User login
New Staples Add to Options for Cesarean Section
SAN DIEGO – Of 500 consecutive cesarean deliveries closed with subcuticular absorbable staples, only one hematoma occurred, and the overall surgical site infection rate was 1.2%, according to study findings.
"This study was surprising," lead investigator Dr. Kirk A. Shibley said in an interview before a poster session at the annual meeting of the American College of Obstetricians and Gynecologists. "I anticipated a low infection rate due to the interrupted and subcuticular nature of the technology, but I did not anticipate that the number of other wound complications would be so low. That is to say that the number of wound hematomas and seromas and separations is almost nonexistent."
Dr. Shibley, who practices obstetrics and gynecology in Edina, Minn., and his associates evaluated data from 500 consecutive cesarean procedures closed with Incisive Surgical’s INSORB absorbable staples. All the operations were performed by five clinicians in a single obstetrics practice during a 4-year period that ended in 2008. The investigators obtained the data from clinic medical records and from 30-day postdischarge infection surveillance programs at two community hospitals.
Dr. Shibley reported that there were only six surgical site infections, for a rate of 1.2%. There was one hematoma, no seromas, and no wound disruptions. In follow-up visits, he said, patients expressed a high level of satisfaction with the results. "The take-home message is that this skin closure technology is fast, and provides low-maintenance, cosmetic wounds with a very low infection and complication rate," he said.
In the poster, Dr. Shibley and his associates postulated that the improved outcomes may be due in part to the fact that the INSORB staples are made of a benign copolymer "of predominately polylactic acid, shown to elicit a very low inflammatory response in animal and human clinical studies, and may account for decreased pain, serous exudate, and other associated complications."
Also, they continued, "absorbable staples are placed entirely within the dermis without the percutaneous insult of metal staples. Absorbable staples avoid the associated patient discomfort and anxiety associated with metal staple removal. The absorbable staples precisely and effectively secure the dermis without tissue strangulation or compression that can occur with ... metal staples."
Dr. Shibley acknowledged that the study is limited as patients were not prospectively randomized to different closure types.
Dr. Shibley disclosed that he is a paid consultant to Incisive Surgical.
SAN DIEGO – Of 500 consecutive cesarean deliveries closed with subcuticular absorbable staples, only one hematoma occurred, and the overall surgical site infection rate was 1.2%, according to study findings.
"This study was surprising," lead investigator Dr. Kirk A. Shibley said in an interview before a poster session at the annual meeting of the American College of Obstetricians and Gynecologists. "I anticipated a low infection rate due to the interrupted and subcuticular nature of the technology, but I did not anticipate that the number of other wound complications would be so low. That is to say that the number of wound hematomas and seromas and separations is almost nonexistent."
Dr. Shibley, who practices obstetrics and gynecology in Edina, Minn., and his associates evaluated data from 500 consecutive cesarean procedures closed with Incisive Surgical’s INSORB absorbable staples. All the operations were performed by five clinicians in a single obstetrics practice during a 4-year period that ended in 2008. The investigators obtained the data from clinic medical records and from 30-day postdischarge infection surveillance programs at two community hospitals.
Dr. Shibley reported that there were only six surgical site infections, for a rate of 1.2%. There was one hematoma, no seromas, and no wound disruptions. In follow-up visits, he said, patients expressed a high level of satisfaction with the results. "The take-home message is that this skin closure technology is fast, and provides low-maintenance, cosmetic wounds with a very low infection and complication rate," he said.
In the poster, Dr. Shibley and his associates postulated that the improved outcomes may be due in part to the fact that the INSORB staples are made of a benign copolymer "of predominately polylactic acid, shown to elicit a very low inflammatory response in animal and human clinical studies, and may account for decreased pain, serous exudate, and other associated complications."
Also, they continued, "absorbable staples are placed entirely within the dermis without the percutaneous insult of metal staples. Absorbable staples avoid the associated patient discomfort and anxiety associated with metal staple removal. The absorbable staples precisely and effectively secure the dermis without tissue strangulation or compression that can occur with ... metal staples."
Dr. Shibley acknowledged that the study is limited as patients were not prospectively randomized to different closure types.
Dr. Shibley disclosed that he is a paid consultant to Incisive Surgical.
SAN DIEGO – Of 500 consecutive cesarean deliveries closed with subcuticular absorbable staples, only one hematoma occurred, and the overall surgical site infection rate was 1.2%, according to study findings.
"This study was surprising," lead investigator Dr. Kirk A. Shibley said in an interview before a poster session at the annual meeting of the American College of Obstetricians and Gynecologists. "I anticipated a low infection rate due to the interrupted and subcuticular nature of the technology, but I did not anticipate that the number of other wound complications would be so low. That is to say that the number of wound hematomas and seromas and separations is almost nonexistent."
Dr. Shibley, who practices obstetrics and gynecology in Edina, Minn., and his associates evaluated data from 500 consecutive cesarean procedures closed with Incisive Surgical’s INSORB absorbable staples. All the operations were performed by five clinicians in a single obstetrics practice during a 4-year period that ended in 2008. The investigators obtained the data from clinic medical records and from 30-day postdischarge infection surveillance programs at two community hospitals.
Dr. Shibley reported that there were only six surgical site infections, for a rate of 1.2%. There was one hematoma, no seromas, and no wound disruptions. In follow-up visits, he said, patients expressed a high level of satisfaction with the results. "The take-home message is that this skin closure technology is fast, and provides low-maintenance, cosmetic wounds with a very low infection and complication rate," he said.
In the poster, Dr. Shibley and his associates postulated that the improved outcomes may be due in part to the fact that the INSORB staples are made of a benign copolymer "of predominately polylactic acid, shown to elicit a very low inflammatory response in animal and human clinical studies, and may account for decreased pain, serous exudate, and other associated complications."
Also, they continued, "absorbable staples are placed entirely within the dermis without the percutaneous insult of metal staples. Absorbable staples avoid the associated patient discomfort and anxiety associated with metal staple removal. The absorbable staples precisely and effectively secure the dermis without tissue strangulation or compression that can occur with ... metal staples."
Dr. Shibley acknowledged that the study is limited as patients were not prospectively randomized to different closure types.
Dr. Shibley disclosed that he is a paid consultant to Incisive Surgical.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF OBSTETRICIANS AND GYNECOLOGISTS
Maternal Age, Obesity Associated With Adverse Pregnancy Outcomes
SAN DIEGO – Although women who delay pregnancy to age 40 years and older face an increased risk for adverse outcomes, reducing prepregnancy body mass index may reduce their risk for cesarean delivery, gestational diabetes, gestational hypertension/preeclampsia, and preterm delivery, results from a large study demonstrated.
"This is an important issue in the United States as more than 13% of pregnant women are now age 35 and older, and nearly 3% are age 40 and older at delivery," Dr. John R. Barton said in an interview prior to a poster session at the annual meeting of the American College of Obstetricians and Gynecologists. "These data underscore the impact of obesity on increasing adverse outcomes in otherwise healthy women."
To examine pregnancy outcomes of healthy nulliparous women aged 40 years or older at delivery, Dr. Barton, director of maternal-fetal medicine at Central Baptist Hospital, Lexington, Ky., and his associates evaluated 53,480 women who were voluntarily enrolled in a pregnancy risk assessment and education program operated by Alere Health between July 1, 2006, and Aug. 1, 2011.
The researchers excluded women who reported heart disease, chronic hypertension, pregestational diabetes, other medical disorders, tobacco use, and conception with assistive reproductive technology. Data were grouped by maternal age (20-29 years or 40 and older) and obesity. Those with a prepregnancy body mass index (PPBMI) of 30 kg/m2 or higher were defined as obese; those with lower BMIs were defined a nonobese.
Within each PPBMI group, nulliparous women 40 years of age and older delivered at a significantly lower gestational age, had a greater incidence of cesarean delivery, gestational diabetes, preterm birth, and both low and very-low birth weight infants, compared with controls aged 20-29 years. In addition, obesity was associated with higher rates of adverse pregnancy outcomes in the group of women aged 40 years and older.
"Throughout the reproductive years, health care providers to women should support and encourage them to maintain a healthy lifestyle and a healthy body habitus," Dr. Barton advised. "In healthy nulliparous women, both advanced maternal age and obesity influence pregnancy outcomes. Women choosing to delay pregnancy until age 40 years and older may modify their risk for cesarean delivery, gestational diabetes, gestational hypertension/preeclampsia, and preterm delivery by reducing their body mass index to nonobese levels prior to conception."
Dr. Barton acknowledged certain limitations of the study, including the fact that while the population included both Medicaid and commercially insured women from across the United States, "we do not know for sure if the same results would be observed in the general population or for those with later prenatal care initiation. It is also important to stress that these women were healthy in that they did not have heart disease, chronic hypertension, diabetes, or other medical disorders such as kidney disease or autoimmune disorders. We also did not include women that reported smoking during pregnancy. The overall rate of obesity was 17.7%, which is not comparable with the general obstetrical population (about 35%) – yet reflects our inclusion/exclusion criteria."
Dr. Barton disclosed that he has received research support from Alere San Diego, a subsidiary of Alere Health. Another study investigator, Dr. Baha M. Sibai, is a consultant for Alere San Diego.
SAN DIEGO – Although women who delay pregnancy to age 40 years and older face an increased risk for adverse outcomes, reducing prepregnancy body mass index may reduce their risk for cesarean delivery, gestational diabetes, gestational hypertension/preeclampsia, and preterm delivery, results from a large study demonstrated.
"This is an important issue in the United States as more than 13% of pregnant women are now age 35 and older, and nearly 3% are age 40 and older at delivery," Dr. John R. Barton said in an interview prior to a poster session at the annual meeting of the American College of Obstetricians and Gynecologists. "These data underscore the impact of obesity on increasing adverse outcomes in otherwise healthy women."
To examine pregnancy outcomes of healthy nulliparous women aged 40 years or older at delivery, Dr. Barton, director of maternal-fetal medicine at Central Baptist Hospital, Lexington, Ky., and his associates evaluated 53,480 women who were voluntarily enrolled in a pregnancy risk assessment and education program operated by Alere Health between July 1, 2006, and Aug. 1, 2011.
The researchers excluded women who reported heart disease, chronic hypertension, pregestational diabetes, other medical disorders, tobacco use, and conception with assistive reproductive technology. Data were grouped by maternal age (20-29 years or 40 and older) and obesity. Those with a prepregnancy body mass index (PPBMI) of 30 kg/m2 or higher were defined as obese; those with lower BMIs were defined a nonobese.
Within each PPBMI group, nulliparous women 40 years of age and older delivered at a significantly lower gestational age, had a greater incidence of cesarean delivery, gestational diabetes, preterm birth, and both low and very-low birth weight infants, compared with controls aged 20-29 years. In addition, obesity was associated with higher rates of adverse pregnancy outcomes in the group of women aged 40 years and older.
"Throughout the reproductive years, health care providers to women should support and encourage them to maintain a healthy lifestyle and a healthy body habitus," Dr. Barton advised. "In healthy nulliparous women, both advanced maternal age and obesity influence pregnancy outcomes. Women choosing to delay pregnancy until age 40 years and older may modify their risk for cesarean delivery, gestational diabetes, gestational hypertension/preeclampsia, and preterm delivery by reducing their body mass index to nonobese levels prior to conception."
Dr. Barton acknowledged certain limitations of the study, including the fact that while the population included both Medicaid and commercially insured women from across the United States, "we do not know for sure if the same results would be observed in the general population or for those with later prenatal care initiation. It is also important to stress that these women were healthy in that they did not have heart disease, chronic hypertension, diabetes, or other medical disorders such as kidney disease or autoimmune disorders. We also did not include women that reported smoking during pregnancy. The overall rate of obesity was 17.7%, which is not comparable with the general obstetrical population (about 35%) – yet reflects our inclusion/exclusion criteria."
Dr. Barton disclosed that he has received research support from Alere San Diego, a subsidiary of Alere Health. Another study investigator, Dr. Baha M. Sibai, is a consultant for Alere San Diego.
SAN DIEGO – Although women who delay pregnancy to age 40 years and older face an increased risk for adverse outcomes, reducing prepregnancy body mass index may reduce their risk for cesarean delivery, gestational diabetes, gestational hypertension/preeclampsia, and preterm delivery, results from a large study demonstrated.
"This is an important issue in the United States as more than 13% of pregnant women are now age 35 and older, and nearly 3% are age 40 and older at delivery," Dr. John R. Barton said in an interview prior to a poster session at the annual meeting of the American College of Obstetricians and Gynecologists. "These data underscore the impact of obesity on increasing adverse outcomes in otherwise healthy women."
To examine pregnancy outcomes of healthy nulliparous women aged 40 years or older at delivery, Dr. Barton, director of maternal-fetal medicine at Central Baptist Hospital, Lexington, Ky., and his associates evaluated 53,480 women who were voluntarily enrolled in a pregnancy risk assessment and education program operated by Alere Health between July 1, 2006, and Aug. 1, 2011.
The researchers excluded women who reported heart disease, chronic hypertension, pregestational diabetes, other medical disorders, tobacco use, and conception with assistive reproductive technology. Data were grouped by maternal age (20-29 years or 40 and older) and obesity. Those with a prepregnancy body mass index (PPBMI) of 30 kg/m2 or higher were defined as obese; those with lower BMIs were defined a nonobese.
Within each PPBMI group, nulliparous women 40 years of age and older delivered at a significantly lower gestational age, had a greater incidence of cesarean delivery, gestational diabetes, preterm birth, and both low and very-low birth weight infants, compared with controls aged 20-29 years. In addition, obesity was associated with higher rates of adverse pregnancy outcomes in the group of women aged 40 years and older.
"Throughout the reproductive years, health care providers to women should support and encourage them to maintain a healthy lifestyle and a healthy body habitus," Dr. Barton advised. "In healthy nulliparous women, both advanced maternal age and obesity influence pregnancy outcomes. Women choosing to delay pregnancy until age 40 years and older may modify their risk for cesarean delivery, gestational diabetes, gestational hypertension/preeclampsia, and preterm delivery by reducing their body mass index to nonobese levels prior to conception."
Dr. Barton acknowledged certain limitations of the study, including the fact that while the population included both Medicaid and commercially insured women from across the United States, "we do not know for sure if the same results would be observed in the general population or for those with later prenatal care initiation. It is also important to stress that these women were healthy in that they did not have heart disease, chronic hypertension, diabetes, or other medical disorders such as kidney disease or autoimmune disorders. We also did not include women that reported smoking during pregnancy. The overall rate of obesity was 17.7%, which is not comparable with the general obstetrical population (about 35%) – yet reflects our inclusion/exclusion criteria."
Dr. Barton disclosed that he has received research support from Alere San Diego, a subsidiary of Alere Health. Another study investigator, Dr. Baha M. Sibai, is a consultant for Alere San Diego.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF OBSTETRICIANS AND GYNECOLOGISTS
Major Finding: A significantly higher proportion of obese women aged 40 years or older underwent primary cesarean delivery, compared with obese women aged 20-29 years (69.3% vs. 47%, respectively). A similar age-related association was observed among nonobese women (55.4% vs. 28.2%).
Data Source: Data were from a study of 53,480 nulliparous women aged either 20-29 years or 40 years and older who were voluntarily enrolled in a pregnancy risk assessment and education program.
Disclosures: Dr. Barton disclosed that he has received research support from Alere San Diego, a subsidiary of Alere Health. Another study investigator, Dr. Baha M. Sibai, is a consultant for Alere San Diego.
Rethinking Obstetric Management in Congenital Heart Disease
CHICAGO – The conventional teaching that pregnant women with congenital heart disease should undergo a passive second stage of labor assisted by forceps or vacuum delivery has been called into question.
Avoidance of active pushing during labor by such patients has long been recommended because of theoretical concerns that the reduction in preload and increased myocardial oxygen requirement that occur with the Valsalva maneuver place women with congenital heart disease at increased risk for cardiac events. But the issue has never actually been studied – until recently, noted Dr. Katherine E. Economy, a maternal-fetal medicine specialist at Brigham and Women’s Hospital, Boston.
"We’ve looked at this in our institution, and what we found is that avoiding Valsalva is associated with worse maternal outcomes: more third- and fourth-degree lacerations and more postpartum hemorrhages. So although the patient numbers were small, this has really encouraged us to move away from doing assisted second stage," she said at the annual meeting of the American College of Cardiology.
Other dogmata widely accepted by cardiologists, obstetricians, and anesthesiologists are that pregnant women with congenital heart disease should routinely be delivered a few weeks early, and by cesarean section. Dr. Economy challenged both notions.
For the Valsalva study, she and her coinvestigators carried out a retrospective cohort study including 112 pregnancies in 65 women with congenital heart disease who were delivered at the hospital during 1998-2005. The focus was on evaluating obstetric outcomes, as the great majority of previous studies of pregnancy outcomes in patients with congenital heart disease addressed maternal cardiac events.
Roughly three-quarters of the women were instructed not to push during the second stage of labor; they underwent a planned assisted delivery in accord with conventional teaching. However, during the study period a shift in practice philosophy occurred in the Boston congenital heart obstetric service, such that women were allowed a trial of pushing on an individualized basis.
Among the 62 pregnancies that reached the second stage of labor, nine (20%) postpartum hemorrhages and seven (16%) third- or fourth-degree lacerations occurred among 45 no-Valsalva patients, compared with none in 17 (0%) women who pushed during labor. The only maternal adverse cardiac event (2%) occurred in a woman who did not do the Valsalva maneuver.
Adverse obstetric events occurred in one-third of women. However, a multivariate analysis didn’t identify any reliable independent predictors for sustaining an adverse obstetric event. Thus, women with congenital heart disease who move forward with pregnancy are at an overall increased risk for adverse obstetric outcomes, but baseline maternal cardiac factors aren’t helpful in picking out a higher-risk subgroup.
The most common adverse obstetric outcome was preterm delivery; the 21% incidence was nearly twice that seen in the general population. Also noteworthy were the 14% incidence of postpartum hemorrhage and the 10% rate of preterm premature rupture of membranes (Int. J. Cardiol. 2010;144:195-9).
Dr. Economy observed that with growing numbers of women with congenital heart disease who survive well into their childbearing years, congenital heart disease now accounts for more than 50% of heart disease in pregnancy. And although maternal deaths due to hemorrhage or venous thromboembolism have fallen sharply over the last 20 years, maternal deaths from cardiovascular disease have risen. Indeed, cardiac disease in pregnancy is now the leading cause of indirect maternal mortality.
"So we think of this in maternal-fetal medicine as a major public health issue," the ob.gyn. said.
She noted that in discussing the possibility of termination in the event of unplanned pregnancy in a patient with congenital heart disease, it’s important to understand that by the second trimester, many of the cardiovascular changes in pregnancy – including a 30%-50% increase in cardiac output, a drop in systemic vascular resistance, and an increase in heart rate – will already have occurred. At that point, the maternal risk may not be altered all that much by terminating.
Managing maternal cardiac risk in patients with congenital aortic root dilatation in accord with joint multispecialty society-backed guidelines (J. Am. Coll. Cardiol. 2010;55:1509-44) entails strict blood pressure control with a beta-blocker, the discontinuation of angiotensin receptor blocker therapy, a monthly or bimonthly echocardiography, an MRI without gadolinium, and delivery in a center with cardiac surgery backup.
In the management of obstetric risk in patients with aortic disease, Dr. Economy recommends a first trimester ultrasound for dating, sequential cervical length measurements beginning at 16 weeks, serious consideration of cerclage placement if the cervix shortens, and ultrasound for fetal growth surveillance.
Interestingly, patients with Marfan syndrome or other connective tissue disorders associated with aortic disease have a high rate of cervical incompetence (Placenta 2009;30:207-15). That’s probably because the cervix is 90% collagen; thus, the cervix may be affected by the same genetic defects that lead to other, more familiar manifestations of disordered connective tissue synthesis and metabolism, she explained.
Timing of delivery is individualized based upon cardiac status, gestational age, Bishop score, and other factors.
"Many of you probably start to lose your nerve a bit at the end and say, ‘Pregnancy is bad for heart disease; we should just deliver.’ But generally speaking, if your patients are doing well in the third trimester, there’s really no reason to induce prematurity," Dr. Economy asserted.
She cited a large multicenter study that has turned heads in the world of maternal-fetal medicine. The study showed significantly increased rates of NICU admission, newborn sepsis, and respiratory complications requiring prolonged intubation with delivery at 37-38 weeks’ gestation, compared with 39 weeks’, in a broad population of pregnant women (N. Engl. J. Med. 2009;360:111-20).
"If your patients are doing well, let them stay pregnant," the ob.gyn. urged.
Cesarean section is really popular in patients with congenital heart disease. The joint guidelines state, "Fetal delivery via cesarean section is reasonable for patients with significant aortic enlargement, dissection, or severe aortic valve regurgitation" (Circulation 2010;121:1544-79). But Dr. Economy pointed out that this recommendation is rated class II, level of evidence C, meaning that it is based solely on expert opinion. And these joint guidelines were drawn up and approved by numerous cardiovascular and imaging societies without the endorsement of any obstetric organizations.
"I would put to you that every time you think about a cesarean section, you stop and remember that cesarean section is worse for women. For all women. C-section is worse for them, okay? It increases the risk of significant blood loss, increases infection risk, and increases the risk of venous thromboembolism," she said.
"My personal opinion is cesarean section should be reserved for obstetric indications – things like failure to progress, breech presentation, or nonreassuring fetal status in labor. The vast majority of patients will be better served by vaginal delivery. Plan on an interdisciplinary effort between obstetrics, cardiology, anesthesiology, and nursing," Dr. Economy advised.
Dr. Economy and her associates reported that they had no relevant financial disclosures.
CHICAGO – The conventional teaching that pregnant women with congenital heart disease should undergo a passive second stage of labor assisted by forceps or vacuum delivery has been called into question.
Avoidance of active pushing during labor by such patients has long been recommended because of theoretical concerns that the reduction in preload and increased myocardial oxygen requirement that occur with the Valsalva maneuver place women with congenital heart disease at increased risk for cardiac events. But the issue has never actually been studied – until recently, noted Dr. Katherine E. Economy, a maternal-fetal medicine specialist at Brigham and Women’s Hospital, Boston.
"We’ve looked at this in our institution, and what we found is that avoiding Valsalva is associated with worse maternal outcomes: more third- and fourth-degree lacerations and more postpartum hemorrhages. So although the patient numbers were small, this has really encouraged us to move away from doing assisted second stage," she said at the annual meeting of the American College of Cardiology.
Other dogmata widely accepted by cardiologists, obstetricians, and anesthesiologists are that pregnant women with congenital heart disease should routinely be delivered a few weeks early, and by cesarean section. Dr. Economy challenged both notions.
For the Valsalva study, she and her coinvestigators carried out a retrospective cohort study including 112 pregnancies in 65 women with congenital heart disease who were delivered at the hospital during 1998-2005. The focus was on evaluating obstetric outcomes, as the great majority of previous studies of pregnancy outcomes in patients with congenital heart disease addressed maternal cardiac events.
Roughly three-quarters of the women were instructed not to push during the second stage of labor; they underwent a planned assisted delivery in accord with conventional teaching. However, during the study period a shift in practice philosophy occurred in the Boston congenital heart obstetric service, such that women were allowed a trial of pushing on an individualized basis.
Among the 62 pregnancies that reached the second stage of labor, nine (20%) postpartum hemorrhages and seven (16%) third- or fourth-degree lacerations occurred among 45 no-Valsalva patients, compared with none in 17 (0%) women who pushed during labor. The only maternal adverse cardiac event (2%) occurred in a woman who did not do the Valsalva maneuver.
Adverse obstetric events occurred in one-third of women. However, a multivariate analysis didn’t identify any reliable independent predictors for sustaining an adverse obstetric event. Thus, women with congenital heart disease who move forward with pregnancy are at an overall increased risk for adverse obstetric outcomes, but baseline maternal cardiac factors aren’t helpful in picking out a higher-risk subgroup.
The most common adverse obstetric outcome was preterm delivery; the 21% incidence was nearly twice that seen in the general population. Also noteworthy were the 14% incidence of postpartum hemorrhage and the 10% rate of preterm premature rupture of membranes (Int. J. Cardiol. 2010;144:195-9).
Dr. Economy observed that with growing numbers of women with congenital heart disease who survive well into their childbearing years, congenital heart disease now accounts for more than 50% of heart disease in pregnancy. And although maternal deaths due to hemorrhage or venous thromboembolism have fallen sharply over the last 20 years, maternal deaths from cardiovascular disease have risen. Indeed, cardiac disease in pregnancy is now the leading cause of indirect maternal mortality.
"So we think of this in maternal-fetal medicine as a major public health issue," the ob.gyn. said.
She noted that in discussing the possibility of termination in the event of unplanned pregnancy in a patient with congenital heart disease, it’s important to understand that by the second trimester, many of the cardiovascular changes in pregnancy – including a 30%-50% increase in cardiac output, a drop in systemic vascular resistance, and an increase in heart rate – will already have occurred. At that point, the maternal risk may not be altered all that much by terminating.
Managing maternal cardiac risk in patients with congenital aortic root dilatation in accord with joint multispecialty society-backed guidelines (J. Am. Coll. Cardiol. 2010;55:1509-44) entails strict blood pressure control with a beta-blocker, the discontinuation of angiotensin receptor blocker therapy, a monthly or bimonthly echocardiography, an MRI without gadolinium, and delivery in a center with cardiac surgery backup.
In the management of obstetric risk in patients with aortic disease, Dr. Economy recommends a first trimester ultrasound for dating, sequential cervical length measurements beginning at 16 weeks, serious consideration of cerclage placement if the cervix shortens, and ultrasound for fetal growth surveillance.
Interestingly, patients with Marfan syndrome or other connective tissue disorders associated with aortic disease have a high rate of cervical incompetence (Placenta 2009;30:207-15). That’s probably because the cervix is 90% collagen; thus, the cervix may be affected by the same genetic defects that lead to other, more familiar manifestations of disordered connective tissue synthesis and metabolism, she explained.
Timing of delivery is individualized based upon cardiac status, gestational age, Bishop score, and other factors.
"Many of you probably start to lose your nerve a bit at the end and say, ‘Pregnancy is bad for heart disease; we should just deliver.’ But generally speaking, if your patients are doing well in the third trimester, there’s really no reason to induce prematurity," Dr. Economy asserted.
She cited a large multicenter study that has turned heads in the world of maternal-fetal medicine. The study showed significantly increased rates of NICU admission, newborn sepsis, and respiratory complications requiring prolonged intubation with delivery at 37-38 weeks’ gestation, compared with 39 weeks’, in a broad population of pregnant women (N. Engl. J. Med. 2009;360:111-20).
"If your patients are doing well, let them stay pregnant," the ob.gyn. urged.
Cesarean section is really popular in patients with congenital heart disease. The joint guidelines state, "Fetal delivery via cesarean section is reasonable for patients with significant aortic enlargement, dissection, or severe aortic valve regurgitation" (Circulation 2010;121:1544-79). But Dr. Economy pointed out that this recommendation is rated class II, level of evidence C, meaning that it is based solely on expert opinion. And these joint guidelines were drawn up and approved by numerous cardiovascular and imaging societies without the endorsement of any obstetric organizations.
"I would put to you that every time you think about a cesarean section, you stop and remember that cesarean section is worse for women. For all women. C-section is worse for them, okay? It increases the risk of significant blood loss, increases infection risk, and increases the risk of venous thromboembolism," she said.
"My personal opinion is cesarean section should be reserved for obstetric indications – things like failure to progress, breech presentation, or nonreassuring fetal status in labor. The vast majority of patients will be better served by vaginal delivery. Plan on an interdisciplinary effort between obstetrics, cardiology, anesthesiology, and nursing," Dr. Economy advised.
Dr. Economy and her associates reported that they had no relevant financial disclosures.
CHICAGO – The conventional teaching that pregnant women with congenital heart disease should undergo a passive second stage of labor assisted by forceps or vacuum delivery has been called into question.
Avoidance of active pushing during labor by such patients has long been recommended because of theoretical concerns that the reduction in preload and increased myocardial oxygen requirement that occur with the Valsalva maneuver place women with congenital heart disease at increased risk for cardiac events. But the issue has never actually been studied – until recently, noted Dr. Katherine E. Economy, a maternal-fetal medicine specialist at Brigham and Women’s Hospital, Boston.
"We’ve looked at this in our institution, and what we found is that avoiding Valsalva is associated with worse maternal outcomes: more third- and fourth-degree lacerations and more postpartum hemorrhages. So although the patient numbers were small, this has really encouraged us to move away from doing assisted second stage," she said at the annual meeting of the American College of Cardiology.
Other dogmata widely accepted by cardiologists, obstetricians, and anesthesiologists are that pregnant women with congenital heart disease should routinely be delivered a few weeks early, and by cesarean section. Dr. Economy challenged both notions.
For the Valsalva study, she and her coinvestigators carried out a retrospective cohort study including 112 pregnancies in 65 women with congenital heart disease who were delivered at the hospital during 1998-2005. The focus was on evaluating obstetric outcomes, as the great majority of previous studies of pregnancy outcomes in patients with congenital heart disease addressed maternal cardiac events.
Roughly three-quarters of the women were instructed not to push during the second stage of labor; they underwent a planned assisted delivery in accord with conventional teaching. However, during the study period a shift in practice philosophy occurred in the Boston congenital heart obstetric service, such that women were allowed a trial of pushing on an individualized basis.
Among the 62 pregnancies that reached the second stage of labor, nine (20%) postpartum hemorrhages and seven (16%) third- or fourth-degree lacerations occurred among 45 no-Valsalva patients, compared with none in 17 (0%) women who pushed during labor. The only maternal adverse cardiac event (2%) occurred in a woman who did not do the Valsalva maneuver.
Adverse obstetric events occurred in one-third of women. However, a multivariate analysis didn’t identify any reliable independent predictors for sustaining an adverse obstetric event. Thus, women with congenital heart disease who move forward with pregnancy are at an overall increased risk for adverse obstetric outcomes, but baseline maternal cardiac factors aren’t helpful in picking out a higher-risk subgroup.
The most common adverse obstetric outcome was preterm delivery; the 21% incidence was nearly twice that seen in the general population. Also noteworthy were the 14% incidence of postpartum hemorrhage and the 10% rate of preterm premature rupture of membranes (Int. J. Cardiol. 2010;144:195-9).
Dr. Economy observed that with growing numbers of women with congenital heart disease who survive well into their childbearing years, congenital heart disease now accounts for more than 50% of heart disease in pregnancy. And although maternal deaths due to hemorrhage or venous thromboembolism have fallen sharply over the last 20 years, maternal deaths from cardiovascular disease have risen. Indeed, cardiac disease in pregnancy is now the leading cause of indirect maternal mortality.
"So we think of this in maternal-fetal medicine as a major public health issue," the ob.gyn. said.
She noted that in discussing the possibility of termination in the event of unplanned pregnancy in a patient with congenital heart disease, it’s important to understand that by the second trimester, many of the cardiovascular changes in pregnancy – including a 30%-50% increase in cardiac output, a drop in systemic vascular resistance, and an increase in heart rate – will already have occurred. At that point, the maternal risk may not be altered all that much by terminating.
Managing maternal cardiac risk in patients with congenital aortic root dilatation in accord with joint multispecialty society-backed guidelines (J. Am. Coll. Cardiol. 2010;55:1509-44) entails strict blood pressure control with a beta-blocker, the discontinuation of angiotensin receptor blocker therapy, a monthly or bimonthly echocardiography, an MRI without gadolinium, and delivery in a center with cardiac surgery backup.
In the management of obstetric risk in patients with aortic disease, Dr. Economy recommends a first trimester ultrasound for dating, sequential cervical length measurements beginning at 16 weeks, serious consideration of cerclage placement if the cervix shortens, and ultrasound for fetal growth surveillance.
Interestingly, patients with Marfan syndrome or other connective tissue disorders associated with aortic disease have a high rate of cervical incompetence (Placenta 2009;30:207-15). That’s probably because the cervix is 90% collagen; thus, the cervix may be affected by the same genetic defects that lead to other, more familiar manifestations of disordered connective tissue synthesis and metabolism, she explained.
Timing of delivery is individualized based upon cardiac status, gestational age, Bishop score, and other factors.
"Many of you probably start to lose your nerve a bit at the end and say, ‘Pregnancy is bad for heart disease; we should just deliver.’ But generally speaking, if your patients are doing well in the third trimester, there’s really no reason to induce prematurity," Dr. Economy asserted.
She cited a large multicenter study that has turned heads in the world of maternal-fetal medicine. The study showed significantly increased rates of NICU admission, newborn sepsis, and respiratory complications requiring prolonged intubation with delivery at 37-38 weeks’ gestation, compared with 39 weeks’, in a broad population of pregnant women (N. Engl. J. Med. 2009;360:111-20).
"If your patients are doing well, let them stay pregnant," the ob.gyn. urged.
Cesarean section is really popular in patients with congenital heart disease. The joint guidelines state, "Fetal delivery via cesarean section is reasonable for patients with significant aortic enlargement, dissection, or severe aortic valve regurgitation" (Circulation 2010;121:1544-79). But Dr. Economy pointed out that this recommendation is rated class II, level of evidence C, meaning that it is based solely on expert opinion. And these joint guidelines were drawn up and approved by numerous cardiovascular and imaging societies without the endorsement of any obstetric organizations.
"I would put to you that every time you think about a cesarean section, you stop and remember that cesarean section is worse for women. For all women. C-section is worse for them, okay? It increases the risk of significant blood loss, increases infection risk, and increases the risk of venous thromboembolism," she said.
"My personal opinion is cesarean section should be reserved for obstetric indications – things like failure to progress, breech presentation, or nonreassuring fetal status in labor. The vast majority of patients will be better served by vaginal delivery. Plan on an interdisciplinary effort between obstetrics, cardiology, anesthesiology, and nursing," Dr. Economy advised.
Dr. Economy and her associates reported that they had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: Nine (20%) postpartum hemorrhages and seven (16%) third- or fourth-degree lacerations occurred among 45 no-Valsalva patients, compared with none in 17 (0%) women who pushed during labor. The only maternal adverse cardiac event (2%) occurred in a woman who did not do the Valsalva maneuver.
Data Source: The researchers conducted a retrospective cohort study including 112 pregnancies in 65 women with congenital heart disease who delivered at the hospital during 1998-2005.
Disclosures: Dr. Economy and her associates reported that they had no relevant financial disclosures.
Pregnancy-Induced Hypertension
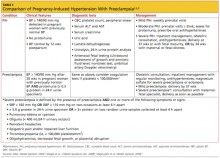
Hypertensive disorders represent one of the most common medical complications of pregnancy.1,2 Based on a nationwide inpatient sample examining more than 36 million deliveries in the United States, the prevalence of associated hypertensive disorders increased from 67.2 per 1,000 deliveries in 1998 to 83.4 per 1,000 deliveries in 2006.3Pregnancy-induced hypertension (also referred to as gestational hypertension or hypertensive disorder of pregnancy)4-6 is estimated to affect 6% to 8% of US pregnancies.1,2
Women who develop severe hypertension during pregnancy may experience adverse effects similar to those associated with mild preeclampsia.2,7,8 In the mother, these may range from elevated liver enzymes to renal dysfunction; and in the fetus, from preterm delivery to intrauterine restriction of fetal growth.7,8
This article will review the risk factors, clinical presentation, diagnosis, and management of pregnancy-induced hypertension. A brief discussion of preeclampsia as it relates to gestational hypertension will be included (see Table 12,6,9).
Classification, Definitions
Pregnancy-induced hypertension (PIH) is classified as mild or severe. Mild PIH is defined as new-onset hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg), occurring after 20 weeks’ gestation. The majority of cases of mild PIH develop beyond 37 weeks’ gestation, and in these cases, pregnancy outcomes are comparable to those of normotensive pregnancies.2,7,8
Severe PIH is defined as sustained elevated blood pressures of ≥ 160 mm Hg systolic and ≥ 110 mm Hg diastolic. In prospective cohort studies in which calcium supplementation and low-dose aspirin use were being investigated for prevention of preeclampsia in healthy pregnant women, those who were severely hypertensive were found to be at increased risk for certain maternal comorbidities (eg, cesarean delivery, renal dysfunction, elevated liver enzymes, placental abruption) and perinatal morbidities (delivery before 37 weeks’ gestation, low birth weight, fetal growth restriction, and neonatal ICU admission), compared with patients who were normotensive or mildly hypertensive.7,8
The diagnosis of PIH may later be amended or replaced by one of the following diagnoses: preeclampsia, if proteinuria (to be defined and discussed later) develops; chronic hypertension, if blood pressure remains elevated past 12 weeks postpartum; or transient hypertension of pregnancy, if blood pressure normalizes by 12 weeks postpartum.5,6,10
Pathophysiology and Risk Factors
Although the pathophysiology of PIH is not well understood, the pathogenesis of preeclampsia likely involves abnormalities in the development, implantation, or perfusion of the placenta, and often leads to impaired maternal organ function.6,11 It is not clear whether PIH and preeclampsia are two different diseases that share a manifestation of elevated blood pressure or whether PIH represents an early stage of preeclampsia.4,12 However, women with preexisting hypertension, especially severe hypertension, are at increased risk for preeclampsia, placental abruption, and fetal growth restriction.2
There are some similarities and some distinct differences among the clinical features and risk factors associated with PIH, compared with those of preeclampsia. Risk factors for PIH include a pre-pregnancy BMI of 25 or greater, PIH and/or preeclampsia in previous pregnancies, and history of renal disease, cardiac disease, or diabetes. The most important risk factors for preeclampsia include preexisting diabetes or nephropathy, chronic hypertension, PIH or preeclampsia in a previous pregnancy, maternal age younger than 18 or older than 34, African-American ethnicity, first pregnancy, multiple pregnancy, history of preeclampsia in the patient’s mother or sister, obesity, autoimmune disease, and an interval between pregnancies longer than 10 years.4-6,13-15
The risk for preeclampsia in patients with PIH is approximately 15% to 25%12,16; according to Magee et al,6 35% of women with PIH onset before 37 weeks’ gestation develop preeclampsia.6,12,17 The risk for recurrence of PIH in subsequent pregnancies is about 26%, whereas women who experience preeclampsia in one pregnancy have a comparable risk for PIH or preeclampsia (about 14% each) in subsequent pregnancies.18
Clinical Presentation and Diagnostic Evaluation
Blood pressure should be measured and recorded at every prenatal visit, using the correct-sized cuff, with the patient in a seated position.5 Gestational hypertension is a clinical diagnosis confirmed by at least two accurate blood pressure measurements in the same arm in women without proteinuria, with readings of ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic. It should then be determined whether the patient’s hypertension is mild or severe (ie, blood pressure > 160/110 mm Hg). The patient with severe PIH should be evaluated for signs of preeclampsia, as discussed below.
Patients with mild PIH are often asymptomatic, and the diagnosis is made at a prenatal visit as a result of routine blood pressure monitoring; this is one of many reasons to encourage early and regular prenatal care. Blood pressure may be higher at night in hypertensive disorders of pregnancy.10
In contrast to patients with mild PIH, the clinical presentation of those with severe PIH or preeclampsia (and the potential for impending eclampsia) may include the following symptoms and signs:
- Generalized edema, including that of the face and hands
- Rapid weight gain
- Blurred vision or scotomata (ie, areas of diminished vision in the visual field)
- Severe, throbbing or pounding headaches
- Epigastric or right upper quadrant pain
- Oliguria (urinary output < 500 mL/d)
- Nausea, with or without vomiting
- Hyperactive reflexes
- Chest pain or tightness
- Shortness of breath.2,6,14
Medical History
Important questions to address in the patient’s medical history relate to risk factors for PIH, such as a history of renal disease, cardiac disease, or diabetes, previous history of PIH and/or preeclampsia, and abuse of cocaine or amphetamines—in addition to the specific aforementioned symptoms and signs of severe preeclampsia.5,6
Physical Examination
The clinician performing the physical exam should be attentive to accurate blood pressure measurements and any signs that suggest preeclampsia. Weight should be measured and BMI calculated at each prenatal visit.
If the patient’s blood pressure is markedly elevated, the focused physical examination should include an ophthalmologic examination for jaundice and for evidence of hypertensive retinopathy or papilledema; pulmonary and cardiac examination; abdominal examination, including palpation of the liver; examination of the face and extremities for edema; and a complete neurologic examination, including assessment of deep tendon reflexes and examination for clonus.
Laboratory Testing
In patients with PIH, laboratory evaluation should be focused to rule out preeclampsia. The potential for proteinuria (defined as ≥ 0.3 g/d in a 24-hour urine sample1,14) must be investigated at diagnosis and at regular visits during the pregnancy.1 At least two random urine samples, collected at least 6 hours apart, should be evaluated for protein. A spot (random) urine sample with a result of 2+ protein or greater is highly suggestive of proteinuria; a 24-hour urine collection is the gold standard by which such findings should be confirmed and protein levels in the urine quantified.1,14
Elevated blood pressure and proteinuria are the hallmarks of preeclampsia.6 Patients affected by these developments must be evaluated for signs and symptoms of severe preeclampsia. However, those with only mild elevations in blood pressure and little or no proteinuria may complain of sudden-onset throbbing or pounding headache, blurry vision, and severe epigastric pain—possibly indicating severe preeclampsia.5,10
In addition to laboratory evaluation for urinary protein excretion, the following tests are recommended by the American College of Obstetricians and Gynecologists (ACOG)14 to assess for end organ involvement, which is consistent with severe preeclampsia:
- Hematocrit, which may be either high, to suggest hemoconcentration; or low, indicating hemolysis
- Platelet count, which is normal in women with PIH and low in those with severe preeclampsia; if results are abnormal, this test should be followed by coagulation testing (international normalized ratio, activated partial thromboplastin time, fibrinogen)
- Renal function testing (blood urea nitrogen and creatinine may be elevated in severe preeclampsia), and random urine testing for proteinuria, as explained earlier
- Liver enzymes (which are elevated in severe preeclampsia), and
- Lactate dehydrogenase (which is elevated in severe preeclampsia).1,14
Additionally, researchers conducting a small cohort study (n = 163) reported in 2009 that in women with PIH, serum uric acid levels exceeding 309 µmol/L were predictive of preeclampsia, with 87.7% sensitivity and 93.3% specificity.19 An increase from first-trimester serum uric acid levels was also a strong prognostic factor for preeclampsia. Earlier this year, a Canadian investigative team reported an increased risk for premature birth (odds ratio, 3.2) and small infant size for gestational age (odds ratio, 2.5) in women with PIH and hyperuricemia.20 While the predictive value of uric acid has been debated to some extent,14 measurement is often included in the workup of patients with hypertensive pregnancies.5,6
The frequency of prenatal visits, laboratory testing, and fetal monitoring should be adjusted according to the severity of PIH. In mildly hypertensive patients, the general recommendation is urine and blood testing at weekly prenatal visits.14 Fetal well-being must be monitored regularly, although neither the type nor frequency of such testing has been well established. Generally, patients should be advised to count daily fetal movements, and they should be scheduled for either a nonstress test (NST) or a biophysical profile as soon as a diagnosis of PIH is made.1,2,6,14
According to a 2010 guidance from the United Kingdom’s National Institute for Health and Clinical Excellence (NICE),13,21 pregnant women with mild to moderate hypertension should undergo an initial ultrasonographic assessment of fetal growth and amniotic fluid volume at the time of diagnosis, then serially every 3 to 4 weeks. If results from initial fetal testing are normal, patients with mild PIH do not require repeat testing after 34 weeks’ gestation, unless conditions change (eg, preeclampsia, worsening hypertension, and/or change in fetal movements).1,2,14 The NICE guidelines also recommend umbilical artery Doppler velocimetry.13,21
In patients with severe PIH, an NST ultrasound assessment of fetal growth and amniotic fluid volume and umbilical artery Doppler velocimetry should be performed at diagnosis to evaluate for placental dysfunction.6,21 If all test results are normal, the ultrasound and umbilical artery Doppler velocimetry need not be repeated more frequently than every two weeks, and the NST no more than once per week.13
Treatment/Management and Follow-Up
Regular prenatal monitoring to assess for worsening of PIH and/or development of preeclampsia is key to management. Figure 12 outlines an algorithm for managing PIH, which is guided by the severity of the condition. Patients with mild PIH can be managed with weekly outpatient visits and assessed for signs and symptoms of preeclampsia, monitoring of fetal movements, weight, blood pressure measurements, and urine and blood tests.2
At each visit, it is important to instruct patients to report immediately any of the following symptoms: new-onset severe headache, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, difficulty breathing or chest tightness, as well as vaginal bleeding, decreased fetal movements, or uterine contractions.6,14
Generally, expectant management with delivery at term is recommended for women with mild PIH.2 Vaginal delivery (or cesarean delivery, if indicated) is recommended at 37 weeks or when fetal maturity is confirmed; and at 34 weeks if fetal or maternal distress is evident.2,6
Findings from the Hypertension and Preeclampsia Intervention Trial At Term (HYPITAT),22 an open-label, randomized clinical trial in women with PIH or mild preeclampsia, suggested an association between induction of labor between 36 and 41 weeks’ gestation and improved maternal outcomes (specifically, reduced risk for severe hypertension), compared with expectant management. Similarly, in a literature review by Caughey et al,23 results from nine randomized controlled trials indicated a reduced risk for cesarean delivery in women who underwent induction of labor, compared with expectant management. Rates of “successful” induction of labor (ie, procedures resulting in vaginal rather than cesarean delivery) were greater in women with higher parity, a favorable cervix, and earlier gestational age.
Based on data from the HYPITAT trial,22 a cost-effectiveness analysis of induction of labor compared with expectant management revealed an 11% reduction in the average cost in delivery that followed induction of labor, compared with expectant management, in women with PIH or mild preeclampsia.24 Caughey et al23 reported similar savings, particularly when induction of labor was performed at 41 weeks’ gestation.
If induction of labor is being considered in a woman with an unfavorable cervix, administration of prostaglandins is recommended to enhance cervical ripening.6
Medication
Pregnant women should be advised to discontinue previously prescribed ACE inhibitors, angiotensin receptor blockers, or thiazide diuretics, which are associated with congenital abnormalities, intrauterine growth restriction, and/or neonatal nephropathy.5,6,13,21
Antihypertensive medication is not recommended for women with mild to moderate PIH, as it does not appear to improve outcomes. Evidence was found insufficient in a 2007 Cochrane review to determine the potential impact of antihypertensive medications for treatment of mild to moderate PIH on clinical outcomes such as preterm birth, infant mortality, and infant size relative to gestational age.25 A similar review conducted in 2011, with primary outcomes that included severe preeclampsia, eclampsia, and maternal death or perinatal death, concluded only that further study was needed to determine how tightly blood pressure must be controlled to improve maternal and fetal outcomes in patients with PIH.26
ACOG14 recommends antihypertensive therapy (eg, hydralazine, labetalol) only for women with diastolic blood pressure of 105 to 110 mm Hg or higher.1,2 There are several recommendations from different organizations regarding the choice of antihypertensive medications for PIH. In the UK’s NICE guidance,21 it is recommended that patients with moderate to severe PIH take oral labetalol as first-line treatment to keep systolic blood pressure below 150 mm Hg and diastolic blood pressure between 80 and 100 mm Hg.
Like severe preeclampsia, severe PIH should be managed in an inpatient setting.6,14 IV labetalol or hydralazine is recommended to lower the blood pressure to less than 160/110 mm Hg, although current evidence is insufficient to identify a target blood pressure.6,26 A 2002 ACOG practice bulletin recommends one of the following:
- Hydralazine 5 to 10 mg IV every 15 to 20 minutes until the desired response is achieved; or
- Labetalol 20 mg IV bolus, followed by 40 mg if not effective within 10 minutes, then 80 mg every 10 minutes with maximum total dose of 220 mg.1,14
In women who have severe PIH or who develop severe preeclampsia or eclampsia, magnesium sulfate is administered to prevent or treat seizures.14 This agent should be used during labor and for at least 24 hours postpartum.2 Dosing of magnesium sulfate for this indication is 4 g IV bolus, followed by infusion of 1 g/h. It is important to monitor treated patients for signs of toxicity, including muscle weakness, loss of patellar reflexes, hypoventilation, pulmonary edema, hypotension, and bradycardia. IV calcium gluconate should be readily available for use as an antidote to life-threatening hypermagnesemia.27,28
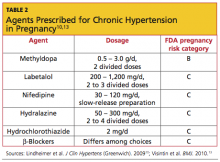
For chronic hypertension in pregnancy, the American Society of Hypertension10 has recommended several agents. There is no consensus on which medication is most appropriate (see Table 210,13).
Patient Education
Patient education is an important aspect of caring for women with PIH. The American Academy of Family Physicians29,30 provides a comprehensive patient education resource that defines the hypertensive disorders in pregnancy, explains the symptoms and signs of severe hypertension or preeclampsia, and describes appropriate diagnostic tests, monitoring, and treatment options. (See http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview.all.html.)
Patients who are considering pregnancy should be counseled to maintain a healthy weight prior to and during pregnancy. Adequate dietary calcium can reduce the risk for PIH, and calcium supplementation has been shown to reduce the risk for preeclampsia, especially in women at high risk.31 The Society of Obstetricians and Gynaecologists of Canada recommends low-dose aspirin (75 mg/d) at bedtime for high-risk women, starting before pregnancy, or upon diagnosis of pregnancy to prevent preeclampsia.6 Although there is insufficient evidence to recommend dietary salt restriction, excess salt can increase fluid retention and possibly blood pressure. Patients should be urged to attend all scheduled prenatal visits and to review the warning signs and symptoms of severe hypertension and preeclampsia at each visit.
Mode of delivery may be discussed and will depend on the severity of hypertension, presence of preeclampsia, and fetal well-being. Vaginal delivery at term is considered optimal unless there are indications for cesarean delivery. Induction of labor may be considered at term in patients with PIH. Those with severe preeclampsia who may require preterm delivery must be prepared for potential issues associated with prematurity.
Follow-Up and Prognosis
Patients with PIH should be evaluated postpartum for persistent hypertension. Blood pressure in patients with PIH usually normalizes by day 7 postpartum.32 If blood pressure elevation persists past 12 weeks postpartum, the patient’s diagnosis is revised to chronic hypertension and managed accordingly.
In the patient with persistent hypertension who chooses breastfeeding, it is important to select an antihypertensive medication with low transfer into breast milk. Many β-adrenergic antagonists and calcium channel antagonists are considered “compatible” with breastfeeding by the American Academy of Pediatrics.33
In addition to the potential for recurrent PIH in subsequent pregnancies, women with PIH are at increased risk for hypertension later in life, and findings from several large cohort studies suggest increased cardiovascular risk in patients with hypertensive pregnancies.16,34 Magnussen et al,9 who followed more than 15,000 mothers of singleton infants for several years postpartum, found that those who experienced hypertensive disorders (particularly recurrent hypertensive disorders) during pregnancy were more likely than normotensive women to subsequently develop diabetes, dyslipidemia, and hypertension. Women who remained normotensive while pregnant generally had lower BMI measurements than those who experienced PIH or preeclampsia.
Conclusion
Hypertensive disorders commonly develop during pregnancy. It is important to diagnose and classify PIH during routine prenatal visits. Once the diagnosis is made, patients must be monitored closely for increasing blood pressure or development of preeclampsia. Urine protein testing is a key clinical test to detect preeclampsia, and positive findings on a random urine protein dipstick should be confirmed and quantified with a 24-hour urine collection.
In addition to undergoing frequent blood pressure measurements and urine protein tests, patients should be asked about signs and symptoms that suggest preeclampsia. Women with mild PIH can be managed as outpatients with prenatal visits at least weekly, followed by delivery at term. Severely hypertensive patients are managed in the hospital with antihypertensive medications and prompt delivery at 34 weeks’ gestation or beyond, should maternal or fetal distress become evident.
1. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.
2. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181-192.
3. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009; 113(6):1299-1306.
4. Villar J, Carroli, G, Wojdyla D, et al; World Health Organization Antenatal Care Trial Research Group. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions. Am J Obstet Gynecol. 2006;194(4):921-931.
5. Leeman L, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2008;78(1):
93-100.
6. Magee LA, Helewa M, Moutquin JM, von Dadelszen P; Hypertension Guideline Committee; Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3 suppl):S1-S48.
7. Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66-71.
8. Hauth JC, Ewell MG, Levine RJ, et al; Calcium for Preeclampsia Prevention Study Group. Pregnancy outcomes in healthy nulliparas who developed hypertension. Obstet Gynecol. 2000;95(1):24-28.
9. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009; 114(5):961-970.
10. Lindheimer MD, Taler SJ, Cunningham FG; American Society of Hypertension (ASH). ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11(4):214-225.
11. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):
1243-1249.
12. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105 (11):1177-1184.
13. Visintin C, Mugglestone MA, Almerie MQ, et al; Guideline Development Group. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207.
14. ACOG Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for Obstetrician–Gynecologists. Diagnosis and management of preeclampsia and eclampsia: ACOG practice bulletin No. 33. Obstet Gynecol. 2002;99:159-167.
15. Parazzini F, Bortolus R, Chatenoud L, et al; Italian Study of Aspirin in Pregnancy Group. Risk factors for pregnancy-induced hypertension in women at high risk for the condition. Epidemiology. 1996;7(3):306-308.
16. Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am J Obstet Gynecol. 2006;194(4):916-920.
17. Barton JR, O’Brien JM, Bergauer NK, et al. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184(5):979-983.
18. Brown MA, Mackenzie C, Dunsmuir W, et al. Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG. 2007; 114(8):984-993.
19. Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension. 2011;58(4):704-708.
20. Hawkins TL, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484-492.
21. National Institute for Health and Clinical Excellence. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. www.nice.org.uk/nicemedia/live/13098/50418/50418.pdf. Accessed April 16, 2012.
22. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
23. Caughey AB, Sundaram V, Kaimal AJ, et al. Maternal and neonatal outcomes of elective induction of labor. Evid Rep Technol Assess (Full Rep). 2009;(176):1-257.
24. Shennan A, Hezelgrave N. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre-eclampsia at term (HYPITAT trial). BJOG. 2010;117(13):1575-1576.
25. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD002252.
26. Nabhan AF, Elsedawy MM. Tight control of mild-moderate pre-existing or non-proteinuric gestational hypertension. Cochrane Database Syst Rev. 2011 Jul 6;(7):CD006907.
27. Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
28. Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005;62(16):1663-1682.
29. FamilyDoctor.org. Pregnancy-induced hypertension (updated 2010). http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview
.all.html. Accessed April 16, 2012.
30. Zamorski MA, Green LA. NHBPEP report on high blood pressure in pregnancy: a summary for family physicians. Am Fam Physician. 2001; 64(2):263-270.
31. Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. BJOG. 2007;114(8): 933-943.
32. Ferrazzani S, De Carolis S, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: relationship with renal impairment and week of delivery. Am J Obstet Gynecol. 1994;171(2):506-512.
33. Beardmore KS, Morris JM, Gallery ED. Excretion of antihypertensive medication into human breast milk: a systematic review. Hypertens Pregnancy. 2002;21(1):85-95.
34. Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845.
Hypertensive disorders represent one of the most common medical complications of pregnancy.1,2 Based on a nationwide inpatient sample examining more than 36 million deliveries in the United States, the prevalence of associated hypertensive disorders increased from 67.2 per 1,000 deliveries in 1998 to 83.4 per 1,000 deliveries in 2006.3Pregnancy-induced hypertension (also referred to as gestational hypertension or hypertensive disorder of pregnancy)4-6 is estimated to affect 6% to 8% of US pregnancies.1,2
Women who develop severe hypertension during pregnancy may experience adverse effects similar to those associated with mild preeclampsia.2,7,8 In the mother, these may range from elevated liver enzymes to renal dysfunction; and in the fetus, from preterm delivery to intrauterine restriction of fetal growth.7,8
This article will review the risk factors, clinical presentation, diagnosis, and management of pregnancy-induced hypertension. A brief discussion of preeclampsia as it relates to gestational hypertension will be included (see Table 12,6,9).
Classification, Definitions
Pregnancy-induced hypertension (PIH) is classified as mild or severe. Mild PIH is defined as new-onset hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg), occurring after 20 weeks’ gestation. The majority of cases of mild PIH develop beyond 37 weeks’ gestation, and in these cases, pregnancy outcomes are comparable to those of normotensive pregnancies.2,7,8
Severe PIH is defined as sustained elevated blood pressures of ≥ 160 mm Hg systolic and ≥ 110 mm Hg diastolic. In prospective cohort studies in which calcium supplementation and low-dose aspirin use were being investigated for prevention of preeclampsia in healthy pregnant women, those who were severely hypertensive were found to be at increased risk for certain maternal comorbidities (eg, cesarean delivery, renal dysfunction, elevated liver enzymes, placental abruption) and perinatal morbidities (delivery before 37 weeks’ gestation, low birth weight, fetal growth restriction, and neonatal ICU admission), compared with patients who were normotensive or mildly hypertensive.7,8
The diagnosis of PIH may later be amended or replaced by one of the following diagnoses: preeclampsia, if proteinuria (to be defined and discussed later) develops; chronic hypertension, if blood pressure remains elevated past 12 weeks postpartum; or transient hypertension of pregnancy, if blood pressure normalizes by 12 weeks postpartum.5,6,10
Pathophysiology and Risk Factors
Although the pathophysiology of PIH is not well understood, the pathogenesis of preeclampsia likely involves abnormalities in the development, implantation, or perfusion of the placenta, and often leads to impaired maternal organ function.6,11 It is not clear whether PIH and preeclampsia are two different diseases that share a manifestation of elevated blood pressure or whether PIH represents an early stage of preeclampsia.4,12 However, women with preexisting hypertension, especially severe hypertension, are at increased risk for preeclampsia, placental abruption, and fetal growth restriction.2
There are some similarities and some distinct differences among the clinical features and risk factors associated with PIH, compared with those of preeclampsia. Risk factors for PIH include a pre-pregnancy BMI of 25 or greater, PIH and/or preeclampsia in previous pregnancies, and history of renal disease, cardiac disease, or diabetes. The most important risk factors for preeclampsia include preexisting diabetes or nephropathy, chronic hypertension, PIH or preeclampsia in a previous pregnancy, maternal age younger than 18 or older than 34, African-American ethnicity, first pregnancy, multiple pregnancy, history of preeclampsia in the patient’s mother or sister, obesity, autoimmune disease, and an interval between pregnancies longer than 10 years.4-6,13-15
The risk for preeclampsia in patients with PIH is approximately 15% to 25%12,16; according to Magee et al,6 35% of women with PIH onset before 37 weeks’ gestation develop preeclampsia.6,12,17 The risk for recurrence of PIH in subsequent pregnancies is about 26%, whereas women who experience preeclampsia in one pregnancy have a comparable risk for PIH or preeclampsia (about 14% each) in subsequent pregnancies.18
Clinical Presentation and Diagnostic Evaluation
Blood pressure should be measured and recorded at every prenatal visit, using the correct-sized cuff, with the patient in a seated position.5 Gestational hypertension is a clinical diagnosis confirmed by at least two accurate blood pressure measurements in the same arm in women without proteinuria, with readings of ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic. It should then be determined whether the patient’s hypertension is mild or severe (ie, blood pressure > 160/110 mm Hg). The patient with severe PIH should be evaluated for signs of preeclampsia, as discussed below.
Patients with mild PIH are often asymptomatic, and the diagnosis is made at a prenatal visit as a result of routine blood pressure monitoring; this is one of many reasons to encourage early and regular prenatal care. Blood pressure may be higher at night in hypertensive disorders of pregnancy.10
In contrast to patients with mild PIH, the clinical presentation of those with severe PIH or preeclampsia (and the potential for impending eclampsia) may include the following symptoms and signs:
- Generalized edema, including that of the face and hands
- Rapid weight gain
- Blurred vision or scotomata (ie, areas of diminished vision in the visual field)
- Severe, throbbing or pounding headaches
- Epigastric or right upper quadrant pain
- Oliguria (urinary output < 500 mL/d)
- Nausea, with or without vomiting
- Hyperactive reflexes
- Chest pain or tightness
- Shortness of breath.2,6,14
Medical History
Important questions to address in the patient’s medical history relate to risk factors for PIH, such as a history of renal disease, cardiac disease, or diabetes, previous history of PIH and/or preeclampsia, and abuse of cocaine or amphetamines—in addition to the specific aforementioned symptoms and signs of severe preeclampsia.5,6
Physical Examination
The clinician performing the physical exam should be attentive to accurate blood pressure measurements and any signs that suggest preeclampsia. Weight should be measured and BMI calculated at each prenatal visit.
If the patient’s blood pressure is markedly elevated, the focused physical examination should include an ophthalmologic examination for jaundice and for evidence of hypertensive retinopathy or papilledema; pulmonary and cardiac examination; abdominal examination, including palpation of the liver; examination of the face and extremities for edema; and a complete neurologic examination, including assessment of deep tendon reflexes and examination for clonus.
Laboratory Testing
In patients with PIH, laboratory evaluation should be focused to rule out preeclampsia. The potential for proteinuria (defined as ≥ 0.3 g/d in a 24-hour urine sample1,14) must be investigated at diagnosis and at regular visits during the pregnancy.1 At least two random urine samples, collected at least 6 hours apart, should be evaluated for protein. A spot (random) urine sample with a result of 2+ protein or greater is highly suggestive of proteinuria; a 24-hour urine collection is the gold standard by which such findings should be confirmed and protein levels in the urine quantified.1,14
Elevated blood pressure and proteinuria are the hallmarks of preeclampsia.6 Patients affected by these developments must be evaluated for signs and symptoms of severe preeclampsia. However, those with only mild elevations in blood pressure and little or no proteinuria may complain of sudden-onset throbbing or pounding headache, blurry vision, and severe epigastric pain—possibly indicating severe preeclampsia.5,10
In addition to laboratory evaluation for urinary protein excretion, the following tests are recommended by the American College of Obstetricians and Gynecologists (ACOG)14 to assess for end organ involvement, which is consistent with severe preeclampsia:
- Hematocrit, which may be either high, to suggest hemoconcentration; or low, indicating hemolysis
- Platelet count, which is normal in women with PIH and low in those with severe preeclampsia; if results are abnormal, this test should be followed by coagulation testing (international normalized ratio, activated partial thromboplastin time, fibrinogen)
- Renal function testing (blood urea nitrogen and creatinine may be elevated in severe preeclampsia), and random urine testing for proteinuria, as explained earlier
- Liver enzymes (which are elevated in severe preeclampsia), and
- Lactate dehydrogenase (which is elevated in severe preeclampsia).1,14
Additionally, researchers conducting a small cohort study (n = 163) reported in 2009 that in women with PIH, serum uric acid levels exceeding 309 µmol/L were predictive of preeclampsia, with 87.7% sensitivity and 93.3% specificity.19 An increase from first-trimester serum uric acid levels was also a strong prognostic factor for preeclampsia. Earlier this year, a Canadian investigative team reported an increased risk for premature birth (odds ratio, 3.2) and small infant size for gestational age (odds ratio, 2.5) in women with PIH and hyperuricemia.20 While the predictive value of uric acid has been debated to some extent,14 measurement is often included in the workup of patients with hypertensive pregnancies.5,6
The frequency of prenatal visits, laboratory testing, and fetal monitoring should be adjusted according to the severity of PIH. In mildly hypertensive patients, the general recommendation is urine and blood testing at weekly prenatal visits.14 Fetal well-being must be monitored regularly, although neither the type nor frequency of such testing has been well established. Generally, patients should be advised to count daily fetal movements, and they should be scheduled for either a nonstress test (NST) or a biophysical profile as soon as a diagnosis of PIH is made.1,2,6,14
According to a 2010 guidance from the United Kingdom’s National Institute for Health and Clinical Excellence (NICE),13,21 pregnant women with mild to moderate hypertension should undergo an initial ultrasonographic assessment of fetal growth and amniotic fluid volume at the time of diagnosis, then serially every 3 to 4 weeks. If results from initial fetal testing are normal, patients with mild PIH do not require repeat testing after 34 weeks’ gestation, unless conditions change (eg, preeclampsia, worsening hypertension, and/or change in fetal movements).1,2,14 The NICE guidelines also recommend umbilical artery Doppler velocimetry.13,21
In patients with severe PIH, an NST ultrasound assessment of fetal growth and amniotic fluid volume and umbilical artery Doppler velocimetry should be performed at diagnosis to evaluate for placental dysfunction.6,21 If all test results are normal, the ultrasound and umbilical artery Doppler velocimetry need not be repeated more frequently than every two weeks, and the NST no more than once per week.13
Treatment/Management and Follow-Up
Regular prenatal monitoring to assess for worsening of PIH and/or development of preeclampsia is key to management. Figure 12 outlines an algorithm for managing PIH, which is guided by the severity of the condition. Patients with mild PIH can be managed with weekly outpatient visits and assessed for signs and symptoms of preeclampsia, monitoring of fetal movements, weight, blood pressure measurements, and urine and blood tests.2
At each visit, it is important to instruct patients to report immediately any of the following symptoms: new-onset severe headache, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, difficulty breathing or chest tightness, as well as vaginal bleeding, decreased fetal movements, or uterine contractions.6,14
Generally, expectant management with delivery at term is recommended for women with mild PIH.2 Vaginal delivery (or cesarean delivery, if indicated) is recommended at 37 weeks or when fetal maturity is confirmed; and at 34 weeks if fetal or maternal distress is evident.2,6
Findings from the Hypertension and Preeclampsia Intervention Trial At Term (HYPITAT),22 an open-label, randomized clinical trial in women with PIH or mild preeclampsia, suggested an association between induction of labor between 36 and 41 weeks’ gestation and improved maternal outcomes (specifically, reduced risk for severe hypertension), compared with expectant management. Similarly, in a literature review by Caughey et al,23 results from nine randomized controlled trials indicated a reduced risk for cesarean delivery in women who underwent induction of labor, compared with expectant management. Rates of “successful” induction of labor (ie, procedures resulting in vaginal rather than cesarean delivery) were greater in women with higher parity, a favorable cervix, and earlier gestational age.
Based on data from the HYPITAT trial,22 a cost-effectiveness analysis of induction of labor compared with expectant management revealed an 11% reduction in the average cost in delivery that followed induction of labor, compared with expectant management, in women with PIH or mild preeclampsia.24 Caughey et al23 reported similar savings, particularly when induction of labor was performed at 41 weeks’ gestation.
If induction of labor is being considered in a woman with an unfavorable cervix, administration of prostaglandins is recommended to enhance cervical ripening.6
Medication
Pregnant women should be advised to discontinue previously prescribed ACE inhibitors, angiotensin receptor blockers, or thiazide diuretics, which are associated with congenital abnormalities, intrauterine growth restriction, and/or neonatal nephropathy.5,6,13,21
Antihypertensive medication is not recommended for women with mild to moderate PIH, as it does not appear to improve outcomes. Evidence was found insufficient in a 2007 Cochrane review to determine the potential impact of antihypertensive medications for treatment of mild to moderate PIH on clinical outcomes such as preterm birth, infant mortality, and infant size relative to gestational age.25 A similar review conducted in 2011, with primary outcomes that included severe preeclampsia, eclampsia, and maternal death or perinatal death, concluded only that further study was needed to determine how tightly blood pressure must be controlled to improve maternal and fetal outcomes in patients with PIH.26
ACOG14 recommends antihypertensive therapy (eg, hydralazine, labetalol) only for women with diastolic blood pressure of 105 to 110 mm Hg or higher.1,2 There are several recommendations from different organizations regarding the choice of antihypertensive medications for PIH. In the UK’s NICE guidance,21 it is recommended that patients with moderate to severe PIH take oral labetalol as first-line treatment to keep systolic blood pressure below 150 mm Hg and diastolic blood pressure between 80 and 100 mm Hg.
Like severe preeclampsia, severe PIH should be managed in an inpatient setting.6,14 IV labetalol or hydralazine is recommended to lower the blood pressure to less than 160/110 mm Hg, although current evidence is insufficient to identify a target blood pressure.6,26 A 2002 ACOG practice bulletin recommends one of the following:
- Hydralazine 5 to 10 mg IV every 15 to 20 minutes until the desired response is achieved; or
- Labetalol 20 mg IV bolus, followed by 40 mg if not effective within 10 minutes, then 80 mg every 10 minutes with maximum total dose of 220 mg.1,14
In women who have severe PIH or who develop severe preeclampsia or eclampsia, magnesium sulfate is administered to prevent or treat seizures.14 This agent should be used during labor and for at least 24 hours postpartum.2 Dosing of magnesium sulfate for this indication is 4 g IV bolus, followed by infusion of 1 g/h. It is important to monitor treated patients for signs of toxicity, including muscle weakness, loss of patellar reflexes, hypoventilation, pulmonary edema, hypotension, and bradycardia. IV calcium gluconate should be readily available for use as an antidote to life-threatening hypermagnesemia.27,28
For chronic hypertension in pregnancy, the American Society of Hypertension10 has recommended several agents. There is no consensus on which medication is most appropriate (see Table 210,13).
Patient Education
Patient education is an important aspect of caring for women with PIH. The American Academy of Family Physicians29,30 provides a comprehensive patient education resource that defines the hypertensive disorders in pregnancy, explains the symptoms and signs of severe hypertension or preeclampsia, and describes appropriate diagnostic tests, monitoring, and treatment options. (See http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview.all.html.)
Patients who are considering pregnancy should be counseled to maintain a healthy weight prior to and during pregnancy. Adequate dietary calcium can reduce the risk for PIH, and calcium supplementation has been shown to reduce the risk for preeclampsia, especially in women at high risk.31 The Society of Obstetricians and Gynaecologists of Canada recommends low-dose aspirin (75 mg/d) at bedtime for high-risk women, starting before pregnancy, or upon diagnosis of pregnancy to prevent preeclampsia.6 Although there is insufficient evidence to recommend dietary salt restriction, excess salt can increase fluid retention and possibly blood pressure. Patients should be urged to attend all scheduled prenatal visits and to review the warning signs and symptoms of severe hypertension and preeclampsia at each visit.
Mode of delivery may be discussed and will depend on the severity of hypertension, presence of preeclampsia, and fetal well-being. Vaginal delivery at term is considered optimal unless there are indications for cesarean delivery. Induction of labor may be considered at term in patients with PIH. Those with severe preeclampsia who may require preterm delivery must be prepared for potential issues associated with prematurity.
Follow-Up and Prognosis
Patients with PIH should be evaluated postpartum for persistent hypertension. Blood pressure in patients with PIH usually normalizes by day 7 postpartum.32 If blood pressure elevation persists past 12 weeks postpartum, the patient’s diagnosis is revised to chronic hypertension and managed accordingly.
In the patient with persistent hypertension who chooses breastfeeding, it is important to select an antihypertensive medication with low transfer into breast milk. Many β-adrenergic antagonists and calcium channel antagonists are considered “compatible” with breastfeeding by the American Academy of Pediatrics.33
In addition to the potential for recurrent PIH in subsequent pregnancies, women with PIH are at increased risk for hypertension later in life, and findings from several large cohort studies suggest increased cardiovascular risk in patients with hypertensive pregnancies.16,34 Magnussen et al,9 who followed more than 15,000 mothers of singleton infants for several years postpartum, found that those who experienced hypertensive disorders (particularly recurrent hypertensive disorders) during pregnancy were more likely than normotensive women to subsequently develop diabetes, dyslipidemia, and hypertension. Women who remained normotensive while pregnant generally had lower BMI measurements than those who experienced PIH or preeclampsia.
Conclusion
Hypertensive disorders commonly develop during pregnancy. It is important to diagnose and classify PIH during routine prenatal visits. Once the diagnosis is made, patients must be monitored closely for increasing blood pressure or development of preeclampsia. Urine protein testing is a key clinical test to detect preeclampsia, and positive findings on a random urine protein dipstick should be confirmed and quantified with a 24-hour urine collection.
In addition to undergoing frequent blood pressure measurements and urine protein tests, patients should be asked about signs and symptoms that suggest preeclampsia. Women with mild PIH can be managed as outpatients with prenatal visits at least weekly, followed by delivery at term. Severely hypertensive patients are managed in the hospital with antihypertensive medications and prompt delivery at 34 weeks’ gestation or beyond, should maternal or fetal distress become evident.
Hypertensive disorders represent one of the most common medical complications of pregnancy.1,2 Based on a nationwide inpatient sample examining more than 36 million deliveries in the United States, the prevalence of associated hypertensive disorders increased from 67.2 per 1,000 deliveries in 1998 to 83.4 per 1,000 deliveries in 2006.3Pregnancy-induced hypertension (also referred to as gestational hypertension or hypertensive disorder of pregnancy)4-6 is estimated to affect 6% to 8% of US pregnancies.1,2
Women who develop severe hypertension during pregnancy may experience adverse effects similar to those associated with mild preeclampsia.2,7,8 In the mother, these may range from elevated liver enzymes to renal dysfunction; and in the fetus, from preterm delivery to intrauterine restriction of fetal growth.7,8
This article will review the risk factors, clinical presentation, diagnosis, and management of pregnancy-induced hypertension. A brief discussion of preeclampsia as it relates to gestational hypertension will be included (see Table 12,6,9).
Classification, Definitions
Pregnancy-induced hypertension (PIH) is classified as mild or severe. Mild PIH is defined as new-onset hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg), occurring after 20 weeks’ gestation. The majority of cases of mild PIH develop beyond 37 weeks’ gestation, and in these cases, pregnancy outcomes are comparable to those of normotensive pregnancies.2,7,8
Severe PIH is defined as sustained elevated blood pressures of ≥ 160 mm Hg systolic and ≥ 110 mm Hg diastolic. In prospective cohort studies in which calcium supplementation and low-dose aspirin use were being investigated for prevention of preeclampsia in healthy pregnant women, those who were severely hypertensive were found to be at increased risk for certain maternal comorbidities (eg, cesarean delivery, renal dysfunction, elevated liver enzymes, placental abruption) and perinatal morbidities (delivery before 37 weeks’ gestation, low birth weight, fetal growth restriction, and neonatal ICU admission), compared with patients who were normotensive or mildly hypertensive.7,8
The diagnosis of PIH may later be amended or replaced by one of the following diagnoses: preeclampsia, if proteinuria (to be defined and discussed later) develops; chronic hypertension, if blood pressure remains elevated past 12 weeks postpartum; or transient hypertension of pregnancy, if blood pressure normalizes by 12 weeks postpartum.5,6,10
Pathophysiology and Risk Factors
Although the pathophysiology of PIH is not well understood, the pathogenesis of preeclampsia likely involves abnormalities in the development, implantation, or perfusion of the placenta, and often leads to impaired maternal organ function.6,11 It is not clear whether PIH and preeclampsia are two different diseases that share a manifestation of elevated blood pressure or whether PIH represents an early stage of preeclampsia.4,12 However, women with preexisting hypertension, especially severe hypertension, are at increased risk for preeclampsia, placental abruption, and fetal growth restriction.2
There are some similarities and some distinct differences among the clinical features and risk factors associated with PIH, compared with those of preeclampsia. Risk factors for PIH include a pre-pregnancy BMI of 25 or greater, PIH and/or preeclampsia in previous pregnancies, and history of renal disease, cardiac disease, or diabetes. The most important risk factors for preeclampsia include preexisting diabetes or nephropathy, chronic hypertension, PIH or preeclampsia in a previous pregnancy, maternal age younger than 18 or older than 34, African-American ethnicity, first pregnancy, multiple pregnancy, history of preeclampsia in the patient’s mother or sister, obesity, autoimmune disease, and an interval between pregnancies longer than 10 years.4-6,13-15
The risk for preeclampsia in patients with PIH is approximately 15% to 25%12,16; according to Magee et al,6 35% of women with PIH onset before 37 weeks’ gestation develop preeclampsia.6,12,17 The risk for recurrence of PIH in subsequent pregnancies is about 26%, whereas women who experience preeclampsia in one pregnancy have a comparable risk for PIH or preeclampsia (about 14% each) in subsequent pregnancies.18
Clinical Presentation and Diagnostic Evaluation
Blood pressure should be measured and recorded at every prenatal visit, using the correct-sized cuff, with the patient in a seated position.5 Gestational hypertension is a clinical diagnosis confirmed by at least two accurate blood pressure measurements in the same arm in women without proteinuria, with readings of ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic. It should then be determined whether the patient’s hypertension is mild or severe (ie, blood pressure > 160/110 mm Hg). The patient with severe PIH should be evaluated for signs of preeclampsia, as discussed below.
Patients with mild PIH are often asymptomatic, and the diagnosis is made at a prenatal visit as a result of routine blood pressure monitoring; this is one of many reasons to encourage early and regular prenatal care. Blood pressure may be higher at night in hypertensive disorders of pregnancy.10
In contrast to patients with mild PIH, the clinical presentation of those with severe PIH or preeclampsia (and the potential for impending eclampsia) may include the following symptoms and signs:
- Generalized edema, including that of the face and hands
- Rapid weight gain
- Blurred vision or scotomata (ie, areas of diminished vision in the visual field)
- Severe, throbbing or pounding headaches
- Epigastric or right upper quadrant pain
- Oliguria (urinary output < 500 mL/d)
- Nausea, with or without vomiting
- Hyperactive reflexes
- Chest pain or tightness
- Shortness of breath.2,6,14
Medical History
Important questions to address in the patient’s medical history relate to risk factors for PIH, such as a history of renal disease, cardiac disease, or diabetes, previous history of PIH and/or preeclampsia, and abuse of cocaine or amphetamines—in addition to the specific aforementioned symptoms and signs of severe preeclampsia.5,6
Physical Examination
The clinician performing the physical exam should be attentive to accurate blood pressure measurements and any signs that suggest preeclampsia. Weight should be measured and BMI calculated at each prenatal visit.
If the patient’s blood pressure is markedly elevated, the focused physical examination should include an ophthalmologic examination for jaundice and for evidence of hypertensive retinopathy or papilledema; pulmonary and cardiac examination; abdominal examination, including palpation of the liver; examination of the face and extremities for edema; and a complete neurologic examination, including assessment of deep tendon reflexes and examination for clonus.
Laboratory Testing
In patients with PIH, laboratory evaluation should be focused to rule out preeclampsia. The potential for proteinuria (defined as ≥ 0.3 g/d in a 24-hour urine sample1,14) must be investigated at diagnosis and at regular visits during the pregnancy.1 At least two random urine samples, collected at least 6 hours apart, should be evaluated for protein. A spot (random) urine sample with a result of 2+ protein or greater is highly suggestive of proteinuria; a 24-hour urine collection is the gold standard by which such findings should be confirmed and protein levels in the urine quantified.1,14
Elevated blood pressure and proteinuria are the hallmarks of preeclampsia.6 Patients affected by these developments must be evaluated for signs and symptoms of severe preeclampsia. However, those with only mild elevations in blood pressure and little or no proteinuria may complain of sudden-onset throbbing or pounding headache, blurry vision, and severe epigastric pain—possibly indicating severe preeclampsia.5,10
In addition to laboratory evaluation for urinary protein excretion, the following tests are recommended by the American College of Obstetricians and Gynecologists (ACOG)14 to assess for end organ involvement, which is consistent with severe preeclampsia:
- Hematocrit, which may be either high, to suggest hemoconcentration; or low, indicating hemolysis
- Platelet count, which is normal in women with PIH and low in those with severe preeclampsia; if results are abnormal, this test should be followed by coagulation testing (international normalized ratio, activated partial thromboplastin time, fibrinogen)
- Renal function testing (blood urea nitrogen and creatinine may be elevated in severe preeclampsia), and random urine testing for proteinuria, as explained earlier
- Liver enzymes (which are elevated in severe preeclampsia), and
- Lactate dehydrogenase (which is elevated in severe preeclampsia).1,14
Additionally, researchers conducting a small cohort study (n = 163) reported in 2009 that in women with PIH, serum uric acid levels exceeding 309 µmol/L were predictive of preeclampsia, with 87.7% sensitivity and 93.3% specificity.19 An increase from first-trimester serum uric acid levels was also a strong prognostic factor for preeclampsia. Earlier this year, a Canadian investigative team reported an increased risk for premature birth (odds ratio, 3.2) and small infant size for gestational age (odds ratio, 2.5) in women with PIH and hyperuricemia.20 While the predictive value of uric acid has been debated to some extent,14 measurement is often included in the workup of patients with hypertensive pregnancies.5,6
The frequency of prenatal visits, laboratory testing, and fetal monitoring should be adjusted according to the severity of PIH. In mildly hypertensive patients, the general recommendation is urine and blood testing at weekly prenatal visits.14 Fetal well-being must be monitored regularly, although neither the type nor frequency of such testing has been well established. Generally, patients should be advised to count daily fetal movements, and they should be scheduled for either a nonstress test (NST) or a biophysical profile as soon as a diagnosis of PIH is made.1,2,6,14
According to a 2010 guidance from the United Kingdom’s National Institute for Health and Clinical Excellence (NICE),13,21 pregnant women with mild to moderate hypertension should undergo an initial ultrasonographic assessment of fetal growth and amniotic fluid volume at the time of diagnosis, then serially every 3 to 4 weeks. If results from initial fetal testing are normal, patients with mild PIH do not require repeat testing after 34 weeks’ gestation, unless conditions change (eg, preeclampsia, worsening hypertension, and/or change in fetal movements).1,2,14 The NICE guidelines also recommend umbilical artery Doppler velocimetry.13,21
In patients with severe PIH, an NST ultrasound assessment of fetal growth and amniotic fluid volume and umbilical artery Doppler velocimetry should be performed at diagnosis to evaluate for placental dysfunction.6,21 If all test results are normal, the ultrasound and umbilical artery Doppler velocimetry need not be repeated more frequently than every two weeks, and the NST no more than once per week.13
Treatment/Management and Follow-Up
Regular prenatal monitoring to assess for worsening of PIH and/or development of preeclampsia is key to management. Figure 12 outlines an algorithm for managing PIH, which is guided by the severity of the condition. Patients with mild PIH can be managed with weekly outpatient visits and assessed for signs and symptoms of preeclampsia, monitoring of fetal movements, weight, blood pressure measurements, and urine and blood tests.2
At each visit, it is important to instruct patients to report immediately any of the following symptoms: new-onset severe headache, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, difficulty breathing or chest tightness, as well as vaginal bleeding, decreased fetal movements, or uterine contractions.6,14
Generally, expectant management with delivery at term is recommended for women with mild PIH.2 Vaginal delivery (or cesarean delivery, if indicated) is recommended at 37 weeks or when fetal maturity is confirmed; and at 34 weeks if fetal or maternal distress is evident.2,6
Findings from the Hypertension and Preeclampsia Intervention Trial At Term (HYPITAT),22 an open-label, randomized clinical trial in women with PIH or mild preeclampsia, suggested an association between induction of labor between 36 and 41 weeks’ gestation and improved maternal outcomes (specifically, reduced risk for severe hypertension), compared with expectant management. Similarly, in a literature review by Caughey et al,23 results from nine randomized controlled trials indicated a reduced risk for cesarean delivery in women who underwent induction of labor, compared with expectant management. Rates of “successful” induction of labor (ie, procedures resulting in vaginal rather than cesarean delivery) were greater in women with higher parity, a favorable cervix, and earlier gestational age.
Based on data from the HYPITAT trial,22 a cost-effectiveness analysis of induction of labor compared with expectant management revealed an 11% reduction in the average cost in delivery that followed induction of labor, compared with expectant management, in women with PIH or mild preeclampsia.24 Caughey et al23 reported similar savings, particularly when induction of labor was performed at 41 weeks’ gestation.
If induction of labor is being considered in a woman with an unfavorable cervix, administration of prostaglandins is recommended to enhance cervical ripening.6
Medication
Pregnant women should be advised to discontinue previously prescribed ACE inhibitors, angiotensin receptor blockers, or thiazide diuretics, which are associated with congenital abnormalities, intrauterine growth restriction, and/or neonatal nephropathy.5,6,13,21
Antihypertensive medication is not recommended for women with mild to moderate PIH, as it does not appear to improve outcomes. Evidence was found insufficient in a 2007 Cochrane review to determine the potential impact of antihypertensive medications for treatment of mild to moderate PIH on clinical outcomes such as preterm birth, infant mortality, and infant size relative to gestational age.25 A similar review conducted in 2011, with primary outcomes that included severe preeclampsia, eclampsia, and maternal death or perinatal death, concluded only that further study was needed to determine how tightly blood pressure must be controlled to improve maternal and fetal outcomes in patients with PIH.26
ACOG14 recommends antihypertensive therapy (eg, hydralazine, labetalol) only for women with diastolic blood pressure of 105 to 110 mm Hg or higher.1,2 There are several recommendations from different organizations regarding the choice of antihypertensive medications for PIH. In the UK’s NICE guidance,21 it is recommended that patients with moderate to severe PIH take oral labetalol as first-line treatment to keep systolic blood pressure below 150 mm Hg and diastolic blood pressure between 80 and 100 mm Hg.
Like severe preeclampsia, severe PIH should be managed in an inpatient setting.6,14 IV labetalol or hydralazine is recommended to lower the blood pressure to less than 160/110 mm Hg, although current evidence is insufficient to identify a target blood pressure.6,26 A 2002 ACOG practice bulletin recommends one of the following:
- Hydralazine 5 to 10 mg IV every 15 to 20 minutes until the desired response is achieved; or
- Labetalol 20 mg IV bolus, followed by 40 mg if not effective within 10 minutes, then 80 mg every 10 minutes with maximum total dose of 220 mg.1,14
In women who have severe PIH or who develop severe preeclampsia or eclampsia, magnesium sulfate is administered to prevent or treat seizures.14 This agent should be used during labor and for at least 24 hours postpartum.2 Dosing of magnesium sulfate for this indication is 4 g IV bolus, followed by infusion of 1 g/h. It is important to monitor treated patients for signs of toxicity, including muscle weakness, loss of patellar reflexes, hypoventilation, pulmonary edema, hypotension, and bradycardia. IV calcium gluconate should be readily available for use as an antidote to life-threatening hypermagnesemia.27,28
For chronic hypertension in pregnancy, the American Society of Hypertension10 has recommended several agents. There is no consensus on which medication is most appropriate (see Table 210,13).
Patient Education
Patient education is an important aspect of caring for women with PIH. The American Academy of Family Physicians29,30 provides a comprehensive patient education resource that defines the hypertensive disorders in pregnancy, explains the symptoms and signs of severe hypertension or preeclampsia, and describes appropriate diagnostic tests, monitoring, and treatment options. (See http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview.all.html.)
Patients who are considering pregnancy should be counseled to maintain a healthy weight prior to and during pregnancy. Adequate dietary calcium can reduce the risk for PIH, and calcium supplementation has been shown to reduce the risk for preeclampsia, especially in women at high risk.31 The Society of Obstetricians and Gynaecologists of Canada recommends low-dose aspirin (75 mg/d) at bedtime for high-risk women, starting before pregnancy, or upon diagnosis of pregnancy to prevent preeclampsia.6 Although there is insufficient evidence to recommend dietary salt restriction, excess salt can increase fluid retention and possibly blood pressure. Patients should be urged to attend all scheduled prenatal visits and to review the warning signs and symptoms of severe hypertension and preeclampsia at each visit.
Mode of delivery may be discussed and will depend on the severity of hypertension, presence of preeclampsia, and fetal well-being. Vaginal delivery at term is considered optimal unless there are indications for cesarean delivery. Induction of labor may be considered at term in patients with PIH. Those with severe preeclampsia who may require preterm delivery must be prepared for potential issues associated with prematurity.
Follow-Up and Prognosis
Patients with PIH should be evaluated postpartum for persistent hypertension. Blood pressure in patients with PIH usually normalizes by day 7 postpartum.32 If blood pressure elevation persists past 12 weeks postpartum, the patient’s diagnosis is revised to chronic hypertension and managed accordingly.
In the patient with persistent hypertension who chooses breastfeeding, it is important to select an antihypertensive medication with low transfer into breast milk. Many β-adrenergic antagonists and calcium channel antagonists are considered “compatible” with breastfeeding by the American Academy of Pediatrics.33
In addition to the potential for recurrent PIH in subsequent pregnancies, women with PIH are at increased risk for hypertension later in life, and findings from several large cohort studies suggest increased cardiovascular risk in patients with hypertensive pregnancies.16,34 Magnussen et al,9 who followed more than 15,000 mothers of singleton infants for several years postpartum, found that those who experienced hypertensive disorders (particularly recurrent hypertensive disorders) during pregnancy were more likely than normotensive women to subsequently develop diabetes, dyslipidemia, and hypertension. Women who remained normotensive while pregnant generally had lower BMI measurements than those who experienced PIH or preeclampsia.
Conclusion
Hypertensive disorders commonly develop during pregnancy. It is important to diagnose and classify PIH during routine prenatal visits. Once the diagnosis is made, patients must be monitored closely for increasing blood pressure or development of preeclampsia. Urine protein testing is a key clinical test to detect preeclampsia, and positive findings on a random urine protein dipstick should be confirmed and quantified with a 24-hour urine collection.
In addition to undergoing frequent blood pressure measurements and urine protein tests, patients should be asked about signs and symptoms that suggest preeclampsia. Women with mild PIH can be managed as outpatients with prenatal visits at least weekly, followed by delivery at term. Severely hypertensive patients are managed in the hospital with antihypertensive medications and prompt delivery at 34 weeks’ gestation or beyond, should maternal or fetal distress become evident.
1. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.
2. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181-192.
3. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009; 113(6):1299-1306.
4. Villar J, Carroli, G, Wojdyla D, et al; World Health Organization Antenatal Care Trial Research Group. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions. Am J Obstet Gynecol. 2006;194(4):921-931.
5. Leeman L, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2008;78(1):
93-100.
6. Magee LA, Helewa M, Moutquin JM, von Dadelszen P; Hypertension Guideline Committee; Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3 suppl):S1-S48.
7. Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66-71.
8. Hauth JC, Ewell MG, Levine RJ, et al; Calcium for Preeclampsia Prevention Study Group. Pregnancy outcomes in healthy nulliparas who developed hypertension. Obstet Gynecol. 2000;95(1):24-28.
9. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009; 114(5):961-970.
10. Lindheimer MD, Taler SJ, Cunningham FG; American Society of Hypertension (ASH). ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11(4):214-225.
11. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):
1243-1249.
12. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105 (11):1177-1184.
13. Visintin C, Mugglestone MA, Almerie MQ, et al; Guideline Development Group. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207.
14. ACOG Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for Obstetrician–Gynecologists. Diagnosis and management of preeclampsia and eclampsia: ACOG practice bulletin No. 33. Obstet Gynecol. 2002;99:159-167.
15. Parazzini F, Bortolus R, Chatenoud L, et al; Italian Study of Aspirin in Pregnancy Group. Risk factors for pregnancy-induced hypertension in women at high risk for the condition. Epidemiology. 1996;7(3):306-308.
16. Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am J Obstet Gynecol. 2006;194(4):916-920.
17. Barton JR, O’Brien JM, Bergauer NK, et al. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184(5):979-983.
18. Brown MA, Mackenzie C, Dunsmuir W, et al. Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG. 2007; 114(8):984-993.
19. Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension. 2011;58(4):704-708.
20. Hawkins TL, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484-492.
21. National Institute for Health and Clinical Excellence. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. www.nice.org.uk/nicemedia/live/13098/50418/50418.pdf. Accessed April 16, 2012.
22. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
23. Caughey AB, Sundaram V, Kaimal AJ, et al. Maternal and neonatal outcomes of elective induction of labor. Evid Rep Technol Assess (Full Rep). 2009;(176):1-257.
24. Shennan A, Hezelgrave N. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre-eclampsia at term (HYPITAT trial). BJOG. 2010;117(13):1575-1576.
25. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD002252.
26. Nabhan AF, Elsedawy MM. Tight control of mild-moderate pre-existing or non-proteinuric gestational hypertension. Cochrane Database Syst Rev. 2011 Jul 6;(7):CD006907.
27. Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
28. Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005;62(16):1663-1682.
29. FamilyDoctor.org. Pregnancy-induced hypertension (updated 2010). http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview
.all.html. Accessed April 16, 2012.
30. Zamorski MA, Green LA. NHBPEP report on high blood pressure in pregnancy: a summary for family physicians. Am Fam Physician. 2001; 64(2):263-270.
31. Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. BJOG. 2007;114(8): 933-943.
32. Ferrazzani S, De Carolis S, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: relationship with renal impairment and week of delivery. Am J Obstet Gynecol. 1994;171(2):506-512.
33. Beardmore KS, Morris JM, Gallery ED. Excretion of antihypertensive medication into human breast milk: a systematic review. Hypertens Pregnancy. 2002;21(1):85-95.
34. Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845.
1. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.
2. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181-192.
3. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009; 113(6):1299-1306.
4. Villar J, Carroli, G, Wojdyla D, et al; World Health Organization Antenatal Care Trial Research Group. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions. Am J Obstet Gynecol. 2006;194(4):921-931.
5. Leeman L, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2008;78(1):
93-100.
6. Magee LA, Helewa M, Moutquin JM, von Dadelszen P; Hypertension Guideline Committee; Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3 suppl):S1-S48.
7. Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66-71.
8. Hauth JC, Ewell MG, Levine RJ, et al; Calcium for Preeclampsia Prevention Study Group. Pregnancy outcomes in healthy nulliparas who developed hypertension. Obstet Gynecol. 2000;95(1):24-28.
9. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009; 114(5):961-970.
10. Lindheimer MD, Taler SJ, Cunningham FG; American Society of Hypertension (ASH). ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11(4):214-225.
11. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):
1243-1249.
12. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105 (11):1177-1184.
13. Visintin C, Mugglestone MA, Almerie MQ, et al; Guideline Development Group. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207.
14. ACOG Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for Obstetrician–Gynecologists. Diagnosis and management of preeclampsia and eclampsia: ACOG practice bulletin No. 33. Obstet Gynecol. 2002;99:159-167.
15. Parazzini F, Bortolus R, Chatenoud L, et al; Italian Study of Aspirin in Pregnancy Group. Risk factors for pregnancy-induced hypertension in women at high risk for the condition. Epidemiology. 1996;7(3):306-308.
16. Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am J Obstet Gynecol. 2006;194(4):916-920.
17. Barton JR, O’Brien JM, Bergauer NK, et al. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184(5):979-983.
18. Brown MA, Mackenzie C, Dunsmuir W, et al. Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG. 2007; 114(8):984-993.
19. Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension. 2011;58(4):704-708.
20. Hawkins TL, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484-492.
21. National Institute for Health and Clinical Excellence. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. www.nice.org.uk/nicemedia/live/13098/50418/50418.pdf. Accessed April 16, 2012.
22. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
23. Caughey AB, Sundaram V, Kaimal AJ, et al. Maternal and neonatal outcomes of elective induction of labor. Evid Rep Technol Assess (Full Rep). 2009;(176):1-257.
24. Shennan A, Hezelgrave N. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre-eclampsia at term (HYPITAT trial). BJOG. 2010;117(13):1575-1576.
25. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD002252.
26. Nabhan AF, Elsedawy MM. Tight control of mild-moderate pre-existing or non-proteinuric gestational hypertension. Cochrane Database Syst Rev. 2011 Jul 6;(7):CD006907.
27. Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
28. Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005;62(16):1663-1682.
29. FamilyDoctor.org. Pregnancy-induced hypertension (updated 2010). http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview
.all.html. Accessed April 16, 2012.
30. Zamorski MA, Green LA. NHBPEP report on high blood pressure in pregnancy: a summary for family physicians. Am Fam Physician. 2001; 64(2):263-270.
31. Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. BJOG. 2007;114(8): 933-943.
32. Ferrazzani S, De Carolis S, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: relationship with renal impairment and week of delivery. Am J Obstet Gynecol. 1994;171(2):506-512.
33. Beardmore KS, Morris JM, Gallery ED. Excretion of antihypertensive medication into human breast milk: a systematic review. Hypertens Pregnancy. 2002;21(1):85-95.
34. Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845.
Lay midwives and the ObGyn: Is collaboration risky?
“We have indeed in America medical practitioners not inferior to the best elsewhere; but there is probably no other country in the world in which there is so great a distance and so fatal a difference between the best, the average, and the worst.”
—Flexner report from 19101
ObGyn is a risky specialty, with no guarantee of a perfect outcome, even with the best education, training, and skills. Does collaboration make it riskier? Or can collaboration help you deliver high-quality care to your patients?
This article explores these questions as they relate to provision of health care in collaboration with midwives—specifically, certified nurse midwives (CNMs), who are approved by the American Midwifery Certification Board, and certified professional midwives (CPMs), who are not. (See thebox for a more detailed discussion of different types of midwives in practice today.)
Got acronym fatigue? Here’s a rundown of the various credentials and certifying organizations.
The American College of Nurse-Midwives (ACNM) is a professional organization established in 1955 for certified nurse midwives and certified midwives. ACNM sets standards for academic preparation and clinical practice. For more information, visit http://www.midwife.org.
The American Midwifery Certification Board (AMCB) is the certification organization affiliated with ACNM. This board was formerly called the ACNM Certification Council (ACC). Certification by AMCB is equivalent to certification by ACC.
In 1997, AMCB opened its national certification exam to non-nurse graduates of midwifery education programs and issued the first certified midwife credential. Since 2010, a graduate degree has been required for entry into clinical practice for both certified nurse midwives and certified midwives. http://www.amcbmidwife.org
Certified midwife (CM). In 1996, the ACNM adopted standards for the certification of direct-entry midwives. These midwives undergo the same certification process as certified nurse midwives, but their training does not include education in nursing. CMs must pass the same certification exam as CNMs and must have a master’s degree.
CMs are licensed in only three states: New Jersey, New York, and Rhode Island. New York had the first CM training program and was the first state to recognize the CM credential. It is the only state that has one unified framework for licensing all midwives—both CNMs and CMs.
Certified nurse midwife (CNM). A midwife who has training in both nursing and midwifery. A master’s degree is required for certification. These midwives typically have prescriptive authority for most drugs; are eligible for third-party reimbursement, including Medicaid; and practice independently or in collaborative practice with physicians.
Certified professional midwife (CPM). In the mid 1990s, the CPM credential was developed jointly by the Midwives Alliance of North America (MANA), the North American Registry of Midwives (NARM), and the Midwifery Education Accreditation Council (MEAC). There is no single standard for education; both apprentice-only–trained midwives and midwives who undergo university-affiliated training use the title CPM.
A CPM can learn through a structured program, through apprenticeship, or through self-study. Another route to the credential is current legal recognition to practice in the United Kingdom. CPMs must pass a written and practical exam for certification.
According to MANA, 24 states recognize the CPM credential as the basis for licensure or use the NARM written exam. Some of these states use a different nomenclature. For example, licensed midwife (LM) is used in California, Idaho, Oregon, and Washington; licensed direct-entry midwife (LDM) is used in Utah; and registered midwife (RM) is used in Colorado.
SOURCE: ACOG10
Moving away from a physician-oriented system
Like it or not, change is under way. Subtle but important shifts are taking place in the way maternity care is provided in your community.
The challenges facing our specialty? Ensuring that the highest levels of patient safety and quality care are maintained. And educating federal and state lawmakers, insurers, and the public accordingly.
Free-standing birth centers are gaining prominence
The Patient Protection and Affordable Care Act (ACA) establishes alternative pathways for maternity care. Congress, state lawmakers, and insurers want to know: Can access to quality maternity care be provided at lower cost outside of hospitals or by nonphysicians? The answer isn’t clear.
Under the ACA, free-standing birth centers are a Medicaid maternity-care choice for low-income women. Birth centers appeal to lawmakers and insurers because of their lower cost. For example, in 2008, the average facility cost for a vaginal delivery in a hospital, with no complications and no newborn charges, was $8,920. In 2010, the average facility cost for a similar delivery at a birth center was $2,277.2,3
We know that dollars alone don’t tell the full story—but they’re easy listening to lawmakers’ ears.
Since 2010, Medicaid payments are allowed to go to state-licensed, free-standing birth centers even if they are not operated by or under the supervision of a physician. Before the ACA became law, Medicaid paid only for services provided in ambulatory centers under the supervision or oversight of a physician.
Another important change: Medicaid now reimburses for the services of any provider who practices in a state-licensed, free-standing birth center as long as that provider is practicing within the state’s scope of practice laws and regulations. That means that if a state allows doulas or lay midwives to provide childbirth care in free-standing birth centers, the federal and state Medicaid programs will pay for this care. This policy is consistent with “any willing provider” rules found elsewhere in Medicaid.
There are 215 birth centers in the United States, with more in development. The number of birth centers has increased more than 20% over the past 5 years; they are regulated in 41 states.4
ACOG’s Guidelines for Perinatal Care asserts: “The hospital, including a birthing center within a hospital complex, or free-standing birthing centers that meet the standards of the Accreditation Association of Birth Centers, provide the safest setting for labor, delivery, and the postpartum period.”5
Reimbursements for nonphysicians are increasing
Beginning in 2011, the Medicare program began reimbursing CNMs, the most highly trained midwives, at 100% of the physician payment rate for obstetric services. Until 2011, CNMs were paid at 65% of the physician’s rate for the same billed services.
In addition, from 2011 through 2015, CNMs whose primary care services account for at least 60% of their Medicare-allowed charges will receive Medicare bonus payments of 10%, reflecting Congress’ concern that our nation faces a serious shortage of primary care providers.
Another important provision goes into effect in 2014: All health plans offered in a state insurance exchange must accept and pay any provider recognized under state law for services covered by that plan. CPMs, some of whom are among the least highly trained providers, are licensed to provide maternity care in 24 states. This provision may put pressure on health insurers to pay for maternity care provided by CPMs, regardless of their training and certification, even if the insurer doesn’t contract with these providers.
“Even a normal pregnancy can become high-risk”
In 2008, the Massachusetts legislature debated expanding childbirth care to encompass less highly trained providers. ACOG President Kenneth L. Noller, MD, MS, cautioned them about the move, saying: “Even a normal pregnancy can become high-risk with little or no warning, and serious, sometimes life-threatening complications may arise for the woman and her fetus.”
He noted that shoulder dystocia occurs in one in every 200 births and listed the frequency of other complications:
- prolapsed umbilical cord: 1 in every 200 births
- life-threatening maternal hemorrhage: 1 in 250
- eclamptic seizures: 1 in 500
- uterine inversion: 1 in 700
- Apgar score of 0–3 at 5 minutes: 1 in 100 to 200.
Three years later, ACOG President Richard A. Waldman, MD, and American College of Nurse Midwives (ACNM) President Holly Powell Kennedy, CNM, PhD, wrote: “Collaborative practice [is] the provision of health care by an interdisciplinary team of professionals who collaborate to accomplish a common goal, and is associated with increased efficiency, improved clinical outcomes, and enhanced provider satisfaction.”5
These messages demonstrate the importance of careful use of collaboration to manage risk and maintain the highest standards of patient care. The questions for ObGyns who are considering collaborative practice:
- What is careful use?
- How do you collaborate carefully, without increasing the risks faced by your patients and your practice?
- How do you make collaboration a success?
- ACOG has taken on these questions and offers sound practical advice.

ACOG recommends high standards and clear practice agreements
ObGyns have a long history of collaboration with our nurse-midwife colleagues—possibly one of the strongest collaborative traditions in medicine. ACOG supports the practice and licensure of trained midwives credentialed by the ACNM. CNMs are well-educated, highly trained, and well-integrated into the health-care system.
In addition to the ACNM standards, ACOG supports the “global standards for midwifery education” established by the International Confederation of Midwives (ICM) in 2010:
- The minimum entry level of students is completion of secondary education
- The minimum length of a direct-entry midwifery education program is 3 years
- The minimum length of a post-nursing/health-care provider program is 18 months
- Standards are congruent with current core ICM documents and position statements.
ACOG strongly encourages that in no case should the professional standards of any maternity provider be less than the standards established or accepted by ACOG or the ACNM.
Effective collaboration depends on clear practice agreements between physicians and CNMs, consistent use of shared practice guidelines, and malpractice insurance coverage of all parties. A collaborative agreement that clearly spells out the mechanism for consultation, collaboration, and referral is essential to assure the best care.
The picture gets a little trickier—and riskier—when we look at less-trained maternity providers.
A majority of CPMs lack adequate training
Few of the nation’s 1,400 CPMs in practice today meet the educational and training standards accepted by ACOG and the ACNM. The educational background of CPMs—known in some states as direct entry or lay midwives—varies widely across the nation. Unlike CNMs, CPMs are not required to have a nursing background. They practice primarily in out-of-hospital settings, including birthing centers and private homes. Many CPMs have no formal academic education or medical training, and their training requirements fall short of internationally established standards for midwives and traditional birth attendants.
Other relevant points:
- A person without a high school degree could be licensed as a CPM if he or she passed the certifying exam, observed 20 deliveries, and participated as the primary attendant in 10
- As a group, CPMs have not adopted home-birth patient-selection criteria that are based on generally accepted medical evidence or public safety
- The curriculum, clinical skills training, and experience of CPMs have not been approved by the American Midwifery Certification Board. Nor are they reviewed by the American Board of Obstetrics and Gynecology or the American Board of Family Medicine—recognized authorities in the certification of knowledge and skills associated with the practice of obstetrics.
- The North American Registry of Midwives’ Portfolio Evaluation Process requires midwives to be the primary care provider during 50 home births and to have 3 years’ experience. The average ObGyn resident gets this much experience in 1 month.
CPMs who lack a high school diploma and are apprentice-trained only (without core curriculum training and formal academic experience) clearly do not meet ACOG standards. Therefore, ACOG cautions its Fellows and the public that, for quality and safety reasons, it “does not support the provision of care by … midwives who are not certified by the American Midwifery Certification Board” [ACNM’s accreditation body]. Certification by this board, then, is a good indication of skill.
Requirements for successful collaborative practice
Where can you look for examples of collaboration that work, and for data on the effects of collaboration on health-care outcomes? Four articles in the September 2011 issue of Obstetrics and Gynecology highlight successful models of collaboration between ObGyns and CNMs in very different, well-established maternity programs.6-9 In each article, the authors describe their collaborative practice model in some detail, offering guidance to others interested in successful collaboration. Common threads run through these narratives:
- trust
- communication
- mutual respect
- administrative support for continuing medical education
- consensus meetings
- common adherence to accepted guidelines
- an established support network for back-up and transfer.
The benefits to ObGyns include greater job satisfaction. Benefits to patients include improved health outcomes, as demonstrated, for example, in a model from Washington State: a high rate of vaginal delivery, low rate of cesarean birth, high rate of successful vaginal birth after cesarean (VBAC), and low rate of repeat cesarean delivery.7
ACOG’s policy on collaborative practice finds its origins just over 100 years ago in the Flexner report, quoted at the beginning of this article, which emphasized the need to ensure that medical care in the United States is of no less quality than in other parts of the world.1
Medical education and quality of care have improved dramatically over the past century. ACOG is working to ensure the highest standards of care for pregnant women, standards no lower than for the rest of the population.
Collaboration is a time-honored tradition in ObGyn. Doing it right is key to patient safety.
- How state budget crises are putting the squeeze on Medicaid (and you)
(February 2012) - Is private ObGyn practice on its way out?
with Janelle Yates (October 2011) - 14 questions (and answers) about health reform and you
with Janelle Yates (August 2010)
Acknowledgment
The author acknowledges and thanks ACOG Executive Vice President Hal C. Lawrence III, MD, for his helpful review and comments
We want to hear from you! Tell us what you think.
1. Flexner A. Medical Education in the United States and Canada. A Report to the Carnegie Foundation for the Advancement of Teaching. Bulletin No. 4. 1910. Boston Mass: D. B. Updike, Merrymount Press; 1972.
2. American Association of Birth Centers Uniform Data Set. 2010 Data. Perkiomenville Pa: ASBC; 2011.
3. Facts and Figures 2008. Healthcare Cost and Utilization Project (HCUP). October 2010. Agency for Healthcare Research and Quality Rockville, MD. www.hcup-us.ahrq.gov/reports/factsandfigures/2008/TOC_2008.jsp. Accessed March 30, 2012.
4. American Association of Birth Centers. http://www.birthcenters.org.Accessed March 30, 2012.
5. Waldman RN, Kennedy HP. Collaborative practice between obstetricians and midwives. Obstet Gynecol. 2011;118(3):503-504.
6. Shaw-Battista J, Fineberg A, Boehler B, Skubic B, Woolley D, Tilton Z. Obstetrician and nurse-midwife collaboration: successful public health and private practice partnership. Obstet Gynecol. 2011;118(3):663-672.
7. Darlington A, McBroom K, Warwick S. A Northwest collaborative practice model. Obstet Gynecol. 2011;118(3):673-677.
8. Hutchison MS, Ennis L, Shaw-Battista J, et al. Great minds don’t think alike: collaborative maternity care at San Francisco General Hospital. Obstet Gynecol. 2011;118(3):678-682.
9. DeJoy S, Burkman RT, Graves BW, et al. Making it work: successful collaborative practice. Obstet Gynecol. 2011;118(3):683-686.
10. American Congress of Obstetricians and Gynecologists Glossary of Midwifery Organizations and Terms. Washington DC; 2010.
“We have indeed in America medical practitioners not inferior to the best elsewhere; but there is probably no other country in the world in which there is so great a distance and so fatal a difference between the best, the average, and the worst.”
—Flexner report from 19101
ObGyn is a risky specialty, with no guarantee of a perfect outcome, even with the best education, training, and skills. Does collaboration make it riskier? Or can collaboration help you deliver high-quality care to your patients?
This article explores these questions as they relate to provision of health care in collaboration with midwives—specifically, certified nurse midwives (CNMs), who are approved by the American Midwifery Certification Board, and certified professional midwives (CPMs), who are not. (See thebox for a more detailed discussion of different types of midwives in practice today.)
Got acronym fatigue? Here’s a rundown of the various credentials and certifying organizations.
The American College of Nurse-Midwives (ACNM) is a professional organization established in 1955 for certified nurse midwives and certified midwives. ACNM sets standards for academic preparation and clinical practice. For more information, visit http://www.midwife.org.
The American Midwifery Certification Board (AMCB) is the certification organization affiliated with ACNM. This board was formerly called the ACNM Certification Council (ACC). Certification by AMCB is equivalent to certification by ACC.
In 1997, AMCB opened its national certification exam to non-nurse graduates of midwifery education programs and issued the first certified midwife credential. Since 2010, a graduate degree has been required for entry into clinical practice for both certified nurse midwives and certified midwives. http://www.amcbmidwife.org
Certified midwife (CM). In 1996, the ACNM adopted standards for the certification of direct-entry midwives. These midwives undergo the same certification process as certified nurse midwives, but their training does not include education in nursing. CMs must pass the same certification exam as CNMs and must have a master’s degree.
CMs are licensed in only three states: New Jersey, New York, and Rhode Island. New York had the first CM training program and was the first state to recognize the CM credential. It is the only state that has one unified framework for licensing all midwives—both CNMs and CMs.
Certified nurse midwife (CNM). A midwife who has training in both nursing and midwifery. A master’s degree is required for certification. These midwives typically have prescriptive authority for most drugs; are eligible for third-party reimbursement, including Medicaid; and practice independently or in collaborative practice with physicians.
Certified professional midwife (CPM). In the mid 1990s, the CPM credential was developed jointly by the Midwives Alliance of North America (MANA), the North American Registry of Midwives (NARM), and the Midwifery Education Accreditation Council (MEAC). There is no single standard for education; both apprentice-only–trained midwives and midwives who undergo university-affiliated training use the title CPM.
A CPM can learn through a structured program, through apprenticeship, or through self-study. Another route to the credential is current legal recognition to practice in the United Kingdom. CPMs must pass a written and practical exam for certification.
According to MANA, 24 states recognize the CPM credential as the basis for licensure or use the NARM written exam. Some of these states use a different nomenclature. For example, licensed midwife (LM) is used in California, Idaho, Oregon, and Washington; licensed direct-entry midwife (LDM) is used in Utah; and registered midwife (RM) is used in Colorado.
SOURCE: ACOG10
Moving away from a physician-oriented system
Like it or not, change is under way. Subtle but important shifts are taking place in the way maternity care is provided in your community.
The challenges facing our specialty? Ensuring that the highest levels of patient safety and quality care are maintained. And educating federal and state lawmakers, insurers, and the public accordingly.
Free-standing birth centers are gaining prominence
The Patient Protection and Affordable Care Act (ACA) establishes alternative pathways for maternity care. Congress, state lawmakers, and insurers want to know: Can access to quality maternity care be provided at lower cost outside of hospitals or by nonphysicians? The answer isn’t clear.
Under the ACA, free-standing birth centers are a Medicaid maternity-care choice for low-income women. Birth centers appeal to lawmakers and insurers because of their lower cost. For example, in 2008, the average facility cost for a vaginal delivery in a hospital, with no complications and no newborn charges, was $8,920. In 2010, the average facility cost for a similar delivery at a birth center was $2,277.2,3
We know that dollars alone don’t tell the full story—but they’re easy listening to lawmakers’ ears.
Since 2010, Medicaid payments are allowed to go to state-licensed, free-standing birth centers even if they are not operated by or under the supervision of a physician. Before the ACA became law, Medicaid paid only for services provided in ambulatory centers under the supervision or oversight of a physician.
Another important change: Medicaid now reimburses for the services of any provider who practices in a state-licensed, free-standing birth center as long as that provider is practicing within the state’s scope of practice laws and regulations. That means that if a state allows doulas or lay midwives to provide childbirth care in free-standing birth centers, the federal and state Medicaid programs will pay for this care. This policy is consistent with “any willing provider” rules found elsewhere in Medicaid.
There are 215 birth centers in the United States, with more in development. The number of birth centers has increased more than 20% over the past 5 years; they are regulated in 41 states.4
ACOG’s Guidelines for Perinatal Care asserts: “The hospital, including a birthing center within a hospital complex, or free-standing birthing centers that meet the standards of the Accreditation Association of Birth Centers, provide the safest setting for labor, delivery, and the postpartum period.”5
Reimbursements for nonphysicians are increasing
Beginning in 2011, the Medicare program began reimbursing CNMs, the most highly trained midwives, at 100% of the physician payment rate for obstetric services. Until 2011, CNMs were paid at 65% of the physician’s rate for the same billed services.
In addition, from 2011 through 2015, CNMs whose primary care services account for at least 60% of their Medicare-allowed charges will receive Medicare bonus payments of 10%, reflecting Congress’ concern that our nation faces a serious shortage of primary care providers.
Another important provision goes into effect in 2014: All health plans offered in a state insurance exchange must accept and pay any provider recognized under state law for services covered by that plan. CPMs, some of whom are among the least highly trained providers, are licensed to provide maternity care in 24 states. This provision may put pressure on health insurers to pay for maternity care provided by CPMs, regardless of their training and certification, even if the insurer doesn’t contract with these providers.
“Even a normal pregnancy can become high-risk”
In 2008, the Massachusetts legislature debated expanding childbirth care to encompass less highly trained providers. ACOG President Kenneth L. Noller, MD, MS, cautioned them about the move, saying: “Even a normal pregnancy can become high-risk with little or no warning, and serious, sometimes life-threatening complications may arise for the woman and her fetus.”
He noted that shoulder dystocia occurs in one in every 200 births and listed the frequency of other complications:
- prolapsed umbilical cord: 1 in every 200 births
- life-threatening maternal hemorrhage: 1 in 250
- eclamptic seizures: 1 in 500
- uterine inversion: 1 in 700
- Apgar score of 0–3 at 5 minutes: 1 in 100 to 200.
Three years later, ACOG President Richard A. Waldman, MD, and American College of Nurse Midwives (ACNM) President Holly Powell Kennedy, CNM, PhD, wrote: “Collaborative practice [is] the provision of health care by an interdisciplinary team of professionals who collaborate to accomplish a common goal, and is associated with increased efficiency, improved clinical outcomes, and enhanced provider satisfaction.”5
These messages demonstrate the importance of careful use of collaboration to manage risk and maintain the highest standards of patient care. The questions for ObGyns who are considering collaborative practice:
- What is careful use?
- How do you collaborate carefully, without increasing the risks faced by your patients and your practice?
- How do you make collaboration a success?
- ACOG has taken on these questions and offers sound practical advice.

ACOG recommends high standards and clear practice agreements
ObGyns have a long history of collaboration with our nurse-midwife colleagues—possibly one of the strongest collaborative traditions in medicine. ACOG supports the practice and licensure of trained midwives credentialed by the ACNM. CNMs are well-educated, highly trained, and well-integrated into the health-care system.
In addition to the ACNM standards, ACOG supports the “global standards for midwifery education” established by the International Confederation of Midwives (ICM) in 2010:
- The minimum entry level of students is completion of secondary education
- The minimum length of a direct-entry midwifery education program is 3 years
- The minimum length of a post-nursing/health-care provider program is 18 months
- Standards are congruent with current core ICM documents and position statements.
ACOG strongly encourages that in no case should the professional standards of any maternity provider be less than the standards established or accepted by ACOG or the ACNM.
Effective collaboration depends on clear practice agreements between physicians and CNMs, consistent use of shared practice guidelines, and malpractice insurance coverage of all parties. A collaborative agreement that clearly spells out the mechanism for consultation, collaboration, and referral is essential to assure the best care.
The picture gets a little trickier—and riskier—when we look at less-trained maternity providers.
A majority of CPMs lack adequate training
Few of the nation’s 1,400 CPMs in practice today meet the educational and training standards accepted by ACOG and the ACNM. The educational background of CPMs—known in some states as direct entry or lay midwives—varies widely across the nation. Unlike CNMs, CPMs are not required to have a nursing background. They practice primarily in out-of-hospital settings, including birthing centers and private homes. Many CPMs have no formal academic education or medical training, and their training requirements fall short of internationally established standards for midwives and traditional birth attendants.
Other relevant points:
- A person without a high school degree could be licensed as a CPM if he or she passed the certifying exam, observed 20 deliveries, and participated as the primary attendant in 10
- As a group, CPMs have not adopted home-birth patient-selection criteria that are based on generally accepted medical evidence or public safety
- The curriculum, clinical skills training, and experience of CPMs have not been approved by the American Midwifery Certification Board. Nor are they reviewed by the American Board of Obstetrics and Gynecology or the American Board of Family Medicine—recognized authorities in the certification of knowledge and skills associated with the practice of obstetrics.
- The North American Registry of Midwives’ Portfolio Evaluation Process requires midwives to be the primary care provider during 50 home births and to have 3 years’ experience. The average ObGyn resident gets this much experience in 1 month.
CPMs who lack a high school diploma and are apprentice-trained only (without core curriculum training and formal academic experience) clearly do not meet ACOG standards. Therefore, ACOG cautions its Fellows and the public that, for quality and safety reasons, it “does not support the provision of care by … midwives who are not certified by the American Midwifery Certification Board” [ACNM’s accreditation body]. Certification by this board, then, is a good indication of skill.
Requirements for successful collaborative practice
Where can you look for examples of collaboration that work, and for data on the effects of collaboration on health-care outcomes? Four articles in the September 2011 issue of Obstetrics and Gynecology highlight successful models of collaboration between ObGyns and CNMs in very different, well-established maternity programs.6-9 In each article, the authors describe their collaborative practice model in some detail, offering guidance to others interested in successful collaboration. Common threads run through these narratives:
- trust
- communication
- mutual respect
- administrative support for continuing medical education
- consensus meetings
- common adherence to accepted guidelines
- an established support network for back-up and transfer.
The benefits to ObGyns include greater job satisfaction. Benefits to patients include improved health outcomes, as demonstrated, for example, in a model from Washington State: a high rate of vaginal delivery, low rate of cesarean birth, high rate of successful vaginal birth after cesarean (VBAC), and low rate of repeat cesarean delivery.7
ACOG’s policy on collaborative practice finds its origins just over 100 years ago in the Flexner report, quoted at the beginning of this article, which emphasized the need to ensure that medical care in the United States is of no less quality than in other parts of the world.1
Medical education and quality of care have improved dramatically over the past century. ACOG is working to ensure the highest standards of care for pregnant women, standards no lower than for the rest of the population.
Collaboration is a time-honored tradition in ObGyn. Doing it right is key to patient safety.
- How state budget crises are putting the squeeze on Medicaid (and you)
(February 2012) - Is private ObGyn practice on its way out?
with Janelle Yates (October 2011) - 14 questions (and answers) about health reform and you
with Janelle Yates (August 2010)
Acknowledgment
The author acknowledges and thanks ACOG Executive Vice President Hal C. Lawrence III, MD, for his helpful review and comments
We want to hear from you! Tell us what you think.
“We have indeed in America medical practitioners not inferior to the best elsewhere; but there is probably no other country in the world in which there is so great a distance and so fatal a difference between the best, the average, and the worst.”
—Flexner report from 19101
ObGyn is a risky specialty, with no guarantee of a perfect outcome, even with the best education, training, and skills. Does collaboration make it riskier? Or can collaboration help you deliver high-quality care to your patients?
This article explores these questions as they relate to provision of health care in collaboration with midwives—specifically, certified nurse midwives (CNMs), who are approved by the American Midwifery Certification Board, and certified professional midwives (CPMs), who are not. (See thebox for a more detailed discussion of different types of midwives in practice today.)
Got acronym fatigue? Here’s a rundown of the various credentials and certifying organizations.
The American College of Nurse-Midwives (ACNM) is a professional organization established in 1955 for certified nurse midwives and certified midwives. ACNM sets standards for academic preparation and clinical practice. For more information, visit http://www.midwife.org.
The American Midwifery Certification Board (AMCB) is the certification organization affiliated with ACNM. This board was formerly called the ACNM Certification Council (ACC). Certification by AMCB is equivalent to certification by ACC.
In 1997, AMCB opened its national certification exam to non-nurse graduates of midwifery education programs and issued the first certified midwife credential. Since 2010, a graduate degree has been required for entry into clinical practice for both certified nurse midwives and certified midwives. http://www.amcbmidwife.org
Certified midwife (CM). In 1996, the ACNM adopted standards for the certification of direct-entry midwives. These midwives undergo the same certification process as certified nurse midwives, but their training does not include education in nursing. CMs must pass the same certification exam as CNMs and must have a master’s degree.
CMs are licensed in only three states: New Jersey, New York, and Rhode Island. New York had the first CM training program and was the first state to recognize the CM credential. It is the only state that has one unified framework for licensing all midwives—both CNMs and CMs.
Certified nurse midwife (CNM). A midwife who has training in both nursing and midwifery. A master’s degree is required for certification. These midwives typically have prescriptive authority for most drugs; are eligible for third-party reimbursement, including Medicaid; and practice independently or in collaborative practice with physicians.
Certified professional midwife (CPM). In the mid 1990s, the CPM credential was developed jointly by the Midwives Alliance of North America (MANA), the North American Registry of Midwives (NARM), and the Midwifery Education Accreditation Council (MEAC). There is no single standard for education; both apprentice-only–trained midwives and midwives who undergo university-affiliated training use the title CPM.
A CPM can learn through a structured program, through apprenticeship, or through self-study. Another route to the credential is current legal recognition to practice in the United Kingdom. CPMs must pass a written and practical exam for certification.
According to MANA, 24 states recognize the CPM credential as the basis for licensure or use the NARM written exam. Some of these states use a different nomenclature. For example, licensed midwife (LM) is used in California, Idaho, Oregon, and Washington; licensed direct-entry midwife (LDM) is used in Utah; and registered midwife (RM) is used in Colorado.
SOURCE: ACOG10
Moving away from a physician-oriented system
Like it or not, change is under way. Subtle but important shifts are taking place in the way maternity care is provided in your community.
The challenges facing our specialty? Ensuring that the highest levels of patient safety and quality care are maintained. And educating federal and state lawmakers, insurers, and the public accordingly.
Free-standing birth centers are gaining prominence
The Patient Protection and Affordable Care Act (ACA) establishes alternative pathways for maternity care. Congress, state lawmakers, and insurers want to know: Can access to quality maternity care be provided at lower cost outside of hospitals or by nonphysicians? The answer isn’t clear.
Under the ACA, free-standing birth centers are a Medicaid maternity-care choice for low-income women. Birth centers appeal to lawmakers and insurers because of their lower cost. For example, in 2008, the average facility cost for a vaginal delivery in a hospital, with no complications and no newborn charges, was $8,920. In 2010, the average facility cost for a similar delivery at a birth center was $2,277.2,3
We know that dollars alone don’t tell the full story—but they’re easy listening to lawmakers’ ears.
Since 2010, Medicaid payments are allowed to go to state-licensed, free-standing birth centers even if they are not operated by or under the supervision of a physician. Before the ACA became law, Medicaid paid only for services provided in ambulatory centers under the supervision or oversight of a physician.
Another important change: Medicaid now reimburses for the services of any provider who practices in a state-licensed, free-standing birth center as long as that provider is practicing within the state’s scope of practice laws and regulations. That means that if a state allows doulas or lay midwives to provide childbirth care in free-standing birth centers, the federal and state Medicaid programs will pay for this care. This policy is consistent with “any willing provider” rules found elsewhere in Medicaid.
There are 215 birth centers in the United States, with more in development. The number of birth centers has increased more than 20% over the past 5 years; they are regulated in 41 states.4
ACOG’s Guidelines for Perinatal Care asserts: “The hospital, including a birthing center within a hospital complex, or free-standing birthing centers that meet the standards of the Accreditation Association of Birth Centers, provide the safest setting for labor, delivery, and the postpartum period.”5
Reimbursements for nonphysicians are increasing
Beginning in 2011, the Medicare program began reimbursing CNMs, the most highly trained midwives, at 100% of the physician payment rate for obstetric services. Until 2011, CNMs were paid at 65% of the physician’s rate for the same billed services.
In addition, from 2011 through 2015, CNMs whose primary care services account for at least 60% of their Medicare-allowed charges will receive Medicare bonus payments of 10%, reflecting Congress’ concern that our nation faces a serious shortage of primary care providers.
Another important provision goes into effect in 2014: All health plans offered in a state insurance exchange must accept and pay any provider recognized under state law for services covered by that plan. CPMs, some of whom are among the least highly trained providers, are licensed to provide maternity care in 24 states. This provision may put pressure on health insurers to pay for maternity care provided by CPMs, regardless of their training and certification, even if the insurer doesn’t contract with these providers.
“Even a normal pregnancy can become high-risk”
In 2008, the Massachusetts legislature debated expanding childbirth care to encompass less highly trained providers. ACOG President Kenneth L. Noller, MD, MS, cautioned them about the move, saying: “Even a normal pregnancy can become high-risk with little or no warning, and serious, sometimes life-threatening complications may arise for the woman and her fetus.”
He noted that shoulder dystocia occurs in one in every 200 births and listed the frequency of other complications:
- prolapsed umbilical cord: 1 in every 200 births
- life-threatening maternal hemorrhage: 1 in 250
- eclamptic seizures: 1 in 500
- uterine inversion: 1 in 700
- Apgar score of 0–3 at 5 minutes: 1 in 100 to 200.
Three years later, ACOG President Richard A. Waldman, MD, and American College of Nurse Midwives (ACNM) President Holly Powell Kennedy, CNM, PhD, wrote: “Collaborative practice [is] the provision of health care by an interdisciplinary team of professionals who collaborate to accomplish a common goal, and is associated with increased efficiency, improved clinical outcomes, and enhanced provider satisfaction.”5
These messages demonstrate the importance of careful use of collaboration to manage risk and maintain the highest standards of patient care. The questions for ObGyns who are considering collaborative practice:
- What is careful use?
- How do you collaborate carefully, without increasing the risks faced by your patients and your practice?
- How do you make collaboration a success?
- ACOG has taken on these questions and offers sound practical advice.

ACOG recommends high standards and clear practice agreements
ObGyns have a long history of collaboration with our nurse-midwife colleagues—possibly one of the strongest collaborative traditions in medicine. ACOG supports the practice and licensure of trained midwives credentialed by the ACNM. CNMs are well-educated, highly trained, and well-integrated into the health-care system.
In addition to the ACNM standards, ACOG supports the “global standards for midwifery education” established by the International Confederation of Midwives (ICM) in 2010:
- The minimum entry level of students is completion of secondary education
- The minimum length of a direct-entry midwifery education program is 3 years
- The minimum length of a post-nursing/health-care provider program is 18 months
- Standards are congruent with current core ICM documents and position statements.
ACOG strongly encourages that in no case should the professional standards of any maternity provider be less than the standards established or accepted by ACOG or the ACNM.
Effective collaboration depends on clear practice agreements between physicians and CNMs, consistent use of shared practice guidelines, and malpractice insurance coverage of all parties. A collaborative agreement that clearly spells out the mechanism for consultation, collaboration, and referral is essential to assure the best care.
The picture gets a little trickier—and riskier—when we look at less-trained maternity providers.
A majority of CPMs lack adequate training
Few of the nation’s 1,400 CPMs in practice today meet the educational and training standards accepted by ACOG and the ACNM. The educational background of CPMs—known in some states as direct entry or lay midwives—varies widely across the nation. Unlike CNMs, CPMs are not required to have a nursing background. They practice primarily in out-of-hospital settings, including birthing centers and private homes. Many CPMs have no formal academic education or medical training, and their training requirements fall short of internationally established standards for midwives and traditional birth attendants.
Other relevant points:
- A person without a high school degree could be licensed as a CPM if he or she passed the certifying exam, observed 20 deliveries, and participated as the primary attendant in 10
- As a group, CPMs have not adopted home-birth patient-selection criteria that are based on generally accepted medical evidence or public safety
- The curriculum, clinical skills training, and experience of CPMs have not been approved by the American Midwifery Certification Board. Nor are they reviewed by the American Board of Obstetrics and Gynecology or the American Board of Family Medicine—recognized authorities in the certification of knowledge and skills associated with the practice of obstetrics.
- The North American Registry of Midwives’ Portfolio Evaluation Process requires midwives to be the primary care provider during 50 home births and to have 3 years’ experience. The average ObGyn resident gets this much experience in 1 month.
CPMs who lack a high school diploma and are apprentice-trained only (without core curriculum training and formal academic experience) clearly do not meet ACOG standards. Therefore, ACOG cautions its Fellows and the public that, for quality and safety reasons, it “does not support the provision of care by … midwives who are not certified by the American Midwifery Certification Board” [ACNM’s accreditation body]. Certification by this board, then, is a good indication of skill.
Requirements for successful collaborative practice
Where can you look for examples of collaboration that work, and for data on the effects of collaboration on health-care outcomes? Four articles in the September 2011 issue of Obstetrics and Gynecology highlight successful models of collaboration between ObGyns and CNMs in very different, well-established maternity programs.6-9 In each article, the authors describe their collaborative practice model in some detail, offering guidance to others interested in successful collaboration. Common threads run through these narratives:
- trust
- communication
- mutual respect
- administrative support for continuing medical education
- consensus meetings
- common adherence to accepted guidelines
- an established support network for back-up and transfer.
The benefits to ObGyns include greater job satisfaction. Benefits to patients include improved health outcomes, as demonstrated, for example, in a model from Washington State: a high rate of vaginal delivery, low rate of cesarean birth, high rate of successful vaginal birth after cesarean (VBAC), and low rate of repeat cesarean delivery.7
ACOG’s policy on collaborative practice finds its origins just over 100 years ago in the Flexner report, quoted at the beginning of this article, which emphasized the need to ensure that medical care in the United States is of no less quality than in other parts of the world.1
Medical education and quality of care have improved dramatically over the past century. ACOG is working to ensure the highest standards of care for pregnant women, standards no lower than for the rest of the population.
Collaboration is a time-honored tradition in ObGyn. Doing it right is key to patient safety.
- How state budget crises are putting the squeeze on Medicaid (and you)
(February 2012) - Is private ObGyn practice on its way out?
with Janelle Yates (October 2011) - 14 questions (and answers) about health reform and you
with Janelle Yates (August 2010)
Acknowledgment
The author acknowledges and thanks ACOG Executive Vice President Hal C. Lawrence III, MD, for his helpful review and comments
We want to hear from you! Tell us what you think.
1. Flexner A. Medical Education in the United States and Canada. A Report to the Carnegie Foundation for the Advancement of Teaching. Bulletin No. 4. 1910. Boston Mass: D. B. Updike, Merrymount Press; 1972.
2. American Association of Birth Centers Uniform Data Set. 2010 Data. Perkiomenville Pa: ASBC; 2011.
3. Facts and Figures 2008. Healthcare Cost and Utilization Project (HCUP). October 2010. Agency for Healthcare Research and Quality Rockville, MD. www.hcup-us.ahrq.gov/reports/factsandfigures/2008/TOC_2008.jsp. Accessed March 30, 2012.
4. American Association of Birth Centers. http://www.birthcenters.org.Accessed March 30, 2012.
5. Waldman RN, Kennedy HP. Collaborative practice between obstetricians and midwives. Obstet Gynecol. 2011;118(3):503-504.
6. Shaw-Battista J, Fineberg A, Boehler B, Skubic B, Woolley D, Tilton Z. Obstetrician and nurse-midwife collaboration: successful public health and private practice partnership. Obstet Gynecol. 2011;118(3):663-672.
7. Darlington A, McBroom K, Warwick S. A Northwest collaborative practice model. Obstet Gynecol. 2011;118(3):673-677.
8. Hutchison MS, Ennis L, Shaw-Battista J, et al. Great minds don’t think alike: collaborative maternity care at San Francisco General Hospital. Obstet Gynecol. 2011;118(3):678-682.
9. DeJoy S, Burkman RT, Graves BW, et al. Making it work: successful collaborative practice. Obstet Gynecol. 2011;118(3):683-686.
10. American Congress of Obstetricians and Gynecologists Glossary of Midwifery Organizations and Terms. Washington DC; 2010.
1. Flexner A. Medical Education in the United States and Canada. A Report to the Carnegie Foundation for the Advancement of Teaching. Bulletin No. 4. 1910. Boston Mass: D. B. Updike, Merrymount Press; 1972.
2. American Association of Birth Centers Uniform Data Set. 2010 Data. Perkiomenville Pa: ASBC; 2011.
3. Facts and Figures 2008. Healthcare Cost and Utilization Project (HCUP). October 2010. Agency for Healthcare Research and Quality Rockville, MD. www.hcup-us.ahrq.gov/reports/factsandfigures/2008/TOC_2008.jsp. Accessed March 30, 2012.
4. American Association of Birth Centers. http://www.birthcenters.org.Accessed March 30, 2012.
5. Waldman RN, Kennedy HP. Collaborative practice between obstetricians and midwives. Obstet Gynecol. 2011;118(3):503-504.
6. Shaw-Battista J, Fineberg A, Boehler B, Skubic B, Woolley D, Tilton Z. Obstetrician and nurse-midwife collaboration: successful public health and private practice partnership. Obstet Gynecol. 2011;118(3):663-672.
7. Darlington A, McBroom K, Warwick S. A Northwest collaborative practice model. Obstet Gynecol. 2011;118(3):673-677.
8. Hutchison MS, Ennis L, Shaw-Battista J, et al. Great minds don’t think alike: collaborative maternity care at San Francisco General Hospital. Obstet Gynecol. 2011;118(3):678-682.
9. DeJoy S, Burkman RT, Graves BW, et al. Making it work: successful collaborative practice. Obstet Gynecol. 2011;118(3):683-686.
10. American Congress of Obstetricians and Gynecologists Glossary of Midwifery Organizations and Terms. Washington DC; 2010.
When macrosomia is suspected at term, does induction of labor lower the risk of cesarean delivery?
Fetal and neonatal macrosomia can lead to morbidity for both mother and infant. Larger babies put the mother at risk of cesarean delivery, severe perineal lacerations, and hemorrhage. The macrosomic fetus faces an elevated risk of birth trauma, shoulder dystocia, and metabolic disorders.
Earlier investigations have concluded that induction of labor does not improve outcomes and may increase the risk of cesarean delivery.1 The American Congress of Obstetricians and Gynecologists (ACOG) does not support suspected fetal macrosomia as an indication for induction of labor.2
Details of the study
The objective of this study was to determine whether women who were carrying a macrosomic fetus and who underwent induction of labor had a higher rate of cesarean delivery than those who were managed expectantly. Using data from the 2003 Vital Statistics Natality birth certificate registry, Cheng and colleagues compared women who underwent induction of labor at 39 weeks with women who were managed expectantly and who delivered at 40, 41, or 42 weeks (by induced or spontaneous labor).
Investigators attempted to adjust for normal gestational growth by assuming a fetal weight gain of 200 g for each additional week of gestation in the women managed expectantly. For instance, one group included women who delivered at 39 weeks (birth weight of 3,875–4,125 g), and they were compared with the group of women who delivered at 40 weeks (birth weight of 4,075–4,325 g), 41 weeks (4,275–4,525 g), and 42 weeks (4,475–4,725 g).
Using this scheme, cesarean delivery was lower in the group of women who underwent induction of labor. The induced groups were also found to have lower odds of composite neonatal morbidity.
Strengths and limitations
Because this was a retrospective study, investigators were able to use known birth weights, rather than estimated birth weights, to overcome misclassifications that can arise with estimates.
Cheng and colleagues refuted the findings of earlier studies that found a higher risk of cesarean delivery with induction of labor. They argued that those investigations compared women who underwent induction of labor with those who experienced spontaneous labor instead of the proper comparison—between women who underwent induction of labor and those who were managed expectantly. Although the comparisons they used in this study alleviate that problem, the retrospective nature of the study necessitated the use of multiple assumptions to allocate each group, creating selection bias.
Group allocations and medical histories cannot be confirmed, and the investigators acknowledge that their conclusions regarding neonatal morbidity lack statistical power.
This study explores an important issue—the prevention of cesarean delivery and poor neonatal outcomes associated with macrosomia. The comparisons in this investigation cast earlier conclusions in question and elucidate potential improvements in neonatal outcomes.
However, because of the numerous assumptions underlying the study groups, I would not recommend induction of labor to reduce the rate of cesarean delivery until further prospective data are available.
Jennifer T. Ahn, MD
We want to hear from you! Tell us what you think.
Fetal and neonatal macrosomia can lead to morbidity for both mother and infant. Larger babies put the mother at risk of cesarean delivery, severe perineal lacerations, and hemorrhage. The macrosomic fetus faces an elevated risk of birth trauma, shoulder dystocia, and metabolic disorders.
Earlier investigations have concluded that induction of labor does not improve outcomes and may increase the risk of cesarean delivery.1 The American Congress of Obstetricians and Gynecologists (ACOG) does not support suspected fetal macrosomia as an indication for induction of labor.2
Details of the study
The objective of this study was to determine whether women who were carrying a macrosomic fetus and who underwent induction of labor had a higher rate of cesarean delivery than those who were managed expectantly. Using data from the 2003 Vital Statistics Natality birth certificate registry, Cheng and colleagues compared women who underwent induction of labor at 39 weeks with women who were managed expectantly and who delivered at 40, 41, or 42 weeks (by induced or spontaneous labor).
Investigators attempted to adjust for normal gestational growth by assuming a fetal weight gain of 200 g for each additional week of gestation in the women managed expectantly. For instance, one group included women who delivered at 39 weeks (birth weight of 3,875–4,125 g), and they were compared with the group of women who delivered at 40 weeks (birth weight of 4,075–4,325 g), 41 weeks (4,275–4,525 g), and 42 weeks (4,475–4,725 g).
Using this scheme, cesarean delivery was lower in the group of women who underwent induction of labor. The induced groups were also found to have lower odds of composite neonatal morbidity.
Strengths and limitations
Because this was a retrospective study, investigators were able to use known birth weights, rather than estimated birth weights, to overcome misclassifications that can arise with estimates.
Cheng and colleagues refuted the findings of earlier studies that found a higher risk of cesarean delivery with induction of labor. They argued that those investigations compared women who underwent induction of labor with those who experienced spontaneous labor instead of the proper comparison—between women who underwent induction of labor and those who were managed expectantly. Although the comparisons they used in this study alleviate that problem, the retrospective nature of the study necessitated the use of multiple assumptions to allocate each group, creating selection bias.
Group allocations and medical histories cannot be confirmed, and the investigators acknowledge that their conclusions regarding neonatal morbidity lack statistical power.
This study explores an important issue—the prevention of cesarean delivery and poor neonatal outcomes associated with macrosomia. The comparisons in this investigation cast earlier conclusions in question and elucidate potential improvements in neonatal outcomes.
However, because of the numerous assumptions underlying the study groups, I would not recommend induction of labor to reduce the rate of cesarean delivery until further prospective data are available.
Jennifer T. Ahn, MD
We want to hear from you! Tell us what you think.
Fetal and neonatal macrosomia can lead to morbidity for both mother and infant. Larger babies put the mother at risk of cesarean delivery, severe perineal lacerations, and hemorrhage. The macrosomic fetus faces an elevated risk of birth trauma, shoulder dystocia, and metabolic disorders.
Earlier investigations have concluded that induction of labor does not improve outcomes and may increase the risk of cesarean delivery.1 The American Congress of Obstetricians and Gynecologists (ACOG) does not support suspected fetal macrosomia as an indication for induction of labor.2
Details of the study
The objective of this study was to determine whether women who were carrying a macrosomic fetus and who underwent induction of labor had a higher rate of cesarean delivery than those who were managed expectantly. Using data from the 2003 Vital Statistics Natality birth certificate registry, Cheng and colleagues compared women who underwent induction of labor at 39 weeks with women who were managed expectantly and who delivered at 40, 41, or 42 weeks (by induced or spontaneous labor).
Investigators attempted to adjust for normal gestational growth by assuming a fetal weight gain of 200 g for each additional week of gestation in the women managed expectantly. For instance, one group included women who delivered at 39 weeks (birth weight of 3,875–4,125 g), and they were compared with the group of women who delivered at 40 weeks (birth weight of 4,075–4,325 g), 41 weeks (4,275–4,525 g), and 42 weeks (4,475–4,725 g).
Using this scheme, cesarean delivery was lower in the group of women who underwent induction of labor. The induced groups were also found to have lower odds of composite neonatal morbidity.
Strengths and limitations
Because this was a retrospective study, investigators were able to use known birth weights, rather than estimated birth weights, to overcome misclassifications that can arise with estimates.
Cheng and colleagues refuted the findings of earlier studies that found a higher risk of cesarean delivery with induction of labor. They argued that those investigations compared women who underwent induction of labor with those who experienced spontaneous labor instead of the proper comparison—between women who underwent induction of labor and those who were managed expectantly. Although the comparisons they used in this study alleviate that problem, the retrospective nature of the study necessitated the use of multiple assumptions to allocate each group, creating selection bias.
Group allocations and medical histories cannot be confirmed, and the investigators acknowledge that their conclusions regarding neonatal morbidity lack statistical power.
This study explores an important issue—the prevention of cesarean delivery and poor neonatal outcomes associated with macrosomia. The comparisons in this investigation cast earlier conclusions in question and elucidate potential improvements in neonatal outcomes.
However, because of the numerous assumptions underlying the study groups, I would not recommend induction of labor to reduce the rate of cesarean delivery until further prospective data are available.
Jennifer T. Ahn, MD
We want to hear from you! Tell us what you think.
Pregnant Teens in Latin America Need Psychiatric Care
BOGOTÁ, COLOMBIA – Adolescent girls who become pregnant and carry to term experience high morbidity, including psychiatric outcomes, numerous studies have established. And, in Latin America, teenage pregnancy rates are rising, and legal abortion is rarely an option.
Learning how to prevent postpartum depression and psychosis among these girls is something "gleaned over years of clinical practice," says Dr. Roberto Chaskel, who has treated such girls for nearly 3 decades. "No one tells you how."
At the fourth International Congress of Medicine and Women’s Mental Health, Dr. Chaskel presented video of an 11-year-old who had just given birth days earlier by cesarean section and was experiencing psychosis. The girl had been referred for psychiatric care only after the birth.
Adolescent pregnancies have risen in many Latin American countries in recent decades, despite declining fertility trends overall, according to a 2007 United Nations Report. One Latin American study of 854,377 girls aged 15 years and younger found pregnancy associated with a fourfold higher risk of a host of adverse pregnancy outcomes, including maternal death, early neonatal death, and anemia, compared with women aged 20 years or older (Am. J. Obstet. Gynecol. 2005;192:342-9).
(Meanwhile, the Centers for Disease Control and Prevention reported recently that teenage pregnancy rates in the United States declined 9% from 2009 to 2010, which means that the rate is at an historic low of 34.3 births/1,000 adolescents aged 15-19 years. The decline was seen across all ethnicities.)
In most Latin American countries, including Colombia – where access to abortion is highly restricted, pregnancies among girls aged 15 years and younger often result in birth. Only three Caribbean or Latin American states permit abortion without regard to reason, according to a 2012 report by the Guttmacher Institute. Illegal procedures are estimated to represent 95% of all abortions performed in the region, according to the same report.
In 2006, in Bogotá there were 22,228 pregnancies reported among teenage girls between 10 and 19 years of age (170/100,000), placing Bogotá’s teenage pregnancy rates nearly on nearly on par with those of Uganda and Sierra Leone, according to a 2010 study by researchers at the Universidad Nacional de Colombia. A tenth of the reported pregnancies were to women aged 14 years and younger.
It is possible to prevent psychotic and depressive episodes related to pregnancy and birth and "offer teenagers and adolescents, and the babies of these girls, the best possible quality of life" through a family practice that seeks to mitigate some of the traumas associated with early sex, pregnancy, and birth, and also to guide the early attachment process between mothers and children, said Dr. Chaskel of the department of psychiatry at the Universidad El Bosque and coordinator of child psychiatry at the Fundación Santa Fe, both in Bogotá, Colombia,.
In a separate presentation at the congress, Dr. Marta B. Rondón, a psychiatrist affiliated with the Universidad Peruana Cayetano Heredia in Lima, Peru, discussed the difficulty of achieving an evidence-based understanding of the mental health impact of abortion and unwanted pregnancy in nations where access is restricted and "generally speaking, a woman cannot choose just to terminate a nondesired pregnancy."
In a restricted legal environment, "the condition of secrecy means the woman has to go a very hard road – this could have negative consequences for her mental health – but we don’t have the numbers," Dr. Rondón said, adding that discussing how to conduct research on abortion and mental health in Latin America would be a priority of next year’s international congress on women’s mental health, which will be held in Lima.
Any attempt to get a perspective on the mental health impact of abortion and unwanted pregnancy means using studies conducted in the United States and Europe – different cultural environments that could produce different results. "We need prospective research with randomized samples in Latin America, which may become possible as abortion is gradually decriminalized," Dr. Rondón said.
Currently, Peru and Colombia allow legal abortion only in the event of a threat to the life or physical health of the woman. In addition, Colombia allows terminations in cases of rape or to preserve a woman’s mental health.
Dr. Chaskel said in an interview that the 11-year-old patient in his video would have been a candidate for legal abortion under Colombian law and that he would have referred her had she presented to him early. However, he said, her obstetrician had judged her ineligible.
Their options might be limited in terms of a choice to terminate, but pregnant adolescents in Latin America do have the benefit of strong family ties, and Dr. Chaskel’s practice taps into the high level of familial support available to most. "You bring grandma and grandpa, and the new uncles and aunts into clinical practice – right into the office," he said in an interview. A pregnancy in adolescence "generates confusion not only for the girl but also on the whole family group: In Latin America, this usually means three generations confused as to how to approach this situation."
The initial goal of the interventions, he said, is to help pregnant adolescents avoid postpartum depression and psychosis. The longer-term goal is to allow them to continue in their adolescence as normally as possible after the birth, while developing a healthy attachment to the child with the support of their families.
"Are they going to keep listening to Lady Gaga? If you have an 18-month-old baby, how do you behave? We’ve really worked on recuperating adolescence for these girls to make sure that they do listen to Lady Gaga," he said. "We try to make sure that if they’re going to have the baby, to know what they want to do – go back to school, go to a friend’s 15th birthday party [Quinceañera], or have one themselves.
"We think it over together."
After the birth, infants and their young mothers are observed and evaluated interacting in-office, where such details as the distance the child crawls away from the mother is measured as a way of gauging healthy attachments. The girls, who are at high risk of having second babies while still in their teenage years, need to be followed up for years.
Neither Dr. Chaskel nor Dr. Rondón reported disclosures.
BOGOTÁ, COLOMBIA – Adolescent girls who become pregnant and carry to term experience high morbidity, including psychiatric outcomes, numerous studies have established. And, in Latin America, teenage pregnancy rates are rising, and legal abortion is rarely an option.
Learning how to prevent postpartum depression and psychosis among these girls is something "gleaned over years of clinical practice," says Dr. Roberto Chaskel, who has treated such girls for nearly 3 decades. "No one tells you how."
At the fourth International Congress of Medicine and Women’s Mental Health, Dr. Chaskel presented video of an 11-year-old who had just given birth days earlier by cesarean section and was experiencing psychosis. The girl had been referred for psychiatric care only after the birth.
Adolescent pregnancies have risen in many Latin American countries in recent decades, despite declining fertility trends overall, according to a 2007 United Nations Report. One Latin American study of 854,377 girls aged 15 years and younger found pregnancy associated with a fourfold higher risk of a host of adverse pregnancy outcomes, including maternal death, early neonatal death, and anemia, compared with women aged 20 years or older (Am. J. Obstet. Gynecol. 2005;192:342-9).
(Meanwhile, the Centers for Disease Control and Prevention reported recently that teenage pregnancy rates in the United States declined 9% from 2009 to 2010, which means that the rate is at an historic low of 34.3 births/1,000 adolescents aged 15-19 years. The decline was seen across all ethnicities.)
In most Latin American countries, including Colombia – where access to abortion is highly restricted, pregnancies among girls aged 15 years and younger often result in birth. Only three Caribbean or Latin American states permit abortion without regard to reason, according to a 2012 report by the Guttmacher Institute. Illegal procedures are estimated to represent 95% of all abortions performed in the region, according to the same report.
In 2006, in Bogotá there were 22,228 pregnancies reported among teenage girls between 10 and 19 years of age (170/100,000), placing Bogotá’s teenage pregnancy rates nearly on nearly on par with those of Uganda and Sierra Leone, according to a 2010 study by researchers at the Universidad Nacional de Colombia. A tenth of the reported pregnancies were to women aged 14 years and younger.
It is possible to prevent psychotic and depressive episodes related to pregnancy and birth and "offer teenagers and adolescents, and the babies of these girls, the best possible quality of life" through a family practice that seeks to mitigate some of the traumas associated with early sex, pregnancy, and birth, and also to guide the early attachment process between mothers and children, said Dr. Chaskel of the department of psychiatry at the Universidad El Bosque and coordinator of child psychiatry at the Fundación Santa Fe, both in Bogotá, Colombia,.
In a separate presentation at the congress, Dr. Marta B. Rondón, a psychiatrist affiliated with the Universidad Peruana Cayetano Heredia in Lima, Peru, discussed the difficulty of achieving an evidence-based understanding of the mental health impact of abortion and unwanted pregnancy in nations where access is restricted and "generally speaking, a woman cannot choose just to terminate a nondesired pregnancy."
In a restricted legal environment, "the condition of secrecy means the woman has to go a very hard road – this could have negative consequences for her mental health – but we don’t have the numbers," Dr. Rondón said, adding that discussing how to conduct research on abortion and mental health in Latin America would be a priority of next year’s international congress on women’s mental health, which will be held in Lima.
Any attempt to get a perspective on the mental health impact of abortion and unwanted pregnancy means using studies conducted in the United States and Europe – different cultural environments that could produce different results. "We need prospective research with randomized samples in Latin America, which may become possible as abortion is gradually decriminalized," Dr. Rondón said.
Currently, Peru and Colombia allow legal abortion only in the event of a threat to the life or physical health of the woman. In addition, Colombia allows terminations in cases of rape or to preserve a woman’s mental health.
Dr. Chaskel said in an interview that the 11-year-old patient in his video would have been a candidate for legal abortion under Colombian law and that he would have referred her had she presented to him early. However, he said, her obstetrician had judged her ineligible.
Their options might be limited in terms of a choice to terminate, but pregnant adolescents in Latin America do have the benefit of strong family ties, and Dr. Chaskel’s practice taps into the high level of familial support available to most. "You bring grandma and grandpa, and the new uncles and aunts into clinical practice – right into the office," he said in an interview. A pregnancy in adolescence "generates confusion not only for the girl but also on the whole family group: In Latin America, this usually means three generations confused as to how to approach this situation."
The initial goal of the interventions, he said, is to help pregnant adolescents avoid postpartum depression and psychosis. The longer-term goal is to allow them to continue in their adolescence as normally as possible after the birth, while developing a healthy attachment to the child with the support of their families.
"Are they going to keep listening to Lady Gaga? If you have an 18-month-old baby, how do you behave? We’ve really worked on recuperating adolescence for these girls to make sure that they do listen to Lady Gaga," he said. "We try to make sure that if they’re going to have the baby, to know what they want to do – go back to school, go to a friend’s 15th birthday party [Quinceañera], or have one themselves.
"We think it over together."
After the birth, infants and their young mothers are observed and evaluated interacting in-office, where such details as the distance the child crawls away from the mother is measured as a way of gauging healthy attachments. The girls, who are at high risk of having second babies while still in their teenage years, need to be followed up for years.
Neither Dr. Chaskel nor Dr. Rondón reported disclosures.
BOGOTÁ, COLOMBIA – Adolescent girls who become pregnant and carry to term experience high morbidity, including psychiatric outcomes, numerous studies have established. And, in Latin America, teenage pregnancy rates are rising, and legal abortion is rarely an option.
Learning how to prevent postpartum depression and psychosis among these girls is something "gleaned over years of clinical practice," says Dr. Roberto Chaskel, who has treated such girls for nearly 3 decades. "No one tells you how."
At the fourth International Congress of Medicine and Women’s Mental Health, Dr. Chaskel presented video of an 11-year-old who had just given birth days earlier by cesarean section and was experiencing psychosis. The girl had been referred for psychiatric care only after the birth.
Adolescent pregnancies have risen in many Latin American countries in recent decades, despite declining fertility trends overall, according to a 2007 United Nations Report. One Latin American study of 854,377 girls aged 15 years and younger found pregnancy associated with a fourfold higher risk of a host of adverse pregnancy outcomes, including maternal death, early neonatal death, and anemia, compared with women aged 20 years or older (Am. J. Obstet. Gynecol. 2005;192:342-9).
(Meanwhile, the Centers for Disease Control and Prevention reported recently that teenage pregnancy rates in the United States declined 9% from 2009 to 2010, which means that the rate is at an historic low of 34.3 births/1,000 adolescents aged 15-19 years. The decline was seen across all ethnicities.)
In most Latin American countries, including Colombia – where access to abortion is highly restricted, pregnancies among girls aged 15 years and younger often result in birth. Only three Caribbean or Latin American states permit abortion without regard to reason, according to a 2012 report by the Guttmacher Institute. Illegal procedures are estimated to represent 95% of all abortions performed in the region, according to the same report.
In 2006, in Bogotá there were 22,228 pregnancies reported among teenage girls between 10 and 19 years of age (170/100,000), placing Bogotá’s teenage pregnancy rates nearly on nearly on par with those of Uganda and Sierra Leone, according to a 2010 study by researchers at the Universidad Nacional de Colombia. A tenth of the reported pregnancies were to women aged 14 years and younger.
It is possible to prevent psychotic and depressive episodes related to pregnancy and birth and "offer teenagers and adolescents, and the babies of these girls, the best possible quality of life" through a family practice that seeks to mitigate some of the traumas associated with early sex, pregnancy, and birth, and also to guide the early attachment process between mothers and children, said Dr. Chaskel of the department of psychiatry at the Universidad El Bosque and coordinator of child psychiatry at the Fundación Santa Fe, both in Bogotá, Colombia,.
In a separate presentation at the congress, Dr. Marta B. Rondón, a psychiatrist affiliated with the Universidad Peruana Cayetano Heredia in Lima, Peru, discussed the difficulty of achieving an evidence-based understanding of the mental health impact of abortion and unwanted pregnancy in nations where access is restricted and "generally speaking, a woman cannot choose just to terminate a nondesired pregnancy."
In a restricted legal environment, "the condition of secrecy means the woman has to go a very hard road – this could have negative consequences for her mental health – but we don’t have the numbers," Dr. Rondón said, adding that discussing how to conduct research on abortion and mental health in Latin America would be a priority of next year’s international congress on women’s mental health, which will be held in Lima.
Any attempt to get a perspective on the mental health impact of abortion and unwanted pregnancy means using studies conducted in the United States and Europe – different cultural environments that could produce different results. "We need prospective research with randomized samples in Latin America, which may become possible as abortion is gradually decriminalized," Dr. Rondón said.
Currently, Peru and Colombia allow legal abortion only in the event of a threat to the life or physical health of the woman. In addition, Colombia allows terminations in cases of rape or to preserve a woman’s mental health.
Dr. Chaskel said in an interview that the 11-year-old patient in his video would have been a candidate for legal abortion under Colombian law and that he would have referred her had she presented to him early. However, he said, her obstetrician had judged her ineligible.
Their options might be limited in terms of a choice to terminate, but pregnant adolescents in Latin America do have the benefit of strong family ties, and Dr. Chaskel’s practice taps into the high level of familial support available to most. "You bring grandma and grandpa, and the new uncles and aunts into clinical practice – right into the office," he said in an interview. A pregnancy in adolescence "generates confusion not only for the girl but also on the whole family group: In Latin America, this usually means three generations confused as to how to approach this situation."
The initial goal of the interventions, he said, is to help pregnant adolescents avoid postpartum depression and psychosis. The longer-term goal is to allow them to continue in their adolescence as normally as possible after the birth, while developing a healthy attachment to the child with the support of their families.
"Are they going to keep listening to Lady Gaga? If you have an 18-month-old baby, how do you behave? We’ve really worked on recuperating adolescence for these girls to make sure that they do listen to Lady Gaga," he said. "We try to make sure that if they’re going to have the baby, to know what they want to do – go back to school, go to a friend’s 15th birthday party [Quinceañera], or have one themselves.
"We think it over together."
After the birth, infants and their young mothers are observed and evaluated interacting in-office, where such details as the distance the child crawls away from the mother is measured as a way of gauging healthy attachments. The girls, who are at high risk of having second babies while still in their teenage years, need to be followed up for years.
Neither Dr. Chaskel nor Dr. Rondón reported disclosures.
EXPERT ANALYSIS FROM THE FOURTH INTERNATIONAL CONGRESS ON MEDICINE AND WOMEN'S HEALTH
Mississippi Law Threatens Abortion Access
Only obstetrician-gynecologists with privileges at local hospitals will be able to perform abortions under a new law in Mississippi.
The law, signed April 16 by Gov. Phil Bryant (R), requires that all physicians who perform abortions at an abortion facility must be board-certified or board-eligible ob.gyns. and must have staff and admitting privileges at a local hospital. The law also requires that a person trained in CPR must be present at the abortion clinic whenever it is open. The law is scheduled to go into effect in July.
The requirements are necessary, Gov. Bryant said, to protect patient safety in the event of a complication during an abortion procedure.
"I believe that all human life is precious, and as governor, I will work to ensure that the lives of the born and unborn are protected in Mississippi," he said in a statement.
Abortion rights advocates say the restrictions are an attempt to eliminate abortions in the state.
Currently, only one abortion clinic, Jackson Womens Health Organization, operates in the state. Shelley Abrams, the clinic’s executive director, said that only one of their physicians currently has admitting privileges at a local hospital.
Going forward, they will attempt to gain privileges for the other physicians who work at the clinic, but Ms. Abrams said that could be difficult. For instance, hospitals generally require that physicians with staff and admitting privileges take call and admit patients. That is a major hurdle for most the clinic’s doctors who live outside of Mississippi, she said.
Ms. Abrams said that she anticipates that hospitals will face pressure from antiabortion activists to deny privileges to the clinic’s physicians. "The antiabortionists in Jackson, Miss., have made the entire state a very inhospitable place for medical practitioners," she said.
If they are unable to gain privileges for their physicians, Ms. Abrams said that they will be forced to challenge the law in court to keep the clinic running.
The state has long been an abortion battleground. Last November, Mississippi voters rejected a "personhood amendment" that would have changed the state’s constitution to grant legal rights to embryos, starting at the time of fertilization.
The Mississippi law was signed just days after Arizona enacted controversial new abortion restrictions that will ban abortions at 20 weeks’ gestation and require that women receive an ultrasound before an abortion can be performed.
Arizona Gov. Jan Brewer (R) praised the law as "common sense" and added that "knowing that abortions become riskier the later they are performed in pregnancy, it only makes sense to prohibit these procedures past 20 weeks."
The Arizona law is one of the most extreme in the country, according to reproductive rights advocates. The Center for Reproductive Rights, which is challenging the law in federal court, noted that 18-20 weeks’ gestation is a time when many women undergo a comprehensive scan to uncover major fetal abnormalities and health risks to the mother.
"Some women at risk of grave complications will be forced to decide whether to proceed with their pregnancies in the dark, before they have all the information they need to arrive at their choices," Nancy Northup, the group’s president and CEO, said in a statement.
Seven other states have similar restrictions on abortions after 20 weeks, according to Gov. Brewer.
Abortion legislation is being considered in many statehouses this year: In the first three months of 2012, 75 abortion restrictions were passed by at least one state legislative chamber, according to an analysis from the Guttmacher Institute. That pace isn’t record setting, but it’s unusually high for an election year, the institute noted. So far, most of the pending legislation has focused on requirements that women receive an ultrasound before an abortion, limiting access to medical abortions, and bans on abortion after a certain gestational age.
Only obstetrician-gynecologists with privileges at local hospitals will be able to perform abortions under a new law in Mississippi.
The law, signed April 16 by Gov. Phil Bryant (R), requires that all physicians who perform abortions at an abortion facility must be board-certified or board-eligible ob.gyns. and must have staff and admitting privileges at a local hospital. The law also requires that a person trained in CPR must be present at the abortion clinic whenever it is open. The law is scheduled to go into effect in July.
The requirements are necessary, Gov. Bryant said, to protect patient safety in the event of a complication during an abortion procedure.
"I believe that all human life is precious, and as governor, I will work to ensure that the lives of the born and unborn are protected in Mississippi," he said in a statement.
Abortion rights advocates say the restrictions are an attempt to eliminate abortions in the state.
Currently, only one abortion clinic, Jackson Womens Health Organization, operates in the state. Shelley Abrams, the clinic’s executive director, said that only one of their physicians currently has admitting privileges at a local hospital.
Going forward, they will attempt to gain privileges for the other physicians who work at the clinic, but Ms. Abrams said that could be difficult. For instance, hospitals generally require that physicians with staff and admitting privileges take call and admit patients. That is a major hurdle for most the clinic’s doctors who live outside of Mississippi, she said.
Ms. Abrams said that she anticipates that hospitals will face pressure from antiabortion activists to deny privileges to the clinic’s physicians. "The antiabortionists in Jackson, Miss., have made the entire state a very inhospitable place for medical practitioners," she said.
If they are unable to gain privileges for their physicians, Ms. Abrams said that they will be forced to challenge the law in court to keep the clinic running.
The state has long been an abortion battleground. Last November, Mississippi voters rejected a "personhood amendment" that would have changed the state’s constitution to grant legal rights to embryos, starting at the time of fertilization.
The Mississippi law was signed just days after Arizona enacted controversial new abortion restrictions that will ban abortions at 20 weeks’ gestation and require that women receive an ultrasound before an abortion can be performed.
Arizona Gov. Jan Brewer (R) praised the law as "common sense" and added that "knowing that abortions become riskier the later they are performed in pregnancy, it only makes sense to prohibit these procedures past 20 weeks."
The Arizona law is one of the most extreme in the country, according to reproductive rights advocates. The Center for Reproductive Rights, which is challenging the law in federal court, noted that 18-20 weeks’ gestation is a time when many women undergo a comprehensive scan to uncover major fetal abnormalities and health risks to the mother.
"Some women at risk of grave complications will be forced to decide whether to proceed with their pregnancies in the dark, before they have all the information they need to arrive at their choices," Nancy Northup, the group’s president and CEO, said in a statement.
Seven other states have similar restrictions on abortions after 20 weeks, according to Gov. Brewer.
Abortion legislation is being considered in many statehouses this year: In the first three months of 2012, 75 abortion restrictions were passed by at least one state legislative chamber, according to an analysis from the Guttmacher Institute. That pace isn’t record setting, but it’s unusually high for an election year, the institute noted. So far, most of the pending legislation has focused on requirements that women receive an ultrasound before an abortion, limiting access to medical abortions, and bans on abortion after a certain gestational age.
Only obstetrician-gynecologists with privileges at local hospitals will be able to perform abortions under a new law in Mississippi.
The law, signed April 16 by Gov. Phil Bryant (R), requires that all physicians who perform abortions at an abortion facility must be board-certified or board-eligible ob.gyns. and must have staff and admitting privileges at a local hospital. The law also requires that a person trained in CPR must be present at the abortion clinic whenever it is open. The law is scheduled to go into effect in July.
The requirements are necessary, Gov. Bryant said, to protect patient safety in the event of a complication during an abortion procedure.
"I believe that all human life is precious, and as governor, I will work to ensure that the lives of the born and unborn are protected in Mississippi," he said in a statement.
Abortion rights advocates say the restrictions are an attempt to eliminate abortions in the state.
Currently, only one abortion clinic, Jackson Womens Health Organization, operates in the state. Shelley Abrams, the clinic’s executive director, said that only one of their physicians currently has admitting privileges at a local hospital.
Going forward, they will attempt to gain privileges for the other physicians who work at the clinic, but Ms. Abrams said that could be difficult. For instance, hospitals generally require that physicians with staff and admitting privileges take call and admit patients. That is a major hurdle for most the clinic’s doctors who live outside of Mississippi, she said.
Ms. Abrams said that she anticipates that hospitals will face pressure from antiabortion activists to deny privileges to the clinic’s physicians. "The antiabortionists in Jackson, Miss., have made the entire state a very inhospitable place for medical practitioners," she said.
If they are unable to gain privileges for their physicians, Ms. Abrams said that they will be forced to challenge the law in court to keep the clinic running.
The state has long been an abortion battleground. Last November, Mississippi voters rejected a "personhood amendment" that would have changed the state’s constitution to grant legal rights to embryos, starting at the time of fertilization.
The Mississippi law was signed just days after Arizona enacted controversial new abortion restrictions that will ban abortions at 20 weeks’ gestation and require that women receive an ultrasound before an abortion can be performed.
Arizona Gov. Jan Brewer (R) praised the law as "common sense" and added that "knowing that abortions become riskier the later they are performed in pregnancy, it only makes sense to prohibit these procedures past 20 weeks."
The Arizona law is one of the most extreme in the country, according to reproductive rights advocates. The Center for Reproductive Rights, which is challenging the law in federal court, noted that 18-20 weeks’ gestation is a time when many women undergo a comprehensive scan to uncover major fetal abnormalities and health risks to the mother.
"Some women at risk of grave complications will be forced to decide whether to proceed with their pregnancies in the dark, before they have all the information they need to arrive at their choices," Nancy Northup, the group’s president and CEO, said in a statement.
Seven other states have similar restrictions on abortions after 20 weeks, according to Gov. Brewer.
Abortion legislation is being considered in many statehouses this year: In the first three months of 2012, 75 abortion restrictions were passed by at least one state legislative chamber, according to an analysis from the Guttmacher Institute. That pace isn’t record setting, but it’s unusually high for an election year, the institute noted. So far, most of the pending legislation has focused on requirements that women receive an ultrasound before an abortion, limiting access to medical abortions, and bans on abortion after a certain gestational age.
Pregnancy and NSAIDs
Nonsteroidal anti-inflammatory drugs are among the most commonly used medications in pregnancy, with 23% of women in the United States recalling the use of one of these products in her first trimester, according to the National Birth Defects Prevention Study (Am. J. Obstet. Gynecol. 2012;206:228e1-8).
Although NSAIDs are typically contraindicated late in pregnancy, first trimester use has not consistently been associated with increased risks for congenital anomalies in human studies. Animal studies have suggested, in particular, that nonselective NSAIDs such as aspirin, ibuprofen, and naproxen – which inhibit both cyclooxegenase-1 and -2 – might be associated with increased risks for midline defects, diaphragmatic hernia, and ventricular septal defects. These animal studies have suggested a stronger association with aspirin and weaker effects with nonaspirin NSAIDs (Birth Defects Res. B Dev. Reprod. Toxicol. 2003;68:5-26).
A recent report from the National Birth Defects Prevention Study – an ongoing, large, U.S. multisite, case-control study of major congenital anomalies and maternal exposures – has suggested that specific NSAIDs might be associated with small to moderate increases in risk for several specific congenital anomalies. Using retrospective maternal interview data collected from 14,915 case and 5,546 control mothers who had due dates between 1997 and 2004, researchers found that 3,173 women (15.5%) reported exposure to NSAIDs and recalled specific frequency of use of the medication in the first trimester. An additional 1,452 women (7.1%) reported using an NSAID "as needed" in the first trimester. Among all women who recalled taking an NSAID, more than 98% reported using one of three NSAID types – aspirin, ibuprofen, or naproxen (Am. J. Obstet. Gynecol. 2012;206:228e1-8).
The results of this study suggested that NSAIDs are not a major cause of birth defects; however, the study did find several small to moderate statistically significant increases in nine specific defects among women who reported a known frequency of NSAID use in the first trimester (adjusted odds ratio range, 1.3-3.5). The defects that occurred with increased frequency included neural tube defects, eye defects, oral clefts, limb reduction defects, amniotic bands/limb body wall defects, and pulmonary valve stenosis. Specific associations varied by type of drug. Estimates of the odds ratios were said to be similar among women who reported "as needed" use of NSAIDs in the first trimester.
The association with oral clefts in this study could be consistent with previous animal studies and one human study from the Swedish Medical Birth Register (Reprod. Toxicol. 2001;15:371-5). However, most human studies have either shown no increased risks for these specific defects (PLoS One 2011;6:e22174), or have suggested a possible increased risk for heart defects, primarily cardiac septal defects (Reprod. Toxicol. 2003;17:255-61; Birth Defects Res. B Dev. Reprod. Toxicol. 2006;77:268-79).
The current study found no association with cardiac defects other than pulmonary valve stenosis.
The strengths of this recent case-control study are the large sample size, including sufficient numbers of specific defects to detect low to moderate risks for certain associations, and large enough numbers to examine risk with exposure to specific NSAID products; maternal interview data regarding actual use of the medications rather than just prescription dispensing; and information on important confounders such as alcohol, tobacco, and folic acid supplement use.
On the other hand, the interview data collection process involved maternal recall up to 24 months after delivery; thus, recollection of mothers – particularly for medications that might be taken sporadically and are available for purchase over the counter – might have been inaccurate. There are also concerns in any retrospective study that recall might be influenced by the mother’s knowledge of the status of her infant regarding birth defects. It is also unknown to what extent the underlying maternal conditions being treated by NSAIDs could have contributed to some of the increased risks that were found in this study.
In addition to these caveats, some or all of the findings in this study could be due to chance, and more studies are needed to follow-up on these concerns. For clinicians, it is important to recognize that the suggested increased risks are for relatively rare congenital anomalies. Thus, even if some of the reported associations are causal, mild to moderate increased risks for these specific defects translate to low absolute risks for the individual patient. For example, if the risk for cleft lip with or without cleft palate is 1 in 1,000 live births in the general population, this study would suggest that for every 1,000 women who take ibuprofen in the first trimester, an additional 0.6 infants would be born with cleft lip.
Nevertheless, given the frequency of use of these medications in pregnant women in the first trimester, this study suggests that caution might be exercised in situations in which casual or indiscriminate use of these medications in early pregnancy could be avoided.
Dr. Chambers is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she had no relevant financial disclosures.
Nonsteroidal anti-inflammatory drugs are among the most commonly used medications in pregnancy, with 23% of women in the United States recalling the use of one of these products in her first trimester, according to the National Birth Defects Prevention Study (Am. J. Obstet. Gynecol. 2012;206:228e1-8).
Although NSAIDs are typically contraindicated late in pregnancy, first trimester use has not consistently been associated with increased risks for congenital anomalies in human studies. Animal studies have suggested, in particular, that nonselective NSAIDs such as aspirin, ibuprofen, and naproxen – which inhibit both cyclooxegenase-1 and -2 – might be associated with increased risks for midline defects, diaphragmatic hernia, and ventricular septal defects. These animal studies have suggested a stronger association with aspirin and weaker effects with nonaspirin NSAIDs (Birth Defects Res. B Dev. Reprod. Toxicol. 2003;68:5-26).
A recent report from the National Birth Defects Prevention Study – an ongoing, large, U.S. multisite, case-control study of major congenital anomalies and maternal exposures – has suggested that specific NSAIDs might be associated with small to moderate increases in risk for several specific congenital anomalies. Using retrospective maternal interview data collected from 14,915 case and 5,546 control mothers who had due dates between 1997 and 2004, researchers found that 3,173 women (15.5%) reported exposure to NSAIDs and recalled specific frequency of use of the medication in the first trimester. An additional 1,452 women (7.1%) reported using an NSAID "as needed" in the first trimester. Among all women who recalled taking an NSAID, more than 98% reported using one of three NSAID types – aspirin, ibuprofen, or naproxen (Am. J. Obstet. Gynecol. 2012;206:228e1-8).
The results of this study suggested that NSAIDs are not a major cause of birth defects; however, the study did find several small to moderate statistically significant increases in nine specific defects among women who reported a known frequency of NSAID use in the first trimester (adjusted odds ratio range, 1.3-3.5). The defects that occurred with increased frequency included neural tube defects, eye defects, oral clefts, limb reduction defects, amniotic bands/limb body wall defects, and pulmonary valve stenosis. Specific associations varied by type of drug. Estimates of the odds ratios were said to be similar among women who reported "as needed" use of NSAIDs in the first trimester.
The association with oral clefts in this study could be consistent with previous animal studies and one human study from the Swedish Medical Birth Register (Reprod. Toxicol. 2001;15:371-5). However, most human studies have either shown no increased risks for these specific defects (PLoS One 2011;6:e22174), or have suggested a possible increased risk for heart defects, primarily cardiac septal defects (Reprod. Toxicol. 2003;17:255-61; Birth Defects Res. B Dev. Reprod. Toxicol. 2006;77:268-79).
The current study found no association with cardiac defects other than pulmonary valve stenosis.
The strengths of this recent case-control study are the large sample size, including sufficient numbers of specific defects to detect low to moderate risks for certain associations, and large enough numbers to examine risk with exposure to specific NSAID products; maternal interview data regarding actual use of the medications rather than just prescription dispensing; and information on important confounders such as alcohol, tobacco, and folic acid supplement use.
On the other hand, the interview data collection process involved maternal recall up to 24 months after delivery; thus, recollection of mothers – particularly for medications that might be taken sporadically and are available for purchase over the counter – might have been inaccurate. There are also concerns in any retrospective study that recall might be influenced by the mother’s knowledge of the status of her infant regarding birth defects. It is also unknown to what extent the underlying maternal conditions being treated by NSAIDs could have contributed to some of the increased risks that were found in this study.
In addition to these caveats, some or all of the findings in this study could be due to chance, and more studies are needed to follow-up on these concerns. For clinicians, it is important to recognize that the suggested increased risks are for relatively rare congenital anomalies. Thus, even if some of the reported associations are causal, mild to moderate increased risks for these specific defects translate to low absolute risks for the individual patient. For example, if the risk for cleft lip with or without cleft palate is 1 in 1,000 live births in the general population, this study would suggest that for every 1,000 women who take ibuprofen in the first trimester, an additional 0.6 infants would be born with cleft lip.
Nevertheless, given the frequency of use of these medications in pregnant women in the first trimester, this study suggests that caution might be exercised in situations in which casual or indiscriminate use of these medications in early pregnancy could be avoided.
Dr. Chambers is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she had no relevant financial disclosures.
Nonsteroidal anti-inflammatory drugs are among the most commonly used medications in pregnancy, with 23% of women in the United States recalling the use of one of these products in her first trimester, according to the National Birth Defects Prevention Study (Am. J. Obstet. Gynecol. 2012;206:228e1-8).
Although NSAIDs are typically contraindicated late in pregnancy, first trimester use has not consistently been associated with increased risks for congenital anomalies in human studies. Animal studies have suggested, in particular, that nonselective NSAIDs such as aspirin, ibuprofen, and naproxen – which inhibit both cyclooxegenase-1 and -2 – might be associated with increased risks for midline defects, diaphragmatic hernia, and ventricular septal defects. These animal studies have suggested a stronger association with aspirin and weaker effects with nonaspirin NSAIDs (Birth Defects Res. B Dev. Reprod. Toxicol. 2003;68:5-26).
A recent report from the National Birth Defects Prevention Study – an ongoing, large, U.S. multisite, case-control study of major congenital anomalies and maternal exposures – has suggested that specific NSAIDs might be associated with small to moderate increases in risk for several specific congenital anomalies. Using retrospective maternal interview data collected from 14,915 case and 5,546 control mothers who had due dates between 1997 and 2004, researchers found that 3,173 women (15.5%) reported exposure to NSAIDs and recalled specific frequency of use of the medication in the first trimester. An additional 1,452 women (7.1%) reported using an NSAID "as needed" in the first trimester. Among all women who recalled taking an NSAID, more than 98% reported using one of three NSAID types – aspirin, ibuprofen, or naproxen (Am. J. Obstet. Gynecol. 2012;206:228e1-8).
The results of this study suggested that NSAIDs are not a major cause of birth defects; however, the study did find several small to moderate statistically significant increases in nine specific defects among women who reported a known frequency of NSAID use in the first trimester (adjusted odds ratio range, 1.3-3.5). The defects that occurred with increased frequency included neural tube defects, eye defects, oral clefts, limb reduction defects, amniotic bands/limb body wall defects, and pulmonary valve stenosis. Specific associations varied by type of drug. Estimates of the odds ratios were said to be similar among women who reported "as needed" use of NSAIDs in the first trimester.
The association with oral clefts in this study could be consistent with previous animal studies and one human study from the Swedish Medical Birth Register (Reprod. Toxicol. 2001;15:371-5). However, most human studies have either shown no increased risks for these specific defects (PLoS One 2011;6:e22174), or have suggested a possible increased risk for heart defects, primarily cardiac septal defects (Reprod. Toxicol. 2003;17:255-61; Birth Defects Res. B Dev. Reprod. Toxicol. 2006;77:268-79).
The current study found no association with cardiac defects other than pulmonary valve stenosis.
The strengths of this recent case-control study are the large sample size, including sufficient numbers of specific defects to detect low to moderate risks for certain associations, and large enough numbers to examine risk with exposure to specific NSAID products; maternal interview data regarding actual use of the medications rather than just prescription dispensing; and information on important confounders such as alcohol, tobacco, and folic acid supplement use.
On the other hand, the interview data collection process involved maternal recall up to 24 months after delivery; thus, recollection of mothers – particularly for medications that might be taken sporadically and are available for purchase over the counter – might have been inaccurate. There are also concerns in any retrospective study that recall might be influenced by the mother’s knowledge of the status of her infant regarding birth defects. It is also unknown to what extent the underlying maternal conditions being treated by NSAIDs could have contributed to some of the increased risks that were found in this study.
In addition to these caveats, some or all of the findings in this study could be due to chance, and more studies are needed to follow-up on these concerns. For clinicians, it is important to recognize that the suggested increased risks are for relatively rare congenital anomalies. Thus, even if some of the reported associations are causal, mild to moderate increased risks for these specific defects translate to low absolute risks for the individual patient. For example, if the risk for cleft lip with or without cleft palate is 1 in 1,000 live births in the general population, this study would suggest that for every 1,000 women who take ibuprofen in the first trimester, an additional 0.6 infants would be born with cleft lip.
Nevertheless, given the frequency of use of these medications in pregnant women in the first trimester, this study suggests that caution might be exercised in situations in which casual or indiscriminate use of these medications in early pregnancy could be avoided.
Dr. Chambers is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she had no relevant financial disclosures.
Telbivudine Cut Mother-to-Infant Hepatitis B Transmission
Telbivudine taken during the second or third trimester of pregnancy reduced the mother-to-infant transmission of hepatitis B to zero in an open-label trial of 88 women who had high viral loads and elevated alanine aminotransferase levels, Dr. Calvin Q. Pan and his colleagues reported in the May issue of Clinical Gastroenterology and Hepatology.
The authors described their study as the first to examine telbivudine therapy in such patients. They found that the vertical transmission rate was 0% with active treatment and 9% in a control group, despite appropriate immunoprophylaxis of the neonate, said Dr. Pan of the division of liver diseases at Mount Sinai Medical Center, New York, and his associates (Clin. Gastroenterol. Hepatol. 2012 Feb. 16 [doi:10.1016/j.cgh.2012.01.019]).
These results support the idea of using antiviral drugs during pregnancy in women who have chronic hepatitis B virus (HBV) infection to reduce rates of immunoprophylaxis failure in their infants, the investigators noted.
The prospective study involved women treated at a single tertiary-care hospital in Nanjing, China, during 2008-2009. The subjects comprised women at 12-30 weeks’ gestation who had high viral loads, high ALT levels, and were positive for both hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg).
The women were not randomly assigned to study groups but instead chose whether to enroll in the antiviral therapy group (53 subjects) or the control group that received only clinical observation (35 subjects). Those in the active treatment group were to receive 600 mg oral telbivudine daily until 28 weeks postpartum.
Mothers who intended to breastfeed were instructed to discontinue the drug before doing so.
The mean duration of exposure to telbivudine was 15 weeks before delivery and 23 weeks postpartum. In all, 13 of the 52 treated mothers (29%) decided to discontinue the antiviral therapy during the first month after delivery, and the rest continued to take it until the study ended at 28 weeks postpartum.
Mean serum HBV DNA levels declined markedly in the treated women but remained steady in the control group. In the intention-to-treat analysis, HBV DNA dropped to undetectable levels in 58% of the telbivudine group but 0% of the control group.
Similarly, ALT levels normalized in 77% of the telbivudine group at 1 month postpartum and in 92% by the end of the study. In comparison, ALT levels normalized in only 49% of the control group at 1 month postpartum and in 71% by the end of the study.
All the infants in the study received hepatitis B immunoglobulin with the HBV vaccine within 6 hours of birth and completed the remainder of the HBV immunizations according to the standard vaccine schedule.
At birth, all infants were HBeAg-positive. Two in the telbivudine group and eight in the control group were HBsAg-positive. And only three infants, all in the control group, had detectable serum levels of HBV DNA.
By week 28, all the infants in the telbivudine group were negative for HBeAg, HBsAg, and HBV DNA. In the control group, three infants remained positive for HBsAg, HBeAg, and HBV DNA, and another five remained positive for HBsAg only.
The primary end point of the study – the rate of mother-to-infant transmission – was 0% (0 of 52 infants) with active treatment and 9% (3 of 32 infants) with no treatment.
In the intention-to-treat analysis, HBV was transmitted to two infants in the telbivudine group (1.9%) and six in the control group (17%).
With regard to safety, there were no significant differences between the two study groups in gestational age at delivery, incidence of maternal peripartum hemorrhage, incidence of premature rupture of membranes, or rate of cesarean delivery. None of the mothers developed hepatitis flares.
The incidence and nature of minor adverse effects among the infants were similar between the two study groups, with no significant differences in birth weight, height, or Apgar scores. There were no congenital deformities and no major adverse effects. All the infants showed normal development through the completion of the study.
During follow-up there were three cases of pneumonia in the telbivudine group and one in the control group. It was not clear whether this difference may have been related to antiviral therapy, the authors said.
They noted that approximately 6% of Asian-American women are HBsAg-positive at perinatal screening, similar to the 7% rate of HBsAg positivity in China. There is no consensus as to the management of these patients because data on antiviral therapy are so limited.
This study lends support to the concept of using antiviral therapy in the second or third trimester in these women, to reduce the failure rate of immunoprophylaxis in their newborns, the researchers said.
Limitations included the relatively small study cohort treated at a single center. A multicenter trial with a larger sample size is warranted, they added.
The study was funded by the Jiangsu Province Department of Health. Dr. Pan reported ties to Gilead, Bristol Myers Squibb, Novartis, Idenix, Roche, Schering Plough, Onyx, Three Rivers, Salix, Genentech, Vertex, and Pharmasset.
Telbivudine taken during the second or third trimester of pregnancy reduced the mother-to-infant transmission of hepatitis B to zero in an open-label trial of 88 women who had high viral loads and elevated alanine aminotransferase levels, Dr. Calvin Q. Pan and his colleagues reported in the May issue of Clinical Gastroenterology and Hepatology.
The authors described their study as the first to examine telbivudine therapy in such patients. They found that the vertical transmission rate was 0% with active treatment and 9% in a control group, despite appropriate immunoprophylaxis of the neonate, said Dr. Pan of the division of liver diseases at Mount Sinai Medical Center, New York, and his associates (Clin. Gastroenterol. Hepatol. 2012 Feb. 16 [doi:10.1016/j.cgh.2012.01.019]).
These results support the idea of using antiviral drugs during pregnancy in women who have chronic hepatitis B virus (HBV) infection to reduce rates of immunoprophylaxis failure in their infants, the investigators noted.
The prospective study involved women treated at a single tertiary-care hospital in Nanjing, China, during 2008-2009. The subjects comprised women at 12-30 weeks’ gestation who had high viral loads, high ALT levels, and were positive for both hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg).
The women were not randomly assigned to study groups but instead chose whether to enroll in the antiviral therapy group (53 subjects) or the control group that received only clinical observation (35 subjects). Those in the active treatment group were to receive 600 mg oral telbivudine daily until 28 weeks postpartum.
Mothers who intended to breastfeed were instructed to discontinue the drug before doing so.
The mean duration of exposure to telbivudine was 15 weeks before delivery and 23 weeks postpartum. In all, 13 of the 52 treated mothers (29%) decided to discontinue the antiviral therapy during the first month after delivery, and the rest continued to take it until the study ended at 28 weeks postpartum.
Mean serum HBV DNA levels declined markedly in the treated women but remained steady in the control group. In the intention-to-treat analysis, HBV DNA dropped to undetectable levels in 58% of the telbivudine group but 0% of the control group.
Similarly, ALT levels normalized in 77% of the telbivudine group at 1 month postpartum and in 92% by the end of the study. In comparison, ALT levels normalized in only 49% of the control group at 1 month postpartum and in 71% by the end of the study.
All the infants in the study received hepatitis B immunoglobulin with the HBV vaccine within 6 hours of birth and completed the remainder of the HBV immunizations according to the standard vaccine schedule.
At birth, all infants were HBeAg-positive. Two in the telbivudine group and eight in the control group were HBsAg-positive. And only three infants, all in the control group, had detectable serum levels of HBV DNA.
By week 28, all the infants in the telbivudine group were negative for HBeAg, HBsAg, and HBV DNA. In the control group, three infants remained positive for HBsAg, HBeAg, and HBV DNA, and another five remained positive for HBsAg only.
The primary end point of the study – the rate of mother-to-infant transmission – was 0% (0 of 52 infants) with active treatment and 9% (3 of 32 infants) with no treatment.
In the intention-to-treat analysis, HBV was transmitted to two infants in the telbivudine group (1.9%) and six in the control group (17%).
With regard to safety, there were no significant differences between the two study groups in gestational age at delivery, incidence of maternal peripartum hemorrhage, incidence of premature rupture of membranes, or rate of cesarean delivery. None of the mothers developed hepatitis flares.
The incidence and nature of minor adverse effects among the infants were similar between the two study groups, with no significant differences in birth weight, height, or Apgar scores. There were no congenital deformities and no major adverse effects. All the infants showed normal development through the completion of the study.
During follow-up there were three cases of pneumonia in the telbivudine group and one in the control group. It was not clear whether this difference may have been related to antiviral therapy, the authors said.
They noted that approximately 6% of Asian-American women are HBsAg-positive at perinatal screening, similar to the 7% rate of HBsAg positivity in China. There is no consensus as to the management of these patients because data on antiviral therapy are so limited.
This study lends support to the concept of using antiviral therapy in the second or third trimester in these women, to reduce the failure rate of immunoprophylaxis in their newborns, the researchers said.
Limitations included the relatively small study cohort treated at a single center. A multicenter trial with a larger sample size is warranted, they added.
The study was funded by the Jiangsu Province Department of Health. Dr. Pan reported ties to Gilead, Bristol Myers Squibb, Novartis, Idenix, Roche, Schering Plough, Onyx, Three Rivers, Salix, Genentech, Vertex, and Pharmasset.
Telbivudine taken during the second or third trimester of pregnancy reduced the mother-to-infant transmission of hepatitis B to zero in an open-label trial of 88 women who had high viral loads and elevated alanine aminotransferase levels, Dr. Calvin Q. Pan and his colleagues reported in the May issue of Clinical Gastroenterology and Hepatology.
The authors described their study as the first to examine telbivudine therapy in such patients. They found that the vertical transmission rate was 0% with active treatment and 9% in a control group, despite appropriate immunoprophylaxis of the neonate, said Dr. Pan of the division of liver diseases at Mount Sinai Medical Center, New York, and his associates (Clin. Gastroenterol. Hepatol. 2012 Feb. 16 [doi:10.1016/j.cgh.2012.01.019]).
These results support the idea of using antiviral drugs during pregnancy in women who have chronic hepatitis B virus (HBV) infection to reduce rates of immunoprophylaxis failure in their infants, the investigators noted.
The prospective study involved women treated at a single tertiary-care hospital in Nanjing, China, during 2008-2009. The subjects comprised women at 12-30 weeks’ gestation who had high viral loads, high ALT levels, and were positive for both hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg).
The women were not randomly assigned to study groups but instead chose whether to enroll in the antiviral therapy group (53 subjects) or the control group that received only clinical observation (35 subjects). Those in the active treatment group were to receive 600 mg oral telbivudine daily until 28 weeks postpartum.
Mothers who intended to breastfeed were instructed to discontinue the drug before doing so.
The mean duration of exposure to telbivudine was 15 weeks before delivery and 23 weeks postpartum. In all, 13 of the 52 treated mothers (29%) decided to discontinue the antiviral therapy during the first month after delivery, and the rest continued to take it until the study ended at 28 weeks postpartum.
Mean serum HBV DNA levels declined markedly in the treated women but remained steady in the control group. In the intention-to-treat analysis, HBV DNA dropped to undetectable levels in 58% of the telbivudine group but 0% of the control group.
Similarly, ALT levels normalized in 77% of the telbivudine group at 1 month postpartum and in 92% by the end of the study. In comparison, ALT levels normalized in only 49% of the control group at 1 month postpartum and in 71% by the end of the study.
All the infants in the study received hepatitis B immunoglobulin with the HBV vaccine within 6 hours of birth and completed the remainder of the HBV immunizations according to the standard vaccine schedule.
At birth, all infants were HBeAg-positive. Two in the telbivudine group and eight in the control group were HBsAg-positive. And only three infants, all in the control group, had detectable serum levels of HBV DNA.
By week 28, all the infants in the telbivudine group were negative for HBeAg, HBsAg, and HBV DNA. In the control group, three infants remained positive for HBsAg, HBeAg, and HBV DNA, and another five remained positive for HBsAg only.
The primary end point of the study – the rate of mother-to-infant transmission – was 0% (0 of 52 infants) with active treatment and 9% (3 of 32 infants) with no treatment.
In the intention-to-treat analysis, HBV was transmitted to two infants in the telbivudine group (1.9%) and six in the control group (17%).
With regard to safety, there were no significant differences between the two study groups in gestational age at delivery, incidence of maternal peripartum hemorrhage, incidence of premature rupture of membranes, or rate of cesarean delivery. None of the mothers developed hepatitis flares.
The incidence and nature of minor adverse effects among the infants were similar between the two study groups, with no significant differences in birth weight, height, or Apgar scores. There were no congenital deformities and no major adverse effects. All the infants showed normal development through the completion of the study.
During follow-up there were three cases of pneumonia in the telbivudine group and one in the control group. It was not clear whether this difference may have been related to antiviral therapy, the authors said.
They noted that approximately 6% of Asian-American women are HBsAg-positive at perinatal screening, similar to the 7% rate of HBsAg positivity in China. There is no consensus as to the management of these patients because data on antiviral therapy are so limited.
This study lends support to the concept of using antiviral therapy in the second or third trimester in these women, to reduce the failure rate of immunoprophylaxis in their newborns, the researchers said.
Limitations included the relatively small study cohort treated at a single center. A multicenter trial with a larger sample size is warranted, they added.
The study was funded by the Jiangsu Province Department of Health. Dr. Pan reported ties to Gilead, Bristol Myers Squibb, Novartis, Idenix, Roche, Schering Plough, Onyx, Three Rivers, Salix, Genentech, Vertex, and Pharmasset.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Major Finding: Women with high viral loads who began telbivudine therapy for HBV at 12-30 weeks gestation had a vertical transmission rate of 0%, vs. 9% in the control group.
Data Source: This was an open-label, single-center trial of 88 women in China.
Disclosures: The study was funded by the Jiangsu Province Department of Health. Dr. Pan reported ties to Gilead, Bristol Myers Squibb, Novartis, Idenix, Roche, Schering Plough, Onyx, Three Rivers, Salix, Genentech, Vertex, and Pharmasset.