User login
Loss of BMD linked to knee OA progression
Longitudinal loss of bone mineral density from the femoral neck was associated with prevalent knee osteoarthritis in an observational cohort study, lending support to the notion that osteoporosis treatments are worth further investigation for the prevention of osteoarthritis progression.
The study, led by Dr. Ji Y. Lee of the division of rheumatology at Tufts Medical Center, Boston, builds on previous research that loss of bone mineral density (BMD) is associated with the progression of radiographic joint space narrowing. The current study is the first to examine the relationship between BMD and knee osteoarthritis (OA) progression as measured by cartilage volume and thickness on MRI, providing a more sensitive measure of changes in knee cartilage (Arthritis Rheum. 2013 March 12 [doi:10.1002/art.37926]).
The study contrasts with previous research, which has shown a positive relationship between BMD and incident or prevalent radiographically measured knee OA. Other past studies have found no relationship between BMD and radiographic progression of OA or a paradoxical opposite relationship in which low BMD predicted radiographic progression, according to Dr. Lee and associates.
Because previous studies included patients who already had OA, there may have been selection bias (collider confounding) in which the variables of interest – BMD and cartilage volume and thickness – were affected by the same factors. Dr. Lee and colleagues hoped to avoid this selection bias by studying the effect of risk factors that change after disease onset – in this case, change in BMD.
The investigators analyzed a cohort of 127 patients with prevalent knee OA, defined as a Kellgren-Lawrence grade of 2 or more. The patients had at least two MRI scans over a 2-year period, but most had three scans: at baseline and at 1 and 2 years. The patients (41% men) had a mean age of 63 years and mean body mass index (BMI) of 30 kg/m2. Baseline BMD averaged across two femoral neck measurements was 0.95 g/cm2.
In multivariate linear regression models – adjusted for baseline values of age, gender, BMI, alignment status, and vitamin D treatment – BMD loss of 0.1 g/cm2 was associated with a 1.25% per year loss of cartilage volume. For patients who lost BMD at a rate considered to be significant (calculated by the investigators to be a loss of at least 4.7% from baseline), cartilage volume loss was 1.02% per year greater than for patients without BMD loss.
The models also showed that a BMD loss of 0.1 g/cm2 was associated with a significant loss of cartilage thickness at the tibia (0.028 mm/year). Those who lost at least 4.7% of BMD lost a mean of 0.021 mm in tibial cartilage thickness per year, compared with those who did not lose BMD, the investigators reported.
Baseline BMD, however, was not significantly associated with any cartilage outcomes in the study.
The biological mechanisms conjectured to link systemic BMD to cartilage loss in knee OA include the possibility that "BMD health might provide an environment that supports optimal subchondral bone turnover and remodeling in response to OA stressors, thus favoring joint stabilization" and the beneficial effect that optimal bone health might have on cartilage health via "humoral mechanisms," Dr. Lee and colleagues wrote. "Systemic BMD could also be a marker for a range of covariates that mediate or confound the relationship, such as systemic inflammation, circulating growth factors or hormones, physical activity, or frailty."
The original trial from which the observational cohort was derived, the Randomized Controlled Trial of Vitamin D for Knee OA, was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. None of the authors had financial disclosures to report.
Dr. M. Kassim Javaid and Dr. Nigel K. Arden commented:
Understanding the association between OA and osteoporosis has proven to
be challenging because of the difficulty in disentangling the effects
of BMD on OA, and, conversely, the effects of OA on BMD and fracture
risk.
The answer might be found in the effects of bone size and
the BMD properties of local subchondral bone on progression of OA.
Patients with OA have been found to have larger bone size but not higher
volumetric BMD (vBMD), as measured by peripheral quantitative CT.
Another study that used CT to measure vBMD of the subchondral bone found
higher vBMD among patients with knee OA, Dr. Javaid and Dr. Arden
wrote.
These factors may be intertwined by characteristics of bone
phenotype that cannot be assessed by measuring BMD, including bone
marrow lesions, metabolic activity, and bone turnover markers.
The
novelty of Dr. Lee and colleagues getting around this problem by
accounting for changes in femoral neck BMD over time helps to shed light
on the true contribution of BMD to OA progression, but it should be
noted that the study "does not address whether changes in [areal] BMD
are a cause vs. effect of cartilage loss. However it is likely the
relationship is bi-directional with bone altering chondrocyte and matrix
properties and vice versa," Dr. Javaid and Dr. Arden said.
They
also noted that the study by Dr. Lee and colleagues is limited by the
fact that only 13% of patients lost enough BMD to be deemed significant
and by the question of whether the knee OA of patients with a
Kellgren-Lawrence grade of 4 could progress.
Further studies will
need to focus on symptomatic progression as a primary outcome rather
than change in cartilage volume. A key randomized controlled trial
appeared to indicate that bisphosphonate treatment had no effect on OA,
but a cartilage-sparing effect of strontium ranelate has been confirmed
in a phase III trial on both symptoms and a structural end point for
knee OA (Ann. Rheum. Dis. 2013;72:179-86), Dr. Javaid and Dr. Arden noted.
Dr.
Javaid and Dr. Arden, both with the University of Oxford (England), did
not report having any conflicts of interest. Their remarks were taken
from an editorial accompanying Dr. Lee’s study (Arthritis Rheum. 2013 March 12 [doi:10.1002/art.37924]).
Dr. M. Kassim Javaid and Dr. Nigel K. Arden commented:
Understanding the association between OA and osteoporosis has proven to
be challenging because of the difficulty in disentangling the effects
of BMD on OA, and, conversely, the effects of OA on BMD and fracture
risk.
The answer might be found in the effects of bone size and
the BMD properties of local subchondral bone on progression of OA.
Patients with OA have been found to have larger bone size but not higher
volumetric BMD (vBMD), as measured by peripheral quantitative CT.
Another study that used CT to measure vBMD of the subchondral bone found
higher vBMD among patients with knee OA, Dr. Javaid and Dr. Arden
wrote.
These factors may be intertwined by characteristics of bone
phenotype that cannot be assessed by measuring BMD, including bone
marrow lesions, metabolic activity, and bone turnover markers.
The
novelty of Dr. Lee and colleagues getting around this problem by
accounting for changes in femoral neck BMD over time helps to shed light
on the true contribution of BMD to OA progression, but it should be
noted that the study "does not address whether changes in [areal] BMD
are a cause vs. effect of cartilage loss. However it is likely the
relationship is bi-directional with bone altering chondrocyte and matrix
properties and vice versa," Dr. Javaid and Dr. Arden said.
They
also noted that the study by Dr. Lee and colleagues is limited by the
fact that only 13% of patients lost enough BMD to be deemed significant
and by the question of whether the knee OA of patients with a
Kellgren-Lawrence grade of 4 could progress.
Further studies will
need to focus on symptomatic progression as a primary outcome rather
than change in cartilage volume. A key randomized controlled trial
appeared to indicate that bisphosphonate treatment had no effect on OA,
but a cartilage-sparing effect of strontium ranelate has been confirmed
in a phase III trial on both symptoms and a structural end point for
knee OA (Ann. Rheum. Dis. 2013;72:179-86), Dr. Javaid and Dr. Arden noted.
Dr.
Javaid and Dr. Arden, both with the University of Oxford (England), did
not report having any conflicts of interest. Their remarks were taken
from an editorial accompanying Dr. Lee’s study (Arthritis Rheum. 2013 March 12 [doi:10.1002/art.37924]).
Dr. M. Kassim Javaid and Dr. Nigel K. Arden commented:
Understanding the association between OA and osteoporosis has proven to
be challenging because of the difficulty in disentangling the effects
of BMD on OA, and, conversely, the effects of OA on BMD and fracture
risk.
The answer might be found in the effects of bone size and
the BMD properties of local subchondral bone on progression of OA.
Patients with OA have been found to have larger bone size but not higher
volumetric BMD (vBMD), as measured by peripheral quantitative CT.
Another study that used CT to measure vBMD of the subchondral bone found
higher vBMD among patients with knee OA, Dr. Javaid and Dr. Arden
wrote.
These factors may be intertwined by characteristics of bone
phenotype that cannot be assessed by measuring BMD, including bone
marrow lesions, metabolic activity, and bone turnover markers.
The
novelty of Dr. Lee and colleagues getting around this problem by
accounting for changes in femoral neck BMD over time helps to shed light
on the true contribution of BMD to OA progression, but it should be
noted that the study "does not address whether changes in [areal] BMD
are a cause vs. effect of cartilage loss. However it is likely the
relationship is bi-directional with bone altering chondrocyte and matrix
properties and vice versa," Dr. Javaid and Dr. Arden said.
They
also noted that the study by Dr. Lee and colleagues is limited by the
fact that only 13% of patients lost enough BMD to be deemed significant
and by the question of whether the knee OA of patients with a
Kellgren-Lawrence grade of 4 could progress.
Further studies will
need to focus on symptomatic progression as a primary outcome rather
than change in cartilage volume. A key randomized controlled trial
appeared to indicate that bisphosphonate treatment had no effect on OA,
but a cartilage-sparing effect of strontium ranelate has been confirmed
in a phase III trial on both symptoms and a structural end point for
knee OA (Ann. Rheum. Dis. 2013;72:179-86), Dr. Javaid and Dr. Arden noted.
Dr.
Javaid and Dr. Arden, both with the University of Oxford (England), did
not report having any conflicts of interest. Their remarks were taken
from an editorial accompanying Dr. Lee’s study (Arthritis Rheum. 2013 March 12 [doi:10.1002/art.37924]).
Longitudinal loss of bone mineral density from the femoral neck was associated with prevalent knee osteoarthritis in an observational cohort study, lending support to the notion that osteoporosis treatments are worth further investigation for the prevention of osteoarthritis progression.
The study, led by Dr. Ji Y. Lee of the division of rheumatology at Tufts Medical Center, Boston, builds on previous research that loss of bone mineral density (BMD) is associated with the progression of radiographic joint space narrowing. The current study is the first to examine the relationship between BMD and knee osteoarthritis (OA) progression as measured by cartilage volume and thickness on MRI, providing a more sensitive measure of changes in knee cartilage (Arthritis Rheum. 2013 March 12 [doi:10.1002/art.37926]).
The study contrasts with previous research, which has shown a positive relationship between BMD and incident or prevalent radiographically measured knee OA. Other past studies have found no relationship between BMD and radiographic progression of OA or a paradoxical opposite relationship in which low BMD predicted radiographic progression, according to Dr. Lee and associates.
Because previous studies included patients who already had OA, there may have been selection bias (collider confounding) in which the variables of interest – BMD and cartilage volume and thickness – were affected by the same factors. Dr. Lee and colleagues hoped to avoid this selection bias by studying the effect of risk factors that change after disease onset – in this case, change in BMD.
The investigators analyzed a cohort of 127 patients with prevalent knee OA, defined as a Kellgren-Lawrence grade of 2 or more. The patients had at least two MRI scans over a 2-year period, but most had three scans: at baseline and at 1 and 2 years. The patients (41% men) had a mean age of 63 years and mean body mass index (BMI) of 30 kg/m2. Baseline BMD averaged across two femoral neck measurements was 0.95 g/cm2.
In multivariate linear regression models – adjusted for baseline values of age, gender, BMI, alignment status, and vitamin D treatment – BMD loss of 0.1 g/cm2 was associated with a 1.25% per year loss of cartilage volume. For patients who lost BMD at a rate considered to be significant (calculated by the investigators to be a loss of at least 4.7% from baseline), cartilage volume loss was 1.02% per year greater than for patients without BMD loss.
The models also showed that a BMD loss of 0.1 g/cm2 was associated with a significant loss of cartilage thickness at the tibia (0.028 mm/year). Those who lost at least 4.7% of BMD lost a mean of 0.021 mm in tibial cartilage thickness per year, compared with those who did not lose BMD, the investigators reported.
Baseline BMD, however, was not significantly associated with any cartilage outcomes in the study.
The biological mechanisms conjectured to link systemic BMD to cartilage loss in knee OA include the possibility that "BMD health might provide an environment that supports optimal subchondral bone turnover and remodeling in response to OA stressors, thus favoring joint stabilization" and the beneficial effect that optimal bone health might have on cartilage health via "humoral mechanisms," Dr. Lee and colleagues wrote. "Systemic BMD could also be a marker for a range of covariates that mediate or confound the relationship, such as systemic inflammation, circulating growth factors or hormones, physical activity, or frailty."
The original trial from which the observational cohort was derived, the Randomized Controlled Trial of Vitamin D for Knee OA, was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. None of the authors had financial disclosures to report.
Longitudinal loss of bone mineral density from the femoral neck was associated with prevalent knee osteoarthritis in an observational cohort study, lending support to the notion that osteoporosis treatments are worth further investigation for the prevention of osteoarthritis progression.
The study, led by Dr. Ji Y. Lee of the division of rheumatology at Tufts Medical Center, Boston, builds on previous research that loss of bone mineral density (BMD) is associated with the progression of radiographic joint space narrowing. The current study is the first to examine the relationship between BMD and knee osteoarthritis (OA) progression as measured by cartilage volume and thickness on MRI, providing a more sensitive measure of changes in knee cartilage (Arthritis Rheum. 2013 March 12 [doi:10.1002/art.37926]).
The study contrasts with previous research, which has shown a positive relationship between BMD and incident or prevalent radiographically measured knee OA. Other past studies have found no relationship between BMD and radiographic progression of OA or a paradoxical opposite relationship in which low BMD predicted radiographic progression, according to Dr. Lee and associates.
Because previous studies included patients who already had OA, there may have been selection bias (collider confounding) in which the variables of interest – BMD and cartilage volume and thickness – were affected by the same factors. Dr. Lee and colleagues hoped to avoid this selection bias by studying the effect of risk factors that change after disease onset – in this case, change in BMD.
The investigators analyzed a cohort of 127 patients with prevalent knee OA, defined as a Kellgren-Lawrence grade of 2 or more. The patients had at least two MRI scans over a 2-year period, but most had three scans: at baseline and at 1 and 2 years. The patients (41% men) had a mean age of 63 years and mean body mass index (BMI) of 30 kg/m2. Baseline BMD averaged across two femoral neck measurements was 0.95 g/cm2.
In multivariate linear regression models – adjusted for baseline values of age, gender, BMI, alignment status, and vitamin D treatment – BMD loss of 0.1 g/cm2 was associated with a 1.25% per year loss of cartilage volume. For patients who lost BMD at a rate considered to be significant (calculated by the investigators to be a loss of at least 4.7% from baseline), cartilage volume loss was 1.02% per year greater than for patients without BMD loss.
The models also showed that a BMD loss of 0.1 g/cm2 was associated with a significant loss of cartilage thickness at the tibia (0.028 mm/year). Those who lost at least 4.7% of BMD lost a mean of 0.021 mm in tibial cartilage thickness per year, compared with those who did not lose BMD, the investigators reported.
Baseline BMD, however, was not significantly associated with any cartilage outcomes in the study.
The biological mechanisms conjectured to link systemic BMD to cartilage loss in knee OA include the possibility that "BMD health might provide an environment that supports optimal subchondral bone turnover and remodeling in response to OA stressors, thus favoring joint stabilization" and the beneficial effect that optimal bone health might have on cartilage health via "humoral mechanisms," Dr. Lee and colleagues wrote. "Systemic BMD could also be a marker for a range of covariates that mediate or confound the relationship, such as systemic inflammation, circulating growth factors or hormones, physical activity, or frailty."
The original trial from which the observational cohort was derived, the Randomized Controlled Trial of Vitamin D for Knee OA, was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. None of the authors had financial disclosures to report.
FROM ARTHRITIS & RHEUMATISM
Major finding: BMD loss of 0.1 g/cm2 was associated with a 1.25% per year loss of cartilage volume.
Data source: An observational cohort study of 127 patients with prevalent knee OA.
Disclosures: The original trial from which the observational cohort was derived, the Randomized Controlled Trial of Vitamin D for Knee OA, was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. None of the authors had financial disclosures to report.
USPSTF recommends against postmenopausal vitamin D/calcium supplementation
The U.S. Preventive Services Task Force has recommended against vitamin D and calcium supplementation in healthy postmenopausal women, citing research showing that such supplementation increases the risk of kidney stones and does not protect against fractures in this population.
Specifically, the USPSTF recommended – with moderate certainty – against supplementation with doses of vitamin D at 400 IU or less, and calcium at 1,000 IU or less in noninstitutionalized postmenopausal women. The evidence with respect to higher doses is insufficient for making a recommendation, Dr. Virginia A. Moyer reported on behalf of the USPSTF.
The evidence is also insufficient to assess the balance of the benefits and harms of combined vitamin D and calcium supplementation for the primary prevention of fractures in men and in premenopausal women, according to the recommendation statement, which was published online in the Feb. 25 issue of Annals of Internal Medicine (doi:10.7326/
0003-4819-158-9-201305070-00605).
Based on previously released recommendation statements, however, the USPSTF does recommend vitamin D supplementation for the prevention of falls in community-dwelling adults aged 65 years or older who are at increased risk for falls, and recommends that women aged 65 years and older be screened for osteoporosis.
Younger women with a fracture risk that is equal to or greater than that of a 65-year-old white woman with no additional risk factors should also be screened.
The new recommendations apply to noninstitutionalized or community-dwelling asymptomatic adults without a history of fractures; they do not apply to persons with osteoporosis or vitamin D deficiency.
The USPSTF acknowledged that the health burden of fractures is substantial in the older population, with nearly half of all women over age 50 years experiencing an osteoporosis-related fracture during their lifetime, but the task force noted that the increased risk of renal stones demonstrated in participants in the Women’s Health Initiative study who were taking supplemental vitamin D and calcium was also substantial.
"One woman was diagnosed with a urinary tract stone for every 273 women who received supplementation over a 7-year follow-up period," according to the recommendation statement.
In developing the recommendations, the USPSTF commissioned two systematic reviews of the evidence from 16 available randomized controlled trials and an updated meta-analysis of vitamin D supplementation with or without calcium supplementation.
The members of the USPSTF said they had no relevant financial disclosures.
The USPSTF’s recommendation "must be interpreted in the light of ongoing disputes about the most effective method for assessing vitamin D deficiency, whether calcium and vitamin D supplements are needed by a large portion of the population, and what level of supplementation might best maximize benefits and minimize risks," according to Marion Nestle, Ph.D., and Malden C. Nesheim, Ph.D.
In particular, they cited the "conflicting perspectives" of the Institute of Medicine (IOM) and the Endocrine Society. Having determined that vitamin D and calcium deficiencies are generally not a serious problem in the United States, the IOM established average adult daily requirements for both, and has expressed concern about the possibility of adverse consequences from oversupplementation. Conversely, because vitamin D is a hormone, and supplementation must be considered a form of hormone replacement therapy, the Endocrine Society in 2011 made intake recommendations from a clinical endocrinology perspective based on the premise that vitamin D deficiencies are common among all age groups.
"The USPSTF’s recommendations can be understood as an attempt to clarify the present situation with respect to one specific outcome of supplementation. In doing so, its recommendations have a substantial advantage. They depend on hard endpoints – fractures – rather than on blood levels of 25-hydroxyvitamin D, at best an indirect measure of vitamin D adequacy. The USPSTF uses the same precautionary approach as did the IOM. In the absence of compelling evidence for benefit, taking supplements is not worth any risk, however small," they wrote.
Dr. Nestle and Dr. Nesheim noted that the USPSTF plans to publish further recommendations on the roles of vitamin D, and they urged the task force to "keep in mind the value of making a single recommendation ... that will encompass all potential benefits and risks" to avoid the confusion of multiple recommendations.
"While we wait for the results of further research, the USPSTF’s cautious, evidence-based advice should encourage clinicians to think carefully before advising calcium and vitamin D supplementation for healthy individuals," they concluded.
Dr. Nestle of New York University and Dr. Nesheim of Cornell University, Ithaca, N.Y., wrote their comments in an editorial responding to the USPSTF’s recommendations. Dr. Nestle disclosed some speakers fees and royalties from published books unrelated to this editorial and Dr. Nesheim disclosed royalties from a book unrelated to this editorial.
The USPSTF’s recommendation "must be interpreted in the light of ongoing disputes about the most effective method for assessing vitamin D deficiency, whether calcium and vitamin D supplements are needed by a large portion of the population, and what level of supplementation might best maximize benefits and minimize risks," according to Marion Nestle, Ph.D., and Malden C. Nesheim, Ph.D.
In particular, they cited the "conflicting perspectives" of the Institute of Medicine (IOM) and the Endocrine Society. Having determined that vitamin D and calcium deficiencies are generally not a serious problem in the United States, the IOM established average adult daily requirements for both, and has expressed concern about the possibility of adverse consequences from oversupplementation. Conversely, because vitamin D is a hormone, and supplementation must be considered a form of hormone replacement therapy, the Endocrine Society in 2011 made intake recommendations from a clinical endocrinology perspective based on the premise that vitamin D deficiencies are common among all age groups.
"The USPSTF’s recommendations can be understood as an attempt to clarify the present situation with respect to one specific outcome of supplementation. In doing so, its recommendations have a substantial advantage. They depend on hard endpoints – fractures – rather than on blood levels of 25-hydroxyvitamin D, at best an indirect measure of vitamin D adequacy. The USPSTF uses the same precautionary approach as did the IOM. In the absence of compelling evidence for benefit, taking supplements is not worth any risk, however small," they wrote.
Dr. Nestle and Dr. Nesheim noted that the USPSTF plans to publish further recommendations on the roles of vitamin D, and they urged the task force to "keep in mind the value of making a single recommendation ... that will encompass all potential benefits and risks" to avoid the confusion of multiple recommendations.
"While we wait for the results of further research, the USPSTF’s cautious, evidence-based advice should encourage clinicians to think carefully before advising calcium and vitamin D supplementation for healthy individuals," they concluded.
Dr. Nestle of New York University and Dr. Nesheim of Cornell University, Ithaca, N.Y., wrote their comments in an editorial responding to the USPSTF’s recommendations. Dr. Nestle disclosed some speakers fees and royalties from published books unrelated to this editorial and Dr. Nesheim disclosed royalties from a book unrelated to this editorial.
The USPSTF’s recommendation "must be interpreted in the light of ongoing disputes about the most effective method for assessing vitamin D deficiency, whether calcium and vitamin D supplements are needed by a large portion of the population, and what level of supplementation might best maximize benefits and minimize risks," according to Marion Nestle, Ph.D., and Malden C. Nesheim, Ph.D.
In particular, they cited the "conflicting perspectives" of the Institute of Medicine (IOM) and the Endocrine Society. Having determined that vitamin D and calcium deficiencies are generally not a serious problem in the United States, the IOM established average adult daily requirements for both, and has expressed concern about the possibility of adverse consequences from oversupplementation. Conversely, because vitamin D is a hormone, and supplementation must be considered a form of hormone replacement therapy, the Endocrine Society in 2011 made intake recommendations from a clinical endocrinology perspective based on the premise that vitamin D deficiencies are common among all age groups.
"The USPSTF’s recommendations can be understood as an attempt to clarify the present situation with respect to one specific outcome of supplementation. In doing so, its recommendations have a substantial advantage. They depend on hard endpoints – fractures – rather than on blood levels of 25-hydroxyvitamin D, at best an indirect measure of vitamin D adequacy. The USPSTF uses the same precautionary approach as did the IOM. In the absence of compelling evidence for benefit, taking supplements is not worth any risk, however small," they wrote.
Dr. Nestle and Dr. Nesheim noted that the USPSTF plans to publish further recommendations on the roles of vitamin D, and they urged the task force to "keep in mind the value of making a single recommendation ... that will encompass all potential benefits and risks" to avoid the confusion of multiple recommendations.
"While we wait for the results of further research, the USPSTF’s cautious, evidence-based advice should encourage clinicians to think carefully before advising calcium and vitamin D supplementation for healthy individuals," they concluded.
Dr. Nestle of New York University and Dr. Nesheim of Cornell University, Ithaca, N.Y., wrote their comments in an editorial responding to the USPSTF’s recommendations. Dr. Nestle disclosed some speakers fees and royalties from published books unrelated to this editorial and Dr. Nesheim disclosed royalties from a book unrelated to this editorial.
The U.S. Preventive Services Task Force has recommended against vitamin D and calcium supplementation in healthy postmenopausal women, citing research showing that such supplementation increases the risk of kidney stones and does not protect against fractures in this population.
Specifically, the USPSTF recommended – with moderate certainty – against supplementation with doses of vitamin D at 400 IU or less, and calcium at 1,000 IU or less in noninstitutionalized postmenopausal women. The evidence with respect to higher doses is insufficient for making a recommendation, Dr. Virginia A. Moyer reported on behalf of the USPSTF.
The evidence is also insufficient to assess the balance of the benefits and harms of combined vitamin D and calcium supplementation for the primary prevention of fractures in men and in premenopausal women, according to the recommendation statement, which was published online in the Feb. 25 issue of Annals of Internal Medicine (doi:10.7326/
0003-4819-158-9-201305070-00605).
Based on previously released recommendation statements, however, the USPSTF does recommend vitamin D supplementation for the prevention of falls in community-dwelling adults aged 65 years or older who are at increased risk for falls, and recommends that women aged 65 years and older be screened for osteoporosis.
Younger women with a fracture risk that is equal to or greater than that of a 65-year-old white woman with no additional risk factors should also be screened.
The new recommendations apply to noninstitutionalized or community-dwelling asymptomatic adults without a history of fractures; they do not apply to persons with osteoporosis or vitamin D deficiency.
The USPSTF acknowledged that the health burden of fractures is substantial in the older population, with nearly half of all women over age 50 years experiencing an osteoporosis-related fracture during their lifetime, but the task force noted that the increased risk of renal stones demonstrated in participants in the Women’s Health Initiative study who were taking supplemental vitamin D and calcium was also substantial.
"One woman was diagnosed with a urinary tract stone for every 273 women who received supplementation over a 7-year follow-up period," according to the recommendation statement.
In developing the recommendations, the USPSTF commissioned two systematic reviews of the evidence from 16 available randomized controlled trials and an updated meta-analysis of vitamin D supplementation with or without calcium supplementation.
The members of the USPSTF said they had no relevant financial disclosures.
The U.S. Preventive Services Task Force has recommended against vitamin D and calcium supplementation in healthy postmenopausal women, citing research showing that such supplementation increases the risk of kidney stones and does not protect against fractures in this population.
Specifically, the USPSTF recommended – with moderate certainty – against supplementation with doses of vitamin D at 400 IU or less, and calcium at 1,000 IU or less in noninstitutionalized postmenopausal women. The evidence with respect to higher doses is insufficient for making a recommendation, Dr. Virginia A. Moyer reported on behalf of the USPSTF.
The evidence is also insufficient to assess the balance of the benefits and harms of combined vitamin D and calcium supplementation for the primary prevention of fractures in men and in premenopausal women, according to the recommendation statement, which was published online in the Feb. 25 issue of Annals of Internal Medicine (doi:10.7326/
0003-4819-158-9-201305070-00605).
Based on previously released recommendation statements, however, the USPSTF does recommend vitamin D supplementation for the prevention of falls in community-dwelling adults aged 65 years or older who are at increased risk for falls, and recommends that women aged 65 years and older be screened for osteoporosis.
Younger women with a fracture risk that is equal to or greater than that of a 65-year-old white woman with no additional risk factors should also be screened.
The new recommendations apply to noninstitutionalized or community-dwelling asymptomatic adults without a history of fractures; they do not apply to persons with osteoporosis or vitamin D deficiency.
The USPSTF acknowledged that the health burden of fractures is substantial in the older population, with nearly half of all women over age 50 years experiencing an osteoporosis-related fracture during their lifetime, but the task force noted that the increased risk of renal stones demonstrated in participants in the Women’s Health Initiative study who were taking supplemental vitamin D and calcium was also substantial.
"One woman was diagnosed with a urinary tract stone for every 273 women who received supplementation over a 7-year follow-up period," according to the recommendation statement.
In developing the recommendations, the USPSTF commissioned two systematic reviews of the evidence from 16 available randomized controlled trials and an updated meta-analysis of vitamin D supplementation with or without calcium supplementation.
The members of the USPSTF said they had no relevant financial disclosures.
FROM ANNALS OF INTERNAL MEDICINE
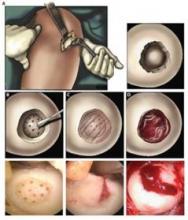
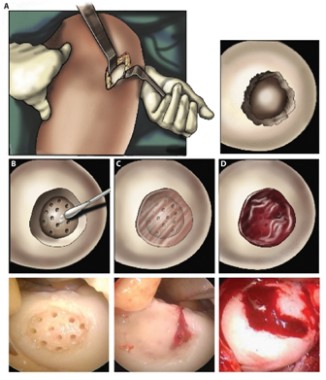
Hydrogel repaired joint defects, sped healing
A light-cured hydrogel combined with microfracture encouraged cartilage repair in knee joints and was associated with reduced pain, a small pilot trial has found.
When applied to a debrided defect in a knee joint, the gel seeped into subchondral microfracture, trapping blood released by microfracture and forming a clot that filled most of the bone defect. Compared with clots formed solely by microfracture, the gel-clot was larger in volume, suggesting that it "may be able to retain more proteins and cells in the defect space ... further augmenting the biological factors that can promote new tissue growth," Blanka Sharma, Ph.D., of Johns Hopkins University, Baltimore, and her colleagues wrote Jan. 9 in Science Translational Medicine.
The authors employed the light-polymerized gel with microfracture in 15 patients with a 2-4 cm2 symptomatic cartilage defect on the medial femoral condyle; three patients with similar defects underwent standard microfracture surgery. The study compared healing, function, and pain over a 6-month postoperative period (Sci. Trans. Med. 2013;5:167ra6).
At 3 months after surgery, MRI scanning in the investigational group showed a combination of clot material, hydrogel, and synovial fluid in the defect. By 6 months, new tissue filled an average of 86% of the defect in the investigational group, compared with an average defect fill of 64% in the microfracture-only group – a significant difference.
Most patients in the investigational group evaluated by MRI (12 of 14) had more than a 75% defect filling, compared with 1 of the 3 control patients.
Most patients who received the hydrogel also experienced significant reductions in pain frequency and severity, with the biggest improvements occurring in the first 3 months after surgery. One patient in this group continued to have pain at 6 months. The probable cause of pain was a misalignment in the treated knee, since the defect was more than 75% filled with new tissue, the authors said.
Control patients experienced similar significant reductions in pain frequency and severity during the first 3 months after surgery. By 6 months, one patient in the group continued to experience improvements in pain; this person "also had a high defect fill (84%), whereas the other two patients had fill values less than 75%," the authors noted.
At 6 months, pain frequency scores were not significantly different between the two groups. There were similar findings in pain severity scores. Knee function improved similarly in both groups.
The study was funded by the National Institutes of Health and the Arthritis Foundation. None of the authors had any financial disclosures.
A light-cured hydrogel combined with microfracture encouraged cartilage repair in knee joints and was associated with reduced pain, a small pilot trial has found.
When applied to a debrided defect in a knee joint, the gel seeped into subchondral microfracture, trapping blood released by microfracture and forming a clot that filled most of the bone defect. Compared with clots formed solely by microfracture, the gel-clot was larger in volume, suggesting that it "may be able to retain more proteins and cells in the defect space ... further augmenting the biological factors that can promote new tissue growth," Blanka Sharma, Ph.D., of Johns Hopkins University, Baltimore, and her colleagues wrote Jan. 9 in Science Translational Medicine.
The authors employed the light-polymerized gel with microfracture in 15 patients with a 2-4 cm2 symptomatic cartilage defect on the medial femoral condyle; three patients with similar defects underwent standard microfracture surgery. The study compared healing, function, and pain over a 6-month postoperative period (Sci. Trans. Med. 2013;5:167ra6).
At 3 months after surgery, MRI scanning in the investigational group showed a combination of clot material, hydrogel, and synovial fluid in the defect. By 6 months, new tissue filled an average of 86% of the defect in the investigational group, compared with an average defect fill of 64% in the microfracture-only group – a significant difference.
Most patients in the investigational group evaluated by MRI (12 of 14) had more than a 75% defect filling, compared with 1 of the 3 control patients.
Most patients who received the hydrogel also experienced significant reductions in pain frequency and severity, with the biggest improvements occurring in the first 3 months after surgery. One patient in this group continued to have pain at 6 months. The probable cause of pain was a misalignment in the treated knee, since the defect was more than 75% filled with new tissue, the authors said.
Control patients experienced similar significant reductions in pain frequency and severity during the first 3 months after surgery. By 6 months, one patient in the group continued to experience improvements in pain; this person "also had a high defect fill (84%), whereas the other two patients had fill values less than 75%," the authors noted.
At 6 months, pain frequency scores were not significantly different between the two groups. There were similar findings in pain severity scores. Knee function improved similarly in both groups.
The study was funded by the National Institutes of Health and the Arthritis Foundation. None of the authors had any financial disclosures.
A light-cured hydrogel combined with microfracture encouraged cartilage repair in knee joints and was associated with reduced pain, a small pilot trial has found.
When applied to a debrided defect in a knee joint, the gel seeped into subchondral microfracture, trapping blood released by microfracture and forming a clot that filled most of the bone defect. Compared with clots formed solely by microfracture, the gel-clot was larger in volume, suggesting that it "may be able to retain more proteins and cells in the defect space ... further augmenting the biological factors that can promote new tissue growth," Blanka Sharma, Ph.D., of Johns Hopkins University, Baltimore, and her colleagues wrote Jan. 9 in Science Translational Medicine.
The authors employed the light-polymerized gel with microfracture in 15 patients with a 2-4 cm2 symptomatic cartilage defect on the medial femoral condyle; three patients with similar defects underwent standard microfracture surgery. The study compared healing, function, and pain over a 6-month postoperative period (Sci. Trans. Med. 2013;5:167ra6).
At 3 months after surgery, MRI scanning in the investigational group showed a combination of clot material, hydrogel, and synovial fluid in the defect. By 6 months, new tissue filled an average of 86% of the defect in the investigational group, compared with an average defect fill of 64% in the microfracture-only group – a significant difference.
Most patients in the investigational group evaluated by MRI (12 of 14) had more than a 75% defect filling, compared with 1 of the 3 control patients.
Most patients who received the hydrogel also experienced significant reductions in pain frequency and severity, with the biggest improvements occurring in the first 3 months after surgery. One patient in this group continued to have pain at 6 months. The probable cause of pain was a misalignment in the treated knee, since the defect was more than 75% filled with new tissue, the authors said.
Control patients experienced similar significant reductions in pain frequency and severity during the first 3 months after surgery. By 6 months, one patient in the group continued to experience improvements in pain; this person "also had a high defect fill (84%), whereas the other two patients had fill values less than 75%," the authors noted.
At 6 months, pain frequency scores were not significantly different between the two groups. There were similar findings in pain severity scores. Knee function improved similarly in both groups.
The study was funded by the National Institutes of Health and the Arthritis Foundation. None of the authors had any financial disclosures.
FROM SCIENCE TRANSLATIONAL MEDICINE
Major Finding: After 6 months, joint defects treated with microfracture plus a photo-polymerized hydrogel were 86% filled, compared with a 64% fill of defects treated with microfracture alone.
Data Source: A pilot study of 18 patients, 15 of whom received the combination treatment.
Disclosures: None of the authors had any financial disclosures. The National Institutes of Health and the Arthritis Foundation funded the study.
Vitamin D lowered aldosterone in heart failure
LOS ANGELES – Oral vitamin D3 may have a future as adjunctive therapy in patients with heart failure, according to Dr. Rebecca S. Boxer.
Six months of oral vitamin D3 therapy at 50,000 IU/wk resulted in a 37% decrease in serum aldosterone level in a randomized, double-blind, placebo-controlled trial conducted in vitamin D–deficient patients with heart failure.
Moreover, parathyroid hormone levels, which were elevated at baseline, fell by 41% in the active treatment arm, she reported at the annual scientific sessions of the American Heart Association.
The study involved 64 patients with New York Heart Association class II-IV heart failure, all having a baseline serum vitamin D level below 20 ng/mL. All participants were on maximum tolerated doses of standard heart failure medications. The patients were randomized to oral vitamin D3 at 50,000 IU/wk plus 400 mg of calcium citrate twice daily for 6 months or to calcium plus placebo.
Mean serum vitamin D levels climbed from 19 ng/mL to 60 ng/mL in treated patients while remaining unchanged over time in controls.
Serum aldosterone levels fell from 10.0 ng/mL to 6.3 ng/dL in the active treatment group and remained static in controls. The mean serum parathyroid hormone level dropped from 62.3 pg/mL to 37.1 pg/mL with vitamin D therapy but stayed steady in controls, said Dr. Boxer of Case Western Reserve University, Cleveland.
The primary endpoint in this randomized trial was change in aerobic capacity; vitamin D supplementation had no effect on this endpoint. Nor did it have any significant impact on heart failure symptoms, echocardiographic findings, body weight, blood pressure, serum renin, or C-reactive protein levels in this relatively small study.
Nonetheless, the finding of a moderate reduction in serum aldosterone level indicates that the effects of vitamin D3 on the renin-angiotensin-aldosterone system (RAAS) in patients with heart failure warrants further study, including assessment of possible long-term clinical benefit.
Dr. Boxer pointed out that serum aldosterone level in patients with heart failure predicts mortality. And aldosterone blockade with an aldosterone antagonist has been shown to improve clinical outcomes in heart failure patients with a depressed left ventricular ejection fraction in RALES, the Randomized Aldactone Evaluation Study (N. Engl. J. Med. 1999;341:709-17) and EPHESUS, the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (N. Engl. J. Med. 2003;348:1309-21). Aldosterone blockade is also actively under study in heart failure patients with preserved systolic function in the ongoing TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) study, due to report results next year.
"There may be certain patient groups that could greatly benefit from vitamin D3 therapy, particularly patients who may not be able to tolerate RAAS blockade or those who have aldosterone escape," she said.
The mechanism by which oral vitamin D3 reduces serum aldosterone is unknown. The mechanism is clearly non–renin-dependent, given that serum renin level remained unchanged. In vitro studies suggest that vitamin D might act through a direct effect on steroidogenesis in adrenal cortical cells, Dr. Boxer said.
The study was funded by the American Heart Association. Dr. Boxer reported having no relevant financial conflicts.
LOS ANGELES – Oral vitamin D3 may have a future as adjunctive therapy in patients with heart failure, according to Dr. Rebecca S. Boxer.
Six months of oral vitamin D3 therapy at 50,000 IU/wk resulted in a 37% decrease in serum aldosterone level in a randomized, double-blind, placebo-controlled trial conducted in vitamin D–deficient patients with heart failure.
Moreover, parathyroid hormone levels, which were elevated at baseline, fell by 41% in the active treatment arm, she reported at the annual scientific sessions of the American Heart Association.
The study involved 64 patients with New York Heart Association class II-IV heart failure, all having a baseline serum vitamin D level below 20 ng/mL. All participants were on maximum tolerated doses of standard heart failure medications. The patients were randomized to oral vitamin D3 at 50,000 IU/wk plus 400 mg of calcium citrate twice daily for 6 months or to calcium plus placebo.
Mean serum vitamin D levels climbed from 19 ng/mL to 60 ng/mL in treated patients while remaining unchanged over time in controls.
Serum aldosterone levels fell from 10.0 ng/mL to 6.3 ng/dL in the active treatment group and remained static in controls. The mean serum parathyroid hormone level dropped from 62.3 pg/mL to 37.1 pg/mL with vitamin D therapy but stayed steady in controls, said Dr. Boxer of Case Western Reserve University, Cleveland.
The primary endpoint in this randomized trial was change in aerobic capacity; vitamin D supplementation had no effect on this endpoint. Nor did it have any significant impact on heart failure symptoms, echocardiographic findings, body weight, blood pressure, serum renin, or C-reactive protein levels in this relatively small study.
Nonetheless, the finding of a moderate reduction in serum aldosterone level indicates that the effects of vitamin D3 on the renin-angiotensin-aldosterone system (RAAS) in patients with heart failure warrants further study, including assessment of possible long-term clinical benefit.
Dr. Boxer pointed out that serum aldosterone level in patients with heart failure predicts mortality. And aldosterone blockade with an aldosterone antagonist has been shown to improve clinical outcomes in heart failure patients with a depressed left ventricular ejection fraction in RALES, the Randomized Aldactone Evaluation Study (N. Engl. J. Med. 1999;341:709-17) and EPHESUS, the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (N. Engl. J. Med. 2003;348:1309-21). Aldosterone blockade is also actively under study in heart failure patients with preserved systolic function in the ongoing TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) study, due to report results next year.
"There may be certain patient groups that could greatly benefit from vitamin D3 therapy, particularly patients who may not be able to tolerate RAAS blockade or those who have aldosterone escape," she said.
The mechanism by which oral vitamin D3 reduces serum aldosterone is unknown. The mechanism is clearly non–renin-dependent, given that serum renin level remained unchanged. In vitro studies suggest that vitamin D might act through a direct effect on steroidogenesis in adrenal cortical cells, Dr. Boxer said.
The study was funded by the American Heart Association. Dr. Boxer reported having no relevant financial conflicts.
LOS ANGELES – Oral vitamin D3 may have a future as adjunctive therapy in patients with heart failure, according to Dr. Rebecca S. Boxer.
Six months of oral vitamin D3 therapy at 50,000 IU/wk resulted in a 37% decrease in serum aldosterone level in a randomized, double-blind, placebo-controlled trial conducted in vitamin D–deficient patients with heart failure.
Moreover, parathyroid hormone levels, which were elevated at baseline, fell by 41% in the active treatment arm, she reported at the annual scientific sessions of the American Heart Association.
The study involved 64 patients with New York Heart Association class II-IV heart failure, all having a baseline serum vitamin D level below 20 ng/mL. All participants were on maximum tolerated doses of standard heart failure medications. The patients were randomized to oral vitamin D3 at 50,000 IU/wk plus 400 mg of calcium citrate twice daily for 6 months or to calcium plus placebo.
Mean serum vitamin D levels climbed from 19 ng/mL to 60 ng/mL in treated patients while remaining unchanged over time in controls.
Serum aldosterone levels fell from 10.0 ng/mL to 6.3 ng/dL in the active treatment group and remained static in controls. The mean serum parathyroid hormone level dropped from 62.3 pg/mL to 37.1 pg/mL with vitamin D therapy but stayed steady in controls, said Dr. Boxer of Case Western Reserve University, Cleveland.
The primary endpoint in this randomized trial was change in aerobic capacity; vitamin D supplementation had no effect on this endpoint. Nor did it have any significant impact on heart failure symptoms, echocardiographic findings, body weight, blood pressure, serum renin, or C-reactive protein levels in this relatively small study.
Nonetheless, the finding of a moderate reduction in serum aldosterone level indicates that the effects of vitamin D3 on the renin-angiotensin-aldosterone system (RAAS) in patients with heart failure warrants further study, including assessment of possible long-term clinical benefit.
Dr. Boxer pointed out that serum aldosterone level in patients with heart failure predicts mortality. And aldosterone blockade with an aldosterone antagonist has been shown to improve clinical outcomes in heart failure patients with a depressed left ventricular ejection fraction in RALES, the Randomized Aldactone Evaluation Study (N. Engl. J. Med. 1999;341:709-17) and EPHESUS, the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (N. Engl. J. Med. 2003;348:1309-21). Aldosterone blockade is also actively under study in heart failure patients with preserved systolic function in the ongoing TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) study, due to report results next year.
"There may be certain patient groups that could greatly benefit from vitamin D3 therapy, particularly patients who may not be able to tolerate RAAS blockade or those who have aldosterone escape," she said.
The mechanism by which oral vitamin D3 reduces serum aldosterone is unknown. The mechanism is clearly non–renin-dependent, given that serum renin level remained unchanged. In vitro studies suggest that vitamin D might act through a direct effect on steroidogenesis in adrenal cortical cells, Dr. Boxer said.
The study was funded by the American Heart Association. Dr. Boxer reported having no relevant financial conflicts.
AT THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: Six months of oral vitamin D3 at 50,000 IU/wk resulted in a 37% reduction in serum aldosterone and a 41% decrease in baseline elevated parathyroid hormone levels in heart failure patients with low serum vitamin D levels.
Data Source: A randomized, double-blind trial of 64 vitamin D–deficient heart failure patients.
Disclosures: The study was funded primarily by the American Heart Association. The presenter reported having no relevant financial conflicts.
Metabolic bone disease markers poor in CKD patients with HF
SAN DIEGO – Levels of calcium, phosphorus, and parathyroid hormone are poorer in patients with heart failure at each stage of chronic kidney disease, results from a large study showed.
The finding "raises more questions than it answers," Dr. Claudine T. Jurkovitz said in an interview during a poster session at Kidney Week 2012. "The question is, are these patients less well managed for their metabolic bone disease than the patients without HF? If so, why? Is it because their HF is so severe, or is it because the nephrologists count on cardiologists or primary care physicians to treat the patients’ metabolic bone disease also? And do cardiologists identify metabolic bone disease in patients with HF?"
Dr. Jurkovitz, a physician scientist with Christiana Care Health System in Newark, Del., and her associates compared the management of CKD-associated metabolic bone disease between patients with and without HF who were treated at a local nephrology practice between 2000 and 2010. They evaluated the medical records of 11,883 patients with CKD stage 3 and above, and excluded dialysis and transplant patients. The researchers calculated average calcium, phosphorus, and intact parathyroid hormone (iPTH) by radioimmunoassay for each patient, and used multilinear regressions to determine the effects of CKD and HF on calcium, phosphorus, and iPTH after controlling for age, race, and gender.
The mean follow-up of the 11,883 patients was 4 years. Of these, nearly one-quarter (24%) had HF at baseline, while 76% had stage 3 CKD, 22% had stage 4 CKD, and 2% had stage 5 CKD. Patients with HF were slightly older, with a mean of 69 years, than were their counterparts without HF, who had a mean 66 years.
Dr. Jurkovitz and her associates found that the adjusted mean for calcium was significantly lower in patients with HF at each CKD stage. The interaction between CKD and HF was statistically significant. The adjusted means for phosphorus and iPTH were significantly higher in patients with HF at each CKD stage, while the interactions between CKD and HF were not significant.
"Physicians need to be concerned about the management of chronic kidney disease in their patients with HF, and the management of metabolic bone disease addressed on a case by case basis in a dialogue between the cardiologists, nephrologists, and primary care physicians," she concluded.
The meeting was sponsored by the American Society of Nephrology. Dr. Jurkovitz said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Levels of calcium, phosphorus, and parathyroid hormone are poorer in patients with heart failure at each stage of chronic kidney disease, results from a large study showed.
The finding "raises more questions than it answers," Dr. Claudine T. Jurkovitz said in an interview during a poster session at Kidney Week 2012. "The question is, are these patients less well managed for their metabolic bone disease than the patients without HF? If so, why? Is it because their HF is so severe, or is it because the nephrologists count on cardiologists or primary care physicians to treat the patients’ metabolic bone disease also? And do cardiologists identify metabolic bone disease in patients with HF?"
Dr. Jurkovitz, a physician scientist with Christiana Care Health System in Newark, Del., and her associates compared the management of CKD-associated metabolic bone disease between patients with and without HF who were treated at a local nephrology practice between 2000 and 2010. They evaluated the medical records of 11,883 patients with CKD stage 3 and above, and excluded dialysis and transplant patients. The researchers calculated average calcium, phosphorus, and intact parathyroid hormone (iPTH) by radioimmunoassay for each patient, and used multilinear regressions to determine the effects of CKD and HF on calcium, phosphorus, and iPTH after controlling for age, race, and gender.
The mean follow-up of the 11,883 patients was 4 years. Of these, nearly one-quarter (24%) had HF at baseline, while 76% had stage 3 CKD, 22% had stage 4 CKD, and 2% had stage 5 CKD. Patients with HF were slightly older, with a mean of 69 years, than were their counterparts without HF, who had a mean 66 years.
Dr. Jurkovitz and her associates found that the adjusted mean for calcium was significantly lower in patients with HF at each CKD stage. The interaction between CKD and HF was statistically significant. The adjusted means for phosphorus and iPTH were significantly higher in patients with HF at each CKD stage, while the interactions between CKD and HF were not significant.
"Physicians need to be concerned about the management of chronic kidney disease in their patients with HF, and the management of metabolic bone disease addressed on a case by case basis in a dialogue between the cardiologists, nephrologists, and primary care physicians," she concluded.
The meeting was sponsored by the American Society of Nephrology. Dr. Jurkovitz said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Levels of calcium, phosphorus, and parathyroid hormone are poorer in patients with heart failure at each stage of chronic kidney disease, results from a large study showed.
The finding "raises more questions than it answers," Dr. Claudine T. Jurkovitz said in an interview during a poster session at Kidney Week 2012. "The question is, are these patients less well managed for their metabolic bone disease than the patients without HF? If so, why? Is it because their HF is so severe, or is it because the nephrologists count on cardiologists or primary care physicians to treat the patients’ metabolic bone disease also? And do cardiologists identify metabolic bone disease in patients with HF?"
Dr. Jurkovitz, a physician scientist with Christiana Care Health System in Newark, Del., and her associates compared the management of CKD-associated metabolic bone disease between patients with and without HF who were treated at a local nephrology practice between 2000 and 2010. They evaluated the medical records of 11,883 patients with CKD stage 3 and above, and excluded dialysis and transplant patients. The researchers calculated average calcium, phosphorus, and intact parathyroid hormone (iPTH) by radioimmunoassay for each patient, and used multilinear regressions to determine the effects of CKD and HF on calcium, phosphorus, and iPTH after controlling for age, race, and gender.
The mean follow-up of the 11,883 patients was 4 years. Of these, nearly one-quarter (24%) had HF at baseline, while 76% had stage 3 CKD, 22% had stage 4 CKD, and 2% had stage 5 CKD. Patients with HF were slightly older, with a mean of 69 years, than were their counterparts without HF, who had a mean 66 years.
Dr. Jurkovitz and her associates found that the adjusted mean for calcium was significantly lower in patients with HF at each CKD stage. The interaction between CKD and HF was statistically significant. The adjusted means for phosphorus and iPTH were significantly higher in patients with HF at each CKD stage, while the interactions between CKD and HF were not significant.
"Physicians need to be concerned about the management of chronic kidney disease in their patients with HF, and the management of metabolic bone disease addressed on a case by case basis in a dialogue between the cardiologists, nephrologists, and primary care physicians," she concluded.
The meeting was sponsored by the American Society of Nephrology. Dr. Jurkovitz said that she had no relevant financial conflicts to disclose.
AT KIDNEY WEEK 2012
Major Finding: The adjusted mean for serum calcium was significantly lower in patients with heart failure at each CKD stage, while the adjusted means for serum phosphorus and parathyroid hormone by immunoassay were significantly higher in patients with HF at each CKD stage.
Data Source: This was a study of 11,883 patients with CKD stage 3 and above who were treated at a single nephrology practice during 2000-2010.
Disclosures: The meeting was sponsored by the American Society of Nephrology. Dr. Jurkovitz said that she had no relevant financial conflicts to disclose.
Bone Risks of Older Antiepileptics Sometimes Unaddressed
SAN DIEGO – The women who were most likely to be on older antiepileptic drugs were the least likely to be counseled about the dangers those drugs pose to bone health in a retrospective study of 756 women with epilepsy.
Being uninsured or having Medicaid coverage was associated with about 50% lower odds for receiving counseling for bone health among women on phenytoin, carbamazepine, or phenobarbital – older antiepileptic drugs that are known to increase bone turnover and cause other bone issues that are especially problematic for women – investigator and second-year medical student Katie Paniszyn reported at the annual meeting of the American Epilepsy Society.
"This is a big problem. You have this huge discrepancy between who’s being prescribed these medications and who’s being counseled. Privately insured patients were less likely to be on these drugs, but more likely to get proper counseling when they were," said Ms. Paniszyn of Brown University, Providence, R.I.
She and her colleagues identified 756 female patients with epilepsy aged 11-60 years who were seen in 2010 at the outpatient academic neurology clinics affiliated with Hasbro Children’s Hospital and Rhode Island Hospital. They analyzed the patients’ clinical notes from 2005 to 2010.
The older antiepileptic drugs were more commonly used in uninsured patients (39 of 59, 66%) and those on Medicaid (52 of 103, 50%) and Medicare (42 of 85, 49%), compared with privately insured women (147 of 509, 29%). That’s not surprising because, as generics, they are more affordable to people with limited income.
What was surprising, however, was that uninsured and Medicaid patients were about half as likely to be encouraged to do weight-bearing exercises, have regular bone density scans, and take vitamin D and calcium supplements while taking the drugs. Bone health counseling was noted in the charts of 64% of Medicare (27 of 42) and 62% of privately insured patients (91 of 147), but in only 31% of Medicaid (16 of 52) and 36% of uninsured patients (14 of 39) (P less than .001). Epilepsy specialists were more likely to provide bone-health counseling than were general neurologists.
The reason for the discrepancy isn’t clear, but could have something to do with the fact that uninsured and Medicaid patients made fewer visits to the neurology clinic so that there were fewer opportunities to counsel them. Advice about compliance and more immediate side effects may have taken precedence, she said.
Whatever the reason is for the discrepancy, the investigators plan to add a flag to their electronic medical record system to remind clinicians about bone health counseling when they prescribe phenytoin, carbamazepine, or phenobarbital. Even "if someone only comes in once or twice, hopefully that will trigger counseling," she said.
While more than half of the women in the study spoke English, many spoke other languages. Because of that, the investigators also plan to print bone health brochures for epilepsy patients in several languages.
Ms. Paniszyn said she had no relevant financial disclosures.
SAN DIEGO – The women who were most likely to be on older antiepileptic drugs were the least likely to be counseled about the dangers those drugs pose to bone health in a retrospective study of 756 women with epilepsy.
Being uninsured or having Medicaid coverage was associated with about 50% lower odds for receiving counseling for bone health among women on phenytoin, carbamazepine, or phenobarbital – older antiepileptic drugs that are known to increase bone turnover and cause other bone issues that are especially problematic for women – investigator and second-year medical student Katie Paniszyn reported at the annual meeting of the American Epilepsy Society.
"This is a big problem. You have this huge discrepancy between who’s being prescribed these medications and who’s being counseled. Privately insured patients were less likely to be on these drugs, but more likely to get proper counseling when they were," said Ms. Paniszyn of Brown University, Providence, R.I.
She and her colleagues identified 756 female patients with epilepsy aged 11-60 years who were seen in 2010 at the outpatient academic neurology clinics affiliated with Hasbro Children’s Hospital and Rhode Island Hospital. They analyzed the patients’ clinical notes from 2005 to 2010.
The older antiepileptic drugs were more commonly used in uninsured patients (39 of 59, 66%) and those on Medicaid (52 of 103, 50%) and Medicare (42 of 85, 49%), compared with privately insured women (147 of 509, 29%). That’s not surprising because, as generics, they are more affordable to people with limited income.
What was surprising, however, was that uninsured and Medicaid patients were about half as likely to be encouraged to do weight-bearing exercises, have regular bone density scans, and take vitamin D and calcium supplements while taking the drugs. Bone health counseling was noted in the charts of 64% of Medicare (27 of 42) and 62% of privately insured patients (91 of 147), but in only 31% of Medicaid (16 of 52) and 36% of uninsured patients (14 of 39) (P less than .001). Epilepsy specialists were more likely to provide bone-health counseling than were general neurologists.
The reason for the discrepancy isn’t clear, but could have something to do with the fact that uninsured and Medicaid patients made fewer visits to the neurology clinic so that there were fewer opportunities to counsel them. Advice about compliance and more immediate side effects may have taken precedence, she said.
Whatever the reason is for the discrepancy, the investigators plan to add a flag to their electronic medical record system to remind clinicians about bone health counseling when they prescribe phenytoin, carbamazepine, or phenobarbital. Even "if someone only comes in once or twice, hopefully that will trigger counseling," she said.
While more than half of the women in the study spoke English, many spoke other languages. Because of that, the investigators also plan to print bone health brochures for epilepsy patients in several languages.
Ms. Paniszyn said she had no relevant financial disclosures.
SAN DIEGO – The women who were most likely to be on older antiepileptic drugs were the least likely to be counseled about the dangers those drugs pose to bone health in a retrospective study of 756 women with epilepsy.
Being uninsured or having Medicaid coverage was associated with about 50% lower odds for receiving counseling for bone health among women on phenytoin, carbamazepine, or phenobarbital – older antiepileptic drugs that are known to increase bone turnover and cause other bone issues that are especially problematic for women – investigator and second-year medical student Katie Paniszyn reported at the annual meeting of the American Epilepsy Society.
"This is a big problem. You have this huge discrepancy between who’s being prescribed these medications and who’s being counseled. Privately insured patients were less likely to be on these drugs, but more likely to get proper counseling when they were," said Ms. Paniszyn of Brown University, Providence, R.I.
She and her colleagues identified 756 female patients with epilepsy aged 11-60 years who were seen in 2010 at the outpatient academic neurology clinics affiliated with Hasbro Children’s Hospital and Rhode Island Hospital. They analyzed the patients’ clinical notes from 2005 to 2010.
The older antiepileptic drugs were more commonly used in uninsured patients (39 of 59, 66%) and those on Medicaid (52 of 103, 50%) and Medicare (42 of 85, 49%), compared with privately insured women (147 of 509, 29%). That’s not surprising because, as generics, they are more affordable to people with limited income.
What was surprising, however, was that uninsured and Medicaid patients were about half as likely to be encouraged to do weight-bearing exercises, have regular bone density scans, and take vitamin D and calcium supplements while taking the drugs. Bone health counseling was noted in the charts of 64% of Medicare (27 of 42) and 62% of privately insured patients (91 of 147), but in only 31% of Medicaid (16 of 52) and 36% of uninsured patients (14 of 39) (P less than .001). Epilepsy specialists were more likely to provide bone-health counseling than were general neurologists.
The reason for the discrepancy isn’t clear, but could have something to do with the fact that uninsured and Medicaid patients made fewer visits to the neurology clinic so that there were fewer opportunities to counsel them. Advice about compliance and more immediate side effects may have taken precedence, she said.
Whatever the reason is for the discrepancy, the investigators plan to add a flag to their electronic medical record system to remind clinicians about bone health counseling when they prescribe phenytoin, carbamazepine, or phenobarbital. Even "if someone only comes in once or twice, hopefully that will trigger counseling," she said.
While more than half of the women in the study spoke English, many spoke other languages. Because of that, the investigators also plan to print bone health brochures for epilepsy patients in several languages.
Ms. Paniszyn said she had no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: Uninsured women and those on Medicaid were about half as likely to be warned of the bone health impacts of older antiepileptic drugs as were those who were on Medicare or had private insurance.
Data Source: Data are from a retrospective chart study of 756 women with epilepsy.
Disclosures: Ms. Paniszyn said she had no relevant financial disclosures.
Osteoporosis, Vertebral Fractures May Flag Heart Risk in RA
WASHINGTON – Low bone mineral density and the presence of vertebral fractures in patients with rheumatoid arthritis not only signal the likely presence of osteoporosis but also appear to signal increased cardiovascular disease risk, according to findings from a cross-sectional study.*
The findings suggest that dual-energy x-ray absorptiometry (DEXA) scans commonly performed in rheumatoid arthritis (RA) patients to assess for osteoporosis could also serve as an assessment of cardiovascular disease risk, Dr. Ausaf Mohammad reported at the annual meeting of the American College of Rheumatology.
Of 603 patients from a convenience sample of adults who met 1987 ACR criteria for RA classification, 230 had at least one documented cardiovascular disease event, and those patients, compared with patients without a cardiovascular disease event, had significantly lower total hip and lumbar spine bone mineral density, and were four times as likely to have osteoporosis (60% vs. 15%) and twice as likely to have a vertebral fracture (24% vs. 12%), said Dr. Mohammad of Galway (Ireland) University Hospitals.
Low bone mineral density and the presence of vertebral fractures were independently associated with cardiovascular disease after adjustment for age, sex, smoking, hypertension, diabetes, and hypercholesterolemia. These measures outperformed traditional risk factors and RA disease activity scores for predicting cardiovascular disease.*
For example, the age- and sex-adjusted odds ratios for osteoporosis and vertebral fractures in those with cardiovascular disease were 2.70 and 2.67; the odds ratios for diabetes mellitus, smoking history, hypertension, hyperlipidemia, C-reactive protein, and Disease Activity Score (DAS-28) indicative of active RA were 1.61, 1.18, 1.58, 1.02, 1.73, and 1.63, respectively.
Patients in the RA cohort included 446 women and 157 men over age 40 years (mean, 56 years) who had a prior DEXA scan with vertebral fracture assessment available for analysis. The scans were evaluated by blinded musculoskeletal radiologists, and osteoporosis diagnoses were made using World Health Organization DEXA criteria; 32% of patients had osteoporosis by bone mineral density criteria, and 13% had one or more vertebral fractures on vertebral fracture assessment.
Cardiovascular events in the cohort included myocardial infarction in 45 patients, stent in 145, heart failure in 33, and stroke in 7.
The findings are of note given that traditional risk factors for cardiovascular disease tend to underperform in patients with RA, and that cardiovascular disease remains the leading cause of mortality in this population, Dr. Mohammad said.
"The risk of cardiovascular disease rises shortly after onset of arthritis, but does not precede it, and the risk appears similar to what is seen in non-RA subjects with diabetes and non-RA subjects who are 5-10 years older, so this tells us we are looking at a very high-risk population," he said.
However, despite a similar distribution in RA and non-RA patients, risk factors for cardiovascular disease "behave differently" in RA patients, and multiple studies have shown that methods for assessing risk, such as the Framingham risk score, underestimate risk in those with RA.
Indeed, the Framingham risk score did not differ significantly between those with and without cardiovascular disease events in the current study, Dr. Mohammad said, adding: "This poses a significant challenge. Therefore we need better markers and better prediction tools."
It appears, based on these findings, that bone mineral density could serve as such a marker, and that DEXA scans could be a good prediction tool, he said.
Since RA patients are also at increased risk for osteoporosis and fractures, many are already referred for DEXA scans.
"So DEXA may provide an opportunity to assess RA subjects at the time of osteoporosis for cardiovascular disease without the need for additional testing," he said.
Although this study has inherent limitations associated with its cross-sectional design, it does have several strengths, including the large cohort size and the fact that participating radiologists were blinded to patient status. Also, the findings are in keeping with those from multiple prior studies in non-RA populations, demonstrating strong associations between osteoporosis and cardiovascular disease, he added, noting that the findings underscore a need for interventions to reduce cardiovascular disease risk in RA patients with osteoporosis.
Dr. Mohammad reported having no relevant financial disclosures.
*Correction: 12/04/2012: An earlier version of this story misstated Dr. Mohammad's findings.
WASHINGTON – Low bone mineral density and the presence of vertebral fractures in patients with rheumatoid arthritis not only signal the likely presence of osteoporosis but also appear to signal increased cardiovascular disease risk, according to findings from a cross-sectional study.*
The findings suggest that dual-energy x-ray absorptiometry (DEXA) scans commonly performed in rheumatoid arthritis (RA) patients to assess for osteoporosis could also serve as an assessment of cardiovascular disease risk, Dr. Ausaf Mohammad reported at the annual meeting of the American College of Rheumatology.
Of 603 patients from a convenience sample of adults who met 1987 ACR criteria for RA classification, 230 had at least one documented cardiovascular disease event, and those patients, compared with patients without a cardiovascular disease event, had significantly lower total hip and lumbar spine bone mineral density, and were four times as likely to have osteoporosis (60% vs. 15%) and twice as likely to have a vertebral fracture (24% vs. 12%), said Dr. Mohammad of Galway (Ireland) University Hospitals.
Low bone mineral density and the presence of vertebral fractures were independently associated with cardiovascular disease after adjustment for age, sex, smoking, hypertension, diabetes, and hypercholesterolemia. These measures outperformed traditional risk factors and RA disease activity scores for predicting cardiovascular disease.*
For example, the age- and sex-adjusted odds ratios for osteoporosis and vertebral fractures in those with cardiovascular disease were 2.70 and 2.67; the odds ratios for diabetes mellitus, smoking history, hypertension, hyperlipidemia, C-reactive protein, and Disease Activity Score (DAS-28) indicative of active RA were 1.61, 1.18, 1.58, 1.02, 1.73, and 1.63, respectively.
Patients in the RA cohort included 446 women and 157 men over age 40 years (mean, 56 years) who had a prior DEXA scan with vertebral fracture assessment available for analysis. The scans were evaluated by blinded musculoskeletal radiologists, and osteoporosis diagnoses were made using World Health Organization DEXA criteria; 32% of patients had osteoporosis by bone mineral density criteria, and 13% had one or more vertebral fractures on vertebral fracture assessment.
Cardiovascular events in the cohort included myocardial infarction in 45 patients, stent in 145, heart failure in 33, and stroke in 7.
The findings are of note given that traditional risk factors for cardiovascular disease tend to underperform in patients with RA, and that cardiovascular disease remains the leading cause of mortality in this population, Dr. Mohammad said.
"The risk of cardiovascular disease rises shortly after onset of arthritis, but does not precede it, and the risk appears similar to what is seen in non-RA subjects with diabetes and non-RA subjects who are 5-10 years older, so this tells us we are looking at a very high-risk population," he said.
However, despite a similar distribution in RA and non-RA patients, risk factors for cardiovascular disease "behave differently" in RA patients, and multiple studies have shown that methods for assessing risk, such as the Framingham risk score, underestimate risk in those with RA.
Indeed, the Framingham risk score did not differ significantly between those with and without cardiovascular disease events in the current study, Dr. Mohammad said, adding: "This poses a significant challenge. Therefore we need better markers and better prediction tools."
It appears, based on these findings, that bone mineral density could serve as such a marker, and that DEXA scans could be a good prediction tool, he said.
Since RA patients are also at increased risk for osteoporosis and fractures, many are already referred for DEXA scans.
"So DEXA may provide an opportunity to assess RA subjects at the time of osteoporosis for cardiovascular disease without the need for additional testing," he said.
Although this study has inherent limitations associated with its cross-sectional design, it does have several strengths, including the large cohort size and the fact that participating radiologists were blinded to patient status. Also, the findings are in keeping with those from multiple prior studies in non-RA populations, demonstrating strong associations between osteoporosis and cardiovascular disease, he added, noting that the findings underscore a need for interventions to reduce cardiovascular disease risk in RA patients with osteoporosis.
Dr. Mohammad reported having no relevant financial disclosures.
*Correction: 12/04/2012: An earlier version of this story misstated Dr. Mohammad's findings.
WASHINGTON – Low bone mineral density and the presence of vertebral fractures in patients with rheumatoid arthritis not only signal the likely presence of osteoporosis but also appear to signal increased cardiovascular disease risk, according to findings from a cross-sectional study.*
The findings suggest that dual-energy x-ray absorptiometry (DEXA) scans commonly performed in rheumatoid arthritis (RA) patients to assess for osteoporosis could also serve as an assessment of cardiovascular disease risk, Dr. Ausaf Mohammad reported at the annual meeting of the American College of Rheumatology.
Of 603 patients from a convenience sample of adults who met 1987 ACR criteria for RA classification, 230 had at least one documented cardiovascular disease event, and those patients, compared with patients without a cardiovascular disease event, had significantly lower total hip and lumbar spine bone mineral density, and were four times as likely to have osteoporosis (60% vs. 15%) and twice as likely to have a vertebral fracture (24% vs. 12%), said Dr. Mohammad of Galway (Ireland) University Hospitals.
Low bone mineral density and the presence of vertebral fractures were independently associated with cardiovascular disease after adjustment for age, sex, smoking, hypertension, diabetes, and hypercholesterolemia. These measures outperformed traditional risk factors and RA disease activity scores for predicting cardiovascular disease.*
For example, the age- and sex-adjusted odds ratios for osteoporosis and vertebral fractures in those with cardiovascular disease were 2.70 and 2.67; the odds ratios for diabetes mellitus, smoking history, hypertension, hyperlipidemia, C-reactive protein, and Disease Activity Score (DAS-28) indicative of active RA were 1.61, 1.18, 1.58, 1.02, 1.73, and 1.63, respectively.
Patients in the RA cohort included 446 women and 157 men over age 40 years (mean, 56 years) who had a prior DEXA scan with vertebral fracture assessment available for analysis. The scans were evaluated by blinded musculoskeletal radiologists, and osteoporosis diagnoses were made using World Health Organization DEXA criteria; 32% of patients had osteoporosis by bone mineral density criteria, and 13% had one or more vertebral fractures on vertebral fracture assessment.
Cardiovascular events in the cohort included myocardial infarction in 45 patients, stent in 145, heart failure in 33, and stroke in 7.
The findings are of note given that traditional risk factors for cardiovascular disease tend to underperform in patients with RA, and that cardiovascular disease remains the leading cause of mortality in this population, Dr. Mohammad said.
"The risk of cardiovascular disease rises shortly after onset of arthritis, but does not precede it, and the risk appears similar to what is seen in non-RA subjects with diabetes and non-RA subjects who are 5-10 years older, so this tells us we are looking at a very high-risk population," he said.
However, despite a similar distribution in RA and non-RA patients, risk factors for cardiovascular disease "behave differently" in RA patients, and multiple studies have shown that methods for assessing risk, such as the Framingham risk score, underestimate risk in those with RA.
Indeed, the Framingham risk score did not differ significantly between those with and without cardiovascular disease events in the current study, Dr. Mohammad said, adding: "This poses a significant challenge. Therefore we need better markers and better prediction tools."
It appears, based on these findings, that bone mineral density could serve as such a marker, and that DEXA scans could be a good prediction tool, he said.
Since RA patients are also at increased risk for osteoporosis and fractures, many are already referred for DEXA scans.
"So DEXA may provide an opportunity to assess RA subjects at the time of osteoporosis for cardiovascular disease without the need for additional testing," he said.
Although this study has inherent limitations associated with its cross-sectional design, it does have several strengths, including the large cohort size and the fact that participating radiologists were blinded to patient status. Also, the findings are in keeping with those from multiple prior studies in non-RA populations, demonstrating strong associations between osteoporosis and cardiovascular disease, he added, noting that the findings underscore a need for interventions to reduce cardiovascular disease risk in RA patients with osteoporosis.
Dr. Mohammad reported having no relevant financial disclosures.
*Correction: 12/04/2012: An earlier version of this story misstated Dr. Mohammad's findings.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: Of 603 patients from a convenience sample of adults who met 1987 ACR criteria for RA classification, 230 had at least one documented cardiovascular disease event, and those patients, compared with patients without a cardiovascular disease event, had significantly lower total hip and lumbar spine bone mineral density, and were four times as likely to have osteoporosis (60% vs. 15%) and twice as likely to have a vertebral fracture (24% vs. 12%).
Data Source: These findings come from a cross-sectional study of 603 adults with RA.
Disclosures: Dr. Mohammad said he had no relevant financial disclosures to report.
Vitamin D Assays Flawed, but Improving
MINNEAPOLIS – Some day physicians will be able to order detailed vitamin D assay panels much like the lipid panels used today to assess cholesterol.
We’re just not there yet.
“Where we’re at today with 25-hydroxyvitamin D measurement is where we were at 50 or 60 years ago with lipid measurement ... but I believe vitamin D assay panels are coming,” said Dr. Neil C. Binkley of the Institute on Aging at the University of Wisconsin, Madison.
Part of the problem with 25-hydroxyvitamin D [25(OH)D] measurement is that it has not yet been standardized, and there is no agreement on which cut points should be used by laboratories and assay manufacturers.
In its 2011 report that set for the first time Recommended Dietary Allowances for calcium and vitamin D, the Institute of Medicine declared an “urgent clinical and public health need” to reassess laboratory ranges for 25(OH)D to avoid problems of overtreatment and undertreatment.
The IOM committee concluded that the prevalence of vitamin D deficiency in North Americans has been overestimated by some because of the use of “inappropriate cut points” that greatly exceed levels identified in the report (J. Clin. Endocrinol. Metab. 2011;96:53-8).
Substantial progress has been made in the past decade, thanks in large part to the work of Dr. Graham Carter and DEQAS, the Vitamin D External Quality Assessment Scheme, Dr. Binkley said at the annual meeting of the American Society for Bone and Mineral Research. DEQAS provides serum samples on a quarterly basis to laboratories that wish to check the accuracy of their assays, and statistically trims the data to produce an all-laboratory trimmed mean (ALTM), standard deviation, and coefficient of variation for all major methodologies. Recent DEQAS data show, however, that ALTMs can vary substantially, even among groups striving to improve the quality of their program, he said.
In 2010, the National Institutes of Health Office of Dietary Supplements also launched the international vitamin D standardization program dedicated to standardizing 25(OH)D measurement to the National Institute of Standards and Technology (NIST) reference procedures. The program recently began quantitating DEQAS materials with real values assigned by NIST, and is also working on standardizing measurements for 25(OH)D2 and D3, the C-3 epimer of 25(OH)D2 and D3, and 24,25(OH)2D, he said.
“This, I think, is a substantial positive development; however, despite these efforts some scatter is going to persist in 25(OH)D measurement,” Dr. Binkley said.
The reason for this is that analyses will pick up components of a sample other than the analyte – a phenomenon known as the matrix effect. Given the number of vitamin D metabolites that exist, it’s not surprising that assays have trouble identifying one analyte from other similar analytes, he explained.
One of those confounders is the C-3 epimer (3-epi) of 25(OH)D3, which was previously thought to exist in infants only and, as such, wasn’t of much concern, but is now known to be present at variable, albeit low, levels in virtually all human serum (J. Clin. Endocrinol. Metab. 2012;97:163-8). Since 3-epi has the same molecular structure and molecular weight as 25(OH)D, it’s not surprising that it can add “noise” to your 25(OH)D result, Dr. Binkley said.
Another confounder is 24,25(OH)2D3, which has historically been considered as simply an inactivating step in the 25(OH)D degradation pathway on the way to calcitroic acid. As such, many feel it isn’t worth measuring, he said. Recent studies, however, have found that 24,25(OH)2D3 possesses physiologic effects on cartilage and bone, and that once again, it is present at variable levels in adults up to about 10% of the 25(OH)D level.
“Three of our current immunoassays that I know of have 100% cross-reactivity with 24,25-dihydroxyvitamin D3, so when you’re measuring 25-hydroxyvitamin D, you are also measuring 24,25-dihydroxyvitamin D3,” he said.
A more recent study reports that patients with high levels of serum 24,25-dihydroxyvitamin D3 may have a less robust response to vitamin D3 (J. Steroid. Biochem. Mol. Biol. 2011;126:72-7).
Finally, two additional studies find that vitamin D3 is more potent at increasing 25(OH)D than is vitamin D2, but what may be more important is whether current assays can measure the two equally, Dr. Binkley said. Indeed, unpublished data he presented clearly show that a newer immunoassay was not up to the task.
“By prescribing ergocalciferol, we clinicians are needlessly making life more difficult for our laboratory colleagues” and unnecessarily confounding assay methodologies, he said.
Dr. Binkley reported that he had no relevant financial disclosures.
MINNEAPOLIS – Some day physicians will be able to order detailed vitamin D assay panels much like the lipid panels used today to assess cholesterol.
We’re just not there yet.
“Where we’re at today with 25-hydroxyvitamin D measurement is where we were at 50 or 60 years ago with lipid measurement ... but I believe vitamin D assay panels are coming,” said Dr. Neil C. Binkley of the Institute on Aging at the University of Wisconsin, Madison.
Part of the problem with 25-hydroxyvitamin D [25(OH)D] measurement is that it has not yet been standardized, and there is no agreement on which cut points should be used by laboratories and assay manufacturers.
In its 2011 report that set for the first time Recommended Dietary Allowances for calcium and vitamin D, the Institute of Medicine declared an “urgent clinical and public health need” to reassess laboratory ranges for 25(OH)D to avoid problems of overtreatment and undertreatment.
The IOM committee concluded that the prevalence of vitamin D deficiency in North Americans has been overestimated by some because of the use of “inappropriate cut points” that greatly exceed levels identified in the report (J. Clin. Endocrinol. Metab. 2011;96:53-8).
Substantial progress has been made in the past decade, thanks in large part to the work of Dr. Graham Carter and DEQAS, the Vitamin D External Quality Assessment Scheme, Dr. Binkley said at the annual meeting of the American Society for Bone and Mineral Research. DEQAS provides serum samples on a quarterly basis to laboratories that wish to check the accuracy of their assays, and statistically trims the data to produce an all-laboratory trimmed mean (ALTM), standard deviation, and coefficient of variation for all major methodologies. Recent DEQAS data show, however, that ALTMs can vary substantially, even among groups striving to improve the quality of their program, he said.
In 2010, the National Institutes of Health Office of Dietary Supplements also launched the international vitamin D standardization program dedicated to standardizing 25(OH)D measurement to the National Institute of Standards and Technology (NIST) reference procedures. The program recently began quantitating DEQAS materials with real values assigned by NIST, and is also working on standardizing measurements for 25(OH)D2 and D3, the C-3 epimer of 25(OH)D2 and D3, and 24,25(OH)2D, he said.
“This, I think, is a substantial positive development; however, despite these efforts some scatter is going to persist in 25(OH)D measurement,” Dr. Binkley said.
The reason for this is that analyses will pick up components of a sample other than the analyte – a phenomenon known as the matrix effect. Given the number of vitamin D metabolites that exist, it’s not surprising that assays have trouble identifying one analyte from other similar analytes, he explained.
One of those confounders is the C-3 epimer (3-epi) of 25(OH)D3, which was previously thought to exist in infants only and, as such, wasn’t of much concern, but is now known to be present at variable, albeit low, levels in virtually all human serum (J. Clin. Endocrinol. Metab. 2012;97:163-8). Since 3-epi has the same molecular structure and molecular weight as 25(OH)D, it’s not surprising that it can add “noise” to your 25(OH)D result, Dr. Binkley said.
Another confounder is 24,25(OH)2D3, which has historically been considered as simply an inactivating step in the 25(OH)D degradation pathway on the way to calcitroic acid. As such, many feel it isn’t worth measuring, he said. Recent studies, however, have found that 24,25(OH)2D3 possesses physiologic effects on cartilage and bone, and that once again, it is present at variable levels in adults up to about 10% of the 25(OH)D level.
“Three of our current immunoassays that I know of have 100% cross-reactivity with 24,25-dihydroxyvitamin D3, so when you’re measuring 25-hydroxyvitamin D, you are also measuring 24,25-dihydroxyvitamin D3,” he said.
A more recent study reports that patients with high levels of serum 24,25-dihydroxyvitamin D3 may have a less robust response to vitamin D3 (J. Steroid. Biochem. Mol. Biol. 2011;126:72-7).
Finally, two additional studies find that vitamin D3 is more potent at increasing 25(OH)D than is vitamin D2, but what may be more important is whether current assays can measure the two equally, Dr. Binkley said. Indeed, unpublished data he presented clearly show that a newer immunoassay was not up to the task.
“By prescribing ergocalciferol, we clinicians are needlessly making life more difficult for our laboratory colleagues” and unnecessarily confounding assay methodologies, he said.
Dr. Binkley reported that he had no relevant financial disclosures.
MINNEAPOLIS – Some day physicians will be able to order detailed vitamin D assay panels much like the lipid panels used today to assess cholesterol.
We’re just not there yet.
“Where we’re at today with 25-hydroxyvitamin D measurement is where we were at 50 or 60 years ago with lipid measurement ... but I believe vitamin D assay panels are coming,” said Dr. Neil C. Binkley of the Institute on Aging at the University of Wisconsin, Madison.
Part of the problem with 25-hydroxyvitamin D [25(OH)D] measurement is that it has not yet been standardized, and there is no agreement on which cut points should be used by laboratories and assay manufacturers.
In its 2011 report that set for the first time Recommended Dietary Allowances for calcium and vitamin D, the Institute of Medicine declared an “urgent clinical and public health need” to reassess laboratory ranges for 25(OH)D to avoid problems of overtreatment and undertreatment.
The IOM committee concluded that the prevalence of vitamin D deficiency in North Americans has been overestimated by some because of the use of “inappropriate cut points” that greatly exceed levels identified in the report (J. Clin. Endocrinol. Metab. 2011;96:53-8).
Substantial progress has been made in the past decade, thanks in large part to the work of Dr. Graham Carter and DEQAS, the Vitamin D External Quality Assessment Scheme, Dr. Binkley said at the annual meeting of the American Society for Bone and Mineral Research. DEQAS provides serum samples on a quarterly basis to laboratories that wish to check the accuracy of their assays, and statistically trims the data to produce an all-laboratory trimmed mean (ALTM), standard deviation, and coefficient of variation for all major methodologies. Recent DEQAS data show, however, that ALTMs can vary substantially, even among groups striving to improve the quality of their program, he said.
In 2010, the National Institutes of Health Office of Dietary Supplements also launched the international vitamin D standardization program dedicated to standardizing 25(OH)D measurement to the National Institute of Standards and Technology (NIST) reference procedures. The program recently began quantitating DEQAS materials with real values assigned by NIST, and is also working on standardizing measurements for 25(OH)D2 and D3, the C-3 epimer of 25(OH)D2 and D3, and 24,25(OH)2D, he said.
“This, I think, is a substantial positive development; however, despite these efforts some scatter is going to persist in 25(OH)D measurement,” Dr. Binkley said.
The reason for this is that analyses will pick up components of a sample other than the analyte – a phenomenon known as the matrix effect. Given the number of vitamin D metabolites that exist, it’s not surprising that assays have trouble identifying one analyte from other similar analytes, he explained.
One of those confounders is the C-3 epimer (3-epi) of 25(OH)D3, which was previously thought to exist in infants only and, as such, wasn’t of much concern, but is now known to be present at variable, albeit low, levels in virtually all human serum (J. Clin. Endocrinol. Metab. 2012;97:163-8). Since 3-epi has the same molecular structure and molecular weight as 25(OH)D, it’s not surprising that it can add “noise” to your 25(OH)D result, Dr. Binkley said.
Another confounder is 24,25(OH)2D3, which has historically been considered as simply an inactivating step in the 25(OH)D degradation pathway on the way to calcitroic acid. As such, many feel it isn’t worth measuring, he said. Recent studies, however, have found that 24,25(OH)2D3 possesses physiologic effects on cartilage and bone, and that once again, it is present at variable levels in adults up to about 10% of the 25(OH)D level.
“Three of our current immunoassays that I know of have 100% cross-reactivity with 24,25-dihydroxyvitamin D3, so when you’re measuring 25-hydroxyvitamin D, you are also measuring 24,25-dihydroxyvitamin D3,” he said.
A more recent study reports that patients with high levels of serum 24,25-dihydroxyvitamin D3 may have a less robust response to vitamin D3 (J. Steroid. Biochem. Mol. Biol. 2011;126:72-7).
Finally, two additional studies find that vitamin D3 is more potent at increasing 25(OH)D than is vitamin D2, but what may be more important is whether current assays can measure the two equally, Dr. Binkley said. Indeed, unpublished data he presented clearly show that a newer immunoassay was not up to the task.
“By prescribing ergocalciferol, we clinicians are needlessly making life more difficult for our laboratory colleagues” and unnecessarily confounding assay methodologies, he said.
Dr. Binkley reported that he had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR BONE AND MINERAL RESEARCH
New Assay Simultaneously Measures Vitamin D Metabolites
MINNEAPOLIS – A new automated assay can measure concentrations of both 24,25-dihydroxyvitamin D2 and D3 in human serum.
Current assays based on competitive binding experiments can differentiate between 24,25-dihydroxyvitamin D3 and D2, but are time consuming and not easily automated. Also, immunoassays fail to differentiate between 25-hydroxyvitamin D and 24,25-dihydrxoxyvitamin D.
The new, high-throughput mass spectrometry–based assay – developed by researchers at the Mayo Clinic – is able to quantify both analytes.
Knowledge of the serum concentrations of both 24,25(OH)2D and 25(OH)D3 is particularly important in establishing reduced activity of the primary vitamin D degradation enzyme CYP24A1 in patients with mutations in the CYP24A1 (cytochrome P450 24-hydroxylase A1) gene.
"A lot of physicians want this metabolite to be measured just in case a person might be a candidate for more extensive genotyping studies ... They also want to know the reference range in normal individuals," lead author and research fellow Hemamalini Ketha, Ph.D., said in an interview.
Researchers at the Roswell Park Cancer Institute in Buffalo, N.Y., recently reported that single nucleotide polymorphisms in CYP24A1 may be related to the higher prevalence of estrogen receptor–negative breast cancer in African American women – a group known to have lower levels of vitamin D (Breast Cancer Res. 2012;14:R58).
Dr. Ketha and her colleagues at the Mayo Clinic in Rochester, Minn., used a liquid chromatography tandem mass spectrometry method to measure serum 24,25(OH)2D concentrations in 92 serum samples from men and women with a wide range of 25(OH)D concentrations.
The limit of detection for 24,25(OH)2D3 was 0.08 ng/mL; the limit for 24,25(OH)2D2 was 0.5 ng/mL, Dr. Ketha reported in a poster at the annual meeting of the American Society for Bone and Mineral Research.
The mean concentration of 25(OH)D in the serum samples was 29.36 ng/mL and was 2.59 ng/mL for 24,25(OH)2D3.
Serum 24,25(OH)2D concentrations were linearly correlated with serum 25(OH)D concentrations (R2 = 0.75), she said.
No correlation was observed, however, between 24,25(OH)2D3 and 1,25(OH)2D3 (R2 = 0.0005).
"For diagnostic purposes, the interpretation of concentrations of serum 24,25(OH)2D3 should therefore take the concomitant 25(OH)D3 concentrations into account," the authors concluded.
Dr. Ketha said the assay is rapid and reproducible, and that the Mayo Clinic is planning to make it commercially available through its endocrinology clinical laboratory.
He said he had no relevant financial disclosures. The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Ketha said she had no relevant financial disclosures.
"I think there is a major need for assays such as this to get into the clinical world, certainly the research world, so we can have additional insight into what is really going on with the relationship of falling vitamin D status today and all of the [associated] outcomes such as falls and fractures," osteoporosis expert Dr. Neil Binkley said in an interview.
"By just measuring 25-hydroxy D, we’re not seeing the whole picture; just like when we measure total cholesterol, we aren’t seeing the whole picture."
Dr. Binkley is associate director of the Institute on Aging at the University of Wisconsin, Madison. He said he had no relevant financial disclosures.
"I think there is a major need for assays such as this to get into the clinical world, certainly the research world, so we can have additional insight into what is really going on with the relationship of falling vitamin D status today and all of the [associated] outcomes such as falls and fractures," osteoporosis expert Dr. Neil Binkley said in an interview.
"By just measuring 25-hydroxy D, we’re not seeing the whole picture; just like when we measure total cholesterol, we aren’t seeing the whole picture."
Dr. Binkley is associate director of the Institute on Aging at the University of Wisconsin, Madison. He said he had no relevant financial disclosures.
"I think there is a major need for assays such as this to get into the clinical world, certainly the research world, so we can have additional insight into what is really going on with the relationship of falling vitamin D status today and all of the [associated] outcomes such as falls and fractures," osteoporosis expert Dr. Neil Binkley said in an interview.
"By just measuring 25-hydroxy D, we’re not seeing the whole picture; just like when we measure total cholesterol, we aren’t seeing the whole picture."
Dr. Binkley is associate director of the Institute on Aging at the University of Wisconsin, Madison. He said he had no relevant financial disclosures.
MINNEAPOLIS – A new automated assay can measure concentrations of both 24,25-dihydroxyvitamin D2 and D3 in human serum.
Current assays based on competitive binding experiments can differentiate between 24,25-dihydroxyvitamin D3 and D2, but are time consuming and not easily automated. Also, immunoassays fail to differentiate between 25-hydroxyvitamin D and 24,25-dihydrxoxyvitamin D.
The new, high-throughput mass spectrometry–based assay – developed by researchers at the Mayo Clinic – is able to quantify both analytes.
Knowledge of the serum concentrations of both 24,25(OH)2D and 25(OH)D3 is particularly important in establishing reduced activity of the primary vitamin D degradation enzyme CYP24A1 in patients with mutations in the CYP24A1 (cytochrome P450 24-hydroxylase A1) gene.
"A lot of physicians want this metabolite to be measured just in case a person might be a candidate for more extensive genotyping studies ... They also want to know the reference range in normal individuals," lead author and research fellow Hemamalini Ketha, Ph.D., said in an interview.
Researchers at the Roswell Park Cancer Institute in Buffalo, N.Y., recently reported that single nucleotide polymorphisms in CYP24A1 may be related to the higher prevalence of estrogen receptor–negative breast cancer in African American women – a group known to have lower levels of vitamin D (Breast Cancer Res. 2012;14:R58).
Dr. Ketha and her colleagues at the Mayo Clinic in Rochester, Minn., used a liquid chromatography tandem mass spectrometry method to measure serum 24,25(OH)2D concentrations in 92 serum samples from men and women with a wide range of 25(OH)D concentrations.
The limit of detection for 24,25(OH)2D3 was 0.08 ng/mL; the limit for 24,25(OH)2D2 was 0.5 ng/mL, Dr. Ketha reported in a poster at the annual meeting of the American Society for Bone and Mineral Research.
The mean concentration of 25(OH)D in the serum samples was 29.36 ng/mL and was 2.59 ng/mL for 24,25(OH)2D3.
Serum 24,25(OH)2D concentrations were linearly correlated with serum 25(OH)D concentrations (R2 = 0.75), she said.
No correlation was observed, however, between 24,25(OH)2D3 and 1,25(OH)2D3 (R2 = 0.0005).
"For diagnostic purposes, the interpretation of concentrations of serum 24,25(OH)2D3 should therefore take the concomitant 25(OH)D3 concentrations into account," the authors concluded.
Dr. Ketha said the assay is rapid and reproducible, and that the Mayo Clinic is planning to make it commercially available through its endocrinology clinical laboratory.
He said he had no relevant financial disclosures. The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Ketha said she had no relevant financial disclosures.
MINNEAPOLIS – A new automated assay can measure concentrations of both 24,25-dihydroxyvitamin D2 and D3 in human serum.
Current assays based on competitive binding experiments can differentiate between 24,25-dihydroxyvitamin D3 and D2, but are time consuming and not easily automated. Also, immunoassays fail to differentiate between 25-hydroxyvitamin D and 24,25-dihydrxoxyvitamin D.
The new, high-throughput mass spectrometry–based assay – developed by researchers at the Mayo Clinic – is able to quantify both analytes.
Knowledge of the serum concentrations of both 24,25(OH)2D and 25(OH)D3 is particularly important in establishing reduced activity of the primary vitamin D degradation enzyme CYP24A1 in patients with mutations in the CYP24A1 (cytochrome P450 24-hydroxylase A1) gene.
"A lot of physicians want this metabolite to be measured just in case a person might be a candidate for more extensive genotyping studies ... They also want to know the reference range in normal individuals," lead author and research fellow Hemamalini Ketha, Ph.D., said in an interview.
Researchers at the Roswell Park Cancer Institute in Buffalo, N.Y., recently reported that single nucleotide polymorphisms in CYP24A1 may be related to the higher prevalence of estrogen receptor–negative breast cancer in African American women – a group known to have lower levels of vitamin D (Breast Cancer Res. 2012;14:R58).
Dr. Ketha and her colleagues at the Mayo Clinic in Rochester, Minn., used a liquid chromatography tandem mass spectrometry method to measure serum 24,25(OH)2D concentrations in 92 serum samples from men and women with a wide range of 25(OH)D concentrations.
The limit of detection for 24,25(OH)2D3 was 0.08 ng/mL; the limit for 24,25(OH)2D2 was 0.5 ng/mL, Dr. Ketha reported in a poster at the annual meeting of the American Society for Bone and Mineral Research.
The mean concentration of 25(OH)D in the serum samples was 29.36 ng/mL and was 2.59 ng/mL for 24,25(OH)2D3.
Serum 24,25(OH)2D concentrations were linearly correlated with serum 25(OH)D concentrations (R2 = 0.75), she said.
No correlation was observed, however, between 24,25(OH)2D3 and 1,25(OH)2D3 (R2 = 0.0005).
"For diagnostic purposes, the interpretation of concentrations of serum 24,25(OH)2D3 should therefore take the concomitant 25(OH)D3 concentrations into account," the authors concluded.
Dr. Ketha said the assay is rapid and reproducible, and that the Mayo Clinic is planning to make it commercially available through its endocrinology clinical laboratory.
He said he had no relevant financial disclosures. The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Ketha said she had no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR BONE AND MINERAL RESEARCH
Major Finding: The limit of detection in the assay for 24,25(OH)2D3 was 0.08 ng/mL; the limit for 24,25(OH)2D2 was 0.5 ng/mL.
Data Source: Data are from an analysis of 92 serum samples in which a liquid chromatography tandem mass spectrometry assay was used.
Disclosures: The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Ketha reported no relevant financial disclosures.
Odanacatib Adds Bone in Alendronate-Pretreated Osteoporosis
MINNEAPOLIS – Odanacatib added bone density in postmenopausal women with osteoporosis previously treated with alendronate, according to results from a study presented at the annual meeting of the American Society for Bone and Mineral Research.
In a phase II trial involving 243 patients, the investigational oral cathepsin K inhibitor began to distinguish itself from placebo at 12 months. At 2 years, the change from baseline in femoral neck bone mineral density (BMD) was significantly increased by 1.73%, compared with a loss of 0.94% for placebo (P less than .001).
BMD changes in the treatment group at 2 years were also significantly different from placebo at the trochanter (1.83% vs. –1.35%) and for the total hip (0.83% vs. –1.87%, both P less than .001).
All of the women studied had received alendronate (Fosamax) for at least 3 years. Patients could have been off the bisphosphonate for up to 3 months prior to enrollment, but many switched directly over to odanacatib, said study coauthor Dr. Albert Leung, executive director of clinical research at Merck Research Laboratories.
"This drug has the potential to give additional benefits when [patients] have been treated with alendronate for a number of years and the treatment effect has reached a plateau and they may need a different treatment," he said in an interview.
In July 2012, a pivotal 16,731-patient, phase III trial of odanacatib was stopped early after an interim analysis showed a "favorable benefit-risk profile" for fracture risk reduction in postmenopausal women with previously untreated osteoporosis.
"It could be quite a population that may benefit from this drug," Dr. Leung remarked.
The study’s data monitoring committee, however, flagged safety concerns in "certain selected areas" for further follow-up. Those safety risks have not been identified as the trial is still closing, but will be monitored along with efficacy in a double-blind, placebo-controlled, extension trial going out to 5 years of treatment, he said.
Merck, which plans to submit regulatory applications for odanacatib in the United States and Europe in the first half of 2013, has high hopes for odanacatib despite competition from generic drugs because of its novel mechanism of action.
Odanacatib inhibits cathepsin K, the primary protease in osteoclasts that breaks down bone collagen during bone resorption. Unlike traditional antiresorptive drugs like bisphosphonates, however, odanacatib does not interfere with the function of the entire osteoclast or reduce the number of osteoclasts. This characteristic is important, as osteoclasts secrete signaling factors to stimulate osteoblasts, the cells responsible for bone formation. As a result, there is greater bone formation with odanacatib, Dr. Leung explained.
The phase II trial enrolled women at least 60 years of age (mean 71 years) with a BMD T score of –2.5 to more than –3.5 at any hip site without a prior fragility fracture or those with a history of fragility fracture (except hip fracture), and a BMD T score of –1.5 and more than –3.5 at any hip site. The women were randomly assigned to odanacatib 50 mg once weekly or placebo for 24 months, as well as 5,600 IU of vitamin D3 per week and calcium at dosages up to 1,200 mg/day. The study was not powered to assess fractures.
At 24 months, the change in BMD at the lumbar spine was significant at 2.28% for odanacatib vs. a loss of 0.30% with placebo (P less than .001).
BMD change was not significant at the distal forearm, with losses of 0.92% vs. 1.14%, respectively.
As expected, urinary collagen type I cross-linked N-telopeptide, a biomarker of bone resorption, increased with placebo, compared with a significant 47% decrease with odanacatib.
The bone formation marker, serum type I procollagen, rose inexplicably with placebo, but this increase was surpassed by a significant gain of 31.2% with odanacatib.
Most unexpected, however, was an increase in the resorption marker collagen type I cross-linked C-telopeptide (sCTx) with once-weekly odanacatib. Dr. Leung said the finding appears to correlate with the bone density changes because sCTx levels remained relatively stable during the first 12 months of treatment before rising in the second year of the study.
Finally, adverse events were similar in both groups. The most common adverse events in the odanacatib and placebo arms were urinary tract infection (11.5% vs. 16.5%, respectively), back pain (11.5% vs. 9.9%), arthralgia (9% vs. 9.9%), and fractures (4.9% vs. 13.2%). Treatment discontinuation rates due to adverse events were 9% vs. 3.3%, he said.
Dr. Leung and several of his coauthors are employees of Merck, the trial sponsor.
MINNEAPOLIS – Odanacatib added bone density in postmenopausal women with osteoporosis previously treated with alendronate, according to results from a study presented at the annual meeting of the American Society for Bone and Mineral Research.
In a phase II trial involving 243 patients, the investigational oral cathepsin K inhibitor began to distinguish itself from placebo at 12 months. At 2 years, the change from baseline in femoral neck bone mineral density (BMD) was significantly increased by 1.73%, compared with a loss of 0.94% for placebo (P less than .001).
BMD changes in the treatment group at 2 years were also significantly different from placebo at the trochanter (1.83% vs. –1.35%) and for the total hip (0.83% vs. –1.87%, both P less than .001).
All of the women studied had received alendronate (Fosamax) for at least 3 years. Patients could have been off the bisphosphonate for up to 3 months prior to enrollment, but many switched directly over to odanacatib, said study coauthor Dr. Albert Leung, executive director of clinical research at Merck Research Laboratories.
"This drug has the potential to give additional benefits when [patients] have been treated with alendronate for a number of years and the treatment effect has reached a plateau and they may need a different treatment," he said in an interview.
In July 2012, a pivotal 16,731-patient, phase III trial of odanacatib was stopped early after an interim analysis showed a "favorable benefit-risk profile" for fracture risk reduction in postmenopausal women with previously untreated osteoporosis.
"It could be quite a population that may benefit from this drug," Dr. Leung remarked.
The study’s data monitoring committee, however, flagged safety concerns in "certain selected areas" for further follow-up. Those safety risks have not been identified as the trial is still closing, but will be monitored along with efficacy in a double-blind, placebo-controlled, extension trial going out to 5 years of treatment, he said.
Merck, which plans to submit regulatory applications for odanacatib in the United States and Europe in the first half of 2013, has high hopes for odanacatib despite competition from generic drugs because of its novel mechanism of action.
Odanacatib inhibits cathepsin K, the primary protease in osteoclasts that breaks down bone collagen during bone resorption. Unlike traditional antiresorptive drugs like bisphosphonates, however, odanacatib does not interfere with the function of the entire osteoclast or reduce the number of osteoclasts. This characteristic is important, as osteoclasts secrete signaling factors to stimulate osteoblasts, the cells responsible for bone formation. As a result, there is greater bone formation with odanacatib, Dr. Leung explained.
The phase II trial enrolled women at least 60 years of age (mean 71 years) with a BMD T score of –2.5 to more than –3.5 at any hip site without a prior fragility fracture or those with a history of fragility fracture (except hip fracture), and a BMD T score of –1.5 and more than –3.5 at any hip site. The women were randomly assigned to odanacatib 50 mg once weekly or placebo for 24 months, as well as 5,600 IU of vitamin D3 per week and calcium at dosages up to 1,200 mg/day. The study was not powered to assess fractures.
At 24 months, the change in BMD at the lumbar spine was significant at 2.28% for odanacatib vs. a loss of 0.30% with placebo (P less than .001).
BMD change was not significant at the distal forearm, with losses of 0.92% vs. 1.14%, respectively.
As expected, urinary collagen type I cross-linked N-telopeptide, a biomarker of bone resorption, increased with placebo, compared with a significant 47% decrease with odanacatib.
The bone formation marker, serum type I procollagen, rose inexplicably with placebo, but this increase was surpassed by a significant gain of 31.2% with odanacatib.
Most unexpected, however, was an increase in the resorption marker collagen type I cross-linked C-telopeptide (sCTx) with once-weekly odanacatib. Dr. Leung said the finding appears to correlate with the bone density changes because sCTx levels remained relatively stable during the first 12 months of treatment before rising in the second year of the study.
Finally, adverse events were similar in both groups. The most common adverse events in the odanacatib and placebo arms were urinary tract infection (11.5% vs. 16.5%, respectively), back pain (11.5% vs. 9.9%), arthralgia (9% vs. 9.9%), and fractures (4.9% vs. 13.2%). Treatment discontinuation rates due to adverse events were 9% vs. 3.3%, he said.
Dr. Leung and several of his coauthors are employees of Merck, the trial sponsor.
MINNEAPOLIS – Odanacatib added bone density in postmenopausal women with osteoporosis previously treated with alendronate, according to results from a study presented at the annual meeting of the American Society for Bone and Mineral Research.
In a phase II trial involving 243 patients, the investigational oral cathepsin K inhibitor began to distinguish itself from placebo at 12 months. At 2 years, the change from baseline in femoral neck bone mineral density (BMD) was significantly increased by 1.73%, compared with a loss of 0.94% for placebo (P less than .001).
BMD changes in the treatment group at 2 years were also significantly different from placebo at the trochanter (1.83% vs. –1.35%) and for the total hip (0.83% vs. –1.87%, both P less than .001).
All of the women studied had received alendronate (Fosamax) for at least 3 years. Patients could have been off the bisphosphonate for up to 3 months prior to enrollment, but many switched directly over to odanacatib, said study coauthor Dr. Albert Leung, executive director of clinical research at Merck Research Laboratories.
"This drug has the potential to give additional benefits when [patients] have been treated with alendronate for a number of years and the treatment effect has reached a plateau and they may need a different treatment," he said in an interview.
In July 2012, a pivotal 16,731-patient, phase III trial of odanacatib was stopped early after an interim analysis showed a "favorable benefit-risk profile" for fracture risk reduction in postmenopausal women with previously untreated osteoporosis.
"It could be quite a population that may benefit from this drug," Dr. Leung remarked.
The study’s data monitoring committee, however, flagged safety concerns in "certain selected areas" for further follow-up. Those safety risks have not been identified as the trial is still closing, but will be monitored along with efficacy in a double-blind, placebo-controlled, extension trial going out to 5 years of treatment, he said.
Merck, which plans to submit regulatory applications for odanacatib in the United States and Europe in the first half of 2013, has high hopes for odanacatib despite competition from generic drugs because of its novel mechanism of action.
Odanacatib inhibits cathepsin K, the primary protease in osteoclasts that breaks down bone collagen during bone resorption. Unlike traditional antiresorptive drugs like bisphosphonates, however, odanacatib does not interfere with the function of the entire osteoclast or reduce the number of osteoclasts. This characteristic is important, as osteoclasts secrete signaling factors to stimulate osteoblasts, the cells responsible for bone formation. As a result, there is greater bone formation with odanacatib, Dr. Leung explained.
The phase II trial enrolled women at least 60 years of age (mean 71 years) with a BMD T score of –2.5 to more than –3.5 at any hip site without a prior fragility fracture or those with a history of fragility fracture (except hip fracture), and a BMD T score of –1.5 and more than –3.5 at any hip site. The women were randomly assigned to odanacatib 50 mg once weekly or placebo for 24 months, as well as 5,600 IU of vitamin D3 per week and calcium at dosages up to 1,200 mg/day. The study was not powered to assess fractures.
At 24 months, the change in BMD at the lumbar spine was significant at 2.28% for odanacatib vs. a loss of 0.30% with placebo (P less than .001).
BMD change was not significant at the distal forearm, with losses of 0.92% vs. 1.14%, respectively.
As expected, urinary collagen type I cross-linked N-telopeptide, a biomarker of bone resorption, increased with placebo, compared with a significant 47% decrease with odanacatib.
The bone formation marker, serum type I procollagen, rose inexplicably with placebo, but this increase was surpassed by a significant gain of 31.2% with odanacatib.
Most unexpected, however, was an increase in the resorption marker collagen type I cross-linked C-telopeptide (sCTx) with once-weekly odanacatib. Dr. Leung said the finding appears to correlate with the bone density changes because sCTx levels remained relatively stable during the first 12 months of treatment before rising in the second year of the study.
Finally, adverse events were similar in both groups. The most common adverse events in the odanacatib and placebo arms were urinary tract infection (11.5% vs. 16.5%, respectively), back pain (11.5% vs. 9.9%), arthralgia (9% vs. 9.9%), and fractures (4.9% vs. 13.2%). Treatment discontinuation rates due to adverse events were 9% vs. 3.3%, he said.
Dr. Leung and several of his coauthors are employees of Merck, the trial sponsor.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR BONE AND MINERAL RESEARCH
Major Finding: The change from baseline in femoral neck bone mineral density at 2 years was +1.73% with odanacatib vs. –0.94% for placebo (P less than .001).
Data Source: This study was a phase II trial in 243 women with postmenopausal osteoporosis previously treated with alendronate.
Disclosures: Dr. Leung and several of his coauthors are employees of Merck, the trial sponsor.