User login
More Than 15% of Reproductive Age Women Use Antidepressants
About 15% of women of childbearing age filled prescriptions for antidepressants at least once a year during 2008-2013, according to a new report from the Centers for Disease Control and Prevention.
Women between the ages of 35 and 44 years made up the largest share of those filling an antidepressant prescription. The most commonly filled prescriptions were for sertraline, bupropion, and citalopram (Morb Mortal Wkly Rep. 2016 Jan 29;65:41-6).
The analysis is based on prescription information from a database of employer-sponsored insurance plans representing an average of 5.8 million women aged 15-44 years for each year of the study.
The findings raise public health concerns given the possible association between some antidepressants and birth defects and the large number of unintended pregnancies, wrote the investigators, led by April L. Dawson of the CDC’s National Center on Birth Defects and Developmental Disabilities.
A recent analysis of the National Birth Defects Prevention Study data found that paroxetine and fluoxetine, both SSRIs, were linked with a higher rate of some birth defects, including cardiac abnormalities, though the increase in absolute risk was small (BMJ 2015;351:h3190).
“Prescribing of antidepressants is common, and research on antidepressant safety during pregnancy needs to be accelerated to provide evidence-based information to health care providers and women about the potential risks for antidepressant exposure before and during pregnancy and between pregnancies,” Ms. Dawson and her colleagues wrote.
The study authors were employees of the CDC or the March of Dimes Foundation.
About 15% of women of childbearing age filled prescriptions for antidepressants at least once a year during 2008-2013, according to a new report from the Centers for Disease Control and Prevention.
Women between the ages of 35 and 44 years made up the largest share of those filling an antidepressant prescription. The most commonly filled prescriptions were for sertraline, bupropion, and citalopram (Morb Mortal Wkly Rep. 2016 Jan 29;65:41-6).
The analysis is based on prescription information from a database of employer-sponsored insurance plans representing an average of 5.8 million women aged 15-44 years for each year of the study.
The findings raise public health concerns given the possible association between some antidepressants and birth defects and the large number of unintended pregnancies, wrote the investigators, led by April L. Dawson of the CDC’s National Center on Birth Defects and Developmental Disabilities.
A recent analysis of the National Birth Defects Prevention Study data found that paroxetine and fluoxetine, both SSRIs, were linked with a higher rate of some birth defects, including cardiac abnormalities, though the increase in absolute risk was small (BMJ 2015;351:h3190).
“Prescribing of antidepressants is common, and research on antidepressant safety during pregnancy needs to be accelerated to provide evidence-based information to health care providers and women about the potential risks for antidepressant exposure before and during pregnancy and between pregnancies,” Ms. Dawson and her colleagues wrote.
The study authors were employees of the CDC or the March of Dimes Foundation.
About 15% of women of childbearing age filled prescriptions for antidepressants at least once a year during 2008-2013, according to a new report from the Centers for Disease Control and Prevention.
Women between the ages of 35 and 44 years made up the largest share of those filling an antidepressant prescription. The most commonly filled prescriptions were for sertraline, bupropion, and citalopram (Morb Mortal Wkly Rep. 2016 Jan 29;65:41-6).
The analysis is based on prescription information from a database of employer-sponsored insurance plans representing an average of 5.8 million women aged 15-44 years for each year of the study.
The findings raise public health concerns given the possible association between some antidepressants and birth defects and the large number of unintended pregnancies, wrote the investigators, led by April L. Dawson of the CDC’s National Center on Birth Defects and Developmental Disabilities.
A recent analysis of the National Birth Defects Prevention Study data found that paroxetine and fluoxetine, both SSRIs, were linked with a higher rate of some birth defects, including cardiac abnormalities, though the increase in absolute risk was small (BMJ 2015;351:h3190).
“Prescribing of antidepressants is common, and research on antidepressant safety during pregnancy needs to be accelerated to provide evidence-based information to health care providers and women about the potential risks for antidepressant exposure before and during pregnancy and between pregnancies,” Ms. Dawson and her colleagues wrote.
The study authors were employees of the CDC or the March of Dimes Foundation.
FROM MMWR
More than 15% of reproductive age women use antidepressants
About 15% of women of childbearing age filled prescriptions for antidepressants at least once a year during 2008-2013, according to a new report from the Centers for Disease Control and Prevention.
Women between the ages of 35 and 44 years made up the largest share of those filling an antidepressant prescription. The most commonly filled prescriptions were for sertraline, bupropion, and citalopram (Morb Mortal Wkly Rep. 2016 Jan 29;65:41-6).
The analysis is based on prescription information from a database of employer-sponsored insurance plans representing an average of 5.8 million women aged 15-44 years for each year of the study.
The findings raise public health concerns given the possible association between some antidepressants and birth defects and the large number of unintended pregnancies, wrote the investigators, led by April L. Dawson of the CDC’s National Center on Birth Defects and Developmental Disabilities.
A recent analysis of the National Birth Defects Prevention Study data found that paroxetine and fluoxetine, both SSRIs, were linked with a higher rate of some birth defects, including cardiac abnormalities, though the increase in absolute risk was small (BMJ 2015;351:h3190).
“Prescribing of antidepressants is common, and research on antidepressant safety during pregnancy needs to be accelerated to provide evidence-based information to health care providers and women about the potential risks for antidepressant exposure before and during pregnancy and between pregnancies,” Ms. Dawson and her colleagues wrote.
The study authors were employees of the CDC or the March of Dimes Foundation.
About 15% of women of childbearing age filled prescriptions for antidepressants at least once a year during 2008-2013, according to a new report from the Centers for Disease Control and Prevention.
Women between the ages of 35 and 44 years made up the largest share of those filling an antidepressant prescription. The most commonly filled prescriptions were for sertraline, bupropion, and citalopram (Morb Mortal Wkly Rep. 2016 Jan 29;65:41-6).
The analysis is based on prescription information from a database of employer-sponsored insurance plans representing an average of 5.8 million women aged 15-44 years for each year of the study.
The findings raise public health concerns given the possible association between some antidepressants and birth defects and the large number of unintended pregnancies, wrote the investigators, led by April L. Dawson of the CDC’s National Center on Birth Defects and Developmental Disabilities.
A recent analysis of the National Birth Defects Prevention Study data found that paroxetine and fluoxetine, both SSRIs, were linked with a higher rate of some birth defects, including cardiac abnormalities, though the increase in absolute risk was small (BMJ 2015;351:h3190).
“Prescribing of antidepressants is common, and research on antidepressant safety during pregnancy needs to be accelerated to provide evidence-based information to health care providers and women about the potential risks for antidepressant exposure before and during pregnancy and between pregnancies,” Ms. Dawson and her colleagues wrote.
The study authors were employees of the CDC or the March of Dimes Foundation.
About 15% of women of childbearing age filled prescriptions for antidepressants at least once a year during 2008-2013, according to a new report from the Centers for Disease Control and Prevention.
Women between the ages of 35 and 44 years made up the largest share of those filling an antidepressant prescription. The most commonly filled prescriptions were for sertraline, bupropion, and citalopram (Morb Mortal Wkly Rep. 2016 Jan 29;65:41-6).
The analysis is based on prescription information from a database of employer-sponsored insurance plans representing an average of 5.8 million women aged 15-44 years for each year of the study.
The findings raise public health concerns given the possible association between some antidepressants and birth defects and the large number of unintended pregnancies, wrote the investigators, led by April L. Dawson of the CDC’s National Center on Birth Defects and Developmental Disabilities.
A recent analysis of the National Birth Defects Prevention Study data found that paroxetine and fluoxetine, both SSRIs, were linked with a higher rate of some birth defects, including cardiac abnormalities, though the increase in absolute risk was small (BMJ 2015;351:h3190).
“Prescribing of antidepressants is common, and research on antidepressant safety during pregnancy needs to be accelerated to provide evidence-based information to health care providers and women about the potential risks for antidepressant exposure before and during pregnancy and between pregnancies,” Ms. Dawson and her colleagues wrote.
The study authors were employees of the CDC or the March of Dimes Foundation.
FROM MMWR
Key clinical point: About 15% of reproductive-age women use antidepressants each year.
Major finding: A total of 15.4% of women aged 15-44 years filled at least one prescription annually for an antidepressant during 2008-2013.
Data source: A retrospective analysis of data from a commercial database that included an average of 5.8 million women with private, employer-sponsored insurance.
Disclosures: Study authors were employees of the CDC or the March of Dimes Foundation.
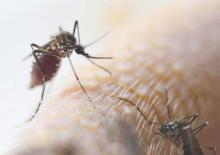
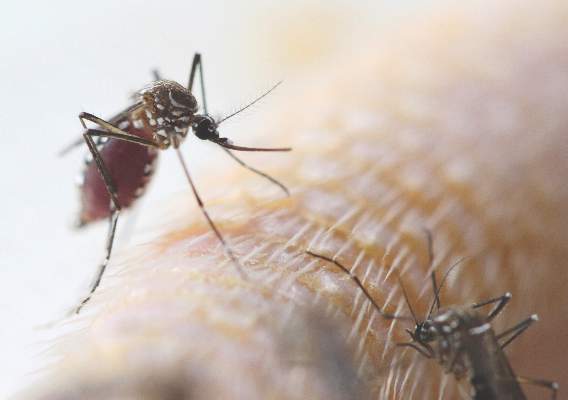
Zika could soon infect 4 million; U.S. impact likely to be much smaller
The Zika virus continues to spread, with the potential to infect up to 4 million people throughout the Americas.
But although locally transmitted infections are all almost certainly inevitable in the United States, federal health officials don’t expect large-scale infections.
“We do expect to see local transmission, but we are not likely to see widespread outbreaks,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said during a press briefing on Jan. 28. “We think these would occur in pockets, similar to what we now see with dengue and chikungunya. We are not being cavalier about this; we are preparing. But large-scale outbreaks are not something we are likely to see, based on our experience with dengue and chikungunya.”
Dr. Fauci’s comments were in contrast to warnings issued simultaneously by the World Health Organization and the Pan American Health Organization (PAHO), WHO’s South American arm.
On Jan. 28, the WHO announced it would convene a meeting of its International Health Regulations Emergency Committee. WHO Director-General Margaret Chan said the virus is now present in 23 countries and territories in the region, as well as in parts of the South Pacific.
“The level of alarm is extremely high,” she said during the meeting. Although a causal link between the virus and outbreaks of microcephaly and Guillain-Barré syndrome is yet unproven, she said it is strongly suspected. “The possible links, only recently suspected, have rapidly changed the risk profile of Zika, from a mild threat to one of alarming proportions.”
Dr. Marcos Espinal, PAHO’s director of communicable diseases and health analysis, outlined the potential spread. His agency expects to see 3-4 million cases throughout North and South America, which could occur in any region that has endemic dengue fever. Both viruses are carried by mosquitoes of the Aedes genus. Two of these, the Asian tiger mosquito and the yellow fever mosquito, are commonly found in the United States, particularly in warmer regions.
Thus far, 31 cases of Zika virus have been confirmed in the United States (11 states and Washington), Dr. Anne Schuchat, principal deputy director for the Centers for Disease Control and Prevention, said in that agency’s briefing. All of these cases have been associated with travel to endemic areas, she said. She was unable to say how many cases had occurred in pregnant women.
There have been 19 confirmed cases in Puerto Rico and 1 in the U.S. Virgin Islands. These could be locally acquired cases, although that has not been confirmed. However, Zika infection is now a notifiable illness and any confirmed cases must be reported to CDC, she said.
Nearly a million cases have been laboratory confirmed in South America since 2015, Dr. Espinal noted. The vast majority of those have caused mild, transient symptoms. But in Brazil alone – the epicenter of the epidemic – there has been a substantial increase in newborn microcephaly. Almost 4,000 cases of this rare birth defect have been identified since October, when tracking began. Although the cases coincide with the rise of Zika infections, no one knows how many were related or what pathology could be mediating the association.
This is just one question muddying the Zika waters right now, Dr. Chan of the WHO said. Several difficult-to-address issues are hampering an effective response, including the widespread range of the mosquito vectors, which allows international spread; a lack of population immunity; and the absence of rapid diagnostic tests, effective treatment, and any vaccine.
Another concern is the potential for illness-potentiating coinfection. In the lab, Zika virus has shown cross-reactions with dengue types 1-4, yellow fever, and West Nile virus – all of which are carried by Aedes mosquitoes.
Scientists at the CDC and National Institutes of Health are working on the problem now, Dr. Fauci said. Efforts include the creation of animal models to study disease transmission and its effect on pre- and postnatal outcomes, and of diagnostic platforms that could easily and quickly identify Zika infections. These would quickly differentiate Zika from dengue infections, which are also caused by a strain of flavivirus.
An effective vaccine will not be quickly forthcoming, he cautioned, although a phase I trial is in the works and could begin later this year. The candidate is a DNA-based vaccine similar to the one now used to protect against dengue. It would almost certainly not be approved in less than 2 years, Dr Fauci added.
Until some of these questions have been answered, or until the epidemic slows, the CDC continues to caution pregnant women against travel to endemic areas. For those who do travel or who live in endemic areas, Dr. Schuchat reiterated CDC’s long-held advice to take precautions against mosquito bites: long-sleeved shirts and long pants; staying inside; and using an effective insect repellent. Those containing DEET are most effective and are safe for pregnant women to use, Dr. Schuchat said.
For now, the CDC’s Zika travel alert includes Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
A transcript of the press briefing is available online.
This article was updated 2/1/2016.
On Twitter @Alz_Gal
The Zika virus continues to spread, with the potential to infect up to 4 million people throughout the Americas.
But although locally transmitted infections are all almost certainly inevitable in the United States, federal health officials don’t expect large-scale infections.
“We do expect to see local transmission, but we are not likely to see widespread outbreaks,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said during a press briefing on Jan. 28. “We think these would occur in pockets, similar to what we now see with dengue and chikungunya. We are not being cavalier about this; we are preparing. But large-scale outbreaks are not something we are likely to see, based on our experience with dengue and chikungunya.”
Dr. Fauci’s comments were in contrast to warnings issued simultaneously by the World Health Organization and the Pan American Health Organization (PAHO), WHO’s South American arm.
On Jan. 28, the WHO announced it would convene a meeting of its International Health Regulations Emergency Committee. WHO Director-General Margaret Chan said the virus is now present in 23 countries and territories in the region, as well as in parts of the South Pacific.
“The level of alarm is extremely high,” she said during the meeting. Although a causal link between the virus and outbreaks of microcephaly and Guillain-Barré syndrome is yet unproven, she said it is strongly suspected. “The possible links, only recently suspected, have rapidly changed the risk profile of Zika, from a mild threat to one of alarming proportions.”
Dr. Marcos Espinal, PAHO’s director of communicable diseases and health analysis, outlined the potential spread. His agency expects to see 3-4 million cases throughout North and South America, which could occur in any region that has endemic dengue fever. Both viruses are carried by mosquitoes of the Aedes genus. Two of these, the Asian tiger mosquito and the yellow fever mosquito, are commonly found in the United States, particularly in warmer regions.
Thus far, 31 cases of Zika virus have been confirmed in the United States (11 states and Washington), Dr. Anne Schuchat, principal deputy director for the Centers for Disease Control and Prevention, said in that agency’s briefing. All of these cases have been associated with travel to endemic areas, she said. She was unable to say how many cases had occurred in pregnant women.
There have been 19 confirmed cases in Puerto Rico and 1 in the U.S. Virgin Islands. These could be locally acquired cases, although that has not been confirmed. However, Zika infection is now a notifiable illness and any confirmed cases must be reported to CDC, she said.
Nearly a million cases have been laboratory confirmed in South America since 2015, Dr. Espinal noted. The vast majority of those have caused mild, transient symptoms. But in Brazil alone – the epicenter of the epidemic – there has been a substantial increase in newborn microcephaly. Almost 4,000 cases of this rare birth defect have been identified since October, when tracking began. Although the cases coincide with the rise of Zika infections, no one knows how many were related or what pathology could be mediating the association.
This is just one question muddying the Zika waters right now, Dr. Chan of the WHO said. Several difficult-to-address issues are hampering an effective response, including the widespread range of the mosquito vectors, which allows international spread; a lack of population immunity; and the absence of rapid diagnostic tests, effective treatment, and any vaccine.
Another concern is the potential for illness-potentiating coinfection. In the lab, Zika virus has shown cross-reactions with dengue types 1-4, yellow fever, and West Nile virus – all of which are carried by Aedes mosquitoes.
Scientists at the CDC and National Institutes of Health are working on the problem now, Dr. Fauci said. Efforts include the creation of animal models to study disease transmission and its effect on pre- and postnatal outcomes, and of diagnostic platforms that could easily and quickly identify Zika infections. These would quickly differentiate Zika from dengue infections, which are also caused by a strain of flavivirus.
An effective vaccine will not be quickly forthcoming, he cautioned, although a phase I trial is in the works and could begin later this year. The candidate is a DNA-based vaccine similar to the one now used to protect against dengue. It would almost certainly not be approved in less than 2 years, Dr Fauci added.
Until some of these questions have been answered, or until the epidemic slows, the CDC continues to caution pregnant women against travel to endemic areas. For those who do travel or who live in endemic areas, Dr. Schuchat reiterated CDC’s long-held advice to take precautions against mosquito bites: long-sleeved shirts and long pants; staying inside; and using an effective insect repellent. Those containing DEET are most effective and are safe for pregnant women to use, Dr. Schuchat said.
For now, the CDC’s Zika travel alert includes Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
A transcript of the press briefing is available online.
This article was updated 2/1/2016.
On Twitter @Alz_Gal
The Zika virus continues to spread, with the potential to infect up to 4 million people throughout the Americas.
But although locally transmitted infections are all almost certainly inevitable in the United States, federal health officials don’t expect large-scale infections.
“We do expect to see local transmission, but we are not likely to see widespread outbreaks,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said during a press briefing on Jan. 28. “We think these would occur in pockets, similar to what we now see with dengue and chikungunya. We are not being cavalier about this; we are preparing. But large-scale outbreaks are not something we are likely to see, based on our experience with dengue and chikungunya.”
Dr. Fauci’s comments were in contrast to warnings issued simultaneously by the World Health Organization and the Pan American Health Organization (PAHO), WHO’s South American arm.
On Jan. 28, the WHO announced it would convene a meeting of its International Health Regulations Emergency Committee. WHO Director-General Margaret Chan said the virus is now present in 23 countries and territories in the region, as well as in parts of the South Pacific.
“The level of alarm is extremely high,” she said during the meeting. Although a causal link between the virus and outbreaks of microcephaly and Guillain-Barré syndrome is yet unproven, she said it is strongly suspected. “The possible links, only recently suspected, have rapidly changed the risk profile of Zika, from a mild threat to one of alarming proportions.”
Dr. Marcos Espinal, PAHO’s director of communicable diseases and health analysis, outlined the potential spread. His agency expects to see 3-4 million cases throughout North and South America, which could occur in any region that has endemic dengue fever. Both viruses are carried by mosquitoes of the Aedes genus. Two of these, the Asian tiger mosquito and the yellow fever mosquito, are commonly found in the United States, particularly in warmer regions.
Thus far, 31 cases of Zika virus have been confirmed in the United States (11 states and Washington), Dr. Anne Schuchat, principal deputy director for the Centers for Disease Control and Prevention, said in that agency’s briefing. All of these cases have been associated with travel to endemic areas, she said. She was unable to say how many cases had occurred in pregnant women.
There have been 19 confirmed cases in Puerto Rico and 1 in the U.S. Virgin Islands. These could be locally acquired cases, although that has not been confirmed. However, Zika infection is now a notifiable illness and any confirmed cases must be reported to CDC, she said.
Nearly a million cases have been laboratory confirmed in South America since 2015, Dr. Espinal noted. The vast majority of those have caused mild, transient symptoms. But in Brazil alone – the epicenter of the epidemic – there has been a substantial increase in newborn microcephaly. Almost 4,000 cases of this rare birth defect have been identified since October, when tracking began. Although the cases coincide with the rise of Zika infections, no one knows how many were related or what pathology could be mediating the association.
This is just one question muddying the Zika waters right now, Dr. Chan of the WHO said. Several difficult-to-address issues are hampering an effective response, including the widespread range of the mosquito vectors, which allows international spread; a lack of population immunity; and the absence of rapid diagnostic tests, effective treatment, and any vaccine.
Another concern is the potential for illness-potentiating coinfection. In the lab, Zika virus has shown cross-reactions with dengue types 1-4, yellow fever, and West Nile virus – all of which are carried by Aedes mosquitoes.
Scientists at the CDC and National Institutes of Health are working on the problem now, Dr. Fauci said. Efforts include the creation of animal models to study disease transmission and its effect on pre- and postnatal outcomes, and of diagnostic platforms that could easily and quickly identify Zika infections. These would quickly differentiate Zika from dengue infections, which are also caused by a strain of flavivirus.
An effective vaccine will not be quickly forthcoming, he cautioned, although a phase I trial is in the works and could begin later this year. The candidate is a DNA-based vaccine similar to the one now used to protect against dengue. It would almost certainly not be approved in less than 2 years, Dr Fauci added.
Until some of these questions have been answered, or until the epidemic slows, the CDC continues to caution pregnant women against travel to endemic areas. For those who do travel or who live in endemic areas, Dr. Schuchat reiterated CDC’s long-held advice to take precautions against mosquito bites: long-sleeved shirts and long pants; staying inside; and using an effective insect repellent. Those containing DEET are most effective and are safe for pregnant women to use, Dr. Schuchat said.
For now, the CDC’s Zika travel alert includes Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
A transcript of the press briefing is available online.
This article was updated 2/1/2016.
On Twitter @Alz_Gal
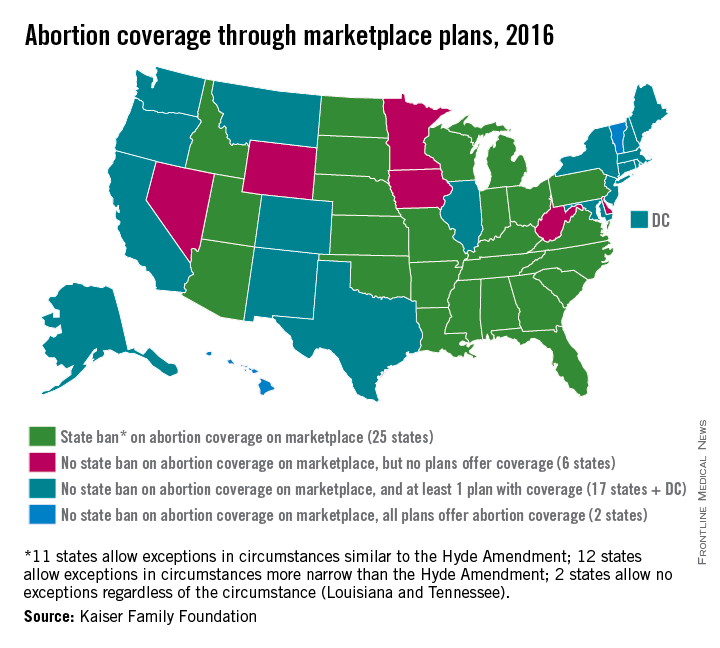
Abortion Coverage Unavailable in 31 State Marketplaces
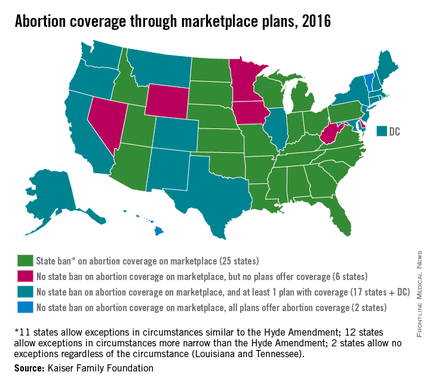
Half of all states currently ban health plans on their ACA marketplaces from providing abortion coverage, while six states allow such coverage but have no plans that offer it, according to an analysis from the Kaiser Family Foundation.
Of the 25 states that prohibit abortion coverage, 11 allow exceptions similar to the Hyde Amendment, which allows for federal funds to be used only for abortions of pregnancies resulting from rape or incest or those that endanger the woman’s life. Another 12 states have exceptions that are more narrow than the Hyde Amendment, and two states, Louisiana and Tennessee, do not have any exceptions, Kaiser reported.
Along with the six states that allow abortion coverage on their marketplaces but have no health plans that offer it, there are 17 states that have at least one plan that makes abortion coverage available and two states, Hawaii and Vermont, where all of the marketplace plans include it, according to Kaiser.
Half of all states currently ban health plans on their ACA marketplaces from providing abortion coverage, while six states allow such coverage but have no plans that offer it, according to an analysis from the Kaiser Family Foundation.
Of the 25 states that prohibit abortion coverage, 11 allow exceptions similar to the Hyde Amendment, which allows for federal funds to be used only for abortions of pregnancies resulting from rape or incest or those that endanger the woman’s life. Another 12 states have exceptions that are more narrow than the Hyde Amendment, and two states, Louisiana and Tennessee, do not have any exceptions, Kaiser reported.
Along with the six states that allow abortion coverage on their marketplaces but have no health plans that offer it, there are 17 states that have at least one plan that makes abortion coverage available and two states, Hawaii and Vermont, where all of the marketplace plans include it, according to Kaiser.

Half of all states currently ban health plans on their ACA marketplaces from providing abortion coverage, while six states allow such coverage but have no plans that offer it, according to an analysis from the Kaiser Family Foundation.
Of the 25 states that prohibit abortion coverage, 11 allow exceptions similar to the Hyde Amendment, which allows for federal funds to be used only for abortions of pregnancies resulting from rape or incest or those that endanger the woman’s life. Another 12 states have exceptions that are more narrow than the Hyde Amendment, and two states, Louisiana and Tennessee, do not have any exceptions, Kaiser reported.
Along with the six states that allow abortion coverage on their marketplaces but have no health plans that offer it, there are 17 states that have at least one plan that makes abortion coverage available and two states, Hawaii and Vermont, where all of the marketplace plans include it, according to Kaiser.

Experts: WHO failing to lead on Zika pandemic
The World Health Organization has failed to act decisively in response to the growing Zika virus outbreak, global health experts said in a JAMA viewpoint article published Jan. 27.
While the global public health dimensions of Zika are quite clear, the WHO is “still not taking a leadership role in the crisis,” wrote coauthors Dr. Daniel R. Lucey of Georgetown University and Lawrence O. Gostin of Georgetown University Law Center, both in Washington. They called on the WHO to convene an emergency committee to advise Director-General Margaret Chan about the conditions necessary to declare a Public Health Emergency of International Concern, a process that would catalyze international attention, funding, and research.
“WHO headquarters has thus far not been proactive, given potentially serious ramifications,” the coauthors said. They encouraged the WHO to learn lessons from its handling of the Ebola epidemic, a key one being the need for an “intermediate-level response to emerging crises,” thus avoiding overreaction while still galvanizing global action.
In the interim, Dr. Lucey and Mr. Gostin assert that the international community cannot wait for the WHO to act, as infectious disease modeling anticipates significant international spread by travelers from Brazil – the epicenter of the current outbreak – to the rest of the Americas, Europe, and Asia. The coauthors emphasize seven key international health system strategies that countries should adopt and fund in preparation for a Zika pandemic. These strategies are critical for countries already affected by the virus and those with significant Aedes mosquito populations – the vectors for Zika virus.
The seven health system strategies recommended by Dr. Lucey and Mr. Gostin are:
• Vector control. Mosquito-borne diseases require reducing source populations, including physical and biological controls. Effective mosquito surveillance is also essential to ensure focused interventions.
• Risk communication. Health information campaigns should advise the public to avoid mosquito exposure.
• Enhanced Zika surveillance. The International Health Regulations (IHR) require countries to report unusual Zika-related cases. Countries must train health workers to observe and report Zika-related disease and create robust systems for collecting and analyzing surveillance data to complement public health strategies.
• Travel advisories. To minimize harm to high-risk travelers, agencies should consider issuing travel advisories, which include guidance on reducing mosquito exposure and greater awareness of symptoms. On returning home, symptomatic individuals should report their travel histories to their physician.
• Clinical management. An estimated 80% of Zika infections are asymptomatic, and most of the remainder are self-limited. No specific antiviral treatment is available and care is supportive, with symptoms usually resolving within 7 days. On Jan. 19, the U.S. Centers for Disease Control and Prevention issued new guidelines for pregnant women with suspected or proven Zika virus infection, including an algorithm for offering laboratory testing.
• Accelerated research and development. When Zika infection was seen as usually asymptomatic and self-limited, the coauthors say researchers had little incentive to develop reliable countermeasures. The emerging data on fetal complications have altered this equation, making research on new vaccines urgent. However, a safe and effective Zika virus vaccine is probably 3-10 years away, even with accelerated research.
• Public health emergency declarations. The coauthors say that a national declaration of a public health emergency could help to focus political attention, while financing a surge in resources. They suggest that countries experiencing major Zika virus outbreaks could invoke heightened emergency powers.
Read the complete viewpoint essay in JAMA (2016 Jan 27. doi: 10.1001/jama.2016.0904)
On Twitter @richpizzi
The World Health Organization has failed to act decisively in response to the growing Zika virus outbreak, global health experts said in a JAMA viewpoint article published Jan. 27.
While the global public health dimensions of Zika are quite clear, the WHO is “still not taking a leadership role in the crisis,” wrote coauthors Dr. Daniel R. Lucey of Georgetown University and Lawrence O. Gostin of Georgetown University Law Center, both in Washington. They called on the WHO to convene an emergency committee to advise Director-General Margaret Chan about the conditions necessary to declare a Public Health Emergency of International Concern, a process that would catalyze international attention, funding, and research.
“WHO headquarters has thus far not been proactive, given potentially serious ramifications,” the coauthors said. They encouraged the WHO to learn lessons from its handling of the Ebola epidemic, a key one being the need for an “intermediate-level response to emerging crises,” thus avoiding overreaction while still galvanizing global action.
In the interim, Dr. Lucey and Mr. Gostin assert that the international community cannot wait for the WHO to act, as infectious disease modeling anticipates significant international spread by travelers from Brazil – the epicenter of the current outbreak – to the rest of the Americas, Europe, and Asia. The coauthors emphasize seven key international health system strategies that countries should adopt and fund in preparation for a Zika pandemic. These strategies are critical for countries already affected by the virus and those with significant Aedes mosquito populations – the vectors for Zika virus.
The seven health system strategies recommended by Dr. Lucey and Mr. Gostin are:
• Vector control. Mosquito-borne diseases require reducing source populations, including physical and biological controls. Effective mosquito surveillance is also essential to ensure focused interventions.
• Risk communication. Health information campaigns should advise the public to avoid mosquito exposure.
• Enhanced Zika surveillance. The International Health Regulations (IHR) require countries to report unusual Zika-related cases. Countries must train health workers to observe and report Zika-related disease and create robust systems for collecting and analyzing surveillance data to complement public health strategies.
• Travel advisories. To minimize harm to high-risk travelers, agencies should consider issuing travel advisories, which include guidance on reducing mosquito exposure and greater awareness of symptoms. On returning home, symptomatic individuals should report their travel histories to their physician.
• Clinical management. An estimated 80% of Zika infections are asymptomatic, and most of the remainder are self-limited. No specific antiviral treatment is available and care is supportive, with symptoms usually resolving within 7 days. On Jan. 19, the U.S. Centers for Disease Control and Prevention issued new guidelines for pregnant women with suspected or proven Zika virus infection, including an algorithm for offering laboratory testing.
• Accelerated research and development. When Zika infection was seen as usually asymptomatic and self-limited, the coauthors say researchers had little incentive to develop reliable countermeasures. The emerging data on fetal complications have altered this equation, making research on new vaccines urgent. However, a safe and effective Zika virus vaccine is probably 3-10 years away, even with accelerated research.
• Public health emergency declarations. The coauthors say that a national declaration of a public health emergency could help to focus political attention, while financing a surge in resources. They suggest that countries experiencing major Zika virus outbreaks could invoke heightened emergency powers.
Read the complete viewpoint essay in JAMA (2016 Jan 27. doi: 10.1001/jama.2016.0904)
On Twitter @richpizzi
The World Health Organization has failed to act decisively in response to the growing Zika virus outbreak, global health experts said in a JAMA viewpoint article published Jan. 27.
While the global public health dimensions of Zika are quite clear, the WHO is “still not taking a leadership role in the crisis,” wrote coauthors Dr. Daniel R. Lucey of Georgetown University and Lawrence O. Gostin of Georgetown University Law Center, both in Washington. They called on the WHO to convene an emergency committee to advise Director-General Margaret Chan about the conditions necessary to declare a Public Health Emergency of International Concern, a process that would catalyze international attention, funding, and research.
“WHO headquarters has thus far not been proactive, given potentially serious ramifications,” the coauthors said. They encouraged the WHO to learn lessons from its handling of the Ebola epidemic, a key one being the need for an “intermediate-level response to emerging crises,” thus avoiding overreaction while still galvanizing global action.
In the interim, Dr. Lucey and Mr. Gostin assert that the international community cannot wait for the WHO to act, as infectious disease modeling anticipates significant international spread by travelers from Brazil – the epicenter of the current outbreak – to the rest of the Americas, Europe, and Asia. The coauthors emphasize seven key international health system strategies that countries should adopt and fund in preparation for a Zika pandemic. These strategies are critical for countries already affected by the virus and those with significant Aedes mosquito populations – the vectors for Zika virus.
The seven health system strategies recommended by Dr. Lucey and Mr. Gostin are:
• Vector control. Mosquito-borne diseases require reducing source populations, including physical and biological controls. Effective mosquito surveillance is also essential to ensure focused interventions.
• Risk communication. Health information campaigns should advise the public to avoid mosquito exposure.
• Enhanced Zika surveillance. The International Health Regulations (IHR) require countries to report unusual Zika-related cases. Countries must train health workers to observe and report Zika-related disease and create robust systems for collecting and analyzing surveillance data to complement public health strategies.
• Travel advisories. To minimize harm to high-risk travelers, agencies should consider issuing travel advisories, which include guidance on reducing mosquito exposure and greater awareness of symptoms. On returning home, symptomatic individuals should report their travel histories to their physician.
• Clinical management. An estimated 80% of Zika infections are asymptomatic, and most of the remainder are self-limited. No specific antiviral treatment is available and care is supportive, with symptoms usually resolving within 7 days. On Jan. 19, the U.S. Centers for Disease Control and Prevention issued new guidelines for pregnant women with suspected or proven Zika virus infection, including an algorithm for offering laboratory testing.
• Accelerated research and development. When Zika infection was seen as usually asymptomatic and self-limited, the coauthors say researchers had little incentive to develop reliable countermeasures. The emerging data on fetal complications have altered this equation, making research on new vaccines urgent. However, a safe and effective Zika virus vaccine is probably 3-10 years away, even with accelerated research.
• Public health emergency declarations. The coauthors say that a national declaration of a public health emergency could help to focus political attention, while financing a surge in resources. They suggest that countries experiencing major Zika virus outbreaks could invoke heightened emergency powers.
Read the complete viewpoint essay in JAMA (2016 Jan 27. doi: 10.1001/jama.2016.0904)
On Twitter @richpizzi
FROM JAMA
Warfarin is best for anticoagulation in prosthetic heart valve pregnancies
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
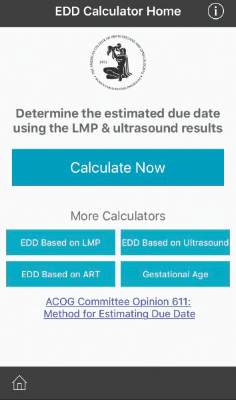
ACOG launches due date calculator app
Is it time to toss out the plastic pregnancy wheel?
The American College of Obstetricians and Gynecologists has launched a new due date calculator application that officials hope will replace the physical wheel and other smartphone apps already on the market.
The Estimated Due Date Calculator (EDD Calculator) incorporates the joint recommendations from ACOG, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine in determining due dates and reconciles the discrepancy in due dates between the first ultrasound and the date of the last menstrual period.
The EDD Calculator – available for free in the Apple and Google Play app stores as part of the ACOG app – also recalculates the due date based on use of assisted reproductive technology and allows users to determine the gestational age at a specific date.
“The EDD Calculator has ACOG guidelines built into the logic, therefore, it is the most accurate tool available for ob.gyns. and their staff,” Dr. Nathaniel DeNicola, ACOG digital and social media expert consultant and senior fellow at the Penn Social Media and Health Innovation Lab, said in a statement.
Although the EDD Calculator is designed for ob.gyns. and other health care providers, it also can be used by patients, according to ACOG.
To get the EDD Calculator, download the ACOG app (www.acog.org/acogapp).
On Twitter @maryellenny
Is it time to toss out the plastic pregnancy wheel?
The American College of Obstetricians and Gynecologists has launched a new due date calculator application that officials hope will replace the physical wheel and other smartphone apps already on the market.
The Estimated Due Date Calculator (EDD Calculator) incorporates the joint recommendations from ACOG, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine in determining due dates and reconciles the discrepancy in due dates between the first ultrasound and the date of the last menstrual period.
The EDD Calculator – available for free in the Apple and Google Play app stores as part of the ACOG app – also recalculates the due date based on use of assisted reproductive technology and allows users to determine the gestational age at a specific date.
“The EDD Calculator has ACOG guidelines built into the logic, therefore, it is the most accurate tool available for ob.gyns. and their staff,” Dr. Nathaniel DeNicola, ACOG digital and social media expert consultant and senior fellow at the Penn Social Media and Health Innovation Lab, said in a statement.
Although the EDD Calculator is designed for ob.gyns. and other health care providers, it also can be used by patients, according to ACOG.
To get the EDD Calculator, download the ACOG app (www.acog.org/acogapp).
On Twitter @maryellenny
Is it time to toss out the plastic pregnancy wheel?
The American College of Obstetricians and Gynecologists has launched a new due date calculator application that officials hope will replace the physical wheel and other smartphone apps already on the market.
The Estimated Due Date Calculator (EDD Calculator) incorporates the joint recommendations from ACOG, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine in determining due dates and reconciles the discrepancy in due dates between the first ultrasound and the date of the last menstrual period.
The EDD Calculator – available for free in the Apple and Google Play app stores as part of the ACOG app – also recalculates the due date based on use of assisted reproductive technology and allows users to determine the gestational age at a specific date.
“The EDD Calculator has ACOG guidelines built into the logic, therefore, it is the most accurate tool available for ob.gyns. and their staff,” Dr. Nathaniel DeNicola, ACOG digital and social media expert consultant and senior fellow at the Penn Social Media and Health Innovation Lab, said in a statement.
Although the EDD Calculator is designed for ob.gyns. and other health care providers, it also can be used by patients, according to ACOG.
To get the EDD Calculator, download the ACOG app (www.acog.org/acogapp).
On Twitter @maryellenny
U.S. Virgin Islands, Dominican Republic added to Zika travel advisory
Officials at the Centers for Disease Control and Prevention have added the U.S. Virgin Islands and the Dominican Republic to the rapidly growing list of areas that are part of the Zika virus travel alert.
The level 2 travel alert cautions travelers to use enhanced precautions to prevent mosquito bites when traveling to areas with ongoing Zika virus transmission. Though the Zika virus is generally mild, with symptoms lasting up to a week, there are more serious risks for pregnant women. Health officials are investigating the connection between microcephaly and other poor outcomes in the babies of pregnant women who were infected with Zika virus.
Until more is known, the CDC is advising pregnant women and those who are trying to become pregnant to postpone travel to these areas. If such travel is necessary, CDC recommends consulting a physician beforehand and strictly following steps to avoid mosquito bites, including wearing long-sleeved shirts and pants, staying indoors, and using insect repellent.
The CDC’s Zika travel alert also includes Puerto Rico (a U.S. territory), Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, and Venezuela.
On Twitter @maryellenny
Officials at the Centers for Disease Control and Prevention have added the U.S. Virgin Islands and the Dominican Republic to the rapidly growing list of areas that are part of the Zika virus travel alert.
The level 2 travel alert cautions travelers to use enhanced precautions to prevent mosquito bites when traveling to areas with ongoing Zika virus transmission. Though the Zika virus is generally mild, with symptoms lasting up to a week, there are more serious risks for pregnant women. Health officials are investigating the connection between microcephaly and other poor outcomes in the babies of pregnant women who were infected with Zika virus.
Until more is known, the CDC is advising pregnant women and those who are trying to become pregnant to postpone travel to these areas. If such travel is necessary, CDC recommends consulting a physician beforehand and strictly following steps to avoid mosquito bites, including wearing long-sleeved shirts and pants, staying indoors, and using insect repellent.
The CDC’s Zika travel alert also includes Puerto Rico (a U.S. territory), Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, and Venezuela.
On Twitter @maryellenny
Officials at the Centers for Disease Control and Prevention have added the U.S. Virgin Islands and the Dominican Republic to the rapidly growing list of areas that are part of the Zika virus travel alert.
The level 2 travel alert cautions travelers to use enhanced precautions to prevent mosquito bites when traveling to areas with ongoing Zika virus transmission. Though the Zika virus is generally mild, with symptoms lasting up to a week, there are more serious risks for pregnant women. Health officials are investigating the connection between microcephaly and other poor outcomes in the babies of pregnant women who were infected with Zika virus.
Until more is known, the CDC is advising pregnant women and those who are trying to become pregnant to postpone travel to these areas. If such travel is necessary, CDC recommends consulting a physician beforehand and strictly following steps to avoid mosquito bites, including wearing long-sleeved shirts and pants, staying indoors, and using insect repellent.
The CDC’s Zika travel alert also includes Puerto Rico (a U.S. territory), Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, and Venezuela.
On Twitter @maryellenny
New testing guidelines for infants with possible Zika virus infection
The Centers for Disease Control and Prevention has released interim guidelines for U.S. clinicians caring for infants born to mothers who traveled to or resided in an area with Zika virus transmission during pregnancy.
The guidelines, released Jan. 26, address the evaluation and testing of infants with possible congenital Zika virus infection, and follow the Jan. 19 release of similar guidelines for the care of pregnant women with possible exposure to the mosquito-borne virus. Most importantly, the new guidelines say Zika virus testing should be performed for infants with microcephaly or intracranial calcifications who are born to women with possible Zika virus exposure during pregnancy, and for infants born to women with positive or inconclusive Zika virus test results.
“Pediatric health are providers should work closely with obstetric providers to identify infants whose mothers were potentially infected with Zika virus during pregnancy (based on travel to or residence in an area with Zika virus transmission),” according to the guidelines, which were published in Morbidity and Mortality Weekly Report (MMWR. 2016 Jan 26;65[Early Release:1-5]).
Infants with laboratory evidence of a possible congenital Zika virus infection should undergo additional clinical evaluation, and state or territorial health departments should be contacted to facilitate testing. Zika virus disease is an arboviral disease and thus is a nationally notifiable condition, according to guideline authors Dr. J. Erin Staples and her colleagues at the CDC, Atlanta.
Both molecular and serologic tests are recommended for infants undergoing evaluation for possible congenital Zika virus infection, they noted.
Serum specimens for reverse-transcription-polymerase chain reaction testing should be collected from the umbilical cord or directly from the infant within 2 days of birth, and cerebrospinal fluid collected for other studies, as well as frozen and fixed placenta obtained at delivery, should also be tested by RT-PCR.
IgM ELISA for Zika virus and dengue virus should also be performed on infant serum, infant CSF, and maternal serum, but results from these assays can be falsely positive because of cross-reacting antibodies, the authors noted.
Other tests that can be considered include a plaque reduction neutralization test to measure virus-specific neutralizing antibodies and to discriminate between cross-reacting antibodies and closely related flaviviruses, and immunohistochemical staining to detect the virus antigen on fixed placenta and umbilical cord tissues.
Further clinical evaluation and laboratory testing is recommended for infants with microcephaly or intracranial calcifications detected prenatally or at birth if the mother was potentially infected during pregnancy, they said.
In infants with possible Zika virus exposure during pregnancy, but without microcephaly or intracranial calcification, subsequent evaluation depends on maternal testing results. Routine care is recommended if maternal test results are negative, and testing for a possible congenital infection is recommended if maternal results are positive or inconclusive.
If all of an infant’s tests are negative for Zika virus infection, no further Zika virus testing or evaluation is recommended. In the event of any positive or inconclusive test, further evaluation and follow-up is recommended.
Other considerations
Abnormal eye findings have been reported in infants with possible congenital infection, therefore an opthalmologic evaluation, including retinal examination is advised during the first month of life, as is a repeat hearing screen at age 6 months – even if the initial screen was normal, the authors said.
The infant should be followed to assess for long-term sequelae, and the case should be reported. Follow-up should include a cranial ultrasound to assess for subclinical findings, unless a third trimester ultrasound showed no brain abnormalities, they added.
No specific antiviral treatment or vaccine exists for Zika virus infection; treatment is supportive and should address specific medical and neurodevelopmental issues, and mothers should be encouraged to breastfeed infants regardless of exposure, as available evidence suggests the benefits of breastfeeding outweigh the theoretical risks of transmission through breast milk, they said.
The authors stressed that prevention of maternal infection is the only way to prevent congenital Zika virus infection and is achieved by avoiding areas with ongoing Zika virus transmission or by strictly following steps to avoid mosquito bites by using air-conditioning or window and door screens, wearing protective clothing, and using insect repellents.
Environmental Protection Agency–registered insect repellents are safe for pregnant women when used according to the product label, they noted.
The Centers for Disease Control and Prevention has released interim guidelines for U.S. clinicians caring for infants born to mothers who traveled to or resided in an area with Zika virus transmission during pregnancy.
The guidelines, released Jan. 26, address the evaluation and testing of infants with possible congenital Zika virus infection, and follow the Jan. 19 release of similar guidelines for the care of pregnant women with possible exposure to the mosquito-borne virus. Most importantly, the new guidelines say Zika virus testing should be performed for infants with microcephaly or intracranial calcifications who are born to women with possible Zika virus exposure during pregnancy, and for infants born to women with positive or inconclusive Zika virus test results.
“Pediatric health are providers should work closely with obstetric providers to identify infants whose mothers were potentially infected with Zika virus during pregnancy (based on travel to or residence in an area with Zika virus transmission),” according to the guidelines, which were published in Morbidity and Mortality Weekly Report (MMWR. 2016 Jan 26;65[Early Release:1-5]).
Infants with laboratory evidence of a possible congenital Zika virus infection should undergo additional clinical evaluation, and state or territorial health departments should be contacted to facilitate testing. Zika virus disease is an arboviral disease and thus is a nationally notifiable condition, according to guideline authors Dr. J. Erin Staples and her colleagues at the CDC, Atlanta.
Both molecular and serologic tests are recommended for infants undergoing evaluation for possible congenital Zika virus infection, they noted.
Serum specimens for reverse-transcription-polymerase chain reaction testing should be collected from the umbilical cord or directly from the infant within 2 days of birth, and cerebrospinal fluid collected for other studies, as well as frozen and fixed placenta obtained at delivery, should also be tested by RT-PCR.
IgM ELISA for Zika virus and dengue virus should also be performed on infant serum, infant CSF, and maternal serum, but results from these assays can be falsely positive because of cross-reacting antibodies, the authors noted.
Other tests that can be considered include a plaque reduction neutralization test to measure virus-specific neutralizing antibodies and to discriminate between cross-reacting antibodies and closely related flaviviruses, and immunohistochemical staining to detect the virus antigen on fixed placenta and umbilical cord tissues.
Further clinical evaluation and laboratory testing is recommended for infants with microcephaly or intracranial calcifications detected prenatally or at birth if the mother was potentially infected during pregnancy, they said.
In infants with possible Zika virus exposure during pregnancy, but without microcephaly or intracranial calcification, subsequent evaluation depends on maternal testing results. Routine care is recommended if maternal test results are negative, and testing for a possible congenital infection is recommended if maternal results are positive or inconclusive.
If all of an infant’s tests are negative for Zika virus infection, no further Zika virus testing or evaluation is recommended. In the event of any positive or inconclusive test, further evaluation and follow-up is recommended.
Other considerations
Abnormal eye findings have been reported in infants with possible congenital infection, therefore an opthalmologic evaluation, including retinal examination is advised during the first month of life, as is a repeat hearing screen at age 6 months – even if the initial screen was normal, the authors said.
The infant should be followed to assess for long-term sequelae, and the case should be reported. Follow-up should include a cranial ultrasound to assess for subclinical findings, unless a third trimester ultrasound showed no brain abnormalities, they added.
No specific antiviral treatment or vaccine exists for Zika virus infection; treatment is supportive and should address specific medical and neurodevelopmental issues, and mothers should be encouraged to breastfeed infants regardless of exposure, as available evidence suggests the benefits of breastfeeding outweigh the theoretical risks of transmission through breast milk, they said.
The authors stressed that prevention of maternal infection is the only way to prevent congenital Zika virus infection and is achieved by avoiding areas with ongoing Zika virus transmission or by strictly following steps to avoid mosquito bites by using air-conditioning or window and door screens, wearing protective clothing, and using insect repellents.
Environmental Protection Agency–registered insect repellents are safe for pregnant women when used according to the product label, they noted.
The Centers for Disease Control and Prevention has released interim guidelines for U.S. clinicians caring for infants born to mothers who traveled to or resided in an area with Zika virus transmission during pregnancy.
The guidelines, released Jan. 26, address the evaluation and testing of infants with possible congenital Zika virus infection, and follow the Jan. 19 release of similar guidelines for the care of pregnant women with possible exposure to the mosquito-borne virus. Most importantly, the new guidelines say Zika virus testing should be performed for infants with microcephaly or intracranial calcifications who are born to women with possible Zika virus exposure during pregnancy, and for infants born to women with positive or inconclusive Zika virus test results.
“Pediatric health are providers should work closely with obstetric providers to identify infants whose mothers were potentially infected with Zika virus during pregnancy (based on travel to or residence in an area with Zika virus transmission),” according to the guidelines, which were published in Morbidity and Mortality Weekly Report (MMWR. 2016 Jan 26;65[Early Release:1-5]).
Infants with laboratory evidence of a possible congenital Zika virus infection should undergo additional clinical evaluation, and state or territorial health departments should be contacted to facilitate testing. Zika virus disease is an arboviral disease and thus is a nationally notifiable condition, according to guideline authors Dr. J. Erin Staples and her colleagues at the CDC, Atlanta.
Both molecular and serologic tests are recommended for infants undergoing evaluation for possible congenital Zika virus infection, they noted.
Serum specimens for reverse-transcription-polymerase chain reaction testing should be collected from the umbilical cord or directly from the infant within 2 days of birth, and cerebrospinal fluid collected for other studies, as well as frozen and fixed placenta obtained at delivery, should also be tested by RT-PCR.
IgM ELISA for Zika virus and dengue virus should also be performed on infant serum, infant CSF, and maternal serum, but results from these assays can be falsely positive because of cross-reacting antibodies, the authors noted.
Other tests that can be considered include a plaque reduction neutralization test to measure virus-specific neutralizing antibodies and to discriminate between cross-reacting antibodies and closely related flaviviruses, and immunohistochemical staining to detect the virus antigen on fixed placenta and umbilical cord tissues.
Further clinical evaluation and laboratory testing is recommended for infants with microcephaly or intracranial calcifications detected prenatally or at birth if the mother was potentially infected during pregnancy, they said.
In infants with possible Zika virus exposure during pregnancy, but without microcephaly or intracranial calcification, subsequent evaluation depends on maternal testing results. Routine care is recommended if maternal test results are negative, and testing for a possible congenital infection is recommended if maternal results are positive or inconclusive.
If all of an infant’s tests are negative for Zika virus infection, no further Zika virus testing or evaluation is recommended. In the event of any positive or inconclusive test, further evaluation and follow-up is recommended.
Other considerations
Abnormal eye findings have been reported in infants with possible congenital infection, therefore an opthalmologic evaluation, including retinal examination is advised during the first month of life, as is a repeat hearing screen at age 6 months – even if the initial screen was normal, the authors said.
The infant should be followed to assess for long-term sequelae, and the case should be reported. Follow-up should include a cranial ultrasound to assess for subclinical findings, unless a third trimester ultrasound showed no brain abnormalities, they added.
No specific antiviral treatment or vaccine exists for Zika virus infection; treatment is supportive and should address specific medical and neurodevelopmental issues, and mothers should be encouraged to breastfeed infants regardless of exposure, as available evidence suggests the benefits of breastfeeding outweigh the theoretical risks of transmission through breast milk, they said.
The authors stressed that prevention of maternal infection is the only way to prevent congenital Zika virus infection and is achieved by avoiding areas with ongoing Zika virus transmission or by strictly following steps to avoid mosquito bites by using air-conditioning or window and door screens, wearing protective clothing, and using insect repellents.
Environmental Protection Agency–registered insect repellents are safe for pregnant women when used according to the product label, they noted.
FROM MMWR
High-dose vitamin D in pregnancy fails to prevent wheezing risk in children
Among pregnant women at high risk for having a child with asthma, high doses of vitamin D administered during the third trimester failed to prevent persistent wheezing illness in their children at age 3, according to two separate reports published online Jan. 26 in JAMA.
Both studies were conducted because vitamin D insufficiency during pregnancy is commonplace and is thought to affect fetal immune programming and to contribute to asthma pathogenesis. In addition, observational studies have found an association between low levels of vitamin D in cord blood and later asthma in the child.
The two randomized, double-blind placebo-controlled clinical trials found that neither 2,800 IU/day nor 4,400 IU/day of vitamin D significantly reduced the risk of persistent wheeze in the offspring through 3 years of age. However, both research groups noted that their studies may have been underpowered to detect a clinically important protective effect, and both recommended longer-term observation of their study participants, as well as further studies using larger sample sizes, higher doses of vitamin D, administration earlier in pregnancy, and postnatal supplementation to establish a definitive result.
In the first study – conducted as part of the Copenhagen Prospective Studies on Asthma in Childhood 2010 cohort – 623 Danish women already taking the standard 400 IU of vitamin D3 during pregnancy were randomly assigned to receive an additional 2,400 IU (315 women) or a matching placebo (308 women) from 22 to 26 weeks’ gestation until delivery. After exclusions, researchers analyzed data on 581 children.
Maternal serum vitamin D levels increased markedly in the active-treatment group. “Correspondingly, the percentage of women with sufficient levels of vitamin D (greater than 30 ng/mL) after the intervention was 81% in the vitamin D group, compared with 44% in the control group,” wrote Dr. Bo L. Chawes of Copenhagen Prospective Studies on Asthma in Childhood, University of Copenhagen, and his associates.
Persistent wheeze developed in 104 (18%) of the 581 children: 47 (16%) in the vitamin D group and 57 (20%) in the control group, a nonsignificant difference. Similarly, asthma was diagnosed in 79 children: 32 (12%) in the vitamin D group and 47 (14%) in the control group, another nonsignificant difference.
Vitamin D supplementation also made no difference in infants’ levels of C-reactive protein, interleukin-6, tumor necrosis factor–alpha, or CXCL8, nor in the number of upper respiratory tract infections (5.2 per year vs 5.3 per year), the number of lower respiratory tract infections (32% vs 33%), the risk of allergic sensitization as measured by skin prick test or specific IgE level, or the development of eczema (23% vs 25%).
However, the risk of persistent wheeze was higher in children whose mothers’ vitamin D levels were lowest, compared with those whose mothers’ vitamin D levels were in the middle and upper tertiles. And high-dose vitamin D was protective with regard to some secondary endpoints, such as preventing more episodes of “troublesome lung symptoms” (5.9 vs. 7.2).
This finding, together with the study’s somewhat reduced statistical power, mean that a clinically important protective effect cannot be ruled out. In addition, the supplementation dose may have been too low or may have been given too late in the course of pregnancy to produce a significant effect, Dr. Chawes and his associates wrote (JAMA. 2016;315[4]:353-61. doi: 10.1001/jama.2015.18318).In the second study – the Vitamin D Antenatal Asthma Reduction Trial – 876 pregnant women in Boston, St. Louis, and San Diego who were already taking the standard 400 IU of vitamin D were randomly assigned to receive either an additional 4,000 IU/day (440 participants) or a matching placebo (436 participants). Maternal levels of vitamin D rose markedly in the active-treatment group (mean, 39.2 ng/mL), compared with the control group (mean, 26.8 ng/mL), and the proportion of women who achieved higher than “inadequate” levels was much greater (74.9% vs 34.0%), reported Dr. Augusto A. Litonjua of Brigham and Women’s Hospital, Boston, and his associates.
A total of 24.3% of the vitamin D group and 30.4% of the control group developed asthma or recurrent wheeze by age 3 years, a nonsignificant difference. However, the incidence of asthma was so much lower than anticipated in both study groups that the study may have lost statistical power to detect a clinically meaningful difference, according to the investigators (JAMA. 2016;315[4]:362-70. doi: 10.1001/jama.2015.18589).
It remains unclear whether vitamin D supplementation during pregnancy will reduce asthma and persistent wheezing in the offspring. “Larger studies and longer follow-up of the children in this study will be needed to answer the question,” the investigators wrote. “If additional studies identify a significant effect, given the high prevalence of low vitamin D levels in pregnant women, the effect of this inexpensive intervention on child health could be substantial.”
The first study was supported by the Copenhagen Prospective Study on Asthma in Childhood, which is funded by private and public research groups. One of the coauthors reported receiving consulting fees from Chiesi. The Vitamin D Antenatal Asthma Reduction Trial was supported by the U.S. National Heart, Lung, and Blood Institute and the National Centers for Advancing Translational Sciences. The lead author, Dr. Litonjua, reported receiving personal fees from UpToDate and Springer Humana Press; his associates reported ties to numerous industry sources.
These are sobering findings. Even if we assume that prenatal vitamin D supplementation will prove more protective as the children in these studies grow older, vitamin D insufficiency still would explain only a small portion of the current asthma epidemic.
But neither study showed any unwanted effects from supplementation, so it seems reasonable for clinicians to prescribe vitamin D to mothers at high risk of having children with asthma by virtue of their own asthma, eczema, or allergic rhinitis – especially if those mothers are deficient in vitamin D. However, the data in these two clinical trials do not support the use of very high-dose vitamin D, since any beneficial effects achieved with 4,400 IU/day were identical to those achieved with approximately half as high a dose.
Dr. Erika von Mutius is at Ludwig Maximilians University, Munich. Dr. Fernando D. Martinez is at the asthma and airway disease research center and the department of pediatrics at the University of Arizona, Tucson. Both reported having no relevant financial disclosures. Their remarks are adapted from an editorial accompanying the two reports (JAMA 2016;315[4]:347-8.).
These are sobering findings. Even if we assume that prenatal vitamin D supplementation will prove more protective as the children in these studies grow older, vitamin D insufficiency still would explain only a small portion of the current asthma epidemic.
But neither study showed any unwanted effects from supplementation, so it seems reasonable for clinicians to prescribe vitamin D to mothers at high risk of having children with asthma by virtue of their own asthma, eczema, or allergic rhinitis – especially if those mothers are deficient in vitamin D. However, the data in these two clinical trials do not support the use of very high-dose vitamin D, since any beneficial effects achieved with 4,400 IU/day were identical to those achieved with approximately half as high a dose.
Dr. Erika von Mutius is at Ludwig Maximilians University, Munich. Dr. Fernando D. Martinez is at the asthma and airway disease research center and the department of pediatrics at the University of Arizona, Tucson. Both reported having no relevant financial disclosures. Their remarks are adapted from an editorial accompanying the two reports (JAMA 2016;315[4]:347-8.).
These are sobering findings. Even if we assume that prenatal vitamin D supplementation will prove more protective as the children in these studies grow older, vitamin D insufficiency still would explain only a small portion of the current asthma epidemic.
But neither study showed any unwanted effects from supplementation, so it seems reasonable for clinicians to prescribe vitamin D to mothers at high risk of having children with asthma by virtue of their own asthma, eczema, or allergic rhinitis – especially if those mothers are deficient in vitamin D. However, the data in these two clinical trials do not support the use of very high-dose vitamin D, since any beneficial effects achieved with 4,400 IU/day were identical to those achieved with approximately half as high a dose.
Dr. Erika von Mutius is at Ludwig Maximilians University, Munich. Dr. Fernando D. Martinez is at the asthma and airway disease research center and the department of pediatrics at the University of Arizona, Tucson. Both reported having no relevant financial disclosures. Their remarks are adapted from an editorial accompanying the two reports (JAMA 2016;315[4]:347-8.).
Among pregnant women at high risk for having a child with asthma, high doses of vitamin D administered during the third trimester failed to prevent persistent wheezing illness in their children at age 3, according to two separate reports published online Jan. 26 in JAMA.
Both studies were conducted because vitamin D insufficiency during pregnancy is commonplace and is thought to affect fetal immune programming and to contribute to asthma pathogenesis. In addition, observational studies have found an association between low levels of vitamin D in cord blood and later asthma in the child.
The two randomized, double-blind placebo-controlled clinical trials found that neither 2,800 IU/day nor 4,400 IU/day of vitamin D significantly reduced the risk of persistent wheeze in the offspring through 3 years of age. However, both research groups noted that their studies may have been underpowered to detect a clinically important protective effect, and both recommended longer-term observation of their study participants, as well as further studies using larger sample sizes, higher doses of vitamin D, administration earlier in pregnancy, and postnatal supplementation to establish a definitive result.
In the first study – conducted as part of the Copenhagen Prospective Studies on Asthma in Childhood 2010 cohort – 623 Danish women already taking the standard 400 IU of vitamin D3 during pregnancy were randomly assigned to receive an additional 2,400 IU (315 women) or a matching placebo (308 women) from 22 to 26 weeks’ gestation until delivery. After exclusions, researchers analyzed data on 581 children.
Maternal serum vitamin D levels increased markedly in the active-treatment group. “Correspondingly, the percentage of women with sufficient levels of vitamin D (greater than 30 ng/mL) after the intervention was 81% in the vitamin D group, compared with 44% in the control group,” wrote Dr. Bo L. Chawes of Copenhagen Prospective Studies on Asthma in Childhood, University of Copenhagen, and his associates.
Persistent wheeze developed in 104 (18%) of the 581 children: 47 (16%) in the vitamin D group and 57 (20%) in the control group, a nonsignificant difference. Similarly, asthma was diagnosed in 79 children: 32 (12%) in the vitamin D group and 47 (14%) in the control group, another nonsignificant difference.
Vitamin D supplementation also made no difference in infants’ levels of C-reactive protein, interleukin-6, tumor necrosis factor–alpha, or CXCL8, nor in the number of upper respiratory tract infections (5.2 per year vs 5.3 per year), the number of lower respiratory tract infections (32% vs 33%), the risk of allergic sensitization as measured by skin prick test or specific IgE level, or the development of eczema (23% vs 25%).
However, the risk of persistent wheeze was higher in children whose mothers’ vitamin D levels were lowest, compared with those whose mothers’ vitamin D levels were in the middle and upper tertiles. And high-dose vitamin D was protective with regard to some secondary endpoints, such as preventing more episodes of “troublesome lung symptoms” (5.9 vs. 7.2).
This finding, together with the study’s somewhat reduced statistical power, mean that a clinically important protective effect cannot be ruled out. In addition, the supplementation dose may have been too low or may have been given too late in the course of pregnancy to produce a significant effect, Dr. Chawes and his associates wrote (JAMA. 2016;315[4]:353-61. doi: 10.1001/jama.2015.18318).In the second study – the Vitamin D Antenatal Asthma Reduction Trial – 876 pregnant women in Boston, St. Louis, and San Diego who were already taking the standard 400 IU of vitamin D were randomly assigned to receive either an additional 4,000 IU/day (440 participants) or a matching placebo (436 participants). Maternal levels of vitamin D rose markedly in the active-treatment group (mean, 39.2 ng/mL), compared with the control group (mean, 26.8 ng/mL), and the proportion of women who achieved higher than “inadequate” levels was much greater (74.9% vs 34.0%), reported Dr. Augusto A. Litonjua of Brigham and Women’s Hospital, Boston, and his associates.
A total of 24.3% of the vitamin D group and 30.4% of the control group developed asthma or recurrent wheeze by age 3 years, a nonsignificant difference. However, the incidence of asthma was so much lower than anticipated in both study groups that the study may have lost statistical power to detect a clinically meaningful difference, according to the investigators (JAMA. 2016;315[4]:362-70. doi: 10.1001/jama.2015.18589).
It remains unclear whether vitamin D supplementation during pregnancy will reduce asthma and persistent wheezing in the offspring. “Larger studies and longer follow-up of the children in this study will be needed to answer the question,” the investigators wrote. “If additional studies identify a significant effect, given the high prevalence of low vitamin D levels in pregnant women, the effect of this inexpensive intervention on child health could be substantial.”
The first study was supported by the Copenhagen Prospective Study on Asthma in Childhood, which is funded by private and public research groups. One of the coauthors reported receiving consulting fees from Chiesi. The Vitamin D Antenatal Asthma Reduction Trial was supported by the U.S. National Heart, Lung, and Blood Institute and the National Centers for Advancing Translational Sciences. The lead author, Dr. Litonjua, reported receiving personal fees from UpToDate and Springer Humana Press; his associates reported ties to numerous industry sources.
Among pregnant women at high risk for having a child with asthma, high doses of vitamin D administered during the third trimester failed to prevent persistent wheezing illness in their children at age 3, according to two separate reports published online Jan. 26 in JAMA.
Both studies were conducted because vitamin D insufficiency during pregnancy is commonplace and is thought to affect fetal immune programming and to contribute to asthma pathogenesis. In addition, observational studies have found an association between low levels of vitamin D in cord blood and later asthma in the child.
The two randomized, double-blind placebo-controlled clinical trials found that neither 2,800 IU/day nor 4,400 IU/day of vitamin D significantly reduced the risk of persistent wheeze in the offspring through 3 years of age. However, both research groups noted that their studies may have been underpowered to detect a clinically important protective effect, and both recommended longer-term observation of their study participants, as well as further studies using larger sample sizes, higher doses of vitamin D, administration earlier in pregnancy, and postnatal supplementation to establish a definitive result.
In the first study – conducted as part of the Copenhagen Prospective Studies on Asthma in Childhood 2010 cohort – 623 Danish women already taking the standard 400 IU of vitamin D3 during pregnancy were randomly assigned to receive an additional 2,400 IU (315 women) or a matching placebo (308 women) from 22 to 26 weeks’ gestation until delivery. After exclusions, researchers analyzed data on 581 children.
Maternal serum vitamin D levels increased markedly in the active-treatment group. “Correspondingly, the percentage of women with sufficient levels of vitamin D (greater than 30 ng/mL) after the intervention was 81% in the vitamin D group, compared with 44% in the control group,” wrote Dr. Bo L. Chawes of Copenhagen Prospective Studies on Asthma in Childhood, University of Copenhagen, and his associates.
Persistent wheeze developed in 104 (18%) of the 581 children: 47 (16%) in the vitamin D group and 57 (20%) in the control group, a nonsignificant difference. Similarly, asthma was diagnosed in 79 children: 32 (12%) in the vitamin D group and 47 (14%) in the control group, another nonsignificant difference.
Vitamin D supplementation also made no difference in infants’ levels of C-reactive protein, interleukin-6, tumor necrosis factor–alpha, or CXCL8, nor in the number of upper respiratory tract infections (5.2 per year vs 5.3 per year), the number of lower respiratory tract infections (32% vs 33%), the risk of allergic sensitization as measured by skin prick test or specific IgE level, or the development of eczema (23% vs 25%).
However, the risk of persistent wheeze was higher in children whose mothers’ vitamin D levels were lowest, compared with those whose mothers’ vitamin D levels were in the middle and upper tertiles. And high-dose vitamin D was protective with regard to some secondary endpoints, such as preventing more episodes of “troublesome lung symptoms” (5.9 vs. 7.2).
This finding, together with the study’s somewhat reduced statistical power, mean that a clinically important protective effect cannot be ruled out. In addition, the supplementation dose may have been too low or may have been given too late in the course of pregnancy to produce a significant effect, Dr. Chawes and his associates wrote (JAMA. 2016;315[4]:353-61. doi: 10.1001/jama.2015.18318).In the second study – the Vitamin D Antenatal Asthma Reduction Trial – 876 pregnant women in Boston, St. Louis, and San Diego who were already taking the standard 400 IU of vitamin D were randomly assigned to receive either an additional 4,000 IU/day (440 participants) or a matching placebo (436 participants). Maternal levels of vitamin D rose markedly in the active-treatment group (mean, 39.2 ng/mL), compared with the control group (mean, 26.8 ng/mL), and the proportion of women who achieved higher than “inadequate” levels was much greater (74.9% vs 34.0%), reported Dr. Augusto A. Litonjua of Brigham and Women’s Hospital, Boston, and his associates.
A total of 24.3% of the vitamin D group and 30.4% of the control group developed asthma or recurrent wheeze by age 3 years, a nonsignificant difference. However, the incidence of asthma was so much lower than anticipated in both study groups that the study may have lost statistical power to detect a clinically meaningful difference, according to the investigators (JAMA. 2016;315[4]:362-70. doi: 10.1001/jama.2015.18589).
It remains unclear whether vitamin D supplementation during pregnancy will reduce asthma and persistent wheezing in the offspring. “Larger studies and longer follow-up of the children in this study will be needed to answer the question,” the investigators wrote. “If additional studies identify a significant effect, given the high prevalence of low vitamin D levels in pregnant women, the effect of this inexpensive intervention on child health could be substantial.”
The first study was supported by the Copenhagen Prospective Study on Asthma in Childhood, which is funded by private and public research groups. One of the coauthors reported receiving consulting fees from Chiesi. The Vitamin D Antenatal Asthma Reduction Trial was supported by the U.S. National Heart, Lung, and Blood Institute and the National Centers for Advancing Translational Sciences. The lead author, Dr. Litonjua, reported receiving personal fees from UpToDate and Springer Humana Press; his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: High doses of vitamin D administered during the third trimester didn’t prevent wheezing in children at age 3.
Major finding: In a study of 581 children, persistent wheeze developed in 16% in the vitamin D group and in 20% in the control group. In a separate trial of 806 infants, there was a 6.1% reduction in asthma or recurrent wheezing at age 3, which was not statistically significant.
Data source: Two separate randomized, double-blind placebo-controlled trials involving a total of nearly 1,500 pregnant women.
Disclosures: The first study was supported by the Copenhagen Prospective Study on Asthma in Childhood, which is funded by private and public research groups. One of the coauthors reported receiving consulting fees from Chiesi. The Vitamin D Antenatal Asthma Reduction Trial was supported by the U.S. National Heart, Lung, and Blood Institute and the National Centers for Advancing Translational Sciences. The lead author, Dr. Litonjua, reported receiving personal fees from UpToDate and Springer Humana Press; his associates reported ties to numerous industry sources.