User login
How ovarian reserve testing can (and cannot) address your patients’ fertility concerns
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.
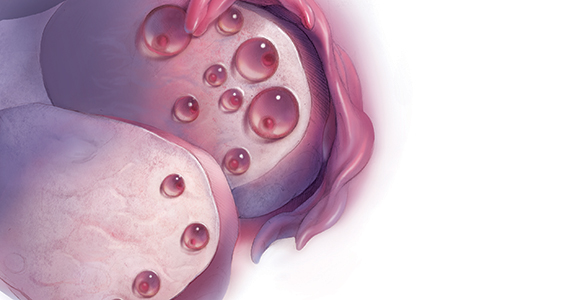
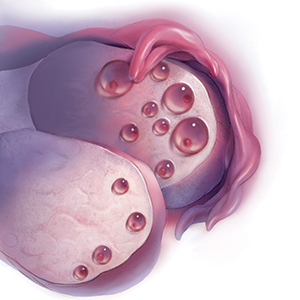
A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
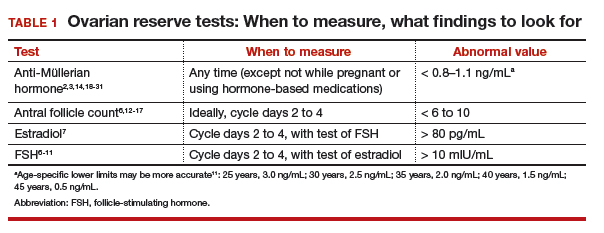
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7
Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
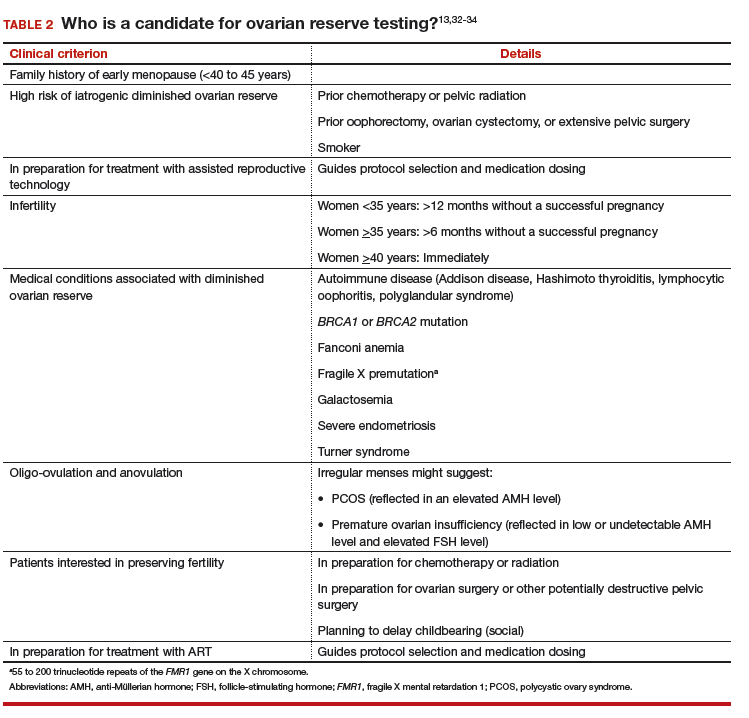
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.
Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.

A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7

Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.

Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.

A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7

Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.

Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.
Subclinical Hypothyroidism in Pregnancy
Vaccine protects against flu-related hospitalizations in pregnancy
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
FROM AN ACIP MEETING
Shorter interpregnancy intervals may increase risk of adverse outcomes
Short interpregnancy intervals carry an increased risk of adverse pregnancy outcomes for women of all ages and increased adverse fetal and infant outcome risks for women between 20 and 34 years old, according to research published in JAMA Internal Medicine.
“This finding may be reassuring particularly for older women who must weigh the competing risks of increasing maternal age with longer interpregnancy intervals (including infertility and chromosomal anomalies) against the risks of short interpregnancy intervals,” wrote Laura Schummers, SD, of the department of epidemiology at Harvard T. H. Chan School of Public Health, Boston, and her colleagues.
The researchers examined 148,544 pregnancies of women in British Columbia who were younger than 20 years old at the index (5%), 20-34 years at the index birth (83%), and 35 years or older (12%). The women had two or more consecutive singleton pregnancies that resulted in a live birth between 2004 and 2014 and were recorded in the British Columbia Perinatal Data Registry. There was a lower number of short interpregnancy intervals, defined as less than 6 months between the index and second pregnancy, among women in the 35-years-or-older group, compared with the 20- to 34-year-old group (4.4% vs. 5.5%); the 35-years-or-older group instead had a higher number of interpregnancy intervals between 6 and 11 months and between 12 and 17 months, compared with the 20- to 34-year-old group (17.7% vs. 16.6%, and 25.2% vs. 22.5%, respectively).
The risk for maternal mortality or severe morbidity was higher in women who were a minimum 35 years old with 6 months between pregnancies (0.62%), compared with women who had 18 months (0.26%) between pregnancies (adjusted relative risk [aRR], 2.39). There was no significant increase in those aged between 20 and 34 years at 6 months, compared with 18 months (0.23% vs. 0.25%; aRR, 0.92). However, the 20- to 34-year-old group did have an increased risk of fetal and infant adverse outcomes at 6 months, compared with 18 months (2.0% vs. 1.4%; aRR, 1.42) and compared with women in the 35-years-or-older group at 6 months and 18 months (2.1% vs. 1.8%; aRR, 1.15).
There was a 5.3% increased risk at 6 months and a 3.2% increased risk at 18 months of spontaneous preterm delivery in the 20- to 34-year-old group (aRR, 1.65), compared with a 5.0% risk at 6 months and 3.6% at 18 months in the 35-years-or-older group (aRR, 1.40). The researchers noted “modest increases” in newborns who were born small for their gestational age and indicated preterm delivery at short intervals that did not differ by age group.
The authors reported no conflicts of interest. Dr Schummers was supported a National Research Service Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and received a grant from the Canadian Institutes for Health Research and the Public Health Agency of Canada Family Planning Public Health Chair Seed Grant. Two of her coauthors were supported by various other awards.
SOURCE: Schummers L et al. JAMA Intern Med. 2018 Oct 29. doi: 10.1001/jamainternmed.2018.4696.
While the findings of Schummers et al. appear to encourage pregnancy spacing among women of all ages, women who are 35 or older should be counseled differently than women aged 20-34 years, Stephanie B. Teal, MD, MPH, and Jeanelle Sheeder, MSPH, PhD, wrote in a related editorial.
“Clinicians should understand that women delivering at age 35 years or later may desire more children and may wish to conceive sooner than recommended,” the authors wrote.
Women who are 35 years old or older may not have 6-12 months to delay pregnancy, the authors explained, and thus should be counseled differently than younger patients. Delaying pregnancy in older women may increase the risk of miscarriage and chromosomal abnormalities, and may cause families to miss out on their desired family size. In addition, spacing out births up to 24 months apart does not significantly diminish the risk of fetal or infant risk among women 35 years and older as it does for younger women, which may make short interpregnancy intervals in this group a “rational choice.”
“Simply telling older women to delay conception is not likely to improve health outcomes, as women are aware of their ‘biological clocks’ and many will value their desire for another child over their physician’s warnings,” Dr. Teal and Dr. Sheeder noted. “Clinicians should use patient-centered counseling and shared decision-making strategies that respect women’s desires for pregnancy, possibly at short intervals in women 35 years or older, and adequately discuss fetal, infant, and maternal risks in this context.”
Dr. Teal and Dr. Sheeder are in the division of family planning in the department of obstetrics and gynecology at the University of Colorado in Aurora. Their their comments were made in an editorial in JAMA Internal Medicine (2018 Oct 29. doi: 10.1001/jamainternmed.2018.4734 ). They reported no conflicts of interest.
While the findings of Schummers et al. appear to encourage pregnancy spacing among women of all ages, women who are 35 or older should be counseled differently than women aged 20-34 years, Stephanie B. Teal, MD, MPH, and Jeanelle Sheeder, MSPH, PhD, wrote in a related editorial.
“Clinicians should understand that women delivering at age 35 years or later may desire more children and may wish to conceive sooner than recommended,” the authors wrote.
Women who are 35 years old or older may not have 6-12 months to delay pregnancy, the authors explained, and thus should be counseled differently than younger patients. Delaying pregnancy in older women may increase the risk of miscarriage and chromosomal abnormalities, and may cause families to miss out on their desired family size. In addition, spacing out births up to 24 months apart does not significantly diminish the risk of fetal or infant risk among women 35 years and older as it does for younger women, which may make short interpregnancy intervals in this group a “rational choice.”
“Simply telling older women to delay conception is not likely to improve health outcomes, as women are aware of their ‘biological clocks’ and many will value their desire for another child over their physician’s warnings,” Dr. Teal and Dr. Sheeder noted. “Clinicians should use patient-centered counseling and shared decision-making strategies that respect women’s desires for pregnancy, possibly at short intervals in women 35 years or older, and adequately discuss fetal, infant, and maternal risks in this context.”
Dr. Teal and Dr. Sheeder are in the division of family planning in the department of obstetrics and gynecology at the University of Colorado in Aurora. Their their comments were made in an editorial in JAMA Internal Medicine (2018 Oct 29. doi: 10.1001/jamainternmed.2018.4734 ). They reported no conflicts of interest.
While the findings of Schummers et al. appear to encourage pregnancy spacing among women of all ages, women who are 35 or older should be counseled differently than women aged 20-34 years, Stephanie B. Teal, MD, MPH, and Jeanelle Sheeder, MSPH, PhD, wrote in a related editorial.
“Clinicians should understand that women delivering at age 35 years or later may desire more children and may wish to conceive sooner than recommended,” the authors wrote.
Women who are 35 years old or older may not have 6-12 months to delay pregnancy, the authors explained, and thus should be counseled differently than younger patients. Delaying pregnancy in older women may increase the risk of miscarriage and chromosomal abnormalities, and may cause families to miss out on their desired family size. In addition, spacing out births up to 24 months apart does not significantly diminish the risk of fetal or infant risk among women 35 years and older as it does for younger women, which may make short interpregnancy intervals in this group a “rational choice.”
“Simply telling older women to delay conception is not likely to improve health outcomes, as women are aware of their ‘biological clocks’ and many will value their desire for another child over their physician’s warnings,” Dr. Teal and Dr. Sheeder noted. “Clinicians should use patient-centered counseling and shared decision-making strategies that respect women’s desires for pregnancy, possibly at short intervals in women 35 years or older, and adequately discuss fetal, infant, and maternal risks in this context.”
Dr. Teal and Dr. Sheeder are in the division of family planning in the department of obstetrics and gynecology at the University of Colorado in Aurora. Their their comments were made in an editorial in JAMA Internal Medicine (2018 Oct 29. doi: 10.1001/jamainternmed.2018.4734 ). They reported no conflicts of interest.
Short interpregnancy intervals carry an increased risk of adverse pregnancy outcomes for women of all ages and increased adverse fetal and infant outcome risks for women between 20 and 34 years old, according to research published in JAMA Internal Medicine.
“This finding may be reassuring particularly for older women who must weigh the competing risks of increasing maternal age with longer interpregnancy intervals (including infertility and chromosomal anomalies) against the risks of short interpregnancy intervals,” wrote Laura Schummers, SD, of the department of epidemiology at Harvard T. H. Chan School of Public Health, Boston, and her colleagues.
The researchers examined 148,544 pregnancies of women in British Columbia who were younger than 20 years old at the index (5%), 20-34 years at the index birth (83%), and 35 years or older (12%). The women had two or more consecutive singleton pregnancies that resulted in a live birth between 2004 and 2014 and were recorded in the British Columbia Perinatal Data Registry. There was a lower number of short interpregnancy intervals, defined as less than 6 months between the index and second pregnancy, among women in the 35-years-or-older group, compared with the 20- to 34-year-old group (4.4% vs. 5.5%); the 35-years-or-older group instead had a higher number of interpregnancy intervals between 6 and 11 months and between 12 and 17 months, compared with the 20- to 34-year-old group (17.7% vs. 16.6%, and 25.2% vs. 22.5%, respectively).
The risk for maternal mortality or severe morbidity was higher in women who were a minimum 35 years old with 6 months between pregnancies (0.62%), compared with women who had 18 months (0.26%) between pregnancies (adjusted relative risk [aRR], 2.39). There was no significant increase in those aged between 20 and 34 years at 6 months, compared with 18 months (0.23% vs. 0.25%; aRR, 0.92). However, the 20- to 34-year-old group did have an increased risk of fetal and infant adverse outcomes at 6 months, compared with 18 months (2.0% vs. 1.4%; aRR, 1.42) and compared with women in the 35-years-or-older group at 6 months and 18 months (2.1% vs. 1.8%; aRR, 1.15).
There was a 5.3% increased risk at 6 months and a 3.2% increased risk at 18 months of spontaneous preterm delivery in the 20- to 34-year-old group (aRR, 1.65), compared with a 5.0% risk at 6 months and 3.6% at 18 months in the 35-years-or-older group (aRR, 1.40). The researchers noted “modest increases” in newborns who were born small for their gestational age and indicated preterm delivery at short intervals that did not differ by age group.
The authors reported no conflicts of interest. Dr Schummers was supported a National Research Service Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and received a grant from the Canadian Institutes for Health Research and the Public Health Agency of Canada Family Planning Public Health Chair Seed Grant. Two of her coauthors were supported by various other awards.
SOURCE: Schummers L et al. JAMA Intern Med. 2018 Oct 29. doi: 10.1001/jamainternmed.2018.4696.
Short interpregnancy intervals carry an increased risk of adverse pregnancy outcomes for women of all ages and increased adverse fetal and infant outcome risks for women between 20 and 34 years old, according to research published in JAMA Internal Medicine.
“This finding may be reassuring particularly for older women who must weigh the competing risks of increasing maternal age with longer interpregnancy intervals (including infertility and chromosomal anomalies) against the risks of short interpregnancy intervals,” wrote Laura Schummers, SD, of the department of epidemiology at Harvard T. H. Chan School of Public Health, Boston, and her colleagues.
The researchers examined 148,544 pregnancies of women in British Columbia who were younger than 20 years old at the index (5%), 20-34 years at the index birth (83%), and 35 years or older (12%). The women had two or more consecutive singleton pregnancies that resulted in a live birth between 2004 and 2014 and were recorded in the British Columbia Perinatal Data Registry. There was a lower number of short interpregnancy intervals, defined as less than 6 months between the index and second pregnancy, among women in the 35-years-or-older group, compared with the 20- to 34-year-old group (4.4% vs. 5.5%); the 35-years-or-older group instead had a higher number of interpregnancy intervals between 6 and 11 months and between 12 and 17 months, compared with the 20- to 34-year-old group (17.7% vs. 16.6%, and 25.2% vs. 22.5%, respectively).
The risk for maternal mortality or severe morbidity was higher in women who were a minimum 35 years old with 6 months between pregnancies (0.62%), compared with women who had 18 months (0.26%) between pregnancies (adjusted relative risk [aRR], 2.39). There was no significant increase in those aged between 20 and 34 years at 6 months, compared with 18 months (0.23% vs. 0.25%; aRR, 0.92). However, the 20- to 34-year-old group did have an increased risk of fetal and infant adverse outcomes at 6 months, compared with 18 months (2.0% vs. 1.4%; aRR, 1.42) and compared with women in the 35-years-or-older group at 6 months and 18 months (2.1% vs. 1.8%; aRR, 1.15).
There was a 5.3% increased risk at 6 months and a 3.2% increased risk at 18 months of spontaneous preterm delivery in the 20- to 34-year-old group (aRR, 1.65), compared with a 5.0% risk at 6 months and 3.6% at 18 months in the 35-years-or-older group (aRR, 1.40). The researchers noted “modest increases” in newborns who were born small for their gestational age and indicated preterm delivery at short intervals that did not differ by age group.
The authors reported no conflicts of interest. Dr Schummers was supported a National Research Service Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and received a grant from the Canadian Institutes for Health Research and the Public Health Agency of Canada Family Planning Public Health Chair Seed Grant. Two of her coauthors were supported by various other awards.
SOURCE: Schummers L et al. JAMA Intern Med. 2018 Oct 29. doi: 10.1001/jamainternmed.2018.4696.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: The risk for maternal mortality or severe morbidity was higher in women who were a minimum 35 years old with 6 months between pregnancies (0.62%), compared with women who had 18 months (0.26%) between pregnancies (adjusted relative risk, 2.39).
Study details: A cohort study of 148,544 pregnancies in Canada between 2004 and 2014.
Disclosures: The authors reported no conflicts of interest. Dr Schummers was supported a National Research Service Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and received a grant from the Canadian Institutes for Health Research and the Public Health Agency of Canada Family Planning Public Health Chair Seed Grant. Two of her coauthors were supported by other awards.
Source: Schummers L et al. JAMA Intern Med. 2018 Oct 29. doi: 10.1001/jamainternmed.2018.4696.
ACR readies first-ever guidelines on managing reproductive health in rheumatology
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM THE ACR ANNUAL MEETING
Understanding hypertensive disorders in pregnancy
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Clarifying the categories of hypertensive disorders in pregnancy
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Low and high BMI tied to higher postpartum depression risk
Women with high and low body mass index in the first trimester of their first pregnancies are at an increased risk of developing postpartum depression, a population-based study of more than 600,000 new mothers shows.
“Our findings show a U-shaped association between BMI extremes and clinically significant depression after childbirth,” Michael E. Silverman, PhD, and his associates reported in the Journal of Affective Disorders. “Specifically, women in the lowest and highest groups were at a significantly increased risk for developing [postpartum depression].”
Dr. Silverman and his associates used the Swedish Medical Birth Register to identify women who delivered first live singleton infants from 1997 to 2008. They then calculated the risk of postpartum depression in relation to each woman’s BMI and history of depression. Postpartum depression was defined as a clinical depression diagnosis within 1 year after delivery, Dr. Silverman and his associates wrote.
The investigators found that women with low BMI (less than 18.5 kg/m2) were at an increased postpartum depression risk (relative risk [RR], 1.52; 95% confidence interval, 1.30-1.78), as were those with high BMI (greater than 35 kg/m2) (RR, 1.23; 95% CI, 1.04-1.45).
In addition, an important difference was found between women with low and high BMI.
“Women in the highest BMI group were only at an increased risk for [postpartum depression] if they had no history of depression, showing for the first time how [postpartum depression] risk factors associated with BMI are modified by maternal depression history,” said Dr. Silverman of the department of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, and his associates.
The investigators cited several limitations. For example, only first births were analyzed, which suggests that the incidence of postpartum depression might have been underestimated. Another limitation is that the registry might not have captured women with mild depression. Nevertheless, they said, the study has important implications.
“ Because pregnant women represent a medically captured population,” they wrote, the findings support implementing preventive strategies for postpartum depression and health literacy for high-risk women.
Dr. Silverman and his associates reported having no conflicts of interest. The study was supported by a grant from the National Institutes of Health.
SOURCE: Silverman ME et al. J Affect Disord. 2018 Nov;240:193-8.
Women with high and low body mass index in the first trimester of their first pregnancies are at an increased risk of developing postpartum depression, a population-based study of more than 600,000 new mothers shows.
“Our findings show a U-shaped association between BMI extremes and clinically significant depression after childbirth,” Michael E. Silverman, PhD, and his associates reported in the Journal of Affective Disorders. “Specifically, women in the lowest and highest groups were at a significantly increased risk for developing [postpartum depression].”
Dr. Silverman and his associates used the Swedish Medical Birth Register to identify women who delivered first live singleton infants from 1997 to 2008. They then calculated the risk of postpartum depression in relation to each woman’s BMI and history of depression. Postpartum depression was defined as a clinical depression diagnosis within 1 year after delivery, Dr. Silverman and his associates wrote.
The investigators found that women with low BMI (less than 18.5 kg/m2) were at an increased postpartum depression risk (relative risk [RR], 1.52; 95% confidence interval, 1.30-1.78), as were those with high BMI (greater than 35 kg/m2) (RR, 1.23; 95% CI, 1.04-1.45).
In addition, an important difference was found between women with low and high BMI.
“Women in the highest BMI group were only at an increased risk for [postpartum depression] if they had no history of depression, showing for the first time how [postpartum depression] risk factors associated with BMI are modified by maternal depression history,” said Dr. Silverman of the department of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, and his associates.
The investigators cited several limitations. For example, only first births were analyzed, which suggests that the incidence of postpartum depression might have been underestimated. Another limitation is that the registry might not have captured women with mild depression. Nevertheless, they said, the study has important implications.
“ Because pregnant women represent a medically captured population,” they wrote, the findings support implementing preventive strategies for postpartum depression and health literacy for high-risk women.
Dr. Silverman and his associates reported having no conflicts of interest. The study was supported by a grant from the National Institutes of Health.
SOURCE: Silverman ME et al. J Affect Disord. 2018 Nov;240:193-8.
Women with high and low body mass index in the first trimester of their first pregnancies are at an increased risk of developing postpartum depression, a population-based study of more than 600,000 new mothers shows.
“Our findings show a U-shaped association between BMI extremes and clinically significant depression after childbirth,” Michael E. Silverman, PhD, and his associates reported in the Journal of Affective Disorders. “Specifically, women in the lowest and highest groups were at a significantly increased risk for developing [postpartum depression].”
Dr. Silverman and his associates used the Swedish Medical Birth Register to identify women who delivered first live singleton infants from 1997 to 2008. They then calculated the risk of postpartum depression in relation to each woman’s BMI and history of depression. Postpartum depression was defined as a clinical depression diagnosis within 1 year after delivery, Dr. Silverman and his associates wrote.
The investigators found that women with low BMI (less than 18.5 kg/m2) were at an increased postpartum depression risk (relative risk [RR], 1.52; 95% confidence interval, 1.30-1.78), as were those with high BMI (greater than 35 kg/m2) (RR, 1.23; 95% CI, 1.04-1.45).
In addition, an important difference was found between women with low and high BMI.
“Women in the highest BMI group were only at an increased risk for [postpartum depression] if they had no history of depression, showing for the first time how [postpartum depression] risk factors associated with BMI are modified by maternal depression history,” said Dr. Silverman of the department of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, and his associates.
The investigators cited several limitations. For example, only first births were analyzed, which suggests that the incidence of postpartum depression might have been underestimated. Another limitation is that the registry might not have captured women with mild depression. Nevertheless, they said, the study has important implications.
“ Because pregnant women represent a medically captured population,” they wrote, the findings support implementing preventive strategies for postpartum depression and health literacy for high-risk women.
Dr. Silverman and his associates reported having no conflicts of interest. The study was supported by a grant from the National Institutes of Health.
SOURCE: Silverman ME et al. J Affect Disord. 2018 Nov;240:193-8.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
HF unlikely in pregnant cancer survivors without history of cardiotoxicity
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Does the preterm birth racial disparity persist among black and white IVF users?
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1
Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1

Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1

Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.