User login
Data Suggest Non-NOF Fragility Fractures Should Be Treated First
Treating patients with non–neck-of-femur (NOF) fragility fractures from osteoporosis before NOF fractures may improve quality-of-life and reduce burden to hospital services and funding, according to a study published the December 2013 Journal of Orthopaedic Surgery and Research.
“Despite the known impact of fragility fractures, osteoporosis still remains unrecognized and untreated in over 50% of patients who present with fragility fractures,” according to authors Tamer Mettyas, Orthopaedic Department of, Royal Brisbane and Women’s Hospital, Australia, and Clare Carpenter, Orthopaedic Department, University Hospital of Wales, Heath Park, Cardiff, United Kingdoms. “Orthopaedic surgeons are often the first to encounter these patients who present with fragility fractures.”
The investigator sought to evaluate current practice of the National Institute for Health and Clinical Excellence (NICE) and British Orthopaedic Association (BOA) guidelines of the secondary prevention of osteoporosis. Patients 50 years and older who were admitted as in-patients with non-NOF fragility fractures between March and September 2008 were included in the study. Mettyas and Carpenter retrospectively evaluated data from March 2008, including risk factors and whether patients were treated; September data was prospectively evaluated after the new trauma admission sheet was introduced. Finally, the authors performed a cross-sectional study assessing services for NOF and non-NOF fragility fractures in September.
Overall, they found that 29% of fragility fractures were non-NOF (mean age, 70 years), and 71% were NOF fractures (mean age, 80). “There is a great difference in the care provided to these patients,” the authors observed. In particular, non-NOF fragility fractures received less attention for osteoporosis assessment (25%) and less interest for investigation by the medical staff (11%), as well as less attention for the treatment of osteoporosis (35%), compared with NOF fracture (35%, 47%, and 71%, respectively). Furthermore, 25% of NOF patients had previous fragility fractures, compared with 6% of non-NOF patients.
“We believe that treating patients with non-NOF fractures from osteoporosis before proceeding to NOF fractures would improve their quality and reduce the burden on hospital services and funding,” the authors concluded.
Mettyas T, Carpenter C. Secondary prevention of osteoporosis in non-neck of femur fractures: is it value for money? A retrospective, prospective and cross-sectional cohort study. J Orthop Surg Res. 2013;8(1):44.
Treating patients with non–neck-of-femur (NOF) fragility fractures from osteoporosis before NOF fractures may improve quality-of-life and reduce burden to hospital services and funding, according to a study published the December 2013 Journal of Orthopaedic Surgery and Research.
“Despite the known impact of fragility fractures, osteoporosis still remains unrecognized and untreated in over 50% of patients who present with fragility fractures,” according to authors Tamer Mettyas, Orthopaedic Department of, Royal Brisbane and Women’s Hospital, Australia, and Clare Carpenter, Orthopaedic Department, University Hospital of Wales, Heath Park, Cardiff, United Kingdoms. “Orthopaedic surgeons are often the first to encounter these patients who present with fragility fractures.”
The investigator sought to evaluate current practice of the National Institute for Health and Clinical Excellence (NICE) and British Orthopaedic Association (BOA) guidelines of the secondary prevention of osteoporosis. Patients 50 years and older who were admitted as in-patients with non-NOF fragility fractures between March and September 2008 were included in the study. Mettyas and Carpenter retrospectively evaluated data from March 2008, including risk factors and whether patients were treated; September data was prospectively evaluated after the new trauma admission sheet was introduced. Finally, the authors performed a cross-sectional study assessing services for NOF and non-NOF fragility fractures in September.
Overall, they found that 29% of fragility fractures were non-NOF (mean age, 70 years), and 71% were NOF fractures (mean age, 80). “There is a great difference in the care provided to these patients,” the authors observed. In particular, non-NOF fragility fractures received less attention for osteoporosis assessment (25%) and less interest for investigation by the medical staff (11%), as well as less attention for the treatment of osteoporosis (35%), compared with NOF fracture (35%, 47%, and 71%, respectively). Furthermore, 25% of NOF patients had previous fragility fractures, compared with 6% of non-NOF patients.
“We believe that treating patients with non-NOF fractures from osteoporosis before proceeding to NOF fractures would improve their quality and reduce the burden on hospital services and funding,” the authors concluded.
Mettyas T, Carpenter C. Secondary prevention of osteoporosis in non-neck of femur fractures: is it value for money? A retrospective, prospective and cross-sectional cohort study. J Orthop Surg Res. 2013;8(1):44.
Treating patients with non–neck-of-femur (NOF) fragility fractures from osteoporosis before NOF fractures may improve quality-of-life and reduce burden to hospital services and funding, according to a study published the December 2013 Journal of Orthopaedic Surgery and Research.
“Despite the known impact of fragility fractures, osteoporosis still remains unrecognized and untreated in over 50% of patients who present with fragility fractures,” according to authors Tamer Mettyas, Orthopaedic Department of, Royal Brisbane and Women’s Hospital, Australia, and Clare Carpenter, Orthopaedic Department, University Hospital of Wales, Heath Park, Cardiff, United Kingdoms. “Orthopaedic surgeons are often the first to encounter these patients who present with fragility fractures.”
The investigator sought to evaluate current practice of the National Institute for Health and Clinical Excellence (NICE) and British Orthopaedic Association (BOA) guidelines of the secondary prevention of osteoporosis. Patients 50 years and older who were admitted as in-patients with non-NOF fragility fractures between March and September 2008 were included in the study. Mettyas and Carpenter retrospectively evaluated data from March 2008, including risk factors and whether patients were treated; September data was prospectively evaluated after the new trauma admission sheet was introduced. Finally, the authors performed a cross-sectional study assessing services for NOF and non-NOF fragility fractures in September.
Overall, they found that 29% of fragility fractures were non-NOF (mean age, 70 years), and 71% were NOF fractures (mean age, 80). “There is a great difference in the care provided to these patients,” the authors observed. In particular, non-NOF fragility fractures received less attention for osteoporosis assessment (25%) and less interest for investigation by the medical staff (11%), as well as less attention for the treatment of osteoporosis (35%), compared with NOF fracture (35%, 47%, and 71%, respectively). Furthermore, 25% of NOF patients had previous fragility fractures, compared with 6% of non-NOF patients.
“We believe that treating patients with non-NOF fractures from osteoporosis before proceeding to NOF fractures would improve their quality and reduce the burden on hospital services and funding,” the authors concluded.
Mettyas T, Carpenter C. Secondary prevention of osteoporosis in non-neck of femur fractures: is it value for money? A retrospective, prospective and cross-sectional cohort study. J Orthop Surg Res. 2013;8(1):44.
miRNAs Play a Role in Bone Health in HIV Patients
In this review, Fabiola E. Del Carpio-Cano and colleagues, from Temple University School of Medicine, Philadelphia, discuss micro-ribonucleic acid (miRNA) in human immunodeficiency virus (HIV)-induced osteoporosis, including basic concepts of bone biology and current research on the role of miRNAs in bone development.
“Increased life expectancy and the need for long-term antiretroviral therapy have brought new challenges to the clinical management of HIV-infected individuals,” the authors explained in a study published online ahead of print October 27, 2013 in the Journal of Osteoporosis. “The prevalence of osteoporosis and fractures is increased in HIV-infected patients; thus optimal strategies for risk management and treatment in this group of patients need to be redefined.”
Del Carpio-Cano and colleagues discuss bone biology, including the 2 distinct phases of bone formation, homeostatic bone remodeling, the role of transforming growth factor-β and bone morphogenic proteins (BMPs) in bone formation, and Runx2. In addition, the authors review pathophysiology of osteoporosis, biology and function of miRNA, the regulatory role of miRNA in bone biology, as well as HIV and bone biology.
“Prevention of bone loss is an important component of HIV care as the HIV population grows older,” the authors concluded. “Understanding the mechanisms by which HIV infection affects bone biology leading to osteoporosis is crucial to delineate potential adjuvant treatment.”
Del Carpio-Cano FE, Dela Cadena RA, Sawaya BE. HIV and Bone Disease: A Perspective of the Role of microRNAs in Bone Biology upon HIV Infection. J Osteoporos. 2013. [Epub ahead of print]
In this review, Fabiola E. Del Carpio-Cano and colleagues, from Temple University School of Medicine, Philadelphia, discuss micro-ribonucleic acid (miRNA) in human immunodeficiency virus (HIV)-induced osteoporosis, including basic concepts of bone biology and current research on the role of miRNAs in bone development.
“Increased life expectancy and the need for long-term antiretroviral therapy have brought new challenges to the clinical management of HIV-infected individuals,” the authors explained in a study published online ahead of print October 27, 2013 in the Journal of Osteoporosis. “The prevalence of osteoporosis and fractures is increased in HIV-infected patients; thus optimal strategies for risk management and treatment in this group of patients need to be redefined.”
Del Carpio-Cano and colleagues discuss bone biology, including the 2 distinct phases of bone formation, homeostatic bone remodeling, the role of transforming growth factor-β and bone morphogenic proteins (BMPs) in bone formation, and Runx2. In addition, the authors review pathophysiology of osteoporosis, biology and function of miRNA, the regulatory role of miRNA in bone biology, as well as HIV and bone biology.
“Prevention of bone loss is an important component of HIV care as the HIV population grows older,” the authors concluded. “Understanding the mechanisms by which HIV infection affects bone biology leading to osteoporosis is crucial to delineate potential adjuvant treatment.”
Del Carpio-Cano FE, Dela Cadena RA, Sawaya BE. HIV and Bone Disease: A Perspective of the Role of microRNAs in Bone Biology upon HIV Infection. J Osteoporos. 2013. [Epub ahead of print]
In this review, Fabiola E. Del Carpio-Cano and colleagues, from Temple University School of Medicine, Philadelphia, discuss micro-ribonucleic acid (miRNA) in human immunodeficiency virus (HIV)-induced osteoporosis, including basic concepts of bone biology and current research on the role of miRNAs in bone development.
“Increased life expectancy and the need for long-term antiretroviral therapy have brought new challenges to the clinical management of HIV-infected individuals,” the authors explained in a study published online ahead of print October 27, 2013 in the Journal of Osteoporosis. “The prevalence of osteoporosis and fractures is increased in HIV-infected patients; thus optimal strategies for risk management and treatment in this group of patients need to be redefined.”
Del Carpio-Cano and colleagues discuss bone biology, including the 2 distinct phases of bone formation, homeostatic bone remodeling, the role of transforming growth factor-β and bone morphogenic proteins (BMPs) in bone formation, and Runx2. In addition, the authors review pathophysiology of osteoporosis, biology and function of miRNA, the regulatory role of miRNA in bone biology, as well as HIV and bone biology.
“Prevention of bone loss is an important component of HIV care as the HIV population grows older,” the authors concluded. “Understanding the mechanisms by which HIV infection affects bone biology leading to osteoporosis is crucial to delineate potential adjuvant treatment.”
Del Carpio-Cano FE, Dela Cadena RA, Sawaya BE. HIV and Bone Disease: A Perspective of the Role of microRNAs in Bone Biology upon HIV Infection. J Osteoporos. 2013. [Epub ahead of print]
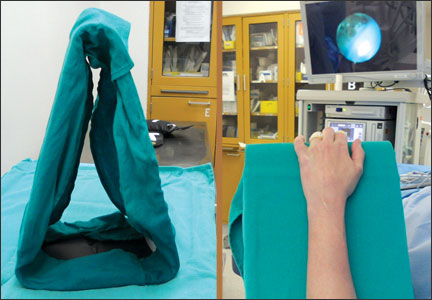
A Simple Wrist Arthroscopy Tower: The Wrist Triangle
Delayed Complete Limb Ischemia Following a Closed Tibial Shaft Fracture
Failure of a Constrained Acetabular Liner Without Reinforcement Ring Disruption
Functional Improvement After Humeral Shaft Nonunion in a Patient With Glenohumeral Ankylosis
100 Most Cited Articles in Fracture Surgery
How to Get Organized and Be Fearless About ICD-10
International Consensus on Periprosthetic Joint Infection: What Was Discussed and Decided?
Periprosthetic joint infection (PJI), with all its disastrous implications, continues to pose a challenge to the orthopedic community. Practicing orthopedic surgeons have invested great efforts to implement strategies to minimize surgical site infection (SSI). Although high-level evidence supports some of these practices, many have little or no scientific foundation. As a result, there is a remarkable variation in practices across the globe for prevention and management of PJI.
Some of the many questions the orthopedic community faces on a daily basis, include:
◾ Should a laminar flow room be used for elective arthroplasty?
◾ How much, and which antibiotic should be added to cement spacers?
◾ What metric should be used to decide on the optimal timing of reimplantation?
◾ What are the indications and contraindications for irrigation and debridement?
◾ How many irrigation and debridement in a joint should
be attempted before resection arthroplasty needs to be considered?
The medical community understands the importance of high-level evidence and engages in the generation of such whenever possible. The community also recognizes that some aspects of medicine will never lend themselves to the generation of high-level evidence nor should it attempt to do so. It is with the recognition of the latter that The International Consensus Meeting on Periprosthetic Joint Infection was organized. Delegates from various disciplines including orthopedic surgery, infectious disease, musculoskeletal pathology, microbiology, anesthesiology, dermatology, nuclear medicine, rheumatology, musculoskeletal radiology, veterinary surgery, pharmacy, and numerous scientists with interest in orthopedic infections came together to evaluate the available evidence, when present, or reach consensus regarding current practices for management of SSI/PJI. The process of generating the consensus has spanned over 10 months. Every stone has been turned in search of evidence for these questions, with over 3,500 related publications evaluated. The evidence, when available, has been assessed. Otherwise, the cumulative wisdom of 400 delegates from 52 countries and over 100 societies has been amassed to reach consensus about practices that lack higher level of evidence. The members of the Musculoskeletal Infection Society (MSIS) and the European Bone and Joint Infection Society (EBJIS), the 2 societies with a mission is to improve care of patients with musculoskeletal infection, have contributed to this initiative immensely.
The delegates have been engaged every step of the way by communicating through a social website generated for this purpose, with over 25,000 communications exchanged. The consensus document has been developed using the Delphi method under the leadership of Dr. Cats-Baril, a world-renowned expert in consensus development. The design of the consensus process was to include as many stakeholders as possible, allow participation in multiple forums, and provide a comprehensive review of the literature. The topics that were covered included the following: mitigation and education on comorbidities associated with increased SSI/PJI, perioperative skin preparation, perioperative antibiotics, operative environment, blood conservation, prosthesis selection, diagnosis of PJI, wound management, spacers, irrigation and debridement, antibiotic treatment and timing of reimplantation, 1-stage versus 2-stage exchange arthroplasty, management of fungal or atypical PJI, oral antibiotic therapy, and prevention of late PJI. Every consensus statement has undergone extreme scrutiny, especially by those with expertise in a specific area, to ensure that implementation of these practices will indeed lead to improvement of patient care.
After synthesizing the literature and assembling a preliminary draft of the consensus statement, over 300 delegates attended a face-to-face meeting in Philadelphia, were involved in active discussions, and voted on the questions/consensus statements. The delegates first met on July 31, 2013, in smaller workgroups, to discuss and resolve any discrepancies and finalize their statements. Then, they met in the general assembly for further discussion of questions and consensus statements. After revising the consensus statements, the finalized consensus statement was assembled and forwarded to the Audience Response System that evening, with voting occurring on the next day. On August 1, 2013 the delegates came into the general assembly and voted on the 207 questions/consensus statements that were being presented. The voting process was conducted using electronic keypads, where one could agree with the consensus statement, disagree with the consensus statement, or abstain from voting. The strength of the consensus was judged by the following scale: 1) Simple Majority: No Consensus (50.1%-59% agreement), 2) Majority: Weak Consensus (60%-65% agreement), 3) Super Majority: Strong Consensus (66%-99% agreement) and 4) Unanimous: 100% agreement. Of the 207 questions, there was unanimous vote for one question (controlling operating room traffic), 202 questions received super majority (strong consensus), 2 questions had weak consensus, and only 3 questions did not achieve any consensus.
The document generated(REF) is the result of innumerable hours of work by the liaisons, leaders and delegates dedicated to this initiative. We are certain that the “best practice guide” set forth by this initiative will serve many of our patients for years to come.
It is essential to state that the information contained in this document is merely a guide to practicing physicians who treat patients with musculoskeletal infection and should not be considered as a standard of care. Clinicians should exercise their wisdom and clinical acumen in making decisions related to each individual patient. In some circumstances this may require implementation of care that differs from what is stated in this document.
Periprosthetic joint infection (PJI), with all its disastrous implications, continues to pose a challenge to the orthopedic community. Practicing orthopedic surgeons have invested great efforts to implement strategies to minimize surgical site infection (SSI). Although high-level evidence supports some of these practices, many have little or no scientific foundation. As a result, there is a remarkable variation in practices across the globe for prevention and management of PJI.
Some of the many questions the orthopedic community faces on a daily basis, include:
◾ Should a laminar flow room be used for elective arthroplasty?
◾ How much, and which antibiotic should be added to cement spacers?
◾ What metric should be used to decide on the optimal timing of reimplantation?
◾ What are the indications and contraindications for irrigation and debridement?
◾ How many irrigation and debridement in a joint should
be attempted before resection arthroplasty needs to be considered?
The medical community understands the importance of high-level evidence and engages in the generation of such whenever possible. The community also recognizes that some aspects of medicine will never lend themselves to the generation of high-level evidence nor should it attempt to do so. It is with the recognition of the latter that The International Consensus Meeting on Periprosthetic Joint Infection was organized. Delegates from various disciplines including orthopedic surgery, infectious disease, musculoskeletal pathology, microbiology, anesthesiology, dermatology, nuclear medicine, rheumatology, musculoskeletal radiology, veterinary surgery, pharmacy, and numerous scientists with interest in orthopedic infections came together to evaluate the available evidence, when present, or reach consensus regarding current practices for management of SSI/PJI. The process of generating the consensus has spanned over 10 months. Every stone has been turned in search of evidence for these questions, with over 3,500 related publications evaluated. The evidence, when available, has been assessed. Otherwise, the cumulative wisdom of 400 delegates from 52 countries and over 100 societies has been amassed to reach consensus about practices that lack higher level of evidence. The members of the Musculoskeletal Infection Society (MSIS) and the European Bone and Joint Infection Society (EBJIS), the 2 societies with a mission is to improve care of patients with musculoskeletal infection, have contributed to this initiative immensely.
The delegates have been engaged every step of the way by communicating through a social website generated for this purpose, with over 25,000 communications exchanged. The consensus document has been developed using the Delphi method under the leadership of Dr. Cats-Baril, a world-renowned expert in consensus development. The design of the consensus process was to include as many stakeholders as possible, allow participation in multiple forums, and provide a comprehensive review of the literature. The topics that were covered included the following: mitigation and education on comorbidities associated with increased SSI/PJI, perioperative skin preparation, perioperative antibiotics, operative environment, blood conservation, prosthesis selection, diagnosis of PJI, wound management, spacers, irrigation and debridement, antibiotic treatment and timing of reimplantation, 1-stage versus 2-stage exchange arthroplasty, management of fungal or atypical PJI, oral antibiotic therapy, and prevention of late PJI. Every consensus statement has undergone extreme scrutiny, especially by those with expertise in a specific area, to ensure that implementation of these practices will indeed lead to improvement of patient care.
After synthesizing the literature and assembling a preliminary draft of the consensus statement, over 300 delegates attended a face-to-face meeting in Philadelphia, were involved in active discussions, and voted on the questions/consensus statements. The delegates first met on July 31, 2013, in smaller workgroups, to discuss and resolve any discrepancies and finalize their statements. Then, they met in the general assembly for further discussion of questions and consensus statements. After revising the consensus statements, the finalized consensus statement was assembled and forwarded to the Audience Response System that evening, with voting occurring on the next day. On August 1, 2013 the delegates came into the general assembly and voted on the 207 questions/consensus statements that were being presented. The voting process was conducted using electronic keypads, where one could agree with the consensus statement, disagree with the consensus statement, or abstain from voting. The strength of the consensus was judged by the following scale: 1) Simple Majority: No Consensus (50.1%-59% agreement), 2) Majority: Weak Consensus (60%-65% agreement), 3) Super Majority: Strong Consensus (66%-99% agreement) and 4) Unanimous: 100% agreement. Of the 207 questions, there was unanimous vote for one question (controlling operating room traffic), 202 questions received super majority (strong consensus), 2 questions had weak consensus, and only 3 questions did not achieve any consensus.
The document generated(REF) is the result of innumerable hours of work by the liaisons, leaders and delegates dedicated to this initiative. We are certain that the “best practice guide” set forth by this initiative will serve many of our patients for years to come.
It is essential to state that the information contained in this document is merely a guide to practicing physicians who treat patients with musculoskeletal infection and should not be considered as a standard of care. Clinicians should exercise their wisdom and clinical acumen in making decisions related to each individual patient. In some circumstances this may require implementation of care that differs from what is stated in this document.
Periprosthetic joint infection (PJI), with all its disastrous implications, continues to pose a challenge to the orthopedic community. Practicing orthopedic surgeons have invested great efforts to implement strategies to minimize surgical site infection (SSI). Although high-level evidence supports some of these practices, many have little or no scientific foundation. As a result, there is a remarkable variation in practices across the globe for prevention and management of PJI.
Some of the many questions the orthopedic community faces on a daily basis, include:
◾ Should a laminar flow room be used for elective arthroplasty?
◾ How much, and which antibiotic should be added to cement spacers?
◾ What metric should be used to decide on the optimal timing of reimplantation?
◾ What are the indications and contraindications for irrigation and debridement?
◾ How many irrigation and debridement in a joint should
be attempted before resection arthroplasty needs to be considered?
The medical community understands the importance of high-level evidence and engages in the generation of such whenever possible. The community also recognizes that some aspects of medicine will never lend themselves to the generation of high-level evidence nor should it attempt to do so. It is with the recognition of the latter that The International Consensus Meeting on Periprosthetic Joint Infection was organized. Delegates from various disciplines including orthopedic surgery, infectious disease, musculoskeletal pathology, microbiology, anesthesiology, dermatology, nuclear medicine, rheumatology, musculoskeletal radiology, veterinary surgery, pharmacy, and numerous scientists with interest in orthopedic infections came together to evaluate the available evidence, when present, or reach consensus regarding current practices for management of SSI/PJI. The process of generating the consensus has spanned over 10 months. Every stone has been turned in search of evidence for these questions, with over 3,500 related publications evaluated. The evidence, when available, has been assessed. Otherwise, the cumulative wisdom of 400 delegates from 52 countries and over 100 societies has been amassed to reach consensus about practices that lack higher level of evidence. The members of the Musculoskeletal Infection Society (MSIS) and the European Bone and Joint Infection Society (EBJIS), the 2 societies with a mission is to improve care of patients with musculoskeletal infection, have contributed to this initiative immensely.
The delegates have been engaged every step of the way by communicating through a social website generated for this purpose, with over 25,000 communications exchanged. The consensus document has been developed using the Delphi method under the leadership of Dr. Cats-Baril, a world-renowned expert in consensus development. The design of the consensus process was to include as many stakeholders as possible, allow participation in multiple forums, and provide a comprehensive review of the literature. The topics that were covered included the following: mitigation and education on comorbidities associated with increased SSI/PJI, perioperative skin preparation, perioperative antibiotics, operative environment, blood conservation, prosthesis selection, diagnosis of PJI, wound management, spacers, irrigation and debridement, antibiotic treatment and timing of reimplantation, 1-stage versus 2-stage exchange arthroplasty, management of fungal or atypical PJI, oral antibiotic therapy, and prevention of late PJI. Every consensus statement has undergone extreme scrutiny, especially by those with expertise in a specific area, to ensure that implementation of these practices will indeed lead to improvement of patient care.
After synthesizing the literature and assembling a preliminary draft of the consensus statement, over 300 delegates attended a face-to-face meeting in Philadelphia, were involved in active discussions, and voted on the questions/consensus statements. The delegates first met on July 31, 2013, in smaller workgroups, to discuss and resolve any discrepancies and finalize their statements. Then, they met in the general assembly for further discussion of questions and consensus statements. After revising the consensus statements, the finalized consensus statement was assembled and forwarded to the Audience Response System that evening, with voting occurring on the next day. On August 1, 2013 the delegates came into the general assembly and voted on the 207 questions/consensus statements that were being presented. The voting process was conducted using electronic keypads, where one could agree with the consensus statement, disagree with the consensus statement, or abstain from voting. The strength of the consensus was judged by the following scale: 1) Simple Majority: No Consensus (50.1%-59% agreement), 2) Majority: Weak Consensus (60%-65% agreement), 3) Super Majority: Strong Consensus (66%-99% agreement) and 4) Unanimous: 100% agreement. Of the 207 questions, there was unanimous vote for one question (controlling operating room traffic), 202 questions received super majority (strong consensus), 2 questions had weak consensus, and only 3 questions did not achieve any consensus.
The document generated(REF) is the result of innumerable hours of work by the liaisons, leaders and delegates dedicated to this initiative. We are certain that the “best practice guide” set forth by this initiative will serve many of our patients for years to come.
It is essential to state that the information contained in this document is merely a guide to practicing physicians who treat patients with musculoskeletal infection and should not be considered as a standard of care. Clinicians should exercise their wisdom and clinical acumen in making decisions related to each individual patient. In some circumstances this may require implementation of care that differs from what is stated in this document.