User login
Patella Alta: A Comprehensive Review of Current Knowledge
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
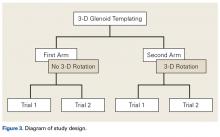
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
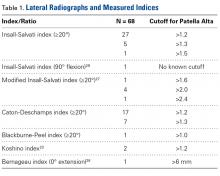
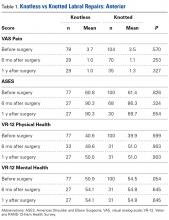
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
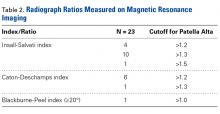
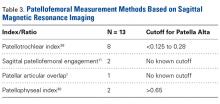
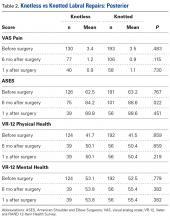
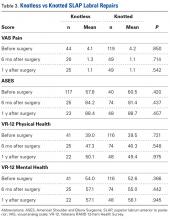
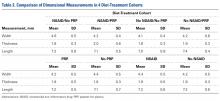
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
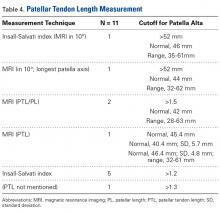
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
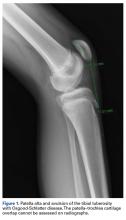
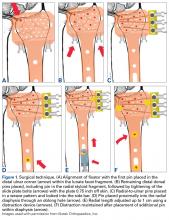
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
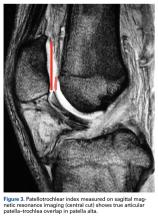
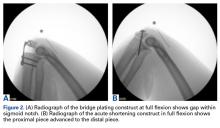
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
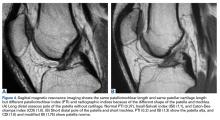
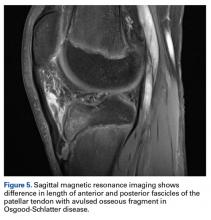
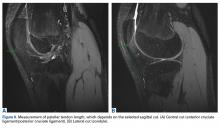
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
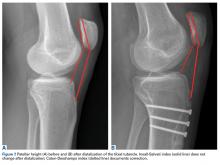
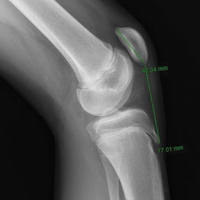
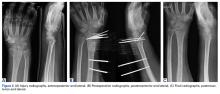
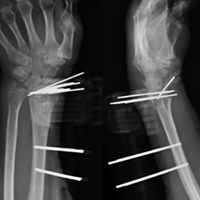
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
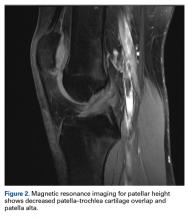
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.
Patient-Reported Outcomes of Knotted and Knotless Glenohumeral Labral Repairs Are Equivalent
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
Update on Internet-Based Orthopedic Registries
Take-Home Points
- PRO data collection can provide feedback for improvements in patient care and physician performance.
- Many options exist for orthopedic physicians to establish clinical data registries.
- Registry systems can help improve patient follow-up with system monitoring and patient reminders.
- Clinical registries can offer many advantages to observational research.
- With registry use becoming more prevalent, work needs to be done to establish standards for validity and reliability.
In a 2012 review of database tools, Lubowitz and Smith1 examined Internet-based applications that arthroscopic surgeons could use to record and monitor patient-reported outcome (PRO) data and potential adverse effects. In this article, we update orthopedic surgeons on the registries and monitoring software mentioned in that earlier publication and in other publications that have since become available.
Most orthopedic surgery candidates are seeking pain relief and improved function. Many patients expect their pain to be completely relieved by surgical intervention and their function to return to what it was before they became stricken.2,3 Therefore, PRO measures (PROMs) are now standard in post-orthopedic surgery outcome reporting.4 PROMs, which include any measurement that assesses a patient’s health, illness, or benefits from the perspective of the patient, are often administered as a questionnaire or survey.5 The collection of PROMs continues to increase and evolve, creating a need for data storage and analysis. Registries, large collections of patient information and outcomes, allow for evaluation of patient outcomes, monitoring of adverse effects, identification of procedure incidence, understanding of predictors of prognosis, generation of feedback for quality of care, monitoring of the safety of implantable devices, and the conducting of hypothesis-driven scientific research.6-9
Orthopedic surgery has registries at regional, national, and international levels. Although the United States has fallen well behind other countries in establishing a national registry,9 it has made some recent progress. The United States now has several national registries, including the American Joint Replacement Registry (AJRR), Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR), the Kaiser Permanente National Total Joint Replacement Registry (TJRR), the Veterans Affairs (VA) and American College of Surgeons (ACS) National Surgical Quality Improvement Programs (NSQIPs), and the National Trauma Data Bank (NTDB).9 AJRR currently has 960 hospitals participating and is tracking 1,084,664 hip and knee replacements.10
These orthopedic registries, however, are limited in 2 ways. First, the majority are joint replacement registries. Second, though registries are established to determine patterns of care and predict patient outcomes, many are not set up to report care data back to healthcare providers.7 For procedures other than joint arthroplasty and for providers interested in tracking their patients’ PROs, systems are available for establishing clinical quality registries in orthopedics.
Registry Systems
CareSense
CareSense (Medtrak) is an Internet-based care management and data collection system designed for patient engagement, which results in fewer missed appointments, increased patient adherence, enhanced patient education, and improved patient satisfaction.11 CareSense features email/text reminders for data entry, custom and standard reports, import and export of electronic medical record (EMR) information, and tools for running research studies.12 CareSense emphasizes care navigation by helping hospitals educate and guide patients through their care by sending exercise videos to patients for home rehabilitation, transferring messages from post-acute care facilities to surgeons and caregivers, and alerting the care team to any potential readmission symptoms.11,13 CareSense is also a Centers for Medicare & Medicaid Services (CMS) approved qualified clinical data registry (QCDR). QCDRs collect data for Merit-Based Incentive Payment System (MIPS) clinicians and submit the data to CMS.12
KareOutcomes
KareOutcomes, a healthcare technology and support firm founded in 2009, advocates transparency and trust among providers and patients, and aims to optimize PROs.14 The KareOutcomes team incorporates patient follow-up personnel, administrators, engineers, physicians, software developers, and technicians. The KareOutcomes software, which is backed by a 6-month guarantee, includes system design and implementation, data collection and entry, methods of submitting data to statewide or nationwide registries and sending standardized and customized surveys, and accessible and meaningful data presentation. KareOutcomes allows patient follow-up through automated reminders by telephone, SMS text message, and email. Patients can respond to surveys or questionnaires whichever way is most convenient—by telephone, Internet, SMS text message, or on paper, either in the office or by mail.
Oberd
Oberd (Universal Research Solutions) offers a comprehensive package of solutions for collecting optimal PRO data. The package has several modules: outcomes, education, registry, operative notes, data import and export, and data reporting.15 Oberd Outcomes allows convenient and engaging data collection. For example, users can send both standardized and customized forms. Oberd Education allows patients to receive information in an interactive, narrated format that is specific to their physician’s techniques and practices. Oberd Registry allows users to input multiple datasets into a registry, compare data, and generate reports with visuals. Like CareSense, Oberd is a CMS-approved QCDR. Oberd’s MIPS Dashboard helps providers collect and report patients’ reported outcomes, and use that information to modify and improve their practice.
Ortech
Ortech is a web-based data registry system that allows physicians and administrators to mine the data they own, track key metrics in their data, and improve reporting.16 Users can collect PROMs, use them to measure and analyze patient progress, and add to their collection of information that helps support their evidence-based decision making. They can capture intraoperative and implant data through barcode scanning, which then registers the data in an implant product code library that allows quick identification of patients with a specific implant in the event of a product recall. Ortech also allows automatic generation of customized operative reports on data entered from the operating room and populated into the EMR. Ortech offers 2 versions of its data collection platform, phiDB and phiDB Lite. The phiDB Lite version is for smaller practices and focuses mainly on PROMs but lacks many of the other features that phiDB offers, such as operating room modules, automated operative reports, barcode scanning, and unlimited data reporting.
Socrates
Socrates (Standardised Orthopaedic Clinical Research and Treatment Evaluation Software; Ortholink) is dedicated orthopedic software that facilitates following patient outcomes and conducting high-quality research.17 Socrates is fully customizable to fit each user’s needs. It allows for tracking of outcome scores, intraoperative details, nonoperative procedures, clinical examinations, therapies, and adverse effects. Users can also create reports from this information, which is inputted to Socrates and can be exported into EMR. Socrates data are stored on the user’s server, on site; the software generates patient summaries, collective summaries, and follow-up reports through its built-in descriptive statistics module. Raw data can be extracted for statistical analysis. Socrates can catalogue images, radiographs, documents, and videos.
Surgical Outcomes System
Surgical Outcomes System (SOS; Arthrex) is a cloud-based orthopedic and sports medicine global registry that focuses on monitoring and evaluating the outcomes of various orthopedic and sports medicine surgical procedures, as well as nonoperative interventions, to contribute to evidence-based protocols for patient treatment.18 SOS can be fully customized with desired PROMs for arthroplasty and for surgical procedures for extremity joints and even the spine. SOS includes real-time reporting on PROs for individual patients, summary PROMs for all of the physician’s patients who are receiving the same treatment, and comparisons with all registry patients (from global de-identified registry data) who had the same treatment or surgery. This real-time analysis provides immediate patient and physician feedback on treatments and products used. A patient portal for education on surgical procedures is also available. SOS is approved for use in 21 countries and is a benefit included with Arthroscopy Association of North America (AANA) membership. SOS is listed on the National Quality Registry Network (NQRN) website and, as a specialized registry as defined by CMS, can accept data generated by EMR technology.
Discussion
Delaunay19 indicated that successful registry management depends on several factors, including “use of a single identifier for each patient to ensure full traceability of all procedures related to a given implant; a long-term funding source; a contemporary, rapid, Internet-based data collection method; and the collection of exhaustive data, at least for innovative implants.” The registry systems reviewed in this article are Internet based and allow healthcare providers to monitor the clinical outcomes of their patients in the hope of improving clinical decision-making and overall patient care. From the provider perspective, many registry systems allow for integration of outcome data reporting into EMRs, including generation of operative reports. In turn, registries can improve documentation efficiency, as it was estimated that a US physician without a registry spends more than 15 hours a week reporting quality measures,20 or almost 800 hours and $15 billion each year.20,21 It remains to be seen whether registry systems will optimize the documentation process, but there is potential improvement in time and cost-efficiency with registry use.
Although the factors involved in management are important, clinical data registries must have systems in place to help ensure patient adherence and minimize selection bias, as adherence is crucial in data accuracy.3 What helps with adherence is the ability to send automated email or SMS text message reminders to patients. According to a review, email reminders increased the completion of PROM datasets by 26%.22 When the new national quality register (NQR) HAKIR (Handkirurgiskt kvalitetsregister) was established in Sweden, it was found that when only 1 type of reminder was used (SMS text message, in this case), only about 30% of participants completed their questionnaires.23 However, after the system was changed to send both SMS text message and email reminders, the response rate increased from 50% to 60%. Using 2 types of automated reminders might minimize lost data more effectively than 1 type alone.
Another benefit of outcome monitoring through a registry is potential reduction of interviewer- related errors. Interviewer bias can occur in many different ways. Interviewers might not follow the same instructions or administer questionnaires or surveys the same way for different patients,24 the interviewer’s presence might cause the patient to alter responses based on social norms,25 and the patient might report better outcomes in the presence of a physician or interviewer.26,27 Given that clinical registries allow electronic capture of self-administered surveys, interviewer bias is reduced because all patients receive a standardized set of questions and instructions. In addition, electronic questionnaires and surveys prompt users to add or fix missed or incorrectly completed items, further reducing potential data inaccuracies.
Healthcare costs continue to rise in the United States. In 2015, the total cost of healthcare expenditure in the United States was $3.2 trillion, or almost 18% of the US gross domestic product.28 In addition, in the first half of 2016, an estimated 16.2% of people under age 65 years were in families that were struggling to pay medical bills.29,30 Healthcare reform provides a financial incentive to healthcare providers to collaborate to reduce unnecessary costs and procedures and improve the quality of healthcare.31 Porter and Teisberg32 defined value as health outcomes achieved per dollar spent. Registry monitoring of PROMs, which are the numerator in this critical value formula, allows providers to track patient outcomes over time to determine which interventions produce the best outcomes.22 Therefore, clinical registries play an important role in improving health outcomes and reducing the cost of healthcare.7
Since the Swedish Knee Arthroplasty Register (SKAR) was established 40 years ago, NQRs have been commonplace in Scandinavian countries, Australia, and the United Kingdom.23 Between 2001 and 2014, the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) documented a decline in the financial burden of hip and knee arthroplasty revision in Australia—in comparison with the United States, which did not have a full national registry at the time and showed a revision rate increase.24 The economic benefit of reducing hip and knee arthroplasty revisions in Australia during that period was an estimated $65 million to $143 million.24 Besides having financial benefits, national registries allow early identification of flawed implantation products and methods, leading to a further reduction in the burden associated with recall and future use of such defective implants—including patient harm.
In addition to monitoring existing techniques and devices, registries can also follow new techniques and, compared with publication in clinical journals, more expeditiously provide clinical data for outcome expectations and treatment methods. This timeliness is particularly valuable given that publication of clinical trials with the usual mandatory 2-year follow-up can take 4 years or longer.33,34 For instance, in the expanding field of hip arthroscopy, data from registries in both Sweden and Denmark are being analyzed.35,36 These data are important in new fields such as hip arthroscopy, in which clinical indications and treatment techniques may vary considerably between locations.35 In 2012, the Danish Hip Arthroscopy Registry (DHAR) was started as a web-based prospective registry.36 Between 2012 and December 2014, DHAR added 2000 procedures, which included all hip arthroscopy procedures performed at 11 centers in Denmark.36 DHAR tracks PROM, surgical procedure, operative, and radiologic data.
Increased use of clinical registries has led to use of their data in clinical research. Registry-based randomized controlled trials (RCTs) are lower in cost than other types of research, allow for rapid enrollment of patients, offer larger population sizes and multi-institutional sampling, and can provide a more diverse patient population.19,37 Although nonregistry RCTs remain the gold standard of clinical research, registry RCTs have several advantages given the abilities and structure of registries. Because of resources and cost, nonregistry RCTs are usually limited in the number of examined exposures and typically focus on only 2.6 Registry RCTs, on the other hand, can monitor multiple exposures, typically at minimal cost difference.6 Another disadvantage of nonregistry RCTs is that they are often performed at institutions providing care that might not be indicative of the quality most patients expect, as these institutions might be selected for a specific clinician or specialty service.
Registry RCTs also have their limitations with respect to clinical research. A major one is their lack of validity standards or accepted benchmarks for accuracy, adherence rates, registry completeness, and data collection.37,38 In addition, lack of standardization across national and international registries could produce conflicting data. Another limitation is that data in most registries are not subjected to any third-party checks or independent auditing.9,39 Furthermore, evaluating the impact of registries is difficult because it is difficult to find comparable outcome data on nonregistry patients.40 A final limitation involves the ethics of including registry data in RCTs. Although data are often added to a registry without patient consent, should the same data be used for research without patient consent? Should patients be able to disallow use of their data for research, or require a notification each time their data are used? These issues must be addressed.
Review Limitations
One limitation of this review of clinical Internet-based outcome systems is that it might not have identified comparable systems. In addition, specific costs associated with each system were not addressed, as they depend on PROM licensing fees, total institutional access, other proprietary costs, and other variables. Another limitation, in terms of creating a national or international registry, continues to be Internet access. The Pew Research Center estimated that 84% of US adults used the Internet in 2015.41 Although 84% represents most of the adult population, the other 16% typically is over age 65 years, where only 58% of adults reported using the Internet, or come from lower income households, where access was <75%. For registries in European countries and North America, where Internet usage typically is >70%, this is not a significant problem. However, worldwide, only 47% of the population used the Internet in 2016.42 Internet usage by Asian and Arab states citizens was 41.6% and 41.9%, respectively, and usage by African citizens was only 25.1%. As a significant benefit of registry use is that researchers can obtain larger sample sizes, it is a problem that some populations—elderly people, people of lower socioeconomic standing, people living where the Internet is unavailable—might be underrepresented in registry data.
As mentioned, patient adherence is an ongoing issue for clinical registries. As adherence tends to decrease as more time passes after a patient’s treatment date, it is important to account for and encourage continued patient participation with outcome monitoring. Missing data lessen the validity and accuracy of a registry, increasing the likelihood that certain groups will be underrepresented. Although registry systems can reduce the cost of following PROMs, doing so requires monitoring and following up on issues of patient adherence. In other words, many clinicians will need the help of a research assistant. Makhni and colleagues21 found that adding a research assistant for this task increased survey adherence from 65% to 94% before surgery, from 65% to 72% 6 months after surgery, and from 38% to 56% 12 months after surgery.
Even though studies continue to use clinical data from registries, there is not much research on the impact of these registries on improvement in healthcare. Again, many factors are involved: lack of standardized benchmarks for accuracy and adherence, lack of an accepted method of data auditing and validation, and difficulty evaluating the impact of registries owing to the difficulty obtaining comparable data on nonregistry patients. Registries must adopt accepted forms of standardization in order to allow better comparisons of registries, because comparing data across registries can be useful in determining the strengths and weaknesses of different registries.27,43 As registries support decision making at clinical, institutional, and governmental levels, it is vital that their clinical data be accurate and reliable.38
Conclusion
Rising healthcare costs, and government and third-party pressures are making patient outcomes collection a standard of care. Going forward, orthopedic surgeons must be proactive, and Internet -based registries provide technological advances that facilitate the process.
1. Lubowitz JH, Smith PA. Current concepts in clinical research: web-based, automated, arthroscopic surgery prospective database registry. Arthroscopy. 2012;28(3):425-428.
2. Ayers DC, Bozic KJ. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res. 2013;471(11):3409-3411.
3. Nwachukwu BU, Fields K, Chang B, Nawabi DH, Kelly BT, Ranawat AS. Preoperative outcome scores are predictive of achieving the minimal clinically important difference after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2017;45(3):612-619.
4. Breckenridge K, Bekker HL, Gibbons E, et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. 2015;30(10):1605-1614.
5. Inacio MC, Paxton EW, Dillon MT. Understanding orthopaedic registry studies: a comparison with clinical studies. J Bone Joint Surg Am. 2016;98(1):e3.
6. Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS One. 2017;12(9):e0183667.
7. Physician Consortium for Performance Improvement. National Quality Registry Network. http://www.thepcpi.org/programs-initiatives/national-quality-registry-network/. Accessed October 5, 2017.
8. Hickey GL, Grant SW, Cosgriff R, et al. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44(4):605-614.
9. Pugely AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopaedic surgery: part 2: clinical registry data. J Bone Joint Surg Am. 2015;97(21):1799-1808.
10. American Joint Replacement Registry. http://www.ajrr.net/. Accessed October 5, 2017.
11. CareSense. https://www.caresense.com/. Accessed October 4, 2017.
12. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Quality Payment Program. Merit-Based Incentive Payment System (MIPS): 2017 CMS-Approved Qualified Clinical Data Registries (QCDRs). https://qpp.cms.gov/docs/QPP_2017_CMS_Approved_QCDRs.pdf. Accessed October 9, 2017.
13. Johnson & Johnson. Johnson & Johnson Medical Devices Companies introduce Orthopaedic Episode of Care Approach, leveraging CareAdvantage capabilities to support better clinical outcomes and reduce the cost of care. https://www.jnj.com/media-center/press-releases/johnson-johnson-medical-devices-companies-introduce-orthopaedic-episode-of-care-approach-leveraging-careadvantage-capabilities-to-support-better-clinical-outcomes-and-reduce-the-cost-of-care. Published January 9, 2017. Accessed October 4, 2017.
14. KareOutcomes. http://www.kareoutcomes.com/. Accessed October 4, 2017.
15. Oberd. http://www.oberd.com/. Accessed October 4, 2017.
16. Ortech Systems. http://www.ortechsystems.com/. Accessed October 4, 2017.
17. Socrates. http://www.socratesortho.com/. Accessed October 4, 2017.
18. Surgical Outcomes System. https://www.surgicaloutcomesystem.com/. Accessed October 4, 2017.
19. Delaunay C. Registries in orthopaedics. Orthop Traumatol Surg Res. 2015;101(1 suppl):S69-S75.
20. Bryan S, Davis J, Broesch J, Doyle-Waters MM, Lewis S, McGrail K. Choosing your partner for the PROM: a review of evidence on patient-reported outcome measures for use in primary and community care. Healthc Policy. 2014;10(2):38-51.
21. Makhni EC, Higgins JD, Hamamoto JT, Cole BJ, Romeo AA, Verma NN. Patient compliance with electronic patient reported outcomes following shoulder arthroscopy [published online ahead of print September 25, 2017]. Arthroscopy. doi:10.1016/j.arthro.2017.06.016.
22. Triplet JJ, Momoh E, Kurowicki J, Villarroel LD, Law T, Levy JC. E-mail reminders improve completion rates of patient-reported outcome measures. JSES Open Access. 2017;1:25-28.
23. Arner M. Developing a national quality registry for hand surgery: challenges and opportunities. EFORT Open Rev. 2016;1(4):100-106.
24. Ngongo CJ, Frick KD, Hightower AW, Mathingau FA, Burke H, Breiman RF. The perils of straying from protocol: sampling bias and interviewer effects. PLoS One. 2015;10(2):e0118025.
25. Hammarstedt JE, Redmond JM, Gupta A, Dunne KF, Vemula SP, Domb BG. Survey mode influence on patient-reported outcome scores in orthopaedic surgery: telephone results may be positively biased. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):50-54.
26. Hoher J, Bach T, Munster A, et al. Does the mode of data collection change results in a subjective knee score? Self-administration versus interview. Am J Sports Med. 1997;25(5):642-647.
27. Lacny S, Bohm E, Hawker G, Powell J, Marshall DA. Assessing the comparability of hip arthroplasty registries in order to improve the recording and monitoring of outcome. Bone Joint J. 2016;98-B(4):442-451.
28. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville, MD: National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services; 2017. DHHS Publication 2017-1232. https://www.cdc.gov/nchs/data/hus/hus16.pdf. Published May 2017. Accessed October 9, 2017.
29. Cohen RA, Zammitti EP. Problems paying medical bills among persons under age 65: early release of estimates from the National Health Interview Survey, 2011-June 2016. National Health Interview Survey Early Release Program, Division of Health Interview Statistics, National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. https://www.cdc.gov/nchs/data/nhis/earlyrelease/probs_paying_medical_bills_jan_2011_jun_2016.pdf. Published November 2016. Accessed October 9, 2017.
30. National Center for Health Statistics. National Health Interview Survey. http://www.cdc.gov/nchs/nhis/releases.htm. Accessed October 5, 2017.
31. Karhade AV, Larsen AMG, Cote DJ, Dubois HM, Smith TR. National databases for neurosurgical outcomes research: options, strengths, and limitations [published online ahead of print August 5, 2017]. Neurosurgery. https://doi.org/10.1093/neuros/nyx408.
32. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
33. Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637.
34. Counsell N, Biri D, Fraczek J, Hackshaw A. Publishing interim results of randomised clinical trials in peer-reviewed journals. Clin Trials. 2017;14(1):67-77.
35. Sansone M, Ahldén M, Jonasson P, et al. A Swedish hip arthroscopy registry: demographics and development. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):774-780.
36. Mygind-Klavsen B, Grønbech Nielsen T, Maagaard N, et al. Danish Hip Arthroscopy Registry: an epidemiologic and perioperative description of the first 2000 procedures. J Hip Preserv Surg. 2016;3(2):138-145.
37. Li G, Sajobi TT, Menon BK, et al; 2016 Symposium on Registry-Based Randomized Controlled Trials in Calgary. Registry-based randomized controlled trials—what are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16-24.
38. Bautista MP, Bonilla GA, Mieth KW. Data quality in institutional arthroplasty registries: description of model of validation and report of preliminary results. J Arthroplasty. 2017;32(7):2065-2069.
39. Tevaearai H, Carrel T. Clinical registries: yes, but then appropriately! Eur J Cardiothorac Surg. 2013;44(4):614-615.
40. Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries: final report. Sydney, Australia: ACSQHC; 2016.
41. Perrin A, Duggan M. Americans’ internet access: 2000-2015. Pew Research Center website. http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015/. Published June 26, 2015. Accessed October 5, 2017.
42. Taylor A. 47 percent of the world’s population now use the internet, study says. https://www.washingtonpost.com/news/worldviews/wp/2016/11/22/47-percent-of-the-worlds-population-now-use-the-internet-users-study-says/. Published November 22, 2016. Accessed October 5, 2017.
43. Romero L, Nieuwenhuijse M, Carr A, Sedrakyan A. Review of clinical outcomes-based anchors of minimum clinically important differences in hip and knee registry–based reports and publications. J Bone Joint Surg Am. 2014;96(suppl 1):98-103.
Take-Home Points
- PRO data collection can provide feedback for improvements in patient care and physician performance.
- Many options exist for orthopedic physicians to establish clinical data registries.
- Registry systems can help improve patient follow-up with system monitoring and patient reminders.
- Clinical registries can offer many advantages to observational research.
- With registry use becoming more prevalent, work needs to be done to establish standards for validity and reliability.
In a 2012 review of database tools, Lubowitz and Smith1 examined Internet-based applications that arthroscopic surgeons could use to record and monitor patient-reported outcome (PRO) data and potential adverse effects. In this article, we update orthopedic surgeons on the registries and monitoring software mentioned in that earlier publication and in other publications that have since become available.
Most orthopedic surgery candidates are seeking pain relief and improved function. Many patients expect their pain to be completely relieved by surgical intervention and their function to return to what it was before they became stricken.2,3 Therefore, PRO measures (PROMs) are now standard in post-orthopedic surgery outcome reporting.4 PROMs, which include any measurement that assesses a patient’s health, illness, or benefits from the perspective of the patient, are often administered as a questionnaire or survey.5 The collection of PROMs continues to increase and evolve, creating a need for data storage and analysis. Registries, large collections of patient information and outcomes, allow for evaluation of patient outcomes, monitoring of adverse effects, identification of procedure incidence, understanding of predictors of prognosis, generation of feedback for quality of care, monitoring of the safety of implantable devices, and the conducting of hypothesis-driven scientific research.6-9
Orthopedic surgery has registries at regional, national, and international levels. Although the United States has fallen well behind other countries in establishing a national registry,9 it has made some recent progress. The United States now has several national registries, including the American Joint Replacement Registry (AJRR), Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR), the Kaiser Permanente National Total Joint Replacement Registry (TJRR), the Veterans Affairs (VA) and American College of Surgeons (ACS) National Surgical Quality Improvement Programs (NSQIPs), and the National Trauma Data Bank (NTDB).9 AJRR currently has 960 hospitals participating and is tracking 1,084,664 hip and knee replacements.10
These orthopedic registries, however, are limited in 2 ways. First, the majority are joint replacement registries. Second, though registries are established to determine patterns of care and predict patient outcomes, many are not set up to report care data back to healthcare providers.7 For procedures other than joint arthroplasty and for providers interested in tracking their patients’ PROs, systems are available for establishing clinical quality registries in orthopedics.
Registry Systems
CareSense
CareSense (Medtrak) is an Internet-based care management and data collection system designed for patient engagement, which results in fewer missed appointments, increased patient adherence, enhanced patient education, and improved patient satisfaction.11 CareSense features email/text reminders for data entry, custom and standard reports, import and export of electronic medical record (EMR) information, and tools for running research studies.12 CareSense emphasizes care navigation by helping hospitals educate and guide patients through their care by sending exercise videos to patients for home rehabilitation, transferring messages from post-acute care facilities to surgeons and caregivers, and alerting the care team to any potential readmission symptoms.11,13 CareSense is also a Centers for Medicare & Medicaid Services (CMS) approved qualified clinical data registry (QCDR). QCDRs collect data for Merit-Based Incentive Payment System (MIPS) clinicians and submit the data to CMS.12
KareOutcomes
KareOutcomes, a healthcare technology and support firm founded in 2009, advocates transparency and trust among providers and patients, and aims to optimize PROs.14 The KareOutcomes team incorporates patient follow-up personnel, administrators, engineers, physicians, software developers, and technicians. The KareOutcomes software, which is backed by a 6-month guarantee, includes system design and implementation, data collection and entry, methods of submitting data to statewide or nationwide registries and sending standardized and customized surveys, and accessible and meaningful data presentation. KareOutcomes allows patient follow-up through automated reminders by telephone, SMS text message, and email. Patients can respond to surveys or questionnaires whichever way is most convenient—by telephone, Internet, SMS text message, or on paper, either in the office or by mail.
Oberd
Oberd (Universal Research Solutions) offers a comprehensive package of solutions for collecting optimal PRO data. The package has several modules: outcomes, education, registry, operative notes, data import and export, and data reporting.15 Oberd Outcomes allows convenient and engaging data collection. For example, users can send both standardized and customized forms. Oberd Education allows patients to receive information in an interactive, narrated format that is specific to their physician’s techniques and practices. Oberd Registry allows users to input multiple datasets into a registry, compare data, and generate reports with visuals. Like CareSense, Oberd is a CMS-approved QCDR. Oberd’s MIPS Dashboard helps providers collect and report patients’ reported outcomes, and use that information to modify and improve their practice.
Ortech
Ortech is a web-based data registry system that allows physicians and administrators to mine the data they own, track key metrics in their data, and improve reporting.16 Users can collect PROMs, use them to measure and analyze patient progress, and add to their collection of information that helps support their evidence-based decision making. They can capture intraoperative and implant data through barcode scanning, which then registers the data in an implant product code library that allows quick identification of patients with a specific implant in the event of a product recall. Ortech also allows automatic generation of customized operative reports on data entered from the operating room and populated into the EMR. Ortech offers 2 versions of its data collection platform, phiDB and phiDB Lite. The phiDB Lite version is for smaller practices and focuses mainly on PROMs but lacks many of the other features that phiDB offers, such as operating room modules, automated operative reports, barcode scanning, and unlimited data reporting.
Socrates
Socrates (Standardised Orthopaedic Clinical Research and Treatment Evaluation Software; Ortholink) is dedicated orthopedic software that facilitates following patient outcomes and conducting high-quality research.17 Socrates is fully customizable to fit each user’s needs. It allows for tracking of outcome scores, intraoperative details, nonoperative procedures, clinical examinations, therapies, and adverse effects. Users can also create reports from this information, which is inputted to Socrates and can be exported into EMR. Socrates data are stored on the user’s server, on site; the software generates patient summaries, collective summaries, and follow-up reports through its built-in descriptive statistics module. Raw data can be extracted for statistical analysis. Socrates can catalogue images, radiographs, documents, and videos.
Surgical Outcomes System
Surgical Outcomes System (SOS; Arthrex) is a cloud-based orthopedic and sports medicine global registry that focuses on monitoring and evaluating the outcomes of various orthopedic and sports medicine surgical procedures, as well as nonoperative interventions, to contribute to evidence-based protocols for patient treatment.18 SOS can be fully customized with desired PROMs for arthroplasty and for surgical procedures for extremity joints and even the spine. SOS includes real-time reporting on PROs for individual patients, summary PROMs for all of the physician’s patients who are receiving the same treatment, and comparisons with all registry patients (from global de-identified registry data) who had the same treatment or surgery. This real-time analysis provides immediate patient and physician feedback on treatments and products used. A patient portal for education on surgical procedures is also available. SOS is approved for use in 21 countries and is a benefit included with Arthroscopy Association of North America (AANA) membership. SOS is listed on the National Quality Registry Network (NQRN) website and, as a specialized registry as defined by CMS, can accept data generated by EMR technology.
Discussion
Delaunay19 indicated that successful registry management depends on several factors, including “use of a single identifier for each patient to ensure full traceability of all procedures related to a given implant; a long-term funding source; a contemporary, rapid, Internet-based data collection method; and the collection of exhaustive data, at least for innovative implants.” The registry systems reviewed in this article are Internet based and allow healthcare providers to monitor the clinical outcomes of their patients in the hope of improving clinical decision-making and overall patient care. From the provider perspective, many registry systems allow for integration of outcome data reporting into EMRs, including generation of operative reports. In turn, registries can improve documentation efficiency, as it was estimated that a US physician without a registry spends more than 15 hours a week reporting quality measures,20 or almost 800 hours and $15 billion each year.20,21 It remains to be seen whether registry systems will optimize the documentation process, but there is potential improvement in time and cost-efficiency with registry use.
Although the factors involved in management are important, clinical data registries must have systems in place to help ensure patient adherence and minimize selection bias, as adherence is crucial in data accuracy.3 What helps with adherence is the ability to send automated email or SMS text message reminders to patients. According to a review, email reminders increased the completion of PROM datasets by 26%.22 When the new national quality register (NQR) HAKIR (Handkirurgiskt kvalitetsregister) was established in Sweden, it was found that when only 1 type of reminder was used (SMS text message, in this case), only about 30% of participants completed their questionnaires.23 However, after the system was changed to send both SMS text message and email reminders, the response rate increased from 50% to 60%. Using 2 types of automated reminders might minimize lost data more effectively than 1 type alone.
Another benefit of outcome monitoring through a registry is potential reduction of interviewer- related errors. Interviewer bias can occur in many different ways. Interviewers might not follow the same instructions or administer questionnaires or surveys the same way for different patients,24 the interviewer’s presence might cause the patient to alter responses based on social norms,25 and the patient might report better outcomes in the presence of a physician or interviewer.26,27 Given that clinical registries allow electronic capture of self-administered surveys, interviewer bias is reduced because all patients receive a standardized set of questions and instructions. In addition, electronic questionnaires and surveys prompt users to add or fix missed or incorrectly completed items, further reducing potential data inaccuracies.
Healthcare costs continue to rise in the United States. In 2015, the total cost of healthcare expenditure in the United States was $3.2 trillion, or almost 18% of the US gross domestic product.28 In addition, in the first half of 2016, an estimated 16.2% of people under age 65 years were in families that were struggling to pay medical bills.29,30 Healthcare reform provides a financial incentive to healthcare providers to collaborate to reduce unnecessary costs and procedures and improve the quality of healthcare.31 Porter and Teisberg32 defined value as health outcomes achieved per dollar spent. Registry monitoring of PROMs, which are the numerator in this critical value formula, allows providers to track patient outcomes over time to determine which interventions produce the best outcomes.22 Therefore, clinical registries play an important role in improving health outcomes and reducing the cost of healthcare.7
Since the Swedish Knee Arthroplasty Register (SKAR) was established 40 years ago, NQRs have been commonplace in Scandinavian countries, Australia, and the United Kingdom.23 Between 2001 and 2014, the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) documented a decline in the financial burden of hip and knee arthroplasty revision in Australia—in comparison with the United States, which did not have a full national registry at the time and showed a revision rate increase.24 The economic benefit of reducing hip and knee arthroplasty revisions in Australia during that period was an estimated $65 million to $143 million.24 Besides having financial benefits, national registries allow early identification of flawed implantation products and methods, leading to a further reduction in the burden associated with recall and future use of such defective implants—including patient harm.
In addition to monitoring existing techniques and devices, registries can also follow new techniques and, compared with publication in clinical journals, more expeditiously provide clinical data for outcome expectations and treatment methods. This timeliness is particularly valuable given that publication of clinical trials with the usual mandatory 2-year follow-up can take 4 years or longer.33,34 For instance, in the expanding field of hip arthroscopy, data from registries in both Sweden and Denmark are being analyzed.35,36 These data are important in new fields such as hip arthroscopy, in which clinical indications and treatment techniques may vary considerably between locations.35 In 2012, the Danish Hip Arthroscopy Registry (DHAR) was started as a web-based prospective registry.36 Between 2012 and December 2014, DHAR added 2000 procedures, which included all hip arthroscopy procedures performed at 11 centers in Denmark.36 DHAR tracks PROM, surgical procedure, operative, and radiologic data.
Increased use of clinical registries has led to use of their data in clinical research. Registry-based randomized controlled trials (RCTs) are lower in cost than other types of research, allow for rapid enrollment of patients, offer larger population sizes and multi-institutional sampling, and can provide a more diverse patient population.19,37 Although nonregistry RCTs remain the gold standard of clinical research, registry RCTs have several advantages given the abilities and structure of registries. Because of resources and cost, nonregistry RCTs are usually limited in the number of examined exposures and typically focus on only 2.6 Registry RCTs, on the other hand, can monitor multiple exposures, typically at minimal cost difference.6 Another disadvantage of nonregistry RCTs is that they are often performed at institutions providing care that might not be indicative of the quality most patients expect, as these institutions might be selected for a specific clinician or specialty service.
Registry RCTs also have their limitations with respect to clinical research. A major one is their lack of validity standards or accepted benchmarks for accuracy, adherence rates, registry completeness, and data collection.37,38 In addition, lack of standardization across national and international registries could produce conflicting data. Another limitation is that data in most registries are not subjected to any third-party checks or independent auditing.9,39 Furthermore, evaluating the impact of registries is difficult because it is difficult to find comparable outcome data on nonregistry patients.40 A final limitation involves the ethics of including registry data in RCTs. Although data are often added to a registry without patient consent, should the same data be used for research without patient consent? Should patients be able to disallow use of their data for research, or require a notification each time their data are used? These issues must be addressed.
Review Limitations
One limitation of this review of clinical Internet-based outcome systems is that it might not have identified comparable systems. In addition, specific costs associated with each system were not addressed, as they depend on PROM licensing fees, total institutional access, other proprietary costs, and other variables. Another limitation, in terms of creating a national or international registry, continues to be Internet access. The Pew Research Center estimated that 84% of US adults used the Internet in 2015.41 Although 84% represents most of the adult population, the other 16% typically is over age 65 years, where only 58% of adults reported using the Internet, or come from lower income households, where access was <75%. For registries in European countries and North America, where Internet usage typically is >70%, this is not a significant problem. However, worldwide, only 47% of the population used the Internet in 2016.42 Internet usage by Asian and Arab states citizens was 41.6% and 41.9%, respectively, and usage by African citizens was only 25.1%. As a significant benefit of registry use is that researchers can obtain larger sample sizes, it is a problem that some populations—elderly people, people of lower socioeconomic standing, people living where the Internet is unavailable—might be underrepresented in registry data.
As mentioned, patient adherence is an ongoing issue for clinical registries. As adherence tends to decrease as more time passes after a patient’s treatment date, it is important to account for and encourage continued patient participation with outcome monitoring. Missing data lessen the validity and accuracy of a registry, increasing the likelihood that certain groups will be underrepresented. Although registry systems can reduce the cost of following PROMs, doing so requires monitoring and following up on issues of patient adherence. In other words, many clinicians will need the help of a research assistant. Makhni and colleagues21 found that adding a research assistant for this task increased survey adherence from 65% to 94% before surgery, from 65% to 72% 6 months after surgery, and from 38% to 56% 12 months after surgery.
Even though studies continue to use clinical data from registries, there is not much research on the impact of these registries on improvement in healthcare. Again, many factors are involved: lack of standardized benchmarks for accuracy and adherence, lack of an accepted method of data auditing and validation, and difficulty evaluating the impact of registries owing to the difficulty obtaining comparable data on nonregistry patients. Registries must adopt accepted forms of standardization in order to allow better comparisons of registries, because comparing data across registries can be useful in determining the strengths and weaknesses of different registries.27,43 As registries support decision making at clinical, institutional, and governmental levels, it is vital that their clinical data be accurate and reliable.38
Conclusion
Rising healthcare costs, and government and third-party pressures are making patient outcomes collection a standard of care. Going forward, orthopedic surgeons must be proactive, and Internet -based registries provide technological advances that facilitate the process.
Take-Home Points
- PRO data collection can provide feedback for improvements in patient care and physician performance.
- Many options exist for orthopedic physicians to establish clinical data registries.
- Registry systems can help improve patient follow-up with system monitoring and patient reminders.
- Clinical registries can offer many advantages to observational research.
- With registry use becoming more prevalent, work needs to be done to establish standards for validity and reliability.
In a 2012 review of database tools, Lubowitz and Smith1 examined Internet-based applications that arthroscopic surgeons could use to record and monitor patient-reported outcome (PRO) data and potential adverse effects. In this article, we update orthopedic surgeons on the registries and monitoring software mentioned in that earlier publication and in other publications that have since become available.
Most orthopedic surgery candidates are seeking pain relief and improved function. Many patients expect their pain to be completely relieved by surgical intervention and their function to return to what it was before they became stricken.2,3 Therefore, PRO measures (PROMs) are now standard in post-orthopedic surgery outcome reporting.4 PROMs, which include any measurement that assesses a patient’s health, illness, or benefits from the perspective of the patient, are often administered as a questionnaire or survey.5 The collection of PROMs continues to increase and evolve, creating a need for data storage and analysis. Registries, large collections of patient information and outcomes, allow for evaluation of patient outcomes, monitoring of adverse effects, identification of procedure incidence, understanding of predictors of prognosis, generation of feedback for quality of care, monitoring of the safety of implantable devices, and the conducting of hypothesis-driven scientific research.6-9
Orthopedic surgery has registries at regional, national, and international levels. Although the United States has fallen well behind other countries in establishing a national registry,9 it has made some recent progress. The United States now has several national registries, including the American Joint Replacement Registry (AJRR), Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR), the Kaiser Permanente National Total Joint Replacement Registry (TJRR), the Veterans Affairs (VA) and American College of Surgeons (ACS) National Surgical Quality Improvement Programs (NSQIPs), and the National Trauma Data Bank (NTDB).9 AJRR currently has 960 hospitals participating and is tracking 1,084,664 hip and knee replacements.10
These orthopedic registries, however, are limited in 2 ways. First, the majority are joint replacement registries. Second, though registries are established to determine patterns of care and predict patient outcomes, many are not set up to report care data back to healthcare providers.7 For procedures other than joint arthroplasty and for providers interested in tracking their patients’ PROs, systems are available for establishing clinical quality registries in orthopedics.
Registry Systems
CareSense
CareSense (Medtrak) is an Internet-based care management and data collection system designed for patient engagement, which results in fewer missed appointments, increased patient adherence, enhanced patient education, and improved patient satisfaction.11 CareSense features email/text reminders for data entry, custom and standard reports, import and export of electronic medical record (EMR) information, and tools for running research studies.12 CareSense emphasizes care navigation by helping hospitals educate and guide patients through their care by sending exercise videos to patients for home rehabilitation, transferring messages from post-acute care facilities to surgeons and caregivers, and alerting the care team to any potential readmission symptoms.11,13 CareSense is also a Centers for Medicare & Medicaid Services (CMS) approved qualified clinical data registry (QCDR). QCDRs collect data for Merit-Based Incentive Payment System (MIPS) clinicians and submit the data to CMS.12
KareOutcomes
KareOutcomes, a healthcare technology and support firm founded in 2009, advocates transparency and trust among providers and patients, and aims to optimize PROs.14 The KareOutcomes team incorporates patient follow-up personnel, administrators, engineers, physicians, software developers, and technicians. The KareOutcomes software, which is backed by a 6-month guarantee, includes system design and implementation, data collection and entry, methods of submitting data to statewide or nationwide registries and sending standardized and customized surveys, and accessible and meaningful data presentation. KareOutcomes allows patient follow-up through automated reminders by telephone, SMS text message, and email. Patients can respond to surveys or questionnaires whichever way is most convenient—by telephone, Internet, SMS text message, or on paper, either in the office or by mail.
Oberd
Oberd (Universal Research Solutions) offers a comprehensive package of solutions for collecting optimal PRO data. The package has several modules: outcomes, education, registry, operative notes, data import and export, and data reporting.15 Oberd Outcomes allows convenient and engaging data collection. For example, users can send both standardized and customized forms. Oberd Education allows patients to receive information in an interactive, narrated format that is specific to their physician’s techniques and practices. Oberd Registry allows users to input multiple datasets into a registry, compare data, and generate reports with visuals. Like CareSense, Oberd is a CMS-approved QCDR. Oberd’s MIPS Dashboard helps providers collect and report patients’ reported outcomes, and use that information to modify and improve their practice.
Ortech
Ortech is a web-based data registry system that allows physicians and administrators to mine the data they own, track key metrics in their data, and improve reporting.16 Users can collect PROMs, use them to measure and analyze patient progress, and add to their collection of information that helps support their evidence-based decision making. They can capture intraoperative and implant data through barcode scanning, which then registers the data in an implant product code library that allows quick identification of patients with a specific implant in the event of a product recall. Ortech also allows automatic generation of customized operative reports on data entered from the operating room and populated into the EMR. Ortech offers 2 versions of its data collection platform, phiDB and phiDB Lite. The phiDB Lite version is for smaller practices and focuses mainly on PROMs but lacks many of the other features that phiDB offers, such as operating room modules, automated operative reports, barcode scanning, and unlimited data reporting.
Socrates
Socrates (Standardised Orthopaedic Clinical Research and Treatment Evaluation Software; Ortholink) is dedicated orthopedic software that facilitates following patient outcomes and conducting high-quality research.17 Socrates is fully customizable to fit each user’s needs. It allows for tracking of outcome scores, intraoperative details, nonoperative procedures, clinical examinations, therapies, and adverse effects. Users can also create reports from this information, which is inputted to Socrates and can be exported into EMR. Socrates data are stored on the user’s server, on site; the software generates patient summaries, collective summaries, and follow-up reports through its built-in descriptive statistics module. Raw data can be extracted for statistical analysis. Socrates can catalogue images, radiographs, documents, and videos.
Surgical Outcomes System
Surgical Outcomes System (SOS; Arthrex) is a cloud-based orthopedic and sports medicine global registry that focuses on monitoring and evaluating the outcomes of various orthopedic and sports medicine surgical procedures, as well as nonoperative interventions, to contribute to evidence-based protocols for patient treatment.18 SOS can be fully customized with desired PROMs for arthroplasty and for surgical procedures for extremity joints and even the spine. SOS includes real-time reporting on PROs for individual patients, summary PROMs for all of the physician’s patients who are receiving the same treatment, and comparisons with all registry patients (from global de-identified registry data) who had the same treatment or surgery. This real-time analysis provides immediate patient and physician feedback on treatments and products used. A patient portal for education on surgical procedures is also available. SOS is approved for use in 21 countries and is a benefit included with Arthroscopy Association of North America (AANA) membership. SOS is listed on the National Quality Registry Network (NQRN) website and, as a specialized registry as defined by CMS, can accept data generated by EMR technology.
Discussion
Delaunay19 indicated that successful registry management depends on several factors, including “use of a single identifier for each patient to ensure full traceability of all procedures related to a given implant; a long-term funding source; a contemporary, rapid, Internet-based data collection method; and the collection of exhaustive data, at least for innovative implants.” The registry systems reviewed in this article are Internet based and allow healthcare providers to monitor the clinical outcomes of their patients in the hope of improving clinical decision-making and overall patient care. From the provider perspective, many registry systems allow for integration of outcome data reporting into EMRs, including generation of operative reports. In turn, registries can improve documentation efficiency, as it was estimated that a US physician without a registry spends more than 15 hours a week reporting quality measures,20 or almost 800 hours and $15 billion each year.20,21 It remains to be seen whether registry systems will optimize the documentation process, but there is potential improvement in time and cost-efficiency with registry use.
Although the factors involved in management are important, clinical data registries must have systems in place to help ensure patient adherence and minimize selection bias, as adherence is crucial in data accuracy.3 What helps with adherence is the ability to send automated email or SMS text message reminders to patients. According to a review, email reminders increased the completion of PROM datasets by 26%.22 When the new national quality register (NQR) HAKIR (Handkirurgiskt kvalitetsregister) was established in Sweden, it was found that when only 1 type of reminder was used (SMS text message, in this case), only about 30% of participants completed their questionnaires.23 However, after the system was changed to send both SMS text message and email reminders, the response rate increased from 50% to 60%. Using 2 types of automated reminders might minimize lost data more effectively than 1 type alone.
Another benefit of outcome monitoring through a registry is potential reduction of interviewer- related errors. Interviewer bias can occur in many different ways. Interviewers might not follow the same instructions or administer questionnaires or surveys the same way for different patients,24 the interviewer’s presence might cause the patient to alter responses based on social norms,25 and the patient might report better outcomes in the presence of a physician or interviewer.26,27 Given that clinical registries allow electronic capture of self-administered surveys, interviewer bias is reduced because all patients receive a standardized set of questions and instructions. In addition, electronic questionnaires and surveys prompt users to add or fix missed or incorrectly completed items, further reducing potential data inaccuracies.
Healthcare costs continue to rise in the United States. In 2015, the total cost of healthcare expenditure in the United States was $3.2 trillion, or almost 18% of the US gross domestic product.28 In addition, in the first half of 2016, an estimated 16.2% of people under age 65 years were in families that were struggling to pay medical bills.29,30 Healthcare reform provides a financial incentive to healthcare providers to collaborate to reduce unnecessary costs and procedures and improve the quality of healthcare.31 Porter and Teisberg32 defined value as health outcomes achieved per dollar spent. Registry monitoring of PROMs, which are the numerator in this critical value formula, allows providers to track patient outcomes over time to determine which interventions produce the best outcomes.22 Therefore, clinical registries play an important role in improving health outcomes and reducing the cost of healthcare.7
Since the Swedish Knee Arthroplasty Register (SKAR) was established 40 years ago, NQRs have been commonplace in Scandinavian countries, Australia, and the United Kingdom.23 Between 2001 and 2014, the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) documented a decline in the financial burden of hip and knee arthroplasty revision in Australia—in comparison with the United States, which did not have a full national registry at the time and showed a revision rate increase.24 The economic benefit of reducing hip and knee arthroplasty revisions in Australia during that period was an estimated $65 million to $143 million.24 Besides having financial benefits, national registries allow early identification of flawed implantation products and methods, leading to a further reduction in the burden associated with recall and future use of such defective implants—including patient harm.
In addition to monitoring existing techniques and devices, registries can also follow new techniques and, compared with publication in clinical journals, more expeditiously provide clinical data for outcome expectations and treatment methods. This timeliness is particularly valuable given that publication of clinical trials with the usual mandatory 2-year follow-up can take 4 years or longer.33,34 For instance, in the expanding field of hip arthroscopy, data from registries in both Sweden and Denmark are being analyzed.35,36 These data are important in new fields such as hip arthroscopy, in which clinical indications and treatment techniques may vary considerably between locations.35 In 2012, the Danish Hip Arthroscopy Registry (DHAR) was started as a web-based prospective registry.36 Between 2012 and December 2014, DHAR added 2000 procedures, which included all hip arthroscopy procedures performed at 11 centers in Denmark.36 DHAR tracks PROM, surgical procedure, operative, and radiologic data.
Increased use of clinical registries has led to use of their data in clinical research. Registry-based randomized controlled trials (RCTs) are lower in cost than other types of research, allow for rapid enrollment of patients, offer larger population sizes and multi-institutional sampling, and can provide a more diverse patient population.19,37 Although nonregistry RCTs remain the gold standard of clinical research, registry RCTs have several advantages given the abilities and structure of registries. Because of resources and cost, nonregistry RCTs are usually limited in the number of examined exposures and typically focus on only 2.6 Registry RCTs, on the other hand, can monitor multiple exposures, typically at minimal cost difference.6 Another disadvantage of nonregistry RCTs is that they are often performed at institutions providing care that might not be indicative of the quality most patients expect, as these institutions might be selected for a specific clinician or specialty service.
Registry RCTs also have their limitations with respect to clinical research. A major one is their lack of validity standards or accepted benchmarks for accuracy, adherence rates, registry completeness, and data collection.37,38 In addition, lack of standardization across national and international registries could produce conflicting data. Another limitation is that data in most registries are not subjected to any third-party checks or independent auditing.9,39 Furthermore, evaluating the impact of registries is difficult because it is difficult to find comparable outcome data on nonregistry patients.40 A final limitation involves the ethics of including registry data in RCTs. Although data are often added to a registry without patient consent, should the same data be used for research without patient consent? Should patients be able to disallow use of their data for research, or require a notification each time their data are used? These issues must be addressed.
Review Limitations
One limitation of this review of clinical Internet-based outcome systems is that it might not have identified comparable systems. In addition, specific costs associated with each system were not addressed, as they depend on PROM licensing fees, total institutional access, other proprietary costs, and other variables. Another limitation, in terms of creating a national or international registry, continues to be Internet access. The Pew Research Center estimated that 84% of US adults used the Internet in 2015.41 Although 84% represents most of the adult population, the other 16% typically is over age 65 years, where only 58% of adults reported using the Internet, or come from lower income households, where access was <75%. For registries in European countries and North America, where Internet usage typically is >70%, this is not a significant problem. However, worldwide, only 47% of the population used the Internet in 2016.42 Internet usage by Asian and Arab states citizens was 41.6% and 41.9%, respectively, and usage by African citizens was only 25.1%. As a significant benefit of registry use is that researchers can obtain larger sample sizes, it is a problem that some populations—elderly people, people of lower socioeconomic standing, people living where the Internet is unavailable—might be underrepresented in registry data.
As mentioned, patient adherence is an ongoing issue for clinical registries. As adherence tends to decrease as more time passes after a patient’s treatment date, it is important to account for and encourage continued patient participation with outcome monitoring. Missing data lessen the validity and accuracy of a registry, increasing the likelihood that certain groups will be underrepresented. Although registry systems can reduce the cost of following PROMs, doing so requires monitoring and following up on issues of patient adherence. In other words, many clinicians will need the help of a research assistant. Makhni and colleagues21 found that adding a research assistant for this task increased survey adherence from 65% to 94% before surgery, from 65% to 72% 6 months after surgery, and from 38% to 56% 12 months after surgery.
Even though studies continue to use clinical data from registries, there is not much research on the impact of these registries on improvement in healthcare. Again, many factors are involved: lack of standardized benchmarks for accuracy and adherence, lack of an accepted method of data auditing and validation, and difficulty evaluating the impact of registries owing to the difficulty obtaining comparable data on nonregistry patients. Registries must adopt accepted forms of standardization in order to allow better comparisons of registries, because comparing data across registries can be useful in determining the strengths and weaknesses of different registries.27,43 As registries support decision making at clinical, institutional, and governmental levels, it is vital that their clinical data be accurate and reliable.38
Conclusion
Rising healthcare costs, and government and third-party pressures are making patient outcomes collection a standard of care. Going forward, orthopedic surgeons must be proactive, and Internet -based registries provide technological advances that facilitate the process.
1. Lubowitz JH, Smith PA. Current concepts in clinical research: web-based, automated, arthroscopic surgery prospective database registry. Arthroscopy. 2012;28(3):425-428.
2. Ayers DC, Bozic KJ. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res. 2013;471(11):3409-3411.
3. Nwachukwu BU, Fields K, Chang B, Nawabi DH, Kelly BT, Ranawat AS. Preoperative outcome scores are predictive of achieving the minimal clinically important difference after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2017;45(3):612-619.
4. Breckenridge K, Bekker HL, Gibbons E, et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. 2015;30(10):1605-1614.
5. Inacio MC, Paxton EW, Dillon MT. Understanding orthopaedic registry studies: a comparison with clinical studies. J Bone Joint Surg Am. 2016;98(1):e3.
6. Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS One. 2017;12(9):e0183667.
7. Physician Consortium for Performance Improvement. National Quality Registry Network. http://www.thepcpi.org/programs-initiatives/national-quality-registry-network/. Accessed October 5, 2017.
8. Hickey GL, Grant SW, Cosgriff R, et al. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44(4):605-614.
9. Pugely AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopaedic surgery: part 2: clinical registry data. J Bone Joint Surg Am. 2015;97(21):1799-1808.
10. American Joint Replacement Registry. http://www.ajrr.net/. Accessed October 5, 2017.
11. CareSense. https://www.caresense.com/. Accessed October 4, 2017.
12. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Quality Payment Program. Merit-Based Incentive Payment System (MIPS): 2017 CMS-Approved Qualified Clinical Data Registries (QCDRs). https://qpp.cms.gov/docs/QPP_2017_CMS_Approved_QCDRs.pdf. Accessed October 9, 2017.
13. Johnson & Johnson. Johnson & Johnson Medical Devices Companies introduce Orthopaedic Episode of Care Approach, leveraging CareAdvantage capabilities to support better clinical outcomes and reduce the cost of care. https://www.jnj.com/media-center/press-releases/johnson-johnson-medical-devices-companies-introduce-orthopaedic-episode-of-care-approach-leveraging-careadvantage-capabilities-to-support-better-clinical-outcomes-and-reduce-the-cost-of-care. Published January 9, 2017. Accessed October 4, 2017.
14. KareOutcomes. http://www.kareoutcomes.com/. Accessed October 4, 2017.
15. Oberd. http://www.oberd.com/. Accessed October 4, 2017.
16. Ortech Systems. http://www.ortechsystems.com/. Accessed October 4, 2017.
17. Socrates. http://www.socratesortho.com/. Accessed October 4, 2017.
18. Surgical Outcomes System. https://www.surgicaloutcomesystem.com/. Accessed October 4, 2017.
19. Delaunay C. Registries in orthopaedics. Orthop Traumatol Surg Res. 2015;101(1 suppl):S69-S75.
20. Bryan S, Davis J, Broesch J, Doyle-Waters MM, Lewis S, McGrail K. Choosing your partner for the PROM: a review of evidence on patient-reported outcome measures for use in primary and community care. Healthc Policy. 2014;10(2):38-51.
21. Makhni EC, Higgins JD, Hamamoto JT, Cole BJ, Romeo AA, Verma NN. Patient compliance with electronic patient reported outcomes following shoulder arthroscopy [published online ahead of print September 25, 2017]. Arthroscopy. doi:10.1016/j.arthro.2017.06.016.
22. Triplet JJ, Momoh E, Kurowicki J, Villarroel LD, Law T, Levy JC. E-mail reminders improve completion rates of patient-reported outcome measures. JSES Open Access. 2017;1:25-28.
23. Arner M. Developing a national quality registry for hand surgery: challenges and opportunities. EFORT Open Rev. 2016;1(4):100-106.
24. Ngongo CJ, Frick KD, Hightower AW, Mathingau FA, Burke H, Breiman RF. The perils of straying from protocol: sampling bias and interviewer effects. PLoS One. 2015;10(2):e0118025.
25. Hammarstedt JE, Redmond JM, Gupta A, Dunne KF, Vemula SP, Domb BG. Survey mode influence on patient-reported outcome scores in orthopaedic surgery: telephone results may be positively biased. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):50-54.
26. Hoher J, Bach T, Munster A, et al. Does the mode of data collection change results in a subjective knee score? Self-administration versus interview. Am J Sports Med. 1997;25(5):642-647.
27. Lacny S, Bohm E, Hawker G, Powell J, Marshall DA. Assessing the comparability of hip arthroplasty registries in order to improve the recording and monitoring of outcome. Bone Joint J. 2016;98-B(4):442-451.
28. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville, MD: National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services; 2017. DHHS Publication 2017-1232. https://www.cdc.gov/nchs/data/hus/hus16.pdf. Published May 2017. Accessed October 9, 2017.
29. Cohen RA, Zammitti EP. Problems paying medical bills among persons under age 65: early release of estimates from the National Health Interview Survey, 2011-June 2016. National Health Interview Survey Early Release Program, Division of Health Interview Statistics, National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. https://www.cdc.gov/nchs/data/nhis/earlyrelease/probs_paying_medical_bills_jan_2011_jun_2016.pdf. Published November 2016. Accessed October 9, 2017.
30. National Center for Health Statistics. National Health Interview Survey. http://www.cdc.gov/nchs/nhis/releases.htm. Accessed October 5, 2017.
31. Karhade AV, Larsen AMG, Cote DJ, Dubois HM, Smith TR. National databases for neurosurgical outcomes research: options, strengths, and limitations [published online ahead of print August 5, 2017]. Neurosurgery. https://doi.org/10.1093/neuros/nyx408.
32. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
33. Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637.
34. Counsell N, Biri D, Fraczek J, Hackshaw A. Publishing interim results of randomised clinical trials in peer-reviewed journals. Clin Trials. 2017;14(1):67-77.
35. Sansone M, Ahldén M, Jonasson P, et al. A Swedish hip arthroscopy registry: demographics and development. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):774-780.
36. Mygind-Klavsen B, Grønbech Nielsen T, Maagaard N, et al. Danish Hip Arthroscopy Registry: an epidemiologic and perioperative description of the first 2000 procedures. J Hip Preserv Surg. 2016;3(2):138-145.
37. Li G, Sajobi TT, Menon BK, et al; 2016 Symposium on Registry-Based Randomized Controlled Trials in Calgary. Registry-based randomized controlled trials—what are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16-24.
38. Bautista MP, Bonilla GA, Mieth KW. Data quality in institutional arthroplasty registries: description of model of validation and report of preliminary results. J Arthroplasty. 2017;32(7):2065-2069.
39. Tevaearai H, Carrel T. Clinical registries: yes, but then appropriately! Eur J Cardiothorac Surg. 2013;44(4):614-615.
40. Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries: final report. Sydney, Australia: ACSQHC; 2016.
41. Perrin A, Duggan M. Americans’ internet access: 2000-2015. Pew Research Center website. http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015/. Published June 26, 2015. Accessed October 5, 2017.
42. Taylor A. 47 percent of the world’s population now use the internet, study says. https://www.washingtonpost.com/news/worldviews/wp/2016/11/22/47-percent-of-the-worlds-population-now-use-the-internet-users-study-says/. Published November 22, 2016. Accessed October 5, 2017.
43. Romero L, Nieuwenhuijse M, Carr A, Sedrakyan A. Review of clinical outcomes-based anchors of minimum clinically important differences in hip and knee registry–based reports and publications. J Bone Joint Surg Am. 2014;96(suppl 1):98-103.
1. Lubowitz JH, Smith PA. Current concepts in clinical research: web-based, automated, arthroscopic surgery prospective database registry. Arthroscopy. 2012;28(3):425-428.
2. Ayers DC, Bozic KJ. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res. 2013;471(11):3409-3411.
3. Nwachukwu BU, Fields K, Chang B, Nawabi DH, Kelly BT, Ranawat AS. Preoperative outcome scores are predictive of achieving the minimal clinically important difference after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2017;45(3):612-619.
4. Breckenridge K, Bekker HL, Gibbons E, et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. 2015;30(10):1605-1614.
5. Inacio MC, Paxton EW, Dillon MT. Understanding orthopaedic registry studies: a comparison with clinical studies. J Bone Joint Surg Am. 2016;98(1):e3.
6. Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS One. 2017;12(9):e0183667.
7. Physician Consortium for Performance Improvement. National Quality Registry Network. http://www.thepcpi.org/programs-initiatives/national-quality-registry-network/. Accessed October 5, 2017.
8. Hickey GL, Grant SW, Cosgriff R, et al. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44(4):605-614.
9. Pugely AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopaedic surgery: part 2: clinical registry data. J Bone Joint Surg Am. 2015;97(21):1799-1808.
10. American Joint Replacement Registry. http://www.ajrr.net/. Accessed October 5, 2017.
11. CareSense. https://www.caresense.com/. Accessed October 4, 2017.
12. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Quality Payment Program. Merit-Based Incentive Payment System (MIPS): 2017 CMS-Approved Qualified Clinical Data Registries (QCDRs). https://qpp.cms.gov/docs/QPP_2017_CMS_Approved_QCDRs.pdf. Accessed October 9, 2017.
13. Johnson & Johnson. Johnson & Johnson Medical Devices Companies introduce Orthopaedic Episode of Care Approach, leveraging CareAdvantage capabilities to support better clinical outcomes and reduce the cost of care. https://www.jnj.com/media-center/press-releases/johnson-johnson-medical-devices-companies-introduce-orthopaedic-episode-of-care-approach-leveraging-careadvantage-capabilities-to-support-better-clinical-outcomes-and-reduce-the-cost-of-care. Published January 9, 2017. Accessed October 4, 2017.
14. KareOutcomes. http://www.kareoutcomes.com/. Accessed October 4, 2017.
15. Oberd. http://www.oberd.com/. Accessed October 4, 2017.
16. Ortech Systems. http://www.ortechsystems.com/. Accessed October 4, 2017.
17. Socrates. http://www.socratesortho.com/. Accessed October 4, 2017.
18. Surgical Outcomes System. https://www.surgicaloutcomesystem.com/. Accessed October 4, 2017.
19. Delaunay C. Registries in orthopaedics. Orthop Traumatol Surg Res. 2015;101(1 suppl):S69-S75.
20. Bryan S, Davis J, Broesch J, Doyle-Waters MM, Lewis S, McGrail K. Choosing your partner for the PROM: a review of evidence on patient-reported outcome measures for use in primary and community care. Healthc Policy. 2014;10(2):38-51.
21. Makhni EC, Higgins JD, Hamamoto JT, Cole BJ, Romeo AA, Verma NN. Patient compliance with electronic patient reported outcomes following shoulder arthroscopy [published online ahead of print September 25, 2017]. Arthroscopy. doi:10.1016/j.arthro.2017.06.016.
22. Triplet JJ, Momoh E, Kurowicki J, Villarroel LD, Law T, Levy JC. E-mail reminders improve completion rates of patient-reported outcome measures. JSES Open Access. 2017;1:25-28.
23. Arner M. Developing a national quality registry for hand surgery: challenges and opportunities. EFORT Open Rev. 2016;1(4):100-106.
24. Ngongo CJ, Frick KD, Hightower AW, Mathingau FA, Burke H, Breiman RF. The perils of straying from protocol: sampling bias and interviewer effects. PLoS One. 2015;10(2):e0118025.
25. Hammarstedt JE, Redmond JM, Gupta A, Dunne KF, Vemula SP, Domb BG. Survey mode influence on patient-reported outcome scores in orthopaedic surgery: telephone results may be positively biased. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):50-54.
26. Hoher J, Bach T, Munster A, et al. Does the mode of data collection change results in a subjective knee score? Self-administration versus interview. Am J Sports Med. 1997;25(5):642-647.
27. Lacny S, Bohm E, Hawker G, Powell J, Marshall DA. Assessing the comparability of hip arthroplasty registries in order to improve the recording and monitoring of outcome. Bone Joint J. 2016;98-B(4):442-451.
28. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville, MD: National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services; 2017. DHHS Publication 2017-1232. https://www.cdc.gov/nchs/data/hus/hus16.pdf. Published May 2017. Accessed October 9, 2017.
29. Cohen RA, Zammitti EP. Problems paying medical bills among persons under age 65: early release of estimates from the National Health Interview Survey, 2011-June 2016. National Health Interview Survey Early Release Program, Division of Health Interview Statistics, National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. https://www.cdc.gov/nchs/data/nhis/earlyrelease/probs_paying_medical_bills_jan_2011_jun_2016.pdf. Published November 2016. Accessed October 9, 2017.
30. National Center for Health Statistics. National Health Interview Survey. http://www.cdc.gov/nchs/nhis/releases.htm. Accessed October 5, 2017.
31. Karhade AV, Larsen AMG, Cote DJ, Dubois HM, Smith TR. National databases for neurosurgical outcomes research: options, strengths, and limitations [published online ahead of print August 5, 2017]. Neurosurgery. https://doi.org/10.1093/neuros/nyx408.
32. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
33. Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637.
34. Counsell N, Biri D, Fraczek J, Hackshaw A. Publishing interim results of randomised clinical trials in peer-reviewed journals. Clin Trials. 2017;14(1):67-77.
35. Sansone M, Ahldén M, Jonasson P, et al. A Swedish hip arthroscopy registry: demographics and development. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):774-780.
36. Mygind-Klavsen B, Grønbech Nielsen T, Maagaard N, et al. Danish Hip Arthroscopy Registry: an epidemiologic and perioperative description of the first 2000 procedures. J Hip Preserv Surg. 2016;3(2):138-145.
37. Li G, Sajobi TT, Menon BK, et al; 2016 Symposium on Registry-Based Randomized Controlled Trials in Calgary. Registry-based randomized controlled trials—what are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16-24.
38. Bautista MP, Bonilla GA, Mieth KW. Data quality in institutional arthroplasty registries: description of model of validation and report of preliminary results. J Arthroplasty. 2017;32(7):2065-2069.
39. Tevaearai H, Carrel T. Clinical registries: yes, but then appropriately! Eur J Cardiothorac Surg. 2013;44(4):614-615.
40. Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries: final report. Sydney, Australia: ACSQHC; 2016.
41. Perrin A, Duggan M. Americans’ internet access: 2000-2015. Pew Research Center website. http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015/. Published June 26, 2015. Accessed October 5, 2017.
42. Taylor A. 47 percent of the world’s population now use the internet, study says. https://www.washingtonpost.com/news/worldviews/wp/2016/11/22/47-percent-of-the-worlds-population-now-use-the-internet-users-study-says/. Published November 22, 2016. Accessed October 5, 2017.
43. Romero L, Nieuwenhuijse M, Carr A, Sedrakyan A. Review of clinical outcomes-based anchors of minimum clinically important differences in hip and knee registry–based reports and publications. J Bone Joint Surg Am. 2014;96(suppl 1):98-103.
Implementing Patient-Reported Outcome Measures in Your Practice: Pearls and Pitfalls
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
Superior Capsular Reconstruction: Clinical Outcomes After Minimum 2-Year Follow-Up
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
Boldly Going (Where No Journal Has Gone Before)
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
Effects of Platelet-Rich Plasma and Indomethacin on Biomechanics of Rotator Cuff Repair
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
Improving Care and Reducing Length of Stay in Patients Undergoing Total Knee Replacement
Many improvements in health care today involve care coordination across the entire health care system. Active management of an orthopedic surgery service from a system perspective allows for improvements that can favorably impact readmissions and length of stay (LOS) for patients.1 The following is an example of a systemwide process improvement in total knee replacement (TKR) surgery that dramatically decreased 30-day readmissions and shortened the LOS during a 12-month period.\
Background
The VA is the largest integrated health care system in the U.S. VA hospitals use the VA Surgical Quality Improvement Program (VASQIP) to monitor surgical services. Initially known as the National Surgery Quality Improvement Program (NSQIP), the program began in 1994 to help provide reliable, valid information on patient presurgical factors, processes of care during surgery, and 30-day morbidity and mortality rates in VA hospitals.2 Since its inception, NSQIP has spread to the private sector and is now widely used throughout the U.S.
Using on-site data acquisition by specially trained and dedicated registered nurses, information on each surgical case is input into a quality program. Quarterly reports are distributed to each hospital, and a comparison of mortality, LOS, 30-day readmissions to the hospital, and other data are analyzed and presented by quarter and rolling 12-month time frames. Use of VASQIP data allows improvement of the structures and processes of care throughout the VA, providing safer surgery for veterans.
At the Phoenix VA Health Care System (PVAHCS) in Arizona, the third quarter 2014 report showed the rolling 12-month average LOS for orthopedic TKR patients was 3.5 days and corresponding 30-day readmissions were 7.9%. Using a systems improvement approach, the authors set a goal of reducing these metrics by 10%.
The orthopedic service engaged members of the hospitalist, anesthesia, physical therapy (PT), nursing, social work, primary care, and pharmacy services, as well as hospital administration. Twelve months later, the LOS for TKR patients declined 20% to 2.8 days. Corresponding 30-day readmissions declined for the patients with knee replacement to 3.4%—a 57% reduction in 1 year. Mortality for these 177 cases was zero.
To accomplish these improvements, the authors divided the surgical procedure into preoperative, perioperative, and postoperative time frames and looked at process improvement during each of these periods. The following is a summary of the various processes that the authors feel contributed to the reduced LOS and 30-day readmission rate. Although some of these interventions were in place before the study period, all the processes were standardized for TKRs through surgeon consensus, and each of the surgeons adopted all the processes during the study period.
Preoperative Processes
In the VA primary care-based model orthopedic surgery is accessed through a consult process in the electronic health record. The orthopedic surgery service reviews each new consult and makes recommendations for optimization at the time the consult was received. This process was used to work closely with primary care providers to preoperatively prepare patients. The orthopedic surgery service advocates smoking cessation, substance abuse treatment, weight loss with an ideal body mass index of ≤ 35, and diabetes mellitus (DM) management with a ≤ 7 hemoglobin A1c value.3-7
This management did not result in fewer patients receiving TKR. In fact, the volume of TKR patients increased by 8% over the study period. Although part of this increase could have been due to increased scheduling efficiency, the orthopedic surgery service worked closely with primary care, nutrition, and medicine services to optimize these patients so they could be placed on the schedule for surgery.
Preoperative Education
Physical therapy and the orthopedic preprocedure clinic provided preoperative education to patients, covering preoperative chlorhexidine body washes, home safety, use of a walker, anticipated LOS, use of ambulatory sequential compressive devices, use of a knee cooling device, as well as PT protocols during hospitalization.8 This helped increase postoperative patient adherence and helped patients anticipate an appropriate LOS. Health care providers worked with patients to understand their home environment and plan for caregivers to assist them in the immediate postoperative period.
Intraoperative Processes
Reducing Blood Loss
The orthopedic surgery service reviewed literature related to the efficacy and safety of tranexamic acid. Based on the literature, the orthopedic surgery service arrived at a consensus agreement to implement a topical tranexamic acid dose of 3 g/100 cc saline for each TKR. Presentation of the pertinent literature to the pharmacy service allowed placement of this medication on the formulary for intraoperative use in the TKR cases.
Specific processes were implemented that involved the orthopedic service ordering tranexamic acid in advance for each patient, pharmacy mixing the solution and having it ready in a timely manner, and the operating room sending a messenger to the pharmacy to pick up a sterile container of the tranexamic acid/saline solution. Postoperative blood loss and transfusions decreased. Less anemia contributed to better performance and less fatigue in PT, which helped move patients down a pathway for quicker discharge.9,10
DVT Mechanical Prophylaxis
The orthopedic surgery service was concerned about adherence with stationary sequential compressive devices for mechanical thromboembolic prophylaxis. Patients had to remove them for PT, ambulation in the halls, and visiting the restroom, and then nurses had to replace them. A literature review examined a mobile compressive device that could be maintained during ambulation, and a demonstration for the orthopedic surgery service was arranged. The orthopedic service decided to change to the newer device, and the mobile compression device was presented to the PVAHCS Therapeutics Committee. Subsequently the new device was implemented after the appropriate in-service of the various clinic, PT, ward, surgery, preoperative, and postoperative personnel.11 The device was initiated in the holding area prior to surgery, continued throughout the hospitalization, and taken home by the patient for 2 weeks of use following surgery. Patients were instructed to return the device to clinic at their 2-week follow-up appointment.
Infection Control
A dilute betadine lavage was instituted for each surgical case, using the pulsatile lavage followed by a lactated Ringer solution rinse prior to TKR implantation. Additionally, the wound was lavaged prior to closure with this dilute betadine solution.12
Pain Control
Immediately before surgery, patients received oral morphine sulfate and celecoxib. A local 2% lidocaine with epinephrine injection was used at the surgical incision and joint after the skin prep and immediately prior to the skin incision. Patients received a mixture of ropivicaine .5%/20 mL, morphine sulfate 10 mg, and toradol 30 mg at the capsular region prior to implantation of the total knee prosthesis. At the end of the procedure, an additional 20 mL of 2% lidocaine was injected into the joint once the capsule was closed. This improved postoperative pain, decreased postoperative opioid dosing, and allowed for earlier ambulation with PT.13
PostOperative Processes
Deep Vein Thrombosis (DVT) Chemoprophylaxis
Once the chest physician guidelines-approved stand-alone mobile compressive devices was implemented, orthopedic surgery service revisited the chemoprophylaxis for routine low-risk patients. Use of subcutaneously daily injections of 2.5 mg fondiparinux was switched to 81 mg enteric-coated aspirin administered orally twice daily. The authors believe this further reduced the postoperative bleeding and transfusion risks. There was not an increase in DVT or pulmonary embolism complications.14,15
Physical Therapy
Partnering with PT, a 2-day LOS protocol was established. Patients were introduced to this protocol in a preoperative PT teaching class, and it was reinforced during the hospital stay. Patients who had earlier cases in the day were seen by PT the day of surgery when staffing and scheduling permitted. Early ambulation contributed significantly to earlier discharge for patients.16 Early ambulation also has been shown to decrease thromboembolic complications in orthopedic total joint patients.
Pain and Nausea Management
Parenteral narcotics were avoided, and oral narcotics were implemented with a graduated dosing based on a 10-point pain scale
Use of a postoperative cooling device that circulated cool water through a pad over the patient’s knee was instituted to assist with pain control. The patient received instruction on this device at the preoperative education sessions and was given the device to continue at home postdischarge.
Hospitalist Comanagement
Comanagement of orthopedic patients with hospitalists has become a standard practice nationally. The orthopedic surgery service works closely with the hospitalist team who see each total joint patient on postoperative admission to the ward. The orthopedic team handles all aspects of PT, wound management, pain control, and DVT prophylaxis. The hospitalist focuses on the remainder of comorbid conditions such as DM, chronic obstructive pulmonary disease, and underlying cardiac conditions.
The American Society of Anesthesiologists (ASA) average score was 2.8 for these procedures. Despite comprehensive preoperative screening, older patients with more comorbidities (higher ASA score) are more prone to emerging complications.17 Integration of the hospitalist team into the care of every orthopedic total joint patient facilitates prompt recognition and mitigation of these complications as they occur, directly reducing overall severity and LOS and allowing safe recovery from the surgical procedure.18,19
Conclusion
At the start of this system improvement, the previous 12-month data showed 164 knee replacements with a 4.9-day VA national LOS and 3.5- day PVAHCS LOS. At the end of the 12-month system improvement, the VA national LOS for TKR was 4.8 days, and at PVAHCS it was 2.8 days.
The 30-day readmission rate was 8.4% nationally and 7.9% at PVAHCS. After the system improvements, the national 30-day readmission rate was 7.1%, while the PVAHCS rate dropped to less than half the national rate: 3.4%.
It is important to note, that the improvements in the aforementioned multiple processes could not have been possible without a dedicated effort from the multiple stakeholders involved. Hospitalists, primary care, PT, pharmacy, operating room staff, anesthesia, preprocedure staff, floor nurses, the Commodities and Therapeutics Committee, and administration all partnered with the orthopedic surgery service to produce the improvements in LOS and corresponding reduction in 30-day readmissions.
These data suggest that there does not need to be an inherent tradeoff between LOS and 30-day readmissions. Rather, both measures can be managed independently to produce improvements across the service. A team approach to process improvement can allow for increased efficiency while providing safer care for patients.
1. Dundon JM, Bosco J, Slover J, Yu S, Sayeed Y, Iorio R. Improvement in total joint replacement quality metrics, year one versus year three of the bundled payments for care improvement initiative. J Bone Joint Surg Am. 2016;98(23):1949-1953.
2. Itani KM. Fifteen years of the National Surgical Quality Improvement Program in review. Am J Surg. 2009;198(suppl 5):S9-S18.
3. Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J. 2016;98-B(3):334-340.
4. Heller S, Rezapoor M, Parvizi J. Minimising the risk of infection: a peri-operative checklist. Bone Joint J. 2016;98-B(1)(suppl A):18-22.
5. Thornqvist C, Gislason GH, Køber L, Jensen PF, Torp-Pedersen C, Andersson C. Body mass index and risk of perioperative cardiovascular adverse events and mortality in 34,744 Danish patients undergoing hip or knee replacement. Acta Orthop. 2014;85(5):456-462.
6. Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am. 2013;95(9):808-814.
7. Akhavan S, Nguyen LC, Chan V, Saleh J, Bozic KJ. Impact of smoking cessation counseling prior to total joint arthroplasty. Orthopedics. 2017;40(2):e323-e328.
8. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1820-1826.
9. Goyal N, Chen DB, Harris IA, Rowden NJ, Kirsh G, MacDessi SJ. Intravenous vs intra-articular tranexamic acid in total knee arthroplasty: a randomized, double-blind trial. J Arthroplasty. 2017;32(1):28-32.
10. Phan DL, Ani F, Schwarzkopf R. Cost analysis of tranexamic acid in anemic total joint arthroplasty patients. J Arthroplasty. 2016;31(3):579-582.
11. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
12. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
13. Fang R, Liu Z, Alijiang A, et al. Efficacy of intra-articular local anesthetics in total knee arthroplasty. Orthopedics. 2015;38(7):e573-e581.
14. Odeh K, Doran J, Yu S, Bolz N, Bosco J, Iorio R. Risk-stratified venous thromboembolism prophylaxis after total joint arthroplasty: aspirin and sequential pneumatic compression devices vs aggressive chemoprophylaxis. J Arthroplasty. 2016;31(suppl 9):78-82.
15. Parvizi J, Huang R, Restrepo C, et al. Low-dose aspirin is effective chemoprophylaxis against clinically important venous thromboembolism following total joint arthroplasty: a preliminary analysis. J Bone Joint Surg Am. 2017;99(2):91-98.
16. Robertson NB, Warganich T, Ghazarossian J, Khatod M. Implementation of an accelerated rehabilitation protocol for total joint arthroplasty in the managed care setting: the experience of one institution. Adv Orthop Surg. 2015;(2015):387197.
17. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
18. Parry MC, Smith AJ, Blom AW. Early death following primary total knee arthroplasty. J Bone Joint Surg Am. 2011;93(10):948-953.
19. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
Many improvements in health care today involve care coordination across the entire health care system. Active management of an orthopedic surgery service from a system perspective allows for improvements that can favorably impact readmissions and length of stay (LOS) for patients.1 The following is an example of a systemwide process improvement in total knee replacement (TKR) surgery that dramatically decreased 30-day readmissions and shortened the LOS during a 12-month period.\
Background
The VA is the largest integrated health care system in the U.S. VA hospitals use the VA Surgical Quality Improvement Program (VASQIP) to monitor surgical services. Initially known as the National Surgery Quality Improvement Program (NSQIP), the program began in 1994 to help provide reliable, valid information on patient presurgical factors, processes of care during surgery, and 30-day morbidity and mortality rates in VA hospitals.2 Since its inception, NSQIP has spread to the private sector and is now widely used throughout the U.S.
Using on-site data acquisition by specially trained and dedicated registered nurses, information on each surgical case is input into a quality program. Quarterly reports are distributed to each hospital, and a comparison of mortality, LOS, 30-day readmissions to the hospital, and other data are analyzed and presented by quarter and rolling 12-month time frames. Use of VASQIP data allows improvement of the structures and processes of care throughout the VA, providing safer surgery for veterans.
At the Phoenix VA Health Care System (PVAHCS) in Arizona, the third quarter 2014 report showed the rolling 12-month average LOS for orthopedic TKR patients was 3.5 days and corresponding 30-day readmissions were 7.9%. Using a systems improvement approach, the authors set a goal of reducing these metrics by 10%.
The orthopedic service engaged members of the hospitalist, anesthesia, physical therapy (PT), nursing, social work, primary care, and pharmacy services, as well as hospital administration. Twelve months later, the LOS for TKR patients declined 20% to 2.8 days. Corresponding 30-day readmissions declined for the patients with knee replacement to 3.4%—a 57% reduction in 1 year. Mortality for these 177 cases was zero.
To accomplish these improvements, the authors divided the surgical procedure into preoperative, perioperative, and postoperative time frames and looked at process improvement during each of these periods. The following is a summary of the various processes that the authors feel contributed to the reduced LOS and 30-day readmission rate. Although some of these interventions were in place before the study period, all the processes were standardized for TKRs through surgeon consensus, and each of the surgeons adopted all the processes during the study period.
Preoperative Processes
In the VA primary care-based model orthopedic surgery is accessed through a consult process in the electronic health record. The orthopedic surgery service reviews each new consult and makes recommendations for optimization at the time the consult was received. This process was used to work closely with primary care providers to preoperatively prepare patients. The orthopedic surgery service advocates smoking cessation, substance abuse treatment, weight loss with an ideal body mass index of ≤ 35, and diabetes mellitus (DM) management with a ≤ 7 hemoglobin A1c value.3-7
This management did not result in fewer patients receiving TKR. In fact, the volume of TKR patients increased by 8% over the study period. Although part of this increase could have been due to increased scheduling efficiency, the orthopedic surgery service worked closely with primary care, nutrition, and medicine services to optimize these patients so they could be placed on the schedule for surgery.
Preoperative Education
Physical therapy and the orthopedic preprocedure clinic provided preoperative education to patients, covering preoperative chlorhexidine body washes, home safety, use of a walker, anticipated LOS, use of ambulatory sequential compressive devices, use of a knee cooling device, as well as PT protocols during hospitalization.8 This helped increase postoperative patient adherence and helped patients anticipate an appropriate LOS. Health care providers worked with patients to understand their home environment and plan for caregivers to assist them in the immediate postoperative period.
Intraoperative Processes
Reducing Blood Loss
The orthopedic surgery service reviewed literature related to the efficacy and safety of tranexamic acid. Based on the literature, the orthopedic surgery service arrived at a consensus agreement to implement a topical tranexamic acid dose of 3 g/100 cc saline for each TKR. Presentation of the pertinent literature to the pharmacy service allowed placement of this medication on the formulary for intraoperative use in the TKR cases.
Specific processes were implemented that involved the orthopedic service ordering tranexamic acid in advance for each patient, pharmacy mixing the solution and having it ready in a timely manner, and the operating room sending a messenger to the pharmacy to pick up a sterile container of the tranexamic acid/saline solution. Postoperative blood loss and transfusions decreased. Less anemia contributed to better performance and less fatigue in PT, which helped move patients down a pathway for quicker discharge.9,10
DVT Mechanical Prophylaxis
The orthopedic surgery service was concerned about adherence with stationary sequential compressive devices for mechanical thromboembolic prophylaxis. Patients had to remove them for PT, ambulation in the halls, and visiting the restroom, and then nurses had to replace them. A literature review examined a mobile compressive device that could be maintained during ambulation, and a demonstration for the orthopedic surgery service was arranged. The orthopedic service decided to change to the newer device, and the mobile compression device was presented to the PVAHCS Therapeutics Committee. Subsequently the new device was implemented after the appropriate in-service of the various clinic, PT, ward, surgery, preoperative, and postoperative personnel.11 The device was initiated in the holding area prior to surgery, continued throughout the hospitalization, and taken home by the patient for 2 weeks of use following surgery. Patients were instructed to return the device to clinic at their 2-week follow-up appointment.
Infection Control
A dilute betadine lavage was instituted for each surgical case, using the pulsatile lavage followed by a lactated Ringer solution rinse prior to TKR implantation. Additionally, the wound was lavaged prior to closure with this dilute betadine solution.12
Pain Control
Immediately before surgery, patients received oral morphine sulfate and celecoxib. A local 2% lidocaine with epinephrine injection was used at the surgical incision and joint after the skin prep and immediately prior to the skin incision. Patients received a mixture of ropivicaine .5%/20 mL, morphine sulfate 10 mg, and toradol 30 mg at the capsular region prior to implantation of the total knee prosthesis. At the end of the procedure, an additional 20 mL of 2% lidocaine was injected into the joint once the capsule was closed. This improved postoperative pain, decreased postoperative opioid dosing, and allowed for earlier ambulation with PT.13
PostOperative Processes
Deep Vein Thrombosis (DVT) Chemoprophylaxis
Once the chest physician guidelines-approved stand-alone mobile compressive devices was implemented, orthopedic surgery service revisited the chemoprophylaxis for routine low-risk patients. Use of subcutaneously daily injections of 2.5 mg fondiparinux was switched to 81 mg enteric-coated aspirin administered orally twice daily. The authors believe this further reduced the postoperative bleeding and transfusion risks. There was not an increase in DVT or pulmonary embolism complications.14,15
Physical Therapy
Partnering with PT, a 2-day LOS protocol was established. Patients were introduced to this protocol in a preoperative PT teaching class, and it was reinforced during the hospital stay. Patients who had earlier cases in the day were seen by PT the day of surgery when staffing and scheduling permitted. Early ambulation contributed significantly to earlier discharge for patients.16 Early ambulation also has been shown to decrease thromboembolic complications in orthopedic total joint patients.
Pain and Nausea Management
Parenteral narcotics were avoided, and oral narcotics were implemented with a graduated dosing based on a 10-point pain scale
Use of a postoperative cooling device that circulated cool water through a pad over the patient’s knee was instituted to assist with pain control. The patient received instruction on this device at the preoperative education sessions and was given the device to continue at home postdischarge.
Hospitalist Comanagement
Comanagement of orthopedic patients with hospitalists has become a standard practice nationally. The orthopedic surgery service works closely with the hospitalist team who see each total joint patient on postoperative admission to the ward. The orthopedic team handles all aspects of PT, wound management, pain control, and DVT prophylaxis. The hospitalist focuses on the remainder of comorbid conditions such as DM, chronic obstructive pulmonary disease, and underlying cardiac conditions.
The American Society of Anesthesiologists (ASA) average score was 2.8 for these procedures. Despite comprehensive preoperative screening, older patients with more comorbidities (higher ASA score) are more prone to emerging complications.17 Integration of the hospitalist team into the care of every orthopedic total joint patient facilitates prompt recognition and mitigation of these complications as they occur, directly reducing overall severity and LOS and allowing safe recovery from the surgical procedure.18,19
Conclusion
At the start of this system improvement, the previous 12-month data showed 164 knee replacements with a 4.9-day VA national LOS and 3.5- day PVAHCS LOS. At the end of the 12-month system improvement, the VA national LOS for TKR was 4.8 days, and at PVAHCS it was 2.8 days.
The 30-day readmission rate was 8.4% nationally and 7.9% at PVAHCS. After the system improvements, the national 30-day readmission rate was 7.1%, while the PVAHCS rate dropped to less than half the national rate: 3.4%.
It is important to note, that the improvements in the aforementioned multiple processes could not have been possible without a dedicated effort from the multiple stakeholders involved. Hospitalists, primary care, PT, pharmacy, operating room staff, anesthesia, preprocedure staff, floor nurses, the Commodities and Therapeutics Committee, and administration all partnered with the orthopedic surgery service to produce the improvements in LOS and corresponding reduction in 30-day readmissions.
These data suggest that there does not need to be an inherent tradeoff between LOS and 30-day readmissions. Rather, both measures can be managed independently to produce improvements across the service. A team approach to process improvement can allow for increased efficiency while providing safer care for patients.
Many improvements in health care today involve care coordination across the entire health care system. Active management of an orthopedic surgery service from a system perspective allows for improvements that can favorably impact readmissions and length of stay (LOS) for patients.1 The following is an example of a systemwide process improvement in total knee replacement (TKR) surgery that dramatically decreased 30-day readmissions and shortened the LOS during a 12-month period.\
Background
The VA is the largest integrated health care system in the U.S. VA hospitals use the VA Surgical Quality Improvement Program (VASQIP) to monitor surgical services. Initially known as the National Surgery Quality Improvement Program (NSQIP), the program began in 1994 to help provide reliable, valid information on patient presurgical factors, processes of care during surgery, and 30-day morbidity and mortality rates in VA hospitals.2 Since its inception, NSQIP has spread to the private sector and is now widely used throughout the U.S.
Using on-site data acquisition by specially trained and dedicated registered nurses, information on each surgical case is input into a quality program. Quarterly reports are distributed to each hospital, and a comparison of mortality, LOS, 30-day readmissions to the hospital, and other data are analyzed and presented by quarter and rolling 12-month time frames. Use of VASQIP data allows improvement of the structures and processes of care throughout the VA, providing safer surgery for veterans.
At the Phoenix VA Health Care System (PVAHCS) in Arizona, the third quarter 2014 report showed the rolling 12-month average LOS for orthopedic TKR patients was 3.5 days and corresponding 30-day readmissions were 7.9%. Using a systems improvement approach, the authors set a goal of reducing these metrics by 10%.
The orthopedic service engaged members of the hospitalist, anesthesia, physical therapy (PT), nursing, social work, primary care, and pharmacy services, as well as hospital administration. Twelve months later, the LOS for TKR patients declined 20% to 2.8 days. Corresponding 30-day readmissions declined for the patients with knee replacement to 3.4%—a 57% reduction in 1 year. Mortality for these 177 cases was zero.
To accomplish these improvements, the authors divided the surgical procedure into preoperative, perioperative, and postoperative time frames and looked at process improvement during each of these periods. The following is a summary of the various processes that the authors feel contributed to the reduced LOS and 30-day readmission rate. Although some of these interventions were in place before the study period, all the processes were standardized for TKRs through surgeon consensus, and each of the surgeons adopted all the processes during the study period.
Preoperative Processes
In the VA primary care-based model orthopedic surgery is accessed through a consult process in the electronic health record. The orthopedic surgery service reviews each new consult and makes recommendations for optimization at the time the consult was received. This process was used to work closely with primary care providers to preoperatively prepare patients. The orthopedic surgery service advocates smoking cessation, substance abuse treatment, weight loss with an ideal body mass index of ≤ 35, and diabetes mellitus (DM) management with a ≤ 7 hemoglobin A1c value.3-7
This management did not result in fewer patients receiving TKR. In fact, the volume of TKR patients increased by 8% over the study period. Although part of this increase could have been due to increased scheduling efficiency, the orthopedic surgery service worked closely with primary care, nutrition, and medicine services to optimize these patients so they could be placed on the schedule for surgery.
Preoperative Education
Physical therapy and the orthopedic preprocedure clinic provided preoperative education to patients, covering preoperative chlorhexidine body washes, home safety, use of a walker, anticipated LOS, use of ambulatory sequential compressive devices, use of a knee cooling device, as well as PT protocols during hospitalization.8 This helped increase postoperative patient adherence and helped patients anticipate an appropriate LOS. Health care providers worked with patients to understand their home environment and plan for caregivers to assist them in the immediate postoperative period.
Intraoperative Processes
Reducing Blood Loss
The orthopedic surgery service reviewed literature related to the efficacy and safety of tranexamic acid. Based on the literature, the orthopedic surgery service arrived at a consensus agreement to implement a topical tranexamic acid dose of 3 g/100 cc saline for each TKR. Presentation of the pertinent literature to the pharmacy service allowed placement of this medication on the formulary for intraoperative use in the TKR cases.
Specific processes were implemented that involved the orthopedic service ordering tranexamic acid in advance for each patient, pharmacy mixing the solution and having it ready in a timely manner, and the operating room sending a messenger to the pharmacy to pick up a sterile container of the tranexamic acid/saline solution. Postoperative blood loss and transfusions decreased. Less anemia contributed to better performance and less fatigue in PT, which helped move patients down a pathway for quicker discharge.9,10
DVT Mechanical Prophylaxis
The orthopedic surgery service was concerned about adherence with stationary sequential compressive devices for mechanical thromboembolic prophylaxis. Patients had to remove them for PT, ambulation in the halls, and visiting the restroom, and then nurses had to replace them. A literature review examined a mobile compressive device that could be maintained during ambulation, and a demonstration for the orthopedic surgery service was arranged. The orthopedic service decided to change to the newer device, and the mobile compression device was presented to the PVAHCS Therapeutics Committee. Subsequently the new device was implemented after the appropriate in-service of the various clinic, PT, ward, surgery, preoperative, and postoperative personnel.11 The device was initiated in the holding area prior to surgery, continued throughout the hospitalization, and taken home by the patient for 2 weeks of use following surgery. Patients were instructed to return the device to clinic at their 2-week follow-up appointment.
Infection Control
A dilute betadine lavage was instituted for each surgical case, using the pulsatile lavage followed by a lactated Ringer solution rinse prior to TKR implantation. Additionally, the wound was lavaged prior to closure with this dilute betadine solution.12
Pain Control
Immediately before surgery, patients received oral morphine sulfate and celecoxib. A local 2% lidocaine with epinephrine injection was used at the surgical incision and joint after the skin prep and immediately prior to the skin incision. Patients received a mixture of ropivicaine .5%/20 mL, morphine sulfate 10 mg, and toradol 30 mg at the capsular region prior to implantation of the total knee prosthesis. At the end of the procedure, an additional 20 mL of 2% lidocaine was injected into the joint once the capsule was closed. This improved postoperative pain, decreased postoperative opioid dosing, and allowed for earlier ambulation with PT.13
PostOperative Processes
Deep Vein Thrombosis (DVT) Chemoprophylaxis
Once the chest physician guidelines-approved stand-alone mobile compressive devices was implemented, orthopedic surgery service revisited the chemoprophylaxis for routine low-risk patients. Use of subcutaneously daily injections of 2.5 mg fondiparinux was switched to 81 mg enteric-coated aspirin administered orally twice daily. The authors believe this further reduced the postoperative bleeding and transfusion risks. There was not an increase in DVT or pulmonary embolism complications.14,15
Physical Therapy
Partnering with PT, a 2-day LOS protocol was established. Patients were introduced to this protocol in a preoperative PT teaching class, and it was reinforced during the hospital stay. Patients who had earlier cases in the day were seen by PT the day of surgery when staffing and scheduling permitted. Early ambulation contributed significantly to earlier discharge for patients.16 Early ambulation also has been shown to decrease thromboembolic complications in orthopedic total joint patients.
Pain and Nausea Management
Parenteral narcotics were avoided, and oral narcotics were implemented with a graduated dosing based on a 10-point pain scale
Use of a postoperative cooling device that circulated cool water through a pad over the patient’s knee was instituted to assist with pain control. The patient received instruction on this device at the preoperative education sessions and was given the device to continue at home postdischarge.
Hospitalist Comanagement
Comanagement of orthopedic patients with hospitalists has become a standard practice nationally. The orthopedic surgery service works closely with the hospitalist team who see each total joint patient on postoperative admission to the ward. The orthopedic team handles all aspects of PT, wound management, pain control, and DVT prophylaxis. The hospitalist focuses on the remainder of comorbid conditions such as DM, chronic obstructive pulmonary disease, and underlying cardiac conditions.
The American Society of Anesthesiologists (ASA) average score was 2.8 for these procedures. Despite comprehensive preoperative screening, older patients with more comorbidities (higher ASA score) are more prone to emerging complications.17 Integration of the hospitalist team into the care of every orthopedic total joint patient facilitates prompt recognition and mitigation of these complications as they occur, directly reducing overall severity and LOS and allowing safe recovery from the surgical procedure.18,19
Conclusion
At the start of this system improvement, the previous 12-month data showed 164 knee replacements with a 4.9-day VA national LOS and 3.5- day PVAHCS LOS. At the end of the 12-month system improvement, the VA national LOS for TKR was 4.8 days, and at PVAHCS it was 2.8 days.
The 30-day readmission rate was 8.4% nationally and 7.9% at PVAHCS. After the system improvements, the national 30-day readmission rate was 7.1%, while the PVAHCS rate dropped to less than half the national rate: 3.4%.
It is important to note, that the improvements in the aforementioned multiple processes could not have been possible without a dedicated effort from the multiple stakeholders involved. Hospitalists, primary care, PT, pharmacy, operating room staff, anesthesia, preprocedure staff, floor nurses, the Commodities and Therapeutics Committee, and administration all partnered with the orthopedic surgery service to produce the improvements in LOS and corresponding reduction in 30-day readmissions.
These data suggest that there does not need to be an inherent tradeoff between LOS and 30-day readmissions. Rather, both measures can be managed independently to produce improvements across the service. A team approach to process improvement can allow for increased efficiency while providing safer care for patients.
1. Dundon JM, Bosco J, Slover J, Yu S, Sayeed Y, Iorio R. Improvement in total joint replacement quality metrics, year one versus year three of the bundled payments for care improvement initiative. J Bone Joint Surg Am. 2016;98(23):1949-1953.
2. Itani KM. Fifteen years of the National Surgical Quality Improvement Program in review. Am J Surg. 2009;198(suppl 5):S9-S18.
3. Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J. 2016;98-B(3):334-340.
4. Heller S, Rezapoor M, Parvizi J. Minimising the risk of infection: a peri-operative checklist. Bone Joint J. 2016;98-B(1)(suppl A):18-22.
5. Thornqvist C, Gislason GH, Køber L, Jensen PF, Torp-Pedersen C, Andersson C. Body mass index and risk of perioperative cardiovascular adverse events and mortality in 34,744 Danish patients undergoing hip or knee replacement. Acta Orthop. 2014;85(5):456-462.
6. Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am. 2013;95(9):808-814.
7. Akhavan S, Nguyen LC, Chan V, Saleh J, Bozic KJ. Impact of smoking cessation counseling prior to total joint arthroplasty. Orthopedics. 2017;40(2):e323-e328.
8. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1820-1826.
9. Goyal N, Chen DB, Harris IA, Rowden NJ, Kirsh G, MacDessi SJ. Intravenous vs intra-articular tranexamic acid in total knee arthroplasty: a randomized, double-blind trial. J Arthroplasty. 2017;32(1):28-32.
10. Phan DL, Ani F, Schwarzkopf R. Cost analysis of tranexamic acid in anemic total joint arthroplasty patients. J Arthroplasty. 2016;31(3):579-582.
11. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
12. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
13. Fang R, Liu Z, Alijiang A, et al. Efficacy of intra-articular local anesthetics in total knee arthroplasty. Orthopedics. 2015;38(7):e573-e581.
14. Odeh K, Doran J, Yu S, Bolz N, Bosco J, Iorio R. Risk-stratified venous thromboembolism prophylaxis after total joint arthroplasty: aspirin and sequential pneumatic compression devices vs aggressive chemoprophylaxis. J Arthroplasty. 2016;31(suppl 9):78-82.
15. Parvizi J, Huang R, Restrepo C, et al. Low-dose aspirin is effective chemoprophylaxis against clinically important venous thromboembolism following total joint arthroplasty: a preliminary analysis. J Bone Joint Surg Am. 2017;99(2):91-98.
16. Robertson NB, Warganich T, Ghazarossian J, Khatod M. Implementation of an accelerated rehabilitation protocol for total joint arthroplasty in the managed care setting: the experience of one institution. Adv Orthop Surg. 2015;(2015):387197.
17. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
18. Parry MC, Smith AJ, Blom AW. Early death following primary total knee arthroplasty. J Bone Joint Surg Am. 2011;93(10):948-953.
19. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
1. Dundon JM, Bosco J, Slover J, Yu S, Sayeed Y, Iorio R. Improvement in total joint replacement quality metrics, year one versus year three of the bundled payments for care improvement initiative. J Bone Joint Surg Am. 2016;98(23):1949-1953.
2. Itani KM. Fifteen years of the National Surgical Quality Improvement Program in review. Am J Surg. 2009;198(suppl 5):S9-S18.
3. Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J. 2016;98-B(3):334-340.
4. Heller S, Rezapoor M, Parvizi J. Minimising the risk of infection: a peri-operative checklist. Bone Joint J. 2016;98-B(1)(suppl A):18-22.
5. Thornqvist C, Gislason GH, Køber L, Jensen PF, Torp-Pedersen C, Andersson C. Body mass index and risk of perioperative cardiovascular adverse events and mortality in 34,744 Danish patients undergoing hip or knee replacement. Acta Orthop. 2014;85(5):456-462.
6. Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am. 2013;95(9):808-814.
7. Akhavan S, Nguyen LC, Chan V, Saleh J, Bozic KJ. Impact of smoking cessation counseling prior to total joint arthroplasty. Orthopedics. 2017;40(2):e323-e328.
8. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1820-1826.
9. Goyal N, Chen DB, Harris IA, Rowden NJ, Kirsh G, MacDessi SJ. Intravenous vs intra-articular tranexamic acid in total knee arthroplasty: a randomized, double-blind trial. J Arthroplasty. 2017;32(1):28-32.
10. Phan DL, Ani F, Schwarzkopf R. Cost analysis of tranexamic acid in anemic total joint arthroplasty patients. J Arthroplasty. 2016;31(3):579-582.
11. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
12. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
13. Fang R, Liu Z, Alijiang A, et al. Efficacy of intra-articular local anesthetics in total knee arthroplasty. Orthopedics. 2015;38(7):e573-e581.
14. Odeh K, Doran J, Yu S, Bolz N, Bosco J, Iorio R. Risk-stratified venous thromboembolism prophylaxis after total joint arthroplasty: aspirin and sequential pneumatic compression devices vs aggressive chemoprophylaxis. J Arthroplasty. 2016;31(suppl 9):78-82.
15. Parvizi J, Huang R, Restrepo C, et al. Low-dose aspirin is effective chemoprophylaxis against clinically important venous thromboembolism following total joint arthroplasty: a preliminary analysis. J Bone Joint Surg Am. 2017;99(2):91-98.
16. Robertson NB, Warganich T, Ghazarossian J, Khatod M. Implementation of an accelerated rehabilitation protocol for total joint arthroplasty in the managed care setting: the experience of one institution. Adv Orthop Surg. 2015;(2015):387197.
17. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
18. Parry MC, Smith AJ, Blom AW. Early death following primary total knee arthroplasty. J Bone Joint Surg Am. 2011;93(10):948-953.
19. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
Acute Shortening Versus Bridging Plate for Highly Comminuted Olecranon Fractures
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
Treating Unstable Distal Radius Fractures With a Nonspanning External Fixation Device: Comparison With Volar Locking Plates in Historical Control Group
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.