User login
Trans-Scaphoid Transcapitate Perilunate Fracture-Dislocation
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
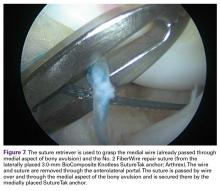
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
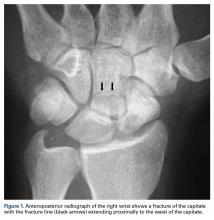
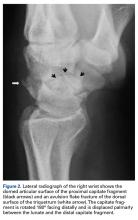
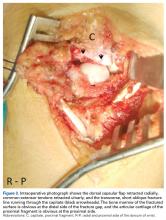
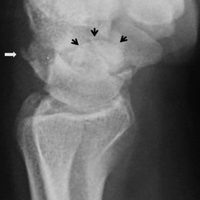
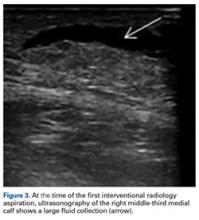
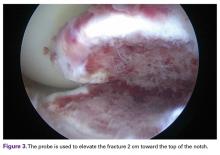
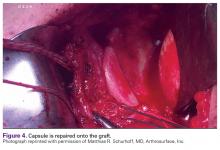
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
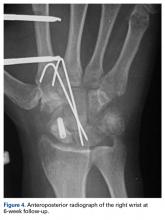
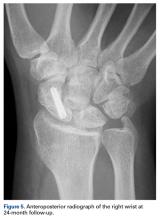
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
Six Steps to Reduce Taxes on Investments: Minimizing What You Pay in a Tough Environment
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Is Simultaneous Bilateral Total Knee Arthroplasty (BTKA) as Safe as Staged BTKA?
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
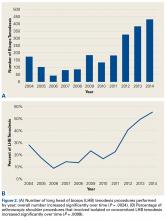
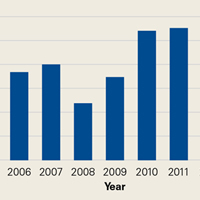
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
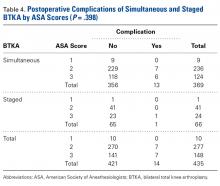
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
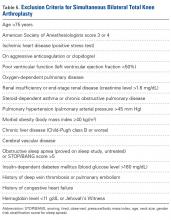
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
VA cohort study: Individualize SSI prophylaxis based on patient factors
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
FROM PLOS MEDICINE
Key clinical point:
Major finding: The SSI incidence was 0.95% vs. 1.48% with combination vs. single agent–therapy in cardiac surgery patients. Acute kidney injuries occurred in 23.75% of all surgery patients receiving combination prophylaxis, compared with 20.79% and 13.93% with vancomycin or a beta-lactam, respectively.
Data source: A retrospective cohort study of more than 70,000 surgical procedures.
Disclosures: This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated grant from Merck Pharmaceuticals in 2013.
Lower Limb Morel-Lavallée Lesion Treated With Short-Stretch Compression Bandaging
Take-Home Points
- Have a high-index of suspicion for MLLs and initiate treatment early.
- Compression needs to occur through short-stretch bandaging over a conventional Ace wrap in order to be successful.
- Apply the short-stretch compression with care to avoid shearing underlying tissue.
- Nonoperative treatment modalities require high patient compliance.
- MLLs need close monitoring until final healing occurs.
Morel-Lavallée lesions (MLLs) are traumatic degloving injuries resulting from separation of subcutaneous fat from underlying fascia. MLLs occur in association with acetabular fractures and are also associated with low-velocity crush injuries.1,2 Shearing creates a “false” space that is filled with hemorrhaged blood, fat, and lymphatic tissue.3 Disruption of the lymphatics leads to cavity formation and, eventually, a fibrotic pseudocapsule.4The pseudocapsule prevents resorption, leading to a chronic fluid collection, which potentiates the risk of infection or tissue necrosis.3,5,6 Skin necrosis may occur through direct-pressure compromise of the dermal vascular plexus.4 Necrotic skin may require multiple débridements, negative-pressure wound therapy or soft-tissue coverage, and may ultimately result in infection. MLLs classically occur in the greater trochanteric region, lateral thigh, buttocks, and back but also appear in the prepatellar region.1,3 Patients present with soft-tissue swelling, bruising, bulging, decreased cutaneous sensation over the region, and a palpable, fluctuant subcutaneous fluid collection with mobile skin.2,4,7 The mechanism of injury may cause a concomitant fracture. Magnetic resonance imaging (MRI), the preferred imaging modality, shows a discrete fluid collection between subcutaneous fat and underlying fascia. Ultrasonography may reveal a thickened capsule surrounding either a hypoechoic area or an anechoic area but its accuracy is user-dependent.7
Large MLLs may be treated with open serial débridement and healing by secondary intention; infection rates, however, are high. Authors have described several other treatment modalities, including percutaneous débridement with a brush followed by use of a large-bore drain and antibiotics; open débridement with meticulous dead-space closure; elastic compression bandaging; aspiration; and doxycycline sclerodesis.1,5,6,8,9 Modifications of short-stretch compression bandaging were recently described in edema control for hindfoot trauma, ankle trauma, and total ankle arthroplasty, but not for MLLs.10,11 Nickerson and colleagues4 retrospectively reviewed 87 MLLs, found that fluid aspirate of >50 mL predicted recurrence and failure with conservative measures, and recommended operative intervention for any MLL with >50 mL of fluid aspirated.
We report the case of an MLL that occurred in an unusual anatomical region, and we describe a novel application of a conservative treatment, which was selected on the basis of its success in lymphedema management. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man was injured when a parked vehicle began moving, pulled him under, and ran over his lower right leg. In the emergency department, no fractures or major injuries were noted (Figures 1A, 1B), and the patient was discharged.
About 10 days after injury, profuse ecchymosis and swelling were noted running from the distal medial thigh to the proximal medial calf (Figures 2A-2C).
Given the size of the MLL, the fluid collection reaccumulated. The patient was evaluated by an orthopedic traumatologist 3 days after the aspiration (17 days after injury).
Another orthopedic traumatologist confirmed the low likelihood that compression would resolve the MLL, given its size (Figures 4A, 4B).
After the second orthopedic consultation, the patient saw a physical therapist trained in complete decongestive therapy. The therapist suggested placing short-stretch bandage wraps over the conventional long-stretch Ace bandage currently being used—a treatment common in lymphedema. The patient was wrapped from toe to groin without an initial layer of padding (Figures 6A, 6B), and the response was immediate.
Nine weeks after injury, the leg was significantly improved, and clinical signs resolved (Figure 7).
Discussion
Short-stretch bandaging has been performed mainly in lymphedema and ulcer management.
Compression bandaging reduces volume in lymphedematous limbs by reducing capillary filtration, shifting fluid into noncompressed parts of the body, increasing lymphatic reabsorption and lymphatic transport stimulation, improving venous pumping, and breaking down fibrosclerotic tissue.15 We think containment, improved venous flow, and enhanced muscle contraction contributed to the effectiveness of short-stretch bandaging as treatment for our patient’s MLL. Because MLLs also contain disrupted lymphatics, lymphedema management strategies (eg, short-stretch bandages) can be used. Our patient rapidly improved after conversion to short-stretch bandages.
These bandages are applied with 50% overlap to ensure even pressures throughout.16 Multiple layers are applied using a combination of spiral and figure-of-8 techniques, first clockwise and then counterclockwise, to avoid shearing underlying tissue.17 This method is very important in MLL treatment, given the degloving involved and the highly mobile skin and subcutaneous fat.
In standard lymphedema management, a foam padding layer is applied before the short-stretch bandage in order to reshape the limb and avoid proximal constrictions.13 In our patient’s case, the short-stretch wrap was applied without padding. Because his condition was acute, and the limb contour was preserved, limb reshaping and thus padding were not necessary.
Given the rapid, high-volume reduction that occurs within the first 1 to 2 weeks, bandages are reapplied daily to effectively adjust for the decreased swelling and altered limb shape.17 Most improvement is expected within the first few weeks—consistent with our patient’s case. Bandages usually are applied to the entire limb. For partial cases, the bandaging must extend past the area of swelling and incorporate the knee to prevent displacement of fluid into the joint.17 Feet and ankles are bandaged in dorsiflexion.17Several factors must be considered with short-stretch wraps. For example, pressure may need to be adjusted in patients with peripheral vascular disease. In patients with ankle-brachial indexes >0.5, it is safe to apply pressure up to 40 mm Hg.12 Reduced pressure is recommended for patients with arterial disease, sensory disturbance, lipoedema, poor mobility, frailty, or palliative needs.13The unusual location of our patient’s MLL accounts for the delay in diagnosis. To our knowledge, no other authors have reported such a large MLL in this location. A few series and case reports have listed MLLs in the calf near the gastrocnemius muscle, in the ankle, in the prepatellar area, and in the suprapatellar region, including the thigh,1,3,18-20 but there are no reports of MLLs running from medial thigh to proximal calf. MLLs of this size classically are treated surgically, but our patient selected nonoperative management.
To our knowledge, there are no earlier reports of using this nonoperative technique to treat MLLs. Conservative treatment with compression has been discussed, but no case involved short-stretch bandages. Large MLLs are thought to require surgery plus some type of drainage. The success of using short-stretch bandages in our patient’s case should prompt further investigation of use in adherent patients—which could ultimately result in reduced surgical needs, improved wound care (surgery is avoided), and a maintained low risk of infection. Although more work is needed to come to a more definitive verdict on this treatment method, it is a promising option that warrants consideration.
Am J Orthop. 2017;46(4):E213-E218. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
2. Tsur A, Galin A, Kogan L, Loberant N. Morel-Lavallee syndrome after crush injury [in Hebrew]. Harefuah. 2006;145(2):111-113.
3. Ciaschini M, Sundaram M. Radiologic case study. Prepatellar Morel-Lavallée lesion. Orthopedics. 2008;31(7):626, 719-721.
4. Nickerson TP, Zielinski MD, Jenkins DH, Schiller HJ. The Mayo Clinic experience with Morel-Lavallée lesions: establishment of a practice management guideline. J Trauma Acute Care Surg. 2014;76(2):493-497.
5. Bansal A, Bhatia N, Singh A, Singh AK. Doxycycline sclerodesis as a treatment option for persistent Morel-Lavallée lesions. Injury. 2013;44(1):66-69.
6. Carlson DA, Simmons J, Sando W, Weber T, Clements B. Morel-Lavalée lesions treated with debridement and meticulous dead space closure: surgical technique. J Orthop Trauma. 2007;21(2):140-144.
7. Miller J, Daggett J, Ambay R, Payne WG. Morel-Lavallée lesion. Eplasty. 2014;14:ic12.
8. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
9. Harma A, Inan M, Ertem K. The Morel-Lavallée lesion: a conservative approach to closed degloving injuries [in Turkish]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
10. Hsu A, Franceschina D, Haddad SL. A novel method of postoperative wound care following total ankle arthroplasty. Foot Ankle Int. 2014;35(7):719-724.
11. Rohner-Spengler M, Frotzler A, Honigmann P, Babst R. Effective treatment of posttraumatic and postoperative edema in patients with ankle and hindfoot fractures: a randomized controlled trial comparing multilayer compression therapy and intermittent impulse compression with the standard treatment with ice. J Bone Joint Surg Am. 2014;96(15):1263-1271.
12. Bjork R. The long and short of it: understanding compression bandaging. Wound Care Advisor. 2013;2(6):12-15.
13. Partsch H. Assessing the effectiveness of multilayer inelastic bandaging. J Lymphoedema. 2007;2(2):55-61.
14. Hafner J, Botonakis I, Burg G. A comparison of multilayer bandage systems during rest, exercise, and over 2 days of wear time. Arch Dermatol. 2000;136(7):857-863.
15. Földi E, Jünger M, Partsch H. The science of lymphoedema bandaging. In: Lymphoedema Bandaging in Practice [European Wound Management Association focus document]. London, England: Medical Education Partnership; 2005:2-4.
16. King TI, Droessler JL. Physical properties of short-stretch compression bandages used to treat lymphedema. Am J Occup Ther. 2001;55(5):573-576.
17. Williams AF, Keller M. Practical guidance on lymphoedema bandaging of the upper and lower limbs. In: Lymphoedema Bandaging in Practice [European Wound Management Association focus document]. London, England: Medical Education Partnership; 2005:10-14.
18. Moriarty JM, Borrero CG, Kavanagh EC. A rare cause of calf swelling: the Morel-Lavallee lesion. Ir J Med Sci. 2011;180(1):265-268.
19. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Knee Morel-Lavallee lesion after a football injury in an 11-year-old boy: case report and review of the literature. Univ Pa Orthop J. 2011;21:56-58.
20. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
Take-Home Points
- Have a high-index of suspicion for MLLs and initiate treatment early.
- Compression needs to occur through short-stretch bandaging over a conventional Ace wrap in order to be successful.
- Apply the short-stretch compression with care to avoid shearing underlying tissue.
- Nonoperative treatment modalities require high patient compliance.
- MLLs need close monitoring until final healing occurs.
Morel-Lavallée lesions (MLLs) are traumatic degloving injuries resulting from separation of subcutaneous fat from underlying fascia. MLLs occur in association with acetabular fractures and are also associated with low-velocity crush injuries.1,2 Shearing creates a “false” space that is filled with hemorrhaged blood, fat, and lymphatic tissue.3 Disruption of the lymphatics leads to cavity formation and, eventually, a fibrotic pseudocapsule.4The pseudocapsule prevents resorption, leading to a chronic fluid collection, which potentiates the risk of infection or tissue necrosis.3,5,6 Skin necrosis may occur through direct-pressure compromise of the dermal vascular plexus.4 Necrotic skin may require multiple débridements, negative-pressure wound therapy or soft-tissue coverage, and may ultimately result in infection. MLLs classically occur in the greater trochanteric region, lateral thigh, buttocks, and back but also appear in the prepatellar region.1,3 Patients present with soft-tissue swelling, bruising, bulging, decreased cutaneous sensation over the region, and a palpable, fluctuant subcutaneous fluid collection with mobile skin.2,4,7 The mechanism of injury may cause a concomitant fracture. Magnetic resonance imaging (MRI), the preferred imaging modality, shows a discrete fluid collection between subcutaneous fat and underlying fascia. Ultrasonography may reveal a thickened capsule surrounding either a hypoechoic area or an anechoic area but its accuracy is user-dependent.7
Large MLLs may be treated with open serial débridement and healing by secondary intention; infection rates, however, are high. Authors have described several other treatment modalities, including percutaneous débridement with a brush followed by use of a large-bore drain and antibiotics; open débridement with meticulous dead-space closure; elastic compression bandaging; aspiration; and doxycycline sclerodesis.1,5,6,8,9 Modifications of short-stretch compression bandaging were recently described in edema control for hindfoot trauma, ankle trauma, and total ankle arthroplasty, but not for MLLs.10,11 Nickerson and colleagues4 retrospectively reviewed 87 MLLs, found that fluid aspirate of >50 mL predicted recurrence and failure with conservative measures, and recommended operative intervention for any MLL with >50 mL of fluid aspirated.
We report the case of an MLL that occurred in an unusual anatomical region, and we describe a novel application of a conservative treatment, which was selected on the basis of its success in lymphedema management. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man was injured when a parked vehicle began moving, pulled him under, and ran over his lower right leg. In the emergency department, no fractures or major injuries were noted (Figures 1A, 1B), and the patient was discharged.
About 10 days after injury, profuse ecchymosis and swelling were noted running from the distal medial thigh to the proximal medial calf (Figures 2A-2C).
Given the size of the MLL, the fluid collection reaccumulated. The patient was evaluated by an orthopedic traumatologist 3 days after the aspiration (17 days after injury).
Another orthopedic traumatologist confirmed the low likelihood that compression would resolve the MLL, given its size (Figures 4A, 4B).
After the second orthopedic consultation, the patient saw a physical therapist trained in complete decongestive therapy. The therapist suggested placing short-stretch bandage wraps over the conventional long-stretch Ace bandage currently being used—a treatment common in lymphedema. The patient was wrapped from toe to groin without an initial layer of padding (Figures 6A, 6B), and the response was immediate.
Nine weeks after injury, the leg was significantly improved, and clinical signs resolved (Figure 7).
Discussion
Short-stretch bandaging has been performed mainly in lymphedema and ulcer management.
Compression bandaging reduces volume in lymphedematous limbs by reducing capillary filtration, shifting fluid into noncompressed parts of the body, increasing lymphatic reabsorption and lymphatic transport stimulation, improving venous pumping, and breaking down fibrosclerotic tissue.15 We think containment, improved venous flow, and enhanced muscle contraction contributed to the effectiveness of short-stretch bandaging as treatment for our patient’s MLL. Because MLLs also contain disrupted lymphatics, lymphedema management strategies (eg, short-stretch bandages) can be used. Our patient rapidly improved after conversion to short-stretch bandages.
These bandages are applied with 50% overlap to ensure even pressures throughout.16 Multiple layers are applied using a combination of spiral and figure-of-8 techniques, first clockwise and then counterclockwise, to avoid shearing underlying tissue.17 This method is very important in MLL treatment, given the degloving involved and the highly mobile skin and subcutaneous fat.
In standard lymphedema management, a foam padding layer is applied before the short-stretch bandage in order to reshape the limb and avoid proximal constrictions.13 In our patient’s case, the short-stretch wrap was applied without padding. Because his condition was acute, and the limb contour was preserved, limb reshaping and thus padding were not necessary.
Given the rapid, high-volume reduction that occurs within the first 1 to 2 weeks, bandages are reapplied daily to effectively adjust for the decreased swelling and altered limb shape.17 Most improvement is expected within the first few weeks—consistent with our patient’s case. Bandages usually are applied to the entire limb. For partial cases, the bandaging must extend past the area of swelling and incorporate the knee to prevent displacement of fluid into the joint.17 Feet and ankles are bandaged in dorsiflexion.17Several factors must be considered with short-stretch wraps. For example, pressure may need to be adjusted in patients with peripheral vascular disease. In patients with ankle-brachial indexes >0.5, it is safe to apply pressure up to 40 mm Hg.12 Reduced pressure is recommended for patients with arterial disease, sensory disturbance, lipoedema, poor mobility, frailty, or palliative needs.13The unusual location of our patient’s MLL accounts for the delay in diagnosis. To our knowledge, no other authors have reported such a large MLL in this location. A few series and case reports have listed MLLs in the calf near the gastrocnemius muscle, in the ankle, in the prepatellar area, and in the suprapatellar region, including the thigh,1,3,18-20 but there are no reports of MLLs running from medial thigh to proximal calf. MLLs of this size classically are treated surgically, but our patient selected nonoperative management.
To our knowledge, there are no earlier reports of using this nonoperative technique to treat MLLs. Conservative treatment with compression has been discussed, but no case involved short-stretch bandages. Large MLLs are thought to require surgery plus some type of drainage. The success of using short-stretch bandages in our patient’s case should prompt further investigation of use in adherent patients—which could ultimately result in reduced surgical needs, improved wound care (surgery is avoided), and a maintained low risk of infection. Although more work is needed to come to a more definitive verdict on this treatment method, it is a promising option that warrants consideration.
Am J Orthop. 2017;46(4):E213-E218. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Have a high-index of suspicion for MLLs and initiate treatment early.
- Compression needs to occur through short-stretch bandaging over a conventional Ace wrap in order to be successful.
- Apply the short-stretch compression with care to avoid shearing underlying tissue.
- Nonoperative treatment modalities require high patient compliance.
- MLLs need close monitoring until final healing occurs.
Morel-Lavallée lesions (MLLs) are traumatic degloving injuries resulting from separation of subcutaneous fat from underlying fascia. MLLs occur in association with acetabular fractures and are also associated with low-velocity crush injuries.1,2 Shearing creates a “false” space that is filled with hemorrhaged blood, fat, and lymphatic tissue.3 Disruption of the lymphatics leads to cavity formation and, eventually, a fibrotic pseudocapsule.4The pseudocapsule prevents resorption, leading to a chronic fluid collection, which potentiates the risk of infection or tissue necrosis.3,5,6 Skin necrosis may occur through direct-pressure compromise of the dermal vascular plexus.4 Necrotic skin may require multiple débridements, negative-pressure wound therapy or soft-tissue coverage, and may ultimately result in infection. MLLs classically occur in the greater trochanteric region, lateral thigh, buttocks, and back but also appear in the prepatellar region.1,3 Patients present with soft-tissue swelling, bruising, bulging, decreased cutaneous sensation over the region, and a palpable, fluctuant subcutaneous fluid collection with mobile skin.2,4,7 The mechanism of injury may cause a concomitant fracture. Magnetic resonance imaging (MRI), the preferred imaging modality, shows a discrete fluid collection between subcutaneous fat and underlying fascia. Ultrasonography may reveal a thickened capsule surrounding either a hypoechoic area or an anechoic area but its accuracy is user-dependent.7
Large MLLs may be treated with open serial débridement and healing by secondary intention; infection rates, however, are high. Authors have described several other treatment modalities, including percutaneous débridement with a brush followed by use of a large-bore drain and antibiotics; open débridement with meticulous dead-space closure; elastic compression bandaging; aspiration; and doxycycline sclerodesis.1,5,6,8,9 Modifications of short-stretch compression bandaging were recently described in edema control for hindfoot trauma, ankle trauma, and total ankle arthroplasty, but not for MLLs.10,11 Nickerson and colleagues4 retrospectively reviewed 87 MLLs, found that fluid aspirate of >50 mL predicted recurrence and failure with conservative measures, and recommended operative intervention for any MLL with >50 mL of fluid aspirated.
We report the case of an MLL that occurred in an unusual anatomical region, and we describe a novel application of a conservative treatment, which was selected on the basis of its success in lymphedema management. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man was injured when a parked vehicle began moving, pulled him under, and ran over his lower right leg. In the emergency department, no fractures or major injuries were noted (Figures 1A, 1B), and the patient was discharged.
About 10 days after injury, profuse ecchymosis and swelling were noted running from the distal medial thigh to the proximal medial calf (Figures 2A-2C).
Given the size of the MLL, the fluid collection reaccumulated. The patient was evaluated by an orthopedic traumatologist 3 days after the aspiration (17 days after injury).
Another orthopedic traumatologist confirmed the low likelihood that compression would resolve the MLL, given its size (Figures 4A, 4B).
After the second orthopedic consultation, the patient saw a physical therapist trained in complete decongestive therapy. The therapist suggested placing short-stretch bandage wraps over the conventional long-stretch Ace bandage currently being used—a treatment common in lymphedema. The patient was wrapped from toe to groin without an initial layer of padding (Figures 6A, 6B), and the response was immediate.
Nine weeks after injury, the leg was significantly improved, and clinical signs resolved (Figure 7).
Discussion
Short-stretch bandaging has been performed mainly in lymphedema and ulcer management.
Compression bandaging reduces volume in lymphedematous limbs by reducing capillary filtration, shifting fluid into noncompressed parts of the body, increasing lymphatic reabsorption and lymphatic transport stimulation, improving venous pumping, and breaking down fibrosclerotic tissue.15 We think containment, improved venous flow, and enhanced muscle contraction contributed to the effectiveness of short-stretch bandaging as treatment for our patient’s MLL. Because MLLs also contain disrupted lymphatics, lymphedema management strategies (eg, short-stretch bandages) can be used. Our patient rapidly improved after conversion to short-stretch bandages.
These bandages are applied with 50% overlap to ensure even pressures throughout.16 Multiple layers are applied using a combination of spiral and figure-of-8 techniques, first clockwise and then counterclockwise, to avoid shearing underlying tissue.17 This method is very important in MLL treatment, given the degloving involved and the highly mobile skin and subcutaneous fat.
In standard lymphedema management, a foam padding layer is applied before the short-stretch bandage in order to reshape the limb and avoid proximal constrictions.13 In our patient’s case, the short-stretch wrap was applied without padding. Because his condition was acute, and the limb contour was preserved, limb reshaping and thus padding were not necessary.
Given the rapid, high-volume reduction that occurs within the first 1 to 2 weeks, bandages are reapplied daily to effectively adjust for the decreased swelling and altered limb shape.17 Most improvement is expected within the first few weeks—consistent with our patient’s case. Bandages usually are applied to the entire limb. For partial cases, the bandaging must extend past the area of swelling and incorporate the knee to prevent displacement of fluid into the joint.17 Feet and ankles are bandaged in dorsiflexion.17Several factors must be considered with short-stretch wraps. For example, pressure may need to be adjusted in patients with peripheral vascular disease. In patients with ankle-brachial indexes >0.5, it is safe to apply pressure up to 40 mm Hg.12 Reduced pressure is recommended for patients with arterial disease, sensory disturbance, lipoedema, poor mobility, frailty, or palliative needs.13The unusual location of our patient’s MLL accounts for the delay in diagnosis. To our knowledge, no other authors have reported such a large MLL in this location. A few series and case reports have listed MLLs in the calf near the gastrocnemius muscle, in the ankle, in the prepatellar area, and in the suprapatellar region, including the thigh,1,3,18-20 but there are no reports of MLLs running from medial thigh to proximal calf. MLLs of this size classically are treated surgically, but our patient selected nonoperative management.
To our knowledge, there are no earlier reports of using this nonoperative technique to treat MLLs. Conservative treatment with compression has been discussed, but no case involved short-stretch bandages. Large MLLs are thought to require surgery plus some type of drainage. The success of using short-stretch bandages in our patient’s case should prompt further investigation of use in adherent patients—which could ultimately result in reduced surgical needs, improved wound care (surgery is avoided), and a maintained low risk of infection. Although more work is needed to come to a more definitive verdict on this treatment method, it is a promising option that warrants consideration.
Am J Orthop. 2017;46(4):E213-E218. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
2. Tsur A, Galin A, Kogan L, Loberant N. Morel-Lavallee syndrome after crush injury [in Hebrew]. Harefuah. 2006;145(2):111-113.
3. Ciaschini M, Sundaram M. Radiologic case study. Prepatellar Morel-Lavallée lesion. Orthopedics. 2008;31(7):626, 719-721.
4. Nickerson TP, Zielinski MD, Jenkins DH, Schiller HJ. The Mayo Clinic experience with Morel-Lavallée lesions: establishment of a practice management guideline. J Trauma Acute Care Surg. 2014;76(2):493-497.
5. Bansal A, Bhatia N, Singh A, Singh AK. Doxycycline sclerodesis as a treatment option for persistent Morel-Lavallée lesions. Injury. 2013;44(1):66-69.
6. Carlson DA, Simmons J, Sando W, Weber T, Clements B. Morel-Lavalée lesions treated with debridement and meticulous dead space closure: surgical technique. J Orthop Trauma. 2007;21(2):140-144.
7. Miller J, Daggett J, Ambay R, Payne WG. Morel-Lavallée lesion. Eplasty. 2014;14:ic12.
8. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
9. Harma A, Inan M, Ertem K. The Morel-Lavallée lesion: a conservative approach to closed degloving injuries [in Turkish]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
10. Hsu A, Franceschina D, Haddad SL. A novel method of postoperative wound care following total ankle arthroplasty. Foot Ankle Int. 2014;35(7):719-724.
11. Rohner-Spengler M, Frotzler A, Honigmann P, Babst R. Effective treatment of posttraumatic and postoperative edema in patients with ankle and hindfoot fractures: a randomized controlled trial comparing multilayer compression therapy and intermittent impulse compression with the standard treatment with ice. J Bone Joint Surg Am. 2014;96(15):1263-1271.
12. Bjork R. The long and short of it: understanding compression bandaging. Wound Care Advisor. 2013;2(6):12-15.
13. Partsch H. Assessing the effectiveness of multilayer inelastic bandaging. J Lymphoedema. 2007;2(2):55-61.
14. Hafner J, Botonakis I, Burg G. A comparison of multilayer bandage systems during rest, exercise, and over 2 days of wear time. Arch Dermatol. 2000;136(7):857-863.
15. Földi E, Jünger M, Partsch H. The science of lymphoedema bandaging. In: Lymphoedema Bandaging in Practice [European Wound Management Association focus document]. London, England: Medical Education Partnership; 2005:2-4.
16. King TI, Droessler JL. Physical properties of short-stretch compression bandages used to treat lymphedema. Am J Occup Ther. 2001;55(5):573-576.
17. Williams AF, Keller M. Practical guidance on lymphoedema bandaging of the upper and lower limbs. In: Lymphoedema Bandaging in Practice [European Wound Management Association focus document]. London, England: Medical Education Partnership; 2005:10-14.
18. Moriarty JM, Borrero CG, Kavanagh EC. A rare cause of calf swelling: the Morel-Lavallee lesion. Ir J Med Sci. 2011;180(1):265-268.
19. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Knee Morel-Lavallee lesion after a football injury in an 11-year-old boy: case report and review of the literature. Univ Pa Orthop J. 2011;21:56-58.
20. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
1. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
2. Tsur A, Galin A, Kogan L, Loberant N. Morel-Lavallee syndrome after crush injury [in Hebrew]. Harefuah. 2006;145(2):111-113.
3. Ciaschini M, Sundaram M. Radiologic case study. Prepatellar Morel-Lavallée lesion. Orthopedics. 2008;31(7):626, 719-721.
4. Nickerson TP, Zielinski MD, Jenkins DH, Schiller HJ. The Mayo Clinic experience with Morel-Lavallée lesions: establishment of a practice management guideline. J Trauma Acute Care Surg. 2014;76(2):493-497.
5. Bansal A, Bhatia N, Singh A, Singh AK. Doxycycline sclerodesis as a treatment option for persistent Morel-Lavallée lesions. Injury. 2013;44(1):66-69.
6. Carlson DA, Simmons J, Sando W, Weber T, Clements B. Morel-Lavalée lesions treated with debridement and meticulous dead space closure: surgical technique. J Orthop Trauma. 2007;21(2):140-144.
7. Miller J, Daggett J, Ambay R, Payne WG. Morel-Lavallée lesion. Eplasty. 2014;14:ic12.
8. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
9. Harma A, Inan M, Ertem K. The Morel-Lavallée lesion: a conservative approach to closed degloving injuries [in Turkish]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
10. Hsu A, Franceschina D, Haddad SL. A novel method of postoperative wound care following total ankle arthroplasty. Foot Ankle Int. 2014;35(7):719-724.
11. Rohner-Spengler M, Frotzler A, Honigmann P, Babst R. Effective treatment of posttraumatic and postoperative edema in patients with ankle and hindfoot fractures: a randomized controlled trial comparing multilayer compression therapy and intermittent impulse compression with the standard treatment with ice. J Bone Joint Surg Am. 2014;96(15):1263-1271.
12. Bjork R. The long and short of it: understanding compression bandaging. Wound Care Advisor. 2013;2(6):12-15.
13. Partsch H. Assessing the effectiveness of multilayer inelastic bandaging. J Lymphoedema. 2007;2(2):55-61.
14. Hafner J, Botonakis I, Burg G. A comparison of multilayer bandage systems during rest, exercise, and over 2 days of wear time. Arch Dermatol. 2000;136(7):857-863.
15. Földi E, Jünger M, Partsch H. The science of lymphoedema bandaging. In: Lymphoedema Bandaging in Practice [European Wound Management Association focus document]. London, England: Medical Education Partnership; 2005:2-4.
16. King TI, Droessler JL. Physical properties of short-stretch compression bandages used to treat lymphedema. Am J Occup Ther. 2001;55(5):573-576.
17. Williams AF, Keller M. Practical guidance on lymphoedema bandaging of the upper and lower limbs. In: Lymphoedema Bandaging in Practice [European Wound Management Association focus document]. London, England: Medical Education Partnership; 2005:10-14.
18. Moriarty JM, Borrero CG, Kavanagh EC. A rare cause of calf swelling: the Morel-Lavallee lesion. Ir J Med Sci. 2011;180(1):265-268.
19. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Knee Morel-Lavallee lesion after a football injury in an 11-year-old boy: case report and review of the literature. Univ Pa Orthop J. 2011;21:56-58.
20. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
Biceps Tenodesis: An Evolution of Treatment
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
Something is Afoot
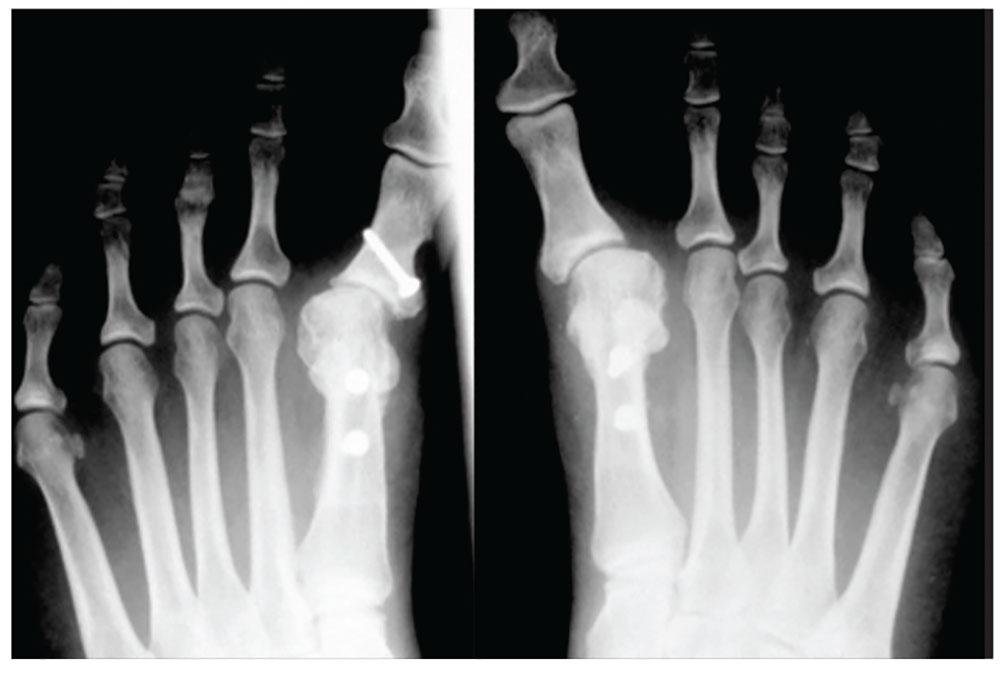
1. A 43-year-old woman presents with progressively worsening bilateral great toe pain that began during pregnancy and increased following the birth of her daughter three years ago. Both of her feet have developed a crescent moon shape, making it painful and difficult to wear normal shoes. This patient has
a) Degenerative arthritis
b) Hallux varus
c) Gout
d) Traumatic sesamoiditis
Diagnosis: Physical exam revealed bilateral hallux varus deformity of the great toe, which was greater on the left foot than on the right (23° and 16°, respectively). The deformities were easily, passively, correctable. Standing radiographs showed evidence of previous proximal osteotomies and well-healed distal first metatarsal osteotomies. Due to unsuccessful nonoperative management, surgical reconstruction was offered.
For further information, see “Bilateral Hallux Varus Deformity Correction With a Suture Button Construct.” Am J Orthop. 2013;42(3):121-124.

2. Following treatment for onychomycosis, this 49-year-old man’s toenails demonstrate inflammation of the medial and lateral nail borders of the hallux and second toes of the left foot. This patient’s diagnosis is
a) Subungual exostosis
b) Primary osteomyelitis of the phalanx
c) Tumors of the nail bed
d) Onychocryptosis
Diagnosis: Onychocryptosis, also known as ingrown toenail, is a rare complication of oral antifungal therapy. As the healthy nail plate advances, it may adhere to the nail bed and cut into the lateral nail folds. In this case, the site of the onychocryptosis corresponded to the proximal clearing of the nail plate. The patient required excision of the nail borders, after which the secondary inflammation resolved. The condition was treated with chemical matrixectomy.
For more information, see “Multiple Onychocryptosis Following Treatment of Onychomycosis With Oral Terbinafine.” Cutis. 2000;66(3):211-212.
3. A 39-year-old man was playing a game of pick-up basketball when he felt a pop, immediately followed by a sharp pain in the back of his ankle and lower leg. He now walks with a limp. The cause is
a) Achilles tendon rupture
b) Medial gastrocnemius tear
c) Calf muscle strain
d) Posterior tibial stress syndrome
Diagnosis: Often diagnosed as an ankle sprain, an Achilles tendon rupture most commonly occurs in middle-aged men from overexertion in sports—usually tennis, racquetball, basketball, or badminton, which involve bursts of jumping, pivoting, and running. Rupture may also occur from a sudden stumble, fall from a significant height, or abrupt step into a hole or off a curb, which causes the tendon to overstretch forcefully.
4. A 60-year-old woman seeks relief for a foot wound of several months’ duration that persists despite use of antibiotics and proper care. The skin over the plantar surface has a full-thickness ulcer, with partial necrosis of the subcutaneous tissue. Her history is significant for diabetes with neuropathy, nephropathy, and retinopathy. The diagnosis is
a) Osteomyelitis
b) Deep venous thrombosis
c) Charcot joint
d) Septic joint
Diagnosis: Charcot joint changes, and an associated stage III pressure ulcer, account for the extensive collapse of the inner arch and “rocker bottom foot” seen on the radiograph. Also known as neurogenic arthropathy, Charcot joint is commonly seen with diabetic neuropathy. In affected patients, secondary degenerative changes to the joints occur with loss of normal muscle tone, proprioception, temperature perception, and pain perception. The joints become loose, enlarged, and boggy. There can be extensive cartilage erosion or osteophyte formation. The normal plantar and heel forces are increased, producing eccentric loading of the foot and leading to microfractures, ligament laxity, and bone destruction.
For more information, see “A disfigured foot with ulcer.” J Fam Pract. 2008;57(5):321-323.

1. A 43-year-old woman presents with progressively worsening bilateral great toe pain that began during pregnancy and increased following the birth of her daughter three years ago. Both of her feet have developed a crescent moon shape, making it painful and difficult to wear normal shoes. This patient has
a) Degenerative arthritis
b) Hallux varus
c) Gout
d) Traumatic sesamoiditis
Diagnosis: Physical exam revealed bilateral hallux varus deformity of the great toe, which was greater on the left foot than on the right (23° and 16°, respectively). The deformities were easily, passively, correctable. Standing radiographs showed evidence of previous proximal osteotomies and well-healed distal first metatarsal osteotomies. Due to unsuccessful nonoperative management, surgical reconstruction was offered.
For further information, see “Bilateral Hallux Varus Deformity Correction With a Suture Button Construct.” Am J Orthop. 2013;42(3):121-124.

2. Following treatment for onychomycosis, this 49-year-old man’s toenails demonstrate inflammation of the medial and lateral nail borders of the hallux and second toes of the left foot. This patient’s diagnosis is
a) Subungual exostosis
b) Primary osteomyelitis of the phalanx
c) Tumors of the nail bed
d) Onychocryptosis
Diagnosis: Onychocryptosis, also known as ingrown toenail, is a rare complication of oral antifungal therapy. As the healthy nail plate advances, it may adhere to the nail bed and cut into the lateral nail folds. In this case, the site of the onychocryptosis corresponded to the proximal clearing of the nail plate. The patient required excision of the nail borders, after which the secondary inflammation resolved. The condition was treated with chemical matrixectomy.
For more information, see “Multiple Onychocryptosis Following Treatment of Onychomycosis With Oral Terbinafine.” Cutis. 2000;66(3):211-212.

3. A 39-year-old man was playing a game of pick-up basketball when he felt a pop, immediately followed by a sharp pain in the back of his ankle and lower leg. He now walks with a limp. The cause is
a) Achilles tendon rupture
b) Medial gastrocnemius tear
c) Calf muscle strain
d) Posterior tibial stress syndrome
Diagnosis: Often diagnosed as an ankle sprain, an Achilles tendon rupture most commonly occurs in middle-aged men from overexertion in sports—usually tennis, racquetball, basketball, or badminton, which involve bursts of jumping, pivoting, and running. Rupture may also occur from a sudden stumble, fall from a significant height, or abrupt step into a hole or off a curb, which causes the tendon to overstretch forcefully.

4. A 60-year-old woman seeks relief for a foot wound of several months’ duration that persists despite use of antibiotics and proper care. The skin over the plantar surface has a full-thickness ulcer, with partial necrosis of the subcutaneous tissue. Her history is significant for diabetes with neuropathy, nephropathy, and retinopathy. The diagnosis is
a) Osteomyelitis
b) Deep venous thrombosis
c) Charcot joint
d) Septic joint
Diagnosis: Charcot joint changes, and an associated stage III pressure ulcer, account for the extensive collapse of the inner arch and “rocker bottom foot” seen on the radiograph. Also known as neurogenic arthropathy, Charcot joint is commonly seen with diabetic neuropathy. In affected patients, secondary degenerative changes to the joints occur with loss of normal muscle tone, proprioception, temperature perception, and pain perception. The joints become loose, enlarged, and boggy. There can be extensive cartilage erosion or osteophyte formation. The normal plantar and heel forces are increased, producing eccentric loading of the foot and leading to microfractures, ligament laxity, and bone destruction.
For more information, see “A disfigured foot with ulcer.” J Fam Pract. 2008;57(5):321-323.

1. A 43-year-old woman presents with progressively worsening bilateral great toe pain that began during pregnancy and increased following the birth of her daughter three years ago. Both of her feet have developed a crescent moon shape, making it painful and difficult to wear normal shoes. This patient has
a) Degenerative arthritis
b) Hallux varus
c) Gout
d) Traumatic sesamoiditis
Diagnosis: Physical exam revealed bilateral hallux varus deformity of the great toe, which was greater on the left foot than on the right (23° and 16°, respectively). The deformities were easily, passively, correctable. Standing radiographs showed evidence of previous proximal osteotomies and well-healed distal first metatarsal osteotomies. Due to unsuccessful nonoperative management, surgical reconstruction was offered.
For further information, see “Bilateral Hallux Varus Deformity Correction With a Suture Button Construct.” Am J Orthop. 2013;42(3):121-124.

2. Following treatment for onychomycosis, this 49-year-old man’s toenails demonstrate inflammation of the medial and lateral nail borders of the hallux and second toes of the left foot. This patient’s diagnosis is
a) Subungual exostosis
b) Primary osteomyelitis of the phalanx
c) Tumors of the nail bed
d) Onychocryptosis
Diagnosis: Onychocryptosis, also known as ingrown toenail, is a rare complication of oral antifungal therapy. As the healthy nail plate advances, it may adhere to the nail bed and cut into the lateral nail folds. In this case, the site of the onychocryptosis corresponded to the proximal clearing of the nail plate. The patient required excision of the nail borders, after which the secondary inflammation resolved. The condition was treated with chemical matrixectomy.
For more information, see “Multiple Onychocryptosis Following Treatment of Onychomycosis With Oral Terbinafine.” Cutis. 2000;66(3):211-212.

3. A 39-year-old man was playing a game of pick-up basketball when he felt a pop, immediately followed by a sharp pain in the back of his ankle and lower leg. He now walks with a limp. The cause is
a) Achilles tendon rupture
b) Medial gastrocnemius tear
c) Calf muscle strain
d) Posterior tibial stress syndrome
Diagnosis: Often diagnosed as an ankle sprain, an Achilles tendon rupture most commonly occurs in middle-aged men from overexertion in sports—usually tennis, racquetball, basketball, or badminton, which involve bursts of jumping, pivoting, and running. Rupture may also occur from a sudden stumble, fall from a significant height, or abrupt step into a hole or off a curb, which causes the tendon to overstretch forcefully.

4. A 60-year-old woman seeks relief for a foot wound of several months’ duration that persists despite use of antibiotics and proper care. The skin over the plantar surface has a full-thickness ulcer, with partial necrosis of the subcutaneous tissue. Her history is significant for diabetes with neuropathy, nephropathy, and retinopathy. The diagnosis is
a) Osteomyelitis
b) Deep venous thrombosis
c) Charcot joint
d) Septic joint
Diagnosis: Charcot joint changes, and an associated stage III pressure ulcer, account for the extensive collapse of the inner arch and “rocker bottom foot” seen on the radiograph. Also known as neurogenic arthropathy, Charcot joint is commonly seen with diabetic neuropathy. In affected patients, secondary degenerative changes to the joints occur with loss of normal muscle tone, proprioception, temperature perception, and pain perception. The joints become loose, enlarged, and boggy. There can be extensive cartilage erosion or osteophyte formation. The normal plantar and heel forces are increased, producing eccentric loading of the foot and leading to microfractures, ligament laxity, and bone destruction.
For more information, see “A disfigured foot with ulcer.” J Fam Pract. 2008;57(5):321-323.
Knotless Arthroscopic Reduction and Internal Fixation of a Displaced Anterior Cruciate Ligament Tibial Eminence Avulsion Fracture
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.
A New Option for Glenoid Reconstruction in Recurrent Anterior Shoulder Instability
Take-Home Points
- Repair anterior bone defect on the glenoid related to recurrent anterior instability with preshaped, predrilled allograft.
- Avoid graft harvest complications related to coracoid (Latarjet) or iliac crest autograft.
- Simple guide system to allow for appropriate graft and screw placement.
- Soft tissues can be repaired to the allograft in predrilled suture holes either inside or outside of the graft
- Position the graft without step at the anterior glenoid.
Anteroinferior glenoid bone loss plays a significant role in recurrent glenohumeral instability. Arthroscopic capsulolabral reconstruction has been associated with a recurrence rate of 4% in the absence of significant glenoid bone loss but 67% in patients with either bone loss of more than 25% of the inferior glenoid diameter or an engaging Hill-Sachs lesion.1,2 Anteroinferior glenoid rim deficiency has been reported in up to 90% of cases of recurrent instability.3 Glenoid reconstruction is therefore recommended in patients with bone loss of more than 25% and in certain revision cases.4 Surgical strategies in these cases include coracoid transfer, iliac crest autograft, and allograft (osteochondral and iliac crest). These procedures all successfully restore stability of the glenohumeral joint. However, they carry the drawbacks of technical complexity with increased operative time or risk of neurovascular damage, or they create a nonanatomical reconstruction, which may contribute to subsequent instability arthropathy. In this article, we introduce a technique in which a preshaped allograft (Glenojet; Arthrosurface, Inc.) is used to match the contour of the glenoid defect. The graft is simple to insert and can reduce operative time.
Graft Preparation
The shaped human tissue cortical bone allograft is usually prepared from proximal or distal tibia or femur. There is no cartilage on the graft. It can be ordered in 2 sizes, 10 mm × 29 mm and 13 mm × 34 mm, for different amounts of bone loss. The more commonly used smaller graft reconstructs defects of 20% to 30% of the glenoid.
The sutures through this allograft can be prepared on the back table while the rest of the equipment is set up. Start by tying a No. 2 FiberWire (or equivalent) over a small thin object, such as a Freer elevator. Once the knot is secure, remove the Freer and trim the knot tails short. Thread another suture through the loop that has been created and pull to make the 2 tails even. Then thread these tails through one of the small holes of the graft, going from the flat side to the concave side. Pull the suture tails all the way through, including through the loop of the prior suture. The knot of the loop prevents the entire construct from pulling through. The suture tails are then able to slide as if attached to an anchor. Repeat these steps for the other 2 small holes to get a total of 3 sutures exiting the concave side of the graft (Figure 1). Alternatively, pass the suture the opposite way, if tying the capsule inside the graft is preferred.
Surgical Technique
A standard deltopectoral approach is used to expose the anterior glenoid. The subscapularis can either be split in line with its fibers or tenotomized with 1 cm to 2 cm attached to the tuberosity for later repair. In either instance, it is important to separate the muscle from the underlying capsule layer, as the capsule is what is directly repaired to the graft.
The capsule is carefully peeled off the anterior glenoid. A Fukuda or similar retractor may be used on the humerus, and a glenoid retractor is placed on the anterior glenoid, under the capsule and subscapularis, for optimal exposure. Once the anterior glenoid surface is exposed, the drill guide is placed flush against the surface of the glenoid.
The guide is removed. The cannulated reamer is introduced and advanced until the guide pin appears in the viewing window of the reamer and hits the stop—approximating the correct amount of bone to remove. This step is repeated for the second guide pin. Reaming flattens the anterior glenoid and allows for maximal stable apposition of the graft to the glenoid. The allograft is then inserted onto the pins in the correct orientation to match the surface of the native glenoid.
The length of the superior guide pin is measured with the depth gauge device. It is then removed, and the appropriate-length 3.5-mm cortical bone screw is inserted (alternatively, the guide pin is removed, and a standard depth gauge is used to measure screw length). Once the superior guide pin is secure, the process is repeated for the inferior guide pin (Figure 3).
Once the graft is secure, the capsule is attached to the graft with the use of a free needle on the suture of the graft (Figure 4).
Outcomes
Coracoid bone transfer or the Bristow-Latarjet technique has become more popular since bone loss was recognized as an important cause of failure of soft-tissue repair for anterior instability. This procedure, however, is not without complications. In a recent systematic review of 45 studies (1904 shoulders), Griesser and colleagues5 found an overall complication rate of 30% and a reoperation rate of 7%.
Given the potential complications of coracoid bone transfer, allograft reconstruction of the anteroinferior glenoid has become increasingly popular and proved successful at short- and medium-term follow-up. Allograft reconstruction avoids the drawbacks of traditional coracoid bone transfer—namely, high rates of neurovascular injury, and nonanatomical reconstruction with high rates of graft resorption and arthritis.5,6 At average 45-month follow-up after fresh distal tibia allograft reconstruction, Provencher and colleagues7 found an 89% radiographic union rate (average lysis, 3%), significantly improved patient-reported outcomes, and no recurrent instability. Similarly, in a study of iliac crest allograft reconstruction in 10 patients with an average 4-year follow-up, Mascarenhas and colleagues8 found an 80% radiographic union rate at 6 months, significantly improved patient-reported outcomes, and no recurrent shoulder instability.
The advantage of Glenojet over other allografts is that it is preshaped and predrilled and saves the surgeon the time and effort of preparing graft in the operating room. The surgical technologist can place the sutures before the patient enters the room. The 2 allograft sizes (10 mm × 29 mm, 13 mm × 34 mm) accommodate the spectrum of bone loss in glenoid deficiency, and graft contour fits the native glenoid well. So far we have implanted this allograft in 15 patients, and at short-term follow-up there are no known cases of recurrent instability.
The potential disadvantages of Glenojet are similar to those of other allografts. Care must be taken with retractor placement to avoid damaging the axillary and musculocutaneous nerves. There are concerns about graft union and subsequent resorption, but this will require long-term follow-up to determine. At 9-month follow-up, we had 1 fracture at the superior corner of the graft, which may have resulted from overtightening the screws in the graft, creating a stress concentration. After removal of this fragment arthroscopically, the patient has done very well clinically with no pain, instability and has returned to all activities. Although the graft does not have an articular surface, the capsular repair covers much of the articular side of the graft, and therefore we do not anticipate that the absence of articular cartilage will contribute to glenohumeral arthritis, though long-term follow-up is lacking. The other question many have is related to the lack of the sling effect since there is no conjoined tendon on the graft. Yamamoto and colleagues9 have reported that the conjoined tendon is the major stabilizing force at time zero in a cadaver model. However, other authors7,8 have successfully reconstructed glenoid defects in these difficult cases without the “sling effect” of the conjoined tendon with excellent clinical results. Our experience has been similar. It is likely that long-term studies will be necessary to answer this question. We have also done some cases with the tendon attached after releasing it from the coracoid, but the series is too small to make any comment about whether this is important or not.
The main limitation of this allograft technique is the lack of long-term outcome studies. However, short-term results are promising, and the ease of the procedure makes it an attractive option for either glenoid reconstruction of bony Bankart lesions or failed bone reconstruction, such as Bristow-Latarjet reconstruction.
Glenojetallograft is a new glenoid reconstruction option that is technically easy and simple to perform in cases of glenoid bone loss, while still creating an anatomical buttress with less surgical dissection than traditional coracoid bone transfer. Short-term outcomes are reassuring, though more research is needed for long-term graft follow-up and recurrent instability.
Am J Orthop. 2017;46(4):199-202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
2. Rowe CR, Sakellarides HT. Factors related to recurrences of anterior dislocations of the shoulder. Clin Orthop. 1961;(20):40-48.
3. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
4. Sayegh ET, Mascarenhas R, Chalmers PN, Cole BJ, Verma NN, Romeo AA. Allograft reconstruction for glenoid bone loss in glenohumeral instability: a systematic review. Arthroscopy. 2014;30(12):1642-1649.
5. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operati ons after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22(2):286-292.
6. Young DC, Rockwood CA Jr. Complications of a failed Bristow procedure and their management. J Bone Joint Surg Am. 1991;73(7):969-981.
7. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
8. Mascarenhas R, Raleigh E, McRae S, Leiter J, Saltzman B, MacDonald PB. Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: surgical technique and results. Int J Shoulder Surg. 2014;8(4):127-132.
9. Yamamoto N, Muraki T, An KN, et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am. 2013;95(15):1390-1397.
Take-Home Points
- Repair anterior bone defect on the glenoid related to recurrent anterior instability with preshaped, predrilled allograft.
- Avoid graft harvest complications related to coracoid (Latarjet) or iliac crest autograft.
- Simple guide system to allow for appropriate graft and screw placement.
- Soft tissues can be repaired to the allograft in predrilled suture holes either inside or outside of the graft
- Position the graft without step at the anterior glenoid.
Anteroinferior glenoid bone loss plays a significant role in recurrent glenohumeral instability. Arthroscopic capsulolabral reconstruction has been associated with a recurrence rate of 4% in the absence of significant glenoid bone loss but 67% in patients with either bone loss of more than 25% of the inferior glenoid diameter or an engaging Hill-Sachs lesion.1,2 Anteroinferior glenoid rim deficiency has been reported in up to 90% of cases of recurrent instability.3 Glenoid reconstruction is therefore recommended in patients with bone loss of more than 25% and in certain revision cases.4 Surgical strategies in these cases include coracoid transfer, iliac crest autograft, and allograft (osteochondral and iliac crest). These procedures all successfully restore stability of the glenohumeral joint. However, they carry the drawbacks of technical complexity with increased operative time or risk of neurovascular damage, or they create a nonanatomical reconstruction, which may contribute to subsequent instability arthropathy. In this article, we introduce a technique in which a preshaped allograft (Glenojet; Arthrosurface, Inc.) is used to match the contour of the glenoid defect. The graft is simple to insert and can reduce operative time.
Graft Preparation
The shaped human tissue cortical bone allograft is usually prepared from proximal or distal tibia or femur. There is no cartilage on the graft. It can be ordered in 2 sizes, 10 mm × 29 mm and 13 mm × 34 mm, for different amounts of bone loss. The more commonly used smaller graft reconstructs defects of 20% to 30% of the glenoid.
The sutures through this allograft can be prepared on the back table while the rest of the equipment is set up. Start by tying a No. 2 FiberWire (or equivalent) over a small thin object, such as a Freer elevator. Once the knot is secure, remove the Freer and trim the knot tails short. Thread another suture through the loop that has been created and pull to make the 2 tails even. Then thread these tails through one of the small holes of the graft, going from the flat side to the concave side. Pull the suture tails all the way through, including through the loop of the prior suture. The knot of the loop prevents the entire construct from pulling through. The suture tails are then able to slide as if attached to an anchor. Repeat these steps for the other 2 small holes to get a total of 3 sutures exiting the concave side of the graft (Figure 1). Alternatively, pass the suture the opposite way, if tying the capsule inside the graft is preferred.
Surgical Technique
A standard deltopectoral approach is used to expose the anterior glenoid. The subscapularis can either be split in line with its fibers or tenotomized with 1 cm to 2 cm attached to the tuberosity for later repair. In either instance, it is important to separate the muscle from the underlying capsule layer, as the capsule is what is directly repaired to the graft.
The capsule is carefully peeled off the anterior glenoid. A Fukuda or similar retractor may be used on the humerus, and a glenoid retractor is placed on the anterior glenoid, under the capsule and subscapularis, for optimal exposure. Once the anterior glenoid surface is exposed, the drill guide is placed flush against the surface of the glenoid.
The guide is removed. The cannulated reamer is introduced and advanced until the guide pin appears in the viewing window of the reamer and hits the stop—approximating the correct amount of bone to remove. This step is repeated for the second guide pin. Reaming flattens the anterior glenoid and allows for maximal stable apposition of the graft to the glenoid. The allograft is then inserted onto the pins in the correct orientation to match the surface of the native glenoid.
The length of the superior guide pin is measured with the depth gauge device. It is then removed, and the appropriate-length 3.5-mm cortical bone screw is inserted (alternatively, the guide pin is removed, and a standard depth gauge is used to measure screw length). Once the superior guide pin is secure, the process is repeated for the inferior guide pin (Figure 3).
Once the graft is secure, the capsule is attached to the graft with the use of a free needle on the suture of the graft (Figure 4).
Outcomes
Coracoid bone transfer or the Bristow-Latarjet technique has become more popular since bone loss was recognized as an important cause of failure of soft-tissue repair for anterior instability. This procedure, however, is not without complications. In a recent systematic review of 45 studies (1904 shoulders), Griesser and colleagues5 found an overall complication rate of 30% and a reoperation rate of 7%.
Given the potential complications of coracoid bone transfer, allograft reconstruction of the anteroinferior glenoid has become increasingly popular and proved successful at short- and medium-term follow-up. Allograft reconstruction avoids the drawbacks of traditional coracoid bone transfer—namely, high rates of neurovascular injury, and nonanatomical reconstruction with high rates of graft resorption and arthritis.5,6 At average 45-month follow-up after fresh distal tibia allograft reconstruction, Provencher and colleagues7 found an 89% radiographic union rate (average lysis, 3%), significantly improved patient-reported outcomes, and no recurrent instability. Similarly, in a study of iliac crest allograft reconstruction in 10 patients with an average 4-year follow-up, Mascarenhas and colleagues8 found an 80% radiographic union rate at 6 months, significantly improved patient-reported outcomes, and no recurrent shoulder instability.
The advantage of Glenojet over other allografts is that it is preshaped and predrilled and saves the surgeon the time and effort of preparing graft in the operating room. The surgical technologist can place the sutures before the patient enters the room. The 2 allograft sizes (10 mm × 29 mm, 13 mm × 34 mm) accommodate the spectrum of bone loss in glenoid deficiency, and graft contour fits the native glenoid well. So far we have implanted this allograft in 15 patients, and at short-term follow-up there are no known cases of recurrent instability.
The potential disadvantages of Glenojet are similar to those of other allografts. Care must be taken with retractor placement to avoid damaging the axillary and musculocutaneous nerves. There are concerns about graft union and subsequent resorption, but this will require long-term follow-up to determine. At 9-month follow-up, we had 1 fracture at the superior corner of the graft, which may have resulted from overtightening the screws in the graft, creating a stress concentration. After removal of this fragment arthroscopically, the patient has done very well clinically with no pain, instability and has returned to all activities. Although the graft does not have an articular surface, the capsular repair covers much of the articular side of the graft, and therefore we do not anticipate that the absence of articular cartilage will contribute to glenohumeral arthritis, though long-term follow-up is lacking. The other question many have is related to the lack of the sling effect since there is no conjoined tendon on the graft. Yamamoto and colleagues9 have reported that the conjoined tendon is the major stabilizing force at time zero in a cadaver model. However, other authors7,8 have successfully reconstructed glenoid defects in these difficult cases without the “sling effect” of the conjoined tendon with excellent clinical results. Our experience has been similar. It is likely that long-term studies will be necessary to answer this question. We have also done some cases with the tendon attached after releasing it from the coracoid, but the series is too small to make any comment about whether this is important or not.
The main limitation of this allograft technique is the lack of long-term outcome studies. However, short-term results are promising, and the ease of the procedure makes it an attractive option for either glenoid reconstruction of bony Bankart lesions or failed bone reconstruction, such as Bristow-Latarjet reconstruction.
Glenojetallograft is a new glenoid reconstruction option that is technically easy and simple to perform in cases of glenoid bone loss, while still creating an anatomical buttress with less surgical dissection than traditional coracoid bone transfer. Short-term outcomes are reassuring, though more research is needed for long-term graft follow-up and recurrent instability.
Am J Orthop. 2017;46(4):199-202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Repair anterior bone defect on the glenoid related to recurrent anterior instability with preshaped, predrilled allograft.
- Avoid graft harvest complications related to coracoid (Latarjet) or iliac crest autograft.
- Simple guide system to allow for appropriate graft and screw placement.
- Soft tissues can be repaired to the allograft in predrilled suture holes either inside or outside of the graft
- Position the graft without step at the anterior glenoid.
Anteroinferior glenoid bone loss plays a significant role in recurrent glenohumeral instability. Arthroscopic capsulolabral reconstruction has been associated with a recurrence rate of 4% in the absence of significant glenoid bone loss but 67% in patients with either bone loss of more than 25% of the inferior glenoid diameter or an engaging Hill-Sachs lesion.1,2 Anteroinferior glenoid rim deficiency has been reported in up to 90% of cases of recurrent instability.3 Glenoid reconstruction is therefore recommended in patients with bone loss of more than 25% and in certain revision cases.4 Surgical strategies in these cases include coracoid transfer, iliac crest autograft, and allograft (osteochondral and iliac crest). These procedures all successfully restore stability of the glenohumeral joint. However, they carry the drawbacks of technical complexity with increased operative time or risk of neurovascular damage, or they create a nonanatomical reconstruction, which may contribute to subsequent instability arthropathy. In this article, we introduce a technique in which a preshaped allograft (Glenojet; Arthrosurface, Inc.) is used to match the contour of the glenoid defect. The graft is simple to insert and can reduce operative time.
Graft Preparation
The shaped human tissue cortical bone allograft is usually prepared from proximal or distal tibia or femur. There is no cartilage on the graft. It can be ordered in 2 sizes, 10 mm × 29 mm and 13 mm × 34 mm, for different amounts of bone loss. The more commonly used smaller graft reconstructs defects of 20% to 30% of the glenoid.
The sutures through this allograft can be prepared on the back table while the rest of the equipment is set up. Start by tying a No. 2 FiberWire (or equivalent) over a small thin object, such as a Freer elevator. Once the knot is secure, remove the Freer and trim the knot tails short. Thread another suture through the loop that has been created and pull to make the 2 tails even. Then thread these tails through one of the small holes of the graft, going from the flat side to the concave side. Pull the suture tails all the way through, including through the loop of the prior suture. The knot of the loop prevents the entire construct from pulling through. The suture tails are then able to slide as if attached to an anchor. Repeat these steps for the other 2 small holes to get a total of 3 sutures exiting the concave side of the graft (Figure 1). Alternatively, pass the suture the opposite way, if tying the capsule inside the graft is preferred.
Surgical Technique
A standard deltopectoral approach is used to expose the anterior glenoid. The subscapularis can either be split in line with its fibers or tenotomized with 1 cm to 2 cm attached to the tuberosity for later repair. In either instance, it is important to separate the muscle from the underlying capsule layer, as the capsule is what is directly repaired to the graft.
The capsule is carefully peeled off the anterior glenoid. A Fukuda or similar retractor may be used on the humerus, and a glenoid retractor is placed on the anterior glenoid, under the capsule and subscapularis, for optimal exposure. Once the anterior glenoid surface is exposed, the drill guide is placed flush against the surface of the glenoid.
The guide is removed. The cannulated reamer is introduced and advanced until the guide pin appears in the viewing window of the reamer and hits the stop—approximating the correct amount of bone to remove. This step is repeated for the second guide pin. Reaming flattens the anterior glenoid and allows for maximal stable apposition of the graft to the glenoid. The allograft is then inserted onto the pins in the correct orientation to match the surface of the native glenoid.
The length of the superior guide pin is measured with the depth gauge device. It is then removed, and the appropriate-length 3.5-mm cortical bone screw is inserted (alternatively, the guide pin is removed, and a standard depth gauge is used to measure screw length). Once the superior guide pin is secure, the process is repeated for the inferior guide pin (Figure 3).
Once the graft is secure, the capsule is attached to the graft with the use of a free needle on the suture of the graft (Figure 4).
Outcomes
Coracoid bone transfer or the Bristow-Latarjet technique has become more popular since bone loss was recognized as an important cause of failure of soft-tissue repair for anterior instability. This procedure, however, is not without complications. In a recent systematic review of 45 studies (1904 shoulders), Griesser and colleagues5 found an overall complication rate of 30% and a reoperation rate of 7%.
Given the potential complications of coracoid bone transfer, allograft reconstruction of the anteroinferior glenoid has become increasingly popular and proved successful at short- and medium-term follow-up. Allograft reconstruction avoids the drawbacks of traditional coracoid bone transfer—namely, high rates of neurovascular injury, and nonanatomical reconstruction with high rates of graft resorption and arthritis.5,6 At average 45-month follow-up after fresh distal tibia allograft reconstruction, Provencher and colleagues7 found an 89% radiographic union rate (average lysis, 3%), significantly improved patient-reported outcomes, and no recurrent instability. Similarly, in a study of iliac crest allograft reconstruction in 10 patients with an average 4-year follow-up, Mascarenhas and colleagues8 found an 80% radiographic union rate at 6 months, significantly improved patient-reported outcomes, and no recurrent shoulder instability.
The advantage of Glenojet over other allografts is that it is preshaped and predrilled and saves the surgeon the time and effort of preparing graft in the operating room. The surgical technologist can place the sutures before the patient enters the room. The 2 allograft sizes (10 mm × 29 mm, 13 mm × 34 mm) accommodate the spectrum of bone loss in glenoid deficiency, and graft contour fits the native glenoid well. So far we have implanted this allograft in 15 patients, and at short-term follow-up there are no known cases of recurrent instability.
The potential disadvantages of Glenojet are similar to those of other allografts. Care must be taken with retractor placement to avoid damaging the axillary and musculocutaneous nerves. There are concerns about graft union and subsequent resorption, but this will require long-term follow-up to determine. At 9-month follow-up, we had 1 fracture at the superior corner of the graft, which may have resulted from overtightening the screws in the graft, creating a stress concentration. After removal of this fragment arthroscopically, the patient has done very well clinically with no pain, instability and has returned to all activities. Although the graft does not have an articular surface, the capsular repair covers much of the articular side of the graft, and therefore we do not anticipate that the absence of articular cartilage will contribute to glenohumeral arthritis, though long-term follow-up is lacking. The other question many have is related to the lack of the sling effect since there is no conjoined tendon on the graft. Yamamoto and colleagues9 have reported that the conjoined tendon is the major stabilizing force at time zero in a cadaver model. However, other authors7,8 have successfully reconstructed glenoid defects in these difficult cases without the “sling effect” of the conjoined tendon with excellent clinical results. Our experience has been similar. It is likely that long-term studies will be necessary to answer this question. We have also done some cases with the tendon attached after releasing it from the coracoid, but the series is too small to make any comment about whether this is important or not.
The main limitation of this allograft technique is the lack of long-term outcome studies. However, short-term results are promising, and the ease of the procedure makes it an attractive option for either glenoid reconstruction of bony Bankart lesions or failed bone reconstruction, such as Bristow-Latarjet reconstruction.
Glenojetallograft is a new glenoid reconstruction option that is technically easy and simple to perform in cases of glenoid bone loss, while still creating an anatomical buttress with less surgical dissection than traditional coracoid bone transfer. Short-term outcomes are reassuring, though more research is needed for long-term graft follow-up and recurrent instability.
Am J Orthop. 2017;46(4):199-202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
2. Rowe CR, Sakellarides HT. Factors related to recurrences of anterior dislocations of the shoulder. Clin Orthop. 1961;(20):40-48.
3. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
4. Sayegh ET, Mascarenhas R, Chalmers PN, Cole BJ, Verma NN, Romeo AA. Allograft reconstruction for glenoid bone loss in glenohumeral instability: a systematic review. Arthroscopy. 2014;30(12):1642-1649.
5. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operati ons after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22(2):286-292.
6. Young DC, Rockwood CA Jr. Complications of a failed Bristow procedure and their management. J Bone Joint Surg Am. 1991;73(7):969-981.
7. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
8. Mascarenhas R, Raleigh E, McRae S, Leiter J, Saltzman B, MacDonald PB. Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: surgical technique and results. Int J Shoulder Surg. 2014;8(4):127-132.
9. Yamamoto N, Muraki T, An KN, et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am. 2013;95(15):1390-1397.
1. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
2. Rowe CR, Sakellarides HT. Factors related to recurrences of anterior dislocations of the shoulder. Clin Orthop. 1961;(20):40-48.
3. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
4. Sayegh ET, Mascarenhas R, Chalmers PN, Cole BJ, Verma NN, Romeo AA. Allograft reconstruction for glenoid bone loss in glenohumeral instability: a systematic review. Arthroscopy. 2014;30(12):1642-1649.
5. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operati ons after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22(2):286-292.
6. Young DC, Rockwood CA Jr. Complications of a failed Bristow procedure and their management. J Bone Joint Surg Am. 1991;73(7):969-981.
7. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
8. Mascarenhas R, Raleigh E, McRae S, Leiter J, Saltzman B, MacDonald PB. Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: surgical technique and results. Int J Shoulder Surg. 2014;8(4):127-132.
9. Yamamoto N, Muraki T, An KN, et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am. 2013;95(15):1390-1397.
Systematic Review of Novel Synovial Fluid Markers and Polymerase Chain Reaction in the Diagnosis of Prosthetic Joint Infection
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.