User login
Breast milk doesn’t contain meaningful levels of certolizumab pegol
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
[email protected]
On Twitter @alz_gal
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
[email protected]
On Twitter @alz_gal
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
[email protected]
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: None of the 137 samples contained more than 0.076 mcg/mL of the drug.
Data source: The 4-week postmarketing study comprised 17 breastfeeding women.
Disclosures: UCB sponsored the study. Dr. Clowse is a consultant for the company.
California bucks trend of rising U.S. maternal mortality
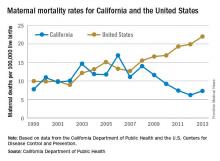
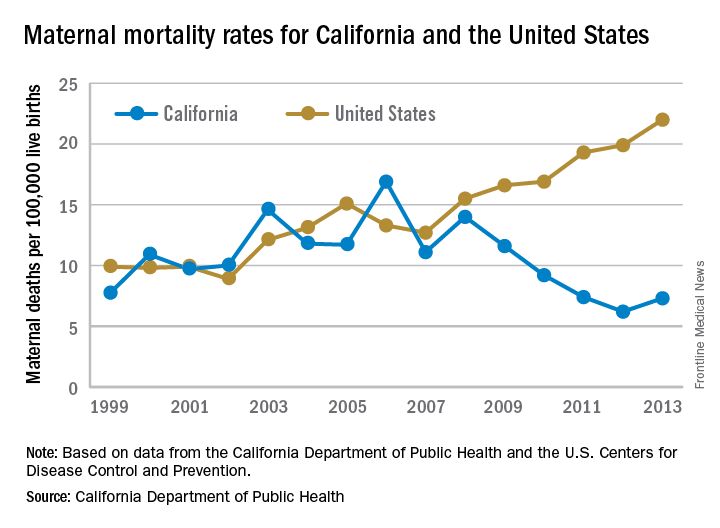
While the United States as a whole is seeing an unsettling rise in maternal mortality, California is on a divergent path.
Maternal mortality in the Golden State was tracking at a similar rate with national figures from 1999-2008 when the trend started to change. By 2013, the U.S. maternal mortality rate had grown to 22.0 deaths per 100,000 live births, while California’s rate had dropped to 7.3 per 100,000, according to data from the California Department of Public Health and the U.S. Centers for Disease Control and Prevention.
“We reviewed every maternal death for almost 10 years and through that process, we learned a lot about practices of care and the opportunities to really have intervened,” Elliott Main, MD, medical director of the Collaborative, said in an interview. “There were certain causes of death that had a pretty high chance of preventability and those were hemorrhage and preeclampsia.”
Identifying risk factors
The Collaborative’s research identified a number of risk factors that were significant contributors to maternal morbidity.
Dr. Main noted that obesity and older maternal age are both associated with an increased likelihood of having a cesarean delivery, which is associated with an increased risk of hemorrhage.
“So there are pathways that can get you into more trouble, but again if you are on top of those, you can be proactive and not necessarily have this high rate of complications,” he added.
With data in hand about what was contributing to the risks of maternal mortality, the Collaborative set out to build a series of toolkits or “bundles” to help guide hospitals in limiting complications and responding to emergencies. These toolkits are based on the state’s own data, as well as best practices identified in the medical literature and national guidelines from organizations such as the American College of Obstetricians and Gynecologists.
For example, the hypertension bundle includes information aimed at readiness, recognition and prevention, response, and reporting/system learning. Other bundles, which the Collaborative helped to develop and which are distributed through the Council on Patient Safety in Women’s Health Care, cover areas such as mental health, thromboembolism, hemorrhage, and safe reduction in primary cesarean births.
“It’s all about the implementation of those and that’s where we spent a lot of time in California with what are called quality collaboratives,” he said. “These are generally state-based [efforts], where you put together a consortium of providers, hospitals, and public health and patient advocates, and you work on improving the care for these certain conditions.”
The bundles are not meant to be cookbook medicine, Dr. Main noted, but rather are designed so that they can be customized based on the resources of an individual hospital, whether the facility handles 300 births a year or 5,000.
“There is no such thing as a national protocol for this,” Dr. Main said. “You have to have some flexibility and differences in the protocols but what we really are striving for is that for emergencies, people have standard protocols for the treatment of that emergency.”
Disparities remain
While California is a success story in terms of its overall drop in maternal mortality, racial disparities remain, particularly for African American women.
Maternal mortality among African Americans has dropped 50% in the state, mirroring the overall decline in the state during the 2008-2013 time period, but it is still three to four times higher than for other racial/ethnic groups.
“African American women do have more risk factors. There is more obesity. There is more hypertension and they have more social stresses and so forth, but none of those are reasons that they should die,” Dr. Main said. “They are reasons that they should have more intensive attention and care. If you have an older African American woman with hypertension, you’ve got to be on your toes when you are taking care of her and be a lot more responsive to warning signs than in a 25-year-old white woman that is perfectly healthy.”
“Minorities represent half of all U.S. persons, yet racial and ethnic minorities suffer higher rates of maternal mortality than whites in this country,” said Dr. Howell, who also chairs ACOG’s work group on reduction of peripartum racial disparities. “In fact, black and African Americans are three to four times more likely to die than whites. This is the largest disparity among population perinatal health measures.”
Native Americans, Asians, and some Latinas also have elevated rates of maternal mortality, compared with white women, she noted.
Depending on the city, those rates could be even higher, she said.
“In New York City, a recent publication by our Department of Health reviewed deaths from 2006 to 2010 and they found that black women were 10 times more likely to die than white women,” Dr. Howell said. “It’s also important to remember that for every maternal death, over 100 women experience severe obstetric morbidity or a life-threatening diagnosis or undergo a lifesaving procedure during their delivery hospitalization.”
But there are care tools available to help address this issue, too. The Alliance for Innovation on Maternal Health, which includes the California collaborative and ACOG, has developed a safety bundle that focuses on themes of shared decision making, implicit bias, continuity of care, provider and patient education, and care fragmentation. It also recommends implementation of a disparity dashboard, meaning that hospitals and health systems would stratify their quality results by race and ethnicity to identify and address gaps in care, Dr. Howell said.
[email protected]
While the United States as a whole is seeing an unsettling rise in maternal mortality, California is on a divergent path.
Maternal mortality in the Golden State was tracking at a similar rate with national figures from 1999-2008 when the trend started to change. By 2013, the U.S. maternal mortality rate had grown to 22.0 deaths per 100,000 live births, while California’s rate had dropped to 7.3 per 100,000, according to data from the California Department of Public Health and the U.S. Centers for Disease Control and Prevention.
“We reviewed every maternal death for almost 10 years and through that process, we learned a lot about practices of care and the opportunities to really have intervened,” Elliott Main, MD, medical director of the Collaborative, said in an interview. “There were certain causes of death that had a pretty high chance of preventability and those were hemorrhage and preeclampsia.”
Identifying risk factors
The Collaborative’s research identified a number of risk factors that were significant contributors to maternal morbidity.
Dr. Main noted that obesity and older maternal age are both associated with an increased likelihood of having a cesarean delivery, which is associated with an increased risk of hemorrhage.
“So there are pathways that can get you into more trouble, but again if you are on top of those, you can be proactive and not necessarily have this high rate of complications,” he added.
With data in hand about what was contributing to the risks of maternal mortality, the Collaborative set out to build a series of toolkits or “bundles” to help guide hospitals in limiting complications and responding to emergencies. These toolkits are based on the state’s own data, as well as best practices identified in the medical literature and national guidelines from organizations such as the American College of Obstetricians and Gynecologists.
For example, the hypertension bundle includes information aimed at readiness, recognition and prevention, response, and reporting/system learning. Other bundles, which the Collaborative helped to develop and which are distributed through the Council on Patient Safety in Women’s Health Care, cover areas such as mental health, thromboembolism, hemorrhage, and safe reduction in primary cesarean births.
“It’s all about the implementation of those and that’s where we spent a lot of time in California with what are called quality collaboratives,” he said. “These are generally state-based [efforts], where you put together a consortium of providers, hospitals, and public health and patient advocates, and you work on improving the care for these certain conditions.”
The bundles are not meant to be cookbook medicine, Dr. Main noted, but rather are designed so that they can be customized based on the resources of an individual hospital, whether the facility handles 300 births a year or 5,000.
“There is no such thing as a national protocol for this,” Dr. Main said. “You have to have some flexibility and differences in the protocols but what we really are striving for is that for emergencies, people have standard protocols for the treatment of that emergency.”
Disparities remain
While California is a success story in terms of its overall drop in maternal mortality, racial disparities remain, particularly for African American women.
Maternal mortality among African Americans has dropped 50% in the state, mirroring the overall decline in the state during the 2008-2013 time period, but it is still three to four times higher than for other racial/ethnic groups.
“African American women do have more risk factors. There is more obesity. There is more hypertension and they have more social stresses and so forth, but none of those are reasons that they should die,” Dr. Main said. “They are reasons that they should have more intensive attention and care. If you have an older African American woman with hypertension, you’ve got to be on your toes when you are taking care of her and be a lot more responsive to warning signs than in a 25-year-old white woman that is perfectly healthy.”
“Minorities represent half of all U.S. persons, yet racial and ethnic minorities suffer higher rates of maternal mortality than whites in this country,” said Dr. Howell, who also chairs ACOG’s work group on reduction of peripartum racial disparities. “In fact, black and African Americans are three to four times more likely to die than whites. This is the largest disparity among population perinatal health measures.”
Native Americans, Asians, and some Latinas also have elevated rates of maternal mortality, compared with white women, she noted.
Depending on the city, those rates could be even higher, she said.
“In New York City, a recent publication by our Department of Health reviewed deaths from 2006 to 2010 and they found that black women were 10 times more likely to die than white women,” Dr. Howell said. “It’s also important to remember that for every maternal death, over 100 women experience severe obstetric morbidity or a life-threatening diagnosis or undergo a lifesaving procedure during their delivery hospitalization.”
But there are care tools available to help address this issue, too. The Alliance for Innovation on Maternal Health, which includes the California collaborative and ACOG, has developed a safety bundle that focuses on themes of shared decision making, implicit bias, continuity of care, provider and patient education, and care fragmentation. It also recommends implementation of a disparity dashboard, meaning that hospitals and health systems would stratify their quality results by race and ethnicity to identify and address gaps in care, Dr. Howell said.
[email protected]
While the United States as a whole is seeing an unsettling rise in maternal mortality, California is on a divergent path.
Maternal mortality in the Golden State was tracking at a similar rate with national figures from 1999-2008 when the trend started to change. By 2013, the U.S. maternal mortality rate had grown to 22.0 deaths per 100,000 live births, while California’s rate had dropped to 7.3 per 100,000, according to data from the California Department of Public Health and the U.S. Centers for Disease Control and Prevention.
“We reviewed every maternal death for almost 10 years and through that process, we learned a lot about practices of care and the opportunities to really have intervened,” Elliott Main, MD, medical director of the Collaborative, said in an interview. “There were certain causes of death that had a pretty high chance of preventability and those were hemorrhage and preeclampsia.”
Identifying risk factors
The Collaborative’s research identified a number of risk factors that were significant contributors to maternal morbidity.
Dr. Main noted that obesity and older maternal age are both associated with an increased likelihood of having a cesarean delivery, which is associated with an increased risk of hemorrhage.
“So there are pathways that can get you into more trouble, but again if you are on top of those, you can be proactive and not necessarily have this high rate of complications,” he added.
With data in hand about what was contributing to the risks of maternal mortality, the Collaborative set out to build a series of toolkits or “bundles” to help guide hospitals in limiting complications and responding to emergencies. These toolkits are based on the state’s own data, as well as best practices identified in the medical literature and national guidelines from organizations such as the American College of Obstetricians and Gynecologists.
For example, the hypertension bundle includes information aimed at readiness, recognition and prevention, response, and reporting/system learning. Other bundles, which the Collaborative helped to develop and which are distributed through the Council on Patient Safety in Women’s Health Care, cover areas such as mental health, thromboembolism, hemorrhage, and safe reduction in primary cesarean births.
“It’s all about the implementation of those and that’s where we spent a lot of time in California with what are called quality collaboratives,” he said. “These are generally state-based [efforts], where you put together a consortium of providers, hospitals, and public health and patient advocates, and you work on improving the care for these certain conditions.”
The bundles are not meant to be cookbook medicine, Dr. Main noted, but rather are designed so that they can be customized based on the resources of an individual hospital, whether the facility handles 300 births a year or 5,000.
“There is no such thing as a national protocol for this,” Dr. Main said. “You have to have some flexibility and differences in the protocols but what we really are striving for is that for emergencies, people have standard protocols for the treatment of that emergency.”
Disparities remain
While California is a success story in terms of its overall drop in maternal mortality, racial disparities remain, particularly for African American women.
Maternal mortality among African Americans has dropped 50% in the state, mirroring the overall decline in the state during the 2008-2013 time period, but it is still three to four times higher than for other racial/ethnic groups.
“African American women do have more risk factors. There is more obesity. There is more hypertension and they have more social stresses and so forth, but none of those are reasons that they should die,” Dr. Main said. “They are reasons that they should have more intensive attention and care. If you have an older African American woman with hypertension, you’ve got to be on your toes when you are taking care of her and be a lot more responsive to warning signs than in a 25-year-old white woman that is perfectly healthy.”
“Minorities represent half of all U.S. persons, yet racial and ethnic minorities suffer higher rates of maternal mortality than whites in this country,” said Dr. Howell, who also chairs ACOG’s work group on reduction of peripartum racial disparities. “In fact, black and African Americans are three to four times more likely to die than whites. This is the largest disparity among population perinatal health measures.”
Native Americans, Asians, and some Latinas also have elevated rates of maternal mortality, compared with white women, she noted.
Depending on the city, those rates could be even higher, she said.
“In New York City, a recent publication by our Department of Health reviewed deaths from 2006 to 2010 and they found that black women were 10 times more likely to die than white women,” Dr. Howell said. “It’s also important to remember that for every maternal death, over 100 women experience severe obstetric morbidity or a life-threatening diagnosis or undergo a lifesaving procedure during their delivery hospitalization.”
But there are care tools available to help address this issue, too. The Alliance for Innovation on Maternal Health, which includes the California collaborative and ACOG, has developed a safety bundle that focuses on themes of shared decision making, implicit bias, continuity of care, provider and patient education, and care fragmentation. It also recommends implementation of a disparity dashboard, meaning that hospitals and health systems would stratify their quality results by race and ethnicity to identify and address gaps in care, Dr. Howell said.
[email protected]
Fewer Zika-infected pregnancies reported for second week in a row
The number of new Zika cases reported among pregnant women in the United States dropped for the second week in a row, according to data from the Centers for Disease Control and Prevention.
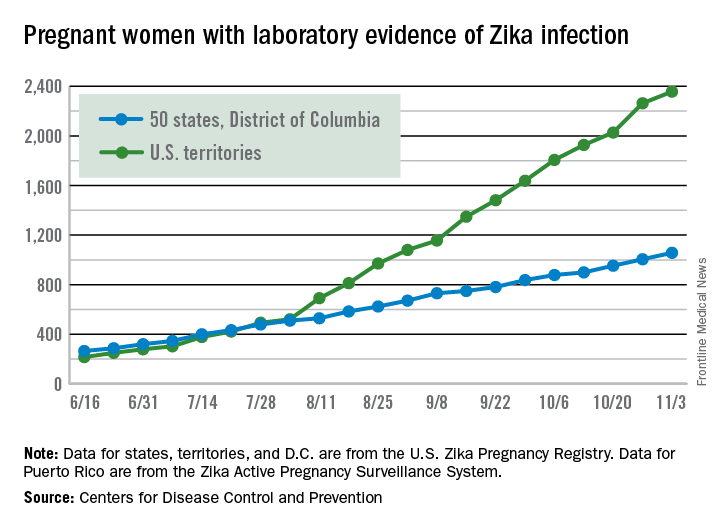
For the week ending Nov. 10, there were 124 new cases of pregnant women with laboratory evidence of Zika virus infection reported to the CDC: 30 in the states and the District of Columbia and 94 in the U.S. territories. The previous week (Nov. 3), the number of new cases was 146, which came on the heels of a new weekly high of 288 for the week of Oct. 27.
The total number of pregnant women with Zika now stands at 3,538 for the year: 1,087 for the states and 2,451 for the territories, the CDC said.
Among all Americans, there have been 36,323 cases of Zika reported to the CDC: 4,255 have occurred in the states/D.C. and 32,068 in the territories. About 98% of territorial cases have occurred in Puerto Rico, the CDC said.
No new cases of infants with Zika-related birth defects were reported for the week ending Nov. 10, so the totals hold at 26 infants born with Zika-related birth defects and five Zika-related pregnancy losses, according to the CDC.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The number of new Zika cases reported among pregnant women in the United States dropped for the second week in a row, according to data from the Centers for Disease Control and Prevention.
For the week ending Nov. 10, there were 124 new cases of pregnant women with laboratory evidence of Zika virus infection reported to the CDC: 30 in the states and the District of Columbia and 94 in the U.S. territories. The previous week (Nov. 3), the number of new cases was 146, which came on the heels of a new weekly high of 288 for the week of Oct. 27.
The total number of pregnant women with Zika now stands at 3,538 for the year: 1,087 for the states and 2,451 for the territories, the CDC said.
Among all Americans, there have been 36,323 cases of Zika reported to the CDC: 4,255 have occurred in the states/D.C. and 32,068 in the territories. About 98% of territorial cases have occurred in Puerto Rico, the CDC said.
No new cases of infants with Zika-related birth defects were reported for the week ending Nov. 10, so the totals hold at 26 infants born with Zika-related birth defects and five Zika-related pregnancy losses, according to the CDC.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The number of new Zika cases reported among pregnant women in the United States dropped for the second week in a row, according to data from the Centers for Disease Control and Prevention.
For the week ending Nov. 10, there were 124 new cases of pregnant women with laboratory evidence of Zika virus infection reported to the CDC: 30 in the states and the District of Columbia and 94 in the U.S. territories. The previous week (Nov. 3), the number of new cases was 146, which came on the heels of a new weekly high of 288 for the week of Oct. 27.
The total number of pregnant women with Zika now stands at 3,538 for the year: 1,087 for the states and 2,451 for the territories, the CDC said.
Among all Americans, there have been 36,323 cases of Zika reported to the CDC: 4,255 have occurred in the states/D.C. and 32,068 in the territories. About 98% of territorial cases have occurred in Puerto Rico, the CDC said.
No new cases of infants with Zika-related birth defects were reported for the week ending Nov. 10, so the totals hold at 26 infants born with Zika-related birth defects and five Zika-related pregnancy losses, according to the CDC.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Nutraceutical cocktail protects against postpartum blues
VIENNA – A dietary supplement blend virtually eliminated postpartum blues in a promising proof-of-concept controlled trial, Yekta Dowlati, PhD, reported during the annual congress of the European College of Neuropsychopharmacology.
The nutraceutical cocktail is designed to compensate for the effects of the early postpartum surge in monoamine oxidase A (MAO-A) activity that her research group previously has reported. They found that as estrogen levels plunge by 100-fold in the first 3 days postpartum, brain MAO-A levels rise by 40% in affect-controlling regions, including the prefrontal cortex and anterior cingulate cortex (Arch Gen Psychiatry. 2010 May;67[5]:468-74).
That suggests a potential causal relationship with postpartum blues, since MAO-A is an enzyme whose effects include promotion of oxidative stress, apoptosis, and metabolizing serotonin, norepinephrine, and dopamine, explained Dr. Dowlati of the department of psychiatry at the University of Toronto.
Postpartum blues is a common prodrome for postpartum depression, the most frequent complication of childbearing, which has an estimated incidence of 13%. Severe postpartum blues is a strong predictor of subsequent postpartum depression. Yet, despite the large burden of illness imposed by postpartum depression, there is no proven preventive strategy. The hypothesis being pursued in developing this nutraceutical is that a safe dietary intervention that prevents postpartum blues also may prevent postpartum depression.
The nutraceutical cocktail developed by Dr. Dowlati and her coinvestigators consists of monoamine precursors: 2 g of tryptophan, 10 g of tyrosine, and blueberry juice plus blueberry extract.
She reported on 41 healthy breast-feeding mothers who on day 5 postpartum, when postpartum blues typically peaks, were assigned to drink the dietary supplement or not. Later that day, they underwent a quantified assessment of their severity of postpartum blues based upon change from baseline in depressed mood scores on a 0-100 visual analog scale after undergoing a standardized sad mood induction procedure. This is a simple protocol widely used by psychiatrists and psychologists researching the neurobiology of mood states. Dr. Dowlati and her colleagues used the Velten protocol, in which subjects read depressing sentences while listening to sad music.
Mean scores on the Visual Analog Scale for sadness following the standardized mood induction procedure jumped by 43.8 points in the control group but were unchanged, with a mere 0.5-point increase, in the 21 women in the active treatment arm.
“The results of the present study, albeit in an open-label trial, reflect by far the most robust effects of a dietary supplement ever seen on postpartum blues. Our effect size was 2.9. Previous trials have reported effect sizes of 0.07-0.28,” she noted.
An effect size of 2.9 means that if a postpartum woman did not experience a plunge in mood after the induction protocol, there was statistically a 98%-99% chance that she had consumed the supplement.
“One explanation to account for an active effect is that the supplement is compensating for the effects of monoamine metabolism and increased oxidative stress by elevated postpartum MAO-A levels,” according to Dr. Dowlati. “Given the effect size of 2.9 and minimal effects of tryptophan and tyrosine supplementation on total levels in breast milk, there is excellent reason to pursue this supplement in a randomized, double-blind, placebo-controlled trial to further assess its effects.”
Before conducting this efficacy study, the investigators evaluated the safety of their planned intervention by randomizing 54 healthy breast-feeding women to single larger doses of oral tyrosine or tryptophan than were used in the nutraceutical cocktail or to no supplements. They found no subsequent increase in total tyrosine or total tryptophan levels in the subjects’ breast milk, although dose-dependent increases were found in free tyrosine and free tryptophan in maternal plasma. Free tyrosine was increased in breast milk; however, the level was significantly lower than what the investigators found in laboratory analysis of a dozen popular brands of infant formula.
The safety and open-label efficacy studies were funded by the Canadian Institutes of Health Research, the Ontario Mental Health Foundation, and university research grants. Dr. Dowlati reported having no financial conflicts of interest.
VIENNA – A dietary supplement blend virtually eliminated postpartum blues in a promising proof-of-concept controlled trial, Yekta Dowlati, PhD, reported during the annual congress of the European College of Neuropsychopharmacology.
The nutraceutical cocktail is designed to compensate for the effects of the early postpartum surge in monoamine oxidase A (MAO-A) activity that her research group previously has reported. They found that as estrogen levels plunge by 100-fold in the first 3 days postpartum, brain MAO-A levels rise by 40% in affect-controlling regions, including the prefrontal cortex and anterior cingulate cortex (Arch Gen Psychiatry. 2010 May;67[5]:468-74).
That suggests a potential causal relationship with postpartum blues, since MAO-A is an enzyme whose effects include promotion of oxidative stress, apoptosis, and metabolizing serotonin, norepinephrine, and dopamine, explained Dr. Dowlati of the department of psychiatry at the University of Toronto.
Postpartum blues is a common prodrome for postpartum depression, the most frequent complication of childbearing, which has an estimated incidence of 13%. Severe postpartum blues is a strong predictor of subsequent postpartum depression. Yet, despite the large burden of illness imposed by postpartum depression, there is no proven preventive strategy. The hypothesis being pursued in developing this nutraceutical is that a safe dietary intervention that prevents postpartum blues also may prevent postpartum depression.
The nutraceutical cocktail developed by Dr. Dowlati and her coinvestigators consists of monoamine precursors: 2 g of tryptophan, 10 g of tyrosine, and blueberry juice plus blueberry extract.
She reported on 41 healthy breast-feeding mothers who on day 5 postpartum, when postpartum blues typically peaks, were assigned to drink the dietary supplement or not. Later that day, they underwent a quantified assessment of their severity of postpartum blues based upon change from baseline in depressed mood scores on a 0-100 visual analog scale after undergoing a standardized sad mood induction procedure. This is a simple protocol widely used by psychiatrists and psychologists researching the neurobiology of mood states. Dr. Dowlati and her colleagues used the Velten protocol, in which subjects read depressing sentences while listening to sad music.
Mean scores on the Visual Analog Scale for sadness following the standardized mood induction procedure jumped by 43.8 points in the control group but were unchanged, with a mere 0.5-point increase, in the 21 women in the active treatment arm.
“The results of the present study, albeit in an open-label trial, reflect by far the most robust effects of a dietary supplement ever seen on postpartum blues. Our effect size was 2.9. Previous trials have reported effect sizes of 0.07-0.28,” she noted.
An effect size of 2.9 means that if a postpartum woman did not experience a plunge in mood after the induction protocol, there was statistically a 98%-99% chance that she had consumed the supplement.
“One explanation to account for an active effect is that the supplement is compensating for the effects of monoamine metabolism and increased oxidative stress by elevated postpartum MAO-A levels,” according to Dr. Dowlati. “Given the effect size of 2.9 and minimal effects of tryptophan and tyrosine supplementation on total levels in breast milk, there is excellent reason to pursue this supplement in a randomized, double-blind, placebo-controlled trial to further assess its effects.”
Before conducting this efficacy study, the investigators evaluated the safety of their planned intervention by randomizing 54 healthy breast-feeding women to single larger doses of oral tyrosine or tryptophan than were used in the nutraceutical cocktail or to no supplements. They found no subsequent increase in total tyrosine or total tryptophan levels in the subjects’ breast milk, although dose-dependent increases were found in free tyrosine and free tryptophan in maternal plasma. Free tyrosine was increased in breast milk; however, the level was significantly lower than what the investigators found in laboratory analysis of a dozen popular brands of infant formula.
The safety and open-label efficacy studies were funded by the Canadian Institutes of Health Research, the Ontario Mental Health Foundation, and university research grants. Dr. Dowlati reported having no financial conflicts of interest.
VIENNA – A dietary supplement blend virtually eliminated postpartum blues in a promising proof-of-concept controlled trial, Yekta Dowlati, PhD, reported during the annual congress of the European College of Neuropsychopharmacology.
The nutraceutical cocktail is designed to compensate for the effects of the early postpartum surge in monoamine oxidase A (MAO-A) activity that her research group previously has reported. They found that as estrogen levels plunge by 100-fold in the first 3 days postpartum, brain MAO-A levels rise by 40% in affect-controlling regions, including the prefrontal cortex and anterior cingulate cortex (Arch Gen Psychiatry. 2010 May;67[5]:468-74).
That suggests a potential causal relationship with postpartum blues, since MAO-A is an enzyme whose effects include promotion of oxidative stress, apoptosis, and metabolizing serotonin, norepinephrine, and dopamine, explained Dr. Dowlati of the department of psychiatry at the University of Toronto.
Postpartum blues is a common prodrome for postpartum depression, the most frequent complication of childbearing, which has an estimated incidence of 13%. Severe postpartum blues is a strong predictor of subsequent postpartum depression. Yet, despite the large burden of illness imposed by postpartum depression, there is no proven preventive strategy. The hypothesis being pursued in developing this nutraceutical is that a safe dietary intervention that prevents postpartum blues also may prevent postpartum depression.
The nutraceutical cocktail developed by Dr. Dowlati and her coinvestigators consists of monoamine precursors: 2 g of tryptophan, 10 g of tyrosine, and blueberry juice plus blueberry extract.
She reported on 41 healthy breast-feeding mothers who on day 5 postpartum, when postpartum blues typically peaks, were assigned to drink the dietary supplement or not. Later that day, they underwent a quantified assessment of their severity of postpartum blues based upon change from baseline in depressed mood scores on a 0-100 visual analog scale after undergoing a standardized sad mood induction procedure. This is a simple protocol widely used by psychiatrists and psychologists researching the neurobiology of mood states. Dr. Dowlati and her colleagues used the Velten protocol, in which subjects read depressing sentences while listening to sad music.
Mean scores on the Visual Analog Scale for sadness following the standardized mood induction procedure jumped by 43.8 points in the control group but were unchanged, with a mere 0.5-point increase, in the 21 women in the active treatment arm.
“The results of the present study, albeit in an open-label trial, reflect by far the most robust effects of a dietary supplement ever seen on postpartum blues. Our effect size was 2.9. Previous trials have reported effect sizes of 0.07-0.28,” she noted.
An effect size of 2.9 means that if a postpartum woman did not experience a plunge in mood after the induction protocol, there was statistically a 98%-99% chance that she had consumed the supplement.
“One explanation to account for an active effect is that the supplement is compensating for the effects of monoamine metabolism and increased oxidative stress by elevated postpartum MAO-A levels,” according to Dr. Dowlati. “Given the effect size of 2.9 and minimal effects of tryptophan and tyrosine supplementation on total levels in breast milk, there is excellent reason to pursue this supplement in a randomized, double-blind, placebo-controlled trial to further assess its effects.”
Before conducting this efficacy study, the investigators evaluated the safety of their planned intervention by randomizing 54 healthy breast-feeding women to single larger doses of oral tyrosine or tryptophan than were used in the nutraceutical cocktail or to no supplements. They found no subsequent increase in total tyrosine or total tryptophan levels in the subjects’ breast milk, although dose-dependent increases were found in free tyrosine and free tryptophan in maternal plasma. Free tyrosine was increased in breast milk; however, the level was significantly lower than what the investigators found in laboratory analysis of a dozen popular brands of infant formula.
The safety and open-label efficacy studies were funded by the Canadian Institutes of Health Research, the Ontario Mental Health Foundation, and university research grants. Dr. Dowlati reported having no financial conflicts of interest.
Key clinical point:
Major finding: Postpartum women who consumed a dietary supplement designed to compensate for the effects of a surge in monoamine oxidase A activity did not experience any lowering of mood after completing a standardized sad mood induction protocol, while a control group showed a steep rise in sadness scores.
Data source: An open-label proof-of-concept study in which 41 women undertook a standardized sad mood induction protocol on day 5 postpartum, after 21 of them had consumed a dietary supplement blend designed to ward off postpartum blues.
Disclosures: The safety and open-label efficacy studies were funded by the Canadian Institutes of Health Research, the Ontario Mental Health Foundation, and university research grants. Dr. Dowlati reported having no financial conflicts of interest.
Weekly number of Zika-infected pregnancies drops by half
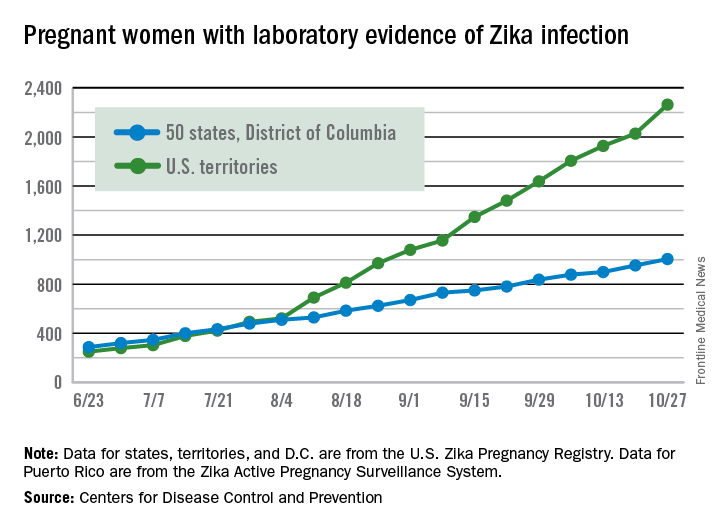
There were 146 new cases of pregnant women with laboratory evidence of Zika infection reported in the United States for the week ending Nov. 3 – just about half of the 288-case increase reported the week before, according to Centers for Disease Control and Prevention.
For the year so far, there have been 1,057 cases of pregnant women with Zika in the states and the District of Columbia – 52 for the week ending Nov. 3 – and 2,357 cases in the territories, the CDC announced, with 94 reported in the most recent week. The total number of U.S. cases – 3,414 – is up by 4.4% over the previous week.
The CDC also reported one new infant born with Zika-related birth defects for the week ending Nov. 3, bringing the total for the year to 26 in the states/D.C. There were no new Zika-related pregnancy losses reported, so the total remains at five. State-level data are not being reported to protect the privacy of affected women and children.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 146 new cases of pregnant women with laboratory evidence of Zika infection reported in the United States for the week ending Nov. 3 – just about half of the 288-case increase reported the week before, according to Centers for Disease Control and Prevention.
For the year so far, there have been 1,057 cases of pregnant women with Zika in the states and the District of Columbia – 52 for the week ending Nov. 3 – and 2,357 cases in the territories, the CDC announced, with 94 reported in the most recent week. The total number of U.S. cases – 3,414 – is up by 4.4% over the previous week.
The CDC also reported one new infant born with Zika-related birth defects for the week ending Nov. 3, bringing the total for the year to 26 in the states/D.C. There were no new Zika-related pregnancy losses reported, so the total remains at five. State-level data are not being reported to protect the privacy of affected women and children.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 146 new cases of pregnant women with laboratory evidence of Zika infection reported in the United States for the week ending Nov. 3 – just about half of the 288-case increase reported the week before, according to Centers for Disease Control and Prevention.
For the year so far, there have been 1,057 cases of pregnant women with Zika in the states and the District of Columbia – 52 for the week ending Nov. 3 – and 2,357 cases in the territories, the CDC announced, with 94 reported in the most recent week. The total number of U.S. cases – 3,414 – is up by 4.4% over the previous week.
The CDC also reported one new infant born with Zika-related birth defects for the week ending Nov. 3, bringing the total for the year to 26 in the states/D.C. There were no new Zika-related pregnancy losses reported, so the total remains at five. State-level data are not being reported to protect the privacy of affected women and children.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Study offers reassuring data on certolizumab use in pregnancy
VIENNA – Data from a large prospective registry of pregnancy outcomes in women on certolizumab are reassuring to date, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This unique dataset of pregnancies exposed to a single agent suggests that exposure is really not a problem, although we’ll continue to collect prospective data and anticipate doing so in women with psoriasis going forward,” said Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
Certolizumab is a tumor necrosis factor–alpha inhibitor currently approved in the United States for the treatment of Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is now in clinical trials for psoriasis, and Dr. Kimball said she expects that it will eventually receive an indication for that disease as well. In the interim, she switches her psoriasis patients who are pregnant or plan to become so to either off-label certolizumab or etanercept (Enbrel). She does the same for her psoriatic arthritis patients, although in that situation certolizumab is on-label therapy.
The reason she turns to etanercept or certolizumab in pregnancy or anticipated pregnancy is that these two biologics, unlike others, don’t cross the placenta in the third trimester.
“I’m concerned about the selective uptake of other monoclonal antibodies in the third trimester. We do see in babies born to moms exposed to these other drugs – like ustekinumab, infliximab, and adalimumab – that the baby’s drug blood levels at birth are higher than the mom’s, so you have potentially put them at some risk for infections unnecessarily and maybe have affected how their immune system develops. So if a woman comes to me who is pregnant and on a biologic agent I would either stop treatment in the second trimester or switch to etanercept or certolizumab,” the dermatologist said.
Of the 256 pregnancies prospectively followed in the UCB certolizumab registry, 80.9% resulted in live births, and 10.2% ended in spontaneous abortion or miscarriage. In addition, the induced abortion rate was 8.6%, and there was a single stillbirth.
Of note, the mean age at pregnancy was 31 years, and 29% of the women became pregnant at age 35 or older. In contrast, the mean age at first pregnancy in the general population is 26, and only about 10% are 35 or older. These data are consistent with Dr. Kimball’s own clinical experience, which is that women with moderate to severe psoriasis or psoriatic arthritis often have trouble conceiving, and if they eventually succeed it’s often at a more advanced age.
The rate of maternal complications in this series was unremarkable: preeclampsia in 3.5%, infection in 3.9%, disease flare in 5.1%, and gestational diabetes in 3.1%. The median gestational age at birth was 39 weeks. The rate of early preterm birth before 32 weeks was 3.5%, with 12.6% of babies arriving at 32-36 weeks. Considering these are older moms being treated for serious underlying systemic inflammatory diseases, these numbers look good, according to Dr. Kimball.
Most women were exposed to certolizumab during the first trimester at least, and many were on the drug throughout pregnancy.
She said about one-third of psoriasis patients experience improvement in their skin diseases during pregnancy.
“If they’re doing really well, I see no reason to keep them on systemic therapy during pregnancy. But I will say that I see a lot of very bad postpartum flares, and I’m quite cautious about that. For the women with psoriatic arthritis there may not be a choice; you may need to continue to treat them all the way through their pregnancy to keep from getting permanent joint destruction,” the dermatologist said.
Dr. Kimball reported receiving research grants from and serving as a consultant to UCB and numerous other pharmaceutical companies.
VIENNA – Data from a large prospective registry of pregnancy outcomes in women on certolizumab are reassuring to date, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This unique dataset of pregnancies exposed to a single agent suggests that exposure is really not a problem, although we’ll continue to collect prospective data and anticipate doing so in women with psoriasis going forward,” said Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
Certolizumab is a tumor necrosis factor–alpha inhibitor currently approved in the United States for the treatment of Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is now in clinical trials for psoriasis, and Dr. Kimball said she expects that it will eventually receive an indication for that disease as well. In the interim, she switches her psoriasis patients who are pregnant or plan to become so to either off-label certolizumab or etanercept (Enbrel). She does the same for her psoriatic arthritis patients, although in that situation certolizumab is on-label therapy.
The reason she turns to etanercept or certolizumab in pregnancy or anticipated pregnancy is that these two biologics, unlike others, don’t cross the placenta in the third trimester.
“I’m concerned about the selective uptake of other monoclonal antibodies in the third trimester. We do see in babies born to moms exposed to these other drugs – like ustekinumab, infliximab, and adalimumab – that the baby’s drug blood levels at birth are higher than the mom’s, so you have potentially put them at some risk for infections unnecessarily and maybe have affected how their immune system develops. So if a woman comes to me who is pregnant and on a biologic agent I would either stop treatment in the second trimester or switch to etanercept or certolizumab,” the dermatologist said.
Of the 256 pregnancies prospectively followed in the UCB certolizumab registry, 80.9% resulted in live births, and 10.2% ended in spontaneous abortion or miscarriage. In addition, the induced abortion rate was 8.6%, and there was a single stillbirth.
Of note, the mean age at pregnancy was 31 years, and 29% of the women became pregnant at age 35 or older. In contrast, the mean age at first pregnancy in the general population is 26, and only about 10% are 35 or older. These data are consistent with Dr. Kimball’s own clinical experience, which is that women with moderate to severe psoriasis or psoriatic arthritis often have trouble conceiving, and if they eventually succeed it’s often at a more advanced age.
The rate of maternal complications in this series was unremarkable: preeclampsia in 3.5%, infection in 3.9%, disease flare in 5.1%, and gestational diabetes in 3.1%. The median gestational age at birth was 39 weeks. The rate of early preterm birth before 32 weeks was 3.5%, with 12.6% of babies arriving at 32-36 weeks. Considering these are older moms being treated for serious underlying systemic inflammatory diseases, these numbers look good, according to Dr. Kimball.
Most women were exposed to certolizumab during the first trimester at least, and many were on the drug throughout pregnancy.
She said about one-third of psoriasis patients experience improvement in their skin diseases during pregnancy.
“If they’re doing really well, I see no reason to keep them on systemic therapy during pregnancy. But I will say that I see a lot of very bad postpartum flares, and I’m quite cautious about that. For the women with psoriatic arthritis there may not be a choice; you may need to continue to treat them all the way through their pregnancy to keep from getting permanent joint destruction,” the dermatologist said.
Dr. Kimball reported receiving research grants from and serving as a consultant to UCB and numerous other pharmaceutical companies.
VIENNA – Data from a large prospective registry of pregnancy outcomes in women on certolizumab are reassuring to date, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This unique dataset of pregnancies exposed to a single agent suggests that exposure is really not a problem, although we’ll continue to collect prospective data and anticipate doing so in women with psoriasis going forward,” said Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
Certolizumab is a tumor necrosis factor–alpha inhibitor currently approved in the United States for the treatment of Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is now in clinical trials for psoriasis, and Dr. Kimball said she expects that it will eventually receive an indication for that disease as well. In the interim, she switches her psoriasis patients who are pregnant or plan to become so to either off-label certolizumab or etanercept (Enbrel). She does the same for her psoriatic arthritis patients, although in that situation certolizumab is on-label therapy.
The reason she turns to etanercept or certolizumab in pregnancy or anticipated pregnancy is that these two biologics, unlike others, don’t cross the placenta in the third trimester.
“I’m concerned about the selective uptake of other monoclonal antibodies in the third trimester. We do see in babies born to moms exposed to these other drugs – like ustekinumab, infliximab, and adalimumab – that the baby’s drug blood levels at birth are higher than the mom’s, so you have potentially put them at some risk for infections unnecessarily and maybe have affected how their immune system develops. So if a woman comes to me who is pregnant and on a biologic agent I would either stop treatment in the second trimester or switch to etanercept or certolizumab,” the dermatologist said.
Of the 256 pregnancies prospectively followed in the UCB certolizumab registry, 80.9% resulted in live births, and 10.2% ended in spontaneous abortion or miscarriage. In addition, the induced abortion rate was 8.6%, and there was a single stillbirth.
Of note, the mean age at pregnancy was 31 years, and 29% of the women became pregnant at age 35 or older. In contrast, the mean age at first pregnancy in the general population is 26, and only about 10% are 35 or older. These data are consistent with Dr. Kimball’s own clinical experience, which is that women with moderate to severe psoriasis or psoriatic arthritis often have trouble conceiving, and if they eventually succeed it’s often at a more advanced age.
The rate of maternal complications in this series was unremarkable: preeclampsia in 3.5%, infection in 3.9%, disease flare in 5.1%, and gestational diabetes in 3.1%. The median gestational age at birth was 39 weeks. The rate of early preterm birth before 32 weeks was 3.5%, with 12.6% of babies arriving at 32-36 weeks. Considering these are older moms being treated for serious underlying systemic inflammatory diseases, these numbers look good, according to Dr. Kimball.
Most women were exposed to certolizumab during the first trimester at least, and many were on the drug throughout pregnancy.
She said about one-third of psoriasis patients experience improvement in their skin diseases during pregnancy.
“If they’re doing really well, I see no reason to keep them on systemic therapy during pregnancy. But I will say that I see a lot of very bad postpartum flares, and I’m quite cautious about that. For the women with psoriatic arthritis there may not be a choice; you may need to continue to treat them all the way through their pregnancy to keep from getting permanent joint destruction,” the dermatologist said.
Dr. Kimball reported receiving research grants from and serving as a consultant to UCB and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point:
Major finding: The rate of major congenital malformations in a large prospective series of pregnancies in women on certolizumab was reassuringly low at 4.2%, with no pattern of malformations being seen.
Data source: This was a report on maternal and fetal outcomes of 256 prospectively followed pregnancies in women on certolizumab.
Disclosures: The presenter reported receiving research funds from and serving as a consultant to UCB, which markets certolizumab and maintains the pregnancy registry.
Another Zika vaccine heads to Phase I trials
Scientists with the U.S. Department of Defense have launched a Phase I clinical trial to test an investigational Zika vaccine that relies on inactivated virus.
The candidate vaccine is known as the Zika Purified Inactivated Virus (ZPIV) vaccine and contains whole but inactivated Zika virus particles to stimulate an immune system response without replicating and causing illness.
“We urgently need a safe and effective vaccine to protect people from Zika virus infection as the virus continues to spread and cause serious public health consequences, particularly for pregnant women and their babies,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said in a statement. “We are pleased to be part of the collaborative effort to advance this promising candidate vaccine into clinical trials.”
The NIAID helped support the preclinical development of the vaccine candidate and is part of a joint research agreement with the Department of Defense and other federal agencies to develop the vaccine for use in humans.
The trial at Walter Reed will enroll 75 individuals, ranging in age from 18-49 years, all of whom should have no history of a flavivirus infection. Twenty-five subjects will be given a pair of either intramuscular ZPIV injections, or a placebo (saline), with 28 days between injections.
The remaining 50 subjects will be divided into two groups of 25, with one group receiving two doses of a Japanese encephalitis virus vaccine and the other getting one dose of a yellow fever vaccine, before they both receive the two-dose ZPIV vaccine regimen.
A subgroup of 30 patients will then receive a third ZPIV dose 1 year later. Across all cohorts, the ZPIV dosage will be 5 micrograms.
In addition to the testing of the ZPIV vaccine, there are Phase I trials of a DNA-based Zika vaccine ongoing at the National Institutes of Health Clinical Center in Bethesda, Md., the Center for Vaccine Development at the University of Maryland in Baltimore, Md., and at Emory University in Atlanta, Ga. Those trials were launched in August 2016.
Scientists with the U.S. Department of Defense have launched a Phase I clinical trial to test an investigational Zika vaccine that relies on inactivated virus.
The candidate vaccine is known as the Zika Purified Inactivated Virus (ZPIV) vaccine and contains whole but inactivated Zika virus particles to stimulate an immune system response without replicating and causing illness.
“We urgently need a safe and effective vaccine to protect people from Zika virus infection as the virus continues to spread and cause serious public health consequences, particularly for pregnant women and their babies,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said in a statement. “We are pleased to be part of the collaborative effort to advance this promising candidate vaccine into clinical trials.”
The NIAID helped support the preclinical development of the vaccine candidate and is part of a joint research agreement with the Department of Defense and other federal agencies to develop the vaccine for use in humans.
The trial at Walter Reed will enroll 75 individuals, ranging in age from 18-49 years, all of whom should have no history of a flavivirus infection. Twenty-five subjects will be given a pair of either intramuscular ZPIV injections, or a placebo (saline), with 28 days between injections.
The remaining 50 subjects will be divided into two groups of 25, with one group receiving two doses of a Japanese encephalitis virus vaccine and the other getting one dose of a yellow fever vaccine, before they both receive the two-dose ZPIV vaccine regimen.
A subgroup of 30 patients will then receive a third ZPIV dose 1 year later. Across all cohorts, the ZPIV dosage will be 5 micrograms.
In addition to the testing of the ZPIV vaccine, there are Phase I trials of a DNA-based Zika vaccine ongoing at the National Institutes of Health Clinical Center in Bethesda, Md., the Center for Vaccine Development at the University of Maryland in Baltimore, Md., and at Emory University in Atlanta, Ga. Those trials were launched in August 2016.
Scientists with the U.S. Department of Defense have launched a Phase I clinical trial to test an investigational Zika vaccine that relies on inactivated virus.
The candidate vaccine is known as the Zika Purified Inactivated Virus (ZPIV) vaccine and contains whole but inactivated Zika virus particles to stimulate an immune system response without replicating and causing illness.
“We urgently need a safe and effective vaccine to protect people from Zika virus infection as the virus continues to spread and cause serious public health consequences, particularly for pregnant women and their babies,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said in a statement. “We are pleased to be part of the collaborative effort to advance this promising candidate vaccine into clinical trials.”
The NIAID helped support the preclinical development of the vaccine candidate and is part of a joint research agreement with the Department of Defense and other federal agencies to develop the vaccine for use in humans.
The trial at Walter Reed will enroll 75 individuals, ranging in age from 18-49 years, all of whom should have no history of a flavivirus infection. Twenty-five subjects will be given a pair of either intramuscular ZPIV injections, or a placebo (saline), with 28 days between injections.
The remaining 50 subjects will be divided into two groups of 25, with one group receiving two doses of a Japanese encephalitis virus vaccine and the other getting one dose of a yellow fever vaccine, before they both receive the two-dose ZPIV vaccine regimen.
A subgroup of 30 patients will then receive a third ZPIV dose 1 year later. Across all cohorts, the ZPIV dosage will be 5 micrograms.
In addition to the testing of the ZPIV vaccine, there are Phase I trials of a DNA-based Zika vaccine ongoing at the National Institutes of Health Clinical Center in Bethesda, Md., the Center for Vaccine Development at the University of Maryland in Baltimore, Md., and at Emory University in Atlanta, Ga. Those trials were launched in August 2016.
Study finds no increase in microcephaly with Tdap vaccine in pregnancy
The combined tetanus, diphtheria, and acellular pertussis (Tdap) vaccine is not associated with an increased risk of microcephaly and other structural birth defects when administered during pregnancy, according to findings from a large, retrospective cohort study.
The U.S. Advisory Committee on Immunization Practices currently recommends administration of the Tdap vaccine between 27 and 36 weeks’ gestation in every pregnancy. However, the overlap of the start of Brazil’s maternal Tdap immunization in November 2014 with the substantial increase in microcephaly cases in 2015 prompted concerns of an association between the vaccine and structural birth defects.
They found that Tdap immunization was not significantly associated with an increased risk for microcephaly during any week of pregnancy (adjusted prevalence ratio, 0.86; 95% CI, 0.60-1.24). They also saw no increased risk of microcephaly when vaccinations occurred before 14 weeks’ gestation (adjusted prevalence ratio, 0.96; 95% CI, 0.36-2.58), or when vaccinations were administered between 27 weeks’ and 36 weeks’ gestation (adjusted prevalence ratio, 1.01; 95% CI, 0.63-1.61). The findings were similar for other structural defects, including congenital heart defects, spina bifida, encephalocele, and anophthalmia (JAMA. 2016;316[17]:1823-5).
“These results expand upon what is known about maternal Tdap vaccination safety to include information about structural birth defects and microcephaly in offspring,” the investigators wrote. “The findings support recommendations for routine Tdap administration during pregnancy.”
However, they noted that the study findings may have been limited by incomplete data on women’s immunization status, birth defects, and defects that may have resulted in pregnancy loss or elective termination.
The study was funded by the Centers for Disease Control and Prevention. The investigators reported having no relevant financial disclosures.
The combined tetanus, diphtheria, and acellular pertussis (Tdap) vaccine is not associated with an increased risk of microcephaly and other structural birth defects when administered during pregnancy, according to findings from a large, retrospective cohort study.
The U.S. Advisory Committee on Immunization Practices currently recommends administration of the Tdap vaccine between 27 and 36 weeks’ gestation in every pregnancy. However, the overlap of the start of Brazil’s maternal Tdap immunization in November 2014 with the substantial increase in microcephaly cases in 2015 prompted concerns of an association between the vaccine and structural birth defects.
They found that Tdap immunization was not significantly associated with an increased risk for microcephaly during any week of pregnancy (adjusted prevalence ratio, 0.86; 95% CI, 0.60-1.24). They also saw no increased risk of microcephaly when vaccinations occurred before 14 weeks’ gestation (adjusted prevalence ratio, 0.96; 95% CI, 0.36-2.58), or when vaccinations were administered between 27 weeks’ and 36 weeks’ gestation (adjusted prevalence ratio, 1.01; 95% CI, 0.63-1.61). The findings were similar for other structural defects, including congenital heart defects, spina bifida, encephalocele, and anophthalmia (JAMA. 2016;316[17]:1823-5).
“These results expand upon what is known about maternal Tdap vaccination safety to include information about structural birth defects and microcephaly in offspring,” the investigators wrote. “The findings support recommendations for routine Tdap administration during pregnancy.”
However, they noted that the study findings may have been limited by incomplete data on women’s immunization status, birth defects, and defects that may have resulted in pregnancy loss or elective termination.
The study was funded by the Centers for Disease Control and Prevention. The investigators reported having no relevant financial disclosures.
The combined tetanus, diphtheria, and acellular pertussis (Tdap) vaccine is not associated with an increased risk of microcephaly and other structural birth defects when administered during pregnancy, according to findings from a large, retrospective cohort study.
The U.S. Advisory Committee on Immunization Practices currently recommends administration of the Tdap vaccine between 27 and 36 weeks’ gestation in every pregnancy. However, the overlap of the start of Brazil’s maternal Tdap immunization in November 2014 with the substantial increase in microcephaly cases in 2015 prompted concerns of an association between the vaccine and structural birth defects.
They found that Tdap immunization was not significantly associated with an increased risk for microcephaly during any week of pregnancy (adjusted prevalence ratio, 0.86; 95% CI, 0.60-1.24). They also saw no increased risk of microcephaly when vaccinations occurred before 14 weeks’ gestation (adjusted prevalence ratio, 0.96; 95% CI, 0.36-2.58), or when vaccinations were administered between 27 weeks’ and 36 weeks’ gestation (adjusted prevalence ratio, 1.01; 95% CI, 0.63-1.61). The findings were similar for other structural defects, including congenital heart defects, spina bifida, encephalocele, and anophthalmia (JAMA. 2016;316[17]:1823-5).
“These results expand upon what is known about maternal Tdap vaccination safety to include information about structural birth defects and microcephaly in offspring,” the investigators wrote. “The findings support recommendations for routine Tdap administration during pregnancy.”
However, they noted that the study findings may have been limited by incomplete data on women’s immunization status, birth defects, and defects that may have resulted in pregnancy loss or elective termination.
The study was funded by the Centers for Disease Control and Prevention. The investigators reported having no relevant financial disclosures.
FROM JAMA
Key clinical point:
Major finding: Tdap immunization was not significantly associated with an increased risk for microcephaly during any week of pregnancy (adjusted prevalence ratio, 0.86; 95% CI, 0.60-1.24).
Data source: A retrospective cohort study in 41,654 singleton infants born to women who received Tdap during pregnancy and a control group of 282,809 babies born to unvaccinated women.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. The investigators reported having no relevant financial disclosures.
Large increase seen in number of Zika-infected pregnancies
The 288 new cases of pregnant women in the United States with laboratory evidence of Zika infection reported for the week ending Oct. 27, 2016, represent the largest weekly increase so far this year, according to new data from the Centers for Disease Control and Prevention.
The 52 new cases in the 50 states and the District of Columbia and the 236 cases in the U.S. territories bring the totals for the year to 1,005 and 2,263, respectively, and to 3,268 for the United States as a whole, the CDC reported.
The week ending Oct. 27 also brought reports of two more infants born with Zika-related birth defects in the 50 states/D.C., but there were no reports of Zika-related pregnancy losses. So far in 2016, there have been 25 liveborn infants with Zika-related birth defects and five pregnancy losses in the states.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The 288 new cases of pregnant women in the United States with laboratory evidence of Zika infection reported for the week ending Oct. 27, 2016, represent the largest weekly increase so far this year, according to new data from the Centers for Disease Control and Prevention.
The 52 new cases in the 50 states and the District of Columbia and the 236 cases in the U.S. territories bring the totals for the year to 1,005 and 2,263, respectively, and to 3,268 for the United States as a whole, the CDC reported.
The week ending Oct. 27 also brought reports of two more infants born with Zika-related birth defects in the 50 states/D.C., but there were no reports of Zika-related pregnancy losses. So far in 2016, there have been 25 liveborn infants with Zika-related birth defects and five pregnancy losses in the states.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The 288 new cases of pregnant women in the United States with laboratory evidence of Zika infection reported for the week ending Oct. 27, 2016, represent the largest weekly increase so far this year, according to new data from the Centers for Disease Control and Prevention.
The 52 new cases in the 50 states and the District of Columbia and the 236 cases in the U.S. territories bring the totals for the year to 1,005 and 2,263, respectively, and to 3,268 for the United States as a whole, the CDC reported.
The week ending Oct. 27 also brought reports of two more infants born with Zika-related birth defects in the 50 states/D.C., but there were no reports of Zika-related pregnancy losses. So far in 2016, there have been 25 liveborn infants with Zika-related birth defects and five pregnancy losses in the states.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
U.S. preterm birth rate rose slightly in 2015
The preterm birth rate in the United States for 2015 increased for the first time in 8 years, according to the March of Dimes.
The national preterm birth rate rose from 9.57% in 2014 to 9.63% last year, earning an overall grade of C on the March of Dimes 2016 Premature Birth Report Card.
The March of Dimes also ranked preterm births in the states by racial/ethnic disparity: Maine had the least disparity, followed by New Hampshire and Utah, while Hawaii had the greatest disparity, just ahead of Pennsylvania and Louisiana. Using an average of the 2012-2014 national preterm birth rates, the March of Dimes calculated that Asian/Pacific Islanders had the lowest preterm birth rate at 8.5%, compared with 9% for whites, 9.1% for Hispanics, 10.4% for American Indian/Alaska Natives, and 13.3% for blacks.
The report card shows “that there is an unfair burden of premature birth among specific racial and ethnic groups as well as geographic areas,” Jennifer L. Howse, PhD, president of the March of Dimes, said in a statement. “Babies in this country have different chances of surviving and thriving simply based on the circumstances of their birth.”
The preterm birth rate in the United States for 2015 increased for the first time in 8 years, according to the March of Dimes.
The national preterm birth rate rose from 9.57% in 2014 to 9.63% last year, earning an overall grade of C on the March of Dimes 2016 Premature Birth Report Card.
The March of Dimes also ranked preterm births in the states by racial/ethnic disparity: Maine had the least disparity, followed by New Hampshire and Utah, while Hawaii had the greatest disparity, just ahead of Pennsylvania and Louisiana. Using an average of the 2012-2014 national preterm birth rates, the March of Dimes calculated that Asian/Pacific Islanders had the lowest preterm birth rate at 8.5%, compared with 9% for whites, 9.1% for Hispanics, 10.4% for American Indian/Alaska Natives, and 13.3% for blacks.
The report card shows “that there is an unfair burden of premature birth among specific racial and ethnic groups as well as geographic areas,” Jennifer L. Howse, PhD, president of the March of Dimes, said in a statement. “Babies in this country have different chances of surviving and thriving simply based on the circumstances of their birth.”
The preterm birth rate in the United States for 2015 increased for the first time in 8 years, according to the March of Dimes.
The national preterm birth rate rose from 9.57% in 2014 to 9.63% last year, earning an overall grade of C on the March of Dimes 2016 Premature Birth Report Card.
The March of Dimes also ranked preterm births in the states by racial/ethnic disparity: Maine had the least disparity, followed by New Hampshire and Utah, while Hawaii had the greatest disparity, just ahead of Pennsylvania and Louisiana. Using an average of the 2012-2014 national preterm birth rates, the March of Dimes calculated that Asian/Pacific Islanders had the lowest preterm birth rate at 8.5%, compared with 9% for whites, 9.1% for Hispanics, 10.4% for American Indian/Alaska Natives, and 13.3% for blacks.
The report card shows “that there is an unfair burden of premature birth among specific racial and ethnic groups as well as geographic areas,” Jennifer L. Howse, PhD, president of the March of Dimes, said in a statement. “Babies in this country have different chances of surviving and thriving simply based on the circumstances of their birth.”