User login
Alarming gaps in gestational diabetes care
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
Prenatal exposure to hydroxychloroquine cuts risk of neonatal cutaneous lupus
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
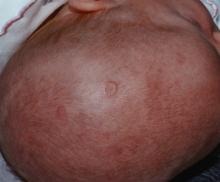
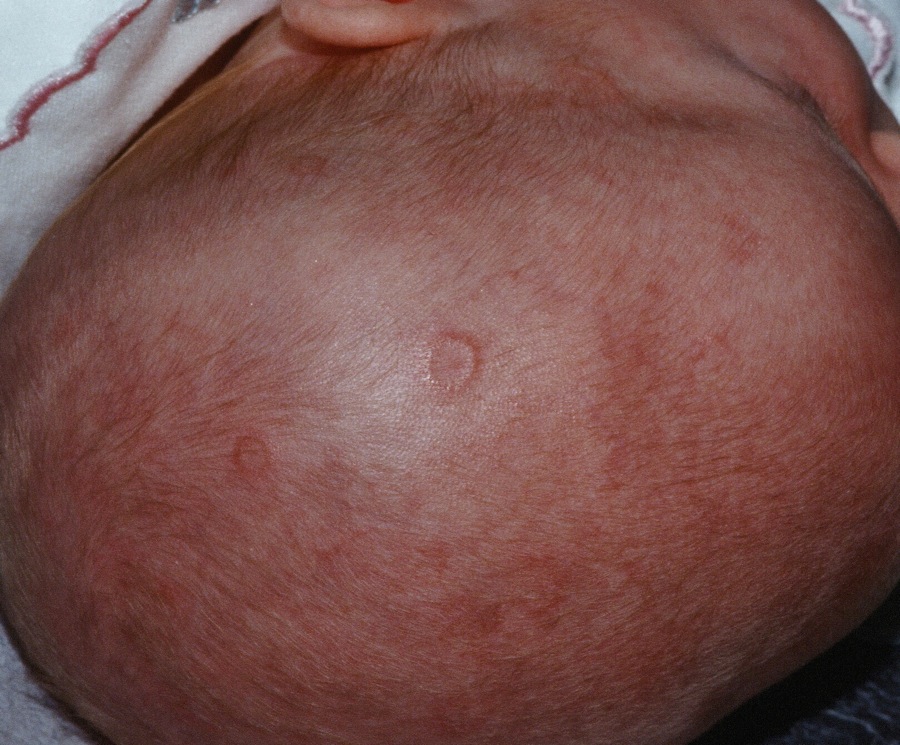
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
[email protected]
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The drug was associated with a 60% decreased risk of developing the disorder.
Data source: The case-control study involved 545 infants.
Disclosures: Dr. Barsalou had no financial disclosures.
Autism risk not increased by maternal influenza infection during pregnancy
Maternal influenza infection during pregnancy does not increase the risk for autism spectrum disorder (ASD) in children, according to Ousseny Zerbo, PhD, and associates.
In a study of 196,929 mother-child pairs (the children were born at Kaiser Permanente Northern California between Jan. 1, 2000, and Dec. 31, 2010), 1.6% of the children were diagnosed with ASD. Influenza was diagnosed in 0.7% of mothers during their pregnancy, and 23% received an influenza vaccination during pregnancy.
Overall, maternal influenza vaccination did not effect likelihood of ASD diagnosis, with 1.7% of children in this group receiving an ASD diagnosis. A small association between ASD diagnosis and maternal influenza vaccination, however, was seen in the first trimester of pregnancy, with an adjusted hazard ratio of 1.2, translating to a potential extra 4 cases of autism per 1,000 births. But further analysis suggested that this could be caused by bias and chance, and “the association was insignificant after statistical correction for multiple comparisons,” the investigators said.
“While we do not advocate changes in vaccine policy or practice, we believe that additional studies are warranted to further evaluate any potential associations between first-trimester maternal influenza vaccination and autism,” the investigators concluded.
Find the full study in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2016.3609).
Maternal influenza infection during pregnancy does not increase the risk for autism spectrum disorder (ASD) in children, according to Ousseny Zerbo, PhD, and associates.
In a study of 196,929 mother-child pairs (the children were born at Kaiser Permanente Northern California between Jan. 1, 2000, and Dec. 31, 2010), 1.6% of the children were diagnosed with ASD. Influenza was diagnosed in 0.7% of mothers during their pregnancy, and 23% received an influenza vaccination during pregnancy.
Overall, maternal influenza vaccination did not effect likelihood of ASD diagnosis, with 1.7% of children in this group receiving an ASD diagnosis. A small association between ASD diagnosis and maternal influenza vaccination, however, was seen in the first trimester of pregnancy, with an adjusted hazard ratio of 1.2, translating to a potential extra 4 cases of autism per 1,000 births. But further analysis suggested that this could be caused by bias and chance, and “the association was insignificant after statistical correction for multiple comparisons,” the investigators said.
“While we do not advocate changes in vaccine policy or practice, we believe that additional studies are warranted to further evaluate any potential associations between first-trimester maternal influenza vaccination and autism,” the investigators concluded.
Find the full study in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2016.3609).
Maternal influenza infection during pregnancy does not increase the risk for autism spectrum disorder (ASD) in children, according to Ousseny Zerbo, PhD, and associates.
In a study of 196,929 mother-child pairs (the children were born at Kaiser Permanente Northern California between Jan. 1, 2000, and Dec. 31, 2010), 1.6% of the children were diagnosed with ASD. Influenza was diagnosed in 0.7% of mothers during their pregnancy, and 23% received an influenza vaccination during pregnancy.
Overall, maternal influenza vaccination did not effect likelihood of ASD diagnosis, with 1.7% of children in this group receiving an ASD diagnosis. A small association between ASD diagnosis and maternal influenza vaccination, however, was seen in the first trimester of pregnancy, with an adjusted hazard ratio of 1.2, translating to a potential extra 4 cases of autism per 1,000 births. But further analysis suggested that this could be caused by bias and chance, and “the association was insignificant after statistical correction for multiple comparisons,” the investigators said.
“While we do not advocate changes in vaccine policy or practice, we believe that additional studies are warranted to further evaluate any potential associations between first-trimester maternal influenza vaccination and autism,” the investigators concluded.
Find the full study in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2016.3609).
FROM JAMA PEDIATRICS
Preventing infection after cesarean delivery: 5 more evidence-based measures to consider
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
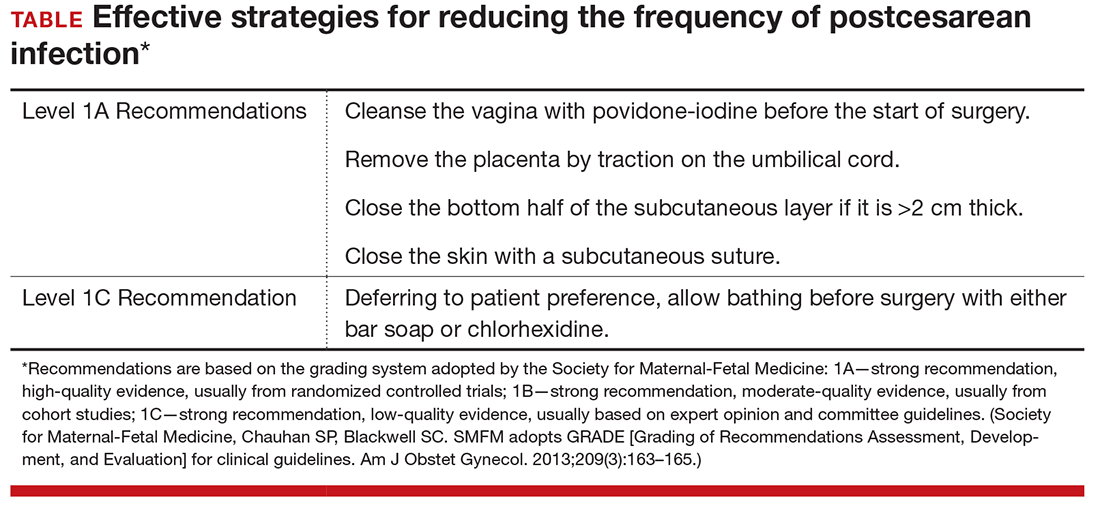
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.
Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.

Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.

Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
Early-onset preeclampsia more likely to occur in women with lupus
WASHINGTON – Women with systemic lupus erythematosus are nine times more likely to develop early-onset preeclampsia during a first pregnancy than are women without the disease, according a study of two Swedish national population-based registries.
The risk of preeclampsia occurring before 34 weeks’ gestation declined with subsequent pregnancies, but it remained significantly elevated above the background risk, Julia F. Simard, ScD, said at the annual meeting of the American College of Rheumatology.
During the study period, 742 births to women with SLE were matched with 10,484 births to women without the disease. The mean age of the patients was 31 years.
Of the women with SLE, 5% had pregestational hypertension and 3% had pregestational diabetes. Antiphospholipid antibodies were present in 2%. Among the controls, less than 1% had pregestational hypertension, and 1.3% had pregestational diabetes. There were no healthy controls with antiphospholipid antibodies.
In the entire cohort, there were 438 cases of preeclampsia: 82 in the SLE group and 356 in the control group. In the fully adjusted model, this translated to nearly a tripling of relative risk (RR, 2.7).
Preeclampsia more commonly occurred in first births, based on 56 cases in the SLE group and 225 in the control group. SLE patients in their first pregnancy also had a tripling of risk (RR, 3.2). Among subsequent births, there were 157 cases: 26 in the SLE group and 131 in the control group. The relative risk for preeclampsia was lower, but still significantly elevated (RR, 2).
There were 87 cases of early-onset preeclampsia: 32 in the SLE group and 55 in the control group. Women with SLE were more than six times more likely to develop the disorder (RR, 6.3). Early-onset preeclampsia was more common in first births for both groups: 24 in the SLE group and 34 in the control group, for a ninefold increased risk (RR, 9.3).
Again, the incidence decreased with subsequent births in both groups: 8 cases in the SLE group and 21 in the control group. But SLE patients still faced a significant threefold increase in risk (RR, 2.8).
“Antiphospholipid antibodies appear to be an important risk factor that needs to more fully understood,” Dr. Simard said. “But the risk seems to be independent of other traditional risk factors, like pregestational hypertension, body mass index, and smoking.”
She and her associates had no financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Women with systemic lupus erythematosus are nine times more likely to develop early-onset preeclampsia during a first pregnancy than are women without the disease, according a study of two Swedish national population-based registries.
The risk of preeclampsia occurring before 34 weeks’ gestation declined with subsequent pregnancies, but it remained significantly elevated above the background risk, Julia F. Simard, ScD, said at the annual meeting of the American College of Rheumatology.
During the study period, 742 births to women with SLE were matched with 10,484 births to women without the disease. The mean age of the patients was 31 years.
Of the women with SLE, 5% had pregestational hypertension and 3% had pregestational diabetes. Antiphospholipid antibodies were present in 2%. Among the controls, less than 1% had pregestational hypertension, and 1.3% had pregestational diabetes. There were no healthy controls with antiphospholipid antibodies.
In the entire cohort, there were 438 cases of preeclampsia: 82 in the SLE group and 356 in the control group. In the fully adjusted model, this translated to nearly a tripling of relative risk (RR, 2.7).
Preeclampsia more commonly occurred in first births, based on 56 cases in the SLE group and 225 in the control group. SLE patients in their first pregnancy also had a tripling of risk (RR, 3.2). Among subsequent births, there were 157 cases: 26 in the SLE group and 131 in the control group. The relative risk for preeclampsia was lower, but still significantly elevated (RR, 2).
There were 87 cases of early-onset preeclampsia: 32 in the SLE group and 55 in the control group. Women with SLE were more than six times more likely to develop the disorder (RR, 6.3). Early-onset preeclampsia was more common in first births for both groups: 24 in the SLE group and 34 in the control group, for a ninefold increased risk (RR, 9.3).
Again, the incidence decreased with subsequent births in both groups: 8 cases in the SLE group and 21 in the control group. But SLE patients still faced a significant threefold increase in risk (RR, 2.8).
“Antiphospholipid antibodies appear to be an important risk factor that needs to more fully understood,” Dr. Simard said. “But the risk seems to be independent of other traditional risk factors, like pregestational hypertension, body mass index, and smoking.”
She and her associates had no financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Women with systemic lupus erythematosus are nine times more likely to develop early-onset preeclampsia during a first pregnancy than are women without the disease, according a study of two Swedish national population-based registries.
The risk of preeclampsia occurring before 34 weeks’ gestation declined with subsequent pregnancies, but it remained significantly elevated above the background risk, Julia F. Simard, ScD, said at the annual meeting of the American College of Rheumatology.
During the study period, 742 births to women with SLE were matched with 10,484 births to women without the disease. The mean age of the patients was 31 years.
Of the women with SLE, 5% had pregestational hypertension and 3% had pregestational diabetes. Antiphospholipid antibodies were present in 2%. Among the controls, less than 1% had pregestational hypertension, and 1.3% had pregestational diabetes. There were no healthy controls with antiphospholipid antibodies.
In the entire cohort, there were 438 cases of preeclampsia: 82 in the SLE group and 356 in the control group. In the fully adjusted model, this translated to nearly a tripling of relative risk (RR, 2.7).
Preeclampsia more commonly occurred in first births, based on 56 cases in the SLE group and 225 in the control group. SLE patients in their first pregnancy also had a tripling of risk (RR, 3.2). Among subsequent births, there were 157 cases: 26 in the SLE group and 131 in the control group. The relative risk for preeclampsia was lower, but still significantly elevated (RR, 2).
There were 87 cases of early-onset preeclampsia: 32 in the SLE group and 55 in the control group. Women with SLE were more than six times more likely to develop the disorder (RR, 6.3). Early-onset preeclampsia was more common in first births for both groups: 24 in the SLE group and 34 in the control group, for a ninefold increased risk (RR, 9.3).
Again, the incidence decreased with subsequent births in both groups: 8 cases in the SLE group and 21 in the control group. But SLE patients still faced a significant threefold increase in risk (RR, 2.8).
“Antiphospholipid antibodies appear to be an important risk factor that needs to more fully understood,” Dr. Simard said. “But the risk seems to be independent of other traditional risk factors, like pregestational hypertension, body mass index, and smoking.”
She and her associates had no financial disclosures.
[email protected]
On Twitter @alz_gal
ACR ANNUAL MEETING
Key clinical point:
Major finding: Women with SLE in a first pregnancy had a ninefold greater risk of early-onset preeclampsia than did healthy control patients in their first pregnancy.
Data source: The data were extracted from two Swedish national population-based registries.
Disclosures: Dr, Simard and her associates had no financial disclosures.
Management of wound complications following obstetric anal sphincter injury (OASIS)
During vaginal delivery spontaneous perineal trauma and extension of episiotomy incisions are common. A severe perineal laceration that extends into or through the anal sphincter complex is referred to as an obstetric anal sphincter injury (OASIS) and requires meticulous repair. Following the repair of an OASIS, serious wound complications, including dehiscence and infection, may occur. In Europe the reported rate of OASIS varies widely among countries, with a rate of 0.1% in Romania, possibly due to underreporting, and 4.9% in Iceland.1 In the United States the rates of 3rd- and 4th-degree lacerations were reported to be 3.3% and 1.1%, respectively.2
Risk factors for OASIS include forceps delivery (odds ratio [OR], 5.50), vacuum-assisted delivery (OR, 3.98), and midline episiotomy (OR, 3.82).3 Additional risk factors for severe perineal injury at vaginal delivery include nulliparity (adjusted odds ratio [aOR], 2.58), delivery from a persistent occiput posterior position (aOR, 2.24), and above-average newborn birth weight (aOR, 1.28).4
In a meta-analysis of randomized trials, the researchers reported that restrictive use of episiotomy reduced the risk of severe perineal trauma (relative risk [RR], 0.67) but increased the risk of anterior perineal trauma (RR, 1.84).5 The American College of Obstetricians and Gynecologists (ACOG) recommends that episiotomy should not be a routine practice and is best restricted to use in a limited number of cases where fetal and maternal benefit is likely.6 In addition, ACOG recommends that if episiotomy is indicated, a mediolateral incision is favored over a midline incision. In my practice I perform only mediolateral episiotomy incisions. However, mediolateral episiotomy may be associated with an increased risk of postpartum perineal pain and dyspareunia.7 Use of warm compresses applied to the perineum during the second stage of labor may reduce the risk of 3rd- and 4th-degree lacerations.8 Techniques to ensure that the fetal head and shoulders are birthed in a slow and controlled fashion may decrease the risk of OASIS.9 See the TABLE, “Four maneuvers to control and slow the birth of the fetal head.”10–14

Related article:
Stop performing median episiotomy!
Wound complications following the repair of a 3rd- or 4th-degree laceration are reported to occur in approximately 5% to 10% of cases.15 The most common wound complications are dehiscence, infection, abscess formation, pain, sexual dysfunction, and anal incontinence. Minor wound complications, including superficial epithelial separation, can be managed expectantly. However, major wound complications need intensive treatment.
In one study of 21 women who had a major wound complication following the repair of a 4th-degree laceration, 53% had dehiscence plus infection, 33% had dehiscence alone, and 14% had infection alone.16 Major wound complications present at a mean of 5 days after delivery, with a wide range from 1 to 17 days following delivery.17 In a study of 144 cases of wound breakdown following a perineal laceration repair, the major risk factors for wound breakdown were episiotomy (aOR, 11.1), smoking (aOR, 6.4), midwife repair of laceration (aOR, 4.7), use of chromic suture (aOR, 3.9), and operative vaginal delivery (aOR, 3.4).18 In one study of 66 women with a wound complication following the repair of a 3rd- or 4th-degree laceration, clinical risk factors for a wound complication were cigarette smoking (OR, 4.04), 4th-degree laceration (OR, 1.89), and operative vaginal delivery (OR, 1.76).19 The use of intrapartum antibiotics appears to be protective (OR, 0.29) against wound complications following a major perineal laceration.19
Approach to the patient with a dehisced and infected perineal wound
Historically, wound dehiscence following surgical repair of a perineal injury was managed by allowing the wound to slowly close. This approach adversely impacts the quality of life of the affected woman because it may take weeks for the wound to heal. One small randomized trial17 and multiple case series20–24 report that an active multistep management algorithm permits early closure of the majority of these wounds, thereby accelerating the patient’s full recovery. Delayed primary (within 72 hours postpartum) or early secondary reconstruction (within 14 days of delivery) has been demonstrated to be safe with acceptable long-term functional out-comes.25 The modern approach to the treatment of a patient with an infected wound dehiscence following a severe perineal injury involves 3 steps.
Related article:
It’s time to restrict the use of episiotomy
Step 1. Restore tissue to health
The dehisced wound is cultured and, if infection is present, treatment is initiated with intravenous antibiotics appropriate for an infection with colorectal flora. One antibiotic option includes a cephalosporin (cefotetan 2 g IV every 6 hours) plus metronidazole (500 mg IV every 8 hours).
In the operating room, the wound should be thoroughly assessed, cleansed, and debrided. This step includes irrigation of the wound with a warm fluid, mechanical debridement, and sharp dissection of necrotic tissue. If the wound is infected, removal of stitches that are visible in the open wound is recommended.
Often more than one session of debridement may be needed to obtain wound edges that are free from exudate and show granulation at the wound margins. Between debridement sessions, wet-to-dry dressings are utilized. Two to 10 days of wound care may be needed before an attempt is made to close the wound. The wound is suitable for repair when there is no infected tissue and granulation tissue is present. Some surgeons prefer a mechanical bowel preparation regimen just before surgically closing the open wound. This may prevent early bowel movements and provide for tissue healing after surgery.26 The same preparations recommended for colonoscopy can be considered prior to surgical repair.
Step 2. Surgically close the wound
The wound is surgically closed in the operating room. If in Step 1 the assessment of the wound shows major trauma, assistance from a urogynecologist may be warranted. Surgical management of a perineal wound dehiscence requires a clear understanding of perineal anatomy and the structures contained between the vagina and the anorectum.
Six key structures may be involved in perineal injury: the anorectal mucosa, internal anal sphincter, external anal sphincter, vaginal wall and perineal skin, bulbocavernosus muscle, and transverse perineal muscles. It is important to definitively identify the individual structures that need to be repaired. Careful dissection is then carried out to mobilize these structures for repair. Additional debridement may be necessary to remove excess granulation tissue.
Anorectal mucosa repair. With repair of a 4th-degree perineal wound dehiscence, the apex of the defect in the anorectal mucosa is identified. The defect is repaired beginning at the apex using closely spaced interrupted sutures or a running suture of 3-0 or 4-0 polyglactin 910. Adequate tissue bites that will resist tearing should be taken. If interrupted sutures are used, tying the knots within the anorectal canal prevents them from being located within the healing wound.
Internal anal sphincter repair. After the anorectal mucosa is closed, attention is turned to reapproximation of the internal anal sphincter. The ends of a torn internal anal sphincter are often located lateral to the anorectal mucosa and appear as shiny gray-white fibrous tissue. The surgeon’s gloved index finger can be placed within the anorectal canal to aid in identification of the internal anal sphincter, as it tends to have a rubbery feel. Additionally, while the surgeon’s gloved index finger is in the anorectal canal, the surgeon’s gloved thumb can be used to retract the anorectal mucosa slightly medial and inferior so that adequate bites of the internal anal sphincter can be taken on each side.
Alternatively, Allis clamps canbe placed on the ends of the retracted internal anal sphincter to facilitate repair. Suture selection for repair of the internal anal sphincter can include 3-0 polyglactin 910 or 3-0 monofilament, delayed-absorbable suture such as polydioxanone sulfate (PDS). Some surgeons prefer delayed-absorbable suture (PDS) for this layer given the internal anal sphincter is constantly contracting and relaxing as it samples stool.26 This layer also can be closed with either interrupted sutures or a running suture.
External anal sphincter repair. After the anorectal mucosa and internal anal sphincter defects are reapproximated, attention is turned to the external anal sphincter. Like the internal anal sphincter, the ends of the external anal sphincter are often retracted laterally and must be definitively identified and mobilized in order to ensure an adequate tension-free repair. It is important to include the fascial sheath in the repair of the external anal sphincter.27 Allis clamps can be used to grasp the ends of the torn muscle after they are identified.
We recommend 0 or 2-0 PDS for repair of the external sphincter. Repair can be performed using either an end-to-end or overlapping technique. An end-to-end repair traditionally involves reapproximating the ends of the torn muscle and its overlying fascial sheath using interrupted sutures placed at four quadrants (12:00, 3:00, 6:00, 9:00).
In contrast, in an overlapping repair, the ends of the muscle are brought together with mattress sutures. Suture is passed top down through the medial aspect of the more superior muscle flap and top down through the inferior muscle flap more laterally. The same suture is then passed bottom up through the inferior muscle flap more laterally and finally bottom up through the medial aspect of the more superior muscle flap. Two to four mattress sutures are usually placed. After all sutures are placed, they are tied securely.
An overlapping repair results in a greater amount of tissue contact between the two torn muscle ends. However, adequate mobility of the external anal sphincter is necessary to perform this type of repair.
Vaginal wall and perineal body repair. After the anal sphincters have been repaired, the vaginal wall and remainder of the perineal body are reconstructed using the same techniques involved in a 2nd-degree laceration repair. Care must be taken to retrieve and reapproximate the torn ends of the bulbocavernosus muscles, which are also often retracted laterally and superiorly. After the bulbocavernosus and transverse perineal muscles are brought together in the midline, the posterior vaginal wall should be perpendicular to the perineum.
An alternative to surgical closure of a 2nd-degree dehiscence is the use of vacuum-assisted wound closure. Disadvantages of this approach include difficulty in maintaining a vacuum seal in the perineal region and the risk of wound contamination with feces. In one case report, 3 weeks of vacuum-assisted wound closure resulted in healing of a 10-cm wound dehiscence that occurred 5 days following a forceps-assisted vaginal delivery with a mediolateral episiotomy.28
Step 3. Ensure complete healing of the wound
Superb postoperative wound care helps to ensure a quick return to full recovery. Wound care should include regularly scheduled sitz baths (at least 3 times daily) followed by drying the perineum. It is preferable to provide a liquid diet that avoids frequent bowel movements in the initial 3 postoperative days. Stool softeners and fiber supplementation are recommended when a full diet is resumed. Some surgeons have found mineral oil (1 to 2 tablespoons daily) effective in producing soft stools that are easy to pass.26 Ensuring soft stool consistency is important to help prevent repair breakdown that may occur with passage of hard stools, fecal impaction, and/or straining during defecation.
We recommend follow-up 1 to 2 weeks after surgery to assess wound healing. No vaginal intercourseis permitted until complete healing is achieved.
Use a surgical checklist
All obstetricians and midwives strive to reduce the risk of OASIS at vaginal birth. When OASIS occurs, it is often useful to use a surgical checklist to ensure the execution of all steps in the management of the repair and recovery process.29 It is heartbreaking to see an OASIS repair breakdown in the week following a vaginal delivery. But by following the 3 steps outlined here, the secondary repair is likely to be successful and will quickly return most patients to full health.
Related article:
Develop and use a checklist for 3rd- and 4th-degree perineal lacerations

The authors report no financial relationships relevant to this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Blondel B, Alexander S, Bjarnadottir RI, et al; Euro-Peristat Scientific Committee. Variations in rates of severe perineal tears and episiotomies in 20 European countries: a study based on routine national data in Euro-Perstat Project. Acta Obstet Gynecol Scand. 2016;95(7):746–754.
- Friedman AM, Ananth CV, Predergast E, D’Alton ME, Wright JD. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125(4):927–937.
- Pergialiotis V, Vlachos D, Protopapas A, Pappa K, Vlachos G. Risk factors for severe perineal lacerations during childbirth. Int J Gynecol Obstet. 2014;125(1):6–14.
- Schmitz T, Alberti C, Andriss B, Moutafoff C, Oury JF, Sibony O. Identification of women at high risk for severe perineal lacerations. Eur J Obstet Gynecol Reprod Biol. 2014;182:11–15.
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.
- ACOG Committee on Practice Bulletins—Obstetrics. Practice bulletin no. 165: Prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2016;128(1):e1–e15.
- Sartore A, De Seta F, Maso G, Pregazzi R, Grimaldi E, Guaschino S. The effects of mediolateral episiotomy on pelvic floor function after vaginal delivery. Obstet Gynecol. 2004;103(4):669–673.
- Aasheim V, Nilsen AB, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2011;(12):CD006672.
- Harvey MA, Pierce M, Alter JE, et al; Society of Obstetricians and Gynaecologists of Canada. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynaecol Can. 2015;37(12):1131–1148.
- Jonsson ER, Elfaghi I, Rydhstrom H, Herbst A. Modified Ritgen’s maneuver for anal sphincter injury at delivery: a randomized controlled trial. Obstet Gynecol. 2008;112(2 pt 1):212–217.
- Williams JW. Obstetrics: A Text-book for the Use of Students and Practitioners. New York, NY: D Appleton and Co; 1903:288.
- Cunningham FG. The Ritgen maneuver: another sacred cow questioned. Obstet Gynecol. 2008;112(2 pt 1):210–211.
- Myrfield K, Brook C, Creedy D. Reducing perineal trauma: implications of flexion and extension of the fetal head during birth. Midwifery. 1997;13:197–201.
- Ostergaard Poulsen M, Lund Madsen M, Skriver-Moller AC, Overgaard C. Does the Finnish intervention prevent obstetrical anal sphincter injuries? A systematic review of the literature. BMJ Open. 2015;5:e008346.
- Kamel A, Khaled M. Episiotomy and obstetric perineal wound dehiscence: beyond soreness. J Obstet Gynaecol. 2014;34(3):215–217.
- Goldaber KG, Wendel PJ, McIntire DD, Wendel GD Jr. Postpartum perineal morbidity after fourth-degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489–493.
- Monberg J, Hammen S. Ruptured episiotomia resutured primarily. Acta Obstet Gynecol Scand. 1987;66(2):163–164.
- Jallad K, Steele SE, Barber MD. Breakdown of perineal laceration repair after vaginal delivery: a case-control study. Female Pelvic Med Reconstr Surg. 2016;22(4):276–279.
- Stock L, Basham E, Gossett DR, Lewicky-Gaupp C. Factors associated with wound complications in women with obstetric anal sphincter injuries (OASIS). Am J Obstet Gynecol. 2013;208(4):327.e1–e8.
- Hauth JC, Gilstrap LC 3rd, Ward SC, Hankins GD. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67(6):806–809.
- Hankins GD, Hauth JC, Gilstrap LC 3rd, Hammond TL, Yeomans ER, Snyder RR. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75(1):48–51.
- Ramin SM, Ramus RM, Little BB, Gilstrap LC 3rd. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167(4 pt 1):1104–1107.
- Arona AJ, Al-Marayati L, Grimes DA, Ballard CA. Early secondary repair of third- and fourth-degree perineal lacerations after outpatient wound preparation. Obstet Gynecol. 1995;86(2):294–296.
- Uygur D, Yesildaglar N, Kis S, Sipahi T. Early repair of episiotomy dehiscence. Aust N Z J Obstet Gynaecol. 2004;44(3):244–246.
- Soerensen MM, Bek KM, Buntzen S, Hojberg KE, Laurberg S. Long-term outcome of delayed primary or early secondary reconstruction of the anal sphincter after obstetrical injury. Dis Colon Rectum. 2008;51(3):312–317.
- Delancey JOL, Berger MB. Surgical approaches to postobstetrical perineal body defects (rectovaginal fistula and chronic third and fourth-degree lacerations). Clin Obstet Gynecol. 2010;53(1):134–144.
- Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician. 2003;68(8):1585–1590.
- Aviki EM, Batalden RP, del Carmen MG, Berkowitz LR. Vacuum-assisted closure for episiotomy dehiscence. Obstet Gynecol. 2015;126(3):530–533.
- Barbieri RL. Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. OBG Manag. 2013;25(8):8–12.
During vaginal delivery spontaneous perineal trauma and extension of episiotomy incisions are common. A severe perineal laceration that extends into or through the anal sphincter complex is referred to as an obstetric anal sphincter injury (OASIS) and requires meticulous repair. Following the repair of an OASIS, serious wound complications, including dehiscence and infection, may occur. In Europe the reported rate of OASIS varies widely among countries, with a rate of 0.1% in Romania, possibly due to underreporting, and 4.9% in Iceland.1 In the United States the rates of 3rd- and 4th-degree lacerations were reported to be 3.3% and 1.1%, respectively.2
Risk factors for OASIS include forceps delivery (odds ratio [OR], 5.50), vacuum-assisted delivery (OR, 3.98), and midline episiotomy (OR, 3.82).3 Additional risk factors for severe perineal injury at vaginal delivery include nulliparity (adjusted odds ratio [aOR], 2.58), delivery from a persistent occiput posterior position (aOR, 2.24), and above-average newborn birth weight (aOR, 1.28).4
In a meta-analysis of randomized trials, the researchers reported that restrictive use of episiotomy reduced the risk of severe perineal trauma (relative risk [RR], 0.67) but increased the risk of anterior perineal trauma (RR, 1.84).5 The American College of Obstetricians and Gynecologists (ACOG) recommends that episiotomy should not be a routine practice and is best restricted to use in a limited number of cases where fetal and maternal benefit is likely.6 In addition, ACOG recommends that if episiotomy is indicated, a mediolateral incision is favored over a midline incision. In my practice I perform only mediolateral episiotomy incisions. However, mediolateral episiotomy may be associated with an increased risk of postpartum perineal pain and dyspareunia.7 Use of warm compresses applied to the perineum during the second stage of labor may reduce the risk of 3rd- and 4th-degree lacerations.8 Techniques to ensure that the fetal head and shoulders are birthed in a slow and controlled fashion may decrease the risk of OASIS.9 See the TABLE, “Four maneuvers to control and slow the birth of the fetal head.”10–14

Related article:
Stop performing median episiotomy!
Wound complications following the repair of a 3rd- or 4th-degree laceration are reported to occur in approximately 5% to 10% of cases.15 The most common wound complications are dehiscence, infection, abscess formation, pain, sexual dysfunction, and anal incontinence. Minor wound complications, including superficial epithelial separation, can be managed expectantly. However, major wound complications need intensive treatment.
In one study of 21 women who had a major wound complication following the repair of a 4th-degree laceration, 53% had dehiscence plus infection, 33% had dehiscence alone, and 14% had infection alone.16 Major wound complications present at a mean of 5 days after delivery, with a wide range from 1 to 17 days following delivery.17 In a study of 144 cases of wound breakdown following a perineal laceration repair, the major risk factors for wound breakdown were episiotomy (aOR, 11.1), smoking (aOR, 6.4), midwife repair of laceration (aOR, 4.7), use of chromic suture (aOR, 3.9), and operative vaginal delivery (aOR, 3.4).18 In one study of 66 women with a wound complication following the repair of a 3rd- or 4th-degree laceration, clinical risk factors for a wound complication were cigarette smoking (OR, 4.04), 4th-degree laceration (OR, 1.89), and operative vaginal delivery (OR, 1.76).19 The use of intrapartum antibiotics appears to be protective (OR, 0.29) against wound complications following a major perineal laceration.19
Approach to the patient with a dehisced and infected perineal wound
Historically, wound dehiscence following surgical repair of a perineal injury was managed by allowing the wound to slowly close. This approach adversely impacts the quality of life of the affected woman because it may take weeks for the wound to heal. One small randomized trial17 and multiple case series20–24 report that an active multistep management algorithm permits early closure of the majority of these wounds, thereby accelerating the patient’s full recovery. Delayed primary (within 72 hours postpartum) or early secondary reconstruction (within 14 days of delivery) has been demonstrated to be safe with acceptable long-term functional out-comes.25 The modern approach to the treatment of a patient with an infected wound dehiscence following a severe perineal injury involves 3 steps.
Related article:
It’s time to restrict the use of episiotomy
Step 1. Restore tissue to health
The dehisced wound is cultured and, if infection is present, treatment is initiated with intravenous antibiotics appropriate for an infection with colorectal flora. One antibiotic option includes a cephalosporin (cefotetan 2 g IV every 6 hours) plus metronidazole (500 mg IV every 8 hours).
In the operating room, the wound should be thoroughly assessed, cleansed, and debrided. This step includes irrigation of the wound with a warm fluid, mechanical debridement, and sharp dissection of necrotic tissue. If the wound is infected, removal of stitches that are visible in the open wound is recommended.
Often more than one session of debridement may be needed to obtain wound edges that are free from exudate and show granulation at the wound margins. Between debridement sessions, wet-to-dry dressings are utilized. Two to 10 days of wound care may be needed before an attempt is made to close the wound. The wound is suitable for repair when there is no infected tissue and granulation tissue is present. Some surgeons prefer a mechanical bowel preparation regimen just before surgically closing the open wound. This may prevent early bowel movements and provide for tissue healing after surgery.26 The same preparations recommended for colonoscopy can be considered prior to surgical repair.
Step 2. Surgically close the wound
The wound is surgically closed in the operating room. If in Step 1 the assessment of the wound shows major trauma, assistance from a urogynecologist may be warranted. Surgical management of a perineal wound dehiscence requires a clear understanding of perineal anatomy and the structures contained between the vagina and the anorectum.
Six key structures may be involved in perineal injury: the anorectal mucosa, internal anal sphincter, external anal sphincter, vaginal wall and perineal skin, bulbocavernosus muscle, and transverse perineal muscles. It is important to definitively identify the individual structures that need to be repaired. Careful dissection is then carried out to mobilize these structures for repair. Additional debridement may be necessary to remove excess granulation tissue.
Anorectal mucosa repair. With repair of a 4th-degree perineal wound dehiscence, the apex of the defect in the anorectal mucosa is identified. The defect is repaired beginning at the apex using closely spaced interrupted sutures or a running suture of 3-0 or 4-0 polyglactin 910. Adequate tissue bites that will resist tearing should be taken. If interrupted sutures are used, tying the knots within the anorectal canal prevents them from being located within the healing wound.
Internal anal sphincter repair. After the anorectal mucosa is closed, attention is turned to reapproximation of the internal anal sphincter. The ends of a torn internal anal sphincter are often located lateral to the anorectal mucosa and appear as shiny gray-white fibrous tissue. The surgeon’s gloved index finger can be placed within the anorectal canal to aid in identification of the internal anal sphincter, as it tends to have a rubbery feel. Additionally, while the surgeon’s gloved index finger is in the anorectal canal, the surgeon’s gloved thumb can be used to retract the anorectal mucosa slightly medial and inferior so that adequate bites of the internal anal sphincter can be taken on each side.
Alternatively, Allis clamps canbe placed on the ends of the retracted internal anal sphincter to facilitate repair. Suture selection for repair of the internal anal sphincter can include 3-0 polyglactin 910 or 3-0 monofilament, delayed-absorbable suture such as polydioxanone sulfate (PDS). Some surgeons prefer delayed-absorbable suture (PDS) for this layer given the internal anal sphincter is constantly contracting and relaxing as it samples stool.26 This layer also can be closed with either interrupted sutures or a running suture.
External anal sphincter repair. After the anorectal mucosa and internal anal sphincter defects are reapproximated, attention is turned to the external anal sphincter. Like the internal anal sphincter, the ends of the external anal sphincter are often retracted laterally and must be definitively identified and mobilized in order to ensure an adequate tension-free repair. It is important to include the fascial sheath in the repair of the external anal sphincter.27 Allis clamps can be used to grasp the ends of the torn muscle after they are identified.
We recommend 0 or 2-0 PDS for repair of the external sphincter. Repair can be performed using either an end-to-end or overlapping technique. An end-to-end repair traditionally involves reapproximating the ends of the torn muscle and its overlying fascial sheath using interrupted sutures placed at four quadrants (12:00, 3:00, 6:00, 9:00).
In contrast, in an overlapping repair, the ends of the muscle are brought together with mattress sutures. Suture is passed top down through the medial aspect of the more superior muscle flap and top down through the inferior muscle flap more laterally. The same suture is then passed bottom up through the inferior muscle flap more laterally and finally bottom up through the medial aspect of the more superior muscle flap. Two to four mattress sutures are usually placed. After all sutures are placed, they are tied securely.
An overlapping repair results in a greater amount of tissue contact between the two torn muscle ends. However, adequate mobility of the external anal sphincter is necessary to perform this type of repair.
Vaginal wall and perineal body repair. After the anal sphincters have been repaired, the vaginal wall and remainder of the perineal body are reconstructed using the same techniques involved in a 2nd-degree laceration repair. Care must be taken to retrieve and reapproximate the torn ends of the bulbocavernosus muscles, which are also often retracted laterally and superiorly. After the bulbocavernosus and transverse perineal muscles are brought together in the midline, the posterior vaginal wall should be perpendicular to the perineum.
An alternative to surgical closure of a 2nd-degree dehiscence is the use of vacuum-assisted wound closure. Disadvantages of this approach include difficulty in maintaining a vacuum seal in the perineal region and the risk of wound contamination with feces. In one case report, 3 weeks of vacuum-assisted wound closure resulted in healing of a 10-cm wound dehiscence that occurred 5 days following a forceps-assisted vaginal delivery with a mediolateral episiotomy.28
Step 3. Ensure complete healing of the wound
Superb postoperative wound care helps to ensure a quick return to full recovery. Wound care should include regularly scheduled sitz baths (at least 3 times daily) followed by drying the perineum. It is preferable to provide a liquid diet that avoids frequent bowel movements in the initial 3 postoperative days. Stool softeners and fiber supplementation are recommended when a full diet is resumed. Some surgeons have found mineral oil (1 to 2 tablespoons daily) effective in producing soft stools that are easy to pass.26 Ensuring soft stool consistency is important to help prevent repair breakdown that may occur with passage of hard stools, fecal impaction, and/or straining during defecation.
We recommend follow-up 1 to 2 weeks after surgery to assess wound healing. No vaginal intercourseis permitted until complete healing is achieved.
Use a surgical checklist
All obstetricians and midwives strive to reduce the risk of OASIS at vaginal birth. When OASIS occurs, it is often useful to use a surgical checklist to ensure the execution of all steps in the management of the repair and recovery process.29 It is heartbreaking to see an OASIS repair breakdown in the week following a vaginal delivery. But by following the 3 steps outlined here, the secondary repair is likely to be successful and will quickly return most patients to full health.
Related article:
Develop and use a checklist for 3rd- and 4th-degree perineal lacerations

The authors report no financial relationships relevant to this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
During vaginal delivery spontaneous perineal trauma and extension of episiotomy incisions are common. A severe perineal laceration that extends into or through the anal sphincter complex is referred to as an obstetric anal sphincter injury (OASIS) and requires meticulous repair. Following the repair of an OASIS, serious wound complications, including dehiscence and infection, may occur. In Europe the reported rate of OASIS varies widely among countries, with a rate of 0.1% in Romania, possibly due to underreporting, and 4.9% in Iceland.1 In the United States the rates of 3rd- and 4th-degree lacerations were reported to be 3.3% and 1.1%, respectively.2
Risk factors for OASIS include forceps delivery (odds ratio [OR], 5.50), vacuum-assisted delivery (OR, 3.98), and midline episiotomy (OR, 3.82).3 Additional risk factors for severe perineal injury at vaginal delivery include nulliparity (adjusted odds ratio [aOR], 2.58), delivery from a persistent occiput posterior position (aOR, 2.24), and above-average newborn birth weight (aOR, 1.28).4
In a meta-analysis of randomized trials, the researchers reported that restrictive use of episiotomy reduced the risk of severe perineal trauma (relative risk [RR], 0.67) but increased the risk of anterior perineal trauma (RR, 1.84).5 The American College of Obstetricians and Gynecologists (ACOG) recommends that episiotomy should not be a routine practice and is best restricted to use in a limited number of cases where fetal and maternal benefit is likely.6 In addition, ACOG recommends that if episiotomy is indicated, a mediolateral incision is favored over a midline incision. In my practice I perform only mediolateral episiotomy incisions. However, mediolateral episiotomy may be associated with an increased risk of postpartum perineal pain and dyspareunia.7 Use of warm compresses applied to the perineum during the second stage of labor may reduce the risk of 3rd- and 4th-degree lacerations.8 Techniques to ensure that the fetal head and shoulders are birthed in a slow and controlled fashion may decrease the risk of OASIS.9 See the TABLE, “Four maneuvers to control and slow the birth of the fetal head.”10–14

Related article:
Stop performing median episiotomy!
Wound complications following the repair of a 3rd- or 4th-degree laceration are reported to occur in approximately 5% to 10% of cases.15 The most common wound complications are dehiscence, infection, abscess formation, pain, sexual dysfunction, and anal incontinence. Minor wound complications, including superficial epithelial separation, can be managed expectantly. However, major wound complications need intensive treatment.
In one study of 21 women who had a major wound complication following the repair of a 4th-degree laceration, 53% had dehiscence plus infection, 33% had dehiscence alone, and 14% had infection alone.16 Major wound complications present at a mean of 5 days after delivery, with a wide range from 1 to 17 days following delivery.17 In a study of 144 cases of wound breakdown following a perineal laceration repair, the major risk factors for wound breakdown were episiotomy (aOR, 11.1), smoking (aOR, 6.4), midwife repair of laceration (aOR, 4.7), use of chromic suture (aOR, 3.9), and operative vaginal delivery (aOR, 3.4).18 In one study of 66 women with a wound complication following the repair of a 3rd- or 4th-degree laceration, clinical risk factors for a wound complication were cigarette smoking (OR, 4.04), 4th-degree laceration (OR, 1.89), and operative vaginal delivery (OR, 1.76).19 The use of intrapartum antibiotics appears to be protective (OR, 0.29) against wound complications following a major perineal laceration.19
Approach to the patient with a dehisced and infected perineal wound
Historically, wound dehiscence following surgical repair of a perineal injury was managed by allowing the wound to slowly close. This approach adversely impacts the quality of life of the affected woman because it may take weeks for the wound to heal. One small randomized trial17 and multiple case series20–24 report that an active multistep management algorithm permits early closure of the majority of these wounds, thereby accelerating the patient’s full recovery. Delayed primary (within 72 hours postpartum) or early secondary reconstruction (within 14 days of delivery) has been demonstrated to be safe with acceptable long-term functional out-comes.25 The modern approach to the treatment of a patient with an infected wound dehiscence following a severe perineal injury involves 3 steps.
Related article:
It’s time to restrict the use of episiotomy
Step 1. Restore tissue to health
The dehisced wound is cultured and, if infection is present, treatment is initiated with intravenous antibiotics appropriate for an infection with colorectal flora. One antibiotic option includes a cephalosporin (cefotetan 2 g IV every 6 hours) plus metronidazole (500 mg IV every 8 hours).
In the operating room, the wound should be thoroughly assessed, cleansed, and debrided. This step includes irrigation of the wound with a warm fluid, mechanical debridement, and sharp dissection of necrotic tissue. If the wound is infected, removal of stitches that are visible in the open wound is recommended.
Often more than one session of debridement may be needed to obtain wound edges that are free from exudate and show granulation at the wound margins. Between debridement sessions, wet-to-dry dressings are utilized. Two to 10 days of wound care may be needed before an attempt is made to close the wound. The wound is suitable for repair when there is no infected tissue and granulation tissue is present. Some surgeons prefer a mechanical bowel preparation regimen just before surgically closing the open wound. This may prevent early bowel movements and provide for tissue healing after surgery.26 The same preparations recommended for colonoscopy can be considered prior to surgical repair.
Step 2. Surgically close the wound
The wound is surgically closed in the operating room. If in Step 1 the assessment of the wound shows major trauma, assistance from a urogynecologist may be warranted. Surgical management of a perineal wound dehiscence requires a clear understanding of perineal anatomy and the structures contained between the vagina and the anorectum.
Six key structures may be involved in perineal injury: the anorectal mucosa, internal anal sphincter, external anal sphincter, vaginal wall and perineal skin, bulbocavernosus muscle, and transverse perineal muscles. It is important to definitively identify the individual structures that need to be repaired. Careful dissection is then carried out to mobilize these structures for repair. Additional debridement may be necessary to remove excess granulation tissue.
Anorectal mucosa repair. With repair of a 4th-degree perineal wound dehiscence, the apex of the defect in the anorectal mucosa is identified. The defect is repaired beginning at the apex using closely spaced interrupted sutures or a running suture of 3-0 or 4-0 polyglactin 910. Adequate tissue bites that will resist tearing should be taken. If interrupted sutures are used, tying the knots within the anorectal canal prevents them from being located within the healing wound.
Internal anal sphincter repair. After the anorectal mucosa is closed, attention is turned to reapproximation of the internal anal sphincter. The ends of a torn internal anal sphincter are often located lateral to the anorectal mucosa and appear as shiny gray-white fibrous tissue. The surgeon’s gloved index finger can be placed within the anorectal canal to aid in identification of the internal anal sphincter, as it tends to have a rubbery feel. Additionally, while the surgeon’s gloved index finger is in the anorectal canal, the surgeon’s gloved thumb can be used to retract the anorectal mucosa slightly medial and inferior so that adequate bites of the internal anal sphincter can be taken on each side.
Alternatively, Allis clamps canbe placed on the ends of the retracted internal anal sphincter to facilitate repair. Suture selection for repair of the internal anal sphincter can include 3-0 polyglactin 910 or 3-0 monofilament, delayed-absorbable suture such as polydioxanone sulfate (PDS). Some surgeons prefer delayed-absorbable suture (PDS) for this layer given the internal anal sphincter is constantly contracting and relaxing as it samples stool.26 This layer also can be closed with either interrupted sutures or a running suture.
External anal sphincter repair. After the anorectal mucosa and internal anal sphincter defects are reapproximated, attention is turned to the external anal sphincter. Like the internal anal sphincter, the ends of the external anal sphincter are often retracted laterally and must be definitively identified and mobilized in order to ensure an adequate tension-free repair. It is important to include the fascial sheath in the repair of the external anal sphincter.27 Allis clamps can be used to grasp the ends of the torn muscle after they are identified.
We recommend 0 or 2-0 PDS for repair of the external sphincter. Repair can be performed using either an end-to-end or overlapping technique. An end-to-end repair traditionally involves reapproximating the ends of the torn muscle and its overlying fascial sheath using interrupted sutures placed at four quadrants (12:00, 3:00, 6:00, 9:00).
In contrast, in an overlapping repair, the ends of the muscle are brought together with mattress sutures. Suture is passed top down through the medial aspect of the more superior muscle flap and top down through the inferior muscle flap more laterally. The same suture is then passed bottom up through the inferior muscle flap more laterally and finally bottom up through the medial aspect of the more superior muscle flap. Two to four mattress sutures are usually placed. After all sutures are placed, they are tied securely.
An overlapping repair results in a greater amount of tissue contact between the two torn muscle ends. However, adequate mobility of the external anal sphincter is necessary to perform this type of repair.
Vaginal wall and perineal body repair. After the anal sphincters have been repaired, the vaginal wall and remainder of the perineal body are reconstructed using the same techniques involved in a 2nd-degree laceration repair. Care must be taken to retrieve and reapproximate the torn ends of the bulbocavernosus muscles, which are also often retracted laterally and superiorly. After the bulbocavernosus and transverse perineal muscles are brought together in the midline, the posterior vaginal wall should be perpendicular to the perineum.
An alternative to surgical closure of a 2nd-degree dehiscence is the use of vacuum-assisted wound closure. Disadvantages of this approach include difficulty in maintaining a vacuum seal in the perineal region and the risk of wound contamination with feces. In one case report, 3 weeks of vacuum-assisted wound closure resulted in healing of a 10-cm wound dehiscence that occurred 5 days following a forceps-assisted vaginal delivery with a mediolateral episiotomy.28
Step 3. Ensure complete healing of the wound
Superb postoperative wound care helps to ensure a quick return to full recovery. Wound care should include regularly scheduled sitz baths (at least 3 times daily) followed by drying the perineum. It is preferable to provide a liquid diet that avoids frequent bowel movements in the initial 3 postoperative days. Stool softeners and fiber supplementation are recommended when a full diet is resumed. Some surgeons have found mineral oil (1 to 2 tablespoons daily) effective in producing soft stools that are easy to pass.26 Ensuring soft stool consistency is important to help prevent repair breakdown that may occur with passage of hard stools, fecal impaction, and/or straining during defecation.
We recommend follow-up 1 to 2 weeks after surgery to assess wound healing. No vaginal intercourseis permitted until complete healing is achieved.
Use a surgical checklist
All obstetricians and midwives strive to reduce the risk of OASIS at vaginal birth. When OASIS occurs, it is often useful to use a surgical checklist to ensure the execution of all steps in the management of the repair and recovery process.29 It is heartbreaking to see an OASIS repair breakdown in the week following a vaginal delivery. But by following the 3 steps outlined here, the secondary repair is likely to be successful and will quickly return most patients to full health.
Related article:
Develop and use a checklist for 3rd- and 4th-degree perineal lacerations

The authors report no financial relationships relevant to this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Blondel B, Alexander S, Bjarnadottir RI, et al; Euro-Peristat Scientific Committee. Variations in rates of severe perineal tears and episiotomies in 20 European countries: a study based on routine national data in Euro-Perstat Project. Acta Obstet Gynecol Scand. 2016;95(7):746–754.
- Friedman AM, Ananth CV, Predergast E, D’Alton ME, Wright JD. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125(4):927–937.
- Pergialiotis V, Vlachos D, Protopapas A, Pappa K, Vlachos G. Risk factors for severe perineal lacerations during childbirth. Int J Gynecol Obstet. 2014;125(1):6–14.
- Schmitz T, Alberti C, Andriss B, Moutafoff C, Oury JF, Sibony O. Identification of women at high risk for severe perineal lacerations. Eur J Obstet Gynecol Reprod Biol. 2014;182:11–15.
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.
- ACOG Committee on Practice Bulletins—Obstetrics. Practice bulletin no. 165: Prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2016;128(1):e1–e15.
- Sartore A, De Seta F, Maso G, Pregazzi R, Grimaldi E, Guaschino S. The effects of mediolateral episiotomy on pelvic floor function after vaginal delivery. Obstet Gynecol. 2004;103(4):669–673.
- Aasheim V, Nilsen AB, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2011;(12):CD006672.
- Harvey MA, Pierce M, Alter JE, et al; Society of Obstetricians and Gynaecologists of Canada. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynaecol Can. 2015;37(12):1131–1148.
- Jonsson ER, Elfaghi I, Rydhstrom H, Herbst A. Modified Ritgen’s maneuver for anal sphincter injury at delivery: a randomized controlled trial. Obstet Gynecol. 2008;112(2 pt 1):212–217.
- Williams JW. Obstetrics: A Text-book for the Use of Students and Practitioners. New York, NY: D Appleton and Co; 1903:288.
- Cunningham FG. The Ritgen maneuver: another sacred cow questioned. Obstet Gynecol. 2008;112(2 pt 1):210–211.
- Myrfield K, Brook C, Creedy D. Reducing perineal trauma: implications of flexion and extension of the fetal head during birth. Midwifery. 1997;13:197–201.
- Ostergaard Poulsen M, Lund Madsen M, Skriver-Moller AC, Overgaard C. Does the Finnish intervention prevent obstetrical anal sphincter injuries? A systematic review of the literature. BMJ Open. 2015;5:e008346.
- Kamel A, Khaled M. Episiotomy and obstetric perineal wound dehiscence: beyond soreness. J Obstet Gynaecol. 2014;34(3):215–217.
- Goldaber KG, Wendel PJ, McIntire DD, Wendel GD Jr. Postpartum perineal morbidity after fourth-degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489–493.
- Monberg J, Hammen S. Ruptured episiotomia resutured primarily. Acta Obstet Gynecol Scand. 1987;66(2):163–164.
- Jallad K, Steele SE, Barber MD. Breakdown of perineal laceration repair after vaginal delivery: a case-control study. Female Pelvic Med Reconstr Surg. 2016;22(4):276–279.
- Stock L, Basham E, Gossett DR, Lewicky-Gaupp C. Factors associated with wound complications in women with obstetric anal sphincter injuries (OASIS). Am J Obstet Gynecol. 2013;208(4):327.e1–e8.
- Hauth JC, Gilstrap LC 3rd, Ward SC, Hankins GD. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67(6):806–809.
- Hankins GD, Hauth JC, Gilstrap LC 3rd, Hammond TL, Yeomans ER, Snyder RR. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75(1):48–51.
- Ramin SM, Ramus RM, Little BB, Gilstrap LC 3rd. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167(4 pt 1):1104–1107.
- Arona AJ, Al-Marayati L, Grimes DA, Ballard CA. Early secondary repair of third- and fourth-degree perineal lacerations after outpatient wound preparation. Obstet Gynecol. 1995;86(2):294–296.
- Uygur D, Yesildaglar N, Kis S, Sipahi T. Early repair of episiotomy dehiscence. Aust N Z J Obstet Gynaecol. 2004;44(3):244–246.
- Soerensen MM, Bek KM, Buntzen S, Hojberg KE, Laurberg S. Long-term outcome of delayed primary or early secondary reconstruction of the anal sphincter after obstetrical injury. Dis Colon Rectum. 2008;51(3):312–317.
- Delancey JOL, Berger MB. Surgical approaches to postobstetrical perineal body defects (rectovaginal fistula and chronic third and fourth-degree lacerations). Clin Obstet Gynecol. 2010;53(1):134–144.
- Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician. 2003;68(8):1585–1590.
- Aviki EM, Batalden RP, del Carmen MG, Berkowitz LR. Vacuum-assisted closure for episiotomy dehiscence. Obstet Gynecol. 2015;126(3):530–533.
- Barbieri RL. Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. OBG Manag. 2013;25(8):8–12.
- Blondel B, Alexander S, Bjarnadottir RI, et al; Euro-Peristat Scientific Committee. Variations in rates of severe perineal tears and episiotomies in 20 European countries: a study based on routine national data in Euro-Perstat Project. Acta Obstet Gynecol Scand. 2016;95(7):746–754.
- Friedman AM, Ananth CV, Predergast E, D’Alton ME, Wright JD. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125(4):927–937.
- Pergialiotis V, Vlachos D, Protopapas A, Pappa K, Vlachos G. Risk factors for severe perineal lacerations during childbirth. Int J Gynecol Obstet. 2014;125(1):6–14.
- Schmitz T, Alberti C, Andriss B, Moutafoff C, Oury JF, Sibony O. Identification of women at high risk for severe perineal lacerations. Eur J Obstet Gynecol Reprod Biol. 2014;182:11–15.
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.
- ACOG Committee on Practice Bulletins—Obstetrics. Practice bulletin no. 165: Prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2016;128(1):e1–e15.
- Sartore A, De Seta F, Maso G, Pregazzi R, Grimaldi E, Guaschino S. The effects of mediolateral episiotomy on pelvic floor function after vaginal delivery. Obstet Gynecol. 2004;103(4):669–673.
- Aasheim V, Nilsen AB, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2011;(12):CD006672.
- Harvey MA, Pierce M, Alter JE, et al; Society of Obstetricians and Gynaecologists of Canada. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynaecol Can. 2015;37(12):1131–1148.
- Jonsson ER, Elfaghi I, Rydhstrom H, Herbst A. Modified Ritgen’s maneuver for anal sphincter injury at delivery: a randomized controlled trial. Obstet Gynecol. 2008;112(2 pt 1):212–217.
- Williams JW. Obstetrics: A Text-book for the Use of Students and Practitioners. New York, NY: D Appleton and Co; 1903:288.
- Cunningham FG. The Ritgen maneuver: another sacred cow questioned. Obstet Gynecol. 2008;112(2 pt 1):210–211.
- Myrfield K, Brook C, Creedy D. Reducing perineal trauma: implications of flexion and extension of the fetal head during birth. Midwifery. 1997;13:197–201.
- Ostergaard Poulsen M, Lund Madsen M, Skriver-Moller AC, Overgaard C. Does the Finnish intervention prevent obstetrical anal sphincter injuries? A systematic review of the literature. BMJ Open. 2015;5:e008346.
- Kamel A, Khaled M. Episiotomy and obstetric perineal wound dehiscence: beyond soreness. J Obstet Gynaecol. 2014;34(3):215–217.
- Goldaber KG, Wendel PJ, McIntire DD, Wendel GD Jr. Postpartum perineal morbidity after fourth-degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489–493.
- Monberg J, Hammen S. Ruptured episiotomia resutured primarily. Acta Obstet Gynecol Scand. 1987;66(2):163–164.
- Jallad K, Steele SE, Barber MD. Breakdown of perineal laceration repair after vaginal delivery: a case-control study. Female Pelvic Med Reconstr Surg. 2016;22(4):276–279.
- Stock L, Basham E, Gossett DR, Lewicky-Gaupp C. Factors associated with wound complications in women with obstetric anal sphincter injuries (OASIS). Am J Obstet Gynecol. 2013;208(4):327.e1–e8.
- Hauth JC, Gilstrap LC 3rd, Ward SC, Hankins GD. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67(6):806–809.
- Hankins GD, Hauth JC, Gilstrap LC 3rd, Hammond TL, Yeomans ER, Snyder RR. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75(1):48–51.
- Ramin SM, Ramus RM, Little BB, Gilstrap LC 3rd. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167(4 pt 1):1104–1107.
- Arona AJ, Al-Marayati L, Grimes DA, Ballard CA. Early secondary repair of third- and fourth-degree perineal lacerations after outpatient wound preparation. Obstet Gynecol. 1995;86(2):294–296.
- Uygur D, Yesildaglar N, Kis S, Sipahi T. Early repair of episiotomy dehiscence. Aust N Z J Obstet Gynaecol. 2004;44(3):244–246.
- Soerensen MM, Bek KM, Buntzen S, Hojberg KE, Laurberg S. Long-term outcome of delayed primary or early secondary reconstruction of the anal sphincter after obstetrical injury. Dis Colon Rectum. 2008;51(3):312–317.
- Delancey JOL, Berger MB. Surgical approaches to postobstetrical perineal body defects (rectovaginal fistula and chronic third and fourth-degree lacerations). Clin Obstet Gynecol. 2010;53(1):134–144.
- Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician. 2003;68(8):1585–1590.
- Aviki EM, Batalden RP, del Carmen MG, Berkowitz LR. Vacuum-assisted closure for episiotomy dehiscence. Obstet Gynecol. 2015;126(3):530–533.
- Barbieri RL. Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. OBG Manag. 2013;25(8):8–12.
Letters to the Editor: Rectal misoprostol for postpartum hemorrhage
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”
ROBERT L. BARBIERI, MD (JULY 2016)
More on rectal misoprostol for postpartum hemorrhage
We applaud Dr. Barbieri’s July Editorial urging providers to stop administering misoprostol rectally for the treatment of postpartum hemorrhage (PPH) given the well-documented evidence and pharmacokinetics that recommend the sublingual route. Confusion among providers may derive from the fact that not all international guidelines, including the American College of Obstetricians and Gynecologists clinical guidelines on the management of PPH, have been updated to reflect the latest evidence.1 Guidelines from the World Health Organization and the International Federation of Gynecology and Obstetrics reflect the latest evidence and clearly recommend the evidence-based regimen of 800 μg misoprostol sublingually for treatment of PPH,2 which has been shown to be comparable to 40 IU oxytocin intravenously in women who receive oxytocin for PPH prophylaxis.3
Although oxytocin remains the first-line treatment for PPH, evidence suggests that sublingual misoprostol should be considered a viable first alternative if oxytocin is not available or fails. There is little evidence on the benefit of methergine or carboprost over misoprostol for PPH treatment, and inclusion of these drugs in treatment guidelines and practice is based on extrapolations from studies on PPH prevention.4 As Dr. Barbieri noted, pyrexia from misoprostol has been cited in the literature; however, contrary to contraindications for methergine, for example, this rare event does not pose serious risks to women, is self-limiting, and appears to be most acute among certain populations.5
It is paramount that safe, effective, and evidence-based PPH treatments be available and known to providers both in the United States and globally in order to provide women with timely treatment. Greater discussion and research is warranted about the hierarchy of use for these drugs and the possible impact of routine use of uterotonics before and during delivery, given that overexposure to uterotonics may in fact be making PPH harder to treat.6
Gillian Burkhardt, MD, and Rasha Dabash, MPH
New York, New York
Dr. Barbieri responds
I thank Drs. Burkhardt and Dabash for sharing their expert perspective with our readers. They advocate for the use of sublingual misoprostol for the treatment of PPH “if oxytocin is not available or fails.” I agree that at a home birth, if oxytocin is not available, sublingual misoprostol would be of great benefit. I remain concerned that misoprostol has little clinical utility for the treatment of PPH in the hospital setting in which oxytocin, methergine, and carboprost are available alternatives. Misoprostol causes fever in many women, and women who develop a postpartum fever due to misoprostol will receive unnecessary antibiotic treatment. I recommend that our readers stop using misoprostol for the treatment of PPH in the hospital setting.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1048. Reaffirmed 2015.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva, Switzerland: World Health Organization; 2012.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind placebo-controlled randomized non-inferiority trial. Lancet. 2010;375(9710):217–223.
- Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG. 2015;122(2):202–210.
- Durocher J, Bynum J, León W, Barrera G, Winikoff B. High fever following postpartum administration of sublingual misoprostol. BJOG. 2010;117(7):845–852.
- Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119(3):552–561.
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”
ROBERT L. BARBIERI, MD (JULY 2016)
More on rectal misoprostol for postpartum hemorrhage
We applaud Dr. Barbieri’s July Editorial urging providers to stop administering misoprostol rectally for the treatment of postpartum hemorrhage (PPH) given the well-documented evidence and pharmacokinetics that recommend the sublingual route. Confusion among providers may derive from the fact that not all international guidelines, including the American College of Obstetricians and Gynecologists clinical guidelines on the management of PPH, have been updated to reflect the latest evidence.1 Guidelines from the World Health Organization and the International Federation of Gynecology and Obstetrics reflect the latest evidence and clearly recommend the evidence-based regimen of 800 μg misoprostol sublingually for treatment of PPH,2 which has been shown to be comparable to 40 IU oxytocin intravenously in women who receive oxytocin for PPH prophylaxis.3
Although oxytocin remains the first-line treatment for PPH, evidence suggests that sublingual misoprostol should be considered a viable first alternative if oxytocin is not available or fails. There is little evidence on the benefit of methergine or carboprost over misoprostol for PPH treatment, and inclusion of these drugs in treatment guidelines and practice is based on extrapolations from studies on PPH prevention.4 As Dr. Barbieri noted, pyrexia from misoprostol has been cited in the literature; however, contrary to contraindications for methergine, for example, this rare event does not pose serious risks to women, is self-limiting, and appears to be most acute among certain populations.5
It is paramount that safe, effective, and evidence-based PPH treatments be available and known to providers both in the United States and globally in order to provide women with timely treatment. Greater discussion and research is warranted about the hierarchy of use for these drugs and the possible impact of routine use of uterotonics before and during delivery, given that overexposure to uterotonics may in fact be making PPH harder to treat.6
Gillian Burkhardt, MD, and Rasha Dabash, MPH
New York, New York
Dr. Barbieri responds
I thank Drs. Burkhardt and Dabash for sharing their expert perspective with our readers. They advocate for the use of sublingual misoprostol for the treatment of PPH “if oxytocin is not available or fails.” I agree that at a home birth, if oxytocin is not available, sublingual misoprostol would be of great benefit. I remain concerned that misoprostol has little clinical utility for the treatment of PPH in the hospital setting in which oxytocin, methergine, and carboprost are available alternatives. Misoprostol causes fever in many women, and women who develop a postpartum fever due to misoprostol will receive unnecessary antibiotic treatment. I recommend that our readers stop using misoprostol for the treatment of PPH in the hospital setting.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”
ROBERT L. BARBIERI, MD (JULY 2016)
More on rectal misoprostol for postpartum hemorrhage
We applaud Dr. Barbieri’s July Editorial urging providers to stop administering misoprostol rectally for the treatment of postpartum hemorrhage (PPH) given the well-documented evidence and pharmacokinetics that recommend the sublingual route. Confusion among providers may derive from the fact that not all international guidelines, including the American College of Obstetricians and Gynecologists clinical guidelines on the management of PPH, have been updated to reflect the latest evidence.1 Guidelines from the World Health Organization and the International Federation of Gynecology and Obstetrics reflect the latest evidence and clearly recommend the evidence-based regimen of 800 μg misoprostol sublingually for treatment of PPH,2 which has been shown to be comparable to 40 IU oxytocin intravenously in women who receive oxytocin for PPH prophylaxis.3
Although oxytocin remains the first-line treatment for PPH, evidence suggests that sublingual misoprostol should be considered a viable first alternative if oxytocin is not available or fails. There is little evidence on the benefit of methergine or carboprost over misoprostol for PPH treatment, and inclusion of these drugs in treatment guidelines and practice is based on extrapolations from studies on PPH prevention.4 As Dr. Barbieri noted, pyrexia from misoprostol has been cited in the literature; however, contrary to contraindications for methergine, for example, this rare event does not pose serious risks to women, is self-limiting, and appears to be most acute among certain populations.5
It is paramount that safe, effective, and evidence-based PPH treatments be available and known to providers both in the United States and globally in order to provide women with timely treatment. Greater discussion and research is warranted about the hierarchy of use for these drugs and the possible impact of routine use of uterotonics before and during delivery, given that overexposure to uterotonics may in fact be making PPH harder to treat.6
Gillian Burkhardt, MD, and Rasha Dabash, MPH
New York, New York
Dr. Barbieri responds
I thank Drs. Burkhardt and Dabash for sharing their expert perspective with our readers. They advocate for the use of sublingual misoprostol for the treatment of PPH “if oxytocin is not available or fails.” I agree that at a home birth, if oxytocin is not available, sublingual misoprostol would be of great benefit. I remain concerned that misoprostol has little clinical utility for the treatment of PPH in the hospital setting in which oxytocin, methergine, and carboprost are available alternatives. Misoprostol causes fever in many women, and women who develop a postpartum fever due to misoprostol will receive unnecessary antibiotic treatment. I recommend that our readers stop using misoprostol for the treatment of PPH in the hospital setting.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1048. Reaffirmed 2015.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva, Switzerland: World Health Organization; 2012.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind placebo-controlled randomized non-inferiority trial. Lancet. 2010;375(9710):217–223.
- Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG. 2015;122(2):202–210.
- Durocher J, Bynum J, León W, Barrera G, Winikoff B. High fever following postpartum administration of sublingual misoprostol. BJOG. 2010;117(7):845–852.
- Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119(3):552–561.
- ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1048. Reaffirmed 2015.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva, Switzerland: World Health Organization; 2012.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind placebo-controlled randomized non-inferiority trial. Lancet. 2010;375(9710):217–223.
- Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG. 2015;122(2):202–210.
- Durocher J, Bynum J, León W, Barrera G, Winikoff B. High fever following postpartum administration of sublingual misoprostol. BJOG. 2010;117(7):845–852.
- Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119(3):552–561.
Texas reports local Zika transmission
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
Infants with congenital Zika born without microcephaly still can still develop it
Infants born with laboratory-confirmed congenital Zika virus but who show no signs of microcephaly at birth may still experience a reduction in cranial size as they grow older, according to the Centers for Disease Control and Prevention’s latest Morbidity and Mortality Weekly Report.
“These findings demonstrate the importance of early neuroimaging for infants exposed to Zika virus prenatally and the need for comprehensive medical and developmental follow-up,” wrote Vanessa van der Linden, MD, of the Association for Assistance of Disabled Children in Recife, Brazil, and her coauthors.
Dr. van der Linden and her coinvestigators examined 13 infants, all of whom were born in Brazil between October 2015 and January 2016, who had confirmed brain abnormalities at birth despite having a normal head size. These abnormalities included ventriculomegaly, subcortical calcifications, cortical malformations, and decreased brain volume. Investigators defined microcephaly as being “head circumference (HC) [that’s] more than 2 [standard deviations] below the mean for gestational age and sex.”
Nine of the infants were male, four were female. Eleven of the infants were born within 37-41 weeks’ of gestation. The remaining two were born at 35-36 weeks’ of gestation, considered “preterm” by the investigators. All infants tested positive for Zika via immunoglobulin M testing of cerebrospinal fluid, serum, or both. Only six of the mothers reported having a rash while pregnant; four reported experiencing it during the first trimester, while the other two said it occurred in the second.
All 13 infants showed a decrease in HC to what was defined as microcephaly within 1 year of birth (October 2016). Neuroimaging showed that all but one had decreased brain volume, all had malformations of cortical development, four had cerebellum or brain-stem hypoplasia, ten had ventriculomegaly, and three had increased extra-axial CSF space.
“More than 60% of infants in this series had epilepsy (likely related to the cortical malformations), and all had significant motor disabilities consistent with mixed cerebral palsy,” the authors noted, adding that the “pathogenesis of postnatal microcephaly from congenital Zika virus infections is [still] not known.”
Infants born with laboratory-confirmed congenital Zika virus but who show no signs of microcephaly at birth may still experience a reduction in cranial size as they grow older, according to the Centers for Disease Control and Prevention’s latest Morbidity and Mortality Weekly Report.
“These findings demonstrate the importance of early neuroimaging for infants exposed to Zika virus prenatally and the need for comprehensive medical and developmental follow-up,” wrote Vanessa van der Linden, MD, of the Association for Assistance of Disabled Children in Recife, Brazil, and her coauthors.
Dr. van der Linden and her coinvestigators examined 13 infants, all of whom were born in Brazil between October 2015 and January 2016, who had confirmed brain abnormalities at birth despite having a normal head size. These abnormalities included ventriculomegaly, subcortical calcifications, cortical malformations, and decreased brain volume. Investigators defined microcephaly as being “head circumference (HC) [that’s] more than 2 [standard deviations] below the mean for gestational age and sex.”
Nine of the infants were male, four were female. Eleven of the infants were born within 37-41 weeks’ of gestation. The remaining two were born at 35-36 weeks’ of gestation, considered “preterm” by the investigators. All infants tested positive for Zika via immunoglobulin M testing of cerebrospinal fluid, serum, or both. Only six of the mothers reported having a rash while pregnant; four reported experiencing it during the first trimester, while the other two said it occurred in the second.
All 13 infants showed a decrease in HC to what was defined as microcephaly within 1 year of birth (October 2016). Neuroimaging showed that all but one had decreased brain volume, all had malformations of cortical development, four had cerebellum or brain-stem hypoplasia, ten had ventriculomegaly, and three had increased extra-axial CSF space.
“More than 60% of infants in this series had epilepsy (likely related to the cortical malformations), and all had significant motor disabilities consistent with mixed cerebral palsy,” the authors noted, adding that the “pathogenesis of postnatal microcephaly from congenital Zika virus infections is [still] not known.”
Infants born with laboratory-confirmed congenital Zika virus but who show no signs of microcephaly at birth may still experience a reduction in cranial size as they grow older, according to the Centers for Disease Control and Prevention’s latest Morbidity and Mortality Weekly Report.
“These findings demonstrate the importance of early neuroimaging for infants exposed to Zika virus prenatally and the need for comprehensive medical and developmental follow-up,” wrote Vanessa van der Linden, MD, of the Association for Assistance of Disabled Children in Recife, Brazil, and her coauthors.
Dr. van der Linden and her coinvestigators examined 13 infants, all of whom were born in Brazil between October 2015 and January 2016, who had confirmed brain abnormalities at birth despite having a normal head size. These abnormalities included ventriculomegaly, subcortical calcifications, cortical malformations, and decreased brain volume. Investigators defined microcephaly as being “head circumference (HC) [that’s] more than 2 [standard deviations] below the mean for gestational age and sex.”
Nine of the infants were male, four were female. Eleven of the infants were born within 37-41 weeks’ of gestation. The remaining two were born at 35-36 weeks’ of gestation, considered “preterm” by the investigators. All infants tested positive for Zika via immunoglobulin M testing of cerebrospinal fluid, serum, or both. Only six of the mothers reported having a rash while pregnant; four reported experiencing it during the first trimester, while the other two said it occurred in the second.
All 13 infants showed a decrease in HC to what was defined as microcephaly within 1 year of birth (October 2016). Neuroimaging showed that all but one had decreased brain volume, all had malformations of cortical development, four had cerebellum or brain-stem hypoplasia, ten had ventriculomegaly, and three had increased extra-axial CSF space.
“More than 60% of infants in this series had epilepsy (likely related to the cortical malformations), and all had significant motor disabilities consistent with mixed cerebral palsy,” the authors noted, adding that the “pathogenesis of postnatal microcephaly from congenital Zika virus infections is [still] not known.”
FROM THE MMWR
Long-acting bupivacaine offers limited benefit in hysterectomy pain
ORLANDO – Port site infiltration during laparoscopic or robot-assisted hysterectomy with extended-release liposomal bupivacaine did not significantly improve most postoperative pain scores, compared with plain 0.25% bupivacaine.
In a randomized trial, the liposomal formulation was associated with 30% less pain on postoperative day 3, a significant difference not seen on postoperative day 1, 2, or 14.
“Liposomal bupivacaine is expected to last about 72 hours but it also comes at a cost,” Kenneth I. Barron, MD, a fellow in advanced minimally invasive gynecologic surgery at Florida Hospital Orlando, said at the meeting sponsored by AAGL. Extended-release bupivacaine costs $280, compared with $1.83 for plain bupivacaine, according to Dr. Barron.
“Based on this study, the routine use of liposomal bupivacaine as a port site local anesthetic in laparoscopic hysterectomy has limited usefulness and is not justified,” he said.
In the blinded study, surgeons at a tertiary-care community hospital performed pre-incision infiltration with undiluted liposomal extended-release bupivacaine for 32 surgery patients and with the short-acting formulation for another 32 surgery patients. All patients underwent either laparoscopic or robot-assisted total hysterectomy for benign indications. They were recruited for the study between July 2015 and January 2016 and there were no significant demographic differences between groups preoperatively.
For the primary outcome measure, investigators called each participant and asked them to rate their average overall pain on postoperative days 1, 2, 3, and 14. They used the Brief Pain Inventory 0-10 scale. There were no significant differences between groups on a composite score of their average and worst pain on days 1, 2, or 14. However, on day 3, the composite score was 3.26 in the extended-release group, compared with 4.83 for those receiving short-acting bupivacaine (P = .009).
“What this shows, if anything, is one method of local anesthetic is probably not enough to make a significant impact,” Dr. Barron said. What is needed instead is “probably more of a global approach to enhance recovery.”
There were no significant differences between groups in the secondary study outcomes: pain scores during the first 24 hours in the hospital, function based on pain interference scores, opioid use, or adverse events.
Dr. Barron reported having no relevant financial disclosures.
ORLANDO – Port site infiltration during laparoscopic or robot-assisted hysterectomy with extended-release liposomal bupivacaine did not significantly improve most postoperative pain scores, compared with plain 0.25% bupivacaine.
In a randomized trial, the liposomal formulation was associated with 30% less pain on postoperative day 3, a significant difference not seen on postoperative day 1, 2, or 14.
“Liposomal bupivacaine is expected to last about 72 hours but it also comes at a cost,” Kenneth I. Barron, MD, a fellow in advanced minimally invasive gynecologic surgery at Florida Hospital Orlando, said at the meeting sponsored by AAGL. Extended-release bupivacaine costs $280, compared with $1.83 for plain bupivacaine, according to Dr. Barron.
“Based on this study, the routine use of liposomal bupivacaine as a port site local anesthetic in laparoscopic hysterectomy has limited usefulness and is not justified,” he said.
In the blinded study, surgeons at a tertiary-care community hospital performed pre-incision infiltration with undiluted liposomal extended-release bupivacaine for 32 surgery patients and with the short-acting formulation for another 32 surgery patients. All patients underwent either laparoscopic or robot-assisted total hysterectomy for benign indications. They were recruited for the study between July 2015 and January 2016 and there were no significant demographic differences between groups preoperatively.
For the primary outcome measure, investigators called each participant and asked them to rate their average overall pain on postoperative days 1, 2, 3, and 14. They used the Brief Pain Inventory 0-10 scale. There were no significant differences between groups on a composite score of their average and worst pain on days 1, 2, or 14. However, on day 3, the composite score was 3.26 in the extended-release group, compared with 4.83 for those receiving short-acting bupivacaine (P = .009).
“What this shows, if anything, is one method of local anesthetic is probably not enough to make a significant impact,” Dr. Barron said. What is needed instead is “probably more of a global approach to enhance recovery.”
There were no significant differences between groups in the secondary study outcomes: pain scores during the first 24 hours in the hospital, function based on pain interference scores, opioid use, or adverse events.
Dr. Barron reported having no relevant financial disclosures.
ORLANDO – Port site infiltration during laparoscopic or robot-assisted hysterectomy with extended-release liposomal bupivacaine did not significantly improve most postoperative pain scores, compared with plain 0.25% bupivacaine.
In a randomized trial, the liposomal formulation was associated with 30% less pain on postoperative day 3, a significant difference not seen on postoperative day 1, 2, or 14.
“Liposomal bupivacaine is expected to last about 72 hours but it also comes at a cost,” Kenneth I. Barron, MD, a fellow in advanced minimally invasive gynecologic surgery at Florida Hospital Orlando, said at the meeting sponsored by AAGL. Extended-release bupivacaine costs $280, compared with $1.83 for plain bupivacaine, according to Dr. Barron.
“Based on this study, the routine use of liposomal bupivacaine as a port site local anesthetic in laparoscopic hysterectomy has limited usefulness and is not justified,” he said.
In the blinded study, surgeons at a tertiary-care community hospital performed pre-incision infiltration with undiluted liposomal extended-release bupivacaine for 32 surgery patients and with the short-acting formulation for another 32 surgery patients. All patients underwent either laparoscopic or robot-assisted total hysterectomy for benign indications. They were recruited for the study between July 2015 and January 2016 and there were no significant demographic differences between groups preoperatively.
For the primary outcome measure, investigators called each participant and asked them to rate their average overall pain on postoperative days 1, 2, 3, and 14. They used the Brief Pain Inventory 0-10 scale. There were no significant differences between groups on a composite score of their average and worst pain on days 1, 2, or 14. However, on day 3, the composite score was 3.26 in the extended-release group, compared with 4.83 for those receiving short-acting bupivacaine (P = .009).
“What this shows, if anything, is one method of local anesthetic is probably not enough to make a significant impact,” Dr. Barron said. What is needed instead is “probably more of a global approach to enhance recovery.”
There were no significant differences between groups in the secondary study outcomes: pain scores during the first 24 hours in the hospital, function based on pain interference scores, opioid use, or adverse events.
Dr. Barron reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS