User login
Traumatic Anterior Shoulder Instability: The US Military Experience
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
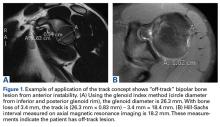
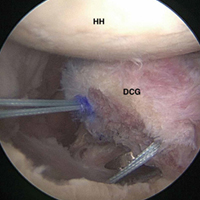
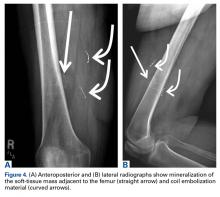
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
Bone Stress Injuries in the Military: Diagnosis, Management, and Prevention
Take-Home Points
- Stress injuries, specifically of the lower extremity, are very common in new military trainees.
- Stress injury can range from benign periosteal reaction to displaced fracture.
- Stress injury should be treated on a case-by-case basis, depending on the severity of injury, the location of the injury, and the likelihood of healing with nonoperative management.
- Modifiable risk factors such as nutritional status, training regiment, and even footwear should be investigated to determine potential causes of injury.
- Prevention is a crucial part of the treatment of these injuries, and early intervention such as careful pre-enrollment physicals and vitamin supplementation can be essential in lowering injury rates.
Bone stress injuries, which are common in military recruits, present in weight-bearing (WB) areas as indolent pain caused by repetitive stress and microtrauma. They were first reported in the metatarsals of Prussian soldiers in 1855.1 Today, stress injuries are increasingly common. One study estimated they account for 10% of patients seen by sports medicine practitioners.2 This injury most commonly affects military members, endurance athletes, and dancers.3-5 Specifically, the incidence of stress fractures in military members has been reported to range from 0.8% to 6.9% for men and from 3.4% to 21.0% for women.4 Because of repetitive vigorous lower extremity loading, stress fractures typically occur in the pelvis, femoral neck, tibial shaft, and metatarsals. Delayed diagnosis and the subsequent duration of treatment required for adequate healing can result in significant morbidity. In a 2009 to 2012 study of US military members, Waterman and colleagues6 found an incidence rate of 5.69 stress fractures per 1000 person-years. Fractures most frequently involved the tibia/fibula (2.26/1000), followed by the metatarsals (0.92/1000) and the femoral neck (0.49/1000).6 In addition, these injuries were most commonly encountered in new recruits, who were less accustomed to the high-volume, high-intensity training required during basic training.4,7 Enlisted junior service members have been reported to account for 77.5% of all stress fractures.6 Age under 20 years or over 40 years and white race have also been found to be risk factors for stress injury.6
The pathogenesis of stress injury is controversial. Stanitski and colleagues8 theorized that multiple submaximal mechanical insults create cumulative stress greater than bone capacity, eventually leading to fracture. Johnson9 conducted a biopsy study and postulated that an accelerated remodeling phase was responsible, whereas Friedenberg10 argued that stress injuries are a form of reduced healing, not an attempt to increase healing, caused by the absence of callous formation in the disease process.
Various other nonmodifiable and modifiable risk factors predispose military service members to stress injury. Nonmodifiable risk factors include sex, bone geometry, limb alignment, race, age, and anatomy. Lower extremity movement biomechanics resulting from dynamic limb alignment during activity may be important. Cameron and colleagues11 examined 1843 patients and found that those with knees in >5° of valgus or >5° of external rotation had higher injury rates. Although variables such as sex and limb alignment cannot be changed, proper identification of modifiable risk factors can assist with injury prevention, and nonmodifiable risk factors can help clinicians and researchers target injury prevention interventions to patients at highest risk.
Metabolic, hormonal, and nutritional status is crucial to overall bone health. Multiple studies have found that low body mass index (BMI) is a significant risk factor for stress fracture.7,12,13 Although low BMI is a concern, patients with abnormally high BMI may also be at increased risk for bone stress injury. In a recently released consensus statement on relative energy deficiency in sport (RED-S), the International Olympic Committee addressed the complex interplay of impairments in physiologic function—including metabolic rate, menstrual function, bone health, immunity, protein synthesis, and cardiovascular health—caused by relative energy deficiency.14 The committee stated that the cause of this syndrome is energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities. This finding reveals that conditions such as stress injury often may represent a much broader systemic deficit that may be influenced by a patient’s overall physiologic imbalance.
Diagnosis
History and Physical Examination
The onset of stress reaction typically is insidious, with the classic presentation being a new military recruit who is experiencing a sudden increase in pain during physical activity.15 Pain typically is initially present only during activity, and is relieved with rest, but with disease progression this evolves to pain at rest. It is crucial that the physician elicit the patient’s history of training and physical activity. Hsu and colleagues7 reported increased prevalence of overweight civilian recruits, indicating an increase in the number of new recruits having limited experience with the repetitive physical activity encountered in basic training. Stress injury should be suspected in the setting of worsening, indolent lower extremity pain that has been present for several days, especially in the higher-risk patient populations mentioned. Diet should be assessed, with specific attention given to the intake of fruits, vegetables, and foods high in vitamin D and calcium and, most important, the energy balance between intake and output.16 Special attention should also be given to female patients, who may experience the female athlete triad, a spectrum of low energy availability, menstrual dysfunction, and impaired bone turnover (high amount of resorption relative to formation). A key part of the RED-S consensus statement14 alerted healthcare providers that metabolic derangements do not solely affect female patients. These types of patients sustain a major insult to the homeostatic balance of the hormones that sustain adequate bone health. Beck and colleagues17 found that women with disrupted menstrual cycles are 2 to 4 times more likely to sustain a stress fracture than women without disrupted menstrual cycles, making this abnormality an important part of the history.
Examination should begin with careful evaluation of limb alignment and specific attention given to varus or valgus alignment of the knees.11 The feet should also be inspected, as pes planus or cavus foot may increase the risk of stress fracture.18 Identification of the area of maximal tenderness is important. The area in question may also be erythematous or warm secondary to the inflammatory response associated with attempted fracture healing. In chronic fractures in superficial areas such as the metatarsals, callus may be palpable. Although there are few specific tests for stress injury, pain may be reproducible with deep palpation and WB.
Laboratory Testing
When a pathology is thought to have a nutritional or metabolic cause, particularly in a low-weight or underweight patient, a laboratory workup should be obtained. Specific laboratory tests that all patients should undergo are 25-hydroxyvitamin D3, complete blood cell count, and basic chemistry panel, including calcium and thyroid-stimulating hormone levels. Although not necessary for diagnosis, phosphate, parathyroid hormone, albumin, and prealbumin should also be considered. Females should undergo testing of follicle stimulating hormone, luteinizing hormone, estradiol, and testosterone and have a urine pregnancy test. In patients with signs of excessive cortisone, a dexamethasone suppression test can be administered.21 In males, low testosterone is a documented risk factor for stress injury.22
Imaging
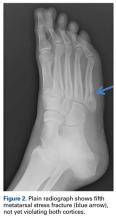
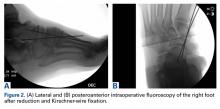
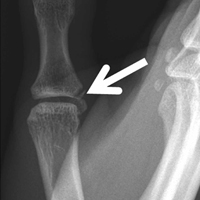
Given their low cost and availability, plain radiographs typically are used for initial examination of a suspected stress injury. However, they often lack sensitivity, particularly in the early stages of stress fracture development (Figure 2).
Management
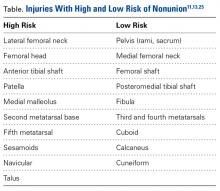
Management of bone stress injury depends on many factors, including symptom duration, fracture location and severity, and risk of progression or nonunion (Table).13
Pelvis
Pelvic stress fractures are rare and represent only 1.6% to 7.1% of all stress fractures.13,27,28 Given the low frequency, physicians must have a high index of suspicion to make the correct diagnosis. These fractures typically occur in marathon runners and other patients who present with persistent pain and a history of high levels of activity. As pelvic stress fractures typically involve the superior or inferior pubic rami, or sacrum, and are at low risk for nonunion,13 most are managed with nonoperative treatment and activity modification for 8 to 12 weeks.27
Femur
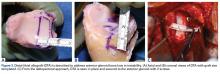
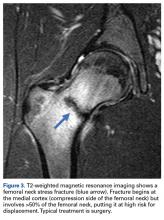
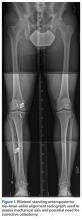
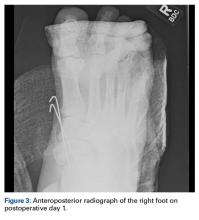
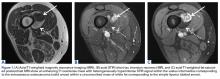
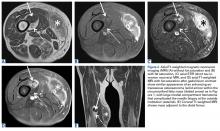
Femoral stress fractures are also relatively uncommon, accounting for about 10% of all stress fractures. Depending on their location, these fractures can be at high risk for progression, nonunion, and significant morbidity.29 Especially concerning are femoral neck stress fractures, which can involve either the tension side (lateral cortex) or the compression side (medial cortex) of the bone. Suspicion of a femoral neck stress fracture should prompt immediate NWB.5 Early recognition of these injuries is crucial because once displacement occurs, their complication and morbidity rates become high.13 Patients with compression-side fractures should undergo NWB treatment for 4 to 6 weeks and then slow progression to WB activity. Most return to light-impact activity by 3 to 4 months. By contrast, tension-side fractures are less likely to heal without operative intervention.11 All tension-side fractures (and any compression-side fractures >50% of the width of the femoral neck) should be treated with percutaneous placement of cannulated screws (Figure 3).
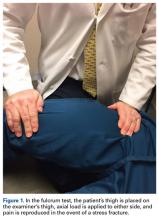
Stress fractures of the femoral shaft are less common than those of the femoral neck and represent as little as 3% of all stress fractures.32 However, femoral shaft stress fractures are more common in military populations. In French military recruits, Niva and colleagues33 found an 18% incidence. Similar to femoral neck fractures, femoral shaft fractures typically are diagnosed with advanced imaging, though the fulcrum test and pain on WB can aid in the diagnosis.19 These injuries are often managed nonoperatively with NWB for a period. Weishaar and colleagues34 described US military cadets treated with progressive rehabilitation who returned to full activity within 12 weeks. Displaced femoral shaft fractures associated with bone stress injury are even less common, and should be managed operatively. Salminen and colleagues35 found an incidence of 1.5 fractures per 100,000 years of military service. Over a 20-year period, they surgically treated 10 of these fractures. Average time from intramedullary nailing to union was 3.5 months.
Tibia
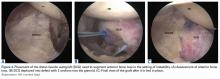
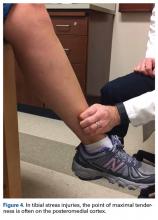
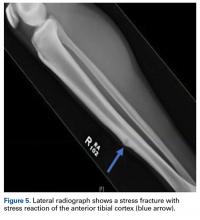
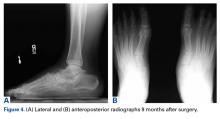
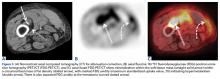
The tibia is one of the more common locations for stress injury and fracture. In a prospective study with members of the military, Giladi and colleagues36 found that 71% of stress fractures were tibia fractures. In addition, a large study of 320 athletes with stress fractures found 49.1% in the tibia.37 Fractures typically are diaphyseal and transverse, usually occurring along the posteromedial cortex, where the bone experiences maximal compressive forces (Figure 4).5,13
Compression-side fractures often heal with nonoperative management, though healing may take several months. Swenson and colleagues40 studied the effects of pneumatic bracing on conservative management and return to play in athletes with tibial stress fractures. Patients with bracing returned to light activity within 7 days and full activity within 21 days, whereas those without bracing returned to light activity within 21 days and full activity within 77 days. Pulsed electromagnetic therapy is of controversial benefit in the management of these injuries. Rettig
Metatarsals
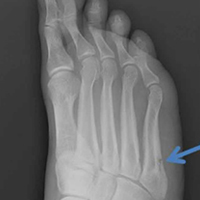
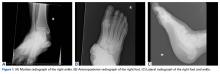
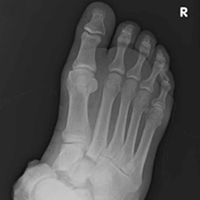
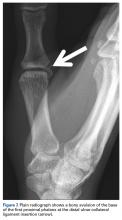
Stress fractures were first discovered by Briethaupt1 in the painful swollen feet of Prussian army members in 1855 and were initially named march fractures. Waterman and colleagues6 reported that metatarsal stress fractures accounted for 16% of all stress fractures in the US military between 2009 and 2012. The second metatarsal neck is the most common location for stress fractures, followed by the third and fourth metatarsals, with the fifth metatarsal being the least common.5 The second metatarsal is thought to sustain these injuries more often than the other metatarsals because of its relative lack of immobility. Donahue and Sharkey43 found that the dorsal aspect of the second metatarsal experiences twice the amount of strain experienced by the fifth metatarsal during gait, and that peak strain in the second metatarsal was further increased by simulated muscle fatigue. The risk of stress fracture can be additionally increased with use of minimalist footwear, as shown by Giuliani and colleagues,44 particularly in the absence of a progressive transition in gait and training volume with a change toward minimalist footwear. In patients with a suspected or confirmed fracture of the second, third, or fourth metatarsal, treatment typically is NWB and immobilization for at least 4 weeks.5 Fifth metatarsal stress injuries (Figure 2) typically are treated differently because of their higher risk of nonunion. Patients with a fifth metatarsal stress fracture complain of lateral midfoot pain with running and jumping. For those who present with this fracture early, acceptable treatment consists of 6 weeks of casting and NWB.5 In cases of failed nonoperative therapy, or presentation with radiographic evidence of nonunion, treatment should be intramedullary screw fixation, with bone graft supplementation based on surgeon preference. DeLee and colleagues45 reported on the results of 10 athletes with fifth metatarsal stress fractures treated with intramedullary screw fixation without bone grafting. All 10 experienced fracture union, at a mean of 7.5 weeks, and returned to sport within 8.5 weeks. One complication with this procedure is pain at the screw insertion site, but this can be successfully managed with footwear modification.45
Prevention
Proper identification of patients at high risk for stress injuries has the potential of reducing the incidence of these injuries. Lappe and colleagues46 prospectively examined female army recruits before and after 8 weeks of basic training and found that those who developed a stress fracture were more likely to have a smoking history, to drink more than 10 alcoholic beverages a week, to have a history of corticosteroid or depot medroxyprogesterone use, and to have lower body weight. In addition, the authors found that a history of prolonged exercise before enrollment was protective against fracture. This finding identifies the importance of having new recruits undergo risk factor screening, which could result in adjusting training regimens to try to reduce injury. The RED-S consensus statement14 offers a comprehensive description of the physiologic factors that can contribute to such injury. Similar to proper risk factor identification, implementation of proper exercise progression programs is a simple, modifiable method of limiting stress injuries. For new recruits or athletes who are resuming activity, injury can be effectively prevented by adjusting the frequency, duration, and intensity of training and the training loads used.47
Vitamin D and calcium supplementation is a simple intervention that can be helpful in injury prevention, and its use has very little downside. A double-blind study found a 20% lower incidence of stress fracture in female navy recruits who took 2000 mg of calcium and 800 IU of vitamin D as daily supplemention.48 Of importance, a meta-analysis of more than 65,000 patients found vitamin D supplementation was effective in reducing fracture risk only when combined with calcium, irrespective of age, sex, or prior fracture.49 In female patients with the female athlete triad, psychological counseling and nutritional consultation are essential in bone health maintenance and long-term prevention.50 Other therapies have been evaluated as well. Use of bisphosphonates is controversial for both treatment and prevention of stress fractures. In a randomized, double-blind study of the potential prophylactic effects of risedronate in 324 new infantry recruits, Milgrom and colleagues51 found no statistically significant differences in tibial, femoral, metatarsal, or total stress fracture incidence between the treatment and placebo groups. Therefore, bisphosphonates are seldom recommended as prevention or in primary management of stress fracture.
In addition to nutritional and pharmacologic therapy, activity modification may have a role in injury prevention. Gait retraining has been identified as a potential intervention for reducing stress fractures in patients with poor biomechanics.47 Crowell and Davis52 investigated the effect of gait retraining on the forces operating in the tibia in runners. After 1 month of gait retraining, tibial acceleration while running decreased by 50%, vertical force loading rate by 30%, and peak vertical force impact by 20%. Such studies indicate the importance of proper mechanics during repetitive activity, especially in patients not as accustomed to the rigorous training methods used with new military recruits. However, whether these reduced loads translate into reduced risk of stress fracture remains unclear. In addition, biomechanical shoe orthoses may lower the stress fracture risk in military recruits by reducing peak tibial strain.53 Warden and colleagues54 found a mechanical loading program was effective in enchaining the structural properties of bone in rats, leading the authors to hypothesize that a similar program aimed at modifying bone structure in humans could help prevent stress fracture. Although there have been no studies of such a strategy in humans, pretraining may be an area for future research, especially for military recruits.
Conclusion
Compared with the general population, members of the military (new recruits in particular) are at increased risk for bone stress injuries. Most of these injuries occur during basic training, when recruits significantly increase their repetitive physical activity. Although the exact pathophysiology of stress injury is debated, nutritional and metabolic abnormalities are contributors. The indolent nature of these injuries, and their high rate of false-negative plain radiographs, may result in a significant delay in diagnosis in the absence of advanced imaging studies. Although a majority of injuries heal with nonoperative management and NWB, several patterns, especially those on the tension side of the bone, are at high risk for progression to fracture and nonunion. These include lateral femoral cortex stress injuries and anterior tibial cortex fractures. There should be a low threshold for operative management in the setting of delayed union or failed nonoperative therapy. Of equal importance to orthopedic management of these injuries is the management of underlying systemic deficits, which may have subjected the patient to injury in the first place. Supplementation with vitamin D and calcium can be an important prophylaxis against stress injury. In addition, military recruits and athletes with underlying metabolic or hormonal deficiencies should receive proper attention with a focus on balancing energy intake and energy expenditure. Stress injury leading to fracture—increasingly common in military populations—often requires a multimodal approach for treatment and subsequent prevention.
Am J Orthop. 2017;46(4):176-183. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Briethaupt MD. Zur Pathologie des menschlichen Fusses [To the pathology of the human foot]. Med Zeitung. 1855;24:169-177.
2. Berger FH, de Jonge MC, Maas M. Stress fractures in the lower extremity. Eur J Radiol. 2007;62(1):16-26.
3. Almeida SA, Williams KM, Shaffer RA, Brodine SK. Epidemiological patterns of musculoskeletal injuries and physical training. Med Sci Sports Exerc. 1999;31(8):1176-1182.
4. Jones BH, Thacker SB, Gilchrist J, Kimsey CD, Sosin DM. Prevention of lower extremity stress fractures in athletes and soldiers: a systematic review. Epidemiol Rev. 2002;24(2):228-247.
5. Jacobs JM, Cameron KL, Bojescul JA. Lower extremity stress fractures in the military. Clin Sports Med. 2014;33(4):591-613.
6. Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181(10):1308-1313.
7. Hsu LL, Nevin RL, Tobler SK, Rubertone MV. Trends in overweight and obesity among 18-year-old applicants to the United States military, 1993–2006. J Adolesc Health. 2007;41(6):610-612.
8. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6(6):391-396.
9. Johnson LC. Histogenesis of stress fractures [annual lecture]. Washington, DC: Armed Forces Institute of Pathology; 1963.
10. Friedenberg ZB. Fatigue fractures of the tibia. Clin Orthop Relat Res. 1971;(76):111-115.
11. Cameron KL, Peck KY, Owens BD, et al. Biomechanical risk factors for lower extremity stress fracture. Orthop J Sports Med. 2013;1(4 suppl).
12. Knapik J, Montain S, McGraw S, Grier T, Ely M, Jones B. Stress fracture risk factors in basic combat training. Int J Sports Med. 2012;33(11):940-946.
13. Behrens SB, Deren ME, Matson A, Fadale PD, Monchik KO. Stress fractures of the pelvis and legs in athletes. Sports Health. 2013;5(2):165-174.
14. Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med. 2014;48(7):491-497.
15. Maitra RS, Johnson DL. Stress fractures. Clinical history and physical examination. Clin Sports Med. 1997;16(2):259-274.
16. Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740-750.
17. Beck BR, Matheson GO, Bergman G, et al. Do capacitively coupled electric fields accelerate tibial stress fracture healing? Am J Sports Med. 2008;36(3):545-553.
18. Simkin A, Leichter I, Giladi M, Stein M, Milgrom C. Combined effect of foot arch structure and an orthotic device on stress fractures. Foot Ankle. 1989;10(1):25-29.
19. Johnson AW, Weiss CB, Wheeler DL. Stress fractures of the femoral shaft in athletes—more common than expected: a new clinical test. Am J Sports Med. 1994;22(2):248-256.
20. Clement D, Ammann W, Taunton J, et al. Exercise-induced stress injuries to the femur. Int J Sports Med. 1993;14(6):347-352.
21. Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem. 1997;34(pt 3):222-229.
22. Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28(2):91-122.
23. Prather JL, Nusynowitz ML, Snowdy HA, Hughes AD, McCartney WH, Bagg RJ. Scintigraphic findings in stress fractures. J Bone Joint Surg Am. 1977;59(7):869-874.
24. Arendt EA, Griffiths HJ. The use of MR imaging in the assessment and clinical management of stress reactions of bone in high-performance athletes. Clin Sports Med. 1997;16(2):291-306.
25. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(6):344-353.
26. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553-561.
27. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
28. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
29. Noakes TD, Smith JA, Lindenberg G, Wills CE. Pelvic stress fractures in long distance runners. Am J Sports Med. 1985;13(2):120-123.
30. Neubauer T, Brand J, Lidder S, Krawany M. Stress fractures of the femoral neck in runners: a review. Res Sports Med. 2016;24(3):283-297.
31. Evans JT, Guyver PM, Kassam AM, Hubble MJW. Displaced femoral neck stress fractures in Royal Marine recruits—management and results of operative treatment. J R Nav Med Serv. 2012;98(2):3-5.
32. Orava S. Stress fractures. Br J Sports Med. 1980;14(1):40-44.
33. Niva MH, Kiuru MJ, Haataja R, Pihlajamäki HK. Fatigue injuries of the femur. J Bone Joint Surg Br. 2005;87(10):1385-1390.
34. Weishaar MD, McMillian DJ, Moore JH. Identification and management of 2 femoral shaft stress injuries. J Orthop Sports Phys Ther. 2005;35(10):665-673.
35. Salminen ST, Pihlajamäki HK, Visuri TI, Böstman OM. Displaced fatigue fractures of the femoral shaft. Clin Orthop Relat Res. 2003;(409):250-259.
36. Giladi M, Ahronson Z, Stein M, Danon YL, Milgrom C. Unusual distribution and onset of stress fractures in soldiers. Clin Orthop Relat Res. 1985;(192):142-146.
37. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
38. Green NE, Rogers RA, Lipscomb AB. Nonunions of stress fractures of the tibia. Am J Sports Med. 1985;13(3):171-176.
39. Orava S, Hulkko A. Stress fracture of the mid-tibial shaft. Acta Orthop Scand. 1984;55(1):35-37.
40. Swenson EJ Jr, DeHaven KE, Sebastianelli WJ, Hanks G, Kalenak A, Lynch JM. The effect of a pneumatic leg brace on return to play in athletes with tibial stress fractures. Am J Sports Med. 1997;25(3):322-328.
41. Rettig AC, Shelbourne KD, McCarroll JR, Bisesi M, Watts J. The natural history and treatment of delayed union stress fractures of the anterior cortex of the tibia. Am J Sports Med. 1988;16(3):250-255.
42. Varner KE, Younas SA, Lintner DM, Marymont JV. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33(7):1071-1076.
43. Donahue SW, Sharkey NA. Strains in the metatarsals during the stance phase of gait: implications for stress fractures. J Bone Joint Surg Am. 1999;81(9):1236-1244.
44. Giuliani J, Masini B, Alitz C, Owens BD. Barefoot-simulating footwear associated with metatarsal stress injury in 2 runners. Orthopedics. 2011;34(7):e320-e323.
45. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
46. Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female army recruits. Osteoporos Int. 2001;12(1):35-42.
47. Friedl KE, Evans RK, Moran DS. Stress fracture and military medical readiness: bridging basic and applied research. Med Sci Sports Exerc. 2008;40(11 suppl):S609-S622.
48. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741-749.
49. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463.
50. Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2(6).
51. Milgrom C, Finestone A, Novack V, et al. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone. 2004;35(2):418-424.
52. Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26(1):78-83.
53. Ekenman I, Milgrom C, Finestone A, et al. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 2002;30(6):866-870.
54. Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20(5):809-816.
Take-Home Points
- Stress injuries, specifically of the lower extremity, are very common in new military trainees.
- Stress injury can range from benign periosteal reaction to displaced fracture.
- Stress injury should be treated on a case-by-case basis, depending on the severity of injury, the location of the injury, and the likelihood of healing with nonoperative management.
- Modifiable risk factors such as nutritional status, training regiment, and even footwear should be investigated to determine potential causes of injury.
- Prevention is a crucial part of the treatment of these injuries, and early intervention such as careful pre-enrollment physicals and vitamin supplementation can be essential in lowering injury rates.
Bone stress injuries, which are common in military recruits, present in weight-bearing (WB) areas as indolent pain caused by repetitive stress and microtrauma. They were first reported in the metatarsals of Prussian soldiers in 1855.1 Today, stress injuries are increasingly common. One study estimated they account for 10% of patients seen by sports medicine practitioners.2 This injury most commonly affects military members, endurance athletes, and dancers.3-5 Specifically, the incidence of stress fractures in military members has been reported to range from 0.8% to 6.9% for men and from 3.4% to 21.0% for women.4 Because of repetitive vigorous lower extremity loading, stress fractures typically occur in the pelvis, femoral neck, tibial shaft, and metatarsals. Delayed diagnosis and the subsequent duration of treatment required for adequate healing can result in significant morbidity. In a 2009 to 2012 study of US military members, Waterman and colleagues6 found an incidence rate of 5.69 stress fractures per 1000 person-years. Fractures most frequently involved the tibia/fibula (2.26/1000), followed by the metatarsals (0.92/1000) and the femoral neck (0.49/1000).6 In addition, these injuries were most commonly encountered in new recruits, who were less accustomed to the high-volume, high-intensity training required during basic training.4,7 Enlisted junior service members have been reported to account for 77.5% of all stress fractures.6 Age under 20 years or over 40 years and white race have also been found to be risk factors for stress injury.6
The pathogenesis of stress injury is controversial. Stanitski and colleagues8 theorized that multiple submaximal mechanical insults create cumulative stress greater than bone capacity, eventually leading to fracture. Johnson9 conducted a biopsy study and postulated that an accelerated remodeling phase was responsible, whereas Friedenberg10 argued that stress injuries are a form of reduced healing, not an attempt to increase healing, caused by the absence of callous formation in the disease process.
Various other nonmodifiable and modifiable risk factors predispose military service members to stress injury. Nonmodifiable risk factors include sex, bone geometry, limb alignment, race, age, and anatomy. Lower extremity movement biomechanics resulting from dynamic limb alignment during activity may be important. Cameron and colleagues11 examined 1843 patients and found that those with knees in >5° of valgus or >5° of external rotation had higher injury rates. Although variables such as sex and limb alignment cannot be changed, proper identification of modifiable risk factors can assist with injury prevention, and nonmodifiable risk factors can help clinicians and researchers target injury prevention interventions to patients at highest risk.
Metabolic, hormonal, and nutritional status is crucial to overall bone health. Multiple studies have found that low body mass index (BMI) is a significant risk factor for stress fracture.7,12,13 Although low BMI is a concern, patients with abnormally high BMI may also be at increased risk for bone stress injury. In a recently released consensus statement on relative energy deficiency in sport (RED-S), the International Olympic Committee addressed the complex interplay of impairments in physiologic function—including metabolic rate, menstrual function, bone health, immunity, protein synthesis, and cardiovascular health—caused by relative energy deficiency.14 The committee stated that the cause of this syndrome is energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities. This finding reveals that conditions such as stress injury often may represent a much broader systemic deficit that may be influenced by a patient’s overall physiologic imbalance.
Diagnosis
History and Physical Examination
The onset of stress reaction typically is insidious, with the classic presentation being a new military recruit who is experiencing a sudden increase in pain during physical activity.15 Pain typically is initially present only during activity, and is relieved with rest, but with disease progression this evolves to pain at rest. It is crucial that the physician elicit the patient’s history of training and physical activity. Hsu and colleagues7 reported increased prevalence of overweight civilian recruits, indicating an increase in the number of new recruits having limited experience with the repetitive physical activity encountered in basic training. Stress injury should be suspected in the setting of worsening, indolent lower extremity pain that has been present for several days, especially in the higher-risk patient populations mentioned. Diet should be assessed, with specific attention given to the intake of fruits, vegetables, and foods high in vitamin D and calcium and, most important, the energy balance between intake and output.16 Special attention should also be given to female patients, who may experience the female athlete triad, a spectrum of low energy availability, menstrual dysfunction, and impaired bone turnover (high amount of resorption relative to formation). A key part of the RED-S consensus statement14 alerted healthcare providers that metabolic derangements do not solely affect female patients. These types of patients sustain a major insult to the homeostatic balance of the hormones that sustain adequate bone health. Beck and colleagues17 found that women with disrupted menstrual cycles are 2 to 4 times more likely to sustain a stress fracture than women without disrupted menstrual cycles, making this abnormality an important part of the history.
Examination should begin with careful evaluation of limb alignment and specific attention given to varus or valgus alignment of the knees.11 The feet should also be inspected, as pes planus or cavus foot may increase the risk of stress fracture.18 Identification of the area of maximal tenderness is important. The area in question may also be erythematous or warm secondary to the inflammatory response associated with attempted fracture healing. In chronic fractures in superficial areas such as the metatarsals, callus may be palpable. Although there are few specific tests for stress injury, pain may be reproducible with deep palpation and WB.
Laboratory Testing
When a pathology is thought to have a nutritional or metabolic cause, particularly in a low-weight or underweight patient, a laboratory workup should be obtained. Specific laboratory tests that all patients should undergo are 25-hydroxyvitamin D3, complete blood cell count, and basic chemistry panel, including calcium and thyroid-stimulating hormone levels. Although not necessary for diagnosis, phosphate, parathyroid hormone, albumin, and prealbumin should also be considered. Females should undergo testing of follicle stimulating hormone, luteinizing hormone, estradiol, and testosterone and have a urine pregnancy test. In patients with signs of excessive cortisone, a dexamethasone suppression test can be administered.21 In males, low testosterone is a documented risk factor for stress injury.22
Imaging
Given their low cost and availability, plain radiographs typically are used for initial examination of a suspected stress injury. However, they often lack sensitivity, particularly in the early stages of stress fracture development (Figure 2).
Management
Management of bone stress injury depends on many factors, including symptom duration, fracture location and severity, and risk of progression or nonunion (Table).13
Pelvis
Pelvic stress fractures are rare and represent only 1.6% to 7.1% of all stress fractures.13,27,28 Given the low frequency, physicians must have a high index of suspicion to make the correct diagnosis. These fractures typically occur in marathon runners and other patients who present with persistent pain and a history of high levels of activity. As pelvic stress fractures typically involve the superior or inferior pubic rami, or sacrum, and are at low risk for nonunion,13 most are managed with nonoperative treatment and activity modification for 8 to 12 weeks.27
Femur
Femoral stress fractures are also relatively uncommon, accounting for about 10% of all stress fractures. Depending on their location, these fractures can be at high risk for progression, nonunion, and significant morbidity.29 Especially concerning are femoral neck stress fractures, which can involve either the tension side (lateral cortex) or the compression side (medial cortex) of the bone. Suspicion of a femoral neck stress fracture should prompt immediate NWB.5 Early recognition of these injuries is crucial because once displacement occurs, their complication and morbidity rates become high.13 Patients with compression-side fractures should undergo NWB treatment for 4 to 6 weeks and then slow progression to WB activity. Most return to light-impact activity by 3 to 4 months. By contrast, tension-side fractures are less likely to heal without operative intervention.11 All tension-side fractures (and any compression-side fractures >50% of the width of the femoral neck) should be treated with percutaneous placement of cannulated screws (Figure 3).
Stress fractures of the femoral shaft are less common than those of the femoral neck and represent as little as 3% of all stress fractures.32 However, femoral shaft stress fractures are more common in military populations. In French military recruits, Niva and colleagues33 found an 18% incidence. Similar to femoral neck fractures, femoral shaft fractures typically are diagnosed with advanced imaging, though the fulcrum test and pain on WB can aid in the diagnosis.19 These injuries are often managed nonoperatively with NWB for a period. Weishaar and colleagues34 described US military cadets treated with progressive rehabilitation who returned to full activity within 12 weeks. Displaced femoral shaft fractures associated with bone stress injury are even less common, and should be managed operatively. Salminen and colleagues35 found an incidence of 1.5 fractures per 100,000 years of military service. Over a 20-year period, they surgically treated 10 of these fractures. Average time from intramedullary nailing to union was 3.5 months.
Tibia
The tibia is one of the more common locations for stress injury and fracture. In a prospective study with members of the military, Giladi and colleagues36 found that 71% of stress fractures were tibia fractures. In addition, a large study of 320 athletes with stress fractures found 49.1% in the tibia.37 Fractures typically are diaphyseal and transverse, usually occurring along the posteromedial cortex, where the bone experiences maximal compressive forces (Figure 4).5,13
Compression-side fractures often heal with nonoperative management, though healing may take several months. Swenson and colleagues40 studied the effects of pneumatic bracing on conservative management and return to play in athletes with tibial stress fractures. Patients with bracing returned to light activity within 7 days and full activity within 21 days, whereas those without bracing returned to light activity within 21 days and full activity within 77 days. Pulsed electromagnetic therapy is of controversial benefit in the management of these injuries. Rettig
Metatarsals
Stress fractures were first discovered by Briethaupt1 in the painful swollen feet of Prussian army members in 1855 and were initially named march fractures. Waterman and colleagues6 reported that metatarsal stress fractures accounted for 16% of all stress fractures in the US military between 2009 and 2012. The second metatarsal neck is the most common location for stress fractures, followed by the third and fourth metatarsals, with the fifth metatarsal being the least common.5 The second metatarsal is thought to sustain these injuries more often than the other metatarsals because of its relative lack of immobility. Donahue and Sharkey43 found that the dorsal aspect of the second metatarsal experiences twice the amount of strain experienced by the fifth metatarsal during gait, and that peak strain in the second metatarsal was further increased by simulated muscle fatigue. The risk of stress fracture can be additionally increased with use of minimalist footwear, as shown by Giuliani and colleagues,44 particularly in the absence of a progressive transition in gait and training volume with a change toward minimalist footwear. In patients with a suspected or confirmed fracture of the second, third, or fourth metatarsal, treatment typically is NWB and immobilization for at least 4 weeks.5 Fifth metatarsal stress injuries (Figure 2) typically are treated differently because of their higher risk of nonunion. Patients with a fifth metatarsal stress fracture complain of lateral midfoot pain with running and jumping. For those who present with this fracture early, acceptable treatment consists of 6 weeks of casting and NWB.5 In cases of failed nonoperative therapy, or presentation with radiographic evidence of nonunion, treatment should be intramedullary screw fixation, with bone graft supplementation based on surgeon preference. DeLee and colleagues45 reported on the results of 10 athletes with fifth metatarsal stress fractures treated with intramedullary screw fixation without bone grafting. All 10 experienced fracture union, at a mean of 7.5 weeks, and returned to sport within 8.5 weeks. One complication with this procedure is pain at the screw insertion site, but this can be successfully managed with footwear modification.45
Prevention
Proper identification of patients at high risk for stress injuries has the potential of reducing the incidence of these injuries. Lappe and colleagues46 prospectively examined female army recruits before and after 8 weeks of basic training and found that those who developed a stress fracture were more likely to have a smoking history, to drink more than 10 alcoholic beverages a week, to have a history of corticosteroid or depot medroxyprogesterone use, and to have lower body weight. In addition, the authors found that a history of prolonged exercise before enrollment was protective against fracture. This finding identifies the importance of having new recruits undergo risk factor screening, which could result in adjusting training regimens to try to reduce injury. The RED-S consensus statement14 offers a comprehensive description of the physiologic factors that can contribute to such injury. Similar to proper risk factor identification, implementation of proper exercise progression programs is a simple, modifiable method of limiting stress injuries. For new recruits or athletes who are resuming activity, injury can be effectively prevented by adjusting the frequency, duration, and intensity of training and the training loads used.47
Vitamin D and calcium supplementation is a simple intervention that can be helpful in injury prevention, and its use has very little downside. A double-blind study found a 20% lower incidence of stress fracture in female navy recruits who took 2000 mg of calcium and 800 IU of vitamin D as daily supplemention.48 Of importance, a meta-analysis of more than 65,000 patients found vitamin D supplementation was effective in reducing fracture risk only when combined with calcium, irrespective of age, sex, or prior fracture.49 In female patients with the female athlete triad, psychological counseling and nutritional consultation are essential in bone health maintenance and long-term prevention.50 Other therapies have been evaluated as well. Use of bisphosphonates is controversial for both treatment and prevention of stress fractures. In a randomized, double-blind study of the potential prophylactic effects of risedronate in 324 new infantry recruits, Milgrom and colleagues51 found no statistically significant differences in tibial, femoral, metatarsal, or total stress fracture incidence between the treatment and placebo groups. Therefore, bisphosphonates are seldom recommended as prevention or in primary management of stress fracture.
In addition to nutritional and pharmacologic therapy, activity modification may have a role in injury prevention. Gait retraining has been identified as a potential intervention for reducing stress fractures in patients with poor biomechanics.47 Crowell and Davis52 investigated the effect of gait retraining on the forces operating in the tibia in runners. After 1 month of gait retraining, tibial acceleration while running decreased by 50%, vertical force loading rate by 30%, and peak vertical force impact by 20%. Such studies indicate the importance of proper mechanics during repetitive activity, especially in patients not as accustomed to the rigorous training methods used with new military recruits. However, whether these reduced loads translate into reduced risk of stress fracture remains unclear. In addition, biomechanical shoe orthoses may lower the stress fracture risk in military recruits by reducing peak tibial strain.53 Warden and colleagues54 found a mechanical loading program was effective in enchaining the structural properties of bone in rats, leading the authors to hypothesize that a similar program aimed at modifying bone structure in humans could help prevent stress fracture. Although there have been no studies of such a strategy in humans, pretraining may be an area for future research, especially for military recruits.
Conclusion
Compared with the general population, members of the military (new recruits in particular) are at increased risk for bone stress injuries. Most of these injuries occur during basic training, when recruits significantly increase their repetitive physical activity. Although the exact pathophysiology of stress injury is debated, nutritional and metabolic abnormalities are contributors. The indolent nature of these injuries, and their high rate of false-negative plain radiographs, may result in a significant delay in diagnosis in the absence of advanced imaging studies. Although a majority of injuries heal with nonoperative management and NWB, several patterns, especially those on the tension side of the bone, are at high risk for progression to fracture and nonunion. These include lateral femoral cortex stress injuries and anterior tibial cortex fractures. There should be a low threshold for operative management in the setting of delayed union or failed nonoperative therapy. Of equal importance to orthopedic management of these injuries is the management of underlying systemic deficits, which may have subjected the patient to injury in the first place. Supplementation with vitamin D and calcium can be an important prophylaxis against stress injury. In addition, military recruits and athletes with underlying metabolic or hormonal deficiencies should receive proper attention with a focus on balancing energy intake and energy expenditure. Stress injury leading to fracture—increasingly common in military populations—often requires a multimodal approach for treatment and subsequent prevention.
Am J Orthop. 2017;46(4):176-183. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Stress injuries, specifically of the lower extremity, are very common in new military trainees.
- Stress injury can range from benign periosteal reaction to displaced fracture.
- Stress injury should be treated on a case-by-case basis, depending on the severity of injury, the location of the injury, and the likelihood of healing with nonoperative management.
- Modifiable risk factors such as nutritional status, training regiment, and even footwear should be investigated to determine potential causes of injury.
- Prevention is a crucial part of the treatment of these injuries, and early intervention such as careful pre-enrollment physicals and vitamin supplementation can be essential in lowering injury rates.
Bone stress injuries, which are common in military recruits, present in weight-bearing (WB) areas as indolent pain caused by repetitive stress and microtrauma. They were first reported in the metatarsals of Prussian soldiers in 1855.1 Today, stress injuries are increasingly common. One study estimated they account for 10% of patients seen by sports medicine practitioners.2 This injury most commonly affects military members, endurance athletes, and dancers.3-5 Specifically, the incidence of stress fractures in military members has been reported to range from 0.8% to 6.9% for men and from 3.4% to 21.0% for women.4 Because of repetitive vigorous lower extremity loading, stress fractures typically occur in the pelvis, femoral neck, tibial shaft, and metatarsals. Delayed diagnosis and the subsequent duration of treatment required for adequate healing can result in significant morbidity. In a 2009 to 2012 study of US military members, Waterman and colleagues6 found an incidence rate of 5.69 stress fractures per 1000 person-years. Fractures most frequently involved the tibia/fibula (2.26/1000), followed by the metatarsals (0.92/1000) and the femoral neck (0.49/1000).6 In addition, these injuries were most commonly encountered in new recruits, who were less accustomed to the high-volume, high-intensity training required during basic training.4,7 Enlisted junior service members have been reported to account for 77.5% of all stress fractures.6 Age under 20 years or over 40 years and white race have also been found to be risk factors for stress injury.6
The pathogenesis of stress injury is controversial. Stanitski and colleagues8 theorized that multiple submaximal mechanical insults create cumulative stress greater than bone capacity, eventually leading to fracture. Johnson9 conducted a biopsy study and postulated that an accelerated remodeling phase was responsible, whereas Friedenberg10 argued that stress injuries are a form of reduced healing, not an attempt to increase healing, caused by the absence of callous formation in the disease process.
Various other nonmodifiable and modifiable risk factors predispose military service members to stress injury. Nonmodifiable risk factors include sex, bone geometry, limb alignment, race, age, and anatomy. Lower extremity movement biomechanics resulting from dynamic limb alignment during activity may be important. Cameron and colleagues11 examined 1843 patients and found that those with knees in >5° of valgus or >5° of external rotation had higher injury rates. Although variables such as sex and limb alignment cannot be changed, proper identification of modifiable risk factors can assist with injury prevention, and nonmodifiable risk factors can help clinicians and researchers target injury prevention interventions to patients at highest risk.
Metabolic, hormonal, and nutritional status is crucial to overall bone health. Multiple studies have found that low body mass index (BMI) is a significant risk factor for stress fracture.7,12,13 Although low BMI is a concern, patients with abnormally high BMI may also be at increased risk for bone stress injury. In a recently released consensus statement on relative energy deficiency in sport (RED-S), the International Olympic Committee addressed the complex interplay of impairments in physiologic function—including metabolic rate, menstrual function, bone health, immunity, protein synthesis, and cardiovascular health—caused by relative energy deficiency.14 The committee stated that the cause of this syndrome is energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities. This finding reveals that conditions such as stress injury often may represent a much broader systemic deficit that may be influenced by a patient’s overall physiologic imbalance.
Diagnosis
History and Physical Examination
The onset of stress reaction typically is insidious, with the classic presentation being a new military recruit who is experiencing a sudden increase in pain during physical activity.15 Pain typically is initially present only during activity, and is relieved with rest, but with disease progression this evolves to pain at rest. It is crucial that the physician elicit the patient’s history of training and physical activity. Hsu and colleagues7 reported increased prevalence of overweight civilian recruits, indicating an increase in the number of new recruits having limited experience with the repetitive physical activity encountered in basic training. Stress injury should be suspected in the setting of worsening, indolent lower extremity pain that has been present for several days, especially in the higher-risk patient populations mentioned. Diet should be assessed, with specific attention given to the intake of fruits, vegetables, and foods high in vitamin D and calcium and, most important, the energy balance between intake and output.16 Special attention should also be given to female patients, who may experience the female athlete triad, a spectrum of low energy availability, menstrual dysfunction, and impaired bone turnover (high amount of resorption relative to formation). A key part of the RED-S consensus statement14 alerted healthcare providers that metabolic derangements do not solely affect female patients. These types of patients sustain a major insult to the homeostatic balance of the hormones that sustain adequate bone health. Beck and colleagues17 found that women with disrupted menstrual cycles are 2 to 4 times more likely to sustain a stress fracture than women without disrupted menstrual cycles, making this abnormality an important part of the history.
Examination should begin with careful evaluation of limb alignment and specific attention given to varus or valgus alignment of the knees.11 The feet should also be inspected, as pes planus or cavus foot may increase the risk of stress fracture.18 Identification of the area of maximal tenderness is important. The area in question may also be erythematous or warm secondary to the inflammatory response associated with attempted fracture healing. In chronic fractures in superficial areas such as the metatarsals, callus may be palpable. Although there are few specific tests for stress injury, pain may be reproducible with deep palpation and WB.
Laboratory Testing
When a pathology is thought to have a nutritional or metabolic cause, particularly in a low-weight or underweight patient, a laboratory workup should be obtained. Specific laboratory tests that all patients should undergo are 25-hydroxyvitamin D3, complete blood cell count, and basic chemistry panel, including calcium and thyroid-stimulating hormone levels. Although not necessary for diagnosis, phosphate, parathyroid hormone, albumin, and prealbumin should also be considered. Females should undergo testing of follicle stimulating hormone, luteinizing hormone, estradiol, and testosterone and have a urine pregnancy test. In patients with signs of excessive cortisone, a dexamethasone suppression test can be administered.21 In males, low testosterone is a documented risk factor for stress injury.22
Imaging
Given their low cost and availability, plain radiographs typically are used for initial examination of a suspected stress injury. However, they often lack sensitivity, particularly in the early stages of stress fracture development (Figure 2).
Management
Management of bone stress injury depends on many factors, including symptom duration, fracture location and severity, and risk of progression or nonunion (Table).13
Pelvis
Pelvic stress fractures are rare and represent only 1.6% to 7.1% of all stress fractures.13,27,28 Given the low frequency, physicians must have a high index of suspicion to make the correct diagnosis. These fractures typically occur in marathon runners and other patients who present with persistent pain and a history of high levels of activity. As pelvic stress fractures typically involve the superior or inferior pubic rami, or sacrum, and are at low risk for nonunion,13 most are managed with nonoperative treatment and activity modification for 8 to 12 weeks.27
Femur
Femoral stress fractures are also relatively uncommon, accounting for about 10% of all stress fractures. Depending on their location, these fractures can be at high risk for progression, nonunion, and significant morbidity.29 Especially concerning are femoral neck stress fractures, which can involve either the tension side (lateral cortex) or the compression side (medial cortex) of the bone. Suspicion of a femoral neck stress fracture should prompt immediate NWB.5 Early recognition of these injuries is crucial because once displacement occurs, their complication and morbidity rates become high.13 Patients with compression-side fractures should undergo NWB treatment for 4 to 6 weeks and then slow progression to WB activity. Most return to light-impact activity by 3 to 4 months. By contrast, tension-side fractures are less likely to heal without operative intervention.11 All tension-side fractures (and any compression-side fractures >50% of the width of the femoral neck) should be treated with percutaneous placement of cannulated screws (Figure 3).
Stress fractures of the femoral shaft are less common than those of the femoral neck and represent as little as 3% of all stress fractures.32 However, femoral shaft stress fractures are more common in military populations. In French military recruits, Niva and colleagues33 found an 18% incidence. Similar to femoral neck fractures, femoral shaft fractures typically are diagnosed with advanced imaging, though the fulcrum test and pain on WB can aid in the diagnosis.19 These injuries are often managed nonoperatively with NWB for a period. Weishaar and colleagues34 described US military cadets treated with progressive rehabilitation who returned to full activity within 12 weeks. Displaced femoral shaft fractures associated with bone stress injury are even less common, and should be managed operatively. Salminen and colleagues35 found an incidence of 1.5 fractures per 100,000 years of military service. Over a 20-year period, they surgically treated 10 of these fractures. Average time from intramedullary nailing to union was 3.5 months.
Tibia
The tibia is one of the more common locations for stress injury and fracture. In a prospective study with members of the military, Giladi and colleagues36 found that 71% of stress fractures were tibia fractures. In addition, a large study of 320 athletes with stress fractures found 49.1% in the tibia.37 Fractures typically are diaphyseal and transverse, usually occurring along the posteromedial cortex, where the bone experiences maximal compressive forces (Figure 4).5,13
Compression-side fractures often heal with nonoperative management, though healing may take several months. Swenson and colleagues40 studied the effects of pneumatic bracing on conservative management and return to play in athletes with tibial stress fractures. Patients with bracing returned to light activity within 7 days and full activity within 21 days, whereas those without bracing returned to light activity within 21 days and full activity within 77 days. Pulsed electromagnetic therapy is of controversial benefit in the management of these injuries. Rettig
Metatarsals
Stress fractures were first discovered by Briethaupt1 in the painful swollen feet of Prussian army members in 1855 and were initially named march fractures. Waterman and colleagues6 reported that metatarsal stress fractures accounted for 16% of all stress fractures in the US military between 2009 and 2012. The second metatarsal neck is the most common location for stress fractures, followed by the third and fourth metatarsals, with the fifth metatarsal being the least common.5 The second metatarsal is thought to sustain these injuries more often than the other metatarsals because of its relative lack of immobility. Donahue and Sharkey43 found that the dorsal aspect of the second metatarsal experiences twice the amount of strain experienced by the fifth metatarsal during gait, and that peak strain in the second metatarsal was further increased by simulated muscle fatigue. The risk of stress fracture can be additionally increased with use of minimalist footwear, as shown by Giuliani and colleagues,44 particularly in the absence of a progressive transition in gait and training volume with a change toward minimalist footwear. In patients with a suspected or confirmed fracture of the second, third, or fourth metatarsal, treatment typically is NWB and immobilization for at least 4 weeks.5 Fifth metatarsal stress injuries (Figure 2) typically are treated differently because of their higher risk of nonunion. Patients with a fifth metatarsal stress fracture complain of lateral midfoot pain with running and jumping. For those who present with this fracture early, acceptable treatment consists of 6 weeks of casting and NWB.5 In cases of failed nonoperative therapy, or presentation with radiographic evidence of nonunion, treatment should be intramedullary screw fixation, with bone graft supplementation based on surgeon preference. DeLee and colleagues45 reported on the results of 10 athletes with fifth metatarsal stress fractures treated with intramedullary screw fixation without bone grafting. All 10 experienced fracture union, at a mean of 7.5 weeks, and returned to sport within 8.5 weeks. One complication with this procedure is pain at the screw insertion site, but this can be successfully managed with footwear modification.45
Prevention
Proper identification of patients at high risk for stress injuries has the potential of reducing the incidence of these injuries. Lappe and colleagues46 prospectively examined female army recruits before and after 8 weeks of basic training and found that those who developed a stress fracture were more likely to have a smoking history, to drink more than 10 alcoholic beverages a week, to have a history of corticosteroid or depot medroxyprogesterone use, and to have lower body weight. In addition, the authors found that a history of prolonged exercise before enrollment was protective against fracture. This finding identifies the importance of having new recruits undergo risk factor screening, which could result in adjusting training regimens to try to reduce injury. The RED-S consensus statement14 offers a comprehensive description of the physiologic factors that can contribute to such injury. Similar to proper risk factor identification, implementation of proper exercise progression programs is a simple, modifiable method of limiting stress injuries. For new recruits or athletes who are resuming activity, injury can be effectively prevented by adjusting the frequency, duration, and intensity of training and the training loads used.47
Vitamin D and calcium supplementation is a simple intervention that can be helpful in injury prevention, and its use has very little downside. A double-blind study found a 20% lower incidence of stress fracture in female navy recruits who took 2000 mg of calcium and 800 IU of vitamin D as daily supplemention.48 Of importance, a meta-analysis of more than 65,000 patients found vitamin D supplementation was effective in reducing fracture risk only when combined with calcium, irrespective of age, sex, or prior fracture.49 In female patients with the female athlete triad, psychological counseling and nutritional consultation are essential in bone health maintenance and long-term prevention.50 Other therapies have been evaluated as well. Use of bisphosphonates is controversial for both treatment and prevention of stress fractures. In a randomized, double-blind study of the potential prophylactic effects of risedronate in 324 new infantry recruits, Milgrom and colleagues51 found no statistically significant differences in tibial, femoral, metatarsal, or total stress fracture incidence between the treatment and placebo groups. Therefore, bisphosphonates are seldom recommended as prevention or in primary management of stress fracture.
In addition to nutritional and pharmacologic therapy, activity modification may have a role in injury prevention. Gait retraining has been identified as a potential intervention for reducing stress fractures in patients with poor biomechanics.47 Crowell and Davis52 investigated the effect of gait retraining on the forces operating in the tibia in runners. After 1 month of gait retraining, tibial acceleration while running decreased by 50%, vertical force loading rate by 30%, and peak vertical force impact by 20%. Such studies indicate the importance of proper mechanics during repetitive activity, especially in patients not as accustomed to the rigorous training methods used with new military recruits. However, whether these reduced loads translate into reduced risk of stress fracture remains unclear. In addition, biomechanical shoe orthoses may lower the stress fracture risk in military recruits by reducing peak tibial strain.53 Warden and colleagues54 found a mechanical loading program was effective in enchaining the structural properties of bone in rats, leading the authors to hypothesize that a similar program aimed at modifying bone structure in humans could help prevent stress fracture. Although there have been no studies of such a strategy in humans, pretraining may be an area for future research, especially for military recruits.
Conclusion
Compared with the general population, members of the military (new recruits in particular) are at increased risk for bone stress injuries. Most of these injuries occur during basic training, when recruits significantly increase their repetitive physical activity. Although the exact pathophysiology of stress injury is debated, nutritional and metabolic abnormalities are contributors. The indolent nature of these injuries, and their high rate of false-negative plain radiographs, may result in a significant delay in diagnosis in the absence of advanced imaging studies. Although a majority of injuries heal with nonoperative management and NWB, several patterns, especially those on the tension side of the bone, are at high risk for progression to fracture and nonunion. These include lateral femoral cortex stress injuries and anterior tibial cortex fractures. There should be a low threshold for operative management in the setting of delayed union or failed nonoperative therapy. Of equal importance to orthopedic management of these injuries is the management of underlying systemic deficits, which may have subjected the patient to injury in the first place. Supplementation with vitamin D and calcium can be an important prophylaxis against stress injury. In addition, military recruits and athletes with underlying metabolic or hormonal deficiencies should receive proper attention with a focus on balancing energy intake and energy expenditure. Stress injury leading to fracture—increasingly common in military populations—often requires a multimodal approach for treatment and subsequent prevention.
Am J Orthop. 2017;46(4):176-183. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Briethaupt MD. Zur Pathologie des menschlichen Fusses [To the pathology of the human foot]. Med Zeitung. 1855;24:169-177.
2. Berger FH, de Jonge MC, Maas M. Stress fractures in the lower extremity. Eur J Radiol. 2007;62(1):16-26.
3. Almeida SA, Williams KM, Shaffer RA, Brodine SK. Epidemiological patterns of musculoskeletal injuries and physical training. Med Sci Sports Exerc. 1999;31(8):1176-1182.
4. Jones BH, Thacker SB, Gilchrist J, Kimsey CD, Sosin DM. Prevention of lower extremity stress fractures in athletes and soldiers: a systematic review. Epidemiol Rev. 2002;24(2):228-247.
5. Jacobs JM, Cameron KL, Bojescul JA. Lower extremity stress fractures in the military. Clin Sports Med. 2014;33(4):591-613.
6. Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181(10):1308-1313.
7. Hsu LL, Nevin RL, Tobler SK, Rubertone MV. Trends in overweight and obesity among 18-year-old applicants to the United States military, 1993–2006. J Adolesc Health. 2007;41(6):610-612.
8. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6(6):391-396.
9. Johnson LC. Histogenesis of stress fractures [annual lecture]. Washington, DC: Armed Forces Institute of Pathology; 1963.
10. Friedenberg ZB. Fatigue fractures of the tibia. Clin Orthop Relat Res. 1971;(76):111-115.
11. Cameron KL, Peck KY, Owens BD, et al. Biomechanical risk factors for lower extremity stress fracture. Orthop J Sports Med. 2013;1(4 suppl).
12. Knapik J, Montain S, McGraw S, Grier T, Ely M, Jones B. Stress fracture risk factors in basic combat training. Int J Sports Med. 2012;33(11):940-946.
13. Behrens SB, Deren ME, Matson A, Fadale PD, Monchik KO. Stress fractures of the pelvis and legs in athletes. Sports Health. 2013;5(2):165-174.
14. Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med. 2014;48(7):491-497.
15. Maitra RS, Johnson DL. Stress fractures. Clinical history and physical examination. Clin Sports Med. 1997;16(2):259-274.
16. Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740-750.
17. Beck BR, Matheson GO, Bergman G, et al. Do capacitively coupled electric fields accelerate tibial stress fracture healing? Am J Sports Med. 2008;36(3):545-553.
18. Simkin A, Leichter I, Giladi M, Stein M, Milgrom C. Combined effect of foot arch structure and an orthotic device on stress fractures. Foot Ankle. 1989;10(1):25-29.
19. Johnson AW, Weiss CB, Wheeler DL. Stress fractures of the femoral shaft in athletes—more common than expected: a new clinical test. Am J Sports Med. 1994;22(2):248-256.
20. Clement D, Ammann W, Taunton J, et al. Exercise-induced stress injuries to the femur. Int J Sports Med. 1993;14(6):347-352.
21. Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem. 1997;34(pt 3):222-229.
22. Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28(2):91-122.
23. Prather JL, Nusynowitz ML, Snowdy HA, Hughes AD, McCartney WH, Bagg RJ. Scintigraphic findings in stress fractures. J Bone Joint Surg Am. 1977;59(7):869-874.
24. Arendt EA, Griffiths HJ. The use of MR imaging in the assessment and clinical management of stress reactions of bone in high-performance athletes. Clin Sports Med. 1997;16(2):291-306.
25. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(6):344-353.
26. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553-561.
27. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
28. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
29. Noakes TD, Smith JA, Lindenberg G, Wills CE. Pelvic stress fractures in long distance runners. Am J Sports Med. 1985;13(2):120-123.
30. Neubauer T, Brand J, Lidder S, Krawany M. Stress fractures of the femoral neck in runners: a review. Res Sports Med. 2016;24(3):283-297.
31. Evans JT, Guyver PM, Kassam AM, Hubble MJW. Displaced femoral neck stress fractures in Royal Marine recruits—management and results of operative treatment. J R Nav Med Serv. 2012;98(2):3-5.
32. Orava S. Stress fractures. Br J Sports Med. 1980;14(1):40-44.
33. Niva MH, Kiuru MJ, Haataja R, Pihlajamäki HK. Fatigue injuries of the femur. J Bone Joint Surg Br. 2005;87(10):1385-1390.
34. Weishaar MD, McMillian DJ, Moore JH. Identification and management of 2 femoral shaft stress injuries. J Orthop Sports Phys Ther. 2005;35(10):665-673.
35. Salminen ST, Pihlajamäki HK, Visuri TI, Böstman OM. Displaced fatigue fractures of the femoral shaft. Clin Orthop Relat Res. 2003;(409):250-259.
36. Giladi M, Ahronson Z, Stein M, Danon YL, Milgrom C. Unusual distribution and onset of stress fractures in soldiers. Clin Orthop Relat Res. 1985;(192):142-146.
37. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
38. Green NE, Rogers RA, Lipscomb AB. Nonunions of stress fractures of the tibia. Am J Sports Med. 1985;13(3):171-176.
39. Orava S, Hulkko A. Stress fracture of the mid-tibial shaft. Acta Orthop Scand. 1984;55(1):35-37.
40. Swenson EJ Jr, DeHaven KE, Sebastianelli WJ, Hanks G, Kalenak A, Lynch JM. The effect of a pneumatic leg brace on return to play in athletes with tibial stress fractures. Am J Sports Med. 1997;25(3):322-328.
41. Rettig AC, Shelbourne KD, McCarroll JR, Bisesi M, Watts J. The natural history and treatment of delayed union stress fractures of the anterior cortex of the tibia. Am J Sports Med. 1988;16(3):250-255.
42. Varner KE, Younas SA, Lintner DM, Marymont JV. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33(7):1071-1076.
43. Donahue SW, Sharkey NA. Strains in the metatarsals during the stance phase of gait: implications for stress fractures. J Bone Joint Surg Am. 1999;81(9):1236-1244.
44. Giuliani J, Masini B, Alitz C, Owens BD. Barefoot-simulating footwear associated with metatarsal stress injury in 2 runners. Orthopedics. 2011;34(7):e320-e323.
45. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
46. Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female army recruits. Osteoporos Int. 2001;12(1):35-42.
47. Friedl KE, Evans RK, Moran DS. Stress fracture and military medical readiness: bridging basic and applied research. Med Sci Sports Exerc. 2008;40(11 suppl):S609-S622.
48. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741-749.
49. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463.
50. Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2(6).
51. Milgrom C, Finestone A, Novack V, et al. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone. 2004;35(2):418-424.
52. Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26(1):78-83.
53. Ekenman I, Milgrom C, Finestone A, et al. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 2002;30(6):866-870.
54. Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20(5):809-816.
1. Briethaupt MD. Zur Pathologie des menschlichen Fusses [To the pathology of the human foot]. Med Zeitung. 1855;24:169-177.
2. Berger FH, de Jonge MC, Maas M. Stress fractures in the lower extremity. Eur J Radiol. 2007;62(1):16-26.
3. Almeida SA, Williams KM, Shaffer RA, Brodine SK. Epidemiological patterns of musculoskeletal injuries and physical training. Med Sci Sports Exerc. 1999;31(8):1176-1182.
4. Jones BH, Thacker SB, Gilchrist J, Kimsey CD, Sosin DM. Prevention of lower extremity stress fractures in athletes and soldiers: a systematic review. Epidemiol Rev. 2002;24(2):228-247.
5. Jacobs JM, Cameron KL, Bojescul JA. Lower extremity stress fractures in the military. Clin Sports Med. 2014;33(4):591-613.
6. Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181(10):1308-1313.
7. Hsu LL, Nevin RL, Tobler SK, Rubertone MV. Trends in overweight and obesity among 18-year-old applicants to the United States military, 1993–2006. J Adolesc Health. 2007;41(6):610-612.
8. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6(6):391-396.
9. Johnson LC. Histogenesis of stress fractures [annual lecture]. Washington, DC: Armed Forces Institute of Pathology; 1963.
10. Friedenberg ZB. Fatigue fractures of the tibia. Clin Orthop Relat Res. 1971;(76):111-115.
11. Cameron KL, Peck KY, Owens BD, et al. Biomechanical risk factors for lower extremity stress fracture. Orthop J Sports Med. 2013;1(4 suppl).
12. Knapik J, Montain S, McGraw S, Grier T, Ely M, Jones B. Stress fracture risk factors in basic combat training. Int J Sports Med. 2012;33(11):940-946.
13. Behrens SB, Deren ME, Matson A, Fadale PD, Monchik KO. Stress fractures of the pelvis and legs in athletes. Sports Health. 2013;5(2):165-174.
14. Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med. 2014;48(7):491-497.
15. Maitra RS, Johnson DL. Stress fractures. Clinical history and physical examination. Clin Sports Med. 1997;16(2):259-274.
16. Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740-750.
17. Beck BR, Matheson GO, Bergman G, et al. Do capacitively coupled electric fields accelerate tibial stress fracture healing? Am J Sports Med. 2008;36(3):545-553.
18. Simkin A, Leichter I, Giladi M, Stein M, Milgrom C. Combined effect of foot arch structure and an orthotic device on stress fractures. Foot Ankle. 1989;10(1):25-29.
19. Johnson AW, Weiss CB, Wheeler DL. Stress fractures of the femoral shaft in athletes—more common than expected: a new clinical test. Am J Sports Med. 1994;22(2):248-256.
20. Clement D, Ammann W, Taunton J, et al. Exercise-induced stress injuries to the femur. Int J Sports Med. 1993;14(6):347-352.
21. Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem. 1997;34(pt 3):222-229.
22. Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28(2):91-122.
23. Prather JL, Nusynowitz ML, Snowdy HA, Hughes AD, McCartney WH, Bagg RJ. Scintigraphic findings in stress fractures. J Bone Joint Surg Am. 1977;59(7):869-874.
24. Arendt EA, Griffiths HJ. The use of MR imaging in the assessment and clinical management of stress reactions of bone in high-performance athletes. Clin Sports Med. 1997;16(2):291-306.
25. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(6):344-353.
26. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553-561.
27. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
28. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
29. Noakes TD, Smith JA, Lindenberg G, Wills CE. Pelvic stress fractures in long distance runners. Am J Sports Med. 1985;13(2):120-123.
30. Neubauer T, Brand J, Lidder S, Krawany M. Stress fractures of the femoral neck in runners: a review. Res Sports Med. 2016;24(3):283-297.
31. Evans JT, Guyver PM, Kassam AM, Hubble MJW. Displaced femoral neck stress fractures in Royal Marine recruits—management and results of operative treatment. J R Nav Med Serv. 2012;98(2):3-5.
32. Orava S. Stress fractures. Br J Sports Med. 1980;14(1):40-44.
33. Niva MH, Kiuru MJ, Haataja R, Pihlajamäki HK. Fatigue injuries of the femur. J Bone Joint Surg Br. 2005;87(10):1385-1390.
34. Weishaar MD, McMillian DJ, Moore JH. Identification and management of 2 femoral shaft stress injuries. J Orthop Sports Phys Ther. 2005;35(10):665-673.
35. Salminen ST, Pihlajamäki HK, Visuri TI, Böstman OM. Displaced fatigue fractures of the femoral shaft. Clin Orthop Relat Res. 2003;(409):250-259.
36. Giladi M, Ahronson Z, Stein M, Danon YL, Milgrom C. Unusual distribution and onset of stress fractures in soldiers. Clin Orthop Relat Res. 1985;(192):142-146.
37. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
38. Green NE, Rogers RA, Lipscomb AB. Nonunions of stress fractures of the tibia. Am J Sports Med. 1985;13(3):171-176.
39. Orava S, Hulkko A. Stress fracture of the mid-tibial shaft. Acta Orthop Scand. 1984;55(1):35-37.
40. Swenson EJ Jr, DeHaven KE, Sebastianelli WJ, Hanks G, Kalenak A, Lynch JM. The effect of a pneumatic leg brace on return to play in athletes with tibial stress fractures. Am J Sports Med. 1997;25(3):322-328.
41. Rettig AC, Shelbourne KD, McCarroll JR, Bisesi M, Watts J. The natural history and treatment of delayed union stress fractures of the anterior cortex of the tibia. Am J Sports Med. 1988;16(3):250-255.
42. Varner KE, Younas SA, Lintner DM, Marymont JV. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33(7):1071-1076.
43. Donahue SW, Sharkey NA. Strains in the metatarsals during the stance phase of gait: implications for stress fractures. J Bone Joint Surg Am. 1999;81(9):1236-1244.
44. Giuliani J, Masini B, Alitz C, Owens BD. Barefoot-simulating footwear associated with metatarsal stress injury in 2 runners. Orthopedics. 2011;34(7):e320-e323.
45. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
46. Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female army recruits. Osteoporos Int. 2001;12(1):35-42.
47. Friedl KE, Evans RK, Moran DS. Stress fracture and military medical readiness: bridging basic and applied research. Med Sci Sports Exerc. 2008;40(11 suppl):S609-S622.
48. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741-749.
49. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463.
50. Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2(6).
51. Milgrom C, Finestone A, Novack V, et al. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone. 2004;35(2):418-424.
52. Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26(1):78-83.
53. Ekenman I, Milgrom C, Finestone A, et al. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 2002;30(6):866-870.
54. Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20(5):809-816.
In Memoriam
Warren R. Kadrmas, MD, COL, MC, USAF
November 6, 1969-May 8, 2014
Matthew T. Provencher, MD, CAPT, MC, USNR, and John M. Tokish, MD
A Selfless Leader and Former Head of Air Force Orthopedics
In 2014, we tragically lost a true friend, outstanding clinician, great family man, and incredible human being. As one of the preeminent sports orthopedists in the military, Dr. Kadrmas was beloved by all and heralded for his many selfless contributions to military musculoskeletal medicine and injury prevention. He was known for his humble nature and steadfast integrity, and served as an exemplary role model whom we all aspired to emulate. We all remember our time with Warren fondly, and he left us all with lasting memories to cherish and countless stories sure to regale.
Warren Kadrmas was born in 1969 in Vermillion, South Dakota and grew up in Sheridan, Wyoming. Dr. Kadrmas graduated with distinction from both the US Air Force Academy in 1992 and Duke University School of Medicine in 1996. He then went on to complete his residency in 2003 at the Hospital for Special Surgery (HSS) in New York City and was recognized with the Jean C. McDaniel Outstanding Resident Award. He began his Air Force orthopedic career at Wilford Hall Ambulatory Surgical Center on the grounds of Lackland Air Force Base in San Antonio, Texas as part of the 59th Medical Wing. Warren was deployed and served as 1 of 5 people on the mobile-field surgical team assigned to the 379th Expeditionary Medical Group. Subsequently, he returned to HSS, where he excelled in sports medicine and shoulder service subspecialty training.
After his fellowship, Warren returned to San Antonio to continue his work as a top military sports surgeon, serving as a mentor, educator, and leader for all of Air Force orthopedics. During this time he served several tours overseas, becoming an invaluable member of the 332nd Expeditionary Medical Group operating out of the Air Force Theater Hospital at Balad Air Base, Iraq. Warren served as the Program Director of the Orthopedic Residency Program at Wilford Hall Ambulatory Surgical Center. He held the position of Head of Orthopedics for the Air Force as Orthopedic Surgery Consultant to the Air Force Surgeon General for 5 years, a role that entailed coordinating all orthopedic assets for the Global War on Terror for the Air Force. Selfless to a fault, he would never ask anything of anyone that he had not done himself. He completed 6 deployments away from family, loved ones, and work in San Antonio.
A true innovator and visionary, Warren was a pioneer in the integration of high-caliber hip arthroscopy, as well as cutting-edge shoulder and knee care for our active-duty military personnel. He was a prominent member of the American Orthopaedic Society for Sports Medicine (AOSSM) and Arthroscopy Association of North America, and was in line to be the incoming President of the Society of Military Orthopaedic Surgeons, after having previously served as the society’s 2nd Vice President. He was selected for and was scheduled to participate in the AOSSM Traveling Fellowship touring Asia just prior to his untimely accident.
One of Warren’s favorite quotes was on the topic of leading from behind. Nelson Mandela said, “It is better to lead from behind and to put others in front, especially when you celebrate victory when nice things occur. You take the front line when there is danger. Then people will appreciate your leadership.” Warren was the embodiment of this quote. He led from the front, and by example, in times of danger to inspire those he led. But he also honed the skill of leading from behind, with quiet self-sacrifice, to celebrate the success of those he led. His tireless dedication was prominent in all the facets of his life, whether as a father, son, brother, surgeon, educator, mentor, or friend. We miss him dearly, and try to embody his spirit by living our lives through what he taught us all.
Brian Allgood, MD, COL
1960-2007
Dean Taylor, MD
An Exemplary Selfless Leader in Orthopedics and Medicine
When people ask me what effective, ethical healthcare leadership looks like, I think of Brian Allgood. Brian was the epitome of leadership. He led quietly, by example and selflessly–always putting the interests of patients and those on his team ahead of his own.
Brian was a 1982 graduate of the United States Military Academy at West Point, and received a Doctor of Medicine degree from the University of Oklahoma. He completed his orthopedic training at Brooke Army Medical Center in San Antonio. I first met Brian in 1994 when he was practicing as an orthopedic surgeon at Womack Army Medical Center at Ft. Bragg, North Carolina, where he also served at the Division Surgeon for the 82nd Airborne Division. At the time, I was extremely impressed with Brian’s outstanding orthopedic skills, and his unwavering commitment to leadership in orthopedics, military medicine, and medicine.
Brian’s role as the 82nd Airborne Division Surgeon was on the leadership track in Army medicine, a track that many of us who enjoyed and were good at patient care shunned because it was structured to limit the amount of time an administrative leader could spend in patient care. Brian was certainly a skilled orthopedic surgeon who loved caring for patients; however, he was courageous enough to put his responsibility to military medicine and the medical profession ahead of his own clinical interests. He realized that he could provide exceptional leadership that would benefit many instead of only those in his sphere of care. And what an exceptional leader he was!
From 2002 to 2004, I saw firsthand Brian’s extraordinary leadership when he served as the hospital commander of Keller Army Community Hospital at West Point. He was the best hospital commander I worked with during my 11 years at West Point. I saw the sacrifices he made for the rest of us. He gave up something he loved–orthopedic surgery–so that he could effectively lead our hospital. While we operated, he occasionally would look longingly through the operating room (OR) windows. When we saw him, we would invite him to scrub in, much to his delight. He would also show up in other services’ ORs and the hospital’s clinics, staying connected to patients and patient care. This patient-centeredness contributed significantly to the beloved leader he was.
Brian’s final assignment was in 2006 as the Command Surgeon of Multi-National Forces, the highest-ranking medical officer in Iraq. On January 20, 2007, Brian Allgood—on the verge of promotion to brigadier general and on the fast track to Surgeon General of the Army—was killed along with 11 other American service members when their helicopter was shot down.
In his life, Brian was an exemplary leader. After his death, he lives on in our memories as an example to which we should all aspire–an ethical, selfless leader who cared for all patients, always striving to do the right thing.
LCpl Benjamin Whetstone Schmidt
1987-2011
David R. Schmidt, MD
A Fallen Hero’s Legacy
On September 11, 2011, LCpl Benjamin Whetstone Schmidt posted on his Facebook page, “I guess you can use today as a reason for us to be here in Afghanistan. Just know I am fighting for myself, but most of all for my friends and family who read this. Everyone, it’s an honor to be your ambassador.”
Benjamin was a Marine Scout Sniper on his second tour to Afghanistan, this time voluntarily. Not one member of his platoon had combat experience. He felt called to lead, to be with his boys. During his first deployment to Afghanistan he was awarded the Navy/USMC Achievement Medal with Valor for his action in combat.
Less than a month later, on October 6, 2011, he was killed while on patrol in Helmand Province. Even now, 6 years after his death, his comrades continue to hail his virtues as a leader, a friend, a patriot, and an inspiration. He was also a fine athlete and a courageous, energetic young man with bold plans for his future.
Other than his family, few knew what Benjamin would inspire in his death. He left $200,000 of his life insurance to establish a scholarship in the History Department at his beloved Texas Christian University (TCU). With a matching gift from his father, orthopedic surgeon David R. Schmidt, MD, and stepmom Teresa, the scholarship provides annual funding for a graduate student. Asked why he chose to support graduate students, Benjamin replied with his signature humor and wisdom, “I wouldn’t invest in a freshman like myself.” Benjamin had spent 2 years at TCU prior to enlisting in the Marine Corps, and intended to return to TCU to complete his undergraduate and graduate degrees.
Certainly not many young men at age 24 years, prior to going to war, have the foresight to envision and implement a legacy bigger than themselves, with the promise of influencing generations into the future. For his actions, Benjamin was a finalist for a Congressional Medal of Honor Society “Citizen Service Before Self” award.
David and Teresa Schmidt subsequently raised $1 million dollars to endow the LCpl Benjamin W. Schmidt Professor of War, Conflict and Society. It is truly inspirational to know that a young man’s selfless vision and his friends’ and family’s support could produce such a lasting legacy.
Warren R. Kadrmas, MD, COL, MC, USAF
November 6, 1969-May 8, 2014
Matthew T. Provencher, MD, CAPT, MC, USNR, and John M. Tokish, MD
A Selfless Leader and Former Head of Air Force Orthopedics
In 2014, we tragically lost a true friend, outstanding clinician, great family man, and incredible human being. As one of the preeminent sports orthopedists in the military, Dr. Kadrmas was beloved by all and heralded for his many selfless contributions to military musculoskeletal medicine and injury prevention. He was known for his humble nature and steadfast integrity, and served as an exemplary role model whom we all aspired to emulate. We all remember our time with Warren fondly, and he left us all with lasting memories to cherish and countless stories sure to regale.
Warren Kadrmas was born in 1969 in Vermillion, South Dakota and grew up in Sheridan, Wyoming. Dr. Kadrmas graduated with distinction from both the US Air Force Academy in 1992 and Duke University School of Medicine in 1996. He then went on to complete his residency in 2003 at the Hospital for Special Surgery (HSS) in New York City and was recognized with the Jean C. McDaniel Outstanding Resident Award. He began his Air Force orthopedic career at Wilford Hall Ambulatory Surgical Center on the grounds of Lackland Air Force Base in San Antonio, Texas as part of the 59th Medical Wing. Warren was deployed and served as 1 of 5 people on the mobile-field surgical team assigned to the 379th Expeditionary Medical Group. Subsequently, he returned to HSS, where he excelled in sports medicine and shoulder service subspecialty training.
After his fellowship, Warren returned to San Antonio to continue his work as a top military sports surgeon, serving as a mentor, educator, and leader for all of Air Force orthopedics. During this time he served several tours overseas, becoming an invaluable member of the 332nd Expeditionary Medical Group operating out of the Air Force Theater Hospital at Balad Air Base, Iraq. Warren served as the Program Director of the Orthopedic Residency Program at Wilford Hall Ambulatory Surgical Center. He held the position of Head of Orthopedics for the Air Force as Orthopedic Surgery Consultant to the Air Force Surgeon General for 5 years, a role that entailed coordinating all orthopedic assets for the Global War on Terror for the Air Force. Selfless to a fault, he would never ask anything of anyone that he had not done himself. He completed 6 deployments away from family, loved ones, and work in San Antonio.
A true innovator and visionary, Warren was a pioneer in the integration of high-caliber hip arthroscopy, as well as cutting-edge shoulder and knee care for our active-duty military personnel. He was a prominent member of the American Orthopaedic Society for Sports Medicine (AOSSM) and Arthroscopy Association of North America, and was in line to be the incoming President of the Society of Military Orthopaedic Surgeons, after having previously served as the society’s 2nd Vice President. He was selected for and was scheduled to participate in the AOSSM Traveling Fellowship touring Asia just prior to his untimely accident.
One of Warren’s favorite quotes was on the topic of leading from behind. Nelson Mandela said, “It is better to lead from behind and to put others in front, especially when you celebrate victory when nice things occur. You take the front line when there is danger. Then people will appreciate your leadership.” Warren was the embodiment of this quote. He led from the front, and by example, in times of danger to inspire those he led. But he also honed the skill of leading from behind, with quiet self-sacrifice, to celebrate the success of those he led. His tireless dedication was prominent in all the facets of his life, whether as a father, son, brother, surgeon, educator, mentor, or friend. We miss him dearly, and try to embody his spirit by living our lives through what he taught us all.
Brian Allgood, MD, COL
1960-2007
Dean Taylor, MD
An Exemplary Selfless Leader in Orthopedics and Medicine
When people ask me what effective, ethical healthcare leadership looks like, I think of Brian Allgood. Brian was the epitome of leadership. He led quietly, by example and selflessly–always putting the interests of patients and those on his team ahead of his own.
Brian was a 1982 graduate of the United States Military Academy at West Point, and received a Doctor of Medicine degree from the University of Oklahoma. He completed his orthopedic training at Brooke Army Medical Center in San Antonio. I first met Brian in 1994 when he was practicing as an orthopedic surgeon at Womack Army Medical Center at Ft. Bragg, North Carolina, where he also served at the Division Surgeon for the 82nd Airborne Division. At the time, I was extremely impressed with Brian’s outstanding orthopedic skills, and his unwavering commitment to leadership in orthopedics, military medicine, and medicine.
Brian’s role as the 82nd Airborne Division Surgeon was on the leadership track in Army medicine, a track that many of us who enjoyed and were good at patient care shunned because it was structured to limit the amount of time an administrative leader could spend in patient care. Brian was certainly a skilled orthopedic surgeon who loved caring for patients; however, he was courageous enough to put his responsibility to military medicine and the medical profession ahead of his own clinical interests. He realized that he could provide exceptional leadership that would benefit many instead of only those in his sphere of care. And what an exceptional leader he was!
From 2002 to 2004, I saw firsthand Brian’s extraordinary leadership when he served as the hospital commander of Keller Army Community Hospital at West Point. He was the best hospital commander I worked with during my 11 years at West Point. I saw the sacrifices he made for the rest of us. He gave up something he loved–orthopedic surgery–so that he could effectively lead our hospital. While we operated, he occasionally would look longingly through the operating room (OR) windows. When we saw him, we would invite him to scrub in, much to his delight. He would also show up in other services’ ORs and the hospital’s clinics, staying connected to patients and patient care. This patient-centeredness contributed significantly to the beloved leader he was.
Brian’s final assignment was in 2006 as the Command Surgeon of Multi-National Forces, the highest-ranking medical officer in Iraq. On January 20, 2007, Brian Allgood—on the verge of promotion to brigadier general and on the fast track to Surgeon General of the Army—was killed along with 11 other American service members when their helicopter was shot down.
In his life, Brian was an exemplary leader. After his death, he lives on in our memories as an example to which we should all aspire–an ethical, selfless leader who cared for all patients, always striving to do the right thing.
LCpl Benjamin Whetstone Schmidt
1987-2011
David R. Schmidt, MD
A Fallen Hero’s Legacy
On September 11, 2011, LCpl Benjamin Whetstone Schmidt posted on his Facebook page, “I guess you can use today as a reason for us to be here in Afghanistan. Just know I am fighting for myself, but most of all for my friends and family who read this. Everyone, it’s an honor to be your ambassador.”
Benjamin was a Marine Scout Sniper on his second tour to Afghanistan, this time voluntarily. Not one member of his platoon had combat experience. He felt called to lead, to be with his boys. During his first deployment to Afghanistan he was awarded the Navy/USMC Achievement Medal with Valor for his action in combat.
Less than a month later, on October 6, 2011, he was killed while on patrol in Helmand Province. Even now, 6 years after his death, his comrades continue to hail his virtues as a leader, a friend, a patriot, and an inspiration. He was also a fine athlete and a courageous, energetic young man with bold plans for his future.
Other than his family, few knew what Benjamin would inspire in his death. He left $200,000 of his life insurance to establish a scholarship in the History Department at his beloved Texas Christian University (TCU). With a matching gift from his father, orthopedic surgeon David R. Schmidt, MD, and stepmom Teresa, the scholarship provides annual funding for a graduate student. Asked why he chose to support graduate students, Benjamin replied with his signature humor and wisdom, “I wouldn’t invest in a freshman like myself.” Benjamin had spent 2 years at TCU prior to enlisting in the Marine Corps, and intended to return to TCU to complete his undergraduate and graduate degrees.
Certainly not many young men at age 24 years, prior to going to war, have the foresight to envision and implement a legacy bigger than themselves, with the promise of influencing generations into the future. For his actions, Benjamin was a finalist for a Congressional Medal of Honor Society “Citizen Service Before Self” award.
David and Teresa Schmidt subsequently raised $1 million dollars to endow the LCpl Benjamin W. Schmidt Professor of War, Conflict and Society. It is truly inspirational to know that a young man’s selfless vision and his friends’ and family’s support could produce such a lasting legacy.
Warren R. Kadrmas, MD, COL, MC, USAF
November 6, 1969-May 8, 2014
Matthew T. Provencher, MD, CAPT, MC, USNR, and John M. Tokish, MD
A Selfless Leader and Former Head of Air Force Orthopedics
In 2014, we tragically lost a true friend, outstanding clinician, great family man, and incredible human being. As one of the preeminent sports orthopedists in the military, Dr. Kadrmas was beloved by all and heralded for his many selfless contributions to military musculoskeletal medicine and injury prevention. He was known for his humble nature and steadfast integrity, and served as an exemplary role model whom we all aspired to emulate. We all remember our time with Warren fondly, and he left us all with lasting memories to cherish and countless stories sure to regale.
Warren Kadrmas was born in 1969 in Vermillion, South Dakota and grew up in Sheridan, Wyoming. Dr. Kadrmas graduated with distinction from both the US Air Force Academy in 1992 and Duke University School of Medicine in 1996. He then went on to complete his residency in 2003 at the Hospital for Special Surgery (HSS) in New York City and was recognized with the Jean C. McDaniel Outstanding Resident Award. He began his Air Force orthopedic career at Wilford Hall Ambulatory Surgical Center on the grounds of Lackland Air Force Base in San Antonio, Texas as part of the 59th Medical Wing. Warren was deployed and served as 1 of 5 people on the mobile-field surgical team assigned to the 379th Expeditionary Medical Group. Subsequently, he returned to HSS, where he excelled in sports medicine and shoulder service subspecialty training.
After his fellowship, Warren returned to San Antonio to continue his work as a top military sports surgeon, serving as a mentor, educator, and leader for all of Air Force orthopedics. During this time he served several tours overseas, becoming an invaluable member of the 332nd Expeditionary Medical Group operating out of the Air Force Theater Hospital at Balad Air Base, Iraq. Warren served as the Program Director of the Orthopedic Residency Program at Wilford Hall Ambulatory Surgical Center. He held the position of Head of Orthopedics for the Air Force as Orthopedic Surgery Consultant to the Air Force Surgeon General for 5 years, a role that entailed coordinating all orthopedic assets for the Global War on Terror for the Air Force. Selfless to a fault, he would never ask anything of anyone that he had not done himself. He completed 6 deployments away from family, loved ones, and work in San Antonio.
A true innovator and visionary, Warren was a pioneer in the integration of high-caliber hip arthroscopy, as well as cutting-edge shoulder and knee care for our active-duty military personnel. He was a prominent member of the American Orthopaedic Society for Sports Medicine (AOSSM) and Arthroscopy Association of North America, and was in line to be the incoming President of the Society of Military Orthopaedic Surgeons, after having previously served as the society’s 2nd Vice President. He was selected for and was scheduled to participate in the AOSSM Traveling Fellowship touring Asia just prior to his untimely accident.
One of Warren’s favorite quotes was on the topic of leading from behind. Nelson Mandela said, “It is better to lead from behind and to put others in front, especially when you celebrate victory when nice things occur. You take the front line when there is danger. Then people will appreciate your leadership.” Warren was the embodiment of this quote. He led from the front, and by example, in times of danger to inspire those he led. But he also honed the skill of leading from behind, with quiet self-sacrifice, to celebrate the success of those he led. His tireless dedication was prominent in all the facets of his life, whether as a father, son, brother, surgeon, educator, mentor, or friend. We miss him dearly, and try to embody his spirit by living our lives through what he taught us all.
Brian Allgood, MD, COL
1960-2007
Dean Taylor, MD
An Exemplary Selfless Leader in Orthopedics and Medicine
When people ask me what effective, ethical healthcare leadership looks like, I think of Brian Allgood. Brian was the epitome of leadership. He led quietly, by example and selflessly–always putting the interests of patients and those on his team ahead of his own.
Brian was a 1982 graduate of the United States Military Academy at West Point, and received a Doctor of Medicine degree from the University of Oklahoma. He completed his orthopedic training at Brooke Army Medical Center in San Antonio. I first met Brian in 1994 when he was practicing as an orthopedic surgeon at Womack Army Medical Center at Ft. Bragg, North Carolina, where he also served at the Division Surgeon for the 82nd Airborne Division. At the time, I was extremely impressed with Brian’s outstanding orthopedic skills, and his unwavering commitment to leadership in orthopedics, military medicine, and medicine.
Brian’s role as the 82nd Airborne Division Surgeon was on the leadership track in Army medicine, a track that many of us who enjoyed and were good at patient care shunned because it was structured to limit the amount of time an administrative leader could spend in patient care. Brian was certainly a skilled orthopedic surgeon who loved caring for patients; however, he was courageous enough to put his responsibility to military medicine and the medical profession ahead of his own clinical interests. He realized that he could provide exceptional leadership that would benefit many instead of only those in his sphere of care. And what an exceptional leader he was!
From 2002 to 2004, I saw firsthand Brian’s extraordinary leadership when he served as the hospital commander of Keller Army Community Hospital at West Point. He was the best hospital commander I worked with during my 11 years at West Point. I saw the sacrifices he made for the rest of us. He gave up something he loved–orthopedic surgery–so that he could effectively lead our hospital. While we operated, he occasionally would look longingly through the operating room (OR) windows. When we saw him, we would invite him to scrub in, much to his delight. He would also show up in other services’ ORs and the hospital’s clinics, staying connected to patients and patient care. This patient-centeredness contributed significantly to the beloved leader he was.
Brian’s final assignment was in 2006 as the Command Surgeon of Multi-National Forces, the highest-ranking medical officer in Iraq. On January 20, 2007, Brian Allgood—on the verge of promotion to brigadier general and on the fast track to Surgeon General of the Army—was killed along with 11 other American service members when their helicopter was shot down.
In his life, Brian was an exemplary leader. After his death, he lives on in our memories as an example to which we should all aspire–an ethical, selfless leader who cared for all patients, always striving to do the right thing.
LCpl Benjamin Whetstone Schmidt
1987-2011
David R. Schmidt, MD
A Fallen Hero’s Legacy
On September 11, 2011, LCpl Benjamin Whetstone Schmidt posted on his Facebook page, “I guess you can use today as a reason for us to be here in Afghanistan. Just know I am fighting for myself, but most of all for my friends and family who read this. Everyone, it’s an honor to be your ambassador.”
Benjamin was a Marine Scout Sniper on his second tour to Afghanistan, this time voluntarily. Not one member of his platoon had combat experience. He felt called to lead, to be with his boys. During his first deployment to Afghanistan he was awarded the Navy/USMC Achievement Medal with Valor for his action in combat.
Less than a month later, on October 6, 2011, he was killed while on patrol in Helmand Province. Even now, 6 years after his death, his comrades continue to hail his virtues as a leader, a friend, a patriot, and an inspiration. He was also a fine athlete and a courageous, energetic young man with bold plans for his future.
Other than his family, few knew what Benjamin would inspire in his death. He left $200,000 of his life insurance to establish a scholarship in the History Department at his beloved Texas Christian University (TCU). With a matching gift from his father, orthopedic surgeon David R. Schmidt, MD, and stepmom Teresa, the scholarship provides annual funding for a graduate student. Asked why he chose to support graduate students, Benjamin replied with his signature humor and wisdom, “I wouldn’t invest in a freshman like myself.” Benjamin had spent 2 years at TCU prior to enlisting in the Marine Corps, and intended to return to TCU to complete his undergraduate and graduate degrees.
Certainly not many young men at age 24 years, prior to going to war, have the foresight to envision and implement a legacy bigger than themselves, with the promise of influencing generations into the future. For his actions, Benjamin was a finalist for a Congressional Medal of Honor Society “Citizen Service Before Self” award.
David and Teresa Schmidt subsequently raised $1 million dollars to endow the LCpl Benjamin W. Schmidt Professor of War, Conflict and Society. It is truly inspirational to know that a young man’s selfless vision and his friends’ and family’s support could produce such a lasting legacy.
Applying Military Strategy to Complex Knee Reconstruction: Tips for Planning and Executing Advanced Surgery
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
Home of the Brave
This Memorial Day, with all that is taking place in the world, it was hard not to think about the brave men and women who have sacrificed so much to preserve our freedom. They are away from their families, sometimes for years at a time, and they operate in the most dangerous places in the world. A safe return is not guaranteed. I am thankful for these intrepid men and women whose sacrifices and commitment to their country allow me to live comfortably at home with my family and practice orthopedic surgery.
A few years ago, my wife and I traveled to Normandy and visited the American cemetery. It was a moving experience that I will never forget. We then toured Pointe du Hoc, the elevated peninsula separating Omaha and Utah beach and the location of German gun emplacements covering both beaches. The bunkers, and even the craters from the bombs, are still there. Army Rangers were tasked with launching an amphibious assault on the beach and then scaling the 100-foot cliffs using grappling hooks, ropes, and ladders. Once at the top, they faced a heavily fortified German force that was dug in. Looking down at the beach and out over the ocean from above, I thought of the troops who landed there and the impossible task they faced. Despite the overwhelming odds stacked against them, the Rangers took Pointe du Hoc in 25 minutes and then repelled multiple counterattacks with their backs against the cliff. In my opinion, it’s one of the greatest testaments to the incredible determination and ability of our military personnel.
Speaking of incredible ability, AJO would like to recognize our military orthopedists. They are often deployed in combat zones and provide the best of care for our soldiers while working in the most stressful of conditions, and doing it all on a government salary. In their spare time, they’ve contributed so much to the orthopedic literature, authoring numerous landmark articles.
In this issue, AJO looks at classic military injuries: shoulder instability, stress fractures, and multi-ligamentous knee injuries. Provencher and colleagues authored a comprehensive review of instability with current guidelines for determining surgical approach. DeBerardino shows our readers how to take a military approach to multi-ligament and complex knee injuries, and Owens and colleagues provide a guide to the diagnosis and treatment of stress injuries to bone.
We also take a moment to recognize 3 members of our military orthopedic family whose lives were tragically cut short. Warren R. Kadrmas, Brian Allgood, and Benjamin Whetstone Schmidt’s memorials are included on the following pages. Benjamin Whetstone Schmidt, son of orthopedist David R. Schmidt from San Antonio, was a Marine Sniper killed in action in Afghanistan after volunteering for a second tour. After his death, the LCpl Benjamin Whetstone Schmidt Endowed Professorship in History was created at the Texas Christian University. Contributions can be made in his memory at www.heartofpurple.com.
As Independence Day is celebrated, AJO is pleased to present “Military Orthopedics” to honor our troops and the military doctors who support them. As you read this issue, take a moment to reflect on the freedoms you enjoy because America is truly the Home of the Brave.
Am J Orthop. 2017;46(4):166. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
This Memorial Day, with all that is taking place in the world, it was hard not to think about the brave men and women who have sacrificed so much to preserve our freedom. They are away from their families, sometimes for years at a time, and they operate in the most dangerous places in the world. A safe return is not guaranteed. I am thankful for these intrepid men and women whose sacrifices and commitment to their country allow me to live comfortably at home with my family and practice orthopedic surgery.
A few years ago, my wife and I traveled to Normandy and visited the American cemetery. It was a moving experience that I will never forget. We then toured Pointe du Hoc, the elevated peninsula separating Omaha and Utah beach and the location of German gun emplacements covering both beaches. The bunkers, and even the craters from the bombs, are still there. Army Rangers were tasked with launching an amphibious assault on the beach and then scaling the 100-foot cliffs using grappling hooks, ropes, and ladders. Once at the top, they faced a heavily fortified German force that was dug in. Looking down at the beach and out over the ocean from above, I thought of the troops who landed there and the impossible task they faced. Despite the overwhelming odds stacked against them, the Rangers took Pointe du Hoc in 25 minutes and then repelled multiple counterattacks with their backs against the cliff. In my opinion, it’s one of the greatest testaments to the incredible determination and ability of our military personnel.
Speaking of incredible ability, AJO would like to recognize our military orthopedists. They are often deployed in combat zones and provide the best of care for our soldiers while working in the most stressful of conditions, and doing it all on a government salary. In their spare time, they’ve contributed so much to the orthopedic literature, authoring numerous landmark articles.
In this issue, AJO looks at classic military injuries: shoulder instability, stress fractures, and multi-ligamentous knee injuries. Provencher and colleagues authored a comprehensive review of instability with current guidelines for determining surgical approach. DeBerardino shows our readers how to take a military approach to multi-ligament and complex knee injuries, and Owens and colleagues provide a guide to the diagnosis and treatment of stress injuries to bone.
We also take a moment to recognize 3 members of our military orthopedic family whose lives were tragically cut short. Warren R. Kadrmas, Brian Allgood, and Benjamin Whetstone Schmidt’s memorials are included on the following pages. Benjamin Whetstone Schmidt, son of orthopedist David R. Schmidt from San Antonio, was a Marine Sniper killed in action in Afghanistan after volunteering for a second tour. After his death, the LCpl Benjamin Whetstone Schmidt Endowed Professorship in History was created at the Texas Christian University. Contributions can be made in his memory at www.heartofpurple.com.
As Independence Day is celebrated, AJO is pleased to present “Military Orthopedics” to honor our troops and the military doctors who support them. As you read this issue, take a moment to reflect on the freedoms you enjoy because America is truly the Home of the Brave.
Am J Orthop. 2017;46(4):166. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
This Memorial Day, with all that is taking place in the world, it was hard not to think about the brave men and women who have sacrificed so much to preserve our freedom. They are away from their families, sometimes for years at a time, and they operate in the most dangerous places in the world. A safe return is not guaranteed. I am thankful for these intrepid men and women whose sacrifices and commitment to their country allow me to live comfortably at home with my family and practice orthopedic surgery.
A few years ago, my wife and I traveled to Normandy and visited the American cemetery. It was a moving experience that I will never forget. We then toured Pointe du Hoc, the elevated peninsula separating Omaha and Utah beach and the location of German gun emplacements covering both beaches. The bunkers, and even the craters from the bombs, are still there. Army Rangers were tasked with launching an amphibious assault on the beach and then scaling the 100-foot cliffs using grappling hooks, ropes, and ladders. Once at the top, they faced a heavily fortified German force that was dug in. Looking down at the beach and out over the ocean from above, I thought of the troops who landed there and the impossible task they faced. Despite the overwhelming odds stacked against them, the Rangers took Pointe du Hoc in 25 minutes and then repelled multiple counterattacks with their backs against the cliff. In my opinion, it’s one of the greatest testaments to the incredible determination and ability of our military personnel.
Speaking of incredible ability, AJO would like to recognize our military orthopedists. They are often deployed in combat zones and provide the best of care for our soldiers while working in the most stressful of conditions, and doing it all on a government salary. In their spare time, they’ve contributed so much to the orthopedic literature, authoring numerous landmark articles.
In this issue, AJO looks at classic military injuries: shoulder instability, stress fractures, and multi-ligamentous knee injuries. Provencher and colleagues authored a comprehensive review of instability with current guidelines for determining surgical approach. DeBerardino shows our readers how to take a military approach to multi-ligament and complex knee injuries, and Owens and colleagues provide a guide to the diagnosis and treatment of stress injuries to bone.
We also take a moment to recognize 3 members of our military orthopedic family whose lives were tragically cut short. Warren R. Kadrmas, Brian Allgood, and Benjamin Whetstone Schmidt’s memorials are included on the following pages. Benjamin Whetstone Schmidt, son of orthopedist David R. Schmidt from San Antonio, was a Marine Sniper killed in action in Afghanistan after volunteering for a second tour. After his death, the LCpl Benjamin Whetstone Schmidt Endowed Professorship in History was created at the Texas Christian University. Contributions can be made in his memory at www.heartofpurple.com.
As Independence Day is celebrated, AJO is pleased to present “Military Orthopedics” to honor our troops and the military doctors who support them. As you read this issue, take a moment to reflect on the freedoms you enjoy because America is truly the Home of the Brave.
Am J Orthop. 2017;46(4):166. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
VIDEO: Hip, knee replacements fall in Danish RA patients
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
[email protected]
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
[email protected]
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: RA patient hip replacements fell from nearly 9/1,000 person-years in 1996 to about 4.5/1,000 person-years in 2011.
Data source: Records from more than 300,000 people in the Danish National Patient Register.
Disclosures: Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
How to prevent secondary posttraumatic knee osteoarthritis
LAS VEGAS – A variety of evidence-based strategies are available for preventing posttraumatic knee osteoarthritis (KOA) in patients who have already sustained an anterior cruciate ligament (ACL) injury. And they’re generally ignored, according to May Arna Risberg, PhD.
“We have a lot of knowledge. We can use secondary prevention strategies. And here I think we, as physical therapists, physicians, and orthopedic surgeons, are doing a lousy job because we are sending these ACL-injured patients back to sports before they have normalized knee function and quadriceps strength,” said Dr. Risberg, professor of sports medicine at the Norwegian School of Sport Sciences in Oslo.
With no proven disease-modifying therapy for KOA available to date, secondary prevention of posttraumatic KOA is worthy of high-priority status, she said at the World Congress on Osteoarthritis. An estimated 250,00 ACL injuries occur annually in the United States, and up to one-half of affected patients, most of whom are young, active people, will experience a second ACL rupture within the first few years after undergoing their initial reconstruction. This second ACL injury greatly increases their risk of developing posttraumatic KOA within 15-20 years, while they are still relatively young, she said.
Moreover, if the second ACL injury involves meniscus surgery, the 5-year risk of posttraumatic KOA roughly triples to up to 48%.
She highlighted a few effective strategies for preventing posttraumatic KOA in patients who already have an ACL injury.
Avoid reinjury
Dr. Risberg was senior author of a recent report from the prospective Delaware-Oslo Cohort Study involving 106 athletes who underwent ACL reconstruction following injury in what she termed level I sports. These are sports that entail lots of pivoting, jumping, and hard cutting, such as basketball, soccer, and handball.
In the first 2 years after ACL repair, 30% of patients who returned to participation in a level 1 sport experienced an ACL reinjury, compared with just 8% who opted for a lower-level sport. Athletes who returned to a level 1 sport had an adjusted 4.3 times greater ACL reinjury rate than those who didn’t, Dr. Risberg noted at the congress sponsored by the Osteoarthritis Research Society International.
The good news is that this sharply increased reinjury risk was mitigated if return to a level 1 sport was delayed for at least 9 months post surgery and if the patient had regained quadriceps strength comparable to the uninjured side. For every month that return to sport was delayed out until 9 months post ACL reconstruction, the knee reinjury rate was reduced by 51% (Br J Sports Med. 2016;50:804-8).
In a meta-analysis by other investigators of 12 studies including 5,707 participants, weakness of the knee extensor muscles was independently associated with a 1.65 times increased risk of developing KOA (Osteoarthritis Cartilage. 2015 Feb;23[2]:171-7).
Attend to BMI
A discussion of the importance of maintaining a healthy body weight is an important aspect of patient education for athletes with knee injuries. In a cohort study of 988 patients who underwent primary ACL reconstruction, being overweight or obese was associated with a significantly increased risk of subsequent meniscal tears and chondral lesions (Am J Sports Med. 2015 Dec;43[12]:2966-73).
Also, it’s well established that obesity is a risk factor for knee OA, and Canadian investigators have shown that young athletes with a sports-related intra-articular knee injury were 3.75 times more likely to be overweight or obese 3-10 years post injury, compared with matched uninjured controls (Osteoarthritis Cartilage. 2015 Jul;23[7]:1122-9).
Consider prehabilitative exercise training
Dr. Risberg and coinvestigators have reported that preoperative quadriceps muscle strength deficits are predictive of impaired knee function, as measured by the Cincinnati Knee Score 2 years post surgery. She said she believes ACL reconstruction shouldn’t be done until quadriceps muscle strength is at least 80% of that in the uninjured limb (Br J Sports Med. 2009 May;43[5]:371-6). She and her coinvestigators have published the details of a 5-week progressive exercise therapy program in which they have shown results in significantly improved early postoperative knee function (J Orthop Sports Phys Ther. 2010 Nov;40[11]:705-21). They now try to have patients complete the twice-weekly, 5-week program before final decisions are reached regarding whether to have ACL reconstruction.
Test all before okaying return to sport
It’s important to know if patients who have undergone ACL reconstruction have gotten full knee function back before determining if they’re ready for full-on sports participation. In the Delaware-Oslo Cohort Study, patients who delayed their return until at least 9 months after surgery and passed the return-to-sports test had a 5.6% reinjury rate within 2 years, while those who failed the return-to-sports criteria had a 38.2% ACL reinjury rate.
The return-to-sports testing utilized in this study entailed isokinetic quadriceps strength testing, the single hop leg test, the 14-item self-rated Knee Outcome Survey–Activities of Daily Living Scale, and a self-rated Global Rating Scale of perceived function on a 0-100 scale. To be cleared for return to sports, a patient had to demonstrate having regained at least 90% of quadriceps muscle strength and hop performance along with scoring in the normative range on both of the self-rating instruments.
Surgical vs. nonsurgical treatment of ACL rupture
The evidence on this score is conflicting, according to Dr. Risberg. While most physical therapists believe ACL reconstruction doesn’t protect against later development of KOA, as reflected in a meta-analysis of published studies (J Bone Joint Surg Am. 2014 Feb 19;96[4]:292-300), a more recent retrospective comparison of 964 patients with an isolated ACL tear and an equal number of matched controls concluded that patients treated nonoperatively were six times more likely to have been diagnosed with KOA and 16.7 times more likely to have undergone total knee replacement at a mean follow-up of 13.7 years than were those treated with ACL reconstruction (Am J Sports Med. 2016 Jul;44[7]:1699-707).
Dr. Risberg’s fellow panelist Jackie Whittaker, PhD, said that, as long as quadriceps muscle strengthening is a priority, it makes sense to strengthen the hamstring as well, particularly if the ACL reconstruction utilized the hamstring tendon.
“Also, I would add that it’s important to develop a relationship with these ACL-injured people, who are often very young. Preventing a disease that they’re going to get 20 years later isn’t a priority for them. You need to develop that relationship and build it up over time. Helping them set realistic expectations is very important. And we need to do what we can to help them find some sort of competitive outlet. A lot of these kids were very competitive, and now they’ve had an injury and can’t compete. They don’t want to go back to playing just any sport. They want to be able to be competitive, and if you don’t help them find another way to express that, they sort of give up on physical activity altogether,” according to Dr. Whittaker of the University of Alberta in Edmonton.
Dr. Risberg and Dr. Whittaker reported having no financial conflicts of interest.
LAS VEGAS – A variety of evidence-based strategies are available for preventing posttraumatic knee osteoarthritis (KOA) in patients who have already sustained an anterior cruciate ligament (ACL) injury. And they’re generally ignored, according to May Arna Risberg, PhD.
“We have a lot of knowledge. We can use secondary prevention strategies. And here I think we, as physical therapists, physicians, and orthopedic surgeons, are doing a lousy job because we are sending these ACL-injured patients back to sports before they have normalized knee function and quadriceps strength,” said Dr. Risberg, professor of sports medicine at the Norwegian School of Sport Sciences in Oslo.
With no proven disease-modifying therapy for KOA available to date, secondary prevention of posttraumatic KOA is worthy of high-priority status, she said at the World Congress on Osteoarthritis. An estimated 250,00 ACL injuries occur annually in the United States, and up to one-half of affected patients, most of whom are young, active people, will experience a second ACL rupture within the first few years after undergoing their initial reconstruction. This second ACL injury greatly increases their risk of developing posttraumatic KOA within 15-20 years, while they are still relatively young, she said.
Moreover, if the second ACL injury involves meniscus surgery, the 5-year risk of posttraumatic KOA roughly triples to up to 48%.
She highlighted a few effective strategies for preventing posttraumatic KOA in patients who already have an ACL injury.
Avoid reinjury
Dr. Risberg was senior author of a recent report from the prospective Delaware-Oslo Cohort Study involving 106 athletes who underwent ACL reconstruction following injury in what she termed level I sports. These are sports that entail lots of pivoting, jumping, and hard cutting, such as basketball, soccer, and handball.
In the first 2 years after ACL repair, 30% of patients who returned to participation in a level 1 sport experienced an ACL reinjury, compared with just 8% who opted for a lower-level sport. Athletes who returned to a level 1 sport had an adjusted 4.3 times greater ACL reinjury rate than those who didn’t, Dr. Risberg noted at the congress sponsored by the Osteoarthritis Research Society International.
The good news is that this sharply increased reinjury risk was mitigated if return to a level 1 sport was delayed for at least 9 months post surgery and if the patient had regained quadriceps strength comparable to the uninjured side. For every month that return to sport was delayed out until 9 months post ACL reconstruction, the knee reinjury rate was reduced by 51% (Br J Sports Med. 2016;50:804-8).
In a meta-analysis by other investigators of 12 studies including 5,707 participants, weakness of the knee extensor muscles was independently associated with a 1.65 times increased risk of developing KOA (Osteoarthritis Cartilage. 2015 Feb;23[2]:171-7).
Attend to BMI
A discussion of the importance of maintaining a healthy body weight is an important aspect of patient education for athletes with knee injuries. In a cohort study of 988 patients who underwent primary ACL reconstruction, being overweight or obese was associated with a significantly increased risk of subsequent meniscal tears and chondral lesions (Am J Sports Med. 2015 Dec;43[12]:2966-73).
Also, it’s well established that obesity is a risk factor for knee OA, and Canadian investigators have shown that young athletes with a sports-related intra-articular knee injury were 3.75 times more likely to be overweight or obese 3-10 years post injury, compared with matched uninjured controls (Osteoarthritis Cartilage. 2015 Jul;23[7]:1122-9).
Consider prehabilitative exercise training
Dr. Risberg and coinvestigators have reported that preoperative quadriceps muscle strength deficits are predictive of impaired knee function, as measured by the Cincinnati Knee Score 2 years post surgery. She said she believes ACL reconstruction shouldn’t be done until quadriceps muscle strength is at least 80% of that in the uninjured limb (Br J Sports Med. 2009 May;43[5]:371-6). She and her coinvestigators have published the details of a 5-week progressive exercise therapy program in which they have shown results in significantly improved early postoperative knee function (J Orthop Sports Phys Ther. 2010 Nov;40[11]:705-21). They now try to have patients complete the twice-weekly, 5-week program before final decisions are reached regarding whether to have ACL reconstruction.
Test all before okaying return to sport
It’s important to know if patients who have undergone ACL reconstruction have gotten full knee function back before determining if they’re ready for full-on sports participation. In the Delaware-Oslo Cohort Study, patients who delayed their return until at least 9 months after surgery and passed the return-to-sports test had a 5.6% reinjury rate within 2 years, while those who failed the return-to-sports criteria had a 38.2% ACL reinjury rate.
The return-to-sports testing utilized in this study entailed isokinetic quadriceps strength testing, the single hop leg test, the 14-item self-rated Knee Outcome Survey–Activities of Daily Living Scale, and a self-rated Global Rating Scale of perceived function on a 0-100 scale. To be cleared for return to sports, a patient had to demonstrate having regained at least 90% of quadriceps muscle strength and hop performance along with scoring in the normative range on both of the self-rating instruments.
Surgical vs. nonsurgical treatment of ACL rupture
The evidence on this score is conflicting, according to Dr. Risberg. While most physical therapists believe ACL reconstruction doesn’t protect against later development of KOA, as reflected in a meta-analysis of published studies (J Bone Joint Surg Am. 2014 Feb 19;96[4]:292-300), a more recent retrospective comparison of 964 patients with an isolated ACL tear and an equal number of matched controls concluded that patients treated nonoperatively were six times more likely to have been diagnosed with KOA and 16.7 times more likely to have undergone total knee replacement at a mean follow-up of 13.7 years than were those treated with ACL reconstruction (Am J Sports Med. 2016 Jul;44[7]:1699-707).
Dr. Risberg’s fellow panelist Jackie Whittaker, PhD, said that, as long as quadriceps muscle strengthening is a priority, it makes sense to strengthen the hamstring as well, particularly if the ACL reconstruction utilized the hamstring tendon.
“Also, I would add that it’s important to develop a relationship with these ACL-injured people, who are often very young. Preventing a disease that they’re going to get 20 years later isn’t a priority for them. You need to develop that relationship and build it up over time. Helping them set realistic expectations is very important. And we need to do what we can to help them find some sort of competitive outlet. A lot of these kids were very competitive, and now they’ve had an injury and can’t compete. They don’t want to go back to playing just any sport. They want to be able to be competitive, and if you don’t help them find another way to express that, they sort of give up on physical activity altogether,” according to Dr. Whittaker of the University of Alberta in Edmonton.
Dr. Risberg and Dr. Whittaker reported having no financial conflicts of interest.
LAS VEGAS – A variety of evidence-based strategies are available for preventing posttraumatic knee osteoarthritis (KOA) in patients who have already sustained an anterior cruciate ligament (ACL) injury. And they’re generally ignored, according to May Arna Risberg, PhD.
“We have a lot of knowledge. We can use secondary prevention strategies. And here I think we, as physical therapists, physicians, and orthopedic surgeons, are doing a lousy job because we are sending these ACL-injured patients back to sports before they have normalized knee function and quadriceps strength,” said Dr. Risberg, professor of sports medicine at the Norwegian School of Sport Sciences in Oslo.
With no proven disease-modifying therapy for KOA available to date, secondary prevention of posttraumatic KOA is worthy of high-priority status, she said at the World Congress on Osteoarthritis. An estimated 250,00 ACL injuries occur annually in the United States, and up to one-half of affected patients, most of whom are young, active people, will experience a second ACL rupture within the first few years after undergoing their initial reconstruction. This second ACL injury greatly increases their risk of developing posttraumatic KOA within 15-20 years, while they are still relatively young, she said.
Moreover, if the second ACL injury involves meniscus surgery, the 5-year risk of posttraumatic KOA roughly triples to up to 48%.
She highlighted a few effective strategies for preventing posttraumatic KOA in patients who already have an ACL injury.
Avoid reinjury
Dr. Risberg was senior author of a recent report from the prospective Delaware-Oslo Cohort Study involving 106 athletes who underwent ACL reconstruction following injury in what she termed level I sports. These are sports that entail lots of pivoting, jumping, and hard cutting, such as basketball, soccer, and handball.
In the first 2 years after ACL repair, 30% of patients who returned to participation in a level 1 sport experienced an ACL reinjury, compared with just 8% who opted for a lower-level sport. Athletes who returned to a level 1 sport had an adjusted 4.3 times greater ACL reinjury rate than those who didn’t, Dr. Risberg noted at the congress sponsored by the Osteoarthritis Research Society International.
The good news is that this sharply increased reinjury risk was mitigated if return to a level 1 sport was delayed for at least 9 months post surgery and if the patient had regained quadriceps strength comparable to the uninjured side. For every month that return to sport was delayed out until 9 months post ACL reconstruction, the knee reinjury rate was reduced by 51% (Br J Sports Med. 2016;50:804-8).
In a meta-analysis by other investigators of 12 studies including 5,707 participants, weakness of the knee extensor muscles was independently associated with a 1.65 times increased risk of developing KOA (Osteoarthritis Cartilage. 2015 Feb;23[2]:171-7).
Attend to BMI
A discussion of the importance of maintaining a healthy body weight is an important aspect of patient education for athletes with knee injuries. In a cohort study of 988 patients who underwent primary ACL reconstruction, being overweight or obese was associated with a significantly increased risk of subsequent meniscal tears and chondral lesions (Am J Sports Med. 2015 Dec;43[12]:2966-73).
Also, it’s well established that obesity is a risk factor for knee OA, and Canadian investigators have shown that young athletes with a sports-related intra-articular knee injury were 3.75 times more likely to be overweight or obese 3-10 years post injury, compared with matched uninjured controls (Osteoarthritis Cartilage. 2015 Jul;23[7]:1122-9).
Consider prehabilitative exercise training
Dr. Risberg and coinvestigators have reported that preoperative quadriceps muscle strength deficits are predictive of impaired knee function, as measured by the Cincinnati Knee Score 2 years post surgery. She said she believes ACL reconstruction shouldn’t be done until quadriceps muscle strength is at least 80% of that in the uninjured limb (Br J Sports Med. 2009 May;43[5]:371-6). She and her coinvestigators have published the details of a 5-week progressive exercise therapy program in which they have shown results in significantly improved early postoperative knee function (J Orthop Sports Phys Ther. 2010 Nov;40[11]:705-21). They now try to have patients complete the twice-weekly, 5-week program before final decisions are reached regarding whether to have ACL reconstruction.
Test all before okaying return to sport
It’s important to know if patients who have undergone ACL reconstruction have gotten full knee function back before determining if they’re ready for full-on sports participation. In the Delaware-Oslo Cohort Study, patients who delayed their return until at least 9 months after surgery and passed the return-to-sports test had a 5.6% reinjury rate within 2 years, while those who failed the return-to-sports criteria had a 38.2% ACL reinjury rate.
The return-to-sports testing utilized in this study entailed isokinetic quadriceps strength testing, the single hop leg test, the 14-item self-rated Knee Outcome Survey–Activities of Daily Living Scale, and a self-rated Global Rating Scale of perceived function on a 0-100 scale. To be cleared for return to sports, a patient had to demonstrate having regained at least 90% of quadriceps muscle strength and hop performance along with scoring in the normative range on both of the self-rating instruments.
Surgical vs. nonsurgical treatment of ACL rupture
The evidence on this score is conflicting, according to Dr. Risberg. While most physical therapists believe ACL reconstruction doesn’t protect against later development of KOA, as reflected in a meta-analysis of published studies (J Bone Joint Surg Am. 2014 Feb 19;96[4]:292-300), a more recent retrospective comparison of 964 patients with an isolated ACL tear and an equal number of matched controls concluded that patients treated nonoperatively were six times more likely to have been diagnosed with KOA and 16.7 times more likely to have undergone total knee replacement at a mean follow-up of 13.7 years than were those treated with ACL reconstruction (Am J Sports Med. 2016 Jul;44[7]:1699-707).
Dr. Risberg’s fellow panelist Jackie Whittaker, PhD, said that, as long as quadriceps muscle strengthening is a priority, it makes sense to strengthen the hamstring as well, particularly if the ACL reconstruction utilized the hamstring tendon.
“Also, I would add that it’s important to develop a relationship with these ACL-injured people, who are often very young. Preventing a disease that they’re going to get 20 years later isn’t a priority for them. You need to develop that relationship and build it up over time. Helping them set realistic expectations is very important. And we need to do what we can to help them find some sort of competitive outlet. A lot of these kids were very competitive, and now they’ve had an injury and can’t compete. They don’t want to go back to playing just any sport. They want to be able to be competitive, and if you don’t help them find another way to express that, they sort of give up on physical activity altogether,” according to Dr. Whittaker of the University of Alberta in Edmonton.
Dr. Risberg and Dr. Whittaker reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM OARSI 2017
Open Navicular Dislocation With Midfoot Dissociation in a 45-Year-Old Man
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
Rare Dual Lesion: Extraskeletal Osteosarcoma Developing Within a Simple Lipoma
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
Multimodality Approach to a Stener Lesion: Radiographic, Ultrasound, Magnetic Resonance Imaging, and Surgical Correlation
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.