User login
Infants Exposed to Minoxidil May Develop Hypertrichosis
OVIEDO, SPAIN – In April 2023, the Navarra Pharmacovigilance Center (NPC) became aware of a case involving an infant who developed progressive hair growth on their back, legs, and thighs (hypertrichosis) over the course of 2 months. During an interview with the family, it was revealed that the infant’s father had been using 5% topical minoxidil to treat androgenic alopecia, and he had taken a leave of absence from work to care for his child. After the medication was discontinued, the infant’s symptoms fully regressed. Specialists from the NPC presented the case at the 13th Spanish Pharmacovigilance Congress in November 2024.
A review of similar cases reported in the Spanish Pharmacovigilance System database identified six additional cases with the same characteristics, all involving infants whose caregivers were using minoxidil. Four more cases were found through the European pharmacovigilance database EudraVigilance and a review of scientific literature, bringing the total to 11 cases.
According to the Navarra Pharmacovigilance Bulletin, these cases are concerning as they highlight the exposure of vulnerable infants to a medication not indicated for their age group. Additionally, the condition can cause significant stress for the families of the affected children.
Mechanism of Transmission Unclear
In the newly identified cases, specialists suspect the drug was transmitted through direct or indirect contact. Accidental ingestion is also a possibility if the infant’s hands touched treated areas on the caregiver’s skin and were then brought to the mouth.
The NPC explained that infants’ skin is more permeable because of the thinner stratum corneum and a higher surface area/body weight ratio, making them more susceptible to absorbing topically applied medications.
Regulatory Changes and Precautions
In light of these findings, the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee concluded that, starting in October 2024, product information for medications containing minoxidil should be updated. Specifically, new information must be added to the package insert warning about the risk for hypertrichosis in infants following accidental exposure to minoxidil.
The NPC emphasizes the importance of caregivers being aware of the risks associated with topical medications like minoxidil. Recommended precautions include thoroughly washing hands after applying the product and covering treated areas to prevent direct contact with infants’ skin.
Healthcare professionals should also be aware of this risk and consider it when diagnosing hypertrichosis in infants. Recognizing the connection can prevent unnecessary testing for the infant and alleviate stress for the family.
This story was translated from Univadis Spain using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
OVIEDO, SPAIN – In April 2023, the Navarra Pharmacovigilance Center (NPC) became aware of a case involving an infant who developed progressive hair growth on their back, legs, and thighs (hypertrichosis) over the course of 2 months. During an interview with the family, it was revealed that the infant’s father had been using 5% topical minoxidil to treat androgenic alopecia, and he had taken a leave of absence from work to care for his child. After the medication was discontinued, the infant’s symptoms fully regressed. Specialists from the NPC presented the case at the 13th Spanish Pharmacovigilance Congress in November 2024.
A review of similar cases reported in the Spanish Pharmacovigilance System database identified six additional cases with the same characteristics, all involving infants whose caregivers were using minoxidil. Four more cases were found through the European pharmacovigilance database EudraVigilance and a review of scientific literature, bringing the total to 11 cases.
According to the Navarra Pharmacovigilance Bulletin, these cases are concerning as they highlight the exposure of vulnerable infants to a medication not indicated for their age group. Additionally, the condition can cause significant stress for the families of the affected children.
Mechanism of Transmission Unclear
In the newly identified cases, specialists suspect the drug was transmitted through direct or indirect contact. Accidental ingestion is also a possibility if the infant’s hands touched treated areas on the caregiver’s skin and were then brought to the mouth.
The NPC explained that infants’ skin is more permeable because of the thinner stratum corneum and a higher surface area/body weight ratio, making them more susceptible to absorbing topically applied medications.
Regulatory Changes and Precautions
In light of these findings, the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee concluded that, starting in October 2024, product information for medications containing minoxidil should be updated. Specifically, new information must be added to the package insert warning about the risk for hypertrichosis in infants following accidental exposure to minoxidil.
The NPC emphasizes the importance of caregivers being aware of the risks associated with topical medications like minoxidil. Recommended precautions include thoroughly washing hands after applying the product and covering treated areas to prevent direct contact with infants’ skin.
Healthcare professionals should also be aware of this risk and consider it when diagnosing hypertrichosis in infants. Recognizing the connection can prevent unnecessary testing for the infant and alleviate stress for the family.
This story was translated from Univadis Spain using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
OVIEDO, SPAIN – In April 2023, the Navarra Pharmacovigilance Center (NPC) became aware of a case involving an infant who developed progressive hair growth on their back, legs, and thighs (hypertrichosis) over the course of 2 months. During an interview with the family, it was revealed that the infant’s father had been using 5% topical minoxidil to treat androgenic alopecia, and he had taken a leave of absence from work to care for his child. After the medication was discontinued, the infant’s symptoms fully regressed. Specialists from the NPC presented the case at the 13th Spanish Pharmacovigilance Congress in November 2024.
A review of similar cases reported in the Spanish Pharmacovigilance System database identified six additional cases with the same characteristics, all involving infants whose caregivers were using minoxidil. Four more cases were found through the European pharmacovigilance database EudraVigilance and a review of scientific literature, bringing the total to 11 cases.
According to the Navarra Pharmacovigilance Bulletin, these cases are concerning as they highlight the exposure of vulnerable infants to a medication not indicated for their age group. Additionally, the condition can cause significant stress for the families of the affected children.
Mechanism of Transmission Unclear
In the newly identified cases, specialists suspect the drug was transmitted through direct or indirect contact. Accidental ingestion is also a possibility if the infant’s hands touched treated areas on the caregiver’s skin and were then brought to the mouth.
The NPC explained that infants’ skin is more permeable because of the thinner stratum corneum and a higher surface area/body weight ratio, making them more susceptible to absorbing topically applied medications.
Regulatory Changes and Precautions
In light of these findings, the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee concluded that, starting in October 2024, product information for medications containing minoxidil should be updated. Specifically, new information must be added to the package insert warning about the risk for hypertrichosis in infants following accidental exposure to minoxidil.
The NPC emphasizes the importance of caregivers being aware of the risks associated with topical medications like minoxidil. Recommended precautions include thoroughly washing hands after applying the product and covering treated areas to prevent direct contact with infants’ skin.
Healthcare professionals should also be aware of this risk and consider it when diagnosing hypertrichosis in infants. Recognizing the connection can prevent unnecessary testing for the infant and alleviate stress for the family.
This story was translated from Univadis Spain using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM THE SPANISH PHARMACOVIGILANCE CONGRESS 2024
Inhaled Insulin Benefits Kids With Diabetes, Too
TOPLINE:
Mannkind expects to submit a request for a supplemental new drug application meeting to the Food and Drug Administration (FDA) for its inhaled human insulin (Afrezza Inhalation Powder). Currently indicated to improve glycemic control in adults with diabetes, the company announced 6-month results from its phase 3 INHALE-1 study of inhaled human insulin in children and adolescents aged 4-17.
METHODOLOGY:
- INHALE-1 is a 26-week, open-label clinical trial that randomized 230 subjects aged 4-17 years with type 1 or type 2 diabetes to either inhaled pre-meal insulin or multiple daily injections (MDI) of rapid-acting insulin analog, both in combination with basal insulin.
- The primary endpoint was a noninferior change in hemoglobin A1c levels, compared with MDI after 26 weeks.
- A 26-week extension phase in which all remaining MDI patients were switched to inhaled insulin is ongoing.
TAKEAWAY:
- In the full intent-to-treat (ITT) population analysis, the between-group difference in mean A1c change over 26 weeks exceeded the prespecified non-inferiority margin of 0.4% (0.435%), but this was largely driven by the variability of a single patient who didn’t adhere to the study protocol.
- A modified ITT analysis excluding that person did not exceed the predetermined threshold of 0.4% (0.370%), thereby establishing noninferiority of inhaled insulin with MDI.
- Over 26 weeks of treatment, there were no differences in lung function parameters between the treatment groups, with mean forced expiratory volume at 1 second (FEV1) at baseline vs 26 weeks of 2.901 liters (99.6% of predicted) vs 2.934 L (96.6%) in the inhaled insulin group and 2.948 L (102.3%) vs 2.957 (98%), respectively, in the MDI group.
- There were no differences between groups or concerns in other safety measures, including hypoglycemia.
IN PRACTICE:
“It was exciting to partner with MannKind and help lead this study to potentially expand the use of inhaled insulin, which is currently used successfully by many adults with diabetes, to a population that hasn’t had a treatment option other than injectable insulin in the history of their care,” said INHALE-1 investigator Roy W. Beck, MD, PhD, founder of the Jaeb Center for Health Research, Tampa, Florida.
“The 6-month results are clinically meaningful and show Afrezza as a potential future treatment option for a growing pediatric population living with type 1 and type 2 diabetes,” Beck added.
SOURCE:
The results of the study were announced at a Mannkind press release on December 16, 2024.
SAFETY INFORMATION:
Inhaled insulin is not recommended for the treatment of diabetic ketoacidosis or in patients who smoke or have recently stopped smoking.
Warning: Risk for acute bronchospasm in patients with chronic lung disease
- Acute bronchospasm has been observed in Afrezza-treated patients with asthma and chronic obstructive pulmonary disease (COPD)
- Afrezza is contraindicated in patients with chronic lung disease such as asthma or COPD
- Before initiating Afrezza, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients
- Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
DISCLOSURES:
This study was funded by MannKind.
A version of this article appeared on Medscape.com.
TOPLINE:
Mannkind expects to submit a request for a supplemental new drug application meeting to the Food and Drug Administration (FDA) for its inhaled human insulin (Afrezza Inhalation Powder). Currently indicated to improve glycemic control in adults with diabetes, the company announced 6-month results from its phase 3 INHALE-1 study of inhaled human insulin in children and adolescents aged 4-17.
METHODOLOGY:
- INHALE-1 is a 26-week, open-label clinical trial that randomized 230 subjects aged 4-17 years with type 1 or type 2 diabetes to either inhaled pre-meal insulin or multiple daily injections (MDI) of rapid-acting insulin analog, both in combination with basal insulin.
- The primary endpoint was a noninferior change in hemoglobin A1c levels, compared with MDI after 26 weeks.
- A 26-week extension phase in which all remaining MDI patients were switched to inhaled insulin is ongoing.
TAKEAWAY:
- In the full intent-to-treat (ITT) population analysis, the between-group difference in mean A1c change over 26 weeks exceeded the prespecified non-inferiority margin of 0.4% (0.435%), but this was largely driven by the variability of a single patient who didn’t adhere to the study protocol.
- A modified ITT analysis excluding that person did not exceed the predetermined threshold of 0.4% (0.370%), thereby establishing noninferiority of inhaled insulin with MDI.
- Over 26 weeks of treatment, there were no differences in lung function parameters between the treatment groups, with mean forced expiratory volume at 1 second (FEV1) at baseline vs 26 weeks of 2.901 liters (99.6% of predicted) vs 2.934 L (96.6%) in the inhaled insulin group and 2.948 L (102.3%) vs 2.957 (98%), respectively, in the MDI group.
- There were no differences between groups or concerns in other safety measures, including hypoglycemia.
IN PRACTICE:
“It was exciting to partner with MannKind and help lead this study to potentially expand the use of inhaled insulin, which is currently used successfully by many adults with diabetes, to a population that hasn’t had a treatment option other than injectable insulin in the history of their care,” said INHALE-1 investigator Roy W. Beck, MD, PhD, founder of the Jaeb Center for Health Research, Tampa, Florida.
“The 6-month results are clinically meaningful and show Afrezza as a potential future treatment option for a growing pediatric population living with type 1 and type 2 diabetes,” Beck added.
SOURCE:
The results of the study were announced at a Mannkind press release on December 16, 2024.
SAFETY INFORMATION:
Inhaled insulin is not recommended for the treatment of diabetic ketoacidosis or in patients who smoke or have recently stopped smoking.
Warning: Risk for acute bronchospasm in patients with chronic lung disease
- Acute bronchospasm has been observed in Afrezza-treated patients with asthma and chronic obstructive pulmonary disease (COPD)
- Afrezza is contraindicated in patients with chronic lung disease such as asthma or COPD
- Before initiating Afrezza, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients
- Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
DISCLOSURES:
This study was funded by MannKind.
A version of this article appeared on Medscape.com.
TOPLINE:
Mannkind expects to submit a request for a supplemental new drug application meeting to the Food and Drug Administration (FDA) for its inhaled human insulin (Afrezza Inhalation Powder). Currently indicated to improve glycemic control in adults with diabetes, the company announced 6-month results from its phase 3 INHALE-1 study of inhaled human insulin in children and adolescents aged 4-17.
METHODOLOGY:
- INHALE-1 is a 26-week, open-label clinical trial that randomized 230 subjects aged 4-17 years with type 1 or type 2 diabetes to either inhaled pre-meal insulin or multiple daily injections (MDI) of rapid-acting insulin analog, both in combination with basal insulin.
- The primary endpoint was a noninferior change in hemoglobin A1c levels, compared with MDI after 26 weeks.
- A 26-week extension phase in which all remaining MDI patients were switched to inhaled insulin is ongoing.
TAKEAWAY:
- In the full intent-to-treat (ITT) population analysis, the between-group difference in mean A1c change over 26 weeks exceeded the prespecified non-inferiority margin of 0.4% (0.435%), but this was largely driven by the variability of a single patient who didn’t adhere to the study protocol.
- A modified ITT analysis excluding that person did not exceed the predetermined threshold of 0.4% (0.370%), thereby establishing noninferiority of inhaled insulin with MDI.
- Over 26 weeks of treatment, there were no differences in lung function parameters between the treatment groups, with mean forced expiratory volume at 1 second (FEV1) at baseline vs 26 weeks of 2.901 liters (99.6% of predicted) vs 2.934 L (96.6%) in the inhaled insulin group and 2.948 L (102.3%) vs 2.957 (98%), respectively, in the MDI group.
- There were no differences between groups or concerns in other safety measures, including hypoglycemia.
IN PRACTICE:
“It was exciting to partner with MannKind and help lead this study to potentially expand the use of inhaled insulin, which is currently used successfully by many adults with diabetes, to a population that hasn’t had a treatment option other than injectable insulin in the history of their care,” said INHALE-1 investigator Roy W. Beck, MD, PhD, founder of the Jaeb Center for Health Research, Tampa, Florida.
“The 6-month results are clinically meaningful and show Afrezza as a potential future treatment option for a growing pediatric population living with type 1 and type 2 diabetes,” Beck added.
SOURCE:
The results of the study were announced at a Mannkind press release on December 16, 2024.
SAFETY INFORMATION:
Inhaled insulin is not recommended for the treatment of diabetic ketoacidosis or in patients who smoke or have recently stopped smoking.
Warning: Risk for acute bronchospasm in patients with chronic lung disease
- Acute bronchospasm has been observed in Afrezza-treated patients with asthma and chronic obstructive pulmonary disease (COPD)
- Afrezza is contraindicated in patients with chronic lung disease such as asthma or COPD
- Before initiating Afrezza, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients
- Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
DISCLOSURES:
This study was funded by MannKind.
A version of this article appeared on Medscape.com.
California Seeks Mental Health Warning Labels on Social Media
In the latest effort to address the mental health crisis among adolescents, legislation in California would require social media platforms to come with a “black box” mental health warning label.
Despite growing evidence linking young people’s use of social media to significant health risks including depression, anxiety, and suicidal thoughts, social media companies have failed to be transparent about the risks, said Assembly member Rebecca Bauer-Kahan (D-Orinda), who introduced Assembly Bill (AB) 56.
“AB 56 ensures that families are armed with clear, actionable information to understand these dangers and make decisions that prioritizes their children’s well-being,” she said in a press release.
Bauer-Kahan noted that 95% of teens report using at least one social media platform and that more than one third say they use social media almost constantly.
“There is a powerful profit motive to keep our young people hooked online and engaged and it is exploiting the human psychology with notifications, likes, endless scrolling, and algorithmic amplification that is harming our children every day,” she said at a press conference on December 9 announcing the bill.
, a sponsor of AB 56, said in a press release.
Speaking at the press conference, Bonta said social media has many “incredible benefits” from giving people an outlet of expression to providing access to critical information but “there is no disputing the fact, it can have an enormously detrimental and dangerous impact on our young people. You cannot debate that. Our children are suffering.”
If AB 56 is successful, he said social media platforms would be required to display a “black box warning” for all users that would appear upon the first use of a platform and weekly thereafter.
The proposed language for the warning label is: “The Surgeon General has advised that there are ample indicators that social media can have a profound risk of harm to the mental health and well-being of children and adolescents.”
“This warning label isn’t a panacea, we know that, but it is another tool in our toolbox. It’s one prong in what has to be a multi-pronged continued, coordinated effort to address this public health crisis,” Bonta said.
Reached for comment, Bonta’s office said sponsorship of the bill was informed by their ongoing work to create a safer online space for children and teens and by the US Surgeon General’s call to Congress to add warning labels to social media.
In June, US Surgeon General Vivek Murthy, MD, said a Surgeon General’s warning label is needed to address the mental health emergency among adolescents and noted that evidence from tobacco studies shows warning labels can increase awareness and change behavior. In September, the attorneys general of 42 states announced their support of the proposal.
Also in September, US Senators John Fetterman (D-PA) and Katie Britt (R-AL) introduced the Stop the Scroll Act to create a mental health warning label requirement for social media platforms.
In a controversial move in November, Australia passed the world’s first law banning social media for children younger than 16 years. The law gives platforms such as TikTok, Facebook, X, Snapchat, and Instagram 1 year to figure out how to implement the ban before facing fines of up 50 million Australian dollars ($33 million) for systemic failures to prevent children younger than 16 years from holding accounts.
‘A Broken Fire Alarm’
“Slapping a warning label on social media is like a broken fire alarm going off with no evidence of smoke. It ignores the reality that most teens view social media as an important outlet for social connection,” Todd O’Boyle, with the tech industry policy group Chamber of Progress, said in a statement on AB 56.
He highlighted a 2022 Pew Research Center survey reporting that most teens credit social media with deepening connections and providing a support network and a 2020 study reporting that social media is not a strong or consistent risk factor for depressive symptoms in US adolescents.
O’Boyle predicted that, without strong evidence, AB 56 will run into the same “First Amendment buzzsaw” that has doomed other California kids’ bills.
Pediatrician Jason Nagata, MD, University of California San Francisco, points out in Bonta’s press release that social media can displace time for other healthful activities including sleep, exercise, and in-person socialization.
“While social media can provide educational content, it can also provide misinformation about health and expose children to content that damages their mental well-being. These are risks that adolescents and their parents should be aware of,” Nagata said.
Indeed, a tearful Victoria Hinks of Larkspur, California, spoke at the press conference of her 16-year-old daughter, Alexandra, who committed suicide 4 months ago after being sucked into social media and served content on self-harm, eating disorders, suicidal ideation, and glamorization of suicide.
“She was led down dark rabbit holes she had no hope of escaping,” Hinks said. “There is not a bone in my body that doubts social media played a role leading her to that final irreversible decision.”
Jim Steyer, CEO and founder of Common Sense Media, applauded California for being the first state to introduce social media warning label legislation. The group plans to lobby for similar proposals in other states he said at the press conference, noting that there are “tens of thousands of Alexandras out there.”
“We have seat belt laws, we have warning labels on cigarettes and alcohol, and that’s what we’re doing here,” Steyer said. “It’s a straightforward simple proposition, which is put your kids and teenagers first, put their self-interest first and hold the largest, most powerful, and wealthy companies in all of our lifetimes accountable for the harms that have happened on their platforms.”
A version of this article first appeared on Medscape.com.
In the latest effort to address the mental health crisis among adolescents, legislation in California would require social media platforms to come with a “black box” mental health warning label.
Despite growing evidence linking young people’s use of social media to significant health risks including depression, anxiety, and suicidal thoughts, social media companies have failed to be transparent about the risks, said Assembly member Rebecca Bauer-Kahan (D-Orinda), who introduced Assembly Bill (AB) 56.
“AB 56 ensures that families are armed with clear, actionable information to understand these dangers and make decisions that prioritizes their children’s well-being,” she said in a press release.
Bauer-Kahan noted that 95% of teens report using at least one social media platform and that more than one third say they use social media almost constantly.
“There is a powerful profit motive to keep our young people hooked online and engaged and it is exploiting the human psychology with notifications, likes, endless scrolling, and algorithmic amplification that is harming our children every day,” she said at a press conference on December 9 announcing the bill.
, a sponsor of AB 56, said in a press release.
Speaking at the press conference, Bonta said social media has many “incredible benefits” from giving people an outlet of expression to providing access to critical information but “there is no disputing the fact, it can have an enormously detrimental and dangerous impact on our young people. You cannot debate that. Our children are suffering.”
If AB 56 is successful, he said social media platforms would be required to display a “black box warning” for all users that would appear upon the first use of a platform and weekly thereafter.
The proposed language for the warning label is: “The Surgeon General has advised that there are ample indicators that social media can have a profound risk of harm to the mental health and well-being of children and adolescents.”
“This warning label isn’t a panacea, we know that, but it is another tool in our toolbox. It’s one prong in what has to be a multi-pronged continued, coordinated effort to address this public health crisis,” Bonta said.
Reached for comment, Bonta’s office said sponsorship of the bill was informed by their ongoing work to create a safer online space for children and teens and by the US Surgeon General’s call to Congress to add warning labels to social media.
In June, US Surgeon General Vivek Murthy, MD, said a Surgeon General’s warning label is needed to address the mental health emergency among adolescents and noted that evidence from tobacco studies shows warning labels can increase awareness and change behavior. In September, the attorneys general of 42 states announced their support of the proposal.
Also in September, US Senators John Fetterman (D-PA) and Katie Britt (R-AL) introduced the Stop the Scroll Act to create a mental health warning label requirement for social media platforms.
In a controversial move in November, Australia passed the world’s first law banning social media for children younger than 16 years. The law gives platforms such as TikTok, Facebook, X, Snapchat, and Instagram 1 year to figure out how to implement the ban before facing fines of up 50 million Australian dollars ($33 million) for systemic failures to prevent children younger than 16 years from holding accounts.
‘A Broken Fire Alarm’
“Slapping a warning label on social media is like a broken fire alarm going off with no evidence of smoke. It ignores the reality that most teens view social media as an important outlet for social connection,” Todd O’Boyle, with the tech industry policy group Chamber of Progress, said in a statement on AB 56.
He highlighted a 2022 Pew Research Center survey reporting that most teens credit social media with deepening connections and providing a support network and a 2020 study reporting that social media is not a strong or consistent risk factor for depressive symptoms in US adolescents.
O’Boyle predicted that, without strong evidence, AB 56 will run into the same “First Amendment buzzsaw” that has doomed other California kids’ bills.
Pediatrician Jason Nagata, MD, University of California San Francisco, points out in Bonta’s press release that social media can displace time for other healthful activities including sleep, exercise, and in-person socialization.
“While social media can provide educational content, it can also provide misinformation about health and expose children to content that damages their mental well-being. These are risks that adolescents and their parents should be aware of,” Nagata said.
Indeed, a tearful Victoria Hinks of Larkspur, California, spoke at the press conference of her 16-year-old daughter, Alexandra, who committed suicide 4 months ago after being sucked into social media and served content on self-harm, eating disorders, suicidal ideation, and glamorization of suicide.
“She was led down dark rabbit holes she had no hope of escaping,” Hinks said. “There is not a bone in my body that doubts social media played a role leading her to that final irreversible decision.”
Jim Steyer, CEO and founder of Common Sense Media, applauded California for being the first state to introduce social media warning label legislation. The group plans to lobby for similar proposals in other states he said at the press conference, noting that there are “tens of thousands of Alexandras out there.”
“We have seat belt laws, we have warning labels on cigarettes and alcohol, and that’s what we’re doing here,” Steyer said. “It’s a straightforward simple proposition, which is put your kids and teenagers first, put their self-interest first and hold the largest, most powerful, and wealthy companies in all of our lifetimes accountable for the harms that have happened on their platforms.”
A version of this article first appeared on Medscape.com.
In the latest effort to address the mental health crisis among adolescents, legislation in California would require social media platforms to come with a “black box” mental health warning label.
Despite growing evidence linking young people’s use of social media to significant health risks including depression, anxiety, and suicidal thoughts, social media companies have failed to be transparent about the risks, said Assembly member Rebecca Bauer-Kahan (D-Orinda), who introduced Assembly Bill (AB) 56.
“AB 56 ensures that families are armed with clear, actionable information to understand these dangers and make decisions that prioritizes their children’s well-being,” she said in a press release.
Bauer-Kahan noted that 95% of teens report using at least one social media platform and that more than one third say they use social media almost constantly.
“There is a powerful profit motive to keep our young people hooked online and engaged and it is exploiting the human psychology with notifications, likes, endless scrolling, and algorithmic amplification that is harming our children every day,” she said at a press conference on December 9 announcing the bill.
, a sponsor of AB 56, said in a press release.
Speaking at the press conference, Bonta said social media has many “incredible benefits” from giving people an outlet of expression to providing access to critical information but “there is no disputing the fact, it can have an enormously detrimental and dangerous impact on our young people. You cannot debate that. Our children are suffering.”
If AB 56 is successful, he said social media platforms would be required to display a “black box warning” for all users that would appear upon the first use of a platform and weekly thereafter.
The proposed language for the warning label is: “The Surgeon General has advised that there are ample indicators that social media can have a profound risk of harm to the mental health and well-being of children and adolescents.”
“This warning label isn’t a panacea, we know that, but it is another tool in our toolbox. It’s one prong in what has to be a multi-pronged continued, coordinated effort to address this public health crisis,” Bonta said.
Reached for comment, Bonta’s office said sponsorship of the bill was informed by their ongoing work to create a safer online space for children and teens and by the US Surgeon General’s call to Congress to add warning labels to social media.
In June, US Surgeon General Vivek Murthy, MD, said a Surgeon General’s warning label is needed to address the mental health emergency among adolescents and noted that evidence from tobacco studies shows warning labels can increase awareness and change behavior. In September, the attorneys general of 42 states announced their support of the proposal.
Also in September, US Senators John Fetterman (D-PA) and Katie Britt (R-AL) introduced the Stop the Scroll Act to create a mental health warning label requirement for social media platforms.
In a controversial move in November, Australia passed the world’s first law banning social media for children younger than 16 years. The law gives platforms such as TikTok, Facebook, X, Snapchat, and Instagram 1 year to figure out how to implement the ban before facing fines of up 50 million Australian dollars ($33 million) for systemic failures to prevent children younger than 16 years from holding accounts.
‘A Broken Fire Alarm’
“Slapping a warning label on social media is like a broken fire alarm going off with no evidence of smoke. It ignores the reality that most teens view social media as an important outlet for social connection,” Todd O’Boyle, with the tech industry policy group Chamber of Progress, said in a statement on AB 56.
He highlighted a 2022 Pew Research Center survey reporting that most teens credit social media with deepening connections and providing a support network and a 2020 study reporting that social media is not a strong or consistent risk factor for depressive symptoms in US adolescents.
O’Boyle predicted that, without strong evidence, AB 56 will run into the same “First Amendment buzzsaw” that has doomed other California kids’ bills.
Pediatrician Jason Nagata, MD, University of California San Francisco, points out in Bonta’s press release that social media can displace time for other healthful activities including sleep, exercise, and in-person socialization.
“While social media can provide educational content, it can also provide misinformation about health and expose children to content that damages their mental well-being. These are risks that adolescents and their parents should be aware of,” Nagata said.
Indeed, a tearful Victoria Hinks of Larkspur, California, spoke at the press conference of her 16-year-old daughter, Alexandra, who committed suicide 4 months ago after being sucked into social media and served content on self-harm, eating disorders, suicidal ideation, and glamorization of suicide.
“She was led down dark rabbit holes she had no hope of escaping,” Hinks said. “There is not a bone in my body that doubts social media played a role leading her to that final irreversible decision.”
Jim Steyer, CEO and founder of Common Sense Media, applauded California for being the first state to introduce social media warning label legislation. The group plans to lobby for similar proposals in other states he said at the press conference, noting that there are “tens of thousands of Alexandras out there.”
“We have seat belt laws, we have warning labels on cigarettes and alcohol, and that’s what we’re doing here,” Steyer said. “It’s a straightforward simple proposition, which is put your kids and teenagers first, put their self-interest first and hold the largest, most powerful, and wealthy companies in all of our lifetimes accountable for the harms that have happened on their platforms.”
A version of this article first appeared on Medscape.com.
Topical Tapinarof Approved for Treating Atopic Dermatitis, Ages 2 and Up
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
Upfront Therapy for ITP in Children: New Drug a Game-Changer?
“This is the first time in 30 years that a new drug is being tested for newly diagnosed pediatric ITP,” said the study’s lead author, Kristin A. Shimano, MD, professor of pediatrics at the Benioff Children’s Hospital, University of California San Francisco, in a press statement for the study, presented at the American Society of Hematology (ASH) 2024 Annual Meeting earlier this month.
“We really think that this has the potential to transform the approach to the management of ITP in the newly diagnosed phase with the use of a therapy that can provide sustained hemostatic platelet counts to bridge the time that patients are at risk of bleeding events with the goal to wean off the medication for patients who have a natural resolution of their disease,” Shimano said in her talk.
While children with ITP, a rare autoimmune blood disorder, very often improve without the need for any treatment, some do require intervention, and the condition can become chronic. First-line therapies for those patients commonly include corticosteroids, intravenous immunoglobulin (IVIg), and anti-D globulin; however, side effects can be undesirable, and with their efficacy often temporary, patients can require monitoring and juggling of treatments.
Eltrombopag, an oral, daily thrombopoietin receptor agonist, was approved by the US Food and Drug Administration for children and adults with chronic ITP in 2015; however, research has been lacking on the benefits of the therapy for newly diagnosed pediatric patients.
To investigate the drug’s efficacy at that stage, Shimano and colleagues with the ITP Consortium of North America launched the prospective, open-label Pediatric ITP Newly diagnosed pts Epag vs Standard therapy (PINES) trial, enrolling 118 patients at 23 institutions between May 2019 and January 2024.
All enrollees had been diagnosed with ITP within 3 months and had been determined by their treating hematologist to require pharmacologic treatment.
Of the patients, about 40% were untreated, and 60% had been treated with at least one medication prior to the trial but did not have a lasting response.
The patients were stratified by age and prior treatment and randomized 2:1 to receive either eltrombopag (n = 78) or the investigator’s choice of one of three standard first-line therapies, including prednisone, IVIg, or anti-D globulin at specified doses (n = 40). Overall, 29 in the standard-of-care arm received prednisone and 11 received IVIg. The patients had a median age of 8 years.
For the study’s primary endpoint, patients in the eltrombopag group had a significantly greater sustained response at 12 weeks, defined as having at least three of four platelet counts > 50 × 109/L during weeks 6-12 without the need for rescue treatment, with a rate of 63% vs 35% in the standard-of-care group (P = .0054).
There were no significant differences between the two groups in terms of the proportion of patients with a high bleeding score at weeks 1-4 and week 12.
However, those in the eltrombopag arm had a significantly lower rate of receiving rescue therapy (18% vs 38% with the standard of care; P = .02).
Both groups showed clinically meaningful improvements from baseline in terms of health-related quality of life, as assessed by parent proxy-reported KIT overall scores.
Twenty adverse events that were grade 3 or higher, including six serious adverse events, occurred in each of the study’s arms, with the most common events including headache and epistaxis.
Treatment-related serious adverse events occurred among six patients in the eltrombopag group and one in the control group, but importantly, no thromboembolic events were reported.
One intracranial hemorrhage occurred in the eltrombopag arm.
With eltrombopag having a slower effect than some other treatments, Shimano cautioned that the therapy is not recommended for patients with severe bleeding.
“Patients with grade 4 or 5 bleeding at the time of screening were specifically excluded from the study, so for patients who have very severe bleeding who need to get their platelets up very quickly, this would not be the ideal therapy for them,” she noted.
On the basis of results, the trial was recommended to close early due to efficacy; however, the participants are being followed for a total of 12 months to determine the durability of the responses, including in terms of bleeding events, quality of life, or the development of chronic ITP.
“We have shown that in pediatric patients with newly diagnosed ITP requiring pharmacologic treatment, eltrombopag resulted in a significant, clinically relevant higher rate of a durable platelet response in the absence of rescue treatment as compared with standard first-line therapies,” Shimano said.
“Eltrombopag could certainly be added to the medication choices hematologists consider as they are making treatment decisions with families, and it is an option that could potentially raise platelets for a more sustained period in children with ITP in the newly diagnosed period, which is one of the most difficult times for patients with regard to the impact of the disease on bleeding symptoms and quality of life,” she added.
Commenting on the study, James B. Bussel, MD, emeritus professor of pediatrics, medicine and obstetrics and gynecology at Weill Cornell Medicine in New York City, commented that “generally, a short-term increase in platelets is the biggest challenge, which is getting the patient to the point of not requiring future treatment to get better.”
“If more children can be shown to be going into remission earlier, that would be great,” he said.
While eltrombopag is known to be effective in chronic ITP, a key caveat of its use in newly diagnosed patients is the question of whether patients will get better on their own and feasibly be able to be spared the cost and burden of treatment in the first place.
However, identifying which patients will fit that profile isn’t always easy.
“Exactly which child needs treatment can be hard to determine, and there is some debate about that,” Bussel noted.
“The theoretic standard is that the platelet count doesn’t matter — only whether the patient is bleeding a lot, and then there is debate over treatment based on bleeding scores,” he said.
Quality-of-life issues, such as patients’ ability to take part in activities, are also a key consideration.
“It would be great if eltrombopag can support children who really need it and provide clear unequivocal benefit beyond just increasing the platelet count, but also leading to better quality of life,” Bussel said.
The new findings are “a very encouraging start, but I’d really like to see what the story is at 1 year.”
The study was funded by Novartis, maker of eltrombopag, and sponsored by the ITP Consortium of North America. Shimano disclosed ties with Sanofi, Sobi, Daiichi Sankyo, Novartis, and Pfizer. Bussel reported a relationship with Novartis that ended more than 2 years ago.
A version of this article first appeared on Medscape.com.
“This is the first time in 30 years that a new drug is being tested for newly diagnosed pediatric ITP,” said the study’s lead author, Kristin A. Shimano, MD, professor of pediatrics at the Benioff Children’s Hospital, University of California San Francisco, in a press statement for the study, presented at the American Society of Hematology (ASH) 2024 Annual Meeting earlier this month.
“We really think that this has the potential to transform the approach to the management of ITP in the newly diagnosed phase with the use of a therapy that can provide sustained hemostatic platelet counts to bridge the time that patients are at risk of bleeding events with the goal to wean off the medication for patients who have a natural resolution of their disease,” Shimano said in her talk.
While children with ITP, a rare autoimmune blood disorder, very often improve without the need for any treatment, some do require intervention, and the condition can become chronic. First-line therapies for those patients commonly include corticosteroids, intravenous immunoglobulin (IVIg), and anti-D globulin; however, side effects can be undesirable, and with their efficacy often temporary, patients can require monitoring and juggling of treatments.
Eltrombopag, an oral, daily thrombopoietin receptor agonist, was approved by the US Food and Drug Administration for children and adults with chronic ITP in 2015; however, research has been lacking on the benefits of the therapy for newly diagnosed pediatric patients.
To investigate the drug’s efficacy at that stage, Shimano and colleagues with the ITP Consortium of North America launched the prospective, open-label Pediatric ITP Newly diagnosed pts Epag vs Standard therapy (PINES) trial, enrolling 118 patients at 23 institutions between May 2019 and January 2024.
All enrollees had been diagnosed with ITP within 3 months and had been determined by their treating hematologist to require pharmacologic treatment.
Of the patients, about 40% were untreated, and 60% had been treated with at least one medication prior to the trial but did not have a lasting response.
The patients were stratified by age and prior treatment and randomized 2:1 to receive either eltrombopag (n = 78) or the investigator’s choice of one of three standard first-line therapies, including prednisone, IVIg, or anti-D globulin at specified doses (n = 40). Overall, 29 in the standard-of-care arm received prednisone and 11 received IVIg. The patients had a median age of 8 years.
For the study’s primary endpoint, patients in the eltrombopag group had a significantly greater sustained response at 12 weeks, defined as having at least three of four platelet counts > 50 × 109/L during weeks 6-12 without the need for rescue treatment, with a rate of 63% vs 35% in the standard-of-care group (P = .0054).
There were no significant differences between the two groups in terms of the proportion of patients with a high bleeding score at weeks 1-4 and week 12.
However, those in the eltrombopag arm had a significantly lower rate of receiving rescue therapy (18% vs 38% with the standard of care; P = .02).
Both groups showed clinically meaningful improvements from baseline in terms of health-related quality of life, as assessed by parent proxy-reported KIT overall scores.
Twenty adverse events that were grade 3 or higher, including six serious adverse events, occurred in each of the study’s arms, with the most common events including headache and epistaxis.
Treatment-related serious adverse events occurred among six patients in the eltrombopag group and one in the control group, but importantly, no thromboembolic events were reported.
One intracranial hemorrhage occurred in the eltrombopag arm.
With eltrombopag having a slower effect than some other treatments, Shimano cautioned that the therapy is not recommended for patients with severe bleeding.
“Patients with grade 4 or 5 bleeding at the time of screening were specifically excluded from the study, so for patients who have very severe bleeding who need to get their platelets up very quickly, this would not be the ideal therapy for them,” she noted.
On the basis of results, the trial was recommended to close early due to efficacy; however, the participants are being followed for a total of 12 months to determine the durability of the responses, including in terms of bleeding events, quality of life, or the development of chronic ITP.
“We have shown that in pediatric patients with newly diagnosed ITP requiring pharmacologic treatment, eltrombopag resulted in a significant, clinically relevant higher rate of a durable platelet response in the absence of rescue treatment as compared with standard first-line therapies,” Shimano said.
“Eltrombopag could certainly be added to the medication choices hematologists consider as they are making treatment decisions with families, and it is an option that could potentially raise platelets for a more sustained period in children with ITP in the newly diagnosed period, which is one of the most difficult times for patients with regard to the impact of the disease on bleeding symptoms and quality of life,” she added.
Commenting on the study, James B. Bussel, MD, emeritus professor of pediatrics, medicine and obstetrics and gynecology at Weill Cornell Medicine in New York City, commented that “generally, a short-term increase in platelets is the biggest challenge, which is getting the patient to the point of not requiring future treatment to get better.”
“If more children can be shown to be going into remission earlier, that would be great,” he said.
While eltrombopag is known to be effective in chronic ITP, a key caveat of its use in newly diagnosed patients is the question of whether patients will get better on their own and feasibly be able to be spared the cost and burden of treatment in the first place.
However, identifying which patients will fit that profile isn’t always easy.
“Exactly which child needs treatment can be hard to determine, and there is some debate about that,” Bussel noted.
“The theoretic standard is that the platelet count doesn’t matter — only whether the patient is bleeding a lot, and then there is debate over treatment based on bleeding scores,” he said.
Quality-of-life issues, such as patients’ ability to take part in activities, are also a key consideration.
“It would be great if eltrombopag can support children who really need it and provide clear unequivocal benefit beyond just increasing the platelet count, but also leading to better quality of life,” Bussel said.
The new findings are “a very encouraging start, but I’d really like to see what the story is at 1 year.”
The study was funded by Novartis, maker of eltrombopag, and sponsored by the ITP Consortium of North America. Shimano disclosed ties with Sanofi, Sobi, Daiichi Sankyo, Novartis, and Pfizer. Bussel reported a relationship with Novartis that ended more than 2 years ago.
A version of this article first appeared on Medscape.com.
“This is the first time in 30 years that a new drug is being tested for newly diagnosed pediatric ITP,” said the study’s lead author, Kristin A. Shimano, MD, professor of pediatrics at the Benioff Children’s Hospital, University of California San Francisco, in a press statement for the study, presented at the American Society of Hematology (ASH) 2024 Annual Meeting earlier this month.
“We really think that this has the potential to transform the approach to the management of ITP in the newly diagnosed phase with the use of a therapy that can provide sustained hemostatic platelet counts to bridge the time that patients are at risk of bleeding events with the goal to wean off the medication for patients who have a natural resolution of their disease,” Shimano said in her talk.
While children with ITP, a rare autoimmune blood disorder, very often improve without the need for any treatment, some do require intervention, and the condition can become chronic. First-line therapies for those patients commonly include corticosteroids, intravenous immunoglobulin (IVIg), and anti-D globulin; however, side effects can be undesirable, and with their efficacy often temporary, patients can require monitoring and juggling of treatments.
Eltrombopag, an oral, daily thrombopoietin receptor agonist, was approved by the US Food and Drug Administration for children and adults with chronic ITP in 2015; however, research has been lacking on the benefits of the therapy for newly diagnosed pediatric patients.
To investigate the drug’s efficacy at that stage, Shimano and colleagues with the ITP Consortium of North America launched the prospective, open-label Pediatric ITP Newly diagnosed pts Epag vs Standard therapy (PINES) trial, enrolling 118 patients at 23 institutions between May 2019 and January 2024.
All enrollees had been diagnosed with ITP within 3 months and had been determined by their treating hematologist to require pharmacologic treatment.
Of the patients, about 40% were untreated, and 60% had been treated with at least one medication prior to the trial but did not have a lasting response.
The patients were stratified by age and prior treatment and randomized 2:1 to receive either eltrombopag (n = 78) or the investigator’s choice of one of three standard first-line therapies, including prednisone, IVIg, or anti-D globulin at specified doses (n = 40). Overall, 29 in the standard-of-care arm received prednisone and 11 received IVIg. The patients had a median age of 8 years.
For the study’s primary endpoint, patients in the eltrombopag group had a significantly greater sustained response at 12 weeks, defined as having at least three of four platelet counts > 50 × 109/L during weeks 6-12 without the need for rescue treatment, with a rate of 63% vs 35% in the standard-of-care group (P = .0054).
There were no significant differences between the two groups in terms of the proportion of patients with a high bleeding score at weeks 1-4 and week 12.
However, those in the eltrombopag arm had a significantly lower rate of receiving rescue therapy (18% vs 38% with the standard of care; P = .02).
Both groups showed clinically meaningful improvements from baseline in terms of health-related quality of life, as assessed by parent proxy-reported KIT overall scores.
Twenty adverse events that were grade 3 or higher, including six serious adverse events, occurred in each of the study’s arms, with the most common events including headache and epistaxis.
Treatment-related serious adverse events occurred among six patients in the eltrombopag group and one in the control group, but importantly, no thromboembolic events were reported.
One intracranial hemorrhage occurred in the eltrombopag arm.
With eltrombopag having a slower effect than some other treatments, Shimano cautioned that the therapy is not recommended for patients with severe bleeding.
“Patients with grade 4 or 5 bleeding at the time of screening were specifically excluded from the study, so for patients who have very severe bleeding who need to get their platelets up very quickly, this would not be the ideal therapy for them,” she noted.
On the basis of results, the trial was recommended to close early due to efficacy; however, the participants are being followed for a total of 12 months to determine the durability of the responses, including in terms of bleeding events, quality of life, or the development of chronic ITP.
“We have shown that in pediatric patients with newly diagnosed ITP requiring pharmacologic treatment, eltrombopag resulted in a significant, clinically relevant higher rate of a durable platelet response in the absence of rescue treatment as compared with standard first-line therapies,” Shimano said.
“Eltrombopag could certainly be added to the medication choices hematologists consider as they are making treatment decisions with families, and it is an option that could potentially raise platelets for a more sustained period in children with ITP in the newly diagnosed period, which is one of the most difficult times for patients with regard to the impact of the disease on bleeding symptoms and quality of life,” she added.
Commenting on the study, James B. Bussel, MD, emeritus professor of pediatrics, medicine and obstetrics and gynecology at Weill Cornell Medicine in New York City, commented that “generally, a short-term increase in platelets is the biggest challenge, which is getting the patient to the point of not requiring future treatment to get better.”
“If more children can be shown to be going into remission earlier, that would be great,” he said.
While eltrombopag is known to be effective in chronic ITP, a key caveat of its use in newly diagnosed patients is the question of whether patients will get better on their own and feasibly be able to be spared the cost and burden of treatment in the first place.
However, identifying which patients will fit that profile isn’t always easy.
“Exactly which child needs treatment can be hard to determine, and there is some debate about that,” Bussel noted.
“The theoretic standard is that the platelet count doesn’t matter — only whether the patient is bleeding a lot, and then there is debate over treatment based on bleeding scores,” he said.
Quality-of-life issues, such as patients’ ability to take part in activities, are also a key consideration.
“It would be great if eltrombopag can support children who really need it and provide clear unequivocal benefit beyond just increasing the platelet count, but also leading to better quality of life,” Bussel said.
The new findings are “a very encouraging start, but I’d really like to see what the story is at 1 year.”
The study was funded by Novartis, maker of eltrombopag, and sponsored by the ITP Consortium of North America. Shimano disclosed ties with Sanofi, Sobi, Daiichi Sankyo, Novartis, and Pfizer. Bussel reported a relationship with Novartis that ended more than 2 years ago.
A version of this article first appeared on Medscape.com.
FROM ASH 2024
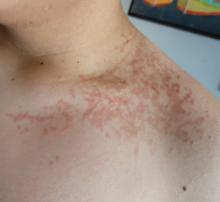
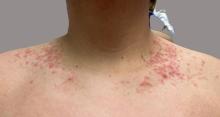
A 16-Year-Old Hispanic Male with a History of Hyperlipidemia Reports a Pruritic Rash on His Neck and Chest
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Case Report
A 16-year-old Hispanic male with a history of hyperlipidemia presents to his pediatrician's office for a routine well-child check-up. He reports a pruritic rash on his neck and chest that has been present for the past 1.5 weeks. The rash is itchy, and although a cream from Mexico initially helped, it has not been effective recently. The patient mentions that he has increased his gym workouts and has been training for basketball. He has a history of obesity but has lost almost 100 pounds in the last 6 months. Most recently, he has stopped consuming carbohydrates and has been fasting in the mornings.
There is no history of eczema or psoriasis, either in the patient or his family.
Physical Examination
The patient weighs 147 pounds, a significant decrease from his previous weight of 270 pounds 6 months ago. Other vital signs are within normal limits.
On physical examination, the patient presents with net-like, pink, scaly plaques on his neck, with no other rashes on the body (see Pictures 1 and 2).
Survey Highlights Trends in Pediatric Cosmetic Dermatology Procedures
TOPLINE:
METHODOLOGY:
- An anonymous online survey conducted with SurveyMonkey targeted healthcare providers who routinely used lasers to treat cutaneous conditions in pediatric patients.
- The survey included members of the Society for Pediatric Dermatology and the American Society for Laser Medicine and Surgery and Surgery, as well as fellowship directors and current fellows of the American Society for Dermatologic Surgery.
- A total of 85 practitioners responded to the survey, with 86% answering all questions; respondents primarily included pediatric dermatologists (77.65%), general dermatologists (18.82%), cosmetic dermatologists (8.24%), and dermatologic/Mohs surgeons (1.18%).
TAKEAWAY:
- Hypertrophic or traumatic scars ranked as the most frequently treated pediatric cosmetic condition (95.29%), followed by acne (89.41%), axillary and facial hyperhidrosis (77.65%), hypertrichosis/hirsutism (67.06%), and pigmented lesion removal (64.71%).
- The most common procedures performed were vascular lasers (77.65%), laser hair removal (50.59%), lasers for pigmentation (28.24%), neuromodulators (25.88%), and laser skin resurfacing (22.35%).
- Additional treatments respondents performed included chemical peels (20.00%), radiofrequency microneedling (16.47%), soft tissue fillers (4.71%), and cryolipolysis/body contouring (4.17%).
- About 50% of respondents said they would start cosmetic treatment of acne, and about 66% said they would start laser hair removal treatment between the ages of 12 and 15 years.
IN PRACTICE:
Noting that the survey results provided insight into the types of cosmetic procedures being performed for pediatric patients, the authors wrote, “These interventions can play a significant role in addressing the emotional and social challenges faced by pediatric patients with cosmetic concerns, allowing them to navigate social interactions more confidently and positively.” Before any procedure, they added, “It is important that any comorbid conditions be addressed,” they added, and “ethical considerations regarding informed consent, patient autonomy, and long-term consequences should be carefully weighed, given the vulnerable nature of pediatric patients.”
SOURCE:
The study was led by Lauren Hoffman, MD, who practices dermatology in Great Neck, New York. It was published online in December 2024 in Dermatologic Surgery.
LIMITATIONS:
The study was subjective in nature and had a small sample size, and the exact number of survey recipients was unclear, hindering an accurate calculation of the response rate. The absolute number of responses accounted for a small portion of the total memberships of the participating societies. Also, the data collection periods varied among the three academic societies, and dermatologists’ practice types may have influenced the range and nature of treated conditions.
DISCLOSURES:
The authors did not disclose funding information. They declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- An anonymous online survey conducted with SurveyMonkey targeted healthcare providers who routinely used lasers to treat cutaneous conditions in pediatric patients.
- The survey included members of the Society for Pediatric Dermatology and the American Society for Laser Medicine and Surgery and Surgery, as well as fellowship directors and current fellows of the American Society for Dermatologic Surgery.
- A total of 85 practitioners responded to the survey, with 86% answering all questions; respondents primarily included pediatric dermatologists (77.65%), general dermatologists (18.82%), cosmetic dermatologists (8.24%), and dermatologic/Mohs surgeons (1.18%).
TAKEAWAY:
- Hypertrophic or traumatic scars ranked as the most frequently treated pediatric cosmetic condition (95.29%), followed by acne (89.41%), axillary and facial hyperhidrosis (77.65%), hypertrichosis/hirsutism (67.06%), and pigmented lesion removal (64.71%).
- The most common procedures performed were vascular lasers (77.65%), laser hair removal (50.59%), lasers for pigmentation (28.24%), neuromodulators (25.88%), and laser skin resurfacing (22.35%).
- Additional treatments respondents performed included chemical peels (20.00%), radiofrequency microneedling (16.47%), soft tissue fillers (4.71%), and cryolipolysis/body contouring (4.17%).
- About 50% of respondents said they would start cosmetic treatment of acne, and about 66% said they would start laser hair removal treatment between the ages of 12 and 15 years.
IN PRACTICE:
Noting that the survey results provided insight into the types of cosmetic procedures being performed for pediatric patients, the authors wrote, “These interventions can play a significant role in addressing the emotional and social challenges faced by pediatric patients with cosmetic concerns, allowing them to navigate social interactions more confidently and positively.” Before any procedure, they added, “It is important that any comorbid conditions be addressed,” they added, and “ethical considerations regarding informed consent, patient autonomy, and long-term consequences should be carefully weighed, given the vulnerable nature of pediatric patients.”
SOURCE:
The study was led by Lauren Hoffman, MD, who practices dermatology in Great Neck, New York. It was published online in December 2024 in Dermatologic Surgery.
LIMITATIONS:
The study was subjective in nature and had a small sample size, and the exact number of survey recipients was unclear, hindering an accurate calculation of the response rate. The absolute number of responses accounted for a small portion of the total memberships of the participating societies. Also, the data collection periods varied among the three academic societies, and dermatologists’ practice types may have influenced the range and nature of treated conditions.
DISCLOSURES:
The authors did not disclose funding information. They declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- An anonymous online survey conducted with SurveyMonkey targeted healthcare providers who routinely used lasers to treat cutaneous conditions in pediatric patients.
- The survey included members of the Society for Pediatric Dermatology and the American Society for Laser Medicine and Surgery and Surgery, as well as fellowship directors and current fellows of the American Society for Dermatologic Surgery.
- A total of 85 practitioners responded to the survey, with 86% answering all questions; respondents primarily included pediatric dermatologists (77.65%), general dermatologists (18.82%), cosmetic dermatologists (8.24%), and dermatologic/Mohs surgeons (1.18%).
TAKEAWAY:
- Hypertrophic or traumatic scars ranked as the most frequently treated pediatric cosmetic condition (95.29%), followed by acne (89.41%), axillary and facial hyperhidrosis (77.65%), hypertrichosis/hirsutism (67.06%), and pigmented lesion removal (64.71%).
- The most common procedures performed were vascular lasers (77.65%), laser hair removal (50.59%), lasers for pigmentation (28.24%), neuromodulators (25.88%), and laser skin resurfacing (22.35%).
- Additional treatments respondents performed included chemical peels (20.00%), radiofrequency microneedling (16.47%), soft tissue fillers (4.71%), and cryolipolysis/body contouring (4.17%).
- About 50% of respondents said they would start cosmetic treatment of acne, and about 66% said they would start laser hair removal treatment between the ages of 12 and 15 years.
IN PRACTICE:
Noting that the survey results provided insight into the types of cosmetic procedures being performed for pediatric patients, the authors wrote, “These interventions can play a significant role in addressing the emotional and social challenges faced by pediatric patients with cosmetic concerns, allowing them to navigate social interactions more confidently and positively.” Before any procedure, they added, “It is important that any comorbid conditions be addressed,” they added, and “ethical considerations regarding informed consent, patient autonomy, and long-term consequences should be carefully weighed, given the vulnerable nature of pediatric patients.”
SOURCE:
The study was led by Lauren Hoffman, MD, who practices dermatology in Great Neck, New York. It was published online in December 2024 in Dermatologic Surgery.
LIMITATIONS:
The study was subjective in nature and had a small sample size, and the exact number of survey recipients was unclear, hindering an accurate calculation of the response rate. The absolute number of responses accounted for a small portion of the total memberships of the participating societies. Also, the data collection periods varied among the three academic societies, and dermatologists’ practice types may have influenced the range and nature of treated conditions.
DISCLOSURES:
The authors did not disclose funding information. They declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Approves IL-31 Inhibitor for Atopic Dermatitis
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
Communicating with Angry Parents
There has been a lot of talk about polarization in America in the past year and how hard it can be to talk with someone with a different world view. It also seems that the COVID pandemic and a move to non–face-to-face communication has eroded social skills, increased disinformation, and made people irritable. As pediatric providers dealing with all kinds of people we have to increasingly deal with difficult communications.
We pediatric providers tend to be a friendly bunch with a mission of helping the health and well-being of children whose problems are no fault of their own. What’s not to like about us? Hence, one of the most difficult situations is a parent approaching us in anger. [All that follows also applies generally to adults, teens, and children but I here focus on parents.]
Health problems are often very frustrating. Parents mainly want their child’s problem fixed, yet that is not easy. Frustration with the health condition is compounded by having to wait, costs for care, life interruption, and confusing information. Anger and aggression are natural results of frustration (remember the Frustration-Aggression paradigm in animals?). Frustration is cumulative — a health problem may be the last straw, especially when social stressors or past trauma, also out of their control, were already present. Individuals with mental disorders or substance use are especially vulnerable to anger reactions.
Allaying Anger
As providers, we don’t know how anger may have been reinforced in the individual’s past. Anger may have scared others into giving in to them and thus became a familiar weapon. Sometimes angry outbursts get people to the front of the line. Expressing anger is also a kind of relief valve for emotions. Acknowledging that their situation is “very upsetting” and “frustrating” and that you will try to get a solution is the first step in effective communication.
Anger also comes from anxiety or fear. Anxiety is often a missed cause of childhood aggressive behavior. Parents are scared about poor short-and long-term outcomes of health problems in their child. Asking “What worries you the most about this illness?” or “What have you heard about this illness?” can elicit reports of fears you may be able to debunk or put in perspective.
Keeping their child healthy and safe are parents’ top priorities, so feeling helpless and out of control when an illness or injury occurs is very disturbing. Being a good parent is partly driven by guilt, which may be unrealistic, or from omission (eg, did not bring the child in sooner), or commission (eg, shouldn’t have taken her to that birthday party where she caught it). This is where you may normalize their actions such as saying “a child really can’t grow up in a bubble” or “symptoms can mean many things. I usually tell parents they don’t need to call unless the temperature is over 101,” for example. If appropriate, you might clarify what actions they should take in the future and provide a handout or instructions for sources of reliable information (and perhaps what is unreliable, such as TikTok!) to give them more power.
Feeling helpless may also evoke memories from the past when, as a child themselves, they were not able to help a loved one suffering from an accident or a health or mental health issue. They may have been helpless in the face of bullying, domestic violence, or racism. Even a hint of helplessness now can tap into the previous emotions, accelerating their reactivity. Promising to “work on this together” lends them your agency.
Of course, the main thing angry parents need is to have their child’s issue resolved. But this may not be a quick fix, especially if they are too incensed to cooperate. But what we can always do is address their need to be heard — both in content and emotion — and to help them regain a sense of control.
Pacing Can Help
One strategy that may seem counterintuitive is called “pacing.” Instead of begging the parent to calm down, which denigrates their strong feelings, we can echo their concerns while mirroring their emotions (within limits) to demonstrate that we understand them. Mirroring emotions may include your physical posture, volume and speed of speech, as well as use of similar sensory modalities. As for the modality component, you may notice that people tend to use visual, auditory or kinesthetic (feeling or action) words. So, for example, for an angry parent “screaming that no one has looked at the lab results yet” we might posture as they do, increase our volume, and use visual words such as saying “You are upset because you don’t see anyone looking over the labs in all this time.” As you hear more, you can continue to paraphrase and summarize what they are saying including their examples or wording. You can then change your own tone and posture progressively downshifting, bringing them along, and establishing rapport as they can tell that you are understanding them. Validating their emotions does not mean you have to agree with what they are saying; it demonstrates that you hear them.
Other strategies to demonstrate listening that can be helpful include sitting down side by side, and taking notes they can see, asking if you are getting the details correct. Using open-ended questions to elicit answers other than yes/no conveys openness to hear their story and may also reveal some of the causes for the upset. Sitting side by side conveys collaboration, whereas face to face implies more confrontation. Keep your arms down and arms and legs uncrossed and your head nodding and tilted somewhat down. These positions and verbal techniques demonstrate that you are listening to both their content and feelings.
Next Steps
The other main component to communication with an angry parent is providing action on the issue, especially involving them in a way that gives them some sense of control. Once they can tell that you understand them, it is then key to stay focused on solutions, bringing the discussion back if it veers off. There may be things they can do or you can do together such as log in to their portal, get on the phone with a relevant staff person such as a referral coordinator, or set a time for a follow-up appointment or call. Any action step they can do, even asking that they record a temperature every 6 hours, reduces helplessness.
It is crucial to elicit the parents’ criteria for satisfaction of their complaint. You may try asking: “What would tell you that your child’s problem is being adequately addressed?” Write down these criteria as part of the overall plan, making sure they are detailed, measurable, feasible, time related, and relevant. Include actions for the parent to take, if possible. By setting criteria and times for follow-up you establish accountability that also gives them a sense of control.
There are certain communications that can make things worse with an angry parent, some of which you may not even know occurred. Their anger may well have sparked a reaction in our staff, who are getting it full force before we even start our visit. Not only you but also your staff need to avoid making excuses for what happened (or didn’t happen) to the patient, blame the family for the child’s issue, imply that the parent’s feelings are invalid, or react as though their anger was a personal affront.
Setting Boundaries
There are certainly times when a parent’s behavior is unacceptable or even dangerous. It is important to have policies about what action to take so that we can verbally refer to these, if needed. We should all avoid threatening expulsion from the practice or calling security. Instead, assertively state the boundaries and rules and tell them what will happen if the behavior continues or exceeds a limit, such as frightening other patients or damaging equipment. It is essential to use respectful language and address them by name and certainly not make comments about them personally or criticize them, as these raise defensiveness. Suggesting they or you “take a break,” give them “some space” for a few minutes in a safe private room, or leave and come back in 15 minutes allows the upset parent to save face and gather themselves. All these things also work with children and teens.
Health care is stressful, especially with short staffing, and is often intensely personal and emotional. The human struggles we deal with may also be present in our own and our staff’s experiences.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
There has been a lot of talk about polarization in America in the past year and how hard it can be to talk with someone with a different world view. It also seems that the COVID pandemic and a move to non–face-to-face communication has eroded social skills, increased disinformation, and made people irritable. As pediatric providers dealing with all kinds of people we have to increasingly deal with difficult communications.
We pediatric providers tend to be a friendly bunch with a mission of helping the health and well-being of children whose problems are no fault of their own. What’s not to like about us? Hence, one of the most difficult situations is a parent approaching us in anger. [All that follows also applies generally to adults, teens, and children but I here focus on parents.]
Health problems are often very frustrating. Parents mainly want their child’s problem fixed, yet that is not easy. Frustration with the health condition is compounded by having to wait, costs for care, life interruption, and confusing information. Anger and aggression are natural results of frustration (remember the Frustration-Aggression paradigm in animals?). Frustration is cumulative — a health problem may be the last straw, especially when social stressors or past trauma, also out of their control, were already present. Individuals with mental disorders or substance use are especially vulnerable to anger reactions.
Allaying Anger
As providers, we don’t know how anger may have been reinforced in the individual’s past. Anger may have scared others into giving in to them and thus became a familiar weapon. Sometimes angry outbursts get people to the front of the line. Expressing anger is also a kind of relief valve for emotions. Acknowledging that their situation is “very upsetting” and “frustrating” and that you will try to get a solution is the first step in effective communication.
Anger also comes from anxiety or fear. Anxiety is often a missed cause of childhood aggressive behavior. Parents are scared about poor short-and long-term outcomes of health problems in their child. Asking “What worries you the most about this illness?” or “What have you heard about this illness?” can elicit reports of fears you may be able to debunk or put in perspective.
Keeping their child healthy and safe are parents’ top priorities, so feeling helpless and out of control when an illness or injury occurs is very disturbing. Being a good parent is partly driven by guilt, which may be unrealistic, or from omission (eg, did not bring the child in sooner), or commission (eg, shouldn’t have taken her to that birthday party where she caught it). This is where you may normalize their actions such as saying “a child really can’t grow up in a bubble” or “symptoms can mean many things. I usually tell parents they don’t need to call unless the temperature is over 101,” for example. If appropriate, you might clarify what actions they should take in the future and provide a handout or instructions for sources of reliable information (and perhaps what is unreliable, such as TikTok!) to give them more power.
Feeling helpless may also evoke memories from the past when, as a child themselves, they were not able to help a loved one suffering from an accident or a health or mental health issue. They may have been helpless in the face of bullying, domestic violence, or racism. Even a hint of helplessness now can tap into the previous emotions, accelerating their reactivity. Promising to “work on this together” lends them your agency.
Of course, the main thing angry parents need is to have their child’s issue resolved. But this may not be a quick fix, especially if they are too incensed to cooperate. But what we can always do is address their need to be heard — both in content and emotion — and to help them regain a sense of control.
Pacing Can Help
One strategy that may seem counterintuitive is called “pacing.” Instead of begging the parent to calm down, which denigrates their strong feelings, we can echo their concerns while mirroring their emotions (within limits) to demonstrate that we understand them. Mirroring emotions may include your physical posture, volume and speed of speech, as well as use of similar sensory modalities. As for the modality component, you may notice that people tend to use visual, auditory or kinesthetic (feeling or action) words. So, for example, for an angry parent “screaming that no one has looked at the lab results yet” we might posture as they do, increase our volume, and use visual words such as saying “You are upset because you don’t see anyone looking over the labs in all this time.” As you hear more, you can continue to paraphrase and summarize what they are saying including their examples or wording. You can then change your own tone and posture progressively downshifting, bringing them along, and establishing rapport as they can tell that you are understanding them. Validating their emotions does not mean you have to agree with what they are saying; it demonstrates that you hear them.
Other strategies to demonstrate listening that can be helpful include sitting down side by side, and taking notes they can see, asking if you are getting the details correct. Using open-ended questions to elicit answers other than yes/no conveys openness to hear their story and may also reveal some of the causes for the upset. Sitting side by side conveys collaboration, whereas face to face implies more confrontation. Keep your arms down and arms and legs uncrossed and your head nodding and tilted somewhat down. These positions and verbal techniques demonstrate that you are listening to both their content and feelings.
Next Steps
The other main component to communication with an angry parent is providing action on the issue, especially involving them in a way that gives them some sense of control. Once they can tell that you understand them, it is then key to stay focused on solutions, bringing the discussion back if it veers off. There may be things they can do or you can do together such as log in to their portal, get on the phone with a relevant staff person such as a referral coordinator, or set a time for a follow-up appointment or call. Any action step they can do, even asking that they record a temperature every 6 hours, reduces helplessness.
It is crucial to elicit the parents’ criteria for satisfaction of their complaint. You may try asking: “What would tell you that your child’s problem is being adequately addressed?” Write down these criteria as part of the overall plan, making sure they are detailed, measurable, feasible, time related, and relevant. Include actions for the parent to take, if possible. By setting criteria and times for follow-up you establish accountability that also gives them a sense of control.
There are certain communications that can make things worse with an angry parent, some of which you may not even know occurred. Their anger may well have sparked a reaction in our staff, who are getting it full force before we even start our visit. Not only you but also your staff need to avoid making excuses for what happened (or didn’t happen) to the patient, blame the family for the child’s issue, imply that the parent’s feelings are invalid, or react as though their anger was a personal affront.
Setting Boundaries
There are certainly times when a parent’s behavior is unacceptable or even dangerous. It is important to have policies about what action to take so that we can verbally refer to these, if needed. We should all avoid threatening expulsion from the practice or calling security. Instead, assertively state the boundaries and rules and tell them what will happen if the behavior continues or exceeds a limit, such as frightening other patients or damaging equipment. It is essential to use respectful language and address them by name and certainly not make comments about them personally or criticize them, as these raise defensiveness. Suggesting they or you “take a break,” give them “some space” for a few minutes in a safe private room, or leave and come back in 15 minutes allows the upset parent to save face and gather themselves. All these things also work with children and teens.
Health care is stressful, especially with short staffing, and is often intensely personal and emotional. The human struggles we deal with may also be present in our own and our staff’s experiences.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
There has been a lot of talk about polarization in America in the past year and how hard it can be to talk with someone with a different world view. It also seems that the COVID pandemic and a move to non–face-to-face communication has eroded social skills, increased disinformation, and made people irritable. As pediatric providers dealing with all kinds of people we have to increasingly deal with difficult communications.
We pediatric providers tend to be a friendly bunch with a mission of helping the health and well-being of children whose problems are no fault of their own. What’s not to like about us? Hence, one of the most difficult situations is a parent approaching us in anger. [All that follows also applies generally to adults, teens, and children but I here focus on parents.]
Health problems are often very frustrating. Parents mainly want their child’s problem fixed, yet that is not easy. Frustration with the health condition is compounded by having to wait, costs for care, life interruption, and confusing information. Anger and aggression are natural results of frustration (remember the Frustration-Aggression paradigm in animals?). Frustration is cumulative — a health problem may be the last straw, especially when social stressors or past trauma, also out of their control, were already present. Individuals with mental disorders or substance use are especially vulnerable to anger reactions.
Allaying Anger
As providers, we don’t know how anger may have been reinforced in the individual’s past. Anger may have scared others into giving in to them and thus became a familiar weapon. Sometimes angry outbursts get people to the front of the line. Expressing anger is also a kind of relief valve for emotions. Acknowledging that their situation is “very upsetting” and “frustrating” and that you will try to get a solution is the first step in effective communication.
Anger also comes from anxiety or fear. Anxiety is often a missed cause of childhood aggressive behavior. Parents are scared about poor short-and long-term outcomes of health problems in their child. Asking “What worries you the most about this illness?” or “What have you heard about this illness?” can elicit reports of fears you may be able to debunk or put in perspective.
Keeping their child healthy and safe are parents’ top priorities, so feeling helpless and out of control when an illness or injury occurs is very disturbing. Being a good parent is partly driven by guilt, which may be unrealistic, or from omission (eg, did not bring the child in sooner), or commission (eg, shouldn’t have taken her to that birthday party where she caught it). This is where you may normalize their actions such as saying “a child really can’t grow up in a bubble” or “symptoms can mean many things. I usually tell parents they don’t need to call unless the temperature is over 101,” for example. If appropriate, you might clarify what actions they should take in the future and provide a handout or instructions for sources of reliable information (and perhaps what is unreliable, such as TikTok!) to give them more power.
Feeling helpless may also evoke memories from the past when, as a child themselves, they were not able to help a loved one suffering from an accident or a health or mental health issue. They may have been helpless in the face of bullying, domestic violence, or racism. Even a hint of helplessness now can tap into the previous emotions, accelerating their reactivity. Promising to “work on this together” lends them your agency.
Of course, the main thing angry parents need is to have their child’s issue resolved. But this may not be a quick fix, especially if they are too incensed to cooperate. But what we can always do is address their need to be heard — both in content and emotion — and to help them regain a sense of control.
Pacing Can Help
One strategy that may seem counterintuitive is called “pacing.” Instead of begging the parent to calm down, which denigrates their strong feelings, we can echo their concerns while mirroring their emotions (within limits) to demonstrate that we understand them. Mirroring emotions may include your physical posture, volume and speed of speech, as well as use of similar sensory modalities. As for the modality component, you may notice that people tend to use visual, auditory or kinesthetic (feeling or action) words. So, for example, for an angry parent “screaming that no one has looked at the lab results yet” we might posture as they do, increase our volume, and use visual words such as saying “You are upset because you don’t see anyone looking over the labs in all this time.” As you hear more, you can continue to paraphrase and summarize what they are saying including their examples or wording. You can then change your own tone and posture progressively downshifting, bringing them along, and establishing rapport as they can tell that you are understanding them. Validating their emotions does not mean you have to agree with what they are saying; it demonstrates that you hear them.
Other strategies to demonstrate listening that can be helpful include sitting down side by side, and taking notes they can see, asking if you are getting the details correct. Using open-ended questions to elicit answers other than yes/no conveys openness to hear their story and may also reveal some of the causes for the upset. Sitting side by side conveys collaboration, whereas face to face implies more confrontation. Keep your arms down and arms and legs uncrossed and your head nodding and tilted somewhat down. These positions and verbal techniques demonstrate that you are listening to both their content and feelings.
Next Steps
The other main component to communication with an angry parent is providing action on the issue, especially involving them in a way that gives them some sense of control. Once they can tell that you understand them, it is then key to stay focused on solutions, bringing the discussion back if it veers off. There may be things they can do or you can do together such as log in to their portal, get on the phone with a relevant staff person such as a referral coordinator, or set a time for a follow-up appointment or call. Any action step they can do, even asking that they record a temperature every 6 hours, reduces helplessness.
It is crucial to elicit the parents’ criteria for satisfaction of their complaint. You may try asking: “What would tell you that your child’s problem is being adequately addressed?” Write down these criteria as part of the overall plan, making sure they are detailed, measurable, feasible, time related, and relevant. Include actions for the parent to take, if possible. By setting criteria and times for follow-up you establish accountability that also gives them a sense of control.
There are certain communications that can make things worse with an angry parent, some of which you may not even know occurred. Their anger may well have sparked a reaction in our staff, who are getting it full force before we even start our visit. Not only you but also your staff need to avoid making excuses for what happened (or didn’t happen) to the patient, blame the family for the child’s issue, imply that the parent’s feelings are invalid, or react as though their anger was a personal affront.
Setting Boundaries
There are certainly times when a parent’s behavior is unacceptable or even dangerous. It is important to have policies about what action to take so that we can verbally refer to these, if needed. We should all avoid threatening expulsion from the practice or calling security. Instead, assertively state the boundaries and rules and tell them what will happen if the behavior continues or exceeds a limit, such as frightening other patients or damaging equipment. It is essential to use respectful language and address them by name and certainly not make comments about them personally or criticize them, as these raise defensiveness. Suggesting they or you “take a break,” give them “some space” for a few minutes in a safe private room, or leave and come back in 15 minutes allows the upset parent to save face and gather themselves. All these things also work with children and teens.
Health care is stressful, especially with short staffing, and is often intensely personal and emotional. The human struggles we deal with may also be present in our own and our staff’s experiences.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Special Considerations Needed in Applying Lupus Nephritis Guideline to Children
WASHINGTON — When the American College of Rheumatology (ACR) released its updated guideline for management of lupus nephritis (LN) at its 2024 Annual Meeting, they included recommendations for managing pediatric LN for the first time.
The pediatric recommendations use the same classification criteria, outcome measures, and treatments as in adults — including the first-line triple therapy recommendation — but there remain important differences between pediatric and adult LN, Mary Beth Son, MD, clinical chief of immunology and section chief of rheumatology at Boston Children’s Hospital in Massachusetts, and an associate professor of pediatrics at Harvard Medical School, also in Boston, told attendees.
“In general, kids and adolescents with lupus are sicker,” Son said. They are more likely to have renal manifestations and neuropsychiatric lupus at diagnosis, compared with adults. Further, “although the disease is the same, it’s happening to kids and adolescents who are undergoing critical periods of growth and development.”
Medication risk profiles also shift for younger patients, Son noted.
“Importantly, they’re at risk for higher cumulative dosing of both glucocorticoids and cyclophosphamide,” Son said. “When we give an adolescent a course of cyclophosphamide, we have to be aware that this might be the first of a few courses over the course of the lifetime disease, and with increasing numbers of cyclophosphamide courses, you have increased risk for infertility and malignancy.”
Son also acknowledged challenges of pediatric literature, including differences in definitions of pediatric lupus, very few randomized controlled trials, and fewer pediatric studies in general, with fewer participants. Given these research gaps, the guideline panels included pediatric rheumatologists and nephrologists, and the patient panel included several patients with childhood-onset disease.
Son also addressed differences in pediatric drug development. Dosing studies also do not always directly translate from adults to children because children have larger drug volume distribution and differences in drug clearance, and they may need different formulations, she said. Children tend to tolerate medications better than adults because they usually have fewer comorbidities, but the assessment of a drug’s safety must take its impact on growth and development into consideration.
During a press conference after the session where the guideline was presented, Linda Hiraki, MD, ScD, a clinician-scientist in rheumatology at the Hospital for Sick Children, Toronto, Ontario, Canada, said the panel took into consideration that pediatric patients receive their diagnosis during a critical time of development, so considerations of medication risks include the fact that children “have much more life to live.”
Triple Therapy Recommended
As with adults, the pediatric LN guideline recommends a triple therapy approach: glucocorticoids plus mycophenolate mofetil and belimumab, in addition to the usual renin-angiotensin-aldosterone system inhibitors and hydroxychloroquine. But Son acknowledged limitations of applying the new guideline to children. For one, voclosporin has not been studied in or approved for pediatric patients, although there exists modest evidence for other calcineurin inhibitors, mainly tacrolimus, in children.
“The other important consideration is that the lower dose of prednisone that’s being offered by the guidelines of 40 mg per day as a starting dose has not been studied in pediatric lupus nephritis patients,” Son said. “However, I would offer that, given that we know that kids get higher doses and longer courses, it’s even more important to consider a lower dose to begin with in the setting of other immunosuppressants.”
Good Practice Statements for Pediatric LN
Son also reviewed three good practice statements for pediatric LN. First, “glucocorticoid regimens should use pediatric-appropriate doses for children, as reduction of human glucocorticoid dosing is critically important given the early age of pediatric lupus onset and attendant comorbidities,” she said.
That statement is based on both common sense and some literature, including awareness that children are more likely to receive higher doses of steroids and that children’s higher damage scores are driven in part by steroid-related toxicity, such as avascular necrosis and cataracts. In addition, glucocorticoids can have profound effects on body mass index, mood, and height attainment.
“This is during a period of emerging self-identity and struggles with appearance; steroids exacerbate that” as well as mood issues already associated with puberty, Son said.
The second good practice statement recommends that clinicians monitor patients “for delayed pubertal onset and decreased growth velocity that can result from disease activity and glucocorticoid treatment and consider referral to pediatric endocrinology if indicated.” The third states that “a structured, intentional transition from pediatric to adult rheumatology care is indicated to avoid poor outcomes during this vulnerable period.”
During the press conference, Hiraki said that pediatric rheumatologists already recognize the need for discussions about transfer to adult care to begin very early, even years before patients are ready to transfer.
“The transition from being a pediatric patient to being an adult patient is very challenging for a number of reasons,” starting with loss of insurance coverage, added Bonnie Bermas, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas, Texas. When adult rheumatologists take on these patients, they may not have had care for 2 or 3 years, she said.
Rebecca Sadun, MD, PhD, an associate professor of pediatrics in rheumatology at Duke University School of Medicine, Durham, North Carolina, and vice-chair of the Systemic Lupus Erythematosus Committee for the Childhood Arthritis and Rheumatology Research Alliance, was not involved in the guideline development process but reviewed the new guideline.
“We appreciate that the ACR took care to involve pediatric rheumatologists, pediatric nephrologists, and patients with childhood-onset lupus in the development of the newest lupus nephritis treatment guidelines,” she said in an interview. She also noted, however, that “the dearth of pediatric-specific clinical trial data means that we continue to wonder when it is appropriate to extrapolate from adult data regarding the efficacy, safety, and dosing of certain medications, including steroids and voclosporin.” She also noted that voclosporin use can increase pill burden and therefore be difficult to use in pediatrics.
“Children, adolescents, and young adults are a unique population with unique challenges, including significant struggles with adherence to complex medication regimens,” she said. Sadun drew attention to two themes from the guideline that she found particularly applicable to management of pediatric LN.
“First, we must remain wary of the serious consequences of long-term, high-dose glucocorticoids, and we should continue to look towards steroid-sparing strategies that will reduce reliance on glucocorticoids,” Sadun said. “Second, we are likely to see better outcomes, including better renal response, when we take advantage of combination immunosuppression earlier in the disease course.”
Son, Bermas, and Sadun had no disclosures. Hiraki has consulted for Janssen. The guideline development did not involve outside funding.
A version of this article first appeared on Medscape.com.
WASHINGTON — When the American College of Rheumatology (ACR) released its updated guideline for management of lupus nephritis (LN) at its 2024 Annual Meeting, they included recommendations for managing pediatric LN for the first time.
The pediatric recommendations use the same classification criteria, outcome measures, and treatments as in adults — including the first-line triple therapy recommendation — but there remain important differences between pediatric and adult LN, Mary Beth Son, MD, clinical chief of immunology and section chief of rheumatology at Boston Children’s Hospital in Massachusetts, and an associate professor of pediatrics at Harvard Medical School, also in Boston, told attendees.
“In general, kids and adolescents with lupus are sicker,” Son said. They are more likely to have renal manifestations and neuropsychiatric lupus at diagnosis, compared with adults. Further, “although the disease is the same, it’s happening to kids and adolescents who are undergoing critical periods of growth and development.”
Medication risk profiles also shift for younger patients, Son noted.
“Importantly, they’re at risk for higher cumulative dosing of both glucocorticoids and cyclophosphamide,” Son said. “When we give an adolescent a course of cyclophosphamide, we have to be aware that this might be the first of a few courses over the course of the lifetime disease, and with increasing numbers of cyclophosphamide courses, you have increased risk for infertility and malignancy.”
Son also acknowledged challenges of pediatric literature, including differences in definitions of pediatric lupus, very few randomized controlled trials, and fewer pediatric studies in general, with fewer participants. Given these research gaps, the guideline panels included pediatric rheumatologists and nephrologists, and the patient panel included several patients with childhood-onset disease.
Son also addressed differences in pediatric drug development. Dosing studies also do not always directly translate from adults to children because children have larger drug volume distribution and differences in drug clearance, and they may need different formulations, she said. Children tend to tolerate medications better than adults because they usually have fewer comorbidities, but the assessment of a drug’s safety must take its impact on growth and development into consideration.
During a press conference after the session where the guideline was presented, Linda Hiraki, MD, ScD, a clinician-scientist in rheumatology at the Hospital for Sick Children, Toronto, Ontario, Canada, said the panel took into consideration that pediatric patients receive their diagnosis during a critical time of development, so considerations of medication risks include the fact that children “have much more life to live.”
Triple Therapy Recommended
As with adults, the pediatric LN guideline recommends a triple therapy approach: glucocorticoids plus mycophenolate mofetil and belimumab, in addition to the usual renin-angiotensin-aldosterone system inhibitors and hydroxychloroquine. But Son acknowledged limitations of applying the new guideline to children. For one, voclosporin has not been studied in or approved for pediatric patients, although there exists modest evidence for other calcineurin inhibitors, mainly tacrolimus, in children.
“The other important consideration is that the lower dose of prednisone that’s being offered by the guidelines of 40 mg per day as a starting dose has not been studied in pediatric lupus nephritis patients,” Son said. “However, I would offer that, given that we know that kids get higher doses and longer courses, it’s even more important to consider a lower dose to begin with in the setting of other immunosuppressants.”
Good Practice Statements for Pediatric LN
Son also reviewed three good practice statements for pediatric LN. First, “glucocorticoid regimens should use pediatric-appropriate doses for children, as reduction of human glucocorticoid dosing is critically important given the early age of pediatric lupus onset and attendant comorbidities,” she said.
That statement is based on both common sense and some literature, including awareness that children are more likely to receive higher doses of steroids and that children’s higher damage scores are driven in part by steroid-related toxicity, such as avascular necrosis and cataracts. In addition, glucocorticoids can have profound effects on body mass index, mood, and height attainment.
“This is during a period of emerging self-identity and struggles with appearance; steroids exacerbate that” as well as mood issues already associated with puberty, Son said.
The second good practice statement recommends that clinicians monitor patients “for delayed pubertal onset and decreased growth velocity that can result from disease activity and glucocorticoid treatment and consider referral to pediatric endocrinology if indicated.” The third states that “a structured, intentional transition from pediatric to adult rheumatology care is indicated to avoid poor outcomes during this vulnerable period.”
During the press conference, Hiraki said that pediatric rheumatologists already recognize the need for discussions about transfer to adult care to begin very early, even years before patients are ready to transfer.
“The transition from being a pediatric patient to being an adult patient is very challenging for a number of reasons,” starting with loss of insurance coverage, added Bonnie Bermas, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas, Texas. When adult rheumatologists take on these patients, they may not have had care for 2 or 3 years, she said.
Rebecca Sadun, MD, PhD, an associate professor of pediatrics in rheumatology at Duke University School of Medicine, Durham, North Carolina, and vice-chair of the Systemic Lupus Erythematosus Committee for the Childhood Arthritis and Rheumatology Research Alliance, was not involved in the guideline development process but reviewed the new guideline.
“We appreciate that the ACR took care to involve pediatric rheumatologists, pediatric nephrologists, and patients with childhood-onset lupus in the development of the newest lupus nephritis treatment guidelines,” she said in an interview. She also noted, however, that “the dearth of pediatric-specific clinical trial data means that we continue to wonder when it is appropriate to extrapolate from adult data regarding the efficacy, safety, and dosing of certain medications, including steroids and voclosporin.” She also noted that voclosporin use can increase pill burden and therefore be difficult to use in pediatrics.
“Children, adolescents, and young adults are a unique population with unique challenges, including significant struggles with adherence to complex medication regimens,” she said. Sadun drew attention to two themes from the guideline that she found particularly applicable to management of pediatric LN.
“First, we must remain wary of the serious consequences of long-term, high-dose glucocorticoids, and we should continue to look towards steroid-sparing strategies that will reduce reliance on glucocorticoids,” Sadun said. “Second, we are likely to see better outcomes, including better renal response, when we take advantage of combination immunosuppression earlier in the disease course.”
Son, Bermas, and Sadun had no disclosures. Hiraki has consulted for Janssen. The guideline development did not involve outside funding.
A version of this article first appeared on Medscape.com.
WASHINGTON — When the American College of Rheumatology (ACR) released its updated guideline for management of lupus nephritis (LN) at its 2024 Annual Meeting, they included recommendations for managing pediatric LN for the first time.
The pediatric recommendations use the same classification criteria, outcome measures, and treatments as in adults — including the first-line triple therapy recommendation — but there remain important differences between pediatric and adult LN, Mary Beth Son, MD, clinical chief of immunology and section chief of rheumatology at Boston Children’s Hospital in Massachusetts, and an associate professor of pediatrics at Harvard Medical School, also in Boston, told attendees.
“In general, kids and adolescents with lupus are sicker,” Son said. They are more likely to have renal manifestations and neuropsychiatric lupus at diagnosis, compared with adults. Further, “although the disease is the same, it’s happening to kids and adolescents who are undergoing critical periods of growth and development.”
Medication risk profiles also shift for younger patients, Son noted.
“Importantly, they’re at risk for higher cumulative dosing of both glucocorticoids and cyclophosphamide,” Son said. “When we give an adolescent a course of cyclophosphamide, we have to be aware that this might be the first of a few courses over the course of the lifetime disease, and with increasing numbers of cyclophosphamide courses, you have increased risk for infertility and malignancy.”
Son also acknowledged challenges of pediatric literature, including differences in definitions of pediatric lupus, very few randomized controlled trials, and fewer pediatric studies in general, with fewer participants. Given these research gaps, the guideline panels included pediatric rheumatologists and nephrologists, and the patient panel included several patients with childhood-onset disease.
Son also addressed differences in pediatric drug development. Dosing studies also do not always directly translate from adults to children because children have larger drug volume distribution and differences in drug clearance, and they may need different formulations, she said. Children tend to tolerate medications better than adults because they usually have fewer comorbidities, but the assessment of a drug’s safety must take its impact on growth and development into consideration.
During a press conference after the session where the guideline was presented, Linda Hiraki, MD, ScD, a clinician-scientist in rheumatology at the Hospital for Sick Children, Toronto, Ontario, Canada, said the panel took into consideration that pediatric patients receive their diagnosis during a critical time of development, so considerations of medication risks include the fact that children “have much more life to live.”
Triple Therapy Recommended
As with adults, the pediatric LN guideline recommends a triple therapy approach: glucocorticoids plus mycophenolate mofetil and belimumab, in addition to the usual renin-angiotensin-aldosterone system inhibitors and hydroxychloroquine. But Son acknowledged limitations of applying the new guideline to children. For one, voclosporin has not been studied in or approved for pediatric patients, although there exists modest evidence for other calcineurin inhibitors, mainly tacrolimus, in children.
“The other important consideration is that the lower dose of prednisone that’s being offered by the guidelines of 40 mg per day as a starting dose has not been studied in pediatric lupus nephritis patients,” Son said. “However, I would offer that, given that we know that kids get higher doses and longer courses, it’s even more important to consider a lower dose to begin with in the setting of other immunosuppressants.”
Good Practice Statements for Pediatric LN
Son also reviewed three good practice statements for pediatric LN. First, “glucocorticoid regimens should use pediatric-appropriate doses for children, as reduction of human glucocorticoid dosing is critically important given the early age of pediatric lupus onset and attendant comorbidities,” she said.
That statement is based on both common sense and some literature, including awareness that children are more likely to receive higher doses of steroids and that children’s higher damage scores are driven in part by steroid-related toxicity, such as avascular necrosis and cataracts. In addition, glucocorticoids can have profound effects on body mass index, mood, and height attainment.
“This is during a period of emerging self-identity and struggles with appearance; steroids exacerbate that” as well as mood issues already associated with puberty, Son said.
The second good practice statement recommends that clinicians monitor patients “for delayed pubertal onset and decreased growth velocity that can result from disease activity and glucocorticoid treatment and consider referral to pediatric endocrinology if indicated.” The third states that “a structured, intentional transition from pediatric to adult rheumatology care is indicated to avoid poor outcomes during this vulnerable period.”
During the press conference, Hiraki said that pediatric rheumatologists already recognize the need for discussions about transfer to adult care to begin very early, even years before patients are ready to transfer.
“The transition from being a pediatric patient to being an adult patient is very challenging for a number of reasons,” starting with loss of insurance coverage, added Bonnie Bermas, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas, Texas. When adult rheumatologists take on these patients, they may not have had care for 2 or 3 years, she said.
Rebecca Sadun, MD, PhD, an associate professor of pediatrics in rheumatology at Duke University School of Medicine, Durham, North Carolina, and vice-chair of the Systemic Lupus Erythematosus Committee for the Childhood Arthritis and Rheumatology Research Alliance, was not involved in the guideline development process but reviewed the new guideline.
“We appreciate that the ACR took care to involve pediatric rheumatologists, pediatric nephrologists, and patients with childhood-onset lupus in the development of the newest lupus nephritis treatment guidelines,” she said in an interview. She also noted, however, that “the dearth of pediatric-specific clinical trial data means that we continue to wonder when it is appropriate to extrapolate from adult data regarding the efficacy, safety, and dosing of certain medications, including steroids and voclosporin.” She also noted that voclosporin use can increase pill burden and therefore be difficult to use in pediatrics.
“Children, adolescents, and young adults are a unique population with unique challenges, including significant struggles with adherence to complex medication regimens,” she said. Sadun drew attention to two themes from the guideline that she found particularly applicable to management of pediatric LN.
“First, we must remain wary of the serious consequences of long-term, high-dose glucocorticoids, and we should continue to look towards steroid-sparing strategies that will reduce reliance on glucocorticoids,” Sadun said. “Second, we are likely to see better outcomes, including better renal response, when we take advantage of combination immunosuppression earlier in the disease course.”
Son, Bermas, and Sadun had no disclosures. Hiraki has consulted for Janssen. The guideline development did not involve outside funding.
A version of this article first appeared on Medscape.com.
FROM ACR 2024