User login
Late to the game: Parenting after 40
As they rolled me down the hallway to the OR, ceiling lights rhythmically passing above, I zoned out into a 1,000-mile stare. How did I get here? I started humming “Swing Low, Sweet Chariot,” praying for a miracle to happen. I thought back to my birth plan, meticulously crafted, a one-pager so that the no-nonsense labor and delivery nurses wouldn›t think me completely off my rocker. No C-section unless medically necessary. Those words laughed back at me – cackling, even. I’d planned out the whole birthing process and here we were, my team almost jogging me to the OR. I lay still, utterly gobsmacked and partially anesthetized.
If I squint my eyes and hallucinate just a bit, that is sort of what motherhood has been like.
It’s about knowing all the things that could go wrong and meeting the unplanned head-on. Motherhood has indeed been a whirlwind – so many physical, psychological, and emotional transformations. And to top it off, the added effort of giving birth in a pandemic. As an over-40 physician, you’d think I would have been better prepared.
I was, but in a sense, I was not. The knowledge, the wisdom, the experience of my medical training surrounded me, but even I panicked at times in the beginning: Am I feeding her correctly? Am I making enough food for her? Am I doing the best that I can for her? What more could I be doing for her?
Over time, I’ve learned to lighten up. Some. In those teachable moments with my daughter Gia, I’ve learned to not sugarcoat reality but encourage the hopeful. If Gia falls on the ground? “You’re okay, sweetie. Now get back up.” If Gia has a tantrum and starts hitting herself? “Honey, our hands are for hugs, not hurting ourselves. Let’s go play.” Eighty percent of motherhood right now is redirection and the other 20% is patience.
I remember this one time I was rushing out the door for work. After getting in the car with my keys, I realized I forgot my coffee back in the house. I left the car, went back in the house to grab the blessed joe, went back to the car, and couldn’t get in because it was locked. I panicked at that moment, went back inside the house, and found Gia playing with my extra key fob. My own daughter locked me out of my car. Of course, it wasn’t her fault. Deep breath and I offered her another kiss while simultaneously taking the key fob from her.
Before Gia could walk, she could climb the stairs in our home. Her father and I sometimes refer to her as “Lil Bamm-Bamm” because she is so strong. One day, Daddy was supposed to be watching her while Mommy was folding laundry upstairs. She was not allowed on the stairs, but what should I hear? Literally, the pitter-patter of little feet, running down the upstairs hallway. Her father had drifted off watching yet another episode of something Star Wars–related. My strong little girl made it up the stairs all by herself and Dad received a strong word. The Force was with me that day.
I would say that I feel like having a child ages you, but what does that really mean when you’re already old? I’ve become acutely aware of my lack of endurance, stamina, and bodily strength. My knees will creak when taking her upstairs to bed, an osseous dirge of a lullaby. Date nights become unintentionally less and less frequent. Friday night dress-up becomes Friday night dress-down. I’ve replaced stiletto heels with comfy sweats.
Once we put Gia down for the night, we are usually exhausted from the day, and the couch and TV are welcome respites. We exhale. As over-40 parents, we knew that having children late in life would bring its challenges. But I’d like to think that we are meeting them the best way that we can. Often I encourage my body to meet Gia at her eye level, see what she sees, play with her on her own terms, and match her energy. She absolutely loves it when I do this. I’m out of breath and my knees are sore by the end of our play session, but I wouldn’t have it any other way.
We are learning from each other. She has a bright and assertive personality, and I am protective of that innocence. Her innocence is without fear. I often wonder what she is thinking when I see her facial expressions. A side-eye, a fleeting giggle. Is she secretly contemplating the chronicity of the cosmos, or is it just gas? I look at her in stolen moments and still can’t believe that I grew a human inside me, and said human was extracted from me and is now walking around my house commanding her bidding. So surreal. The unromanticized, scientific ingredients that are at play from conception to delivery are nothing short of miraculous. And the miracles of parenting over 40 are present every day.
Dr. Tolliver is a family medicine physician at The Ohio State University Wexner Medical Center in Columbus. A version of this article first appeared on Medscape.com.
As they rolled me down the hallway to the OR, ceiling lights rhythmically passing above, I zoned out into a 1,000-mile stare. How did I get here? I started humming “Swing Low, Sweet Chariot,” praying for a miracle to happen. I thought back to my birth plan, meticulously crafted, a one-pager so that the no-nonsense labor and delivery nurses wouldn›t think me completely off my rocker. No C-section unless medically necessary. Those words laughed back at me – cackling, even. I’d planned out the whole birthing process and here we were, my team almost jogging me to the OR. I lay still, utterly gobsmacked and partially anesthetized.
If I squint my eyes and hallucinate just a bit, that is sort of what motherhood has been like.
It’s about knowing all the things that could go wrong and meeting the unplanned head-on. Motherhood has indeed been a whirlwind – so many physical, psychological, and emotional transformations. And to top it off, the added effort of giving birth in a pandemic. As an over-40 physician, you’d think I would have been better prepared.
I was, but in a sense, I was not. The knowledge, the wisdom, the experience of my medical training surrounded me, but even I panicked at times in the beginning: Am I feeding her correctly? Am I making enough food for her? Am I doing the best that I can for her? What more could I be doing for her?
Over time, I’ve learned to lighten up. Some. In those teachable moments with my daughter Gia, I’ve learned to not sugarcoat reality but encourage the hopeful. If Gia falls on the ground? “You’re okay, sweetie. Now get back up.” If Gia has a tantrum and starts hitting herself? “Honey, our hands are for hugs, not hurting ourselves. Let’s go play.” Eighty percent of motherhood right now is redirection and the other 20% is patience.
I remember this one time I was rushing out the door for work. After getting in the car with my keys, I realized I forgot my coffee back in the house. I left the car, went back in the house to grab the blessed joe, went back to the car, and couldn’t get in because it was locked. I panicked at that moment, went back inside the house, and found Gia playing with my extra key fob. My own daughter locked me out of my car. Of course, it wasn’t her fault. Deep breath and I offered her another kiss while simultaneously taking the key fob from her.
Before Gia could walk, she could climb the stairs in our home. Her father and I sometimes refer to her as “Lil Bamm-Bamm” because she is so strong. One day, Daddy was supposed to be watching her while Mommy was folding laundry upstairs. She was not allowed on the stairs, but what should I hear? Literally, the pitter-patter of little feet, running down the upstairs hallway. Her father had drifted off watching yet another episode of something Star Wars–related. My strong little girl made it up the stairs all by herself and Dad received a strong word. The Force was with me that day.
I would say that I feel like having a child ages you, but what does that really mean when you’re already old? I’ve become acutely aware of my lack of endurance, stamina, and bodily strength. My knees will creak when taking her upstairs to bed, an osseous dirge of a lullaby. Date nights become unintentionally less and less frequent. Friday night dress-up becomes Friday night dress-down. I’ve replaced stiletto heels with comfy sweats.
Once we put Gia down for the night, we are usually exhausted from the day, and the couch and TV are welcome respites. We exhale. As over-40 parents, we knew that having children late in life would bring its challenges. But I’d like to think that we are meeting them the best way that we can. Often I encourage my body to meet Gia at her eye level, see what she sees, play with her on her own terms, and match her energy. She absolutely loves it when I do this. I’m out of breath and my knees are sore by the end of our play session, but I wouldn’t have it any other way.
We are learning from each other. She has a bright and assertive personality, and I am protective of that innocence. Her innocence is without fear. I often wonder what she is thinking when I see her facial expressions. A side-eye, a fleeting giggle. Is she secretly contemplating the chronicity of the cosmos, or is it just gas? I look at her in stolen moments and still can’t believe that I grew a human inside me, and said human was extracted from me and is now walking around my house commanding her bidding. So surreal. The unromanticized, scientific ingredients that are at play from conception to delivery are nothing short of miraculous. And the miracles of parenting over 40 are present every day.
Dr. Tolliver is a family medicine physician at The Ohio State University Wexner Medical Center in Columbus. A version of this article first appeared on Medscape.com.
As they rolled me down the hallway to the OR, ceiling lights rhythmically passing above, I zoned out into a 1,000-mile stare. How did I get here? I started humming “Swing Low, Sweet Chariot,” praying for a miracle to happen. I thought back to my birth plan, meticulously crafted, a one-pager so that the no-nonsense labor and delivery nurses wouldn›t think me completely off my rocker. No C-section unless medically necessary. Those words laughed back at me – cackling, even. I’d planned out the whole birthing process and here we were, my team almost jogging me to the OR. I lay still, utterly gobsmacked and partially anesthetized.
If I squint my eyes and hallucinate just a bit, that is sort of what motherhood has been like.
It’s about knowing all the things that could go wrong and meeting the unplanned head-on. Motherhood has indeed been a whirlwind – so many physical, psychological, and emotional transformations. And to top it off, the added effort of giving birth in a pandemic. As an over-40 physician, you’d think I would have been better prepared.
I was, but in a sense, I was not. The knowledge, the wisdom, the experience of my medical training surrounded me, but even I panicked at times in the beginning: Am I feeding her correctly? Am I making enough food for her? Am I doing the best that I can for her? What more could I be doing for her?
Over time, I’ve learned to lighten up. Some. In those teachable moments with my daughter Gia, I’ve learned to not sugarcoat reality but encourage the hopeful. If Gia falls on the ground? “You’re okay, sweetie. Now get back up.” If Gia has a tantrum and starts hitting herself? “Honey, our hands are for hugs, not hurting ourselves. Let’s go play.” Eighty percent of motherhood right now is redirection and the other 20% is patience.
I remember this one time I was rushing out the door for work. After getting in the car with my keys, I realized I forgot my coffee back in the house. I left the car, went back in the house to grab the blessed joe, went back to the car, and couldn’t get in because it was locked. I panicked at that moment, went back inside the house, and found Gia playing with my extra key fob. My own daughter locked me out of my car. Of course, it wasn’t her fault. Deep breath and I offered her another kiss while simultaneously taking the key fob from her.
Before Gia could walk, she could climb the stairs in our home. Her father and I sometimes refer to her as “Lil Bamm-Bamm” because she is so strong. One day, Daddy was supposed to be watching her while Mommy was folding laundry upstairs. She was not allowed on the stairs, but what should I hear? Literally, the pitter-patter of little feet, running down the upstairs hallway. Her father had drifted off watching yet another episode of something Star Wars–related. My strong little girl made it up the stairs all by herself and Dad received a strong word. The Force was with me that day.
I would say that I feel like having a child ages you, but what does that really mean when you’re already old? I’ve become acutely aware of my lack of endurance, stamina, and bodily strength. My knees will creak when taking her upstairs to bed, an osseous dirge of a lullaby. Date nights become unintentionally less and less frequent. Friday night dress-up becomes Friday night dress-down. I’ve replaced stiletto heels with comfy sweats.
Once we put Gia down for the night, we are usually exhausted from the day, and the couch and TV are welcome respites. We exhale. As over-40 parents, we knew that having children late in life would bring its challenges. But I’d like to think that we are meeting them the best way that we can. Often I encourage my body to meet Gia at her eye level, see what she sees, play with her on her own terms, and match her energy. She absolutely loves it when I do this. I’m out of breath and my knees are sore by the end of our play session, but I wouldn’t have it any other way.
We are learning from each other. She has a bright and assertive personality, and I am protective of that innocence. Her innocence is without fear. I often wonder what she is thinking when I see her facial expressions. A side-eye, a fleeting giggle. Is she secretly contemplating the chronicity of the cosmos, or is it just gas? I look at her in stolen moments and still can’t believe that I grew a human inside me, and said human was extracted from me and is now walking around my house commanding her bidding. So surreal. The unromanticized, scientific ingredients that are at play from conception to delivery are nothing short of miraculous. And the miracles of parenting over 40 are present every day.
Dr. Tolliver is a family medicine physician at The Ohio State University Wexner Medical Center in Columbus. A version of this article first appeared on Medscape.com.
Metformin use linked to birth defects in boys
researchers have found.
The association appears to involve the effects of metformin on the development of sperm during a critical window prior to conception. Female offspring were not affected. Although previous studies have linked diabetes with fertility problems in men, the latest study is the first to show that these problems can result from treatment rather than the disease itself, according to the researchers, whose findings appear in Annals of Internal Medicine.
“This is the first data to suggest that paternal metformin [use] may be associated with birth defects in children. As such, it would be early to begin to alter clinical practice,” Michael Eisenberg, MD, director of male reproductive medicine and surgery, department of urology, Stanford (Calif.) University, who is a coauthor of the study, said in an interview. “However, if it is confirmed in other populations, then it may begin to enter counseling discussions.”
Dr. Eisenberg added that eating a nutritious diet, exercising, and maintaining a healthy body weight “can improve a man’s health and likely his fertility as well.”
For the new study, Dr. Eisenberg and colleagues analyzed records in a registry of all 1.25 million births that occurred in Denmark between 1997 and 2016. The registry included information on birth defects and parental drug prescriptions.
Offspring were considered exposed to a diabetes drug if a father had filled one or more prescriptions for the medications during the 3 months prior to conception, when the fertilizing sperm would have been produced.
The final analysis included 1,116,779 offspring – all singleton births to women without a history of diabetes or essential hypertension – of whom 7,029 were exposed to diabetes drugs via the father, and 3.3% (n = 36,585) had one or more major birth defects.
Among male offspring whose fathers had taken metformin (n = 1,451), there was a 3.4-fold greater incidence of major genitourinary birth defects, according to the researchers. The study failed to find associations between birth defects and the use of insulin. Although a signal did emerge for sulfonylurea-based drugs, it did not reach statistical significance.
The risk associated with metformin did not appear for men who were prescribed the drug in the year before or after sperm development. Nor was it evident in siblings of the boys with birth defects who were not considered to have been exposed to the medication, the researchers reported.
In an editorial accompanying the journal article, Germaine Buck Louis, PhD, a reproductive and perinatal epidemiologist, wrote: “Given the prevalence of metformin use as first-line therapy for type 2 diabetes, corroboration of these findings is urgently needed.”
Dr. Louis, dean of the College of Health and Human Services at George Mason University, Washington, said a key limitation of the research is the lack of data on how well men in the study adhered to their diabetes treatment. Nevertheless, “clinical guidance is needed to help couples planning pregnancy weigh the risks and benefits of paternal metformin use relative to other medications.”
The researchers received funding from the National Institutes of Health and the Centers for Disease Control and Prevention.
A version of this article first appeared on Medscape.com.
researchers have found.
The association appears to involve the effects of metformin on the development of sperm during a critical window prior to conception. Female offspring were not affected. Although previous studies have linked diabetes with fertility problems in men, the latest study is the first to show that these problems can result from treatment rather than the disease itself, according to the researchers, whose findings appear in Annals of Internal Medicine.
“This is the first data to suggest that paternal metformin [use] may be associated with birth defects in children. As such, it would be early to begin to alter clinical practice,” Michael Eisenberg, MD, director of male reproductive medicine and surgery, department of urology, Stanford (Calif.) University, who is a coauthor of the study, said in an interview. “However, if it is confirmed in other populations, then it may begin to enter counseling discussions.”
Dr. Eisenberg added that eating a nutritious diet, exercising, and maintaining a healthy body weight “can improve a man’s health and likely his fertility as well.”
For the new study, Dr. Eisenberg and colleagues analyzed records in a registry of all 1.25 million births that occurred in Denmark between 1997 and 2016. The registry included information on birth defects and parental drug prescriptions.
Offspring were considered exposed to a diabetes drug if a father had filled one or more prescriptions for the medications during the 3 months prior to conception, when the fertilizing sperm would have been produced.
The final analysis included 1,116,779 offspring – all singleton births to women without a history of diabetes or essential hypertension – of whom 7,029 were exposed to diabetes drugs via the father, and 3.3% (n = 36,585) had one or more major birth defects.
Among male offspring whose fathers had taken metformin (n = 1,451), there was a 3.4-fold greater incidence of major genitourinary birth defects, according to the researchers. The study failed to find associations between birth defects and the use of insulin. Although a signal did emerge for sulfonylurea-based drugs, it did not reach statistical significance.
The risk associated with metformin did not appear for men who were prescribed the drug in the year before or after sperm development. Nor was it evident in siblings of the boys with birth defects who were not considered to have been exposed to the medication, the researchers reported.
In an editorial accompanying the journal article, Germaine Buck Louis, PhD, a reproductive and perinatal epidemiologist, wrote: “Given the prevalence of metformin use as first-line therapy for type 2 diabetes, corroboration of these findings is urgently needed.”
Dr. Louis, dean of the College of Health and Human Services at George Mason University, Washington, said a key limitation of the research is the lack of data on how well men in the study adhered to their diabetes treatment. Nevertheless, “clinical guidance is needed to help couples planning pregnancy weigh the risks and benefits of paternal metformin use relative to other medications.”
The researchers received funding from the National Institutes of Health and the Centers for Disease Control and Prevention.
A version of this article first appeared on Medscape.com.
researchers have found.
The association appears to involve the effects of metformin on the development of sperm during a critical window prior to conception. Female offspring were not affected. Although previous studies have linked diabetes with fertility problems in men, the latest study is the first to show that these problems can result from treatment rather than the disease itself, according to the researchers, whose findings appear in Annals of Internal Medicine.
“This is the first data to suggest that paternal metformin [use] may be associated with birth defects in children. As such, it would be early to begin to alter clinical practice,” Michael Eisenberg, MD, director of male reproductive medicine and surgery, department of urology, Stanford (Calif.) University, who is a coauthor of the study, said in an interview. “However, if it is confirmed in other populations, then it may begin to enter counseling discussions.”
Dr. Eisenberg added that eating a nutritious diet, exercising, and maintaining a healthy body weight “can improve a man’s health and likely his fertility as well.”
For the new study, Dr. Eisenberg and colleagues analyzed records in a registry of all 1.25 million births that occurred in Denmark between 1997 and 2016. The registry included information on birth defects and parental drug prescriptions.
Offspring were considered exposed to a diabetes drug if a father had filled one or more prescriptions for the medications during the 3 months prior to conception, when the fertilizing sperm would have been produced.
The final analysis included 1,116,779 offspring – all singleton births to women without a history of diabetes or essential hypertension – of whom 7,029 were exposed to diabetes drugs via the father, and 3.3% (n = 36,585) had one or more major birth defects.
Among male offspring whose fathers had taken metformin (n = 1,451), there was a 3.4-fold greater incidence of major genitourinary birth defects, according to the researchers. The study failed to find associations between birth defects and the use of insulin. Although a signal did emerge for sulfonylurea-based drugs, it did not reach statistical significance.
The risk associated with metformin did not appear for men who were prescribed the drug in the year before or after sperm development. Nor was it evident in siblings of the boys with birth defects who were not considered to have been exposed to the medication, the researchers reported.
In an editorial accompanying the journal article, Germaine Buck Louis, PhD, a reproductive and perinatal epidemiologist, wrote: “Given the prevalence of metformin use as first-line therapy for type 2 diabetes, corroboration of these findings is urgently needed.”
Dr. Louis, dean of the College of Health and Human Services at George Mason University, Washington, said a key limitation of the research is the lack of data on how well men in the study adhered to their diabetes treatment. Nevertheless, “clinical guidance is needed to help couples planning pregnancy weigh the risks and benefits of paternal metformin use relative to other medications.”
The researchers received funding from the National Institutes of Health and the Centers for Disease Control and Prevention.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
ADHD link to prenatal opioid exposure shifts with other substances
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
FROM JAMA NETWORK OPEN
Does the U.S. have enough abortion providers?
A small, and likely decreasing, number of health care providers in the United States perform abortions, and there is a risk that the count will be shrinking in the face of legislative attacks on the service, researchers have found.
Until now, producing an accurate count of abortion service providers in the United States has been difficult, leaving researchers to rely on indirect assessments of abortion clinics rather than counts of physicians who perform the procedure.
But the authors of a research letter published in JAMA Internal Medicine have come up with a number: Roughly 3,550 clinicians provide procedural and medication abortions, while 22,001 manage pregnancy loss with the same procedures and medications. More than half of all abortions in the United States now are achieved by medication.
The small number of providers is a cause for concern as a growing number of states move to restrict access to abortions, experts say.
“Abortions are only available if clinicians provide them,” said Julia Strasser, DrPH, MPH, senior research scientist at the George Washington University Milken Institute School of Public Health, Washington, D.C., who led the research. “This study finds that a variety of clinician types provide abortion care. But the number of abortion providers is low, and increasing restrictions will only make this worse.”
For their census, Dr. Strasser and her colleagues evaluated medical claims covering a full year from a private data company. They focused on two sets of services: medications (misoprostol and mifepristone) used in abortion care and pregnancy loss and procedures such as dilation and curettage and dilation and evacuation. Services were categorized as induced abortion or management by pregnancy loss on the basis of medical coding.
The researchers found that there were 3,550 abortion providers and 22,001 clinicians who managed pregnancy loss. Of those who induced abortions, 88% were physicians and 12% were advanced practice clinicians.
The clinicians who most frequently provided induced abortions were ob/gyns (72%), followed by family physicians (9%), advanced practice registered nurses (8%), and nurse midwives (3%). Several other specialists performed about 1% of abortions each.
Dr. Strasser said that 3,550 is an undercount because many providers do offer abortions but cannot or do not bill for them. Even so, the number likely will fall because fewer medical students are being trained for abortion procedures, according to Kaiser Health News.
Despite recommendations from the American College of Obstetricians and Gynecologists for standardized training on abortion care during medical residency, the number of programs that prohibit that training has surged in recent years, the report notes.
Restrictions looming
Compounding the problem, the researchers say, is the recent spate of state-level legislation regarding access to abortion. The U.S. Supreme Court is due to rule soon on a Mississippi law banning all abortions over 15 weeks’ gestational age, except in medical emergencies and in the case of severe fetal abnormalities.
Last May, Texas passed a law outlawing termination of pregnancy after 6 weeks of gestation – before many women know they’re pregnant. The law created a bounty system that permits essentially anyone in the United States to sue a woman in the state who seeks an abortion outside the law or anyone who assists her – including health care professionals. The Supreme Court in December refused to overturn the law – which reportedly has triggered a surge in women seeking abortion services in neighboring Oklahoma.
The legal environment is greatly increasing the risk that more clinicians will drop out of the workforce, Dr. Strasser told this news organization.
“As this happens, abortion care will undoubtedly become harder to access, especially for vulnerable populations,” she said. “Patients will have to travel farther, pay more money, or forgo necessary care.”
Another major variable is insurance coverage, the researchers found. Abortion coverage is highly restricted under private insurance and Medicaid, they note. Beyond increasingly restrictive payment issues, policies seen as punitive toward clinicians may cause many to stop offering medication and procedural services, Dr. Strasser said.
“The national political climate will likely see more barriers and less access to care in the coming months and years,” she told this news organization. “However, some states are taking concrete steps to protect abortion access for their residents and for others out of state. In supportive environments like these, enhanced training, expanded scope of practice, and improved reimbursement policies can increase access.”
If the Supreme Court overturns Roe v. Wade, Kaiser Health News reported, 26 states would likely ban abortion, triggering a flood of patients to states where the procedure remains more widely available.
According to the Center for Reproductive Rights, states that have expanded access are Washington, Oregon, California, New York, Vermont, Connecticut, and New Jersey. Another 12 states offer protected access, in which abortion is likely to remain legal even if Roe v. Wade is overturned, since in many of them, abortion is protected under their state constitutions.
Pivot to telehealth?
Another study, published in the same issue of JAMA Internal Medicine, evaluated health outcomes for 3,779 women. That study found that eligibility screening for medical abortions by history alone, without pelvic examination or ultrasonography, was safe and effective. That study found that medications were either dispensed in person or through the mail.
Taken together, the two studies suggest that more abortion services may shift toward telehealth, which could expand the number of health care professionals performing such services. Providers could include nurse practitioners, midwives, and physician assistants, said Melissa Grant, chief operating officer of carafem, a reproductive health and abortion service provider.
The service, which has offices in Atlanta, Chicago, Nashville, and Washington, D.C., has found that many patients prefer online appointments, especially if they live in rural areas, Ms. Grant said. The pandemic created a push toward online services.
“Even before the current breadth of restrictive legislation, we were seeing in increase because of COVID,” she said. “Most likely, abortion providers will continue to be pushed out of the profession, so having an option that’s widely available no matter where you live is essential. The United States is moving toward a system where the ZIP code you live in will foretell what care you get. That’s chilling.”
Those who currently provide abortion care have two advantages over what was available previously, Ms. Grant said. First, medical abortion is much more common, and data show that it is safe and effective for most pregnant people, as long as they undergo a health screening. Second, the boom in telehealth during the pandemic means providers are much more experienced in this type of service than before.
As more services such as carafem crop up, costs will drop, since a telehealth clinic – even one that uses health care professionals – has fewer expenses, such as for rent and equipment, than a physical facility.
“Because of the stigma around abortion, this is not likely to prompt a big rush of start-ups, but I do think we’re going to see a shake-up in the way services are being offered, and both patients and providers will likely turn toward technology,” Ms. Grant said. “An environment like this will require flexibility, innovation, and some real grit. We may take some time to get there, but it’s possible this moment is a pivot point in how abortion care is provided.”
Some of the researchers received grants from the Susan T. Buffett Foundation.
A version of this article first appeared on Medscape.com.
A small, and likely decreasing, number of health care providers in the United States perform abortions, and there is a risk that the count will be shrinking in the face of legislative attacks on the service, researchers have found.
Until now, producing an accurate count of abortion service providers in the United States has been difficult, leaving researchers to rely on indirect assessments of abortion clinics rather than counts of physicians who perform the procedure.
But the authors of a research letter published in JAMA Internal Medicine have come up with a number: Roughly 3,550 clinicians provide procedural and medication abortions, while 22,001 manage pregnancy loss with the same procedures and medications. More than half of all abortions in the United States now are achieved by medication.
The small number of providers is a cause for concern as a growing number of states move to restrict access to abortions, experts say.
“Abortions are only available if clinicians provide them,” said Julia Strasser, DrPH, MPH, senior research scientist at the George Washington University Milken Institute School of Public Health, Washington, D.C., who led the research. “This study finds that a variety of clinician types provide abortion care. But the number of abortion providers is low, and increasing restrictions will only make this worse.”
For their census, Dr. Strasser and her colleagues evaluated medical claims covering a full year from a private data company. They focused on two sets of services: medications (misoprostol and mifepristone) used in abortion care and pregnancy loss and procedures such as dilation and curettage and dilation and evacuation. Services were categorized as induced abortion or management by pregnancy loss on the basis of medical coding.
The researchers found that there were 3,550 abortion providers and 22,001 clinicians who managed pregnancy loss. Of those who induced abortions, 88% were physicians and 12% were advanced practice clinicians.
The clinicians who most frequently provided induced abortions were ob/gyns (72%), followed by family physicians (9%), advanced practice registered nurses (8%), and nurse midwives (3%). Several other specialists performed about 1% of abortions each.
Dr. Strasser said that 3,550 is an undercount because many providers do offer abortions but cannot or do not bill for them. Even so, the number likely will fall because fewer medical students are being trained for abortion procedures, according to Kaiser Health News.
Despite recommendations from the American College of Obstetricians and Gynecologists for standardized training on abortion care during medical residency, the number of programs that prohibit that training has surged in recent years, the report notes.
Restrictions looming
Compounding the problem, the researchers say, is the recent spate of state-level legislation regarding access to abortion. The U.S. Supreme Court is due to rule soon on a Mississippi law banning all abortions over 15 weeks’ gestational age, except in medical emergencies and in the case of severe fetal abnormalities.
Last May, Texas passed a law outlawing termination of pregnancy after 6 weeks of gestation – before many women know they’re pregnant. The law created a bounty system that permits essentially anyone in the United States to sue a woman in the state who seeks an abortion outside the law or anyone who assists her – including health care professionals. The Supreme Court in December refused to overturn the law – which reportedly has triggered a surge in women seeking abortion services in neighboring Oklahoma.
The legal environment is greatly increasing the risk that more clinicians will drop out of the workforce, Dr. Strasser told this news organization.
“As this happens, abortion care will undoubtedly become harder to access, especially for vulnerable populations,” she said. “Patients will have to travel farther, pay more money, or forgo necessary care.”
Another major variable is insurance coverage, the researchers found. Abortion coverage is highly restricted under private insurance and Medicaid, they note. Beyond increasingly restrictive payment issues, policies seen as punitive toward clinicians may cause many to stop offering medication and procedural services, Dr. Strasser said.
“The national political climate will likely see more barriers and less access to care in the coming months and years,” she told this news organization. “However, some states are taking concrete steps to protect abortion access for their residents and for others out of state. In supportive environments like these, enhanced training, expanded scope of practice, and improved reimbursement policies can increase access.”
If the Supreme Court overturns Roe v. Wade, Kaiser Health News reported, 26 states would likely ban abortion, triggering a flood of patients to states where the procedure remains more widely available.
According to the Center for Reproductive Rights, states that have expanded access are Washington, Oregon, California, New York, Vermont, Connecticut, and New Jersey. Another 12 states offer protected access, in which abortion is likely to remain legal even if Roe v. Wade is overturned, since in many of them, abortion is protected under their state constitutions.
Pivot to telehealth?
Another study, published in the same issue of JAMA Internal Medicine, evaluated health outcomes for 3,779 women. That study found that eligibility screening for medical abortions by history alone, without pelvic examination or ultrasonography, was safe and effective. That study found that medications were either dispensed in person or through the mail.
Taken together, the two studies suggest that more abortion services may shift toward telehealth, which could expand the number of health care professionals performing such services. Providers could include nurse practitioners, midwives, and physician assistants, said Melissa Grant, chief operating officer of carafem, a reproductive health and abortion service provider.
The service, which has offices in Atlanta, Chicago, Nashville, and Washington, D.C., has found that many patients prefer online appointments, especially if they live in rural areas, Ms. Grant said. The pandemic created a push toward online services.
“Even before the current breadth of restrictive legislation, we were seeing in increase because of COVID,” she said. “Most likely, abortion providers will continue to be pushed out of the profession, so having an option that’s widely available no matter where you live is essential. The United States is moving toward a system where the ZIP code you live in will foretell what care you get. That’s chilling.”
Those who currently provide abortion care have two advantages over what was available previously, Ms. Grant said. First, medical abortion is much more common, and data show that it is safe and effective for most pregnant people, as long as they undergo a health screening. Second, the boom in telehealth during the pandemic means providers are much more experienced in this type of service than before.
As more services such as carafem crop up, costs will drop, since a telehealth clinic – even one that uses health care professionals – has fewer expenses, such as for rent and equipment, than a physical facility.
“Because of the stigma around abortion, this is not likely to prompt a big rush of start-ups, but I do think we’re going to see a shake-up in the way services are being offered, and both patients and providers will likely turn toward technology,” Ms. Grant said. “An environment like this will require flexibility, innovation, and some real grit. We may take some time to get there, but it’s possible this moment is a pivot point in how abortion care is provided.”
Some of the researchers received grants from the Susan T. Buffett Foundation.
A version of this article first appeared on Medscape.com.
A small, and likely decreasing, number of health care providers in the United States perform abortions, and there is a risk that the count will be shrinking in the face of legislative attacks on the service, researchers have found.
Until now, producing an accurate count of abortion service providers in the United States has been difficult, leaving researchers to rely on indirect assessments of abortion clinics rather than counts of physicians who perform the procedure.
But the authors of a research letter published in JAMA Internal Medicine have come up with a number: Roughly 3,550 clinicians provide procedural and medication abortions, while 22,001 manage pregnancy loss with the same procedures and medications. More than half of all abortions in the United States now are achieved by medication.
The small number of providers is a cause for concern as a growing number of states move to restrict access to abortions, experts say.
“Abortions are only available if clinicians provide them,” said Julia Strasser, DrPH, MPH, senior research scientist at the George Washington University Milken Institute School of Public Health, Washington, D.C., who led the research. “This study finds that a variety of clinician types provide abortion care. But the number of abortion providers is low, and increasing restrictions will only make this worse.”
For their census, Dr. Strasser and her colleagues evaluated medical claims covering a full year from a private data company. They focused on two sets of services: medications (misoprostol and mifepristone) used in abortion care and pregnancy loss and procedures such as dilation and curettage and dilation and evacuation. Services were categorized as induced abortion or management by pregnancy loss on the basis of medical coding.
The researchers found that there were 3,550 abortion providers and 22,001 clinicians who managed pregnancy loss. Of those who induced abortions, 88% were physicians and 12% were advanced practice clinicians.
The clinicians who most frequently provided induced abortions were ob/gyns (72%), followed by family physicians (9%), advanced practice registered nurses (8%), and nurse midwives (3%). Several other specialists performed about 1% of abortions each.
Dr. Strasser said that 3,550 is an undercount because many providers do offer abortions but cannot or do not bill for them. Even so, the number likely will fall because fewer medical students are being trained for abortion procedures, according to Kaiser Health News.
Despite recommendations from the American College of Obstetricians and Gynecologists for standardized training on abortion care during medical residency, the number of programs that prohibit that training has surged in recent years, the report notes.
Restrictions looming
Compounding the problem, the researchers say, is the recent spate of state-level legislation regarding access to abortion. The U.S. Supreme Court is due to rule soon on a Mississippi law banning all abortions over 15 weeks’ gestational age, except in medical emergencies and in the case of severe fetal abnormalities.
Last May, Texas passed a law outlawing termination of pregnancy after 6 weeks of gestation – before many women know they’re pregnant. The law created a bounty system that permits essentially anyone in the United States to sue a woman in the state who seeks an abortion outside the law or anyone who assists her – including health care professionals. The Supreme Court in December refused to overturn the law – which reportedly has triggered a surge in women seeking abortion services in neighboring Oklahoma.
The legal environment is greatly increasing the risk that more clinicians will drop out of the workforce, Dr. Strasser told this news organization.
“As this happens, abortion care will undoubtedly become harder to access, especially for vulnerable populations,” she said. “Patients will have to travel farther, pay more money, or forgo necessary care.”
Another major variable is insurance coverage, the researchers found. Abortion coverage is highly restricted under private insurance and Medicaid, they note. Beyond increasingly restrictive payment issues, policies seen as punitive toward clinicians may cause many to stop offering medication and procedural services, Dr. Strasser said.
“The national political climate will likely see more barriers and less access to care in the coming months and years,” she told this news organization. “However, some states are taking concrete steps to protect abortion access for their residents and for others out of state. In supportive environments like these, enhanced training, expanded scope of practice, and improved reimbursement policies can increase access.”
If the Supreme Court overturns Roe v. Wade, Kaiser Health News reported, 26 states would likely ban abortion, triggering a flood of patients to states where the procedure remains more widely available.
According to the Center for Reproductive Rights, states that have expanded access are Washington, Oregon, California, New York, Vermont, Connecticut, and New Jersey. Another 12 states offer protected access, in which abortion is likely to remain legal even if Roe v. Wade is overturned, since in many of them, abortion is protected under their state constitutions.
Pivot to telehealth?
Another study, published in the same issue of JAMA Internal Medicine, evaluated health outcomes for 3,779 women. That study found that eligibility screening for medical abortions by history alone, without pelvic examination or ultrasonography, was safe and effective. That study found that medications were either dispensed in person or through the mail.
Taken together, the two studies suggest that more abortion services may shift toward telehealth, which could expand the number of health care professionals performing such services. Providers could include nurse practitioners, midwives, and physician assistants, said Melissa Grant, chief operating officer of carafem, a reproductive health and abortion service provider.
The service, which has offices in Atlanta, Chicago, Nashville, and Washington, D.C., has found that many patients prefer online appointments, especially if they live in rural areas, Ms. Grant said. The pandemic created a push toward online services.
“Even before the current breadth of restrictive legislation, we were seeing in increase because of COVID,” she said. “Most likely, abortion providers will continue to be pushed out of the profession, so having an option that’s widely available no matter where you live is essential. The United States is moving toward a system where the ZIP code you live in will foretell what care you get. That’s chilling.”
Those who currently provide abortion care have two advantages over what was available previously, Ms. Grant said. First, medical abortion is much more common, and data show that it is safe and effective for most pregnant people, as long as they undergo a health screening. Second, the boom in telehealth during the pandemic means providers are much more experienced in this type of service than before.
As more services such as carafem crop up, costs will drop, since a telehealth clinic – even one that uses health care professionals – has fewer expenses, such as for rent and equipment, than a physical facility.
“Because of the stigma around abortion, this is not likely to prompt a big rush of start-ups, but I do think we’re going to see a shake-up in the way services are being offered, and both patients and providers will likely turn toward technology,” Ms. Grant said. “An environment like this will require flexibility, innovation, and some real grit. We may take some time to get there, but it’s possible this moment is a pivot point in how abortion care is provided.”
Some of the researchers received grants from the Susan T. Buffett Foundation.
A version of this article first appeared on Medscape.com.
Family Physician: Abortion care is health and primary care
I am aware of how intersecting social, economic, familial, and environmental factors influence what is best for patient’s lives, and I consider having this awareness to be part of being a family medicine physician.
People being able to make choices about their reproductive health and their reproductive futures without unnecessary and harmful barriers is a part of a person’s overall health that family medicine physicians should recognize and prioritize. Helping people achieve their reproductive health care goals includes helping patients access abortion care if that is the care that they decide that they need.
According to the Guttmacher Institute, 2021 was “the worst year for abortion rights in almost half a century” as 108 abortion restrictions were enacted throughout the country. The most damaging restriction was introduced in Texas in the fall of 2021 called SB8, which has virtually stopped all abortion care in person for any person with a pregnancy greater than 6 weeks’ gestation. Now, in 2022 we are seeing several other states, including Idaho and Oklahoma, set to pass similar laws that will essentially halt most abortion care in the clinical setting in those states.
Abortion access had already been a problem in much of the country prior to 2021 because of burdensome and not medically necessary restrictions. Based on current political trends we are getting to a place where it is not hard to imagine that up to half of the states in this country will not allow their communities to access abortion care in the clinical setting at all in the very near future. This is not reproductive freedom, and I am outraged that people are being forced to travel hundreds of miles for their abortion care, forced to continue pregnancies that they don’t want, or forced to find other ways to obtain medication abortion pills.
While obtaining medication abortion pills online and managing the abortion process at home is safe and recognized as safe by the World Health Organization, no one should be forced to utilize a certain type of care based on their state of residence, in my opinion.
Providing evidence-based medicine to patients is ‘my duty’
Abortion care is health care and is primary care. One in four women will have an abortion by the age of 45, and we know that transgender, nonbinary, and gender-expansive people also have abortions. That means on any given day as family medicine physicians we are likely taking care of more than one person who has had an abortion, will have an abortion, and/or is considering an abortion. Therefore, all family medicine physicians need to be prepared to counsel patients about all of their pregnancy options, answer questions about pregnancy and abortion, and help people get the compassionate care that they deserve.
Our patients turn to us as trusted sources of information. When they reach out to us, I consider providing evidence-based medicine to patients – that includes factual information about abortion care if and when our patients need it – to be my duty as a family medicine physician.
Resources on abortion care for family medicine physicians
For family medicine physicians who did not have adequate exposure to abortion care during residency, there are many evidence-based resources to review in order to become more knowledgeable in abortion care.
In many areas of medicine, we have to continue to learn and seek out references, and abortion care is no different. One could argue that understanding abortion care and providing patients with factual information about their options and abortion care is even more important because of stigma surrounding abortion care and the rampant lies about abortion care that are easily accessible and that even other medical professionals and politicians spread. There are even fake clinics, often called “crisis pregnancy centers”, that intimidate, lie about abortion, and coerce patients to make decisions that are against their desires. Thus, being that trusted source of factual information about abortion care is even more important in the face of so many lies.
There are several organizations that are dedicated to education surrounding abortion care, in particular within the primary care setting. The Reproductive Health Access Project (RHAP), Reproductive Health Education in Family Medicine (RHEDI), and Training in Early Abortion for Comprehensive Healthcare (TEACH) all provide free resources on abortion care, how to incorporate abortion care into primary care, and how to teach medical students and residents about abortion care.
In addition, the National Network of Abortion Funds connects people to community-led organizations that provide assistance related to direct financial and logistical support for obtaining abortion care. I believe it is critical that we familiarize ourselves with our local abortion funds and share what we learn about these resources with our patients.
As abortion access continues to be further stripped away from many people that we take care of, I think standing up for what is right and what is our duty as physicians becomes more important. That duty is to provide our patients with evidence-based medicine and compassionate care so that our communities can obtain reproductive health outcomes and freedom that are best for their lives.
Dr. Lockley is a family physician currently living in Harlem, N.Y., and a member of the editorial advisory board of Family Practice News. She currently works for Public Health Solutions’ Sexual and Reproductive Health Centers in Brooklyn, providing primary care and reproductive health care services there, and as an abortion provider throughout the New York region. She completed both medical school and residency in Philadelphia and then did a fellowship in reproductive health care and advocacy through the Family Health Center of Harlem and the Reproductive Health Access Project. She can be reached at [email protected].
I am aware of how intersecting social, economic, familial, and environmental factors influence what is best for patient’s lives, and I consider having this awareness to be part of being a family medicine physician.
People being able to make choices about their reproductive health and their reproductive futures without unnecessary and harmful barriers is a part of a person’s overall health that family medicine physicians should recognize and prioritize. Helping people achieve their reproductive health care goals includes helping patients access abortion care if that is the care that they decide that they need.
According to the Guttmacher Institute, 2021 was “the worst year for abortion rights in almost half a century” as 108 abortion restrictions were enacted throughout the country. The most damaging restriction was introduced in Texas in the fall of 2021 called SB8, which has virtually stopped all abortion care in person for any person with a pregnancy greater than 6 weeks’ gestation. Now, in 2022 we are seeing several other states, including Idaho and Oklahoma, set to pass similar laws that will essentially halt most abortion care in the clinical setting in those states.
Abortion access had already been a problem in much of the country prior to 2021 because of burdensome and not medically necessary restrictions. Based on current political trends we are getting to a place where it is not hard to imagine that up to half of the states in this country will not allow their communities to access abortion care in the clinical setting at all in the very near future. This is not reproductive freedom, and I am outraged that people are being forced to travel hundreds of miles for their abortion care, forced to continue pregnancies that they don’t want, or forced to find other ways to obtain medication abortion pills.
While obtaining medication abortion pills online and managing the abortion process at home is safe and recognized as safe by the World Health Organization, no one should be forced to utilize a certain type of care based on their state of residence, in my opinion.
Providing evidence-based medicine to patients is ‘my duty’
Abortion care is health care and is primary care. One in four women will have an abortion by the age of 45, and we know that transgender, nonbinary, and gender-expansive people also have abortions. That means on any given day as family medicine physicians we are likely taking care of more than one person who has had an abortion, will have an abortion, and/or is considering an abortion. Therefore, all family medicine physicians need to be prepared to counsel patients about all of their pregnancy options, answer questions about pregnancy and abortion, and help people get the compassionate care that they deserve.
Our patients turn to us as trusted sources of information. When they reach out to us, I consider providing evidence-based medicine to patients – that includes factual information about abortion care if and when our patients need it – to be my duty as a family medicine physician.
Resources on abortion care for family medicine physicians
For family medicine physicians who did not have adequate exposure to abortion care during residency, there are many evidence-based resources to review in order to become more knowledgeable in abortion care.
In many areas of medicine, we have to continue to learn and seek out references, and abortion care is no different. One could argue that understanding abortion care and providing patients with factual information about their options and abortion care is even more important because of stigma surrounding abortion care and the rampant lies about abortion care that are easily accessible and that even other medical professionals and politicians spread. There are even fake clinics, often called “crisis pregnancy centers”, that intimidate, lie about abortion, and coerce patients to make decisions that are against their desires. Thus, being that trusted source of factual information about abortion care is even more important in the face of so many lies.
There are several organizations that are dedicated to education surrounding abortion care, in particular within the primary care setting. The Reproductive Health Access Project (RHAP), Reproductive Health Education in Family Medicine (RHEDI), and Training in Early Abortion for Comprehensive Healthcare (TEACH) all provide free resources on abortion care, how to incorporate abortion care into primary care, and how to teach medical students and residents about abortion care.
In addition, the National Network of Abortion Funds connects people to community-led organizations that provide assistance related to direct financial and logistical support for obtaining abortion care. I believe it is critical that we familiarize ourselves with our local abortion funds and share what we learn about these resources with our patients.
As abortion access continues to be further stripped away from many people that we take care of, I think standing up for what is right and what is our duty as physicians becomes more important. That duty is to provide our patients with evidence-based medicine and compassionate care so that our communities can obtain reproductive health outcomes and freedom that are best for their lives.
Dr. Lockley is a family physician currently living in Harlem, N.Y., and a member of the editorial advisory board of Family Practice News. She currently works for Public Health Solutions’ Sexual and Reproductive Health Centers in Brooklyn, providing primary care and reproductive health care services there, and as an abortion provider throughout the New York region. She completed both medical school and residency in Philadelphia and then did a fellowship in reproductive health care and advocacy through the Family Health Center of Harlem and the Reproductive Health Access Project. She can be reached at [email protected].
I am aware of how intersecting social, economic, familial, and environmental factors influence what is best for patient’s lives, and I consider having this awareness to be part of being a family medicine physician.
People being able to make choices about their reproductive health and their reproductive futures without unnecessary and harmful barriers is a part of a person’s overall health that family medicine physicians should recognize and prioritize. Helping people achieve their reproductive health care goals includes helping patients access abortion care if that is the care that they decide that they need.
According to the Guttmacher Institute, 2021 was “the worst year for abortion rights in almost half a century” as 108 abortion restrictions were enacted throughout the country. The most damaging restriction was introduced in Texas in the fall of 2021 called SB8, which has virtually stopped all abortion care in person for any person with a pregnancy greater than 6 weeks’ gestation. Now, in 2022 we are seeing several other states, including Idaho and Oklahoma, set to pass similar laws that will essentially halt most abortion care in the clinical setting in those states.
Abortion access had already been a problem in much of the country prior to 2021 because of burdensome and not medically necessary restrictions. Based on current political trends we are getting to a place where it is not hard to imagine that up to half of the states in this country will not allow their communities to access abortion care in the clinical setting at all in the very near future. This is not reproductive freedom, and I am outraged that people are being forced to travel hundreds of miles for their abortion care, forced to continue pregnancies that they don’t want, or forced to find other ways to obtain medication abortion pills.
While obtaining medication abortion pills online and managing the abortion process at home is safe and recognized as safe by the World Health Organization, no one should be forced to utilize a certain type of care based on their state of residence, in my opinion.
Providing evidence-based medicine to patients is ‘my duty’
Abortion care is health care and is primary care. One in four women will have an abortion by the age of 45, and we know that transgender, nonbinary, and gender-expansive people also have abortions. That means on any given day as family medicine physicians we are likely taking care of more than one person who has had an abortion, will have an abortion, and/or is considering an abortion. Therefore, all family medicine physicians need to be prepared to counsel patients about all of their pregnancy options, answer questions about pregnancy and abortion, and help people get the compassionate care that they deserve.
Our patients turn to us as trusted sources of information. When they reach out to us, I consider providing evidence-based medicine to patients – that includes factual information about abortion care if and when our patients need it – to be my duty as a family medicine physician.
Resources on abortion care for family medicine physicians
For family medicine physicians who did not have adequate exposure to abortion care during residency, there are many evidence-based resources to review in order to become more knowledgeable in abortion care.
In many areas of medicine, we have to continue to learn and seek out references, and abortion care is no different. One could argue that understanding abortion care and providing patients with factual information about their options and abortion care is even more important because of stigma surrounding abortion care and the rampant lies about abortion care that are easily accessible and that even other medical professionals and politicians spread. There are even fake clinics, often called “crisis pregnancy centers”, that intimidate, lie about abortion, and coerce patients to make decisions that are against their desires. Thus, being that trusted source of factual information about abortion care is even more important in the face of so many lies.
There are several organizations that are dedicated to education surrounding abortion care, in particular within the primary care setting. The Reproductive Health Access Project (RHAP), Reproductive Health Education in Family Medicine (RHEDI), and Training in Early Abortion for Comprehensive Healthcare (TEACH) all provide free resources on abortion care, how to incorporate abortion care into primary care, and how to teach medical students and residents about abortion care.
In addition, the National Network of Abortion Funds connects people to community-led organizations that provide assistance related to direct financial and logistical support for obtaining abortion care. I believe it is critical that we familiarize ourselves with our local abortion funds and share what we learn about these resources with our patients.
As abortion access continues to be further stripped away from many people that we take care of, I think standing up for what is right and what is our duty as physicians becomes more important. That duty is to provide our patients with evidence-based medicine and compassionate care so that our communities can obtain reproductive health outcomes and freedom that are best for their lives.
Dr. Lockley is a family physician currently living in Harlem, N.Y., and a member of the editorial advisory board of Family Practice News. She currently works for Public Health Solutions’ Sexual and Reproductive Health Centers in Brooklyn, providing primary care and reproductive health care services there, and as an abortion provider throughout the New York region. She completed both medical school and residency in Philadelphia and then did a fellowship in reproductive health care and advocacy through the Family Health Center of Harlem and the Reproductive Health Access Project. She can be reached at [email protected].
Maternal obesity promotes risk of perinatal death
The infants of obese pregnant women had a 55% higher adjusted perinatal death rate, compared with those of normal-weight pregnant women, but lower gestational age had a mediating effect, based on data from nearly 400,000 women-infant pairs.
“While some obesity-related causes of fetal death are known, the exact pathophysiology behind the effects of obesity on perinatal death are not completely understood,” Jeffrey N. Bone, MD, of the University of British Columbia, Vancouver, and colleagues wrote. Higher body mass index prior to pregnancy also is associated with preterm delivery, but the effect of gestational age on the association between BMI and infant mortality has not been well explored.
In a study published in PLOS ONE, the researchers reviewed data from nearly 400,000 women obtained through the British Columbia Perinatal Database Registry, which collects obstetric and neonatal data from hospital charts and from delivery records of home births. Births at less than 20 weeks’ gestation and late pregnancy terminations were excluded.
BMI was based on self-reported prepregnancy height and weight; of the 392,820 included women, 12.8% were classified as obese, 20.6% were overweight, 60.6% were normal weight, and 6.0% were underweight. Infants of women with higher BMI had a lower gestational age at delivery. Perinatal mortality occurred in 1,834 pregnancies (0.5%). In adjusted analysis, infant perinatal death was significantly more likely for obese women (adjusted odds ratio, 1.55) and overweight women (aOR, 1.22).
However, 63.1% of this association in obese women was mediated by gestational age of the infant at delivery, with aORs of 1.32 and 1.18 for natural indirect and natural direct effects, respectively, compared with that of normal-weight women. Similar, but lesser effects were noted for overweight women, with aORs of 1.11 and 1.10, respectively. “Direct effects were higher, and mediation was lower for stillbirth than for neonatal death, where the total effect was entirely indirect,” but the confidence intervals remained consistent with the primary analyses, the researchers noted.
The increased perinatal death rates of infants of obese and overweight women reflect data from previous studies, but the current study’s use of mediation analysis offers new insight on the mechanism by which perinatal death rates increase with higher maternal BMI, the researchers wrote.
The study findings were limited by several factors including the need to consider potential common risk factors for both perinatal death and early delivery that would be affected by maternal obesity, the researchers noted. Other limitations included the use of gestational age at stillbirth, which represents an approximation of fetal death in some cases, and the use of self-reports for prepregnancy maternal BMI.
However, the results were strengthened by the large, population-based design and information on potential confounding variables, and suggest that early gestational age at delivery may play a role in maternal obesity-related perinatal death risk.
“To better inform the pregnancy management in obese women, further studies should continue to disentangle the causal pathways under which obesity increases the risk of perinatal death, including, for example, gestational diabetes and other obesity-related pregnancy complications,” they concluded.
More testing and counseling are needed
The current study is important because obesity rates continue to increase in the reproductive-age population, Marissa Platner, MD, of Emory University, Atlanta, said in an interview. “Obesity has become a known risk factor for adverse pregnancy outcomes, specifically the risk of stillbirth and perinatal death. However, the authors correctly point out that the underlying cause of these perinatal deaths in women with obesity is unclear. Additionally, ACOG recently updated their clinical guidelines to recommend routine antenatal testing for women with obesity due to these increased rates of stillbirth.
“I was not surprised by these findings; similar to previous literature, the risks of perinatal death seem to have a dose-response relationship with increasing BMI. We know that women with prepregnancy obesity are also at higher risk of perinatal complications in the preterm period, which would increase the risk of perinatal death,” Dr. Platner said
“I think the take-home message for clinicians is twofold,” Dr. Platner said. First, “we need to take the updated antenatal testing guidelines from ACOG very seriously and implement these in our practices.” Second, “in the preconception or early antepartum period, these patients should be thoroughly counseled on the associated risks of pregnancy and discuss appropriate gestational weight gain guidelines and lifestyle modifications.”
However, “additional research is needed in a U.S. population with higher rates of obesity to determine the true effects of obesity on perinatal deaths and to further elucidate the underlying pathophysiology and disease processes that may lead to increased risk of both stillbirth and perinatal deaths,” Dr. Platner emphasized.
*This story was updated on March 23, 2022.
The study was supported by the Sick Kids Foundation and the Canadian Institute of Health Research. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
The infants of obese pregnant women had a 55% higher adjusted perinatal death rate, compared with those of normal-weight pregnant women, but lower gestational age had a mediating effect, based on data from nearly 400,000 women-infant pairs.
“While some obesity-related causes of fetal death are known, the exact pathophysiology behind the effects of obesity on perinatal death are not completely understood,” Jeffrey N. Bone, MD, of the University of British Columbia, Vancouver, and colleagues wrote. Higher body mass index prior to pregnancy also is associated with preterm delivery, but the effect of gestational age on the association between BMI and infant mortality has not been well explored.
In a study published in PLOS ONE, the researchers reviewed data from nearly 400,000 women obtained through the British Columbia Perinatal Database Registry, which collects obstetric and neonatal data from hospital charts and from delivery records of home births. Births at less than 20 weeks’ gestation and late pregnancy terminations were excluded.
BMI was based on self-reported prepregnancy height and weight; of the 392,820 included women, 12.8% were classified as obese, 20.6% were overweight, 60.6% were normal weight, and 6.0% were underweight. Infants of women with higher BMI had a lower gestational age at delivery. Perinatal mortality occurred in 1,834 pregnancies (0.5%). In adjusted analysis, infant perinatal death was significantly more likely for obese women (adjusted odds ratio, 1.55) and overweight women (aOR, 1.22).
However, 63.1% of this association in obese women was mediated by gestational age of the infant at delivery, with aORs of 1.32 and 1.18 for natural indirect and natural direct effects, respectively, compared with that of normal-weight women. Similar, but lesser effects were noted for overweight women, with aORs of 1.11 and 1.10, respectively. “Direct effects were higher, and mediation was lower for stillbirth than for neonatal death, where the total effect was entirely indirect,” but the confidence intervals remained consistent with the primary analyses, the researchers noted.
The increased perinatal death rates of infants of obese and overweight women reflect data from previous studies, but the current study’s use of mediation analysis offers new insight on the mechanism by which perinatal death rates increase with higher maternal BMI, the researchers wrote.
The study findings were limited by several factors including the need to consider potential common risk factors for both perinatal death and early delivery that would be affected by maternal obesity, the researchers noted. Other limitations included the use of gestational age at stillbirth, which represents an approximation of fetal death in some cases, and the use of self-reports for prepregnancy maternal BMI.
However, the results were strengthened by the large, population-based design and information on potential confounding variables, and suggest that early gestational age at delivery may play a role in maternal obesity-related perinatal death risk.
“To better inform the pregnancy management in obese women, further studies should continue to disentangle the causal pathways under which obesity increases the risk of perinatal death, including, for example, gestational diabetes and other obesity-related pregnancy complications,” they concluded.
More testing and counseling are needed
The current study is important because obesity rates continue to increase in the reproductive-age population, Marissa Platner, MD, of Emory University, Atlanta, said in an interview. “Obesity has become a known risk factor for adverse pregnancy outcomes, specifically the risk of stillbirth and perinatal death. However, the authors correctly point out that the underlying cause of these perinatal deaths in women with obesity is unclear. Additionally, ACOG recently updated their clinical guidelines to recommend routine antenatal testing for women with obesity due to these increased rates of stillbirth.
“I was not surprised by these findings; similar to previous literature, the risks of perinatal death seem to have a dose-response relationship with increasing BMI. We know that women with prepregnancy obesity are also at higher risk of perinatal complications in the preterm period, which would increase the risk of perinatal death,” Dr. Platner said
“I think the take-home message for clinicians is twofold,” Dr. Platner said. First, “we need to take the updated antenatal testing guidelines from ACOG very seriously and implement these in our practices.” Second, “in the preconception or early antepartum period, these patients should be thoroughly counseled on the associated risks of pregnancy and discuss appropriate gestational weight gain guidelines and lifestyle modifications.”
However, “additional research is needed in a U.S. population with higher rates of obesity to determine the true effects of obesity on perinatal deaths and to further elucidate the underlying pathophysiology and disease processes that may lead to increased risk of both stillbirth and perinatal deaths,” Dr. Platner emphasized.
*This story was updated on March 23, 2022.
The study was supported by the Sick Kids Foundation and the Canadian Institute of Health Research. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
The infants of obese pregnant women had a 55% higher adjusted perinatal death rate, compared with those of normal-weight pregnant women, but lower gestational age had a mediating effect, based on data from nearly 400,000 women-infant pairs.
“While some obesity-related causes of fetal death are known, the exact pathophysiology behind the effects of obesity on perinatal death are not completely understood,” Jeffrey N. Bone, MD, of the University of British Columbia, Vancouver, and colleagues wrote. Higher body mass index prior to pregnancy also is associated with preterm delivery, but the effect of gestational age on the association between BMI and infant mortality has not been well explored.
In a study published in PLOS ONE, the researchers reviewed data from nearly 400,000 women obtained through the British Columbia Perinatal Database Registry, which collects obstetric and neonatal data from hospital charts and from delivery records of home births. Births at less than 20 weeks’ gestation and late pregnancy terminations were excluded.
BMI was based on self-reported prepregnancy height and weight; of the 392,820 included women, 12.8% were classified as obese, 20.6% were overweight, 60.6% were normal weight, and 6.0% were underweight. Infants of women with higher BMI had a lower gestational age at delivery. Perinatal mortality occurred in 1,834 pregnancies (0.5%). In adjusted analysis, infant perinatal death was significantly more likely for obese women (adjusted odds ratio, 1.55) and overweight women (aOR, 1.22).
However, 63.1% of this association in obese women was mediated by gestational age of the infant at delivery, with aORs of 1.32 and 1.18 for natural indirect and natural direct effects, respectively, compared with that of normal-weight women. Similar, but lesser effects were noted for overweight women, with aORs of 1.11 and 1.10, respectively. “Direct effects were higher, and mediation was lower for stillbirth than for neonatal death, where the total effect was entirely indirect,” but the confidence intervals remained consistent with the primary analyses, the researchers noted.
The increased perinatal death rates of infants of obese and overweight women reflect data from previous studies, but the current study’s use of mediation analysis offers new insight on the mechanism by which perinatal death rates increase with higher maternal BMI, the researchers wrote.
The study findings were limited by several factors including the need to consider potential common risk factors for both perinatal death and early delivery that would be affected by maternal obesity, the researchers noted. Other limitations included the use of gestational age at stillbirth, which represents an approximation of fetal death in some cases, and the use of self-reports for prepregnancy maternal BMI.
However, the results were strengthened by the large, population-based design and information on potential confounding variables, and suggest that early gestational age at delivery may play a role in maternal obesity-related perinatal death risk.
“To better inform the pregnancy management in obese women, further studies should continue to disentangle the causal pathways under which obesity increases the risk of perinatal death, including, for example, gestational diabetes and other obesity-related pregnancy complications,” they concluded.
More testing and counseling are needed
The current study is important because obesity rates continue to increase in the reproductive-age population, Marissa Platner, MD, of Emory University, Atlanta, said in an interview. “Obesity has become a known risk factor for adverse pregnancy outcomes, specifically the risk of stillbirth and perinatal death. However, the authors correctly point out that the underlying cause of these perinatal deaths in women with obesity is unclear. Additionally, ACOG recently updated their clinical guidelines to recommend routine antenatal testing for women with obesity due to these increased rates of stillbirth.
“I was not surprised by these findings; similar to previous literature, the risks of perinatal death seem to have a dose-response relationship with increasing BMI. We know that women with prepregnancy obesity are also at higher risk of perinatal complications in the preterm period, which would increase the risk of perinatal death,” Dr. Platner said
“I think the take-home message for clinicians is twofold,” Dr. Platner said. First, “we need to take the updated antenatal testing guidelines from ACOG very seriously and implement these in our practices.” Second, “in the preconception or early antepartum period, these patients should be thoroughly counseled on the associated risks of pregnancy and discuss appropriate gestational weight gain guidelines and lifestyle modifications.”
However, “additional research is needed in a U.S. population with higher rates of obesity to determine the true effects of obesity on perinatal deaths and to further elucidate the underlying pathophysiology and disease processes that may lead to increased risk of both stillbirth and perinatal deaths,” Dr. Platner emphasized.
*This story was updated on March 23, 2022.
The study was supported by the Sick Kids Foundation and the Canadian Institute of Health Research. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
FROM PLOS ONE
Antiretroviral therapy associated with less risk of preterm birth
Over the past decade, data have suggested that antiretroviral therapy (ART) may be associated with an increased risk for adverse pregnancy outcomes, namely, preterm birth (PTB). But a combination of methodologic challenges, demographic gaps, and spotty clinical data has left the question unresolved, especially for pregnant women with HIV who reside in developed countries.
“Given that a lot of the emerging data has come out of resource-limited settings where patient and clinical characteristics are different from developed world settings like the United States, we felt that this was an important question to address,” Kartik Venkatesh, MD, PhD, a high-risk obstetrician and perinatal epidemiologist at the Ohio State Wexner Medical Center, Columbus, told this news organization.
In a prospective cohort study of U.S. women with or at risk for HIV, Dr. Venkatesh and his colleagues found that ART exposure (including highly active antiretroviral therapy [HAART]) was associated with as much as an 80% decline in the likelihood of PTB (defined as birth less than 34 weeks). The study was published in HIV Medicine.
24 years of data analyzed
Dr. Venkatesh and his team analyzed self-reported birth data of women with singleton live-born pregnancies enrolled in the ongoing, multicenter, prospective observational Women’s Interagency HIV Study (WIHS) from Oct. 1, 1995, to March 31, 2019.
“We first looked at women with HIV versus without HIV, [who were] matched on many clinical and sociodemographic characteristics and at similarly high risk of some of these obstetrical outcomes like PTB,” explained Dr. Venkatesh. “We then looked at the relative impact of antiretroviral therapy amongst women living with HIV compared to no antiretroviral therapy.”
ART regimens were classified as none, monotherapy, dual therapy, or HAART. (HAART was defined as more than three antiretrovirals, including at least one protease inhibitor [PI], nonnucleoside reverse transcriptase inhibitor, integrase inhibitor, or entry inhibitor.) In this cohort, for 63.5% of women receiving ART, therapy was initiated before pregnancy (mean duration of HAART, 6 years), and most were virally suppressed.
Among the 4,944 women assessed in the WIHS trial, 74% (3,646) had HIV. In total, 383 women had 488 singleton deliveries, including 218 women with HIV (272 deliveries) and 165 without HIV (216 deliveries). Sociodemographics in both cohorts were well matched. For most participants, the mean age was 40-41 years at delivery, most were non-Hispanic Black persons, and the mean pregnancy body mass index was greater than or equal to 29 kg/m2. Of the women with HIV, 33% had chronic hypertension; of those without HIV, 42.1% had chronic hypertension; 4.7% and 5.0%, respectively, had pregestational diabetes.
The findings showed that PTB risk less than 34 weeks was similar between women with (10%) and without (8%) HIV (adjusted risk ratio, 1.30; 95% confidence interval, 0.74-2.31). Among deliveries to women with HIV who were receiving ART, PTB risk less than 34 weeks was lower with HAART (7%), compared with not receiving ART (26%) (aRR, 0.19), as well as with monotherapy or dual therapy (3% vs. no ART) (aRR, 0.12). Notably, 67% of deliveries to women receiving HAART included a PI-containing regimen, but these women were not significantly more likely to have a PTB less than 34 weeks, compared with women taking non-PI HAART regimens (aRR, 2.61; 95% CI, 0.65-10.59). Results were similar for secondary outcomes (PTB less than 28 weeks, less than 37 weeks).
Filling in the gaps toward the safest regimen
“This study spans 25 years, so it covers a lot of the history of HIV in pregnancy and is reassuring around using ART in pregnancy,” Shahin Lockman, MD, told this news organization. Dr. Lockman is an associate professor of infectious diseases at Brigham and Women’s Hospital and a co-PI of the Botswana Clinical Trials Unit at the Botswana Harvard AIDS Institute Partnership. She was not involved in the study. “One of the worst things for a mother and for pregnancy outcomes, for the fetus and baby’s health and development, is uncontrolled maternal HIV,’’ she said.
Dr. Lockman also noted potential confounders that drive poor birth outcomes in Southern African women, compared with U.S. women, making comparisons between this and other observational studies difficult. Still, she said that the question is not whether women should be receiving treatment but whether or not there are differences between antiretroviral regimens.
“One of the areas that we did not go deeper into was the subtype of antiretroviral therapy, given the relatively small study numbers [did not] allow us to do a robust analysis,” Dr. Venkatesh said.
Rather, he emphasized that the findings might lend more weight to speculation that immunologic characteristics associated with HIV status and immunotherapy – such as low CD4 cell counts prior to delivery, or duration of HIV infection – may be important drivers of adverse birth outcomes among women with HIV taking ART.
And at least in this cohort, many of these characteristics were similar between the treatment groups.
Both researchers agree that the findings – while reassuring – highlight the importance of collecting robust obstetric and safety data as part of prospective databases of individuals living with HIV, not only in resource-limited settings but also among the domestic U.S. population.
“We’ve learned a lot over the last 10 years,” Dr. Lockman said. “Some regimens (like lopinavir/ritonavir or nevirapine) are associated with significantly worse birth outcomes, whereas efavirenz doesn’t seem to be, or less so, and dolutegravir seems to be associated with even better outcomes. So, I think that where we are moving is to regimens that are the safest.”
Moving forward, Dr. Venkatesh explained, not only should researchers focus on exploring which antiretrovirals are safest in this context but also if the use of preexposure prophylaxis during conception periods affects birth outcomes.
Dr. Venkatesh and Dr. Lockman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over the past decade, data have suggested that antiretroviral therapy (ART) may be associated with an increased risk for adverse pregnancy outcomes, namely, preterm birth (PTB). But a combination of methodologic challenges, demographic gaps, and spotty clinical data has left the question unresolved, especially for pregnant women with HIV who reside in developed countries.
“Given that a lot of the emerging data has come out of resource-limited settings where patient and clinical characteristics are different from developed world settings like the United States, we felt that this was an important question to address,” Kartik Venkatesh, MD, PhD, a high-risk obstetrician and perinatal epidemiologist at the Ohio State Wexner Medical Center, Columbus, told this news organization.
In a prospective cohort study of U.S. women with or at risk for HIV, Dr. Venkatesh and his colleagues found that ART exposure (including highly active antiretroviral therapy [HAART]) was associated with as much as an 80% decline in the likelihood of PTB (defined as birth less than 34 weeks). The study was published in HIV Medicine.
24 years of data analyzed
Dr. Venkatesh and his team analyzed self-reported birth data of women with singleton live-born pregnancies enrolled in the ongoing, multicenter, prospective observational Women’s Interagency HIV Study (WIHS) from Oct. 1, 1995, to March 31, 2019.
“We first looked at women with HIV versus without HIV, [who were] matched on many clinical and sociodemographic characteristics and at similarly high risk of some of these obstetrical outcomes like PTB,” explained Dr. Venkatesh. “We then looked at the relative impact of antiretroviral therapy amongst women living with HIV compared to no antiretroviral therapy.”
ART regimens were classified as none, monotherapy, dual therapy, or HAART. (HAART was defined as more than three antiretrovirals, including at least one protease inhibitor [PI], nonnucleoside reverse transcriptase inhibitor, integrase inhibitor, or entry inhibitor.) In this cohort, for 63.5% of women receiving ART, therapy was initiated before pregnancy (mean duration of HAART, 6 years), and most were virally suppressed.
Among the 4,944 women assessed in the WIHS trial, 74% (3,646) had HIV. In total, 383 women had 488 singleton deliveries, including 218 women with HIV (272 deliveries) and 165 without HIV (216 deliveries). Sociodemographics in both cohorts were well matched. For most participants, the mean age was 40-41 years at delivery, most were non-Hispanic Black persons, and the mean pregnancy body mass index was greater than or equal to 29 kg/m2. Of the women with HIV, 33% had chronic hypertension; of those without HIV, 42.1% had chronic hypertension; 4.7% and 5.0%, respectively, had pregestational diabetes.
The findings showed that PTB risk less than 34 weeks was similar between women with (10%) and without (8%) HIV (adjusted risk ratio, 1.30; 95% confidence interval, 0.74-2.31). Among deliveries to women with HIV who were receiving ART, PTB risk less than 34 weeks was lower with HAART (7%), compared with not receiving ART (26%) (aRR, 0.19), as well as with monotherapy or dual therapy (3% vs. no ART) (aRR, 0.12). Notably, 67% of deliveries to women receiving HAART included a PI-containing regimen, but these women were not significantly more likely to have a PTB less than 34 weeks, compared with women taking non-PI HAART regimens (aRR, 2.61; 95% CI, 0.65-10.59). Results were similar for secondary outcomes (PTB less than 28 weeks, less than 37 weeks).
Filling in the gaps toward the safest regimen
“This study spans 25 years, so it covers a lot of the history of HIV in pregnancy and is reassuring around using ART in pregnancy,” Shahin Lockman, MD, told this news organization. Dr. Lockman is an associate professor of infectious diseases at Brigham and Women’s Hospital and a co-PI of the Botswana Clinical Trials Unit at the Botswana Harvard AIDS Institute Partnership. She was not involved in the study. “One of the worst things for a mother and for pregnancy outcomes, for the fetus and baby’s health and development, is uncontrolled maternal HIV,’’ she said.
Dr. Lockman also noted potential confounders that drive poor birth outcomes in Southern African women, compared with U.S. women, making comparisons between this and other observational studies difficult. Still, she said that the question is not whether women should be receiving treatment but whether or not there are differences between antiretroviral regimens.
“One of the areas that we did not go deeper into was the subtype of antiretroviral therapy, given the relatively small study numbers [did not] allow us to do a robust analysis,” Dr. Venkatesh said.
Rather, he emphasized that the findings might lend more weight to speculation that immunologic characteristics associated with HIV status and immunotherapy – such as low CD4 cell counts prior to delivery, or duration of HIV infection – may be important drivers of adverse birth outcomes among women with HIV taking ART.
And at least in this cohort, many of these characteristics were similar between the treatment groups.
Both researchers agree that the findings – while reassuring – highlight the importance of collecting robust obstetric and safety data as part of prospective databases of individuals living with HIV, not only in resource-limited settings but also among the domestic U.S. population.
“We’ve learned a lot over the last 10 years,” Dr. Lockman said. “Some regimens (like lopinavir/ritonavir or nevirapine) are associated with significantly worse birth outcomes, whereas efavirenz doesn’t seem to be, or less so, and dolutegravir seems to be associated with even better outcomes. So, I think that where we are moving is to regimens that are the safest.”
Moving forward, Dr. Venkatesh explained, not only should researchers focus on exploring which antiretrovirals are safest in this context but also if the use of preexposure prophylaxis during conception periods affects birth outcomes.
Dr. Venkatesh and Dr. Lockman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over the past decade, data have suggested that antiretroviral therapy (ART) may be associated with an increased risk for adverse pregnancy outcomes, namely, preterm birth (PTB). But a combination of methodologic challenges, demographic gaps, and spotty clinical data has left the question unresolved, especially for pregnant women with HIV who reside in developed countries.
“Given that a lot of the emerging data has come out of resource-limited settings where patient and clinical characteristics are different from developed world settings like the United States, we felt that this was an important question to address,” Kartik Venkatesh, MD, PhD, a high-risk obstetrician and perinatal epidemiologist at the Ohio State Wexner Medical Center, Columbus, told this news organization.
In a prospective cohort study of U.S. women with or at risk for HIV, Dr. Venkatesh and his colleagues found that ART exposure (including highly active antiretroviral therapy [HAART]) was associated with as much as an 80% decline in the likelihood of PTB (defined as birth less than 34 weeks). The study was published in HIV Medicine.
24 years of data analyzed
Dr. Venkatesh and his team analyzed self-reported birth data of women with singleton live-born pregnancies enrolled in the ongoing, multicenter, prospective observational Women’s Interagency HIV Study (WIHS) from Oct. 1, 1995, to March 31, 2019.
“We first looked at women with HIV versus without HIV, [who were] matched on many clinical and sociodemographic characteristics and at similarly high risk of some of these obstetrical outcomes like PTB,” explained Dr. Venkatesh. “We then looked at the relative impact of antiretroviral therapy amongst women living with HIV compared to no antiretroviral therapy.”
ART regimens were classified as none, monotherapy, dual therapy, or HAART. (HAART was defined as more than three antiretrovirals, including at least one protease inhibitor [PI], nonnucleoside reverse transcriptase inhibitor, integrase inhibitor, or entry inhibitor.) In this cohort, for 63.5% of women receiving ART, therapy was initiated before pregnancy (mean duration of HAART, 6 years), and most were virally suppressed.
Among the 4,944 women assessed in the WIHS trial, 74% (3,646) had HIV. In total, 383 women had 488 singleton deliveries, including 218 women with HIV (272 deliveries) and 165 without HIV (216 deliveries). Sociodemographics in both cohorts were well matched. For most participants, the mean age was 40-41 years at delivery, most were non-Hispanic Black persons, and the mean pregnancy body mass index was greater than or equal to 29 kg/m2. Of the women with HIV, 33% had chronic hypertension; of those without HIV, 42.1% had chronic hypertension; 4.7% and 5.0%, respectively, had pregestational diabetes.
The findings showed that PTB risk less than 34 weeks was similar between women with (10%) and without (8%) HIV (adjusted risk ratio, 1.30; 95% confidence interval, 0.74-2.31). Among deliveries to women with HIV who were receiving ART, PTB risk less than 34 weeks was lower with HAART (7%), compared with not receiving ART (26%) (aRR, 0.19), as well as with monotherapy or dual therapy (3% vs. no ART) (aRR, 0.12). Notably, 67% of deliveries to women receiving HAART included a PI-containing regimen, but these women were not significantly more likely to have a PTB less than 34 weeks, compared with women taking non-PI HAART regimens (aRR, 2.61; 95% CI, 0.65-10.59). Results were similar for secondary outcomes (PTB less than 28 weeks, less than 37 weeks).
Filling in the gaps toward the safest regimen
“This study spans 25 years, so it covers a lot of the history of HIV in pregnancy and is reassuring around using ART in pregnancy,” Shahin Lockman, MD, told this news organization. Dr. Lockman is an associate professor of infectious diseases at Brigham and Women’s Hospital and a co-PI of the Botswana Clinical Trials Unit at the Botswana Harvard AIDS Institute Partnership. She was not involved in the study. “One of the worst things for a mother and for pregnancy outcomes, for the fetus and baby’s health and development, is uncontrolled maternal HIV,’’ she said.
Dr. Lockman also noted potential confounders that drive poor birth outcomes in Southern African women, compared with U.S. women, making comparisons between this and other observational studies difficult. Still, she said that the question is not whether women should be receiving treatment but whether or not there are differences between antiretroviral regimens.
“One of the areas that we did not go deeper into was the subtype of antiretroviral therapy, given the relatively small study numbers [did not] allow us to do a robust analysis,” Dr. Venkatesh said.
Rather, he emphasized that the findings might lend more weight to speculation that immunologic characteristics associated with HIV status and immunotherapy – such as low CD4 cell counts prior to delivery, or duration of HIV infection – may be important drivers of adverse birth outcomes among women with HIV taking ART.
And at least in this cohort, many of these characteristics were similar between the treatment groups.
Both researchers agree that the findings – while reassuring – highlight the importance of collecting robust obstetric and safety data as part of prospective databases of individuals living with HIV, not only in resource-limited settings but also among the domestic U.S. population.
“We’ve learned a lot over the last 10 years,” Dr. Lockman said. “Some regimens (like lopinavir/ritonavir or nevirapine) are associated with significantly worse birth outcomes, whereas efavirenz doesn’t seem to be, or less so, and dolutegravir seems to be associated with even better outcomes. So, I think that where we are moving is to regimens that are the safest.”
Moving forward, Dr. Venkatesh explained, not only should researchers focus on exploring which antiretrovirals are safest in this context but also if the use of preexposure prophylaxis during conception periods affects birth outcomes.
Dr. Venkatesh and Dr. Lockman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonstress test and maximal vertical pocket vs the biophysical profile: Equivocal or equivalent?
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2
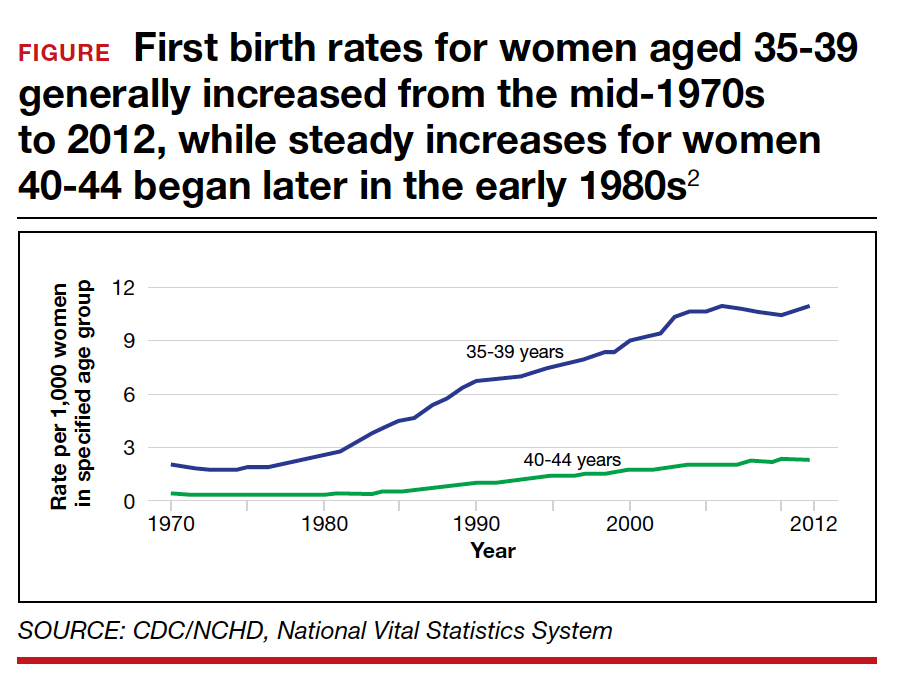
Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2

Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2

Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.
Is the United States addressing maternal mortality rates from preeclampsia/eclampsia and chronic hypertension?
Ananth CV, Brandt JS, Hill J, et al. Historical and recent changes in maternal mortality due to hypertensive disorders in the United States, 1979 to 2018. Hypertension. 2021;78:1414–1422. doi: 10.1161/HYPERTENSIONAHA.121.17661.
EXPERT COMMENTARY
Maternal mortality is a pressing public health issue and is largely preventable. Up to 10% of all US pregnancies are complicated by a hypertensive disorder, and rates of chronic hypertension and severe preeclampsia have steadily increased over the last 4 decades. However, maternal mortality is an outcome in a population with advancing maternal age, increasing obesity, and undermanaged chronic disease. The MMR due to hypertension is substantially higher among Black women compared with White women. Countless studies attribute systemic racism to these disparities.
Details of the study
Spanning 40 years, a recent study by Ananth and colleagues included live births across all 50 United States and Washington, DC. Of the 1.5 million live births examined, there were 3,287 hypertension-related maternal deaths.
Data were deidentified and available in the public domain. The researchers compiled mortality data and live births among women aged 15 to 49. The MMR was considered the death of a woman during pregnancy or within the 42 days following a live birth.
Key points of the study included:
- An estimated two-thirds of maternal deaths are preventable.
- The hypertension-related MMR was 2.1 per 100,000 live births.
- Preeclampsia-related MMR decreased, while hypertension-related MMR increased.
- The MMR from chronic hypertension has increased annually by 9.2%.
- Pregnancies among women with advanced maternal age have grown, especially among those over age 40.
- The MMR due to hypertension increases with age and is highest among women age 45 to 49.
Study strengths and limitations
A major strength of this study is the sheer size of the sample. This is one of the largest studies that examined changes in the MMR in the United States.
As with any study that spans a long period, a primary limitation is inconsistencies in the data collected. In 2003, the US death certificate was revised to include a set of “pregnancy checkboxes” indicating pregnancy at the time of death.
There also have been shifts in diagnostic coding and criteria for preeclampsia.
Classification of race and ethnicity has improved and broadened over time. Despite these limitations, the overarching trends are compelling. ●
This study’s authors note that maternal mortality is largely preventable. Patients need to be aware of their health and how to adopt healthy behaviors long before pregnancy is even a consideration. Primary and secondary prevention are essential for reducing the MMR.
Clinicians who care for women have an opportunity to emphasize cardiac health at every visit. This includes strict blood pressure control through modifiable behaviors like diet and exercise. The busy clinician could consider a 1- to 2-minute pitch to emphasize that heart disease is the leading cause of death in women both during pregnancy and later in life. A tool from the American Heart Association, Life’s Simple 7 (https://www.heart .org/en/healthy-living/healthy-lifestyle/my-life-check--lifes-sim ple-7), can help guide this language.
In office and clinical settings, consider strategies to raise awareness among staff and colleagues about cultural sensitivities to improve the health of all patients. Addressing systemic racism in the US health care system is critical to mitigate racial inequities in the rates of MMR. An editorial in The New England Journal of Medicine urges clinicians to observe patient color rather than be “color blind.”1 The editorialists note that “physician-citizens must recognize the harm inflicted by discrimination and racism and consider this environmental agent of disease as a vital sign— alongside blood pressure, pulse, weight, and temperature—that provides important information about a patient’s condition.”1
LAUREN B. GOLFER, WHNP-BC, AND MARY L. ROSSER, MD, PHD
- Evans MK, Rosenbaum L, Malina D, et al. Diagnosing and treating systemic racism. N Engl J Med. 2020;383:274-276. https://www.nejm.org/doi/full/10.1056/NEJMe2021693. Accessed February 24, 2022.
Ananth CV, Brandt JS, Hill J, et al. Historical and recent changes in maternal mortality due to hypertensive disorders in the United States, 1979 to 2018. Hypertension. 2021;78:1414–1422. doi: 10.1161/HYPERTENSIONAHA.121.17661.
EXPERT COMMENTARY
Maternal mortality is a pressing public health issue and is largely preventable. Up to 10% of all US pregnancies are complicated by a hypertensive disorder, and rates of chronic hypertension and severe preeclampsia have steadily increased over the last 4 decades. However, maternal mortality is an outcome in a population with advancing maternal age, increasing obesity, and undermanaged chronic disease. The MMR due to hypertension is substantially higher among Black women compared with White women. Countless studies attribute systemic racism to these disparities.
Details of the study
Spanning 40 years, a recent study by Ananth and colleagues included live births across all 50 United States and Washington, DC. Of the 1.5 million live births examined, there were 3,287 hypertension-related maternal deaths.
Data were deidentified and available in the public domain. The researchers compiled mortality data and live births among women aged 15 to 49. The MMR was considered the death of a woman during pregnancy or within the 42 days following a live birth.
Key points of the study included:
- An estimated two-thirds of maternal deaths are preventable.
- The hypertension-related MMR was 2.1 per 100,000 live births.
- Preeclampsia-related MMR decreased, while hypertension-related MMR increased.
- The MMR from chronic hypertension has increased annually by 9.2%.
- Pregnancies among women with advanced maternal age have grown, especially among those over age 40.
- The MMR due to hypertension increases with age and is highest among women age 45 to 49.
Study strengths and limitations
A major strength of this study is the sheer size of the sample. This is one of the largest studies that examined changes in the MMR in the United States.
As with any study that spans a long period, a primary limitation is inconsistencies in the data collected. In 2003, the US death certificate was revised to include a set of “pregnancy checkboxes” indicating pregnancy at the time of death.
There also have been shifts in diagnostic coding and criteria for preeclampsia.
Classification of race and ethnicity has improved and broadened over time. Despite these limitations, the overarching trends are compelling. ●
This study’s authors note that maternal mortality is largely preventable. Patients need to be aware of their health and how to adopt healthy behaviors long before pregnancy is even a consideration. Primary and secondary prevention are essential for reducing the MMR.
Clinicians who care for women have an opportunity to emphasize cardiac health at every visit. This includes strict blood pressure control through modifiable behaviors like diet and exercise. The busy clinician could consider a 1- to 2-minute pitch to emphasize that heart disease is the leading cause of death in women both during pregnancy and later in life. A tool from the American Heart Association, Life’s Simple 7 (https://www.heart .org/en/healthy-living/healthy-lifestyle/my-life-check--lifes-sim ple-7), can help guide this language.
In office and clinical settings, consider strategies to raise awareness among staff and colleagues about cultural sensitivities to improve the health of all patients. Addressing systemic racism in the US health care system is critical to mitigate racial inequities in the rates of MMR. An editorial in The New England Journal of Medicine urges clinicians to observe patient color rather than be “color blind.”1 The editorialists note that “physician-citizens must recognize the harm inflicted by discrimination and racism and consider this environmental agent of disease as a vital sign— alongside blood pressure, pulse, weight, and temperature—that provides important information about a patient’s condition.”1
LAUREN B. GOLFER, WHNP-BC, AND MARY L. ROSSER, MD, PHD
Ananth CV, Brandt JS, Hill J, et al. Historical and recent changes in maternal mortality due to hypertensive disorders in the United States, 1979 to 2018. Hypertension. 2021;78:1414–1422. doi: 10.1161/HYPERTENSIONAHA.121.17661.
EXPERT COMMENTARY
Maternal mortality is a pressing public health issue and is largely preventable. Up to 10% of all US pregnancies are complicated by a hypertensive disorder, and rates of chronic hypertension and severe preeclampsia have steadily increased over the last 4 decades. However, maternal mortality is an outcome in a population with advancing maternal age, increasing obesity, and undermanaged chronic disease. The MMR due to hypertension is substantially higher among Black women compared with White women. Countless studies attribute systemic racism to these disparities.
Details of the study
Spanning 40 years, a recent study by Ananth and colleagues included live births across all 50 United States and Washington, DC. Of the 1.5 million live births examined, there were 3,287 hypertension-related maternal deaths.
Data were deidentified and available in the public domain. The researchers compiled mortality data and live births among women aged 15 to 49. The MMR was considered the death of a woman during pregnancy or within the 42 days following a live birth.
Key points of the study included:
- An estimated two-thirds of maternal deaths are preventable.
- The hypertension-related MMR was 2.1 per 100,000 live births.
- Preeclampsia-related MMR decreased, while hypertension-related MMR increased.
- The MMR from chronic hypertension has increased annually by 9.2%.
- Pregnancies among women with advanced maternal age have grown, especially among those over age 40.
- The MMR due to hypertension increases with age and is highest among women age 45 to 49.
Study strengths and limitations
A major strength of this study is the sheer size of the sample. This is one of the largest studies that examined changes in the MMR in the United States.
As with any study that spans a long period, a primary limitation is inconsistencies in the data collected. In 2003, the US death certificate was revised to include a set of “pregnancy checkboxes” indicating pregnancy at the time of death.
There also have been shifts in diagnostic coding and criteria for preeclampsia.
Classification of race and ethnicity has improved and broadened over time. Despite these limitations, the overarching trends are compelling. ●
This study’s authors note that maternal mortality is largely preventable. Patients need to be aware of their health and how to adopt healthy behaviors long before pregnancy is even a consideration. Primary and secondary prevention are essential for reducing the MMR.
Clinicians who care for women have an opportunity to emphasize cardiac health at every visit. This includes strict blood pressure control through modifiable behaviors like diet and exercise. The busy clinician could consider a 1- to 2-minute pitch to emphasize that heart disease is the leading cause of death in women both during pregnancy and later in life. A tool from the American Heart Association, Life’s Simple 7 (https://www.heart .org/en/healthy-living/healthy-lifestyle/my-life-check--lifes-sim ple-7), can help guide this language.
In office and clinical settings, consider strategies to raise awareness among staff and colleagues about cultural sensitivities to improve the health of all patients. Addressing systemic racism in the US health care system is critical to mitigate racial inequities in the rates of MMR. An editorial in The New England Journal of Medicine urges clinicians to observe patient color rather than be “color blind.”1 The editorialists note that “physician-citizens must recognize the harm inflicted by discrimination and racism and consider this environmental agent of disease as a vital sign— alongside blood pressure, pulse, weight, and temperature—that provides important information about a patient’s condition.”1
LAUREN B. GOLFER, WHNP-BC, AND MARY L. ROSSER, MD, PHD
- Evans MK, Rosenbaum L, Malina D, et al. Diagnosing and treating systemic racism. N Engl J Med. 2020;383:274-276. https://www.nejm.org/doi/full/10.1056/NEJMe2021693. Accessed February 24, 2022.
- Evans MK, Rosenbaum L, Malina D, et al. Diagnosing and treating systemic racism. N Engl J Med. 2020;383:274-276. https://www.nejm.org/doi/full/10.1056/NEJMe2021693. Accessed February 24, 2022.
DMTs tied to lower MS relapse during reproductive therapy
WEST PALM BEACH, FLA. – , new research suggests. In a cohort study of women undergoing ART, those who did not receive DMTs had a significantly higher relapse risk than their peers who were treated with the drugs.
In addition, the likelihood of achieving pregnancy through ART while having MS appeared favorable, researchers noted.
“In this modern case series and the largest cohort to date, we identified a lower risk of relapses after ART than previously reported,” Edith L. Graham, MD, of the department of neurology, Northwestern University, Chicago, and colleagues wrote. “Importantly, continuing DMT during ART may reduce risk of relapse during this period of marked hormonal fluctuations and stressors,” they added.
The findings were presented at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Study details
Previous research shows a wide range of relapse risk in patients with MS undergoing ART.
To investigate the potential role of DMTs in mitigating relapse risk, the researchers evaluated data on 37 women with either relapsing-remitting MS (n = 31) or clinically isolated syndrome (CIS; n = 6) who underwent ART. The women all had low disability, with a median Expanded Disability Status Scale (EDSS) score of 1.0. All participants had undergone one to five cycles of reproductive therapy between 2010 and 2021.
Most (78%) were receiving ART because of infertility or a need for preimplantation genetic testing, whereas 22% were undergoing the treatment for the preservation of fertility. Average age of the participants was 35 years and average disease duration was 7.4 years.
Among 19 of the 37 patients who were taking DMTs prior to ART, 10 remained on the medication throughout ovarian hyperstimulation.
In those who received DMTs in the 12 months prior to ART, treatment included glatiramer acetate (n = 9), interferons (n = 3), and dimethyl fumarate (n = 1). Three participants received B-cell–depleting agents.
In addition, three women received medication in response to a rebound after discontinuation. Of these, two received fingolimod and one natalizumab.
Five patients (13.5%) experienced MS relapses in the 12 months following ART therapy. Among those experiencing relapse, none were treated with DMTs during the preceding 12 months.
Of the relapses, three occurred within 3 months of the ART treatment, one within 6 months, and one within 12 months.
High rate of successful pregnancy
Overall, 24 of 29 women (83%) underwent in vitro fertilization (IVF) with embryo transfer as part of ART achieved pregnancy. The remaining five patients were undergoing egg cryopreservation.
Although 14 of the 24 who achieved pregnancy were on DMTs and 2 of 5 who did not achieve pregnancy were on the therapies, Dr. Graham noted, “these numbers seem too small to draw conclusions.”
In particular, patients may benefit from treatment with rituximab or ocrelizumab 3-6 months prior to ART, “which gives better protection during ART cycle with low risk of fetal exposure,” she said.
“Treatment does not need to be discontinued if undergoing embryo banking only,” Dr. Graham added. “The risk to the fetus occurs only after embryo transfer.”
Although there is a lack of research examining whether MS relapse lowers the chance of pregnancy, Dr. Graham noted, “in theory, relapsing MS may compromise ART success because [patients] may have a narrower window to undergo ART treatments if they are trying to mitigate DMT exposure to the fetus.”
However, the study’s results generally suggest favorable outcomes with ART among women with MS, she added. “We found that overall ART is actually very successful among people with MS. I was actually very surprised by this high rate of successful pregnancy,” Dr. Graham said.
She noted that as women with MS increasingly undergo IVF as well as egg cryopreservation, research on these issues is gaining importance for clinicians. “This is going to be something that MS specialists need to know more about, particularly the safety of ART in their patients,” said Dr. Graham.
“What’s important is there are no [formal] recommendations along these lines, so this represents an opportunity to get the word out to clinicians that you want to make sure patients with MS are protected throughout the ART cycle and that you’re not discontinuing their DMT too early,” she added.
Protective against relapse?
Commenting on the study, Jiwon Oh, MD, PhD, medical director of the Barlo Multiple Sclerosis Program at St. Michael’s Hospital, University of Toronto, noted that, while there are many guidelines/recommendations regarding use of older DMTs peripregnancy, data on many newer therapies is more limited.
“Often, when people do not have definitive evidence, they tend to take a conservative approach, which is why there is likely reluctance to keep patients on DMTs during ART as well as in early pregnancy,” said Dr. Oh, who was not involved in the research.
Importantly, there is also no definitive evidence of a relationship between MS relapses and ART success or pregnancy outcomes, she noted. However, “from a common-sense perspective, most clinicians worry that extreme stress or disability may negatively affect both ART and pregnancy outcomes,” she added.
Dr. Oh agreed that ocrelizumab is an appropriate choice in terms of preventing relapse during ART. “Ocrevus is one of our highest-efficacy DMTs and is only dosed every 6 months. So this allows for ART cycles and conception without worrying about fetal drug exposure and the drug affecting ART cycles,” she said.
She noted the study’s findings “are in keeping with some prior studies, but not others, demonstrating there may be a higher risk of relapse with ART” in patients who are not taking a DMT.
“However, in my mind the most important conclusion from this study is that being on a DMT seems to be protective of relapse risk, which is an important point that will be useful to provide patients with clinical guidance,” Dr. Oh said.
Dr. Graham reported having received consulting fees from Genentech. Dr. Oh reported having received consulting or speaking fees from Alexion, Biogen Idec, BMS, EMD Serono, Genzyme, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
WEST PALM BEACH, FLA. – , new research suggests. In a cohort study of women undergoing ART, those who did not receive DMTs had a significantly higher relapse risk than their peers who were treated with the drugs.
In addition, the likelihood of achieving pregnancy through ART while having MS appeared favorable, researchers noted.
“In this modern case series and the largest cohort to date, we identified a lower risk of relapses after ART than previously reported,” Edith L. Graham, MD, of the department of neurology, Northwestern University, Chicago, and colleagues wrote. “Importantly, continuing DMT during ART may reduce risk of relapse during this period of marked hormonal fluctuations and stressors,” they added.
The findings were presented at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Study details
Previous research shows a wide range of relapse risk in patients with MS undergoing ART.
To investigate the potential role of DMTs in mitigating relapse risk, the researchers evaluated data on 37 women with either relapsing-remitting MS (n = 31) or clinically isolated syndrome (CIS; n = 6) who underwent ART. The women all had low disability, with a median Expanded Disability Status Scale (EDSS) score of 1.0. All participants had undergone one to five cycles of reproductive therapy between 2010 and 2021.
Most (78%) were receiving ART because of infertility or a need for preimplantation genetic testing, whereas 22% were undergoing the treatment for the preservation of fertility. Average age of the participants was 35 years and average disease duration was 7.4 years.
Among 19 of the 37 patients who were taking DMTs prior to ART, 10 remained on the medication throughout ovarian hyperstimulation.
In those who received DMTs in the 12 months prior to ART, treatment included glatiramer acetate (n = 9), interferons (n = 3), and dimethyl fumarate (n = 1). Three participants received B-cell–depleting agents.
In addition, three women received medication in response to a rebound after discontinuation. Of these, two received fingolimod and one natalizumab.
Five patients (13.5%) experienced MS relapses in the 12 months following ART therapy. Among those experiencing relapse, none were treated with DMTs during the preceding 12 months.
Of the relapses, three occurred within 3 months of the ART treatment, one within 6 months, and one within 12 months.
High rate of successful pregnancy
Overall, 24 of 29 women (83%) underwent in vitro fertilization (IVF) with embryo transfer as part of ART achieved pregnancy. The remaining five patients were undergoing egg cryopreservation.
Although 14 of the 24 who achieved pregnancy were on DMTs and 2 of 5 who did not achieve pregnancy were on the therapies, Dr. Graham noted, “these numbers seem too small to draw conclusions.”
In particular, patients may benefit from treatment with rituximab or ocrelizumab 3-6 months prior to ART, “which gives better protection during ART cycle with low risk of fetal exposure,” she said.
“Treatment does not need to be discontinued if undergoing embryo banking only,” Dr. Graham added. “The risk to the fetus occurs only after embryo transfer.”
Although there is a lack of research examining whether MS relapse lowers the chance of pregnancy, Dr. Graham noted, “in theory, relapsing MS may compromise ART success because [patients] may have a narrower window to undergo ART treatments if they are trying to mitigate DMT exposure to the fetus.”
However, the study’s results generally suggest favorable outcomes with ART among women with MS, she added. “We found that overall ART is actually very successful among people with MS. I was actually very surprised by this high rate of successful pregnancy,” Dr. Graham said.
She noted that as women with MS increasingly undergo IVF as well as egg cryopreservation, research on these issues is gaining importance for clinicians. “This is going to be something that MS specialists need to know more about, particularly the safety of ART in their patients,” said Dr. Graham.
“What’s important is there are no [formal] recommendations along these lines, so this represents an opportunity to get the word out to clinicians that you want to make sure patients with MS are protected throughout the ART cycle and that you’re not discontinuing their DMT too early,” she added.
Protective against relapse?
Commenting on the study, Jiwon Oh, MD, PhD, medical director of the Barlo Multiple Sclerosis Program at St. Michael’s Hospital, University of Toronto, noted that, while there are many guidelines/recommendations regarding use of older DMTs peripregnancy, data on many newer therapies is more limited.
“Often, when people do not have definitive evidence, they tend to take a conservative approach, which is why there is likely reluctance to keep patients on DMTs during ART as well as in early pregnancy,” said Dr. Oh, who was not involved in the research.
Importantly, there is also no definitive evidence of a relationship between MS relapses and ART success or pregnancy outcomes, she noted. However, “from a common-sense perspective, most clinicians worry that extreme stress or disability may negatively affect both ART and pregnancy outcomes,” she added.
Dr. Oh agreed that ocrelizumab is an appropriate choice in terms of preventing relapse during ART. “Ocrevus is one of our highest-efficacy DMTs and is only dosed every 6 months. So this allows for ART cycles and conception without worrying about fetal drug exposure and the drug affecting ART cycles,” she said.
She noted the study’s findings “are in keeping with some prior studies, but not others, demonstrating there may be a higher risk of relapse with ART” in patients who are not taking a DMT.
“However, in my mind the most important conclusion from this study is that being on a DMT seems to be protective of relapse risk, which is an important point that will be useful to provide patients with clinical guidance,” Dr. Oh said.
Dr. Graham reported having received consulting fees from Genentech. Dr. Oh reported having received consulting or speaking fees from Alexion, Biogen Idec, BMS, EMD Serono, Genzyme, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
WEST PALM BEACH, FLA. – , new research suggests. In a cohort study of women undergoing ART, those who did not receive DMTs had a significantly higher relapse risk than their peers who were treated with the drugs.
In addition, the likelihood of achieving pregnancy through ART while having MS appeared favorable, researchers noted.
“In this modern case series and the largest cohort to date, we identified a lower risk of relapses after ART than previously reported,” Edith L. Graham, MD, of the department of neurology, Northwestern University, Chicago, and colleagues wrote. “Importantly, continuing DMT during ART may reduce risk of relapse during this period of marked hormonal fluctuations and stressors,” they added.
The findings were presented at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Study details
Previous research shows a wide range of relapse risk in patients with MS undergoing ART.
To investigate the potential role of DMTs in mitigating relapse risk, the researchers evaluated data on 37 women with either relapsing-remitting MS (n = 31) or clinically isolated syndrome (CIS; n = 6) who underwent ART. The women all had low disability, with a median Expanded Disability Status Scale (EDSS) score of 1.0. All participants had undergone one to five cycles of reproductive therapy between 2010 and 2021.
Most (78%) were receiving ART because of infertility or a need for preimplantation genetic testing, whereas 22% were undergoing the treatment for the preservation of fertility. Average age of the participants was 35 years and average disease duration was 7.4 years.
Among 19 of the 37 patients who were taking DMTs prior to ART, 10 remained on the medication throughout ovarian hyperstimulation.
In those who received DMTs in the 12 months prior to ART, treatment included glatiramer acetate (n = 9), interferons (n = 3), and dimethyl fumarate (n = 1). Three participants received B-cell–depleting agents.
In addition, three women received medication in response to a rebound after discontinuation. Of these, two received fingolimod and one natalizumab.
Five patients (13.5%) experienced MS relapses in the 12 months following ART therapy. Among those experiencing relapse, none were treated with DMTs during the preceding 12 months.
Of the relapses, three occurred within 3 months of the ART treatment, one within 6 months, and one within 12 months.
High rate of successful pregnancy
Overall, 24 of 29 women (83%) underwent in vitro fertilization (IVF) with embryo transfer as part of ART achieved pregnancy. The remaining five patients were undergoing egg cryopreservation.
Although 14 of the 24 who achieved pregnancy were on DMTs and 2 of 5 who did not achieve pregnancy were on the therapies, Dr. Graham noted, “these numbers seem too small to draw conclusions.”
In particular, patients may benefit from treatment with rituximab or ocrelizumab 3-6 months prior to ART, “which gives better protection during ART cycle with low risk of fetal exposure,” she said.
“Treatment does not need to be discontinued if undergoing embryo banking only,” Dr. Graham added. “The risk to the fetus occurs only after embryo transfer.”
Although there is a lack of research examining whether MS relapse lowers the chance of pregnancy, Dr. Graham noted, “in theory, relapsing MS may compromise ART success because [patients] may have a narrower window to undergo ART treatments if they are trying to mitigate DMT exposure to the fetus.”
However, the study’s results generally suggest favorable outcomes with ART among women with MS, she added. “We found that overall ART is actually very successful among people with MS. I was actually very surprised by this high rate of successful pregnancy,” Dr. Graham said.
She noted that as women with MS increasingly undergo IVF as well as egg cryopreservation, research on these issues is gaining importance for clinicians. “This is going to be something that MS specialists need to know more about, particularly the safety of ART in their patients,” said Dr. Graham.
“What’s important is there are no [formal] recommendations along these lines, so this represents an opportunity to get the word out to clinicians that you want to make sure patients with MS are protected throughout the ART cycle and that you’re not discontinuing their DMT too early,” she added.
Protective against relapse?
Commenting on the study, Jiwon Oh, MD, PhD, medical director of the Barlo Multiple Sclerosis Program at St. Michael’s Hospital, University of Toronto, noted that, while there are many guidelines/recommendations regarding use of older DMTs peripregnancy, data on many newer therapies is more limited.
“Often, when people do not have definitive evidence, they tend to take a conservative approach, which is why there is likely reluctance to keep patients on DMTs during ART as well as in early pregnancy,” said Dr. Oh, who was not involved in the research.
Importantly, there is also no definitive evidence of a relationship between MS relapses and ART success or pregnancy outcomes, she noted. However, “from a common-sense perspective, most clinicians worry that extreme stress or disability may negatively affect both ART and pregnancy outcomes,” she added.
Dr. Oh agreed that ocrelizumab is an appropriate choice in terms of preventing relapse during ART. “Ocrevus is one of our highest-efficacy DMTs and is only dosed every 6 months. So this allows for ART cycles and conception without worrying about fetal drug exposure and the drug affecting ART cycles,” she said.
She noted the study’s findings “are in keeping with some prior studies, but not others, demonstrating there may be a higher risk of relapse with ART” in patients who are not taking a DMT.
“However, in my mind the most important conclusion from this study is that being on a DMT seems to be protective of relapse risk, which is an important point that will be useful to provide patients with clinical guidance,” Dr. Oh said.
Dr. Graham reported having received consulting fees from Genentech. Dr. Oh reported having received consulting or speaking fees from Alexion, Biogen Idec, BMS, EMD Serono, Genzyme, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
REPORTING FROM ACTRIMS FORUM 2022