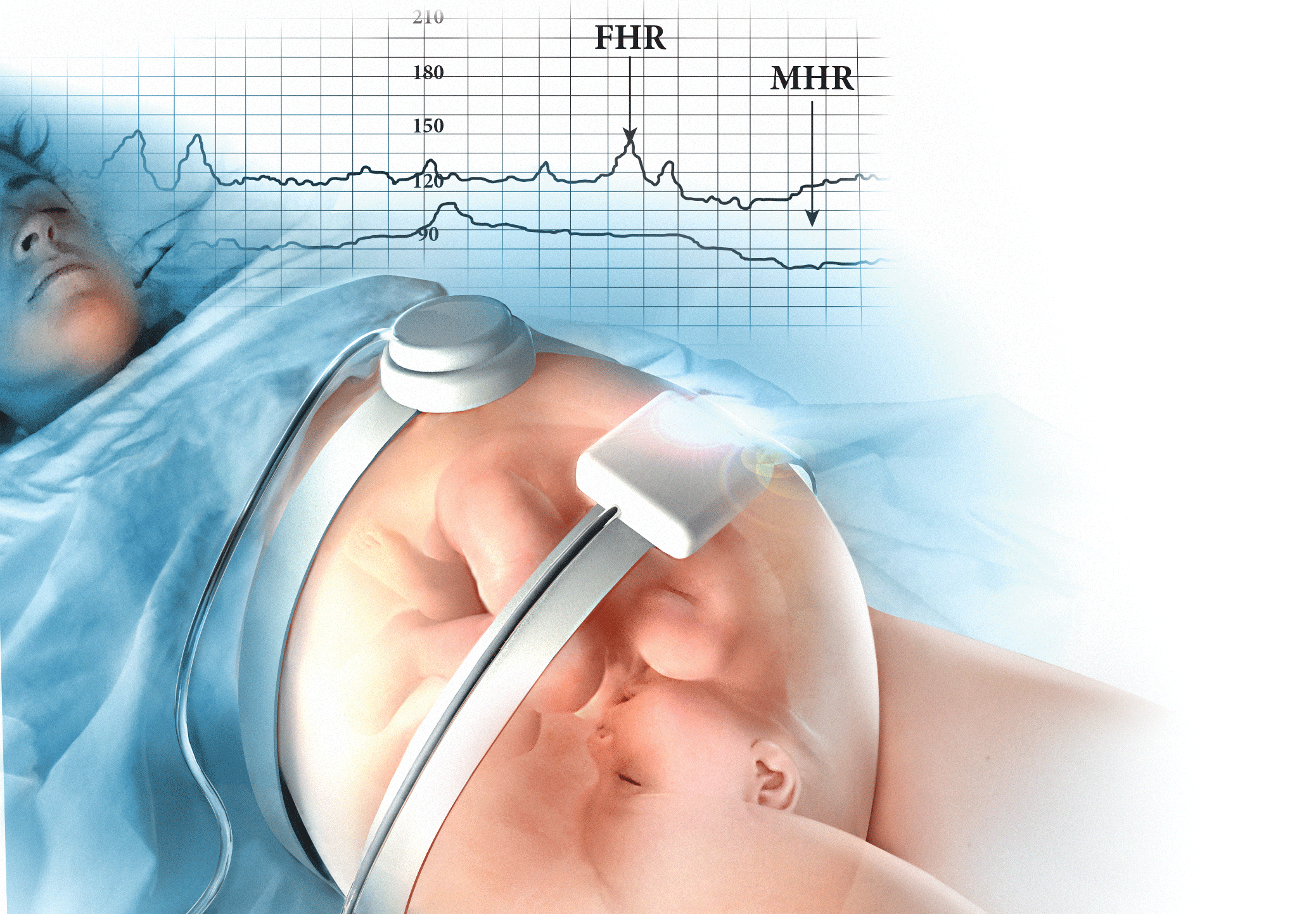
User login
CDC: Zika virus urine testing preferable to serum
New data showing that Zika virus can be found at higher levels or for longer duration in urine than serum, has prompted the Centers for Disease Control and Prevention to update its interim diagnostic testing guidance for the virus in public health laboratories.
The CDC now recommends that Zika virus real-time reverse transcription–polymerase chain reaction (rRT-PCR) be performed on urine collected less than 14 days after the onset of symptoms in patients with suspected Zika virus disease. The rRT-PCR is the preferred test for Zika infection because it can be performed rapidly and is highly specific, the CDC notes, and currently, the CDC Trioplex rRT-PCR assay is the only diagnostic tool authorized by the Food and Drug Administration for Zika virus testing of urine (MMWR. 2016 May 10. doi:10.15585/mmwr.mm6518e1).
In most patients, Zika virus RNA is unlikely to be detected in serum after the first week of illness, the CDC said, but adaptations of previously published diagnostic methods suggest that Zika virus RNA can be detected in urine for at least 2 weeks after onset of symptoms. However, the CDC affirmed that Zika virus rRT-PCR testing of urine should be performed in conjunction with serum testing if using specimens collected less than 7 days after symptom onset, and a positive result in either specimen type provides evidence of Zika virus infection.
The CDC added that, because viremia decreases over time and dates of illness onset may not be accurately reported, a negative rRT-PCR does not exclude Zika virus infection, and IgM antibody testing should be performed. The agency also said other laboratory-developed tests will need in-house validations to adequately characterize the performance of the assay and meet Clinical Laboratory Improvement Amendments requirements.
Read the full report here.
On Twitter @richpizzi
New data showing that Zika virus can be found at higher levels or for longer duration in urine than serum, has prompted the Centers for Disease Control and Prevention to update its interim diagnostic testing guidance for the virus in public health laboratories.
The CDC now recommends that Zika virus real-time reverse transcription–polymerase chain reaction (rRT-PCR) be performed on urine collected less than 14 days after the onset of symptoms in patients with suspected Zika virus disease. The rRT-PCR is the preferred test for Zika infection because it can be performed rapidly and is highly specific, the CDC notes, and currently, the CDC Trioplex rRT-PCR assay is the only diagnostic tool authorized by the Food and Drug Administration for Zika virus testing of urine (MMWR. 2016 May 10. doi:10.15585/mmwr.mm6518e1).
In most patients, Zika virus RNA is unlikely to be detected in serum after the first week of illness, the CDC said, but adaptations of previously published diagnostic methods suggest that Zika virus RNA can be detected in urine for at least 2 weeks after onset of symptoms. However, the CDC affirmed that Zika virus rRT-PCR testing of urine should be performed in conjunction with serum testing if using specimens collected less than 7 days after symptom onset, and a positive result in either specimen type provides evidence of Zika virus infection.
The CDC added that, because viremia decreases over time and dates of illness onset may not be accurately reported, a negative rRT-PCR does not exclude Zika virus infection, and IgM antibody testing should be performed. The agency also said other laboratory-developed tests will need in-house validations to adequately characterize the performance of the assay and meet Clinical Laboratory Improvement Amendments requirements.
Read the full report here.
On Twitter @richpizzi
New data showing that Zika virus can be found at higher levels or for longer duration in urine than serum, has prompted the Centers for Disease Control and Prevention to update its interim diagnostic testing guidance for the virus in public health laboratories.
The CDC now recommends that Zika virus real-time reverse transcription–polymerase chain reaction (rRT-PCR) be performed on urine collected less than 14 days after the onset of symptoms in patients with suspected Zika virus disease. The rRT-PCR is the preferred test for Zika infection because it can be performed rapidly and is highly specific, the CDC notes, and currently, the CDC Trioplex rRT-PCR assay is the only diagnostic tool authorized by the Food and Drug Administration for Zika virus testing of urine (MMWR. 2016 May 10. doi:10.15585/mmwr.mm6518e1).
In most patients, Zika virus RNA is unlikely to be detected in serum after the first week of illness, the CDC said, but adaptations of previously published diagnostic methods suggest that Zika virus RNA can be detected in urine for at least 2 weeks after onset of symptoms. However, the CDC affirmed that Zika virus rRT-PCR testing of urine should be performed in conjunction with serum testing if using specimens collected less than 7 days after symptom onset, and a positive result in either specimen type provides evidence of Zika virus infection.
The CDC added that, because viremia decreases over time and dates of illness onset may not be accurately reported, a negative rRT-PCR does not exclude Zika virus infection, and IgM antibody testing should be performed. The agency also said other laboratory-developed tests will need in-house validations to adequately characterize the performance of the assay and meet Clinical Laboratory Improvement Amendments requirements.
Read the full report here.
On Twitter @richpizzi
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
USPSTF recommends daily folic acid supplements for women of childbearing age
All women who are capable of getting pregnant should take a daily supplement containing 400-800 micrograms of folic acid to prevent neural tube defects in early pregnancy, according to a draft recommendation from the U.S. Preventive Services Task Force.
The grade A draft recommendation, issued May 10, reaffirms the Task Force’s 2009 recommendation on folic acid supplementation in women of childbearing age.
The critical period for supplementation occurs 1 month before conception and continues through the first 2-3 months of pregnancy, according to the draft recommendation. Although folic acid is found naturally in many fruits and vegetables, and many cereals and breads are fortified with folic acid, most women still fall short of the daily recommended dose of 400 micrograms of folic acid.
In the evidence review, the USPSTF evaluated one randomized controlled trial, two cohort studies, and eight case-control studies for evidence of effectiveness of folic acid supplementation. The Task Force found no substantial new evidence on benefits and harms from folic acid supplementation to change its 2009 recommendation.
“The USPSTF concludes with high certainty that the net benefit of daily folic acid supplementation to prevent neural tube defects in the developing fetus is substantial for women who are planning or capable of pregnancy,” the statement noted.
The draft recommendation is open for public comment on the USPSTF website until June 6.
All women who are capable of getting pregnant should take a daily supplement containing 400-800 micrograms of folic acid to prevent neural tube defects in early pregnancy, according to a draft recommendation from the U.S. Preventive Services Task Force.
The grade A draft recommendation, issued May 10, reaffirms the Task Force’s 2009 recommendation on folic acid supplementation in women of childbearing age.
The critical period for supplementation occurs 1 month before conception and continues through the first 2-3 months of pregnancy, according to the draft recommendation. Although folic acid is found naturally in many fruits and vegetables, and many cereals and breads are fortified with folic acid, most women still fall short of the daily recommended dose of 400 micrograms of folic acid.
In the evidence review, the USPSTF evaluated one randomized controlled trial, two cohort studies, and eight case-control studies for evidence of effectiveness of folic acid supplementation. The Task Force found no substantial new evidence on benefits and harms from folic acid supplementation to change its 2009 recommendation.
“The USPSTF concludes with high certainty that the net benefit of daily folic acid supplementation to prevent neural tube defects in the developing fetus is substantial for women who are planning or capable of pregnancy,” the statement noted.
The draft recommendation is open for public comment on the USPSTF website until June 6.
All women who are capable of getting pregnant should take a daily supplement containing 400-800 micrograms of folic acid to prevent neural tube defects in early pregnancy, according to a draft recommendation from the U.S. Preventive Services Task Force.
The grade A draft recommendation, issued May 10, reaffirms the Task Force’s 2009 recommendation on folic acid supplementation in women of childbearing age.
The critical period for supplementation occurs 1 month before conception and continues through the first 2-3 months of pregnancy, according to the draft recommendation. Although folic acid is found naturally in many fruits and vegetables, and many cereals and breads are fortified with folic acid, most women still fall short of the daily recommended dose of 400 micrograms of folic acid.
In the evidence review, the USPSTF evaluated one randomized controlled trial, two cohort studies, and eight case-control studies for evidence of effectiveness of folic acid supplementation. The Task Force found no substantial new evidence on benefits and harms from folic acid supplementation to change its 2009 recommendation.
“The USPSTF concludes with high certainty that the net benefit of daily folic acid supplementation to prevent neural tube defects in the developing fetus is substantial for women who are planning or capable of pregnancy,” the statement noted.
The draft recommendation is open for public comment on the USPSTF website until June 6.
Combatting misperceptions in prenatal exposures
It’s clear that for pregnant women and the physicians who care for them, the risk of using medications in pregnancy is a significant issue. Unfortunately, sometimes the perception of that risk is much greater than the reality and drives behavior that can harm women and their babies.
Before the tragedy of thalidomide, the medical community held the general belief that drugs and chemicals do not cross the placenta, so there was no need to fear fetal malformations from medication use in pregnancy. In 1961, thalidomide became a formative event that changed everyone’s perception, with many people adopting the belief that every drug could be dangerous. In reality, though, very few medications prescribed today are known teratogens that cause malformations.
In recent years, an increasing number of drugs have been shown to be “safe.” The issue with the term safe is that there can always be more cases and more studies showing some very small risk that was previously unknown. But, in general, there are more reassuring studies in the literature than ones showing drugs to be dangerous in pregnancy.
The Bendectin example
In the highly charged medicolegal atmosphere in which we practice, physicians are afraid to be sued. If you remember that about 3% of babies are born with malformations just by chance, and that mothers will likely be taking some type of medication, there is always the possibility of a bad outcome that could cast blame on a drug.
In the 1970s, a lot of that litigation centered around the morning sickness drug Bendectin – originally formulated with doxylamine succinate, pyridoxine HCl, and dicyclomine HCl, and later reformulated without the dicyclomine. The drug was taken off the U.S. market by the manufacturer in 1983 because the company couldn’t afford the high cost of litigation and insurance, despite the fact that a panel convened by the Food and Drug Administration said there was no association between Bendectin and human birth defects.
It took nearly 20 years before the FDA declared that the drug had been withdrawn from the market for reasons unrelated to safety and effectiveness. In the meantime, American women remained without an FDA-approved medication to treat morning sickness, and there was a more than twofold increase in hospitalization rates for pregnant women with hyperemesis gravidarum (Can J Public Health. 1995 Jan-Feb;86[1]:66-70). The lesson here is that perceptions in the absence of evidence can lead to grave outcomes.
Exaggeration of risk
Over the years, my colleagues and I have studied how pregnant women perceive drug risk by simply asking them to estimate the risk to their baby from the medication they are currently taking. What we discovered was that women exposed to nonteratogenic drugs consider themselves at a risk of about 25% for having a child with a major malformation. In reality, the risk is between 1% and 3% and has nothing to do with the drug itself. It became clear that there is a huge perception of risk when women are exposed to drugs that should not increase that risk (Am J Obstet Gynecol. 1989 May;160[5 Pt 1]:1190-4).
The same study also showed that many of the women who gave exaggerated risk assessments said they would consider termination of the pregnancy. Even after hearing the drug is safe, some women were still considering termination.
Sadly, women terminating a pregnancy because of a perceived risk for malformation is not unique to this study. In the 1980s, following the explosion at Chernobyl in the Ukraine, women in Athens were told that they had a high risk for malformation in their children because of radiation exposure. Statistics show that during that month, nearly a quarter of early pregnancies in Athens were terminated (Br Med J [Clin Res Ed] 1987;295:1100).
We have further found that women exposed to radiation for diagnostic purposes estimate a high risk of malformation. This type of estimate is likely influenced by the effects of radiation at Hiroshima and Nagasaki, but there is no comparison between the extremely high amounts of radiation in those incidents, compared with the very low amounts in diagnostic tests. Still, we found that women again considered termination because of their perceived risk from radiation.
Social economics are also part of this. Women who are single mothers are more likely to terminate a pregnancy, or consider termination, after exposure to a drug in pregnancy. Women with psychiatric conditions have a similar tendency. On the other hand, women with chronic diseases – who may be used to the effects of a certain medications – are less likely to suggest termination because of perceived risk.
Communicating risk
These are important concepts to consider in the context of the emerging threat of Zika virus and the news from the Centers for Disease Control and Prevention that it is a definitive cause of microcephaly and other severe fetal malformations. While there is a real risk for pregnant women, both through mosquitoes and sexual contact, women are likely to perceive the highest level of risk. In South America, where therapeutic abortion is often not an option, accurate risk communication is critical.
When medications are prescribed during pregnancy, the first step is determining that a drug is truly needed, often in consultation with a specialist. Once that determination is made, it’s key to ensure that women and their families are familiar with the known risk or the lack of risk based on the best available data. There are resources for physicians to help understand and communicate about drug risks in pregnancy, including information from the Organization of Teratology Information Specialists. It’s also important to note that in every pregnancy, there is a 1%-3% risk of major malformations, even if the drug itself is safe. And it can’t hurt to think defensively and document that conversation and that the patient appears to have understood the concept of risk.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having been a paid consultant for Novartis and for Duchesnay, which makes Diclegis to treat nausea and vomiting in pregnancy.
It’s clear that for pregnant women and the physicians who care for them, the risk of using medications in pregnancy is a significant issue. Unfortunately, sometimes the perception of that risk is much greater than the reality and drives behavior that can harm women and their babies.
Before the tragedy of thalidomide, the medical community held the general belief that drugs and chemicals do not cross the placenta, so there was no need to fear fetal malformations from medication use in pregnancy. In 1961, thalidomide became a formative event that changed everyone’s perception, with many people adopting the belief that every drug could be dangerous. In reality, though, very few medications prescribed today are known teratogens that cause malformations.
In recent years, an increasing number of drugs have been shown to be “safe.” The issue with the term safe is that there can always be more cases and more studies showing some very small risk that was previously unknown. But, in general, there are more reassuring studies in the literature than ones showing drugs to be dangerous in pregnancy.
The Bendectin example
In the highly charged medicolegal atmosphere in which we practice, physicians are afraid to be sued. If you remember that about 3% of babies are born with malformations just by chance, and that mothers will likely be taking some type of medication, there is always the possibility of a bad outcome that could cast blame on a drug.
In the 1970s, a lot of that litigation centered around the morning sickness drug Bendectin – originally formulated with doxylamine succinate, pyridoxine HCl, and dicyclomine HCl, and later reformulated without the dicyclomine. The drug was taken off the U.S. market by the manufacturer in 1983 because the company couldn’t afford the high cost of litigation and insurance, despite the fact that a panel convened by the Food and Drug Administration said there was no association between Bendectin and human birth defects.
It took nearly 20 years before the FDA declared that the drug had been withdrawn from the market for reasons unrelated to safety and effectiveness. In the meantime, American women remained without an FDA-approved medication to treat morning sickness, and there was a more than twofold increase in hospitalization rates for pregnant women with hyperemesis gravidarum (Can J Public Health. 1995 Jan-Feb;86[1]:66-70). The lesson here is that perceptions in the absence of evidence can lead to grave outcomes.
Exaggeration of risk
Over the years, my colleagues and I have studied how pregnant women perceive drug risk by simply asking them to estimate the risk to their baby from the medication they are currently taking. What we discovered was that women exposed to nonteratogenic drugs consider themselves at a risk of about 25% for having a child with a major malformation. In reality, the risk is between 1% and 3% and has nothing to do with the drug itself. It became clear that there is a huge perception of risk when women are exposed to drugs that should not increase that risk (Am J Obstet Gynecol. 1989 May;160[5 Pt 1]:1190-4).
The same study also showed that many of the women who gave exaggerated risk assessments said they would consider termination of the pregnancy. Even after hearing the drug is safe, some women were still considering termination.
Sadly, women terminating a pregnancy because of a perceived risk for malformation is not unique to this study. In the 1980s, following the explosion at Chernobyl in the Ukraine, women in Athens were told that they had a high risk for malformation in their children because of radiation exposure. Statistics show that during that month, nearly a quarter of early pregnancies in Athens were terminated (Br Med J [Clin Res Ed] 1987;295:1100).
We have further found that women exposed to radiation for diagnostic purposes estimate a high risk of malformation. This type of estimate is likely influenced by the effects of radiation at Hiroshima and Nagasaki, but there is no comparison between the extremely high amounts of radiation in those incidents, compared with the very low amounts in diagnostic tests. Still, we found that women again considered termination because of their perceived risk from radiation.
Social economics are also part of this. Women who are single mothers are more likely to terminate a pregnancy, or consider termination, after exposure to a drug in pregnancy. Women with psychiatric conditions have a similar tendency. On the other hand, women with chronic diseases – who may be used to the effects of a certain medications – are less likely to suggest termination because of perceived risk.
Communicating risk
These are important concepts to consider in the context of the emerging threat of Zika virus and the news from the Centers for Disease Control and Prevention that it is a definitive cause of microcephaly and other severe fetal malformations. While there is a real risk for pregnant women, both through mosquitoes and sexual contact, women are likely to perceive the highest level of risk. In South America, where therapeutic abortion is often not an option, accurate risk communication is critical.
When medications are prescribed during pregnancy, the first step is determining that a drug is truly needed, often in consultation with a specialist. Once that determination is made, it’s key to ensure that women and their families are familiar with the known risk or the lack of risk based on the best available data. There are resources for physicians to help understand and communicate about drug risks in pregnancy, including information from the Organization of Teratology Information Specialists. It’s also important to note that in every pregnancy, there is a 1%-3% risk of major malformations, even if the drug itself is safe. And it can’t hurt to think defensively and document that conversation and that the patient appears to have understood the concept of risk.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having been a paid consultant for Novartis and for Duchesnay, which makes Diclegis to treat nausea and vomiting in pregnancy.
It’s clear that for pregnant women and the physicians who care for them, the risk of using medications in pregnancy is a significant issue. Unfortunately, sometimes the perception of that risk is much greater than the reality and drives behavior that can harm women and their babies.
Before the tragedy of thalidomide, the medical community held the general belief that drugs and chemicals do not cross the placenta, so there was no need to fear fetal malformations from medication use in pregnancy. In 1961, thalidomide became a formative event that changed everyone’s perception, with many people adopting the belief that every drug could be dangerous. In reality, though, very few medications prescribed today are known teratogens that cause malformations.
In recent years, an increasing number of drugs have been shown to be “safe.” The issue with the term safe is that there can always be more cases and more studies showing some very small risk that was previously unknown. But, in general, there are more reassuring studies in the literature than ones showing drugs to be dangerous in pregnancy.
The Bendectin example
In the highly charged medicolegal atmosphere in which we practice, physicians are afraid to be sued. If you remember that about 3% of babies are born with malformations just by chance, and that mothers will likely be taking some type of medication, there is always the possibility of a bad outcome that could cast blame on a drug.
In the 1970s, a lot of that litigation centered around the morning sickness drug Bendectin – originally formulated with doxylamine succinate, pyridoxine HCl, and dicyclomine HCl, and later reformulated without the dicyclomine. The drug was taken off the U.S. market by the manufacturer in 1983 because the company couldn’t afford the high cost of litigation and insurance, despite the fact that a panel convened by the Food and Drug Administration said there was no association between Bendectin and human birth defects.
It took nearly 20 years before the FDA declared that the drug had been withdrawn from the market for reasons unrelated to safety and effectiveness. In the meantime, American women remained without an FDA-approved medication to treat morning sickness, and there was a more than twofold increase in hospitalization rates for pregnant women with hyperemesis gravidarum (Can J Public Health. 1995 Jan-Feb;86[1]:66-70). The lesson here is that perceptions in the absence of evidence can lead to grave outcomes.
Exaggeration of risk
Over the years, my colleagues and I have studied how pregnant women perceive drug risk by simply asking them to estimate the risk to their baby from the medication they are currently taking. What we discovered was that women exposed to nonteratogenic drugs consider themselves at a risk of about 25% for having a child with a major malformation. In reality, the risk is between 1% and 3% and has nothing to do with the drug itself. It became clear that there is a huge perception of risk when women are exposed to drugs that should not increase that risk (Am J Obstet Gynecol. 1989 May;160[5 Pt 1]:1190-4).
The same study also showed that many of the women who gave exaggerated risk assessments said they would consider termination of the pregnancy. Even after hearing the drug is safe, some women were still considering termination.
Sadly, women terminating a pregnancy because of a perceived risk for malformation is not unique to this study. In the 1980s, following the explosion at Chernobyl in the Ukraine, women in Athens were told that they had a high risk for malformation in their children because of radiation exposure. Statistics show that during that month, nearly a quarter of early pregnancies in Athens were terminated (Br Med J [Clin Res Ed] 1987;295:1100).
We have further found that women exposed to radiation for diagnostic purposes estimate a high risk of malformation. This type of estimate is likely influenced by the effects of radiation at Hiroshima and Nagasaki, but there is no comparison between the extremely high amounts of radiation in those incidents, compared with the very low amounts in diagnostic tests. Still, we found that women again considered termination because of their perceived risk from radiation.
Social economics are also part of this. Women who are single mothers are more likely to terminate a pregnancy, or consider termination, after exposure to a drug in pregnancy. Women with psychiatric conditions have a similar tendency. On the other hand, women with chronic diseases – who may be used to the effects of a certain medications – are less likely to suggest termination because of perceived risk.
Communicating risk
These are important concepts to consider in the context of the emerging threat of Zika virus and the news from the Centers for Disease Control and Prevention that it is a definitive cause of microcephaly and other severe fetal malformations. While there is a real risk for pregnant women, both through mosquitoes and sexual contact, women are likely to perceive the highest level of risk. In South America, where therapeutic abortion is often not an option, accurate risk communication is critical.
When medications are prescribed during pregnancy, the first step is determining that a drug is truly needed, often in consultation with a specialist. Once that determination is made, it’s key to ensure that women and their families are familiar with the known risk or the lack of risk based on the best available data. There are resources for physicians to help understand and communicate about drug risks in pregnancy, including information from the Organization of Teratology Information Specialists. It’s also important to note that in every pregnancy, there is a 1%-3% risk of major malformations, even if the drug itself is safe. And it can’t hurt to think defensively and document that conversation and that the patient appears to have understood the concept of risk.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having been a paid consultant for Novartis and for Duchesnay, which makes Diclegis to treat nausea and vomiting in pregnancy.
US Official Raises Concerns Over Zika Readiness
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
U.S. official raises concerns over Zika readiness
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
On Twitter @whitneymcknight
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
On Twitter @whitneymcknight
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
On Twitter @whitneymcknight
Fistula developed after delivery: $50M verdict
Fistula developed after delivery: $50M verdict
During delivery of a 31-year-old woman's baby, a nuchal cord was encountered. In order to safely deliver the child, the ObGyn performed an episiotomy.
After delivery, the patient reported an odorous vaginal discharge. The ObGyn explained that the condition was a natural byproduct of delivery and suggested that it would resolve without treatment.
The patient became pregnant a second time shortly after her first delivery and was evaluated by a midwife. The patient again reported the odorous discharge, but the condition was not addressed. At delivery of her second child, the ObGyn determined that the patient had a rectovaginal fistula. The patient underwent 13 repair operations.
PATIENT’S CLAIM:
The fistula was a byproduct of the episiotomy performed during the first delivery. The episiotomy should not have been performed. The ObGyn should have diagnosed and treated the fistula prior to delivery of the second child and performed a cesarean delivery.
DEFENDANT'S DEFENSE:
The ObGyn reported that the patient's medical records showed that she did not report the odorous discharge until after her second delivery.
VERDICT:
A New York $50 million verdict was returned.
Related article:
Management of wound complications following obstetric anal sphincter injury (OASIS)
Abdominal wall hematoma during pregnancy: $2.5M award
At 35 weeks' gestation, a 38-year-old woman presented to the emergency department (ED) with right upper abdominal pain. Her pregnancy was at high risk because of her age and the fact that she had thrombophilia involving both factor V and protein S deficiency. During pregnancy she was anticoagulated. She had been coughing from bronchitis, which was treated with antibiotics and an inhaler.
In the ED, laboratory testing determined that her blood was not properly clotting. Upper abdominal ultrasonography (US) showed an abdominal wall hematoma and gall stones. The ED physician, after contacting the on-call ObGyn, told the patient that nothing further could be done until after the baby's birth and prescribed medications for nausea and pain. The patient was discharged.
Thirty-three hours later, the patient was rushed to the hospital after she was found barely responsive, pale, and in severe pain. US results showed that the hematoma had grown extensively. The patient was in hypovolemic shock having lost more than 50% of her blood volume. She was admitted to the intensive care unit.
After induced labor, a stillborn son was delivered. The autopsy report revealed that the child died from either asphyxiation or an hypoxic ischemic event that occurred when the mother went into shock.
PATIENT’S CLAIM:
The ED physician and staff were negligent. Once the hematoma was identified, the standard of care is to monitor the hematoma with regular US. Instead, the ED physician discharged the patient. The ED physician contacted the on-call ObGyn but did not ask for a consult. The patient should have been admitted for monitoring.
DEFENDANT'S DEFENSE:
The ED physician met the standard of care. The mother's condition would likely have been detected during a nonstress test scheduled for the following day but the mother missed the prenatal exam because she had just left the hospital.
VERDICT:
A $2.5 million Missouri verdict was returned.
Incorrect due date, child with brain injuries: $1.2M
When a pregnant woman presented for her first prenatal visit, she was unsure of the date of her last menstrual period. During subsequent prenatal visits, she underwent 3 ultrasounds.
Labor was induced on August 1 because she reported gastrointestinal reflux. The infant appeared healthy at birth but soon went into respiratory distress. He was slow to meet developmental goals and was believed to be autistic. At age 5 years, he was given a diagnosis of periventricular leukomalacia.
PARENT’S CLAIM:
The child, 11 years old at the time of trial, has permanent brain injuries due to premature delivery. The mother's due date should have been projected as August 25 according to prenatal US measurements. The ObGyn misinterpreted the US data and estimated a due date of August 15. Therefore induction on August 1st caused him to be premature.
PHYSICIAN’S DEFENSE:
The standard of care was met. Gestational age evaluation using US is an estimate based on the child's size at specific time points, not an exact calculation, especially if the mother is not sure about the date of her last menses.
VERDICT:
A $1.2 million New Jersey verdict was returned.
Related article:
Three good apps for calculating the date of delivery
Bacterial infection blamed for birth injury
A woman was at 28 weeks' gestation when her membranes ruptured on September 28. She began to leak amniotic fluid and was put on bed rest. She saw her ObGyn on October 13 with signs of a bacterial infection of her membranes. The ObGyn decided to induce labor; a baby girl was born 11 hours later. The child had meningitis at birth and other infection-related complications including a brain hemorrhage. She continues to have permanent neurologic deficits.
PARENT’S CLAIM:
The ObGyn was negligent in not immediately delivering the child via cesarean delivery on October 13. The delay exposed the baby to infection for 11 more hours; the extended exposure led to her permanent injury.
PHYSICIAN’S DEFENSE:
The patient's treatment met the standard of care.
VERDICT:
A Virginia defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Fistula developed after delivery: $50M verdict
During delivery of a 31-year-old woman's baby, a nuchal cord was encountered. In order to safely deliver the child, the ObGyn performed an episiotomy.
After delivery, the patient reported an odorous vaginal discharge. The ObGyn explained that the condition was a natural byproduct of delivery and suggested that it would resolve without treatment.
The patient became pregnant a second time shortly after her first delivery and was evaluated by a midwife. The patient again reported the odorous discharge, but the condition was not addressed. At delivery of her second child, the ObGyn determined that the patient had a rectovaginal fistula. The patient underwent 13 repair operations.
PATIENT’S CLAIM:
The fistula was a byproduct of the episiotomy performed during the first delivery. The episiotomy should not have been performed. The ObGyn should have diagnosed and treated the fistula prior to delivery of the second child and performed a cesarean delivery.
DEFENDANT'S DEFENSE:
The ObGyn reported that the patient's medical records showed that she did not report the odorous discharge until after her second delivery.
VERDICT:
A New York $50 million verdict was returned.
Related article:
Management of wound complications following obstetric anal sphincter injury (OASIS)
Abdominal wall hematoma during pregnancy: $2.5M award
At 35 weeks' gestation, a 38-year-old woman presented to the emergency department (ED) with right upper abdominal pain. Her pregnancy was at high risk because of her age and the fact that she had thrombophilia involving both factor V and protein S deficiency. During pregnancy she was anticoagulated. She had been coughing from bronchitis, which was treated with antibiotics and an inhaler.
In the ED, laboratory testing determined that her blood was not properly clotting. Upper abdominal ultrasonography (US) showed an abdominal wall hematoma and gall stones. The ED physician, after contacting the on-call ObGyn, told the patient that nothing further could be done until after the baby's birth and prescribed medications for nausea and pain. The patient was discharged.
Thirty-three hours later, the patient was rushed to the hospital after she was found barely responsive, pale, and in severe pain. US results showed that the hematoma had grown extensively. The patient was in hypovolemic shock having lost more than 50% of her blood volume. She was admitted to the intensive care unit.
After induced labor, a stillborn son was delivered. The autopsy report revealed that the child died from either asphyxiation or an hypoxic ischemic event that occurred when the mother went into shock.
PATIENT’S CLAIM:
The ED physician and staff were negligent. Once the hematoma was identified, the standard of care is to monitor the hematoma with regular US. Instead, the ED physician discharged the patient. The ED physician contacted the on-call ObGyn but did not ask for a consult. The patient should have been admitted for monitoring.
DEFENDANT'S DEFENSE:
The ED physician met the standard of care. The mother's condition would likely have been detected during a nonstress test scheduled for the following day but the mother missed the prenatal exam because she had just left the hospital.
VERDICT:
A $2.5 million Missouri verdict was returned.
Incorrect due date, child with brain injuries: $1.2M
When a pregnant woman presented for her first prenatal visit, she was unsure of the date of her last menstrual period. During subsequent prenatal visits, she underwent 3 ultrasounds.
Labor was induced on August 1 because she reported gastrointestinal reflux. The infant appeared healthy at birth but soon went into respiratory distress. He was slow to meet developmental goals and was believed to be autistic. At age 5 years, he was given a diagnosis of periventricular leukomalacia.
PARENT’S CLAIM:
The child, 11 years old at the time of trial, has permanent brain injuries due to premature delivery. The mother's due date should have been projected as August 25 according to prenatal US measurements. The ObGyn misinterpreted the US data and estimated a due date of August 15. Therefore induction on August 1st caused him to be premature.
PHYSICIAN’S DEFENSE:
The standard of care was met. Gestational age evaluation using US is an estimate based on the child's size at specific time points, not an exact calculation, especially if the mother is not sure about the date of her last menses.
VERDICT:
A $1.2 million New Jersey verdict was returned.
Related article:
Three good apps for calculating the date of delivery
Bacterial infection blamed for birth injury
A woman was at 28 weeks' gestation when her membranes ruptured on September 28. She began to leak amniotic fluid and was put on bed rest. She saw her ObGyn on October 13 with signs of a bacterial infection of her membranes. The ObGyn decided to induce labor; a baby girl was born 11 hours later. The child had meningitis at birth and other infection-related complications including a brain hemorrhage. She continues to have permanent neurologic deficits.
PARENT’S CLAIM:
The ObGyn was negligent in not immediately delivering the child via cesarean delivery on October 13. The delay exposed the baby to infection for 11 more hours; the extended exposure led to her permanent injury.
PHYSICIAN’S DEFENSE:
The patient's treatment met the standard of care.
VERDICT:
A Virginia defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Fistula developed after delivery: $50M verdict
During delivery of a 31-year-old woman's baby, a nuchal cord was encountered. In order to safely deliver the child, the ObGyn performed an episiotomy.
After delivery, the patient reported an odorous vaginal discharge. The ObGyn explained that the condition was a natural byproduct of delivery and suggested that it would resolve without treatment.
The patient became pregnant a second time shortly after her first delivery and was evaluated by a midwife. The patient again reported the odorous discharge, but the condition was not addressed. At delivery of her second child, the ObGyn determined that the patient had a rectovaginal fistula. The patient underwent 13 repair operations.
PATIENT’S CLAIM:
The fistula was a byproduct of the episiotomy performed during the first delivery. The episiotomy should not have been performed. The ObGyn should have diagnosed and treated the fistula prior to delivery of the second child and performed a cesarean delivery.
DEFENDANT'S DEFENSE:
The ObGyn reported that the patient's medical records showed that she did not report the odorous discharge until after her second delivery.
VERDICT:
A New York $50 million verdict was returned.
Related article:
Management of wound complications following obstetric anal sphincter injury (OASIS)
Abdominal wall hematoma during pregnancy: $2.5M award
At 35 weeks' gestation, a 38-year-old woman presented to the emergency department (ED) with right upper abdominal pain. Her pregnancy was at high risk because of her age and the fact that she had thrombophilia involving both factor V and protein S deficiency. During pregnancy she was anticoagulated. She had been coughing from bronchitis, which was treated with antibiotics and an inhaler.
In the ED, laboratory testing determined that her blood was not properly clotting. Upper abdominal ultrasonography (US) showed an abdominal wall hematoma and gall stones. The ED physician, after contacting the on-call ObGyn, told the patient that nothing further could be done until after the baby's birth and prescribed medications for nausea and pain. The patient was discharged.
Thirty-three hours later, the patient was rushed to the hospital after she was found barely responsive, pale, and in severe pain. US results showed that the hematoma had grown extensively. The patient was in hypovolemic shock having lost more than 50% of her blood volume. She was admitted to the intensive care unit.
After induced labor, a stillborn son was delivered. The autopsy report revealed that the child died from either asphyxiation or an hypoxic ischemic event that occurred when the mother went into shock.
PATIENT’S CLAIM:
The ED physician and staff were negligent. Once the hematoma was identified, the standard of care is to monitor the hematoma with regular US. Instead, the ED physician discharged the patient. The ED physician contacted the on-call ObGyn but did not ask for a consult. The patient should have been admitted for monitoring.
DEFENDANT'S DEFENSE:
The ED physician met the standard of care. The mother's condition would likely have been detected during a nonstress test scheduled for the following day but the mother missed the prenatal exam because she had just left the hospital.
VERDICT:
A $2.5 million Missouri verdict was returned.
Incorrect due date, child with brain injuries: $1.2M
When a pregnant woman presented for her first prenatal visit, she was unsure of the date of her last menstrual period. During subsequent prenatal visits, she underwent 3 ultrasounds.
Labor was induced on August 1 because she reported gastrointestinal reflux. The infant appeared healthy at birth but soon went into respiratory distress. He was slow to meet developmental goals and was believed to be autistic. At age 5 years, he was given a diagnosis of periventricular leukomalacia.
PARENT’S CLAIM:
The child, 11 years old at the time of trial, has permanent brain injuries due to premature delivery. The mother's due date should have been projected as August 25 according to prenatal US measurements. The ObGyn misinterpreted the US data and estimated a due date of August 15. Therefore induction on August 1st caused him to be premature.
PHYSICIAN’S DEFENSE:
The standard of care was met. Gestational age evaluation using US is an estimate based on the child's size at specific time points, not an exact calculation, especially if the mother is not sure about the date of her last menses.
VERDICT:
A $1.2 million New Jersey verdict was returned.
Related article:
Three good apps for calculating the date of delivery
Bacterial infection blamed for birth injury
A woman was at 28 weeks' gestation when her membranes ruptured on September 28. She began to leak amniotic fluid and was put on bed rest. She saw her ObGyn on October 13 with signs of a bacterial infection of her membranes. The ObGyn decided to induce labor; a baby girl was born 11 hours later. The child had meningitis at birth and other infection-related complications including a brain hemorrhage. She continues to have permanent neurologic deficits.
PARENT’S CLAIM:
The ObGyn was negligent in not immediately delivering the child via cesarean delivery on October 13. The delay exposed the baby to infection for 11 more hours; the extended exposure led to her permanent injury.
PHYSICIAN’S DEFENSE:
The patient's treatment met the standard of care.
VERDICT:
A Virginia defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Study reaffirms that maternal flu immunization reduces infants’ risk of flu
Infants born to mothers who received flu immunization during pregnancy were 70% less likely to contract lab-confirmed influenza and 81% less likely to be hospitalized for flu before age 6 months, compared with infants of unimmunized mothers, a study reaffirmed.
Yet just one in ten pregnant women received the vaccine, a proportion that has steadily risen since the 2009-2010 H1N1 flu season.
“The results of this large retrospective study support the conclusions of prospective studies regarding the protective benefit of maternal influenza immunization during pregnancy,” reported Dr. Julie H. Shakib of the University of Utah, Salt Lake City, and her associates (Pediatrics. 2016 May 3. doi: 10.1542/peds.2015-2360). “Interventions that target both healthy pregnant women and those with chronic conditions are needed to increase vaccine uptake,” they wrote.
The researchers analyzed self-reported seasonal influenza immunization uptake in the 245,386 women who gave birth between December 2005 and March 2014 in the Intermountain Healthcare facilities in Utah and Idaho. Although 10% of the women overall received flu vaccinations, just 2.2% of the women who delivered before the 2009-2010 H1N1 pandemic had received them. That number rose after the pandemic to 21% (P < .001). More than half (52%) of the women giving birth during the 2013-2014 flu season reported getting the seasonal flu vaccine.
Among the women’s 249,387 infants, 866 had at least one influenza-like illness (ILI), including 32 born to vaccinated women and 834 born to unvaccinated women. The infants born to women receiving the flu vaccine during pregnancy were 64% less likely to develop an ILI, with illnesses in 1.34 per 1,000 born to vaccinated women and 3.7 per 1,000 born to unvaccinated women (relative risk, 0.36).
The rates of laboratory-confirmed influenza in the 658 children who contracted it were 0.84 per 1,000 children born to vaccinated mothers and 2.83 per 1,000 children born to unvaccinated mothers, translating to a 70% lower risk of flu in those born to vaccinated mothers (RR, 0.30). Similarly, infants born to vaccinated mothers were 81% less likely to be hospitalized for lab-confirmed influenza (RR, 0.19, P = .005). Just 3 of 151 hospitalized infants had been born to mothers who received the flu vaccine, for a rate of 0.13 per 1,000 for children of vaccinated mothers and 0.66 per 1,000 for children of unvaccinated mothers.
Pregnant women with public insurance or no insurance were less likely to report getting the seasonal vaccine than were privately insured women, but those with chronic conditions were more likely to be vaccinated. Uptake also was lower among women with incomes below the federal poverty level and among women living in either rural or frontier areas or in the Urban South or Southwest Intermountain regions.
“The Intermountain Urban South region includes Utah County, 1 of 30 U.S. counties with the largest estimated numbers of unvaccinated children from 1995-2001 CDC National Immunization Surveys (NIS) data,” the authors wrote. “It is possible that factors leading to parental vaccine hesitancy in children may similarly affect pregnant women considering maternal immunization during pregnancy.”
Because of widespread testing for respiratory viruses at Intermountain facilities, the researchers also could determine that flu vaccine receipt among the mothers did not affect incidence of RSV.
“Our study strengthens the evidence that maternal immunization provides passive protection against influenza to infants during the vulnerable period before they are old enough to receive active immunization,” Dr. Shakib and her associates wrote.
The research was funded by the National Institutes of Health, the University of Utah Children’s Health Research Center and the Pediatric Clinical and Translational Scholar Program, the H.A. and Edna Benning Presidential Endowment, and the University of Utah Center for Clinical and Translational Science through the National Center for Research Resources and the National Center for Advancing Translational Sciences. The authors reported no disclosures.
Infants born to mothers who received flu immunization during pregnancy were 70% less likely to contract lab-confirmed influenza and 81% less likely to be hospitalized for flu before age 6 months, compared with infants of unimmunized mothers, a study reaffirmed.
Yet just one in ten pregnant women received the vaccine, a proportion that has steadily risen since the 2009-2010 H1N1 flu season.
“The results of this large retrospective study support the conclusions of prospective studies regarding the protective benefit of maternal influenza immunization during pregnancy,” reported Dr. Julie H. Shakib of the University of Utah, Salt Lake City, and her associates (Pediatrics. 2016 May 3. doi: 10.1542/peds.2015-2360). “Interventions that target both healthy pregnant women and those with chronic conditions are needed to increase vaccine uptake,” they wrote.
The researchers analyzed self-reported seasonal influenza immunization uptake in the 245,386 women who gave birth between December 2005 and March 2014 in the Intermountain Healthcare facilities in Utah and Idaho. Although 10% of the women overall received flu vaccinations, just 2.2% of the women who delivered before the 2009-2010 H1N1 pandemic had received them. That number rose after the pandemic to 21% (P < .001). More than half (52%) of the women giving birth during the 2013-2014 flu season reported getting the seasonal flu vaccine.
Among the women’s 249,387 infants, 866 had at least one influenza-like illness (ILI), including 32 born to vaccinated women and 834 born to unvaccinated women. The infants born to women receiving the flu vaccine during pregnancy were 64% less likely to develop an ILI, with illnesses in 1.34 per 1,000 born to vaccinated women and 3.7 per 1,000 born to unvaccinated women (relative risk, 0.36).
The rates of laboratory-confirmed influenza in the 658 children who contracted it were 0.84 per 1,000 children born to vaccinated mothers and 2.83 per 1,000 children born to unvaccinated mothers, translating to a 70% lower risk of flu in those born to vaccinated mothers (RR, 0.30). Similarly, infants born to vaccinated mothers were 81% less likely to be hospitalized for lab-confirmed influenza (RR, 0.19, P = .005). Just 3 of 151 hospitalized infants had been born to mothers who received the flu vaccine, for a rate of 0.13 per 1,000 for children of vaccinated mothers and 0.66 per 1,000 for children of unvaccinated mothers.
Pregnant women with public insurance or no insurance were less likely to report getting the seasonal vaccine than were privately insured women, but those with chronic conditions were more likely to be vaccinated. Uptake also was lower among women with incomes below the federal poverty level and among women living in either rural or frontier areas or in the Urban South or Southwest Intermountain regions.
“The Intermountain Urban South region includes Utah County, 1 of 30 U.S. counties with the largest estimated numbers of unvaccinated children from 1995-2001 CDC National Immunization Surveys (NIS) data,” the authors wrote. “It is possible that factors leading to parental vaccine hesitancy in children may similarly affect pregnant women considering maternal immunization during pregnancy.”
Because of widespread testing for respiratory viruses at Intermountain facilities, the researchers also could determine that flu vaccine receipt among the mothers did not affect incidence of RSV.
“Our study strengthens the evidence that maternal immunization provides passive protection against influenza to infants during the vulnerable period before they are old enough to receive active immunization,” Dr. Shakib and her associates wrote.
The research was funded by the National Institutes of Health, the University of Utah Children’s Health Research Center and the Pediatric Clinical and Translational Scholar Program, the H.A. and Edna Benning Presidential Endowment, and the University of Utah Center for Clinical and Translational Science through the National Center for Research Resources and the National Center for Advancing Translational Sciences. The authors reported no disclosures.
Infants born to mothers who received flu immunization during pregnancy were 70% less likely to contract lab-confirmed influenza and 81% less likely to be hospitalized for flu before age 6 months, compared with infants of unimmunized mothers, a study reaffirmed.
Yet just one in ten pregnant women received the vaccine, a proportion that has steadily risen since the 2009-2010 H1N1 flu season.
“The results of this large retrospective study support the conclusions of prospective studies regarding the protective benefit of maternal influenza immunization during pregnancy,” reported Dr. Julie H. Shakib of the University of Utah, Salt Lake City, and her associates (Pediatrics. 2016 May 3. doi: 10.1542/peds.2015-2360). “Interventions that target both healthy pregnant women and those with chronic conditions are needed to increase vaccine uptake,” they wrote.
The researchers analyzed self-reported seasonal influenza immunization uptake in the 245,386 women who gave birth between December 2005 and March 2014 in the Intermountain Healthcare facilities in Utah and Idaho. Although 10% of the women overall received flu vaccinations, just 2.2% of the women who delivered before the 2009-2010 H1N1 pandemic had received them. That number rose after the pandemic to 21% (P < .001). More than half (52%) of the women giving birth during the 2013-2014 flu season reported getting the seasonal flu vaccine.
Among the women’s 249,387 infants, 866 had at least one influenza-like illness (ILI), including 32 born to vaccinated women and 834 born to unvaccinated women. The infants born to women receiving the flu vaccine during pregnancy were 64% less likely to develop an ILI, with illnesses in 1.34 per 1,000 born to vaccinated women and 3.7 per 1,000 born to unvaccinated women (relative risk, 0.36).
The rates of laboratory-confirmed influenza in the 658 children who contracted it were 0.84 per 1,000 children born to vaccinated mothers and 2.83 per 1,000 children born to unvaccinated mothers, translating to a 70% lower risk of flu in those born to vaccinated mothers (RR, 0.30). Similarly, infants born to vaccinated mothers were 81% less likely to be hospitalized for lab-confirmed influenza (RR, 0.19, P = .005). Just 3 of 151 hospitalized infants had been born to mothers who received the flu vaccine, for a rate of 0.13 per 1,000 for children of vaccinated mothers and 0.66 per 1,000 for children of unvaccinated mothers.
Pregnant women with public insurance or no insurance were less likely to report getting the seasonal vaccine than were privately insured women, but those with chronic conditions were more likely to be vaccinated. Uptake also was lower among women with incomes below the federal poverty level and among women living in either rural or frontier areas or in the Urban South or Southwest Intermountain regions.
“The Intermountain Urban South region includes Utah County, 1 of 30 U.S. counties with the largest estimated numbers of unvaccinated children from 1995-2001 CDC National Immunization Surveys (NIS) data,” the authors wrote. “It is possible that factors leading to parental vaccine hesitancy in children may similarly affect pregnant women considering maternal immunization during pregnancy.”
Because of widespread testing for respiratory viruses at Intermountain facilities, the researchers also could determine that flu vaccine receipt among the mothers did not affect incidence of RSV.
“Our study strengthens the evidence that maternal immunization provides passive protection against influenza to infants during the vulnerable period before they are old enough to receive active immunization,” Dr. Shakib and her associates wrote.
The research was funded by the National Institutes of Health, the University of Utah Children’s Health Research Center and the Pediatric Clinical and Translational Scholar Program, the H.A. and Edna Benning Presidential Endowment, and the University of Utah Center for Clinical and Translational Science through the National Center for Research Resources and the National Center for Advancing Translational Sciences. The authors reported no disclosures.
FROM PEDIATRICS
Key clinical point: Young infants born to mothers receiving the flu vaccine were less likely to develop the flu and serious complications.
Major finding: Infants under 6 months old were 64% less likely to develop influenza-like illness, 70% less likely to develop lab-confirmed flu, and 81% less likely to be hospitalized for flu if born to vaccinated mothers.
Data source: The findings are based on a retrospective cohort study of 245,386 women who gave birth to 249,387 infants between December 2005 and March 2014 in the Intermountain Healthcare facilities in Utah and Idaho.
Disclosures: The research was funded by the National Institutes of Health, the University of Utah Children’s Health Research Center and the Pediatric Clinical and Translational Scholar Program, the H.A. and Edna Benning Presidential Endowment, and the University of Utah Center for Clinical and Translational Science through the National Center for Research Resources and the National Center for Advancing Translational Sciences. The authors reported no disclosures.
Preeclampsia test cancelled: $5M settlement, and more
Preeclampsia test cancelled: $5M settlement
A 35-year-old woman was pregnant with her first child. Prior to and during her pregnancy, she took medication for chronic hypertension. Although another ObGyn had ordered a 24-hour urinalysis to test for preeclampsia, the ObGyn who saw the mother in early May for a third trimester visit cancelled the test.
The mother delivered the child by cesarean delivery when the fetal heart-rate monitor indicated fetal distress. After birth, the child received a diagnosis of cerebral palsy, spastic quadriplegia, and dystonia.
Parents' claim: The decision by the second ObGyn to cancel the 24-hour urinalysis eliminated the opportunity to diagnose preeclampsia superimposed on chronic hypertension. Over time, preeclampsia impaired blood flow to the placenta and fetus. If the mother had been assessed in early May, the injury could have been prevented.
Defendants' defense: The case was settled during trial.
Verdict: A $5,000,000 Illinois settlement was reached through mediation with the hospital physicians’ group and 2 ObGyns.
Umbilical cord damaged at delivery: $1.5M settlement
A mother at full term presented to the hospital in labor. During delivery, the umbilical cord was severed during maneuvers to address shoulder dystocia. The fetus was stillborn.
Parents' claim: The patient told the nurses that shoulder dystocia had been encountered during a previous delivery. Shoulder dystocia maneuvers were not performed correctly. Cesarean delivery was never offered.
Hospital's Defense: The nurses called the certified nurse midwife who was managing labor and delivery to alert her of the patient’s history. The midwife denied receiving such a call. The case was settled during trial.
Verdict: A $1.5 million Illinois settlement was reached.
What caused sepsis after oophorectomy?
A woman had a cyst on her left ovary. The ObGyn began surgery laparoscopically but converted to open salpingo-oophorectomy because of extensive adhesions. Four days after surgery, the patient received a diagnosis of peritonitis and sepsis due to spillage from the sigmoid colon. She required a second surgery to repair the damage, followed by a long recovery.
Patient's claim: The ObGyn should not have attempted laparoscopic surgery; he knew of her extensive surgical history and should have anticipated the presence of adhesions. If the laparoscopic entry site had been examined properly intraoperatively, the injury could have been repaired immediately.
Physician's defense: The ObGyn had no reason to believe the patient would have adhesions in the umbilical area; prior surgeries occurred in the upper abdomen. Laparoscopic surgery with Veress needle access is an accepted method used by obstetric surgeons. The ObGyn carefully irrigated and inspected the abdomen before closing. Injury to the sigmoid colon is a known complication of left oophorectomy.
At the time of surgery, the patient was likely suffering from diverticulosis, a long-term condition that can lead to a leak in the large colon. The weakness in the patient’s colon caused a postsurgical leak; signs and symptoms did not appear until 4 days after surgery.
Verdict: A California defense verdict was returned.
Ectopic pregnancy misdiagnosed
A 39-year-old woman reported abdominal pain to her ObGyn. After ultrasonography (US), she was given a diagnosis of ectopic pregnancy. The ObGyn administered methotrexate to terminate the pregnancy. Five days later, repeat US showed a viable uterine pregnancy. Based on the risks posed by methotrexate, the patient terminated the pregnancy.
Patient's claim: The ObGyn misdiagnosed the pregnancy as ectopic.
Hospital's Defense: The case was settled during trial.
Verdict: A $625,000 Illinois settlement was reached.
Infant dies. was it fetal hydrops?
A woman was admitted to the hospital in full-term labor. She was cared for by a team of residents and nurses supervised by an attending ObGyn. During labor, the staff documented late, variable decelerations with periods of minimal or undetectable variability on the fetal heart-rate monitor. The fetal heart rate, however, was reported as being reassuring overall.
After 90 minutes, fetal heart-rate tracings became non-reassuring. Because the baby's head was crowning, the ObGyn used vacuum extraction for delivery. The infant was born without signs of life. A neonatologist thought the infant appeared hydropic with generalized edema, ascites, and pleural effusion. Efforts at resuscitation were unsuccessful until the neonatologist performed thoracentesis. The infant died several hours later. Cause of death has charted as hypoxic ischemic encephalopathy and multisystem organ failure.
Estate's claim: The hospital staff deviated from the standard of care by failing to appropriately communicate, failing to recognize fetal distress, and failing to perform a cesarean delivery when tracings were nonreassuring. An expert neonatologist claimed that failure to react to fetal distress caused the fetus to develop severe intrauterine hypoxic ischemia causing death.
Defendants' defense: Overall, the fetal heart-rate tracings were reassuring. The team communicated appropriately and kept the attending ObGyn alerted to the status. Delivery was expedited when fetal distress was evident.
Fetal hydrops was the end result of a serious problem in utero that could not have developed during the hours of labor and delivery; it most likely arose days to weeks before delivery. Nothing that occurred during labor and delivery caused hypoxic ischemic encephalopathy.
Verdict: An Illinois defense verdict was returned.
Child has permanent shoulder injury: $1M verdict
A mother was admitted to a hospital in full-term labor. During delivery, anterior shoulder dystocia was encountered.
The child received a diagnosis of a left brachial plexus injury and extracranial and intracranial bleeding. She underwent 3 surgeries to reattach nerve roots and move muscles and tendons in her shoulder and forearm in an effort to improve function in her left arm, wrist, and hand. She has undergone extensive physical and occupational therapy. Her left arm is smaller than her right arm and she has minimal strength, dexterity, and only 20% functionality of her left arm.
Parent's claim: The ObGyn exerted excessive traction when delivering the child. Alternate methods should have been used to manage shoulder dystocia. The hospital nurses should not have used fundal pressure.
Defendants' defense: The suit was brought against the ObGyn, his practice, and the hospital. The ObGyn claimed that he used several maneuvers to manage shoulder dystocia. The child’s injuries were a result of the maternal forces of labor and were not caused by negligence on the part of the ObGyn or nurses. The nurses denied using fundal pressure; they were trained to use suprapubic pressure.
Verdict: A $1,012,00 Illinois verdict was returned, finding the ObGyn’s practice 100% liable.
Parvovirus exposure: Fetal death
When a woman first saw an ObGyn, ultrasonography (US) indicated that her fetus was at 8 to 9 weeks’ gestation. One month later, she told the same ObGyn that she had been exposed to Fifth disease. Because blood work was positive for parvovirus B19, the ObGyn ordered the patient to undergo US every 2 weeks for the next 10 weeks. Two weeks later, the patient saw a second ObGyn at the same clinic. Although the first ObGyn had ordered US, none was performed; the patient’s next appointment was scheduled in 4 weeks. At that time, the patient saw a third ObGyn, who ordered US. He noted in her chart that the fetus had a nuchal fold, indicating Down syndrome. He told the patient to return in 2 weeks for a follow-up US. The results of that US showed that the fetus had died. Fetal cord blood tested positive for parvovirus B19.
Parent's claim: All 3 ObGyns failed to react properly to indications of parvovirus infection. Regular US should have been performed, as suggested by the first ObGyn. The mother should have been referred to a perinatologist or other maternal-fetal specialist when blood work was positive for parvovirus B19. A specialist could have provided treatment for the virus.
Physician's defense: The ObGyns denied any breach in the standard of care. They claimed that results would have been the same if they had referred the patient to a specialist.
Verdict: An Alabama defense verdict was returned.
Preeclampsia test cancelled: $5M settlement
A 35-year-old woman was pregnant with her first child. Prior to and during her pregnancy, she took medication for chronic hypertension. Although another ObGyn had ordered a 24-hour urinalysis to test for preeclampsia, the ObGyn who saw the mother in early May for a third trimester visit cancelled the test.
The mother delivered the child by cesarean delivery when the fetal heart-rate monitor indicated fetal distress. After birth, the child received a diagnosis of cerebral palsy, spastic quadriplegia, and dystonia.
Parents' claim: The decision by the second ObGyn to cancel the 24-hour urinalysis eliminated the opportunity to diagnose preeclampsia superimposed on chronic hypertension. Over time, preeclampsia impaired blood flow to the placenta and fetus. If the mother had been assessed in early May, the injury could have been prevented.
Defendants' defense: The case was settled during trial.
Verdict: A $5,000,000 Illinois settlement was reached through mediation with the hospital physicians’ group and 2 ObGyns.
Umbilical cord damaged at delivery: $1.5M settlement
A mother at full term presented to the hospital in labor. During delivery, the umbilical cord was severed during maneuvers to address shoulder dystocia. The fetus was stillborn.
Parents' claim: The patient told the nurses that shoulder dystocia had been encountered during a previous delivery. Shoulder dystocia maneuvers were not performed correctly. Cesarean delivery was never offered.
Hospital's Defense: The nurses called the certified nurse midwife who was managing labor and delivery to alert her of the patient’s history. The midwife denied receiving such a call. The case was settled during trial.
Verdict: A $1.5 million Illinois settlement was reached.
What caused sepsis after oophorectomy?
A woman had a cyst on her left ovary. The ObGyn began surgery laparoscopically but converted to open salpingo-oophorectomy because of extensive adhesions. Four days after surgery, the patient received a diagnosis of peritonitis and sepsis due to spillage from the sigmoid colon. She required a second surgery to repair the damage, followed by a long recovery.
Patient's claim: The ObGyn should not have attempted laparoscopic surgery; he knew of her extensive surgical history and should have anticipated the presence of adhesions. If the laparoscopic entry site had been examined properly intraoperatively, the injury could have been repaired immediately.
Physician's defense: The ObGyn had no reason to believe the patient would have adhesions in the umbilical area; prior surgeries occurred in the upper abdomen. Laparoscopic surgery with Veress needle access is an accepted method used by obstetric surgeons. The ObGyn carefully irrigated and inspected the abdomen before closing. Injury to the sigmoid colon is a known complication of left oophorectomy.
At the time of surgery, the patient was likely suffering from diverticulosis, a long-term condition that can lead to a leak in the large colon. The weakness in the patient’s colon caused a postsurgical leak; signs and symptoms did not appear until 4 days after surgery.
Verdict: A California defense verdict was returned.
Ectopic pregnancy misdiagnosed
A 39-year-old woman reported abdominal pain to her ObGyn. After ultrasonography (US), she was given a diagnosis of ectopic pregnancy. The ObGyn administered methotrexate to terminate the pregnancy. Five days later, repeat US showed a viable uterine pregnancy. Based on the risks posed by methotrexate, the patient terminated the pregnancy.
Patient's claim: The ObGyn misdiagnosed the pregnancy as ectopic.
Hospital's Defense: The case was settled during trial.
Verdict: A $625,000 Illinois settlement was reached.
Infant dies. was it fetal hydrops?
A woman was admitted to the hospital in full-term labor. She was cared for by a team of residents and nurses supervised by an attending ObGyn. During labor, the staff documented late, variable decelerations with periods of minimal or undetectable variability on the fetal heart-rate monitor. The fetal heart rate, however, was reported as being reassuring overall.
After 90 minutes, fetal heart-rate tracings became non-reassuring. Because the baby's head was crowning, the ObGyn used vacuum extraction for delivery. The infant was born without signs of life. A neonatologist thought the infant appeared hydropic with generalized edema, ascites, and pleural effusion. Efforts at resuscitation were unsuccessful until the neonatologist performed thoracentesis. The infant died several hours later. Cause of death has charted as hypoxic ischemic encephalopathy and multisystem organ failure.
Estate's claim: The hospital staff deviated from the standard of care by failing to appropriately communicate, failing to recognize fetal distress, and failing to perform a cesarean delivery when tracings were nonreassuring. An expert neonatologist claimed that failure to react to fetal distress caused the fetus to develop severe intrauterine hypoxic ischemia causing death.
Defendants' defense: Overall, the fetal heart-rate tracings were reassuring. The team communicated appropriately and kept the attending ObGyn alerted to the status. Delivery was expedited when fetal distress was evident.
Fetal hydrops was the end result of a serious problem in utero that could not have developed during the hours of labor and delivery; it most likely arose days to weeks before delivery. Nothing that occurred during labor and delivery caused hypoxic ischemic encephalopathy.
Verdict: An Illinois defense verdict was returned.
Child has permanent shoulder injury: $1M verdict
A mother was admitted to a hospital in full-term labor. During delivery, anterior shoulder dystocia was encountered.
The child received a diagnosis of a left brachial plexus injury and extracranial and intracranial bleeding. She underwent 3 surgeries to reattach nerve roots and move muscles and tendons in her shoulder and forearm in an effort to improve function in her left arm, wrist, and hand. She has undergone extensive physical and occupational therapy. Her left arm is smaller than her right arm and she has minimal strength, dexterity, and only 20% functionality of her left arm.
Parent's claim: The ObGyn exerted excessive traction when delivering the child. Alternate methods should have been used to manage shoulder dystocia. The hospital nurses should not have used fundal pressure.
Defendants' defense: The suit was brought against the ObGyn, his practice, and the hospital. The ObGyn claimed that he used several maneuvers to manage shoulder dystocia. The child’s injuries were a result of the maternal forces of labor and were not caused by negligence on the part of the ObGyn or nurses. The nurses denied using fundal pressure; they were trained to use suprapubic pressure.
Verdict: A $1,012,00 Illinois verdict was returned, finding the ObGyn’s practice 100% liable.
Parvovirus exposure: Fetal death
When a woman first saw an ObGyn, ultrasonography (US) indicated that her fetus was at 8 to 9 weeks’ gestation. One month later, she told the same ObGyn that she had been exposed to Fifth disease. Because blood work was positive for parvovirus B19, the ObGyn ordered the patient to undergo US every 2 weeks for the next 10 weeks. Two weeks later, the patient saw a second ObGyn at the same clinic. Although the first ObGyn had ordered US, none was performed; the patient’s next appointment was scheduled in 4 weeks. At that time, the patient saw a third ObGyn, who ordered US. He noted in her chart that the fetus had a nuchal fold, indicating Down syndrome. He told the patient to return in 2 weeks for a follow-up US. The results of that US showed that the fetus had died. Fetal cord blood tested positive for parvovirus B19.
Parent's claim: All 3 ObGyns failed to react properly to indications of parvovirus infection. Regular US should have been performed, as suggested by the first ObGyn. The mother should have been referred to a perinatologist or other maternal-fetal specialist when blood work was positive for parvovirus B19. A specialist could have provided treatment for the virus.
Physician's defense: The ObGyns denied any breach in the standard of care. They claimed that results would have been the same if they had referred the patient to a specialist.
Verdict: An Alabama defense verdict was returned.
Preeclampsia test cancelled: $5M settlement
A 35-year-old woman was pregnant with her first child. Prior to and during her pregnancy, she took medication for chronic hypertension. Although another ObGyn had ordered a 24-hour urinalysis to test for preeclampsia, the ObGyn who saw the mother in early May for a third trimester visit cancelled the test.
The mother delivered the child by cesarean delivery when the fetal heart-rate monitor indicated fetal distress. After birth, the child received a diagnosis of cerebral palsy, spastic quadriplegia, and dystonia.
Parents' claim: The decision by the second ObGyn to cancel the 24-hour urinalysis eliminated the opportunity to diagnose preeclampsia superimposed on chronic hypertension. Over time, preeclampsia impaired blood flow to the placenta and fetus. If the mother had been assessed in early May, the injury could have been prevented.
Defendants' defense: The case was settled during trial.
Verdict: A $5,000,000 Illinois settlement was reached through mediation with the hospital physicians’ group and 2 ObGyns.
Umbilical cord damaged at delivery: $1.5M settlement
A mother at full term presented to the hospital in labor. During delivery, the umbilical cord was severed during maneuvers to address shoulder dystocia. The fetus was stillborn.
Parents' claim: The patient told the nurses that shoulder dystocia had been encountered during a previous delivery. Shoulder dystocia maneuvers were not performed correctly. Cesarean delivery was never offered.
Hospital's Defense: The nurses called the certified nurse midwife who was managing labor and delivery to alert her of the patient’s history. The midwife denied receiving such a call. The case was settled during trial.
Verdict: A $1.5 million Illinois settlement was reached.
What caused sepsis after oophorectomy?
A woman had a cyst on her left ovary. The ObGyn began surgery laparoscopically but converted to open salpingo-oophorectomy because of extensive adhesions. Four days after surgery, the patient received a diagnosis of peritonitis and sepsis due to spillage from the sigmoid colon. She required a second surgery to repair the damage, followed by a long recovery.
Patient's claim: The ObGyn should not have attempted laparoscopic surgery; he knew of her extensive surgical history and should have anticipated the presence of adhesions. If the laparoscopic entry site had been examined properly intraoperatively, the injury could have been repaired immediately.
Physician's defense: The ObGyn had no reason to believe the patient would have adhesions in the umbilical area; prior surgeries occurred in the upper abdomen. Laparoscopic surgery with Veress needle access is an accepted method used by obstetric surgeons. The ObGyn carefully irrigated and inspected the abdomen before closing. Injury to the sigmoid colon is a known complication of left oophorectomy.
At the time of surgery, the patient was likely suffering from diverticulosis, a long-term condition that can lead to a leak in the large colon. The weakness in the patient’s colon caused a postsurgical leak; signs and symptoms did not appear until 4 days after surgery.
Verdict: A California defense verdict was returned.
Ectopic pregnancy misdiagnosed
A 39-year-old woman reported abdominal pain to her ObGyn. After ultrasonography (US), she was given a diagnosis of ectopic pregnancy. The ObGyn administered methotrexate to terminate the pregnancy. Five days later, repeat US showed a viable uterine pregnancy. Based on the risks posed by methotrexate, the patient terminated the pregnancy.
Patient's claim: The ObGyn misdiagnosed the pregnancy as ectopic.
Hospital's Defense: The case was settled during trial.
Verdict: A $625,000 Illinois settlement was reached.
Infant dies. was it fetal hydrops?
A woman was admitted to the hospital in full-term labor. She was cared for by a team of residents and nurses supervised by an attending ObGyn. During labor, the staff documented late, variable decelerations with periods of minimal or undetectable variability on the fetal heart-rate monitor. The fetal heart rate, however, was reported as being reassuring overall.
After 90 minutes, fetal heart-rate tracings became non-reassuring. Because the baby's head was crowning, the ObGyn used vacuum extraction for delivery. The infant was born without signs of life. A neonatologist thought the infant appeared hydropic with generalized edema, ascites, and pleural effusion. Efforts at resuscitation were unsuccessful until the neonatologist performed thoracentesis. The infant died several hours later. Cause of death has charted as hypoxic ischemic encephalopathy and multisystem organ failure.
Estate's claim: The hospital staff deviated from the standard of care by failing to appropriately communicate, failing to recognize fetal distress, and failing to perform a cesarean delivery when tracings were nonreassuring. An expert neonatologist claimed that failure to react to fetal distress caused the fetus to develop severe intrauterine hypoxic ischemia causing death.
Defendants' defense: Overall, the fetal heart-rate tracings were reassuring. The team communicated appropriately and kept the attending ObGyn alerted to the status. Delivery was expedited when fetal distress was evident.
Fetal hydrops was the end result of a serious problem in utero that could not have developed during the hours of labor and delivery; it most likely arose days to weeks before delivery. Nothing that occurred during labor and delivery caused hypoxic ischemic encephalopathy.
Verdict: An Illinois defense verdict was returned.
Child has permanent shoulder injury: $1M verdict
A mother was admitted to a hospital in full-term labor. During delivery, anterior shoulder dystocia was encountered.
The child received a diagnosis of a left brachial plexus injury and extracranial and intracranial bleeding. She underwent 3 surgeries to reattach nerve roots and move muscles and tendons in her shoulder and forearm in an effort to improve function in her left arm, wrist, and hand. She has undergone extensive physical and occupational therapy. Her left arm is smaller than her right arm and she has minimal strength, dexterity, and only 20% functionality of her left arm.
Parent's claim: The ObGyn exerted excessive traction when delivering the child. Alternate methods should have been used to manage shoulder dystocia. The hospital nurses should not have used fundal pressure.
Defendants' defense: The suit was brought against the ObGyn, his practice, and the hospital. The ObGyn claimed that he used several maneuvers to manage shoulder dystocia. The child’s injuries were a result of the maternal forces of labor and were not caused by negligence on the part of the ObGyn or nurses. The nurses denied using fundal pressure; they were trained to use suprapubic pressure.
Verdict: A $1,012,00 Illinois verdict was returned, finding the ObGyn’s practice 100% liable.
Parvovirus exposure: Fetal death
When a woman first saw an ObGyn, ultrasonography (US) indicated that her fetus was at 8 to 9 weeks’ gestation. One month later, she told the same ObGyn that she had been exposed to Fifth disease. Because blood work was positive for parvovirus B19, the ObGyn ordered the patient to undergo US every 2 weeks for the next 10 weeks. Two weeks later, the patient saw a second ObGyn at the same clinic. Although the first ObGyn had ordered US, none was performed; the patient’s next appointment was scheduled in 4 weeks. At that time, the patient saw a third ObGyn, who ordered US. He noted in her chart that the fetus had a nuchal fold, indicating Down syndrome. He told the patient to return in 2 weeks for a follow-up US. The results of that US showed that the fetus had died. Fetal cord blood tested positive for parvovirus B19.
Parent's claim: All 3 ObGyns failed to react properly to indications of parvovirus infection. Regular US should have been performed, as suggested by the first ObGyn. The mother should have been referred to a perinatologist or other maternal-fetal specialist when blood work was positive for parvovirus B19. A specialist could have provided treatment for the virus.
Physician's defense: The ObGyns denied any breach in the standard of care. They claimed that results would have been the same if they had referred the patient to a specialist.
Verdict: An Alabama defense verdict was returned.
Additional Medical Verdicts
• Umbilical cord damaged at delivery: $1.5M settlement
• What caused sepsis after oophorectomy?
• Ectopic pregnancy misdiagnosed
• Infant dies. Was it fetal hydrops?
• Child has permanent shoulder injury: $1M verdict
• Parvovirus exposure: fetal death
10 tips for overcoming common challenges of intrapartum fetal monitoring
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||
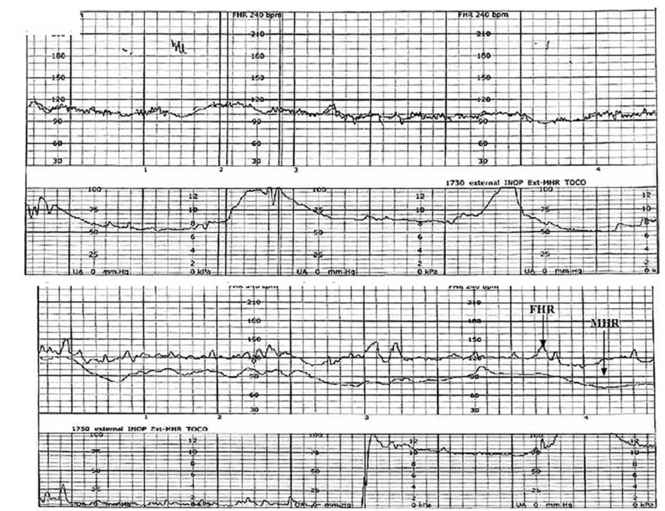
| ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||
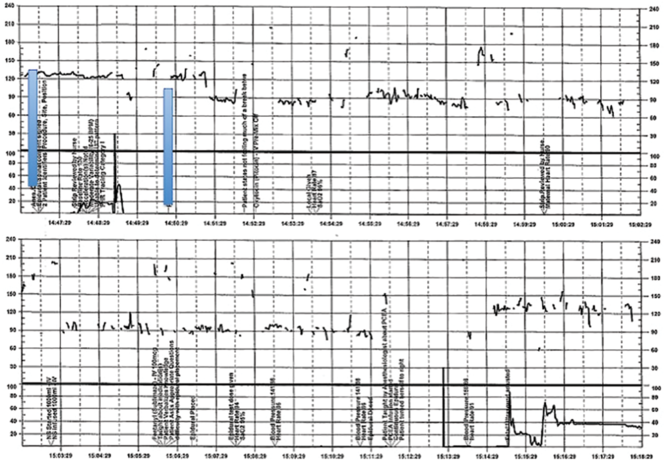
| ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||
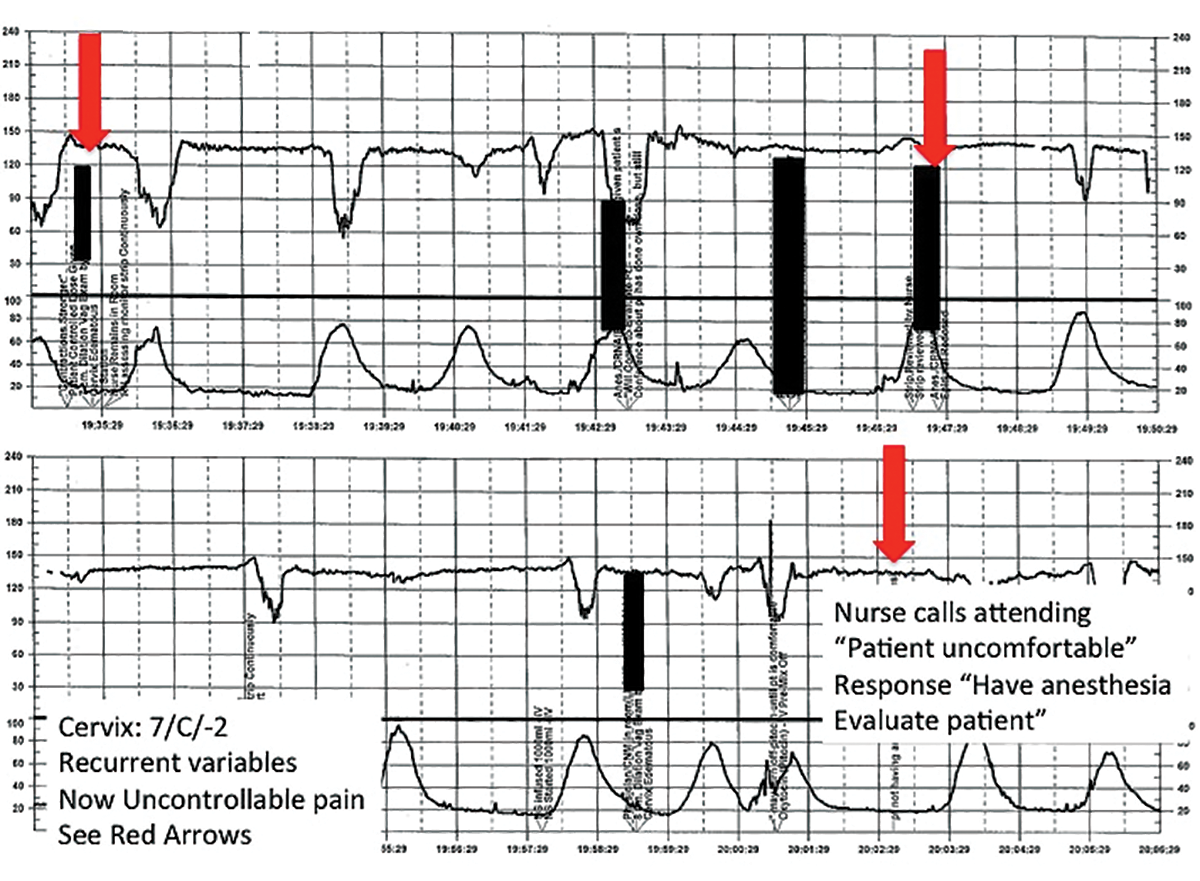
| ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||
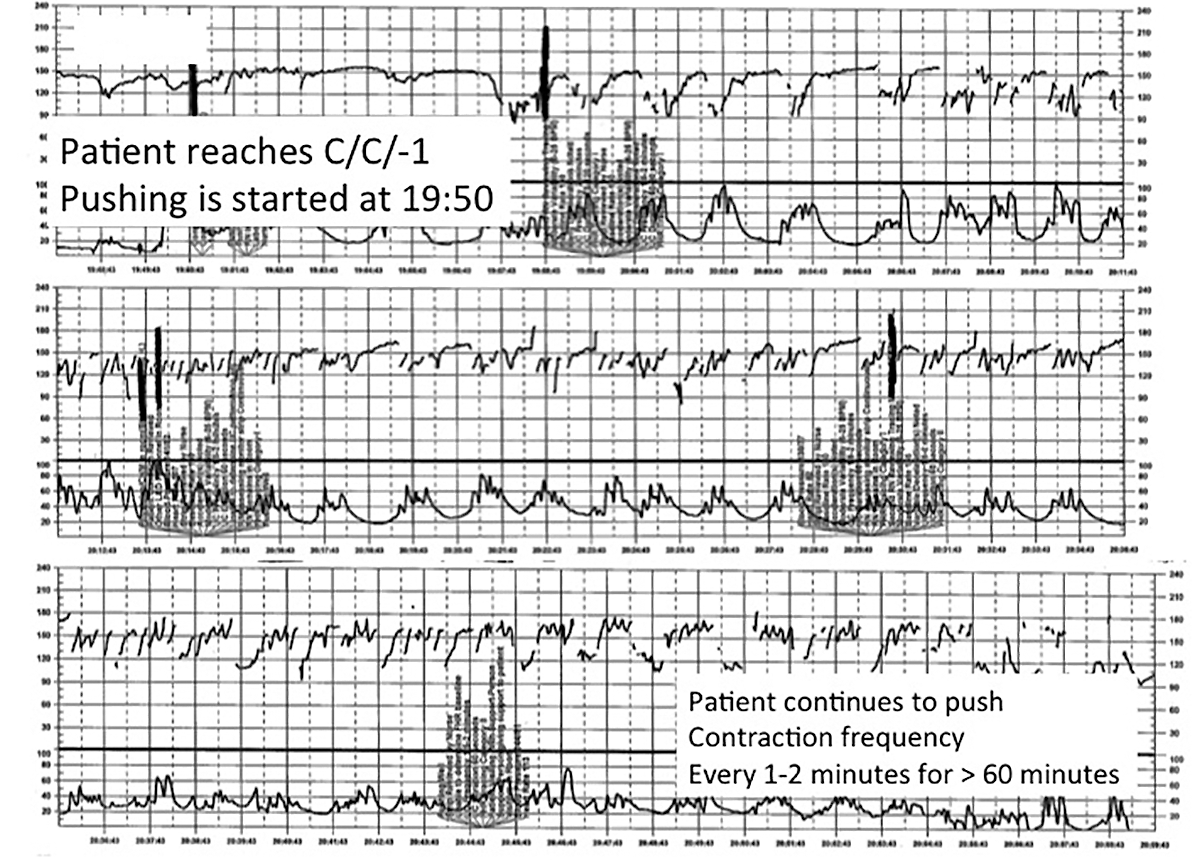
| ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||
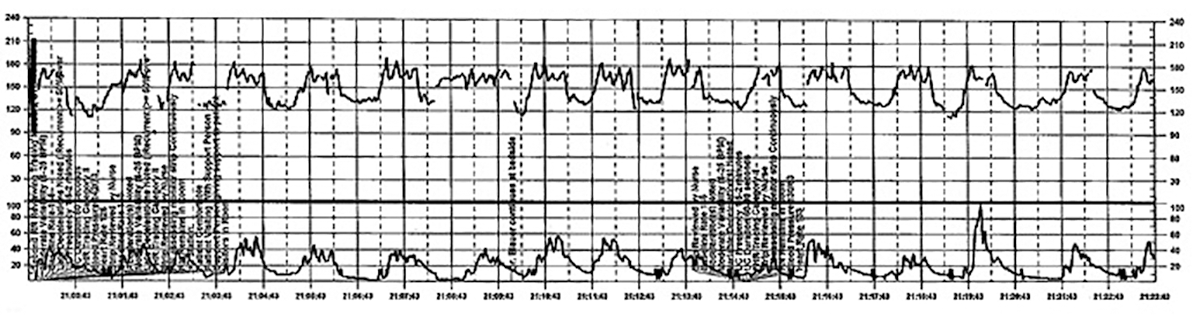
| ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||
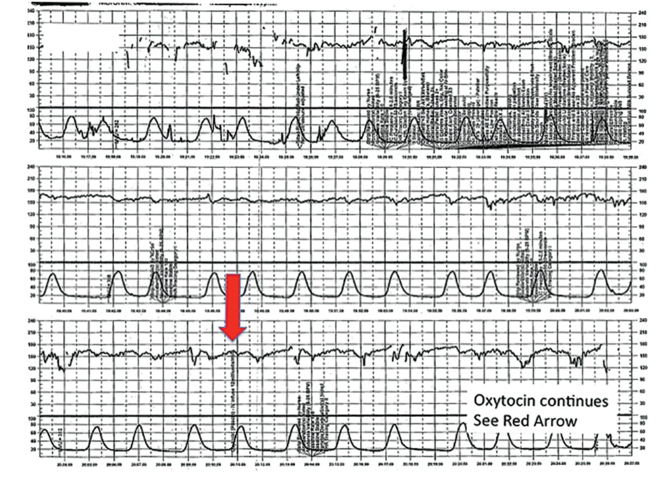
| ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||

| ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||

| ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||

| ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||

| ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||

| ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||

| ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||

| ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||

| ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||

| ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||

| ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||

| ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||

| ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
In this article
• Communicate urgency
• Situational awareness
Start offering aspirin to pregnant women at high risk for preeclampsia
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7
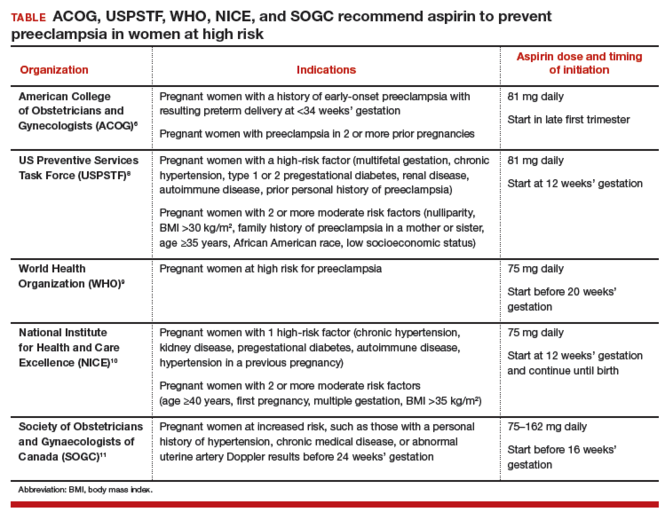
An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7

An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7

An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.