User login
National initiative cuts incidence of birth hypoxia by 25%
Although it’s not a new trend, ObGyns are changing how they practice medicine as a direct result of the high cost or availability of liability insurance. From 2009 through 2011 about 18% of practicing obstetricians decreased the number of high-risk patients they were treating, 15% increased the number of cesarean deliveries they performed, 13.5% stopped offering vaginal birth after cesarean, and 5% stopped practicing obstetrics altogether, according to the American Congress of Obstetrics and Gynecology.1 Liability exposure, in part, also has resulted in fewer hospitals across the United States offering birthing services.2
The Premier Perinatal Safety Initiative (PPSI) is a national endeavor, involving 14 hospitals, designed to 1) lower the incidence of preventable adverse birth events, such as birth asphyxia and neonatal neurologic disability, 2) better define preventable perinatal harm, 3) identify measures to improve outcomes, and 4) evaluate the effect of harm reductions on liability claims and pay-outs.2
Reduced adverse events
In 2 years, PPSI hospitals reduced, on average2:
- birth hypoxia and asphyxia by 25%
- neonatal birth trauma by 22%
- complications from administering anesthesia during labor and delivery by 15%
- postpartum hemorrhage by 5.4%.
The adverse outcome index rate, which measures the number of patients with one or more of the identified adverse events as a proportion of total deliveries, was reduced by 7.5%, or 144 fewer adverse events from 2008 to 2010. All hospitals scored below the 2008 Agency for Healthcare Research and Quality (AHRQ) Provider Rate, a national comparative rate measuring perinatal harm.2
Reduced liability claims
In addition, liability claims and payouts decreased by 39% from 2006 to 2010, versus 10% at nonparticipating hospitals. All PPSI hospitals averaged 18 liability claims per year at baseline, but that number dropped to 10 in 2009 and is trending to 8 in 2010 (final claims losses are not yet available because it typically takes 2 years or longer for a claim to be filed).2
Strategies to achieve best outcomes
The best outcomes were achieved with two factors: An increased adherence to evidence-based care bundles in participating hospitals, and enhanced communication and teamwork among hospital staff.2
Increased adherence to evidence-based care bundles. Grouping essential processes together in care bundles helped clinical staff remember to take all of the necessary steps to provide optimal care. For a care bundle to be considered adhered to, staff were scored as “all or none,” meaning that all elements of the care bundle must have been observed for credit to have been given. For instance, the augmenting care bundle included four essential steps. If fetal weight was not calculated before oxytocin was administered, no credit was given for the care provided.2
PPSI hospitals significantly improved compliance with care bundles from 2008 to 2010. On average2:
- Elective induction bundle compliance increased from 58% to 88%.
- Augmentation bundle compliance increased from 33% to 72%.
- Vacuum bundle compliance increased from 9% to 51%.
High-reliability teams. PPSI hospitals implemented proven strategies for certain high-risk protocols known to enhance communication and teamwork, including2:
- TeamSTEPPS®. Developed by AHRQ, TeamSTEPPS produces highly effective medical teams that optimize the use of information, people, and resources to achieve the best clinical outcomes.
- Situation Background Assessment Recommendation (SBAR). An effective situational briefing strategy used by the US Navy helps people communicate relevant case facts in a respectful, focused, and effective manner.
- Simulation drills. Practice exercises feature actresses and mannequins reacting as real patients during the birthing process.
Data regarding outcomes for these communication and teamwork strategies in the PPSI hospitals continues to be evaluated, and will be available in fall 2013.
Study information
Baseline data was completed in a retrospective study of harm outcome data from 2006 and 2007. During Phase 1, health-care teams implemented interventions and worked on improving performance and perinatal safety improvement across approximately 145,000 births. Phase 2 began in January 2011 and will be completed in December 2012.2
The 14 participating hospitals include 4 with small birth volume (1,000 to 2,499 births per year), 8 with medium birth volume (2,500 to 5,000 births per year), and 2 with large birth volume (5,000 or more births per year) in 10 states: Illinois, Kentucky, Massachusetts, Minnesota, New Mexico, Ohio, Tennessee, Texas, Washington, and Wisconsin. Six of 14 hospitals have academic teaching status.2
We want to hear from you! Tell us what you think.
1. 2012 ACOG survey on professional liability results. The American Congress of Obstetricians and Gynecologists Web site. http://www.acog.org/About_ACOG/ACOG_Departments/Professional_Liability/2012_Survey_Results. Accessed December 11, 2012.
2. Reducing preventable birth injuries and liability claims through evidence-based care, enhanced teamwork. Premier Perinatal Safety Initiative Phase 1 Summary, 2008–2010. PPSI_member_white_paper_Nov2012_FINAL.pdf. Published December 2010. Accessed December 11, 2012.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Antidepressants linked to pregnancy risks in infertility treatment</para></item> <item><para>Highlights from 41st Annual AAGL Meeting in Las Vegas</para></item> <item><para>In a study of compliance, a new contraceptive patch tops the pill</para></item> <item><para>Breast cancer genome analysis highlights 4 subtypes, link to ovarian cancer</para></item> <item><para>ObGyns’ status of Maintenance of Certification now public</para></item> <item><para>VTE risk varies by hormone therapy formulation</para></item> </list>
Although it’s not a new trend, ObGyns are changing how they practice medicine as a direct result of the high cost or availability of liability insurance. From 2009 through 2011 about 18% of practicing obstetricians decreased the number of high-risk patients they were treating, 15% increased the number of cesarean deliveries they performed, 13.5% stopped offering vaginal birth after cesarean, and 5% stopped practicing obstetrics altogether, according to the American Congress of Obstetrics and Gynecology.1 Liability exposure, in part, also has resulted in fewer hospitals across the United States offering birthing services.2
The Premier Perinatal Safety Initiative (PPSI) is a national endeavor, involving 14 hospitals, designed to 1) lower the incidence of preventable adverse birth events, such as birth asphyxia and neonatal neurologic disability, 2) better define preventable perinatal harm, 3) identify measures to improve outcomes, and 4) evaluate the effect of harm reductions on liability claims and pay-outs.2
Reduced adverse events
In 2 years, PPSI hospitals reduced, on average2:
- birth hypoxia and asphyxia by 25%
- neonatal birth trauma by 22%
- complications from administering anesthesia during labor and delivery by 15%
- postpartum hemorrhage by 5.4%.
The adverse outcome index rate, which measures the number of patients with one or more of the identified adverse events as a proportion of total deliveries, was reduced by 7.5%, or 144 fewer adverse events from 2008 to 2010. All hospitals scored below the 2008 Agency for Healthcare Research and Quality (AHRQ) Provider Rate, a national comparative rate measuring perinatal harm.2
Reduced liability claims
In addition, liability claims and payouts decreased by 39% from 2006 to 2010, versus 10% at nonparticipating hospitals. All PPSI hospitals averaged 18 liability claims per year at baseline, but that number dropped to 10 in 2009 and is trending to 8 in 2010 (final claims losses are not yet available because it typically takes 2 years or longer for a claim to be filed).2
Strategies to achieve best outcomes
The best outcomes were achieved with two factors: An increased adherence to evidence-based care bundles in participating hospitals, and enhanced communication and teamwork among hospital staff.2
Increased adherence to evidence-based care bundles. Grouping essential processes together in care bundles helped clinical staff remember to take all of the necessary steps to provide optimal care. For a care bundle to be considered adhered to, staff were scored as “all or none,” meaning that all elements of the care bundle must have been observed for credit to have been given. For instance, the augmenting care bundle included four essential steps. If fetal weight was not calculated before oxytocin was administered, no credit was given for the care provided.2
PPSI hospitals significantly improved compliance with care bundles from 2008 to 2010. On average2:
- Elective induction bundle compliance increased from 58% to 88%.
- Augmentation bundle compliance increased from 33% to 72%.
- Vacuum bundle compliance increased from 9% to 51%.
High-reliability teams. PPSI hospitals implemented proven strategies for certain high-risk protocols known to enhance communication and teamwork, including2:
- TeamSTEPPS®. Developed by AHRQ, TeamSTEPPS produces highly effective medical teams that optimize the use of information, people, and resources to achieve the best clinical outcomes.
- Situation Background Assessment Recommendation (SBAR). An effective situational briefing strategy used by the US Navy helps people communicate relevant case facts in a respectful, focused, and effective manner.
- Simulation drills. Practice exercises feature actresses and mannequins reacting as real patients during the birthing process.
Data regarding outcomes for these communication and teamwork strategies in the PPSI hospitals continues to be evaluated, and will be available in fall 2013.
Study information
Baseline data was completed in a retrospective study of harm outcome data from 2006 and 2007. During Phase 1, health-care teams implemented interventions and worked on improving performance and perinatal safety improvement across approximately 145,000 births. Phase 2 began in January 2011 and will be completed in December 2012.2
The 14 participating hospitals include 4 with small birth volume (1,000 to 2,499 births per year), 8 with medium birth volume (2,500 to 5,000 births per year), and 2 with large birth volume (5,000 or more births per year) in 10 states: Illinois, Kentucky, Massachusetts, Minnesota, New Mexico, Ohio, Tennessee, Texas, Washington, and Wisconsin. Six of 14 hospitals have academic teaching status.2
We want to hear from you! Tell us what you think.
Although it’s not a new trend, ObGyns are changing how they practice medicine as a direct result of the high cost or availability of liability insurance. From 2009 through 2011 about 18% of practicing obstetricians decreased the number of high-risk patients they were treating, 15% increased the number of cesarean deliveries they performed, 13.5% stopped offering vaginal birth after cesarean, and 5% stopped practicing obstetrics altogether, according to the American Congress of Obstetrics and Gynecology.1 Liability exposure, in part, also has resulted in fewer hospitals across the United States offering birthing services.2
The Premier Perinatal Safety Initiative (PPSI) is a national endeavor, involving 14 hospitals, designed to 1) lower the incidence of preventable adverse birth events, such as birth asphyxia and neonatal neurologic disability, 2) better define preventable perinatal harm, 3) identify measures to improve outcomes, and 4) evaluate the effect of harm reductions on liability claims and pay-outs.2
Reduced adverse events
In 2 years, PPSI hospitals reduced, on average2:
- birth hypoxia and asphyxia by 25%
- neonatal birth trauma by 22%
- complications from administering anesthesia during labor and delivery by 15%
- postpartum hemorrhage by 5.4%.
The adverse outcome index rate, which measures the number of patients with one or more of the identified adverse events as a proportion of total deliveries, was reduced by 7.5%, or 144 fewer adverse events from 2008 to 2010. All hospitals scored below the 2008 Agency for Healthcare Research and Quality (AHRQ) Provider Rate, a national comparative rate measuring perinatal harm.2
Reduced liability claims
In addition, liability claims and payouts decreased by 39% from 2006 to 2010, versus 10% at nonparticipating hospitals. All PPSI hospitals averaged 18 liability claims per year at baseline, but that number dropped to 10 in 2009 and is trending to 8 in 2010 (final claims losses are not yet available because it typically takes 2 years or longer for a claim to be filed).2
Strategies to achieve best outcomes
The best outcomes were achieved with two factors: An increased adherence to evidence-based care bundles in participating hospitals, and enhanced communication and teamwork among hospital staff.2
Increased adherence to evidence-based care bundles. Grouping essential processes together in care bundles helped clinical staff remember to take all of the necessary steps to provide optimal care. For a care bundle to be considered adhered to, staff were scored as “all or none,” meaning that all elements of the care bundle must have been observed for credit to have been given. For instance, the augmenting care bundle included four essential steps. If fetal weight was not calculated before oxytocin was administered, no credit was given for the care provided.2
PPSI hospitals significantly improved compliance with care bundles from 2008 to 2010. On average2:
- Elective induction bundle compliance increased from 58% to 88%.
- Augmentation bundle compliance increased from 33% to 72%.
- Vacuum bundle compliance increased from 9% to 51%.
High-reliability teams. PPSI hospitals implemented proven strategies for certain high-risk protocols known to enhance communication and teamwork, including2:
- TeamSTEPPS®. Developed by AHRQ, TeamSTEPPS produces highly effective medical teams that optimize the use of information, people, and resources to achieve the best clinical outcomes.
- Situation Background Assessment Recommendation (SBAR). An effective situational briefing strategy used by the US Navy helps people communicate relevant case facts in a respectful, focused, and effective manner.
- Simulation drills. Practice exercises feature actresses and mannequins reacting as real patients during the birthing process.
Data regarding outcomes for these communication and teamwork strategies in the PPSI hospitals continues to be evaluated, and will be available in fall 2013.
Study information
Baseline data was completed in a retrospective study of harm outcome data from 2006 and 2007. During Phase 1, health-care teams implemented interventions and worked on improving performance and perinatal safety improvement across approximately 145,000 births. Phase 2 began in January 2011 and will be completed in December 2012.2
The 14 participating hospitals include 4 with small birth volume (1,000 to 2,499 births per year), 8 with medium birth volume (2,500 to 5,000 births per year), and 2 with large birth volume (5,000 or more births per year) in 10 states: Illinois, Kentucky, Massachusetts, Minnesota, New Mexico, Ohio, Tennessee, Texas, Washington, and Wisconsin. Six of 14 hospitals have academic teaching status.2
We want to hear from you! Tell us what you think.
1. 2012 ACOG survey on professional liability results. The American Congress of Obstetricians and Gynecologists Web site. http://www.acog.org/About_ACOG/ACOG_Departments/Professional_Liability/2012_Survey_Results. Accessed December 11, 2012.
2. Reducing preventable birth injuries and liability claims through evidence-based care, enhanced teamwork. Premier Perinatal Safety Initiative Phase 1 Summary, 2008–2010. PPSI_member_white_paper_Nov2012_FINAL.pdf. Published December 2010. Accessed December 11, 2012.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Antidepressants linked to pregnancy risks in infertility treatment</para></item> <item><para>Highlights from 41st Annual AAGL Meeting in Las Vegas</para></item> <item><para>In a study of compliance, a new contraceptive patch tops the pill</para></item> <item><para>Breast cancer genome analysis highlights 4 subtypes, link to ovarian cancer</para></item> <item><para>ObGyns’ status of Maintenance of Certification now public</para></item> <item><para>VTE risk varies by hormone therapy formulation</para></item> </list>
1. 2012 ACOG survey on professional liability results. The American Congress of Obstetricians and Gynecologists Web site. http://www.acog.org/About_ACOG/ACOG_Departments/Professional_Liability/2012_Survey_Results. Accessed December 11, 2012.
2. Reducing preventable birth injuries and liability claims through evidence-based care, enhanced teamwork. Premier Perinatal Safety Initiative Phase 1 Summary, 2008–2010. PPSI_member_white_paper_Nov2012_FINAL.pdf. Published December 2010. Accessed December 11, 2012.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Antidepressants linked to pregnancy risks in infertility treatment</para></item> <item><para>Highlights from 41st Annual AAGL Meeting in Las Vegas</para></item> <item><para>In a study of compliance, a new contraceptive patch tops the pill</para></item> <item><para>Breast cancer genome analysis highlights 4 subtypes, link to ovarian cancer</para></item> <item><para>ObGyns’ status of Maintenance of Certification now public</para></item> <item><para>VTE risk varies by hormone therapy formulation</para></item> </list>
Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia?
The optimal management of gestational hypertension and mild preeclampsia at term has been a subject of great debate over the past decade. The controversy centers on the timing of delivery—induction of labor versus expectant management.
Proponents of immediate induction raise the valid concern that maternal disease may worsen if pregnancy is allowed to continue. Conversely, proponents of expectant management point to the possibility that the rate of cesarean delivery will be increased with immediate induction; they also cite concerns that neonatal morbidity may be increased with an early term delivery.
To shed light on this debate, investigators in the well-known HYPITAT trial randomly assigned 756 women who had gestational hypertension or mild preeclampsia at term to induction of labor (n = 377) or expectant management (n = 379). All women were carrying a singleton fetus that was 36 to 41 weeks old, with cephalic presentation. The main findings of the trial, published in Lancet, were that induction of labor produced fewer “high-risk situations” (relative risk [RR], 0.71; 95% confidence interval [CI], 0.59–0.86), with no increase in the risk of cesarean delivery (RR, 0.75; 95% CI, 0.55–1.04) or adverse neonatal outcomes (RR, 0.75; 95% CI, 0.45–1.26).1
Although these findings are important, one question lingered in the minds of many obstetricians: Should the choice between induction of labor and expectant management hinge on the favorability of the cervix?
That is the question addressed by Tajik and colleagues.
Zooming in on cervical status
In their secondary analysis from the HYPITAT trial, Tajik and colleagues reanalyzed the association between induction of labor and expectant management, focusing on the same outcomes (high-risk situations, cesarean delivery, adverse neonatal outcomes), but they stratified their data by cervical status. As stated above, their findings are surprising and seemingly counterintuitive:
- Among women who underwent immediate induction of labor, cervical length was not associated with a higher probability of high-risk situations
- The beneficial effect of induction of labor—in terms of reducing the rate of cesarean delivery—was greater among women who had an unfavorable cervix.
Strengths and limitations of the trial
Overall, this was a well-conducted secondary analysis that tackled an important issue. It featured 1) a robust dataset, with all variables of interest collected, and 2) a thoughtful approach to data analysis.
However, the analysis also raises a question: Is it possible that some of its negative findings (composite neonatal morbidity) are due to insufficient power? This is a question I ask whenever I encounter a secondary analysis of a randomized, controlled trial. The answer here: Possibly.
This study provides additional evidence that induction of labor is the optimal approach to gestational hypertension or mild preeclampsia in a pregnancy at 36 weeks or beyond—regardless of cervical status. I would expect clinicians to embrace the findings of the HYPITAT trial, including the secondary analysis, and incorporate this management strategy in their practice.
GEORGE MACONES, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
Reference
1. Koopmans CM, Bijlenga D, Groen H, et al. HYPITAT Study Group. Induction of labour versus expectant monitoring for gestational hypertension or mild preeclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
The optimal management of gestational hypertension and mild preeclampsia at term has been a subject of great debate over the past decade. The controversy centers on the timing of delivery—induction of labor versus expectant management.
Proponents of immediate induction raise the valid concern that maternal disease may worsen if pregnancy is allowed to continue. Conversely, proponents of expectant management point to the possibility that the rate of cesarean delivery will be increased with immediate induction; they also cite concerns that neonatal morbidity may be increased with an early term delivery.
To shed light on this debate, investigators in the well-known HYPITAT trial randomly assigned 756 women who had gestational hypertension or mild preeclampsia at term to induction of labor (n = 377) or expectant management (n = 379). All women were carrying a singleton fetus that was 36 to 41 weeks old, with cephalic presentation. The main findings of the trial, published in Lancet, were that induction of labor produced fewer “high-risk situations” (relative risk [RR], 0.71; 95% confidence interval [CI], 0.59–0.86), with no increase in the risk of cesarean delivery (RR, 0.75; 95% CI, 0.55–1.04) or adverse neonatal outcomes (RR, 0.75; 95% CI, 0.45–1.26).1
Although these findings are important, one question lingered in the minds of many obstetricians: Should the choice between induction of labor and expectant management hinge on the favorability of the cervix?
That is the question addressed by Tajik and colleagues.
Zooming in on cervical status
In their secondary analysis from the HYPITAT trial, Tajik and colleagues reanalyzed the association between induction of labor and expectant management, focusing on the same outcomes (high-risk situations, cesarean delivery, adverse neonatal outcomes), but they stratified their data by cervical status. As stated above, their findings are surprising and seemingly counterintuitive:
- Among women who underwent immediate induction of labor, cervical length was not associated with a higher probability of high-risk situations
- The beneficial effect of induction of labor—in terms of reducing the rate of cesarean delivery—was greater among women who had an unfavorable cervix.
Strengths and limitations of the trial
Overall, this was a well-conducted secondary analysis that tackled an important issue. It featured 1) a robust dataset, with all variables of interest collected, and 2) a thoughtful approach to data analysis.
However, the analysis also raises a question: Is it possible that some of its negative findings (composite neonatal morbidity) are due to insufficient power? This is a question I ask whenever I encounter a secondary analysis of a randomized, controlled trial. The answer here: Possibly.
This study provides additional evidence that induction of labor is the optimal approach to gestational hypertension or mild preeclampsia in a pregnancy at 36 weeks or beyond—regardless of cervical status. I would expect clinicians to embrace the findings of the HYPITAT trial, including the secondary analysis, and incorporate this management strategy in their practice.
GEORGE MACONES, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
The optimal management of gestational hypertension and mild preeclampsia at term has been a subject of great debate over the past decade. The controversy centers on the timing of delivery—induction of labor versus expectant management.
Proponents of immediate induction raise the valid concern that maternal disease may worsen if pregnancy is allowed to continue. Conversely, proponents of expectant management point to the possibility that the rate of cesarean delivery will be increased with immediate induction; they also cite concerns that neonatal morbidity may be increased with an early term delivery.
To shed light on this debate, investigators in the well-known HYPITAT trial randomly assigned 756 women who had gestational hypertension or mild preeclampsia at term to induction of labor (n = 377) or expectant management (n = 379). All women were carrying a singleton fetus that was 36 to 41 weeks old, with cephalic presentation. The main findings of the trial, published in Lancet, were that induction of labor produced fewer “high-risk situations” (relative risk [RR], 0.71; 95% confidence interval [CI], 0.59–0.86), with no increase in the risk of cesarean delivery (RR, 0.75; 95% CI, 0.55–1.04) or adverse neonatal outcomes (RR, 0.75; 95% CI, 0.45–1.26).1
Although these findings are important, one question lingered in the minds of many obstetricians: Should the choice between induction of labor and expectant management hinge on the favorability of the cervix?
That is the question addressed by Tajik and colleagues.
Zooming in on cervical status
In their secondary analysis from the HYPITAT trial, Tajik and colleagues reanalyzed the association between induction of labor and expectant management, focusing on the same outcomes (high-risk situations, cesarean delivery, adverse neonatal outcomes), but they stratified their data by cervical status. As stated above, their findings are surprising and seemingly counterintuitive:
- Among women who underwent immediate induction of labor, cervical length was not associated with a higher probability of high-risk situations
- The beneficial effect of induction of labor—in terms of reducing the rate of cesarean delivery—was greater among women who had an unfavorable cervix.
Strengths and limitations of the trial
Overall, this was a well-conducted secondary analysis that tackled an important issue. It featured 1) a robust dataset, with all variables of interest collected, and 2) a thoughtful approach to data analysis.
However, the analysis also raises a question: Is it possible that some of its negative findings (composite neonatal morbidity) are due to insufficient power? This is a question I ask whenever I encounter a secondary analysis of a randomized, controlled trial. The answer here: Possibly.
This study provides additional evidence that induction of labor is the optimal approach to gestational hypertension or mild preeclampsia in a pregnancy at 36 weeks or beyond—regardless of cervical status. I would expect clinicians to embrace the findings of the HYPITAT trial, including the secondary analysis, and incorporate this management strategy in their practice.
GEORGE MACONES, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
Reference
1. Koopmans CM, Bijlenga D, Groen H, et al. HYPITAT Study Group. Induction of labour versus expectant monitoring for gestational hypertension or mild preeclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
Reference
1. Koopmans CM, Bijlenga D, Groen H, et al. HYPITAT Study Group. Induction of labour versus expectant monitoring for gestational hypertension or mild preeclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
A stitch in time: The B-Lynch, Hayman, and Pereira uterine compression sutures
CLICK HERE to access 6 articles about treating postpartum hemorrhage published in OBG Management in 2011 and 2012.
CASE You are performing a cesarean delivery for a 30-year-old G1P0 woman who presented in labor with a breech fetus at term. Earlier in the pregnancy an external version was unsuccessful in achieving a cephalic presentation. The breech delivery of the newborn is uncomplicated but, immediately following delivery of the placenta, you note excessive uterine bleeding and diagnose a postpartum hemorrhage (PPH) due to uterine atony. Manual massage of the uterus and administration of oxytocin, misoprostol, carboprost tromethamine (Hemabate), and methergine do not result in resolution of the hemorrhage. Your assistant suggests a uterine compression suture to treat the PPH.
What uterine compression suture would you choose?
The management of PPH can be conveniently described using one algorithm for cases that follow a vaginal delivery, and another algorithm for PPH that occurs during cesarean delivery (see “Managing PPH following vaginal and cesarean delivery”). If PPH does not respond to initial treatment steps, more invasive and resource-intensive steps should be performed quickly. Time is critical; delay in initiating escalating steps in the treatment algorithm should be minimized.
PPH at cesarean: Remember your suture options!
In the algorithm for the treatment of PPH occurring at the time of cesarean delivery, the uterine compression suture is an important option.
In 1997, Christopher B-Lynch reported1 on the first widely utilized uterine compression suture. Alternative compression sutures have been reported by Hayman,2 Pereira,3 and others. Every obstetrician should be proficient with the placement of at least one uterine compression suture for the treatment of PPH caused by uterine atony.
Consider the hysterotomy
When it’s open. When PPH caused by uterine atony occurs at cesarean delivery and the hysterotomy incision is open, the B-Lynch suture (FIGURE 1) is a common selection by obstetricians.
When it’s closed. When the hysterotomy is already closed when PPH is noted, the Hayman or Pereira suture(s) are often selected by obstetricians (FIGURES 2 AND 3). Both of these compression sutures also could be applied when the hysterotomy is open.
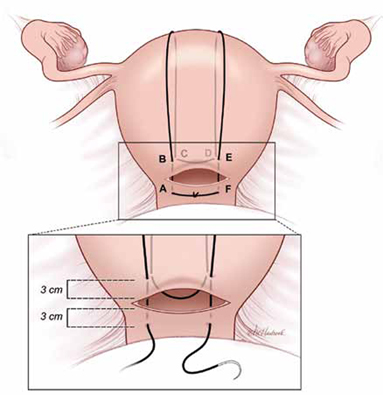
FIGURE 1 B-Lynch suture
The B-Lynch suture as seen from the anterior uterine wall.
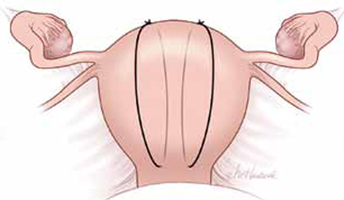
FIGURE 2 Hayman suture
The Hayman suture passes directly from the anterior uterine wall through the posterior uterine wall. Two to four longitudinal sutures can be placed. Two longitudinal sutures are pictured in this figure. A transverse cervicoisthmic suture also can be placed, if needed, to control bleeding from the lower uterine segment.
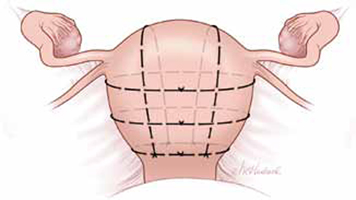
FIGURE 3 Pereira sutures
The Pereira sutures combine longitudinal and transverse sutures placed as a series of bites into the submucosal myometrium. The sutures do not enter the uterine cavity. The longitudinal sutures begin and end at the level of the transverse suture closest to the cervix. Avoid damage to blood vessels and the ureters when placing the transverse sutures. Two longitudinal sutures and three transverse sutures are pictured in this figure.
Combination treatment
Consider combining a uterine compression suture with either:
- placement of an intrauterine balloon, the so-called uterine sandwich,4 or
- uterine devascularization sutures (O’Leary ligation of branches of uterine artery and ligation of the uterine-ovarian arteries).
It’s important to note that the combination of a uterine compression suture with devascularization sutures may be associated with a higher rate of uterine ischemia and myometrial necrosis than the combination of compression sutures with an intrauterine balloon.5
Placing the B-Lynch suture
The B-Lynch suture (FIGURE 1) is placed with the following steps:
1) Take bites on either side of the right edge of the hysterotomy incision (A and B). These bites are placed approximately 3 cm from the edge of the hysterotomy incision.
2) Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly below point B.
3) Pull the suture tightly, but do not tear into the myometrium.
4) Exit the posterior wall of the uterus through point D.
5) Loop the suture over the uterine fundus.
6) Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (E and F).
7) Pull the two ends of the suture tight while an assistant simultaneously squeezes the uterus to aid compression.
8) Place a surgical knot while the assistant continues to compress the uterus.
9) Close the lower uterine segment in the usual manner. B-Lynch1 advised that if there is excessive bleeding from a specific area of the uterus (possible placenta accreta) that a figure-of-8 stitch should be placed through that area of the uterus prior to placing the compression suture.
Choose suture material wisely
In the original description of the B-Lynch suture, a chromic suture was used.1 In a later report, a No. 1 poliglecaprone-25 suture (Monocryl) was utilized.6
I prefer a #1 chromic suture on a large curved needle (Ethicon GL30, 65-mm tapered needle, 30” looped suture) because the uterine compression suture only needs to maintain suture integrity for a few days to be effective. As the uterus involutes to a nonpregnant size, delayed absorption sutures may result in long “rabbit ear” loops separated from the uterus that theoretically could trap intra-abdominal tissue. It is important to ensure that the suture selected is sufficiently long to complete the encirclement of the uterus and with sufficient residual length to facilitate tying the knot.
Evaluate for postop complications
Following recovery from a PPH treated with a uterine compression suture, some women develop uterine complications such as:
- hematometra
- pyometra
- Asherman’s syndrome
- localized areas of uterine necrosis and full-thickness defects in the lower uterine segment or uterine fundus.
Some experts recommend that, for women considering a future pregnancy, the uterine cavity be evaluated, preferably with hysteroscopy.7,8 Hysterosalpingogram, hysterosonography, and MRI are alternative options for evaluating the uterus.
Sutures are effective when used
When PPH is caused by uterine atony, compression sutures have been reported to effectively manage the hemorrhage in about 80% to 90% of cases if the suture is placed in an expedient manner.9-11 The introduction of uterine compression sutures has helped to significantly reduce the number of women who undergo hysterectomy following a PPH. The uterine compression suture represents a significant advance in obstetric care. Every obstetrician should be facile in placing at least one type of compression suture.
The sequential treatment of PPH can be conveniently divided into two algorithms:
1. PPH following vaginal delivery
2. PPH at cesarean delivery.
In both situations, administration of uterotonics; uterine massage; aggressive replacement of red blood cells and clotting factors (fresh frozen plasma, cryoprecipitate, Riastap-lyophilized fibrinogen concentrate), and platelets and monitoring of coagulation effectiveness are critically important. Eliciting the aid of additional obstetricians, anesthesiologists, and nursing staff is also essential.
| Managing PPH following vaginal delivery | Managing PPH at cesarean delivery |
|---|---|
|
|
INSTANT POLL: What are the most important clinical pearls concerning the placement of uterine compression sutures? Click here to share your experience.
1. B-Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104(3):372-375.
2. Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99(3):502-506.
3. Pereira A, Nunes F, Pedroso S, Saraiva J, Retto H, Meirinho M. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106(3):569-572.
4. Diemert A, Ortmeyer G, Hollwitz B, et al. The combination of intrauterine balloon tamponade and the B-Lynch procedure for the treatment of severe postpartum hemorrhage. Am J Obstet Gynecol. 2012;206(1):65.e1-e4.
5. Fotopoulou C, Dudenhausen JW. Uterine compression sutures for preserving fertility in severe postpartum hemorrhage: an overview 13 years after the first description. J Obstet Gynaecol. 2010;30(4):339-349.
6. Price N, B-Lynch C. Technical description of the B-Lynch brace suture for treatment of massive postpartum hemorrhage and review of published cases. Int J Fertil Womens Med. 2005;50(4):148-163.
7. Poujade O, Grossetti A, Mougel L, Ceccaldi PF, Ducarme G, Luton D. Risk of synechiae following uterine compression sutures in the management of major postpartum haemorrhage. BJOG. 2011;118(4):433-439.
8. Amorim-Costa C, Mota R, Rebelo C, Silva PT. Uterine compression sutures for postpartum hemorrhage: is routine postoperative cavity evaluation needed? ACTA Obstet Gynecolog Scand. 2011;90(7):701-706.
9. Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007;62(8):540-547.
10. Mallappa Saroja CS, Nankani A, El-Hamamy E. Uterine compression sutures an update: review of efficacy, safety and complications of B-Lynch suture and other uterine compression techniques for postpartum hemorrhage. Arch Gynecol Obstet. 2010;281(4):581-588.
11. Kayem G, Kurinczuk JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M. UK Obstetric Surveillance System (UKOSS). Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. 2011;117(1):14-20.
CLICK HERE to access 6 articles about treating postpartum hemorrhage published in OBG Management in 2011 and 2012.
CASE You are performing a cesarean delivery for a 30-year-old G1P0 woman who presented in labor with a breech fetus at term. Earlier in the pregnancy an external version was unsuccessful in achieving a cephalic presentation. The breech delivery of the newborn is uncomplicated but, immediately following delivery of the placenta, you note excessive uterine bleeding and diagnose a postpartum hemorrhage (PPH) due to uterine atony. Manual massage of the uterus and administration of oxytocin, misoprostol, carboprost tromethamine (Hemabate), and methergine do not result in resolution of the hemorrhage. Your assistant suggests a uterine compression suture to treat the PPH.
What uterine compression suture would you choose?
The management of PPH can be conveniently described using one algorithm for cases that follow a vaginal delivery, and another algorithm for PPH that occurs during cesarean delivery (see “Managing PPH following vaginal and cesarean delivery”). If PPH does not respond to initial treatment steps, more invasive and resource-intensive steps should be performed quickly. Time is critical; delay in initiating escalating steps in the treatment algorithm should be minimized.
PPH at cesarean: Remember your suture options!
In the algorithm for the treatment of PPH occurring at the time of cesarean delivery, the uterine compression suture is an important option.
In 1997, Christopher B-Lynch reported1 on the first widely utilized uterine compression suture. Alternative compression sutures have been reported by Hayman,2 Pereira,3 and others. Every obstetrician should be proficient with the placement of at least one uterine compression suture for the treatment of PPH caused by uterine atony.
Consider the hysterotomy
When it’s open. When PPH caused by uterine atony occurs at cesarean delivery and the hysterotomy incision is open, the B-Lynch suture (FIGURE 1) is a common selection by obstetricians.
When it’s closed. When the hysterotomy is already closed when PPH is noted, the Hayman or Pereira suture(s) are often selected by obstetricians (FIGURES 2 AND 3). Both of these compression sutures also could be applied when the hysterotomy is open.

FIGURE 1 B-Lynch suture
The B-Lynch suture as seen from the anterior uterine wall.

FIGURE 2 Hayman suture
The Hayman suture passes directly from the anterior uterine wall through the posterior uterine wall. Two to four longitudinal sutures can be placed. Two longitudinal sutures are pictured in this figure. A transverse cervicoisthmic suture also can be placed, if needed, to control bleeding from the lower uterine segment.

FIGURE 3 Pereira sutures
The Pereira sutures combine longitudinal and transverse sutures placed as a series of bites into the submucosal myometrium. The sutures do not enter the uterine cavity. The longitudinal sutures begin and end at the level of the transverse suture closest to the cervix. Avoid damage to blood vessels and the ureters when placing the transverse sutures. Two longitudinal sutures and three transverse sutures are pictured in this figure.
Combination treatment
Consider combining a uterine compression suture with either:
- placement of an intrauterine balloon, the so-called uterine sandwich,4 or
- uterine devascularization sutures (O’Leary ligation of branches of uterine artery and ligation of the uterine-ovarian arteries).
It’s important to note that the combination of a uterine compression suture with devascularization sutures may be associated with a higher rate of uterine ischemia and myometrial necrosis than the combination of compression sutures with an intrauterine balloon.5
Placing the B-Lynch suture
The B-Lynch suture (FIGURE 1) is placed with the following steps:
1) Take bites on either side of the right edge of the hysterotomy incision (A and B). These bites are placed approximately 3 cm from the edge of the hysterotomy incision.
2) Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly below point B.
3) Pull the suture tightly, but do not tear into the myometrium.
4) Exit the posterior wall of the uterus through point D.
5) Loop the suture over the uterine fundus.
6) Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (E and F).
7) Pull the two ends of the suture tight while an assistant simultaneously squeezes the uterus to aid compression.
8) Place a surgical knot while the assistant continues to compress the uterus.
9) Close the lower uterine segment in the usual manner. B-Lynch1 advised that if there is excessive bleeding from a specific area of the uterus (possible placenta accreta) that a figure-of-8 stitch should be placed through that area of the uterus prior to placing the compression suture.
Choose suture material wisely
In the original description of the B-Lynch suture, a chromic suture was used.1 In a later report, a No. 1 poliglecaprone-25 suture (Monocryl) was utilized.6
I prefer a #1 chromic suture on a large curved needle (Ethicon GL30, 65-mm tapered needle, 30” looped suture) because the uterine compression suture only needs to maintain suture integrity for a few days to be effective. As the uterus involutes to a nonpregnant size, delayed absorption sutures may result in long “rabbit ear” loops separated from the uterus that theoretically could trap intra-abdominal tissue. It is important to ensure that the suture selected is sufficiently long to complete the encirclement of the uterus and with sufficient residual length to facilitate tying the knot.
Evaluate for postop complications
Following recovery from a PPH treated with a uterine compression suture, some women develop uterine complications such as:
- hematometra
- pyometra
- Asherman’s syndrome
- localized areas of uterine necrosis and full-thickness defects in the lower uterine segment or uterine fundus.
Some experts recommend that, for women considering a future pregnancy, the uterine cavity be evaluated, preferably with hysteroscopy.7,8 Hysterosalpingogram, hysterosonography, and MRI are alternative options for evaluating the uterus.
Sutures are effective when used
When PPH is caused by uterine atony, compression sutures have been reported to effectively manage the hemorrhage in about 80% to 90% of cases if the suture is placed in an expedient manner.9-11 The introduction of uterine compression sutures has helped to significantly reduce the number of women who undergo hysterectomy following a PPH. The uterine compression suture represents a significant advance in obstetric care. Every obstetrician should be facile in placing at least one type of compression suture.
The sequential treatment of PPH can be conveniently divided into two algorithms:
1. PPH following vaginal delivery
2. PPH at cesarean delivery.
In both situations, administration of uterotonics; uterine massage; aggressive replacement of red blood cells and clotting factors (fresh frozen plasma, cryoprecipitate, Riastap-lyophilized fibrinogen concentrate), and platelets and monitoring of coagulation effectiveness are critically important. Eliciting the aid of additional obstetricians, anesthesiologists, and nursing staff is also essential.
| Managing PPH following vaginal delivery | Managing PPH at cesarean delivery |
|---|---|
|
|
INSTANT POLL: What are the most important clinical pearls concerning the placement of uterine compression sutures? Click here to share your experience.
CLICK HERE to access 6 articles about treating postpartum hemorrhage published in OBG Management in 2011 and 2012.
CASE You are performing a cesarean delivery for a 30-year-old G1P0 woman who presented in labor with a breech fetus at term. Earlier in the pregnancy an external version was unsuccessful in achieving a cephalic presentation. The breech delivery of the newborn is uncomplicated but, immediately following delivery of the placenta, you note excessive uterine bleeding and diagnose a postpartum hemorrhage (PPH) due to uterine atony. Manual massage of the uterus and administration of oxytocin, misoprostol, carboprost tromethamine (Hemabate), and methergine do not result in resolution of the hemorrhage. Your assistant suggests a uterine compression suture to treat the PPH.
What uterine compression suture would you choose?
The management of PPH can be conveniently described using one algorithm for cases that follow a vaginal delivery, and another algorithm for PPH that occurs during cesarean delivery (see “Managing PPH following vaginal and cesarean delivery”). If PPH does not respond to initial treatment steps, more invasive and resource-intensive steps should be performed quickly. Time is critical; delay in initiating escalating steps in the treatment algorithm should be minimized.
PPH at cesarean: Remember your suture options!
In the algorithm for the treatment of PPH occurring at the time of cesarean delivery, the uterine compression suture is an important option.
In 1997, Christopher B-Lynch reported1 on the first widely utilized uterine compression suture. Alternative compression sutures have been reported by Hayman,2 Pereira,3 and others. Every obstetrician should be proficient with the placement of at least one uterine compression suture for the treatment of PPH caused by uterine atony.
Consider the hysterotomy
When it’s open. When PPH caused by uterine atony occurs at cesarean delivery and the hysterotomy incision is open, the B-Lynch suture (FIGURE 1) is a common selection by obstetricians.
When it’s closed. When the hysterotomy is already closed when PPH is noted, the Hayman or Pereira suture(s) are often selected by obstetricians (FIGURES 2 AND 3). Both of these compression sutures also could be applied when the hysterotomy is open.

FIGURE 1 B-Lynch suture
The B-Lynch suture as seen from the anterior uterine wall.

FIGURE 2 Hayman suture
The Hayman suture passes directly from the anterior uterine wall through the posterior uterine wall. Two to four longitudinal sutures can be placed. Two longitudinal sutures are pictured in this figure. A transverse cervicoisthmic suture also can be placed, if needed, to control bleeding from the lower uterine segment.

FIGURE 3 Pereira sutures
The Pereira sutures combine longitudinal and transverse sutures placed as a series of bites into the submucosal myometrium. The sutures do not enter the uterine cavity. The longitudinal sutures begin and end at the level of the transverse suture closest to the cervix. Avoid damage to blood vessels and the ureters when placing the transverse sutures. Two longitudinal sutures and three transverse sutures are pictured in this figure.
Combination treatment
Consider combining a uterine compression suture with either:
- placement of an intrauterine balloon, the so-called uterine sandwich,4 or
- uterine devascularization sutures (O’Leary ligation of branches of uterine artery and ligation of the uterine-ovarian arteries).
It’s important to note that the combination of a uterine compression suture with devascularization sutures may be associated with a higher rate of uterine ischemia and myometrial necrosis than the combination of compression sutures with an intrauterine balloon.5
Placing the B-Lynch suture
The B-Lynch suture (FIGURE 1) is placed with the following steps:
1) Take bites on either side of the right edge of the hysterotomy incision (A and B). These bites are placed approximately 3 cm from the edge of the hysterotomy incision.
2) Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly below point B.
3) Pull the suture tightly, but do not tear into the myometrium.
4) Exit the posterior wall of the uterus through point D.
5) Loop the suture over the uterine fundus.
6) Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (E and F).
7) Pull the two ends of the suture tight while an assistant simultaneously squeezes the uterus to aid compression.
8) Place a surgical knot while the assistant continues to compress the uterus.
9) Close the lower uterine segment in the usual manner. B-Lynch1 advised that if there is excessive bleeding from a specific area of the uterus (possible placenta accreta) that a figure-of-8 stitch should be placed through that area of the uterus prior to placing the compression suture.
Choose suture material wisely
In the original description of the B-Lynch suture, a chromic suture was used.1 In a later report, a No. 1 poliglecaprone-25 suture (Monocryl) was utilized.6
I prefer a #1 chromic suture on a large curved needle (Ethicon GL30, 65-mm tapered needle, 30” looped suture) because the uterine compression suture only needs to maintain suture integrity for a few days to be effective. As the uterus involutes to a nonpregnant size, delayed absorption sutures may result in long “rabbit ear” loops separated from the uterus that theoretically could trap intra-abdominal tissue. It is important to ensure that the suture selected is sufficiently long to complete the encirclement of the uterus and with sufficient residual length to facilitate tying the knot.
Evaluate for postop complications
Following recovery from a PPH treated with a uterine compression suture, some women develop uterine complications such as:
- hematometra
- pyometra
- Asherman’s syndrome
- localized areas of uterine necrosis and full-thickness defects in the lower uterine segment or uterine fundus.
Some experts recommend that, for women considering a future pregnancy, the uterine cavity be evaluated, preferably with hysteroscopy.7,8 Hysterosalpingogram, hysterosonography, and MRI are alternative options for evaluating the uterus.
Sutures are effective when used
When PPH is caused by uterine atony, compression sutures have been reported to effectively manage the hemorrhage in about 80% to 90% of cases if the suture is placed in an expedient manner.9-11 The introduction of uterine compression sutures has helped to significantly reduce the number of women who undergo hysterectomy following a PPH. The uterine compression suture represents a significant advance in obstetric care. Every obstetrician should be facile in placing at least one type of compression suture.
The sequential treatment of PPH can be conveniently divided into two algorithms:
1. PPH following vaginal delivery
2. PPH at cesarean delivery.
In both situations, administration of uterotonics; uterine massage; aggressive replacement of red blood cells and clotting factors (fresh frozen plasma, cryoprecipitate, Riastap-lyophilized fibrinogen concentrate), and platelets and monitoring of coagulation effectiveness are critically important. Eliciting the aid of additional obstetricians, anesthesiologists, and nursing staff is also essential.
| Managing PPH following vaginal delivery | Managing PPH at cesarean delivery |
|---|---|
|
|
INSTANT POLL: What are the most important clinical pearls concerning the placement of uterine compression sutures? Click here to share your experience.
1. B-Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104(3):372-375.
2. Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99(3):502-506.
3. Pereira A, Nunes F, Pedroso S, Saraiva J, Retto H, Meirinho M. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106(3):569-572.
4. Diemert A, Ortmeyer G, Hollwitz B, et al. The combination of intrauterine balloon tamponade and the B-Lynch procedure for the treatment of severe postpartum hemorrhage. Am J Obstet Gynecol. 2012;206(1):65.e1-e4.
5. Fotopoulou C, Dudenhausen JW. Uterine compression sutures for preserving fertility in severe postpartum hemorrhage: an overview 13 years after the first description. J Obstet Gynaecol. 2010;30(4):339-349.
6. Price N, B-Lynch C. Technical description of the B-Lynch brace suture for treatment of massive postpartum hemorrhage and review of published cases. Int J Fertil Womens Med. 2005;50(4):148-163.
7. Poujade O, Grossetti A, Mougel L, Ceccaldi PF, Ducarme G, Luton D. Risk of synechiae following uterine compression sutures in the management of major postpartum haemorrhage. BJOG. 2011;118(4):433-439.
8. Amorim-Costa C, Mota R, Rebelo C, Silva PT. Uterine compression sutures for postpartum hemorrhage: is routine postoperative cavity evaluation needed? ACTA Obstet Gynecolog Scand. 2011;90(7):701-706.
9. Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007;62(8):540-547.
10. Mallappa Saroja CS, Nankani A, El-Hamamy E. Uterine compression sutures an update: review of efficacy, safety and complications of B-Lynch suture and other uterine compression techniques for postpartum hemorrhage. Arch Gynecol Obstet. 2010;281(4):581-588.
11. Kayem G, Kurinczuk JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M. UK Obstetric Surveillance System (UKOSS). Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. 2011;117(1):14-20.
1. B-Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104(3):372-375.
2. Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99(3):502-506.
3. Pereira A, Nunes F, Pedroso S, Saraiva J, Retto H, Meirinho M. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106(3):569-572.
4. Diemert A, Ortmeyer G, Hollwitz B, et al. The combination of intrauterine balloon tamponade and the B-Lynch procedure for the treatment of severe postpartum hemorrhage. Am J Obstet Gynecol. 2012;206(1):65.e1-e4.
5. Fotopoulou C, Dudenhausen JW. Uterine compression sutures for preserving fertility in severe postpartum hemorrhage: an overview 13 years after the first description. J Obstet Gynaecol. 2010;30(4):339-349.
6. Price N, B-Lynch C. Technical description of the B-Lynch brace suture for treatment of massive postpartum hemorrhage and review of published cases. Int J Fertil Womens Med. 2005;50(4):148-163.
7. Poujade O, Grossetti A, Mougel L, Ceccaldi PF, Ducarme G, Luton D. Risk of synechiae following uterine compression sutures in the management of major postpartum haemorrhage. BJOG. 2011;118(4):433-439.
8. Amorim-Costa C, Mota R, Rebelo C, Silva PT. Uterine compression sutures for postpartum hemorrhage: is routine postoperative cavity evaluation needed? ACTA Obstet Gynecolog Scand. 2011;90(7):701-706.
9. Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007;62(8):540-547.
10. Mallappa Saroja CS, Nankani A, El-Hamamy E. Uterine compression sutures an update: review of efficacy, safety and complications of B-Lynch suture and other uterine compression techniques for postpartum hemorrhage. Arch Gynecol Obstet. 2010;281(4):581-588.
11. Kayem G, Kurinczuk JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M. UK Obstetric Surveillance System (UKOSS). Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. 2011;117(1):14-20.
Pregnancy Loss Boosts Multiple Atherosclerotic Risks
LOS ANGELES – Pregnancy loss is strongly associated with increased risks of three different clinical forms of atherosclerotic disease over the subsequent 15 years, a study of more than 1 million Danish pregnant women has shown.
"This is the largest-ever study on the occurrence of atherosclerotic disease after pregnancy loss. This study, taken together with previous studies, implies a possible common underlying pathology linking pregnancy losses and atherosclerosis," said Dr. Mattis F. Ranthe of the Statens Serum Institute, Copenhagen.
The study used Denmark’s comprehensive national cradle-to-the-grave health care registry to track all 1,031,279 Danes who were free of a history of cardiovascular disease at the time they became pregnant during 1977-1988. A total of 8,191 women had one or more stillbirths. There were 151,808 women with one miscarriage, 28,398 with two miscarriages, 5,979 with three, and 2,406 women with four or more miscarriages.
The three expressions of atherosclerosis under study were acute MI, cerebral infarction, and renovascular hypertension. During more than 15 million person-years of follow-up through the registry, there were 2,798 cases of MI, 4,053 cerebral infarcts, and 1,269 diagnoses of renovascular hypertension, Dr. Ranthe reported at the annual scientific sessions of the American Heart Association.
A history of even a single stillbirth was associated with a 2.69-fold increased incidence rate ratio for subsequent MI, a 1.74-fold increase in cerebral infarction, and a 2.42-fold increase in renovascular hypertension after adjustment for age, number of live births, and calendar year.
A robust dose-response relationship was evident between the number of miscarriages and atherosclerotic disease risk. Women with a history of a single miscarriage had an adjusted 1.11-fold increased risk of MI, a 1.13-fold increase in cerebral infarction, and a 1.15-fold greater risk of developing renovascular hypertension during follow-up than did women with no miscarriages. These 11%-15% increases in relative risk were all strongly significant, given the large numbers.
With two miscarriages, the risks of MI, cerebral infarct, and renovascular hypertension were increased 1.18-fold, 1.22-fold, and 1.12-fold, respectively. With a history of three miscarriages, the risks were 0.85, 1.43, and 1.78. And with 4 or more miscarriages, the incidence rate ratio for MI was increased 2.08-fold, that for cerebral infarct was 1.89-fold, and for renovascular hypertension it was 3.78-fold greater than in women with no miscarriages.
Further adjustment for diabetes, smoking, thrombophilia, and polycystic ovarian syndrome left these estimates unchanged.
The risk of each of the three forms of atherosclerosis climbed by 10%-20% with each additional miscarriage. However, the risk wasn’t evenly spread across all age groups. Rather, the risk of developing atherosclerotic disease within the next 15 years was greatest in the youngest women who miscarried. The risk associated with miscarriage late in the period of childbearing potential was far less, the physician noted.
That observation raised a red flag for one audience member.
"If you have younger women of childbearing years having myocardial infarctions earlier on, one tends to think that mechanistically it may be atherosclerosis, but it may actually be due to other issues involving connective tissue diseases. I’d be cautious in using atherosclerosis as a broad pathophysiologic explanation," she said.
Dr. Ranthe agreed.
He reported having no financial conflicts related to this study, which was funded by the Danish Heart Foundation.
LOS ANGELES – Pregnancy loss is strongly associated with increased risks of three different clinical forms of atherosclerotic disease over the subsequent 15 years, a study of more than 1 million Danish pregnant women has shown.
"This is the largest-ever study on the occurrence of atherosclerotic disease after pregnancy loss. This study, taken together with previous studies, implies a possible common underlying pathology linking pregnancy losses and atherosclerosis," said Dr. Mattis F. Ranthe of the Statens Serum Institute, Copenhagen.
The study used Denmark’s comprehensive national cradle-to-the-grave health care registry to track all 1,031,279 Danes who were free of a history of cardiovascular disease at the time they became pregnant during 1977-1988. A total of 8,191 women had one or more stillbirths. There were 151,808 women with one miscarriage, 28,398 with two miscarriages, 5,979 with three, and 2,406 women with four or more miscarriages.
The three expressions of atherosclerosis under study were acute MI, cerebral infarction, and renovascular hypertension. During more than 15 million person-years of follow-up through the registry, there were 2,798 cases of MI, 4,053 cerebral infarcts, and 1,269 diagnoses of renovascular hypertension, Dr. Ranthe reported at the annual scientific sessions of the American Heart Association.
A history of even a single stillbirth was associated with a 2.69-fold increased incidence rate ratio for subsequent MI, a 1.74-fold increase in cerebral infarction, and a 2.42-fold increase in renovascular hypertension after adjustment for age, number of live births, and calendar year.
A robust dose-response relationship was evident between the number of miscarriages and atherosclerotic disease risk. Women with a history of a single miscarriage had an adjusted 1.11-fold increased risk of MI, a 1.13-fold increase in cerebral infarction, and a 1.15-fold greater risk of developing renovascular hypertension during follow-up than did women with no miscarriages. These 11%-15% increases in relative risk were all strongly significant, given the large numbers.
With two miscarriages, the risks of MI, cerebral infarct, and renovascular hypertension were increased 1.18-fold, 1.22-fold, and 1.12-fold, respectively. With a history of three miscarriages, the risks were 0.85, 1.43, and 1.78. And with 4 or more miscarriages, the incidence rate ratio for MI was increased 2.08-fold, that for cerebral infarct was 1.89-fold, and for renovascular hypertension it was 3.78-fold greater than in women with no miscarriages.
Further adjustment for diabetes, smoking, thrombophilia, and polycystic ovarian syndrome left these estimates unchanged.
The risk of each of the three forms of atherosclerosis climbed by 10%-20% with each additional miscarriage. However, the risk wasn’t evenly spread across all age groups. Rather, the risk of developing atherosclerotic disease within the next 15 years was greatest in the youngest women who miscarried. The risk associated with miscarriage late in the period of childbearing potential was far less, the physician noted.
That observation raised a red flag for one audience member.
"If you have younger women of childbearing years having myocardial infarctions earlier on, one tends to think that mechanistically it may be atherosclerosis, but it may actually be due to other issues involving connective tissue diseases. I’d be cautious in using atherosclerosis as a broad pathophysiologic explanation," she said.
Dr. Ranthe agreed.
He reported having no financial conflicts related to this study, which was funded by the Danish Heart Foundation.
LOS ANGELES – Pregnancy loss is strongly associated with increased risks of three different clinical forms of atherosclerotic disease over the subsequent 15 years, a study of more than 1 million Danish pregnant women has shown.
"This is the largest-ever study on the occurrence of atherosclerotic disease after pregnancy loss. This study, taken together with previous studies, implies a possible common underlying pathology linking pregnancy losses and atherosclerosis," said Dr. Mattis F. Ranthe of the Statens Serum Institute, Copenhagen.
The study used Denmark’s comprehensive national cradle-to-the-grave health care registry to track all 1,031,279 Danes who were free of a history of cardiovascular disease at the time they became pregnant during 1977-1988. A total of 8,191 women had one or more stillbirths. There were 151,808 women with one miscarriage, 28,398 with two miscarriages, 5,979 with three, and 2,406 women with four or more miscarriages.
The three expressions of atherosclerosis under study were acute MI, cerebral infarction, and renovascular hypertension. During more than 15 million person-years of follow-up through the registry, there were 2,798 cases of MI, 4,053 cerebral infarcts, and 1,269 diagnoses of renovascular hypertension, Dr. Ranthe reported at the annual scientific sessions of the American Heart Association.
A history of even a single stillbirth was associated with a 2.69-fold increased incidence rate ratio for subsequent MI, a 1.74-fold increase in cerebral infarction, and a 2.42-fold increase in renovascular hypertension after adjustment for age, number of live births, and calendar year.
A robust dose-response relationship was evident between the number of miscarriages and atherosclerotic disease risk. Women with a history of a single miscarriage had an adjusted 1.11-fold increased risk of MI, a 1.13-fold increase in cerebral infarction, and a 1.15-fold greater risk of developing renovascular hypertension during follow-up than did women with no miscarriages. These 11%-15% increases in relative risk were all strongly significant, given the large numbers.
With two miscarriages, the risks of MI, cerebral infarct, and renovascular hypertension were increased 1.18-fold, 1.22-fold, and 1.12-fold, respectively. With a history of three miscarriages, the risks were 0.85, 1.43, and 1.78. And with 4 or more miscarriages, the incidence rate ratio for MI was increased 2.08-fold, that for cerebral infarct was 1.89-fold, and for renovascular hypertension it was 3.78-fold greater than in women with no miscarriages.
Further adjustment for diabetes, smoking, thrombophilia, and polycystic ovarian syndrome left these estimates unchanged.
The risk of each of the three forms of atherosclerosis climbed by 10%-20% with each additional miscarriage. However, the risk wasn’t evenly spread across all age groups. Rather, the risk of developing atherosclerotic disease within the next 15 years was greatest in the youngest women who miscarried. The risk associated with miscarriage late in the period of childbearing potential was far less, the physician noted.
That observation raised a red flag for one audience member.
"If you have younger women of childbearing years having myocardial infarctions earlier on, one tends to think that mechanistically it may be atherosclerosis, but it may actually be due to other issues involving connective tissue diseases. I’d be cautious in using atherosclerosis as a broad pathophysiologic explanation," she said.
Dr. Ranthe agreed.
He reported having no financial conflicts related to this study, which was funded by the Danish Heart Foundation.
AT THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: Women with a history of one stillbirth or at least four miscarriages have at least a twofold increased risk of having an acute MI, having a cerebral infarct, or being diagnosed with renovascular hypertension in the subsequent 15 years, compared with women who have only live births.
Data Source: Data are from a retrospective Danish national health care registry study of more than 1 million Danes pregnant in 1977-2008, with a total follow-up in excess of 15 million person-years.
Disclosures: This study was funded by the Danish Heart Foundation. The presenter said he had no relevant financial conflicts.
Kidney Disease a Risk Factor for Death in Pregnancy
SAN DIEGO – Pregnant women with kidney disease face an increased risk of adverse maternal outcomes including maternal mortality independent of underlying comorbid conditions that can occur with kidney disease, according to Dr. Shailendra Sharma.
"Any degree of kidney disease during pregnancy should be recognized and should be treated promptly with respect because we now know that can lead to bad outcomes down the road," Dr. Sharma said in an interview during a poster session at the Kidney Week 2012. "This is not something that should be underestimated."
Dr. Sharma, a second-year renal fellow at the University of Colorado, Aurora, and his associates retrospectively studied the records of 646 women with kidney disease who gave birth in Colorado and Utah between 2000 and 2011 at facilities operated by Intermountain Health Care. For comparison, the researchers randomly selected the records of 62,757 pregnancies from women without kidney disease.
Kidney disease was defined by ICD-9 code, and adverse maternal outcomes were defined as preterm delivery (prior to 37 weeks’ gestation), delivery by cesarean section, length of hospital stay, and maternal death. The researchers used multivariate logistic regression analysis to examine the association between kidney disease and adverse maternal outcomes. Covariates included in the fully adjusted model were maternal age, race, history of diabetes, chronic hypertension, liver disease, and connective tissue disorders.
The mean age of patients was 28 years. Compared with women who did not have kidney disease, those who did were significantly more likely to have comorbid conditions including diabetes (12% vs. 1%, respectively); chronic hypertension (2% vs. 7%); liver disease (9% vs. 1%); and connective tissue disorders (7% vs. 0.4%). They also were more likely to have preeclampsia/eclampsia (11% vs. 3%), to have a longer hospital stay (a mean of 3 vs. 2 days), and to give birth to a lower-weight infant (a mean of 3,067 g vs. 3,325 g).
After the investigators adjusted for age, race, history of diabetes, hypertension, liver disease, and connective tissue disorders, Dr. Sharma and his associates found that pregnant women with kidney disease had a significantly increased risk of death (OR, 3.38); preterm delivery (OR, 1.95); delivery via C-section (OR, 1.38); and longer length of hospital stay (OR, 1.39). "The most striking finding was the association of kidney disease with maternal mortality," Dr. Sharma said at the meeting, which was sponsored by the American Society of Nephrology. "The magnitude of this association surprised us."
He said that the retrospective design of the study is a limitation. "If there’s a prospective study moving forward, specifically designed to answer these questions, then it probably would help us establish the causality."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Sharma said he had no relevant financial conflicts to disclose.
SAN DIEGO – Pregnant women with kidney disease face an increased risk of adverse maternal outcomes including maternal mortality independent of underlying comorbid conditions that can occur with kidney disease, according to Dr. Shailendra Sharma.
"Any degree of kidney disease during pregnancy should be recognized and should be treated promptly with respect because we now know that can lead to bad outcomes down the road," Dr. Sharma said in an interview during a poster session at the Kidney Week 2012. "This is not something that should be underestimated."
Dr. Sharma, a second-year renal fellow at the University of Colorado, Aurora, and his associates retrospectively studied the records of 646 women with kidney disease who gave birth in Colorado and Utah between 2000 and 2011 at facilities operated by Intermountain Health Care. For comparison, the researchers randomly selected the records of 62,757 pregnancies from women without kidney disease.
Kidney disease was defined by ICD-9 code, and adverse maternal outcomes were defined as preterm delivery (prior to 37 weeks’ gestation), delivery by cesarean section, length of hospital stay, and maternal death. The researchers used multivariate logistic regression analysis to examine the association between kidney disease and adverse maternal outcomes. Covariates included in the fully adjusted model were maternal age, race, history of diabetes, chronic hypertension, liver disease, and connective tissue disorders.
The mean age of patients was 28 years. Compared with women who did not have kidney disease, those who did were significantly more likely to have comorbid conditions including diabetes (12% vs. 1%, respectively); chronic hypertension (2% vs. 7%); liver disease (9% vs. 1%); and connective tissue disorders (7% vs. 0.4%). They also were more likely to have preeclampsia/eclampsia (11% vs. 3%), to have a longer hospital stay (a mean of 3 vs. 2 days), and to give birth to a lower-weight infant (a mean of 3,067 g vs. 3,325 g).
After the investigators adjusted for age, race, history of diabetes, hypertension, liver disease, and connective tissue disorders, Dr. Sharma and his associates found that pregnant women with kidney disease had a significantly increased risk of death (OR, 3.38); preterm delivery (OR, 1.95); delivery via C-section (OR, 1.38); and longer length of hospital stay (OR, 1.39). "The most striking finding was the association of kidney disease with maternal mortality," Dr. Sharma said at the meeting, which was sponsored by the American Society of Nephrology. "The magnitude of this association surprised us."
He said that the retrospective design of the study is a limitation. "If there’s a prospective study moving forward, specifically designed to answer these questions, then it probably would help us establish the causality."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Sharma said he had no relevant financial conflicts to disclose.
SAN DIEGO – Pregnant women with kidney disease face an increased risk of adverse maternal outcomes including maternal mortality independent of underlying comorbid conditions that can occur with kidney disease, according to Dr. Shailendra Sharma.
"Any degree of kidney disease during pregnancy should be recognized and should be treated promptly with respect because we now know that can lead to bad outcomes down the road," Dr. Sharma said in an interview during a poster session at the Kidney Week 2012. "This is not something that should be underestimated."
Dr. Sharma, a second-year renal fellow at the University of Colorado, Aurora, and his associates retrospectively studied the records of 646 women with kidney disease who gave birth in Colorado and Utah between 2000 and 2011 at facilities operated by Intermountain Health Care. For comparison, the researchers randomly selected the records of 62,757 pregnancies from women without kidney disease.
Kidney disease was defined by ICD-9 code, and adverse maternal outcomes were defined as preterm delivery (prior to 37 weeks’ gestation), delivery by cesarean section, length of hospital stay, and maternal death. The researchers used multivariate logistic regression analysis to examine the association between kidney disease and adverse maternal outcomes. Covariates included in the fully adjusted model were maternal age, race, history of diabetes, chronic hypertension, liver disease, and connective tissue disorders.
The mean age of patients was 28 years. Compared with women who did not have kidney disease, those who did were significantly more likely to have comorbid conditions including diabetes (12% vs. 1%, respectively); chronic hypertension (2% vs. 7%); liver disease (9% vs. 1%); and connective tissue disorders (7% vs. 0.4%). They also were more likely to have preeclampsia/eclampsia (11% vs. 3%), to have a longer hospital stay (a mean of 3 vs. 2 days), and to give birth to a lower-weight infant (a mean of 3,067 g vs. 3,325 g).
After the investigators adjusted for age, race, history of diabetes, hypertension, liver disease, and connective tissue disorders, Dr. Sharma and his associates found that pregnant women with kidney disease had a significantly increased risk of death (OR, 3.38); preterm delivery (OR, 1.95); delivery via C-section (OR, 1.38); and longer length of hospital stay (OR, 1.39). "The most striking finding was the association of kidney disease with maternal mortality," Dr. Sharma said at the meeting, which was sponsored by the American Society of Nephrology. "The magnitude of this association surprised us."
He said that the retrospective design of the study is a limitation. "If there’s a prospective study moving forward, specifically designed to answer these questions, then it probably would help us establish the causality."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Sharma said he had no relevant financial conflicts to disclose.
AT KIDNEY WEEK 2012
Major Finding: Pregnant women with kidney disease had a significantly increased risk of death (OR, 3.38), preterm delivery (OR, 1.95), delivery via C-section (OR, 1.38), and longer length of hospital stay (OR, 1.39), compared with pregnant women who did not have kidney disease.
Data Source: Data are from a retrospective study comparing 646 women with kidney disease who gave birth in Colorado and Utah between 2000 and 2011 with 62,757 pregnancies from women without kidney disease. The women all gave birth at Intermountain Health Care.
Disclosures: The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Sharma said he had no relevant financial conflicts to disclose.
Is the rate of progress the same for induced and spontaneous labors?
Induction of labor is warranted when the benefits of delivery (for the mother or fetus) outweigh the advantages of continuing the pregnancy. Common indications include membrane rupture, gestational hypertension, nonreassuring fetal status, and various maternal medical or fetal conditions.
Induction involves the stimulation of contractions in the absence of spontaneous labor (with or without ruptured membranes), whereas augmentation refers to stimulation of preexisting spontaneous contractions that are considered inadequate because of failed or inadequate cervical dilation and fetal descent.
Women who undergo induction of labor—particularly if nulliparous—are more likely to require cesarean delivery than those who enter labor spontaneously. As the authors of this study point out, it is unclear why induction of labor is associated with an increased risk of cesarean delivery, but it may be related, in part, to the way induced labors are managed.
The incidence of labor induction in the United States more than doubled over the past 20 years. In 2007, more than 20% of all labors were induced in the United States.1 When augmented labors are added to the equation, the sum likely represents half of all pregnancies, so this subject is important to us all.
Details of the study
Enter Harper and colleagues, who focused on women who 1) carried a singleton pregnancy in vertex presentation, 2) reached 10 cm of dilation, and 3) had an umbilical cord gas obtained at delivery. The women were admitted for labor from July 2004 to June 2008 at Washington University Medical Center in St. Louis, Missouri. They had a minimum gestational age of 37 weeks and reached the second stage of labor. Labor and delivery records included information on medications, type of labor, times of cervical examination, extent of cervical dilation, station, duration and curves of the first stage of labor, length of the stages of labor, mode of delivery, and postpartum status.
Of 5,388 women in the cohort, 2,021 entered labor spontaneously, 1,720 had labor augmented, and 1,647 had labor induced. After adjustments for race, obesity, macrosomia, and Bishop score, women who underwent induction of labor spent a significantly longer total time in labor than did women who entered labor spontaneously.
Among nulliparous women, the median (95th percentile) time to progress from 4 cm to 10 cm was 5.5 (16.8) hours when labor was induced versus 3.8 (11.8) hours for spontaneous labors. Among multiparous women, the figures were 4.4 (16.2) hours and 2.4 (8.8) hours, respectively.
The time it took for dilation to increase 1 cm in latent labor (<6 cm dilation) was significantly longer in induced labors, compared with spontaneous labors. However, the time it took for dilation to increase 1 cm in active labor (≥6 cm dilation) was similar between groups.
Strengths and weaknesses of the trial
Induced labor in this cohort was significantly slower than currently accepted definitions of protraction (dilation <1 cm/hr for 4 hr) and arrest disorders (no cervical dilation for 2 hr). And the active phase of labor (defined as an increased rate of cervical dilation) began at 6 cm in this study, much later than previously accepted definitions of 3 to 4 cm.2 If the traditional definitions of active-phase arrest are applied to women whose labors are induced, a significant number of cesarean deliveries may be performed prematurely for arrest disorders.
A strength of this investigation is the large size of the cohort. Patient-level data, including patient characteristics and medication details, enabled the investigators to reconstruct labor curves while adjusting for relevant confounding variables. Methods of cervical ripening (prostaglandins, Foley balloon) were documented, as were indications for induction, making this study generalizable to a wide population.
Harper and colleagues did not stratify their findings by favorability of the cervix at the time of induction. Women who required cervical ripening had a slower labor than did women in spontaneous labor until they reached 6 cm, at which point labor patterns converged. Of interest, women who had a favorable cervix at the time of induction had a faster labor than did women in spontaneous labor, largely as a result of shorter times to reach 6 cm.
As for the women who underwent labor augmentation, the progress of labor before 6 cm was very similar to progress among those whose labor was induced. This finding may reflect misclassification of women between the induction and augmentation groups, or misdiagnosis of labor at the time of admission.
Women were excluded from this study if they did not reach the second stage of labor, because investigators were interested in examining the normal course of labor rather than the need for cesarean delivery. However, this exclusion could have caused selection bias.
Analysis did not begin until women reached 3 cm of dilation, largely because women in spontaneous labor were typically admitted when their cervix had dilated at least 3 cm. The period before 3 cm of dilation seems to be longest when induction of labor occurs in the presence of an unfavorable cervix.
Harper and colleagues confirm a commonly held perception that women undergoing induction of labor spend a longer total time in labor than women who enter labor spontaneously.3,4 Before 6 cm, women undergoing induction of labor may take as long as 10 hours to achieve each centimeter of dilation. This pattern suggests that a diagnosis of arrest of labor before 6 cm of dilation needs to be scrutinized carefully to prevent unnecessary cesarean delivery.
William F. Rayburn, MD, MBA
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Does maternal exposure to magnesium sulfate affect fetal heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
When macrosomia is suspected at term, does induction of labor lower the risk of cesarean delivery?
Jennifer T. Ahn, MD (May 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
1. Induction of labor. ACOG Practice Bulletin #107. August 2009. Obstet Gynecol. 2009;114(2 Pt 1):386-397.
2. Dystocia and augmentation of labor. ACOG Practice Bulletin #49. December 2003. Obstet Gynecol. 2003;102(6):1445-1454.
3. Rinehart BK, Terrone DA, Hudson C, Isler CM, Larmon JE, Perry KG, Jr. Lack of utility of standard labor curves in the prediction of progression during labor induction. Am J Obstet Gynecol. 2000;182(6):1520-1526.
4. Cheng YW, Delaney SS, Hopkins LM, Caughey AB. The association between the length of first stage of labor, mode of delivery, and perinatal outcomes in women undergoing induction of labor. Am J Obstet Gynecol. 2009;201(5):477.e1-e7.
Induction of labor is warranted when the benefits of delivery (for the mother or fetus) outweigh the advantages of continuing the pregnancy. Common indications include membrane rupture, gestational hypertension, nonreassuring fetal status, and various maternal medical or fetal conditions.
Induction involves the stimulation of contractions in the absence of spontaneous labor (with or without ruptured membranes), whereas augmentation refers to stimulation of preexisting spontaneous contractions that are considered inadequate because of failed or inadequate cervical dilation and fetal descent.
Women who undergo induction of labor—particularly if nulliparous—are more likely to require cesarean delivery than those who enter labor spontaneously. As the authors of this study point out, it is unclear why induction of labor is associated with an increased risk of cesarean delivery, but it may be related, in part, to the way induced labors are managed.
The incidence of labor induction in the United States more than doubled over the past 20 years. In 2007, more than 20% of all labors were induced in the United States.1 When augmented labors are added to the equation, the sum likely represents half of all pregnancies, so this subject is important to us all.
Details of the study
Enter Harper and colleagues, who focused on women who 1) carried a singleton pregnancy in vertex presentation, 2) reached 10 cm of dilation, and 3) had an umbilical cord gas obtained at delivery. The women were admitted for labor from July 2004 to June 2008 at Washington University Medical Center in St. Louis, Missouri. They had a minimum gestational age of 37 weeks and reached the second stage of labor. Labor and delivery records included information on medications, type of labor, times of cervical examination, extent of cervical dilation, station, duration and curves of the first stage of labor, length of the stages of labor, mode of delivery, and postpartum status.
Of 5,388 women in the cohort, 2,021 entered labor spontaneously, 1,720 had labor augmented, and 1,647 had labor induced. After adjustments for race, obesity, macrosomia, and Bishop score, women who underwent induction of labor spent a significantly longer total time in labor than did women who entered labor spontaneously.
Among nulliparous women, the median (95th percentile) time to progress from 4 cm to 10 cm was 5.5 (16.8) hours when labor was induced versus 3.8 (11.8) hours for spontaneous labors. Among multiparous women, the figures were 4.4 (16.2) hours and 2.4 (8.8) hours, respectively.
The time it took for dilation to increase 1 cm in latent labor (<6 cm dilation) was significantly longer in induced labors, compared with spontaneous labors. However, the time it took for dilation to increase 1 cm in active labor (≥6 cm dilation) was similar between groups.
Strengths and weaknesses of the trial
Induced labor in this cohort was significantly slower than currently accepted definitions of protraction (dilation <1 cm/hr for 4 hr) and arrest disorders (no cervical dilation for 2 hr). And the active phase of labor (defined as an increased rate of cervical dilation) began at 6 cm in this study, much later than previously accepted definitions of 3 to 4 cm.2 If the traditional definitions of active-phase arrest are applied to women whose labors are induced, a significant number of cesarean deliveries may be performed prematurely for arrest disorders.
A strength of this investigation is the large size of the cohort. Patient-level data, including patient characteristics and medication details, enabled the investigators to reconstruct labor curves while adjusting for relevant confounding variables. Methods of cervical ripening (prostaglandins, Foley balloon) were documented, as were indications for induction, making this study generalizable to a wide population.
Harper and colleagues did not stratify their findings by favorability of the cervix at the time of induction. Women who required cervical ripening had a slower labor than did women in spontaneous labor until they reached 6 cm, at which point labor patterns converged. Of interest, women who had a favorable cervix at the time of induction had a faster labor than did women in spontaneous labor, largely as a result of shorter times to reach 6 cm.
As for the women who underwent labor augmentation, the progress of labor before 6 cm was very similar to progress among those whose labor was induced. This finding may reflect misclassification of women between the induction and augmentation groups, or misdiagnosis of labor at the time of admission.
Women were excluded from this study if they did not reach the second stage of labor, because investigators were interested in examining the normal course of labor rather than the need for cesarean delivery. However, this exclusion could have caused selection bias.
Analysis did not begin until women reached 3 cm of dilation, largely because women in spontaneous labor were typically admitted when their cervix had dilated at least 3 cm. The period before 3 cm of dilation seems to be longest when induction of labor occurs in the presence of an unfavorable cervix.
Harper and colleagues confirm a commonly held perception that women undergoing induction of labor spend a longer total time in labor than women who enter labor spontaneously.3,4 Before 6 cm, women undergoing induction of labor may take as long as 10 hours to achieve each centimeter of dilation. This pattern suggests that a diagnosis of arrest of labor before 6 cm of dilation needs to be scrutinized carefully to prevent unnecessary cesarean delivery.
William F. Rayburn, MD, MBA
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Does maternal exposure to magnesium sulfate affect fetal heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
When macrosomia is suspected at term, does induction of labor lower the risk of cesarean delivery?
Jennifer T. Ahn, MD (May 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
Induction of labor is warranted when the benefits of delivery (for the mother or fetus) outweigh the advantages of continuing the pregnancy. Common indications include membrane rupture, gestational hypertension, nonreassuring fetal status, and various maternal medical or fetal conditions.
Induction involves the stimulation of contractions in the absence of spontaneous labor (with or without ruptured membranes), whereas augmentation refers to stimulation of preexisting spontaneous contractions that are considered inadequate because of failed or inadequate cervical dilation and fetal descent.
Women who undergo induction of labor—particularly if nulliparous—are more likely to require cesarean delivery than those who enter labor spontaneously. As the authors of this study point out, it is unclear why induction of labor is associated with an increased risk of cesarean delivery, but it may be related, in part, to the way induced labors are managed.
The incidence of labor induction in the United States more than doubled over the past 20 years. In 2007, more than 20% of all labors were induced in the United States.1 When augmented labors are added to the equation, the sum likely represents half of all pregnancies, so this subject is important to us all.
Details of the study
Enter Harper and colleagues, who focused on women who 1) carried a singleton pregnancy in vertex presentation, 2) reached 10 cm of dilation, and 3) had an umbilical cord gas obtained at delivery. The women were admitted for labor from July 2004 to June 2008 at Washington University Medical Center in St. Louis, Missouri. They had a minimum gestational age of 37 weeks and reached the second stage of labor. Labor and delivery records included information on medications, type of labor, times of cervical examination, extent of cervical dilation, station, duration and curves of the first stage of labor, length of the stages of labor, mode of delivery, and postpartum status.
Of 5,388 women in the cohort, 2,021 entered labor spontaneously, 1,720 had labor augmented, and 1,647 had labor induced. After adjustments for race, obesity, macrosomia, and Bishop score, women who underwent induction of labor spent a significantly longer total time in labor than did women who entered labor spontaneously.
Among nulliparous women, the median (95th percentile) time to progress from 4 cm to 10 cm was 5.5 (16.8) hours when labor was induced versus 3.8 (11.8) hours for spontaneous labors. Among multiparous women, the figures were 4.4 (16.2) hours and 2.4 (8.8) hours, respectively.
The time it took for dilation to increase 1 cm in latent labor (<6 cm dilation) was significantly longer in induced labors, compared with spontaneous labors. However, the time it took for dilation to increase 1 cm in active labor (≥6 cm dilation) was similar between groups.
Strengths and weaknesses of the trial
Induced labor in this cohort was significantly slower than currently accepted definitions of protraction (dilation <1 cm/hr for 4 hr) and arrest disorders (no cervical dilation for 2 hr). And the active phase of labor (defined as an increased rate of cervical dilation) began at 6 cm in this study, much later than previously accepted definitions of 3 to 4 cm.2 If the traditional definitions of active-phase arrest are applied to women whose labors are induced, a significant number of cesarean deliveries may be performed prematurely for arrest disorders.
A strength of this investigation is the large size of the cohort. Patient-level data, including patient characteristics and medication details, enabled the investigators to reconstruct labor curves while adjusting for relevant confounding variables. Methods of cervical ripening (prostaglandins, Foley balloon) were documented, as were indications for induction, making this study generalizable to a wide population.
Harper and colleagues did not stratify their findings by favorability of the cervix at the time of induction. Women who required cervical ripening had a slower labor than did women in spontaneous labor until they reached 6 cm, at which point labor patterns converged. Of interest, women who had a favorable cervix at the time of induction had a faster labor than did women in spontaneous labor, largely as a result of shorter times to reach 6 cm.
As for the women who underwent labor augmentation, the progress of labor before 6 cm was very similar to progress among those whose labor was induced. This finding may reflect misclassification of women between the induction and augmentation groups, or misdiagnosis of labor at the time of admission.
Women were excluded from this study if they did not reach the second stage of labor, because investigators were interested in examining the normal course of labor rather than the need for cesarean delivery. However, this exclusion could have caused selection bias.
Analysis did not begin until women reached 3 cm of dilation, largely because women in spontaneous labor were typically admitted when their cervix had dilated at least 3 cm. The period before 3 cm of dilation seems to be longest when induction of labor occurs in the presence of an unfavorable cervix.
Harper and colleagues confirm a commonly held perception that women undergoing induction of labor spend a longer total time in labor than women who enter labor spontaneously.3,4 Before 6 cm, women undergoing induction of labor may take as long as 10 hours to achieve each centimeter of dilation. This pattern suggests that a diagnosis of arrest of labor before 6 cm of dilation needs to be scrutinized carefully to prevent unnecessary cesarean delivery.
William F. Rayburn, MD, MBA
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Does maternal exposure to magnesium sulfate affect fetal heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
When macrosomia is suspected at term, does induction of labor lower the risk of cesarean delivery?
Jennifer T. Ahn, MD (May 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
1. Induction of labor. ACOG Practice Bulletin #107. August 2009. Obstet Gynecol. 2009;114(2 Pt 1):386-397.
2. Dystocia and augmentation of labor. ACOG Practice Bulletin #49. December 2003. Obstet Gynecol. 2003;102(6):1445-1454.
3. Rinehart BK, Terrone DA, Hudson C, Isler CM, Larmon JE, Perry KG, Jr. Lack of utility of standard labor curves in the prediction of progression during labor induction. Am J Obstet Gynecol. 2000;182(6):1520-1526.
4. Cheng YW, Delaney SS, Hopkins LM, Caughey AB. The association between the length of first stage of labor, mode of delivery, and perinatal outcomes in women undergoing induction of labor. Am J Obstet Gynecol. 2009;201(5):477.e1-e7.
1. Induction of labor. ACOG Practice Bulletin #107. August 2009. Obstet Gynecol. 2009;114(2 Pt 1):386-397.
2. Dystocia and augmentation of labor. ACOG Practice Bulletin #49. December 2003. Obstet Gynecol. 2003;102(6):1445-1454.
3. Rinehart BK, Terrone DA, Hudson C, Isler CM, Larmon JE, Perry KG, Jr. Lack of utility of standard labor curves in the prediction of progression during labor induction. Am J Obstet Gynecol. 2000;182(6):1520-1526.
4. Cheng YW, Delaney SS, Hopkins LM, Caughey AB. The association between the length of first stage of labor, mode of delivery, and perinatal outcomes in women undergoing induction of labor. Am J Obstet Gynecol. 2009;201(5):477.e1-e7.
Assessing Fetal Heart Rate
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
Postpartum Tdap Acceptance Impacts Infant Vaccine Rates
SAN DIEGO – Maternal acceptance of influenza vaccine during pregnancy and postpartum Tdap immunization both increase infant vaccination rates, results from a large single-center study showed.
"The postpartum period may be an optimal time for education of current threats related to vaccine-preventable infection for mothers and infants," lead study author Gina Calarco said in an interview after IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. "Getting obstetricians and pediatricians actively discussing and educating their patients during this critical period appears to benefit both maternal and infant vaccination rates."
Ms. Calarco and her coinvestigators enrolled 900 postpartum women-infant dyads cared for at Shawnee Mission (Kan.) Medical Center in a prospective study intended to evaluate Tdap vaccine acceptance. This particular hospital was targeted as the research site because of its "location in a relatively affluent area with higher education and socioeconomic status and the highest birth rates in the bistate area," explained Ms. Calarco, who led the study during her tenure as infectious disease study coordinator at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. "In past studies this type of demographic has been known to be vaccine hesitant and this is why we chose this facility. We compared maternal acceptance of Tdap to the infant’s 3-month vaccine record to determine if there are any early indicators of vaccine-hesitant parents. Our overall question was to determine if mom declined vaccination of herself, is she more likely to not fully vaccinate her infant, or conversely if a mom accepts Tdap, does that impact her infant’s vaccine status at 3 months of age."
The mean age of the study participants was 28 years, 83% were white, 66% were married, and 67% had private insurance. Ms. Calarco, who is now a clinical project manager with Quintiles in Overland Park, Kan., reported that 597 of the mothers (66%) received Tdap prior to discharge and 269 (30%) declined the vaccine, while 34 (4%) had received Tdap within 2 years and thus were not currently eligible to receive the vaccine prior to discharge.
A total of 757 maternal Tdap and infant vaccine records and 740 maternal influenza and infant vaccine records were available for analysis. The records revealed that mothers who accepted Tdap were 13.5 times more likely to fully immunize their infant than not to do so. In addition, mothers who received the influenza vaccine were 1.85 times more likely to fully immunize their children than were mothers who did not receive the influenza vaccine.
When the researchers adjusted for maternal age, they found that mothers older than 28 years had a 6% per year increase in having a fully vaccinated infant (OR, 1.06) However, the 34 mothers who had Tdap within the previous 2 years were less likely to have a fully vaccinated infant, compared with other mothers in the study (OR, 0.28). "This was surprising in that these women had accepted Tdap for themselves previously yet were not as likely to immunize their infant," Ms. Calarco commented. "We haven’t explored this finding further, but it may be that the education supplied when they received Tdap was too far removed from them vaccinating their infant, or maybe they received a required tetanus booster vaccine due to an accident and this wasn’t as much [of] a choice."
The study was supported by a grant from the Kenneth and Eva Smith Foundation. Ms. Calarco said she had no relevant financial disclosures.
SAN DIEGO – Maternal acceptance of influenza vaccine during pregnancy and postpartum Tdap immunization both increase infant vaccination rates, results from a large single-center study showed.
"The postpartum period may be an optimal time for education of current threats related to vaccine-preventable infection for mothers and infants," lead study author Gina Calarco said in an interview after IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. "Getting obstetricians and pediatricians actively discussing and educating their patients during this critical period appears to benefit both maternal and infant vaccination rates."
Ms. Calarco and her coinvestigators enrolled 900 postpartum women-infant dyads cared for at Shawnee Mission (Kan.) Medical Center in a prospective study intended to evaluate Tdap vaccine acceptance. This particular hospital was targeted as the research site because of its "location in a relatively affluent area with higher education and socioeconomic status and the highest birth rates in the bistate area," explained Ms. Calarco, who led the study during her tenure as infectious disease study coordinator at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. "In past studies this type of demographic has been known to be vaccine hesitant and this is why we chose this facility. We compared maternal acceptance of Tdap to the infant’s 3-month vaccine record to determine if there are any early indicators of vaccine-hesitant parents. Our overall question was to determine if mom declined vaccination of herself, is she more likely to not fully vaccinate her infant, or conversely if a mom accepts Tdap, does that impact her infant’s vaccine status at 3 months of age."
The mean age of the study participants was 28 years, 83% were white, 66% were married, and 67% had private insurance. Ms. Calarco, who is now a clinical project manager with Quintiles in Overland Park, Kan., reported that 597 of the mothers (66%) received Tdap prior to discharge and 269 (30%) declined the vaccine, while 34 (4%) had received Tdap within 2 years and thus were not currently eligible to receive the vaccine prior to discharge.
A total of 757 maternal Tdap and infant vaccine records and 740 maternal influenza and infant vaccine records were available for analysis. The records revealed that mothers who accepted Tdap were 13.5 times more likely to fully immunize their infant than not to do so. In addition, mothers who received the influenza vaccine were 1.85 times more likely to fully immunize their children than were mothers who did not receive the influenza vaccine.
When the researchers adjusted for maternal age, they found that mothers older than 28 years had a 6% per year increase in having a fully vaccinated infant (OR, 1.06) However, the 34 mothers who had Tdap within the previous 2 years were less likely to have a fully vaccinated infant, compared with other mothers in the study (OR, 0.28). "This was surprising in that these women had accepted Tdap for themselves previously yet were not as likely to immunize their infant," Ms. Calarco commented. "We haven’t explored this finding further, but it may be that the education supplied when they received Tdap was too far removed from them vaccinating their infant, or maybe they received a required tetanus booster vaccine due to an accident and this wasn’t as much [of] a choice."
The study was supported by a grant from the Kenneth and Eva Smith Foundation. Ms. Calarco said she had no relevant financial disclosures.
SAN DIEGO – Maternal acceptance of influenza vaccine during pregnancy and postpartum Tdap immunization both increase infant vaccination rates, results from a large single-center study showed.
"The postpartum period may be an optimal time for education of current threats related to vaccine-preventable infection for mothers and infants," lead study author Gina Calarco said in an interview after IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. "Getting obstetricians and pediatricians actively discussing and educating their patients during this critical period appears to benefit both maternal and infant vaccination rates."
Ms. Calarco and her coinvestigators enrolled 900 postpartum women-infant dyads cared for at Shawnee Mission (Kan.) Medical Center in a prospective study intended to evaluate Tdap vaccine acceptance. This particular hospital was targeted as the research site because of its "location in a relatively affluent area with higher education and socioeconomic status and the highest birth rates in the bistate area," explained Ms. Calarco, who led the study during her tenure as infectious disease study coordinator at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. "In past studies this type of demographic has been known to be vaccine hesitant and this is why we chose this facility. We compared maternal acceptance of Tdap to the infant’s 3-month vaccine record to determine if there are any early indicators of vaccine-hesitant parents. Our overall question was to determine if mom declined vaccination of herself, is she more likely to not fully vaccinate her infant, or conversely if a mom accepts Tdap, does that impact her infant’s vaccine status at 3 months of age."
The mean age of the study participants was 28 years, 83% were white, 66% were married, and 67% had private insurance. Ms. Calarco, who is now a clinical project manager with Quintiles in Overland Park, Kan., reported that 597 of the mothers (66%) received Tdap prior to discharge and 269 (30%) declined the vaccine, while 34 (4%) had received Tdap within 2 years and thus were not currently eligible to receive the vaccine prior to discharge.
A total of 757 maternal Tdap and infant vaccine records and 740 maternal influenza and infant vaccine records were available for analysis. The records revealed that mothers who accepted Tdap were 13.5 times more likely to fully immunize their infant than not to do so. In addition, mothers who received the influenza vaccine were 1.85 times more likely to fully immunize their children than were mothers who did not receive the influenza vaccine.
When the researchers adjusted for maternal age, they found that mothers older than 28 years had a 6% per year increase in having a fully vaccinated infant (OR, 1.06) However, the 34 mothers who had Tdap within the previous 2 years were less likely to have a fully vaccinated infant, compared with other mothers in the study (OR, 0.28). "This was surprising in that these women had accepted Tdap for themselves previously yet were not as likely to immunize their infant," Ms. Calarco commented. "We haven’t explored this finding further, but it may be that the education supplied when they received Tdap was too far removed from them vaccinating their infant, or maybe they received a required tetanus booster vaccine due to an accident and this wasn’t as much [of] a choice."
The study was supported by a grant from the Kenneth and Eva Smith Foundation. Ms. Calarco said she had no relevant financial disclosures.
AT IDWEEK 2012
Major Finding: Mothers who accepted the Tdap vaccine were 13.5 times more likely to fully immunize their infant than not to do so. Also, mothers who received the influenza vaccine were 1.85 times more likely to fully immunize their infant than were mothers who did not receive the influenza vaccine.
Data Source: Data are from a prospective study of 900 postpartum women-infant dyads at a single community hospital that evaluated Tdap vaccine acceptance.
Disclosures: The study was supported by a grant from the Kenneth and Eva Smith Foundation. Ms. Calarco said she had no relevant financial disclosures.
Pregnancy Registries: Advantages and Disadvantages
Despite the fact that prescription medications are commonly used by pregnant women, for most products and for new drugs in particular, there is typically little to no human safety information available to aid clinicians and patients in managing risk. As randomized clinical trials are usually not considered ethical to perform in pregnancy, observational epidemiologic studies are often the next best option to address human pregnancy exposure. An increasingly common approach to gathering human safety data is postmarketing pregnancy registries.
These pregnancy registries are initiated many times by agreement between the manufacturer and a regulatory agency as a postmarketing commitment or requirement following shortly after drug approval. Furthermore, because use of a new drug in pregnant women might be relatively rare, a pregnancy registry may be the only feasible method for gathering preliminary safety information as quickly as possible so that potential signals might be detected and clinical decision making can be better informed.
Pregnancy registries vary in design, but all involve collection of data on exposure to the drug of interest in pregnant women, and collection of outcome data for those pregnancies. The primary outcome of interest is typically major congenital anomalies; some registries also collect outcome data on fetal/infant growth, preterm delivery, pregnancy loss, specific neonatal outcomes, and postnatal longer term growth and development. The rates of these outcomes can be compared with general population reference rates, or rates occurring in a specific comparison group that might be more similar to the exposed women, for example, in terms of the underlying maternal condition being treated by the drug.
In addition to early information on a new drug, some of the major advantages of many pregnancy registry designs are the ability to collect information on the exposure and other pregnancy details before the mother knows what the outcome of her pregnancy will be; direct collection of exposure information from the mother herself, so that important factors such as drug and alcohol use, dose, and exact timing of exposure to the drug of interest; information on important other factors such as tobacco, alcohol, and multivitamin use.
The most challenging aspect of pregnancy registries is recruitment, for which registries largely depend on obstetric providers and other specialty physicians. Although low numbers of recruited pregnancies may be caused by limited use of a new drug, clearly most pregnancy registries enroll a very small fraction of all exposed pregnancies that are in existence. A second, related issue is that there may be bias in the self-selection of women who do find out about the registry and agree to participate, thus raising questions about the generalizability of the findings. A third issue is that many registries experience high rates of "lost to follow-up," in which outcome information is unobtainable from the health care provider or the pregnant woman – in some cases as high as 40%. There is also a concern about bias involved with the timing in gestation when a pregnancy enters a registry, such as the later in gestation a pregnancy is enrolled, the more likely that prenatal diagnosis, pregnancy loss, or other adverse outcomes have already occurred – thus making the enrollment essentially retrospective.
Another concern is that few registries have a concurrently enrolled group of unexposed women for purposes of comparison. Thus, their findings are commonly compared to external reference statistics which may not be the most appropriate. Finally, in some registries, the absence of information on individual dose and specific timing in gestation of exposure may preclude evaluating the biological plausibility of any registry findings. All of these issues can lead to long delays in accumulation of sufficient information to draw meaningful inferences, and potential concerns about interpretation of results.
How can awareness of pregnancy registries and more representative enrollment of exposed women be improved? A variety of methods are used to inform physicians and their patients about existing pregnancy registries for the purpose of encouraging referrals, including the Food and Drug Administration website, information in product labeling and on product websites, direct to provider or direct to consumer advertising, and in commonly used resources for clinicians such as this column and Reprotox, an information system developed by the Reproductive Toxicology Center. However, with the rapidly increasing number of registries, it is challenging for physicians to remain current on which medications are being monitored through a registry, what the criteria for enrollment are, and how a physician or patient can find out more. Pregnancy registry designs that are disease based – such as encompassing all medications used to treat a specific disease in pregnancy – help simplify the referral process by broadening the criteria for enrollment. Particularly for specialty physicians, this can ease the burden of identifying eligible women for enrollment.
How can enrollment be accomplished as early as possible in pregnancy (after exposure, but before the outcome is known), and how can more complete ascertainment of exposure and outcome be improved? An approach that some registries have used to address this is by "direct to consumer" campaigns. Registries such as the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Registry require that the pregnant woman herself enroll in the study, and therefore, the study is marketed directly to those women, although physician referral is encouraged. At least in this case, this has led to low rates of lost to follow-up (less than 5%), recruitment timing that is typically before the seventh or eighth week of gestation, and collection of specific information on dose and timing in gestation of exposure.
Multiple drug, disease-based, multiple sponsor registries such as the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drugs in Pregnancy Registry (patients call 888-233-2334), and the National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) offer distinct advantages but are not always feasible for a specific product. A national pregnancy registry for all new drugs has been suggested as another solution to many of the challenges facing single product registries and to streamline referral and follow-up. In addition, including pregnant women in selected preapproval studies has several advantages. Finally, creative new technologies for earlier and more complete ascertainment and referral, such as use of electronic medical records, should be fully explored. The need for safety information on new drugs is urgent.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. Dr. Cohen directs the perinatal psychiatry program at Massachusetts General Hospital, which provides information about pregnancy and mental health. Dr. Koren is professor of pediatrics, pharmacology, pharmacy, and medical genetics at the University of Toronto. He heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk program. Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; a clinical professor of pharmacy at the University of California, San Francisco; and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation." Dr. Cohen is the principal investigator on the National Pregnancy Registry for Atypical Antipsychotics, which is sponsored by multiple atypical antipsychotic manufacturers.
Dr. Chambers, Dr. Koren, and Mr. Briggs said they had no relevant financial disclosures.
Despite the fact that prescription medications are commonly used by pregnant women, for most products and for new drugs in particular, there is typically little to no human safety information available to aid clinicians and patients in managing risk. As randomized clinical trials are usually not considered ethical to perform in pregnancy, observational epidemiologic studies are often the next best option to address human pregnancy exposure. An increasingly common approach to gathering human safety data is postmarketing pregnancy registries.
These pregnancy registries are initiated many times by agreement between the manufacturer and a regulatory agency as a postmarketing commitment or requirement following shortly after drug approval. Furthermore, because use of a new drug in pregnant women might be relatively rare, a pregnancy registry may be the only feasible method for gathering preliminary safety information as quickly as possible so that potential signals might be detected and clinical decision making can be better informed.
Pregnancy registries vary in design, but all involve collection of data on exposure to the drug of interest in pregnant women, and collection of outcome data for those pregnancies. The primary outcome of interest is typically major congenital anomalies; some registries also collect outcome data on fetal/infant growth, preterm delivery, pregnancy loss, specific neonatal outcomes, and postnatal longer term growth and development. The rates of these outcomes can be compared with general population reference rates, or rates occurring in a specific comparison group that might be more similar to the exposed women, for example, in terms of the underlying maternal condition being treated by the drug.
In addition to early information on a new drug, some of the major advantages of many pregnancy registry designs are the ability to collect information on the exposure and other pregnancy details before the mother knows what the outcome of her pregnancy will be; direct collection of exposure information from the mother herself, so that important factors such as drug and alcohol use, dose, and exact timing of exposure to the drug of interest; information on important other factors such as tobacco, alcohol, and multivitamin use.
The most challenging aspect of pregnancy registries is recruitment, for which registries largely depend on obstetric providers and other specialty physicians. Although low numbers of recruited pregnancies may be caused by limited use of a new drug, clearly most pregnancy registries enroll a very small fraction of all exposed pregnancies that are in existence. A second, related issue is that there may be bias in the self-selection of women who do find out about the registry and agree to participate, thus raising questions about the generalizability of the findings. A third issue is that many registries experience high rates of "lost to follow-up," in which outcome information is unobtainable from the health care provider or the pregnant woman – in some cases as high as 40%. There is also a concern about bias involved with the timing in gestation when a pregnancy enters a registry, such as the later in gestation a pregnancy is enrolled, the more likely that prenatal diagnosis, pregnancy loss, or other adverse outcomes have already occurred – thus making the enrollment essentially retrospective.
Another concern is that few registries have a concurrently enrolled group of unexposed women for purposes of comparison. Thus, their findings are commonly compared to external reference statistics which may not be the most appropriate. Finally, in some registries, the absence of information on individual dose and specific timing in gestation of exposure may preclude evaluating the biological plausibility of any registry findings. All of these issues can lead to long delays in accumulation of sufficient information to draw meaningful inferences, and potential concerns about interpretation of results.
How can awareness of pregnancy registries and more representative enrollment of exposed women be improved? A variety of methods are used to inform physicians and their patients about existing pregnancy registries for the purpose of encouraging referrals, including the Food and Drug Administration website, information in product labeling and on product websites, direct to provider or direct to consumer advertising, and in commonly used resources for clinicians such as this column and Reprotox, an information system developed by the Reproductive Toxicology Center. However, with the rapidly increasing number of registries, it is challenging for physicians to remain current on which medications are being monitored through a registry, what the criteria for enrollment are, and how a physician or patient can find out more. Pregnancy registry designs that are disease based – such as encompassing all medications used to treat a specific disease in pregnancy – help simplify the referral process by broadening the criteria for enrollment. Particularly for specialty physicians, this can ease the burden of identifying eligible women for enrollment.
How can enrollment be accomplished as early as possible in pregnancy (after exposure, but before the outcome is known), and how can more complete ascertainment of exposure and outcome be improved? An approach that some registries have used to address this is by "direct to consumer" campaigns. Registries such as the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Registry require that the pregnant woman herself enroll in the study, and therefore, the study is marketed directly to those women, although physician referral is encouraged. At least in this case, this has led to low rates of lost to follow-up (less than 5%), recruitment timing that is typically before the seventh or eighth week of gestation, and collection of specific information on dose and timing in gestation of exposure.
Multiple drug, disease-based, multiple sponsor registries such as the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drugs in Pregnancy Registry (patients call 888-233-2334), and the National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) offer distinct advantages but are not always feasible for a specific product. A national pregnancy registry for all new drugs has been suggested as another solution to many of the challenges facing single product registries and to streamline referral and follow-up. In addition, including pregnant women in selected preapproval studies has several advantages. Finally, creative new technologies for earlier and more complete ascertainment and referral, such as use of electronic medical records, should be fully explored. The need for safety information on new drugs is urgent.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. Dr. Cohen directs the perinatal psychiatry program at Massachusetts General Hospital, which provides information about pregnancy and mental health. Dr. Koren is professor of pediatrics, pharmacology, pharmacy, and medical genetics at the University of Toronto. He heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk program. Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; a clinical professor of pharmacy at the University of California, San Francisco; and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation." Dr. Cohen is the principal investigator on the National Pregnancy Registry for Atypical Antipsychotics, which is sponsored by multiple atypical antipsychotic manufacturers.
Dr. Chambers, Dr. Koren, and Mr. Briggs said they had no relevant financial disclosures.
Despite the fact that prescription medications are commonly used by pregnant women, for most products and for new drugs in particular, there is typically little to no human safety information available to aid clinicians and patients in managing risk. As randomized clinical trials are usually not considered ethical to perform in pregnancy, observational epidemiologic studies are often the next best option to address human pregnancy exposure. An increasingly common approach to gathering human safety data is postmarketing pregnancy registries.
These pregnancy registries are initiated many times by agreement between the manufacturer and a regulatory agency as a postmarketing commitment or requirement following shortly after drug approval. Furthermore, because use of a new drug in pregnant women might be relatively rare, a pregnancy registry may be the only feasible method for gathering preliminary safety information as quickly as possible so that potential signals might be detected and clinical decision making can be better informed.
Pregnancy registries vary in design, but all involve collection of data on exposure to the drug of interest in pregnant women, and collection of outcome data for those pregnancies. The primary outcome of interest is typically major congenital anomalies; some registries also collect outcome data on fetal/infant growth, preterm delivery, pregnancy loss, specific neonatal outcomes, and postnatal longer term growth and development. The rates of these outcomes can be compared with general population reference rates, or rates occurring in a specific comparison group that might be more similar to the exposed women, for example, in terms of the underlying maternal condition being treated by the drug.
In addition to early information on a new drug, some of the major advantages of many pregnancy registry designs are the ability to collect information on the exposure and other pregnancy details before the mother knows what the outcome of her pregnancy will be; direct collection of exposure information from the mother herself, so that important factors such as drug and alcohol use, dose, and exact timing of exposure to the drug of interest; information on important other factors such as tobacco, alcohol, and multivitamin use.
The most challenging aspect of pregnancy registries is recruitment, for which registries largely depend on obstetric providers and other specialty physicians. Although low numbers of recruited pregnancies may be caused by limited use of a new drug, clearly most pregnancy registries enroll a very small fraction of all exposed pregnancies that are in existence. A second, related issue is that there may be bias in the self-selection of women who do find out about the registry and agree to participate, thus raising questions about the generalizability of the findings. A third issue is that many registries experience high rates of "lost to follow-up," in which outcome information is unobtainable from the health care provider or the pregnant woman – in some cases as high as 40%. There is also a concern about bias involved with the timing in gestation when a pregnancy enters a registry, such as the later in gestation a pregnancy is enrolled, the more likely that prenatal diagnosis, pregnancy loss, or other adverse outcomes have already occurred – thus making the enrollment essentially retrospective.
Another concern is that few registries have a concurrently enrolled group of unexposed women for purposes of comparison. Thus, their findings are commonly compared to external reference statistics which may not be the most appropriate. Finally, in some registries, the absence of information on individual dose and specific timing in gestation of exposure may preclude evaluating the biological plausibility of any registry findings. All of these issues can lead to long delays in accumulation of sufficient information to draw meaningful inferences, and potential concerns about interpretation of results.
How can awareness of pregnancy registries and more representative enrollment of exposed women be improved? A variety of methods are used to inform physicians and their patients about existing pregnancy registries for the purpose of encouraging referrals, including the Food and Drug Administration website, information in product labeling and on product websites, direct to provider or direct to consumer advertising, and in commonly used resources for clinicians such as this column and Reprotox, an information system developed by the Reproductive Toxicology Center. However, with the rapidly increasing number of registries, it is challenging for physicians to remain current on which medications are being monitored through a registry, what the criteria for enrollment are, and how a physician or patient can find out more. Pregnancy registry designs that are disease based – such as encompassing all medications used to treat a specific disease in pregnancy – help simplify the referral process by broadening the criteria for enrollment. Particularly for specialty physicians, this can ease the burden of identifying eligible women for enrollment.
How can enrollment be accomplished as early as possible in pregnancy (after exposure, but before the outcome is known), and how can more complete ascertainment of exposure and outcome be improved? An approach that some registries have used to address this is by "direct to consumer" campaigns. Registries such as the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Registry require that the pregnant woman herself enroll in the study, and therefore, the study is marketed directly to those women, although physician referral is encouraged. At least in this case, this has led to low rates of lost to follow-up (less than 5%), recruitment timing that is typically before the seventh or eighth week of gestation, and collection of specific information on dose and timing in gestation of exposure.
Multiple drug, disease-based, multiple sponsor registries such as the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drugs in Pregnancy Registry (patients call 888-233-2334), and the National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) offer distinct advantages but are not always feasible for a specific product. A national pregnancy registry for all new drugs has been suggested as another solution to many of the challenges facing single product registries and to streamline referral and follow-up. In addition, including pregnant women in selected preapproval studies has several advantages. Finally, creative new technologies for earlier and more complete ascertainment and referral, such as use of electronic medical records, should be fully explored. The need for safety information on new drugs is urgent.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. Dr. Cohen directs the perinatal psychiatry program at Massachusetts General Hospital, which provides information about pregnancy and mental health. Dr. Koren is professor of pediatrics, pharmacology, pharmacy, and medical genetics at the University of Toronto. He heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk program. Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; a clinical professor of pharmacy at the University of California, San Francisco; and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation." Dr. Cohen is the principal investigator on the National Pregnancy Registry for Atypical Antipsychotics, which is sponsored by multiple atypical antipsychotic manufacturers.
Dr. Chambers, Dr. Koren, and Mr. Briggs said they had no relevant financial disclosures.
CDC Panel Backs Tdap Vaccination During Every Pregnancy
All pregnant women should receive a dose of the Tdap vaccine, whether she has received the vaccine previously, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended.
At a meeting on Oct. 24, the committee voted 14-0, with one abstention, to support the recommendation proposed by the ACIP pertussis vaccine work group that providers of prenatal care implement a Tdap immunization program for all pregnant women and that health care personnel "should administer a dose of Tdap during each pregnancy, irrespective of the patient’s prior history of receiving Tdap." If not administered, during pregnancy, "Tdap should be administered immediately post partum," according to the recommendation.
The recommendation is a change from the previous recommendation made by ACIP in June 2011, which says that that Tdap should be administered during pregnancy "only to women who have not previously received Tdap." The current recommendation also states that women who do not receive the vaccine during pregnancy should be vaccinated immediately post partum.
Optimally, women should receive Tdap between 27 and 36 weeks’ gestation, to maximize the maternal antibody response and passive transfer of antibodies to the infant, said Dr. Jennifer Liang, the lead CDC member of the ACIP pertussis vaccine work group. Based on work group’s review, the evidence is "reassuring" – that two doses of Tdap are safe, she said.
Dr. Liang said that only about 2.6% of pregnant women are vaccinated with Tdap during pregnancy and that the work group is attempting to remove barriers to improve the uptake of the vaccine.
The work group concluded that Tdap maternal pertussis antibodies would wane greatly between subsequent pregnancies, and that a single Tdap dose during one pregnancy was not sufficient to provide adequate protection for subsequent pregnancies. Considering that the number of children born per woman in the United States is about two, a "very small proportion of women – about 5% – would receive four or more Tdap doses, she said.
The number of expected pertussis cases in the United States this year is expected to be the highest since 1959, with more than 32,000 cases reported to date.* There have been 16 deaths so far, and most were in infants, who also have the highest rates of hospitalization, according to the CDC.
In the United States, the rate overall is 10.6 cases/100,000 population, but the rate varies considerably by state. In infants under age 1 year, the rate ranges from 20-100 cases/100,000.
The work group has requested that the CDC conduct safety studies to address the potential increase in severe adverse events when Tdap is given in subsequent pregnancies, she said.
"This is a great opportunity for obstetricians to help their patients protect their newborns and themselves. I urge all obstetricians to recommend and give Tdap vaccine to their pregnant patients," Dr. Richard Beigi, a member of the American College of Obstetricians and Gynecologists immunization work group and the ACIP pertussis vaccines work group said after the vote. Dr. Beigi was not at the meeting.
There are 15 experts in immunization-related fields on the ACIP committee, which develops written recommendations for the routine administration of vaccines to children and adults in the civilian population.
* This story was updated on 10/26/2012.
All pregnant women should receive a dose of the Tdap vaccine, whether she has received the vaccine previously, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended.
At a meeting on Oct. 24, the committee voted 14-0, with one abstention, to support the recommendation proposed by the ACIP pertussis vaccine work group that providers of prenatal care implement a Tdap immunization program for all pregnant women and that health care personnel "should administer a dose of Tdap during each pregnancy, irrespective of the patient’s prior history of receiving Tdap." If not administered, during pregnancy, "Tdap should be administered immediately post partum," according to the recommendation.
The recommendation is a change from the previous recommendation made by ACIP in June 2011, which says that that Tdap should be administered during pregnancy "only to women who have not previously received Tdap." The current recommendation also states that women who do not receive the vaccine during pregnancy should be vaccinated immediately post partum.
Optimally, women should receive Tdap between 27 and 36 weeks’ gestation, to maximize the maternal antibody response and passive transfer of antibodies to the infant, said Dr. Jennifer Liang, the lead CDC member of the ACIP pertussis vaccine work group. Based on work group’s review, the evidence is "reassuring" – that two doses of Tdap are safe, she said.
Dr. Liang said that only about 2.6% of pregnant women are vaccinated with Tdap during pregnancy and that the work group is attempting to remove barriers to improve the uptake of the vaccine.
The work group concluded that Tdap maternal pertussis antibodies would wane greatly between subsequent pregnancies, and that a single Tdap dose during one pregnancy was not sufficient to provide adequate protection for subsequent pregnancies. Considering that the number of children born per woman in the United States is about two, a "very small proportion of women – about 5% – would receive four or more Tdap doses, she said.
The number of expected pertussis cases in the United States this year is expected to be the highest since 1959, with more than 32,000 cases reported to date.* There have been 16 deaths so far, and most were in infants, who also have the highest rates of hospitalization, according to the CDC.
In the United States, the rate overall is 10.6 cases/100,000 population, but the rate varies considerably by state. In infants under age 1 year, the rate ranges from 20-100 cases/100,000.
The work group has requested that the CDC conduct safety studies to address the potential increase in severe adverse events when Tdap is given in subsequent pregnancies, she said.
"This is a great opportunity for obstetricians to help their patients protect their newborns and themselves. I urge all obstetricians to recommend and give Tdap vaccine to their pregnant patients," Dr. Richard Beigi, a member of the American College of Obstetricians and Gynecologists immunization work group and the ACIP pertussis vaccines work group said after the vote. Dr. Beigi was not at the meeting.
There are 15 experts in immunization-related fields on the ACIP committee, which develops written recommendations for the routine administration of vaccines to children and adults in the civilian population.
* This story was updated on 10/26/2012.
All pregnant women should receive a dose of the Tdap vaccine, whether she has received the vaccine previously, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended.
At a meeting on Oct. 24, the committee voted 14-0, with one abstention, to support the recommendation proposed by the ACIP pertussis vaccine work group that providers of prenatal care implement a Tdap immunization program for all pregnant women and that health care personnel "should administer a dose of Tdap during each pregnancy, irrespective of the patient’s prior history of receiving Tdap." If not administered, during pregnancy, "Tdap should be administered immediately post partum," according to the recommendation.
The recommendation is a change from the previous recommendation made by ACIP in June 2011, which says that that Tdap should be administered during pregnancy "only to women who have not previously received Tdap." The current recommendation also states that women who do not receive the vaccine during pregnancy should be vaccinated immediately post partum.
Optimally, women should receive Tdap between 27 and 36 weeks’ gestation, to maximize the maternal antibody response and passive transfer of antibodies to the infant, said Dr. Jennifer Liang, the lead CDC member of the ACIP pertussis vaccine work group. Based on work group’s review, the evidence is "reassuring" – that two doses of Tdap are safe, she said.
Dr. Liang said that only about 2.6% of pregnant women are vaccinated with Tdap during pregnancy and that the work group is attempting to remove barriers to improve the uptake of the vaccine.
The work group concluded that Tdap maternal pertussis antibodies would wane greatly between subsequent pregnancies, and that a single Tdap dose during one pregnancy was not sufficient to provide adequate protection for subsequent pregnancies. Considering that the number of children born per woman in the United States is about two, a "very small proportion of women – about 5% – would receive four or more Tdap doses, she said.
The number of expected pertussis cases in the United States this year is expected to be the highest since 1959, with more than 32,000 cases reported to date.* There have been 16 deaths so far, and most were in infants, who also have the highest rates of hospitalization, according to the CDC.
In the United States, the rate overall is 10.6 cases/100,000 population, but the rate varies considerably by state. In infants under age 1 year, the rate ranges from 20-100 cases/100,000.
The work group has requested that the CDC conduct safety studies to address the potential increase in severe adverse events when Tdap is given in subsequent pregnancies, she said.
"This is a great opportunity for obstetricians to help their patients protect their newborns and themselves. I urge all obstetricians to recommend and give Tdap vaccine to their pregnant patients," Dr. Richard Beigi, a member of the American College of Obstetricians and Gynecologists immunization work group and the ACIP pertussis vaccines work group said after the vote. Dr. Beigi was not at the meeting.
There are 15 experts in immunization-related fields on the ACIP committee, which develops written recommendations for the routine administration of vaccines to children and adults in the civilian population.
* This story was updated on 10/26/2012.
FROM A MEETING OF THE CENTERS FOR DISEASE CONTROL AND PREVENTION'S ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES