User login
Smart Mattress to Reduce SUDEP?
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AES 2024
Sleep Apnea Linked to Heightened Mortality in Epilepsy
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM AES 2024
Noise and Artificial Light
If you’ve ever taken a red-eye flight you have probably received a little packet of items the airline hopes will make your night flight more comfortable. If you had shelled out for “extra leg room” or “more comfort” seating, your little kit may have included some one-size-never-fits-all socks, a toothbrush large enough to brush one tooth at a time, and a miniature tube of toothpaste the GEICO gecko would laugh at. I have no personal knowledge what the folks in first class are getting, but I suspect it comes in a calf skin Gucci pouch. But, regardless of where you are sitting, at a minimum your night comfort kit will come with an eye mask and ear plugs. Unfortunately, these freebies are wasted on me because I already use a sleep mask every night and simply turn off my hearing aids to mute the noise. But I appreciate their effort.
Light and sound are well-known sleep disruptors. Temperature gets less attention, but is nonetheless a potent contributor to a poor night’s sleep in my experience. Just by chance while I was recovering from my most recent jet lag, I encountered two papers from investigators who were curious about the association between healthy sleep and ambient light and noise.
The first paper looked at the relationship between artificial light at night (ALAN) and the incidence of insomnia. Looking at more than 300 Chinese cities, the investigators measured ALAN using satellite images and correlated the data with insomnia-related posts on social media. The researchers found when ALAN increased insomnia, related posts also increased. Not surprisingly, this relationship was greater in less populated cities during extreme temperatures and when air quality was poor.
The second paper came from University of Texas at Houston. Using Fitbit data from more than 3000 adolescents, the researchers looked for correlations between blood pressure, sleep health, and “median nighttime anthropogenic noise levels by ZIP code.” Turns out the Federal Highway Administration has a readily available map of these noise levels.
What the investigators found was that adequate sleep significantly reduces the risk of hypertension in adolescents. Not an unexpected finding to an ex-pediatrician like myself who is obsessed with the importance of sleep deprivation. However, the investigators and I were surprised that they had found no association between neighborhood noise alone or in combination with sleep health. I still suspect there is an association lurking there in the weeds of their data, but obviously it is not robust enough to float to the surface. It may be that in an acute situation noise can contribute to hypertension, but over time individuals adjust to the new sound level and their blood pressure settles down. Sleep is such a critical factor that it is not something our cardiovascular system can adapt to so easily. For various reasons most of us may already be functioning at the margins of sleep deprivation.
How then do we respond to observations by these two research teams? Do we take an approach similar to that the airlines have taken and prescribe, hand out, or sell ear plugs and sleep masks to every patient, or at least those with hypertension? This is what we could call the put-the-onus-on-the-patient approach, which seems to be the default when we lack the political will to take a bolder step.
The other path we could call the socio-environmental approach. The airlines have made a passing attempt at this by turning the cabin lights down on red-eye flights. I recently wrote about the “exposome,” which some investigators define as the total non-genetic exposures an individual endures during a lifetime and which in many situations has a negative effect on the individual’s health. These two papers clearly demonstrate that noise and nighttime artificial light are potent features of an uncountable number of individuals’ exposomes.
Unfortunately, it is going to require something far beyond these two relatively obscure studies to move the needle in the direction of a healthier population. It’s is not a stretch to put obesity and the attention deficit phenomenon under this same umbrella where our society needs to look at itself for the answers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If you’ve ever taken a red-eye flight you have probably received a little packet of items the airline hopes will make your night flight more comfortable. If you had shelled out for “extra leg room” or “more comfort” seating, your little kit may have included some one-size-never-fits-all socks, a toothbrush large enough to brush one tooth at a time, and a miniature tube of toothpaste the GEICO gecko would laugh at. I have no personal knowledge what the folks in first class are getting, but I suspect it comes in a calf skin Gucci pouch. But, regardless of where you are sitting, at a minimum your night comfort kit will come with an eye mask and ear plugs. Unfortunately, these freebies are wasted on me because I already use a sleep mask every night and simply turn off my hearing aids to mute the noise. But I appreciate their effort.
Light and sound are well-known sleep disruptors. Temperature gets less attention, but is nonetheless a potent contributor to a poor night’s sleep in my experience. Just by chance while I was recovering from my most recent jet lag, I encountered two papers from investigators who were curious about the association between healthy sleep and ambient light and noise.
The first paper looked at the relationship between artificial light at night (ALAN) and the incidence of insomnia. Looking at more than 300 Chinese cities, the investigators measured ALAN using satellite images and correlated the data with insomnia-related posts on social media. The researchers found when ALAN increased insomnia, related posts also increased. Not surprisingly, this relationship was greater in less populated cities during extreme temperatures and when air quality was poor.
The second paper came from University of Texas at Houston. Using Fitbit data from more than 3000 adolescents, the researchers looked for correlations between blood pressure, sleep health, and “median nighttime anthropogenic noise levels by ZIP code.” Turns out the Federal Highway Administration has a readily available map of these noise levels.
What the investigators found was that adequate sleep significantly reduces the risk of hypertension in adolescents. Not an unexpected finding to an ex-pediatrician like myself who is obsessed with the importance of sleep deprivation. However, the investigators and I were surprised that they had found no association between neighborhood noise alone or in combination with sleep health. I still suspect there is an association lurking there in the weeds of their data, but obviously it is not robust enough to float to the surface. It may be that in an acute situation noise can contribute to hypertension, but over time individuals adjust to the new sound level and their blood pressure settles down. Sleep is such a critical factor that it is not something our cardiovascular system can adapt to so easily. For various reasons most of us may already be functioning at the margins of sleep deprivation.
How then do we respond to observations by these two research teams? Do we take an approach similar to that the airlines have taken and prescribe, hand out, or sell ear plugs and sleep masks to every patient, or at least those with hypertension? This is what we could call the put-the-onus-on-the-patient approach, which seems to be the default when we lack the political will to take a bolder step.
The other path we could call the socio-environmental approach. The airlines have made a passing attempt at this by turning the cabin lights down on red-eye flights. I recently wrote about the “exposome,” which some investigators define as the total non-genetic exposures an individual endures during a lifetime and which in many situations has a negative effect on the individual’s health. These two papers clearly demonstrate that noise and nighttime artificial light are potent features of an uncountable number of individuals’ exposomes.
Unfortunately, it is going to require something far beyond these two relatively obscure studies to move the needle in the direction of a healthier population. It’s is not a stretch to put obesity and the attention deficit phenomenon under this same umbrella where our society needs to look at itself for the answers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If you’ve ever taken a red-eye flight you have probably received a little packet of items the airline hopes will make your night flight more comfortable. If you had shelled out for “extra leg room” or “more comfort” seating, your little kit may have included some one-size-never-fits-all socks, a toothbrush large enough to brush one tooth at a time, and a miniature tube of toothpaste the GEICO gecko would laugh at. I have no personal knowledge what the folks in first class are getting, but I suspect it comes in a calf skin Gucci pouch. But, regardless of where you are sitting, at a minimum your night comfort kit will come with an eye mask and ear plugs. Unfortunately, these freebies are wasted on me because I already use a sleep mask every night and simply turn off my hearing aids to mute the noise. But I appreciate their effort.
Light and sound are well-known sleep disruptors. Temperature gets less attention, but is nonetheless a potent contributor to a poor night’s sleep in my experience. Just by chance while I was recovering from my most recent jet lag, I encountered two papers from investigators who were curious about the association between healthy sleep and ambient light and noise.
The first paper looked at the relationship between artificial light at night (ALAN) and the incidence of insomnia. Looking at more than 300 Chinese cities, the investigators measured ALAN using satellite images and correlated the data with insomnia-related posts on social media. The researchers found when ALAN increased insomnia, related posts also increased. Not surprisingly, this relationship was greater in less populated cities during extreme temperatures and when air quality was poor.
The second paper came from University of Texas at Houston. Using Fitbit data from more than 3000 adolescents, the researchers looked for correlations between blood pressure, sleep health, and “median nighttime anthropogenic noise levels by ZIP code.” Turns out the Federal Highway Administration has a readily available map of these noise levels.
What the investigators found was that adequate sleep significantly reduces the risk of hypertension in adolescents. Not an unexpected finding to an ex-pediatrician like myself who is obsessed with the importance of sleep deprivation. However, the investigators and I were surprised that they had found no association between neighborhood noise alone or in combination with sleep health. I still suspect there is an association lurking there in the weeds of their data, but obviously it is not robust enough to float to the surface. It may be that in an acute situation noise can contribute to hypertension, but over time individuals adjust to the new sound level and their blood pressure settles down. Sleep is such a critical factor that it is not something our cardiovascular system can adapt to so easily. For various reasons most of us may already be functioning at the margins of sleep deprivation.
How then do we respond to observations by these two research teams? Do we take an approach similar to that the airlines have taken and prescribe, hand out, or sell ear plugs and sleep masks to every patient, or at least those with hypertension? This is what we could call the put-the-onus-on-the-patient approach, which seems to be the default when we lack the political will to take a bolder step.
The other path we could call the socio-environmental approach. The airlines have made a passing attempt at this by turning the cabin lights down on red-eye flights. I recently wrote about the “exposome,” which some investigators define as the total non-genetic exposures an individual endures during a lifetime and which in many situations has a negative effect on the individual’s health. These two papers clearly demonstrate that noise and nighttime artificial light are potent features of an uncountable number of individuals’ exposomes.
Unfortunately, it is going to require something far beyond these two relatively obscure studies to move the needle in the direction of a healthier population. It’s is not a stretch to put obesity and the attention deficit phenomenon under this same umbrella where our society needs to look at itself for the answers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
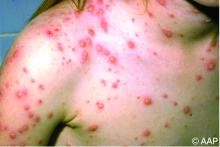
Childhood Atopic Dermatitis Doesn’t Delay Puberty
TOPLINE:
METHODOLOGY:
- Investigators conducted a nationwide cohort study among 15,534 children in Denmark whose pubertal development was assessed every 6 months with a web-based questionnaire starting at the age of 11 years.
- The children were classified into three groups: No atopic dermatitis; self-reported doctor-diagnosed atopic dermatitis (maternal report of a doctor diagnosis at 6 months, 18 months, and/or 7 years of age); hospital-diagnosed atopic dermatitis (registry data showing it as the primary reason for hospital contact up to the age of 8 years), representing mainly severe cases.
- The main outcome was the age difference averaged across a range of pubertal milestones (attainment of Tanner stages; development of axillary hair, acne, and voice break; and occurrence of first ejaculation and menarche).
TAKEAWAY:
- Overall, 21.5% of the children had self-reported doctor-diagnosed atopic dermatitis and 0.7% had hospital-diagnosed atopic dermatitis.
- Relative to girls without atopic dermatitis, girls with self-reported doctor-diagnosed atopic dermatitis reached the milestones at the same age, with a mean difference of 0.0 months, and girls with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- Relative to boys without atopic dermatitis, boys with self-reported doctor-diagnosed atopic dermatitis reached the milestones a mean of 0.1 month later and boys with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- A more stringent definition of atopic dermatitis — persistent or recurrent atopic dermatitis at 7 years of age (assumed more likely to affect sleep and disrupt the skin barrier near the start of puberty) — was also not associated with delayed pubertal development.
IN PRACTICE:
“Previous studies on atopic dermatitis and puberty are limited, some suggest a link between atopic dermatitis and delayed puberty, akin to other chronic inflammatory diseases in childhood,” the authors wrote. “The results of the present study are reassuring for young patients with atopic dermatitis approaching puberty and reproductive health in adult life,” they concluded.
SOURCE:
The study was led by Camilla Lomholt Kjersgaard, MD, Aarhus University, Aarhus, Denmark, and was published online in JAAD International.
LIMITATIONS:
Limitations included a lack of information on treatment, the use of analyses that did not address missing data, and a possible misclassification of self-reported pubertal development.
DISCLOSURES:
The study was funded by the Danish Council for Independent Research; Aarhus University; and Fonden af Fam. Kjærsgaard, Sunds; and was cofunded by the European Union. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators conducted a nationwide cohort study among 15,534 children in Denmark whose pubertal development was assessed every 6 months with a web-based questionnaire starting at the age of 11 years.
- The children were classified into three groups: No atopic dermatitis; self-reported doctor-diagnosed atopic dermatitis (maternal report of a doctor diagnosis at 6 months, 18 months, and/or 7 years of age); hospital-diagnosed atopic dermatitis (registry data showing it as the primary reason for hospital contact up to the age of 8 years), representing mainly severe cases.
- The main outcome was the age difference averaged across a range of pubertal milestones (attainment of Tanner stages; development of axillary hair, acne, and voice break; and occurrence of first ejaculation and menarche).
TAKEAWAY:
- Overall, 21.5% of the children had self-reported doctor-diagnosed atopic dermatitis and 0.7% had hospital-diagnosed atopic dermatitis.
- Relative to girls without atopic dermatitis, girls with self-reported doctor-diagnosed atopic dermatitis reached the milestones at the same age, with a mean difference of 0.0 months, and girls with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- Relative to boys without atopic dermatitis, boys with self-reported doctor-diagnosed atopic dermatitis reached the milestones a mean of 0.1 month later and boys with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- A more stringent definition of atopic dermatitis — persistent or recurrent atopic dermatitis at 7 years of age (assumed more likely to affect sleep and disrupt the skin barrier near the start of puberty) — was also not associated with delayed pubertal development.
IN PRACTICE:
“Previous studies on atopic dermatitis and puberty are limited, some suggest a link between atopic dermatitis and delayed puberty, akin to other chronic inflammatory diseases in childhood,” the authors wrote. “The results of the present study are reassuring for young patients with atopic dermatitis approaching puberty and reproductive health in adult life,” they concluded.
SOURCE:
The study was led by Camilla Lomholt Kjersgaard, MD, Aarhus University, Aarhus, Denmark, and was published online in JAAD International.
LIMITATIONS:
Limitations included a lack of information on treatment, the use of analyses that did not address missing data, and a possible misclassification of self-reported pubertal development.
DISCLOSURES:
The study was funded by the Danish Council for Independent Research; Aarhus University; and Fonden af Fam. Kjærsgaard, Sunds; and was cofunded by the European Union. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators conducted a nationwide cohort study among 15,534 children in Denmark whose pubertal development was assessed every 6 months with a web-based questionnaire starting at the age of 11 years.
- The children were classified into three groups: No atopic dermatitis; self-reported doctor-diagnosed atopic dermatitis (maternal report of a doctor diagnosis at 6 months, 18 months, and/or 7 years of age); hospital-diagnosed atopic dermatitis (registry data showing it as the primary reason for hospital contact up to the age of 8 years), representing mainly severe cases.
- The main outcome was the age difference averaged across a range of pubertal milestones (attainment of Tanner stages; development of axillary hair, acne, and voice break; and occurrence of first ejaculation and menarche).
TAKEAWAY:
- Overall, 21.5% of the children had self-reported doctor-diagnosed atopic dermatitis and 0.7% had hospital-diagnosed atopic dermatitis.
- Relative to girls without atopic dermatitis, girls with self-reported doctor-diagnosed atopic dermatitis reached the milestones at the same age, with a mean difference of 0.0 months, and girls with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- Relative to boys without atopic dermatitis, boys with self-reported doctor-diagnosed atopic dermatitis reached the milestones a mean of 0.1 month later and boys with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- A more stringent definition of atopic dermatitis — persistent or recurrent atopic dermatitis at 7 years of age (assumed more likely to affect sleep and disrupt the skin barrier near the start of puberty) — was also not associated with delayed pubertal development.
IN PRACTICE:
“Previous studies on atopic dermatitis and puberty are limited, some suggest a link between atopic dermatitis and delayed puberty, akin to other chronic inflammatory diseases in childhood,” the authors wrote. “The results of the present study are reassuring for young patients with atopic dermatitis approaching puberty and reproductive health in adult life,” they concluded.
SOURCE:
The study was led by Camilla Lomholt Kjersgaard, MD, Aarhus University, Aarhus, Denmark, and was published online in JAAD International.
LIMITATIONS:
Limitations included a lack of information on treatment, the use of analyses that did not address missing data, and a possible misclassification of self-reported pubertal development.
DISCLOSURES:
The study was funded by the Danish Council for Independent Research; Aarhus University; and Fonden af Fam. Kjærsgaard, Sunds; and was cofunded by the European Union. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Measurement-Based Treatment to Target Approaches
Clinical Scenario
Lilly is a 15-year-old girl in her sophomore year of high school. Over the course of a month after a romantic and then a friend-group breakup, her parents have been concerned about her increasing tearfulness every day and retreat from activities to avoid social interactions with others that she once enjoyed so much. She has been missing more and more school, saying that she can’t bear to go, and staying in bed during the days, even on weekends. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment in 2 weeks with you and then again in another 2 weeks. Her parents and she tell you, “I thought she would be better by now.” You feel stuck with how to proceed in the visit. You have correctly identified the problem as depression, started the recommended evidence-based treatments, but the parents and Lilly are looking to you for something more or different. There are not many or other local resources. When and how do you all determine what “better” looks and feels like? Where do you go from here?
Metrics Can Guide Next Steps
This clinical scenario is not uncommon. As a psychiatrist consultant in primary care, I often encounter the following comment and question: “Someone isn’t feeling better. I have them taking an SSRI and doing psychotherapy. What is the next thing to do?” In discussions with supervisees and in training residents, I often say that you will know that your consultations have made a real impact on providers’ practices when these questions shift from “what’s the next medication or treatment” to a more robust baseline and follow-up inventory of symptoms via common and available metrics (PHQ9A, PSC-17 or 30, SCARED) shared with you at the start, the middle, and at other times of treatment. Such metrics can more meaningfully guide your collaborative clinical discussions and decisions.
Tracking baseline metrics and follow-up with treatment interventions is a transformative approach to clinical care. But, in primary care, it’s common that the question around mental health care may not receive the same robust screening and tracking of symptoms which have the power to more thoughtfully guide decision-making, even though this is common in other forms of patient care which have more routine use of more objective data.
Measurement-based treatment to target approaches are well-studied, but not often or always implemented. They involve providing a baseline metric (PHQ9A, Pediatric Symptom Checklist 17 or 30, GAD7, or SCARED), and tracking that metric for response over time using specific scores for decision points.
An Alternative Clinical Scenario
Consider the following alternative scenario for the above patient using a measurement-based treatment to target approach:
Lilly is a 15-year-old girl in her sophomore year of high school with symptoms concerning for depression. A PHQ9A is administered in your appointment, and she scores 20 out of 30, exceeding the threshold score for 11 for depression. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment with you in 2 weeks and then again in another 2 weeks. You obtain a PHQ9A at each appointment, and track the change with her and her parents over time.
You share with her and the family that it is common that there will be fluctuations in measurements, and you know that a score change on the PHQ9A greater than 7 is considered a clinically significant, reliable change. So, a PHQ9 score reduction from 20 to 13 would be meaningful progress. While seeking a score within the normal and non-clinical range, the progress can be tracked in a way that allows a more robust monitoring of treatment response. If the scores do not improve, you can see that and act accordingly. This way of using metrics shifts the conversation from “how are you feeling now and today” to tracking symptoms more broadly and tracking those individual symptoms over time, some of which may improve and some which may be trickier to target.
Such a way of tracking common mental health symptoms with a focus on having data at baseline and throughout treatment allows a provider to change or adapt interventions, and to not chase something that can feel ephemeral, such as “feeling happy or looking better.”
For additional information on the measurement-based treatment to target approach, there are resources that share in more depth the research informing this approach, and other and broader real ways to integrate these practices into your own visits:
- Is Treatment Working? Detecting Real Change in the Treatment of Child and Adolescent Depression
- AACAP Clinical Update: Collaborative Mental Health Care for Children and Adolescents in Primary Care
Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
Clinical Scenario
Lilly is a 15-year-old girl in her sophomore year of high school. Over the course of a month after a romantic and then a friend-group breakup, her parents have been concerned about her increasing tearfulness every day and retreat from activities to avoid social interactions with others that she once enjoyed so much. She has been missing more and more school, saying that she can’t bear to go, and staying in bed during the days, even on weekends. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment in 2 weeks with you and then again in another 2 weeks. Her parents and she tell you, “I thought she would be better by now.” You feel stuck with how to proceed in the visit. You have correctly identified the problem as depression, started the recommended evidence-based treatments, but the parents and Lilly are looking to you for something more or different. There are not many or other local resources. When and how do you all determine what “better” looks and feels like? Where do you go from here?
Metrics Can Guide Next Steps
This clinical scenario is not uncommon. As a psychiatrist consultant in primary care, I often encounter the following comment and question: “Someone isn’t feeling better. I have them taking an SSRI and doing psychotherapy. What is the next thing to do?” In discussions with supervisees and in training residents, I often say that you will know that your consultations have made a real impact on providers’ practices when these questions shift from “what’s the next medication or treatment” to a more robust baseline and follow-up inventory of symptoms via common and available metrics (PHQ9A, PSC-17 or 30, SCARED) shared with you at the start, the middle, and at other times of treatment. Such metrics can more meaningfully guide your collaborative clinical discussions and decisions.
Tracking baseline metrics and follow-up with treatment interventions is a transformative approach to clinical care. But, in primary care, it’s common that the question around mental health care may not receive the same robust screening and tracking of symptoms which have the power to more thoughtfully guide decision-making, even though this is common in other forms of patient care which have more routine use of more objective data.
Measurement-based treatment to target approaches are well-studied, but not often or always implemented. They involve providing a baseline metric (PHQ9A, Pediatric Symptom Checklist 17 or 30, GAD7, or SCARED), and tracking that metric for response over time using specific scores for decision points.
An Alternative Clinical Scenario
Consider the following alternative scenario for the above patient using a measurement-based treatment to target approach:
Lilly is a 15-year-old girl in her sophomore year of high school with symptoms concerning for depression. A PHQ9A is administered in your appointment, and she scores 20 out of 30, exceeding the threshold score for 11 for depression. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment with you in 2 weeks and then again in another 2 weeks. You obtain a PHQ9A at each appointment, and track the change with her and her parents over time.
You share with her and the family that it is common that there will be fluctuations in measurements, and you know that a score change on the PHQ9A greater than 7 is considered a clinically significant, reliable change. So, a PHQ9 score reduction from 20 to 13 would be meaningful progress. While seeking a score within the normal and non-clinical range, the progress can be tracked in a way that allows a more robust monitoring of treatment response. If the scores do not improve, you can see that and act accordingly. This way of using metrics shifts the conversation from “how are you feeling now and today” to tracking symptoms more broadly and tracking those individual symptoms over time, some of which may improve and some which may be trickier to target.
Such a way of tracking common mental health symptoms with a focus on having data at baseline and throughout treatment allows a provider to change or adapt interventions, and to not chase something that can feel ephemeral, such as “feeling happy or looking better.”
For additional information on the measurement-based treatment to target approach, there are resources that share in more depth the research informing this approach, and other and broader real ways to integrate these practices into your own visits:
- Is Treatment Working? Detecting Real Change in the Treatment of Child and Adolescent Depression
- AACAP Clinical Update: Collaborative Mental Health Care for Children and Adolescents in Primary Care
Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
Clinical Scenario
Lilly is a 15-year-old girl in her sophomore year of high school. Over the course of a month after a romantic and then a friend-group breakup, her parents have been concerned about her increasing tearfulness every day and retreat from activities to avoid social interactions with others that she once enjoyed so much. She has been missing more and more school, saying that she can’t bear to go, and staying in bed during the days, even on weekends. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment in 2 weeks with you and then again in another 2 weeks. Her parents and she tell you, “I thought she would be better by now.” You feel stuck with how to proceed in the visit. You have correctly identified the problem as depression, started the recommended evidence-based treatments, but the parents and Lilly are looking to you for something more or different. There are not many or other local resources. When and how do you all determine what “better” looks and feels like? Where do you go from here?
Metrics Can Guide Next Steps
This clinical scenario is not uncommon. As a psychiatrist consultant in primary care, I often encounter the following comment and question: “Someone isn’t feeling better. I have them taking an SSRI and doing psychotherapy. What is the next thing to do?” In discussions with supervisees and in training residents, I often say that you will know that your consultations have made a real impact on providers’ practices when these questions shift from “what’s the next medication or treatment” to a more robust baseline and follow-up inventory of symptoms via common and available metrics (PHQ9A, PSC-17 or 30, SCARED) shared with you at the start, the middle, and at other times of treatment. Such metrics can more meaningfully guide your collaborative clinical discussions and decisions.
Tracking baseline metrics and follow-up with treatment interventions is a transformative approach to clinical care. But, in primary care, it’s common that the question around mental health care may not receive the same robust screening and tracking of symptoms which have the power to more thoughtfully guide decision-making, even though this is common in other forms of patient care which have more routine use of more objective data.
Measurement-based treatment to target approaches are well-studied, but not often or always implemented. They involve providing a baseline metric (PHQ9A, Pediatric Symptom Checklist 17 or 30, GAD7, or SCARED), and tracking that metric for response over time using specific scores for decision points.
An Alternative Clinical Scenario
Consider the following alternative scenario for the above patient using a measurement-based treatment to target approach:
Lilly is a 15-year-old girl in her sophomore year of high school with symptoms concerning for depression. A PHQ9A is administered in your appointment, and she scores 20 out of 30, exceeding the threshold score for 11 for depression. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment with you in 2 weeks and then again in another 2 weeks. You obtain a PHQ9A at each appointment, and track the change with her and her parents over time.
You share with her and the family that it is common that there will be fluctuations in measurements, and you know that a score change on the PHQ9A greater than 7 is considered a clinically significant, reliable change. So, a PHQ9 score reduction from 20 to 13 would be meaningful progress. While seeking a score within the normal and non-clinical range, the progress can be tracked in a way that allows a more robust monitoring of treatment response. If the scores do not improve, you can see that and act accordingly. This way of using metrics shifts the conversation from “how are you feeling now and today” to tracking symptoms more broadly and tracking those individual symptoms over time, some of which may improve and some which may be trickier to target.
Such a way of tracking common mental health symptoms with a focus on having data at baseline and throughout treatment allows a provider to change or adapt interventions, and to not chase something that can feel ephemeral, such as “feeling happy or looking better.”
For additional information on the measurement-based treatment to target approach, there are resources that share in more depth the research informing this approach, and other and broader real ways to integrate these practices into your own visits:
- Is Treatment Working? Detecting Real Change in the Treatment of Child and Adolescent Depression
- AACAP Clinical Update: Collaborative Mental Health Care for Children and Adolescents in Primary Care
Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
Rheumatology Match: Less than Half of Pediatric Positions Filled, Worsening Existing Trend
Over half of pediatric rheumatology fellowship positions went unfilled in 2024, according to the National Resident Matching Program (NRMP). Comparatively, nearly all adult rheumatology positions were filled.
Across all 39 subspecialties in internal medicine and pediatrics, there was an 86% fill rate. In pediatric subspecialties, the fill rate was 78%. There were more than 10,200 applicants in this year’s medicine and pediatric specialties match — a 9% increase from 2023 — and 81% matched to a position.
The NRMP reported that adult rheumatology filled 129 (97.7%) of 132 programs, with 284 (99%) out of 287 positions filled. In 2024, there were five new programs and 11 more fellowship positions available compared with the previous year.
In pediatric rheumatology, 16 (44%) of 36 programs were filled, with 27 (49%) of 55 positions filled. This is a notable decrease from 2023, where pediatric rheumatology filled 21 of 38 programs (55%) and 32 (62%) of 52 positions.
This year, 27 of 30 applicants preferring pediatric rheumatology matched to a program, while in 2023 all 32 applicants that preferred pediatric rheumatology matched.
“It’s a little disappointing that our overall number of applicants have not gone up,” Jay Mehta, MD, the program director of the Children’s Hospital of Philadelphia’s pediatric rheumatology fellowship said in an interview with Medscape Medical News. “It’s an especially exciting time in pediatric rheumatology, with really fantastic breakthroughs in terms of treatments and diagnostics. Unfortunately, that excitement hasn’t necessarily translated into more interest in our field.”
Mehta noted that the number of applicants to pediatric rheumatology fellowships have remained relatively consistent. Since 2019, the number of applicants has ranged from 28 to 33.
“Given the low number of overall positions/programs it is hard to read too much into year-to-year differences,” added Kristen Hayward, MD, a pediatric rheumatologist at Seattle Children’s in Washington. “While this total number of applicants per year is steady, this number is insufficient to build an adequate workforce for our current needs, much less for the future.”
This year, matched applicants to pediatric rheumatology included 13 MD graduates, eight DO graduates, five foreign applicants, and one US citizen international medical graduate.
In adult rheumatology, matched applicants included 108 MD graduates, 97 foreign applicants, 41 DO graduates, and 38 US citizen international medical graduates. A total of 365 applicants preferred the specialty, and 76% matched to rheumatology. Seven applicants matched to another specialty, and the remaining 79 did not match to any program.
Rheumatology was one of several specialties offering at least 150 positions with a fill rate of over 98%. The other specialties included allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, endocrinology, gastroenterology, and hematology and oncology.
While some pediatric subspecialties like critical care medicine and cardiology had fill rates over 90%, many “cognitive subspecialties” beyond pediatric rheumatology also struggled to fill spots, Hayward noted. Only 37% of pediatric nephrology positions and 48% of pediatric infectious disease positions were filled this year, in addition to a decline in filled pediatric-residency positions overall, she added.
Mehta had no relevant disclosures. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer.
A version of this article first appeared on Medscape.com.
Over half of pediatric rheumatology fellowship positions went unfilled in 2024, according to the National Resident Matching Program (NRMP). Comparatively, nearly all adult rheumatology positions were filled.
Across all 39 subspecialties in internal medicine and pediatrics, there was an 86% fill rate. In pediatric subspecialties, the fill rate was 78%. There were more than 10,200 applicants in this year’s medicine and pediatric specialties match — a 9% increase from 2023 — and 81% matched to a position.
The NRMP reported that adult rheumatology filled 129 (97.7%) of 132 programs, with 284 (99%) out of 287 positions filled. In 2024, there were five new programs and 11 more fellowship positions available compared with the previous year.
In pediatric rheumatology, 16 (44%) of 36 programs were filled, with 27 (49%) of 55 positions filled. This is a notable decrease from 2023, where pediatric rheumatology filled 21 of 38 programs (55%) and 32 (62%) of 52 positions.
This year, 27 of 30 applicants preferring pediatric rheumatology matched to a program, while in 2023 all 32 applicants that preferred pediatric rheumatology matched.
“It’s a little disappointing that our overall number of applicants have not gone up,” Jay Mehta, MD, the program director of the Children’s Hospital of Philadelphia’s pediatric rheumatology fellowship said in an interview with Medscape Medical News. “It’s an especially exciting time in pediatric rheumatology, with really fantastic breakthroughs in terms of treatments and diagnostics. Unfortunately, that excitement hasn’t necessarily translated into more interest in our field.”
Mehta noted that the number of applicants to pediatric rheumatology fellowships have remained relatively consistent. Since 2019, the number of applicants has ranged from 28 to 33.
“Given the low number of overall positions/programs it is hard to read too much into year-to-year differences,” added Kristen Hayward, MD, a pediatric rheumatologist at Seattle Children’s in Washington. “While this total number of applicants per year is steady, this number is insufficient to build an adequate workforce for our current needs, much less for the future.”
This year, matched applicants to pediatric rheumatology included 13 MD graduates, eight DO graduates, five foreign applicants, and one US citizen international medical graduate.
In adult rheumatology, matched applicants included 108 MD graduates, 97 foreign applicants, 41 DO graduates, and 38 US citizen international medical graduates. A total of 365 applicants preferred the specialty, and 76% matched to rheumatology. Seven applicants matched to another specialty, and the remaining 79 did not match to any program.
Rheumatology was one of several specialties offering at least 150 positions with a fill rate of over 98%. The other specialties included allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, endocrinology, gastroenterology, and hematology and oncology.
While some pediatric subspecialties like critical care medicine and cardiology had fill rates over 90%, many “cognitive subspecialties” beyond pediatric rheumatology also struggled to fill spots, Hayward noted. Only 37% of pediatric nephrology positions and 48% of pediatric infectious disease positions were filled this year, in addition to a decline in filled pediatric-residency positions overall, she added.
Mehta had no relevant disclosures. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer.
A version of this article first appeared on Medscape.com.
Over half of pediatric rheumatology fellowship positions went unfilled in 2024, according to the National Resident Matching Program (NRMP). Comparatively, nearly all adult rheumatology positions were filled.
Across all 39 subspecialties in internal medicine and pediatrics, there was an 86% fill rate. In pediatric subspecialties, the fill rate was 78%. There were more than 10,200 applicants in this year’s medicine and pediatric specialties match — a 9% increase from 2023 — and 81% matched to a position.
The NRMP reported that adult rheumatology filled 129 (97.7%) of 132 programs, with 284 (99%) out of 287 positions filled. In 2024, there were five new programs and 11 more fellowship positions available compared with the previous year.
In pediatric rheumatology, 16 (44%) of 36 programs were filled, with 27 (49%) of 55 positions filled. This is a notable decrease from 2023, where pediatric rheumatology filled 21 of 38 programs (55%) and 32 (62%) of 52 positions.
This year, 27 of 30 applicants preferring pediatric rheumatology matched to a program, while in 2023 all 32 applicants that preferred pediatric rheumatology matched.
“It’s a little disappointing that our overall number of applicants have not gone up,” Jay Mehta, MD, the program director of the Children’s Hospital of Philadelphia’s pediatric rheumatology fellowship said in an interview with Medscape Medical News. “It’s an especially exciting time in pediatric rheumatology, with really fantastic breakthroughs in terms of treatments and diagnostics. Unfortunately, that excitement hasn’t necessarily translated into more interest in our field.”
Mehta noted that the number of applicants to pediatric rheumatology fellowships have remained relatively consistent. Since 2019, the number of applicants has ranged from 28 to 33.
“Given the low number of overall positions/programs it is hard to read too much into year-to-year differences,” added Kristen Hayward, MD, a pediatric rheumatologist at Seattle Children’s in Washington. “While this total number of applicants per year is steady, this number is insufficient to build an adequate workforce for our current needs, much less for the future.”
This year, matched applicants to pediatric rheumatology included 13 MD graduates, eight DO graduates, five foreign applicants, and one US citizen international medical graduate.
In adult rheumatology, matched applicants included 108 MD graduates, 97 foreign applicants, 41 DO graduates, and 38 US citizen international medical graduates. A total of 365 applicants preferred the specialty, and 76% matched to rheumatology. Seven applicants matched to another specialty, and the remaining 79 did not match to any program.
Rheumatology was one of several specialties offering at least 150 positions with a fill rate of over 98%. The other specialties included allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, endocrinology, gastroenterology, and hematology and oncology.
While some pediatric subspecialties like critical care medicine and cardiology had fill rates over 90%, many “cognitive subspecialties” beyond pediatric rheumatology also struggled to fill spots, Hayward noted. Only 37% of pediatric nephrology positions and 48% of pediatric infectious disease positions were filled this year, in addition to a decline in filled pediatric-residency positions overall, she added.
Mehta had no relevant disclosures. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer.
A version of this article first appeared on Medscape.com.
Project’s Improvement in JIA Outcome Disparities Sets Stage for Further Interventions
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Could Biomarkers Help to Detect Lung Disease Earlier in Systemic JIA?
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
FROM ACR 2024
With Chemo, Blinatumomab Boosts DFS in Pediatric B-ALL
Among pediatric patients with B-ALL followed for a mean of 2.5 years (1.6-3.2 years), 718 patients in the blinatumomab-plus-chemotherapy group had a 3-year DFS of 96.0 ± 1.2%, compared with 87.9 ± 2.1% of the 722 patients in the chemotherapy-only group, researchers reported at the American Society of Hematology (ASH) 2024 Annual Meeting.
“Our results demonstrate that blinatumomab added to chemotherapy represents a new treatment standard for most patients with NCI [National Cancer Institute] standard-risk [B-ALL],” said first author Rachel E. Rau, MD, Seattle Children’s Hospital, University of Washington, during a news briefing.
As Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute, noted in a news briefing: “B-cell ALL is the most common childhood cancer and one of the most treatable. However, some children still relapse following standard chemotherapy treatments and then have a much grimmer outcome.”
The AALL1731 study was initiated in 2019 with a recruitment goal of 2245 participants. The patients were over age 1 and less than 10 years, with an initial white blood cell count of < 50,000/μL and were considered to be standard risk–high or standard risk–average.
The control group received standard-intensity chemotherapy (standard risk–average patients) or augmented Berlin-Frankfurt-Münster–based chemotherapy (standard risk–high patients). In addition, the blinatumomab groups received two cycles of the drug.
Randomization was terminated in 2024 at 1440 patients because of the positive results. Patients had a median age of 4.3 years (2.8-6.4), 52.6% were boys, 26% were Hispanic, and 5% were non-Hispanic Black.
The addition of blinatumomab improved DFS by 61% (hazard ratio, 0.39; 95% CI, 0.24-0.64; P < .0001).
In the group of standard risk–average patients, 3-year DFS was 97.5±1.3% in the blinatumomab group vs 90.2±2.3% in the control group (HR, 0.33; 95% CI, 0.15-0.69). For standard risk–high patients, 3-year DFS was 94.1 ± 2.5% and 84.8 ± 3.8%, respectively.
Six deaths occurred in remission, all in standard risk–high patients and none during blinatumomab cycles. Out of first courses of blinatumomab, 0.3% were associated with Grade 3 or higher cytokine release syndrome and 0.7% with seizures.
“We did note higher rates of subsequent sepsis and catheter-related infections in our standard risk–average patients who received blinatumomab,” Rau said.
“The improvement in disease survival was secondary to significant reduction in bone marrow relapse,” Rau added. “We did not see a similar reduction in the more rare event of an isolated central nervous system relapse. This finding was not surprising given blinatumomab’s known limited activity in the central nervous system.”
Rau noted that there are two challenges in terms of access to blinatumomab: its cost, at about $225,000 per a 2023 report, and its administration. The drug is administered via 4-week-long infusions. “The delivery method is very cumbersome,” she said.
“These are big problems that are going to take the combined efforts of pediatric oncologist cancer consortia and pharmaceutical industry partners as well as government agencies,” she said. Fortunately, she said, in June 2024 the Food and Drug Administration approved blinatumomab for adult and pediatric patients 1 month and older with CD19-positive Philadelphia chromosome–negative B-ALL in the consolidation phase of multiphase chemotherapy.
“So it’s relatively easy, at least, to prescribe blinatumomab in the United States for our patients that we feel would benefit from it,” she said.
As for method of delivery, Rau said easier-to-deliver formulations are in development.
Rau has disclosed spousal employment (AbbVie), serving on advisory boards (Servier, Jazz), consulting, and receiving honoraria (Jazz). Other study authors report various disclosures including ties with Amgen, the maker of blinatumomab. Dunbar has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among pediatric patients with B-ALL followed for a mean of 2.5 years (1.6-3.2 years), 718 patients in the blinatumomab-plus-chemotherapy group had a 3-year DFS of 96.0 ± 1.2%, compared with 87.9 ± 2.1% of the 722 patients in the chemotherapy-only group, researchers reported at the American Society of Hematology (ASH) 2024 Annual Meeting.
“Our results demonstrate that blinatumomab added to chemotherapy represents a new treatment standard for most patients with NCI [National Cancer Institute] standard-risk [B-ALL],” said first author Rachel E. Rau, MD, Seattle Children’s Hospital, University of Washington, during a news briefing.
As Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute, noted in a news briefing: “B-cell ALL is the most common childhood cancer and one of the most treatable. However, some children still relapse following standard chemotherapy treatments and then have a much grimmer outcome.”
The AALL1731 study was initiated in 2019 with a recruitment goal of 2245 participants. The patients were over age 1 and less than 10 years, with an initial white blood cell count of < 50,000/μL and were considered to be standard risk–high or standard risk–average.
The control group received standard-intensity chemotherapy (standard risk–average patients) or augmented Berlin-Frankfurt-Münster–based chemotherapy (standard risk–high patients). In addition, the blinatumomab groups received two cycles of the drug.
Randomization was terminated in 2024 at 1440 patients because of the positive results. Patients had a median age of 4.3 years (2.8-6.4), 52.6% were boys, 26% were Hispanic, and 5% were non-Hispanic Black.
The addition of blinatumomab improved DFS by 61% (hazard ratio, 0.39; 95% CI, 0.24-0.64; P < .0001).
In the group of standard risk–average patients, 3-year DFS was 97.5±1.3% in the blinatumomab group vs 90.2±2.3% in the control group (HR, 0.33; 95% CI, 0.15-0.69). For standard risk–high patients, 3-year DFS was 94.1 ± 2.5% and 84.8 ± 3.8%, respectively.
Six deaths occurred in remission, all in standard risk–high patients and none during blinatumomab cycles. Out of first courses of blinatumomab, 0.3% were associated with Grade 3 or higher cytokine release syndrome and 0.7% with seizures.
“We did note higher rates of subsequent sepsis and catheter-related infections in our standard risk–average patients who received blinatumomab,” Rau said.
“The improvement in disease survival was secondary to significant reduction in bone marrow relapse,” Rau added. “We did not see a similar reduction in the more rare event of an isolated central nervous system relapse. This finding was not surprising given blinatumomab’s known limited activity in the central nervous system.”
Rau noted that there are two challenges in terms of access to blinatumomab: its cost, at about $225,000 per a 2023 report, and its administration. The drug is administered via 4-week-long infusions. “The delivery method is very cumbersome,” she said.
“These are big problems that are going to take the combined efforts of pediatric oncologist cancer consortia and pharmaceutical industry partners as well as government agencies,” she said. Fortunately, she said, in June 2024 the Food and Drug Administration approved blinatumomab for adult and pediatric patients 1 month and older with CD19-positive Philadelphia chromosome–negative B-ALL in the consolidation phase of multiphase chemotherapy.
“So it’s relatively easy, at least, to prescribe blinatumomab in the United States for our patients that we feel would benefit from it,” she said.
As for method of delivery, Rau said easier-to-deliver formulations are in development.
Rau has disclosed spousal employment (AbbVie), serving on advisory boards (Servier, Jazz), consulting, and receiving honoraria (Jazz). Other study authors report various disclosures including ties with Amgen, the maker of blinatumomab. Dunbar has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among pediatric patients with B-ALL followed for a mean of 2.5 years (1.6-3.2 years), 718 patients in the blinatumomab-plus-chemotherapy group had a 3-year DFS of 96.0 ± 1.2%, compared with 87.9 ± 2.1% of the 722 patients in the chemotherapy-only group, researchers reported at the American Society of Hematology (ASH) 2024 Annual Meeting.
“Our results demonstrate that blinatumomab added to chemotherapy represents a new treatment standard for most patients with NCI [National Cancer Institute] standard-risk [B-ALL],” said first author Rachel E. Rau, MD, Seattle Children’s Hospital, University of Washington, during a news briefing.
As Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute, noted in a news briefing: “B-cell ALL is the most common childhood cancer and one of the most treatable. However, some children still relapse following standard chemotherapy treatments and then have a much grimmer outcome.”
The AALL1731 study was initiated in 2019 with a recruitment goal of 2245 participants. The patients were over age 1 and less than 10 years, with an initial white blood cell count of < 50,000/μL and were considered to be standard risk–high or standard risk–average.
The control group received standard-intensity chemotherapy (standard risk–average patients) or augmented Berlin-Frankfurt-Münster–based chemotherapy (standard risk–high patients). In addition, the blinatumomab groups received two cycles of the drug.
Randomization was terminated in 2024 at 1440 patients because of the positive results. Patients had a median age of 4.3 years (2.8-6.4), 52.6% were boys, 26% were Hispanic, and 5% were non-Hispanic Black.
The addition of blinatumomab improved DFS by 61% (hazard ratio, 0.39; 95% CI, 0.24-0.64; P < .0001).
In the group of standard risk–average patients, 3-year DFS was 97.5±1.3% in the blinatumomab group vs 90.2±2.3% in the control group (HR, 0.33; 95% CI, 0.15-0.69). For standard risk–high patients, 3-year DFS was 94.1 ± 2.5% and 84.8 ± 3.8%, respectively.
Six deaths occurred in remission, all in standard risk–high patients and none during blinatumomab cycles. Out of first courses of blinatumomab, 0.3% were associated with Grade 3 or higher cytokine release syndrome and 0.7% with seizures.
“We did note higher rates of subsequent sepsis and catheter-related infections in our standard risk–average patients who received blinatumomab,” Rau said.
“The improvement in disease survival was secondary to significant reduction in bone marrow relapse,” Rau added. “We did not see a similar reduction in the more rare event of an isolated central nervous system relapse. This finding was not surprising given blinatumomab’s known limited activity in the central nervous system.”
Rau noted that there are two challenges in terms of access to blinatumomab: its cost, at about $225,000 per a 2023 report, and its administration. The drug is administered via 4-week-long infusions. “The delivery method is very cumbersome,” she said.
“These are big problems that are going to take the combined efforts of pediatric oncologist cancer consortia and pharmaceutical industry partners as well as government agencies,” she said. Fortunately, she said, in June 2024 the Food and Drug Administration approved blinatumomab for adult and pediatric patients 1 month and older with CD19-positive Philadelphia chromosome–negative B-ALL in the consolidation phase of multiphase chemotherapy.
“So it’s relatively easy, at least, to prescribe blinatumomab in the United States for our patients that we feel would benefit from it,” she said.
As for method of delivery, Rau said easier-to-deliver formulations are in development.
Rau has disclosed spousal employment (AbbVie), serving on advisory boards (Servier, Jazz), consulting, and receiving honoraria (Jazz). Other study authors report various disclosures including ties with Amgen, the maker of blinatumomab. Dunbar has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASH 2024
Varicella Outbreaks: 2022-2024
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.
As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.

As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.

As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.