User login
When and for whom is telehealth OCD treatment appropriate?
It is no secret that the COVID-19 pandemic resulted in widespread disruptions in health care services. While providers and resources were limited and many patients were apprehensive to present to health care settings out of concern of disease contraction, telehealth services did offer some relief.
Compared to other specialty care services, mental health care providers were well equipped to handle the expansion of telehealth services, as extensive treatment literature provides strong support for the use of psychotherapeutic interventions over telehealth mediums.1 This holds true in the context of obsessive-compulsive disorder (OCD), where an impressive literature supports the use of telehealth delivery for the gold-standard psychotherapeutic, exposure and response prevention (ERP).2,3,4
Through ERP, patients work with a clinician to systematically expose themselves to anxiety-providing triggers while actively resisting compulsive behaviors to learn the distress does go away with time and/or the distress is within their ability to cope. This intervention is conceptually similar to repeatedly watching a scary film, by which continued exposure results in less pronounced emotional reaction with subsequent viewings.
Fortunately for patients and providers, the expansion of telehealth ERP across different treatment settings has had many unintended benefits, including increased access to care, lower no-show rates due to the ease of attending appointments, and the ability to offer higher levels of care, including intensive outpatient programs, over telehealth mediums. Anecdotally, our clinic has been able to increase patient reach by providing telehealth ERP to those who historically would not have been able to access care due to geography. Even for those living within driving distance to our clinic, the ease of joining a video visit for a 45-minute appointment far outweighs driving into the clinic, in many circumstances. With these benefits, the delivery of ERP over telehealth appears likely to stay, although OCD providers delivering ERP will need to consider when and for whom this medium may not be appropriate.
To this end, we recently conducted a study examining ERP providers perceptions of telehealth and in-person ERP, patient characteristics best suited for telehealth services, and provider ability to identify and address factors that adversely impact the course of treatment (e.g., substance use, limited symptom insight, distractions during ERP, etc.).5 Providers reported lower feasibility ratings for telehealth compared to in-person ERP for younger patients (aged under 13 years), and patients with more severe OCD presentations. Providers also reported more difficulty identifying and addressing ERP interfering factors over telehealth relative to in-person. The findings from our research do not necessarily speak to the effectiveness of telehealth ERP, which has repeatedly been documented in treatment literature, but rather our findings highlight that ERP providers endorse reservations about the feasibility of ERP for certain OCD patient profiles, and that telehealth ERP may not be appropriate for all patients with OCD.
Mental health care providers, including those delivering ERP, should consider when telehealth is and is not appropriate. Importantly, telehealth offers a limited field of view compared to in-person, and providers can only observe what is captured by the camera. In the context of telehealth ERP, patients may engage in subtle avoidant behaviors that are more difficult to observe, which may prevent them from experiencing necessary anxiety during exposure practice. Many providers may have firsthand experience with this, or patients who appear distracted over telehealth mediums because of environmental factors that can be controlled for during in-person services.
As telehealth treatment options appear increasingly likely to stay, ERP providers and intervention researchers should continue identifying patient characteristics that are more and less appropriate for telehealth settings in order to maximize treatment outcomes. Providers should share concerns with patients when delivering telehealth ERP and work to address interfering factors impacting the course of treatment. In circumstances where this is not possible, such as when the patients age or symptom severity prevents effective telehealth ERP, or when treatment progress stalls, providers should speak with patients to determine if it would be beneficial to transition to in-person services.
Both in-person and telehealth ERP are fundamentally the same, however it does appear that subtle differences across these modalities may have differential impacts on treatment outcomes for certain OCD patient presentations. , however appropriate caution should be exhibited, and providers should use clinical judgment when offering telehealth services.
Dr. Wiese is a clinical psychologist in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston, Texas. He is primarily focused on conducting research on OCD and related disorders and providing empirically supported treatments to individuals diagnosed with these conditions.
References
1. Fernandez E et al. Live psychotherapy by video versus in‐person: A meta‐analysis of efficacy and its relationship to types and targets of treatment. Clin Psychol Psychother. 2021 Nov;28(6):1535-49. doi: 10.1002/cpp.2594.
2. Storch EA et al. Preliminary investigation of web-camera delivered cognitive-behavioral therapy for youth with obsessive-compulsive disorder. Psychiatry Res. 2011 Oct 30;189(3):407-12. doi: 10.1016/j.psychres.2011.05.047.
3. Fletcher TL et al. A pilot open trial of video telehealth-delivered exposure and response prevention for obsessive-compulsive disorder in rural Veterans. Mil Psychol. 2021 Oct 28;34(1):83-90. doi: 10.1080/08995605.2021.1970983.
4. Wootton BM. Remote cognitive–behavior therapy for obsessive–compulsive symptoms: a meta-analysis. Clin Psychol Rev. 2016 Feb;43:103-13. doi: 10.1016/j.cpr.2015.10.001.
5. Wiese AD et al. Provider perceptions of telehealth and in-person exposure and response prevention for obsessive–compulsive disorder. Psychiatry Res. 2022 Jul;313:114610. doi: 10.1016/j.psychres.2022.114610.
It is no secret that the COVID-19 pandemic resulted in widespread disruptions in health care services. While providers and resources were limited and many patients were apprehensive to present to health care settings out of concern of disease contraction, telehealth services did offer some relief.
Compared to other specialty care services, mental health care providers were well equipped to handle the expansion of telehealth services, as extensive treatment literature provides strong support for the use of psychotherapeutic interventions over telehealth mediums.1 This holds true in the context of obsessive-compulsive disorder (OCD), where an impressive literature supports the use of telehealth delivery for the gold-standard psychotherapeutic, exposure and response prevention (ERP).2,3,4
Through ERP, patients work with a clinician to systematically expose themselves to anxiety-providing triggers while actively resisting compulsive behaviors to learn the distress does go away with time and/or the distress is within their ability to cope. This intervention is conceptually similar to repeatedly watching a scary film, by which continued exposure results in less pronounced emotional reaction with subsequent viewings.
Fortunately for patients and providers, the expansion of telehealth ERP across different treatment settings has had many unintended benefits, including increased access to care, lower no-show rates due to the ease of attending appointments, and the ability to offer higher levels of care, including intensive outpatient programs, over telehealth mediums. Anecdotally, our clinic has been able to increase patient reach by providing telehealth ERP to those who historically would not have been able to access care due to geography. Even for those living within driving distance to our clinic, the ease of joining a video visit for a 45-minute appointment far outweighs driving into the clinic, in many circumstances. With these benefits, the delivery of ERP over telehealth appears likely to stay, although OCD providers delivering ERP will need to consider when and for whom this medium may not be appropriate.
To this end, we recently conducted a study examining ERP providers perceptions of telehealth and in-person ERP, patient characteristics best suited for telehealth services, and provider ability to identify and address factors that adversely impact the course of treatment (e.g., substance use, limited symptom insight, distractions during ERP, etc.).5 Providers reported lower feasibility ratings for telehealth compared to in-person ERP for younger patients (aged under 13 years), and patients with more severe OCD presentations. Providers also reported more difficulty identifying and addressing ERP interfering factors over telehealth relative to in-person. The findings from our research do not necessarily speak to the effectiveness of telehealth ERP, which has repeatedly been documented in treatment literature, but rather our findings highlight that ERP providers endorse reservations about the feasibility of ERP for certain OCD patient profiles, and that telehealth ERP may not be appropriate for all patients with OCD.
Mental health care providers, including those delivering ERP, should consider when telehealth is and is not appropriate. Importantly, telehealth offers a limited field of view compared to in-person, and providers can only observe what is captured by the camera. In the context of telehealth ERP, patients may engage in subtle avoidant behaviors that are more difficult to observe, which may prevent them from experiencing necessary anxiety during exposure practice. Many providers may have firsthand experience with this, or patients who appear distracted over telehealth mediums because of environmental factors that can be controlled for during in-person services.
As telehealth treatment options appear increasingly likely to stay, ERP providers and intervention researchers should continue identifying patient characteristics that are more and less appropriate for telehealth settings in order to maximize treatment outcomes. Providers should share concerns with patients when delivering telehealth ERP and work to address interfering factors impacting the course of treatment. In circumstances where this is not possible, such as when the patients age or symptom severity prevents effective telehealth ERP, or when treatment progress stalls, providers should speak with patients to determine if it would be beneficial to transition to in-person services.
Both in-person and telehealth ERP are fundamentally the same, however it does appear that subtle differences across these modalities may have differential impacts on treatment outcomes for certain OCD patient presentations. , however appropriate caution should be exhibited, and providers should use clinical judgment when offering telehealth services.
Dr. Wiese is a clinical psychologist in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston, Texas. He is primarily focused on conducting research on OCD and related disorders and providing empirically supported treatments to individuals diagnosed with these conditions.
References
1. Fernandez E et al. Live psychotherapy by video versus in‐person: A meta‐analysis of efficacy and its relationship to types and targets of treatment. Clin Psychol Psychother. 2021 Nov;28(6):1535-49. doi: 10.1002/cpp.2594.
2. Storch EA et al. Preliminary investigation of web-camera delivered cognitive-behavioral therapy for youth with obsessive-compulsive disorder. Psychiatry Res. 2011 Oct 30;189(3):407-12. doi: 10.1016/j.psychres.2011.05.047.
3. Fletcher TL et al. A pilot open trial of video telehealth-delivered exposure and response prevention for obsessive-compulsive disorder in rural Veterans. Mil Psychol. 2021 Oct 28;34(1):83-90. doi: 10.1080/08995605.2021.1970983.
4. Wootton BM. Remote cognitive–behavior therapy for obsessive–compulsive symptoms: a meta-analysis. Clin Psychol Rev. 2016 Feb;43:103-13. doi: 10.1016/j.cpr.2015.10.001.
5. Wiese AD et al. Provider perceptions of telehealth and in-person exposure and response prevention for obsessive–compulsive disorder. Psychiatry Res. 2022 Jul;313:114610. doi: 10.1016/j.psychres.2022.114610.
It is no secret that the COVID-19 pandemic resulted in widespread disruptions in health care services. While providers and resources were limited and many patients were apprehensive to present to health care settings out of concern of disease contraction, telehealth services did offer some relief.
Compared to other specialty care services, mental health care providers were well equipped to handle the expansion of telehealth services, as extensive treatment literature provides strong support for the use of psychotherapeutic interventions over telehealth mediums.1 This holds true in the context of obsessive-compulsive disorder (OCD), where an impressive literature supports the use of telehealth delivery for the gold-standard psychotherapeutic, exposure and response prevention (ERP).2,3,4
Through ERP, patients work with a clinician to systematically expose themselves to anxiety-providing triggers while actively resisting compulsive behaviors to learn the distress does go away with time and/or the distress is within their ability to cope. This intervention is conceptually similar to repeatedly watching a scary film, by which continued exposure results in less pronounced emotional reaction with subsequent viewings.
Fortunately for patients and providers, the expansion of telehealth ERP across different treatment settings has had many unintended benefits, including increased access to care, lower no-show rates due to the ease of attending appointments, and the ability to offer higher levels of care, including intensive outpatient programs, over telehealth mediums. Anecdotally, our clinic has been able to increase patient reach by providing telehealth ERP to those who historically would not have been able to access care due to geography. Even for those living within driving distance to our clinic, the ease of joining a video visit for a 45-minute appointment far outweighs driving into the clinic, in many circumstances. With these benefits, the delivery of ERP over telehealth appears likely to stay, although OCD providers delivering ERP will need to consider when and for whom this medium may not be appropriate.
To this end, we recently conducted a study examining ERP providers perceptions of telehealth and in-person ERP, patient characteristics best suited for telehealth services, and provider ability to identify and address factors that adversely impact the course of treatment (e.g., substance use, limited symptom insight, distractions during ERP, etc.).5 Providers reported lower feasibility ratings for telehealth compared to in-person ERP for younger patients (aged under 13 years), and patients with more severe OCD presentations. Providers also reported more difficulty identifying and addressing ERP interfering factors over telehealth relative to in-person. The findings from our research do not necessarily speak to the effectiveness of telehealth ERP, which has repeatedly been documented in treatment literature, but rather our findings highlight that ERP providers endorse reservations about the feasibility of ERP for certain OCD patient profiles, and that telehealth ERP may not be appropriate for all patients with OCD.
Mental health care providers, including those delivering ERP, should consider when telehealth is and is not appropriate. Importantly, telehealth offers a limited field of view compared to in-person, and providers can only observe what is captured by the camera. In the context of telehealth ERP, patients may engage in subtle avoidant behaviors that are more difficult to observe, which may prevent them from experiencing necessary anxiety during exposure practice. Many providers may have firsthand experience with this, or patients who appear distracted over telehealth mediums because of environmental factors that can be controlled for during in-person services.
As telehealth treatment options appear increasingly likely to stay, ERP providers and intervention researchers should continue identifying patient characteristics that are more and less appropriate for telehealth settings in order to maximize treatment outcomes. Providers should share concerns with patients when delivering telehealth ERP and work to address interfering factors impacting the course of treatment. In circumstances where this is not possible, such as when the patients age or symptom severity prevents effective telehealth ERP, or when treatment progress stalls, providers should speak with patients to determine if it would be beneficial to transition to in-person services.
Both in-person and telehealth ERP are fundamentally the same, however it does appear that subtle differences across these modalities may have differential impacts on treatment outcomes for certain OCD patient presentations. , however appropriate caution should be exhibited, and providers should use clinical judgment when offering telehealth services.
Dr. Wiese is a clinical psychologist in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston, Texas. He is primarily focused on conducting research on OCD and related disorders and providing empirically supported treatments to individuals diagnosed with these conditions.
References
1. Fernandez E et al. Live psychotherapy by video versus in‐person: A meta‐analysis of efficacy and its relationship to types and targets of treatment. Clin Psychol Psychother. 2021 Nov;28(6):1535-49. doi: 10.1002/cpp.2594.
2. Storch EA et al. Preliminary investigation of web-camera delivered cognitive-behavioral therapy for youth with obsessive-compulsive disorder. Psychiatry Res. 2011 Oct 30;189(3):407-12. doi: 10.1016/j.psychres.2011.05.047.
3. Fletcher TL et al. A pilot open trial of video telehealth-delivered exposure and response prevention for obsessive-compulsive disorder in rural Veterans. Mil Psychol. 2021 Oct 28;34(1):83-90. doi: 10.1080/08995605.2021.1970983.
4. Wootton BM. Remote cognitive–behavior therapy for obsessive–compulsive symptoms: a meta-analysis. Clin Psychol Rev. 2016 Feb;43:103-13. doi: 10.1016/j.cpr.2015.10.001.
5. Wiese AD et al. Provider perceptions of telehealth and in-person exposure and response prevention for obsessive–compulsive disorder. Psychiatry Res. 2022 Jul;313:114610. doi: 10.1016/j.psychres.2022.114610.
Compulsivity contributes to poor outcomes in body-focused repetitive behaviors
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Longer circadian rhythms linked to severe depression in teens
, according to results from a European study.
A range of psychiatric symptoms and conditions has been linked to sleep pathologies, wrote Liisa Kuula, PhD, of the University of Helsinki, Finland, and colleagues. Some research suggests that late circadian rhythms and irregular sleep patterns increase the risk for psychiatric conditions, but the association has not been well studied, especially in adolescents, although the onset of psychiatric problems often occurs at this age, they said.
In a study published in the Journal of Psychiatric Research (2022 Apr 4. doi: 10.1016/j.jpsychires.2022.03.056.), the investigators reviewed data from 342 adolescents who were part of SleepHelsinki! a large cohort study of delayed sleep phase disorder (DSPD) in adolescents. The mean age of the participants was 17.4 years, and 70% were female.
The participants completed the Mini International Neuropsychiatric Interview (MINI) and wore temperature loggers for 3 days to assess circadian rhythms. The primary outcome was the impact of circadian dynamics on different psychiatric problems. Delayed Sleep Phase (DSP) behavior was defined as going to sleep later than 1 a.m. at least three times a week.
Circadian length was determined through the temperature loggers worn for 3 days. Most participants also completed 1-week GeneActiv Original actigraphy measurements (wearing the actigraph for 1 week) and responded to the Morningness-Eveningness Questionnaire, which divided participants into three circadian preference groups: morning, intermediate, and evening. Sleep duration was calculated as total sleep time, sleep quality was estimated by sleep efficiency, and sleep timing was assessed by the midpoint of the sleep period.
Overall, the MINI interview results suggested that approximately one-third (36%) of the teens had at least one psychiatric problem, and 21% had comorbid conditions.
Severe depression was significantly associated with a longer circadian period (P = .002), while suicidality was significantly associated with a later midpoint and more irregular sleep (P = .007 for both).
Participants with agoraphobia slept longer than did those without, the researchers noted (P = .013). However, sleep duration was not significantly associated with other psychiatric conditions.
Manic episodes and psychotic disorders were associated with irregular sleep timing (P < .018 and P < .017, respectively).
When the researchers examined DSP and circadian preferences, they found that 21.5% of individuals with suicidality had characteristics of DSP, as did 21.5% of those with panic disorder.
Individuals with a preference for eveningness were significantly more likely to meet criteria for severe depression, panic disorder, generalized anxiety disorder, and obsessive-compulsive disorder than were those without a preference for eveningness, the researchers noted.
“Our findings are the first to encompass diverse circadian measures alongside an array of psychiatric symptoms in such a focused age range,” the researchers wrote in their discussion. The data reflect results from other studies and extend the likely role of circadian patterns in mental wellbeing, they said.
The study findings were limited by several factors including the lack of actual diagnoses from medical records and use of self-reported symptoms, the researchers noted. Other limitations included the lack of polysomnography data and small size of subgroups of the study sample.
However, the results were strengthened by the heterogenous study population and use of multiple measures to examine sleep and circadian rhythms, as well as consideration of personal circadian preferences, the researchers said.
“The importance of overall synchronization with environment is perhaps best highlighted by response to treatment: most psychopathologic symptoms benefit from sleep-targeted therapeutic approaches,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
, according to results from a European study.
A range of psychiatric symptoms and conditions has been linked to sleep pathologies, wrote Liisa Kuula, PhD, of the University of Helsinki, Finland, and colleagues. Some research suggests that late circadian rhythms and irregular sleep patterns increase the risk for psychiatric conditions, but the association has not been well studied, especially in adolescents, although the onset of psychiatric problems often occurs at this age, they said.
In a study published in the Journal of Psychiatric Research (2022 Apr 4. doi: 10.1016/j.jpsychires.2022.03.056.), the investigators reviewed data from 342 adolescents who were part of SleepHelsinki! a large cohort study of delayed sleep phase disorder (DSPD) in adolescents. The mean age of the participants was 17.4 years, and 70% were female.
The participants completed the Mini International Neuropsychiatric Interview (MINI) and wore temperature loggers for 3 days to assess circadian rhythms. The primary outcome was the impact of circadian dynamics on different psychiatric problems. Delayed Sleep Phase (DSP) behavior was defined as going to sleep later than 1 a.m. at least three times a week.
Circadian length was determined through the temperature loggers worn for 3 days. Most participants also completed 1-week GeneActiv Original actigraphy measurements (wearing the actigraph for 1 week) and responded to the Morningness-Eveningness Questionnaire, which divided participants into three circadian preference groups: morning, intermediate, and evening. Sleep duration was calculated as total sleep time, sleep quality was estimated by sleep efficiency, and sleep timing was assessed by the midpoint of the sleep period.
Overall, the MINI interview results suggested that approximately one-third (36%) of the teens had at least one psychiatric problem, and 21% had comorbid conditions.
Severe depression was significantly associated with a longer circadian period (P = .002), while suicidality was significantly associated with a later midpoint and more irregular sleep (P = .007 for both).
Participants with agoraphobia slept longer than did those without, the researchers noted (P = .013). However, sleep duration was not significantly associated with other psychiatric conditions.
Manic episodes and psychotic disorders were associated with irregular sleep timing (P < .018 and P < .017, respectively).
When the researchers examined DSP and circadian preferences, they found that 21.5% of individuals with suicidality had characteristics of DSP, as did 21.5% of those with panic disorder.
Individuals with a preference for eveningness were significantly more likely to meet criteria for severe depression, panic disorder, generalized anxiety disorder, and obsessive-compulsive disorder than were those without a preference for eveningness, the researchers noted.
“Our findings are the first to encompass diverse circadian measures alongside an array of psychiatric symptoms in such a focused age range,” the researchers wrote in their discussion. The data reflect results from other studies and extend the likely role of circadian patterns in mental wellbeing, they said.
The study findings were limited by several factors including the lack of actual diagnoses from medical records and use of self-reported symptoms, the researchers noted. Other limitations included the lack of polysomnography data and small size of subgroups of the study sample.
However, the results were strengthened by the heterogenous study population and use of multiple measures to examine sleep and circadian rhythms, as well as consideration of personal circadian preferences, the researchers said.
“The importance of overall synchronization with environment is perhaps best highlighted by response to treatment: most psychopathologic symptoms benefit from sleep-targeted therapeutic approaches,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
, according to results from a European study.
A range of psychiatric symptoms and conditions has been linked to sleep pathologies, wrote Liisa Kuula, PhD, of the University of Helsinki, Finland, and colleagues. Some research suggests that late circadian rhythms and irregular sleep patterns increase the risk for psychiatric conditions, but the association has not been well studied, especially in adolescents, although the onset of psychiatric problems often occurs at this age, they said.
In a study published in the Journal of Psychiatric Research (2022 Apr 4. doi: 10.1016/j.jpsychires.2022.03.056.), the investigators reviewed data from 342 adolescents who were part of SleepHelsinki! a large cohort study of delayed sleep phase disorder (DSPD) in adolescents. The mean age of the participants was 17.4 years, and 70% were female.
The participants completed the Mini International Neuropsychiatric Interview (MINI) and wore temperature loggers for 3 days to assess circadian rhythms. The primary outcome was the impact of circadian dynamics on different psychiatric problems. Delayed Sleep Phase (DSP) behavior was defined as going to sleep later than 1 a.m. at least three times a week.
Circadian length was determined through the temperature loggers worn for 3 days. Most participants also completed 1-week GeneActiv Original actigraphy measurements (wearing the actigraph for 1 week) and responded to the Morningness-Eveningness Questionnaire, which divided participants into three circadian preference groups: morning, intermediate, and evening. Sleep duration was calculated as total sleep time, sleep quality was estimated by sleep efficiency, and sleep timing was assessed by the midpoint of the sleep period.
Overall, the MINI interview results suggested that approximately one-third (36%) of the teens had at least one psychiatric problem, and 21% had comorbid conditions.
Severe depression was significantly associated with a longer circadian period (P = .002), while suicidality was significantly associated with a later midpoint and more irregular sleep (P = .007 for both).
Participants with agoraphobia slept longer than did those without, the researchers noted (P = .013). However, sleep duration was not significantly associated with other psychiatric conditions.
Manic episodes and psychotic disorders were associated with irregular sleep timing (P < .018 and P < .017, respectively).
When the researchers examined DSP and circadian preferences, they found that 21.5% of individuals with suicidality had characteristics of DSP, as did 21.5% of those with panic disorder.
Individuals with a preference for eveningness were significantly more likely to meet criteria for severe depression, panic disorder, generalized anxiety disorder, and obsessive-compulsive disorder than were those without a preference for eveningness, the researchers noted.
“Our findings are the first to encompass diverse circadian measures alongside an array of psychiatric symptoms in such a focused age range,” the researchers wrote in their discussion. The data reflect results from other studies and extend the likely role of circadian patterns in mental wellbeing, they said.
The study findings were limited by several factors including the lack of actual diagnoses from medical records and use of self-reported symptoms, the researchers noted. Other limitations included the lack of polysomnography data and small size of subgroups of the study sample.
However, the results were strengthened by the heterogenous study population and use of multiple measures to examine sleep and circadian rhythms, as well as consideration of personal circadian preferences, the researchers said.
“The importance of overall synchronization with environment is perhaps best highlighted by response to treatment: most psychopathologic symptoms benefit from sleep-targeted therapeutic approaches,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Multiple mental health woes? Blame it on genetics
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
Cluttered consciousness: The mental effects of growing up with a hoarder
Many of us are reluctant to throw things out.
We buy. We accumulate. We collect. Eventually our attics are packed with dusty heirlooms that we rarely, if ever, look at. Eventually we’re forced to pare down and head to the Goodwill.
But not all of us.
Hoarding – or the prolonged difficulty of discarding unneeded possessions – is pervasive in our culture, affecting nearly 3% of the population. This compulsive collecting, and unwillingness to part with “stuff,” is even the subject of multiple popular television series.
How do you conceptualize hoarding behavior?
The core feature of hoarding is the inability to throw things away. This can be due to many different reasons, whether there’s a strong sentimental attachment or the belief that you will need these items one day. Compulsive buying is often involved, and inevitable clutter.
How was hoarding first conceptualized among psychiatrists and psychologists? And when did the term first enter the lexicon?
It was originally conceptualized as a difficult-to-treat subtype of obsessive-compulsive disorder (OCD). A lot of that work identifying this subgroup was going on in the late 1980s and early 1990s. There was a small but growing group of researchers demonstrating that this is fundamentally different from OCD in several ways.
In terms of the clinical presentation, the comorbidity patterns are different from those for OCD. And the course is a little bit different; we see a progressive development across the lifespan, as opposed to a clear-cut diagnosis earlier in life, as is typically seen with OCD. By the time a lot of people seek treatment, they’re often being brought in by, say, family members when they’re a little bit older. With hoarding, there is also this consistent pattern of poor treatment response across the board, whether to selective serotonin reuptake inhibitors or behavioral therapy.
A lot of this work together led to advocacy for recognizing hoarding as an independent diagnosis in the DSM-5. I think official recognition by our “big book” prompted more attention to this population. Previously these patients probably would have been diagnosed with OCD, and it really isn’t appropriate to think of hoarding as purely an anxiety disorder.
Hoarding exposure and future mental health
You have a new study, published in Annals of Clinical Psychiatry, looking at mental health among adult children of parents with hoarding problems. Can you tell us what inspired you to run this study, and what you found?
There were a couple of factors.
We’d seen a lot of folks with hoarding in OCD specialty clinics, so my clinical experiences with this population certainly drew me to this general area. But then, at the same time, I have this broad training in child mental health. And childhood trauma or adverse childhood experiences, which can include being around hoarding, can be a very difficult thing to live through and deal with. And here I have to give a lot of credit to Suzanne Chabaud, PhD, of the OCD Institute of Greater New Orleans, who’s one of the coauthors on the paper. She’s been beating the drum of thinking about the family and kids of people with hoarding disorders for years. My interests came from some of those experiences, but she had the good idea of really looking at this problem in a detailed way.
Prior to your paper, had there been research on the prevalence of mental illnesses such as anxiety and depression in the children of people with hoarding behaviors?
That particular question was new to our paper. It was the first time anyone, to my knowledge, had looked at a validated assessment of anxiety and depression in this population.
How did you assess their symptoms and what did you find?
We asked study participants to think back on how they felt throughout their teenage years and gauged their responses with the Patient Health Questionnaire (PHQ), a measure of mental health disorders. I should say up front that we didn’t have a control group. But we found that among our 414 study participants, somewhere between 30% and 50% reported clinically significant anxiety or depressive symptoms, far higher than you’d expect in the normal population. So when looking back on how they were feeling as teenagers in that environment, they were struggling, and they often felt rejected by their parents.
We also found that almost 10% of participants were threatened with eviction at some point in their childhood; 15% had to live outside of their home at some point, because of the clutter; and 2% had involvement from child protective services and were removed from the home.
I know you recruited patients from online forums established by the children of hoarding parents. Presumably, these are the people most affected by this phenomenon. How does this play out in people who simply like to, say, collect something? Is this a continuum of behavior, with a breaking point at which it becomes a pathology?
I think it’s safe to conceptualize collecting and hoarding as a continuum, and you’ve got to draw a line somewhere in terms of clinical significance.
Did you assess whether the children of hoarders were more likely to hoard themselves as adults?
This is our follow-up paper; we haven’t looked at it yet.
But in looking at preliminary data, the prevalence seems pretty low, actually, at least in our sample. And as you mentioned, in our study there were folks who were seeking support specifically because they grew up in a really cluttered home.
Management
How do mental health providers typically address and treat hoarding?
To my knowledge, there are no current Food and Drug Administration–approved medications for hoarding, though psychiatrists will prescribe SSRIs and try to treat co-occurring problems such as depression and anxiety symptoms.
I can speak to cognitive-behavioral therapy (CBT) in a bit more detail. A number of randomized controlled trials support CBT for hoarding. I mentioned before that when we as a field treated hoarding akin to OCD and did exposure and response prevention therapy, we didn’t really target the specific features of hoarding. People didn’t do that well.
But now researchers are focusing on CBT interventions focused on discarding tasks that really address hoarding. You can create different categories for different items: Patients can either keep them, throw them out, or donate them. You can explore what thoughts or expectations are associated with these items and try to address them. Clinicians can help patients look at, say, different areas of their house and discuss what they might be willing to part with or at least think about parting with. You find their internal motivations for keeping things.
This sort of therapy generally takes longer than it does for, say, OCD. It can be a little bit slower, particularly if someone has a lot of stuff. And often it can involve doing home visits. In the age of Zoom this is a little bit easier because home visits aren’t always feasible.
What role does family play in managing hoarding? I imagine that including loved ones and friends in the process could be quite helpful.
Yes, absolutely. And social support, more broadly.
A colleague I worked with did a really interesting study where she looked at psychologist-delivered versus peer-delivered CBT for hoarding. They found that the biggest predictor of improved outcomes was having what they called a “clutter buddy,” which follows the Alcoholics Anonymous sponsor model. This would be somebody else struggling with the same problem who’s an accountability partner helping a patient follow through with their goals related to discarding. I think that finding underscores how important that social support is.
Any final thoughts for our audience of clinicians and researchers on how to approach hoarding?
I think there’s been a stigma – at least in psychology circles – that it’s not really treatable because of that earlier work with OCD. But on the CBT side, there’s now good reason to believe that people can live much happier lives and overcome this problem. CBT does seem to work for a lot of people with hoarding. That’s what I’d like to emphasize.
Dr. Stetka is executive editor for Medscape. A version of this article first appeared on Medscape.com.
Many of us are reluctant to throw things out.
We buy. We accumulate. We collect. Eventually our attics are packed with dusty heirlooms that we rarely, if ever, look at. Eventually we’re forced to pare down and head to the Goodwill.
But not all of us.
Hoarding – or the prolonged difficulty of discarding unneeded possessions – is pervasive in our culture, affecting nearly 3% of the population. This compulsive collecting, and unwillingness to part with “stuff,” is even the subject of multiple popular television series.
How do you conceptualize hoarding behavior?
The core feature of hoarding is the inability to throw things away. This can be due to many different reasons, whether there’s a strong sentimental attachment or the belief that you will need these items one day. Compulsive buying is often involved, and inevitable clutter.
How was hoarding first conceptualized among psychiatrists and psychologists? And when did the term first enter the lexicon?
It was originally conceptualized as a difficult-to-treat subtype of obsessive-compulsive disorder (OCD). A lot of that work identifying this subgroup was going on in the late 1980s and early 1990s. There was a small but growing group of researchers demonstrating that this is fundamentally different from OCD in several ways.
In terms of the clinical presentation, the comorbidity patterns are different from those for OCD. And the course is a little bit different; we see a progressive development across the lifespan, as opposed to a clear-cut diagnosis earlier in life, as is typically seen with OCD. By the time a lot of people seek treatment, they’re often being brought in by, say, family members when they’re a little bit older. With hoarding, there is also this consistent pattern of poor treatment response across the board, whether to selective serotonin reuptake inhibitors or behavioral therapy.
A lot of this work together led to advocacy for recognizing hoarding as an independent diagnosis in the DSM-5. I think official recognition by our “big book” prompted more attention to this population. Previously these patients probably would have been diagnosed with OCD, and it really isn’t appropriate to think of hoarding as purely an anxiety disorder.
Hoarding exposure and future mental health
You have a new study, published in Annals of Clinical Psychiatry, looking at mental health among adult children of parents with hoarding problems. Can you tell us what inspired you to run this study, and what you found?
There were a couple of factors.
We’d seen a lot of folks with hoarding in OCD specialty clinics, so my clinical experiences with this population certainly drew me to this general area. But then, at the same time, I have this broad training in child mental health. And childhood trauma or adverse childhood experiences, which can include being around hoarding, can be a very difficult thing to live through and deal with. And here I have to give a lot of credit to Suzanne Chabaud, PhD, of the OCD Institute of Greater New Orleans, who’s one of the coauthors on the paper. She’s been beating the drum of thinking about the family and kids of people with hoarding disorders for years. My interests came from some of those experiences, but she had the good idea of really looking at this problem in a detailed way.
Prior to your paper, had there been research on the prevalence of mental illnesses such as anxiety and depression in the children of people with hoarding behaviors?
That particular question was new to our paper. It was the first time anyone, to my knowledge, had looked at a validated assessment of anxiety and depression in this population.
How did you assess their symptoms and what did you find?
We asked study participants to think back on how they felt throughout their teenage years and gauged their responses with the Patient Health Questionnaire (PHQ), a measure of mental health disorders. I should say up front that we didn’t have a control group. But we found that among our 414 study participants, somewhere between 30% and 50% reported clinically significant anxiety or depressive symptoms, far higher than you’d expect in the normal population. So when looking back on how they were feeling as teenagers in that environment, they were struggling, and they often felt rejected by their parents.
We also found that almost 10% of participants were threatened with eviction at some point in their childhood; 15% had to live outside of their home at some point, because of the clutter; and 2% had involvement from child protective services and were removed from the home.
I know you recruited patients from online forums established by the children of hoarding parents. Presumably, these are the people most affected by this phenomenon. How does this play out in people who simply like to, say, collect something? Is this a continuum of behavior, with a breaking point at which it becomes a pathology?
I think it’s safe to conceptualize collecting and hoarding as a continuum, and you’ve got to draw a line somewhere in terms of clinical significance.
Did you assess whether the children of hoarders were more likely to hoard themselves as adults?
This is our follow-up paper; we haven’t looked at it yet.
But in looking at preliminary data, the prevalence seems pretty low, actually, at least in our sample. And as you mentioned, in our study there were folks who were seeking support specifically because they grew up in a really cluttered home.
Management
How do mental health providers typically address and treat hoarding?
To my knowledge, there are no current Food and Drug Administration–approved medications for hoarding, though psychiatrists will prescribe SSRIs and try to treat co-occurring problems such as depression and anxiety symptoms.
I can speak to cognitive-behavioral therapy (CBT) in a bit more detail. A number of randomized controlled trials support CBT for hoarding. I mentioned before that when we as a field treated hoarding akin to OCD and did exposure and response prevention therapy, we didn’t really target the specific features of hoarding. People didn’t do that well.
But now researchers are focusing on CBT interventions focused on discarding tasks that really address hoarding. You can create different categories for different items: Patients can either keep them, throw them out, or donate them. You can explore what thoughts or expectations are associated with these items and try to address them. Clinicians can help patients look at, say, different areas of their house and discuss what they might be willing to part with or at least think about parting with. You find their internal motivations for keeping things.
This sort of therapy generally takes longer than it does for, say, OCD. It can be a little bit slower, particularly if someone has a lot of stuff. And often it can involve doing home visits. In the age of Zoom this is a little bit easier because home visits aren’t always feasible.
What role does family play in managing hoarding? I imagine that including loved ones and friends in the process could be quite helpful.
Yes, absolutely. And social support, more broadly.
A colleague I worked with did a really interesting study where she looked at psychologist-delivered versus peer-delivered CBT for hoarding. They found that the biggest predictor of improved outcomes was having what they called a “clutter buddy,” which follows the Alcoholics Anonymous sponsor model. This would be somebody else struggling with the same problem who’s an accountability partner helping a patient follow through with their goals related to discarding. I think that finding underscores how important that social support is.
Any final thoughts for our audience of clinicians and researchers on how to approach hoarding?
I think there’s been a stigma – at least in psychology circles – that it’s not really treatable because of that earlier work with OCD. But on the CBT side, there’s now good reason to believe that people can live much happier lives and overcome this problem. CBT does seem to work for a lot of people with hoarding. That’s what I’d like to emphasize.
Dr. Stetka is executive editor for Medscape. A version of this article first appeared on Medscape.com.
Many of us are reluctant to throw things out.
We buy. We accumulate. We collect. Eventually our attics are packed with dusty heirlooms that we rarely, if ever, look at. Eventually we’re forced to pare down and head to the Goodwill.
But not all of us.
Hoarding – or the prolonged difficulty of discarding unneeded possessions – is pervasive in our culture, affecting nearly 3% of the population. This compulsive collecting, and unwillingness to part with “stuff,” is even the subject of multiple popular television series.
How do you conceptualize hoarding behavior?
The core feature of hoarding is the inability to throw things away. This can be due to many different reasons, whether there’s a strong sentimental attachment or the belief that you will need these items one day. Compulsive buying is often involved, and inevitable clutter.
How was hoarding first conceptualized among psychiatrists and psychologists? And when did the term first enter the lexicon?
It was originally conceptualized as a difficult-to-treat subtype of obsessive-compulsive disorder (OCD). A lot of that work identifying this subgroup was going on in the late 1980s and early 1990s. There was a small but growing group of researchers demonstrating that this is fundamentally different from OCD in several ways.
In terms of the clinical presentation, the comorbidity patterns are different from those for OCD. And the course is a little bit different; we see a progressive development across the lifespan, as opposed to a clear-cut diagnosis earlier in life, as is typically seen with OCD. By the time a lot of people seek treatment, they’re often being brought in by, say, family members when they’re a little bit older. With hoarding, there is also this consistent pattern of poor treatment response across the board, whether to selective serotonin reuptake inhibitors or behavioral therapy.
A lot of this work together led to advocacy for recognizing hoarding as an independent diagnosis in the DSM-5. I think official recognition by our “big book” prompted more attention to this population. Previously these patients probably would have been diagnosed with OCD, and it really isn’t appropriate to think of hoarding as purely an anxiety disorder.
Hoarding exposure and future mental health
You have a new study, published in Annals of Clinical Psychiatry, looking at mental health among adult children of parents with hoarding problems. Can you tell us what inspired you to run this study, and what you found?
There were a couple of factors.
We’d seen a lot of folks with hoarding in OCD specialty clinics, so my clinical experiences with this population certainly drew me to this general area. But then, at the same time, I have this broad training in child mental health. And childhood trauma or adverse childhood experiences, which can include being around hoarding, can be a very difficult thing to live through and deal with. And here I have to give a lot of credit to Suzanne Chabaud, PhD, of the OCD Institute of Greater New Orleans, who’s one of the coauthors on the paper. She’s been beating the drum of thinking about the family and kids of people with hoarding disorders for years. My interests came from some of those experiences, but she had the good idea of really looking at this problem in a detailed way.
Prior to your paper, had there been research on the prevalence of mental illnesses such as anxiety and depression in the children of people with hoarding behaviors?
That particular question was new to our paper. It was the first time anyone, to my knowledge, had looked at a validated assessment of anxiety and depression in this population.
How did you assess their symptoms and what did you find?
We asked study participants to think back on how they felt throughout their teenage years and gauged their responses with the Patient Health Questionnaire (PHQ), a measure of mental health disorders. I should say up front that we didn’t have a control group. But we found that among our 414 study participants, somewhere between 30% and 50% reported clinically significant anxiety or depressive symptoms, far higher than you’d expect in the normal population. So when looking back on how they were feeling as teenagers in that environment, they were struggling, and they often felt rejected by their parents.
We also found that almost 10% of participants were threatened with eviction at some point in their childhood; 15% had to live outside of their home at some point, because of the clutter; and 2% had involvement from child protective services and were removed from the home.
I know you recruited patients from online forums established by the children of hoarding parents. Presumably, these are the people most affected by this phenomenon. How does this play out in people who simply like to, say, collect something? Is this a continuum of behavior, with a breaking point at which it becomes a pathology?
I think it’s safe to conceptualize collecting and hoarding as a continuum, and you’ve got to draw a line somewhere in terms of clinical significance.
Did you assess whether the children of hoarders were more likely to hoard themselves as adults?
This is our follow-up paper; we haven’t looked at it yet.
But in looking at preliminary data, the prevalence seems pretty low, actually, at least in our sample. And as you mentioned, in our study there were folks who were seeking support specifically because they grew up in a really cluttered home.
Management
How do mental health providers typically address and treat hoarding?
To my knowledge, there are no current Food and Drug Administration–approved medications for hoarding, though psychiatrists will prescribe SSRIs and try to treat co-occurring problems such as depression and anxiety symptoms.
I can speak to cognitive-behavioral therapy (CBT) in a bit more detail. A number of randomized controlled trials support CBT for hoarding. I mentioned before that when we as a field treated hoarding akin to OCD and did exposure and response prevention therapy, we didn’t really target the specific features of hoarding. People didn’t do that well.
But now researchers are focusing on CBT interventions focused on discarding tasks that really address hoarding. You can create different categories for different items: Patients can either keep them, throw them out, or donate them. You can explore what thoughts or expectations are associated with these items and try to address them. Clinicians can help patients look at, say, different areas of their house and discuss what they might be willing to part with or at least think about parting with. You find their internal motivations for keeping things.
This sort of therapy generally takes longer than it does for, say, OCD. It can be a little bit slower, particularly if someone has a lot of stuff. And often it can involve doing home visits. In the age of Zoom this is a little bit easier because home visits aren’t always feasible.
What role does family play in managing hoarding? I imagine that including loved ones and friends in the process could be quite helpful.
Yes, absolutely. And social support, more broadly.
A colleague I worked with did a really interesting study where she looked at psychologist-delivered versus peer-delivered CBT for hoarding. They found that the biggest predictor of improved outcomes was having what they called a “clutter buddy,” which follows the Alcoholics Anonymous sponsor model. This would be somebody else struggling with the same problem who’s an accountability partner helping a patient follow through with their goals related to discarding. I think that finding underscores how important that social support is.
Any final thoughts for our audience of clinicians and researchers on how to approach hoarding?
I think there’s been a stigma – at least in psychology circles – that it’s not really treatable because of that earlier work with OCD. But on the CBT side, there’s now good reason to believe that people can live much happier lives and overcome this problem. CBT does seem to work for a lot of people with hoarding. That’s what I’d like to emphasize.
Dr. Stetka is executive editor for Medscape. A version of this article first appeared on Medscape.com.
Trichotillomania: What you should know about this common hair-pulling disorder
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Treatment augmentation strategies for OCD: A review of 8 studies
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.
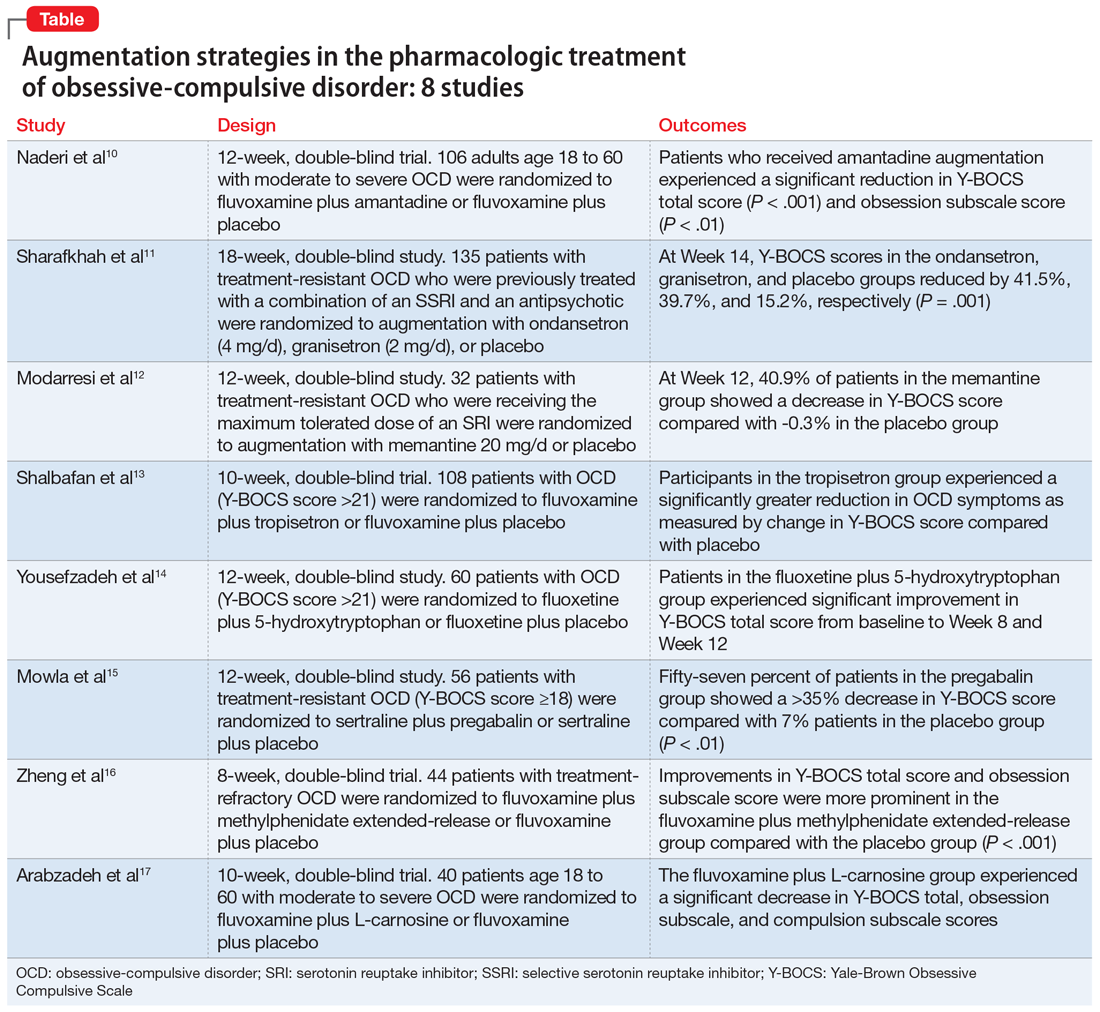
Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.

Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.

Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
DSM-5 update: What’s new?
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Psychiatry and semantics
I am a psychiatrist, which means I am a mental health professional, which means I work with people with mental illness. Sometimes people with mental health conditions who suffer from mental illness need to take a day off work – also called a mental health day – because they are too symptomatic to work, and sometimes people who don’t have a mental illness need to take a day off work, also called a mental health day, because they are feeling stressed.
Sometimes professional athletes don’t do things they agreed to do in their contracts because they realize that doing these things is very upsetting and will be detrimental to their mental health, or maybe they have a mental illness and doing these things will worsen their mental health condition, which is, in fact, a mental illness. Other times people with mental health conditions need to have pets travel with them because this mitigates the symptoms of their mental illness or perhaps it’s just good for their mental health. And finally, some people suffer from mental illnesses, or sometimes from learning problems, which are severe enough that a person with these conditions has a disability and needs special accommodations to function optimally in educational or occupational settings, or needs public financial support because their difficulties disable them to the point that they can’t work at all.
Is your head spinning yet? who we serve, and differentiating the fact that what someone with a psychiatric disorder needs to do to function or to alleviate emotional suffering may be entirely different from the things that everyone needs to do, regardless of whether they have a psychiatric disorder, to feel their emotional best.
The National Alliance on Mental Illness tells us that one in five Americans are suffering from a mental illness, while the Epidemiologic Catchment Area Program revealed that half of people will meet criteria for a mental illness at some point in their lives. We hear about “the mentally ill” constantly in the news – often in relation to mass shooters or homelessness – yet even psychiatrists might be pressed to define who exactly the “mentally ill” are. And how many of us could not somehow, at some time, find ourselves in 1 of the 157 disorders that DSM-5 lists – down from 365 disorders in the DSM-IV-TR?
Differentiating mental health from mental illness is just the beginning of our semantic confusion. As psychiatrists we treat major depression, and yet the illness “depression,” a syndromic constellation of symptoms, includes the key symptom of sadness. People often say they are “depressed” when they mean they are sad or demoralized, and yet, if their sadness persists in the absence of other symptoms, they may well want, or feel they “should” have medications, even in the absence of a disorder. And maybe those medications help them feel better, so that the presence or absence of a verified illness doesn’t really matter. But if the medications cause adverse reactions, then psychiatry might have done a better job by that person’s sadness. Melancholia, or perhaps any designation than “depression,” with its multiple meanings, might better serve our patients and our profession. This is only one example, as the number of people who tell me they have obsessive-compulsive disorder – or more often announce, “I’m OCD!” because they are well organized in a productive way is remarkable. And while I have treated only a few people who meet the criteria for narcissistic personality disorder, from general conversation it would seem that they are at every dinner table and by every water cooler.
Does it matter? A diagnostic lexicon can be so helpful when it guides treatment, provides a heterogeneous group of patients for research studies, and allows for an understanding of the etiology, course, and prognosis of a given condition. When someone is so depressed that they can’t get out of bed, or is so disorganized that they can’t perform their job and might cause a disturbance in their workplace, it is good to instruct them to take time off work and send them back well with a doctor’s note. But this is different from the person who doesn’t want to face a difficult situation, who simply doesn’t like their job or their boss, or who wants their pet declared an emotional support animal to avoid the fee the airlines charge to bring an animal on board if one does not have a psychiatric diagnosis. Sometimes these lines are blurry – if someone does not want to do something because it makes them anxious, does it matter how deep the pit in their stomach is, or if they are having full-blown panic attacks? When do we agree that their distress is reason to allow them to avoid responsibilities without repercussions versus a violation of their obligations and an infringement on others?
Diagnoses offer solace to some patients: There is a name for their suffering, available treatment, and often others with the same condition to look to for guidance and community. For others, a psychiatric diagnosis is a source of shame, a label they see as damaging to their character and sometimes to their careers – including in medicine – where we have been particularly unsympathetic to those who announce a psychiatric history.
In some cultures, the label itself decreases someone’s attractiveness as a potential marriage partner. We would all like to see the stigma of mental illness vanish, but we have a long way to go.
Psychiatric diagnoses move over time and with our politics and culture. This is good; we don’t hold on to what we learn to be untrue. But they may well add to issues of inequity. Those who can afford to pay for expensive educational assessments can request educational accommodations, including untimed standardized tests. This advantage may not be available to those without the resources to pay for these evaluations, and one might wonder why all comers can’t take untimed tests so as not to favor the privileged. Psychiatry has long been accused of diagnosing people of color with poor prognosis illnesses and women with conditions that imply emotional weakness.
While our diagnoses have clinical utility, it is unfortunate that they have come to be about reimbursement. A diagnosis needs to be assigned for insurers to pay for care, and so we create diagnostic categories to allow for treatment. Is this reasonable? Do we need to say that someone who is suffering after the death of a loved one has a mental illness in order to allow them to seek relief from their suffering? It leads us to believe that all suffering is about pathology, that we should expect pain-free emotional lives. Perhaps we need a diagnostic category of psychic pain, not otherwise specified, to allow for treatment for those who simply ache.
Mental illness is about interventions to alleviate the suffering of those with disorders. Mental health is about interventions that may benefit everyone, whether they suffer from a mental illness or not. Sleep, nutrition, exercise, sunlight, nature, entertainment and escape, yoga, meditation, vacations in beautiful places with loving people – these are things that potentially help us all whether we do or do not have an illness. With so much confusion about what it is we do, and about who “should” get help, who can get help, who might want help, and where they should go to seek help, perhaps it would be better if our lingo were more precise.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). The has a private practice and is assistant professor of psychiatry ad behavioral sciences at Johns Hopkins University, both in Baltimore. She has no disclosures.
I am a psychiatrist, which means I am a mental health professional, which means I work with people with mental illness. Sometimes people with mental health conditions who suffer from mental illness need to take a day off work – also called a mental health day – because they are too symptomatic to work, and sometimes people who don’t have a mental illness need to take a day off work, also called a mental health day, because they are feeling stressed.
Sometimes professional athletes don’t do things they agreed to do in their contracts because they realize that doing these things is very upsetting and will be detrimental to their mental health, or maybe they have a mental illness and doing these things will worsen their mental health condition, which is, in fact, a mental illness. Other times people with mental health conditions need to have pets travel with them because this mitigates the symptoms of their mental illness or perhaps it’s just good for their mental health. And finally, some people suffer from mental illnesses, or sometimes from learning problems, which are severe enough that a person with these conditions has a disability and needs special accommodations to function optimally in educational or occupational settings, or needs public financial support because their difficulties disable them to the point that they can’t work at all.
Is your head spinning yet? who we serve, and differentiating the fact that what someone with a psychiatric disorder needs to do to function or to alleviate emotional suffering may be entirely different from the things that everyone needs to do, regardless of whether they have a psychiatric disorder, to feel their emotional best.
The National Alliance on Mental Illness tells us that one in five Americans are suffering from a mental illness, while the Epidemiologic Catchment Area Program revealed that half of people will meet criteria for a mental illness at some point in their lives. We hear about “the mentally ill” constantly in the news – often in relation to mass shooters or homelessness – yet even psychiatrists might be pressed to define who exactly the “mentally ill” are. And how many of us could not somehow, at some time, find ourselves in 1 of the 157 disorders that DSM-5 lists – down from 365 disorders in the DSM-IV-TR?
Differentiating mental health from mental illness is just the beginning of our semantic confusion. As psychiatrists we treat major depression, and yet the illness “depression,” a syndromic constellation of symptoms, includes the key symptom of sadness. People often say they are “depressed” when they mean they are sad or demoralized, and yet, if their sadness persists in the absence of other symptoms, they may well want, or feel they “should” have medications, even in the absence of a disorder. And maybe those medications help them feel better, so that the presence or absence of a verified illness doesn’t really matter. But if the medications cause adverse reactions, then psychiatry might have done a better job by that person’s sadness. Melancholia, or perhaps any designation than “depression,” with its multiple meanings, might better serve our patients and our profession. This is only one example, as the number of people who tell me they have obsessive-compulsive disorder – or more often announce, “I’m OCD!” because they are well organized in a productive way is remarkable. And while I have treated only a few people who meet the criteria for narcissistic personality disorder, from general conversation it would seem that they are at every dinner table and by every water cooler.
Does it matter? A diagnostic lexicon can be so helpful when it guides treatment, provides a heterogeneous group of patients for research studies, and allows for an understanding of the etiology, course, and prognosis of a given condition. When someone is so depressed that they can’t get out of bed, or is so disorganized that they can’t perform their job and might cause a disturbance in their workplace, it is good to instruct them to take time off work and send them back well with a doctor’s note. But this is different from the person who doesn’t want to face a difficult situation, who simply doesn’t like their job or their boss, or who wants their pet declared an emotional support animal to avoid the fee the airlines charge to bring an animal on board if one does not have a psychiatric diagnosis. Sometimes these lines are blurry – if someone does not want to do something because it makes them anxious, does it matter how deep the pit in their stomach is, or if they are having full-blown panic attacks? When do we agree that their distress is reason to allow them to avoid responsibilities without repercussions versus a violation of their obligations and an infringement on others?
Diagnoses offer solace to some patients: There is a name for their suffering, available treatment, and often others with the same condition to look to for guidance and community. For others, a psychiatric diagnosis is a source of shame, a label they see as damaging to their character and sometimes to their careers – including in medicine – where we have been particularly unsympathetic to those who announce a psychiatric history.
In some cultures, the label itself decreases someone’s attractiveness as a potential marriage partner. We would all like to see the stigma of mental illness vanish, but we have a long way to go.
Psychiatric diagnoses move over time and with our politics and culture. This is good; we don’t hold on to what we learn to be untrue. But they may well add to issues of inequity. Those who can afford to pay for expensive educational assessments can request educational accommodations, including untimed standardized tests. This advantage may not be available to those without the resources to pay for these evaluations, and one might wonder why all comers can’t take untimed tests so as not to favor the privileged. Psychiatry has long been accused of diagnosing people of color with poor prognosis illnesses and women with conditions that imply emotional weakness.
While our diagnoses have clinical utility, it is unfortunate that they have come to be about reimbursement. A diagnosis needs to be assigned for insurers to pay for care, and so we create diagnostic categories to allow for treatment. Is this reasonable? Do we need to say that someone who is suffering after the death of a loved one has a mental illness in order to allow them to seek relief from their suffering? It leads us to believe that all suffering is about pathology, that we should expect pain-free emotional lives. Perhaps we need a diagnostic category of psychic pain, not otherwise specified, to allow for treatment for those who simply ache.
Mental illness is about interventions to alleviate the suffering of those with disorders. Mental health is about interventions that may benefit everyone, whether they suffer from a mental illness or not. Sleep, nutrition, exercise, sunlight, nature, entertainment and escape, yoga, meditation, vacations in beautiful places with loving people – these are things that potentially help us all whether we do or do not have an illness. With so much confusion about what it is we do, and about who “should” get help, who can get help, who might want help, and where they should go to seek help, perhaps it would be better if our lingo were more precise.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). The has a private practice and is assistant professor of psychiatry ad behavioral sciences at Johns Hopkins University, both in Baltimore. She has no disclosures.
I am a psychiatrist, which means I am a mental health professional, which means I work with people with mental illness. Sometimes people with mental health conditions who suffer from mental illness need to take a day off work – also called a mental health day – because they are too symptomatic to work, and sometimes people who don’t have a mental illness need to take a day off work, also called a mental health day, because they are feeling stressed.
Sometimes professional athletes don’t do things they agreed to do in their contracts because they realize that doing these things is very upsetting and will be detrimental to their mental health, or maybe they have a mental illness and doing these things will worsen their mental health condition, which is, in fact, a mental illness. Other times people with mental health conditions need to have pets travel with them because this mitigates the symptoms of their mental illness or perhaps it’s just good for their mental health. And finally, some people suffer from mental illnesses, or sometimes from learning problems, which are severe enough that a person with these conditions has a disability and needs special accommodations to function optimally in educational or occupational settings, or needs public financial support because their difficulties disable them to the point that they can’t work at all.
Is your head spinning yet? who we serve, and differentiating the fact that what someone with a psychiatric disorder needs to do to function or to alleviate emotional suffering may be entirely different from the things that everyone needs to do, regardless of whether they have a psychiatric disorder, to feel their emotional best.
The National Alliance on Mental Illness tells us that one in five Americans are suffering from a mental illness, while the Epidemiologic Catchment Area Program revealed that half of people will meet criteria for a mental illness at some point in their lives. We hear about “the mentally ill” constantly in the news – often in relation to mass shooters or homelessness – yet even psychiatrists might be pressed to define who exactly the “mentally ill” are. And how many of us could not somehow, at some time, find ourselves in 1 of the 157 disorders that DSM-5 lists – down from 365 disorders in the DSM-IV-TR?
Differentiating mental health from mental illness is just the beginning of our semantic confusion. As psychiatrists we treat major depression, and yet the illness “depression,” a syndromic constellation of symptoms, includes the key symptom of sadness. People often say they are “depressed” when they mean they are sad or demoralized, and yet, if their sadness persists in the absence of other symptoms, they may well want, or feel they “should” have medications, even in the absence of a disorder. And maybe those medications help them feel better, so that the presence or absence of a verified illness doesn’t really matter. But if the medications cause adverse reactions, then psychiatry might have done a better job by that person’s sadness. Melancholia, or perhaps any designation than “depression,” with its multiple meanings, might better serve our patients and our profession. This is only one example, as the number of people who tell me they have obsessive-compulsive disorder – or more often announce, “I’m OCD!” because they are well organized in a productive way is remarkable. And while I have treated only a few people who meet the criteria for narcissistic personality disorder, from general conversation it would seem that they are at every dinner table and by every water cooler.
Does it matter? A diagnostic lexicon can be so helpful when it guides treatment, provides a heterogeneous group of patients for research studies, and allows for an understanding of the etiology, course, and prognosis of a given condition. When someone is so depressed that they can’t get out of bed, or is so disorganized that they can’t perform their job and might cause a disturbance in their workplace, it is good to instruct them to take time off work and send them back well with a doctor’s note. But this is different from the person who doesn’t want to face a difficult situation, who simply doesn’t like their job or their boss, or who wants their pet declared an emotional support animal to avoid the fee the airlines charge to bring an animal on board if one does not have a psychiatric diagnosis. Sometimes these lines are blurry – if someone does not want to do something because it makes them anxious, does it matter how deep the pit in their stomach is, or if they are having full-blown panic attacks? When do we agree that their distress is reason to allow them to avoid responsibilities without repercussions versus a violation of their obligations and an infringement on others?
Diagnoses offer solace to some patients: There is a name for their suffering, available treatment, and often others with the same condition to look to for guidance and community. For others, a psychiatric diagnosis is a source of shame, a label they see as damaging to their character and sometimes to their careers – including in medicine – where we have been particularly unsympathetic to those who announce a psychiatric history.
In some cultures, the label itself decreases someone’s attractiveness as a potential marriage partner. We would all like to see the stigma of mental illness vanish, but we have a long way to go.
Psychiatric diagnoses move over time and with our politics and culture. This is good; we don’t hold on to what we learn to be untrue. But they may well add to issues of inequity. Those who can afford to pay for expensive educational assessments can request educational accommodations, including untimed standardized tests. This advantage may not be available to those without the resources to pay for these evaluations, and one might wonder why all comers can’t take untimed tests so as not to favor the privileged. Psychiatry has long been accused of diagnosing people of color with poor prognosis illnesses and women with conditions that imply emotional weakness.
While our diagnoses have clinical utility, it is unfortunate that they have come to be about reimbursement. A diagnosis needs to be assigned for insurers to pay for care, and so we create diagnostic categories to allow for treatment. Is this reasonable? Do we need to say that someone who is suffering after the death of a loved one has a mental illness in order to allow them to seek relief from their suffering? It leads us to believe that all suffering is about pathology, that we should expect pain-free emotional lives. Perhaps we need a diagnostic category of psychic pain, not otherwise specified, to allow for treatment for those who simply ache.
Mental illness is about interventions to alleviate the suffering of those with disorders. Mental health is about interventions that may benefit everyone, whether they suffer from a mental illness or not. Sleep, nutrition, exercise, sunlight, nature, entertainment and escape, yoga, meditation, vacations in beautiful places with loving people – these are things that potentially help us all whether we do or do not have an illness. With so much confusion about what it is we do, and about who “should” get help, who can get help, who might want help, and where they should go to seek help, perhaps it would be better if our lingo were more precise.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). The has a private practice and is assistant professor of psychiatry ad behavioral sciences at Johns Hopkins University, both in Baltimore. She has no disclosures.
SCAMP: Assessing body-focused repetitive behaviors
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.














