User login
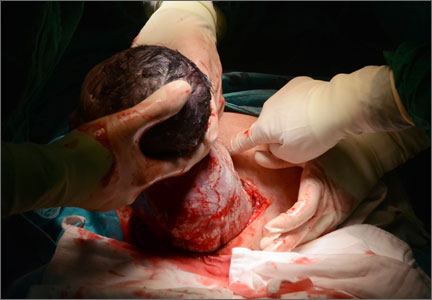
Hysterotomy incision and repair: Many options, many personal preferences
CASE: Your colleague’s hysterotomy practices vary from yours
You are in the hospital on a weekend inducing labor in your patient with hypertension. A colleague asks you to assist at a primary cesarean delivery for failure to progress in the second stage. You are glad to help. During the cesarean delivery, your colleague does not create a bladder flap, makes a superficial incision in the uterus and enters the uterine cavity bluntly with her index finger, uses blunt cephalad-caudad expansion of the uterine incision, and closes the uterine incision in a single-layer of continuous suture.
In your practice your general preference is to routinely dissect a bladder flap, enter the uterus using Allis clamps and sharp dissection; use blunt transverse expansion of the uterine incision; and close the uterine incision in two layers, locking the first layer. You wonder, is there any evidence that there is one best approach to managing the hysterotomy incision?
For many obstetrician-gynecologists, cesarean delivery is the major operation we perform most frequently. In planning and performing a cesarean delivery there are many technical surgical decision points, each with many options. A recent Cochrane review concluded that for most surgical options for uterine incision and closure, short-term maternal outcomes were similar among the options and that surgeons should use the techniques that they prefer and are comfortable performing.1 However, other authorities believe that the available evidence indicates that certain surgical techniques are associated with better maternal outcomes.2,3
In this editorial I focus on the varying surgical options available when performing a low transverse hysterotomy during cesarean delivery and the impact of these choices on maternal outcomes.
The bladder flap—surgeon’s choice
Theoretically, dissecting a bladder flap moves the dome of the bladder away from the anterior surface of the lower uterine segment, thereby protecting it from injury during the hysterotomy incision and repair. Three randomized trials have evaluated maternal outcomes following a hysterotomy with or without a bladder flap. All three trials reported that maternal outcomes were similar whether or not a bladder flap was created.4–6 In one trial, the creation of a bladder flap during a primary cesarean delivery was associated with increased adhesions between the parietal and visceral peritoneum and between the bladder and uterus at a repeat cesarean delivery.5
Some authorities have concluded that in most cesarean deliveries it is not necessary to create a bladder flap because the evidence does not indicate that it improves surgical outcomes.3 However, there may be clinical situations where a bladder flap is warranted. For example, during a repeat cesarean delivery, if the bladder is observed to be advanced high on the anterior uterine wall because of previous uterine surgery, a bladder flap may be helpful to ensure that the hysterotomy incision is performed in the lower uterine segment and not in the thickest, most muscular part of the uterine wall.
A second example is a case of arrested labor in the second stage with a deep transverse arrest of a macrosomic fetus. Lower segment lacerations may occur in this scenario, and some clinicians elect to dissect a bladder flap in anticipation of the risk of multiple extensions and a difficult hysterotomy repair. Since bladder injury occurs in less than 1% of cesarean deliveries, it would be difficult to perform a study with sufficient statistical power to determine whether creating a bladder flap influences the rate of bladder injury.7
Entering the uterine cavity—Try blunt entry
There are few clinical trial data to guide the technique for entering the uterine cavity. A major goal is to minimize the risk of a fetal laceration. One technique to reduce this risk is to superficially incise the uterus with a scalpel and then enter the uterus bluntly with a finger. Both the Misgav Ladach and modified Joel-Cohen techniques for cesarean delivery advocate the use of a superficial incision of the lower uterine segment with blunt entry into the uterine cavity.8,9 Other surgical options for entering the uterine cavity with minimal risk to the fetus include:
- Superficially incise the uterus with a scalpel and then apply Allis clamps to the upper and lower incision. Pull the tissue away from the underlying fetus before incising the final layer of uterine tissue and entering the cavity.10
- Apply the tip of the suction tubing with suction on and gently elevate the tissue trapped in the suction tip, incising the tissue to enter the uterus.
- Use a surgical device designed to reduce fetal lacerations (such as C-SAFE, CooperSurgical) to enter the uterus and extend the hysterotomy incision.11
Expanding the uterine incision—Use blunt expansion
Authors of a recent Cochrane meta-analysis analyzed five randomized controlled trials, involving
2,141 women, that evaluated blunt versus sharp expansion of a low transverse uterine incision.1 There was no difference in maternal febrile morbidity or major morbidity between the two techniques. However, blunt expansion of the uterine incision was associated with slightly less maternal blood loss and a lower risk of maternal blood transfusion than sharp incision (0.7% vs 3.1%).1 In another meta-analysis blunt expansion of the uterine incision with the surgeon’s fingers resulted in a smaller decrease in hematocrit and hemoglobin levels and fewer unintended extensions, but no difference in the rate of blood transfusion.12 Based on these findings some authorities recommend using blunt expansion of the uterine incision when a lower uterine segment incision is performed.3
One study, involving 811 women, compared cephalad-caudad blunt expansion versus transverse blunt expansion of the uterine incision.13 Cephalad-caudad blunt expansion compared with transverse blunt expansion resulted in a trend to less blood loss (398 mL versus 440 mL; P = .09), a significantly lower rate of unintended extension of the uterine incision (3.7% vs 7.4%, P = .03) and fewer cases with blood loss greater than 1,500 mL (0.2% vs 2.0%, P = .04). However, there was no difference in the rate of transfusion (0.7% vs 0.7%, P = 1.0) between cephalad-caudad versus transverse blunt expansion. Based on the results from this one trial, some authorities recommend that cephalad-caudad blunt extension be utilized rather than transverse blunt extension.3
Closing the uterine incision—One or two layers?
In the recent Cochrane meta-analysis, researchers compared outcomes of single-layer and two-layer closure of the uterine incision in 14 studies involving 13,890 women.1 There was no difference in rates of febrile morbidity (5.0% vs 5.1%), wound infection (9.4% vs 9.5%), or blood transfusion (2.1% vs 2.4%) between the two techniques. Authors of another systematic review of 20 trials of single- versus double-layer closure of the uterine incision concluded that, based on the available evidence from randomized trials, single- and double-layer closure appeared to produce similar outcomes.14 These authors cautioned, however, that based on nonrandomized studies, single layer closure might be associated with an increased risk of uterine rupture in a subsequent pregnancy.15,16
A uterine incision that was closed with a locked single-layer closure may be at an especially high risk of rupture during a subsequent trial of labor. In one analysis of relevant reports with heterogeneous study designs, the risk of uterine rupture during a trial of labor after a prior cesarean was 1.8% with a double-layer closure, 3.5% with an unlocked single-layer closure, and 6.2% with a locked single-layer closure.17 My perspective is that a double-layer closure generally is preferred because in a future pregnancy with a planned vaginal delivery, the double-layer closure may be associated with a lower rate of uterine rupture.
Some authorities recommend single-layer uterine closure if the patient is sure that she has no future plans to conceive. For example, a woman who is undergoing a tubal ligation at the time of cesarean delivery may be an optimal candidate for single-layer closure.3
Individualization and innovation in surgical care
Surgeons advance their skills by continually using the best evidence and advice from colleagues to guide changes in their practice. Many clinical situations present unique combinations of medical and anatomic problems, and surgeons need to use both creativity and expert judgment to solve these unique problems. Surgical choices that are guided by both the best evidence and hard-won clinical experience will result in optimal patient outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Dodd JM, Anderson ER, Gates S, Grivell RM. Surgical techniques for uterine incision and uterine closure at the time of cesarean section. Cochrane Database Sys Rev. 2014;7(3):CD004732.
2. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607–1617.
3. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: an updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
4. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98(6):1089–1092.
5. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in caesarean section on repeat caesarean delivery Eur J Obstet Gynecol. 2011;159(2):300–304.
6. Tuuli MG, Obido AO, Fogertey P, Roehl K, Stamilio D, Macones GA. Utility of the bladder flap at cesarean delivery. A randomized controlled trial. Obstet Gynecol. 2012;119(4):815–821.
7. Cahill AG, Stout MJ, Stamillo DM, Odibo AO, Peipert JF, Macones GA. Risk factors for bladder injury in patients with a prior hysterotomy. Obstet Gynecol. 2008;112(1):116–120.
8. Holmgren G, Sjoholm L, Stark M. The Misgav-Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78(7):615–621.
9. Wallin G, Fall O. Modified Joel-Cohen technique for cesarean delivery. Br J Obstet Gynaecol. 1999;106(3):221–226.
10. Gilstrap LC, Cunningham FG, Van Dorsten JP, eds. Operative Obstetrics, 2nd ed. New York, NY: McGraw Hill; 2002.
11. C SAFE. http://www.csafe.us/. Trumbull, CT: CooperSurgical, Inc.
12. Saad AF, Rahman M, Costantine MM, Saade GR. Blunt versus sharp uterine incision expansion during low transverse cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2014;211(6):684.e1–e11.
13. Cromi A, Ghezzi F, Di Naro E, Siesto G, Loverro G, Bolis P. Blunt expansion of the low transverse uterine incision at cesarean delivery: a randomized comparison of 2 techniques. Am J Obstet Gynecol. 2008;199(3):292.e1–e6.
14. Roberge S, Demers S, Berghella V, Chaillet N, Moore L, Bujold E. Impact of single- and double-layer closure on adverse outcomes and uterine scar defect: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014; 211(5):453–460.
15. Yasmin S, Sadaf J, Fatima N. Impact of methods for uterine incision closure on repeat cesarean section scar of lower uterine segment. J Coll Physicians Surg Pak. 2011;21(9): 522–526.
16. Bujold E, Mehta SH, Bujold C, Gauthier RJ. Interdelivery interval and uterine rupture. Am J Obstet Gynecol. 2002;187(5): 1199–1202.
17. Roberge S, Chaillet N, Boutin A, et al. Single- versus double-layer closure of the hysterotomy incision during cesarean delivery and risk of uterine rupture. Int J Gynaecol Obstet. 2011;115(1): 5–10.
CASE: Your colleague’s hysterotomy practices vary from yours
You are in the hospital on a weekend inducing labor in your patient with hypertension. A colleague asks you to assist at a primary cesarean delivery for failure to progress in the second stage. You are glad to help. During the cesarean delivery, your colleague does not create a bladder flap, makes a superficial incision in the uterus and enters the uterine cavity bluntly with her index finger, uses blunt cephalad-caudad expansion of the uterine incision, and closes the uterine incision in a single-layer of continuous suture.
In your practice your general preference is to routinely dissect a bladder flap, enter the uterus using Allis clamps and sharp dissection; use blunt transverse expansion of the uterine incision; and close the uterine incision in two layers, locking the first layer. You wonder, is there any evidence that there is one best approach to managing the hysterotomy incision?
For many obstetrician-gynecologists, cesarean delivery is the major operation we perform most frequently. In planning and performing a cesarean delivery there are many technical surgical decision points, each with many options. A recent Cochrane review concluded that for most surgical options for uterine incision and closure, short-term maternal outcomes were similar among the options and that surgeons should use the techniques that they prefer and are comfortable performing.1 However, other authorities believe that the available evidence indicates that certain surgical techniques are associated with better maternal outcomes.2,3
In this editorial I focus on the varying surgical options available when performing a low transverse hysterotomy during cesarean delivery and the impact of these choices on maternal outcomes.
The bladder flap—surgeon’s choice
Theoretically, dissecting a bladder flap moves the dome of the bladder away from the anterior surface of the lower uterine segment, thereby protecting it from injury during the hysterotomy incision and repair. Three randomized trials have evaluated maternal outcomes following a hysterotomy with or without a bladder flap. All three trials reported that maternal outcomes were similar whether or not a bladder flap was created.4–6 In one trial, the creation of a bladder flap during a primary cesarean delivery was associated with increased adhesions between the parietal and visceral peritoneum and between the bladder and uterus at a repeat cesarean delivery.5
Some authorities have concluded that in most cesarean deliveries it is not necessary to create a bladder flap because the evidence does not indicate that it improves surgical outcomes.3 However, there may be clinical situations where a bladder flap is warranted. For example, during a repeat cesarean delivery, if the bladder is observed to be advanced high on the anterior uterine wall because of previous uterine surgery, a bladder flap may be helpful to ensure that the hysterotomy incision is performed in the lower uterine segment and not in the thickest, most muscular part of the uterine wall.
A second example is a case of arrested labor in the second stage with a deep transverse arrest of a macrosomic fetus. Lower segment lacerations may occur in this scenario, and some clinicians elect to dissect a bladder flap in anticipation of the risk of multiple extensions and a difficult hysterotomy repair. Since bladder injury occurs in less than 1% of cesarean deliveries, it would be difficult to perform a study with sufficient statistical power to determine whether creating a bladder flap influences the rate of bladder injury.7
Entering the uterine cavity—Try blunt entry
There are few clinical trial data to guide the technique for entering the uterine cavity. A major goal is to minimize the risk of a fetal laceration. One technique to reduce this risk is to superficially incise the uterus with a scalpel and then enter the uterus bluntly with a finger. Both the Misgav Ladach and modified Joel-Cohen techniques for cesarean delivery advocate the use of a superficial incision of the lower uterine segment with blunt entry into the uterine cavity.8,9 Other surgical options for entering the uterine cavity with minimal risk to the fetus include:
- Superficially incise the uterus with a scalpel and then apply Allis clamps to the upper and lower incision. Pull the tissue away from the underlying fetus before incising the final layer of uterine tissue and entering the cavity.10
- Apply the tip of the suction tubing with suction on and gently elevate the tissue trapped in the suction tip, incising the tissue to enter the uterus.
- Use a surgical device designed to reduce fetal lacerations (such as C-SAFE, CooperSurgical) to enter the uterus and extend the hysterotomy incision.11
Expanding the uterine incision—Use blunt expansion
Authors of a recent Cochrane meta-analysis analyzed five randomized controlled trials, involving
2,141 women, that evaluated blunt versus sharp expansion of a low transverse uterine incision.1 There was no difference in maternal febrile morbidity or major morbidity between the two techniques. However, blunt expansion of the uterine incision was associated with slightly less maternal blood loss and a lower risk of maternal blood transfusion than sharp incision (0.7% vs 3.1%).1 In another meta-analysis blunt expansion of the uterine incision with the surgeon’s fingers resulted in a smaller decrease in hematocrit and hemoglobin levels and fewer unintended extensions, but no difference in the rate of blood transfusion.12 Based on these findings some authorities recommend using blunt expansion of the uterine incision when a lower uterine segment incision is performed.3
One study, involving 811 women, compared cephalad-caudad blunt expansion versus transverse blunt expansion of the uterine incision.13 Cephalad-caudad blunt expansion compared with transverse blunt expansion resulted in a trend to less blood loss (398 mL versus 440 mL; P = .09), a significantly lower rate of unintended extension of the uterine incision (3.7% vs 7.4%, P = .03) and fewer cases with blood loss greater than 1,500 mL (0.2% vs 2.0%, P = .04). However, there was no difference in the rate of transfusion (0.7% vs 0.7%, P = 1.0) between cephalad-caudad versus transverse blunt expansion. Based on the results from this one trial, some authorities recommend that cephalad-caudad blunt extension be utilized rather than transverse blunt extension.3
Closing the uterine incision—One or two layers?
In the recent Cochrane meta-analysis, researchers compared outcomes of single-layer and two-layer closure of the uterine incision in 14 studies involving 13,890 women.1 There was no difference in rates of febrile morbidity (5.0% vs 5.1%), wound infection (9.4% vs 9.5%), or blood transfusion (2.1% vs 2.4%) between the two techniques. Authors of another systematic review of 20 trials of single- versus double-layer closure of the uterine incision concluded that, based on the available evidence from randomized trials, single- and double-layer closure appeared to produce similar outcomes.14 These authors cautioned, however, that based on nonrandomized studies, single layer closure might be associated with an increased risk of uterine rupture in a subsequent pregnancy.15,16
A uterine incision that was closed with a locked single-layer closure may be at an especially high risk of rupture during a subsequent trial of labor. In one analysis of relevant reports with heterogeneous study designs, the risk of uterine rupture during a trial of labor after a prior cesarean was 1.8% with a double-layer closure, 3.5% with an unlocked single-layer closure, and 6.2% with a locked single-layer closure.17 My perspective is that a double-layer closure generally is preferred because in a future pregnancy with a planned vaginal delivery, the double-layer closure may be associated with a lower rate of uterine rupture.
Some authorities recommend single-layer uterine closure if the patient is sure that she has no future plans to conceive. For example, a woman who is undergoing a tubal ligation at the time of cesarean delivery may be an optimal candidate for single-layer closure.3
Individualization and innovation in surgical care
Surgeons advance their skills by continually using the best evidence and advice from colleagues to guide changes in their practice. Many clinical situations present unique combinations of medical and anatomic problems, and surgeons need to use both creativity and expert judgment to solve these unique problems. Surgical choices that are guided by both the best evidence and hard-won clinical experience will result in optimal patient outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Your colleague’s hysterotomy practices vary from yours
You are in the hospital on a weekend inducing labor in your patient with hypertension. A colleague asks you to assist at a primary cesarean delivery for failure to progress in the second stage. You are glad to help. During the cesarean delivery, your colleague does not create a bladder flap, makes a superficial incision in the uterus and enters the uterine cavity bluntly with her index finger, uses blunt cephalad-caudad expansion of the uterine incision, and closes the uterine incision in a single-layer of continuous suture.
In your practice your general preference is to routinely dissect a bladder flap, enter the uterus using Allis clamps and sharp dissection; use blunt transverse expansion of the uterine incision; and close the uterine incision in two layers, locking the first layer. You wonder, is there any evidence that there is one best approach to managing the hysterotomy incision?
For many obstetrician-gynecologists, cesarean delivery is the major operation we perform most frequently. In planning and performing a cesarean delivery there are many technical surgical decision points, each with many options. A recent Cochrane review concluded that for most surgical options for uterine incision and closure, short-term maternal outcomes were similar among the options and that surgeons should use the techniques that they prefer and are comfortable performing.1 However, other authorities believe that the available evidence indicates that certain surgical techniques are associated with better maternal outcomes.2,3
In this editorial I focus on the varying surgical options available when performing a low transverse hysterotomy during cesarean delivery and the impact of these choices on maternal outcomes.
The bladder flap—surgeon’s choice
Theoretically, dissecting a bladder flap moves the dome of the bladder away from the anterior surface of the lower uterine segment, thereby protecting it from injury during the hysterotomy incision and repair. Three randomized trials have evaluated maternal outcomes following a hysterotomy with or without a bladder flap. All three trials reported that maternal outcomes were similar whether or not a bladder flap was created.4–6 In one trial, the creation of a bladder flap during a primary cesarean delivery was associated with increased adhesions between the parietal and visceral peritoneum and between the bladder and uterus at a repeat cesarean delivery.5
Some authorities have concluded that in most cesarean deliveries it is not necessary to create a bladder flap because the evidence does not indicate that it improves surgical outcomes.3 However, there may be clinical situations where a bladder flap is warranted. For example, during a repeat cesarean delivery, if the bladder is observed to be advanced high on the anterior uterine wall because of previous uterine surgery, a bladder flap may be helpful to ensure that the hysterotomy incision is performed in the lower uterine segment and not in the thickest, most muscular part of the uterine wall.
A second example is a case of arrested labor in the second stage with a deep transverse arrest of a macrosomic fetus. Lower segment lacerations may occur in this scenario, and some clinicians elect to dissect a bladder flap in anticipation of the risk of multiple extensions and a difficult hysterotomy repair. Since bladder injury occurs in less than 1% of cesarean deliveries, it would be difficult to perform a study with sufficient statistical power to determine whether creating a bladder flap influences the rate of bladder injury.7
Entering the uterine cavity—Try blunt entry
There are few clinical trial data to guide the technique for entering the uterine cavity. A major goal is to minimize the risk of a fetal laceration. One technique to reduce this risk is to superficially incise the uterus with a scalpel and then enter the uterus bluntly with a finger. Both the Misgav Ladach and modified Joel-Cohen techniques for cesarean delivery advocate the use of a superficial incision of the lower uterine segment with blunt entry into the uterine cavity.8,9 Other surgical options for entering the uterine cavity with minimal risk to the fetus include:
- Superficially incise the uterus with a scalpel and then apply Allis clamps to the upper and lower incision. Pull the tissue away from the underlying fetus before incising the final layer of uterine tissue and entering the cavity.10
- Apply the tip of the suction tubing with suction on and gently elevate the tissue trapped in the suction tip, incising the tissue to enter the uterus.
- Use a surgical device designed to reduce fetal lacerations (such as C-SAFE, CooperSurgical) to enter the uterus and extend the hysterotomy incision.11
Expanding the uterine incision—Use blunt expansion
Authors of a recent Cochrane meta-analysis analyzed five randomized controlled trials, involving
2,141 women, that evaluated blunt versus sharp expansion of a low transverse uterine incision.1 There was no difference in maternal febrile morbidity or major morbidity between the two techniques. However, blunt expansion of the uterine incision was associated with slightly less maternal blood loss and a lower risk of maternal blood transfusion than sharp incision (0.7% vs 3.1%).1 In another meta-analysis blunt expansion of the uterine incision with the surgeon’s fingers resulted in a smaller decrease in hematocrit and hemoglobin levels and fewer unintended extensions, but no difference in the rate of blood transfusion.12 Based on these findings some authorities recommend using blunt expansion of the uterine incision when a lower uterine segment incision is performed.3
One study, involving 811 women, compared cephalad-caudad blunt expansion versus transverse blunt expansion of the uterine incision.13 Cephalad-caudad blunt expansion compared with transverse blunt expansion resulted in a trend to less blood loss (398 mL versus 440 mL; P = .09), a significantly lower rate of unintended extension of the uterine incision (3.7% vs 7.4%, P = .03) and fewer cases with blood loss greater than 1,500 mL (0.2% vs 2.0%, P = .04). However, there was no difference in the rate of transfusion (0.7% vs 0.7%, P = 1.0) between cephalad-caudad versus transverse blunt expansion. Based on the results from this one trial, some authorities recommend that cephalad-caudad blunt extension be utilized rather than transverse blunt extension.3
Closing the uterine incision—One or two layers?
In the recent Cochrane meta-analysis, researchers compared outcomes of single-layer and two-layer closure of the uterine incision in 14 studies involving 13,890 women.1 There was no difference in rates of febrile morbidity (5.0% vs 5.1%), wound infection (9.4% vs 9.5%), or blood transfusion (2.1% vs 2.4%) between the two techniques. Authors of another systematic review of 20 trials of single- versus double-layer closure of the uterine incision concluded that, based on the available evidence from randomized trials, single- and double-layer closure appeared to produce similar outcomes.14 These authors cautioned, however, that based on nonrandomized studies, single layer closure might be associated with an increased risk of uterine rupture in a subsequent pregnancy.15,16
A uterine incision that was closed with a locked single-layer closure may be at an especially high risk of rupture during a subsequent trial of labor. In one analysis of relevant reports with heterogeneous study designs, the risk of uterine rupture during a trial of labor after a prior cesarean was 1.8% with a double-layer closure, 3.5% with an unlocked single-layer closure, and 6.2% with a locked single-layer closure.17 My perspective is that a double-layer closure generally is preferred because in a future pregnancy with a planned vaginal delivery, the double-layer closure may be associated with a lower rate of uterine rupture.
Some authorities recommend single-layer uterine closure if the patient is sure that she has no future plans to conceive. For example, a woman who is undergoing a tubal ligation at the time of cesarean delivery may be an optimal candidate for single-layer closure.3
Individualization and innovation in surgical care
Surgeons advance their skills by continually using the best evidence and advice from colleagues to guide changes in their practice. Many clinical situations present unique combinations of medical and anatomic problems, and surgeons need to use both creativity and expert judgment to solve these unique problems. Surgical choices that are guided by both the best evidence and hard-won clinical experience will result in optimal patient outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Dodd JM, Anderson ER, Gates S, Grivell RM. Surgical techniques for uterine incision and uterine closure at the time of cesarean section. Cochrane Database Sys Rev. 2014;7(3):CD004732.
2. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607–1617.
3. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: an updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
4. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98(6):1089–1092.
5. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in caesarean section on repeat caesarean delivery Eur J Obstet Gynecol. 2011;159(2):300–304.
6. Tuuli MG, Obido AO, Fogertey P, Roehl K, Stamilio D, Macones GA. Utility of the bladder flap at cesarean delivery. A randomized controlled trial. Obstet Gynecol. 2012;119(4):815–821.
7. Cahill AG, Stout MJ, Stamillo DM, Odibo AO, Peipert JF, Macones GA. Risk factors for bladder injury in patients with a prior hysterotomy. Obstet Gynecol. 2008;112(1):116–120.
8. Holmgren G, Sjoholm L, Stark M. The Misgav-Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78(7):615–621.
9. Wallin G, Fall O. Modified Joel-Cohen technique for cesarean delivery. Br J Obstet Gynaecol. 1999;106(3):221–226.
10. Gilstrap LC, Cunningham FG, Van Dorsten JP, eds. Operative Obstetrics, 2nd ed. New York, NY: McGraw Hill; 2002.
11. C SAFE. http://www.csafe.us/. Trumbull, CT: CooperSurgical, Inc.
12. Saad AF, Rahman M, Costantine MM, Saade GR. Blunt versus sharp uterine incision expansion during low transverse cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2014;211(6):684.e1–e11.
13. Cromi A, Ghezzi F, Di Naro E, Siesto G, Loverro G, Bolis P. Blunt expansion of the low transverse uterine incision at cesarean delivery: a randomized comparison of 2 techniques. Am J Obstet Gynecol. 2008;199(3):292.e1–e6.
14. Roberge S, Demers S, Berghella V, Chaillet N, Moore L, Bujold E. Impact of single- and double-layer closure on adverse outcomes and uterine scar defect: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014; 211(5):453–460.
15. Yasmin S, Sadaf J, Fatima N. Impact of methods for uterine incision closure on repeat cesarean section scar of lower uterine segment. J Coll Physicians Surg Pak. 2011;21(9): 522–526.
16. Bujold E, Mehta SH, Bujold C, Gauthier RJ. Interdelivery interval and uterine rupture. Am J Obstet Gynecol. 2002;187(5): 1199–1202.
17. Roberge S, Chaillet N, Boutin A, et al. Single- versus double-layer closure of the hysterotomy incision during cesarean delivery and risk of uterine rupture. Int J Gynaecol Obstet. 2011;115(1): 5–10.
1. Dodd JM, Anderson ER, Gates S, Grivell RM. Surgical techniques for uterine incision and uterine closure at the time of cesarean section. Cochrane Database Sys Rev. 2014;7(3):CD004732.
2. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607–1617.
3. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: an updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
4. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98(6):1089–1092.
5. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in caesarean section on repeat caesarean delivery Eur J Obstet Gynecol. 2011;159(2):300–304.
6. Tuuli MG, Obido AO, Fogertey P, Roehl K, Stamilio D, Macones GA. Utility of the bladder flap at cesarean delivery. A randomized controlled trial. Obstet Gynecol. 2012;119(4):815–821.
7. Cahill AG, Stout MJ, Stamillo DM, Odibo AO, Peipert JF, Macones GA. Risk factors for bladder injury in patients with a prior hysterotomy. Obstet Gynecol. 2008;112(1):116–120.
8. Holmgren G, Sjoholm L, Stark M. The Misgav-Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78(7):615–621.
9. Wallin G, Fall O. Modified Joel-Cohen technique for cesarean delivery. Br J Obstet Gynaecol. 1999;106(3):221–226.
10. Gilstrap LC, Cunningham FG, Van Dorsten JP, eds. Operative Obstetrics, 2nd ed. New York, NY: McGraw Hill; 2002.
11. C SAFE. http://www.csafe.us/. Trumbull, CT: CooperSurgical, Inc.
12. Saad AF, Rahman M, Costantine MM, Saade GR. Blunt versus sharp uterine incision expansion during low transverse cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2014;211(6):684.e1–e11.
13. Cromi A, Ghezzi F, Di Naro E, Siesto G, Loverro G, Bolis P. Blunt expansion of the low transverse uterine incision at cesarean delivery: a randomized comparison of 2 techniques. Am J Obstet Gynecol. 2008;199(3):292.e1–e6.
14. Roberge S, Demers S, Berghella V, Chaillet N, Moore L, Bujold E. Impact of single- and double-layer closure on adverse outcomes and uterine scar defect: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014; 211(5):453–460.
15. Yasmin S, Sadaf J, Fatima N. Impact of methods for uterine incision closure on repeat cesarean section scar of lower uterine segment. J Coll Physicians Surg Pak. 2011;21(9): 522–526.
16. Bujold E, Mehta SH, Bujold C, Gauthier RJ. Interdelivery interval and uterine rupture. Am J Obstet Gynecol. 2002;187(5): 1199–1202.
17. Roberge S, Chaillet N, Boutin A, et al. Single- versus double-layer closure of the hysterotomy incision during cesarean delivery and risk of uterine rupture. Int J Gynaecol Obstet. 2011;115(1): 5–10.
Defining “abnormal rise” is the elephant in the room
“STOP USING THE HCG DISCRIMINATORY ZONE OF 1,500 TO
2,000 mIU/mL TO GUIDE INTERVENTION DURING EARLY PREGNANCY”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2015)
Defining “abnormal rise” is the elephant in the room
It was refreshing to read Dr. Barbieri’srecent editorial on the management of ectopic pregnancies based on human chorionic gonadotropin (hCG) levels. However, I feel there’s an elephant in the room. The phrase “abnormal rise” is not clearly defined, even though countless texts and review articles continue to use the phrase. In fact, many authors include a flow chart in which an abnormal rise usually is followed by a recommendation to empty the uterus in an attempt to find chorionic villi.1,2 The most recent versions of several widely used textbooks advocate this practice,3–5 and a current UpToDate article includes a flow chart suggesting that a “suboptimal rise” is sufficient for diagnosis of an abnormal pregnancy requiring treatment.6
Kadar and colleagues originally suggested that 85% of viable intrauterine pregnancies (IUPs) will have a rise in hCG levels of at least 66% every 48 hours, leading to what is commonly called the “doubling rule.”7 Barnhart and colleagues showed that the slowest recorded hCG rise for a viable pregnancy in 48 hours was 53%, demonstrating that the hCG level does not double in 48 to 72 hours as traditionally expected.8 In their final model, the authors calculated that 99% percent of normal IUPs should have an increase of at least 53% in 2 days, moving the bar for a “normal” hCG rise even lower. This also implies that 1% of viable IUPs may have a rise of less than 53% over 2 days. I have to wonder how many viable, wanted pregnancies have been interrupted by the misguided use of discriminatory zones or abnormal rises resulting in the emptying of the pregnant uterus?
Clinical assessment and ultrasonography should continue to be the mainstay of management of ectopic pregnancy. When surgical diagnosis is needed, laparoscopy should be considered. In this day and age, when we are being more cautious about emptying a uterus based on ultrasound measurements of the fetus or gestational sac, why are we still so eager to base that decision on laboratory values? Are we really willing to accept that 1% of the time we will be terminating a viable pregnancy? We should stop the continued propagation of flow charts and strategies that use hCG discriminatory zones or abnormal rises to determine viability and when to evacuate the uterus. A contemporary update to address the issue is needed.
Devin Namaky, MD
Cincinnati, Ohio
References
1. Seeber B, Barnhart K. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107(2):399–413.
2. Mukul LV, Teal SB. Current management of ectopic pregnancy. Obstet Gynecol Clin North Am. 2007;34(3):403–419.
3. Ectopic pregnancy. In: Hoffman BL, Schorge JP, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill Professional; 2012:198–218.
4. Lobo RA. Ectopic pregnancy. In: Lentz GM, ed. Comprehensive Gynecology. 6th ed. Philadelphia, PA: Elsevier Mosby; 2012:361–382 .
5. Damario MA, Rock JA. Ectopic pregnancy. In: Rock JA, Jones HW III, eds. Te Linde’s Operative Gynecology. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:798–824.
6. Tulandi T. Ectopic pregnancy: clinical manifestations and diagnosis. UpToDate Web site. http://www.uptodate.com/contents/ectopic-pregnancy-clinical-manifestations-and-diag nosis. Updated October 22, 2014. Accessed February 18, 2015.
7. Kadar N, Caldwell BV, Romera R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol. 1981;58(2):162–166.
8. Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104(1):50–55.
Why not use serum progesterone to determine early pregnancy viability?
I read with attention Dr. Barbieri’s recent editorial about evaluation of early pregnancy. My question is: Why is serum progesterone not recommended or used to assess the health of an early pregnancy?
In my practice, I always use a serum progesterone test along with hCG measurements to ascertain if an early pregnancy is healthy. I have observed, as biased as this observation can be, as I have not done any study about it, that a serum progesterone level above 12 ng/dL correlates quite well with a healthy pregnancy. I only follow more intensively with serial ultrasonography and hCG measurements in a patient whose progesterone level is below 11 ng/dL.
When a patient’s only symptom is bleeding, if the serum progesterone level is below 11 ng/dL, and her serum quantitative hCG level does not double as appropriate, I consider the pregnancy not normal and counsel the patient about continuing observation or terminating the pregnancy.
In the same laboratory scenario but with a patient who has bleeding and pain, and even moreso if the progesterone level is below 8 ng/dL, I strongly consider an ectopic pregnancy until it is proven otherwise. In this case, I offer treatment with methotrexate, as it’s my experience that the vast majority of these patients are carrying an abnormal pregnancy— either a spontaneous abortion or an ectopic pregnancy. Methotrexate therapy will prevent further complications without causing the loss of normal pregnancies.
For me, progesterone levels add one more piece to the puzzle. A patient with pain, vaginal bleeding, a quantitative hCG value of
500 mIU/mL, and a serum progesterone level of 20 ng/dL will have a normal pregnancy and is not a candidate for any intervention besides a follow-up hCG measurement to ascertain “doubling” of the analyte as expected in a normal pregnancy.
An asymptomatic patient whose quantitative hCG measurement is 1,000 mIU/mL and progesterone level is 4 ng/dL is carrying an abnormal pregnancy. If this quantitative hCG does not double appropriately in the follow-up, I counsel the patient about the greater chance of a spontaneous abortion or ectopic pregnancy.
Is this approach faulty?
Tomas Hernandez, MD
Pasco, Washington
Know the sensitivity of the HCG test
I found Dr. Barbieri’s editorial very timely. It brings some clarity to the use of the hCG discriminatory zone in symptomatic pregnant patients.
An additional important point is that it is the clinician’s obligation to know the sensitivity of the test the laboratory uses. Serum tests use the threshold for a negative result of either less than 1 mIU/mL or less than 5 mIU/mL. If possible, serial hCG measurements should be performed in the same laboratory, because the result may not represent a true change in the hCG concentration if the second test is performed at a different laboratory.1 This is especially important when the clinician is considering the use of methotrexate to treat a suspected ectopic pregnancy.
Magdalen E. Hull, MD, MPH
Great River, New York
Reference
1. Meriko Mori K, Lurain, JR. Human chorionic gonadotropin: testing in pregnancy and gestational trophoblastic disease and causes of low persistent levels. UpToDate. http://www.uptodate.com/contents/human-chorionic-gonadotropin-testing-in-pregnancy-and-gesta tional-trophoblastic-disease-and-causes-of-low-persistent-levels. Published October 23, 2013. Accessed January 29, 2015.
Further thoughts on HCG, ultrasound, and early pregnancy diagnosis
I want to thank Dr. Barbieri for his important, timely message about suspected nonviable pregnancy. I agree with virtually all of his excellent suggestions. In fact, I was part of the consensus panel that developed the findings diagnostic of and suggestive of IUP failure.1 There are a few points, however, that I believe the readership of OBG Management should know.
The endothelial heart tube, the first organ system to form, folds in on itself and begins to beat at 21 days postconception. Thus, it is present and beating prior to our ability to image it on transvaginal ultrasound. Yet new guidelines1 now say not to call an IUP failed until there is a crown-rump length of 7 mm or greater with no cardiac activity. In the past, many clinicians used 4 or 5 mm. In fact, one of the most recent studies utilizing an 8-mHz transducer found all cardiac activity was visualized by 3.1 mm embryonic size!2
So why has the number been increased to 7 mm? I’ve given this a lot of thought. Most clinical trials, from which guidelines were derived in the past, were well-designed, tightly controlled, and performed by better-trained clinicians, often with state-of-the-art equipment. In contrast, well-meaning health-care providers practicing in the field are often without the same level of quality control, equipment, or expertise but are still expected to duplicate the data from the trials used to create the guidelines. The reason this is relevant pertains to the statement that 94% of 291 cases of ectopic pregnancy had an adnexal mass.3 This is an excellent study, done at one of the nation’s most outstanding academic institutions by world-class sonologists. I do not believe that well-meaning clinicians in the field will be able to achieve this level of detection.
Dr. Barbieri also discusses an inability to see an IUP even when hCG levels are greater than 1,500 or 2,000 mIU/mL or above. He mentions obesity, fibroids, and adenomyosis as increasing the risk of an ultrasound failing to detect an early IUP. This point needs to be expanded upon. Twenty-seven years ago, we claimed a discriminatory level of 1,025 mIU/mL of hCG if the gestational sac was normal and the uterus was normal with normal echo patterns. We had three cases with markedly greater hCG levels (one, in fact, as high as 5,544 mIU/mL)with coexisting fibroids.4
I would also submit that it is the axial uterus that is a very important source of potential error. The closer the beam of sound coming off of the footprint is to a right angle with the endometrium (as it will be in a markedly anteverted or retroverted uterus), the better the resulting image. With an axial uterus, the endometrium is in the same plane as the beam of sound, and this diminishes imaging capability. Furthermore, twin pregnancies are a potential confounder in attempting to correlate hCG levels in transvaginal ultrasound findings.
I wholeheartedly agree with Dr. Barbieri: There is virtually no role to administer methotrexate based on a single hCG determination in a hemodynamically stable patient.
Steven R. Goldstein, MD
New York, New York
References
1. Doubilet PM, Benson CB, Bourne T, Blaivas M; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
2. Abaid LN, As-Sanie S, Wolfe HM. Relationship between crown-rump length and early detection of cardiac activity. J Reprod Med. 2007;52(5):375–378.
3. Frates MC, Doubilet PM, Peters HE, Benson CR. Adnexal sonographic findings in ectopic pregnancy and their correlation with tubal rupture and human chorionic gonadotropin levels. J Ultrasound Med. 2014;33(4):697–703.
4. Goldstein SR, Snyder JR, Watson C, Danon M. Very early pregnancy detection with endovaginal ultrasound. Obstet Gynecol. 1988;72(2):200–204.
Dr. Barbieri responds
Dr. Namaky, Dr. Hernandez, Dr. Hull, and Dr. Goldstein provide great clinical advice for readers. I agree with Dr. Namaky that the mainstays of managing a pregnancy of unknown location in a stable patient are clinical assessment and ultrasonography. Dr. Hernandez recommends using the serum progesterone measurement to help guide clinical decisions in a pregnancy of unknown location. I also use serum progesterone in my practice because a very low progesterone level helps the patient to understand that she has a failed pregnancy, and facilitates her acceptance of timely intervention. Many of my colleagues do not use progesterone measurement because they prefer to rely on clinical assessment, serial hCG measurement, and ultrasound results to guide their treatment. I agree with Dr. Hull that serial hCG measurements are most useful when the same laboratory performs all the tests. Dr. Goldstein, a world class expert in the evaluation and management of early pregnancy problems, provides great advice for all readers on how to best integrate ultrasonography in their practices to optimize patient care.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“STOP USING THE HCG DISCRIMINATORY ZONE OF 1,500 TO
2,000 mIU/mL TO GUIDE INTERVENTION DURING EARLY PREGNANCY”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2015)
Defining “abnormal rise” is the elephant in the room
It was refreshing to read Dr. Barbieri’srecent editorial on the management of ectopic pregnancies based on human chorionic gonadotropin (hCG) levels. However, I feel there’s an elephant in the room. The phrase “abnormal rise” is not clearly defined, even though countless texts and review articles continue to use the phrase. In fact, many authors include a flow chart in which an abnormal rise usually is followed by a recommendation to empty the uterus in an attempt to find chorionic villi.1,2 The most recent versions of several widely used textbooks advocate this practice,3–5 and a current UpToDate article includes a flow chart suggesting that a “suboptimal rise” is sufficient for diagnosis of an abnormal pregnancy requiring treatment.6
Kadar and colleagues originally suggested that 85% of viable intrauterine pregnancies (IUPs) will have a rise in hCG levels of at least 66% every 48 hours, leading to what is commonly called the “doubling rule.”7 Barnhart and colleagues showed that the slowest recorded hCG rise for a viable pregnancy in 48 hours was 53%, demonstrating that the hCG level does not double in 48 to 72 hours as traditionally expected.8 In their final model, the authors calculated that 99% percent of normal IUPs should have an increase of at least 53% in 2 days, moving the bar for a “normal” hCG rise even lower. This also implies that 1% of viable IUPs may have a rise of less than 53% over 2 days. I have to wonder how many viable, wanted pregnancies have been interrupted by the misguided use of discriminatory zones or abnormal rises resulting in the emptying of the pregnant uterus?
Clinical assessment and ultrasonography should continue to be the mainstay of management of ectopic pregnancy. When surgical diagnosis is needed, laparoscopy should be considered. In this day and age, when we are being more cautious about emptying a uterus based on ultrasound measurements of the fetus or gestational sac, why are we still so eager to base that decision on laboratory values? Are we really willing to accept that 1% of the time we will be terminating a viable pregnancy? We should stop the continued propagation of flow charts and strategies that use hCG discriminatory zones or abnormal rises to determine viability and when to evacuate the uterus. A contemporary update to address the issue is needed.
Devin Namaky, MD
Cincinnati, Ohio
References
1. Seeber B, Barnhart K. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107(2):399–413.
2. Mukul LV, Teal SB. Current management of ectopic pregnancy. Obstet Gynecol Clin North Am. 2007;34(3):403–419.
3. Ectopic pregnancy. In: Hoffman BL, Schorge JP, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill Professional; 2012:198–218.
4. Lobo RA. Ectopic pregnancy. In: Lentz GM, ed. Comprehensive Gynecology. 6th ed. Philadelphia, PA: Elsevier Mosby; 2012:361–382 .
5. Damario MA, Rock JA. Ectopic pregnancy. In: Rock JA, Jones HW III, eds. Te Linde’s Operative Gynecology. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:798–824.
6. Tulandi T. Ectopic pregnancy: clinical manifestations and diagnosis. UpToDate Web site. http://www.uptodate.com/contents/ectopic-pregnancy-clinical-manifestations-and-diag nosis. Updated October 22, 2014. Accessed February 18, 2015.
7. Kadar N, Caldwell BV, Romera R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol. 1981;58(2):162–166.
8. Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104(1):50–55.
Why not use serum progesterone to determine early pregnancy viability?
I read with attention Dr. Barbieri’s recent editorial about evaluation of early pregnancy. My question is: Why is serum progesterone not recommended or used to assess the health of an early pregnancy?
In my practice, I always use a serum progesterone test along with hCG measurements to ascertain if an early pregnancy is healthy. I have observed, as biased as this observation can be, as I have not done any study about it, that a serum progesterone level above 12 ng/dL correlates quite well with a healthy pregnancy. I only follow more intensively with serial ultrasonography and hCG measurements in a patient whose progesterone level is below 11 ng/dL.
When a patient’s only symptom is bleeding, if the serum progesterone level is below 11 ng/dL, and her serum quantitative hCG level does not double as appropriate, I consider the pregnancy not normal and counsel the patient about continuing observation or terminating the pregnancy.
In the same laboratory scenario but with a patient who has bleeding and pain, and even moreso if the progesterone level is below 8 ng/dL, I strongly consider an ectopic pregnancy until it is proven otherwise. In this case, I offer treatment with methotrexate, as it’s my experience that the vast majority of these patients are carrying an abnormal pregnancy— either a spontaneous abortion or an ectopic pregnancy. Methotrexate therapy will prevent further complications without causing the loss of normal pregnancies.
For me, progesterone levels add one more piece to the puzzle. A patient with pain, vaginal bleeding, a quantitative hCG value of
500 mIU/mL, and a serum progesterone level of 20 ng/dL will have a normal pregnancy and is not a candidate for any intervention besides a follow-up hCG measurement to ascertain “doubling” of the analyte as expected in a normal pregnancy.
An asymptomatic patient whose quantitative hCG measurement is 1,000 mIU/mL and progesterone level is 4 ng/dL is carrying an abnormal pregnancy. If this quantitative hCG does not double appropriately in the follow-up, I counsel the patient about the greater chance of a spontaneous abortion or ectopic pregnancy.
Is this approach faulty?
Tomas Hernandez, MD
Pasco, Washington
Know the sensitivity of the HCG test
I found Dr. Barbieri’s editorial very timely. It brings some clarity to the use of the hCG discriminatory zone in symptomatic pregnant patients.
An additional important point is that it is the clinician’s obligation to know the sensitivity of the test the laboratory uses. Serum tests use the threshold for a negative result of either less than 1 mIU/mL or less than 5 mIU/mL. If possible, serial hCG measurements should be performed in the same laboratory, because the result may not represent a true change in the hCG concentration if the second test is performed at a different laboratory.1 This is especially important when the clinician is considering the use of methotrexate to treat a suspected ectopic pregnancy.
Magdalen E. Hull, MD, MPH
Great River, New York
Reference
1. Meriko Mori K, Lurain, JR. Human chorionic gonadotropin: testing in pregnancy and gestational trophoblastic disease and causes of low persistent levels. UpToDate. http://www.uptodate.com/contents/human-chorionic-gonadotropin-testing-in-pregnancy-and-gesta tional-trophoblastic-disease-and-causes-of-low-persistent-levels. Published October 23, 2013. Accessed January 29, 2015.
Further thoughts on HCG, ultrasound, and early pregnancy diagnosis
I want to thank Dr. Barbieri for his important, timely message about suspected nonviable pregnancy. I agree with virtually all of his excellent suggestions. In fact, I was part of the consensus panel that developed the findings diagnostic of and suggestive of IUP failure.1 There are a few points, however, that I believe the readership of OBG Management should know.
The endothelial heart tube, the first organ system to form, folds in on itself and begins to beat at 21 days postconception. Thus, it is present and beating prior to our ability to image it on transvaginal ultrasound. Yet new guidelines1 now say not to call an IUP failed until there is a crown-rump length of 7 mm or greater with no cardiac activity. In the past, many clinicians used 4 or 5 mm. In fact, one of the most recent studies utilizing an 8-mHz transducer found all cardiac activity was visualized by 3.1 mm embryonic size!2
So why has the number been increased to 7 mm? I’ve given this a lot of thought. Most clinical trials, from which guidelines were derived in the past, were well-designed, tightly controlled, and performed by better-trained clinicians, often with state-of-the-art equipment. In contrast, well-meaning health-care providers practicing in the field are often without the same level of quality control, equipment, or expertise but are still expected to duplicate the data from the trials used to create the guidelines. The reason this is relevant pertains to the statement that 94% of 291 cases of ectopic pregnancy had an adnexal mass.3 This is an excellent study, done at one of the nation’s most outstanding academic institutions by world-class sonologists. I do not believe that well-meaning clinicians in the field will be able to achieve this level of detection.
Dr. Barbieri also discusses an inability to see an IUP even when hCG levels are greater than 1,500 or 2,000 mIU/mL or above. He mentions obesity, fibroids, and adenomyosis as increasing the risk of an ultrasound failing to detect an early IUP. This point needs to be expanded upon. Twenty-seven years ago, we claimed a discriminatory level of 1,025 mIU/mL of hCG if the gestational sac was normal and the uterus was normal with normal echo patterns. We had three cases with markedly greater hCG levels (one, in fact, as high as 5,544 mIU/mL)with coexisting fibroids.4
I would also submit that it is the axial uterus that is a very important source of potential error. The closer the beam of sound coming off of the footprint is to a right angle with the endometrium (as it will be in a markedly anteverted or retroverted uterus), the better the resulting image. With an axial uterus, the endometrium is in the same plane as the beam of sound, and this diminishes imaging capability. Furthermore, twin pregnancies are a potential confounder in attempting to correlate hCG levels in transvaginal ultrasound findings.
I wholeheartedly agree with Dr. Barbieri: There is virtually no role to administer methotrexate based on a single hCG determination in a hemodynamically stable patient.
Steven R. Goldstein, MD
New York, New York
References
1. Doubilet PM, Benson CB, Bourne T, Blaivas M; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
2. Abaid LN, As-Sanie S, Wolfe HM. Relationship between crown-rump length and early detection of cardiac activity. J Reprod Med. 2007;52(5):375–378.
3. Frates MC, Doubilet PM, Peters HE, Benson CR. Adnexal sonographic findings in ectopic pregnancy and their correlation with tubal rupture and human chorionic gonadotropin levels. J Ultrasound Med. 2014;33(4):697–703.
4. Goldstein SR, Snyder JR, Watson C, Danon M. Very early pregnancy detection with endovaginal ultrasound. Obstet Gynecol. 1988;72(2):200–204.
Dr. Barbieri responds
Dr. Namaky, Dr. Hernandez, Dr. Hull, and Dr. Goldstein provide great clinical advice for readers. I agree with Dr. Namaky that the mainstays of managing a pregnancy of unknown location in a stable patient are clinical assessment and ultrasonography. Dr. Hernandez recommends using the serum progesterone measurement to help guide clinical decisions in a pregnancy of unknown location. I also use serum progesterone in my practice because a very low progesterone level helps the patient to understand that she has a failed pregnancy, and facilitates her acceptance of timely intervention. Many of my colleagues do not use progesterone measurement because they prefer to rely on clinical assessment, serial hCG measurement, and ultrasound results to guide their treatment. I agree with Dr. Hull that serial hCG measurements are most useful when the same laboratory performs all the tests. Dr. Goldstein, a world class expert in the evaluation and management of early pregnancy problems, provides great advice for all readers on how to best integrate ultrasonography in their practices to optimize patient care.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“STOP USING THE HCG DISCRIMINATORY ZONE OF 1,500 TO
2,000 mIU/mL TO GUIDE INTERVENTION DURING EARLY PREGNANCY”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2015)
Defining “abnormal rise” is the elephant in the room
It was refreshing to read Dr. Barbieri’srecent editorial on the management of ectopic pregnancies based on human chorionic gonadotropin (hCG) levels. However, I feel there’s an elephant in the room. The phrase “abnormal rise” is not clearly defined, even though countless texts and review articles continue to use the phrase. In fact, many authors include a flow chart in which an abnormal rise usually is followed by a recommendation to empty the uterus in an attempt to find chorionic villi.1,2 The most recent versions of several widely used textbooks advocate this practice,3–5 and a current UpToDate article includes a flow chart suggesting that a “suboptimal rise” is sufficient for diagnosis of an abnormal pregnancy requiring treatment.6
Kadar and colleagues originally suggested that 85% of viable intrauterine pregnancies (IUPs) will have a rise in hCG levels of at least 66% every 48 hours, leading to what is commonly called the “doubling rule.”7 Barnhart and colleagues showed that the slowest recorded hCG rise for a viable pregnancy in 48 hours was 53%, demonstrating that the hCG level does not double in 48 to 72 hours as traditionally expected.8 In their final model, the authors calculated that 99% percent of normal IUPs should have an increase of at least 53% in 2 days, moving the bar for a “normal” hCG rise even lower. This also implies that 1% of viable IUPs may have a rise of less than 53% over 2 days. I have to wonder how many viable, wanted pregnancies have been interrupted by the misguided use of discriminatory zones or abnormal rises resulting in the emptying of the pregnant uterus?
Clinical assessment and ultrasonography should continue to be the mainstay of management of ectopic pregnancy. When surgical diagnosis is needed, laparoscopy should be considered. In this day and age, when we are being more cautious about emptying a uterus based on ultrasound measurements of the fetus or gestational sac, why are we still so eager to base that decision on laboratory values? Are we really willing to accept that 1% of the time we will be terminating a viable pregnancy? We should stop the continued propagation of flow charts and strategies that use hCG discriminatory zones or abnormal rises to determine viability and when to evacuate the uterus. A contemporary update to address the issue is needed.
Devin Namaky, MD
Cincinnati, Ohio
References
1. Seeber B, Barnhart K. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107(2):399–413.
2. Mukul LV, Teal SB. Current management of ectopic pregnancy. Obstet Gynecol Clin North Am. 2007;34(3):403–419.
3. Ectopic pregnancy. In: Hoffman BL, Schorge JP, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill Professional; 2012:198–218.
4. Lobo RA. Ectopic pregnancy. In: Lentz GM, ed. Comprehensive Gynecology. 6th ed. Philadelphia, PA: Elsevier Mosby; 2012:361–382 .
5. Damario MA, Rock JA. Ectopic pregnancy. In: Rock JA, Jones HW III, eds. Te Linde’s Operative Gynecology. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:798–824.
6. Tulandi T. Ectopic pregnancy: clinical manifestations and diagnosis. UpToDate Web site. http://www.uptodate.com/contents/ectopic-pregnancy-clinical-manifestations-and-diag nosis. Updated October 22, 2014. Accessed February 18, 2015.
7. Kadar N, Caldwell BV, Romera R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol. 1981;58(2):162–166.
8. Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104(1):50–55.
Why not use serum progesterone to determine early pregnancy viability?
I read with attention Dr. Barbieri’s recent editorial about evaluation of early pregnancy. My question is: Why is serum progesterone not recommended or used to assess the health of an early pregnancy?
In my practice, I always use a serum progesterone test along with hCG measurements to ascertain if an early pregnancy is healthy. I have observed, as biased as this observation can be, as I have not done any study about it, that a serum progesterone level above 12 ng/dL correlates quite well with a healthy pregnancy. I only follow more intensively with serial ultrasonography and hCG measurements in a patient whose progesterone level is below 11 ng/dL.
When a patient’s only symptom is bleeding, if the serum progesterone level is below 11 ng/dL, and her serum quantitative hCG level does not double as appropriate, I consider the pregnancy not normal and counsel the patient about continuing observation or terminating the pregnancy.
In the same laboratory scenario but with a patient who has bleeding and pain, and even moreso if the progesterone level is below 8 ng/dL, I strongly consider an ectopic pregnancy until it is proven otherwise. In this case, I offer treatment with methotrexate, as it’s my experience that the vast majority of these patients are carrying an abnormal pregnancy— either a spontaneous abortion or an ectopic pregnancy. Methotrexate therapy will prevent further complications without causing the loss of normal pregnancies.
For me, progesterone levels add one more piece to the puzzle. A patient with pain, vaginal bleeding, a quantitative hCG value of
500 mIU/mL, and a serum progesterone level of 20 ng/dL will have a normal pregnancy and is not a candidate for any intervention besides a follow-up hCG measurement to ascertain “doubling” of the analyte as expected in a normal pregnancy.
An asymptomatic patient whose quantitative hCG measurement is 1,000 mIU/mL and progesterone level is 4 ng/dL is carrying an abnormal pregnancy. If this quantitative hCG does not double appropriately in the follow-up, I counsel the patient about the greater chance of a spontaneous abortion or ectopic pregnancy.
Is this approach faulty?
Tomas Hernandez, MD
Pasco, Washington
Know the sensitivity of the HCG test
I found Dr. Barbieri’s editorial very timely. It brings some clarity to the use of the hCG discriminatory zone in symptomatic pregnant patients.
An additional important point is that it is the clinician’s obligation to know the sensitivity of the test the laboratory uses. Serum tests use the threshold for a negative result of either less than 1 mIU/mL or less than 5 mIU/mL. If possible, serial hCG measurements should be performed in the same laboratory, because the result may not represent a true change in the hCG concentration if the second test is performed at a different laboratory.1 This is especially important when the clinician is considering the use of methotrexate to treat a suspected ectopic pregnancy.
Magdalen E. Hull, MD, MPH
Great River, New York
Reference
1. Meriko Mori K, Lurain, JR. Human chorionic gonadotropin: testing in pregnancy and gestational trophoblastic disease and causes of low persistent levels. UpToDate. http://www.uptodate.com/contents/human-chorionic-gonadotropin-testing-in-pregnancy-and-gesta tional-trophoblastic-disease-and-causes-of-low-persistent-levels. Published October 23, 2013. Accessed January 29, 2015.
Further thoughts on HCG, ultrasound, and early pregnancy diagnosis
I want to thank Dr. Barbieri for his important, timely message about suspected nonviable pregnancy. I agree with virtually all of his excellent suggestions. In fact, I was part of the consensus panel that developed the findings diagnostic of and suggestive of IUP failure.1 There are a few points, however, that I believe the readership of OBG Management should know.
The endothelial heart tube, the first organ system to form, folds in on itself and begins to beat at 21 days postconception. Thus, it is present and beating prior to our ability to image it on transvaginal ultrasound. Yet new guidelines1 now say not to call an IUP failed until there is a crown-rump length of 7 mm or greater with no cardiac activity. In the past, many clinicians used 4 or 5 mm. In fact, one of the most recent studies utilizing an 8-mHz transducer found all cardiac activity was visualized by 3.1 mm embryonic size!2
So why has the number been increased to 7 mm? I’ve given this a lot of thought. Most clinical trials, from which guidelines were derived in the past, were well-designed, tightly controlled, and performed by better-trained clinicians, often with state-of-the-art equipment. In contrast, well-meaning health-care providers practicing in the field are often without the same level of quality control, equipment, or expertise but are still expected to duplicate the data from the trials used to create the guidelines. The reason this is relevant pertains to the statement that 94% of 291 cases of ectopic pregnancy had an adnexal mass.3 This is an excellent study, done at one of the nation’s most outstanding academic institutions by world-class sonologists. I do not believe that well-meaning clinicians in the field will be able to achieve this level of detection.
Dr. Barbieri also discusses an inability to see an IUP even when hCG levels are greater than 1,500 or 2,000 mIU/mL or above. He mentions obesity, fibroids, and adenomyosis as increasing the risk of an ultrasound failing to detect an early IUP. This point needs to be expanded upon. Twenty-seven years ago, we claimed a discriminatory level of 1,025 mIU/mL of hCG if the gestational sac was normal and the uterus was normal with normal echo patterns. We had three cases with markedly greater hCG levels (one, in fact, as high as 5,544 mIU/mL)with coexisting fibroids.4
I would also submit that it is the axial uterus that is a very important source of potential error. The closer the beam of sound coming off of the footprint is to a right angle with the endometrium (as it will be in a markedly anteverted or retroverted uterus), the better the resulting image. With an axial uterus, the endometrium is in the same plane as the beam of sound, and this diminishes imaging capability. Furthermore, twin pregnancies are a potential confounder in attempting to correlate hCG levels in transvaginal ultrasound findings.
I wholeheartedly agree with Dr. Barbieri: There is virtually no role to administer methotrexate based on a single hCG determination in a hemodynamically stable patient.
Steven R. Goldstein, MD
New York, New York
References
1. Doubilet PM, Benson CB, Bourne T, Blaivas M; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
2. Abaid LN, As-Sanie S, Wolfe HM. Relationship between crown-rump length and early detection of cardiac activity. J Reprod Med. 2007;52(5):375–378.
3. Frates MC, Doubilet PM, Peters HE, Benson CR. Adnexal sonographic findings in ectopic pregnancy and their correlation with tubal rupture and human chorionic gonadotropin levels. J Ultrasound Med. 2014;33(4):697–703.
4. Goldstein SR, Snyder JR, Watson C, Danon M. Very early pregnancy detection with endovaginal ultrasound. Obstet Gynecol. 1988;72(2):200–204.
Dr. Barbieri responds
Dr. Namaky, Dr. Hernandez, Dr. Hull, and Dr. Goldstein provide great clinical advice for readers. I agree with Dr. Namaky that the mainstays of managing a pregnancy of unknown location in a stable patient are clinical assessment and ultrasonography. Dr. Hernandez recommends using the serum progesterone measurement to help guide clinical decisions in a pregnancy of unknown location. I also use serum progesterone in my practice because a very low progesterone level helps the patient to understand that she has a failed pregnancy, and facilitates her acceptance of timely intervention. Many of my colleagues do not use progesterone measurement because they prefer to rely on clinical assessment, serial hCG measurement, and ultrasound results to guide their treatment. I agree with Dr. Hull that serial hCG measurements are most useful when the same laboratory performs all the tests. Dr. Goldstein, a world class expert in the evaluation and management of early pregnancy problems, provides great advice for all readers on how to best integrate ultrasonography in their practices to optimize patient care.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Pregnancy outcomes mixed after bariatric surgery
A history of bariatric surgery appears to both positively and negatively influence pregnancy outcomes, according to a prospective, nationwide cohort study published in the New England Journal of Medicine.
Pregnancies after bariatric surgery, as compared with control pregnancies matched for presurgery body mass index, were associated with a significantly lower risk of gestational diabetes (1.9% vs. 6.8%; odds ratio, 0.25; P < .001) and large-for-gestational age infants (8.6% vs. 22.4%; OR, 0.33; P < .001).
“However, increased surveillance during pregnancy and the neonatal period is warranted, since a history of bariatric surgery was also associated with small-for-gestational-age infants (15.6% vs. 7.6%; OR, 2.20; P < .001), shorter gestation (273 days vs. 277.5 days; P < .001), and potentially an increased risk of stillbirth or neonatal death [1.7% vs. 0.7%; OR, 2.39; P: 0.06],” study author Kari Johansson, Ph.D., from the Karolinska Institute in Stockholm, suggested in the study (N. Engl. J. Med. 2015;372:814-24 [doi:10.1056/NEJMoa1405789]).
The study, thought to be the largest to date comparing 2,952 pregnancy outcomes between women with and without a history of bariatric surgery, found no difference in the risk of congenital malformations between groups. The median time from surgery to conception was 1.1 years.
Dr. Johansson reported support from the Swedish Research Council and a young investigator award from the Obesity Society. Her coauthors reported support from the Swedish Research Council, Stockholm County Council, and consulting fees from Itrim and Strategic Health Resources.
A history of bariatric surgery appears to both positively and negatively influence pregnancy outcomes, according to a prospective, nationwide cohort study published in the New England Journal of Medicine.
Pregnancies after bariatric surgery, as compared with control pregnancies matched for presurgery body mass index, were associated with a significantly lower risk of gestational diabetes (1.9% vs. 6.8%; odds ratio, 0.25; P < .001) and large-for-gestational age infants (8.6% vs. 22.4%; OR, 0.33; P < .001).
“However, increased surveillance during pregnancy and the neonatal period is warranted, since a history of bariatric surgery was also associated with small-for-gestational-age infants (15.6% vs. 7.6%; OR, 2.20; P < .001), shorter gestation (273 days vs. 277.5 days; P < .001), and potentially an increased risk of stillbirth or neonatal death [1.7% vs. 0.7%; OR, 2.39; P: 0.06],” study author Kari Johansson, Ph.D., from the Karolinska Institute in Stockholm, suggested in the study (N. Engl. J. Med. 2015;372:814-24 [doi:10.1056/NEJMoa1405789]).
The study, thought to be the largest to date comparing 2,952 pregnancy outcomes between women with and without a history of bariatric surgery, found no difference in the risk of congenital malformations between groups. The median time from surgery to conception was 1.1 years.
Dr. Johansson reported support from the Swedish Research Council and a young investigator award from the Obesity Society. Her coauthors reported support from the Swedish Research Council, Stockholm County Council, and consulting fees from Itrim and Strategic Health Resources.
A history of bariatric surgery appears to both positively and negatively influence pregnancy outcomes, according to a prospective, nationwide cohort study published in the New England Journal of Medicine.
Pregnancies after bariatric surgery, as compared with control pregnancies matched for presurgery body mass index, were associated with a significantly lower risk of gestational diabetes (1.9% vs. 6.8%; odds ratio, 0.25; P < .001) and large-for-gestational age infants (8.6% vs. 22.4%; OR, 0.33; P < .001).
“However, increased surveillance during pregnancy and the neonatal period is warranted, since a history of bariatric surgery was also associated with small-for-gestational-age infants (15.6% vs. 7.6%; OR, 2.20; P < .001), shorter gestation (273 days vs. 277.5 days; P < .001), and potentially an increased risk of stillbirth or neonatal death [1.7% vs. 0.7%; OR, 2.39; P: 0.06],” study author Kari Johansson, Ph.D., from the Karolinska Institute in Stockholm, suggested in the study (N. Engl. J. Med. 2015;372:814-24 [doi:10.1056/NEJMoa1405789]).
The study, thought to be the largest to date comparing 2,952 pregnancy outcomes between women with and without a history of bariatric surgery, found no difference in the risk of congenital malformations between groups. The median time from surgery to conception was 1.1 years.
Dr. Johansson reported support from the Swedish Research Council and a young investigator award from the Obesity Society. Her coauthors reported support from the Swedish Research Council, Stockholm County Council, and consulting fees from Itrim and Strategic Health Resources.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Most of the findings are consistent with those from smaller studies and meta-analyses, although an association between bariatric surgery and subsequent perinatal mortality has not been previously suggested, Dr. Aaron B. Caughey noted in an accompanying editorial (N. Engl. J. Med. 2015;372:877-8 [doi:10.1056/nejme1500230]).
If anything, a recent meta-analysis showed that higher body mass index was associated with an increased risk of stillbirth and neonatal mortality (JAMA 2014;311:1536-46).
“The current data, combined with previous reports, suggest that it may be prudent to monitor fetal growth in women who have undergone bariatric surgery, particularly in those who have had gastric bypass surgery. I would not recommend that all such women undergo antepartum fetal surveillance on the basis of the current study, since evidence to indicate such care would improve outcomes is lacking,” Dr. Caughey wrote.
Dr. Aaron B. Caughey is chair of obstetrics and gynecology and associate dean for women’s health research and policy at the Oregon Health & Science University School of Medicine in Portland. He reported having no financial disclosures.
Feedback device helped shorten second stage of labor
SAN DIEGO – An investigational system that provides precise measurement and real-time maternal feedback of descent during the second stage of labor shortened the second stage in pregnant women who received epidural anesthesia, a randomized, controlled study demonstrated.
The system also reduced composite adverse maternal outcomes and reduced neonatal ICU admissions, Dr. Merlin B. Fausett reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The length of second-stage labor is highly correlated with the incidence of maternal morbidities and the incidence of neonatal ICU admissions,” said Dr. Fausett, a maternal-fetal medicine specialist in Missoula, Montana. “The majority of laboring women in the U.S. have epidurals during labor for labor pain management. In some studies, the length of second-stage labor is increased in women receiving regional anesthesia, compared with women without. This increased length of labor may be due to the lack of maternal sensation, resulting in decreased physiologic feedback that inhibits learning and decreases the efficacy of maternal expulsive efforts.”
Recognizing the difficulties with a long second stage of labor, clinicians have developed several nonoperative methods of attempting to reduce its length, including coached pushing, allowing periods of passive descent, and waiting for the laboring women to develop an urge to push. Some physicians also provide maternal visual feedback with a mirror.
“These methods have proven to be of limited benefit at best,” Dr. Fausett said. “With coached pushing, for example, frequent or continuous vaginal examinations may cause genital trauma, perinatal edema, and increased rates of infection. This method also requires a significant amount of the provider’s time. Finally, the use of mirrors is not well accepted by many patients and is only effective if the baby is already low enough in the pelvis to be visible to the mother with the mirror.”
Dr. Fausett and his colleagues hypothesized that using an experimental metrology device and software to give real-time quantitative maternal feedback of fetal descent during labor could result in more effective expulsion efforts, reduce the length of second stage, and improve perinatal outcomes.
They designed a prospective randomized, nonblinded trial to assess the clinical impact of providing laboring women real-time audiovisual feedback regarding fetal descent during second stage. The primary outcomes were a comparison of the length of time from initiation of pushing until delivery of the baby and a comparison of a composite of clinical outcomes.
“The pushing time comparison was to be made between study and control group subjects who had a spontaneous vaginal delivery, thus excluding those whose second stage was short with an operational or cesarean delivery,” Dr. Fausett said. “This comparison was also to control for fetal station at the initiation of pushing, as well as neonatal birth weight.”
The composite outcome was defined as a comparison of the number of women in the study versus control group who had any one of the following outcomes: cesarean delivery, operative vaginal delivery, third- or fourth-degree lacerations, an intra-amniotic infection, or NICU admission.
Developed by OB Technologies, the metrology device consisted of a support arm attached to the maternal sacral area using tape. This support arm extended through the gluteal cleft and terminated distal to the perineum. An optical sensor was then attached to the distal center of the support arm. A standard needle scalp electrode was modified by placing a semirigid wire at the base of the attachment where the spiral electrode attached to the needle head.
“This wire extended from the scalp across the optical sensor, and thus movement of the fetal head relative to the maternal pelvis was detected,” Dr. Fausett explained. “The instrument allowed detection of movements as small as 50 micrometers. Fetal station relative to the position in the pelvis was recorded. For feedback subjects, the movement was conveyed to them graphically and audibly using piano tones that occurred as often as every 150 milliseconds. The feedback was related to both the velocity and the distance of descent.”
Dr. Fausett reported results from 69 laboring nulliparous women with singleton pregnancies who were at least 36 weeks’ gestational age with epidural anesthesia.
Women in the study group received feedback on descent during the second stage by way of a laptop that displayed graphics and sounded musical notes corresponding to the movement of the fetus relative to the maternal bony pelvis.
Control subjects were not provided the visual and auditory feedback but the descent information was recorded with the same equipment. Normal labor and delivery procedures, including pelvic examination and nurse-provided feedback to the patient, were allowed in both groups. On postpartum day one, study subjects were asked to complete an anonymous survey about the use of the feedback device.
The study was halted after the planned interim analysis because the researchers identified a difference in the composite outcome between study and controls that was substantially above the threshold prescribed by the study design (more than 30%), Dr. Fausett said.
He presented findings from 46 women in the feedback group and 23 women in the control group. There were no differences between the groups in birth weights, mean station at the initiation of pushing, or in the maternal body mass indexes. However, the incidence of the composite adverse outcome was 9% in the feedback group, compared with 33% in the control group (P = .011). This corresponded to a 73% reduction in the composite outcome in the feedback group.
With regard to pushing time, the median pushing time was 77 minutes versus 58 minutes for control and study groups, respectively (P = .016).
The researchers did not identify any statistical differences in the incidence of cesarean delivery or intra-amniotic infections between the study and control groups. However, in the feedback group there were significant reductions in the incidences of operative vaginal deliveries (6.6% vs. 25%; P = .035), third- and fourth-degree lacerations (0% vs. 17%; P = .005), and neonatal ICU admission (0% vs. 13%; P = .015).
Among the 35 subjects that responded to a survey about the device on postpartum day one, “the majority strongly agreed that the device was comfortable, it helped them to push better, and they felt it gave them an increased sense of control during pushing,” he said.
Dr. Fausett acknowledged certain limitations of the study, including the potential for bias since it was not a blinded trial.
“We also did not address the optimal method or methods of feedback in this study,” he said. “Additional studies should evaluate and validate our findings as well as focus on fine-tuning the methods of graphic, auditory, and haptic feedback. If such feedback continues to prove effective, there are significant potential benefits on perinatal outcomes with important medical and economic consequences.”
Dr. Fausett disclosed that he is a shareholder in OB Technologies.
On Twitter @dougbrunk
SAN DIEGO – An investigational system that provides precise measurement and real-time maternal feedback of descent during the second stage of labor shortened the second stage in pregnant women who received epidural anesthesia, a randomized, controlled study demonstrated.
The system also reduced composite adverse maternal outcomes and reduced neonatal ICU admissions, Dr. Merlin B. Fausett reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The length of second-stage labor is highly correlated with the incidence of maternal morbidities and the incidence of neonatal ICU admissions,” said Dr. Fausett, a maternal-fetal medicine specialist in Missoula, Montana. “The majority of laboring women in the U.S. have epidurals during labor for labor pain management. In some studies, the length of second-stage labor is increased in women receiving regional anesthesia, compared with women without. This increased length of labor may be due to the lack of maternal sensation, resulting in decreased physiologic feedback that inhibits learning and decreases the efficacy of maternal expulsive efforts.”
Recognizing the difficulties with a long second stage of labor, clinicians have developed several nonoperative methods of attempting to reduce its length, including coached pushing, allowing periods of passive descent, and waiting for the laboring women to develop an urge to push. Some physicians also provide maternal visual feedback with a mirror.
“These methods have proven to be of limited benefit at best,” Dr. Fausett said. “With coached pushing, for example, frequent or continuous vaginal examinations may cause genital trauma, perinatal edema, and increased rates of infection. This method also requires a significant amount of the provider’s time. Finally, the use of mirrors is not well accepted by many patients and is only effective if the baby is already low enough in the pelvis to be visible to the mother with the mirror.”
Dr. Fausett and his colleagues hypothesized that using an experimental metrology device and software to give real-time quantitative maternal feedback of fetal descent during labor could result in more effective expulsion efforts, reduce the length of second stage, and improve perinatal outcomes.
They designed a prospective randomized, nonblinded trial to assess the clinical impact of providing laboring women real-time audiovisual feedback regarding fetal descent during second stage. The primary outcomes were a comparison of the length of time from initiation of pushing until delivery of the baby and a comparison of a composite of clinical outcomes.
“The pushing time comparison was to be made between study and control group subjects who had a spontaneous vaginal delivery, thus excluding those whose second stage was short with an operational or cesarean delivery,” Dr. Fausett said. “This comparison was also to control for fetal station at the initiation of pushing, as well as neonatal birth weight.”
The composite outcome was defined as a comparison of the number of women in the study versus control group who had any one of the following outcomes: cesarean delivery, operative vaginal delivery, third- or fourth-degree lacerations, an intra-amniotic infection, or NICU admission.
Developed by OB Technologies, the metrology device consisted of a support arm attached to the maternal sacral area using tape. This support arm extended through the gluteal cleft and terminated distal to the perineum. An optical sensor was then attached to the distal center of the support arm. A standard needle scalp electrode was modified by placing a semirigid wire at the base of the attachment where the spiral electrode attached to the needle head.
“This wire extended from the scalp across the optical sensor, and thus movement of the fetal head relative to the maternal pelvis was detected,” Dr. Fausett explained. “The instrument allowed detection of movements as small as 50 micrometers. Fetal station relative to the position in the pelvis was recorded. For feedback subjects, the movement was conveyed to them graphically and audibly using piano tones that occurred as often as every 150 milliseconds. The feedback was related to both the velocity and the distance of descent.”
Dr. Fausett reported results from 69 laboring nulliparous women with singleton pregnancies who were at least 36 weeks’ gestational age with epidural anesthesia.
Women in the study group received feedback on descent during the second stage by way of a laptop that displayed graphics and sounded musical notes corresponding to the movement of the fetus relative to the maternal bony pelvis.
Control subjects were not provided the visual and auditory feedback but the descent information was recorded with the same equipment. Normal labor and delivery procedures, including pelvic examination and nurse-provided feedback to the patient, were allowed in both groups. On postpartum day one, study subjects were asked to complete an anonymous survey about the use of the feedback device.
The study was halted after the planned interim analysis because the researchers identified a difference in the composite outcome between study and controls that was substantially above the threshold prescribed by the study design (more than 30%), Dr. Fausett said.
He presented findings from 46 women in the feedback group and 23 women in the control group. There were no differences between the groups in birth weights, mean station at the initiation of pushing, or in the maternal body mass indexes. However, the incidence of the composite adverse outcome was 9% in the feedback group, compared with 33% in the control group (P = .011). This corresponded to a 73% reduction in the composite outcome in the feedback group.
With regard to pushing time, the median pushing time was 77 minutes versus 58 minutes for control and study groups, respectively (P = .016).
The researchers did not identify any statistical differences in the incidence of cesarean delivery or intra-amniotic infections between the study and control groups. However, in the feedback group there were significant reductions in the incidences of operative vaginal deliveries (6.6% vs. 25%; P = .035), third- and fourth-degree lacerations (0% vs. 17%; P = .005), and neonatal ICU admission (0% vs. 13%; P = .015).
Among the 35 subjects that responded to a survey about the device on postpartum day one, “the majority strongly agreed that the device was comfortable, it helped them to push better, and they felt it gave them an increased sense of control during pushing,” he said.
Dr. Fausett acknowledged certain limitations of the study, including the potential for bias since it was not a blinded trial.
“We also did not address the optimal method or methods of feedback in this study,” he said. “Additional studies should evaluate and validate our findings as well as focus on fine-tuning the methods of graphic, auditory, and haptic feedback. If such feedback continues to prove effective, there are significant potential benefits on perinatal outcomes with important medical and economic consequences.”
Dr. Fausett disclosed that he is a shareholder in OB Technologies.
On Twitter @dougbrunk
SAN DIEGO – An investigational system that provides precise measurement and real-time maternal feedback of descent during the second stage of labor shortened the second stage in pregnant women who received epidural anesthesia, a randomized, controlled study demonstrated.
The system also reduced composite adverse maternal outcomes and reduced neonatal ICU admissions, Dr. Merlin B. Fausett reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The length of second-stage labor is highly correlated with the incidence of maternal morbidities and the incidence of neonatal ICU admissions,” said Dr. Fausett, a maternal-fetal medicine specialist in Missoula, Montana. “The majority of laboring women in the U.S. have epidurals during labor for labor pain management. In some studies, the length of second-stage labor is increased in women receiving regional anesthesia, compared with women without. This increased length of labor may be due to the lack of maternal sensation, resulting in decreased physiologic feedback that inhibits learning and decreases the efficacy of maternal expulsive efforts.”
Recognizing the difficulties with a long second stage of labor, clinicians have developed several nonoperative methods of attempting to reduce its length, including coached pushing, allowing periods of passive descent, and waiting for the laboring women to develop an urge to push. Some physicians also provide maternal visual feedback with a mirror.
“These methods have proven to be of limited benefit at best,” Dr. Fausett said. “With coached pushing, for example, frequent or continuous vaginal examinations may cause genital trauma, perinatal edema, and increased rates of infection. This method also requires a significant amount of the provider’s time. Finally, the use of mirrors is not well accepted by many patients and is only effective if the baby is already low enough in the pelvis to be visible to the mother with the mirror.”
Dr. Fausett and his colleagues hypothesized that using an experimental metrology device and software to give real-time quantitative maternal feedback of fetal descent during labor could result in more effective expulsion efforts, reduce the length of second stage, and improve perinatal outcomes.
They designed a prospective randomized, nonblinded trial to assess the clinical impact of providing laboring women real-time audiovisual feedback regarding fetal descent during second stage. The primary outcomes were a comparison of the length of time from initiation of pushing until delivery of the baby and a comparison of a composite of clinical outcomes.
“The pushing time comparison was to be made between study and control group subjects who had a spontaneous vaginal delivery, thus excluding those whose second stage was short with an operational or cesarean delivery,” Dr. Fausett said. “This comparison was also to control for fetal station at the initiation of pushing, as well as neonatal birth weight.”
The composite outcome was defined as a comparison of the number of women in the study versus control group who had any one of the following outcomes: cesarean delivery, operative vaginal delivery, third- or fourth-degree lacerations, an intra-amniotic infection, or NICU admission.
Developed by OB Technologies, the metrology device consisted of a support arm attached to the maternal sacral area using tape. This support arm extended through the gluteal cleft and terminated distal to the perineum. An optical sensor was then attached to the distal center of the support arm. A standard needle scalp electrode was modified by placing a semirigid wire at the base of the attachment where the spiral electrode attached to the needle head.
“This wire extended from the scalp across the optical sensor, and thus movement of the fetal head relative to the maternal pelvis was detected,” Dr. Fausett explained. “The instrument allowed detection of movements as small as 50 micrometers. Fetal station relative to the position in the pelvis was recorded. For feedback subjects, the movement was conveyed to them graphically and audibly using piano tones that occurred as often as every 150 milliseconds. The feedback was related to both the velocity and the distance of descent.”
Dr. Fausett reported results from 69 laboring nulliparous women with singleton pregnancies who were at least 36 weeks’ gestational age with epidural anesthesia.
Women in the study group received feedback on descent during the second stage by way of a laptop that displayed graphics and sounded musical notes corresponding to the movement of the fetus relative to the maternal bony pelvis.
Control subjects were not provided the visual and auditory feedback but the descent information was recorded with the same equipment. Normal labor and delivery procedures, including pelvic examination and nurse-provided feedback to the patient, were allowed in both groups. On postpartum day one, study subjects were asked to complete an anonymous survey about the use of the feedback device.
The study was halted after the planned interim analysis because the researchers identified a difference in the composite outcome between study and controls that was substantially above the threshold prescribed by the study design (more than 30%), Dr. Fausett said.
He presented findings from 46 women in the feedback group and 23 women in the control group. There were no differences between the groups in birth weights, mean station at the initiation of pushing, or in the maternal body mass indexes. However, the incidence of the composite adverse outcome was 9% in the feedback group, compared with 33% in the control group (P = .011). This corresponded to a 73% reduction in the composite outcome in the feedback group.
With regard to pushing time, the median pushing time was 77 minutes versus 58 minutes for control and study groups, respectively (P = .016).
The researchers did not identify any statistical differences in the incidence of cesarean delivery or intra-amniotic infections between the study and control groups. However, in the feedback group there were significant reductions in the incidences of operative vaginal deliveries (6.6% vs. 25%; P = .035), third- and fourth-degree lacerations (0% vs. 17%; P = .005), and neonatal ICU admission (0% vs. 13%; P = .015).
Among the 35 subjects that responded to a survey about the device on postpartum day one, “the majority strongly agreed that the device was comfortable, it helped them to push better, and they felt it gave them an increased sense of control during pushing,” he said.
Dr. Fausett acknowledged certain limitations of the study, including the potential for bias since it was not a blinded trial.
“We also did not address the optimal method or methods of feedback in this study,” he said. “Additional studies should evaluate and validate our findings as well as focus on fine-tuning the methods of graphic, auditory, and haptic feedback. If such feedback continues to prove effective, there are significant potential benefits on perinatal outcomes with important medical and economic consequences.”
Dr. Fausett disclosed that he is a shareholder in OB Technologies.
On Twitter @dougbrunk
AT THE PREGNANCY MEETING
Breastfeeding-related changes in gut bacteria protect against childhood allergy
HOUSTON – Findings from a cohort study of mothers and babies point to breastfeeding as influencing infants’ gut microbiome in a way that protects them from developing allergic disease.
In findings presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, epidemiologist Christine Cole Johnson, Ph.D., of the Henry Ford Health System in Detroit described a correlation between certain maternal and birth characteristics that had previously been shown to relate to allergic response, and measurable differences in the bacterial profiles of the study infants’ stools.
Using data from the WHEALS (Wayne County [Michigan] Health, Environment, Allergy, and Asthma Longitudinal Study) cohort, Dr. Johnson and her colleagues looked at stool samples from 298 children at 1 and 6 months of age. The investigators also collected dust samples from the infants’ homes, obtained medical records, and conducted interviews with the families (J. Allergy Clin. Immunol. 2015 [http://dx.doi.org/10.1016/j.jaci.2014.12.1443]).
The presence of household pets, the body mass index of mothers before delivery, the mode of delivery, household smoke exposure, marital status, income, race, and maternal education were all found to be significantly correlated to different gut bacterial profiles.
“Environmental and lifestyle variables that we’ve been working on related to childhood asthma and allergy seem to be associated – at least in our study – with the child’s gut microbiome,” Dr. Johnson said. “These factors vary a lot by whether those stool samples were collected at 1 or 6 months,” she said, noting that the infant gut microbiome is shaped rapidly in the first year.
But, at both 1 and 6 months of age, breastfeeding was seen as the dominant factor influencing gut bacterial composition.
At 6 months, breastfed infants had bacterial profiles showing overwhelming dominance of Bifidobacteriaceae, but vastly lower levels of other families of bacteria, notably Lachnospiraceae, which were prominent in the guts of non-breastfed babies.
In a related study that used the same cohort to explore whether the influence of breastfeeding on gut bacterial composition correlated to the development of allergic symptoms at 4 years old, Alexandra R. Sitarik, also of the Henry Ford Health System in Detroit, reported that babies being breastfed at 1 month of age had a significantly lower risk of developing allergiclike symptoms to pets by age 4 years (P = .028) (J. Allergy Clin. Immunol. 2015 [http://dx.doi.org/10.1016/j.jaci.2014.12.1444]).
Both breastfeeding and allergiclike response to pets were significantly related to compositional variation in gut microbiome (P < .001 and P = .023, respectively), Ms. Sitarik reported.
Of the 109 types of bacteria significantly associated with both breastfeeding and allergiclike response to pets, 71% were negatively associated with breastfeeding but positively associated with allergiclike response to pets.
This subset of risk-increasing bacteria suppressed by breastfeeding were predominantly members of the family Lachnospiraceae, the researchers found.
Lachnospiraceae are common adult gut colonizers, Ms. Sitarik said, and as people age the relative abundance of Lachnospiraceae increases. “What we think might be happening in terms of the gut microbiome is that maybe breastfeeding is preventing this premature shift into adulthood,” she said.
Dr. Johnson and Ms. Sitarik reported having no financial disclosures.
HOUSTON – Findings from a cohort study of mothers and babies point to breastfeeding as influencing infants’ gut microbiome in a way that protects them from developing allergic disease.
In findings presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, epidemiologist Christine Cole Johnson, Ph.D., of the Henry Ford Health System in Detroit described a correlation between certain maternal and birth characteristics that had previously been shown to relate to allergic response, and measurable differences in the bacterial profiles of the study infants’ stools.
Using data from the WHEALS (Wayne County [Michigan] Health, Environment, Allergy, and Asthma Longitudinal Study) cohort, Dr. Johnson and her colleagues looked at stool samples from 298 children at 1 and 6 months of age. The investigators also collected dust samples from the infants’ homes, obtained medical records, and conducted interviews with the families (J. Allergy Clin. Immunol. 2015 [http://dx.doi.org/10.1016/j.jaci.2014.12.1443]).
The presence of household pets, the body mass index of mothers before delivery, the mode of delivery, household smoke exposure, marital status, income, race, and maternal education were all found to be significantly correlated to different gut bacterial profiles.
“Environmental and lifestyle variables that we’ve been working on related to childhood asthma and allergy seem to be associated – at least in our study – with the child’s gut microbiome,” Dr. Johnson said. “These factors vary a lot by whether those stool samples were collected at 1 or 6 months,” she said, noting that the infant gut microbiome is shaped rapidly in the first year.
But, at both 1 and 6 months of age, breastfeeding was seen as the dominant factor influencing gut bacterial composition.
At 6 months, breastfed infants had bacterial profiles showing overwhelming dominance of Bifidobacteriaceae, but vastly lower levels of other families of bacteria, notably Lachnospiraceae, which were prominent in the guts of non-breastfed babies.
In a related study that used the same cohort to explore whether the influence of breastfeeding on gut bacterial composition correlated to the development of allergic symptoms at 4 years old, Alexandra R. Sitarik, also of the Henry Ford Health System in Detroit, reported that babies being breastfed at 1 month of age had a significantly lower risk of developing allergiclike symptoms to pets by age 4 years (P = .028) (J. Allergy Clin. Immunol. 2015 [http://dx.doi.org/10.1016/j.jaci.2014.12.1444]).
Both breastfeeding and allergiclike response to pets were significantly related to compositional variation in gut microbiome (P < .001 and P = .023, respectively), Ms. Sitarik reported.
Of the 109 types of bacteria significantly associated with both breastfeeding and allergiclike response to pets, 71% were negatively associated with breastfeeding but positively associated with allergiclike response to pets.
This subset of risk-increasing bacteria suppressed by breastfeeding were predominantly members of the family Lachnospiraceae, the researchers found.
Lachnospiraceae are common adult gut colonizers, Ms. Sitarik said, and as people age the relative abundance of Lachnospiraceae increases. “What we think might be happening in terms of the gut microbiome is that maybe breastfeeding is preventing this premature shift into adulthood,” she said.
Dr. Johnson and Ms. Sitarik reported having no financial disclosures.
HOUSTON – Findings from a cohort study of mothers and babies point to breastfeeding as influencing infants’ gut microbiome in a way that protects them from developing allergic disease.
In findings presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, epidemiologist Christine Cole Johnson, Ph.D., of the Henry Ford Health System in Detroit described a correlation between certain maternal and birth characteristics that had previously been shown to relate to allergic response, and measurable differences in the bacterial profiles of the study infants’ stools.
Using data from the WHEALS (Wayne County [Michigan] Health, Environment, Allergy, and Asthma Longitudinal Study) cohort, Dr. Johnson and her colleagues looked at stool samples from 298 children at 1 and 6 months of age. The investigators also collected dust samples from the infants’ homes, obtained medical records, and conducted interviews with the families (J. Allergy Clin. Immunol. 2015 [http://dx.doi.org/10.1016/j.jaci.2014.12.1443]).
The presence of household pets, the body mass index of mothers before delivery, the mode of delivery, household smoke exposure, marital status, income, race, and maternal education were all found to be significantly correlated to different gut bacterial profiles.
“Environmental and lifestyle variables that we’ve been working on related to childhood asthma and allergy seem to be associated – at least in our study – with the child’s gut microbiome,” Dr. Johnson said. “These factors vary a lot by whether those stool samples were collected at 1 or 6 months,” she said, noting that the infant gut microbiome is shaped rapidly in the first year.
But, at both 1 and 6 months of age, breastfeeding was seen as the dominant factor influencing gut bacterial composition.
At 6 months, breastfed infants had bacterial profiles showing overwhelming dominance of Bifidobacteriaceae, but vastly lower levels of other families of bacteria, notably Lachnospiraceae, which were prominent in the guts of non-breastfed babies.
In a related study that used the same cohort to explore whether the influence of breastfeeding on gut bacterial composition correlated to the development of allergic symptoms at 4 years old, Alexandra R. Sitarik, also of the Henry Ford Health System in Detroit, reported that babies being breastfed at 1 month of age had a significantly lower risk of developing allergiclike symptoms to pets by age 4 years (P = .028) (J. Allergy Clin. Immunol. 2015 [http://dx.doi.org/10.1016/j.jaci.2014.12.1444]).
Both breastfeeding and allergiclike response to pets were significantly related to compositional variation in gut microbiome (P < .001 and P = .023, respectively), Ms. Sitarik reported.
Of the 109 types of bacteria significantly associated with both breastfeeding and allergiclike response to pets, 71% were negatively associated with breastfeeding but positively associated with allergiclike response to pets.
This subset of risk-increasing bacteria suppressed by breastfeeding were predominantly members of the family Lachnospiraceae, the researchers found.
Lachnospiraceae are common adult gut colonizers, Ms. Sitarik said, and as people age the relative abundance of Lachnospiraceae increases. “What we think might be happening in terms of the gut microbiome is that maybe breastfeeding is preventing this premature shift into adulthood,” she said.
Dr. Johnson and Ms. Sitarik reported having no financial disclosures.
AT THE 2015 AAAAI ANNUAL MEETING
Key clinical point: Breastfeeding helps to alter the gut microbiome of infants, protecting them from pet allergies in childhood.
Major finding: Infants breastfed at 1 month of age had a significantly lower risk of being allergic to household pets at age 4 years (P =.028).
Data source: Data and stool samples from 298 infants enrolled in the Wayne County [Michigan] Health, Environment, Allergy, and Asthma Longitudinal Study (WHEALS) study.
Disclosures: The investigators reported having no financial disclosures.
Maternal age, cardioseptal defects are major risk factors for peripartum thrombosis
NASHVILLE, TENN. – The risk of a peripartum thrombotic event is rare, but significantly increased for women who have a cardioseptal defect. In a large national sample, the rate of thrombotic events was seven times higher among women with an atrial or ventral septal defect.
Advanced maternal age also was a significant independent predictor of this complication; among more than 7,000 women who developed a thrombotic complication, 81% were older than 45 years.
Dr. Ali Razmara of the University of Southern California, Los Angeles, mined the National Inpatient Sample for data linking peripartum thrombotic events to patient demographics and medical comorbidities. His cohort comprised 4.3 million normal vaginal and cesarean deliveries from 2000 to 2010. Events of interest included transient ischemic attack, ischemic stroke, hemorrhagic stroke, acute MI, and venous thromboembolism.
There were 7,242 peripartum thrombotic events (0.17%).The majority occurred in women who were older than 45 years (81%); white (58%); and admitted through the emergency department (67%). Women with thrombotic events were more likely to have hypertension (52% vs. 2%), dyslipidemia (26% vs. 0.52%), diabetes (20% vs. 2%), atrial fibrillation (10% vs. 0.23%), and heart failure (10% vs. 0.26%), he said at the International Stroke Conference.
A multivariate regression model controlled for patient demographics and comorbidities, including, among others, preeclampsia, hypercoagulable states, chorioamnionitis, renal and liver disease, hypertension, diabetes, and cardiovascular disorders including atrial fibrillation, heart failure, and atrial/ventral septal defects.
In a multivariate regression analysis, maternal age shook out as the most powerful independent risk factor; the rate of thrombosis was 91 times greater among women older than age 45 years.
Other significant independent predictors included emergency vs. routine admission (RR 3.3), cardiac septal defect (RR 7), preeclampsia (RR 3.3), and hypercoagulability (RR 3).
Dyslipidemia and hypertension doubled the rate of a thrombotic event. Hypertension, migraine, renal disease, heart disease, atrial fibrillation, and heart failure were also significant factors, increasing the rate of thrombosis by 40%-50%, Dr. Razmara said at the meeting, which was sponsored by the American Heart Association.
“Our goal is development of targeted interventions for screening, prevention, and treatment of thrombosis related to pregnancy.”
Dr. Razmara had no relevant financial disclosures.
NASHVILLE, TENN. – The risk of a peripartum thrombotic event is rare, but significantly increased for women who have a cardioseptal defect. In a large national sample, the rate of thrombotic events was seven times higher among women with an atrial or ventral septal defect.
Advanced maternal age also was a significant independent predictor of this complication; among more than 7,000 women who developed a thrombotic complication, 81% were older than 45 years.
Dr. Ali Razmara of the University of Southern California, Los Angeles, mined the National Inpatient Sample for data linking peripartum thrombotic events to patient demographics and medical comorbidities. His cohort comprised 4.3 million normal vaginal and cesarean deliveries from 2000 to 2010. Events of interest included transient ischemic attack, ischemic stroke, hemorrhagic stroke, acute MI, and venous thromboembolism.
There were 7,242 peripartum thrombotic events (0.17%).The majority occurred in women who were older than 45 years (81%); white (58%); and admitted through the emergency department (67%). Women with thrombotic events were more likely to have hypertension (52% vs. 2%), dyslipidemia (26% vs. 0.52%), diabetes (20% vs. 2%), atrial fibrillation (10% vs. 0.23%), and heart failure (10% vs. 0.26%), he said at the International Stroke Conference.
A multivariate regression model controlled for patient demographics and comorbidities, including, among others, preeclampsia, hypercoagulable states, chorioamnionitis, renal and liver disease, hypertension, diabetes, and cardiovascular disorders including atrial fibrillation, heart failure, and atrial/ventral septal defects.
In a multivariate regression analysis, maternal age shook out as the most powerful independent risk factor; the rate of thrombosis was 91 times greater among women older than age 45 years.
Other significant independent predictors included emergency vs. routine admission (RR 3.3), cardiac septal defect (RR 7), preeclampsia (RR 3.3), and hypercoagulability (RR 3).
Dyslipidemia and hypertension doubled the rate of a thrombotic event. Hypertension, migraine, renal disease, heart disease, atrial fibrillation, and heart failure were also significant factors, increasing the rate of thrombosis by 40%-50%, Dr. Razmara said at the meeting, which was sponsored by the American Heart Association.
“Our goal is development of targeted interventions for screening, prevention, and treatment of thrombosis related to pregnancy.”
Dr. Razmara had no relevant financial disclosures.
NASHVILLE, TENN. – The risk of a peripartum thrombotic event is rare, but significantly increased for women who have a cardioseptal defect. In a large national sample, the rate of thrombotic events was seven times higher among women with an atrial or ventral septal defect.
Advanced maternal age also was a significant independent predictor of this complication; among more than 7,000 women who developed a thrombotic complication, 81% were older than 45 years.
Dr. Ali Razmara of the University of Southern California, Los Angeles, mined the National Inpatient Sample for data linking peripartum thrombotic events to patient demographics and medical comorbidities. His cohort comprised 4.3 million normal vaginal and cesarean deliveries from 2000 to 2010. Events of interest included transient ischemic attack, ischemic stroke, hemorrhagic stroke, acute MI, and venous thromboembolism.
There were 7,242 peripartum thrombotic events (0.17%).The majority occurred in women who were older than 45 years (81%); white (58%); and admitted through the emergency department (67%). Women with thrombotic events were more likely to have hypertension (52% vs. 2%), dyslipidemia (26% vs. 0.52%), diabetes (20% vs. 2%), atrial fibrillation (10% vs. 0.23%), and heart failure (10% vs. 0.26%), he said at the International Stroke Conference.
A multivariate regression model controlled for patient demographics and comorbidities, including, among others, preeclampsia, hypercoagulable states, chorioamnionitis, renal and liver disease, hypertension, diabetes, and cardiovascular disorders including atrial fibrillation, heart failure, and atrial/ventral septal defects.
In a multivariate regression analysis, maternal age shook out as the most powerful independent risk factor; the rate of thrombosis was 91 times greater among women older than age 45 years.
Other significant independent predictors included emergency vs. routine admission (RR 3.3), cardiac septal defect (RR 7), preeclampsia (RR 3.3), and hypercoagulability (RR 3).
Dyslipidemia and hypertension doubled the rate of a thrombotic event. Hypertension, migraine, renal disease, heart disease, atrial fibrillation, and heart failure were also significant factors, increasing the rate of thrombosis by 40%-50%, Dr. Razmara said at the meeting, which was sponsored by the American Heart Association.
“Our goal is development of targeted interventions for screening, prevention, and treatment of thrombosis related to pregnancy.”
Dr. Razmara had no relevant financial disclosures.
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Advanced maternal age and a cardioseptal defect increase the risk of a peripartum thrombotic event.
Major finding: The rate of peripartum thrombotic events was 0.17%; cardioseptal defects increased the rate of a peripartum thombotic event by more than seven times.
Data source: A sample that comprised 4.5 million deliveries during 2000-2010.
Disclosures: Dr. Razmara had no relevant disclosures.
EXPECT: Omalizumab pregnancy safety data called ‘reassuring’
HOUSTON – Babies born to mothers who took omalizumab just before or during pregnancy exhibited birth defects that are in line with the general population, according to data from the EXPECT registry.
EXPECT (the Xolair Pregnancy Registry) is an ongoing, prospective observational study of pregnant women who received at least 1 dose of omalizumab within 2 months of conception or during their pregnancies.
Dr. Jennifer Namazy of the Scripps Clinic in La Jolla, Calif., presented data from women enrolled between September 2006 and November 2013 at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The registry aims to enroll 250 women.
Dr. Namazy reported on 186 of the 207 expectant mothers for whom outcomes are recorded. Asthma severity was available for 164. Most (105 or 64%) had severe asthma, while 55 (34%) had moderate asthma, and 4 (2.4%) had mild disease. Each received omalizumab during the first trimester of pregnancy.
A total of 178 births were recorded, 174 of which were live. Of the 170 singletons, 24 (14%) were born at less than 37 weeks’ gestation and of those 3 (13%) were considered small for gestational age (weight less than 10th percentile for gestational age).
Of 140 single-born infants who were carried to full term and also had relevant weight data, 4 (3%) had low birth weight (less than 2.5 kg) and 16 (11%) were considered small for gestational age. Overall, 27 (15%) infants had confirmed congenital anomalies and 11 (6.2%) had a major birth defect.
“This is all very reassuring data,” Dr. Namazy said in an interview. “In terms of the outcomes – major defects and conditional defects – these results are not too unusual, [compared with] the general population. But when we’re looking at infant outcomes, it’s not too different from the severe asthma population.”
She noted, however, that “it’s hard to make large-scale conclusions from this sample size. When you’re talking about congenital malformations, they’re so rare and uncommon that you need a very large population in order to reach any kind of conclusion. But looking at these [data], we’re reassured because there isn’t any consistent information that’s disconcerting; it’s all over the spectrum, and so there’s no significant increase in the rate of birth rate defects that we’re seeing.”
Omalizumab is marketed by Genentech as Xolair. The EXPECT study is funded by Genentech and Novartis Pharma AG. Dr. Namazy is affiliated with Genentech.
HOUSTON – Babies born to mothers who took omalizumab just before or during pregnancy exhibited birth defects that are in line with the general population, according to data from the EXPECT registry.
EXPECT (the Xolair Pregnancy Registry) is an ongoing, prospective observational study of pregnant women who received at least 1 dose of omalizumab within 2 months of conception or during their pregnancies.
Dr. Jennifer Namazy of the Scripps Clinic in La Jolla, Calif., presented data from women enrolled between September 2006 and November 2013 at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The registry aims to enroll 250 women.
Dr. Namazy reported on 186 of the 207 expectant mothers for whom outcomes are recorded. Asthma severity was available for 164. Most (105 or 64%) had severe asthma, while 55 (34%) had moderate asthma, and 4 (2.4%) had mild disease. Each received omalizumab during the first trimester of pregnancy.
A total of 178 births were recorded, 174 of which were live. Of the 170 singletons, 24 (14%) were born at less than 37 weeks’ gestation and of those 3 (13%) were considered small for gestational age (weight less than 10th percentile for gestational age).
Of 140 single-born infants who were carried to full term and also had relevant weight data, 4 (3%) had low birth weight (less than 2.5 kg) and 16 (11%) were considered small for gestational age. Overall, 27 (15%) infants had confirmed congenital anomalies and 11 (6.2%) had a major birth defect.
“This is all very reassuring data,” Dr. Namazy said in an interview. “In terms of the outcomes – major defects and conditional defects – these results are not too unusual, [compared with] the general population. But when we’re looking at infant outcomes, it’s not too different from the severe asthma population.”
She noted, however, that “it’s hard to make large-scale conclusions from this sample size. When you’re talking about congenital malformations, they’re so rare and uncommon that you need a very large population in order to reach any kind of conclusion. But looking at these [data], we’re reassured because there isn’t any consistent information that’s disconcerting; it’s all over the spectrum, and so there’s no significant increase in the rate of birth rate defects that we’re seeing.”
Omalizumab is marketed by Genentech as Xolair. The EXPECT study is funded by Genentech and Novartis Pharma AG. Dr. Namazy is affiliated with Genentech.
HOUSTON – Babies born to mothers who took omalizumab just before or during pregnancy exhibited birth defects that are in line with the general population, according to data from the EXPECT registry.
EXPECT (the Xolair Pregnancy Registry) is an ongoing, prospective observational study of pregnant women who received at least 1 dose of omalizumab within 2 months of conception or during their pregnancies.
Dr. Jennifer Namazy of the Scripps Clinic in La Jolla, Calif., presented data from women enrolled between September 2006 and November 2013 at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The registry aims to enroll 250 women.
Dr. Namazy reported on 186 of the 207 expectant mothers for whom outcomes are recorded. Asthma severity was available for 164. Most (105 or 64%) had severe asthma, while 55 (34%) had moderate asthma, and 4 (2.4%) had mild disease. Each received omalizumab during the first trimester of pregnancy.
A total of 178 births were recorded, 174 of which were live. Of the 170 singletons, 24 (14%) were born at less than 37 weeks’ gestation and of those 3 (13%) were considered small for gestational age (weight less than 10th percentile for gestational age).
Of 140 single-born infants who were carried to full term and also had relevant weight data, 4 (3%) had low birth weight (less than 2.5 kg) and 16 (11%) were considered small for gestational age. Overall, 27 (15%) infants had confirmed congenital anomalies and 11 (6.2%) had a major birth defect.
“This is all very reassuring data,” Dr. Namazy said in an interview. “In terms of the outcomes – major defects and conditional defects – these results are not too unusual, [compared with] the general population. But when we’re looking at infant outcomes, it’s not too different from the severe asthma population.”
She noted, however, that “it’s hard to make large-scale conclusions from this sample size. When you’re talking about congenital malformations, they’re so rare and uncommon that you need a very large population in order to reach any kind of conclusion. But looking at these [data], we’re reassured because there isn’t any consistent information that’s disconcerting; it’s all over the spectrum, and so there’s no significant increase in the rate of birth rate defects that we’re seeing.”
Omalizumab is marketed by Genentech as Xolair. The EXPECT study is funded by Genentech and Novartis Pharma AG. Dr. Namazy is affiliated with Genentech.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Omalizumab does not appear to increase the risk of birth defects or adverse outcomes in pregnant women and their babies.
Major finding: Of 140 singleton infants carried to full term and for whom relevant weight data was available, 4 (2.9%) had low birth weight and 16 (11.4%) were considered small for gestational age, 27 (15.2%) had congenital anomalies, and 11 (6.2%) had a major birth defect.
Data source: EXPECT trial – an ongoing observational study of 207 prospectively enrolled women.
Disclosures: EXPECT is funded by Genentech and Novartis Pharma AG. Dr. Namazy is affiliated with Genentech.
Link found between substance use, teen pregnancy
Pregnant adolescents were more likely than their nonpregnant peers to use alcohol and illicit drugs, report Christopher P. Salas-Wright, Ph.D., and coauthors from the School of Social Work at the University of Texas at Austin.
A study of 97,850 female respondents aged 12-17 years who completed the National Survey on Drug Use and Health between 2002 and 2012 found that pregnant teens were significantly more likely to have used alcohol (odds ratio, 1.44; 95% confidence interval, 1.18-1.76), cannabis (OR, 1.79; 95% CI, 1.45-2.20), and other substances in the previous 12 months, the investigators wrote. Pregnant adolescents also were more likely to meet the criteria for substance abuse disorders involving alcohol (OR, 1.65; 95% CI, 1.26-2.17), cannabis (OR, 2.29; 95% CI, 1.72-3.04), and other illicit drugs (OR, 2.84; 95% CI, 1.92-4.19).
“Our results point not only to a relationship between pregnancy and prior substance use, but also suggest that substance use continues for many teens during pregnancy,” Dr. Salas-Wright and his colleagues said in the report.
Read the full article in Addictive Behaviors.
Pregnant adolescents were more likely than their nonpregnant peers to use alcohol and illicit drugs, report Christopher P. Salas-Wright, Ph.D., and coauthors from the School of Social Work at the University of Texas at Austin.
A study of 97,850 female respondents aged 12-17 years who completed the National Survey on Drug Use and Health between 2002 and 2012 found that pregnant teens were significantly more likely to have used alcohol (odds ratio, 1.44; 95% confidence interval, 1.18-1.76), cannabis (OR, 1.79; 95% CI, 1.45-2.20), and other substances in the previous 12 months, the investigators wrote. Pregnant adolescents also were more likely to meet the criteria for substance abuse disorders involving alcohol (OR, 1.65; 95% CI, 1.26-2.17), cannabis (OR, 2.29; 95% CI, 1.72-3.04), and other illicit drugs (OR, 2.84; 95% CI, 1.92-4.19).
“Our results point not only to a relationship between pregnancy and prior substance use, but also suggest that substance use continues for many teens during pregnancy,” Dr. Salas-Wright and his colleagues said in the report.
Read the full article in Addictive Behaviors.
Pregnant adolescents were more likely than their nonpregnant peers to use alcohol and illicit drugs, report Christopher P. Salas-Wright, Ph.D., and coauthors from the School of Social Work at the University of Texas at Austin.
A study of 97,850 female respondents aged 12-17 years who completed the National Survey on Drug Use and Health between 2002 and 2012 found that pregnant teens were significantly more likely to have used alcohol (odds ratio, 1.44; 95% confidence interval, 1.18-1.76), cannabis (OR, 1.79; 95% CI, 1.45-2.20), and other substances in the previous 12 months, the investigators wrote. Pregnant adolescents also were more likely to meet the criteria for substance abuse disorders involving alcohol (OR, 1.65; 95% CI, 1.26-2.17), cannabis (OR, 2.29; 95% CI, 1.72-3.04), and other illicit drugs (OR, 2.84; 95% CI, 1.92-4.19).
“Our results point not only to a relationship between pregnancy and prior substance use, but also suggest that substance use continues for many teens during pregnancy,” Dr. Salas-Wright and his colleagues said in the report.
Read the full article in Addictive Behaviors.
Maternal obesity, not mild GDM, affects childhood BMI
SAN DIEGO – Maternal glycemia was associated with anthropometric measures of obesity in offspring during childhood, but not with childhood body mass index, fasting glucose, or insulin resistance in a secondary analysis of a long-term follow-up study of women with mild gestational diabetes mellitus.
Baseline maternal body mass index (BMI), maternal weight gain, and Hispanic ethnicity, however, were consistently related to childhood BMI (P < .01) in the study of 236 children born to women who had untreated mild gestational diabetes mellitus (GDM) and 480 children whose mothers had a normal oral glucose tolerance test during pregnancy.
The findings underscore the importance of reducing obesity prior to pregnancy, and suggest that obesity reduction efforts should be emphasized in clinical practice, Dr. Mark B. Landon said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The obesity epidemic certainly has not spared the pediatric population,” said Dr. Landon, chair of obstetrics and gynecology at the Ohio State University in Columbus.
And a serious concern is that overweight and obesity are associated with a downstream risk of both metabolic and cardiovascular abnormalities into adulthood, he added.
Hispanic ethnicity and maternal BMI were also associated with childhood homeostatic model assessment-insulin resistance (HOMA-IR), and Hispanic ethnicity was associated with fasting glucose, Dr. Landon reported on behalf of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network.
There was a significant correlation between fasting, 1-hour, 2-hour, and 3-hour maternal glucose and subscapular/triceps skin fold ratio (Spearman correlation coefficients, 0.10, 0.08, 0.11, and 0.12, respectively). Similar correlations were seen between maternal glucose and HOMA-IR, and maternal glucose and sum of skin folds.
Fasting maternal glucose and fasting child glucose were also significantly correlated (Spearman correlation coefficients, 0.09).
On multivariable regression analysis – after controlling for maternal and neonatal factors – the only significant correlations between maternal glycemia and childhood outcomes were for 1-hour, 2-hour, and 3-hour maternal glucose measures, sum of skin folds, and subscapular/triceps skin fold ratio, he said.
Study subjects were the offspring of women who underwent a 3-hour oral glucose tolerance test between 27 and 31 weeks’ gestation, and childhood outcomes were assessed between the ages of 5 and 10 years.
Prior to the widespread recognition of the concept of fetal programming over 3 decades ago, Dr. Norbert Freinkel proposed that mild forms of diabetes, such as GDM, might exaggerate the normal dependency of the fetus on maternal fuels and that the concept of teratogenesis should be expanded to include alterations which could have long-range effects on behavioral, anthropometric, and metabolic functions, Dr. Landon said.
Multiple subsequent studies have demonstrated a link between in utero exposure to maternal hyperglycemia and the development of obesity in childhood – findings that are independent of both birth weight and maternal obesity.
A 2013 study showed that GDM was associated with both overweight status in childhood and childhood obesity (Diabet. Med. 2013;30:1449-56). Also, the landmark Hyperglycemia and Adverse Pregnancy Outcomes study showed that with increasing maternal glucose levels lower than the threshold for GDM diagnosis, the frequency of large-for-gestational-age infants increased proportionately – and this risk also remained after adjustment for maternal BMI and other confounders (N. Engl. J. Med. 2008;358:1991-2002).
“We have similarly reported a monotonic relationship between maternal glucose levels and the frequency of large-for-gestational-age infants in a secondary analysis from the Mild GDM Network randomized controlled trial. Recognizing that maternal glycemia clearly contributes to fetal growth, we sought to determine in this analysis whether the degree of maternal glucose tolerance during pregnancy affects the risk of childhood obesity and metabolic dysfunction,” Dr. Landon said.
Although the findings do not address the potential long-term effects of more significant hyperglycemia during pregnancy, they do confirm that maternal obesity has a greater impact on childhood obesity risk than exposure to mild hyperglycemia during pregnancy, he said in an interview.
Dr. Landon reported having no financial disclosures.
SAN DIEGO – Maternal glycemia was associated with anthropometric measures of obesity in offspring during childhood, but not with childhood body mass index, fasting glucose, or insulin resistance in a secondary analysis of a long-term follow-up study of women with mild gestational diabetes mellitus.
Baseline maternal body mass index (BMI), maternal weight gain, and Hispanic ethnicity, however, were consistently related to childhood BMI (P < .01) in the study of 236 children born to women who had untreated mild gestational diabetes mellitus (GDM) and 480 children whose mothers had a normal oral glucose tolerance test during pregnancy.
The findings underscore the importance of reducing obesity prior to pregnancy, and suggest that obesity reduction efforts should be emphasized in clinical practice, Dr. Mark B. Landon said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The obesity epidemic certainly has not spared the pediatric population,” said Dr. Landon, chair of obstetrics and gynecology at the Ohio State University in Columbus.
And a serious concern is that overweight and obesity are associated with a downstream risk of both metabolic and cardiovascular abnormalities into adulthood, he added.
Hispanic ethnicity and maternal BMI were also associated with childhood homeostatic model assessment-insulin resistance (HOMA-IR), and Hispanic ethnicity was associated with fasting glucose, Dr. Landon reported on behalf of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network.
There was a significant correlation between fasting, 1-hour, 2-hour, and 3-hour maternal glucose and subscapular/triceps skin fold ratio (Spearman correlation coefficients, 0.10, 0.08, 0.11, and 0.12, respectively). Similar correlations were seen between maternal glucose and HOMA-IR, and maternal glucose and sum of skin folds.
Fasting maternal glucose and fasting child glucose were also significantly correlated (Spearman correlation coefficients, 0.09).
On multivariable regression analysis – after controlling for maternal and neonatal factors – the only significant correlations between maternal glycemia and childhood outcomes were for 1-hour, 2-hour, and 3-hour maternal glucose measures, sum of skin folds, and subscapular/triceps skin fold ratio, he said.
Study subjects were the offspring of women who underwent a 3-hour oral glucose tolerance test between 27 and 31 weeks’ gestation, and childhood outcomes were assessed between the ages of 5 and 10 years.
Prior to the widespread recognition of the concept of fetal programming over 3 decades ago, Dr. Norbert Freinkel proposed that mild forms of diabetes, such as GDM, might exaggerate the normal dependency of the fetus on maternal fuels and that the concept of teratogenesis should be expanded to include alterations which could have long-range effects on behavioral, anthropometric, and metabolic functions, Dr. Landon said.
Multiple subsequent studies have demonstrated a link between in utero exposure to maternal hyperglycemia and the development of obesity in childhood – findings that are independent of both birth weight and maternal obesity.
A 2013 study showed that GDM was associated with both overweight status in childhood and childhood obesity (Diabet. Med. 2013;30:1449-56). Also, the landmark Hyperglycemia and Adverse Pregnancy Outcomes study showed that with increasing maternal glucose levels lower than the threshold for GDM diagnosis, the frequency of large-for-gestational-age infants increased proportionately – and this risk also remained after adjustment for maternal BMI and other confounders (N. Engl. J. Med. 2008;358:1991-2002).
“We have similarly reported a monotonic relationship between maternal glucose levels and the frequency of large-for-gestational-age infants in a secondary analysis from the Mild GDM Network randomized controlled trial. Recognizing that maternal glycemia clearly contributes to fetal growth, we sought to determine in this analysis whether the degree of maternal glucose tolerance during pregnancy affects the risk of childhood obesity and metabolic dysfunction,” Dr. Landon said.
Although the findings do not address the potential long-term effects of more significant hyperglycemia during pregnancy, they do confirm that maternal obesity has a greater impact on childhood obesity risk than exposure to mild hyperglycemia during pregnancy, he said in an interview.
Dr. Landon reported having no financial disclosures.
SAN DIEGO – Maternal glycemia was associated with anthropometric measures of obesity in offspring during childhood, but not with childhood body mass index, fasting glucose, or insulin resistance in a secondary analysis of a long-term follow-up study of women with mild gestational diabetes mellitus.
Baseline maternal body mass index (BMI), maternal weight gain, and Hispanic ethnicity, however, were consistently related to childhood BMI (P < .01) in the study of 236 children born to women who had untreated mild gestational diabetes mellitus (GDM) and 480 children whose mothers had a normal oral glucose tolerance test during pregnancy.
The findings underscore the importance of reducing obesity prior to pregnancy, and suggest that obesity reduction efforts should be emphasized in clinical practice, Dr. Mark B. Landon said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The obesity epidemic certainly has not spared the pediatric population,” said Dr. Landon, chair of obstetrics and gynecology at the Ohio State University in Columbus.
And a serious concern is that overweight and obesity are associated with a downstream risk of both metabolic and cardiovascular abnormalities into adulthood, he added.
Hispanic ethnicity and maternal BMI were also associated with childhood homeostatic model assessment-insulin resistance (HOMA-IR), and Hispanic ethnicity was associated with fasting glucose, Dr. Landon reported on behalf of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network.
There was a significant correlation between fasting, 1-hour, 2-hour, and 3-hour maternal glucose and subscapular/triceps skin fold ratio (Spearman correlation coefficients, 0.10, 0.08, 0.11, and 0.12, respectively). Similar correlations were seen between maternal glucose and HOMA-IR, and maternal glucose and sum of skin folds.
Fasting maternal glucose and fasting child glucose were also significantly correlated (Spearman correlation coefficients, 0.09).
On multivariable regression analysis – after controlling for maternal and neonatal factors – the only significant correlations between maternal glycemia and childhood outcomes were for 1-hour, 2-hour, and 3-hour maternal glucose measures, sum of skin folds, and subscapular/triceps skin fold ratio, he said.
Study subjects were the offspring of women who underwent a 3-hour oral glucose tolerance test between 27 and 31 weeks’ gestation, and childhood outcomes were assessed between the ages of 5 and 10 years.
Prior to the widespread recognition of the concept of fetal programming over 3 decades ago, Dr. Norbert Freinkel proposed that mild forms of diabetes, such as GDM, might exaggerate the normal dependency of the fetus on maternal fuels and that the concept of teratogenesis should be expanded to include alterations which could have long-range effects on behavioral, anthropometric, and metabolic functions, Dr. Landon said.
Multiple subsequent studies have demonstrated a link between in utero exposure to maternal hyperglycemia and the development of obesity in childhood – findings that are independent of both birth weight and maternal obesity.
A 2013 study showed that GDM was associated with both overweight status in childhood and childhood obesity (Diabet. Med. 2013;30:1449-56). Also, the landmark Hyperglycemia and Adverse Pregnancy Outcomes study showed that with increasing maternal glucose levels lower than the threshold for GDM diagnosis, the frequency of large-for-gestational-age infants increased proportionately – and this risk also remained after adjustment for maternal BMI and other confounders (N. Engl. J. Med. 2008;358:1991-2002).
“We have similarly reported a monotonic relationship between maternal glucose levels and the frequency of large-for-gestational-age infants in a secondary analysis from the Mild GDM Network randomized controlled trial. Recognizing that maternal glycemia clearly contributes to fetal growth, we sought to determine in this analysis whether the degree of maternal glucose tolerance during pregnancy affects the risk of childhood obesity and metabolic dysfunction,” Dr. Landon said.
Although the findings do not address the potential long-term effects of more significant hyperglycemia during pregnancy, they do confirm that maternal obesity has a greater impact on childhood obesity risk than exposure to mild hyperglycemia during pregnancy, he said in an interview.
Dr. Landon reported having no financial disclosures.
AT THE PREGNANCY MEETING
Key clinical point: Maternal obesity is associated with childhood body mass index and should be addressed in clinical practice.
Major finding: Baseline maternal BMI, weight gain, and Hispanic ethnicity – but not maternal glycemia – were consistently related to childhood BMI (P < 0.01).
Data source: A secondary analysis of a long-term follow-up study of women with mild gestational diabetes mellitus and 716 of their offspring.
Disclosures: Dr. Landon reported having no financial disclosures.
Is midwifery the key to laborist model success?
SAN DIEGO – A shift from a conventional private practice model to a 24-hour obstetrician and midwifery model was associated with a dramatic decrease in the nulliparous term single vertex cesarean delivery rate, and an increase in the vaginal birth after cesarean delivery rate among privately insured women who were delivered at a community hospital in California.
But in a larger cross-sectional population-based study involving multiple community hospitals, no difference was seen in the primary cesarean rate, the successful vaginal birth after cesarean (VBAC) rate, or maternal morbidity for laboring women who gave birth in hospitals with an obstetrician available around the clock versus hospitals not using a laborist model.
Researchers who worked on the two studies said the differences might be explained by multiple factors, including the midwifery component in the single-center study, and a lack of information about exactly how laborists functioned at the various centers included in the larger, multicenter study.
In the single-center prospective cohort study, conducted at Marin General Hospital in Greenbrae, Calif., the nulliparous term single vertex cesarean delivery (NTSV CD) rate among privately insured women fell from 32.2% prior to the model change, to 25% after the switch. There was an immediate 5% decrease, followed by a nearly 2% decrease each year thereafter.
Prior to the switch to a laborist model, the NTSV CD rate had been increasing by 0.6% annually, similar to national trends, Dr. Melissa Rosenstein of the University of California, San Francisco, reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Further, the VBAC rate, which had been decreasing slightly each year before the change, increased from 13% to 22% after the change, and increased by about 8% per year thereafter, she said.
Previously, privately insured women were managed by their individual obstetricians; only publicly insured women utilized the laborist model at the hospital.
The rates of NTSV CD and VBAC among publicly insured women did not change significantly during the study period – the NTSV CD rates were 15.7% and 15.8% before and after the change, and VBAC rates were 33.9% and 27.9% before and after the change, she said.
The study included all singleton term deliveries at the community hospital between January 2005 and April 2014. The model shift occurred in April 2011. Overall, 3,684 NTSV deliveries and 1,375 deliveries in women with a prior cesarean delivery were included in the analysis.
“The changes were seen to a statistically significant degree only in the group of women exposed to the practice change, suggesting causation rather than secular trends for other hospitalwide interventions,” Dr. Rosenstein said.
But a larger, multicenter study found no difference between 43 hospitals with laborists and 139 without laborists on a range of outcomes, including the primary cesarean delivery rate (13.9% vs. 14.1%), the rate of maternal composite morbidity (10.3% vs. 10.8%), the rate of severe maternal complications (1.25% vs. 1.07%), and the successful VBAC rate (55.8% vs. 59.8%).
The researchers relied on structured 1-hour interviews conducted with labor and delivery nurse managers from 182 community hospitals with 221,247 deliveries in California between November 2012 and January 2014. They also considered discharge data and information regarding hospital policies and practices.
So why were the findings so different? Dr. Rosenstein, who worked on the single-center study, stressed the importance of the midwives in the laborist model used at Marin General Hospital.
“The other study didn’t include the midwifery component, which I think is a very important part of the Marin General Hospital experience,” Dr. Rosenstein said. “Not only does our hospital have midwives, but they work closely and collaboratively with physicians and participate in twice-daily rounds to discuss patient management.”
The principal investigator in the multicenter study, Dr. Kimberly Gregory, director of the division of maternal-fetal medicine at Cedars-Sinai Medical Center in Los Angeles, agreed, noting that midwifery is already known to make a difference.
“What we don’t know is what model of laborists, if any, makes a difference,” she said.
Other models that employ both midwives and dedicated obstetricians in labor and delivery, while having separate physicians for gynecologic and emergency care, are showing promise, Dr. Gregory said.
Another major difference between the two studies was the lack of categorization of hospitals by “how laborists actually functioned on the unit” in her study, Dr. Gregory said.
Since the multicenter study didn’t look at the specific roles of laborists on the hospital unit, it’s unclear whether they took care of just a few patients, or whether they managed all of the obstetrics patients, Dr. Rosenstein said.
Additionally, the multicenter study defined a laborist hospital as one where there was an obstetrician present 24/7. By that definition, the Marin General Hospital, where the single-center study was conducted, would have been considered a laborist hospital both before and after the intervention, she said.
Dr. Gregory also pointed out that all of the patients in the single-hospital study were exposed to the same “culture,” whereas patients in the multicenter study were subject to varying approaches and cultures.
The findings of both studies are of value for identifying the best approach to improving outcomes, Dr. Rosenstein said.
“Studies at the hospital level and at the population level are both important to determine the most optimal labor and delivery staffing pattern,” she said.
The single-center study was funded by the National Institutes of Health and the Prima Medical Foundation. The multicenter study was funded by the Agency for Healthcare Research and Quality, the American College of Obstetricians and Gynecologists, and the March of Dimes. The researchers reported having no relevant financial disclosures.
SAN DIEGO – A shift from a conventional private practice model to a 24-hour obstetrician and midwifery model was associated with a dramatic decrease in the nulliparous term single vertex cesarean delivery rate, and an increase in the vaginal birth after cesarean delivery rate among privately insured women who were delivered at a community hospital in California.
But in a larger cross-sectional population-based study involving multiple community hospitals, no difference was seen in the primary cesarean rate, the successful vaginal birth after cesarean (VBAC) rate, or maternal morbidity for laboring women who gave birth in hospitals with an obstetrician available around the clock versus hospitals not using a laborist model.
Researchers who worked on the two studies said the differences might be explained by multiple factors, including the midwifery component in the single-center study, and a lack of information about exactly how laborists functioned at the various centers included in the larger, multicenter study.
In the single-center prospective cohort study, conducted at Marin General Hospital in Greenbrae, Calif., the nulliparous term single vertex cesarean delivery (NTSV CD) rate among privately insured women fell from 32.2% prior to the model change, to 25% after the switch. There was an immediate 5% decrease, followed by a nearly 2% decrease each year thereafter.
Prior to the switch to a laborist model, the NTSV CD rate had been increasing by 0.6% annually, similar to national trends, Dr. Melissa Rosenstein of the University of California, San Francisco, reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Further, the VBAC rate, which had been decreasing slightly each year before the change, increased from 13% to 22% after the change, and increased by about 8% per year thereafter, she said.
Previously, privately insured women were managed by their individual obstetricians; only publicly insured women utilized the laborist model at the hospital.
The rates of NTSV CD and VBAC among publicly insured women did not change significantly during the study period – the NTSV CD rates were 15.7% and 15.8% before and after the change, and VBAC rates were 33.9% and 27.9% before and after the change, she said.
The study included all singleton term deliveries at the community hospital between January 2005 and April 2014. The model shift occurred in April 2011. Overall, 3,684 NTSV deliveries and 1,375 deliveries in women with a prior cesarean delivery were included in the analysis.
“The changes were seen to a statistically significant degree only in the group of women exposed to the practice change, suggesting causation rather than secular trends for other hospitalwide interventions,” Dr. Rosenstein said.
But a larger, multicenter study found no difference between 43 hospitals with laborists and 139 without laborists on a range of outcomes, including the primary cesarean delivery rate (13.9% vs. 14.1%), the rate of maternal composite morbidity (10.3% vs. 10.8%), the rate of severe maternal complications (1.25% vs. 1.07%), and the successful VBAC rate (55.8% vs. 59.8%).
The researchers relied on structured 1-hour interviews conducted with labor and delivery nurse managers from 182 community hospitals with 221,247 deliveries in California between November 2012 and January 2014. They also considered discharge data and information regarding hospital policies and practices.
So why were the findings so different? Dr. Rosenstein, who worked on the single-center study, stressed the importance of the midwives in the laborist model used at Marin General Hospital.
“The other study didn’t include the midwifery component, which I think is a very important part of the Marin General Hospital experience,” Dr. Rosenstein said. “Not only does our hospital have midwives, but they work closely and collaboratively with physicians and participate in twice-daily rounds to discuss patient management.”
The principal investigator in the multicenter study, Dr. Kimberly Gregory, director of the division of maternal-fetal medicine at Cedars-Sinai Medical Center in Los Angeles, agreed, noting that midwifery is already known to make a difference.
“What we don’t know is what model of laborists, if any, makes a difference,” she said.
Other models that employ both midwives and dedicated obstetricians in labor and delivery, while having separate physicians for gynecologic and emergency care, are showing promise, Dr. Gregory said.
Another major difference between the two studies was the lack of categorization of hospitals by “how laborists actually functioned on the unit” in her study, Dr. Gregory said.
Since the multicenter study didn’t look at the specific roles of laborists on the hospital unit, it’s unclear whether they took care of just a few patients, or whether they managed all of the obstetrics patients, Dr. Rosenstein said.
Additionally, the multicenter study defined a laborist hospital as one where there was an obstetrician present 24/7. By that definition, the Marin General Hospital, where the single-center study was conducted, would have been considered a laborist hospital both before and after the intervention, she said.
Dr. Gregory also pointed out that all of the patients in the single-hospital study were exposed to the same “culture,” whereas patients in the multicenter study were subject to varying approaches and cultures.
The findings of both studies are of value for identifying the best approach to improving outcomes, Dr. Rosenstein said.
“Studies at the hospital level and at the population level are both important to determine the most optimal labor and delivery staffing pattern,” she said.
The single-center study was funded by the National Institutes of Health and the Prima Medical Foundation. The multicenter study was funded by the Agency for Healthcare Research and Quality, the American College of Obstetricians and Gynecologists, and the March of Dimes. The researchers reported having no relevant financial disclosures.
SAN DIEGO – A shift from a conventional private practice model to a 24-hour obstetrician and midwifery model was associated with a dramatic decrease in the nulliparous term single vertex cesarean delivery rate, and an increase in the vaginal birth after cesarean delivery rate among privately insured women who were delivered at a community hospital in California.
But in a larger cross-sectional population-based study involving multiple community hospitals, no difference was seen in the primary cesarean rate, the successful vaginal birth after cesarean (VBAC) rate, or maternal morbidity for laboring women who gave birth in hospitals with an obstetrician available around the clock versus hospitals not using a laborist model.
Researchers who worked on the two studies said the differences might be explained by multiple factors, including the midwifery component in the single-center study, and a lack of information about exactly how laborists functioned at the various centers included in the larger, multicenter study.
In the single-center prospective cohort study, conducted at Marin General Hospital in Greenbrae, Calif., the nulliparous term single vertex cesarean delivery (NTSV CD) rate among privately insured women fell from 32.2% prior to the model change, to 25% after the switch. There was an immediate 5% decrease, followed by a nearly 2% decrease each year thereafter.
Prior to the switch to a laborist model, the NTSV CD rate had been increasing by 0.6% annually, similar to national trends, Dr. Melissa Rosenstein of the University of California, San Francisco, reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Further, the VBAC rate, which had been decreasing slightly each year before the change, increased from 13% to 22% after the change, and increased by about 8% per year thereafter, she said.
Previously, privately insured women were managed by their individual obstetricians; only publicly insured women utilized the laborist model at the hospital.
The rates of NTSV CD and VBAC among publicly insured women did not change significantly during the study period – the NTSV CD rates were 15.7% and 15.8% before and after the change, and VBAC rates were 33.9% and 27.9% before and after the change, she said.
The study included all singleton term deliveries at the community hospital between January 2005 and April 2014. The model shift occurred in April 2011. Overall, 3,684 NTSV deliveries and 1,375 deliveries in women with a prior cesarean delivery were included in the analysis.
“The changes were seen to a statistically significant degree only in the group of women exposed to the practice change, suggesting causation rather than secular trends for other hospitalwide interventions,” Dr. Rosenstein said.
But a larger, multicenter study found no difference between 43 hospitals with laborists and 139 without laborists on a range of outcomes, including the primary cesarean delivery rate (13.9% vs. 14.1%), the rate of maternal composite morbidity (10.3% vs. 10.8%), the rate of severe maternal complications (1.25% vs. 1.07%), and the successful VBAC rate (55.8% vs. 59.8%).
The researchers relied on structured 1-hour interviews conducted with labor and delivery nurse managers from 182 community hospitals with 221,247 deliveries in California between November 2012 and January 2014. They also considered discharge data and information regarding hospital policies and practices.
So why were the findings so different? Dr. Rosenstein, who worked on the single-center study, stressed the importance of the midwives in the laborist model used at Marin General Hospital.
“The other study didn’t include the midwifery component, which I think is a very important part of the Marin General Hospital experience,” Dr. Rosenstein said. “Not only does our hospital have midwives, but they work closely and collaboratively with physicians and participate in twice-daily rounds to discuss patient management.”
The principal investigator in the multicenter study, Dr. Kimberly Gregory, director of the division of maternal-fetal medicine at Cedars-Sinai Medical Center in Los Angeles, agreed, noting that midwifery is already known to make a difference.
“What we don’t know is what model of laborists, if any, makes a difference,” she said.
Other models that employ both midwives and dedicated obstetricians in labor and delivery, while having separate physicians for gynecologic and emergency care, are showing promise, Dr. Gregory said.
Another major difference between the two studies was the lack of categorization of hospitals by “how laborists actually functioned on the unit” in her study, Dr. Gregory said.
Since the multicenter study didn’t look at the specific roles of laborists on the hospital unit, it’s unclear whether they took care of just a few patients, or whether they managed all of the obstetrics patients, Dr. Rosenstein said.
Additionally, the multicenter study defined a laborist hospital as one where there was an obstetrician present 24/7. By that definition, the Marin General Hospital, where the single-center study was conducted, would have been considered a laborist hospital both before and after the intervention, she said.
Dr. Gregory also pointed out that all of the patients in the single-hospital study were exposed to the same “culture,” whereas patients in the multicenter study were subject to varying approaches and cultures.
The findings of both studies are of value for identifying the best approach to improving outcomes, Dr. Rosenstein said.
“Studies at the hospital level and at the population level are both important to determine the most optimal labor and delivery staffing pattern,” she said.
The single-center study was funded by the National Institutes of Health and the Prima Medical Foundation. The multicenter study was funded by the Agency for Healthcare Research and Quality, the American College of Obstetricians and Gynecologists, and the March of Dimes. The researchers reported having no relevant financial disclosures.
AT THE PREGNANCY MEETING