User login
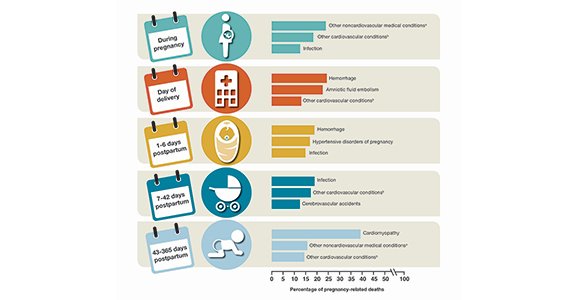
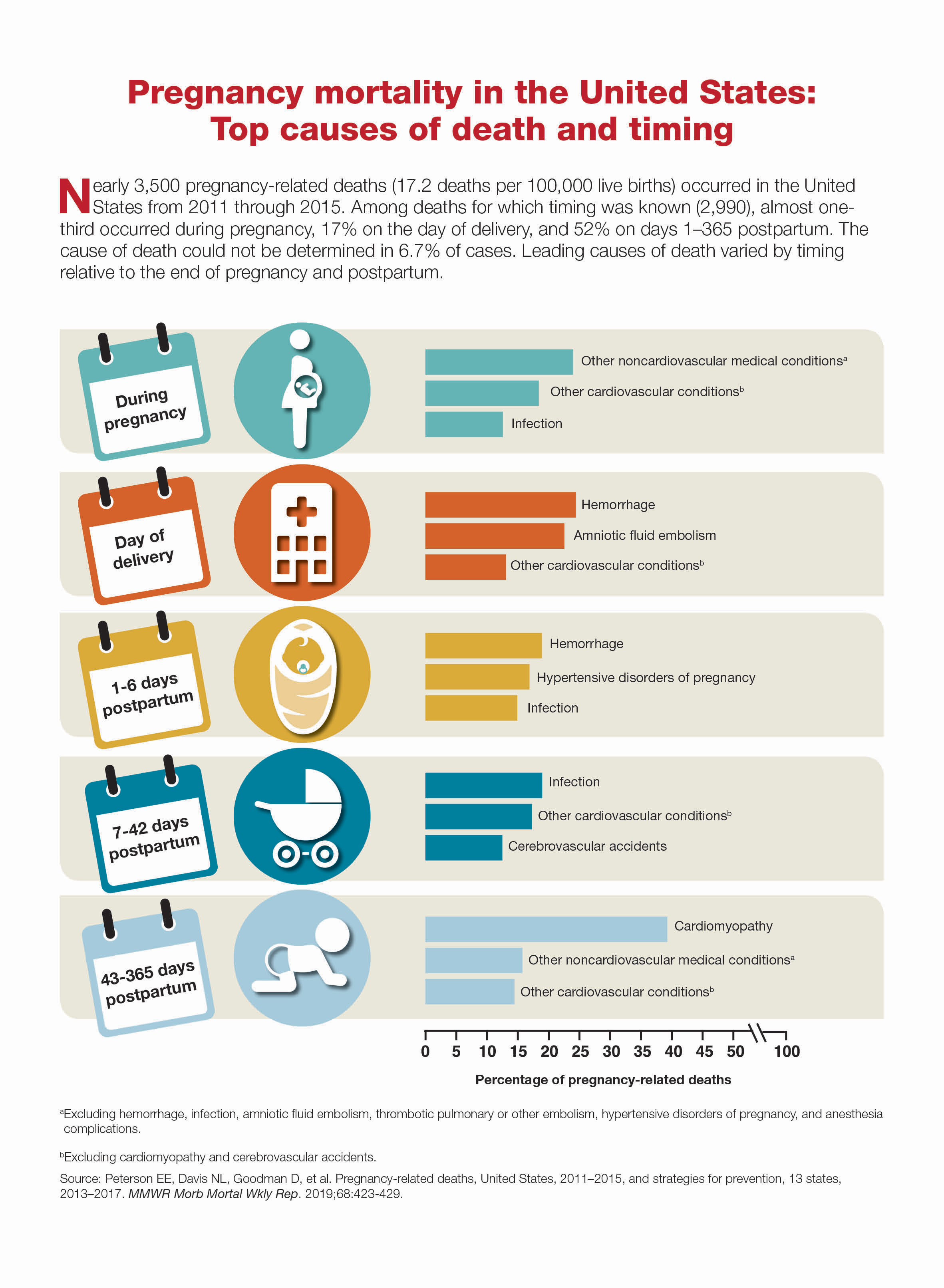
Pregnancy mortality in the United States: Top causes of death and timing


FDA: Vinpocetine associated with fetal harms, miscarriage
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
What’s in store for ObGyn reimbursement in the EHR age and beyond
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
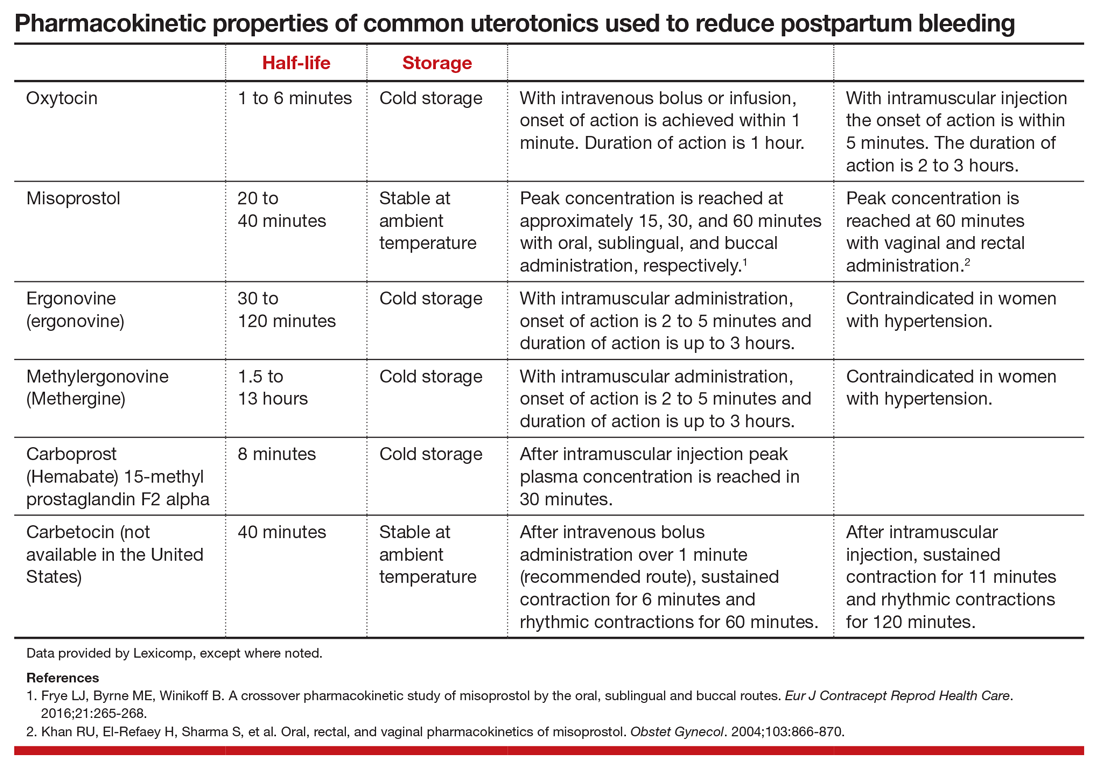
One versus two uterotonics: Which is better for minimizing postpartum blood loss?
Excessive postpartum bleeding is a major cause of maternal morbidity and mortality. Worldwide, obstetric hemorrhage is the most common cause of maternal death.1,2 Medications reported to reduce postpartum bleeding include oxytocin, misoprostol, ergonovine, methylergonovine, carboprost, and tranexamic acid. A recent Cochrane network meta-analysis of 196 trials, including 135,559 women, distilled in 1,361 pages of analysis, reported on the medications associated with the greatest reduction in postpartum bleeding.3 Surprisingly, for preventing blood loss ≥ 500 mL, misoprostol plus oxytocin and ergonovine plus oxytocin were the highest ranked interventions. This evidence is summarized here.
Misoprostol plus oxytocin
After newborn delivery, active management of the third stage of labor, including uterotonic administration, is strongly recommended because it will reduce postpartum blood loss, decreasing the rate of postpartum hemorrhage (PPH).4 Both oxytocin and misoprostol are effective uterotonics. However, the combination of oxytocin plus misoprostol appears to be more effective than oxytocin alone in reducing the frequency of postpartum blood loss greater than 500 mL.3 To understand the clinical efficacy and adverse effects (AEs) of combined oxytocin plus misoprostol a meta-analysis was performed for both vaginal and cesarean deliveries (CDs).
Efficacy and AEs during vaginal delivery. In the meta-analysis, about 6,000 vaginal deliveries were analyzed, with no significant differences for misoprostol plus oxytocin versus oxytocin alone found for the following outcomes: maternal death, intensive care unit admissions, and rate of blood loss ≥ 1,000 mL (1.7% for both uterotonics vs 2.2% for oxytocin alone).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (0.95% vs 2.5%), reduced risk of blood loss ≥ 500 mL (5.9% vs 8.0%), reduced risk of requiring an additional uterotonic (3.6% vs 5.8%), and a smaller decrease in hemoglobin concentration from pre- to postdelivery (-0.89 g/L).3
In my opinion, the difference in hemoglobin concentration, although statistically significant, is not of clinical significance. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (2.4% vs 0.66%), vomiting (3.1% vs 0.86%), and fever (21% vs 3.9%).3 A weakness of this meta-analysis is that the trials used a wide range of misoprostol dosages (200 to 600 µg) and multiple routes of administration, including sublingual (under the tongue), buccal, and rectal. This makes it impossible to identify a best misoprostol dosage and administration route.
Efficacy and AEs during CD. In the same meta-analysis about 2,000 CDs were analyzed, with no significant difference for misoprostol plus oxytocin versus oxytocin alone for the following outcomes: maternal death, intensive care unit admissions, and PPH ≥ 1,000 mL blood loss (6.2% vs 6.5%).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (2.6% vs 5.4%), reduced risk of blood loss ≥ 500 mL (32% vs 47%), reduced risk of requiring an additional uterotonic (14% vs 28%), and a smaller decrease in hemoglobin concentration from before to after delivery (-4.0 g/L).3 In my opinion, the statistically significant difference in hemoglobin concentration is not clinically significant. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (12% vs 6.1%), vomiting (8.1% vs 5.4%), shivering (13% vs 7%), and fever (7.7% vs 4.0%).3
Continue to: Ergonovine plus oxytocin...
Ergonovine plus oxytocin
Ergonovine is an ergot derivative that causes uterine contractions and has been shown to effectively reduce blood loss at delivery. In the United States a methyl-derivative of ergonovine, methylergonovine, is widely available. In a meta-analysis with mostly vaginal deliveries, there were no significant differences for ergonovine plus oxytocin versus oxytocin alone for the following outcomes: death, intensive care unit admission, rate of blood loss ≥ 1,000 mL(2.0% vs 2.7%), blood transfusion, administration of an additional uterotonic, change in hemoglobin from pre- to postdelivery, nausea, hypertension, shivering, and fever.3 However, ergonovine plus oxytocin, compared with oxytocin alone, resulted in a significantly reduced rate of blood loss ≥ 500 mL (8.3% vs 10.2%) and an increased rate of vomiting (8.1% vs 1.6%).3 In these trials women with a blood pressure ≥ 150/100 mm Hg were generally excluded from receiving ergonovine because of its hypertensive effect.
Clinical practice options
Given the Cochrane meta-analysis results, ObGyns have two approaches for optimizing PPH reduction.
Option 1: Use a single uterotonic to reduce postpartum blood loss. If excess bleeding occurs, rapidly administer a second uterotonic agent. Currently, monotherapy with intravenous or intramuscular oxytocin is the standard for reducing postpartum blood loss.5,6 Advantages of this approach compared with dual agent therapy include simplification of care and minimization of AEs. However, oxytocin monotherapy for minimizing postpartum bleeding may be suboptimal. In the largest trial ever performed (involving 29,645 women) when oxytocin was administered postpartum, the rates of estimated blood loss ≥ 500 mL and ≥ 1,000 mL were 9.1% and 1.45%, respectively.5 Is 9% an optimal rate for blood loss ≥ 500 mL following a vaginal delivery? Or should we try to achieve a lower rate?
Given the “high” rate of blood loss ≥ 500 mL with oxytocin alone, it is important for clinicians using the one-uterotonic approach to promptly recognize patients who have excessive bleeding and transition rapidly from prevention to treatment. When PPH cases are reviewed, a common finding is that the clinicians did not timely recognize excess bleeding, delaying transition to treatment with additional uterotonics and other interventions. When routinely using oxytocin monotherapy, lowering the threshold for administering a second uterotonic (methylergonovine, carboprost, misoprostol, or tranexamic acid) may help decrease the frequency of excess postpartum blood loss.
Option 2: Administer two uterotonics to reduce postpartum blood loss at all deliveries. Given the “high” rate of excess postpartum blood loss with oxytocin monotherapy, an alternative is to administer two uterotonics at all births or at births with a high risk of excess blood loss. As discussed, administering two uterotonics, oxytocin plus misoprostol or oxytocin plus ergonovine, has been reported to be more effective than oxytocin alone for reducing postpartum bleeding ≥ 500 mL.3 In the Cochrane meta-analysis, per 1,000 women given oxytocin following a vaginal birth, 122 would have blood loss ≥ 500 mL, compared with 85 given oxytocin plus misoprostol or oxytocin plus ergonovine.3
Misoprostol is administered sublingually, buccally, or rectally, and methylergonovine is administered by intramuscular injection. Although dual uterotonic therapy is more effective than monotherapy, dual therapy is associated with more AEs. As noted, compared with oxytocin monotherapy, the combination of oxytocin plus misoprostol is associated with more nausea, vomiting, shivering, and fever. Oxytocin plus ergonovine is associated with a higher rate of vomiting than oxytocin monotherapy. In my practice I prefer using intramuscular methylergonovine as the second agent to avoid the high rate of fever associated with misoprostol.
For dual agent therapy, one approach is to administer misoprostol 200 µg or 400 µg through the buccal7,8 or sublingual9,10 routes. Higher dosages of misoprostol (600 µg to 800 µg) have been used11,12 but are likely associated with higher rates of nausea, vomiting,shivering, and fever than the lower dosages. Methylergonovine 0.2 mg is administered intramuscularly.
Continue to: The bottom line...
The bottom line
PPH is a major cause of maternal morbidity, and in low-resource settings, mortality. Oxytocin is the standard for reducing postpartum blood loss, but rates of blood loss ≥ 500 mL are high following this monotherapy. To reduce postpartum blood loss beyond what is possible with oxytocin alone, clinicians can more rapidly transition to administering a second uterotonic when they suspect blood loss is becoming excessive or they can use two uterotonic agents with all births or in those at high risk for excess bleeding. If blood loss does become excessive, clinicians need to pivot rapidly from prevention with oxytocin to treatment with our entire therapeutic armamentarium.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Slomski A. Why do hundreds of US women die annually in childbirth? JAMA. 2019;321:1239-1241.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD011689.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- Widmer M, Piaggio G, Nguyen TM, et al; WHO Champion Trial Group. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379:743-752.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ. 2018;362:k3546.
- Hamm J, Russell Z, Botha T, et al. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005;192:1404-1406.
- Bhullar A, Carlan SJ, Hamm J, et al. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol. 2004;104:1282-1288.
- Hofmeyr GJ, Fawole B, Mugerwa K, et al. Administration of 400 µg of misoprostol to augment routine active management of the third stage of labor. Int J Gynaecol Obstet. 2011;112:98-102.
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum hemorrhage. Int J Gynaecol Obstet. 2016;132:191-195.
- Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labor: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:210-216.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:217-223.
Excessive postpartum bleeding is a major cause of maternal morbidity and mortality. Worldwide, obstetric hemorrhage is the most common cause of maternal death.1,2 Medications reported to reduce postpartum bleeding include oxytocin, misoprostol, ergonovine, methylergonovine, carboprost, and tranexamic acid. A recent Cochrane network meta-analysis of 196 trials, including 135,559 women, distilled in 1,361 pages of analysis, reported on the medications associated with the greatest reduction in postpartum bleeding.3 Surprisingly, for preventing blood loss ≥ 500 mL, misoprostol plus oxytocin and ergonovine plus oxytocin were the highest ranked interventions. This evidence is summarized here.
Misoprostol plus oxytocin
After newborn delivery, active management of the third stage of labor, including uterotonic administration, is strongly recommended because it will reduce postpartum blood loss, decreasing the rate of postpartum hemorrhage (PPH).4 Both oxytocin and misoprostol are effective uterotonics. However, the combination of oxytocin plus misoprostol appears to be more effective than oxytocin alone in reducing the frequency of postpartum blood loss greater than 500 mL.3 To understand the clinical efficacy and adverse effects (AEs) of combined oxytocin plus misoprostol a meta-analysis was performed for both vaginal and cesarean deliveries (CDs).
Efficacy and AEs during vaginal delivery. In the meta-analysis, about 6,000 vaginal deliveries were analyzed, with no significant differences for misoprostol plus oxytocin versus oxytocin alone found for the following outcomes: maternal death, intensive care unit admissions, and rate of blood loss ≥ 1,000 mL (1.7% for both uterotonics vs 2.2% for oxytocin alone).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (0.95% vs 2.5%), reduced risk of blood loss ≥ 500 mL (5.9% vs 8.0%), reduced risk of requiring an additional uterotonic (3.6% vs 5.8%), and a smaller decrease in hemoglobin concentration from pre- to postdelivery (-0.89 g/L).3
In my opinion, the difference in hemoglobin concentration, although statistically significant, is not of clinical significance. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (2.4% vs 0.66%), vomiting (3.1% vs 0.86%), and fever (21% vs 3.9%).3 A weakness of this meta-analysis is that the trials used a wide range of misoprostol dosages (200 to 600 µg) and multiple routes of administration, including sublingual (under the tongue), buccal, and rectal. This makes it impossible to identify a best misoprostol dosage and administration route.
Efficacy and AEs during CD. In the same meta-analysis about 2,000 CDs were analyzed, with no significant difference for misoprostol plus oxytocin versus oxytocin alone for the following outcomes: maternal death, intensive care unit admissions, and PPH ≥ 1,000 mL blood loss (6.2% vs 6.5%).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (2.6% vs 5.4%), reduced risk of blood loss ≥ 500 mL (32% vs 47%), reduced risk of requiring an additional uterotonic (14% vs 28%), and a smaller decrease in hemoglobin concentration from before to after delivery (-4.0 g/L).3 In my opinion, the statistically significant difference in hemoglobin concentration is not clinically significant. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (12% vs 6.1%), vomiting (8.1% vs 5.4%), shivering (13% vs 7%), and fever (7.7% vs 4.0%).3
Continue to: Ergonovine plus oxytocin...
Ergonovine plus oxytocin
Ergonovine is an ergot derivative that causes uterine contractions and has been shown to effectively reduce blood loss at delivery. In the United States a methyl-derivative of ergonovine, methylergonovine, is widely available. In a meta-analysis with mostly vaginal deliveries, there were no significant differences for ergonovine plus oxytocin versus oxytocin alone for the following outcomes: death, intensive care unit admission, rate of blood loss ≥ 1,000 mL(2.0% vs 2.7%), blood transfusion, administration of an additional uterotonic, change in hemoglobin from pre- to postdelivery, nausea, hypertension, shivering, and fever.3 However, ergonovine plus oxytocin, compared with oxytocin alone, resulted in a significantly reduced rate of blood loss ≥ 500 mL (8.3% vs 10.2%) and an increased rate of vomiting (8.1% vs 1.6%).3 In these trials women with a blood pressure ≥ 150/100 mm Hg were generally excluded from receiving ergonovine because of its hypertensive effect.

Clinical practice options
Given the Cochrane meta-analysis results, ObGyns have two approaches for optimizing PPH reduction.
Option 1: Use a single uterotonic to reduce postpartum blood loss. If excess bleeding occurs, rapidly administer a second uterotonic agent. Currently, monotherapy with intravenous or intramuscular oxytocin is the standard for reducing postpartum blood loss.5,6 Advantages of this approach compared with dual agent therapy include simplification of care and minimization of AEs. However, oxytocin monotherapy for minimizing postpartum bleeding may be suboptimal. In the largest trial ever performed (involving 29,645 women) when oxytocin was administered postpartum, the rates of estimated blood loss ≥ 500 mL and ≥ 1,000 mL were 9.1% and 1.45%, respectively.5 Is 9% an optimal rate for blood loss ≥ 500 mL following a vaginal delivery? Or should we try to achieve a lower rate?
Given the “high” rate of blood loss ≥ 500 mL with oxytocin alone, it is important for clinicians using the one-uterotonic approach to promptly recognize patients who have excessive bleeding and transition rapidly from prevention to treatment. When PPH cases are reviewed, a common finding is that the clinicians did not timely recognize excess bleeding, delaying transition to treatment with additional uterotonics and other interventions. When routinely using oxytocin monotherapy, lowering the threshold for administering a second uterotonic (methylergonovine, carboprost, misoprostol, or tranexamic acid) may help decrease the frequency of excess postpartum blood loss.
Option 2: Administer two uterotonics to reduce postpartum blood loss at all deliveries. Given the “high” rate of excess postpartum blood loss with oxytocin monotherapy, an alternative is to administer two uterotonics at all births or at births with a high risk of excess blood loss. As discussed, administering two uterotonics, oxytocin plus misoprostol or oxytocin plus ergonovine, has been reported to be more effective than oxytocin alone for reducing postpartum bleeding ≥ 500 mL.3 In the Cochrane meta-analysis, per 1,000 women given oxytocin following a vaginal birth, 122 would have blood loss ≥ 500 mL, compared with 85 given oxytocin plus misoprostol or oxytocin plus ergonovine.3
Misoprostol is administered sublingually, buccally, or rectally, and methylergonovine is administered by intramuscular injection. Although dual uterotonic therapy is more effective than monotherapy, dual therapy is associated with more AEs. As noted, compared with oxytocin monotherapy, the combination of oxytocin plus misoprostol is associated with more nausea, vomiting, shivering, and fever. Oxytocin plus ergonovine is associated with a higher rate of vomiting than oxytocin monotherapy. In my practice I prefer using intramuscular methylergonovine as the second agent to avoid the high rate of fever associated with misoprostol.
For dual agent therapy, one approach is to administer misoprostol 200 µg or 400 µg through the buccal7,8 or sublingual9,10 routes. Higher dosages of misoprostol (600 µg to 800 µg) have been used11,12 but are likely associated with higher rates of nausea, vomiting,shivering, and fever than the lower dosages. Methylergonovine 0.2 mg is administered intramuscularly.
Continue to: The bottom line...
The bottom line
PPH is a major cause of maternal morbidity, and in low-resource settings, mortality. Oxytocin is the standard for reducing postpartum blood loss, but rates of blood loss ≥ 500 mL are high following this monotherapy. To reduce postpartum blood loss beyond what is possible with oxytocin alone, clinicians can more rapidly transition to administering a second uterotonic when they suspect blood loss is becoming excessive or they can use two uterotonic agents with all births or in those at high risk for excess bleeding. If blood loss does become excessive, clinicians need to pivot rapidly from prevention with oxytocin to treatment with our entire therapeutic armamentarium.
Excessive postpartum bleeding is a major cause of maternal morbidity and mortality. Worldwide, obstetric hemorrhage is the most common cause of maternal death.1,2 Medications reported to reduce postpartum bleeding include oxytocin, misoprostol, ergonovine, methylergonovine, carboprost, and tranexamic acid. A recent Cochrane network meta-analysis of 196 trials, including 135,559 women, distilled in 1,361 pages of analysis, reported on the medications associated with the greatest reduction in postpartum bleeding.3 Surprisingly, for preventing blood loss ≥ 500 mL, misoprostol plus oxytocin and ergonovine plus oxytocin were the highest ranked interventions. This evidence is summarized here.
Misoprostol plus oxytocin
After newborn delivery, active management of the third stage of labor, including uterotonic administration, is strongly recommended because it will reduce postpartum blood loss, decreasing the rate of postpartum hemorrhage (PPH).4 Both oxytocin and misoprostol are effective uterotonics. However, the combination of oxytocin plus misoprostol appears to be more effective than oxytocin alone in reducing the frequency of postpartum blood loss greater than 500 mL.3 To understand the clinical efficacy and adverse effects (AEs) of combined oxytocin plus misoprostol a meta-analysis was performed for both vaginal and cesarean deliveries (CDs).
Efficacy and AEs during vaginal delivery. In the meta-analysis, about 6,000 vaginal deliveries were analyzed, with no significant differences for misoprostol plus oxytocin versus oxytocin alone found for the following outcomes: maternal death, intensive care unit admissions, and rate of blood loss ≥ 1,000 mL (1.7% for both uterotonics vs 2.2% for oxytocin alone).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (0.95% vs 2.5%), reduced risk of blood loss ≥ 500 mL (5.9% vs 8.0%), reduced risk of requiring an additional uterotonic (3.6% vs 5.8%), and a smaller decrease in hemoglobin concentration from pre- to postdelivery (-0.89 g/L).3
In my opinion, the difference in hemoglobin concentration, although statistically significant, is not of clinical significance. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (2.4% vs 0.66%), vomiting (3.1% vs 0.86%), and fever (21% vs 3.9%).3 A weakness of this meta-analysis is that the trials used a wide range of misoprostol dosages (200 to 600 µg) and multiple routes of administration, including sublingual (under the tongue), buccal, and rectal. This makes it impossible to identify a best misoprostol dosage and administration route.
Efficacy and AEs during CD. In the same meta-analysis about 2,000 CDs were analyzed, with no significant difference for misoprostol plus oxytocin versus oxytocin alone for the following outcomes: maternal death, intensive care unit admissions, and PPH ≥ 1,000 mL blood loss (6.2% vs 6.5%).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (2.6% vs 5.4%), reduced risk of blood loss ≥ 500 mL (32% vs 47%), reduced risk of requiring an additional uterotonic (14% vs 28%), and a smaller decrease in hemoglobin concentration from before to after delivery (-4.0 g/L).3 In my opinion, the statistically significant difference in hemoglobin concentration is not clinically significant. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (12% vs 6.1%), vomiting (8.1% vs 5.4%), shivering (13% vs 7%), and fever (7.7% vs 4.0%).3
Continue to: Ergonovine plus oxytocin...
Ergonovine plus oxytocin
Ergonovine is an ergot derivative that causes uterine contractions and has been shown to effectively reduce blood loss at delivery. In the United States a methyl-derivative of ergonovine, methylergonovine, is widely available. In a meta-analysis with mostly vaginal deliveries, there were no significant differences for ergonovine plus oxytocin versus oxytocin alone for the following outcomes: death, intensive care unit admission, rate of blood loss ≥ 1,000 mL(2.0% vs 2.7%), blood transfusion, administration of an additional uterotonic, change in hemoglobin from pre- to postdelivery, nausea, hypertension, shivering, and fever.3 However, ergonovine plus oxytocin, compared with oxytocin alone, resulted in a significantly reduced rate of blood loss ≥ 500 mL (8.3% vs 10.2%) and an increased rate of vomiting (8.1% vs 1.6%).3 In these trials women with a blood pressure ≥ 150/100 mm Hg were generally excluded from receiving ergonovine because of its hypertensive effect.

Clinical practice options
Given the Cochrane meta-analysis results, ObGyns have two approaches for optimizing PPH reduction.
Option 1: Use a single uterotonic to reduce postpartum blood loss. If excess bleeding occurs, rapidly administer a second uterotonic agent. Currently, monotherapy with intravenous or intramuscular oxytocin is the standard for reducing postpartum blood loss.5,6 Advantages of this approach compared with dual agent therapy include simplification of care and minimization of AEs. However, oxytocin monotherapy for minimizing postpartum bleeding may be suboptimal. In the largest trial ever performed (involving 29,645 women) when oxytocin was administered postpartum, the rates of estimated blood loss ≥ 500 mL and ≥ 1,000 mL were 9.1% and 1.45%, respectively.5 Is 9% an optimal rate for blood loss ≥ 500 mL following a vaginal delivery? Or should we try to achieve a lower rate?
Given the “high” rate of blood loss ≥ 500 mL with oxytocin alone, it is important for clinicians using the one-uterotonic approach to promptly recognize patients who have excessive bleeding and transition rapidly from prevention to treatment. When PPH cases are reviewed, a common finding is that the clinicians did not timely recognize excess bleeding, delaying transition to treatment with additional uterotonics and other interventions. When routinely using oxytocin monotherapy, lowering the threshold for administering a second uterotonic (methylergonovine, carboprost, misoprostol, or tranexamic acid) may help decrease the frequency of excess postpartum blood loss.
Option 2: Administer two uterotonics to reduce postpartum blood loss at all deliveries. Given the “high” rate of excess postpartum blood loss with oxytocin monotherapy, an alternative is to administer two uterotonics at all births or at births with a high risk of excess blood loss. As discussed, administering two uterotonics, oxytocin plus misoprostol or oxytocin plus ergonovine, has been reported to be more effective than oxytocin alone for reducing postpartum bleeding ≥ 500 mL.3 In the Cochrane meta-analysis, per 1,000 women given oxytocin following a vaginal birth, 122 would have blood loss ≥ 500 mL, compared with 85 given oxytocin plus misoprostol or oxytocin plus ergonovine.3
Misoprostol is administered sublingually, buccally, or rectally, and methylergonovine is administered by intramuscular injection. Although dual uterotonic therapy is more effective than monotherapy, dual therapy is associated with more AEs. As noted, compared with oxytocin monotherapy, the combination of oxytocin plus misoprostol is associated with more nausea, vomiting, shivering, and fever. Oxytocin plus ergonovine is associated with a higher rate of vomiting than oxytocin monotherapy. In my practice I prefer using intramuscular methylergonovine as the second agent to avoid the high rate of fever associated with misoprostol.
For dual agent therapy, one approach is to administer misoprostol 200 µg or 400 µg through the buccal7,8 or sublingual9,10 routes. Higher dosages of misoprostol (600 µg to 800 µg) have been used11,12 but are likely associated with higher rates of nausea, vomiting,shivering, and fever than the lower dosages. Methylergonovine 0.2 mg is administered intramuscularly.
Continue to: The bottom line...
The bottom line
PPH is a major cause of maternal morbidity, and in low-resource settings, mortality. Oxytocin is the standard for reducing postpartum blood loss, but rates of blood loss ≥ 500 mL are high following this monotherapy. To reduce postpartum blood loss beyond what is possible with oxytocin alone, clinicians can more rapidly transition to administering a second uterotonic when they suspect blood loss is becoming excessive or they can use two uterotonic agents with all births or in those at high risk for excess bleeding. If blood loss does become excessive, clinicians need to pivot rapidly from prevention with oxytocin to treatment with our entire therapeutic armamentarium.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Slomski A. Why do hundreds of US women die annually in childbirth? JAMA. 2019;321:1239-1241.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD011689.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- Widmer M, Piaggio G, Nguyen TM, et al; WHO Champion Trial Group. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379:743-752.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ. 2018;362:k3546.
- Hamm J, Russell Z, Botha T, et al. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005;192:1404-1406.
- Bhullar A, Carlan SJ, Hamm J, et al. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol. 2004;104:1282-1288.
- Hofmeyr GJ, Fawole B, Mugerwa K, et al. Administration of 400 µg of misoprostol to augment routine active management of the third stage of labor. Int J Gynaecol Obstet. 2011;112:98-102.
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum hemorrhage. Int J Gynaecol Obstet. 2016;132:191-195.
- Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labor: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:210-216.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:217-223.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Slomski A. Why do hundreds of US women die annually in childbirth? JAMA. 2019;321:1239-1241.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD011689.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- Widmer M, Piaggio G, Nguyen TM, et al; WHO Champion Trial Group. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379:743-752.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ. 2018;362:k3546.
- Hamm J, Russell Z, Botha T, et al. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005;192:1404-1406.
- Bhullar A, Carlan SJ, Hamm J, et al. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol. 2004;104:1282-1288.
- Hofmeyr GJ, Fawole B, Mugerwa K, et al. Administration of 400 µg of misoprostol to augment routine active management of the third stage of labor. Int J Gynaecol Obstet. 2011;112:98-102.
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum hemorrhage. Int J Gynaecol Obstet. 2016;132:191-195.
- Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labor: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:210-216.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:217-223.
In women with late preterm mild hypertensive disorders, does immediate delivery versus expectant management differ in terms of neonatal neurodevelopmental outcomes?
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
Systematic review indicates cutaneous laser therapy may be safe during pregnancy
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
FROM DERMATOLOGIC SURGERY
Even if successful, IVF may boost relapses in MS
SEATTLE – Failed in-vitro fertilization (IVF) treatment appears to boost the risk of relapse in women with multiple sclerosis (MS). Does successful IVF have the same effect? The preliminary results of a new study suggests it does, a finding that may influence how physicians track patients during pregnancy.
“We found that IVF can still cause a relapse even if it is successful,” study lead author Maria Claudia Manieri, a graduate student at Harvard Medical School’s Partners MS Center, said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where she presented the findings.
Multiple studies have linked infertility treatment in women with MS to relapses. In a 2013 review, researchers analyzed several papers, and “all of them reported an increase in annualized relapse rate after ART [assisted reproductive treatment]. Furthermore, in a recent study, clinical worsening was associated with an increase in MRI activity” (Clin Immunol. 2013 Nov;149(2):219-24).
For the new report, based on statistics from the New England Multiple Sclerosis Pregnancy Prospective Cohort Study, Ms. Manieri and colleagues collected data on 91 women (mean age = 33). Eleven were unsuccessful in conceiving, and 80 successfully conceived.
Three of the 91 women (3%) used intrauterine insemination as a fertility treatment. Another 9 (10%) relied on ART; all used IVF except for 1 who underwent intracytoplasmic sperm injection.
The new report is a preliminary analysis of early data, Ms. Manieri said. The study has recruited about one-sixth of its participants, she said, and will track women beyond pregnancy to explore long-term outcomes in their children.
Eleven women relapsed during pregnancy, including 9 who were using fertility treatment (P = .003). Of those 9, 7 women (78%) used ART.
No other factor other than fertility treatment predicted intrapartum relapses. The relapses during pregnancy started at 21 weeks (± 12 weeks) of gestational age and lasted for 4 weeks (± 2 weeks).
Of those who successfully conceived, 4 of 5 (80%) who used fertility treatment relapsed, compared with 7 of 64 (11%) who didn’t use fertility treatment. Of women who did not successfully conceive, 2 of 3 (67%) relapsed among those who used fertility treatment vs. 1 of 7 (14%) of those who didn’t.
It’s not clear how infertility treatment may be boosting MS relapse in women, but the 2013 review offered these possibilities: “temporary interruption of disease modified therapies, stressful events associated with infertility, and immunological changes induced by hormones such as increase in pro-inflammatory cytokines and anti-MOG antibodies, as well as an increase in immune cell migration across the blood-brain-barrier.”
MS tends to improve during pregnancy, and it’s common for neurologists to not see patients for extended periods, Ms. Manieri said. In light of the findings, she said, it may be wise for neurologists to continue follow-up appointments during pregnancy. “Avoid delaying care and keep monitoring the patient,” she advised.
The study was funded by Sanofi Genzyme and a gift from Michelle and Christopher Rondeau. The study authors report no relevant disclosures.
SEATTLE – Failed in-vitro fertilization (IVF) treatment appears to boost the risk of relapse in women with multiple sclerosis (MS). Does successful IVF have the same effect? The preliminary results of a new study suggests it does, a finding that may influence how physicians track patients during pregnancy.
“We found that IVF can still cause a relapse even if it is successful,” study lead author Maria Claudia Manieri, a graduate student at Harvard Medical School’s Partners MS Center, said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where she presented the findings.
Multiple studies have linked infertility treatment in women with MS to relapses. In a 2013 review, researchers analyzed several papers, and “all of them reported an increase in annualized relapse rate after ART [assisted reproductive treatment]. Furthermore, in a recent study, clinical worsening was associated with an increase in MRI activity” (Clin Immunol. 2013 Nov;149(2):219-24).
For the new report, based on statistics from the New England Multiple Sclerosis Pregnancy Prospective Cohort Study, Ms. Manieri and colleagues collected data on 91 women (mean age = 33). Eleven were unsuccessful in conceiving, and 80 successfully conceived.
Three of the 91 women (3%) used intrauterine insemination as a fertility treatment. Another 9 (10%) relied on ART; all used IVF except for 1 who underwent intracytoplasmic sperm injection.
The new report is a preliminary analysis of early data, Ms. Manieri said. The study has recruited about one-sixth of its participants, she said, and will track women beyond pregnancy to explore long-term outcomes in their children.
Eleven women relapsed during pregnancy, including 9 who were using fertility treatment (P = .003). Of those 9, 7 women (78%) used ART.
No other factor other than fertility treatment predicted intrapartum relapses. The relapses during pregnancy started at 21 weeks (± 12 weeks) of gestational age and lasted for 4 weeks (± 2 weeks).
Of those who successfully conceived, 4 of 5 (80%) who used fertility treatment relapsed, compared with 7 of 64 (11%) who didn’t use fertility treatment. Of women who did not successfully conceive, 2 of 3 (67%) relapsed among those who used fertility treatment vs. 1 of 7 (14%) of those who didn’t.
It’s not clear how infertility treatment may be boosting MS relapse in women, but the 2013 review offered these possibilities: “temporary interruption of disease modified therapies, stressful events associated with infertility, and immunological changes induced by hormones such as increase in pro-inflammatory cytokines and anti-MOG antibodies, as well as an increase in immune cell migration across the blood-brain-barrier.”
MS tends to improve during pregnancy, and it’s common for neurologists to not see patients for extended periods, Ms. Manieri said. In light of the findings, she said, it may be wise for neurologists to continue follow-up appointments during pregnancy. “Avoid delaying care and keep monitoring the patient,” she advised.
The study was funded by Sanofi Genzyme and a gift from Michelle and Christopher Rondeau. The study authors report no relevant disclosures.
SEATTLE – Failed in-vitro fertilization (IVF) treatment appears to boost the risk of relapse in women with multiple sclerosis (MS). Does successful IVF have the same effect? The preliminary results of a new study suggests it does, a finding that may influence how physicians track patients during pregnancy.
“We found that IVF can still cause a relapse even if it is successful,” study lead author Maria Claudia Manieri, a graduate student at Harvard Medical School’s Partners MS Center, said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where she presented the findings.
Multiple studies have linked infertility treatment in women with MS to relapses. In a 2013 review, researchers analyzed several papers, and “all of them reported an increase in annualized relapse rate after ART [assisted reproductive treatment]. Furthermore, in a recent study, clinical worsening was associated with an increase in MRI activity” (Clin Immunol. 2013 Nov;149(2):219-24).
For the new report, based on statistics from the New England Multiple Sclerosis Pregnancy Prospective Cohort Study, Ms. Manieri and colleagues collected data on 91 women (mean age = 33). Eleven were unsuccessful in conceiving, and 80 successfully conceived.
Three of the 91 women (3%) used intrauterine insemination as a fertility treatment. Another 9 (10%) relied on ART; all used IVF except for 1 who underwent intracytoplasmic sperm injection.
The new report is a preliminary analysis of early data, Ms. Manieri said. The study has recruited about one-sixth of its participants, she said, and will track women beyond pregnancy to explore long-term outcomes in their children.
Eleven women relapsed during pregnancy, including 9 who were using fertility treatment (P = .003). Of those 9, 7 women (78%) used ART.
No other factor other than fertility treatment predicted intrapartum relapses. The relapses during pregnancy started at 21 weeks (± 12 weeks) of gestational age and lasted for 4 weeks (± 2 weeks).
Of those who successfully conceived, 4 of 5 (80%) who used fertility treatment relapsed, compared with 7 of 64 (11%) who didn’t use fertility treatment. Of women who did not successfully conceive, 2 of 3 (67%) relapsed among those who used fertility treatment vs. 1 of 7 (14%) of those who didn’t.
It’s not clear how infertility treatment may be boosting MS relapse in women, but the 2013 review offered these possibilities: “temporary interruption of disease modified therapies, stressful events associated with infertility, and immunological changes induced by hormones such as increase in pro-inflammatory cytokines and anti-MOG antibodies, as well as an increase in immune cell migration across the blood-brain-barrier.”
MS tends to improve during pregnancy, and it’s common for neurologists to not see patients for extended periods, Ms. Manieri said. In light of the findings, she said, it may be wise for neurologists to continue follow-up appointments during pregnancy. “Avoid delaying care and keep monitoring the patient,” she advised.
The study was funded by Sanofi Genzyme and a gift from Michelle and Christopher Rondeau. The study authors report no relevant disclosures.
REPORTING FROM CMSC 2019
Obesity doesn’t hamper flu vaccine response in pregnancy
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
REPORTING FROM ESPID 2019
Key clinical point: High BMI doesn’t impair influenza vaccine responses in pregnant women.
Major finding: Protective antibody levels against all three vaccine antigens were documented 1 month post vaccination in 92% of the obese and 74% of the nonobese mothers.
Study details: This was a prospective observational study of 90 women vaccinated against influenza during pregnancy, 24 of whom were obese.
Disclosures: The study was supported by the University of Adelaide Women’s and Children’s Hospital Research Foundation.
Check for complementopathies in lupus pregnancy
SAN FRANCISCO – It’s important to check for complementopathies in pregnant women with lupus, according to Michelle Petri, MD, a professor of rheumatology at Johns Hopkins University, Baltimore.
A new diagnosis being developed at Hopkins and elsewhere, complementopathies involve an inappropriate activation of the alternative pathway of complement (APC), either from a mutation in a complement control protein, or, in the case of lupus, an autoantibody against one. They’ve been implicated as a major cause of hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, a condition to which women with lupus are particularly prone.
Hopkins has developed a serum test to diagnose inappropriate APC activation in a few hours, the modified Ham test. When HELLP develops in a woman with a complementopathy, the complement inhibitor eculizumab (Soliris) is proving to be a safe alternative to pregnancy termination.
“I urge you to think about using the modified Ham test, because if it is positive, you can treat HELLP without having to stop the pregnancy,” said Dr. Petri, also codirector of the Hopkins Lupus Pregnancy Center.
Lupus management has come a long way from the days when women were counseled to avoid or terminate pregnancy. Risks remain, “but many pregnancies are successful. I think that for every woman with lupus, we do want to offer the possibility of successful pregnancy,” she said.
Disease control is key. Preterm birth, the most common adverse outcome in lupus, correlates closely with disease activity, and disease activity can be controlled with hydroxychloroquine, and, when needed, azathioprine and tacrolimus for renal flairs.
“But these kinds of basic lessons – we need hydroxychloroquine in pregnancy; we must control disease activity – are not heard out in the real world. Claims data have shown that pregnant women with lupus actually take fewer prescribed medications, and they have fewer rheumatology visits.” It’s a problem that needs to be addressed, Dr. Petri said.
Vitamin D is also important. Hopkins has shown that supplementation to hit a level of 40 ng/mL reduces both global and renal disease activity without toxicity; studies in the general population have shown reduced preeclampsia, preterm birth, and low birth weight, all concerns in lupus.
“I haven’t convinced the world of lupus how important vitamin D is,” but “I actually love it just as much as I love hydroxychloroquine,” Dr. Petri said.
Cosupplementation with calcium complicates matters. Together, they seem to reduce the risk of preeclampsia, but increase the risk of preterm birth. More needs to be known, so “for all of us with pregnancy cohorts, it’s time to start to record vitamin D and calcium levels so we can look at this,” she said.
Dr. Petri has worked with numerous companies.
SAN FRANCISCO – It’s important to check for complementopathies in pregnant women with lupus, according to Michelle Petri, MD, a professor of rheumatology at Johns Hopkins University, Baltimore.
A new diagnosis being developed at Hopkins and elsewhere, complementopathies involve an inappropriate activation of the alternative pathway of complement (APC), either from a mutation in a complement control protein, or, in the case of lupus, an autoantibody against one. They’ve been implicated as a major cause of hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, a condition to which women with lupus are particularly prone.
Hopkins has developed a serum test to diagnose inappropriate APC activation in a few hours, the modified Ham test. When HELLP develops in a woman with a complementopathy, the complement inhibitor eculizumab (Soliris) is proving to be a safe alternative to pregnancy termination.
“I urge you to think about using the modified Ham test, because if it is positive, you can treat HELLP without having to stop the pregnancy,” said Dr. Petri, also codirector of the Hopkins Lupus Pregnancy Center.
Lupus management has come a long way from the days when women were counseled to avoid or terminate pregnancy. Risks remain, “but many pregnancies are successful. I think that for every woman with lupus, we do want to offer the possibility of successful pregnancy,” she said.
Disease control is key. Preterm birth, the most common adverse outcome in lupus, correlates closely with disease activity, and disease activity can be controlled with hydroxychloroquine, and, when needed, azathioprine and tacrolimus for renal flairs.
“But these kinds of basic lessons – we need hydroxychloroquine in pregnancy; we must control disease activity – are not heard out in the real world. Claims data have shown that pregnant women with lupus actually take fewer prescribed medications, and they have fewer rheumatology visits.” It’s a problem that needs to be addressed, Dr. Petri said.
Vitamin D is also important. Hopkins has shown that supplementation to hit a level of 40 ng/mL reduces both global and renal disease activity without toxicity; studies in the general population have shown reduced preeclampsia, preterm birth, and low birth weight, all concerns in lupus.
“I haven’t convinced the world of lupus how important vitamin D is,” but “I actually love it just as much as I love hydroxychloroquine,” Dr. Petri said.
Cosupplementation with calcium complicates matters. Together, they seem to reduce the risk of preeclampsia, but increase the risk of preterm birth. More needs to be known, so “for all of us with pregnancy cohorts, it’s time to start to record vitamin D and calcium levels so we can look at this,” she said.
Dr. Petri has worked with numerous companies.
SAN FRANCISCO – It’s important to check for complementopathies in pregnant women with lupus, according to Michelle Petri, MD, a professor of rheumatology at Johns Hopkins University, Baltimore.
A new diagnosis being developed at Hopkins and elsewhere, complementopathies involve an inappropriate activation of the alternative pathway of complement (APC), either from a mutation in a complement control protein, or, in the case of lupus, an autoantibody against one. They’ve been implicated as a major cause of hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, a condition to which women with lupus are particularly prone.
Hopkins has developed a serum test to diagnose inappropriate APC activation in a few hours, the modified Ham test. When HELLP develops in a woman with a complementopathy, the complement inhibitor eculizumab (Soliris) is proving to be a safe alternative to pregnancy termination.
“I urge you to think about using the modified Ham test, because if it is positive, you can treat HELLP without having to stop the pregnancy,” said Dr. Petri, also codirector of the Hopkins Lupus Pregnancy Center.
Lupus management has come a long way from the days when women were counseled to avoid or terminate pregnancy. Risks remain, “but many pregnancies are successful. I think that for every woman with lupus, we do want to offer the possibility of successful pregnancy,” she said.
Disease control is key. Preterm birth, the most common adverse outcome in lupus, correlates closely with disease activity, and disease activity can be controlled with hydroxychloroquine, and, when needed, azathioprine and tacrolimus for renal flairs.
“But these kinds of basic lessons – we need hydroxychloroquine in pregnancy; we must control disease activity – are not heard out in the real world. Claims data have shown that pregnant women with lupus actually take fewer prescribed medications, and they have fewer rheumatology visits.” It’s a problem that needs to be addressed, Dr. Petri said.
Vitamin D is also important. Hopkins has shown that supplementation to hit a level of 40 ng/mL reduces both global and renal disease activity without toxicity; studies in the general population have shown reduced preeclampsia, preterm birth, and low birth weight, all concerns in lupus.
“I haven’t convinced the world of lupus how important vitamin D is,” but “I actually love it just as much as I love hydroxychloroquine,” Dr. Petri said.
Cosupplementation with calcium complicates matters. Together, they seem to reduce the risk of preeclampsia, but increase the risk of preterm birth. More needs to be known, so “for all of us with pregnancy cohorts, it’s time to start to record vitamin D and calcium levels so we can look at this,” she said.
Dr. Petri has worked with numerous companies.
EXPERT ANALYSIS FROM LUPUS 2019
C-section linked to serious infection in preschoolers
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
REPORTING FROM ESPID 2019