User login
Prenatal triple ART arrests HIV transmission
A triple-drug antiretroviral therapy given to HIV-infected pregnant women significantly reduced transmission of the disease to their newborns, but with greater risk of adverse outcomes for mothers and infants, a study showed.
The findings were based on data from three treatment regimens in approximately 3,500 women and infant sets. The three treatments were zidovudine plus intrapartum single-dose nevirapine with 6-14 days of tenofovir and emtricitabine post partum (zidovudine alone); zidovudine, lamivudine, and lopinavir–ritonavir (zidovudine-based antiretroviral therapy [ART]); or tenofovir, emtricitabine, and lopinavir–ritonavir (tenofovir-based ART). All infants received nevirapine once daily, and infants of mothers coinfected with hepatitis B also received hepatitis B vaccination.
The Promoting Maternal and Infant Survival Everywhere (PROMISE) trial included patients at 14 sites in seven countries (India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe). The current study presented findings from women with a CD4 count of at least 350 cells per cubic millimeter who were randomized at 14 weeks’ gestation to one of the three treatment regimens.
Maternal adverse events (grade 2 or higher) were significantly more common in the zidovudine-based ART group than in the zidovudine-only group (21.1% vs. 17.3%), as was the rate of grade 2 or higher abnormal blood chemical values (5.8% vs. 1.3%).
In addition, rates of abnormal blood chemical values grade 2 or higher were significantly more common in women treated with tenofovir-based ART than with zidovudine alone (2.9% vs. 0.8%).
Low birth weight (less than 2,500 g) was significantly more likely for infants of mothers in the zidovudine-based ART group, compared with the zidovudine-only group (23.0% vs. 12.0%) and in the tenofovir-based ART group, compared with the zidovudine-only group (16.9% vs. 8.9%). Preterm delivery and early infant death rates were significantly more likely in the tenofovir-based ART group than in the zidovudine-based ART group. Overall, the rate of HIV-free survival was highest among infants whose mothers received zidovudine-based ART, the investigators reported.
The findings were limited by several factors, and the safest and most effective regimens have yet to be determined, the researchers said. “Our findings emphasize the need for continued research to assess ART in pregnancy to ensure safer pregnancies for HIV-infected women and healthier outcomes for their uninfected infants,” they wrote.
A study coauthor reported receiving grant support from Gilead Sciences and ViiV Healthcare, and consulting fees from Janssen, paid directly to her institution. None of the other researchers, disclosed any financial conflicts.
A triple-drug antiretroviral therapy given to HIV-infected pregnant women significantly reduced transmission of the disease to their newborns, but with greater risk of adverse outcomes for mothers and infants, a study showed.
The findings were based on data from three treatment regimens in approximately 3,500 women and infant sets. The three treatments were zidovudine plus intrapartum single-dose nevirapine with 6-14 days of tenofovir and emtricitabine post partum (zidovudine alone); zidovudine, lamivudine, and lopinavir–ritonavir (zidovudine-based antiretroviral therapy [ART]); or tenofovir, emtricitabine, and lopinavir–ritonavir (tenofovir-based ART). All infants received nevirapine once daily, and infants of mothers coinfected with hepatitis B also received hepatitis B vaccination.
The Promoting Maternal and Infant Survival Everywhere (PROMISE) trial included patients at 14 sites in seven countries (India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe). The current study presented findings from women with a CD4 count of at least 350 cells per cubic millimeter who were randomized at 14 weeks’ gestation to one of the three treatment regimens.
Maternal adverse events (grade 2 or higher) were significantly more common in the zidovudine-based ART group than in the zidovudine-only group (21.1% vs. 17.3%), as was the rate of grade 2 or higher abnormal blood chemical values (5.8% vs. 1.3%).
In addition, rates of abnormal blood chemical values grade 2 or higher were significantly more common in women treated with tenofovir-based ART than with zidovudine alone (2.9% vs. 0.8%).
Low birth weight (less than 2,500 g) was significantly more likely for infants of mothers in the zidovudine-based ART group, compared with the zidovudine-only group (23.0% vs. 12.0%) and in the tenofovir-based ART group, compared with the zidovudine-only group (16.9% vs. 8.9%). Preterm delivery and early infant death rates were significantly more likely in the tenofovir-based ART group than in the zidovudine-based ART group. Overall, the rate of HIV-free survival was highest among infants whose mothers received zidovudine-based ART, the investigators reported.
The findings were limited by several factors, and the safest and most effective regimens have yet to be determined, the researchers said. “Our findings emphasize the need for continued research to assess ART in pregnancy to ensure safer pregnancies for HIV-infected women and healthier outcomes for their uninfected infants,” they wrote.
A study coauthor reported receiving grant support from Gilead Sciences and ViiV Healthcare, and consulting fees from Janssen, paid directly to her institution. None of the other researchers, disclosed any financial conflicts.
A triple-drug antiretroviral therapy given to HIV-infected pregnant women significantly reduced transmission of the disease to their newborns, but with greater risk of adverse outcomes for mothers and infants, a study showed.
The findings were based on data from three treatment regimens in approximately 3,500 women and infant sets. The three treatments were zidovudine plus intrapartum single-dose nevirapine with 6-14 days of tenofovir and emtricitabine post partum (zidovudine alone); zidovudine, lamivudine, and lopinavir–ritonavir (zidovudine-based antiretroviral therapy [ART]); or tenofovir, emtricitabine, and lopinavir–ritonavir (tenofovir-based ART). All infants received nevirapine once daily, and infants of mothers coinfected with hepatitis B also received hepatitis B vaccination.
The Promoting Maternal and Infant Survival Everywhere (PROMISE) trial included patients at 14 sites in seven countries (India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe). The current study presented findings from women with a CD4 count of at least 350 cells per cubic millimeter who were randomized at 14 weeks’ gestation to one of the three treatment regimens.
Maternal adverse events (grade 2 or higher) were significantly more common in the zidovudine-based ART group than in the zidovudine-only group (21.1% vs. 17.3%), as was the rate of grade 2 or higher abnormal blood chemical values (5.8% vs. 1.3%).
In addition, rates of abnormal blood chemical values grade 2 or higher were significantly more common in women treated with tenofovir-based ART than with zidovudine alone (2.9% vs. 0.8%).
Low birth weight (less than 2,500 g) was significantly more likely for infants of mothers in the zidovudine-based ART group, compared with the zidovudine-only group (23.0% vs. 12.0%) and in the tenofovir-based ART group, compared with the zidovudine-only group (16.9% vs. 8.9%). Preterm delivery and early infant death rates were significantly more likely in the tenofovir-based ART group than in the zidovudine-based ART group. Overall, the rate of HIV-free survival was highest among infants whose mothers received zidovudine-based ART, the investigators reported.
The findings were limited by several factors, and the safest and most effective regimens have yet to be determined, the researchers said. “Our findings emphasize the need for continued research to assess ART in pregnancy to ensure safer pregnancies for HIV-infected women and healthier outcomes for their uninfected infants,” they wrote.
A study coauthor reported receiving grant support from Gilead Sciences and ViiV Healthcare, and consulting fees from Janssen, paid directly to her institution. None of the other researchers, disclosed any financial conflicts.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Prenatal ART significantly lowered rates of early HIV transmission from HIV-infected pregnant women to their newborns, compared with zidovudine alone.
Major finding: The transmission rate for HIV was significantly lower in patients who underwent ART, compared with zidovudine alone (0.5% vs. 1.8%).
Data source: A randomized trial including 3,529 HIV-positive pregnant women at at least 14 weeks’ gestation.
Disclosures: A study coauthor reported receiving grant support from Gilead Sciences and ViiV Healthcare, and consulting fees from Janssen, paid directly to her institution. None of the other researchers disclosed any financial conflicts.
Syphilis testing before and after stillbirth is suboptimal
ATLANTA – Physicians are falling short on syphilis testing in both the prenatal period and at the time of delivery, suggest the findings of a study examining insurance claims from nearly 10,000 women who experienced stillbirths.
Overall, less than 10% of women in the study were tested for syphilis following a stillbirth delivery, while less than two-thirds of women who experienced a stillbirth had received prenatal syphilis testing.
Dr. Patel and his coinvestigators examined data from the Truven Health MarketScan Medicaid and commercial claims database to evaluate the proportion of women who had syphilis testing within at least 1 week before and 1 week after a stillbirth delivery.
The investigators identified women aged 15-44 years who had a stillbirth delivery in 2013. Stillbirths were identified via ICD-9 codes and these codes were also used to track prenatal syphilis testing, as well as syphilis testing, placental examination and complete blood count (CBC) performed at the time of delivery.
In total, there were 3,731 women enrolled in Medicaid and 6,096 commercially-insured women who experienced stillbirths and were included in the study. Of these women, 65.5% of Medicaid-covered women and 56.6% of commercially-insured women received prenatal syphilis testing. At delivery, 6.5% of Medicaid-insured women and 9.3% of commercially-insured women received syphilis testing.
Most women in the study were receiving prenatal care. In all, 73.2% of Medicaid-covered women and 76.5% of commercially-insured women received it. Placental examination at the time of delivery occurred for 61.5% of Medicaid-covered women and 58.0% of commercially-insured women, while CBC was performed in 31.2% and 35.8% of women, respectively.
“Overall, prenatal syphilis testing was significantly higher than syphilis testing at the time of delivery,” Dr. Patel said. “Women with prenatal syphilis testing were more likely to be tested for syphilis at delivery than those not tested, regardless of [their] insurance.”
Dr. Patel did not report information on financial disclosures.
ATLANTA – Physicians are falling short on syphilis testing in both the prenatal period and at the time of delivery, suggest the findings of a study examining insurance claims from nearly 10,000 women who experienced stillbirths.
Overall, less than 10% of women in the study were tested for syphilis following a stillbirth delivery, while less than two-thirds of women who experienced a stillbirth had received prenatal syphilis testing.
Dr. Patel and his coinvestigators examined data from the Truven Health MarketScan Medicaid and commercial claims database to evaluate the proportion of women who had syphilis testing within at least 1 week before and 1 week after a stillbirth delivery.
The investigators identified women aged 15-44 years who had a stillbirth delivery in 2013. Stillbirths were identified via ICD-9 codes and these codes were also used to track prenatal syphilis testing, as well as syphilis testing, placental examination and complete blood count (CBC) performed at the time of delivery.
In total, there were 3,731 women enrolled in Medicaid and 6,096 commercially-insured women who experienced stillbirths and were included in the study. Of these women, 65.5% of Medicaid-covered women and 56.6% of commercially-insured women received prenatal syphilis testing. At delivery, 6.5% of Medicaid-insured women and 9.3% of commercially-insured women received syphilis testing.
Most women in the study were receiving prenatal care. In all, 73.2% of Medicaid-covered women and 76.5% of commercially-insured women received it. Placental examination at the time of delivery occurred for 61.5% of Medicaid-covered women and 58.0% of commercially-insured women, while CBC was performed in 31.2% and 35.8% of women, respectively.
“Overall, prenatal syphilis testing was significantly higher than syphilis testing at the time of delivery,” Dr. Patel said. “Women with prenatal syphilis testing were more likely to be tested for syphilis at delivery than those not tested, regardless of [their] insurance.”
Dr. Patel did not report information on financial disclosures.
ATLANTA – Physicians are falling short on syphilis testing in both the prenatal period and at the time of delivery, suggest the findings of a study examining insurance claims from nearly 10,000 women who experienced stillbirths.
Overall, less than 10% of women in the study were tested for syphilis following a stillbirth delivery, while less than two-thirds of women who experienced a stillbirth had received prenatal syphilis testing.
Dr. Patel and his coinvestigators examined data from the Truven Health MarketScan Medicaid and commercial claims database to evaluate the proportion of women who had syphilis testing within at least 1 week before and 1 week after a stillbirth delivery.
The investigators identified women aged 15-44 years who had a stillbirth delivery in 2013. Stillbirths were identified via ICD-9 codes and these codes were also used to track prenatal syphilis testing, as well as syphilis testing, placental examination and complete blood count (CBC) performed at the time of delivery.
In total, there were 3,731 women enrolled in Medicaid and 6,096 commercially-insured women who experienced stillbirths and were included in the study. Of these women, 65.5% of Medicaid-covered women and 56.6% of commercially-insured women received prenatal syphilis testing. At delivery, 6.5% of Medicaid-insured women and 9.3% of commercially-insured women received syphilis testing.
Most women in the study were receiving prenatal care. In all, 73.2% of Medicaid-covered women and 76.5% of commercially-insured women received it. Placental examination at the time of delivery occurred for 61.5% of Medicaid-covered women and 58.0% of commercially-insured women, while CBC was performed in 31.2% and 35.8% of women, respectively.
“Overall, prenatal syphilis testing was significantly higher than syphilis testing at the time of delivery,” Dr. Patel said. “Women with prenatal syphilis testing were more likely to be tested for syphilis at delivery than those not tested, regardless of [their] insurance.”
Dr. Patel did not report information on financial disclosures.
AT THE 2016 STD PREVENTION CONFERENCE
Key clinical point:
Major finding: A total of 65.5% of Medicaid-covered women and 56.6% of commercially-insured women received prenatal syphilis testing. At delivery, 6.5% of Medicaid-covered women and 9.3% of commercially-insured women received syphilis testing.
Data source: Review of claims data from 3,731 women enrolled in Medicaid and 6,096 commercially-insured women who had stillbirth deliveries in 2013.
Disclosures: Dr. Patel did not report information on financial disclosures.
The 50-year quest for better pregnancy data
Editor’s note: As Ob.Gyn. News celebrates its 50th anniversary, we wanted to know how far the medical community has come in identifying and mitigating drug risks during pregnancy and in the postpartum period. In this article, our four expert columnists share their experiences trying to find and interpret critical pregnancy data, as well as how they weigh the potential risks and benefits for their patients.
The search for information
The biggest advance in the past 50 years is the availability of information, even though limited, relating to the effects of drugs in pregnancy and lactation. In the first few years of this period, it was a daunting task to obtain this information. I can recall spending hours in the hospital’s medical library going through huge volumes of Index Medicus to obtain references that the library could order for me. The appearance of Thomas H. Shepard’s first edition (Catalog of Teratogenic Agents) in 1973 was a step forward and in 1977, O.P. Heinonen and colleagues’ book (Birth Defects and Drugs in Pregnancy) was helpful.
Although all of the above sources were helpful, any book in an evolving field will not have the newest information. Two important services, TERIS and Reprotox, were started to allow clinicians to contact them for up-to-date data. Nevertheless, the biggest change was the availability of current information from the U.S. National Library of Medicine via Toxnet, PubMed, and LactMed, relating to the effects of drugs in pregnancy and lactation.
My method is to ask three questions. First, are there other drugs with a similar mechanism of action that have some human data? In most cases, the answer to this question is no, but even when there are data, it is typically very limited. Second, does the drug cross the human placenta? The answer is typically based on the molecular weight. Any drug with a molecular weight less than 1,000 daltons probably crosses. In the second half of pregnancy, especially in the third trimester, almost every drug crosses. Third, do the animal pregnancy data predict embryo/fetal risk? It was thought that it could if the dose causing harm was less than or equal to 10 times the human dose based on BSA or AUC and there were no signs of maternal toxicity. However, using data from my 10th edition, I and eight coauthors, all of whom are knowledgeable on the effects of drugs in pregnancy, found that the animal data for 311 drugs raised the possibility of human embryo-fetal harm that current data confirmed in only 75 (24%) of the drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5).
The system needs to be fixed. One method is to give the Food and Drug Administration the authority to require manufacturers of drugs likely to be used in pregnancy to gather and publish data on their use in pregnancy. That sounds reasonable, but will it ever occur?
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Learning the lessons of the past
During the last 50 years, two of the most potent known human teratogens, thalidomide and isotretinoin, became available for prescription in the United States. Thanks to the efforts of Frances Kelsey, MD, PhD, at the FDA, the initial application for approval of thalidomide in the United States was denied in the early 1960s. Subsequently, based on evidence from other countries where thalidomide was marketed that the drug can cause a pattern of serious birth defects, a very strict pregnancy prevention program was implemented when the drug was finally approved in the United States in 2006.
Over the last 50 years, we have also seen an important evolution in our ability to conduct pregnancy exposure safety studies. Though we still have limited ability to conduct clinical trials in pregnant women, the need for good quality observational studies has become more widely accepted. The Centers for Disease Control and Prevention’s National Birth Defects Prevention Study (now in its most recent iteration known as BD STEPS) has been one very important source of data on the safety of a wide variety of medications. Using a case-control study design, women who have delivered infants with specific birth defects and comparison women who have delivered non-malformed infants are interviewed about their exposures in pregnancy. These data have been extremely helpful in generating new hypotheses, confirming or refuting findings from other studies, and in testing hypotheses regarding the safety of medications widely used in women of reproductive age. These analyses, for example, have contributed to the large body of literature now available on the safety of antidepressant medications in pregnancy.
At the same time, in the last 30 years, we have seen a tremendous increase in the number of pregnancy registries required or recommended upon approval of a new drug in the United States. These registry studies, while challenging to complete in a timely manner, have steadily improved in terms of rigor, and several disease-based pregnancy exposure studies have been implemented, which have allowed us to better understand the comparative risks or safety of anticonvulsants and antiretroviral drugs, to name a few.
It is important to note that with all these advances in the last 50 years, we still have a huge gap in knowledge about medication safety in pregnancy and lactation. Recent reviews suggest that more than 80% of drugs currently marketed have insufficient or no data available. If we include over-the-counter medications, the knowledge gap grows larger. With the 2014 approval of the long-awaited Pregnancy and Lactation Labeling Rule, clinicians are now beginning to experience the elimination of the old A-B-C-D-X category system for pregnancy safety. In its place, data-driven product labels are required. These are expected to provide the clinician with a clear summary of the relevant studies for a given medication, and to place these in the context of the background risks for the underlying maternal disease being treated, as well as the population risks. However, it is painfully clear that we have a long way to go to generate the needed, high-quality data, to populate those labels.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, San Diego, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
Moving toward personalized medicine
Nowhere is a lack of actionable data more pronounced than in the impact of mental health drugs in pregnancy.
As Dr. Briggs and Dr. Chambers have outlined, the quality of data regarding the reproductive safety of medications across the therapeutic spectrum has historically been fair at best. The methodology and the rigor has been sparse and to a large extent, in psychiatry, we were only able to look for signals of concern. Prior to the late 1980s and early 1990s, there was little to guide clinicians on the safety of even very commonly used psychiatric medications during pregnancy. The health implications for women of reproductive age are extraordinary and yet that urgency was not matched by the level of investigation until more recently.
In psychiatry, we have rapidly improving data informing women about the risk for major congenital malformations. The clinical dilemma of weighing the necessity to stay on a medication to prevent relapse of a psychiatric disorder with the potential risk of malformation in the fetus is a wrenching one for the mother-to-be. Only good information can help patients, together with their physician, make collaborative decisions that make sense for them. Given the same information and the same severity of illness, women will make different decisions, and that’s a good thing. The calculus couples use to make these private decisions is unique to those involved. But they are able to move through the process because they have a platform of high-quality information.
So where do we go in the future? We need to get beyond the question of risk of major malformations and move toward understanding the long-term neurodevelopmental implications of prenatal exposures – whether such exposures confer risk or are even potentially salutary. One needs only look at the vast body of literature regarding fetal exposure to selective serotonin reuptake inhibitors (SSRIs) to observe the realization of this trend. When it comes to SSRIs, a fairly clear picture has emerged that they pose little absolute risk in terms of congenital malformations. What is missing is how SSRIs impact a child’s learning and development at age 3, 5, and 10. There have been a few studies in this area, but not a single, large prospective study that accurately quantifies both exposure to SSRIs and maternal psychiatric illness during pregnancy.
I expect that the future will also bring a greater understanding of the impact of untreated mental illness on the risk for obstetrical, neonatal, and longer-term neurodevelopmental outcomes. Most of the safety concerns have centered around the effect of fetal exposure to medications, but we also need to better understand how untreated psychiatric disorders impact the spectrum of relevant outcomes.
Getting back to the dilemma faced by pregnant women who really need medication to sustain emotional well-being, there simply is no perfect answer. No decision is perfect or risk free. What we can hope is that we’ll have personalized approaches that take into account the best available data and the patient’s individual situation and wishes. We’ve already come a long way toward meeting that goal, and I’m optimistic about where we’re going.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Perception of risk
Every year, numerous new medicines are approved by the FDA without data in pregnancy. Animal studies may show a problem that doesn’t appear in humans, or as was the case with thalidomide, the problem may not be apparent in animals and show up later in humans. There are many drugs that are safe in pregnancy, but women are understandably afraid of the potential impact on fetal development.
While my colleagues have presented the advances we’ve made in understanding the actual risks of medications during the prenatal period, it’s also important to focus on the perception of risk and to recognize that the reality and the perception can be vastly different.
At the same time, we began to ask women, using a visual analog scale, what would be their trend toward continuing or terminating pregnancy? Over several studies, we found that the likelihood of termination was high, and certainly much higher than was supported by the evidence of actual harm to the fetus. Specifically, if a woman received information about the safety of the drug and she still gave more than a 50% probability of terminating the pregnancy when surveyed, there was a good chance that she would terminate the pregnancy.
When you consider that most of the drugs that women are commonly prescribed in pregnancy – from most painkillers to antidepressants – are not known to cause malformations in pregnancy, you begin to see how problematic an inflated perception of risk can become.
But we see different trends in women with serious and chronic health problems, such as lupus or epilepsy. These women are typically under the care of a subspecialist, who in many cases has developed a significant knowledge base and comfort level around prescribing the drugs in this area and is able to communicate more clearly to patients both the risks to the fetus and the consequences of failure to treat their condition.
So clearly, the role of the physician and the ob.gyn. in particular is critical. It’s no secret that physicians face a negative legal climate that encourages defensive medicine and that they are often hesitant to tell women, without reservation, that it is okay to take a drug. But we must all remember that it is very easy to cause a woman not to take a medication in pregnancy and often that’s not what’s best for her health. Many women now postpone the age of starting a family and more have chronic conditions that require treatment. The idea of not treating certain conditions for the length of a pregnancy is not always a viable option. Yet there are quite a few women who would consider termination “just to be on the safe side.” That must be taken very seriously by the medical profession.
Dr. Koren is a professor of physiology/pharmacology at Western University, London, Ont., and a professor of medicine at Tel Aviv University. He is the founder of the Motherisk Program. He reported being a paid consultant for Duchesnay and Novartis.
Editor’s note: As Ob.Gyn. News celebrates its 50th anniversary, we wanted to know how far the medical community has come in identifying and mitigating drug risks during pregnancy and in the postpartum period. In this article, our four expert columnists share their experiences trying to find and interpret critical pregnancy data, as well as how they weigh the potential risks and benefits for their patients.
The search for information
The biggest advance in the past 50 years is the availability of information, even though limited, relating to the effects of drugs in pregnancy and lactation. In the first few years of this period, it was a daunting task to obtain this information. I can recall spending hours in the hospital’s medical library going through huge volumes of Index Medicus to obtain references that the library could order for me. The appearance of Thomas H. Shepard’s first edition (Catalog of Teratogenic Agents) in 1973 was a step forward and in 1977, O.P. Heinonen and colleagues’ book (Birth Defects and Drugs in Pregnancy) was helpful.
Although all of the above sources were helpful, any book in an evolving field will not have the newest information. Two important services, TERIS and Reprotox, were started to allow clinicians to contact them for up-to-date data. Nevertheless, the biggest change was the availability of current information from the U.S. National Library of Medicine via Toxnet, PubMed, and LactMed, relating to the effects of drugs in pregnancy and lactation.
My method is to ask three questions. First, are there other drugs with a similar mechanism of action that have some human data? In most cases, the answer to this question is no, but even when there are data, it is typically very limited. Second, does the drug cross the human placenta? The answer is typically based on the molecular weight. Any drug with a molecular weight less than 1,000 daltons probably crosses. In the second half of pregnancy, especially in the third trimester, almost every drug crosses. Third, do the animal pregnancy data predict embryo/fetal risk? It was thought that it could if the dose causing harm was less than or equal to 10 times the human dose based on BSA or AUC and there were no signs of maternal toxicity. However, using data from my 10th edition, I and eight coauthors, all of whom are knowledgeable on the effects of drugs in pregnancy, found that the animal data for 311 drugs raised the possibility of human embryo-fetal harm that current data confirmed in only 75 (24%) of the drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5).
The system needs to be fixed. One method is to give the Food and Drug Administration the authority to require manufacturers of drugs likely to be used in pregnancy to gather and publish data on their use in pregnancy. That sounds reasonable, but will it ever occur?
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Learning the lessons of the past
During the last 50 years, two of the most potent known human teratogens, thalidomide and isotretinoin, became available for prescription in the United States. Thanks to the efforts of Frances Kelsey, MD, PhD, at the FDA, the initial application for approval of thalidomide in the United States was denied in the early 1960s. Subsequently, based on evidence from other countries where thalidomide was marketed that the drug can cause a pattern of serious birth defects, a very strict pregnancy prevention program was implemented when the drug was finally approved in the United States in 2006.
Over the last 50 years, we have also seen an important evolution in our ability to conduct pregnancy exposure safety studies. Though we still have limited ability to conduct clinical trials in pregnant women, the need for good quality observational studies has become more widely accepted. The Centers for Disease Control and Prevention’s National Birth Defects Prevention Study (now in its most recent iteration known as BD STEPS) has been one very important source of data on the safety of a wide variety of medications. Using a case-control study design, women who have delivered infants with specific birth defects and comparison women who have delivered non-malformed infants are interviewed about their exposures in pregnancy. These data have been extremely helpful in generating new hypotheses, confirming or refuting findings from other studies, and in testing hypotheses regarding the safety of medications widely used in women of reproductive age. These analyses, for example, have contributed to the large body of literature now available on the safety of antidepressant medications in pregnancy.
At the same time, in the last 30 years, we have seen a tremendous increase in the number of pregnancy registries required or recommended upon approval of a new drug in the United States. These registry studies, while challenging to complete in a timely manner, have steadily improved in terms of rigor, and several disease-based pregnancy exposure studies have been implemented, which have allowed us to better understand the comparative risks or safety of anticonvulsants and antiretroviral drugs, to name a few.
It is important to note that with all these advances in the last 50 years, we still have a huge gap in knowledge about medication safety in pregnancy and lactation. Recent reviews suggest that more than 80% of drugs currently marketed have insufficient or no data available. If we include over-the-counter medications, the knowledge gap grows larger. With the 2014 approval of the long-awaited Pregnancy and Lactation Labeling Rule, clinicians are now beginning to experience the elimination of the old A-B-C-D-X category system for pregnancy safety. In its place, data-driven product labels are required. These are expected to provide the clinician with a clear summary of the relevant studies for a given medication, and to place these in the context of the background risks for the underlying maternal disease being treated, as well as the population risks. However, it is painfully clear that we have a long way to go to generate the needed, high-quality data, to populate those labels.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, San Diego, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
Moving toward personalized medicine
Nowhere is a lack of actionable data more pronounced than in the impact of mental health drugs in pregnancy.
As Dr. Briggs and Dr. Chambers have outlined, the quality of data regarding the reproductive safety of medications across the therapeutic spectrum has historically been fair at best. The methodology and the rigor has been sparse and to a large extent, in psychiatry, we were only able to look for signals of concern. Prior to the late 1980s and early 1990s, there was little to guide clinicians on the safety of even very commonly used psychiatric medications during pregnancy. The health implications for women of reproductive age are extraordinary and yet that urgency was not matched by the level of investigation until more recently.
In psychiatry, we have rapidly improving data informing women about the risk for major congenital malformations. The clinical dilemma of weighing the necessity to stay on a medication to prevent relapse of a psychiatric disorder with the potential risk of malformation in the fetus is a wrenching one for the mother-to-be. Only good information can help patients, together with their physician, make collaborative decisions that make sense for them. Given the same information and the same severity of illness, women will make different decisions, and that’s a good thing. The calculus couples use to make these private decisions is unique to those involved. But they are able to move through the process because they have a platform of high-quality information.
So where do we go in the future? We need to get beyond the question of risk of major malformations and move toward understanding the long-term neurodevelopmental implications of prenatal exposures – whether such exposures confer risk or are even potentially salutary. One needs only look at the vast body of literature regarding fetal exposure to selective serotonin reuptake inhibitors (SSRIs) to observe the realization of this trend. When it comes to SSRIs, a fairly clear picture has emerged that they pose little absolute risk in terms of congenital malformations. What is missing is how SSRIs impact a child’s learning and development at age 3, 5, and 10. There have been a few studies in this area, but not a single, large prospective study that accurately quantifies both exposure to SSRIs and maternal psychiatric illness during pregnancy.
I expect that the future will also bring a greater understanding of the impact of untreated mental illness on the risk for obstetrical, neonatal, and longer-term neurodevelopmental outcomes. Most of the safety concerns have centered around the effect of fetal exposure to medications, but we also need to better understand how untreated psychiatric disorders impact the spectrum of relevant outcomes.
Getting back to the dilemma faced by pregnant women who really need medication to sustain emotional well-being, there simply is no perfect answer. No decision is perfect or risk free. What we can hope is that we’ll have personalized approaches that take into account the best available data and the patient’s individual situation and wishes. We’ve already come a long way toward meeting that goal, and I’m optimistic about where we’re going.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Perception of risk
Every year, numerous new medicines are approved by the FDA without data in pregnancy. Animal studies may show a problem that doesn’t appear in humans, or as was the case with thalidomide, the problem may not be apparent in animals and show up later in humans. There are many drugs that are safe in pregnancy, but women are understandably afraid of the potential impact on fetal development.
While my colleagues have presented the advances we’ve made in understanding the actual risks of medications during the prenatal period, it’s also important to focus on the perception of risk and to recognize that the reality and the perception can be vastly different.
At the same time, we began to ask women, using a visual analog scale, what would be their trend toward continuing or terminating pregnancy? Over several studies, we found that the likelihood of termination was high, and certainly much higher than was supported by the evidence of actual harm to the fetus. Specifically, if a woman received information about the safety of the drug and she still gave more than a 50% probability of terminating the pregnancy when surveyed, there was a good chance that she would terminate the pregnancy.
When you consider that most of the drugs that women are commonly prescribed in pregnancy – from most painkillers to antidepressants – are not known to cause malformations in pregnancy, you begin to see how problematic an inflated perception of risk can become.
But we see different trends in women with serious and chronic health problems, such as lupus or epilepsy. These women are typically under the care of a subspecialist, who in many cases has developed a significant knowledge base and comfort level around prescribing the drugs in this area and is able to communicate more clearly to patients both the risks to the fetus and the consequences of failure to treat their condition.
So clearly, the role of the physician and the ob.gyn. in particular is critical. It’s no secret that physicians face a negative legal climate that encourages defensive medicine and that they are often hesitant to tell women, without reservation, that it is okay to take a drug. But we must all remember that it is very easy to cause a woman not to take a medication in pregnancy and often that’s not what’s best for her health. Many women now postpone the age of starting a family and more have chronic conditions that require treatment. The idea of not treating certain conditions for the length of a pregnancy is not always a viable option. Yet there are quite a few women who would consider termination “just to be on the safe side.” That must be taken very seriously by the medical profession.
Dr. Koren is a professor of physiology/pharmacology at Western University, London, Ont., and a professor of medicine at Tel Aviv University. He is the founder of the Motherisk Program. He reported being a paid consultant for Duchesnay and Novartis.
Editor’s note: As Ob.Gyn. News celebrates its 50th anniversary, we wanted to know how far the medical community has come in identifying and mitigating drug risks during pregnancy and in the postpartum period. In this article, our four expert columnists share their experiences trying to find and interpret critical pregnancy data, as well as how they weigh the potential risks and benefits for their patients.
The search for information
The biggest advance in the past 50 years is the availability of information, even though limited, relating to the effects of drugs in pregnancy and lactation. In the first few years of this period, it was a daunting task to obtain this information. I can recall spending hours in the hospital’s medical library going through huge volumes of Index Medicus to obtain references that the library could order for me. The appearance of Thomas H. Shepard’s first edition (Catalog of Teratogenic Agents) in 1973 was a step forward and in 1977, O.P. Heinonen and colleagues’ book (Birth Defects and Drugs in Pregnancy) was helpful.
Although all of the above sources were helpful, any book in an evolving field will not have the newest information. Two important services, TERIS and Reprotox, were started to allow clinicians to contact them for up-to-date data. Nevertheless, the biggest change was the availability of current information from the U.S. National Library of Medicine via Toxnet, PubMed, and LactMed, relating to the effects of drugs in pregnancy and lactation.
My method is to ask three questions. First, are there other drugs with a similar mechanism of action that have some human data? In most cases, the answer to this question is no, but even when there are data, it is typically very limited. Second, does the drug cross the human placenta? The answer is typically based on the molecular weight. Any drug with a molecular weight less than 1,000 daltons probably crosses. In the second half of pregnancy, especially in the third trimester, almost every drug crosses. Third, do the animal pregnancy data predict embryo/fetal risk? It was thought that it could if the dose causing harm was less than or equal to 10 times the human dose based on BSA or AUC and there were no signs of maternal toxicity. However, using data from my 10th edition, I and eight coauthors, all of whom are knowledgeable on the effects of drugs in pregnancy, found that the animal data for 311 drugs raised the possibility of human embryo-fetal harm that current data confirmed in only 75 (24%) of the drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5).
The system needs to be fixed. One method is to give the Food and Drug Administration the authority to require manufacturers of drugs likely to be used in pregnancy to gather and publish data on their use in pregnancy. That sounds reasonable, but will it ever occur?
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Learning the lessons of the past
During the last 50 years, two of the most potent known human teratogens, thalidomide and isotretinoin, became available for prescription in the United States. Thanks to the efforts of Frances Kelsey, MD, PhD, at the FDA, the initial application for approval of thalidomide in the United States was denied in the early 1960s. Subsequently, based on evidence from other countries where thalidomide was marketed that the drug can cause a pattern of serious birth defects, a very strict pregnancy prevention program was implemented when the drug was finally approved in the United States in 2006.
Over the last 50 years, we have also seen an important evolution in our ability to conduct pregnancy exposure safety studies. Though we still have limited ability to conduct clinical trials in pregnant women, the need for good quality observational studies has become more widely accepted. The Centers for Disease Control and Prevention’s National Birth Defects Prevention Study (now in its most recent iteration known as BD STEPS) has been one very important source of data on the safety of a wide variety of medications. Using a case-control study design, women who have delivered infants with specific birth defects and comparison women who have delivered non-malformed infants are interviewed about their exposures in pregnancy. These data have been extremely helpful in generating new hypotheses, confirming or refuting findings from other studies, and in testing hypotheses regarding the safety of medications widely used in women of reproductive age. These analyses, for example, have contributed to the large body of literature now available on the safety of antidepressant medications in pregnancy.
At the same time, in the last 30 years, we have seen a tremendous increase in the number of pregnancy registries required or recommended upon approval of a new drug in the United States. These registry studies, while challenging to complete in a timely manner, have steadily improved in terms of rigor, and several disease-based pregnancy exposure studies have been implemented, which have allowed us to better understand the comparative risks or safety of anticonvulsants and antiretroviral drugs, to name a few.
It is important to note that with all these advances in the last 50 years, we still have a huge gap in knowledge about medication safety in pregnancy and lactation. Recent reviews suggest that more than 80% of drugs currently marketed have insufficient or no data available. If we include over-the-counter medications, the knowledge gap grows larger. With the 2014 approval of the long-awaited Pregnancy and Lactation Labeling Rule, clinicians are now beginning to experience the elimination of the old A-B-C-D-X category system for pregnancy safety. In its place, data-driven product labels are required. These are expected to provide the clinician with a clear summary of the relevant studies for a given medication, and to place these in the context of the background risks for the underlying maternal disease being treated, as well as the population risks. However, it is painfully clear that we have a long way to go to generate the needed, high-quality data, to populate those labels.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, San Diego, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
Moving toward personalized medicine
Nowhere is a lack of actionable data more pronounced than in the impact of mental health drugs in pregnancy.
As Dr. Briggs and Dr. Chambers have outlined, the quality of data regarding the reproductive safety of medications across the therapeutic spectrum has historically been fair at best. The methodology and the rigor has been sparse and to a large extent, in psychiatry, we were only able to look for signals of concern. Prior to the late 1980s and early 1990s, there was little to guide clinicians on the safety of even very commonly used psychiatric medications during pregnancy. The health implications for women of reproductive age are extraordinary and yet that urgency was not matched by the level of investigation until more recently.
In psychiatry, we have rapidly improving data informing women about the risk for major congenital malformations. The clinical dilemma of weighing the necessity to stay on a medication to prevent relapse of a psychiatric disorder with the potential risk of malformation in the fetus is a wrenching one for the mother-to-be. Only good information can help patients, together with their physician, make collaborative decisions that make sense for them. Given the same information and the same severity of illness, women will make different decisions, and that’s a good thing. The calculus couples use to make these private decisions is unique to those involved. But they are able to move through the process because they have a platform of high-quality information.
So where do we go in the future? We need to get beyond the question of risk of major malformations and move toward understanding the long-term neurodevelopmental implications of prenatal exposures – whether such exposures confer risk or are even potentially salutary. One needs only look at the vast body of literature regarding fetal exposure to selective serotonin reuptake inhibitors (SSRIs) to observe the realization of this trend. When it comes to SSRIs, a fairly clear picture has emerged that they pose little absolute risk in terms of congenital malformations. What is missing is how SSRIs impact a child’s learning and development at age 3, 5, and 10. There have been a few studies in this area, but not a single, large prospective study that accurately quantifies both exposure to SSRIs and maternal psychiatric illness during pregnancy.
I expect that the future will also bring a greater understanding of the impact of untreated mental illness on the risk for obstetrical, neonatal, and longer-term neurodevelopmental outcomes. Most of the safety concerns have centered around the effect of fetal exposure to medications, but we also need to better understand how untreated psychiatric disorders impact the spectrum of relevant outcomes.
Getting back to the dilemma faced by pregnant women who really need medication to sustain emotional well-being, there simply is no perfect answer. No decision is perfect or risk free. What we can hope is that we’ll have personalized approaches that take into account the best available data and the patient’s individual situation and wishes. We’ve already come a long way toward meeting that goal, and I’m optimistic about where we’re going.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Perception of risk
Every year, numerous new medicines are approved by the FDA without data in pregnancy. Animal studies may show a problem that doesn’t appear in humans, or as was the case with thalidomide, the problem may not be apparent in animals and show up later in humans. There are many drugs that are safe in pregnancy, but women are understandably afraid of the potential impact on fetal development.
While my colleagues have presented the advances we’ve made in understanding the actual risks of medications during the prenatal period, it’s also important to focus on the perception of risk and to recognize that the reality and the perception can be vastly different.
At the same time, we began to ask women, using a visual analog scale, what would be their trend toward continuing or terminating pregnancy? Over several studies, we found that the likelihood of termination was high, and certainly much higher than was supported by the evidence of actual harm to the fetus. Specifically, if a woman received information about the safety of the drug and she still gave more than a 50% probability of terminating the pregnancy when surveyed, there was a good chance that she would terminate the pregnancy.
When you consider that most of the drugs that women are commonly prescribed in pregnancy – from most painkillers to antidepressants – are not known to cause malformations in pregnancy, you begin to see how problematic an inflated perception of risk can become.
But we see different trends in women with serious and chronic health problems, such as lupus or epilepsy. These women are typically under the care of a subspecialist, who in many cases has developed a significant knowledge base and comfort level around prescribing the drugs in this area and is able to communicate more clearly to patients both the risks to the fetus and the consequences of failure to treat their condition.
So clearly, the role of the physician and the ob.gyn. in particular is critical. It’s no secret that physicians face a negative legal climate that encourages defensive medicine and that they are often hesitant to tell women, without reservation, that it is okay to take a drug. But we must all remember that it is very easy to cause a woman not to take a medication in pregnancy and often that’s not what’s best for her health. Many women now postpone the age of starting a family and more have chronic conditions that require treatment. The idea of not treating certain conditions for the length of a pregnancy is not always a viable option. Yet there are quite a few women who would consider termination “just to be on the safe side.” That must be taken very seriously by the medical profession.
Dr. Koren is a professor of physiology/pharmacology at Western University, London, Ont., and a professor of medicine at Tel Aviv University. He is the founder of the Motherisk Program. He reported being a paid consultant for Duchesnay and Novartis.
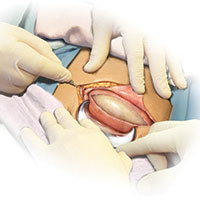
Preventing infection after cesarean delivery: Evidence-based guidance
Cesarean delivery is now the most commonly performed major operation in hospitals across the United States. Approximately 30% of the 4 million deliveries that occur each year are by cesarean. Endometritis and wound infection (superficial and deep surgical site infection) are the most common postoperative complications of cesarean delivery. These 2 infections usually can be treated in a straightforward manner with antibiotics or surgical drainage. In some cases, however, they can lead to serious sequelae, such as pelvic abscess, septic pelvic vein thrombophlebitis, and wound dehiscence/evisceration, thereby prolonging the patient’s hospitalization and significantly increasing medical expenses.
Accordingly, in the past 50 years many investigators have proposed various specific measures to reduce the risk of postcesarean infection. In this article, we critically evaluate 2 of these major interventions: methods of skin preparation and administration of prophylactic antibiotics. In part 2 of this series next month, we will review the evidence regarding preoperative bathing with an antiseptic, preoperative vaginal cleansing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
CASE Cesarean delivery required for nonprogressing labor
A 26-year-old obese primigravid woman, body mass index (BMI) 37 kg m2, at 40 weeks’ gestation has been in labor for 20 hours. Her membranes have been ruptured for 16 hours. Her cervix is completely effaced and is 7 cm dilated. The fetal head is at −1 cm station. Her cervical examination findings have not changed in 4 hours despite adequate uterine contractility documented by intrauterine pressure catheter. You are now ready to proceed with cesarean delivery, and you want to do everything possible to prevent the patient from developing a postoperative infection.
What are the best practices for postcesarean infection prevention in this patient?
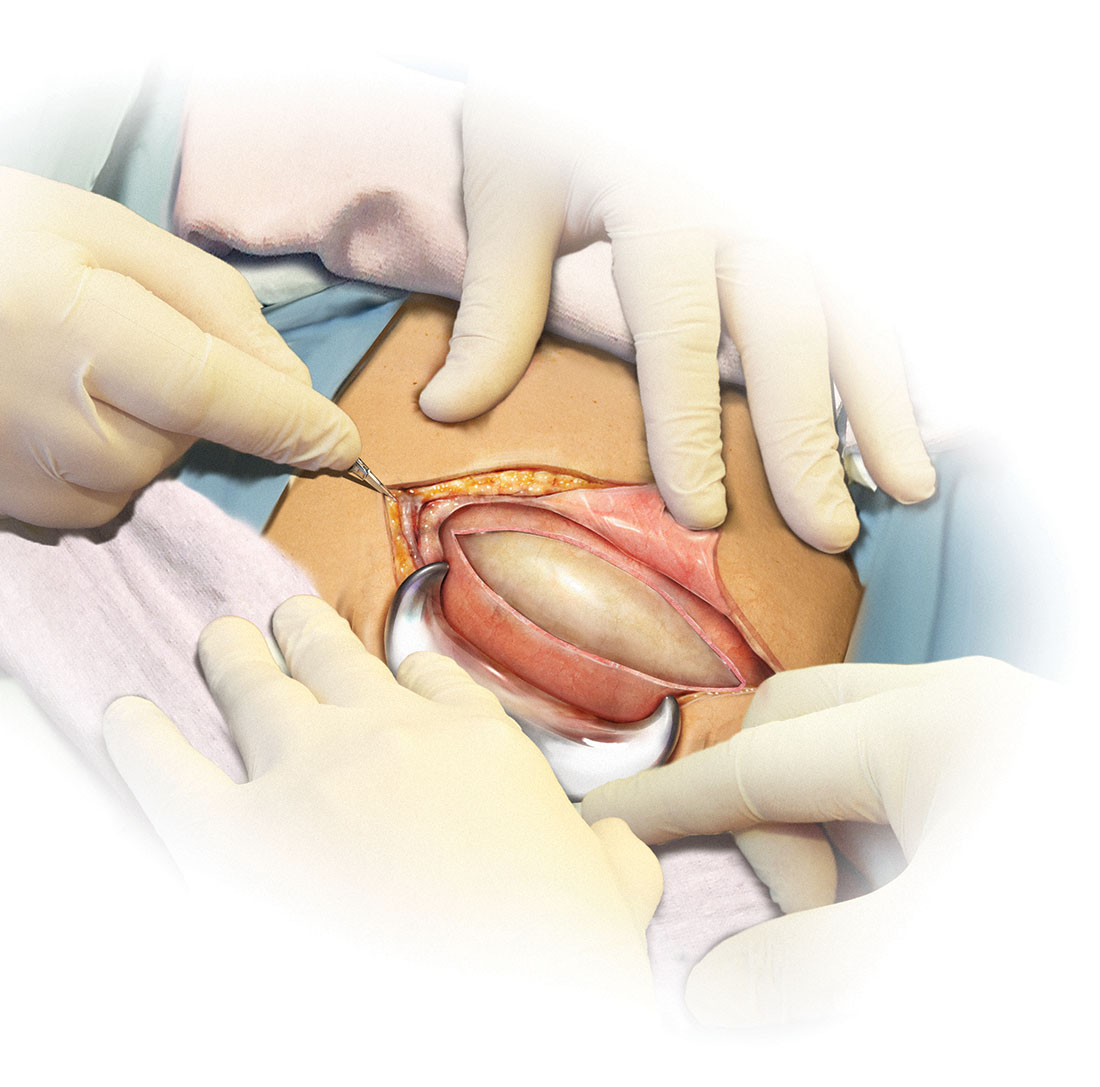
Skin preparation
Adequate preoperative skin preparation is an important first step in preventing post‑ cesarean infection.
How should you prepare the patient’s skin for surgery?
Two issues to address when preparing the abdominal wall for surgery are hair removal and skin cleansing. More than 40 years ago, Cruse and Foord definitively answered the question about hair removal.1 In a landmark cohort investigation of more than 23,000 patients having many different types of operative procedures, they demonstrated that shaving the hair on the evening before surgery resulted in a higher rate of wound infection than clipping the hair, removing the hair with a depilatory cream just before surgery, or not removing the hair at all.
Three recent investigations have thoughtfully addressed the issue of skin cleansing. Darouiche and colleagues conducted a prospective, randomized, multicenter trial comparing chlorhexidine-alcohol with povidone-iodine for skin preparation before surgery.2 Their investigation included 849 patients having many different types of surgical procedures, only a minority of which were in obstetric and gynecologic patients. They demonstrated fewer superficial wound infections in patients in the chlorhexidine-alcohol group (4.2% vs 8.6%, P = .008). Of even greater importance, patients in the chlorhexidine-alcohol group had fewer deep wound infections (1% vs 3%, P = .005).
Ngai and co-workers recently reported the results of a randomized controlled trial (RCT) in which women undergoing nonurgent cesarean delivery had their skin cleansed with povidone-iodine with alcohol, chlorhexidine with alcohol, or the sequential combination of both solutions.3 The overall rate of surgical site infection was just 4.3%. The 3 groups had comparable infection rates and, accordingly, the authors were unable to conclude that one type of skin preparation was superior to the other.
The most informative recent investigation was by Tuuli and colleagues, who evaluated 1,147 patients having cesarean delivery assigned to undergo skin preparation with either chlorhexidine-alcohol or iodine-alcohol.4 Unlike the study by Ngai and co-workers, in this study approximately 40% of the patients in each treatment arm had unscheduled, urgent cesarean deliveries.3,4 Overall, the rate of infection in the chlorhexidine-alcohol group was 4.0% compared with 7.3% in the iodine-alcohol group (relative risk [RR], 0.55; 95% confidence interval [CI], 0.34–0.90, P = .02).
What the evidence says
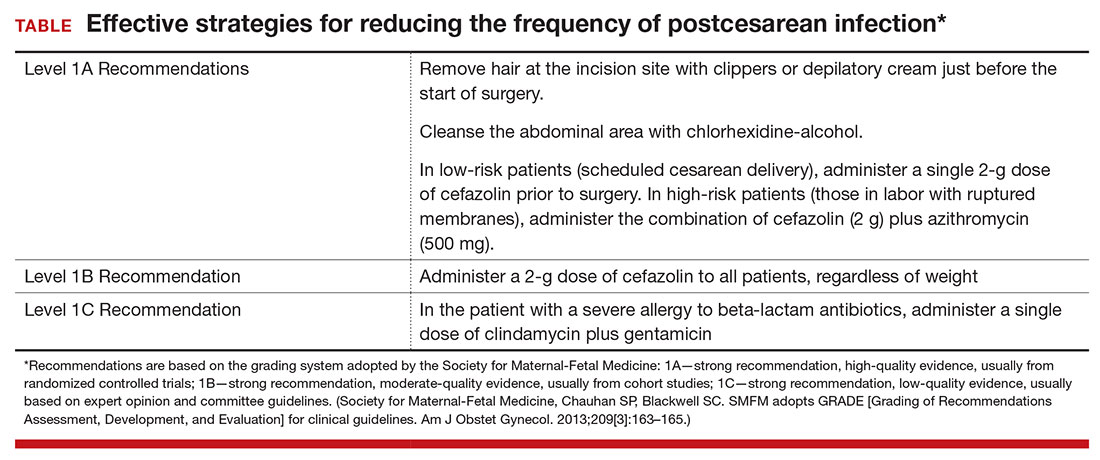
Based on the evidence cited above, we advise removing hair at the incision site with clippers or depilatory cream just before the start of surgery. The abdomen should then be cleansed with a chlorhexidine-alcohol solution (Level I Evidence, Level 1A Recommendation; TABLE).
Antibiotic prophylaxis
Questions to consider regarding antibiotic prophylaxis for cesarean delivery include appropriateness of treatment, antibiotic(s) selection, timing of administration, dose, and special circumstances.
Should you give the patient prophylactic antibiotics?
Prophylactic antibiotics are justified for surgical procedures whenever 3 major criteria are met5:
- the surgical site is inevitably contaminated with bacteria
- in the absence of prophylaxis, the frequency of infection at the operative site is unacceptably high
- operative site infections have the potential to lead to serious, potentially life-threatening sequelae.
Without a doubt, all 3 of these criteria are fulfilled when considering either urgent or nonurgent cesarean delivery. When cesarean delivery follows a long labor complicated by ruptured membranes, multiple internal vaginal examinations, and internal fetal monitoring, the operative site is inevitably contaminated with hundreds of thousands of pathogenic bacteria. Even when cesarean delivery is scheduled to occur before the onset of labor and ruptured membranes, a high concentration of vaginal organisms is introduced into the uterine and pelvic cavities coincident with making the hysterotomy incision.6
In the era before prophylactic antibiotics were used routinely, postoperative infection rates in some highly indigent patient populations approached 85%.5 Finally, as noted previously, postcesarean endometritis may progress to pelvic abscess formation, septic pelvic vein thrombophlebitis, and septic shock; wound infections may be complicated by dehiscence and evisceration.
When should you administer antibiotics: Before the surgical incision or after cord clamping?
More than 50 years ago, Burke conducted the classic sequence of basic science experiments that forms the foundation for use of prophylactic antibiotics.7 Using a guinea pig model, he showed that prophylactic antibiotics exert their most pronounced effect when they are administered before the surgical incision is made and before bacterial contamination occurs. Prophylaxis that is delayed more than 4 hours after the start of surgery will likely be ineffective.
Interestingly, however, when clinicians first began using prophylactic antibiotics for cesarean delivery, some investigators expressed concern about the possible exposure of the neonate to antibiotics just before delivery—specifically, whether this exposure would increase the frequency of evaluations for suspected sepsis or would promote resistance among organisms that would make neonatal sepsis more difficult to treat.
Gordon and colleagues published an important report in 1979 that showed that preoperative administration of ampicillin did not increase the frequency of immediate or delayed neonatal infections.8 However, delaying the administration of ampicillin until after the umbilical cord was clamped was just as effective in preventing post‑cesarean endometritis. Subsequently, Cunningham and co-workers showed that preoperative administration of prophylactic antibiotics significantly increased the frequency of sepsis workups in exposed neonates compared with infants with no preoperative antibiotic exposure (28% vs 15%; P<.025).9 Based on these 2 reports, obstetricians adopted a policy of delaying antibiotic administration until after the infant’s umbilical cord was clamped.
In 2007, Sullivan and colleagues challenged this long-standing practice.10 In a carefully designed prospective, randomized, double-blind trial, they showed that patients who received preoperative cefazolin had a significant reduction in the frequency of endometritis compared with women who received the same antibiotic after cord clamping (1% vs 5%; RR, 0.2; 95% CI, 0.2–0.94). The rate of wound infection was lower in the preoperative antibiotic group (3% vs 5%), but this difference did not reach statistical significance. The total infection-related morbidity was significantly reduced in women who received antibiotics preoperatively (4.0% vs 11.5%; RR, 0.4; 95% CI, 0.18–0.87). Additionally, there was no increase in the frequency of proven or suspected neonatal infection in the infants exposed to antibiotics before delivery.
Subsequent to the publication by Sullivan and colleagues, other reports have confirmed that administration of antibiotics prior to surgery is superior to administration after clamping of the umbilical cord.10–12 Thus, we have come full circle back to Burke’s principle established more than a half century ago.7
Which antibiotic(s) should you administer for prophylaxis, and how many doses?
In an earlier review, one of us (PD) examined the evidence regarding choice of antibiotics and number of doses, concluding that a single dose of a first-generation cephalosporin, such as cefazolin, was the preferred regimen.5 The single dose was comparable in effectiveness to 2- or 3-dose regimens and to single- or multiple-dose regimens of broader-spectrum agents. For more than 20 years now, the standard of care for antibiotic prophylaxis has been a single 1- to 2-g dose of cefazolin.
Several recent reports, however, have raised the question of whether the prophylactic effect could be enhanced if the spectrum of activity of the antibiotic regimen was broadened to include an agent effective against Ureaplasma species.
Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection; they showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis.13 In a follow-up report from the same institution, Tita and co-workers demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.14 In both of these investigations, the antibiotics were administered after cord clamping.
In a subsequent report, Ward and Duff15 showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a very low rate of both endometritis and wound infection in a population similar to that studied by Tita et al.13,14
Very recently, Tita and associates published the results of the Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial conducted at 14 US hospitals.16 This study included 2,013 women undergoing cesarean delivery during labor or after membrane rupture who were randomly assigned to receive intravenous azithromycin 500 mg (n = 1,019) or placebo (n = 994). All women also received standard antibiotic prophylaxis with cefazolin. The primary outcome (a composite of endometritis, wound infection, or other infection within 6 weeks) was significantly lower in the azithromycin group than in the placebo group (6.1% vs 12.0%, P<.001). In addition, there were significant differences between the treatment groups in the rates of endometritis (3.8% in the azithromycin group vs 6.1% in the placebo group, P = .02) as well as in the rates of wound infection (2.4% vs 6.6%, respectively, P<.001). Of additional note, there were no differences between the 2 groups in the composite neonatal outcome of death and serious neonatal complications (14.3% vs 13.6%, P = .63).The investigators concluded that extended-spectrum prophylaxis with adjunctive azithromycin safely reduces infection rates without raising the risk of neonatal adverse outcomes.
What the evidence says
We conclude that all patients, even those having a scheduled cesarean before the onset of labor or ruptured membranes, should receive prophylactic antibiotics in a single dose administered preoperatively rather than after cord clamping (Level I Evidence, Level 1A Recommendation; TABLE). In high-risk populations (eg, women in labor with ruptured membranes who are having an urgent cesarean), for whom the baseline risk of infection is high, administer the combination of cefazolin plus azithromycin in lieu of cefazolin alone (Level I Evidence, Level 1A Recommendation; TABLE).
If the patient has a history of an immediate hypersensitivity reaction to beta-lactam antibiotics, we recommend the combination of clindamycin (900 mg) plus gentamicin (1.5 mg/kg) as a single infusion prior to surgery. We base this recommendation on the need to provide reasonable coverage against a broad range of pathogens. Clindamycin covers gram-positive aerobes, such as staphylococci species and group B streptococci, and anaerobes; gentamicin covers aerobic gram-negative bacilli. A single agent, such as clindamycin or metronidazole, does not provide the broad-based coverage necessary for effective prophylaxis (Level III Evidence, Level 1C Recommendation; TABLE).
If the patient is overweight or obese, should you modify the antibiotic dose?
The prevalence of obesity in the United States continues to increase. One-third of all US reproductive-aged women are obese, and 6% of women are extremely obese.17 Obesity increases the risk of postcesarean infection 3- to 5- fold.18 Because both pregnancy and obesity increase the total volume of a drug’s distribution, achieving adequate antibiotic tissue concentrations may be hindered by a dilutional effect. Furthermore, pharmacokinetic studies consistently have shown that the tissue concentration of an antibiotic—which, ideally, should be above the minimum inhibitory concentration (MIC) for common bacteria—determines the susceptibility of those tissues to infection, regardless of whether the serum concentration of the antibiotic is in the therapeutic range.19
These concerns have led to several recent investigations evaluating different doses of cefazolin for obese patients. Pevzner and colleagues conducted a prospective cohort study of 29 women having a scheduled cesarean delivery.20 The patients were divided into 3 groups: lean (BMI <30 kg m2), obese (BMI 30.0–39.9 kg m2), and extremely obese (BMI >40 kg m2). All women received a 2-g dose of cefazolin 30 to 60 minutes before surgery. Cefazolin concentrations in adipose tissue obtained at the time of skin incision were inversely proportional to maternal BMI (r, −0.67; P<.001). All specimens demonstrated a therapeutic concentration (>1 µg/g) of cefazolin for gram-positive cocci, but 20% of the obese women and 33% of the extremely obese women did not achieve the MIC (>4 µg/g) for gram-negative bacilli (P = .29 and P = .14, respectively). At the time of skin closure, 20% of obese women and 44% of extremely obese women did not have tissue concentrations that exceeded the MIC for gram-negative bacteria.
Swank and associates conducted a prospective cohort study that included 28 women.18 They demonstrated that, after a 2-g dose of cefazolin, only 20% of the obese women (BMI 30–40 kg m2) and 0% of the extremely obese women (BMI >40 kg m2) achieved an adipose tissue concentration that exceeded the MIC for gram-negative rods (8 µg/mL). However, 100% and 71.4%, respectively, achieved such a tissue concentration after a 3-g dose. When the women were stratified by actual weight, there was a statistically significant difference between those who weighed less than 120 kg and those who weighed more than 120 kg. Seventy-nine percent of the former had a tissue concentration of cefazolin greater than 8 µg/mL compared with 0% of the women who weighed more than 120 kg. Based on these observations, the authors recommended a 3-g dose of cefazolin for women who weigh more than 120 kg.
In a double-blind RCT with 26 obese women (BMI ≥30 kg m2), Young and colleagues demonstrated that, at the time of hysterotomy and fascial closure, significantly higher concentrations of cefazolin were found in the adipose tissue of obese women who received a 3-g dose of antibiotic compared with those who received a 2-g dose.21 However, all concentrations of cefazolin were consistently above the MIC of cefazolin for gram-positive cocci (1 µg/g) and gram-negative bacilli (4 µg/g). Further, Maggio and co-workers conducted a double-blind RCT comparing a 2-g dose of cefazolin versus a 3-g dose in 57 obese women (BMI ≥30 kg m2).22 They found no statistically significant difference in the percentage of women who had tissue concentrations of cefazolin greater than the MIC for gram-positive cocci (8 µg/g). All samples were above the MIC of cefazolin for gram-negative bacilli (2 µg/g). Based on these data, these investigators did not recommend increasing the dose of cefazolin from 2 g to 3 g in obese patients.21,22
The studies discussed above are difficult to compare for 3 reasons. First, each study used a different MIC of cefazolin for both gram-positive and gram-negative bacteria. Second, the authors sampled different maternal tissues or serum at varying times during the cesarean delivery. Third, the studies did not specifically investigate, or were not powered sufficiently to address, the more important clinical outcome of surgical site infection. In a recent historical cohort study, Ward and Duff were unable to show that increasing the dose of cefazolin to 2 g in all women with a BMI <30 kg m2 and to 3 g in all women with a BMI >30 kg m2 reduced the rate of endometritis and wound infection below the level already achieved with combined prophylaxis with cefazolin (1 g) plus azithromycin (500 mg).15
Sutton and colleagues recently assessed the pharmacokinetics of azithromycin when used as prophylaxis for cesarean delivery.23 They studied 30 women who had a scheduled cesarean delivery and who received a 500-mg intravenous dose of azithromycin that was initiated 15, 30, or 60 minutes before the surgical incision and then infused over 1 hour. They obtained maternal plasma samples multiple times during the first 8 hours after surgery. They also obtained samples of amniotic fluid, placenta, myometrium, adipose tissue, and umbilical cord blood intraoperatively. The median concentration of azithromycin in adipose tissue was 102 ng/g, which is below the MIC50 for Ureaplasma species (250 ng/mL). The median concentration in myometrial tissue was 402 ng/g. The concentration in maternal plasma consistently exceeded the MIC50 for Ureaplasma species.
What the evidence says
All women, regardless of weight,
CASE Resolved
For the 26-year-old obese laboring patient about to undergo cesarean delivery, reasonable steps for prevention of infection include removing the hair at the incision site with clippers or depilatory cream immediately prior to the start of surgery; cleansing the abdomen with a chlorhexidine-alcohol solution; and administering cefazolin (2 g) plus azithromycin (500 mg) preoperatively.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cruse PJ, Foord R. A five‑year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107(2):206–210.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine‑alcohol versus povidone‑iodine for surgical‑site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery. Obstet Gynecol. 2015;126(6):1251–1257.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost‑effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 pt 1):794–798.
- Dinsmoor MJ, Gilbert S, Landon MB, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal‑Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168.
- Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstet Gynecol. 1979;53(2):151–156.
- Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151–154.
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1–e5.
- Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1–e6.
- Owens SM, Brozanski BS, Meyn LA, Wiesenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended‑spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51–56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended‑spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1–e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1–e4.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegel KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006:295(13):1549–1555.
- Swank ML, Wing DA, Nicolau DP, McNulty JA. Increased 3‑gram cefazolin dosing for cesarean delivery prophylaxis in obese women. Am J Obstet Gynecol. 2015;213(3):415.e1–e8.
- Liu P, Derendorf H. Antimicrobial tissue concentrations. Infect Dis Clin North Am. 2003:17(3):599–613.
- Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
- Young OM, Shaik IH, Twedt R, et al. Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol. 2015;213(4):541.e1–e7.
- Maggio L, Nicolau DP, DaCosta M, Rouse DJ, Hughes BL. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1205–1210.
- Sutton AL, Acosta EP, Larson KB, Kerstner‑Wood CD, Tita AT, Biggio JR. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol. 2015;212(6):812. e1–e6.
Cesarean delivery is now the most commonly performed major operation in hospitals across the United States. Approximately 30% of the 4 million deliveries that occur each year are by cesarean. Endometritis and wound infection (superficial and deep surgical site infection) are the most common postoperative complications of cesarean delivery. These 2 infections usually can be treated in a straightforward manner with antibiotics or surgical drainage. In some cases, however, they can lead to serious sequelae, such as pelvic abscess, septic pelvic vein thrombophlebitis, and wound dehiscence/evisceration, thereby prolonging the patient’s hospitalization and significantly increasing medical expenses.
Accordingly, in the past 50 years many investigators have proposed various specific measures to reduce the risk of postcesarean infection. In this article, we critically evaluate 2 of these major interventions: methods of skin preparation and administration of prophylactic antibiotics. In part 2 of this series next month, we will review the evidence regarding preoperative bathing with an antiseptic, preoperative vaginal cleansing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
CASE Cesarean delivery required for nonprogressing labor
A 26-year-old obese primigravid woman, body mass index (BMI) 37 kg m2, at 40 weeks’ gestation has been in labor for 20 hours. Her membranes have been ruptured for 16 hours. Her cervix is completely effaced and is 7 cm dilated. The fetal head is at −1 cm station. Her cervical examination findings have not changed in 4 hours despite adequate uterine contractility documented by intrauterine pressure catheter. You are now ready to proceed with cesarean delivery, and you want to do everything possible to prevent the patient from developing a postoperative infection.
What are the best practices for postcesarean infection prevention in this patient?

Skin preparation
Adequate preoperative skin preparation is an important first step in preventing post‑ cesarean infection.
How should you prepare the patient’s skin for surgery?
Two issues to address when preparing the abdominal wall for surgery are hair removal and skin cleansing. More than 40 years ago, Cruse and Foord definitively answered the question about hair removal.1 In a landmark cohort investigation of more than 23,000 patients having many different types of operative procedures, they demonstrated that shaving the hair on the evening before surgery resulted in a higher rate of wound infection than clipping the hair, removing the hair with a depilatory cream just before surgery, or not removing the hair at all.
Three recent investigations have thoughtfully addressed the issue of skin cleansing. Darouiche and colleagues conducted a prospective, randomized, multicenter trial comparing chlorhexidine-alcohol with povidone-iodine for skin preparation before surgery.2 Their investigation included 849 patients having many different types of surgical procedures, only a minority of which were in obstetric and gynecologic patients. They demonstrated fewer superficial wound infections in patients in the chlorhexidine-alcohol group (4.2% vs 8.6%, P = .008). Of even greater importance, patients in the chlorhexidine-alcohol group had fewer deep wound infections (1% vs 3%, P = .005).
Ngai and co-workers recently reported the results of a randomized controlled trial (RCT) in which women undergoing nonurgent cesarean delivery had their skin cleansed with povidone-iodine with alcohol, chlorhexidine with alcohol, or the sequential combination of both solutions.3 The overall rate of surgical site infection was just 4.3%. The 3 groups had comparable infection rates and, accordingly, the authors were unable to conclude that one type of skin preparation was superior to the other.
The most informative recent investigation was by Tuuli and colleagues, who evaluated 1,147 patients having cesarean delivery assigned to undergo skin preparation with either chlorhexidine-alcohol or iodine-alcohol.4 Unlike the study by Ngai and co-workers, in this study approximately 40% of the patients in each treatment arm had unscheduled, urgent cesarean deliveries.3,4 Overall, the rate of infection in the chlorhexidine-alcohol group was 4.0% compared with 7.3% in the iodine-alcohol group (relative risk [RR], 0.55; 95% confidence interval [CI], 0.34–0.90, P = .02).
What the evidence says
Based on the evidence cited above, we advise removing hair at the incision site with clippers or depilatory cream just before the start of surgery. The abdomen should then be cleansed with a chlorhexidine-alcohol solution (Level I Evidence, Level 1A Recommendation; TABLE).

Antibiotic prophylaxis
Questions to consider regarding antibiotic prophylaxis for cesarean delivery include appropriateness of treatment, antibiotic(s) selection, timing of administration, dose, and special circumstances.
Should you give the patient prophylactic antibiotics?
Prophylactic antibiotics are justified for surgical procedures whenever 3 major criteria are met5:
- the surgical site is inevitably contaminated with bacteria
- in the absence of prophylaxis, the frequency of infection at the operative site is unacceptably high
- operative site infections have the potential to lead to serious, potentially life-threatening sequelae.
Without a doubt, all 3 of these criteria are fulfilled when considering either urgent or nonurgent cesarean delivery. When cesarean delivery follows a long labor complicated by ruptured membranes, multiple internal vaginal examinations, and internal fetal monitoring, the operative site is inevitably contaminated with hundreds of thousands of pathogenic bacteria. Even when cesarean delivery is scheduled to occur before the onset of labor and ruptured membranes, a high concentration of vaginal organisms is introduced into the uterine and pelvic cavities coincident with making the hysterotomy incision.6
In the era before prophylactic antibiotics were used routinely, postoperative infection rates in some highly indigent patient populations approached 85%.5 Finally, as noted previously, postcesarean endometritis may progress to pelvic abscess formation, septic pelvic vein thrombophlebitis, and septic shock; wound infections may be complicated by dehiscence and evisceration.
When should you administer antibiotics: Before the surgical incision or after cord clamping?
More than 50 years ago, Burke conducted the classic sequence of basic science experiments that forms the foundation for use of prophylactic antibiotics.7 Using a guinea pig model, he showed that prophylactic antibiotics exert their most pronounced effect when they are administered before the surgical incision is made and before bacterial contamination occurs. Prophylaxis that is delayed more than 4 hours after the start of surgery will likely be ineffective.
Interestingly, however, when clinicians first began using prophylactic antibiotics for cesarean delivery, some investigators expressed concern about the possible exposure of the neonate to antibiotics just before delivery—specifically, whether this exposure would increase the frequency of evaluations for suspected sepsis or would promote resistance among organisms that would make neonatal sepsis more difficult to treat.
Gordon and colleagues published an important report in 1979 that showed that preoperative administration of ampicillin did not increase the frequency of immediate or delayed neonatal infections.8 However, delaying the administration of ampicillin until after the umbilical cord was clamped was just as effective in preventing post‑cesarean endometritis. Subsequently, Cunningham and co-workers showed that preoperative administration of prophylactic antibiotics significantly increased the frequency of sepsis workups in exposed neonates compared with infants with no preoperative antibiotic exposure (28% vs 15%; P<.025).9 Based on these 2 reports, obstetricians adopted a policy of delaying antibiotic administration until after the infant’s umbilical cord was clamped.
In 2007, Sullivan and colleagues challenged this long-standing practice.10 In a carefully designed prospective, randomized, double-blind trial, they showed that patients who received preoperative cefazolin had a significant reduction in the frequency of endometritis compared with women who received the same antibiotic after cord clamping (1% vs 5%; RR, 0.2; 95% CI, 0.2–0.94). The rate of wound infection was lower in the preoperative antibiotic group (3% vs 5%), but this difference did not reach statistical significance. The total infection-related morbidity was significantly reduced in women who received antibiotics preoperatively (4.0% vs 11.5%; RR, 0.4; 95% CI, 0.18–0.87). Additionally, there was no increase in the frequency of proven or suspected neonatal infection in the infants exposed to antibiotics before delivery.
Subsequent to the publication by Sullivan and colleagues, other reports have confirmed that administration of antibiotics prior to surgery is superior to administration after clamping of the umbilical cord.10–12 Thus, we have come full circle back to Burke’s principle established more than a half century ago.7
Which antibiotic(s) should you administer for prophylaxis, and how many doses?
In an earlier review, one of us (PD) examined the evidence regarding choice of antibiotics and number of doses, concluding that a single dose of a first-generation cephalosporin, such as cefazolin, was the preferred regimen.5 The single dose was comparable in effectiveness to 2- or 3-dose regimens and to single- or multiple-dose regimens of broader-spectrum agents. For more than 20 years now, the standard of care for antibiotic prophylaxis has been a single 1- to 2-g dose of cefazolin.
Several recent reports, however, have raised the question of whether the prophylactic effect could be enhanced if the spectrum of activity of the antibiotic regimen was broadened to include an agent effective against Ureaplasma species.
Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection; they showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis.13 In a follow-up report from the same institution, Tita and co-workers demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.14 In both of these investigations, the antibiotics were administered after cord clamping.
In a subsequent report, Ward and Duff15 showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a very low rate of both endometritis and wound infection in a population similar to that studied by Tita et al.13,14
Very recently, Tita and associates published the results of the Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial conducted at 14 US hospitals.16 This study included 2,013 women undergoing cesarean delivery during labor or after membrane rupture who were randomly assigned to receive intravenous azithromycin 500 mg (n = 1,019) or placebo (n = 994). All women also received standard antibiotic prophylaxis with cefazolin. The primary outcome (a composite of endometritis, wound infection, or other infection within 6 weeks) was significantly lower in the azithromycin group than in the placebo group (6.1% vs 12.0%, P<.001). In addition, there were significant differences between the treatment groups in the rates of endometritis (3.8% in the azithromycin group vs 6.1% in the placebo group, P = .02) as well as in the rates of wound infection (2.4% vs 6.6%, respectively, P<.001). Of additional note, there were no differences between the 2 groups in the composite neonatal outcome of death and serious neonatal complications (14.3% vs 13.6%, P = .63).The investigators concluded that extended-spectrum prophylaxis with adjunctive azithromycin safely reduces infection rates without raising the risk of neonatal adverse outcomes.
What the evidence says
We conclude that all patients, even those having a scheduled cesarean before the onset of labor or ruptured membranes, should receive prophylactic antibiotics in a single dose administered preoperatively rather than after cord clamping (Level I Evidence, Level 1A Recommendation; TABLE). In high-risk populations (eg, women in labor with ruptured membranes who are having an urgent cesarean), for whom the baseline risk of infection is high, administer the combination of cefazolin plus azithromycin in lieu of cefazolin alone (Level I Evidence, Level 1A Recommendation; TABLE).
If the patient has a history of an immediate hypersensitivity reaction to beta-lactam antibiotics, we recommend the combination of clindamycin (900 mg) plus gentamicin (1.5 mg/kg) as a single infusion prior to surgery. We base this recommendation on the need to provide reasonable coverage against a broad range of pathogens. Clindamycin covers gram-positive aerobes, such as staphylococci species and group B streptococci, and anaerobes; gentamicin covers aerobic gram-negative bacilli. A single agent, such as clindamycin or metronidazole, does not provide the broad-based coverage necessary for effective prophylaxis (Level III Evidence, Level 1C Recommendation; TABLE).
If the patient is overweight or obese, should you modify the antibiotic dose?
The prevalence of obesity in the United States continues to increase. One-third of all US reproductive-aged women are obese, and 6% of women are extremely obese.17 Obesity increases the risk of postcesarean infection 3- to 5- fold.18 Because both pregnancy and obesity increase the total volume of a drug’s distribution, achieving adequate antibiotic tissue concentrations may be hindered by a dilutional effect. Furthermore, pharmacokinetic studies consistently have shown that the tissue concentration of an antibiotic—which, ideally, should be above the minimum inhibitory concentration (MIC) for common bacteria—determines the susceptibility of those tissues to infection, regardless of whether the serum concentration of the antibiotic is in the therapeutic range.19
These concerns have led to several recent investigations evaluating different doses of cefazolin for obese patients. Pevzner and colleagues conducted a prospective cohort study of 29 women having a scheduled cesarean delivery.20 The patients were divided into 3 groups: lean (BMI <30 kg m2), obese (BMI 30.0–39.9 kg m2), and extremely obese (BMI >40 kg m2). All women received a 2-g dose of cefazolin 30 to 60 minutes before surgery. Cefazolin concentrations in adipose tissue obtained at the time of skin incision were inversely proportional to maternal BMI (r, −0.67; P<.001). All specimens demonstrated a therapeutic concentration (>1 µg/g) of cefazolin for gram-positive cocci, but 20% of the obese women and 33% of the extremely obese women did not achieve the MIC (>4 µg/g) for gram-negative bacilli (P = .29 and P = .14, respectively). At the time of skin closure, 20% of obese women and 44% of extremely obese women did not have tissue concentrations that exceeded the MIC for gram-negative bacteria.
Swank and associates conducted a prospective cohort study that included 28 women.18 They demonstrated that, after a 2-g dose of cefazolin, only 20% of the obese women (BMI 30–40 kg m2) and 0% of the extremely obese women (BMI >40 kg m2) achieved an adipose tissue concentration that exceeded the MIC for gram-negative rods (8 µg/mL). However, 100% and 71.4%, respectively, achieved such a tissue concentration after a 3-g dose. When the women were stratified by actual weight, there was a statistically significant difference between those who weighed less than 120 kg and those who weighed more than 120 kg. Seventy-nine percent of the former had a tissue concentration of cefazolin greater than 8 µg/mL compared with 0% of the women who weighed more than 120 kg. Based on these observations, the authors recommended a 3-g dose of cefazolin for women who weigh more than 120 kg.
In a double-blind RCT with 26 obese women (BMI ≥30 kg m2), Young and colleagues demonstrated that, at the time of hysterotomy and fascial closure, significantly higher concentrations of cefazolin were found in the adipose tissue of obese women who received a 3-g dose of antibiotic compared with those who received a 2-g dose.21 However, all concentrations of cefazolin were consistently above the MIC of cefazolin for gram-positive cocci (1 µg/g) and gram-negative bacilli (4 µg/g). Further, Maggio and co-workers conducted a double-blind RCT comparing a 2-g dose of cefazolin versus a 3-g dose in 57 obese women (BMI ≥30 kg m2).22 They found no statistically significant difference in the percentage of women who had tissue concentrations of cefazolin greater than the MIC for gram-positive cocci (8 µg/g). All samples were above the MIC of cefazolin for gram-negative bacilli (2 µg/g). Based on these data, these investigators did not recommend increasing the dose of cefazolin from 2 g to 3 g in obese patients.21,22
The studies discussed above are difficult to compare for 3 reasons. First, each study used a different MIC of cefazolin for both gram-positive and gram-negative bacteria. Second, the authors sampled different maternal tissues or serum at varying times during the cesarean delivery. Third, the studies did not specifically investigate, or were not powered sufficiently to address, the more important clinical outcome of surgical site infection. In a recent historical cohort study, Ward and Duff were unable to show that increasing the dose of cefazolin to 2 g in all women with a BMI <30 kg m2 and to 3 g in all women with a BMI >30 kg m2 reduced the rate of endometritis and wound infection below the level already achieved with combined prophylaxis with cefazolin (1 g) plus azithromycin (500 mg).15
Sutton and colleagues recently assessed the pharmacokinetics of azithromycin when used as prophylaxis for cesarean delivery.23 They studied 30 women who had a scheduled cesarean delivery and who received a 500-mg intravenous dose of azithromycin that was initiated 15, 30, or 60 minutes before the surgical incision and then infused over 1 hour. They obtained maternal plasma samples multiple times during the first 8 hours after surgery. They also obtained samples of amniotic fluid, placenta, myometrium, adipose tissue, and umbilical cord blood intraoperatively. The median concentration of azithromycin in adipose tissue was 102 ng/g, which is below the MIC50 for Ureaplasma species (250 ng/mL). The median concentration in myometrial tissue was 402 ng/g. The concentration in maternal plasma consistently exceeded the MIC50 for Ureaplasma species.
What the evidence says
All women, regardless of weight,
CASE Resolved
For the 26-year-old obese laboring patient about to undergo cesarean delivery, reasonable steps for prevention of infection include removing the hair at the incision site with clippers or depilatory cream immediately prior to the start of surgery; cleansing the abdomen with a chlorhexidine-alcohol solution; and administering cefazolin (2 g) plus azithromycin (500 mg) preoperatively.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cesarean delivery is now the most commonly performed major operation in hospitals across the United States. Approximately 30% of the 4 million deliveries that occur each year are by cesarean. Endometritis and wound infection (superficial and deep surgical site infection) are the most common postoperative complications of cesarean delivery. These 2 infections usually can be treated in a straightforward manner with antibiotics or surgical drainage. In some cases, however, they can lead to serious sequelae, such as pelvic abscess, septic pelvic vein thrombophlebitis, and wound dehiscence/evisceration, thereby prolonging the patient’s hospitalization and significantly increasing medical expenses.
Accordingly, in the past 50 years many investigators have proposed various specific measures to reduce the risk of postcesarean infection. In this article, we critically evaluate 2 of these major interventions: methods of skin preparation and administration of prophylactic antibiotics. In part 2 of this series next month, we will review the evidence regarding preoperative bathing with an antiseptic, preoperative vaginal cleansing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
CASE Cesarean delivery required for nonprogressing labor
A 26-year-old obese primigravid woman, body mass index (BMI) 37 kg m2, at 40 weeks’ gestation has been in labor for 20 hours. Her membranes have been ruptured for 16 hours. Her cervix is completely effaced and is 7 cm dilated. The fetal head is at −1 cm station. Her cervical examination findings have not changed in 4 hours despite adequate uterine contractility documented by intrauterine pressure catheter. You are now ready to proceed with cesarean delivery, and you want to do everything possible to prevent the patient from developing a postoperative infection.
What are the best practices for postcesarean infection prevention in this patient?

Skin preparation
Adequate preoperative skin preparation is an important first step in preventing post‑ cesarean infection.
How should you prepare the patient’s skin for surgery?
Two issues to address when preparing the abdominal wall for surgery are hair removal and skin cleansing. More than 40 years ago, Cruse and Foord definitively answered the question about hair removal.1 In a landmark cohort investigation of more than 23,000 patients having many different types of operative procedures, they demonstrated that shaving the hair on the evening before surgery resulted in a higher rate of wound infection than clipping the hair, removing the hair with a depilatory cream just before surgery, or not removing the hair at all.
Three recent investigations have thoughtfully addressed the issue of skin cleansing. Darouiche and colleagues conducted a prospective, randomized, multicenter trial comparing chlorhexidine-alcohol with povidone-iodine for skin preparation before surgery.2 Their investigation included 849 patients having many different types of surgical procedures, only a minority of which were in obstetric and gynecologic patients. They demonstrated fewer superficial wound infections in patients in the chlorhexidine-alcohol group (4.2% vs 8.6%, P = .008). Of even greater importance, patients in the chlorhexidine-alcohol group had fewer deep wound infections (1% vs 3%, P = .005).
Ngai and co-workers recently reported the results of a randomized controlled trial (RCT) in which women undergoing nonurgent cesarean delivery had their skin cleansed with povidone-iodine with alcohol, chlorhexidine with alcohol, or the sequential combination of both solutions.3 The overall rate of surgical site infection was just 4.3%. The 3 groups had comparable infection rates and, accordingly, the authors were unable to conclude that one type of skin preparation was superior to the other.
The most informative recent investigation was by Tuuli and colleagues, who evaluated 1,147 patients having cesarean delivery assigned to undergo skin preparation with either chlorhexidine-alcohol or iodine-alcohol.4 Unlike the study by Ngai and co-workers, in this study approximately 40% of the patients in each treatment arm had unscheduled, urgent cesarean deliveries.3,4 Overall, the rate of infection in the chlorhexidine-alcohol group was 4.0% compared with 7.3% in the iodine-alcohol group (relative risk [RR], 0.55; 95% confidence interval [CI], 0.34–0.90, P = .02).
What the evidence says
Based on the evidence cited above, we advise removing hair at the incision site with clippers or depilatory cream just before the start of surgery. The abdomen should then be cleansed with a chlorhexidine-alcohol solution (Level I Evidence, Level 1A Recommendation; TABLE).

Antibiotic prophylaxis
Questions to consider regarding antibiotic prophylaxis for cesarean delivery include appropriateness of treatment, antibiotic(s) selection, timing of administration, dose, and special circumstances.
Should you give the patient prophylactic antibiotics?
Prophylactic antibiotics are justified for surgical procedures whenever 3 major criteria are met5:
- the surgical site is inevitably contaminated with bacteria
- in the absence of prophylaxis, the frequency of infection at the operative site is unacceptably high
- operative site infections have the potential to lead to serious, potentially life-threatening sequelae.
Without a doubt, all 3 of these criteria are fulfilled when considering either urgent or nonurgent cesarean delivery. When cesarean delivery follows a long labor complicated by ruptured membranes, multiple internal vaginal examinations, and internal fetal monitoring, the operative site is inevitably contaminated with hundreds of thousands of pathogenic bacteria. Even when cesarean delivery is scheduled to occur before the onset of labor and ruptured membranes, a high concentration of vaginal organisms is introduced into the uterine and pelvic cavities coincident with making the hysterotomy incision.6
In the era before prophylactic antibiotics were used routinely, postoperative infection rates in some highly indigent patient populations approached 85%.5 Finally, as noted previously, postcesarean endometritis may progress to pelvic abscess formation, septic pelvic vein thrombophlebitis, and septic shock; wound infections may be complicated by dehiscence and evisceration.
When should you administer antibiotics: Before the surgical incision or after cord clamping?
More than 50 years ago, Burke conducted the classic sequence of basic science experiments that forms the foundation for use of prophylactic antibiotics.7 Using a guinea pig model, he showed that prophylactic antibiotics exert their most pronounced effect when they are administered before the surgical incision is made and before bacterial contamination occurs. Prophylaxis that is delayed more than 4 hours after the start of surgery will likely be ineffective.
Interestingly, however, when clinicians first began using prophylactic antibiotics for cesarean delivery, some investigators expressed concern about the possible exposure of the neonate to antibiotics just before delivery—specifically, whether this exposure would increase the frequency of evaluations for suspected sepsis or would promote resistance among organisms that would make neonatal sepsis more difficult to treat.
Gordon and colleagues published an important report in 1979 that showed that preoperative administration of ampicillin did not increase the frequency of immediate or delayed neonatal infections.8 However, delaying the administration of ampicillin until after the umbilical cord was clamped was just as effective in preventing post‑cesarean endometritis. Subsequently, Cunningham and co-workers showed that preoperative administration of prophylactic antibiotics significantly increased the frequency of sepsis workups in exposed neonates compared with infants with no preoperative antibiotic exposure (28% vs 15%; P<.025).9 Based on these 2 reports, obstetricians adopted a policy of delaying antibiotic administration until after the infant’s umbilical cord was clamped.
In 2007, Sullivan and colleagues challenged this long-standing practice.10 In a carefully designed prospective, randomized, double-blind trial, they showed that patients who received preoperative cefazolin had a significant reduction in the frequency of endometritis compared with women who received the same antibiotic after cord clamping (1% vs 5%; RR, 0.2; 95% CI, 0.2–0.94). The rate of wound infection was lower in the preoperative antibiotic group (3% vs 5%), but this difference did not reach statistical significance. The total infection-related morbidity was significantly reduced in women who received antibiotics preoperatively (4.0% vs 11.5%; RR, 0.4; 95% CI, 0.18–0.87). Additionally, there was no increase in the frequency of proven or suspected neonatal infection in the infants exposed to antibiotics before delivery.
Subsequent to the publication by Sullivan and colleagues, other reports have confirmed that administration of antibiotics prior to surgery is superior to administration after clamping of the umbilical cord.10–12 Thus, we have come full circle back to Burke’s principle established more than a half century ago.7
Which antibiotic(s) should you administer for prophylaxis, and how many doses?
In an earlier review, one of us (PD) examined the evidence regarding choice of antibiotics and number of doses, concluding that a single dose of a first-generation cephalosporin, such as cefazolin, was the preferred regimen.5 The single dose was comparable in effectiveness to 2- or 3-dose regimens and to single- or multiple-dose regimens of broader-spectrum agents. For more than 20 years now, the standard of care for antibiotic prophylaxis has been a single 1- to 2-g dose of cefazolin.
Several recent reports, however, have raised the question of whether the prophylactic effect could be enhanced if the spectrum of activity of the antibiotic regimen was broadened to include an agent effective against Ureaplasma species.
Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection; they showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis.13 In a follow-up report from the same institution, Tita and co-workers demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.14 In both of these investigations, the antibiotics were administered after cord clamping.
In a subsequent report, Ward and Duff15 showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a very low rate of both endometritis and wound infection in a population similar to that studied by Tita et al.13,14
Very recently, Tita and associates published the results of the Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial conducted at 14 US hospitals.16 This study included 2,013 women undergoing cesarean delivery during labor or after membrane rupture who were randomly assigned to receive intravenous azithromycin 500 mg (n = 1,019) or placebo (n = 994). All women also received standard antibiotic prophylaxis with cefazolin. The primary outcome (a composite of endometritis, wound infection, or other infection within 6 weeks) was significantly lower in the azithromycin group than in the placebo group (6.1% vs 12.0%, P<.001). In addition, there were significant differences between the treatment groups in the rates of endometritis (3.8% in the azithromycin group vs 6.1% in the placebo group, P = .02) as well as in the rates of wound infection (2.4% vs 6.6%, respectively, P<.001). Of additional note, there were no differences between the 2 groups in the composite neonatal outcome of death and serious neonatal complications (14.3% vs 13.6%, P = .63).The investigators concluded that extended-spectrum prophylaxis with adjunctive azithromycin safely reduces infection rates without raising the risk of neonatal adverse outcomes.
What the evidence says
We conclude that all patients, even those having a scheduled cesarean before the onset of labor or ruptured membranes, should receive prophylactic antibiotics in a single dose administered preoperatively rather than after cord clamping (Level I Evidence, Level 1A Recommendation; TABLE). In high-risk populations (eg, women in labor with ruptured membranes who are having an urgent cesarean), for whom the baseline risk of infection is high, administer the combination of cefazolin plus azithromycin in lieu of cefazolin alone (Level I Evidence, Level 1A Recommendation; TABLE).
If the patient has a history of an immediate hypersensitivity reaction to beta-lactam antibiotics, we recommend the combination of clindamycin (900 mg) plus gentamicin (1.5 mg/kg) as a single infusion prior to surgery. We base this recommendation on the need to provide reasonable coverage against a broad range of pathogens. Clindamycin covers gram-positive aerobes, such as staphylococci species and group B streptococci, and anaerobes; gentamicin covers aerobic gram-negative bacilli. A single agent, such as clindamycin or metronidazole, does not provide the broad-based coverage necessary for effective prophylaxis (Level III Evidence, Level 1C Recommendation; TABLE).
If the patient is overweight or obese, should you modify the antibiotic dose?
The prevalence of obesity in the United States continues to increase. One-third of all US reproductive-aged women are obese, and 6% of women are extremely obese.17 Obesity increases the risk of postcesarean infection 3- to 5- fold.18 Because both pregnancy and obesity increase the total volume of a drug’s distribution, achieving adequate antibiotic tissue concentrations may be hindered by a dilutional effect. Furthermore, pharmacokinetic studies consistently have shown that the tissue concentration of an antibiotic—which, ideally, should be above the minimum inhibitory concentration (MIC) for common bacteria—determines the susceptibility of those tissues to infection, regardless of whether the serum concentration of the antibiotic is in the therapeutic range.19
These concerns have led to several recent investigations evaluating different doses of cefazolin for obese patients. Pevzner and colleagues conducted a prospective cohort study of 29 women having a scheduled cesarean delivery.20 The patients were divided into 3 groups: lean (BMI <30 kg m2), obese (BMI 30.0–39.9 kg m2), and extremely obese (BMI >40 kg m2). All women received a 2-g dose of cefazolin 30 to 60 minutes before surgery. Cefazolin concentrations in adipose tissue obtained at the time of skin incision were inversely proportional to maternal BMI (r, −0.67; P<.001). All specimens demonstrated a therapeutic concentration (>1 µg/g) of cefazolin for gram-positive cocci, but 20% of the obese women and 33% of the extremely obese women did not achieve the MIC (>4 µg/g) for gram-negative bacilli (P = .29 and P = .14, respectively). At the time of skin closure, 20% of obese women and 44% of extremely obese women did not have tissue concentrations that exceeded the MIC for gram-negative bacteria.
Swank and associates conducted a prospective cohort study that included 28 women.18 They demonstrated that, after a 2-g dose of cefazolin, only 20% of the obese women (BMI 30–40 kg m2) and 0% of the extremely obese women (BMI >40 kg m2) achieved an adipose tissue concentration that exceeded the MIC for gram-negative rods (8 µg/mL). However, 100% and 71.4%, respectively, achieved such a tissue concentration after a 3-g dose. When the women were stratified by actual weight, there was a statistically significant difference between those who weighed less than 120 kg and those who weighed more than 120 kg. Seventy-nine percent of the former had a tissue concentration of cefazolin greater than 8 µg/mL compared with 0% of the women who weighed more than 120 kg. Based on these observations, the authors recommended a 3-g dose of cefazolin for women who weigh more than 120 kg.
In a double-blind RCT with 26 obese women (BMI ≥30 kg m2), Young and colleagues demonstrated that, at the time of hysterotomy and fascial closure, significantly higher concentrations of cefazolin were found in the adipose tissue of obese women who received a 3-g dose of antibiotic compared with those who received a 2-g dose.21 However, all concentrations of cefazolin were consistently above the MIC of cefazolin for gram-positive cocci (1 µg/g) and gram-negative bacilli (4 µg/g). Further, Maggio and co-workers conducted a double-blind RCT comparing a 2-g dose of cefazolin versus a 3-g dose in 57 obese women (BMI ≥30 kg m2).22 They found no statistically significant difference in the percentage of women who had tissue concentrations of cefazolin greater than the MIC for gram-positive cocci (8 µg/g). All samples were above the MIC of cefazolin for gram-negative bacilli (2 µg/g). Based on these data, these investigators did not recommend increasing the dose of cefazolin from 2 g to 3 g in obese patients.21,22
The studies discussed above are difficult to compare for 3 reasons. First, each study used a different MIC of cefazolin for both gram-positive and gram-negative bacteria. Second, the authors sampled different maternal tissues or serum at varying times during the cesarean delivery. Third, the studies did not specifically investigate, or were not powered sufficiently to address, the more important clinical outcome of surgical site infection. In a recent historical cohort study, Ward and Duff were unable to show that increasing the dose of cefazolin to 2 g in all women with a BMI <30 kg m2 and to 3 g in all women with a BMI >30 kg m2 reduced the rate of endometritis and wound infection below the level already achieved with combined prophylaxis with cefazolin (1 g) plus azithromycin (500 mg).15
Sutton and colleagues recently assessed the pharmacokinetics of azithromycin when used as prophylaxis for cesarean delivery.23 They studied 30 women who had a scheduled cesarean delivery and who received a 500-mg intravenous dose of azithromycin that was initiated 15, 30, or 60 minutes before the surgical incision and then infused over 1 hour. They obtained maternal plasma samples multiple times during the first 8 hours after surgery. They also obtained samples of amniotic fluid, placenta, myometrium, adipose tissue, and umbilical cord blood intraoperatively. The median concentration of azithromycin in adipose tissue was 102 ng/g, which is below the MIC50 for Ureaplasma species (250 ng/mL). The median concentration in myometrial tissue was 402 ng/g. The concentration in maternal plasma consistently exceeded the MIC50 for Ureaplasma species.
What the evidence says
All women, regardless of weight,
CASE Resolved
For the 26-year-old obese laboring patient about to undergo cesarean delivery, reasonable steps for prevention of infection include removing the hair at the incision site with clippers or depilatory cream immediately prior to the start of surgery; cleansing the abdomen with a chlorhexidine-alcohol solution; and administering cefazolin (2 g) plus azithromycin (500 mg) preoperatively.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cruse PJ, Foord R. A five‑year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107(2):206–210.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine‑alcohol versus povidone‑iodine for surgical‑site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery. Obstet Gynecol. 2015;126(6):1251–1257.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost‑effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 pt 1):794–798.
- Dinsmoor MJ, Gilbert S, Landon MB, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal‑Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168.
- Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstet Gynecol. 1979;53(2):151–156.
- Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151–154.
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1–e5.
- Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1–e6.
- Owens SM, Brozanski BS, Meyn LA, Wiesenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended‑spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51–56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended‑spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1–e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1–e4.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegel KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006:295(13):1549–1555.
- Swank ML, Wing DA, Nicolau DP, McNulty JA. Increased 3‑gram cefazolin dosing for cesarean delivery prophylaxis in obese women. Am J Obstet Gynecol. 2015;213(3):415.e1–e8.
- Liu P, Derendorf H. Antimicrobial tissue concentrations. Infect Dis Clin North Am. 2003:17(3):599–613.
- Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
- Young OM, Shaik IH, Twedt R, et al. Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol. 2015;213(4):541.e1–e7.
- Maggio L, Nicolau DP, DaCosta M, Rouse DJ, Hughes BL. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1205–1210.
- Sutton AL, Acosta EP, Larson KB, Kerstner‑Wood CD, Tita AT, Biggio JR. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol. 2015;212(6):812. e1–e6.
- Cruse PJ, Foord R. A five‑year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107(2):206–210.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine‑alcohol versus povidone‑iodine for surgical‑site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery. Obstet Gynecol. 2015;126(6):1251–1257.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost‑effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157(4 pt 1):794–798.
- Dinsmoor MJ, Gilbert S, Landon MB, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal‑Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168.
- Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstet Gynecol. 1979;53(2):151–156.
- Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151–154.
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1–e5.
- Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1–e6.
- Owens SM, Brozanski BS, Meyn LA, Wiesenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended‑spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51–56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended‑spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1–e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1–e4.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegel KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006:295(13):1549–1555.
- Swank ML, Wing DA, Nicolau DP, McNulty JA. Increased 3‑gram cefazolin dosing for cesarean delivery prophylaxis in obese women. Am J Obstet Gynecol. 2015;213(3):415.e1–e8.
- Liu P, Derendorf H. Antimicrobial tissue concentrations. Infect Dis Clin North Am. 2003:17(3):599–613.
- Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
- Young OM, Shaik IH, Twedt R, et al. Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol. 2015;213(4):541.e1–e7.
- Maggio L, Nicolau DP, DaCosta M, Rouse DJ, Hughes BL. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1205–1210.
- Sutton AL, Acosta EP, Larson KB, Kerstner‑Wood CD, Tita AT, Biggio JR. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol. 2015;212(6):812. e1–e6.
In this Article
- Prepping the skin for surgery
- Selecting the antibiotic(s) for infection prevention
- Prophylaxis for the obese patient
Does one particular cesarean technique confer better maternal and neonatal outcomes?
EXPERT COMMENTARY
John M. Thorp Jr, MD, McAllister Distinguished Professor, Division Director, General Obstetrics and Gynecology, Vice Chair of Research, Department of Ob-Gyn, University of North Carolina Schools of Medicine and Public Health, Chapel Hill.
Five years ago one of our interns operating with the director of labor and delivery challenged him as to why we were not using evidenced-based surgical techniques for cesarean delivery. Bruised by the formidable (and at times misleading) club of “evidence-based medicine” that is held as sacrosanct by the modern obstetrician, the director responded to the charge by researching a systematic review on abdominal delivery that amalgamated studies of poor quality with precious few trials. He unilaterally decided that we needed an opening in the transparent portion of the drape overlying the incision site so that we might use “evidence” to prevent operative site infection. The end result: No change in the incidence of wound infections, and adhesive drapes that did not adhere well, thereby displacing the effluent of amniotic fluid and blood that are part of a cesarean delivery back into the first assistant’s socks, shoes, and clothing. It was as if the clock had been turned back to my early years as an attending when we had cloth drapes. So much for having an evidence-based protocol. I was thus elated at reading the results of the CORONIS trial.
Details of the study
The CORONIS trial, in which investigators randomly assigned almost 16,000 women from 7 countries (Argentina, Chile, Ghana, India, Kenya, Pakistan, and Sudan), used a sophisticated factorial design and followed up 13,153 (84%) of the women for 3 years. The investigators tested an array of technical questions about 5 intervention pairs used during abdominal delivery and reported the main outcomes of interest for each intervention, including:
- blunt versus sharp abdominal entry—no evidence of a difference in risk of abdominal hernias (adjusted risk ratio [RR], 0.66; 95% confidence interval [CI], 0.39–1.11)
- exteriorization of the uterus versus intra-abdominal repair—no evidence of a difference in risk of infertility (RR, 0.91; 95% CI, 0.71–1.18) or of ectopic pregnancy (RR, 0.50; CI, 0.15–1.66)
- single- versus double-layer closure of the uterus—no evidence of a difference in maternal death (RR, 0.78; 95% CI, 0.46–1.32) or a composite of pregnancy complications (RR, 1.20; 95% CI, 0.75–1.90)
- closure versus nonclosure of the peritoneum—no evidence of a difference in any outcomes relating to symptoms associated with pelvic adhesions, such as infertility (RR, 0.8; 95% CI, 0.61–1.06)
- chromic catgut versus polyglactin-910 sutures—no evidence of a difference in the main comparisons for adverse pregnancy outcomes in a subsequent pregnancy, such as uterine rupture (RR, 3.05; 95% CI, 0.32–29.29).
Strengths and limitations. The CORONIS trial included a large number of participants and had comprehensive follow-up, a rigorous data collection process, and the participation of many countries. The trial’s participating centers, however, were mostly large referral hospitals with high research interest; adverse outcomes might have been higher in other settings. As well, a lower incidence of subsequent pregnancy among participants limited the study’s power to detect differences in outcomes between the intervention pairs.
Conclusions. None of the alternative techniques produced any real benefits despite syntheses-suggested benefit reported in systematic reviews. Surgeon preference for cesarean delivery techniques likely will continue to guide clinical practice along with economic and institution factors.
A word to the wise: Evidence is not created equally, and pushing it into lumps does not increase its value.
--John M. Thorp Jr, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
John M. Thorp Jr, MD, McAllister Distinguished Professor, Division Director, General Obstetrics and Gynecology, Vice Chair of Research, Department of Ob-Gyn, University of North Carolina Schools of Medicine and Public Health, Chapel Hill.
Five years ago one of our interns operating with the director of labor and delivery challenged him as to why we were not using evidenced-based surgical techniques for cesarean delivery. Bruised by the formidable (and at times misleading) club of “evidence-based medicine” that is held as sacrosanct by the modern obstetrician, the director responded to the charge by researching a systematic review on abdominal delivery that amalgamated studies of poor quality with precious few trials. He unilaterally decided that we needed an opening in the transparent portion of the drape overlying the incision site so that we might use “evidence” to prevent operative site infection. The end result: No change in the incidence of wound infections, and adhesive drapes that did not adhere well, thereby displacing the effluent of amniotic fluid and blood that are part of a cesarean delivery back into the first assistant’s socks, shoes, and clothing. It was as if the clock had been turned back to my early years as an attending when we had cloth drapes. So much for having an evidence-based protocol. I was thus elated at reading the results of the CORONIS trial.
Details of the study
The CORONIS trial, in which investigators randomly assigned almost 16,000 women from 7 countries (Argentina, Chile, Ghana, India, Kenya, Pakistan, and Sudan), used a sophisticated factorial design and followed up 13,153 (84%) of the women for 3 years. The investigators tested an array of technical questions about 5 intervention pairs used during abdominal delivery and reported the main outcomes of interest for each intervention, including:
- blunt versus sharp abdominal entry—no evidence of a difference in risk of abdominal hernias (adjusted risk ratio [RR], 0.66; 95% confidence interval [CI], 0.39–1.11)
- exteriorization of the uterus versus intra-abdominal repair—no evidence of a difference in risk of infertility (RR, 0.91; 95% CI, 0.71–1.18) or of ectopic pregnancy (RR, 0.50; CI, 0.15–1.66)
- single- versus double-layer closure of the uterus—no evidence of a difference in maternal death (RR, 0.78; 95% CI, 0.46–1.32) or a composite of pregnancy complications (RR, 1.20; 95% CI, 0.75–1.90)
- closure versus nonclosure of the peritoneum—no evidence of a difference in any outcomes relating to symptoms associated with pelvic adhesions, such as infertility (RR, 0.8; 95% CI, 0.61–1.06)
- chromic catgut versus polyglactin-910 sutures—no evidence of a difference in the main comparisons for adverse pregnancy outcomes in a subsequent pregnancy, such as uterine rupture (RR, 3.05; 95% CI, 0.32–29.29).
Strengths and limitations. The CORONIS trial included a large number of participants and had comprehensive follow-up, a rigorous data collection process, and the participation of many countries. The trial’s participating centers, however, were mostly large referral hospitals with high research interest; adverse outcomes might have been higher in other settings. As well, a lower incidence of subsequent pregnancy among participants limited the study’s power to detect differences in outcomes between the intervention pairs.
Conclusions. None of the alternative techniques produced any real benefits despite syntheses-suggested benefit reported in systematic reviews. Surgeon preference for cesarean delivery techniques likely will continue to guide clinical practice along with economic and institution factors.
A word to the wise: Evidence is not created equally, and pushing it into lumps does not increase its value.
--John M. Thorp Jr, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
John M. Thorp Jr, MD, McAllister Distinguished Professor, Division Director, General Obstetrics and Gynecology, Vice Chair of Research, Department of Ob-Gyn, University of North Carolina Schools of Medicine and Public Health, Chapel Hill.
Five years ago one of our interns operating with the director of labor and delivery challenged him as to why we were not using evidenced-based surgical techniques for cesarean delivery. Bruised by the formidable (and at times misleading) club of “evidence-based medicine” that is held as sacrosanct by the modern obstetrician, the director responded to the charge by researching a systematic review on abdominal delivery that amalgamated studies of poor quality with precious few trials. He unilaterally decided that we needed an opening in the transparent portion of the drape overlying the incision site so that we might use “evidence” to prevent operative site infection. The end result: No change in the incidence of wound infections, and adhesive drapes that did not adhere well, thereby displacing the effluent of amniotic fluid and blood that are part of a cesarean delivery back into the first assistant’s socks, shoes, and clothing. It was as if the clock had been turned back to my early years as an attending when we had cloth drapes. So much for having an evidence-based protocol. I was thus elated at reading the results of the CORONIS trial.
Details of the study
The CORONIS trial, in which investigators randomly assigned almost 16,000 women from 7 countries (Argentina, Chile, Ghana, India, Kenya, Pakistan, and Sudan), used a sophisticated factorial design and followed up 13,153 (84%) of the women for 3 years. The investigators tested an array of technical questions about 5 intervention pairs used during abdominal delivery and reported the main outcomes of interest for each intervention, including:
- blunt versus sharp abdominal entry—no evidence of a difference in risk of abdominal hernias (adjusted risk ratio [RR], 0.66; 95% confidence interval [CI], 0.39–1.11)
- exteriorization of the uterus versus intra-abdominal repair—no evidence of a difference in risk of infertility (RR, 0.91; 95% CI, 0.71–1.18) or of ectopic pregnancy (RR, 0.50; CI, 0.15–1.66)
- single- versus double-layer closure of the uterus—no evidence of a difference in maternal death (RR, 0.78; 95% CI, 0.46–1.32) or a composite of pregnancy complications (RR, 1.20; 95% CI, 0.75–1.90)
- closure versus nonclosure of the peritoneum—no evidence of a difference in any outcomes relating to symptoms associated with pelvic adhesions, such as infertility (RR, 0.8; 95% CI, 0.61–1.06)
- chromic catgut versus polyglactin-910 sutures—no evidence of a difference in the main comparisons for adverse pregnancy outcomes in a subsequent pregnancy, such as uterine rupture (RR, 3.05; 95% CI, 0.32–29.29).
Strengths and limitations. The CORONIS trial included a large number of participants and had comprehensive follow-up, a rigorous data collection process, and the participation of many countries. The trial’s participating centers, however, were mostly large referral hospitals with high research interest; adverse outcomes might have been higher in other settings. As well, a lower incidence of subsequent pregnancy among participants limited the study’s power to detect differences in outcomes between the intervention pairs.
Conclusions. None of the alternative techniques produced any real benefits despite syntheses-suggested benefit reported in systematic reviews. Surgeon preference for cesarean delivery techniques likely will continue to guide clinical practice along with economic and institution factors.
A word to the wise: Evidence is not created equally, and pushing it into lumps does not increase its value.
--John M. Thorp Jr, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Do you utilize vasopressin in your difficult cesarean delivery surgeries?
Vasopressin is often used to reduce blood loss in gynecologic surgery. Results of randomized clinical trials indicate that its use reduces blood loss in many gynecologic surgery procedures, including hysterectomy, myomectomy, cervical conization, and second trimester pregnancy termination.1−7 In contrast to the widespread use of dilute vasopressin injection in gynecology surgery, obstetricians in the United States seldom use vasopressin to reduce blood loss in difficult cesarean delivery surgery. Although there is very little direct evidence from clinical trials on the value of vasopressin in obstetric surgery, high-quality evidence from relevant gynecologic surgery and case reports from obstetricians support its use during difficult cesarean delivery surgery.
Biology of oxytocin and vasopressin
Oxytocin and vasopressin are fraternal twin nanopeptides that differ by only two amino acids and are secreted from the posterior pituitary. The human uterus contains both oxytocin and vasopressin receptors; stimulation of either receptor causes uterine contraction. Vasopressin receptor activation also causes vasoconstriction and platelet activation.
Given the similar biochemistry of oxytocin and vasopressin it is not surprising that each hormone is capable of binding to both oxytocin and vasopressin receptors. The affinity of oxytocin for the oxytocin and vasopressin receptors as expressed as an inhibition constant is 6.8 nM and 35 nM, respectively. Vasopressin’s affinity for the oxytocin and vasopressin V1a receptors is 48 nM and 1.4 nM, respectively.8
Administering vasopressin into the uterus will achieve a high concentration of the hormone, which stimulates both the oxytocin and vasopressin receptors, resulting in uterine contraction, vasoconstriction, and platelet activation. Of particular importance to obstetricians is that following a prolonged labor or administration of oxytocin, myometrial oxytocin receptors may be downregulated, but vasopressin receptors may remain functional.9,10
Vasopressin regulates plasma volume, blood pressure, osmolality, and uterine contractility. The vasopressin V1a receptor is present on vascular smooth muscle cells, platelets, and uterine myocytes. Activating this receptor causes vasoconstriction, platelet activation, and uterine contraction.
Vasopressin reduces surgical blood loss in two ways. The first major mechanism is through vasoconstriction.11 Second, in uterine surgery specifically, vasopressin stimulates uterine contraction. The hormone exerts its antidiuretic action through the V2 receptor in the kidney.
Optimal vasopressin dose
In gynecologic surgery, the vasopressin doses utilized to reduce blood loss range from 5 U to 20 U diluted in 20 mL to 200 mL of saline. Randomized trial results indicate that a vasopressin dose of 4 U is effective in reducing blood loss during second trimester pregnancy termination,7 and a dose of 3 U is effective in reducing blood loss during cervical conization.5,6 There is insufficient obstetric literature to determine the optimal dose of vasopressin to reduce blood loss in difficult cesarean delivery sur- gery, but doses similar to those used in gynecologic surgery should be considered.
Possible effects of vasopressin overdosing. In gynecologic surgery, injection of vasopressin has been reported to cause bradycardia, hypotension, myocardial infarction, and cardiovascular collapse.12 Given that multiple vasoactive medications may be given to a patient undergoing a complex cesarean delivery, including oxytocin, methergine, and ephedrine, it is important for the obstetrician to use the lowest effective dose of vasopressin necessary to facilitate control of blood loss. The obstetrician needs to communicate with the anesthesiologist and coordinate the use of dilute vasopressin with other vasoactive medications.
Avoid intravascular injection of vasopressin. I prefer to inject vasopressin in the subserosa of the uterus rather than to inject it in a highly vascular area such as the subendometrium or near the uterine artery and vein.
Vasopressin reduces blood loss during hysterectomy
One randomized trial has reported that the administration of 10 U of vasopressin diluted in saline into the lower uterine segment reduced blood loss at abdominal hysterectomy in nonpregnant women compared with an injection of saline alone (445 mL vs 748 mL of blood loss, respectively).1 There are no clinical trials of the use of vasopressin in cesarean hysterectomy. However, abdominal hysterectomy procedures and cesarean hysterectomy are similar, and vasopressin likely helps to reduce blood loss at cesarean hysterectomy.
Vasopressin reduces blood loss during myomectomy
Authors of 3 small, randomized clinical trials in nonpregnant women have reported that the intramyometrial injection of dilute vasopressin reduces blood loss during myomectomy surgery.2−4 The vasopressin doses in the 3 trials ranged from 5 U of vasopressin in 100 mL of saline to 20 U of vasopressin in 20 mL of saline. A Cochrane meta-analyis of the 3 studies concluded that, at myomectomy, the intramyometrial injection of dilute vasopressin was associated with a significant reduction in blood loss compared with placebo (246 mL vs 483 mL, respectively).13
There are great similarities between myomectomy in the nonpregnant and pregnant uterus. Given the clinical trials data that support the use of vasopressin to reduce blood loss during myomectomy in the nonpregnant uterus, it is likely that vasopressin also would reduce blood loss during myomectomy performed at the time of a cesarean delivery.
At cesarean delivery, elective myomectomy of intramural fibroids is generally not recommended because of the risk of massive blood loss. Clinicians often remove large pedunculated fibroids because this surgery does not usually cause massive bleeding. However, on occasion it may be necessary to perform a myomectomy on intramural myoma(s) in order to close a hysterotomy incision.
For myomectomy surgery performed at the time of cesarean delivery, many techniques have been utilized to reduce blood loss, including:
- intravenous oxytocin infusion14,15
- injection of oxytocin into the myoma pseudocapsule15
- electrosurgery16−18
- argon beam coagulator19
- uterine tourniquet20
- premyomectomy placement of a uterine U stitch21 or purse string suture22
- O’Leary sutures23,24
- temporary balloon occlusion of pelvic arteries25
- vasopressin injection.26
Given the widespread use of vasopressin injection in gynecologic surgery to reduce blood loss at myomectomy, obstetricians should consider using vasopressin in their cesarean myomectomy surgery.
Use of vasopressin during cesarean delivery for placenta previa may reduce blood loss
Women with a complete placenta previa require a cesarean delivery to safely birth their baby. Cesarean deliveries performed for this indication are associated with an increased risk of hemorrhage. In one case series of 59 patients with placenta previa undergoing cesarean delivery, 4 U of vasopressin diluted in 20 mL of saline was injected into the placental implantation site to reduce blood loss. Among the patients receiving vasopressin in- jection, the blood loss was 1,149 mL. Among 50 women with placenta previa who did not receive vasopressin injection, the blood loss was 1,634 mL.27
Obstetric surgery and vasopressin: The time has come
As obstetricians and gynecologists we constantly strive to improve the effectiveness of our surgical procedures and reduce adverse outcomes, including infection and blood loss. The use of vasopressin is widely accepted in gynecologic surgery as an adjuvant that reduces blood loss. The time has come to expand the use of vasopressin in difficult obstetric surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97:867–872.
- Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Brit J Obstet Gynaecol. 1994;101:435–437.
- Assaf A. Adhesions after laparoscopic myomectomy effect of the technique used. Gynaecol Endosc. 1999;8(4):225–229.
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertil Steril. 2011;95(2):762–766.
- Sabol ED, Gibson JL, Bowes WA Jr. Vasopressin injection in cervical conization. A double-blind study. Obstet Gynecol. 1971;37(4):596–601.
- Martin-Hirsch PP, Keep SL, Bryant A. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;(6):CD001421.
- Schulz KE, Grimes DA, Christensen DD. Vasopressin reduces blood loss from second trimester dilatation and evacuation abortion. Lancet. 1985;2(8451):353–356.
- Akerlund M, Bossmar T, Brouard R, et al. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects in isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999;106(10):1047–1053.
- Akerlund M. Involvement of oxytocin and vasopressin in the pathophysiology of preterm labor and primary dysmenorrhea. Prog Brain Res. 2002;139:359–365.
- Helmer H, Hacki T, Schneeberger C, et al. Oxytocinand vasopressin 1a receptor gene expression in the cycling or pregnant human uterus. Am J Obstet Gynecol. 1998;179(6 pt 1):1572–1578.
- Wing DA, Goharkhay N, Felix JC, Rostamkhani M, Naidu YM, Kovacs BW. Expression of the oxytocin and V1a vasopressin receptors in human myometrium during differing physiological states and following misoprostol administration. Gynecol Obstet Invest. 2006;62(4):181–185.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014; (8):CD005355.
- Tinelli A, Malvasi A, Mynbaev OA, et al. The surgical outcome of intracapsular cesarean myomectomy. A match control study. J Matern Fetal Neonatal Med. 2014;27(1):66–71.
- Brown D, Fletcher HM, Myrie MO, Reid M. Caesarean myomectomy—a safe procedure. A retrospective case controlled study. J Obstet Gynaecol. 1999;19(2):139–141.
- Kaymak O, Ustunyrt E, Okyay RE, Kalyoncu S, Mollamahmutoglu L. Myomectomy during cesarean section. Int J Gynaecol Obstet. 2005;89(2):90–93.
- Park BJ, Kim YW. Safety of cesarean myomectomy. J Obstet Gynaecol Res. 2009;35(5):906–911.
- Kim YS, Choi SD, Bae DH. Risk factors for complications in patients undergoing myomectomy at the time of cesarean section. J Obstet Gynaecol Res. 2010;36(3):550–554.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynecol Obstet. 1999;67(3):189–190.
- Incebiyik A, Hilali NG, Camuzcuoglu A, Vural M, Camuzcuoglu H. Myomectomy during caesarean: a retrospective evaluation of 6 cases. Arch Gynecol Obstet. 2014;289(3):569–573.
- Cobellis L, Pecori E, Cobellis G. Hemostatic technique for myomectomy during cesarean section. Int J Gynaecol Obstet. 2002;79(3):261–262.
- Lee JH, Cho DH. Myomectomy using purse-string suture during cesarean section. Arch Gynecol Obstet. 2011;283(suppl 1):S35–S37.
- Desai BR, Patted SS, Pujar YV, Sheriqar BY, Das SR, Ruge JC. A novel technique of selective uterine devascularization before myomectomy at the time of cesarean section: a pilot study. Fertil Steril. 2010;94(1):362–364.
- Sapmaz E, Celik H, Altungul A. Bilateral ascending uterine artery ligation vs. tourniquet use for hemostasis in cesarean myomectomy. A comparison. J Reprod Med. 2003;48(12):950–954.
- Sparic R, Malvasi A, Kadija S, Babovic I, Nejkovic L, Tinelli A. Cesarean myomectomy trends and controveries: an appraisal [published online ahead of print July 17, 2016]. J Matern Fetal Neonatal Med. doi:10.1080/14767058.2016.1205024.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing cesarean section. J Obstet Gynaecol Res. 2010;36(2):284–290.
- Kato S, Tanabe A, Kanki K, et al. Local injection of vasopressin reduces the blood loss during cesarean section in placenta previa. J Obstet Gynaecol Res. 2014;40(5):1249–1256.
Vasopressin is often used to reduce blood loss in gynecologic surgery. Results of randomized clinical trials indicate that its use reduces blood loss in many gynecologic surgery procedures, including hysterectomy, myomectomy, cervical conization, and second trimester pregnancy termination.1−7 In contrast to the widespread use of dilute vasopressin injection in gynecology surgery, obstetricians in the United States seldom use vasopressin to reduce blood loss in difficult cesarean delivery surgery. Although there is very little direct evidence from clinical trials on the value of vasopressin in obstetric surgery, high-quality evidence from relevant gynecologic surgery and case reports from obstetricians support its use during difficult cesarean delivery surgery.
Biology of oxytocin and vasopressin
Oxytocin and vasopressin are fraternal twin nanopeptides that differ by only two amino acids and are secreted from the posterior pituitary. The human uterus contains both oxytocin and vasopressin receptors; stimulation of either receptor causes uterine contraction. Vasopressin receptor activation also causes vasoconstriction and platelet activation.
Given the similar biochemistry of oxytocin and vasopressin it is not surprising that each hormone is capable of binding to both oxytocin and vasopressin receptors. The affinity of oxytocin for the oxytocin and vasopressin receptors as expressed as an inhibition constant is 6.8 nM and 35 nM, respectively. Vasopressin’s affinity for the oxytocin and vasopressin V1a receptors is 48 nM and 1.4 nM, respectively.8
Administering vasopressin into the uterus will achieve a high concentration of the hormone, which stimulates both the oxytocin and vasopressin receptors, resulting in uterine contraction, vasoconstriction, and platelet activation. Of particular importance to obstetricians is that following a prolonged labor or administration of oxytocin, myometrial oxytocin receptors may be downregulated, but vasopressin receptors may remain functional.9,10
Vasopressin regulates plasma volume, blood pressure, osmolality, and uterine contractility. The vasopressin V1a receptor is present on vascular smooth muscle cells, platelets, and uterine myocytes. Activating this receptor causes vasoconstriction, platelet activation, and uterine contraction.
Vasopressin reduces surgical blood loss in two ways. The first major mechanism is through vasoconstriction.11 Second, in uterine surgery specifically, vasopressin stimulates uterine contraction. The hormone exerts its antidiuretic action through the V2 receptor in the kidney.
Optimal vasopressin dose
In gynecologic surgery, the vasopressin doses utilized to reduce blood loss range from 5 U to 20 U diluted in 20 mL to 200 mL of saline. Randomized trial results indicate that a vasopressin dose of 4 U is effective in reducing blood loss during second trimester pregnancy termination,7 and a dose of 3 U is effective in reducing blood loss during cervical conization.5,6 There is insufficient obstetric literature to determine the optimal dose of vasopressin to reduce blood loss in difficult cesarean delivery sur- gery, but doses similar to those used in gynecologic surgery should be considered.
Possible effects of vasopressin overdosing. In gynecologic surgery, injection of vasopressin has been reported to cause bradycardia, hypotension, myocardial infarction, and cardiovascular collapse.12 Given that multiple vasoactive medications may be given to a patient undergoing a complex cesarean delivery, including oxytocin, methergine, and ephedrine, it is important for the obstetrician to use the lowest effective dose of vasopressin necessary to facilitate control of blood loss. The obstetrician needs to communicate with the anesthesiologist and coordinate the use of dilute vasopressin with other vasoactive medications.
Avoid intravascular injection of vasopressin. I prefer to inject vasopressin in the subserosa of the uterus rather than to inject it in a highly vascular area such as the subendometrium or near the uterine artery and vein.
Vasopressin reduces blood loss during hysterectomy
One randomized trial has reported that the administration of 10 U of vasopressin diluted in saline into the lower uterine segment reduced blood loss at abdominal hysterectomy in nonpregnant women compared with an injection of saline alone (445 mL vs 748 mL of blood loss, respectively).1 There are no clinical trials of the use of vasopressin in cesarean hysterectomy. However, abdominal hysterectomy procedures and cesarean hysterectomy are similar, and vasopressin likely helps to reduce blood loss at cesarean hysterectomy.
Vasopressin reduces blood loss during myomectomy
Authors of 3 small, randomized clinical trials in nonpregnant women have reported that the intramyometrial injection of dilute vasopressin reduces blood loss during myomectomy surgery.2−4 The vasopressin doses in the 3 trials ranged from 5 U of vasopressin in 100 mL of saline to 20 U of vasopressin in 20 mL of saline. A Cochrane meta-analyis of the 3 studies concluded that, at myomectomy, the intramyometrial injection of dilute vasopressin was associated with a significant reduction in blood loss compared with placebo (246 mL vs 483 mL, respectively).13
There are great similarities between myomectomy in the nonpregnant and pregnant uterus. Given the clinical trials data that support the use of vasopressin to reduce blood loss during myomectomy in the nonpregnant uterus, it is likely that vasopressin also would reduce blood loss during myomectomy performed at the time of a cesarean delivery.
At cesarean delivery, elective myomectomy of intramural fibroids is generally not recommended because of the risk of massive blood loss. Clinicians often remove large pedunculated fibroids because this surgery does not usually cause massive bleeding. However, on occasion it may be necessary to perform a myomectomy on intramural myoma(s) in order to close a hysterotomy incision.
For myomectomy surgery performed at the time of cesarean delivery, many techniques have been utilized to reduce blood loss, including:
- intravenous oxytocin infusion14,15
- injection of oxytocin into the myoma pseudocapsule15
- electrosurgery16−18
- argon beam coagulator19
- uterine tourniquet20
- premyomectomy placement of a uterine U stitch21 or purse string suture22
- O’Leary sutures23,24
- temporary balloon occlusion of pelvic arteries25
- vasopressin injection.26
Given the widespread use of vasopressin injection in gynecologic surgery to reduce blood loss at myomectomy, obstetricians should consider using vasopressin in their cesarean myomectomy surgery.
Use of vasopressin during cesarean delivery for placenta previa may reduce blood loss
Women with a complete placenta previa require a cesarean delivery to safely birth their baby. Cesarean deliveries performed for this indication are associated with an increased risk of hemorrhage. In one case series of 59 patients with placenta previa undergoing cesarean delivery, 4 U of vasopressin diluted in 20 mL of saline was injected into the placental implantation site to reduce blood loss. Among the patients receiving vasopressin in- jection, the blood loss was 1,149 mL. Among 50 women with placenta previa who did not receive vasopressin injection, the blood loss was 1,634 mL.27
Obstetric surgery and vasopressin: The time has come
As obstetricians and gynecologists we constantly strive to improve the effectiveness of our surgical procedures and reduce adverse outcomes, including infection and blood loss. The use of vasopressin is widely accepted in gynecologic surgery as an adjuvant that reduces blood loss. The time has come to expand the use of vasopressin in difficult obstetric surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Vasopressin is often used to reduce blood loss in gynecologic surgery. Results of randomized clinical trials indicate that its use reduces blood loss in many gynecologic surgery procedures, including hysterectomy, myomectomy, cervical conization, and second trimester pregnancy termination.1−7 In contrast to the widespread use of dilute vasopressin injection in gynecology surgery, obstetricians in the United States seldom use vasopressin to reduce blood loss in difficult cesarean delivery surgery. Although there is very little direct evidence from clinical trials on the value of vasopressin in obstetric surgery, high-quality evidence from relevant gynecologic surgery and case reports from obstetricians support its use during difficult cesarean delivery surgery.
Biology of oxytocin and vasopressin
Oxytocin and vasopressin are fraternal twin nanopeptides that differ by only two amino acids and are secreted from the posterior pituitary. The human uterus contains both oxytocin and vasopressin receptors; stimulation of either receptor causes uterine contraction. Vasopressin receptor activation also causes vasoconstriction and platelet activation.
Given the similar biochemistry of oxytocin and vasopressin it is not surprising that each hormone is capable of binding to both oxytocin and vasopressin receptors. The affinity of oxytocin for the oxytocin and vasopressin receptors as expressed as an inhibition constant is 6.8 nM and 35 nM, respectively. Vasopressin’s affinity for the oxytocin and vasopressin V1a receptors is 48 nM and 1.4 nM, respectively.8
Administering vasopressin into the uterus will achieve a high concentration of the hormone, which stimulates both the oxytocin and vasopressin receptors, resulting in uterine contraction, vasoconstriction, and platelet activation. Of particular importance to obstetricians is that following a prolonged labor or administration of oxytocin, myometrial oxytocin receptors may be downregulated, but vasopressin receptors may remain functional.9,10
Vasopressin regulates plasma volume, blood pressure, osmolality, and uterine contractility. The vasopressin V1a receptor is present on vascular smooth muscle cells, platelets, and uterine myocytes. Activating this receptor causes vasoconstriction, platelet activation, and uterine contraction.
Vasopressin reduces surgical blood loss in two ways. The first major mechanism is through vasoconstriction.11 Second, in uterine surgery specifically, vasopressin stimulates uterine contraction. The hormone exerts its antidiuretic action through the V2 receptor in the kidney.
Optimal vasopressin dose
In gynecologic surgery, the vasopressin doses utilized to reduce blood loss range from 5 U to 20 U diluted in 20 mL to 200 mL of saline. Randomized trial results indicate that a vasopressin dose of 4 U is effective in reducing blood loss during second trimester pregnancy termination,7 and a dose of 3 U is effective in reducing blood loss during cervical conization.5,6 There is insufficient obstetric literature to determine the optimal dose of vasopressin to reduce blood loss in difficult cesarean delivery sur- gery, but doses similar to those used in gynecologic surgery should be considered.
Possible effects of vasopressin overdosing. In gynecologic surgery, injection of vasopressin has been reported to cause bradycardia, hypotension, myocardial infarction, and cardiovascular collapse.12 Given that multiple vasoactive medications may be given to a patient undergoing a complex cesarean delivery, including oxytocin, methergine, and ephedrine, it is important for the obstetrician to use the lowest effective dose of vasopressin necessary to facilitate control of blood loss. The obstetrician needs to communicate with the anesthesiologist and coordinate the use of dilute vasopressin with other vasoactive medications.
Avoid intravascular injection of vasopressin. I prefer to inject vasopressin in the subserosa of the uterus rather than to inject it in a highly vascular area such as the subendometrium or near the uterine artery and vein.
Vasopressin reduces blood loss during hysterectomy
One randomized trial has reported that the administration of 10 U of vasopressin diluted in saline into the lower uterine segment reduced blood loss at abdominal hysterectomy in nonpregnant women compared with an injection of saline alone (445 mL vs 748 mL of blood loss, respectively).1 There are no clinical trials of the use of vasopressin in cesarean hysterectomy. However, abdominal hysterectomy procedures and cesarean hysterectomy are similar, and vasopressin likely helps to reduce blood loss at cesarean hysterectomy.
Vasopressin reduces blood loss during myomectomy
Authors of 3 small, randomized clinical trials in nonpregnant women have reported that the intramyometrial injection of dilute vasopressin reduces blood loss during myomectomy surgery.2−4 The vasopressin doses in the 3 trials ranged from 5 U of vasopressin in 100 mL of saline to 20 U of vasopressin in 20 mL of saline. A Cochrane meta-analyis of the 3 studies concluded that, at myomectomy, the intramyometrial injection of dilute vasopressin was associated with a significant reduction in blood loss compared with placebo (246 mL vs 483 mL, respectively).13
There are great similarities between myomectomy in the nonpregnant and pregnant uterus. Given the clinical trials data that support the use of vasopressin to reduce blood loss during myomectomy in the nonpregnant uterus, it is likely that vasopressin also would reduce blood loss during myomectomy performed at the time of a cesarean delivery.
At cesarean delivery, elective myomectomy of intramural fibroids is generally not recommended because of the risk of massive blood loss. Clinicians often remove large pedunculated fibroids because this surgery does not usually cause massive bleeding. However, on occasion it may be necessary to perform a myomectomy on intramural myoma(s) in order to close a hysterotomy incision.
For myomectomy surgery performed at the time of cesarean delivery, many techniques have been utilized to reduce blood loss, including:
- intravenous oxytocin infusion14,15
- injection of oxytocin into the myoma pseudocapsule15
- electrosurgery16−18
- argon beam coagulator19
- uterine tourniquet20
- premyomectomy placement of a uterine U stitch21 or purse string suture22
- O’Leary sutures23,24
- temporary balloon occlusion of pelvic arteries25
- vasopressin injection.26
Given the widespread use of vasopressin injection in gynecologic surgery to reduce blood loss at myomectomy, obstetricians should consider using vasopressin in their cesarean myomectomy surgery.
Use of vasopressin during cesarean delivery for placenta previa may reduce blood loss
Women with a complete placenta previa require a cesarean delivery to safely birth their baby. Cesarean deliveries performed for this indication are associated with an increased risk of hemorrhage. In one case series of 59 patients with placenta previa undergoing cesarean delivery, 4 U of vasopressin diluted in 20 mL of saline was injected into the placental implantation site to reduce blood loss. Among the patients receiving vasopressin in- jection, the blood loss was 1,149 mL. Among 50 women with placenta previa who did not receive vasopressin injection, the blood loss was 1,634 mL.27
Obstetric surgery and vasopressin: The time has come
As obstetricians and gynecologists we constantly strive to improve the effectiveness of our surgical procedures and reduce adverse outcomes, including infection and blood loss. The use of vasopressin is widely accepted in gynecologic surgery as an adjuvant that reduces blood loss. The time has come to expand the use of vasopressin in difficult obstetric surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97:867–872.
- Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Brit J Obstet Gynaecol. 1994;101:435–437.
- Assaf A. Adhesions after laparoscopic myomectomy effect of the technique used. Gynaecol Endosc. 1999;8(4):225–229.
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertil Steril. 2011;95(2):762–766.
- Sabol ED, Gibson JL, Bowes WA Jr. Vasopressin injection in cervical conization. A double-blind study. Obstet Gynecol. 1971;37(4):596–601.
- Martin-Hirsch PP, Keep SL, Bryant A. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;(6):CD001421.
- Schulz KE, Grimes DA, Christensen DD. Vasopressin reduces blood loss from second trimester dilatation and evacuation abortion. Lancet. 1985;2(8451):353–356.
- Akerlund M, Bossmar T, Brouard R, et al. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects in isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999;106(10):1047–1053.
- Akerlund M. Involvement of oxytocin and vasopressin in the pathophysiology of preterm labor and primary dysmenorrhea. Prog Brain Res. 2002;139:359–365.
- Helmer H, Hacki T, Schneeberger C, et al. Oxytocinand vasopressin 1a receptor gene expression in the cycling or pregnant human uterus. Am J Obstet Gynecol. 1998;179(6 pt 1):1572–1578.
- Wing DA, Goharkhay N, Felix JC, Rostamkhani M, Naidu YM, Kovacs BW. Expression of the oxytocin and V1a vasopressin receptors in human myometrium during differing physiological states and following misoprostol administration. Gynecol Obstet Invest. 2006;62(4):181–185.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014; (8):CD005355.
- Tinelli A, Malvasi A, Mynbaev OA, et al. The surgical outcome of intracapsular cesarean myomectomy. A match control study. J Matern Fetal Neonatal Med. 2014;27(1):66–71.
- Brown D, Fletcher HM, Myrie MO, Reid M. Caesarean myomectomy—a safe procedure. A retrospective case controlled study. J Obstet Gynaecol. 1999;19(2):139–141.
- Kaymak O, Ustunyrt E, Okyay RE, Kalyoncu S, Mollamahmutoglu L. Myomectomy during cesarean section. Int J Gynaecol Obstet. 2005;89(2):90–93.
- Park BJ, Kim YW. Safety of cesarean myomectomy. J Obstet Gynaecol Res. 2009;35(5):906–911.
- Kim YS, Choi SD, Bae DH. Risk factors for complications in patients undergoing myomectomy at the time of cesarean section. J Obstet Gynaecol Res. 2010;36(3):550–554.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynecol Obstet. 1999;67(3):189–190.
- Incebiyik A, Hilali NG, Camuzcuoglu A, Vural M, Camuzcuoglu H. Myomectomy during caesarean: a retrospective evaluation of 6 cases. Arch Gynecol Obstet. 2014;289(3):569–573.
- Cobellis L, Pecori E, Cobellis G. Hemostatic technique for myomectomy during cesarean section. Int J Gynaecol Obstet. 2002;79(3):261–262.
- Lee JH, Cho DH. Myomectomy using purse-string suture during cesarean section. Arch Gynecol Obstet. 2011;283(suppl 1):S35–S37.
- Desai BR, Patted SS, Pujar YV, Sheriqar BY, Das SR, Ruge JC. A novel technique of selective uterine devascularization before myomectomy at the time of cesarean section: a pilot study. Fertil Steril. 2010;94(1):362–364.
- Sapmaz E, Celik H, Altungul A. Bilateral ascending uterine artery ligation vs. tourniquet use for hemostasis in cesarean myomectomy. A comparison. J Reprod Med. 2003;48(12):950–954.
- Sparic R, Malvasi A, Kadija S, Babovic I, Nejkovic L, Tinelli A. Cesarean myomectomy trends and controveries: an appraisal [published online ahead of print July 17, 2016]. J Matern Fetal Neonatal Med. doi:10.1080/14767058.2016.1205024.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing cesarean section. J Obstet Gynaecol Res. 2010;36(2):284–290.
- Kato S, Tanabe A, Kanki K, et al. Local injection of vasopressin reduces the blood loss during cesarean section in placenta previa. J Obstet Gynaecol Res. 2014;40(5):1249–1256.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97:867–872.
- Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Brit J Obstet Gynaecol. 1994;101:435–437.
- Assaf A. Adhesions after laparoscopic myomectomy effect of the technique used. Gynaecol Endosc. 1999;8(4):225–229.
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertil Steril. 2011;95(2):762–766.
- Sabol ED, Gibson JL, Bowes WA Jr. Vasopressin injection in cervical conization. A double-blind study. Obstet Gynecol. 1971;37(4):596–601.
- Martin-Hirsch PP, Keep SL, Bryant A. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;(6):CD001421.
- Schulz KE, Grimes DA, Christensen DD. Vasopressin reduces blood loss from second trimester dilatation and evacuation abortion. Lancet. 1985;2(8451):353–356.
- Akerlund M, Bossmar T, Brouard R, et al. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects in isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999;106(10):1047–1053.
- Akerlund M. Involvement of oxytocin and vasopressin in the pathophysiology of preterm labor and primary dysmenorrhea. Prog Brain Res. 2002;139:359–365.
- Helmer H, Hacki T, Schneeberger C, et al. Oxytocinand vasopressin 1a receptor gene expression in the cycling or pregnant human uterus. Am J Obstet Gynecol. 1998;179(6 pt 1):1572–1578.
- Wing DA, Goharkhay N, Felix JC, Rostamkhani M, Naidu YM, Kovacs BW. Expression of the oxytocin and V1a vasopressin receptors in human myometrium during differing physiological states and following misoprostol administration. Gynecol Obstet Invest. 2006;62(4):181–185.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014; (8):CD005355.
- Tinelli A, Malvasi A, Mynbaev OA, et al. The surgical outcome of intracapsular cesarean myomectomy. A match control study. J Matern Fetal Neonatal Med. 2014;27(1):66–71.
- Brown D, Fletcher HM, Myrie MO, Reid M. Caesarean myomectomy—a safe procedure. A retrospective case controlled study. J Obstet Gynaecol. 1999;19(2):139–141.
- Kaymak O, Ustunyrt E, Okyay RE, Kalyoncu S, Mollamahmutoglu L. Myomectomy during cesarean section. Int J Gynaecol Obstet. 2005;89(2):90–93.
- Park BJ, Kim YW. Safety of cesarean myomectomy. J Obstet Gynaecol Res. 2009;35(5):906–911.
- Kim YS, Choi SD, Bae DH. Risk factors for complications in patients undergoing myomectomy at the time of cesarean section. J Obstet Gynaecol Res. 2010;36(3):550–554.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynecol Obstet. 1999;67(3):189–190.
- Incebiyik A, Hilali NG, Camuzcuoglu A, Vural M, Camuzcuoglu H. Myomectomy during caesarean: a retrospective evaluation of 6 cases. Arch Gynecol Obstet. 2014;289(3):569–573.
- Cobellis L, Pecori E, Cobellis G. Hemostatic technique for myomectomy during cesarean section. Int J Gynaecol Obstet. 2002;79(3):261–262.
- Lee JH, Cho DH. Myomectomy using purse-string suture during cesarean section. Arch Gynecol Obstet. 2011;283(suppl 1):S35–S37.
- Desai BR, Patted SS, Pujar YV, Sheriqar BY, Das SR, Ruge JC. A novel technique of selective uterine devascularization before myomectomy at the time of cesarean section: a pilot study. Fertil Steril. 2010;94(1):362–364.
- Sapmaz E, Celik H, Altungul A. Bilateral ascending uterine artery ligation vs. tourniquet use for hemostasis in cesarean myomectomy. A comparison. J Reprod Med. 2003;48(12):950–954.
- Sparic R, Malvasi A, Kadija S, Babovic I, Nejkovic L, Tinelli A. Cesarean myomectomy trends and controveries: an appraisal [published online ahead of print July 17, 2016]. J Matern Fetal Neonatal Med. doi:10.1080/14767058.2016.1205024.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing cesarean section. J Obstet Gynaecol Res. 2010;36(2):284–290.
- Kato S, Tanabe A, Kanki K, et al. Local injection of vasopressin reduces the blood loss during cesarean section in placenta previa. J Obstet Gynaecol Res. 2014;40(5):1249–1256.
MIs in pregnancy have worse prognosis
ROME – It’s fortunate that pregnancy-associated acute MIs are infrequent, because the associated in-hospital mortality is markedly higher than in similar-age nonchildbearing women, Reza Masoomi, MD, said at the annual congress of the European Society of Cardiology.
One likely contributor to the disparity in outcome is that current management appears to feature underutilization of percutaneous intervention in women who experience pregnancy-associated MI, according to Dr. Masoomi of the University of Kansas in Kansas City.
He presented an analysis of the U.S. National Inpatient Sample database for the years 2008-2012. The NIS is a nationally representative sample of hospitalizations drawn from all of the country’s nonfederal acute-care hospitals.
A total of 55,315 hospitalizations with a discharge diagnosis of acute MI were recorded in women aged 15-54 years during the study years, of which 453 involved an ante- or postpartum MI. Extrapolating from those figures, nearly 262,000 women aged 15-54 years across the U.S. had an acute MI during the study years, of whom an estimated 2,153 experienced a pregnancy-associated MI.
In-hospital mortality among women with peripregnancy MI was 7.2%, significantly higher than the 5.2% rate in women who weren’t pregnant.
Women with peripregnancy MI had a significantly higher rate of ST-elevation MI (STEMI) than did nonpregnant women with MI in their reproductive years, by a margin of 35.3% to 32.8%. They were younger, too: an average age of 34.9 years, compared with 47.3 years in nonpregnant patients with an MI. Nearly two-thirds of women with peripregnancy MI were nonwhite, compared with 47.3% of the comparison group.
Regardless of whether women with peripregnancy MI had a STEMI or non-STEMI, they had significantly lower rates of diagnostic coronary angiography and percutaneous intervention. They were also far less likely to receive drug-eluting stents.
Diagnostic coronary angiography was performed in 59% of women with pregnancy-associated STEMI, compared with 73% of nonpregnant women with a STEMI. Only 34% of patients with peripregnancy STEMI underwent PCI, compared with 61% of nonpregnant women with a STEMI. Drug-eluting stents were implanted in 12% of peripregnancy STEMI patients and in 35% of nonpregnant patients. In contrast, 10% of patients with a pregnancy-related STEMI underwent coronary artery bypass surgery, compared with 5% of nonpregnant women with a STEMI.
The PCI rate among women with a peripregnancy non-STEMI was 7.8%, compared with 28.7% in nonpregnant women with a non-STEMI. However, CABG was utilized less frequently in the peripregnancy non-STEMI group, by a margin of 4.4% to 5.9%.
Dr. Masoomi reported having no financial conflicts regarding his study.
ROME – It’s fortunate that pregnancy-associated acute MIs are infrequent, because the associated in-hospital mortality is markedly higher than in similar-age nonchildbearing women, Reza Masoomi, MD, said at the annual congress of the European Society of Cardiology.
One likely contributor to the disparity in outcome is that current management appears to feature underutilization of percutaneous intervention in women who experience pregnancy-associated MI, according to Dr. Masoomi of the University of Kansas in Kansas City.
He presented an analysis of the U.S. National Inpatient Sample database for the years 2008-2012. The NIS is a nationally representative sample of hospitalizations drawn from all of the country’s nonfederal acute-care hospitals.
A total of 55,315 hospitalizations with a discharge diagnosis of acute MI were recorded in women aged 15-54 years during the study years, of which 453 involved an ante- or postpartum MI. Extrapolating from those figures, nearly 262,000 women aged 15-54 years across the U.S. had an acute MI during the study years, of whom an estimated 2,153 experienced a pregnancy-associated MI.
In-hospital mortality among women with peripregnancy MI was 7.2%, significantly higher than the 5.2% rate in women who weren’t pregnant.
Women with peripregnancy MI had a significantly higher rate of ST-elevation MI (STEMI) than did nonpregnant women with MI in their reproductive years, by a margin of 35.3% to 32.8%. They were younger, too: an average age of 34.9 years, compared with 47.3 years in nonpregnant patients with an MI. Nearly two-thirds of women with peripregnancy MI were nonwhite, compared with 47.3% of the comparison group.
Regardless of whether women with peripregnancy MI had a STEMI or non-STEMI, they had significantly lower rates of diagnostic coronary angiography and percutaneous intervention. They were also far less likely to receive drug-eluting stents.
Diagnostic coronary angiography was performed in 59% of women with pregnancy-associated STEMI, compared with 73% of nonpregnant women with a STEMI. Only 34% of patients with peripregnancy STEMI underwent PCI, compared with 61% of nonpregnant women with a STEMI. Drug-eluting stents were implanted in 12% of peripregnancy STEMI patients and in 35% of nonpregnant patients. In contrast, 10% of patients with a pregnancy-related STEMI underwent coronary artery bypass surgery, compared with 5% of nonpregnant women with a STEMI.
The PCI rate among women with a peripregnancy non-STEMI was 7.8%, compared with 28.7% in nonpregnant women with a non-STEMI. However, CABG was utilized less frequently in the peripregnancy non-STEMI group, by a margin of 4.4% to 5.9%.
Dr. Masoomi reported having no financial conflicts regarding his study.
ROME – It’s fortunate that pregnancy-associated acute MIs are infrequent, because the associated in-hospital mortality is markedly higher than in similar-age nonchildbearing women, Reza Masoomi, MD, said at the annual congress of the European Society of Cardiology.
One likely contributor to the disparity in outcome is that current management appears to feature underutilization of percutaneous intervention in women who experience pregnancy-associated MI, according to Dr. Masoomi of the University of Kansas in Kansas City.
He presented an analysis of the U.S. National Inpatient Sample database for the years 2008-2012. The NIS is a nationally representative sample of hospitalizations drawn from all of the country’s nonfederal acute-care hospitals.
A total of 55,315 hospitalizations with a discharge diagnosis of acute MI were recorded in women aged 15-54 years during the study years, of which 453 involved an ante- or postpartum MI. Extrapolating from those figures, nearly 262,000 women aged 15-54 years across the U.S. had an acute MI during the study years, of whom an estimated 2,153 experienced a pregnancy-associated MI.
In-hospital mortality among women with peripregnancy MI was 7.2%, significantly higher than the 5.2% rate in women who weren’t pregnant.
Women with peripregnancy MI had a significantly higher rate of ST-elevation MI (STEMI) than did nonpregnant women with MI in their reproductive years, by a margin of 35.3% to 32.8%. They were younger, too: an average age of 34.9 years, compared with 47.3 years in nonpregnant patients with an MI. Nearly two-thirds of women with peripregnancy MI were nonwhite, compared with 47.3% of the comparison group.
Regardless of whether women with peripregnancy MI had a STEMI or non-STEMI, they had significantly lower rates of diagnostic coronary angiography and percutaneous intervention. They were also far less likely to receive drug-eluting stents.
Diagnostic coronary angiography was performed in 59% of women with pregnancy-associated STEMI, compared with 73% of nonpregnant women with a STEMI. Only 34% of patients with peripregnancy STEMI underwent PCI, compared with 61% of nonpregnant women with a STEMI. Drug-eluting stents were implanted in 12% of peripregnancy STEMI patients and in 35% of nonpregnant patients. In contrast, 10% of patients with a pregnancy-related STEMI underwent coronary artery bypass surgery, compared with 5% of nonpregnant women with a STEMI.
The PCI rate among women with a peripregnancy non-STEMI was 7.8%, compared with 28.7% in nonpregnant women with a non-STEMI. However, CABG was utilized less frequently in the peripregnancy non-STEMI group, by a margin of 4.4% to 5.9%.
Dr. Masoomi reported having no financial conflicts regarding his study.
Key clinical point:
Major finding: In-hospital mortality among U.S. women with peripregnancy MI was 7.2% during 2008-2012, significantly higher than the 5.2% rate in women of reproductive age who weren’t pregnant.
Data source: This analysis of data from the U.S. National Inpatient Sample concluded that of an estimated 261,806 U.S. women aged 15-54 years who had an acute MI during 2008-2012, a total of 2,153 of them had an ante- or postpartum-associated MI.
Disclosures: The study presenter reported having no financial conflicts of interest.
United States nears 3,000 Zika-infected pregnancies
There were 54 new cases of pregnant women with laboratory evidence of Zika virus in the 50 states and the District of Columbia reported during the week ending Oct. 20 – the largest weekly increase in a month, according to the Centers for Disease Control and Prevention.
The number of cases reported in the U.S. territories, 100, was lower for the second week in a row, however, so the U.S. total for the week was a fairly average 154. The total number of pregnant women with laboratory evidence of Zika virus infection is now 2,980 for the year: 953 in the states/D.C. and 2,027 in the territories, the CDC reported.
Among all Americans, the number of cases for 2015-2016 is now up to 32,814, with 1,396 new cases reported for the week ending Oct. 26: 75 in the states/D.C. and 1,321 in the territories. Almost all (98%) of the territorial cases have occurred in Puerto Rico, which continues to retroactively report cases, the CDC Arboviral Disease Branch noted.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 54 new cases of pregnant women with laboratory evidence of Zika virus in the 50 states and the District of Columbia reported during the week ending Oct. 20 – the largest weekly increase in a month, according to the Centers for Disease Control and Prevention.
The number of cases reported in the U.S. territories, 100, was lower for the second week in a row, however, so the U.S. total for the week was a fairly average 154. The total number of pregnant women with laboratory evidence of Zika virus infection is now 2,980 for the year: 953 in the states/D.C. and 2,027 in the territories, the CDC reported.
Among all Americans, the number of cases for 2015-2016 is now up to 32,814, with 1,396 new cases reported for the week ending Oct. 26: 75 in the states/D.C. and 1,321 in the territories. Almost all (98%) of the territorial cases have occurred in Puerto Rico, which continues to retroactively report cases, the CDC Arboviral Disease Branch noted.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 54 new cases of pregnant women with laboratory evidence of Zika virus in the 50 states and the District of Columbia reported during the week ending Oct. 20 – the largest weekly increase in a month, according to the Centers for Disease Control and Prevention.
The number of cases reported in the U.S. territories, 100, was lower for the second week in a row, however, so the U.S. total for the week was a fairly average 154. The total number of pregnant women with laboratory evidence of Zika virus infection is now 2,980 for the year: 953 in the states/D.C. and 2,027 in the territories, the CDC reported.
Among all Americans, the number of cases for 2015-2016 is now up to 32,814, with 1,396 new cases reported for the week ending Oct. 26: 75 in the states/D.C. and 1,321 in the territories. Almost all (98%) of the territorial cases have occurred in Puerto Rico, which continues to retroactively report cases, the CDC Arboviral Disease Branch noted.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
USPSTF gives breastfeeding support a ‘B’ grade
The U.S. Preventive Services Task Force (USPSTF) has issued a B-level recommendation for interventions given during pregnancy and after birth to support breastfeeding.
The Task Force cites “adequate” evidence that breastfeeding provides substantial health benefits for children and moderate health benefits for women. While they found evidence to support individual-level interventions, such as education and psychosocial support, system-level interventions were not shown to be effective. The recommendation appears online in JAMA (2016 Oct 25;316[16]:1688-93).
The recommendation updates a previous one issued in 2008, in which the USPSTF also recommended breastfeeding support interventions with a grade of B. The new recommendation is based on a review of 43 studies of individual-level primary care interventions in support of breastfeeding, and nine system-level interventions. The authors evaluated the available evidence on breastfeeding initiation, duration, and exclusivity, as well as breastfeeding’s effects on child and maternal health outcomes. They determined that support from a professional lactation consultant or a peer group was effective in producing any or exclusive breastfeeding, while systemwide interventions, such as the World Health Organization’s Baby-Friendly Hospital Initiative offered inconsistent benefits. The evidence review was also published in JAMA (2016;316[16]:1694-1705).
The Initiative’s “Ten Steps to Successful Breastfeeding” program, in particular, presents a potential risk, they noted. The program recommends counseling parents to avoid use of pacifiers in the newborn period to support breastfeeding, but evidence is growing that avoiding pacifiers is not associated with any breastfeeding outcomes and pacifier use may be protective against sudden infant death syndrome.
“U.S. institutions will need to disengage from the Ten Steps if they conclude that the scientific evidence that conflicts with them is valid,” Dr. Flaherman and Dr. Von Kohorn wrote.
The practice of recommending that mothers do not supplement breast-milk feedings with formula is also of concern, based on mixed evidence. Since not all mothers produce adequate milk supplies during the first week postpartum, not supplementing with formula could run the risk that the infant suffers dehydration, hyperbilirubinemia, or other complications. With up to 2% of all newborns in the United States requiring hospital readmission – the risk is doubled for breastfed infants – Dr. Flaherman and Dr. Von Kohorn suggest that strict adherence to a “breast-milk only” policy has the potential to be harmful, especially given that current evidence doesn’t show that exclusive breastfeeding in the newborn period improves breastfeeding duration.
“Individual clinical judgment may be more valuable than a single rigid rule for exclusive breastfeeding for the first 6 months,” Dr. Flaherman and Dr. Von Kohorn wrote.
Based on the evidence, the USPSTF advised clinicians that they can support women before and after childbirth by promoting the benefits of breastfeeding during monthly visits, providing practical guidance on how to breastfeed, and offering psychosocial support.
“Although there is moderate certainty that breastfeeding is of moderate net benefit to women and their infants and children, not all women choose to or are able to breastfeed. Clinicians should, as with any preventive service, respect the autonomy of women and their families to make decisions that fit their specific situation, values, and preferences,” the USPSTF members wrote.
The USPSTF members reported receiving travel reimbursement and honorarium for participating in USPSTF meetings. Dr. Flaherman and Dr. Von Kohorn reported having no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
The key to successful office-based health promotion and prevention programs is to prioritize evidence-based practices and recommendations that parents already have a predilection to follow. That is, of the myriad of topics one might discuss, why not start with the ones that caregivers are most interested in. Breastfeeding is surely one of those.
The USPSTF estimates the number needed to treat when offering breastfeeding support is 30. That is, if 30 women are offered it, 1 additional woman will breastfeed for 6 full months. This compares favorably with the NNT for antibiotics for otitis media for fever and pain reduction at 48 hours in children older than 2 years, (NNT, 20) especially when one considers the comparable benefits of each. And the breastfeeding NNT could be reduced even further if there were structural changes to workplaces and communities that helped support breastfeeding mothers.
Dimitri A. Christakis, MD, MPH , of Seattle Children’s Research Institute, is an associate editor for JAMA Pediatrics. His comments are adapted from an editorial published in JAMA Pediatrics (2016 Oct 25. doi: 10.1001/jamapediatrics.2016.3390). He reported having no relevant financial disclosures.
The key to successful office-based health promotion and prevention programs is to prioritize evidence-based practices and recommendations that parents already have a predilection to follow. That is, of the myriad of topics one might discuss, why not start with the ones that caregivers are most interested in. Breastfeeding is surely one of those.
The USPSTF estimates the number needed to treat when offering breastfeeding support is 30. That is, if 30 women are offered it, 1 additional woman will breastfeed for 6 full months. This compares favorably with the NNT for antibiotics for otitis media for fever and pain reduction at 48 hours in children older than 2 years, (NNT, 20) especially when one considers the comparable benefits of each. And the breastfeeding NNT could be reduced even further if there were structural changes to workplaces and communities that helped support breastfeeding mothers.
Dimitri A. Christakis, MD, MPH , of Seattle Children’s Research Institute, is an associate editor for JAMA Pediatrics. His comments are adapted from an editorial published in JAMA Pediatrics (2016 Oct 25. doi: 10.1001/jamapediatrics.2016.3390). He reported having no relevant financial disclosures.
The key to successful office-based health promotion and prevention programs is to prioritize evidence-based practices and recommendations that parents already have a predilection to follow. That is, of the myriad of topics one might discuss, why not start with the ones that caregivers are most interested in. Breastfeeding is surely one of those.
The USPSTF estimates the number needed to treat when offering breastfeeding support is 30. That is, if 30 women are offered it, 1 additional woman will breastfeed for 6 full months. This compares favorably with the NNT for antibiotics for otitis media for fever and pain reduction at 48 hours in children older than 2 years, (NNT, 20) especially when one considers the comparable benefits of each. And the breastfeeding NNT could be reduced even further if there were structural changes to workplaces and communities that helped support breastfeeding mothers.
Dimitri A. Christakis, MD, MPH , of Seattle Children’s Research Institute, is an associate editor for JAMA Pediatrics. His comments are adapted from an editorial published in JAMA Pediatrics (2016 Oct 25. doi: 10.1001/jamapediatrics.2016.3390). He reported having no relevant financial disclosures.
The U.S. Preventive Services Task Force (USPSTF) has issued a B-level recommendation for interventions given during pregnancy and after birth to support breastfeeding.
The Task Force cites “adequate” evidence that breastfeeding provides substantial health benefits for children and moderate health benefits for women. While they found evidence to support individual-level interventions, such as education and psychosocial support, system-level interventions were not shown to be effective. The recommendation appears online in JAMA (2016 Oct 25;316[16]:1688-93).
The recommendation updates a previous one issued in 2008, in which the USPSTF also recommended breastfeeding support interventions with a grade of B. The new recommendation is based on a review of 43 studies of individual-level primary care interventions in support of breastfeeding, and nine system-level interventions. The authors evaluated the available evidence on breastfeeding initiation, duration, and exclusivity, as well as breastfeeding’s effects on child and maternal health outcomes. They determined that support from a professional lactation consultant or a peer group was effective in producing any or exclusive breastfeeding, while systemwide interventions, such as the World Health Organization’s Baby-Friendly Hospital Initiative offered inconsistent benefits. The evidence review was also published in JAMA (2016;316[16]:1694-1705).
The Initiative’s “Ten Steps to Successful Breastfeeding” program, in particular, presents a potential risk, they noted. The program recommends counseling parents to avoid use of pacifiers in the newborn period to support breastfeeding, but evidence is growing that avoiding pacifiers is not associated with any breastfeeding outcomes and pacifier use may be protective against sudden infant death syndrome.
“U.S. institutions will need to disengage from the Ten Steps if they conclude that the scientific evidence that conflicts with them is valid,” Dr. Flaherman and Dr. Von Kohorn wrote.
The practice of recommending that mothers do not supplement breast-milk feedings with formula is also of concern, based on mixed evidence. Since not all mothers produce adequate milk supplies during the first week postpartum, not supplementing with formula could run the risk that the infant suffers dehydration, hyperbilirubinemia, or other complications. With up to 2% of all newborns in the United States requiring hospital readmission – the risk is doubled for breastfed infants – Dr. Flaherman and Dr. Von Kohorn suggest that strict adherence to a “breast-milk only” policy has the potential to be harmful, especially given that current evidence doesn’t show that exclusive breastfeeding in the newborn period improves breastfeeding duration.
“Individual clinical judgment may be more valuable than a single rigid rule for exclusive breastfeeding for the first 6 months,” Dr. Flaherman and Dr. Von Kohorn wrote.
Based on the evidence, the USPSTF advised clinicians that they can support women before and after childbirth by promoting the benefits of breastfeeding during monthly visits, providing practical guidance on how to breastfeed, and offering psychosocial support.
“Although there is moderate certainty that breastfeeding is of moderate net benefit to women and their infants and children, not all women choose to or are able to breastfeed. Clinicians should, as with any preventive service, respect the autonomy of women and their families to make decisions that fit their specific situation, values, and preferences,” the USPSTF members wrote.
The USPSTF members reported receiving travel reimbursement and honorarium for participating in USPSTF meetings. Dr. Flaherman and Dr. Von Kohorn reported having no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
The U.S. Preventive Services Task Force (USPSTF) has issued a B-level recommendation for interventions given during pregnancy and after birth to support breastfeeding.
The Task Force cites “adequate” evidence that breastfeeding provides substantial health benefits for children and moderate health benefits for women. While they found evidence to support individual-level interventions, such as education and psychosocial support, system-level interventions were not shown to be effective. The recommendation appears online in JAMA (2016 Oct 25;316[16]:1688-93).
The recommendation updates a previous one issued in 2008, in which the USPSTF also recommended breastfeeding support interventions with a grade of B. The new recommendation is based on a review of 43 studies of individual-level primary care interventions in support of breastfeeding, and nine system-level interventions. The authors evaluated the available evidence on breastfeeding initiation, duration, and exclusivity, as well as breastfeeding’s effects on child and maternal health outcomes. They determined that support from a professional lactation consultant or a peer group was effective in producing any or exclusive breastfeeding, while systemwide interventions, such as the World Health Organization’s Baby-Friendly Hospital Initiative offered inconsistent benefits. The evidence review was also published in JAMA (2016;316[16]:1694-1705).
The Initiative’s “Ten Steps to Successful Breastfeeding” program, in particular, presents a potential risk, they noted. The program recommends counseling parents to avoid use of pacifiers in the newborn period to support breastfeeding, but evidence is growing that avoiding pacifiers is not associated with any breastfeeding outcomes and pacifier use may be protective against sudden infant death syndrome.
“U.S. institutions will need to disengage from the Ten Steps if they conclude that the scientific evidence that conflicts with them is valid,” Dr. Flaherman and Dr. Von Kohorn wrote.
The practice of recommending that mothers do not supplement breast-milk feedings with formula is also of concern, based on mixed evidence. Since not all mothers produce adequate milk supplies during the first week postpartum, not supplementing with formula could run the risk that the infant suffers dehydration, hyperbilirubinemia, or other complications. With up to 2% of all newborns in the United States requiring hospital readmission – the risk is doubled for breastfed infants – Dr. Flaherman and Dr. Von Kohorn suggest that strict adherence to a “breast-milk only” policy has the potential to be harmful, especially given that current evidence doesn’t show that exclusive breastfeeding in the newborn period improves breastfeeding duration.
“Individual clinical judgment may be more valuable than a single rigid rule for exclusive breastfeeding for the first 6 months,” Dr. Flaherman and Dr. Von Kohorn wrote.
Based on the evidence, the USPSTF advised clinicians that they can support women before and after childbirth by promoting the benefits of breastfeeding during monthly visits, providing practical guidance on how to breastfeed, and offering psychosocial support.
“Although there is moderate certainty that breastfeeding is of moderate net benefit to women and their infants and children, not all women choose to or are able to breastfeed. Clinicians should, as with any preventive service, respect the autonomy of women and their families to make decisions that fit their specific situation, values, and preferences,” the USPSTF members wrote.
The USPSTF members reported receiving travel reimbursement and honorarium for participating in USPSTF meetings. Dr. Flaherman and Dr. Von Kohorn reported having no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
Zika increase slows slightly in pregnant women
The total number of pregnant women in the United States and its territories with laboratory evidence of Zika infection rose by 142 for the week ending Oct. 13 – the smallest increase since early September, according to the Centers for Disease Control and Prevention.
There were 121 new cases of Zika virus infection in pregnant women reported in the U.S. territories and 21 new cases in the states and the District of Columbia, bringing the corresponding totals for the year to 1,927 cases in the territories and 899 in the states/D.C. – a total of 2,826 pregnant women with Zika in the United States, the CDC reported Oct. 20.
The Zika caseload among all Americans was 31,418 as of Oct. 19. An additional 80 cases reported from Oct. 14 through Oct. 19 brought the state/D.C. total to 4,016, and 1,447 new cases for the week brings the territorial total to 27,402 for 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The total number of pregnant women in the United States and its territories with laboratory evidence of Zika infection rose by 142 for the week ending Oct. 13 – the smallest increase since early September, according to the Centers for Disease Control and Prevention.
There were 121 new cases of Zika virus infection in pregnant women reported in the U.S. territories and 21 new cases in the states and the District of Columbia, bringing the corresponding totals for the year to 1,927 cases in the territories and 899 in the states/D.C. – a total of 2,826 pregnant women with Zika in the United States, the CDC reported Oct. 20.
The Zika caseload among all Americans was 31,418 as of Oct. 19. An additional 80 cases reported from Oct. 14 through Oct. 19 brought the state/D.C. total to 4,016, and 1,447 new cases for the week brings the territorial total to 27,402 for 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The total number of pregnant women in the United States and its territories with laboratory evidence of Zika infection rose by 142 for the week ending Oct. 13 – the smallest increase since early September, according to the Centers for Disease Control and Prevention.
There were 121 new cases of Zika virus infection in pregnant women reported in the U.S. territories and 21 new cases in the states and the District of Columbia, bringing the corresponding totals for the year to 1,927 cases in the territories and 899 in the states/D.C. – a total of 2,826 pregnant women with Zika in the United States, the CDC reported Oct. 20.
The Zika caseload among all Americans was 31,418 as of Oct. 19. An additional 80 cases reported from Oct. 14 through Oct. 19 brought the state/D.C. total to 4,016, and 1,447 new cases for the week brings the territorial total to 27,402 for 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.