. Forty of the studies used nabiximols, an oromucosal spray that is derived from the cannabis sativa plant and approved for use in multiple countries but not yet in the US.
According to Breward, some of the other treatments included dried cannabis that is smoked or eaten and cannabidiol that’s typically delivered with tetrahydrocannabinol (THC) either oromucosally or as an oral capsule.
MS symptoms treated in the studies included spasticity (n = 29), pain (n = 8), and cognition (n = 6). The researchers considered 22 studies to be poor quality, 14 to be fair quality, and 24 to be good/excellent quality.
The researchers found that the cannabis-based medicine “significantly reduced spasticity and pain in several individual good-quality studies,” Breward said. The drugs seem to work by inhibiting neurotransmitter release via cannabinoids. “However, the variability in study quality—and in the products and regimens studied—make it hard to draw any conclusions about specific products and doses that may have the most potential benefit,” she added.
“Further research should focus on the use of different products and formulations of cannabis-based medicine such as cannabis oil and cannabidiol-prominent products, as no studies have focused on this area,” she said. “Research should also look at the potential of cannabis-based medicine for the treatment of disease progression, as cannabinoids are anti-inflammatory and immunomodulatory. Finally, more research regarding the potentially synergistic effects of cannabis-based medicine administered with current MS medications would also be useful.”
Randy Dotinga, MDedge.com/neurology
Brain Volumes After TBI Correlate With Clinical Features
Brain volumes of specific regions of interest can be used to classify traumatic brain injury subjects that fall into predetermined symptom categories, according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computed cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI.
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite (surfer.nmr.mgh.harvard.edu), which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations. Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction.
In an abstract, the researchers noted that their study is ongoing. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc; BrainBox Solutions, Inc; and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc and has received research support from BrainBox Solutions. The other authors reported no other disclosures.
Glenn S. Williams, MDedge.com/neurology
What Other Drugs Do Patients Take When They Start MS Therapy?
Concomitant medication use is common when patients with multiple sclerosis (MS) start disease-modifying drugs (DMDs), according to research presented at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA Real-World Data Adjudicated Claims–US database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with ≥ 2 MS diagnosis claims and at least 1 DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18 to 63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th revisions, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson Comorbidity Index was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged ≥ 55 years. Hyperlipidemia, hypertension, and diabetes mellitus were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
Jake Rem
Depression, Fatigue, Pain, and Anxiety Are Common in the Year After MS Diagnosis
In the 12 months after diagnosis, pain, fatigue, depression, and anxiety are common among patients with multiple sclerosis (MS), researchers reported at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and about 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for 1 condition, 21% for 2, 19% for 3, and 17% for all 4. Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
Jake Remaly, MDedge.com/neurology
Experts Propose New Definition and Recommendations for Alzheimer-like Disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain. “As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer, are seen in > 20% of individuals aged > 80 years, according to large, community-based autopsy series. It coexists with Alzheimer disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and colleagues noted.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer disease neuropathologic changes.
The authors proposed a 3-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus. The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E ∑ 4 allele, known to be a risk factor for Alzheimer disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report. LATE also could obscure the effects of potentially disease-modifying agents being tested in Alzheimer disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote. Dr. Nelson and coauthors reported no author disclosures.
Source: Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527.
Andrew D. Bowser, MDedge.com/neurology
Proton Pump Inhibitor Use and Risk of Dementia in the Veteran Population (FULL)
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.