User login
Could retinal changes be a harbinger of Parkinson’s?
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
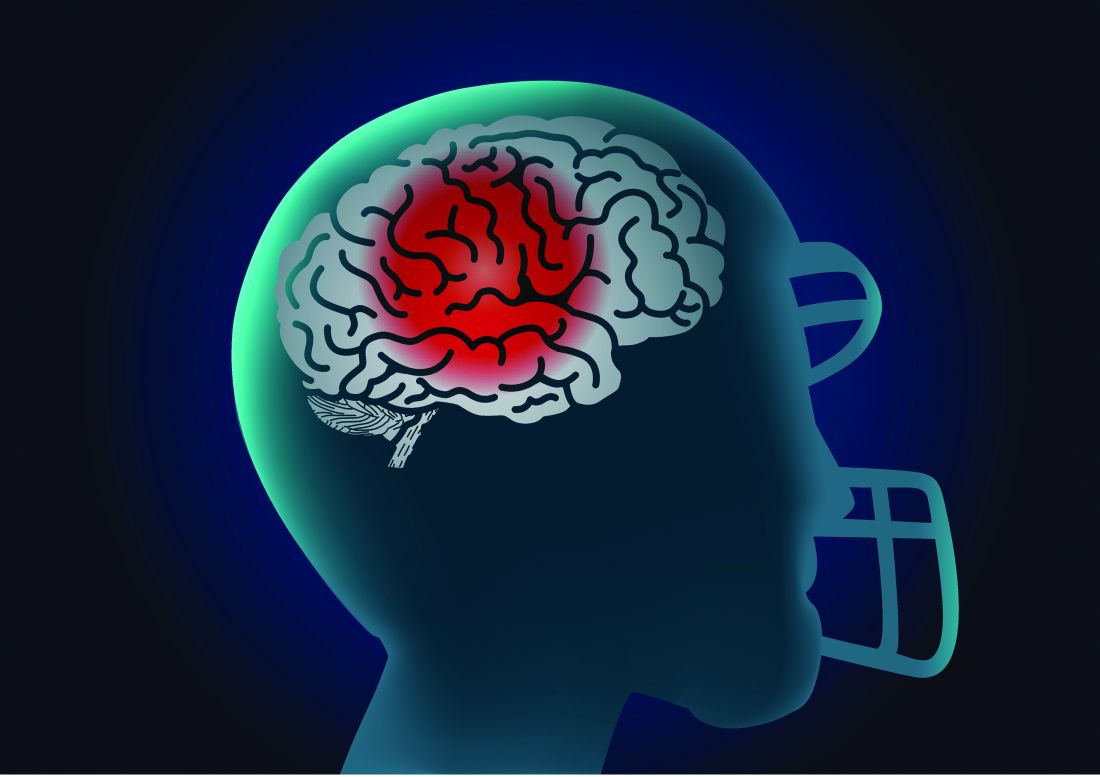
Playing football linked to higher Parkinson’s risk
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Why genetic testing may be our best shot at progress in Parkinson’s disease
In 2017, Sanofi Genzyme launched a phase 2 clinical trial of a drug designed to target a specific genetic mutation in some patients with Parkinson’s disease. Researchers hoped the drug would slow or even stop disease progression.
Like many before it, the trial yielded disappointing results and the company shut it down in 2021. It was the latest in a string of unsuccessful clinical trials testing disease-modifying Parkinson’s disease drugs.
Although it failed, the Sanofi Genzyme study was different: It was the first to enroll patients with Parkinson’s disease who had a specific genotype and marked the earliest days of precision medicine and gene-specific drug development for the disease.
Once thought to play only a small role in a small number of patients with Parkinson’s disease,
“We’re about to enter this era of precision medicine for Parkinson’s disease, which makes genetic testing important,” said James Beck, PhD, senior vice president and chief scientific officer for the Parkinson’s Foundation.
“A number of companies have clinical trials or are in preparation for clinical trials to test some specific therapies that would depend upon people having a specific genetic mutation,” he said.
Today, at least four clinical trials of drugs that target specific Parkinson’s disease-related gene variants on LRRK2 and GBA are under way, and more are in the pipeline. Whether these drugs will be effective at modifying the course of the disease remains to be seen. First, the trials must enroll enough patients. And therein lies the challenge: Genetic testing isn’t part of routine Parkinson’s disease care and isn’t covered by most insurance policies. Most patients don’t know their genotype.
It’s a significant roadblock to the future of a precision medicine approach that is based on a patient’s individual genotype, which some experts argue offers the best shot at slowing disease progression.
“To enroll in clinical trials for precision drugs people with Parkinson’s disease have to be aware of their genetic status,” said Roy N. Alcalay, MD, chief of the movement disorders division at Tel Aviv Medical Center in Israel and part-time associate professor at Columbia University in New York. “How can a person with Parkinson’s and a LRRK2 mutation join a precision medicine trial for LRRK2 if she does not know she is a LRRK2 carrier?”
Free genetic testing
Previous studies have shown that some genetic variants increase the risk for Parkinson’s disease after exposure to environmental factors such as pesticides. Research has also shown that a patient’s genotype can predict survival time and that certain medications may prove more effective at slowing disease progression in patients with specific genotypes. All of this points to a significant role for genetics in a disorder that is rapidly increasing.
This makes expanding patient access to genetic testing even more important, Dr. Alcalay said, noting that it’s equally important that patients are informed of their genotype, something that doesn’t usually happen in blinded clinical trials.
To that end, Dr. Alcalay hopes a national genetics study he is leading will address access and need-to-know issues. PD GENEration, a project launched in 2019 by the Parkinson’s Foundation, offers patients free genetic testing for seven clinically relevant Parkinson’s disease-related genes.
Testing is done at home or in a nearby clinic and the results are shared with patients during a free genetic counseling session and with site investigators. Patient samples are stored in a genetic data bank that is open to researchers around the world.
“We surveyed clinical trialists in the Parkinson’s disease field prior to initiation of PD GENEration and estimated that over 90% of people with Parkinson’s disease prior to the effort were not aware of their genetic status,” Dr. Alcalay said.
“I think precision medicine in Parkinson’s disease will not happen without PD GENEration or similar efforts.”
‘Overwhelming’ patient interest
Participants in the study are screened for variants in seven genes known to be involved in Parkinson’s disease risk: GBA, LRRK2, PRKN, PINK1, SNCA, PARK7, and VPS35.
In less than 3 years, the study has already produced what is thought to be the largest genetic data bank of sequenced sets of Parkinson’s disease-risk genes made accessible to patients. Since the end of 2020, the first year of patient enrollment, the number of participants has increased from 676 to 10,515 and the number of participating clinical sites rose from 12 to 101.
The foundation has spent nearly $20 million on the project so far and plans to spend another $10 million to reach a goal of 15,000 patients. The study, which is funded by private donors, is so successful that the foundation has had to scale back enrollment.
“When we were at a peak, we had over 700 participants enrolling each month,” Dr. Beck said. Beginning in April, the program capped new sign-ups to 200 patients per month and created a waiting list for future enrollment. The waiting list is hundreds of patients long.
“The participants’ response to enroll in PD GENEration demonstrates there is an overwhelming interest by people with Parkinson’s disease to learn more about their genetic risk factors,” Dr. Alcalay said.
A research driver
Nearly 60% of participants enrolled so far are male and close to 80% are White. The average age is 69 years and 44% were diagnosed in the past 5 years. Close to 75% had never participated in a clinical trial.
Nearly 13% have tested positive for mutations on at least one of the seven target genes. Previous studies had suggested genetics were involved in only about 10% of cases.
The majority of those with positive results had early-onset Parkinson’s disease, high-risk ancestry, or a first-degree relative with the disease. However, 9% of people who tested positive weren’t in any of those categories.
Genetic information collected by the project is shared with the Global Parkinson’s Genetics Program (GP2), a resource program of the Aligning Science Across Parkinson’s initiative that is focused on the disease’s genetic architecture. Researchers around the world have access to GP2 data to study known gene variants and identify new ones.
PD GENEration participants can choose to be notified if they are carriers of gene variants discovered in the future.
“All DNA samples shared by participants are undergoing research-grade testing,” Dr. Beck said. “Not only do we want to be able to inform people with Parkinson’s disease about their genetic status, but we also want to be able to use this precious resource to further drive research into the genetics of Parkinson’s disease.”
Early success
Patient recruitment has long been one of the biggest challenges to any clinical trial’s success. Research suggests that 90% of all clinical trials fail to reach recruitment milestones in their allotted time frame and two-thirds of multicenter trials fold because too few patients sign up. Data from the Parkinson’s Foundation show that only about 1% of all patients with Parkinson’s disease participate in clinical trials.
Increasing those numbers is the primary goal of PD GENEration, Dr. Beck said. And there’s evidence it’s already paying off.
Earlier this year, one of the program’s participating clinical sites, Intermountain Health, in Salt Lake City, Utah, joined a phase 2 clinical trial of an experimental drug that targets a mutation on the GBA1 gene.
“One of the reasons we were able to participate was when we got the call about joining, we were able to say that we had patients with that specific gene mutation, and we could only say that because the patients had been genotyped through PD GENEration,” said Kathleen E. McKee, MD, director of movement disorders, associate medical director of neurosciences research, and PD GENEration principal investigator at Intermountain Health.
Since 2021, Dr. McKee has enrolled hundreds of patients in the foundation’s gene study and hopes to enroll even more. Few patients turn down the opportunity to participate, she added. Knowing their genotype has proven empowering for her patients, most of whom could not afford genetic testing on their own.
“Previously I would tell patients this is not going to change your immediate management,” Dr. McKee said. “Now I tell my patients that these trials are out there, it may actually change how I treat you and what I recommend.”
A version of this article appeared on Medscape.com.
In 2017, Sanofi Genzyme launched a phase 2 clinical trial of a drug designed to target a specific genetic mutation in some patients with Parkinson’s disease. Researchers hoped the drug would slow or even stop disease progression.
Like many before it, the trial yielded disappointing results and the company shut it down in 2021. It was the latest in a string of unsuccessful clinical trials testing disease-modifying Parkinson’s disease drugs.
Although it failed, the Sanofi Genzyme study was different: It was the first to enroll patients with Parkinson’s disease who had a specific genotype and marked the earliest days of precision medicine and gene-specific drug development for the disease.
Once thought to play only a small role in a small number of patients with Parkinson’s disease,
“We’re about to enter this era of precision medicine for Parkinson’s disease, which makes genetic testing important,” said James Beck, PhD, senior vice president and chief scientific officer for the Parkinson’s Foundation.
“A number of companies have clinical trials or are in preparation for clinical trials to test some specific therapies that would depend upon people having a specific genetic mutation,” he said.
Today, at least four clinical trials of drugs that target specific Parkinson’s disease-related gene variants on LRRK2 and GBA are under way, and more are in the pipeline. Whether these drugs will be effective at modifying the course of the disease remains to be seen. First, the trials must enroll enough patients. And therein lies the challenge: Genetic testing isn’t part of routine Parkinson’s disease care and isn’t covered by most insurance policies. Most patients don’t know their genotype.
It’s a significant roadblock to the future of a precision medicine approach that is based on a patient’s individual genotype, which some experts argue offers the best shot at slowing disease progression.
“To enroll in clinical trials for precision drugs people with Parkinson’s disease have to be aware of their genetic status,” said Roy N. Alcalay, MD, chief of the movement disorders division at Tel Aviv Medical Center in Israel and part-time associate professor at Columbia University in New York. “How can a person with Parkinson’s and a LRRK2 mutation join a precision medicine trial for LRRK2 if she does not know she is a LRRK2 carrier?”
Free genetic testing
Previous studies have shown that some genetic variants increase the risk for Parkinson’s disease after exposure to environmental factors such as pesticides. Research has also shown that a patient’s genotype can predict survival time and that certain medications may prove more effective at slowing disease progression in patients with specific genotypes. All of this points to a significant role for genetics in a disorder that is rapidly increasing.
This makes expanding patient access to genetic testing even more important, Dr. Alcalay said, noting that it’s equally important that patients are informed of their genotype, something that doesn’t usually happen in blinded clinical trials.
To that end, Dr. Alcalay hopes a national genetics study he is leading will address access and need-to-know issues. PD GENEration, a project launched in 2019 by the Parkinson’s Foundation, offers patients free genetic testing for seven clinically relevant Parkinson’s disease-related genes.
Testing is done at home or in a nearby clinic and the results are shared with patients during a free genetic counseling session and with site investigators. Patient samples are stored in a genetic data bank that is open to researchers around the world.
“We surveyed clinical trialists in the Parkinson’s disease field prior to initiation of PD GENEration and estimated that over 90% of people with Parkinson’s disease prior to the effort were not aware of their genetic status,” Dr. Alcalay said.
“I think precision medicine in Parkinson’s disease will not happen without PD GENEration or similar efforts.”
‘Overwhelming’ patient interest
Participants in the study are screened for variants in seven genes known to be involved in Parkinson’s disease risk: GBA, LRRK2, PRKN, PINK1, SNCA, PARK7, and VPS35.
In less than 3 years, the study has already produced what is thought to be the largest genetic data bank of sequenced sets of Parkinson’s disease-risk genes made accessible to patients. Since the end of 2020, the first year of patient enrollment, the number of participants has increased from 676 to 10,515 and the number of participating clinical sites rose from 12 to 101.
The foundation has spent nearly $20 million on the project so far and plans to spend another $10 million to reach a goal of 15,000 patients. The study, which is funded by private donors, is so successful that the foundation has had to scale back enrollment.
“When we were at a peak, we had over 700 participants enrolling each month,” Dr. Beck said. Beginning in April, the program capped new sign-ups to 200 patients per month and created a waiting list for future enrollment. The waiting list is hundreds of patients long.
“The participants’ response to enroll in PD GENEration demonstrates there is an overwhelming interest by people with Parkinson’s disease to learn more about their genetic risk factors,” Dr. Alcalay said.
A research driver
Nearly 60% of participants enrolled so far are male and close to 80% are White. The average age is 69 years and 44% were diagnosed in the past 5 years. Close to 75% had never participated in a clinical trial.
Nearly 13% have tested positive for mutations on at least one of the seven target genes. Previous studies had suggested genetics were involved in only about 10% of cases.
The majority of those with positive results had early-onset Parkinson’s disease, high-risk ancestry, or a first-degree relative with the disease. However, 9% of people who tested positive weren’t in any of those categories.
Genetic information collected by the project is shared with the Global Parkinson’s Genetics Program (GP2), a resource program of the Aligning Science Across Parkinson’s initiative that is focused on the disease’s genetic architecture. Researchers around the world have access to GP2 data to study known gene variants and identify new ones.
PD GENEration participants can choose to be notified if they are carriers of gene variants discovered in the future.
“All DNA samples shared by participants are undergoing research-grade testing,” Dr. Beck said. “Not only do we want to be able to inform people with Parkinson’s disease about their genetic status, but we also want to be able to use this precious resource to further drive research into the genetics of Parkinson’s disease.”
Early success
Patient recruitment has long been one of the biggest challenges to any clinical trial’s success. Research suggests that 90% of all clinical trials fail to reach recruitment milestones in their allotted time frame and two-thirds of multicenter trials fold because too few patients sign up. Data from the Parkinson’s Foundation show that only about 1% of all patients with Parkinson’s disease participate in clinical trials.
Increasing those numbers is the primary goal of PD GENEration, Dr. Beck said. And there’s evidence it’s already paying off.
Earlier this year, one of the program’s participating clinical sites, Intermountain Health, in Salt Lake City, Utah, joined a phase 2 clinical trial of an experimental drug that targets a mutation on the GBA1 gene.
“One of the reasons we were able to participate was when we got the call about joining, we were able to say that we had patients with that specific gene mutation, and we could only say that because the patients had been genotyped through PD GENEration,” said Kathleen E. McKee, MD, director of movement disorders, associate medical director of neurosciences research, and PD GENEration principal investigator at Intermountain Health.
Since 2021, Dr. McKee has enrolled hundreds of patients in the foundation’s gene study and hopes to enroll even more. Few patients turn down the opportunity to participate, she added. Knowing their genotype has proven empowering for her patients, most of whom could not afford genetic testing on their own.
“Previously I would tell patients this is not going to change your immediate management,” Dr. McKee said. “Now I tell my patients that these trials are out there, it may actually change how I treat you and what I recommend.”
A version of this article appeared on Medscape.com.
In 2017, Sanofi Genzyme launched a phase 2 clinical trial of a drug designed to target a specific genetic mutation in some patients with Parkinson’s disease. Researchers hoped the drug would slow or even stop disease progression.
Like many before it, the trial yielded disappointing results and the company shut it down in 2021. It was the latest in a string of unsuccessful clinical trials testing disease-modifying Parkinson’s disease drugs.
Although it failed, the Sanofi Genzyme study was different: It was the first to enroll patients with Parkinson’s disease who had a specific genotype and marked the earliest days of precision medicine and gene-specific drug development for the disease.
Once thought to play only a small role in a small number of patients with Parkinson’s disease,
“We’re about to enter this era of precision medicine for Parkinson’s disease, which makes genetic testing important,” said James Beck, PhD, senior vice president and chief scientific officer for the Parkinson’s Foundation.
“A number of companies have clinical trials or are in preparation for clinical trials to test some specific therapies that would depend upon people having a specific genetic mutation,” he said.
Today, at least four clinical trials of drugs that target specific Parkinson’s disease-related gene variants on LRRK2 and GBA are under way, and more are in the pipeline. Whether these drugs will be effective at modifying the course of the disease remains to be seen. First, the trials must enroll enough patients. And therein lies the challenge: Genetic testing isn’t part of routine Parkinson’s disease care and isn’t covered by most insurance policies. Most patients don’t know their genotype.
It’s a significant roadblock to the future of a precision medicine approach that is based on a patient’s individual genotype, which some experts argue offers the best shot at slowing disease progression.
“To enroll in clinical trials for precision drugs people with Parkinson’s disease have to be aware of their genetic status,” said Roy N. Alcalay, MD, chief of the movement disorders division at Tel Aviv Medical Center in Israel and part-time associate professor at Columbia University in New York. “How can a person with Parkinson’s and a LRRK2 mutation join a precision medicine trial for LRRK2 if she does not know she is a LRRK2 carrier?”
Free genetic testing
Previous studies have shown that some genetic variants increase the risk for Parkinson’s disease after exposure to environmental factors such as pesticides. Research has also shown that a patient’s genotype can predict survival time and that certain medications may prove more effective at slowing disease progression in patients with specific genotypes. All of this points to a significant role for genetics in a disorder that is rapidly increasing.
This makes expanding patient access to genetic testing even more important, Dr. Alcalay said, noting that it’s equally important that patients are informed of their genotype, something that doesn’t usually happen in blinded clinical trials.
To that end, Dr. Alcalay hopes a national genetics study he is leading will address access and need-to-know issues. PD GENEration, a project launched in 2019 by the Parkinson’s Foundation, offers patients free genetic testing for seven clinically relevant Parkinson’s disease-related genes.
Testing is done at home or in a nearby clinic and the results are shared with patients during a free genetic counseling session and with site investigators. Patient samples are stored in a genetic data bank that is open to researchers around the world.
“We surveyed clinical trialists in the Parkinson’s disease field prior to initiation of PD GENEration and estimated that over 90% of people with Parkinson’s disease prior to the effort were not aware of their genetic status,” Dr. Alcalay said.
“I think precision medicine in Parkinson’s disease will not happen without PD GENEration or similar efforts.”
‘Overwhelming’ patient interest
Participants in the study are screened for variants in seven genes known to be involved in Parkinson’s disease risk: GBA, LRRK2, PRKN, PINK1, SNCA, PARK7, and VPS35.
In less than 3 years, the study has already produced what is thought to be the largest genetic data bank of sequenced sets of Parkinson’s disease-risk genes made accessible to patients. Since the end of 2020, the first year of patient enrollment, the number of participants has increased from 676 to 10,515 and the number of participating clinical sites rose from 12 to 101.
The foundation has spent nearly $20 million on the project so far and plans to spend another $10 million to reach a goal of 15,000 patients. The study, which is funded by private donors, is so successful that the foundation has had to scale back enrollment.
“When we were at a peak, we had over 700 participants enrolling each month,” Dr. Beck said. Beginning in April, the program capped new sign-ups to 200 patients per month and created a waiting list for future enrollment. The waiting list is hundreds of patients long.
“The participants’ response to enroll in PD GENEration demonstrates there is an overwhelming interest by people with Parkinson’s disease to learn more about their genetic risk factors,” Dr. Alcalay said.
A research driver
Nearly 60% of participants enrolled so far are male and close to 80% are White. The average age is 69 years and 44% were diagnosed in the past 5 years. Close to 75% had never participated in a clinical trial.
Nearly 13% have tested positive for mutations on at least one of the seven target genes. Previous studies had suggested genetics were involved in only about 10% of cases.
The majority of those with positive results had early-onset Parkinson’s disease, high-risk ancestry, or a first-degree relative with the disease. However, 9% of people who tested positive weren’t in any of those categories.
Genetic information collected by the project is shared with the Global Parkinson’s Genetics Program (GP2), a resource program of the Aligning Science Across Parkinson’s initiative that is focused on the disease’s genetic architecture. Researchers around the world have access to GP2 data to study known gene variants and identify new ones.
PD GENEration participants can choose to be notified if they are carriers of gene variants discovered in the future.
“All DNA samples shared by participants are undergoing research-grade testing,” Dr. Beck said. “Not only do we want to be able to inform people with Parkinson’s disease about their genetic status, but we also want to be able to use this precious resource to further drive research into the genetics of Parkinson’s disease.”
Early success
Patient recruitment has long been one of the biggest challenges to any clinical trial’s success. Research suggests that 90% of all clinical trials fail to reach recruitment milestones in their allotted time frame and two-thirds of multicenter trials fold because too few patients sign up. Data from the Parkinson’s Foundation show that only about 1% of all patients with Parkinson’s disease participate in clinical trials.
Increasing those numbers is the primary goal of PD GENEration, Dr. Beck said. And there’s evidence it’s already paying off.
Earlier this year, one of the program’s participating clinical sites, Intermountain Health, in Salt Lake City, Utah, joined a phase 2 clinical trial of an experimental drug that targets a mutation on the GBA1 gene.
“One of the reasons we were able to participate was when we got the call about joining, we were able to say that we had patients with that specific gene mutation, and we could only say that because the patients had been genotyped through PD GENEration,” said Kathleen E. McKee, MD, director of movement disorders, associate medical director of neurosciences research, and PD GENEration principal investigator at Intermountain Health.
Since 2021, Dr. McKee has enrolled hundreds of patients in the foundation’s gene study and hopes to enroll even more. Few patients turn down the opportunity to participate, she added. Knowing their genotype has proven empowering for her patients, most of whom could not afford genetic testing on their own.
“Previously I would tell patients this is not going to change your immediate management,” Dr. McKee said. “Now I tell my patients that these trials are out there, it may actually change how I treat you and what I recommend.”
A version of this article appeared on Medscape.com.
Global burden of brain disorders surpasses cardiovascular disease and cancer
– at huge cost to health care systems and society, an analysis of data from the most recent Global Burden of Disease (GBD) study shows.
“The burden of brain conditions will increase as populations continue to grow and age,” said study presenter Shayla Smith, MPH, an epidemiologist at the Institute for Health Metrics and Evaluation, the University of Washington, Seattle, in a press release.
“By 2050, more than 50 million people will be aged 65-79,” she explained, adding that the COVID-19 pandemic “has also influenced the prevalence of mental disorders globally, as people were forced to isolate and social networks broke down.”
Other factors related to brain disorders, she noted, include education level, obesity, and smoking.
“There’s still research to be done on what is the most effective way to maintain brain health, but some literature suggests a healthy brain can be achieved through a healthy lifestyle of managing conditions such as high blood pressure and diabetes, limiting alcohol consumption and smoking, prioritizing sleep, eating healthy, and staying physically and mentally active,” said Ms. Smith.
The findings were presented at the annual meeting of the Congress of the European Academy of Neurology.
An ‘ambitious exercise’
Coinvestigator Xaviera Steele, also from the IHME, told press conference attendees that the institute was established at the University of Washington in 2007 with the aim of “standardizing the measurement of health outcomes around the world and for all health conditions.”
A central part of that is the GBD study, “which is a very ambitious exercise in descriptive epidemiology in an effort to systematically quantify health loss” due to disease, injury, and risk factors over time, stratified by country, region, age, and sex. In addition, researchers are mapping and projecting trends over the next century and are estimating disease expenditure by country, by type of expense, and by condition “to derive a health care access and quality score for each health system in the world,” Ms. Steele said.
They are also estimating exposure to risk factors, how those risk factors contribute to health burden, and associated health outcomes by race and ethnicity to reflect the “disparities that we know are very prevalent in countries such as the United States.” From that work, Ms. Steele said that brain health and related conditions “do emerge as one of the more pressing challenges of the 21st century.”
Increase in dementia, mental health conditions
The data, which were gathered from 200,000 sources by the IHME, indicate that the number of individuals aged 65 years or older will increase by 350% by 2100. Ms. Steele underlined that “policy action will be needed to help families, who will struggle to provide high-quality care for their loved ones with dementia at a reasonable cost.”
The IHME calculates that in Europe health care spending on Alzheimer’s disease will increase by 226% between 2015 and 2040.
Turning to other conditions, Ms. Steele showed that since 1990, the number of individuals living with anxiety in the European region has increased by 14%, while the number living with depressive disorders has gone up by 13%.
Worldwide, the figures are even starker. Depression is estimated to affect 300 million people across the globe, which represents a 71% increase since 1990. The number of strokes increased by 95% over the same period.
Nevertheless, the “impact of brain conditions such as stroke has decreased since the 1990s due to improved treatments available,” Ms. Smith noted in the press release.
To estimate the toll caused by brain conditions, including neurologic disorders, mental disorders, cerebrovascular disease, brain cancer, brain injuries, and select infectious conditions, the researchers calculated disability-adjusted life years (DALYs).
This, Ms. Smith explained in her presentation, “captures the morbidity and mortality associated with brain conditions” and is adjusted for patient location, age, and sex.
The investigators found that, globally, brain conditions accounted for more than 15% of all health loss in 2021, at 406 DALYs – more than the 206 million DALYs that were associated with cancer, and the 402 million that were linked to cardiovascular disease.
This health loss is associated with a $1.22 trillion loss in income for people living with health disorders worldwide and accounts for $1.14 trillion in direct health care costs.
The burden of mental disorders, neurologic conditions, and stroke is expected to increase dramatically between now and 2050, said Ms. Smith, who noted that health loss linked to brain conditions is higher in younger patients. This will create “new challenges for health systems, employers, patients, and families,” she said in the press release.
“Our goal is to see an improved prevention and treatment landscape for other brain conditions and reverse the growing health loss that we are currently forecasting.”
Worrying increase in stroke
Jurgita Valaikiene, MD, PhD, center of neurology, clinic of neurology and neurosurgery, Vilnius (Lithuania) University Faculty of Medicine, who chaired the session, was taken aback by the findings, particularly by the worldwide increase in stroke cases.
“I work in stroke,” she said, and “we spend a lot of time on the diagnosis of stroke” and its prevention. “We try to be faster, to catch asymptomatic stenosis in the neck or head, and to apply the best medical treatment to avoid a stroke. But despite that, the numbers are increasing. I understand the population is getting older ... but still it’s a huge number.”
Dr. Valaikiene pointed out that stroke is not necessarily a condition of aging, insofar as increasing age “is not related directly to stenosis in the neck. “For example, we can have healthier vessels in older age and unhealthy vessels, with high-grade stenosis, in someone aged 30 or 40 years.”
“There are a lot of risk factors, such as smoking, physical activity, and so on. It depends on the individual,” she added.
The study was funded by the Institute for Health Metrics and Evaluation at the University of Washington. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– at huge cost to health care systems and society, an analysis of data from the most recent Global Burden of Disease (GBD) study shows.
“The burden of brain conditions will increase as populations continue to grow and age,” said study presenter Shayla Smith, MPH, an epidemiologist at the Institute for Health Metrics and Evaluation, the University of Washington, Seattle, in a press release.
“By 2050, more than 50 million people will be aged 65-79,” she explained, adding that the COVID-19 pandemic “has also influenced the prevalence of mental disorders globally, as people were forced to isolate and social networks broke down.”
Other factors related to brain disorders, she noted, include education level, obesity, and smoking.
“There’s still research to be done on what is the most effective way to maintain brain health, but some literature suggests a healthy brain can be achieved through a healthy lifestyle of managing conditions such as high blood pressure and diabetes, limiting alcohol consumption and smoking, prioritizing sleep, eating healthy, and staying physically and mentally active,” said Ms. Smith.
The findings were presented at the annual meeting of the Congress of the European Academy of Neurology.
An ‘ambitious exercise’
Coinvestigator Xaviera Steele, also from the IHME, told press conference attendees that the institute was established at the University of Washington in 2007 with the aim of “standardizing the measurement of health outcomes around the world and for all health conditions.”
A central part of that is the GBD study, “which is a very ambitious exercise in descriptive epidemiology in an effort to systematically quantify health loss” due to disease, injury, and risk factors over time, stratified by country, region, age, and sex. In addition, researchers are mapping and projecting trends over the next century and are estimating disease expenditure by country, by type of expense, and by condition “to derive a health care access and quality score for each health system in the world,” Ms. Steele said.
They are also estimating exposure to risk factors, how those risk factors contribute to health burden, and associated health outcomes by race and ethnicity to reflect the “disparities that we know are very prevalent in countries such as the United States.” From that work, Ms. Steele said that brain health and related conditions “do emerge as one of the more pressing challenges of the 21st century.”
Increase in dementia, mental health conditions
The data, which were gathered from 200,000 sources by the IHME, indicate that the number of individuals aged 65 years or older will increase by 350% by 2100. Ms. Steele underlined that “policy action will be needed to help families, who will struggle to provide high-quality care for their loved ones with dementia at a reasonable cost.”
The IHME calculates that in Europe health care spending on Alzheimer’s disease will increase by 226% between 2015 and 2040.
Turning to other conditions, Ms. Steele showed that since 1990, the number of individuals living with anxiety in the European region has increased by 14%, while the number living with depressive disorders has gone up by 13%.
Worldwide, the figures are even starker. Depression is estimated to affect 300 million people across the globe, which represents a 71% increase since 1990. The number of strokes increased by 95% over the same period.
Nevertheless, the “impact of brain conditions such as stroke has decreased since the 1990s due to improved treatments available,” Ms. Smith noted in the press release.
To estimate the toll caused by brain conditions, including neurologic disorders, mental disorders, cerebrovascular disease, brain cancer, brain injuries, and select infectious conditions, the researchers calculated disability-adjusted life years (DALYs).
This, Ms. Smith explained in her presentation, “captures the morbidity and mortality associated with brain conditions” and is adjusted for patient location, age, and sex.
The investigators found that, globally, brain conditions accounted for more than 15% of all health loss in 2021, at 406 DALYs – more than the 206 million DALYs that were associated with cancer, and the 402 million that were linked to cardiovascular disease.
This health loss is associated with a $1.22 trillion loss in income for people living with health disorders worldwide and accounts for $1.14 trillion in direct health care costs.
The burden of mental disorders, neurologic conditions, and stroke is expected to increase dramatically between now and 2050, said Ms. Smith, who noted that health loss linked to brain conditions is higher in younger patients. This will create “new challenges for health systems, employers, patients, and families,” she said in the press release.
“Our goal is to see an improved prevention and treatment landscape for other brain conditions and reverse the growing health loss that we are currently forecasting.”
Worrying increase in stroke
Jurgita Valaikiene, MD, PhD, center of neurology, clinic of neurology and neurosurgery, Vilnius (Lithuania) University Faculty of Medicine, who chaired the session, was taken aback by the findings, particularly by the worldwide increase in stroke cases.
“I work in stroke,” she said, and “we spend a lot of time on the diagnosis of stroke” and its prevention. “We try to be faster, to catch asymptomatic stenosis in the neck or head, and to apply the best medical treatment to avoid a stroke. But despite that, the numbers are increasing. I understand the population is getting older ... but still it’s a huge number.”
Dr. Valaikiene pointed out that stroke is not necessarily a condition of aging, insofar as increasing age “is not related directly to stenosis in the neck. “For example, we can have healthier vessels in older age and unhealthy vessels, with high-grade stenosis, in someone aged 30 or 40 years.”
“There are a lot of risk factors, such as smoking, physical activity, and so on. It depends on the individual,” she added.
The study was funded by the Institute for Health Metrics and Evaluation at the University of Washington. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– at huge cost to health care systems and society, an analysis of data from the most recent Global Burden of Disease (GBD) study shows.
“The burden of brain conditions will increase as populations continue to grow and age,” said study presenter Shayla Smith, MPH, an epidemiologist at the Institute for Health Metrics and Evaluation, the University of Washington, Seattle, in a press release.
“By 2050, more than 50 million people will be aged 65-79,” she explained, adding that the COVID-19 pandemic “has also influenced the prevalence of mental disorders globally, as people were forced to isolate and social networks broke down.”
Other factors related to brain disorders, she noted, include education level, obesity, and smoking.
“There’s still research to be done on what is the most effective way to maintain brain health, but some literature suggests a healthy brain can be achieved through a healthy lifestyle of managing conditions such as high blood pressure and diabetes, limiting alcohol consumption and smoking, prioritizing sleep, eating healthy, and staying physically and mentally active,” said Ms. Smith.
The findings were presented at the annual meeting of the Congress of the European Academy of Neurology.
An ‘ambitious exercise’
Coinvestigator Xaviera Steele, also from the IHME, told press conference attendees that the institute was established at the University of Washington in 2007 with the aim of “standardizing the measurement of health outcomes around the world and for all health conditions.”
A central part of that is the GBD study, “which is a very ambitious exercise in descriptive epidemiology in an effort to systematically quantify health loss” due to disease, injury, and risk factors over time, stratified by country, region, age, and sex. In addition, researchers are mapping and projecting trends over the next century and are estimating disease expenditure by country, by type of expense, and by condition “to derive a health care access and quality score for each health system in the world,” Ms. Steele said.
They are also estimating exposure to risk factors, how those risk factors contribute to health burden, and associated health outcomes by race and ethnicity to reflect the “disparities that we know are very prevalent in countries such as the United States.” From that work, Ms. Steele said that brain health and related conditions “do emerge as one of the more pressing challenges of the 21st century.”
Increase in dementia, mental health conditions
The data, which were gathered from 200,000 sources by the IHME, indicate that the number of individuals aged 65 years or older will increase by 350% by 2100. Ms. Steele underlined that “policy action will be needed to help families, who will struggle to provide high-quality care for their loved ones with dementia at a reasonable cost.”
The IHME calculates that in Europe health care spending on Alzheimer’s disease will increase by 226% between 2015 and 2040.
Turning to other conditions, Ms. Steele showed that since 1990, the number of individuals living with anxiety in the European region has increased by 14%, while the number living with depressive disorders has gone up by 13%.
Worldwide, the figures are even starker. Depression is estimated to affect 300 million people across the globe, which represents a 71% increase since 1990. The number of strokes increased by 95% over the same period.
Nevertheless, the “impact of brain conditions such as stroke has decreased since the 1990s due to improved treatments available,” Ms. Smith noted in the press release.
To estimate the toll caused by brain conditions, including neurologic disorders, mental disorders, cerebrovascular disease, brain cancer, brain injuries, and select infectious conditions, the researchers calculated disability-adjusted life years (DALYs).
This, Ms. Smith explained in her presentation, “captures the morbidity and mortality associated with brain conditions” and is adjusted for patient location, age, and sex.
The investigators found that, globally, brain conditions accounted for more than 15% of all health loss in 2021, at 406 DALYs – more than the 206 million DALYs that were associated with cancer, and the 402 million that were linked to cardiovascular disease.
This health loss is associated with a $1.22 trillion loss in income for people living with health disorders worldwide and accounts for $1.14 trillion in direct health care costs.
The burden of mental disorders, neurologic conditions, and stroke is expected to increase dramatically between now and 2050, said Ms. Smith, who noted that health loss linked to brain conditions is higher in younger patients. This will create “new challenges for health systems, employers, patients, and families,” she said in the press release.
“Our goal is to see an improved prevention and treatment landscape for other brain conditions and reverse the growing health loss that we are currently forecasting.”
Worrying increase in stroke
Jurgita Valaikiene, MD, PhD, center of neurology, clinic of neurology and neurosurgery, Vilnius (Lithuania) University Faculty of Medicine, who chaired the session, was taken aback by the findings, particularly by the worldwide increase in stroke cases.
“I work in stroke,” she said, and “we spend a lot of time on the diagnosis of stroke” and its prevention. “We try to be faster, to catch asymptomatic stenosis in the neck or head, and to apply the best medical treatment to avoid a stroke. But despite that, the numbers are increasing. I understand the population is getting older ... but still it’s a huge number.”
Dr. Valaikiene pointed out that stroke is not necessarily a condition of aging, insofar as increasing age “is not related directly to stenosis in the neck. “For example, we can have healthier vessels in older age and unhealthy vessels, with high-grade stenosis, in someone aged 30 or 40 years.”
“There are a lot of risk factors, such as smoking, physical activity, and so on. It depends on the individual,” she added.
The study was funded by the Institute for Health Metrics and Evaluation at the University of Washington. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Smartwatches able to detect very early signs of Parkinson’s
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
FROM NATURE MEDICINE
Can a repurposed Parkinson’s drug slow ALS progression?
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL STEM CELL
Gout linked to smaller brain volume, higher likelihood of neurodegenerative diseases
Patients with gout may have smaller brain volumes and higher brain iron markers than people without gout, and also be more likely to develop Parkinson’s disease, probable essential tremor, and dementia, researchers in the United Kingdom report.
“We were surprised about the regions of the brain affected by gout, several of which are important for motor function. The other intriguing finding was that the risk of dementia amongst gout patients was strongly time-dependent: highest in the first 3 years after their gout diagnosis,” lead study author Anya Topiwala, BMBCh, DPhil, said in an interview.
“Our combination of traditional and genetic approaches increases the confidence that gout is causing the brain findings,” said Dr. Topiwala, a clinical research fellow and consultant psychiatrist in the Nuffield Department of Population Health at the University of Oxford, England.
“We suggest that clinicians be vigilant for cognitive and motor problems after gout diagnosis, particularly in the early stages,” she added.
Links between gout and neurodegenerative diseases debated in earlier studies
Gout, the most common inflammatory arthritis, affects around 1%-4% of people, the authors wrote, with monosodium urate crystal deposits causing acute flares of pain and swelling in joints and periarticular tissues.
Whether and how gout may affect the brain has been debated in the literature. Gout and hyperuricemia have been linked with elevated stroke risk; and although observational studies have linked hyperuricemia with lower dementia risk, especially Alzheimer’s disease, Mendelian randomization studies have had conflicting results in Alzheimer’s disease.
A novel approach that analyzes brain structure and genetics
In a study published in Nature Communications, Dr. Topiwala and her colleagues combined observational and Mendelian randomization techniques to explore relationships between gout and neurodegenerative diseases. They analyzed data from over 303,000 volunteer participants between 40 and 69 years of age recruited between 2006 and 2010 to contribute their detailed genetic and health information to the U.K. Biobank, a large-scale biomedical database and research resource.
Patients with gout tended to be older and male. At baseline, all participants’ serum urate levels were measured, and 30.8% of patients with gout reported that they currently used urate-lowering therapy.
MRI shows brain changes in patients with gout
In what the authors said is the first investigation of neuroimaging markers in patients with gout, they compared differences in gray matter volumes found in the 1,165 participants with gout and the 32,202 controls without gout who had MRI data.
They found no marked sex differences in associations. Urate was inversely linked with global brain volume and with gray and white matter volumes, and gout appeared to age global gray matter by 2 years.
Patients with gout and higher urate showed significant differences in regional gray matter volumes, especially in the cerebellum, pons, and midbrain, as well as subcortical differences in the nucleus accumbens, putamen, and caudate. They also showed significant differences in white matter tract microstructure in the fornix.
Patients with gout were more likely to develop dementia (average hazard ratio [HR] over study = 1.60), especially in the first 3 years after gout diagnosis (HR = 7.40). They were also at higher risk for vascular dementia (average HR = 2.41), compared with all-cause dementia, but not for Alzheimer’s disease (average HR = 1.62).
In asymptomatic participants though, urate and dementia were inversely linked (HR = 0.85), with no time dependence.
Gout was linked with higher incidence of Parkinson’s disease (HR = 1.43) and probable essential tremor (HR = 6.75). In asymptomatic participants, urate and Parkinson’s disease (HR = 0.89), but not probable essential tremor, were inversely linked.
Genetic analyses reinforce MRI results
Using Mendelian randomization estimates, the authors found that genetic links generally reflected their observational findings. Both genetically predicted gout and serum urate were significantly linked with regional gray matter volumes, including cerebellar, midbrain, pons, and brainstem.
They also found significant links with higher magnetic susceptibility in the putamen and caudate, markers of higher iron. But while genetically predicted gout was significantly linked with global gray matter volume, urate was not.
In males, but not in females, urate was positively linked with alcohol intake and lower socioeconomic status.
Dr. Topiwala acknowledged several limitations to the study, writing that “the results from the volunteer participants may not apply to other populations; the cross-sectional serum urate measurements may not reflect chronic exposure; and Parkinson’s disease and essential tremor may have been diagnostically confounded.”
A novel approach that suggests further related research
Asked to comment on the study, Puja Khanna, MD, MPH, a rheumatologist and clinical associate professor of medicine at the University of Michigan, Ann Arbor, called its novel use of neuroimaging interesting.
Dr. Khanna, who was not involved in the study, said she would like to know more about the role that horizontal pleiotropy – one genetic variant having independent effects on multiple traits – plays in this disease process, and about the impact of the antioxidative properties of urate in maintaining neuroprotection.
“[The] U.K. Biobank is an excellent database to look at questions of association,” John D. FitzGerald, MD, PhD, MPH, MBA, professor and clinical chief of rheumatology at the University of California, Los Angeles, said in an interview.
“This is a fairly rigorous study,” added Dr. FitzGerald, also not involved in the study. “While it has lots of strengths,” including its large sample size and Mendelian randomization, it also has “abundant weaknesses,” he added. “It is largely cross-sectional, with single urate measurement and single brain MRI.”
“Causation is the big question,” Dr. FitzGerald noted. “Does treating gout (or urate) help prevent dementia or neurodegenerative decline?”
Early diagnosis benefits patients
Dr. Khanna and Dr. FitzGerald joined the authors in advising doctors to monitor their gout patients for cognitive and motor symptoms of neurodegenerative disease.
“It is clearly important to pay close attention to the neurologic exam and history in gout, especially because it is a disease of the aging population,” Dr. Khanna advised. “Addressing dementia when gout is diagnosed can lead to prompt mitigation strategies that can hugely impact patients.”
Dr. Topiwala and her colleagues would like to investigate why the dementia risk was time-dependent. “Is this because of the acute inflammatory response in gout, or could it just be that patients with gout visit their doctors more frequently, so any cognitive problems are picked up sooner?” she asked.
The authors, and Dr. Khanna and Dr. FitzGerald, report no relevant financial relationships. The Wellcome Trust; the U.K. Medical Research Council; the European Commission Horizon 2020 research and innovation program; the British Heart Foundation; the U.S. National Institutes of Health; the Engineering and Physical Sciences Research Council; and the National Institute for Health and Care Research funded the study.
Patients with gout may have smaller brain volumes and higher brain iron markers than people without gout, and also be more likely to develop Parkinson’s disease, probable essential tremor, and dementia, researchers in the United Kingdom report.
“We were surprised about the regions of the brain affected by gout, several of which are important for motor function. The other intriguing finding was that the risk of dementia amongst gout patients was strongly time-dependent: highest in the first 3 years after their gout diagnosis,” lead study author Anya Topiwala, BMBCh, DPhil, said in an interview.
“Our combination of traditional and genetic approaches increases the confidence that gout is causing the brain findings,” said Dr. Topiwala, a clinical research fellow and consultant psychiatrist in the Nuffield Department of Population Health at the University of Oxford, England.
“We suggest that clinicians be vigilant for cognitive and motor problems after gout diagnosis, particularly in the early stages,” she added.
Links between gout and neurodegenerative diseases debated in earlier studies
Gout, the most common inflammatory arthritis, affects around 1%-4% of people, the authors wrote, with monosodium urate crystal deposits causing acute flares of pain and swelling in joints and periarticular tissues.
Whether and how gout may affect the brain has been debated in the literature. Gout and hyperuricemia have been linked with elevated stroke risk; and although observational studies have linked hyperuricemia with lower dementia risk, especially Alzheimer’s disease, Mendelian randomization studies have had conflicting results in Alzheimer’s disease.
A novel approach that analyzes brain structure and genetics
In a study published in Nature Communications, Dr. Topiwala and her colleagues combined observational and Mendelian randomization techniques to explore relationships between gout and neurodegenerative diseases. They analyzed data from over 303,000 volunteer participants between 40 and 69 years of age recruited between 2006 and 2010 to contribute their detailed genetic and health information to the U.K. Biobank, a large-scale biomedical database and research resource.
Patients with gout tended to be older and male. At baseline, all participants’ serum urate levels were measured, and 30.8% of patients with gout reported that they currently used urate-lowering therapy.
MRI shows brain changes in patients with gout
In what the authors said is the first investigation of neuroimaging markers in patients with gout, they compared differences in gray matter volumes found in the 1,165 participants with gout and the 32,202 controls without gout who had MRI data.
They found no marked sex differences in associations. Urate was inversely linked with global brain volume and with gray and white matter volumes, and gout appeared to age global gray matter by 2 years.
Patients with gout and higher urate showed significant differences in regional gray matter volumes, especially in the cerebellum, pons, and midbrain, as well as subcortical differences in the nucleus accumbens, putamen, and caudate. They also showed significant differences in white matter tract microstructure in the fornix.
Patients with gout were more likely to develop dementia (average hazard ratio [HR] over study = 1.60), especially in the first 3 years after gout diagnosis (HR = 7.40). They were also at higher risk for vascular dementia (average HR = 2.41), compared with all-cause dementia, but not for Alzheimer’s disease (average HR = 1.62).
In asymptomatic participants though, urate and dementia were inversely linked (HR = 0.85), with no time dependence.
Gout was linked with higher incidence of Parkinson’s disease (HR = 1.43) and probable essential tremor (HR = 6.75). In asymptomatic participants, urate and Parkinson’s disease (HR = 0.89), but not probable essential tremor, were inversely linked.
Genetic analyses reinforce MRI results
Using Mendelian randomization estimates, the authors found that genetic links generally reflected their observational findings. Both genetically predicted gout and serum urate were significantly linked with regional gray matter volumes, including cerebellar, midbrain, pons, and brainstem.
They also found significant links with higher magnetic susceptibility in the putamen and caudate, markers of higher iron. But while genetically predicted gout was significantly linked with global gray matter volume, urate was not.
In males, but not in females, urate was positively linked with alcohol intake and lower socioeconomic status.
Dr. Topiwala acknowledged several limitations to the study, writing that “the results from the volunteer participants may not apply to other populations; the cross-sectional serum urate measurements may not reflect chronic exposure; and Parkinson’s disease and essential tremor may have been diagnostically confounded.”
A novel approach that suggests further related research
Asked to comment on the study, Puja Khanna, MD, MPH, a rheumatologist and clinical associate professor of medicine at the University of Michigan, Ann Arbor, called its novel use of neuroimaging interesting.
Dr. Khanna, who was not involved in the study, said she would like to know more about the role that horizontal pleiotropy – one genetic variant having independent effects on multiple traits – plays in this disease process, and about the impact of the antioxidative properties of urate in maintaining neuroprotection.
“[The] U.K. Biobank is an excellent database to look at questions of association,” John D. FitzGerald, MD, PhD, MPH, MBA, professor and clinical chief of rheumatology at the University of California, Los Angeles, said in an interview.
“This is a fairly rigorous study,” added Dr. FitzGerald, also not involved in the study. “While it has lots of strengths,” including its large sample size and Mendelian randomization, it also has “abundant weaknesses,” he added. “It is largely cross-sectional, with single urate measurement and single brain MRI.”
“Causation is the big question,” Dr. FitzGerald noted. “Does treating gout (or urate) help prevent dementia or neurodegenerative decline?”
Early diagnosis benefits patients
Dr. Khanna and Dr. FitzGerald joined the authors in advising doctors to monitor their gout patients for cognitive and motor symptoms of neurodegenerative disease.
“It is clearly important to pay close attention to the neurologic exam and history in gout, especially because it is a disease of the aging population,” Dr. Khanna advised. “Addressing dementia when gout is diagnosed can lead to prompt mitigation strategies that can hugely impact patients.”
Dr. Topiwala and her colleagues would like to investigate why the dementia risk was time-dependent. “Is this because of the acute inflammatory response in gout, or could it just be that patients with gout visit their doctors more frequently, so any cognitive problems are picked up sooner?” she asked.
The authors, and Dr. Khanna and Dr. FitzGerald, report no relevant financial relationships. The Wellcome Trust; the U.K. Medical Research Council; the European Commission Horizon 2020 research and innovation program; the British Heart Foundation; the U.S. National Institutes of Health; the Engineering and Physical Sciences Research Council; and the National Institute for Health and Care Research funded the study.
Patients with gout may have smaller brain volumes and higher brain iron markers than people without gout, and also be more likely to develop Parkinson’s disease, probable essential tremor, and dementia, researchers in the United Kingdom report.
“We were surprised about the regions of the brain affected by gout, several of which are important for motor function. The other intriguing finding was that the risk of dementia amongst gout patients was strongly time-dependent: highest in the first 3 years after their gout diagnosis,” lead study author Anya Topiwala, BMBCh, DPhil, said in an interview.
“Our combination of traditional and genetic approaches increases the confidence that gout is causing the brain findings,” said Dr. Topiwala, a clinical research fellow and consultant psychiatrist in the Nuffield Department of Population Health at the University of Oxford, England.
“We suggest that clinicians be vigilant for cognitive and motor problems after gout diagnosis, particularly in the early stages,” she added.
Links between gout and neurodegenerative diseases debated in earlier studies
Gout, the most common inflammatory arthritis, affects around 1%-4% of people, the authors wrote, with monosodium urate crystal deposits causing acute flares of pain and swelling in joints and periarticular tissues.
Whether and how gout may affect the brain has been debated in the literature. Gout and hyperuricemia have been linked with elevated stroke risk; and although observational studies have linked hyperuricemia with lower dementia risk, especially Alzheimer’s disease, Mendelian randomization studies have had conflicting results in Alzheimer’s disease.
A novel approach that analyzes brain structure and genetics
In a study published in Nature Communications, Dr. Topiwala and her colleagues combined observational and Mendelian randomization techniques to explore relationships between gout and neurodegenerative diseases. They analyzed data from over 303,000 volunteer participants between 40 and 69 years of age recruited between 2006 and 2010 to contribute their detailed genetic and health information to the U.K. Biobank, a large-scale biomedical database and research resource.
Patients with gout tended to be older and male. At baseline, all participants’ serum urate levels were measured, and 30.8% of patients with gout reported that they currently used urate-lowering therapy.
MRI shows brain changes in patients with gout
In what the authors said is the first investigation of neuroimaging markers in patients with gout, they compared differences in gray matter volumes found in the 1,165 participants with gout and the 32,202 controls without gout who had MRI data.
They found no marked sex differences in associations. Urate was inversely linked with global brain volume and with gray and white matter volumes, and gout appeared to age global gray matter by 2 years.
Patients with gout and higher urate showed significant differences in regional gray matter volumes, especially in the cerebellum, pons, and midbrain, as well as subcortical differences in the nucleus accumbens, putamen, and caudate. They also showed significant differences in white matter tract microstructure in the fornix.
Patients with gout were more likely to develop dementia (average hazard ratio [HR] over study = 1.60), especially in the first 3 years after gout diagnosis (HR = 7.40). They were also at higher risk for vascular dementia (average HR = 2.41), compared with all-cause dementia, but not for Alzheimer’s disease (average HR = 1.62).
In asymptomatic participants though, urate and dementia were inversely linked (HR = 0.85), with no time dependence.
Gout was linked with higher incidence of Parkinson’s disease (HR = 1.43) and probable essential tremor (HR = 6.75). In asymptomatic participants, urate and Parkinson’s disease (HR = 0.89), but not probable essential tremor, were inversely linked.
Genetic analyses reinforce MRI results
Using Mendelian randomization estimates, the authors found that genetic links generally reflected their observational findings. Both genetically predicted gout and serum urate were significantly linked with regional gray matter volumes, including cerebellar, midbrain, pons, and brainstem.
They also found significant links with higher magnetic susceptibility in the putamen and caudate, markers of higher iron. But while genetically predicted gout was significantly linked with global gray matter volume, urate was not.
In males, but not in females, urate was positively linked with alcohol intake and lower socioeconomic status.
Dr. Topiwala acknowledged several limitations to the study, writing that “the results from the volunteer participants may not apply to other populations; the cross-sectional serum urate measurements may not reflect chronic exposure; and Parkinson’s disease and essential tremor may have been diagnostically confounded.”
A novel approach that suggests further related research
Asked to comment on the study, Puja Khanna, MD, MPH, a rheumatologist and clinical associate professor of medicine at the University of Michigan, Ann Arbor, called its novel use of neuroimaging interesting.
Dr. Khanna, who was not involved in the study, said she would like to know more about the role that horizontal pleiotropy – one genetic variant having independent effects on multiple traits – plays in this disease process, and about the impact of the antioxidative properties of urate in maintaining neuroprotection.
“[The] U.K. Biobank is an excellent database to look at questions of association,” John D. FitzGerald, MD, PhD, MPH, MBA, professor and clinical chief of rheumatology at the University of California, Los Angeles, said in an interview.
“This is a fairly rigorous study,” added Dr. FitzGerald, also not involved in the study. “While it has lots of strengths,” including its large sample size and Mendelian randomization, it also has “abundant weaknesses,” he added. “It is largely cross-sectional, with single urate measurement and single brain MRI.”
“Causation is the big question,” Dr. FitzGerald noted. “Does treating gout (or urate) help prevent dementia or neurodegenerative decline?”
Early diagnosis benefits patients
Dr. Khanna and Dr. FitzGerald joined the authors in advising doctors to monitor their gout patients for cognitive and motor symptoms of neurodegenerative disease.
“It is clearly important to pay close attention to the neurologic exam and history in gout, especially because it is a disease of the aging population,” Dr. Khanna advised. “Addressing dementia when gout is diagnosed can lead to prompt mitigation strategies that can hugely impact patients.”
Dr. Topiwala and her colleagues would like to investigate why the dementia risk was time-dependent. “Is this because of the acute inflammatory response in gout, or could it just be that patients with gout visit their doctors more frequently, so any cognitive problems are picked up sooner?” she asked.
The authors, and Dr. Khanna and Dr. FitzGerald, report no relevant financial relationships. The Wellcome Trust; the U.K. Medical Research Council; the European Commission Horizon 2020 research and innovation program; the British Heart Foundation; the U.S. National Institutes of Health; the Engineering and Physical Sciences Research Council; and the National Institute for Health and Care Research funded the study.
FROM NATURE COMMUNICATIONS
Potential new treatment for REM sleep behavior disorder
Dual orexin receptor antagonists (DORAs), a class of drugs approved to treat insomnia, may also be effective for rapid eye movement sleep behavior disorder (RBD), a study suggests.
About 3 million people in the United States have RBD, which is often a precursor to Parkinson’s disease. People with the disorder act out their dreams by talking, flailing their arms and legs, punching, kicking, and exhibiting other behaviors while asleep.
Researchers used an animal model for the study, which they say is the first to identify a new form of treatment for RBD.
“REM behavior disorder is difficult to treat, and the treatments are mostly limited to clonazepam and melatonin,” which may have side effects, senior investigator Andrew Varga, MD, PhD, associate professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai, New York, told this news organization. “We’re using something completely different, which raises the possibility this might be something useful for REM behavior disorders.”
The findings, with Mount Sinai assistant professor Korey Kam, PhD, as lead author, were published online in the Journal of Neuroscience.
A new model for RBD?
RBD can signal risk for synucleinopathies, a group of neurological conditions such as Parkinson’s disease that involve the formation of clumps of alpha-synuclein protein in the brain.
Prior research on RBD was done in synucleinopathy mouse models. For this study, however, researchers used a tauopathy mouse model to investigate how the abnormal accumulation of tau protein might affect RBD.
Researchers collected data on biophysical properties when the mice were awake and in REM and non-REM sleep. They examined length of sleep, transitions from waking to sleep, and how some factors are related to age.
Nearly a third of the older animals showed behaviors similar to REM sleep behavior disorder in humans, including chewing and limb extension.
But after researchers administered a DORA medication twice during a 24-hour period, they noted that the medication not only helped the animals fall asleep faster and for longer, it also reduced levels of dream enactment that are a hallmark of RBD.
The ‘bigger highlight’
Finding RBD behaviors in a tauopathy animal model was surprising, Dr. Varga said, because RBD has been previously linked to synucleinopathies. There was no known correlation between RBD and abnormal accumulation of tau.
Another unexpected finding was the detection of RBD in some of the younger animals, who had not yet shown evidence of tau accumulation.
“It appears to be a biomarker or a signature of something that’s going on that predicts the impending tauopathy at a time where there is very little, or no, tau pathology going on in the brain,” Dr. Varga said.
If RBD is an early predictor of future tau accumulation, the model could guide future prevention and treatment. However, the more important finding is the potential new treatment for the condition.
“The bigger highlight here is less about what’s causing the RBD [than about] what you can do to make it better,” he said.
The next step in the work is to study whether the effect of DORAs on RBD seen in this tauopathy mouse model is evidenced in other animals and whether it is effective in humans with RBD, Dr. Varga said.
The study was funded by the Alzheimer’s Association and Merck Investigator Studies Program. Dr. Kam, Dr. Varga, and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dual orexin receptor antagonists (DORAs), a class of drugs approved to treat insomnia, may also be effective for rapid eye movement sleep behavior disorder (RBD), a study suggests.
About 3 million people in the United States have RBD, which is often a precursor to Parkinson’s disease. People with the disorder act out their dreams by talking, flailing their arms and legs, punching, kicking, and exhibiting other behaviors while asleep.
Researchers used an animal model for the study, which they say is the first to identify a new form of treatment for RBD.
“REM behavior disorder is difficult to treat, and the treatments are mostly limited to clonazepam and melatonin,” which may have side effects, senior investigator Andrew Varga, MD, PhD, associate professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai, New York, told this news organization. “We’re using something completely different, which raises the possibility this might be something useful for REM behavior disorders.”
The findings, with Mount Sinai assistant professor Korey Kam, PhD, as lead author, were published online in the Journal of Neuroscience.
A new model for RBD?
RBD can signal risk for synucleinopathies, a group of neurological conditions such as Parkinson’s disease that involve the formation of clumps of alpha-synuclein protein in the brain.
Prior research on RBD was done in synucleinopathy mouse models. For this study, however, researchers used a tauopathy mouse model to investigate how the abnormal accumulation of tau protein might affect RBD.
Researchers collected data on biophysical properties when the mice were awake and in REM and non-REM sleep. They examined length of sleep, transitions from waking to sleep, and how some factors are related to age.
Nearly a third of the older animals showed behaviors similar to REM sleep behavior disorder in humans, including chewing and limb extension.
But after researchers administered a DORA medication twice during a 24-hour period, they noted that the medication not only helped the animals fall asleep faster and for longer, it also reduced levels of dream enactment that are a hallmark of RBD.
The ‘bigger highlight’
Finding RBD behaviors in a tauopathy animal model was surprising, Dr. Varga said, because RBD has been previously linked to synucleinopathies. There was no known correlation between RBD and abnormal accumulation of tau.
Another unexpected finding was the detection of RBD in some of the younger animals, who had not yet shown evidence of tau accumulation.
“It appears to be a biomarker or a signature of something that’s going on that predicts the impending tauopathy at a time where there is very little, or no, tau pathology going on in the brain,” Dr. Varga said.
If RBD is an early predictor of future tau accumulation, the model could guide future prevention and treatment. However, the more important finding is the potential new treatment for the condition.
“The bigger highlight here is less about what’s causing the RBD [than about] what you can do to make it better,” he said.
The next step in the work is to study whether the effect of DORAs on RBD seen in this tauopathy mouse model is evidenced in other animals and whether it is effective in humans with RBD, Dr. Varga said.
The study was funded by the Alzheimer’s Association and Merck Investigator Studies Program. Dr. Kam, Dr. Varga, and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dual orexin receptor antagonists (DORAs), a class of drugs approved to treat insomnia, may also be effective for rapid eye movement sleep behavior disorder (RBD), a study suggests.
About 3 million people in the United States have RBD, which is often a precursor to Parkinson’s disease. People with the disorder act out their dreams by talking, flailing their arms and legs, punching, kicking, and exhibiting other behaviors while asleep.
Researchers used an animal model for the study, which they say is the first to identify a new form of treatment for RBD.
“REM behavior disorder is difficult to treat, and the treatments are mostly limited to clonazepam and melatonin,” which may have side effects, senior investigator Andrew Varga, MD, PhD, associate professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai, New York, told this news organization. “We’re using something completely different, which raises the possibility this might be something useful for REM behavior disorders.”
The findings, with Mount Sinai assistant professor Korey Kam, PhD, as lead author, were published online in the Journal of Neuroscience.
A new model for RBD?
RBD can signal risk for synucleinopathies, a group of neurological conditions such as Parkinson’s disease that involve the formation of clumps of alpha-synuclein protein in the brain.
Prior research on RBD was done in synucleinopathy mouse models. For this study, however, researchers used a tauopathy mouse model to investigate how the abnormal accumulation of tau protein might affect RBD.
Researchers collected data on biophysical properties when the mice were awake and in REM and non-REM sleep. They examined length of sleep, transitions from waking to sleep, and how some factors are related to age.
Nearly a third of the older animals showed behaviors similar to REM sleep behavior disorder in humans, including chewing and limb extension.
But after researchers administered a DORA medication twice during a 24-hour period, they noted that the medication not only helped the animals fall asleep faster and for longer, it also reduced levels of dream enactment that are a hallmark of RBD.
The ‘bigger highlight’
Finding RBD behaviors in a tauopathy animal model was surprising, Dr. Varga said, because RBD has been previously linked to synucleinopathies. There was no known correlation between RBD and abnormal accumulation of tau.
Another unexpected finding was the detection of RBD in some of the younger animals, who had not yet shown evidence of tau accumulation.
“It appears to be a biomarker or a signature of something that’s going on that predicts the impending tauopathy at a time where there is very little, or no, tau pathology going on in the brain,” Dr. Varga said.
If RBD is an early predictor of future tau accumulation, the model could guide future prevention and treatment. However, the more important finding is the potential new treatment for the condition.
“The bigger highlight here is less about what’s causing the RBD [than about] what you can do to make it better,” he said.
The next step in the work is to study whether the effect of DORAs on RBD seen in this tauopathy mouse model is evidenced in other animals and whether it is effective in humans with RBD, Dr. Varga said.
The study was funded by the Alzheimer’s Association and Merck Investigator Studies Program. Dr. Kam, Dr. Varga, and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROSCIENCE
‘Robust evidence’ that exercise cuts Parkinson’s risk in women
Investigators found that among almost 99,000 women participating in the ongoing E3N study, those who exercised the most frequently had up to a 25% lower risk for PD than their less-active counterparts.
The results highlight the importance of exercising early in mid-life to prevent PD later on, study investigator Alexis Elbaz, MD, PhD, research director, French Institute of Health and Medical Research (Inserm), Paris, said in an interview.
This is especially critical since there is no cure nor disease-modifying treatments. The medications that are available are aimed at symptom reduction.
“Finding ways to prevent or delay the onset of Parkinson’s is really important, and physical activity seems to be one of the possible strategies to reduce the risk,” Dr. Elbaz said.
The study was published online in Neurology.
Direct protective effect?
Results from previous research examining the relationship physical activity and PD has been inconsistent. One meta-analysis showed a statistically significant association among men but a nonsignificant link in women.
The investigators noted that some of the findings from previous studies may have been affected by reverse causation. As nonmotor symptoms such as constipation and subtle motor signs such as tremor and balance issues can present years before a PD diagnosis, patients may reduce their physical activity because of such symptoms.
To address this potential confounder, the researchers used “lag” analyses, where data on physical activity levels in the years close to a PD diagnosis are omitted.
The study relied on data from the E3N, an ongoing cohort study of 98,995 women, born between 1925 and 1950 and recruited in 1990, who were affiliated with a French national health insurance plan that primarily covers teachers. Participants completed a questionnaire on lifestyle and medical history at baseline and follow-up questionnaires every 2-3 years.
In six of the questionnaires, participants provided details about various recreational, sports, and household activities – for example, walking, climbing stairs, gardening, and cleaning. The authors attributed metabolic equivalent of task (MET) values to each activity and multiplied METs by their frequency and duration to obtain a physical activity score.
Definite and probable PD cases were determined through self-reported physician diagnoses, anti-parkinsonian drug claims, and medical records, with diagnoses verified by an expert panel.
Researchers investigated the relationship between physical activity and PD onset in a nested-case control study that included 25,075 women (1,196 PD cases and 23,879 controls) with a mean age of 71.9 years. They found physical activity was significantly lower in cases than in controls throughout follow-up.
The difference between cases and controls began to increase at 10 years before diagnosis (P-interaction = .003). “When we looked at the trajectories of physical activity in PD patients and in controls, we saw that in the 10 years before the diagnosis, physical activity declined at a steeper rate in controls. We think this is because those subtle prodromal symptoms cause people to exercise less,” said Dr. Elbaz.
In the main analysis, which had a 10-year lag, 1,074 women developed incident PD during a mean follow-up of 17.2 years. Those in the highest quartile of physical activity had a 25% lower risk for PD vs. those in the lowest quartile (adjusted hazard ratio [HR], 0.75, 95% confidence interval [CI], 0.63-0.89).
The risk for PD decreased with increasing levels of physical activity in a linear fashion, noted Dr. Elbaz. “So doing even a little bit of physical activity is better than doing nothing at all.”
Analyses that included 15-year and 20-year lag times had similar findings.
Sensitivity analyses that adjusted for the Mediterranean diet and caffeine and dairy intake also yielded comparable results. This was also true for analyses that adjusted for comorbidities such as body mass index, hypertension, hypercholesterolemia, diabetes, and cardiovascular disease, all of which can affect PD risk.
“This gives weight to the idea that diabetes or cardiovascular diseases do not explain the relationship between physical activity and PD, which means the most likely hypothesis is that physical activity has a direct protective effect on the brain,” said Dr. Elbaz.
Studies have shown that physical activity affects brain plasticity and can reduce oxidative stress in the brain – a key mechanism involved in PD, he added.
Physical activity is a low-risk, inexpensive, and accessible intervention. But the study was not designed to determine the types of physical activity that are most protective against PD.
The study’s main limitation is that it used self-reported physical activity rather than objective measures such as accelerometers. In addition, the participants were not necessarily representative of the general population.
Robust evidence
In an accompanying editorial, Lana M. Chahine, MD, associate professor in the department of neurology at the University of Pittsburgh, and Sirwan K. L. Darweesh, MD, PhD, Radboud University Medical Center, Donders Institute for Brain, Cognition and Behaviour, Center of Expertise for Parkinson and Movement Disorders, Nijmegen, the Netherlands, said the study “provides robust evidence” that physical activity reduces risk for PD in women.
“These results show that the field is moving in the right direction and provide a clear rationale for exercise trials to prevent or delay the onset of manifest PD in at-risk individuals” they wrote.
The study highlights “gaps” in knowledge that merit closer attention and that “further insight is warranted on how much the effects on PD vary by type, intensity, frequency, and duration of physical activity,” the editorialists noted.
Another gap is how the accuracy of assessment of physical activity can be improved beyond self-report. “Wearable sensor technology now offers the potential to assess physical activity remotely and objectively in prevention trials,” they added.
Other areas that need exploring relate to mechanisms by which physical activity reduces PD risk, and to what extent effects of physical activity vary between individuals, Dr. Chahine and Dr. Darweesh noted.
Commenting for this article, Michael S. Okun, MD, executive director of the Fixel Institute for Neurological Diseases at University of Florida Health, and medical adviser for the Parkinson’s Foundation, said the findings are “significant and important.”
Based on only a handful of previous studies, it was assumed that physical activity was associated with reduced Parkinson’s diagnosis only in men, said Dr. Okun. “The current dataset was larger and included longer-term outcomes, and it informs the field that exercise may be important for reducing the risk of Parkinson’s disease in men as well as in women.”
The investigators, the editorialists, and Dr. Okun reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators found that among almost 99,000 women participating in the ongoing E3N study, those who exercised the most frequently had up to a 25% lower risk for PD than their less-active counterparts.
The results highlight the importance of exercising early in mid-life to prevent PD later on, study investigator Alexis Elbaz, MD, PhD, research director, French Institute of Health and Medical Research (Inserm), Paris, said in an interview.
This is especially critical since there is no cure nor disease-modifying treatments. The medications that are available are aimed at symptom reduction.
“Finding ways to prevent or delay the onset of Parkinson’s is really important, and physical activity seems to be one of the possible strategies to reduce the risk,” Dr. Elbaz said.
The study was published online in Neurology.
Direct protective effect?
Results from previous research examining the relationship physical activity and PD has been inconsistent. One meta-analysis showed a statistically significant association among men but a nonsignificant link in women.
The investigators noted that some of the findings from previous studies may have been affected by reverse causation. As nonmotor symptoms such as constipation and subtle motor signs such as tremor and balance issues can present years before a PD diagnosis, patients may reduce their physical activity because of such symptoms.
To address this potential confounder, the researchers used “lag” analyses, where data on physical activity levels in the years close to a PD diagnosis are omitted.
The study relied on data from the E3N, an ongoing cohort study of 98,995 women, born between 1925 and 1950 and recruited in 1990, who were affiliated with a French national health insurance plan that primarily covers teachers. Participants completed a questionnaire on lifestyle and medical history at baseline and follow-up questionnaires every 2-3 years.
In six of the questionnaires, participants provided details about various recreational, sports, and household activities – for example, walking, climbing stairs, gardening, and cleaning. The authors attributed metabolic equivalent of task (MET) values to each activity and multiplied METs by their frequency and duration to obtain a physical activity score.
Definite and probable PD cases were determined through self-reported physician diagnoses, anti-parkinsonian drug claims, and medical records, with diagnoses verified by an expert panel.
Researchers investigated the relationship between physical activity and PD onset in a nested-case control study that included 25,075 women (1,196 PD cases and 23,879 controls) with a mean age of 71.9 years. They found physical activity was significantly lower in cases than in controls throughout follow-up.
The difference between cases and controls began to increase at 10 years before diagnosis (P-interaction = .003). “When we looked at the trajectories of physical activity in PD patients and in controls, we saw that in the 10 years before the diagnosis, physical activity declined at a steeper rate in controls. We think this is because those subtle prodromal symptoms cause people to exercise less,” said Dr. Elbaz.
In the main analysis, which had a 10-year lag, 1,074 women developed incident PD during a mean follow-up of 17.2 years. Those in the highest quartile of physical activity had a 25% lower risk for PD vs. those in the lowest quartile (adjusted hazard ratio [HR], 0.75, 95% confidence interval [CI], 0.63-0.89).
The risk for PD decreased with increasing levels of physical activity in a linear fashion, noted Dr. Elbaz. “So doing even a little bit of physical activity is better than doing nothing at all.”
Analyses that included 15-year and 20-year lag times had similar findings.
Sensitivity analyses that adjusted for the Mediterranean diet and caffeine and dairy intake also yielded comparable results. This was also true for analyses that adjusted for comorbidities such as body mass index, hypertension, hypercholesterolemia, diabetes, and cardiovascular disease, all of which can affect PD risk.
“This gives weight to the idea that diabetes or cardiovascular diseases do not explain the relationship between physical activity and PD, which means the most likely hypothesis is that physical activity has a direct protective effect on the brain,” said Dr. Elbaz.
Studies have shown that physical activity affects brain plasticity and can reduce oxidative stress in the brain – a key mechanism involved in PD, he added.
Physical activity is a low-risk, inexpensive, and accessible intervention. But the study was not designed to determine the types of physical activity that are most protective against PD.
The study’s main limitation is that it used self-reported physical activity rather than objective measures such as accelerometers. In addition, the participants were not necessarily representative of the general population.
Robust evidence
In an accompanying editorial, Lana M. Chahine, MD, associate professor in the department of neurology at the University of Pittsburgh, and Sirwan K. L. Darweesh, MD, PhD, Radboud University Medical Center, Donders Institute for Brain, Cognition and Behaviour, Center of Expertise for Parkinson and Movement Disorders, Nijmegen, the Netherlands, said the study “provides robust evidence” that physical activity reduces risk for PD in women.
“These results show that the field is moving in the right direction and provide a clear rationale for exercise trials to prevent or delay the onset of manifest PD in at-risk individuals” they wrote.
The study highlights “gaps” in knowledge that merit closer attention and that “further insight is warranted on how much the effects on PD vary by type, intensity, frequency, and duration of physical activity,” the editorialists noted.
Another gap is how the accuracy of assessment of physical activity can be improved beyond self-report. “Wearable sensor technology now offers the potential to assess physical activity remotely and objectively in prevention trials,” they added.
Other areas that need exploring relate to mechanisms by which physical activity reduces PD risk, and to what extent effects of physical activity vary between individuals, Dr. Chahine and Dr. Darweesh noted.
Commenting for this article, Michael S. Okun, MD, executive director of the Fixel Institute for Neurological Diseases at University of Florida Health, and medical adviser for the Parkinson’s Foundation, said the findings are “significant and important.”
Based on only a handful of previous studies, it was assumed that physical activity was associated with reduced Parkinson’s diagnosis only in men, said Dr. Okun. “The current dataset was larger and included longer-term outcomes, and it informs the field that exercise may be important for reducing the risk of Parkinson’s disease in men as well as in women.”
The investigators, the editorialists, and Dr. Okun reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators found that among almost 99,000 women participating in the ongoing E3N study, those who exercised the most frequently had up to a 25% lower risk for PD than their less-active counterparts.
The results highlight the importance of exercising early in mid-life to prevent PD later on, study investigator Alexis Elbaz, MD, PhD, research director, French Institute of Health and Medical Research (Inserm), Paris, said in an interview.
This is especially critical since there is no cure nor disease-modifying treatments. The medications that are available are aimed at symptom reduction.
“Finding ways to prevent or delay the onset of Parkinson’s is really important, and physical activity seems to be one of the possible strategies to reduce the risk,” Dr. Elbaz said.
The study was published online in Neurology.
Direct protective effect?
Results from previous research examining the relationship physical activity and PD has been inconsistent. One meta-analysis showed a statistically significant association among men but a nonsignificant link in women.
The investigators noted that some of the findings from previous studies may have been affected by reverse causation. As nonmotor symptoms such as constipation and subtle motor signs such as tremor and balance issues can present years before a PD diagnosis, patients may reduce their physical activity because of such symptoms.
To address this potential confounder, the researchers used “lag” analyses, where data on physical activity levels in the years close to a PD diagnosis are omitted.
The study relied on data from the E3N, an ongoing cohort study of 98,995 women, born between 1925 and 1950 and recruited in 1990, who were affiliated with a French national health insurance plan that primarily covers teachers. Participants completed a questionnaire on lifestyle and medical history at baseline and follow-up questionnaires every 2-3 years.
In six of the questionnaires, participants provided details about various recreational, sports, and household activities – for example, walking, climbing stairs, gardening, and cleaning. The authors attributed metabolic equivalent of task (MET) values to each activity and multiplied METs by their frequency and duration to obtain a physical activity score.
Definite and probable PD cases were determined through self-reported physician diagnoses, anti-parkinsonian drug claims, and medical records, with diagnoses verified by an expert panel.
Researchers investigated the relationship between physical activity and PD onset in a nested-case control study that included 25,075 women (1,196 PD cases and 23,879 controls) with a mean age of 71.9 years. They found physical activity was significantly lower in cases than in controls throughout follow-up.
The difference between cases and controls began to increase at 10 years before diagnosis (P-interaction = .003). “When we looked at the trajectories of physical activity in PD patients and in controls, we saw that in the 10 years before the diagnosis, physical activity declined at a steeper rate in controls. We think this is because those subtle prodromal symptoms cause people to exercise less,” said Dr. Elbaz.
In the main analysis, which had a 10-year lag, 1,074 women developed incident PD during a mean follow-up of 17.2 years. Those in the highest quartile of physical activity had a 25% lower risk for PD vs. those in the lowest quartile (adjusted hazard ratio [HR], 0.75, 95% confidence interval [CI], 0.63-0.89).
The risk for PD decreased with increasing levels of physical activity in a linear fashion, noted Dr. Elbaz. “So doing even a little bit of physical activity is better than doing nothing at all.”
Analyses that included 15-year and 20-year lag times had similar findings.
Sensitivity analyses that adjusted for the Mediterranean diet and caffeine and dairy intake also yielded comparable results. This was also true for analyses that adjusted for comorbidities such as body mass index, hypertension, hypercholesterolemia, diabetes, and cardiovascular disease, all of which can affect PD risk.
“This gives weight to the idea that diabetes or cardiovascular diseases do not explain the relationship between physical activity and PD, which means the most likely hypothesis is that physical activity has a direct protective effect on the brain,” said Dr. Elbaz.
Studies have shown that physical activity affects brain plasticity and can reduce oxidative stress in the brain – a key mechanism involved in PD, he added.
Physical activity is a low-risk, inexpensive, and accessible intervention. But the study was not designed to determine the types of physical activity that are most protective against PD.
The study’s main limitation is that it used self-reported physical activity rather than objective measures such as accelerometers. In addition, the participants were not necessarily representative of the general population.
Robust evidence
In an accompanying editorial, Lana M. Chahine, MD, associate professor in the department of neurology at the University of Pittsburgh, and Sirwan K. L. Darweesh, MD, PhD, Radboud University Medical Center, Donders Institute for Brain, Cognition and Behaviour, Center of Expertise for Parkinson and Movement Disorders, Nijmegen, the Netherlands, said the study “provides robust evidence” that physical activity reduces risk for PD in women.
“These results show that the field is moving in the right direction and provide a clear rationale for exercise trials to prevent or delay the onset of manifest PD in at-risk individuals” they wrote.
The study highlights “gaps” in knowledge that merit closer attention and that “further insight is warranted on how much the effects on PD vary by type, intensity, frequency, and duration of physical activity,” the editorialists noted.
Another gap is how the accuracy of assessment of physical activity can be improved beyond self-report. “Wearable sensor technology now offers the potential to assess physical activity remotely and objectively in prevention trials,” they added.
Other areas that need exploring relate to mechanisms by which physical activity reduces PD risk, and to what extent effects of physical activity vary between individuals, Dr. Chahine and Dr. Darweesh noted.
Commenting for this article, Michael S. Okun, MD, executive director of the Fixel Institute for Neurological Diseases at University of Florida Health, and medical adviser for the Parkinson’s Foundation, said the findings are “significant and important.”
Based on only a handful of previous studies, it was assumed that physical activity was associated with reduced Parkinson’s diagnosis only in men, said Dr. Okun. “The current dataset was larger and included longer-term outcomes, and it informs the field that exercise may be important for reducing the risk of Parkinson’s disease in men as well as in women.”
The investigators, the editorialists, and Dr. Okun reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY
Parkinson’s in Marines linked to toxic drinking water at Camp Lejeune
in Jacksonville, N.C.
In one of the best-documented, large-scale contaminations in U.S. history, the drinking water at the Marine Corps base was contaminated with TCE and other volatile organic compounds from about 1953 to 1987.
The new study of more than 340,000 service members found the risk of PD was 70% higher in Marines stationed at Camp Lejeune in North Carolina during the years 1975-1985, compared with Marines stationed at Camp Pendleton in Oceanside, Calif.
“This is by far the largest study to look at the association of TCE and PD and the evidence is pretty strong,” lead investigator Samuel M. Goldman, MD, MPH, with University of California, San Francisco, said in an interview.
The link is supported by animal models that show that TCE can induce a neurodegenerative syndrome that is “very similar pathologically to what we see in PD,” Dr. Goldman said.
The study was published online in JAMA Neurology.
‘Hundreds of thousands’ at risk
At Camp Lejeune during the years 1975-1985, the period of maximal contamination, the estimated monthly median TCE level was more than 70-fold the Environmental Protection Agency maximum contaminant level. Maximum contaminant levels were also exceeded for perchloroethylene (PCE) and vinyl chloride.
Dr. Goldman and colleagues had health data on 158,122 veterans – 84,824 from Camp Lejeune and 73,298 from Camp Pendleton – who served for at least 3 months between 1975 and 1985, with follow up from Jan. 1, 1997, to Feb. 17, 2021.
Demographic characteristics were similar between the two groups; most were White men with an average age of 59 years.
A total of 430 veterans had PD: 279 from Camp Lejeune (prevalence, 0.33%) and 151 from Camp Pendleton (prevalence, 0.21%).
In multivariable models, Camp Lejeune veterans had a 70% higher risk for PD (odds ratio, 1.70; 95% confidence interval, 1.39-2.07; P < .001).
“Remarkably,” the researchers noted, among veterans without PD, residence at Camp Lejeune was also associated with a significantly higher risk of having several well-established prodromal features of PD, including tremor, suggesting they may be in a prediagnostic phase of evolving PD pathology.
Importantly, they added, in addition to the exposed service members, “hundreds of thousands of family members and civilian workers exposed to contaminated water at Camp Lejeune may also be at increased risk of PD, cancers, and other health consequences. Continued prospective follow-up of this population is essential.”
‘An unreasonable risk’
The new study supports a prior, and much smaller, study by Dr. Goldman and colleagues showing TCE exposure was associated with a sixfold increased risk for PD.
TCE is a ubiquitous environmental contaminant. The EPA Toxics Release Inventory estimates 2.05 million pounds of TCE was released into the environment from industrial sites in 2017.
In an accompanying editorial, E. Ray Dorsey, MD, with the University of Rochester (N.Y.) and coauthors noted the work of Dr. Goldman and colleagues “increases the certainty” that environmental exposure to TCE and the similar compound PCE “contribute importantly to the cause of the world’s fastest-growing brain disease.”
In December, the EPA found that PCE posed “an unreasonable risk” to human health, and 1 month later, it reached the same conclusion for TCE.
“These actions could lay the foundation for increased regulation and possibly a ban of these two chemicals that have contributed to immeasurable death and disability for generations,” Dr. Dorsey and colleagues noted.
“A U.S. ban would be a step forward but would not address the tens of thousands of TCE/PCE-contaminated sites in the U.S. and around the world or the rising global use of the toxic solvents,” they added.
This research was supported by Department of Veterans Affairs. Dr. Goldman reported no relevant financial relationships. Dr. Dorsey has received personal fees from organizations including the American Neurological Association, Elsevier, International Parkinson and Movement Disorder Society, Massachusetts Medical Society, Michael J. Fox Foundation, National Institutes of Health, and WebMD, as well as numerous pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
in Jacksonville, N.C.
In one of the best-documented, large-scale contaminations in U.S. history, the drinking water at the Marine Corps base was contaminated with TCE and other volatile organic compounds from about 1953 to 1987.
The new study of more than 340,000 service members found the risk of PD was 70% higher in Marines stationed at Camp Lejeune in North Carolina during the years 1975-1985, compared with Marines stationed at Camp Pendleton in Oceanside, Calif.
“This is by far the largest study to look at the association of TCE and PD and the evidence is pretty strong,” lead investigator Samuel M. Goldman, MD, MPH, with University of California, San Francisco, said in an interview.
The link is supported by animal models that show that TCE can induce a neurodegenerative syndrome that is “very similar pathologically to what we see in PD,” Dr. Goldman said.
The study was published online in JAMA Neurology.
‘Hundreds of thousands’ at risk
At Camp Lejeune during the years 1975-1985, the period of maximal contamination, the estimated monthly median TCE level was more than 70-fold the Environmental Protection Agency maximum contaminant level. Maximum contaminant levels were also exceeded for perchloroethylene (PCE) and vinyl chloride.
Dr. Goldman and colleagues had health data on 158,122 veterans – 84,824 from Camp Lejeune and 73,298 from Camp Pendleton – who served for at least 3 months between 1975 and 1985, with follow up from Jan. 1, 1997, to Feb. 17, 2021.
Demographic characteristics were similar between the two groups; most were White men with an average age of 59 years.
A total of 430 veterans had PD: 279 from Camp Lejeune (prevalence, 0.33%) and 151 from Camp Pendleton (prevalence, 0.21%).
In multivariable models, Camp Lejeune veterans had a 70% higher risk for PD (odds ratio, 1.70; 95% confidence interval, 1.39-2.07; P < .001).
“Remarkably,” the researchers noted, among veterans without PD, residence at Camp Lejeune was also associated with a significantly higher risk of having several well-established prodromal features of PD, including tremor, suggesting they may be in a prediagnostic phase of evolving PD pathology.
Importantly, they added, in addition to the exposed service members, “hundreds of thousands of family members and civilian workers exposed to contaminated water at Camp Lejeune may also be at increased risk of PD, cancers, and other health consequences. Continued prospective follow-up of this population is essential.”
‘An unreasonable risk’
The new study supports a prior, and much smaller, study by Dr. Goldman and colleagues showing TCE exposure was associated with a sixfold increased risk for PD.
TCE is a ubiquitous environmental contaminant. The EPA Toxics Release Inventory estimates 2.05 million pounds of TCE was released into the environment from industrial sites in 2017.
In an accompanying editorial, E. Ray Dorsey, MD, with the University of Rochester (N.Y.) and coauthors noted the work of Dr. Goldman and colleagues “increases the certainty” that environmental exposure to TCE and the similar compound PCE “contribute importantly to the cause of the world’s fastest-growing brain disease.”
In December, the EPA found that PCE posed “an unreasonable risk” to human health, and 1 month later, it reached the same conclusion for TCE.
“These actions could lay the foundation for increased regulation and possibly a ban of these two chemicals that have contributed to immeasurable death and disability for generations,” Dr. Dorsey and colleagues noted.
“A U.S. ban would be a step forward but would not address the tens of thousands of TCE/PCE-contaminated sites in the U.S. and around the world or the rising global use of the toxic solvents,” they added.
This research was supported by Department of Veterans Affairs. Dr. Goldman reported no relevant financial relationships. Dr. Dorsey has received personal fees from organizations including the American Neurological Association, Elsevier, International Parkinson and Movement Disorder Society, Massachusetts Medical Society, Michael J. Fox Foundation, National Institutes of Health, and WebMD, as well as numerous pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
in Jacksonville, N.C.
In one of the best-documented, large-scale contaminations in U.S. history, the drinking water at the Marine Corps base was contaminated with TCE and other volatile organic compounds from about 1953 to 1987.
The new study of more than 340,000 service members found the risk of PD was 70% higher in Marines stationed at Camp Lejeune in North Carolina during the years 1975-1985, compared with Marines stationed at Camp Pendleton in Oceanside, Calif.
“This is by far the largest study to look at the association of TCE and PD and the evidence is pretty strong,” lead investigator Samuel M. Goldman, MD, MPH, with University of California, San Francisco, said in an interview.
The link is supported by animal models that show that TCE can induce a neurodegenerative syndrome that is “very similar pathologically to what we see in PD,” Dr. Goldman said.
The study was published online in JAMA Neurology.
‘Hundreds of thousands’ at risk
At Camp Lejeune during the years 1975-1985, the period of maximal contamination, the estimated monthly median TCE level was more than 70-fold the Environmental Protection Agency maximum contaminant level. Maximum contaminant levels were also exceeded for perchloroethylene (PCE) and vinyl chloride.
Dr. Goldman and colleagues had health data on 158,122 veterans – 84,824 from Camp Lejeune and 73,298 from Camp Pendleton – who served for at least 3 months between 1975 and 1985, with follow up from Jan. 1, 1997, to Feb. 17, 2021.
Demographic characteristics were similar between the two groups; most were White men with an average age of 59 years.
A total of 430 veterans had PD: 279 from Camp Lejeune (prevalence, 0.33%) and 151 from Camp Pendleton (prevalence, 0.21%).
In multivariable models, Camp Lejeune veterans had a 70% higher risk for PD (odds ratio, 1.70; 95% confidence interval, 1.39-2.07; P < .001).
“Remarkably,” the researchers noted, among veterans without PD, residence at Camp Lejeune was also associated with a significantly higher risk of having several well-established prodromal features of PD, including tremor, suggesting they may be in a prediagnostic phase of evolving PD pathology.
Importantly, they added, in addition to the exposed service members, “hundreds of thousands of family members and civilian workers exposed to contaminated water at Camp Lejeune may also be at increased risk of PD, cancers, and other health consequences. Continued prospective follow-up of this population is essential.”
‘An unreasonable risk’
The new study supports a prior, and much smaller, study by Dr. Goldman and colleagues showing TCE exposure was associated with a sixfold increased risk for PD.
TCE is a ubiquitous environmental contaminant. The EPA Toxics Release Inventory estimates 2.05 million pounds of TCE was released into the environment from industrial sites in 2017.
In an accompanying editorial, E. Ray Dorsey, MD, with the University of Rochester (N.Y.) and coauthors noted the work of Dr. Goldman and colleagues “increases the certainty” that environmental exposure to TCE and the similar compound PCE “contribute importantly to the cause of the world’s fastest-growing brain disease.”
In December, the EPA found that PCE posed “an unreasonable risk” to human health, and 1 month later, it reached the same conclusion for TCE.
“These actions could lay the foundation for increased regulation and possibly a ban of these two chemicals that have contributed to immeasurable death and disability for generations,” Dr. Dorsey and colleagues noted.
“A U.S. ban would be a step forward but would not address the tens of thousands of TCE/PCE-contaminated sites in the U.S. and around the world or the rising global use of the toxic solvents,” they added.
This research was supported by Department of Veterans Affairs. Dr. Goldman reported no relevant financial relationships. Dr. Dorsey has received personal fees from organizations including the American Neurological Association, Elsevier, International Parkinson and Movement Disorder Society, Massachusetts Medical Society, Michael J. Fox Foundation, National Institutes of Health, and WebMD, as well as numerous pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY