User login
Sexually transmitted Zika case confirmed in Texas
A report of sexually transmitted Zika virus infection has been confirmed in Texas, according to officials at the Dallas County Health and Human Services Department (DCHHS).
The patient was infected with Zika virus via sexual contact with an individual who returned home after becoming ill in a country where the virus is present. The department received confirmation from the U.S. Centers for Disease Control and Prevention on Feb. 2.
“Now that we know Zika virus can be transmitted through sex, this increases our awareness campaign in educating the public about protecting themselves and others,” Zachary Thompson, DCHHS director, said in a statement.
See the full statement here.
On Twitter @denisefulton
A report of sexually transmitted Zika virus infection has been confirmed in Texas, according to officials at the Dallas County Health and Human Services Department (DCHHS).
The patient was infected with Zika virus via sexual contact with an individual who returned home after becoming ill in a country where the virus is present. The department received confirmation from the U.S. Centers for Disease Control and Prevention on Feb. 2.
“Now that we know Zika virus can be transmitted through sex, this increases our awareness campaign in educating the public about protecting themselves and others,” Zachary Thompson, DCHHS director, said in a statement.
See the full statement here.
On Twitter @denisefulton
A report of sexually transmitted Zika virus infection has been confirmed in Texas, according to officials at the Dallas County Health and Human Services Department (DCHHS).
The patient was infected with Zika virus via sexual contact with an individual who returned home after becoming ill in a country where the virus is present. The department received confirmation from the U.S. Centers for Disease Control and Prevention on Feb. 2.
“Now that we know Zika virus can be transmitted through sex, this increases our awareness campaign in educating the public about protecting themselves and others,” Zachary Thompson, DCHHS director, said in a statement.
See the full statement here.
On Twitter @denisefulton
Heightened emphasis on sex-specific cardiovascular risk factors
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
CDC: Screen women for alcohol, birth control use
An estimated 3.3 million U.S. women aged 15-44 years risk conceiving children with fetal alcohol spectrum disorders by using alcohol but not birth control.
The finding has officials at the Centers for Disease Control and Prevention urging physicians to screen this group for concomitant drinking and nonuse of contraception. The data come from an analysis of 4,303 nonpregnant, nonsterile women ages 15-44 years from the 2011-2013 National Survey of Family Growth, conducted by the CDC (MMWR. 2016;65:1-7.).
“Alcohol can permanently harm a developing baby before a woman knows she is pregnant,” Dr. Anne Schuchat, the CDC’s principal deputy director, said during a media briefing on Feb. 2. “About half of all pregnancies in the United States are unplanned, and even if planned, most women won’t know they are pregnant for the first month or so, when they might still be drinking. The risk is real. Why take the chance?”
Fetal alcohol spectrum disorders (FASD) can include physical, behavioral, and intellectual disabilities that can last for a child’s lifetime. Dr, Schuchat said the CDC estimates that as many as 1 in 20 U.S. schoolchildren may have FASD. Currently, there are no data on what amounts of alcohol are safe for a woman to drink at any stage of pregnancy.
“Not drinking alcohol is one of the best things you can do to ensure the health of your baby,” Dr. Schuchat said.
For the study, a woman was considered at risk for an alcohol-exposed pregnancy during the past month if she was nonsterile and had sex with a nonsterile male, drank any alcohol, and did not use contraception in the past month. The CDC found the weighted prevalence of alcohol-exposed pregnancy risk among U.S. women aged 15-44 years was 7.3%.
During a 1-month period, approximately 3.3 million U.S. women were at risk for an alcohol-exposed pregnancy. The highest risk group – at 10.4% – were women aged 25-29 years. The lowest risk group were those aged 15-20 years, at 2.2%.
Neither race nor ethnicity were found to be risk factors, although the risk for an alcohol-exposed pregnancy was higher among married and cohabitating women at 11.7% and 13.6% respectively, compared with their single counterparts (2.3%).
The study also found that three-quarters of women who want to get pregnant as soon as possible do not stop drinking alcohol after discontinuing contraception.
Physicians and other health care providers should advise women who want to become pregnant to stop drinking alcohol as soon as they stop using birth control, Dr. Schuchat said.
She added that physicians should screen all adults for alcohol use, not just women, even though that is not currently standard practice. “We think it should be more common to do on a regular basis.” Dr. Schuchat said the federal government requires most health plans to cover alcohol screening without cost to the patient.
The CDC recommends that physicians:
• Screen all adult female patients for alcohol use annually.
• Advise women to cease all alcohol intake if there is any chance at all that she could be pregnant.
• Counsel, refer, and follow-up with patients who need additional support to not drink while pregnant.
• Use correct billing codes to be reimbursed for screening and counseling.
The American College of Obstetricians and Gynecologists, which recommends that women completely abstain from alcohol during pregnancy, praised the CDC’s guidance that physicians routinely screen women regarding their alcohol use.
Dr. Mark S. DeFrancesco, ACOG president, said the other important message from the CDC report is that physicians should counsel women about contraception use.
“As the CDC notes, roughly half of all pregnancies in the United States are unintended. In many cases of unintended pregnancy, women inadvertently expose their fetuses to alcohol and its teratogenic effects prior to discovering that they are pregnant,” he said in statement. “This is just another reason why it’s so important that health care providers counsel women about how to prevent unintended pregnancy through use of the contraceptive method that is right for them. There are many benefits to helping women become pregnant only when they are ready, and avoiding alcohol exposure is one of them.”
On Twitter @whitneymcknight
An estimated 3.3 million U.S. women aged 15-44 years risk conceiving children with fetal alcohol spectrum disorders by using alcohol but not birth control.
The finding has officials at the Centers for Disease Control and Prevention urging physicians to screen this group for concomitant drinking and nonuse of contraception. The data come from an analysis of 4,303 nonpregnant, nonsterile women ages 15-44 years from the 2011-2013 National Survey of Family Growth, conducted by the CDC (MMWR. 2016;65:1-7.).
“Alcohol can permanently harm a developing baby before a woman knows she is pregnant,” Dr. Anne Schuchat, the CDC’s principal deputy director, said during a media briefing on Feb. 2. “About half of all pregnancies in the United States are unplanned, and even if planned, most women won’t know they are pregnant for the first month or so, when they might still be drinking. The risk is real. Why take the chance?”
Fetal alcohol spectrum disorders (FASD) can include physical, behavioral, and intellectual disabilities that can last for a child’s lifetime. Dr, Schuchat said the CDC estimates that as many as 1 in 20 U.S. schoolchildren may have FASD. Currently, there are no data on what amounts of alcohol are safe for a woman to drink at any stage of pregnancy.
“Not drinking alcohol is one of the best things you can do to ensure the health of your baby,” Dr. Schuchat said.
For the study, a woman was considered at risk for an alcohol-exposed pregnancy during the past month if she was nonsterile and had sex with a nonsterile male, drank any alcohol, and did not use contraception in the past month. The CDC found the weighted prevalence of alcohol-exposed pregnancy risk among U.S. women aged 15-44 years was 7.3%.
During a 1-month period, approximately 3.3 million U.S. women were at risk for an alcohol-exposed pregnancy. The highest risk group – at 10.4% – were women aged 25-29 years. The lowest risk group were those aged 15-20 years, at 2.2%.
Neither race nor ethnicity were found to be risk factors, although the risk for an alcohol-exposed pregnancy was higher among married and cohabitating women at 11.7% and 13.6% respectively, compared with their single counterparts (2.3%).
The study also found that three-quarters of women who want to get pregnant as soon as possible do not stop drinking alcohol after discontinuing contraception.
Physicians and other health care providers should advise women who want to become pregnant to stop drinking alcohol as soon as they stop using birth control, Dr. Schuchat said.
She added that physicians should screen all adults for alcohol use, not just women, even though that is not currently standard practice. “We think it should be more common to do on a regular basis.” Dr. Schuchat said the federal government requires most health plans to cover alcohol screening without cost to the patient.
The CDC recommends that physicians:
• Screen all adult female patients for alcohol use annually.
• Advise women to cease all alcohol intake if there is any chance at all that she could be pregnant.
• Counsel, refer, and follow-up with patients who need additional support to not drink while pregnant.
• Use correct billing codes to be reimbursed for screening and counseling.
The American College of Obstetricians and Gynecologists, which recommends that women completely abstain from alcohol during pregnancy, praised the CDC’s guidance that physicians routinely screen women regarding their alcohol use.
Dr. Mark S. DeFrancesco, ACOG president, said the other important message from the CDC report is that physicians should counsel women about contraception use.
“As the CDC notes, roughly half of all pregnancies in the United States are unintended. In many cases of unintended pregnancy, women inadvertently expose their fetuses to alcohol and its teratogenic effects prior to discovering that they are pregnant,” he said in statement. “This is just another reason why it’s so important that health care providers counsel women about how to prevent unintended pregnancy through use of the contraceptive method that is right for them. There are many benefits to helping women become pregnant only when they are ready, and avoiding alcohol exposure is one of them.”
On Twitter @whitneymcknight
An estimated 3.3 million U.S. women aged 15-44 years risk conceiving children with fetal alcohol spectrum disorders by using alcohol but not birth control.
The finding has officials at the Centers for Disease Control and Prevention urging physicians to screen this group for concomitant drinking and nonuse of contraception. The data come from an analysis of 4,303 nonpregnant, nonsterile women ages 15-44 years from the 2011-2013 National Survey of Family Growth, conducted by the CDC (MMWR. 2016;65:1-7.).
“Alcohol can permanently harm a developing baby before a woman knows she is pregnant,” Dr. Anne Schuchat, the CDC’s principal deputy director, said during a media briefing on Feb. 2. “About half of all pregnancies in the United States are unplanned, and even if planned, most women won’t know they are pregnant for the first month or so, when they might still be drinking. The risk is real. Why take the chance?”
Fetal alcohol spectrum disorders (FASD) can include physical, behavioral, and intellectual disabilities that can last for a child’s lifetime. Dr, Schuchat said the CDC estimates that as many as 1 in 20 U.S. schoolchildren may have FASD. Currently, there are no data on what amounts of alcohol are safe for a woman to drink at any stage of pregnancy.
“Not drinking alcohol is one of the best things you can do to ensure the health of your baby,” Dr. Schuchat said.
For the study, a woman was considered at risk for an alcohol-exposed pregnancy during the past month if she was nonsterile and had sex with a nonsterile male, drank any alcohol, and did not use contraception in the past month. The CDC found the weighted prevalence of alcohol-exposed pregnancy risk among U.S. women aged 15-44 years was 7.3%.
During a 1-month period, approximately 3.3 million U.S. women were at risk for an alcohol-exposed pregnancy. The highest risk group – at 10.4% – were women aged 25-29 years. The lowest risk group were those aged 15-20 years, at 2.2%.
Neither race nor ethnicity were found to be risk factors, although the risk for an alcohol-exposed pregnancy was higher among married and cohabitating women at 11.7% and 13.6% respectively, compared with their single counterparts (2.3%).
The study also found that three-quarters of women who want to get pregnant as soon as possible do not stop drinking alcohol after discontinuing contraception.
Physicians and other health care providers should advise women who want to become pregnant to stop drinking alcohol as soon as they stop using birth control, Dr. Schuchat said.
She added that physicians should screen all adults for alcohol use, not just women, even though that is not currently standard practice. “We think it should be more common to do on a regular basis.” Dr. Schuchat said the federal government requires most health plans to cover alcohol screening without cost to the patient.
The CDC recommends that physicians:
• Screen all adult female patients for alcohol use annually.
• Advise women to cease all alcohol intake if there is any chance at all that she could be pregnant.
• Counsel, refer, and follow-up with patients who need additional support to not drink while pregnant.
• Use correct billing codes to be reimbursed for screening and counseling.
The American College of Obstetricians and Gynecologists, which recommends that women completely abstain from alcohol during pregnancy, praised the CDC’s guidance that physicians routinely screen women regarding their alcohol use.
Dr. Mark S. DeFrancesco, ACOG president, said the other important message from the CDC report is that physicians should counsel women about contraception use.
“As the CDC notes, roughly half of all pregnancies in the United States are unintended. In many cases of unintended pregnancy, women inadvertently expose their fetuses to alcohol and its teratogenic effects prior to discovering that they are pregnant,” he said in statement. “This is just another reason why it’s so important that health care providers counsel women about how to prevent unintended pregnancy through use of the contraceptive method that is right for them. There are many benefits to helping women become pregnant only when they are ready, and avoiding alcohol exposure is one of them.”
On Twitter @whitneymcknight
FROM THE MMWR
Key clinical point: The CDC advises physicians to screen women for alcohol use and provide contraception counseling.
Major finding: A total of 3.3 million women aged 15-44 years risk conceiving a child with FASD by using alcohol and having unprotected sex.
Data source: Data on 4,303 nonpregnant, nonsterile women aged 15-44 years from the 2011-2013 National Survey of Family Growth.
Disclosures: The researchers did not report having any financial disclosures.
CDC expands Zika virus travel warnings again
Officials at the Centers for Disease Control and Prevention have added four more destinations to their Zika virus travel alert – American Samoa, Costa Rica, Curacao, and Nicaragua.
The Level 2 travel alert means that individuals are urged to take enhanced precautions against mosquito bites while in these regions to minimize their chances of contracting the Zika virus. Pregnant women are being advised to consider postponing travel to areas where Zika virus transmission in ongoing. Pregnant women and those trying to become pregnant who must travel to these areas are advised to consult with their physician before traveling and take steps to prevent mosquito bites.
The CDC has already issued a Level 2 travel alert for these areas where Zika virus transmission is ongoing: Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny
Officials at the Centers for Disease Control and Prevention have added four more destinations to their Zika virus travel alert – American Samoa, Costa Rica, Curacao, and Nicaragua.
The Level 2 travel alert means that individuals are urged to take enhanced precautions against mosquito bites while in these regions to minimize their chances of contracting the Zika virus. Pregnant women are being advised to consider postponing travel to areas where Zika virus transmission in ongoing. Pregnant women and those trying to become pregnant who must travel to these areas are advised to consult with their physician before traveling and take steps to prevent mosquito bites.
The CDC has already issued a Level 2 travel alert for these areas where Zika virus transmission is ongoing: Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny
Officials at the Centers for Disease Control and Prevention have added four more destinations to their Zika virus travel alert – American Samoa, Costa Rica, Curacao, and Nicaragua.
The Level 2 travel alert means that individuals are urged to take enhanced precautions against mosquito bites while in these regions to minimize their chances of contracting the Zika virus. Pregnant women are being advised to consider postponing travel to areas where Zika virus transmission in ongoing. Pregnant women and those trying to become pregnant who must travel to these areas are advised to consult with their physician before traveling and take steps to prevent mosquito bites.
The CDC has already issued a Level 2 travel alert for these areas where Zika virus transmission is ongoing: Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny
WHO Declares "Public Health Emergency" for Microcephaly Linked to Zika Virus
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
WHO declares ‘public health emergency’ for microcephaly linked to Zika virus
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
On Twitter @maryellenny
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
On Twitter @maryellenny
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
On Twitter @maryellenny
Managing Diabetes in Women of Childbearing Age
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
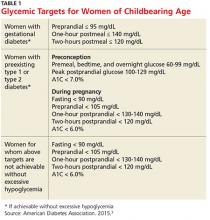
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
Aneuploidy Screening: Newer Noninvasive Test Gains Traction
PRACTICE CHANGER
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multicenter cohort studies.1,2
A 28-year-old woman (gravida 2, para 1001) at 10 weeks’ gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities, and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk for fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments.
It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011, after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However, until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are two large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The two groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the firstline approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA be discussed with all patients.8
Continue for study summaries >>
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2,042 pregnant patients ages 18 to 49 (mean, 29.6) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes, thyroid disorders, and other comorbidities. cfDNA testing was done on 1,909 maternal blood samples for trisomy 21 and 1,905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and nonlive births, the incidence of trisomy 21 was 5 of 1,909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1,905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly 1 in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false-positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false-positive rate was 0.3% compared to 3.6% for standard screening (P < .001); for trisomy 18, the cfDNA false-positive rate was 0.2% compared to 0.6% for standard screening (P = .03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in six countries.
This study enrolled 18,955 women ages 18 to 48 (mean, 31) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1 – specificity; < .700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages < 35).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P = .001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P < .001) and a lower false-positive rate (0.06% vs 5.4%; P < .001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing versus standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false-positive rates for cfDNA, compared with traditional screening.
WHAT’S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false-positive rates than standard fetal aneuploidy screening for the general obstetric population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetric population, according to ACOG and SMFM, the two groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
Continue for caveats >>
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to FDA approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false-negative results. Further testing has shown that such false-negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false-positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing, such as chorionic villus sampling or amniocentesis, before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Clinicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family practice clinicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, clinicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
References
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372: 1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011; 342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207: 137.e1-e8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 640: Cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126:e31-e37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013; 33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(1):49-52.
PRACTICE CHANGER
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multicenter cohort studies.1,2
A 28-year-old woman (gravida 2, para 1001) at 10 weeks’ gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities, and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk for fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments.
It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011, after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However, until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are two large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The two groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the firstline approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA be discussed with all patients.8
Continue for study summaries >>
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2,042 pregnant patients ages 18 to 49 (mean, 29.6) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes, thyroid disorders, and other comorbidities. cfDNA testing was done on 1,909 maternal blood samples for trisomy 21 and 1,905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and nonlive births, the incidence of trisomy 21 was 5 of 1,909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1,905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly 1 in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false-positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false-positive rate was 0.3% compared to 3.6% for standard screening (P < .001); for trisomy 18, the cfDNA false-positive rate was 0.2% compared to 0.6% for standard screening (P = .03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in six countries.
This study enrolled 18,955 women ages 18 to 48 (mean, 31) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1 – specificity; < .700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages < 35).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P = .001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P < .001) and a lower false-positive rate (0.06% vs 5.4%; P < .001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing versus standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false-positive rates for cfDNA, compared with traditional screening.
WHAT’S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false-positive rates than standard fetal aneuploidy screening for the general obstetric population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetric population, according to ACOG and SMFM, the two groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
Continue for caveats >>
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to FDA approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false-negative results. Further testing has shown that such false-negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false-positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing, such as chorionic villus sampling or amniocentesis, before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Clinicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family practice clinicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, clinicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
References
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372: 1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011; 342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207: 137.e1-e8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 640: Cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126:e31-e37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013; 33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(1):49-52.
PRACTICE CHANGER
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multicenter cohort studies.1,2
A 28-year-old woman (gravida 2, para 1001) at 10 weeks’ gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities, and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk for fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments.
It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011, after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However, until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are two large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The two groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the firstline approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA be discussed with all patients.8
Continue for study summaries >>
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2,042 pregnant patients ages 18 to 49 (mean, 29.6) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes, thyroid disorders, and other comorbidities. cfDNA testing was done on 1,909 maternal blood samples for trisomy 21 and 1,905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and nonlive births, the incidence of trisomy 21 was 5 of 1,909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1,905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly 1 in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false-positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false-positive rate was 0.3% compared to 3.6% for standard screening (P < .001); for trisomy 18, the cfDNA false-positive rate was 0.2% compared to 0.6% for standard screening (P = .03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in six countries.
This study enrolled 18,955 women ages 18 to 48 (mean, 31) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1 – specificity; < .700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages < 35).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P = .001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P < .001) and a lower false-positive rate (0.06% vs 5.4%; P < .001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing versus standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false-positive rates for cfDNA, compared with traditional screening.
WHAT’S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false-positive rates than standard fetal aneuploidy screening for the general obstetric population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetric population, according to ACOG and SMFM, the two groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
Continue for caveats >>
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to FDA approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false-negative results. Further testing has shown that such false-negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false-positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing, such as chorionic villus sampling or amniocentesis, before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Clinicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family practice clinicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, clinicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
References
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372: 1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011; 342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207: 137.e1-e8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 640: Cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126:e31-e37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013; 33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(1):49-52.
VIDEO: Dr. Anthony S. Fauci addresses the Zika virus situation
WASHINGTON – The outbreak of Zika virus infection in scores of countries and territories in Central and South America has raised many questions for physicians and their patients.
Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, explained the current situation to business leaders at a meeting held by the Economic Club and took the time to answer some concerns of practicing physicians. Dr. Fauci was joined on the dais by David Rubenstein, president of the Economic Club.
See Dr. Fauci’s comments here.
On Twitter @denisefulton
WASHINGTON – The outbreak of Zika virus infection in scores of countries and territories in Central and South America has raised many questions for physicians and their patients.
Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, explained the current situation to business leaders at a meeting held by the Economic Club and took the time to answer some concerns of practicing physicians. Dr. Fauci was joined on the dais by David Rubenstein, president of the Economic Club.
See Dr. Fauci’s comments here.
On Twitter @denisefulton
WASHINGTON – The outbreak of Zika virus infection in scores of countries and territories in Central and South America has raised many questions for physicians and their patients.
Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, explained the current situation to business leaders at a meeting held by the Economic Club and took the time to answer some concerns of practicing physicians. Dr. Fauci was joined on the dais by David Rubenstein, president of the Economic Club.
See Dr. Fauci’s comments here.
On Twitter @denisefulton
AT A MEETING OF THE ECONOMIC CLUB OF WASHINGTON, D.C.
Endometrial cancer after unopposed estrogen: $7.5M
Endometrial cancer after unopposed estrogen: $7.5M
A 42-year-old woman took unopposed estrogen as treatment for reported perimenopausal symptoms from October 2010 through October 2012, although she still had her uterus.
In December 2013, the patient was diagnosed with Stage 3 endometrial cancer. She underwent a radical hysterectomy followed by several rounds of chemotherapy. Despite treatment, the cancer metastasized, leaving the patient with a decreased life expectancy.
Patient’s claim Use of unopposed estrogens by a woman who still has her uterus significantly increases her risk of developing endometrial cancer. The gynecologist was negligent for prescribing the drug.
Physician’s defense The case was settled during the trial.
Verdict A $7.5 million Illinois settlement was reached
Preeclampsia treatment delayed because BP machine failed: $5M
At 31 4/7 weeks’ gestation, a woman went to the hospital. She reported burning pain in her chest, headache, and vomiting.
Two nurses cared for the mother: one who was completing her shift (Nurse A) and another who was beginning her shift (Nurse B). Nurse A stated that the blood pressure (BP) machine was not working correctly when she attempted to assess the patient at admission. When Nurse B took the patient’s BP, it indicated preeclampsia. Shortly thereafter, fetal heart-rate monitoring showed a concerning pattern. Nurse B notified the ObGyn 75 minutes after the mother’s arrival. The ObGyn ordered intervention to treat the baby’s concerning heart rate and, when those efforts failed, an emergency cesarean delivery was performed. The baby was found to have brain damage caused by hypoxia.
Parents’ claim Because the BP machine was not working properly when the mother arrived at the hospital, intervention for preeclampsia was delayed. Preeclampsia caused the baby’s injuries. An earlier cesarean delivery should have been performed.
Defendant’s defense The patient was adequately treated. The injury likely occurred before the mother arrived at the hospital. The case was settled during the trial.
Verdict A $5 million Massachusetts settlement was reached with the hospital and ObGyn.
Standard prenatal scan missed congenital syndrome: $3.75M
A woman receiving prenatal care at a medical center requested an ultrasonographic anatomical fetal scan. A limited ultrasound (US) was performed, and no abnormalities were detected.
Upon birth, the child was found to have Dandy Walker syndrome, a malformation of the brain affecting mobility. The child requires full-time nursing care.
Parents’ claim If a complete prenatal anatomical survey had been performed when requested, the abnormality would have been detected. The mother would have terminated the pregnancy.
Defendant’s defense It is the medical center’s policy to perform complete anatomical surveys only on women with high-risk pregnancies, which this was not. The woman switched health care providers during her pregnancy. A subsequent US performed by the new health care provider did not show a fetal abnormality. The case was settled during the trial.
Verdict A $3.75 million New Jersey settlement was reached with the medical center.
Should mother have been discharged? $700,000 settlement
Due to elevated fetal heart rate, a woman was admitted to the hospital for fetal heart-rate monitoring and then discharged a few hours later. After 2 days, the mother was readmitted for induction of labor, but she was discharged the following day. The next day, she was readmitted when she noticed lack of fetal movement. The infant was stillborn.
Parents’ claim The mother and fetus were not properly monitored; she should not have been sent home after induction of labor. The hospital was negligent for not properly monitoring labor, for not assigning an ObGyn to care for the mother, and for not performing cesarean delivery.
Hospital’s defense The case was settled during the trial.
Verdict A $700,000 Illinois settlement was reached with the hospital.
Endometrial cancer after unopposed estrogen: $7.5M
A 42-year-old woman took unopposed estrogen as treatment for reported perimenopausal symptoms from October 2010 through October 2012, although she still had her uterus.
In December 2013, the patient was diagnosed with Stage 3 endometrial cancer. She underwent a radical hysterectomy followed by several rounds of chemotherapy. Despite treatment, the cancer metastasized, leaving the patient with a decreased life expectancy.
Patient’s claim Use of unopposed estrogens by a woman who still has her uterus significantly increases her risk of developing endometrial cancer. The gynecologist was negligent for prescribing the drug.
Physician’s defense The case was settled during the trial.
Verdict A $7.5 million Illinois settlement was reached
Preeclampsia treatment delayed because BP machine failed: $5M
At 31 4/7 weeks’ gestation, a woman went to the hospital. She reported burning pain in her chest, headache, and vomiting.
Two nurses cared for the mother: one who was completing her shift (Nurse A) and another who was beginning her shift (Nurse B). Nurse A stated that the blood pressure (BP) machine was not working correctly when she attempted to assess the patient at admission. When Nurse B took the patient’s BP, it indicated preeclampsia. Shortly thereafter, fetal heart-rate monitoring showed a concerning pattern. Nurse B notified the ObGyn 75 minutes after the mother’s arrival. The ObGyn ordered intervention to treat the baby’s concerning heart rate and, when those efforts failed, an emergency cesarean delivery was performed. The baby was found to have brain damage caused by hypoxia.
Parents’ claim Because the BP machine was not working properly when the mother arrived at the hospital, intervention for preeclampsia was delayed. Preeclampsia caused the baby’s injuries. An earlier cesarean delivery should have been performed.
Defendant’s defense The patient was adequately treated. The injury likely occurred before the mother arrived at the hospital. The case was settled during the trial.
Verdict A $5 million Massachusetts settlement was reached with the hospital and ObGyn.
Standard prenatal scan missed congenital syndrome: $3.75M
A woman receiving prenatal care at a medical center requested an ultrasonographic anatomical fetal scan. A limited ultrasound (US) was performed, and no abnormalities were detected.
Upon birth, the child was found to have Dandy Walker syndrome, a malformation of the brain affecting mobility. The child requires full-time nursing care.
Parents’ claim If a complete prenatal anatomical survey had been performed when requested, the abnormality would have been detected. The mother would have terminated the pregnancy.
Defendant’s defense It is the medical center’s policy to perform complete anatomical surveys only on women with high-risk pregnancies, which this was not. The woman switched health care providers during her pregnancy. A subsequent US performed by the new health care provider did not show a fetal abnormality. The case was settled during the trial.
Verdict A $3.75 million New Jersey settlement was reached with the medical center.
Should mother have been discharged? $700,000 settlement
Due to elevated fetal heart rate, a woman was admitted to the hospital for fetal heart-rate monitoring and then discharged a few hours later. After 2 days, the mother was readmitted for induction of labor, but she was discharged the following day. The next day, she was readmitted when she noticed lack of fetal movement. The infant was stillborn.
Parents’ claim The mother and fetus were not properly monitored; she should not have been sent home after induction of labor. The hospital was negligent for not properly monitoring labor, for not assigning an ObGyn to care for the mother, and for not performing cesarean delivery.
Hospital’s defense The case was settled during the trial.
Verdict A $700,000 Illinois settlement was reached with the hospital.
Endometrial cancer after unopposed estrogen: $7.5M
A 42-year-old woman took unopposed estrogen as treatment for reported perimenopausal symptoms from October 2010 through October 2012, although she still had her uterus.
In December 2013, the patient was diagnosed with Stage 3 endometrial cancer. She underwent a radical hysterectomy followed by several rounds of chemotherapy. Despite treatment, the cancer metastasized, leaving the patient with a decreased life expectancy.
Patient’s claim Use of unopposed estrogens by a woman who still has her uterus significantly increases her risk of developing endometrial cancer. The gynecologist was negligent for prescribing the drug.
Physician’s defense The case was settled during the trial.
Verdict A $7.5 million Illinois settlement was reached
Preeclampsia treatment delayed because BP machine failed: $5M
At 31 4/7 weeks’ gestation, a woman went to the hospital. She reported burning pain in her chest, headache, and vomiting.
Two nurses cared for the mother: one who was completing her shift (Nurse A) and another who was beginning her shift (Nurse B). Nurse A stated that the blood pressure (BP) machine was not working correctly when she attempted to assess the patient at admission. When Nurse B took the patient’s BP, it indicated preeclampsia. Shortly thereafter, fetal heart-rate monitoring showed a concerning pattern. Nurse B notified the ObGyn 75 minutes after the mother’s arrival. The ObGyn ordered intervention to treat the baby’s concerning heart rate and, when those efforts failed, an emergency cesarean delivery was performed. The baby was found to have brain damage caused by hypoxia.
Parents’ claim Because the BP machine was not working properly when the mother arrived at the hospital, intervention for preeclampsia was delayed. Preeclampsia caused the baby’s injuries. An earlier cesarean delivery should have been performed.
Defendant’s defense The patient was adequately treated. The injury likely occurred before the mother arrived at the hospital. The case was settled during the trial.
Verdict A $5 million Massachusetts settlement was reached with the hospital and ObGyn.
Standard prenatal scan missed congenital syndrome: $3.75M
A woman receiving prenatal care at a medical center requested an ultrasonographic anatomical fetal scan. A limited ultrasound (US) was performed, and no abnormalities were detected.
Upon birth, the child was found to have Dandy Walker syndrome, a malformation of the brain affecting mobility. The child requires full-time nursing care.
Parents’ claim If a complete prenatal anatomical survey had been performed when requested, the abnormality would have been detected. The mother would have terminated the pregnancy.
Defendant’s defense It is the medical center’s policy to perform complete anatomical surveys only on women with high-risk pregnancies, which this was not. The woman switched health care providers during her pregnancy. A subsequent US performed by the new health care provider did not show a fetal abnormality. The case was settled during the trial.
Verdict A $3.75 million New Jersey settlement was reached with the medical center.
Should mother have been discharged? $700,000 settlement
Due to elevated fetal heart rate, a woman was admitted to the hospital for fetal heart-rate monitoring and then discharged a few hours later. After 2 days, the mother was readmitted for induction of labor, but she was discharged the following day. The next day, she was readmitted when she noticed lack of fetal movement. The infant was stillborn.
Parents’ claim The mother and fetus were not properly monitored; she should not have been sent home after induction of labor. The hospital was negligent for not properly monitoring labor, for not assigning an ObGyn to care for the mother, and for not performing cesarean delivery.
Hospital’s defense The case was settled during the trial.
Verdict A $700,000 Illinois settlement was reached with the hospital.
ADDITIONAL MEDICAL VERDICTS CASES:
Stroke during delivery: $35.4M verdict: OBG Manag. 2016;28(2):46.
Failure to find breast cancer; later diagnosed at Stage 3: OBG Manag. 2016;28(2):48.